Abstract
Control of visceral leishmaniasis (VL) caused by Leishmania donovani requires interferon-γ production by CD4+ T cells. In VL patients, antiparasitic CD4+ T-cell responses are ineffective for unknown reasons. In this study, we measured the expression of genes associated with various immune functions in these cells from VL patients and compared them to CD4+ T cells from the same patients after drug treatment and from endemic controls. We found reduced GATA3, RORC, and FOXP3 gene expression in CD4+ T cells of VL patients, associated with reduced Th2, Th17, and FOXP3+CD4+ T regulatory cell frequencies in VL patient blood. Interleukin 2 (IL-2) was an important upstream regulator of CD4+ T cells from VL patients, and functional studies demonstrated the therapeutic potential of IL-2 for improving antiparasitic immunity. Together, these results provide new insights into the characteristics of CD4+ T cells from VL patients that can be used to improve antiparasitic immune responses.
Keywords: visceral leishmaniasis, Leishmania donovani, CD4+ T cells, IL-2
Transcriptional changes in CD4+ T cells during visceral leishmaniasis caused by Leishmania donovani infection were examined, and interleukin 2 (IL-2) was identified as an important upstream regulator of these cells. Functional studies demonstrated the therapeutic potential of IL-2 for improving antiparasitic immunity.
CD4+ T cells play key roles in immunity against many different pathogens [1]. Intracellular protozoan parasites such as Leishmania donovani are controlled by interferon (IFN)–γ+TBET+CD4+ T cells (T-helper [Th] 1 cells) [2]. The proinflammatory cytokines produced by Th1 cells help phagocytes kill captured or resident parasites by directly stimulating antimicrobial pathways [3, 4]. However, if infection persists, a juxtaposing pattern of inflammation and immune regulation can be established, resulting in CD4+ T-cell dysfunction and associated disease [5, 6].
Interleukin 2 (IL-2) is an important cytokine that influences T-cell behavior [7]. It is required for the survival, proliferation, and differentiation of CD4+ T cells, CD8+ T cells, and natural killer (NK) cells [8], by binding to either the high-affinity trimeric IL-2 receptor (IL-2R), made up of IL-2Rα (CD25), IL-2Rβ (CD122), and IL-2Rγ (CD132), or the dimeric IL-2R (comprising β and γ chains) [9]. The trimeric IL-2R receptor is highly expressed on activated CD4+ T cells and Foxp3+CD4+ T regulatory (Treg) cells, while memory CD8+ T cells and NK cells express high levels of the dimeric IL-2R [7].
Leishmania donovani–infected mice receiving IL-2-blocking monoclonal antibodies (mAbs) failed to control hepatic parasite growth, associated with impaired granuloma development, whereas infected mice treated with exogenous IL-2 had reduced liver parasite burdens and increased granuloma development, relative to controls [10]. Similarly, intranodular injection of recombinant IL-2 in patients with disseminated cutaneous leishmaniasis reduced parasite numbers, associated with CD4+ T-cell infiltration [11]. However, the therapeutic application of IL-2 has had limitations because of its short half-life [12] and adverse side effects [13].
Combining recombinant IL-2 with certain IL-2–reactive mAbs can preserve IL-2 signaling capacity and enhance cytokine half-life in vivo [14]. In addition, different IL-2 mAbs expose different IL-2R binding sites when bound to recombinant IL-2 [15]. For example, injecting IL-2 conjugated to the JES6.1A12 anti–IL-2 mAb (IL-2Jc) into mice led to selective stimulation and expansion of CD25+ T cells, but not CD25– T cells [15].
In this study, we measured the expression of a defined set of genes in peripheral blood CD4+ T cells from patients with visceral leishmaniasis (VL) infected with L. donovani on presentation to clinic and 30 days after drug treatment, as well as in the same cells from endemic controls (ECs). We identified IL-2 as a major upstream signaling molecule in CD4+ T cells from VL patients with active disease, and examined whether this cytokine signaling pathway could be manipulated for therapeutic advantage.
MATERIALS AND METHODS
Sample Collection
All patients presented with symptoms of VL at the Kala-Azar Medical Research Centre, Muzaffarpur, Bihar, India, for diagnosis and treatment. Diagnosis was performed by detection of anti-rK39 antibodies in the serum and/or amastigotes in splenic biopsies. Patients were treated with a single dose of AmBisome (10 mg/kg) administered intravenously. Blood was collected on admission to hospital and 30 days after drug treatment. The study was approved by the Institute of Medical Sciences, Banaras Hindu University Ethics Committee, and all subjects provided written informed consent. Clinical data for all subjects enrolled in the study are presented in Supplementary Table 1. Heparinized venous blood was collected from patients (n = 82) and endemic healthy subjects (n = 68). All patients were human immunodeficiency virus negative and >12 years of age.
Isolation of CD4+ T-Cell RNA, Quality Control, and Gene Expression Analysis
CD4+ T cells were isolated from peripheral blood mononuclear cells (PBMCs), RNA isolated, quality controlled, and subjected to gene expression analysis on the NanoString gene expression platform (NanoString Technologies) using a code set consisting of a gene panel related to T-cell biology, activation, differentiation, and regulation, as previously described [16] (Supplementary Table 2). Gene expression data were normalized for each sample prior to differential gene expression analysis [16]. In brief, after quality control assessment and background subtraction, count values were normalized using the geometric mean of housekeeping genes (ACTB, B2M, GAPDH, HPRT1, and RPLP0). Comparisons between 2 groups were made using a paired t test for day 0 and day 30 comparisons, or an unpaired t test for day 0 or day 30 comparisons to ECs. P values were adjusted for multiple testing using the Benjamini–Hochberg method where the false discovery rate (FDR) (Q value; P value FDR) considered significant was Q ≤ .05.
Real-Time Polymerase Chain Reaction
Real-time quantitative polymerase chain reaction was performed using TaqMan-based chemistries with 6-carboxyfluorescein (FAM) XXX (MGB)–labeled primer/probes to measure messenger RNA expression, while VIC-MGB–labeled 18S ribosomal RNA was used as an endogenous control, as previously described [17].
Antibodies
Fluorescently conjugated antibodies against CD4 (RPA-T4), CD3ε (UCHT1), FOXP3 (236A/E7), GATA3 (L50-823), RORγt (Q21-559), TBET (4B10), CTLA4 (BNI3), CD96 (6F9), CCR6 (11A9), CCR4 (1G1), CD38 (HIT2), CD40L (89-76), and IFN-γR1 (GIR-208) (BD Biosciences), as well as Aqua Zombie viable dye (Biolegend), were used for flow cytometry studies on human samples.
