Abstract
Regulation of protein synthesis is critical for maintaining cellular homeostasis. In mammalian systems, translational regulatory networks have been elucidated in considerable detail. In plants, however, regulation occurs through different mechanisms that remain largely elusive. In this study, we present evidence that the Arabidopsis thaliana energy sensing kinase SnRK1, a homologue of mammalian AMPK and yeast SNF1, inhibits translation by phosphorylating the cap binding proteins eIF4E and eIFiso4E. We establish that eIF4E and eIFiso4E contain two deeply conserved SnRK1 consensus target sites and that both interact with SnRK1 in vivo. We then demonstrate that SnRK1 phosphorylation inhibits the ability of Arabidopsis eIF4E and eIFiso4E to complement a yeast strain lacking endogenous eIF4E, and that inhibition correlates with repression of polysome formation. Finally, we show that SnRK1 over-expression in Nicotiana benthamiana plants reduces polysome formation, and that this effect can be counteracted by transient expression of eIF4E or mutant eIF4E containing non-phosphorylatable SnRK1 target residues, but not by a phosphomimic eIF4E. Together, these studies elucidate a novel and direct pathway for translational control in plant cells. In light of previous findings that SnRK1 conditions an innate antiviral defense and is inhibited by geminivirus pathogenicity factors, we speculate that phosphorylation of cap binding proteins may be a component of the resistance mechanism.
Keywords: SnRK1, eIF4E, eIFiso4E, translation, geminivirus
Graphical Abstract
Regulation of protein synthesis is critical for maintaining cellular homeostasis. In this study, we present evidence that the Arabidopsis thaliana energy sensing kinase SnRK1, a homologue of mammalian AMPK and yeast SNF1, inhibits translation by phosphorylating the cap binding proteins eIF4E and eIFiso4E at two specific target residues. These studies suggest a novel and direct pathway for translational control in plant cells.
Introduction
Protein synthesis lies at the core of life’s processes, and its regulation is critical to maintain cellular homeostasis and for appropriate responses to environmental fluctuations and pathogen attack. However, while mechanisms that control translation in mammalian systems are reasonably well understood, plants employ different mechanisms that remain elusive. In previous work, we found that geminivirus pathogenicity factors AL2 and L2 interact with and inhibit SNF1-related kinase 1 (SnRK1), and showed that SnRK1 conditions an innate defense effective against the DNA-containing geminiviruses [1]. From this apparent defense-counterdefense interaction, we speculated that SnRK1 may be involved in regulation of protein synthesis, and asked whether it might target key translation factors. Based on work described in this report, we suggest that SnRK1 phosphorylates eukaryotic initiation factor 4E (eIF4E) and the plant-specific eukaryotic initiation factor iso4E (eIFiso4E) to inhibit translation initiation.
SnRK1 belongs to a conserved family of serine-threonine kinases that includes sucrose non-fermenting 1 (SNF1) in yeast and AMP-activated kinase (AMPK) in animals. These kinases are key metabolic regulators that maintain homeostasis in response to fluctuations in cellular energy levels [2–8]. In the case of AMPK and SNF1, cellular energy charge is sensed by relative AMP/ADP/ATP concentration. AMP allosterically activates AMPK, and both SNF1 and AMPK bind low energy adenylates that act to sustain kinase activity. In contrast, SnRK1 does not appear to bind AMP [9], although AMP has been shown to prevent SnRK1 inactivation in vitro [10]. Further, adenosine kinase (ADK), which converts adenosine to 5’-AMP, forms a complex with SnRK1 in vivo that could increase local AMP concentration and sustain kinase activity [11]. In this context, it is interesting to note that geminivirus AL2 and L2 proteins interact with and inactivate both SnRK1 and ADK [1, 12]. While mechanisms by which SnRK1 might be activated in response to ATP depletion remain unclear, sugar-phosphates, including trehalose-6-phosphate, have been identified as allosteric inhibitors of SnRK1 [13–15]. Regardless of how energy status is sensed, conditions that reduce cellular energy levels stimulate SNF1/AMPK/SnRK1 to switch on energy generating catabolic pathways and switch off energy consuming anabolic pathways. In plants, SnRK1 has been shown to impact diverse pathways involved in nitrogen assimilation, starch synthesis, sterol synthesis, stress responses, and pathogen defense by mechanisms that include direct phosphorylation of key enzymes, down-regulation of TOR (target of rapamycin) kinase, and transcriptional reprogramming [4–6, 8, 16].
SNF1/AMPK/SnRK1 exist as a heterotrimers consisting of α, β, and γ-subunits. The β and γ-subunits play regulatory and structural roles, while the kinase activity resides in the α subunit [3, 6, 7]. In plants, SNF1-related kinases have expanded into SnRK1, SnRK2, and SnRK3 subfamilies, comprising in total more than 30 enzymes in the reference plant Arabidopsis thaliana. Of these, the SnRK1 subfamily is most closely related to SNF1 and AMPK, and members are able to complement yeast SNF1 deletion mutants [1, 17]. The Arabidopsis genome encodes three SnRK1 α-subunits, but only SnRK1.1 and SnRK1.2 (At3G01090 and At3G29160, also known as AKIN10 and AKIN11, respectively) are expressed to appreciable levels [18]. Hereafter, SnRK1 will refer to SnRK1.2, unless otherwise specified. These α-subunits associate with one of three β-subunits as well as one atypical βγ-subunit, which replaces of the γ-subunit found in SNF1 and AMPK complexes [9, 19]. A predicted SnRK1 γ-subunit is expressed in Arabidopsis, however, it does not appear to associate with the heterotrimer and its function is unknown.
The mRNA cap-binding proteins eIF4E and eIFiso4E, the latter found only in flowering plants [20], are critical components of the translation initiation complex. They connect the mRNA cap to the eIF4G or eIFiso4G scaffolding proteins, which bind additional proteins including poly(A) binding protein to circularize the mRNA and initiate translation [21, 22]. eIFiso4E appears to be expressed to higher levels than eIF4E [23, 24]. However, in Arabidopsis, canonical eIF4E (At4g18040) and eIFiso4E (At5g35620) can be individually lost with little phenotypic impact, but disruption of both genes is lethal [20]. This suggests at least partially overlapping roles for the two proteins, and further indicates that two additional eIF4E isoforms with restricted expression patterns cannot substitute for the canonical proteins [25]. Since translation is largely controlled at the level of initiation [21], eIF4E and eIFiso4E are ideal targets for regulation. In many organisms, eIF4E activity is modulated by eIF4E binding proteins (4E-BPs), which repress protein synthesis by sequestering eIF4E from the translation machinery [26, 27]. Conversely, C-terminal phosphorylation by MAP kinase-interacting kinases Mnk1 and Mnk2 promotes eIF4E activity and stimulates translation of selected mRNAs [28, 29]. As plants lack 4E-BPs and Mnk1/2 homologues, and the C-terminal phosphorylation sites are not present in eIF4E and eIFiso4E, plant cap-binding activities are likely regulated by other mechanisms. Arabidopsis proteins lipoxigenase 2 (Lox2) and basic transcription factor 3 (BTF3) have been shown to interact with eIF4E in vitro or in yeast two-hybrid experiments [30, 31], and more recently conserved binding of eIF4E1 (CBE1) was identified as a component of cap-binding complexes in vivo [32]. However, apart from the observation that CBE1-deficient plants accumulate high levels of mRNAs for proteins related to mitosis, the biological significance of these eIF4E interactions are unknown.
Here, we show that Arabidopsis SnRK1 phosphorylates eIFiso4E in vitro, and also interacts with eIF4E and eIFiso4E in a yeast two-hybrid system and in vivo. We also present evidence that eIFiso4E is phosphorylated by SnRK1 in vivo. Using a reconstructed system comprised of Arabidopsis proteins in yeast, we further demonstrate that SnRK1 phosphorylation of eIF4E and eIFiso4E inhibits translation. We also present evidence that SnRK1 inhibits translation in Nicotiana benthamiana plants, which can be complemented by over-expression of eIF4E or a mutant derivative containing non-phosphorylatable SnRK1 target residues, but not by one containing an aspartate residue at a target site (phosphomimic mutant). The results support our hypothesis that SnRK1 is directly involved in control of protein synthesis, and suggest a novel mechanism for translation regulation in response to stress.
Results
eIF4E and eIFiso4E contain two deeply conserved SnRK1 target sites
Translation initiation factors were scanned for a consensus target sequence (M/L/V/I/F-x-R/K/H-x-x-S/T-x-x-x-M/L/V/I/F) shared by SNF1/AMPK/SnRK1 [33]. We found that canonical Arabidopsis eIF4E and eIFiso4E each contain two instances of the SnRK1 consensus. Further investigation revealed that these sites are conserved throughout the plant kingdom and also in diverse organisms such as flies, worms, and yeast. However, while the consensus sequence remains relatively intact in vertebrates, the target serine/threonine residues are notably absent (Fig. 1A). The previously solved structure of Triticum aestivum (wheat) eIF4E was used to identify the locations of the potential SnRK1 phosphorylation sites [34]. Both are solvent exposed on the surface of the protein, and therefore accessible for modification (Fig. 1B).
Fig. 1.

Plant eIF4E and eIFiso4E contain two deeply conserved SnRK1 phosphorylation sites. (A) Partial alignment of canonical eIF4E and eIFiso4E from selected plants, fungi, invertebrates, and vertebrates reveals two consensus SNF1/AMPK/ SnRK1 sites. eIFiso4E is found only in higher plants. Phosphorylation target residues are highlighted in red. Notably, vertebrate lineages lack serine or threonine target residues, despite strong conservation of surrounding sequence. (B) Structure of wheat eIF4E with target threonine residues highlighted in yellow and green. A cap analogue structure is shown in black, blue, and red (modified from [34]).
Recombinant proteins were generated to test whether SnRK1 could phosphorylate the predicted residues in vitro. For these and many subsequent experiments, the kinase domain of the SnRK1.2 α-subunit (amino acids 1–342, hereafter SnRK1-KD) was used. The kinase domain is commonly employed in SNF1/AMPK/SnRK1 research due to its significantly increased activity and ease of expression compared to the full-length protein [35–37]. SnRK1-KD was expressed in N. benthamiana leaves as an N-terminal double hemagglutinin peptide, 6-histidine (HA2His6) fusion protein using a TMV-based TRBO vector [11, 38]. A catalytically inactive derivative, SnRK1-KD-K49R (SnRK1-KDKR), containing an arginine for lysine substitution in the ATP binding site, was similarly expressed [1, 39]. Arabidopsis eIFiso4E (At eIFiso4E) and Homo sapiens eIF4E (Hs eIF4E, which lacks SnRK1 target residues) were expressed in E. coli as 6-histidine, Xpress tag fusions (His6Xpress). Glutathione-S-transferase (GST, negative control) and GST-SAMS (positive control) were also expressed in E. coli. The SAMS peptide is an established target of SNF1/AMPK/SnRK1 [11, 40, 41].
SnRK1-KD displayed strong autophosphorylation activity in kinase assays. Additionally, SnRK1-KD, but not SnRK1-KDKR, phosphorylated At eIFiso4E and GST-SAMS. Neither SnRK1-KD nor SnRK1-KDKR phosphorylated GST or Hs eIF4E (Fig. 2A and 2B). The SnRK1-KD construct used in these experiments was derived from Arabidopsis SnRK1.2. However, we also found that the kinase domain of the Arabidopsis SnRK1.1 α-subunit has similar activity and phosphorylated GST-SAMS and At eIFiso4E, but not Hs eIF4E (Fig. 2C).
Fig. 2.
