Abstract
Background
Outcomes of younger acute myeloid leukaemia patients have moderately improved. Blocking PD-1/PD-L1 pathways enhances anti-leukaemia responses by enabling T-cells in murine models. We hypothesized that adding nivolumab to frontline therapy may decrease the risk of relapse.
Methods
Patients aged 18–60 years with newly diagnosed acute myeloid leukaemia (or >60 years if suitable for intensive chemotherapy) were enrolled. Induction included cytarabine 1·5 g/m2 by 24-hours continuous infusion daily for four days (three days in patients >60 years) and idarubicin 12 mg/m2 daily for three days. Nivolumab 3 mg/kg was started on day 24±2 and continued every two weeks for up to a year in responders. Responders received either up to five consolidation cycles of attenuated doses of idarubicin and cytarabine, or allogeneic stem cell transplantation if eligible. The primary endpoint was event-free survival per protocol. This ongoing trial was registered with ClinicalTrials.gov ().
Findings
Forty-four patients were enrolled of whom 22 (50%) had adverse genetic risk by European Leukaemia Network classification. All patients were evaluable for safety and efficacy. At a median follow-up of 17.25 months, median event-free survival was not reached. The median relapse-free survival of responders was 18·54 months (IQR, 1·7–25·6). Early mortality was 5%. Six patients had seven grade 3/4 immune-related events with rash (n=2), colitis (n=2), transaminitis, pancreatitis and cholecystitis (n=1; each). Nineteen responders of the 44 patients (43%) proceeded to allogeneic stem cell transplantation with grade 3/4 graft-versus-host disease seen in 5. Correlative studies conducted on baseline bone marrow specimens showed that non-responders had significantly higher percentage of CD4+ T-effectors co-expressing PD-1/TIM-3 (p=0·01) and PD-1/LAG-3 (p=0·04) compared to responders.
Interpretation
Addition of nivolumab to induction chemotherapy is feasible. Post-transplant severe graft-versus-host disease is not prohibitive. Studies with earlier initiation of checkpoint inhibitor therapy are planned.
Keywords: AML, induction, idarubicin, cytarabine, nivolumab, T-cells, immune exhaustion
INTRODUCTION
The combination of an anthracycline and cytarabine has remained, for more than four decades, the standard induction regimen for the treatment of patients with newly diagnosed acute myeloid leukaemia (AML).1 This strategy produces remission rates approaching 80% and a cure rate of only 30–50% in younger patients.2,3 Various manipulations and refinements of this combination have been evaluated, including different cytarabine and anthracycline doses and schedules, and incorporation of a third investigational agent. Unfortunately, these efforts have in general produced limited effects on the response rates and overall survival.4–8
Based on early studies by Plunkett and Gandhi demonstrating the maximal effect of cytarabine at 1–2 g/m2, and an improved survival in younger patients with intensification of induction chemotherapy using higher doses of anthracyclines or cytarabine,9,10 we have adopted a combination of high-dose cytarabine at 1·5 g/m2 daily for four days and idarubicin at a dose of 12 mg/m2 daily for three days as the backbone of our induction regimen in younger patients with AML.
Prior preclinical studies have demonstrated that cytarabine injection suppresses the expression of the T-cell inhibitory receptor programmed death-1 (PD-1) on the surface of myeloblasts, subjecting them to enhanced killing by cytotoxic T-lymphocytes (CTLs).11 Furthermore, the release of antigens and potentially neoantigens following cytotoxic chemotherapy may help to prime these CTLs and boost antitumor efficacy further. In parallel, blocking PD-1 and its ligand-1 (PD-L1) pathway augments cytotoxic anti-leukaemia responses to conventional chemotherapy.12 This response was attributed to enhanced CD8+ T-cell activity. PD-1+ CD8+ T-cells were reported to be increased in the bone marrow of AML patients compared to healthy individuals,13 prompting the evaluation of this pathway in AML. As a single agent, PD-1 inhibition has shown limited activity in a pilot study of patients with various advanced hematological malignancies including AML.14 We therefore hypothesized that the addition of nivolumab to idarubicin and cytarabine may improve outcomes by decreasing risk of relapse. We designed a phase II study to evaluate the feasibility, safety and efficacy of this combination in patients with newly diagnosed AML and high-risk myelodysplastic syndrome (MDS).
METHODS
Study design and participants
This was an open-label, single-arm, phase II part of the phase I/II study of nivolumab in combination with idarubicin and cytarabine conducted at the University of Texas MD Anderson Cancer Centre. Patients aged 18–60 years with treatment-naïve de-novo or secondary AML (excluding acute promyelocytic and core-binding factor leukaemias) per WHO criteria or high-risk MDS were enrolled.15 Patients older than 60 years who are deemed fit to receive intensive chemotherapy were also eligible. High-risk MDS was defined as Intermediate-2 or high-risk by International Prognostic Scoring System16 or having >10% blasts in the bone marrow. Other eligibility criteria included Eastern cooperative Oncology Group performance status of 0–2, normal cardiac ejection fraction (≥50%) and adequate renal (creatinine <1·5 mg/dl) and hepatic (total bilirubin <1·5 mg/dL, unless due to hemolysis and transaminases <2·5x ULN) functions. Exclusion criteria included prior therapy for AML other than temporary measures such as apheresis or hydroxyurea or one dose of cytarabine ≤ 2 grams for control of hyperleukocytosis prior to enrollment. Prior therapy for MDS was allowed. Patients with active, known or suspected autoimmune disease were excluded. Pregnant or lactating women and patients with any uncontrolled clinically significant illness or infection were also excluded. Infection prophylaxis was administered per institutional standards. The trial was approved by the Institutional Review Board and all patients provided written informed consent before enrollment according to institutional policies and the declaration of Helsinki. The trial was registered in ClinicalTrials.gov, number .
