Abstract
A defining characteristic of the hypothalamus-pituitary-gonad reproductive endocrine axis is the episodic secretion of the pituitary gonadotropin hormones LH and FSH by the anterior pituitary gonadotropes. Hormone secretion is dictated by pulsatile stimulation, with GnRH released by hypothalamic neurons that bind and activate the G protein–coupled GnRH receptor expressed by gonadotropes. Hormone secretion and synthesis of gonadotropins are influenced by the amplitude and frequency of GnRH stimulation; variation in either affects the proportion of LH and FSH secreted and the differential regulation of hormone subunit gene expression. Therefore, proper decoding of GnRH signals is essential for appropriate gonadotropin synthesis and secretion. The GnRH receptor robustly activates downstream signaling cascades to facilitate exocytosis and stimulate gene expression and protein synthesis. It is necessary to rapidly quench signaling to preserve sensitivity and adaptability to changing pulse patterns. Reactive oxygen species (ROS) generated by receptor-activated oxidases fulfill the role of rapid signaling intermediates that facilitate robust and transient signaling. However, excess ROS can be detrimental and, unchecked, can confuse signal interpretation. We demonstrate that sulfiredoxin (SRXN1), an ATP-dependent reductase, is essential for normal responses to GnRH receptor signaling and plays a central role in resolution of ROS induced by GnRH stimulation. SRXN1 expression is mitogen-activated protein kinase dependent, and knockdown reduces Lhb and Fshb glycoprotein hormone subunit mRNA and promoter activity. Loss of SRXN1 leads to increased basal and GnRH-stimulated ROS levels. We conclude that SRXN1 is essential for normal responses to GnRH stimulation and plays an important role in ROS management.
The distinct phases of the ovulatory cycle in mammals are characterized by unique pituitary gonadotropin secretion patterns that ultimately are controlled by different patterns of GnRH pulse amplitude and frequency (1–4). Interpretation of varying pulsatile GnRH signals by gonadotropes is necessary to appropriately respond with differential synthesis and secretion of LH and FSH, but the details of pulse decoding have been difficult to define (2, 3, 5–9). It is essential that gonadotropes remain sensitive to repeated GnRH stimulation to appropriately decode the varying GnRH pulse frequencies and amplitude as they change (9). A unique feature of GnRH receptor signaling is the robust and rapid activation and subsequent inactivation of the mitogen-activated protein kinase (MAPK) cascades that target transcription and translation regulatory factors controlling gonadotropin gene expression (8, 10). The re-establishment of signal cascade sensitivity may serve as a mechanism for maintaining pulse pattern vigilance. Stimulation of gonadotropes with GnRH results in generation of reactive oxygen species (ROS) by the nicotinamide adenine dinucleotide phosphate oxidase (NADPH)/dual-specificity oxidases (NOX/DUOXs), and activation of the MAPK signaling cascades (11). ROS serve as transient diffusible signal intermediates that are ideally suited for the role of a rapid-acting participant in a robust cellular responses to external stimulation (12). Although ROS have useful signaling properties, they also exhibit cytotoxic effects that must be minimized or eliminated (13, 14). This is accomplished through a complex reduction system involving peroxiredoxins (PRDXs), which reduce cellular peroxides and shuttle reactive oxygen to the cellular reducing machinery (15, 16). PRDXs are, themselves, susceptible to hyperoxidation and are inactivated by excess ROS. Hyperoxidized PRDX intermediates are recycled through action of sulfiredoxin (SRXN1), an ATP-dependent reductase that reduces hyperoxidized PRDX species and returns them to the redox protein pool (15, 16). Here, we examine GnRH-induced hyperoxidation of PRDX and the regulation of the resolving factor SRXN1, a major redox regulator in mammalian cells, and demonstrate the necessity of redox management in regulation of gonadotropin gene expression. Our results support the role of redox signaling in GnRH stimulation of gonadotropes.
Materials and Methods
Cell culture and transient transfection
The LβT2 cell line (17, 18) was initially reported as derived from a male mouse (19) but has recently been shown to be of female origin (20). Cells were cultured in high-glucose HEPES-buffered DMEM supplemented with penicillin/streptomycin and 10% fetal bovine serum (FBS) and maintained at 37°C in a humidified atmosphere of 5% CO2. For gonadotropin β-subunit gene promoter activity analysis, LβT2 cells were seeded into 12-well plates with 3 × 105 cells per well for 1 day and then transfected with a pGL3-basic luciferase reporter gene containing either −398 bp mouse Fshb or −1.8 kbp rat Lhb promoters using FuGENE 6 transfection reagent (Roche Molecular Biochemicals, Indianapolis, IN) following the manufacturer’s protocol. The cells were cotransfected with a pGL3-basic reporter plasmid containing the herpesvirus thymidine kinase promoter and a substitution of the luciferase coding sequence with β-galactosidase (10, 21). Cells were then were preincubated with serum-free DMEM for up to 16 hours, as previously reported (2, 10, 11, 22–24), and then treated with vehicle (PBS) or 10 nM GnRH (Sigma Aldrich, St. Louis, MO) for the times indicated. Inhibitors of NOX/DUOX or MAPK signaling cascades were applied to cultures 30 minutes before GnRH treatment. Diphenyleneiodonium (DPI), SP600125, SB203580, PD98059, and U0126 were purchased from Calbiochem (San Diego, CA). N-acetyl-l-cysteine (NAC) was purchased from Sigma-Aldrich. Cells were lysed with Reporter Lysis Buffer (Promega, Madison, WI) and assayed using the luciferase assay kit (Promega) and Galacto-Light Plus kit (Applied Biosystems, Foster City, CA) following the manufacturer’s protocol. Luminescence was measured by a Veritas microplate luminometer (Turner BioSystems, Sunnyvale, CA).
GnRH pulse stimulation and LH secretion analysis
Perifusion culture was performed as previously described (23) with noted modifications. Briefly, 2 × 107 LβT2 cells were seeded on 10-cm plates containing a 0.5-mL bed volume of Cytodex 3 microcarrier beads (GE Healthcare, Buckinghamshire, United Kingdom). After 24 hours, cell medium was changed to 5-mL serum-free DMEM with antibiotics for 16 hours. Microcarriers were then loaded into perifusion columns and equilibrated for 40 minutes in serum-free DMEM at a flow rate of 200 µL/min. Subsequently, cells were pulsed for 2 minutes with 10 nM GnRH with a 58-minute interpulse interval for 4 hours (23). After perifusion, microcarriers were harvested and split into two fractions. Protein was harvested using radio-immunoprecipitation assay lysis buffer containing proteinase inhibitor cocktail (Sigma-Aldrich). mRNA was harvested using Trizol LS according to manufacturer’s instructions.
Reverse transcription-PCR and quantitative PCR
Total RNA was extracted from LβT2 cells using Trizol reagent (Thermo Fisher Scientific, Carlsbad, CA) according to the manufacturer’s instructions. Isolated total RNA (1 µg per sample) was then reverse transcribed with qScript cDNA SuperMix (Quanta Biosciences, Gaithersburg, MD) according to the manufacturer’s instructions. Quantitative PCR (qPCR) was performed with KAPA SYBR Green (KAPA Biosystems, Boston, MA) using the MyIQ Single-Color Real-Time Detection System (Bio-Rad Laboratories, Hercules, CA). Specific primers used were previously described (11, 22, 25) and are listed in Table 1. Relative levels of gene expression were calculated using the comparative threshold cycle method, as previously described (26). Gapdh expression was used to normalize each sample.
Table 1.
Primer Sequences for qPCR
| Gene Target | Forward | Reverse |
|---|---|---|
| Srxn1 | 5′-AGCCTGGTGGACACGATC-3′ | 5′-AGGAATAGTAGTAGTCGCCA-3′ |
| Prdx1 | 5′-CACCCAAGAAACAAGGAGGA-3′ | 5′-TGGTCCAGTGCTCACTTCTG-3′ |
| Prdx2 | 5′-AGGACTTCCGAAAGCTAGGC-3′ | 5′-CCTTGCTGTCATCCACATTG-3′ |
| Sod1 | 5′-AAGGCCGTGTGCGTGCTGAA-3′ | 5′-GGTTCACCGCTTGCCTTCTGCT-3′ |
| Sod2 | 5′-CGCGCTGGAGCCGCACATTA-3′ | 5′-TCACGTAGGTCGCGTGGTGC-3′ |
| Cat | 5′-CCAGGGCCGCCTTTTTGCTT-3′ | 5′-GCCATCGCGCTGGTAGTTGG-3′ |
| Gpx1 | 5′-GAATGCCTTGCCAGCACCCA-3′ | 5′-CGCACTGGAACACCGTCTGG-3′ |
| Lhb | 5′-TGTCCTAGCATGGTCCGAGT-3′ | 5′-CCCCCACAGTCAGAGCTACT-3′ |
| Fshb | 5′-GCCGTTTCTGCATAAGC-3′ | 5′-CAATCTTACGGTCTCGTATACC-3′ |
| Gapdh | 5′-TGCACCACCAACTGCTTAG-3′ | 5′-CCGTCTGCTAGGTAGATCATCC-3′ |
Knockdown of Srxn1 gene expression
To establish LβT2 cells with stable knockdown of Srxn1, validated lentiviral short hairpin RNA (shRNA) particles for mouse Srxn1 (shSrxn1; catalog no. SHCLNV-NM_029688, catalog no. TRCN0000085872), or pLKO.1-puro control transduction particles (shCtrl; catalog no. SHC001V) were purchased from Sigma-Aldrich. Alternatively, lentivirus particles for transduction of shRNA expression vectors were prepared using Lenti-Xpackaging system (Takara Bio USA, Mountain View, CA) with pLKO.1 plasmid (Sigma-Aldrich). The shRNA-encoding pLKO.1 plasmid encoding shCtrl or shSrxn1 for mouse Srxn1 mRNA was transfected to Lenti-X™ 293T cell (Takara Bio USA) in complete DMEM with 10% FBS, as well as control lentiviral particles bearing a nontargeting shRNA (catalog no. SHC002; Sigma-Aldrich). Supernatants were collected 48 and 72 hours after transfection, filtered through a 0.45-μm polyethersulfone syringe filter and concentrated by 10% polyethylene glycol 8000 incubation for 16 hours and centrifugation at 1600 g for 1 hour at 4°C. Virus titer was checked by Lenti-X GoStix (Takara Bio USA).
