Abstract
Preclinical studies have shown effects of chronic exposure to addictive drugs on glutamatergic-mediated neuroplasticity in frontostriatal circuitry. These initial findings have been paralleled by human fMRI research demonstrating weaker frontostriatal resting-state functional connectivity (rsFC) among individuals with psychostimulant use disorders. However, there is a dearth of human imaging literature describing associations between long-term prescription opioid use, frontostriatal rsFC, and brain morphology among chronic pain patients. We hypothesized that prescription opioid users with chronic pain, as compared to healthy control subjects, would evidence weaker frontostriatal rsFC coupled with less frontostriatal gray matter volume (GMV). Further, that those opioid use-related deficits in frontostriatal circuitry would be associated with the negative affect and drug misuse. Prescription opioid users with chronic pain (n = 31) and drug-free healthy controls (n =30) underwent a high-resolution anatomical and an eyes-closed resting-state functional scan. The opioid group, relative to controls, exhibited weaker frontostriatal rsFC and less frontostriatal GMV in both L.NAc and L.vmPFC. Frontostriatal rsFC partially mediated group differences in negative affect. Within opioid users, L.NAc GMV predicted opioid misuse severity. The current study revealed that prescription opioid use in the context of chronic pain is associated with functional and structural abnormalities in frontostriatal circuitry. These results suggest that opioid use-related abnormalities in frontostriatal circuitry may undergird disturbances in affect that may contribute to the ongoing maintenance of opioid use and misuse. These findings warrant further examination of interventions to treat opioid pathophysiology in frontostriatal circuitry over the course of treatment.
Keywords: resting-state, rsFC, VBM, fMRI, gray matter, corticostriatal
INTRODUCTION
Opioid misuse and addiction are endemic in the U.S. and adversely impact public health and economic productivity [1, 2]. Long-term use and misuse of opioids in the context of chronic pain may dysregulate affective processes [3], significantly elevating risk of opioid use disorder (OUD) [4] and overdose-related mortality and morbidity [1]. Although current first line FDA-approved medication-assisted treatments effectively attenuate withdrawal symptom severity (e.g., Buprenorphine, Methadone), it remains questionable as to whether or not they treat the underlying neuropathology of OUD [5], as evidenced by >50% of participants relapsing after discontinuing opioid maintenance therapy [6]. Therefore, further mechanistic research on prolonged opioid use among individuals with and without chronic pain is needed to elucidate neurobiological mechanisms to be targeted by new treatment approaches.
The extant literature on animal models of addiction reveals that chronic exposure to drugs of abuse produces glutamatergic neuroplasticity in frontostriatal circuitry [7], suggesting a transdiagnostic mechanism in addiction and therefore a potential target for treating substance use disorders. Across drugs of abuse, transition into the compulsive stage of addiction is associated with glutamatergic-mediated, synaptic- and meta-plasticity in frontostriatal circuity [7, 8] comprising the medial prefrontal cortex (mPFC)—a region coding for the appraisal of environmental stimuli [9]—and the nucleus accumbens (NAc) —a region coding for the predictive value of a rewarding stimulus (e.g., food, sex, drugs) [10]. Opiates [11], nicotine [12, 13] and cocaine [14] each dysregulate glutamatergic homeostasis in the NAc, which mediates the reinstatement of drug-seeking behavior upon exposure to conditioned drug cues in animal models [15–18].
Analogous to electrophysiological methods used in preclinical models, resting-state functional connectivity (rsFC) of neural networks [19] has provided utility in characterizing substance use disorder pathophysiology (for review, see [20, 21]). Paralleling preclinical findings, human fMRI research has demonstrated weaker frontostriatal rsFC among individuals with nicotine-use disorder [22] and polysubstance abuse [23]; however, findings are mixed among treatment-seeking [24–26] and non-treatment-seeking opioid use disorder (OUD) [27] samples. The prior failure to replicate findings across studies of opioid addiction may be, in part, due to participants within- and across-samples varying with regard to stage of use vs. abstinence, use of opioid-replacement therapy, small sample sizes and presence vs. absence of chronic pain. Therefore, the present investigation was designed to address these limitations by studying a larger sample drawn from a population at risk of developing OUD [2] – i.e., chronic pain patients engaged in long-term use of prescription opioid analgesics – in order to ascertain the extent to which prolonged opioid use in the context of chronic pain is associated with disruptions in frontostriatal circuitry similar to those observed in other substance use disorders.
Regarding brain morphology, chronic opioid use has been linked to reductions in total cerebrum volume [28] and, among regions containing monosynaptic inputs to the nucleus accumbens, decreased gray matter volume (GMV), for example the: amygdala [27, 29], hippocampus[29], and vmPFC [30]. However, to the best of our knowledge, no studies have reported on associations between prescription opioid use, frontostriatal rsFC, morphology and clinical variables relevant to chronic pain (i.e., affect, opioid misuse, opioid dosing, and pain severity/interference). The current study utilized functional and structural imaging techniques to compare a group of prescription opioid users with chronic pain (OUG) (N=31) to drug-free healthy controls (N=30). We hypothesized that the OUG, as compared to healthy control subjects, would evidence weaker frontostriatal rsFC and less frontostriatal GMV that would in turn be significantly associated with the magnitude of affective disturbances and opioid misuse.
MATERIALS AND METHODS
Participants
Participants (N = 63) were recruited via community advertisements and referrals from primary care and pain clinics, attended an in-person screening visit to determine eligibility and then an experimental fMRI visit. Participants gave written informed consent and received financial compensation for study participation. Inclusion criteria were: being age 18 years or higher and being able to comply with protocol requirements (e.g. fMRI compatibility, indicate having reliable transportation to the laboratory). Among the opioid use group (OUG: n = 33), current use of prescription opioids for >3 consecutive months for treating chronic pain and a positive urine drug screen for opioids were also inclusion criteria, and participants were instructed to maintain their typical use of opioids over the course of the study and not abstain from using on the day of the lab visit. Exclusion criteria were: presence of unstable medical illness, history of major neurological illness or significant head injury with resultant loss of consciousness, any contraindication to MRI, current cancer diagnosis, use of illicit substances within the last month as evidenced by a positive drug screen (other than opioids among the OUG), and among females, a positive urine pregnancy test. Additional exclusion criteria for the healthy control group included: history of chronic opioid use (i.e., self-report history of ≥ 3 months of opioid use) or current use within the past 3 months, history of chronic pain or self-report of pain lasting ≥ 3 months [31]. The study was approved by the institutional review boards of the Medical University of South Carolina and the University of Utah. Individuals meeting criteria for OUG (n=33) and a healthy control group (n=30) were scanned on Siemens 3T MRI scanners at the University of Utah (n = 20) and University of South Carolina systems (n=43). One subject was excluded due to poor structural image quality and another for in-scanner motion, resulting in a final N of 61 (OUG = 31, control = 30).
