Xylan biosynthetic proteins and products localize to distinct structural and functional domains in the abundant Golgi stacks during secondary cell wall production.
Abstract
Secondary cell wall (SCW) production during xylem development requires massive up-regulation of hemicellulose (e.g. glucuronoxylan) biosynthesis in the Golgi. Although mutant studies have revealed much of the xylan biosynthetic machinery, the precise arrangement of these proteins and their products in the Golgi apparatus is largely unknown. We used a fluorescently tagged xylan backbone biosynthetic protein (IRREGULAR XYLEM9; IRX9) as a marker of xylan production in the Golgi of developing protoxylem tracheary elements in Arabidopsis (Arabidopsis thaliana). Both live-cell confocal and transmission electron microscopy (TEM) revealed SCW deposition is accompanied by a significant proliferation of Golgi stacks. Furthermore, although Golgi stacks were randomly distributed, the organization of the cytoplasm ensured their close proximity to developing SCWs. Quantitative immuno-TEM revealed IRX9 is present in a specific subdomain of the Golgi stack and was most abundant in the ring of the inner margins of medial cisternae where fenestrations are abundant. Conversely, the xylan product accumulated in swollen trans cisternal margins and the Trans-Golgi network (TGN). The irx9 mutant lacked this expansion for both the cisternal margins and the TGN, whereas Golgi stack proliferation was unaffected. Golgi in irx9 also displayed dramatic changes in their structure, with increases in cisternal fenestration and tubulation. Our data support a new model where xylan biosynthesis and packaging into secretory vesicles are localized in distinct structural and functional domains of the Golgi. Rather than polysaccharide biosynthesis occurring in the center of the cisternae, IRX9 and the xylan product are arranged in successive concentric rings in Golgi cisternae.
The successful colonization of land by plants has been ascribed in part to the development of secondary cell walls (SCWs), which reinforce water-conducting vessel elements and supportive fibers. Branched polysaccharides, termed hemicelluloses, are essential chemical components of SCWs that can account for as much as 30% to 50% of the wall by weight (Scheller and Ulvskov, 2010). In eudicots, the major SCW hemicellulose is a xylan characterized by a Xyl backbone decorated with side chains of GlcA and methyl and acetyl substitutions (Ebringerová, 2005). Xylan is synthesized in the Golgi by a suite of glycosyltransferases working in concert with other transferases as well as substrate biosynthetic proteins and transporters. Among these proteins, IRREGULAR XYLEM 9 (IRX9) is a type-II transmembrane protein proposed to form part of a xylan backbone biosynthetic complex (Rennie and Scheller, 2014). Loss of IRX9 function results in reduced growth, collapsed xylem vessels, and reduced xylan content and xylosyltransferase activity, collectively supporting a role for IRX9 in SCW xylan biosynthesis (Lee et al., 2007; Lee et al., 2010; Wu et al., 2010). The proposed xylan backbone biosynthetic complex includes not only IRX9, but also IRX9-LIKE (IRX9L; Lee et al., 2010; Wu et al., 2010), as well as IRX10/IRX10L (Brown et al., 2009; Wu et al., 2009), and IRX14/IRX14L (Keppler and Showalter, 2010). The xylan backbone precursor, UDP-Xyl, must be transported into the Golgi by UDP-Xyl Transporters (UXTs), primarily UXT1 (Ebert et al., 2015). In addition, the xylan backbone is decorated with GlcA by xylan glucuronosyltransferases of the GUX family (Mortimer et al., 2010; Rennie et al., 2012), whereas methylation of the GlcA residues is catalyzed by glucuronoxylan methyltransferases (Lee et al., 2012; Urbanowicz et al., 2012). Xylan is acetylated primarily by the protein ESK1TBL29 (Xiong et al., 2013; Yuan et al., 2013; Urbanowicz et al., 2014), although other Trichome Birefringence-Like (TBL) proteins also acetylate xylan at different positions and with various affinities (Yuan et al., 2016a, 2016b; Zhong et al., 2017a). Despite the identification of each of these proteins, little is known about how they are organized in the Golgi and how they work together to synthesize the xylan that is ultimately deposited in the SCW.
The Golgi apparatus is a central component of the endomembrane system involved in protein processing and trafficking in addition to synthesis of cell wall polysaccharides like xylan. During primary cell wall (PCW) production, increased demand for Golgi products is met, in part, by a proliferation in the number of Golgi stacks making up the Golgi apparatus (Garcia-Herdugo et al., 1988; Seguí-Simarro and Staehelin, 2006; Young et al., 2008; Toyooka et al., 2014). Whether the onset of xylan synthesis during SCW development results in similar increases in Golgi number is unknown. Furthermore, during SCW synthesis, secretion of xylan from the Golgi must be targeted to specific plasma membrane domains to produce the patterned SCWs characteristic of cells like protoxylem tracheary elements (Chou et al., 2018). This targeting may be facilitated by a close association between Golgi and the forming SCWs, as suggested by live-cell imaging of Golgi containing fluorescently tagged SCW CELLULOSE SYNTHASES (CESAs; Schneider et al., 2017). However, it is unclear if the proximity of Golgi to SCWs observed in these experiments is due to preferential targeting or random chance.
Each individual Golgi body is composed of a stack of flattened membrane-bound cisternae arranged in a polar fashion from cis to trans. Proteins enter the Golgi at the cis-most cisterna via the endoplasmic reticulum (ER; Brandizzi and Barlowe, 2013), and Golgi products typically exit at the trans cisterna and associated Trans Golgi Network (TGN; Kang et al., 2011; Uemura, 2016; Wang et al., 2017). Golgi-resident proteins that carry out polysaccharide synthesis and modification are maintained in Golgi cisternae, whereas their products transit through the Golgi stack. The cisternal maturation model posits that cisternae change over time from cis to medial and then trans cisternae (reviewed by Glick and Luini, 2011). This model is supported by experiments in yeast (Saccharomyces cerevisiae) showing the population of resident proteins in a cisterna changes as cis-type residents are replaced by more trans-type residents via retrograde trafficking from later to earlier cisternae (Losev et al., 2006; Matsuura-Tokita et al., 2006). In mammalian cells, COAT PROTEIN I (COPI)-coated vesicles are important for this intra-Golgi retrograde transport of Golgi resident proteins, and COPI tubules can mediate transport in both the anterograde and retrograde directions (Yang et al., 2011; Park et al., 2015). Although COPI-coated tubules have not yet been reported in plant Golgi, intra-Golgi COPI vesicles have been identified (Donohoe et al., 2007), and they have been hypothesized to play a role in glycosyltransferase recycling (Donohoe et al., 2007; Kang et al., 2011). This is supported by studies showing that disruption of COPI vesicle machinery using the inhibitor brefeldin A or mutations leads to mislocalization of a heterologously expressed Golgi resident protein (Nebenführ et al., 2002; Naramoto et al., 2014). In the context of xylan biosynthesis, cisternal maturation implies xylan moves through the Golgi with the maturation of the cisternae, whereas the xylan biosynthetic enzymes are actively recycled to previous cisternae to maintain their position in the Golgi. Because all reports of xylan biosynthetic proteins demonstrate strict Golgi localization, these proteins must also be sequestered from the xylan product no later than the trans most cisternae, where this final flattened cisterna matures into TGN/secretory vesicle clusters (Kang et al., 2011; Toyooka et al., 2014), although the mechanism by which this occurs has not been resolved. In addition to the cis-trans polarity of Golgi stacks, each cisterna also varies in structure and composition between the flat center of the cisternae and their swollen outer margins with associated vesicles and fenestrations (Mogelsvang et al., 2003; Mogelsvang et al., 2004; Koga and Ushiki, 2006; Kang and Staehelin, 2008; Kang et al., 2011; Donohoe et al., 2013). Freeze-fracture images through the center of Golgi cisternae in root cells reveals arrays of integral membrane proteins proposed to be glycosyltransferases producing cell wall polysaccharides (Staehelin et al., 1990). However, the hypothesis that the flattened center of the Golgi is the site of cell wall biosynthesis has not been directly tested. The goal of this study was to examine both the cis-to-trans and the center-to-margins distribution of a xylan biosynthetic protein (IRX9) at its native expression level during SCW synthesis.
Direct localization of Golgi-resident proteins like glycosyltransferases is difficult because their abundance is often too low to detect (Fukuda et al., 1996). The few studies localizing glycosyltransferases within the Golgi used overexpression of genes in heterologous systems (Chevalier et al., 2010), which may not reflect the localization found with native expression levels. The production of large amounts of glucuronoxylan during SCW biosynthesis therefore provides an excellent opportunity to study the cell wall production machinery in the Golgi. SCW biosynthesis can be experimentally triggered in an Arabidopsis (Arabidopsis thaliana) model system where an inducible version of the master transcription factor VASCULAR RELATED NAC-DOMAIN7 (VND7) initiates transdifferentiation of cells throughout the plant into protoxylem tracheary elements with thick helical SCWs (Kubo et al., 2005; Yamaguchi et al., 2010a). Furthermore, during SCW synthesis, IRX9 gene expression naturally increases over 4-fold, and production of large amounts of glucuronoxylan in the Golgi is triggered (Yamaguchi et al., 2010b; Yamaguchi et al., 2010a).
To locate IRX9-containing Golgi, we generated a functional, fluorescently tagged version of IRX9 driven by its native promoter, and introduced this into the VND7-induction system. We then used a combination of confocal and transmission electron microscopy (TEM) to show that the number of Golgi stacks increases significantly with the onset of SCW production. Within individual Golgi stacks, IRX9 and its polysaccharide product were localized to concentric circles within medial and trans Golgi cisternae, respectively. The greatest amount of IRX9 was in the fenestrated region of the cisternae, whereas the xylan product correlated with swollen cisternal edges and the TGN. The localization of a required SCW biosynthetic protein, and its product, in successive concentric rings in the Golgi cisternae generates new hypotheses about the mechanisms driving Golgi protein recycling and xylan product packaging in the maturing, dynamic, and structurally complex Golgi cisternae.
RESULTS
Live-Cell Imaging of Functional Fluorescently Tagged IRX9 Driven by the Native Promoter
To test if a fluorescently tagged IRX9 is still functional, a C-terminal fusion of IRX9-GFP was introduced into the irx9-2 T-DNA insertional mutant line (Lee et al., 2010; Wu et al., 2010). The dwarf growth phenotype of the mutant was restored to wild-type levels when irx9 was transformed with proIRX9:IRX9-GFP (Supplemental Fig. S1A). Wild-type vascular bundles have large, open tracheary elements in toluidine blue-stained stem cross-sections (Supplemental Fig. S1B), which are frequently collapsed in irx9 (Supplemental Fig. S1C). Restoration of open vessel elements was apparent in the complemented lines (Supplemental Fig. S1D). Complementation of the mutant phenotypes indicates the GFP-tagged IRX9 was functional.
In live-cell imaging of seedling roots from plants containing proIRX9:IRX9-GFP, GFP signal was detected in the paired cell files of the developing root protoxylem tracheary elements (Supplemental Fig. S1E). Although the native promoter drove expression in the appropriate cell type, imaging the tagged proteins in these cells demonstrated the typical low resolution associated with imaging native tracheary elements deep in an organ. Furthermore, the rapid programmed cell death of protoxylem tracheary elements meant only a few cells per root could be imaged. To overcome these inherent limitations, the VND7-VP16-GR experimental system was adopted.
Plants carrying the proIRX9:IRX9-GFP in both the wild-type and irx9-2 backgrounds were transformed with a proUBQ10-driven version of VND7-VP16-GR. Upon induction of SCW synthesis in this complemented line, IRX9-GFP localized to numerous punctae which streamed through the cytoplasm, consistent with Golgi localization (Supplemental Fig. S1F). Similar results were obtained when proIRX9:IRX9-GFP was introduced into a 35S:VND7-VP16-GR line in a wild-type background in which a very large number of cells undergo transdifferentiation. To control for the effect of IRX9 copy number, the subcellular localization of IRX9-GFP was compared with the irx9-complemented line irx9/proIRX9:IRX9-GFP/proUBQ10:VND7-VP16-GR, and no obvious differences in IRX9-GFP localization were apparent (Supplemental Fig. S1G). This suggests the presence of wild-type IRX9 did not alter the localization of IRX9-GFP. Introduction of a functional IRX9-GFP into a system with inducible transdifferentiation of cells into protoxylem tracheary elements permitted investigation of IRX9-GFP in the Golgi apparatus as a whole and within each Golgi stack.
The Number of Golgi Stacks Increases with Onset of SCW Deposition
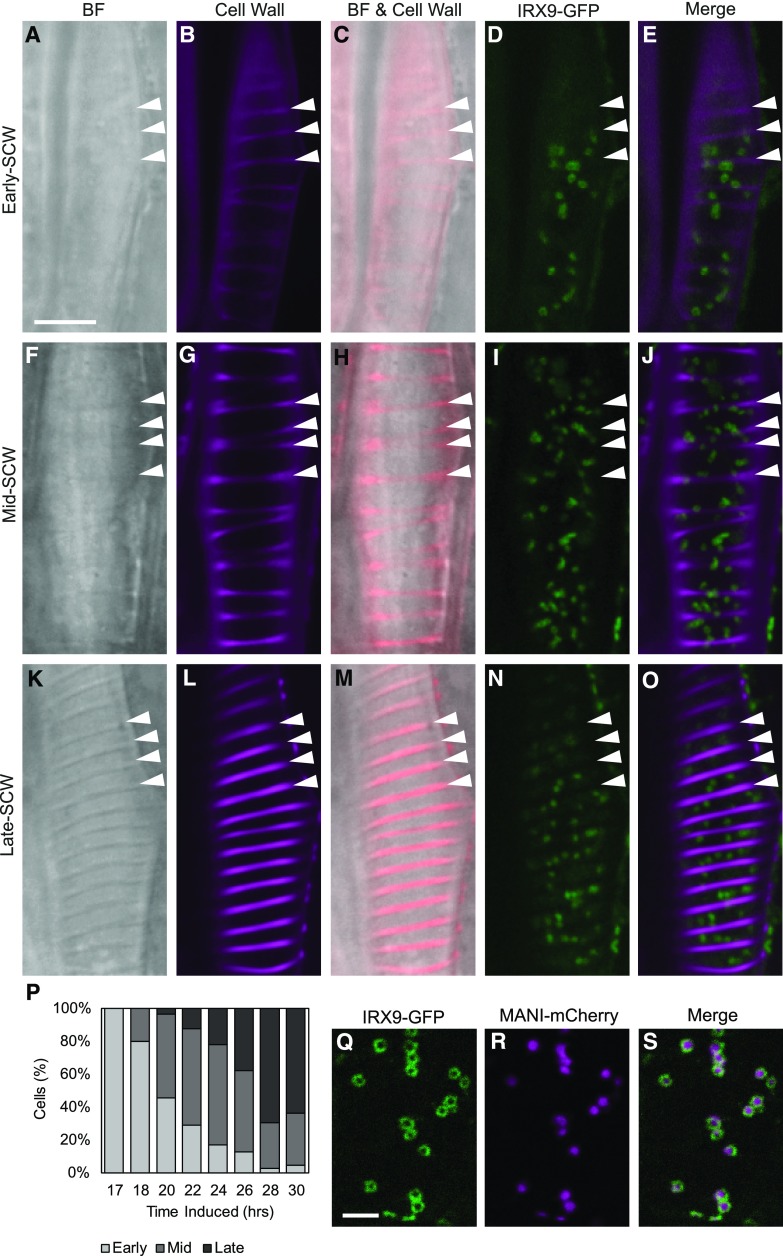
To test the hypothesis that Golgi stack proliferation coincides with the surge of glucuronoxylan biosynthesis during SCW production, it was necessary to compare Golgi numbers at well-defined xylem developmental stages. As timing of transdifferentiation between cells and seedlings can be variable, the timing of xylem differentiation in each cell was characterized using brightfield and confocal microscopy of hypocotyls from 4- to 7-d-old seedlings, 17 to 30 h after induction of VND7. The presence of IRX9-GFP signal and the prominence of SCW bands in brightfield and after propidium iodide staining were used to define the stage of SCW deposition. Cells in the early-SCW stage (Fig. 1, A–E) had no visible SCW in brightfield (Fig. 1A), although faint SCW bands were sometimes seen with propidium iodide staining (Fig. 1, B and C), and IRX9-GFP expression was detectable (Fig. 1, D and E). The mid-SCW stage was characterized by the first appearance of SCW in brightfield, increasing propidium iodide stain (Fig. 1, F–H), and strong IRX9-GFP signal in punctae (Fig. 1, I and J). By the late-SCW stage the SCW was conspicuous in brightfield, stained intensely with propidium iodide, and IRX9-GFP signal in punctae was prominent (Fig. 1, K–O). According to these definitions, the early-SCW stage was most abundant 17–20 h following VND7 induction, whereas the mid-SCW stage was more prevalent at 20–26 h, and the late-SCW stage at 26–30 h (Fig. 1P). This characterization of early-, mid-, and late-SCW stages during transdifferentiation of protoxylem tracheary elements identified 17–30 h as the period of maximum SCW deposition, and this was then used to guide subsequent experiments.
