Abstract
The molecular mechanisms underlying the development and progression of colorectal cancer (CRC) have not been clarified. The purpose of the present study was to identify key genes that may serve as novel therapeutic targets or prognostic predictors in patients with CRC using bioinformatics analysis. Four gene expression datasets were downloaded from the Gene Expression Omnibus database, which revealed 19 upregulated and 34 downregulated differentially expressed genes (DEGs). The downregulated DEGs were significantly enriched in eight pathways according to Kyoto Encyclopedia of Genes and Genomes pathway enrichment analysis. A protein-protein interaction network was constructed with 52 DEGs and 458 edges. Ten key genes were identified according to the degree value, betweenness centrality and closeness centrality. Survival analysis revealed that low expression of four of the ten genes, carcinoembryonic antigen related cell adhesion molecule 7 (CEACAM7), solute carrier family 4 member 4 (SLC4A4), glucagon (GCG) and chloride channel accessory 1 (CLCA1) genes, were associated with unfavorable prognosis in CRC. Furthermore, gene set enrichment analysis revealed that two pathways were significantly enriched in the CEACAM7 low-expression group. Thus, CEACAM7, SLC4A4, GCG and CLCA1 may be prognostic markers or therapeutic targets of CRC. Low CEACAM7 expression may be associated with the activation of glycosaminoglycan biosynthesis-chondroitin sulfate and extracellular matrix receptor interaction pathways and may affect the prognosis of CRC.
Keywords: bioinformatics, genes, prognosis, colorectal cancer
Introduction
Colorectal cancer (CRC), which includes colon cancer and rectum cancer, is the third most common type of cancer and was the second leading cause of cancer-associated mortality for men and women worldwide in 2018 (1). It was estimated that there were more than 1.8 million new cases and over 0.8 million mortalities worldwide due to CRC in 2018 (1). In the USA, the number of newly diagnosed CRC cases in 2019 was ~145,600, accounting for 8.3% of all new cancer cases (2). Furthermore, the number of CRC-associated deaths in 2019 was ~51,020 in the USA, accounting for 8.4% of all cancer-associated deaths (2). The survival and prognosis of patients with CRC are closely associated with the staging of the tumor. If the tumors are diagnosed early and removed, the disease may be curable. The 5-year survival rate of patients with localized CRC was ~90% in the USA between 2001 and 2007 (3). However, in CRC cases at regional and distant stages, the 5-year survival rates are only ~70 and ~11%, respectively (3). Unfortunately, in developed countries including the USA, only ~40% of patients with CRC are diagnosed at early stages (4). Therefore, the identification of diagnostic and prognostic molecular markers for early detection and the prediction of prognosis for patients with CRC is clinically important.
The development of CRC involves interconnections between environmental and genetic factors. In recent decades, great progress has been made in understanding the molecular pathogenesis of CRC, which includes four main mechanisms of molecular pathogenesis: Adenoma-carcinoma sequence, inherited forms, mismatch repair deficiency, and high-level microsatellite instability (5). However, the precise molecular mechanisms underlying the development of CRC have not been fully elucidated. With the rapid development of bioinformatics and high-throughput platforms for detecting gene expression, screening key genes for CRC based on publicly available databases provides a strategy to clarify the molecular mechanisms of CRC. Large numbers of gene expression datasets for CRC are available in the Gene Expression Omnibus (GEO) database, and numerous studies have used these datasets for the identification of differentially expressed genes (DEGs) in CRC (6–9). Previous studies have revealed the prognostic value of certain DEGs in CRC (10–13). However, the results of these individual studies varied and the studies demonstrated differences in sample collection, platform types and analysis methods. Furthermore, large-scale studies on the prognostic value of the DEGs in CRC are lacking. In addition, the enrichment pathways, gene set enrichment analysis, Gene Ontology (GO) functions and the interaction network involved in the DEGs remain to be clarified.
In order to overcome these shortcomings, the present study integrated and reanalyzed four online GEO datasets of CRC using bioinformatics analysis methods. DEGs between CRC samples and noncancerous samples were determined, and the interaction network among these DEGs was constructed. Enrichment analysis for these DEGs was conducted using the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathways and GO functions. Through network analysis, gene expression confirmation and overall survival analysis, four key genes that were associated with the prognosis of CRC were identified. These four genes may provide valuable information for the identification of potential prognostic markers of CRC and to elucidate the molecular mechanism of CRC.
Materials and methods
Data source and identification of DEGs
In order to compensate for the limitation of small sample size and result offset in a single cohort study, four gene expression profiles (GSE113513, GSE87211, GSE35279 and GSE24551) were acquired from the GEO database (http://www.ncbi.nlm.nih.gov/geo/) between January 1 2010 and August 31 2018. These profiles included both CRC samples and noncancerous samples, and all datasets contained at least five samples in each group (Table I). GSE113513 (unpublished, 2018; http://www.ncbi.nlm.nih.gov/geo/query/acc.cgi?acc=GSE113513) was based on the platform GPL15207 [(PrimeView) Affymetrix Human Gene Expression Array], GSE87211 was based on the platform GPL13497 (Agilent-026652 Whole Human Genome Microarray 4×44K v2) (6), GSE35279 was based on the platform GPL6480 (Agilent-014850 Whole Human Genome Microarray 4×44K G4112F) (7), and GSE24551 was based on the platform GPL5175 [(HuEx-1_0-st) Affymetrix Human Exon 1.0 ST Array] [transcript (gene) version] (8).
