Reproduction in the bacterial kingdom predominantly occurs through binary fission—a process in which one parental cell is divided into two similarly sized daughter cells. How cell division, in conjunction with cell elongation and chromosome segregation, is orchestrated by a multitude of proteins has been an active area of research spanning the past few decades.
KEYWORDS: DivIVA, EzrA, FtsZ, GpsB, PBP, ParA/ParB, Ser/Thr kinase, cell division, cell shape, cell wall
ABSTRACT
Reproduction in the bacterial kingdom predominantly occurs through binary fission—a process in which one parental cell is divided into two similarly sized daughter cells. How cell division, in conjunction with cell elongation and chromosome segregation, is orchestrated by a multitude of proteins has been an active area of research spanning the past few decades. Together, the monumental endeavors of multiple laboratories have identified several cell division and cell shape regulators as well as their underlying regulatory mechanisms in rod-shaped Escherichia coli and Bacillus subtilis, which serve as model organisms for Gram-negative and Gram-positive bacteria, respectively. Yet our understanding of bacterial cell division and morphology regulation is far from complete, especially in noncanonical and non-rod-shaped organisms. In this review, we focus on two proteins that are highly conserved in Gram-positive organisms, DivIVA and its homolog GpsB, and attempt to summarize the recent advances in this area of research and discuss their various roles in cell division, cell growth, and chromosome segregation in addition to their interactome and posttranslational regulation.
INTRODUCTION
Cell division in bacteria usually gives rise to two similarly sized progenies, although exceptions to this rule exist (1–3). On the basis of the presence of nutrients and/or stress signals, a multiprotein complex, called the divisome, orchestrates the division process (4). Division in bacterial cells is precisely timed by regulators tied to cell size and the status of DNA replication and segregation (5). The identity and nature of many of the factors involved in regulating cell size, shape, and division have been discovered in the Gram-negative organisms Escherichia coli and Caulobacter crescentus and in the Gram-positive organism Bacillus subtilis (4, 5). However, there are still gaps in our knowledge of these model organisms (6), and a remarkable increase in studies of nontraditional model organisms of various cell shapes and division modes has recently highlighted the presence of diverse regulators and a multitude of underpinning regulatory mechanisms (3, 4). In this review, we focus on two highly conserved proteins in Gram-positive organisms—DivIVA and a DivIVA-like-domain-containing protein, GpsB. DivIVA is a coiled-coil protein broadly conserved in Gram-positive Firmicutes and Actinobacteria and also, to a limited extent, in the phyla Cyanobacteria (7), Tenericutes (8), Deltaproteobacteria (9, 10), and Deinococcus (11, 12). Unlike DivIVA, GpsB is present only among Firmicutes and was likely acquired via divIVA gene duplication (13–15). We aim to shine light on the diverse roles performed by DivIVA and GpsB in B. subtilis, in which they were first characterized, and summarize their roles, known interaction partners, and posttranslational regulation in other organisms (Fig. 1). A list of interaction partners (including negative results) and of the methods used to test the interactions is provided in Table S1 in the supplemental material.
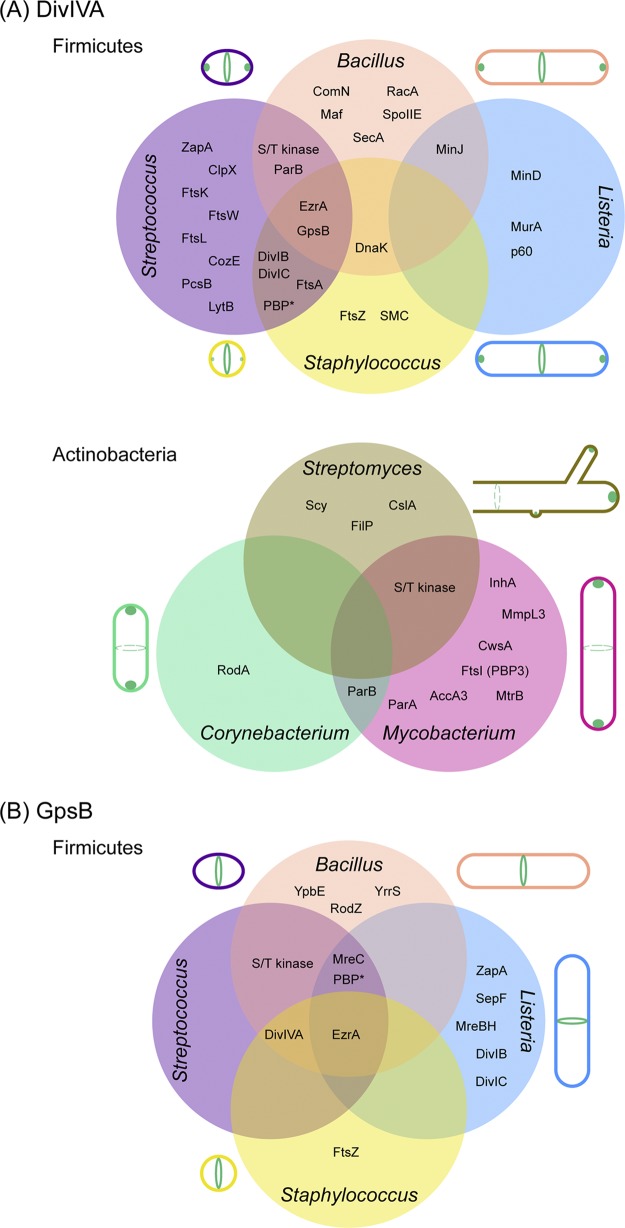
FIG 1.
Localization of DivIVA and GpsB and the overlapping network of their interaction partners in Gram-positive bacteria. Venn diagrams show the interaction partners of DivIVA (A) and GpsB (B) discussed in this article. (A) (Top panel) Clockwise from the top right, localization patterns of DivIVA in actively dividing cells of B. subtilis, L. monocytogenes, S. aureus, and S. pneumoniae are shown. (Bottom panel) Clockwise from the top, localization patterns of DivIVA in actively growing cells of Streptomyces, Mycobacterium, and Corynebacterium species are depicted. Dashed circles indicate alternate sites of localization. The Streptomyces cell is depicted in an open-ended manner to indicate the mycelial growth pattern. (B) Clockwise from the top right, localization patterns of GpsB in actively dividing cells of B. subtilis, L. monocytogenes, S. aureus, and S. pneumoniae are presented. The subcellular locations of DivIVA and GpsB are illustrated in green. A comprehensive list of DivIVA and GpsB partners and methods used to test interactions, including negative results, is provided in Table S1. *, the specific names of penicillin-binding proteins are shown in Table S1.
Discovery of DivIVA.
Studies of cell division mutants in B. subtilis isolated in the early 1970s (div IV-A1 and div IV-B1 [16–18]) led to the eventual mapping and discovery of the divIVA gene more than 2 decades later (19, 20). The name “DivIVA” was chosen on the basis of the recommendation that division mutants that exhibited abnormal off-center septum placement be named Div IV mutants (21). The mutation in another Div IV mutant, div IV-B1, which mapped to the minCD cluster (adjoining mreBCD genes encode cell shape-determining proteins), specifically disrupted the minD gene (22–24). MinCD, together with the help of the spatial determinant DivIVA (and of MinJ), regulate the assembly of the FtsZ ring (Z-ring), a central component of the cell division machinery required for cell division (25–27).
DIVERSE ROLES OF DivIVA IN FIRMICUTES
Bacillus subtilis.
DivIVA is capable of self-association and forms large multimeric structures (28, 29). Subsequent structural analysis showed that DivIVA is a tetramer (30). Genetic and crystallographic studies have pinpointed a key phenylalanine residue (F17) as critical for DivIVA-membrane association (30). Several other mutations that disrupt DivIVA localization and/or function have been described in many published studies; we have provided the summary of reported DivIVA (and GpsB) mutations in B. subtilis and other organisms in Table S2 in the supplemental material. In B. subtilis, DivIVA is capable of localization by sensing negative (concave) membrane curvature within the cell, presumably independently of the action of FtsZ at cell poles (31, 32), and at sites where the division septum intersects the lateral cell surface (33, 34). In fact, an earlier report indicated that DivIVA targets the sites of cytokinesis even when artificially expressed in unrelated organisms—E. coli and fission yeast Schizosaccharomyces pombe (35). It is possible that DivIVA is able to localize to nascent division sites by recognizing negative curvature elicited by the constriction of Z-rings (27, 31). Superresolution microscopy revealed that DivIVA localizes and forms two rings at midcell, likely sandwiching a constricting Z-ring (27). DivIVA localization appears to be dynamic in actively dividing cells, and transfer of molecules between the old and the nascent division sites has been observed (36). DivIVA is localized at division sites during vegetative growth and prevents aberrant assembly of Z-rings immediately adjacent to newly formed septa (27, 37–39). Furthermore, localization of DivIVA, a peripheral membrane protein, appears to depend on the presence of a translocase subunit, SecA, of the protein secretion apparatus (40).
DivIVA is a versatile protein which performs multiple roles by interacting with several partners at various points during the cell cycle and stress response (41) (Table S1A). At the onset of endospore formation (sporulation), DivIVA prelocalizes to the cell poles and interacts with RacA and the Soj/Spo0J (ParA/ParB) system to anchor the origins of the segregating chromosomes to the cell poles (42–49). DivIVA facilitates the asymmetric positioning of the septum at the onset of sporulation, where it localizes and sequesters SpoIIE, a regulatory phosphatase (50), to allow the subsequent compartment-specific differential gene expression (51). When B. subtilis cells enter a competent state, DivIVA sequesters the posttranscriptional regulator ComN (52, 53) and interacts with Maf to stall cell division (54). Additional interactions between ComN-MinJ and ComN-MinD (45) highlight the influence of the competence pathway on cell division in B. subtilis. Cells lacking divIVA exhibit impaired swarming motility, most likely due to inefficient cell division (37).
Other than the interactions described above, DivIVA was previously shown in bacterial two-hybrid screens to interact with PrkC, a division septum-localized S/T kinase (55–57), EzrA, a cell division protein (58), and GpsB (56). Although S109 of DivIVA is capable of being phosphorylated (59), based on in vitro experiments, PrkC may not be the kinase responsible (56). An arginine kinase, McsB, may phosphorylate R102 of DivIVA (60). Investigations involving phosphomimetic (R102E) and phosphoablative (R102K) variants of DivIVA suggested that the arginine in this position, irrespective of the type of mutation, is important for the role played by DivIVA in sporulation (possibly for RacA binding) but not for vegetative cell division (61). Thus, the physiological significance of DivIVA phosphorylation by an S/T kinase or McsB remains to be elucidated. A tyrosine kinase, PtkA, is able to interact with MinD, localize to cell poles in a MinD-dependent manner, and phosphorylate DivIVA (62). Another tyrosine kinase involved in the sporulation process, YabT, is able to phosphorylate RacA, while SpoIIE is able to dephosphorylate it (62)—the last two, as described earlier, are known interaction partners of DivIVA. Additionally, the DnaK chaperone, another substrate of PtkA (63), appears to protect B. subtilis DivIVA from degradation based on protein turnover analysis (64).
