Abstract
Gut microbial research has recently opened new frontiers in neuroscience and potentiated novel therapies for mental health problems (Mayer, et al., 2014). Much of our understanding of the gut microbiome’s role in brain function and behavior, however, has been largely derived from research on nonhuman animals. Even less is known about how the development of the gut microbiome influences critical periods of neural and behavioral development, particularly adolescence. In this review, we first discuss why the gut microbiome has become increasingly relevant to developmental cognitive neuroscience and provide a synopsis of the known connections of the gut microbiome with social–affective brain function and behavior, specifically highlighting human developmental work when possible. We then focus on adolescence, a key period of neurobiological and social–affective development. Specifically, we review the links between the gut microbiome and six overarching domains of change during adolescence: (a) social processes, (b) motivation and behavior, (c) neural development, (d) cognition, (e) neuroendocrine function, and (f) physical health and wellness. Using a developmental science perspective, we summarize key changes across these six domains to underscore the promise for the gut microbiome to bidirectionally influence and transform adolescent development.
Keywords: adolescence, development cognitive neuroscience, gut microbiome, gut-brain axis, social
1 |. INTRODUCTION
Scientific and media coverage on the “gut–brain” axis has dramatically increased in recent years, as we rapidly accrue knowledge about how the gut microbiome and central nervous system (CNS) bidirectionally communicate through neurobiological and immunological pathways to influence behavior. Indeed, gut microbial research has opened new frontiers in neuroscience and potentiated novel therapies for mental health disorders (Mayer, Knight, Mazmanian, Cryan, & Tillisch, 2014; Sarkar et al., 2016). The gut microbiome helps cultivate healthy neurobiological programming in early development and continues to associate with brain function and behavior into adulthood (Borre et al., 2014). For example, altering the gut microbiome can change social and affective behavior, and in preliminary rodent and adult studies, reverse psychiatric symptoms (Akkasheh et al., 2016; Dinan & Cryan, 2016). Despite the promise of these dramatic findings, we possess relatively limited insight into the development and function of the microbiome beyond early childhood. This is particularly true of adolescence, which is an important time of neurobiological and behavioral development, including an emergent risk for psychopathology.
Adolescence is increasingly understood to represent more than a transitional period between child and adulthood, but rather a critical period of neurobiological programming, social–affective processing, and explorative behavior that continues to shape our neurobiological and social and emotional processing into adulthood (Dahl, Allen, Wilbrecht, & Suleiman, 2018). This also means that adolescence is a vulnerable period for a heightened risk for the emergence of psychiatric symptoms and disorders that persist across the life span. Despite the growing emphasis on adolescence as a “flux” point in development within developmental cognitive neurosciences, and the parallel growing emphasis on the gut microbiome’s role in neurobiological, social-emotional, explorative, and psychiatric symptom development, there is a relative dearth of research into the gut microbiome’s role during this critical period of developmental programming. In this review, we first highlight why gut microbiome research is becoming increasingly relevant to psychology and the cognitive neurosciences, then focus on the microbiome’s specific connections to social–affective processing, and lastly, synopsize six domains of change that are undergoing unique programming during adolescence and that are particularly relevant to the microbiome.
2 |. PART I. THE GUT MICROBIOME’S GROWING CONNECTION WITH PSYCHOLOGY AND THE COGNITIVE NEUROSCIENCES
The gut microbiome consists of a diverse community of microorganisms “microbes” and their genes that resides in the gastrointestinal tract, including bacteria, fungi, archaea, and viruses. Our number of microbial cells are now believed to closely match our number of human cells (approximately 1:1 ratio; Sender, Fuchs, & Milo, 2016), with the largest microbial population residing in the gastrointestinal tract. The composition and function of the gut microbiome is dynamic and malleable to the environment across the life span, including changes in diet, geography, stress, illness, or use of antibiotics. The gut microbiome interacts with the enteric nervous system (ENS): a branch of the autonomic nervous system (ANS) that can also operate independently to regulate the gastrointestinal system. The ENS contains an estimated 70% of body’s immune cells and produces an estimated 95% of the body’s serotonin (Mayer, 2011). The connections between the ENS and the brain (i.e., the gut–brain axis) were first discovered centuries ago; however, the microbiota’s role in this gut–brain communication has only recently emerged (and contributes to the moniker of the “microbiome”–gut–brain axis; Zhu et al., 2017).
The microbiome performs myriad functions. It regulates immune function activity, gut motility, nutrient absorption, fat distribution, and maintains homeostasis of the intestinal barrier (Carabotti, Scirocco, Maselli, & Severi, 2015). The microbiome also communicates with the CNS through bidirectional signaling along the autonomic nervous system, and neuroimmune and neuroendocrine pathways. For example, the microbiome–gut–brain axis operates through microbial sourced metabolites (e.g., short-chain fatty acids, peptides, neurotransmitters), vagal nerve innervation, hormonal signaling, and immune cells (see Figure 1; see also Sharon, Sampson, Geschwind, & Mazmanian, 2016 for a review of connection mechanisms). Microbiome colonization also directly overlaps with the first critical period of neurobiological development, beginning during prenatal development through the mother’s placenta and continuing through the first 3–4 years of life (Collado, Rautava, Aakko, Isolauri, & Salminen, 2016). In fact, the microbiome helps form the blood–brain barrier and is necessary for normative brain development, immune function, and hypothalamus–pituitary–adrenal (HPA) axis programming (Borre et al., 2014). Although the mechanistic connections have been predominantly modeled in nonhuman vertebrate animals, these discoveries have raised the potential that the microbiome–gut–brain axis can transform how we understand and approach our models of human behavior.
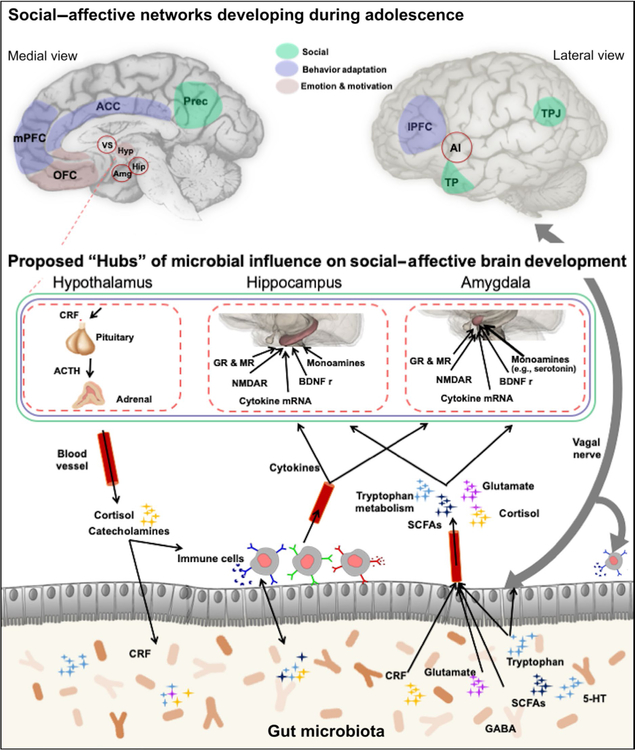
FIGURE 1.
Proposed Critical Hubs of Microbiome–Gut–Brain Axis. Figure 1 depicts the bidirectional pathways between the gut microbiome and both the central and the peripheral nervous systems. Social, Behavioral Adaptation, and Emotion & Motivation are important networks that undergo significant restructuring during adolescence. Proposed brain “hubs” of microbial influence on social-affective brain development include the hypothalamus, hippocampus, and amygdala. Animal literature documents microbial changes influence brain structure and function through changes in MR concentrations, NMDAR, cytokine mRNA, BDNF, and monoamines, such as serotonin in the hippocampus and the amygdala. This is not intended to highlight every region that has been correlated with the gut microbiome, rather we specifically highlight brain regions implicated social–affective, explorative learning and self-processes. Note: Open circle indicates region is subcortical. 5-HT: serotonin; ACTH: adrenocorticotrophic-releasing hormone; AI: anterior insula; Amg: amygdala; BDNF r: brain-derived neurotrophic factor receptors; CRF: corticotrophin-releasing factor; GABA: gamma-aminobutyric acid; GR: glucocorticoid receptors; Hip: hippocampus; Hyp: hypothalamus; lPFC: lateral prefrontal cortex; mPFC: medial prefrontal cortex; MR: mineralocorticoid receptors; NMDAR: N-methyl-D-aspartate receptor; OFC: orbital frontal cortex; SCFAs: short-chain fatty acids. Brain regions; TP: temporal pole; TPJ: temporal parietal junction; VS: ventral striatum. Figure partly adopted from Cryan and Dinan (2012)
2.1 |. A novel treatment method
Emergent gut microbial interventions in medicine highlight the microbiome’s unique potential to individualize treatment in psychology. One of the most commonly used microbial interventions is probiotic administration. By definition, probiotics are live bacteria that, when administered in precise quantities, provide a health benefit to the host (Sanders, 2008). Studies have used probiotics to treat a range of both gastrointestinal and mental health symptoms. For example, in a population of adults with comorbid irritable bowel syndrome (IBS) and anxiety/depression, a group that took a probiotic for ten weeks had more individuals that showed declines in depression symptoms (64%) than a group that took a placebo (32%; Pinto-Sanchez et al., 2017). A recent systematic review supported the positive effects of probiotics on depression symptoms, but reported that additional randomized control trials are needed before clinical recommendations can be made (Pirbaglou et al., 2016; Wallace & Milev, 2017). However, the most recent meta-analysis of those reports only found support for a beneficial effect of probiotics in mildly depressed, compared to not depressed, subjects (there was no effect of probiotics on clinical depression symptoms; Ng, Peters, Ho, Lim, & Yeo, 2018). No meta-analyses have yet focused on clinical anxiety symptoms.