Table I.
Sample numbers in the four GSE datasets.
| Dataset ID | CRC | Noncancerous tissues | Total |
|---|---|---|---|
| GSE113513 | 14 | 14 | 28 |
| GSE87211 | 203 | 160 | 363 |
| GSE35279 | 74 | 5 | 79 |
| GSE24551 | 160 | 13 | 173 |
CRC, colorectal cancer.
The DEGs between CRC samples and noncancerous samples in the four GEO series were filtered using the online GEO2R tool (https://www.ncbi.nlm.nih.gov/geo/geo2r/) (14,15). Genes that satisfied the threshold [|log2fold change (FC)| ≥2.0; adjusted P<0.05] were classified as DEGs. Statistical analysis was performed for each dataset. The overlapping genes among the four profiles were determined and presented by the online tool Venny 2.1.0 (http://bioinfogp.cnb.csic.es/tools/venny/index.html) (16). The expression of the upregulated and downregulated DEGs in each dataset was visualized using a volcano plot generated by Sanger Box (version 0.0.9; http://sangerbox.com/).
GO and KEGG enrichment analysis
GO analysis is commonly performed for functional enrichment analysis, in which gene function is classified into biological process (BP), molecular function (MF) and cellular component (CC) terms (17,18). KEGG is frequently used for exploring the advanced functions and mechanisms involved in the biological system at the molecular level (19). In the present study, GO and KEGG analysis was completed with the Database for Annotation, Visualization and Integrated Discovery (DAVID) platform (version 6.8; http://david.ncifcrf.gov/) (20,21). Statistical significance was set as P<0.05.
Construction of a protein-protein interaction (PPI) network and identification of key genes
The GeneMANIA (http://genemania.org/) prediction server was designed to assess the PPI network (22). The network was analyzed and visualized by Cytoscape 3.6.1 (http://www.cytoscape.org/). In the network, a high degree value indicated a more essential role for that gene. The degree value of each gene was calculated by the network analyzer tool that was built in the Cytoscape software. The genes whose degree value, closeness centrality and betweenness centrality were greater than the median value were identified as key genes.
Confirmation of key genes and overall survival analysis
The expression of key genes in CRC samples and noncancerous samples were further examined using the Gene Expression Profiling Interactive Analysis (GEPIA) platform (http://gepia.cancer-pku.cn) (23). The expression profiles of key genes between tumor samples and adjacent normal samples of colon adenocarcinoma (COAD) and rectum adenocarcinoma (READ) were obtained from The Cancer Genome Atlas (TCGA) database by GEPIA (http://gepia.cancer-pku.cn) (23). Student's unpaired t-test was used to determine the statistical significance of the calculated differential expression. The fold change was defined as 2 and the P-value of significance was set at 0.01.
Overall survival analysis was performed in GEPIA by log-rank test based on gene expression. The overall survival analysis plot also contained the Cox proportional hazard ratio and the 95% confidence interval. The patients with CRC were divided into low-expression and high-expression groups, based on the median value of mRNA expression of the ten key genes. The differences between the two groups were evaluated separately for each of the ten key genes. P<0.05 was considered to indicate a statistically significant result.
Gene set enrichment analysis (GSEA) of prognosis-associated key genes
The pre-processed level 3 RNA-seq data and corresponding clinical information of patients with CRC were collected from the TCGA-COAD and TCGA-READ datasets (https://cancergenome.nih.gov/, updated in September 2018). GSEA (http://www.broadinstitute.org/gsea/index.jsp) (24,25) for the prognosis-associated key genes was performed on the TCGA datasets. The c2.cp.kegg.v6.0.symbols.gmt dataset was obtained from the molecular signatures database (MSigDB) v6.0 on the GSEA website. The CRC samples obtained from the TCGA database were divided into high- and low-expression groups according to the median expression level of prognosis-associated genes. The samples were analyzed by default weighted enrichment statistics using the GSEA 3.0 software. In the present study, the gene sets satisfying nominal P<0.05 and false discovery rate (FDR)<0.25 were considered to be significantly enriched. The enrichment analysis was carried out by default weighted enrichment statistics, and the number of random permutations was set to 1,000 times.
