Abstract
The circadian and seasonal actions of melatonin are mediated by high affinity G-protein coupled receptors (melatonin receptors, MTRs), classified into phylogenetically distinct subtypes based on sequence divergence and pharmacological characteristics. Three vertebrate MTR subtypes are currently described: MT1 (MTNR1A), MT2 (MTNR1B), and Mel1c (MTNR1C / GPR50), which exhibit distinct affinities, tissue distributions and signaling properties. We present phylogenetic and comparative genomic analyses supporting a revised classification of the vertebrate MTR family. We demonstrate four ancestral vertebrate MTRs, including a novel molecule hereafter named Mel1d. We reconstructed the evolution of each vertebrate MTR, detailing genetic losses in addition to gains resulting from whole genome duplication events in teleost fishes. We show that Mel1d was lost separately in mammals and birds and has been previously mistaken for an MT1 paralogue. The genetic and functional diversity of vertebrate MTRs is more complex than appreciated, with implications for our understanding of melatonin actions in different taxa. The significance of our findings, including the existence of Mel1d, are discussed in an evolutionary and functional context accommodating a robust phylogenetic assignment of MTR gene family structure.
Keywords: Melatonin receptor, Mel1d, vertebrate evolution, phylogenetic analysis, comparative genomics, conserved synteny, teleost fish
Melatonin is an ancient eukaryotic signaling molecule that regulates diverse biological functions. While best known as a regulator of biological rhythms in humans, this hormone also regulates energy balance, temperature, behavior, blood pressure, and seasonal reproduction. Melatonin is secreted by the pineal gland and targets the brain as well as peripheral tissues (Hardeland et al. 2011, Slominski et al. 2012), but is also produced by several tissues, eliciting paracrine effects (Weaver and Reppert 1990). The actions of melatonin depend on the spatiotemporal expression of high-affinity melatonin receptors (MTR), representing a specific class of G protein-coupled receptor (GPCR).
Three paralogous MTR family members have been characterized in jawed vertebrates, namely MT1 (Mel1a / MTNR1A), MT2 (Mel1b / MTNR1B), and Mel1c (MTNR1C / GPR50 in mammals) (Reppert et al. 1994, 1995a, 1995b). Despite showing overlap in expression, these different MTRs have evolved unique functions. MT1 has a higher affinity for melatonin than MT2 (Dubocovich and Markowska 2005), and in mammals, Mel1c has lost the ability to bind melatonin (Dufourny et al. 2008), though it does modulate melatonin signaling via its association with MT1 (Levoye et al. 2006). While MT1 associates with a range of G proteins to activate several distinct signaling pathways, eliciting wide-ranging cellular effects (Witt-Enderby et al. 2003), MT2 associates with a single G protein (Jockers et al. 2008). Owing to such functional divergence, different MTRs may have very distinct biological effects, even when expressed in the same cell types (e.g., Dubocovich and Markowska 2005).
A past study demonstrated melatonin binding in the brain of jawed vertebrates and lamprey, but not in hagfishes or amphioxus (Vernadakis et al. 1998). Thus, it is likely that high-affinity MTRs were present in the vertebrate ancestor, and were secondarily lost in some jawless fishes, as noted for several other traits (e.g., reduction of vertebrae-like elements - Ota et al. 2011; Dlx genes - Sugahara et al. 2013; reviewed in Kuraku 2013). MTR-like GPCR genes have also been discovered in urochordates, cephalochordates, hemichordates and echinoderms (Kamesh et al. 2008, Nordström et al. 2008, Krishnan et al. 2013), but their evolutionary affinity to the vertebrate MTRs remains ambiguous. The distinct MTRs of jawed vertebrates potentially originated during two rounds (2R) of whole genome duplication (WGD) at the stem of vertebrate evolution (e.g., Dehal and Boore 2005), though this is yet to be established. Additional expansions in the MTR family of fishes (e.g., Shang & Zhdanova 2007; Hong et al. 2014) may owe to a further round of teleost-specific WGD (‘Ts3R’) in the common teleost ancestor, or additional lineage-specific WGD events in some lineages, e.g., the salmonid-specific 4R (‘Ss4R’) (Macqueen and Johnston 2014; Lien et al. 2016), though, again, this has not been properly explored.
The overarching goal of this study was to re-examine the evolutionary history of vertebrate MTRs, using data in publically-available sequence databases for robust phylogenetic and comparative genomic reconstructions. Our findings concretely demonstrate a fourth ancestral MTR (‘Mel1d’), along with teleost-specific expansions in MTR diversity, likely owing to Ts3R and Ss4R. With a new evolutionary framework in place we reinterpret findings on vertebrate MTR sequence divergence and expression from past studies. Overall, this study highlights substantial unexplored diversity in MTR signaling within vertebrates, pointing to new lines of investigation.
Materials and Methods
Sequence and phylogenetic analyses
Amino acid sequences encoded by MTR or FAT protocadherin family member genes were collected from representative jawed vertebrate species with high-quality genome assemblies. Details of these sequences are given in Table S1 (MTR) and Table S2 (FAT), which include database accession numbers and nomenclature matching the findings of our phylogenetic analyses. As a start point for the analysis, MTR/FAT proteins of human (i.e., MT1/MT2/Mel1c/GPR50 or FAT1/2/3) were used as queries in BLASTp (Altschul et al. 1997) searches to identify homologs within the NCBI database (https://www.ncbi.nlm.nih.gov/). We also used the Ensembl genome browser (https://www.ensembl.org/) to collect MTR family proteins from several species, using the EnsemblCompara method (Vilella et al. 2009).
The sequences were aligned using MAFFT v.7 (Katoh and Standley 2013) with default settings and subjected to quality filtering using GBlocks with default settings (Talavera and Castresana 2007). Final alignments of 300 (MTR) and 2,540 (FAT) amino acid positions (Additional Dataset 1) were used for tree building, done using Bayesian (BY) and maximum likelihood (ML) (MTR) or just ML (FAT) methods. ML trees were generated using IQ-TREE (Nguyen et al. 2015) via the IQ-TREE webserver (Trifinopoulos et al. 2016), employing the best-fitting amino acid substitution model selected with ModelFinder (Kalyaanamoorthy et al. 2017) under the Bayesian information criterion. The best fit models were JTT+F+I+ G4 for MTR and JTT+G4+I for FAT, where ‘JTT’ is the general matrix of Jones et al. (1992), ‘+I’ includes empirical estimation of the proportion of invariant sites, ‘+F’ includes empirical estimation of amino acid frequencies and ‘+G4’ denotes estimation of the gamma distribution parameter with 4 rate classes. The stability of branching in the ML trees was assessed using 1,000 ultrafast bootstrap replicates, (Hoang et al. 2018). The BY analysis (MTR dataset) was done in BEAST v1.8.3 (Drummond et al. 2012), employing an uncorrelated relaxed clock model (Drummond et al. 2017) and a Yule speciation prior (Gernhard 2008), along with the best-fitting substitution model selected by IQ-TREE. A Markov chain Monte Carlo (MCMC) chain of 50 million generations was generated and sampled every 5,000 generations. Convergence of the MCMC chain was assessed using Tracer v1.7.1 http://beast.bio.ed.ac.uk/tracer). A maximum clade credibility tree was generated in TreeAnnotator (Drummond et al. 2012) after removal of the first 10% sampled trees.
Comparative genomic and sequence analyses
Synteny analyses were performed using Ensembl genome browser annotations via the Genomicus platform (Nguyen et al. 2018). These analyses were supplemented with data from NCBI GenBank for species not available in Ensembl. Gene prediction and annotation for Lethenteron camtschaticum was performed using FGENESH (Solovyev et al. 2006). Comparative analyses of MTR family amino acid sequences was done using the final alignment described above (note: the Gblocks filtering step served to remove flanking regions outside the transmembrane/loop regions, which were unaltered). The sequence similarity of the proposed vestigial MTR-like pseudogenes identified in our synteny analyses was established using BLASTx within the NCBI database.
