Abstract
Rationale: Chronic obstructive pulmonary disease (COPD) has been associated with numerous genetic variants, yet the extent to which its genetic risk is mediated by variation in lung structure remains unknown.
Objectives: To characterize associations between a genetic risk score (GRS) associated with COPD susceptibility and lung structure on computed tomography (CT).
Methods: We analyzed data from MESA Lung (Multi-Ethnic Study of Atherosclerosis Lung Study), a U.S. general population–based cohort, and SPIROMICS (Subpopulations and Intermediate Outcome Measures in COPD Study). A weighted GRS was calculated from 83 SNPs that were previously associated with lung function. Lung density, spatially matched airway dimensions, and airway counts were assessed on full-lung CT. Generalized linear models were adjusted for age, age squared, sex, height, principal components of genetic ancestry, smoking status, pack-years, CT model, milliamperes, and total lung volume.
Measurements and Main Results: MESA Lung and SPIROMICS contributed 2,517 and 2,339 participants, respectively. Higher GRS was associated with lower lung function and increased COPD risk, as well as lower lung density, smaller airway lumens, and fewer small airways, without effect modification by smoking. Adjustment for CT lung structure, particularly small airway measures, attenuated associations between the GRS and FEV1/FVC by 100% and 60% in MESA and SPIROMICS, respectively. Lung structure (P < 0.0001), but not the GRS (P > 0.10), improved discrimination of moderate-to-severe COPD cases relative to clinical factors alone.
Conclusions: A GRS associated with COPD susceptibility was associated with CT lung structure. Lung structure may be an important mediator of heritability and determinant of personalized COPD risk.
Keywords: spirometry, emphysema, airway remodeling
At a Glance Commentary
Scientific Knowledge on the Subject
Chronic obstructive pulmonary disease (COPD) has been associated with numerous genetic variants, yet the extent to which its genetic risk is mediated by variation in lung structure remains unknown.
What This Study Adds to the Field
This is the first study to demonstrate that a genetic risk score associated with COPD susceptibility was also associated with variation in lung structure on computed tomography. Our findings suggest that elements of lung structure, particularly those relating to the small airways, may be important mediators of COPD heritability and major determinants of personalized risk for COPD.
Chronic obstructive pulmonary disease (COPD), which is defined by airflow limitation that is incompletely reversible (1), is the third leading cause of death worldwide (2, 3). Prediction and prevention of COPD are particularly important given the lack of medical therapies that have been proved to reduce COPD-related mortality (4, 5).
Numerous genetic variants have been associated with low lung function and COPD, providing a promising avenue for estimation of personalized COPD risk. A genome-wide association study (GWAS) of participants at extremes of lung function in the UK Biobank found 43 new signals for lung function (6). Combined with 54 previously reported signals, 97 SNPs were estimated to account for up to 14% of the SNP-based heritability of lung function traits, which is equivalent to one-third of the total estimated heritability (7, 8). Based on 95 of 97 SNPs, a genetic risk score (GRS) was developed and found to be associated with COPD status in several COPD case–control studies, a lung resection cohort, and two community-based studies (6). Furthermore, the GRS was associated with lung function in children (6), suggesting that it may index developmental factors relevant to risk of spirometry-defined COPD in adulthood (9–11).
Variations in lung structure are increasingly recognized as risk determinants for COPD (12–19). We tested the hypothesis that the GRS would be associated with quantitative measures from lung computed tomography (CT) in adults included in a general U.S. population–based cohort and a large case–control study of COPD. Furthermore, to understand the potential importance of genetic testing and CT scanning in COPD risk prediction, we tested the contributions of the GRS and CT lung structure to discrimination of moderate-to-severe COPD. Some of the results have been previously reported in the form of abstracts (20, 21).
Methods
Samples
MESA (Multi-Ethnic Study of Atherosclerosis) enrolled 6,814 participants, 45–84 years old, who self-reported non-Hispanic white, African American, Hispanic/Latino, or Asian American race/ethnicity in 2000–2002. The exclusion criteria were a history of clinical cardiovascular disease, weight > 136 kg, and impediments to long-term participation (22). MESA Lung (23) comprised a random subsample with baseline endothelial function measurement, genetic consent, and spirometry in 2004–2006; participants in MESA Air, which recruited 257 participants in 2006–2007 using MESA inclusion criteria (24); and a random sample of participants undergoing cardiac magnetic resonance imaging in 2010–2012. The present analysis included all MESA Lung participants who underwent chest CT in 2010–2012.
SPIROMICS (Subpopulations and Intermediate Outcome Measures in COPD Study) recruited individuals with and without COPD, 40–80 years old, with ≥20 pack-years of smoking, in 2010–2015 (25). The exclusion criteria included other chronic lung diseases except asthma, body mass index > 40 kg/m2, prior lung resection, metal in chest, and pregnancy. A small number of SPIROMICS nonsmokers (<1 pack-year) were excluded from the current analysis.
Institutional review board approval was obtained at each clinical site for both studies. Written informed consent was obtained from all participants.
Genotyping
Genotyping was performed in MESA using the Affymetrix Human SNP array 6.0 (Affymetrix Inc.), and in SPIROMICS via the Illumina HumanOmniExpressExome BeadChip and BeadStudio (Illumina, Inc.). A total of 897,981 SNPs passed study-specific quality control, and an additional ∼2 million SNPs were imputed in each racial/ethnic group via 1,000 Genomes imputation (Phase 3, v5) and the Haplotype Reference Consortium.
The GRS was calculated and risk alleles were weighted based on the prior publication (6). SNPs that were not available in the imputation files or had low imputation quality (R2 ≤ 0.5) were not used (Table E1 in the online supplement). An unweighted GRS was used for sensitivity analyses.
Genotype data were also used to estimate principal components of genetic ancestry (26).
Lung Function
Spirometry was performed in accordance with American Thoracic Society recommendations (27) on a dry-rolling-seal spirometer in MESA Lung and a pneumotachograph in SPIROMICS. Exams with fewer than two acceptable measures repeatable within 200 ml were excluded. Predicted values and limits of normal were calculated using reference equations (28). COPD was defined as a post-bronchodilator FEV1/FVC < 0.70 (1). Moderate-to-severe COPD was defined by an FEV1% predicted < 80% (1).
Lung Structure
Participants underwent full-lung inspiratory CT on 64-slice or 128-slice helical scanners (120 kVp, 0.625- to 0.75-mm slice thickness, and 0.5-s rotation time) via the same protocol in both studies (29).
Percent emphysema was defined on inspiratory scans as lung voxels with attenuation < −950 Hounsfield units (HU) divided by total imaged lung voxels × 100 (30). The upper limit of normal (ULN) for percent emphysema was defined by reference equations (31).
In SPIROMICS, paired expiratory scans were also obtained, permitting parametric response mapping (PRM) analysis to define functional small airway disease (PRMfSAD) as areas of lung >−950 HU on inspiration and <−856 HU on expiration (32).
Airway dimensions were assessed on inspiratory scans at a single reading center for both studies blinded to other participant information (33). The central airway tree was identified using Apollo Software (VIDA Diagnostics). Airways were labeled anatomically from the trachea to subsegmental bronchi along five prespecified paths (RB1, RB4, RB10, LB1, and LB10). Segmentation and labeling were visually verified by a dedicated image analyst and all labeled airways were assigned a generation number based on the number of branch points from the trachea (generation 0). The small airway count was defined as the sum of airway counts for generations 6 or greater (34). All paths were counted in MESA Lung, whereas only five paths were measured in SPIROMICS.