Fluorescence-Activated Cell Sorting Analysis of CD4+ T Cells
PBMCs were stained for cell surface and intracellular markers as previously described. Cells were analyzed using CellQuest Pro (BD Biosciences) and FlowJo software (Tree Star). Gates were set using fluorescence minus one controls. Analysis was performed by gating on CD3ε+CD4+ T cells and then measuring the molecule of interest.
Ex Vivo Whole Blood Assay
Recombinant human IL-2 (1 μg/mL) (R&D Systems) or vehicle (phosphate-buffered saline) was added to whole blood assays [17], as indicated in the figure legends. IFN-γ and interleukin 10 (IL-10) levels in cell culture supernatants were measured by enzyme-linked immunosorbent assay [18].
L. donovani Infections of C57BL/6 Mice
Leishmania donovani (LV9; MHOM/ET/67/HU3) [19] amastigotes (2 × 107) were injected intravenously into experimental mice. Hepatic parasite burdens were expressed in Leishman–Donovan units, as previously described [20]. Splenic parasite burden was determined by limiting dilution assay [18].
Mice
Female C57BL/6 mice were purchased from the Animal Resource Centre (Canning Vale, Australia). B6.Foxp3.DTR mice [21] were bred and at QIMR Berghofer. Mice were age- and sex-matched and maintained under pathogen-free conditions. All procedures were conducted with approval of the QIMR Animal Ethics Committee (A02-634M), and in accordance with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes (National Health and Medical Research Council, Canberra, Australia).
Administration of IL-2/Anti–IL-2 Complexes to Mice
Antimouse IL-2 (JES6-1A12) was purified from cell culture supernatant by protein G column chromatography (Amersham), followed by endotoxin removal (Mustang Membranes, Pall Life Sciences). Recombinant murine IL-2, 1.5 µg (eBioscience), was incubated with 50 µg of JES6-1A12 mAb in saline for 30 minutes at 37°C, as previously reported [15], prior to intraperitoneal administration in a volume of 200 µL on days 14 and 21 postinfection (p.i.).
CD4+ T-Cell Depletion in Mice
CD4+ T cells were depleted as described previously [22]. Foxp3+ Treg cells in B6.Foxp3.DTR animals were depleted via intraperitoneal administration of diphtheria toxin [23]. Efficiency of cell depletion was >95% for CD4+ T cells and Treg cells, as previously reported [22, 23].
Statistical Analysis
Statistical analysis was performed using GraphPad Prism 6 (GraphPad Software). Analysis of human cellular assays was performed using Wilcoxon matched-pairs signed-rank test or nonparametric Mann–Whitney tests, as appropriate. Analysis of mouse data used Mann–Whitney tests for comparisons between 2 groups, and a 1-way analysis of variance to assess >2 groups within an experiment. P < .05 was considered significant.
RESULTS
A CD4+ T-Cell Gene Signature From VL Patients
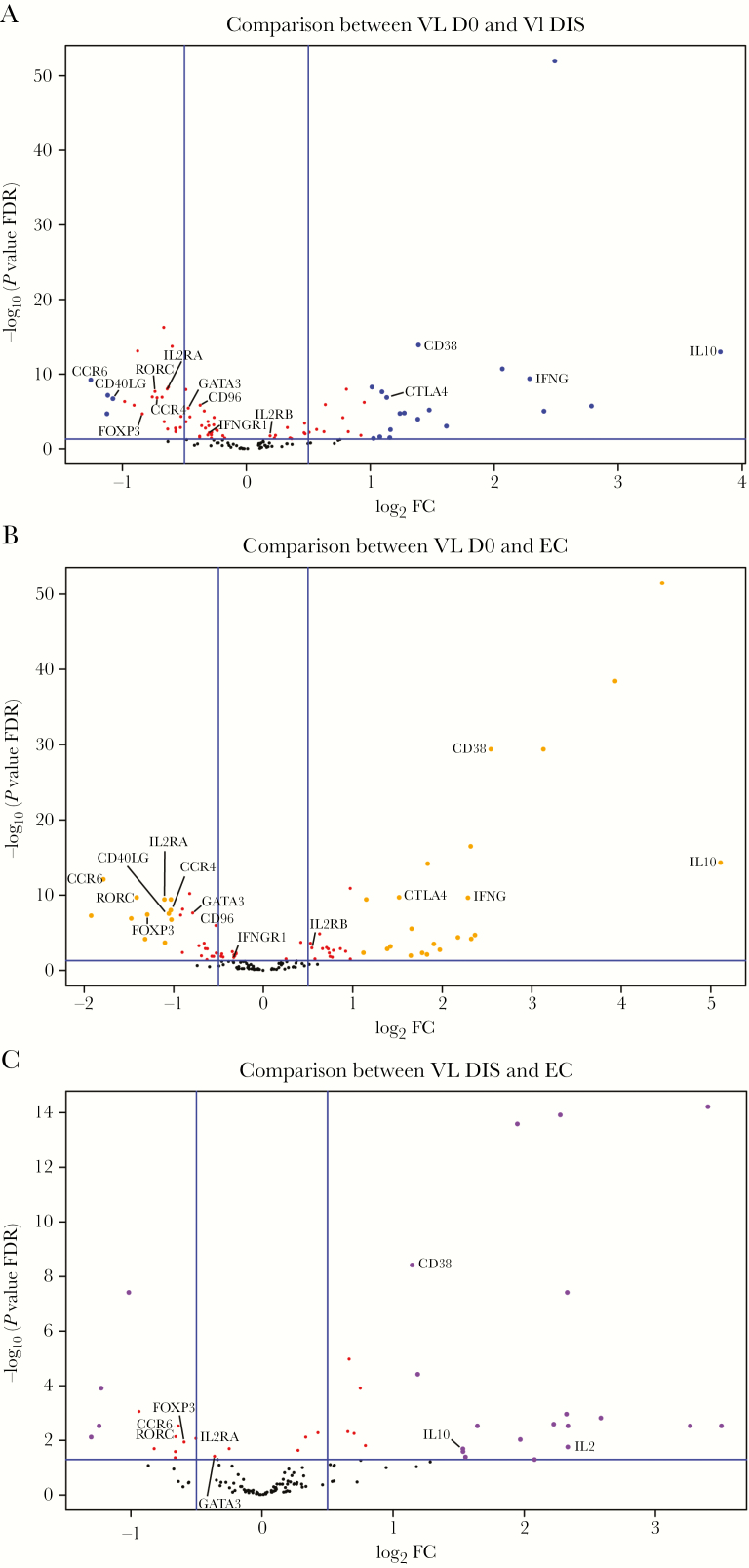
Peripheral blood CD4+ T cells were isolated from VL patients prior to antiparasitic drug treatment (day 0), from the same patients 30 days after commencement of drug treatment at hospital discharge (day 30), and from healthy ECs. RNA was isolated, and targeted transcriptional profiling of 130 genes associated with T-cell activation, differentiation, effector functions, and immune regulation was performed as previously described [16]. Interestingly, we found more transcriptionally down-regulated than up-regulated genes when we compared CD4+ T cells from VL patients before drug treatment with time of discharge (Figure 1A), similar numbers of down- and up-regulated genes when compared with CD4+ T cells from ECs (Figure 1B), and more transcriptionally up-regulated than down-regulated genes when we compared CD4+ T cells from VL patients at time of discharge with ECs (Figure 1C; Supplementary Figure 1).