Data availability
Fig. S1. ML phylogenetic analysis of MTRs in vertebrates. This analysis was done using IQ-TREE with a high-confidence alignment of eighty MTRs (300 amino acid positions; Additional Dataset 1) and the best-fitting amino acid substitution model (JTT+F+I+G4). Numbers on branches are bootstrap support values. Other details as in the Figure 1 legend (see main text) Table S1 provides details of all protein sequences used for phylogenetic analyses of the vertebrate MTR family. Table S2 provides details of all sequences used for phylogenetic analyses of the vertebrate FAT protocadherin family
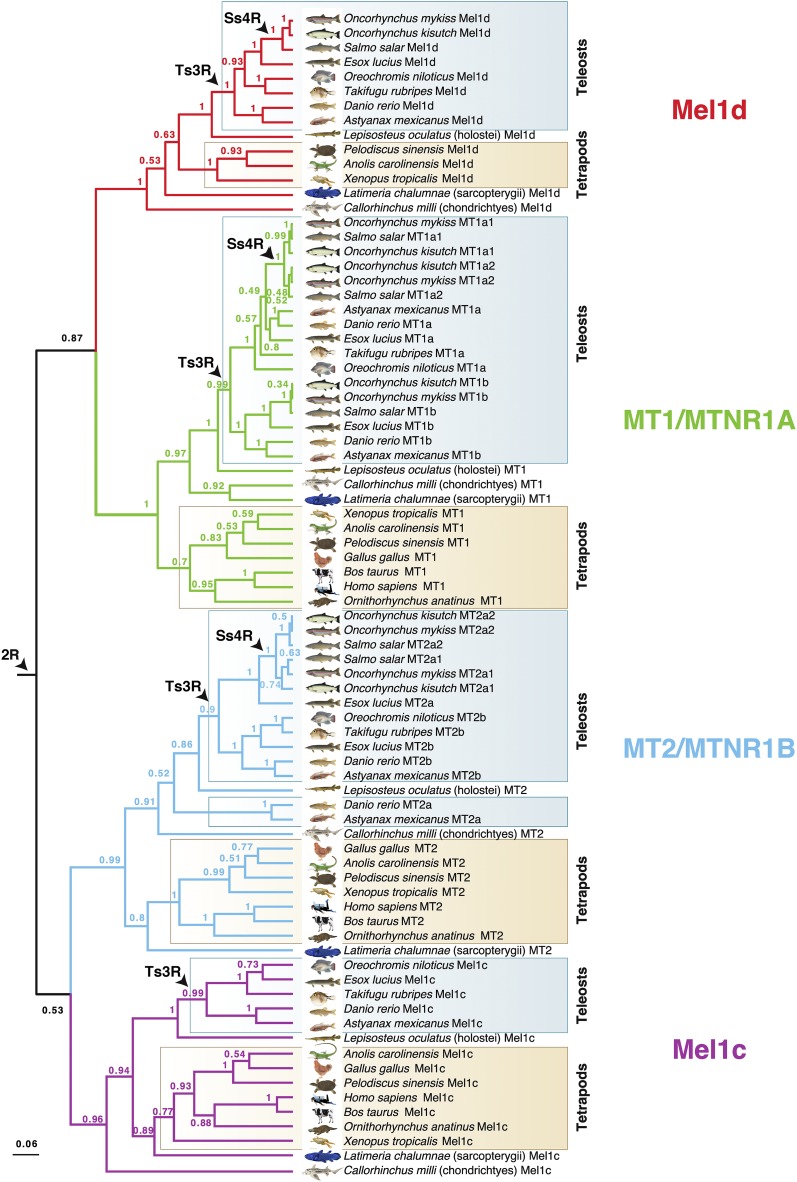
Figure 1.
Bayesian phylogenetic tree of MTR family evolution in jawed vertebrates. The analysis was done using BEAST with a high-confidence alignment of eighty MTRs (300 amino acid positions; Additional Dataset 1), an uncorrelated relaxed molecular clock model and the best-fitting amino acid substitution model (JTT+F+I+G4). Numbers on branches are posterior probability support. Three WGD events in vertebrate evolution are shown (2R - ancestral to vertebrates; Ts3R - ancestral to teleosts; Ss4R - ancestral to salmonids). A ML tree was performed using the same data and is provided in Fig. S1.
Additional Dataset 1 is the MTR sequence alignment used for phylogenetic analysis and comparative sequence analysis. Additional Dataset 2 is the FAT alignment used for phylogenetic analysis. Supplemental material available at FigShare: https://doi.org/10.25387/g3.7823630.
Results
Four MTRs are retained in jawed vertebrates
We identified eighty unique MTR family member proteins in sequence databases representing a standardized set of eighteen jawed vertebrate lineages (see MATERIALS AND METHODS). A phylogenetic analysis was done using a BY method (Figure 1) incorporating a relaxed molecular clock model, which allows estimation of the most plausible root location in the tree (Drummond et al. 2006; e.g., Macqueen and Wilcox 2014; Redmond et al. 2018). Four distinct MTR clades (Figure 1) had strong statistical support (posterior probability, PP: >0.96), and each was represented by cartilaginous fish, as well as ray-finned and lobe-finned fish lineages, with branching patterns closely matching expected species phylogeny (Figure 1). Three of these clades correspond to known ancestral vertebrate MTR family members (e.g., Dufourny et al. 2008). The fourth clade is hereafter named ‘Mel1d’. The same four clades were strongly supported in an unrooted ML phylogenetic analysis (bootstrap support: >96%) congruent with the BY tree (Fig. S1).
These analyses indicate that four distinct MTRs existed in the jawed vertebrate ancestor. However, the phylogenetic affinity of the four MTRs remains equivocal in the BY analysis, with moderate support for Mel1d/MT1 (PP: 0.87) and MT2/Mel1C (PP: 0.53) being paralogues, which can be explained parsimoniously by 2R (Figure 1).
Evolutionary history of individual vertebrate MTRs
Expanding on the above findings, we reconstructed a more detailed evolutionary history for each ancestral MTR in jawed vertebrates, accommodating gene losses, in addition to gains resulting from WGD events in teleosts (summarized in Figure 2).
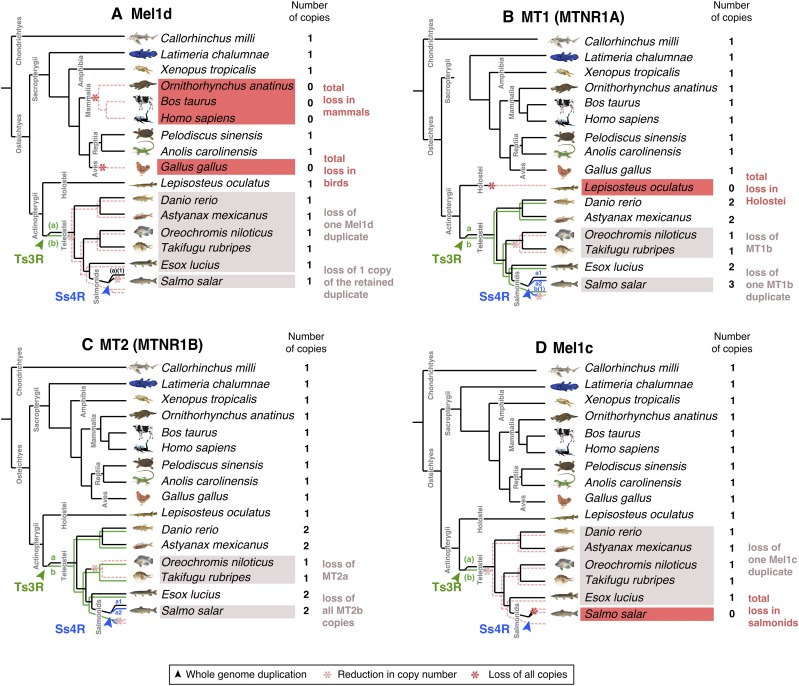
Figure 2.
Proposed evolutionary history of each MTR family member, considering (a) Mel1d, (b) MT1, (c) MT2, and (d) Mel1c. Species inferred to have lost all copies of a MTR gene are highlighted in dark red. Teleost species inferred to have lost paralogues of MTR genes arising from the Ts3R and Ss4R events are highlighted in light red.
Mel1d was encoded by a single gene in all represented species (Figure 1, Figure 2a) including teleosts, consistent with the loss of any paralogues created during Ts3R and Ss4R. In lobe-finned fish, Mel1d was identified in a coelacanth, an amphibian, and two reptiles, but was not identified in the mammals and birds represented in our trees (Figure 2a). As only a small number of bird and mammals were included, we decided to search more broadly for Mel1d orthologs. Hence, BLAST searches of the complete set of proteins stored in NCBI for mammals (∼4.6 million) and birds (∼2.8 million) were done using reptile Mel1d orthologs as the query. Though hundreds of bird and mammal genomes are available in NCBI with protein-level annotations (spanning the diversity of each lineage), the top mammal/bird hit for reptile Mel1D was always MT1/MTNR1A (not shown). Considering our current understanding of amniote phylogeny (e.g., Chiari et al. 2012), our data requires that independent losses of Mel1d occurred in the ancestors to birds and mammals.
For all studied vertebrate species outside teleosts, we identified one copy of MT1, barring spotted gar, where MT1 was not identified (Figure 1, Figure 2b); its trace was retrieved in the genome after further analyses (see section below), representing a sequence annotated as a pseudogene. Several teleost species retain two or more ancestral MT1 copies (PP: 0.99, Figure 1), which can be explained by Ts3R. These duplicates have been annotated in zebrafish within the ZFIN database as “Mtnr1aa” and “Mtnr1ab” (Howe et al. 2013). Consequently, we maintained the same ‘a’ and ‘b’ nomenclature in all species according to inferences of orthology with zebrafish (Figure 1). The two teleost-specific MT1 paralogues were not present in all teleost lineages, with MT1b absent from the studied acanthopterygians (tilapia and pufferfish). Salmonid-specific paralogues of MT1a (MT1a1 and 1a2) were identified, ancestral to three salmonid species (PP: 1.0, Figure 2b), consistent with retention from Ss4R, though only a single copy of MT1b was retained in the same three species, suggesting ancestral loss following Ss4R (Figure 1, Figure 2b).
