Abstract
The number of patients dialyzed for ESKD exceeds 500,000 in the United States and more than 2.6 million people worldwide, with the expectation that the worldwide number will double by 2030. The human cost of health and societal financial cost of ESKD is substantial. Dialytic therapy is associated with an unacceptably high morbidity and mortality rate and poor quality of life. Although innovation in many areas of science has been transformative, there has been little innovation in dialysis or alternatives for kidney replacement therapy (KRT) since its introduction approximately 70 years ago. Advances in kidney biology, stem cells and kidney cell differentiation protocols, biomaterials, sensors, nano/microtechnology, sorbents and engineering, and interdisciplinary approaches and collaborations can lead to disruptive innovation. The Kidney Health Initiative, a public–private partnership between the American Society of Nephrology and the US Food and Drug Administration, has convened a multidisciplinary group to create a technology roadmap for innovative approaches to KRT to address patients’ needs. The Roadmap is a living document. It identifies the design criteria that must be considered to replace the myriad functions of the kidney, as well as scientific, technical, regulatory, and payor milestones required to commercialize and provide patient access to KRT alternatives. Various embodiments of potential solutions are discussed, but the Roadmap is agnostic to any particular solution set. System enablers are identified, including vascular access, biomaterial development, biologic and immunologic modulation, function, and safety monitoring. Important Roadmap supporting activities include regulatory alignment and innovative financial incentives and payment pathways. The Roadmap provides estimated timelines for replacement of specific kidney functions so that approaches can be conceptualized in ways that are actionable and attract talented innovators from multiple disciplines. The Roadmap has been used to guide the selection of KidneyX prizes for innovation in KRT.
Keywords: dialysis, kidney bioengineering, xenotransplantation, kidney chimeras, proximal tubule, toxicity, secretion, RRT access, blood filtration, electrolyte homeostasis, kidney organoid, nephrology, renal dialysis, kidney dialysis, biocompatible materials, microtechnology, United States Food and Drug Administration, motivation, public-private sector partnerships, quality of life, chronic kidney failure, renal replacement therapy, kidney, urinary tract physiological phenomena; stem cells
Introduction
Statement of the Problem
CKD affects >10% of the world’s population (1). Often, CKD leads to ESKD requiring kidney replacement therapy (KRT) in the form of dialysis (hemo or peritoneal) and/or (in the case of a small subgroup) transplantation. More than 500,000 people in the United States (2) and 2.6 million worldwide receive dialysis as KRT (3), and at least 5 million people (by conservative estimate) are actually in need of KRT today. It is projected that worldwide use of KRT will more than double to an estimated 5.4 million people by 2030 (3). Rapid economic growth and epidemics of diabetes and hypertension in the large emerging economies of China, India, and Brazil will substantially increase global dialysis numbers.
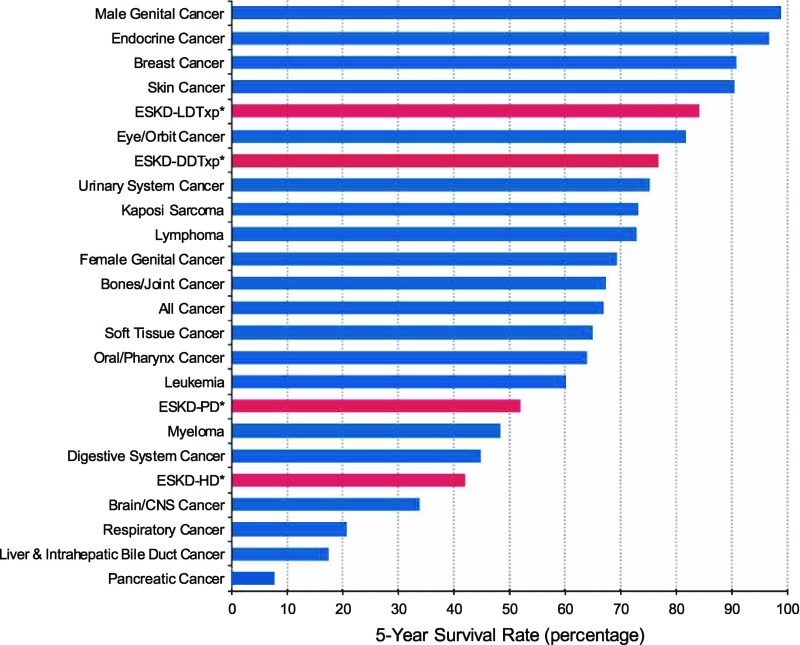
Despite some refinements in dialytic devices, innovation has been modest, especially when compared with the technological advancements in many other areas of medicine and society in general. Although transformative communication technologies such as cell phones and the internet have revolutionized daily lives, dialysis for KRT has changed little since it was introduced approximately 70 years ago. This stagnation has occurred despite unacceptably high morbidity and mortality rates, very high cardiovascular risk, infectious and hematologic complications, hospitalizations, and poor quality of life (4,5). Patients treated with dialysis have a life expectancy that is shorter than that associated with most cancers (Figure 1). In contrast to KRT, there have been a number of novel therapeutic approaches to various cancers (6) and in many other areas of medicine. It is encouraging that there are data that increasing frequency of dialysis can improve quality of life (7), indicating that patients with ESKD can respond to innovative changes in dialytic approaches such as continuous use products (wearable or implantable artificial kidneys) or xenotransplantation.