Figure 1.
Nanostring analysis of immune-related genes in CD4+ T cells reveals more up- than down-regulated genes. The volcano plots show the distribution of immune-related genes in comparisons between visceral leishmaniasis (VL; n = 27) patients during infection (D0) and VL patients after treatment (DIS) (A), D0 and endemic controls (ECs; n = 15) (B), and DIS and ECs (C). Genes were determined to be up-regulated or down-regulated in the condition listed first in the title above each panel. Vertical lines in panels indicate absolute log2 fold-change (FC) = 0.5, and the horizontal lines indicate -log10 (P value false discovery rate [FDR]) = 1.3 (ie, FDR < 0.05). Genes of interest that were found to be differentially expressed (-log10 [FDR > 1.3], ie, FDR < 0.05) are indicated.
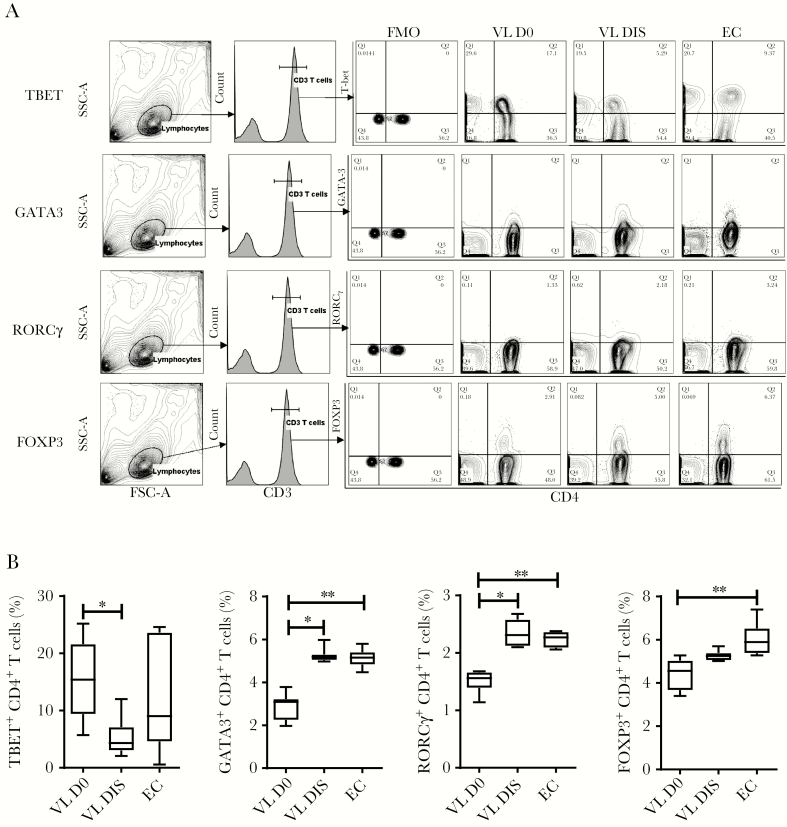
Genes encoding several important CD4+ T-cell lineage–defining transcription factors had decreased expression in CD4+ T cells from VL patients, compared with cells from the same patients after drug treatment and from ECs. These differentially expressed genes (DEGs) included GATA3, RORC, and FOXP3, associated with Th2, Th17, and Treg cells, respectively. Consistent with transcription data, we found reduced frequencies of GATA3+, RORCγ+ (encoded by RORC), and FOXP3+CD4+ T cells in the peripheral blood of VL patients, compared with CD4+ T cells from the same patients after drug treatment and from ECs, although the decrease in FOXP3+CD4+ T cells did not always reach statistical significance (Figure 2A and 2B). Interestingly, despite no significant differences in CD4+ T-cell TBX21 gene (encoding TBET) expression between groups, we found significantly higher frequencies of TBET+CD4+ T cells in the peripheral blood of VL patients, compared to the same patients after drug treatment, but no difference compared to ECs (Figure 2A and 2B). These data show that Indian VL patients have reduced peripheral blood Th2, Th17, and Treg cell frequencies, and a decreased Th1 cell frequency in VL patients following drug treatment.
Figure 2.
Changes in peripheral blood CD4+ T-cell subsets. The fluorescence-activated cell sorting plots show the gating strategy used to identify TBET+, GATA3+, RORCγ+, and FOXP3+ CD4+ T cells (A), and the frequency of these subsets among CD4+ T cells between visceral leishmaniasis (VL) patients during infection (D0; n = 7), VL patients after treatment (n = 7), and endemic controls (n = 6), median + minimum and maximum (B). *P < .05, **P < .01, Wilcoxon matched-pairs signed-rank test. Abbreviations: DIS, visceral leishmaniasis patients after treatment; D0, day zero; EC, endemic control; FMO, fluorescence minus one; FSC, forward scatter; SSC-A, side scatter-A; VL, visceral leishmaniasis.