We identified one copy of MT2 in non-teleost vertebrate lineages, and evidence for teleost-specific paralogues (Figure 2c). Two MT2 paralogues were identified in Ostariophysi members (zebrafish and Mexican cavefish) and northern pike (Protacanthoptergii); however, only one MT2 copy was identified in Acanthopterygii members (Nile tilapia and pufferfish) (Figure 1, Figure 2c). Branching patterns among these duplicates were not well resolved when considering species phylogeny. An ancestral teleost duplication event (e.g., Ts3R) predicts two paralogous MT2 teleost clades, each containing teleosts branching after expected species relationships (as seen for MT1a/b). However, a clade containing zebrafish “Mtnr1ba” (ZFIN 2008, “ZMel1b2” in Shang & Zhdanova 2007) branched outside other fish (including the non-teleost spotted gar) in both the BY and ML trees (Figure 1 and S1). Internal to the spotted gar, there were two teleost MT2 clades, one containing zebrafish “Mtnr1bb” (ZFIN 2008, “ZMel1b1” in Shang & Zhdanova 2007) and other teleost lineages (northern pike and Acanthopterygii members), while the other contained a separate northern pike sequence and all MT2 sequences from salmonids. Given the strong support for the clade containing zebrafish “Mtnr1bb” (PP:1.0, Bootstrap support: 100%), we considered all sequences therein to be orthologous, and named them MT2b (to maintain the zebrafish “b” nomenclature) (Figure 2c). We named the remaining teleost MT2 sequences as MT2a (Figure 2c), under the hypothesis that orthology to zebrafish MT2a was obscured by a long-branch attraction artifact (note the long-branch length leading to Ostariophysi members for MT2a; Fig. S1). This scenario is parsimonious, as it allows for a single ancestral teleost duplication (e.g., Ts3R) rather than several lineage-specific MT2 gains. Accordingly, we propose that MT2a was lost in the ancestor to Oreochromis and Takifugu, while two salmonid duplicates of MT2a (MT2a1 and 2a2) were retained from Ss4R (Figure 1 and S1, Figure 2c). No copies of MT2b were identified in salmonids, suggesting a loss in the common salmonid ancestor (Figure 2c).
As shown elsewhere (Dufourny et al. 2008), Mel1c and mammalian GPR50 proteins grouped together in a well-supported clade (Figure 1). A single Mel1c copy was identified in all teleosts barring salmonids, which evidently lack Mel1c (Figure 2d). This is consistent with a scenario where one Mel1c paralogue was lost early following Ts3R, and an additional loss occurred in the common salmonid ancestor (Figure 2d).
Synteny analysis supports phylogenetic assignment of vertebrate MTRs
Next, to gain an independent line of evidence to support our phylogenetic reconstructions, we compared the genomic regions harboring MTR-encoding genes among a range of vertebrate lineages. The local gene neighborhood containing each MTR family member was more or less conserved across jawed vertebrate evolution, defining identifiable synteny groups specific to each ancestral MTR (Figure 3), including teleost and salmonid-specific paralogues (Figure 4). The genomic neighborhood containing the single MTR locus of lampreys did not conserve synteny with an equivalent region containing any single MTR gene in gnathostomes. Instead, the genes surrounding the single MTR locus of lampreys showed notable similarity to a combination of genes located around the various gnathostome MTRs (Figure 4e). This lends support to an ancestral origin of MTRs in the vertebrate lineage, but does not allow us to pinpoint the relationship of lamprey MTR to the four MTR family members of jawed vertebrates. One possible interpretation is that the duplications generating four gnathostome MTR genes occurred after the cyclostomes and gnathostomes split, with the lamprey genomic neighborhood reflecting a derived representation of the ancestral vertebrate state. However, the current consensus is that at least one round of WGD is shared by cyclostomes and gnathostomes (e.g., Kuraku et al. 2009, Stadler et al. 2004). In this scenario, conserved synteny between a single genomic region in the former to multiple blocks in the latter may be explained by one or more shared duplications followed by lineage-specific rediploidization, as proposed by Robertson et al. (2017).
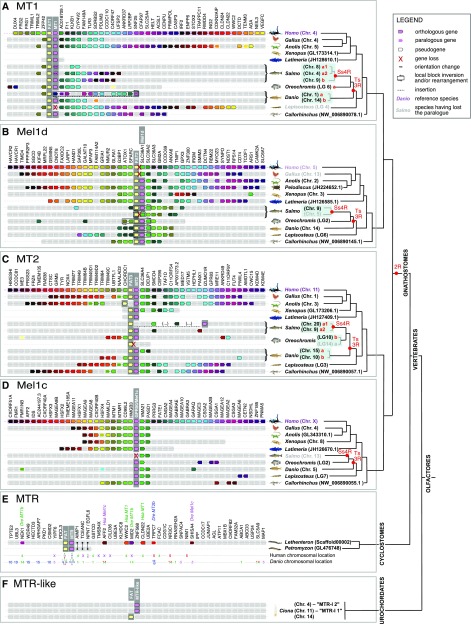
Figure 3.
Conserved synteny between the genomic neighborhood containing MTR orthologs of different lineages, shown for (a) jawed vertebrate MT1, (b) jawed vertebrate Mel1d, (c) jawed vertebrate MT2, (d) jawed vertebrate Mel1c, (e) comparing MTR from two lamprey species with jawed vertebrates, and (f) comparing a urochordate with vertebrates.
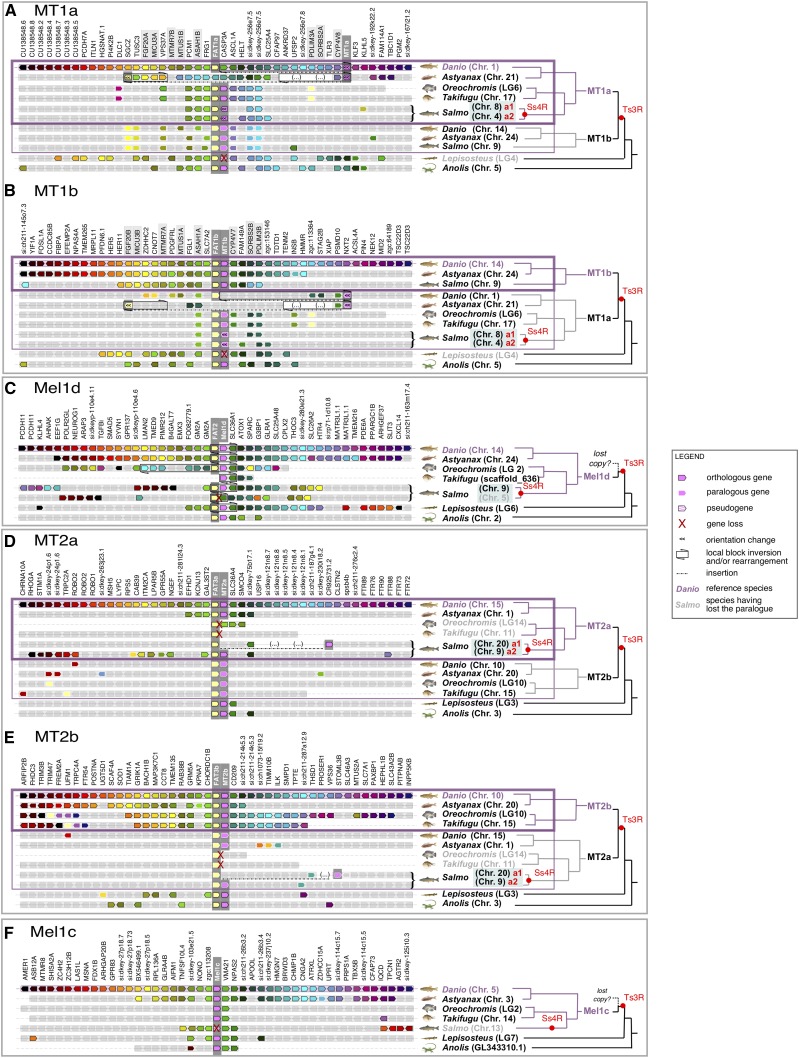
Figure 4.
Conserved synteny between the genomic neighborhood containing MTR paralogues retained from Ts3R and Ss4R, shown for (a) MT1a, (b) MT1b, (c) Mel1d, (d) MT2a, (e) MT2b, and (f) Mel1c.
Genetic linkage between MTR and FAT genes
Tandem-linked MTR and FAT protocadherin gene family members are strongly conserved in all vertebrates (Figure 3, Figure 4). Specifically, MT1, Mel1d, and MT2 were almost always in tandem with FAT1, 2, and 3, respectively (Figure 3, Figure 4). This association was absent for Mel1c, in addition to MTR co-orthologs from a sea squirt (Figure 3f) and the Florida lancelet (not shown), defining this as a vertebrate-specific feature. Past studies have noted genetic linkage between MTR and FAT genes. For example, the FAT3-MT2 locus is involved in diabetes risk, with several SNPs involved in disease located between the two genes, implying potential functional links (e.g., Prokopenko et al. 2009, Dupuis et al. 2010). While, the reason for co-evolution of these loci is yet to be determined, the tandem organization of FAT and MTR genes indicates selective pressure to maintain an association that may be underpinned by a conserved feature of vertebrate physiology.
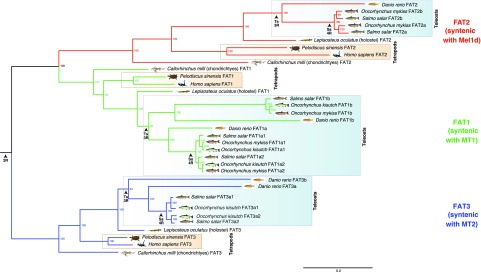
FAT family sequences also provide an independent source of phylogenetic information that may help reconstruct the evolution of the genomic regions containing linked MTR genes. In an ML analysis performed with FAT proteins from representative vertebrate species, we observed three clades (FAT1, 2 and 3) that branched according to expected species relationships (Figure 5). When the ML tree was midpoint rooted, FAT1 (linked to MT1) and FAT2 (linked to Mel1d) were sister groups (Figure 5), consistent with the sister relationship of MT1 and Mel1d recovered by the MTR phylogeny. Further, the teleost duplications observed for MTR genes were clearly identifiable in the respective tandem FAT genes (Figure 5). Finally, the well-supported branching of salmonid FAT3a sequences with zebrafish FAT3a (i.e., linked to the MT2a gene, Figure 3c) adds weight to the hypothesis that salmonid/pike MT2 sequences are orthologous to zebrafish MT2a (Figure 2c).
Figure 5.
