Abstract
For the management of obesity in childhood and adolescence, non-operative approaches have limited efficacy, including community-based and behavioral interventions and pharmacotherapy approved for use in adults. Roux-en-Y gastric bypass and laparoscopic sleeve gastrectomy are efficacious in reducing weight, body mass index, and comorbidities in adolescents. Understanding the phenotype associated with obesity provides an opportunity to individualize patients’ treatments directed at the brain-gut axis. These phenotypes include rapid gastric emptying, increased fasting gastric volume, reduced postprandial incretins, and central mechanisms that impact appetite and satiation including hedonic eating and affective disorders. Further studies are required in adolescents. Identifying phenotypes could enhance efficacy with behavioral, dietary, and pharmacotherapeutic interventions alone or in combination in children and adolescents.
Keywords: pharmacotherapy, bariatric, phenotypes, gastric, incretins
Objectives and Background
The objectives of this article are to review the general strategies for the management of obesity in children and adolescents, review the brain-gut axis in obesity as a foundation for understanding different phenotypes in obesity, and discuss the potential for designing individualized treatment approaches, whether as single or as combination therapies.
There are several complex biological, environmental and societal factors contributing to obesity: Biological factors include genetics, epigenetics, internal clocks, inflammation, medications, weight cycling characterized by weight loss and regain, adipose tissue distribution, signals to the brain from adipose tissue, gut, liver and pancreas, the brain reward system, neurocircuits of appetite and satiety regulation, psychiatric diseases, addiction and microbiota. In addition, several environmental and societal factors contribute to the development of obesity such as eating culture, food marketing, work factors (workplace, shift work, stress), media (social and TV), smoking, recreational drug use, and computer games (1). Ultimately, all these factors impact food intake, metabolism, energy expenditure, and physical activity. The current prevalence and the increase in prevalence of obesity over time are well documented (1).
Although a genome-wide polygenic score can quantify inherited susceptibility to obesity and the polygenic score demonstrates potential effect of genetic variation on weight that emerges early in life and increases into adulthood and is associated with a strong risk for severe obesity and associated diseases (2), monogenic obesity mutations (e.g. in MC4R gene, leptin and leptin receptor deficiency, pro-opiomelanocortin deficiency) are rare. There are recognized secondary causes of obesity; however these are relatively rare and include, in addition to the monogenic disorders: endocrine (e.g. hypothyroidism and growth hormone deficiency), specific syndromes (e.g. Prader-Willi or Bardet-Biedl), neurological (e.g. cranial irridiation and hypothalamic obesity), and drug-induced disorders (e.g. steroids, psychotropic agents, sulfonylureas) (3).
Thus, the general strategy recommended for obesity in children or adults is based on diet, physical activity, and behavioral programs.
General Strategy for Managing Obesity in Children or Adults
The general strategy recognizes three phases in management of obesity: weight loss, maintenance of weight loss, and prevention of weight regain. For this to be achieved, it is recommended that a multidisciplinary team should be involved: physicians (whose primary discipline should be obesity medicine, whether they are endocrinologists or pediatric or adult gastroenterologists) and other professionals that provide a comprehensive assessment and intervention. These include bariatric surgeon or endoscopist, a mid-level provider (physician assistant, nurse practitioner, or nurse), a registered dietitian nutritionist, a behavioral therapist (e.g., psychiatric social worker, psychiatrist, or psychologist), a physical therapist and medical assistants.
From the initial contact with the patient and through the continuum of care, it is recommended that the team should embrace obesity as a chronic medical problem, provide respectful care, and foster motivation and inspiration to achieve the proposed goals. These are the essential principles communicated in the American Gastroenterological Association’s Practice Guide on Obesity and Weight Management, Education, and Resources (POWER) (4). The importance of teamwork and of inclusion of nutrition specialist and behavioralist for pediatric obesity is illustrated by the meta-regression analysis of BMI at 4 time points from 3–6 months to >2 years (5).
From an analysis and synthesis of the recommendations of 17 guidelines for nutritional management of pediatric obesity (6), the summary is provided in Table 1 and recent reviews (3). The importance of addressing over-weight or obesity in children aged 0 to 6 years old is emphasized by a meta-regression demonstrating the risk of adult metabolic syndrome with childhood obesity (7).
Table 1.
Summary of Recommendations for Nutritional Management in Obesity
| Nutritional Management | Examples of details assessed |
|---|---|
| 1. Nutritional assessment | Diagnosis based on World Health Organization or national definition |
| Plot anthropometric measurements on BMI and growth curves | |
| Food/nutrition history: quantity, quality, meal cotext/emnvironment | |
| Emotional eating signals | |
| Body signals: hunger, satiety | |
| Screen for eating disorders | |
| Physical activity | |
| Personal history: co-morbidity, mental health | |
| Family history including mother’s pregnancy, obesity | |
| Social and Cultural History: Socioeconomic status, ethnicity | |
| Motivation for behavioral change | |
| Medications: steroids, psychotropics | |
| Biochemical Test results: blood pressure, fasting glucose, aspartate aminotransferase (AST), alanine aminotransferase (ALT), lipids | |
| 2. Nutrition diagnosis | Overweight or obesity |
| Excessive or undesirable food intake | |
| Disordered eating pattern | |
| Physical inactivity | |
| Inability to manage self-care | |
| 3. Nutritional intervention | Daily food balance: >5 portions of fruits/vegetables; high fiber, reduce added sugar, avoid processed food and sweetened beverages |
| Food structure and environment: 3 meals, 2 snacks; avoid other snacking; eat with family at table | |
| Portion size adjusted to age, gender, physical activity | |
| Read food labels to educate regarding content | |
| Physical activity | |
| Behavioral change including setting goals, self-monitoring | |
| Involve the family including positive reinforcement | |
| Coordination with entire obesity management team | |
| 4. Nutritional management and evaluation | Individual or group therapy adapted to child’s situation and needs |
| Monitor anthropometric measurements: weight, BMI, growth | |
| Review diet, weight goals, activity goals | |
| Establish goals for weight stabilization or progressive weight loss | |
| If needed, refer for pharmacotherapy, bariatric surgery | |
Approaches to Reverse Obesity in Children and Adolescents
A. Community based and behavioral interventions
A systematic review and meta-regression analysis of 21 programs which were heterogeneous in nature (including length, number and frequency of sessions, parent-involvement and technology involvement) showed reduction in adolescent body mass index (BMI) z score (age and sex standardized body mass index) ranged from 2 to 9% post-program and from 2 to 11% after varied lengths of follow-up (8). In addition, there was no clear relationship between the dose of the behavioral interventions and the weight-related outcomes (9).
