Abstract
Introduction
Low muscle strength is common and important in geriatric syndromes including frailty and sarcopenia. The epidemiology of grip strength of older people under long-term care facilities has been little explored.
Purpose
The aim of this study was to assess handgrip strength of older women and men covered by institutional care and to analyse the associations between HGS and mobility, leg strength, flexibility, and postural balance.
Materials and Methods
This is a cross-sectional study carried out at care homes in southeastern Poland. After considering the inclusion criteria, 209 older people aged 65 to 85 were included in the study. Sociodemographic data were collected, and tests of muscular strength, mobility, flexibility, and postural balance were carried out by the use of the stabilometric platform CQ Stab 2P.
Results
The average handgrip strength in the study group amounted to 19.8 kg, including 14.8 kg in women and 25.9 kg in men. Low grip strength was found in 67.83% women and 52.13% men in institutional care. A negative correlation between handgrip strength (HGS) and the Timed Up and Go (TUG) test was demonstrated, both with and without cognitive task and strength of lower limbs. Gait speed and dynamic balance were positively correlated with HGS. A negative correlation was found between the total length of the centre of pressure (COP) path, the length of the COP path in the lateral-medial direction, and the sway area delimited by the COP and HGS for the dominant hand. Speaking of women, gait speed was most strongly associated with HGS, while among men, it was upper limb flexibility.
Conclusion
Regardless of gender, HGS is associated with mobility, strength of the lower limbs, and dynamic balance. By means of simple tools, early diagnosis will facilitate the planning of appropriate interventions in order to prevent disability and mortality in long-term care facilities.
1. Introduction
Aging is associated with progressive loss of muscle mass with a simultaneous increase in fat mass [1]. A decrease in skeletal muscle mass takes place at the rate of 3–8% for a decade and begins after 30 years of age [2]. Its loss is also accompanied by a significant decrease in muscle strength amounting to more than 15% per decade [3].
Loss of muscle strength is a key indicator for many geriatric syndromes, including weakness syndrome, sarcopenia, mobility impairments, and falls [4]. Weaker grip strength is tightly associated with multiple morbidities [5] and poorer self-rated health [6]. Epidemiological studies have shown that it is an important indicator of the risk of cognitive impairment, dementia, and depression in older people [7, 8]. It was also found that age, gender, body mass index (BMI), and nutritional state correlate with HGS [9, 10].
Muscle strength is an important determinant of healthy aging [11]. Low handgrip strength (HGS) is a strong predictor of mobility impairment, both in women and men [12]. The decrease in the grip strength associated with aging reduces the independence of the older people, leading to the need to use family support or caregivers [13]. It may impair manual dexterity of upper limbs, as well as affect the ability to maintain postural balance and gait independence [14]. It is used to predict disability, morbidity, and mortality in the future [15]. Early detection of low muscle strength can help identify people at risk of significant mobility restrictions and increased bedtime [16].
The European Working Group on Sarcopenia in Older People (EWGSOP) recognizes that strength is a better measurement than muscle mass in predicting loss of independence or need for long-term care placement. EWGSOP recommends using a range of tools in the assessment of older people, which includes handgrip strength, chair stand, gait speed, TUG test, and balance assessment. For individual measurements, cutoff points for diagnostic variables for people at risk of weakness are specified [17]. However, there are few data assessing the correlation between HGS and other feasible measures of mobility, leg strength, flexibility, and postural balance in the population of older people covered by institutional care.
Functional disability, in the face of demographic changes, is a challenge for public health. Due to the fact that the average grip strength varies depending on the geographic regions of the world, the extension of reference values among older women and men receiving institutional care in Poland is important for clinical practice [18]. The aim of the study was to assess the strength of the handgrip and identify factors associated with it among older women and men in long-term care facilities.
2. Materials and Methods
2.1. Study Setting
It is a cross-sectional study carried out in randomly selected 9 residential care homes in the southeast of Poland.
2.2. Participants
The study involved older people who lead a sedentary lifestyle staying in residential long-term care facilities in southeastern Poland. The criteria for inclusion in the study were age from 65 to 85 years, a normal cognitive status or a mild impairment in the field of orientation and memory examined by Mini-Mental State Examination (MMSE) from 30 to 19 points, no or moderate depression in the Geriatric Depression Scale (GDS) below 11 points, a level of physical performance enabling the subject to take a standing position on the stabilometric platform, and physically inactive—performing activities in a sitting position, such as reading and watching TV, for a minimum of 4 hours a day/6-7 days a week. Exclusion criteria were vestibular and neurological disorders, dizziness, taking drugs significantly affecting the body's balance, injuries of the lower limbs during the last 6 months, paresis or deformities in the upper limbs, and severe systemic diseases. Regarding 784 residents of care homes and after considering inclusion criteria and receiving written consent of the residents to participate in the project, 209 people were included in the study. A flow diagram shows participant selection and dropout (Figure 1).
Figure 1.
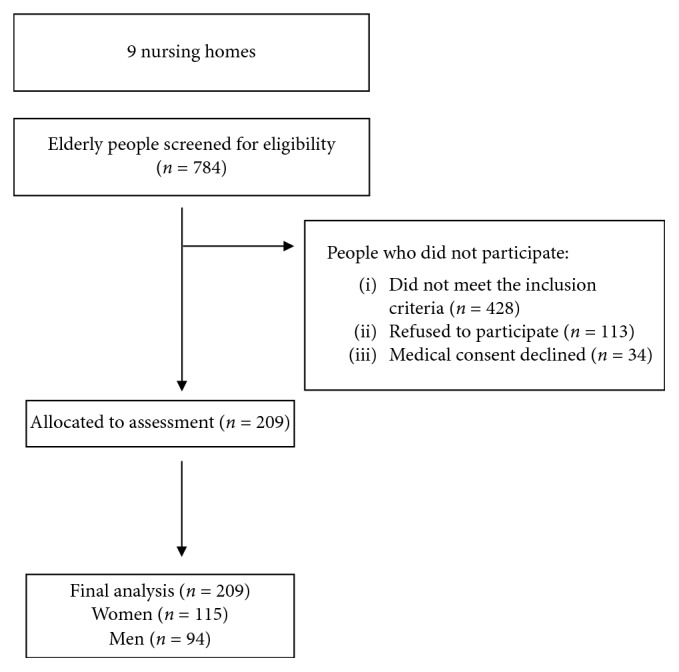
Flow diagram of participants through the study.