Enrolled patients were followed with complete blood counts and chemistries at least twice weekly during remission induction, then every 1–4 weeks during maintenance therapy. Bone marrow examination to assess response was done on day 21–28 of cycle 1, then aspirate every 1–2 weeks as required until CR, after that every 3–6 months as indicated at the discretion of the treating physician. Per protocol, minimal residual disease (MRD) studies by flow cytometry were performed on pre-treatment bone marrow and all subsequent bone marrow exams.
Bone marrow specimens were obtained at diagnosis and evaluated for presence of cytogenetic and molecular aberrations. For documentation of mutations, the entire coding sequences of 81 genes known to be frequently mutated in myeloid hematologic malignancies including ABL1, ASXL1, BRAF, DNMT3A, EGFR, EZH2, FLT3, GATA1, GATA2, HRAS, IDH1, IDH2, KIT, KRAS, MDM2, IKZF2, JAK2, MLL, MPL, MYD88, NOTCH1, NPM1, NRAS, PTPN11, RUNX1, TET2, TP53, and WT1 were sequenced using the Illumina MiSeq platform as previously described.17
Treatment plan
Induction therapy consisted of cytarabine at 1·5 g/m2 given over 24 hours daily on days 1–4 (days 1–3 for patients older than 60 years) and idarubicin at 12 mg/m2 daily on days 1–3. Nivolumab 3 mg/kg was started on day 24±2 of chemotherapy and continued at approximately 2-weeks interval for up to a year in patients achieving complete response (CR), CR with incomplete count recovery (CRi) or platelets recovery (CRp). Due to limited clinical experience with the administration of nivolumab with chemotherapy, we first performed a run-in phase in patients with relapsed disease in which nivolumab was administered at 1mg/kg with IA. As no specific toxicity was observed with the first three patients, nivolumab was then administered at a dose of 3 mg/kg. Patients who did not achieve CR/CRi/CRp following the first induction cycle could receive a second course at same doses. Responding patients received up to 5 consolidation cycles of attenuated doses of idarubicin (8 mg/m2 daily for two days) and cytarabine (0·75 g/m2 over 24 hours daily for three days) in 4–6 weeks cycles, depending on response, counts recovery and resolution of drug-related toxicities. Dose modifications and delays were allowed for persistent cytopenias or non-hematological toxicities. Eligible responders were also offered allogeneic stem cell transplant (allo-SCT) at any time during or after consolidation based on the availability of a suitable donor and at the discretion of the treating physician. Therapy with nivolumab was discontinued at least one week prior to the procedure. Solumedrol 50 mg or equivalent were used with cytarabine to prevent fever and rash. All patients aged ≥50 years were admitted to a laminar air flow room for an average of 28 days of induction therapy.
Outcomes
The primary endpoint was event-free survival (EFS) with events defined as treatment failure, relapse or death. Responses were categorized based on the revised International Working Group criteria for AML18. MRD was assessed by multi-parameter flow cytometry (MFC) using an 8-color panel on BM samples at the time of CR/CRi/CRp as previously described, with assay sensitivity of 0·01%.19 The presence of MRD was defined as a cluster of ≥20 cells with altered expression of ≥2 antigens. Treatment failure was defined as not achieving CR/CRi/CRp after two cycles of therapy. Secondary endpoints included relapse-free survival (RFS) and overall survival.
The trial was overseen by the MD Anderson investigational new drugs office. Adverse events were classified according to the common terminology criteria for adverse events (CTCAE) version 4·03.
Statistical Considerations
Summary statistics were used to describe the continuous variables of the study population. Categorical endpoints were summarized using frequencies and percentages. Fisher’s exact and Wilcoxon rank tests were used in univariate analyses of categorical and continuous variables, respectively. The primary objective of this study was to assess EFS, calculated from the time of starting therapy to off-study date for treatment failure, relapse or death; patients without an event at their last follow-up were censored on that date. Using the Bayesian sequential monitoring method of Thall, Simon and Estey,20 we assumed that EFS followed an exponential distribution and the trial would stop if the median EFS was less than seven months at the time of analysis. The operating characteristics of this rule were performed for a maximum recruited number of 75 patients, computed assuming a 5-patient per month accrual rate. The trial would also stop if clinically significant non-haematological toxicity attributable to nivolumab was greater than 10% at 12 months. Overall survival was calculated from the start of treatment to death or date of last follow-up; patients who were alive at their last follow-up were censored on that date. RFS estimated the time from treatment response to date of relapse or death, whichever occurred first. EFS, overall survival, RFS were estimated using Kaplan-Meier method and were not censored for allo-SCT in the primary analysis. Survival estimates were compared with the log-rank test. The analysis of all secondary endpoints was descriptive. All subgroup analyses by baseline characteristics were exploratory. Statistical analysis was performed using Stata/SE version 14·1 (Stata Corp. LP, College Station, TX) and GraphPad Prism 8 (GraphPad Software, Inc., La Jolla, CA).
Correlative Studies
As specified in the protocol, MFC studies were conducted on baseline BM and peripheral blood samples to assess T-cell repertoire, including CD4+ and CD8+ T-cells and expression of costimulatory and inhibitory receptors and ligands on T-cells and leukaemic blasts, respectively.
Role of funding source
This study was funded by BMS pharmaceuticals, the MD Anderson Cancer Centre Support Grant CA016672, the MD Anderson Cancer Centre Leukaemia SPORE CA100632 from the National Cancer Institute and the Charif Souki Cancer Research Fund. The funding agencies had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to study data and the final responsibility to submit for publication.
