Abstract
Study Objectives:
Limited evidence exists on the cost-effectiveness of mandibular advancement device (MAD) compared to continuous positive airway pressure (CPAP) therapy in moderate obstructive sleep apnea (OSA). Therefore, this study compares the clinical and cost-effectiveness of MAD therapy with CPAP therapy in moderate OSA.
Methods:
In a multicentre randomized controlled trial, patients with an apnea-hypopnea index (AHI) of 15 to 30 events/h were randomized to either MAD or CPAP. Incremental cost-effectiveness and cost-utility ratios (ICER/ICUR, in terms of AHI reduction and quality-adjusted life-years [QALYs, based on the EuroQol Five-Dimension Quality of Life questionnaire]) were calculated after 12 months, all from a societal perspective.
Results:
In the 85 randomized patients (n = 42 CPAP, n = 43 MAD), AHI reduction was significantly greater with CPAP (median reduction AHI 18.3 [14.8–22.6] events/h) than with MAD therapy (median reduction AHI 13.5 [8.5–18.4] events/h) after 12 months. Societal costs after 12 months were higher for MAD than for CPAP (mean difference €2.156). MAD was less cost-effective than CPAP after 12 months (ICER −€305 [−€3.003 to €1.572] per AHI point improvement). However, in terms of QALY, MAD performed better than CPAP after 12 months (€33.701 [−€191.106 to €562.271] per QALY gained).
Conclusions:
CPAP was more clinically effective (in terms of AHI reduction) and cost-effective than MAD. However, costs per QALY was better with MAD as compared to CPAP. Therefore, CPAP is the first-choice treatment option in moderate OSA and MAD may be a good alternative.
Clinical Trial Registration:
Registry: ClinicalTrials.gov; Identifier: NCT01588275.
Citation:
de Vries GE, Hoekema A, Vermeulen KM, Claessen J, JacobsW, van derMaten J, van der Hoeven JH, Stegenga B, Kerstjens HAM,Wijkstra PJ. Clinicaland cost-effectiveness of a mandibular advancement device versus continuous positive airway pressure in moderate obstructive sleep apnea. J Clin Sleep Med. 2019;15(10):1477-1485.
Keywords: costs and cost analysis, randomized controlled trial, sleep apnea
BRIEF SUMMARY
Current Knowledge/Study Rationale: This randomized controlled trial compares the clinical- and cost-effectiveness of a mandibular advancement device (MAD) versus continuous positive airway pressure (CPAP) in moderate obstructive sleep apnea (OSA) as there is little evidence to aid in choosing between both therapies in this specific subgroup of OSA severity.
Study Impact: CPAP therapy is the first-choice treatment option in moderate OSA and MAD therapy may be a good alternative, particularly when patients refuse CPAP or prefer MAD therapy because of the less invasive nature of the device. Future research should focus on long-term quality of life and cardiovascular outcomes in order to provide justified treatment advice, also taking into account the initial preference of the patient to offer personalised medical care in patients with moderate OSA.
INTRODUCTION
Obstructive sleep apnea (OSA) is a common sleep-related breathing disorder characterized by recurrent upper airway obstructions during sleep, resulting in limited airflow and intermittent hypoxia.1 The resulting poor quality of sleep can lead to excessive daytime sleepiness (EDS), impaired quality of life, sick leave, and work disability.2,3 Ultimately, cardiovascular consequences may include an increased risk of the development of systemic hypertension4–6 and cardiovascular diseases, such as myocardial infarction, cardiac arrhythmias, and stroke.7–15
Because OSA largely affects individual health and societal costs, it is important that patients receive appropriate treatment in order to reduce symptoms, comorbidities, and economic burden. Continuous positive airway pressure (CPAP) is the gold standard in treating moderate to severe OSA.16 CPAP substantially reduces the number of apneas and hypopneas, and the occurrence of EDS.16 Furthermore, it improves health-related quality of life, and may reduce the risk of cardiovascular diseases and implications.9,16 Some patients, however, do report discomfort with CPAP, which might result in low adherence rates.
Oral appliance therapy has emerged as an attractive alternative to CPAP, especially for the treatment of mild and moderate OSA.17 Benefits of MAD therapy include substantial improvements in quality of life, daytime sleepiness, and sleep quality of both patient and bedpartner. Side effects in the early phase are usually related to the forced ventral position of the mandible and are mild and of transient nature. Long-term side effects might involve small changes in dental occlusion.18
Although MAD is generally considered less effective than CPAP,19–23 in a previous study it was shown not to be inferior in nonsevere OSA and some patients reported greater satisfaction with MAD.17 Therefore, in moderate OSA (apnea-hypopnea index [AHI] 15 to 30 events/h) both MAD and CPAP therapy can be considered as primary interventions.24,25 To date, cost-effectiveness studies assessed different types of oral appliances, also including less efficacious tongue retaining and rigid advancement devices, and included not solely patients with moderate OSA.19,26,27 Therefore, limited evidence exists on the cost-effectiveness of MAD when directly compared to CPAP in moderate OSA. Overall, the rationale to advise decision-makers in prescribing MAD or CPAP therapy in moderate OSA is limited and unconvincing given the lack of randomized controlled trial (RCT) data when considering costs in combination with health-related quality of life. Therefore, the objective of this study was to evaluate the cost-effectiveness of one commonly used adjustable type of MAD compared to CPAP therapy alongside a clinical RCT. Interventions were evaluated from a societal perspective in terms of the incremental cost per additional point of AHI reduction and per utility in patients with moderate OSA.