Streptococcus spp.
On the basis of phylogenetic analysis, coccoid cell morphology is the result of mutations in rod-shaped bacteria (65). For instance, non-rod-shaped Firmicutes typically lack the Min system; however, they have retained the B. subtilis Min system regulatory protein DivIVA (66), as is the case in the ovococcoid Streptococcus pneumoniae. Note that Streptococcus spp. lack the cell elongation factor MreB (66–68). The deletion phenotypes of genes contained within the division and cell wall (dcw) cluster region suggest that DivIVA is important for cell division, morphology, and nucleoid segregation in S. pneumoniae (69).
DivIVA localizes to the midcell and cell poles in S. pneumoniae, resembling the pattern seen in B. subtilis (70, 71). As in other organisms, pneumococcal DivIVA also directly interacts with a cohort of cell division proteins (70, 72) (Fig. 1; see also Table S1A), including GpsB and EzrA, to help regulate cell elongation and cell separation (70, 73). In Streptococcus suis, cells lacking divIVA divide along multiple planes, implying a role for DivIVA in division plane selection in this organism (74). Regarding DivIVA turnover, clues from a study in Streptococcus mutans strongly suggest the involvement of ClpXP protease machinery (75); however, how DivIVA is recognized as a substrate remains to be determined.
Chromosome segregation also plays a role in the determination of the cell division site in S. pneumoniae (76), and a prior report indicated that S. pneumoniae DivIVA interacts with Spo0J/ParB (70). In addition to exhibiting a cell separation defect (70, 73), the divIVA null strain also produces anucleate cells (70). Given that ParB and SMC (structural maintenance of chromosomes) condensin facilitate nucleoid segregation (77), pneumococcal DivIVA may play an additional role in nucleoid segregation similar to that played by B. subtilis DivIVA (44, 45); however, this possibility remains to be further evaluated. Of note, unlike certain model organisms, S. pneumoniae lacks the ParA ATPase component of the chromosome segregation machinery (78).
Streptococcal StkP, an S/T kinase, regulates cell elongation and division in multiple species, and DivIVA is one of its prominent substrates (71, 74, 79–81). Although phosphorylation of residue T201 of pneumococcal DivIVA was shown to be important for the maintenance of cell morphology (82), it appears to be dependent on the strain type (83, 84). Another substrate of StkP, MapZ/LocZ, is key for streptococcal division site selection (85–87), as originally proposed (88, 89). Finally, in another ovococcoid organism, Enterococcus faecalis, which also lacks the Min system, DivIVA is essential and plays a role in division site selection, cell separation, and chromosome segregation (90).
Other Firmicutes.
In the rod-shaped bacterium Listeria monocytogenes, the absence of DivIVA leads to a cell separation defect (91) which mimics the chaining phenotype seen in a S. pneumoniae divIVA null strain (70, 73). Defective cell separation was attributed to the dependency of autolysins p60 and MurA on DivIVA for subsequent secretion via the SecA2 pathway (91) (Table S1A). Furthermore, an L. monocytogenes strain lacking divIVA is unable to swarm (a process which is independent of MinJ, unlike the case in B. subtilis [37]) or build biofilms and exhibits attenuated virulence (91, 92). DivIVA also appears to play a role in cell division in L. monocytogenes by directly interacting with MinD, thereby circumventing the need for MinJ (93); the direct MinD-DivIVA interaction has also been documented in Clostridium spp. (94).
In the spherical bacterium Staphylococcus aureus (which also lacks min genes, similarly to other non-rod-shaped bacteria), the absence of DivIVA does not lead to significant changes in cell morphology or produce cell division defects (95), even though S. aureus DivIVA localizes to division sites (64, 95). A role for DivIVA in chromosome segregation, possibly through a direct interaction with SMC condensin, was observed previously (64). Bacterial two-hybrid and protein abundance analyses revealed that DivIVA interacts with and is stabilized by DnaK (64). Additional DivIVA-interacting proteins include the cell division proteins FtsZ, FtsA, EzrA, DivIC, penicillin-binding protein 1 (PBP1), and GpsB (64) (Fig. 1; see also Table S1A).
MULTIPLE FUNCTIONS OF DivIVA IN ACTINOBACTERIA
Streptomyces coelicolor.
In Streptomyces coelicolor, a filamentous bacterium that undergoes polar growth, DivIVA is an essential protein and localizes to the tips of the hyphae and sites of branching and plays a role in promoting peptidoglycan synthesis at cell poles (96, 97). Time-lapse microscopy showed that hyphal-tip-localized DivIVA could detach to mark future branching sites (98). The site of the germ tube (vegetative cell) emerging from germinating spores is also marked by DivIVA (96). As observed in some Firmicutes, S. coelicolor DivIVA is also phosphorylated by at least one of the 34 S/T kinases encoded by this organism, AfsK, and the colocalization of DivIVA and AfsK has been reported previously (99). DivIVA of S. coelicolor interacts with other polar-localized intermediate filament-like proteins, Scy and FilP (100, 101) (see Table S1B in the supplemental material). In Streptomyces venezuelae, FilP displays enhanced localization at regions proximal to the tips of growing hyphae (which is similar to S. coelicolor [101]), and lack of filP results in an irregular growth pattern that is likely a consequence of the presence of differing levels of DivIVA accumulation at poles (102), suggesting a crucial role for FilP and DivIVA in hyphal growth. Consistent with the chromosome tethering/segregation functions described in Firmicutes, DivIVA appears to be involved in anchoring the origin of the linear S. coelicolor chromosomes via ParA indirectly (103, 104). An interaction between S. coelicolor DivIVA and ParB proteins was observed in E. coli and by the use of multiple in vitro experiments (105); however, this interaction was not confirmed in its natural host (103).
Corynebacterium glutamicum.
DivIVA is essential in Corynebacterium glutamicum (previously referred to as Brevibacterium lactofermentum), and its overproduction leads to polar bulging and other morphological defects (106). DivIVA localizes to the cell poles and division sites subsequent to septation in this bacterium (106, 107). To support robust polar growth, DivIVA recruits RodA, a lipid II flippase, to the cell poles (108, 109) (Table S1B). Thus, in Corynebacterium, which does not encode actin-like cytoskeletal proteins (110, 111), DivIVA appears to play a major role in cell shape regulation. As in other organisms, chromosome segregation and cell division are intertwined in C. glutamicum (112, 113). In this organism, the origins of the replicated chromosomes are tethered to cell poles via the ParAB machinery (114). While absence of ParA, PldP (a ParA-like protein), or ParB results in the production of minicells lacking nucleoids, overexpression of ParA or PldP leads to increased cell length (114). In fact, a direct interaction between FtsZ and ParB was noted through bacterial two-hybrid assay (114). At least when artificially expressed in E. coli, DivIVA and ParB of C. glutamicum interact with each other (105). However, the physiological function of DivIVA in chromosome segregation in this organism remains to be elucidated.
Although there is no evidence for the phosphorylation of DivIVA in this organism (109, 111, 115), there are reports that indicate a role for S/T kinases in cell division and growth (116–118). Interestingly, a coiled-coil domain containing cell shape morphogenetic protein RsmP (which is overexpressed upon DivIVA depletion [115]) is phosphorylated by at least one of the 4 S/T kinases encoded by C. glutamicum (117), namely, PknA, and the phosphorylation leads to its accumulation at cell poles (115). How RsmP influences peptidoglycan synthesis at poles and whether RsmP works together with DivIVA for this purpose need to be evaluated.
Mycobacterium spp.
DivIVA, also known as Wag31, was initially characterized as the highly immunogenic antigen Ag84 in Mycobacterium tuberculosis and Mycobacterium leprae strains (119, 120). In Mycobacterium species (which, unlike Firmicutes, undergo apical growth [121]), DivIVA is essential (122–124) and localizes to the septum, cell poles, and branching sites (124–127). Overproduction of DivIVA leads to polar bulging and branching in Mycobacterium smegmatis (125), a fast-growing cousin of M. tuberculosis, where DivIVA dictates the site of peptidoglycan insertion (128, 129). An indirect link between DivIVA and Z-ring assembly was revealed by the elucidation of direct interactions between DivIVA and a cell wall synthesis protein (CwsA) and FtsZ-interacting FtsI/PBP3 (130–133) (Table S1B). Localization of DivIVA depends on Ami1 amidase, as there was a higher rate of unusual lateral budding in an Ami1 amidase mutant due to DivIVA mislocalization to the sites of lateral branching (134), which resembles the localization pattern of DivIVA in Streptomyces. In addition to assisting in cell wall synthesis, DivIVA also interacts with proteins involved in the biosynthesis of mycolic acids (128, 135, 136). Mycolic acids are an integral part of the mycomembrane and play a crucial role in pathogenesis (137).
A role for S/T kinases and cognate phosphatases in regulating cell shape and division in M. tuberculosis has been observed previously (138, 139). Of the 11 S/T kinases encoded by this organism (140), at least one of them, PknA, phosphorylates DivIVA at T73 (138, 141). It was also elucidated that a phosphomimetic variant of DivIVA displays an increased rate of peptidoglycan synthesis compared to the phosphoablative mutant (126). Furthermore, the phosphorylation status of DivIVA is also regulated by the essential MtrAB two-component system and FtsI (133). Similarly to DivIVA in other organisms, M. smegmatis DivIVA also interacts with the chromosome segregation machinery—in this case, with the ParA ATPase component (142) and possibly with ParB as well (105). In M. smegmatis, which lacks the Min system, a paralog of DivIVA, SepIVA, is involved in cell division regulation (143). Apart from the functions described above, there are additional roles for DivIVA in oxidative stress response (130) and bacteriophage infection (144).
PROCESSES INFLUENCED BY GpsB
Bacillus subtilis.
In 2008, two independent groups explored the possible roles of B. subtilis GpsB (formerly YpsB), a DivIVA-like protein conserved in Firmicutes (14, 15). A synthetic-lethal screen to identify genes that become essential in cells that lack a nonessential cell division/elongation factor, EzrA, led to the discovery of GpsB, and its role in guiding PBP1 (a bifunctional penicillin-binding protein) shuttling from cell poles to division sites to regulate cell wall synthesis was uncovered (15). Bacterial two-hybrid analysis revealed the interactions between GpsB and MreC, a known cell shape regulator (145), EzrA, and PBP1 (15) (see Table S1C in the supplemental material). B. subtilis GpsB was also independently investigated by another group, and their data showed that GpsB localizes to division sites and becomes synthetically essential in the absence of FtsA, an FtsZ-anchoring protein (14). Although GpsB becomes important in the absence of EzrA or FtsA, both of which directly interact with FtsZ, it is believed that the predominant role of GpsB is in regulating peptidoglycan synthesis (58).