ML phylogenetic analysis of FAT atypical protocadherins in jawed vertebrates. The analysis was done using IQ-TREE with a high-confidence alignment of thirty-five FAT proteins (2,540 amino acid positions; Additional Dataset 2) and the best-fitting amino acid substitution model (JTT+G+I). Numbers on branches are bootstrap support values. Other details are as in the Fig. 1 legend.
Synteny analyses support MTR losses
The conservation of synteny across vertebrate taxa in genomic regions containing MTR genes provides useful information on MTR genes inferred to be absent in sequence databases. In this respect, we observed that the genomic regions containing Mel1d in reptiles, frogs and fishes have matched syntenic regions in the human and chicken genomes (Figure 3d). Consequently, the regions predicted to contain Mel1d in human and chicken have been properly assembled and are otherwise well annotated, consistent with bone-fide genetic losses of Mel1d in these species. The same approach allowed us to detect a pseudogene likely to be a vestige of Mel1c in Atlantic salmon (LOC106568030) (Figure 3d), and a gene annotated as ‘non-coding’ bearing similarity with MT1 (according to BLAST) at the predicted MT1 locus in spotted gar (LOC107077181) (Figure 3a). Further, a second FAT2 paralogue was detected in Atlantic salmon, supporting our previous conclusion of an ancestral loss of one Mel1d copy following Ss4R. Similarly, a second FAT3 paralogue was detected in Oreochromis, non-paired with an MTR2 gene (Figure 3c), confirming the loss of MT2a in this species.
Comparative sequence analysis of Mel1d With other MTRs
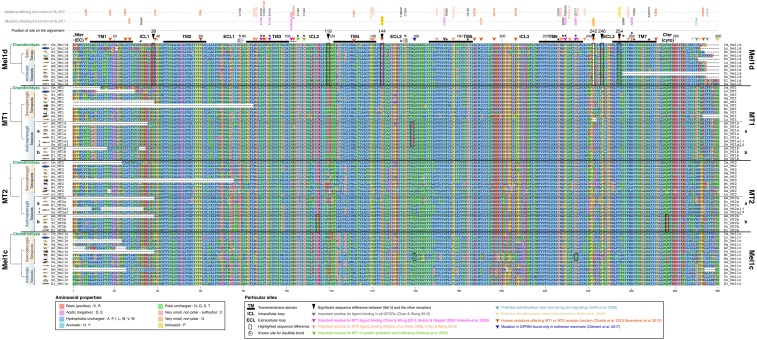
Having established that Mel1d is an ancestral vertebrate MTR, we sought to compare the primary amino acid sequence of this molecule to other MTR family members, hoping to gain clues on its function considering existing literature (Figure 6).
Figure 6.
Alignment used to compare amino acid positions among vertebrate MTR proteins (matching to the alignment used for phylogenetic analysis; Additional Dataset S1). Species abbreviations: Ac = Anolis carolensis (green anole lizard); Am = Astyanax mexicanus (Mexican cavefish); Bt = Bos taurus (cattle); Cm = Callorhinchus milli (elephant shark); Dr = Danio rerio (zebrafish); El = Esox lucius (northern pike); Gg = Gallus gallus (chicken); Hs = Homo sapiens (human); Lc = Latimeria chalumnae (coelacanth); Lo = Lepisosteus oculatus (spotted gar); Oa = Ornithorhynchus anatinus (platypus); On = Oreochromis niloticus (Nile tilapia); Ps = Pelodiscus sinensis (Chinese softshell turtle); Tr = Takifugu rubripes (tiger pufferfish); Xt = Xenopus tropicalis (western clawed frog). Detailed annotation of sequences flagged up in the main text are provided within the figure.
We first examined the MTR transmembrane domains and ligand-binding residues, which have known functional importance. The characteristic seven transmembrane domain structure (TMDs) of all MTRs, critical for GPCR structure and ligand binding (Baldwin 1994), were conserved in Mel1d, MT1, MT2 and Mel1c (Figure 6). Indeed, most of the residues identified as key for melatonin binding are readily identifiable in the Mel1d transmembrane domains (Figure 6), in particular TM3, 6 and 7 (Gubitz & Reppert 2000, Kokkola et al. 2003, Mazna et al. 2005, 2008, Chan & Wong 2013). The only notable difference in the TMDs was that several Mel1d orthologs had threonine replacements at position 254, specific to this MTR. This position is important for melatonin binding in MT2 (valine-291 on human MT2), which was not reported for MT1 (Mazna et al. 2005). Outside the TMDs, two additional melatonin binding residues (asparagine-102 of the conserved NRY motif and alanine-238) were conserved in Mel1d (Figure 6). Interestingly, a mutation in the second extracellular loop of GPR50 linked to the loss of melatonin binding function in mammals (Clément et al. 2018) was absent in Mel1d (Figure 6).
Other key sites conserved in Mel1d included cysteine-78 and cysteine-155, responsible for a conserved disulfide bridge essential to MTR structure (Figure 6). In addition, residues important for G protein activation and trafficking of MT1 (Kokkola et al. 2005) were all conserved in Mel1d (green arrows on Figure 6). Putative palmitoylation site in MT1 and MT2 (cysteine-314 in MT1 and cysteine- 332 in MT2, Sethi et al. 2008) required for G-protein interaction (light blue arrow on Figure 6) were either not identified (MT2 cysteine-332) in Mel1d or absent from most species (MT1 cysteine-314). However a proximal conserved cysteine in position 294 of Mel1d (Figure 6) may fulfill a similar function. Several phosphorylation sites have been suggested in the C-terminal cytoplasmic tail of MT1 and MT2, which might be important for β-arrestin-dependent receptor internalization (Ebisawa et al. 1994, Sethi et al. 2008, yellow arrows on Figure 6). One of these sites is present on Mel1d, at position 288, however only in coelacanth and tetrapods. None of the other phosphorylation sites are present because of the shorter length of Mel1d, and this could be linked to differences in phosphorylation properties.
Residue changes distinguishing Mel1d from other MTRs
The above analyses confirm that Mel1d has most of the canonical residues for melatonin binding and MTR structure/function. We next sought to identify conserved differences between Mel1d and the other MTRs, as candidates to impart functional properties unique to Mel1d.
Five extracellular or intracellular positions in Mel1d show substantial differences with either one or all other MTRs (Figure 6). In most Mel1d orthologs, the first extracellular loop contains lysine (positive charged) at position 38, which is typically asparagine (neutrally charged) in the other MTRs. At position 144, which is almost always fixed as glycine in MT1, MT2 and Mel1c, Mel1d orthologs retain glutamic acid or aspartic acid. This replacement is presumed functionally significant, as glycine provides high conformational flexibility (Betts and Russell 2003), while glutamic and aspartic acid are highly negatively charged. At position 246, MTRs usually conserve proline (except for the two derived GPR50 from mammals), but Mel1d shows a high diversity of residues with diverse functional properties, suggesting a distinct mode of selective pressure. In the same loop (position 242), a gap is observed in all Mel1d sequences at an amino acid position that is variable among the other MTRs. Finally, a notable difference between Mel1d and MT1 is observed in position 119, in the second intracellular loop. Most MT1 sequences have aspartic acid at this position, while Mel1d conserves asparagine or serine, leading to a major difference in charge.
Discussion
Our unequivocal demonstration of a new ancestral vertebrate MTR forces a revision of current models for the origin and diversity of MTRs, and has biological implications for vertebrate lineages conserving distinct MTR gene repertoires.
It seems important to ask why Mel1d has previously been missed as a unique MTR, when the gene is readily detectable in sequence databases. This is likely partly due to a historic assumption that the MTR gene family structure of birds and mammals (i.e., MT1, MT2 and Mel1c) is representative for all vertebrates. Mel1d has high similarity with MT1, and has tended to be named ‘mtnr1a-like’ in genome databases. In addition, previous phylogenetic studies of MTRs have been based on small datasets (e.g., Reppert et al. 1995a; Mazurais et al. 1999; Park et al. 2006, 2007a,b; Shang & Zhdanova 2007; Hong et al. 2014), with biases in the taxa sampled, and could not by design distinguish Mel1d and MT1. A single past study noted a Xenopus MTR sequence that did not group with MT1, MT2 or Mel1c and concluded the existence of a novel MTR (Mel1d) (Shiu et al. 1996); correctly according to our findings. Our study benefits from a much broader survey of vertebrate MTR sequences, allowing us to conclude that Mel1d is at least 450 million years old, having been present in the jawed vertebrate ancestor.
Our phylogenetic reconstruction of MTRs will help the field going forward, as researchers can be certain of which family member (including teleost-specific paralogues) they are studying, allowing more reliable conclusions in comparative studies of function and gene expression. We show that teleost-specific paralogues of MT1 are easily distinguished from Mel1d and provide a scheme to allow researchers to match teleost MTRs formerly named under several nomenclature systems to a single phylogenetically-assigned naming system accommodating orthologs and paralogues (Table 1).
