Abstract
The toxicity of dietary E 171, a food grade titanium dioxide was evaluated. A recent study reported rats receiving E 171 in water developed inflammation and aberrant crypt foci (ACF) in the gastrointestinal tract, here, rats received food containing E 171 (7 or 100 days). The 100-day study included feeding E 171 after dimethylhydrazine (DMH) or vehicle only pretreatment. Food consumption was similar between treatment groups with maximum total cumulative E 171 exposure being 2617 mg/kg in 7 days and 29,400 mg/kg in 100 days. No differences were observed due to E 171 in the percentage of dendritic, CD4+ T or Treg cells within Peyer’s patches or the periphery, or in cytokine production in plasma, sections of jejunum, and colon in 7- or 100-day E 171 alone fed rats. Differences were observed for IL-17A in colon (400 ppm E 171+DMH) and IL-12p70 in plasma (40 ppm E 171+DMH). E 171 had no effect on histopathologic evaluations of small and large intestines, liver, spleen, lungs, or testes, and no effects on ACF, goblet cell numbers, or colonic gland length. Dietary E 171 administration (7- or 100-day), even at high doses, produced no effect on the immune parameters or tissue morphology.
Keywords: E 171, Dietary titanium dioxide, Gastrointestinal inflammation, Tregulatory cells, Intestinal carcinogenesis
1. Introduction
Additives are routinely incorporated into food products to enhance properties such as texture, taste, and color with potential applications including protection from microbial contamination and oxidation. However, consumer awareness of food additives is currently heightened and as such, there is much interest in the health impacts of such additives. Titanium dioxide (TiO2) is a naturally occurring metal oxide that has been used as a food additive for more than 50 years. It confers a naturally white opaqueness to substances to which it is added due to its high refractive index. In the US, the Food and Drug Administration allows up to 1% by weight of TiO2 as a food additive [1] while in the European Union (EU) there is no established threshold as long as levels are not higher than what is required for the intended purpose (e.g., coloring) [2].
Food grade TiO2 can be in either the rutile or anatase form or a mixture of both and is primarily larger than 100nm in particle diameter [3–5]. Transmission electron microscopy (TEM) of several lots of E 171, the EU designation for food-grade TiO2, revealed an average frequency of 17-35% of particles by number falling below 100nm in diameter (nano-fraction) [4]. More relevantly, TEM analysis of various chewing gum coatings revealed that on average, 4.2% of TiO2 particles were of the nano fraction [6, 7]. TiO2 has been extensively studied for potential hazard to human health, and it is generally accepted that inhalation of high doses of nano-sized TiO2 presents a toxicological risk, especially in an occupational setting [8–13]. In rodent models, orally ingested TiO2 was largely innocuous, even when administered in the diet at levels of 50,000 ppm (5.0% of the diet) to rats and mice for up to two years [14]. Adverse effects were only reported in study animals dosed with high amounts of entirely nano-sized particles [15–23]. However, while it is generally accepted that >99% of orally ingested TiO2 particles are passed in the feces [24–26], it has been shown that TiO2 particles can accumulate in intestinal crypts [27, 28], bind in vitro and in vivo to phagocytic microfold cells (M-cells) [28, 29], and pass through the gut into the lamina propria [27–29]. Further, intestinal dendritic cells can directly internalize TiO2 particles [27, 29–31]. Many of these studies were performed with TiO2 particles of a diameter less than 100nm [32]. It has also been reported that TiO2 particle-uptake can provoke inflammatory responses as TiO2 nano-particles induced inflammasome activation in vitro [23, 33]. It has been proposed that these effects could potentially provoke or exacerbate gut inflammatory disorders such as inflammatory bowel disease [23, 34].
Given the potential for TiO2 particles to accumulate in the gastrointestinal tract and interact with the gut immune system, it is critical to examine the effects of relevant oral TiO2 exposure on gut immune homeostasis. While TiO2 has been studied extensively, many of these studies were not relevant to the human route of exposure, oral dietary ingestion, instead relying on inhalation, direct injection, oral gavage, or administration in drinking water with purified TiO2 particles of various size fractions (See [32] for review). Further, it has been shown that TiO2 particles routinely incorporate surrounding macromolecules onto the surface architecture of their particle structure, and depending on the types of molecules, the physical properties of TiO2 particles, such as absorption, aggregation, and tissue uptake can be drastically altered [35–41]. Food-grade TiO2, E 171, is consumed by humans already in food preparations, allowing for the incorporation of food macromolecules onto the surfaces of E 171 particles. Further, food passes slowly through the digestive system compared to liquids such as water, which is absorbed primarily in the small intestine [42]. Given that TiO2 particles are insoluble in water and tend to aggregate, delivery of E 171 particles in water may not recapitulate the biology of TiO2 particles already incorporated into food products.
In addition to possible immunologic effects, recent publications have raised concerns about a possible carcinogenic effect on the intestines [43–45]. A two-year bioassay of dietary TiO2 to F344 rats and B6C3F1 mice showed no evidence of proliferative or neoplastic effects on the gastrointestinal tract or neoplastic effects in any tissues [14]. Recently, studies have focused on shorter-term evaluations of aberrant crypt foci (ACF) and goblet cells as indicators of possible carcinogenic effects. However, these utilized administration of TiO2 by oral gavage or in drinking water rather than diet, with potential agglomeration of particles occurring, altering their interaction with host tissues [44, 46, 47].
The objectives of the present study were to evaluate the acute (7 days) and long term (100 days) effects of dietary E 171 exposure on the immune system of the gastrointestinal (GI) tract and periphery as well as to evaluate chronic exposure either alone or after pre-administration of a known intestinal genotoxic carcinogen, dimethylhydrazine (DMH). Following the 7- and 100-day feeding periods, rats were euthanized and measurements of inflammatory cytokines and phenotyping of immune cells in the periphery and GI tract were performed. Peyer’s patches, peripheral blood mononuclear cells (PBMC), and spleen cells were analyzed for inflammatory and regulatory T-cell responses directly ex vivo or after in vitro stimulation (7 days). Histopathology, ACF, and goblet cell evaluations were performed on rats in the 100-day study. All tissues were collected from well-defined areas, and measurements, procedures, and evaluations were performed in a standardized and blinded manner. In addition, evaluations of possible proliferative or tumor enhancing effects were performed focusing on histopathology, assessment of ACF (also evaluated in a blinded manner), and quantification of goblet cells in the distal large intestine.
2. Materials and Methods
2.1. Chemicals
Food grade sample E171-E was supplied by the Titanium Dioxide Manufacturers Association based on an assessment and characterization of the different grades of E171 placed on the market. Chemical analysis, including particle size distribution, was performed and diets (control and E171 containing) were analyzed for TiO2 content and homogeneity.. It was stored at room temperature. N, N’-dimethylhydrazine dihydrochloride (DMH · 2HCl) (98% purity) was purchased from Sigma Aldrich (St. Louis, MO) and stored at room temperature.
2.2. E171 Chemical Analysis
Chemical analysis of the particle size and particle size distribution of the respective lot of E171 used in food preparations containing TiO2 were performed by 2 independent contract laboratories (Kronos; VENATOR). Analyses were performed using 2 independent techniques, electron microscopy and direct volume/mass based methods. Consistent with published findings, the specific method used to quantify E171 particle size and distribution yielded noticeably different results [48]. For example, analysis using scanning electron microscopy (SEM) showed 36% of TiO2 particles were <100nm in diameter with the average diameter recorded being between 110-115nm. Volume/mass based approaches showed similar average particle diameter (150nm) but showed only 1-2% or particles falling below 100nm in diameter. In spite of the differences observed between the two analytical methods in the percentage of particles possessing a diameter below 100nm, the results obtained by the two independent laboratories were similar with each of the two methodologies, demonstrating good concordance between the two laboratories.
2.3. E171 Food Preparation
All diets containing E 171 were prepared by Dyets, Inc. (Bethlehem, PA). E 171 was administered in irradiated and pelleted Certified Purina 5002R33 (LabDiet, Richmond, IN) at a concentration of 40, 400 or 5,000 ppm and containing 2% maltose dextrin throughout the 7- and 100-day studies. Two batches of diet were prepared. The first batch of diet was fed throughout the 7-day study and through week 10 of the 100-day study. The second batch of diet was fed during the remainder of the 100-day study. Food and water were provided ad libitum to all animals during the quarantine and acclimation period and the 7- and 100-day exposure periods. The homogeneity and concentration of E171 in the test diets were analyzed by Eurofins Food Chemistry Testing US, Inc. (Madison, WI). Analysis of triplicate samples from top, middle and bottom of the diet preparations confirmed homogeneity (data not presented). The concentrations of E171 in the first and second batches of diets based on the mean of six determinations per diet ± S.D. were found to be, respectively: 0 ppm dose (22.3 ± 1.2, 23.7±1.8 ppm); 40 ppm dose (59.6 ±1.1, 61.0±2.6 ppm); 400 ppm dose (384 ± 8, 387±13 ppm) and the 5000 ppm dose (4310 ± 132, 4610±160 ppm).
2.4. Animals
Six-week-old male Wistar Han IGS (Crl:WI (Han)) rats were purchased from Charles River Laboratories (Raleigh, NC) for all studies. Animals were housed in facilities fully accredited by the American Association for Accreditation of Laboratory Animal Care (AAALAC). The level of care provided to the animals is based on federal regulations including the U.S. Government Principles for the Utilization and Care of Vertebrate Animals Used in Testing, Research and Training, USDA implementing regulations, (9CFR), of the Animal Welfare Act and U.S. Public Health Service Policy Assurance for the Humane Care and Use of Laboratory Animals negotiated with the Office of Laboratory Animal Welfare, OLAW. The 7-day study protocol was approved by the Michigan State University Institutional Animal Care & Use Committee (IACUC) and performed at Michigan State University. The 100-day study protocol was approved by the University of Nebraska Medical Center (UNMC) IACUC and performed at UNMC. Rats were housed individually during replicate 7-day studies and 2/cage during the 100-day study in plastic cages with dry corn-cob bedding containing environmental enrichment.
2.5. Study design
2.5.1. 7-day study
Animals were acclimated for 12 days prior to the start of two replicate feeding studies. At the initiation of each of the replicate studies, rats were randomized into 4 groups of 5 animals each using an in-house weight-based randomization program [49]. Total food and water consumption were determined at the end of each study. Body weights were determined at the start and end of the 7-day exposure period at the time of euthanasia.
2.5.2. 100-day study
Note that all doses are for dimethylhydrazine dihydrochloride not dimethylhydrazine. After acclimation for 7 days, the animals were randomized as described above, into 8 groups of 16 animals each. Animals in groups 1-4 were treated with a single intraperitoneal (i.p.) injection of a sterile dose of 180 mg/kg BW dimethylhydrazine dihydrochloride (DMH · 2HCl) in 1.5% EDTA-0.9% NaCl, pH 6.5. Animals in groups 5-8 were treated with a single sterile dose of 1.5% EDTA-0.9% NaCl, pH 6.5. Seven days after i.p. injection, dietary administration of 0, 40, 400, or 5000 ppm E171, respectively, was initiated in groups 1-4 and 5-8 and continued for 100 days. The dose of DMH · 2HCl was chosen as the highest dose of DMH · 2HCl that could be administered at pH 6.5 with no signs of toxicity based on 3 pilot studies. During the first 2 pilot studies, rats exhibited signs of extreme toxicity including death, when administered i.p. doses of 90, 130, 180 or 398 mg/kg BW DMH · 2HCl (no pH adjustment) which correspond to DMH doses of 41, 59, 81, and 180 mg/kg BW, respectively. In a third pilot study, there was a statistically significant decrease in body weights for rats administered i.p. doses of 287 and 398 mg/kg BW DMH · 2HCl, pH 6.5. A dose of 287 mg/kg BW DMH · 2HCl, pH 6.5, corresponds to a DMH dose of 130 mg/kg BW. There were no signs of toxicity at i.p. doses of 90, 130 and 180 mg/kg BW DMH · 2HCl, pH 6.5. Animals were checked daily for moribundity and mortality, and detailed clinical observations were performed during study weeks 8 and 13. Body weights were determined weekly beginning on day 0 of the study and just prior to euthanasia. Food consumption was determined weekly beginning with administration of the E171 supplemented diets. Water consumption was determined during weeks 3, 8 and 13 of the study.
2.6. Blood and Tissue Collection, Processing and Analysis
At the end of the 7- and 100-day studies, rats were euthanized by administration of a lethal dose (150 mg/kg) of Fatal-Plus® (Vortech Pharmaceuticals, Dearborn, MI) by intraperitoneal injection. All samples were blinded prior to analysis except for samples from four control animals in each study that were used to calibrate the flow cytometry analyses, and samples from three control animals in the 100-day study that were used to establish the normal histopathology for this strain of rat and samples used for goblet cell analysis.