The cross-sectional airway wall area and wall thickness, as well as the lumen area, diameter, and perimeter, were measured perpendicular to the local airway segment’s long axis using a subvoxel resolution algorithm, within an image plane, and measurements were averaged along the middle third of each labeled airway segment (33, 35, 36). Percent wall area was defined as (airway wall area/total cross-sectional area) × 100.
Covariates
Age, sex, race/ethnicity, and tobacco use were self-reported. Never-smokers in MESA were defined by lifetime smoking of <100 cigarettes, and current smokers were defined by cigarette use within the past 30 days, with biochemical verification in a subset (24). Pack-years were calculated as (cigarettes per day/20) × years smoked. Height was measured using standard techniques.
Statistical Analysis
Effect estimates for the GRS were reported per SD and per quintile. All models were stratified by study.
Associations between the GRS and lung function (FEV1, FVC, and FEV1/FVC), lung density (log-transformed percent emphysema and PRMfSAD), and small airway count were analyzed in linear regression models. Associations with moderate-to-severe COPD were tested by logistic regression. Generalized estimating equations accounting for repeated measures within subjects were used to test associations with airway dimensions.
As in the study that derived the GRS (6), models were adjusted for age, age squared, sex, height, smoking status, pack-years, principal components of ancestry 1–10, and site. Models for CT lung structure endpoints were additionally adjusted for CT model, milliamperes, and total imaged lung volume. In sensitivity analyses, the impact of additional adjustment for lung function was assessed, and differential associations by age, racial/ethnic group, and smoking status were tested in stratified models and via multiplicative interaction terms.
Based on our hypotheses (Figure E1), we assessed the potential mediation of the association between the GRS and low lung function (37). First, the effect estimate for the GRS with respect to lung function was assessed in models adjusted for the standard covariates mentioned above, yielding βGRS,total. Next, the effect estimate for the GRS was recalculated after additional adjustment for elements of lung structure, which were treated as mediators, yielding βGRS,unmediated. Percent mediation was defined as (βGRS,total − βGRS,unmediated)/βGRS,total × 100. The Sobel test was used to test the significance of potential mediation effects.
With respect to discrimination of cases of moderate-to-severe COPD, logistic regression models including clinical risk factors only versus those additionally including the GRS and/or lung structure were contrasted using concordance (c) statistics, or rank correlations between predicted probabilities of moderate-to-severe COPD versus the observed disease status (38), and receiver operating characteristic curves, compared using DeLong’s test (39).
Analyses were performed in SAS, version 9.4 or R.
Results
Characteristics
The analytic sample included 2,517 MESA Lung participants and 2,339 SPIROMICS participants (Table 1). The average age was 69 years for MESA Lung participants and 64 years for SPIROMICS participants, and the proportion of male participants was 48% and 54%, respectively.
Table 1.
Baseline Characteristics of the Participants
| MESA Lung (N = 2,517) | SPIROMICS (N = 2,339) |
||
|---|---|---|---|
| Control Subjects (n = 781) | Cases (n = 1,558) | ||
| Genetic risk score, mean (SD) | 87.3 (6.6) | 88.0 (6.6) | 88.7 (6.5) |
| Age, mean (SD), yr | 69.1 (9.3) | 60.5 (9.6) | 65.3 (8.0) |
| Height, mean (SD), cm | 165.4 (9.9) | 169.5 (9.3) | 170.2 (9.6) |
| Body mass index, mean (SD), kg/m2 | 28.4 (5.5) | 29.0 (5.1) | 27.3 (5.3) |
| Male, n (%) | 1,206 (47.9) | 374 (47.9) | 894 (57.4) |
| Race/ethnicity, n (%) | |||
| Non-Hispanic white | 995 (39.5) | 557 (71.3) | 1,292 (82.9) |
| African American | 617 (24.5) | 206 (26.4) | 238 (15.3) |
| Hispanic/Latino | 551 (21.9) | 0 (0) | 0 (0) |
| Asian American | 354 (14.1) | 4 (0.5) | 19 (1.2) |
| Smoking status, n (%) | |||
| Never | 1,228 (48.8) | 0 (0) | 0 (0) |
| Former | 1,126 (44.7) | 382 (48.9) | 1,018 (65.3) |
| Current | 163 (6.5) | 399 (51.1) | 540 (34.7) |
| Pack-years, median (Q1–Q3)* | 12 (2–30) | 37.5 (30.0–49.5) | 47.0 (35.0–63.0) |
| Lung function | |||
| FEV1, mean (SD), percent predicted† | 95.5 (23.0) | 90.8 (14.3) | 53.9 (22.4) |
| FEV1/FVC, mean (SD), percent | 74.1 (8.8) | 74.0 (5.5) | 49.7 (12.9) |
| COPD, n (%) | |||
| GOLD 1 | 89 (4.2) | 11 (1.4) | 340 (21.9) |
| GOLD 2 | 62 (2.9) | 9 (1.2) | 699 (44.9) |
| GOLD 3 | 9 (0.4) | 0 (0) | 363 (23.3) |
| GOLD 4 | 8 (0.4) | 0 (0) | 148 (9.5) |
| Lung structure | |||
| Lung density | |||
| Percent emphysema, median (Q1–Q3), percent | 1.44 (0.58–3.02) | 0.98 (0.47–2.06) | 6.70 (2.34–15.46) |
| Percent emphysema > ULN, n (%) | 207 (8.2) | 59 (7.6) | 836 (53.7) |
| PRMfSAD, median (Q1–Q3), percent | 6.0 (2.0–11.0) | 27.0 (16.0–37.0) | |
| Airway dimensions | |||
| Inner airway diameter, median (Q1–Q3), mm | 4.3 (3.6–5.0) | 4.4 (3.1–6.2) | 4.1 (2.7–5.9) |
| Average wall area, median (Q1–Q3), mm2 | 30.3 (24.2–37.1) | 51.7 (29.1–87.9) | 47.3 (25.2–82.6) |
| Percent wall area, median (Q1–Q3), percent | 61.5 (58.2–64.4) | 60.2 (53.6–65.0) | 61.8 (55.0–66.3) |
| Small airway count, n (SD) | 186 (124–250) | 18 (17–19) | 17 (14–18) |
Definition of abbreviations: COPD = chronic obstructive pulmonary disease; GOLD = Global Initiative for Chronic Obstructive Lung Disease; MESA = Multi-Ethnic Study of Atherosclerosis; PRMfSAD = parametric response mapping of functional small airway disease; Q = quartile; SPIROMICS = Subpopulations and Intermediate Outcome Measures in COPD Study; ULN = upper limit of normal.
Excludes participants without valid measurement of genetic risk score for COPD susceptibility and/or valid measurements of lung function and structure.
In ever-smokers.
Prebronchodilator.
With respect to self-reported race/ethnicity, 40% of MESA Lung participants were non-Hispanic white, 25% were African American, 22% were Hispanic/Latino, and 14% were Asian American. In SPIROMICS, 80% were non-Hispanic white and 19% were African American. GRS distributions varied slightly by race/ethnicity: compared with non-Hispanic white participants, African American and Hispanic/Latino participants had higher average GRS, whereas Asian American participants had lower average GRS (Figure E2).
