Abstract
Nepal and many developing countries are currently suffering from increased prevalence of obesity, type 2 diabetes, and other metabolic disorders. Unhealthy dietary habits and physical inactivity are traditionally considered as responsible factors for these disorders. The relatively new concept of foetal programming suggests that development of metabolic diseases later in life may be associated with poor nutritional status in utero, and such phenomenon could be amplified by subsequent exposure to unhealthy diets after birth. We suggest that foetal programming and mismatched nutritional situations during foetal and postnatal life are important causative factors for increased prevalence of obesity and metabolic disorders in Nepal. Issues highlighted in this paper may also be relevant to other developing countries with similar socioeconomic status. Undernutrition in foetal life can predispose for visceral fat deposition and may alter dietary preferences towards unhealthy diets, amplifying the risk of nutritional mismatch after birth; this can lead to metabolic disturbances in a number of pathways including glucose and lipid metabolism. Providing attention to early life nutrition could therefore be an important tool to reduce the prevalence of lifestyle diseases in Nepal. Future national health policies should thus include changes in research and intervention activities towards preventing averse early life nutritional programming. Availability of free-of-cost and mandatory nutritional education and medical services to pregnant women and their families and better management of national health care systems including digitalization of national health data could be viable strategies to achieve these goals.
Keywords: foetal programming, metabolic disorder, nutrition, Nepal, obesity
INTRODUCTION
The epidemic-like development of obesity and other associated so-called welfare diseases are causing global rises in adverse effects on human life quality. Generally, obesity and metabolic disorders are considered public health issues of developed countries. However, reports suggest that over the last decades issues related to obesity are not limited to the developed world per se but are also expanding towards many underdeveloped countries, affecting people of all age groups (Misra and Khurana, 2008). Obesity is therefore becoming more common and is considered a serious public health concern both in the developed and developing world (Ng et al., 2014; Prentice, 2006).
Low- and middle-income countries are now facing a double disease burden, infectious diseases as well as non-communicable diseases, such as cardiovascular diseases and diabetes, which are on the rise as increased urbanization and lifestyle changes give rise to over-eating and reduced levels of physical activity (Vaidya et al., 2010). In these countries, public health issues have primarily focused on communicable and specific nutrient-deficiency related diseases, whereas health problems, like obesity and type 2 diabetes, are still being ignored with a common belief that “being fatter is healthier”. Although extensive research or surveys on the prevalence of obesity and type 2 diabetes are limited, some studies have highlighted that the risk of obesity and metabolic disorders are a growing concern in the developing world and are rising at an alarming rate (Ellulu et al., 2014; Hossain et al., 2007; Misra and Bhardwaj, 2014). These studies suggest that this is particularly associated with changes in the pattern of nutritional compositions of diets (Bhurosy and Jeewon, 2014; Popkin, 2001).
Identifying the underlying mechanisms behind the current increase in prevalence of obesity and associated metabolic disorders is crucial to limit and even prevent a further increase of such health problems. It is therefore important to be aware that development of obesity in developing countries may not only be associated with lack of physical activity or over- or unhealthy nutrition following improvements in socio-economic status, but could also be associated with the nutritional status in utero, i.e. before birth. In fact, previous epidemiological and experimental animal studies have shown that exposure to adverse nutrition or other environmental factors during foetal life can predispose for obesity and associated metabolic disorders later in life (Dyer and Rosenfeld, 2011; Khanal et al., 2014; Taylor and Poston, 2007); this may be due to altered phenotypic expression of the genome as a consequence of a phenomenon, known as ‘foetal metabolic programming’.
This review highlights the potential, and yet not fully recognized, role of foetal programming as a causative factor for the rising prevalence of obesity and associated metabolic disorders, such as obesity, type 2 diabetes, and cardiovascular diseases, in Nepal. Nepal is a developing country in South-East Asia, however the issues raised and associated recommendations may potentially reflect situations prevailing in other developing countries across the world.
OVERWEIGHT AND OBESITY TRENDS IN NEPAL
Obesity, hypertension (Dhungana et al., 2016; Hasan et al., 2018), and type 2 diabetes (Gyawali et al., 2015) are high-burden diseases in Nepal and affect people of all ages and genders (Table 1). Among children and adolescents, there is a global trend in population body mass index (BMI) towards overweight and obesity; this increase has accelerated in many countries, including South Asia (Abarca-Gómez et al., 2017).
Table 1.
Prevalence of obesity, overweight, and metabolic disorders in Nepal
| Study conditions and methods | Study subjects | Study location | Major observations | References |
|---|---|---|---|---|
| Cross-sectional study; Self-administered questionnaire with parents; Height and weight measurements and body-mass- index (BMI) calculations in children | Both genders aged 6 13 yrs; 986 subjects | Latilpur district (Urban district) | 26% children overweight or obese | Koirala et al., 2015 |
| Screening with a physical examination and blood tests | Both genders (62% females) aged 20 100 yrs; 17,425 subjects | Eastern Nepal | 28% overweight; 32% obesity; 22.5% metabolic syndrome | Sharma et al., 2011 |
| Cross-sectional questionnaire survey among civil servants; Height and weight recording | Both genders (80% male): young (<45 yrs) versus old (>45 yrs); 341 subjects | Urban district around Kathmandu valley | 33.4% prevalence of overweight/obesity | Simkhada et al., 2011 |
| Cross-sectional study; Self-administered questionnaire survey and anthropometric measurements | Both genders (52.8% female) aged 16 19 yrs; urban school adolescents; 360 subjects | Lalitpur sub-metropolitan city | 12.2% overweight | Piryani et al., 2016 |
| Cross-sectional study; Self-administered questionnaire survey; Height and weight measurements and BMI and weight-to-hip circumference ratio (WHR) | Both genders aged 17 24 yrs; undergraduate students; 384 subjects | Institute of Medicine (IOM), Kathmandu | 32.5% overweight and 11.4% obese; this figure increased to 46.35% when considering WHR as parameter for obesity | Nepal et al., 2018 |
| Nationally representative cross-sectional data from three Demographic and Health Surveys | Women of child bearing age from 15 49 yrs; 7,900, 10,079, and 5,873 subjects in years 2001, 2006, and 2011, respectively | National survey | 27.4% and 11.8% overweight in urban and rural areas, respectively | Kinnunen and Neupane, 2014 |
About one third of adults in a diverse study population with 14,425 subjects in Nepal suffered from hypertension, overweight, or obesity, and high prevalence of these disorders was associated with lower educational level and working at home, and there was a gender bias towards increased prevalence among females (Sharma et al., 2011). It is a general picture across developing countries in Asia that the prevalence of obesity and overweight is increasing, mainly affecting women in the reproductive age (15~49 years) (Balarajan and Villamor, 2009). About one third of civil servants from urban district areas were also found to be overweight or obese in Nepal (Simkhada et al., 2011).