2.3. Procedure
The study was conducted by a research team in two stages. On the first day, sociodemographic data were collected and anthropometric measurements were carried out, whereas on the second day, functional tests were carried out.
Data regarding age, sex, education, and marital status were collected on the basis of records kept by care homes and an interview with the researched people. Data considering chronic diseases were collected from medical records kept by doctors in care homes. The diseases were categorized and divided into 4 main groups: cardiovascular, neurological, musculoskeletal, and urinary tract diseases. Information on the number of taken medications was also collected.
Body height measurements were recorded to the nearest centimetre by the use of a stadiometer, and body weight was measured to the nearest kilogram by the use of a digital weigh scale. Body mass index (BMI) was calculated as weight in kg divided by height in meter squared and classified according to World Health Organization categories [19].
The assessment of cognitive abilities was carried out using the MMSE [20] and mood assessment using GDS [21]. The preferred form of spending free time was determined by asking the question of how much time the participant spent in sitting position
2.3.1. Handgrip Strength (HGS)
The assessment was carried out by the use of a hand dynamometer (JAMAR PLUS + Digital Hand Dynamometer, Patterson Medical) calibrated by the manufacturer. The measurement was performed in a sitting position, on a chair without armrests, with the feet of the examined person resting flat on the floor, arms set along the torso, the elbow flexed at 90 degrees, the forearm in a neutral position, and the wrist in 0 degrees to 30 degrees extension following the recommendations of the American Society of Hand Therapists [22]. The subject was instructed to clench the hand maximally and hold for 6 seconds. The procedure was repeated three times for the dominant hand, with a one-minute rest between the tests. The average of three measurements (in kilograms) was recorded. Normal and low grip strength values were established according to the criteria proposed by the EWGSOP [17].
2.3.2. Timed Up and Go (TUG)
Mobility assessment of the subject was made on the basis of a specific sequence of movements: getting up from the chair (height 41 cm with back support), walking a distance of 3 meters, rotating 180 degrees, covering the distance back to the chair, and sitting again [23]. The test was carried out in three attempts. The sample with the shortest time (s) was selected for the assessment.
2.3.3. Timed Up and Go Cognitive (TUG cog)
The test was performed the same way as in the TUG assessment, but while the test was being performed, the cognitive task was added. The older person was asked to subtract constantly the number 3 starting from the number indicated by the tester.
2.3.4. Gait Speed
Assessment of the walking speed was carried out using a 10-meter corridor test. The test assessed the time taken by an elderly person to cover a distance of 10 meters. The test was carried out in two attempts. The first attempt was getting to know the test, and the second (proper) consisted in fast (but safe) gait reaching the destination [24]. Walking speed was calculated by dividing the distance by the time needed to cover the distance (m/s).
2.3.5. Lower Limb Strength
Lower limb strength was assessed by the use of the chair stand test [25]. The elderly people were ordered to stand up from the chair 5 times and sit on it without the help of upper limbs, at the fastest possible pace. The time needed to perform the test (s) was measured.
2.3.6. Upper Body Flexibility
Upper body flexibility was assessed by the use of the back stretch test [26]. The study was carried out in the standing position. The elderly were asked to stretch one hand up and over the shoulder and reach down the back and the other hand behind and reach up the back, with the intention of meeting the hands in the middle of the back, between the shoulder blades. The distance between the tips of the middle fingers of both hands was measured in centimetres: if the fingertips just barely touched, the score was zero; the distance of overlapping fingertips was recorded as a plus (+) score; the distance between the tips of the middle fingers was recorded as a minus (‐) score if they did not touch.
2.3.7. Lower Body Flexibility
Lower body flexibility was assessed by the use of the chair sit and reach test [27]. The participants were asked to sit on the edge of a chair. One leg stayed flat on the floor, and the other leg was extended as straight as possible in front of the hip with the heel placed on the floor and with the ankle flexed at approximately 90 degrees. Participants were asked to stretch out the arms with overlapping hands and slowly bend forward at the hip joint reaching as far forward as possible toward or past the toes. The assistant measured the distance from the middle fingertips to the top of the toes in centimetres: if the fingertips touched the toes, then the score was zero; if they did not touch at this point, the distance was recorded as a minus (‐) score; if they overlapped, the distance was recorded as a plus (+) score.
2.3.8. Postural Stability
Assessment of the postural stability was performed by the use of a two-plate stability platform CQ Stab 2P (CQ Elektronik System, Poland). Each of the platform plates had 3 force sensors that determined the displacement of the centre of pressure on the support plane. During the measurements, the values describing the static balance were recorded. The platform plates were placed parallel, 2 m from the wall of the room where there was a marker for fixing eyesight during the test with open eyes. Each time before the measurements were taken, the device was calibrated. The test consisted of a 30-second sample performed with eyes open and eyes closed. The subjects were instructed to remove shoes and take a free-standing position on the platform plates with their arms set along the trunk [28]. The higher the value of the parameters recorded by the platform, the more the COP displacement was on the support plane [29].
The following parameters were used in the analysis:
SP: total path length measures on the XY axes in mm
SPAP: statokinesiogram path length measured in the Y axis direction in mm
SPML: statokinesiogram path length measured in the X axis direction in mm
MA: mean COP displacement (radius) in mm
MAAP: mean COP displacement from point 0 in the Y axisdirection in mm
MAML: mean COP displacement from point 0 in the X axis direction in mm
MaxAP: maximal COP displacement from point 0 in the Y axis direction in mm
MaxML: maximal COP displacement from point 0 in the X axis direction in mm.
2.4. Ethical Approval and Informed Consent
In accordance with the Declaration of Helsinki, the participants were informed about the aim and the course of the study and gave their informed consent to take part. Due to representative nature of the results obtained in the study, they allowed us to gain knowledge about a large community by examining its representation. The study design was approved by the Bioethical Committee of the University of Rzeszow (No. 6/06/2015).
2.5. Statistical Analysis
The collected data were analysed with the use of TIBCO Software Inc. (2017) Statistica (data analysis software system), version 13. The preliminary analysis used the measurements of descriptive statistics. Pearson's correlation coefficient was calculated in order to assess two-dimensional correlation between examined parameters. Regression with dependent variables was used to determine the relationship between HGS and parameters assessing leg strength, flexibility, and body balance after adjusting for age, sex, and BMI. Statistical significance was set at p < 0.05.