2.6.1. 7-day study
Whole blood was collected by cardiac puncture from euthanized rats using a heparin coated needle and syringe. Red blood cells (RBC) in whole blood were lysed using ACK lysis buffer, washed twice with HBSS and resuspended in RPMI containing 10% FBS (Hyclone, Logan, UT) and 100 U/ml of penicillin and streptomycin (Gibco Life Technologies, Grand Island, NY). Leukocytes were either phenotyped by flow cytometry or PBMCs were isolated by layering the cell suspension onto Lympholyte-Rat (CederLane, Burlington, NC) and centrifuged at 1500 x g for 20 min, washed twice with HBSS and resuspended in RPMI containing 10% FBS (Hyclone, Logan, UT) and 100 U/ml of penicillin and streptomycin (Gibco Life Technologies, Grand Island, NY) and activated using anti-CD3/anti-CD28 antibodies to induce cytokine production ex vivo (see below). Plasma was collected from the remaining whole blood by centrifugation at 500 x g and stored at −80°C for cytokine quantification. A 1-2 cm segment of jejunum, approximately 15 cm from the cecum and a 1-2 cm segment of colon, approximately 10 cm from the anus were harvested from each rat for evaluation of immunologic parameters. The tissues were mechanically disrupted in 1 ml of RIPA buffer (0.5% deoxycholate, 0.1% SDS, and 1% Igepal in TBS) containing complete protease inhibitor cocktail (Roche Diagnostics, Mannheim, Germany) for determination of inflammatory cytokines within the gastrointestinal track. Cytokine levels were normalized based on total protein concentrations as determined by a BCA kit (Sigma, St. Louis, MO) following manufacturers protocol. Peyer’s patches were removed along the jejunum and ileum and leukocytes were isolated for phenotyping and activated using anti-CD3/ CD28 antibodies to induce cytokine production ex vivo (see below). Briefly, isolated jejunum and ileum Peyer’s patches were washed 4 times with HBSS. After the last wash, 2ml of Spleen Dissociation Medium (StemCell Technologies, Cambridge, MA) were added to the harvested Peyer’s patches and incubated at RT for 30 min. After incubation, EDTA was added to a final concentration of 10 mM. Cell suspensions were filtered through a 70 μm nylon mesh strainer and then layered onto Lympholyte-Rat (CederLane, Burlington, NC) and centrifuged at 1500 x g for 20 min. Rat spleens were excised, weighed and cut transversely. One half of the spleen was frozen at −80°C, and the other half was made into a single cell suspension, washed with HBSS and resuspended in RPMI containing 10% FBS and 100 U/ml of penicillin and streptomycin. Splenocytes were either used for phenotyping by flow cytometry or for cytokine quantification after ex vivo activation (see below). The whole liver was removed, weighed and frozen at −80°C.
2.6.2. 100-day study
All blood and tissue samples for determination of immunological parameters by flow cytometry were collected, processed and analyzed as described in Section 2.6.1 above. For evaluation of aberrant crypt foci (ACF) analysis, three 2 cm sections were removed from the colon: 1) one from the distal end, 2) one approximately 7 cm from the distal end (midportion), and 3) one at the proximal end. Each segment of colon for ACF analysis was placed separately between 2 strips of dental wax and fixed in 10% buffered formalin prior to being processed for ACF evaluation. For evaluation of histopathology and goblet cells, a 1 cm transverse segment approximately 2 cm from the distal end of the colon was removed and fixed in 10% buffered formalin. The spleen, liver and 1 testis were removed and weighed. Sections from the liver, lung, spleen, duodenum, jejunum and ileum were placed in 10% buffered formalin. The testis was fixed in modified Davidson’s solution. All fixed tissues were paraffin embedded in the Tissue Sciences Facility, UNMC. Approximately 4–5 micron sections were stained with hematoxylin and eosin and examined histopathologically (Cohen et al., 2007). The second testis was removed and along with sections from the lungs, colon, and liver were frozen at −20° C.
2.7. Anti-Rat CD3/CD28 Activation
Leukocytes from whole blood, Peyer’s patches and spleen were counted on a Coulter Counter Z1 (Beckman Coulter, FL) and adjusted to a cell concentration of 1x106 cells/ml. Leukocytes from whole blood and spleen were placed into 24-well tissue culture plates while leukocytes from the Peyer’s patches were placed in 96 well flat bottom culture plates (Corning, Corning, NY) that were precoated with anti-rat CD3 (5 μg/ml) (Biolegend, CA). Immediately after addition of cells to the 96-well culture plates, anti-rat CD28 (5 μg/ml) (Biolegend, CA) was added to each well. After a 4-day incubation at 37° C with 5% CO2, supernatants were collected and stored at −80°C for quantification of cytokines.
2.8. Flow Cytometry
Phenotyping of leukocytes from whole blood, Peyer’s patches and spleen was performed by flow cytometry using antibodies directed against rat CD45 (OX-1, Biolegend, San Diego, CA) to identify rat leukocytes. Antibodies directed against rat CD103 (OX-62, Biolegend), MHC-II (RT1B)(OX-6, Biolegend) and CD11b/c (OX-42, Biolegend) were used to quantify dendritic cells (DC); CD4 (W3/25, Biolegend) to quantify Thelper cells, CD25 (OX-39, Biolegend) to quantify activated Thelper cells. Similarly antibodies directed against rat FoxP3 (FJK-16s, eBioscience) was used to identify total Tregulatory (Treg) (CD4+, FoxP3+) cells and activated Treg (CD4+, CD25+ and FoxP3+). Flow cytometry data were collected on a Canto II (BD Biosciences, San Jose, CA) and the collected data were analyzed using Flowjo v10.2 (Tree Star Inc., Ashland, OR).
2.9. Quantification of Cytokines
Cytokines in plasma, tissue extracts and cell culture supernatants were quantified using the LEGENDplex rat inflammation panel (Biolegend, San Diego, CA) for IL-10, IFN-γ, CXCL1 (KC), MCP-1, TNF-α, GM-CSF, IL-18, IL-12 (p70), IL-17A, IL-33, IL-1α, IL-1β and IL-6 per the manufacturer’s protocol.
2.10. ACF analysis
Colon sections were removed from 10% buffered formalin one at a time and rinsed briefly in 0.9% saline. Each section was stained for 30 seconds in 0.5% toluidine blue in 0.5% sodium borate. Excess dye was removed by rinsing in clean 0.9% saline. The section was placed on a glass slide, lumen side up that had previously been divided on the back-side into 25 sections with dimensions of approximately 4 mm2 each so that ACF would not be counted more than once. The stained colon tissue section was covered with a few drops of aqueous dry mounting medium and cover slipped. The number of ACF in each 4 mm2 and the number of aberrant -crypts (ABC)/foci was determined by light microscopic examination at 40X. If necessary for confirmation of identification of ABC, the 4 mm2 section was examined at 400X.
2.11. Goblet cell analysis
Beginning with the lowest numbered animal in each group, Hemolysin & Eosin stained slides of colon tissue were evaluated for acceptability to assess goblet cells, which included no evidence of dilatation of the lumen (secondary to presence of feces) and no evidence of autolysis. The first five colons from each group that met those criteria were evaluated. Two glands were randomly chosen for which the entire length of the gland from the colonic luminal surface to the base of the gland could be identified, and the glandular lumen was present the entire length of the gland. As close as possible, glands were chosen that were perpendicular to the intestinal lumen and perpendicular to the underlying connective tissue to avoid tangential cutting artifact. For the glands that were evaluated, the length of the gland from the epithelial luminal surface to the base of the crypt was measured using an ocular micrometer. The number of goblet cells in that gland was then evaluated. Two glands were evaluated for each colon.
2.12. Statistical analysis
Prism 6 (GraphPad, San Diego, CA) software was used to determine statistical significance. Data was first assessed for homogeneity using a Bartlett’s test. Homogenous data was then evaluated for significant differences from comparative controls using a one-way ANOVA (Dunnett’s multiple comparisons test). For data sets deemed nonhomogeneous per the Bartlett’s test, values were log transformed to meet the variance of the standard deviation and then tested using a one-way analysis of variance ANOVA (Dunnett’s multiple comparisons test). For data sets that still did not pass the variance test (i.e., Bartlett’s test), an outlier test was performed (Rout test) followed by a reassessment of variance and then a one-way ANOVA. The only data set for which the outlier test was applied was the 100-day plasma IL-6 cytokine levels. For statistical analysis of the ACF, a value of 0.5 was added to all samples so a log transformation could be performed to replace values of “0” in all data sets [50]. Statistical analysis was performed using SAS for Windows, version 9.4. Group means for body weights, food and water consumptions, goblet cell analysis, and organ tissue weights were evaluated using ANOVA, followed by Duncan’s multiple range test for group-wise comparisons. Histopathology was compared using the 2-tailed, Fisher’s Exact test. A p value of < 0.05 was deemed statistically significant.
3. Results
3.1. 7- and 100-day studies: In life parameters
In order to investigate the effects of E 171 delivered in a relevant manner, 6-week-old Wistar Hans rats were fed diets containing E 171 (0, 40, 400 and 5,000 ppm) for 7 and 100 days. As shown in Table 1, rats in the study groups had comparable body weights by the end of the 7 and 100 day feeding studies. Rats fed acute and chronic high-content dietary E 171 did not demonstrate significantly different food or water consumption (Tables 2 and 3). It is noteworthy that despite comparable body weight in rats receiving 400 or 5000 ppm dietary E 171, there was a trend toward increased food consumption in rats receiving 5000 ppm E 171. At the 5000 ppm dose, rats consumed approximately 10 more grams of food during the 7 day study compared to rats receiving the control diet (Table 2A). However, the difference in food consumption by weight was not significantly different between any of the treatment groups and this trend was not present in the 100 day study at any time point, including after 7 days (Table 2B).
Table 1.
Body weights of male Wistar rats treated with E 171 for 100 days with or without DMH · 2HCl pretreatment
| Body Weight (g) Mean ± S.E.a |
|||||
|---|---|---|---|---|---|
| Group | Treatment | Week 1 | Week 5 | Week 10 | Week 15 |
| 1 | 0 ppm E171 | 244.0 ± 3.9 | 353.0 ± 7.5 | 416.4 ± 10.1 | 443.7 ± 11.1 |
| 2 | 40 ppm E 171 | 237.3 ± 2.6 | 341.2 ± 5.4 | 408.1 ± 8.3 | 439.9 ± 10.1 |
| 3 | 400 ppm E 171 | 241.4 ± 3.4 | 355.8 ± 6.9 | 426.4 ± 9.6 | 454.3 ± 10.7 |
| 4 | 5000 ppm E 171 | 240.8 ± 3.3 | 348.3 ± 6.2 | 415.4 ± 7.8 | 446.4 ± 9.1 |
| 5 | 180 mg/kg DMH · 2HCl | 224.3 ± 2.9b | 330.7 ± 4.9b | 393.9 ± 6.7 | 423.7 ± 7.2 |
| 6 | 180 mg/kg DMH · 2HCl + 40 ppm E 171 | 226.4 ± 3.2c | 337.8 ± 6.1 | 406.6 ± 8.1 | 436.8 ± 9.6 |
| 7 | 180 mg/kg DMH · 2HCl + 400 ppm E 171 | 221.5 ± 3.5d | 330.9 ± 6.3d | 398.6 ± 8.2d | 428.5 ± 8.7d |
| 8 | 180 mg/kg DMH · 2HCl + 5000 ppm E 171 | 223.3 ± 3.3e | 335.6 ± 8.4 | 405.5 ± 10.9 | 435.4 ± 12.7 |
N=16 for all groups and all time points
Significantly different compared to 0 ppm E 171 group, p<0.05
Significantly different compared to 40 ppm E 171 group, p<0.05
Significantly different compared to 400 ppm E 171 group, p<0.05
Significantly different compared to 5000 ppm E 171 group, p<0.05
Table 2.
