Visual Abstract
Keywords: acute renal failure; dialysis; ultrafiltration; child; infant; infant, newborn; adult; humans; critical illness; diuretics; retrospective studies; grassland; renal replacement therapy; kidney replacement therapy; acute kidney injury; renal dialysis; kidney dialysis; chronic kidney failure; kidney failure, chronic; heart failure; hemofiltration; demography
Abstract
Background and objectives
Provision of kidney replacement therapy (KRT) to manage kidney injury and volume overload in critically ill neonates and small children is technically challenging. The use of machines designed for adult-sized patients, necessitates large catheters, a high extracorporeal volume relative to patient size, and need for blood priming. The Aquadex FlexFlow System (CHF Solutions Inc., Eden Prairie, MN) is an ultrafiltration device designed for fluid removal in adults with diuretic resistant heart failure. It has an extracorporeal volume of 33 ml, which can potentially mitigate some complications seen at onset of KRT in smaller infants.
Design, setting, participants, & measurements
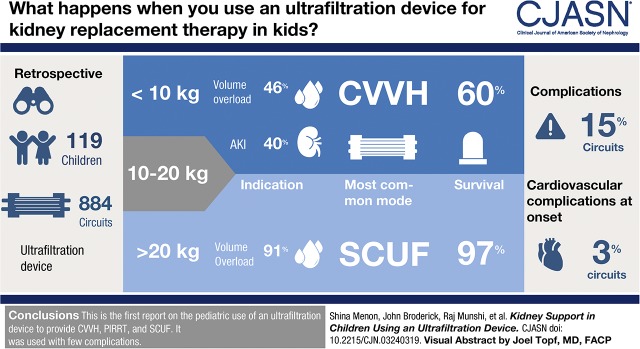
In this multicenter, retrospective case series of children who received KRT with an ultrafiltration device (n=119 admissions, 884 circuits), we report demographics, circuit characteristics, complications, and short- and long-term outcomes. Patients were grouped according to weight (<10, 10–20, and >20 kg), and received one of three modalities: slow continuous ultrafiltration, continuous venovenous hemofiltration (CVVH), or prolonged intermittent KRT. Our primary outcome was survival to end of KRT.
Results
Treatment patterns and outcomes varied between the groups. In patients who weighed <10 kg, the primary indication was AKI in 40%, volume overload in 46%, and ESKD in 14%. These patients primarily received CVVH (66%, n=48) and prolonged intermittent KRT (21%, n=15). In the group weighing >20 kg, volume overload was the primary indication in 91% and slow continuous ultrafiltration was the most common modality. Patients <10 kg had lower KRT survival than those >20 kg (60% versus 97%), more volume overload at onset, and received KRT for a longer duration. Cardiovascular complications at initiation were seen in 3% of treatments and none were severe. Complications during therapy were seen in 15% treatments and most were vascular access–related.
Conclusions
We report the first pediatric experience using an ultrafiltration device to provide a range of therapies, including CVVH, prolonged intermittent KRT, and slow continuous ultrafiltration. We were able to initiate KRT with minimal complications, particularly in critically ill neonates. There is an unmet need for devices specifically designed for younger patients. Having size-appropriate machines will improve the care of smaller children who require kidney support.
Introduction
AKI and volume overload are common and are associated with morbidity and mortality in critically ill neonates and children (1–3). In recent years, continuous renal replacement therapy (CRRT) has emerged as the preferred modality to provide kidney support to such children (4). CRRT is used sparingly in neonates and is associated with worse outcomes compared with larger children (5,6).
The main reasons for the lower use of CRRT in infants are that it is technically challenging and has higher risk of complications than in older and larger children (weighing >10 kg). Machines designed for adult-sized patients require larger catheters, tubing, and filters, which result in a high extracorporeal volume relative to patient size (6,7). In the United States, there are no CRRT machines approved by the US Food and Drug Administration (FDA) for use in children <20 kg; nevertheless, existing CRRT machines approved for use in older children are used off-label in small children. To reduce the complications at KRT initiation from large extracorporeal volume, most centers prime the circuit with blood when the extracorporeal volume is >10% of total blood volume. Although blood primes are generally safe, they can result in hypocalcemia, hyperkalemia, acidosis, thrombocytopenia, and coagulopathy around initiation. A recent single study evaluation of hemodynamic stability in neonates (eight patients, 70 sessions) after CRRT initiation showed that 55% of sessions had intradialytic hypotension, most of which occurred shortly after CRRT initiation (8).
Furthermore, dialysis machines with smaller extracorporeal volume may provide an option for KRT to neonates with ESKD who are unable to initiate peritoneal dialysis (PD) because of their small size, recent abdominal surgery, or abdominal wall defect.
Novel machines designed for neonates are used in a few centers in Europe, but are not currently available in the United States (9,10). The Aquadex FlexFlow System (Aquadex; CHF Solutions Inc., Eden Prairie, MN) is FDA-approved for isolated ultrafiltration (UF) in adults with diuretic-resistant congestive heart failure (11,12). With an extracorporeal volume of 33 ml, it can mitigate some complications seen at CRRT initiation in smaller infants. Askenazi et al. (7) published a case series of continuous venovenous hemofiltration (CVVH) in 12 infants and small children by adapting the device. They were able to initiate CVVH with minimal complications (7).