Figure 1.
IRX9-GFP localizes to the Golgi throughout SCW deposition. A to O, IRX9-GFP and SCW localization at the early-SCW, mid-SCW, and late-SCW stages. SCW bands are visible in brightfield (BF) in mid-SCW and late-SCW stages, and are sometimes also apparent when early-SCW cells are stained with propidium iodide (Cell Wall). IRX9-GFP is found in Golgi-like punctae in all stages. Cell wall images were acquired using different sensitivities. Images are representative single optical sections through the cell cortex. Arrowheads = SCW bands. Scale bar = 10 µm. P, The stage of SCW development varies among populations of transdifferentiating cells. The percent of IRX9-GFP expressing cells assigned to the early, mid, or late stages of SCW-deposition at each time point following treatment with DEX. Included per induction time point were 60–204 cells, across 4 replicate experiments. Q to S, IRX9-GFP and MANI-mCherry colocalize in the Golgi apparatus. Spinning disk confocal colocalization of the xylan biosynthetic IRX9-GFP (Q) and the Golgi-marker MANI-mCherry (R). The co-occurrence of the proteins in every Golgi stack is apparent in the merged image (S). Distinct distributions within the Golgi are visible as ring-shaped IRX9-GFP and solid MANI-mCherry signals. Scale bar = 5 µm.
Within each Golgi stack, the IRX9-GFP signal displayed a ring-shaped localization (Fig. 1Q). To verify the ring-shaped fluorescent bodies observed in confocal microscopy of IRX9-GFP represent Golgi bodies, a well-characterized Golgi marker, proUBQ10:MANI-mCherry (Nebenführ et al., 1999) was introduced, and colocalization with IRX9-GFP was assessed. In every cell expressing IRX9-GFP and MANI-mCherry, both markers were present in all structures (Fig. 1, Q–S). When the Mander’s colocalization coefficient (Manders et al., 1993) was averaged for 26 cells from 5 seedlings, 73.3% of IRX9-GFP signal overlapped with MANI-mCherry signal, confirming the Golgi localization of IRX9-GFP. Interestingly, the MANI-mCherry signal was distributed throughout the center of Golgi stacks (Fig. 1R) where IRX9-GFP signal was absent (Fig. 1S) resulting in only 39.6% of MANI-mCherry signal overlapping with IRX9-GFP, and a Pearson correlation coefficient of 0.322. Despite these differences in distribution of MANI-mCherry and IRX9-GFP within individual Golgi bodies, they were always found together, suggesting all stacks in the Golgi apparatus were participating in xylan production.
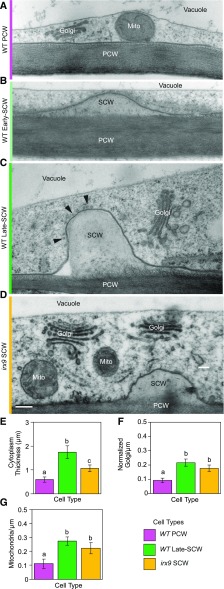
Confocal imaging of Golgi bodies does not provide the context of the surrounding unlabeled cytoplasm; therefore, the same stages of SCW deposition were examined in developing tracheary elements using TEM (Fig. 2, A–C). The time following VND7 induction was selected based on the predominant stage of SCW development according to the live cell imaging (Fig. 1P) to enrich for cells at the early-SCW (17–20 h) or late-SCW (22–30 h) stages. Seedlings were high-pressure-frozen/freeze-substituted and embedded in Spurr’s resin for morphological characterization. Plants lacking the VND7-induction construct, and thus producing only PCWs, were imaged as a control. These PCW-producing cells were dominated by a large central vacuole (Fig. 2A). After SCW deposition was triggered, small SCW intrusions (more electron-lucent than the PCW) were visible at repeating intervals along the cell periphery at the early-SCW stage (Fig. 2B). By the late-SCW stage, the SCWs protruded into the cell (Fig. 2C) and bundles of microtubules were visible lining the plasma membrane at SCW-domains (Fig. 2C, arrowheads). To understand how xylan production contributes to the cellular changes occurring with the onset of SCW synthesis, irx9-2 mutant lines transformed with proUBQ10:VND7-VP16-GR were similarly prepared for TEM imaging. This mutant produces significantly less xylan than wild type (Brown et al., 2007; Lee et al., 2010; Wu et al., 2010). Transdifferentiating irx9-2 cells continued to produce patterned SCW thickenings of approximately the same width, but the SCW did not extend into the cell to the same extent as the wild-type late-SCW cells (Fig. 2, C and D). Integrating this characterization of SCW deposition in TEM with the stages of development outlined using confocal microscopy provides a multiscale platform, which was used to further examine the Golgi during SCW biosynthesis.
Figure 2.
Golgi abundance increases with SCW deposition in wild-type and irx9 cells. A to D, TEM images of cell cortex in wild-type cells before deposition (A; wild-type [WT] PCW), early in SCW deposition (B; wild-type Early-SCW), late in SCW deposition (C; wild-type Late-SCW), and in irx9 during SCW deposition (D; irx9 SCW). Representative images are from 2 to 3 replicate high-pressure freezing experiments. Mito, mitochondrion; arrowheads = microtubules lining SCW domain. Scale bar = 200 nm. E to G, Quantification of cellular features observed in TEM revealed increased thickness of cytoplasm (E), and Golgi (F) and mitochondrial abundance per cell perimeter (G) in cross-sections during SCW synthesis. Golgi abundance has been normalized by average cisternal diameter in each type of cell. Means ± 95% CI. Different letters (a to c) in (E to G) indicate statistically significant differences. Statistics = one-way ANOVAs with Tukey HSD post hoc tests (P < 0.05); wild-type PCW and wild-type late-SCW (n = 31 cells, 4 to 5 seedlings, 4 high-pressure freezing experiments); irx9 SCW (n = 17 cells, 7 seedlings, 2 high-pressure freezing experiments).
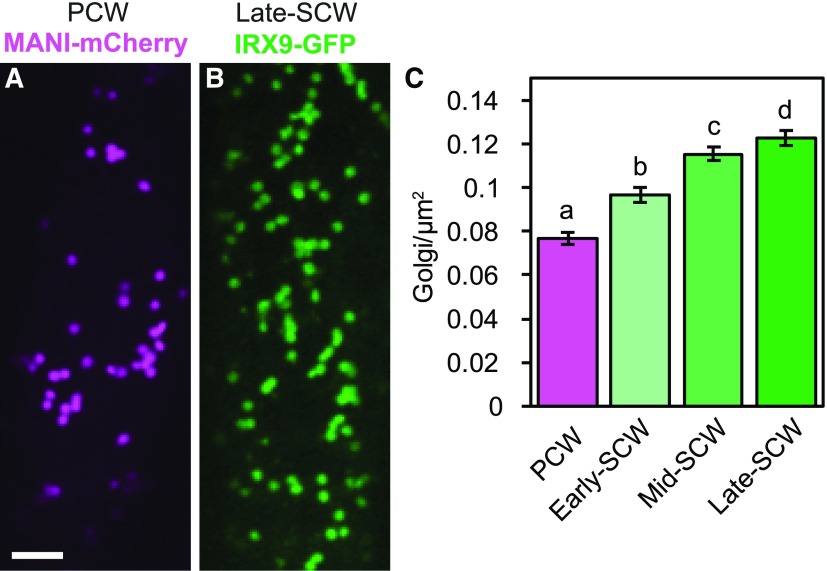
Given the rapid deposition of SCWs over a 12-h period (Fig. 1P), the abundance of Golgi stacks was quantified to determine if this shift in demand for Golgi products correlated with an increase in the number of Golgi stacks in the cell. The number of Golgi stacks was quantified in a TEM survey of the cortical cytoplasm surrounding the large central vacuole before SCW deposition (wild-type PCW), and after SCW deposition had commenced (wild-type SCW; Fig. 2, A and C). The number of mitochondria was also examined for comparison purposes. The number of Golgi in cells producing SCW increased compared with cells producing only PCW (Supplemental Fig. S2). However, cells producing SCWs had significantly thicker cortical cytoplasm from tonoplast to plasma membrane (Fig. 2E). To isolate the change in organelle abundance from this increase in cortical cytoplasm, Golgi and mitochondrial abundance was calculated per cell perimeter (counts/micron), rather than per cytoplasmic area (counts/micron squared; Supplemental Fig. S2). Golgi counts were also adjusted for differences in Golgi width to correct for overestimation of Golgi abundance in SCW-producing cells due to more frequent detection of larger Golgi in thin sections (Supplemental Fig. S2). With use of this method, the number of Golgi per cell perimeter was found to increase significantly in wild-type SCW cells (Fig. 2F). Interestingly, the number of mitochondria per cell perimeter also increased significantly with SCW production (Fig. 2G). To test if xylan production contributes to the increased Golgi abundance during SCW deposition, these analyses were repeated in the irx9 mutant background. Golgi and mitochondrial abundances in irx9 SCW cells were not different than those in wild-type SCW cells (Fig. 2, F and G), suggesting Golgi and mitochondrial proliferation during SCW synthesis is independent of xylan biosynthesis. The increase in Golgi abundance was also quantified using confocal imaging of the Golgi marker MANI-mCherry and IRX9-GFP during PCW and SCW production, respectively (Fig. 3, A and B). Dividing cells into early-, mid-, and late-SCW stages revealed a significant, consistent, and gradual increase in Golgi abundance in the cortical cytoplasm over the course of SCW deposition (Fig. 3C). This significant increase in Golgi density with the onset of SCW synthesis was also observed in plants in the irx9 mutant background with IRX9-GFP complementation (Supplemental Fig. S3). Together, the TEM and confocal quantification of the Golgi indicate the absolute number of Golgi stacks increased during SCW production, and this was not influenced by loss of IRX9-dependent production of xylan in the Golgi.
Figure 3.
IRX9-GFP-labeled Golgi increase in density as SCW deposition progresses. A and B, Golgi in the cell cortex in a PCW-producing cell with the Golgi marker proUBQ10:MANI-mCherry (A) or a late-SCW cell expressing proIRX9:IRX9-GFP (B). Scale bar = 5 μm. C, Quantification of Golgi density in the cell cortex before SCW deposition (PCW) and at early, mid, and late stages of SCW deposition. Means ± 95% CI. Different letters (a to d) indicate statistically significant differences. Statistics = one-way ANOVA and Tukey HSD post hoc test (P < 0.05). For each stage n = 223–342 cells from 31 to 78 plants in 4–6 replicate confocal experiments.