Food consumption by male Wistar rats treated with E 171
| A. 7-day Study | |||
|---|---|---|---|
| Food Consumption Mean ± S.E.a |
|||
| Group | Treatment | g/animal/day | g/kg BW/day |
| 1 | 0 ppm E 171 | 21.4 ± 0.6 | 75.8 ± 1.8 |
| 2 | 40 ppm E 171 | 22.1 ± 0.4 | 78.9 ± 2.0 |
| 3 | 400 ppm E 171 | 22.3 ± 0.5 | 81.6 ± 3.0 |
| 4 | 5000 ppm E 171 | 23.0 ± 0.3 | 83.8 ± 1.8b |
| B. 100-day Study | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Food Consumption Mean ± S.E.a |
|||||||||
| Week 2 | Week 5 | Week 10 | Week 15 | ||||||
| Group | Treatment | g/animal/day | g/kg BW/day | g/animal/day | g/kg BW/day | g/animal/day | g/kg BW/day | g/animal/day | g/kg BW/day |
| 1 | 0 ppm E 171 | 23.9 ± 1.4 | 86.5 ± 5.2 | 22.8 ± 0.6 | 64.7 ± 1.7 | 22.4 ± 0.6 | 53.8 ± 1.4 | 22.0 ± 0.5 | 49.5 ± 1.2 |
| 2 | 40 ppm E 171 | 22.9 ± 0.4 | 85.3 ± 1.6 | 22.7 ± 0.2 | 66.7 ± 0.7 | 21.3 ± 0.4 | 52.2 ± 0.9 | 21.4 ± 0.3 | 48.7 ± 0.8 |
| 3 | 400 ppm E 171 | 23.1 ± 0.5 | 83.1 ± 1.8 | 24.8 ± 0.6b | 69.8 ± 1.6b | 22.6 ± 0.6 | 52.9 ± 1.4 | 22.1 ± 0.8 | 48.7 ± 1.7 |
| 4 | 5000 ppm E 171 | 23.3 ± 0.2 | 84.9 ± 0.8 | 24.7 ± 0.3b | 70.9 ± 0.8b | 22.7 ± 0.4 | 54.7 ± 1.1 | 22.3 ± 0.4 | 49.9 ± 0.9 |
| 5 | 180 mg/kg DMH · 2HCl | 22.3 ± 0.3 | 85.5 ± 1.2 | 22.0 ± 0.4 | 66.5 ± 1.2 | 21.5 ± 0.3 | 54.6 ± 0.9 | 21.4 ± 0.3 | 50.6 ± 0.7 |
| 6 | 180 mg/kg DMH · 2HCl + 40 ppm E 171 | 23.4 ± 0.5 | 89.1 ± 1.7 | 23.2 ± 0.5 | 68.8 ± 1.6 | 22.6 ± 0.5 | 55.5 ± 1.3 | 21.7 ± 0.5 | 49.7 ± 1.2 |
| 7 | 180 mg/kg DMH · 2HCl + 400 ppm E 171 | 22.1 ± 0.5 | 85.8 ± 1.9 | 22.3 ± 0.4c | 67.4 ± 1.3 | 21.5 ± 0.3 | 53.9 ± 0.9 | 21.0 ± 0.3 | 47.6 ± 0.7 |
| 8 | 180 mg/kg DMH · 2HCl + 5000 ppm E 171 | 23.5 ± 0.8 | 89.6 ± 3.2 | 24.0 ± 0.8d | 71.6 ± 2.3 | 22.2 ± 0.8 | 54.7 ± 1.8 | 22.2 ± 0.8 | 51.0 ± 1.8 |
N=10 for all groups
Significantly different compared to 0 ppm E 171 group p<0.05
N=8 for all groups and all time points
Significantly different compared to 0 ppm E 171 group, p<0.05
Significantly different compared to 400 ppm E 171 group, p<0.05
Significantly different compared to 180 mg/kg DMH · 2HCl group, p<0.05
Table 3.
Water consumption by male Wistar rats treated with E 171
| A. 7-day Study | |||
|---|---|---|---|
| Water Consumption Mean ± S.E.a |
|||
| Group | Treatment | g/animal/day | g/kg BW/day |
| 1 | 0 ppm E171 | 68.1 ± 1.3 | 241.1 ± 4.7 |
| 2 | 40 ppm E 171 | 66.3 ± 1.7 | 235.9 ± 5.6 |
| 3 | 400 ppm E 171 | 68.9 ± 1.3 | 252.9 ± 8.3 |
| 4 | 5000 ppm E 171 | 69.4 ± 1.4 | 253.3 ± 6.6 |
| B. 100-day Study | |||||||
|---|---|---|---|---|---|---|---|
| Water Consumption Mean ± S.E.a |
|||||||
| Week 3 |
Week 8 |
Week 13 |
|||||
| Group | Treatment | g/animal/day | g/kg BW/day | g/animal/day | g/kg BW/day | g/animal/day | g/kg BW/day |
| 1 | 0 ppm E 171 | 37.3 ± 1.6 | 122.1 ± 5.1 | 35.6 ± 1.0 | 90.0 ± 2.6 | 31.2 ± 1.3 | 71.5 ± 3.0 |
| 2 | 40 ppm E 171 | 37.9 ± 3.2 | 127.4 ± 10.8 | 34.8 ± 1.6 | 90.0 ± 4.2 | 35.2 ± 2.7 | 81.8 ± 6.3 |
| 3 | 400 ppm E 171 | 34.9 ± 1.0 | 113.2 ± 3.3 | 37.9 ± 2.0 | 94.1 ± 5.0 | 32.1 ± 1.1 | 71.8 ± 2.5 |
| 4 | 5000 ppm E 171 | 38.8 ± 1.4 | 128.2 ± 4.6 | 35.9 ± 2.1 | 91.9 ± 5.4 | 33.2 ± 1.8 | 75.8 ± 4.1 |
| 5 | 180 mg/kg DMH · 2HCl | 37.1 ± 1.4 | 128.5 ± 4.7 | 35.5 ± 1.3 | 95.4 ± 3.4 | 31.8 ± 1.2 | 76.5 ± 2.9 |
| 6 | 180 mg/kg DMH · 2HCl + 40 ppm E 171 | 35.6 ± 1.6 | 121.3 ± 5.5 | 35.1 ± 2.1 | 91.3 ± 5.3 | 32.7 ± 2.1 | 76.6 ± 4.9 |
| 7 | 180 mg/kg DMH · 2HCl + 400 ppm E 171 | 37.0 ± 1.6 | 129.1 ± 5.5b | 35.6 ± 1.3 | 94.9 ± 3.4 | 32.0 ± 1.8 | 76.3 ± 4.4 |
| 8 | 180 mg/kg DMH · 2HCl + 5000 ppm E 171 | 36.5 ± 1.8 | 124.2 ± 6.2 | 37.7 ± 2.5 | 98.3 ± 6.4 | 32.6 ± 2.3 | 76.5 ± 5.3 |
N=7 for groups 2 and 8, Week 3. For all other groups and all other time points, N=8.
Significantly different compared to 400 ppm E 171 group, p<0.05
Based on the weight of food consumed, the amount of E 171 each group received was calculated and is shown in Table 4 for both the 7- and 100-day studies. For the 100-day study, two lots of E 171 containing food were used and E 171 content and exposure is broken down by respective lot used (Table 4B). Rats in the highest E 171 treatment groups consumed a total of approximately 361.3 mg of E 171 per kilogram of bodyweight per day during the 7- and 100-day studies (Table 4). Rats in the next highest E 171 group consumed approximately 10-fold less E 171 during the 7- and 100-day studies compared to the 5000 ppm E 171 group (Table 4). To this end, the 5000 ppm E 171 group received an average dietary exposure of E 171 per animal that is orders of magnitude higher than the average reported human exposure of 1.4 mg/kg [32]. Together these results demonstrate that high acute and chronic E 171 intake did not alter the in life measures of study animals.
Table 4.
Consumption of E 171
| A. 7-day Study | |||
|---|---|---|---|
| E 171 Consumptiona |
|||
| Group | Treatment | mg E 171/rat/day | mg E 171/kg BW/day |
| 1 | 0 ppm E171 | 0.51 | 1.81 |
| 2 | 40 ppm E 171 | 1.33 | 4.76 |
| 3 | 400 ppm E 171 | 8.58 | 31.43 |
| 4 | 5000 ppm E 171 | 102.5 | 373.86 |
| B. 100-day Study | |||||
|---|---|---|---|---|---|
| E 171 Consumption - First Batch of Dieta Weeks 2-10 | E 171 Consumption - Second Batch of Dieta Weeks 11-15 | ||||
| Group | Treatment | mg E 171/rat/day | mg E 171/kg BW/day | mg E 171/rat/day | mg E 171/kg BW/day |
| 1 | 0 ppm E 171 | 0.5 | 1.5 | 0.5 | 1.1 |
| 2 | 40 ppm E 171 | 1.3 | 3.9 | 1.3 | 3.0 |
| 3 | 400 ppm E 171 | 9.1 | 25.5 | 8.5 | 19.0 |
| 4 | 5000 ppm E 171 | 103 | 294 | 103 | 236 |
| 5 | 180 mg/kg DMH · 2HCl | 0.5 | 1.5 | 0.5 | 1.1 |
| 6 | 180 mg/kg DMH · 2HCl + 40 ppm E 171 | 1.4 | 4.1 | 1.3 | 3.1 |
| 7 | 180 mg/kg DMH · 2HCl + 400 ppm E 171 | 8.6 | 25.7 | 8.1 | 19.2 |
| 8 | 180 mg/kg DMH · 2HCl + 5000 ppm E 171 | 102 | 300 | 101 | 237 |
Consumption of E 171 was determined based on the concentration of E 171 measured in the diets and on body weights and food consumption
Previous studies have shown that high-dose TiO2 administration in albino mice resulted in increased liver weight and cellularity due to the infiltration of nano-sized TiO2 particles into the sinuses of the liver [18]. Another study suggested that TiO2 can induce systemic inflammation [43]. As part of a general assessment of acute toxicity due to dietary E 171, liver and spleen weights were also determined. As shown in Table 5, E 171 did not alter the liver to body weight ratio in any of the 7- or 100-day study groups. Similarly, no change in the spleen to body weight ratio in any experimental groups with the exception of the 400 ppm E 171+DMH treatment group, which was modestly increased compared to 400 ppm E 171. There were no changes in spleen cellularity across any of the treatment groups (Table 5). Collectively, these results suggest that dietary E 171 produced no general signs of overt toxicity even at doses as high as 5000 ppm of E 171 over 100-days.
Table 5.
Terminal body and tissue weights of male Wistar rats treated with E 171
| A. 7-day Study | |||||||
|---|---|---|---|---|---|---|---|
| Mean ± S.E.a |
|||||||
| Group | Treatment | Body Weight (g) | Spleen Weight (g) | Relative Spleen Weight (mg/g) | Splenocyte Cellularity (106 cells/mg) | Liver Weight (g) | Relative Liver Weight (mg/g) |
| 1 | 0 ppm E 171 | 282.8 ± 5.5 | 0.77 ± 0.04 | 2.73 ± 0.15 | 0.23 ± 0.04 | 12.1 ± 0.2 | 43.0 ± 1.0 |
| 2 | 40 ppm E 171 | 281.5 ± 6.3 | 0.73 ± 0.04 | 2.60 ± 0.14 | 0.27 ± 0.03 | 12.0 ± 0.4 | 42.7 ± 1.3 |
| 3 | 400 ppm E 171 | 273.7 ± 5.9 | 0.74 ± 0.04 | 2.72 ± 0.17 | 0.28 ± 0.06 | 11.9 ± 0.3 | 43.4 ± 0.8 |
| 4 | 5000 ppm E 171 | 275.0 ± 6.1 | 0.69 ± 0.05 | 2.53 ± 0.19 | 0.31 ± 0.04 | 11.6 ± 0.4 | 42.2 ± 0.9 |
| B. 100-day Study | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mean ± S.E.a |
|||||||||
| Group | Treatment | Body Weight (g) | Spleen Weight (g) | Relative Spleen Weight (mg/g) | Splenocyte Cellularity (106 cells/mg) | Liver Weight (g) | Relative Liver Weight (mg/g) | Testes Weight (g) | Relative Testes Weight (mg/g) |
| 1 | 0 ppm E171 | 451.6 ± 12.2 | 0.86 ± 0.04 | 1.91 ± 0.07 | 0.56 ± 0.06 | 14.2 ± 0.5 | 31.4 ± 0.6 | 3.69 ± 0.10 | 8.21 ± 0.19 |
| 2 | 40 ppm E 171 | 445.7 ± 11.1 | 0.83 ± 0.04 | 1.85 ± 0.07 | 0.53 ± 0.06 | 13.9 ± 0.4 | 31.2 ± 0.4 | 3.60 ± 0.09 | 8.10 ± 0.17 |
| 3 | 400 ppm E 171 | 461.3 ± 11.9 | 0.90 ± 0.03 | 1.95 ± 0.06 | 0.51 ± 0.04 | 13.9 ± 0.4 | 30.3 ± 0.6 | 3.80 ± 0.10 | 8.31 ± 0.30 |
| 4 | 5000 ppm E 171 | 451.0 ± 10.0 | 0.94 ± 0.04 | 2.08 ± 0.09 | 0.45 ± 0.04 | 13.8 ± 0.4 | 30.6 ± 0.5 | 3.64 ± 0.09 | 8.13 ± 0.27 |
| 5 | 180 mg/kg DMH · 2HCl | 430.1 ± 7.7 | 0.86 ± 0.03 | 1.99 ± 0.06 | 0.50 ± 0.06 | 13.2 ± 0.5 | 30.6 ± 0.7 | 3.46 ± 0.08 | 8.07 ± 0.20 |
| 6 | 180 mg/kg DMH · 2HCl + 40 ppm E 171 | 440.1 ± 9.9 | 0.87 ± 0.04 | 1.97 ± 0.07 | 0.48 ± 0.05 | 13.7 ± 0.4 | 31.1 ± 0.7 | 3.74 ± 0.12 | 8.51 ± 0.22 |
| 7 | 180 mg/kg DMH · 2HCl + 400 ppm E 171 | 425.0 ± 11.3b | 0.90 ± 0.03 | 2.13 ± 0.07b | 0.49 ± 0.03 | 13.1 ± 0.4 | 30.9 ± 0.9 | 3.76 ± 0.10 | 8.95 ± 0.36 |
| 8 | 180 mg/kg DMH · 2HCl + 5000 ppm E 171 | 443.2 ± 13.7 | 0.85 ± 0.03 | 1.92 ± 0.06 | 0.39 ± 0.05 | 13.4 ± 0.6 | 30.2 ± 0.7 | 3.69 ± 0.12 | 8.39 ± 0.34 |
N =10 for all data
N=15 for all data
Significantly different compared to 400 ppm E 171 group, p<0.05
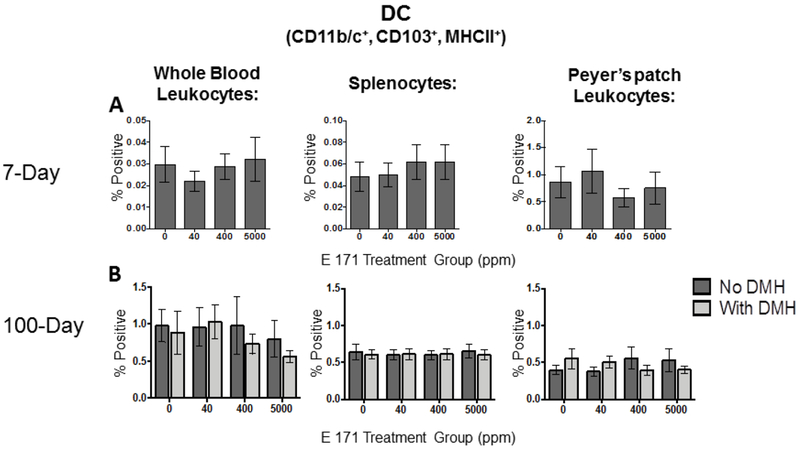
3.2. Effects of acute and chronic dietary E 171 exposure on CD103+ dendritic cell (DC) populations in the periphery and in the gut.