RESULTS
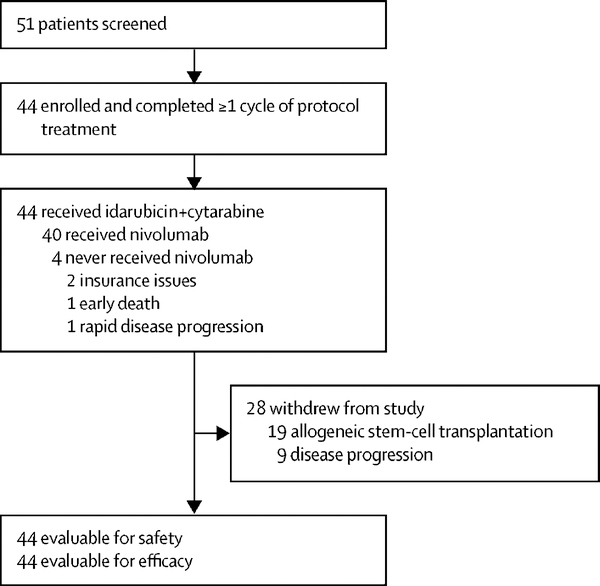
Between August 7, 2015 and June 2, 2018, 44 patients were enrolled (Figure 1). Three patients with relapsed AML were initially treated at a run-in phase with IA induction as above in combination with nivolumab 1 mg/kg without specific drug-related toxicity. Subsequently, 44 previously untreated patients received chemotherapy and nivolumab 3 mg/kg as above. Patients’ baseline characteristics are summarized in Table 1. Their median age was 54 years (IQR, 26–66), and 10/44 (23%) were older than 60 years. Forty-two of 44 (96%) patients had AML (32 de novo, 7 secondary and 3 therapy-related) and 2/44 (4%) had high-risk MDS. According to European Leukaemia Network (ELN) criteria, the genetic risk was favorable in 5/44 (11%) patients, intermediate in 17/44 (39%) and adverse in 22/44 (50%). Targeted gene sequencing was performed on all 44 patients. The median number of mutations per patient was 2 (IQR, 0–5) and 22/44 (50%) of patients had more than one mutation. The most common mutations were RAS (10/44; 23%), IDH2 (8/44; 18%), TP53 (8/44; 18%), DNMT3A (7/44; 16%), and NPM1 (6/44; 14%).
Figure 1:
Trial design
Table 1:
Baseline patients’ characteristics
| Characteristics (N = 44) | N (%); Median [IQR] |
|---|---|
| Age (years) | 54 [26 – 66] |
| >60 years | 9 (20) |
| Female | 27 (61) |
| WBC (× 109/L) | 4.8 [0.4 – 46.1] |
| Platelets (× 109/L) | 39 [6 – 308] |
| Hemoglobin (g/dL) | 9.6 [5.9 – 14.1] |
| Creatinine (mg/dL) | 0.8 [0.51 – 1.31] |
| Bilirubin | 0.7 [0.2 – 2.5] |
| Bone marrow Blast (%) | 42 [15 – 96] |
| AML/MDS | |
| De novo AML | 32 (73) |
| AML from antecedent hematological disorder | 7 (16) |
| Therapy-related AML | 3 (7) |
| High-risk MDS (≥ 10% blasts) | 2 (4) |
| Prior therapy for MDS | |
| None | 38 (86) |
| Hypomethylating agents | 6 (14) |
| Cytogenetics | |
| Diploid | 10 (23) |
| Other intermediate | 13 (30) |
| −7/7q−/−5/complex | 16 (36) |
| Insufficient | 5 (11) |
| ELN | |
| Favorable | 5 (11) |
| Intermediate | 17 (39) |
| Adverse | 22 (50) |
| Mutations | |
| ASXL1 | 3 (7) |
| DNMT3A | 7 (16) |
| FLT3-ITD | 4 (9) |
| FLT3-D835 | 3 (7) |
| IDH1 | 2 (5) |
| IDH2 | 8 (18) |
| KRAS/NRAS | 10 (23) |
| NPM1 | 6 (14) |
| RUNX1 | 5 (11) |
| TET2 | 5 (11) |
| TP53 | 8 (18) |
Data are expressed as n (%) or median [IQR]. WBC: white blood cell count; AML: acute myeloid leukaemia; MDS: myelodysplastic syndrome; ELN: European Leukaemia Network.
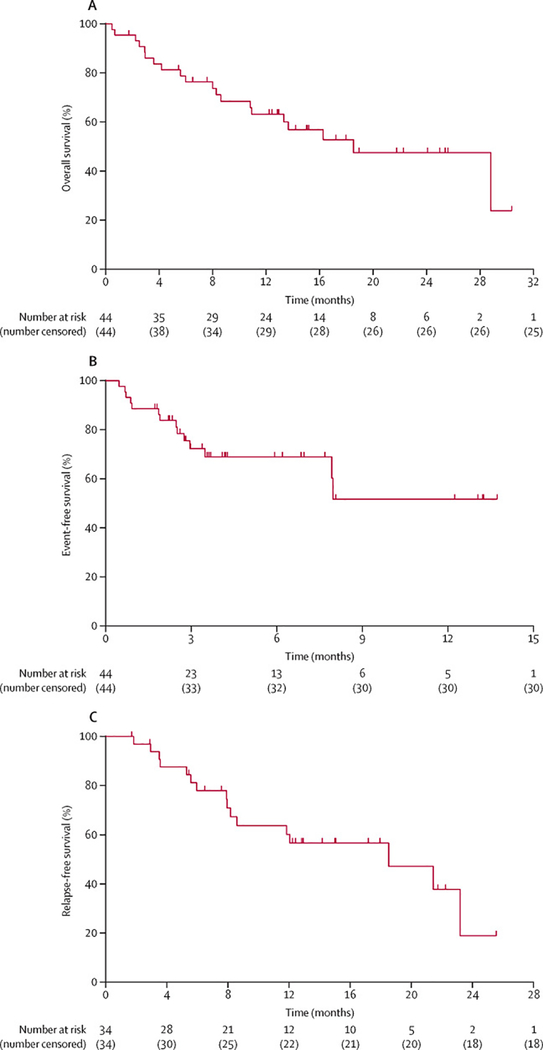
At a median follow-up of 17·25 months (IQR, 0·5–30·4), 24/44 (55%) patients remain alive. The median overall survival was 18·54 months (95% CI [10.81–28.81]) (Figure 2A) while the median EFS was not reached (Figure 2B). The 34 patients who achieved CR/CRi/CRp had a median RFS of 18·5 months (95% CI [8.20–23.22]) (Figure 2C) with a total of six patients dying in CR.