Results
Identification of DEGs
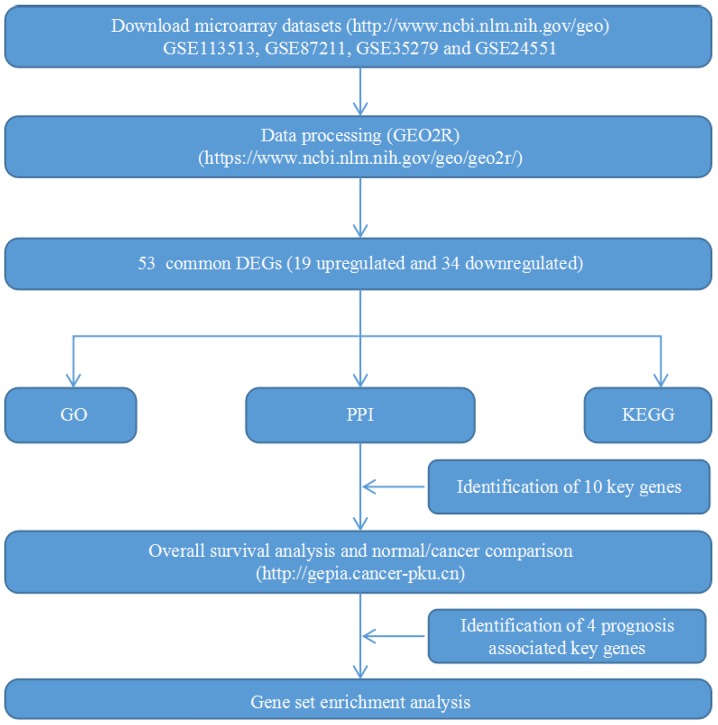
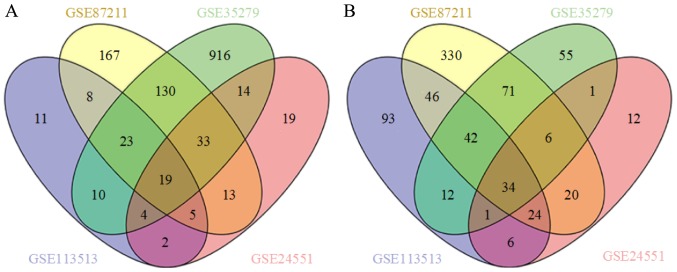
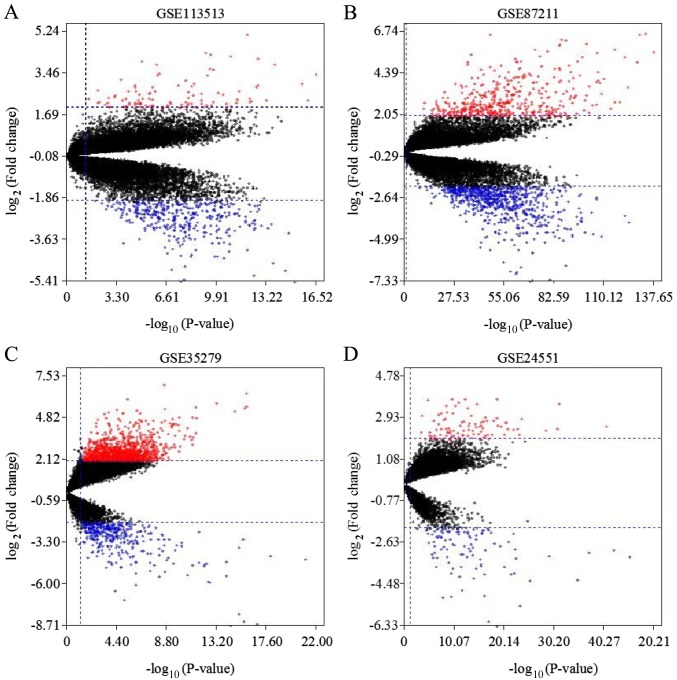
A flow chart of the present study design is presented in Fig. 1. Four GEO datasets (GSE113513, GSE87211, GSE35279 and GSE24551) were downloaded. The numbers of CRC and noncancerous samples in each dataset are presented in Table I. GSE113513 consisted of 14 CRC samples and 14 noncancerous tissues samples; GSE87211 included 203 CRC samples and 160 noncancerous tissues samples; GSE35279 contained 74 CRC samples and 5 noncancerous tissues samples; and GSE24551 included 160 CRC samples and 13 noncancerous tissues samples. GSE113513 comprised 340 DEGs, 258 of which were upregulated genes and 82 were downregulated genes. GSE87211 comprised 971 DEGs, including 573 upregulated genes and 398 downregulated genes. GSE35279 included 1371 DEGs, with 222 upregulated genes and 1149 downregulated genes. GSE24551 comprised 213 DEGs, with 109 upregulated and 104 downregulated genes. The intersection of the DEGs is presented in Venn diagrams (Fig. 2). The DEGs in each dataset are presented in volcano plots (Fig. 3). In total, 53 common DEGs (19 upregulated and 34 downregulated) were identified among all four datasets.
Figure 1.
Flow chart of the present study. GEO, Gene Expression Omnibus; GO, Gene Ontology; PPI, protein-protein interaction; KEGG, Kyoto Encyclopedia of Genes and Genomes; DEGs, differentially expressed genes.
Figure 2.
Venn diagram of DEGs in datasets GSE113513, GSE87211, GSE35279 and GSE24551. (A) A total of 19 upregulated common DEGs were extracted from datasets GSE113513, GSE87211, GSE35279 and GSE24551 with a threshold of |log2FC|≥2.0 and adjusted P<0.05. (B) In total, 34 downregulated common DEGs were extracted from datasets GSE113513, GSE87211, GSE35279 and GSE24551 with a threshold of |log2FC|≥2.0 and adjusted P<0.05. FC, fold change; DEGs, differentially expressed genes.
Figure 3.
Volcano plot of DEGs in datasets GSE113513, GSE87211, GSE35279 and GSE24551. Red dots represent upregulated DEGs and blue dots represent downregulated DEGs with a threshold of |log2FC|≥2.0 and adjusted P<0.05 in datasets (A) GSE113513, (B) GSE87211, (C) GSE35279 and (D) GSE24551. DEGs, differentially expressed genes; FC, fold change.