Self-Report Measures
All participants completed the 20-item positive and negative affect schedule (PANAS) [32]. Among the OUG, daily Morphine Milligram Equivalence (MME) was calculated via participant report [33]; pain (over the past 24 hours) was assessed with the Brief Pain Inventory (BPI) [34]; opioid misuse assessed with the Current Opioid Misuse Measure (COMM) [35]; and self-reported craving for opioids using a 10-point visual analog scale.
Neuroimaging Data Acquisition
At each site, a high-resolution 3-dimensional, spoiled gradient recalled acquisition structural sequence (3D-MPRAGE) was acquired, followed by an eyes-closed, resting-state scan using an echo-planar gradient-echo pulse fMRI-BOLD sequence (Supplemental Methods).
Image Processing
All functional and structural images were preprocessed using the computational anatomy 12 (CAT12 - www.neuro.uni-jena.de/cat) and statistical parametric mapping 12 (SPM12 - http://www.fil.ion.ucl.ac.uk/spm/software/spm12/) MATLAB 2018a toolboxes according to a standard pipeline employing default settings (Supplemental Methods). Structural quality-control was performed visually and quantitatively by using CAT12’s homogeneity of covariance function across unsmoothed gray matter volumetric maps, excluding those that exceeded 3 SD from the mean sample covariance. One subject’s data was omitted due to poor structural imaging data quality.
Pre-processing of resting-state fMRI images included: slice-time correction (for non-multiband data) and realignment; motion outlier detection (framewise displacement > 1mm) www.nitrc.org/projects/artifact_detect) and correction (via nearest-neighbor interpolation); coregistration of functional images to structural image; warping to MNI space using forward deformations and resampling to (1.5mm)3 voxel size (i.e., 3.375µL). Art toolbox (https://www.nitrc.org/projects/artifact_detect/) was used to define “rapid motion” as motion across a volume with a frame-wise displacement exceeding 1mm. Volumes identified by art were then “corrected” via ArtRepair toolbox via nearest neighbor interpolation (https://www.nitrc.org/projects/art_repair/). Exclusion threshold for rapid motion was 20% of run length; one subject was excluded for excessive head motion. All preprocessed fMRI images and segmented tissue maps were smoothed with a (10mm)3 FWHM Gaussian filter prior to modeling (Supplemental Methods). Publication images were created using MRIcroGL (http://www.mccauslandcenter.sc.edu/mricrogl/home).
Frontostriatal Network rsFC Mask
The primary rsFC hypotheses of this study were tested within a regions of interest (ROI) mask derived from Wake Forest University (WFU) PickAtlas (http://fmri.wfubmc.edu/software/PickAtlas) (Figure 1). This included bilateral ROIs for anterior and middle cingulate cortices, rectus and olfactory gyri, medial-superior prefrontal cortices (PFC), and mid-frontal orbital PFC, along with 3mm-radius sphere around the nucleus accumbens (±12, 8, −8) [36, 37] (http://marsbar.sourceforge.net/).
Figure 1. Frontostriatal Network rsFC Mask.
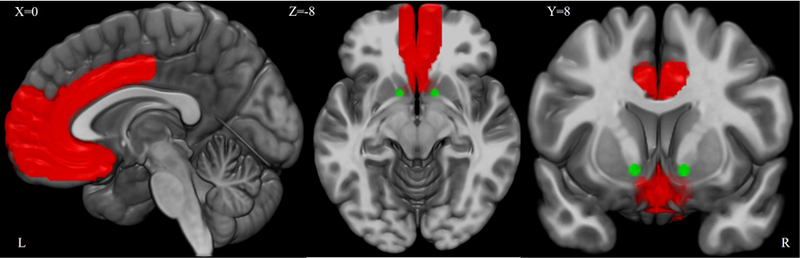
Frontostriatal network mask for resting-state functional connectivity. Medial prefrontal regions above are shaded red while the bilateral nucleus accumbens spheres are shaded green.
Statistical Analyses
General Statistical Considerations.
Statistical significance was defined at α = .05 for all tests. Homogeneity of covariance was assessed via Levene’s test. Marginal means were reported from ANCOVA. Cohen’s d statistic was computed for each ANCOVA using an online calculator (https://bit.ly/2tUiLLc). Post hoc ‘observed power’ from SPSS is reported where applicable for the convenience of the reader. Given the use of covariates as described below, t- and F-statistics are presented with degrees of freedom and significant regression findings further include unstandardized beta coefficients. Mean (M) and standard error (±) reported where appropriate.
Covariates.
For the purposes of cluster detection, the second-level, seed-to-voxel, rsFC model included each subject’s image-quality rating (IQR), derived from CAT12 structural-image processing, as a nuisance covariate. This indicator is an aggregate, weighted measure of image and preprocessing quality, including measures of resolution, noise and bias [38]. Controlling for IQR allows for the removal of variance related to scanner field homogeneity and any influences of structural image quality on the functional data normalization process. To account for potentially confounding scanner effects, the following series of analyses were performed. First, IQR was assessed via ANOVA and, as expected, shown to differ as a function of Scanner (F(1,59)=7.76, p = 0.007) but did not differ by Group (F(1,59)=1.59, p = 0.212) nor associated with rsFC (r=.062, p=.637). Next, an ANOVA was conducted to examine the effects of each scanner—irrespective of participant group—on rsFC and no significant effect of scanner was observed (F(1,58)=.176, p=0.676). Next, to rule out the potential confound associated with the control group being scanned only at one site (MUSC), a within opioid group only ANOVA was conducted comparing rsFC in the opioid group data collected at MUSC as compared to the opioid group collected from outside MUSC, revealing no significant differences in rsFC as a function of scanner F(1,30) = 1.01, p=0.323. Despite no observed significant effects of scanner on rsFC differences, IQR was included as a nuisance covariate to control for scanner variance in image quality. Overall, OUG subjects tended to be older, less educated, and exhibited lower total intracranial volume (TIV) than the control group but sexes were evenly matched between groups (Table 1). All subsequent between-subjects statistical models included age and education as covariates; additionally, models including brain findings (i.e., rsFC and VBM) included IQR as a nuisance covariate, and VBM models further included TIV [38].
Table 1.