While studies have demonstrated that >99% of TiO2 particles ingested in food pass through the digestive system within feces, it has been shown that gut DC, which are CD103 positive, can actively take up TiO2 particles from the gut lumen [31, 33]. TiO2 particles have also been shown to be reactive, particularly in inducing cellular reactive oxygen species [51]. Further, despite the low levels of TiO2 retention in the host gut, the possibility of CD103+ DC recruitment to the gut and uptake of E 171 over time by DC in Peyer’s patches exists. Therefore, the number of CD103+ DC in the periphery and in Peyer’s patches was quantified.
After 7- and 100-days of dietary exposure to E 171, rats were euthanized and blood, spleens, and Peyer’s patches were isolated and CD103+ DC evaluated. Peripherally, CD103+ DC made up less than 1% of the total leukocyte population in peripheral blood as well as in spleen (Fig.1A) and these percentages were not affected by the E 171-containing diet. These results were unsurprising as CD103-expressing DC are typically found in tissue parenchyma such as the lung and the GI tract [52]. As such, CD103+ DC made up a substantially larger percentage of the total leukocyte population in the Peyer’s patches from the small intestine, accounting for approximately 1% of the total leukocyte pool (Fig. 1A). Likewise, consumption of dietary E 171 did not alter the percent of CD103+ DC Peyer’s patches. When we quantified CD103+ DC in rats fed E 171 for 100-days, we again did not observe an effect with increasing E 171 consumption in any of the tissues analyzed (Fig. 1B), although the frequency of CD103+ DC increased modestly in the peripheral blood and spleen compared to the 7-day study (Fig. 1). Surprisingly, pretreatment with DMH, an inducer of GI tract inflammation, had no effect on CD103+ DC content, with or without E 171 consumption (Fig. 1B). Together these results suggest that there was no change in the percentage of CD103+ DC in peripheral blood, spleen or Peyer’s patches due to acute or chronic dietary E 171 consumption.
Figure 1. Dietary exposure to E 171 did not significantly alter systemic or gastrointestinal tract resident CD103+ dendritic cell frequency.
The frequency of gut resident and circulating CD103+ DC was determined in rats fed dietary E 171 for 7 or 100 consecutive days. Rats in the 100 day study were pretreated with 180 mg/kg of DMH or Vh control by i.p. injection. Following the indicated feeding period, animals were euthanized and PBMC, spleen, and Peyer’s patches were collected and processed into single cell suspension. Cells were then stained for CD11b/c, CD103, and MHCII and analyzed by flow cytometry to quantify CD103+ DC populations. CD103+ DC from the 7 day study are shown in panel A and from the 100 day study are shown in panel B. Data are presented as a percentage of the live cells within the lymphocyte gate. Data were analyzed for statistical significance using a one-way ANOVA with a Dunnett’s multiple comparisons test when there were no significant differences in the variance of the standard deviation.
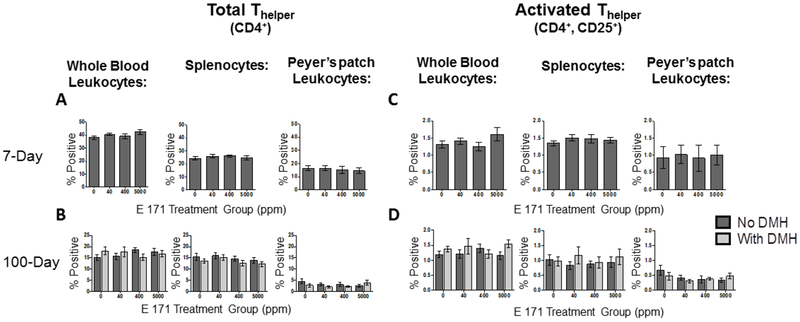
3.3. Effect of Dietary E 171 on CD4+ Thelper populations in the periphery and locally in Peyer’s patches.
Based on a previous report suggesting CD4+ T cells were affected in response to E 171 administration in drinking water, CD4+ T cells were quantified after dietary administration of E 171 for 7- or 100-days. Specifically, the total percentage of CD4+ Thelper cells and the percentage of Thelper cells expressing CD25, an indicator of Thelper activation, were quantified in peripheral blood, spleen, and Peyer’s patches [43].
Activated CD4+ Thelper cells traffic in the peripheral blood from lymphoid organs such as the spleen or draining lymph nodes to areas of inflammation or toward chemokine gradients where they exert their effector functions, typically in the form of inflammatory cytokine release [53]. We first examined the total CD4+ T cell population in PBMC, spleen, and Peyer’s patches. As shown in figure 2, a robust population of CD4+ T cells was detected in all tissues examined from all treatment groups. When the relative proportion of CD4+ T cells was compared to animals exposed to E 171 for 7 or 100 days, we did not observe any E 171 mediated alterations in this population of cells (Fig. 2A and 2B). Since the potential exists for E 171 to alter activation state, and subsequently effector cytokine release, we next determined if E 171 modulated CD4+ Thelper activation. As such, PBMC were examined to determine if dietary E 171 altered the activation status of these cells. As shown in figure 2C and 2D, a sizable percentage of activated CD4+ Thelper cells were not detected in the peripheral blood of rats fed E 171 acutely or chronically. Likewise, a small proportion of activated CD4+ Thelper cells were detected in the spleen, a major site for T cell recruitment and activation, at 7- or 100-days (Fig. 2C and 2D). These results were not surprising as in the absence of an immunogenic stimuli, one would not expect a high proportion of activated Thelper cells [54]. Furthermore, rats fed E 171 for 100 days exhibited no difference in the percentage of activated CD4+ Thelper cells in peripheral blood or the spleen, compared to controls, suggesting that E 171 does not induce activation of this population of cells in the periphery (Fig. 2C and 2D). However, there remains the potential that activated CD4+ Thelper cells may have been recruited to the gut in response to local immune activation. Counter to this possibility, no significant difference in the percentage of activated CD4+ Thelper cells was observed in the Peyer’s patches of rats fed E 171 containing food compared to rats fed a control diet for either 7- or 100-days (Fig. 2). DMH pretreatment also did not alter the percentage of activated Thelper cells in any of the tissues analyzed in the 100-day feeding study, despite the predicted increase in gut inflammation [55] (Fig. 2D). Moreover, we made the unexpected observation that DMH and E 171 appeared to modestly reduce Thelper cell activation in Peyer’s patches contrary to previously reported results [43]. Collectively, these data suggest that despite the potential for accumulation of E 171 particles locally in the gut or in gut-resident DC, there was no detectable impact on the percentage of activated CD4+ Thelper cells either peripherally or locally in the Peyer’s patches of rats fed E 171 containing diets for 7- or 100-days.
Figure 2. Dietary E 171 exposure did not change the frequency of CD4+ Thelper cells systemically or in intestinal Peyer’s patches.
The frequency of gut resident and circulating total and activated Thelper cells were determined in rats fed dietary E 171 for 7 or 100 days. Rats in the 100 day study were pretreated with 180 mg/kg of DMH or Vh control by i.p. injection. After the feeding period, animals were euthanized and PBMC, spleen, and Peyer’s patches were collected and processed into single cell suspensions. Cells were then stained for CD4 and CD25 and analyzed by flow cytometry. Activated Thelper cells were identified as being CD4/CD25 double positive. Total Thelper from 7 (A) and 100 (C) day animals and activated Thelper from 7 (B) and 100 (D) day studies are shown. Data are presented as a percentage of the live cells in the lymphocyte gate staining positive. Data were analyzed for statistical significance using a one-way ANOVA with a Dunnett’s multiple comparisons test when there were no significant differences in the variance of the standard deviation.
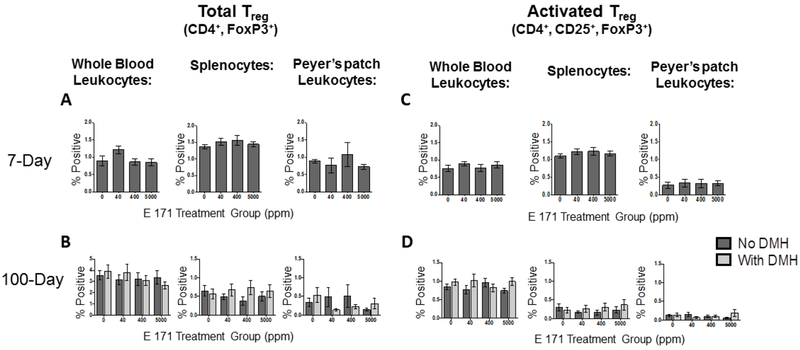
3.4. Effect of dietary E 171 on the percentage of Tregulatory (Treg) cells in the periphery and locally in Peyer’s patches.
We did not observe an impact of dietary E 171 at any of the dose levels on CD103+ DC populations or on the total percentage of CD4+ Thelper or activated Thelper cells. However, there remains the potential that E 171 may alter the percentage of Treg cells, a critical mediator of local and systemic inflammation, which could lead to a low level inflammatory response in the absence of increased inflammatory cells [56]. To test this possibility, the percentage of leukocytes staining positive for CD4, CD25 and FoxP3, the critical transcription factor and marker of Treg cells [57], was quantified in PBMC, spleen, and Peyer’s patches from rats fed a control or E 171-containing diet for 7 or 100 days. We first determined if there were gross differences in the total Treg population (CD4+ FoxP3+) in whole blood, spleen, and Peyer’s patches. As shown in figure 3A and 3B, we did not observe any significant alterations of the total Treg population due to E 171 administration in any of the tissues examined. Pretreatment with DMH also did not impact the gross Treg population in the periphery (Fig. 3B).
Figure 3. Acute or chronic exposure to dietary E 171 did not significantly affect the frequency of circulating or GI-resident Tregulatory (Treg) cells.
The frequency of gut resident and circulating total and activated Treg cells were determined in rats fed dietary E 171 for a total of 7 or 100 days. Rats in the 100 day study were pretreated with 180 mg/kg of DMH or Vh control by i.p. injection. Following the 7- and 100-day feeding period, animals were euthanized and PBMC, spleen, and Peyer’s patches were collected and processed into single cell suspensions. Cells were then surface stained for CD4 and CD25 and subsequently stained intracellularly for FoxP3. Following antibody stains, cells were analyzed via flow cytometry to identify Treg cell populations. Data are presented as a percentage of the live cells falling in the lymphocyte gate. Total Treg from 7 (A) and 100 (C) as well as activated Treg from 7 (B) and 100 (D) day studies are shown. Data were analyzed for statistical significance using a one-way ANOVA with a Dunnett’s multiple comparisons test when there were no significant differences in the variance of the standard deviation.
However, as with CD4+ Thelper cells, Treg cells express CD25 when they are activated and exert suppressive effector function. As such, one would not expect to find Treg cells in excess either in the periphery or locally within the tissue in the absence of an immune reaction [56, 57]. Indeed, studies have shown that the percentage of T cells comprised by Treg cells steadily increases as inflammation increases [58]. Corroborating this notion, less than 1% of PBMC and 2% of splenocytes (Fig. 3C) were identified as being of the Treg population suggesting no evidence of systemic inflammation. Likewise, rats fed the E 171-containing diet for 100 days did not have significant differences in the percentages of peripheral leukocytes staining positive for FoxP3 compared to controls (Fig. 3D).
As with activated CD4+ Thelper cells, the local distribution of Treg cells can be as critical in mediating biological activity through local exertion of immune suppressive functions [57]. To this end, the percentages of Treg and activated Treg cells in the gut Peyer’s patches were determined for both the acute and chronic feeding studies. As observed peripherally, no significant differences were detected in the percentage of total or activated Treg cells in the Peyer’s patches of rats fed a control diet compared to rats fed the E 171 containing diets for 7 days (Fig. 3A and 3C). Likewise, the percentage of total and activated Treg cells in the Peyer’s patches of rats receiving dietary E 171 chronically for 100 days was unaffected compared to rats receiving the control diet (Fig.3B and 3D). DMH pretreatment also did not alter activation state or tissue accumulation of Treg cells (Fig. 3B and 3D). Collectively, these results suggest that E 171 consumption does not alter T cell-mediated mechanisms of immune control. Further, these data are in agreement with the results obtained in figure 2, where no increase in the percentage of activated CD4+ Thelper cells in Peyer’s patches of animals fed E 171 was observed. As stated previously, there is a direct correlation between the level of inflammation and the number of Treg cells controlling normal immunological homeostasis. In total, the results presented in figures 2 and 3 suggest that dietary E 171 is not pro-inflammatory in the context of either promoting inflammatory CD4+ Thelper cell activation or in reducing the percentage of anti-inflammatory Treg cells.