GO and KEGG enrichment analysis
GO and KEGG analyses of the DEGs were performed using DAVID and the results are summarized in Table II. BP analysis indicated that upregulated genes were enriched in proteolysis, response to tumor necrosis factor, and positive regulation of gene expression, whereas the downregulated genes were enriched in seven terms comprised ‘bicarbonate transport’, ‘one-carbon metabolic process’, ‘regulation of chloride transport’, ‘positive regulation of cellular pH reduction’, ‘chloride transmembrane transport’, ‘ethanol oxidation’ and ‘positive regulation of synaptic transmission’. Analysis of the CC function indicated that the main enriched functions of upregulated genes involved the proteinaceous extracellular matrix and extracellular space, whereas the downregulated genes involved the seven terms ‘apical plasma membrane’, ‘basolateral plasma membrane’, ‘extracellular exosome’, ‘anchored component of membrane’, ‘plasma membrane’, ‘zymogen granule membrane’ and ‘integral component of membrane’. Analysis of the MF, identified calcium ion binding as a significantly enriched term of upregulated genes, whereas nine terms were significantly enriched in downregulated genes, including ‘carbonate dehydratase activity’, ‘chloride channel activity’, ‘zinc-dependent alcohol dehydrogenase activity’, ‘arylesterase activity’, ‘alcohol dehydrogenase (NAD) activity’, ‘zinc ion binding’, ‘intracellular calcium activated chloride channel activity’, ‘retinol dehydrogenase activity’ and ‘carbohydrate binding’. Furthermore, KEGG analysis revealed that downregulated genes were significantly enriched in nitrogen metabolism, proximal tubule bicarbonate reclamation and six other pathways.
Table II.
Enriched GO terms and KEGG pathways of DEGs.
| A, Upregulated expression | ||
|---|---|---|
| Category | Description | P-value |
| BP | GO:0006508-proteolysis | 1.10×10−2 |
| BP | GO:0034612-response to tumor necrosis factor | 2.36×10−2 |
| BP | GO:0010628-positive regulation of gene expression | 2.52×10−2 |
| CC | GO:0005578-proteinaceous extracellular matrix | 2.18×10−3 |
| CC | GO:0005615-extracellular space | 8.32×10−3 |
| MF | GO:0005509-calcium ion binding | 3.87×10−2 |
| B, Downregulated expression | ||
| Category | Description | P-value |
| BP | GO:0015701-bicarbonate transport | 1.11×10−8 |
| BP | GO:0006730-one-carbon metabolic process | 1.91×10−7 |
| BP | GO:2001225-regulation of chloride transport | 3.45×10−3 |
| BP | GO:0032849-positive regulation of cellular pH reduction | 6.89×10−3 |
| BP | GO:1902476-chloride transmembrane transport | 1.12×10−2 |
| BP | GO:0006069-ethanol oxidation | 2.05×10−2 |
| BP | GO:0032230-positive regulation of synaptic transmission, GABAergic | 2.05×10−2 |
| CC | GO:0016324-apical plasma membrane | 1.61×10−3 |
| CC | GO:0016323-basolateral plasma membrane | 3.81×10−3 |
| CC | GO:0070062-extracellular exosome | 6.42×10−3 |
| CC | GO:0031225-anchored component of membrane | 1.67×10−2 |
| CC | GO:0005886-plasma membrane | 1.71×10−2 |
| CC | GO:0042589-zymogen granule membrane | 1.92×10−2 |
| CC | GO:0016021-integral component of membrane | 4.47×10−2 |
| MF | GO:0004089-carbonate dehydratase activity | 8.01×10−9 |
| MF | GO:0005254-chloride channel activity | 4.13×10−3 |
| MF | GO:0004024-alcohol dehydrogenase activity, zinc-dependent | 1.06×10−2 |
| MF | GO:0004064-arylesterase activity | 1.06×10−2 |
| MF | GO:0004022-alcohol dehydrogenase (NAD) activity | 1.24×10−2 |
| MF | GO:0008270-zinc ion binding | 1.54×10−2 |
| MF | GO:0005229-intracellular calcium activated chloride channel activity | 2.81×10−2 |
| MF | GO:0004745-retinol dehydrogenase activity | 3.15×10−2 |
| MF | GO:0030246-carbohydrate binding | 4.72×10−2 |
| KEGG | hsa00910-nitrogen metabolism | 1.49×10−7 |
| KEGG | hsa04964-proximal tubule bicarbonate reclamation | 4.18×10−5 |
| KEGG | hsa04972-pancreatic secretion | 2.67×10−3 |
| KEGG | hsa00830-retinol metabolism | 1.60×10−2 |
| KEGG | hsa00010-glycolysis/Gluconeogenesis | 1.74×10−2 |
| KEGG | hsa00982-drug metabolism-cytochrome P450 | 1.79×10−2 |
| KEGG | hsa00980-metabolism of xenobiotics by cytochrome P450 | 2.10×10−2 |
| KEGG | hsa05204-chemical carcinogenesis | 2.43×10−2 |
GO, Gene Ontology; KEGG, Kyoto Encyclopedia of Genes and Genomes; DEGs, differentially expressed genes; BP, biological process; CC, cellular component; MF, molecular function; GABA, gamma aminobutyric acid.