Table 1. Phylogenetic assignment of teleost MTRs to a standardized nomenclature system.
| Receptors attributed from literature vs. orthology group assignment from this study | References | |||||||
|---|---|---|---|---|---|---|---|---|
| MT1 | MT2 | Mel1c | Mel1d | |||||
| Species | MT1a | MT1b | MT2a | MT2b | ||||
| Dr | Danio rerio (zebrafish) | Z1.7 (U31822.1) | Z2.6 (U31824.1) | Z1.4 (U31823.1) | Reppert et al. (1995b) | |||
| zMel1a1, Z1.7-4, mtnr1aa (NM_131393.1) | zMel1a3 (XM_688989.6) | zMel1b2, Z6.2, Mel1b-19, mtnr1ba (NM_131395.1) | zMel1b1, Z2.6-4, mtnr1bb (NM_131394.1) | zMel1c, Z2.3, mtnr1c (NM_001161484.1) | zMel1a2, Z1.4, mtnr1al (NM_001159909.1) | Shang & Zhdanova (2007) | ||
| Om | Oncorhynchus mykiss (rainbow trout) | R1.7 (AF156262.1) = MT1a2 | R1.4 (AF178538.1) | Mazurais et al. (1999) | ||||
| El | Esox lucius (northern pike) | P2.6 (AF188871.1) | P1.4 (XM_010903666.1) | Gaildrat and Falcón, (2000) | ||||
| Oke | Oncorhynchus keta (chum salmon) | mel1a (AY356364.1) = MT1a2 | mel1b (AY356365.1) | Shi et al. (2004) | ||||
| Sg | Siganus guttatus (golden rabbitfish) | Mel1a (DQ768087.1) | Mel1c (DQ768088.1) | Mel1b (DQ522314.1) | Park et al. (2006), (2007a,b), (2014) | |||
| Ca | Carassius auratus (goldfish) | Mel1a1.7 (AB378058.1) | Mel1b (AB378059.1) | Mel1c (AB378060.1) | Mel1a1.4 (AB378057.1) | Ikegami et al. (2009) | ||
| G1.7 (AB481372.1) | G6.2 (AB481374.1) | G2.6 type1 (AB481373.1) | Mel1c (AB481375.1) | G1.4 (AB481371.1) | Saito, unpublished | |||
| Dl | Dicentrarchus labrax (sea bass) | dlMT1 (EU378918.1) | dlMT2 (EU378919.1) | dlMel1c (EU378920.1) | Sauzet et al. (2008) Herrera-Pérez et al. (2010) | |||
| Sse | Solea senegalensis (Senegal sole) | ssMT2 (FM213464.1) | ssMel1c (FM213465.1) | ssMT1 (FM213463.1) | Confente et al. (2010) | |||
| On | Oreochromis niloticus (Nile tilapia) | mel1a (AY569971.1) | Jin et al. (2013) | |||||
| Ec | Epinephelus coioides (orange-spotted grouper) | MT1 (JX524508.1) | MT2 (JX524509.1) | Chai et al. (2013) | ||||
| Bp | Boleophthalmus pectinirostris (mudskipper) | Mtnr1a1.7 (KC622030.1) | Mtrn1b (KC622031.1) | Mtnr1c (KC622032.1) | Mtnr1a1.4 (KC622029.1) | Hong et al. (2014) | ||
| Tn | Takifugu niphobles (grass puffer) | mel1a1.7 (AB492764.1) | mel1b (AB492765.1) | mel1c (AB492766.1) | mel1a1.4 (AB492763.1) | Ikegami et al. (2015) | ||
| Pn | Porichthys notatus (plainfin midshipman - “singing” fish) | mtnr1A1.7 (HQ007044) | Mel1b (KT878765.1) | mtnr1a1.4 (HQ007045) | Feng & Bass, 2016, Feng unpublished | |||
| Amel | Amphiprion melanopus (cinnamon clownfish) | MT-R1 (HM107821.1) | Choi et al. (2016) | |||||
Phylogenetic assignment according to findings of this study; previous publications using distinct nomenclature systems are provided. Sequences in red signal a significant change in assignment. N.B.: the R2.6 gene (AF178929.1) is a chimera between Mel1d and MT2b2.
Insights into Mel1d function: reinterpreting expression data in teleosts
While not being previously recognized as a unique vertebrate MTR, Mel1d has already been studied in various teleosts (Table 1). These past studies demonstrate that the Mel1d transcript is abundantly expressed in a manner like other MTR family members, but showing differences that may underlie unique functions. A pattern seems conserved across multiple species, where Mel1d and MT1a expression is higher in brain and retina, respectively (e.g., Park et al. 2006, 2007a,b; Ikegami et al. 2009: Confente et al. 2010; Hong et al. 2014). Mel1d tends to be more strongly expressed in brain regions associated with visual perception (e.g., Mazurais et al. 1999; Gaildrat and Falcón 2000; Shi et al. 2004; Confente et al. 2010; Hong et al. 2014). Many peripheral tissues were reported to express Mel1d with species-specific differences and in a distinct manner to other MTRs (Park et al. 2006, 2007a,b; Ikegami et al. 2009; Confente et al. 2010; Hong et al. 2014). Such data suggests involvement of Mel1d in photoreceptive processes, along with broader regulatory roles in the physiological functions of peripheral organs.
Rhythmical oscillations in the expression of Mel1d have also been reported, with variations depending on species, organ and season. In zebrafish, a day/night oscillation of MTR brain gene expression (peaking at night) was noted for all six MTR paralogues, including Mel1d, with further expression upregulation in response to melatonin administration (Shang & Zhdanova 2007). In golden rabbitfish, MT1a, Mel1d and Mel1c expression was higher at night for brain and retina, with Mel1d levels peaking at different times (Park et al. 2006, 2007a,b 2014). In goldfish, Mel1d was the only MTR showing rhythmical oscillations in optic tectum expression, while the same was true for MT1a in retina, both peaking at the night-day transition (Ikegami et al. 2009). In a marine pufferfish, Mel1d, MT1a and Mel1c showed synchronous daily cycling of expression in the pineal gland with a nocturnal peak (Ikegami et al. 2015). Conversely, in golden rabbitfish pineal gland, oscillations were desynchronized for the same three MTRs (Park et al. 2006, 2007a,b). Daily rhythmicity in Mel1d expression has also been observed in peripheral tissues (liver and kidney) of golden rabbitfish, with higher expression during the day, opposite to the brain/retina (Park et al. 2006, 2007b). In addition to daily variation in regulation, Mel1d expression is regulated by other cycles, for example showing semilunar oscillation in the diencephalon of mudskipper (Hong et al. 2014) and ultradiurnal oscillation in a marine pufferfish, which may be circatidal (Ikegami et al. 2015). Mel1d expression in the Senegalese sole exhibited stronger day-to-night and seasonal variation than other MTR family members, with reciprocal differences recorded between retina and optic tectum (Confente et al. 2010). Therefore, past work shows that Mel1d is regulated during multiple biological cycles in teleosts, showing variations distinct from other MTRs, implying functional distinctiveness.
Functional divergence between Mel1d and MT1?
High protein-level similarity between Mel1d and MT1, taken with the conservation of all key residues in the MTR transmembrane domains, strongly implies that Mel1d binds melatonin. Notably, residues showing conserved replacements between Mel1d and MT1 are all located in extracellular or cytoplasmic loops, which is predicted to impact interactions with other proteins, in particular signaling partners, rather than melatonin. Strikingly, one of these sites corresponds to a documented human MT1 mutation studied in vitro (Chaste et al. 2010). The replacement of glycine-144 (MT1) with glutamic acid or aspartic acid corresponds to a G166E mutation in human MT1, associated with impaired activation of cAMP signaling, despite retention of strong melatonin binding (Chaste et al. 2010). The elephant shark retains glutamic acid at this position in both MT1 and Mel1d, suggesting this represents the ancestral state, with functional divergence arising in the common ancestor to lobe and ray-finned fishes. It is also intriguing to observe that Mel1d of two tetrapods have apparently reverted to glycine in this position, indicating selection toward the ancestral residue.
Why was Mel1d lost in mammals and birds?
Further work will be needed to establish the extent of conservation in Mel1d function and regulation across different vertebrate lineages. This should focus on reptiles and amphibians, where the function of this gene has not been studied experimentally. Such studies may help explain the specific biological requirements for Mel1d, and reveal why the gene was lost independently in mammals and birds. It is notable that mammals and birds stand out from other vertebrates when considering their melatonin-dependent light detection and clock systems. Mammals have lost extraocular light perception and relocated control of their biological clock away from melatonin-producing pinealocytes to the suprachiasmatic nucleus (Falcón et al. 2009). Birds have both the ancestral pineal clock and melatonin production system, but also independently developed a clock system in the homolog of the suprachiasmatic nucleus and use retinal detection (Cassone 1991, Falcón et al. 2009). Another distinguishing feature specific to both groups is homeothermy, with modulatory effects of melatonin on body temperature regulation reported in humans (Cagnacci et al. 1992; Viswanathan et al. 1990) and Japanese quail (Underwood and Edmonds 1995). Extrinsic temperature variation appears a less important zeitgeber for the circadian clock of homeotherms relative to poikilotherms (Rensing and Ruoff 2002), which are known to use melatonin to regulate behavioral thermoregulation (Lutterschmidt et al. 2003). In addition, birds and mammals are the only vertebrates that have evolved (through convergent mechanisms) stereotypical slow wave and rapid eye movement sleep phases, linked to melatonin regulation in mammals (Lesku et al. 2011). Such changes in the physiological role of melatonin and consequent re-organization of melatonin response pathways, may have been the ultimate driver for Mel1d redundancy and gene loss through relaxation of purifying selection.
Another melatonin-associated function that is present in vertebrate lineages retaining Mel1d (in addition to lamprey), but lost in both mammals and birds, is the negative regulation of pigmentation development in the dark, known as the “body-blanching response” (Hamasaki and Eder 1977, Norris and Carr 2013). In fishes, melatonin is thought to regulate chromatosome aggregation in different kinds of chromatophores (Fujii 2000); Mel1d is expressed in the skin of mudskipper (together with MT1 - Hong et al. 2014), the goldfish (together with MT2 and Mel1c - Ikegami et al. 2008) and the sole (together with MT2 - Confente et al. 2010). In addition, in sole skin, Mel1d is the only MTR to be up-regulated at night. It is therefore possible that Mel1d is involved in skin physiology and pigment regulation in fish chromatophores.
