Abstract
Background.
Certain headache characteristics and associated symptoms are commonly attributed to increased intracranial pressure, but they have not been systematically studied among children in the context of revised diagnostic criteria for pseudotumor cerebri syndrome (PTCS).
Methods.
We performed a retrospective cohort study of patients treated for suspected or confirmed PTCS. Charts were reviewed for PTCS and headache diagnostic criteria and associated characteristics. Chi-squared or Fisher’s exact tests were used to compare the frequency of headache characteristics between groups.
Results.
One hundred and twenty-seven individuals were identified: 61 had definite PTCS, 10 had probable PTCS, 31 had elevated opening pressure (OP) without papilledema, and 25 had normal OP without papilledema. Eleven children had no headache (6 with definite PTCS, 5 with probable PTCS). Headache pattern was episodic in 49% (95% CI: 34–64%) of those with definite PTCS, 18% (95% CI 6–37%) of those with elevated OP without papilledema, and 16% (5–36%) of those with normal OP without papilledema. Headache location was more likely to involve the head along with neck or shoulders in those with definite PTCS compared with elevated OP without papilledema (OR = 7.2, 95% CI: 1.9–27.6) and normal OP (OR = 4.5, 95% CI: 1.3–15.6) groups.
Discussion.
While missing data and small cohort size are limitations, this study suggests that headache in PTCS is more likely to involve the head along with neck/shoulders, and that headache in PTCS may be episodic or constant. Headache is occasionally absent in PTCS.
Keywords: pseudotumor cerebri, headache, migraine, opening pressure, idiopathic intracranial hypertension, children
INTRODUCTION
Headache is the most common symptom of the pseudotumor cerebri syndrome (PTCS), occurring in 63–98% of both pediatric1–7 and adult8 patients. Headaches attributed to increased intracranial pressure are classically thought to be constant and/or daily, worsened with recumbence, on awakening, and Valsalva maneuvers, and associated with visual symptoms and pulsatile tinnitus.9 Revised diagnostic criteria for adults and children with PTCS were recently published.10 In contrast to prior criteria which required elevated intracranial pressure in the presence of related signs (ie papilledema) or symptoms (ie headache or visual changes),11–13 both papilledema and elevated cerebrospinal fluid (CSF) pressure are now required for the diagnosis of definite PTCS. Patients with papilledema or sixth nerve palsy but with normal opening pressure14 may be given a diagnosis of probable PTCS.10 However, the criteria do not confer a diagnosis of PTCS to patients with headache and increased CSF pressure who do not have papilledema or other objective signs. These patients are thought not to be at risk for vision loss given the absence of papilledema,15,16 but whether they more closely resemble PTCS or non-PTCS headache disorders in their clinical features and treatment responsiveness is not known.
During the initial evaluation of a patient with headaches, especially if papilledema is either absent or difficult to ascertain, clinicians may use the presence or absence of certain headache characteristics to guide the decision of whether to obtain a lumbar puncture for the measurement of CSF opening pressure. However, there are limitations to the use of headache characteristics as a screening or diagnostic tool in these patients. One case series found the triad of daily headache, diffuse/non-pulsating pain, and aggravation by Valsalva in only 36.6% of patients with PTCS.17 While headache was indeed constant or daily in 51% of the Idiopathic Intracranial Hypertension Treatment Trial population at the time of enrollment,18 about 40% of adults19 and 35% of children20 seen in subspecialty headache clinics also have chronic daily headaches. In early studies of adults with PTCS, postural aggravating factors were rare,8 and the discriminative value of associated visual and auditory symptoms is confounded by the high prevalence of comorbid primary headache disorders (specifically migraine, which can independently produce similar symptoms) in these patients.7,16
While pediatric case series have reported on the presence of headache, few have examined features of the headache. None of these studies have addressed the question of whether the headaches in children with elevated OP without papilledema are more similar to those with papilledema or to controls with normal OP. In this retrospective cohort study, we compare the clinical and headache-specific characteristics of children with definite PTCS, probable PTCS, elevated OP without papilledema, and normal OP without papilledema.