3. Results
The study included 115 women (55.02%) and 94 men (44.98%). The average age of the entire study group was 74.6 years, while the average age of women was significantly higher than men. This is in line with the general population trend in Poland. Most of the participants were widows or widowers (40.67%). Over 60% of people had primary or vocational education. Generally, the study group was dominated by people with normal body weight (39.71%) and obesity (39.23%), with women in majority having normal weight, while men were mostly obese. Over 40% of studied people had normal cognitive status. Most patients did not have depression (66.51%). Cardiovascular and musculoskeletal diseases dominated in the study group. The average number of drugs taken in the study group was on average 4 items. The data on the state of health did not differentiate the researched women and men. The average strength of handgrip in the studied group was 19.8 kg, and for women, it was 14.8 kg and for men 25.9 kg. Over 60% of the researched people were characterized by reduced handgrip strength (including 67.83% women and 52.13% men). There were differences between sexes for age, body mass, height, marital status, handgrip, mobility, gait speed, right upper limb flexibility, and postural balance variables describing the mean and maximal COP displacement in the anteroposterior direction. Characteristics of participants are shown in Table 1.
Table 1.
Characteristics of the studied group.
| Women | Men | Total | p value | |
|---|---|---|---|---|
| Age (years) | 76.2 (7.8) | 72.7 (8.1) | 74.6 (8.1) | 0.002 |
| Body mass (kg) | 65.9 (15.6) | 78.5 (15.0) | 71.6 (16.5) | <0.001 |
| Height (cm) | 158.4 (8.2) | 171.3 (7.6) | 164.2 (10.2) | <0.001 |
| BMI (kg/m2) | 0.275 | |||
| Underweight | 3 (2.61) | 4 (4.26) | 7 (3.35) | |
| Normal body weight | 51 (44.35) | 32 (34.04) | 83 (39.71) | |
| Overweight | 16 (13.91) | 21 (22.34) | 37 (17.71) | |
| Obesity | 45 (39.13) | 37 (39.36) | 82 (39.23) | |
| Marital status | <0.001 | |||
| Married | 3 (2.61) | 17 (18.09) | 20 (9.57) | |
| Widow/widower | 56 (48.70) | 29 (30.85) | 85 (40.67) | |
| Divorced | 16 (13.91) | 17 (18.09) | 33 (15.79) | |
| Single | 40 (34.78) | 31 (32.98) | 71 (33.97) | |
| Education | 0.084 | |||
| Basic | 35 (30.43) | 41 (43.62) | 76 (36.36) | |
| Vocational | 35 (30.43) | 30 (31.91) | 65 (31.10) | |
| Secondary | 42 (36.53) | 20 (21.28) | 62 (29.67) | |
| Higher | 3 (2.61) | 3 (3.19) | 6 (2.87) | |
| Chronic disease | ||||
| Cardiovascular | 100 (89.96) | 81 (86.17) | 181 (86.60) | 0.868 |
| Musculoskeletal | 76 (66.09) | 52 (55.32) | 128 (61.24) | 0.112 |
| Neurological | 25 (21.74) | 19 (20.21) | 44 (21.05) | 0.787 |
| Pulmonary | 61 (53.04) | 43 (45.74) | 104 (49.76) | 0.294 |
| Urinary system | 20 (17.29) | 16 (17.02) | 36 (17.22) | 0.944 |
| GDS | 0.458 | |||
| No depression | 79 (68.70) | 60 (63.83) | 139 (66.51) | |
| Moderate depression | 36 (31.30) | 34 (36.17) | 70 (33.49) | |
| MMSE | 0.710 | |||
| No cognitive impairment | 45 (39.13) | 39 (41.49) | 84 (40.19) | |
| Cognitive impairment without dementia | 34 (29.57) | 23 (24.47) | 57 (27.27) | |
| Mild dementia | 36 (31.30) | 32 (34.04) | 68 (32.54) | |
| Number of drugs | 3.9 (1.7) | 4.1 (1.6) | 4.0 (1.7) | 0.513 |
| Number of falls | 0.8 (1.1) | 0.6 (1.0) | 0.7 (1.0) | 0.180 |
| Strength | ||||
| Handgrip dominant (kg) | 14.8 (6.4) | 25.9 (9.8) | 19.8 (9.8) | <0.001 |
| Cutoff points handgrip strength dominant (kg) | 78 (67.83) | 49 (52.13) | 127 (60.77) | |
| Chair stand (s) | 22.83 (9.83) | 20.36 (8.82) | 21.72 (9.45) | 0.083 |
| Mobility | ||||
| TUG (s) | 20.44 (9.77) | 17.88 (9.17) | 19.28 (9.56) | 0.009 |
| TUG cog (s) | 25.04 (12.28) | 21.44 (10.14) | 23.42 (11.48) | 0.019 |
| Gait speed (m/s) | 0.60 (0.25) | 0.69 (0.27) | 0.64 (0.26) | 0.007 |
| Flexibility | ||||
| Upper limb flexibilityR (cm) | –27.68 (14.10) | –33.30 (14.30) | –30.20 (14.42) | 0.002 |
| Upper limb flexibilityL (cm) | –31.92 (14.96) | –32.27 (14.76) | –32.07 (14.83) | 0.868 |
| Lower limb flexibilityR (cm) | –10.56 (12.87) | –12.51 (14.05) | –11.43 (13.41) | 0.286 |
| Lower limb flexibilityL (cm) | –11.04 (13.23) | –13.53 (13.35) | –12.15 (13.30) | 0.116 |
| Body balance | ||||
| BERG | 34 (13) | 36 (13) | 35 (13) | 0.214 |
| Postural balance (eyes open) | ||||
| SP (mm) | 485.00 (330.78) | 567.48 (498.83) | 522.10 (415.83) | 0.132 |
| SPAP (mm) | 385.27 (293.035) | 455.01 (409.59) | 416.64 (351.11) | 0.083 |
| SPML (mm) | 211.79 (138.64) | 243.06 (251.78) | 225.86 (197.80) | 0.278 |
| MA (mm) | 5.46 (3.14) | 6.29 (4.02) | 5.84 (3.58) | 0.039 |
| MAAP (mm) | 3.82 (1.92) | 4.85 (3.32) | 4.28 (2.69) | 0.004 |
| MAML (mm) | 2.99 (2.72) | 3.00 (2.31) | 3.00 (2.54) | 0.456 |
| MaxAP (mm) | 16.59 (11.08) | 19.69 (15.68) | 17.98 (13.40 | 0.030 |