LβT2 cells were seeded into 24-well plates at a density of 2.5 × 105 cells per well in complete DMEM with 10% FBS; the next day, lentivirus was added in serial dilutions with 4 µg/mL polybrene and incubated for 6 hours at 37°C, followed by a change to complete DMEM with 10% FBS. After 48 hours’ culture at 37°C, the transduced cells were selected by 0.5µg/mL puromycin in complete DMEM with 10% FBS. Cultures transduced at dilutions that promoted survival were propagated further under selection. Medium with 0.5 µg/mL puromycin was changed every 2 to 3 days. Cells were maintained in complete DMEM with 10% FBS supplemented with 0.25 µg/mL puromycin. Levels of Srxn1 expression in shRNA-expressing cells were analyzed by qPCR and protein levels confirmed by western blot analysis.
Pituitary primary culture and immunofluorescence
Whole pituitary glands were dissected from wild-type C57BL/6 female mice at 9 to 10 weeks of age. Mice were cohoused but not assessed for estrous cycle stage at harvest. Mice were handled in accordance with University of California, San Diego, Institutional Animal Care and Use Committee regulations under an approved protocol. Dispersed primary pituitary cells were seeded on poly-l-lysine–coated chamber slides at a density of 50,000 cells/cm2 and cultured in complete DMEM with 10% FBS. After serum starving overnight, cells were treated with 10 nM GnRH for 4 hours. Cells were fixed with 4% formaldehyde in PBS for 15 minutes, then washed in PBS. Afterward, LH was detected using anti-LH antibody (27) at 1:2000 dilution or anti-SRXN1 antibody (28) at 1:1000 dilution. Secondary staining and detection were accomplished using goat anti-guinea pig Alexa Fluor 568 (29) or anti-mouse Alexa Fluor 488 (30), respectively, at 1:800 dilution. Slides were mounted with Vectashield mounting medium with 4′, 6-diamidino-2-phenylindole (DAPI). Wide-field fluorescence images were obtained as described in the next paragraph. SRXN1-staining intensity was compared between cells positive for LH and cells not showing LH immunoreactivity in the same fields.
Detection of intracellular ROS
To detect intracellular ROS accumulation, LβT2 cells were cultured at a density of 1.5 × 105 cells per well in four-chamber slides (Laboratory-Tek, Rochester, NY) for 24 hours, then transfected with pLV-mCherry2-N1 plasmid (Addgene, Watertown, NY) and shCtrl plasmid or shSrxn1 pLKO.1 plasmid (Sigma-Aldrich) with Poly-Jet reagent (SignaGen Laboratories, Gaithersburg, MD). After 24-hour posttransfection, medium was replaced with serum-free DMEM medium for 16 hours, then cells were stained with CellRox (Thermo Fisher Scientific, Waltham, MA) for 30 minutes, fixed in 4% formaldehyde, and coverslips applied with Vectashield mounting medium with DAPI (Vector Laboratories, Burlingame, CA). Wide-field fluorescence images were obtained using a Nikon TE2000-U microscope (Nikon America, Melville, NY) equipped with an X-Cite 120PC collimated light source (Lumen Dynamics Group, Mississauga, ON, Canada) and DAPI-1160A, FITC-5050A-000, and mCherry-c filter sets (Semrock, Rochester, NY) using a CoolSnap EZ monochrome camera (Photometrics, Tucson, AZ). Images were captured and analyzed using NIS-Elements software (Nikon America).
Flow cytometry
To detect cell ROS production, 1 × 106 shCtrl- or shSrxn1-transduced cells were incubated for 15 minutes at 37°C in PBS containing 5 μM CellRox Green Reagent (Thermo Fisher Scientific) and 1:800 dilution of Zombie NIR Live/Dead fixable stain (Biolegend, San Diego, CA) and fixed with 4% paraformaldehyde for 20 minutes. Samples were washed three times with PBS and harvested with 0.25% trypsin and fixed with 1% paraformaldehyde in PBS. Samples were run on a FACSCanto II flow cytometer (BD Biosciences, Franklin Lakes, NJ) and analyzed with FlowJo Software (Treestar, Ashland, OR).
Western blotting
LβT2 and HeLa cells were lysed with radio-immunoprecipitation assay lysis buffer containing proteinase inhibitor cocktail (Sigma-Aldrich). Isolated proteins were separated by electrophoresis on 14% (for SRXN1) or 12% (for PRDX-SO2/3) SDS polyacrylamide gels (Mini-PROTEAN; Bio-Rad Laboratories), and transferred to nitrocellulose membranes. Membranes were blocked with 5% fat-free milk (for SRXN1) or 3% BSA (for PRDX-SO2/3) in Tris-buffered (pH 8.0) saline containing 0.05% Tween 20 (TBS-T) for 1 hour at room temperature and then probed with primary antibody in blocking solution.
Primary antibodies for SRXN1 (28), PRDX-SO2/3 (31), and β-actin (32) were diluted 1:1000 in blocking solution prior to use. After three washes with TBS-T for 10 minutes, membranes were incubated in horseradish peroxidase–conjugated goat anti-mouse (33) or goat anti-rabbit (34) secondary antibody at 1:2000 dilution. Membranes were rinsed three times with TBS-T, and antibody-antigen complexes were detected by Chemiglow chemiluminescence (Protein Simple, San Jose, CA) and the FluorChem Q Imaging System (Cell Biosciences, Santa Clara, CA). Densitometry and quantification were performed using AlphaView Q software (Cell Biosciences). Membranes were stripped with Restore plus western blotting stripping buffer (Thermo Fisher Scientific) and probed with anti–β-actin antibody (32) to control for protein loading.
Results
GnRH induces PRDX oxidation
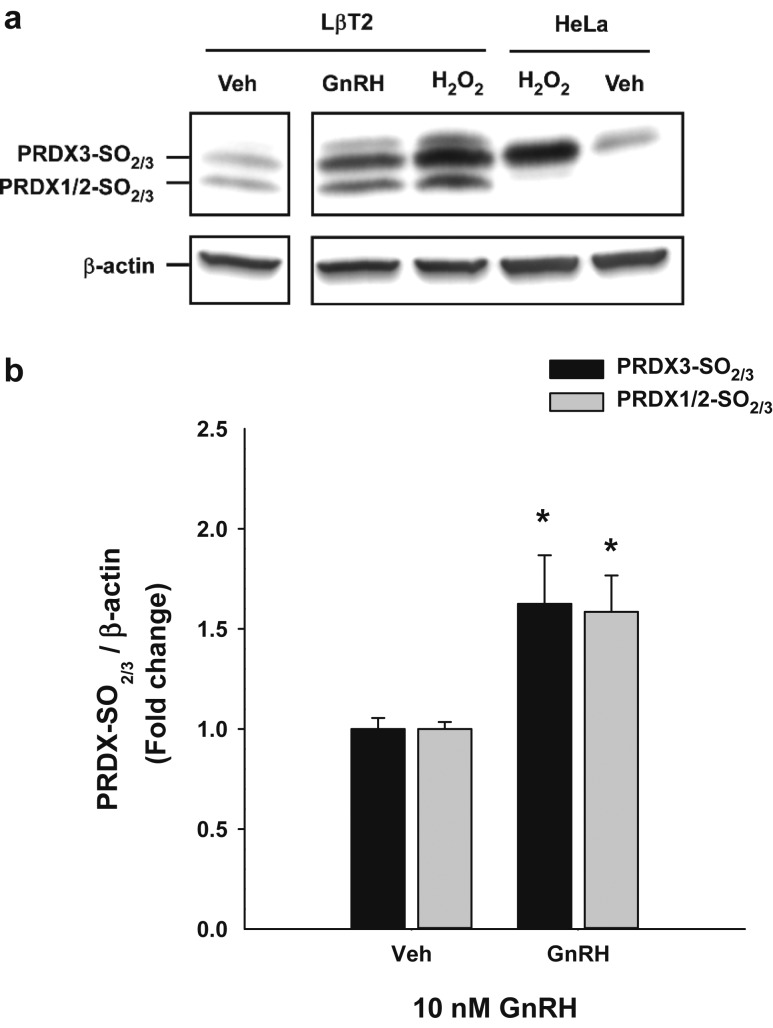
We examined whether PRDX isoforms are oxidized by GnRH treatment in the LβT2 gonadotrope cell line. We treated cells with 10 nM GnRH and examined PRDX oxidation by western blot using antibody that detects both sulfinic and sulfonic PRDX intermediates. Treatment of the LβT2 gonadotrope cells with 10 nM GnRH for up to 6 hours led to >50% increase in oxidation of both the cytosolic PRDX 1/2 and mitochondrial PRDX 3 isoforms (Fig. 1). In contrast, direct treatment with H2O2 directly caused strong oxidation of PRDX 3 in HeLa cells, and of PRDX 1-3 in LβT2 cells, indicating differences in expression of PRDX isoforms or in localized management of ROS.