METHODS
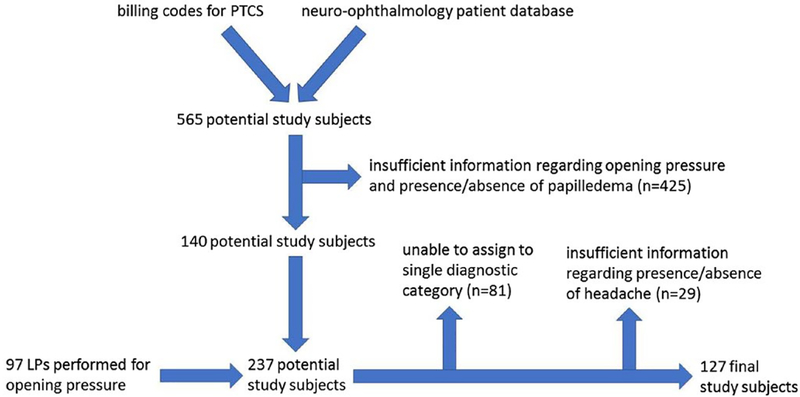
This is a retrospective cohort study of patients treated at the Children’s Hospital of Philadelphia for suspected or confirmed PTCS. No a priori power calculation was conducted to guide sample size. Children who were given a diagnosis of PTCS between July 1, 1993 and April 16, 2013 were identified using ICD-9 billing codes and a neuro-ophthalmology patient database (GTL). This yielded 565 potential study subjects. These charts were reviewed as previously described,21 and those who had sufficient information regarding the presence or absence of papilledema and opening pressure were identified (n = 140). In addition, to expand the comparison groups of subjects with elevated and normal opening pressure, procedure notes for any outpatient lumbar puncture performed in the departments of neurology or interventional radiology between January 1, 2009 and April 20, 2015 were reviewed, and those subjects were included if the primary indication for lumbar puncture was measurement of opening pressure (OP). This yielded an additional 97 subjects. In total, 237 subjects were identified for inclusion in the study. The study was approved by the local institutional review board; because the study involved retrospective chart review, informed consent was not required.
Once subjects were identified, their inpatient and outpatient medical records were reviewed. The case report form that was utilized to collect data was modeled after that used during the Idiopathic Intracranial Hypertension Treatment Trial.22 Using recently revised diagnostic criteria,10 subjects were classified as having one of the following: definite PTCS, probable PTCS, elevated OP without papilledema, and normal OP without papilledema. Definite PTCS was diagnosed if a subject had documented papilledema confirmed by a neuro-ophthalmologist (GTL), a normal neurologic exam except for cranial nerve VI palsy, normal brain MRI, normal CSF constituents, and an elevated lumbar puncture opening pressure (which was defined as ≥28 cm if the patient was obese or sedated for the procedure and ≥25 cm if non-obese and non-sedated).14 Those with opening pressure exceeding the manometer’s measurement capability were recorded as having OP at the manometer’s maximum measurement, usually 37 cm. Per the revised diagnostic criteria, normal venous imaging was also required for the diagnosis of PTCS in non-obese and male subjects. Probable PTCS was diagnosed if a subject had normal opening pressure but otherwise met all other diagnostic criteria for definite PTCS such as the presence of papilledema confirmed by a neuro-ophthalmologist. Elevated OP without papilledema was diagnosed if a subject had opening pressure above the reference range with normal CSF constituents but no papilledema on funduscopic examination by either a general neurologist or neuro-ophthalmologist. Those with papilledema suspected by another clinician but not confirmed by a neuro-ophthalmologist were excluded due to the high rate of misdiagnosis demonstrated in a prior study.23 None of the subjects in the elevated OP without papilledema group had other objective findings of PTCS, such as cranial nerve VI palsy, or MRI findings of PTCS (3 of 4 of empty sella, flattening of posterior aspect of globe, distention of perioptic subarachnoid space, transverse sinus stenosis). Normal opening pressure without papilledema was diagnosed when a subject had CSF pressure within the reference range and did not have papilledema on funduscopic examination by either a general neurologist or neuro-ophthalmologist.
Lumbar punctures were usually performed by a supervised neurology or pediatrics resident in the outpatient neurology clinic or sedation unit, in the emergency department, or on the inpatient neurology floor. Some of the lumbar punctures were performed by interventional radiology, usually if a prior attempt was unsuccessful. Lumbar punctures for opening pressure at our institution are standardly performed in the lateral decubitus position, and the opening pressure measurement is usually obtained with the legs extended. However, since 2010 when a small cohort study at our institution found that patient position resulted in only a small magnitude difference in opening pressure,24 flexed position may have been used in some cases. While the presence or absence of sedation was not directly recorded in our database, that information had been obtained during chart review in order to classify an opening pressure as elevated or not. In cases where subjects had more than one lumbar puncture, the results of the first lumbar puncture were used for diagnostic classification.