Another novel treatment that involves an indirect manipulation of gastrointestinal bacteria is administration of prebiotics. By definition, prebiotics are dietary compounds used by bacteria to yield a positive benefit to the host (e.g., fructooligosaccharides and galactooligosaccharides; Gibson et al., 2017). Some studies have administered such prebiotics either to healthy volunteers, showing a reduction in neuroendocrine activity associated with stress regulation (Schmidt et al., 2015) or to rodents, showing positive outcomes on several stress-associated outcomes, including anxiety and depression (Burokas et al., 2017). Currently, there are no meta-analyses of prebiotic utility in depression or anxiety.
Microbial interventions have extended to several areas of medicine (Xu et al., 2015). By profiling the gut microbiome, for example, immunotherapies and clinical drug trials are beginning to identify individual differences in the course of optimal chemotherapy and drug metabolism (Petrosino, 2018). Pilot studies in adults have also sparked promise that microbial transplants or “fecal pills” which have been used for GI disorders, may be able to alleviate symptoms of depression or autism spectrum disorder (Kang et al., 2017; Zheng et al., 2016). As the field expands, it may also mean that psychological interventions could individualize psychotherapeutic treatments based on microbial profiles, or that the microbiome could provide meaningful biomarkers for pre- and postpsychological interventions. Most of these medical treatments are not yet commercially available, nor sophisticated enough for psychological interventions, but with the rise in technological advancements, they may not be far off.
2.2 |. Advancing technology
Owing in large part to recent advances in DNA sequencing technology and analytical methodology, our ability to study the gut micro- biome’s connections to neurobiology and behaviors has dramatically improved. Over the past decade, DNA sequencing procedures have been streamlined and commercialized, resulting in lower costs for generating DNA sequences from a microbiome sample. This has afforded new avenues of discovery. For example, targeted sequencing of the bacterial 16S locus enables relatively rapid, comprehensive, and precise determination of the taxonomic composition of a microbiome sample (Kuczynski et al., 2011). A rapidly growing body of research additionally employs an approach termed shotgun metagenomics, which seeks to sequence the entire genome of microbes that comprise a community (Sharpton, 2014). These advanced methods are beginning to uncover the metabolic pathways encoded in these genomes and link these pathways to physiological or behavioral processes, such as CNS functioning, neuroimmunology, and neuroendocrinology (Arnold, Roach, & Azcarate-Peril, 2016).
Innovations in study design are also made possible thanks to initiatives such as American Gut, which has created the first open-source, community-generated database of the human microbiome for individuals to participate or analyze the microbiome on a large scale (McDonald et al., 2018). Companies have also developed commercialized collection kits for researchers to conduct their own studies. The advent of such kits and standard operating procedures for microbial collection and analyses (http://www.microbiome-standards.org/index.html) has opened new avenues for studying high-risk or hard-to-reach developmental populations (particularly within the United States) by reducing participant burden, providing flexible stool storage options, and enabling the delivery of samples through U.S. mail transport (Anderson et al., 2016). However, online gut microbial repositories are limited in terms of their ability to measure cognitive processes and to provide diagnostic information. As we seek to bridge disciplines, rigorous measures of psychopathology and brain structure, function, or connectivity will still require some portion of an in-laboratory or in-person study design.
3 |. PART II. EVIDENCE LINKING THE GUT MICROBIOME WITH SOCIAL–AFFECTIVE NEURAL PROCESSES
The role of the microbiome in social–affective brain function is a relatively recent area of inquiry, especially within human subjects. Nonhuman studies, however, can provide some initial insight about the microbiome’s role in social–affective brain development. We begin by highlighting known links between the gut microbiome and molecular or structural changes in rodent models, focusing on the hippocampus and amygdala—key regions in learning and social–affective processing and potential “hubs” for microbial influence on brain function. Next, we summarize initial work in humans, which also includes other associated regions, such as the perigenual anterior cingulate cortex (pACC), anterior midcingulate cortex (aMCC), insula, and precuneus.
3.1 |. Rodent models
The hippocampus and the amygdala are two limbic regions centrally involved in social–affective learning and memory that rely on input from the gut microbiome for normative development. Furthermore, the amygdala and hippocampus are probable “hubs” for the gut microbiome’s influence on brain function and cognition (for full review of this theory, see Cowan et al., n.d.). Briefly, manipulating the presence or composition of the microbiome changes the molecular and structural development of the hippocampus and amygdala (Foster, Rinaman, & Cryan, 2017). For example, the absence of a microbiome (e.g., via animals raised without a microbiome) or the disturbance of the microbiome (e.g., via antibiotic treatment) alters amygdala and hippocampal gene expression involved in neural plasticity (i.e., brain-derived neurotrophic factor [BDNF] and Finkel–Biskis–Jinkins murine osteosarcoma viral oncogene homolog B [FosB]), synaptic plasticity involved with learning and memory (i.e., N-methyl-d-aspartate receptor [NMDAR], and the neurotransmission of monoamines, such as dopamine, norepinephrine, and serotonin (Bercik et al., 2011; Neufeld, Kang, Bienenstock, & Foster, 2011). In addition, the absence of the microbiome results in an enlarged amygdala volume and dendritic hypertrophy in the basolateral amygdala (Luczynski et al., 2016). Although these regions are also central in stress regulation, and the pathway may be through reduced glucocorticoids (GC) and proinflammatory cytokines, some evidence suggests these effects can occur independent of decreased GC, or anxiety levels (Gareau et al., 2011; Savignac, Tramullas, Kiely, Dinan, & Cryan, 2015). Such neural alterations to these limbic regions can impair social–affective learning and memory and may influence the later development of the hippocampus and amygdala’s functional connectivity to “top-down” regions (e.g., prefrontal cortex) involved in social–affective regulation in adolescence (for review, see Tottenham, 2015).
3.2 |. Human models
Promising work in adults demonstrates the microbiome’s role in neural function extends to functional brain reactivity. These connections were first discovered in adults with gastrointestinal disorders, such as IBS, in which the microbiome’s composition characteristically differs from that of individuals not manifesting the syndrome (Mayer, Naliboff, & Craig, 2006). Compared to controls, adult patients with IBS displayed altered functional activity in regions involved in attention, sensation, and emotional arousal, including the amygdala, pACC, aMCC, and somatosensory cortex (Tillisch, Mayer, & Labus, 2011), possibly as a function of microbial alterations in the composition and/or stability over time.
The microbiome’s link to brain activity has also been observed in healthy adults. For example, in a pre- and post-fMRI design, compared to baseline activity, women who received a 4-week probiotic treatment had reduced resting-state functional activity in regions involved in emotion and sensation processing, including the insula, somatosensory cortex, as well as the prefrontal cortex, parahippocampal gyrus, precuneus, and basal ganglia (Tillisch et al., 2013). In follow-up experiments, Tillisch et al. (2017) also demonstrated that an individual’s functional and structural connectivity could be characterized by the type of prominent bacteria in their microbiome (a clustering analysis typically referred to as “enterotyping”; see Costea et al. (2017) for a review on the debate surrounding this methodology). Women in the microbial cluster predominated by Prevotella (vs. Bacteroides) had decreased hippocampal activity and increased functional brain connectivity between brain regions involved in emotional and attentional sensory processing. At the same time, structural connectivity patterns obtained from white and gray matter tracts correctly categorized women into those two predominant bacterial groups with 66.7% and 87.2% accuracy, respectively (Tillisch et al., 2017). Together, these data provide a foundation for thinking about how various sensory, attentional, and emotional processes may be altered by microbiome–gut–brain axis communication.
3.3 |. Gut microbiome development and links to early psychosocial behavior
Currently, the research on gut microbial programming has largely excluded psychosocial development, with some notable exceptions that focus on early childhood. Two foundational longitudinal studies in early childhood revealed that the gut microbiome predicted child cognitive performance at 12 months old (Carlson et al., 2018) and temperament at 24 months old (Christian et al., 2015), suggesting that the gut microbiome is already playing a role in shaping cognition and social–affective behavior from an early age.