Figure 1.
Comparison of survival of patients with ESKD compared with survival of patients with many forms of cancer. Patients with ESKD were divided into four categories: those with a living related transplant, deceased donor transplant or those on hemodialysis or peritoneal dialysis. *Reference population: patients with incident ESKD, 2011. Adjusted for age, sex, race, Hispanic ethnicity, and primary diagnosis. ESKD-DDTxp, ESKD received deceased donor transplant; ESKD-PD, ESKD receiving peritoneal dialysis; ESKD-HD, ESKD receiving hemodialysis; ESKD-LDTxp, ESKD received living donor transplant (40,41).
The societal financial cost of ESKD is substantial. Medicare spending for beneficiaries with ESKD was $35.4 billion in 2016 (approximately 7.2% of the overall Medicare paid claims costs [8]). The amount spent on transplant patients was $3.4 billion. When adding $79 billion dollars of expenditures for CKD costs, total annual Medicare costs for advanced kidney disease exceeded $114 billion (8). These numbers do not include costs incurred by non-Medicare sources, nor do they include lost work and other lost opportunities for patients, families, and caregivers. Costs are expected to rise significantly as the prevalence of the risk factors for ESKD (diabetes, hypertension, etc.) continues to rise. This projected financial situation is unsustainable. We can and must do better for our patients and our health care systems. Financial investment to find cures or preventive approaches have represented only a small fraction of these clinical costs. National Institutes of Health funding for kidney research was only $564 million dollars in 2015 (<1% of the Medicare funds spent on the care of kidney patients). We are long overdue in seeking innovative alternatives. Although we are certainly too late for many, we owe it to our growing population of patients with CKD to provide a brighter future. Developing the Roadmap itself draws attention to the US Food and Drug Administration (FDA)’s and Kidney Health Initiative (KHI)’s vision of requirements necessary to achieve a better state and encourage innovation that will ultimately bring better therapies and an improved quality of life. Many patients are also altruistic enough to appreciate that the enthusiasm and focus will help their successors if not them.
KHI Commitment to Development of a Technology Roadmap
To address the great need for innovation in KRT, the KHI, a public–private partnership between the American Society of Nephrology (ASN) and the FDA (9), including over 100 companies and organizations representing patients, care partners, health care professionals, industry, and government, submitted a commitment statement at the White House Summit on Organ Donation in June 2016 (10). The stated goals were endorsed: “Identify the scientific, technical, and regulatory milestones needed to achieve the goal of creating a bio-artificial or bioengineered alternative to dialysis as RRT.” In other words, create a scientific and regulatory roadmap to spur the development of innovative products to improve the quality of life of people on dialysis.
To create the Technology Roadmap for Innovative Approaches to RRT (11), KHI solicited the input of a diverse and international group of stakeholders, including patients, clinicians, research scientists, entrepreneurs, leaders of dialysis, pharma and biotechnology companies, regulators, and payors (Supplemental Table 1). The goals of the Roadmap, listed in Table 1, reflect patient input, in keeping with KHI’s core value and strategic priority (12,13). Table 2 provides examples of patient-identified goals that would improve their quality of life. The Roadmap is intended to drive “disruptive” innovation in the development of new solutions without being prescriptive at this time. The Roadmap has already demonstrated utility as it has served to guide the first round of KidneyX prizes—a process that resulted in 165 applications for 15 prizes provided by the ASN and the US Department Health and Human Services (14).
Table 1.
Goals of the KHI KRT Technology Roadmap
| Goals |
|---|
| Spur innovation in KRT |
| Attract industry, academic, and governmental investment in KRT solutions |
| Encourage international, multidisciplinary approaches to solutions |
| Accelerate the availability and adoption of commercially viable solutions |
| Ensure that patient and care partner preferences are incorporated throughout the KRT solution development life cycle |
| Define regulatory agency considerations |
| Optimize reimbursement models |
Taken with minor modifications from the Roadmap (Supplemental Appendix 1). KHI, Kidney Health Initiative; KRT, kidney replacement therapy.
Table 2.
Examples of patient-focused goals of novel kidney replacement therapies
| Decrease time on dialysis |
|---|
| Reduce restrictions on diet/fluids |
| Alleviate symptoms: |
| Dialysis associated dizziness, headaches, muscle cramps |
| Nausea and vomiting |
| Dry, itchy skin |
| Sleep disturbances |
| Depression |
| Fatigue |
| Weakness |
| Mental status changes |
| Improve: |
| BP control |
| Ability to work |
| Ability to travel |
| Anemia |
| Bone disease |
Taken with minor modifications from the Roadmap (Supplemental Appendix 1).