Figure 1.
GnRH stimulation increased oxidized PRDX isoforms in LβT2 cells. Cultured LβT2 or HeLa cells were incubated for 24 h in DMEM/10% FBS and then serum starved for 12 to 16 h. Cultures were subsequently treated with vehicle (Veh) or tonic 10 nM GnRH for 4 h or H2O2 for 30 min. Cells were lysed and subjected to SDS-PAGE and western blotted using antibodies against PRDX-SO2/3 and β-actin. Band intensities for PRDX3-SO2/3 and PRDX1/2-SO2/3 were measured by quantitative chemiluminescence and normalized for β-actin. (a) A representative blot from three independent experiments. (b) Mean values ± SEM normalized to vehicles of four independent determinations. *Significant difference from the respective time-zero expression levels as determined by ANOVA and post hoc testing with Dunnett comparison with control test.
PRDX recycling factor SRXN1 is specifically induced by GnRH
The presence of oxidized PRDX in response to tonic GnRH treatment implies that PRDX oxidation or hyperoxidation occurs and requires resolution. In our previous studies of pulsatile and tonic GnRH treatment of LβT2 cells in perifusion culture, Srxn1, also known as Npn3 for Neoplastic Progression 3 (38), was identified as highly responsive to both pulsatile and tonic GnRH treatment, and overall response levels were proportional to the amplitude and frequency of GnRH stimulation (Table 2).
Table 2.
GnRH-Induced Increase of Srxn1 Gene Expression of in LβT2 Cells
| GnRH Pulse Concentration, 10 nM | GnRH Pulse Concentration, 100 nM | |||
|---|---|---|---|---|
| GnRH Pulse Interval, min | Fold Response | P | Fold Response | P |
| 240 | 0.97 | NS | 2.62 | <0.001 |
| 120 | 2.90 | <0.001 | 6.07 | <0.001 |
| 60 | 7.34 | <0.001 | 11.73 | <0.001 |
| 30 | 10.40 | <0.001 | 15.92 | <0.001 |
| Tonic | ND | ND | 21.70 | <0.001 |
Extract of Srxn1 (Npn3) expression measurements as reported by Lawson et al. (2) and Mistry et al. (3). Perifusion cultures of LβT2 cells were pulsed at 10 nM and 100 nM peak GnRH for 4 h at the intervals indicated. Cells were harvested and processed for analysis by Affymetrix MU74AV2 expression array. Resultant signal data were evaluated using VAMPIRE Bayesian variance modeling and significance based on αbonf < 0.05, as described previously (2). Srxn1/Npn3 expression is shown as fold change relative to vehicle control and exhibits significant increases based on frequency and amplitude of stimulation. Data were extracted from the analyzed data set reported by Lawson et al. (2). Significance was determined by difference from the corresponding vehicle treatment as ascertained by Student t test.
Abbreviations: ND, no data; NS, not significant.
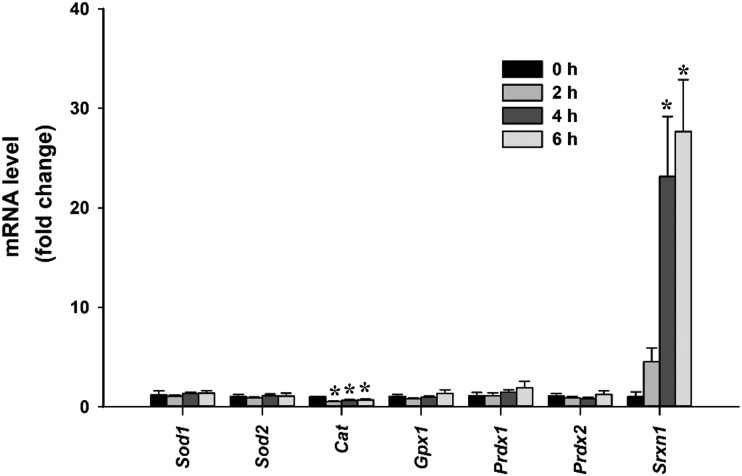
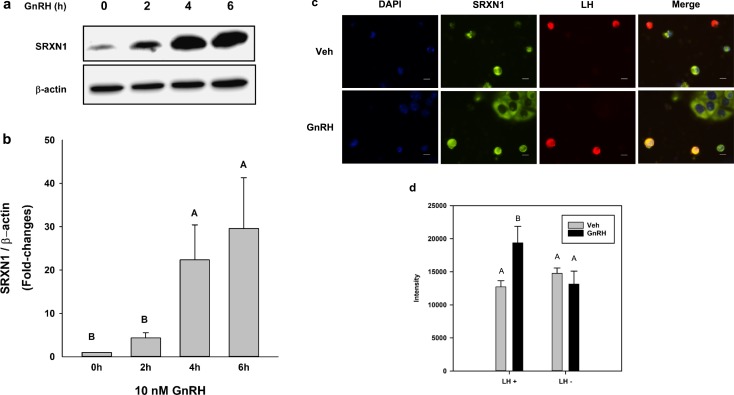
We examined the regulation of Srxn1 and other redox management factors in LβT2 cells under similar conditions using qPCR measurement. Tonic treatment of LβT2 cells for up to 6 hours with 10 nM GnRH did not result in significant changes in superoxide dismutase 1, 2, (Sod1, 2), catalase (Cat), glutathione peroxidase 1 (Gpx1), or Prdx1 and Prdx2 mRNA. However, Srxn1 mRNA responded robustly to ∼25-fold over vehicle treated values (Fig. 2). At 4 hours, Srxn1 mRNA peaked to levels similar to those reported in our observations of pulsatile stimulation of LβT2 cells, which was determined using microarray expression analysis (Table 2). Similarly, SRXN1 protein levels peaked at 25 to 30 times untreated time-zero values within 4 hours under tonic 10 nM GnRH (Fig. 3a). The 4- and 6-hour response levels were significantly higher than those of baseline and 2-hour stimulation. (Fig. 3b).
Figure 2.
GnRH induced Srxn1 mRNA but not other redox genes in LβT2 cells. LβT2 cells were cultured for 24 h in DMEM/10% FBS and then serum starved for 12 to 16 hours. Cultures were subsequently treated with vehicle (control) or tonic 10 nM GnRH for the indicated times. Total RNA was extracted and relative mRNA expression of Sod1, Sod2, Gpx1, Cat, Prdx1, Prdx2, and Srxn1 was determined by qPCR. The relative levels of each mRNA were normalized to Gapdh mRNA and normalized to respective time-zero levels. Data are reported as mean ± SEM of at least four independent experiments. *Significant differences from time-zero control (P < 0.05) by one-way ANOVA followed by post hoc testing with the Tukey multiple-comparison test.
Figure 3.
Tonic GnRH treatment increased SRXN1 in LβT2 cells. Cultures of LβT2 cells were incubated for 24 h in DMEM/10% FBS, serum starved for 12 to 16 h, and then treated with tonic 10 nM GnRH for the indicated times, harvested, and subjected to SDS-PAGE and western blot analysis using antibodies against SRXN1 and β-actin. Band intensities were measured using quantitative chemiluminescence, and SRXN1 values were normalized to β-actin. (a) A representative blot from three independent experiments. (b) Mean values ± SEM normalized to time zero of four independent experiments. Different letters indicate a significant difference between groups (P < 0.05) by one-way ANOVA followed by post hoc testing with the Tukey multiple-comparison test. Dispersed primary pituitary cells from adult female mice were plated in lysine-coated chamber slides and cultured overnight. Cells were treated with vehicle (Veh) or GnRH for 4 h and then fixed and stained for the presence of SRXN1 protein (green) or LH (red). (c) Nuclei were visualized by inclusion of DAPI in mounting medium. (d) SRXN1 staining intensity was compared between cells positive for LH and cells not showing LH immunoreactivity in the same fields. Scale bar, 10 µm. A summary of staining intensity is presented. Different letters indicate significant differences between groups (P < 0.05) as determined by two-factor ANOVA and post hoc testing with the Tukey multiple-comparison test.