Cytoplasmic Geometry Constrains Randomly Positioned Golgi Stacks to Regions near SCW Domains
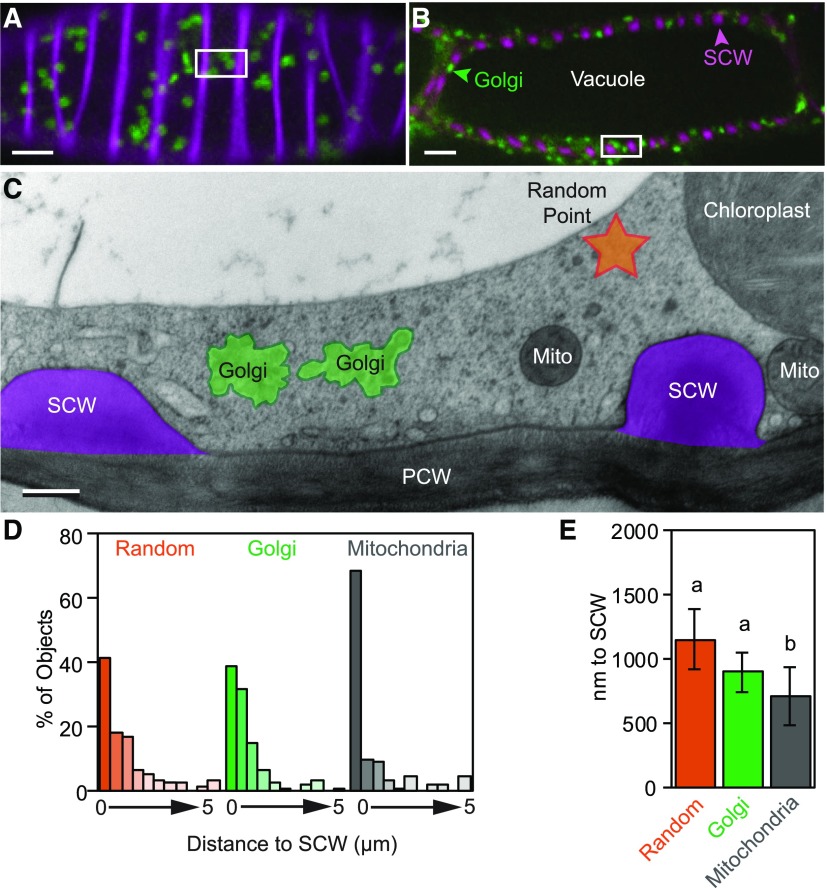
In live-cell imaging of Golgi labeled with a YFP-tagged SCW CESA7, Golgi have been observed to pause at SCW-domains (Watanabe et al., 2015), and 85% of Golgi were found ‘underneath’ SCW bands (Schneider et al., 2017). These results suggest Golgi may preferentially associate with SCWs, perhaps to facilitate targeting of secretion to these domains. Confocal imaging of IRX9-GFP in optical sections through the cell cortex (Fig. 4A) or through the cell center (Fig. 4B) illustrated how the large central vacuole restricts the Golgi to the cell periphery. Cross-sections through the cell using TEM (Fig. 4C) revealed a very thin layer of cortical cytoplasm that accommodated mitochondria, chloroplasts, ER bodies, the nucleus, and other organelles, in addition to Golgi. To test the hypothesis that Golgi preferentially associate with SCWs, the distance between Golgi stacks and the nearest SCW was measured (Fig. 4C). These values were then compared with the predicted results of a random distribution of Golgi using random points in the available cytoplasm. The distance between mitochondria and the SCW was also analyzed for comparison, as mitochondria are a similar size to Golgi in these cells. The majority of Golgi and random points were found within 1 µm of a SCW (Fig. 4D). A similar percentage of Golgi (38.6%) and random points (41.7%) were found within 500 nm of a SCW, whereas a larger proportion of mitochondria (68.4%) were found within this distance (Fig. 4D). As distance from the SCW increased, the abundance of all three dropped exponentially. The average distance of Golgi stacks to the SCW was not significantly different from that of random points in the cytoplasm (Fig. 4E). This is in contrast with the distribution of mitochondria, which are found significantly nearer to SCWs than both random points and Golgi stacks (Fig. 4E). This confirms this analysis is capable of detecting whether organelles are significantly closer to SCWs than random. As such, the random distribution of Golgi during SCW synthesis indicates most of the free cytoplasmic space is near SCWs in these cells; therefore, Golgi are naturally more abundant in these regions. Thus, although Golgi are found in close proximity to SCWs, they do not appear to preferentially associate with the plasma membrane lining domains of SCW secretion, unlike mitochondria.
Figure 4.
Cytoplasmic geometry constrains randomly distributed Golgi stacks to regions near SCWs. A and B, Optical sections through the cell cortex (A) and more endoplasmic regions (B) in cells expressing IRX9-GFP (green) and with SCWs stained with propidium iodide (magenta). The dark center of the cell in (B) is dominated by the vacuole, which is unlabeled. White boxes give an approximation of the size of the field of view in (C). Scale bars = 5 µm. C, TEM cross-section of cortical cytoplasm showing the position of Golgi stacks (green), mitochondria (Mito), and a random point (orange) relative to the SCW (magenta). Scale bar = 500 nm. D, Histogram showing the distance to the SCW in TEM images of random points, Golgi, and mitochondria using 500 nm bins. E, Average distance to SCW from random points, Golgi, or mitochondria. Means ± 95% CI. Different letters (a to b) indicate statistically significant differences. Statistics = one-way ANOVA with Tukey HSD post hoc (P < 0.05) for square-root transformed distances; n = 168 (Random, Golgi) and 114 (Mitochondria) from 16 cells, 5 seedlings, and 3 high-pressure freezing experiments.
Golgi Become Wider, and Cisternal Margins Expand, during SCW Synthesis
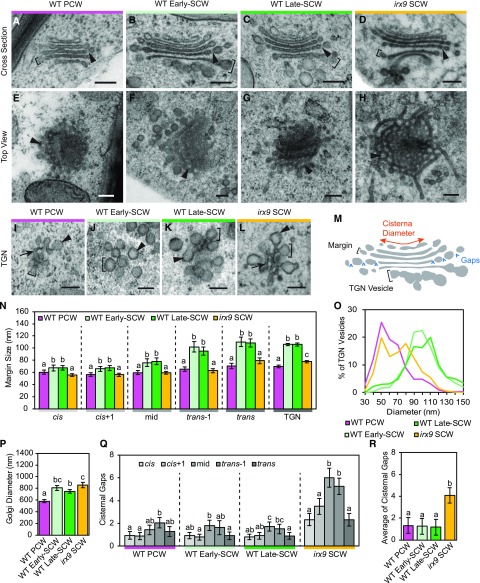
The onset of SCW synthesis during differentiation of protoxylem tracheary elements requires a shift in Golgi function, from PCW production to SCW production (Meents et al., 2018; Watanabe et al., 2018). With the intense demands on the Golgi during rapid SCW synthesis, we hypothesized the diameter of Golgi and the size of cisternal margins and TGN vesicles would increase. Golgi ultrastructure was characterized in TEM images of cryo-fixed wild type (wild-type PCW) and wild type/35S:VND7-VP16-GR (wild-type early-SCW and wild-type late-SCW) seedlings. To assess the specific impact of xylan biosynthesis on Golgi structure, Golgi were also examined in xylan-deficient irx9 mutant plants carrying the VND7 construct (irx9/proUBQ10:VND7-VP16-GR; irx9 SCW). VND7 activity was induced for 22–28 h, except for the wild-type early-SCW plants, which were exposed for 17–20 h (Fig. 1P). With extensive sampling, the different planes of section of Golgi were identified (Supplemental Fig. S4), and cross-sections of Golgi cisternae were used for measurements of Golgi size and structure (Supplemental Fig. S4, A–C). Clear qualitative differences were observed in the structure of Golgi stacks (Fig. 5, A–H) and TGN (Fig. 5, I–L) during SCW biosynthesis. Golgi features were quantified to provide a more nuanced characterization of the observed structural changes within individual Golgi stacks and across the different genotypes (Fig. 5M). Cisternae were categorized as cis, cis+1, mid, trans-1, and trans, based on their position in the stack and their physical features (as described in the “Materials and Methods”). Most Golgi contained 5 or 6 cisternae, and the number of cisternae did not change significantly with the onset of SCW synthesis or in the irx9 mutant (Supplemental Fig. S5B).
Figure 5.
Cisternal structure changes with the onset of xylan biosynthesis and SCW deposition. A to D, Golgi cross-sections for wild-type (WT) PCW (A), wild-type Early-SCW (B), wild-type Late-SCW (C), and irx9 SCW (D) showing gaps in cisternae (arrowheads) and examples of size measurements of cisternal margins (brackets). Scale bars = 200 nm. E to H, Sections through tilted ‘Top View’ Golgi showing fenestrations (arrowheads) in the cisternae from wild-type PCW (E), wild-type Early-SCW (F), wild-type Late-SCW (G), and irx9 SCW cells (H). Scale bars = 200 nm. I to L, TGN from wild-type PCW (I), wild-type Early-SCW (J), wild-type Late-SCW (K), and irx9 SCW cells (L) showing the formation of large vesicles (arrowheads), small tubules (arrows), and examples of vesicle bud size measurements (brackets). Scale bars = 200 nm. M, Diagram illustrating Golgi ultrastructural features quantified. The diameter (red arrow), margin, and TGN vesicle size (brackets) and number of gaps (blue arrowheads) were quantified for every cisterna in each stack. N, The diameter of cisternal margins and TGN vesicle buds in wild-type PCW, wild-type Early-SCW, wild-type Late-SCW, and irx9 SCW Golgi, measured as indicated in (M). Means ± 95% CI. Statistics = separate Kruskal-Wallis and post hoc analysis (P < 0.05); n = 35–55 Golgi and 327–640 TGN vesicles in 5–7 seedlings from 2 to 3 high-pressure freezing experiments. O, Distribution of TGN vesicle-bud sizes; n = 327–640 TGN vesicle buds in 5–7 seedlings from 2 to 3 high-pressure freezing experiments. P, Golgi diameter in wild-type PCW, wild-type Early-SCW, wild-type Late-SCW, and irx9 SCW cells. Golgi diameter was calculated by averaging the diameter of cis+1 to trans-1 cisternae. Means ± 95% CI. Statistics = one-way ANOVA and Tukey HSD post hoc analysis (P < 0.05); n = 40–55 Golgi and in 5–7 seedlings from 2 to 3 high-pressure freezing experiments. Q and R, Number of cisternal gaps in each cisterna (Q) and averaged across all cisternae (R) in each stack in wild-type PCW, wild-type Early-SCW, wild-type Late-SCW, and irx9 SCW Golgi. Means ± 95% CI. Statistics = separate Kruskal-Wallis and post hoc analysis (P < 0.05); n = 35–44 Golgi in 5–7 seedlings from 2 to 3 high-pressure freezing experiments. Different letters (a to c) in (N and P to R) indicate statistically significant differences.
Golgi cisterna have a flattened central region with margins (i.e. the rounded profiles at the edge of each cisterna) that increase in size from cis to trans. In other cell types, increases in cisternal margins have been attributed to accumulation of polysaccharide cargo, culminating in the production of secretory vesicles at the TGN (Zhang and Staehelin, 1992; Young et al., 2008; Wang et al., 2017). Analysis of margin size in this study found a similar trend with a significant increase in the size of more trans cisternal margins, regardless of stage of SCW deposition or genotype (Supplemental Fig. S5). Furthermore, the onset of SCW production resulted in a significant increase in margin size compared with wild-type PCW cells, with an ∼18% increase in the margins of cis-cisternae, up to a dramatic ∼65% increase of trans cisterna margins (Fig. 5N). The size of irx9 SCW Golgi margins was significantly smaller than Golgi in wild-type SCW-producing cells (Fig. 5N), consistent with the hypothesis that cell wall polysaccharide accumulation drives the expansion of Golgi margins.
The TGN is composed of interconnected clusters of tubules, secretory vesicle clusters, and clathrin-coated vesicle buds (Young et al., 2008; Toyooka et al., 2009; Kang et al., 2011; Boutté et al., 2013). As the size of these buds can vary depending on the type of vesicle and its contents, the size of these swollen membrane regions, termed ‘TGN vesicles’, was quantified. For all sample types, the size of TGN vesicles was not significantly different than the size of the trans cisterna margins, supporting a model where the transmost cisterna matures into the TGN (Supplemental Fig. S5C). A large increase in the size of TGN vesicles accompanied the onset of SCW production, shifting from ∼60 nm in wild-type PCW TGN up to ∼100 nm in wild-type early-SCW and late-SCW cells (Fig. 5N). This change was also apparent in the distribution of vesicle sizes at each stage (Fig. 5O). Reduced xylan biosynthesis in irx9 SCW cells resulted in an intermediate TGN vesicle size with a mean of ∼75 nm (Fig. 5N). The irx9 SCW TGN also had a more hybrid structure, with tubular regions like wild-type PCW TGN and regions with large vesicle buds more like the wild-type SCW TGN (Fig. 5, I–L). These different structures are reflected in a bimodal distribution of irx9 TGN vesicle sizes (Fig. 5O). These data show that although the packaging of xylan in secretory vesicles contributes to increased TGN vesicle size during SCW production, other SCW cargo likely contribute as well.
In each Golgi stack, the length of each cisterna from edge to edge was measured in Golgi cross-sections (Fig. 5, A–D). The largest cisternae in both wild-type PCW and wild-type SCW Golgi were the mid- and trans-1 cisternae, and there was a significant (10% to 20%) decrease in diameter from the trans-1 to trans cisterna (Supplemental Fig. S5D). There was a significant (2-fold) change in cisternal diameter from the smallest to largest cisternae for all sample types (Supplemental Fig. S5D). Averaging the diameter of cis+1 to trans-1 cisternae provided an estimate of Golgi size that was used to compare Golgi width in the different samples. SCW production was accompanied by an ∼50% increase in Golgi width in wild-type early-SCW and late-SCW cells (Fig. 5P). This increase in cisternal diameter could have been driven by the accumulation of xylan, in a similar fashion as the increase in margin size. However, this appears not to be the case, as a similar increase in Golgi width was observed in irx9 SCW cells as in the wild-type SCW cells (Fig. 5P). These data indicate that the onset of SCW production is accompanied by an increase in the overall width of Golgi cisternae; however, this increase appears to be independent of xylan accumulation.
Golgi in irx9 Mutants Have Abundant Tubules and Fenestrae
Another key feature of many Golgi cisternae are fenestrations, which often appear as gaps in cisternal edges in Golgi cross-sections (Staehelin and Kang, 2008; Kang et al., 2011; Donohoe et al., 2013). Despite their ubiquity, the function of Golgi fenestrations has not been resolved. The most fenestrated cisternae in the Golgi stacks were the mid and trans-1 cisternae, regardless of developmental stage or genotype (Fig. 5Q). Surprisingly, top-down views of irx9 SCW Golgi showed highly tubulated, web-like cisternae with large and numerous fenestrations (Fig. 5H). To quantify this increase in fenestrations, the number of fenestrations in Golgi cisterna was determined by counting the number of gaps in cisternal cross-sections (Fig. 5Q). As predicted, the irx9 SCW Golgi had a significant 3-fold increase in the number of fenestrations compared with all other samples (Fig. 5R).
Fine-scale quantification of cisternal architecture confirmed that altering Golgi function by triggering SCW synthesis changes Golgi structure with increased SCW production correlating with increased cisternal length, margin width, and TGN vesicle size. Conversely, decreased xylan production in the irx9 mutant led to a reduction in the size of the cisternal margins and TGN vesicles, indicating xylan accumulation contributes substantially to these aspects of Golgi structure but not to Golgi diameter. Surprisingly, the irx9 Golgi cisternae were also highly tubulated and displayed a significant increase in the number of fenestrations. The presence of IRX9 thus also promotes the formation of smooth cisternal sheets at the expense of fenestrations and tubules.