| MaxML (mm) | 14.59 (17.80) | 14.25 (16.91) | 14.43 (17.36) | 0.816 |
| Postural balance (eyes closed) | ||||
| SP (mm) | 559.85 (525.71) | 558.53 (445.76) | 559.25 (490.16) | 0.904 |
| SPAP (mm) | 474.64 (472.58) | 470.44 (402.63) | 472.75 (441.51) | 0.982 |
| SPML (mm) | 210.88 (172.25) | 210.78 (166.38) | 210.83 (169.22) | 0.418 |
| MA (mm) | 4.77 (2.73) | 5.13 (2.88) | 4.93 (2.79) | 0.403 |
| MAAP (mm) | 3.91 (2.42) | 4.10 (2.34) | 3.99 (2.37) | 0.462 |
| MAML (mm) | 1.97 (1.29) | 2.23 (1.69) | 2.08 (1.48) | 0.470 |
| MaxAP (mm) | 17.37 (12.43) | 17.03 (11.14) | 17.21 (11.84) | 0.872 |
| MaxML (mm) | 7.77 (5.79) | 10.54 (15.13) | 9.01 (11.06) | 0.565 |
N, number; SD, standard deviation; BMI, body mass index; GDS, Geriatric Depression Scale; MMSE, Mini-Mental State Examination; TUG, Timed Up and Go; TUG cog, Timed Up and Go cognitive; SP, total path length; SPAP, statokinesiogram path length; SPML, statokinesiogram path length; MA: mean COP displacement; MAAP, mean COP displacement from point 0 in the Y direction; MAML, mean COP displacement from point 0 in the X direction; MaxAP, maximal COP displacement from point 0 in the Y direction; MaxML, maximal COP displacement from point 0 in the X direction.
Considering received data negative correlation was found between HGS and age, TUG test, both with and without a cognitive task and the lower limb strength. BMI, gait speed, and dynamic balance were positively correlated with HGS. Futhermore, there was no correlation between upper and lower body flexibility with HGS for the dominant hand. A negative correlation was identified between the total path length COP, the length of the COP path in the lateral-medial direction, and the sway area delimited by the COP and HGS for the dominant hand. In addition, among women, gait speed and lower limb flexibility were positively correlated with HGS, whereas lower limb strength and the total path length COP and the length of the COP path in the lateral-medial direction were correlated negatively. Among men, upper right hand flexibility and left lower limb flexibility were positively associated with HGS, while age, lower limb strength, total path length COP, and the length of the COP path in the anterior-posterior and lateral-medial direction were negatively correlated (Table 2).
Table 2.
Correlation between HGS and different variables among older adults by sex.
| Handgrip (kg) | ||||||
|---|---|---|---|---|---|---|
| Women | p value | Men | p value | Total | p value | |
| Age (years) | –0.18 | 0.053 | –0.11 | 0.029 | –0.23 | 0.001 |
| BMI (kg/m2) | 0.15 | 0.102 | 0.14 | 0.165 | 0.16 | 0.022 |
| Mobility | ||||||
| TUG (s) | –0.17 | 0.073 | –0.11 | 0.295 | –0.18 | 0.008 |
| TUG cog (s) | –0.15 | 0.119 | –0.07 | 0.532 | –0.17 | 0.014 |
| Gait speed (m/s) | 0.30 | 0.001 | 0.10 | 0.319 | 0.24 | <0.001 |
| Strength | ||||||
| Chair stand (s) | –0.27 | 0.004 | –0.24 | 0.018 | –0.27 | <0.001 |
| Flexibility | ||||||
| Upper limb flexibilityR (cm) | 0.16 | 0.098 | 0.42 | <0.001 | 0.13 | 0.060 |
| Upper limb flexibilityL (cm) | –0.04 | 0.703 | –0.18 | 0.185 | –0.08 | 0.245 |
| Lower limb flexibilityR (cm) | 0.24 | 0.010 | 0.15 | 0.137 | 0.11 | 0.102 |
| Lower limb flexibilityL (cm) | 0.20 | 0.035 | 0.25 | 0.012 | 0.13 | 0.655 |
| Body balance | ||||||
| BERG | 0.18 | 0.051 | 0.09 | 0.397 | 0.15 | 0.030 |
| Postural balance (eyes open) | ||||||
| SP (mm) | –0.19 | 0.046 | –0.30 | 0.003 | –0.16 | 0.023 |
| SPAP (mm) | –0.16 | 0.080 | –0.27 | 0.010 | –0.13 | 0.058 |
| SPML (mm) | –0.22 | 0.017 | –0.30 | 0.003 | –0.18 | 0.008 |
| MA (mm) | –0.14 | 0.129 | –0.14 | 0.184 | –0.05 | 0.483 |
| MAAP (mm) | –0.10 | 0.282 | –0.12 | 0.237 | 0.01 | 0.831 |
| MAML (mm) | –0.15 | 0.111 | –0.14 | 0.174 | –0.11 | 0.103 |
| MaxAP (mm) | –0.05 | 0.576 | –0.05 | 0.615 | 0.02 | 0.752 |
| MaxML (mm) | –0.12 | 0.213 | –0.13 | 0.207 | –0.11 | 0.128 |
| Postural balance (eyes closed) | ||||||
| SP (mm) | –0.09 | 0.326 | 0.17 | 0.111 | 0.03 | 0.653 |
| SPAP (mm) | –0.09 | 0.334 | 0.18 | 0.089 | 0.04 | 0.616 |
| SPML (mm) | –0.10 | 0.304 | 0.09 | 0.388 | 0.00 | 0.950 |
| MA (mm) | 0.09 | 0.338 | 0.15 | 0.160 | 0.14 | 0.053 |
| MAAP (mm) | 0.13 | 0.167 | 0.15 | 0.146 | 0.14 | 0.051 |
| MAML (mm) | –0.05 | 0.582 | 0.08 | 0.422 | 0.08 | 0.274 |
| MaxAP (mm) | 0.03 | 0.733 | 0.18 | 0.079 | 0.08 | 0.244 |
| MaxML (mm) | 0.13 | 0.154 | 0.14 | 0.170 | 0.16 | 0.026 |
BMI, body mass index; TUG, Timed Up and Go; TUG cog, Timed Up and Go cognitive; SP, total path length; SPAP, statokinesiogram path length; SPML, statokinesiogram path length; MA: mean COP displacement; MAAP, mean COP displacement from point 0 in the Y direction; MAML, mean COP displacement from point 0 in the X direction; MaxAP, maximal COP displacement from point 0 in the Y direction; MaxML, maximal COP displacement from point 0 in the X direction.