PPI network and key genes
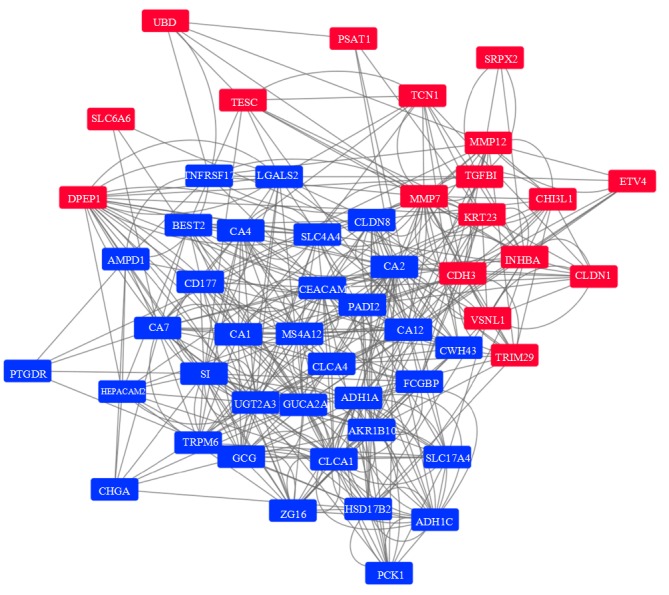
The PPI network of the 53 DEGs was generated with the GeneMANIA platform (22) and visualized by Cytoscape (26) (Fig. 4). Following the removal of the single epoxide hydrolase 4 gene that had no connections in the network, the network contained 52 DEGs and 458 edges. The connections between the DEGs included physical interactions, co-expression and co-localization. Among the DEGs, ten genes were selected as key genes associated with CRC. The carbonic anhydrase 2 (CA2) gene was the most connected gene (degree, 44), followed by the guanylate cyclase activator 2A gene (GUCA2A; degree, 35), and carcinoembryonic antigen-related cell adhesion molecule 7 gene (CEACAM7; degree, 34), among others (Table III). Only one gene, matrix metalloprotease 7 (MMP7), was upregulated in the CRC samples; the other nine genes were downregulated.
Figure 4.
Protein-protein interaction network among the DEGs. Red nodes indicate upregulated DEGs while blue nodes indicate downregulated DEGs. The grey lines represent the connections between the DEGs. DEGs, differentially expressed genes.
Table III.
Degree value of ten key genes.
| Gene symbol | Description | Degree | Betweenness centrality | Closeness centrality |
|---|---|---|---|---|
| CA2 | Carbonic anhydrase 2 | 44 | 7.61×10−2 | 6.80×10−1 |
| GUCA2A | Guanylate cyclase activator 2A | 35 | 8.12×10−2 | 6.80×10−1 |
| CEACAM7 | Carcinoembryonic antigen-related cell adhesion molecule 7 | 34 | 4.07×10−2 | 6.30×10−1 |
| MMP7 | Matrix metalloproteinase-7 | 33 | 4.37×10−2 | 6.07×10−1 |
| SLC4A4 | Solute carrier family 4 member 4 | 26 | 2.90×10−2 | 5.86×10−1 |
| CA12 | Carbonic anhydrase 12 | 25 | 3.27×10−2 | 6.14×10−1 |
| GCG | Glucagon | 25 | 1.79×10−2 | 6.00×10−1 |
| MS4A12 | Membrane-spanning 4-domains subfamily A member 12 | 24 | 1.04×10−2 | 5.93×10−1 |
| CA1 | Carbonic anhydrase 1 | 24 | 9.63×10−3 | 5.93×10−1 |
| CLCA1 | Calcium-activated chloride channel regulator 1 | 24 | 8.05×10−3 | 5.73×10−1 |
CA2, carbonic anhydrase 2; GUCA2A, guanylate cyclase activator 2A; CEACAM7, carcinoembryonic antigen-related cell adhesion molecule 7; MMP7, matrix metalloproteinase-7; SLC4A4, solute carrier family 4 member 4; CA, carbonic anhydrase 12; GCG, glucagon; MS4A12, membrane-spanning 4-domains subfamily A member 12; CLCA1, calcium-activated chloride channel regulator 1.
Confirmation and survival analysis of ten key genes
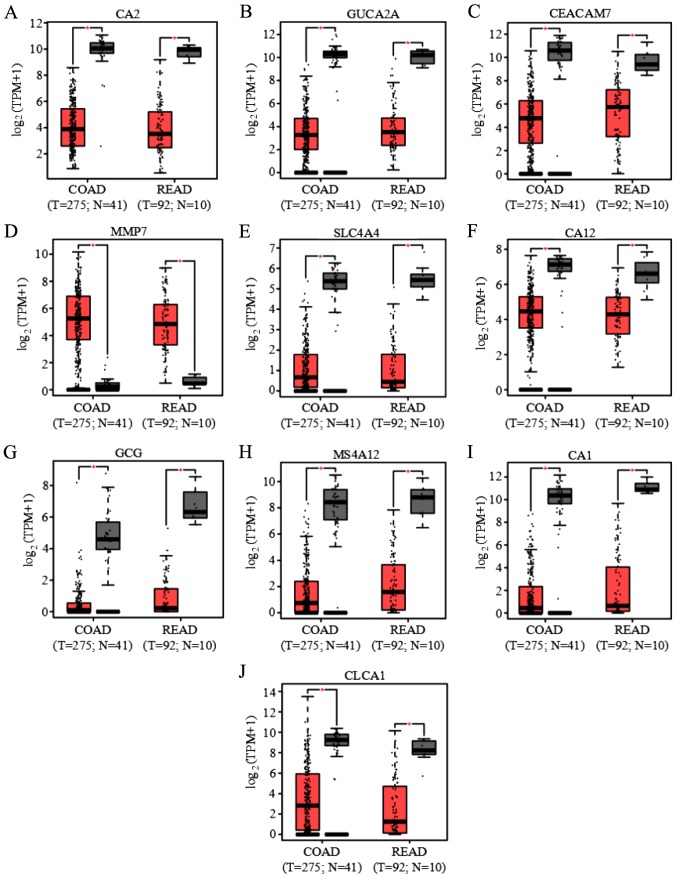
GEPIA (23) was used to compare the expression levels of the ten key genes between CRC tumor samples and adjacent normal samples in COAD and READ obtained from the TCGA database. A total of 275 tumor samples and 41 adjacent normal samples in COAD and 92 tumor samples and ten adjacent normal samples in READ were available for analyses. Among the ten genes, only MMP7 was significantly upregulated in tumor samples compared with control samples, whereas the other nine genes were significantly downregulated in tumor samples (Fig. 5). The differential expression of the ten key genes in the present study was confirmed in GEPIA.
