Abstract
Hippocampal lesions are a defining pathology of Alzheimer’s disease (AD). However, the molecular mechanisms that underlie hippocampal synaptic injury in AD have not been fully elucidated. Current therapeutic efforts for AD treatment are not effective in correcting hippocampal synaptic deficits. Growth hormone secretagogue receptor 1α (GHSR1α) is critical for hippocampal synaptic physiology. Here, we report that GHSR1α interaction with β-amyloid (Aβ) suppresses GHSR1α activation, leading to compromised GHSR1α regulation of dopamine receptor D1 (DRD1) in the hippocampus from patients with AD. The simultaneous application of the selective GHSR1α agonist MK0677 with the selective DRD1 agonist SKF81297 rescued Ghsr1α function from Aβ inhibition, mitigating hippocampal synaptic injury and improving spatial memory in an AD mouse model. Our data reveal a mechanism of hippocampal vulnerability in AD and suggest that a combined activation of GHSR1α and DRD1 may be a promising approach for treating AD.
INTRODUCTION
Hippocampal lesions are an early and defining pathology of Alzheimer’s disease (AD) and underlie decline in cognitive ability (1). Currently, no effective therapy exists to correct these hippocampal synaptic deficits (2), and the precise mechanisms of hippocampal vulnerability in this neurodegenerative disorder are not completely understood. Growth hormone secretagogue receptor 1α (GHSR1α), also known as ghrelin receptor, is a member of the class A G protein–coupled receptor (GPCR) family. Aside from its abundance in the pituitary gland and hypothalamus, GHSR1α is expressed in the hippocampus, both in the dentate gyrus and Ammon’s horn (3-5), indicating its relevance to hippocampal function. Several studies have demonstrated specific roles for hippocampal ghrelin/GHSR1α signaling in learning, motivational, and hedonic components of eating (6, 7). Emerging evidence suggests a role for GHSR1α signaling in hippocampal synaptic physiology through regulation of dopamine receptor D1 (DRD1) (8-12). The modulation of DRD1 signaling by GHSR1α is critical for initiating hippocampal synaptic reorganization via the noncanonical Gαq-Ca2+ signaling pathway that results in activation of Ca2+/calmodulin-dependent protein kinase II (CaMKII) (8, 9). This pivotal role of GHSR1α in hippocampal synaptic function raises the question of whether GHSR1α dysfunction contributes to hippocampal synaptic deficits in AD.
Previous studies revealed inconsistent effects of GHSR1α activation on AD phenotypes in patients (10) and in AD animal and cell models (11). Our own recent study in patients with mild cognitive impairment (MCI) showed a negative correlation between cognitive performance and circulating acylated ghrelin (12). These results suggest that, in AD, hippocampal GHSR1α may become insensitive to activation by exogenous and endogenous ligands. Thus, understanding the functional status of hippocampal GHSR1α in AD-related conditions might provide insights into the molecular mechanisms of AD hippocampal pathology.
Here, we report increased GHSR1α expression and a direct interaction of GHSR1α with β-amyloid (Aβ) in the hippocampus of patients with AD and in a mouse model that mimics AD brain amyloidopathy with hippocampal synaptic injury (5×FAD mice). GHSR1α interaction with Aβ inhibited its activation and prevented GHSR1α/DRD1 heterodimerization. Loss of Ghsr1α in mice replicated hippocampal synaptic stress and cognitive impairment seen in 5×FAD mice. Furthermore, our results showed that the combined activation of Ghsr1α and Drd1 with their selective agonists MK0677 and SKF81297, respectively, rescued hippocampal synaptic function and cognition in 5×FAD mice.
RESULTS
Aβ physically interacts with GHSR1α
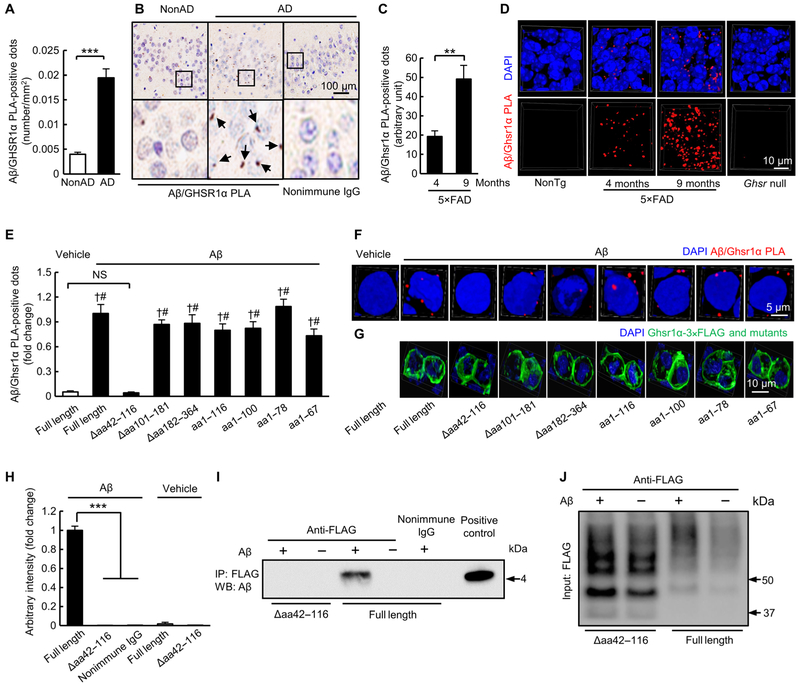
To determine whether GHSR1α expression is changed in the hippocampus in AD, we performed immunohistochemical and membrane blotting assays in postmortem hippocampal tissues from four subjects with AD and four nondemented healthy donors (nonAD). We observed increased GHSR1α expression in hippocampal tissues from patients with AD (fig. S1, A and B, and table S1), which positively correlated with the amounts of soluble Aβ40 and Aβ42 in hippocampi (fig. S1, C and D). Increased hippocampal Ghsr1α expression was prominent in 5×FAD mice especially at 9 months old (fig. S1, E and F) when the mice demonstrate heavy brain amyloidopathy with severe hippocampal lesions (13). These results suggest a potential relationship between GHSR1α expression and Aβ toxicity. Because Aβ binds to multiple proteins (14-16), we next explored whether GHSR1α is an Aβ binding target. To examine the interaction between GHSR1α and Aβ, we labeled GHSR1α and Aβ with their specific antibodies and ran Duolink proximity ligation assay (PLA), which is a sensitive method to visualize and quantify direct protein interactions in situ (17), on hippocampal tissues from four subjects with AD and four healthy donors. We observed Aβ/GHSR1α complexes in hippocampi from patients with AD (Fig. 1, A and B). Moreover, 5×FAD mice at 4 and 9 months old exhibited increased hippocampal Aβ/Ghsr1α complexes in an age-dependent manner (Fig. 1, C and D). To validate the specificity of this interaction, we expressed full-length mouse Ghsr1α or its truncating mutants (figs. S2 and S3) in otherwise non–Ghsr1α-expressing human embryonic kidney (HEK) 293T cells, and the cells exhibited similar expression of FLAG-tagged Ghsr1α and its mutants (Fig. 1G and fig. S4). HEK 293T cells expressing full-length mouse Ghsr1α or its mutants were exposed to 5 μM oligomeric Aβ42 for 24 hours followed by Duolink PLA to detect Aβ/Ghsr1α complexes. In contrast to full-length Ghsr1α and other tested Ghsr1α mutants (Fig. 1, E and F), Ghsr1α mutant devoid of amino acid (aa)42–116 (Ghsr1αΔaa42–116) showed no interaction with oligomeric Aβ42 (Fig. 1, E and F). The Duolink PLA results were further validated by using coimmuno-precipitation (Co-IP) (Fig. 1, H to J, and fig. S5, A and B). These results confirm the interaction between the two proteins as seen in AD and further suggest that aa42-116 residues on Ghsr1α are critical for Aβ binding. Together, our findings indicate that Aβ physically interacts with GHSR1α in AD.
Fig. 1. Aβ physically interacts with GHSR1α.
(A) Measurement of PLA-positive dots for Aβ/GHSR1α complex in hippocampi from subjects with AD. ***P < 0.001, unpaired Student’s t test. n = 4 healthy donors or subjects with AD. (B) Representative images of quantification in (A). Arrows indicate Aβ/GHSR1α PLA-positive dots. (C) Analysis of Aβ/Ghsr1α PLA-positive dots in the hippocampal region from 4- and 9-month-old 5×FAD mice. **P < 0.01, unpaired Student’s t test. n = 4 mice per group. (D) Representative three-dimensional (3D) reconstructed images. The slices from 9-month-old Ghsr null mice were used as negative control. (E to G) Analysis of Ghsr1α/Aβ PLA-positive dots in HEK 293T cells expressing different forms of Ghsr1α treated with vehicle or 5 μM oligomeric Aβ42 for 24 hours. Anti-FLAG antibody was used to detect Ghsr1α and its mutants. †P < 0.001 versus cells expressing full-length Ghsr1α without oligomeric Aβ42 treatment and #P < 0.001 versus cells expressing Ghsr1α Δaa42–116 with oligomeric Aβ42 treatment, unpaired Student’s t test. n = 4 to 7. (F) Representative 3D reconstructed images of Ghsr1α/Aβ PLA-positive dots in HEK 293T cells expressing different forms of Ghsr1α treated with vehicle or oligomeric Aβ42 (top panels) and (G) representative 3D reconstructed images of immunofluorescent staining of different forms of Ghsr1α (bottom panels) recognized by anti-FLAG antibody. (H) Densitometry of all immunoreactive bands generated from Co-IP on HEK 293T cells expressing different forms of Ghsr1α treated with vehicle or 5 μM oligomeric Aβ42 for 24 hours. ***P < 0.001, one-way ANOVA followed by Bonferroni post hoc analysis. Data were collected from three independent experiments. n (from left to right) = 3, 5, 2, 3, and 3. Nonimmune immunoglobulin G (IgG) to replace specific FLAG antibody was used for examining specificity of Co-IP. (I) Representative immunoblots showing the interaction of oligomeric Aβ42 with Ghsr1α and Ghsr1α Δaa42–116. (J) Representative immunoblots showing the input of Ghsr1α and Ghsr1α Δaa42–116. DAPI, 4′,6-diamidino-2-phenylindole; NS, not significant; IP, immunoprecipitation; WB, Western blot.