Volume overload is common in critically ill children (13,14). Although volume overload and AKI are often interrelated and have similar deleterious effects, they may not always occur together. Volume overload may be seen without significant kidney dysfunction, as in patients with congestive heart failure or nephrotic syndrome. These children are often managed conservatively with diuretics and an ultrafiltration device has not been widely used (15). Given that volume overload, with or without AKI, is a common indication for kidney support in children, the paucity of data on its use in pediatrics is surprising.
We performed a multicenter study to report the characteristics of children who received kidney support with Aquadex. We describe demographics and short- and long-term outcomes in these patients. We also report differences in KRT modality (slow continuous ultrafiltration versus CVVH versus prolonged intermittent kidney replacement therapy), circuit characteristics, and complications during KRT.
Materials and Methods
Study Design and Population
This is a multicenter, descriptive, retrospective case series of children aged 0–20 years, who received KRT with an ultrafiltration device from January 2012 to March 2018 at three United States pediatric hospitals (Seattle Children’s Hospital [SCH], Seattle, WA; Children’s of Alabama [COA], Birmingham, AL, and Cincinnati Children’s Hospital Medical Center [CCHMC], Cincinnati, OH). Patients were included from the intensive care units, inpatient floors, and chronic dialysis units. For each patient, the decision to initiate KRT and the modality prescribed was per clinician discretion, after reviewing the options of care with the primary team and family. Clinical informed caregiver consent for KRT was obtained in accordance with policies for all acute KRT procedures (hemodialysis, PD, CRRT) at each institution. Local institutional review boards at each center approved the study, with a waiver of informed research consent.
Demographic, clinical, and circuit data were collected through medical record review, and from institutional CRRT clinical quality improvement databases. Data included sex, age/weight at admission, primary indication for KRT (AKI, electrolyte abnormalities, volume overload, and ESKD), percent fluid overload (volume overload%), and catheter location and size.
Volume overload% was calculated with the formula described by Selewski et al. (16):
 |
Circuit data included maximal blood pump flow rate, complications at initiation and during therapy, and anticoagulation method. We looked at overall circuit life and the proportion of CVVH circuits that survived to 60 hours. For the latter metric, we censored circuits that were disconnected for procedures or other patient-related issues as has been described previously by the Prospective Pediatric CRRT (ppCRRT) registry (17).
The primary outcome was patient survival to end of KRT using the ultrafiltration device, i.e., the patient no longer needed KRT or was transitioned to another modality. Secondary outcomes included survival and kidney function at 1 year or last follow up.
Patients were grouped by weight (<10, 10–20, and >20 kg) for analyses. We chose these categories on the basis of previous studies that have looked at CRRT in patients <10 kg, and because CRRT machines are FDA-approved for children >20 kg (5,6,18).
Treatment Modality
Kidney support modalities included CVVH, prolonged intermittent KRT, or slow continuous ultrafiltration (Supplemental Table 1). The three centers differed in their choice of therapy. CVVH was primarily used at SCH and COA, prolonged intermittent KRT was primarily used at CCHMC, and slow continuous ultrafiltration was performed at all three.
CVVH was provided using replacement fluids as previously described (7). Briefly, this device uses the UF 500 filter set, which has a polysulfone membrane with a 0.12 m2 surface area and a 33 ml ECV. The set has pre- and postfilter pigtail ports, an air detector, and a blood-leak detector. The blood pump can run at 10–40 ml/min (at intervals of five). The effluent dose used for CVVH was 24 ml/kg per h (at COA) or 2000 ml/1.73 m2 per hour (at SCH and CCHMC), which is within the maximum UF rate of the device. The UF pump has a range of 0–500 ml/h (at intervals of 10 ml/h) and an accuracy of ±10% of setting.
Pre- or postfilter replacement fluid was infused with an Alaris (Becton, Dickinson and Company, Franklin Lakes, NJ) fluid infusion system via the proximal or distal pigtail, respectively. Prismasol or phoxillum (Gambro Renal Products, Inc., Daytona Beach, FL) was used as replacement fluid. UF rates were on the basis of the hourly fluid intake and overall desired fluid balance. CVVH circuits were changed at least every 72 hours per manufacturer protocol. During CVVH or prolonged intermittent KRT, heparin anticoagulation was infused through a y-connector on the withdrawal (access) line. Laboratory analysis to assess anticoagulation was drawn from the postfilter pigtail. A blood warmer (AstoFlo Plus Stihler Electronic; Stuttgart, Germany or HOTLINE Blood and Fluid Warmer; Smiths Medical, Dublin, OH) was placed on the infusion line if required.
Slow continuous ultrafiltration was used to treat isolated volume overload. This did not involve provision of replacement fluid and the duration of therapy was typically 6 hours. Therapy was labeled as prolonged intermittent KRT when hemofiltration (similar to CVVH above) was performed for an extended period of 6–8 hours, either daily or multiple times per week. The dose used for prolonged intermittent KRT was 2000 ml/1.73 m2 per hour for 6–8 hours. The typical blood pump speed (Qb) was 30–40 ml/min.