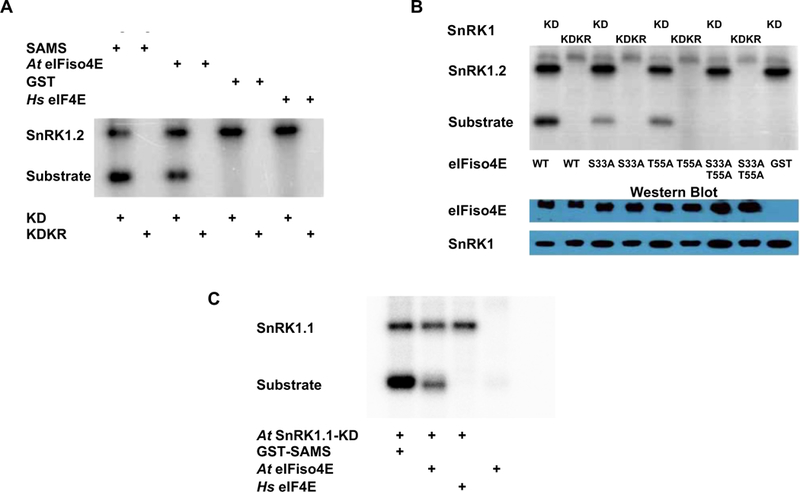
SnRK1 phosphorylates At eIFiso4E at serine 33 and threonine 55 in vitro. (A) Autoradiogram shows that the Arabidopsis SnRK1 kinase domain (KD, derived from SnRK1.2), but not inactive mutant SnRK-KDK49R (KDKR), is capable of autophosphorylation and phosphorylation of Arabidopsis eIFiso4E (At eIFiso4E) and positive control GST-SAMS, but not negative control proteins GST and human eIF4E (Hs eIF4E). HA2His6-tagged SnRK1-KD and SnRK1-KDKR were expressed in N. benthamiana, and the His6Xpress-tagged substrates At eIFiso4E and Hs eIF4E were expressed in E. coli. The indicated proteins were incubated together with γ32P-ATP, fractionated on SDS-containing polyacrylamide gels (SDS-PAGE), and exposed to a phosphor-imager to detect labeled proteins. (B) Autoradiogram shows that SnRK1-KD can phosphorylate wild type (WT) At eIFiso4E and single substitution mutants S33A and T55A, but not the double mutant S33A/T55A. Inactive SnRK1-KDKR and GST were included as negative controls. For loading controls, Western immunoblots were probed with anti-HA to detect the kinases, and anti-Xpress to detect At eIFiso4E and mutant derivatives. The autoradiogram shown is representative of three independent experiments, one of which included only the wild type and double mutant proteins. (C) Autoradiogram shows that SnRK1-KD derived from Arabidopsis SnRK1.1 is capable of autophosphorylation and also phosphorylates eIFiso4E and GST-SAMS, but not human eIF4E (Hs eIF4E).
To confirm that At eIFiso4E is phosphorylated at the predicted sites, the protein was modified so that one or both targets were changed to a non-phosphorylatable residue. When these proteins were used in the in vitro SnRK1 kinase assay, single mutants with either S33 or T55 substituted by alanine showed approximately half the phosphorylation signal of wild type protein. The double mutant with both S33 and T55 substituted by alanine showed no phosphorylation signal (Fig. 2B). Thus, SnRK1-KD phosphorylates At eIFiso4E at both predicted sites in vitro, and does not phosphorylate the protein elsewhere.
Multiple attempts were made to phosphorylate At eIF4E in vitro. The protein was expressed in both N. benthamiana and E. coli with a variety of different tags, including a GST tag that could be cleaved with thrombin to yield an untagged eIF4E. The kinase assay was also performed in the presence of methyl-7-GTP to simulate eIF4E cap binding. None of these resulted in in vitro phosphorylation of At eIF4E. Therefore, peptide fragments containing the individual phosphorylation sites and flanking sequence were expressed and tested. This resulted in phosphorylation of an At eIF4E peptide (amino acids 23–85) containing the first target site (T67), although a peptide (amino acids 73–139) harboring the second site (T91) still was not phosphorylated. The reasons for these technical difficulties remain unclear. Nevertheless, our in vivo experiments provide strong evidence that At eIF4E interacts with and is phosphorylated at the predicted sites by At SnRK1-KD and full-length At SnRK1 (see below).
SnRK1 interacts with eIF4E and eIFiso4E in yeast and plant cells
Standard assays were used to investigate potential interactions between At SnRK1 and At eIF4E and At eIFiso4E. We first tested interaction using a yeast two-hybrid system that relies on HIS3 and ADE2 reporter genes to detect interaction between test proteins fused to the β-galactosidase (GAL4) DNA binding domain (BD, bait) or the GAL4 activation domain (AD, prey). We found that the full-length SnRK1 α-subunit interacts with both eIF4E and eIFiso4E, allowing robust growth on synthetic complete media lacking histidine and adenine regardless of which protein served as bait or prey. Positive control interactions between SnRK1 and Tomato golden mosaic virus AL2 (TGMV AL2), previously shown to interact with SnRK1 [1], and known interaction partners Dicer-like 4 (DCL4) and double-stranded RNA binding protein 4 (DRB4) [42, 43], also promoted growth. However, DRB4 and DCL4 (negative controls) did not interact with SnRK1 (Fig. 3A).
Fig. 3.
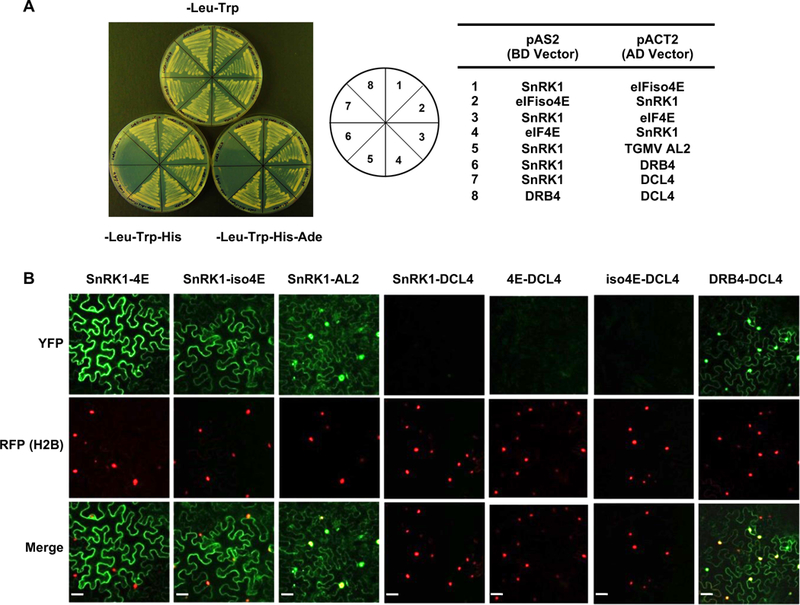
SnRK1 and eIF4E/iso4E interact in yeast and plant cells. (A) Yeast two-hybrid interaction. Full-length At SnRK1, At eIF4E, and At eIFiso4E were expressed as GAL4 DNA binding domain fusions (BD, bait) or GAL4 activation domain fusions (AD, prey) in PJ649A yeast cells. TGMV AL2 was included as a positive control, and interaction partners DRB4 and DCL4 were negative controls. Growth on media lacking leucine (-Leu) and tryptophan (-Trp) indicates maintenance of activation domain and binding domain plasmids, respectively. Interaction is indicated by growth on plates also lacking histidine (-His) or histidine and adenine (-His-Ade). (B) BiFC interaction. Constructs expressing full-length SnRK1 and eIF4E or eIFiso4E fused to the N- or C-terminal portions of YFP were delivered to N. benthamiana leaf cells by agroinfiltration. Cells were photographed 48 hours later using a confocal microscope at 40 X magnification. Representative images of epidermal cells, which have an irregular shape and a large central vacuole that restricts the cytoplasm to the cell periphery, are shown. RFP-histone H2B was a marker for the nucleus. TGMV AL2 and the interaction partners DCL4 and DRB4 were control proteins. Co-expressed proteins are indicated above the photographs. Scale bars = 100 μm.
Arabidopsis SnRK1 α-subunit is found in the nucleus and the cytoplasm [44]. eIF4E is also present in both compartments, although its relative abundance in the nucleus and cytoplasm depends on the stage of cell growth. eIFiso4E is mostly cytoplasmic, although it is also present in the nucleus [24, 45]. To determine whether and where interaction between these proteins might occur in vivo, bimolecular fluorescence complementation (BiFC) experiments were performed. Constructs designed to express test proteins fused to the N- or C-terminus of yellow fluorescent protein (YFP) were introduced into N. benthamiana leaves by agroinfiltration, and epidermal cells were viewed under a confocal microscope. DCL4 and DRB4, which interact in both the nucleus and the cytoplasm [43], served as controls, and histone H2B fused with red fluorescent protein (RFP) was a marker for the nucleus. TGMV AL2, present in both the nucleus and cytoplasm of infected cells [12], was an additional control. More specifically, in previous BiFC experiments AL2 was found to interact with itself (dimerize) in the nucleus and with ADK in the cytoplasm [46]. Test proteins were examined in all possible combinations before it was judged that a particular pair did not interact.
No signal was observed when full-length SnRK1, eIF4E, or eIFiso4E were co-expressed with DCL4 (negative control), whereas YFP fluorescence indicative of complex formation was seen in both the nucleus and the cytoplasm when DCL4 and DRB4 proteins were co-expressed, verifying that these control proteins were expressed and properly folded in these experiments (Fig. 3B). We also confirmed that AL2 and SnRK1 are present and showed for the first time that they interact in both the nucleus and cytoplasm. However, fluorescent signal indicative of interaction was confined to the cytoplasm in cells expressing full-length SnRK1 and eIF4E, or SnRK1 and eIFiso4E. We concluded that SnRK1 forms mainly cytoplasmic complexes with the cap-binding proteins in plant cells. Taken together, the results of yeast two-hybrid and BiFC experiments provide strong evidence for in vivo and in planta interaction between full-length SnRK1 and eIF4E/iso4E.
SnRK1 phosphorylates eIFiso4E in vivo
To determine whether interaction results in in vivo phosphorylation, eIFiso4E was transiently co-expressed in N. benthamiana leaves with SnRK1-KD or negative control EML1 (EMSY-like 1), a histone reader protein [47]. This was accomplished by infiltrating leaves with Agrobacterium cultures designed to deliver TRBO vectors containing HA2His6 tagged genes of interest. Immunoprecipitation was performed with extracts from infiltration zones using an antibody that binds phosphorylated serine, threonine, or tyrosine residues (phospho-antibody) to detect phosphorylated eIFiso4E.
Protein expression was verified by Western blot analysis with HA antibody, which showed that eIFiso4E accumulated to significantly higher levels than SnRK1-KD or EML1 (Fig. 4A). Phosphoproteins were immunoprecipitated (IP) from the extracts using phospho-antibody. At picogram level sensitivity (Fig. 4B), phosphorylated eIFiso4E was detected following co-expression with SnRK1-KD, but not with EML1. However, at femtogram level sensitivity (Fig. 4C), phosphorylated eIFiso4E was also detected following co-expression with EML. We speculate that this low-level phosphorylation is due to an endogenous kinase activity, likely SnRK1. Based on this evidence and BiFC data above (Fig. 3B), we conclude that SnRK1 both interacts with and phosphorylates eIFiso4E in plant cells.
Fig. 4.
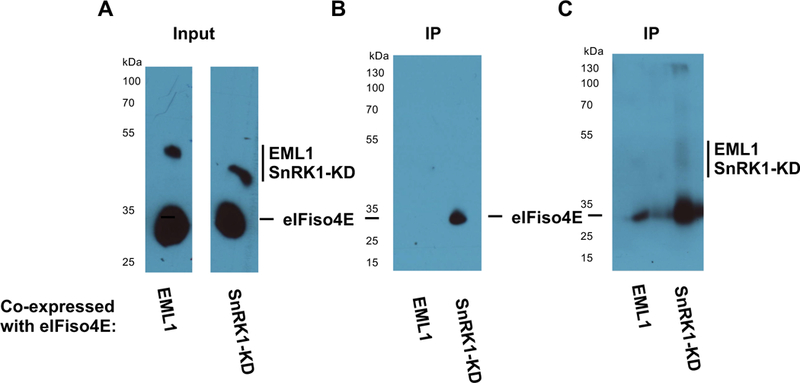
SnRK1 phosphorylates eIFiso4E in vivo.