IRX9-GFP and Xylan Localize to Different Regions of Golgi Stacks
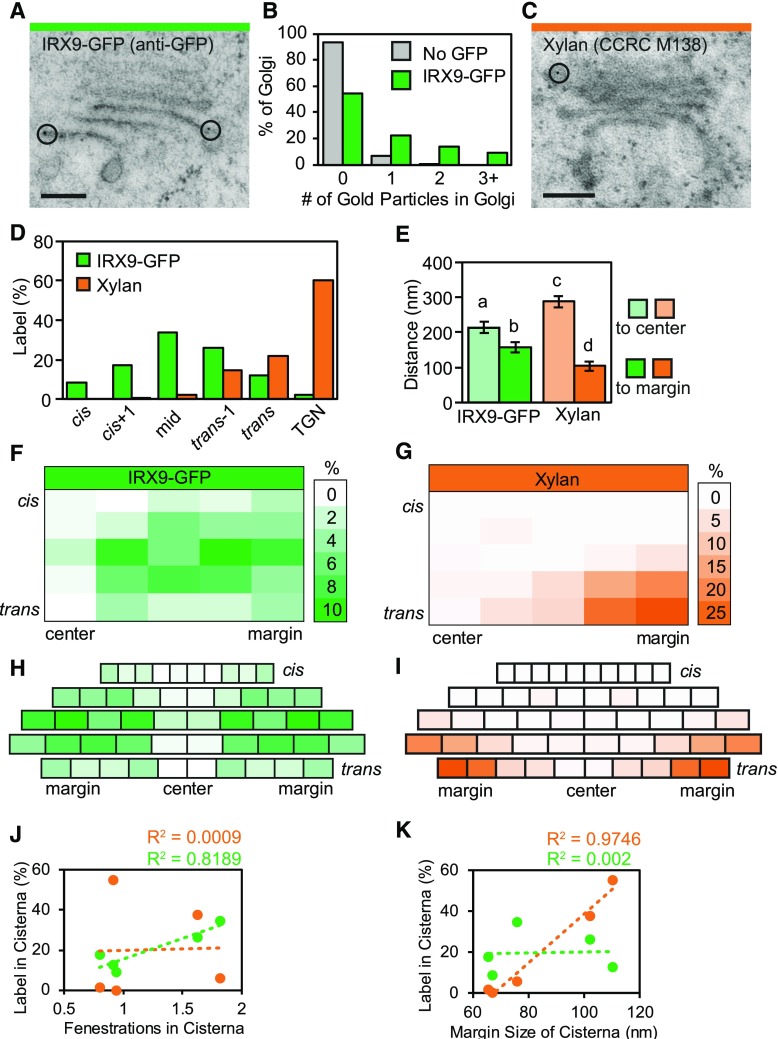
In live-cell imaging, IRX9-GFP is found in a ring around the center of the Golgi stack (Fig. 1Q), but current models based on freeze-fracture data propose these types of Golgi-localized biosynthetic proteins reside in the cisternal centers (Staehelin et al., 1990). To reconcile this difference, and test the existing model, the distributions of the Golgi-resident protein IRX9 and its product, xylan, were quantified across different domains of Golgi cisternae. Immuno-TEM localization of IRX9-GFP and xylan, via anti-GFP and anti-xylan (CCRC M138) antibodies, respectively, was conducted on high-pressure frozen/freeze-substituted proIRX9:IRX9-GFP/35S:VND7-VP16-GR samples where VND7 activity was induced for 22–26 h.
To map the distribution of IRX9 in Golgi and TGN, seedlings containing proIRX9:IRX9-GFP were prepared for immuno-TEM using anti-GFP antibodies. The anti-GFP antibody consistently labeled the Golgi in IRX9-GFP SCW cells (Fig. 6A), with very little nonspecific label in wild-type SCW cells from plants lacking GFP (Supplemental Fig. S6, A and B). When anti-GFP binding was quantified in IRX9-GFP lines, 46% of Golgi or TGN contained one or more gold particles, whereas only 7% of Golgi were labeled in the plants not containing GFP (Fig. 6B). The average number of gold particles per Golgi/TGN increased 92% from 0.07 gold particle per Golgi in wild-type cells lacking IRX9-GFP to 0.83 in IRX9-GFP cells. The gold label in IRX9-GFP Golgi was significantly different than that of background label, according to a Χ2 test (P < 0.001). When mapped to distinct regions of the Golgi, IRX9-GFP label was found in all cisternae with peak label in the midcisternae (33.9%), tapering to 8.6% in cis cisternae, and 12.4% in trans cisternae (Fig. 6D). A very small proportion of label was found in the TGN (2.2%).
Figure 6.
IRX9-GFP and xylan are spatially segregated in different regions of golgi cisternae. A, Anti-GFP labeling of Golgi in SCW-producing cells in a plant containing IRX9-GFP. Black circles outline gold particles. Scale bar = 200 nm. B, Anti-GFP signal above background label. Distribution of gold label among Golgi in SCW-producing negative controls that lack IRX9-GFP (No GFP) and in similar cells in plants containing IRX9-GFP is shown; No GFP (n = 20 gold particles, 277 Golgi, 19 cells, 4 seedlings, 2 high-pressure freezing experiments); IRX9-GFP (n = 410 gold particles, 493 Golgi, 39 cells, 4 seedlings, 2 high-pressure freezing experiments). C, Anti-xylan (CCRC M138) labeling of a Golgi in a SCW-producing cell in a plant containing IRX9-GFP. Black circle outlines a gold particle. Scale bar = 200 nm. D, Percent of gold particles in Golgi cisternae from cis cisternae through to the TGN for anti-GFP (IRX9-GFP) or CCRC-M138 anti-xylan (Xylan). E, Average distance from gold label to the cisterna center and margin. All gold particles were pooled, regardless of cisterna labeled. Means ± 95% CI. Different letters (a to d) indicate statistically significant differences. Statistics = one-way ANOVA with Tukey HSD post hoc (P < 0.05). F and G, Heat map of gold distribution across the Golgi from cis to trans and in 10% blocks of cisternal diameter from the center to the edge of each cisterna. Distribution of IRX9-GFP (F) and xylan (G). H and I, Distributions of IRX9-GFP (H) and xylan (I) adjusted for changes in cisternal width from cis to trans using the average cisternal diameters of early-SCW Golgi. Blocks represent 10% of the cisternal diameter. J and K, Correlation of number of fenestrations (J) or cisternal margin size (K) in each cisterna with amount of GFP (green) or xylan (orange) label in each cisterna; Xylan (n = 136 gold particles, 17 cells); IRX9-GFP (n = 182 gold particles, 33 cells). Data are from 4 seedlings and 2 high-pressure freezing experiments.
To map the product of IRX9 during SCW biosynthesis, xylan distribution was quantified in Golgi and TGN using anti-xylan antibodies. Five antibodies were tested, including LM10 (McCartney et al., 2005; Kim and Daniel, 2012), CCRC-M138, CCRC-M147, CCRC-M149, and CCRC-M153 (Pattathil et al., 2010). Although LM10 labeled the SCW as previously reported (McCartney et al., 2005; Kim and Daniel, 2012), the Golgi label in high-pressure frozen and freeze substituted samples was too sparse for quantification. Of the other antibodies tested, CCRC-M138 had the strongest signal and was therefore chosen for xylan quantification. The CCRC-M138 antibody recognizes unsubstituted xylopentaose units (Peralta et al., 2017) and was used previously in immunofluorescence experiments to label xylan in Arabidopsis SCWs (Pattathil et al., 2010). CCRC-M138 binding was detected with a secondary antibody conjugated to colloidal gold and imaged with TEM. CCRC-M138 signal was found in Golgi, TGN, and SCWs in all cells undergoing SCW deposition (Fig. 6C; Supplemental Fig. S6, C–E). In total, 57% of Golgi and 57% of TGN were labeled with one or more gold particles, averaging 1.17 gold particles per Golgi and 1.36 gold particles per TGN. The secondary antibody was specific to CCRC-M138, as no gold labeling was seen in the Golgi, TGN, or SCW in the absence of the primary antibody (Supplemental Fig. S6, F–H). Furthermore, gold labeling was never observed in cells not producing SCWs (Supplemental Fig. S6I), suggesting CCRC-M138 is specific to an epitope present during SCW deposition, which is consistent with xylan specificity. Mapping of xylan revealed 60% of the label in the TGN and 40% in the Golgi, with label first appearing in small quantities in the medial cisternae (2.3%) and becoming much more abundant in trans cisternae (21.9%; Fig. 6D).
Lateral mapping of IRX9-GFP and xylan across the width of the cisternae was conducted by measuring the distance from each gold particle to the edge and center of the cisterna. Both GFP and xylan label was present significantly closer to the margin of cisternae than the centers, although the xylan label was significantly closer to the margin than GFP (104 nm vs 157 nm, respectively; Fig. 6E). To better visualize the distribution of label across Golgi cisternae, every cisterna was divided into ten equal regions, giving 5 blocks for each half cisterna from the cisternal center to the margin (Fig. 6, F and G). The number of gold particles falling into each block was then tallied and the distribution visualized using a heat map (Fig. 6, F and G). As suggested by the previous analysis, IRX9-GFP and xylan label only overlapped slightly. IRX9-GFP label was most abundant in cis+1, mid, and trans-1 cisternae adjacent to the cisternal centers (Fig. 6F), whereas xylan label was found predominantly in the outermost margins of trans-1 and trans cisternae (Fig. 6G). Combining these labeling patterns with the quantified architecture of Golgi stacks from the TEM (Fig. 5) allows scaling of the heat maps to account for the changing diameter of Golgi cisternae in wild-type SCW-producing cells (Supplemental Fig. S5D). Both IRX9-GFP and xylan label are low in the center of cisternae, and each is found in spatially distinct regions of the Golgi (Fig. 6, H and I). The IRX9-GFP label appeared to be abundant in regions of the Golgi that are highly fenestrated (Fig. 6H, and refer to Fig. 5Q). Indeed, the amount of IRX9-GFP label in each cisterna was strongly correlated with the average number of cisternal fenestrations via Pearson’s r [r(3) = 0.99, P < 0.01], which was not the case for xylan label [r(3) = 0.04, P > 0.05; Fig. 6J]. Conversely, xylan label highly correlated with the size of cisternal margins [r(3) = 0.91, P < 0.05; Fig. 6I, and refer to Supplemental Fig. S5C], whereas IRX9-GFP label did not [r(3) = 0.03, P > 0.05; Fig. 6K]. These results clearly demonstrate IRX9-GFP and its xylan product localize to distinct subdomains of the Golgi in both the cis-to-trans and centers-to-margins dimensions. These distributions also correlate with different structural features of the Golgi with xylan abundance correlating with the size of the cisternal margins and IRX9-GFP labeling with the number of fenestrations.
DISCUSSION
A Concentric Circles Model Defines Functional Domains of Polysaccharide Production in Golgi Stacks
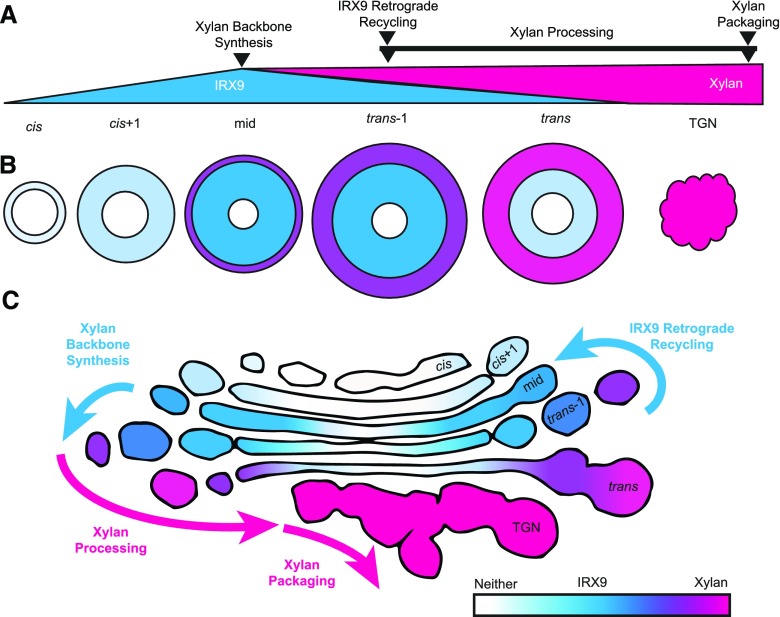
During SCW biosynthesis, the xylan biosynthetic protein IRX9 and its xylan product were detected in concentric circles in the Golgi cisternae. The live-cell imaging of Golgi ring-like structures, as well as quantitative immuno-TEM mapping of IRX9 and xylan to well-defined Golgi structures, support this model of hemicellulose production in the Golgi (Fig. 7). Importantly, this concentric circles model emphasizes the enrichment of biosynthetic proteins and products in specific functional Golgi domains with concomitant structural features. In this model we propose (1) xylan backbone synthesis occurs in a ring in the inner-margin of fenestrated, medial cisternae where IRX9-GFP is localized and not in cisternal centers; (2) xylan accumulates in the outer-margins of trans-1 and trans cisternae, which become increasingly swollen as the cisternae mature; (3) IRX9 maintains its appropriate localization in medial cisternae while IRX9 is depleted from trans cisternae, suggesting active retrograde recycling; and (4) xylan is packaged into large secretory vesicle buds at the TGN for export to SCW domains. This model is consistent with the cisternal maturation model of Golgi processing (Glick and Luini, 2011) and the proposed formation of xylan biosynthetic complexes containing IRX9 (Zeng et al., 2010; Jiang et al., 2016; Zeng et al., 2016).
Figure 7.
Organization of xylan biosynthetic machinery and products in concentric circles defines functional regions of Golgi cisternae. A, Proposed steps of xylan synthesis in Golgi cisternae and TGN in comparison with a schematic of the relative abundance of IRX9 and xylan in each compartment. Backbone synthesis is proposed to predominate in mid and trans-1 cisternae where IRX9 is most abundant. IRX9 must be removed from cisternae during maturation of trans-1 into trans cisternae as it is much depleted in the trans cisternae. IRX9 is predicted to recycle via retrograde trafficking to cis+1 or mid cisternae, where IRX9 first appears in substantial amounts. After backbone synthesis, xylan processing, including addition of GlcA side chains, methylation, acetylation and deacetylation, likely occurs during maturation of trans-1 cisternae through to TGN. Final packaging of xylan into secretory vesicles occurs at the TGN before secretion to the SCW. B, Top-down view of simplified cisternae showing the concentric circle organization of xylan synthesis with an accumulation of xylan in outer cisternal margins, abundant IRX9 in inner-margins, and the absence of both IRX9 and xylan in cisternal centers. The size of each cisternae and the TGN is adjusted to reflect their size in a wild-type early-SCW Golgi. C, Distribution of IRX9 (blue) and xylan (magenta) abundance from Figure 6, superimposed on the outline of a representative wild-type early-SCW Golgi cross-section from a TEM micrograph. IRX9 is abundant in more central regions of fenestrated mid and trans-1 cisternae, whereas xylan accumulates in the swollen outer margins of trans cisternae and the TGN. The collapse of the flattened cisternal structure in the TGN is proposed to accompany the packaging of xylan into secretory vesicles.