The interaction with Aβ induces GHSR1α dysfunction
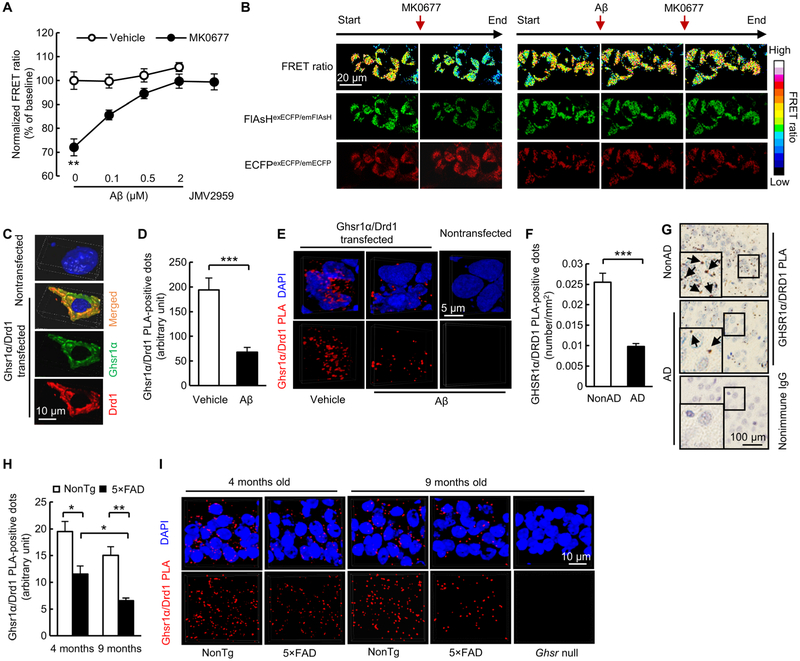
To determine whether Aβ’s interaction affects the function of Ghsr1α, we examined Ghsr1α activity in Aβ-enriched environments using fluorescein arsenical hairpin binder (FlAsH)–based fluorescence resonance energy transfer (FRET) assay, which is advantageous for GPCR activity assay because of its minimally perturbing effect on GPCR function (18). FlAsH-based FRET was created by structural dynamics of Ghsr1α with FlAsH in the intercellular loop 3 and enhanced cyan fluorescent protein (ECFP) in the C terminus (Ghsr1αFIAsH/ECFP). Ghsr1αFIAsH/ECFP was expressed in HEK 293T cells, and changes in FRET ratio (FFIAsH/FECFP) were monitored for the measurement of agonist-induced Ghsr1α activation. No change in FRET ratio was detected in vehicle-treated Ghsr1αFIAsH/ECFP-expressing HEK 293T cells, whereas the administration of MK0677 (50 μM) induced a decrease in FRET ratio (Fig. 2, A and B). Ghsr1α antagonist JMV2959 (50 μM) diminished MK0677-mediated FRET ratio change (Fig. 2A). These results indicate that agonist-induced Ghsr1α activation results in less energy transfer between ECFP and FlAsH (fig. S6). Oligomeric Aβ42 at indicated concentrations was applied on Ghsr1αFIAsH/ECFP-expressing HEK 293T cells for 5 min (preincubation) followed by coincubation with vehicle or Ghsr1α agonist MK0677. Although oligomeric Aβ42 had no impact on FRET ratio in vehicle-treated cells, agonist-induced Ghsr1α activation was suppressed by oligomeric Aβ42 (Fig. 2, A and B), suggesting a Ghsr1α antagonist-like effect of oligomeric Aβ42. Therefore, oligomeric Aβ42 reduced the response of Ghsr1α to activation (fig. S6) and that this effect was likely to be due, at least partially, to Aβ/Ghsr1α interaction.
Fig. 2. Interaction with Aβ disrupts GHSR1α activity.
(A to C) Impact of oligomeric Aβ42 (2 μM, 5-min pretreatment) on Ghsr1α FlAsH-FRET response in the presence or absence of MK0677 (50 μM). (A) FRET ratio quantified from data collected from a microplate reader. The effect of Ghsr1α antagonist JMV2959 (50 μM) against MK0677-induced Ghsr1α activation was used as positive control. **P < 0.01 compared with other groups, two-way ANOVA followed by Bonferroni post hoc analysis. n = 12 per group. (B) Representative confocal microscopy images for FRET pseudo-color ratio (FlAsHexECFP/emFIAsH/ECFPexECFP/emECFP) (top), FlAsHexECFP/emFIAsH (middle, green) and ECFPexECFP/emECFP (bottom, red). (C) Representative 3D reconstructed images for Ghsr1α and Drd1 expression in Ghsr1α/Drd1 coexpressing HEK 293T cells. (D) Analysis of Ghsr1α/Drd1 PLA-positive dot intensity in Ghsr1α/Drd1 coexpressing HEK 293T cells. ***P < 0.001, unpaired Student’s t test. Data were collected from three independent experiments. n = 78 cells for vehicle-treated group and n = 60 cells for the group with oligomeric Aβ42 treatment (5 μM, 24 hours). Anti-Ghsr1α and anti-Drd1 antibodies were used in this experiment. (E) Representative 3D reconstructed images for Ghsr1α/Drd1 PLA-positive dots in Ghsr1α/Drd1 coexpressing HEK 293T cells. (F) Analysis of GHSR1α/DRD1 PLA-positive dots in hippocampal sections from patients with AD and healthy controls. ***P < 0.001, unpaired Student’s t test. n = 5 per group. (G) Representative images of GHSR1α/DRD1 PLA dots. Arrows indicate GHSR1α/DRD1 PLA-positive dots. (H) Analysis of Ghsr1α/Drd1 PLA-positive dots in hippocampal CA1 region in 4- and 9-month-old 5×FAD mice. *P < 0.05 and **P < 0.01, unpaired Student’s t test. n = 3 for each group. (I) Representative 3D reconstructed images of Ghsr1α/Drd1 PLA-positive dots in the hippocampus of 4- and 9-month-old nonTg and 5×FAD mice. Ghsr null mice at 9 months old were used as critical negative control.
GHSR1α forms complex with DRD1 to regulate DRD1-mediated hippocampal synaptic strength and memory (8). To fully evaluate the influence of Aβ on GHSR1α, we next examined whether oligomeric Aβ42 affects the heterodimerization of GHSR1α and DRD1 by using HEK 293T cells coexpressing Ghsr1α and Drd1 (Fig. 2C and fig. S2). After an incubation with oligomeric Aβ42 (5 μM) for 24 hours, the interaction between Ghsr1α and Drd1 determined by Duolink PLA was suppressed (Fig. 2, D and E), indicating the inhibitory effect of oligomeric Aβ42 on Ghsr1α/Drd1 heterodimerization. To test whether GHSR1α/DRD1 interaction was modulated in a clinical setting, we examined hippocampal tissues from five patients with AD and compared with tissues from five healthy controls. Analysis of Duolink PLA data showed a reduction of GHSR1α/DRD1 complexes in hippocampi from subjects with AD compared with those from control subjects (Fig. 2, F and G), with a negative correlation between GHSR1α/DRD1 complex density and the amounts of hippocampal soluble Aβ40 and Aβ42 (fig. S7, A and B). Decreased Ghsr1α/Drd1 heterodimerization also occurred in 5×FAD mice, and this effect was exacerbated with age (Fig. 2, H and I). The preserved expression of DRD1 in hippocampi from subjects with AD (fig. S8, A and B) and 5×FAD mice (fig. S8, C and D) suggests that decreased GHSR1α/DRD1 interaction in AD-relevant pathological settings is not due to DRD1 loss. FlAsH-based FRET assay was performed to examine the effect of oligomeric Aβ42 (2 μM, 5 min preincubation) on agonist (SKF81297, 100 μM)-induced Drd1 activity in HEK 293T cells expressing Drd1 with FlAsH in the intercellular loop 3 and ECFP in the C terminus (Drd1FIAsH/ECFP). Unaltered agonist-induced Drd1 activation examined by FlAsH-based FRET indicates no impact of oligomeric Aβ42 on Drd1 activation (fig. S9A). Moreover, Aβ/DRD1 complexes were not observed in hippocampi from patients with AD (fig. S9B) by using Duolink PLA or oligomeric Aβ42 (5 μM, 24 hours)-exposed Drd1-expressing HEK 293T cells by using Duolink PLA (fig. S9, C and D) and Co-IP assays (fig. S9E). Therefore, decreased hippocampal GHSR1α/DRD1 interaction in AD-relevant conditions most likely results from Aβ-mediated GHSR1α deregulation.
Loss of Ghsr1α induces AD-like hippocampal synaptic stress and memory deficits
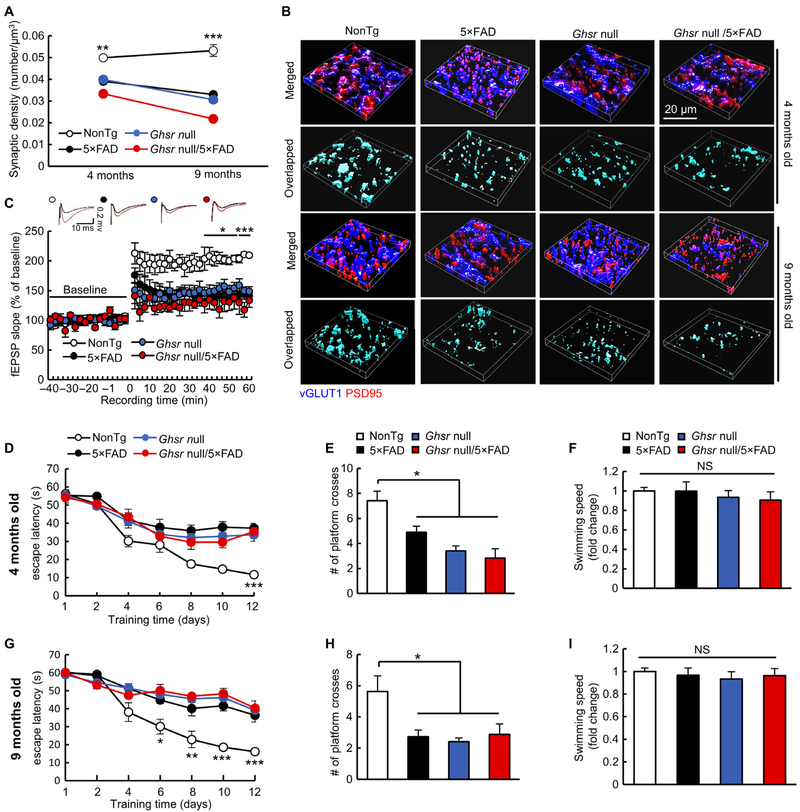
Because GHSR1α function is critical for hippocampal synaptic physiology (19), we hypothesized that Ghsr1α-deficient mice would display AD-like synaptic loss in the hippocampus and cognitive impairment. To test this hypothesis, we measured synaptic density in hippocampal slices from Ghsr null (20), nontransgenic (nonTg), 5×FAD, and Ghsr null/5×FAD mice at 4 and 9 months old. Ghsr null mice demonstrated reduced synapse density (Fig. 3, A and B) in the hippocampal CA1 region, one of the areas afflicted in AD with Ghsr1α abundance (5, 21). The effect of the lack of Ghsr signaling on synaptic density is in agreement with a previous report (19). We next examined long-term potentiation (LTP) in the hippocampal CA3-CA1 pathway (15) to determine the effect of Ghsr loss on synaptic strength. We found impairments of stimulus-evoked LTP on hippocampal slices from 9-month-old Ghsr null mice demonstrated by decreased field excitatory postsynaptic potential (fEPSP) slope after theta burst stimuli at 40 to 60 min (Fig. 3C) without altering baseline input-output relationships of the evoked responses (fig. S10). Moreover, loss of Ghsr impaired hippocampus-dependent spatial navigation in the Morris water maze test (Fig. 3, D, E, G, and H) without affecting mouse swimming speed (Fig. 3, F and I). These changes in Ghsr null mice were in line with phenotypic alterations observed in age- and gender-matched 5×FAD mice (Fig. 3, D to I). Ghsr null/5×FAD showed no major differences in the tested parameters relative to their age- and gender-matched 5×FAD littermates (Fig. 3, A to I) and no differences in hippocampal Aβ loading (fig. S11) or serum ghrelin (fig. S12). These results suggest that the impairments observed in Ghsr null and in 5×FAD mice might be mediated by overlapping mechanisms. The regulation of DRD1 by GHSR1α is pivotal for hippocampal synaptic plasticity, which is modulated through the activation of CaMKII downstream of DRD1 signaling (8). To determine whether impaired CaMKII activation due to Ghsr1α deregulation is involved in hippocampal synaptic deficits in 5×FAD mice, we examined CaMKII phosphorylation at Thr286 (P-CaMKIIα Thr286), an activated form of CaMKII (22), in postsynaptic densities isolated from mouse hippocampus by immunoblotting to reflect CaMKIIα activation at hippocampal postsynapses. Decreased CaMKII phosphorylation at Thr286 was detected in hippocampal postsynaptic densities from 9-month-old Ghsr null, 5×FAD, and Ghsr null/5×FAD mice as compared with their nonTg littermates (fig. S13). Ghsr1α deficiency did not affect the expression of hippocampal Drd1, regardless of Aβ overexpression (fig. S8, C and D). These results implicate the detrimental effect of Ghsr1α deregulation on Drd1-related CaMKII signaling in 5×FAD mice. Together, our findings support the idea that Aβ-induced Ghsr1α deregulation underpins hippocampal synaptic deficits and cognitive decline in 5×FAD mice.
