Abstract
Irritable bowel syndrome (IBS) is a common gastrointestinal (GI) condition involving numerous potential causative factors (e.g. alterations in gut microbiota, motility, brain–gut axis). Several interventions are available for the management of patients with IBS, but no universal management algorithm currently exists. The aim of this article is to review interventions that may be considered in the management of patients with IBS with diarrhea (IBS-D). Nonpharmacological interventions include dietary and lifestyle modification, which are generally used as first-line therapy. Probiotics have demonstrated efficacy and safety in patients with IBS, but studies are inconsistent in strains examined, dosing, and treatment duration. Psychological therapies (e.g. cognitive behavioral therapy, hypnotherapy) also may improve IBS symptoms. Pharmacological interventions for the management of IBS-D include the US Food and Drug Administration–approved agents eluxadoline, rifaximin, and alosetron, as well as loperamide, smooth muscle antispasmodics, bile acid sequestrants, and antidepressants (i.e. tricyclic antidepressants, selective serotonin reuptake inhibitors). Eluxadoline and rifaximin have been shown to improve abdominal pain and stool consistency in patients with IBS-D. In addition, data indicate that alosetron improves IBS symptoms; however, it is approved only for women with severe IBS-D. Of the three approved agents, rifaximin has the most favorable safety profile. The risk–benefit ratio is an important consideration with every medication, but is especially important in the treatment of functional GI disorders such as IBS-D. Thus, the most troublesome symptoms, quality of life, symptom intensity, and individual patient preferences should be considered when formulating a management plan for patients with IBS-D.
Keywords: alosetron, diarrhea, efficacy, eluxadoline, irritable bowel syndrome, rifaximin, safety, treatment
Introduction
Irritable bowel syndrome (IBS) is a common functional gastrointestinal (GI) disorder characterized by recurrent abdominal pain associated with changes in stool frequency and form.1 IBS is differentiated further by the predominant bowel habit pattern, such as IBS with diarrhea (IBS-D), IBS with constipation (IBS-C), and IBS with mixed bowel habits (IBS-M).1 Patients with IBS commonly experience abdominal bloating and distention; these symptoms are supportive of IBS although not required for diagnosis, per Rome IV criteria.1 In fact, abdominal cramping, bloating, and abdominal pain/discomfort were the three most bothersome symptoms reported in patients with IBS in a study by Ringel and colleagues, in which more than 300 patients were surveyed (IBS-M, 45.1%; IBS-D, 39.2%; IBS-C, 15.7%).2 Further, patients with IBS often experience impaired quality of life (QOL), and their symptoms can have a significant negative impact on work and activities of daily living.3–5 Survey data indicated that patients with IBS-D (n = 1102) experienced significantly greater impairment of health-related QOL compared with a control group (n = 65,389; p < 0.001).3 In another study, 76.5% of patients with IBS (n = 179; IBS-M, 58.7%; IBS-D, 31.8%; IBS-C, 9.5%) reported impairment in ⩾5 of 10 domains of daily life (social activity [80%], eating alone [80%], job/school performance [72%], physical activity [68%], eating in groups [65%], leisure activity [63%], household activities [54%], sexual activity [54%], physical appearance [53%], and travel [50%]).4 Moreover, a greater percentage of patients with IBS (subtype(s) not specified; n = 48) included in a single-center study indicated having poor sleep quality compared with healthy individuals (72% versus 39%, respectively).5 Survey data also indicated that IBS can negatively affect the ability to work, such that patients with IBS-D reported more work absenteeism compared with controls (5.1% versus 2.9%, respectively; p = 0.004) and had significantly greater loss of overall work productivity (20.7% versus 13.2%, respectively; p < 0.001).3
The pathophysiology of IBS is unclear, but may include alterations in the gut microbiota, GI motility, visceral sensation, intestinal permeability, and the brain–gut axis, and may also occur as the consequence of infection or psychological stressors.6 Given the multifactorial nature of IBS, there is currently no single management strategy for a particular IBS subtype that has been universally adopted.6 The aim of this narrative review is to provide a summary of nonpharmacological and pharmacological treatments for the management of IBS-D, and to review the data supporting their use.
Nonpharmacological management options
Dietary and lifestyle modification
A US study published in 2018 reported that more than half of patients with IBS ‘usually’ (45.6%) or ‘almost always’ (13.6%) perceive diet to play a role in clinical symptoms, according to a majority of US gastroenterologists who were surveyed (n = 1562).7 Lending support to this notion, a French study published in 2018 demonstrated a greater likelihood of developing IBS with an increasing percentage of ultraprocessed food in the diet (p < 0.0001).8 Further, a Western diet (containing greater amounts of fatty and sugary foods, soda, salty snacks) was associated with an increased risk of IBS in the same French cohort [adjusted odds ratio (OR) = 1.2; 95% confidence interval (CI): 1.1–1.4; adjusted p-trend = 0.001].9 Additionally, self-reported vegetarianism was associated with IBS (adjusted OR = 2.6; 95% CI: 1.4–4.9), including IBS-D (adjusted OR = 2.8; 95% CI: 1.01–7.6).10
A survey of US gastroenterologists published in 2018 also indicated that more than half (52.6%) of patients with IBS ‘usually’ or ‘almost always’ tried to self-manage symptoms before seeking care from a specialist.7 Gastroenterologists stated that diets ‘usually’ or ‘almost always’ tried by patients included trial and error (50%), reduced lactose (33%), gluten-free (24%), low-fat (6%), and low fermentable oligosaccharides, disaccharides, monosaccharides, and polyols (FODMAPs; 2%).7 More than half of survey respondents indicated that dietary modification was a primary management strategy for IBS; providers reported ‘usually’ or ‘almost always’ recommending low-FODMAP (77%), high-fiber (45%), reduced-lactose (45%), low-fat (18%), and gluten-free (12%) diets.7 Gastroenterologists reported that they considered a low FODMAP diet to be ‘very effective’ (20%) or ‘somewhat effective’ (65%) for their patients.7 Results of a meta-analysis of two randomized controlled trials (RCTs) of adults with IBS [n = 111; subtype(s) not specified] showed that patients with a previous response to a gluten-free diet who were then randomly assigned to receive a diet ‘spiked’ with gluten were more likely to experience a worsening of global IBS symptoms compared with patients assigned to continue a gluten-free diet [relative risk (RR) = 0.4; 95% CI: 0.1–1.6], although results were not significant.11 A RCT of 45 patients with IBS-D reported that those given a gluten-containing diet (n = 22) for 4 weeks had significantly more bowel movements per day compared with patients adhering to a gluten-free diet (n = 23; p = 0.04).12 Further, alterations in small intestinal barrier function were greater in patients receiving a gluten-containing diet than in those assigned to a gluten-free diet, as determined by the total urinary mannitol excretion (p = 0.03) and lactulose to mannitol ratio (p = 0.001).12 Overall, these study findings suggest that gluten, in particular, may be an exacerbating factor in the pathophysiology of IBS-D.
In a meta-analysis of seven RCTs of adults with IBS, a low FODMAP diet was associated with a decrease in global IBS symptoms compared with control diets (i.e. alternative diet, high FODMAP diet, usual diet, or placebo; persistence of IBS symptoms: 43.2% versus 61.6%, respectively; RR = 0.7; 95% CI: 0.5–0.9).11 In a RCT by Zahedi and colleagues, patients with IBS-D randomly assigned to a low FODMAP diet (n = 55) experienced improvements in abdominal pain intensity (p = 0.001) and frequency (p = 0.02), abdominal distention (p < 0.001), dissatisfaction with intestinal transit (p = 0.001), and interference with daily life (p = 0.005) compared with general dietary advice (n = 55) after 6 weeks.13 In addition, a low FODMAP diet was shown to improve IBS-QOL scores from baseline compared with a modified diet (recommended by the National Institute for Health and Care Excellence) after 4 weeks (15.9 versus 5.0 points, respectively; 95% CI: –17.4 to ‒4.3) in a RCT of 84 patients with IBS-D by Eswaran and colleagues.14 Further, a significantly greater percentage of patients achieved a clinical response (i.e. ⩾14-point improvement from baseline in IBS-QOL score) with a low FODMAP diet after 4 weeks (52% versus 21%, respectively; 95% CI: ‒0.52 to ‒0.08).14 Taken together, these data suggest that adherence to a low FODMAP diet may be a reasonable dietary approach for patients with IBS-D. Although no validated treatment algorithm for IBS-D exists, this intervention is frequently the first employed by primary care providers and gastroenterologists. Further, it should be noted that many gluten-containing foods are considered high FODMAP foods.