To assess the effects of diet, physical activity and behavior changing interventions (BCI) for the treatment of overweight or obese adolescents, aged 12 to 17 years, a Cochrane review included 44 completed randomized, controlled trials (4781 participants) and 50 ongoing studies, with length of follow-up 6 to 24 months. The change in BMI at the longest follow-up period in favor of BCI was −1.18 (95% CI −1.67 to −0.69) kg/m2; BCI lowered the change in BMI z score by −0.13 units (−0.21 to −0.05) and body weight by −3.67 (−5.21 to −2.13) kg (1993 participants; 20 trials; moderate quality evidence). The effect on weight measures persisted in trials with 18 to 24 months’ follow-up for both BMI (−1.49, −2.56 to −0.41 kg/m2) and BMI z score (−0.34, −0.66 to −0.02) (10). In children aged 6–11 years (11), based on 70 randomized controlled trials (RCTs) with a total of 8461 participants randomized to either the intervention or control groups, the mean difference in BMI was −0.53 (−0.82 to −0.24) kg/m2, and BMI z score −0.06 (−0.10 to −0.02) units.
B. Pharmacotherapy
1. Metformin
On the basis of 14 RCTs, metformin provides a statistically significant, but very modest reduction in BMI when combined with lifestyle interventions over the short term. For BMI, moderate-strength evidence indicated a reduction of −1.38 (95% CI, −1.93 to −0.82) from baseline compared with control at 6 months. However, 26% reported a gastrointestinal adverse event compared with 13% in control groups (relative risk, 2.05; 95% CI, 1.19–3.54) (12). In addition, despite several randomized clinical trials evaluating the potential impact of metformin on body weight and insulin resistance in children, its efficacy in treating pediatric obesity with normal glucose tolerance remains controversial (13,14).
2. Other drug interventions for adolescent obesity
A Cochrane review (15), included trials that evaluated metformin (11 trials), sibutramine (six trials), orlistat (four trials), and one trial arm investigated the combination of metformin and fluoxetine. In addition, ongoing trials evaluated metformin (four trials), topiramate (two trials) and exenatide (two trials). A total of 2484 people participated in the included trials, with 1478 participants randomized to drug intervention and 904 to comparator groups (18 used placebo as comparator). Intervention versus comparator mean difference in BMI change was −1.3 (−1.9 to −0.8) kg/m2, and change in weight −3.9 (95% CI −5.9 to −1.9) kg, with beneficial effect on weight with sibutramine, metformin and orlistat. Serious adverse events occurred in 2.7% participants in the intervention groups versus 1.7% in the comparator groups; 5.0% participants in the intervention groups versus 2.7% in the comparator groups discontinued the trial because of adverse events. The most common adverse events in orlistat and metformin trials were gastrointestinal (such as diarrhea, mild abdominal pain or discomfort, fatty stools). The most frequent adverse events in sibutramine trials included tachycardia, constipation and hypertension (13).
The safety and efficacy of pharmacotherapy for obesity in adults is well documented, based on 28 randomized clinical trials with 29,018 patients (16). Among overweight or obese adults, orlistat, lorcaserin, naltrexone-bupropion, phentermine-topiramate, and liraglutide, compared with placebo, were each associated with achieving at least 5% weight loss at 52 weeks. Phentermine-topiramate and liraglutide were associated with the highest odds of achieving at least 5% weight loss (16). However, orlistat is the only medication currently approved by the Food and Drug Administration for the treatment of obesity in adolescents (age >12 years). The efficacy of orlistat is modest: 1-y placebo-subtracted change in BMI is less than 1 kg/m2.
C. Weight Loss in Adolescents after Bariatric Surgery
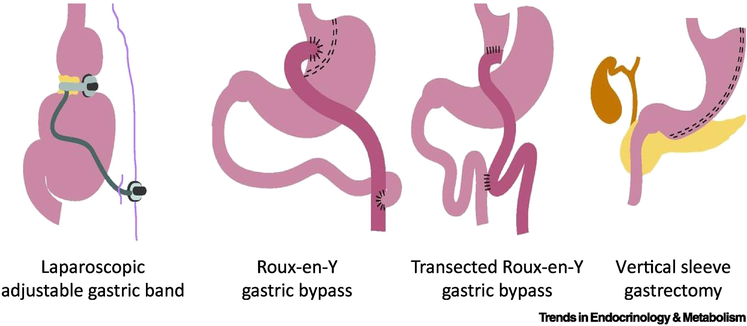
In general, bariatric surgery is considered as an option in adolescents with BMI >40kg/m2 or BMI >35 kg/m2 with related comorbidities who fail to achieve sufficient weight loss through behavioral interventions (with or without pharmacotherapy) (17,18). As in adults, there has been a shift in recent years from Roux-en-Y gastric bypass to sleeve gastrectomy (19). Figure 1 shows the most commonly performed bariatric surgeries. Several systematic reviews and meta-analyses document the efficacy of bariatric surgery in adolescents.