KRT Technology Roadmap
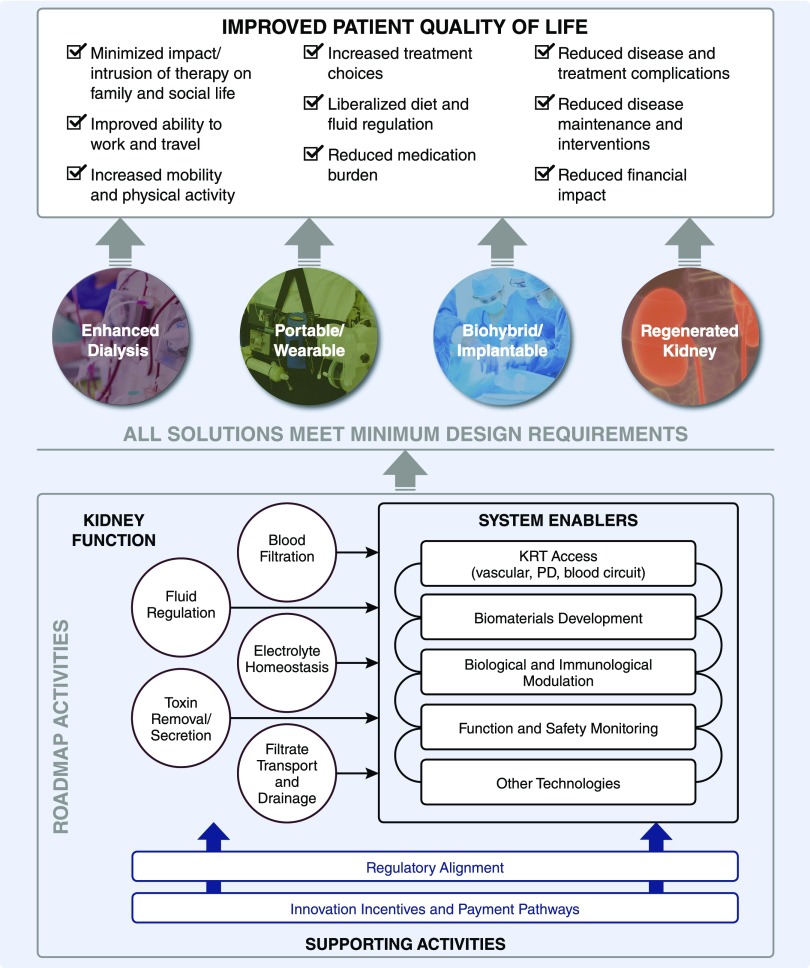
An overview of the approach and considerations incorporated into the Roadmap is depicted in Figure 2. Four potential broad approaches were identified in order of complexity: enhanced dialysis (improvements on current approaches), a portable or wearable solution, biohybrid/implantable kidney (including xenotransplantation), and a regenerated kidney. Current dialysis, whether hemo or peritoneal, is intermittent and addresses primarily the kidney’s filtration physiology with tubular functional replacement modeled by passive diffusion. By contrast, the continuous filtration and reabsorptive and secretory properties of the kidney tubules, refined over millions of years of evolution, provide precise regulation of the internal milieu to clear wastes and maintain fluid, electrolyte, and hormonal balance that is adaptive to wide changes in intake and metabolic processes and demands. Although a therapy that is continuous may be better tolerated physiologically, the ultimate arbiter of the best approach will be the one that proves to be safe, effective, and improves patients’ lives. A potential solution should not be bound by preconceived biases influenced by current dialytic therapy or even the anatomic/physiologic features of the kidney. Accordingly, functional replacement should drive the design of the solution.
Figure 2.
An overview of the approach and considerations incorporated into the Roadmap. Milestones were identified in order of complexity: enhanced dialysis (improvements on current approaches), a portable or wearable solution, biohybrid/implantable kidney (including xenotransplantation), and a regenerated kidney. Kidney functions that would have to be replaced were grouped into five categories, including: blood filtration, fluid regulation, electrolyte homeostasis, toxin removal/secretion, and filtrate transport and drainage. How replacement would occur was not prescribed, but rather left to the ingenuity of the community. A number of “system enablers” could facilitate progress. These include, but are not limited to, kidney replacement access advances, developments in biomaterials, new insights into biologic and immunologic modulation, current advances in function, and safety monitoring. It was recognized that alignment with regulatory agencies throughout this development process was critical. It was also recognized that financial incentives and novel payment pathways would be very important to incentivize the innovation needed to accomplish the goals of the Roadmap. Figure is taken, with minor modifications, from the Roadmap (Supplemental Appendix 1). PD, peritoneal dialysis.
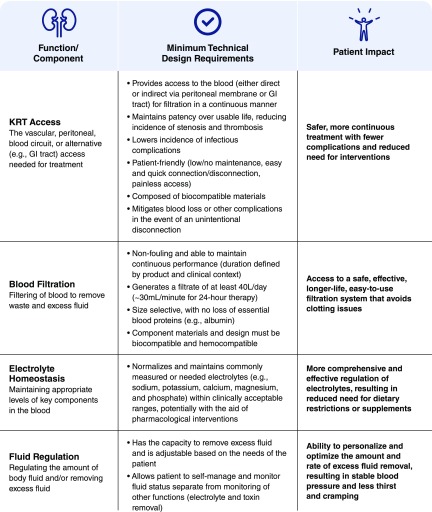
The stakeholder group discussed a variety of design criteria for an innovative KRT product or approach (Figure 3). It is important to be clear about which kidney functions are most important to replace. Prioritization and subdivisions of the tasks can make the goals more achievable and highlight the need to define a minimal set of criteria and milestones that will be clinically meaningful to patients and caregivers. For example, fluid balance might dictate removal of up to 3 L of fluid per day to allow reasonable fluid intake. The system would have to accommodate the drainage of fluid and waste products. Small molecules, such as urea, as well as other larger “uremic toxins” and drugs would need to be removed. Effective blood clearance goals might be at least 30–40 L/d for small solutes. Electrolyte homeostasis and pH regulation are important although some of this control may be facilitated by sensors and the use of orally or closed-loop administered alkali or specific binders. Although solute secretion or sorbent adsorption could theoretically reduce the demand on clearance, the secretory functions or adsorption characteristics would have to be very expansive to eliminate uremic solutes, some of which remain unknown, as well as the ever-growing numbers of drugs that rely on the kidney for excretion by secretion. Supplemental Figure 1, taken from the Roadmap, includes a list of activities that could lead to solutions to replace various components of kidney function, including blood filtration, electrolyte homeostasis, fluid regulation, toxin removal, and filtrate transport and drainage. In Supplemental Figure 1 we also include estimated timelines for achieving the goals stated, assuming that adequate financial resources are available. We also predict whether the activities will require mechanical/physiochemical-based solutions, cellular-based solutions, or combined biohybrid solutions.