However, patients with IBS have reported that the low FODMAP diet can be burdensome, citing an impact on themselves as well as family members and friends (e.g. changes in routine of shared meals).15 Patients also indicated that the expense of ingredients and time required to prepare meals were taxing. Further, while economic cost data for the low FODMAP diet in patients with IBS are limited, one study published in 2015 estimated per-patient costs for group education (12 patient limit; initial session approximately 90 min) and one-on-one education (initial session approximately 60 min) of US$115 and US$238, respectively.16
The American College of Gastroenterology (ACG) guidelines provide a weak recommendation for exercise in the treatment of IBS symptoms, although this recommendation is based on limited data and well-designed clinical studies are needed in this regard.17 A meta-analysis of various forms of exercise (e.g. yoga, walking, cycling, swimming, and running) reported improvements in GI symptoms, QOL, and anxiety in patients with IBS (n = 683), although the studies differed in patient demographics, study design, and study size, thereby limiting generalizability of the results.18
Yoga utilizes stretching and breathing exercises as a means to relieve stress. Although its mechanism of action in patients with IBS is unclear, it may affect the brain–gut axis, improve sleep and QOL, or alter gut microbiota, particularly in patients participating in concomitant dietary interventions.19 Patients with IBS (n = 30; IBS-D, 43.3%; IBS-M, 30.0%; IBS-C, 23.3%) randomly assigned to attend two 75-min yoga classes per week for 12 weeks, with encouragement to practice yoga at home, experienced significant improvement in overall IBS symptoms at weeks 12 and 24 (p < 0.001, for both comparisons).20 Further, patients practicing yoga achieved significant improvements in pain duration from baseline at week 12 (p = 0.04) and in both bowel satisfaction and interference with life at weeks 12 and 24 (bowel satisfaction: p = 0.02 and p = 0.001, respectively; interference with life: p < 0.001, for both time points).20 Although additional studies on the potential benefits of yoga are needed, limited data to date suggest that the practice of yoga may significantly improve GI symptoms and QOL for some patients with IBS.21,22
Acupuncture involves the manipulation of needles at 1 or more of 361 designated points along 14 meridians that are believed to correspond to internal organs.23 The premise of acupuncture is to restore energy balance and normalize organ function.23 A meta-analysis of four RCTs of patients with IBS (n = 343) demonstrated that acupuncture significantly improved IBS symptoms compared with placebo (OR = 7.7; 95% CI: 3.8–16.0); of interest, sham (placebo) acupuncture also significantly improved IBS symptoms compared with placebo (OR = 4.7; 95% CI: 2.0–11.0).24 However, another meta-analysis of five RCTs reported no significant benefit of acupuncture on IBS symptom severity or QOL compared with sham acupuncture.25 In a study by MacPherson and colleagues, patients who were randomly assigned to receive up to 10 weekly sessions of acupuncture plus usual care (n = 116) achieved significantly greater improvement in overall IBS symptoms compared with patients receiving usual care alone (n = 117) at 1 year follow-up (p < 0.05); however, differences were not sustained at 2 years of follow-up.26 Despite the lack of robust data supporting the use of acupuncture in the treatment of IBS-D, much like diet and exercise, it is a seemingly safe and reasonable therapy to consider in patients with IBS-D who favor a nonmedicinal approach to treating their symptoms.
Prebiotics/probiotics/synbiotics
Prebiotics, probiotics, and synbiotics (combinations of prebiotics and probiotics) have been considered in the treatment of IBS as they may, in theory, modulate the gut microbiota.17 ACG guidelines provide a weak recommendation for use of probiotics in the treatment of global IBS symptoms, including bloating and flatulence.17 A meta-analysis of 21 RCTs of patients with IBS (n = 1931) reported that combination probiotic products significantly decreased the persistence of IBS symptoms compared with placebo (RR = 0.8; 95% CI: 0.7–0.9; p = 0.001).27 The trend toward a reduction in symptoms favored specific strains of probiotics (i.e. Lactobacillus, Saccharomyces, Bifidobacterium) over control treatment, but the difference between groups did not achieve statistical significance.27 In 19 trials (n = 1341) assessing the improvement of global symptoms or abdominal pain scores, probiotics were significantly more efficacious than placebo [standardized mean difference (SMD) = –0.3; 95% CI: ‒0.4 to ‒0.2; p < 0.00001].27 In 24 separate studies (n = 2256), combination probiotics were associated with significantly decreased flatulence compared with placebo (11 trials; SMD = –0.3; 95% CI: ‒0.5 to ‒0.1; p = 0.01); combination probiotics also decreased bloating scores compared with placebo, but the difference was not statistically significant (17 trials; SMD = –0.2; 95% CI: ‒0.3 to 0.01; p = 0.07).27 The safety of probiotics was examined in 36 studies in this same meta-analysis (n = 4183 patients) and was found to be generally comparable to that of placebo (RR = 1.1; 95% CI: 0.9–1.3).27 However, it remains to be elucidated which bacterial strain(s), doses, and treatment durations are most effective for patients with IBS.28
A secondary analysis of a double-blind RCT of patients with IBS-D receiving a combination probiotic product (i.e. BIO-25 LR, Supherb Ltd., Nazareth Ilit, Israel; n = 51) containing 25 billion bacteria/capsule from 11 different strains, reported that the number of daily bowel movements and relative abundance of Lactobacillus citreum spp. were negatively correlated as compared with placebo (n = 46) after 8 weeks of treatment.29 Further, 41.2% and 38.9% of probiotic clinical responders achieved improvements in abdominal pain (mean improvement, 53.9%) and bloating scores (mean improvement, 49.2%), respectively.29 Additionally, a double-blind, placebo-controlled RCT of patients with moderate-to-severe IBS-D receiving a combination probiotic [i.e. Bio-Kult®, Probiotics International Ltd. (Protexin), Somerset, United Kingdom; n = 181], containing 2 billion colony-forming units/capsule from 14 different bacterial strains, demonstrated that IBS symptoms were mild in intensity after 16 weeks in a significantly greater percentage of patients receiving the probiotic product compared with placebo (n = 179; 52.5% versus 39.1%, respectively; p < 0.001).30 Further, a significantly greater percentage of patients receiving probiotics were symptom-free compared with placebo after 16 weeks (33.7% versus 12.8%, respectively; p < 0.001).30
The European Society for Primary Care Gastroenterology has recommended use of probiotics for improvement of global IBS symptoms in some patients, based on findings of a meta-analysis of 23 studies (n = 3112 patients with IBS) that included 19 different probiotics (high grade of evidence).31 A similar recommendation was made for probiotic use in patients with IBS-D specifically, based on data from seven studies (n = 495 patients with IBS-D; low grade of evidence).31 Of note, a 2018 RCT assessing the use of the probiotic Clostridium butyricum (ATaiNing, Qingdao Eastsea Pharmaceutical Co., Ltd., Qingdao, China) in patients with IBS-D noted that IBS symptoms were significantly improved (based on a decrease in IBS symptom severity scale scores from baseline) with C. butyricum (n = 105) compared with placebo (n = 95) after 4 weeks (p = 0.04).32 Response was significantly greater in patients with moderate-to-severe symptoms receiving C. butyricum compared with placebo (54.2% versus 32.9%, respectively; p = 0.007).32 Overall, numerous studies have demonstrated modest benefit for the use of probiotics to treat IBS, including patients with IBS-D, though generalizability of these results is limited by the great diversity of strains and doses that have been studied to date. Still, probiotics appear to be another safe nonpharmacologic treatment option that may improve symptoms of IBS-D, including bowel frequency, abdominal pain, or bloating. They are perhaps best suited for use for patients with mild-to-moderate symptoms, given the somewhat underwhelming data with regard to their efficacy as a whole.
A meta-analysis of two RCTs reported that synbiotics did not significantly improve IBS symptoms compared with placebo.27 However, a small study of patients with IBS-D (n = 10) receiving the synbiotic OMNi-BiOTiC® STRESS Repair (Institut Allergosan, Graz, Austria) twice daily reported a significant improvement from baseline in symptom severity after 4 weeks of treatment (improvement scores, 237 versus 54, respectively; p = 0.002).33 Given the lack of robust data, ACG guidelines currently provide only a weak recommendation for treatment with synbiotics and prebiotics in patients with IBS.17
Serum-derived bovine immunoglobulin
Results of a small randomized, double-blind, placebo-controlled study of nutritional supplementation with serum-derived bovine immunoglobulin (SBI) 5 g/day (n = 15) and 10 g/day (n = 15) in patients with IBS-D showed significant improvement from baseline in the mean number of days per week with any symptom from week 2 to week 6 (p = 0.01 and p < 0.01, respectively); patients receiving placebo (n = 13) experienced no significant difference in mean number of days with any symptom during this time period (p = 0.3).34 At week 6, patients receiving SBI 5 g/day had significant improvement from baseline in flatulence (p = 0.04) and incomplete evacuation (p < 0.05). Further, at week 6, patients receiving SBI 10 g/day experienced significant improvement from baseline to week 6 in the number of days with any symptom (p < 0.01), abdominal pain (p < 0.01), bloating (p < 0.05), flatulence (p < 0.01), loose stools (p = 0.01), and urgency (p = 0.05). Patients receiving placebo had no significant improvement from baseline to week 6 in symptoms. No statistical comparisons between SBI and placebo were conducted. The safety profile of SBI was generally comparable with placebo, although mean corpuscular hemoglobin increased significantly, while remaining within the normal range, from baseline to week 6 in patients receiving SBI 10 g/day (p = 0.02). An open-label noncomparative study in 15 patients with IBS-D reported that SBI 5 g/day twice daily significantly improved bowel function from baseline to week 8, including mean number of stools per day (2.4–1.8; p < 0.001), mean stool ease of passage per day (4.7–4.4; p = 0.04), and percentage with incomplete evacuation per day (37–30%; p = 0.004).35 However, no significant improvements from baseline were noted for any abdominal pain severity assessment. Despite the aforementioned data, SBI is not used widely in the treatment of IBS-D because data from large, prospective, randomized, placebo-controlled trials are lacking. Further research is needed before SBI can be routinely considered a treatment option.
Psychological therapies
In a meta-analysis of 36 individual RCTs comparing psychological therapies with control treatment [i.e. symptom monitoring (n = 18 studies), usual care (n = 15), supportive treatment (n = 2), and placebo (n = 1)], a smaller percentage of patients with IBS did not experience improvement of symptoms with psychological therapies compared with control therapy [52.2% (n = 1407 patients) versus 75.9% (n = 1080), respectively; RR = 0.7; 95% CI: 0.6–0.8; Table 1].36 In the meta-analysis, efficacy outcomes were assessed for each specific psychological therapy (Table 1); each intervention was associated with improvement of IBS symptoms compared with control therapy, although the degree of benefit differed among therapies.36 Of note, the overall number needed to treat (NNT) for psychological therapies was four (Table 2).17,34,36–42 Combined with the little-to-no adverse risk profile of psychological therapies, the low NNT makes this entity a particularly intriguing treatment option for patients with IBS.
Table 1.
