ABSTRACT
Axis specification is a fundamental developmental process. Despite this, the mechanisms by which it is controlled across insect taxa are strikingly different. An excellent example of this is terminal patterning, which in Diptera such as Drosophila melanogaster occurs via the localized activation of the receptor tyrosine kinase Torso. In Hymenoptera, however, the same process appears to be achieved via localized mRNA. How these mechanisms evolved and what they evolved from remains largely unexplored. Here, we show that torso-like, known for its role in Drosophila terminal patterning, is instead required for the integrity of the vitelline membrane in the hymenopteran wasp Nasonia vitripennis. We find that other genes known to be involved in Drosophila terminal patterning, such as torso and Ptth, also do not function in Nasonia embryonic development. These findings extended to orthologues of Drosophila vitelline membrane proteins known to play a role in localizing Torso-like in Drosophila; in Nasonia these are instead required for dorso–ventral patterning, gastrulation and potentially terminal patterning. Our data underscore the importance of the vitelline membrane in insect development, and implies phenotypes caused by knockdown of torso-like must be interpreted in light of its function in the vitelline membrane. In addition, our data imply that the signalling components of the Drosophila terminal patterning systems were co-opted from roles in regulating moulting, and co-option into terminal patterning involved the evolution of a novel interaction with the vitelline membrane protein Torso-like.
This article has an associated First Person interview with the first author of the paper.
KEY WORDS: Vitelline membrane, Terminal patterning, Axis formation, Nasonia, Drosophila, Evolution of development
Summary: In the parasitic wasp Nasonia, Tsl, a key component of the process that defines the termini of the embryo of Drosophila, has a function in the structure of the vitelline membrane.
INTRODUCTION
Establishment of the major axes and termini are some of the first events to take place during insect embryonic development. In Drosophila, the termini are patterned via the localized activation of the receptor tyrosine kinase Torso (Tor) by its ligand Trunk (Trk) which results in expression of the transcription factors Tailless (Tll) (Klingler et al., 1988; Strecker et al., 1989) and Huckebein (Bronner and Jackle, 1991, 1996) at the anterior and posterior of the embryo.
While localized activation of tailless is required at the termini, neither trk nor torso expression is localized (Casanova et al., 1995; Sprenger et al., 1989). Localization of signalling is provided by torso-like (tsl) (Savant-Bhonsale and Montell, 1993; Stevens et al., 1990), which encodes a protein present only at the termini of the embryo (Stevens et al., 2003). Tsl is expressed in subpopulations of follicle cells present at the anterior and posterior poles of the developing oocyte (Furriols et al., 2007; Stevens et al., 1990) and is secreted into the extracellular space between the follicle cells and oocyte where it associates with the vitelline membrane (VM) (Jiménez et al., 2002; Stevens et al., 2003). At the onset of embryogenesis, Tsl is proposed to transfer from the inner VM to the terminal plasma membrane of the embryo (Mineo et al., 2015; Stevens et al., 2003). Ectopic expression of tsl in all follicle cells causes the protein to be transported to the entire plasma membrane (Mineo et al., 2015) and loss of proteins that stabilize Tsl in the VM cause terminal pattering defects in addition to VM integrity problems (Ventura et al., 2010). The long-standing model is that Tsl localizes the activity of the Tor ligand (Trk) to the terminal regions, possibly via a mechanism involving the regulation of Trk secretion (Johnson et al., 2015), although other models have also been proposed (Amarnath et al., 2017; Mineo et al., 2018a,b).
The Torso pathway has other roles during development, such as in the initiation of metamorphosis (Rewitz et al., 2009). In this role, Tor responds to a different ligand, but one similar to Trk, prothoracicotropic hormone (PTTH). Tsl also has a role in regulating metamorphosis, however this is independent of Tor/PTTH signalling, and appears to be mediated via an effect on insulin signalling (Grillo et al., 2012; Henstridge et al., 2018; Johnson et al., 2013). Thus, the mechanism that controls Tor activation in the embryo is distinct from the one used to initiate metamorphosis. This implies that upstream factors involved in Tor activation (which we call the Tor activation cassette, or TAC) could be readily gained and lost during evolution.
Aside from these roles, tsl is also known to act in the development of the Drosophila immune system (Forbes-Beadle et al. 2016) and in morphogenesis (Johnson et al. 2017). In the latter, maternal tsl (but not tor) is required for formation of the ventral furrow during gastrulation.
Beyond Drosophila, the functions of tsl, and the other TAC genes, are unclear. In Tribolium, tsl, tor and trk are required for posterior terminal patterning (Schoppmeier and Schroder 2005, Grillo et al., 2012). Tsl protein and RNA are expressed at the termini of the oocyte (Schoppmeier and Schroder 2005; Baumer et al. 2012), and (like Drosophila) no localized expression of tor or trk has been reported. In hymenoptera (bees, wasps, ants and others), trk is missing from multiple sequenced genomes implying its loss early in hymenopteran evolution (Skelly et al., 2018). PTTH is missing from a more limited subset often correlating with loss of tor. Terminal patterning, via tll activation, instead relies on maternal localization of orthodenticle and/or tailless RNA (Lynch et al., 2006a,b; Wilson and Dearden, 2009, 2011). In the honeybee, where PTTH, trk and tor are absent (Dearden et al., 2006), maternal localization of posterior tll patterns the posterior terminal, while anteriorly expressed otd activates anterior tll expression (Wilson and Dearden, 2009). The parasitoid wasp, Nasonia (which lacks only trk), patterns its termini via localized maternal otd at both ends of the embryo (Lynch et al., 2006a,b). Since its genome contains a tsl gene, but one unlikely to be involved in terminal patterning, Nasonia provides an opportunity to explore conserved roles for tsl and may help us to understand how this gene has come to be involved in terminal patterning in Drosophila.
RESULTS
Nasonia tsl is expressed during oogenesis and in developing embryos
A nasonia tsl sequence was identified and its phylogenetic position assessed with respect to pan-arthropod Tsl proteins in (Skelly et al., 2018). We refer to this gene (NCBI LOC100118858) as Nv-tsl.
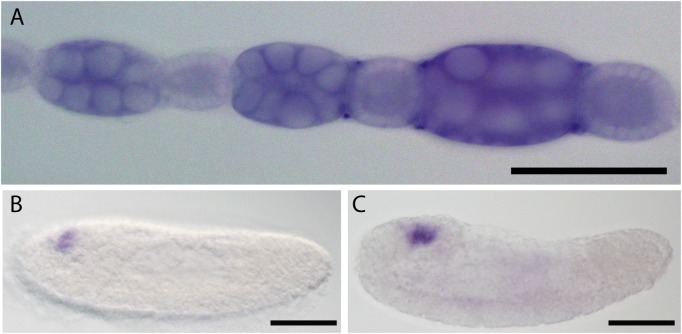
To determine if Nv-tsl is expressed in a pattern related to axis formation we investigated the expression of Nv-tsl RNA in Nasonia ovaries and embryos. In the ovary, Nv-tsl RNA is detected strongly in nurse cells, and weakly but ubiquitously in the oocyte and follicle cells, in a non-stage-specific manner (Fig. 1A). There is no specific localization of Nv-tsl RNA at the embryo termini, however we cannot rule out that protein is localized to the terminal regions. In the early embryo, Nv-tsl RNA is not expressed at the termini. Instead it is expressed in the embryonic brain during germband elongation (Fig. 1B,C). This expression pattern is consistent with tsl expression in the visual anlagen in Drosophila and near the cephalic lobe in aphids (Duncan et al., 2013) and may indicate a conserved role for tsl in brain function.
Fig. 1.