It is further alarming that overweight and obesity are also becoming common and more prevalent among adolescents and young adults in South Asia, as the younger generations adopt more unhealthy food habits (Jayawardena et al., 2017). Among Nepalese school children in city areas, 26% of the children aged 6~13 years were found to be overweight or obese (Koirala et al., 2015). A cross-sectional study involving school adolescents from an urban area (Lalitpur area) also revealed that 12.2% of the adolescent studied were overweight, and this was associated with higher socioeconomic status, longer time of television watching as well as consuming less fruits (Piryani et al., 2016). Even higher obesity prevalence has been reported among young university students aged 17~24 years (up to ~45% obesity) (Nepal et al., 2018).
CAUSES OF THE OBESITY PROBLEM IN NEPAL
Obesity and the metabolic syndrome are multifactorial diseases. In Nepal, obesity and associated metabolic disorders, such as cardiovascular diseases, hypertension, and type 2 diabetes, are linked to socioeconomic factors including geography, education levels, physical activity, and gender (Sharma et al., 2011). For example, a national survey revealed that a higher prevalence of overweight or obesity was found in the residents of lower-lying hill vs. mountain regions, and it was also higher for people in urban vs. rural areas in Nepal (Aryal et al., 2015). Evaluation of data from three Demographic and Health Surveys suggested that the prevalence of overweight was distinctly higher in women of child-bearing age from urban areas compared to those from rural areas; socioeconomic status was a major factor explaining this difference (Kinnunen and Neupane, 2014). Differences in socioeconomic status among people in developing countries are therefore related to eating behaviour (junk food vs. healthy heating) and physical activity levels (sedentary vs. active daily lifestyles). In agreement with this, increased urbanization, nutrition transition, and lowered physical activity have been identified as key players behind the rising prevalence of obesity and metabolic syndrome across the developing world (Misra and Khurana, 2008). Hence, eating patterns, consumption of energy-rich junk foods, and the trend towards a more sedentary lifestyle with less physical activity have been a prime focus in the past to explain the rise in obesity and prevalence of associated metabolic disorders.
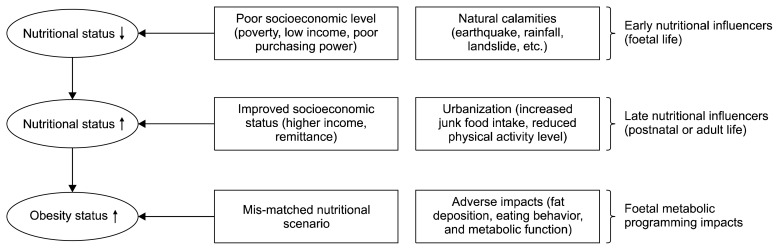
However, nutritional transitions in the developing world have also created a mismatched nutritional scenario in terms of nutritional exposure (quantitatively and qualitatively) during foetal life as compared to the nutrition exposure in postnatal life. In Nepal, poverty and poor socioeconomic status have been widespread for decades, with natural calamities including earthquakes and landslides causing serious food shortage which lead to poor nutritional statuses for developing foetuses (Fig. 1). Scarcity of food and undernutrition, which has often been common for adults including pregnant women, may still occur following natural disasters despite overall improvements in the socioeconomic status of South Asia.
Fig. 1.
Consequences of foetal malnutrition and foetal metabolic programming that can contribute to predispose for obesity and associated health disorders in Nepal and other developing countries.
These factors could explain a recent World Health Organization report that suggested a higher percentage (28%) of infants born are born with a lower birth weight and a small gestational age in South Asia compared with the global average (15%) (WHO, 2014). In many Southeast Asian countries like Nepal the majority of deliveries is performed at home particularly in poor families and with only help from family and relatives (Montagu et al., 2011). It is therefore likely that the percentage of babies born with low birth weights may be higher than those reflected by the official numbers, particularly over previous decades, since no official anthropometric recordings are performed for babies born at home (Singh et al., 2017) and the demographic bias is towards poorer socioeconomic status in rural areas.
The nutritional situation of Nepal has changed in recent years due to rapid urbanization, migration from rural to urban areas, and general economic development. In 2014, it was estimated that about 27% of the total Nepalese population lived in city areas, however the urban population was rapidly increasing at a rate of ~8% per year, about 6 times that of the national population growth (Subedi, 2014). In addition, mass migration of labour to foreign countries, particularly from low- or middle-class families, had become a tradition, facilitating a dramatic rise in the inflow of remittances, leading to improved livelihood and economic security (Knerr, 2017). This has further promoted rural-urban migration. Remittance inflow, higher purchasing power, and higher rate of urbanization are therefore recent factors promoting more sedentary lifestyles and a dietary shift towards consumption of more Westernized junk and energy-dense foods (Fig. 1).
This transition of dietary habits, which is observed in many developing countries including Nepal, has resulted in mismatched nutritional scenario during very early life, including the foetal period, as compared to later in postnatal life (Fig. 1). Interestingly, such nutritional mismatches have been observed in human epidemiological and animal experimental studies and these predispose to the welfare diseases that are increasing in Nepal (Cleal et al., 2007; Gluckman et al., 2007; Khanal et al., 2014; Nielsen et al., 2013; Painter et al., 2005). The underlying reason is that malnutrition during foetal life can alter the function of our genome and thus alter sensitivity towards unhealthy nutritional exposures later in life. This is due to an underlying phenomenon, known as “foetal metabolic programming” (FMP). We will now present scientific evidence for the causative role of FMP in predisposing to obesity and associated metabolic diseases, which has been largely overlooked from the perspective of the developing world.