Fig. 3. Loss of Ghsr replicates AD-like phenotypes.
(A) Analysis of synaptic density in CA1 regions from 4- and 9-month-old mice. **P < 0.001 and ***P < 0.001 nonTg versus other groups at the same age, one-way ANOVA followed by Bonferroni post hoc analysis. Four-month-old mice: nonTg, n = 4; 5×FAD, n = 7; Ghsr null mice, n = 5; and Ghsr null/5×FAD, n = 4. Nine-month-old mice: nonTg, n = 4, 5×FAD, n = 4, Ghsr null mice, n = 4, and Ghsr null/5×FAD, n = 3. (B) Representative 3D reconstructed images of synapse staining. Vesicular glutamate transporter 1 (vGLUT1, blue) and postsynaptic density 95 (PSD95, red) were used to visualize pre- and postsynaptic terminals, respectively. The overlapped staining of vGLUT1 and PSD95 indicates synapses. (C) Time course of LTP and representative fEPSP responses during the baseline period (black trace) and 30 s after theta burst simulation (red trace) in four groups of mice at 9 months old. *P < 0.05 and ***P < 0.001 nonTg versus other groups, one-way ANOVA followed by Bonferroni post hoc analysis. nonTg, n = 5; 5×FAD, n = 4; Ghsr null, n = 5; and Ghsr null/5×FAD, n = 5. (D to I) Spatial navigation of four groups of mice in the Morris water maze test. (D and G) Spatial learning of four groups of mice at 4 (D) and 9 (G) months old. *P < 0.05, **P < 0.01, and ***P < 0.001, nonTg versus other groups on the same day, one-way ANOVA followed by Bonferroni post hoc analysis. (E and H) Spatial reference memory of different groups of mice at 4 (E) and 9 (H) months of age. *P < 0.05, one-way ANOVA followed by Bonferroni post hoc analysis. (F and I) Swimming speed of four groups of mice at 4 (F) and 9 (I) months old. Four-month-old mice: nonTg, n = 7; 5×FAD, n = 9; Ghsr null mice, n = 10; and Ghsr null/5×FAD, n = 6. Nine-month-old mice: nonTg, n = 8; 5×FAD, n = 11; Ghsr null mice, n = 5; and Ghsr null/5×FAD, n = 8.
Reduced Ghsr1α/Drd1 interaction contributes to Aβ-induced hippocampal synaptic injury
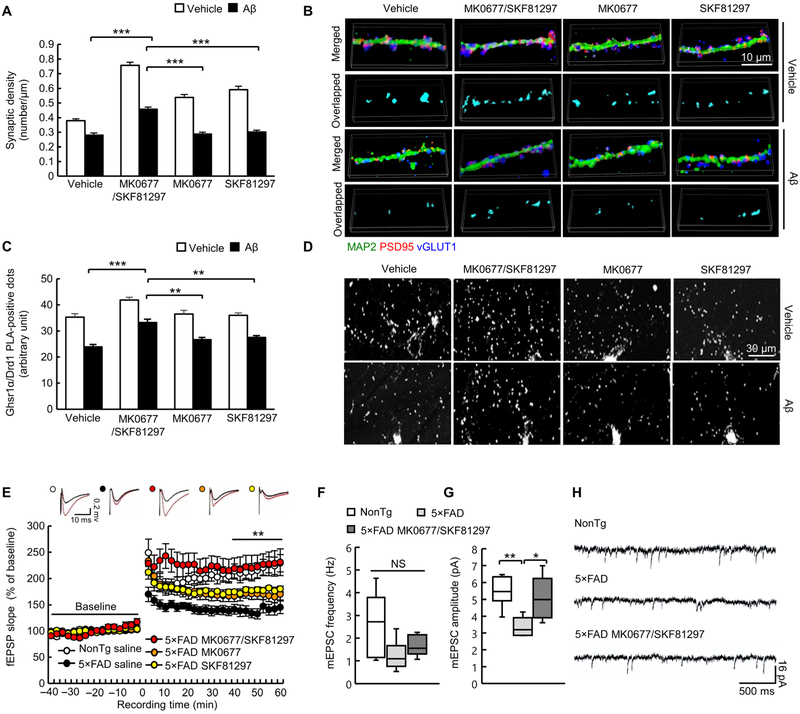
Given the deleterious influence of the GHSR1α deficiency on hippocampal synapses, we asked whether GHSR1α activation could restore synaptic function in Aβ-rich environments. To directly test this, we applied the Ghsr1α agonist MK0677 to oligomeric Aβ42-exposed hippocampal neuron cultures. Our interest in MK0677 derives from the translational potential of MK0677, a nonpeptide ghrelin mimetic compound with higher potency than ghrelin (23). The agonist MK0677 alone at doses greater than 1.5 μM substantially promoted synaptic formation demonstrated by increased synaptic density (fig. S14A), suggesting that Ghsr1α activity can induce synaptogenesis. However, administration of MK0677 (1.5 μM) did not mitigate oligomeric Aβ42 (1 μM, 24 hours)-induced synapse loss in hippocampal neuron cultures (Fig. 4, A and B), which is consistent with our finding that oligomeric Aβ42 blunts MK0677-mediated Ghsr1α activation. Because MK0677 alone was not protective against oligomeric Aβ42-induced synapse loss, we further explored the importance of Ghsr1α/Drd1 interaction. We sought to promote Ghsr1α regulation of Drd1 by increasing Ghsr1α/Drd1 heterodimerization through simultaneously activating Ghsr1α and Drd1 in cultured mouse hippocampal neurons. The optimal dose for the selective Drd1 agonist SKF81297 (2 μM) was determined on the basis of its capability in promoting synapse formation in cultured mouse hippocampal neurons (fig. S14B). Same as MK0677, SKF81297 itself increased synaptic density in cultured mouse hippocampal neurons but failed to alleviate oligomeric Aβ42 (1 μM, 24 hours)-induced synapse loss in hippocampal neuron cultures (Fig. 4, A and B). In contrast to MK0677 or SKF81297 alone, the simultaneous stimulation of Ghsr1α and Drd1 using both compounds (1.5 μM MK0677 and 2 μM SKF81297) increased synaptic density in oligomeric Aβ42 (1 μM, 24 hours)-treated mouse hippocampal neuron cultures (Fig. 4, A and B). Consistent with this observation, coapplication of MK0677 and SKF81297, but not the agonists alone, preserved Ghsr1α/Drd1 complex formation from oligomeric Aβ42 (1 μM, 24 hours) (Fig. 4, C and D). These results indicate that interaction of Ghsr1α/Drd1 promotes synaptic formation in hippocampal neurons, which is in agreement with previous reports (8), and they further support the hypothesis that perturbed GHSR1α regulation of DRD1 contributes to synaptic injury in AD.
Fig. 4. Combined Ghsr1α/Drd1 activation rescues hippocampal synapse in vitro.
(A) Effect of different treatments (1.5 μM MK0677, 2 μM SKF81297, or in combination) on synaptic density in hippocampal neurons in the presence or absence of oligomeric Aβ42 (1 μM, 24 hours). ***P < 0.001 vehicle-treated versus oligomeric Aβ42–treated groups, two-way ANOVA followed by Bonferroni post hoc analysis. Data were collected from three independent experiments. n = 30 to 48 neurites. (B) Representative 3D reconstructed images of synapse staining. vGLUT1 (blue) and PSD95 (red) were used to visualize pre- and postsynaptic terminals, respectively. The dendrites were stained with MAP2 (green). The overlaid staining of vGLUT1/PSD95 identifies synapses. (C) Effect of different treatments (1.5 μM MK0677, 2 μM SKF81297, or in combination) on Ghsr1α/Drd1 complex in hippocampal neurons in the presence or absence of oligomeric Aβ42 (1 μM, 24 hours). **P < 0.01 and ***P < 0.001 vehicle-treated versus oligomeric Aβ42-treated groups, two-way ANOVA followed by Bonferroni post hoc analysis. Data were collected from three independent experiments. n = 8 to 10 neurons. (D) Representative images of Ghsr1α/Drd1 PLA-positive dots. (E) Time course of LTP and representative fEPSP responses during the baseline period (black trace) and 30 s after theta burst simulation (red trace) in five treatment groups at 4 months of age. **P < 0.01 5×FAD MK0677/SKF81297 versus 5×FAD saline, one-way ANOVA followed by Bonferroni post hoc analysis. nonTg saline, n = 9; 5×FAD saline, n = 10; 5×FAD MK0677/SKF81297, n = 7; 5×FAD MK0677, n = 9; and 5×FAD SKF81297, n = 9. (F to H) mEPSC frequency (F) and amplitude (G) in the indicated groups of 4-month-old mice. *P < 0.05 and **P < 0.01, one-way ANOVA followed by Bonferroni post hoc analysis. n = 6. (H) Representative traces of mEPSC recordings.