After adjusting for age, sex, and BMI and gender interaction, a relationship between handgrip strength and mobility has been demonstrated with and without cognitive task, as well as gait speed, lower limb strength, and dynamic body balance. No effect of gender interaction with HGS has been shown on these dependent variables. Significantly higher values of the parameters of the total COP path length and lateral-medial COP path length under visual control have been found in men than in women. The relationship between HGS and mobility, leg strength, and postural balance after age adjustment, sex, and BMI is shown in Table 3.
Table 3.
Association between HGS and all outcomes after adjusting for age, sex, and BMI with interaction between sex and HGS.
| β | Standard error | p value | |
|---|---|---|---|
| TUG | |||
| HGS | –0.47 | 0.13 | <0.001 |
| Age | 0.28 | 0.08 | <0.001 |
| Sex (M vs W) | –3.12 | 3.26 | 0.34 |
| HGS: sex (M vs W) | 0.2 | 0.16 | 0.203 |
| BMI | –0.03 | 0.12 | 0.825 |
| TUG cog | |||
| HGS | –0.49 | 0.16 | 0.002 |
| Age | 0.30 | 0.09 | 0.002 |
| Sex (M vs W) | –1.36 | 3.97 | 0.732 |
| HGS: sex (M vs W) | 0.14 | 0.19 | 0.451 |
| BMI | –0.15 | 0.14 | 0.304 |
| Gait speed | |||
| HGS | –0.45 | 0.14 | 0.001 |
| Age | 0.29 | 0.08 | 0.001 |
| Sex (M vs W) | –4.92 | 3.47 | 0.158 |
| HGS: sex (M vs W) | 0.25 | 0.17 | 0.139 |
| BMI | –0.09 | 0.12 | 0.482 |
| Chair stand | |||
| HGS | –0.35 | 0.13 | 0.010 |
| Age | 0.26 | 0.08 | 0.002 |
| Sex (M vs W) | –1.68 | 3.38 | 0.619 |
| HGS: sex (M vs W) | 0.15 | 0.16 | 0.343 |
| BMI | –0.06 | 0.12 | 0.640 |
| BERG | |||
| HGS | 0.53 | 0.17 | 0.002 |
| Age | –0.35 | 0.1 | 0.001 |
| Sex (M vs W) | –1.54 | 4.36 | 0.724 |
| HGS: sex (M vs W) | –0.09 | 0.21 | 0.658 |
| BMI | 0.05 | 0.16 | 0.734 |
| SP-EO | |||
| HGS | –7.54 | 5.95 | 0.207 |
| Age | 5.14 | 3.57 | 0.152 |
| Sex (M vs W) | 360.87 | 151.32 | 0.018 |
| HGS: sex (M vs W) | –6.63 | 7.26 | 0.362 |
| BMI | –8.05 | 5.43 | 0.140 |
| SPML-EO | |||
| HGS | –3.66 | 2.79 | 0.191 |
| Age | 1.19 | 1.67 | 0.476 |
| Sex (M vs W) | 168.53 | 70.91 | 0.018 |
| HGS: sex (M vs W) | –3.38 | 3.4 | 0.322 |
| BMI | –7.5 | 2.54 | 0.004 |
| MaxML-EC | |||
| HGS | 0.13 | 0.17 | 0.438 |
| Age | 0.11 | 0.1 | 0.289 |
| Sex (M vs W) | 2.88 | 4.32 | 0.506 |
| HGS: sex (M vs W) | –0.15 | 0.21 | 0.458 |
| BMI | –0.04 | 0.15 | 0.813 |
HGS, hand grip strength; BMI, Body mass index; TUG, Timed Up and Go; TUG cog, Timed Up and Go cognitive; M, men; W, women; SP, total path length; SPML, statokinesiogram path length; MaxML, maximal COP displacement from point 0 in the X direction; EO, eyes open; EC, eyes closed.
4. Discussion
The research showed that regarding women, gait speed was the most strongly associated with HGS, while in men, it was the flexibility of the upper limb, gait speed, strength of the lower limbs, and dynamic body balance with reference to older people living in residential care homes.
HGS is a good indicator determining the risk of disability and mortality. Therefore, there is an increasing interest in its assessment in clinical settings. In our own research, it was shown that the average strength of the handgrip in the study group amounted to 19.8 kg, including 14.8 kg for women and 25.9 kg for men. Al Snih et al. showed that 42% of older women with a handgrip strength of less than 14 kg and 38% men with handgrip strength less than 22 kg died within 5 years [30]. A recent study containing normative data from the FNIH Sarcopenia Project regarding the strength of handgrip and further life course indicated that the limit for the occurrence of the weakness syndrome is HGS for women under 16 kg and for men under 27 kg [31]. The own research showed that 67.83% of women and 52.13% of men had reduced muscle strength. This percentage is much higher than the observations carried out in the older population living in community [32]. The data considering HGS in the general population were carried out in many countries. However, there are no data on the incidence of reduced muscle strength in older people in long-term care facilities [33].
As a result of the analyses, a statistically significant negative correlation between age and HGS was observed in the entire study group. Similar results were obtained by Silva Nde et al. [34]. For each year, over 60 years of age, there is a decrease in the mean handgrip strength by 0.1 kg [33]. A longitudinal study among the Danish older population indicated that men were losing HGS faster than women, but they remained independent in their daily activities for longer [35].