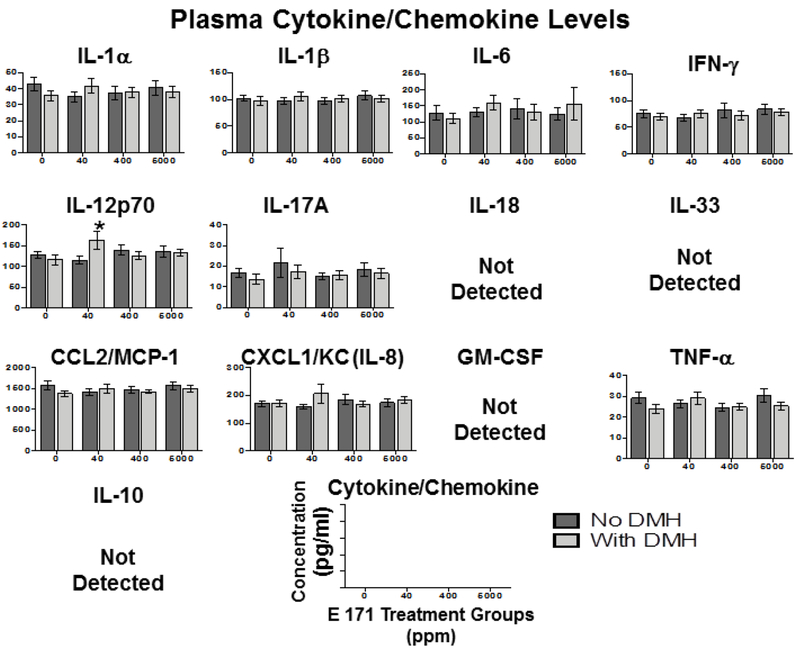
3.5. Dietary E 171 did not significantly alter inflammatory cytokine levels in plasma, small intestine, or colon.
While the safety of E 171 as a food additive has been studied extensively for the past 50+ years, recent studies have suggested that E 171 and other micro-particles can trigger intestinal inflammation [23, 33, 34], inflammasome activation [23, 33], and the presence of TiO2 particles weakly associates with the development of inflammatory bowel disease (IBD) [34]. More recently, a study by Bettini and colleagues suggested that TiO2 intake resulted in increased inflammatory cytokine profiles strikingly similar to those found in IBD patients [43]. As stated previously, the method of delivery of TiO2 particles can change the properties of the particles themselves and lead to differential interactions between TiO2 particles and host tissues [36–38]. To this end, we attempted to recapitulate the findings of Bettini et al. using a model of TiO2 delivery in food containing E 171.
Six-week-old Wistar Han rats were fed a diet consisting of either control chow or chow with increasing concentrations of E 171 for 7 or 100 days. Rats in the 100-day study were pretreated with either DMH or vehicle (Vh) before being fed E 171 containing diets. Following the feeding period rats were euthanized and colon, small intestine, and plasma were collected. Peyer’s patches from rats acutely fed E 171 were also collected for ex vivo analysis of anti-CD3/CD28 induced Thelper cell inflammatory cytokine release. A rat inflammatory cytokine bead array was used to quantify tissue levels of IL-1α, IL-1β, IL-6, interferon γ (IFNγ), IL-12p70, IL-17A, IL-18, IL-33. CCL2/MCP-1, CXCL1/KC (IL-8), GM-CSF, and tissue necrosis factor α (TNFα), many of which have been implicated in gut inflammatory disorders such as IBD [59, 60].
First, changes in circulating inflammatory cytokine levels were examined in response to acute exposure to dietary E 171. Two of the cytokines examined were below the limit of detection (TNFα and IL-17) while the others were not altered by dietary E 171 in any of the treatment groups, when compared to control diet (Supplemental Figure 1), suggesting that acute exposure to dietary E 171 did not alter the levels of circulating inflammatory cytokines.
We also investigated the possibility of local inflammatory cytokine production in the GI tract, specifically in the small intestine and colon where inflammatory cytokine production has been shown to correspond with inflammatory GI disorders [59, 60]. Of the inflammatory cytokines that were detected in both tissues, no statistical differences were observed in the concentration of these cytokines between any of the E 171 treatment groups (Supplemental Figure 2 and 3) suggesting 7 days of dietary E 171 did not induce GI inflammation. Likewise, the anti-inflammatory cytokine, IL-10, which increases in concentration in response to local inflammation [57], was not altered in plasma, intestine, or colon further suggesting that inflammation was not induced by consumption of dietary E 171 even in the 5,000 ppm treatment group (Supplemental Figures 1, 2, and 3). Together these data suggest that dietary E 171 does not induce inflammation peripherally or in the GI tract.
As no significant effect of acute dietary E 171 was observed compared to controls on the levels of inflammatory cytokines in the periphery or GI tract, additional studies were performed to explore the possibility that E 171 might alter the effector cytokine profile of Thelper cells in lymphoid tissue or circulation, which may not be manifest without T cell specific stimuli. To address this potential, lymphocytes were isolated from peripheral blood, spleen, and Peyer’s patches of rats E 171 and activated ex vivo with anti-CD3/anti-CD28 for 4 days to induce Thelper cell cytokine production. Following the 4-day culture period, supernatants were collected and assayed for inflammatory cytokine production. As shown in supplemental figures 4, 5, and 6, we did not observe any acute effects of E 171 exposure on any of the induced cytokines produced from ex vivo stimulated Thelper cells, which is contrary to a previous study [43].
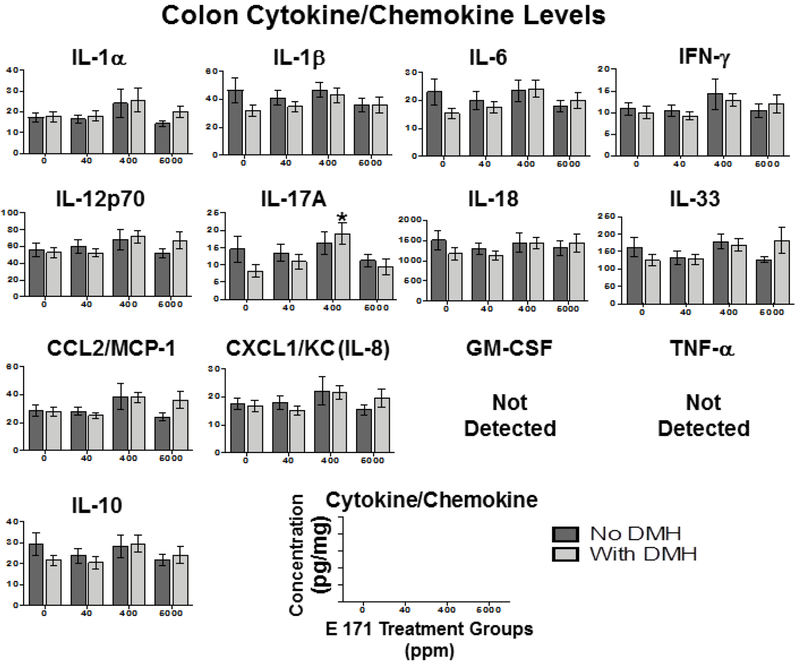
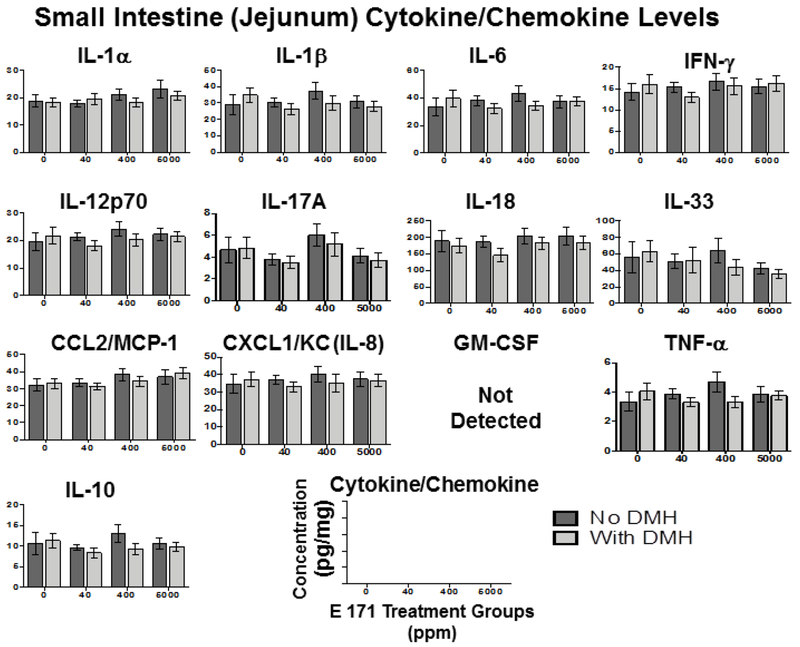
While there were no overt effects of acute E 171 administration, we also examined the inflammatory cytokine profile in rats chronically exposed to E 171 as alterations in immune homeostasis may become evident later. As noted in the 7-day feeding study, we did not observe any significant E 171-mediated alteration of any of the 13 cytokines assayed in any of the tissues examined (Fig. 4–6). Furthermore, DMH pretreatment also did not significantly alter tissue specific cytokine release, with the sole exceptions being elevated IL-12p70 in plasma (Fig.4) and IL-17A in the colon (Fig.6). These results were surprising as we would have expected more inflammatory cytokine release in the GI tract in response to DMH, a known inflammagen [55, 61].
Figure 4. Chronic dietary E 171 exposure did not alter systemic inflammation as evidenced by inflammatory cytokine accumulation.
Rats were pretreated with 180 mg/kg DMH or Vh control by i.p. injection and fed dietary E 171 for a total of 100 days. Following the 100 day feeding period, animals were euthanized and plasma was collected and assayed for inflammatory cytokines and chemokines using the LEGENDplex rat inflammation panel. Data are shown as cytokine/chemokine concentration (pg/ml) for plasma.. Data were analyzed for statistical significance using a one-way ANOVA with a Dunnett’s multiple comparisons test when there were no significant differences in the variance of the standard deviation. * = p<0.05 indicates significance compared to no E 171 control.
Figure 6. Dietary E 171 exposure did not alter colonic inflammation as evidenced by inflammatory cytokine accumulation.
Rats were pretreated with 180 mg/kg DMH or Vh control by i.p. injection and fed dietary E 171 for a total of 100 days. Following the 100 day feeding period, animals were euthanized and plasma was collected and assayed for inflammatory cytokines and chemokines using the LEGENDplex rat inflammation panel. Data are shown as cytokine/chemokine concentration (pg/ml) for colon. Cytokine protein levels were normalized to total protein levels for each sample of colon. Data were analyzed for statistical significance using a one-way ANOVA with a Dunnett’s multiple comparisons test when there were no significant differences in the variance of the standard deviation. *=p<0.05 indicates significance compared to respective no E 171 control.
These results suggest that E 171, fed acutely or chronically, does not alter inflammatory cytokine production either peripherally or locally in the GI tract. However, it is noteworthy that the increase in IL-12p70 occurred in the 40 ppm E 171+DMH treatment group (Fig.4), and there was no indication of an E 171 dose-dependent effect rendering this finding difficult to interpret. Conversely, while increased IL-17A was detected in the colons of DMH treated rats fed 400 ppm E 171 (Fig.6), this effect was not observed in the 5,000 ppm + DMH treatment group. The biological significance of increased IL-17A in the colon at only 400 ppm E 171+DMH is unclear, especially in light of the fact IL17A levels in this treatment group were not different from background levels (no treatment control) in the colon.
3.6. Effect of 100-day dietary administration of E 171 on histopathology
As prior studies have suggested that chronic E 171 exposure resulted in increased neoplastic lesions [43], we next used histopathological analysis to determine any gross abnormalities associated with E 171 exposure in various tissues. Histologic evaluation of the duodenum, jejunum, ileum, spleen, liver, lung, and testes was normal for nearly all animals. Occasional animals had mononuclear infiltrate in the livers, which is a normal finding in this strain of rats [62]. There were no significant differences in the incidences between groups. In the spleen, a single animal in the 5000 ppm E 171 group showed possible decreased lymphoid tissue based only on a visual proportion of lymphoid to non-lymphoid tissue, but this could be well within the normal range for this strain of rats. The testes were all normal except for one atrophic testis with focal tubular calcification in one rat in the 5000 ppm E 171 group, a common lesion in rats even at this age [62–66]. There were no histologic changes in any of the segments of the small intestine.