Figure 3.
Design criteria for innovative kidney replacement product or approach. Minimum technical design criteria were identified in six areas: KRT access, blood filtration, electrolyte homeostasis, fluid regulation, toxin removal/secretion, and filtrate transport and drainage. Figure is taken, with minor modifications, from the Roadmap (Supplemental Appendix 1). GI, gastrointestinal; KRT, kidney replacement therapy.
In the Roadmap, there are proposed a number of “system enablers” that represent technologies that would facilitate the development of viable solution(s) (Supplemental Figure 2). Vascular access, a stable, safe, and reliable connection to the circulation, is an important consideration. Advances in biomaterials should enable filtration without clotting, specific adsorption characteristics of sorbents, and substrates upon which cells can thrive and remain functionally active. The KRT product ideally would be small and lightweight. In cases where a solution contains living cells, biologic and immunologic technologies will enable longer survival and functional integrity. Advances in sensor technologies can enable function and safety monitoring so that the solution can be personalized to the needs of the patient.
Early products might be exclusively mechanical, dealing primarily with filtration and exposure of filtrate to sorbents to remove specific components before returning the nonexcreted filtrate to the circulation. Regardless of the characteristics of the product, it must have sufficient durability or be easily replaced or recharged. It must satisfy the requirements of regulatory agencies for sterility, biocompatibility, and overall safety. It is, therefore, critically important that the FDA be involved as progress is made. Strong continued involvement of the regulatory agencies will also help to derisk investment made by the private sector. Likewise it is important to define perspectives of the payors so that private investments will be financially prudent. Finally, any product must be commercially viable and available in both the industrialized and the developing world. Making the initiative particularly challenging are the number of important knowledge gaps in kidney disease understanding that are very relevant to KRT strategies (Table 3).
Table 3.
Examples of knowledge gaps in kidney disease relevant to kidney replacement strategies
| Strategies |
|---|
| Patient therapy preferences (setting, duration, etc.) |
| Patient-centric measures of adequate KRT |
| Biomarkers of adequate KRT |
| Amount of KRT needed to minimize uremic symptoms and consequences |
| Adequate animal uremic models that model human ESKD to test new products for safety and efficacy |
| Identification of critical kidney functions that must be replaced and which ones can be treated with drugs or managed in other ways |
Taken with minor modifications from the Roadmap (Supplemental Appendix 1). KRT, kidney replacement therapy.
State of the Science and Technology
There has been substantial progress in multiple areas of technology and cell and integrative biology that make it more realistic to conceive of breakthrough innovation in KRT. Nevertheless there remain significant technical challenges. Kidney functions are complex and reproducing any one of these functions within a tenable timeline will take a great deal of focus, ingenuity, increase in recognition of the need, and multidisciplinary collaborations among content experts in disparate disciplines. Although it is appropriate to have as a long-term goal the development of a system that can recapitulate the precise regulation that our normally functioning kidneys provide, it is also important that we establish realistic short-term milestones and divide the overall challenge into subunits with associated milestones that can be tackled by groups of innovators in an aggressive time frame. Shorter-term milestones should bring some relief to patients sooner, deemed as partial success by patients, funders, regulatory agencies, and payors. Knowledge gaps (Table 3) should not prevent us from defining goals achievable in the short term. Although it may have been a long-term goal of John F. Kennedy, the 35th President of the United States, to engage in intergalactic space flight, the short-term achievable milestone was placing a man on the moon and bringing him back alive. Likewise, although achieving the Roadmap goals may require iterations, additional approaches must allow for the “disruptive,” leveraging of new technologies. The Roadmap becomes “granular” in defining many of the specific activities that can guide product development for replacement of each of the categories of kidney function (blood filtration, electrolyte homeostasis, fluid regulation, toxin removal, and filtrate transport and drainage) with an estimated timeline (near term, within 4 years; midterm, 5–7 years; long term, >7 years) (Supplemental Figure 1). In the next sections we will include examples of potential technologies that can be applied.
Mechanical/Physiochemical/Acellular
Material science (15), sensor technologies (16,17), microelectromechanical systems, and biochips (18) comprise some of the most rapidly growing areas of technology. A filtering device could be integrated with sensors, which can be used to modify the amount of filtration, dependent upon the patient’s needs. Although a device that only provides filtration may be very useful for patients whose primary need is volume regulation, it may also provide some convective clearance and could reduce the total number of conventional dialysis treatments needed.