(A) N. benthamiana leaves were infiltrated with agrobacterium cultures to co-express HA-His tagged eIFiso4E with similarly tagged SnRK1-KD or negative control protein EML1. Protein expression was verified by Western blot with HA antibody. (B) Phosphoproteins were immunoprecipitated from extracts using a phospho-antibody, and eIFiso4E was detected in immunoprecipitates with HA antibody. eIFiso4E was observed to immunoprecipitate with phospho-antibody when co-expressed with SnRK1-KD, but not with negative control protein EML1 when detection was performed using a picogram-level detection system. (C) The same blot shown in (B) was imaged using a femtogram-level detection system. Under these conditions, low-level phosphorylation of eIFiso4E was observed on co-expression with EML1, likely the result of endogenous kinase activity. Samples were loaded in every other lane, and signal in lanes flanking the eIFiso4E-SnRK1-KD is due to spill-over. The experiment shown is representative of three independent trials.
SnRK1-KD expression inhibits At eIF4E/iso4E complementation of an eIF4E-deficient yeast strain
To explore the functional consequences of eIF4E/iso4E phosphorylation, a complemented yeast system was established. In addition to rapid and facile genetic analysis, Saccharomyces cerevisiae offers several advantages over plant systems. Yeast has only one eIF4E gene [48, 49] and one SNF1 gene [50], and plant eIF4E/iso4E and SnRK1/SnRK1-KD and can complement loss of their yeast counterparts [1, 51]. In contrast, plants have both canonical eIF4E/iso4E genes as well as non-canonical versions, multiple SnRK1 genes, and a plethora of SnRK2 and SnRK3 genes, many of which are not well-characterized and thought to have somewhat overlapping functions [5, 25]. We reasoned that a yeast system would greatly simplify experiments and data interpretation.
To investigate our hypothesis that SnRK1 phosphorylation inhibits eIF4E/iso4E activity, we obtained a yeast strain containing a disrupted eIF4E gene (Jo56, cdc33Δ) in which to express Arabidopsis proteins. The viability of a modified cdc33Δ strain (Jo55) is maintained by a plasmid containing URA3 as a selectable marker and expressing human eIF4E (Hs eIF4E) from the glucose-repressible, galactose-inducible GAL promoter [52, 53]. Following transformation of Jo55 cells with plasmids expressing At eIF4E or At eIFiso4E, we observed that both could support growth on glucose media, confirming that the Arabidopsis proteins can complement yeast cdc33Δ. However, as reported previously [51], complementation with At eIF4E was more robust, as colonies were first observed after 3 days for cells expressing At eIF4E, and after 4–5 days with cells transformed with eIFiso4E (data not shown).
Strain Jo55 cells were next transformed with pRS403-based integrative vector plasmids harboring At eIF4E, At eIFiso4E, or Hs eIF4E driven by the constitutive glyceraldehyde-3-phosphate dehydrogenase (GPD) promoter. Subsequently, the original URA3 plasmid containing Hs eIF4E was evicted using 5-fluoroorotic acid (5-FOA), which is non-toxic to yeast unless converted to 5-fluorouracil by the URA3 gene product [54]. The resulting strains were designated Jo55-At4E, Jo55-iso4E, and Jo55-Hs4E. These were transformed with pRS414CUP1-based plasmids to express either SnRK1-KD or catalytically inactive SnRK1-KDKR from a copper-inducible CUP1 promoter, generating strains Jo55-At4E-KD, Jo55-At4E-KDKR, Jo55-iso4E-KD, Jo55-iso4E-KDKR, Jo55-Hs4E-KD, and Jo55-Hs4E-KDKR.
To assess the effect of SnRK1-KD on growth of these strains, yeast cells were grown in minimal media to an OD of 0.5, spotted in a dilution series, and allowed to grow at 30°C for 3 (eIF4E) or 4 (eIFiso4E) days. In the absence of copper, no difference in growth was observed between yeast containing plasmids harboring SnRK1-KD or SnRK1-KDKR. However, when the media was supplemented with 200 μM CuSO4 to induce SnRK1-KD expression, strains Jo55-At4E-KD and Jo55-iso4E-KD showed significant growth inhibition compared to Jo55-At4E-KDKR and Jo55-iso4E-KDKR, respectively. In contrast, copper induction had comparatively little impact on growth of Jo55-Hs4E-KD, which lacks SnRK1 target residues (Fig. 5A). These results suggest that SnRK1 phosphorylation of eIF4E/iso4E has an inhibitory effect on growth, likely through inhibition of eIF4E/iso4E function.
Fig. 5.
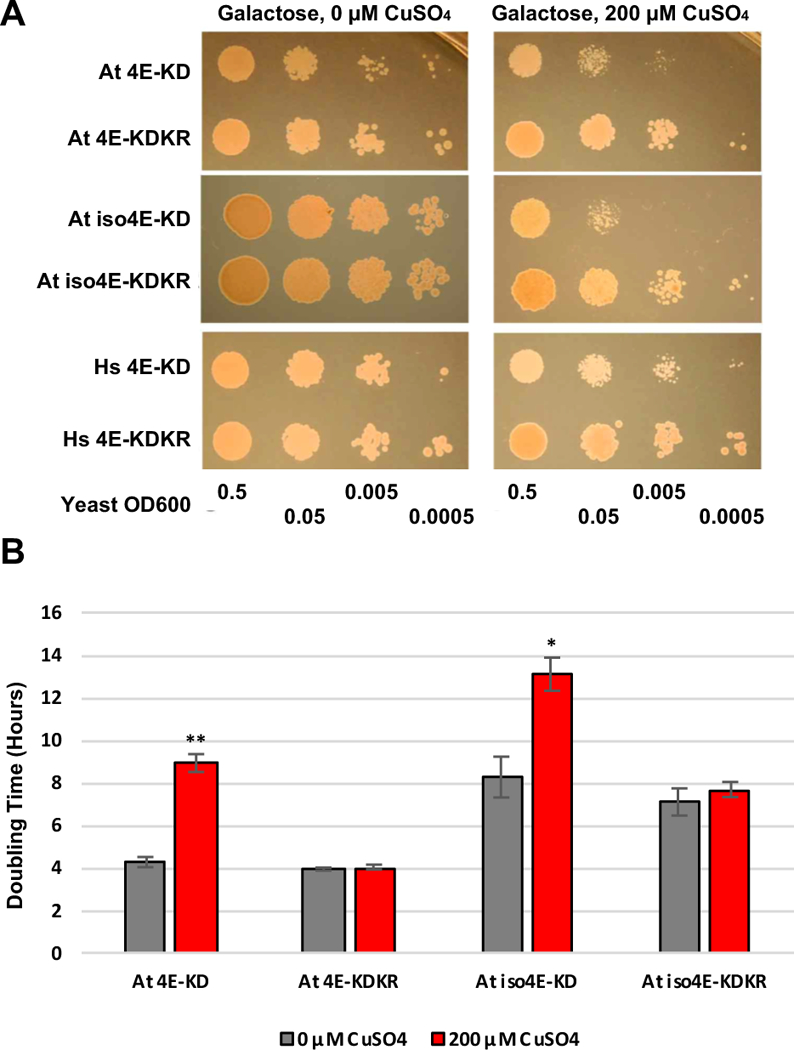
Yeast complementation by eIF4E/iso4E is inhibited by SnRK1-KD expression. (A) Yeast strains lacking endogenous eIF4E (cdc33Δ) and complemented with At eIF4E, At iso4E, or Hs eIF4E were transformed with plasmids to express SnRK1-KD or inactive SnRK1-KD-K49R (KDKR) from a copper inducible promoter. Cells were spotted in a dilution series on minimal media containing galactose or minimal media containing galactose and 200 μM CuSO4. (B) The doubling times of yeast strains expressing At 4E or At iso4E and either SnRK1-KD (At4E-KD) or inactive SnRK1-KDKR (At4E-KDKR) were determined in liquid culture in the presence and absence of copper. Data were compiled from four independent experiments with At 4E and three with At iso4E. Bars indicate standard error of the mean. Asterisk indicates differences in growth rates between copper induced and uninduced cells of the same cell line are significant at the p < 0.05 level, while double asterisk indicates significance at the p < 0.01 level.
To quantitate the effect on cells complemented with At eIF4E and At eIFiso4E, growth rates were monitored in liquid culture by optical density and doubling time was calculated from the resulting growth curves. We observed that the doubling time of Jo55-At4E-KD cells increased from an average of 4.27 ± 0.24 hours without copper to an average of 8.93 ± 0.42 hours upon SnRK1-KD induction by 200 μM CuSO4 (> 2-fold change). In comparison, the average doubling time of Jo55-At4E-KDKR cells was essentially unaffected, increasing from 3.94 ± 0.07 hours to only 4.03 ± 0.12 hours in the presence of copper (Fig. 5B). Similarly, Jo55-Atiso4E-KD cells increased from an average of 8.27 ± 0.95 hours to 13.10 ± 0.78 hours upon SnRK1-KD induction, while Jo55-Atiso4E-KDKR cells were essentially unaffected, increasing from 7.10 ± 0.64 hours to 7.68 ± 0.35 hours (Fig. 5B). The difference in doubling time between Jo55-At4E-KD when SnRK1-KD was expressed (when CuSO4 was present) and when it was not was highly significant (p = 7.21 × 10−5), as determined by two-tailed, two-sample Student’s t-test. Jo55-Atiso4E-KD also showed a statistically significant reduction in growth rate (p = 0.0174). Neither Jo55-At4E-KDKR nor Jo55-Atiso4E-KDKR showed a significant change in growth upon SnRK1-KDKR induction. We concluded that expression of SnRK1-KD results in a significant reduction in the growth of yeast cdc33Δ cells complemented by Arabidopsis eIF4E or eIFiso4E.
Identification of a non-phosphorylatable At eIF4E mutant to complement eIF4E-deficient yeast
Non-phosphorylatable eIF4E mutants are compelling controls to link phenotypes observed upon SnRK1 expression to SnRK1 phosphorylation sites. While previous experiments employed Hs eIF4E for this purpose, a non-phosphorylatable At eIF4E was desired. Unfortunately, we found that a double alanine mutant (At eIF4E T67A/T91A) was unable to efficiently complement Jo55 cdc33Δ cells due to the T67A substitution (Fig. 6). Thus, we set out to find a suitable At eIF4E derivative. Mutagenesis primers were constructed that contained degenerate bases at the T67 codon, allowing any amino acid except serine or threonine at this position. Site-directed mutagenesis on an eIF4E T67A substrate generated a library of T67 mutants, which were transformed into Jo55 cdc33Δ cells and selected for efficient complementation. This strategy identified non-phosphorylatable At eIF4E-T67V/T91A (hereafter At eIF4E-VA) (Fig. 6), and this mutant was included as a control in subsequent experiments.
Fig. 6.
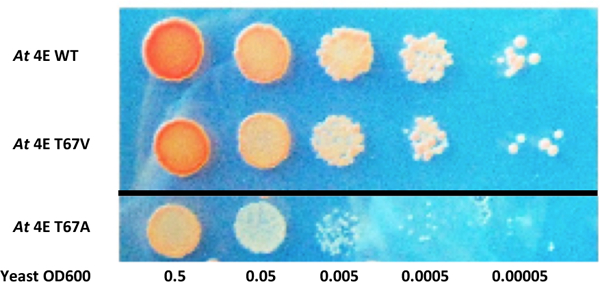
eIF4E-VA complements a yeast eIF4E null mutant. Spot plate assay shows that Jo55 cdc33Δ cells lacking endogenous eIF4E are only weakly complemented by Arabidopsis eIF4E containing an alanine substitution at the first SnRK1 target site (At eIF4E T67A). Complementation by eIF4E with valine at this site (At eIF4E T67V) is similar to wild type protein. The dark line indicates the experiment was done simultaneously across two separate plates due to space constraints. The plates contained the same nutrients and were exposed to the same environmental conditions. Agar appears blue due to lighting conditions.