Participant demographics and baseline self-report
| Measure | Opioid Group | Control Group | Test Statistic | p-value |
|---|---|---|---|---|
| N (%) | 31 (52%) | 30 (48%) | ||
| Female, N (%) | 15 (45%) | 16 (53%) | χ2 = 0.407 | 0.52 |
| Age, M ± SD | 51.5 ± 11.1 | 36.9 ± 11.9 | t = 4.9 | < 0.001** |
| Race, N (%) | ||||
| African American | 5 (16%) | 9 (30%) | ||
| White | 25 (81%) | 18 (60%) | ||
| Asian/Pacific Islander | - | 3 (10%) | ||
| Other | 1 (3%) | - | ||
| Years Education, M ± SD | 14.4 ± 2.2 | 15.7 ± 2.4 | t= 2.17 | 0.034* |
| PANAS: Positive M ± SD | 29.97 ± 8.8 | 31.8 ± 7.2 | t = .869 | 0.39 |
| PANAS: Negative M ± SD | 18.0 ± 8.2 | 11.2 ± 1.9 | t = 4.45 | < 0.001** |
| Opioid Craving (VAS scale: 1=low, 10 high) | 2.87 ± 3.47 | |||
| Total Intracranial Volume | 1448.95 (142.92) | 1538.98 (132.75) | t = 2.55 | 0.013* |
| Primary Pain Location, N (%) | ||||
| Low back | 12 (39%) | |||
| Back | 6 (19%) | |||
| Neck | 5 (16%) | |||
| Joint | 2 (6%) | |||
| Other | 6 (19%) | |||
| Pain Severity (BPI), M ± SD | 5.4 ± 1.81 | |||
| Pain Interferences (BPI), M ± SD | 5.95 ± 2.41 | |||
| Primary Opioid Type | ||||
| Oxycodone | 11 (35%) | |||
| Hydrocodone | 10 (32%) | |||
| Morphine | 3 (10%) | |||
| Other | 7 (23%) | |||
| Morphine Milligram Equivalent Daily Dose, M ± SD | 105.3 ± 163.9 | |||
| Opioid Misuse Risk (COMM), M ± SD | 14.1 ± 8.3 | |||
COMM = Current Opioid Misuse Measure; BPI = Brief Pain Inventory; PANAS = Positive and Negative Affect Schedule, M = Mean; ± SD = Standard Deviation
Resting-State Functional Connectivity.
Preprocessed resting-state fMRI data were uploaded into the CONN functional connectivity toolbox (http://www.nitrc.org/projects/conn) for denoising and connectivity analysis, along with unsmoothed, segmented tissue images and functionally-defined regions of interest (ROIs), i.e., bilateral nucleus accumbens (NAc) spheres (http://marsbar.sourceforge.net) (Supplemental Methods). Frontostriatal rsFC was assessed using a seed (NAc)-to-voxel (within a vmPFC search mask) approach separately for the right and left NAc seed ROIs, and then modeled voxel-wise using a nonparametric, HRF-weighted GLM. Results were thresholded using cluster-level inference at pvoxel < 0.001, pcluster < 0.05 FWE using a priori directional hypothesis t-test (Control > OUG). Fisher-transformed correlation coefficients (rZ values) were extracted from the significant target cluster and imported into SPSS for further analyses via ANCOVA, regression and exploratory path-analysis.
Voxel-Based Morphometry.
A priori L.NAc seed and significant target cluster from the rsFC analyses were used to derive absolute, weighted-mean GMV values from each significant region of interest (ROI), converted from proportion per voxel to µL per voxel by multiplying the volume of the voxel by the percent of gray matter per voxel to yield an absolute volumetric measure of gray matter within a voxel or as an average over a cluster in microliters [39].
Brain-Behavior and Post-hoc Analyses.
Planned a priori and additional unplanned post-hoc brain-behavior relationships were explored in SPSS v25 and PROCESS v2.16.3 (processmacro.org/) via ANCOVA, correlation, regression, and path analysis. All tests are two-tailed unless otherwise specified. Mediation analyses were performed to test the post-hoc hypothesis that the strength of frontostriatal rsFC mediates group differences in trait negative affect using bootstrapping (10,000) with bias-corrected and accelerated (BCa) 95% CI.
RESULTS
Behavioral Measures
The OUG reported significantly higher negative affect than the control group (F(1,57) = 14.79, p < 0.001, d = .98; OUG Mean (M) = 18.25 ±1.21, control group M = 10.98 ±1.24). No significant main effect of Group was observed for positive affect (F(1,57) = .482, p = 0.49). Among the OUG, self-reported negative affect was neither associated with craving (r = .184, p = .323) nor with pain severity (r=.056, p=.767).
Frontostriatal Resting-State Functional Connectivity
A main effect of Group (F(1,56) = 8.69, p = 0.005, d = .76) was observed for the left frontostriatal pathway—between the L.NAc and L.vmPFC (Figure 2, Table 2), revealing significantly weaker rsFC among the OUG (M = .009 ±.03) as compared to the control group (M= .137 ±.03). Connectivity within the OUG group was not significantly different from zero (t(30) = .282, p = 0.78). No significant effects were observed for the R.NAc-vmPFC pathway.
Figure 2. Group Differences in Frontostriatal rsFC.
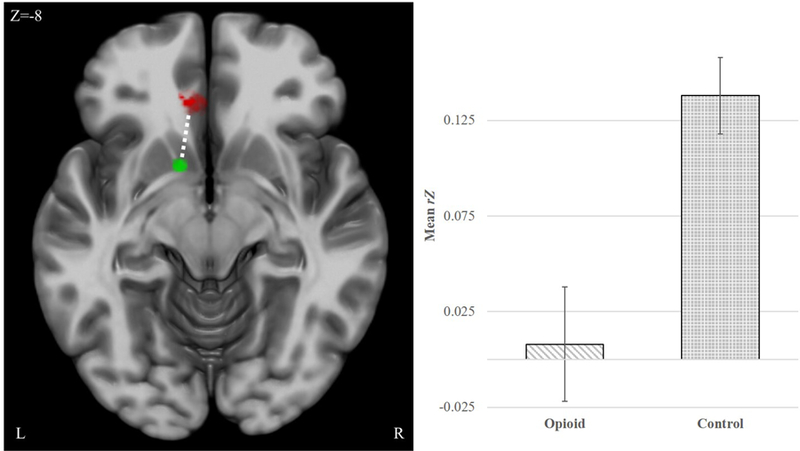
The opioid use group (OUG), relative to control group, evidenced weaker resting-state functional connectivity (rsFC) between the L.Nucleus Accumbens [NAc] (SEED; green sphere) and the L.ventromedial prefrontal cortex [vmPFC] (TARGET; red cluster). Error bars = +/− 1 SE of the mean. Note that the OUG connectivity within this pathway is not significantly different from zero.
Table 2.
Main effect of Group on frontostriatal resting-state functional connectivity
| Seed1 | Target(s)2 | MNI (x,y,z) | SPM t-threshold | Cluster size mm3 | Control 3 | Opioid 3 | F(1,56)4 | p-value | d 5 |
|---|---|---|---|---|---|---|---|---|---|
| L.NAcc | L.vmPFC | −12 32 −12 | pvoxel <.001, pcluster < .05 FWE | 870.75 | .14 (.03) | .01 (.03) | 8.69 | 0.005 | 0.76 |
| R.NAcc | none | ||||||||
Note. Standard error reported in parentheses next to mean where applicable.