B. subtilis GpsB is a hexamer (146), and further structural analysis of GpsB and its binding partner PBP1 uncovered the molecular basis of their interaction (147). On the basis of a signature sequence motif, GpsB was discovered to interact with two lesser-known nonessential membrane proteins, YpbE and YrrS, with links to peptidoglycan binding and/or synthesis (147). Similarly to what was observed for DivIVA (51), GpsB also localizes to asymmetric division sites during sporulation, at least when produced under the control of an inducible system (14). The precise function of GpsB during sporulation, which may be mediated by YrrS activity, remains to be investigated (147). However, sporulation progresses in an unaffected manner in the absence of GpsB (14).
Similarly to its homolog, DivIVA, links have been established between the S/T kinase PrkC and GpsB. Phosphorylation of GpsB by PrkC apparently occurs at residue T75, and the unphosphorylated form of GpsB enhances the autophosphorylation activity of PrkC and its kinase activity toward its other substrates (56). Additionally, the same study showed that GpsB, regardless of the status of phosphorylation, interacts with PrkC, EzrA, and DivIVA (Table S1C). Thus, it appears that GpsB, via PrkC, is the main driver connecting cell division and other processes (57) and that PrpC, the cognate phosphatase of PrkC, could reset this process through dephosphorylating GpsB during different stages of the cell cycle (56).
Listeria monocytogenes.
The Halbedel group was the first to report that GpsB is dispensable in L. monocytogenes under conditions of growth at 30°C and 37°C (146). However, at 37°C, cell lysis and increases in cell width and length were observed in cells lacking gpsB. At 42°C, GpsB was deemed to be essential for growth (146). Roles for GpsB in virulence (146) and in lysozyme resistance for immune evasion (148) have also been noted. Deletion of both gpsB and divIVA leads to cell elongation and decreased cell width (146). In fact, similarly to the GpsB counterparts in B. subtilis and S. pneumoniae, listerial GpsB also binds a class A bifunctional PBP (PBP A1) and, based on genetic analysis, GpsB appears to negatively regulate the activity of PBP A1 (146, 147). Isolation of suppressor mutants that were able to overcome the growth defect at 42°C appears to have confirmed the GpsB-mediated regulation of PBP A1 (149). Full-length GpsB of L. monocytogenes functions as a hexamer (146), and this hexameric structure could allow GpsB to bind multiple PBP1-like molecules (150). Apart from the well-studied interaction with PBP A1, listerial GpsB is also capable of binding multiple partners, including the known FtsZ-tethering proteins ZapA, SepF, and EzrA; cell shape regulators MreC and MreBH; late-arriving divisomal proteins DivIB and DivIC; and several penicillin-binding proteins—PBP A2, PBP B1, PBP B2, and PBP B3 (147) (Table S1C). As seen with the other organisms described here, the S/T kinase PrkA is important for maintaining cell shape and virulence in L. monocytogenes (151). Whether GpsB interacts with or is phosphorylated by PrkA remains to be determined.
Streptococcus pneumoniae.
GpsB is essential in S. pneumoniae, but accumulation of suppressor mutations that allow GpsB to become nonessential in some strain backgrounds has been clearly delineated (73, 84). Depletion of GpsB results in cell elongation and formation of constriction-deficient Z-rings (152). In a different strain background, deletion of gpsB resulted in cell elongation because of improper helical localization of division proteins and concomitant peptidoglycan insertion (73). This phenotype appears to be corrected in cells in which divIVA is also absent, highlighting that coordination of cell wall synthesis in pneumococcus requires both DivIVA and GpsB (73). However, the corrective effect was not reproducible in other strain types (84).
The interaction between GpsB and PBP2a has been established by genetic, biochemical, and structural methods, and the residues involved in the interaction between GpsB and PBP2a are similar to those of B. subtilis GpsB and PBP1 (84, 147). In addition, GpsB was found to be in a complex with EzrA, MreC, StkP, and multiple penicillin-binding proteins: PBP1a, PBP2b, and PBP2x (84, 147) (Table S1C). The interaction between GpsB and PBP2b observed via coimmunoprecipitation analysis may not be direct, as the interaction was not seen in bacterial two-hybrid or fluorescence polarization assays (147). Together, multiple lines of evidence indicate that GpsB plays a vital role in linking peptidoglycan synthesis at division sites for septation and cell periphery for cell elongation (84).
The S/T kinase StkP plays a critical role in cell wall synthesis and virulence in multiple Streptococcus species (153–155). Known StkP substrates include GpsB, DivIVA, FtsA, EzrA, MapZ, and FtsZ (153, 155). Bacterial two-hybrid and surface plasmon resonance experiments revealed that pneumococcal GpsB interacts with both EzrA and DivIVA and that GpsB is required for proper localization and autophosphorylation of StkP (73). In a different pneumococcal strain, however, the interaction between GpsB and DivIVA (based on coimmunoprecipitation assay) and GpsB-mediated localization of StkP were not evident (84). StkP autophosphorylation and phosphorylation of its substrates, on the other hand, are dependent on GpsB in multiple strain backgrounds (84).
Staphylococcus aureus.
A genome-wide transposon mutagenesis experiment indicated that transposon insertion was not tolerated at certain parts of gpsB (corresponding to the region coding for DivIVA-like N-terminal domain); therefore, it was determined that GpsB is a domain-essential protein in S. aureus (156). Bacterial two-hybrid analysis led to the finding that S. aureus GpsB self-interacts and also interacts with EzrA (157), similarly to the GpsB of other organisms discussed here (Table S1C). Our group showed that overproduction of GpsB results in cell enlargement in S. aureus (158), indicative of cell division inhibition and improper incorporation of peptidoglycan along the cell periphery (159). Using superresolution microscopy, we observed that GpsB preferentially localized to the leading edge of the invaginating membrane during septation (158). It appears that the localization of FtsZ and GpsB are codependent in S. aureus. Furthermore, using multiple in vitro analyses, we noted that GpsB transiently bundles FtsZ polymers and subsequently enhances the GTPase activity of FtsZ to promote its depolymerization. We speculate that this function of GpsB may be important for kick-starting FtsZ treadmilling—a process in which the concerted action of Z-ring constriction and peptidoglycan synthesis allows the creation of division septum (160–162). This role of GpsB may be broadly conserved in other bacteria, albeit with the help of FtsZ-tethering proteins such as EzrA (163). Given that teichoic acid (TA) synthesis impairment significantly affects division site positioning in staphylococcal cell division (164) and that the EzrA interacts with many of the key TA synthesis machinery components (165), it is likely that EzrA, together with its interaction partners, acts as a transducer of signals between multiple processes such as cell wall/TA synthesis and cell division (157, 166).
In S. aureus, the S/T kinase, Stk (also known as PknB and Stk1), is targeted to the division septum (167) and, together with its cognate phosphatase, Stp, plays a role in the regulation of peptidoglycan and capsule biosynthesis (168–170). Although a direct interaction between Stk and GpsB is absent, a prominent interaction between EzrA and Stk (and also between EzrA and other binding partners of Stk) has been noted (169). Nevertheless, GpsB of S. aureus is phosphorylated at multiple S and T residues as well as at least one R residue, including the following: T21, T75, S76, R77, S85, T93, and S105 (171). It is therefore conceivable that the phosphorylation status of GpsB in S. aureus may be used to regulate and hook Z-ring constriction and the peptidoglycan synthesis machinery either directly or indirectly via EzrA-like protein partners.
CONCLUDING THOUGHTS
Studies over the past 2 decades have uncovered multiple critical roles played by DivIVA and GpsB in Gram-positive bacteria. Although their function in peptidoglycan synthesis regulation is well documented, much remains to be elucidated in regard to other processes.
(i) Chromosome segregation.
Although the link between DivIVA and DNA segregation is relatively well understood in B. subtilis (45), how DivIVA affects DNA segregation in other organisms needs further clarification. Apart from the organisms that belong to the Firmicutes and Actinobacteria phyla, interactions between DivIVA and chromosome segregation machinery components have also been in noted in Deinococcus radiodurans (172). Identification of new factors that link cell division and chromosome segregation may shed more light on the interplay between these two processes (173).
(ii) Protein secretion.
It would be interesting to see if the link between DivIVA and SecA-mediated protein secretion that is seen in B. subtilis and L. monocytogenes (40, 91) is also conserved in other species.
(iii) Regulation of FtsZ.
Although the role of GpsB in regulating peptidoglycan incorporation became increasingly clear in recent years, whether the function of GpsB in FtsZ regulation that was identified previously in S. aureus (158) is also conserved in other organisms remains to be investigated. Given the conserved nature of the binding partners of GpsB, it appears that this function may operate in an indirect manner via EzrA (FtsA, SepF, and/or ZapA). It is also possible that GpsB (together with other proteins) acts as an anchor to link the Z-ring and septal cell wall biosynthesis machinery for FtsZ treadmilling during septation.
(iv) Posttranslational regulation.
While the emerging trend suggests that DivIVA and GpsB utilize S/T kinase-mediated phosphorylation, or vice versa, to direct cell wall synthesis at appropriate sites in multiple organisms, more biochemical and structural studies are needed to fully appreciate their precise mechanisms of action and modes of regulation. In B. subtilis, several lines of evidence point to a role for PrkC phosphorylation-mediated regulation of lipoteichoic acid synthesis, carbon metabolism, stress response, and cell wall synthesis (174–178). Given that GpsB regulates the phosphorylation status of PrkC in B. subtilis and StkP in S. pneumoniae (56, 57, 73), it is possible that GpsB performs a larger role in linking multiple processes by modulating the S/T kinase activity. Indeed, this may be the case in S. pneumoniae, as a mutation that disrupts the phpP phosphatase was shown previously to render gpsB nonessential (84). The significance of arginine phosphorylation in DivIVA/GpsB (60, 171) and the role of tyrosine kinases in chromosome segregation and cell division (62, 179) warrant further investigation.
(v) Future directions.
Further research to reveal and validate the complete set of interaction partners of DivIVA and GpsB during various stages of cell cycle and under different types of stress conditions would provide us a better understanding of the versatility of these two proteins. Solving the multicomplex structure(s) of GpsB, DivIVA, EzrA, FtsA, FtsZ, S/T kinase, PBP(s), and other protein partners involved in division/growth would give us the critically needed molecular details of the protein-protein interactions. Similar structures were recently reported for lipopolysaccharide export in E. coli by the use of advanced structural biology techniques (180, 181).
Given the ominous rise in antibiotic resistance, particularly among Gram-positive pathogens (182, 183), more in-depth understanding of the regulation of cell division and cell wall synthesis is urgently needed to reveal novel drug targets. Thus, in addition to enhancing our fundamental comprehension of basic bacterial processes, knowledge of the key players involved in cell growth and division would be immensely beneficial for the targeted development of new lines of antibiotics and antibiotic adjuvants.
Supplementary Material
ACKNOWLEDGMENTS
We thank our laboratory members for their comments on this article.
This work was funded by a start-up grant from the University of South Florida and National Institute of General Medical Sciences (NIH/NIGMS) grant R35GM133617 (to P.J.E.).
ADDENDUM
While the manuscript was in preparation, a complementary review article discussing the structural features of DivIVA and GpsB and the nature of their interaction with multiple partners was published (184).
Footnotes
Supplemental material for this article may be found at https://doi.org/10.1128/JB.00245-19.