There is, however, mounting evidence that the microbiome continues to develop beyond the initially presumed first few years of life. It turns out that children (ages 1–7 years) distinctly separated from adults on a principal component analysis (PCA), showing less diverse microbiomes, and at the genus level, showing higher abundance of Bifidobacterium, Clostridium, and lower Prevotella and Sutterella (Hopkins, Sharp, & Macfarlane, 2001). In another study spanning from childhood through pre-adolescence (ages 7–12 years), children also had more similar microbiomes to one another than to adults (Hollister et al., 2015). Specifically, children differed in the relative abundance of the taxa that comprise the gut microbiome (i.e., increased Bifidobacterium spp., Faecalibacterium spp., and members of the Lachnospiraceae, while adults harbored greater abundances of Bacteroides spp.). Furthermore, the authors concluded that children harbor microbiome metabolic pathways that promote ongoing neural development (e.g., vitamin B12 synthesis, de novo synthesis of folate and oxidative phosphorylation and lipopolysaccharide biosynthesis), whereas adult functional metabolic pathways were more associated with inflammation and obesity (e.g., lipopolysaccharide biosynthesis, chemotaxis, and triggering mucosal inflammation; Hollister et al., 2015). These findings suggest that not only does the microbiome continue to change with age, but also its constitution and functionality are specifically tailored to relevant developmental processes.
4 |. PART III. ADOLESCENCE AS A SENSITIVE PERIOD OF MICROBIAL CHANGE
While human studies of the adolescent gut microbiome are still relatively sparse, rodent models suggest adolescence represents a critical window during which the microbiome’s colonization of the gut impacts parts of the CNS’s wiring that are linked to stress-associated behaviors (Foster & McVey Neufeld, 2013; McVey Neufeld, Luczynski, Dinan, & Cryan, 2016). For example, germ-free mice that were colonized with a microbiome either at birth or 3 weeks old showed normalized stress-associated behaviors, whereas germ-free mice that were colonized with a microbiome at 10 weeks old did not show this effect (Heijtz et al., 2011; Neulfeld et al., 2011, Clarke et al., 2013). Furthermore, emerging evidence demonstrates the gut microbiome plays a critical role in monoamine and neuromodulator function in hippocampus and amygdala specifically during adolescence. For example, antibiotic depletion of the gut microbiome during adolescence led to changed hippocampal monoamine concentrations (i.e., noradrenaline, but not serotonin or dopamine); metabolites, such as increased tryptophan and decreased kynurenine; reduced hippocampal BDNF expression; and reduced neuropeptides (oxytocin and vasopressin) into adulthood (Desbonnet et al., 2015). Furthermore, antibiotic-treated adolescent rats also showed changes in amygdala amino acid (increased levodopa; L-DOPA) and catecholamine metabolite synthesis (increased homovanillic acid; Desbonnet et al., 2015). In other words, rodent models suggest changes to the microbiome prior to the end of adolescence can be a unique opportunity to shape CNS development (see Figure 1).
Initial case studies in humans also suggested the microbiome had distinct features in adolescence compared with childhood or adulthood, although the small sample sizes could not rule out that individual variability accounted for the differences (Paliy, Kenche, Abernathy, & Michail, 2009; Schloss, Iverson, Petrosino, & Schloss, 2014). One study confirmed these pilot studies by showing that adolescents’ (11–18 years old) microbiomes were distinguishable from adults (22–61 years old) by specific taxa and relative abundance of taxa in the gut (Agans et al., 2011). Collectively, these studies provide some of the first evidence that the health and function of the adolescent microbiome is distinct from adults and should therefore be studied and interpreted through a developmental lens. Large-scale studies with the aim to characterize normative developmental changes in the microbiome are currently underway through the NIH initiative human microbiome project (GNIH HPC Group et al., 2009).
Uncertainty remains regarding the extent to which adolescence is a unique period of gut microbial development. The environment plays a key role in shaping gut and brain function across development, but it may be particularly relevant in adolescence. Adolescence is arguably the second largest environmental shift in development (following early life) due to concurrent changes across multiple domains of behavior and neurobiology (Dahl et al., 2018). We therefore propose adolescence reflects a sensitivity along the microbiome–gut–brain axis, in which the microbiome may spark change in developing neurobiological systems and behavior. At the same time, the number of changes in environment, neurobiology, and behavior during adolescence may ignite transformation in the microbiome.
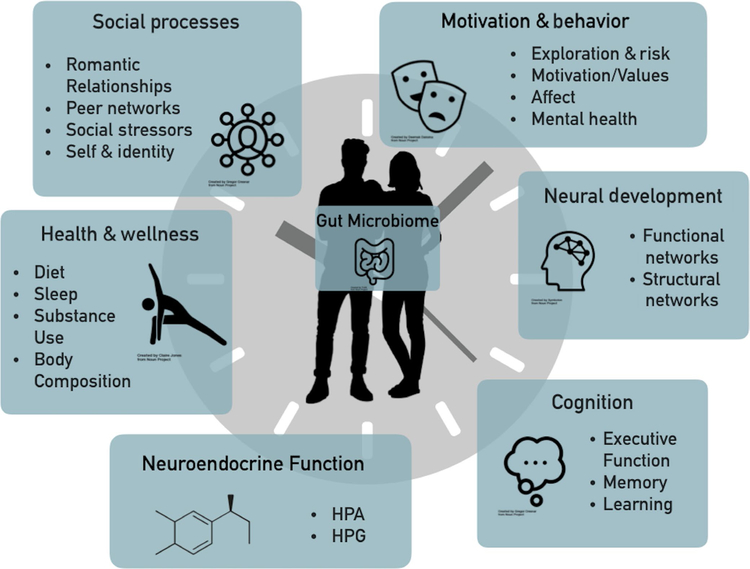
Taking a developmental science approach, we argue that several broad domains of adolescent change—specifically (a) social processes, (b) motivation and behavior, (c) neural development, (d) cognition, (e) neuroendocrine function, and (f) physical health and wellness—have clear bidirectional connections to the microbiome. We specifically focus on these domains as they represent a sensitive period of developmental programming that can have long-term implications for functioning into adulthood (Dahl et al., 2018). Below, we briefly summarize how these domains link to the microbiome and the potential implications of such linkages to understanding adolescent psychobiological development (see Figure 2). These categories are not intended to be exhaustive, nor expected to be independent from one another, but are intended to highlight some fruitful directions for future interdisciplinary work.
FIGURE 2.
Developmental timing. Figure 2 depicts the six overarching domains of change that occurs during adolescence that we propose are also associated with the gut microbiome. These categories are not intended to be exhaustive, nor expected to be independent from one another, rather they are intended to highlight some fruitful directions for future interdisciplinary work
4.1 |. Social processes
Adolescence is a time of self-identity development and social exploration both within and outside of one’s family (Pfeifer & Berkman, 2018). This also means it is a time of novel microbial exposure to new places and increased direct microbial exchange with others. As social dynamics change within families, friendships, and romantic relationships, peers become more salient, romantic relationships burgeon, and social stressors become particularly potent (Blakemore & Mills, 2014). Structurally, adolescence is also a time of increased autonomy outside the home and an opportunity for new social interactions. Within the United States, this typically includes transitioning from grade school into middle and high school, where there are an increased number of classrooms and peer interactions. At the same time, adolescents spend an increasing amount of time alone and remain heavily influenced by their parents and their home structure (Larson & Richards, 1991). This period of budding independence sets a unique stage for the existing familial identity and the expanding self and social identities to interact.
These social developmental changes likely have important implications for the developing microbiome. In general, social behaviors can guide the degree of novel microbial environment and microbial exchange that occurs by increasing or decreasing one’s level of exploration and exposure to the environment. At the same time, rodent studies suggest the microbiome modifies the initiation of these social behaviors (e.g., altering novel exploration and desire to choose social vs. nonsocial interactions; see Münger, Montiel-Castro, Langhans, & Pacheco-López, 2018 for full review of the bidirectional social–microbiome relationship). Microbial studies of autism spectrum disorder (ASD) demonstrate the reciprocal relationship between social behaviors and the microbiome. Individuals with ASD, traditionally characterized by altered social behavior and communication, show alterations in their gut microbiomes and exhibit significantly higher rates of gastrointestinal problems as compared to neurotypical controls (Chaidez, Hansen, & Hertz-Picciotto, 2014; Strati et al., 2017). For example, germ-free mice showed altered social behavior, characterized as a preference for an object over another mouse (Foster et al., 2017); and maternal immune activation (MIA) mice offspring showed disruption to their GI barrier and behavioral features consistent with ASD that could be reversed with probiotic treatment (Hsiao et al., 2013). In preliminary human experimental manipulations of the microbiome (i.e., treated with probiotics or fecal transplants), children with ASD engaged in more social behavior following the interventions (Kang et al., 2013, 2017; West et al., 2014), although findings are still inconsistent, within relatively small sample sizes, and warrant further replication (for review on ASD links with the microbiome, see Li, Han, Dy, & Hagerman, 2017). These findings reveal a potential mutually reinforcing relationship between the gut microbiome and social behavior that should be further explored during adolescence.
4.2 |. Motivational and behavior change
In addition to the changes that occur in social development, adolescents have unique developmental goals that influence their motivations and explorative behaviors (Crone & Dahl, 2012). One of the developmental goals for adolescents is to prepare for adult roles and relationships. In service of this goal, adolescents demonstrate a greater tolerance of ambiguity (compared to adults) in order to approach novel experiences (van den Bos & Hertwig, 2017). This pattern of increased exploration inherently involves increased risking-taking and sensitivity to reward (Romer, 2010) and commonly interacts with social development (e.g., influence of peers on risk-taking behavior; Braams, Duijvenvoorde, Peper, & Crone, 2015). While the microbiome has been linked to behavioral exploration, this connection has not been studied during adolescence. For example, germ-free mice exhibit less environmental exploration and a diminished ability to distinguish novel stimuli (Desbonnet et al., 2015; Heijtz et al., 2011). The microbiome’s influence on explorative behavior could have important implications for the type of experiences that adolescents engage in. At the same time, the environments they immerse themselves in also influence their microbial exposure.