SnRK1-KD phosphorylation of eIF4E/iso4E inhibits yeast translation
To directly link SnRK1 phosphorylation of eIF4E/iso4E to translation inhibition, we turned to polysome profiling. Under conditions that allow protein synthesis, multiple ribosomes typically accumulate on each mRNA transcript, forming polysomes. However, as initiation is inhibited, fewer ribosomes associate with each transcript, and 80S monosomes as well as 40S and 60S ribosomal subunits accumulate [52, 55, 56]. Therefore, the relative abundance of ribosomes in each state (the polysome:non-polysome ratio, P:NP) can be used as an indicator of the cellular translation status.
Polysome profiling experiments employed complemented Jo55 cdc33Δ yeast strains Jo55-At4E-KD, Jo55-At4E-KDKR, Jo55-iso4E-KD, Jo55-iso4E-KDKR, Jo55-At4E-VA-KD, and Jo55-Hs4E-KD. Cells were cultured in the presence of CuSO4 to induce SnRK1-KD or SnRK1-KDKR expression. After 8 to 12 hours of induction, cell extracts were fractionated on sucrose density gradients. We observed that in cells complemented with wild type At eIF4E or At eIFiso4E, SnRK1-KD induction caused dramatic reductions in P:NP ratio of 50.4% and 57.6%, respectively (Fig. 7 and Table 1). Induction of SnRK1-KDKR, on the other hand, resulted in more modest reductions of 17.3% in cells complemented with At eIF4E and 34.7% in cells complemented with eIFiso4E. In cells complemented with At eIF4E-VA or Hs eIF4E (neither of which have SnRK1 phosphorylation sites), SnRK1-KD expression had minimal impact on polysome formation, with only 6.2% and 18.6% reductions in P:NP ratio, respectively. A two-sample Student’s t-test revealed that the difference in P:NP ratio between induced and uninduced Jo55-At4E-KD was statistically significant at the p < 0.01 level, while differences for Jo55-At4E-KDKR, Jo55-At4E-VA-KD, and Jo55-Hs4E-KD were not statistically significant. Due to the difficulties arising from the slow growth rate of At eIFiso4E complemented yeast, only one experiment was performed with Jo55-iso4E-KD and Jo55-iso4E-KDKR.
Fig. 7.
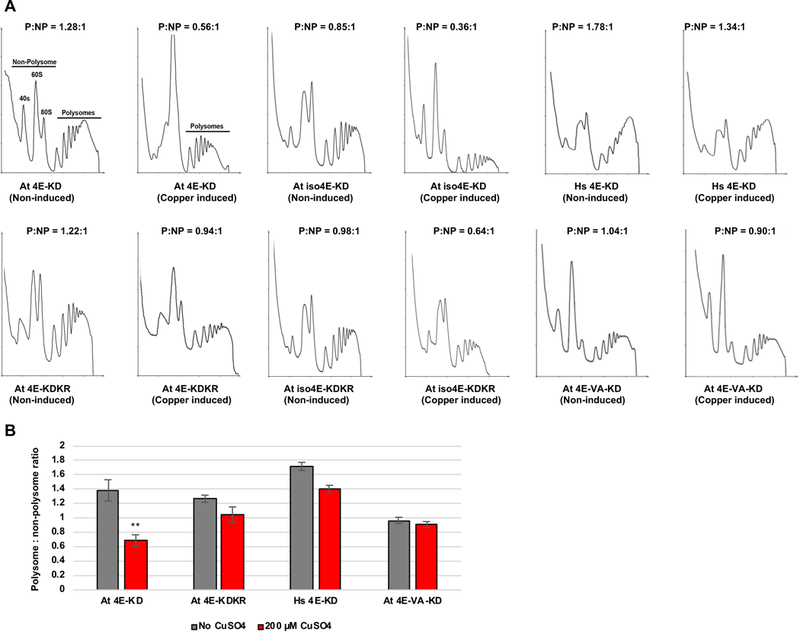
SnRK1-KD expression inhibits polysome formation in complemented yeast cells in a phosphorylation site-dependent manner. (A) The indicated Jo55 cdc33Δ-based yeast strains lacking endogenous eIF4E were incubated in the absence or presence of 200 μM CuSO4 to induce SnRK1-KD or SnRK1-KDKR (control) expression, and cell extracts were analyzed on sucrose density gradients to obtain polysome profiles. A polysome:non-polysome ratio (P:NP) was calculated for each sample. Representative traces are shown. Dramatic reductions in P:NP ratio were observed following SnRK1-KD induction in complemented strains At 4E-KD and At iso4E-KD, but not At 4E-VA-KD or Hs 4E-KD. (B) Histogram shows average P:NP ratios for At 4E-KD and At 4E-KDKR, Hs 4E-KD, and At4E-VA-KD obtained with and without copper induction. Error bars represent standard error of the mean. Asterisks indicate the difference between uninduced and copper induced At 4E-KD samples is significant at the p < 0.01 level, as determined by Student’s t-test.
Table 1.
SnRK1-KD expression inhibits polysome formation. Indicated strains were grown in the absence or presence of CuSO4 to induce kinase expression, and polysome profiles obtained and quantified as described in Fig. 7. Average polysome:non-polysome ratios (P:NP) are displayed along with standard error of the mean (SEM), number of independent replicates (n), and % reduction in P:NP ratio between the uninduced and induced samples.
| Strain | Copper Induction |
P:NP Ratio | SEM | n | % Reduction |
|---|---|---|---|---|---|
| Jo55-At 4E-KD | − | 1.39 | 0.15 | 6 | 50.38 |
| Jo55-At 4E-KD | + | 0.69 | 0.08 | 5 | |
| Jo55-At 4E-KDKR | − | 1.27 | 0.05 | 2 | 17.32 |
| Jo55-At 4E-KDKR | + | 1.05 | 0.11 | 2 | |
| Jo55-Hs 4E-KD | − | 1.72 | 0.06 | 2 | 18.60 |
| Jo55-Hs 4E-KD | + | 1.40 | 0.06 | 2 | |
| Jo55-At 4E-VA-KD | − | 0.97 | 0.04 | 2 | 6.19 |
| Jo55-At 4E-VA-KD | + | 0.91 | 0.04 | 2 | |
| Jo55-At iso4E-KD | − | 0.85 | N/A | 1 | 57.65 |
| Jo55-At iso4E-KD | + | 0.36 | N/A | 1 | |
| Jo55-At iso4E-KDKR | − | 0.98 | N/A | 1 | 34.69 |
| Jo55-At iso4E-KDKR | + | 0.64 | N/A | 1 |
Indicated strains were grown in the absence or presence of CuSO4 to induce kinase expression, and polysome profiles obtained and quantified as described in Fig. 7. Average polysome:non-polysome ratios (P:NP) are displayed along with standard error of the mean (SEM), number of independent replicates (n), and % reduction in P:NP ratio between the uninduced and induced samples.
Because SnRK1 functions in a variety of pathways, it is perhaps not surprising that expression of this kinase in inactive form, which may retain the ability to interact with substrates, inhibits translation to some degree whether or not the complementing eIF4E/iso4E is a SnRK1 phosphorylation target. However, dramatically decreased polysome accumulation following SnRK1-KD expression in cells complemented by At eIF4E and At eIFiso4E, but not SnRK1-insensitive Hs eIF4E or At eIF4E-VA, clearly links phosphorylation of eIF4E/iso4E to translation inhibition.
SnRK1 inhibits translation in plant cells
We next asked whether SnRK1 expression also inhibits translation in planta. Because polysome profiling in plants is technically challenging, we adopted a simplified 10%/35% sucrose step-gradient approach to separate polysomes from non-polysomes. Following ultracentrifugation of cell extracts, polysomes pellet at the bottom of the tube, while monosomes, free ribosomes, ribosomal subunits, and other ribonucleoproteins collect at the interface between the two layers [57–59]. RNA from these locations was purified and the amount of 18S ribosomal RNA (rRNA) measured by quantitative reverse transcription PCR (RT-qPCR). As in yeast polysome experiments, a reduction in polysome:non-polysome ratio indicates inhibition of translation initiation. That these gradients separate polysomes from smaller complexes is apparent from the near absence of 18S rRNA from the pellet fraction in gradients containing EDTA, which disrupts polysomes. However, 18S rRNA remains detectable at the interface where ribosome components are expected to accumulate, hence the P:NP ratio is reduced to near zero (Fig. 8A).
Fig. 8.
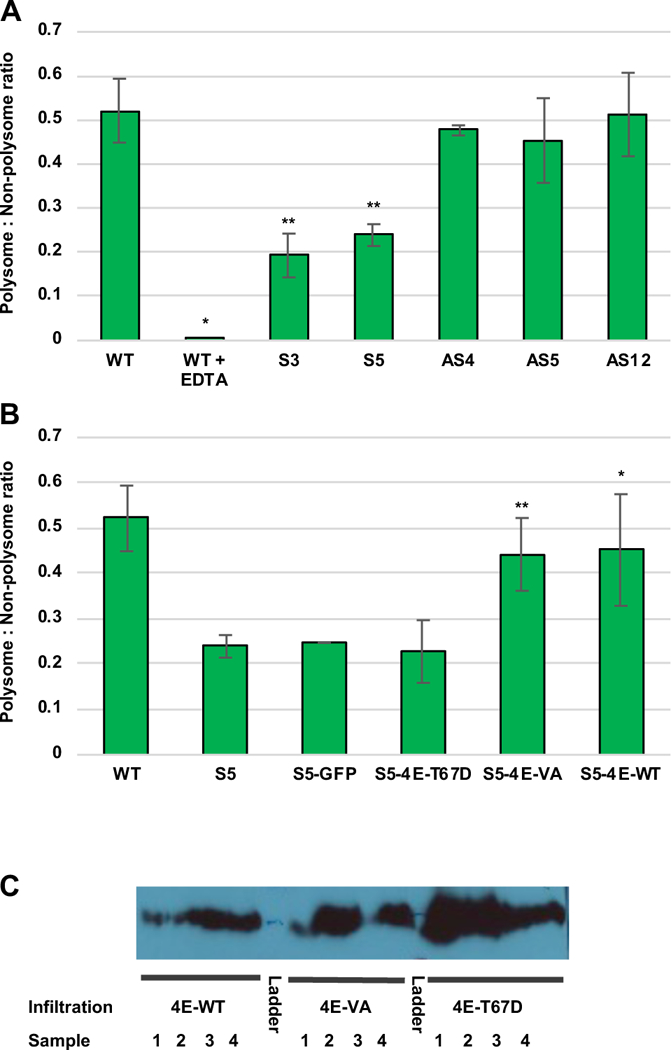
Transgenic expression of SnRK1 depletes polysomes in N. benthamiana cells. (A) Extracts from wild type plants (n = 26) and transgenic lines expressing full-length Arabidopsis SnRK1 in sense (S3, n = 11; S5, n = 20) or antisense (AS4, n = 2; AS5, n = 3; AS12, n = 9) orientation were analyzed on 10%/35% sucrose step gradients to obtain polysome:non-polysome (P:NP) ratios, as determined from 18S rRNA levels in the pellet (polysome) and interface (non-polysome) fractions. 18S rRNA levels were determined by RT-qPCR. Negative control samples from wild type plants (n = 3) contained EDTA (50 mM) to disrupt polysomes. Error bars represent standard error of the mean. Double asterisks indicate differences between wild type plants and SnRK1 over-expression lines S3 and S5 are significant at the p < 0.01 level (p = 0.0078 and 0.0019, respectively), while the single asterisk indicates that differences between EDTA treated wild type samples and those not treated with EDTA were significant at the p < 0.05 level (p = 0.0234). (B) Leaves of transgenic S5 plants were agroinfiltrated with cultures to express At 4E (wild type, n = 3), At4E-VA (n = 9), At-4E T67D (phosphomimetic mutant, n = 3) or GFP (negative control, n = 2), and extracts from infiltration zones were used to determine P:NP ratios. Data for N. benthamiana wild type and S5 plants is from (A). Bars indicate standard error of the mean. Double asterisks indicate differences between 4E-VA and line S5 were significantly different at the p < 0.01 level (p = 0.004), while the single asterisk indicates that differences between 4E-WT samples and line S5 were significant at the p < 0.05 level (p = 0.011). (C). Representative immunoblot using HA antibody confirms expression of At 4E, At 4E-VA, and At 4E-T67D in infiltration zones.