METHODS
Study Procedures and Patients
Baseline polysomnographic outcomes were those obtained at the time of diagnosis. When the diagnostic sleep study was a polygraphy, polysomnography (PSG) was performed before inclusion. All consecutive patients aged 18 years or older with an AHI of 15 to 30 events/h based on PSG (type I in-laboratory in one center, and type II home-based in two centers), and fulfilling the inclusion and exclusion criteria were scheduled for a baseline visit, which included questionnaire evaluation (Epworth Sleepiness Scale [ESS],28 36-Item Short Form health survey [SF-36],29 the Functional Outcomes of Sleep Questionnaire [FOSQ],30 and the Hospital Anxiety and Depression Scale [HADS]31).
Subsequently, patients were randomized to either MAD or CPAP therapy using a computer program, thereby concealing the allocation sequence from the investigators. Patients could not be blinded to the intervention they received.
Patients returned 3, 6, and 12 months after the start of therapy for follow-up measurements. A PSG was performed after 3 months. In case of unsuccessful treatment (ie, < 50% AHI reduction), adjustments to the therapy were made and a second PSG was scheduled (approximately 6 months after the start of therapy). After 12 months, a final PSG was performed. For each patient individually the same type of PSG (in-laboratory/home-based) was performed during follow-up as on baseline.
Patients switching to the other therapy (randomized therapy not being effective or patient unable to comply with randomized therapy) remained part of the study and were analyzed according to their initial therapy (intention-to-treat analysis).
The study was approved by the ethical committee of the University Medical Center Groningen (number NL34138.042.10; NCT01588275, ClinicalTrials.gov). All patients provided informed consent.
Interventions
Mandibular Advancement Device
Patients randomized to the MAD group were treated with a custom-made titratable bibloc MAD (SomnoDent MAS, SomnoMed Australia/Europe AG). To start, the mandible was set at approximately 60% to 70% of the patient’s maximum advancement.
Continuous Positive Airway Pressure
Patients randomized to the CPAP group were treated with auto-adjusting CPAP (Philips Respironics REMstar Auto A-Flex, provided by VitalAire BV The Netherlands) for 3 weeks, after which the appropriate fixed CPAP pressure for each individual patient was set by a skilled, specialized nurse (ie, highest pressure derived from the Hoffstein formula32 or the 90% criterion (mean pressure ≤ 90% of the time) of the auto-adjusting CPAP). During the study, patients were allowed to change their mask and to use chinstraps or a humidifier if desired.
Outcomes
Cost-Effectiveness and Cost-Utility Analysis
The incremental cost-effectiveness and cost-utility ratios (ICER/ICUR) were calculated after 12 months. ICER was based on the incremental costs and the effects on AHI reduction of MAD versus CPAP (MAD considered the alternative and CPAP the control/reference intervention). ICUR was based on the incremental costs and the effects on utility scores EuroQol Five-Dimension Quality of Life questionnaire, three levels (EQ-5D-3L). The answers on the five domains of the EQ-5D-3L, can be converted into a single index value (also called utility value) between 0 and 1 (with 1 being the optimal health status). Different algorithms to calculate the utility values have been obtained using representative samples of the general population, thereby representing the societal perspective. For this study the Dolan algorithm was used as it is frequently used in international literature and studies, thereby facilitating international comparisons.33 Quality-adjusted life-year (QALY) was calculated using the utility values multiplied with the survival time (in this analysis 1 year). Bootstrap resampling (5,000 replications) was performed on the cost and effect pairs to calculate confidence intervals and to depict cost-effectiveness planes. Furthermore, cost-effectiveness acceptability curves were plotted to illustrate the probability of interventions studied being more cost-effective than the other therapy over a range of thresholds. In the Netherlands, no formal threshold for cost-effectiveness exists.
Costs
Assuming that both MAD and CPAP have a lifespan of 5 years, device costs were uniformly depreciated over a 5-year period. Costs were studied from a societal perspective, which means that all costs are included regardless of who pays them. Therefore, the following cost components were taken into account in the economic evaluation: direct medical costs, such as costs of treatment (including PSG), outpatient hospital visits, visits to general practitioner and other health care providers, and hospital stay. Direct costs outside the health care sector (direct nonmedical costs) included travel expenses and parking costs. Indirect costs included income missed from being absent from paid work. In case patients switched to the other therapy, costs were calculated for both therapies together. The time horizon of this study encompassed 1 year, using 2015 as the reference year; therefore, no discounting was applied on costs and effects. Cost components were scored according to the Dutch standard guidelines for economic evaluations.34
Additional detail on the inclusion and exclusion criteria, study procedures, questionnaires, interventions, and cost components taken into account in the economic evaluation, is provided in the supplemental material.
Statistical Analysis
Descriptive statistics for continuous variables are presented as means and standard deviations (normal distribution) or medians and interquartile ranges (skewed distribution). Categorical variables are presented in terms of proportions.
Differences between baseline and follow-up variables within the groups were compared using the paired t test or Wilcoxon signed-rank test for variables with skewed distributions. Differences between treatment groups were compared using the independent t test or Mann–Whitney U test for variables with skewed distributions.
“Intention-to-treat” and “per protocol” analyses were performed on the primary endpoints. The power analysis was based on a test on the difference between two independent means. Based on an estimated AHI reduction of 12.4 ± 8.5 points with MAD treatment and 17.4 ± 6.1 points with CPAP treatment (based on literature17,35–37 and own data of regular care), an alpha of 0.05 and power of 0.8, 36 patients were required per treatment group. It was expected that 10% to 15% of patients from each group would drop out.17 Therefore, the estimated number for this RCT was 43 patients per group, resulting in a total of 86 patients.
Differences were considered to be statistically significant when P < .05. Statistical analyses were performed using IBM SPSS Statistics 23 (IBM, Armonk, New York, USA).