Adolescence is also a period of normative change in affective reactivity and regulation, as well as a period of heightened risk for the emergence of mental health disorders. For example, as of 2016 in the United States, 1 in 4 adolescents had a mental health diagnosis and 3.1 million adolescents ages 12–17 had at least one depressive episode, with 70% having a severe impairment (NIMH). Although there is mounting evidence that the microbiome is associated with social and affective disorders, and early disruption to the microbiome may lead to greater vulnerability of social and affective disorder later in life (Dinan & Cryan, 2017), the causal mechanisms are still poorly understood and the microbiome–behavior relationship is likely bidirectionally reinforced. As discussed earlier, in adult humans and other vertebrate animals, experimental manipulations such as transplantation and pre/probiotic treatment are being tested for mental health disorders such as depression and anxiety (Sarkar et al., 2016). These studies demonstrate that the gut microbiome is not just altered with these disorders but prompts these phenotypes. Mental health disorders are also intimately tied to changes in social behaviors (Thoits, 2011), suggesting there is likely a complex interaction between the microbiome, social development, and affective and explorative behaviors. For example, one mechanism could be that a disrupted microbiome profile initiates depressive behavior via encouraging restricted social and explorative behavior. Concurrently, depressive behaviors, such as restricted environment and/or altered diet and weight, may maintain the alteration in the microbiome.
4.3 |. Brain development
Adolescence is a well-known sensitive period of structural and functional brain development (Dahl et al., 2018). There is limited information, however, on how these neurodevelopmental changes influence microbiome–gut–brain axis communication, particularly regarding known social, motivational, explorative, and affective behavioral changes.
Key brain regions implicated in social–affective processing that also have known connections with the gut microbiome exhibit dramatic changes during adolescence. For example, regions implicated in emotional processing and learning (e.g., amygdala, hippocampus, the dorsolateral prefrontal cortex [dlPFC], and ventral medial prefrontal cortex [vmPFC]) are functionally and structurally changing to reflect the importance of the adolescent environment and developmental stage (Flannery, Giuliani, Flournoy, & Pfeifer, 2017; Mills et al., 2016). Connectivity between limbic regions such as the amygdala, and regulatory regions like the prefrontal cortex, begins to shift to reflect adult-like patterns of top-down regulation (Gee et al., 2013). This period of sensitivity also means greater vulnerability to perturbations. Mental health disorders during adolescence are associated with alterations in brain activity, structure, and connectivity (Lee et al., 2014). Depression during adolescence, for example, is associated with decreased connectivity among regions involved in emotion regulation such as the amygdala, hippocampus, insula, and prefrontal cortex (Connolly et al., 2013; Cullen et al., 2014; Davey et al., 2015; Pannekoek et al., 2014). Given the gut microbiome’s established links to several social–affective processes, it is imperative we understand their relationship during this time of developmental flux in said systems.
Despite the microbiome’s link to social and explorative behaviors, the microbiome has not been connected to brain processes or networks centrally involved in social and explorative behaviors. Notably, however, regions within these networks have been linked to the gut microbiome (e.g., precuneus and ACC). Compared to adults, adolescents show more activity in regions implicated in reward processing when they are making risky choices (e.g., picking an ambiguous option; see review Sherman, Steinberg, & Chein, 2017; Silverman, Jedd, & Luciana, 2015), and learning a task (e.g., learning pattern to improve; see review Romer, Reyna, & Satterthwaite, 2017). In addition, regions associated with social processing (i.e., the “social brain network”) undergo significant functional and structural changes during adolescence (see review Blakemore & Mills, 2014) as well significant changes in functional activation and connectivity between midline structures involved in self-processing (see review Pfeifer & Berkman, 2018). A future avenue of interdisciplinary work should seek to identify gut–brain correlates of social and explorative behaviors during adolescence.
4.4 |. Cognition
Cognitive development heavily intertwines with the adolescent-specific changes in synaptic density in the prefrontal cortex. This time of prolonged maturation through adolescence represents a unique period of developmental programming in higher-order cognitive processes, such as decision-making, working memory, and learning (Juraska & Willing, 2017; Peverill, McLaughlin, Finn, & Sheridan, 2016; Zhou et al., 2016).
Cognitive impairments following alterations in the microbiome have led to the theory of a microbiome–cognition connection. Specifically, the microbiome may be necessary for proper fear learning. For example, germ-free mice show impaired maintenance of fear stimuli–response associations, which was partially reversed with microbial colonization and fully reversed with targeted changes to amygdala gene expression (Hoban et al., 2018). These findings suggest the microbiome influences normative gene expression in the amygdala.
Antibiotics can also produce decreased spatial learning ability, increased anxiety (Wang et al., 2015), reduced memory, and impaired novel object recognition (Fröhlich et al., 2016; Vázquez et al., 2015). Antibiotics change BDNF mRNA expression in critical regions of cognition, including the medial prefrontal cortex, hippocampus, and hypothalamus, possibly by altering tight junction proteins and cytokine mRNA expression (Fröhlich et al., 2016). Alternatively, mice provided with a probiotic (e.g., milk oligosaccharides) or prebiotics showed increased memory and reinforcement learning and cognitive flexibility (attentional set shifting; Gareau et al., 2011; Wang et al., 2015). These effects were similarly reflected in changes in BDNF, and increased mineralocorticoids and NMDA (Gareau et al., 2011; Wang et al., 2015). Notably, there are still inconsistencies in these effects, particularly within humans (Sarkar et al., 2018) that warrant further investigation. Future work should specifically seek to test the microbiome–cognition connection during adolescence when cognition is actively rewiring.
4.5 |. Neuroendocrine development
One possible mechanism of change in microbiome–gut–brain axis communication during adolescence is neuroendocrine development. Adolescence is typically defined by the onset of puberty, including changes in the hypothalamus–pituitary–gonadal (HPG) axis, but also in the hypothalamus–pituitary–adrenal (HPA) axis (Shirtcliff et al., 2015). Although these systems change across the life span, adolescence is a well-documented period of developmental programming for the HPA and HPG axis. These neuroendocrine changes are associated and interact with changes in brain structure and function, secondary sexual characteristics, and physiological stress responses, all of which can influence adolescent behavior and the microbiome (Shirtcliff et al., 2015; Siervogel et al., 2003).
Changes in the HPG axis are associated with changes in sex hormones, such as estrogen, progesterone, and testosterone production (which associate with brain structure and function, see Vijayakumar, Op de Macks, Shirtcliff, & Pfeifer, 2018). There is also growing evidence that sex hormones change the vaginal microbiome, in particular that menarche is likely associated with reprogramming of the vaginal microbiome (Brotman, Ravel, Bavoil, Gravitt, & Ghanem, 2014). Further preliminary evidence suggests hormonal changes that occur across a female’s menstrual cycle additionally alter the gut microbiome (Chen et al., 2017). Pubertal development, specifically, may represent another sensitive period in reprogramming of both the vaginal and gut microbiome; yet to date, this theory has not been tested. Pubertal development has also been linked to microbial changes in other areas, such as subgingival, skin, and nares, demonstrating it may be a time of widespread change in microbial exposure and malleability (Gusberti, Mombelli, Lang, & Minder, 1990; Oh, Conlan, Polley, Segre, & Kong, 2012).
During adolescence, there are also changes to the HPA axis, the primary stress response system. For example, cortisol normatively changes in adolescence, resulting in a prolonged HPA axis response compared to adults (Bingham et al., 2011). The HPA axis is involved in normative microbiome–gut–brain axis communication (Bailey et al., 2010). For example, the microbiome can shape how an organism responds to stress in early development (O’Mahony, Clarke, Dinan, & Cryan, 2017). Germ-free mice display a hyper-reactive HPA axis response (Sudo et al., 2004), but the introduction of specific microbes such as Bifidobacterium can reverse the hyper-reactive HPA axis response (Sudo et al., 2004). Similarly, the HPA axis can change the microbiome. The HPA axis potentiates the release of catecholamines that are metabolized by the microbiome (Moloney et al., 2016). However, under conditions of social and chronic stress, increased cortisol, the end product of the HPA axis, can lead to increased permeability of the intestinal wall (Kelly et al., 2015; Rogers et al., 2016). Stress disruptions to the microbial composition can lead to disruptions in microbiome–gut–brain axis, resulting in low-grade inflammation and depression (see review Farzi, Fröhlich, & Holzer, 2018). Adolescence, therefore, provides the opportunity to positively influence the relationship between the HPA axis and the gut microbiome through normative change in HPA axis function and the microbiome’s sensitivity to change during adolescence. On the other hand, the increased vulnerability to social stressors and malleability in these systems during adolescence may also lead to disruptions along the microbiome–gut–brain axis.