Nucleus Accumbens
Ventromedial Prefrontal Cortex
group mean (SE) cluster rZ value
covariates included: age, education, image quality rating (IQR)
cohen’s d calculated based on Control and Opioid group marginal means
Association between frontostriatal rsFC and behavior measures.
Self-reported affect.
Strength of frontostriatal rsFC as associated with lower self-reported negative affect (t (56) = −2.9, β = −16.71, p = 0.005). No significant associations were found between frontostriatal rsFC and positive affect (t (56) = .98, p = 0.33).
Opioid Misuse and Craving.
Frontostriatal rsFC was not associated with COMM scores (t (28) = .246, p = 0.81); opioid dose (MME) (t (28) = −.166, p = 0.88); nor with craving (r=.150, p=.421).
Pain.
Frontostriatal rsFC was not associated with BPI pain severity (t (28) = .454, p = 0.65) nor pain interference (t (28) = −.172, p = 0.87).
Frontostriatal Morphology
The opioid user group showed significantly less average GMV (µL / voxel) than the control group across both rsFC ROIs: L.NAc (F(1,55) = 10.89, p = 0.002, d = .85; OUG M = 1.77 ±0.04, Control = 1.96 ±.04); and, L.vmPFC (F(1,55) = 140.70, p < 0.001, d = 3.04; OUG M = 1.4 ±0.03, Control = 1.8 ±.03).
Association between frontostriatal morphology and behavior measures.
Self-reported affect.
L.vmPFC volume was negatively associated with self-reported negative affect (t (55) = −2.22, β = −25.38, p = 0.03) while L.NAc volume was not associated with negative affect (t (55) = −.559, p = 0.58). Neither L.vmPFC (t(55) = .75, p = .45) nor L.NAc (t(55) = .74, p = 0.46) were independently associated with positive affect.
Opioid Misuse.
L.NAc volume was positively associated with greater opioid misuse severity (COMM scores) (t (27) = 2.08, β = 56.77, p = 0.047). vmPFC volume was not associated with COMM scores (t (27) = .945, p = 0.353). Neither L.NAc (t (27) = .232, p = 0.82) nor L.vmPFC (t (27) = −.69, p = 0.5) were associated with opioid dose.
Pain.
L.NAc volume was positively associated with both BPI pain severity (t (27) = 2.24, β = 12.69, p = 0.034) and BPI pain interference (t (27) = 2.49, β = 18.84, p = 0.019). L.vmPFC volume was positively associated with BPI pain interference (t (27) = 2.3, β = 21.03, p = 0.031), but not with BPI pain severity (t (27) = .59, p = 0.56).
Post hoc Analyses.
Morphology and Connectonomic Relationships.
Across all subjects, L.vmPFC volume was positively associated with frontostriatal rsFC (t (55) = 2.81, β = .68, p = 0.007), while L.NAc volume was not (t (55) = 1.12, p = 0.266).
Mediation.
Path-analysis revealed a partial mediation effect whereby strength of frontostriatal rsFC (L.NAc and L.vmPFC: β = 1.26, SE = .92, CI [.04 – 3.92]) mediated the association between opioid use and heightened negative affect (Figure 3). Connectivity explained 19.4% of the group differences in negative affect (ratio of indirect/total effect).
Figure 3. Frontostriatal mediation of groups differences in negative affect.
The opioid-user group (OUG) reported greater negative affect than the healthy control group (F(1,57) = 14.79, p < .001, d = .98; OUG M = 18.25 ±1.21, Control = 10.98 ±1.24). The greater reported negative affect by the OUG was partially mediated by weaker frontostriatal resting-state functional connectivity (rsFC) between the left nucleus accumbens (NAc) and the left ventromedial prefrontal cortex (vmPFC) (β = 1.26, SE = .92, CI [.04 – 3.92]). Level of confidence intervals was 95% and error bars represent ± standard error (SE).
DISCUSSION.
In the current study, we examined associations between prescription opioid use in the context of chronic pain and functional/structural abnormalities in frontostriatal circuitry through a combination of fMRI resting-state functional connectivity and MRI voxel-based morphometry. Results of this study demonstrate that prescription opioid use among chronic pain patients is associated with both deficits in frontostriatal connectivity and reduced frontostriatal GMV. Moreover, exploratory analyses revealed that reduced frontostriatal rsFC mediated the association between chronic opioid use and negative affect, whereas frontostriatal morphology was associated with opioid misuse and pain severity.
Opioid users evidenced weaker frontostriatal rsFC.
Repeated exposure to drugs of abuse produces glutamatergic neuroplasticity in frontostriatal circuitry which serves to bias reward processing towards drug consumption and away from the pursuit of natural rewards [40–43]. Resting-state studies have also implicated the frontostriatal vmPFC → NAc pathway in impulsivity [44, 45], compulsivity [44, 46], adaptive decision making [47–49] and the regulation of hedonic tone [50–53] and pain [54]. Weaker frontostriatal resting-state functional connectivity has previously been shown among individuals with tobacco [22, 36], opioid [27, 55], cocaine [56] and polysubstance [23] use disorder- but other studies have reported evidence of hyperconnectivity [57] in the context of cocaine [44] and opioid use [24–26, 58–61], some of which were confounded by abstinence [24, 25, 60, 61] or ongoing methadone treatment [25, 26, 58, 59]. In the present study, which controlled for treatment-related confounds (i.e. abstinence, medication assisted treatment of OUD), frontostriatal rsFC partially mediated the association between opioid use in the context of chronic pain and elevated negative affect, suggesting that frontostriatal dysconnectivity may undergird disruptions in hedonic tone and emotion regulation evident in opioid (mis)using chronic pain patients [62].
Frontostriatal rsFC partially mediated opioid users’ increased negative affect.
Negative emotionality is included as one of the core domains in the newly developed Addictions Neuroclinical Assessment [41] and is considered as one of the primary drivers of relapse during withdrawal [40]. Negative affect is traditionally considered to be mediated through frontolimbic pathways (i.e., anterior cingulate cortex/vmPFC → amygdala) with frontostriatal circuitry involved in ventral-striatal inhibition; yet, previous fMRI investigations into associations between frontostriatal activation, connectivity and affect in addiction have primarily focused on appetitive processing [63] and revealed associations with positive affect (and the lack thereof, i.e., anhedonia or hypo-hedonia) [36, 40, 64–67]. Here, the opioid user group reported elevated negative affect relative to the control group. Post-hoc analyses revealed that the increased negative affect observed in opioid users was partially mediated by the strength of frontostriatal connectivity. These findings are consistent with the literature that highlights vmPFC as a central node in negative affect regulation [49, 68–72] and may inform the ongoing scientific debate regarding the role of vmPFC in the acquisition, maintenance and extinction of opioid vs. psychostimulant (e.g., nicotine, cocaine) drug-seeking behaviors [73].