Figure 2:
Kaplan-Meier curves showing (A) overall survival (OS), (B) event-free survival (EFS) and (C) relapse-free survival (RFS) rates of the patients treated with IA and nivolumab.
The 19 patients who received allo-SCT had a median overall survival of 25 months (IQR 1–26) while the EFS had not been reached (Figure 1, appendix p2). Compared to responding patients who continued idarubicin+cytarabine and nivolumab beyond remission (n=16), those who underwent allo-SCT (n=19) had similar overall survival (p = 0·534, HR=0.70, 95% CI [0.22 – 2.23]) and EFS (p=0·459, HR= 0.657; 95% CI [0.19 – 2.21]) and tended to have worse RFS (p=0·184; HR=0.519; 95% CI [0.19 – 1.41]). (Figure 2, appendix pp 3–5).
All patients were evaluable for response. The median duration of treatment was 3·2 months (IQR, 0·5–13·7). The proportion of patients who achieved a response (CR+CRi+CRp) was 34/44 (78%) including 28/44 CR (64%), 5/44 CRp (12%) and 1/44 CRi (2%). Furthermore, one patient responded with partial remission (PR), for an ORR of 80%. Of the 34 patients with CR/CRi/CRp, 18/34 (53%) achieved undetectable MRD by MFC following induction, and of the remaining 16 patients who had either residual or indeterminate MRD at time of response, 9/16 (56%) converted to MRD-negative status after an additional 1–3 months of follow-up, during which time they received nivolumab. Eight of 44 patients (18%) received two induction cycles and four responded (three with CR and one with CRp).
The median number of total cycles received was 2 (IQR, 1–6). Counting maintenance schedule, the median number of nivolumab doses received was 2 (IQR, 0–25) with 15/44 patients (34%) receiving ≥4 doses. Four patients, however, did not receive nivolumab because of insurance issues (n=2), early death (n=1) and rapid disease progression (n=1). Responders completed a median of 3 cycles of idarubicin+cytarabine+nivolumab therapy (IQR, 1–6) with a median duration of response of 12 months (IQR, 1·7–30·4). Of the 22 patients with adverse ELN, 16 (73%) achieved CR+CRi+CRp. A poor-risk mutation profile (defined as the presence of ASXL1, RUNX1, TP53, or wild-type NPM1 with FLT3-ITD mutation with an allelic ratio >0·521) was present in 12 patients (27%), of whom 8 (67%) achieved a CR. All six NPM1-mutated patients achieved CR (n=4) and CRp (n=2) with negative MRD by flow cytometry. Longitudinal follow-up by RQ-PCR was available for 4 of these 6 patients (67%) who achieved and sustained an undetectable molecular MRD. Of the 6 patients with prior MDS treated with HMA, 4 (67%) responded with CR (n=2), CRp (n=1) and PR (n=1). Comparison of characteristics between CR/CRi/CRp patients and non-responders showed that non-responders tended to have more TP53 mutations (4/10 [40%] vs 4/34 [12%]; p=0·06) and more secondary and therapy-related AML (4/10 [40%] vs 6/34 [18%]; p=0·20) (table 1, appendix p1). However, cytogenetics and ELN genetic risk did not have an impact on response.
Nineteen responders (43%) later proceeded to allo-SCT in first remission (Table 2). Donor source was matched-related in 3, matched-unrelated in 12 and haplo-identical in 4 patients. Of these, 12 patients received fludarabine+busulfan-based conditioning regimen while 7 had fludarabine+melphalan-based therapy. Of the 19 transplanted patients, 13 (68%) developed graft-versus-host-disease (GVHD) (grades 1/2 in eight and 3/4 in five patients), which responded to treatment in eight. At a median follow-up of 12·6 months post allo-SCT (IQR, 1–26), 12/19 (63%) patients remain in CR, four died in CR and three had relapsed.
Table 2:
characteristics and outcomes of patients who underwent allogeneic stem cell transplantation in remission
| Characteristic (N = 19) | Number (%); median [IQR] |
|---|---|
| Donor source | |
| Matched related | 3 (16) |
| Matched unrelated | 12 (63) |
| Haplo-identical sibling | 4 (21) |
| Conditioning regimen | |
| Fludarabine + Busulfan | 12 (63) |
| Fludarabine + Melphalan | 7 (37) |
| Graft-versus-host-disease | 13 (68) |
| Grade I - II | 8 (42) |
| Grade III - IV | 5 (26) |
| Median follow-up post-SCT (months) | 12.6 [1 – 26] |
| Outcomes | |
| Death in CR | 4 (21) |
| Relapse | 3 (16) |
| Continuous CR | 12 (63) |
Data are expressed as n (%) or median [IQR].
SCT: stem cell transplantation; CR: complete remission
All 44 patients were eligible for toxicity evaluation (Table 3). Following induction therapy, myelosuppression was universal with one patient taken off-study because of prolonged myelosuppression. Twenty-eight patients achieved CR with recovery of normal blood counts at a median of 16 days (IQR, 9–36), including 3 who necessitated reinduction therapy. Another 6 patients achieved CRp (n=5) and CRi (n=1) as best response with one patient reaching CRp following re-induction. Of these 34 responders, 18 proceeded to allo-SCT without major delay, including 14 patients in CR, 3 in CRp and 1 in CRi. Therefore, 15 patients in CR continued idarubicin+cytarabine+nivolumab beyond remission and the overall median duration of post-remission cytopenias was 12 days (IQR, 8–26). The most common grade 3/4 non-hematological toxicities irrespective of attribution were febrile neutropenia (14/44; 32%) and diarrhea (7/44; 16%). Other grade 3/4 adverse events were considered immune-related including rash (n=2), colitis (n=2), transaminitis and pancreatitis (n=1; each). Grade 3 cholecystitis was also possibly attributed to nivolumab in one patient. All patients were treated with steroids and nivolumab interruption and were successfully re-challenged with nivolumab. Two of 44 (5%) patients died during the early induction period (≤28 days; on days 14 and 20 from pneumonia and intracranial hemorrhage, respectively), and a total of three (5%) patients within the first eight weeks.