Circuits were primed with packed red blood cells (pRBCs) or crystalloid. Crystalloid primes were used if the extracorporeal volume was <10% of total blood volume, and the patient was clinically stable. If extracorporeal volume ≥10% of total blood volume, the circuit was primed with pRBCs per each institution’s protocol. At SCH, pRBCs are mixed 1:1 with 5% albumin for the prime. At COA, prime includes pRBCs and sodium bicarbonate in equal parts, and CCHMC uses pRBCs only. Calcium gluconate was given for all initiations at COA, and at SCH and CCHMC was given per clinician decision. If a blood primed circuit was undergoing a planned circuit change, the new circuit was crossprimed with blood from the expiring circuit, when possible.
Statistics
The Shapiro–Wilk test was used to check for normal distribution. Data were not normally distributed, so continuous variables are reported as median (with interquartile range [IQR]). Categorical variables are reported as number and percentage. For analysis, R program was used (R Core Team, 2018; https://www.R-project.org/.)
Results
Patient Characteristics
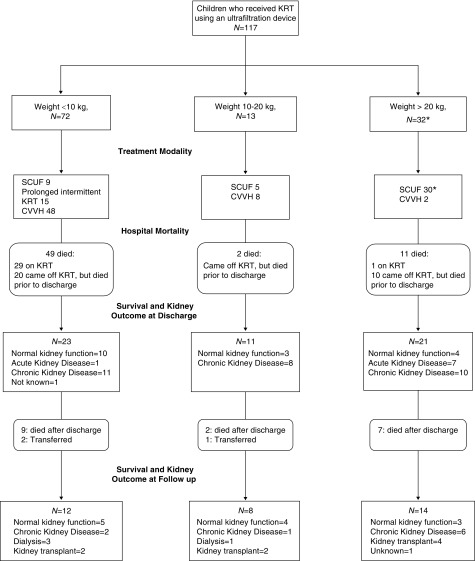
We present data on 117 unique patients (Figure 1). Patients were divided into three groups by weight (<10 kg, n=72; 10–20 kg, n=13; >20 kg, n=32). Two patients in the >20 kg group had a repeat admission requiring KRT with the ultrafiltration device >12 months after the first admission; thus there were a total of 119 separate hospital encounters (Table 1). For the short-term outcomes, we evaluated all 119 encounters individually; for long-term outcomes, we looked at 117 patients and considered the first encounter.
Figure 1.
Flow diagram of all children who initiated kidney replacement therapy using an ultrafiltration device, according to their weight at initiation, through hospital discharge and follow-up. *Two patients had two admissions >12 months apart. For this figure, their first admission was used. KRT, kidney replacement therapy; SCUF, slow continuous ultrafiltration; CVVH, continuous venovenous hemofiltration.
Table 1.
Patient characteristics
| Characteristic | Group 1 (Weight <10 kgs) n=72 | Group 2 (Weight 10–20 kgs) n=13 | Group 3 (Weight >20 kgs) n=34a |
|---|---|---|---|
| Age at first KRT | 19 (7, 87) d | 26 (17, 38) mo | 190 (158, 258) mo |
| Patient location | |||
| Neonatal ICU | 46 (64) | 1 (8) | 0 |
| Cardiac ICU | 13 (18) | 3 (23) | 12 (35) |
| Pediatric ICU | 12 (17) | 8 (62) | 16 (47) |
| Dialysis unit | 1 (1) | 1 (8) | 4 (12) |
| Inpatient floor | 0 | 0 | 2 (6) |
| Base weight, kg | 3.2 (2.6, 4.3) | 12.3 (11.5, 13.7) | 58.7 (37.1, 77) |
| Weight at onset of therapy, kg | 4.1 (3.1, 5.6) | 15.1 (12.9, 15.9) | 60.1 (38.2, 79.2) |
| Female | 34 (47) | 3 (23) | 20 (59) |
| Primary disease | |||
| Cardiac | 21 (29) | 2 (15) | 13 (38) |
| Kidney | 31 (43) | 7 (54) | 8 (24) |
| Other | 20 (28) | 4 (31) | 13 (38) |
| Primary indication | |||
| Volume overload | 33 (46) | 7 (54) | 31 (91) |
| AKI and electrolyte abnormalities | 18 (25) | 2 (15) | 0 |
| AKI and volume overload | 11 (15) | 1(8) | 2 (6) |
| ESKD | 10 (14) | 3 (23) | 1 (3) |
| Treatment modality | |||
| Slow continuous ultrafiltration | 9 (12) | 5 (38) | 32 (94) |
| Prolonged intermittent KRT | 15 (21) | 0 | 0 |
| CVVH | 48 (67) | 8 (62) | 2 (6) |
| Volume overload at onset of KRT (%) | 20 (5, 40) | 20 (8, 23) | 7 (3, 11) |
| Days in hospital before KRT | 13 (3, 28) | 21 (7, 88) | 11 (3, 35) |
| Days in ICU before KRT | 12 (2, 23) | 11 (3, 97) | 6 (2, 20) |
| Days on Ultrafiltration Device | 9 (3, 33) | 7 (5, 14) | 1 (1, 3) |
| Number of circuits | 4 (2, 10) | 3 (2, 6) | 2 (1, 3) |
| Initial catheter site | |||
| Right internal jugular | 46 (64) | 6 (46) | 19 (56) |
| Femoral | 19 (26) | 3 (23) | 7 (21) |
| Other | 7 (10) | 4 (31) | 8 (24) |
| Initial catheter size | |||
| 6 Fr | 25 (35) | 3 (23) | 1 (3) |
| 7–8 Fr | 37 (51) | 7 (54) | 5 (15) |
| 9–10 Fr | 5 (7) | 2 (15) | 8 (23) |
| 12–14 Fr | 0 | 1 (8) | 20 (59) |
| Other | 5 (7) | 0 | 0 |
| Treatment course survival | 43 (60) | 13 (100) | 33 (97) |
| Hospital survival | 23 (32) | 11 (85) | 23 (68) |
| Kidney outcome at discharge among survivors | (n=23) | (n=11) | n=23 |
| Normal kidney function | 10 | 3 | 4 |
| Acute kidney disease | 1 | 0 | 7 |
| CKD | 11 | 8 | 12 |
| Not known | 1 | 0 | 0 |
Continuous variables are shown as median (interquartile range) and categorical variables as number (percentage). The percentages may not add up to 100 because of rounding. KRT, kidney replacement therapy; d, days; mo, months; ICU, intensive care unit; CVVH, continuous venovenous hemofiltration.