To verify this hypothesis in Aβ overexpression-mediated model of synaptic injury, we applied MK0677 (1.5 μM) and SKF81297 (2 μM), alone or in combination, to hippocampal slices from 4-month-old nonTg and 5×FAD mice and examined LTP to reflect synaptic strength in the hippocampal CA3-CA1 pathway. Previous studies (13) showed that 5×FAD mice present early hippocampal synaptic lesions at 4 months of age. Vehicle-treated 5×FAD hippocampal slices exhibited impairments of stimulus-evoked LTP (Fig. 4E), indicating decreased synaptic strength. The treatment of MK0677 or SKF81297 alone had no effect on hippocampal LTP in 5×FAD hippocampal slices (Fig. 4E). In contrast, simultaneous application of MK0677 and SKF81297 markedly mitigated impairments of stimulus-evoked LTP in 5×FAD hippocampal slices (Fig. 4E). The treatment of MK0677 or SKF81297 alone or in combination had no effect on hippocampal LTP in brain slices from nonTg mice (fig. S15). In view of damaged excitatory synaptic transmission in AD (24), we next examined the effect of the MK0677/SKF81297 mixture on excitatory synaptic transmission in the hippocampus of 5×FAD mice at 4 months old by performing whole-cell recordings of miniature excitatory postsynaptic currents (mEPSCs). Although no genotypic effect on mEPSC frequency was observed (Fig. 4, F and H), CA1 neurons from 5×FAD mice demonstrated a decrease in mEPSC amplitude, which was protected by the MK0677/SKF81297 mixture (Fig. 4, G and H). Because mEPSC frequency primarily represents the probability of presynaptic release and mEPSC amplitude is largely associated with the conductance of postsynaptic receptors (25), the results suggest improved postsynaptic receptor function in MK0677/SKF81297-treated 5×FAD CA1 neurons. Together, these findings strongly suggest a role for dysfunctional GHSR1α regulation of DRD1 in AD hippocampal synaptic failure and also indicate that combined stimulation of GHSR1α and DRD1 can rescue Aβ-induced hippocampal synaptic deficits.
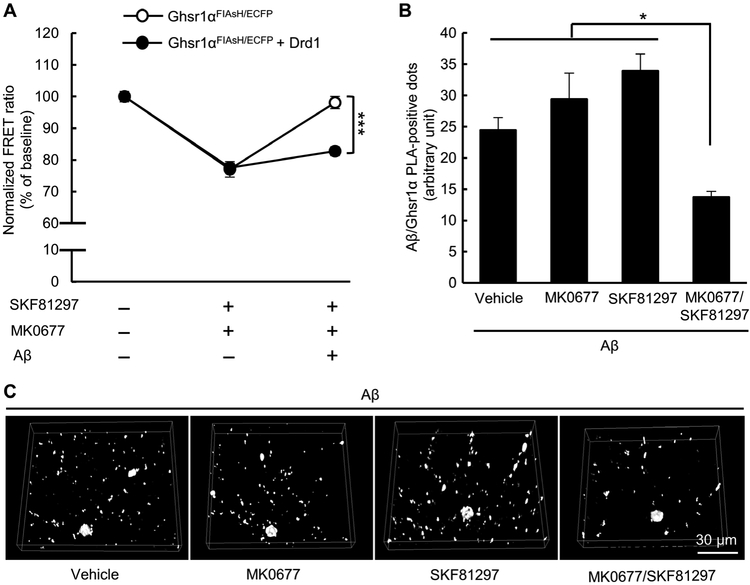
Ghsr1α/Drd1 coactivation rescues Ghsr1α function from Aβ toxicity
To assess whether the protective effects of Ghsr1α and Drd1 coactivation were mediated by Ghsr1α activity, we measured Ghsr1α activity by FlAsH-based FRET on HEK 293T cells either expressing Ghsr1αFIAsH/ECFP alone or coexpressed with Drd1. The cells were exposed to vehicle treatment or the mixture of MK0677 (50 μM) and SKF81297 (100 μM) in the presence or absence of a 5-min pretreatment of 2 μM oligomeric Aβ42. Without oligomeric Aβ42, the two types of cells exhibited similar response to the combined treatment (Fig. 5A), indicating that Ghsr1α was activated by its agonist regardless of Drd1 expression. The inhibitory effect of oligomeric Aβ42 on Ghsr1α activation was diminished in MK0677/SKF81297 mixture-treated Ghsr1α/Drd1 coexpressing cells (Fig. 5A). These results suggest that coactivation of Ghsr1α and Drd1 can prevent Aβ-induced effects on Ghsr1α. In further support of this hypothesis, the MK0677/SKF81297 mixture, but not MK0677 or SKF81297 alone, alleviated Aβ/Ghsr1α complex formation in oligomeric Aβ42 (1 μM, 24 hours)-treated mouse hippocampal neuron cultures (Fig. 5, B and C). Together, these results suggest that Ghsr1α/Drd1 coactivation preserves Ghsr1α activity by reducing the interaction between Aβ and Ghsr1α.
Fig. 5. Coactivation of Ghsr1α and Drd1 preserves Ghsr1α activity from Aβ toxicity.
(A) Effect of SKF81297 (100 μM) and MK0677 (50 μM) alone or in combination on Ghsr1α FIAsH-FRET response in the presence or absence of oligomeric Aβ42 (2 μM, 5-min pretreatment). Cells expressing Ghsr1αFIAsH/ECFP alone or coexpressed with Drd1 were used. Data were collected from a microplate reader. ***P < 0.001, two-way ANOVA followed by Bonferroni post hoc analysis. Data were collected from three independent experiments. n = 9 to 25 samples. (B and C) Effect of different treatments (1.5 μM MK0677, 2 μM SKF81297, or in combination) on Aβ/Ghsr1α complex in oligomeric Aβ42 (1 μM, 24 hours)-treated hippocampal neurons. *P < 0.05, one-way ANOVA followed by Bonferroni post hoc analysis. Data were collected from three independent experiments. n = 10 neurons per group.
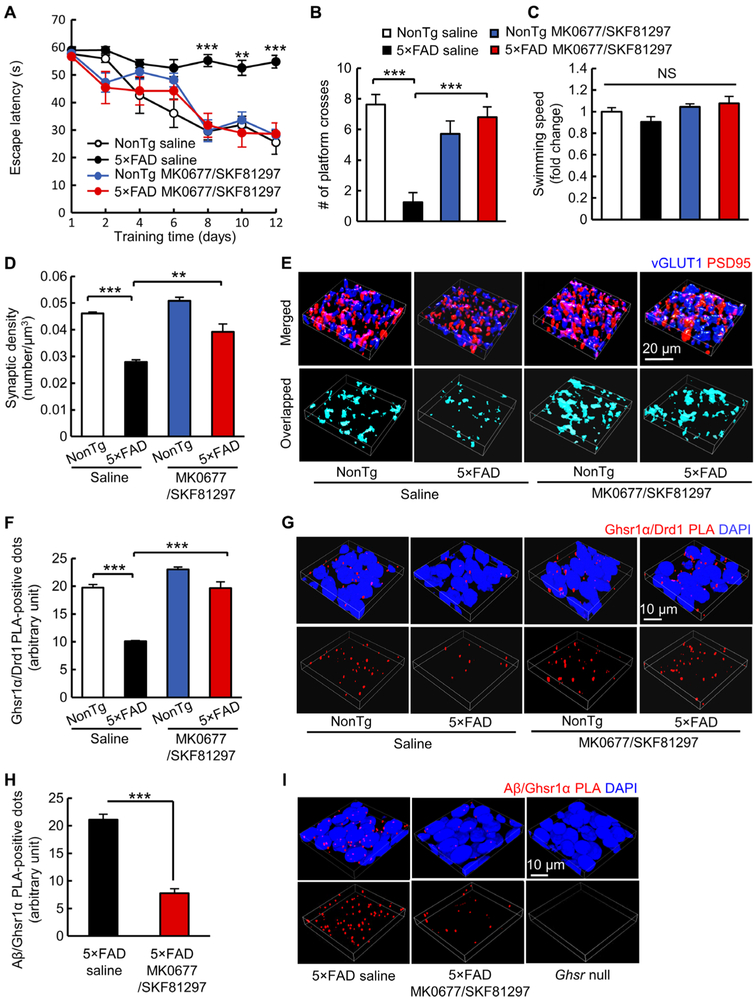
Ghsr1α/Drd1 coactivation rescues synaptic density and memory in 5×FAD mice
Next, we attempted to replicate our findings in vivo. Because 5×FAD mice begin to exhibit compromised spatial learning and memory at 4 to 5 months old (26-28), we expected that Ghsr1α/Drd1 coactivation would restore hippocampal synaptic function and improve behavior in young 5×FAD mice at 4 to 5 months old when hippocampal lesions are limited and Aβ accumulation is low (26-28). Age- and gender-matched nonTg and presymptomatic 5×FAD mice (“presymptomatic” refers to unaffected spatial learning and memory) at 3 months old received daily intraperitoneal injections of the MK0677/SKF81297 combination therapy [MK0677 (1 mg/kg) and SKF81297 (1.5 mg/kg)] for 30 days followed by behavioral experiments at 4 months of age. These treatment regimens were optimized on the basis of preliminary experiments that took into account the influence on body weight (fig. S16, A and C), serum ghrelin (fig. S16, B and D), and behavioral performance (Fig. 6, A to C, and fig.S16, E to G), as well as previous reports (29, 30). Saline-treated 5×FAD mice demonstrated memory defects in the Morris water maze test, which were prevented by treatment with MK0677/SKF81297 (Fig. 6, A to C). Moreover, mice that received MK0677/SKF81297 treatment maintained body weight (fig. S16A) and serum ghrelin amount (fig. S16B) and showed unaffected cell density in the liver, kidney, and brain (fig. S17). 5×FAD mice treated with MK0677/SKF81297 showed considerably less hippocampal CA1 synapse loss (Fig. 6, D and E), preserved Ghsr1α/Drd1 heterodimerization (Fig. 6, F and G), and fewer Aβ/Ghsr1α complexes (Fig. 6, H and I) as compared with their vehicle-treated counterparts. In addition, because Ghsr1α activation has been shown to improve hippocampal neurogenesis in 5×FAD mice (31), we examined neurogenesis in the dentate gyrus by performing immunocytochemistry staining of adult brain neurogenesis marker, doublecortin (DCX) (32). Compared with their vehicle-treated counterparts, MK0677/SKF81297-treated 5×FAD mice exhibited increased DCX-positive neurons in their dentate gyrus (fig. S18).