Table 3:
Adverse events regardless of causality
| Adverse Event (N=44) | Grade 1–2; N (%) | Grade 3; N (%) | Grade 4; N (%) |
|---|---|---|---|
| Nausea | 1 (2) | 1 (2) | 0 |
| Diarrhea | 3 (5) | 7 (16) | 0 |
| Mucositis/Stomatitis | 1 (2) | 0 | 0 |
| Muscle weakness | 0 | 1 (2) | 0 |
| Syncope | 0 | 1 (2) | 0 |
| Elevated transaminases | 3 (5) | 1 (2) | 0 |
| Elevated bilirubin | 0 | 1 (2) | 0 |
| Febrile neutropenia | 1 (2) | 13 (30) | 1 (2) |
| Rash | 1 (2) | 2 (5) | 0 |
| Pneumonitis | 1 (2) | 0 | 0 |
| Colitis | 1 (2) | 1 (2) | 1 (2) |
| Pancreatitis | 1 (2) | 1 (2) | 0 |
| Cholecystitis | 0 | 1 (2) | 0 |
| Small bowel obstruction | 0 | 1 (2) | 0 |
| Thrombosis/embolism | 1 (2) | 0 | 0 |
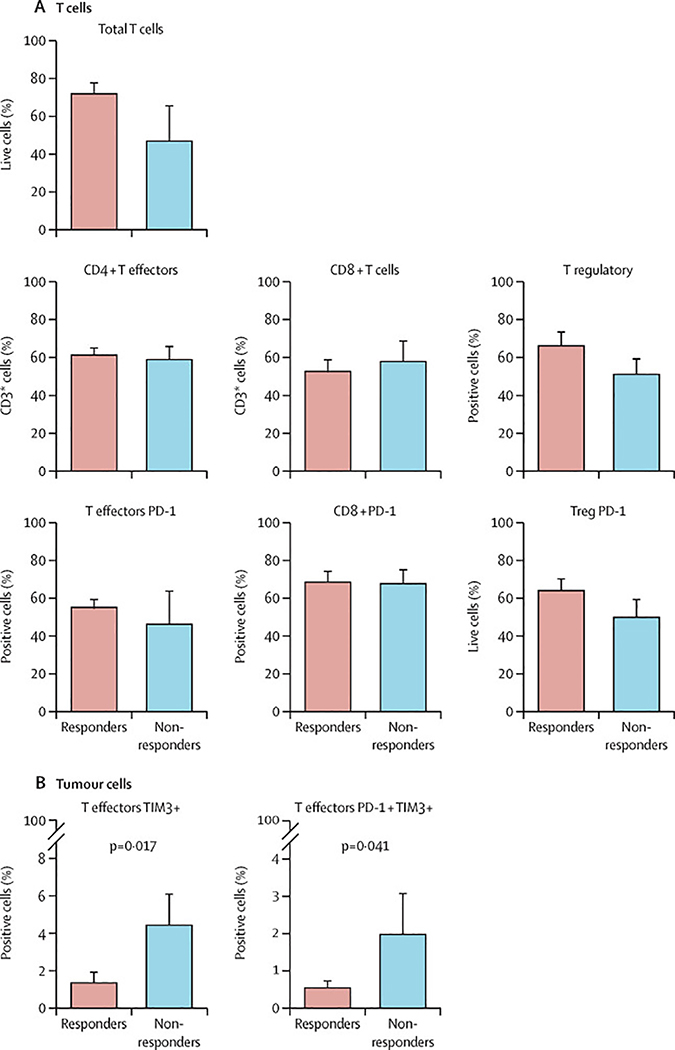
MFC studies were conducted on baseline (prior to first nivolumab dose) bone marrow aspirate and peripheral blood, to assess T-cell repertoire and expression of co-stimulatory and inhibitory receptors and ligands on T-cells and leukaemic blasts, respectively. Baseline BM was evaluated on 19 responders and five non-responders. Although, at diagnosis, total T-cell population was increased in the patients achieving CR, this was not statistically significant. Furthermore, there was no significant difference between T-cell subsets among the patients who did or did not achieve CR. However, patients who achieved CR/CRi/CRp had a higher CD3+ T-cell infiltrate compared to non-responders which was not statistically significant (Figure 3A).
Figure 3:
A: T-cell subtypes and PD-1 expression pre nivolumab dose in responders compared to non-responders; B: Percentage of TIM-3+ and PD-1/TIM-3 double positive in responders compared to non-responders.
We then evaluated expression of immune markers on T-cell subsets: CD4+ T-effectors: CD3+CD4+CD127lo/+Foxp3−, CD4+ T-regulatory: CD3+CD4+CD127−Foxp3+, and CD8+ T-cells. At baseline, BM of non-responders had significantly higher percentages of CD4+ T-effectors co-expressing PD-1/TIM-3 (p=0·0421) (Figure 3B) and a trend towards higher percentage of CD4+ T-effector cells co-expressing PD-1/LAG-3 compared to responders (data not shown). We did not evaluate the change in T-cell subsets and their markers following nivolumab. Using Cytof mass cytometry, we quantified leukaemic progenitor cells and T-cells and demonstrated clearance of progenitors and reconstitution of T-cells in patients achieving CR during and after consolidation therapy (figure 3, appendix pp 5–6).