The presence of xylan biosynthetic proteins in the inner margin of medial cisternae is consistent with the ring-shaped distribution of IRX9-GFP in confocal live-cell imaging and was demonstrated quantitatively using high-resolution immuno-TEM mapping. Although the distribution of IRX9-GFP in quantitative immuno-TEM may be skewed somewhat by the presence of wild-type IRX9 in these plants, the pattern observed is consistent with the ring-shaped localization of IRX9-GFP in the mutant irx9 background from confocal imaging, providing confidence the IRX9-GFP distribution seen in Figure 6 is indicative of the true localization of IRX9 in the Golgi. Other Golgi-localized proteins have been found in similar localization patterns in confocal imaging, including IRX9L (Zhang et al., 2016) and several COPI-related proteins (Ritzenthaler et al., 2002). Experiments in a variety of eukaryotes suggest oligomerization may influence protein distribution across Golgi cisternae from the center to the margins. In plants, the ring-shaped distribution of the PCW cellulose synthase CESA3 in the Golgi was lost in the stello mutant background, where CESA3 shifted toward a more central cisternal localization, coinciding with a decrease in the formation of CESA complexes (Zhang et al., 2016). In mammalian cells, induced aggregation of engineered Golgi-resident proteins caused them to shift toward cisternal centers, whereas disaggregation returned them to the rims (Rizzo et al., 2013). Additionally, localization of these proteins in the cisternal rims was required to maintain their position in appropriate cisternae, suggesting aggregated Golgi-residents were excluded from retrograde trafficking and thus carried downstream with cisternal maturation. Furthermore, aggregation of membrane-associated cargo proteins led to aggregate accumulation in more central regions of the cisternae, whereas aggregations of soluble cargo were detected in Golgi edges (Lavieu et al., 2013). The formation of a large xylan biosynthetic complex may similarly shift localization of these proteins toward the center of cisternae while allowing the soluble cargo to accumulate in the outermost margins. The xylan biosynthetic complex may then dissociate as cisternae mature, allowing individual proteins to move transiently to the margins where they become available for retrograde trafficking to earlier cisterna where the complex reforms and the next round of xylan biosynthesis begins.
Mapping of IRX9 to a ring in the inner-margin of medial cisternae suggests synthesis of the xylan backbone is occurring in these domains. Production of the xylan backbone for Arabidopsis SCWs requires three sets of proteins: IRX9/IRX9L (Lee et al., 2010; Wu et al., 2010), IRX10/IRX10L (Brown et al., 2009; Wu et al., 2009), and IRX14/IRX14L (Keppler and Showalter, 2010). The other proteins involved in backbone synthesis may be similarly localized, as a number of experiments suggest xylan is synthesized by a biosynthetic complex. Coimmunoprecipitation and bimolecular fluorescence complementation studies during PCW production of arabinoxylans in wheat (Triticum aestivum) and asparagus (Asparagus officianalis) have demonstrated in planta heterodimerization of xylan biosynthetic proteins orthologous to the Arabidopsis proteins (Zeng et al., 2010; Jiang et al., 2016; Zeng et al., 2016). Each protein was also shown to homodimerize in planta, further increasing the size of the proposed xylan biosynthetic complex to at least six members (Zeng et al., 2016). Proteins involved in decorating the xylan backbone may also be incorporated into the protein complex, as a PCW xylan biosynthetic complex isolated from wheat also showed arabinosyltransferase and glucuronosyltransferase activity necessary for side chain addition (Zeng et al., 2010). Furthermore, because the UDP-Xyl substrate for xylan backbone synthesis is synthesized in the cytosol (Kuang et al., 2016; Zhong et al., 2017b), the Golgi-localized UDP-Xyl transporters, especially UXT1, are hypothesized to be associated with the xylan biosynthetic complex in the Golgi to ensure adequate substrate availability (Ebert et al., 2015).
Some degree of sequential processing in different Golgi cisternae is likely occurring during xylan biosynthesis. Following backbone production and addition of side chains, methylation and acetylation are likely occurring in different downstream regions of the Golgi. Analysis of xylans in various xylan biosynthetic mutants suggests the methylation rate of GlcA residues is independent of the rate of xylan synthesis, which is consistent with a model where methylation occurs following backbone synthesis (Zhong et al., 2005; Peña et al., 2007; Kuang et al., 2016). Xylans are also acetylated in the Golgi (Urbanowicz et al., 2014) and then deacetylated before deposition in the wall (Zhang et al., 2017). The resulting pattern of acetylation was essential for xylan interaction with cellulose (Grantham et al., 2017). Xylan acetylation and deacetylation almost certainly occur in different regions of the Golgi. With the localization of xylan backbone synthesis to the medial Golgi, we can now formulate testable hypotheses about the relative location of other steps in xylan biosynthesis. For example, the conserved reducing-end oligosaccharide (REO) found in many SCW xylans (Peña et al., 2016) has been hypothesized to be either a primer for consecutive xylan synthesis or a terminator of synthesis transferred en bloc to the completed xylan backbone (York and O’Neill, 2008). If the REO is a primer for sequential xylan biosynthesis, then we would expect the enzymes producing REOs to appear in cis-cisterna. However, if the REO is a xylan terminator the biosynthetic enzymes may appear in more-trans cisternae. The mapping of IRX9 therefore allows the medial Golgi to be used as a benchmark to guide mapping of the various steps of xylan biosynthesis.
Immuno-TEM labeling showed xylan was abundant in the margins of later cisternae and in the TGN, and the swelling of the cisternal margins and large TGN vesicles was strongly correlated with the amount of xylan label in each cisterna. The increased accumulation of polysaccharide cargo in the cisternal periphery and TGN vesicles is also supported by the reduction in margin size in irx9 Golgi. The decrease in margin size and TGN in irx9 is analogous to a similar decrease observed during Arabidopsis seed coat development in a mutant with substantially reduced pectin biosynthesis (Young et al., 2008). There is a population of intermediate-sized TGN vesicle buds in irx9 mutants that are likely important for trafficking other cargo, including cellulose synthases. The ixr9 plants still produce patterned SCWs containing cellulose (Petersen et al., 2012); therefore, the CESAs must still be trafficked to, and subsequently from, the SCW domains.
The interpretation of the anti-xylan antibody labeling in this study depends on our understanding of the epitope the antibody recognizes, as well as potential masking by other cell wall components such as the GlcA substitutions on the xylan backbone. Because the CCRC-M138 anti-xylan antibody used recognizes unsubstituted xylopentaose (Peralta et al., 2017), and the xylan backbone in Arabidopsis is substituted with GlcA at intervals greater than five Xyl residues (Bromley et al., 2013), CCRC-M138 labeling is likely minimally affected by GlcA substitutions. However, we cannot exclude the possibility that the xylan label detected in our study reflects a more mature, partially deacetylated xylan. This could be investigated by examining anti-xylan labeling in plants defective in xylan acetylation, such as the eskimo1 (esk1) mutant (Yuan et al., 2013; Urbanowicz et al., 2014). Immuno-TEM mapping using other anti-xylan antibodies (Ruprecht et al., 2017) may also shed light on the distribution of various populations of xylans in the Golgi.
Abundant Golgi Fenestration and Tubulation in the Absence of IRX9
The regions of the Golgi in which IRX9-GFP labeling was strongest also contained the largest number of fenestrations. The presence of fenestrations is well documented in plant (Kang et al., 2011; Donohoe et al., 2013), mammalian (Mogelsvang et al., 2004; Koga and Ushiki, 2006), and yeast Golgi (Mogelsvang et al., 2003). Despite conservation of this Golgi feature across eukaryotes, their function is unknown. There was a surprising and dramatic increase in fenestrations in the irx9 mutant. The mechanism by which IRX9 inhibits fenestration formation is unclear. Several functions have been proposed for fenestrations, including acting as ‘spot welds’ to control cisternal swelling, increasing membrane curvature to facilitate the formation of vesicle buds, and providing pathways for movement of cytoplasm through cisternae to aid diffusion and/or Golgi movement (Ladinsky et al., 1999). This first hypothesis seems unlikely, as fenestration abundance increased in the irx9 mutant, despite decreased swelling of cisternal margins. Alternately, fenestrations could be important for increasing the membrane area of specific regions of the Golgi to accommodate abundant membrane-associated Golgi resident proteins. Regions with fenestrations are predicted to have high surface area:volume ratios, in contrast with the swollen cisternal margins that must contain large volumes of secretory cargo.
One model of fenestration formation requires homotypic fusion of tubules protruding from cisternal margins and looping around to reconnect with the originating cisterna (Weidman et al., 1993). This model is consistent with the increased tubulation and fenestration seen in irx9 Golgi. In irx9, the Golgi stacks are lacking the large quantities of xylan, which normally accumulate in the margins, but they are also lacking the Golgi resident protein IRX9. Furthermore, in transient expression of an IRX9 homolog in Nicotiana benthamiana, IRX9 did not localize to the Golgi unless also expressed with IRX10 and IRX14 (Zeng et al., 2016). The absence of IRX9 from the cell in this study may therefore similarly prevent Golgi localization of other xylan biosynthetic proteins. To what extent can we then attribute the changes in Golgi structure in irx9 to loss of Golgi polysaccharide cargo versus Golgi residents? Given that irx9 Golgi are of a similar size to wild-type Golgi, it is apparent that the additional fenestrations in irx9 occur closer to the center of the Golgi cisternae where IRX9-GFP is usually observed and xylan is absent. The increased abundance of fenestrations in more central regions of irx9 cisternae is therefore more likely to be a result of the lack of IRX9, rather than xylan, which is not abundant in these regions. Further experiments will be required to elucidate the mechanism by which IRX9 may inhibit Golgi fenestration. One possibility is that a large xylan biosynthetic complex in the Golgi lumen physically prevents spontaneous fusion of the membrane on either side of the lumen.
Cytoplasmic Geometry Constrains Golgi during SCW Production
Confocal imaging of Golgi in the cell cortex allows some characterization of the relationship between the Golgi and the SCW but gives little information about the organization of other cellular components. TEM imaging takes into account the volume and organization of the cytoplasm and other organelles, allowing us to conclude the close proximity of Golgi to SCWs in confocal imaging (Watanabe et al., 2015; Schneider et al., 2017) is due to a concentration of free cytoplasmic space in this area. In any plant cell, the organization of Golgi stacks at any given time is determined by the contribution of both moving and stationary Golgi. This ‘stop-and-go’ behavior has been well-characterized in several cell types, including Nicotiana tabacum BY2 cell cultures (Nebenführ et al., 1999), Nicotiana clevelandii pavement cells (Boevink et al., 1998), and Arabidopsis trichomes (Lu et al., 2005). In native root tracheary elements, Golgi were similarly said to ‘pause’ in their rapid cytoplasmic streaming at SCW domains (Wightman and Turner, 2008). This was also demonstrated in the VND7-induction system, where Golgi pausing near SCWs lasted anywhere from 15 s to 3 min (Watanabe et al., 2015). However, Golgi paused at SCW domains may represent a small proportion of the population of Golgi stacks, which may explain why the larger population of Golgi bodies remains randomly distributed in the narrow cortical cytoplasm.
Multitasking of the Golgi Apparatus during SCW Synthesis
Deposition of SCWs requires abundant production of xylan in the plant Golgi apparatus, as well as targeted secretion of Golgi products to the plasma membrane lining SCW domains. Accomplishing these tasks requires the concerted action of the population of Golgi stacks streaming rapidly through the cytoplasm. In this study, we demonstrate the onset of SCW production is accompanied by significant proliferation in the number of Golgi stacks. Increase in the abundance of Golgi stacks also occurs during the onset of mucilage production in Arabidopsis seed epidermal cells (Young et al., 2008) and during mitosis (Garcia-Herdugo et al., 1988; Seguí-Simarro and Staehelin, 2006; Toyooka et al., 2014). The prevailing model for Golgi biogenesis in plants proposes Golgi stacks increase in diameter before cisternal fission, creating two new stacks with the same number of cisterna as the ‘parent’ Golgi (Ito et al., 2014). Indeed, electron microscopy in various cell types have captured Golgi at what could be a ‘mid-division’ stage (Hirose and Komamine, 1989; Langhans et al., 2007; Staehelin and Kang, 2008). Our data are consistent with this model, as Golgi proliferation during SCW synthesis was accompanied by a significant increase in Golgi diameter. Similar increases in Golgi size have been observed in the transition from root meristem to columella cells (Staehelin et al., 1990).
The observation that all Golgi stacks in the cell contained both IRX9-GFP and the Golgi marker MANI-mCherry demonstrates the entire population of Golgi stacks in the cell are likely working in concert to produce xylan for SCWs. The Golgi marker MANI is involved in the early stages of glycoprotein processing in the Golgi (Schoberer and Strasser, 2011), implying each individual Golgi stack is carrying out both glycoprotein processing and xylan biosynthesis simultaneously. This is consistent with previous work showing Golgi stacks can produce both pectin and xyloglucan concurrently (Zhang and Staehelin, 1992; Young et al., 2008) and perform polysaccharide biosynthesis at the same time as glycoprotein processing (Moore et al., 1991). The conservation of Golgi stack multitasking across tissue types and species strongly suggests multitasking is a common feature of plant Golgi.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
All Arabidopsis (Arabidopsis thaliana) seeds were sterilized using chlorine gas in a sealed container containing 100 mL of bleach and 3 mL of concentrated HCl for 3–6 h Seeds were then plated on Germination Media (1× Murashige and Skoog 1% [w/v] Suc, 1× Gamborg’s Vitamin mix, 0.05% [w/v] MES, 0.8% [w/v] agar at pH 5.8) and then transferred to growth conditions. Plants were grown under long-day conditions (16-h light/8-h dark) at 21°C in a vertical position. The irx9-2 (irx9) mutant line (AT2G37090, SALK_057033) was obtained from the Arabidopsis Biological Resource Center (The Ohio State University, Columbus; ABRC). The 35S:VND7-VP16-GR seeds and plasmid were kindly donated by Taku Demura (Yamaguchi et al., 2010a). To induce VND7-VP16-GR activity, 4- to 7-d-old seedlings were treated with 10 mL of 10 μm dexamethasone (DEX; Sigma) for 17–36 h, depending on the experiment. The Arabidopsis Columbia-0 ecotype was used to generate all transgenic lines using Agrobacterium tumefaciens (strain GV3101) and the floral dip method. Primary transformants were selected by plating on Germination Media plates containing 50 µg/mL kanamycin, 25 µg/mL hygromycin, or 25 µg/mL Basta.