A weak positive correlation between HGS and BMI was observed in the examined group of people. The results obtained in the study suggest that the occurrence of overweight or obesity may be a factor determining greater muscle strength. The results of previous studies were varied. Underweight was associated with low HGS and obesity with a high parameter [6, 36]. Wearing et al. did not show any relations between BMI and HGS [37]. The results discrepancy may be due to the lack of optimal BMI reference values for the older population [38].
The results of our own research presented that the mobility (TUG) with and without the cognitive task, regardless of sex, was negatively correlated with HGS. This means that people with lower handgrip strength were characterized by a longer duration of the task. Porta et al. also showed correlations between TUG and muscle strength [39]. The authors indicted that all TUG parameters were significantly correlated with HGS. Lino et al. also observed that cognitive impairment was significantly associated with weaker handgrip strength [40]. The TUG test reflects physiological changes occurring with age [41]. A slow decrease in reaction time, reduction of nerve conduction velocity, and reduction of sensorimotor responses may lead to balance disorders, postural abnormalities, and then mobility and walking speed restrictions [42].
The measure of gait speed and grip strength is accurate and specific [43]. As a result of the performed research, it was shown that the gait speed was positively correlated with HGS. Muscle strength affects the variability of gait speed [44]. With regards to older people, there is a decrease in gait parameters. A decrease in self-efficiency, balance disorders, and fear of falls influences the phenomenon of a more cautious and slower gait pattern, which transfers to its speed [45].
The results of our own research showed a correlation between dynamic body balance and HGS. Fujita et al. also showed a significantly lower handgrip strength in the case of greater balance disorders [46]. In order to maintain vertical balance, the centre of body mass should remain within the limits of the quadrilateral support defined by the foot contour. Moving the COP beyond the limits of foot support causes loss of balance, which is interfaced with the motor response [47]. A decrease in muscle strength, delay in muscle activation, and slower reaction time affect greater balance disorders [48]. Body balance is considered to be a crucial element of many everyday activities, starting from maintaining a calm position to more complex activities, such as walking during a conversation or a change of the walking direction [49]. Detection of existing balance disorders is important for preventing falls and planning improvement strategies.
The research showed that HGS correlated with the flexibility of the lower limbs in women and the right upper and lower left limbs in men. Muscle flexibility is important because its limitations have a large impact on the movement performance [50]. Silva Nde et al. obtained different results and pointed out that the reasons for the lack of a linear correlation between flexibility and muscular strength in older people are not clear and recommend conducting further analyses [34].
Our own research indicated the existence of a strong relationship between HGS and the strength of the lower limbs in both genders. The regression result shows that the strength of the lower limbs depends on HGS and age. Fragala et al. also observed a significant correlation between grip strength and leg extension strength [51]. HGS correlates with the knee extension strength of both the ipsilateral and contralateral sides, which favours the use of handgrip strength as a measure of global strength [52, 53].
The measurement of screening test to the needs of clinical practice can be a measure of handgrip strength with the help of a dynamometer. The implementation requires minimal staff training, the duration of the test is about 2 minutes, and the cost of measuring equipment is affordable for most medical facilities. Lower HGS was significantly associated with weaker muscular strength of the lower limbs, gait speed, and balance, and thus, it is a predictive measure to determine the general functional status of an older person. Quick identification of people with weakness syndrome gives the opportunity to optimize health management and implement appropriate rehabilitation exercises.
Our research has some limitations. First of all, no data considering the diet of older people were collected as well as body mass composition was not assessed, which could be a better indicator than BMI. Secondly, due to cross-sectional data, the study does not permit considerations on causality.
5. Conclusion
Low grip strength was found in 67.83% women and 52.13% men in institutional care. HGS, regardless of gender, is associated with mobility, strength of the lower limbs, and dynamic balance. HGS assessment can be a simple, fast, and inexpensive way to assess the prevalence of mobility limitations and functional performance. Early diagnosis will facilitate the planning and application of appropriate interventions in order to prevent disability and mortality in long-term care facilities.
Data Availability
The datasets used and analysed in the current study are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare that they have no conflicts of interest.
References
- 1.Roubenoff R., Castaneda C. Sarcopenia-understanding the dynamics of aging muscle. JAMA. 2001;286(10):1230–1231. doi: 10.1001/jama.286.10.1230. [DOI] [PubMed] [Google Scholar]
- 2.Cheung C.-L., Nguyen U.-S, Au E., Tan K. C., Kung A. W. Association of handgrip strength with chronic diseases and multimorbidity. AGE. 2013;35(3):929–941. doi: 10.1007/s11357-012-9385-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Keller K., Engelhardt M. Strength and muscle mass loss with aging process. Age and strength loss. Muscle Ligaments and Tendons Journal. 2019;3(4):346–350. doi: 10.32098/mltj.04.2013.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roberts H. C., Syddall H. E., Sparkes J., et al. Grip strength and its determinants among older people in different healthcare settings. Age and Ageing. 2014;43(2):241–246. doi: 10.1093/ageing/aft118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vancampfort D., Stubbs B., Firth J., Koyanagi A. Handgrip strength, chronic physical conditions and physical multimorbidity in middle-aged and older adults in six low- and middle income countries. European Journal of Internal Medicine. 2019;61:96–102. doi: 10.1016/j.ejim.2018.11.007. [DOI] [PubMed] [Google Scholar]