Figure 1.
Most frequently performed bariatric surgery
Black et al. showed a significant BMI loss of 13.5 kg/m2 (95%CI, −14.1, −11.9) at 1 year after bariatric surgery in adolescents with obesity (20). Furthermore, the authors showed the superiority of RYGB (−17.2 kg/m2) to laparoscopic sleeve gastrectomy (−14.5 kg/m2) and adjustable gastric banding (−10.5 kg/m2).
Another meta-analysis of 37 studies similarly showed a BMI loss of 11.6 kg/m2 for AGB, 16.6 kg/ m2 (21).
A third systematic review and meta-analysis of bariatric surgery in 950 morbidly obese adolescents assessed long-term outcome with >3 years follow-up and documented average BMI reduction of −13.3 kg/m2 (95%CI −11.9, −14.7) (22). There was a weight regain <5 kg/m2 between 5 and 6 years of follow-up. Removal, exchange, or conversion of the previous band to RYGB constituted the majority of 53 revision procedures.
In 24 studies in adolescent patients (age≤19) totaling 29 surgical subgroup populations and 1928 patients (gastric band: 1010, gastric sleeve: 139, gastric bypass: 779), the short-term weight loss, measured as BMI (kg/m2) at 6 months, was −5.4 (95%CI −3.0, −7.8) after gastric band, −11.5 (−8.8, −14.2) after gastric sleeve, and −18.8 (−10.9, −26.6) after gastric bypass. BMI reduction at 36 months was −10.3 (−7.0, −13.7) kg/m2 after gastric band, −13.0 (−11.0, −15.0) after gastric sleeve, and −15.0 (−13.5, −16.5) after gastric bypass (23). At 36 months, excess weight loss was −58.8% (95%CI −40.9, −76.8%) after gastric band, −75.9% (−67.6, −84.2%) after gastric sleeve, and −54.8% (−50.4, −59.3%) after gastric bypass.
Adolescents achieved the same weight loss benefits as adults 5 years after gastric bypass surgery, and remission rates of diabetes and hypertension were greater in adolescents than in adults (24). It was also demonstrated that increased weight loss, female sex, and younger age predicted a higher probability of resolution of specific cardiovascular disease risk factors (25), and it has been proposed that this may lead to refinements in patient selection and optimal timing of adolescent bariatric surgery designed to improve clinical outcomes.
D. Endoscopic Procedures for Weight Loss in Adolescents
Several studies have documented the effects and safety of endoscopic procedures specifically in adolescents.
In 12 severely obese adolescents with an intragastric 500mL balloon placed for 6 months as an adjunct to a lifestyle support program, there were short-term, highly variable effects on weight change (e.g., 7.05 kg±7.13 at the time of balloon removal at 6 months, and 3.05kg±14.69 in 9 patients at 12 months). Improvements in psychosocial health, physical activity and cardiorespiratory fitness were maintained at 12 months, with varying results at 24 months (26). In a separate study of a liquid-filled intragastric balloon of 600–700mL volume along with a multidisciplinary weight loss program, 27 adolescents experienced total percent weight loss at 6–7 months (time of removal of balloon) of 16.35+9.6% (mean body weight from 102.21 to 86.23 kg), with significant positive correlation of the weight loss to adherence to the multidisciplinary program (27). Both studies demonstrated that the balloons were safe. In addition, clinically relevant improvements in blood pressure, insulin (glucose metabolism), liver function, and sleep apnea were observed at 6 months, although improvements were not sustained at 2 years except for diastolic blood pressure, HBA1c, insulin area under the curve (AUC) which demonstrated longer-term improvement despite weight regain, and bone mass accrual showed age appropriate increases (28).
Preliminary reports documented the efficacy and safety of endoscopic sleeve gastroplasty in 55 pediatric patients (29). Mean percent excess weight loss at one (n=45), three (n=31), six (n=24), nine (n=13), and twelve months (n=5) was 46.7±29.6%, 58.7±39.2%, 66.0±42.2%, 79.2±49.5%, and 60.0±48.3%, respectively. Postoperative pain and nausea required treatment, but there were no hospital admissions, mortality, or significant morbidity.
Given the relative efficacy of bariatric surgery on weight loss and cardiovascular and metabolic risks, as well as endoscopic procedures on weight loss in adolescents compared to more conservative approaches, a new approach is required to enhance the efficacy of obesity management in children and adolescents beyond diet, behavioral approaches, and orlistat.
The Brain-Gut Axis and Appetite
There is evidence that there are alterations of the brain-gut axis in the context of obesity. The concept of satiety implies the absence of appetite to ingest food, and it can be measured by the number of calories ingested at an ad libitum meal after the ingestion of a standard meal, typically 4 hours after a 300 kilocalorie meal. In contrast, satiation is a measure of food intake at the time of meal termination and this is conveniently measured by the volume to fullness or the maximum tolerated volume, as well as intra- and postprandial symptoms during a nutrient drink test. Appetite is, therefore, related to the rate of stomach emptying, as well as the volume that can be accommodated in the stomach after a meal.
Gut functions and satiation involve a variety of neurohormonal mechanisms: food ingestion stimulates the vagus nerve, and distention of the stomach activates circuits that stimulate the emptying of food from the stomach. The arrival of food in the intestine results in distension and chemical stimulation, with hormonal mediators released in the proximal or distal small intestine. In the distal small bowel, glucagon-like peptide-1, oxyntomodulin, peptide YY, and neurotensin result in inhibition of proximal gastrointestinal motor function and are termed the jejunal and ileal brakes. However, they also affect hypothalamic centers involved in appetite.