Nv-tsl expression in the Nasonia ovary and embryo as detected by in-situ hybridization. (A) Nv-tsl RNA is present in all cell types of the Nasonia ovary. Staining is darker in nurse cells than the oocyte, consistent with maternal expression and RNA transport into oocytes. Dark spots at the outer boundary of oocyte and nurse cell clusters are an artifact due to probe trapping. (B,C) Nv-tsl is expressed in the embryonic brain in Nasonia embryos after germband extension. Negative controls shown in Fig. S3. Scale bars: 100 μm.
Knockdown of Nv-tsl causes egg failure
The maternal expression of Nv-tsl implies that it has a function in the ovary in Nasonia. To investigate this, we reduced Nv-tsl expression using maternal RNAi. This method has been used previously in Nasonia to investigate early axis formation (Lynch et al., 2006; Lynch and Desplan, 2006) and ovarian development (Lynch and Desplan 2006). Embryos collected from Nv-tsl RNAi-treated wasps (Nv-tsl knockdown) had a severe phenotype where many failed to form a cuticle, and thus embryonic structures, after 24 h (Fig. 2A,B). This implies that the embryos do not survive to form a cuticle, and reduction of Nv-tsl has an early, lethal effect on development. Surviving embryos were morphologically indistinguishable from wild type and are likely unaffected by RNAi, which is incompletely penetrant in Nasonia (Lynch and Desplan, 2006). Supporting this, Nv-tsl expression in Nv-tsl knockdown wasp ovaries was approximately half that of control wasps (see Fig. S1, P=0.0031, unpaired t-test, effect size=5.2, Cohen's d-test). Given the significant lethal effect of Nv-tsl parental RNAi, and the presumption that parental RNAi effects do not last all through embryogenesis (Rosenberg et al. 2014), we cannot determine in this experiment whether Nv-tsl acts in later developmental processes such as egg activation, terminal patterning or gastrulation. Nv-tsl may have roles in these processes that are masked by early lethality prior to the formation of cuticular pattern elements.
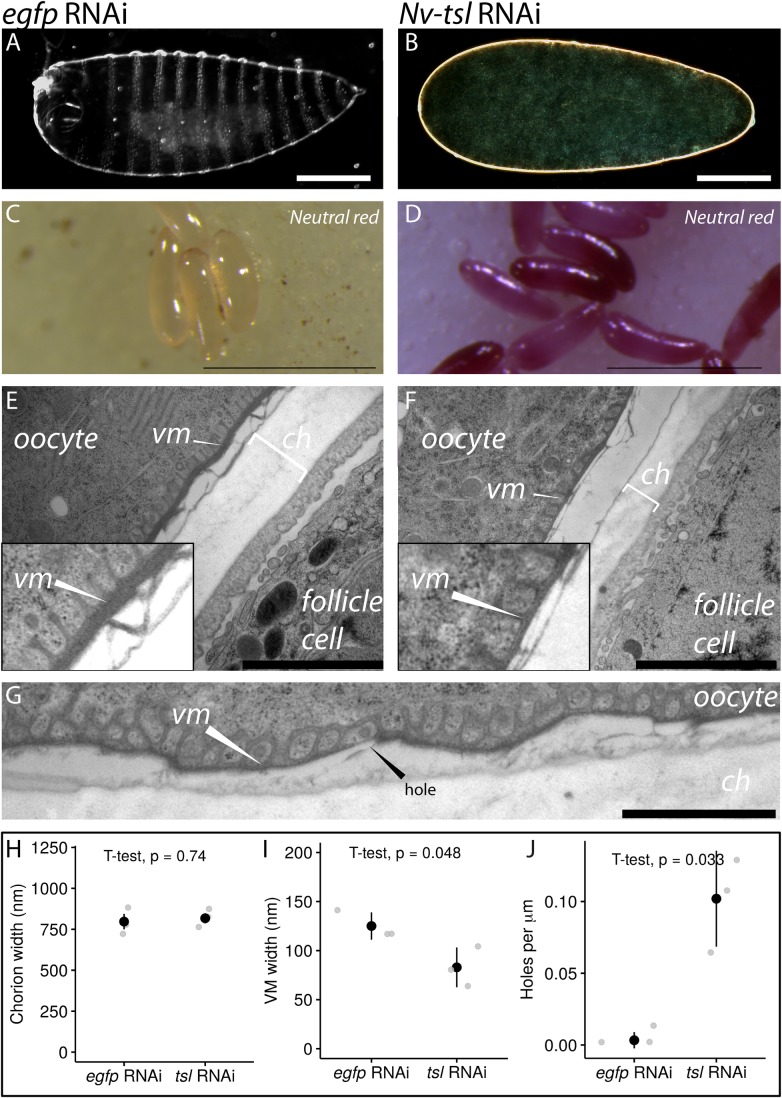
Fig. 2.
Nv-tsl is necessary for VM integrity in Nasonia. (A,B) Cuticle preparations of egfp RNAi (A) and Nv-tsl RNAi (B) embryos. Scale bars: 100 μm. (C,D) Embryos from RNAi-injected females following Neutral Red assay. Egfp RNAi embryos (C) show no appreciable staining with Neutral Red, while Nv-tsl RNAi (D) embryos stain consistently. Scale bars: 500 µm. (E,F) Transmission electron micrographs of egfp RNAi (E) and Nv-tsl RNAi (F) ovaries. Scale bars: 2000 nm. ch, chorion (clear region); vm, vitelline membrane (electron dense region). The gap between the chorion and VM is due to the oocyte peeling away from the follicle cells. Insets show the location and thickness of the vitelline membrane in each treatment. (G) Transmission electron micrograph of Nv-tsl RNAi ovary showing thin chorion with a hole (marked). Scale bar: 1000 nm. (H) Quantification of chorion width for egfp and Nv-tsl RNAi-treated ovaries. For each image, the mean chorion width was measured. Data from 2–11 images (technical replicates) were averaged for each biological replicate. There is no significant difference between chorion width between egfp and Nv-tsl RNAi-treated ovaries. (I) Quantification of VM width for egfp and Nv-tsl RNAi-treated ovaries. For each image, the mean VM width was measured. Data from 2–11 images (technical replicates) were averaged for each biological replicate. VM width differs significantly between egfp and Nv-tsl RNAi-treated ovaries. (J) Quantification of gaps in the VM for egfp and Nv-tsl RNAi-treated ovaries. The number of gaps per micron in each technical replicate was averaged to produce the mean number per biological replicate. Egfp RNAi-treated ovaries have significantly fewer gaps than those treated by Nv-tsl RNAi.
We infer that the lack of cuticle structure formation after 24 h indicates embryos were dying early in development. Indeed, some early (0–4 h old) embryos that we collected were flaccid to the touch. This phenotype is characteristic of a defect in one of the membranes surrounding the egg, the VM, a proteinaceous, heavily cross-linked matrix surrounding the oocyte (Waring, 2000). To further investigate this, we exposed embryos to Neutral Red, a vital dye used to reveal VM integrity problems in Drosophila (LeMosy and Hashimoto, 2000; Ventura et al., 2010; Waring, 2000) and eggshell integrity in Aedes aegypti (Isoe et al., 2019). Embryos with impaired eggshell integrity take up the dye and stain bright red. When Nv-tsl knockdown embryos were exposed to Neutral Red, 63% became stained, compared to 4.5% of embryos from control wasps injected with double-stranded RNA against the gene encoding enhanced green fluorescence protein (egfp RNAi) (Fig. 2C,D). This implies that Nv-tsl may be required for an aspect of eggshell biogenesis. Two membranes surround insect oocytes; outside the plasma membrane of the egg is the VM and beyond it lies the chorion. Since the chorions of embryos were not removed prior to Neutral Red staining, (as this is very difficult in Nasonia), our assay cannot distinguish between defects in the chorion and VM in Nasonia.