In MESA Lung, 49% of the participants were never-smokers and 7% were current smokers. Among ever-smokers, the median pack-years smoked was 12. By contrast, 40% of the SPIROMICS participants were current smokers, and the median pack-years smoked was 44. Although participants in higher quintiles of the GRS for COPD included a higher proportion of current smokers (Tables E2 and E3), these differences were not statistically significant within racial/ethnic strata.
Moderate-to-severe COPD was present in 4% (n = 79) of MESA Lung participants, in contrast to 52% (n = 1,219) of SPIROMICS participants (Table 1). In both cohorts, lower lung function and more cases of moderate-to-severe COPD were observed in higher GRS quintiles (Tables E2 and E3).
Percent emphysema > ULN was found in 8% and 38% of MESA Lung and SPIROMICS participants, respectively (Table 1). Airway measurements were similar across cohorts, except for the small airway count, which was expected given the differences in the airways measured. Participants in higher GRS quintiles demonstrated more percent emphysema > ULN, fewer small airways, thinner airway lumens, and thinner airway walls (Tables E2 and E3).
Lung Function
In adjusted models, higher GRS was associated with lower FEV1 and FEV1/FVC (Table 2). Effect estimates were larger in SPIROMICS than in MESA Lung, yet the results were statistically significant in both cohorts; associations with FVC were nonsignificant. One SD greater GRS was associated with an odds ratio for moderate-to-severe COPD of 1.44 in MESA Lung and 1.28 in SPIROMICS. There was no evidence for effect modification by race/ethnicity (Pinteraction > 0.20).
Table 2.
Associations between the Genetic Risk Score and Lung Function
| Quintile of Genetic Risk Score |
Per SD (95% CI) | P Value* | |||||
|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | |||
| FEV1, ml | |||||||
| MESA Lung | Ref | 12.3 | −19.1 | −50.3 | −56.2 | −22.1 (−40.3 to −3.9) | 0.0173 |
| SPIROMICS | Ref | −10.4 | −58.4 | −48.8 | −173.1† | −56.0 (−86.5 to −26.0) | 0.0003 |
| FVC, ml | |||||||
| MESA Lung | Ref | 30.3 | 28.3 | 10.8 | 21.8 | 7.1 (−14.9 to 29.2) | 0.53 |
| SPIROMICS | Ref | 28.7 | 0.1 | 21.0 | −70.1 | −21.5 (−50.1 to 7.8) | 0.15 |
| FEV1/FVC, percent | |||||||
| MESA Lung | Ref | −0.52 | −1.28‡ | −1.83† | −2.21† | −0.80 (−1.13 to −0.46) | <0.0001 |
| SPIROMICS | Ref | −0.73 | −1.71 | −1.74 | −4.32† | −1.37 (−1.95 to −0.78) | <0.0001 |
| Moderate-to-severe COPD | |||||||
| MESA Lung | Ref | 0.90 | 1.57 | 2.23 | 2.14 | 1.44 (1.12 to 1.86) | 0.0051 |
| SPIROMICS | Ref | 1.11‡ | 1.32 | 1.50 | 2.01† | 1.28 (1.17 to 1.40) | <0.0001 |
Definition of abbreviations: CI = confidence interval; COPD = chronic obstructive pulmonary disease; MESA = Multi-Ethnic Study of Atherosclerosis; Q = quintile; Ref = reference; SPIROMICS = Subpopulations and Intermediate Outcome Measures in COPD Study.
One SD is equivalent to 6.6 in MESA Lung and 6.5 in SPIROMICS. The linear regression models were adjusted for age, age2, sex, height, smoking status, pack-years, principal components of ancestry 1–10, and site.
Significance for linear model of continuous genetic risk score.
Significance for quintiles of genetic risk score: P < 0.001.
Significance for quintiles of genetic risk score: P < 0.05.
Lung Structure
Higher GRS was associated with lower lung density (Table 3). In MESA Lung, per SD, the GRS for COPD was associated with 5% greater percent emphysema. In SPIROMICS, the GRS was not significantly associated with percent emphysema, but was associated with 6% greater PRMfSAD per SD.
Table 3.
Associations between the Genetic Risk Score and Lung Structure on Computed Tomography
| Quintile of Genetic Risk Score |
Per SD (95% CI) | P Value* | |||||
|---|---|---|---|---|---|---|---|
| Q1 | Q2 | Q3 | Q4 | Q5 | |||
| Lung density | |||||||
| Log-transformed % emphysema | |||||||
| MESA Lung | Ref | 1.14† | 1.13† | 1.15† | 1.14† | 1.05 (1.02 to 1.09) | 0.0010 |
| SPIROMICS | Ref | 1.00 | 1.03 | 1.02 | 1.10 | 1.03 (0.99 to 1.08) | 0.147 |
| Log-transformed % PRMfSAD | |||||||
| SPIROMICS | Ref | 0.98 | 1.03 | 1.06 | 1.15† | 1.06 (1.02 to 1.10) | 0.0027 |
| Small airway count‡ | |||||||
| MESA Lung | Ref | −9.59 | −22.16§ | −27.07§ | −34.18§ | −13.65 (−17.06 to −10.25) | <0.0001 |
| SPIROMICS | Ref | −0.44 | −0.49 | −0.41 | −0.83† | −0.28 (−0.48 to −0.09) | 0.0037 |
| Airway dimensions | |||||||
| Inner airway diameter | |||||||
| MESA Lung | Ref | 0.04 | −0.01 | −0.04 | −0.09§ | −0.07 (−0.09 to −0.05) | <0.0001 |
| SPIROMICS | Ref | 0.05 | 0.00 | −0.01 | −0.09§ | −0.06 (−0.08 to −0.03) | <0.0001 |
| Average wall area | |||||||
| MESA Lung | Ref | 0.26 | −0.15 | −0.25 | −0.63† | −0.47 (−0.65 to −0.29) | <0.0001 |
| SPIROMICS | Ref | 1.11† | 0.07 | −0.42 | −2.14§ | −1.31 (−1.85 to −0.78) | <0.0001 |
| Percent wall area | |||||||
| MESA Lung | Ref | −0.25† | 0.04 | 0.18 | 0.42§ | 0.32 (0.21 to 0.42) | <0.0001 |
| SPIROMICS | Ref | −0.05 | −0.06 | 0.04 | 0.29§ | 0.17 (0.09 to 0.26) | <0.0001 |
Definition of abbreviations: CI = confidence interval; MESA = Multi-Ethnic Study of Atherosclerosis; PRMfSAD = functional small airway disease; Q = quintile; Ref = reference; SPIROMICS = Subpopulations and Intermediate Outcome Measures in COPD Study.
One SD is equivalent to 6.6 in MESA and 6.5 in SPIROMICS. Number of participants: 2,517 in MESA analyses and 2,339 in SPIROMICS analyses. The linear regression model was adjusted for age, age2, sex, height, smoking status, pack-years, principal components of ancestry 1–10, site, computed tomography scanner model, and body mass index high/low; airway count and dimension analyses were additionally adjusted for total lung volume and lobe.
Significance for linear model of continuous genetic risk score.
Significance for quintiles of genetic risk score: P < 0.05.