Expansion of the MTR repertoire of teleosts
Contrary to mammals/birds, there has been a trend toward evolutionary expansion in the MTR repertoire of teleosts, as observed in many gene families with paralogues retained from Ts3R (Glasauer and Neuhauss 2014) and Ss4R (Houston and Macqueen 2019). Interestingly, not all MTR family members were affected equally. While we identified multiple paralogous copies of MT1 and MT2 - presumed to have been retained from Ts3R and Ss4R - Mel1c and Mel1d were always single copy, requiring repeated losses of paralogues generated during gene duplication or WGD events. This is compatible with a hypothesis where the functions or expression-level regulation of MT1 and MT2 can be divided among paralogous copies, following the well-established subfunctionalization model, or potentially reflects fixation of new adaptive functions among MT1/MT2 paralogues (Stoltzfus 1999 and Force et al. 1999). In this respect, we observed several amino acid substitutions between MT1a vs. MT1b and MT2a vs. MT2b (Figure 6), consistent with protein-level functional divergence. Conversely, selection has operated in a distinct manner for Mel1c and Mel1d, with any duplicates generated being quickly purged by selection for reasons that remain to be established, but potentially linked to dosage constraints, or a mechanism of regulation that cannot be divided across distinct loci.
Conclusions
Mel1d is one of four ancestral vertebrate MTRs that shows a wide phylogenetic distribution but has been lost in mammals and birds. Compared to MT1, MT2 and Mel1c, Mel1d has many conserved, but also divergent characteristics, both in terms of protein sequence and spatio-temporal expression patterns of relevance to chronobiological traits. Additional work is needed to characterize the functional distinctiveness of Mel1d compared to other MTRs and to explain why unique MTR repertoires have been conserved in different vertebrate lineages.
Acknowledgments
This work was funded by the Frimedbio (Fri prosjektstøtte for medisin, helse og biologi) program of the Research Council of Norway (grant number 241016 - “Light & Salt - Thyroid hormone deiodinase paralogues & the evolution of complex life-history strategy in salmonids”). DJM was supported from BBSRC Institute Strategic Program funding to The Roslin Institute (grant ref: BBS/E/D/10002071). The study was initiated by DH, LOEE and DJM. Sequence collection, alignment and phylogenetic analyses was done by DJM. Comparative genomic and sequence analyses were done by ED. The manuscript was written by ED and DJM, with critical inputs from DH and LOEE.
Footnotes
Supplemental material available at FigShare: https://doi.org/10.25387/g3.7823630.
Communicating editor: A. Whitehead
Literature Cited
- Altschul S. F., Madden T. L., Schäffer A. A., Zhang J., Zhang Z. et al. , 1997. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 25: 3389–3402. 10.1093/nar/25.17.3389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baldwin J. M., 1994. Structure and function of receptors coupled to G proteins. Curr. Opin. Cell Biol. 6: 180–190. 10.1016/0955-0674(94)90134-1 [DOI] [PubMed] [Google Scholar]
- Betts M. J., and Russell R. B., 2003. Amino acid properties and consequences of subsitutions, Bioinformatics for Geneticists, edited by Barnes M. R., and Gray I. C.. Wiley, New Jersey: 10.1002/0470867302.ch14 [DOI] [Google Scholar]
- Cagnacci A., Elliott J. A., and Yen S. S., 1992. Melatonin: a major regulator of the circadian rhythm of core temperature in humans. J. Clin. Endocrinol. Metab. 75: 447–452. [DOI] [PubMed] [Google Scholar]
- Cassone V. M., 1991. Melatonin and suprachiasmatic nucleus function, pp. 309–323 in Suprachiasmatic Nucleus: The Mind’s Clock, edited by Klein D. C., Moore R. Y., and Reppert S. M., Oxford Univ. Press, New York. [Google Scholar]
- Chan K. H., and Wong Y. H., 2013. A molecular and chemical perspective in defining MTR subtype selectivity. Int. J. Mol. Sci. 14: 18385–18406. 10.3390/ijms140918385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai K., Liu X., Zhang Y., and Lin H., 2013. Day-night and reproductive cycle profiles of melatonin receptor, kiss, and gnrh expression in orange-spotted grouper (Epinephelus coioides). Mol. Reprod. Dev. 80: 535–548. 10.1002/mrd.22191 [DOI] [PubMed] [Google Scholar]
- Chaste P., Clément N., Mercati O., Guillaume J. L., Delorme R. et al. , 2010. Identification of pathway-biased and deleterious MTR mutants in autism spectrum disorders and in the general population. PLoS One 5: e11495 10.1371/journal.pone.0011495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiari Y., Cahais V., Galtier N., and Delsuc F., 2012. Phylogenomic analyses support the position of turtles as the sister group of birds and crocodiles (Archosauria). BMC Biol. 10: 65 10.1186/1741-7007-10-65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y. J., Habibi H. R., and Choi C. Y., 2016. Profiles of gonadotropin-inhibitory hormone and melatonin during the sex change and maturation of cinnamon clownfish, Amphiprion melanopus. Biochem. Biophys. Res. Commun. 475: 189–193. 10.1016/j.bbrc.2016.05.073 [DOI] [PubMed] [Google Scholar]
- Clément N., Renault N., Guillaume J. L., Cecon E., Journé A. S. et al. , 2018. Importance of the second extracellular loop for melatonin MT1 receptor function and absence of melatonin binding in GPR50. Br. J. Pharmacol. 175: 3281–3297. 10.1111/bph.14029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Confente F., Rendón M., Besseau L., Falcón J., and Muñoz-Cueto J. A., 2010. Melatonin receptors in a pleuronectiform species, Solea senegalensis: Cloning, tissue expression, day-night and seasonal variations. Gen. Comp. Endocrinol. 167: 202–214. 10.1016/j.ygcen.2010.03.006 [DOI] [PubMed] [Google Scholar]
- Dehal P., and Boore J. L., 2005. Two rounds of whole genome duplication in the ancestral vertebrate. PLoS Biol. 3: e314 10.1371/journal.pbio.0030314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond A. J., Ho S. Y. W., Phillips M. J., and Rambaut A., 2006. Relaxed phylogenetics and dating with confidence. PLoS Biol. 4: e88 10.1371/journal.pbio.0040088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond A. J., Suchard M. A., Xie D., and Rambaut A., 2012. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol. Biol. Evol. 29: 1969–1973. 10.1093/molbev/mss075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubocovich M. L., and Markowska M., 2005. Functional MT1 and MT2 melatonin receptors in Mammals. Endocrine 27: 101–110. 10.1385/ENDO:27:2:101 [DOI] [PubMed] [Google Scholar]
- Dufourny L., Levasseur A., Migaud M., Callebaut I., Pontarotti P. et al. , 2008. GPR50 is the mammalian ortholog of Mel1c: Evidence of rapid evolution in mammals. BMC Evol. Biol. 8: 105 10.1186/1471-2148-8-105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dupuis J., Langenberg C., Prokopenko I., Saxena R., Soranzo N. et al. , 2010. New genetic loci implicated in fasting glucose homeostasis and their impact on type 2 diabetes risk. Nat. Genet. 42: 105–116. :464. 10.1038/ng.520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebisawa T., Karne S., Lerner M. R., and Reppert S. M., 1994. Expression cloning of a high-affinity melatonin receptor from Xenopus dermal melanophores. Proc. Natl. Acad. Sci. USA 91: 6133–6137. 10.1073/pnas.91.13.6133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falcón J., Besseau L., Fuentès M., Sauzet S., Magnanou E. et al. , 2009. Structural and functional evolution of the pineal melatonin system in vertebrates. Ann. N. Y. Acad. Sci. 1163: 101–111. 10.1111/j.1749-6632.2009.04435.x [DOI] [PubMed] [Google Scholar]
- Feng N. Y., and Bass A. H., 2016. “Singing” Fish Rely on Circadian Rhythm and Melatonin for the Timing of Nocturnal Courtship Vocalization. Curr. Biol. 26: 2681–2689. 10.1016/j.cub.2016.07.079 [DOI] [PubMed] [Google Scholar]
- Force A., Lynch M., Pickett F. B., Amores A., Yan Y. L. et al. , 1999. Preservation of duplicate genes by complementary, degenerative mutations. Genetics 151: 1531–1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii R., 2000. The regulation of motile activity in fish chromatophores. Pigment Cell Res. 13: 300–319. 10.1034/j.1600-0749.2000.130502.x [DOI] [PubMed] [Google Scholar]
- Gaildrat P., and Falcón J., 2000. Melatonin receptors in the pituitary of a teleost fish: mRNA expression, 2-[(125)I]iodomelatonin binding and cyclic AMP response. Neuroendocrinology 72: 57–66. 10.1159/000054571 [DOI] [PubMed] [Google Scholar]
- Gernhard T., 2008. The conditioned reconstructed process. J. Theor. Biol. 253: 769–778. 10.1016/j.jtbi.2008.04.005 [DOI] [PubMed] [Google Scholar]
- Glasauer S. M., and Neuhauss S. C., 2014. Whole-genome duplication in teleost fishes and its evolutionary consequences. Mol. Genet. Genomics 289: 1045–1060. 10.1007/s00438-014-0889-2 [DOI] [PubMed] [Google Scholar]