Nv-tsl knockdown oocytes have defective VMs
As Nv-tsl RNAi embryos have early lethal phenotypes, we examined the ultrastructure of the ovary after RNAi against Nv-tsl. No morphological differences were detected between Nv-tsl knockdown and egfp knockdown using confocal microscopy after staining for actin and DNA (see Fig. S1). All key cell types; the follicle cells, nurse cells and oocytes, are indistinguishable from controls. In addition, there are no visible holes in the eggshell, and the oocyte nucleus is properly positioned. This implies that Nv-tsl does not interfere with gross ovary structure.
We therefore reasoned that the lack of visible eggshell defects might be due to insufficient resolution. To determine if Nv-tsl is required for VM or chorion integrity, we used high-pressure freezing and imaged Nv-tsl knockdown and egfp knockdown Nasonia ovaries using transmission electron microscopy.
Under electron microscopy, the chorion appears as a thick, electron-lucent (pale) layer. Comparing between Nv-tsl RNAi-treated and controls we found no significant differences in thickness of the chorion (Fig. 2G, t-test, P=0.72), nor were there any noticeable structural defects.
We next looked to see whether VM integrity might be compromised in tsl RNAi embryos.
The VM is a thin, electron-dense layer closely associated with the eggshell (Richards, 1969). Projections from the VM protrude towards the oocyte (Fig. 2E,F). We first noted that the VM of mature Nv-tsl RNAi oocytes appeared considerably thinner than that of controls. In addition, regions of the Nv-tsl knockdown VM contained holes that were rarely observed in control ovaries of equivalent stage (Fig. 2G).
To confirm this, we measured the VM thickness in micrographs where the VM appeared as a straight line, as we found a high degree of variability in regions where the VM curves around the oocyte surface. The average VM width of control ovaries was 125±35 nm, whereas the Nv-tsl knockdown VM was significantly thinner at 83±50 nm (P=0.048, effect size=−2.4, unpaired t-test and Cohen's d-test, n=3) (Fig. 2I). As another measure of VM integrity, we also counted the number of holes in the VMs of these wasp eggs (per micron of VM). We found that Nv-tsl knockdown eggs had significantly more VM holes than controls (P=0.033, unpaired t-test, effect size=4.1, Cohen's d test, n=3) (Fig. 2J). Taken together, our data imply that Nv-tsl knockdown in ovaries causes eggshell permeability via compromised VM integrity.
Overexpression of Nv-tsl in Drosophila produces a spliced phenotype but does not rescue a tsl null mutation
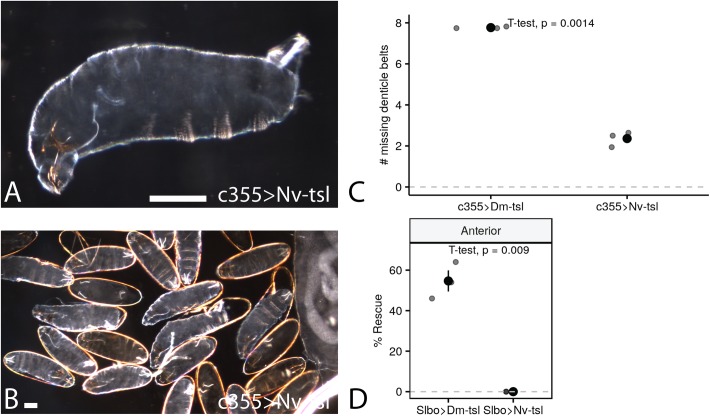
To determine if Nv-tsl has a different biochemical function to Drosophila tsl, consistent with its different biological effects when knocked down in Nasonia, we overexpressed Nv-tsl in Drosophila ovary tissue using the Gal4/UAS system (Brand et al., 1994) (Fig. 3A,B). When overexpressed from all follicle cells (via c355-Gal4) Nv-tsl causes loss of approximately two denticle belts on average per embryo (Fig. 3C), though to a lesser degree than Dm-tsl (loss of 7.8 denticle belts per embryo; Fig. 3C). We note that denticle belt loss is never observed in control embryos (c355-Gal4 alone), and that the denticle belt loss in both Dm-tsl and Nv-tsl overexpression embryos is statistically different to zero (Dm-tsl: P=2.40e-5; Nv-tsl: P=0.0162, one-sample t-test, n=3, mu=0, Bonferroni error correction). This denticle belt loss, known as a ‘spliced’ phenotype, results from expansion of the termini at the expense of central segments and is diagnostic of non-localized activation of terminal patterning (Klingler et al., 1988; Savant-Bhonsale and Montell, 1993). We further found that Nv-tsl is unable to rescue anterior terminal patterning in a Drosophila tsl null mutant background (Fig. 3D) when expressed using a strong anterior follicle cell driver (slbo-Gal4, P=0.18, one-sample t-test, n=3, mu=0, Bonferroni error correction). In this situation, Dm-tsl expression fully rescues more than half of the tested embryos (Fig. 3D, P=0.009, one-sample t-test, n=3, mu=0, Bonferroni error correction). This implies that Nv-tsl has a biochemical function similar to, but less effective than, Dm-tsl when placed in the Dm terminal system.
Fig. 3.
Overexpression of Nv-tsl in Drosophila indicates some conservation of function. (A) ‘Spliced’ Drosophila larva produced by overexpression of Nv-tsl using the c335 GAL4 driver, which expresses in Drosophila follicle cells. (B) Wide view of the results of larvae produced by overexpression of Nv-tsl using the c335 GAL4 driver to show range and variation of phenotypes. Scale bars: 100 μm. (C) Comparison of numbers of missing denticle belts from overexpression of Drosophila tsl and Nv-tsl using the c335 GAL4 driver. Nv-tsl expression causes the loss of fewer denticle belts than Drosophila tsl. (D) Rescue of a tsl deletion via expression of Drosophila tsl or Nv-tsl using the Slbo GAL4 line. Drosophila tsl rescues a deletion in the anterior of the embryos while Nv-tsl does not.
Nv-tsl knockdown embryos do not phenocopy other putative VM proteins, which produce a contracted germband phenotype
In Drosophila, Tsl is localized to the VM by the Drosophila VM proteins encoded by female sterile (1) Nasrat [fs(1)N], Female sterile (1) M3 [also called Female sterile (1) polehole) [fs(1)M3] and Closca (Clos), which stabilize the axis formation proteins Nudel and Tsl in the VM (Mineo et al., 2018a; Stevens et al., 2003; Ventura et al., 2010). To investigate potential terminal patterning functions of VM proteins we next determined the roles of Nasonia orthologues of fs(1)N, fs(1)M3, and clos in Nasonia embryonic development.
We identified Nasonia orthologues of Drosophila fs(1)N, fs(1)M3, clos using reciprocal blast. Phylograms of Maximum likelihood phylogeny inference (see Table S3 for alignment, and Fig. S2 for phylogram) indicate that LOC100169973 (XP_016843824) is the orthologue of fs(1)N [here named Nv-fs(1)N]; LOC103317810 (XP_008215462) is the Nasonia orthologue of fs(1)M3 [here named Nv-fs(1)M3], and LOC100678101 (XP_016839710) is the Nasonia orthologue of clos (here named Nv-clos).