To confirm that SRXN1 was induced by GnRH in gonadotropes, we examined the expression of SRXN1 in female mouse primary pituitary cultures. After harvest, dispersion, and culture for 24 hours, cells were serum starved overnight, then treated with10 nM GnRH for 4 hours. Gonadotropes identified by anti-LH antibody immunoreactivity were examined for SRXN1 levels by anti-SRXN1 immunoreactivity in comparison with cells negative for LH (Fig. 3c). Non-LH cells had varying levels of SRXN1 reactivity that was unaltered by GnRH treatment. Anti–LH-reactive cells showed a consistent increase in SRXN1 reactivity that was increased by GnRH treatment (Fig. 3d).
GnRH-induced SRXN1 expression requires NOX/DUOX activity and ROS
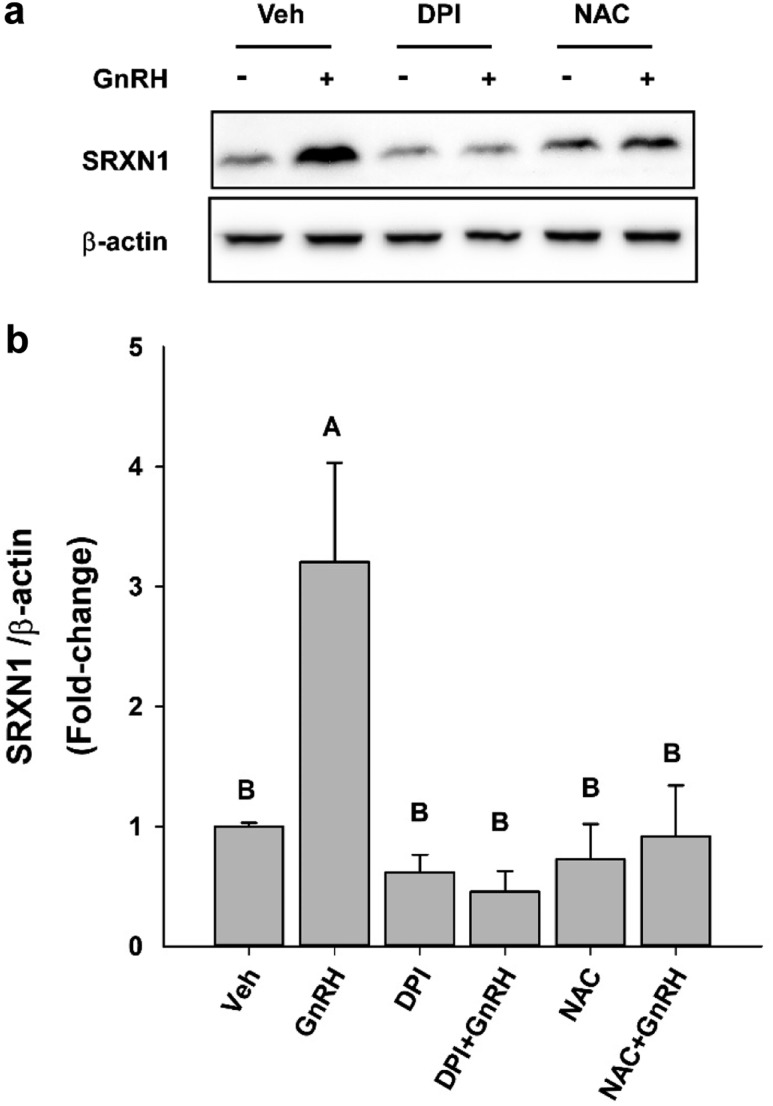
Transcriptional activation of the Srxn1 gene is highly responsive to oxidative stress (25). Both AP1 and NRF2 regulate Srxn1 through an antioxidant response element in the promoter (15, 16). Under normal physiological conditions, AP1 activity is inhibited by constitutive nuclear export by XPO1 (CRM1 in yeast) (39). Oxidation of the cysteine-rich domain of both FOS and JUN blocks export and facilitates interaction with target gene promoters. Similarly, NRF2 resides in the cytosol, where it is targeted for degradation by KEAP1. Oxidation of KEAP permits increased nuclear translocation and interaction of NRF2 with target genes bearing antioxidant response elements. Regulation by two oxidation-sensitive factors suggests potential robust activation of Srxn1 expression in response to oxidative stress (25, 40). In gonadotropes, the NOX/DUOX are major sources of intracellular ROS produced in response to GnRH stimulation (11). Inhibition of NOX/DUOX activity with the inhibitor DPI prevents the GnRH-induced increase in SRXN1 (Fig. 4a). Furthermore, the ROS scavenger NAC also limits increased SRXN1 protein levels. Both DPI and NAC prevent a substantial increase in SRXN1 in response to GnRH (Fig. 4b), supporting the role of ROS produced by GnRH stimulation in the overall increased expression of SRXN1.
Figure 4.
DPI and NAC attenuated GnRH pulse–mediated SRXN1 induction. LβT2 cells were cultured for 24 h in DMEM/10% FBS and then serum starved for 12 to 16 h. Cultures were then preincubated with the NADPH oxidase inhibitor DPI at 5 μM or the reactive oxygen scavenger NAC at 20 mM for 30 min, and then stimulated with 10 nM GnRH for 4 h. Cells were harvested and subjected to SDS-PAGE and western blot analysis using antibodies against SRXN1 and β-actin. Intensity values were measured by quantitative chemiluminescence. (a) A representative blot from three independent experiments. (b) The chart represents mean values ± SEM normalized to vehicle (Veh) of four independent determinations. Both DPI and NAC inhibited GnRH-induced increase in SRXN1. Different letters indicate a significant difference between groups (P < 0.05) as determined by one-way ANOVA followed by post hoc testing with the Tukey multiple-comparison test.
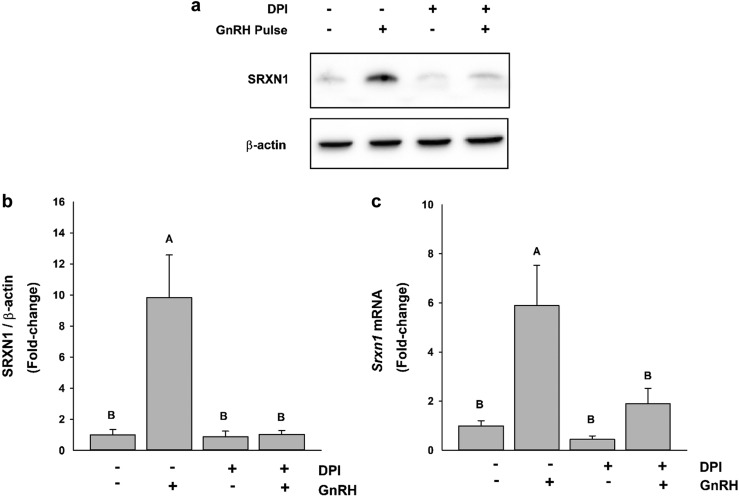
In vivo, GnRH stimulates gonadotropes through episodic pulses arriving via the hypophyseal portal circulation that dictate gene expression and hormone secretion. To simulate this, LβT2 cells were cultured in an in vitro perifusion system that permits the introduction of pulsatile GnRH and given hourly pulses of GnRH at 10 nM peak value for 4 hours. Then SRXN1 levels were examined by western blot (Fig. 5a). Pulsatile stimulation induced a ninefold induction of SRXN1 protein (Fig. 5b) and a sixfold induction of Srxn1 mRNA (Fig. 5c). The increase of both protein and mRNA were blocked by DPI treatment, indicating that PRDX oxidation and SRXN1 response to GnRH depends on signaling-induced ROS.
Figure 5.
DPI attenuated GnRH pulse–mediated SRXN1 and PRDX3 induction. LβT2 cells (n = 1 × 107) were cultured on Cytodex microcarriers for 24 h in DMEM/10% FBS with antibiotics. The cells were changed into serum-free medium and incubated for an additional 16 h. Cells were pretreated with vehicle or 5 μM DPI for 30 min and then pulsed for 2 min at 58-min intervals with vehicle or 10 nM GnRH for 4 h. After pulse stimulation, total RNA and protein were harvested. Protein was analyzed by western blot to detect (a) SRXN1 protein levels and (b) PRDX3 oxidation. (c) Intensity values for SRXN1 were measured by quantitative chemiluminescence and charted. (d) Isolated RNA was evaluated by qPCR for Srxn1 levels; charts represent mean values ± SEM. Different letters indicate a significant difference between groups (P < 0.05) by two-way ANOVA and post hoc testing with the Tukey multiple-comparison test.
GnRH-induced SRXN1 expression requires MAPK1/3 activity
The oxidative stress response generally operates through an MAPK8-10/c-JUN/AP1 signaling pathway that leads to activation of target genes that participate in the oxidative or stress response. Stress signaling is strongly associated with activation of the MAPK signaling pathway that includes MAPK8-10 activation. However, we have found that GnRH-induced oxidative signaling primarily affects the MAPK1/3 pathway. Similarly, in cardiac progenitor cells, SRXN1 is also associated with MAPK1/3 activation (41).