Second, intrinsic circuits within the gastrointestinal tract are inhibited by vagal and hormonal functions and are, in turn, stimulated by hormones released in response to nutrients. Thus osmotic, pH, and fat in the meal trigger the release of cholecystokinin and secretin, whereas amino acids and carbohydrates stimulate the release of gastrin and glucose-stimulated insulinotropic peptide (GIP).
Third, hypothalamic centers and peptidergic circuits stimulated by vagal afferents, as well as the hormones released from the small intestine and colon in response to nutrients, provide input to the vagal motor nuclei to alter gastrointestinal functions. In addition, specific nuclei in the hypothalamus, such as the arcuate and paraventricular nuclei, respond to incoming stimuli to activate orexigenic or anorexgenic waves that either stimulate (orexin) or inhibit (melanocortin) the lateral hypothalamic nuclei and, subsequently, affect feeding behavior. Extensive reviews have documented the drugs in development for obesity and their targets, which include central neuropeptide signaling, monoamine neurotransmission, as well as the peripheral targets such as intestinal peptide hormone signaling and pancreatic hormone signaling (30,31).
A Novel Approach: Phenotyping Gastrointestinal and Psychologic Traits to Individualize Obesity Therapy
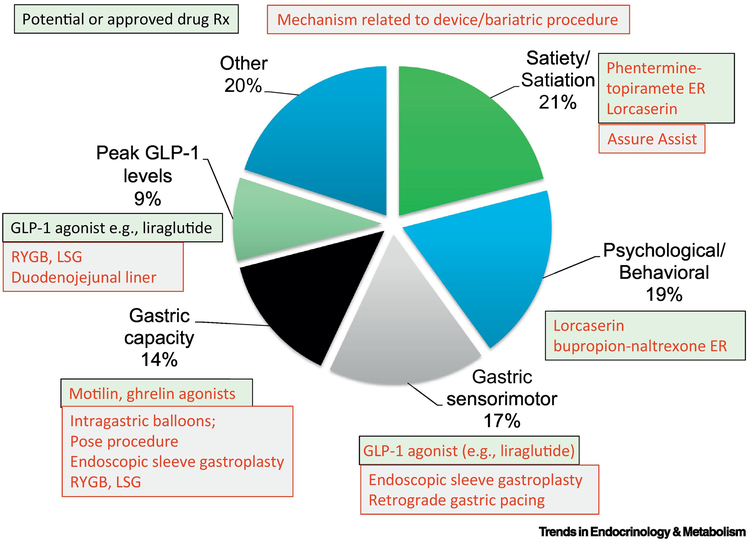
Based on a study of 507 overweight, obese, or normal weight participants, obesity was associated with larger fasting gastric volume, accelerated gastric emptying of solids and liquids, lower postprandial levels of satiation-associated hormones, particularly peptide YY (PYY), higher volume of liquid calories ingested to achieve comfortable postprandial fullness, and larger calorie intake in a buffet meal. Using a principal component analysis, we identified latent dimensions that accounted for approximately 81% of the variation among overweight and obese participants, with the following breakdown: satiety or satiation (21%), gastric motility (14%), behavioral factors (13%), and gastric sensorimotor factors (11%). The methods to measure these phenotypes are described briefly.
In the satiation test, a nutrient drink (1kcal/mL) is ingested at a rate of 30kcal/minute (32,33); the volume to fullness is increased in proportion to the degree of obesity. Thus, there is an overall trend to increased volume of Ensure® to feel comfortably full, with a significant increase of calories ingested in obesity. In class II and III obesity, patients ingest 150kcal more than normal weight adults at the point of usual fullness. Gastric emptying of solids and liquids in obesity has been measured by means of scintigraphy using radioisotopes for each phase of the meal (33). In these studies, overweight and obese individuals were shown to have acceleration of both liquid and solid phases of the meal.
The measurement of gastric volume is based on a radioisotope injection and single photon emission computed tomography. With this method, it was demonstrated that class II and III obesity is associated with larger fasting gastric volume, although the accommodation volume after a 300mL Ensure® meal was normal in overweight and obesity (33).
Whereas, there are multiple targets for anti-obesity drugs in the central nervous system and the gastrointestinal tract, the significant efficacy of bariatric surgery suggests that further attention to gastrointestinal mediators of appetite and satiation is warranted. Opportunities in the GI tract (34) include targeting gastrointestinal motor functions such as gastric emptying, inducing fat malabsorption, or modulating got hormone mediators of appetite and glycemic control [Figure 2 (35)]. Examples of these targets for obesity therapy include glucagon-like peptide-1 (GLP-1) receptor analogs or agonists such as exenatide and liraglutide, orlistat, and pramlintide, which is an amylin agonist. In addition, several of the gut hormones impact energy intake, and these include GIP, GLP-1, glucagon, oxyntomodulin, ghrelin and PYY.
Figure 2. Obesity phenotypes useful to personalize therapy and related treatments.