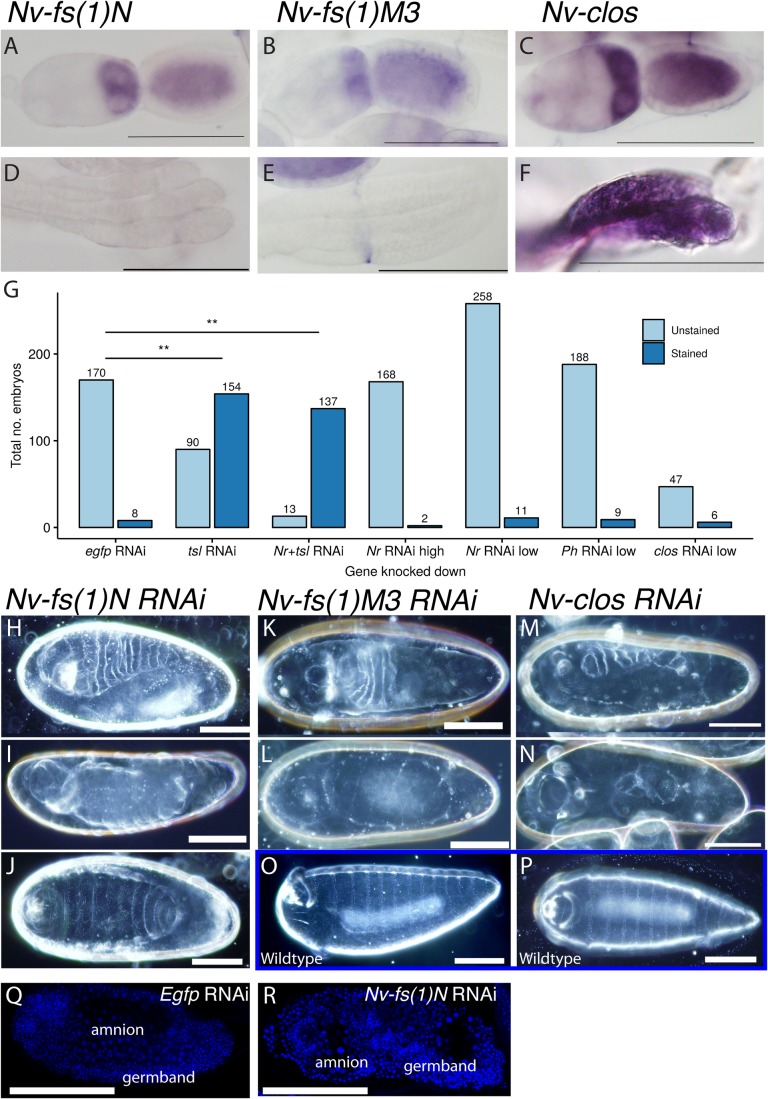
We examined the expression of Nv-fs(1)N, Nv-fs(1)M3 and Nv-clos by in situ hybridization in the Nasonia embryos and ovaries, but were only able to detect expression in the latter. Here, Nv-fs(1)N, Nv-fs(1)M3 and Nv-clos RNA are present in the oocyte and nurse cells, but are not detected in follicle cells (see Fig. 4A–C). Nv-clos, but not Nv-fs(1)N or Nv-fs(1)M3, is also detectable in the germarium (Fig. 4D–F). These data are consistent with the notion that Nv-fs(1)Nr, Nv-fs(1)M3 and Nv-clos could encode structural constituents of the VM in Nasonia, as they do in Drosophila.
Fig. 4.
Nv-fs(1)N, Nv-fs(1)M3 and Nv-clos are expressed in the ovary and cause embryonic defects when knocked down. (A–F) Nv-fs(1)N (A,D), Nv-fs(1)M3 (B,E) and Nv-clos (C,F) are provided maternally to oocytes as detected by in situ hybridization. Nv-fs(1)N (D) and Nv-fs(1)M3 (E) expression is not detectable in the germarium of the ovary while Nv-clos (F) is. Negative controls shown in Fig. S3. (G) Quantification of embryos from females treated with various RNAi injections stained with Neutral Red to demonstrate integrity of embryo membranes. Gene names abbreviated for clarity. Tsl, Nv-tsl; Nr, Nv-fs(1)N; Ph, Nv-fs(1) M3; and clos, Nv-clos. Nr+tsl refers to injection with both Nv-tsl and Nv-fs(1)N. High RNAi refers to dsRNA concentrations ∼1000 ng/µl. Low RNAi refers to dsRNA concentrations 100–300 ng/µl. **P=1.596752e-32 between egfp RNAi and tsl RNAi; 5.560025e-54 between egfp RNAi and Nr+tsl RNAi. (H–N) Knockdown of Nv-fs(1)N (H–J), Nv-fs(1)M3 (K,L) or Nv-clos (M,N) causes a range of phenotypes including contracted germband phenotypes (H,K,M) and apparent dorsal–ventral phenotypes (I,L,N). (J) ∼1% of embryos from females treated with Nv-fs(1)N RNAi exhibit loss of anterior and posterior segments. (O,P) Wild-type cuticles for comparison. (Q,R) Confocal section of DAPI-stained Egfp RNAi (O) or Nv-fs(1)N RNAi showing a twisted germband phenotype caused by maternal treatment of Nv-fs(1)N RNAi. Scale bars: 100 µm.
Using RNAi, we examined the impact of knocking-down expression of Nv-fs(1)N, Nv-fs(1)M3 and Nv-clos maternally. We examined the possibility that Nv-fs(1)N, Nv-fs(1)M3 and Nv-clos might be necessary for VM integrity in the same way that Nv-tsl is. Embryos from maternal RNAi for Nv-fs(1)N, Nv-fs(1)M3 or Nv-clos are not permeable to Neutral Red (Fig. 4G), indicating their eggshells are intact.
Unlike the phenotype observed for Nv-tsl RNAi, these embryos developed through to late stages and it was possible to examine their cuticles. Maternal knockdown of Nv-fs(1)N, Nv-fs(1)M3 or Nv-clos all cause severe embryonic defects (Fig. 4H–P). Knockdown of any one of these three genes appeared to cause embryonic defects of the germband (Fig. 4H,K,M), and possibly ventral specification (Fig. 4I,L,N). We also observed phenotypes similar to terminal defects in Nv-fs(1)N knockdown embryos (Fig. 4J). Nv-fs(1)N RNAi phenotype also causes the germband to be wrapped around the embryo on an angle, rather than oriented along the dorso–ventral axis (Fig. 4Q,R). None of these knockdown experiments give phenotypes similar to that of Nv-tsl, implying that these genes act in different processes. It is possible that Nv-fs(1)N has a function that influences terminal patterning but overall these data indicate that Nv-fs(1)N, Nv-fs(1)M3 and Nv-clos may not be critical components of the VM in Nasonia as they are in Drosophila.
To investigate whether it is possible that the VM function of Nv-Tsl might be exacerbated or ameliorated by knockdown of orthologues of Drosophila VM genes, we performed double knockdown of Nv-tsl and Nv-fs(1)N. Nv-tsl, Nv-fs(1)N double-knockdown embryos were permeable to Neutral Red (Fig. 4G), indicating that these genes are unlikely to act in the same pathway.
Other TAC components do not give terminal phenotypes, nor phenocopy Nv-tsl knockdowns
In Nasonia, Nv-tsl is necessary for VM integrity (Fig. 2). To investigate whether this function is coupled to the canonical terminal patterning system, we investigated the function of orthologues of the remaining TAC genes, PTTH and its receptor torso (previously identified by Skelly et al., 2018). Based on these data, we name LOC103315910 as Nv-PTTH and LOC103316970 as Nv-torso. Knockdown of either of Nv-PTTH or Nv-torso caused no overt cuticle phenotype (Fig. 5C,D), despite using very high concentrations of dsRNA (1000 ng/µl) in this experiment. Taken together, these data imply that Nv-tor and Nv-PTTH are not required for embryonic development in Nasonia, and that the TAC is neither involved in terminal patterning nor in mediating the VM function of Nv-Tsl.
Fig. 5.