Two patients had repeat admissions >12 months apart requiring KRT and are counted as separate.
The median age and weight at onset of KRT are shown in Table 1. In the <10 kg group, 44 (61%) were <30 days old and 58 (82%) weighed <5 kg.
Most patients had an underlying kidney or cardiac disease; however, the indication for KRT was different between the groups. In <10 kg group, the primary indication was AKI and electrolyte abnormalities in 25%, volume overload in 46%, AKI and volume overload in 15%, and ESKD (with relative or absolute contraindication for PD) in 14%. These patients primarily received CVVH (66%, n=48) and prolonged intermittent KRT (21%, n=15). In patients weighing >20 kg, volume overload was the primary indication in most (91%, n=31) and slow continuous ultrafiltration was the most common modality (94%, n=32).
Primary Outcome
Of the 72 patients <10 kg, 43 (60%) patients survived to end of KRT with the ultrafiltration device or transitioned to another modality of kidney support, and 23 (32%) survived to hospital discharge. Among patients >20 kg, 33 (97%) survived to KRT discontinuation and 23 (68%) survived to hospital discharge.
Secondary Outcomes
We looked at survival and kidney outcomes at last follow-up for patients who were discharged from the hospital (Table 2). In the <10 kg group, of the 23 who survived to hospital discharge, two were transferred to another facility and 12 survived to last follow-up. The median follow-up duration was 13 months (IQR, 12–24) and age at last follow-up was 16 months (IQR, 12–25). In this group, five patients had ESKD, including two who subsequently received a kidney transplant. Of those, three patients had initiated KRT for underlying CKD and two progressed to ESKD from AKI. After excluding those with ESKD, median eGFR at follow-up was 111 ml/min per 1.73 m2 (IQR, 102–131).
Table 2.
Outcomes at last follow-up of unique patients who survived to hospital discharge
| Characteristics | Group 1 (Weight <10 kg), n=23 | Group 2 (Weight 10–20 kg), n=11 | Group 3 (Weight >20 kg), n=21a |
|---|---|---|---|
| Outcome at last follow up | Expired=9 | Expired=2 | Expired=7 |
| Alive=12 | Alive=8 | Alive=14 | |
| Transferred=2 | Transferred=1 | Transferred=1 | |
| Age, mo | 16 (12, 25) | 41 (35, 57) | 221 (192, 259) |
| Median follow-up duration, months | 13 (12, 24) | 20 (19, 26) | 27 (22, 46) |
| Kidney outcome | n=12 | n=8 | n=14 |
| eGFR>150 | 1 | 0 | 0 |
| eGFR 90–150 | 5 | 4 | 3 |
| eGFR 60–90 | 0 | 1 | 2 |
| eGFR<60 | 1 | 0 | 4 |
| eGFR<15 | 3 | 1 | 0 |
| Kidney transplant | 2 | 2 | 4 |
| Unknown | 0 | 0 | 1 |
| Median eGFR at last follow up b | 111 (102, 131) | 98 (94, 124) | 66 (53, 102) |
eGFR, estimated Glomerular Filtration Rate. eGFR is reported in ml/min per 1.73 m2.
Two patients had repeat admissions requiring kidney replacement therapy. Follow-up data are from their first admission.
After excluding those with eGFR<15.
In the entire cohort, hypertension was present in 15 (60%) patients and proteinuria (>0.2 mg protein/mg creatinine on a spot sample) was seen in six patients at last follow-up.
Circuit Characteristics and Outcomes
We report on 884 circuits used over 2338 days. Of these, 157 circuits were for slow continuous ultrafiltration, 190 for prolonged intermittent KRT, and 537 for CVVH (Supplemental Table 2). We looked at complications at initiation and during KRT, according to weight (Table 3).
Table 3.