REFERENCES
- 1.Angert ER. 2005. Alternatives to binary fission in bacteria. Nat Rev Microbiol 3:214–224. doi: 10.1038/nrmicro1096. [DOI] [PubMed] [Google Scholar]
- 2.Kysela DT, Brown PJ, Huang KC, Brun YV. 2013. Biological consequences and advantages of asymmetric bacterial growth. Annu Rev Microbiol 67:417–435. doi: 10.1146/annurev-micro-092412-155622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eswara PJ, Ramamurthi KS. 2017. Bacterial cell division: nonmodels poised to take the spotlight. Annu Rev Microbiol 71:393–411. doi: 10.1146/annurev-micro-102215-095657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haeusser DP, Margolin W. 2016. Splitsville: structural and functional insights into the dynamic bacterial Z ring. Nat Rev Microbiol 14:305–319. doi: 10.1038/nrmicro.2016.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Westfall CS, Levin PA. 2017. Bacterial cell size: multifactorial and multifaceted. Annu Rev Microbiol 71:499–517. doi: 10.1146/annurev-micro-090816-093803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hajduk IV, Rodrigues CD, Harry EJ. 2016. Connecting the dots of the bacterial cell cycle: coordinating chromosome replication and segregation with cell division. Semin Cell Dev Biol 53:2–9. doi: 10.1016/j.semcdb.2015.11.012. [DOI] [PubMed] [Google Scholar]
- 7.Miyagishima SY, Wolk CP, Osteryoung KW. 2005. Identification of cyanobacterial cell division genes by comparative and mutational analyses. Mol Microbiol 56:126–143. doi: 10.1111/j.1365-2958.2005.04548.x. [DOI] [PubMed] [Google Scholar]
- 8.Danchin A, Fang G. 2016. Unknown unknowns: essential genes in quest for function. Microb Biotechnol 9:530–540. doi: 10.1111/1751-7915.12384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akiyama T, Inouye S, Komano T. 2003. Novel developmental genes, fruCD, of Myxococcus xanthus: involvement of a cell division protein in multicellular development. J Bacteriol 185:3317–3324. doi: 10.1128/jb.185.11.3317-3324.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lambert C, Chang CY, Capeness MJ, Sockett RE. 2010. The first bite–profiling the predatosome in the bacterial pathogen Bdellovibrio. PLoS One 5:e8599. doi: 10.1371/journal.pone.0008599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maurya GK, Modi K, Misra HS. 2016. Divisome and segrosome components of Deinococcus radiodurans interact through cell division regulatory proteins. Microbiology 162:1321–1334. doi: 10.1099/mic.0.000330. [DOI] [PubMed] [Google Scholar]
- 12.Maurya GK, Modi K, Banerjee M, Chaudhary R, Rajpurohit YS, Misra HS. 2018. Phosphorylation of FtsZ and FtsA by a DNA damage-responsive Ser/Thr protein kinase affects their functional interactions in Deinococcus radiodurans. mSphere 3:e00325-18. doi: 10.1128/mSphere.00325-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brzozowski RS, Huber M, Burroughs AM, Graham G, Walker M, Alva SA, Aravind L, Eswara PJ. 2019. Deciphering the role of a SLOG superfamily protein YpsA in Gram-positive bacteria. Front Microbiol 10:623. doi: 10.3389/fmicb.2019.00623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tavares JR, de Souza RF, Meira GL, Gueiros-Filho FJ. 2008. Cytological characterization of YpsB, a novel component of the Bacillus subtilis divisome. J Bacteriol 190:7096–7107. doi: 10.1128/JB.00064-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Claessen D, Emmins R, Hamoen LW, Daniel RA, Errington J, Edwards DH. 2008. Control of the cell elongation-division cycle by shuttling of PBP1 protein in Bacillus subtilis. Mol Microbiol 68:1029–1046. doi: 10.1111/j.1365-2958.2008.06210.x. [DOI] [PubMed] [Google Scholar]
- 16.Van Alstyne D, Simon MI. 1971. Division mutants of Bacillus subtilis: isolation and PBS1 transduction of division-specific markers. J Bacteriol 108:1366–1379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reeve JN, Mendelson NH, Coyne SI, Hallock LL, Cole RM. 1973. Minicells of Bacillus subtilis. J Bacteriol 114:860–873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mendelson NH. 1975. Cell division suppression in the Bacillus subtilis div IC-A1 minicell-producing mutant. J Bacteriol 121:1166–1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cha JH, Stewart GC. 1997. The divIVA minicell locus of Bacillus subtilis. J Bacteriol 179:1671–1683. doi: 10.1128/jb.179.5.1671-1683.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Edwards DH, Errington J. 1997. The Bacillus subtilis DivIVA protein targets to the division septum and controls the site specificity of cell division. Mol Microbiol 24:905–915. doi: 10.1046/j.1365-2958.1997.3811764.x. [DOI] [PubMed] [Google Scholar]
- 21.Young FE, Wilson GA. 1972. Genetics of Bacillus subtilis and other gram-positive sporulating bacilli, p 77–106. In Halvorson HO, Hanson R, Campbell LL (ed), Spores V. American Society for Microbiology, Washington, DC. [Google Scholar]
- 22.Levin PA, Margolis PS, Setlow P, Losick R, Sun D. 1992. Identification of Bacillus subtilis genes for septum placement and shape determination. J Bacteriol 174:6717–6728. doi: 10.1128/jb.174.21.6717-6728.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varley AW, Stewart GC. 1992. The divIVB region of the Bacillus subtilis chromosome encodes homologs of Escherichia coli septum placement (minCD) and cell shape (mreBCD) determinants. J Bacteriol 174:6729–6742. doi: 10.1128/jb.174.21.6729-6742.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee S, Price CW. 1993. The minCD locus of Bacillus subtilis lacks the minE determinant that provides topological specificity to cell division. Mol Microbiol 7:601–610. doi: 10.1111/j.1365-2958.1993.tb01151.x. [DOI] [PubMed] [Google Scholar]
- 25.Marston AL, Errington J. 1999. Selection of the midcell division site in Bacillus subtilis through MinD-dependent polar localization and activation of MinC. Mol Microbiol 33:84–96. doi: 10.1046/j.1365-2958.1999.01450.x. [DOI] [PubMed] [Google Scholar]
- 26.Gregory JA, Becker EC, Pogliano K. 2008. Bacillus subtilis MinC destabilizes FtsZ-rings at new cell poles and contributes to the timing of cell division. Genes Dev 22:3475–3488. doi: 10.1101/gad.1732408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eswaramoorthy P, Erb ML, Gregory JA, Silverman J, Pogliano K, Pogliano J, Ramamurthi KS. 2011. Cellular architecture mediates DivIVA ultrastructure and regulates min activity in Bacillus subtilis. mBio 2:e00257-11. doi: 10.1128/mBio.00257-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stahlberg H, Kutejova E, Muchova K, Gregorini M, Lustig A, Muller SA, Olivieri V, Engel A, Wilkinson AJ, Barak I. 2004. Oligomeric structure of the Bacillus subtilis cell division protein DivIVA determined by transmission electron microscopy. Mol Microbiol 52:1281–1290. doi: 10.1111/j.1365-2958.2004.04074.x. [DOI] [PubMed] [Google Scholar]
- 29.Muchova K, Kutejova E, Scott DJ, Brannigan JA, Lewis RJ, Wilkinson AJ, Barak I. 2002. Oligomerization of the Bacillus subtilis division protein DivIVA. Microbiology 148:807–813. doi: 10.1099/00221287-148-3-807. [DOI] [PubMed] [Google Scholar]
- 30.Oliva MA, Halbedel S, Freund SM, Dutow P, Leonard TA, Veprintsev DB, Hamoen LW, Lowe J. 2010. Features critical for membrane binding revealed by DivIVA crystal structure. EMBO J 29:1988–2001. doi: 10.1038/emboj.2010.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harry EJ, Lewis PJ. 2003. Early targeting of Min proteins to the cell poles in germinated spores of Bacillus subtilis: evidence for division apparatus-independent recruitment of Min proteins to the division site. Mol Microbiol 47:37–48. doi: 10.1046/j.1365-2958.2003.03253.x. [DOI] [PubMed] [Google Scholar]
- 32.Renner LD, Eswaramoorthy P, Ramamurthi KS, Weibel DB. 2013. Studying biomolecule localization by engineering bacterial cell wall curvature. PLoS One 8:e84143. doi: 10.1371/journal.pone.0084143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lenarcic R, Halbedel S, Visser L, Shaw M, Wu LJ, Errington J, Marenduzzo D, Hamoen LW. 2009. Localisation of DivIVA by targeting to negatively curved membranes. EMBO J 28:2272–2282. doi: 10.1038/emboj.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ramamurthi KS, Losick R. 2009. Negative membrane curvature as a cue for subcellular localization of a bacterial protein. Proc Natl Acad Sci U S A 106:13541–13545. doi: 10.1073/pnas.0906851106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Edwards DH, Thomaides HB, Errington J. 2000. Promiscuous targeting of Bacillus subtilis cell division protein DivIVA to division sites in Escherichia coli and fission yeast. EMBO J 19:2719–2727. doi: 10.1093/emboj/19.11.2719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bach JN, Albrecht N, Bramkamp M. 2014. Imaging DivIVA dynamics using photo-convertible and activatable fluorophores in Bacillus subtilis. Front Microbiol 5:59. doi: 10.3389/fmicb.2014.00059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Patrick JE, Kearns DB. 2008. MinJ (YvjD) is a topological determinant of cell division in Bacillus subtilis. Mol Microbiol 70:1166–1179. doi: 10.1111/j.1365-2958.2008.06469.x. [DOI] [PubMed] [Google Scholar]
- 38.Bramkamp M, Emmins R, Weston L, Donovan C, Daniel RA, Errington J. 2008. A novel component of the division-site selection system of Bacillus subtilis and a new mode of action for the division inhibitor MinCD. Mol Microbiol 70:1556–1569. doi: 10.1111/j.1365-2958.2008.06501.x. [DOI] [PubMed] [Google Scholar]
- 39.van Baarle S, Bramkamp M. 2010. The MinCDJ system in Bacillus subtilis prevents minicell formation by promoting divisome disassembly. PLoS One 5:e9850. doi: 10.1371/journal.pone.0009850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Halbedel S, Kawai M, Breitling R, Hamoen LW. 2014. SecA is required for membrane targeting of the cell division protein DivIVA in vivo. Front Microbiol 5:58. doi: 10.3389/fmicb.2014.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kaval KG, Halbedel S. 2012. Architecturally the same, but playing a different game: the diverse species-specific roles of DivIVA proteins. Virulence 3:406–407. doi: 10.4161/viru.20747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ben-Yehuda S, Rudner DZ, Losick R. 2003. RacA, a bacterial protein that anchors chromosomes to the cell poles. Science 299:532–536. doi: 10.1126/science.1079914. [DOI] [PubMed] [Google Scholar]