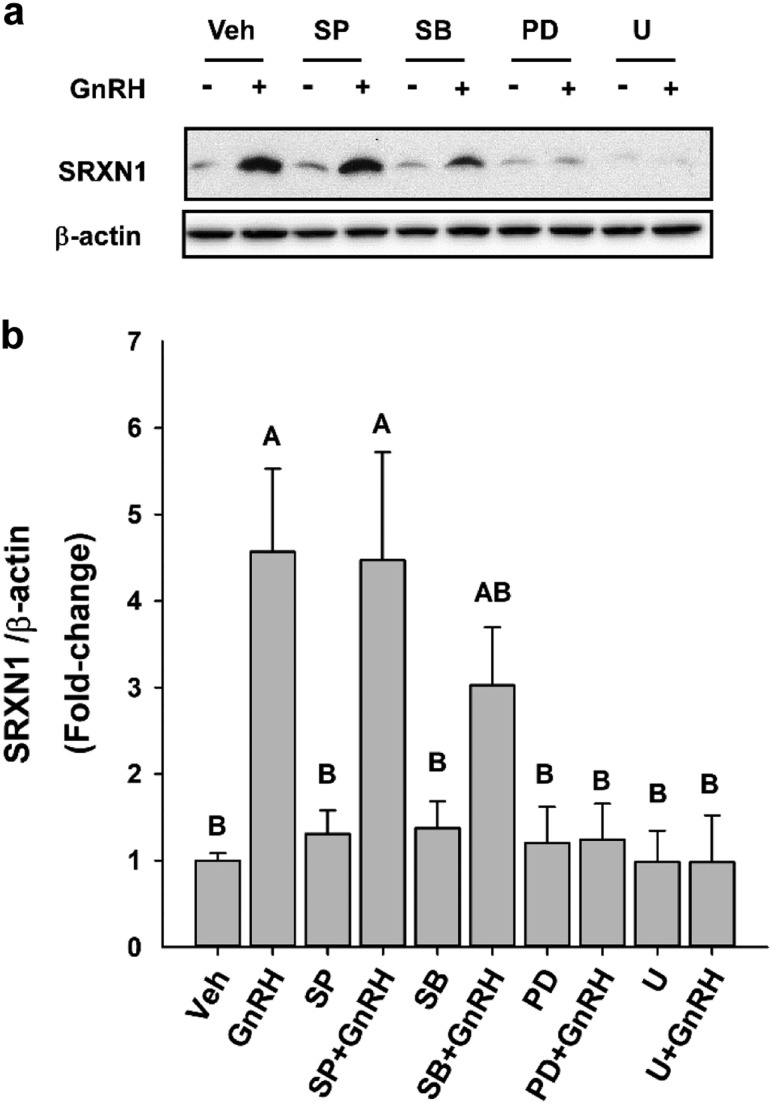
We examined the relative impact of the MAPK11-14 (p38), MAPK8-10 (JUN), and MAPK1/3 cascades on SRXN1 protein increases in LβT2 cells in response to GnRH by inhibiting each cascade (Fig. 6a). Inhibition of MAPK1/3 activation by U0126 and PD098059 had the most profound impact on SRXN1 increases in LβT2 cells by eliminating all response (Fig. 6b). Inhibition of MAPK8-10 activation by SP600125 did not significantly inhibit SRXN1 accumulation, reducing the response from 4.5- to threefold. Blockade of MAPK11-14 activation by SP203580 had no effect on GNRH-induced SRXN1 accumulation. The dependence of SRXN1 in response to GnRH-induced ROS production on NOX/DUOX activity and MAPK1/3 activation indicates that the antioxidant response is directly targeted by GnRH signaling mechanisms and is an integral part of the regulatory cascade mediating GnRH regulatory effects.
Figure 6.
MAPK1/3 inhibitors reduced the SRXN1 response to GnRH stimulation. Cultured LβT2 cells were incubated for 24 h in DMEM/10% FBS; serum starved for 12 to 16 hours; pretreated with vehicle (Veh; dimethyl sulfoxide), 10 µM SP600125 (SP), 20 µM SB202190 (SB), 30 µM PD98059 (PD), or 2 µM U0126 (U) for 30 min; and then subsequently treated with 10 nM GnRH for 4 h. Cells were lysed and subjected to SDS-PAGE and western blot analysis using antibodies against SRXN1 and β-actin. Band intensities were measured using quantitative chemiluminescence, and SRXN1 values were normalized to β-actin. (a) A representative blot from three independent experiments. The chart represents mean values ± SEM, normalized to Veh, of four independent determinations. Different letters indicate a significant difference between groups (P < 0.05) by one-way ANOVA and post hoc testing with the Tukey multiple-comparison test.
SRXN1 knockdown inhibits GnRH-induced Lhb and Fshb gene expression
The increased cellular ROS and oxidized PRDX species that occurs after GnRH stimulation coupled with the induction of Srxn1 gene expression suggests that GnRH signaling actively modulates ROS production. Srxn1 gene expression is targeted by the MAPK1/3 pathway, which is also central to regulation of gonadotropin gene expression. GnRH-induced expression of both Lhb and Fshb requires ROS and inhibition or reduced expression of NOX/DUOX enzymes attenuates Lhb and Fshb response to GnRH (11). The loss of SRXN1 can lead to a general toxic effect due to unresolved oxidation or altered gene expression through dysregulation of proteins sensitive to redox modification (12, 15, 36). This has been reported for MAPK1/3, which is altered through loss of negative feedback by dual-specificity protein phosphatase (DUSP) activity due to redox-sensitive oxidation of active-site thiol residues and inappropriate nuclear localization due to redox-sensitive nuclear transport.
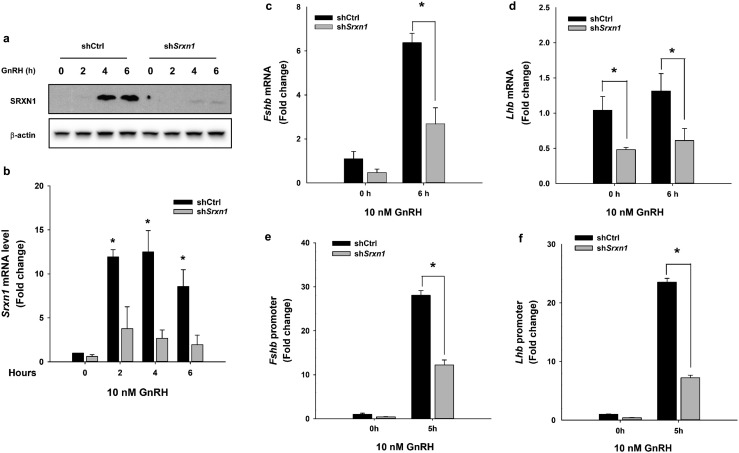
We examined the impact of SRXN1 protein knockdown on gonadotropin gene expression under unstimulated and GnRH-stimulated conditions. Cells transduced with shSrxn1-expressing lentiviruses were treated with 10 nM GnRH to examine the consequences of depletion of SRXN1 on the GnRH-induced increases in gonadotropin subunit mRNA. Transduced cells were treated with GnRH for up to 6 hours to induce SRXN1 protein. In shSrxn1-transduced cells, the GnRH-induced increase in SRXN1 was nearly completely abolished (Fig. 7a). In addition, the baseline and GnRH-induced levels of Srxn1 mRNA were reduced to 40% of shCtrl levels (Fig. 7b). In cells transduced with shSrxn1 lentivirus, baseline levels of both Lhb and Fshb mRNA were reduced (Fig. 7c and 7d). The GnRH-induced increase in both Lhb and Fshb mRNA also was attenuated, suggesting that the loss of SRXN1 may affect both MAPK1/3 activation, which targets Lhb expression via the immediate-early transcription factor EGR1, and c-JUN activation, which targets Fshb transcription through the redox-sensitive transcription factor AP1.
Figure 7.
Knockdown of shSrxn1 in LβT2 cells reduced GnRH-induced gonadotropin gene expression and reporter gene responses. LβT2 cells transduced with lentiviral vectors expressing shRNA for shCtrl or shSrxn1 were generated and selected with puromycin. The serum-starved shCtrl and shSrxn1 cells were treated with or without 10 nM GnRH for the indicated times. Total RNA and protein were harvested and analyzed by western blot or qPCR to evaluate SRXN1 protein and Srxn1 mRNA, respectively. (a) A representative blot from three independent experiments. (b) The chart represents mean Srxn1 values ± SEM normalized to vehicle control from four independent determinations. *P < 0.05 from time zero by Student t test. For analysis of Fshb and Lhb mRNA expression, serum-starved shCtrl and shSrxn1 cell lines were treated with 10 nM GnRH for 6 h. qPCR was performed using specific primers for (c) Fshb, and (d) Lhb. Data shown are the mean ± SEM values from three independent experiments. *P < 0.05, Student t test. Serum-starved shCtrl and shSrxn1 cells were transiently transfected with (e) a −396 bp mouse Fshb promoter or (f) −1.8 kb rat Lhb promoter luciferase reporter plasmid. Transfected cells were treated with 10 nM GnRH for 5 h, the time of maximal reporter gene response. Luciferase values were internally normalized using a cotransfected β-galactosidase reporter to control for transfection efficiency. Data are reported as mean ± SEM from three independent experiments. *P < 0.05, by Student t test.