4.6 |. Physical health and wellness
All the changes mentioned above have potential influences on physical health and well-being across adolescence, which can culminate in alterations in diet, substance use, body composition, body mass index (BMI), exercise, and sleep (Dahl et al., 2018; Winpenny et al., 2017; Worthman & Trang, 2018). This is particularly critical, given the gut microbiome’s primary role and extensive links to these processes across species.
Physically, adolescents are undergoing major changes. In concert with the emergence of secondary sex characteristics, body composition changes, fat distribution changes, and obesity rates can increase (Loomba-Albrecht & Styne, 2009). Exercise habits that are formed in adolescence can promote hippocampal BDNF levels and alter microbial composition (in rodents) in a way that does not occur in childhood or adulthood (Hopkins, Nitecki, & Bucci, 2011). Adolescents choose more of their own meals with less parental regulation of their diet. These dietary changes are often associated with an increased percentage of high fat and refined sugars, and heightened risk for increased BMI (Andrade, Previdelli, Cesar, Marchioni, & Fisberg, 2016). In Western cultures, the school structure can also heavily influence dietary choices (e.g., vending machines and limited healthy options; Driessen, Cameron, Thornton, Lai, & Barnett, 2014). The school structure is also at odds with the sleep patterns of adolescence, which are associated with a change in diurnal cycling (Colrain & Baker, 2011). Adolescents begin to stay up later, but still need to get up early for school, which can result in a pattern of inadequate sleep, with periods of “catch up” on the weekends (Carskadon, 2011). Sleep deprivation during adolescence is linked to several behavioral risks, including poor diet; increased stimulant use, such as caffeine; substance use, such as alcohol and cigarette use; lower mood; and high-risk behaviors (McKnight-Eily et al., 2011). Every single one of these risks has been associated with the microbiome.
Alterations to physical health, such as those described above, represent one of the more extensively studied areas in regard to the gut microbiome which suggests adolescence is a particularly critical period of change (see McVey Neufeld et al., 2016). As one of the primary functions, the gut microbiome diversifies in accordance with the nutrients it obtains through diet. For example, individuals with a vegan or omnivore diet need different enzymes to break down their food, and they will produce different nutrients for both the host and the microbes along the gastrointestinal tract (David et al., 2014). The microbiome is also highly sensitive, so changes in diet within an individual will change the individual’s microbiome (David et al., 2014). Dietary changes in adolescence, therefore, may have several implications. For example, in rodents, a diet high in fat was associated with elevated anxiety and depression, increased HPA axis response to a stressor, and increased intestinal permeability (de Sousa Rodrigues et al., 2017). There is also preliminary evidence in mice that dietary changes can normalize the gut microbiome following early life stress and altered stress response system, suggesting a change in diet may have a circular effect on said systems (Foster et al., 2017). Thus, one prominent hypothesis is that dietary decisions influence brain and behavior via the gut microbiome.
Food choices are also not the only source of dietary change in adolescence, as this period is partly characterized by the increase in alcohol and drug experimentation. Alcohol use, as well as several recreational drugs, can induce changes in microbial composition and metabolic function and, in some cases, can lead to increased intestinal permeability (Gorky & Schwaber, 2016). On the other hand, the microbiome may also influence initial cravings and has been linked to addictions, though this is still under investigation (de Timary, Leclercq, Stärkel, & Delzenne, 2016). Lastly, while there is preliminary evidence that the microbiome may be directly altered by changes in sleep profiles (Thompson et al., 2017), the connection between the two may be more easily interpreted within the context of the vast consequences of altered sleep on diet, and digestion. Overall, these data suggest drastic changes can occur during adolescence in several health and wellness domains that are closely linked to the function of the gut microbiome. Therefore, not only is there a strong reason to believe these indices of physical health and wellness change the microbiome, but also that the microbiome is likely a prominent player in the function and changes that occur in this domain during adolescence.
5 |. CONCLUSION
Adolescence needs to be interrogated as a sensitive period of microbiome–gut–brain axis communication. We propose that adolescence reflects not only a period of sensitivity to alter the development of the microbiome, but also a period wherein the microbiome may drive other behavioral and neurobiological changes. As such, it is imperative that researchers incorporate the microbiome into conceptual models and measures of adolescent psychobiological development. We wish to emphasize that studying the microbiome in addition to other methodologies, rather than as a replacement for them, should provide maximal insight about adolescent development. As psychologists continue to embrace the gut microbiome as a new methodology to understand psychological development, it is important to understand the causal limitations of any one biological system on development and to avoid bold, oversimplified claims. Multidisciplinary collaborations into the several novel domains summarized here should provide excellent forays to pursue the interactive role of the microbiome-gut-brain axis within adolescent development.
REFERENCES
- Agans R, Rigsbee L, Kenche H, Michail S, Khamis HJ, & Paliy O (2011). Distal gut microbiota of adolescent children is different from that of adults. FEMS Microbiology Ecology, 77(2), 404–412. 10.1111/j.1574-6941.2011.01120.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akkasheh G, Kashani-Poor Z, Tajabadi-Ebrahimi M, Jafari P, Akbari H, Taghizadeh M, … Esmaillzadeh A (2016). Clinical and metabolic response to probiotic administration in patients with major depressive disorder: A randomized, double-blind, placebo-controlled trial. Nutrition, 32(3), 315–320. 10.1016/j.nut.2015.09.003 [DOI] [PubMed] [Google Scholar]
- Anderson EL, Li W, Klitgord N, Highlander SK, Dayrit M, Seguritan V, … Jones MB (2016). A robust ambient temperature collection and stabilization strategy: Enabling worldwide functional studies of the human microbiome. Scientific Reports, 6, 31731 10.1038/srep31731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold JW, Roach J, & Azcarate-Peril MA (2016). Emerging technologies for gut microbiome research. Trends in Microbiology, 24(11), 887–901. 10.1016/j.tim.2016.06.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey MT, Dowd SE, Parry NMA, Galley JD, Schauer DB, & Lyte M (2010). Stressor exposure disrupts commensal microbial populations in the intestines and leads to increased colonization by Citrobacter rodentium. Infection and Immunity, 78(4), 1509–1519. 10.1128/IAI.00862-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bercik P, Denou E, Collins J, Jackson W, Lu J, Jury J, … Collins SM. (2011). The intestinal microbiota affect central levels of brain-derived neurotropic factor and behavior in mice. Gastroenterology, 141(2), 599–609, 609.e1–3. 10.1053/j.gastro.2011.04.052 [DOI] [PubMed] [Google Scholar]
- Bingham B, McFadden K, Zhang X, Bhatnagar S, Beck S, & Valentino R (2011). Early adolescence as a critical window during which social stress distinctly alters behavior and brain norepinephrine activity. Neuropsychopharmacology, 36(4), 896–909. 10.1038/npp.2010.229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blakemore S-J, & Mills KL (2014). Is adolescence a sensitive period for sociocultural processing? Annual Review of Psychology, 65(1), 187–207. 10.1146/annurev-psych-010213-115202 [DOI] [PubMed] [Google Scholar]
- Borre YE, O’Keeffe GW, Clarke G, Stanton C, Dinan TG, & Cryan JF (2014). Microbiota and neurodevelopmental windows: Implications for brain disorders. Trends in Molecular Medicine, 20(9), 509–518. 10.1016/j.molmed.2014.05.002 [DOI] [PubMed] [Google Scholar]
- Braams BR, van Duijvenvoorde ACK, Peper JS, & Crone EA (2015). Longitudinal changes in adolescent risk-taking: A comprehensive study of neural responses to rewards, pubertal development, and risk-taking behavior. Journal of Neuroscience, 35(18), 7226–7238. 10.1523/JNEUROSCI.4764-14.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brotman RM, Ravel J, Bavoil PM, Gravitt PE, & Ghanem KG (2014). Microbiome, sex hormones, and immune responses in the reproductive tract: Challenges for vaccine development against sexually transmitted infections. Vaccine, 32(14), 1543–1552. 10.1016/j.vaccine.2013.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burokas A, Arboleya S, Moloney RD, Peterson VL, Murphy K, Clarke G, … Cryan JF (2017). Targeting the microbiota-gut-brain axis: Prebiotics have anxiolytic and antidepressant-like effects and reverse the impact of chronic stress in mice. Biological Psychiatry, 82(7), 472–487. 10.1016/j.biopsych.2016.12.031 [DOI] [PubMed] [Google Scholar]