- 43.Wu LJ, Errington J. 2003. RacA and the Soj-Spo0J system combine to effect polar chromosome segregation in sporulating Bacillus subtilis. Mol Microbiol 49:1463–1475. doi: 10.1046/j.1365-2958.2003.03643.x. [DOI] [PubMed] [Google Scholar]
- 44.Perry SE, Edwards DH. 2006. The Bacillus subtilis DivIVA protein has a sporulation-specific proximity to Spo0J. J Bacteriol 188:6039–6043. doi: 10.1128/JB.01750-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kloosterman TG, Lenarcic R, Willis CR, Roberts DM, Hamoen LW, Errington J, Wu LJ. 2016. Complex polar machinery required for proper chromosome segregation in vegetative and sporulating cells of Bacillus subtilis. Mol Microbiol 101:333–350. doi: 10.1111/mmi.13393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schumacher MA, Lee J, Zeng W. 2016. Molecular insights into DNA binding and anchoring by the Bacillus subtilis sporulation kinetochore-like RacA protein. Nucleic Acids Res 44:5438–5449. doi: 10.1093/nar/gkw248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Radzinski NP, Besprozvannaya M, McLean EL, Talwalkar A, Burton BM. 2019. DNA-membrane anchor facilitates efficient chromosome translocation at a distance in Bacillus subtilis. mBio 10:e01117-19. doi: 10.1128/mBio.01117-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Thomaides HB, Freeman M, El Karoui M, Errington J. 2001. Division site selection protein DivIVA of Bacillus subtilis has a second distinct function in chromosome segregation during sporulation. Genes Dev 15:1662–1673. doi: 10.1101/gad.197501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perry SE, Edwards DH. 2004. Identification of a polar targeting determinant for Bacillus subtilis DivIVA. Mol Microbiol 54:1237–1249. doi: 10.1111/j.1365-2958.2004.04363.x. [DOI] [PubMed] [Google Scholar]
- 50.Bradshaw N, Losick R. 2015. Asymmetric division triggers cell-specific gene expression through coupled capture and stabilization of a phosphatase. Elife 4:e08145. doi: 10.7554/eLife.08145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Eswaramoorthy P, Winter PW, Wawrzusin P, York AG, Shroff H, Ramamurthi KS. 2014. Asymmetric division and differential gene expression during a bacterial developmental program requires DivIVA. PLoS Genet 10:e1004526. doi: 10.1371/journal.pgen.1004526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ogura M, Tanaka T. 2009. The Bacillus subtilis late competence operon comE is transcriptionally regulated by yutB and under post-transcription initiation control by comN (yrzD). J Bacteriol 191:949–958. doi: 10.1128/JB.01429-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.dos Santos VT, Bisson-Filho AW, Gueiros-Filho FJ. 2012. DivIVA-mediated polar localization of ComN, a posttranscriptional regulator of Bacillus subtilis. J Bacteriol 194:3661–3669. doi: 10.1128/JB.05879-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Briley K Jr, Prepiak P, Dias MJ, Hahn J, Dubnau D. 2011. Maf acts downstream of ComGA to arrest cell division in competent cells of B. subtilis. Mol Microbiol 81:23–39. doi: 10.1111/j.1365-2958.2011.07695.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Dworkin J. 2015. Ser/Thr phosphorylation as a regulatory mechanism in bacteria. Curr Opin Microbiol 24:47–52. doi: 10.1016/j.mib.2015.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pompeo F, Foulquier E, Serrano B, Grangeasse C, Galinier A. 2015. Phosphorylation of the cell division protein GpsB regulates PrkC kinase activity through a negative feedback loop in Bacillus subtilis. Mol Microbiol 97:139–150. doi: 10.1111/mmi.13015. [DOI] [PubMed] [Google Scholar]
- 57.Pompeo F, Byrne D, Mengin-Lecreulx D, Galinier A. 2018. Dual regulation of activity and intracellular localization of the PASTA kinase PrkC during Bacillus subtilis growth. Sci Rep 8:1660. doi: 10.1038/s41598-018-20145-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Errington J, Wu LJ. 2017. Cell cycle machinery in Bacillus subtilis. Subcell Biochem 84:67–101. doi: 10.1007/978-3-319-53047-5_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ravikumar V, Shi L, Krug K, Derouiche A, Jers C, Cousin C, Kobir A, Mijakovic I, Macek B. 2014. Quantitative phosphoproteome analysis of Bacillus subtilis reveals novel substrates of the kinase PrkC and phosphatase PrpC. Mol Cell Proteomics 13:1965–1978. doi: 10.1074/mcp.M113.035949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Elsholz AK, Turgay K, Michalik S, Hessling B, Gronau K, Oertel D, Mader U, Bernhardt J, Becher D, Hecker M, Gerth U. 2012. Global impact of protein arginine phosphorylation on the physiology of Bacillus subtilis. Proc Natl Acad Sci U S A 109:7451–7456. doi: 10.1073/pnas.1117483109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Baarle S, Celik IN, Kaval KG, Bramkamp M, Hamoen LW, Halbedel S. 2013. Protein-protein interaction domains of Bacillus subtilis DivIVA. J Bacteriol 195:1012–1021. doi: 10.1128/JB.02171-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Shi L, Pigeonneau N, Ventroux M, Derouiche A, Bidnenko V, Mijakovic I, Noirot-Gros MF. 2014. Protein-tyrosine phosphorylation interaction network in Bacillus subtilis reveals new substrates, kinase activators and kinase cross-talk. Front Microbiol 5:538. doi: 10.3389/fmicb.2014.00538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shi L, Ravikumar V, Derouiche A, Macek B, Mijakovic I. 2016. Tyrosine 601 of Bacillus subtilis DnaK undergoes phosphorylation and is crucial for chaperone activity and heat shock survival. Front Microbiol 7:533. doi: 10.3389/fmicb.2016.00533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bottomley AL, Liew ATF, Kusuma KD, Peterson E, Seidel L, Foster SJ, Harry EJ. 2017. Coordination of chromosome segregation and cell division in Staphylococcus aureus. Front Microbiol 8:1575. doi: 10.3389/fmicb.2017.01575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Siefert JL, Fox GE. 1998. Phylogenetic mapping of bacterial morphology. Microbiology 144:2803–2808. doi: 10.1099/00221287-144-10-2803. [DOI] [PubMed] [Google Scholar]
- 66.Pinho MG, Kjos M, Veening JW. 2013. How to get (a)round: mechanisms controlling growth and division of coccoid bacteria. Nat Rev Microbiol 11:601–614. doi: 10.1038/nrmicro3088. [DOI] [PubMed] [Google Scholar]
- 67.Busiek KK, Margolin W. 2015. Bacterial actin and tubulin homologs in cell growth and division. Curr Biol 25:R243–R254. doi: 10.1016/j.cub.2015.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Massidda O, Novakova L, Vollmer W. 2013. From models to pathogens: how much have we learned about Streptococcus pneumoniae cell division? Environ Microbiol 15:3133–3157. doi: 10.1111/1462-2920.12189. [DOI] [PubMed] [Google Scholar]
- 69.Fadda D, Pischedda C, Caldara F, Whalen MB, Anderluzzi D, Domenici E, Massidda O. 2003. Characterization of divIVA and other genes located in the chromosomal region downstream of the dcw cluster in Streptococcus pneumoniae. J Bacteriol 185:6209–6214. doi: 10.1128/jb.185.20.6209-6214.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Fadda D, Santona A, D'Ulisse V, Ghelardini P, Ennas MG, Whalen MB, Massidda O. 2007. Streptococcus pneumoniae DivIVA: localization and interactions in a MinCD-free context. J Bacteriol 189:1288–1298. doi: 10.1128/JB.01168-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Beilharz K, Novakova L, Fadda D, Branny P, Massidda O, Veening JW. 2012. Control of cell division in Streptococcus pneumoniae by the conserved Ser/Thr protein kinase StkP. Proc Natl Acad Sci U S A 109:E905–E913. doi: 10.1073/pnas.1119172109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Vicente M, Garcia-Ovalle M. 2007. Making a point: the role of DivIVA in streptococcal polar anatomy. J Bacteriol 189:1185–1188. doi: 10.1128/JB.01710-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Fleurie A, Manuse S, Zhao C, Campo N, Cluzel C, Lavergne JP, Freton C, Combet C, Guiral S, Soufi B, Macek B, Kuru E, VanNieuwenhze MS, Brun YV, Di Guilmi AM, Claverys JP, Galinier A, Grangeasse C. 2014. Interplay of the serine/threonine-kinase StkP and the paralogs DivIVA and GpsB in pneumococcal cell elongation and division. PLoS Genet 10:e1004275. doi: 10.1371/journal.pgen.1004275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ni H, Fan W, Li C, Wu Q, Hou H, Hu D, Zheng F, Zhu X, Wang C, Cao X, Shao ZQ, Pan X. 2018. Streptococcus suis DivIVA protein is a substrate of Ser/Thr kinase STK and involved in cell division regulation. Front Cell Infect Microbiol 8:85. doi: 10.3389/fcimb.2018.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jana B, Tao L, Biswas I. 2016. Strain-dependent recognition of a unique degradation motif by ClpXP in Streptococcus mutans. mSphere 1:e00287-16. doi: 10.1128/mSphere.00287-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.van Raaphorst R, Kjos M, Veening JW. 2017. Chromosome segregation drives division site selection in Streptococcus pneumoniae. Proc Natl Acad Sci U S A 114:E5959–E5968. doi: 10.1073/pnas.1620608114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Minnen A, Attaiech L, Thon M, Gruber S, Veening JW. 2011. SMC is recruited to oriC by ParB and promotes chromosome segregation in Streptococcus pneumoniae. Mol Microbiol 81:676–688. doi: 10.1111/j.1365-2958.2011.07722.x. [DOI] [PubMed] [Google Scholar]
- 78.Kjos M, Veening JW. 2014. Tracking of chromosome dynamics in live Streptococcus pneumoniae reveals that transcription promotes chromosome segregation. Mol Microbiol 91:1088–1105. doi: 10.1111/mmi.12517. [DOI] [PubMed] [Google Scholar]