Generation of Constructs
Constructs were primarily generated using Gateway cloning (Invitrogen), including PCR amplification with attB sequences, Gateway Clonase-mediated insertion into a donor vector, and then transfer to the destination vector. PCR was conducted with Phusion High Fidelity DNA polymerase (New England Biolabs) followed by two-step adapter PCR to incorporate the full attB sequences for Gateway cloning. The native promoter used for IRX9 (AT2G37090) consisted of the 2045 bp intergenic sequences upstream of the start codon as in published promoter-GUS experiments (Wu et al., 2010). ProIRX9:IRX9 was amplified from genomic DNA, cloned into pDONRzeo, and then into pMDC107 (Curtis and Grossniklaus, 2003) to incorporate an in-frame C-terminal GFP and stop codon, thereby generating proIRX9:IRX9-GFP. Seeds and plasmids containing a 35S-driven version of soybean (Glycine max) MANI-mCherry were available, but signal was largely absent due to silencing. To address this, the UBIQUITIN10 (UBQ10; AT4G05320) promoter was used to generate proUBQ10:MANI-mCherry by amplifying MANI-mCherry from the published 35S:MANI-mCherry construct (Nelson et al., 2007), cloning this into pDONRzeo, and then pUB-DEST to add the proUBQ10 promoter (Grefen et al., 2010). Alternate versions of the published 35S:VND7-VP16-GR (Kanamycin resistant; KanR) construct (Yamaguchi et al., 2010a) were generated to reduce ongoing silencing problems believed to be exacerbated by the 35S promoter and to provide an alternate plant selectable-marker. VND7-VP16-GR was amplified from the existing plasmid and cloned into pDONRzeo and then into pUB-DEST to generate proUBQ10:VND7-VP16-GR (Basta resistant; BasR) or pB2GW7 to generate pro35S:VND7-VP16-GR (BasR).
Complementation Testing
Proper function of proIRX9:IRX9-GFP was tested by transforming the irx9 mutant and looking for complementation of its dwarf and collapsed xylem phenotypes (Lee et al., 2010; Wu et al., 2010). Restoration of wild-type height was measured in 40-d-old plants and averaged for 5 plants in each of the wild type, mutant, and complemented lines. Complementation of the irregular xylem phenotype was observed in hand sections from the bottom 1 cm of mature wild type, mutant, and complemented inflorescence stems stained with 0.01% toluidine blue (Ted Pella) for 5 min and imaged on a Leica DMR microscope equipped with a QICAM digital camera (QIMAGING). Images were prepared in Image J.
Native Expression of proIRX9:IRX9-GFP
Live-cell imaging of irx9/proIRX9:IRX9-GFP in native roots was conducted on a Perkin-Elmer UltraView VoX spinning disk confocal mounted on a Leica DMI6000 inverted microscope with a Hamamatsu 9100-02 CCD camera. Samples were mounted in water, and expression in developing tracheary elements in the root was observed using a glycerol 20× objective (aperture 0.7), 488 nm excitation filter, and 525 emission filter (GFP).
Subcellular Localization in the VND7-Induction System
Subcellular localization of IRX9-GFP was assessed in the complemented irx9 and in wild-type backgrounds. The complemented irx9/proIRX9:IRX9-GFP was transformed with proUBQ10:VND7-VP16-GR (BasR), whereas the wild-type line was generated by transforming 35S:VND7-VP16-GR (KanR) with proIRX9:IRX9-GFP. The wild-type line was used in subsequent experiments, except where indicated otherwise. The developmental time-course of proIRX9:IRX9-GFP expression and subcellular localization was determined by imaging plants at 2-h increments from 17 to 30 h following induction with DEX. Cells were categorized as ‘early’ if a secondary cell wall was not visible in brightfield imaging but IRX9-GFP was expressed. ‘Mid’-development coincided with the appearance of faint SCWs in brightfield, and ‘Late’ development was defined by obvious SCWs. The differences in deposition of the SCW during these stages was validated by separate experiments where imaging of IRX9-GFP and SCWs in brightfield coincided with staining of the cell wall with 10 µg/mL propidium iodide for 5 min. Cells were observed on the Perkin-Elmer spinning disk set-up, described above, using a 63× objective (aperture 1.2).
Colocalization of IRX9-GFP and MANI-mCherry
Colocalization of IRX9-GFP and MANI-mCherry was conducted in F1 crosses of proIRX9:IRX9-GFP/35S:VND7-VP16-GR with proUBQ10:MANI-mCherry. Plants were imaged on a Perkin-Elmer UltraView VoX spinning disk confocal mounted on a Leica DMI DMi8 and a Hamamatsu 9100-02 CCD camera and an immersion oil 100× objective (aperture 1.47) and the following excitation and emission filters: GFP (488 nm, 525 nm), RFP (561 nm, 595 nm). Images of 26 cells from five seedlings were analyzed for quantification of colocalization via Pearson’s Correlation Coefficient (PCC) and Mander’s overlap coefficients (Manders et al., 1993) in Volocity image analysis software (Improvision). All confocal images were acquired using Volocity and prepared in Image J.
Golgi Stack Abundance using Confocal Microscopy
To quantify Golgi density throughout SCW deposition, plant cells expressing Golgi-localized proteins were imaged on a Perkin-Elmer UltraView VoX spinning disk confocal mounted on a Leica DMI DMi8 and a Hamamatsu VOXC9100-23B camera with a glycerol 63× objective (aperture 1.2) and the following excitation and emission filters: GFP (488 nm, 525 nm), RFP (561 nm, 595 nm). Because IRX9 is not expressed during PCW biosynthesis in Arabidopsis, MANI-mCherry lines were used to count Golgi numbers before SCW deposition. Golgi density during PCW synthesis was assessed using the proUBQ10:MANI-mCherry line and compared with Golgi density in SCW-producing cells using the proIRX9:IRX9-GFP lines described above. All plants were treated with DEX for 17–30 h before imaging, and at least three plants were imaged per time point for each experiment. For each cell analyzed, a single optical section through the cell cortex was selected and the number of Golgi-like puncta was divided by the cell area in the section to calculate the Golgi density. Because stage of development varies from cell to cell in any one plant, the appearance of the SCW in brightfield imaging was used to assign the stage of SCW deposition, according to the definitions laid out in the previous time-course experiments. Approximately 10 cells per plant were analyzed. All images were processed using Volocity image analysis software (Improvision) and Image J. PCW data were collected from 319 cells from 31 seedlings across six replicate experiments. Golgi density in the IRX9-GFP line in the wild-type background was measured for 795 cells from 78 seedlings across four replicate experiments. Data from the complemented line was collected from 150 cells across ten seedlings in two replicate experiments.
TEM Imaging
For TEM analysis, hypocotyls and petioles of 4-d-old seedlings were high-pressure frozen, freeze-substituted, sectioned, and prepared for TEM as previously described (McFarlane et al., 2008). Briefly, dissected samples were loaded into copper hats (Ted Pella) with 1-hexadecene as a cryoprotectant and frozen using a Leica HPM-100 high-pressure freezer. Samples were transferred to freeze-substitution media containing 2% (w/v) osmium tetroxide and 8% (v/v) dimethoxypropane in anhydrous acetone for morphological analysis, or 0.25% (v/v) glutaraldehyde, 0.1% (w/v) uranyl acetate, and 8% (v/v) dimethoxypropane in acetone for immunolabeling. Freeze substitution was performed for 5 d at −80°C using an acetone/dry ice slush. Samples were transferred to −20°C overnight, followed by 4°C for 2 h, and then transferred to room temperature. Samples were removed from the sample holders and rinsed in anhydrous acetone several times before slowly infiltrating over the course of 4 d with increasing concentrations of Spurr’s resin (Electron Microscopy Services) for analysis of morphology or LR White for immunolabeling (London Resin Company). Following polymerization at 60°C, samples were sectioned using a Leica Ultracut UCT Ultramicrotome (Leica Microsystems). Thin sections (70–80 nm thick) were poststained using 2% uranyl acetate in 70% methanol and Reynolds lead citrate and viewed on a Hitachi H7600 PC-TEM at an accelerating voltage of 80 kV. Photographs were taken using an ATM Advantage HR digital CCD camera and images analyzed in Image J.
TEM Ultrastructure Analysis
Golgi ultrastructure was quantified in TEM images of DEX-treated wild type (wild-type PCW), wild type/35S:VND7-VP16-GR (wild-type SCW), and irx9/proUB10:VND7-VP16-GR (irx9 SCW) seedlings. For wild type/35S:VND7-VP16-GR, Golgi were analyzed from both 16- to 18-h–induced (wild-type Early-SCW) and 22- to 30-h–induced (wild-type Late-SCW) plants. For each of wild-type PCW, wild-type Early-SCW, wild-type Late-SCW, and irx9 SCW, quantification data were collected from Golgi cross-sections in cells with visible SCWs from replicate seedlings (8, 5, 8, 7, respectively) and experiments (3, 2, 3, 2, respectively).
The ‘cis’ cisternae were identified by lighter staining, visible lumen in the cisternal center, and smaller margins than cisternae on the opposite side of the stack (Staehelin et al., 1990; Samuels et al., 2002). The ‘trans’ cisterna was defined as the last structure on the trans face with a flattened, cisterna-like region. Cisternae adjacent to cis and trans were designated ‘cis+1’ and ‘trans-1’, respectively, and the remaining intervening cisterna(e) classified as ‘mid’. The number of fenestrations in each cisterna was estimated by counting cisternal ‘gaps’ in Golgi cross-sections for each developmental stage or genotype. These gaps, defined as breaks in a cisterna or regions of lighter staining corresponding with indentations in the cisternal membrane, were assumed to be fenestrations based on published Golgi tomography and transverse-sections of cisternae (Mollenhauer and Morré, 1998; Donohoe et al., 2013). Cisternal diameters were determined by measuring the length of each cisterna from one edge to the other. Golgi width was calculated by averaging cisternal diameter for cis+1, mid and trans-1 cisternae. Cis and trans cisternae were excluded as their structures were assumed to be changing rapidly as the cisterna forms or collapses, respectively. Cisternal margins are known to become swollen in more-trans cisternae (Staehelin et al., 1990; Samuels et al., 2002). Margin size across cisternae was therefore quantified by measuring the diameter of the margins of each cisterna. The diameter of round profiles on TGN was also quantified to estimate the size of budding or secretory vesicles. All measurements were conducted in ImageJ.
Golgi Abundance in TEM
For each cell quantified, the number of Golgi or mitochondria (termed ‘counts’) were summed from nonoverlapping images of the cell cortex in wild-type PCW, wild-type Late-SCW, and irx9 SCW cells. For the purposes of quantification of organelle abundance in TEM, Golgi counts included vesicle aggregations, as sections through cisternal margins could not be easily distinguished from the TGN at lower magnifications. Counts/perimeter was then calculated by dividing the number of counts in each cell by the length of the cell perimeter in the images from which the counts were obtained. The area of cytoplasm in each image was then determined (excluding the vacuole, cell wall, plastids, nucleus, and ER bodies ∼1 μm2 or larger) and summed for all images in each cell. Counts/area was calculated by dividing the number of counts in each cell by this summed area. Increases in Golgi diameter would increase the probability of sectioning through individual Golgi in a cell, thereby artificially increasing Golgi counts using this method. To account for this, the percent increase in Golgi width between wild-type PCW and wild-type Late-SCW or irx9 SCW was divided from the respective Golgi/perimeter and Golgi/area averages, thereby normalizing them to the width of wild-type PCW Golgi. Average cytoplasm thickness was determined by dividing the measured perimeter length from the cytoplasmic area. For all parameters quantified, values were averaged for 31 cells taken from four or five seedlings prepared in four replicate high-pressure freezing experiments (wild-type Pre-SCW and wild-type Late-SCW) or 17 cells from seven seedlings in two experiments (irx9 SCW).
Golgi Position in TEM
A subset of the images from the wild-type Late-SCW TEM organelle abundance quantifications were used to estimate the distance to the forming SCW from Golgi/TGN, mitochondria, or random positions in the cytoplasm. A 500-nm grid was laid over each image, and Excel’s random number generator used to select random points on the grid that a Golgi or mitochondrion could be found (i.e. excluding the vacuole, cell wall, nucleus, plastids, and large ER bodies). The shortest distance between each Golgi, mitochondrion, and random point and the nearest SCW was then measured. A total of 114 mitochondria, and 168 random points and Golgi, were quantified from sixteen cells, from five seedlings, in three replicate experiments. Values were square root transformed before statistical analysis to achieve a normal distribution.
TEM Immunogold Labeling and Mapping
Primary antibodies included the anti-xylan antibody CCRC M138 (Carbosource) and a polyclonal anti-GFP (Torrey Pine Biolabs). Secondary antibodies included goat antimouse (CCRC M138) and goat antirabbit (anti-GFP) antibodies conjugated to 10-nm colloidal gold (Ted Pella). For immunogold labeling, wild-type Late-SCW or IRX9-GFP Late-SCW (proIRX9:IRX9-GFP/35S:VND7-VP16-GR) samples were induced for 22–24 h before high-pressure freezing, freeze-substitution, and embedding in LR-white, as described above. Formvar-coated nickel grids with 70-nm sections were blocked with 5% (w/v) nonfat dry milk (NFDM) in 1× Tris-buffered saline/ 0.1% (v/v) Tween 20 (TBST) for 20 min. After removal of excess blocking solution by blotting, grids were transferred to the primary antibody, which was undiluted (CCRC M138) or diluted 1:50 with 1% (w/v) NFDM in 1× TBST (Torrey Pines anti-GFP) for 60 min. After being rinsed with 1× TBST, grids were transferred to appropriate secondary antibodies diluted 1:100 in 1% (w/v) NFDM in 1x TBST for 60 min, rinsed again with 1 x TBST and then thoroughly washed with distilled water. Grids were poststained for 5 min in uranyl acetate and 2 min in lead citrate before imaging as described above.
Ideal antibodies for Golgi mapping would have sufficient label in the Golgi of the cells making SCWs, but little or no label in negative controls. To assess this signal-to-noise ratio, anti-xylan labeling in IRX9-GFP Late-SCW cells with SCWs was compared with cells in the same seedling not making SCWs and with cells with SCWs but not exposed to the primary antibody. Anti-GFP antibodies were tested by comparing labeling in IRX9-GFP Late-SCW to similar cells in the wild-type Late-SCW samples, as well as IRX9-GFP Late-SCW samples not exposed to the primary antibody. Quantification of gold label in anti-xylan negative controls and anti-GFP no-primary-antibody controls was unnecessary as they contained virtually no gold label. For comparison of anti-GFP label in IRX9-GFP for Late-SCW and wild-type Late-SCW, all Golgi and TGN in each cell examined were imaged and the number of gold particles in each Golgi was counted. For this purpose, 493 Golgi/TGN in 39 cells (IRX9-GFP Late-SCW) and 277 Golgi/TGN in 19 cells (wild-type Late-SCW) were imaged from four seedlings in two replicate high-pressure freezing experiments. The difference between anti-GFP label and nonspecific binding was demonstrated by comparing the number of gold particles labeling Golgi in IRX9-GFP Late-SCW and wild-type Late-SCW.