Fig. 6. Combined Ghsr1α/Drd1 activation protects hippocampal synapse and cognition in vivo.
(A to C) Spatial navigation analysis in four groups of mice treated with vehicle (saline) or MK0677/SKF81297 (MK0677, 1 mg/kg and SKF81297, 1.5 mg/kg) performing the Morris water maze test. (A) Spatial learning. **P < 0.01 and ***P < 0.001 5×FAD saline versus other groups, two-way ANOVA followed by Bonferroni post hoc analysis. (B) Spatial reference memory. ***P < 0.001 5×FAD saline versus other groups, two-way ANOVA followed by Bonferroni post hoc analysis. (C) Swimming speed. nonTg saline, n = 8; 5×FAD saline, n = 8; nonTg MK0677/SKF81297, n = 7; and 5×FAD MK0677/SKF81297, n = 5. (D and E) Analysis of synaptic density in the hippocampal CA1 region. **P < 0.01 and ***P < 0.001 5×FAD saline versus other groups, two-way ANOVA followed by Bonferroni post hoc analysis. nonTg saline, n = 3; 5×FAD saline, n = 4; nonTg MK0677/SKF81297, n = 4; and 5×FAD MK0677/SKF81297, n = 4. (E) Representative 3D reconstructed images of synapse staining in the CA1 region. vGLUT1 (blue) and PSD95 (red) were used to visualize pre- and postsynaptic components, respectively. The overlaid staining of vGLUT1 and PSD95 indicates synapses. (F and G) Analysis of Ghsr1α/Drd1 complex in the CA1 region. ***P < 0.001 5×FAD saline versus other groups, two-way ANOVA followed by Bonferroni post hoc analysis. n = 4 per group. (G) Representative 3D reconstructed images of Ghsr1α/Drd1 PLA-positive dots (red). Nuclei were stained with DAPI. (H and I) Analysis of Aβ/Ghsr1α PLA-positive dots in CA1 region. ***P < 0.001, unpaired Student’s t test. n = 4 per group. (I) Representative 3D reconstructed images of Aβ/Ghsr1α PLA dots (red). Nuclei were stained with DAPI.
Ghsr1α/Drd1 coactivation does not affect hippocampal amyloidosis or tau pathology in 5×FAD mice
To determine whether synaptic function and memory improvement in MK0677/SKF81297-treated 5×FAD mice were associated with altered Aβ production and deposition, we examined hippocampal tissues from MK0677/SKF81297- and vehicle-treated 5×FAD mice. Immunoblotting assay using antibody against amyloid precursor protein (APP) was performed to examine APP expression in hippocampal homogenates (Fig. 7A). Immunohistochemical staining using antibody against Aβ was performed to detect Aβ load on hippocampal slices (Fig. 7B). The amounts of soluble Aβ40 and Aβ42 in hippocampal homogenates were measured by enzyme-linked immunosorbent assay (Fig. 7C). Moreover, because 5×FAD mice have intraneuronal Aβ deposition in addition to extracellular Aβ plaques mimicking AD brain amyloidosis (13), we further examined intraneuronal Aβ deposition in hippocampal CA1 neurons demonstrated by the overlapping staining of Aβ and class III β-tubulin, a specific neuronal marker (Fig. 7D) (33), as well as extracellular amyloid plaques in the hippocampus determined by Congo red–positive staining (Fig. 7E). No difference in these parameters was observed between MK0677/SKF81297- and vehicle-treated 5×FAD mice (Fig. 7, A to E). In addition, to determine whether MK0677/SKF81297 treatment affects tau pathology, we analyzed tau phosphorylation by performing immunoblotting on mice hippocampal homogenates. Tau phosphorylation at multiple phosphorylation sites including Ser202/Thr205, Ser396, and Ser404 or total tau in 5×FAD mice were not changed by MK0677/SKF81297 treatment (Fig. 7F). Therefore, the protective effects of MK0677/SKF81297 treatment do not result from modulation on brain amyloidosis or tau pathology.
Fig. 7. Hippocampal amyloidosis and tau phosphorylation remain unaltered in treated 5×FAD mice.
(A) Analysis of APP expression level in the hippocampus by using immunoblotting. Unpaired Student’s t test. n = 4 per group. The right panel shows representative images of immunoblotting. β-Actin was used as the loading control. (B) Aβ deposition in the hippocampal region was measured and analyzed by immunostaining using Aβ antibody. Unpaired Student’s t test. 5×FAD saline mice, n = 6 and 5×FAD MK0677/SKF81297 mice, n = 5. The right panel shows representative images of Aβ staining (red). The neurons were identified by the staining of NeuN (green). (C) Soluble Aβ40 and Aβ42 amounts in hippocampal homogenate were detected by ELISA assay. Unpaired Student’s t test. 5×FAD saline mice, n = 6 and 5×FAD MK0677/SKF81297 mice, n = 5. (D) Analysis of intracellular Aβ in hippocampal CA1 neurons. Unpaired Student’s t test. 5×FAD saline mice, n = 8 and 5×FAD MK0677/SKF81297 mice, n = 5. The right panel shows representative images. Aβ was recognized by anti-Aβ antibody (red). Neurons were labeled by anti–β-III-tubulin (green). Nuclei were identified by the staining of DAPI (blue color). The overlaid staining of Aβ and β-III-tubulin indicates intraneuronal Aβ. Scale bar, 20 μm. (E) Congo red staining was used to label extracellular parenchymal Aβ plaques. Unpaired Student’s t test. 5×FAD saline mice, n = 8 and 5×FAD MK0677/SKF81297 mice, n = 5. The right panel shows representative images of Aβ plaque staining. (F) Immunoblotting analysis of tau phosphorylation at different motifs and total tau in mouse hippocampal tissues. Unpaired Student’s t test. n = 4 per group. The lower panel shows representative images of immunoblotting. β-Actin was used as the loading control. P-tau stands for phosphorylated tau, and T-tau stands for total tau.
DISCUSSION
Recent studies highlighted the importance of GHSR1α in hippocampal synaptic physiology (8, 19, 34), but the functional status of GHSR1α in AD remains largely unknown. In this study, we found elevated expression of GHSR1α in the hippocampus from patients with AD and in 5×FAD mice. Our recent observation of an inverse relationship between serum acylated ghrelin amounts and cognitive function in MCI (12), as well as our findings here showing that Aβ alters the response of Ghsr1α to its agonist and that a strong correlation of GHSR1α expression with soluble Aβ amounts in subjects with AD exists, seem to suggest that increased GHSR1α expression in hippocampi from patients with AD might reflect a compensatory response to Aβ toxicity. Our results are in disagreement with a previous report showing decreased GHSR1α mRNA in temporal gyri from patients with AD (35). This difference may result from different mechanisms of regulation of GHSR1α at the pre- and posttranscriptional steps and/or a brain region–specific response to Aβ toxicity. Because GHSR1α expression is relatively low in the neocortex (5), it is unclear whether the decreased GHSR1α mRNA expression in the neocortical temporal lobe contributes to AD. Furthermore, it should be noted that GHSR1β, a truncated splice variant of GHSR1α, blocks GHSR1α function (36), and the aforementioned study reported increased GHSR1β mRNA in neocortical temporal tissues from subjects with AD (35). Therefore, GHSR1β may also contribute to hippocampal GHSR1α deregulation in AD. Additional studies are needed to address these questions and to understand the contribution of the GHSR/ghrelin system in AD pathogenesis. Here, we show that hippocampal GHSR1α deregulation can be induced through a physical interaction with Aβ, and we established a link between Ghsr1α deregulation, hippocampal synaptic injury, and cognitive impairments in mice. The alterations in Ghsr1α in young 5×FAD mice and the abnormal increase of acylated ghrelin in patients with MCI (12) seem to suggest that GHSR1α deregulation may develop in prodromal or early stages of the disease. Studies of postmortem tissues from preclinical patients with AD could help to address this possibility.
Substantial efforts are currently directed toward the development of new AD treatments, especially toward the development of disease-modifying therapies (37). However, current AD interventions, including acetylcholinesterase inhibitors or N-methyl-d-aspartate receptor blockers, do not target the underlying mechanisms that cause synaptic injury and thus have limited efficacy (2, 38, 39). Moreover, ongoing attempts to remove Aβ or ameliorate tau pathology have yet to prove effective (40, 41). Our results identify GHSR1α, and particularly its interaction with DRD1, as a target for AD treatment with translational potential. Studies that previously explored GHSR1α agonism for the treatment of AD produced inconsistent results (10, 11). GHSR1α agonists such as MK0677 and LY444711 provided protection in animal and cell models (42-45); however, a clinical trial of MK0677 in patients with AD failed to show clinical benefits (10). Although this trial was originally designed to enhance brain Aβ clearance by augmenting insulin-like growth factor 1 release (10), its negative outcome discouraged further attempts to target GHSR1α in AD. We speculate here that a potential explanation for this clinical trial’s failure may be that GHSR1α becomes insensitive to its agonists in AD. Previous studies found ghrelin (44) or acylated ghrelin (46) is protective against acute Aβ-induced synaptic dysfunction, cognitive impairments, and neuroinflammation. Discrepancies between these studies and ours may reflect differences in the degree of Aβ exposure, as Aβ overexpression in transgenic mouse models of AD exerts a more insidious and sustained deleterious effect than transient Aβ exposure (47). In contrast to our observation of no effect of the treatment on hippocampal Aβ load in 5×FAD mice, a recent study did report that MK0677 treatment lowered neocortical Aβ plaques in young 5×FAD mice (43). A higher dose of MK0677 used in that study in comparison to ours may partially explain this discrepancy in the effect of Ghsr1α activation on Aβ load. In addition, a previous study from this group found that systemic ghrelin treatment did not affect hippocampal Aβ load in 5×FAD mice (31). We cannot rule out that MK0677 may affect Aβ production and/or clearance in a dose-dependent manner. This idea requires further investigation, taking into account the potential systemic effects on body weight and glucose regulation of high doses of this drug because obesity is a risk factor for AD (48).