Summary of meta-analysis findings on psychological therapies for patients with irritable bowel syndrome.36
| Psychological therapy, pts (n) | Control therapy, pts (n) | Studies, n | IBS symptoms not improving with active versus control therapy, % | RR of IBS symptoms not improving with active treatment (95% CI) | NNT (95% CI) |
|---|---|---|---|---|---|
| All therapies (n = 1407) | All controls (n = 1080) involving symptom monitoring (18 studies), ‘usual management’ (15 studies), supportive therapy (2 studies), or placebo (1 study) | 36 | 52.2% versus 75.9% | 0.7 (0.6–0.8) | 4 (3.5–5.5) |
| Cognitive behavioral therapy (n = 349) | n= 261 | 9 | 41.5% versus 63.6% | 0.6 (0.4–0.8) | 4 (3–9) |
| Self-administered or minimal contact cognitive behavioral therapy (n = 73) | n = 71 | 3 | 46.6% versus 88.7% | 0.5 (0.2–1.7) | Not reported |
| Cognitive behavioral therapy delivered by internet (n = 71) | n = 69 | 2 | 71.8% versus 98.6% | 0.8 (0.5–1.2) | Not reported |
| Relaxation training or therapy (n = 185) | n = 175 | 8 | 68.1% versus 84.0% | 0.8 (0.7–1.0) | 6 (3–60) |
| Hypnotherapy (n = 141) | n = 137 | 5 | 54.6% versus 77.4% | 0.6 (0.6–0.9) | 5 (3.5–10) |
| Stress management (n = 80) | n = 62 | 3 | 46.3% versus 69.4% | 0.7 (0.4–1.2) | Not reported |
| Dynamic psychotherapy (n = 138) | n = 135 | 2 | 44.2% versus 70.4% | 0.6 (0.4–0.9) | 4 (2–20) |
| Mindfulness meditation training (n = 79) | n = 86 | 2 | 55.7% versus 67.4% | 0.8 (0.4–1.4) | Not reported |
| Multicomponent psychological therapy (n = 168) | n = 167 | 5 | 57.1% versus 80.8% | 0.7 (0.6–0.8) | 4 (3–7) |
| Multicomponent psychological therapy delivered by | Not reported | 1 | Not reported | Not reported | |
| • Phone | 0.8 (0.6–0.9) | ||||
| • Contingency management | 0.4 (0.3–0.8) | ||||
| • Emotional awareness and expression training | 0.5 (0.3–0.9) |
CI, confidence interval; IBS, irritable bowel syndrome; NNT, number needed to treat; pts, patients; RR, relative risk.
Table 2.
Number needed to treat and number needed to harm for interventions considered for symptom management of irritable bowel syndrome with diarrhea.a, 17,34,36–42
| Intervention | NNT (efficacy) | NNH (safety) |
|---|---|---|
| Nonpharmacological interventions | ||
| Dietary modification (low FODMAP) | 5 | NA |
| Lifestyle modification (exercise) | NA | NA |
| Prebiotics | NA | NA |
| Probiotics | 7 | 35 |
| Synbiotics | NA | NA |
| Serum-derived bovine immunoglobulin | NA | NA |
| Psychological therapies | 4 | NA |
| Cognitive behavioral therapy | 4 | NA |
| Hypnotherapy | 5 | NA |
| Multicomponent therapy | 4 | NA |
| Relaxation training or therapy | 6 | NA |
| Pharmacological agents indicated for adults with IBS-D | ||
| Eluxadoline | 75 mg: 10–15 100 mg: 9–13 |
75 mg: 25 100 mg: 23 |
| Rifaximin | 8 and 11 | 8971 |
| Alosetron | 8 and 6 | 10 and 19 |
| Other pharmacological agents | ||
| Loperamide | NA | NA |
| Diphenoxylate/atropine | NA | NA |
| Smooth muscle antispasmodicsb | 5 | 22 |
| Peppermint oil | 4 | NA |
| Bile acid sequestrants | NA | NA |
| Antidepressants | 4 | 8.5 |
| Tricyclic antidepressants | 4.5 and 8 | 18 |
| Selective serotonin reuptake inhibitors | 5 | NA |
| Ondansetron | NA | NA |
| Crofelemer | NA | NA |
Multiple values for NNT and NNH are presented when available.
Includes cimetropium, dicyclomine, drotaverine, hyoscine, otilonium, and pinaverium.
FODMAP, fermentable oligosaccharides, disaccharides, monosaccharides, and polyols; IBS-D, irritable bowel syndrome with diarrhea; NA, not available; NNH, number needed to harm; NNT, number needed to treat.
Cognitive behavioral therapy (CBT) includes numerous structured techniques (e.g. psychoeducation, relaxation, cognitive restructuring, problem-solving skills) administered by a trained therapist that are aimed at modifying specific forms of negative thinking (e.g. catastrophizing, excessive worrying) and are believed to affect IBS symptom expression.43,44 A meta-analysis of nine RCTs found that significantly fewer patients with IBS receiving CBT had no symptom improvement compared with control therapy (41.5% versus 63.6%, respectively; RR = 0.6; 95% CI: 0.4–0.8).36 CBT was significantly more effective than control therapy (i.e. symptom monitoring/waiting list control, standard care, medical treatment, other psychological treatments) for improvement of symptom scores in a pooled analysis of 16 studies (n = 1380) involving patients with IBS (SMD = 0.7; 95% CI: 0.4–0.9).45 A subsequent meta-analysis of six studies of patients with IBS (n = 638) reported that CBT had significantly greater efficacy for improving IBS symptom severity (medium-to-large effect) compared with its effect on psychosocial distress (low-to-medium effect; p = 0.02).46 Further, a significantly greater percentage of patients with moderate-to-severe IBS randomly assigned to receive home-based CBT with minimal therapist contact (four clinic visits during 10 weeks) achieved global IBS symptom improvement (based on Clinical Global Impressions-Improvement Scale) compared with patients receiving standard IBS education (four sessions during 10 weeks) at week 10 (55.7% versus 40.4%, respectively; p < 0.01), and efficacy of home-based CBT was comparable to that of standard (clinic-based) CBT (10 sessions) at week 10.47
In GI-focused hypnotherapy, patients with IBS receive repeated suggestions regarding the control of GI function, while in a hypnotic state, in an effort to achieve symptom and psychological improvement.48 A study of 1000 patients with IBS symptoms refractory to other treatments (i.e. dietary modification, antidiarrheal or laxative agents, antispasmodics, antidepressants) demonstrated that 76% of patients who received ⩽12 1-h sessions of GI-focused hypnotherapy (over 3 months) achieved a ⩾50-point decrease from baseline in overall IBS Symptom Severity Score (IBS-SSS) post-treatment (p < 0.001). Further, 58% and 42% of patients achieved ⩾100- and ⩾150-point decreases from baseline, respectively, in IBS-SSS post-treatment.49 Similarly, in a smaller study of patients with IBS (n = 85) undergoing once-weekly sessions of GI-focused hypnotherapy for 12 weeks, 58% of patients achieved a ⩾50-point decrease from baseline in the overall IBS-SSS at week 12 (p < 0.0001); female sex was predictive of response to hypnotherapy (p = 0.003) and no adverse events (AEs) were reported.50 A meta-analysis of five RCTs (n = 278) reported that significantly fewer patients with IBS receiving hypnotherapy experienced no improvement in IBS symptoms compared with patients receiving control therapy (54.6% versus 77.4%, respectively; RR = 0.7; 95% CI: 0.6–0.9); the NNT for hypnotherapy was 5 (95% CI: 3.5–10).36 Given the success of psychological therapies, including CBT and gut-directed hypnotherapy, in patients with IBS, the American Gastroenterological Association recommends that gastroenterologists consider incorporating mental health providers into the patient care model.51 Thus, psychological therapies, such as CBT and hypnotherapy, should be considered safe, alternative treatment options for patients with IBS-D, especially patients with concomitant psychiatric disorders or those who do not respond adequately to other treatment modalities. Given the safety and favorable treatment profiles, psychological therapies should be offered early in the course of therapy and not saved as a ‘last ditch’ treatment option.
Pharmacological therapies
Opioid receptor agonists/antagonists
Loperamide and diphenoxylate/atropine
ACG guidelines currently recommend against the use of the antidiarrheal agent loperamide for overall symptom improvement in patients with IBS.17 Loperamide was no more effective than placebo in a pooled analysis of two RCTs (n = 42; RR = 0.4; 95% CI: 0.1–1.4).17 In one study by Cann and colleagues, not included in the aforementioned pooled analysis, loperamide improved daily stool frequency compared with placebo after 5 weeks of treatment (1.3 versus 1.9 stools/day, respectively); however, loperamide did not improve the number of days per week that patients experienced abdominal pain compared with placebo (3.5 versus 3.4 days).52 Additionally, in a randomized, double-blind study of patients with acute nonspecific diarrhea (n = 493), a 2-day treatment with loperamide 2 mg alone (maximum daily dose, 8 mg) was not as effective as the combination of loperamide 2 mg and simethicone 125 mg for the relief of gas-related symptoms (e.g. pain and bloating); the median time to complete relief of gas-related abdominal discomfort was 42 h with loperamide alone and 12 h with loperamide and simethicone combined versus 48 h with placebo (p < 0.001, for loperamide and simethicone combined versus other groups).53 Similarly, in a subsequent randomized, double-blind study of patients with acute diarrhea (n = 483), the median time to complete relief of gas-related abdominal discomfort (e.g. pain and bloating) was significantly shorter with a 2-day treatment of loperamide 2 mg and simethicone 125 mg combination compared with loperamide 2 mg alone (maximum daily dose, 8 mg) or placebo (12 versus 24 and 23.5 h, respectively; p = 0.0001, for both comparisons).54 In a randomized, double-blind study of patients with IBS (n = 69), treatment with loperamide 2 mg once daily (adjusted as needed to 4 mg/day for 5 days, and up to 6 mg/day for the next 4 days) did not improve abdominal pain from baseline, or compared with placebo, after 5 weeks.55 In a randomized, double-blind crossover study (n = 30) that included patients with IBS-D (n = 21), the antidiarrheal agent diphenoxylate 5 mg/atropine sulfate 0.025 mg was associated with a significantly smaller percentage of solid stools compared with loperamide 2 mg or codeine 45 mg after 4 weeks of treatment (36.3% versus 67.8% and 58.4%, respectively; p < 0.01 for diphenoxylate versus loperamide and versus codeine).56 Further, diphenoxylate treatment was associated with significantly more AEs than loperamide treatment (p < 0.05), including central effects (i.e. depression, dizziness, drowsiness, nausea/vomiting; p < 0.01).56 In summary, although commonly used in clinical practice, loperamide is not effective at treating two of the most bothersome symptoms of IBS-D, namely abdominal pain and bloating. Other agents that improve multiple symptoms of IBS are preferable.