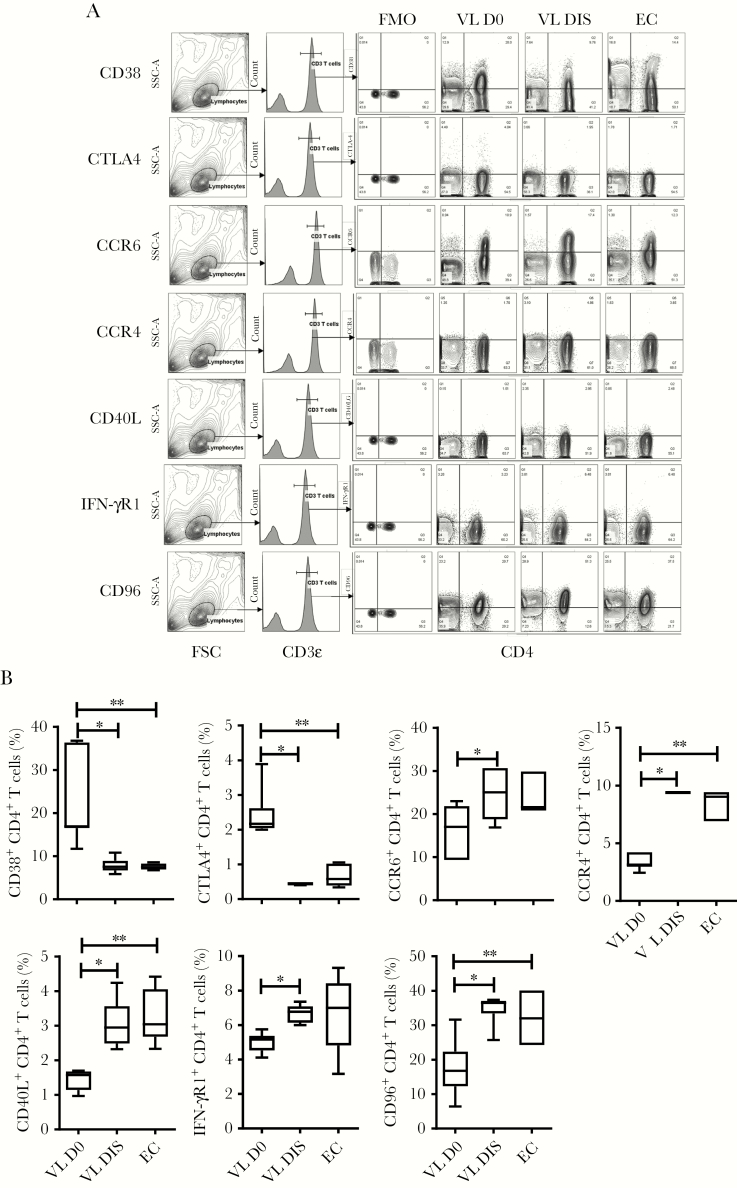
Consistent with DEG data, we found an increased frequency of CD4+ T cells from VL patients expressing the immunoregulatory molecules CD38 and CTLA4, compared to CD4+ T cells from the same patients 30 days after drug treatment and ECs (Figure 3A and 3B). In contrast, but again consistent with DEG data, we found a decreased frequency of CD4+ T cells from VL patients expressing the chemokine receptors CCR4 and CCR6, as well as the important cell signaling molecules CD40LG, IFNGR1, and CD96, compared to control groups (Figure 3A and 3B). Thus, CD4+ T cells from VL patients had DEGs encoding a broad range of immunoregulatory and inflammatory molecules.
Figure 3.
Changes in immune molecule expression by CD4+ T cells. The fluorescence-activated cell sorting plots show the gating strategy used to identify CD38+, CTLA4+, CCR6+, CCR4+, CD40L+, IFN-γR1+, and CD96+ CD4+ T cells (A), and the frequency of these subsets among CD4+ T cells between visceral leishmaniasis (VL) patients during infection (D0; n = 6), VL patients after treatment (n = 6), and endemic controls (n = 6), median + minimum and maximum (B). *P < .05, **P < .01, Wilcoxon matched-pairs signed-rank test. Abbreviations: DIS, visceral leishmaniasis patients after treatment; D0, day zero; EC, endemic control; FMO, fluorescence minus one; FSC, forward scatter; IFN, interferon; SSC-A, side scatter-A; VL, visceral leishmaniasis.
IL-2 Is a Major Upstream Regulator of CD4+ T Cells From VL Patients
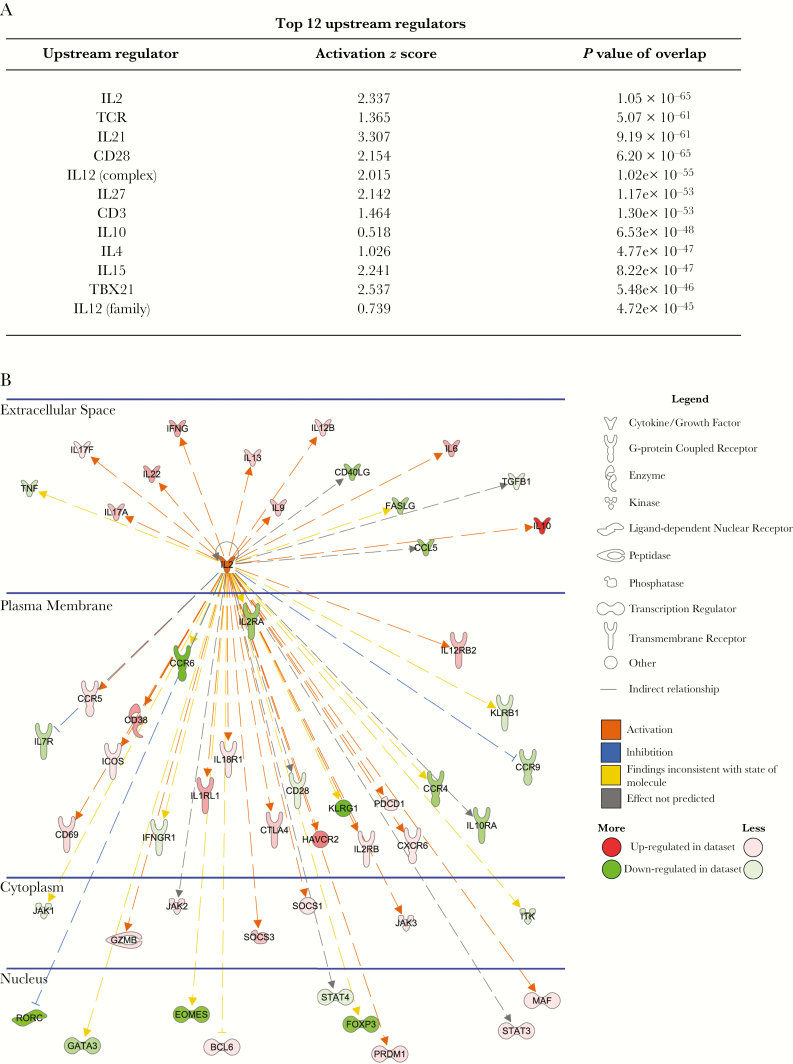
We next compared DEGs in CD4+ T cells from VL patients before drug treatment and from ECs using ingenuity pathways analysis. The top upstream regulator of genes in VL patients’ CD4+ T cells, based on statistical significance, was IL-2 (Figure 4A). IL-2 was predicted to regulate genes with various immune functions located in the plasma membrane, cytoplasm, and nucleus, as well as secreted CD4+ T-cell products (Figure 4B). Therefore, we next investigated the therapeutic potential of IL-2 for treating VL.
Figure 4.
Interleukin 2 (IL-2) is the top upstream regulator of immune-related genes in visceral leishmaniasis patients. The table lists the top 12 upstream regulators (sorted by P value of overlap) that were identified by ingenuity pathway analysis (A). The network shows all up-regulated (red) and down-regulated (green) genes within the dataset that were predicted to be regulated by IL-2, and illustrates the predicted relationship between IL-2 and these genes (B).