While we did not observe any gross pathologies in the E 171 treated animals, rats that were pretreated with DMH displayed several histopathologic abnormalities, with or without E 171 exposure. There were two invasive adenocarcinomas in one animal in the 180 mg/kg DMH · 2HCl + 0 ppm E 171 group, and single adenomas in one animal in the 180 mg/kg DMH · 2HCl + 40 ppm E 171 group and one animal in the 180 mg/kg DMH · 2HCl + 400 ppm E 171 group (Data not shown). There were no other histologic changes in the large intestines of the other animals treated with E 171 with or without pretreatment with DMH · 2HCl. One rat in the 180 mg/kg DMH · 2HCl + 0 ppm E 171 group and one rat in the 400 ppm group had subpleural lymphocytes in the lung, but without any evidence of acute inflammatory changes or hyperplasia. Collectively, these data suggest that in the absence of a known GI carcinogen, that E 171 does not induce gross or histopathologic changes or abnormalities.
3.7. Effect of 100-day dietary E 171 on formation of aberrant crypt foci (ACF) and aberrant crypts (ABC)
While many of the observed histopathological abnormalities occurred in DMH pretreated rats, there exists the possibility that 100 days is not adequate to observe the full transition to cancer. As such, we quantified ACF and ABC as described precursor to GI tract carcinogenesis [67, 68]. Unfortunately, much of the epithelial surface of the colon samples (proximal, middle and distal) were obscured when observed by light microscopy, therefore we were unable to examine the entire surface of the colon samples (Table 6). This was not observed in a previous study performed in mice [69]. In an effort to determine if the epithelial surface was obscured due to mucus, we dehydrated a portion of one section in an ascending series of alcohols (70-100%) [70]; however, this did not increase the area of visible epithelium compared to an undehydrated portion of the same section. It is also possible this obscuring of the surface was the result of autolysis due to delayed fixation, which was a result of the processing of the colonic tissue during necropsy, although this was not apparent by histopathology. The results for the areas of epithelium that were observed show that there is the expected significant increase in ACF/cm2 and ABC/cm2 in groups 5-8 that were pretreated with 180 mg/kg BW DMH · 2HCl compared to the groups that were not pretreated with DMH (Table 6). E 171 treatment administered after DMH did not result in statistically significant increases in ACF or ABC (Table 6). No change in the number of AFC and ABC were observed due to E 171 exposure alone in the absence of DMH.
Table 6.
Evaluation of E 171 treatment for 100 days with or without pretreatment with DMH · 2HCl on the induction of colonic aberrant crypt foci (ACF) and aberrant crypts (ABC) in male Wistar rats
| Group | Treatment | Total cm2 Evaluated | Mean No. of ACF/cm2,a | Mean No. of ABC/cm2,a |
|---|---|---|---|---|
| 1 | 0 ppm E 171 | 25.4 | 0.8 ± 0.5 | 1.9 ± 1.1 |
| 2 | 40 ppm E 171 | 19.7 | 0.1 ± 0.1 | 0.2 ± 0.2 |
| 3 | 400 ppm E 171 | 27.6 | 0.9 ± 0.4 | 2.1 ± 1.1 |
| 4 | 5000 ppm E 171 | 23.9 | 0.9 ± 0.5 | 2.7 ± 1.6 |
| 5 | 180 mg/kg DMH · 2HCl | 24.2 | 5.4 ± 1.2b | 17.1 ± 4.1b |
| 6 | 180 mg/kg DMH · 2HCl + 40 ppm E 171 |
27.3 | 5.3 ± 1.3c | 14.8 ± 3.9c |
| 7 | 180 mg/kg DMH · 2HCl + 400 ppm E 171 | 30.4 | 7.2 ± 1.3d | 19.7 ± 3.6d |
| 8 | 180 mg/kg DMH · 2HCl + 5000 ppm E 171 | 26.9 | 10.1 ± 2.6e | 28.4 ± 6.6e |
Results expressed as mean ± S.E. N=15 for groups 1 and 6-8 and 14 for groups 2-5
Significantly different compared to 0 ppm E 171 group, p<0.05
Significantly different compared to 40 ppm E 171 group, p<0.05
Significantly different compared to 400 ppm E 171 group, p<0.05
Significantly different compared to 5000 ppm E 171 group, p<0.05
3.8. Evaluation of goblet cells
As expressed above, dietary E 171 consumption by rats pretreated with or without DMH did not increase the incidence of aberrant crypts or tumorgenesis. However, 100 days may not be an adequate amount of time for this transition. It has been reported that an early contributing event to intestinal inflammation, morphologic changes, and eventual tumor formation is the loss of mucin secretion by intestinal goblet cells [71]. These cells produce mucins, particularly mucin 2, which forms a barrier that maintains intestinal immune homeostasis by segregating commensal bacteria from the intestinal epithelia [71, 72]. It has been reported that a loss of goblet cells, mucin secretion, and a lengthening of the colonic gland precede intestinal inflammation and tumor formation [71, 73]. Furthermore, another study also suggested that these cells could preferentially take up nanoparticles, such as TiO2, from intestinal M cells, allowing for accumulation [74]. As such, we evaluated colonic gland length and enumerated gland goblet cell content as an early indicator of intestinal inflammation as a result of dietary E 171. First, we measured the colonic gland length in defined sections of colon from rats fed E 171 with or without DMH pretreatment. The length of the colonic glands examined were not significantly different in any treatment group, with the exception of a significant lengthening of colonic gland length in DMH pretreated rats receiving no E 171 (Table 7). Next we quantified the number of goblet cells per colonic gland (unit) to determine if dietary E 171 resulted in a loss of these cells. As shown in Table 7,the number of goblet cells/unit was similar in all treatment groups. Our results suggest that dietary E 171, with or without treatment with DMH, for 100 days had no effect on the length of the colonic glands examined or the number of goblet cells/unit.
Table 7.
Evaluation of colonic goblet cells in male Wistar rats treated with E 171 for 100 days with or without pretreatment with DMH · 2HCl
| Group | Treatment | Mean Length of Gland (units)a,b | Mean No. of Goblet Cells/Unitb |
|---|---|---|---|
| 1 | 0 ppm E 171 | 4.6 ± 0.1 | 6.5 ± 0.5 |
| 2 | 40 ppm E 171 | 5.2 ± 0.2 | 5.9 ± 0.2 |
| 3 | 400 ppm E 171 | 4.9 ± 0.3 | 6.4 ± 0.3 |
| 4 | 5000 ppm E 171 | 5.3 ± 0.2 | 6.2 ± 0.3 |
| 5 | 180 mg/kg DMH · 2HCl | 5.5 ± 0.3c | 6.2 ± 0.4 |
| 6 | 180 mg/kg DMH · 2HCl + 40 ppm E 171 |
5.0 ± 0.2 | 5.9 ± 0.4 |
| 7 | 180 mg/kg DMH · 2HCl + 400 ppm E 171 | 5.3 ± 0.3 | 6.0 ± 0.4 |
| 8 | 180 mg/kg DMH · 2HCl + 5000 ppm E 171 | 5.5 ± 0.2 | 6.2 ± 0.2 |
The length of the gland from the epithelial luminal surface to the base of the crypt was measured using an ocular micrometer.
Results expressed as mean ± S.E. N=5 for all groups except 3 and 8. Only 4 animals in these groups met the criteria for inclusion in goblet cell evaluation
Significantly different compared to 0 ppm E 171 group, p<0.05
4.0. Discussion
Food-grade TiO2, otherwise referred to as E 171 in the European Union, is a widely used food additive that is ingested daily by human consumption of numerous manufactured food products. Despite the extensive risk assessment performed on E 171 over the course of the past 5 decades, it has undergone renewed scrutiny as recent reports have suggested that E 171 can bind intestinal cells, pass through intestinal lumen, and induce inflammatory cytokine production in the GI tract [15, 23, 26–31]. However, few in vivo studies have used E 171 as a dietary component at relevant levels with relevant characteristics, i.e. >100 nm diameter TiO2 particle size [32]. Here we show that acute and chronic dietary consumption of E 171 at increasing doses (40-5000 ppm) resulted in no significant effects on either peripheral or GI tract immune homeostasis as evidenced by immune cell phenotyping or inflammatory cytokine analysis. The sole exceptions to this were significantly elevated IL-17A from colon DMH+400ppm E 171 treated rats and IL-12p70 from plasma of DMH+40ppm E 171 treated rats (Figs. 4&6). Furthermore, there were no effects of E 171 on histopathologic evaluations of small and large intestine, liver, spleen, lungs, or testes, as well as no effects on ACF, colonic goblet cell numbers, or colonic gland length, which is in contrast to recent reports [43, 44] but is consistent with the lack of preneoplastic or neoplastic changes in rats or mice in a two-year bioassay at dietary doses as high as 50,000 ppm (5%) [14].
Recently, it was reported that acute exposure to TiO2 preparations, including E 171, by intragastric gavage for 7 days resulted in altered frequency of GI resident DC, Thelper cells, and Treg cells [43]. Specifically, it was reported that the frequency of CD103+ DC increased in the Peyer’s patches of rats fed E 171, while the frequencies of Thelper and Treg cells decreased [43]. By contrast, we observed no changes in the frequency of local gut DC, Thelper, or Treg cells in either the 7- or 100-day feeding studies. The authors did not propose a direct mechanism for why GI resident DC populations would expand with TiO2 treatment, other than to note this could be compensatory to the local inflammation induced by TiO2 exposure. However, they did not detect increased frequency of inflammatory Thelper cells or anti-inflammatory Treg cells. This finding would be counter to the hypothesis of increased local inflammation. Indeed, our own findings suggest no difference in peripheral or local tissue levels of inflammatory cytokines or tissue frequency of Thelper cells, even at dietary E 171 levels greater than those evaluated by Bettini et al [43]. While we did observe significantly elevated levels of IL-17A in the colons and increased circulating IL-12p70 in plasma of DMH+400ppm E 171 treated rats in the 100-day study, this is in stark contrast to published findings where IL-17A was significantly increased in as little as 7 days following E 171 exposure alone [43]. Further, the same study did not identify significant increases in IL-17A at the tissue level, but rather from peripheral splenocytes [43] highlighting substantial differences. Despite the elevated levels of inflammatory IL-17A in the colon of DMH+400ppm E 171 treated rats, it is important to emphasize that this elevation was in comparison to DMH alone, which was lower than background levels of IL-17A (i.e., no treatment control). Moreover, the IL-17A levels in DMH + 400ppm E 171 treated rats were not different from the no E 171, mock treatment control group. Furthermore, studies have linked increased IL-17A to tumorogenesis in colitis-associated cancer models [75]. Our finding that IL-17A levels were not significantly elevated compared to control animals strongly argues against the ability of E 171 to induce colitis or tumorogenesis. Likewise, there were no changes in the percentage of Treg isolated from the Peyer’s patch nor were there any histological features indicative of colonic inflammation. Interestingly, DMH did not significantly alter any of the immune parameters that were evaluated, whether in the intestines, spleen or peripheral blood [55]. However, there was a general trend in that the colon associated inflammatory cytokine levels in the DMH only control treatment group showed lower levels compared to the no treatment control group, including IL-1β, IL-6, IL-17A, IL-18 and IL-33.
While altered basal inflammation could be responsible for the increases observed in GI dendritic cells, it would not explain the decreased number of gut-associated Thelper cells. The aforementioned study ascribed the effect of decreased Thelper cell frequency in the Peyer’s patches to decreased Thelper cell differentiation [43]. However, the Peyer’s patch is restricted to the types of T lymphocytes that can be recruited and maintained compared to the spleen, which is less selective [76]. Splenic selectivity could also account for the observed differences between Thelper cells from Peyer’s patches and spleen stimulated ex vivo with a non-specific activator or all T cells with anti-CD3/anti-CD28 stimulation. Yet, this would not explain the differences observed in our study compared to recent reports as we did not observe any difference in Thelper frequency in GI-associated tissue or in the spleens of animals exposed to E 171 in either the 7- or 100-day studies.
Another explanation for the observed difference between our study and the one conducted by Bettini and colleagues [43] could potentially be how E 171 was delivered. In the aforementioned study E 171, which was suspended in water, was administered directly into the stomach of rats by intragastric gavage in a 7-day study. Further, in a 100-day study, they administered E 171 in the drinking water. These modes of exposure are strikingly different from the typical mode of human exposure in which E 171 is delivered to the GI tract as a constituent of food preparations. As stated previously, the surface structure of TiO2 molecules is rather malleable to alteration by surrounding macromolecules, resulting in widely different coronas depending on the preparation of TiO2 [77, 78], which could have impacted the way TiO2 particles interact with the host gut environment in our respective studies.
Equally important, it is well established that TiO2 is water insoluble [32]. In the Bettini study [43], the E 171 used for gavage was delivered in a water suspension [79]. Delivery of TiO2 in water can greatly change the nature of the particles themselves, as TiO2 tends to aggregate and agglomerate in water suspension [32], raising the possibility that delivery of E 171 in an aqueous solution could lead to clumps of TiO2 passing through to the gut environment. As mentioned above, the aforementioned study administered, E 171 in drinking water, which requires sonication, a process with unknown effects on the form of TiO2. Agglomeration and particle settling could also occur over time. This would represent a key difference in how TiO2 is seen by the host when consumed in food, where more particles would be part of the food matrix resulting in a more uniform profile of single particles as compared to clumps [35]. In fact, some studies utilizing differing routes of delivery for TiO2 nanoparticles have suggested that the aggregation/agglomeration of TiO2 particles can impact their interface with the host immune system [80, 81].