Eluxadoline
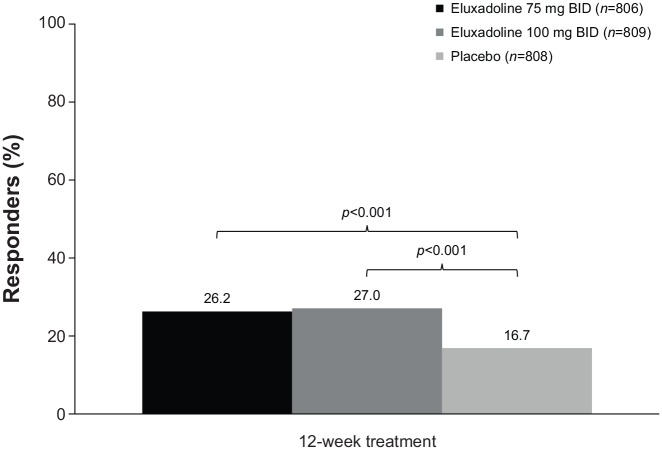
The mixed µ-opioid receptor agonist/delta opioid receptor antagonist eluxadoline is indicated for the treatment of adults with IBS-D.57 The prescribing information for eluxadoline recommends dosing of 75 mg or 100 mg twice daily, depending on concomitant medication use, tolerability, and hepatic impairment.57 Eluxadoline is contraindicated in patients without a gallbladder,57 due to an increased risk of developing pancreatitis.58 Eluxadoline is also contraindicated in patients with a history of alcoholism, alcohol abuse, or addiction, and in those who consume more than three alcohol-containing beverages per day.57 A significantly greater percentage of patients with IBS-D receiving eluxadoline 75 mg (n = 806) and 100 mg (n = 809) twice daily achieved a composite response for abdominal pain and stool consistency (decrease of ⩾30% in worst abdominal pain for ⩾50% of days, and, on the same days, a Bristol Stool Scale score <5) compared with placebo (n = 808) after 12 weeks of treatment in a pooled analysis of two randomized, double-blind, placebo-controlled studies (response rates of 26.2% and 27.0%, versus 16.7%, respectively; p < 0.001 for both treatments versus placebo; Figure 1).38 Further, the percentage of composite responders after 26 weeks was significantly greater with eluxadoline 75 mg or 100 mg than with placebo (26.7% and 31.0%, respectively, versus 19.5%; p < 0.001 for both treatments versus placebo).38 A pooled subgroup analysis of these two studies showed that for patients <65 years of age, the percentage of composite responders with eluxadoline 75 mg (n = 743) or 100 mg (n = 732) was greater versus placebo (n = 707; 24.6% and 30.1% versus 19.5%, respectively; p values not provided).59 For patients ⩾65 years of age, a greater percentage of composite responders was observed with eluxadoline 75 mg (n = 65; 50.8%) and eluxadoline 100 mg (n = 74; 40.5%) versus placebo (n = 102; 19.6%; p values not provided).59 In addition, the efficacy of eluxadoline was not affected by sex or race, although 86.0% of the patients in the pooled analysis were White.59
Figure 1.
Percentage of responders to 12 weeks of daily eluxadoline.38 Responders were defined as patients with a ⩾30% decrease from baseline in worst abdominal pain for ⩾50% of days, and, on the same days, a Bristol Stool Scale score <5.
BID, twice daily.
The most common AEs reported by patients receiving 26–52 weeks of eluxadoline 75 mg or 100 mg (pooled treatment groups versus placebo) were constipation (8.0% versus 2.5%), nausea (7.7% versus 5.1%), and abdominal pain (6.5% versus 4.1%).38 Some patients discontinued the study due to constipation (eluxadoline 75 mg or 100 mg versus placebo: 1.1% or 1.7%, respectively, versus 0.2%) and nausea (0.6% or 0%, respectively, versus 0.5%). Serious AEs of pancreatitis and sphincter of Oddi dysfunction were reported in five (0.3%) and eight (0.5%) patients receiving eluxadoline 75 mg or 100 mg, respectively; these AEs did not occur in any patients receiving placebo.38 Of the 597 reports to the Federal Adverse Events Reporting System between July 2015 and September 2016 involving eluxadoline use, 98 reports (16.4%) were for pancreatitis, and 30 (5.0%) involved sphincter of Oddi dysfunction.60 Thus, eluxadoline may be a reasonable pharmacologic treatment option, especially in patients who have failed more conservative measures, but selecting the appropriate patient population is of great importance.
Smooth muscle antispasmodics
The ACG provides a weak recommendation for smooth muscle antispasmodics, such as cimetropium, dicyclomine, drotaverine, hyoscine, otilonium, and pinaverium, for the improvement of IBS symptoms, including acute abdominal pain, based on evidence from 26 randomized controlled studies (n = 2811 patients).17 Overall, antispasmodics were significantly more effective in improving IBS symptoms compared with placebo (RR of IBS symptoms not improving = 0.6; 95% CI: 0.6–0.8).17 However, the small number of studies for individual agents examined within this group limited the conclusions that could be made regarding any specific antispasmodic agent.17 Of note, antispasmodics were associated with a significantly greater risk of AEs compared with placebo, based on a pooled analysis of 17 studies (RR = 1.6; 95% CI: 1.2–2.2), such as dry mouth, dizziness, and blurred vision, although no serious AEs were reported in any of the studies.17
Peppermint oil
Peppermint oil is an antispasmodic agent shown to significantly improve IBS symptoms compared with placebo based on an analysis of seven RCTs (n = 634 patients; RR of IBS symptoms not improving = 0.5; 95% CI: 0.4–0.8).17 Further, peppermint oil seems to be a relatively safe treatment option, as a pooled safety analysis of six RCTs noted that the incidence of AEs was comparable between peppermint oil and placebo (RR = 1.9; 95% CI: 0.8–4.5).17 Peppermint oil may therefore be a practical treatment option, especially among patients seeking a ‘natural’ remedy.
Bile acid sequestrants
Bile acid malabsorption was shown to occur in 28.1% (95% CI: 22.6%–34%) of patients with IBS-D in a pooled analysis of six studies (n = 908).61 A trial of bile acid sequestrant therapy (e.g. cholestyramine) therefore may be a reasonable approach for management of patients with IBS-D; however, data on the use of these agents in patients with IBS-D are limited. Although a gold-standard diagnostic test for bile acid malabsorption is currently lacking,61 retention of 75Se-labeled homocholic acid-taurine (75SeHCAT) has been used to diagnose bile acid malabsorption.62 In an open-label study of patients with IBS with bile acid malabsorption (determined by 75SeHCAT retention levels), 15 of 27 patients (55%) achieved a response to colestipol (response defined as adequate relief of symptoms from baseline of ⩾50% during at least 2 of the last 4 weeks of the 8-week treatment period).62 Additionally, a surrogate marker for bile acid malabsorption is the measurement of serum 7α-hydroxy-4-cholesten-3-one (7C4) levels.63,64 Results of a single-center study suggested that serum 7C4 levels were elevated in randomly selected patients with IBS-D (n = 22) compared with patients with IBS-C (n = 26; p = 0.02) or healthy volunteers (n = 23; p = 0.01).63 A small, open-label study of patients with IBS-D with bile acid malabsorption (n = 12) reported significant improvement from baseline in stool consistency (according to Bristol Stool Scale scores) following a 10-day course of colesevelam treatment (from 4.8 to 4.4; p = 0.04); fecal bile acid excretion (determined by 7C4 serum levels) was also increased from baseline after 10 days (from 1558 to 3496 µmol; p = 0.01).64 In summary, a trial of a bile acid sequestrant may be considered in patients with IBS-D, especially those who have undergone cholecystectomy, as >25% of patients with IBS-D may have concomitant bile acid malabsorption.61 However, care must be taken with the timing of dosing, as bile acid sequestrants can interfere with the absorption of other medications.
Antidepressants
A meta-analysis of 18 RCTs of antidepressants for the treatment of IBS symptoms [tricyclic antidepressants (TCAs): n = 11; selective serotonin reuptake inhibitors (SSRIs): n = 6; both TCAs and SSRIs: n = 1] found that 43.5% of treated patients (n = 612) did not experience improvement of symptoms, compared with 66.0% of patients receiving placebo (n = 515; RR = 0.7; 95% CI: 0.6–0.8).36 Based on the findings of eight RCTs, AEs were reported in a greater percentage of patients receiving antidepressants for IBS symptoms (n = 228) compared with placebo (n = 223; 36.4% versus 21.1%, respectively; RR of any AE = 1.6; 95% CI: 1.2–2.0).36 However, no serious AEs were reported, and the number needed to harm (NNH) for antidepressants was 8.5 (95% CI: 5–21).36
Tricyclic antidepressants
A meta-analysis of 12 RCTs of TCAs found that 42.7% of 436 patients receiving these drugs for treatment of IBS symptoms did not experience improvement, compared with 63.8% of 351 patients receiving placebo (RR = 0.6; 95% CI: 0.6–0.8).36 However, studies included in the meta-analysis differed in the definition of response, and most studies were conducted prior to the 2012 recommended FDA guidance regarding efficacy endpoints in clinical studies of IBS.36,65 The NNT for TCAs was 4.5 (95% CI: 3.5–7).36 A meta-analysis of six RCTs of TCAs reported that the risk of any AE was significantly greater with TCAs than with placebo (RR = 1.6; 95% CI: 1.2–2.1); the most common reported adverse events were drowsiness and dry mouth.36 However, constipation can also occur as a side effect with use of TCAs. Therefore, TCAs may be a particularly useful treatment option for IBS-D, given the perceived beneficial effects of TCAs on visceral hypersensitivity and the brain–gut axis, but also the potentially advantageous consequence of slowing GI motility in patients with diarrhea.