- 6.Forrest K. Y. Z., Williams A. M., Leeds M. J., Robare J. F., Bechard T. J. Patterns and correlates of grip strength in older Americans. Current Aging Science. 2018;11(1):63–70. doi: 10.2174/1874609810666171116164000. [DOI] [PubMed] [Google Scholar]
- 7.Vancampfort D., Stubbs B., Firth J., Smith L., Swinnen N., Koyanagi A. Associations between handgrip strength and mild cognitive impairment in middle-aged and older adults in six low- and middle-income countries. International Journal of Geriatric Psychiatry. 2019;34(4):609–616. doi: 10.1002/gps.5061. [DOI] [PubMed] [Google Scholar]
- 8.Lee M. R., Jung S. M., Bang H., Kim H. S., Kim Y. B. The association between muscular strength and depression in Korean adults: a cross-sectional analysis of the sixth Korea National Health and Nutrition Examination Survey (KNHANES VI) 2014. BMC Public Health. 2018;18(1):p. 1123. doi: 10.1186/s12889-018-6030-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pengpid S., Peltzer K. Hand grip strength and its sociodemographic and health correlates among older adult men and women (50 years and older) in Indonesia. Current Gerontology and Geriatrics Research. 2018;2018:8. doi: 10.1155/2018/3265041.3265041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barrea L., Muscogiuri G., Di Somma C., et al. Association between Mediterranean diet and hand grip strength in older adult women. Clinical Nutrition. 2019;38(2):721–729. doi: 10.1016/j.clnu.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 11.McLeod M., Breen L., Hamilton D. L., Philp A. Live strong and prosper: the importance of skeletal muscle strength for healthy ageing. Biogerontology. 2016;17(3):497–510. doi: 10.1007/s10522-015-9631-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mclean R. R., Shardell M. D., Alley D. E., et al. Criteria for clinically relevant weakness and low lean mass and their longitudinal association with incident mobility impairment and mortality: the foundation for the National Institutes of Health (FNIH) Sarcopenia Project. The Journals of Gerontology: Series A. 2014;69(5):576–583. doi: 10.1093/gerona/glu012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szanton S. L., Roth J., Nkimbeng M., Savage J., Klimmek R. Improving unsafe environments to support aging independence with limited resources. Nursing Clinics of North America. 2014;49(2):133–145. doi: 10.1016/j.cnur.2014.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stevens P. J., Syddall H. E., Patel H. P., Martin H. J., Cooper C., Aihie Sayer A. Is grip strength a good marker of physical performance among community-dwelling older people? The Journal of Nutrition, Health & Aging. 2012;16(9):769–774. doi: 10.1007/s12603-012-0388-2. [DOI] [PubMed] [Google Scholar]
- 15.Sayer A. A., Kirkwood T. B. L. Grip strength and mortality: a biomarker of ageing? The Lancet. 2015;386(9990):226–227. doi: 10.1016/s0140-6736(14)62349-7. [DOI] [PubMed] [Google Scholar]
- 16.Clark B. C., Manini T. M. Functional consequences of sarcopenia and dynapenia in the elderly. Current Opinion in Clinical Nutrition and Metabolic Care. 2010;13(3):271–276. doi: 10.1097/mco.0b013e328337819e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cruz-Jentoft A. J., Bahat G., Bauer J., et al. Sarcopenia: revised European consensus on definition and diagnosis. Age and Ageing. 2019;48(1):16–31. doi: 10.1093/ageing/afy169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leong D. P., Teo K. K., Rangarajan S., et al. Reference ranges of handgrip strength from 125,462 healthy adults in 21 countries: a prospective urban rural epidemiologic (PURE) study. Journal of Cachexia, Sarcopenia and Muscle. 2016;7(5):535–546. doi: 10.1002/jcsm.12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.World Health Organization. Geneva, Switzerland: World Health Organization; Global database on body mass index (BMI) 2015, https://www.who.int/nutrition/databases/bmi/en/ [Google Scholar]
- 20.Fryderyk-Łukasik M. Comprehensive geriatric assessment in everyday geriatric and caring practice. Geriatria i Opieka Długoterminowa. 2015;1(1):1–6. [Google Scholar]
- 21.Albiński R., Kleszczewska-Albińska A., Bedyńska S. Geriatric Depression Scale (GDS). Validity and reliability of different versions of the scale-review. Psychiatria Polska. 2011;45(4):555–562. [PubMed] [Google Scholar]
- 22.Trampisch U. S., Franke J., Jedamzik N., Hinrichs T., Platen P. Optimal Jamar dynamometer handle position to assess maximal isometric hand grip strength in epidemiological studies. The Journal of Hand Surgery. 2012;37(11):2368–2373. doi: 10.1016/j.jhsa.2012.08.014. [DOI] [PubMed] [Google Scholar]
- 23.Giladi N., Herman T., Reider-Groswasser I. I., Gurevich T., Hausdorff J. M. Clinical characteristics of elderly patients with a cautious gait of unknown origin. Journal of Neurology. 2005;252(3):300–306. doi: 10.1007/s00415-005-0641-2. [DOI] [PubMed] [Google Scholar]
- 24.Wolf S. L., Catlin P. A., Gage K., Gurucharri K., Robertson R., Stephen K. Establishing the reliability and validity of measurements of walking time using the emory functional ambulation profile. Physical Therapy. 1999;79(12):1122–1133. [PubMed] [Google Scholar]
- 25.Schaubert K. L., Bohannon R. W. Reliability and validity of three strength measures obtained from community-dwelling elderly persons. Journal of Strength and Conditioning Research. 2005;19(3):717–720. doi: 10.1519/00124278-200508000-00038. [DOI] [PubMed] [Google Scholar]
- 26.Konopack J. F., Marquez D. X., Hu L., Elavsky S., McAuley E., Kramer A. F. Correlates of functional fitness in older adults. International Journal of Behavioral Medicine. 2008;15(4):311–318. doi: 10.1080/10705500802365557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Belza B., Shumway-Cook A., Phelan E., Williams B., Snyder S. J., LoGerfo J. P. The effects of a community-based exercise program on function and health in older adults: the Enhance Fitness Program. Journal of Applied Gerontology. 2006;25(4):291–306. doi: 10.1177/0733464806290934. [DOI] [Google Scholar]
- 28.Rachwał M., Drzał-Grabiec J., Walicka-Cuprys K., Truszczyńska A. Quantitative analysis of static equilibrium in women after mastectomy. International Journal on Disability and Human Development. 2015;14(1):81–87. [Google Scholar]
- 29.Wyszomirska I., Kaczmarczyk K., Zdrodowska A., Błażkiewicz M., Ilnicka L., Marciniak T. Evaluation of static and dynamic postural stability in young, elderly and with vision loss women. Advances in Rehabilitation. 2013;3:33–39. doi: 10.2478/rehab-2014-0019. [DOI] [Google Scholar]