Quantitative mapping in IRX9-GFP was completed using CCRC M138 for xylan labeling and Torrey Pines anti-GFP for IRX9-GFP labeling. Every gold particle in a cell labeling the Golgi or TGN was quantified, and the position of gold particles in the Golgi was assessed when plane-of-section and sample preservation permitted. The results shown consist of data from 24 cells (xylan mapping) and 39 cells (GFP mapping) from four seedlings and two high-pressure freezing experiments. High-resolution mapping of gold label in appropriately oriented sections through Golgi stacks resulted in quantification of 136 gold particles in 17 cells (xylan mapping) and 182 gold particles in 33 cells (GFP mapping).
The cisterna with gold label was identified according to the parameters outlined in the ultrastructure characterization section above. The distance between the gold particle and the cisternal edge, as well was the diameter of the cisterna, was measured. The distance to the cisternal center was calculated by dividing the cisternal width in two and subtracting the distance to the cisternal edge. To compare the cisternal position of xylan and GFP label, the average distance to cisternal centers and cisternal edges was calculated, irrespective of the cisterna in which the gold particle was found. To map the distribution of gold label across Golgi width, the distance to the cisternal edge was converted into a percent of Golgi width for each gold particle. Gold particles mapping to the same cisterna, and the same 10% block of cisternal width, were binned together for generation of the labeling heat maps. Although mapping of gold label to the TGN was largely independent of plane of section and preservation quality, only ∼50% Golgi label could be accurately mapped to a specific region of individual cisternae. As such, the amount of TGN label could not be directly compared with the Golgi-mapping frequencies. Instead, the proportion of TGN vs Golgi label before mapping was used to scale down the amount of TGN label to coincide with the amount of Golgi label that was actually mapped. Heat map colors were generated in Excel (Microsoft) based on these final distributions. For easier comparison of Golgi structure and the mapping distributions, the heat map colors were then manually transferred to a realistic Golgi outline based on Golgi ultrastructure imaging.
Statistical Analysis
Statistical analysis was conducted using SPSS 25 (IBM). Before comparison of means, normality was assessed visually using histograms and Q-Q plots, supplemented with Kolmogorov-Smirnov and Shapiro-Wilkes tests. If the data were approximately normally distributed, a t test for independent means was used to compare two means or a one-way ANOVA was used to compare more than two means. If the ANOVA showed a significant difference, Tukey HSD post hoc analysis was conducted to identify significant differences. If the data were non-normal, data transformations were applied as indicated in the results, and normality retested as described above. If normality was achieved, the above parametric procedures were followed. If the data continued to be non-normal, nonparametric tests were performed with either the Mann-Whitney test (U) for two means or the Kruskal-Wallis test (H) for more than two means followed by post hoc analysis using Dunn’s procedure.
Accession Numbers
Sequence data from this article can be found using the following accession numbers: IRX9, AT2G37090; VND7, AT1G71930; UBQ10, AT4G05320; MANI, AT1G51590.
SUPPLEMENTAL DATA
The following supplemental materials are available.
Supplemental Figure S1. ProIRX9:IRX9-GFP complements the irx9 mutant phenotypes and is expressed in native root protoxylem tracheary elements.
Supplemental Figure S2. Comparing methods for quantifying the abundance of Golgi and mitochondria in WT PCW, WT Late-SCW and irx9 SCW cells from TEM cross-sections.
Supplemental Figure S3. In the complemented irx9 background, IRX9-GFP-labeled Golgi punctae increase in density as SCW deposition progresses compared to MANI-mCherry-labeled Golgi in PCW-producing cells.
Supplemental Figure S4. Identifying cross-sections of Golgi stacks for ultrastructure characterization.
Supplemental Figure S5. Additional quantification of Golgi structural features.
Supplemental Figure S6. Controls for immuno-TEM antibody labeling of xylan and IRX9-GFP.
ACKNOWLEDGMENTS
The authors thank the staff of the UBC Bioimaging Facility for technical support. Taku Demura from the Nara Institute of Science and Technology kindly donated the 35S:VND7-VP16-GR seeds and plasmid.
Footnotes
This work was supported by the Gouvernement of Canada | Natural Sciences and Engineering Research Council of Canada (Conseil de recherches en sciences naturelles et en génie du Canada) (discovery grants to S.D.M. and A.L.S.); Canada postgraduate scholarships (to M.J.M.); and the Faculty of Graduate Studies, University of British Columbia (four-year fellowship to M.J.M.). The authors declare no conflicts of interest.
Articles can be viewed without a subscription.
References
- Boevink P, Oparka K, Santa Cruz S, Martin B, Betteridge A, Hawes C (1998) Stacks on tracks: The plant Golgi apparatus traffics on an actin/ER network. Plant J 15: 441–447 [DOI] [PubMed] [Google Scholar]
- Boutté Y, Jonsson K, McFarlane HE, Johnson E, Gendre D, Swarup R, Friml J, Samuels L, Robert S, Bhalerao RP (2013) ECHIDNA-mediated post-Golgi trafficking of auxin carriers for differential cell elongation. Proc Natl Acad Sci USA 110: 16259–16264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandizzi F, Barlowe C (2013) Organization of the ER-Golgi interface for membrane traffic control. Nat Rev Mol Cell Biol 14: 382–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bromley JR, Busse-Wicher M, Tryfona T, Mortimer JC, Zhang Z, Brown DM, Dupree P (2013) GUX1 and GUX2 glucuronyltransferases decorate distinct domains of glucuronoxylan with different substitution patterns. Plant J 74: 423–434 [DOI] [PubMed] [Google Scholar]
- Brown DM, Goubet F, Wong VW, Goodacre R, Stephens E, Dupree P, Turner SR (2007) Comparison of five xylan synthesis mutants reveals new insight into the mechanisms of xylan synthesis. Plant J 52: 1154–1168 [DOI] [PubMed] [Google Scholar]
- Brown DM, Zhang Z, Stephens E, Dupree P, Turner SR (2009) Characterization of IRX10 and IRX10-like reveals an essential role in glucuronoxylan biosynthesis in Arabidopsis. Plant J 57: 732–746 [DOI] [PubMed] [Google Scholar]
- Chevalier L, Bernard S, Ramdani Y, Lamour R, Bardor M, Lerouge P, Follet-Gueye M-L, Driouich A (2010) Subcompartment localization of the side chain xyloglucan-synthesizing enzymes within Golgi stacks of tobacco suspension-cultured cells. Plant J 64: 977–989 [DOI] [PubMed] [Google Scholar]
- Chou EY, Schuetz M, Hoffmann N, Watanabe Y, Sibout R, Samuels AL (2018) Distribution, mobility, and anchoring of lignin-related oxidative enzymes in Arabidopsis secondary cell walls. J Exp Bot 69: 1849–1859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis MD, Grossniklaus U (2003) A gateway cloning vector set for high-throughput functional analysis of genes in planta. Plant Physiol 133: 462–469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohoe BS, Kang B-H, Gerl MJ, Gergely ZR, McMichael CM, Bednarek SY, Staehelin LA (2013) Cis-Golgi cisternal assembly and biosynthetic activation occur sequentially in plants and algae. Traffic 14: 551–567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donohoe BS, Kang B-H, Staehelin LA (2007) Identification and characterization of COPIa- and COPIb-type vesicle classes associated with plant and algal Golgi. Proc Natl Acad Sci USA 104: 163–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebert B, Rautengarten C, Guo X, Xiong G, Stonebloom S, Smith-Moritz AM, Herter T, Chan LJG, Adams PD, Petzold CJ, et al. (2015) Identification and characterization of a Golgi-localized UDP-xylose transporter family from Arabidopsis. Plant Cell 27: 1218–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebringerová A. (2005) Structural diversity and application potential of hemicelluloses. Macromol Symp 232: 1–12 [Google Scholar]
- Fukuda M, Bierhuizen MF, Nakayama J (1996) Expression cloning of glycosyltransferases. Glycobiology 6: 683–689 [DOI] [PubMed] [Google Scholar]
- Garcia-Herdugo G, González-Reyes JA, Gracia-Navarro F, Navas P (1988) Growth kinetics of the Golgi apparatus during the cell cycle in onion root meristems. Planta 175: 305–312 [DOI] [PubMed] [Google Scholar]
- Glick BS, Luini A (2011) Models for Golgi traffic: A critical assessment. Cold Spring Harb Perspect Biol 3: a005215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grantham NJ, Wurman-Rodrich J, Terrett OM, Lyczakowski JJ, Stott K, Iuga D, Simmons TJ, Durand-Tardif M, Brown SP, Dupree R, et al. (2017) An even pattern of xylan substitution is critical for interaction with cellulose in plant cell walls. Nat Plants 3: 859–865 [DOI] [PubMed] [Google Scholar]
- Grefen C, Donald N, Hashimoto K, Kudla J, Schumacher K, Blatt MR (2010) A ubiquitin-10 promoter-based vector set for fluorescent protein tagging facilitates temporal stability and native protein distribution in transient and stable expression studies. Plant J 64: 355–365 [DOI] [PubMed] [Google Scholar]
- Hirose S, Komamine A (1989) Changes in ultrastructure of Golgi apparatus during the cell cycle in a synchronous culture of Catharanthus roseus. New Phytol 111: 599–605 [DOI] [PubMed] [Google Scholar]
- Ito Y, Uemura T, Nakano A (2014) Formation and maintenance of the Golgi apparatus in plant cells. Int Rev Cell Mol Biol 310: 221–287 [DOI] [PubMed] [Google Scholar]
- Jiang N, Wiemels RE, Soya A, Whitley R, Held M, Faik A (2016) Composition, assembly, and trafficking of a wheat xylan synthase complex. Plant Physiol 170: 1999–2023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang B-H, Nielsen E, Preuss ML, Mastronarde D, Staehelin LA (2011) Electron tomography of RabA4b- and PI-4Kβ1-labeled trans Golgi network compartments in Arabidopsis. Traffic 12: 313–329 [DOI] [PubMed] [Google Scholar]
- Kang B-H, Staehelin LA (2008) ER-to-Golgi transport by COPII vesicles in Arabidopsis involves a ribosome-excluding scaffold that is transferred with the vesicles to the Golgi matrix. Protoplasma 234: 51–64 [DOI] [PubMed] [Google Scholar]
- Keppler BD, Showalter AM (2010) IRX14 and IRX14-LIKE, two glycosyl transferases involved in glucuronoxylan biosynthesis and drought tolerance in Arabidopsis. Mol Plant 3: 834–841 [DOI] [PubMed] [Google Scholar]
- Kim JS, Daniel G (2012) Immunolocalization of hemicelluloses in Arabidopsis thaliana stem. Part I: Temporal and spatial distribution of xylans. Planta 236: 1275–1288 [DOI] [PubMed] [Google Scholar]
- Koga D, Ushiki T (2006) Three-dimensional ultrastructure of the Golgi apparatus in different cells: High-resolution scanning electron microscopy of osmium-macerated tissues. Arch Histol Cytol 69: 357–374 [DOI] [PubMed] [Google Scholar]
- Kuang B, Zhao X, Zhou C, Zeng W, Ren J, Ebert B, Beahan CT, Deng X, Zeng Q, Zhou G, et al. (2016) The role of UDP-glucuronic acid decarboxylase (UXS) in xylan biosynthesis in Arabidopsis. Mol Plant 9: 1119–1131 [DOI] [PubMed] [Google Scholar]
- Kubo M, Udagawa M, Nishikubo N, Horiguchi G, Yamaguchi M, Ito J, Mimura T, Fukuda H, Demura T (2005) Transcription switches for protoxylem and metaxylem vessel formation. Genes Dev 19: 1855–1860 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladinsky MS, Mastronarde DN, McIntosh JR, Howell KE, Staehelin LA (1999) Golgi structure in three dimensions: Functional insights from the normal rat kidney cell. J Cell Biol 144: 1135–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langhans M, Hawes C, Hillmer S, Hummel E, Robinson DG (2007) Golgi regeneration after brefeldin A treatment in BY-2 cells entails stack enlargement and cisternal growth followed by division. Plant Physiol 145: 527–538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavieu G, Zheng H, Rothman JE (2013) Stapled Golgi cisternae remain in place as cargo passes through the stack. eLife 2: e00558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, O’Neill MA, Tsumuraya Y, Darvill AG, Ye ZH (2007) The irregular xylem9 mutant is deficient in xylan xylosyltransferase activity. Plant Cell Physiol 48: 1624–1634 [DOI] [PubMed] [Google Scholar]
- Lee C, Teng Q, Huang W, Zhong R, Ye Z-H (2010) The Arabidopsis family GT43 glycosyltransferases form two functionally nonredundant groups essential for the elongation of glucuronoxylan backbone. Plant Physiol 153: 526–541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee C, Teng Q, Zhong R, Yuan Y, Haghighat M, Ye Z-H (2012) Three Arabidopsis DUF579 domain-containing GXM proteins are methyltransferases catalyzing 4-o-methylation of glucuronic acid on xylan. Plant Cell Physiol 53: 1934–1949 [DOI] [PubMed] [Google Scholar]
- Losev E, Reinke CA, Jellen J, Strongin DE, Bevis BJ, Glick BS (2006) Golgi maturation visualized in living yeast. Nature 441: 1002–1006 [DOI] [PubMed] [Google Scholar]
- Lu L, Lee Y-RJ, Pan R, Maloof JN, Liu B (2005) An internal motor kinesin is associated with the Golgi apparatus and plays a role in trichome morphogenesis in Arabidopsis. Mol Biol Cell 16: 811–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manders EMM, Verbeek FJ, Aten JA (1993) Measurement of co-localization of objects in dual-colour confocal images. J Microsc 169: 375–382 [DOI] [PubMed] [Google Scholar]
- Matsuura-Tokita K, Takeuchi M, Ichihara A, Mikuriya K, Nakano A (2006) Live imaging of yeast Golgi cisternal maturation. Nature 441: 1007–1010 [DOI] [PubMed] [Google Scholar]
- McCartney L, Marcus SE, Knox JP (2005) Monoclonal antibodies to plant cell wall xylans and arabinoxylans. J Histochem Cytochem 53: 543–546 [DOI] [PubMed] [Google Scholar]
- McFarlane HE, Young RE, Wasteneys GO, Samuels AL (2008) Cortical microtubules mark the mucilage secretion domain of the plasma membrane in Arabidopsis seed coat cells. Planta 227: 1363–1375 [DOI] [PubMed] [Google Scholar]