Selective serotonin reuptake inhibitors
A meta-analysis of seven RCTs of SSRIs for treatment of IBS symptoms found that 45.5% of 176 treated patients reported no improvement of their symptoms, compared with 67.2% of 180 patients receiving placebo (RR = 0.7; 95% CI: 0.5–0.9).36 The NNT for SSRIs was 5 (95% CI: 3–16.5), although it should be noted that there was significant heterogeneity among studies (i.e. patient populations, methods).36 Based on low quality of evidence, ACG guidelines weakly recommend SSRIs for improvement of symptoms in patients with IBS.36 However, it should be noted that SSRIs can cause diarrhea, and may therefore be a more appropriate treatment option for patients with IBS with constipation.
Rifaximin
The nonsystemic antibiotic rifaximin is indicated for the treatment of adults with IBS-D.66 It has been presumed that rifaximin alters the gut microbiota through its actions as an antibiotic, although analysis of fecal samples from patients with IBS-D receiving a 2-week treatment with rifaximin showed only modest, transient changes in gut microbiota. Therefore, the positive effect of rifaximin in the treatment of IBS-D may be related to other mechanisms that have yet to be elucidated.67 Fortunately, rifaximin use does not appear to cause antibiotic resistance, as skin Staphylococcus and fecal samples from patients with IBS-D receiving 2-week courses of repeat rifaximin treatment showed no evidence of antibiotic resistance to 11 different antibiotics.68,69
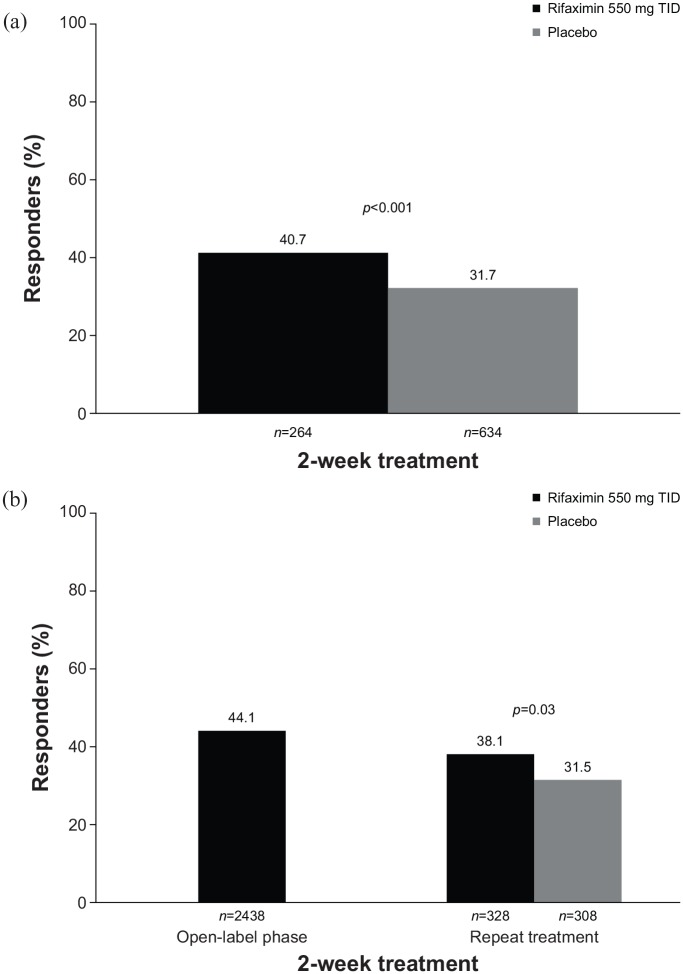
A 2-week course of rifaximin 550 mg three times daily was shown to improve global IBS symptoms for ⩾2 of the first 4 weeks post-treatment (primary efficacy endpoint) in two identically designed, randomized, double-blind, placebo-controlled phase III studies of patients with nonconstipation IBS (all treated patients had IBS-D70) compared with placebo (pooled analysis, 40.7% versus 31.7%, respectively; p < 0.001; Figure 2a).71 A significantly greater percentage of patients receiving a 2-week course of rifaximin achieved adequate relief of bloating for ⩾2 of the first 4 weeks post-treatment (key secondary efficacy endpoint) compared with placebo (pooled analysis, 40.2% versus 30.3%; p < 0.001).71 The therapeutic gain with a 2-week course of rifaximin versus placebo was approximately 10% in this study, and this gain was maintained for at least 10 treatment-free weeks. Of note, the safety profile of rifaximin was comparable to that of placebo, with headache (6.1% versus 6.6%, respectively), upper respiratory tract infection (5.6% versus 6.2%), abdominal pain (4.6% versus 5.5%), nausea (4.3% versus 3.8%), diarrhea (4.3% versus 3.5%), and nasopharyngitis (3.0% versus 5.4%) the most common AEs reported. No patients in either of these trials experienced Clostridium difficile infection or ischemic colitis.
Figure 2.
Percentage of responders to a 2-week course of rifaximin.71,72 (a) Responders were defined as patients with adequate relief of global IBS symptoms (determined by ‘yes’ or ‘no’ response to the weekly question ‘In regard to all your symptoms of IBS, as compared with the way you felt before you started the study medication, have you, in the past 7 days, had adequate relief of your IBS symptoms?’) for ⩾2 of the first 4 weeks post-treatment. (b) Responders were defined as patients with ⩾30% decrease from baseline in mean weekly pain score and ⩾50% decrease from baseline in the number of days/week with Bristol Stool Scale type 6 or 7 stool for ⩾2 of the first 4 weeks post-treatment.
IBS, irritable bowel syndrome; TID, three times daily.
In addition, a phase III repeat-treatment study evaluated the efficacy and safety of up to three 2-week courses of rifaximin treatment to manage IBS-D symptom recurrence.72 Among 2438 evaluable patients with IBS-D who received a 2-week course of open-label rifaximin 550 mg three times daily, 1074 (44.1%) achieved a response with regard to abdominal pain and stool consistency (i.e. ⩾30% decrease from baseline in mean weekly pain score and ⩾50% decrease from baseline in the number of days/week with Bristol Stool Scale type 6 or 7 stool for ⩾2 of the first 4 weeks posttreatment; Figure 2b). Responders (n = 636) with subsequent IBS symptom recurrence within 18 weeks of treatment-free follow up then entered a randomized, double-blind, placebo-controlled phase examining the efficacy and safety of repeat rifaximin treatment. A significantly greater percentage of patients receiving repeat treatment with rifaximin (n = 328) achieved response (primary endpoint) compared with placebo (n = 308) for ⩾2 of the first 4 weeks post-treatment (38.1% versus 31.5%, respectively; p = 0.03; Figure 2b).72 In this study, up to three courses of rifaximin were well tolerated, with a generally comparable incidence of AEs among treatment groups (double-blind phase: rifaximin, 42.7%; placebo, 45.5%). The most common AEs with repeat rifaximin treatment compared with placebo were nausea (3.7% versus 2.3%, respectively), upper respiratory tract infection (3.7% versus 2.6%), urinary tract infection (3.4% versus 4.9%), and nasopharyngitis (3.0% versus 2.9%). Serious AEs occurred in four rifaximin-treated patients [1.2%; breast cancer (n = 1), Clostridium difficile colitis (n = 1), dyspnea (n = 1), fall (n = 1)] and four placebo-treated patients [1.3%; moderate noncardiac chest pain and severe coronary artery occlusion (n = 1), moderate transient ischemic attack (n = 1), severe cellulitis (n = 1), severe hypertension and severe transient ischemic attack (n = 1)] during the double-blind treatment phase of the study; none were considered to be treatment-related. Thus, rifaximin appears to be a relatively safe and well-accepted nonsystemic option for the treatment of IBS-D. One advantage, compared with other agents that need to be used daily, is that a 2-week course of rifaximin may improve symptoms in some patients for up to 10 weeks. However, many patients note a recurrence of symptoms, and, thus, retreatment will likely be required.