Circuit characteristics by patient weight
| Characteristic | Group 1 (Weight <10 kg), n=711 | Group 2 (Weight 10–20 kg), n=66 | Group 3 (Weight >20 kg), n=107 |
|---|---|---|---|
| Blood flow rate, ml/min | 40 (30, 40) | 40 (30, 40) | 40 (40, 40) |
| Cardiorespiratory support at initiation (or with complications at initiation) | 24 (3) | 4 (7) | 0 |
| Type of support neededa | |||
| Normal saline | 4 | 1 | — |
| Inotrope | 8 | — | — |
| Calcium chloride | 1 | 2 | — |
| Albumin | 8 | 1 | — |
| Sodium bicarbonate | 2 | — | — |
| Blood transfusion | 5 | ||
| Circuits with complications during therapy | 106 (15) | 12 (18) | 15 (14) |
| Type of complicationa | |||
| Hypothermia | 4 | — | — |
| Bleeding | 17 | — | — |
| Hypotensionb | 30 | 3 | 4 |
| Clot | 37 | 9 | 6 |
| Thrombocytopenia | 2 | — | — |
| Seizure | — | — | 2 |
| Catheter malfunction | 12 | — | — |
| Other | 8 | — | 3 |
| Anticoagulation | |||
| Heparin | 616 | 47 | 68 |
| Citrate | 2 | — | — |
| Argatroban | 0 | 11 | 3 |
| None | 91 | 5 | 30 |
| Unknown/other | 2 | 3 | 6 |
Continuous variables are shown as median (interquartile range) and categorical data as number (%). —, zero.
Some patients needed more than one intervention or had multiple complications.
Hypotension was defined as needing bolus of fluid, new inotrope requirement, or an increase in rate of existing inotrope infusion.
The right internal jugular vein was the most common location for vascular access, followed by the femoral vein. Heparin was used in >80% circuits (n=731). No anticoagulation was used in 126 circuits (14%). Citrate anticoagulation was attempted in one patient (two circuits). However, it was not successful because of the need for multiple lines and a nonintegrated system.
The median circuit life for the 537 CVVH circuits was 66 hours (IQR, 30–71). Of the CVVH circuits, 71 out of 537 (13%) were discontinued because of patient issues (withdrawal of cardiopulmonary support, elective therapeutic plasmapheresis, radiologic and/or surgical procedures, or improvement in patient condition and trial off CVVH) and ten out of 537 (2%) circuits underwent planned change before 60 hours for staff scheduling reasons. After censoring circuits that were changed because of patient issues or scheduling, we evaluated the remaining 456 out of 537 (85%) circuits and found that 309 (68%) of these circuits lasted for ≥60 hours, whereas 147 out of 309 (32%) had circuit loss, defined as unplanned, early termination at <60 hours. The reasons for early termination include clot in the circuit (98 out of 147, 67%), problems with vascular access (32 out of 147, 22%), machine malfunction (14 out of 147, 9%), and unknown (three out of 147, 2.0%).
Overall, only 28 out of 884 (3%) circuit initiations required an increase in cardiovascular support, and most (24 out of 28, 86%) were in the group weighing ≤10 kg (Table 3). In this group, the interventions included extra volume of saline, 5% albumin, or pRBCs in 17 out of 24 (70%), and a new inotrope or an increase in dose of inotropes in eight patients. Four patients in the group weighing 10–20 kg had cardiorespiratory complications at circuit initiation. No complications at circuit initiation were seen in the >20 kg group (Table 3). None had severe decompensation associated with circuit initiation.
Overall, complications during therapy were seen in 132 out of 884 treatments (15%). In the group weighing <10 kg, complications were seen in 106 treatments (15%) and were primarily vascular access–related, with a clot in 37 and catheter malfunction in 12. Bleeding was seen in 17, primarily oozing from catheter insertion sites that improved with modification of anticoagulation. None had known cerebral or pulmonary hemorrhage attributed to KRT. Hypothermia was seen in four treatments in this group. Clot in the line and hypotension during treatment were the commonest complications in patients weighing 10–20 kg and >20 kg. Other rare complications (seen in four or fewer treatments each across the entire cohort) included thrombocytopenia, seizure, arrhythmias, acidosis, and hypomagnesemia.
Discussion
We report a three-center experience with an ultrafiltration device, in children. We used it to provide a range of kidney supportive therapies, including CVVH, prolonged intermittent KRT, and slow continuous ultrafiltration. Adapting this system to provide CVVH allowed us to initiate KRT with minimal complications in critically ill neonates. More than 50% of infants weighing <10 kg survived to come off KRT with this device or transition to another modality of kidney support. We were also able to provide kidney support to neonates with ESKD who were unable to receive PD. In patients weighing >20 kg, who primarily received slow continuous ultrafiltration, 97% survived to end of therapy. In addition, we were able to manage a small group of patients with volume overload in the outpatient dialysis unit.
Many of our patients who received CVVH were small, young, and critically ill. Because of the challenges previously discussed, a majority of these may not have traditionally received KRT (2,6). The main advantage to the use of this device is the ability to initiate therapy with excellent hemodynamic stability. Fewer than 4% treatments had any complications reported at initiation, and none were serious enough to interrupt initiation. The incidence of cardiopulmonary complications at circuit initiation in infants using other machines has not been widely reported. A recent study reported hypotension at onset in 55% of CRRT sessions in neonates with 25% showing recovery within 60 minutes (8).