Nanomaterials have unique size-dependent properties, which can confer high conductivity and modifiable adsorptive properties (18). Thus it is also possible that sorbents could be incorporated into a mechanical device that selectively absorb and/or remove electrolytes, toxins, etc., thereby contributing to maintenance of biochemical regulation of the blood. Some newer materials, such as carbon nanotubes, have antimicrobial properties (18). Development of “smart membranes” with sufficient capacity and analyte “coverage” is unlikely to be possible in the short term because the composition of the blood is complex and changes with physiologic state, dietary intake, and the increasing number of medications, but progress can be iterative. There will remain several challenges with mechanical or physiochemical approaches for dialysis devices, including a lack of current consensus on which uremic toxins should be targeted for removal. As devices get smaller and filtration surface area is maximized, there is increased chance for fouling and obstruction to flow and filtration. Thus, there will be great demands for biocompatibility of the devices so that the surfaces will not activate blood cells or factors in the clotting system. In addition, it is essential that systems be developed that either prevent or rapidly remove air or generated carbon dioxide from entering the blood circuit.
Cellular
Benefits of cellular products include the harnessing of cell energetics (ATP, etc.) to enable movement of molecules. There has been important progress in generation of kidney cells and structures from human pluripotent stem cells. Human induced pluripotent stem cells allow for a personalized approach to development of kidney cells and tissue from blood cells of patients who need kidney replacement, thus reducing some immunologic barriers. As an example, a model of the glomerular filtration barrier has been generated by placing podocytes derived from human pluripotent stem cells on one side of a laminin-coated, flexible, porous elastomer membrane with primary cultured glomerular endothelial cells placed on the other side of the membrane (19). Proximal tubule epithelial cells perform the bulk of water and electrolyte reabsorption of the glomerular filtrate and secretion of organic cations and anions. Proximal tubules actively secrete charged potentially toxic xenobiotics from blood into the kidney tubule lumen and into the urine. Proximal tubules also play key roles in vitamin D metabolism. Engineered systems that include stable cells, which maintain characteristics of proximal tubules ex vivo, together with perfusion on their luminal surface with filtrate of the plasma and on their basolateral side by blood containing oxygen and nutrients, could recapitulate the reabsorptive and secretory properties of proximal tubules in vivo (20). Maintenance of proximal tubule cells ex vivo with differentiated properties mimicking those in vivo has been an elusive goal. More recently, however, kidney organoid technology has progressed to a state of development where various nephron structures, derived from human pluripotent stem cells, form polarized epithelia and express proteins and other characteristics of proximal and distal tubules and collecting ducts, although the functional characteristics of the cells are not yet fully defined (21–23).
Xenotransplantation and Chimeric Organs
Although not discussed in depth in version 1.0 of the Roadmap, a cellular solution could include xenotransplantation (24) or chimeras (25). Efforts to generate an animal-derived organ for human kidney xenotransplantation have benefited from advances in gene-editing technology and immunosuppression regimens. Challenges include immunologic barriers for transplantation, coagulation system incompatibilities, and the risk of transmission and recombination of infectious agents. The development of gene-editing technologies such as CRISPR-Cas9 has made it more feasible to perform multiplex gene-editing, which can be used to simultaneously eliminate multiple xenoantigens or introduce genes to minimize activation of the human complement and coagulation systems (26,27). Recent studies have also shown the effectiveness of new approaches to inactivate the porcine endogenous retroviruses in epithelial cells using CRISPR-Cas9 approaches (28), potentially reducing the risk to public health of transplanting porcine organs into humans.
Another approach to producing a replacement organ is through chimera formation, in which genetically distinct cells from more than one species grow together in a single animal, resulting in an organ that is composed of both human and nonhuman cells. Several studies have been published on interspecies blastocyst complementation where xenogeneic pluripotent stem cells or nephron progenitor cells are introduced into a blastocyst that has a defective gene critical for organogenesis to generate a chimeric organ in the host animal (29–31).
Regulatory Considerations
The FDA recognizes the lack of innovation in ESKD, and understands that ESKD management is costly and negatively affects the patient’s quality of life. Because of the need to advance medical product development for areas of high need, FDA has developed both specific programs targeted at this area, as well as a number of expedited programs. In an attempt to spur innovation in this product area, the FDA’s Center for Devices and Radiologic Health announced the “Innovation Challenge: ESRD” in 2012 (32). This program introduced FDA to novel ESKD devices, including wearable and implantable systems at various stages of development. Novel ESKD technologies underscore the complexities of both the technological challenges and regulatory requirements involved in bringing innovative ESKD products to market. Additionally, it became apparent to FDA that much of the critical scientific, engineering, product development, and clinical expertise exists outside of FDA, and that the KHI public–private partnership model is uniquely suited to unite the different stakeholders to further the effort of facilitating innovation in ESKD technologies (33). As a member of KHI, FDA is actively engaged in the innovative KRT roadmap project and recognizes the importance of advancing this collaborative effort to enable the development of novel products for ESKD.
FDA has also implemented expedited and breakthrough programs to facilitate and expedite the development and review of promising new therapies with the potential to address unmet medical needs in the treatment of serious or life-threatening, persistent, recurrent, or irreversibly debilitating diseases or conditions. Products for the replacement of kidney function may be eligible for these programs that are intended to speed patient access to new and innovative medical products, provided the products meet the qualifying criteria for the expedited programs. Examples include the Breakthrough Devices Program (34) Expedited Programs for Serious Conditions for drugs and biologics (35), and the Regenerative Medicine Advanced Therapy designation program created under the provisions of the 21st Century Cures Act (36). These programs preserve the FDA’s statutory standards of safety and efficacy for marketing authorization while helping patients get more timely access to medical products. FDA guidance documents on cellular therapy and xenotransplantation products (37–39) could provide useful information related to the design and manufacturing, preclinical testing, and clinical evaluation of innovative KRT products.