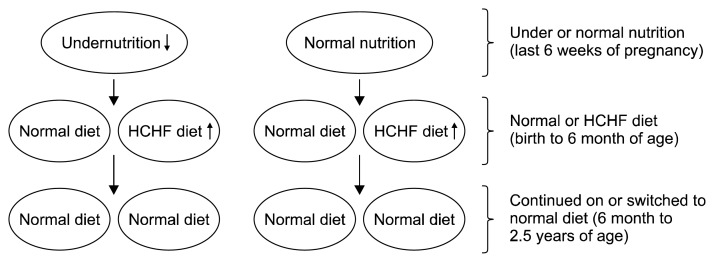
We developed the Copenhagen sheep model (Khanal et al., 2014; Nielsen et al., 2013) to evaluate how exposure to mismatched nutritional situations in foetal life vs. early postnatal life impact body functions that may cause non-communicable diseases prevailing in many developing countries (Fig. 2). In the following sections, we will highlight the mechanisms behind the predisposition for development of obesity in response to FMP, with a particular focus on 1) fat deposition patterns, 2) eating preferences, and 3) altered metabolic function (Fig. 3).
Fig. 2.
Copenhagen sheep model mimicking situations of pre- and postnatal mismatched nutritional status (Khanal et al., 2014; Nielsen et al., 2013). HCHF, high-carbohydrate-high-fat.
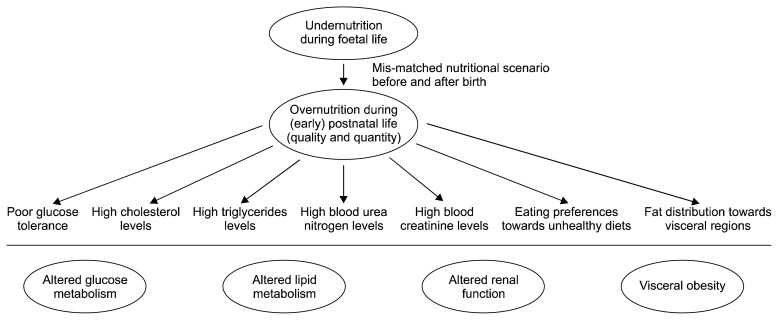
Fig. 3.
Major physiological impacts of mismatched nutritional scenarios during foetal and (early) postnatal life in the developing countries across the world, including Nepal (Cleal et al., 2007; Gluckman et al., 2007; Khanal et al., 2015; Khanal et al., 2014; Khanal et al., 2016; Nielsen et al., 2013).
FMP AND POSTNATAL ADIPOSE TISSUE DEPOSITION
Obesity is a state of abnormal or excessive accumulation of fat in adipose tissue depots to the extent that health may be impaired (WHO, 2000). In various adipose tissue depots, increased fat deposition in abdominal or visceral regions (abdominal obesity) is a marker of dysfunctional adipose tissues; this has been emphasized as the central indicator and predisposing condition for development of the metabolic syndrome (Després and Lemieux, 2006). The developmental origin of obesity in humans has been convincingly demonstrated. Children born to mothers that were in early pregnancy during the Dutch famine (1944~1945) developed higher BMI and waist circumferences in adulthood (Ravelli et al., 1999).
Various controlled animal experimental studies using rodents (Bellinger et al., 2006), pigs (Kind et al., 2002), or sheep (Gopalakrishnan et al., 2004; Khanal et al., 2014) have confirmed these findings. In our Copenhagen sheep model, we found that the increased predisposition to deposit fat in the abdominal region of lambs exposed to undernutrition in late foetal life (last six weeks of pregnancy) was associated with a reduced ability to deposit fat in subcutaneous adipose tissue (Khanal et al., 2014; Nielsen et al., 2013). There is accumulating evidence that subcutaneous fat may be considered a healthy fat, since accumulation of fat here rather in the abdominal region is associated with healthier metabolic profiles; storage of extra energy reserves in this fat also protects from overflow of nutrients into fat depots in the visceral region (Tran et al., 2008; Wronska and Kmiec, 2012). Thus, limitations to the lipid storing ability of subcutaneous fat can lead to increased risk of fat deposition in regions such as the abdomen, which negatively impacts visceral organs such as the pancreas. We in fact showed the increased direction of lipid deposition in the visceral region due to poorer ability to store fat in subcutaneous adipose tissues in lambs subjected to foetal undernutrition that gave rise to extreme hypertrophy of perirenal adipocytes, when the lambs were exposed to an energy dense, high-fat diet in postnatal life (Khanal and Nielsen, 2017). In addition, we observed higher occurrence of very small adipocytes in subcutaneous adipose tissue along with increased collagen infiltration in lambs that had been subjected to prenatal undernutrition (Nielsen et al., 2016). Increased collagen infiltration can in itself limit the expandability (lipid storing ability) of subcutaneous adipose tissue, and results from studies with obese human patients suggests that the existence of a subpopulation of very small adipocytes in subcutaneous adipose tissue appears to be linked to lowered whole body insulin sensitivity (McLaughlin et al., 2014). Our studies in the Copenhagen sheep model suggest that occurrence of very small adipocytes may have a foetal origin. It is noteworthy that South Asians appear to be more susceptible to development of central obesity and associated undesirable outcomes (adipose tissue overflow hypothesis) (Sniderman et al., 2007). Based on findings by ourselves and others, we suggest that the increased susceptibility of South Asians to develop visceral obesity in part could have a foetal origin, and South Asians may therefore be particularly sensitive to suboptimal nutrition exposures during the critical period of foetal development.