GHSR1α and DRD1 are abundantly coexpressed in the hippocampus and are believed to serve important roles in hippocampal function (19, 49). Kern and colleagues have determined a mechanism that links GHSR1α and DRD1 in the regulation of hippocampal synaptic function. They found that activated GHSR1α shifts DRD1 from a Gαs to a Gαq state via the formation of GHSR1α/DRD1 heterodimers, which allows dopamine to activate hippocampal synaptic activity-related Ca2+ signaling (8). This pivotal role of the GHSR1α/DRD1 heterodimer in hippocampal synaptic physiology reinforces considering coactivation of GHSR1α and DRD1 for restoring synaptic defects. A further relevant finding from our study is that coactivation of Ghsr1α/Drd1 protects Ghsr1α from Aβ toxicity. We propose that this resistance to Aβ is conferred through a Ghsr1α conformational change that arises via its interaction with Drd1. This observation provides another potential explanation for why using a GHSR1α agonist in isolation lacked clinical efficacy. In support of this hypothesis, previous studies revealed that simultaneous use of agonists of different GPCR family members can induce allosteric interactions and alterations in functional properties (50, 51). Moreover, although we did not observe DRD1 alterations in AD hippocampi, we cannot conclude that DRD1 function is intact in AD. In addition to the observation that DRD1 B2 allele is an AD risk factor (52), previous studies showed damage in the locus coeruleus in patients with AD (53). Because tyrosine hydroxylase–expressing neurons in the locus coeruleus project to the hippocampus, which enhances synaptic activity and hippocampus-related memory via D1-type dopamine receptors (54), it is therefore possible that dopaminergic input to the hippocampus is altered in AD. In this regard, the impaired regulation of DRD1 by GHSR1α in AD-related conditions may also result from insufficient supply of dopamine, DRD1’s natural ligand, in the hippocampus, which could be mitigated by the supplementation of DRD1 agonist. Last, in this study, we used young 5×FAD mice, which have relatively mild synaptic lesions and no hippocampal neuron loss (13). Whether MK0677/SKF81297 benefits older 5×FAD mice with more pervasive hippocampal lesions remains untested and need further investigation. Nevertheless, this proof-of-concept study shows the potential protective effects of this AD dual GPCR agonist intervention. MK0677 is approved by Food and Drug Administration, and although SKF81297 is not, other DRD1 agonists including levodopa and pergolide are clinically available for clinical use (55).
Another question related to the protective effects of the treatment merits discussion is the role of neurogenesis. A previous study suggested that ghrelin attenuates hippocampal pathology in 5×FAD mice by potentiating hippocampal neurogenesis (31). Similarly, in our study, we found that coactivation of Ghsr1α and Drd1 promotes neurogenesis in the dentate gyrus of mixture-treated 5×FAD mice. The neurogenic effect of GHSR1α has been linked to its role in hippocampal energy metabolism (56). However, the impact of altered neurogenesis on cognitive impairments in AD and whether neurogenesis can correct synaptic and brain network injury remained unclear (57).
Previous studies directly attributed hippocampal synaptic injury that was observed in AD to Aβ toxicity and/or tauopathy (58). Although our current study explored Ghsr1α defects in the context of an Aβ-rich environment, potential broader effects of Ghsr1α deregulation on hippocampal metabolic processes and calcium signaling should not be overlooked. It is well documented that alterations in metabolic hormones such as ghrelin, leptin, and insulin can affect feeding behavior and nutrient availability, culminating in alterations of brain energy homeostasis and synapse remodeling (19, 59). Therefore, ghrelin system perturbations could cause brain and systemic metabolic deregulation, which is strongly associated with deficits in synaptic activity and hippocampus-dependent memory in both aging and AD (19). Moreover, the hypothalamus, a target of many metabolic hormones, plays a crucial role in maintaining brain metabolic homeostasis and hippocampal synaptic physiology (60). Hypothalamic pathology occurs in patients with AD (61). Therefore, given GHSR1α’s role in hypothalamic function (62), it is possible that GHSR1α deregulation may affect hypothalamic function and indirectly drive hippocampal damage. We cannot rule out the possibility that the treatment-derived neuroprotection in our study may, at least in part, reflect improvements in hippocampal energy metabolism that arises secondary to effects on hypothalamic GHSR1α. Moreover, in view of the influence of GHSR1α on DRD1-mediated Ca2+ signaling pathway related to hippocampal synaptic plasticity (8), GHSR1α deregulation may also represent a mechanism of calcium signaling–associated selective neuronal vulnerability of AD hippocampi (63). In this context, altered hypothalamic-hippocampal connectivity and perturbed calcium signaling at synapses that result from GHSR1α dysfunction might act as critical Aβ-independent metabolic and calcium-related mechanisms of hippocampal synaptic failure in AD.
In summary, we have demonstrated a mechanism of hippocampal pathology through GHSR1α deregulation and showed that restoring Ghsr1α/Drd1 activity prevented AD-mediated synaptic abnormalities and behavioral impairments in a mouse model of AD. The most parsimonious interpretation of our findings is that GHSR1α deregulation mediates hippocampal damage and that targeting this pathological mechanism might therefore prove therapeutically useful.
MATERIALS AND METHODS
Study design
The objective of this study was to determine the role of GHSR1α in AD pathology and develop a strategy for preventing AD phenotype in a mouse model. Hippocampal tissues from patients with AD and nonAD controls were analyzed to determine GHSR1α expression, Aβ/GHSR1α interaction, and GHSR1α/DRD1 complexes. The transgenic 5×FAD mouse model was used to mimic AD amyloidopathy (27). Ghsr null (20) and Ghsr null/5×FAD mice were used to explore the role of GHSR1α deficiency in an AD-like environment. To mimic MCI and later stage AD, respectively, the studies were performed using 4- and 9-month-old mice. Both male and female mice were used. The investigators performing the experiments did not allocate the mice.
For all the experiments, sample sizes were determined by our previous data, prior literature, and power calculation to ensure sufficient sample sizes to allow the detection of statistically significant differences. Sample exclusion was not permitted. The number of unique replicates for each experiment is specified in the figure legends. Mice were randomized by genotype and gender during behavioral testing. For the behavioral, electrophysiological, pathological, and Duolink PLAs, experimenters were blinded during data acquisition and unblinded for data analysis. Raw data are provided in table S2 (separate Excel file).
Statistical analysis
Statistical comparisons were performed using GraphPad Prism 5 software. One-way or two-way analysis of variance (ANOVA) followed by Bonferroni post hoc analysis or unpaired two-way Student’s t test were applied in data analysis. Pearson’s correlation coefficient was used for correlation testing. Numbers of replicates and P values are stated in each figure legend. All data were expressed as means ± SEM except for the box plots, which were shown as maximum, median, and minimum. Significance was concluded when the *P value was less than 0.05. Significance was indicated by *P < 0.05, **P < 0.010, ***P < 0.001, #P < 0.001, and †P < 0.001. NS (not significant) denotes P > 0.05.
SUPPLEMENTARY MATERIALS
Fig. S1. Expression of GHSR1α increased in hippocampi from subjects with AD and 9-month-old 5×FAD mice.
Fig. S2. Expressions of Ghsr1α and Drd1 were validated in transfected HEK 293T cells.
Fig. S3. Schematic diagram shows the sequence of full-length Ghsr1α and its truncating mutants.
Fig. S4. FLAG-tagged Ghsr1α and its truncating mutants were similarly expressed in HEK 293T cells.
Fig. S5. The interaction between Ghsr1α mutants and Aβ42 was assessed by using Co-IP.
Fig. S6. Schematic diagram represents FlAsH-FRET assay for Ghsr1α activity and the impact of oligomeric Aβ42 on Ghsr1α activation.
Fig. S7. GHSR1α/DRD1 complex density was negatively correlated with hippocampal soluble Aβ40 or Aβ42 amounts in subjects with AD.
Fig. S8. Expression of hippocampal DRD1 remained unaltered in hippocampi from subjects with AD and 5×FAD mice.
Fig. S9. Oligomeric Aβ42 did not affect agonist-induced activation of Drd1 or form complex with Drd1.
Fig. S10. Input/output curves of fEPSPs were similar in four types of transgenic mice.
Fig. S11. Aβ deposition in the hippocampus remained unchanged in Ghsr null/5×FAD mice as compared with their 5×FAD littermates.
Fig. S12. Serum ghrelin amounts were similar in four types of transgenic mice.
Fig. S13. Loss of Ghsr1α suppressed postsynaptic CaMKII activation in the hippocampus.
Fig. S14. The optimal doses of MK0677 and SKF81297 were determined by their augmenting effect on synaptogenesis in cultured hippocampal neurons.
Fig. S15. Time course of LTP and fEPSP amplitudes was not changed by different treatments on hippocampal slices from nonTg mice.
Fig. S16. The doses of MK0677/SKF81297 treatment were optimized on the basis of the influence on body weight, serum ghrelin, and behavioral performance.
Fig. S17. MK0677/SKF81297 [MK0677 (1 mg/kg) and SKF81297 (1.5 mg/kg)] treatment on mice had no effect on hepatic, renal, and hippocampal cell density.
Fig. S18. MK0677/SKF81297 [MK0677 (1 mg/kg) and SKF81297 (1.5 mg/kg)] treatment improved neurogenesis in the dentate gyrus of 5×FAD mice.
Table S1. Human brain tissue information.
Table S2. Raw data (provided as separate Excel file).
Acknowledgments
Funding: This study was supported by research funding from NIH (R00AG037716, R01AG053588, and R01AG059753 to H.D. and P30 AG035982 to KUMC), Alzheimer’s Association (AARG-16-442863 to H.D.), and China Scholarship Council (201606220203 and 201706220265 to S.S. and Q.W).
Footnotes
Competing interests: H.D. is an inventor on patent/patent application (USPTO serial no. 62/769,428) held/submitted by the University of Texas at Dallas that covers “Composition and Method for Treatment of Hippocampal Synapse Dysfunction and Cognitive Deficits in Alzheimer’s Disease.” All other authors declare that they have no competing interests to declare.
Data and materials availability: All data associated with this study are in the paper or the Supplementary Materials.