Targeting the High-Affinity IL-2R to Enhance Antiparasitic Immunity During Established Experimental VL
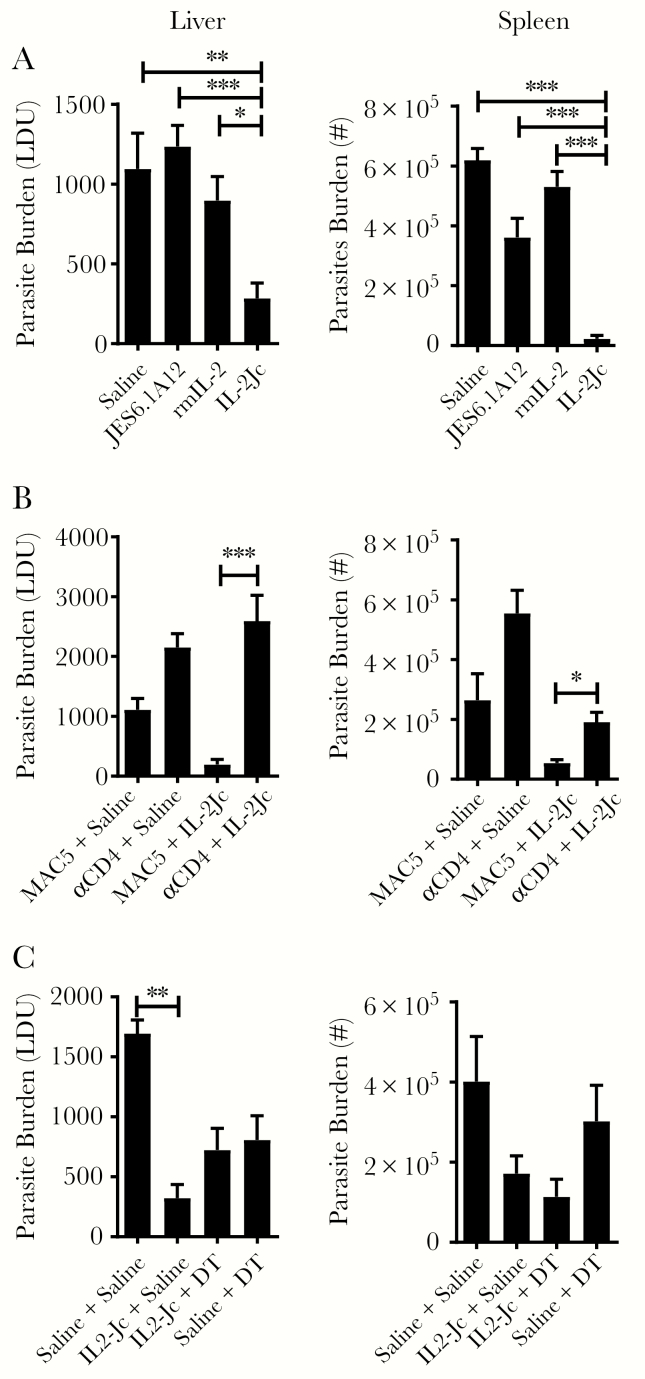
First, we used a preclinical model of VL caused by infection of C57BL/6 with L. donovani. Previous work identified combinations of recombinant IL-2 and anti–IL-2 mAbs that could selectively target high- or low-affinity IL-2 receptors [15]. We chose to target the high-affinity IL-2R during established infection using recombinant IL-2 complexed with the JES6.1A12 anti–IL-2 mAb (IL-2Jc) because activated CD4+ T cells express the high affinity IL-2R [7] and play a critical role in the control of parasite growth in this model [22, 24]. Infected mice were treated on days 14 and 21 p.i., and the impact on parasite burden was assessed at day 28 p.i. Animals treated with IL-2Jc had significantly lower liver and spleen parasite burdens, compared with control mice treated with vehicle (saline), JES6.1A12 anti–IL-2 mAb alone, or recombinant IL-2 alone (Figure 5A). However, no changes in NK, CD4+ T-cell or CD8+ T-cell numbers, or their ability to produce IFN-γ, in the liver and spleen at the time of parasite burden measurement (day 28 p.i.) was found (data not shown), suggesting a potent but transient effect of IL-2Jc administration.
Figure 5.
Mouse interleukin 2 (IL-2)/antibody complexes stimulate CD4+ T-cell–dependent antiparasitic immunity in mice infected with Leishmania donovani. C57BL/6 mice were infected with parasites for 14 days and then administered saline, anti–IL-2 monoclonal antibody (mAb) (JES6.1A12), recombinant mouse IL-2 (rmIL-2), or anti–IL-2 mAb complexed with rmIL-2 (IL-2Jc) on days 14 and 21 postinfection (p.i.), as indicated, and parasite burdens in the liver and spleen were measured 14 days later (day 28 p.i.) (A). Leishmania donovani–infected mice receiving saline or IL-2Jc were treated with control mAb (MAC5) or anti-CD4 mAb every 3 days, as indicated, over the same time period, and parasite burdens (expressed in Leishman–Donovan units [LDU]) in the liver and spleen were measured at day 28 p.i. (B). Leishmania donovani–infected B6.Foxp3.DTR mice receiving saline or IL-2Jc were treated with saline or diphtheria toxin (DT) every 3 days, as indicated, over the same time period, and parasite burdens in the liver and spleen were measured at day 28 p.i. (C). All values are mean ± standard error of the mean of at least 2 independent experiments, n = 5 mice per group. *P < .05, **P < .01, ***P < .001; significance assessed by 1-way analysis of variance.
To establish whether CD4+ T cells were needed for the therapeutic effects of IL-2Jc, we depleted this cell population during IL-2Jc treatment, and found that the positive therapeutic response was lost (Figure 5B). Although this result does not show that CD4+ T cells were a direct target for IL-2Jc treatment, it indicates that CD4+ T cells were critical for the antiparasitic effect of IL-2Jc in this preclinical model of VL (Figure 5B). Treg cells also express high levels of the high-affinity IL-2R and can be an important cell target for IL-2/antibody complexes [7, 15]. Therefore, we next treated L. donovani–infected B6.Foxp3.DTR mice with diphtheria toxin to deplete Treg cells during IL-2Jc treatment to test whether they influenced treatment outcome. However, we found no significant impact of Treg cell depletion on the antiparasitic effects of IL-2Jc treatment, compared with controls (Figure 5C). Together, these data establish the therapeutic potential of targeting the high-affinity IL-2R in experimental VL, and identify conventional CD4+ T cells as critical for the antiparasitic effects of this treatment.
IL-2 Improves Antiparasitic Proinflammatory, but Not Regulatory Responses, in VL Patient Blood Cells.