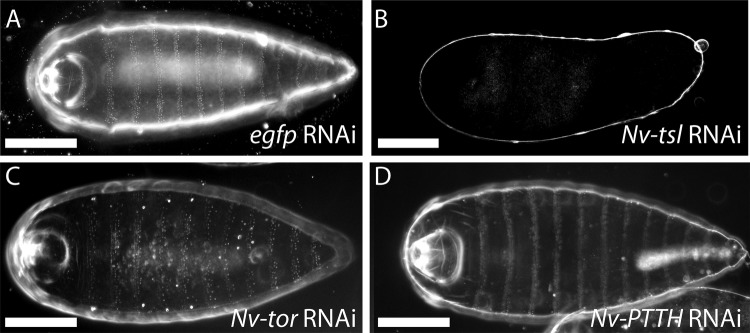
Maternal RNAi knockdown of remaining Nasonia TAC components causes no embryonic phenotype. (A) Example of a wild-type embryo from a female treated with egfp RNAi. (B) Example of an affected embryo from a female treated with Nv-tsl RNAi (representative of 100 embryos). (C,D) Example embryos from a Nv-tor-treated female (C, representative of 35 embryos), and a Nv-PTTH-treated female (D, representative of 41 embryos) both with wild-type patterns. Scale bars: 100 μm.
DISCUSSION
Here, we show that Nv-tsl is necessary for VM integrity in Nasonia. Functional evidence for this is twofold. (1) Embryos with reduced maternal Nv-tsl have permeable eggshells. (2) Electron microscopy reveals that the VM is thinner in Nv-tsl knockdown wasps compared to controls, potentially explaining the VM defect. Alongside this, Drosophila genes involved in VM function, fs(1)N, fs(1)M3 and clos, cause embryonic patterning defects when knocked down in Nasonia, but not defects in the VM. Thus, the function of Nv-tsl in the VM is likely independent of Nv-fs(1)N, Nv-fs(1)M3 and Nv-clos. Despite the difference in phenotype from reducing Nv-tsl in Nasonia compared to tsl mutants in Drosophila, the biochemical function of Nv-tsl is able to produce a ‘spliced’ phenotype when overexpressed in Drosophila follicle cells. This implies that while the biochemical function of Tsl may have remained similar over this evolutionary period, the consequences of that function have changed.
Our knockdown experiments involve maternal injection in Nasonia, which is thought to be unable to knock down zygotically acting developmental genes (Rosenberg et al. 2014). As the severe VM phenotype caused by Nv-tsl knockdown makes it impossible to detect other phenotypes of Nv-tsl knockdown, we cannot rule out a role for Nv-tsl in terminal patterning. Such a function seems unlikely, as all embryos collected from Nv-tsl knockdown mothers were either completely wild type or dead: there were no intermediate phenotypes from embryos with an intact VM but embryonic Nv-tsl knockdown. In addition, other Nasonia TAC genes do not act in Nasonia terminal patterning, and instead terminal patterning is known to be achieved via maternal localization of otd (Lynch et al., 2006a,b).
Ancestral roles of tsl
The ancestral role of tsl is unclear. The gene is present throughout the Pancrustacea and is present in genomes lacking other TAC genes (Skelly et al. 2018). This implies it likely has an ancestral role independent from terminal patterning as carried out in Drosophila and from the other TAC genes. In support of this, the functional data presented here and from other studies imply that the ancestral role of tsl is not terminal patterning. In the most distantly related species to Drosophila examined, the hemipteran Oncopeltus, reduction of tsl expression causes germband invagination defects, but these appear to be caused by altered expression of hunchback and giant, but not tll or huckbein (Weisbrod et al. 2013).
Our data underscore the importance of the VM in early insect development. In Drosophila, both dorso–ventral and terminal axes are formed via proteins anchored in the VM. The terminal signal is provided by tsl, which is anchored in the VM by Fs(1)N, Fs(1)M3 and Clos (Stevens et al., 2003). Nudel, required for the dorso–ventral axis, is also stabilized by these proteins in the VM (Mineo et al., 2017). The blastoderm embryos of both Tribolium and Drosophila are attached via integrins to the VM, ensuring the tissue expands asymmetrically during gastrulation (Münster et al., 2019).
The function of Tsl in the VM described here may be an ancestral one. The VM is laid down by the follicle cells during oogenesis (Cummings et al., 1971) and tsl is expressed in the follicle cells of every insect species surveyed to date, except the honeybee (Duncan et al., 2013). In addition, Tsl is known to be a component of the VM in Drosophila, the only other species where such evidence is available (Stevens et al. 2003). Therefore, tsl is plausibly present in the VM of most insects. Proposing this allows the reinterpretation for functional data from other taxa. Recent findings have implicated that the VM has an essential role in gastrulation (Münster et al., 2019), so it is plausible that a role of tsl in the structural integrity of the VM explains gastrulation phenotypes of tsl in Drosophila (Johnson et al. 2017) and Oncopeltus (Weisbrod et al. 2013).
If the ancestral function of tsl is in the insect VM, and it is present in the Drosophila VM, why do Drosophila not have the VM defects seen in Nasonia RNAi knockdown experiments? Our overexpression experiments imply that Drosophila and Nasonia Tsl share biochemical functions, so loss of Drosophila Tsl might be expected to cause VM holes. One possibility is that the change in function of Tsl from expression throughout the VM to just localization at the termini means that the knockdown phenotype is less severe in Drosophila and allows uncovering of the terminal (and other embryonic functions) activity of this gene.
The evolution of canonical terminal patterning
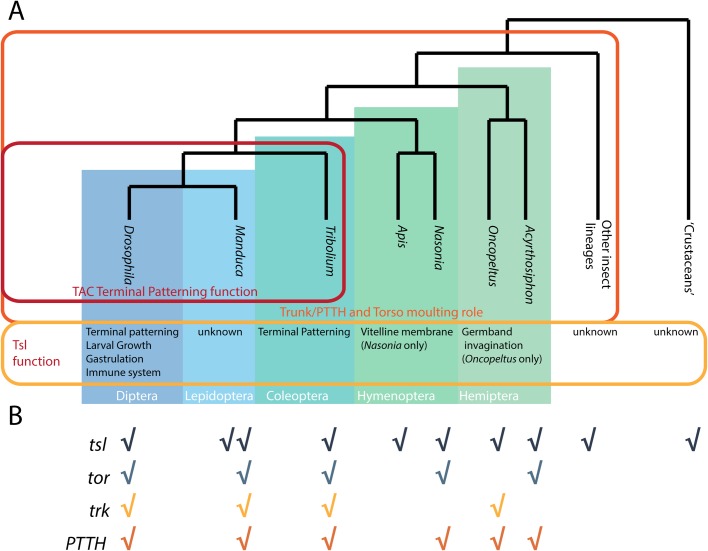
Our data support the hypothesis that the TAC genes were co-opted from other roles into terminal patterning (Duncan et al., 2013). Trunk/PTTH and Torso seem to have been co-opted from a more ancient role in moulting control (Duncan et al., 2013; Skelly et al., 2018). Our data from Nasonia, [a hymenopteran insect, the sister group to the rest of the holometabola (Krauss et al., 2004; Savard et al., 2006; Zdobnov and Bork, 2007)], implies an ancestral role of tsl could have been to ensure VM integrity (Fig. 6).
Fig. 6.
Evolution of the Torso activation cassette. (A) Phylogeny of arthropods with roles of TAC components, and particularly Tsl, marked. (B) Presence/absence of TAC components for the species listed in A, data from Skelly et al. (2018).
Co-option of Tsl into terminal patterning may be related to a change in the regulation of the tsl gene, and novel protein–protein interactions with Fs(1)N, Fs(1)M3, and Clos. In Drosophila Fs(1)N and Fs(1)M3 mutants, the terminal expression domain of tsl in the VM is expanded, the total amount of tsl present is decreased, but localized expression is not completely lost (Stevens et al., 2003). Loss of fs(1)N, fs(1)M3 and clos also reduces/abolishes Tsl in the VM of oocytes (Jiménez et al., 2002; Ventura et al., 2010). Thus, Fs(1)N, Fs(1)M3 and Clos-mediated localization of Tsl in the VM is necessary, but perhaps not sufficient, for Tsl stabilization in the VM.