DISCUSSION
In this pilot phase II trial, we combined nivolumab with idarubicin and high-dose cytarabine for frontline therapy in AML and high-risk MDS, on the basis of compilation of preclinical data with single-agent nivolumab14 and a potential synergistic antileukaemia effect with cytarabine.11 Nevertheless, this modulation could be dependent on cytarabine concentration in the plasma Because one of the primary rationales for the incorporation of nivolumab into AML regimens is through potentiation of cytotoxic activity especially that of cytarabine, it is possible that incremental improvements in outcomes would be obtained with intensification of the current regimen using higher doses of cytarabine during consolidation. The SWOG S1203 trial highlighted the importance of such intensification, as lack of improvement in outcomes with IA compared to 7+3 could be attributed to the relatively light consolidation doses used with IA (cytarabine at a dose of 2.25 g/m2 compared to 18 g/m2 per consolidation).
Because of limited clinical experience with the combination, the study started with an initial run-in phase with no specific or cumulative toxicities. Furthermore, our study did not meet the stopping boundaries for EFS.
The adverse events profile seen on the study was as expected with prominent gastrointestinal toxicity and grade 3/4 febrile neutropenia, all managed with standard of care. Myelosuppression was universal with one patient taken off-study. The combination did not produce excess grade immune-related adverse events (irAEs) and the induction mortality was low (5%).
The combination of idarubicin, cytarabine, and nivolumab was also active, leading to an ORR of 80% including 64% CR and 14% CRi/CRp in spite of the high proportion of patients with poor prognostic features, such as adverse ELN genetic risk (50%) and TP53 mutations (18%).
We also assessed MRD using an 8-color MFC assay. Of the 34 patients who achieved CR/CRi/CRp, 18 had undetectable MRD following induction and another nine converted to MRD-negative status within 1–3 months. It is generally believed that initial cytotoxic chemotherapy removes the leukaemic mass, leaving the pre-leukaemic and leukaemic stem and progenitor cells (LSPC) which persist in morphological CR due to their quiescence and resistance to apoptosis.22 These LSPC are implicated as the source of relapse. We hypothesized that an induced antileukaemic immune response using nivolumab may be effective in prolonging response. Furthermore, nivolumab could eradicate MRD.
Due to their high risk disease profile, a significant number of patients proceeded to allo-SCT after achieving a response. The use of nivolumab did not result in increased incidence of complications with SCT, particularly grade 3/4 GVHD, despite the use of various donor sources and conditioning regimens. The outcomes of these patients were promising as median overall survival and EFS were still not reached at the time of data analysis. Interestingly, responding patients who continued idarubicin, cytarabine and nivolumab beyond remission had similar overall survival and EFS compared to those bridged to allo-SCT, suggesting the possibility of nivolumab’s efficacy in restoring the host antitumor immune surveillance.
Implementing successful immunotherapeutic strategies that depend on manipulating antineoplastic T-cells largely relies on the presence of an adequately preserved CD4+CD8+ T-cell repertoire in the tumor microenvironment (TME).23 We explored the T-cell subsets and the expression of co-stimulatory and inhibitory receptors and ligands on T-cells and leukaemic blasts, as well as the expression of immune markers on T-cell subsets. Prior to administration of therapy, a higher rate of live CD3+ T-cell in the BM appears to predict for response, while non-responders had significantly higher percentage of CD4+ T-effectors co-expressing PD-1/TIM-3 and PD-1/LAG-3. A previous report suggested that T-cells of newly diagnosed patients with AML express higher levels of TIM-3 compared to those of healthy controls, and higher levels predicted a poorer prognosis.24,25 In xenograft models, TIM-3 blockade reduced leukaemic burden and TIM-3 may be a potential clinical target for immunotherapy.26 TIM-3/LAG-3 co-expression on PD-1+ T-cells has been linked to an exhausted immune phenotype in AML.27 T-cell exhaustion is thought to be of paramount importance in cancer whereby malignant cells become resistant to immune recognition, and it is potentially salvaged by blocking immune checkpoints (ICPs) such as PD-1, CTLA-4, TIM-3 and LAG-3.28,29 Since the TME is enriched in multiple subsets of ICPs on exhausted T-cells, inhibition of more than one is likely necessary to elicit an enhanced antitumor response and improved survival compared to inhibition of any individual pathway alone; therefore, potential future strategies may involve the combined blockade of PD-1 and TIM-3.27,30
Our study is limited by a small sample size, short follow-up and lack of a prospective comparator population, which would affect the conclusions drawn for some subgroups. Furthermore, the choice of backbone chemotherapy could be debatable but the decision to pursue this regimen will depend on a careful discussion between the patient and his physician.
CONCLUSION
Addition of nivolumab to idarubicin+cytarabine induction therapy is feasible, and safe in younger AML patients. The risk of post-transplant severe GVHD is not prohibitive. In non-responders, CD4+ T-effector cells displayed exhausted phenotype that could explain mechanisms of resistance to therapy and can be potentially reversed. This study serves to demonstrate the feasibility of this approach and future larger randomized trials are needed to determine the efficacy of this approach.
Supplementary Material
Research in context.
Evidence before this study
Acute myeloid leukaemia is a clonal stem cell disorder characterized by complex molecular and cytogenetic architecture, with limited treatment options. Despite improvement of outcomes of younger patients, disease relapse remains the most common cause of treatment failure and death, and therefore, strategies to improve remission duration are highly needed.
Immune checkpoint blockade using monoclonal antibodies has recently revolutionized the field of cancer immunotherapy and underscores the practical capability to harness our immune system and awaken an antitumor response. Immune checkpoint inhibitors could offer the exciting advantages of survival improvement and potentially durable response rates along with manageable adverse events. The rationale for developing immunotherapeutic agents in acute myeloid leukaemia is strongly built on the impact of immune surveillance in the development and progression of leukaemia, as well as the role of graft-versus-leukemia effect from allogeneic stem cell transplantation which proved to be curative of several haematological malignancies. Evidence from a thorough literature review on Pubmed using keywords such as AML/MDS, PD-1/PD-L1, T-cells, suggests that the PD-1/PD-L1 immune checkpoint pathway is exploited by myeloblasts for immune evasion, and that blocking this pathway enhances cytotoxic T-cell activity and improves anti-leukaemia responses to conventional chemotherapy. While CD8+ T-cells overexpressing PD-1 are increased in the bone marrow of acute myeloid leukaemia patients compared to healthy individuals, PD-1 inhibition has limited activity as single agent in this setting.