RESULTS
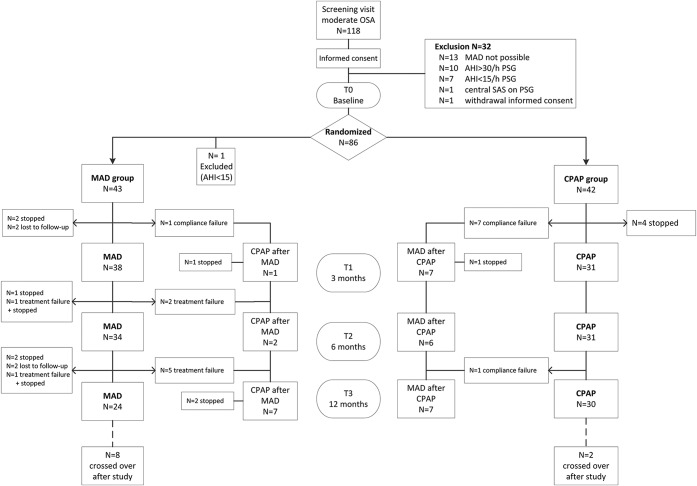
Between June 2012 and September 2016, 118 patients were screened. Thirty-two patients were excluded after the screening visit (Figure 1). Eighty-six patients were randomized (44 MAD, 42 CPAP), of which one patient (randomized to MAD) was excluded after unjustified randomization due to having mild OSA at baseline (AHI 8.6 events/h based on PSG).
Figure 1. Flowchart showing allocation, switching and dropout of patients.
AHI = apnea-hypopnea index, CPAP = continuous positive airway pressure, MAD = mandibular advancement device, OSA = obstructive sleep apnea, PSG = polysomnography, SAS = sleep apnea syndrome.
Of the 85 patients (50.7 ± 9.7 years, BMI 30.2 ± 4.9 kg/m2, men/women 70/15) with AHI 15 to 30 events/h (mean AHI 20.9 ± 4.5 events/h), 18 switched to the other therapy (10 from MAD to CPAP, 8 from CPAP to MAD) and 19 patients dropped out during the study (14 MAD, 5 CPAP), of which 6 dropped out after switching to the other therapy (5 MAD, 1 CPAP). In total, 54 patients (24 MAD; 30 CPAP) completed the study period receiving the therapy to which they were initially randomized (per protocol group).
There were no significant baseline differences in age, AHI, BMI and ESS scores between dropouts and “per protocol” patients. Furthermore, no significant baseline differences in age, AHI at baseline (20.9 ± 4.4 for MAD and 21.0 ± 4.7 for CPAP), BMI, and ESS scores were observed between patients randomized to MAD versus CPAP therapy. Of note, minimal oxygen saturation (SpO2) at baseline was significantly lower in patients randomized to CPAP therapy.
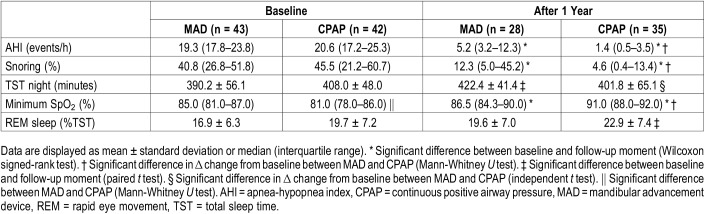
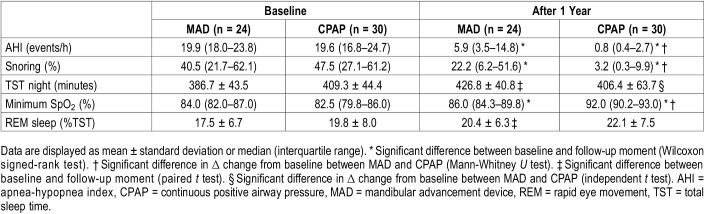
During the follow-up period, in total 71 PSG tests were performed in the group randomized to MAD and 72 PSG tests in the group randomized to CPAP therapy. Both devices significantly reduced AHI after 12 months (MAD from median [interquartile range, IQR] 19.3 [17.8–23.8] to 5.2 [3.2–12.3] and CPAP from 20.6 [17.2–25.3] to 1.4 [0.5–3.5]) based on the intention-to-treat analysis. The reduction in AHI was significantly greater (P < .01) with CPAP (median reduction AHI 18.3 [14.8–22.6] events/h after 12 months) as compared to MAD therapy (median reduction AHI 13.5 [8.5–18.4] events/h [Table 1]). Results from the per-protocol analysis were not substantially different for AHI (Table 2).
Table 1.
Polysomnographic outcomes (intention-to-treat analysis n = 85).
Table 2.
Polysomnographic outcomes (per protocol analysis n = 54).
After 12 months, in total 14 patients (50%) randomized to MAD therapy (of the 28 with PSG after 12 months) could be classified as having no OSA (AHI < 5 events/h), 8 patients (29%) as having mild OSA (AHI 5 to 15), and 6 patients (21%) had an AHI > 15 events/h. In total, 30 patients (86%) randomized to CPAP therapy (of the 35 with PSG after 12 months) had no OSA, 4 patients (11%) had mild OSA, and 1 patient (3%) had an AHI > 15 events/h.