- 79.Novakova L, Bezouskova S, Pompach P, Spidlova P, Saskova L, Weiser J, Branny P. 2010. Identification of multiple substrates of the StkP Ser/Thr protein kinase in Streptococcus pneumoniae. J Bacteriol 192:3629–3638. doi: 10.1128/JB.01564-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Silvestroni A, Jewell KA, Lin WJ, Connelly JE, Ivancic MM, Tao WA, Rajagopal L. 2009. Identification of serine/threonine kinase substrates in the human pathogen group B streptococcus. J Proteome Res 8:2563–2574. doi: 10.1021/pr900069n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Sun X, Ge F, Xiao CL, Yin XF, Ge R, Zhang LH, He QY. 2010. Phosphoproteomic analysis reveals the multiple roles of phosphorylation in pathogenic bacterium Streptococcus pneumoniae. J Proteome Res 9:275–282. doi: 10.1021/pr900612v. [DOI] [PubMed] [Google Scholar]
- 82.Fleurie A, Cluzel C, Guiral S, Freton C, Galisson F, Zanella-Cleon I, Di Guilmi AM, Grangeasse C. 2012. Mutational dissection of the S/T-kinase StkP reveals crucial roles in cell division of Streptococcus pneumoniae. Mol Microbiol 83:746–758. doi: 10.1111/j.1365-2958.2011.07962.x. [DOI] [PubMed] [Google Scholar]
- 83.Straume D, Stamsas GA, Berg KH, Salehian Z, Havarstein LS. 2017. Identification of pneumococcal proteins that are functionally linked to penicillin-binding protein 2b (PBP2b). Mol Microbiol 103:99–116. doi: 10.1111/mmi.13543. [DOI] [PubMed] [Google Scholar]
- 84.Rued BE, Zheng JJ, Mura A, Tsui HT, Boersma MJ, Mazny JL, Corona F, Perez AJ, Fadda D, Doubravova L, Buriankova K, Branny P, Massidda O, Winkler ME. 2017. Suppression and synthetic-lethal genetic relationships of DeltagpsB mutations indicate that GpsB mediates protein phosphorylation and penicillin-binding protein interactions in Streptococcus pneumoniae D39. Mol Microbiol 103:931–957. doi: 10.1111/mmi.13613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Perez AJ, Cesbron Y, Shaw SL, Bazan Villicana J, Tsui HT, Boersma MJ, Ye ZA, Tovpeko Y, Dekker C, Holden S, Winkler ME. 2019. Movement dynamics of divisome proteins and PBP2x:FtsW in cells of Streptococcus pneumoniae. Proc Natl Acad Sci U S A 116:3211–3220. doi: 10.1073/pnas.1816018116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Feng Z, Zhang J, Xu D, Jiang YL, Zhou CZ, Chen Y. 2019. Multi-functional regulator MapZ controls both positioning and timing of FtsZ polymerization. Biochem J 476:1433–1444. doi: 10.1042/BCJ20190138. [DOI] [PubMed] [Google Scholar]
- 87.Li Y, Shao S, Xu X, Su X, Sun Y, Wei S. 2018. MapZ forms a stable ring structure that acts as a nanotrack for FtsZ treadmilling in Streptococcus mutans. ACS Nano 12:6137–6146. doi: 10.1021/acsnano.8b02469. [DOI] [PubMed] [Google Scholar]
- 88.Holečková N, Doubravová L, Massidda O, Molle V, Buriánková K, Benada O, Kofroňová O, Ulrych A, Branny P. 2014. LocZ is a new cell division protein involved in proper septum placement in Streptococcus pneumoniae. mBio 6:e01700. doi: 10.1128/mBio.01700-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Fleurie A, Lesterlin C, Manuse S, Zhao C, Cluzel C, Lavergne JP, Franz-Wachtel M, Macek B, Combet C, Kuru E, VanNieuwenhze MS, Brun YV, Sherratt D, Grangeasse C. 2014. MapZ marks the division sites and positions FtsZ rings in Streptococcus pneumoniae. Nature 516:259–262. doi: 10.1038/nature13966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ramirez-Arcos S, Liao M, Marthaler S, Rigden M, Dillon JA. 2005. Enterococcus faecalis divIVA: an essential gene involved in cell division, cell growth and chromosome segregation. Microbiology 151:1381–1393. doi: 10.1099/mic.0.27718-0. [DOI] [PubMed] [Google Scholar]
- 91.Halbedel S, Hahn B, Daniel RA, Flieger A. 2012. DivIVA affects secretion of virulence-related autolysins in Listeria monocytogenes. Mol Microbiol 83:821–839. doi: 10.1111/j.1365-2958.2012.07969.x. [DOI] [PubMed] [Google Scholar]
- 92.Kaval KG, Hauf S, Rismondo J, Hahn B, Halbedel S. 2017. Genetic dissection of DivIVA functions in Listeria monocytogenes. J Bacteriol 199:e00421-17. doi: 10.1128/JB.00421-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kaval KG, Rismondo J, Halbedel S. 2014. A function of DivIVA in Listeria monocytogenes division site selection. Mol Microbiol 94:637–654. doi: 10.1111/mmi.12784. [DOI] [PubMed] [Google Scholar]
- 94.Valencikova R, Krascsenitsova E, Labajova N, Makroczyova J, Barak I. 2018. Clostridial DivIVA and MinD interact in the absence of MinJ. Anaerobe 50:22–31. doi: 10.1016/j.anaerobe.2018.01.013. [DOI] [PubMed] [Google Scholar]
- 95.Pinho MG, Errington J. 2004. A divIVA null mutant of Staphylococcus aureus undergoes normal cell division. FEMS Microbiol Lett 240:145–149. doi: 10.1016/j.femsle.2004.09.038. [DOI] [PubMed] [Google Scholar]
- 96.Flardh K. 2003. Essential role of DivIVA in polar growth and morphogenesis in Streptomyces coelicolor A3(2). Mol Microbiol 49:1523–1536. doi: 10.1046/j.1365-2958.2003.03660.x. [DOI] [PubMed] [Google Scholar]
- 97.Hempel AM, Wang SB, Letek M, Gil JA, Flardh K. 2008. Assemblies of DivIVA mark sites for hyphal branching and can establish new zones of cell wall growth in Streptomyces coelicolor. J Bacteriol 190:7579–7583. doi: 10.1128/JB.00839-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Richards DM, Hempel AM, Flardh K, Buttner MJ, Howard M. 2012. Mechanistic basis of branch-site selection in filamentous bacteria. PLoS Comput Biol 8:e1002423. doi: 10.1371/journal.pcbi.1002423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hempel AM, Cantlay S, Molle V, Wang SB, Naldrett MJ, Parker JL, Richards DM, Jung YG, Buttner MJ, Flardh K. 2012. The Ser/Thr protein kinase AfsK regulates polar growth and hyphal branching in the filamentous bacteria Streptomyces. Proc Natl Acad Sci U S A 109:E2371–E2379. doi: 10.1073/pnas.1207409109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Holmes NA, Walshaw J, Leggett RM, Thibessard A, Dalton KA, Gillespie MD, Hemmings AM, Gust B, Kelemen GH. 2013. Coiled-coil protein Scy is a key component of a multiprotein assembly controlling polarized growth in Streptomyces. Proc Natl Acad Sci U S A 110:E397–E406. doi: 10.1073/pnas.1210657110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Fuchino K, Bagchi S, Cantlay S, Sandblad L, Wu D, Bergman J, Kamali-Moghaddam M, Flärdh K, Ausmees N. 2013. Dynamic gradients of an intermediate filament-like cytoskeleton are recruited by a polarity landmark during apical growth. Proc Natl Acad Sci U S A 110:E1889–E1897. doi: 10.1073/pnas.1305358110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Frojd MJ, Flardh K. 15 April 2019, posting date. Apical assemblies of intermediate filament-like protein FilP are highly dynamic and affect polar growth determinant DivIVA in Streptomyces venezuelae. Mol Microbiol doi: 10.1111/mmi.14253. [DOI] [PubMed] [Google Scholar]
- 103.Kois-Ostrowska A, Strzałka A, Lipietta N, Tilley E, Zakrzewska-Czerwińska J, Herron P, Jakimowicz D. 2016. Unique function of the bacterial chromosome segregation machinery in apically growing Streptomyces—targeting the chromosome to new hyphal tubes and its anchorage at the tips. PLoS Genet 12:e1006488. doi: 10.1371/journal.pgen.1006488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ditkowski B, Holmes N, Rydzak J, Donczew M, Bezulska M, Ginda K, Kedzierski P, Zakrzewska-Czerwińska J, Kelemen GH, Jakimowicz D. 2013. Dynamic interplay of ParA with the polarity protein, Scy, coordinates the growth with chromosome segregation in Streptomyces coelicolor. Open Biol 3:130006. doi: 10.1098/rsob.130006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Donovan C, Sieger B, Kramer R, Bramkamp M. 2012. A synthetic Escherichia coli system identifies a conserved origin tethering factor in Actinobacteria. Mol Microbiol 84:105–116. doi: 10.1111/j.1365-2958.2012.08011.x. [DOI] [PubMed] [Google Scholar]
- 106.Ramos A, Honrubia MP, Valbuena N, Vaquera J, Mateos LM, Gil JA. 2003. Involvement of DivIVA in the morphology of the rod-shaped actinomycete Brevibacterium lactofermentum. Microbiology 149:3531–3542. doi: 10.1099/mic.0.26653-0. [DOI] [PubMed] [Google Scholar]
- 107.Letek M, Ordonez E, Vaquera J, Margolin W, Flardh K, Mateos LM, Gil JA. 2008. DivIVA is required for polar growth in the MreB-lacking rod-shaped actinomycete Corynebacterium glutamicum. J Bacteriol 190:3283–3292. doi: 10.1128/JB.01934-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sieger B, Schubert K, Donovan C, Bramkamp M. 2013. The lipid II flippase RodA determines morphology and growth in Corynebacterium glutamicum. Mol Microbiol 90:966–982. doi: 10.1111/mmi.12411. [DOI] [PubMed] [Google Scholar]
- 109.Sieger B, Bramkamp M. 2014. Interaction sites of DivIVA and RodA from Corynebacterium glutamicum. Front Microbiol 5:738. doi: 10.3389/fmicb.2014.00738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Margolin W. 2009. Sculpting the bacterial cell. Curr Biol 19:R812–R822. doi: 10.1016/j.cub.2009.06.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Letek M, Fiuza M, Villadangos AF, Mateos LM, Gil JA. 2012. Cytoskeletal proteins of actinobacteria. Int J Cell Biol 2012:905832. doi: 10.1155/2012/905832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Donovan C, Schauss A, Kramer R, Bramkamp M. 2013. Chromosome segregation impacts on cell growth and division site selection in Corynebacterium glutamicum. PLoS One 8:e55078. doi: 10.1371/journal.pone.0055078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Donovan C, Bramkamp M. 2014. Cell division in Corynebacterineae. Front Microbiol 5:132. doi: 10.3389/fmicb.2014.00132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Donovan C, Schwaiger A, Kramer R, Bramkamp M. 2010. Subcellular localization and characterization of the ParAB system from Corynebacterium glutamicum. J Bacteriol 192:3441–3451. doi: 10.1128/JB.00214-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fiuza M, Letek M, Leiba J, Villadangos AF, Vaquera J, Zanella-Cleon I, Mateos LM, Molle V, Gil JA. 2010. Phosphorylation of a novel cytoskeletal protein (RsmP) regulates rod-shaped morphology in Corynebacterium glutamicum. J Biol Chem 285:29387–29397. doi: 10.1074/jbc.M110.154427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Fiuza M, Canova MJ, Patin D, Letek M, Zanella-Cleon I, Becchi M, Mateos LM, Mengin-Lecreulx D, Molle V, Gil JA. 2008. The MurC ligase essential for peptidoglycan biosynthesis is regulated by the serine/threonine protein kinase PknA in Corynebacterium glutamicum. J Biol Chem 283:36553–36563. doi: 10.1074/jbc.M807175200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Fiuza M, Canova MJ, Zanella-Cleon I, Becchi M, Cozzone AJ, Mateos LM, Kremer L, Gil JA, Molle V. 2008. From the characterization of the four serine/threonine protein kinases (PknA/B/G/L) of Corynebacterium glutamicum toward the role of PknA and PknB in cell division. J Biol Chem 283:18099–18112. doi: 10.1074/jbc.M802615200. [DOI] [PubMed] [Google Scholar]