- Gubitz A. K., and Reppert S. M., 2000. Chimeric and point-mutated receptors reveal that a single glycine residue in transmembrane domain 6 is critical for high affinity melatonin binding. Endocrinology 141: 1236–1244. 10.1210/endo.141.3.7356 [DOI] [PubMed] [Google Scholar]
- Hardeland R., Cardinali D. P., Srinivasan V., Spence D. W., Brown G. M. et al. , 2011. Melatonin–a pleiotropic, orchestrating regulator molecule. Prog. Neurobiol. 93: 350–384. 10.1016/j.pneurobio.2010.12.004 [DOI] [PubMed] [Google Scholar]
- Hamasaki D. I., and Eder D. J., 1977. Adaptive radiation of the pineal system, Vol. VII/5, pp. 497–548 in Handbook of sensory physiology - The Visual System in Vertebrates, edited by Crescitelli F. Springer-Verlag, New York. [Google Scholar]
- Herrera-Pérez P., Del Carmen Rendón M., Besseau L., Sauzet S., Falcón J. et al. , 2010. Melatonin receptors in the brain of the European sea bass: An in situ hybridization and autoradiographic study. J. Comp. Neurol. 518: 3495–3511. 10.1002/cne.22408 [DOI] [PubMed] [Google Scholar]
- Hoang D. T., Chernomor O., von Haeseler A., Minh B. Q., and Vinh L. S., 2018. UFBoot2: Improving the ultrafast bootstrap approximation. Mol. Biol. Evol. 35: 518–522. 10.1093/molbev/msx281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong L. Y., Hong W. S., Zhu W. B., Shi Q., You X. X. et al. , 2014. Cloning and expression of melatonin receptors in the mudskipper Boleophthalmus pectinirostris: their role in synchronizing its semilunar spawning rhythm. Gen. Comp. Endocrinol. 195: 138–150. 10.1016/j.ygcen.2013.11.004 [DOI] [PubMed] [Google Scholar]
- Houston R. D., and Macqueen D. J., 2019. Atlantic salmon (Salmo salar L.) genetics in the 21st century: taking leaps forward in aquaculture and biological understanding. Anim. Genet. 50: 3–14. 10.1111/age.12748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegami T., Azuma K., Nakamura M., Suzuki N., Hattori A. et al. , 2009. Diurnal expressions of four subtypes of melatonin receptor genes in the optic tectum and retina of goldfish. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 152: 219–224. 10.1016/j.cbpa.2008.09.030 [DOI] [PubMed] [Google Scholar]
- Ikegami T., Maruyama Y., Doi H., Hattori A., and Ando H., 2015. Ultradian oscillation in expression of four melatonin receptor subtype genes in the pineal gland of the grass puffer, a semilunar-synchronized spawner, under constant darkness. Front. Neurosci. 9: 9 10.3389/fnins.2015.00009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin Y. H., Park J. W., Kim J. H., and Kwon J. Y., 2013. The Expression Pattern of Melatonin Receptor 1a Gene during Early Life Stages in the Nile tilapia (Oreochromis niloticus). Dev. Reprod. 17: 45–53. 10.12717/DR.2013.17.1.045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jockers R., Maurice P., Boutin J. A., and Delagrange P., 2008. Melatonin receptors, heterodimerization, signal transduction and binding sites: what’s new? Br. J. Pharmacol. 154: 1182–1195. 10.1038/bjp.2008.184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D. T., Taylor W. R., and Thornton J. M., 1992. The rapid generation of mutation data matrices from protein sequences. Bioinformatics 8: 275–282. 10.1093/bioinformatics/8.3.275 [DOI] [PubMed] [Google Scholar]
- Kalyaanamoorthy S., Minh B. Q., Wong T. K. F., von Haeseler A., and Jermiin L. S., 2017. ModelFinder: fast model selection for accurate phylogenetic estimates. Nat. Methods 14: 587–589. 10.1038/nmeth.4285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamesh N., Aradhyam G. K., and Manoj N., 2008. The repertoire of G protein-coupled receptors in the sea squirt Ciona intestinalis. BMC Evol. Biol. 8: 129 10.1186/1471-2148-8-129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh K., and Standley D. M., 2013. MAFFT multiple sequence alignment software version 7: improvements in performance and usability. Mol. Biol. Evol. 30: 772–780. 10.1093/molbev/mst010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokkola T., Foord S. M., Watson M. A., Vakkuri O., and Laitinen J. T., 2003. Important amino acids for the function of the human MT1 melatonin receptor. Biochem. Pharmacol. 65: 1463–1471. 10.1016/S0006-2952(03)00113-8 [DOI] [PubMed] [Google Scholar]
- Kokkola T., Salo O. M., Poso A., and Laitinen J. T., 2005. The functional role of cysteines adjacent to the NRY motif of the human MT1 melatonin receptor. J. Pineal Res. 39: 1–11. 10.1111/j.1600-079X.2004.00204.x [DOI] [PubMed] [Google Scholar]
- Krishnan A., Almén M. S., Fredriksson R., and Schiöth H. B., 2013. Remarkable similarities between the hemichordate (Saccoglossus kowalevskii) and vertebrate GPCR repertoire. Gene 526: 122–133. 10.1016/j.gene.2013.05.005 [DOI] [PubMed] [Google Scholar]
- Kuraku S., 2013. Impact of asymmetric gene repertoire between cyclostomes and gnathostomes. Semin. Cell Dev. Biol. 24: 119–127. 10.1016/j.semcdb.2012.12.009 [DOI] [PubMed] [Google Scholar]
- Kuraku S., Meyer A., and Kuratani S., 2009. Timing of Genome Duplications Relative to the Origin of the Vertebrates: Did Cyclostomes Diverge before or after? Mol. Biol. Evol. 26: 47–59. 10.1093/molbev/msn222 [DOI] [PubMed] [Google Scholar]
- Lesku J. A., Vyssotski A. L., Martinez-Gonzalez D., Wilzeck C., and Rattenborg N. C., 2011. Local sleep homeostasis in the avian brain: convergence of sleep function in mammals and birds? Proc. Biol. Sci. 278: 2419–2428. 10.1098/rspb.2010.2316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levoye A., Dam J., Ayoub M. A., Guillaume J. L., Couturier C. et al. , 2006. The orphan GPR50 receptor specifically inhibits MT1 melatonin receptor function through heterodimerization. EMBO J. 25: 3012–3023. 10.1038/sj.emboj.7601193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lien S., Koop B. F., Sandve S. R., Miller J. R., Kent M. P. et al. , 2016. The Atlantic salmon genome provides insights into rediploidization. Nature 533: 200–205. 10.1038/nature17164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutterschmidt D. I., Lutterschmidt W. I., and Hutchison V. H., 2003. Melatonin and thermoregulation in ectothermic vertebrates: a review. Can. J. Zool. 81: 1–13. 10.1139/z02-189 [DOI] [Google Scholar]
- Macqueen D. J., and Johnston I. A., 2014. A well-constrained estimate for the timing of the salmonid whole genome duplication reveals major decoupling from species diversification. Proc. Biol. Sci. 281: 20132881 10.1098/rspb.2013.2881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macqueen D. J., and Wilcox A. H., 2014. Characterization of the definitive classical calpain family of vertebrates using phylogenetic, evolutionary and expression analyses. Open Biol. 4: 130219 10.1098/rsob.130219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazna P., Grycova L., Balik A., Zemkova H., Friedlova E. et al. , 2008. The role of proline residues in the structure and function of human MT2 melatonin receptor. J. Pineal Res. 45: 361–372. 10.1111/j.1600-079X.2008.00598.x [DOI] [PubMed] [Google Scholar]
- Mazna P., Berka K., Jelinkova I., Balik A., Svoboda P. et al. , 2005. Ligand binding to the human MT2 melatonin receptor: the role of residues in transmembrane domains 3, 6, and 7. Biochem. Biophys. Res. Commun. 332: 726–734. 10.1016/j.bbrc.2005.05.017 [DOI] [PubMed] [Google Scholar]
- Mazurais D., Brierley I., Anglade I., Drew J., Randall C. et al. , 1999. Central melatonin receptors in the rainbow trout: comparative distribution of ligand binding and gene expression. J. Comp. Neurol. 409: 313–324. [DOI] [PubMed] [Google Scholar]
- Norris D. O., and Carr J. A., 2013. Vertebrate endocrinology, Academic Press, Massachusetts. [Google Scholar]
- Nordström K. J., Fredriksson R., and Schioth H. B., 2008. The amphioxus Branchiostoma floridae genome contains a highly diversified set of G protein-coupled receptors. BMC Evol. Biol. 8: 9 10.1186/1471-2148-8-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen L.-T., Schmidt H. A., von Haeseler A., and Minh B. Q., 2015. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum likelihood phylogenies. Mol. Biol. Evol. 32: 268–274. 10.1093/molbev/msu300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen N. T. T., Vincens P., Roest Crollius H., and Louis A., 2018. Genomicus 2018: karyotype evolutionary trees and on-the-fly synteny computing. Nucleic Acids Res. 46: D816–D822. 10.1093/nar/gkx1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ota K. G., Fujimoto S., Oisi Y., and Kuratani S., 2011. Identification of vertebra-like elements and their possible differentiation from sclerotomes in the hagfish. Nat. Commun. 2: 373 10.1038/ncomms1355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park Y. J., Park J. G., Takeuchi Y., Hur S. P., Lee Y. D. et al. , 2014. Influence of moonlight on mRNA expression patterns of melatonin receptor subtypes in the pineal organ of a tropical fish. Mar. Genomics 14: 67–70. 10.1016/j.margen.2013.10.006 [DOI] [PubMed] [Google Scholar]