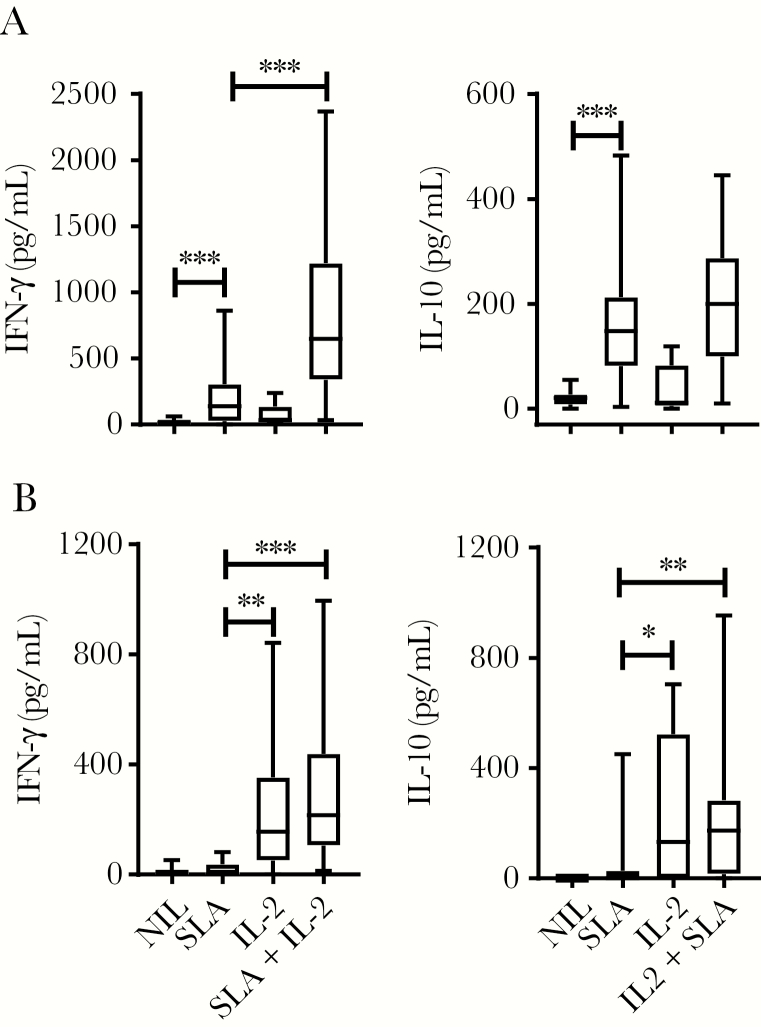
We next tested used recombinant human IL-2 in an ex vivo antigen-stimulated whole blood assay [17], rationalizing that the relatively short-term nature of these assays (<24 hours) would not require the extended half-life of cytokine activity provided by the cytokine complexed with mAb. When blood from VL patients was stimulated with parasite antigen, we found a significant increase in IFN-γ and IL-10 production, compared with blood cultured without antigen (Figure 6A), as previously reported [18]. The addition of IL-2 to blood cultures resulted in a significant increase in antigen-specific IFN-γ, but not IL-10 production (Figure 6A), indicating a selective effect on IFN-γ production. In contrast, addition of IL-2 alone to EC blood cells increased IFN-γ and IL-10 production, independent of antigen stimulation (Figure 6B), possibly reflecting the autocrine growth promoting properties of IL-2 and the effect of the cytokine on circulating effector CD4+ T cells. Thus, these data show that IL-2 can be used to selectively promote antigen-specific IFN-γ production by blood cells from VL patients, thereby providing support for targeting the IL-2 signaling pathway for therapeutic advantage in this disease.
Figure 6.
Human interleukin 2 (IL-2) stimulates antigen-specific interferon gamma (IFN-γ), but not interleukin 10 (IL-10) production by whole blood cells from patients with visceral leishmaniasis (VL). Antigen-specific IFN-γ and IL-10 production was measured in whole blood cells from VL patients on admission to clinic (n = 8–26) (A) and from endemic controls (n = 10–24) (B) cultured for 24 hours with media alone (NIL), soluble leishmania antigen (SLA), recombinant human IL-2, or SLA + IL-2, as indicated. Median + minimum and maximum, *P < .05, **P < .01, ***P < .001; significance assessed by Wilcoxon matched-pairs signed-rank test.
DISCUSSION
We identified a molecular signature associated with peripheral blood CD4+ T cells from VL patients, including reduced transcription of GATA3, RORC, and FOXP3 genes, associated with reduced frequencies of blood Th2, Th17, and Treg cells, respectively. We also found that blood TBET+CD4+ (Th1) cell frequency was reduced in VL patients 30 days after they commenced antiparasitic drug treatment, despite no differences in CD4+ T-cell TBX21 gene expression. Elevated levels of cytokines associated with Th1 and Th2 cell responses have been reported in VL patients [25–28], as well as in splenic transcripts encoding these cytokines in a hamster model of VL [29]. However, the human studies measured either plasma cytokine levels or focused on IL-10 as the main Th2 cytokine, which we now know is produced by Th1 cells in both humans and mice [28, 30, 31]. Thus, Th2 cell frequency in VL patient blood may have been overestimated, although we cannot exclude the possibility they accumulate in infected tissues. Consistent with our finding of reduced frequencies of Th17 cells in VL patients, a report from Sudan found that Th17 cell induction was strongly associated with protection against VL [32]. Hence, reduced Th17 cell frequencies in Indian VL patients may contribute to disease development. Studies on Treg cells in VL patients have been inconsistent [33–35], and this is in part attributed to different markers used to define these cells. Here, we relied only on FOXP3 expression to identify Treg cells, and further studies using a more definitive range of human Treg cell markers are needed to establish their role in VL patients.
We also found increased expression of immune checkpoint molecule genes in VL patients’ CD4+ T cells, including LAG3, CD38, and CTLA4, all of which can influence CD4+ T-cell behavior [36]. In addition, we found decreased expression of CCR4 and CCR6, which promote migration of CD4+ T cells to the skin and liver, respectively [37, 38]. Given that parasites reside in both tissues, impaired CD4+ T-cell migration caused by their down-regulation may contribute to establishment and/or persistence of infection in these sites. Furthermore, decreased expression of CD40L by VL patients’ CD4+ T cells is likely to impact their ability to help dendritic cells, B cells, and other CD40-expressing immune cells perform their functions [39]. Similarly, decreased expression of IFN-γR1 by VL patients’ CD4+ T cells may impact the maintenance of Th1 cells during infection, and contribute to the altered composition of CD4+ T-cell subsets, as well as expression of IFN-regulated genes and associated immune functions [40]. However, increased expression of proinflammatory molecules such as IFN-γ was found, suggesting that VL patients’ blood comprises a heterogeneous population of CD4+ T cells with different functions, possibly reflecting genetic diversity among patients and ECs, and different histories of pathogen exposure.