Count of airway generations 6–9. All paths were counted in MESA, whereas only five paths were measured in SPIROMICS.
Significance for quintiles of genetic risk score: P < 0.001.
Furthermore, higher GRS was associated with lower small airway counts and smaller airway lumens in both cohorts, with similar effect estimates. Higher GRS was associated with thinner airway walls but a greater percent wall area, consistent with a proportionately greater decrement in lumen size than in wall area.
Results according to GRS quintile showed monotonic associations without strong evidence for nonlinear associations.
Sensitivity Analyses
Among never-smokers in MESA Lung (n = 1,228), significant associations with the GRS were observed for all lung structural elements, and there was no evidence of effect modification by smoking status in either cohort (Pinteraction > 0.20; Table E4 and Figures E3–E5).
Compared with participants <65 years old, elderly SPIROMICS participants demonstrated a larger effect estimate for small airway count (Pinteraction = 0.0066) and, notably, demonstrated the same effect estimate for percent emphysema as was observed in elderly MESA Lung participants (1.05 per SD of the GRS; Table E4 and Figures E3–E5).
Associations between the GRS and lung structure on CT were similar across strata of race/ethnicity in MESA Lung (Table E4 and Figures E3–E5). In SPIROMICS, African American participants demonstrated weaker GRS–structure associations, but no definite evidence for effect modification was observed (Pinteraction = 0.054–0.91).
Further adjustment for lung function substantially attenuated associations between the GRS and PRMfSAD in SPIROMICS (Table E5). By contrast, associations between the GRS and percent emphysema were independent of lung function in MESA Lung, as were associations with airway dimensions and airway counts in both cohorts.
Similar results were obtained using the unweighted GRS (Tables E6 and E7).
Mediation
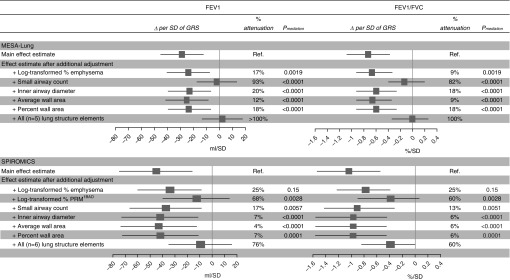
Associations between the GRS and FEV1 and FEV1/FVC were attenuated by adjustment for lung structure on CT (Figure 1), consistent with substantial mediation of GRS–lung function associations by lung structure. In MESA Lung, the small airway count alone attenuated 93% of the association with FEV1 and 82% of the association with FEV1/FVC. In SPIROMICS, the greatest attenuation was observed for PRMfSAD: 68% for FEV1 and 60% for FEV1/FVC. Percent emphysema was associated with a significant attenuation in MESA Lung, but not in SPIROMICS. In both cohorts, small airway dimensions were associated with a modest but significant attenuation. Adjustment for all CT lung structure elements in the same model resulted in full attenuation of the associations in MESA Lung, and 60–76% attenuation in SPIROMICS.
Figure 1.
Associations between the genetic risk score (GRS) and lung function, sequentially adjusted by features of lung structure on computed tomography (CT). Number of participants: 2,517 in MESA (Multi-Ethnic Study of Atherosclerosis) analyses and 2,339 in SPIROMICS (Subpopulations and Intermediate Outcome Measures in COPD Study) analyses. The linear regression model was adjusted for age, age2, sex, height, smoking status, pack-years, principal components of ancestry 1–10, site, CT model, body mass index high/low, total imaged lung volume, and lobe. The percent attenuation of the main effect estimate after additional adjustment for CT lung structural element(s) is calculated as [(original effect estimate − adjusted effect estimate)/original effect estimate] × 100. The statistical significance of potential mediational effects was evaluated by the Sobel (cross-product) method. COPD = chronic obstructive pulmonary disease; PRMfSAD = parametric response mapping of functional small airway disease; Ref. = reference.
Discrimination of Moderate-to-Severe COPD
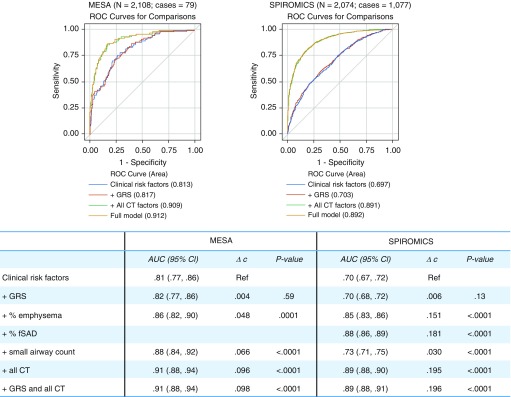
Despite strong and statistically significant associations with moderate-to-severe COPD, the contribution of the GRS to discrimination of cases was low: the c-statistic increased 0.004 in MESA Lung and 0.006 in SPIROMICS (P > 0.10 for both; Figure 2). By contrast, percent emphysema, percent PRMfSAD, and small airway count all significantly improved discrimination of cases. The combination of these lung structure features increased the c-statistic by 0.096 in MESA Lung and 0.195 in SPIROMICS, for a total area under the curve of 0.91 in MESA Lung and 0.89 in SPIROMICS.
Figure 2.
Receiver operating characteristic (ROC) curves for moderate-to-severe chronic obstructive pulmonary disease according to clinical risk factors, lung structure, and genetic risk score (GRS). Clinical risk factors included in the referent model were age, age2, sex, height, smoking status, pack-years, site, and principal components of ancestry 1–10. To this model, the following factors were added individually: the GRS for COPD, percent emphysema, percent functional small airway disease (fSAD, where available), and small airway count. Then, all CT factors (percent emphysema, percent fSAD, and small airway count) were added as a group. Lastly, a full model including clinical risk factors, the GRS, and CT factors was compared. Participants with missing post-bronchodilator spirometry data in MESA (Multi-Ethnic Study of Atherosclerosis) Lung or missing parametric response mapping of fSAD data in SPIROMICS (Subpopulations and Intermediate Outcome Measures in COPD Study) were excluded. AUC = area under the curve; CI = confidence interval; COPD = chronic obstructive pulmonary disease; CT = computed tomography; Ref = reference.
Discussion
In a general U.S. population–based sample, as well as in a case–control study of COPD, we found that a GRS associated with COPD susceptibility was significantly associated with greater percent emphysema and PRMfSAD, fewer small airways, smaller airway lumens, and thinner airway walls. Elements of lung structure on CT, particularly the small airway count and PRMfSAD, strongly attenuated the associations between the GRS and lower lung function, consistent with substantial mediation. We also observed that lung structure on CT provided greater discrimination of moderate-to-severe COPD cases than the GRS. Our results suggest that variation in lung structure on CT may be an important mediator of COPD heritability and may be suitable for personalized prediction of COPD.
Risk of COPD has been associated with impairments in peak lung function and accelerated lung function decline (9). Peak lung function is determined by developmental and early life factors (40), whereas the most well-established cause of accelerated lung function decline is smoking (41, 42). Current evidence suggests that genetic variants associated with low lung function are more likely to be associated with peak lung function than with accelerated decline (43). For example, previous genetic studies of lung function have implicated pathways (e.g., Hedgehog signaling) critical to early development (6, 12), and demonstrated a shared genetic architecture for lung function among never- and heavy smokers (44). To date, strong gene–environment interactions with respect to smoking and lung function decline have remained elusive, although this may be due to inadequate statistical power (45). Consistent with the hypothesis that a GRS derived from a GWAS of lung function traits, not clinical COPD, would predominantly index developmental rather than acquired risk factors, we found similar GRS performance in never- and ever-smokers.