Dysregulated hedonic tone (i.e. anhedonia), a major feature of mood and addiction disorders [74], has been linked with morphological and functional changes in the NAc, such as volumetric reductions [71], blunted responsivity to natural rewards [71] and abnormal connectonomic profiles [53]; but, there has been an ongoing debate as to whether or not anhedonia results from a reduced capacity for experiencing pleasure [71] or rather reflects an underlying neurobiological deficit in sustaining positive affect over time [51]. Blunted responsivity to natural rewards has been observed among prescription opioid misusers with chronic pain [3, 75]. However, heightened negative affect has also been observed in this population, with opioid craving mediating the association between negative affect and opioid misuse [76]. Relationships between positive and negative affect, anhedonia and pain are tightly intertwined, making for a complex phenotype in prescription opioid using chronic pain patients [77].
Opioid users exhibited less frontostriatal gray matter volume.
In line with prior findings, less vmPFC [30] and NAc [78, 79] GMV was observed in opioid using chronic pain patients. The vmPFC is not a discrete anatomical structure but rather heterogeneous in terms of cytoarchitecture, function and structural connectivity [49]. Domains most frequently associated with vmPFC include: (a) reward-processing and value-based decision making (via frontostriatal circuitry) (b) negative affect generation and regulation (via amygdalofrontal circuitry) and (c) placebo- and expectancy-manipulated pain alleviation (amygdala-periaqueductal grey [PAG], anterior cingulate cortex [ACC])[49]. Negative affect generation is primarily mediated through vmPFC projections to the amygdala [49] although complementary inhibition within frontostriatal circuitry is also thought to be involved. In primates, lesions to vmPFC gray matter, while sparing white matter, were shown to increase negative affective responses to aversive cues [80]. Clinical research with lesion patients has revealed further evidence of vmPFC-mediated frontostriatal dysfunction whereby vmPFC lesions were shown to correlate positively with attenuated striatal response during reward anticipation and inversely with NAc GMV [47]. In addition, opioid use—in the form of codeine-containing cough syrup—has been shown to be associated with less vmPFC GMV and aberrant connectivity with the default mode network that were associated with higher impulsivity. The current study findings, when placed in context of the broader literature, suggest that compromised frontostriatal morphology among prescription opioid users with chronic pain may serve as a neuroendophenotype of opioid dependence in this population and also as a marker of dysregulated negative affective processing and behavior control over drug use.
Nucleus accumbens morphology predicts opioid misuse and pain.
Dissociating effects of opioid use from chronic pain has proven challenging because neural circuits for pain regulation overlap those involved in drug addiction – including the frontostriatal pathway [81–83]. The present study was not designed to disentangle these phenomena, but instead aimed to examine the neural correlates of the comorbidity of chronic pain and prolonged opioid use. Findings from the current study revealed that opioid using pain patients, as compared to the control group, exhibited less GMV in bilateral nucleus accumbens and vmPFC. This is consistent with prior literature that reported chronic pain plus opioid treatment, compared to chronic pain alone, was associated with atrophy in the vmPFC [84]; and findings [85] that opioid users exhibit less L.NAc volume than controls but within the opioid group, increased individual NAc volume predicted greater pain severity, interference, and opioid misuse. The findings form Jahari et al, [84] are consistent with the relationship between L.NAc volume, pain, and opioid misuse observed in the present study. Thus, striatal hypertrophy may index escalating appetitive reactivity to opioids, compensatory pain regulatory processes in the attempt to maintain hedonic homeostasis, and/or an Opioid X Pain interaction. Initial prescription opioid use targets pain alleviation but chronic use has not been proven effective in long-term, non-cancer pain management [86, 87] and may actually potentiate hyperalgesia, particularly in patients prone to misuse [88].
In conclusion, long-term prescription opioid use among chronic pain patients is associated with aberrant frontostriatal circuit morphology and function. Importantly, frontostriatal rsFC partially mediated group differences in negative affect, thus providing a potential neural biomarker for evaluating the efficacy of pharmaco-behavioral treatments aimed at ameliorating negative affective precipitants of prescription opioid misuse and OUD. While the specific molecular pathways affected during the entrenchment of addiction differ substantially between drugs of abuse, these results, in accordance with current scientific thinking, support the tenet that allostatic neuroplasticity in glutamatergic, ventromedial prefrontal cortex-mediated signaling pathways represents a common pathway that drives addiction maintenance [89]. The primary findings of this study are largely consistent with our hypotheses and the broader addiction literature suggesting that frontostriatal resting-state functional connectivity deficits may be a transdiagnostic biomarker of addiction and/or addiction vulnerability. Despite numerous design strengths, such as multimodal neuroimaging, a matched control group, and a homogenous, actively-using opioid-user group -- this study was not without limitations, including: the lack of a chronic pain group not receiving opioid therapy and data acquisition across different scanner sites influenced image quality; however, analysis of site did not reveal significant differences in neural outcomes and, in terms of ecological validity, we find this to be a strength.
These limitations notwithstanding, findings from the current study support the hypothesis that prolonged prescription opioid use in the context of chronic pain is marked by deficits in frontostriatal morphology and resting-state functional connectivity, similar to chronic use of other drugs with significant abuse liability. These findings will help guide further research into frontostriatal-targeted pharmacotherapies producing glutamate homeostasis (e.g., N-acetylcysteine[90]) and/or behavioral interventions for substance use disorders [91, 92]) that may modulate frontostriatal circuitry function [93]. For example, in a double-blind, placebo (PBO)-controlled N-acetylcysteine (NAC) study in smoking-abstinent, nicotine dependent smokers, NAC was associated with stronger frontostriatal rsFC, less craving and affective disturbances, and remaining smoking abstinent over the medication period [36]. If indeed corticostriatal rsFC is a transdiagnostic indicator of substance use disorder pathophysiology, then research on pharmacotherapies for treating glutamatergic corticostriatal circuitry in OUD is warranted. Further, studies of Mindfulness-oriented recovery enhancement (MORE)—a manualized behavioral treatment for opioid misuse and OUD in chronic pain patients, has been shown to decrease opioid attentional bias [94] and opioid misuse related deficits in autonomic response (i.e., heart-rate variability, HRV) [75], a proxy of frontostriatal function [95], were demonstrably remediated following MORE treatment [96]. Finally, future research is needed to evaluate more spatially-distributed, systems-level neurodynamics beyond the a priori hypothesized frontostriatal pathway in the current study among samples of opioid using chronic pain patients, as well as to systematically evaluate whether modulating frontostriatal circuitry translates to improved treatment outcomes.