In addition, use of machines with smaller filters (and corresponding tubing sets with lower extracorporeal volume) help reduce the frequent exposure to blood products for blood prime and allow for smaller vascular access and lower blood flow rates (18–20). Using the rule that anyone with extracorporeal volume >10% receives a blood prime, the smallest available filter most commonly used in the United States (Prismaflex M60; extracorporeal volume 93 ml) necessitates blood prime for anyone weighing <11.5 kg. By using the ultrafiltration device, we were able to avoid blood prime for 23 patients weighing between 4 and 11.5 kg.
Complications during therapy were also infrequent, and mostly related to vascular access (clot or catheter malfunction) or minor bleeding at catheter insertion sites. Hypothermia was reported in four treatments and all were before the use of an in-line blood warmer. Once again, there are limited reports on complications of CRRT in infants.
This ultrafiltration device has not been widely used in children even for its traditional indication of slow continuous ultrafiltration (15). Although it is FDA-approved in patients weighing >20 kg, we included these patients in our study to highlight the clinical use of this device in the pediatric setting, particularly in patients with congenital heart disease and cardiomyopathies with associated heart failure. In our study, 50 patients (40%) underwent 157 treatments for UF only. Of these, six patients were able to receive treatment in the dialysis unit. The ease of doing UF and a low rate of complications suggests a promising role for this therapy in the ambulatory management of patients with diuretic-resistant volume overload, such as those with steroid-resistant nephrotic syndrome or congestive heart failure.
Our intensive care unit and hospital survival rate for patients <10 kg was 32%, which is lower than some previous studies. Symons et al. (5) reported 38% survival to intensive care unit discharge in children <10 kg; however, in the ≤3kg subgroup the survival was only 25%. Data from the ppCRRT registry reported an intensive care unit survival rate of 44% in children ≤5 kg (6). A more recent study showed a higher intensive care unit survival rate (57%) in infants <10 kg receiving CVVH (18). However, 28% of their cohort had inborn errors of metabolism, which typically has better outcomes. They also did not include patients with complex cardiac problems who have higher mortality and the smallest patient weighed 2 kg. The lower survival in our cohort likely reflects the fact that our patients were more premature, had lower weight, and more comorbidity. In our cohort, six patients weighed <2 kg and the smallest patient was 1.4 kg. KRT using existing dialysis machines would have been difficult and they may not have received KRT without this adapted system.
Our 1-year survival for children <10 kg was 52%; in the subset of patients who were ≤30 days at initiation of KRT, it was 16%. This low rate was also secondary to factors mentioned above. Limited data are available on the long-term outcomes of children who start KRT at a younger age. In the multinational study by van Stralen et al. (21), children with ESKD who started KRT in the neonatal period had an estimated 2-year survival of 81% and 5-year survival of 76%. They did not include children in whom kidney support was withheld or withdrawn early because of various reasons, or those who died shortly after initiation, thus potentially overestimating their survival rates. Another study reported 65.3% (32 out of 49) mortality in neonates who started CRRT in the first 30 days of life (22). The median age of death was 22 days, with 56% of the total deaths occurring within the first 30 days of life, and 94% within the first year. The authors did not comment on long term kidney outcomes (20). Nishimi et al. (8) reported 75% mortality in neonates who underwent CRRT; five out of eight died within 3 months, and one died at 2 years.
Although we have been able to successfully provide CVVH using an ultrafiltration device, it has certain limitations. As it was designed for slow continuous ultrafiltration, it does not have a pump to provide countercurrent dialysis and can only provide CVVH. The replacement fluid and heparin infusions have to be given through separate flow regulators external to the device that are not integrated with it. This requires extra vigilance on the part of the nursing staff in case either the CRRT pump or the replacement fluid flow regulator is not running. The accuracy of the UF pump is ±10% of setting, which can be a clinically significant volume change for smaller patients. Additionally, although the extracorporeal volume is low (33 ml), infants weighing <4 kg will still require a blood prime (for extracorporeal volume >10%).
The ultrafiltration device has a sensor that allows noninvasive monitoring of hematocrit and can be used to guide UF. Changes in hematocrit are inversely proportional to changes in patient blood volume. A typical setting alerts the clinician when the patient’s blood volume change exceeds 10%. This technology has been previously used in the maintenance hemodialysis setting to reduce intradialytic events related to UF (23,24). There are limited data on its use in acute KRT. We used noninvasive monitoring in some of our older patients to guide UF. However, we do not have data on this.
These problems can be addressed by machines specifically developed for providing kidney support to small children. The Cardio-Renal Pediatric Dialysis Emergency Machine (CARPEDIEM) is a pump-driven machine with filters available in three sizes (ranging in volume from 27.2 to 41.5 ml) (9). It can be used with catheters as small as 4 Fr to provide blood flow rates of 5–50 ml/min (25). The Newcastle Infant Dialysis and Ultrafiltration System (NIDUS) has been designed to provide continuous venovenous hemodialysis to infants between 800 g and 8 kg (10). It is a syringe-driven system (extracorporeal volume of 10 ml) that withdraws 5–12.5 ml of blood and passes it through a dialysis filter before returning to the patient. NIDUS can be used with a 4 Fr single-lumen catheter.