Conclusions
In conclusion, with the aid of patients, care partners, and multiple academic, private, and public communities, the KHI has developed a technology roadmap for innovation in KRT with a primary focus on the professed needs and preferences of patients with advanced kidney disease. With increased awareness by disparate technological communities of opportunities to make transformative contributions, together with additional interest and investment from the corporate, philanthropic, and venture capital communities, it is hoped that this Roadmap will facilitate the achievement of brighter future for the growing population of patients worldwide with kidney disease. A copy of the entire Roadmap is included as Supplemental Appendix 1.
Disclosures
Dr. Bonventre reports serving as a consultant for Abbvie, Aldeyra, Astellas, Biomarin, Boehringer Ingelheim, Catabasis, Cerespir, ECB Celltech, Eleven, Eli Lilly, Eloxx, Medimmune, Merck, Mitobridge, and PTC. Dr. Bonventre also reports ownership equity in Dicerna, DXNow, Goldfinch, Goldilocks, Innoviva, Medibeacon, Medssenger, Rubius, Sensor-Kinesis, Sentien, Theravance, and Thrasos; he also reports grant funding from Astellas, Boehringer Ingelheim and the National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases and National Center for Advancing Translational Science (R37 DK39773, RO1 DK072381, UG TR002155). In addition, Dr. Bonventre is coinventor on KIM-1 and human kidney organoid patents assigned to Partners Healthcare. Dr. Roy-Chaudhury reports serving as a consultant for Akebia, Bayer, BD, Cormedix, Medtronic, Reata, Relypsa, and WL Gore and reports ownership equity in Inovasc LLC; all disclosures are outside the submitted work. Ms. West discloses that she is employed by the American Society of Nephrology. Dr. Hurst, Dr. Sheldon, and Dr. Wu have nothing to disclose.
Supplementary Material
Acknowledgments
This work was supported by the Kidney Health Initiative (KHI), a public–private partnership of the American Society of Nephrology, US Food and Drug Administration, and >100 member not-for-profit and for-profit organizations. KHI funds were used to defray costs, including project management costs. There was no remuneration to KHI workgroup members. The authors are fully responsible for the content of this work. KHI makes every effort to avoid actual, potential, or perceived conflicts of interest among members of the workgroup. More information on the KHI, or conflict of interest policy, can be found at www.kidneyhealthinitiative.org.
The views and opinions expressed in this manuscript are those of the authors and do not necessarily represent the official views or policies of any KHI member organization, or the US Department of Health and Human Services. No mention of trade names, organizations, or reference to particular technologies implies endorsement by the KHI, or any US Government agency.
Footnotes
Published online ahead of print. Publication date available at www.cjasn.org.
Supplemental Material
This article contains the following supplemental material online at http://cjasn.asnjournals.org/lookup/suppl/doi:10.2215/CJN.02570319/-/DCSupplemental.
Supplemental Table 1. List of participants who provided sustained input on the Roadmap.
Supplemental Figure 1. Kidney function replacement activities.
Supplemental Figure 2. System enabler activities.
Supplemental Appendix 1. Technology roadmap for innovative approaches to RRT.
References
- 1.Levin A, Tonelli M, Bonventre J, Coresh J, Donner JA, Fogo AB, Fox CS, Gansevoort RT, Heerspink HJL, Jardine M, Kasiske B, Köttgen A, Kretzler M, Levey AS, Luyckx VA, Mehta R, Moe O, Obrador G, Pannu N, Parikh CR, Perkovic V, Pollock C, Stenvinkel P, Tuttle KR, Wheeler DC, Eckardt KU; ISN Global Kidney Health Summit participants: Global kidney health 2017 and beyond: A roadmap for closing gaps in care, research, and policy. Lancet 390: 1888–1917, 2017 [DOI] [PubMed] [Google Scholar]
- 2.US Renal Data System: 2018 Annual Data Report: Epidemiology of kidney disease in the US. NIH, NIDDK, Bethesda MD [DOI] [PMC free article] [PubMed]
- 3.Liyanage T, Ninomiya T, Jha V, Neal B, Patrice HM, Okpechi I, Zhao MH, Lv J, Garg AX, Knight J, Rodgers A, Gallagher M, Kotwal S, Cass A, Perkovic V: Worldwide access to treatment for end-stage kidney disease: A systematic review. Lancet 385: 1975–1982, 2015 [DOI] [PubMed] [Google Scholar]
- 4.Jaar BG, Chang A, Plantinga L: Can we improve quality of life of patients on dialysis? Clin J Am Soc Nephrol 8: 1–4, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Wyld M, Morton RL, Hayen A, Howard K, Webster AC: A systematic review and meta-analysis of utility-based quality of life in chronic kidney disease treatments. PLoS Med 9: e1001307, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pal S, Miller MJ, Agarwal N, Chang SM, Chavez-MacGregor M, Cohen E, Cole S, Dale W, Magid CS Diefenbach, Disis ML, Dreicer R, Graham DL, Henry NL, Jones J, Keedy V, Klepin HD, Markham MJ, Mittendorf EA, Rodriguez-Galindo C, Sabel MS, Schilsky RL, Sznol M, Tap MD, Westin SN, Johnson BE: Clinical Cancer Advances 2019 : Annual Report on Progress Against Cancer From the American Society of Clinical Oncology. J Clin Oncol 37: 834–849, 2019 [DOI] [PubMed] [Google Scholar]