The discontinuous gradient technique was applied to extracts from wild type N. benthamiana plants as well as previously characterized lines harboring transgenes to augment or reduce SnRK1 expression [1]. Specifically, the transgenic plants contained the full-length Arabidopsis SnRK1 α-subunit transcribed from the constitutive CaMV 35S promoter in either sense (over-expression) or antisense (knock-down) orientation. Transgene expression in each line was verified by RNA blot analysis. The SnRK1 overexpression lines display a slow growth phenotype and are smaller than nontransgenic plants, however, they exhibit resistance to geminiviruses, such that much greater inoculum doses are required to elicit an infection. Conversely, SnRK1 knock-down lines are morphologically normal but are hypersensitive to geminivirus infection, as evidenced by a more rapid onset of disease symptoms compared to nontransgenic plants [1]. Extracts from over-expression (sense) lines S3 and S5 were subsequently shown to have SnRK1 activities >1.5-fold and >2.5-fold greater than nontransgenic plants, respectively, as measured by phosphorylation of test substrate GST-SAMS. In knock-down (antisense) lines AS-4 and AS-12, SnRK1 activity is reduced ~50% [11].
In extracts from wild type N. benthamiana, the amount of 18S rRNA recovered in the polysome fraction was approximately half that detected in the non-polysome fraction (average P:NP ratio = 0.521 ± 0.072). In control experiments which included EDTA to disrupt polysomes, the ratio was drastically reduced (P:NP = 0.0016 ± 0.0002). However, in independent SnRK1 over-expressing lines S3 and S5, the average P:NP ratio was observed to decrease to 0.193 ± 0.050 and 0.239 ± 0.025, respectively. Analysis of SnRK1 knock-down N. benthamiana lines revealed P:NP ratios that were not significantly different from wild type plants: 0.477 ± 0.011, 0.454 ± 0.096, and 0.512 ± 0.095 for antisense lines AS4, AS5, and AS12, respectively (Fig. 8A). Possibly the reduction of SnRK1 activity in these lines was insufficient to alter P:NP ratios. In contrast, two-sample Student’s t-test comparing the P:NP ratios of SnRK1 over-expressing lines to wild type N. benthamiana plants yielded highly significant p values of 0.0079 (S3) and 0.0019 (S5). Thus, SnRK1 overexpression inhibits translation in N. benthamiana plants under standard growth conditions.
To link reduced translation caused by SnRK1 over-expression to eIF4E, we investigated the effect of transiently overexpressing eIF4E in transgenic line S5. This was accomplished by infiltrating S5 leaves with agrobacterium cultures to deliver TMV-based expression vectors (TRBO) containing HA2His6-tagged genes of interest. Cell extracts were prepared from infiltration zones and subjected to step gradient polysome analysis as described above (Fig. 8B). Western immunoblot analysis using HA antibody confirmed transient expression of infiltrated genes (Fig. 8C). Expression of GFP (negative control) had no discernable effect on P:NP ratio. Remarkably, however, expression of wild type At eIF4E or non-phosphorylatable At eIF4E-VA increased the P:NP ratio, restoring it to near wild type levels (0.452 ± 0.123 and 0.442 ± 0.080, respectively). Two sample Student’s t-test showed these changes were significant, with p values of 0.011 for At eIF4E-WT and 0.004 for At eIF4E-VA when compared with line S5. Notably, expression of phosphomimic At eIF4E-T67D had no impact on polysome formation, consistent with T67 being a SnRK1 target (Fig. 8B). Thus, the negative effect of SnRK1 overexpression on polysome formation can be rescued by augmenting eIF4E levels (as wild type eIF4E or SnRK1-insensitive eIF4E-VA), likely overwhelming the ability of SnRK1 to suppress translation. Further, the inability of phosphomimic eIF4E-T67D to complement links reduced polysome accumulation to SnRK1 phosphorylation activity.
Discussion
In mammalian systems, mechanisms that regulate translation are reasonably well understood. However, apart from the fact that regulation occurs differently in plants, comparatively little is known about the mechanisms by which it is controlled. In this study, we present evidence that the energy sensing kinase SnRK1, a homologue of mammalian AMPK and yeast SNF1, can inhibit translation by phosphorylating the cap binding proteins eIF4E and eIFiso4E, suggesting a novel pathway for translational control in plants.
We observed that eIF4E/iso4E and homologues from a variety of non-vertebrate species contain two SNF1/AMPK/SnRK1 consensus phosphorylation sites, and that Arabidopsis SnRK1 kinase domain can phosphorylate these sites within At eIFiso4E in vitro. Yeast two-hybrid and BiFC assays confirmed that full-length SnRK1 interacts with both At eIF4E and At eIFiso4E in yeast and predominantly in the cytoplasm of plant cells, and phosphorylation of At eIFiso4E by SnRK1-KD was also demonstrated in vivo. Using a reconstructed system based on a yeast strain lacking eIF4E (cdc33Δ), we found that SnRK1-KD expression inhibited growth more strongly in cells complemented with At eIF4E or At eIFiso4E than in those expressing non-phosphorylatable Hs eIF4E, lending support to our hypothesis that SnRK1 phosphorylation is inhibitory. Further support was obtained by polysome profiling, which demonstrated that SnRK1-KD expression dramatically inhibits translation in yeast cdc33Δ cells complemented with At eIF4E or eIFiso4E, but has comparatively little effect on translation in cells complemented with At eIF4E-VA or Hs eIF4E, which lack SnRK1 target sites. Finally, we demonstrated that SnRK1 over-expression in transgenic N. benthamiana plants also significantly reduces polysome formation, and that translation is restored to near normal levels by transient expression of At eIF4E or At eIF4E-VA, but not phosphomimic At eIF4E-T67D. Thus, we conclude that much of the reduction in translation observed in N. benthamiana is due to SnRK1 phosphorylation of eIF4E and eIFiso4E. SnRK1 has been shown to control a wide variety of cellular processes, but to our knowledge this is the first evidence that this kinase can directly inhibit translation, providing a new mechanism for plant response to stress.
The majority of global translation regulation occurs at the initiation step. Vertebrates are known to regulate translation under a variety of conditions through inhibitory phosphorylation of eIF2α. Vertebrates also have eIF4E binding proteins (4E-BPs) that prevent eIF4E from forming functional complexes with eIF4G [27]. Hence it is not surprising to find eIF4E at the center of a plant translational regulatory mechanism. Since 4E-BPs have not been found in plants, we propose that direct phosphorylation of eIF4E by SnRK1 may serve a similar purpose. In addition to 4E-BPs, eIF4E is regulated by phosphorylation at a C-terminal serine (S209 in humans) by MAP-kinase interacting kinases Mnk1 and Mnk2 [28, 29]. The functional consequences of this remain somewhat controversial, but this post-translational mark correlates with increased translation of selected proteins. Plant eIF4E and eIFiso4E lack both an equivalent S209 to serve as a Mnk target, as well as the eIF4G C-terminal motif needed for Mnk recruitment. However, an S209 equivalent residue appears to be conserved in most animals, leaving non-vertebrates as an interesting edge case in which there is potential for both AMPK and Mnk phosphorylation of eIF4E (Fig. 1).
In a recent phosphoproteomics study [44], Arabidopsis plants lacking both SnRK1 α-subunits due to inducible expression of artificial miRNAs targeting SnRK1.1 and 1.2 were shown to have increased phosphorylation of eukaryotic initiation factor 5A (eIF5A). Interestingly, eIF5A functions in both translation initiation and elongation, although the exact mechanisms are unclear [60]. The functional significance of this phosphorylation event is unknown. It was also found that ribosomal protein S6 (RPS6) had significantly greater phosphorylation in the SnRK1 α-subunit double mutants than in wild type plants, and showed decreased phosphorylation levels in SnRK1 over-expressing plants [44]. RPS6 phosphorylation is known to correlate with TOR activity [61, 62]. Additionally, SnRK1 was shown to interact with and phosphorylate Arabidopsis Raptor [44]. This is likely analogous to AMPK phosphorylation of mammalian Raptor, a key component of the growth stimulating mTORC1 (mammalian target of rapamycin complex 1), which leads to complex inhibition and engages a metabolic checkpoint [63]. Taken together, this data indicates indirect control of translation by SnRK1, most likely through modulation of the TOR kinase cascade [64]. However, our study is the first to show a direct link from SnRK1 to translation initiation.
Other recent phosphoproteomics studies reported that Arabidopsis eIFiso4G1 and eIFiso4G2 are phosphorylated by SnRK1.1 in response to submergence stress [65, 66]. eIF4G and eIFiso4G are major scaffolding proteins critical to translation initiation, and are direct interaction partners of eIF4E and eIFiso4E. Interestingly, phosphorylation of eIFiso4G1 affects hypoxia tolerance by enhancing translation of specific mRNAs. However, phosphorylation does not appear to impact global translation levels, and may be restricted to Brassicaceae. The same study did not observe changes in eIF4E and eIFiso4E phosphorylation levels in response to submergence either in the presence or absence of SnRK1.1 [65]. This may be due to a limitation of the methods employed (eIF4E/iso4E peptides were not reported), or it may indicate that SnRK1 phosphorylation of eIF4E/iso4E is specific to biotic stressors. It is also possible that phosphorylation of eIF4E/iso4E leads to degradation, which has been observed for other SnRK1 targets [67–69].
At SnRK1.1 and SnRK1.2 share 80% overall amino acid identity, with 88% identity in the kinase domain. However, they are very differently regulated. SnRK1.1 is broadly expressed whereas SnRK1.2 expression is more limited and tissue restricted under normal conditions [18, 70]. However, SnRK1.2 expression is significantly induced by trehalose [70], which is thought to be an inducer of both biotic and abiotic plant stress responses [71]. SnRK1.2 transcript levels are also increased by geminivirus infection (G. Sunter, unpublished observations). Therefore, we speculate that SnRK1.2 is specialized to respond to stress, and its functions may include mounting an antiviral defense by inhibiting initiation of translation.
Although our data indicate that phosphorylation of eIF4E/iso4E by SnRK1 impairs translation, the mechanism of inhibition is not known. Binding between eIF4E and the eIF4G scaffolding protein is a critical step in translation initiation. The recently reported structure of Cucumis melo (muskmelon) eIF4E in complex with a cognate eIF4G peptide shows that the binding interaction is bipartite, with eIF4G engaging the dorsal surface of eIF4E through a “canonical” (C) binding motif (YXXXXL, where is hydrophobic) and the lateral surface via a “non-canonical” (NC) motif consisting of a series of hydrophobic residues [72]. Similar bipartite binding occurs between metazoan 4E-BPs and Drosophila eIF4E-eIF4G [73–75]. The SnRK1 α-subunit lacks the C motif characteristic of many eIF4E binding proteins, but hydrophobic patches resembling the NC motif are present in the N-terminal, C-terminal, and central regions. Interestingly, SnRK1 target sites lie within the lateral surface of eIF4E that is engaged by the NC motif [72], suggesting that phosphorylation might disrupt eIF4E-eIF4G interaction. This possibility is currently under investigation.
The lateral surface is also interesting in light of interaction between eIF4E and VPg proteins that substitute for the cap structure at the 5’ end of potyvirus RNAs. Certain mutations in eIF4E disrupt the interaction and render plants resistant to potyviruses [76, 77]. In non-susceptible eIF4E alleles, one cluster of mutations lies within the lateral surface of the protein. Further, VPg exemplifies a class of eIF4E binding proteins that apparently lack a C motif but appear to contain a hydrophobic NC motif [72].