- Carabotti M, Scirocco A, Maselli MA, & Severi C (2015). The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Annals of Gastroenterology, 28(2), 203–209. [PMC free article] [PubMed] [Google Scholar]
- Carlson AL, Xia K, Azcarate-Peril MA, Goldman BD, Ahn M, Styner MA, … Knickmeyer RC (2018). Infant gut microbiome associated with cognitive development. Biological Psychiatry, 83(2), 148–159. 10.1016/j.biopsych.2017.06.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carskadon MA (2011). Sleep in adolescents: The perfect storm. Pediatric Clinics of North America, 58(3), 637–647. 10.1016/j.pcl.2011.03.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaidez V, Hansen RL, & Hertz-Picciotto I (2014). Gastrointestinal problems in children with autism, developmental delays or typical development. Journal of Autism and Developmental Disorders, 44(5), 1117–1127. 10.1007/s10803-013-1973-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Song X, Wei W, Zhong H, Dai J, Lan Z, … Jia H (2017). The microbiota continuum along the female reproductive tract and its relation to uterine-related diseases. Nature Communications, 8(1), 875 10.1038/s41467-017-00901-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian LM, Galley JD, Hade EM, Schoppe-Sullivan S, Kamp Dush C, & Bailey MT (2015). Gut microbiome composition is associated with temperament during early childhood. Brain, Behavior, and Immunity, 45, 118–127. 10.1016/j.bbi.2014.10.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke G, Grenham S, Scully P, Fitzgerald P, Moloney RD, Shanahan F, … Cryan JF. (2013). The microbiome-gut-brain axis during early life regulates the hippocampal serotonergic system in a sex-dependent manner. Molecular Psychiatry, 18(6), 666 10.1038/mp.2012.77 [DOI] [PubMed] [Google Scholar]
- Collado MC, Rautava S, Aakko J, Isolauri E, & Salminen S (2016). Human gut colonisation may be initiated in utero by distinct microbial communities in the placenta and amniotic fluid. Scientific Reports, 6, 23129 10.1038/srep23129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colrain IM, & Baker FC (2011). Changes in sleep as a function of adolescent development. Neuropsychology Review, 21(1), 5–21. 10.1007/s11065-010-9155-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly CG, Wu J, Ho TC, Hoeft F, Wolkowitz O, Eisendrath S, … Yang TT (2013). Resting-state functional connectivity of subgenual anterior cingulate cortex in depressed adolescents. Biological Psychiatry, 74(12), 898–907. 10.1016/j.biopsych.2013.05.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costea PI, Coelho LP, Sunagawa S, Munch R, Huerta-Cepas J, Forslund K, … Bork P. (2017). Subspecies in the global human gut microbiome. Molecular Systems Biology, 13(12), 960. 10.15252/msb.20177589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowan CSM, Hoban AE, Ventura-Silva AP, Dinan TG, Clarke G, & Cryan JF (n.d.). Gutsy Moves: The Amygdala as a Critical Node in Microbiota to Brain Signaling. BioEssays, 40(1), 1700172 10.1002/bies.201700172 [DOI] [PubMed] [Google Scholar]
- Crone EA, & Dahl RE (2012). Understanding adolescence as a period of social–affective engagement and goal flexibility. Nature Reviews Neuroscience, 13(9), 636–650. 10.1038/nrn3313 [DOI] [PubMed] [Google Scholar]
- Cryan JF, & Dinan TG (2012). Mind-altering microorganisms: The impact of the gut microbiota on brain and behaviour. Nature Reviews Neuroscience, 13(10), 701–712. 10.1038/nrn3346. [DOI] [PubMed] [Google Scholar]
- Cullen KR, Westlund MK, Klimes-Dougan B, Mueller BA, Houri A, Eberly LE, & Lim KO (2014). Abnormal Amygdala resting-state functional connectivity in adolescent depression. JAMA Psychiatry, 71(10), 1138–1147. 10.1001/jamapsychiatry.2014.1087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl RE, Allen NB, Wilbrecht L, & Suleiman AB (2018). Importance of investing in adolescence from a developmental science perspective. Nature, 554(7693), 441–450. 10.1038/nature25770 [DOI] [PubMed] [Google Scholar]
- Davey CG, Whittle S, Harrison BJ, Simmons JG, Byrne ML, Schwartz OS, & Allen NB (2015). Functional brain-imaging correlates of negative affectivity and the onset of first-episode depression. Psychological Medicine, 45(5), 1001–1009. 10.1017/S0033291714002001 [DOI] [PubMed] [Google Scholar]
- David LA, Maurice CF, Carmody RN, Gootenberg DB, Button JE, Wolfe BE, … Turnbaugh PJ (2014). Diet rapidly and reproducibly alters the human gut microbiome. Nature, 505(7484), 559–563. 10.1038/nature12820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Andrade SC, Previdelli ÁN, Cesar CLG, Marchioni DML, & Fisberg RM (2016). Trends in diet quality among adolescents, adults and older adults: A population-based study. Preventive Medicine Reports, 4, 391–396. 10.1016/j.pmedr.2016.07.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Sousa Rodrigues ME, Bekhbat M, Houser MC, Chang J, Walker DI, Jones DP, … Tansey MG (2017). Chronic psychological stress and high-fat high-fructose diet disrupt metabolic and inflammatory gene networks in the brain, liver, and gut and promote behavioral deficits in mice. Brain, Behavior, and Immunity, 59, 158–172. 10.1016/j.bbi.2016.08.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Timary P, Leclercq S, Stärkel P, & Delzenne N (2016). A dysbiotic subpopulation of alcohol-dependent subjects. Gut Microbes, 6(6), 388–391. 10.1080/19490976.2015.1107696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desbonnet L, Clarke G, Traplin A, O’Sullivan O, Crispie F, Moloney RD, … Cryan JF (2015). Gut microbiota depletion from early adolescence in mice: Implications for brain and behaviour. Brain, Behavior, and Immunity, 48, 165–173. 10.1016/j.bbi.2015.04.004 [DOI] [PubMed] [Google Scholar]
- Dinan TG, & Cryan JF (2016). Mood by microbe: Towards clinical translation. Genome Medicine, 8, 36 10.1186/s13073-016-0292-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinan TG, & Cryan JF (2017). Brain–gut–microbiota axis — mood, metabolism and behaviour. Nature Reviews Gastroenterology and Hepatology, 14(2), 69–70. 10.1038/nrgastro.2016.200 [DOI] [PubMed] [Google Scholar]
- Driessen CE, Cameron AJ, Thornton LE, Lai SK, & Barnett LM (2014). Effect of changes to the school food environment on eating behaviours and/or body weight in children: A systematic review. Obesity Reviews, 15(12), 968–982. 10.1111/obr.12224 [DOI] [PubMed] [Google Scholar]
- Farzi A, Fröhlich EE, & Holzer P (2018). Gut microbiota and the neuroendocrine system. Neurotherapeutics, 15(1), 5–22. 10.1007/s13311-017-0600-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flannery JE, Giuliani NR, Flournoy JC, & Pfeifer JH (2017). Neurodevelopmental changes across adolescence in viewing and labeling dynamic peer emotions. Developmental Cognitive Neuroscience, 25, 113–127. 10.1016/j.dcn.2017.02.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster JA, & McVey Neufeld K-A (2013). Gut–brain axis: How the microbiome influences anxiety and depression. Trends in Neurosciences, 36(5), 305–312. 10.1016/j.tins.2013.01.005 [DOI] [PubMed] [Google Scholar]
- Foster JA, Rinaman L, & Cryan,Schloss PD, Iverson KD, Petrosino JF, & Schloss SJ. (2014). The J. F. (2017). Stress & the gut-brain axis: Regulation by the microbiome. Neurobiology of Stress, 7, 124–136. 10.1016/j.ynstr.2017.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fröhlich EE, Farzi A, Mayerhofer R, Reichmann F, Jačan A, Wagner B, … Holzer P (2016). Cognitive impairment by antibiotic-induced gut dysbiosis: Analysis of gut microbiota-brain communication. Brain, Behavior, and Immunity, 56, 140–155. 10.1016/j.bbi.2016.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gareau MG, Wine E, Rodrigues DM, Cho JH, Whary MT, Philpott DJ, … Sherman PM (2011). Bacterial infection causes stress-induced memory dysfunction in mice. Gut, 60(3), 307–317. 10.1136/gut.2009.202515 [DOI] [PubMed] [Google Scholar]
- Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M, … Tottenham N (2013). A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. Journal of Neuroscience, 33(10), 4584–4593. 10.1523/JNEUROSCI.3446-12.2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, … Reid G (2017). Expert consensus document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nature Reviews Gastroenterology and Hepatology, 14(8), 491–502. 10.1038/nrgastro.2017.75 [DOI] [PubMed] [Google Scholar]
- Gorky J, & Schwaber J (2016). The role of the gut–brain axis in alcohol use disorders. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 65, 234–241. 10.1016/j.pnpbp.2015.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Group TNHW, Peterson J, Garges S, Giovanni M, McInnes P, Wang L, … Guyer M (2009). The NIH human microbiome project. Genome Research, 19(12), 2317–2323. 10.1101/gr.096651.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusberti FA, Mombelli A, Lang NP, & Minder CE (1990). Changes in subgingival microbiota during puberty. A 4-year longitudinal study. Journal of Clinical Periodontology, 17(10), 685–692. 10.1111/j.1600-051X.1990.tb01054.x [DOI] [PubMed] [Google Scholar]