5-HT3 antagonists
Alosetron
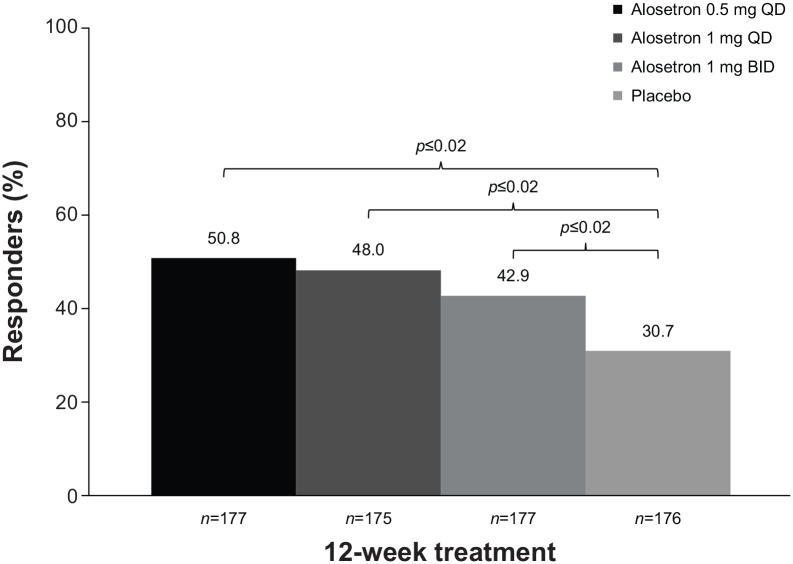
The selective serotonin 5-HT3 antagonist alosetron is indicated for the treatment of women with severe IBS-D who have failed conventional therapy. The recommended starting dose is 0.5 mg twice daily, which may be increased to 1 mg twice daily after 4 weeks.73 In a randomized, double-blind, placebo-controlled study, a significantly greater percentage of women with severe IBS-D (n = 705) receiving alosetron 0.5 mg or 1 mg once daily, or 1 mg twice daily, achieved response (moderate or substantial improvement in global IBS symptoms over the past 4 weeks), with rates of 50.8% (0.5 mg once daily), 48.0% (1 mg once daily), and 42.9% (1 mg twice daily), compared with placebo (30.7%; p ⩽ 0.02 for all) after 12 weeks (Figure 3).74 Constipation was the most common AE reported by patients receiving alosetron 0.5 mg (9%) or 1 mg (16%) once daily, or 1 mg twice daily (19%); constipation occurred in 5% of patients receiving placebo. Most (75%) patients experiencing constipation with alosetron treatment reported one episode during the first 2 weeks of treatment. The percentage of patients who experienced serious AEs was 4% each for alosetron 0.5 mg and 1 mg once daily, 3% for 1 mg twice daily, and 2% for placebo. One patient receiving alosetron 0.5 mg once daily developed ischemic colitis after 31 days of treatment and was discontinued from the study. Two patients had possible complications of constipation [bowel obstruction (n = 1) and fecal impaction (n = 1)] following treatment with alosetron 0.5 mg once daily and 1 mg twice daily, respectively; both events were considered to be treatment related.
Figure 3.
Percentage of women with severe IBS-D with response to 12 weeks of daily alosetron.74 Responders were defined as patients with either moderate or substantial improvement in global IBS symptoms, as determined by response to the question ‘Compared to the way you usually felt during the 3 months before you entered the study, are your IBS symptoms over the past 4 weeks substantially worse, moderately worse, slightly worse, no change, slightly improved, moderately improved, or substantially improved?’
BID, twice daily; IBS, irritable bowel syndrome; QD, once daily.
In an open-label study, 45% of 105 women with severe IBS-D were composite responders to alosetron (defined as a decrease from baseline of ⩾30% in weekly abdominal pain score and a decrease from baseline of ⩾50% in the number of days/week with at least one stool with Bristol Stool Scale score type 6 or 7, for ⩾6 of 12 weeks of treatment).75 Alosetron dosing was initiated at 0.5 mg twice daily, and the study allowed for dose escalation to 1 mg twice daily if the initial dose was well tolerated after 4 weeks. Alosetron is still marketed under a Risk Evaluation and Mitigation Strategy program with the aim of decreasing the occurrence of serious AEs, such as ischemic colitis and complications of constipation.73,76 However, a 9-year analysis (i.e. November 2002 to December 2011) of postmarketing reports of AEs in the alosetron safety database found that the incidence rates of probable and possible cases of ischemic colitis and complications of constipation were low at 1.03/1000 patient-years and 0.25/1000 patient-years, respectively.77 In summary, alosetron is a reasonable consideration for the treatment of women with severe IBS-D symptoms, and dose titration may help reduce the risk of adverse effects.
Ondansetron
In a randomized, double-blind, crossover study of 120 adults with IBS-D, the 5-HT3 antagonist ondansetron significantly improved stool form compared with placebo for the last 2 weeks of a 5-week treatment period (p < 0.001).42 In this study, compared with placebo, ondansetron significantly decreased the number of days with urgency (p < 0.001) and bloating (p = 0.002), as well as stool frequency (11% decrease; p = 0.001), but did not affect the number of days with pain (p = 0.2) or the average pain score (p = 0.1). Ondansetron significantly reduced the mean urgency score compared with placebo (p < 0.001). For patients included in the ITT analysis (n = 98), constipation was the most common AE with ondansetron versus placebo (9% versus 2%, respectively). Other AEs reported with ondansetron versus placebo included headache (n = 2 in each group), rectal bleeding (n = 2 in each group; none due to ischemic colitis), abdominal pain (n = 2 versus n = 1, respectively), and backache (n = 1 in each group). Despite these data, ondansetron is not widely used as a treatment for IBS-D.
Crofelemer
The efficacy and safety of crofelemer, a plant-derived product, was evaluated in a randomized, double-blind, placebo-controlled study of 244 adults with IBS-D.41 Crofelemer did not improve stool consistency response [primary efficacy outcome; defined as a decrease from baseline ⩾0.5 point in the stool consistency score (scale range, 1–5)] compared with placebo after 3 months.41 However, crofelemer 500 mg twice daily significantly decreased the daily stool frequency from baseline versus placebo after 3 months (change from baseline, ‒0.4; p = 0.03).41 Further, crofelemer 500 mg twice daily significantly improved the number of pain-free days compared with placebo at month 3 (24.3% improvement; p = 0.03).41 The most common AEs with crofelemer 500 mg twice daily versus placebo were abdominal pain (5% versus 2%, respectively), headache (6% versus 8%), IBS (5% versus 3%), and nausea (5% versus 7%).41 Like ondansetron, crofelemer is not widely used in the treatment of IBS-D. Large, prospective, randomized, placebo-controlled trials using the Rome IV criteria and new FDA and European Medicines Agency guidelines that define responders are needed to support its use.
Discussion and conclusions
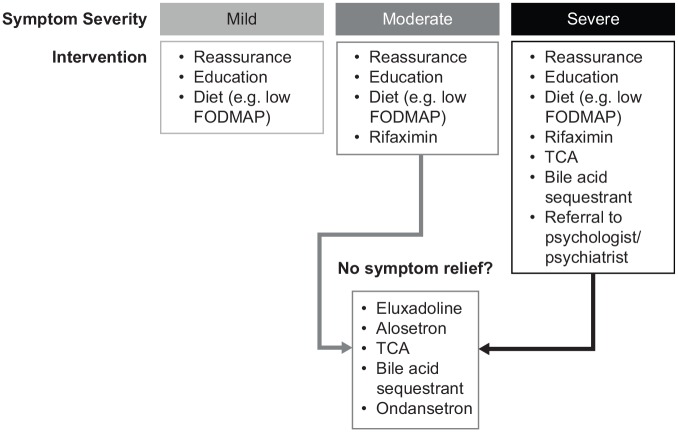
A number of different therapeutic interventions are available for the management of patients with IBS, including approved pharmacological agents (i.e. eluxadoline, rifaximin, and alosetron) and other interventions (e.g. dietary modification, psychological interventions). Treatment of patients with IBS should be personalized, taking into consideration the most troublesome symptoms, symptom intensity, and patient preferences regarding treatment goals and acceptable risks with treatment (Figure 4). Of note, a survey of patients with IBS (n = 182) reported that patients were willing to accept a median 1% risk of sudden mortality associated with use of a hypothetical medication, if they had a 99% chance of cure of IBS symptoms.78 Efficacy and safety profiles of therapies are important factors to consider when making management decisions. NNT and NNH values, when available, can provide guidance regarding the potential benefits and risks associated with each treatment. Unfortunately, variability across clinical study populations (e.g. criteria for IBS, sample size) and stringency of clinical trial endpoints limit comparisons that can be made across interventions.
Figure 4.
Proposed treatment algorithm for management of patients with irritable bowel syndrome with diarrhea.
FODMAP, fermentable oligosaccharides, disaccharides, monosaccharides, and polyols; TCA, tricyclic antidepressant.
Dietary modification is commonly recommended to patients with IBS to improve overall symptoms, although the quality of evidence for such a recommendation is low because of variability in study designs and the small numbers of patients included in individual studies. Psychological therapies, such as CBT and hypnotherapy, are intriguing treatment options given their demonstrated efficacy in managing IBS symptoms, low NNT, and minimal-to-no negative adverse effect profile.
Bile acid malabsorption is estimated to occur in more than one-quarter of patients with IBS-D. Therefore, empiric treatment with a bile acid sequestrant can be considered, as there is currently no standard diagnostic test for bile acid malabsorption. Loperamide is effective for the treatment of acute diarrhea but not for abdominal pain and bloating, which patients often consider to be among the most bothersome IBS symptoms. Eluxadoline improves symptoms in patients with IBS-D, but is contraindicated in patients with prior cholecystectomy and in those with a history of alcohol abuse. Three large studies have demonstrated that 2-week courses of rifaximin are efficacious and well tolerated in patients with IBS-D, though therapeutic gain over placebo was modest and retreatment is common. Limited data suggest that SBI, ondansetron, and crofelemer may be efficacious in improving some symptoms associated with IBS-D, though these agents are currently not widely used to treat this condition. Finally, antidepressants have been proven effective in the treatment of symptoms of IBS-D, although potential adverse effects need to be considered with their use. In conclusion, a number of effective pharmacological and nonpharmacological interventions are available for the management of adults with IBS-D, and there is no universally accepted algorithm to guide management. Therefore, management should be individualized and the benefit–risk ratio for each therapy, as well as individual patient preference, should be prioritized in formulating a treatment plan for patients with IBS-D.
Acknowledgments
Technical editorial and medical writing assistance was provided under the direction of the authors by Mary Beth Moncrief and Sophie Bolick, Synchrony Medical Communications, LLC, West Chester, PA. Funding for this support was provided by Salix Pharmaceuticals.
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and publication of this article: Funding for technical editorial and medical writing assistance was provided by Salix Pharmaceuticals.
Conflict of interest statement: DJC reports having nothing to disclose.
BEL reports serving as an advisory board member for Ironwood Pharmaceuticals, Inc., Salix Pharmaceuticals, and Forest Laboratories, a subsidiary of Allergan plc.