To put our studies in context, we previously found that SnRK1 conditions an innate antiviral defense [1], and that SnRK1 and ADK interact in a manner that stimulates ADK activity [11]. ADK phosphorylates adenosine to generate AMP, which is implicated in maintaining SnRK1 activity. Additionally, geminivirus pathogenicity proteins AL2 and L2 inhibit both SnRK1 and ADK [1, 12], indicating a defense-counterdefense interaction. Here, we show that SnRK1 phosphorylation of eIF4E and eIFiso4E inhibits translation, demonstrating a novel mechanism for direct control of translation initiation. We further speculate that eIF4E/iso4E phosphorylation is a component of SnRK1-mediated antiviral defense.
Materials and methods
Gene cloning
The SnRK1.2 cDNA has been previously described [1], and SnRK1.1 cDNA was obtained from Arabidopsis thaliana ecotype Col-0 by RT-PCR. At eIF4E (U12635) and At eIFiso4E (U16070) cDNAs were obtained from the Arabidopsis Biological Resource Center (ABRC) at The Ohio State University. Primers to amplify SnRK1.1, SnRK1.2, eIF4E, and eIFiso4E cDNA added a PacI site at the 5’ end and AscI site to the 3’ end. PCR products were subsequently digested with PacI and AscI and ligated into a pUC18 plasmid. The cDNAs were then subcloned into various vectors, noted below, using PacI and AscI (New England Biolabs, Ipswitch, MA, USA). Site-directed mutagenesis was performed to generate the mutants At eIF4E T67A, T91A, and T67D, and mutants At eIFiso4E S33A and T55A. The T67V mutant was created using degenerate primers to generate all possible sense codons at this position except serine and threonine. Primers used in this study are available on request.
Yeast strain construction
Yeast strains used in this study are listed in Table 2. Methods used to construct S. cerevisiae strains based on Jo55 (cdc33Δ) complemented by Arabidopsis eIF4E/iso4E and mutant derivatives, or human eIF4E, are outlined in Results. Yeast transformation was performed using the Frozen-EZ yeast transformation kit (Zymo Research, Irvine, CA, USA). Strains were grown on synthetic defined media to maintain plasmids.
Table 2.
Yeast strains used in this study.
| Yeast Strain | Genetic Background | Plasmid 1 | Plasmid 2 |
|---|---|---|---|
| Jo56 | cdc33: :LEU2 his3 ura3 trp1 ade2 | pYCpTrp-hu4E, TRP1 | n/a |
| Jo55.5 | cdc33: :LEU2 his3 ura3 trp1 ade2 | pYCpTrp-hu4E, TRP1 | pYCpSupex-hu4E, URA3 |
| Jo55 | cdc33: :LEU2 his3 ura3 trp1 ade2 | n/a | pYCpSupex-hu4E, URA3 |
| Jo55.5-At4E | cdc33: :LEU2 his3 ura3 trp1 ade2 | pRS403GPD-At4E, HIS3 | pYCpSupex-hu4E, URA3 |
| Jo55.5-iso4E | cdc33: :LEU2 his3 ura3 trp1 ade2 | pRS403GPD-iso4E, HIS3 | pYCpSupex-hu4E, URA3 |
| Jo55.5-At4E-VA | cdc33: :LEU2 his3 ura3 trp1 ade2 | pRS403GPD-At4E-VA, HIS3 | pYCpSupex-hu4E, URA3 |
| Jo55.5-hu4E | cdc33: :LEU2 his3 ura3 trp1 ade2 | pRS403GPD-hu4E, HIS3 | pYCpSupex-hu4E, URA3 |
| Jo55-At4E | cdc33: :LEU2 his3 ura3 trp1 ade2 | pRS403GPD-At4E,HIS3 | n/a |
| Jo55-iso4E | cdc33: :LEU2 his3 ura3 trp1 ade2 | pRS403GPD-iso4E, HIS3 | n/a |
| Jo55-At4E-VA | cdc33: :LEU2 his3 ura3 trp1 ade2 | pRS403GPD-At4E-VA,HIS3 | n/a |
| Jo55-Hs4E | cdc33: :LEU2 his3 ura3 trp1 ade2 | pRS403GPD-hu4E, HIS3 | n/a |
| Jo55-At4E-KD | cdc33: :LEU2 his3 ura3 trp1 ade2 | pRS403GPD-At4E,HIS3 | pRS414CUP1-KD, TRP1 |
| Jo55-At4E-KD-K49R | cdc33: :LEU2 his3 ura3 trp1 ade2 | pRS403GPD-At4E,HIS3 | pRS414CUP1-KDKR, TRP1 |
| Jo55-iso4E-KD | cdc33: :LEU2 his3 ura3 trp1 ade2 | pRS403GPD-iso4E, HIS3 | pRS414CUP1-KD, TRP1 |
| Jo55-iso4E-KD-K49R | cdc33: :LEU2 his3 ura3 trp1 ade2 | pRS403GPD-iso4E, HIS3 | pRS414CUP1-KDKR, TRP1 |
| Jo55-At4E-VA-KD | cdc33: :LEU2 his3 ura3 trp1 ade2 | pRS403GPD-At4E-VA,HIS3 | pRS414CUP1-KD, TRP1 |
| Jo55-At4E-VA-KDKR | cdc33: :LEU2 his3 ura3 trp1 ade2 | pRS403GPD-At4E-VA,HIS3 | pRS414CUP1-KDKR, TRP1 |
| Jo55-Hs4E-KD | cdc33: :LEU2 his3 ura3 trp1 ade2 | pRS403GPD-hu4E, HIS3 | pRS414CUP1-KD, TRP1 |
| Jo55-Hs4E-KD-K49R | cdc33: :LEU2 his3 ura3 trp1 ade2 | pRS403GPD-hu4E, HIS3 | pRS414CUP1-KDKR, TRP1 |
| snf1Δ APY192 | snf1::LEU2 ade2 can1 his3 leu2 trp1 ura3 | n/a | n/a |
| cdc33-E72G (ts) | his3 ura3 leu2 met1 | n/a | n/a |
Protein expression in N. benthamiana
SnRK1-KD and SnRK1-KD-K49R (SnRK1-KDKR) were expressed in N. benthamiana leaves and purified as previously described [11]. Briefly, cDNAs were cloned into the pJL50 TRBO vector, a TMV based expression vector [38], modified to generate N-terminal double hemagglutinin peptide-six-histidine (HA2His6) fusion proteins. Plasmids were transformed into Agrobacterium tumefaciens C58C1, and cultures used to infiltrate N. benthamiana leaves as described [78]. Infiltrated leaf tissue was collected ~5 days post-infiltration and proteins were extracted and partially purified using nickel nitrilotriacetic acid agarose columns (Ni-NTA agarose) (Invitrogen, Waltham, MA, USA) [11]. Protein concentrations were estimated using the Bradford assay (Bio-Rad, Hercules, CA, USA), and by comparison with BSA standards on SDS-polyacrylamide gels stained using Coomassie brilliant blue.
Protein expression in E. coli
GST-SAMS was expressed and purified by glutathione agarose chromatography as described [11]. Arabidopsis eIF4E, eIFiso4E, DRB3, and DRB4 cDNAs were cloned into a pRSET-B vector (Invitrogen) to generate His6-Xpress tag fusion proteins. Plasmids were transformed into E. coli BL21 cells and expressed proteins purified using Ni-NTA agarose. Protein concentrations were estimated as described above.
Yeast two-hybrid analysis
Interactions between SnRK1 and At eIF4E or At eIFiso4E were detected using a yeast two hybrid system as described [79]. SnRK1, At eIF4E, and At eIFiso4E were each inserted into pAS2 and pACT2 containing the GAL4 DNA binding domain (bait) and GAL4 activation domain (prey), respectively. Constructs were co-transformed into PJ649A cells using Zymo Research Frozen-EZ Yeast Transformation II Kit. Transformants were selected on yeast synthetic drop-out media lacking leucine and tryptophan. Interaction between proteins was indicated by growth on media lacking leucine, tryptophan, histidine, and adenine.
BiFC analysis
Bimolecular fluorescence complementation assays were performed using previously described vectors and methods [46]. The SnRK1, At eIF4E, At eIFiso4E, and control genes were cloned into BiFC expression vectors pYN, pYC, p2YN, and p2YC. Plasmids were transformed into A. tumefaciens, and cultures used to infiltrate N. benthamiana leaves. Leaf tissue was examined by confocal microscopy approximately 36 hours post-infiltration. To record YFP fluorescence, a band-pass emission filter (EM515/30HQ) with a 450 to 490 nm excitation wavelength and 515 nm emission wavelength was used. To record RFP fluorescence, a 565 nm long-pass filter (E565LP) was used. Images were captured using Simple PCI Software and compiled with Adobe Photoshop.
Phosphoprotein immunoprecipitation
N. benthamiana plants were co-infiltrated with agrobacterium containing HA-His tagged eIFiso4E along with either SnRK1-KD or negative control protein EML1 in TRBO vectors. Two days later the same leaves were infiltrated with a solution of 100 μM MG132 (Sigma-Aldrich) and 1 mM NaF to reduce protein degradation and dephosphorylation. Tissue was harvested six hours later. Cells were lysed in 50 mM Tris pH 7.5, 100 mM NaCl, 10% glycerol, 0.125% Triton X-100, 0.125% NP-40, 1 mM EGTA, 1 mM DTT, 0.1 mM PMSF, 100 μM MG132, 1 mM NaF, and protease inhibitor cocktail (Sigma-Aldrich, St. Louis, MO, USA). The lysate was then cleared by centrifugation. The supernatants were then pre-cleared with 50 μL protein A beads (Genscript, Piscataway, NJ, USA). Phosphoproteins were immunoprecipitated using a phospho-amino acid antibody (Invitrogen) and protein A beads (Genscript). Beads were washed with lysis buffer six times. Proteins were eluted using SDS-PAGE buffer at 95° C and analyzed by Western blot using a monoclonal HA antibody (Millipore, St. Louis, MO, USA) and SuperSignal West Pico PLUS, or SuperSignal West Femto Maximum Sensitivity detection systems (ThermoFisher Scientific, Waltham, MA, USA).
Kinase assays
Assays were performed essentially as described by Celenza and Carlson [39]. Reactions containing 10 to 15 ng of SnRK1-KD or SnRK1-KDKR, 3 μg of substrate proteins, and 0.5 μl of γ−32P-ATP (3000 Ci/mmol, Perkin Elmer) were mixed in 20 μl reaction buffer (50 mM Tris-HCl pH 7.5, 10 mM MgCl2, 1 mM DTT, 1% Triton X-100) and incubated at 30° C for 30 min. The γ−32P-ATP labeled proteins were separated on SDS–PAGE gels and the radioactive signal was recorded using a phosphorimager (Bio-Rad). Images were generated by a Bio-Rad Personal Molecular Imager System (PMI) or a GE Typhoon 8600 System. Phosphorylation signal values (in arbitrary units) were obtained by measuring band signal intensities on the phosphor image using Bio-Rad Quantity One software or GE Typhoon software.
Polysome profiling
Yeast cells were grown in 100 ml of liquid medium to mid-log phase (OD600 0.4 to 0.6). Yeast cultures were kept in ice for 5 min, and cycloheximide (Sigma-Aldrich) was added to a final concentration of 0.1 mg/ml. Cells were centrifuged at 6000 rpm for 4 min at 4°C, then washed twice in 1.5 ml of lysis buffer containing 20 mM Tris HCl (pH 8.0), 140 mM KCl, 10 mM MgCl2, 0.5 mM DTT, 0.1 mg/ml cycloheximide and 1 mg/ml heparin (Sigma-Aldrich). Cells were subsequently lysed in 0.7 ml of lysis buffer with 0.5 ml of chilled glass beads (0.45–0.55 mm diameter) by vigorous vortex in a cold room (4°C) for 5 min. Lysates were clarified by centrifugation at 4700 rpm for 4 min at 4°C. The concentration of total RNA was measured using a NanoDrop Spectrophotometer (ThermoScientific). Ten OD260 units of each lysate was loaded on a 7%−47% sucrose gradient in a 12 ml polycarbonate ultracentrifuge tube (Beckman Coulter, Atlanta, GA, USA) and centrifuged at 35,000 rpm for 2.5 hours at 4°C in an SW41 rotor. The mRNA-ribosome suspensions were fractionated using a UA-5 UV detector and model 185 ISCO gradient fractionator with the following settings: pump speed 0.75 ml/min, fraction time 1.2 min/fraction, chart speed 60 cm/hour.