(adapted from ref. 35, Camilleri, M. et al. (2016) Gastrointestinal traits: individualizing therapy for obesity with drugs and devices. Gastrointest. Endosc. 83, 48–56). GLP-1=glucagon-like peptide-1; RYGB=Roux-en-Y gastric bypass; LSG=laparoscopic sleeve gastrectomy
In fact, it is likely that many of these potential therapeutic targets actually affect the phenotypes that modulate appetite, energy intake, and satiation in obesity. Proof of this concept is provided by two recent small clinical trials. In the first trial, phentermine and topiramate extended release were tested in comparison to placebo in these patients. As expected, over the two-week, randomized, controlled trial, there was significantly greater weight loss with phentermine and topiramate compared to placebo. However the body weight change was dependent on the calorie intake at a satiety buffet meal prior to entry into the study. Thus, patients who were able to ingest more than 900kcal had a significant benefit with phentermine-topiramate compared to placebo, whereas patients who ingested less than 900kcal did not benefit with the active drug compared to the placebo (33).
A second illustration of the potential individualization of pharmacotherapy for obesity is demonstrated by the effects of the GLP-1 receptor agonist, exenatide, 5 μg twice daily, on gastric emptying and weight loss in obese patients with previously documented accelerated gastric emptying (36). In the group treated with exenatide, the mean gastric emptying t1/2 was 187 minutes, compared to 86 minutes on placebo. In the group receiving exenatide, there was a significant difference in weight compared to baseline; such a difference was not observed in the placebo treated patients.
A third study assessed the relationship between liraglutide-induced gastric emptying delay and degree of weight loss. When assessed at the end of 5 weeks, during which the liraglutide dose was being escalated in accordance with regulatory guidance, there was a strong correlation between the change in gastric emptying from baseline and the degree of weight loss over the 5 weeks. A significant correlation was similarly observed at the end of 16 weeks of liraglutide treatment, with the majority of participants having received the target dose of 3.0 mg liraglutide for at least 10 weeks (37). Although this study did not assess whether the baseline phenotype of accelerated gastric emptying resulted in greater weight loss in response to liraglutide, it suggests that slowing gastric emptying complements the reduction of appetite via effects on hypothalamic appetite and other brain centers (38).
The role of gastric emptying in mediating weight loss is also illustrated by the relationship observed after endoscopic sleeve gastroplasty (39). Thus, gastric retention in a proximal gastric pouch following sleeve gastroplasty is associated with greater weight loss. In one study, delayed gastric emptying was proposed to be the mechanism of action leading to weight loss during intra-gastric balloon therapy (40). In a systematic review and meta-analysis, sleeve gastrectomy reduced gastric emptying t1/2, whereas fluid-filled balloons significantly increased gastric emptying t1/2. Air-filled balloons do not significantly change the time of gastric emptying, which could account for their low efficacy. Antral botulinum toxin injections produced small temporary increases in gastric emptying time, which was associated with weight loss. The overall conclusion was that changes in gastric emptying time after surgical and endoscopic bariatric interventions correlate with weight loss (41).
Other potential approaches to obesity, based on the viscosity, constituents, and physical characteristics of ingested food, have been proposed and may have effects on gastric emptying, gastrointestinal hormones, as well as glycemic control (42–47). These substances include guar, cellulose, soluble fiber and, possibly, alginate (48), although the effects of alginate on gastric motor functions were not confirmed in another study (49).
More recently, Gelesis 100 has been proposed as a novel, nonsystemic, super absorbent hydrogel that modifies several gastrointestinal functions (50). For example, the modified cellulose cross linked with citric acid creates a three-dimensional matrix and, after administration before a meal, the particles rapidly absorb water in the stomach and are homogeneously mixed with ingested foods. Thus, Gelesis 100 occupies about 25% of stomach volume, resulting in firm consistency similar to that of ingested vegetables, but without adding caloric value. This approach has been associated with important levels of weight loss over a 6-month trial, including significant increases in the percentage of patients achieving greater than 7.5 and 10% weight loss, as well as improvement in pre-diabetes.
Finally it is worth noting that combination therapies are being developed to enhance weight loss in obesity, and these approaches are reviewed in greater detail elsewhere (51). With all combination therapies, it is important to emphasize the roles of intensive behavioral and dietetic therapies, as has been demonstrated with liraglutide, 3.0 mg, in a randomized, controlled study (52). Interestingly, several of the combination therapies include agents, mechanisms or interventions that modify gastrointestinal functions or phenotypes, which re-emphasizes the potential to individualize treatment. The best examples would appear to relate to accelerated gastric emptying since so many treatments retard gastric emptying (including amylin, pramlintide, GLP-1 analogs or agonists, PYY, and sleeve gastroplasty) or alter gastric capacity (e.g. balloons, sleeve gastrectomy, reduced size of gastric remnant after Roux-en-Y gastric bypass) or a combination of both (balloon plus liraglutide). Thus, examples of combination therapies include the combinations of pramlintide and phentermine (53); amylin and bupropion-naltrexone (54); exenatide with dapagliflozin (55,56); incretin and pancreatic hormones that both generally inhibit upper gastrointestinal motor functions, specifically GLP-1, with glucagon (57), as well as a unimolecular dual incretin of PEGylated GLP-1/GIP co-agonist (58); combined infusions of GLP-1, PYY, and oxyntomodulin (59); endoscopic intervention for Roux-en-Y gastric bypass when there is weight regain; combination of intragastric balloon plus the GLP-1 analog, liraglutide (60); pramlintide with phentermine (53); endoscopic intervention (suturing or sclerosant injection into gastric pouch remnant) for Roux-en-Y gastric bypass after weight regain (61–63); combination of diet with repeated placement of intragastric balloon for the permitted 6 month period (64); combination of intragastric balloon plus the GLP-1 analog, liraglutide (65); and combined sleeve gastrectomy with duodenojejunal bypass (66).