Despite the reported effects of E 171 on immune homeostasis, the most pertinent finding by Bettini and colleagues was the increase in ACF by E 171 in a model of DMH-induced carcinogenesis, suggesting that E 171 increased intestinal carcinogenesis [43]. However, no significant differences between groups administered E 171 at any of the three concentrations evaluated, whether fed alone or after pretreatment with DMH, were observed in ACF or ABC. Although there was a weak trend observed between increasing E 171 exposure and increased ACF/ABC in DMH-treated animals, this was not statistically significant. The failure to reach significance is critical as we utilized 15 rats per group to increase statistical power of the study. Further, evaluation of the effects of E 171 on proliferative lesions of the intestine involved histopathology, ACF numbers and size, large intestinal goblet cell number, and gland length in defined areas of intestine that were evaluated in a blinded manner (except for goblet cell number and gland length), a key factor in performing reproducible scientific experiments [82].
Urrutia-Ortega et al. [44] reported that they detected decreases in goblet cells in the distal colon, and suggested that the decreased mucin could increase the risk of colon cancer. It was also reported by Talbot et al.[83] that TiO2 was trapped by intestinal mucus but no effect on mucin O-glycosylation or short-chain fatty acid synthesis, concluding that TiO2 did not alter the intestinal barrier protection under healthy conditions. However we did not observe any differences between treatment groups in numbers of goblet cells per gland, the size of the glands, or number of goblet cells per unit length of the glands. The one exception to this was a significant lengthening of colonic glands in rats pretreated with DMH only (Table 7). As with other parameters, we used well-defined tissue samples, methods, and criteria to evaluate the goblet cells. We especially were careful to only evaluate glands from segments of colons that were not dilated because of the presence of feces; such dilation distorts the shape and size of the glands and can affect the number of goblet cells. Importantly, no lesions were observed in rats administered E 171 alone. Thus our data suggests that even under pathologic conditions in the intestine (DMH pretreatment) that dietary E 171 is innocuous in regards to reported effects on intestinal goblet cells and mucosal barrier maintenance.
In rats pretreated with DMH, one rat in the DMH + control group developed two separate invasive adenocarcinomas, one of which could be identified as arising from an adenoma. In each of the DMH + low-dose E 171 and DMH + mid-dose E 171, there was an adenoma. In the DMH + high-dose E 171 group there were no tumors or any abnormalities of the intestine detected histopathologically. No abnormalities in the small or large intestines were observed in rats treated with E 171 without DMH pretreatment.
While DMH is a known, potent, genotoxic intestinal carcinogen in rodents, variability in numbers and types of lesions in the intestine are usually seen in bioassays of DMH and other carcinogens, particularly when a relatively low dose is administered as in our experiments [84, 85]. There is also considerable variability in the number and size of ACF that are induced by DMH, as seen in the present experiment with the inter-individual variability seen within each group. In the rats fed E 171 without pretreatment with DMH, there were few ACF in any group, a typical finding for control rats. We did not observe the increase in ACF reported by Bettini et al. [43]. In their study, there were no groups administered only E 171; they only evaluated groups pre-treated with DMH. In their publication, they did not mention whether they neutralized the highly acidic DMH-2HCI, but they almost certainly did as the acid without neutralization is highly irritating and highly toxic to the rats when injected ip. Also, they stated that they injected 180 mg/kg of DMH. However, they used DMH-2HCI as did we, and at that level of DMH (which would be 398 mg/kg DMH-2HCI, there is severe toxicity. Thus, it is likely that they used 180 mg DMH-2HCI, which is 81 mg/kg DMH, the dose that we used.
Based on our results it is unlikely that E 171 affects intestinal carcinogenesis in a DMH model of colonic cancer.. In agreement with this finding, E 171 showed no evidence of direct carcinogenesis in a National Toxicology Program bioassay in either rats or mice when fed at levels of 2.5 or 5.0% of the diet [14], doses considerably higher than those used by Bettini et al. [43] Urrutia-Ortega et al. [44], or in the study presented here. Also, it is well known that non-genotoxic substances that increase intestinal tumorigenesis show increased proliferative lesions in short term studies when administered alone, without pretreatment with a genotoxic carcinogen like DMH [85–88]. Furthermore, non-genotoxic substances that enhance intestinal carcinogenesis will increase intestinal tumor incidences when administered alone in a two-year bioassay [14]. Thus, it is highly unlikely that TiO2 is an intestinal carcinogen or enhances intestinal carcinogenesis.
Lastly, histopathology of liver, spleen, lungs, and testes did not show evidence of E 171 treatment-related effects. Subpleural lymphocytes in the lung occur occasionally in this strain of rats, and mononuclear foci in the liver commonly occur [62]. The finding of atrophy in 1 testis also is not unusual for this strain of rats, even by 21 weeks of age as in our experiment.
In conclusion, we report the finding that acute and chronic dietary exposure to E 171 in food preparation did not result in significant alteration of gut immune homeostasis as evidenced by altered frequency of intestinal DC, Thelper, or Treg cells. Despite changes at a single dose in colonic IL-17A and plasma levels of IL-12p70 in DMH+E 171 treated rats, no other changes in intestinal tissue or blood inflammatory cytokine production was observed. Furthermore, no E 171 effects on intestinal histopathology, ACF, or goblet cells were observed alone or after pretreatment with the genotoxic intestinal carcinogen DMH. Assessment of E 171 delivered via food represents a critical model of human TiO2 exposure, ingestion through food consumption, and had notably different effects as compared to recent studies utilizing different models of TiO2 delivery to experimental animals with other modes of administration. Given the varied nature of reports of adverse health effects with TiO2 in specific contexts, this would suggest that the matrix in which TiO2 is received when administered orally might be one important determinant, as E 171 administered in food does not appear to affect peripheral or GI tract immune homeostasis or intestinal carcinogenesis.
Supplementary Material
Figure 5. Chronic dietary E 171 exposure did not modulate small intestine inflammatory cytokine accumulation.
Rats were pretreated with 180 mg/kg DMH or Vh control by i.p. injection and fed dietary E 171 for a total of 100 days. Following the 100 day feeding period, animals were euthanized and plasma was collected and assayed for inflammatory cytokines and chemokines using the LEGENDplex rat inflammation panel. Data are shown as cytokine/chemokine concentration (pg/ml) for small intestine. Cytokine protein levels were normalized to total protein levels for each sample of small intestine. Data were analyzed for statistical significance using a one-way ANOVA with a Dunnett’s multiple comparisons test when there were no significant differences in the variance of the standard deviation.
Highlights:
To determine the immunological and pathological effects of dietary E 171 consumption
Dietary E 171 did not change immune cell profile in Peyer’s patches or peripherally
Dietary E 171 did not alter inflammatory cytokine profile in GI tract or circulation
Dietary E 171 did not increase colonic proliferative lesions
Dietary E 171 did not induce histopathologic changes in small and large intestine, liver, spleen, lungs, or testes
Acknowledgements
We gratefully acknowledge the technical expertise of the Tissue Sciences Facility at UNMC in the preparation of H&E (Haemototoxylin and Eosin) slides for histopathology and goblet cell examination, and the assistance of Stephanie Henkel in the preparation of this manuscript.
Financial support
This work was supported by the Center for Research on Ingredient Safety, Michigan State University, the Grocery Manufacturers Association, the Titanium Dioxide Manufacturers Association, the International Association of Color Manufacturers, and the Fred and Pamela Buffett Cancer Center Tissue Sciences Facility Shared Resource, supported by the National Cancer Institute under award number P30 CA036727.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Declaration of interests
X The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Code of Federal Regulations. Listing of color additives exempt from certification. 2018; Available from: https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfcfr/cfrsearch.cfm?fr=73.575.
- 2.Regulation (EC) No 1333/2008 of the European Parliament and of the Council of 16 December 2008 on food additives. 2018. December/20/18]; Available from: https://eurlex.europa.eu/eli/reg/2008/1333/2014-04-14.
- 3.Diebold U, The surface science of titanium dioxide. Surface Science Reports, 2003. 48(5-8): p. 53–229. [Google Scholar]
- 4.Yang Y, et al. , Characterization of food-grade titanium dioxide: the presence of nanosized particles. Environ Sci Technol, 2014. 48(11): p. 6391–400. [DOI] [PubMed] [Google Scholar]
- 5.Theissmann R, Kluwig M, and Koch T, A reproducible number-based sizing method for pigment-grade titanium dioxide. Beilstein J Nanotechnol, 2014. 5: p. 1815–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dudefoi W, et al. , Evaluation of the content of TiO2 nanoparticles in the coatings of chewing gums. Food Addit Contam Part A Chem Anal Control Expo Risk Assess, 2018. 35(2): p. 211–221. [DOI] [PubMed] [Google Scholar]
- 7.Dudefoi W, et al. , Criteria to define a more relevant reference sample of titanium dioxide in the context of food: a multiscale approach. Food Addit Contam Part A Chem Anal Control Expo Risk Assess, 2017. 34(5): p. 653–665. [DOI] [PubMed] [Google Scholar]
- 8.Oberdorster G, Ferin J, and Lehnert BE, Correlation between particle size, in vivo particle persistence, and lung injury. Environ Health Perspect, 1994. 102 Suppl 5: p. 173–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Warheit DB, et al. , Pulmonary instillation studies with nanoscale TiO2 rods and dots in rats: toxicity is not dependent upon particle size and surface area. Toxicol Sci, 2006. 91(1): p. 227–36. [DOI] [PubMed] [Google Scholar]
- 10.Sager TM, Kommineni C, and Castranova V, Pulmonary response to intratracheal instillation of ultrafine versus fine titanium dioxide: role of particle surface area. Part Fibre Toxicol, 2008. 5: p.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baan R, et al. , Carcinogenicity of carbon black, titanium dioxide, and talc. Lancet Oncol, 2006. 7(4): p. 295–6. [DOI] [PubMed] [Google Scholar]
- 12.Lee KP, Trochimowicz HJ, and Reinhardt CF, Pulmonary response of rats exposed to titanium dioxide (TiO2) by inhalation for two years. Toxicol Appl Pharmacol, 1985. 79(2): p. 179–92. [DOI] [PubMed] [Google Scholar]
- 13.Humans, I.W.G.o.t.E.o.C.R.t., Carbon black, titanium dioxide, and talc. IARC Monogr Eval Carcinog Risks Hum, 2010. 93: p. 1–413. [PMC free article] [PubMed] [Google Scholar]
- 14.National Toxicology P., Bioassay of titanium dioxide for possible carcinogenicity. Natl Cancer Inst Carcinog Tech Rep Ser, 1979. 97: p. 1–123. [PubMed] [Google Scholar]
- 15.Wang J, et al. , Acute toxicity and biodistribution of different sized titanium dioxide particles in mice after oral administration. Toxicol Lett, 2007. 168(2): p. 176–85. [DOI] [PubMed] [Google Scholar]
- 16.Tassinari R, et al. , Oral, short-term exposure to titanium dioxide nanoparticles in Sprague-Dawley rat: focus on reproductive and endocrine systems and spleen. Nanotoxicology, 2014. 8(6): p. 654–62. [DOI] [PubMed] [Google Scholar]
- 17.Mohamed HR, Estimation of TiO(2) nanoparticle-induced genotoxicity persistence and possible chronic gastritis-induction in mice. Food Chem Toxicol, 2015. 83: p. 76–83. [DOI] [PubMed] [Google Scholar]
- 18.Shukla RK, et al. , TiO(2) nanoparticles induce oxidative DNA damage and apoptosis in human liver cells. Nanotoxicology, 2013. 7(1): p. 48–60. [DOI] [PubMed] [Google Scholar]
- 19.Azim SA, et al. , Amelioration of titanium dioxide nanoparticles-induced liver injury in mice: possible role of some antioxidants. Exp Toxicol Pathol, 2015. 67(4): p. 305–14. [DOI] [PubMed] [Google Scholar]
- 20.Orazizadeh M, et al. , Effect of glycyrrhizic acid on titanium dioxide nanoparticles-induced hepatotoxicity in rats. Chem Biol Interact, 2014. 220: p. 214–21. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, et al. , Susceptibility of young and adult rats to the oral toxicity of titanium dioxide nanoparticles. Small, 2013. 9(9-10): p. 1742–52. [DOI] [PubMed] [Google Scholar]