FMP AND EATING PREFERENCES
The development of obesity and other metabolic disorders is closely linked to eating habits and food preferences. It is therefore disturbing that exposure to undernutrition during foetal life can induce unwanted changes in postnatal eating preferences and energy consumption (Portella et al., 2012). In rats, offspring born to dams exposed to a low protein diet during pregnancy had increased preferences for a high-fat diets and reduced preferences for high-carbohydrate diets in young adulthood compared to offspring that were not exposed to gestational protein undernutrition (Bellinger et al., 2004). Similarly, another study showed that extreme foetal undernutrition (30% of requirements) of Wistar rats leads to postnatal hyperphagia for a hypercaloric diet (30% fat), resulting in development of obesity and associated hyperleptineamia and hyperinsulinemia (Vickers et al., 2000). In addition, lambs exposed to foetal maternal undernutrition (50% of protein and energy requirements) in late gestation had increased neonatal appetites for fat-rich rather than carbohydrate-rich diets (Nielsen et al., 2013). To our knowledge, there are no reports on the direct consequences of prenatal nutrition for postnatal eating behaviour in human, however some observations indirectly indicate that eating behaviour may be altered by suboptimal nutritional environments in utero. Thus, young Ethiopians subjected to famine, showed higher prevalence of diabetes within 4 years of migration to Israel, associated with consumption of large amounts of refined carbohydrates instead of traditional Ethiopian bread (injera) and spicy stews (Cohen et al., 1988).
Overall, although evidence by direct human studies are limited, nutritional programming due to maternal undernourishment during foetal development may alter postnatal appetite and shift dietary preferences in favour of high-fat high-calorie ‘junk’ foods. This may lead to foetuses predisposed to altered fat distributions and visceral obesity development (Fig. 3).
FMP AND METABOLIC FUNCTION
Both epidemiological and animal studies show that undernutrition exposure in utero results in metabolic disturbances in various metabolic pathways, including glucose and lipid metabolism (Fig. 3). For example, prenatal exposure to famine during late gestation leads to decreased glucose tolerance in human adults and permanent changes in insulin-glucose metabolism (Ravelli et al., 1998). We and others have demonstrated that maternal undernutrition in sheep during late gestation leads to reduced insulin sensitivity and poorer glucose tolerances of offspring in later life (Gardner et al., 2005; Kongsted et al., 2014). These studies underline that the glucose-insulin axis is a major target of foetal programming caused by maternal undernutrition during pregnancy. Many studies also suggests that lipid metabolic pathways are targets of early life nutritional programming. For example, people exposed to famine in early gestation have undesirable plasma lipid profiles (lower high-density-lipoproteins and, higher total and low-density-lipoprotein levels) in adulthood (Roseboom et al., 2000), and adolescent lambs exposed to gestation undernutrition develop hypercholesterolemia upon exposure to high-carbohydrate high-fat diets in early postnatal life (Khanal et al., 2015). Hypercholesterolemia may persist into adulthood, even after the diet had been corrected to normal diet for 2 years (Khanal et al., 2016). These studies show that cholesterol metabolism and hepatic function may permanently be affected by exposure to maternal undernutrition during foetal life.
Accumulating evidence suggests that foetal nutritional programming can influence a number of other important endocrine systems and regulatory pathways, such as the hypothalamic-pituitary-adrenal axis (Phillips et al., 2005), leptin (McMillen et al., 2006), and the hypothalamic-pituitary-thyroid hormone axis (Johnsen et al., 2013). Given the nutritional and socioeconomic transition in Nepal and other developing countries during the past decades, it is relevant to draw attention to the potential causative role of nutritional mismatch between foetal and postnatal life in the development of major non-communicable diseases: obesity, diabetes, hypertension, etc.
FUTURE PERSPECTIVES/RECOMMENDATIONS
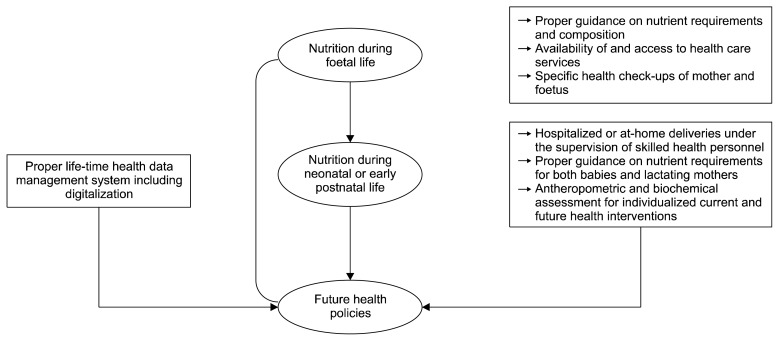
In the following sections, we will propose future strategies required to prevent or cope with unfortunate consequences of early life nutritional programming at the public or policy level to overcome undesirable impacts of lifestyle diseases with potential foetal origins in Nepal (Fig. 4).
Fig. 4.
Important considerations for the formulation of future health care policies in Nepal.
RESEARCH FOCUS
In any society, research serves as an important tool for making improvements to (public) health care systems. As is the case for most developing countries, the number of scientific studies and published reports on nutrition and health status in Nepal is remarkably low, and these focus on a limited number of topics (Simkhada et al., 2010). The few studies that exist examining non-communicable diseases have primarily focused on malnutrition or specific nutrient insufficiencies, and only a few have focused on obesity, type 2 diabetes, and other metabolic disorders. Moreover, a few Nepalese studies relating to obesity and metabolic disorders have mainly been limited to the adult population. Due to accumulating evidence demonstrating a strong association between early life nutrition and risks of developing diseases/metabolic disorders later in life, there is a need for a change of focus to include the possible association between prenatal and childhood nutrition in relation to obesity development. Proper epidemiological research should be conducted to design better nutritional strategies for children who have had significant nutritional transitions and lifestyle changes in early life and who’s families have had changes in socioeconomic status.