Sucrose step gradient analysis and RT-qPCR
Wild type and transgenic N. benthamiana plants were grown in 16-hour days at 22° C in a growth room. For samples in which eIF4E and derivatives were transiently expressed, tissue was collected from agroinfiltration zones four days post-infiltration. Harvested tissue (0.30 g) was ground in liquid nitrogen, and the powder resuspended in 250 μL of lysis buffer (200 mM Tris pH 9, 400 mM KCl, 35 mM MgCl2, 200 mM sucrose, 1% Triton X-100, 1% Tween-20, 5 mM DTT 1 mM PMSF, 100 μg/mL chloramphenicol, 50 μg/mL cycloheximide). The slurry was incubated with gentle rocking for 30 minutes at 4°, then spun at 16,100 x g for 8 minutes at 4° C in a table top microcentrifuge. The supernatant was transferred to a new tube, and the centrifugation and transfer were repeated two more times. Sucrose step gradients (10%/35%) were prepared essentially as described [57–59]. The gradient buffer included 50 mM Tris pH 7.5, 10 mM KCl, 10 mM MgCl2, 150 mM NaCl, 2 mM DTT, and 200 μg/mL cycloheximide. In control experiments, EDTA (50 mM) was included in both the lysis and gradient buffers. Clarified extracts (200 μl) were loaded on step gradients in a 2.5 mL ultracentrifuge tube (Beckman Coulter), and centrifuged in a Sorvall Discovery M120 SE ultracentrifuge (Thermo Scientific) with an S55S-1123 rotor at 189,000 x g for one hour. 400 μl of sample was taken from the interface between the 10% and 35% sucrose layers, and the pellet was resuspended in 300 μL DEPC treated water. RNA from each fraction was purified using the Zymo Research Direct-zol RNA MiniPrep Plus kit (Zymo Research). 18S ribosomal RNA in pellet and interface fractions was quantified by quantitative reverse transcriptase (RT-qPCR) essentially as described [47]. Reverse transcription was performed using Superscript IV reverse transcriptase (Invitrogen) following the manufacturer’s instructions using a reverse primer specific for 18S rRNA. To ensure that no DNA contamination was present, a control lacking reverse transcriptase was included for each sample. Primers were designed to produce a 108 bp PCR product from 18S cDNA without creating primer dimers. The success of reverse transcription was tested by PCR, and showed the expected band in samples containing reverse transcriptase, and no band in those without the enzyme. qPCR was then performed using Bullseye EvaGreen qPCR mastermix (Midwest Scientific, Valley Park, MO, USA) with a Bio-Rad CFX96 Real-Time C1000 Thermal cycler.
Acknowledegements
We thank members of the Bisaro laboratory for assistance and technical support, and especially Shannon Phillips for assistance with eIF4E cloning. We also thank Dr. Paul Herman for advice on yeast methods and Dr. Dan Schoenberg for advice on polysome analysis. Work was supported by grants from the U.S. Department of Agriculture, National Institute of Food and Agriculture (USDA/NIFA 2015-6703-22999), and the National Science Foundation (NSF MCB-1158262 and IOS-1354636). Support for A.N.B. was provided by the Cellular, Molecular, and Biochemical Sciences Training Program, National Institute for General Medical Sciences, National Institutes of Health (NIH T32-GM-086252), and by the OSU Center for Applied Plant Sciences (CAPS). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations
- SnRK1
SNF1-related kinase 1
- SNF1
sucrose non-fermenting 1
- AMPK
AMP-activated kinase
- eIF4E
eukaryotic initiation factor 4E
- eIFiso4E
eukaryotic initiation factor iso4E
- SnRK1-KD
SnRK1 kinase domain
- SnRK1-KDKR
SnRK1 kinase domain inactive
Footnotes
Conflict of Interest
The authors declare no conflict of interest
References
- 1.Hao L, Wang H, Sunter G & Bisaro DM (2003) Geminivirus AL2 and L2 proteins interact with and inactivate SNF1 kinase. Plant Cell 15, 1034–1048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hardie DG (2007) AMP-activated/SNF1 protein kinases: conserved guardians of cellular energy. Nature Rev 8, 774–785. [DOI] [PubMed] [Google Scholar]
- 3.Polge C & Thomas M (2007) SNF1/AMPK/SnRK1 kinases, global regulators at the heart of energy control? Trends Plant Sci 12, 20–28. [DOI] [PubMed] [Google Scholar]
- 4.Baena-Gonzalez E & Sheen J (2008) Convergent energy and stress signaling. Trends Plant Sci 13, 474–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Halford N & Hey SJ (2009) Snf1-related protein kinases (SnRKs) act within an intricate network that links metabolic and stress signalling in plants. Biochem J 419, 247–259. [DOI] [PubMed] [Google Scholar]
- 6.Broeckx T, Hulsmans S & Rolland F (2016) The plant energy sensor: evolutionary conservation and divergence in SnRK1 structure, regulation, and function. J Exp Bot 67, 6215–6252. [DOI] [PubMed] [Google Scholar]
- 7.Emanuelle S, Doblin MS, Stapleton DI, Bacic A & Gooley PR (2016) Molecular insights into the enigmatic metabolic regulator, SnRK1. Trends Plant Sci 21, 341–353. [DOI] [PubMed] [Google Scholar]
- 8.Hulsmans S, Rodriguez M, De Coninck B & Rolland F (2016) The SnRK1 energy sensor in plant biotic interactions. Trends Plant Sci 21, 648–661. [DOI] [PubMed] [Google Scholar]
- 9.Emanuelle S, Hossain MI, Moller IE, Pedersen HL, van de Meene AML, Doblin MS, Koay A, Oakhill JS, Scott JW, Willats WGT, Kemp BE, Bacic A, Gooley PR & Stapleton DI (2015) SnRK1 from Arabidopsis thaliana is an atypical AMPK. Plant J 82, 183–192. [DOI] [PubMed] [Google Scholar]
- 10.Sugden C, Crawford RM, Halford NG & Hardie DG (1999) Regulation of spinach SNF1-related (SnRK1) kinases by protein kinases and phosphatases is associated with phosphorylation of the T loop and is regulated by 5’-AMP. Plant J 19, 433–439. [DOI] [PubMed] [Google Scholar]
- 11.Mohannath G, Jackel JN, Lee YH, Buchmann RC, Wang H, Patil V, Adams AK & Bisaro DM (2014) A complex containing SNF1-related kinase (SnRK1) and adenosine kinase in Arabidopsis. PLoS ONE 9, e87592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang H, Hao L, Shung CY, Sunter G & Bisaro DM (2003) Adenosine kinase is inactivated by geminivirus AL2 and L2 proteins. Plant Cell 15, 3020–3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Toroser D, Plaut Z & Huber SC (2000) Regulation of plant SNF1-related protein kinase by glucose-6-phosphate. Plant Physiol 123, 403–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Primavesi LF, Jhurreea D, Andralojc PJ, Mitchell RAC, Powers SJ, Schluepmann H, Delatte T, Wingler A & Paul MJ (2009) Inhibition of SNF1-related protein kinase1 activity and regulation of metabolic pathways by trehalose-6-phosphate. Plant Physiol 149, 1860–1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nunes C, Primavesi LF, Patel MK, Martinez-Barajas E, Powers SJ, Sagar R, Fevereiro PS, Davis BG & Paul MJ (2013) Inhibition of SnRK1 by metabolites: Tissue-dependent effects and cooperative inhibition by glucose 1-phosphate in combination with trehalose 6-phosphate. Plant Physiol Biochem 63, 89–98. [DOI] [PubMed] [Google Scholar]
- 16.Pedrotti L, Weiste C, Nägele T, Wolf E, Lorenzin F, Dietrich K, Mair A, Weckwerth W, Teige M, Baena-González E & Dröge-Laser W (2018) Snf1-related kinase 1-controlled C/S1-bZIP signaling activates alternative mitochondrial metabolic pathways to ensure plant survival in extended darkness. Plant Cell 30, 495–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alderson A, Sabelli PA, Dickinson JR, Cole D, Richardson M, Kreis M, Shewry PR & Halford N (1991) Complementation of snf1, a mutation affecting global regulation of carbon metabolism in yeast, by a plant protein kinase cDNA. Proc Natl Acad Sci USA 88, 8602–8605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fragoso S, Espíndola L, Páez-Valencia J, Gamboa A, Camacho Y, Martínez-Barajas E & Coello P (2009) SnRK1 isoforms AKIN10 and AKIN11 are differentially regulated in Arabidopsis plants under phosphate starvation. Plant Physiol 149, 1906–1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ramon M, Ruelens P, Li Y, Sheen J, Geuten K & Rolland F (2013) The hybrid Four-CBS-Domain KIN subunit functions as the canonical subunit of the plant energy sensor SnRK1. Plant J 75, 11–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Patrick RM & Browning KS (2012) The eIF4F and eIFiso4F complexes of plants: an evolutionary perspective. Comp Func Genomics 2012, 287814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jackson RJ, Hellen CUT & Pestova TV (2010) The mechanism of eukaryotic translation initation and principles of its regulation. Nature Rev Mol Cell Biol 10, 113–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Browning KS & Bailey-Serres J (2015) Mechanism of cytoplasmic mRNA translation. Arabidopsis Book 13, e0176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Browning KS, Humphreys J, Hobbs W, Smith GB & Ravel JM (1990) Determination of the amounts of the protein synthesis initiation and elongation factors in wheat germ. J Biol Chem 1990, 17967–17973. [PubMed] [Google Scholar]
- 24.Beauchemin C, Boutet N & Laliberté J-F (2007) Visualization of the interaction between the precursors of VPg, the viral protein linked to the genome of Turnip mosaic virus, and the translation eukaryotic initiation factor iso 4E in planta. J Virol 81, 775–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patrick RM, Mayberry LK, Choy G, Woodard LE, Liu JS, White A, Mullen RA, Tanavan TM, Latz CA & Browning KS (2014) Two Arabidopsis loci encode novel eukaryotic initiation factor 4E isofoms that are functionally distinct from the conserved plant eukaryotic initiation factor 4E. Plant Physiol 164, 1820–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pause A, Belsham GJ, Gingras AC, Donzé O, Lin TA, Lawrence JC Jr & Sonenberg N (1994) Insulin-dependent stimulation of protein synthesis by phosphorylation of a regulator of 5’-cap function. Nature 371, 762–767. [DOI] [PubMed] [Google Scholar]
- 27.Gebauer F & Hentze MW (2004) Molecular mechanisms of translation control. Nature Rev Mol Cell Biol 5, 827–835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Proud CG (2015) Mnks, eIF4E phosphorylation and cancer. Biochim Biophys Acta 1849, 766–773. [DOI] [PubMed] [Google Scholar]
- 29.Bramham CR, Jensen KB & Proud CG (2016) Tuning specific translation in cancer metastasis and synaptic memory: control at the MNK-eIF4E axis. Trends Biochem Sci 41, 847–858. [DOI] [PubMed] [Google Scholar]
- 30.Frere MA, Tourneur C, Granier F, Camonis J, El Amrani A, Browning KS & Robaglia C (2000) Plant lipoxigenase 2 is a translation initiation factor-4E binding protein. Plant Mol Biol 44, 129–140. [DOI] [PubMed] [Google Scholar]
- 31.Freire MA (2005) Translation initiation factor (iso) 4E interacts with BTF3, the beta subunit of the nascent polypeptide-associated complex. Gene 345, 271–277. [DOI] [PubMed] [Google Scholar]
- 32.Patrick RM, Lee JCH, Teetsel JRJ, Yang S-H, Choi GS & Browning KS (2018) Discovery and characterization of conserved binding of eIF4E1 (CBE1), a eukaryotic translation initiation factor 4E-binding plant protein. J Biol Chem 293, 17240–17247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Halford N & Hardie DG (1998) SNF1-related protein kinases: global regulators of carbon metabolism in plants? Plant Mol Biol 37, 735–748. [DOI] [PubMed] [Google Scholar]