Concluding Remarks and Future Perspectives
There are diverse obesity phenotypes, as have been demonstrated in adults. Essential next steps are documentation of these diverse phenotypes in children and adolescents and application of individual or combination therapies based on baseline phenotype measurements and comparisons with empiric therapy to ascertain whether there is, in fact, an advantage to individualizing treatment of obesity [as is being tested in adults (NCT03374956)] in all age groups. Given the prevalence and magnitude of the public health issues, the lack of approved nonsurgical approaches for children and adolescents, and the potential adverse effects of previously approved centrally acting agents such as rimonabant and sibutramine, a new approach is warranted to identify alternatives to bariatric surgery and endoscopy which are indicated in individuals with severe obesity, but cannot realistically be offered to the vast majority of pediatric patients. We propose that individualization based on obesity phenotype is one approach that deserves consideration and prioritization.
See Outstanding Questions Box.
Outstanding Questions.
-
How can community, behavioral or dietetic approaches become more effective in the management of pediatric obesity?
Explanation: In the long term, these approaches are the mainstays of management alone or in combination with other treatments for obesity.
-
Are the phenotypes associated with altered appetite that are identified in obese adults also recognized in the pediatric age group?
Explanation: If accelerated gastric emptying and reduced “satiety” incretin hormones are confirmed, or increased gastric volume with reduced satiation is confirmed, they would help in the selection of treatment strategy.
-
Orlistat is the only medication approved for treatment of obesity in children. Which medications currently approved in adults are efficacious in children?
Explanation: there is a need for controlled clinical trials of medications approved in adults such as liraglutide, bupropion-naltrexone, phentermine-topiramate, and lorcaserin.
-
What is the most efficacious and safe approach to combine non-surgical approaches to the treatment of obesity in the pediatric age group?
Explanation: There is no evidence to support combined treatments or to prioritize selection of treatments for pediatric obesity.
-
Given the efficacy of bariatric surgery and its proven mechanisms (gastric reservoir, increased incretin levels), is it possible to achieve the same effects without bariatric surgery and, if so, does the baseline phenotype impact the optimal approach?
Explanation: As in adults, these remain unachieved goals; however, knowing the baseline pathophysiology may assist prioritization of diverse treatments in combination with diet and behavioral treatment.
Trends.
Bariatric surgery represents the most efficacious weight loss treatment for obesity, with targets including reduced gastric capacity and fasting ghrelin and increased postprandial incretin levels.
Behavioral and dietetic treatment is the mainstay of long-term management alone or in combination with other treatments.
There are essentially no approved pharmacological treatments for obesity in the pediatric age group.
Different phenotypes that modify appetite and satiation are identified in obesity.
Identifying such phenotypes provides opportunities for individualizing treatment by targeting the putative mechanisms that increase appetite or reduce satiation in pediatric obesity.
Acknowledgments
Funding: Dr. Camilleri is supported by grant R01-DK67071 from National Institutes of Health.
Footnotes
Disclosures: Dr. Camilleri: partner in Phenomix Sciences; advisor to Kallyope; drugs donated for pilot clinical trials provided by Vivus, Inc and NovoNordisk
Dr. Staiano: clinical investigator for Aboca, Nestlé; consultant for Aboca and Nestlé; was consultant for D.M.G. Italy, data safety monitoring board member for Sucampo AG, and speaker for Aboca, Angelini, D.M.G. Italy, Valeas
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Blüher M (2019) Obesity: global epidemiology and pathogenesis. Nat. Rev. Endocrinol 15, 288–298 [DOI] [PubMed] [Google Scholar]
- 2.Khera AV et al. (2019) Polygenic prediction of weight and obesity trajectories from birth to adulthood. Cell 177, 587–596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kumar S et al. (2017) Review of childhood obesity: from epidemiology, etiology, and comorbidities to clinical assessment and treatment. Mayo Clin. Proc 92, 251–265 [DOI] [PubMed] [Google Scholar]
- 4.Acosta A et al. (2017) White Paper AGA: POWER - Practice guide on obesity and weight management, education, and resources. Clin. Gastroenterol. Hepatol 15, 631–649, e10 [DOI] [PubMed] [Google Scholar]
- 5.Thompson KL et al. (2019) The effectiveness of nutrition specialists on pediatric weight management outcomes in multicomponent pediatric weight management interventions: a systematic review and exploratory meta-analysis. J. Acad. Nutr. Diet 119, 799–817, e43 [DOI] [PubMed] [Google Scholar]
- 6.Pfeifflé S et al. (2019) Current recommendations for nutritional management of overweight and obesity in children and adolescents: a structured framework. Nutrients 11, pii.E362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kim J et al. (2017) Overweight or obesity in children aged 0 to 6 and the risk of adult metabolic syndrome: a systematic review and meta-analysis. J. Clin. Nurs 26, 3869–3880 [DOI] [PubMed] [Google Scholar]
- 8.Moores CJ et al. (2018) A systematic review of community-based interventions for the treatment of adolescents with overweight and obesity. Obes. Rev 19, 698–715 [DOI] [PubMed] [Google Scholar]