In Nepal, the traditional ‘more teaching and less research’ healthcare institutions, such as hospitals or universities, should become more research-oriented and research-based teaching institutions. This can be achieved by intra- and inter-university research collaborations that are active in the field of life sciences in Nepal. More importantly, South Asian nations that share regional similarities and common health problems should seek for mutual collaboration to develop new health research projects (Sadana et al., 2004). For example, medical science and health related institutions could establish collaborative research with universities dealing with animal and veterinary science programs to develop relevant animal models and undertake controlled research trials in various animal species. Animal studies focusing on the impacts of early life nutrition generate insight into underlying causative factors of health problems; this could be capitalized to design nutritional strategies at policy levels or to conduct awareness programs for the public. To the best of our knowledge, advanced research facilities to conduct cutting-edge researches on early life nutrition do not currently exist in Nepal, but this could be changed by establishing Early Life Nutrition Research Centres within existing universities or as independent research institutes. In addition to changing research focus, specific dietary or nutritional strategies should be formulated in Nepal during pregnancy, at birth and during early postnatal period as highlighted below.
NUTRITIONAL STRATEGIES DURING PREGNANCY, AT BIRTH AND DURING EARLY POSTNATAL LIFE
Nepal has secured a constitutional right to provide health services free of cost. However, in reality, this has not been properly implemented, and therefore parts of the population, particularly in more remote areas, are vulnerable and are unable to access quality national health services and this also applies to pregnant women and new-born babies. A targeted strategy in Nepal for better nutrition and health management during pregnancy and early postnatal life is needed to prevent unfortunate foetal programming and the associated long-lasting consequences. In Nepal, it is important to create an environment where women can attend nearby health facilities from when pregnancy is first detected. Thus, specific health intervention strategies should be formulated to ensure pregnant women receive regular check-ups to assess the health of both the mother and developing foetus, and to ensure they are provided guidance in daily nutritional requirements and diet compositions. One of the best ways to successfully implement this could be to provide compulsory awareness programs on health and nutrition education for pregnant women, their families and relatives, which would bring significant changes, such as identifying local sources of nutrients and getting acquainted with healthy cooking techniques, to minimize unwanted nutrient loss during the cooking process. Thus, incorporation of free-of-cost and mandatory a) health and nutrition program to pregnant mothers and their family members and b) regular check-up of mother and developing foetus, is crucial to ensure proper nutritional status of pregnant mothers and to ensure healthy deliveries.
When close to term, women and their families should be advised and encouraged to deliver at local health posts, hospitals, designated health, etc. or at-home under the supervision and assistance of skilled health professionals such as midwifes, nurses, etc. Some innovative health care policies, such as the national free delivery policy, have to some extend been successful in increasing the number of people visiting health care facilities (Witter et al., 2011); however, these policies still need to be implemented in a more effective and sustainable manner to cover the wide range of the targeted population. Another major aspect is the quality of maternal and child health facilities; in some cases, it is crucial to improve the quality of current health services rather than just increasing the number and expansion of such services and outreach activities (Acharya and Cleland, 2000). Additionally, major morphometric measurements of new-borns should be recorded and blood samples should be taken; this information could be valuable for relating early life health status with later disease outcomes. More importantly, these assessments could also be important for identifying if a newborn has been exposed to adverse conditions during foetal life, and for professionals to design specific nutritional strategies for these children, if needed. A major important concern in Nepal is for lactating mothers to avoid unhealthy dietary regimes, which affect neonatal babies. Lactating mothers are traditionally supplied with specific foods rich in fat, and this may influence the nutritional composition of the mother’s milk and potentially the babies’ health.
To sum up, provision of free and mandatory at-hospital/clinic-deliveries, morphometric, and biochemical assessments at birth, and development of proper nutritional strategies for both mothers and their babies during foetal and neonatal periods and during early postnatal life are some of the key issues recommended to be incorporated into national health policy programs in Nepal.
NATIONAL HEALTH DATA MANAGEMENT SYSTEM
Proper management of national health data is a major concern in Nepal when it comes to formulating new and effective health policy plans. In Nepal, the national health data management system is not advanced; most national health data is not yet digitalized and this creates obstacles for designing pharmacological or nutritional strategies. Digitalization of all national health data is of foremost important, as it enables data to be visible and available for networks of national health institutions. Digital data are valuable research sources in all countries, and can be utilized to design better and targeted health care services, including for mothers and their babies during pregnancy and lactation.
Longitudinal epidemiological studies focusing on the impacts of foetal programming on human health require careful evaluation of data over a long period of time i.e. from birth to adulthood. Proper management of health data is thus extremely important to correlate early life nutritional status to incidences of disease later in life. Proper management and digitalization of national health data should be a top priority, in addition to formulating future health policies in Nepal. Local health posts, district and regional health care centres, and national hospitals could be important locations for collecting and storing the digital health data, in order to provide medical services with valuable data to sustain research on human health in Nepal.
CONCLUSION
Nepal, alike many developing countries, is suffering from traditional nutritional deficiency diseases and the economic burden of increased prevalence of lifestyle diseases, such as obesity, type 2 diabetes, and other metabolic disorders. The increased prevalence of lifestyle diseases in Nepal could be associated with foetal programming resulting from a mismatched nutrition scenarios: undernutrition in foetal life followed by exposure to unhealthy ‘calorie rich junk’ foods after birth. Foetal programming predisposes for increased visceral fat deposition and changes of dietary preferences towards unhealthy high fat or high-energy diets, and can lead to metabolic disturbances in a number of regulatory pathways involved in glucose and lipid metabolism. Providing proper nutrition in early life could therefore be important for reducing the prevalence of lifestyle diseases in Nepal. However, for this to occur, future national healthcare policies must include a) research focus on early life nutritional programming, b) free-of-cost and mandatory nutritional education and health-care services to pregnant women and their families, and c) improved national health data systems, including the use of digitalization. We believe that the issues highlighted here could be equally valid in many other developing countries across the world with similar socioeconomic statuses as Nepal.
ACKNOWLEDGEMENTS
The authors wish to thank all the members and laboratory technicians involved in the studies based on the Copenhagen sheep model at the Department of Veterinary and Animal Sciences, University of Copenhagen, Denmark.
Footnotes
FUNDING
The research activities involving the Copenhagen sheep model were supported by the Danish Council for Strategic Research through the research programme of the Centre for Foetal Programming (CFP), Denmark.