Supplementary Material
Acknowledgments
Funding/Support: This research was supported by NIDA grants R01DA033459 (Dr. Froeliger), R01DA042033 (Dr. Garland) and NCCIH grant R61AT009296 (Drs. Garland and Zubieta); in part by pilot research funding, Hollings Cancer Center’s Cancer Center Support Grant P30 CA138313 at the Medical University of South Carolina; data analysis and manuscript preparation by R01DA033459 and R01DA038700 (Dr. Froeliger)
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication
Footnotes
Conflict of Interest Disclosures: Consultant: Promentis Pharmaceuticals, Inc. (BF) and Alkermes, Inc. (JKZ), for work unrelated to the content of the manuscript. The remaining authors have nothing to disclose.
REFERENCES
- 1.CDC/NCHS, National Vital Statistics System, Mortality, Wonder C, Editor. 2017, US Department of Health and Human Services: Atlanta, GA. [Google Scholar]
- 2.Volkow ND, Koob GF, and McLellan AT, Neurobiologic advances from the brain disease model of addiction. New England Journal of Medicine, 2016. 374(4): p. 363–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garland EL, et al. , Deficits in autonomic indices of emotion regulation and reward processing associated with prescription opioid use and misuse. Psychopharmacology (Berl), 2017. 234(4): p. 621–629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Garland EL, et al. , The downward spiral of chronic pain, prescription opioid misuse, and addiction: cognitive, affective, and neuropsychopharmacologic pathways. Neurosci Biobehav Rev, 2013. 37(10 Pt 2): p. 2597–607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hickman M, et al. , The impact of buprenorphine and methadone on mortality: a primary care cohort study in the United Kingdom. Addiction, 2018. [DOI] [PMC free article] [PubMed]
- 6.Bentzley BS, et al. , Discontinuation of buprenorphine maintenance therapy: perspectives and outcomes. J Subst Abuse Treat, 2015. 52: p. 48–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kalivas PW, The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci, 2009. 10(8): p. 561–72. [DOI] [PubMed] [Google Scholar]
- 8.Neuhofer D and Kalivas P, Metaplasticity at the addicted tetrapartite synapse: A common denominator of drug induced adaptations and potential treatment target for addiction. Neurobiology of learning and memory, 2018. [DOI] [PMC free article] [PubMed]
- 9.Abraham A, The world according to me: personal relevance and the medial prefrontal cortex. Front Hum Neurosci, 2013. 7: p. 341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Knutson B and Gibbs SE, Linking nucleus accumbens dopamine and blood oxygenation. Psychopharmacology (Berl), 2007. 191(3): p. 813–22. [DOI] [PubMed] [Google Scholar]
- 11.Shen HW, et al. , Synaptic glutamate spillover due to impaired glutamate uptake mediates heroin relapse. J Neurosci, 2014. 34(16): p. 5649–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gipson CD, et al. , Reinstatement of nicotine seeking is mediated by glutamatergic plasticity. Proc Natl Acad Sci U S A, 2013. 110(22): p. 9124–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Knackstedt LA, et al. , The role of cystine-glutamate exchange in nicotine dependence in rats and humans. Biol Psychiatry, 2009. 65(10): p. 841–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Baker DA, et al. , Neuroadaptations in cystine-glutamate exchange underlie cocaine relapse. Nat Neurosci, 2003. 6(7): p. 743–9. [DOI] [PubMed] [Google Scholar]
- 15.Baker DA, et al. , N-acetyl cysteine-induced blockade of cocaine-induced reinstatement. Annals of the New York Academy of Sciences, 2003. 1003: p. 349–51. [DOI] [PubMed] [Google Scholar]
- 16.Pierce RC, et al. , Repeated cocaine augments excitatory amino acid transmission in the nucleus accumbens only in rats having developed behavioral sensitization. The Journal of neuroscience : the official journal of the Society for Neuroscience, 1996. 16(4): p. 1550–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Berglind WJ, et al. , A single intra-PFC infusion of BDNF prevents cocaine-induced alterations in extracellular glutamate within the nucleus accumbens. The Journal of neuroscience : the official journal of the Society for Neuroscience, 2009. 29(12): p. 3715–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.LaLumiere RT and Kalivas PW, Glutamate release in the nucleus accumbens core is necessary for heroin seeking. J Neurosci, 2008. 28(12): p. 3170–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shmuel A and Leopold DA, Neuronal correlates of spontaneous fluctuations in fMRI signals in monkey visual cortex: Implications for functional connectivity at rest. Hum Brain Mapp, 2008. 29(7): p. 751–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lu H and Stein EA, Resting state functional connectivity: Its physiological basis and application in neuropharmacology. Neuropharmacology, 2013. 84: p. 79–89. [DOI] [PubMed] [Google Scholar]
- 21.Wilcox CE, Abbott CC, and Calhoun VD, Alterations in resting-state functional connectivity in substance use disorders and treatment implications. Prog Neuropsychopharmacol Biol Psychiatry, 2018. [DOI] [PMC free article] [PubMed]
- 22.Hong LE, et al. , Association of nicotine addiction and nicotine’s actions with separate cingulate cortex functional circuits. Arch Gen Psychiatry, 2009. 66(4): p. 431–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Motzkin JC, et al. , Neural correlates of substance abuse: Reduced functional connectivity between areas underlying reward and cognitive control. Hum Brain Mapp, 2014. 35(9): p. 4282–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhai T, et al. , Nature of functional links in valuation networks differentiates impulsive behaviors between abstinent heroin-dependent subjects and nondrug-using subjects. Neuroimage, 2015. 115: p. 76–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma N, et al. , Addiction related alteration in resting-state brain connectivity. NeuroImage, 2010. 49(1): p. 738–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang PW, et al. , Abnormal interhemispheric resting state functional connectivity of the insula in heroin users under methadone maintenance treatment. Psychiatry Res Neuroimaging, 2016. 255: p. 9–14. [DOI] [PubMed] [Google Scholar]
- 27.Upadhyay J, et al. , Alterations in brain structure and functional connectivity in prescription opioid-dependent patients. Brain, 2010. 133(Pt 7): p. 2098–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kivisaari R, et al. , Cerebral measurements and their correlation with the onset age and the duration of opioid abuse. J Opioid Manag, 2010. 6(6): p. 423–9. [DOI] [PubMed] [Google Scholar]
- 29.Younger JW, et al. , Prescription opioid analgesics rapidly change the human brain. Pain, 2011. 152(8): p. 1803–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qiu YW, et al. , Reduced ventral medial prefrontal cortex (vmPFC) volume and impaired vmPFC-default mode network integration in codeine-containing cough syrups users. Drug Alcohol Depend, 2014. 134: p. 314–321. [DOI] [PubMed] [Google Scholar]