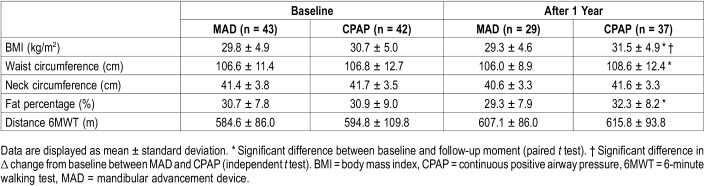
Both devices significantly reduced the percentage of snoring (MAD from median [IQR] 40.8 [26.8–51.8] to 12.3 [5.0–45.2], (P < .01) and CPAP from 45.5 [21.2–60.7] to 4.6 [0.4–13.4], [P < .001]). The reduction in percentage of snoring was significantly greater with CPAP than with MAD therapy (P < .01). Minimal oxygen saturation (SpO2) increased with both MAD (median [IQR] 85.0 [81.0–87.0] to 86.5 [84.3–90.0]) and CPAP therapy (median [IQR] 81.0 [78.0–86.0] to 91.0 [88.0–92.0]). The difference between MAD and CPAP therapy in minimal oxygen saturation change from baseline was significant (P < .001) after 12 months, possibly due to the fact that at baseline minimal oxygen saturation was significantly lower in patients randomized to CPAP therapy. BMI, waist circumference, and fat percentage increased during 12 months in the CPAP group; no changes were observed with MAD therapy (Table 3). Daytime sleepiness, measured with the ESS, was significantly reduced with both MAD and CPAP therapy (MAD 10.3 ± 5.3 to 7.1 ± 5.2, P < .001) and CPAP (9.8 ± 4.1 to 5.3 ± 3.9, P < .001). Also, sleep-related functioning and quality of life measures (FOSQ and SF-36) improved with both MAD and CPAP therapy (Table 4).
Table 3.
Physical measures (intention-to-treat analysis n = 85).
Table 4.
Neurobehavioral outcomes (intention-to-treat analysis n = 85).
After 12 months, self-reported adherence with treatment did not differ significantly between patients randomized to MAD and patients randomized to CPAP. Daily objective adherence of both MAD and CPAP was monitored in two of the three participating centers (n = 59). Of those, 40 patients (68%; MAD n = 17, CPAP n = 23) completed the study with the therapy to which they were randomly assigned. The median [IQR] objective adherence (h/night) in the third month was 7.4 [5.2–8.2] for MAD and 6.8 [5.7–7.6] for CPAP (P = .41). In the 12th month, MAD was used for 6.9 [3.5–7.9] h/night and CPAP was used for 6.8 [5.2–7.6] h/night (P = .85). When applying a worst-case scenario (ie, adherence after dropout and crossover was scored as zero), the intention-to-treat analysis showed no significant differences between MAD and CPAP in median h/night.
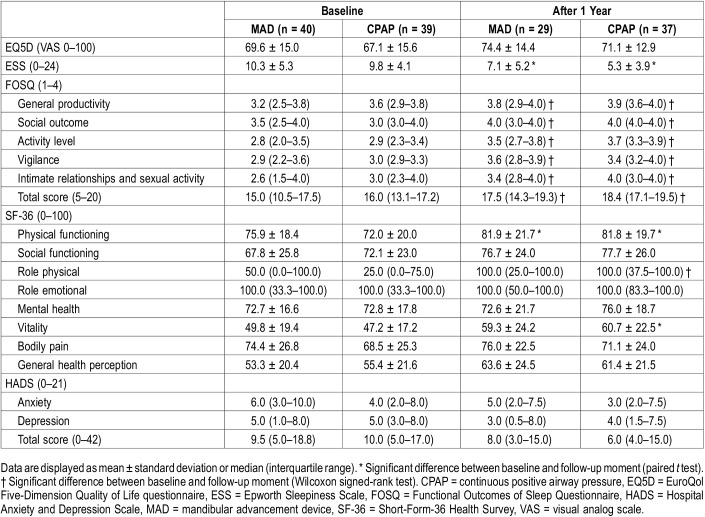
After 12 months, societal costs, including direct medical and nonmedical costs and indirect costs, were higher for MAD than for CPAP therapy (mean difference €2,156). Thus, in addition to MAD therapy being less effective than CPAP therapy after 12 months, it was less cost-effective as well (ICER of −€305 [−€3,003 to €1,572] per AHI point improvement) (Figure 2). Additional data on the direct medical, direct nonmedical, and indirect costs, based on the trial data before bootstrapping, are provided in the supplemental material (Table S2).
Figure 2. Incremental cost-effectiveness ratio after 12 months.
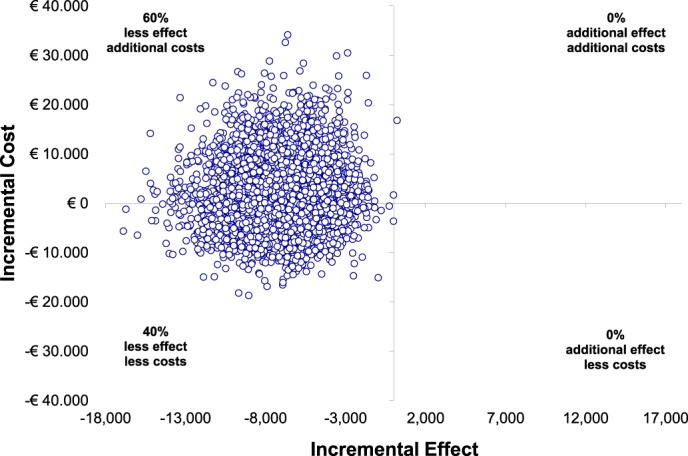
Incremental cost-effectiveness plane. Scatterplot displaying the cost and effect (ie, AHI reduction) pairs for MAD versus CPAP therapy resulting from bootstrapping, with MAD considered the alternative and CPAP therapy the control intervention. AHI = apnea-hypopnea index, CPAP = continuous positive airway pressure, MAD = mandibular advancement device.