- Heijtz RD, Wang S, Anuar F, Qian Y, Björkholm B, Samuelsson A, … Pettersson S (2011). Normal gut microbiota modulates brain development and behavior. Proceedings of the National Academy of Sciences, 108(7), 3047–3052. 10.1073/pnas.1010529108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoban AE, Stilling RM, Moloney G, Shanahan F, Dinan TG, Clarke G, & Cryan JF (2018). The microbiome regulates amygdala-dependent fear recall. Molecular Psychiatry, 23(5), 1134–1144. 10.1038/mp.2017.100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollister EB, Riehle K, Luna RA, Weidler EM, Rubio-Gonzales M, Mistretta T-A, … Versalovic J (2015). Structure and function of the healthy pre-adolescent pediatric gut microbiome. Microbiome, 3, 36 10.1186/s40168-015-0101-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins ME, Nitecki R, & Bucci DJ (2011). Physical exercise during adolescence versus adulthood: Differential effects on object recognition memory and BDNF levels. Neuroscience, 194, 84–94. 10.1016/j.neuroscience.2011.07.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins M, Sharp R, & Macfarlane G (2001). Age and disease related changes in intestinal bacterial populations assessed by cell culture, 16S rRNA abundance, and community cellular fatty acid profiles. Gut, 48(2), 198–205. 10.1136/gut.48.2.198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao EY, McBride SW, Hsien S, Sharon G, Hyde ER, McCue T, … Mazmanian SK. (2013). Microbiota modulate behavioral and physiological abnormalities associated with neurodevelopmental disorders. Cell, 155(7), 1451–1463. 10.1016/j.cell.2013.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juraska JM, & Willing J (2017). Pubertal onset as a critical transition for neural development and cognition. Brain Research, 1654, 87–94. 10.1016/j.brainres.2016.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang D-W, Adams JB, Gregory AC, Borody T, Chittick L, Fasano A, … Krajmalnik-Brown R (2017). Microbiota Transfer Therapy alters gut ecosystem and improves gastrointestinal and autism symptoms: An open-label study. Microbiome, 5, 10 10.1186/s40168-016-0225-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang D-W, Park JG, Ilhan ZE, Wallstrom G, LaBaer J, Adams JB, & Krajmalnik-Brown R (2013). Reduced incidence of prevotella and other fermenters in intestinal microflora of autistic children. PLoS ONE, 8(7). 10.1371/journal.pone.0068322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly JR, Kennedy PJ, Cryan JF, Dinan TG, Clarke G, & Hyland NP (2015). Breaking down the barriers: The gut microbiome, intestinal permeability and stress-related psychiatric disorders. Frontiers in Cellular Neuroscience, 9, 392 10.3389/fncel.2015.00392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuczynski J, Lauber CL, Walters WA, Parfrey LW, Clemente JC, Gevers D, & Knight R (2011). Experimental and analytical tools for studying the human microbiome. Nature Reviews. Genetics, 13(1), 47–58. 10.1038/nrg3129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson R, & Richards MH (1991). Daily companionship in late childhood and early adolescence: Changing developmental contexts. Child Development, 62(2), 284–300. 10.1111/j.1467-8624.1991.tb01531.x [DOI] [PubMed] [Google Scholar]
- Lee FS, Heimer H, Giedd JN, Lein ES, Šestan N, Weinberger DR, & Casey BJ (2014). Adolescent mental health—Opportunity and obligation. Science, 346(6209), 547–549. 10.1126/science.1260497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Q, Han Y, Dy ABC, & Hagerman RJ (2017). The gut microbiota and autism spectrum disorders. Frontiers in Cellular Neuroscience, 11, 120 10.3389/fncel.2017.00120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loomba-Albrecht LA, & Styne DM (2009). Effect of puberty on body composition. Current Opinion in Endocrinology, Diabetes, and Obesity, 16(1), 10–15. 10.1097/MED.0b013e328320d54c [DOI] [PubMed] [Google Scholar]
- Luczynski P, Whelan SO, O’Sullivan C, Clarke G, Shanahan F, Dinan TG, & Cryan JF (2016). Adult microbiota-deficient mice have distinct dendritic morphological changes: Differential effects in the amygdala and hippocampus. European Journal of Neuroscience, 44(9), 2654–2666. 10.1111/ejn.13291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer EA (2011). Gut feelings: tHe emerging biology of gut–brain communication. Nature Reviews Neuroscience, 12(8), 453–466. 10.1038/nrn3071 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer EA, Knight R, Mazmanian SK, Cryan JF, & Tillisch K (2014). Gut microbes and the brain: Paradigm shift in neuroscience. Journal of Neuroscience, 34(46), 15490–15496. 10.1523/JNEUROSCI.3299-14.2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayer EA, Naliboff BD, & Craig ADB (2006). Neuroimaging of the brain-gut axis: From basic understanding to treatment of functional GI disorders. Gastroenterology, 131(6), 1925–1942. 10.1053/j.gastro.2006.10.026 [DOI] [PubMed] [Google Scholar]
- McDonald D, Hyde ER, Debelius JW, Morton JT, Gonzalez A, Ackermann G, Knight R (2018). American gut: An open platform for citizen-science microbiome research. bioRxiv, 3(3), e00031–18. 10.1101/277970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight-Eily LR, Eaton DK, Lowry R, Croft JB, Presley-Cantrell L, & Perry GS (2011). Relationships between hours of sleep and health-risk behaviors in US adolescent students. Preventive Medicine, 53(4), 271–273. 10.1016/j.ypmed.2011.06.020 [DOI] [PubMed] [Google Scholar]
- McVey Neufeld K-A, Luczynski P, Dinan TG, & Cryan JF (2016). Reframing the teenage wasteland: Adolescent microbiota-gut-brain axis. Canadian Journal of Psychiatry. Revue Canadienne De Psychiatrie, 61(4), 214–221. 10.1177/0706743716635536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills KL, Goddings A-L, Herting MM, Meuwese R, Blakemore S-J, Crone EA, … Tamnes CK (2016). Structural brain development between childhood and adulthood: Convergence across four longitudinal samples. NeuroImage, 141, 273–281. 10.1016/j.neuroimage.2016.07.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moloney RD, Johnson AC, O’Mahony SM, Dinan TG, Meerveld B-G-V, & Cryan JF (2016). Stress and the Microbiota–Gut–brain axis in visceral pain: Relevance to irritable bowel syndrome. CNS Neuroscience and Therapeutics, 22(2), 102–117. 10.1111/cns.12490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münger E, Montiel-Castro AJ, Langhans W, & Pacheco-López G (2018). Reciprocal interactions between gut microbiota and host social behavior. Frontiers in Integrative Neuroscience, 12, 21 10.3389/fnint.2018.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufeld KM, Kang N, Bienenstock J, & Foster JA (2011). Reduced anxiety-like behavior and central neurochemical change in germ-free mice. Neurogastroenterology and Motility: The Official Journal of the European Gastrointestinal Motility Society, 23(3), 255–264, e119. 10.1111/j.1365-2982.2010.01620.x [DOI] [PubMed] [Google Scholar]
- Ng QX, Peters C, Ho CYX, Lim DY, & Yeo W-S (2018). A meta-analysis of the use of probiotics to alleviate depressive symptoms. Journal of Affective Disorders, 228, 13–19. 10.1016/j.jad.2017.11.063 [DOI] [PubMed] [Google Scholar]
- O’Mahony SM, Clarke G, Dinan TG, & Cryan JF (2017). Early-life adversity and brain development: Is the microbiome a missing piece of the puzzle? Neuroscience, 342, 37–54. 10.1016/j.neuroscience.2015.09.068 [DOI] [PubMed] [Google Scholar]
- Oh J, Conlan S, Polley EC, Segre JA, & Kong HH (2012). Shifts in human skin and nares microbiota of healthy children and adults. Genome Medicine, 4(10), 77 10.1186/gm378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paliy O, Kenche H, Abernathy F, & Michail S (2009). High-through-put quantitative analysis of the human intestinal microbiota with a phylogenetic microarray. Applied and Environmental Microbiology, 75(11), 3572–3579. 10.1128/AEM.02764-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannekoek JN, van der Werff S, Meens PHF, van den Bulk BG, Jolles DD, Veer IM, … Vermeiren RRJM (2014). Aberrant resting-state functional connectivity in limbic and salience networks in treatment-naïve clinically depressed adolescents. Journal of Child Psychology and Psychiatry, 55(12), 1317–1327. 10.1111/jcpp.12266 [DOI] [PubMed] [Google Scholar]
- Petrosino JF (2018). The microbiome in precision medicine: The way forward. Genome Medicine, 10, 12 10.1186/s13073-018-0525-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peverill M, McLaughlin KA, Finn AS, & Sheridan MA (2016). Working memory filtering continues to develop into late adolescence. Developmental Cognitive Neuroscience, 18, 78–88. 10.1016/j.dcn.2016.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeifer JH, & Berkman ET (2018). The Development of self and identity in adolescence: Neural evidence and implications for a value- based choice perspective on motivated behavior. Child Development Perspectives, 12(3), 158–164. 10.1111/cdep.12279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto-Sanchez MI, Hall GB, Ghajar K, Nardelli A, Bolino C, Lau JT, … Bercik P (2017). Probiotic Bifidobacterium longum NCC3001 reduces depression scores and alters brain activity: A pilot study in patients with irritable bowel syndrome. Gastroenterology, 153(2), 448–459.e8. 10.1053/j.gastro.2017.05.003 [DOI] [PubMed] [Google Scholar]