The differences in terminal patterning between Nasonia and Drosophila provide some insight into the evolution of axis formation mechanisms. Both, unsurprisingly, rely on asymmetries set up maternally, either in the localization of some function of Tsl, allowing Torso activation in limited domains, or by localized placement of maternal RNA. It seems that the signals by which asymmetry is interpreted by the embryo to trigger axis formation are rapidly evolving and can involve many factors, including subtle regional differences in the VM.
The Drosophila terminal patterning system appears to have evolved through the coming together of two systems into the TAC. Our data provides a novel function for the only localized component of this system, tsl, in VM integrity. Existing hypotheses (Duncan et al., 2013) imply that the rest of the TAC, Tor, Trk and PTTH, are co-opted regulators of moulting. We propose the canonical terminal pathway evolved via co-option from two different processes (moulting and VM integrity/control) requiring novel interactions between moulting proteins and the ancestral VM protein Tsl.
MATERIALS AND METHODS
RNA interference and cuticle preparation
Nasonia were reared on Lucilia sericata blowflies (www.biosuppliers.com) at 25°C, after Werren and Loehlin (2009). For maternal RNAi, dsRNA was produced using run-off transcription from linearized plasmid (See Table S1 for plasmid sequences). This was carried out using the Invitrogen MEGAscript RNAi kit, per the manufacturer's instructions. Pupal microinjection and cuticle preparation were performed following Lynch and Desplan (2006) and Werren and Loehlin (2009). Concentrations of 150–500 ng/μl dsRNA were used for Nv-tsl, Nv-fs(1) N (Nasrat), fs(1) ph (pole hole) and Nv-clos (closca) knockdowns, and 1000 ng/μl for Nv-torso and Nv-PTTH.
In situ hybridization
Dioxygenin-labelled probes for in situ hybridization were produced via run-off transcription. Ovaries for hybridization were dissected in PBS and placed into a 1:1 mix of 4% formaldehyde mix and heptane on ice, and fixed for 25 min before being stored in 100% methanol. Embryos were collected from vials with hosts placed into modified plugs (Lynch and Desplan, 2006). Hosts were cracked open and dipped into a 15 ml falcon tube containing 5 ml heptane, 4.5 ml PBS and 0.5 ml 37% formaldehyde (Invitrogen), and fixed for 8 h or overnight (Buchta et al., 2013). Ovary in situ hybridization was performed following Osborne and Dearden (2005) with ovaries digested for 12 min in proteinase K. Ovarioles were not separated before in situ hybridization. Embryo in situ hybridization was performed in the same manner, except embryos were digested for 5 min in 0.4 µl of proteinase K and hybridization was performed at 60°C.
DAPI staining of embryos
Embryos were fixed as for in situ hybridization and stored in methanol without peeling. Embryos were then dehydrated through a 75%-50%-25% Methanol:PTw series for 5 min each, before being incubated with 1 µl/ml DAPI [4,6-Diamidino-2-Phenylindole, dihydrochloride (Invitrogen)] for 15 min, mounting in glycerol, and imaging under the FV1000 confocal microscope and 405 nm laser.
Tsl overexpression and rescue in Drosophila
The following Drosophila stocks were used: c355-Gal4 (BL3750), slbo-Gal4 UAS-GFP (BL6458), tslΔ and UAS-tsl (Johnson et al., 2013). To generate UAS-Nv-tsl transgenic lines, the open reading frame from Nv-Tsl (accession XM_001602685) was synthesized (Genscript) and ligated into pUASTattB via NotI/XbaI. Transgenic lines were generated by phiC31-mediated integration into the ZH-51C attP landing site (Bischof et al., 2007). Crosses were conducted at 25°C on standard fly media and adults were allowed to lay on media containing apple juice and agar supplemented with yeast paste at 25°C for 24 h before being removed. Embryos were developed for a further 24 h before dechorionation in 50% vol/vol bleach and mounting on slides in a mixture of 1:1 Hoyer's solution: lactic acid. Slides were incubated overnight at 65°C and imaged using dark field optics (Leica). Fifty individuals were scored per replicate and the data reported as the average number of denticle belts missing.
qPCR
For qPCR, 1-day old wasp ovaries (∼8 days following RNAi) were dissected onto dry ice and stored at −80°C. RNA was extracted using the Qiagen RNeasy kit following the manufacturer's instructions. cDNA was produced using the Invitrogen SuperScriptIII kit. qPCR was performed after Duncan et al. (2013). The reference genes used were Rpn1 and L23 (NCBI LOC100115795, LOC100114985) and data was normalized to the mean Cq value of these genes. Data was analysed using R version 3.4.4 and the ‘pcr’ package version 1.1.2 (Ahmed 2018). See Table S2 for PCR primer sequences and efficiencies.
Electron microscopy
Nasonia wasps were anaesthetized in fly vials on ice. Ovaries containing oocytes were dissected while submerged in ambient temperature PBS buffer and then transferred into Wohlwend #1093 copper gold-coated EMPACT membrane carriers (with a cavity diameter of 1450 µm and depth of 200 µm) and filled with 10% Ficoll (GE Healthcare 17-0310-10 Ficoll PM70) in 0.1 M Sorensen's phosphate buffer. Samples were frozen with a Leica EMPACT 2 High Pressure Freezer and stored in liquid nitrogen for 10 days.
Samples were transferred into a Leica EM AFS2 automatic freeze-substitution device (Leica Microsystems GmbH, Vienna, Austria). Tissue was stained with a freeze substitution medium consisting of 0.1% uranyl acetate, 1% glutaraldehyde and 1% osmium tetroxide (Electron Microscopy Science, Hatfield, USA) in acetone for 30 min at −130°C. Over 2 h the temperature was increased to −90°C. The freeze substitution medium was refreshed and held at −90°C for 44 h and 30 min. The temperature was then increased by 5°C per h until reaching −50°C where it was held for 8 h. The temperature was then increased by 10°C per h and held at −20°C for 2 h. Finally, the temperature was increased to 0°C over 1 h. The reagent baths containing the samples were removed from the AFS and all remaining infiltration steps were performed at room temperature.
Samples were infiltrated with a mixture of acetone with increasing concentrations of EMBED 812 epoxy resin [EMBED 812 Resin 14900 Electron Microscopy Sciences with medium hardness using accelerator BDMA (N-Benzyldimethylamine), 11400-25 Electron Microscopy Sciences] in the following series, 2:1, 1:1, 1:2 for 1 h each. Samples were then infiltrated with resin overnight with three resin changes. Blocks were polymerized for 48 h at 60°C.
Sections were cut on a Leica UC6 Ultramicrotome (Leica Microsystems, Germany) at ∼85 nm and mounted on formvar-coated copper slot grids (Electron Microscopy Science, Hatfield, USA). Grids were post-stained with uranyl acetate (Agar Scientific, Essex, UK) and lead citrate (cycle=UA, 20 min at 25°C, LC, 3 min at 25°C) with an LKB 2168 Ultrostain grid stainer (LKB-Produkter AB, Bromma, Sweden).
Sections were imaged with a Philips CM100 BioTWIN transmission electron microscope with LaB6 emitter (Philips/FEI Corporation, Eindhoven, The Netherlands) fitted with MegaView lll digital camera (Olympus Soft Imaging Solutions GmbH, Münster, Germany).