Figure 5.
Boxplots of the expression of ten key genes in COAD and READ. Gene expression in COAD and READ of (A) CA2, (B) GUCA2A, (C) CEACAM7, (D) MMP7, (E) SLC4A4, (F) CA12, (G) GCG, (H) MS4A12, (I) CA1 and (J) CLCA1. *P<0.01 vs. the noncancerous group. COAD, colon adenocarcinoma; READ, rectum adenocarcinoma; T, tumor; N, normal; CA2, carbonic anhydrase 2; GUCA2A, guanylate cyclase activator 2A; CEACAM7, carcinoembryonic antigen-related cell adhesion molecule 7; MMP7, matrix metalloproteinase-7; SLC4A4, solute carrier family 4 member 4; CA2, carbonic anhydrase 2; GCG, glucagon; MS4A12, membrane-spanning 4-domains subfamily A member 12; CLCA1, calcium-activated chloride channel regulator 1; TPM, transcripts per million.
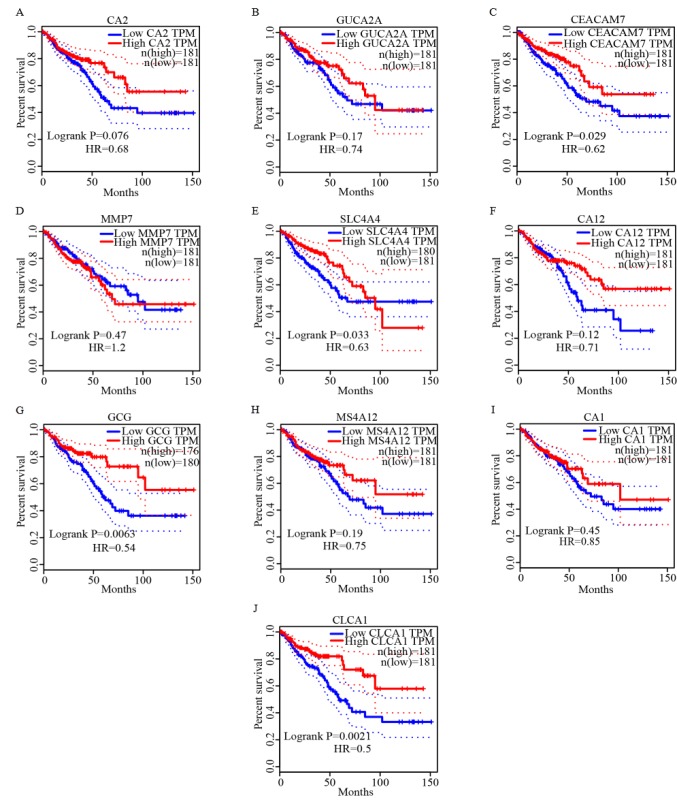
The overall survival analysis of the ten genes was also obtained from GEPIA. Among these key genes, low expression of CEACAM7, solute carrier family 4 member 4 (SLC4A4), glucagon (GCG) and chloride channel accessory 1 (CLCA1) genes were significantly associated with an unfavorable outcome of CRC (Fig. 6).
Figure 6.
Survival curves of overall survival analysis of ten key genes. Survival curves of overall survival time of (A) CA2, (B) GUCA2A, (C) CEACAM7, (D) MM7, (E) SLC4A4, (F) CA12, (G) GCG, (H) MS4A12, (I) CA1 and (J) CLCA1. CA2, carbonic anhydrase 2; GUCA2A, guanylate cyclase activator 2A; CEACAM7, carcinoembryonic antigen-related cell adhesion molecule 7; MMP7, matrix metalloproteinase-7; SLC4A4, solute carrier family 4 member 4; GCG, glucagon; MS4A12, membrane-spanning 4-domains subfamily A member 12; CA2, carbonic anhydrase 2; CLCA1, calcium-activated chloride channel regulator 1; TPM, transcripts per million.