Despite being able to provide kidney support successfully to a large cohort, we acknowledge certain limitations in our study. Being a retrospective study, we were dependent on data available in our quality improvement database and medical records. In certain cases where the reason for escalation of inotropic support was not specified, we assumed it to be secondary to CRRT initiation. Information on complications of access placement, including the need for multiple catheters was not collected and included in this analysis. Furthermore, as a retrospective study, the data are subject to center and patient selection bias.
In conclusion, we report the first large experience in pediatrics using an ultrafiltration device, both for UF and for modified CVVH. This study speaks to the unmet need for devices specifically designed for younger patients. With the use of a machine with small extracorporeal volume, we could initiate CRRT safely without significant cardiovascular decompensation. Having more size-appropriate machines will shift the benefit–risk equation such that small children can get the benefit of kidney support to a level that is closer to larger children and older patients.
Disclosures
Dr. Askenazi is a consultant for and reports an education grant for neonatal AKI from CHF Solutions Inc. Dr. Goldstein reports personal fees from and a position as a consultant to CHF Solutions Inc., which manufactures the Aquadex device. Dr. Goldstein also consults for Medtronic Inc. (Minneapolis, MN) which manufactures a CRRT device. Dr. Goldstein receives grant funding from and serves as a consultant and on a Speaker Bureau for Baxter Healthcare, Inc. (McGaw Park, IL), which manufacturers a CRRT device. Dr. Askenazi is on a Speaker Bureau for Baxter Healthcare, Inc. (McGaw Park, IL). Dr. Broderick, Dr. Claes, Dr. DePaoli, Ms. Dill, Dr. Menon, Dr. Munshi, and Dr. Fathallah-Shaykh have nothing to disclose.
Supplementary Material
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.03240319/-/DCSupplemental.
Supplemental Table 1. Description of the kidney replacement therapy modality used.
Supplemental Table 2. Circuit characteristics by treatment modality.
References
- 1.Kaddourah A, Basu RK, Bagshaw SM, Goldstein SL; AWARE Investigators : Epidemiology of acute kidney injury in critically ill children and young adults. N Engl J Med 376: 11–20, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jetton JG, Boohaker LJ, Sethi SK, Wazir S, Rohatgi S, Soranno DE, Chishti AS, Woroniecki R, Mammen C, Swanson JR, Sridhar S, Wong CS, Kupferman JC, Griffin RL, Askenazi DJ; Neonatal Kidney Collaborative (NKC) : Incidence and outcomes of neonatal acute kidney injury (AWAKEN): A multicentre, multinational, observational cohort study. Lancet Child Adolesc Health 1: 184–194, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Menon S, Goldstein SL, Mottes T, Fei L, Kaddourah A, Terrell T, Arnold P, Bennett MR, Basu RK: Urinary biomarker incorporation into the renal angina index early in intensive care unit admission optimizes acute kidney injury prediction in critically ill children: A prospective cohort study. Nephrol Dial Transplant 31: 586–594, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sutherland SM, Alexander SR: Continuous renal replacement therapy in children. Pediatr Nephrol 27: 2007–2016, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Symons JM, Brophy PD, Gregory MJ, McAfee N, Somers MJ, Bunchman TE, Goldstein SL: Continuous renal replacement therapy in children up to 10 kg. Am J Kidney Dis 41: 984–989, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Askenazi DJ, Goldstein SL, Koralkar R, Fortenberry J, Baum M, Hackbarth R, Blowey D, Bunchman TE, Brophy PD, Symons J, Chua A, Flores F, Somers MJ: Continuous renal replacement therapy for children ≤10 kg: A report from the Prospective Pediatric Continuous Renal Replacement Therapy Registry. J Pediatr 162: 587–592.e3, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Askenazi D, Ingram D, White S, Cramer M, Borasino S, Coghill C, Dill L, Tenney F, Feig D, Fathallah-Shaykh S: Smaller circuits for smaller patients: Improving renal support therapy with Aquadex™. Pediatr Nephrol 31: 853–860, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nishimi S, Sugawara H, Onodera C, Toya Y, Furukawa H, Konishi Y, Sotodate G, Matsumoto A, Ishikawa K, Oyama K: Complications during continuous renal replacement therapy in critically ill neonates. Blood Purif 47[Suppl 2]: 74–80, 2019 [DOI] [PubMed] [Google Scholar]
- 9.Ronco C, Garzotto F, Brendolan A, Zanella M, Bellettato M, Vedovato S, Chiarenza F, Ricci Z, Goldstein SL: Continuous renal replacement therapy in neonates and small infants: Development and first-in-human use of a miniaturised machine (CARPEDIEM). Lancet 383: 1807–1813, 2014 [DOI] [PubMed] [Google Scholar]