To calculate mean VM and chorion widths an ImageJ macro was used (originally http://imagej.1557.x6.nabble.com/Distance-Between-Lines-a-plugin-for-ImageJ-td3701802.html, also available on github https://github.com/Shannon-E-Taylor/tsl-project-scripts). In ImageJ, the two edges of the membrane being measured were traced manually, and a parallel line drawn between them. The macro then measured the distance between the traced lines, using the parallel line as a guide. A custom R script was used to calculate the mean membrane width for each image, and for each biological replicate (single ovary) (https://github.com/Shannon-E-Taylor/tsl-project-scripts).
Neutral Red assay
0–4 h old embryos were collected into a ceramic staining dish in PTx (phosphate buffer solution+0.1% Triton 100), and washed three times in PTx. The PTx was then replaced with 5 mg/ml Neutral Red (Sigma-Aldrich) in PBS and incubated for 15 min at room temperature. Embryos were rinsed three times in PTx before being imaged.
Supplementary Material
Acknowledgements
The authors wish to thank M. Lovegrove for support and P.M. Dearden for critical reading of this manuscript.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
Conceptualization: P.K.D.; Methodology: S.E.T., S.L.; Software: S.E.T.; Investigation: S.E.T., J.T., D.B., S.L., T.K.J., P.K.D.; Resources: P.K.D.; Data curation: S.E.T., T.K.J., P.K.D.; Writing - original draft: S.E.T., P.K.D.; Writing - review & editing: S.E.T., C.G.W., T.K.J., P.K.D.; Supervision: C.G.W., T.K.J., P.K.D.; Project administration: P.K.D.; Funding acquisition: P.K.D.
Funding
This work was funded by a New Zealand Ministry of Business Innovation and Employment grant (grant UOOX1707) to P.K.D.
Data availability
All sequence data is available in Genbank. Embryo images and scripts are available at https://github.com/Shannon-E-Taylor/tsl-project-scripts.
Supplementary information
Supplementary information available online at http://bio.biologists.org/lookup/doi/10.1242/bio.046284.supplemental
References
- Ahmed M. (2018). pcr: Analysing Real-Time Quantitative PCR data.
- Amarnath S., Stevens L. M. and Stein D. S. (2017). Reconstitution of Torso signaling in cultured cells suggests a role for both Trunk and Torso-like in receptor activation. Development 144, 677-686. 10.1242/dev.146076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumer D., Strohlein N. M. and Schoppmeier M. (2012). Opposing effects of Notch-signaling in maintaining the proliferative state of follicle cells in the telotrophic ovary of the beetle Tribolium. Front Zool 9, 15 10.1186/1742-9994-9-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bischof J., Maeda R. K., Hediger M., Karch F. and Basler K. (2007). An optimized transgenesis system for Drosophila using germ-line-specific phiC31 integrases. Proc. Natl. Acad. Sci. USA 104, 3312-3317. 10.1073/pnas.0611511104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand A. H., Manoukian A. S. and Perrimon N. (1994). Chapter 33 ectopic expression in Drosophila. Methods Cell Biol. 44, 635-654. 10.1016/S0091-679X(08)60936-X [DOI] [PubMed] [Google Scholar]
- Bronner G. and Jackle H. (1991). Control and function of terminal gap gene activity in the posterior pole region of the Drosophila embryo. Mech. Dev. 35, 205-211. 10.1016/0925-4773(91)90019-3 [DOI] [PubMed] [Google Scholar]
- Bronner G. and Jackle H. (1996). Regulation and function of the terminal gap gene huckebein in the Drosophila blastoderm. Int. J. Dev. Biol. 40, 157-165. [PubMed] [Google Scholar]
- Buchta T., Özüak O., Stappert D., Roth S. and Lynch J. A. (2013). Patterning the dorsal-ventral axis of the wasp Nasonia vitripennis. Dev. Biol. 381, 189-202. 10.1016/j.ydbio.2013.05.026 [DOI] [PubMed] [Google Scholar]
- Casanova J., Furriols M., McCormick C. A. and Struhl G. (1995). Similarities between trunk and spätzle, putative extracellular ligands specifying body pattern in Drosophila. Genes Dev. 9, 2539-2544. 10.1101/gad.9.20.2539 [DOI] [PubMed] [Google Scholar]
- Cummings M. R., Brown N. M. and King R. C. (1971). The cytology of the vitellogenic stages of oogenesis in Drosophila melanogaster. Z. Zellforsch. Mikrosk. Anat. 118, 482-492. 10.1007/BF00324615 [DOI] [PubMed] [Google Scholar]
- Dearden P. K., Wilson M. J., Sablan L., Osborne P. W., Havler M., McNaughton E., Kimura K., Milshina N. V., Hasselmann M., Gempe T. et al. (2006). Patterns of conservation and change in honey bee developmental genes. Genome Res. 16, 1376-1384. 10.1101/gr.5108606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan E. J., Benton M. A. and Dearden P. K. (2013). Canonical terminal patterning is an evolutionary novelty. Dev. Biol. 377, 245-261. 10.1016/j.ydbio.2013.02.010 [DOI] [PubMed] [Google Scholar]
- Forbes-Beadle L., Crossman T., Johnson T. K., Burke R., Warr C. G. and Whisstock J. C. (2016). Development of the Cellular Immune System of Drosophila Requires the Membrane Attack Complex/Perforin-Like Protein Torso-Like. Genetics 204, 675–681. 10.1534/genetics.115.185462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furriols M., Ventura G. and Casanova J. (2007). Two distinct but convergent groups of cells trigger Torso receptor tyrosine kinase activation by independently expressing torso-like. Proc. Natl. Acad. Sci. USA 104, 11660-11665. 10.1073/pnas.0700991104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillo M., Furriols M., de Miguel C., Franch-Marro X. and Casanova J. (2012). Conserved and divergent elements in Torso RTK activation in Drosophila development. Sci. Rep. 2, 762 10.1038/srep00762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henstridge M. A., Aulsebrook L., Koyama T., Johnson T. K., Whisstock J. C., Tiganis T., Mirth C. K. and Warr C. G. (2018). Torso-like is a component of the hemolymph and regulates the insulin signaling pathway in Drosophila. Genetics 208, 1523-1533. 10.1534/genetics.117.300601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isoe J., Koch L. E., Isoe Y. E., Rascón A. A. Jr, Brown H. E., Massani B. B. and Miesfeld R. L. (2019). Identification and characterization of a mosquito-specific eggshell organizing factor in Aedes aegypti mosquitoes. PLoS. Biol. 17, e3000068 10.1371/journal.pbio.3000068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiménez G., González-Reyes A. and Casanova J. (2002). Cell surface proteins Nasrat and Polehole stabilize the Torso-like extracellular determinant in Drosophila oogenesis. Genes Dev. 16, 913-918. 10.1101/gad.223902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson T. K., Crossman T., Foote K. A., Henstridge M. A., Saligari M. J., Forbes Beadle L., Herr A., Whisstock J. C. and Warr C. G. (2013). Torso-like functions independently of Torso to regulate Drosophila growth and developmental timing. Proc. Natl Acad. Sci. USA 110, 14688-14692. 10.1073/pnas.1309780110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson T. K., Henstridge M. A., Herr A., Moore K. A., Whisstock J. C. and Warr C. G. (2015). Torso-like mediates extracellular accumulation of Furin-cleaved Trunk to pattern the Drosophila embryo termini. Nat. Commun. 6, 8759 10.1038/ncomms9759 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson T. K., Moore K. A., Whisstock J. C. and Warr C. G. (2017). Maternal Torso-Like Coordinates Tissue Folding During Drosophila Gastrulation. Genetics 206, 1459–1468. 10.1534/genetics.117.200576 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klingler M., Erdélyi M., Szabad J. and Nüsslein-Volhard C. (1988). Function of torso in determining the terminal anlagen of the Drosophila embryo. Nature 335, 275-277. 10.1038/335275a0 [DOI] [PubMed] [Google Scholar]