AUTHOR DISCLOSURE STATEMENT
The authors declare no conflict of interest.
AUTHORS’ CONTRIBUTIONS
PK wrote the manuscript, MON provided critical revisions of the manuscript and both authors read and approved the final version of the manuscript.
REFERENCES
- Abarca-Gómez L, Abdeen ZA, Hamid ZA, Abu-Rmeileh NM, Acosta-Cazares B, Acuin C, et al. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128·9 million children, adolescents, and adults. Lancet. 2017;390:2627–2642. doi: 10.1016/S0140-6736(17)32129-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acharya LB, Cleland J. Maternal and child health services in rural Nepal: does access or quality matter more? Health Policy Plan. 2000;15:223–229. doi: 10.1093/heapol/15.2.223. [DOI] [PubMed] [Google Scholar]
- Aryal KK, Mehata S, Neupane S, Vaidya A, Dhimal M, Dhakal P, et al. The burden and determinants of non communicable diseases risk factors in Nepal: findings from a nationwide STEPS survey. PLoS One. 2015;10:e0134834. doi: 10.1371/journal.pone.0134834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balarajan Y, Villamor E. Nationally representative surveys show recent increases in the prevalence of overweight and obesity among women of reproductive age in Bangladesh, Nepal, and India. J Nutr. 2009;139:2139–2144. doi: 10.3945/jn.109.112029. [DOI] [PubMed] [Google Scholar]
- Bellinger L, Lilley C, Langley-Evans SC. Prenatal exposure to a maternal low-protein diet programmes a preference for high-fat foods in the young adult rat. Br J Nutr. 2004;92:513–520. doi: 10.1079/BJN20041224. [DOI] [PubMed] [Google Scholar]
- Bellinger L, Sculley DV, Langley-Evans SC. Exposure to undernutrition in fetal life determines fat distribution, locomotor activity and food intake in ageing rats. Int J Obes. 2006;30:729–738. doi: 10.1038/sj.ijo.0803205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhurosy T, Jeewon R. Overweight and obesity epidemic in developing countries: a problem with diet, physical activity, or socioeconomic status? Sci World J. 2014;2014 doi: 10.1155/2014/964236. 964236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleal JK, Poore KR, Boullin JP, Khan O, Chau R, Hambidge O, et al. Mismatched pre- and postnatal nutrition leads to cardiovascular dysfunction and altered renal function in adulthood. Proc Natl Acad Sci USA. 2007;104:9529–9533. doi: 10.1073/pnas.0610373104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen MP, Stern E, Rusecki Y, Zeidler A. High prevalence of diabetes in young adult Ethiopian immigrants to Israel. Diabetes. 1988;37:824–828. doi: 10.2337/diab.37.6.824. [DOI] [PubMed] [Google Scholar]
- Després JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444:881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- Dhungana RR, Pandey AR, Bista B, Joshi S, Devkota S. Prevalence and associated factors of hypertension: a community-based cross-sectional study in municipalities of Kathmandu, Nepal. Int J Hypertens. 2016;2016 doi: 10.1155/2016/1656938. 1656938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyer JS, Rosenfeld CR. Metabolic imprinting by prenatal, perinatal, and postnatal overnutrition: a review. Semin Reprod Med. 2011;29:266–276. doi: 10.1055/s-0031-1275521. [DOI] [PubMed] [Google Scholar]
- Ellulu M, Abed Y, Rahmat A, Ranneh Y, Ali F. Epidemiology of obesity in developing countries: challenges and prevention. Glob Epidemic Obes. 2014 doi: 10.7243/2052-5966-2-2. doi: 10.7243/2052-5966-2-2. Available from: [DOI]
- Gardner DS, Tingey K, Van Bon BW, Ozanne SE, Wilson V, Dandrea J, et al. Programming of glucose-insulin metabolism in adult sheep after maternal undernutrition. Am J Physiol Regul Integr Comp Physiol. 2005;289:R947–R954. doi: 10.1152/ajpregu.00120.2005. [DOI] [PubMed] [Google Scholar]
- Gluckman PD, Hanson MA, Beedle AS. Early life events and their consequences for later disease: a life history and evolutionary perspective. Am J Hum Biol. 2007;19:1–19. doi: 10.1002/ajhb.20590. [DOI] [PubMed] [Google Scholar]
- Gopalakrishnan GS, Gardner DS, Rhind SM, Rae MT, Kyle CE, Brooks AN, et al. Programming of adult cardiovascular function after early maternal undernutrition in sheep. Am J Physiol Regul Integr Comp Physiol. 2004;287:R12–R20. doi: 10.1152/ajpregu.00687.2003. [DOI] [PubMed] [Google Scholar]
- Gyawali B, Sharma R, Neupane D, Mishra SR, van Teijlingen E, Kallestrup P. Prevalence of type 2 diabetes in Nepal: a systematic review and meta-analysis from 2000 to 2014. Glob Health Action. 2015;8:29088. doi: 10.3402/gha.v8.29088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan M, Sutradhar I, Akter T, Das Gupta R, Joshi H, Haider MR, et al. Prevalence and determinants of hypertension among adult population in Nepal: data from Nepal Demographic and Health Survey 2016. PLoS One. 2018;13:e0198028. doi: 10.1371/journal.pone.0198028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossain P, Kawar B, El Nahas M. Obesity and diabetes in the developing world–a growing challenge. N Engl J Med. 2007;356:213–215. doi: 10.1056/NEJMp068177. [DOI] [PubMed] [Google Scholar]
- Jayawardena R, Ranasinghe P, Wijayabandara M, Hills AP, Misra A. Nutrition transition and obesity among teenagers and young adults in South Asia. Curr Diabetes Rev. 2017;13:444–451. doi: 10.2174/1573399812666160808100211. [DOI] [PubMed] [Google Scholar]