Added value of this study
This phase II trial investigated the addition of nivolumab, a fully human IgG4 anti-PD-1 monoclonal antibody, to acute myeloid leukaemia induction therapy.
The trial met its primary endpoint where median event-free survival was not reached at a median follow-up of 17.25 months. The early mortality was 5%. Additionally, the combination was well-tolerated with few grade 3/4 immune-related events and no treatment-related deaths. Furthermore, patients were able to proceed to an allogeneic stem cell transplant without a marked increase in the incidence of graft-versus-host disease. But more importantly, we demonstrated that patients with an “exhausted” immune phenotype in bone marrow T-cells may be less likely to respond to induction and may benefit from such immune modulation. Indeed, non-responders had significantly higher percentage of CD4+ T-effectors co-expressing PD-1/TIM-3 (p=0·01) and PD-1/LAG-3 (p=0·04) compared to responders.
Implications of all the available evidence
The results of our study suggest that the addition of nivolumab to cytarabine and idarubicin induction is feasible. Immune mediated adverse events were readily managed using steroids and other appropriate measures. Larger randomized studies should be therefore conducted and other strategies such as earlier introduction of checkpoint inhibitor therapy or use of combined checkpoint inhibitors should be investigated.
Acknowledgments
Funding source: The MD Anderson Cancer Centre Support Grant CA016672, and the MD Anderson Cancer Centre Leukaemia SPORE CA100632 from the National Cancer Institute and Bristol Myers Squibb.
Footnotes
Conflicts of Interest: Research funding from Bristol Myers Squibb. FR received honoraria from Bristol Myers Squibb
Trial Registration ID: Clinicaltrials.gov identifier:
Contributor Information
Farhad Ravandi, Department of Leukaemia, the University of Texas MD Anderson Cancer Centre, Houston, United States.
Rita Assi, Department of Leukaemia, the University of Texas MD Anderson Cancer Centre, Houston, United States; Lebanese American University, Gilbert and Rose-Marie Chagoury School of Medicine, Lebanon; Lebanese American University Medical Center-Rizk Hospital, Beirut, Lebanon.
Naval Daver, Department of Leukaemia, the University of Texas MD Anderson Cancer Centre, Houston, United States.
Christopher B Benton, Department of Leukaemia, the University of Texas MD Anderson Cancer Centre, Houston, United States.
Tapan Kadia, Department of Leukaemia, the University of Texas MD Anderson Cancer Centre, Houston, United States.
Philip A Thompson, Department of Leukaemia, the University of Texas MD Anderson Cancer Centre, Houston, United States.
Gautam Borthakur, Department of Leukaemia, the University of Texas MD Anderson Cancer Centre, Houston, United States.
Yesid Alvarado, Department of Leukaemia, the University of Texas MD Anderson Cancer Centre, Houston, United States.
Elias J. Jabbour, Department of Leukaemia, the University of Texas MD Anderson Cancer Centre, Houston, United States.
Marina Konopleva, Department of Leukaemia, the University of Texas MD Anderson Cancer Centre, Houston, United States.
Koichi Takahashi, Department of Leukaemia, the University of Texas MD Anderson Cancer Centre, Houston, United States.
Steven Kornblau, Department of Leukaemia, the University of Texas MD Anderson Cancer Centre, Houston, United States.
Courtney D. DiNardo, Department of Leukaemia, the University of Texas MD Anderson Cancer Centre, Houston, United States.
Zeev Estrov, Department of Leukaemia, the University of Texas MD Anderson Cancer Centre, Houston, United States.
Wilmer Flores, Department of Leukaemia, the University of Texas MD Anderson Cancer Centre, Houston, United States.
Sreyashi Basu, Department of Leukaemia, the University of Texas MD Anderson Cancer Centre, Houston, United States.
James Allison, Department of Leukaemia, the University of Texas MD Anderson Cancer Centre, Houston, United States.
Padmanee Sharma, Department of Leukaemia, the University of Texas MD Anderson Cancer Centre, Houston, United States.
Sherry Pierce, Department of Leukaemia, the University of Texas MD Anderson Cancer Centre, Houston, United States.
Allison Pike, Department of Leukaemia, the University of Texas MD Anderson Cancer Centre, Houston, United States.
Jorge E. Cortes, Department of Leukaemia, the University of Texas MD Anderson Cancer Centre, Houston, United States.
Guillermo Garcia-Manero, Department of Leukaemia, the University of Texas MD Anderson Cancer Centre, Houston, United States.
Hagop M. Kantarjian, Department of Leukaemia, the University of Texas MD Anderson Cancer Centre, Houston, United States.