GSEA of the four prognosis-associated key genes
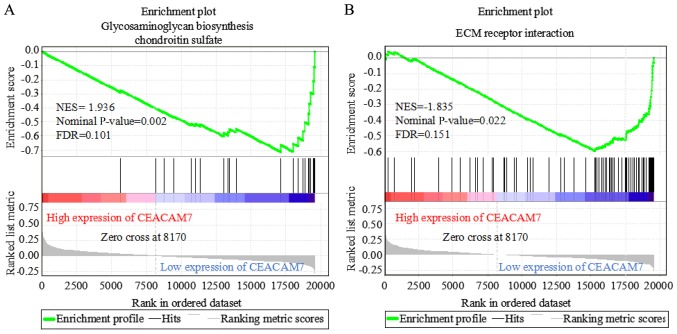
The mechanism of the four prognosis-associated genes (CEACAM7, SLC4A4, GCG and CLCA1) was further investigated by examining the associated pathways via GSEA. The expression matrix of CRC from the TCGA database was divided into high-expression (323 samples) and low-expression (324 samples) groups, according to the median expression level of CEACAM7, SLC4A4, GCG and CLCA1. In the low CEACAM7 expression group, two significantly enriched KEGG pathways at nominal P<0.05 and FDR<0.25 were identified, including glycosaminoglycan biosynthesis-chondroitin sulfate (nominal P=0.002; FDR, 0.101) and extracellular matrix-receptor interaction (nominal P=0.022; FDR, 0.151) (Fig. 7). However, no pathway was significantly enriched in the low SLC4A4, GCG and CLCA1 expression groups.
Figure 7.
Gene set enrichment analysis of CEACAM7. Two significantly enriched pathways were identified in the low CEACAM7 expression group of patients with CRC at nominal P<0.05 and FDR<0.25, including (A) glycosaminoglycan biosynthesis-chondroitin sulfate (nominal P=0.002; FDR, 0.101) and (B) ECM receptor interaction (nominal P=0.022; FDR, 0.151). CEACAM7, carcinoembryonic antigen-related cell adhesion molecule 7; CRC, colorectal cancer; NES, normalized enrichment score; FDR, false discovery rate; ECM, extracellular matrix.
Discussion
In the present study, 53 DEGs were identified in CRC samples compared with noncancerous tissues samples, including 19 upregulated genes and 34 downregulated genes. The downregulated DEGs were significantly enriched in eight KEGG pathways, including nitrogen metabolism, proximal tubule bicarbonate reclamation and six other pathways. The upregulated DEGs were associated with six GO terms: Proteolysis, response to tumor necrosis factor, positive regulation of gene expression, proteinaceous extracellular matrix, extracellular space and calcium ion binding. The downregulated DEGs were associated with 23 GO terms such as bicarbonate transport, one-carbon metabolic process and regulation of chloride transport, among others. A PPI network was constructed, consisting of 52 nodes and 458 edges, to evaluate the interactions among these DEGs. The ten key genes identified were CA2, GUCA2A, CEACAM7, MMP7, SLC4A4, CA12, GCG, membrane spanning 4-domains A12, CA1 and CLCA1. Only the MMP7 gene was upregulated in patients with CRC, whereas the other nine genes were downregulated. Confirmation and survival analyses of these genes were performed using GEPIA. Survival analysis revealed that low expressions of CEACAM7, SLC4A4, GCG and CLCA1 genes were significantly associated with unfavorable prognosis in patients with CRC. Furthermore, GSEA results showed that the glycosaminoglycan biosynthesis-chondroitin sulfate and extracellular matrix-receptor interaction pathways were significantly enriched in the low CEACAM7 expression group of patients with CRC.
Genetic factors serve a critical role in the pathogenesis of CRC (27). CEACAM7, also termed CGM2, is a member of the carcinoembryonic antigen family and is expressed on highly differentiated colorectal epithelial cells and within ducts of pancreas epithelial cells (28). CEACAM7 sequences were detected only in human cDNA libraries of pancreas, pancreatic islets, colonic tumors and colon (29). The very narrow expression spectrum of CEACAM7 in pancreatic and colonic epithelial cells indicated a highly specialized function. Thompson et al (30) reported that CEACAM7 was downregulated in colorectal carcinoma. Messick et al (31) showed that CEACAM7 was significantly decreased in rectal cancer and considered a predictor for the recurrence of rectal cancer. Schölzel et al (28) identified that CEACAM7 was downregulated in hyperplastic polyps as well as early adenomas, which indicated early detected subtleties at the molecular level that lead to CRC.
The present study demonstrated that the glycosaminoglycan biosynthesis-chondroitin sulfate and extracellular matrix receptor interaction pathways were significantly enriched in the group with low expression of CEACAM7. The extracellular matrix components closely interact with cell surface receptors, growth factors and cytokines, supporting a substantial role for the extracellular matrix in the morphogenesis of tissues and organs and in maintaining the structure and function of cells and tissues. In addition, the functional macromolecules of extracellular matrix are involved in regulating the properties and function of cells. Notably, surface molecules of matrix such as synaptophysin itself can also act as cell receptors or co-receptors. Consequently, the components of the extracellular matrix were closely associated with the cellular and molecular mechanisms of malignant cells (32). Recent studies have reported various roles of matrix molecules in tissue development, homeostasis and pathological processes (33–35). Glycosaminoglycans, a type of matrix molecule, affect the growth and progression of tumors by interacting with growth factors, cytokines and growth factor receptors (36). Furthermore, chondroitin sulfate is also a critical molecule in cancer progression (37). However, the association between CEACAM7 and glycosaminoglycan biosynthesis-chondroitin sulfate and extracellular matrix receptor interaction pathways has not yet been investigated. Further reports are required to clarify this potential connection.
SLC4A4 may regulate bicarbonate influx and efflux in the basolateral membrane of cells and regulate intracellular pH (38–42). Certain studies have shown that SLC4A4 is significantly downregulated in CRC (43–45). However, the mechanism of SLC4A4 in affecting the prognosis of CRC is not well studied, and future studies should examine the potential function of SLC4A4 in CRC.