Patient records were further reviewed to determine if a patient experienced headaches. If headache was present around the time of diagnosis, additional information regarding headache quality, severity, location, frequency, chronicity, and associated symptoms was abstracted. Additional information regarding headache features commonly attributed to intracranial hypertension, such as worsening with recumbence or on awakening and pulsatile tinnitus, was also collected when documented.
Statistical Analyses.
Statistical analysis was performed using STATA version 14 (College Station, TX). For each clinical characteristic, an overall comparison was first performed to determine whether there were any differences among groups, then exploratory pairwise comparisons were performed. Overall group comparisons were considered significant when P < .01, which was determined by adjusting the standard P-value of .05 for multiple comparisons using the Bonferroni method. For group comparisons that were significant at that level, pair-wise comparisons were considered significant at the level of 0.0125. Fisher’s exact tests were used to compare the proportion of subjects in different PTCS groups with different headache features where the count for at least one group was ≤5. Chi-squared tests were used in pairwise comparisons among definite PTCS, elevated OP, and normal OP groups where counts for all groups were >5. Odds ratios (OR) were calculated by 2 × 2 contingency tables. The Kruskal–Wallis and Mann–Whitney tests were used to compare the medians of continuous variables. Any variable with >20% missing data was dropped. As a result, planned comparisons of diagnosis of migraine vs tension-type headache, positional exacerbation, effect of lumbar puncture, presence of pulsatile tinnitus, pain quality, laterality, effects of Valsalva maneuvers and activity, and associated photophobia and phonophobia were not included in the results.
RESULTS
Of the 237 subjects initially identified for inclusion in the study, 81 did not meet criteria for one of the specified diagnostic categories. Of the remaining 156 subjects, 29 had insufficient information regarding the presence or absence of headaches to be included in the study. Of the excluded 29 subjects with insufficient information, age and proportion female were similar to that of the final 127 subjects. However, whereas 57% of the cohort with sufficient information had seen neurology, only 4 (14%) of those with insufficient information had seen neurology. See Figure 1.
Fig. 1.—
Flow diagram of study subjects.
A final sample size of 127 subjects was used in the analysis. Of these 127 subjects, the median age was 13.6 years (25–75%ile: 10.2–16), and 64.6% (95% CI: 56–73%) were female. Self-reported race was identified as African American for 19.7%, Caucasian for 72.4%, Asian for 1.6% and not identified in the medical record for 6.3%. Sixty-one subjects were classified as having definite PTCS, 10 subjects had probable PTCS, 31 subjects had elevated OP without papilledema, and 25 had normal OP without papilledema. Table 1 lists the basic clinical and demographic characteristics of the study population by diagnostic category. A secondary cause for PTCS was found in 12 cases of definite PTCS (eight with tetracycline use, two with renal failure, one with lithium use, and one with corticosteroid withdrawal). The remainder had primary PTCS, or idiopathic intracranial hypertension (IIH) (n = 49). Subjects with definite PTCS had significantly higher opening pressure compared to those with elevated OP without papilledema (P = .007). Both of these groups had significantly higher BMI Z-score than those with normal OP without papilledema (P = .006 and P = .004, respectively). The median BMI Z-score of the probable PTCS group was similar in magnitude to that of the definite PTCS group but was not statistically significantly different from the other groups. Subjects without headache showed no significant difference in age, gender, or BMI Z-score when compared to subjects with definite or probable PTCS with headache.