- Krauss V., Pecyna M., Kurz K. and Sass H. (2004). Phylogenetic mapping of intron positions: a case study of translation initiation factor eIF2γ. Mol. Biol. Evol. 10.1093/molbev/msh255 [DOI] [PubMed] [Google Scholar]
- LeMosy E. K. and Hashimoto C. (2000). The nudel protease of Drosophila is required for eggshell biogenesis in addition to embryonic patterning. Dev. Biol. 217, 352-361. 10.1006/dbio.1999.9562 [DOI] [PubMed] [Google Scholar]
- Lynch J. A. and Desplan C. (2006). A method for parental RNA interference in the wasp Nasonia vitripennis. Nat. Protoc. 1, 486-494. 10.1038/nprot.2006.70 [DOI] [PubMed] [Google Scholar]
- Lynch J. A., Brent A. E., Leaf D. S., Pultz M. A. and Desplan C. (2006a). Localized maternal orthodenticle patterns anterior and posterior in the long germ wasp Nasonia. Nature 439, 728-732. 10.1038/nature04445 [DOI] [PubMed] [Google Scholar]
- Lynch J. A., Olesnicky E. C. and Desplan C. (2006b). Regulation and function of tailless in the long germ wasp Nasonia vitripennis. Dev. Genes Evol. 216, 493-498. 10.1007/s00427-006-0076-5 [DOI] [PubMed] [Google Scholar]
- Mineo A., Furriols M. and Casanova J. (2015). Accumulation of the Drosophila Torso-like protein at the blastoderm plasma membrane suggests that it translocates from the eggshell. Development 142, 1299-1304. 10.1242/dev.117630 [DOI] [PubMed] [Google Scholar]
- Mineo A., Furriols M. and Casanova J. (2017). Transfer of dorsoventral and terminal information from the ovary to the embryo by a common group of eggshell proteins in Drosophila. Genetics 205, 1529-1536. 10.1534/genetics.116.197574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineo A., Fuentes E., Furriols M. and Casanova J. (2018a). Holes in the plasma membrane mimic torso-like perforin in torso tyrosine kinase receptor activation in the Drosophila embryo. Genetics 210, 257-262. 10.1534/genetics.118.301397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineo A., Furriols M. and Casanova J. (2018b). The trigger (and the restriction) of Torso RTK activation. Open Biol. 8, 180180 10.1098/rsob.180180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Münster S., Jain A., Mietke A., Pavlopoulos A., Grill S. W. and Tomancak P. (2019). Attachment of the blastoderm to the vitelline envelope affects gastrulation of insects. Nature 568, 395-399. 10.1038/s41586-019-1044-3 [DOI] [PubMed] [Google Scholar]
- Osborne P. and Dearden P. K. (2005). Non-radioactive in-situ hybridisation to honeybee embryos and ovaries. Apidologie 36, 113-118. 10.1051/apido:2004075 [DOI] [Google Scholar]
- Rewitz K. F., Yamanaka N., Gilbert L. I. and O'Connor M. B. (2009). The insect neuropeptide PTTH activates receptor tyrosine kinase torso to initiate metamorphosis. Science 326, 1403-1405. 10.1126/science.1176450 [DOI] [PubMed] [Google Scholar]
- Richards J. G. (1969). The structure and formation of the egg membranes in Nasonia vitripennis (Walker) (Hymenoptera, Pteromalidæ). J. Microsc. 89, 43-53. 10.1111/j.1365-2818.1969.tb00648.x [DOI] [PubMed] [Google Scholar]
- Rosenberg M. I., Brent A. E., Payre F. and Desplan C. (2014). Dual mode of embryonic development is highlighted by expression and function of Nasonia pair-rule genes. Elife 3, e01440 10.7554/elife.01440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savant-Bhonsale S. and Montell D. J. (1993). torso-like encodes the localized determinant of Drosophila terminal pattern formation. Genes Dev. 7, 2548-2555. 10.1101/gad.7.12b.2548 [DOI] [PubMed] [Google Scholar]
- Savard J., Tautz D., Richards S., Weinstock G. M., Gibbs R. A., Werren J. H., Tettelin H. and Lercher M. J. (2006). Phylogenomic analysis reveals bees and wasps (Hymenoptera) at the base of the radiation of Holometabolous insects. Genome Res. 16, 1334-1338. 10.1101/gr.5204306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoppmeier M. and Schroder R. (2005). Maternal torso signaling controls body axis elongation in a short germ insect. Current biology : CB 15, 2131–2136. 10.1016/j.cub.2005.10.036 [DOI] [PubMed] [Google Scholar]
- Skelly J., Pushparajan C., Duncan E. J. and Dearden P. K. (2018). Evolution of the Torso activation cassette, a pathway required for terminal patterning and moulting. Insect Mol. Biol. 10.1111/imb.12560 [DOI] [PubMed] [Google Scholar]
- Sprenger F., Stevens L. M. and Nüsslein-Volhard C. (1989). The Drosophila gene torso encodes a putative receptor tyrosine kinase. Nature 338, 478-483. 10.1038/338478a0 [DOI] [PubMed] [Google Scholar]
- Stevens L. M., Frohnhöfer H. G., Klingler M. and Nusslein-Volhard C. (1990). Localized requirement for torso-like expression in follicle cells for development of terminal anlagen of the Drosophila embryo. Nature 346, 660-663. 10.1038/346660a0 [DOI] [PubMed] [Google Scholar]
- Stevens L. M., Beuchle D., Jurcsak J., Tong X. and Stein D. (2003). The Drosophila embryonic patterning determinant torsolike is a component of the eggshell. Curr. Biol. 13, 1058-1063. 10.1016/S0960-9822(03)00379-8 [DOI] [PubMed] [Google Scholar]
- Strecker T. R., Halsell S. R., Fisher W. W. and Lipshitz H. D. (1989). Reciprocal effects of hyper- and hypoactivity mutations in the Drosophila pattern gene torso. Science 243, 1062-1066. 10.1126/science.2922596 [DOI] [PubMed] [Google Scholar]
- Ventura G., Furriols M., Martín N., Barbosa V. and Casanova J. (2010). closca, a new gene required for both Torso RTK activation and vitelline membrane integrity. germline proteins contribute to Drosophila eggshell composition. Dev. Biol. 344, 224-232. 10.1016/j.ydbio.2010.05.002 [DOI] [PubMed] [Google Scholar]
- Waring G. L. (2000). Morphogenesis of the eggshells in Drosophila. In International Review of Cytology (ed. Jeon K. W.), pp. 67-108. Elsevier. [DOI] [PubMed] [Google Scholar]
- Weisbrod A., Cohen M. and Chipman A. D. (2013). Evolution of the insect terminal patterning system--insights from the milkweed bug, Oncopeltus fasciatus. Developmental biology 380, 125–131. 10.1016/j.ydbio.2013.04.030 [DOI] [PubMed] [Google Scholar]
- Werren J. H. and Loehlin D. W. (2009). The parasitoid wasp Nasonia: an emerging model system with haploid male genetics. Cold. Spring. Harbor. Protoc. 2009, emo134 10.1101/pdb.emo134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M. J. and Dearden P. K. (2009). Tailless patterning functions are conserved in the honeybee even in the absence of Torso signaling. Dev. Biol. 335, 276-287. 10.1016/j.ydbio.2009.09.002 [DOI] [PubMed] [Google Scholar]
- Wilson M. J. and Dearden P. K. (2011). Diversity in insect axis formation: two orthodenticle genes and hunchback act in anterior patterning and influence dorsoventral organization in the honeybee (Apis mellifera). Development 138, 3497-3507. 10.1242/dev.067926 [DOI] [PubMed] [Google Scholar]
- Zdobnov E. M. and Bork P. (2007). Quantification of insect genome divergence. Trends Genet. 23, 16-20. 10.1016/j.tig.2006.10.004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.