- 118.Schultz C, Niebisch A, Schwaiger A, Viets U, Metzger S, Bramkamp M, Bott M. 2009. Genetic and biochemical analysis of the serine/threonine protein kinases PknA, PknB, PknG and PknL of Corynebacterium glutamicum: evidence for non-essentiality and for phosphorylation of OdhI and FtsZ by multiple kinases. Mol Microbiol 74:724–741. doi: 10.1111/j.1365-2958.2009.06897.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hermans PW, Abebe F, Kuteyi VI, Kolk AH, Thole JE, Harboe M. 1995. Molecular and immunological characterization of the highly conserved antigen 84 from Mycobacterium tuberculosis and Mycobacterium leprae. Infect Immun 63:954–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Samten B, Fannin S, Sarva K, Yi N, Madiraju M, Rajagopalan M. 2016. Modulation of human T cell cytokines by the Mycobacterium tuberculosis-secreted protein Wag31. Tuberculosis (Edinb) 101S:S99–S104. doi: 10.1016/j.tube.2016.09.017. [DOI] [PubMed] [Google Scholar]
- 121.Cameron TA, Zupan JR, Zambryski PC. 2015. The essential features and modes of bacterial polar growth. Trends Microbiol 23:347–353. doi: 10.1016/j.tim.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 122.Sassetti CM, Boyd DH, Rubin EJ. 2003. Genes required for mycobacterial growth defined by high density mutagenesis. Mol Microbiol 48:77–84. doi: 10.1046/j.1365-2958.2003.03425.x. [DOI] [PubMed] [Google Scholar]
- 123.Griffin JE, Gawronski JD, Dejesus MA, Ioerger TR, Akerley BJ, Sassetti CM. 2011. High-resolution phenotypic profiling defines genes essential for mycobacterial growth and cholesterol catabolism. PLoS Pathog 7:e1002251. doi: 10.1371/journal.ppat.1002251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kang CM, Nyayapathy S, Lee JY, Suh JW, Husson RN. 2008. Wag31, a homologue of the cell division protein DivIVA, regulates growth, morphology and polar cell wall synthesis in mycobacteria. Microbiology 154:725–735. doi: 10.1099/mic.0.2007/014076-0. [DOI] [PubMed] [Google Scholar]
- 125.Nguyen L, Scherr N, Gatfield J, Walburger A, Pieters J, Thompson CJ. 2007. Antigen 84, an effector of pleiomorphism in Mycobacterium smegmatis. J Bacteriol 189:7896–7910. doi: 10.1128/JB.00726-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jani C, Eoh H, Lee JJ, Hamasha K, Sahana MB, Han JS, Nyayapathy S, Lee JY, Suh JW, Lee SH, Rehse SJ, Crick DC, Kang CM. 2010. Regulation of polar peptidoglycan biosynthesis by Wag31 phosphorylation in mycobacteria. BMC Microbiol 10:327. doi: 10.1186/1471-2180-10-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Scherr N, Nguyen L. 2009. Mycobacterium versus Streptomyces–we are different, we are the same. Curr Opin Microbiol 12:699–707. doi: 10.1016/j.mib.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 128.Meniche X, Otten R, Siegrist MS, Baer CE, Murphy KC, Bertozzi CR, Sassetti CM. 2014. Subpolar addition of new cell wall is directed by DivIVA in mycobacteria. Proc Natl Acad Sci U S A 111:E3243–E3251. doi: 10.1073/pnas.1402158111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Melzer ES, Sein CE, Chambers JJ, Sloan Siegrist M. 2018. DivIVA concentrates mycobacterial cell envelope assembly for initiation and stabilization of polar growth. Cytoskeleton (Hoboken) 75:498–507. doi: 10.1002/cm.21490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Mukherjee P, Sureka K, Datta P, Hossain T, Barik S, Das KP, Kundu M, Basu J. 2009. Novel role of Wag31 in protection of mycobacteria under oxidative stress. Mol Microbiol 73:103–119. doi: 10.1111/j.1365-2958.2009.06750.x. [DOI] [PubMed] [Google Scholar]
- 131.Plocinski P, Arora N, Sarva K, Blaszczyk E, Qin H, Das N, Plocinska R, Ziolkiewicz M, Dziadek J, Kiran M, Gorla P, Cross TA, Madiraju M, Rajagopalan M. 2012. Mycobacterium tuberculosis CwsA interacts with CrgA and Wag31, and the CrgA-CwsA complex is involved in peptidoglycan synthesis and cell shape determination. J Bacteriol 194:6398–6409. doi: 10.1128/JB.01005-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Plocinski P, Martinez L, Sarva K, Plocinska R, Madiraju M, Rajagopalan M. 2013. Mycobacterium tuberculosis CwsA overproduction modulates cell division and cell wall synthesis. Tuberculosis (Edinb) 93(Suppl):S21–S27. doi: 10.1016/S1472-9792(13)70006-4. [DOI] [PubMed] [Google Scholar]
- 133.Plocinska R, Martinez L, Gorla P, Pandeeti E, Sarva K, Blaszczyk E, Dziadek J, Madiraju MV, Rajagopalan M. 2014. Mycobacterium tuberculosis MtrB sensor kinase interactions with FtsI and Wag31 proteins reveal a role for MtrB distinct from that regulating MtrA activities. J Bacteriol 196:4120–4129. doi: 10.1128/JB.01795-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Senzani S, Li D, Bhaskar A, Ealand C, Chang J, Rimal B, Liu C, Joon Kim S, Dhar N, Kana B. 2017. An Amidase_3 domain-containing N-acetylmuramyl-l-alanine amidase is required for mycobacterial cell division. Sci Rep 7:1140. doi: 10.1038/s41598-017-01184-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Xu WX, Zhang L, Mai JT, Peng RC, Yang EZ, Peng C, Wang HH. 2014. The Wag31 protein interacts with AccA3 and coordinates cell wall lipid permeability and lipophilic drug resistance in Mycobacterium smegmatis. Biochem Biophys Res Commun 448:255–260. doi: 10.1016/j.bbrc.2014.04.116. [DOI] [PubMed] [Google Scholar]
- 136.Carel C, Nukdee K, Cantaloube S, Bonne M, Diagne CT, Laval F, Daffe M, Zerbib D. 2014. Mycobacterium tuberculosis proteins involved in mycolic acid synthesis and transport localize dynamically to the old growing pole and septum. PLoS One 9:e97148. doi: 10.1371/journal.pone.0097148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Marrakchi H, Laneelle MA, Daffe M. 2014. Mycolic acids: structures, biosynthesis, and beyond. Chem Biol 21:67–85. doi: 10.1016/j.chembiol.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 138.Kang CM, Abbott DW, Park ST, Dascher CC, Cantley LC, Husson RN. 2005. The Mycobacterium tuberculosis serine/threonine kinases PknA and PknB: substrate identification and regulation of cell shape. Genes Dev 19:1692–1704. doi: 10.1101/gad.1311105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Sharma AK, Arora D, Singh LK, Gangwal A, Sajid A, Molle V, Singh Y, Nandicoori VK. 2016. Serine/threonine protein phosphatase PstP of Mycobacterium tuberculosis is necessary for accurate cell division and survival of pathogen. J Biol Chem 291:24215–24230. doi: 10.1074/jbc.M116.754531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Av-Gay Y, Everett M. 2000. The eukaryotic-like Ser/Thr protein kinases of Mycobacterium tuberculosis. Trends Microbiol 8:238–244. doi: 10.1016/S0966-842X(00)01734-0. [DOI] [PubMed] [Google Scholar]
- 141.Lee JJ, Kan CM, Lee JH, Park KS, Jeon JH, Lee SH. 2014. Phosphorylation-dependent interaction between a serine/threonine kinase PknA and a putative cell division protein Wag31 in Mycobacterium tuberculosis. New Microbiol 37:525–533. [PubMed] [Google Scholar]
- 142.Ginda K, Bezulska M, Ziółkiewicz M, Dziadek J, Zakrzewska-Czerwińska J, Jakimowicz D. 2013. ParA of Mycobacterium smegmatis co-ordinates chromosome segregation with the cell cycle and interacts with the polar growth determinant DivIVA. Mol Microbiol 87:998–1012. doi: 10.1111/mmi.12146. [DOI] [PubMed] [Google Scholar]
- 143.Wu KJ, Zhang J, Baranowski C, Leung V, Rego EH, Morita YS, Rubin EJ, Boutte CC. 2018. Characterization of conserved and novel septal factors in Mycobacterium smegmatis. J Bacteriol 200:e00649-17. doi: 10.1128/JB.00649-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Ko CC, Hatfull GF. 2018. Mycobacteriophage Fruitloop gp52 inactivates Wag31 (DivIVA) to prevent heterotypic superinfection. Mol Microbiol 108:443–460. doi: 10.1111/mmi.13946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Leaver M, Errington J. 2005. Roles for MreC and MreD proteins in helical growth of the cylindrical cell wall in Bacillus subtilis. Mol Microbiol 57:1196–1209. doi: 10.1111/j.1365-2958.2005.04736.x. [DOI] [PubMed] [Google Scholar]
- 146.Rismondo J, Cleverley RM, Lane HV, Großhennig S, Steglich A, Möller L, Mannala GK, Hain T, Lewis RJ, Halbedel S. 2016. Structure of the bacterial cell division determinant GpsB and its interaction with penicillin-binding proteins. Mol Microbiol 99:978–998. doi: 10.1111/mmi.13279. [DOI] [PubMed] [Google Scholar]
- 147.Cleverley RM, Rutter ZJ, Rismondo J, Corona F, Tsui HT, Alatawi FA, Daniel RA, Halbedel S, Massidda O, Winkler ME, Lewis RJ. 2019. The cell cycle regulator GpsB functions as cytosolic adaptor for multiple cell wall enzymes. Nat Commun 10:261. doi: 10.1038/s41467-018-08056-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rismondo J, Wamp S, Aldridge C, Vollmer W, Halbedel S. 2018. Stimulation of PgdA-dependent peptidoglycan N-deacetylation by GpsB-PBP A1 in Listeria monocytogenes. Mol Microbiol 107:472–487. doi: 10.1111/mmi.13893. [DOI] [PubMed] [Google Scholar]