Table 1.—
Demographic Characteristics of Patients by PTCS Classification
| Definite PTCS (n = 61) |
Probable PTCS (n = 10) |
Elevated OP without Papilledema (n = 31) |
Normal OP (reference) (n = 25) |
P-value | |
|---|---|---|---|---|---|
| Age in years (median, IQR) | 13.6(10.5–16.3) | 11.5(8.4–13.9) | 14.7 (10.6–16.2) | 14.2(11.0–15.4) | .37 |
| Female | 41 (67.2%) | 6 (60%) | 19 (61.3%) | 16 (64%) | .91 |
| Race | .17 | ||||
| Asian | 0 | 0 | 1 | 1 | |
| African American | 15 | 0 | 7 | 3 | |
| Caucasian | 39 | 10 | 23 | 20 | |
| Not Specified | 7 | 0 | 0 | 1 | |
| Opening pressure in cm CSF (median, IQR)* | 39 (35–49) | 24 (21–26)* | 35 (31–37) | 23 (18–25)* | Overall P < .001; Definite PTCS vs Probable, Elevated OP, & Normal OP, P < .001; Elevated OP vs Normal OP P < .001 |
| BMI Z-score (median, IQR) | 1.6(0.8–2.1) | 1.6 (0.7–1.8) | 1.2(0.8–1.7) | 0.5 (−0.1 – 1.2) | Overall P = .017; Definite PTCS vs Normal OP, P = .006; Elevated OP vs Normal OP, P = .004 |
P-values are from Fisher’s exact test to compare frequencies and Kruskal–Wallis or Mann–Whitney test to compare medians of continuous variables.
Three subjects in the Probable PTCS group and 5 in the Normal OP group had opening pressure between 25 and 28 cm but were classified as Normal OP because the subject was either sedated and/or obese.
Eleven subjects did not have headache (6 with definite PTCS and 5 with probable PTCS). The absence of headache was significantly more common in probable PTCS compared to definite PTCS (odds ratio [OR] = 9.2, 95% confidence interval [CI]: 2–41). Headache pattern was episodic in 49% (95% CI: 34–64%) of those with definite PTCS, 18% (95% CI: 6–37%) of those with elevated OP without papilledema (P = .008 compared to Definite), and 16% (5–36%) of those with normal OP without papilledema (P = .006, compared to Definite).
Specific headache features and associated symptoms were then considered. There was no significant difference in pain severity nor presence of nausea between groups. Headache location was more likely to involve the head along with neck or shoulders in those with definite PTCS compared with elevated OP without papilledema (OR = 7.2, 95% CI: 1.9–27.6) and normal OP (OR = 4.5, 95% CI: 1.3–15.6) groups. The presence of subjective vision changes (including both vision loss and positive visual phenomena) was not significantly different between groups. See Table 2 for full details.
Table 2.—
Frequency of Headache Characteristics and Associated Symptoms by PTCS Classification
| Definite PTCS (n = 61 total, 55 with headache) |
Probable PTCS (n=10 total, 5 with headache) |
Elevated OP without Papilledema (n = 31) |
Normal OP (reference) (n = 25) |
P-value | |
|---|---|---|---|---|---|
| Headache Characteristics | |||||
| Pattern, Constant/Daily | 21 | 0 | 14 | 17 | Overall P = .001; Definite PTCS vs Elevated OP, P = .005; Definite PTCS vs Normal OP, P = .015 |
| Episodic+ | 24 | 2 | 5 | 4 | |
| Variable− | 4 | 2 | 9 | 4 | |
| Not Specified | 6 | 1 | 3 | 0 | |
| Severity, Mild | 2 | 0 | 0 | 0 | .73 |
| Moderate | 12 | 2 | 9 | 10 | |
| Severe | 26 | 2 | 18 | 13 | |
| Not Specified | 15 | 1 | 4 | 2 | |
| Location Focal | 17 | 2 | 16 | 14 | Overall P = .013; Definite PTCS vs Elevated OP, P = .003 |
| All Over Head− | 5 | 0 | 9 | 7 | |
| Head + Neck/ Shoulder+ | 19 | 2 | 3 | 4 | |
| Not Specified | 14 | 1 | 3 | 0 | |
| Other Symptoms (includes subject without headache) | |||||
| Vision Present | 47 | 5 | 16 | 14 | .05 |
| Change Absent | 13 | 5 | 12 | 11 | |
| Not Specified | 1 | 0 | 3 | 0 | |
| Nausea Present | 39 | 3 | 20 | 14 | .213 |
| Absent | 22 | 7 | 10 | 9 | |
| Not Specified | 0 | 0 | 1 | 2 | |
P-values are from Fisher’s exact test to compare frequencies.
Considered as present for binary comparisons specified in text.
Considered as absent for binary comparisons specified in text.