In terms of QALY, MAD performed better than CPAP (ICUR €33,701 [-€191,106 to €562,271]) (Figure 3A). The cost and effect pairs are now predominantly located in the Northeast and Southeast-quadrants of Figure 3A, showing additional effects in terms of QALY. The acceptability curve (Figure 3B) shows the probability that MAD therapy is cost effective compared to CPAP therapy over a range of thresholds, up to €50,000 per QALY gained. At a value of around €30,000 the probability exceeds 50%; however, it will not even exceed 65% at the €50,000 threshold.
Figure 3. Incremental cost-utility ratio after 12 months.
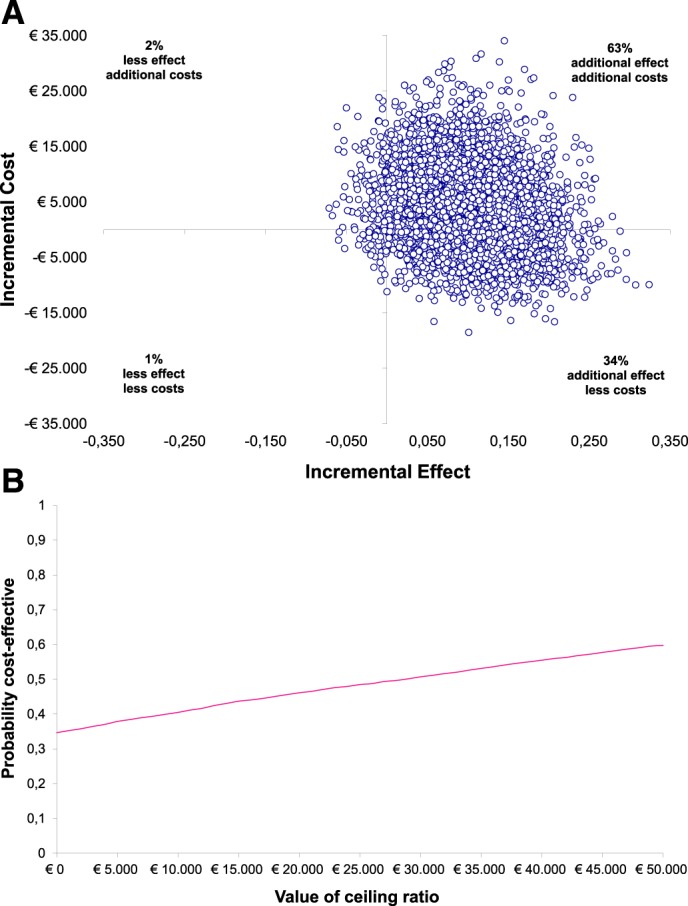
(A) Incremental cost-utility plane. Scatterplot displaying the cost and effect (ie, QALY, based on EQ-5D-3L, index values 0–1) pairs for MAD versus CPAP therapy resulting from bootstrapping, with MAD considered the alternative and CPAP therapy the control intervention. (B) Cost-utility acceptability curve. The x-axis displays the “cost effectiveness threshold” and the y-axis the “probability of MAD being cost-effective compared to CPAP therapy.” Results are expressed as a function of societal willingness to pay for additional units of health (QALY gained). CPAP = continuous positive airway pressure, EQ-5D-3L = EuroQol Five-Dimension Quality of Life Questionnaire, three levels, MAD = mandibular advancement device, QALY = quality-adjusted life-years.
DISCUSSION
The strength of this study is the direct comparison of MAD versus CPAP therapy in a randomized trial, specifically following up with patients with moderate OSA over a period of 12 months. In this RCT one commonly and globally used MAD, proven to be effective, was used. A broad range of clinical measures as well as direct medical, direct nonmedical, and indirect costs (societal perspective) were assessed.
The main results from our RCT demonstrate that although both MAD and CPAP therapy significantly reduced AHI after 12 months, CPAP therapy was clinically more effective. These findings are in accordance with the results of other studies comparing the effectiveness of MAD and CPAP therapy.35–37
In line with our data, previously performed economic studies in patient populations with OSA19,26,27 suggested that both CPAP and MAD therapy are cost-effective compared to no treatment, with CPAP therapy being the most cost-effective.
The main difference between aforementioned studies and our current study is that we exclusively assessed patients with moderate OSA and that we chose AHI instead of ESS as the primary clinical outcome measure. We believe that ESS is less appropriate because of the limited correlation with the severity and consequences of OSA. Furthermore, Sadatsafavi et al26 and McDaid et al27 assessed the cost-effectiveness of different types of oral appliances simultaneously and all studies assessed a broader range than only patients with moderate OSA.19,26,27 From a societal perspective, MAD therapy was less cost-effective, driven by the difference in AHI reduction. Conversely, in terms of improvement in QALY, MAD therapy was the better treatment option. This difference in conclusion can be attributed to the choice of outcome measure. To date, QALY has become an important component in cost-effectiveness studies as it allows comparison across different interventions and settings by using a common unit of measure (costs per QALYs gained). A downside of using QALY is that it is difficult to generate a value for health status as it is perceived by different individuals and societies. Furthermore, thresholds do vary largely between countries, and most are informal. Therefore, it is difficult to directly compare thresholds. In the United Kingdom the National Institute for Health and Care Excellence (NICE) uses a threshold range of £20,000 to £30,000 per QALY gained for a cost-effective intervention.38 In North America, a similar amount of $50,000 per QALY gained is often used.