- 149.Rismondo J, Bender JK, Halbedel S. 2017. Suppressor mutations linking gpsB with the first committed step of peptidoglycan biosynthesis in Listeria monocytogenes. J Bacteriol 199:e00393-16. doi: 10.1128/JB.00393-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cleverley RM, Rismondo J, Lockhart-Cairns MP, Van Bentum PT, Egan AJ, Vollmer W, Halbedel S, Baldock C, Breukink E, Lewis RJ. 2016. Subunit arrangement in GpsB, a regulator of cell wall biosynthesis. Microb Drug Resist 22:446–460. doi: 10.1089/mdr.2016.0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Pensinger DA, Boldon KM, Chen GY, Vincent WJ, Sherman K, Xiong M, Schaenzer AJ, Forster ER, Coers J, Striker R, Sauer JD. 2016. The Listeria monocytogenes PASTA kinase PrkA and its substrate YvcK are required for cell wall homeostasis, metabolism, and virulence. PLoS Pathog 12:e1006001. doi: 10.1371/journal.ppat.1006001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Land AD, Tsui HC, Kocaoglu O, Vella SA, Shaw SL, Keen SK, Sham LT, Carlson EE, Winkler ME. 2013. Requirement of essential Pbp2x and GpsB for septal ring closure in Streptococcus pneumoniae D39. Mol Microbiol 90:939–955. doi: 10.1111/mmi.12408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Manuse S, Fleurie A, Zucchini L, Lesterlin C, Grangeasse C. 2016. Role of eukaryotic-like serine/threonine kinases in bacterial cell division and morphogenesis. FEMS Microbiol Rev 40:41–56. doi: 10.1093/femsre/fuv041. [DOI] [PubMed] [Google Scholar]
- 154.Banu LD, Conrads G, Rehrauer H, Hussain H, Allan E, van der Ploeg JR. 2010. The Streptococcus mutans serine/threonine kinase, PknB, regulates competence development, bacteriocin production, and cell wall metabolism. Infect Immun 78:2209–2220. doi: 10.1128/IAI.01167-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Burnside K, Lembo A, Harrell MI, Gurney M, Xue L, BinhTran NT, Connelly JE, Jewell KA, Schmidt BZ, de los Reyes M, Tao WA, Doran KS, Rajagopal L. 2011. Serine/threonine phosphatase Stp1 mediates post-transcriptional regulation of hemolysin, autolysis, and virulence of group B Streptococcus. J Biol Chem 286:44197–44210. doi: 10.1074/jbc.M111.313486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Santiago M, Matano LM, Moussa SH, Gilmore MS, Walker S, Meredith TC. 2015. A new platform for ultra-high density Staphylococcus aureus transposon libraries. BMC Genomics 16:252. doi: 10.1186/s12864-015-1361-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Steele VR, Bottomley AL, Garcia-Lara J, Kasturiarachchi J, Foster SJ. 2011. Multiple essential roles for EzrA in cell division of Staphylococcus aureus. Mol Microbiol 80:542–555. doi: 10.1111/j.1365-2958.2011.07591.x. [DOI] [PubMed] [Google Scholar]
- 158.Eswara PJ, Brzozowski RS, Viola MG, Graham G, Spanoudis C, Trebino C, Jha J, Aubee JI, Thompson KM, Camberg JL, Ramamurthi KS. 2018. An essential Staphylococcus aureus cell division protein directly regulates FtsZ dynamics. Elife 7:e38856. doi: 10.7554/eLife.38856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Pinho MG, Errington J. 2003. Dispersed mode of Staphylococcus aureus cell wall synthesis in the absence of the division machinery. Mol Microbiol 50:871–881. doi: 10.1046/j.1365-2958.2003.03719.x. [DOI] [PubMed] [Google Scholar]
- 160.Bisson-Filho AW, Hsu YP, Squyres GR, Kuru E, Wu F, Jukes C, Sun Y, Dekker C, Holden S, VanNieuwenhze MS, Brun YV, Garner EC. 2017. Treadmilling by FtsZ filaments drives peptidoglycan synthesis and bacterial cell division. Science 355:739–743. doi: 10.1126/science.aak9973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Yang X, Lyu Z, Miguel A, McQuillen R, Huang KC, Xiao J. 2017. GTPase activity-coupled treadmilling of the bacterial tubulin FtsZ organizes septal cell wall synthesis. Science 355:744–747. doi: 10.1126/science.aak9995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Monteiro JM, Pereira AR, Reichmann NT, Saraiva BM, Fernandes PB, Veiga H, Tavares AC, Santos M, Ferreira MT, Macario V, VanNieuwenhze MS, Filipe SR, Pinho MG. 2018. Peptidoglycan synthesis drives an FtsZ-treadmilling-independent step of cytokinesis. Nature 554:528–532. doi: 10.1038/nature25506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Cleverley RM, Barrett JR, Basle A, Bui NK, Hewitt L, Solovyova A, Xu ZQ, Daniel RA, Dixon NE, Harry EJ, Oakley AJ, Vollmer W, Lewis RJ. 2014. Structure and function of a spectrin-like regulator of bacterial cytokinesis. Nat Commun 5:5421. doi: 10.1038/ncomms6421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Santa Maria JP Jr, Sadaka A, Moussa SH, Brown S, Zhang YJ, Rubin EJ, Gilmore MS, Walker S. 2014. Compound-gene interaction mapping reveals distinct roles for Staphylococcus aureus teichoic acids. Proc Natl Acad Sci U S A 111:12510–12515. doi: 10.1073/pnas.1404099111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Reichmann NT, Piçarra Cassona C, Monteiro JM, Bottomley AL, Corrigan RM, Foster SJ, Pinho MG, Gründling A. 2014. Differential localization of LTA synthesis proteins and their interaction with the cell division machinery in Staphylococcus aureus. Mol Microbiol 92:273–286. doi: 10.1111/mmi.12551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Jorge AM, Hoiczyk E, Gomes JP, Pinho MG. 2011. EzrA contributes to the regulation of cell size in Staphylococcus aureus. PLoS One 6:e27542. doi: 10.1371/journal.pone.0027542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Hardt P, Engels I, Rausch M, Gajdiss M, Ulm H, Sass P, Ohlsen K, Sahl HG, Bierbaum G, Schneider T, Grein F. 2017. The cell wall precursor lipid II acts as a molecular signal for the Ser/Thr kinase PknB of Staphylococcus aureus. Int J Med Microbiol 307:1–10. doi: 10.1016/j.ijmm.2016.12.001. [DOI] [PubMed] [Google Scholar]
- 168.Beltramini AM, Mukhopadhyay CD, Pancholi V. 2009. Modulation of cell wall structure and antimicrobial susceptibility by a Staphylococcus aureus eukaryote-like serine/threonine kinase and phosphatase. Infect Immun 77:1406–1416. doi: 10.1128/IAI.01499-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Jarick M, Bertsche U, Stahl M, Schultz D, Methling K, Lalk M, Stigloher C, Steger M, Schlosser A, Ohlsen K. 2018. The serine/threonine kinase Stk and the phosphatase Stp regulate cell wall synthesis in Staphylococcus aureus. Sci Rep 8:13693. doi: 10.1038/s41598-018-32109-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Rausch M, Deisinger JP, Ulm H, Muller A, Li W, Hardt P, Wang X, Li X, Sylvester M, Engeser M, Vollmer W, Muller CE, Sahl HG, Lee JC, Schneider T. 2019. Coordination of capsule assembly and cell wall biosynthesis in Staphylococcus aureus. Nat Commun 10:1404. doi: 10.1038/s41467-019-09356-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Junker S, Maaβ S, Otto A, Michalik S, Morgenroth F, Gerth U, Hecker M, Becher D. 2018. Spectral library based analysis of arginine phosphorylations in Staphylococcus aureus. Mol Cell Proteomics 17:335–348. doi: 10.1074/mcp.RA117.000378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Chaudhary R, Gupta A, Kota S, Misra HS. 2019. N-terminal domain of DivIVA contributes to its dimerization and interaction with genome segregation proteins in a radioresistant bacterium Deinococcus radiodurans. Int J Biol Macromol 128:12–21. doi: 10.1016/j.ijbiomac.2019.01.085. [DOI] [PubMed] [Google Scholar]
- 173.Mercy C, Ducret A, Slager J, Lavergne JP, Freton C, Nagarajan SN, Garcia PS, Noirot-Gros MF, Dubarry N, Nourikyan J, Veening JW, Grangeasse C. 2019. RocS drives chromosome segregation and nucleoid protection in Streptococcus pneumoniae. Nat Microbiol doi: 10.1038/s41564-019-0472-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Foulquier E, Pompeo F, Freton C, Cordier B, Grangeasse C, Galinier A. 2014. PrkC-mediated phosphorylation of overexpressed YvcK protein regulates PBP1 protein localization in Bacillus subtilis mreB mutant cells. J Biol Chem 289:23662–23669. doi: 10.1074/jbc.M114.562496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Patel V, Wu Q, Chandrangsu P, Helmann JD. 2018. A metabolic checkpoint protein GlmR is important for diverting carbon into peptidoglycan biosynthesis in Bacillus subtilis. PLoS Genet 14:e1007689. doi: 10.1371/journal.pgen.1007689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Pompeo F, Rismondo J, Grundling A, Galinier A. 2018. Investigation of the phosphorylation of Bacillus subtilis LTA synthases by the serine/threonine kinase PrkC. Sci Rep 8:17344. doi: 10.1038/s41598-018-35696-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Absalon C, Obuchowski M, Madec E, Delattre D, Holland IB, Seror SJ. 2009. CpgA, EF-Tu and the stressosome protein YezB are substrates of the Ser/Thr kinase/phosphatase couple, PrkC/PrpC, in Bacillus subtilis. Microbiology 155:932–943. doi: 10.1099/mic.0.022475-0. [DOI] [PubMed] [Google Scholar]
- 178.Pietack N, Becher D, Schmidl SR, Saier MH, Hecker M, Commichau FM, Stulke J. 2010. In vitro phosphorylation of key metabolic enzymes from Bacillus subtilis: PrkC phosphorylates enzymes from different branches of basic metabolism. J Mol Microbiol Biotechnol 18:129–140. doi: 10.1159/000308512. [DOI] [PubMed] [Google Scholar]
- 179.Grangeasse C. 2016. Rewiring the pneumococcal cell cycle with serine/threonine- and tyrosine-kinases. Trends Microbiol 24:713–724. doi: 10.1016/j.tim.2016.04.004. [DOI] [PubMed] [Google Scholar]
- 180.Li Y, Orlando BJ, Liao M. 2019. Structural basis of lipopolysaccharide extraction by the LptB2FGC complex. Nature 567:486–490. doi: 10.1038/s41586-019-1025-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Owens TW, Taylor RJ, Pahil KS, Bertani BR, Ruiz N, Kruse AC, Kahne D. 2019. Structural basis of unidirectional export of lipopolysaccharide to the cell surface. Nature 567:550–553. doi: 10.1038/s41586-019-1039-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Munita JM, Bayer AS, Arias CA. 2015. Evolving resistance among Gram-positive pathogens. Clin Infect Dis 61(Suppl 2):S48–S57. doi: 10.1093/cid/civ523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Fonseca JD, Knight GM, McHugh TD. 2015. The complex evolution of antibiotic resistance in Mycobacterium tuberculosis. Int J Infect Dis 32:94–100. doi: 10.1016/j.ijid.2015.01.014. [DOI] [PubMed] [Google Scholar]
- 184.Halbedel S, Lewis RJ. 2019. Structural basis for interaction of DivIVA/GpsB proteins with their ligands. Mol Microbiol doi: 10.1111/mmi.14244. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.