REFERENCES AND NOTES
- 1.Scheff SW, Price DA, Schmitt FA, Mufson EJ, Hippocampal synaptic loss in early Alzheimer’s disease and mild cognitive impairment. Neurobiol. Aging 27, 1372–1384 (2006). [DOI] [PubMed] [Google Scholar]
- 2.Graham WV, Bonito-Oliva A, Sakmar TP, Update on Alzheimer’s Disease Therapy and Prevention Strategies. Annu. Rev. Med 68, 413–430 (2017). [DOI] [PubMed] [Google Scholar]
- 3.Mani BK, Walker AK, Lopez Soto EJ, Raingo J, Lee CE, Perelló M, Andrews ZB, Zigman JM, Neuroanatomical characterization of a growth hormone secretagogue receptor-green fluorescent protein reporter mouse. J. Comp. Neurol 522, 3644–3666 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hsu TM, Hahn JD, Konanur VR, Noble EE, Suarez AN, Thai J, Nakamoto EM, Kanoski SE, Hippocampus ghrelin signaling mediates appetite through lateral hypothalamic orexin pathways. eLife 4, e11190 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mani BK, Osborne-Lawrence S, Mequinion M, Lawrence S, Gautron L, Andrews ZB, Zigman JM, The role of ghrelin-responsive mediobasal hypothalamic neurons in mediating feeding responses to fasting. Mol. Metab 6, 882–896 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kanoski SE, Fortin SM, Ricks KM, Grill HJ, Ghrelin signaling in the ventral hippocampus stimulates learned and motivational aspects of feeding via PI3K-Akt signaling. Biol. Psychiatry 73, 915–923 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hsu TM, Noble EE, Reiner DJ, Liu CM, Suarez AN, Konanur VR, Hayes MR, Kanoski SE, Hippocampus ghrelin receptor signaling promotes socially-mediated learned food preference. Neuropharmacology 131, 487–496 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kern A, Mavrikaki M, Ullrich C, Albarran-Zeckler R, Brantley AF, Smith RG, Hippocampal dopamine/DRD1 signaling dependent on the ghrelin receptor. Cell 163, 1176–1190 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kern A, Grande C, Smith RG, Apo-ghrelin receptor (apo-GHSR1a) regulates dopamine signaling in the brain. Front. Endocrinol 5, 129 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sevigny JJ, Ryan JM, van Dyck CH, Peng Y, Lines CR, Nessly ML, Growth hormone secretagogue MK-677: No clinical effect on AD progression in a randomized trial. Neurology 71, 1702–1708 (2008). [DOI] [PubMed] [Google Scholar]
- 11.Seminara RS, Jeet C, Biswas S, Kanwal B, Iftikhar W, Sakibuzzaman M, Rutkofsky IH, The neurocognitive effects of ghrelin-induced signaling on the hippocampus: A promising approach to Alzheimer’s disease. Cureus 10, e3285 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cao X, Zhu M, He Y, Chu W, Du Y, Du H, Increased serum acylated ghrelin levels in patients with mild cognitive impairment. J. Alzheimers Dis 61, 545–552 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Eimer WA, Vassar R, Neuron loss in the 5×FAD mouse model of Alzheimer’s disease correlates with intraneuronal Aβ42 accumulation and caspase-3 activation. Mol. Neurodegener 8, 2 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manczak M, Calkins MJ, Reddy PH, Impaired mitochondrial dynamics and abnormal interaction of amyloid beta with mitochondrial protein Drp1 in neurons from patients with Alzheimer’s disease: Implications for neuronal damage. Hum. Mol. Genet 20, 2495–2509 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beck SJ, Guo L, Phensy A, Tian J, Wang L, Tandon N, Gauba E, Lu L, Pascual JM, Kroener S, Du H, Deregulation of mitochondrial F1FO-ATP synthase via OSCP in Alzheimer’s disease. Nat. Commun 7, 11483 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sadowski M, Pankiewicz J, Scholtzova H, Ripellino JA, Li Y, Schmidt SD, Mathews PM, Fryer JD, Holtzman DM, Sigurdsson EM, Wisniewski T, A synthetic peptide blocking the apolipoprotein E/β-amyloid binding mitigates β-amyloid toxicity and fibril formation in vitro and reduces β-amyloid plaques in transgenic mice. Am. J. Pathol 165, 937–948 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lutz MI, Schwaiger C, Hochreiter B, Kovacs GG, Schmid JA, Novel approach for accurate tissue-based protein colocalization and proximity microscopy. Sci. Rep 7, 2668 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hoffmann C, Gaietta G, Bünemann M, Adams SR, Oberdorff-Maass S, Behr B, Vilardaga JP, Tsien RY, Ellisman MH, Lohse MJ, A FlAsH-based FRET approach to determine G protein-coupled receptor activation in living cells. Nat. Methods. 2, 171–176 (2005). [DOI] [PubMed] [Google Scholar]
- 19.Diano S, Farr SA, Benoit SC, McNay EC, da Silva I, Horvath B, Gaskin FS, Nonaka N, Jaeger LB, Banks WA, Morley JE, Pinto S, Sherwin RS, Xu L, Yamada KA, Sleeman MW, Tschop MH, Horvath TL, Ghrelin controls hippocampal spine synapse density and memory performance. Nat. Neurosci 9, 381–388 (2006). [DOI] [PubMed] [Google Scholar]
- 20.Zigman JM, Nakano Y, Coppari R, Balthasar N, Marcus JN, Lee CE, Jones JE, Deysher AE, Waxman AR, White RD, Williams TD, Lachey JL, Seeley RJ, Lowell BB, Elmquist JK, Mice lacking ghrelin receptors resist the development of diet-induced obesity. J. Clin. Invest 115, 3564–3572 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kerchner GA, Hess CP, Hammond-Rosenbluth KE, Xu D, Rabinovici GD, Kelley DAC, Vigneron DB, Nelson SJ, Miller BL, Hippocampal CA1 apical neuropil atrophy in mild Alzheimer disease visualized with 7-T MRI. Neurology 75, 1381–1387 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Huang B, Yang C-S, Wojton J, Huang N-J, Chen C, Soderblom EJ, Zhang L, Kornbluth S, Metabolic control of Ca2+/calmodulin-dependent protein kinase II (CaMKII)-mediated caspase-2 suppression by the B55β/protein phosphatase 2A (PP2A). J. Biol. Chem 289, 35882–35890 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Patchett AA, Nargund RP, Tata JR, Chen MH, Barakat KJ, Johnston DB, Cheng K, Chan WW, Butler B, Hickey G, Design and biological activities of L-163,191 (MK-0677): A potent, orally active growth hormone secretagogue. Proc. Natl. Acad. Sci. U.S.A 92, 7001–7005 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Paula-Lima AC, Brito-Moreira J, Ferreira ST, Deregulation of excitatory neurotransmission underlying synapse failure in Alzheimer’s disease. J. Neurochem 126, 191–202 (2013). [DOI] [PubMed] [Google Scholar]
- 25.Malkin SL, Kim KK, Tikhonov DB, Zaitsev AV, Properties of spontaneous and miniature excitatory postsynaptic currents of rat prefrontal cortex neurons. J. Evol. Biochem. Physiol 50, 506–514 (2014). [PubMed] [Google Scholar]
- 26.Wang L, Guo L, Lu L, Sun H, Shao M, Beck SJ, Li L, Ramachandran J, Du Y, Du H, Synaptosomal mitochondrial dysfunction in 5×FAD mouse model of Alzheimer’s disease. PLOS ONE 11, e0150441 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oakley H, Cole SL, Logan S, Maus E, Shao P, Craft J, Guillozet-Bongaarts A, Ohno M, Disterhoft J, Van Eldik L, Berry R, Vassar R, Intraneuronal β-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer’s disease mutations: Potential factors in amyloid plaque formation. J. Neurosci 26, 10129–10140 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lu L, Guo L, Gauba E, Tian J, Wang L, Tandon N, Shankar M, Beck SJ, Du Y, Du H, Transient cerebral ischemia promotes brain mitochondrial dysfunction and exacerbates cognitive impairments in young 5×FAD mice. PLOS ONE 10, e0144068 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zheng H, Bailey A, Jiang M-H, Honda K, Chen HY, Trumbauer ME, Van der Ploeg LHT, Schaeffer JM, Leng G, Smith RG, Somatostatin receptor subtype 2 knockout mice are refractory to growth hormone-negative feedback on arcuate neurons. Mol. Endocrinol 11, 1709–1717 (1997). [DOI] [PubMed] [Google Scholar]
- 30.Xu M, Koeltzow TE, Santiago GT, Moratalla R, Cooper DC, Hu XT, White NM, Graybiel AM, White FJ, Tonegawa S, Dopamine D3 receptor mutant mice exhibit increased behavioral sensitivity to concurrent stimulation of D1 and D2 receptors. Neuron 19, 837–848 (1997). [DOI] [PubMed] [Google Scholar]
- 31.Moon M, Cha M-Y, Mook-Jung I, Impaired hippocampal neurogenesis and its enhancement with ghrelin in 5×FAD mice. J. Alzheimers Dis 41, 233–241 (2014). [DOI] [PubMed] [Google Scholar]
- 32.Couillard-Despres S, Winner B, Schaubeck S, Aigner R, Vroemen M, Weidner N, Bogdahn U, Winkler J, Kuhn H-G, Aigner L, Doublecortin expression levels in adult brain reflect neurogenesis. Eur. J. Neurosci 21, 1–14 (2005). [DOI] [PubMed] [Google Scholar]
- 33.Menezes JR, Luskin MB, Expression of neuron-specific tubulin defines a novel population in the proliferative layers of the developing telencephalon. J. Neurosci 14, 5399–5416 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ribeiro LF, Catarino T, Santos SD, Benoist M, van Leeuwen JF, Esteban JA, Carvalho AL, Ghrelin triggers the synaptic incorporation of AMPA receptors in the hippocampus. Proc. Natl. Acad. Sci. U.S.A 111, E149–E158 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gahete MD, Rubio A, Córdoba-Chacón J, Gracia-Navarro F, Kineman RD, Avila J, Luque RM, Castaño JP, Expression of the ghrelin and neurotensin systems is altered in the temporal lobe of Alzheimer’s disease patients. J. Alzheimers Dis 22, 819–828 (2010). [DOI] [PubMed] [Google Scholar]
- 36.Navarro G, Aguinaga D, Angelats E, Medrano M, Moreno E, Mallol J, Cortes A, Canela EI, Casadó V, McCormick PJ, Lluis C, Ferre S, A significant role of the truncated ghrelin receptor GHS-R1b in ghrelin-induced signaling in neurons. J. Biol. Chem 291, 13048–13062 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gauthier SG, Alzheimer’s disease: The benefits of early treatment. Eur. J. Neurol 12 (Suppl. 3), 11–16 (2005). [DOI] [PubMed] [Google Scholar]
- 38.Boada-Rovira M, Brodaty H, Cras P, Baloyannis S, Emre M, Zhang R, Bahra R, Efficacy and safety of donepezil in patients with Alzheimer’s disease: Results of a global, multinational, clinical experience study. Drugs Aging 21, 43–53 (2004). [DOI] [PubMed] [Google Scholar]