ORCID iD: Brian E. Lacy  https://orcid.org/0000-0003-4121-7970
https://orcid.org/0000-0003-4121-7970
Contributor Information
David J. Cangemi, Division of Gastroenterology and Hepatology, Section of Gastroenterology, Mayo Clinic, Jacksonville, FL, USA
Brian E. Lacy, Division of Gastroenterology and Hepatology, Section of Gastroenterology, Mayo Clinic, 4500 San Pablo Road, Jacksonville, FL 32224, USA.
References
- 1. Lacy BE, Mearin F, Chang L, et al. Bowel disorders. Gastroenterology 2016; 150: 1393–1407. [DOI] [PubMed] [Google Scholar]
- 2. Ringel Y, Williams RE, Kalilani L, et al. Prevalence, characteristics, and impact of bloating symptoms in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol 2009; 7: 68–72. [DOI] [PubMed] [Google Scholar]
- 3. Buono JL, Carson RT, Flores NM. Health-related quality of life, work productivity, and indirect costs among patients with irritable bowel syndrome with diarrhea. Health Qual Life Outcomes 2017; 15: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ballou S, Keefer L. The impact of irritable bowel syndrome on daily functioning: characterizing and understanding daily consequences of IBS. Neurogastroenterol Motil 2017; 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Ballou S, Alhassan E, Hon E, et al. Sleep disturbances are commonly reported among patients presenting to a gastroenterology clinic. Dig Dis Sci 2018; 63: 2983–2991. [DOI] [PubMed] [Google Scholar]
- 6. Ford AC, Lacy BE, Talley NJ. Irritable bowel syndrome. N Engl J Med 2017; 376: 2566–2578. [DOI] [PubMed] [Google Scholar]
- 7. Lenhart A, Ferch C, Shaw M, et al. Use of dietary management in irritable bowel syndrome: results of a survey of over 1500 United States gastroenterologists. J Neurogastroenterol Motil 2018; 24: 437–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schnabel L, Buscail C, Sabate JM, et al. Association between ultra-processed food consumption and functional gastrointestinal disorders: results from the French NutriNet-Santé cohort. Am J Gastroenterol 2018; 113: 1217–1228. [DOI] [PubMed] [Google Scholar]
- 9. Buscail C, Sabate JM, Bouchoucha M, et al. Western dietary pattern is associated with irritable bowel syndrome in the French NutriNet cohort. Nutrients 2017; 9: 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Buscail C, Sabate JM, Bouchoucha M, et al. Association between self-reported vegetarian diet and the irritable bowel syndrome in the French NutriNet cohort. PLoS One 2017; 12: e0183039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dionne J, Ford AC, Yuan Y, et al. A systematic review and meta-analysis evaluating the efficacy of a gluten-free diet and a low FODMAPs diet in treating symptoms of irritable bowel syndrome. Am J Gastroenterol 2018; 113: 1290–1300. [DOI] [PubMed] [Google Scholar]
- 12. Vazquez-Roque MI, Camilleri M, Smyrk T, et al. A controlled trial of gluten-free diet in patients with irritable bowel syndrome-diarrhea: effects on bowel frequency and intestinal function. Gastroenterology 2013; 144: 903–911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zahedi MJ, Behrouz V, Azimi M. Low fermentable oligo-di-mono-saccharides and polyols diet versus general dietary advice in patients with diarrhea-predominant irritable bowel syndrome: a randomized controlled trial. J Gastroenterol Hepatol 2018; 33: 1192–1199. [DOI] [PubMed] [Google Scholar]
- 14. Eswaran S, Chey WD, Jackson K, et al. A diet low in fermentable oligo-, di-, and mono-saccharides and polyols improves quality of life and reduces activity impairment in patients with irritable bowel syndrome and diarrhea. Clin Gastroenterol Hepatol 2017; 15: 1890–1899. [DOI] [PubMed] [Google Scholar]
- 15. Trott N, Aziz I, Rej A, et al. How patients with IBS use low FODMAP dietary information provided by general practitioners and gastroenterologists: a qualitative study. Nutrients 2019; 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Whigham L, Joyce T, Harper G, et al. Clinical effectiveness and economic costs of group versus one-to-one education for short-chain fermentable carbohydrate restriction (low FODMAP diet) in the management of irritable bowel syndrome. J Hum Nutr Diet 2015; 28: 687–696. [DOI] [PubMed] [Google Scholar]
- 17. Ford AC, Moayyedi P, Chey WD, et al. American College of Gastroenterology monograph on management of irritable bowel syndrome. Am J Gastroenterol 2018; 113: 1–18. [DOI] [PubMed] [Google Scholar]
- 18. Zhou C, Zhao E, Li Y, et al. Exercise therapy of patients with irritable bowel syndrome: a systematic review of randomized controlled trials. Neurogastroenterol Motil 2018: e13461. [DOI] [PubMed] [Google Scholar]
- 19. Patel N, Lacy B. Does yoga help patients with irritable bowel syndrome? Clin Gastroenterol Hepatol 2016; 14: 1732–1734. [DOI] [PubMed] [Google Scholar]
- 20. Schumann D, Langhorst J, Dobos G, et al. Randomised clinical trial: yoga vs a low-FODMAP diet in patients with irritable bowel syndrome. Aliment Pharmacol Ther 2018; 47: 203–211. [DOI] [PubMed] [Google Scholar]
- 21. Schumann D, Cramer H. Editorial: irritable bowel syndrome—in addition to having properly-trained dietitians, is it time to add a yoga teacher to our multidisciplinary team? Authors’ reply. Aliment Pharmacol Ther 2018; 47: 433–434. [DOI] [PubMed] [Google Scholar]
- 22. Muir JG, Iacovou M. Editorial: irritable bowel syndrome—in addition to having properly-trained dietitians, is it time to add a yoga teacher to our multidisciplinary team? Aliment Pharmacol Ther 2018; 47: 432–433. [DOI] [PubMed] [Google Scholar]
- 23. Ouyang H, Chen JD. Review article: therapeutic roles of acupuncture in functional gastrointestinal disorders. Aliment Pharmacol Ther 2004; 20: 831–841. [DOI] [PubMed] [Google Scholar]
- 24. Zhu L, Ma Y, Ye S, et al. Acupuncture for diarrhoea-predominant irritable bowel syndrome: a network meta-analysis. Evid Based Complement Alternat Med 2018; 2018: 2890465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Manheimer E, Wieland LS, Cheng K, et al. Acupuncture for irritable bowel syndrome: systematic review and meta-analysis. Am J Gastroenterol 2012; 107: 835–847; quiz 848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. MacPherson H, Tilbrook H, Agbedjro D, et al. Acupuncture for irritable bowel syndrome: 2-year follow-up of a randomised controlled trial. Acupunct Med 2017; 35: 17–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Ford AC, Harris LA, Lacy BE, et al. Systematic review with meta-analysis: the efficacy of prebiotics, probiotics, synbiotics and antibiotics in irritable bowel syndrome. Aliment Pharmacol Ther 2018; 48: 1044–1060. [DOI] [PubMed] [Google Scholar]
- 28. Shapiro J, Bernica J, Hernaez R. Risk of bias analysis of systematic reviews of probiotics for treatment of irritable bowel syndrome. Clin Gastroenterol Hepatol 2019; 17: 784–785. [DOI] [PubMed] [Google Scholar]
- 29. Hod K, Dekel R, Aviv Cohen N, et al. The effect of a multispecies probiotic on microbiota composition in a clinical trial of patients with diarrhea-predominant irritable bowel syndrome. Neurogastroenterol Motil 2018: e13456. [DOI] [PubMed] [Google Scholar]
- 30. Ishaque SM, Khosruzzaman SM, Ahmed DS, et al. A randomized placebo-controlled clinical trial of a multi-strain probiotic formulation (Bio-Kult®) in the management of diarrhea-predominant irritable bowel syndrome. BMC Gastroenterol 2018; 18: 71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Hungin APS, Mitchell CR, Whorwell P, et al. Systematic review: probiotics in the management of lower gastrointestinal symptoms-an updated evidence-based international consensus. Aliment Pharmacol Ther 2018; 47: 1054–1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sun YY, Li M, Li YY, et al. The effect of Clostridium butyricum on symptoms and fecal microbiota in diarrhea-dominant irritable bowel syndrome: a randomized, double-blind, placebo-controlled trial. Sci Rep 2018; 8: 2964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Moser AM, Spindelboeck W, Halwachs B, et al. Effects of an oral synbiotic on the gastrointestinal immune system and microbiota in patients with diarrhea-predominant irritable bowel syndrome. Eur J Nutr. Epub ahead of print 24 September 2018. DOI: 10.1007/s00394-018-1826-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Wilson D, Evans M, Weaver E, et al. Evaluation of serum-derived bovine immunoglobulin protein isolate in subjects with diarrhea-predominant irritable bowel syndrome. Clin Med Insights Gastroenterol 2013; 6: 49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Oliva EM, Christopher ML, Wells D, et al. Opioid overdose education and naloxone distribution: development of the Veterans Health Administration’s national program. J Am Pharm Assoc (2003) 2017; 57: S168–S179 e4. [DOI] [PubMed] [Google Scholar]
- 36. Ford AC, Lacy BE, Harris L, et al. Effect of antidepressants and psychological therapies in irritable bowel syndrome: an updated systematic review and meta-analysis. Am J Gastroenterol 2019; 114: 21–39. [DOI] [PubMed] [Google Scholar]
- 37. Shah E, Kim S, Chong K, et al. Evaluation of harm in the pharmacotherapy of irritable bowel syndrome. Am J Med 2012; 125: 381–393. [DOI] [PubMed] [Google Scholar]
- 38. Lembo AJ, Lacy BE, Zuckerman MJ, et al. Eluxadoline for irritable bowel syndrome with diarrhea. N Engl J Med 2016; 374: 242–253. [DOI] [PubMed] [Google Scholar]