Peak lung function is contingent on adequate lung structural development (46, 47). Impairments in lung growth have been linked to early life infections and environmental exposures, such as parental smoking and air pollution (10, 48–51). Although lung CT has not been used extensively in healthy children and young adults, it has demonstrated marked differences among adults with respect to lung density and airway dimensions, which have been validated against pathological samples and associated with COPD incidence and progression (16–19, 52–54). By correlating adult structural variation with variation in genetic risk, this work provides evidence that variation in lung structure may be a biologically plausible mechanism for increased genetic susceptibility to COPD, and suggests that CT lung structure in middle and old age may be informative with respect to developmental differences and peak lung development.
In particular, our work suggests that small airway development and dysfunction may be important mediators of lung function heritability. The small airway count was found to be a potentially major mediator of genetic risk in the population-based MESA sample, although less so in SPIROMICS. This may be due to methodological differences or selection biases, particularly relating to the “healthy smokers” recruited in SPIROMICS (55). Nonetheless, it is interesting to consider that prior work that associated the total airway count with accelerated lung function decline and COPD severity was performed in another population-based sample with a low burden of smoking and clinical disease (56). This suggests that a low number of small airways may precede other structural abnormalities. The possibility that a deficit in small airway development reflects a heritable, high-risk phenotype warrants further investigation. Meanwhile, among smokers in SPIROMICS, an index of small airway dysfunction, PRMfSAD, was the strongest potential mediator of genetic risk. In addition to correlating with small airway disease, PRMfSAD has been hypothesized to precede percent emphysema (57), which was associated with the GRS in MESA and among elderly SPIROMICS participants. Hence, in addition to the emphasis on variation in small airways, our results suggest that genetic risk may be associated with, and partially mediated by, the development of emphysematous lung deterioration, consistent with the enrichment of elastic fiber pathways identified among the variants in the GRS (6).
Our findings suggest that lung-structure measures enhance discrimination of COPD cases to a greater extent than the GRS. This is not unexpected, as lung structural variation is not fully explained by genetic variation and strongly associates with smoking intensity and accelerated lung function decline (16–18, 36, 56), both of which are major COPD risk factors that are not associated with variation in this GRS (6, 43, 58). An updated GRS based on a larger BioBank general population that includes 184 additional variants could add to the GRS’s discriminative performance, but this is unlikely to alter our main conclusions; in fact, relative to clinical factors, the updated GRS was reported to increase the c-statistic for moderate-to-severe COPD by only 0.02 (58). This increment is approximately five times what we observed in our study, but only 10–20% of the increment provided by CT measures.
The strengths of the present work include the application of a rigorously defined GRS, quantitative measurement of lung structure elements using full-lung CT, and the comparison of results from two large, highly characterized studies that are informative regarding COPD risk in both the general population and in heavy smokers. Nonetheless, certain limitations must be considered.
Our results are consistent with the hypothesis that variation in lung structure substantially mediates the genetic risk of low lung function; however, alternative hypotheses must be considered. Our hypothesis is predicated on the assumption that changes in lung structure precede and predict loss of lung function and COPD, which is supported by recent studies (16–18, 56) and the robustness of our findings in a population-based sample of individuals with relatively preserved lung function. Nonetheless, spirometric obstruction can also influence lung structure. Indeed, the extent of potential mediation we observed, particularly in MESA, was greater than would be expected based on the number of genetic variants in the GRS without clear linkages to lung structural development (6). With respect to potential bidirectional relationships between structure and function, we found that lung function attenuated the GRS–structure associations to a lesser extent than lung structure attenuated the GRS–function associations. Interestingly, the observation that GRS–structure associations were independent of lung function could be consistent with lung structure indexing “preobstructive” disease (16, 17) and/or nonobstructive emphysema or the “symptomatic smoking” phenotype (14, 35, 59). Future studies should examine causal pathways more precisely by leveraging structure-specific GWAS signals, subclassified COPD endotypes, and longitudinal measurements of disease incidence and progression (60, 61).
Both the original GRS (6) and updated GRS (58) were developed in persons of European ancestry. In the current report, we did not find any definite evidence for effect modification of the GRS–structure associations by race/ethnicity. However, we found that the average GRS was higher in African American and Hispanic/Latino participants, among whom some GRS–structure associations were of lower magnitude. This is consistent with some previous work (58) and reflects, at least in part, varying allele frequencies of lung function loci across individuals from different racial/ethnic groups with varying ancestral backgrounds (62). Other considerations that could not be definitively resolved include selection bias, imprecision, or differential associations. We anticipate that incorporating genetic risk determinants in non-European ancestries (31, 63) may be important to optimize the performance of the GRS in ethnically diverse populations.
CT measures may be differentially misclassified in the context of current smoking, which increases lung inflammation and thereby artifactually decreases estimates of percent emphysema and gas trapping (64). Nonetheless, the models were adjusted for smoking status and pack-years history, no significant effect modification by smoking status was observed, and results were similar in both the general population–based and heavy-smoking samples.
Lastly, MESA Lung and SPIROMICS measured lung function and lung structure later in life; hence our inferences regarding associations between genetic risk and peak lung function attainment in early adulthood are based on indirect evidence. This highlights the need to develop additional cohorts of young adults with measurements of lung structure.
In conclusion, a GRS associated with COPD susceptibility was significantly associated with lower lung density, fewer smaller airways, and altered airway dimensions on CT, independently of smoking history. Lung structure substantially mediated associations between the GRS and lung function, and also, unlike the GRS, improved discrimination of COPD cases. This is the first study to use imaging to demonstrate that a GRS obtained by a general-population GWAS of lung function may partially exert its effects through mechanisms resulting in lung structure abnormalities. The findings suggest that lung structure may be an important mediator of heritability and determinant of personalized risk for COPD.