- 9.Heerman WJ et al. (2017) The dose of behavioral interventions to prevent and treat childhood obesity: a systematic review and meta-regression. Int. J. Behav. Nutr. Phys. Act 14, 157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Al-Khudairy L et al. (2017) Diet, physical activity and behavioural interventions for the treatment of overweight or obese adolescents aged 12 to 17 years. Cochrane Database Syst. Rev 6, CD012691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mead E et al. (2017) Diet, physical activity and behavioural interventions for the treatment of overweight or obese children from the age of 6 to 11 years. Cochrane Database Syst. Rev 6, CD012651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McDonagh MS et al. (2014) Systematic review of the benefits and risks of metformin in treating obesity in children aged 18 years and younger. JAMA Pediatr. 168, 178–184 [DOI] [PubMed] [Google Scholar]
- 13.Brufani C et al. (2013) Systematic review of metformin use in obese nondiabetic children and adolescents. Horm. Res. Paediatr 80, 78–85 [DOI] [PubMed] [Google Scholar]
- 14.Khokhar A et al. (2017) Metformin use in children and adolescents with prediabetes. Pediatr. Clin. North Am 64, 1341–1353 [DOI] [PubMed] [Google Scholar]
- 15.Mead E et al. (2016) Drug interventions for the treatment of obesity in children and adolescents. Cochrane Database Syst. Rev 11, CD012436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khera R et al. (2016) Association of pharmacological treatments for obesity with weight loss and adverse events: a systematic review and meta-analysis. JAMA 315, 2424–2434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pratt JSA et al. (2018) ASMBS pediatric metabolic and bariatric surgery guidelines, 2018. Surg. Obes. Relat. Dis 14, 882–901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thenappan A et al. (2019) Bariatric surgery in children: indications, types, and outcomes. Curr. Gastroenterol. Rep 21, 24. [DOI] [PubMed] [Google Scholar]
- 19.Arafat M et al. (2019) Characterizing bariatric surgery utilization and complication rates in the adolescent population. J. Pediatr. Surg 54, 288–292 [DOI] [PubMed] [Google Scholar]
- 20.Black JA et al. (2013) Bariatric surgery for obese children and adolescents: a systematic review and meta-analysis. Obes. Rev 14, 634–644 [DOI] [PubMed] [Google Scholar]
- 21.Paulus GF et al. (2015) Bariatric surgery in morbidly obese adolescents: a systematic review and meta-analysis. Obes. Surg 25, 860–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shoar S et al. (2017) Long-term outcome of bariatric surgery in morbidly obese adolescents: a systematic review and meta-analysis of 950 patients with a minimum of 3 years follow-up. Obes. Surg 27, 3110–3117 [DOI] [PubMed] [Google Scholar]
- 23.Pedroso FE et al. (2018) Weight loss after bariatric surgery in obese adolescents: a systematic review and meta-analysis. Surg. Obes. Related Dis 14, 413–423 [DOI] [PubMed] [Google Scholar]
- 24.Inge TH et al. (2019) Five-year outcomes of gastric bypass in adolescents as compared with adults. N. Engl. J. Med 380, 2136–2145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Michalsky MP et al. (2018) Cardiovascular risk factors after adolescent bariatric surgery. Pediatrics 141, pii: e20172485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Reece LJ et al. (2017) Intra-gastric balloon as an adjunct to lifestyle support in severely obese adolescents; impact on weight, physical activity, cardiorespiratory fitness and psychosocial well-being. Int. J. Obes. (Lond.) 41, 591–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fittipaldi-Fernandez RJ et al. (2017) Efficacy of intragastric balloon treatment for adolescent obesity. Obes. Surg 27, 2546–2551 [DOI] [PubMed] [Google Scholar]
- 28.Sachdev P et al. (2018) Intragastric balloon as an adjunct to lifestyle programme in severely obese adolescents: impact on biomedical outcomes and skeletal health. Int. J. Obes. (Lond.) 42, 115–118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alqahtani AR et al. (2018) Endoscopic sleeve gastroplasty in children and adolescents with obesity: outcomes during the first year. SOARD 14 (Issue 11, Supplement), S53 [Google Scholar]
- 30.Valentino MA et al. (2010) Central and peripheral molecular targets for antiobesity pharmacotherapy. Clin. Pharmacol. Ther 87, 652–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colon-Gonzalez F et al. (2013) Obesity pharmacotherapy: what is next? Mol. Aspects Med 34, 71–83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chial HJ, et al. (2002) A nutrient drink test to assess maximum tolerated volume and postprandial symptoms: effects of gender, body mass index and age in health. Neurogastroenterol. Motil 14, 249–253 [DOI] [PubMed] [Google Scholar]
- 33.Acosta A et al. (2015) Quantitative gastrointestinal and psychological traits associated with obesity and response to weight-loss therapy. Gastroenterology 148, 537–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bray GA et al. (2016) Management of obesity. Lancet 387, 1947–1956 [DOI] [PubMed] [Google Scholar]
- 35.Camilleri M et al. (2016) Gastrointestinal traits: individualizing therapy for obesity with drugs and devices. Gastrointest. Endosc 83, 48–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Acosta A et al. (2015) Exenatide in obesity with accelerated gastric emptying: a randomized, pharmacodynamics study. Physiol. Rep 3, pii: e12610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Halawi H et al. (2017) Effects of liraglutide on weight, satiation, and gastric functions in obesity: a randomised, placebo-controlled pilot trial. Lancet Gastroenterol. Hepatol 2, 890–899 [DOI] [PubMed] [Google Scholar]
- 38.Farr OM et al. (2016) GLP-1 receptors exist in the parietal cortex, hypothalamus and medulla of human brains and the GLP-1 analogue liraglutide alters brain activity related to highly desirable food cues in individuals with diabetes: a crossover, randomised, placebo-controlled trial. Diabetologia 59, 954–965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Abu Dayyeh BK et al. (2017) Endoscopic sleeve gastroplasty alters gastric physiology and induces loss of body weight in obese individuals. Clin. Gastroenterol. Hepatol 15, 37–43, e1 [DOI] [PubMed] [Google Scholar]