- 30.Al Snih S., Markides K. S., Ray L., Ostir G. V., Goodwin J. S. Handgrip strength and mortality in older Mexican Americans. Journal of the American Geriatrics Society. 2002;50(7):1250–1256. doi: 10.1046/j.1532-5415.2002.50312.x. [DOI] [PubMed] [Google Scholar]
- 31.Alley D. E., Shardell M. D., Peters K. W., et al. Grip strength cutpoints for the identification of clinically relevant weakness. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2014;69(5):559–566. doi: 10.1093/gerona/glu011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dodds R. M., Syddall H. E., Cooper R., et al. Grip strength across the life course: normative data from twelve British studies. PLoS One. 2014;9(12) doi: 10.1371/journal.pone.0113637.e113637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dodds R. M., Syddall H. E., Cooper R., Kuh D., Cooper C., Sayer A. A. Global variation in grip strength: a systematic review and meta-analysis of normative data. Age and Ageing. 2016;45(2):209–216. doi: 10.1093/ageing/afv192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silva Nde A., Menezes T. N., Melo R. L., Pedraza D. F. Handgrip strength and flexibility and their association with anthropometric variables in the elderly. Revista da Associação Médica Brasileira. 2013;59(2):128–135. doi: 10.1016/s2255-4823(13)70445-4. [DOI] [PubMed] [Google Scholar]
- 35.Oksuzyan A., Maier H., McGue M., Vaupel J. W., Christensen K. Sex differences in the level and rate of change of physical function and grip strength in the Danish 1905-cohort study. Journal of Aging and Health. 2010;22(5):589–610. doi: 10.1177/0898264310366752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Su L. Q., Yin Z. X., Wang X. C., et al. Study on handgrip strength of elderly ≥60 years old from longevity areas in China. Zhonghua Yu Fang Yi Xue Za Zhi. 2017;51(11):1007–1011. doi: 10.3760/cma.j.issn.0253-9624.2017.11.010. [DOI] [PubMed] [Google Scholar]
- 37.Wearing J., Konings P., Stokes M., de Bruin E. D. Handgrip strength in old and oldest old Swiss adults—a cross-sectional study. BMC Geriatrics. 2018;18(1):p. 266. doi: 10.1186/s12877-018-0959-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cederholm T., Bosaeus I., Barazzoni R., et al. Diagnostic criteria for malnutrition—an ESPEN consensus statement. Clinical Nutrition. 2015;34(3):335–340. doi: 10.1016/j.clnu.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 39.Porta M., Pilloni G., Corona F., et al. Relationships between objectively assessed functional mobility and handgrip strength in healthy older adults. European Geriatric Medicine. 2018;9(2):201–209. doi: 10.1007/s41999-018-0025-7. [DOI] [PubMed] [Google Scholar]
- 40.Lino V. T., Rodrigues N. C., O’Dwyer G., Andrade M. K., Mattos I. E., Portela M. C. Handgrip strength and factors associated in poor elderly assisted at a primary care unit in rio de Janeiro, Brazil. PLoS One. 2016;11(11) doi: 10.1371/journal.pone.0166373.e0166373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bergland A., Jørgensen L., Emaus N., Strand B. H. Mobility as a predictor of all-cause mortality in older men and women: 11.8 year follow-up in the Tromsø study. BMC Health Services Research. 2017;17(1) doi: 10.1186/s12913-016-1950-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khant N., Dani V. B., Patel P., Rathod R. Establishing the reference value for “timed up-and-go” test in healthy adults of Gujarat, India. Journal of Education and Health Promotion. 2018;7(1):p. 62. doi: 10.4103/jehp.jehp_12_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee L., Patel T., Costa A., et al. Screening for frailty in primary care: accuracy of gait speed and hand-grip strength. Canadian Family Physician Médecin de Famille Canadien. 2017;63(1):e51–e57. [PMC free article] [PubMed] [Google Scholar]
- 44.Purser J. L., Pieper C. F., Poole C., Morey M. Trajectories of leg strength and gait speed among sedentary older adults: longitudinal pattern of dose response. The Journals of Gerontology Series A: Biological Sciences and Medical Sciences. 2003;58(12):M1125–M1134. doi: 10.1093/gerona/58.12.m1125. [DOI] [PubMed] [Google Scholar]
- 45.Wijlhuizen G. J., Chorus A. M. J., Hopman-Rock M. Fragility, fear of falling, physical activity and falls among older persons: some theoretical considerations to interpret mediation. Preventive Medicine. 2008;46(6):612–614. doi: 10.1016/j.ypmed.2008.01.016. [DOI] [PubMed] [Google Scholar]
- 46.Fujita K., Kaburagi H., Nimura A., et al. Lower grip strength and dynamic body balance in women with distal radial fractures. Osteoporosis International. 2019;30(5):949–956. doi: 10.1007/s00198-018-04816-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Rath R., Wade M. G. The two faces of postural control in older adults: stability and function. EBioMedicine. 2017;21:5–6. doi: 10.1016/j.ebiom.2017.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Martinikorena I., Martínez-Ramírez A., Gómez M., et al. Gait variability related to muscle quality and muscle power output in frail nonagenarian older adults. Journal of the American Medical Directors Association. 2016;17(2):162–167. doi: 10.1016/j.jamda.2015.09.015. [DOI] [PubMed] [Google Scholar]
- 49.Karimi M. T., Solomonidis S. The relationship between parameters of static and dynamic stability tests. Journal of Research in Medical Sciences. 2011;16(4):530–535. [PMC free article] [PubMed] [Google Scholar]
- 50.Cristopoliski F., Sarraf T. A., Dezan V. H., Provensi C. L. G., Rodacki A. L. F. Efeito transiente de exercícios de flexibilidade na articulação do quadril sobre a marcha de idosas. Revista Brasileira de Medicina do Esporte. 2008;14(2):139–144. doi: 10.1590/s1517-86922008000200011. [DOI] [Google Scholar]
- 51.Fragala M. S., Alley D. E., Shardell M. D., et al. Comparison of handgrip and leg extension strength in predicting slow gait speed in older adults. Journal of the American Geriatrics Society. 2016;64(1):144–150. doi: 10.1111/jgs.13871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alonso A. C., Ribeiro S. M., Luna N. M. S., et al. Association between handgrip strength, balance, and knee flexion/extension strength in older adults. PLoS One. 2018;13(6) doi: 10.1371/journal.pone.0198185.e0198185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bohannon R. W., Magasi S. R., Bubela D. J., Wang Y.-C., Gershon R. C. Grip and knee extension muscle strength reflect a common construct among adults. Muscle & Nerve. 2012;46(4):555–558. doi: 10.1002/mus.23350. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and analysed in the current study are available from the corresponding author on reasonable request.