Supplementary Material
Acknowledgments
Acknowledgment
The authors thank the SPIROMICS participants and participating physicians, investigators, and staff for making this research possible. The authors also thank the other investigators, the staff, and the participants of the MESA study for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
Footnotes
Primarily supported by NHLBI grants K23-HL130627, K08-HL118128, R01-HL077612, R01-HL093081 and R01-HL130506. MESA (Multi-Ethnic Study of Atherosclerosis), MESA Lung, and the MESA SHARe project are conducted and supported by the NHLBI in collaboration with MESA investigators. Support for MESA is provided by contracts HHSN268201500003I, N01-HC-95159, N01-HC-95160, N01-HC-95161, N01-HC-95162, N01-HC-95163, N01-HC-95164, N01-HC-95165, N01-HC-95166, N01-HC-95167, N01-HC-95168, N01-HC-95169, UL1-TR-000040, UL1-TR-001079, UL1-TR-001420, UL1-TR-001881, and DK063491. The MESA Lung Study was supported by R01-HL077612 and R01-HL093081. Development of the MESA COPD genetic risk score was supported by NHLBI grant R01-HL131565. Funding for SHARe genotyping was provided by NHLBI contract N02-HL-64278. Genotyping was performed at Affymetrix and the Broad Institute of Harvard and MIT using the Affymetrix Genome-Wide Human SNP Array 6.0. This publication was developed under STAR research assistance agreements RD831697 (MESA Air) and RD-83830001 (MESA Air Next Stage) awarded by the U.S. Environmental Protection Agency (EPA). It has not been formally reviewed by the EPA. The views expressed in this document are solely those of the authors and the EPA does not endorse any products or commercial services mentioned in this publication. SPIROMICS (Subpopulations and Intermediate Outcome Measures in COPD Study) was supported by contracts from the NIH and NHLBI (HHSN268200900013C, HHSN268200900014C, HHSN268200900015C, HHSN268200900016C, HHSN268200900017C, HHSN268200900018C, HHSN268200900019C, and HHSN268200900020C), which were supplemented by contributions made through the Foundation for the NIH from AstraZeneca, Bellerophon Therapeutics, Boehringer Ingelheim Pharmaceuticals Inc., Chiesi Farmaceutici SpA, Forest Research Institute Inc., GlaxoSmithKline, Grifols Therapeutics Inc., Ikaria Inc., Nycomed GmbH, Takeda Pharmaceutical Co., Novartis Pharmaceuticals Corporation, Regeneron Pharmaceuticals Inc., and Sanofi. Neither the NHLBI nor the EPA was involved in collection, analysis, or interpretation of the data; writing of this report; or the decision to submit for publication.
Author Contributions: E.C.O.: study design, data quality control and harmonization, data analysis, and manuscript preparation. V.E.O.: data quality control and harmonization, calculation of genetic risk score, data analysis, and critical review of the manuscript. B.M.S. and E.A.H.: study design, measurement of lung structure, and critical review of the manuscript. J.N.N., A.W.M., X.G., K.D.T., and S.P.: calculation of genetic risk score and critical review of the manuscript. P.G.W., D.J.C., N.N.H., F.J.M., R.P., M.K.H., C.C., M.T.D., G.C., J.A.K., R.B., and R.G.B.: study design, data collection, and critical review of the manuscript. E.R.B. and D.A.M.: data collection, calculation of genetic risk score, and critical review of the manuscript. S.S.R. and J.I.R.: study design, data collection, calculation of genetic risk score, and critical review of the manuscript. E.C.O. had full access to all of the data in the studies and had final responsibility for the decision to submit for publication.
This article has an online supplement, which is accessible from this issue’s table of contents at www.atsjournals.org.
Originally Published in Press as DOI: 10.1164/rccm.201812-2355OC on March 29, 2019
Author disclosures are available with the text of this article at www.atsjournals.org.
References
- 1.Vogelmeier CF, Criner GJ, Martinez FJ, Anzueto A, Barnes PJ, Bourbeau J, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease 2017 report: GOLD executive summary. Am J Respir Crit Care Med. 2017;195:557–582. doi: 10.1164/rccm.201701-0218PP. [DOI] [PubMed] [Google Scholar]
- 2.World Health Organization. Global Health Observatory (GHO) data. Top 10 causes of death. [accessed 2018 Dec 12]. Available from: http://www.who.int/gho/mortality_burden_disease/causes_death/top_10/en/
- 3.GBD 2016 Causes of Death Collaborators. Global, regional, and national age-sex specific mortality for 264 causes of death, 1980-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2017;390:1151–1210. doi: 10.1016/S0140-6736(17)32152-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kiley J, Gibbons G. Developing a research agenda for primary prevention of chronic lung diseases: an NHLBI perspective. Am J Respir Crit Care Med. 2014;189:762–763. doi: 10.1164/rccm.201402-0380ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wain LV, Shrine N, Artigas MS, Erzurumluoglu AM, Noyvert B, Bossini-Castillo L, et al. Understanding Society Scientific Group. Genome-wide association analyses for lung function and chronic obstructive pulmonary disease identify new loci and potential druggable targets. Nat Genet. 2017;49:416–425. doi: 10.1038/ng.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rennard SI, Drummond MB. Early chronic obstructive pulmonary disease: definition, assessment, and prevention. Lancet. 2015;385:1778–1788. doi: 10.1016/S0140-6736(15)60647-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilk JB, Djousse L, Arnett DK, Rich SS, Province MA, Hunt SC, et al. Evidence for major genes influencing pulmonary function in the NHLBI family heart study. Genet Epidemiol. 2000;19:81–94. doi: 10.1002/1098-2272(200007)19:1<81::AID-GEPI6>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 8.Palmer LJ, Knuiman MW, Divitini ML, Burton PR, James AL, Bartholomew HC, et al. Familial aggregation and heritability of adult lung function: results from the Busselton Health Study. Eur Respir J. 2001;17:696–702. doi: 10.1183/09031936.01.17406960. [DOI] [PubMed] [Google Scholar]
- 9.Lange P, Celli B, Agustí A, Boje Jensen G, Divo M, Faner R, et al. Lung-function trajectories leading to chronic obstructive pulmonary disease. N Engl J Med. 2015;373:111–122. doi: 10.1056/NEJMoa1411532. [DOI] [PubMed] [Google Scholar]
- 10.McGeachie MJ, Yates KP, Zhou X, Guo F, Sternberg AL, Van Natta ML, et al. Patterns of growth and decline in lung function in persistent childhood asthma. N Engl J Med. 2016;374:1842–1852. doi: 10.1056/NEJMoa1513737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kalhan R, Arynchyn A, Colangelo LA, Dransfield MT, Gerald LB, Smith LJ. Lung function in young adults predicts airflow obstruction 20 years later. Am J Med. 2010;123 doi: 10.1016/j.amjmed.2009.07.037. 468, e1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smith BM, Traboulsi H, Austin JHM, Manichaikul A, Hoffman EA, Bleecker ER, et al. MESA Lung and SPIROMICS Investigators. Human airway branch variation and chronic obstructive pulmonary disease. Proc Natl Acad Sci USA. 2018;115:E974–E981. doi: 10.1073/pnas.1715564115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bodduluri S, Puliyakote ASK, Gerard SE, Reinhardt JM, Hoffman EA, Newell JD, Jr, et al. COPDGene Investigators. Airway fractal dimension predicts respiratory morbidity and mortality in COPD. J Clin Invest. 