- 34.Monzingo AF, Dhaliwal S, Dutt-Chaudhuri A, Lyon A, Sadow JH, Hoffman DW, Robertus JD & Browning KS (2007) The structure of eukaryotic translation initiation factor-4E from wheat reveals a novel disulfide bond. Plant Physiol 143, 1504–1518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yang CS, Lam CKL, Chari M, Cheung GWC, Kokorovic A, Gao S, Leclerc I, Rutter GA & Lam TKT (2010) Hypothalmic AMP-activated protein kinase regulates glucose production. Diabetes 59, 2435–2443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shen W, Dallas MB, Goshe MB & Hanley-Bowdoin L (2014) SnRK1 phopshorylation of AL2 delays Cabbage leaf curl virus infection in Arabidopsis. J Virol 88, 10598–10612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Son S, Oh CJ & An CS (2014) Arabidopsis thaliana remorins interact with SnRK1 and play a role in susceptibility to Beet curly top virus and Beet severe curly top virus. Plant Pathol J 30, 269–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lindbo JA (2007) TRBO: A high-efficiency Tobacco mosiac virus RNA-based overexpression vector. Plant Physiol 145, 1232–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Celenza JL & Carlson M (1989) Mutational analysis of the Saccharomyces cerevisiae SNF1 protein kinase and evidence for functional interaction with the SNF4 protein. Mol Cell Biol 9, 5034–5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Davies SP, Carling D & Hardie DG (1989) Tissue distribution of AMP-activated protein kinase, and lack of activation by cyclic AMP-dependent protein kinase, studied using a specific and sensitive peptide assay. Eur J Biochem 186, 123–128. [DOI] [PubMed] [Google Scholar]
- 41.Kishimoto A, Ogura T & Esumi H (2006) A pull-down assay for 5’AMP-activated protein kinase activity using the GST-fused protein. Mol Biotechnol 32, 17–21. [DOI] [PubMed] [Google Scholar]
- 42.Hiraguri A, Itoh R, Kondo N, Nomura Y, Aizawa D, Murai Y, Koiwa H, Seki M, Shinozaki K & Fukuhara T (2005) Specific interacions between Dicer-like proteins and HYL1/DRB-family dsRNA-binding proteins in Arabidopsis thaliana. Plant Mol Biol 57, 173–188. [DOI] [PubMed] [Google Scholar]
- 43.Raja P, Jackel JN, Li S, Heard IM & Bisaro DM (2014) Arabidopsis double-stranded RNA binding protein DRB3 participates in methylation-mediated defense against geminiviruses. J Virol 88, 2611–2622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nukarinen E, Nägele T, Pedrotti L, Wurzinger B, Mair A, Landgraf R, Börnke F, Hanson J, Teige M, Baena-Gonzalez E, Dröge-Laser W & Weckwerth W (2016) Quantitative phosphoproteomics reveals the role of the AMPK plant ortholog SnRK1 as a metabolic master regulator under energy deprivation. Sci Rep 6, 31697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bush MS, Hutchins AP, Jones AME, Naldrett MJ, Jarmolowski A, Lloyd CW & Doonan JH (2009) Selective recruitment of proteins to 5’ cap complexes during the growth cycle in Arabidopsis. Plant J 59, 400–412. [DOI] [PubMed] [Google Scholar]
- 46.Yang X, Baliji S, Buchmann RC, Wang H, Lindbo JA, Sunter G & Bisaro DM (2007) Functional modulation of the geminivirus AL2 transcription factor and silencing suppressor by self-interaction. J Virol 81, 11972–11981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Coursey T, Milutinovic M, Regedanz E, Brkljacic J & Bisaro DM (2018) Arabidopsis histone reader EMSY-like 1 binds H3K36 and suppresses geminivirus infection. J Virol 92, e00219–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Altmann M, Handschin C & Trachsel H (1987) mRNA cap-binding protein: cloning of the gene encoding protein synthesis initiation factor eIF-4E from Saccharomyces cerevisiae, Mol Cell Biol 7, 998–1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Brenner C, Nakayama N, Goebl M, Tanaka K, Toh-e A & Matsumoto K (1988) CDC33 encodes mRNA cap-binding protein eIF-4E of Saccharomyces cerevisiae. Mol Cell Biol 8, 3556–3559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Celenza JL & Carlson M (1986) A yeast gene that is essential for release from glucose repression encodes a protein kinase. Science 233, 1175–1180. [DOI] [PubMed] [Google Scholar]
- 51.Rodriguez CM, Freire MA, Camilleri C & Robaglia C (1998) The Arabidopsis thaliana cDNAs coding for eIF4E and eIF(iso)4E are not functionally equivalent for yeast complemenation and are differentially expressed during plant development. Plant J 13, 465–473. [DOI] [PubMed] [Google Scholar]
- 52.Hughes JMX, Ptushkina M, Karim MM, Koloteva N, von der Haar T & McCarthy JEG (1999) Translational repression by human 4E-BP1 in yeast specifically requires human eIF4E as target. J Biol Chem 274, 3261–3264. [DOI] [PubMed] [Google Scholar]
- 53.German-Retana S, Walter J, Doublet B, Roudet-Tavert G, Nicaise V, Lecampion C, Houvenaghel M-C, Robaglia C, Michon T & Le Gall O (2008) Mutational analysis of plant cap-binding protein eIF4E reveals key amino acids involved in biochemical functions and potyvirus infection. J Virol 82, 7601–7612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boeke JD, Trueheart J, Natsoulis G & Fink GR (1987) 5-Fluoroorotic acid as a selective agent in yeast molecular genetics. Methods Enzymol 154, 164–175. [DOI] [PubMed] [Google Scholar]
- 55.Kuhn KM, DeRisi JL, Brown PO & Sarnow P (2001) Global and specific translational regulation in the genomic response of Saccharomyces cerevisiae to a rapid transfer from a fermentable to a nonfermentable carbon source. Mol Cell Biol 21, 916–927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Arava Y, Wang Y, Storey JD, Long Liu C, Brown PO & Herschlag D (2003) Genome-wide analysis of mRNA translation profiles in Saccharomyces cerevisiae. Proc Natl Acad Sci USA 100, 3889–3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.McGrew LL, Dworkin-Rasti E, Dworkin MB & Richter JD (1989) Poly(A) elongation during Xenopus oocyte maturation is required for translational recruitment and is mediated by a short sequence element. Genes Dev 3, 803–815. [DOI] [PubMed] [Google Scholar]
- 58.Cunningham KS, Hanson MN & Schoenberg DR (2001) Polysomal ribonuclease 1 exists in a latent form on polysomes prior to estrogen activation of mRNA decay. Nucleic Acids Res 29, 1156–1162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yang F & Schoenberg DR (2004) Endonuclease-mediated mRNA decay involves selective targeting of PMR1 to polyribosome-bound substrate mRNA. Mol Cell 14, 435–445. [DOI] [PubMed] [Google Scholar]
- 60.Mathews MB & Hershey JWB (2015) The translation factor eIF5A and human cancer. Biochim Biophys Acta 1849, 836–844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ruvinsky I & Meyuhas O (2006) Ribosomal protein S6 phosphorylation: from protein synthesis to cell size. Trends Biochem Sci 31, 342–348. [DOI] [PubMed] [Google Scholar]
- 62.Meyuhas O (2015) Ribosomal protein S6 phosphorylation: four decades of research. Int Rev Cell Mol Biol 320, 41–73. [DOI] [PubMed] [Google Scholar]
- 63.Gwinn DM, Shackelford DB, Egan DF, Mihaylova MM, Mery A, Vasquez DS, Turk BE & Shaw RJ (2008) AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol Cell 30, 214–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Baena-Gonzalez E & Hanson J (2017) Shaping plant development through SnRK1-TOR metabolic regulators. Curr Opin Plant Biol 35, 152–157. [DOI] [PubMed] [Google Scholar]
- 65.Cho H-Y, Wen T-N, Wang Y-T & Shih M-C (2016) Quantitative phosphoproteomics of protein kinase SnRK1 regulated protein phosphorylation in Arabidopsis under submergence. J Exp Bot 67, 2745–2760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cho H-Y, Lu M-Y & Shih M-C (2018) The SnRK1-eIFiso4G signaling relay regulates the ranslation of specific mRNAs in Arabidopsis under submergence. New Phytol 222, 366–381. [DOI] [PubMed] [Google Scholar]
- 67.Im JH, Cho YH, Kang GH, Hong JW & Yoo SD (2014) Inverse modulation of the energy sensor Snf1-related protein kinase 1 on hypoxia adaptation and salt stress tolerance in Arabidopsis thaliana. Plant Cell Environ 37, 2303–2312. [DOI] [PubMed] [Google Scholar]
- 68.Liu XJ, An XH, Liu X, Hu DG, Wang XF, You CX & Hao YJ (2017) MdSnRK1.1 interacts with MdJAZ18 to regulate sucrose-induced anthocyanin and proanthocyanidin accumulation in apple. J Exp Bot 68, 2977–2990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu XJ, Liu X, An XH, Han PL, You CX & Hao YJ (2017) An apple protein kinase MdSnRK1.1 interacts with MdCAIP1 to regulate ABA sensitivity. Plant Cell Physiol 58, 1631–1641. [DOI] [PubMed] [Google Scholar]
- 70.Williams SP, Rangarajan P, Donahue JL, Hess JE & Gillaspy GE (2014) Regulation of sucrose non-fermenting related kinase 1 genes in Arabidopsis thaliana. Front Plant Sci 5, 324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Fernandez O, Béthencourt L, Quero A, Sangwan RS & Clément C (2010) Trehalose and plant stress responses: friend or foe? Trends Plant Sci 15, 409–417. [DOI] [PubMed] [Google Scholar]
- 72.Miras M, Truniger V, Silva C, Verdaguer N, Aranda MA & Querol-Audí J (2017) Structure of eIF4E in complex with an eIF4G peptide supports a universal bipartite binding mode for protein translation. Plant Physiol 174, 1476–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mader S, Lee H, Pause A & Sonenberg N (1995) The translation initiation factor eIF-4E binds to a common motif shared by tranlation factor eIF-4 gamma and the translational repressors 4E-binding proteins. Mol Cell Biol 15, 4990–4997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Marcotrigiano J, Gingras AC, Sonenberg N & Burley SK (1999) Cap-dependent translation initiation in eukaryotes is regulated by a molecular mimic of of eIF4G. Mol Cell 3, 707–716. [DOI] [PubMed] [Google Scholar]
- 75.Gruner S, Peter D, Weber R, Wohlbold L, Chung M-Y, Weichenreider O, Valkov E, Igreja C & Izaurralde E (2016) The structures of eIF4E-eIF4G complexes reveal an extended interface to regulate translation. Mol Cell 64, 467–479. [DOI] [PubMed] [Google Scholar]
- 76.Robaglia C & Caranta C (2006) Translation initiation factors: a weak link in plant RNA virus infection. Trends Plant Sci 11, 40–45. [DOI] [PubMed] [Google Scholar]
- 77.Sanfaçon H (2015) Plant translation factors and virus resistance. Viruses 7, 3392–3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Wang H, Buckley KJ, Yang X, Buchmann RC & Bisaro DM (2005) Adenosine kinase inhibition and suppression of RNA silencing by geminivirus AL2 and L2 proteins. J Virol 79, 7410–7418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang X, Xie Y, Raja P, Li S, Wolf JN, Shen Q, Bisaro DM & Zhou X (2011) Suppression of methylation-mediated transcriptional gene silencing by βC1-SAHH protein interaction during geminivirus-betasatellite infection. PLoS Pathog 7, e1002329. [DOI] [PMC free article] [PubMed] [Google Scholar]