- 39.Egan MF, Kost J, Voss T, Mukai Y, Aisen PS, Cummings JL, Tariot PN, Vellas B, van Dyck CH, Boada M, Zhang Y, Li W, Furtek C, Mahoney E, Harper Mozley L, Mo Y, Sur C, Michelson D, Randomized trial of Verubecestat for prodromal Alzheimer’s disease. N. Engl. J. Med 380, 1408–1420 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gauthier S, Feldman HH, Schneider LS, Wilcock GK, Frisoni GB, Hardlund JH, Moebius HJ, Bentham P, Kook KA, Wischik DJ, Schelter BO, Davis CS, Staff RT, Bracoud L, Shamsi K, Storey JM, Harrington CR, Wischik CM, Efficacy and safety of tau-aggregation inhibitor therapy in patients with mild or moderate Alzheimer’s disease: A randomised, controlled, double-blind, parallel-arm, phase 3 trial. Lancet 388, 2873–2884 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Honig LS, Vellas B, Woodward M, Boada M, Bullock R, Borrie M, Hager K, Andreasen N, Scarpini E, Liu-Seifert H, Case M, Dean RA, Hake A, Sundell K, Poole Hoffmann V, Carlson C, Khanna R, Mintun M, DeMattos R, Selzler KJ, Siemers E, Trial of solanezumab for mild dementia due to Alzheimer’s disease. N. Engl. J. Med 378, 321–330 (2018). [DOI] [PubMed] [Google Scholar]
- 42.Kunath N, van Groen T, Allison DB, Kumar A, Dozier-Sharpe M, Kadish I, Ghrelin agonist does not foster insulin resistance but improves cognition in an Alzheimer’s disease mouse model. Sci. Rep 5, 11452 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jeong Y.-o., Shin SJ, Park JY, Ku BK, Song JS, Kim J-J, Jeon SG, Lee SM, Moon M, MK-0677, a ghrelin agonist, alleviates amyloid beta-related pathology in 5×FAD Mice, an animal model of Alzheimer’s disease. Int. J. Mol. Sci 19, 1800 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Eslami M, Sadeghi B, Goshadrou F, Chronic ghrelin administration restores hippocampal long-term potentiation and ameliorates memory impairment in rat model of Alzheimer’s disease. Hippocampus 28, 724–734 (2018). [DOI] [PubMed] [Google Scholar]
- 45.Dhurandhar EJ, Allison DB, van Groen T, Kadish I, Hunger in the absence of caloric restriction improves cognition and attenuates Alzheimer’s disease pathology in a mouse model. PLOS ONE 8, e60437 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Santos VV, Stark R, Rial D, Silva HB, Bayliss JA, Lemus MB, Davies JS, Cunha RA, Prediger RD, Andrews ZB, Acyl ghrelin improves cognition, synaptic plasticity deficits and neuroinflammation following amyloid β (Aβ1–40) administration in mice. J. Neuroendocrinol 29, 1–11 (2017). [DOI] [PubMed] [Google Scholar]
- 47.LaFerla FM, Green KN, Animal models of Alzheimer disease. Cold Spring Harb. Perspect. Med 2, a006320 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gustafson D, Rothenberg E, Blennow K, Steen B, Skoog I, An 18-year follow-up of overweight and risk of Alzheimer disease. Arch. Intern. Med 163, 1524–1528 (2003). [DOI] [PubMed] [Google Scholar]
- 49.O’Carroll CM, Martin SJ, Sandin J, Frenguelli B, Morris RGM, Dopaminergic modulation of the persistence of one-trial hippocampus-dependent memory. Learn. Mem 13, 760–769 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lee MJ, Dohlman HG, Coactivation of G protein signaling by cell-surface receptors and an intracellular exchange factor. Curr. Biol 18, 211–215 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jin J, Kunapuli SP, Coactivation of two different G protein-coupled receptors is essential for ADP-induced platelet aggregation. Proc. Natl. Acad. Sci. U.S.A 95, 8070–8074 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Holmes C, Smith H, Ganderton R, Arranz M, Collier D, Powell J, Lovestone S, Psychosis and aggression in Alzheimer’s disease: The effect of dopamine receptor gene variation. J. Neurol. Neurosurg. Psychiatry 71, 777–779 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Braak H, Del Tredici K, The pathological process underlying Alzheimer’s disease in individuals under thirty. Acta Neuropathol. 121, 171–181 (2011). [DOI] [PubMed] [Google Scholar]
- 54.Takeuchi T, Duszkiewicz AJ, Sonneborn A, Spooner PA, Yamasaki M, Watanabe M, Smith CC, Fernández G, Deisseroth K, Greene RW, Morris RGM, Locus coeruleus and dopaminergic consolidation of everyday memory. Nature 537, 357–362 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Crispo JAG, Fortin Y, Thibault DP, Emons M, Bjerre LM, Kohen DE, Perez-Lloret S, Mattison D, Willis AW, Krewski D, Trends in inpatient antiparkinson drug use in the USA, 2001–2012. Eur. J. Clin. Pharmacol 71, 1011–1019 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hornsby AKE, Redhead YT, Rees DJ, Ratcliff MS, Reichenbach A, Wells T, Francis L, Amstalden K, Andrews ZB, Davies JS, Short-term calorie restriction enhances adult hippocampal neurogenesis and remote fear memory in a Ghsr-dependent manner. Psychoneuroendocrinology 63, 198–207 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Martinez-Canabal A, Reconsidering hippocampal neurogenesis in Alzheimer’s disease. Front. Neurosci 8, 147 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Forner S, Baglietto-Vargas D, Martini AC, Trujillo-Estrada L, LaFerla FM, Synaptic impairment in Alzheimer’s Disease: A dysregulated symphony. Trends Neurosci. 40, 347–357 (2017). [DOI] [PubMed] [Google Scholar]
- 59.Kim JJ, Diamond DM, The stressed hippocampus, synaptic plasticity and lost memories. Nat. Rev. Neurosci 3, 453–462 (2002). [DOI] [PubMed] [Google Scholar]
- 60.Dietrich MO, Horvath TL, Hypothalamic control of energy balance: Insights into the role of synaptic plasticity. Trends Neurosci. 36, 65–73 (2013). [DOI] [PubMed] [Google Scholar]
- 61.Baloyannis SJ, Mavroudis I, Mitilineos D, Baloyannis IS, Costa VG, The hypothalamus in Alzheimer’s disease: A Golgi and electron microscope study. Am. J. Alzheimers Dis. Other Demen 30, 478–487 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Yin Y, Li Y, Zhang W, The growth hormone secretagogue receptor: Its intracellular signaling and regulation. Int. J. Mol. Sci 15, 4837–4855 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Alzheimer’s Association Calcium Hypothesis Workgroup, Calcium hypothesis of Alzheimer’s disease and brain aging: A framework for integrating new evidence into a comprehensive theory of pathogenesis. Alzheimers Dement. 13, 178–182.e17 (2017). [DOI] [PubMed] [Google Scholar]
- 64.Uhlen M, Bandrowski A, Carr S, Edwards A, Ellenberg J, Lundberg E, Rimm DL, Rodriguez H, Hiltke T, Snyder M, Yamamoto T, A proposal for validation of antibodies. Nat. Methods 13, 823–827 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gomes I, Sierra S, Devi LA, Detection of Receptor Heteromerization Using In Situ Proximity Ligation Assay. Curr. Protoc. Pharmacol 75, 2.16.1–2.16.31 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Muczynski V, Bazaa A, Loubière C, Harel A, Cherel G, Denis CV, Lenting PJ, Christophe OD, Macrophage receptor SR-AI is crucial to maintain normal plasma levels of coagulation factor X. Blood 127, 778–786 (2016). [DOI] [PubMed] [Google Scholar]
- 67.Wang Q, Tian J, Chen H, Du H, Guo L, Amyloid beta-mediated KIF5A deficiency disrupts anterograde axonal mitochondrial movement. Neurobiol. Dis 127, 410–418 (2019). [DOI] [PubMed] [Google Scholar]
- 68.Vilardaga J-P, Nikolaev VO, Lorenz K, Ferrandon S, Zhuang Z, Lohse MJ, Conformational cross-talk between α2A-adrenergic and μ-opioid receptors controls cell signaling. Nat. Chem. Biol 4, 126–131 (2008). [DOI] [PubMed] [Google Scholar]
- 69.Morris RGM, Morris water maze. Scholarpedia 3, 6315 (2008). [Google Scholar]
- 70.Lehner I, Niehof M, Borlak J, An optimized method for the isolation and identification of membrane proteins. Electrophoresis 24, 1795–1808 (2003). [DOI] [PubMed] [Google Scholar]
- 71.Kato AS, Zhou W, Milstein AD, Knierman MD, Siuda ER, Dotzlaf JE, Yu H, Hale JE, Nisenbaum ES, Nicoll RA, Bredt DS, New transmembrane AMPA receptor regulatory protein isoform, γ-7, differentially regulates AMPA receptors. J. Neurosci 27, 4969–4977 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Wilcock DM, Gordon MN, Morgan D, Quantification of cerebral amyloid angiopathy and parenchymal amyloid plaques with Congo red histochemical stain. Nat. Protoc 1, 1591–1595 (2006). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Expression of GHSR1α increased in hippocampi from subjects with AD and 9-month-old 5×FAD mice.
Fig. S2. Expressions of Ghsr1α and Drd1 were validated in transfected HEK 293T cells.
Fig. S3. Schematic diagram shows the sequence of full-length Ghsr1α and its truncating mutants.
Fig. S4. FLAG-tagged Ghsr1α and its truncating mutants were similarly expressed in HEK 293T cells.
Fig. S5. The interaction between Ghsr1α mutants and Aβ42 was assessed by using Co-IP.
Fig. S6. Schematic diagram represents FlAsH-FRET assay for Ghsr1α activity and the impact of oligomeric Aβ42 on Ghsr1α activation.
Fig. S7. GHSR1α/DRD1 complex density was negatively correlated with hippocampal soluble Aβ40 or Aβ42 amounts in subjects with AD.
Fig. S8. Expression of hippocampal DRD1 remained unaltered in hippocampi from subjects with AD and 5×FAD mice.
Fig. S9. Oligomeric Aβ42 did not affect agonist-induced activation of Drd1 or form complex with Drd1.
Fig. S10. Input/output curves of fEPSPs were similar in four types of transgenic mice.
Fig. S11. Aβ deposition in the hippocampus remained unchanged in Ghsr null/5×FAD mice as compared with their 5×FAD littermates.
Fig. S12. Serum ghrelin amounts were similar in four types of transgenic mice.
Fig. S13. Loss of Ghsr1α suppressed postsynaptic CaMKII activation in the hippocampus.
Fig. S14. The optimal doses of MK0677 and SKF81297 were determined by their augmenting effect on synaptogenesis in cultured hippocampal neurons.
Fig. S15. Time course of LTP and fEPSP amplitudes was not changed by different treatments on hippocampal slices from nonTg mice.
Fig. S16. The doses of MK0677/SKF81297 treatment were optimized on the basis of the influence on body weight, serum ghrelin, and behavioral performance.
Fig. S17. MK0677/SKF81297 [MK0677 (1 mg/kg) and SKF81297 (1.5 mg/kg)] treatment on mice had no effect on hepatic, renal, and hippocampal cell density.
Fig. S18. MK0677/SKF81297 [MK0677 (1 mg/kg) and SKF81297 (1.5 mg/kg)] treatment improved neurogenesis in the dentate gyrus of 5×FAD mice.
Table S1. Human brain tissue information.
Table S2. Raw data (provided as separate Excel file).