IL-2 was identified as a major regulator of VL patients’ CD4+ T-cell genes, and given our desire to target CD4+ T cells, we decided to target the trimeric, high-affinity IL-2R expressed by activated CD4+ T cells in mice. In C57BL/6 mice, the liver is a site of acute, resolving infection where highly effective antiparasitic CD4+ T cells develop, whereas the spleen is a site of chronic infection characterized by dysfunctional CD4+ T-cell responses [41]. Previous studies demonstrated the utility of IL-2Jc in treating inflammation and autoimmune disease in mice [42, 43]. Our results showed that IL-2Jc can also be used for therapeutic advantage in an experimental model of VL with antiparasitic effects in both liver and spleen. As expected, the antiparasitic effect of IL-2Jc was dependent on CD4+ T cells. Despite the potential for IL-2Jc to expand Treg cells [15], we found no impact of this regulatory T-cell subset in IL-2Jc–treated animals, suggesting that conventional, activated CD4+ T cells were the main target of this treatment. It will be useful in future to investigate the potential for adjunctive therapy with conventional antiparasitic drugs, as this is the likely way such a treatment would be used for patients with VL.
Previous studies using whole blood transcriptional profiling found that IL-2 activation and signaling pathways were down-regulated in VL patients infected with Leishmania infantum [44], suggesting that these pathways may be differentially regulated in different lymphocyte subsets. An earlier study also showed that the addition of recombinant human IL-2 to Indian VL patients’ PBMCs stimulated with parasite antigen did not rescue proliferative responses [45]. Although cell proliferation was not measured in our studies, we showed that exogenous IL-2 enhanced parasite-specific IFN-γ production by VL patients’ blood cells. Remarkably, there was limited antigen-specific effect on IL-10 production, possibly reflecting differential expression of the trimeric IL-2R on IFN-γ– and IL-10–producing CD4+ T-cell populations. The impact of IL-2 on the expression of immunoregulatory/exhaustion markers should be investigated in future studies. Nevertheless, these results indicate that selective improvement of IFN-γ production by CD4+ T cells can be achieved in VL patients without accompanying IL-10 production and associated suppressive effects on antiparasitic immunity [18, 30]. There have been significant advancements in engineering IL-2 for extended half-life and targeting IL-2R expressed by specific immune cell subsets [46]. Future studies should test the therapeutic potential of these next-generation IL-2 molecules in VL.
In summary, we identified a distinct pattern of gene expression in CD4+ T cells from patients with VL. No clear association with any specific CD4+ T-cell subset was recognized, although we found a transcriptional signature consistent with our finding of reduced Th2, Th17, and Treg cell frequencies in the blood of VL patients after drug treatment. IL-2 was identified as a major upstream regulator of CD4+ T cells from VL patients and the therapeutic potential of this cytokine was shown. Future studies should test recently developed IL-2 molecules with favorable therapeutic properties that allow safe targeting of specific CD4+ T-cell subsets with antiparasitic activity.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgments. We thank the staff at the Kala-Azar Medical Research Centre, Muzaffarpur, Bihar, India, for help in collecting blood samples, as well as patients and volunteers for allowing the use of blood samples. We also thank the QIMR Berghofer Animal Facility for their help. Shashi Chauhan was supported by a Junior Research Fellowship from the Indian Council of Medical Research.
Financial support. This work was supported by the National Institutes of Health Tropical Medicine Research Center (grant number U19 AI074321) and the Queensland State Government. This research was also supported by grants and fellowships from the National Health and Medical Research Council of Australia; Australian Postgraduate Awards through the Queensland University of Technology, Institute of Health and Biomedical Innovation and Griffith University, School of Natural Sciences; and an INSPIRE Fellowship to Rajiv Kumar provided by the Indian government’s Department of Science and Technology.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Zhu J, Yamane H, Paul WE. Differentiation of effector CD4 T cell populations (*). Annu Rev Immunol 2010; 28:445–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rosas LE, Snider HM, Barbi J, et al. . Cutting edge: STAT1 and T-bet play distinct roles in determining outcome of visceral leishmaniasis caused by Leishmania donovani. J Immunol 2006; 177:22–5. [DOI] [PubMed] [Google Scholar]
- 3. Janeway CA Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol 2002; 20:197–216. [DOI] [PubMed] [Google Scholar]
- 4. Tubo NJ, Jenkins MK. CD4+ T cells: guardians of the phagosome. Clin Microbiol Rev 2014; 27:200–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sakaguchi S, Yamaguchi T, Nomura T, Ono M. Regulatory T cells and immune tolerance. Cell 2008; 133:775–87. [DOI] [PubMed] [Google Scholar]
- 6. Engwerda CR, Ng SS, Bunn PT. The regulation of CD4(+) T cell responses during protozoan infections. Front Immunol 2014; 5:498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Spolski R, Li P, Leonard WJ. Biology and regulation of IL-2: from molecular mechanisms to human therapy. Nat Rev Immunol 2018; 18:648–59. [DOI] [PubMed] [Google Scholar]
- 8. Smith KA. Interleukin-2: inception, impact, and implications. Science 1988; 240:1169–76. [DOI] [PubMed] [Google Scholar]
- 9. Smith KA. The interleukin 2 receptor. Adv Immunol 1988; 42:165–79. [DOI] [PubMed] [Google Scholar]
- 10. Murray HW, Miralles GD, Stoeckle MY, McDermott DF. Role and effect of IL-2 in experimental visceral leishmaniasis. J Immunol 1993; 151:929–38. [PubMed] [Google Scholar]
- 11. Akuffo H, Kaplan G, Kiessling R, et al. . Administration of recombinant interleukin-2 reduces the local parasite load of patients with disseminated cutaneous leishmaniasis. J Infect Dis 1990; 161:775–80. [DOI] [PubMed] [Google Scholar]
- 12. Lotze MT, Matory YL, Ettinghausen SE, et al. . In vivo administration of purified human interleukin 2. II. Half life, immunologic effects, and expansion of peripheral lymphoid cells in vivo with recombinant IL 2. J Immunol 1985; 135:2865–75. [PubMed] [Google Scholar]
- 13. McDermott DF, Atkins MB. Application of IL-2 and other cytokines in renal cancer. Expert Opin Biol Ther 2004; 4:455–68. [DOI] [PubMed] [Google Scholar]
- 14. Létourneau S, van Leeuwen EM, Krieg C, et al. . IL-2/anti-IL-2 antibody complexes show strong biological activity by avoiding interaction with IL-2 receptor alpha subunit CD25. Proc Natl Acad Sci U S A 2010; 107:2171–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science 2006; 311:1924–7. [DOI] [PubMed] [Google Scholar]