- Johnsen L, Kongsted AH, Nielsen MO. Prenatal undernutrition and postnatal overnutrition alter thyroid hormone axis function in sheep. J Endocrinol. 2013;216:389–402. doi: 10.1530/JOE-12-0389. [DOI] [PubMed] [Google Scholar]
- Khanal P, Axel AM, Kongsted AH, Husted SV, Johnsen L, Pandey D, et al. Late gestation under- and overnutrition have differential impacts when combined with a post-natal obesogenic diet on glucose-lactate-insulin adaptations during metabolic challenges in adolescent sheep. Acta Physiol. 2015;213:519–536. doi: 10.1111/apha.12391. [DOI] [PubMed] [Google Scholar]
- Khanal P, Husted SV, Axel AM, Johnsen L, Pedersen KL, Mortensen MS, et al. Late gestation over- and undernutrition predispose for visceral adiposity in response to a post-natal obesogenic diet, but with differential impacts on glucose-insulin adaptations during fasting in lambs. Acta Physiol. 2014;210:110–126. doi: 10.1111/apha.12129. [DOI] [PubMed] [Google Scholar]
- Khanal P, Johnsen L, Axel AM, Hansen PW, Kongsted AH, Lyckegaard NB, et al. Long-term impacts of foetal malnutrition followed by early postnatal obesity on fat distribution pattern and metabolic adaptability in adult sheep. PLoS One. 2016;11:e0156700. doi: 10.1371/journal.pone.0156700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khanal P, Nielsen MO. Maternal undernutrition and visceral adiposity. In: Rajendram R, Preedy V, Patel V, editors. Diet, Nutrition, and Fetal Programming. Humana Press; New York, NY, USA: 2017. pp. 91–105. [DOI] [Google Scholar]
- Kind KL, Simonetta G, Clifton PM, Robinson JS, Owens JA. Effect of maternal feed restriction on blood pressure in the adult guinea pig. Exp Physiol. 2002;87:469–477. doi: 10.1111/j.1469-445X.2002.tb00060.x. [DOI] [PubMed] [Google Scholar]
- Kinnunen TI, Neupane S. Prevalence of overweight among women of childbearing age in Nepal: trends from 2001 to 2011 and associations with socio-demographic factors. Matern Child Health J. 2014;18:1846–1853. doi: 10.1007/s10995-013-1428-1. [DOI] [PubMed] [Google Scholar]
- Knerr B. International labour migration and remittances in Nepal: an overview. In: Knerr B, editor. International Labor Migration and Livelihood Security in Nepal–Considering the Household Level. Kassel University Press GmbH; Kassel, Germany: 2017. p. 63. [Google Scholar]
- Koirala M, Khatri RB, Khanal V, Amatya A. Prevalence and factors associated with childhood overweight/obesity of private school children in Nepal. Obes Res Clin Pract. 2015;9:220–227. doi: 10.1016/j.orcp.2014.10.219. [DOI] [PubMed] [Google Scholar]
- Kongsted AH, Tygesen MP, Husted SV, Oliver MH, Tolver A, Christensen VG, et al. Programming of glucose-insulin homoeostasis: long-term consequences of pre-natal versus early postnatal nutrition insults. Evidence from a sheep model. Acta Physiol. 2014;210:84–98. doi: 10.1111/apha.12080. [DOI] [PubMed] [Google Scholar]
- McLaughlin T, Lamendola C, Coghlan N, Liu TC, Lerner K, Sherman A, et al. Subcutaneous adipose cell size and distribution: relationship to insulin resistance and body fat. Obesity. 2014;22:673–680. doi: 10.1002/oby.20209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillen IC, Edwards LJ, Duffield J, Muhlhausler BS. Regulation of leptin synthesis and secretion before birth: implications for the early programming of adult obesity. Reproduction. 2006;131:415–427. doi: 10.1530/rep.1.00303. [DOI] [PubMed] [Google Scholar]
- Misra A, Bhardwaj S. Obesity and the metabolic syndrome in developing countries: focus on South Asians. In: Black RE, Singhal A, Uauy R, editors. International Nutrition: Achieving Millennium Goals and Beyond. Vol. 78. Karger Publishers; Basel, Switzerland: 2014. pp. 133–140. [DOI] [PubMed] [Google Scholar]
- Misra A, Khurana L. Obesity and the metabolic syndrome in developing countries. J Clin Endocrinol Metab. 2008;93:S9–S30. doi: 10.1210/jc.2008-1595. [DOI] [PubMed] [Google Scholar]
- Montagu D, Yamey G, Visconti A, Harding A, Yoong J. Where do poor women in developing countries give birth? A multi-country analysis of Demographic and Health Survey Data. PLoS One. 2011;6:e17155. doi: 10.1371/journal.pone.0017155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nepal G, Tuladhar ET, Dahal S, Ahamad ST, Adhikari S, Kandel A. Lifestyle practices and obesity in Nepalese youth: a cross-sectional study. Cureus. 2018;10:e2209. doi: 10.7759/cureus.2209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng M, Fleming T, Robinson M, Thomson B, Graetz N, Margono C, et al. Global, regional, and national prevalence of overweight and obesity in children and adults during 1980–2013 a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2014;384:766–781. doi: 10.1016/S0140-6736(14)60460-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen MO, Hou L, Johnsen L, Khanal P, Bechshøft CL, Kongsted AH, et al. Do very small adipocytes in subcutaneous adipose tissue (a proposed risk factor for insulin insensitivity) have a fetal origin? Clin Nutr Exp. 2016;8:9–24. doi: 10.1016/j.yclnex.2016.05.003. [DOI] [Google Scholar]
- Nielsen MO, Kongsted AH, Thygesen MP, Strathe AB, Caddy S, Quistorff B, et al. Late gestation undernutrition can predispose for visceral adiposity by altering fat distribution patterns and increasing the preference for a high-fat diet in early postnatal life. Br J Nutr. 2013;109:2098–2110. doi: 10.1017/S0007114512004199. [DOI] [PubMed] [Google Scholar]