- 40.Gómez V et al. (2016) Delayed gastric emptying as a proposed mechanism of action during intragastric balloon therapy: results of a prospective study. Obesity 24, 1849–1853 [DOI] [PubMed] [Google Scholar]
- 41.Vargas EJ et al. (2019) Changes in time of gastric emptying after surgical and endoscopic bariatrics and weight loss: a systematic review and meta-analysis. Clin. Gastroenterol. Hepatol pii: S1542–3565(19)30363–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Russo A et al. (2003) Guar attenuates fall in postprandial blood pressure and slows gastric emptying of oral glucose in type 2 diabetes. Dig. Dis. Sci 48, 1221–1229 [DOI] [PubMed] [Google Scholar]
- 43.Hoad CL et al. (2004) In vivo imaging of intragastric gelation and its effect on satiety in humans. J. Nutr 134, 2293–2300 [DOI] [PubMed] [Google Scholar]
- 44.Jenkins DJ et al. (1988) Manipulation of gut hormone response to food by soluble fiber and alpha-glucosidase inhibition. Am. J. Gastroenterol 83, 393–397 [PubMed] [Google Scholar]
- 45.Jenkins DJ et al. (1979) Combined use of guar and acarbose in reduction of postprandial glycaemia. Lancet 2, 924–927 [DOI] [PubMed] [Google Scholar]
- 46.Williams JA et al. (2004) Inclusion of guar gum and alginate into a crispy bar improves postprandial glycemia in humans. J. Nutr 134, 886–889 [DOI] [PubMed] [Google Scholar]
- 47.Berthold HK et al. (2008) Effect of a cellulose-containing weight-loss supplement on gastric emptying and sensory functions. Obesity 16, 2272–2280 [DOI] [PubMed] [Google Scholar]
- 48.Krakamp B et al. (2001) Adipositastherapie—Plazebokontrollierte Doppelblindstudie zur Wirksamkeit von CM3. Kassenarzt 11, 45–47 [Google Scholar]
- 49.Odunsi ST et al. (2010) Effect of alginate on satiation, appetite, gastric function and selected gut satiety hormones in overweight and obesity. Obesity 18, 1579–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Greenway FL et al. (2019) A randomized, double-blind, placebo-controlled study of Gelesis100: a novel nonsystemic oral hydrogel for weight loss. Obesity 27, 205–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Camilleri M et al. (2018) Combination therapies for obesity. Metab. Syndr. Relat. Disord 16, 390–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wadden TA et al. (2019) Intensive behavioral therapy for obesity combined with liraglutide 3.0 mg: a randomized controlled trial. Obesity 27, 75–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Aronne LJ et al. (2010) Enhanced weight loss following coadministration of pramlintide with sibutramine or phentermine in a multicenter trial. Obesity 18, 1739–1746 [DOI] [PubMed] [Google Scholar]
- 54.Clapper JR et al. (2013) Effects of amylin and bupropion/naltrexone on food intake and body weight are interactive in rodent models. Eur. J. Pharmacol 698, 292–298 [DOI] [PubMed] [Google Scholar]
- 55.Frías JP et al. (2016) Exenatide once weekly plus dapagliflozin once daily versus exenatide or dapagliflozin alone in patients with type 2 diabetes inadequately controlled with metformin monotherapy (DURATION-8): a 28 week, multicentre, double-blind, phase 3, randomised controlled trial. Lancet Diabetes Endocrinol. 4, 1004–1016 [DOI] [PubMed] [Google Scholar]
- 56.Jabbour SA et al. (2018) Effects of exenatide once weekly plus dapagliflozin, exenatide once weekly, or dapagliflozin, added to metformin monotherapy, on body weight, systolic blood pressure, and triglycerides in patients with type 2 diabetes in the DURATION-8 study. Diabetes Obes. Metab 20, 1515–1519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tan TM et al. (2013) Coadministration of glucagon-like peptide-1 during glucagon infusion in humans results in increased energy expenditure and amelioration of hyperglycemia. Diabetes 62, 1131–1138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Finan B et al. (2013) Unimolecular dual incretins maximize metabolic benefits in rodents, monkeys, and humans. Sci. Transl. Med 5, 209ra151. [DOI] [PubMed] [Google Scholar]
- 59.Tan T et al. (2017) The effect of a subcutaneous infusion of GLP-1, OXM, and PYY on energy intake and expenditure in obese volunteers. J. Clin. Endocrinol. Metab 102, 2364–2372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mosli MM et al. (2017) Does combining liraglutide with intragastric balloon insertion improve sustained weight reduction? Saudi J. Gastroenterol 23, 117–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thompson CC et al. (2013) Endoscopic suturing for transoral outlet reduction increases weight loss after Roux-en-Y gastric bypass surgery. Gastroenterology 145, 129–137, e3 [DOI] [PubMed] [Google Scholar]
- 62.Kumar N et al. (2016) Transoral outlet reduction for weight regain after gastric bypass: long-term follow-up. Gastrointest. Endosc 83, 776–779 [DOI] [PubMed] [Google Scholar]
- 63.Riva P et al. (2017) Weight regain following RYGB can be effectively treated using a combination of endoscopic suturing and sclerotherapy. Surg. Endosc 31, 1891–1895 [DOI] [PubMed] [Google Scholar]
- 64.Genco A et al. (2010) Intragastric balloon followed by diet vs intragastric balloon followed by another balloon: a prospective study on 100 patients. Obes. Surg 20, 1496–1500 [DOI] [PubMed] [Google Scholar]
- 65.Mosli MM et al. (2017) Does combining liraglutide with intragastric balloon insertion improve sustained weight reduction? Saudi J. Gastroenterol 23, 117–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Seki Y et al. (2017) Five-year results of laparoscopic sleeve gastrectomy with duodenojejunal bypass for weight loss and type 2 diabetes mellitus. Obes. Surg 27, 795–801 [DOI] [PubMed] [Google Scholar]