To assess whether loss of SRXN1 directly affects activity of gonadotropin gene promoters, reporter gene assays were conducted in shRNA-transduced LβT2 cells that were further transfected with either an Fshb or Lhb reporter gene vector. Basal and GnRH-induced fold changes of Fshb and Lhb reporter gene activity were attenuated in cells expressing Srxn1-targeted shRNA (Fig. 7e and 7f). The impact on both gonadotropin subunit genes supports a model of generalized attenuation of signaling responses.
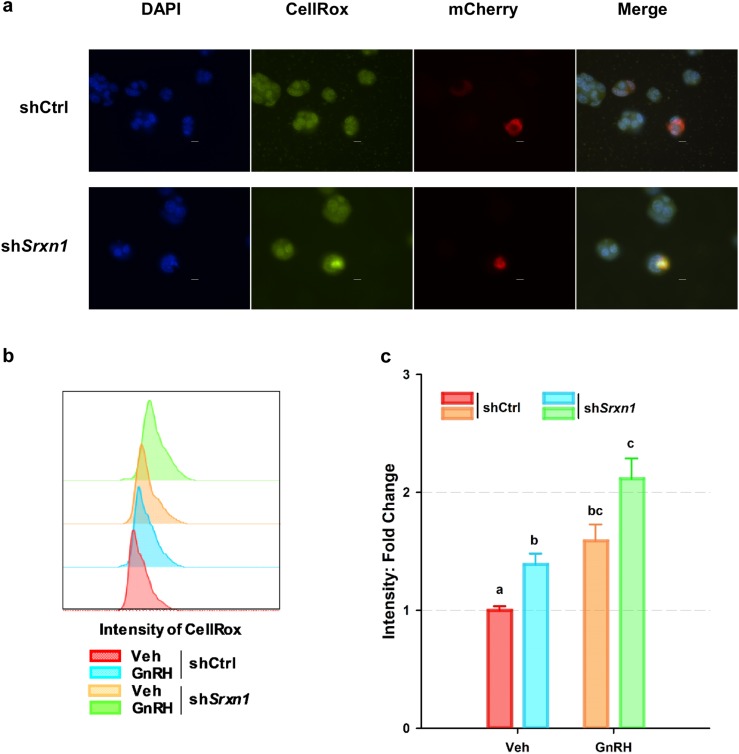
To confirm that loss of SRXN1 affects the management of ROS in gonadotropes, LβT2 cells were cotransfected with shCtrl or shSrxn1 expression plasmids with an mCherry GFP expression vector to mark transfected cells. Cells cotransfected with the shSrxn1 expression vector had higher levels of ROS production, as determined by intensity of fluorescence of cotransfected cells after treatment with the intracellular ROS-activated dye CellRox green (Fig. 8a). The impact of GnRH treatment was further examined using LβT2 cell lines transduced with lentivirus expressing shCtrl or shSrxn1 and selected with puromycin to remove untransduced cells. Approximately 2 million cells were briefly treated with GnRH to induce SRXN1, then restimulated with GnRH to increase ROS production and treated with the ROS-activated dye CellRox. Cells were then subjected to flow cytometry to quantify intensity levels (Fig. 8b). The shSrxn1 cell line had higher levels of ROS production in the untreated state than did GnRH-induced shCtrl cells, and this was substantially increased by GnRH stimulation (Fig. 8c). Overall, these findings indicate management of GnRH-induced ROS requires the presence of SRXN1.
Figure 8.
Knockdown of shSrxn1 enhanced ROS production in LβT2 cells. LβT2 cells were transiently transfected with shCtrl or shSrxn1 expression vectors and pLV-mCherry to mark transfected cells (red). At 48 h posttransfection, cells were serum starved and treated with 10 nM GnRH for 4 h and then incubated with CellRox (green) to detect the presence of ROS. Cells were fixed with 4% formaldehyde, and nuclei were stained with DAPI (blue) to illuminate nuclei. (a) Representative micrographs are shown. The shCtrl and shSrxn1 cell lines were treated with or without GnRH for 4 h and given a 1-h rest to allow induction of SRXN1 expression. After resting, cells were retreated with a single pulse of vehicle or GnRH for 30 min and changed to normal serum-free medium and incubated an additional 30 min. Cells were incubated with CellRox for 15 min followed by 4% formaldehyde fixation for 20 min. Scale bar, 10 µm. (b) Cells were evaluated by flow cytometry, and fluorescein isothiocyanate–A frequency distribution of intensity was compared between shCtrl and shSrxn1 cells with or without GnRH. (c) Geometric means of fluorescein isothiocyanate–A signals for CellRox were extracted and normalized to the ratio of shCtrl cells treated with vehicle. Error bars denote SEM from at least four set samples in each. Means were compared using two-factor ANOVA and post hoc testing with Dunnett comparison with control test using ShCtrl cells treated with vehicle (Veh) as the control. Groups with different letters are significantly different (P < 0.05).
Discussion
Eukaryotic cells have evolved a complex system for management of peroxides and oxidized macromolecules through the action of PRDX family members (35, 36). Their most notable difference in expression is their subcellular localization to either the cytosol (PRDXs 1, 2, and 4) or to mitochondria (PRDX 3) (37). All PRDX species contain an active-site cysteine residue, Cp, which rapidly reduces peroxides through oxidation of Cp-SH to sulfenic acid, Cp-SOH. A neighboring Cys, Cr, is also conserved among the major 2-Cys family of PRDXs (15, 36). The oxidized sulfenic intermediate reacts with Cr to form an intramolecular disulfide bridge that is then reduced by thioredoxin (15, 37). In mammalian cells, a unique feature of the 2-Cys PRDXs is their rapid inactivation by hyperoxidation through further reaction of the Cp-SOH sulfenic acid with a second peroxide, resulting in hyperoxidized sulfinic acid Cp-SOOH, or sulfonic acid Cp-SOOOH (15). These hyperoxidized forms are resistant to reduction by glutathione or thioredoxin and are lost from the PRDX redox buffering pool. The ATP-dependent reduction of hyperoxidized PRDX is catalyzed by SRXN1, which recycles regenerated PRDX back into the redox buffering pool (16).
In gonadotropes, GnRH stimulation causes increased ROS production and this is essential for the rapid activation of MAPKs 1 and 3 [MAPK1/3 (also called ERK 2 and ERK 1, respectively)], and MAPK8-10 (also known as JNK 1-3), which mediate both transcriptional and translational responses to GnRH stimulation (11). However, resolution of the GnRH receptor signaling response must be accomplished to restore sensitivity to subsequent stimulation.
It is generally understood that ROS is an important but potentially toxic byproduct of biological processes that must be effectively managed (13, 14). Multiple biochemical reactions produce ROS as a side product of catalysis or oxidation, and these products are detrimental to cellular macromolecules (13). Oxidation of catalytic sites or structural components of proteins leads to their dysregulation or inactivation, and oxidation of nucleic acids can lead to misregulation of gene expression and mutation (12, 13, 35). Although potentially hazardous, reactive oxygen in the form of H2O2 is increasingly appreciated as an important signaling molecule that participates in signaling cascades regulating growth, differentiation, and differentiated cell functions (12).
The participation of H2O2 in signaling then implies that peroxide generation must be controlled in such a way that facilitates the desirable signaling activities and minimizes undesirable or off-target affects (12). This can be accomplished through regulated production and spatiotemporal confinement to areas where signal propagation and detection are necessary. The 2-Cys PRDXs are unique in that they can participate in the reduction of peroxides but have the unique characteristic that hyperoxidation is favored if thioredoxin-mediated reduction is saturated or delayed (36). Hyperoxidation of PRDX family members is resolved by ATP-dependent reduction by SRXN1 (16). It has been proposed that this unique two-step recycling in which hyperoxidation of PRDX is kinetically facilitated and subsequently reduced by SRXN1 provides a mechanism for buffering low levels of peroxide generated by cellular processes while providing a process by which transient, elevated levels are permitted to facilitate signal propagation (12, 15). Thus the 2-cys PRDXs conform to a high-pass filter model of signal propagation that readily mitigates low-level or nonspecific signals but allows signal propagation via rapid, elevated ROS that outpaces PRDX reduction (15, 36). SRXN1 is then tasked with reduction of excess ROS once the short-term capacity of PRDX is surpassed.
We recently proposed the role of SRXN1 in feedback control of GnRH signaling (42). Here, we provide evidence for SRXN1 in the regulation of the gonadotropin subunit genes Lhb and Fshb. GnRH stimulation of gonadotropes results in rapid production of ROS through the activity of the NOX/DUOX enzymes. Both MAPK and calcium signaling activation, as well as gonadotropin subunit gene expression, depend on activity of these enzymes (11, 43). We demonstrate that pulsatile or tonic GnRH results in the rapid increase of Srxn1 mRNA and SRXN1 protein, and this is sensitive to inhibition of the NOX/DUOX enzymes by DPI and the ROS scavenger NAC. Inclusion of SRXN1 in the regulatory GnRH signaling cascade provides a mechanism for resolution of GnRH-induced ROS and fulfills the role of a feedback regulator of redox signaling.