2018;128:5374–5382. doi: 10.1172/JCI120693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oelsner EC, Hoffman EA, Folsom AR, Carr JJ, Enright PL, Kawut SM, et al. Association between emphysema-like lung on cardiac computed tomography and mortality in persons without airflow obstruction: a cohort study. Ann Intern Med. 2014;161:863–873. doi: 10.7326/M13-2570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oelsner EC, Carr JJ, Enright PL, Hoffman EA, Folsom AR, Kawut SM, et al. Per cent emphysema is associated with respiratory and lung cancer mortality in the general population: a cohort study. Thorax. 2016;71:624–632. doi: 10.1136/thoraxjnl-2015-207822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oelsner EC, Smith BM, Hoffman EA, Kalhan R, Donohue KM, Kaufman JD, et al. Prognostic significance of large airway dimensions on computed tomography in the general population: the Multi-Ethnic Study of Atherosclerosis (MESA) Lung Study. Ann Am Thorac Soc. 2018;15:718–727. doi: 10.1513/AnnalsATS.201710-820OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Oelsner EC, Smith BM, Hoffman EA, Folsom AR, Kawut SM, Kaufman JD, et al. Associations between emphysema-like lung on CT and incident airflow limitation: a general population-based cohort study. Thorax. 2018;73:486–488. doi: 10.1136/thoraxjnl-2017-210842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bhatt SP, Bodduluri S, Hoffman EA, Newell JD, Jr, Sieren JC, Dransfield MT, et al. COPDGene Investigators. Computed tomography measure of lung at risk and lung function decline in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2017;196:569–576. doi: 10.1164/rccm.201701-0050OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bhatt SP, Soler X, Wang X, Murray S, Anzueto AR, Beaty TH, et al. COPDGene Investigators. Association between functional small airway disease and FEV1 decline in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2016;194:178–184. doi: 10.1164/rccm.201511-2219OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Oelsner EC, Nguyen J, Manichaikul A, Ortega VE, Smith BM, Hoffman EA, et al. Associations between a genetic risk score for COPD susceptibility and lung function and structure on computed tomography in a multiethnic US population-based sample: the MESA Lung Study [abstract] Am J Respir Crit Care Med. 2018;197:A7407. [Google Scholar]
- 21.Oelsner EC, Smith BM, Nguyen J, Manichaikul A, Hoffman EA, Ampleford E, et al. Associations between a COPD genetic risk score and lung structure on computed tomography (CT): SPIROMICS. Eur Respir J. 2018;52:OA2187. [Google Scholar]
- 22.Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, et al. Multi-Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. doi: 10.1093/aje/kwf113. [DOI] [PubMed] [Google Scholar]
- 23.Kaufman JD, Adar SD, Allen RW, Barr RG, Budoff MJ, Burke GL, et al. Prospective study of particulate air pollution exposures, subclinical atherosclerosis, and clinical cardiovascular disease: the Multi-Ethnic Study of Atherosclerosis and Air Pollution (MESA Air) Am J Epidemiol. 2012;176:825–837. doi: 10.1093/aje/kws169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rodriguez J, Jiang R, Johnson WC, MacKenzie BA, Smith LJ, Barr RG. The association of pipe and cigar use with cotinine levels, lung function, and airflow obstruction: a cross-sectional study. Ann Intern Med. 2010;152:201–210. doi: 10.1059/0003-4819-152-4-201002160-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Couper D, LaVange LM, Han M, Barr RG, Bleecker E, Hoffman EA, et al. SPIROMICS Research Group. Design of the subpopulations and intermediate outcomes in COPD study (SPIROMICS) Thorax. 2014;69:491–494. doi: 10.1136/thoraxjnl-2013-203897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Powell R, Davidson D, Divers J, Manichaikul A, Carr JJ, Detrano R, et al. Genetic ancestry and the relationship of cigarette smoking to lung function and per cent emphysema in four race/ethnic groups: a cross-sectional study. Thorax. 2013;68:634–642. doi: 10.1136/thoraxjnl-2012-202116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, et al. ATS/ERS Task Force. Standardisation of spirometry. Eur Respir J. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 28.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med. 1999;159:179–187. doi: 10.1164/ajrccm.159.1.9712108. [DOI] [PubMed] [Google Scholar]
- 29.Sieren JP, Newell JD, Jr, Barr RG, Bleecker ER, Burnette N, Carretta EE, et al. SPIROMICS Research Group. SPIROMICS protocol for multicenter quantitative computed tomography to phenotype the lungs. Am J Respir Crit Care Med. 2016;194:794–806. doi: 10.1164/rccm.201506-1208PP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoffman EA, Jiang R, Baumhauer H, Brooks MA, Carr JJ, Detrano R, et al. Reproducibility and validity of lung density measures from cardiac CT scans: the Multi-Ethnic Study of Atherosclerosis (MESA) Lung Study. Acad Radiol. 2009;16:689–699. doi: 10.1016/j.acra.2008.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Manichaikul A, Hoffman EA, Smolonska J, Gao W, Cho MH, Baumhauer H, et al. Genome-wide study of percent emphysema on computed tomography in the general population: the Multi-Ethnic Study of Atherosclerosis Lung/SNP Health Association Resource Study. Am J Respir Crit Care Med. 2014;189:408–418. doi: 10.1164/rccm.201306-1061OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Galbán CJ, Han MK, Boes JL, Chughtai KA, Meyer CR, Johnson TD, et al. Computed tomography-based biomarker provides unique signature for diagnosis of COPD phenotypes and disease progression. Nat Med. 2012;18:1711–1715. doi: 10.1038/nm.2971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith BM, Hoffman EA, Rabinowitz D, Bleecker E, Christenson S, Couper D, et al. The Multi-Ethnic Study of Atherosclerosis (MESA) COPD Study and the Subpopulations and Intermediate Outcomes in COPD Study (SPIROMICS) Comparison of spatially matched airways reveals thinner airway walls in COPD. Thorax. 2014;69:987–996. doi: 10.1136/thoraxjnl-2014-205160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hogg JC, Chu F, Utokaparch S, Woods R, Elliott WM, Buzatu L, et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N Engl J Med. 2004;350:2645–2653. doi: 10.1056/NEJMoa032158. [DOI] [PubMed] [Google Scholar]
- 35.Donohue KM, Hoffman EA, Baumhauer H, Guo J, Ahmed FS, Lovasi GS, et al. Asthma and lung structure on computed tomography: the Multi-Ethnic Study of Atherosclerosis Lung Study. J Allergy Clin Immunol. 2013;131:361–368, e1–11. doi: 10.1016/j.jaci.2012.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Donohue KM, Hoffman EA, Baumhauer H, Guo J, Budoff M, Austin JH, et al. Cigarette smoking and airway wall thickness on CT scan in a multi-ethnic cohort: the MESA Lung Study. Respir Med. 2012;106:1655–1664. doi: 10.1016/j.rmed.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Baron RM, Kenny DA. The moderator-mediator variable distinction in social psychological research: conceptual, strategic, and statistical considerations. J Pers Soc Psychol. 1986;51:1173–1182. doi: 10.1037//0022-3514.51.6.1173. [DOI] [PubMed] [Google Scholar]
- 38.Bamber D. The area above the ordinal dominance graph and the area below the receiver operating characteristic graph. J Math Psychol. 1975;12:387–415. [Google Scholar]
- 39.DeLong ER, DeLong DM, Clarke-Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: a nonparametric approach. Biometrics. 1988;44:837–845. [PubMed] [Google Scholar]