- 31.Turk DC and Rudy TE, IASP taxonomy of chronic pain syndromes: preliminary assessment of reliability. Pain, 1987. 30(2): p. 177–89. [DOI] [PubMed] [Google Scholar]
- 32.Watson D, Clark LA, and Tellegen A, Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol, 1988. 54(6): p. 1063–70. [DOI] [PubMed] [Google Scholar]
- 33.Services USD o.H.a.H. and C.f.D.C.a. Prevention, CALCULATING TOTAL DAILY DOSE OF OPIOIDS FOR SAFER DOSAGE
- 34.Cleeland CS and Ryan KM, Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore, 1994. 23(2): p. 129–38. [PubMed] [Google Scholar]
- 35.Butler SF, et al. , Development and validation of the Current Opioid Misuse Measure. Pain, 2007. 130(1–2): p. 144–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Froeliger B, et al. , The effects of N-Acetylcysteine on frontostriatal resting-state functional connectivity, withdrawal symptoms and smoking abstinence: A double-blind, placebo-controlled fMRI pilot study. Drug & Alcohol Dependence, 2015. 156: p. 234–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zink CF, et al. , Human striatal response to salient nonrewarding stimuli. J Neurosci, 2003. 23(22): p. 8092–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gaser C and Kurth F, Manual Computational Anatomy Toolbox-CAT12. Structural Brain Mapping Group at the Departments of Psychiatry and Neurology, University of Jena, 2017. [Google Scholar]
- 39.Radua J, et al. , Validity of modulation and optimal settings for advanced voxel-based morphometry. Neuroimage, 2014. 86: p. 81–90. [DOI] [PubMed] [Google Scholar]
- 40.Koob GF, The Dark Side of Addiction: The Horsley Gantt to Joseph Brady Connection. The Journal of nervous and mental disease, 2017. 205(4): p. 270–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kwako LE, Bickel WK, and Goldman D, Addiction Biomarkers: Dimensional Approaches to Understanding Addiction. Trends in molecular medicine, 2018. 24(2): p. 121–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chen SA, et al. , Unlimited access to heroin self-administration: independent motivational markers of opiate dependence. Neuropsychopharmacology, 2006. 31(12): p. 2692. [DOI] [PubMed] [Google Scholar]
- 43.Lubman D, et al. , Electrophysiological evidence that drug cues have greater salience than other affective stimuli in opiate addiction. Journal of psychopharmacology, 2008. 22(8): p. 836–842. [DOI] [PubMed] [Google Scholar]
- 44.Hu Y, et al. , Impaired functional connectivity within and between frontostriatal circuits and its association with compulsive drug use and trait impulsivity in cocaine addiction. JAMA psychiatry, 2015. 72(6): p. 584–592. [DOI] [PubMed] [Google Scholar]
- 45.Qiu Y, et al. , Reduced regional homogeneity in bilateral frontostriatal system relates to higher impulsivity behavior in codeine-containing cough syrups dependent individuals. PLoS One, 2013. 8(11): p. e78738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meunier D, et al. , Brain functional connectivity in stimulant drug dependence and obsessive–compulsive disorder. Neuroimage, 2012. 59(2): p. 1461–1468. [DOI] [PubMed] [Google Scholar]
- 47.Pujara MS, et al. , Ventromedial prefrontal cortex damage is associated with decreased ventral striatum volume and response to reward. Journal of Neuroscience, 2016. 36(18): p. 5047–5054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pujara M and Koenigs M, Mechanisms of reward circuit dysfunction in psychiatric illness: prefrontal–striatal interactions. The Neuroscientist, 2014. 20(1): p. 82–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hiser J and Koenigs M, The multifaceted role of ventromedial prefrontal cortex in emotion, decision-making, social cognition, and psychopathology. Biological psychiatry, 2017. 83(8): p. 638–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Heller AS, et al. , Relationships between changes in sustained fronto-striatal connectivity and positive affect in major depression resulting from antidepressant treatment. American Journal of Psychiatry, 2013. 170(2): p. 197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Heller AS, et al. , Reduced capacity to sustain positive emotion in major depression reflects diminished maintenance of fronto-striatal brain activation. Proceedings of the National Academy of Sciences, 2009. 106(52): p. 22445–22450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Admon R and Pizzagalli DA, Corticostriatal pathways contribute to the natural time course of positive mood. Nature communications, 2015. 6: p. 10065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Drysdale AT, et al. , Resting-state connectivity biomarkers define neurophysiological subtypes of depression. Nature medicine, 2017. 23(1): p. 28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wiech K, Deconstructing the sensation of pain: The influence of cognitive processes on pain perception. Science, 2016. 354(6312): p. 584–587. [DOI] [PubMed] [Google Scholar]
- 55.Wang W, et al. , Changes in functional connectivity of ventral anterior cingulate cortex in heroin abusers. Chinese medical journal, 2010. 123(12): p. 1582–1588. [PubMed] [Google Scholar]
- 56.Zhang S and Li C-SR, Ventral striatal dysfunction in cocaine dependence–difference mapping for subregional resting state functional connectivity. Translational psychiatry, 2018. 8(1): p. 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sutherland MT, et al. , Resting state functional connectivity in addiction: lessons learned and a road ahead. Neuroimage, 2012. 62(4): p. 2281–2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang Y, et al. , Granger causality reveals a dominant role of memory circuit in chronic opioid dependence. Addiction biology, 2017. 22(4): p. 1068–1080. [DOI] [PubMed] [Google Scholar]
- 59.Fareed A, et al. , Effect of heroin use on changes of brain functions as measured by functional magnetic resonance imaging, a systematic review. Journal of addictive diseases, 2017. 36(2): p. 105–116. [DOI] [PubMed] [Google Scholar]
- 60.Liu J, et al. , Dysfunctional connectivity patterns in chronic heroin users: an fMRI study. Neuroscience letters, 2009. 460(1): p. 72–77. [DOI] [PubMed] [Google Scholar]
- 61.Xie C, et al. , Imbalanced functional link between valuation networks in abstinent heroin-dependent subjects. Molecular psychiatry, 2014. 19(1): p. 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Garland EL, et al. , Emotion dysregulation in addiction, in Oxford Handbook of Emotion Dysregulation, Beauchaine SCT, Editor. 2017, Oscfor University: New York. [Google Scholar]
- 63.Limbrick-Oldfield EH, van Holst RJ, and Clark L, Fronto-striatal dysregulation in drug addiction and pathological gambling: consistent inconsistencies? NeuroImage: Clinical, 2013. 2: p. 385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Koob GF and Le Moal M, Drug addiction, dysregulation of reward, and allostasis. Neuropsychopharmacology, 2001. 24(2): p. 97. [DOI] [PubMed] [Google Scholar]