GnRH activation of MAPK1/3 and MAPK8-10 is characterized by a rapid increase in protein phosphorylation that is followed by an equally marked decline, such that individual GnRH pulses provide a robust but rapidly resolved signal (44, 45). This pattern contrasts with activation by other extracellular signals such as insulin or epidermal growth factor, which manifest a slower and lower maximal stimulation. The rapid production of ROS by NOX/DUOX activation and the buffering capacity provided by the PRDX proteins and SRXN1 recycling provide a potential mechanism for this unique burst pattern of activation and resolution (15, 36). The DUSPs are highly expressed in LβT2 cells and may provide a constant repression of MAPK activation by phosphorylation (46). Gonadotropin gene expression is sensitive to DUSP1 levels and loss of DUSP1 results in elevated MAPK1/3 phosphorylation and Lhb gene expression (10). Other DUSP family members are regulated by GnRH stimulation and at different developmental and estrous cycle stages in vivo (47). DUSP family members are rapidly inactivated by active-site cysteine oxidation, potentially resulting in unrestrained accumulation of phosphor-activated MAPKs(48). The activity of PRDXs provide a mechanism for recycling inactive, oxidized DUSP species. SRXN1 is also rapidly increased in response to GnRH, providing a pathway for recycling and maintenance of the PRDX pool. Therefore, rapid activation of MAPK family members could be achieved through ROS-mediated inactivation of negative feedback by DUSPs coupled with positive feed-forward phosphorylation through the ras-raf-MEK cascade (42, 48, 49). Elevated ROS also trigger increased SRXN1, which then restores the buffering capacity of PRDX, allowing rapid resolution of MAPK activation by recycling of DUSPs, promoting dephosphorylation of activated kinases. Although the general outlines of this mechanism are consistent with our observations, additional testing of the details of this model is necessary.
In general, excess ROS causes oxidative stress that leads to cellular and mitochondrial damage and can eventually cause cell death (50, 51). GnRH also induces prohibitin gene expression, which can facilitate synthesis of BAX and promote apoptosis, indicating that GnRH stimulation promotes both stimulatory signals via ROS that exert differentiated cell function but also primes the cell for apoptosis (52).
It is of interest that Srxn1 expression is also associated with neoplastic progression. This association was first noted prior to knowledge of the function of Srxn1, hence its initial designation as Npn3 (38). GnRH receptor expression and GnRH signaling are associated with growth of transformed cells including breast, prostate, and ovarian cancers. Managing high levels of oxidative stress is an essential survival strategy of transformed cells. The role of GnRH in elevating SRXN1 levels may explain the widely reported observation that GnRH can promote the growth of transformed cells that express the GnRH receptor (53). Furthermore, the use of GnRH as a receptor agonist to repress the production of gonadotropins in treating reproductive cancers may also promote tumor growth through stimulation of SRXN1, potentially limiting the effectiveness of this therapeutic approach.
The role of oxidative stress is well recognized as a contributing factor to reduced fertility in men and women (54, 55). Polycystic ovary syndrome, one of the most common reproductive endocrine pathologies, is also associated with oxidative stress, dyslipidemia, and obesity (56, 57). The demonstration that ROS is an integral component of GnRH signaling and that compromise of redox buffering results in decreased expression of gonadotropins implies a role in the obesity-related decline in gonadotropin production or general dysregulation of gonadotropes through disruption of pulsatile signal interpretation. ROS production through fatty acid metabolism in LβT2 cells and gonadotropes limits gene expression and gonadotropin secretion in vitro, further supporting this possibility (24). Continued studies of the role of ROS in GnRH-mediated regulation of gonadotropin production may shed light on the normal and pathophysiological consequences of ROS on reproduction.
Acknowledgments
Financial Support: This work was supported by National Institutes of Health (NIH) Grants R01 HD 037568, R25 GM083275, P50 HD012303, and R01 HD082567 (to M.A.L.); Grant T32 HD007203 (to T.K.); and Grant K12 GM068524 (to D.A.N.). T.T. was supported by the Uehara Memorial Foundation. D.A.N. also was supported by the University of California President’s Postdoctoral Fellowship Program. V.S.K. was supported by NIH Grant R25 GM083275 (to M.A.L.).
Additional Information
Disclosure Summary: The authors have nothing to disclose.
Data Availability: All data generated or analyzed during this study are included in this published article or in the data repositories listed in References.
Glossary
Abbreviations:
- DAPI
4', 6-diamidino-2-phenylindole
- DPI
diphenyleneiodonium
- DUSP
dual-specificity protein phosphatase
- FBS
fetal bovine serum
- MAPK
mitogen-activated protein kinase
- NAC
N-acetyl-l-cysteine
- NADPH
nicotinamide adenine dinucleotide phosphate oxidase
- NOX/DUOX
NADPH/dual-specificity oxidase
- PRDX
peroxiredoxin
- ROS
reactive oxygen species
- qPCR
quantitative PCR
- shCtrl
short hairpin control
- shRNA
short hairpin RNA
- shSrxn1
short hairpin Srxn1
- SRXN1
sulfiredoxin
- TBS-T
Tris-buffered (pH 8.0) saline containing 0.05% Tween 20
References and Notes
- 1. Marshall JC, Dalkin AC, Haisenleder DJ, Griffin ML, Kelch RP. GnRH pulses--the regulators of human reproduction. Trans Am Clin Climatol Assoc. 1993;104:31–46. [PMC free article] [PubMed] [Google Scholar]
- 2. Lawson MA, Tsutsumi R, Zhang H, Talukdar I, Butler BK, Santos SJ, Mellon PL, Webster NJ. Pulse sensitivity of the luteinizing hormone beta promoter is determined by a negative feedback loop Involving early growth response-1 and Ngfi-A binding protein 1 and 2. Mol Endocrinol. 2007;21(5):1175–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mistry DS, Tsutsumi R, Fernandez M, Sharma S, Cardenas SA, Lawson MA, Webster NJ. Gonadotropin-releasing hormone pulse sensitivity of follicle-stimulating hormone-beta gene is mediated by differential expression of positive regulatory activator protein 1 factors and corepressors SKIL and TGIF1. Mol Endocrinol. 2011;25(8):1387–1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kanasaki H, Bedecarrats GY, Kam KY, Xu S, Kaiser UB. Gonadotropin-releasing hormone pulse frequency-dependent activation of extracellular signal-regulated kinase pathways in perifused LbetaT2 cells. Endocrinology. 2005;146(12):5503–5513. [DOI] [PubMed] [Google Scholar]
- 5. Armstrong SP, Caunt CJ, Fowkes RC, Tsaneva-Atanasova K, McArdle CA. Pulsatile and sustained gonadotropin-releasing hormone (GnRH) receptor signaling: does the Ca2+/NFAT signaling pathway decode GnRH pulse frequency? J Biol Chem. 2009;284(51):35746–35757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Armstrong SP, Caunt CJ, Fowkes RC, Tsaneva-Atanasova K, McArdle CA. Pulsatile and sustained gonadotropin-releasing hormone (GnRH) receptor signaling: does the ERK signaling pathway decode GnRH pulse frequency? J Biol Chem. 2010;285(32):24360–24371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Choi SG, Jia J, Pfeffer RL, Sealfon SC. G proteins and autocrine signaling differentially regulate gonadotropin subunit expression in pituitary gonadotrope. J Biol Chem. 2012;287(25):21550–21560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Perrett RM, Voliotis M, Armstrong SP, Fowkes RC, Pope GR, Tsaneva-Atanasova K, McArdle CA. Pulsatile hormonal signaling to extracellular signal-regulated kinase: exploring system sensitivity to gonadotropin-releasing hormone pulse frequency and width. J Biol Chem. 2014;289(11):7873–7883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Pratap A, Garner KL, Voliotis M, Tsaneva-Atanasova K, McArdle CA. Mathematical modeling of gonadotropin-releasing hormone signaling. Mol Cell Endocrinol. 2017;449:42–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Nguyen KA, Intriago RE, Upadhyay HC, Santos SJ, Webster NJ, Lawson MA. Modulation of gonadotropin-releasing hormone-induced extracellular signal-regulated kinase activation by dual-specificity protein phosphatase 1 in LbetaT2 gonadotropes. Endocrinology. 2010;151(10):4882–4893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kim T, Lawson MA. GnRH regulates gonadotropin gene expression through NADPH/dual oxidase-derived reactive oxygen species. Endocrinology. 2015;156(6):2185–2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rhee SG. Cell signaling. H2O2, a necessary evil for cell signaling. Science. 2006;312(5782):1882–1883. [DOI] [PubMed] [Google Scholar]
- 13. Finkel T. Reactive oxygen species and signal transduction. IUBMB Life. 2001;52(1-2):3–6. [DOI] [PubMed] [Google Scholar]
- 14. Jiang F, Zhang Y, Dusting GJ. NADPH oxidase-mediated redox signaling: roles in cellular stress response, stress tolerance, and tissue repair. Pharmacol Rev. 2011;63(1):218–242. [DOI] [PubMed] [Google Scholar]
- 15. Abbas K, Riquier S, Drapier JC. Peroxiredoxins and sulfiredoxin at the crossroads of the NO and H2O2 signaling pathways. Methods Enzymol. 2013;527:113–128. [DOI] [PubMed] [Google Scholar]
- 16. Jeong W, Bae SH, Toledano MB, Rhee SG. Role of sulfiredoxin as a regulator of peroxiredoxin function and regulation of its expression. Free Radic Biol Med. 2012;53(3):447–456. [DOI] [PubMed] [Google Scholar]
- 17. RRID:CVCL_0398, https://scicrunch.org/resolver/CVCL_0398.