- Meents MJ, Watanabe Y, Samuels AL (2018) The cell biology of secondary cell wall biosynthesis. Ann Bot 121: 1107–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogelsvang S, Gomez-Ospina N, Soderholm J, Glick BS, Staehelin LA (2003) Tomographic evidence for continuous turnover of Golgi cisternae in Pichia pastoris. Mol Biol Cell 14: 2277–2291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mogelsvang S, Marsh BJ, Ladinsky MS, Howell KE (2004) Predicting function from structure: 3D structure studies of the mammalian Golgi complex. Traffic 5: 338–345 [DOI] [PubMed] [Google Scholar]
- Mollenhauer HH, Morré DJ (1998) The tubular network of the Golgi apparatus. Histochem Cell Biol 109: 533–543 [DOI] [PubMed] [Google Scholar]
- Moore PJ, Swords KMM, Lynch MA, Staehelin LA (1991) Spatial organization of the assembly pathways of glycoproteins and complex polysaccharides in the Golgi apparatus of plants. J Cell Biol 112: 589–602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer JC, Miles GP, Brown DM, Zhang Z, Segura MP, Weimar T, Yu X, Seffen KA, Stephens E, Turner SR, et al. (2010) Absence of branches from xylan in Arabidopsis gux mutants reveals potential for simplification of lignocellulosic biomass. Proc Natl Acad Sci USA 107: 17409–17414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naramoto S, Otegui MS, Kutsuna N, de Rycke R, Dainobu T, Karampelias M, Fujimoto M, Feraru E, Miki D, Fukuda H, et al. (2014) Insights into the localization and function of the membrane trafficking regulator GNOM ARF-GEF at the Golgi apparatus in Arabidopsis. Plant Cell 26: 3062–3076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebenführ A, Gallagher LA, Dunahay TG, Frohlick JA, Mazurkiewicz AM, Meehl JB, Staehelin LA (1999) Stop-and-go movements of plant Golgi stacks are mediated by the acto-myosin system. Plant Physiol 121: 1127–1142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebenführ A, Ritzenthaler C, Robinson DG (2002) Brefeldin A: Deciphering an enigmatic inhibitor of secretion. Plant Physiol 130: 1102–1108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson BK, Cai X, Nebenführ A (2007) A multicolored set of in vivo organelle markers for co-localization studies in Arabidopsis and other plants. Plant J 51: 1126–1136 [DOI] [PubMed] [Google Scholar]
- Park S-Y, Yang J-S, Schmider AB, Soberman RJ, Hsu VW (2015) Coordinated regulation of bidirectional COPI transport at the Golgi by CDC42. Nature 521: 529–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattathil S, Avci U, Baldwin D, Swennes AG, McGill JA, Popper Z, Bootten T, Albert A, Davis RH, Chennareddy C, et al. (2010) A comprehensive toolkit of plant cell wall glycan-directed monoclonal antibodies. Plant Physiol 153: 514–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña MJ, Kulkarni AR, Backe J, Boyd M, O’Neill MA, York WS (2016) Structural diversity of xylans in the cell walls of monocots. Planta 244: 589–606 [DOI] [PubMed] [Google Scholar]
- Peña MJ, Zhong R, Zhou G-K, Richardson EA, O’Neill MA, Darvill AG, York WS, Ye Z-H (2007) Arabidopsis irregular xylem8 and irregular xylem9: Implications for the complexity of glucuronoxylan biosynthesis. Plant Cell 19: 549–563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peralta AG, Venkatachalam S, Stone SC, Pattathil S (2017) Xylan epitope profiling: An enhanced approach to study organ development-dependent changes in xylan structure, biosynthesis, and deposition in plant cell walls. Biotechnol Biofuels 10: 245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen PD, Lau J, Ebert B, Yang F, Verhertbruggen Y, Kim JS, Varanasi P, Suttangkakul A, Auer M, Loqué D, Scheller HV (2012) Engineering of plants with improved properties as biofuels feedstocks by vessel-specific complementation of xylan biosynthesis mutants. Biotechnol Biofuels 5: 84–102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennie EA, Hansen SF, Baidoo EEK, Hadi MZ, Keasling JD, Scheller HV (2012) Three members of the Arabidopsis glycosyltransferase family 8 are xylan glucuronosyltransferases. Plant Physiol 159: 1408–1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rennie EA, Scheller HV (2014) Xylan biosynthesis. Curr Opin Biotechnol 26: 100–107 [DOI] [PubMed] [Google Scholar]
- Ritzenthaler C, Nebenführ A, Movafeghi A, Stussi-Garaud C, Behnia L, Pimpl P, Staehelin LA, Robinson DG (2002) Reevaluation of the effects of brefeldin A on plant cells using tobacco Bright Yellow 2 cells expressing Golgi-targeted green fluorescent protein and COPI antisera. Plant Cell 14: 237–261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rizzo R, Parashuraman S, Mirabelli P, Puri C, Lucocq J, Luini A (2013) The dynamics of engineered resident proteins in the mammalian Golgi complex relies on cisternal maturation. J Cell Biol 201: 1027–1036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruprecht C, Bartetzko MP, Senf D, Dallabernadina P, Boos I, Andersen MCF, Kotake T, Knox JP, Hahn MG, Clausen MH, Pfrengle F (2017) A synthetic glycan microarray enables epitope mapping of plant cell wall glycan-directed antibodies. Plant Physiol 175: 1094–1104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samuels AL, Rensing KH, Douglas CJ, Mansfield SD, Dharmawardhana DP, Ellis BE (2002) Cellular machinery of wood production: Differentiation of secondary xylem in Pinus contorta var. latifolia. Planta 216: 72–82 [DOI] [PubMed] [Google Scholar]
- Scheller HV, Ulvskov P (2010) Hemicelluloses. Annu Rev Plant Biol 61: 263–289 [DOI] [PubMed] [Google Scholar]
- Schneider R, Tang L, Lampugnani ER, Barkwill S, Lathe R, Zhang Y, McFarlane HE, Pesquet E, Niittyla T, Mansfield SD, Zhou Y, Persson S (2017) Two complementary mechanisms underpin cell wall patterning during xylem vessel development. Plant Cell 29: 2433–2449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoberer J, Strasser R (2011) Sub-compartmental organization of Golgi-resident N-glycan processing enzymes in plants. Mol Plant 4: 220–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seguí-Simarro JM, Staehelin LA (2006) Cell cycle-dependent changes in Golgi stacks, vacuoles, clathrin-coated vesicles and multivesicular bodies in meristematic cells of Arabidopsis thaliana: A quantitative and spatial analysis. Planta 223: 223–236 [DOI] [PubMed] [Google Scholar]
- Staehelin LA, Giddings TH Jr., Kiss JZ, Sack FD (1990) Macromolecular differentiation of Golgi stacks in root tips of Arabidopsis and Nicotiana seedlings as visualized in high pressure frozen and freeze-substituted samples. Protoplasma 157: 75–91 [DOI] [PubMed] [Google Scholar]
- Staehelin LA, Kang B-H (2008) Nanoscale architecture of endoplasmic reticulum export sites and of Golgi membranes as determined by electron tomography. Plant Physiol 147: 1454–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyooka K, Goto Y, Asatsuma S, Koizumi M, Mitsui T, Matsuoka K (2009) A mobile secretory vesicle cluster involved in mass transport from the Golgi to the plant cell exterior. Plant Cell 21: 1212–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toyooka K, Sato M, Kutsuna N, Higaki T, Sawaki F, Wakazaki M, Goto Y, Hasezawa S, Nagata N, Matsuoka K (2014) Wide-range high-resolution transmission electron microscopy reveals morphological and distributional changes of endomembrane compartments during log to stationary transition of growth phase in tobacco BY-2 cells. Plant Cell Physiol 55: 1544–1555 [DOI] [PubMed] [Google Scholar]
- Uemura T. (2016) Physiological roles of plant post-golgi transport pathways in membrane trafficking. Plant Cell Physiol 57: 2013–2019 [DOI] [PubMed] [Google Scholar]
- Urbanowicz BR, Peña MJ, Moniz HA, Moremen KW, York WS (2014) Two Arabidopsis proteins synthesize acetylated xylan in vitro. Plant J 80: 197–206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urbanowicz BR, Peña MJ, Ratnaparkhe S, Avci U, Backe J, Steet HF, Foston M, Li H, O’Neill MA, Ragauskas AJ, et al. (2012) 4-O-methylation of glucuronic acid in Arabidopsis glucuronoxylan is catalyzed by a domain of unknown function family 579 protein. Proc Natl Acad Sci USA 109: 14253–14258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang P, Chen X, Goldbeck C, Chung E, Kang B-H (2017) A distinct class of vesicles derived from the trans-Golgi mediates secretion of xylogalacturonan in the root border cell. Plant J 92: 596–610 [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Meents MJ, McDonnell LM, Barkwill S, Sampathkumar A, Cartwright HN, Demura T, Ehrhardt DW, Samuels AL, Mansfield SD (2015) Visualization of cellulose synthases in Arabidopsis secondary cell walls. Science 350: 198–203 [DOI] [PubMed] [Google Scholar]
- Watanabe Y, Schneider R, Barkwill S, Gonzales-Vigil E, Hill JL Jr., Samuels AL, Persson S, Mansfield SD (2018) Cellulose synthase complexes display distinct dynamic behaviors during xylem transdifferentiation. Proc Natl Acad Sci USA 115: E6366–E6374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidman P, Roth R, Heuser J (1993) Golgi membrane dynamics imaged by freeze-etch electron microscopy: Views of different membrane coatings involved in tubulation versus vesiculation. Cell 75: 123–133 [PubMed] [Google Scholar]
- Wightman R, Turner SR (2008) The roles of the cytoskeleton during cellulose deposition at the secondary cell wall. Plant J 54: 794–805 [DOI] [PubMed] [Google Scholar]
- Wu A-M, Hörnblad E, Voxeur A, Gerber L, Rihouey C, Lerouge P, Marchant A (2010) Analysis of the Arabidopsis IRX9/IRX9-L and IRX14/IRX14-L pairs of glycosyltransferase genes reveals critical contributions to biosynthesis of the hemicellulose glucuronoxylan. Plant Physiol 153: 542–554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu A-M, Rihouey C, Seveno M, Hörnblad E, Singh SK, Matsunaga T, Ishii T, Lerouge P, Marchant A (2009) The Arabidopsis IRX10 and IRX10-LIKE glycosyltransferases are critical for glucuronoxylan biosynthesis during secondary cell wall formation. Plant J 57: 718–731 [DOI] [PubMed] [Google Scholar]
- Xiong G, Cheng K, Pauly M (2013) Xylan O-acetylation impacts xylem development and enzymatic recalcitrance as indicated by the Arabidopsis mutant tbl29. Mol Plant 6: 1373–1375 [DOI] [PubMed] [Google Scholar]
- Yamaguchi M, Goué N, Igarashi H, Ohtani M, Nakano Y, Mortimer JC, Nishikubo N, Kubo M, Katayama Y, Kakegawa K, Dupree P, Demura T (2010a) VASCULAR-RELATED NAC-DOMAIN6 and VASCULAR-RELATED NAC-DOMAIN7 effectively induce transdifferentiation into xylem vessel elements under control of an induction system. Plant Physiol 153: 906–914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi M, Ohtani M, Mitsuda N, Kubo M, Ohme-Takagi M, Fukuda H, Demura T (2010b) VND-INTERACTING2, a NAC domain transcription factor, negatively regulates xylem vessel formation in Arabidopsis. Plant Cell 22: 1249–1263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J-S, Valente C, Polishchuk RS, Turacchio G, Layre E, Moody DB, Leslie CC, Gelb MH, Brown WJ, Corda D, Luini A, Hsu VW (2011) COPI acts in both vesicular and tubular transport. Nat Cell Biol 13: 996–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- York WS, O’Neill MA (2008) Biochemical control of xylan biosynthesis - which end is up? Curr Opin Plant Biol 11: 258–265 [DOI] [PubMed] [Google Scholar]
- Young RE, McFarlane HE, Hahn MG, Western TL, Haughn GW, Samuels AL (2008) Analysis of the Golgi apparatus in Arabidopsis seed coat cells during polarized secretion of pectin-rich mucilage. Plant Cell 20: 1623–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan Y, Teng Q, Zhong R, Ye Z-H (2013) The Arabidopsis DUF231 domain-containing protein ESK1 mediates 2-O- and 3-O-acetylation of xylosyl residues in xylan. Plant Cell Physiol 54: 1186–1199 [DOI] [PubMed] [Google Scholar]
- Yuan Y, Teng Q, Zhong R, Ye Z-H (2016a) Roles of Arabidopsis TBL34 and TBL35 in xylan acetylation and plant growth. Plant Sci 243: 120–130 [DOI] [PubMed] [Google Scholar]
- Yuan Y, Teng Q, Zhong R, Ye ZH (2016b) TBL3 and TBL31, two Arabidopsis DUF231 domain proteins, are required for 3-O-monoacetylation of xylan. Plant Cell Physiol 57: 35–45 [DOI] [PubMed] [Google Scholar]
- Zeng W, Jiang N, Nadella R, Killen TL, Nadella V, Faik A (2010) A glucurono(arabino)xylan synthase complex from wheat contains members of the GT43, GT47, and GT75 families and functions cooperatively. Plant Physiol 154: 78–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng W, Lampugnani ER, Picard KL, Song L, Wu AM, Farion IM, Zhao J, Ford K, Doblin MS, Bacic A (2016) Asparagus IRX9, IRX10, and IRX14A are components of an active xylan backbone synthase complex that rorms in the Golgi apparatus. Plant Physiol 171: 93–109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Zhang L, Li F, Zhang D, Liu X, Wang H, Xu Z, Chu C, Zhou Y (2017) Control of secondary cell wall patterning involves xylan deacetylation by a GDSL esterase. Nat Plants 3: 17017. [DOI] [PubMed] [Google Scholar]
- Zhang GF, Staehelin LA (1992) Functional compartmentation of the Golgi apparatus of plant cells: Immunocytochemical analysis of high-pressure frozen- and freeze-substituted sycamore maple suspension culture cells. Plant Physiol 99: 1070–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Nikolovski N, Sorieul M, Vellosillo T, McFarlane HE, Dupree R, Kesten C, Schneider R, Driemeier C, Lathe R, et al. (2016) Golgi-localized STELLO proteins regulate the assembly and trafficking of cellulose synthase complexes in Arabidopsis. Nat Commun 7: 11656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong R, Cui D, Ye Z-H (2017a) Regiospecific acetylation of xylan is mediated by a group of DUF231-containing O-acetyltransferases. Plant Cell Physiol 58: 2126–2138 [DOI] [PubMed] [Google Scholar]
- Zhong R, Peña MJ, Zhou G-K, Nairn CJ, Wood-Jones A, Richardson EA, Morrison WH III, Darvill AG, York WS, Ye ZH (2005) Arabidopsis fragile fiber8, which encodes a putative glucuronyltransferase, is essential for normal secondary wall synthesis. Plant Cell 17: 3390–3408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong R, Teng Q, Haghighat M, Yuan Y, Furey ST, Dasher RL, Ye Z-H (2017b) Cytosol-localized UDP-xylose synthases provide the major source of UDP-xylose for the biosynthesis of xylan and xyloglucan. Plant Cell Physiol 58: 156–174 [DOI] [PubMed] [Google Scholar]