DISCUSSION
In this retrospective cohort study, we report the headache characteristics of a large series of children evaluated for suspected PTCS, including those with definite and probable PTCS, elevated OP without papilledema, and normal OP. Ten percent (95% CI: 4–20%) of those with definite PTCS and 50% (95% CI: 19–81%) of those with probable PTCS did not report headache. The higher portion of children with absence of headache in the probable PTCS group (with normal opening pressure) contrasts with prior studies where children with PTCS without headache had similarly elevated opening pressures compared to those with headache.4,6,25 Some of these differences could be due to the use of different diagnostic criteria for PTCS. For example, when papilledema was required for the diagnosis of PTCS, the reported prevalence of headache was lower1–3 than when it was not required.4–7 While intracranial pressure has not clearly correlated with headache severity in both experimental and observational studies of adults,22,26 the fact that the absence of headache in PTCS was more frequently encountered in the setting of normal rather than elevated opening pressure in our study suggests that intracranial hyper-tension plays at least a partial role in the pathogenesis of headache in PTCS. Alternatively, the absence of both headache and intracranial hypertension in a subset of the probable PTCS group is similar to other studies,27 and raises questions regarding the diagnostic accuracy of papilledema vs pseudopapilledema in these patients. Subtle MRI findings may aid in making this distinction,28 though given that this is a rare occurrence, further studies are needed.
In tertiary headache centers, as many as 10–15% of patients with chronic migraine may have elevated opening pressure without papilledema.16,29 The precise threshold CSF pressure required for papilledema to develop is unclear and likely to depend on individual patient characteristics. In patients with intracranial hypertension without papilledema, it may be that their papilledema threshold lies higher than their measured CSF opening pressure.15 Indeed, among adult patients with opening pressure above the upper limit of normal, those with papilledema have been found to have higher opening pressures than those without papilledema,30 and the data here confirmed this finding in a pediatric population. Because a small cup-to-disk ratio is known to be a risk factor for PTCS, some have suggested that patients with intracranial hypertension without papilledema are more likely to have a larger cup-to-disk ratio, which may protect against the development of papilledema.31 The absence of papilledema is reported with similar frequency in children as in adults. However, in contrast to adults, the degree of opening pressure elevation does not appear to be associated with the presence of papilledema in children.7
Children with PTCS had similar frequencies of episodic and chronic headache patterns, consistent with prior studies2 but unlike studies of adults, where a constant or daily pattern is much more common.32 Many children without PTCS had constant or daily headaches. This reflects the fact that the reference group was limited to patients who underwent lumbar puncture and were found to have normal opening pressure; specifically, clinicians may be more likely to consider the diagnosis of PTCS and order lumbar puncture in children with constant or daily (but not episodic) headaches. The reference group is therefore not representative of the general pediatric headache population. Neck and shoulder pain was also more common in PTCS, possibly due to distension of spinal root dural sheaths under increased pressure.
A major limitation of our study was missing data, as this was a retrospective study where the data regarding headache characteristics were limited to those collected as part of routine clinical care. Furthermore, the amount of missing data was non-random. Patients with definite and probable PTCS were more likely to be lacking data on headache characteristics, which reflects that they were more likely to be seen only in neuro-ophthalmology clinic, where less detailed information about headache and more information about visual symptoms is collected. Consequently, prospectively collected studies are needed to characterize headache in PTCS more thoroughly. Another potential source of bias was the classification of normal or elevated opening pressure based on a single lumbar puncture, as prior studies have shown that opening pressure can be normal on an initial lumbar puncture and then elevated on subsequent evaluations3,5 or during continuous CSF pressure monitoring.32–34 Furthermore, while the presence of papilledema in subjects with definite and probable PTCS was confirmed by a neuro-ophthalmologist, the absence of papilledema in subjects with elevated or normal OP without papilledema was sometimes determined by a general neurologist and was not always confirmed by a neuro-ophthalmologist. Thus, it is possible that some of these patients may have actually had definite or probable PTCS, though this is relatively unlikely as studies have shown that the misdiagnosis of PTCS is typically caused by false positives (diagnosing papilledema in its absence) rather than false negatives (failing to diagnose papilledema in its presence)23. Finally, since normal venous imaging was required only for atypical patients, ie, those who were male or not obese, it is possible that female, obese patients could have been misclassified as PTCS when in fact the symptoms of increased ICP were due to a sinus venous thrombosis.