- 18. Alarid ET, Windle JJ, Whyte DB, Mellon PL. Immortalization of pituitary cells at discrete stages of development by directed oncogenesis in transgenic mice. Development. 1996;122(10):3319–3329. [DOI] [PubMed] [Google Scholar]
- 19. Turgeon JL, Kimura Y, Waring DW, Mellon PL. Steroid and pulsatile gonadotropin-releasing hormone (GnRH) regulation of luteinizing hormone and GnRH receptor in a novel gonadotrope cell line. Mol Endocrinol. 1996;10(4):439–450. [DOI] [PubMed] [Google Scholar]
- 20. Ruf-Zamojski F, Ge Y, Pincas H, Shan J, Song Y, Hines N, Kelley K, Montagna C, Nair P, Toufaily C, Bernard DJ, Mellon PL, Nair V, Turgeon JL, Sealfon SC. Cytogenetic, genomic, and functional characterization of pituitary gonadotrope cell lines. J Endocr Soc. 2019;3(5):902–920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Breen KM, Thackray VG, Coss D, Mellon PL. Runt-related transcription factors impair activin induction of the follicle-stimulating hormone beta-subunit gene. Endocrinology. 2010;151(6):2669–2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Do MT, Kim T, He F, Dave H, Intriago RE, Astorga UA, Jain S, Lawson MA. Polyribosome and ribonucleoprotein complex redistribution of mRNA induced by GnRH involves both EIF2AK3 and MAPK signaling. Mol Cell Endocrinol. 2014;382(1):346–357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Do MH, Santos SJ, Lawson MA. GNRH induces the unfolded protein response in the LbetaT2 pituitary gonadotrope cell line. Mol Endocrinol. 2009;23(1):100–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Li S, Mbong EF, John DT, Terasaka T, Li D, Lawson MA. Induction of stress signaling in vitro and suppression of gonadotropin secretion by free fatty acids in female mouse gonadotropes. Endocrinology. 2018;159(2):1074–1087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kim H, Jung Y, Shin BS, Kim H, Song H, Bae SH, Rhee SG, Jeong W. Redox regulation of lipopolysaccharide-mediated sulfiredoxin induction, which depends on both AP-1 and Nrf2. J Biol Chem. 2010;285(45):34419–34428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods. 2001;25(4):402–408. [DOI] [PubMed] [Google Scholar]
- 27. RRID:AB_2784507, https://scicrunch.org/resolver/AB_2784507.
- 28. RRID:AB_2286615, https://scicrunch.org/resolver/AB_2286615.
- 29. RRID:AB_141954, https://scicrunch.org/resolver/AB_141954.
- 30. RRID:AB_2534069, https://scicrunch.org/resolver/AB_2534069.
- 31. RRID:AB_443491, https://scicrunch.org/resolver/AB_443491.
- 32. RRID:AB_476744, https://scicrunch.org/resolver/AB_476744.
- 33. RRID:AB_631736, https://scicrunch.org/resolver/AB_631736.
- 34. RRID:AB_631746, https://scicrunch.org/resolver/AB_631746.
- 35. Forman HJ, Torres M. Reactive oxygen species and cell signaling: respiratory burst in macrophage signaling. Am J Respir Crit Care Med. 2002;166(12 Pt 2)S4–S8. [DOI] [PubMed] [Google Scholar]
- 36. Rhee SG, Woo HA, Kil IS, Bae SH. Peroxiredoxin functions as a peroxidase and a regulator and sensor of local peroxides. J Biol Chem. 2012;287(7):4403–4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wood ZA, Schröder E, Robin Harris J, Poole LB. Structure, mechanism and regulation of peroxiredoxins. Trends Biochem Sci. 2003;28(1):32–40. [DOI] [PubMed] [Google Scholar]
- 38. Kwak MK, Wakabayashi N, Itoh K, Motohashi H, Yamamoto M, Kensler TW. Modulation of gene expression by cancer chemopreventive dithiolethiones through the Keap1-Nrf2 pathway. Identification of novel gene clusters for cell survival. J Biol Chem. 2003;278(10):8135–8145. [DOI] [PubMed] [Google Scholar]
- 39. Yan C, Lee LH, Davis LI. Crm1p mediates regulated nuclear export of a yeast AP-1-like transcription factor. EMBO J. 1998;17(24):7416–7429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Soriano FX, Baxter P, Murray LM, Sporn MB, Gillingwater TH, Hardingham GE. Transcriptional regulation of the AP-1 and Nrf2 target gene sulfiredoxin. Mol Cells. 2009;27(3):279–282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Li X, He P, Wang XL, Zhang S, Devejian N, Bennett E, Cai C. Sulfiredoxin-1 enhances cardiac progenitor cell survival against oxidative stress via the upregulation of the ERK/NRF2 signal pathway. Free Radic Biol Med. 2018;123:8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Terasaka T, Adakama ME, Li S, Kim T, Terasaka E, Li D, Lawson MA. Reactive oxygen species link gonadotropin-releasing hormone receptor signaling cascades in the gonadotrope. Front Endocrinol (Lausanne). 2017;8:286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Dang AK, Chaplin NL, Murtazina DA, Boehm U, Clay CM, Amberg GC. Subplasmalemmal hydrogen peroxide triggers calcium influx in gonadotropes. J Biol Chem. 2018;293(41):16028–16042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Harris D, Bonfil D, Chuderland D, Kraus S, Seger R, Naor Z. Activation of MAPK cascades by GnRH: ERK and Jun N-terminal kinase are involved in basal and GnRH-stimulated activity of the glycoprotein hormone LHbeta-subunit promoter. Endocrinology. 2002;143(3):1018–1025. [DOI] [PubMed] [Google Scholar]
- 45. Burger LL, Haisenleder DJ, Aylor KW, Marshall JC. Regulation of intracellular signaling cascades by GNRH pulse frequency in the rat pituitary: roles for CaMK II, ERK, and JNK activation. Biol Reprod. 2008;79(5):947–953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Nguyen KA, Santos SJ, Kreidel MK, Diaz AL, Rey R, Lawson MA. Acute regulation of translation initiation by gonadotropin-releasing hormone in the gonadotrope cell line LbetaT2. Mol Endocrinol. 2004;18(5):1301–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Qiao S, Nordström K, Muijs L, Gasparoni G, Tierling S, Krause E, Walter J, Boehm U. Molecular plasticity of male and female murine gonadotropes revealed by mRNA sequencing. Endocrinology. 2016;157(3):1082–1093. [DOI] [PubMed] [Google Scholar]
- 48. Kondoh K, Nishida E. Regulation of MAP kinases by MAP kinase phosphatases. Biochim Biophys Acta. 2007;1773(8):1227–1237. [DOI] [PubMed] [Google Scholar]
- 49. Keyse SM, Emslie EA. Oxidative stress and heat shock induce a human gene encoding a protein-tyrosine phosphatase. Nature. 1992;359(6396):644–647. [DOI] [PubMed] [Google Scholar]
- 50. Hampton MB, Orrenius S. Dual regulation of caspase activity by hydrogen peroxide: implications for apoptosis. FEBS Lett. 1997;414(3):552–556. [DOI] [PubMed] [Google Scholar]
- 51. Kannan K, Jain SK. Oxidative stress and apoptosis. Pathophysiology. 2000;7(3):153–163. [DOI] [PubMed] [Google Scholar]
- 52. Savulescu D, Feng J, Ping YS, Mai O, Boehm U, He B, O’Malley BW, Melamed P. Gonadotropin-releasing hormone-regulated prohibitin mediates apoptosis of the gonadotrope cells. Mol Endocrinol. 2013;27(11):1856–1870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Gründker C, Emons G. The role of gonadotropin-releasing hormone in cancer cell proliferation and metastasis. Front Endocrinol (Lausanne). 2017;8:187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Agarwal A, Aponte-Mellado A, Premkumar BJ, Shaman A, Gupta S. The effects of oxidative stress on female reproduction: a review. Reprod Biol Endocrinol. 2012;10(1):49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Aitken RJ, Smith TB, Jobling MS, Baker MA, De Iuliis GN. Oxidative stress and male reproductive health. Asian J Androl. 2014;16(1):31–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Liu J, Zhang D. The role of oxidative stress in the pathogenesis of polycystic ovary syndrome [in Chinese]. Sichuan Da Xue Xue Bao Yi Xue Ban. 2012;43(2):187–190. [PubMed] [Google Scholar]
- 57. Barber TM, Vojtechova P, Franks S. The impact of hyperandrogenism in female obesity and cardiometabolic diseases associated with polycystic ovary syndrome. Horm Mol Biol Clin Investig. 2013;15(3):91–103. [DOI] [PubMed] [Google Scholar]