- 7.Garg AX, Suri RS, Eggers P, Finkelstein FO, Greene T, Kimmel PL, Kliger AS, Larive B, Lindsay RM, Pierratos A, Unruh M, Chertow GM; Frequent Hemodialysis Network Trial Investigators: Patients receiving frequent hemodialysis have better health-related quality of life compared to patients receiving conventional hemodialysis. Kidney Int 91: 746–754, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.US Renal Data System: 2018 Annual Data Report: Epidemiology of kidney disease in the US. NIH, NIDDK, Bethesda, MD. Ref Table K.2: Medicare Expenditures for Persons with ESRD, 2016
- 9.Archdeacon P, Shaffer RN, Winkelmayer WC, Falk RJ, Roy-Chaudhury P: Fostering innovation, advancing patient safety: The kidney health initiative. Clin J Am Soc Nephrol 8: 1609–1617, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meswala A, Erickson J, Barbero R: Saving Lives and Giving Hope by Reducing the Organ Waiting List, 2016. Available at: https://obamawhitehouse.archives.gov/blog/2016/06/13/saving-lives-and-giving-hope-reducing-organ-waiting-list. Accessed September 5, 2019 [Google Scholar]
- 11.Kidney Health Initiative: Technology Roadmap for Innovative Approaches to Renal Replacement, 2018. Available at: www.kidneyhealthinitiative.org/rrtroadmap. Accessed September 5, 2019.
- 12.Linde PG, Archdeacon P, Breyer MD, Ibrahim T, Inrig JK, Kewalramani R, Lee CC, Neuland CY, Roy-Chaudhury P, Sloand JA, Meyer R, Smith KA, Snook J, West M, Falk RJ: Overcoming barriers in kidney health-forging a platform for innovation. J Am Soc Nephrol 27: 1902–1910, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hurst FP, Chianchiano D, Upchurch L, Fisher BR, Flythe JE, Castillo Lee C, Hill T, Neuland CY: Stimulating patient engagement in medical device development in kidney disease: A report of a kidney health initiative workshop. Am J Kidney Dis 70: 561–569, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.American Society of Nephrology: HHS and the American Society of Nephrology Award KidneyX Redesign Dialysis Phase 1 Winners, 2019. Available at: https://www.asn-online.org/about/press/releases/ASN_PR_20190429_KidneyX_4.30.19221L.pdf. Accessed September 5, 2019
- 15.Cormode DP, Gao L, Koo H: Emerging biomedical applications of enzyme-like catalytic nanomaterials. Trends Biotechnol 36: 15–29, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heikenfeld J, Jajack A, Rogers J, Gutruf P, Tian L, Pan T, Li R, Khine M, Kim J, Wang J, Kim J: Wearable sensors: Modalities, challenges, and prospects. Lab Chip 18: 217–248, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ledet EH, D’Lima D, Westerhoff P, Szivek JA, Wachs RA, Bergmann G: Implantable sensor technology: From research to clinical practice. J Am Acad Orthop Surg 20: 383–392, 2012 [DOI] [PubMed] [Google Scholar]
- 18.Gehrke I, Geiser A, Somborn-Schulz A: Innovations in nanotechnology for water treatment. Nanotechnol Sci Appl 8: 1–17, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Musah S, Mammoto A, Ferrante TC, Jeanty SSF, Hirano-Kobayashi M, Mammoto T, Roberts K, Chung S, Novak R, Ingram M, Fatanat-Didar T, Koshy S, Weaver JC, Church GM, Ingber DE: Mature induced-pluripotent-stem-cell-derived human podocytes reconstitute kidney glomerular-capillary-wall function on a chip. Nat Biomed Eng 1: 0069, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Homan KA, Kolesky DB, Skylar-Scott MA, Herrmann J, Obuobi H, Moisan A, Lewis JA: Bioprinting of 3D convoluted renal proximal tubules on perfusable chips. Sci Rep 6: 34845, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Freedman BS, Brooks CR, Lam AQ, Fu H, Morizane R, Agrawal V, Saad AF, Li MK, Hughes MR, Werff RV, Peters DT, Lu J, Baccei A, Siedlecki AM, Valerius MT, Musunuru K, McNagny KM, Steinman TI, Zhou J, Lerou PH, Bonventre JV: Modelling kidney disease with CRISPR-mutant kidney organoids derived from human pluripotent epiblast spheroids. Nat Commun 6: 8715, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morizane R, Lam AQ, Freedman BS, Kishi S, Valerius MT, Bonventre JV: Nephron organoids derived from human pluripotent stem cells model kidney development and injury. Nat Biotechnol 33: 1193–1200, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Takasato M, Er PX, Chiu HS, Maier B, Baillie GJ, Ferguson C, Parton RG, Wolvetang EJ, Roost MS, Chuva de Sousa Lopes SM, Little MH: Kidney organoids from human iPS cells contain multiple lineages and model human nephrogenesis. Nature 526: 564–568, 2015 [DOI] [PubMed] [Google Scholar]