However, the fact that MAD therapy outperformed CPAP therapy when considering costs per QALY gained implies that patients receiving MAD therapy experience a better health status, which could have important health (care) consequences in the long run. We believe that patients with moderate OSA should be advised to start CPAP. When CPAP fails, MAD therapy is currently the next best option. In addition, for patients who refuse CPAP therapy, a MAD can be a primary option as it reduces AHI and excessive daytime sleepiness, and improves health-related quality of life. The discontinuation and dropout rates in our study were higher than expected. Eighteen patients (21%) switched to the other therapy (10 from MAD to CPAP, 8 from CPAP to MAD). In total, five patients randomized to MAD needed extra PSG measurements versus two patients randomized to CPAP. There was a major difference in the rationale for crossing over between therapies; all patients switching from CPAP to MAD therapy could not comply with CPAP therapy (in seven patients, CPAP therapy failed within 3 months; patient-driven crossover), whereas patients switching from MAD to CPAP therapy in general experienced treatment failures, meaning MAD therapy was not adequately effective in reducing AHI (study/physician driven crossover). A total of 19 patients (22%) dropped out during the study: dropout rates were higher in the MAD (n = 14) than in the CPAP group (n = 5). Although more patients than anticipated dropped out, resulting in a lower number of patients than estimated necessary based on the a priori power analysis (36 per group), the differential effect was more pronounced and the main results were statistically significant (actual power of 0.89).
The current study has several limitations. First, cardiovascular measures were not included in our cost-effectiveness analysis. All aforementioned previous cost-effectiveness studies19,26,27 used economic models having the additional value of merging cardiovascular data associated with OSA. However, a paucity of data on (long-term) cardiovascular effects of CPAP and especially MAD therapy still exists39 and the long-term clinical implications of OSA remain unclear. Therefore, cardiovascular effects were not included in our cost-effectiveness analyses.
Second, our study had a follow-up period of 12 months. Even though surpassing follow-up periods of most RCTs, an even longer follow-up would have been more desirable in assessing cost-effectiveness. For example, most costs for MAD therapy are made in the first months, as the device is custom-made. Maintenance costs for MAD are low and relatively high for CPAP therapy after the first year, which could influence cost-effectiveness when considering long-term therapy.
Third, results from this study only apply to patients with moderate OSA willing to be randomized to either MAD or CPAP therapy. This may be conducive to selection bias, thereby reducing the generalizability of this study to regular care settings (all comers) where patients have free treatment choice and can express their a priori preference. In fact, several patients were not willing to participate because they had a clear preference for either MAD or CPAP therapy and therefore received the preferred therapy outside the study setting. Unfortunately, patient therapy preference (excluding them from participation) was not systematically assessed. Nevertheless, retrospectively, the percentage of patients with a priori preference was similar for both therapies (50% and 50%). Treatment preference has been assessed in some short-term crossover studies,35,36,40,41 mostly indicating that most patients preferred MAD over CPAP therapy.35,36,41 Long-term studies on treatment preference are currently lacking.
One type of MAD device was used in the current study, thereby limiting the variation in the costs and potentially limiting the generalizability to other devices. However, because prices of other devices are not substantially different, large effects on the current results are not to be expected. However, in other countries prices of different devices might differ. Furthermore, it is important to state that the costs of health care consumption used in this study apply to the Dutch health system, and that those costs need to be put into perspective in countries with different health systems.
The larger positive effects of CPAP therapy on AHI and oxygen saturation suggest better long-term outcomes for CPAP therapy. In accordance, Doff and colleagues18 described significantly better improvements on AHI and the lowest oxyhemoglobin saturation with CPAP compared to MAD therapy in patients with mild, moderate, and severe OSA (AHI ≥ 5 events/h).
Currently, there is debate on which parameter to use for effect measurement of OSA treatment. The oxygen desaturation index could potentially provide more predictive information on cardiovascular effects in patients with OSA, as oxygen desaturation index scores the number of events of reduction in blood oxygen levels irrespective of whether reduction in airflow is taking place.
In summary, results of this RCT suggest that CPAP therapy is the first-choice treatment option in moderate OSA and that MAD therapy may be a good alternative, particularly when patients refuse CPAP therapy or prefer MAD therapy because of the less-invasive nature of the device. Future research should focus on long-term quality of life and cardiovascular outcomes in order to provide justified treatment advice, also taking into account the initial preference of the patient and to offer personalised medical care in patients with moderate OSA.
DISCLOSURE STATEMENT
All authors have seen and approved the manuscript. This study was funded by SomnoMed Goedegebuure and VitalAire Nederland BV. GEV, AH, and PJW report grants from SomnoMed Goedegebuure and from VitalAire Nederland BV, during the conduct of the study. AH reports personal fees for being Medical advisor from Somnomed, Airway Management, and Zephyr Sleep Technologies, outside the submitted work. PJW reports grants and personal fees from Philips, grants and personal fees from RESMED, grants from VIVISOL, personal fees from Synapse, and from Bresotec, outside the submitted work. The other authors report no conflicts of interest.