- Pirbaglou M, Katz J, de Souza RJ, Stearns JC, Motamed M, & Ritvo P (2016). Probiotic supplementation can positively affect anxiety and depressive symptoms: A systematic review of randomized controlled trials. Nutrition Research, 36(9), 889–898. 10.1016/j.nutres.2016.06.009 [DOI] [PubMed] [Google Scholar]
- Rogers GB, Keating DJ, Young RL, Wong M-L, Licinio J, & Wesselingh S (2016). From gut dysbiosis to altered brain function and mental illness: Mechanisms and pathways. Molecular Psychiatry, 21(6), 738–748. 10.1038/mp.2016.50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer D (2010). Adolescent risk taking, impulsivity, and brain development: Implications for prevention. Developmental Psychobiology, 52(3), 263–276. 10.1002/dev.20442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer D, Reyna VF, & Satterthwaite TD (2017). Beyond stereotypes of adolescent risk taking: Placing the adolescent brain in developmental context. Developmental Cognitive Neuroscience, 27, 19–34. 10.1016/j.dcn.2017.07.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders ME (2008). Probiotics: Definition, sources, selection, and uses. Clinical Infectious Diseases, 46(Supp. 2), S58–S61. 10.1086/523341 [DOI] [PubMed] [Google Scholar]
- Sarkar A, Lehto SM, Harty S, Dinan TG, Cryan JF, & Burnet PWJ (2016). Psychobiotics and the manipulation of bacteria–gut–brain signals. Trends in Neurosciences, 39(11), 763–781. 10.1016/j.tins.2016.09.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar A, Harty S, Lehto SM, Moeller AH, Dinan TG, Dunbar RIM, … Burnet PWJ (2018). The microbiome in psychology and cognitive neuroscience. Trends in Cognitive Sciences, 22(7), 611–636. 10.1016/j.tics.2018.04.006 [DOI] [PubMed] [Google Scholar]
- Savignac HM, Tramullas M, Kiely B, Dinan TG, & Cryan JF (2015). Bifidobacteria modulate cognitive processes in an anxious mouse strain. Behavioural Brain Research, 287, 59–72. 10.1016/j.bbr.2015.02.044 [DOI] [PubMed] [Google Scholar]
- Schloss PD, Iverson KD, Petrosino JF, & Schloss SJ (2014). The dynamics of a family’s gut microbiota reveal variations on a theme. Microbiome, 2, 25 10.1186/2049-2618-2-25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt K, Cowen PJ, Harmer CJ, Tzortzis G, Errington S, & Burnet PWJ (2015). Prebiotic intake reduces the waking cortisol response and alters emotional bias in healthy volunteers. Psychopharmacology, 232(10), 1793–1801. 10.1007/s00213-014-3810-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sender R, Fuchs S, & Milo R (2016). Are we really vastly outnumbered? Revisiting the ratio of bacterial to host cells in humans. Cell, 164(3), 337–340. 10.1016/j.cell.2016.01.013 [DOI] [PubMed] [Google Scholar]
- Sharon G, Sampson TR, Geschwind DH, & Mazmanian SK (2016). The Central nervous system and the gut microbiome. Cell, 167(4), 915–932. 10.1016/j.cell.2016.10.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharpton TJ (2014). An introduction to the analysis of shotgun metagenomic data. Frontiers in Plant Science, 5, 209 10.3389/fpls.2014.00209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman L, Steinberg L, & Chein J (2017). Connecting brain responsivity and real-world risk taking: Strengths and limitations of current methodological approaches. Developmental Cognitive Neuroscience, 33, 27–41. 10.1016/j.dcn.2017.05.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Dismukes AR, Marceau KP, Ruttle P, Simmons J, & Han G (2015). A dual-axis approach to understanding neuroendocrine development. Developmental Psychobiology, 57(6), 643–653. 10.1002/dev.21337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siervogel RM, Demerath EW, Schubert C, Remsberg KE, Chumlea WC, Sun S, … Towne B (2003). Puberty and body composition. Hormone Research in Paediatrics, 60(Suppl. 1), 36–45. 10.1159/000071224 [DOI] [PubMed] [Google Scholar]
- Silverman MH, Jedd K, & Luciana M (2015). Neural networks involved in adolescent reward processing: An activation likelihood estimation meta-analysis of functional neuroimaging studies. NeuroImage, 122, 427–439. 10.1016/j.neuroimage.2015.07.083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strati F, Cavalieri D, Albanese D, De Felice C, Donati C, Hayek J, … De Filippo C (2017). New evidences on the altered gut microbiota in autism spectrum disorders. Microbiome, 5(1), 24 10.1186/s40168-017-0242-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sudo N, Chida Y, Aiba Y, Sonoda J, Oyama N, Yu X-N, … Koga Y (2004). Postnatal microbial colonization programs the hypothalamic–pituitary–adrenal system for stress response in mice. The Journal of Physiology, 558(1), 263–275. 10.1113/jphysiol.2004.063388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoits PA (2011). Mechanisms linking social ties and support to physical and mental health. Journal of Health and Social Behavior, 52(2), 145–161. 10.1177/0022146510395592 [DOI] [PubMed] [Google Scholar]
- Thompson RS, Roller R, Mika A, Greenwood BN, Knight R, Chichlowski M, … Fleshner M (2017). Dietary prebiotics and bioactive milk fractions improve NREM sleep, enhance REM sleep rebound and attenuate the stress-induced decrease in diurnal temperature and gut microbial alpha diversity. Frontiers in Behavioral Neuroscience, 10 10.3389/fnbeh.2016.00240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillisch K, Labus J, Kilpatrick L, Jiang Z, Strains J, Ebrat B, Mayer EA (2013). Consumption of fermented milk product with probiotic modulates brain activity. Gastroenterology, 144(7), 1394–1401.e4. 10.1053/j.gastro.2013.02.043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillisch K, Mayer EA, & Labus JS (2011). Quantitative meta-analysis identifies brain regions activated during rectal distension in irritable bowel syndrome. Gastroenterology, 140(1), 91–100. 10.1053/j.gastro.2010.07.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillisch K, Mayer EA, Gupta A, Gill Z, Brazeilles R, Le Nevé B, … Labus JS (2017). Brain structure and response to emotional stimuli as related to gut microbial profiles in healthy women. Psychosomatic Medicine, 79(8), 905 10.1097/PSY.0000000000000493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tottenham N (2015). Social scaffolding of human amygdala-mPFC circuit development. Social Neuroscience, 10(5), 489–499. 10.1080/17470919.2015.1087424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Bos W, & Hertwig R (2017). Adolescents display distinctive tolerance to ambiguity and to uncertainty during risky decision making. Scientific Reports, 7, 40962 10.1038/srep40962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayakumar N, Op de Macks Z, Shirtcliff EA, & Pfeifer JH (2018). Puberty and the human brain: Insights into adolescent development. Neuroscience and Biobehavioral Reviews, 92, 417–436. 10.1016/j.neubiorev.2018.06.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace CJK, & Milev R (2017). The effects of probiotics on depressive symptoms in humans: A systematic review. Annals of General Psychiatry, 16, 14 10.1186/s12991-017-0138-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang T, Hu X, Liang S, Li W, Wu X, Wang L, & Jin F (2015). Lactobacillus fermentum NS9 restores the antibiotic induced physiological and psychological abnormalities in rats. Beneficial Microbes, 6(5), 707–717. 10.3920/BM2014.0177 [DOI] [PubMed] [Google Scholar]
- West PR, Amaral DG, Bais P, Smith AM, Egnash LA, Ross ME, … Burrier RE. (2014). Metabolomics as a tool for discovery of biomarkers of autism spectrum disorder in the blood plasma of children. PLOS ONE, 9(11), e112445 10.1371/journal.pone.0112445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winpenny EM, Penney TL, Corder K, White M, & van Sluijs EMF (2017). Change in diet in the period from adolescence to early adulthood: A systematic scoping review of longitudinal studies. The International Journal of Behavioral Nutrition and Physical Activity, 14(1), 60 10.1186/s12966-017-0518-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthman CM, & Trang K (2018). Dynamics of body time, social time and life history at adolescence. Nature, 554(7693), 451–457. 10.1038/nature25750 [DOI] [PubMed] [Google Scholar]
- Xu M-Q, Cao H-L, Wang W-Q, Wang S, Cao X-C, Yan F, & Wang B-M (2015). Fecal microbiota transplantation broadening its application beyond intestinal disorders. World Journal of Gastroenterology, 21(1), 102–111. 10.3748/wjg.v21.i1.102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng P, Zeng B, Zhou C, Liu M, Fang Z, Xu X, … Xie P (2016). Gut microbiome remodeling induces depressive-like behaviors through a pathway mediated by the host’s metabolism. Molecular Psychiatry, 21(6), 786 10.1038/mp.2016.44 [DOI] [PubMed] [Google Scholar]
- Zhou X, Zhu D, Qi X-L, Li S, King SG, Salinas E, … Constantinidis C (2016). Neural correlates of working memory development in adolescent primates. Nature Communications, 7, 13423 10.1038/ncomms13423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu X, Han Y, Du J, Liu R, Jin K, & Yi W (2017). Microbiota-gut-brain axis and the central nervous system. Oncotarget, 8(32), 53829–53838. 10.18632/oncotarget.17754 [DOI] [PMC free article] [PubMed] [Google Scholar]