In summary, in this single-center retrospective cohort study, headache in children with PTCS was more likely to involve the neck and shoulders but less likely to be constant or daily compared to those without PTCS. When headache was absent in PTCS, opening pressure was more likely to be normal than abnormal. Prospective studies are needed to further characterize headache burden in PTCS and limit the effects of recall bias.
CLINICAL IMPLICATIONS.
Pseudotumor cerebri syndrome (PTCS) is frequently accompanied by headache in both children and adults. PTCS also carries a risk of vision loss, thus it is important to distinguish children with PTCS and non-PTCS headache disorders.
Common headache patterns in children with PTCS include both episodic and constant/daily, and common locations include focal pain and head/neck/shoulders.
Financial disclosure:
Grant Liu was a former consultant for Ipsen. Christina Szperka has grant support from Pfizer and Amgen. The remaining authors have no financial relationships relevant to this article to disclose.
Funding source: Dr. McCormack is supported by NIH grant, K12 DK094723–03/K23 DK102658.
Abbreviations:
- PTCS
pseudotumor cerebri syndrome
- OP
opening pressure
- CSF
cerebrospinal fluid
- IIH
idiopathic intracranial hypertension
- OR
odds ratio
- CI
confidence interval
REFERENCES
- 1.Ravid S, Shahar E, Schif A, et al. Visual outcome and recurrence rate in children with idiopathic intracranial hypertension. J Child Neurol. 2015;30:1448–1452. [DOI] [PubMed] [Google Scholar]
- 2.Per H, Canpolat M, Gumus H, et al. Clinical spectrum of the pseudotumor cerebri in children: Etiological, clinical features, treatment and prognosis. Brain Dev. 2013;35:561–568. [DOI] [PubMed] [Google Scholar]
- 3.Distelmaier F, Sengler U, Messing-Juenger M, et al. Pseudotumor cerebri as an important differential diagnosis of papilledema in children. Brain Dev. 2006;28:190–195. [DOI] [PubMed] [Google Scholar]
- 4.Lim M, Kurian M, Penn A, et al. Visual failure without headache in idiopathic intracranial hypertension. Arch Dis Child. 2005;90:206–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aylward SC, Waslo CS, Au JN, et al. Manifestations of pediatric intracranial hypertension from the intracranial hypertension registry. Pediatr Neurol. 2016;61:76–82. [DOI] [PubMed] [Google Scholar]
- 6.Aylward SC, Aronowitz C, Reem R, et al. Intracranial hypertension without headache in children. J Child Neurol. 2015;30:703–706. [DOI] [PubMed] [Google Scholar]
- 7.Aylward SC, Aronowitz C, Roach ES. Intracranial hypertension without papilledema in children. J Child Neurol. 2016;31:177–183. [DOI] [PubMed] [Google Scholar]
- 8.Wall M. The headache profile of idiopathic intracranial hypertension. Cephalalgia. 1990;10:331–335. [DOI] [PubMed] [Google Scholar]
- 9.Mallery RM, Friedman DI, Liu GT. Headache and the pseudotumor cerebri syndrome. Curr Pain Headache Rep. 2014;18:446. [DOI] [PubMed] [Google Scholar]
- 10.Friedman DI, Liu GT, Digre KB. Revised diagnostic criteria for the pseudotumor cerebri syndrome in adults and children. Neurology. 2013;81:1159–1165. [DOI] [PubMed] [Google Scholar]
- 11.Rangwala LM, Liu GT. Pediatric idiopathic intracranial hypertension. Surv Ophthalmol. 2007;52:597–617. [DOI] [PubMed] [Google Scholar]
- 12.Friedman DI, Jacobson DM. Diagnostic criteria for idiopathic intracranial hypertension. Neurology. 2002;59:1492–1495. [DOI] [PubMed] [Google Scholar]
- 13.Smith JL. Whence pseudotumor cerebri? J Clin Neuroophthalmol. 1985;5:55–56. [PubMed] [Google Scholar]
- 14.Avery RA, Shah SS, Licht DJ, et al. Reference range for cerebrospinal fluid opening pressure in children. N Engl J Med. 2010;363:891–893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marcelis J, Silberstein SD. Idiopathic intracranial hypertension without papilledema. Arch Neurol. 1991;48:392–399. [DOI] [PubMed] [Google Scholar]