REFERENCES
- 1.Yates JW, Wallace HJ, Ellison RR, Holland JF. Cytosine arabinoside (NSC-63878) and daunorubicin (NSC-83142) therapy in acute nonlymphocytic leukemia. Cancer Chemother Rep 1973; 57(4): 485–8. [PubMed] [Google Scholar]
- 2.Stein EM, Tallman MS. Emerging therapeutic drugs for AML. Blood 2016; 127(1): 71–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Walter RB, Othus M, Burnett AK, et al. Resistance prediction in AML: analysis of 4601 patients from MRC/NCRI, HOVON/SAKK, SWOG and MD Anderson Cancer Center. Leukemia 2015; 29(2): 312–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Holowiecki J, Grosicki S, Robak T, et al. Addition of cladribine to daunorubicin and cytarabine increases complete remission rate after a single course of induction treatment in acute myeloid leukemia. Multicenter, phase III study. Leukemia 2004; 18(5): 989–97. [DOI] [PubMed] [Google Scholar]
- 5.Kolitz JE, George SL, Dodge RK, et al. Dose escalation studies of cytarabine, daunorubicin, and etoposide with and without multidrug resistance modulation with PSC-833 in untreated adults with acute myeloid leukemia younger than 60 years: final induction results of Cancer and Leukemia Group B Study 9621. J Clin Oncol 2004; 22(21): 4290–301. [DOI] [PubMed] [Google Scholar]
- 6.Burnett AK, Hills RK, Milligan DW, et al. Attempts to optimize induction and consolidation treatment in acute myeloid leukemia: results of the MRC AML12 trial. J Clin Oncol 2010; 28(4): 586–95. [DOI] [PubMed] [Google Scholar]
- 7.Garcia-Manero G, Tambaro FP, Bekele NB, et al. Phase II trial of vorinostat with idarubicin and cytarabine for patients with newly diagnosed acute myelogenous leukemia or myelodysplastic syndrome. J Clin Oncol 2012; 30(18): 2204–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jabbour E, Short NJ, Ravandi F, et al. A randomized phase 2 study of idarubicin and cytarabine with clofarabine or fludarabine in patients with newly diagnosed acute myeloid leukemia. Cancer 2017; 123(22): 4430–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Burnett AK, Russell NH, Hills RK, et al. Optimization of chemotherapy for younger patients with acute myeloid leukemia: results of the medical research council AML15 trial. J Clin Oncol 2013; 31(27): 3360–8. [DOI] [PubMed] [Google Scholar]
- 10.Willemze R, Suciu S, Meloni G, et al. High-dose cytarabine in induction treatment improves the outcome of adult patients younger than age 46 years with acute myeloid leukemia: results of the EORTC-GIMEMA AML-12 trial. J Clin Oncol 2014; 32(3): 219–28. [DOI] [PubMed] [Google Scholar]
- 11.Vereecque R, Saudemont A, Quesnel B. Cytosine arabinoside induces costimulatory molecule expression in acute myeloid leukemia cells. Leukemia 2004; 18(7): 1223–30. [DOI] [PubMed] [Google Scholar]
- 12.Zhang L, Gajewski TF, Kline J. PD-1/PD-L1 interactions inhibit antitumor immune responses in a murine acute myeloid leukemia model. Blood 2009; 114(8): 1545–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Williams P, Basu S, Garcia-Manero G, et al. Checkpoint Expression By Acute Myeloid Leukemia (AML) and the Immune Microenvironment Suppresses Adaptive Immunity. Blood 2017; 130(Suppl 1): 185-. [Google Scholar]
- 14.Berger R, Rotem-Yehudar R, Slama G, et al. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin Cancer Res 2008; 14(10): 3044–51. [DOI] [PubMed] [Google Scholar]
- 15.Arber DA, Orazi A, Hasserjian R, et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 2016; 127(20): 2391–405. [DOI] [PubMed] [Google Scholar]
- 16.Greenberg PL, Tuechler H, Schanz J, et al. Revised international prognostic scoring system for myelodysplastic syndromes. Blood 2012; 120(12): 2454–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Luthra R, Patel KP, Reddy NG, et al. Next-generation sequencing-based multigene mutational screening for acute myeloid leukemia using MiSeq: applicability for diagnostics and disease monitoring. Haematologica 2014; 99(3): 465–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheson BD, Greenberg PL, Bennett JM, et al. Clinical application and proposal for modification of the International Working Group (IWG) response criteria in myelodysplasia. Blood 2006; 108(2): 419–25. [DOI] [PubMed] [Google Scholar]
- 19.Ravandi F, Jorgensen J, Borthakur G, et al. Persistence of minimal residual disease assessed by multiparameter flow cytometry is highly prognostic in younger patients with acute myeloid leukemia. Cancer 2017; 123(3): 426–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thall PF, Simon RM, Estey EH. Bayesian sequential monitoring designs for single-arm clinical trials with multiple outcomes. Stat Med 1995; 14(4): 357–79. [DOI] [PubMed] [Google Scholar]
- 21.Dohner H, Estey E, Grimwade D, et al. Diagnosis and management of AML in adults: 2017 ELN recommendations from an international expert panel. Blood 2017; 129(4): 424–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shlush LI, Zandi S, Mitchell A, et al. Identification of pre-leukaemic haematopoietic stem cells in acute leukaemia. Nature 2014; 506(7488): 328–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vidriales MB, Orfao A, Lopez-Berges MC, et al. Lymphoid subsets in acute myeloid leukemias: increased number of cells with NK phenotype and normal T-cell distribution. Ann Hematol 1993; 67(5): 217–22. [DOI] [PubMed] [Google Scholar]
- 24.Jan M, Chao MP, Cha AC, et al. Prospective separation of normal and leukemic stem cells based on differential expression of TIM3, a human acute myeloid leukemia stem cell marker. Proc Natl Acad Sci U S A 2011; 108(12): 5009–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li C, Chen X, Yu X, et al. Tim-3 is highly expressed in T cells in acute myeloid leukemia and associated with clinicopathological prognostic stratification. Int J Clin Exp Pathol 2014; 7(10): 6880–8. [PMC free article] [PubMed] [Google Scholar]
- 26.Kikushige Y, Shima T, Takayanagi S, et al. TIM-3 is a promising target to selectively kill acute myeloid leukemia stem cells. Cell Stem Cell 2010; 7(6): 708–17. [DOI] [PubMed] [Google Scholar]
- 27.Zhou Q, Munger ME, Veenstra RG, et al. Coexpression of Tim-3 and PD-1 identifies a CD8+ T-cell exhaustion phenotype in mice with disseminated acute myelogenous leukemia. Blood 2011; 117(17): 4501–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang AC, Postow MA, Orlowski RJ, et al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature 2017; 545(7652): 60–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kamphorst AO, Wieland A, Nasti T, et al. Rescue of exhausted CD8 T cells by PD-1-targeted therapies is CD28-dependent. Science 2017; 355(6332): 1423–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kong Y, Zhang J, Claxton DF, et al. PD-1(hi)TIM-3(+) T cells associate with and predict leukemia relapse in AML patients post allogeneic stem cell transplantation. Blood Cancer J 2015; 5: e330. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.