- Park Y. J., Park J. G., Jeong H. B., Takeuchi Y., Kim S. J. et al. , 2007a Expression of the melatonin receptor Mel(1c) in neural tissues of the reef fish Siganus guttatus. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 147: 103–111. 10.1016/j.cbpa.2006.11.027 [DOI] [PubMed] [Google Scholar]
- Park Y. J., Park J. G., Hiyakawa N., Lee Y. D., Kim S. J. et al. , 2007b Diurnal and circadian regulation of a melatonin receptor, MT1, in the golden rabbitfish, Siganus guttatus. Gen. Comp. Endocrinol. 150: 253–262. 10.1016/j.ygcen.2006.08.011 [DOI] [PubMed] [Google Scholar]
- Park Y. J., Park J. G., Kim S. J., Lee Y. D., Saydur Rahman M. et al. , 2006. Melatonin receptor of a reef fish with lunar-related rhythmicity: cloning and daily variations. J. Pineal Res. 41: 166–174. 10.1111/j.1600-079X.2006.00350.x [DOI] [PubMed] [Google Scholar]
- Prokopenko I., Langenberg C., Florez J. C., Saxena R., Soranzo N. et al. , 2009. Variants in MTNR1B influence fasting glucose levels. Nat. Genet. 41: 77–81. 10.1038/ng.290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redmond A. K., Macqueen D. J., and Dooley H., 2018. Phylotranscriptomics suggests the jawed vertebrate ancestor could generate diverse helper and regulatory T cell subsets. BMC Evol. Biol. 18: 169 10.1186/s12862-018-1290-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rensing L., and Ruoff P., 2002. Temperature effect on entrainment, phase shifting, and amplitude of circadian clocks and its molecular bases. Chronobiol. Int. 19: 807–864. 10.1081/CBI-120014569 [DOI] [PubMed] [Google Scholar]
- Reppert S. M., Weaver D. R., and Ebisawa T., 1994. Cloning and characterization of a mammalian melatonin receptor that mediates reproductive and circadian responses. Neuron 13: 1177–1185. 10.1016/0896-6273(94)90055-8 [DOI] [PubMed] [Google Scholar]
- Reppert S. M., Godson C., Mahle C. D., Weaver D. R., Slaugenhaupt S. A. et al. , 1995a Molecular characterization of a second melatonin receptor expressed in human retina and brain: the Mel1b melatonin receptor. Proc. Natl. Acad. Sci. USA 92: 8734–8738. 10.1073/pnas.92.19.8734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reppert S. M., Weaver D. R., Cassone V. M., Godson C., and Kolakowski L. F. Jr, 1995b Melatonin receptors are for the birds: molecular analysis of two receptor subtypes differentially expressed in chick brain. Neuron 15: 1003–1015. 10.1016/0896-6273(95)90090-X [DOI] [PubMed] [Google Scholar]
- Robertson F. M., Gundappa M. K., Grammes F., Hvidsten T. R., Redmond A. K. et al. , 2017. Lineage-specific rediploidization is a mechanism to explain time-lags between genome duplication and evolutionary diversification. Genome Biol. 18: 111 10.1186/s13059-017-1241-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauzet S., Besseau L., Herrera Perez P., Covès D., Chatain B. et al. , 2008. Cloning and retinal expression of melatonin receptors in the European sea bass, Dicentrarchus labrax. Gen. Comp. Endocrinol. 157: 186–195. 10.1016/j.ygcen.2008.04.008 [DOI] [PubMed] [Google Scholar]
- Sethi S., Adams W., Pollock J., and Witt-Enderby P. A., 2008. C-terminal domains within human MT1 and MT2 melatonin receptors are involved in internalization processes. J. Pineal Res. 45: 212–218. 10.1111/j.1600-079X.2008.00579.x [DOI] [PubMed] [Google Scholar]
- Shang E. H., and Zhdanova I. V., 2007. The circadian system is a target and modulator of prenatal cocaine effects. PLoS One 2: e587 10.1371/journal.pone.0000587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Q., Ando H., Coon S. L., Sato S., Ban M. et al. , 2004. Embryonic and post-embryonic expression of arylalkylamine N-acetyltransferase and melatonin receptor genes in the eye and brain of chum salmon (Oncorhynchus keta). Gen. Comp. Endocrinol. 136: 311–321. 10.1016/j.ygcen.2004.01.004 [DOI] [PubMed] [Google Scholar]
- Shiu S. Y., Ng N., and Pang S. F., 1996. A molecular perspective of the genetic relationships of G-protein coupled melatonin receptor subtypes. J. Pineal Res. 20: 198–204. 10.1111/j.1600-079X.1996.tb00259.x [DOI] [PubMed] [Google Scholar]
- Slominski R. M., Reiter R. J., Schlabritz-Loutsevitch N., Ostrom R. S., and Slominski A. T., 2012. Melatonin membrane receptors in peripheral tissues: distribution and functions. Mol. Cell. Endocrinol. 351: 152–166. 10.1016/j.mce.2012.01.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solovyev V., Kosarev P., Seledsov I., Vorobyev D., 2006. Automatic annotation of eukaryotic genes, pseudogenes and promoters. Genome Biol. 7: 10.1–10.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler P. F., Fried C., Prohaska S. J., Bailey W. J., Misof B. Y. et al. , 2004. Evidence for independent Hox gene duplications in the hagfish lineage: a PCR-based gene inventory of Eptatretus stoutii. Mol. Phylogenet. Evol. 32: 686–694. 10.1016/j.ympev.2004.03.015 [DOI] [PubMed] [Google Scholar]
- Stoltzfus A., 1999. On the possibility of constructive neutral evolution. J. Mol. Evol. 49: 169–181. 10.1007/PL00006540 [DOI] [PubMed] [Google Scholar]
- Sugahara F., Murakami Y., Adachi N., and Kuratani S., 2013. Evolution of the regionalization and patterning of the vertebrate telencephalon: what can we learn from cyclostomes? Curr. Opin. Genet. Dev. 23: 475–483. 10.1016/j.gde.2013.02.008 [DOI] [PubMed] [Google Scholar]
- Talavera G., and Castresana J., 2007. Improvement of phylogenies after removing divergent and ambiguously aligned blocks from protein sequence alignments. Syst. Biol. 56: 564–577. 10.1080/10635150701472164 [DOI] [PubMed] [Google Scholar]
- Trifinopoulos J., Nguyen L. T., von Haeseler A., and Minh B. Q., 2016. W-IQ-TREE: a fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 44: W232–W235. 10.1093/nar/gkw256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underwood H., and Edmonds K., 1995. The circadian rhythm of thermoregulation in Japanese quail: III. Effects of melatonin administration. J. Biol. Rhythms 10: 284–298. 10.1177/074873049501000402 [DOI] [PubMed] [Google Scholar]
- Vernadakis A. J., Bemis W. E., and Bittman E. L., 1998. Localization and partial characterization of MTRs in amphioxus, hagfish, lamprey, and skate. Gen. Comp. Endocrinol. 110: 67–78. 10.1006/gcen.1997.7042 [DOI] [PubMed] [Google Scholar]
- Vilella A. J., Severin J., Ureta-Vidal A., Heng L., Durbin R. et al. , 2009. EnsemblCompara GeneTrees: Complete, duplication-aware phylogenetic trees in vertebrates. Genome Res. 19: 327–335. 10.1101/gr.073585.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan M., Laitinen J. T., and Saavedra J. M., 1990. Expression of melatonin receptors in arteries involved in thermoregulation. Proc. Natl. Acad. Sci. USA 87: 6200–6203. 10.1073/pnas.87.16.6200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver D. R., and Reppert S. M., 1990. Melatonin receptors are present in the ferret pars tuberalis and pars distalis, but not in brain. Endocrinology 127: 2607–2609. 10.1210/endo-127-5-2607 [DOI] [PubMed] [Google Scholar]
- Witt-Enderby P. A., Bennett J., Jarzynka M. J., Firestine S., and Melan M. A., 2003. Melatonin receptors and their regulation: biochemical and structural mechanisms. Life Sci. 72: 2183–2198. 10.1016/S0024-3205(03)00098-5 [DOI] [PubMed] [Google Scholar]
- ZFIN , 2013. The Zebrafish Model Organism Database: increased support for mutants and transgenics. Nucleic Acids Res. 41(Database issue): D854-60 https://zfin.org
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Fig. S1. ML phylogenetic analysis of MTRs in vertebrates. This analysis was done using IQ-TREE with a high-confidence alignment of eighty MTRs (300 amino acid positions; Additional Dataset 1) and the best-fitting amino acid substitution model (JTT+F+I+G4). Numbers on branches are bootstrap support values. Other details as in the Figure 1 legend (see main text) Table S1 provides details of all protein sequences used for phylogenetic analyses of the vertebrate MTR family. Table S2 provides details of all sequences used for phylogenetic analyses of the vertebrate FAT protocadherin family
Figure 1.
Bayesian phylogenetic tree of MTR family evolution in jawed vertebrates. The analysis was done using BEAST with a high-confidence alignment of eighty MTRs (300 amino acid positions; Additional Dataset 1), an uncorrelated relaxed molecular clock model and the best-fitting amino acid substitution model (JTT+F+I+G4). Numbers on branches are posterior probability support. Three WGD events in vertebrate evolution are shown (2R - ancestral to vertebrates; Ts3R - ancestral to teleosts; Ss4R - ancestral to salmonids). A ML tree was performed using the same data and is provided in Fig. S1.
Additional Dataset 1 is the MTR sequence alignment used for phylogenetic analysis and comparative sequence analysis. Additional Dataset 2 is the FAT alignment used for phylogenetic analysis. Supplemental material available at FigShare: https://doi.org/10.25387/g3.7823630.