- 65.Koob GF and Le Moal M, Neurobiological mechanisms for opponent motivational processes in addiction. Philosophical Transactions of the Royal Society of London B: Biological Sciences, 2008. 363(1507): p. 3113–3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Volkow N, et al. , Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature, 1997. 386(6627): p. 830. [DOI] [PubMed] [Google Scholar]
- 67.Crits-Christoph P, et al. , Symptoms of anhedonia, not depression, predict the outcome of treatment of cocaine dependence. Journal of Substance Abuse Treatment, 2018. [DOI] [PMC free article] [PubMed]
- 68.Roy M, Shohamy D, and Wager TD, Ventromedial prefrontal-subcortical systems and the generation of affective meaning. Trends in cognitive sciences, 2012. 16(3): p. 147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zald DH, Mattson DL, and Pardo JV, Brain activity in ventromedial prefrontal cortex correlates with individual differences in negative affect. Proceedings of the National Academy of Sciences, 2002. 99(4): p. 2450–2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Urry HL, et al. , Amygdala and ventromedial prefrontal cortex are inversely coupled during regulation of negative affect and predict the diurnal pattern of cortisol secretion among older adults. Journal of Neuroscience, 2006. 26(16): p. 4415–4425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wacker J, Dillon DG, and Pizzagalli DA, The role of the nucleus accumbens and rostral anterior cingulate cortex in anhedonia: integration of resting EEG, fMRI, and volumetric techniques. Neuroimage, 2009. 46(1): p. 327–337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Shackman AJ, et al. , The integration of negative affect, pain and cognitive control in the cingulate cortex. Nature Reviews Neuroscience, 2011. 12(3): p. 154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Peters J, Pattij T, and De Vries TJ, Targeting cocaine versus heroin memories: divergent roles within ventromedial prefrontal cortex. Trends in pharmacological sciences, 2013. 34(12): p. 689–695. [DOI] [PubMed] [Google Scholar]
- 74.Der-Avakian A and Pizzagalli DA, Translational Assessments of Reward and Anhedonia. Biological psychiatry, 2018. 83(11): p. 932–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Garland EL, Froeliger B, and Howard MO, Allostatic dysregulation of natural reward processing in prescription opioid misuse: autonomic and attentional evidence. Biol Psychol, 2015. 105: p. 124–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Martel MO, et al. , The association between negative affect and prescription opioid misuse in patients with chronic pain: the mediating role of opioid craving. J Pain, 2014. 15(1): p. 90–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Finan PH and Garland EL, The role of positive affect in pain and its treatment. The Clinical journal of pain, 2015. 31(2): p. 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Seifert CL, et al. , Reduced volume of the nucleus accumbens in heroin addiction. European archives of psychiatry and clinical neuroscience, 2015. 265(8): p. 637–645. [DOI] [PubMed] [Google Scholar]
- 79.Schuch-Goi SB, et al. , Accumbens volumes are reduced among crack-cocaine users. Neuroscience letters, 2017. 645: p. 86–89. [DOI] [PubMed] [Google Scholar]
- 80.Shiba Y, et al. , Lesions of either anterior orbitofrontal cortex or ventrolateral prefrontal cortex in marmoset monkeys heighten innate fear and attenuate active coping behaviors to predator threat. Frontiers in systems neuroscience, 2015. 8: p. 250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Becker S, Gandhi W, and Schweinhardt P, Cerebral interactions of pain and reward and their relevance for chronic pain. Neuroscience letters, 2012. 520(2): p. 182–187. [DOI] [PubMed] [Google Scholar]
- 82.Elman I and Borsook D, Common brain mechanisms of chronic pain and addiction. Neuron, 2016. 89(1): p. 11–36. [DOI] [PubMed] [Google Scholar]
- 83.Taylor AM, Corticolimbic circuitry in the modulation of chronic pain and substance abuse. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 2017. [DOI] [PMC free article] [PubMed]
- 84.Qiu Y.w., et al. , Intrinsic brain network abnormalities in codeine‐containing cough syrup‐dependent male individuals revealed in resting‐state fMRI. Journal of Magnetic Resonance Imaging, 2017. 45(1): p. 177–186. [DOI] [PubMed] [Google Scholar]
- 85.Jarrahi B, Johnson K, and Mackey S, Effect of opioids on brain morphometrics in patients with chronic low back pain: a pilot MRI study. The Journal of Pain, 2018. 19(3): p. S7. [Google Scholar]
- 86.Grider JS and Ackerman WE, Opioid-induced hyperalgesia and tolerance: understanding opioid side effects. Expert review of clinical pharmacology, 2008. 1(2): p. 291–297. [DOI] [PubMed] [Google Scholar]
- 87.Davis MP, Opioid tolerance and hyperalgesia: basic mechanisms and management in review. Progress in Palliative Care, 2011. 19(2): p. 73–86. [Google Scholar]
- 88.Edwards RR, et al. , Elevated pain sensitivity in chronic pain patients at risk for opioid misuse. The Journal of Pain, 2011. 12(9): p. 953–963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hearing M, et al. , Opioid and Psychostimulant Plasticity: Targeting Overlap in Nucleus Accumbens Glutamate Signaling. Trends in pharmacological sciences, 2018. 39(3): p. 276–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Tomko RL, et al. , N-acetylcysteine: A potential treatment for substance use disorders. Current psychiatry, 2018. 17(6): p. 30–36. [PMC free article] [PubMed] [Google Scholar]
- 91.McConnell PA and Froeliger B, Mindfulness, Mechanisms and Meaning: Perspectives from the Cognitive Neuroscience of Addiction. Psychol Inq, 2015. 26(4): p. 349–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Garland EL, Restructuring reward processing with Mindfulness‐Oriented Recovery Enhancement: novel therapeutic mechanisms to remediate hedonic dysregulation in addiction, stress, and pain. Annals of the New York Academy of Sciences, 2016. 1373(1): p. 25–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Froeliger B, et al. , Restructuring Reward Mechanisms in Nicotine Addiction: A Pilot fMRI Study of Mindfulness-Oriented Recovery Enhancement for Cigarette Smokers. Evid Based Complement Alternat Med, 2017. 2017: p. 7018014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Garland EL, Baker AK, and Howard MO, Mindfulness-Oriented Recovery Enhancement Reduces Opioid Attentional Bias Among Prescription Opioid-Treated Chronic Pain Patients. Journal of the Society for Social Work and Research, 2017. 8(4): p. 493–509. [Google Scholar]
- 95.Thayer JF, et al. , A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neurosci Biobehav Rev, 2012. 36(2): p. 747–56. [DOI] [PubMed] [Google Scholar]
- 96.Garland EL, Froeliger B, and Howard MO, Effects of Mindfulness-Oriented Recovery Enhancement on reward responsiveness and opioid cue-reactivity. Psychopharmacology (Berl), 2014. 231(16): p. 3229–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.