- 16. Edwards CL, Ng SS, Corvino D, et al. . Early changes in CD4+ T-cell activation during blood-stage Plasmodium falciparum infection. J Infect Dis 2018; 218:1119–29. [DOI] [PubMed] [Google Scholar]
- 17. Ansari NA, Kumar R, Gautam S, et al. . IL-27 and IL-21 are associated with T cell IL-10 responses in human visceral leishmaniasis. J Immunol 2011; 186:3977–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kumar R, Singh N, Gautam S, et al. . Leishmania specific CD4 T cells release IFNγ that limits parasite replication in patients with visceral leishmaniasis. PLoS Negl Trop Dis 2014; 8:e3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bradley DJ, Kirkley J. Regulation of Leishmania populations within the host. I. the variable course of Leishmania donovani infections in mice. Clin Exp Immunol 1977; 30:119–29. [PMC free article] [PubMed] [Google Scholar]
- 20. Stanley AC, Dalton JE, Rossotti SH, et al. . VCAM-1 and VLA-4 modulate dendritic cell IL-12p40 production in experimental visceral leishmaniasis. PLoS Pathog 2008; 4:e1000158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lahl K, Loddenkemper C, Drouin C, et al. . Selective depletion of Foxp3+ regulatory T cells induces a scurfy-like disease. J Exp Med 2007; 204:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bunn PT, Stanley AC, de Labastida Rivera F, et al. . Tissue requirements for establishing long-term CD4+ T cell-mediated immunity following Leishmania donovani infection. J Immunol 2014; 192:3709–18. [DOI] [PubMed] [Google Scholar]
- 23. Haque A, Best SE, Amante FH, et al. . CD4+ natural regulatory T cells prevent experimental cerebral malaria via CTLA-4 when expanded in vivo. PLoS Pathog 2010; 6:e1001221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Murray HW, Hariprashad J, Fichtl RE. Models of relapse of experimental visceral leishmaniasis. J Infect Dis 1996; 173:1041–3. [DOI] [PubMed] [Google Scholar]
- 25. Karp CL, el-Safi SH, Wynn TA, et al. . In vivo cytokine profiles in patients with kala-azar. Marked elevation of both interleukin-10 and interferon-gamma. J Clin Invest 1993; 91:1644–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ghalib HW, Piuvezam MR, Skeiky YA, et al. . Interleukin 10 production correlates with pathology in human Leishmania donovani infections. J Clin Invest 1993; 92:324–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Holaday BJ, Pompeu MM, Jeronimo S, et al. . Potential role for interleukin-10 in the immunosuppression associated with kala azar. J Clin Invest 1993; 92:2626–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nylén S, Maurya R, Eidsmo L, Manandhar KD, Sundar S, Sacks D. Splenic accumulation of IL-10 mRNA in T cells distinct from CD4+CD25+ (Foxp3) regulatory T cells in human visceral leishmaniasis. J Exp Med 2007; 204:805–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Medina-Colorado AA, Osorio EY, Saldarriaga OA, et al. . Splenic CD4+ T cells in progressive visceral leishmaniasis show a mixed effector-regulatory phenotype and impair macrophage effector function through inhibitory receptor expression. PLoS One 2017; 12:e0169496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Montes de Oca M, Kumar R, de Labastida Rivera F, et al. . Blimp-1-dependent IL-10 production by Tr1 cells regulates TNF-mediated tissue pathology. PLoS Pathog 2016; 12:e1005398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Stäger S, Maroof A, Zubairi S, Sanos SL, Kopf M, Kaye PM. Distinct roles for IL-6 and IL-12p40 in mediating protection against Leishmania donovani and the expansion of IL-10+ CD4+ T cells. Eur J Immunol 2006; 36:1764–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Pitta MG, Romano A, Cabantous S, et al. . IL-17 and IL-22 are associated with protection against human kala azar caused by Leishmania donovani. J Clin Invest 2009; 119:2379–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Bhattacharya P, Ghosh S, Ejazi SA, et al. . Induction of IL-10 and TGFβ from CD4+CD25+FoxP3+ T cells correlates with parasite load in Indian Kala-azar patients infected with Leishmania donovani. PLoS Negl Trop Dis 2016; 10:e0004422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Rai AK, Thakur CP, Singh A, et al. . Regulatory T cells suppress T cell activation at the pathologic site of human visceral leishmaniasis. PLoS One 2012; 7:e31551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Saha S, Mondal S, Ravindran R, et al. . IL-10- and TGF-beta-mediated susceptibility in kala-azar and post-kala-azar dermal leishmaniasis: the significance of amphotericin B in the control of Leishmania donovani infection in India. J Immunol 2007; 179:5592–603. [DOI] [PubMed] [Google Scholar]
- 36. Kumar R, Chauhan SB, Ng SS, Sundar S, Engwerda CR. Immune checkpoint targets for host-directed therapy to prevent and treat leishmaniasis. Front Immunol 2017; 8:1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Acosta-Rodriguez EV, Rivino L, Geginat J, et al. . Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol 2007; 8:639–46. [DOI] [PubMed] [Google Scholar]
- 38. Campbell JJ, Haraldsen G, Pan J, et al. . The chemokine receptor CCR4 in vascular recognition by cutaneous but not intestinal memory T cells. Nature 1999; 400:776–80. [DOI] [PubMed] [Google Scholar]
- 39. Elgueta R, Benson MJ, de Vries VC, Wasiuk A, Guo Y, Noelle RJ. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol Rev 2009; 229:152–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Castro F, Cardoso AP, Gonçalves RM, Serre K, Oliveira MJ. Interferon-gamma at the crossroads of tumor immune surveillance or evasion. Front Immunol 2018; 9:847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Engwerda CR, Kaye PM. Organ-specific immune responses associated with infectious disease. Immunol Today 2000; 21:73–8. [DOI] [PubMed] [Google Scholar]
- 42. Lee SY, Cho ML, Oh HJ, et al. . Interleukin-2/anti-interleukin-2 monoclonal antibody immune complex suppresses collagen-induced arthritis in mice by fortifying interleukin-2/STAT5 signalling pathways. Immunology 2012; 137: 305–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Webster KE, Walters S, Kohler RE, et al. . In vivo expansion of T reg cells with IL-2-mAb complexes: induction of resistance to EAE and long-term acceptance of islet allografts without immunosuppression. J Exp Med 2009; 206: 751–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gardinassi LG, Garcia GR, Costa CH, Costa Silva V, de Miranda Santos IK. blood transcriptional profiling reveals immunological signatures of distinct states of infection of humans with Leishmania infantum. PLoS Negl Trop Dis 2016; 10:e0005123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sacks DL, Lal SL, Shrivastava SN, Blackwell J, Neva FA. An analysis of T cell responsiveness in Indian kala-azar. J Immunol 1987; 138:908–13. [PubMed] [Google Scholar]
- 46. Mitra S, Ring AM, Amarnath S, et al. . Interleukin-2 activity can be fine tuned with engineered receptor signaling clamps. Immunity 2015; 42:826–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.