- 22.Jia F, et al. , Effect of pubertal nano-TiO2 exposure on testosterone synthesis and spermatogenesis in mice. Arch Toxicol, 2014. 88(3): p. 781–8. [DOI] [PubMed] [Google Scholar]
- 23.Ruiz PA, et al. , Titanium dioxide nanoparticles exacerbate DSS-induced colitis: role of the NLRP3 inflammasome. Gut, 2017. 66(7): p. 1216–1224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kreyling WG, et al. , Quantitative biokinetics of titanium dioxide nanoparticles after intravenous injection in rats: Part 1. Nanotoxicology, 2017. 11(4): p. 434–442. [DOI] [PubMed] [Google Scholar]
- 25.Kreyling WG, et al. , Quantitative biokinetics of titanium dioxide nanoparticles after intratracheal instillation in rats: Part 3. Nanotoxicology, 2017. 11(4): p. 454–464. [DOI] [PubMed] [Google Scholar]
- 26.Kreyling WG, et al. , Quantitative biokinetics of titanium dioxide nanoparticles after oral application in rats: Part 2. Nanotoxicology, 2017. 11(4): p. 443–453. [DOI] [PubMed] [Google Scholar]
- 27.Brun E, et al. , Titanium dioxide nanoparticle impact and translocation through ex vivo, in vivo and in vitro gut epithelia. Part Fibre Toxicol, 2014. 11: p. 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Powell JJ, et al. , Origin and fate of dietary nanoparticles and microparticles in the gastrointestinal tract. J Autoimmun, 2010. 34(3): p. J226–33. [DOI] [PubMed] [Google Scholar]
- 29.Powell JJ, et al. , Characterisation of inorganic microparticles in pigment cells of human gut associated lymphoid tissue. Gut, 1996. 38(3): p. 390–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Powell JJ, et al. , An endogenous nanomineral chaperones luminal antigen and peptidoglycan to intestinal immune cells. Nat Nanotechnol, 2015. 10(4): p. 361–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manolova V, et al. , Nanoparticles target distinct dendritic cell populations according to their size. Eur J Immunol, 2008. 38(5): p. 1404–13. [DOI] [PubMed] [Google Scholar]
- 32.Winkler HC, et al. , Critical review of the safety assessment of titanium dioxide additives in food. J Nanobiotechnology, 2018. 16(1): p. 51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Winter M, et al. , Activation of the inflammasome by amorphous silica and TiO2 nanoparticles in murine dendritic cells. Nanotoxicology, 2011. 5(3): p. 326–40. [DOI] [PubMed] [Google Scholar]
- 34.Hummel TZ, et al. , Exogenous pigment in Peyer patches of children suspected of having IBD. J Pediatr Gastroenterol Nutr, 2014. 58(4): p. 477–80. [DOI] [PubMed] [Google Scholar]
- 35.McCracken C, et al. , Minimal intestinal epithelial cell toxicity in response to short- and long-term food-relevant inorganic nanoparticle exposure. Chem Res Toxicol, 2013. 26(10): p. 1514–25. [DOI] [PubMed] [Google Scholar]
- 36.Nel AE, et al. , Understanding biophysicochemical interactions at the nano-bio interface. Nat Mater, 2009. 8(7): p. 543–57. [DOI] [PubMed] [Google Scholar]
- 37.Ruh H, et al. , Identification of serum proteins bound to industrial nanomaterials. Toxicol Lett, 2012. 208(1): p. 41–50. [DOI] [PubMed] [Google Scholar]
- 38.Walkey CD and Chan WC, Understanding and controlling the interaction of nanomaterials with proteins in a physiological environment. Chem Soc Rev, 2012. 41(7): p. 2780–99. [DOI] [PubMed] [Google Scholar]
- 39.DeLoid GM, et al. , An integrated methodology for assessing the impact of food matrix and gastrointestinal effects on the biokinetics and cellular toxicity of ingested engineered nanomaterials. Part Fibre Toxicol, 2017. 14(1): p. 40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nagayama S, et al. , Time-dependent changes in opsonin amount associated on nanoparticles alter their hepatic uptake characteristics. Int J Pharm, 2007. 342(1-2): p. 215–21. [DOI] [PubMed] [Google Scholar]
- 41.McClements DJ, et al. , The Role of the Food Matrix and Gastrointestinal Tract in the assessment of biological properties of ingested engineered nanomaterials (iENMs): State of the science and knowledge gaps. NanoImpact, 2016. 3-4: p. 47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Boland M, Human digestion--a processing perspective. J Sci Food Agric, 2016. 96(7): p. 2275–83. [DOI] [PubMed] [Google Scholar]
- 43.Bettini S, et al. , Food-grade TiO2 impairs intestinal and systemic immune homeostasis, initiates preneoplastic lesions and promotes aberrant crypt development in the rat colon. Sci Rep, 2017. 7: p. 40373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Urrutia-Ortega IM, et al. , Food-grade titanium dioxide exposure exacerbates tumor formation in colitis associated cancer model. Food Chem Toxicol, 2016. 93: p. 20–31. [DOI] [PubMed] [Google Scholar]
- 45.Proquin H, et al. , Gene expression profiling in colon of mice exposed to food additive titanium dioxide (E171). Food Chem Toxicol, 2018. 111: p. 153–165. [DOI] [PubMed] [Google Scholar]
- 46.Additives, E.Panel o.F. and N.S.a.t. Food, Re-evaluation of titanium dioxide (E171) as a food additive. EFSA Journal, 2016. 14(9): p. e04545. [Google Scholar]
- 47.Additives, E.Panel o.F., et al. , Evaluation of four new studies on the potential toxicity of titanium dioxide used as a food additive (E171). EFSA Journal, 2018. 16(7): p. e05366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ortlieb M, White giant or white dwarf?: Particle size distribution measurements of TiO2. GIT Lab J Eur, 2010. 14: p. 42–43. [Google Scholar]
- 49.Martin RA, et al. , Randomization of Animals by Computer-Program for Toxicity Studies. Journal of the American College of Toxicology, 1984. 3(1): p. 1–11. [PubMed] [Google Scholar]
- 50.Yamamura K, Transformation using (x+0.5) to stabilize the variance of populations. Researches on Population Ecology, 1999. 41(3): p. 229–234. [Google Scholar]
- 51.Sayes CM, et al. , Correlating nanoscale titania structure with toxicity: a cytotoxicity and inflammatory response study with human dermal fibroblasts and human lung epithelial cells. Toxicol Sci, 2006. 92(1): p. 174–85. [DOI] [PubMed] [Google Scholar]
- 52.Scott CL, Aumeunier AM, and Mowat AM, Intestinal CD103+ dendritic cells: master regulators of tolerance? Trends Immunol, 2011. 32(9): p. 412–9. [DOI] [PubMed] [Google Scholar]
- 53.Hirahara K and Nakayama T, CD4+ T-cell subsets in inflammatory diseases: beyond the Th1/Th2 paradigm. Int Immunol, 2016. 28(4): p. 163–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Schmitt N, Bentebibel SE, and Ueno H, Phenotype and functions of memory Tfh cells in human blood. Trends Immunol, 2014. 35(9): p. 436–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yoshimi N, et al. , Expression of cytokines, TNF-alpha and IL-1 alpha, in MAM acetate and 1-hydroxyanthraquinone-induced colon carcinogenesis of rats. Carcinogenesis, 1994. 15(4): p. 783–5. [DOI] [PubMed] [Google Scholar]
- 56.Luu M, Steinhoff U, and Visekruna A, Functional heterogeneity of gut-resident regulatory T cells. Clin Transl Immunology, 2017. 6(9): p. e156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bluestone JA and Tang Q, How do CD4+CD25+ regulatory Tcells control autoimmunity? Curr Opin Immunol, 2005. 17(6): p. 638–42. [DOI] [PubMed] [Google Scholar]
- 58.Joosse ME, et al. , Tipping the balance: inhibitory checkpoints in intestinal homeostasis. Mucosal Immunol, 2019. 12(1): p. 21–35. [DOI] [PubMed] [Google Scholar]
- 59.Neurath MF, Cytokines in inflammatory bowel disease. Nat Rev Immunol, 2014. 14(5): p. 329–42. [DOI] [PubMed] [Google Scholar]
- 60.Francescone R, Hou V, and Grivennikov SI, Cytokines, IBD, and colitis-associated cancer. Inflamm Bowel Dis, 2015. 21(2): p. 409–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hamiza OO, et al. , Amelioration of 1,2 Dimethylhydrazine (DMH) induced colon oxidative stress, inflammation and tumor promotion response by tannic acid in Wistar rats. Asian Pac J Cancer Prev, 2012. 13(9): p. 4393–402. [DOI] [PubMed] [Google Scholar]
- 62.Thoolen B, et al. , Proliferative and nonproliferative lesions of the rat and mouse hepatobiliary system. Toxicol Pathol, 2010. 38(7 Suppl): p. 5S–81S. [DOI] [PubMed] [Google Scholar]
- 63.Wright JR Jr., et al. , Testicular atrophy in the spontaneously diabetic BB Wistar rat. Am J Pathol, 1982. 108(1): p. 72–9. [PMC free article] [PubMed] [Google Scholar]
- 64.Foley GL, Overview of male reproductive pathology. Toxicol Pathol, 2001. 29(1): p. 49–63. [DOI] [PubMed] [Google Scholar]
- 65.James RW and Heywood R, Age-related variations in the testes and prostate of beagle dogs. Toxicology, 1979. 12(3): p. 273–9. [DOI] [PubMed] [Google Scholar]
- 66.James RW and Heywood R, Age-Related Variations in the Testes of Sprague-Dawley Rats. Toxicology Letters, 1979. 4(4): p. 257–261. [DOI] [PubMed] [Google Scholar]
- 67.Corpet DE and Tache S, Most effective colon cancer chemopreventive agents in rats: a systematic review of aberrant crypt foci and tumor data, ranked by potency. Nutr Cancer, 2002. 43(1): p. 1–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wargovich MJ, Brown VR, and Morris J, Aberrant crypt foci: the case for inclusion as a biomarker for colon cancer. Cancers (Basel), 2010. 2(3): p. 1705–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhou L, et al. , Suggestive evidence for the induction of colonic aberrant crypts in mice fed sodium nitrite. Nutr Cancer, 2016. 68(1): p. 105–12. [DOI] [PubMed] [Google Scholar]
- 70.Strugala V, et al. , Colonic mucin: methods of measuring mucus thickness. Proc Nutr Soc, 2003. 62(1): p. 237–43. [DOI] [PubMed] [Google Scholar]
- 71.Van der Sluis M, et al. , Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology, 2006. 131(1): p. 117–29. [DOI] [PubMed] [Google Scholar]
- 72.Pelaseyed T, et al. , The mucus and mucins of the goblet cells and enterocytes provide the first defense line of the gastrointestinal tract and interact with the immune system. Immunol Rev, 2014. 260(1): p. 8–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Velcich A, et al. , Colorectal cancer in mice genetically deficient in the mucin Muc2. Science, 2002. 295(5560): p. 1726–9. [DOI] [PubMed] [Google Scholar]
- 74.Hansen GH, et al. , Endocytic trafficking from the small intestinal brush border probed with FM dye. Am J Physiol Gastrointest Liver Physiol, 2009. 297(4): p. G708–15. [DOI] [PubMed] [Google Scholar]
- 75.Hyun YS, et al. , Role of IL-17A in the development of colitis-associated cancer. Carcinogenesis, 2012. 33(4): p. 931–6. [DOI] [PubMed] [Google Scholar]
- 76.Williams MB and Butcher EC, Homing of naive and memory T lymphocyte subsets to Peyer’s patches, lymph nodes, and spleen. J Immunol, 1997. 159(4): p. 1746–52. [PubMed] [Google Scholar]
- 77.Lundqvist M, et al. , Nanoparticle size and surface properties determine the protein corona with possible implications for biological impacts. Proc Natl Acad Sci U S A, 2008. 105(38): p. 14265–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cedervall T, et al. , Understanding the nanoparticle-protein corona using methods to quantify exchange rates and affinities of proteins for nanoparticles. Proc Natl Acad Sci U S A, 2007. 104(7): p. 2050–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Cho WS, et al. , Comparative absorption, distribution, and excretion of titanium dioxide and zinc oxide nanoparticles after repeated oral administration. Part Fibre Toxicol, 2013. 10: p. 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fu Y, et al. , Systemic immune effects of titanium dioxide nanoparticles after repeated intratracheal instillation in rat. Int J Mol Sci, 2014. 15(4): p. 6961–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kobayashi N, et al. , Comparative pulmonary toxicity study of nano-TiO(2) particles of different sizes and agglomerations in rats: different short- and long-term post-instillation results. Toxicology, 2009. 264(1-2): p. 110–8. [DOI] [PubMed] [Google Scholar]
- 82.Begley CG, Six red flags for suspect work. Nature, 2013. 497(7450): p. 433–4. [DOI] [PubMed] [Google Scholar]
- 83.Talbot P, et al. , Food-grade TiO2 is trapped by intestinal mucus in vitro but does not impair mucin O-glycosylation and short-chain fatty acid synthesis in vivo: implications for gut barrier protection. J Nanobiotechnology, 2018. 16(1): p. 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Perse M, The dimethylhydrazine induced colorectal tumours in rat - experimental colorectal carcinogenesis. Radiol Oncol, 2005. 39(1). [Google Scholar]
- 85.Rosenberg DW, Giardina C, and Tanaka T, Mouse models for the study of colon carcinogenesis. Carcinogenesis, 2009. 30(2): p. 183–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Cooper HS, et al. , Dysplasia and cancer in the dextran sulfate sodium mouse colitis model. Relevance to colitis-associated neoplasia in the human: a study of histopathology, B-catenin and p53 expression and the role of inflammation. Carcinogenesis, 2000. 21(4): p. 757–68. [DOI] [PubMed] [Google Scholar]
- 87.Kozoni V, et al. , The effect of lithocholic acid on proliferation and apoptosis during the early stages of colon carcinogenesis: differential effect on apoptosis in the presence of a colon carcinogen. Carcinogenesis, 2000. 21(5): p. 999–1005. [DOI] [PubMed] [Google Scholar]
- 88.Redston M, Carcinogenesis in the GI tract: from morphology to genetics and back again. Mod Pathol, 2001. 14(3): p. 236–45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.