- 24.US Department of Health and Human Services; Food and Drug Administration, Center for Biologics Evaluation and Research (CBER): Author Guidance for Industry: Source Animal, Product, Preclinical, and Clinical Issues Concerning the Use of Xenotransplantation Products in Humans, 2016. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/source-animal-product-preclinical-and-clinical-issues-concerning-use-xenotransplantation-products. Accessed September 9, 2019
- 25.Gitler C, Zarmi B, Kalef E, Meller R, Zor U, Goldman R: Calcium-dependent oxidation of thioredoxin during cellular growth initiation. Biochem Biophys Res Commun 290: 624–628, 2002 [DOI] [PubMed] [Google Scholar]
- 26.Estrada JL, Martens G, Li P, Adams A, Newell KA, Ford ML, Butler JR, Sidner R, Tector M, Tector J: Evaluation of human and non-human primate antibody binding to pig cells lacking GGTA1/CMAH/β4GalNT2 genes. Xenotransplantation 22: 194–202, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hryhorowicz M, Zeyland J, Słomski R, Lipiński D: Genetically modified pigs as organ donors for xenotransplantation. Mol Biotechnol 59: 435–444, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang L, Güell M, Niu D, George H, Lesha E, Grishin D, Aach J, Shrock E, Xu W, Poci J, Cortazio R, Wilkinson RA, Fishman JA, Church G: Genome-wide inactivation of porcine endogenous retroviruses (PERVs). Science 350: 1101–1104, 2015 [DOI] [PubMed] [Google Scholar]
- 29.Mascetti VL, Pedersen RA: Contributions of mammalian chimeras to pluripotent stem cell research. Cell Stem Cell 19: 163–175, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Längin M, Mayr T, Reichart B, Michel S, Buchholz S, Guethoff S, Dashkevich A, Baehr A, Egerer S, Bauer A, Mihalj M, Panelli A, Issl L, Ying J, Fresch AK, Buttgereit I, Mokelke M, Radan J, Werner F, Lutzmann I, Steen S, Sjöberg T, Paskevicius A, Qiuming L, Sfriso R, Rieben R, Dahlhoff M, Kessler B, Kemter E, Kurome M, Zakhartchenko V, Klett K, Hinkel R, Kupatt C, Falkenau A, Reu S, Ellgass R, Herzog R, Binder U, Wich G, Skerra A, Ayares D, Kind A, Schönmann U, Kaup FJ, Hagl C, Wolf E, Klymiuk N, Brenner P, Abicht JM: Consistent success in life-supporting porcine cardiac xenotransplantation. Nature 564: 430–433, 2018 [DOI] [PubMed] [Google Scholar]
- 31.Goto T, Hara H, Sanbo M, Masaki H, Sato H, Yamaguchi T, Hochi S, Kobayashi T, Nakauchi H, Hirabayashi M: Generation of pluripotent stem cell-derived mouse kidneys in Sall1-targeted anephric rats. Nat Commun 10: 451, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.US Food and Drug Administration/Center for Devices and Radiological Health- Innovation Challenge: End-Stage Renal Disease, 2012. Available at: https://wayback.archive-it.org/7993/20170111191653/http://www.fda.gov/AboutFDA/CentersOffices/OfficeofMedicalProductsandTobacco/CDRH/CDRHInnovation/InnovationPathway/ucm286140.htm. Accessed September 9, 2019
- 33.Wu I, Hurst FP: Making Innovation Reality in Renal Replacement Therapy, 2017. Available at: https://www.kidneynews.org/careers/advancing-research-and-innovation/making-innovation-reality-in-renal-replacement-therapy. Accessed September 9, 2019
- 34.US Food and Drug Administration: Breakthrough Devices Program. Available at: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/breakthrough-devices-program. Accessed September 9, 2019
- 35.US Food and Drug Administration: Guidance for Industry: Expedited Programs for Serious Conditions – Drugs and Biologics, 2014. Available at: https://www.fda.gov/files/drugs/published/Expedited-Programs-for-Serious-Conditions-Drugs-and-Biologics.pdf. Accessed September 5, 2019
- 36.US Food and Drug Administration: Guidance for Industry: Expedited Programs for Regenerative Medicine Therapies for Serious Conditions, 2017. Available at: https://www.fda.gov/media/120267/download. Accessed September 5, 2019
- 37.US Food and Drug Administration: Guidance for Industry: Considerations for the Design of Early-Phase Clinical Trials of Cellular and Gene Therapy Products, 2015. Available at: https://www.fda.gov/media/106369/download. Accessed September 5, 2019
- 38.US Food and Drug Administration: Guidance for Industry: Source Animal, Product, Preclinical, and Clinical Issues Concerning the Use of Xenotransplantation Products in Humans, 2016. Available at: https://www.fda.gov/media/102126/download. Accessed September 5, 2019
- 39.US Food and Drug Administration: Guidance for Industry: Preclinical Assessment of Investigational Cellular and Gene Therapy Products, 2013. Available at: https://www.fda.gov/media/87564/download. Accessed September 5, 2019
- 40.Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ, Cronin KA (eds): SEER Cancer Statistics Review, 1975-2014, National Cancer Institute. Bethesda, MD, Available at: https://seer.cancer.gov/csr/1975_2014/. Accessed September 8, 2019 [Google Scholar]
- 41.United States Renal Data System: 2016 USRDS annual data report: Epidemiology of kidney disease in the United States. National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, Bethesda, MD, 2016
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.