- Painter RC, Roseboom TJ, Bleker OP. Prenatal exposure to the Dutch famine and disease in later life: an overview. Reprod Toxicol. 2005;20:345–352. doi: 10.1016/j.reprotox.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Phillips DI, Bennett FI, Wilks R, Thame M, Boyne M, Osmond C, et al. Maternal body composition, offspring blood pressure and the hypothalamic-pituitary-adrenal axis. Paediatr Perinat Epidemiol. 2005;9:294–302. doi: 10.1111/j.1365-3016.2005.00661.x. [DOI] [PubMed] [Google Scholar]
- Piryani S, Baral KP, Pradhan B, Poudyal AK, Piryani RM. Overweight and its associated risk factors among urban school adolescents in Nepal: a cross-sectional study. BMJ Open. 2016;6:e010335. doi: 10.1136/bmjopen-2015-010335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popkin BM. The nutrition transition and obesity in the developing world. J Nutr. 2001;131:871S–873S. doi: 10.1093/jn/131.3.871S. [DOI] [PubMed] [Google Scholar]
- Portella AK, Kajantie E, Hovi P, Desai M, Ross MG, Goldani MZ, et al. Effects of in utero conditions on adult feeding preferences. J Dev Orig Health Dis. 2012;3:140–152. doi: 10.1017/S2040174412000062. [DOI] [PubMed] [Google Scholar]
- Prentice AM. The emerging epidemic of obesity in developing countries. Int J Epidemiol. 2006;35:93–99. doi: 10.1093/ije/dyi272. [DOI] [PubMed] [Google Scholar]
- Ravelli AC, van der Meulen JH, Michels RP, Osmond C, Barker DJ, Hales CN, et al. Glucose tolerance in adults after prenatal exposure to famine. Lancet. 1998;351:173–177. doi: 10.1016/S0140-6736(97)07244-9. [DOI] [PubMed] [Google Scholar]
- Ravelli AC, van Der Meulen JH, Osmond C, Barker DJ, Bleker OP. Obesity at the age of 50 y in men and women exposed to famine prenatally. Am J Clin Nutr. 1999;70:811–816. doi: 10.1093/ajcn/70.5.811. [DOI] [PubMed] [Google Scholar]
- Roseboom TJ, van der Meulen JH, Osmond C, Barker DJ, Ravelli AC, Bleker OP. Plasma lipid profiles in adults after prenatal exposure to the Dutch famine. Am J Clin Nutr. 2000;72:1101–1106. doi: 10.1093/ajcn/72.5.1101. [DOI] [PubMed] [Google Scholar]
- Sadana R, D’Souza C, Hyder AA, Chowdhury AM. Importance of health research in South Asia. BMJ. 2004;328:826–830. doi: 10.1136/bmj.328.7443.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma SK, Ghimire A, Radhakrishnan J, Thapa L, Shrestha NR, Paudel N, et al. Prevalence of hypertension, obesity, diabetes, and metabolic syndrome in Nepal. Int J Hypertens. 2011;2011 doi: 10.4061/2011/821971. 821971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simkhada P, Poobalan A, Simkhada PP, Amalraj R, Aucott L. Knowledge, attitude, and prevalence of overweight and obesity among civil servants in Nepal. Asia Pac J Public Health. 2011;23:507–517. doi: 10.1177/1010539509348662. [DOI] [PubMed] [Google Scholar]
- Simkhada PP, Baral YR, van Teijlingen ER. Health and medical research in Nepal: a bibliometric review. Asia Pac J Public Health. 2010;22:492–500. doi: 10.1177/1010539510371020. [DOI] [PubMed] [Google Scholar]
- Singh U, Ueranantasun A, Kuning M. Factors associated with low birth weight in Nepal using multiple imputation. BMC Pregnancy Childbirth. 2017;17:67. doi: 10.1186/s12884-017-1252-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sniderman AD, Bhopal R, Prabhakaran D, Sarrafzadegan N, Tchernof A. Why might South Asians be so susceptible to central obesity and its atherogenic consequences? The adipose tissue overflow hypothesis. Int J Epidemiol. 2007;36:220–225. doi: 10.1093/ije/dyl245. [DOI] [PubMed] [Google Scholar]
- Subedi BP. Urbanization in Nepal: spatial pattern, social demography and development. Central Bureau of Statistics; Kathmandu, Nepal: 2014. pp. 95–154. [Google Scholar]
- Taylor PD, Poston L. Developmental programming of obesity in mammals. Exp Physiol. 2007;92:287–298. doi: 10.1113/expphysiol.2005.032854. [DOI] [PubMed] [Google Scholar]
- Tran TT, Yamamoto Y, Gesta S, Kahn CR. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab. 2008;7:410–420. doi: 10.1016/j.cmet.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya A, Shakya S, Krettek A. Obesity prevalence in Nepal: public health challenges in a low-income nation during an alarming worldwide trend. Int J Environ Res Public Health. 2010;7:2726–2744. doi: 10.3390/ijerph7062726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers MH, Breier BH, Cutfield WS, Hofman PL, Gluckman PD. Fetal origins of hyperphagia, obesity, and hypertension and postnatal amplification by hypercaloric nutrition. Am J Physiol Endocrinol Metab. 2000;279:E83–E87. doi: 10.1152/ajpendo.2000.279.1.E83. [DOI] [PubMed] [Google Scholar]
- WHO. Global nutrition targets 2025: low birth weight policy brief. World Health Organization; Geneva, Switzerland: 2014. pp. 1–8. [Google Scholar]
- WHO. Obesity: preventing and managing–the global epidemic Report of a WHO Consultation (WHO technical report series 894) World Health Organization; Geneva, Switzerland: 2000. p. 6. [PubMed] [Google Scholar]
- Witter S, Khadka S, Nath H, Tiwari S. The national free delivery policy in Nepal: early evidence of its effects on health facilities. Health Policy Plan. 2011;26:ii84–ii91. doi: 10.1093/heapol/czr066. [DOI] [PubMed] [Google Scholar]
- Wronska A, Kmiec Z. Structural and biochemical characteristics of various white adipose tissue depots. Acta Physiol. 2012;205:194–208. doi: 10.1111/j.1748-1716.2012.02409.x. [DOI] [PubMed] [Google Scholar]