ACKNOWLEDGMENTS
Author contributions: Figures: GEDV, KMV, PJW; study design: GEDV, AH, BS, HAMK, PJW; data collection: GEDV, JQPJC, WJ, JVDM, JHVDH, PJW; data analysis: GEDV, AH, KMV, BS, HAMK, PJW; data interpretation: GEDV, AH, KMV, HAMK, PJW; writing: GEDV, AH, KMV, JQPJC, WJ, JVDM, JHVDH, BS, HAMK, PJW.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- BMI
body mass index
- CPAP
continuous positive airway pressure
- EDS
excessive daytime sleepiness
- EQ-5D-3L
EuroQol Five-Dimension Quality of Life Questionnaire, three levels
- ESS
Epworth Sleepiness Scale
- FOSQ
Functional Outcomes of Sleep Questionnaire
- HADS
Hospital Anxiety and Depression Scale
- ICER
incremental cost-effectiveness ratio
- ICUR
incremental cost-utility ratio
- MAD
mandibular advancement device
- OSA
obstructive sleep apnea
- PSG
polysomnography
- QALY
quality-adjusted life-years
- SF-36
Short Form-36 Health Survey
- VAS
visual analog scale
REFERENCES
- 1.Peppard PE, Young T, Barnet JH, Palta M, Hagen EW, Hla KM. Increased prevalence of sleep-disordered breathing in adults. Am J Epidemiol. 2013;177(9):1006–1014. doi: 10.1093/aje/kws342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sivertsen B, Overland S, Glozier N, Bjorvatn B, Maeland JG, Mykletun A. The effect of OSAS on sick leave and work disability. Eur Respir J. 2008;32(6):1497–1503. doi: 10.1183/09031936.00044908. [DOI] [PubMed] [Google Scholar]
- 3.Sjösten N, Kivimaki M, Oksanen T, et al. Obstructive sleep apnoea syndrome as a predictor of work disability. Respir Med. 2009;103(7):1047–1055. doi: 10.1016/j.rmed.2009.01.014. [DOI] [PubMed] [Google Scholar]
- 4.Pedrosa RP, Drager LF, Gonzaga CC, et al. Obstructive sleep apnea: the most common secondary cause of hypertension associated with resistant hypertension. Hypertension. 2011;58(5):811–817. doi: 10.1161/HYPERTENSIONAHA.111.179788. [DOI] [PubMed] [Google Scholar]
- 5.Marin JM, Agusti A, Villar I, et al. Association between treated and untreated obstructive sleep apnea and risk of hypertension. JAMA. 2012;307(20):2169–2176. doi: 10.1001/jama.2012.3418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Floras JS. Hypertension and sleep apnea. Can J Cardiol. 2015;31(7):889–897. doi: 10.1016/j.cjca.2015.05.003. [DOI] [PubMed] [Google Scholar]
- 7.Peker Y, Hedner J, Norum J, Kraiczi H, Carlson J. Increased incidence of cardiovascular disease in middle-aged men with obstructive sleep apnea: a 7-year follow-up. Am J Respir Crit Care Med. 2002;166(2):159–165. doi: 10.1164/rccm.2105124. [DOI] [PubMed] [Google Scholar]
- 8.Bradley TD, Floras JS. Sleep apnea and heart failure: part I: obstructive sleep apnea. Circulation. 2003;107(12):1671–1678. doi: 10.1161/01.CIR.0000061757.12581.15. [DOI] [PubMed] [Google Scholar]
- 9.Marin JM, Carrizo SJ, Vicente E, Agusti AG. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: An observational study. Lancet. 2005;365(9464):1046–1053. doi: 10.1016/S0140-6736(05)71141-7. [DOI] [PubMed] [Google Scholar]
- 10.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353(19):2034–2041. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 11.Mehra R, Benjamin EJ, Shahar E, et al. Association of nocturnal arrhythmias with sleep-disordered breathing: The sleep heart health study. Am J Respir Crit Care Med. 2006;173(8):910–916. doi: 10.1164/rccm.200509-1442OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Somers VK, White DP, Amin R, et al. Sleep apnea and cardiovascular disease: an American Heart Association/American College of Cardiology Foundation Scientific Statement from the American Heart Association Council for High Blood Pressure Research Professional Education Committee, Council on Clinical Cardiology, Stroke Council, and Council on Cardiovascular Nursing. In collaboration with the National Heart, Lung, and Blood Institute National Center on Sleep Disorders Research (National Institutes of Health) Circulation. 2008;118(10):1080–1111. doi: 10.1161/CIRCULATIONAHA.107.189375. [DOI] [PubMed] [Google Scholar]
- 13.Bradley TD, Floras JS. Obstructive sleep apnoea and its cardiovascular consequences. Lancet. 2009;373(9657):82–93. doi: 10.1016/S0140-6736(08)61622-0. [DOI] [PubMed] [Google Scholar]
- 14.Kasai T, Floras JS, Bradley TD. Sleep apnea and cardiovascular disease: A bidirectional relationship. Circulation. 2012;126(12):1495–1510. doi: 10.1161/CIRCULATIONAHA.111.070813. [DOI] [PubMed] [Google Scholar]
- 15.Al-Falahi Z, Williamson J, Dimitri H. Atrial fibrillation and sleep apnoea: Guilt by association? Heart Lung Circ. 2017;26(9):902–910. doi: 10.1016/j.hlc.2017.05.127. [DOI] [PubMed] [Google Scholar]
- 16.Giles TL, Lasserson TJ, Smith BH, White J, Wright J, Cates CJ. Continuous positive airways pressure for obstructive sleep apnoea in adults. Cochrane Database Syst Rev. 2006;3:1469–1493. doi: 10.1002/14651858.CD001106.pub3. [DOI] [PubMed] [Google Scholar]
- 17.Hoekema A, Stegenga B, Wijkstra PJ, van der Hoeven JH, Meinesz AF, de Bont LG. Obstructive sleep apnea therapy. J Dent Res. 2008;87(9):882–887. doi: 10.1177/154405910808700917. [DOI] [PubMed] [Google Scholar]
- 18.Doff MH, Hoekema A, Wijkstra PJ, et al. Oral appliance versus continuous positive airway pressure in obstructive sleep apnea syndrome: A 2-year follow-up. Sleep. 2013;36(9):1289–1296. doi: 10.5665/sleep.2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sharples L, Glover M, Clutterbuck-James A, et al. Clinical effectiveness and cost-effectiveness results from the randomised controlled trial of oral mandibular advancement devices for obstructive sleep apnoea-hypopnoea (TOMADO) and long-term economic analysis of oral devices and continuous positive airway pressure. Health Technol Assess. 2014;18(67):1–296. doi: 10.3310/hta18670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sutherland K, Phillips CL, Cistulli PA. Efficacy versus effectiveness in the treatment of obstructive sleep apnea: CPAP and oral appliances. J Dent Sleep Med. 2015;2(4):175–181. [Google Scholar]