- 16.Mathew NT, Ravishankar K, Sanin LC. Coexistence of migraine and idiopathic intracranial hypertension without papilledema. Neurology. 1996;46:1226–1230. [DOI] [PubMed] [Google Scholar]
- 17.D’Amico D, Curone M, Farago G, et al. Headache in patients with idiopathic intracranial hypertension: A pilot study to assess applicability of ICHD-2 diagnostic criteria. Neurol Sci. 2012;33(Suppl 1):S189–191. [DOI] [PubMed] [Google Scholar]
- 18.Wall M, Kupersmith MJ, Kieburtz KD, et al. The idiopathic intracranial hypertension treatment trial: Clinical profile at baseline. JAMA Neurol. 2014;71:693–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pascual J, Colas R, Castillo J. Epidemiology of chronic daily headache. Curr Pain Headache Rep. 2001;5:529–536. [DOI] [PubMed] [Google Scholar]
- 20.Hershey AD, Powers SW, Bentti AL, et al. Characterization of chronic daily headaches in children in a multidisciplinary headache center. Neurology. 2001;56: 1032–1037. [DOI] [PubMed] [Google Scholar]
- 21.Paley GL, Sheldon CA, Burrows EK, et al. Overweight and obesity in pediatric secondary pseudotumor cerebri syndrome. Am J Ophthalmol. 2015;159:e341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Friedman DI, Quiros P, Subramanian PS, et al. Headache Outcomes in the Idiopathic Intracranial Hypertension Treatment Trial (IIHTT) In: American Headache Society 59th Annual Scientific Meeting; 2017; Boston, MA: Headache: The Journal of Head & Face Pain; 2017. [Google Scholar]
- 23.Fisayo A, Bruce BB, Newman NJ, et al. Overdiagnosis of idiopathic intracranial hypertension. Neurology. 2016;86:341–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Avery RA, Mistry RD, Shah SS, et al. Patient position during lumbar puncture has no meaningful effect on cerebrospinal fluid opening pressure in children. J Child Neurol. 2010;25:616–619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lessell S Pediatric pseudotumor cerebri (idiopathic intracranial hypertension). Surv Ophthalmol. 1992;37:155–166. [DOI] [PubMed] [Google Scholar]
- 26.Johnston I, Paterson A. Benign intracranial hypertension. II. CSF pressure and circulationBrain. 1974;97:301–312. [DOI] [PubMed] [Google Scholar]
- 27.Tibussek D, Distelmaier F, Karenfort M, et al. Probable pseudotumor cerebri complex in 25 children. Further support of a concept. Eur J Paediatr Neurol. 2016;21:280–285. [DOI] [PubMed] [Google Scholar]
- 28.Digre KB. Imaging characteristics of IIH: Are they reliable? Cephalalgia. 2013;33:1067–1069. [DOI] [PubMed] [Google Scholar]
- 29.Vieira DS, Masruha MR, Goncalves AL, et al. Idiopathic intracranial hypertension with and without papilloedema in a consecutive series of patients with chronic migraine. Cephalalgia. 2008;28:609–613. [DOI] [PubMed] [Google Scholar]
- 30.Digre KB, Nakamoto BK, Warner JE, et al. A comparison of idiopathic intracranial hypertension with and without papilledema. Headache. 2009;49:185–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dai S, Trimboli C, Buncic JR. The optic disc is minimal in children with idiopathic intracranial hypertension. J Child Neurol. 2013;28:1245–1249. [DOI] [PubMed] [Google Scholar]
- 32.Torbey MT, Geocadin RG, Razumovsky AY, et al. Utility of CSF pressure monitoring to identify idiopathic intracranial hypertension without papilledema in patients with chronic daily headache. Cephalalgia. 2004;24:495–502. [DOI] [PubMed] [Google Scholar]
- 33.Friedman DI. Idiopathic intracranial hypertension with Dan and beyond: The 2010 Jacobson Lecture. J Neuroophthalmol. 2010;30:380–385. [DOI] [PubMed] [Google Scholar]
- 34.Bono F, Salvino D, Tallarico T, et al. Abnormal pressure waves in headache sufferers with bilateral transverse sinus stenosis. Cephalalgia. 2010;30:1419–1425. [DOI] [PubMed] [Google Scholar]