- 39. Lacy BE, Dove S, Andrae D, et al. Robustness of eluxadoline for the treatment of irritable bowel syndrome with diarrhea: results from phase 3 composite endpoint assessments (Abstract no. Su1378). Gastroenterology 2015; 148: S–491. [Google Scholar]
- 40. Valentin N, Camilleri M, Carlson P, et al. Potential mechanisms of effects of serum-derived bovine immunoglobulin/protein isolate therapy in patients with diarrhea-predominant irritable bowel syndrome. Physiol Rep 2017; 5 pii: e13170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Mangel AW, Chaturvedi P. Evaluation of crofelemer in the treatment of diarrhea-predominant irritable bowel syndrome patients. Digestion 2008; 78: 180–186. [DOI] [PubMed] [Google Scholar]
- 42. Garsed K, Chernova J, Hastings M, et al. A randomised trial of ondansetron for the treatment of irritable bowel syndrome with diarrhoea. Gut 2014; 63: 1617–1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Radziwon CD, Lackner JM. Cognitive behavioral therapy for IBS: how useful, how often, and how does it work? Curr Gastroenterol Rep 2017; 19: 49. [DOI] [PubMed] [Google Scholar]
- 44. Kinsinger SW. Cognitive-behavioral therapy for patients with irritable bowel syndrome: current insights. Psychol Res Behav Manag 2017; 10: 231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Li L, Xiong L, Zhang S, et al. Cognitive-behavioral therapy for irritable bowel syndrome: a meta-analysis. J Psychosom Res 2014; 77: 1–12. [DOI] [PubMed] [Google Scholar]
- 46. Radu M, Moldovan R, Pintea S, et al. Predictors of outcome in cognitive and behavioural interventions for irritable bowel syndrome. A meta-analysis. J Gastrointestin Liver Dis 2018; 27: 257–263. [DOI] [PubMed] [Google Scholar]
- 47. Lackner JM, Jaccard J, Keefer L, et al. Improvement in gastrointestinal symptoms after cognitive behavior therapy for refractory irritable bowel syndrome. Gastroenterology 2018; 155: 47–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Peters SL, Muir JG, Gibson PR. Review article: gut-directed hypnotherapy in the management of irritable bowel syndrome and inflammatory bowel disease. Aliment Pharmacol Ther 2015; 41: 1104–1115. [DOI] [PubMed] [Google Scholar]
- 49. Miller V, Carruthers HR, Morris J, et al. Hypnotherapy for irritable bowel syndrome: an audit of one thousand adult patients. Aliment Pharmacol Ther 2015; 41: 844–855. [DOI] [PubMed] [Google Scholar]
- 50. Lövdahl J, Ringström G, Agerforz P, et al. Nurse-administered, gut-directed hypnotherapy in IBS: efficacy and factors predicting a positive response. Am J Clin Hypn 2015; 58: 100–114. [DOI] [PubMed] [Google Scholar]
- 51. Keefer L, Palsson OS, Pandolfino JE. Best practice update: incorporating psychogastroenterology into management of digestive disorders. Gastroenterology 2018; 154: 1249–1257. [DOI] [PubMed] [Google Scholar]
- 52. Cann PA, Read NW, Holdsworth CD, et al. Role of loperamide and placebo in management of irritable bowel syndrome (IBS). Dig Dis Sci 1984; 29: 239–247. [DOI] [PubMed] [Google Scholar]
- 53. Kaplan MA, Prior MJ, Ash RR, et al. Loperamide-simethicone vs loperamide alone, simethicone alone, and placebo in the treatment of acute diarrhea with gas-related abdominal discomfort. A randomized controlled trial. Arch Fam Med 1999; 8: 243–248. [DOI] [PubMed] [Google Scholar]
- 54. Hanauer SB, DuPont HL, Cooper KM, et al. Randomized, double-blind, placebo-controlled clinical trial of loperamide plus simethicone versus loperamide alone and simethicone alone in the treatment of acute diarrhea with gas-related abdominal discomfort. Curr Med Res Opin 2007; 23: 1033–1043. [DOI] [PubMed] [Google Scholar]
- 55. Efskind PS, Bernklev T, Vatn MH. A double-blind placebo-controlled trial with loperamide in irritable bowel syndrome. Scand J Gastroenterol 1996; 31: 463–468. [DOI] [PubMed] [Google Scholar]
- 56. Palmer KR, Corbett CL, Holdsworth CD. Double-blind cross-over study comparing loperamide, codeine and diphenoxylate in the treatment of chronic diarrhea. Gastroenterology 1980; 79: 1272–1275. [PubMed] [Google Scholar]
- 57. Viberzi (eluxadoline) tablets, for oral use, CIV [package insert]. Madison, NJ: Allergan USA, Inc, 2018. [Google Scholar]
- 58. US Food and Drug Administration. FDA drug safety communication: FDA warns about increased risk of serious pancreatitis with irritable bowel drug Viberzi (eluxadoline) in patients without a gallbladder, https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-warns-about-increased-risk-serious-pancreatitis-irritable-bowel (2017).
- 59. Lacy BE, Harris LA, Chang L, et al. Impact of patient and disease characteristics on the efficacy and safety of eluxadoline for IBS-D: a subgroup analysis of phase III trials. Therap Adv Gastroenterol 2019; 12: 1756284819841290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Gawron AJ, Bielefeldt K. Risk of pancreatitis following treatment of irritable bowel syndrome with eluxadoline. Clin Gastroenterol Hepatol 2018; 16: 378–384. [DOI] [PubMed] [Google Scholar]
- 61. Slattery SA, Niaz O, Aziz Q, et al. Systematic review with meta-analysis: the prevalence of bile acid malabsorption in the irritable bowel syndrome with diarrhoea. Aliment Pharmacol Ther 2015; 42: 3–11. [DOI] [PubMed] [Google Scholar]
- 62. Bajor A, Törnblom H, Rudling M, et al. Increased colonic bile acid exposure: a relevant factor for symptoms and treatment in IBS. Gut 2015; 64: 84–92. [DOI] [PubMed] [Google Scholar]
- 63. Wong BS, Camilleri M, Carlson P, et al. Increased bile acid biosynthesis is associated with irritable bowel syndrome with diarrhea. Clin Gastroenterol Hepatol 2012; 10: 1009–1015 e1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Camilleri M, Acosta A, Busciglio I, et al. Effect of colesevelam on faecal bile acids and bowel functions in diarrhoea-predominant irritable bowel syndrome. Aliment Pharmacol Ther 2015; 41: 438–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. U.S. Department of Health and Human Services, Food and Drug Administration and Center for Drug Evaluation and Research. Guidance for industry: irritable bowel syndrome — clinical evaluation of drugs for treatment. Silver Spring, MD: Center for Drug Evaluation and Research (CDER), 2012. [Google Scholar]
- 66. Xifaxan® (rifaximin) tablets, for oral use [package insert]. Bridgewater, NJ: Salix Pharmaceuticals, 2018. [Google Scholar]
- 67. Fodor AA, Pimentel M, Chey WD, et al. Rifaximin is associated with modest, transient decreases in multiple taxa in the gut microbiota of patients with diarrhoea-predominant irritable bowel syndrome. Gut Microbes 2019; 10: 22–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Pimentel M, Cash BD, Lembo A, et al. Repeat rifaximin for irritable bowel syndrome: no clinically significant changes in stool microbial antibiotic sensitivity. Dig Dis Sci 2017; 62: 2455–2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. DuPont HL, Wolf RA, Israel RJ, et al. Antimicrobial susceptibility of Staphylococcus isolates from the skin of patients with diarrhea-predominant irritable bowel syndrome treated with repeat courses of rifaximin. Antimicrob Agents Chemother 2017; 61: e02165–e02116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Schoenfeld P, Pimentel M, Chang L, et al. Safety and tolerability of rifaximin for the treatment of irritable bowel syndrome without constipation: a pooled analysis of randomised, double-blind, placebo-controlled trials. Aliment Pharmacol Ther 2014; 39: 1161–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Pimentel M, Lembo A, Chey WD, et al. Rifaximin therapy for patients with irritable bowel syndrome without constipation. N Engl J Med 2011; 364: 22–32. [DOI] [PubMed] [Google Scholar]
- 72. Lembo A, Pimentel M, Rao SS, et al. Repeat treatment with rifaximin is safe and effective in patients with diarrhea-predominant irritable bowel syndrome. Gastroenterology 2016; 151: 1113–1121. [DOI] [PubMed] [Google Scholar]
- 73. Lotronex® (alosetron hydrochloride) tablets [package insert]. Roswell, GA: Sebela Pharmaceuticals Inc, 2016. [Google Scholar]
- 74. Krause R, Ameen V, Gordon SH, et al. A randomized, double-blind, placebo-controlled study to assess efficacy and safety of 0.5 mg and 1 mg alosetron in women with severe diarrhea-predominant IBS. Am J Gastroenterol 2007; 102: 1709–1719. [DOI] [PubMed] [Google Scholar]
- 75. Lacy BE, Nicandro JP, Chuang E, et al. Alosetron use in clinical practice: significant improvement in irritable bowel syndrome symptoms evaluated using the US Food and Drug Administration composite endpoint. Ther Adv Gastroenterol 2018; 11: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Understanding the benefits and risks: the LOTRONEX REMS program prescriber education slide deck. Sebela Pharmaceuticals Inc., https://lotronexrems.com/pdf/REMS_Prgm_Slide_Deck_FINAL_03232016.pdf (2016).
- 77. Tong K, Nicandro JP, Shringarpure R, et al. A 9-year evaluation of temporal trends in alosetron postmarketing safety under the risk management program. Ther Adv Gastroenterol 2013; 6: 344–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Lacy BE, Everhart KK, Weiser KT, et al. IBS patients’ willingness to take risks with medications. Am J Gastroenterol 2012; 107: 804–809. [DOI] [PubMed] [Google Scholar]