- 21.Sharples LD, Clutterbuck-James AL, Glover MJ, et al. Meta-analysis of randomised controlled trials of oral mandibular advancement devices and continuous positive airway pressure for obstructive sleep apnoea-hypopnoea. Sleep Med Rev. 2016;27:108–124. doi: 10.1016/j.smrv.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marklund M. Update on oral appliance therapy for OSA. Curr Sleep Med Rep. 2017;3(3):143–151. doi: 10.1007/s40675-017-0080-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schwartz M, Acosta L, Hung YL, Padilla M, Enciso R. Effects of CPAP and mandibular advancement device treatment in obstructive sleep apnea patients: A systematic review and meta-analysis. Sleep Breath. 2018;22(3):555–568. doi: 10.1007/s11325-017-1590-6. [DOI] [PubMed] [Google Scholar]
- 24.Zhu Y, Long H, Jian F, et al. The effectiveness of oral appliances for obstructive sleep apnea syndrome: A meta-analysis. J Dent. 2015;43(12):1394–1402. doi: 10.1016/j.jdent.2015.10.008. [DOI] [PubMed] [Google Scholar]
- 25.Serra-Torres S, Bellot-Arcis C, Montiel-Company JM, Marco-Algarra J, Almerich-Silla JM. Effectiveness of mandibular advancement appliances in treating obstructive sleep apnea syndrome: A systematic review. Laryngoscope. 2016;126(2):507–514. doi: 10.1002/lary.25505. [DOI] [PubMed] [Google Scholar]
- 26.Sadatsafavi M, Marra CA, Ayas NT, Stradling J, Fleetham J. Cost-effectiveness of oral appliances in the treatment of obstructive sleep apnoea-hypopnoea. Sleep Breath. 2009;13(3):241–252. doi: 10.1007/s11325-009-0248-4. [DOI] [PubMed] [Google Scholar]
- 27.McDaid C, Griffin S, Weatherly H, et al. Continuous positive airway pressure devices for the treatment of obstructive sleep apnoea-hypopnoea syndrome: A systematic review and economic analysis. Health Technol Assess. 2009;13(4) doi: 10.3310/hta13040. [DOI] [PubMed] [Google Scholar]
- 28.Johns MW. A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep. 1991;14(6):540–545. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 29.Ware JE, Jr, Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. conceptual framework and item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- 30.Weaver TE, Laizner AM, Evans LK, et al. An instrument to measure functional status outcomes for disorders of excessive sleepiness. Sleep. 1997;20(10):835–843. [PubMed] [Google Scholar]
- 31.Zigmond AS, Snaith RP. The hospital anxiety and depression scale. Acta Psychiatr Scand. 1983;67(6):361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 32.Miljeteig H, Hoffstein V. Determinants of continuous positive airway pressure level for treatment of obstructive sleep apnea. Am Rev Respir Dis. 1993;147(6 Pt 1):1526–1530. doi: 10.1164/ajrccm/147.6_Pt_1.1526. [DOI] [PubMed] [Google Scholar]
- 33.Dolan P. Modeling valuations for EuroQol health states. Med Care. 1997;35(11):1095–1108. doi: 10.1097/00005650-199711000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Zorginstituut Nederland . Kostenhandleiding: Methodologie van kostenonderzoek en referentieprijzen voor economische evaluaties in de gezondheidszorg. Diemen, Netherlands: Zorginstituut Nederland; 2015. [Google Scholar]
- 35.Clark GT, Blumenfeld I, Yoffe N, Peled E, Lavie P. A crossover study comparing the efficacy of continuous positive airway pressure with anterior mandibular positioning devices on patients with obstructive sleep apnea. Chest. 1996;109(6):1477–1483. doi: 10.1378/chest.109.6.1477. [DOI] [PubMed] [Google Scholar]
- 36.Ferguson KA, Ono T, Lowe AA, al Majed S, Love LL, Fleetham JA. A short-term controlled trial of an adjustable oral appliance for the treatment of mild to moderate obstructive sleep apnoea. Thorax. 1997;52(4):362–368. doi: 10.1136/thx.52.4.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aarab G, Lobbezoo F, Heymans MW, Hamburger HL, Naeije M. Long-term follow-up of a randomized controlled trial of oral appliance therapy in obstructive sleep apnea. Respiration. 2011;82(2):162–168. doi: 10.1159/000324580. [DOI] [PubMed] [Google Scholar]
- 38.National Institute for Health and Care Excellence How NICE measures value for money in relation to public health interventions. https://www.nice.org.uk/Media/Default/guidance/LGB10-Briefing-20150126.pdf. Accessed May 13, 2019.
- 39.de Vries GE, Wijkstra PJ, Houwerzijl EJ, Kerstjens HAM, Hoekema A. Cardiovascular effects of oral appliance therapy in obstructive sleep apnea: A systematic review and meta-analysis. Sleep Med Rev. 2018;40:55–68. doi: 10.1016/j.smrv.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 40.Engleman HM, McDonald JP, Graham D, et al. Randomized crossover trial of two treatments for sleep apnea/hypopnea syndrome: Continuous positive airway pressure and mandibular repositioning splint. Am J Respir Crit Care Med. 2002;166(6):855–859. doi: 10.1164/rccm.2109023. [DOI] [PubMed] [Google Scholar]
- 41.White DP, Shafazand S. Mandibular advancement device vs. CPAP in the treatment of obstructive sleep apnea: Are they equally effective in short term health outcomes? J Clin Sleep Med. 2013;9(9):971–972. doi: 10.5664/jcsm.3008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.