- 40.Allinson JP, Hardy R, Donaldson GC, Shaheen SO, Kuh D, Wedzicha JA. Combined impact of smoking and early-life exposures on adult lung function trajectories. Am J Respir Crit Care Med. 2017;196:1021–1030. doi: 10.1164/rccm.201703-0506OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Anthonisen NR, Connett JE, Kiley JP, Altose MD, Bailey WC, Buist AS, et al. Effects of smoking intervention and the use of an inhaled anticholinergic bronchodilator on the rate of decline of FEV1: the Lung Health Study. JAMA. 1994;272:1497–1505. [PubMed] [Google Scholar]
- 42.Fletcher C, Peto R. The natural history of chronic airflow obstruction. BMJ. 1977;1:1645–1648. doi: 10.1136/bmj.1.6077.1645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.John C, Soler Artigas M, Hui J, Nielsen SF, Rafaels N, Paré PD, et al. Genetic variants affecting cross-sectional lung function in adults show little or no effect on longitudinal lung function decline. Thorax. 2017;72:400–408. doi: 10.1136/thoraxjnl-2016-208448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wain LV, Shrine N, Miller S, Jackson VE, Ntalla I, Soler Artigas M, et al. UK Brain Expression Consortium (UKBEC); OxGSK Consortium. Novel insights into the genetics of smoking behaviour, lung function, and chronic obstructive pulmonary disease (UK BiLEVE): a genetic association study in UK Biobank. Lancet Respir Med. 2015;3:769–781. doi: 10.1016/S2213-2600(15)00283-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Aschard H, Tobin MD, Hancock DB, Skurnik D, Sood A, James A, et al. Understanding Society Scientific Group. Evidence for large-scale gene-by-smoking interaction effects on pulmonary function. Int J Epidemiol. 2017;46:894–904. doi: 10.1093/ije/dyw318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zeltner TB, Burri PH. The postnatal development and growth of the human lung: II. Morphology. Respir Physiol. 1987;67:269–282. doi: 10.1016/0034-5687(87)90058-2. [DOI] [PubMed] [Google Scholar]
- 47.Zeltner TB, Caduff JH, Gehr P, Pfenninger J, Burri PH. The postnatal development and growth of the human lung: I. Morphometry. Respir Physiol. 1987;67:247–267. doi: 10.1016/0034-5687(87)90057-0. [DOI] [PubMed] [Google Scholar]
- 48.Belgrave DCM, Granell R, Turner SW, Curtin JA, Buchan IE, Le Souëf PN, et al. Lung function trajectories from pre-school age to adulthood and their associations with early life factors: a retrospective analysis of three population-based birth cohort studies. Lancet Respir Med. 2018;6:526–534. doi: 10.1016/S2213-2600(18)30099-7. [DOI] [PubMed] [Google Scholar]
- 49.Bui DS, Lodge CJ, Burgess JA, Lowe AJ, Perret J, Bui MQ, et al. Childhood predictors of lung function trajectories and future COPD risk: a prospective cohort study from the first to the sixth decade of life. Lancet Respir Med. 2018;6:535–544. doi: 10.1016/S2213-2600(18)30100-0. [DOI] [PubMed] [Google Scholar]
- 50.Bui DS, Walters HE, Burgess JA, Perret JL, Bui MQ, Bowatte G, et al. Childhood respiratory risk factor profiles and middle-age lung function: a prospective cohort study from the first to sixth decade. Ann Am Thorac Soc. 2018;15:1057–1066. doi: 10.1513/AnnalsATS.201806-374OC. [DOI] [PubMed] [Google Scholar]
- 51.Berry CE, Billheimer D, Jenkins IC, Lu ZJ, Stern DA, Gerald LB, et al. A distinct low lung function trajectory from childhood to the fourth decade of life. Am J Respir Crit Care Med. 2016;194:607–612. doi: 10.1164/rccm.201604-0753OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gevenois PA, de Maertelaer V, De Vuyst P, Zanen J, Yernault JC. Comparison of computed density and macroscopic morphometry in pulmonary emphysema. Am J Respir Crit Care Med. 1995;152:653–657. doi: 10.1164/ajrccm.152.2.7633722. [DOI] [PubMed] [Google Scholar]
- 53.Gevenois PA, Zanen J, de Maertelaer V, De Vuyst P, Dumortier P, Yernault JC. Macroscopic assessment of pulmonary emphysema by image analysis. J Clin Pathol. 1995;48:318–322. doi: 10.1136/jcp.48.4.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Nakano Y, Wong JC, de Jong PA, Buzatu L, Nagao T, Coxson HO, et al. The prediction of small airway dimensions using computed tomography. Am J Respir Crit Care Med. 2005;171:142–146. doi: 10.1164/rccm.200407-874OC. [DOI] [PubMed] [Google Scholar]
- 55.Becklake MR, Lalloo U. The ‘healthy smoker’: a phenomenon of health selection? Respiration. 1990;57:137–144. doi: 10.1159/000195837. [DOI] [PubMed] [Google Scholar]
- 56.Kirby M, Tanabe N, Tan WC, Zhou G, Obeidat M, Hague CJ, et al. CanCOLD Collaborative Research Group; Canadian Respiratory Research Network; CanCOLD Collaborative Research Group, the Canadian Respiratory Research Network. Total airway count on computed tomography and the risk of chronic obstructive pulmonary disease progression: findings from a population-based study. Am J Respir Crit Care Med. 2018;197:56–65. doi: 10.1164/rccm.201704-0692OC. [DOI] [PubMed] [Google Scholar]
- 57.Boes JL, Hoff BA, Bule M, Johnson TD, Rehemtulla A, Chamberlain R, et al. Parametric response mapping monitors temporal changes on lung CT scans in the Subpopulations and Intermediate Outcome Measures in COPD Study (SPIROMICS) Acad Radiol. 2015;22:186–194. doi: 10.1016/j.acra.2014.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Shrine N, Guyatt AL, Erzurumluoglu AM, Jackson VE, Hobbs BD, Melbourne CA, et al. Understanding Society Scientific Group New genetic signals for lung function highlight pathways and chronic obstructive pulmonary disease associations across multiple ancestries Nat Genet 2019;51481–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Woodruff PG, Barr RG, Bleecker E, Christenson SA, Couper D, Curtis JL, et al. SPIROMICS Research Group. Clinical significance of symptoms in smokers with preserved pulmonary function. N Engl J Med. 2016;374:1811–1821. doi: 10.1056/NEJMoa1505971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.van der Plaat DA, Vonk JM, Lahousse L, de Jong K, Faiz A, Nedeljkovic I, et al. Limited overlap in significant hits between genome-wide association studies on two airflow obstruction definitions in the same population. BMC Pulm Med. 2019;19:58. doi: 10.1186/s12890-019-0811-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sakornsakolpat P, Prokopenko D, Lamontagne M, Reeve NF, Guyatt AL, Jackson VE, et al. SpiroMeta Consortium; International COPD Genetics Consortium. Genetic landscape of chronic obstructive pulmonary disease identifies heterogeneous cell-type and phenotype associations. Nat Genet. 2019;51:494–505. doi: 10.1038/s41588-018-0342-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ortega VE, Kumar R. The effect of ancestry and genetic variation on lung function predictions: what is “normal” lung function in diverse human populations? Curr Allergy Asthma Rep. 2015;15:16. doi: 10.1007/s11882-015-0516-2. [DOI] [PubMed] [Google Scholar]
- 63.Burkart KM, Sofer T, London SJ, Manichaikul A, Hartwig FP, Yan Q, et al. The Hispanic Community Health Study/Study of Latinos. A genome-wide association study in Hispanics/Latinos identifies novel signals for lung function. Am J Respir Crit Care Med. 2018;198:208–219. doi: 10.1164/rccm.201707-1493OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zach JA, Williams A, Jou SS, Yagihashi K, Everett D, Hokanson JE, et al. COPDGene Investigators. Current smoking status is associated with lower quantitative CT measures of emphysema and gas trapping. J Thorac Imaging. 2016;31:29–36. doi: 10.1097/RTI.0000000000000181. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.