- 10.Coulthard MG, Crosier J, Griffiths C, Smith J, Drinnan M, Whitaker M, Beckwith R, Matthews JN, Flecknell P, Lambert HJ: Haemodialysing babies weighing <8 kg with the Newcastle infant dialysis and ultrafiltration system (Nidus): Comparison with peritoneal and conventional haemodialysis. Pediatr Nephrol 29: 1873–1881, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Costanzo MR, Negoianu D, Jaski BE, Bart BA, Heywood JT, Anand IS, Smelser JM, Kaneshige AM, Chomsky DB, Adler ED, Haas GJ, Watts JA, Nabut JL, Schollmeyer MP, Fonarow GC: Aquapheresis versus intravenous diuretics and hospitalizations for heart failure. JACC Heart Fail 4: 95–105, 2016 [DOI] [PubMed] [Google Scholar]
- 12.Armand P, Boutarin D, Gerbaaï R: [Aquapheresis, an innovative technique in cardiology]. Rev Infirm 209: 31–32, 2015 [DOI] [PubMed] [Google Scholar]
- 13.Selewski DT, Goldstein SL: The role of fluid overload in the prediction of outcome in acute kidney injury. Pediatr Nephrol 33: 13–24, 2018 [DOI] [PubMed] [Google Scholar]
- 14.Arikan AA, Zappitelli M, Goldstein SL, Naipaul A, Jefferson LS, Loftis LL: Fluid overload is associated with impaired oxygenation and morbidity in critically ill children. Pediatr Crit Care Med 13: 253–258, 2012 [DOI] [PubMed] [Google Scholar]
- 15.Chakravarti S, Al-Qaqaa Y, Faulkner M, Bhatla P, Argilla M, Ramirez M: Novel use of an ultrafiltration device as an alternative method for fluid removal in critically ill pediatric patients with cardiac disease: A case series. Pediatr Rep 8: 6596, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Selewski DT, Cornell TT, Lombel RM, Blatt NB, Han YY, Mottes T, Kommareddi M, Kershaw DB, Shanley TP, Heung M: Weight-based determination of fluid overload status and mortality in pediatric intensive care unit patients requiring continuous renal replacement therapy. Intensive Care Med 37: 1166–1173, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brophy PD, Somers MJ, Baum MA, Symons JM, McAfee N, Fortenberry JD, Rogers K, Barnett J, Blowey D, Baker C, Bunchman TE, Goldstein SL: Multi-centre evaluation of anticoagulation in patients receiving continuous renal replacement therapy (CRRT). Nephrol Dial Transplant 20: 1416–1421, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Kaempfen S, Dutta-Kukreja P, Mok Q: Continuous venovenous hemofiltration in children less than or equal to 10 kg: A single-center experience. Pediatr Crit Care Med 18: e70–e76, 2017 [DOI] [PubMed] [Google Scholar]
- 19.Goldstein SL, Hackbarth R, Bunchman TE, Blowey D, Brophy PD; Prospective Pediatric CRRT Registry Group Houston : Evaluation of the PRISMA M10 circuit in critically ill infants with acute kidney injury: A report from the Prospective Pediatric CRRT Registry Group. Int J Artif Organs 29: 1105–1108, 2006 [DOI] [PubMed] [Google Scholar]
- 20.Rödl S, Marschitz I, Mache CJ, Koestenberger M, Madler G, Rehak T, Zobel G: One-year safe use of the Prismaflex HF20(®) disposable set in infants in 220 renal replacement treatment sessions. Intensive Care Med 37: 884–885, 2011 [DOI] [PubMed] [Google Scholar]
- 21.van Stralen KJ, Borzych-Dużalka D, Hataya H, Kennedy SE, Jager KJ, Verrina E, Inward C, Rönnholm K, Vondrak K, Warady BA, Zurowska AM, Schaefer F, Cochat P; ESPN/ERA-EDTA registry; IPPN registry; ANZDATA registry; Japanese RRT registry : Survival and clinical outcomes of children starting renal replacement therapy in the neonatal period. Kidney Int 86: 168–174, 2014 [DOI] [PubMed] [Google Scholar]
- 22.Maizlin II, Shroyer MC, Perger L, Chen MK, Beierle EA, Martin CA, Anderson SA, Mortellaro VE, Rogers DA, Russell RT: Outcome assessment of renal replacement therapy in neonates. J Surg Res 204: 34–38, 2016 [DOI] [PubMed] [Google Scholar]
- 23.Patel HP, Goldstein SL, Mahan JD, Smith B, Fried CB, Currier H, Flynn JT: A standard, noninvasive monitoring of hematocrit algorithm improves blood pressure control in pediatric hemodialysis patients. Clin J Am Soc Nephrol 2: 252–257, 2007 [DOI] [PubMed] [Google Scholar]
- 24.Michael M, Brewer ED, Goldstein SL: Blood volume monitoring to achieve target weight in pediatric hemodialysis patients. Pediatr Nephrol 19: 432–437, 2004 [DOI] [PubMed] [Google Scholar]
- 25.Lorenzin A, Garzotto F, Alghisi A, Neri M, Galeano D, Aresu S, Pani A, Vidal E, Ricci Z, Murer L, Goldstein SL, Ronco C: CVVHD treatment with CARPEDIEM: Small solute clearance at different blood and dialysate flows with three different surface area filter configurations. Pediatr Nephrol 31: 1659–1665, 2016 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.