GCG serves a key role in glucose metabolism and homeostasis. Much attention has been drawn to its low expression in CRC tissues (44,46–48). GCG is cleaved into glucagon-like peptide (GLP)-1, GLP-2 and other small peptides in intestinal endocrine cells and brain neurons (49). Moreover, GLP-1 and its analogs have become an effective therapeutic strategy for numerous patients with type 2 diabetes (50). Notably, CRC is more common in diabetic patients than in the non-diabetic population (51–54). Furthermore, Zanders et al (55) demonstrated that diabetes affects the presentation, treatment and outcome of CRC. Patients with both CRC and diabetes are likely to have a lower survival rate compared with patients with CRC without diabetes. In a study by Koehler et al (27), GLP-1 receptor activation decreased proliferation and survival of CT26 colon cancer cells that expressed the endogenous classical GLP-1 receptor. Hence, more studies on the associations between GLP-1 and CRC are required, particularly for patients with both diabetes and CRC. GLP-2, a nutrient-responsive neuropeptide and intestinal hormone, functions in promoting cell proliferation and survival (56,57). Previous studies have demonstrated the therapeutic potential of GLP-2 in surgical resections and ulcerative colitis (58,59). However, the function of GLP2 was shown to be controversial. A histopathological analysis in one study showed a significant increase in tumor load of mice treated with Gly2-GLP-2, which indicated that GLP2 promoted the development of CRC (60). These findings appear to be inconsistent with the present study, which revealed shorter overall survival time associated with low expression of GCG in patients with CRC. Therefore, further investigation is required to elucidate whether GLP2 promotes intestinal healing or accelerates the development of CRC.
CLCA1 is the first reported member of the CLCA family and is mainly expressed in the colon, small intestine and appendix (61). Yang et al (62) revealed that CLCA1 is expressed in differentiated, growth-arrested mammalian epithelial cells but is downregulated during tumor progression. CLCA1 has been identified as a regulator of the transition from proliferation to differentiation in Caco-2 cells. Further investigations demonstrated that low expression levels of CLCA1 predicted lower survival in patients with CRC (63). Li et al (64) demonstrated that increased expression levels of CLCA1 could suppress the aggressiveness of CRC via inhibiting the epithelial-mesenchymal transition process and the Wnt/β-catenin signaling pathway. Hence, CLCA1 is associated with CRC prognosis and may be a tumor suppressor in CRC.
Overall, the present study identified four prognosis-associated key genes, CEACAM7, SLC4A4, GCG and CLCA1, in CRC using bioinformatics analysis. All four genes were downregulated in patients with CRC. Differential expressions of these genes were also observed in CRC tumor samples. Low expression of these genes appeared to be associated with adverse clinical outcome in patients with CRC. These four genes may be potential prognosis markers or therapeutic targets of CRC. Low expression of CEACAM7 may affect the prognosis of patients with CRC via activating glycosaminoglycan biosynthesis-chondroitin sulfate and extracellular matrix receptor interaction pathways. It is speculated that CLCA1 is a potential prognosis predictor and therapeutic target of CRC. Further study is required to verify and investigate the molecular mechanisms of these genes in CRC in vitro and in vivo.
Acknowledgements
The authors would like to thank Dr Shaohua Lei, Dr Min Oh, Dr Mohammad Shabbir Hasan and Dr Saima Sultana Tithi (Zhang Lab, Virginia Tech) for their helpful discussions. The authors would also like to thank to Dr Gustavo Arango and Dr Dhoha Abid (Virginia Tech) for their guidance to improve the manuscript.
Funding
This study was funded by the National Natural Science Foundation of China (grant nos. 81630104 and 81973748), the China National Funds for Distinguished Young Scientists (grant no. 30825046) and the China Scholarship Council (grant no. 201706550003).
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
QB drafted the manuscript. JXC and LZ designed the study. WQ, CP and FD collected the data. MS and XS performed data analyses. YL designed the figures and tables, and helped QB to interpret the results. JBC helped with statistical analysis. QB and LZ revised the manuscript. JXC contributed to funding acquisition. JXC and LZ supervised the study.
Ethics approval and consent to participate
Not applicable.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394–424. doi: 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- 2.SEER Cancer Statistics Factsheets, corp-author. Colon and Rectum Cancer. National Cancer Institute; Bethesda, MD: 2018. [Feb;2018 ]. [Google Scholar]
- 3.Siegel R, DeSantis C, Virgo K, Stein K, Mariotto A, Smith T, Cooper D, Gansler T, Lerro C, Fedewa S, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J Clin. 2012;62:220–241. doi: 10.3322/caac.20138. [DOI] [PubMed] [Google Scholar]
- 4.American Cancer Society, Colorectal Cancer Facts & Figures 2014–2016, corp-author. American Cancer Society; Atlanta, Ga, USA: 2014. p. 7. [Google Scholar]
- 5.Brenner H, Kloor M, Pox CP. Colorectal cancer. Lancet. 2014;383:1490–1502. doi: 10.1016/S0140-6736(13)61649-9. [DOI] [PubMed] [Google Scholar]
- 6.Hu Y, Gaedcke J, Emons G, Beissbarth T, Grade M, Jo P, Yeager M, Chanock SJ, Wolff H, Camps J, et al. Colorectal cancer susceptibility loci as predictive markers of rectal cancer prognosis after surgery. Genes Chromosomes Cancer. 2018;57:140–149. doi: 10.1002/gcc.22512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kagawa Y, Matsumoto S, Kamioka Y, Mimori K, Naito Y, Ishii T, Okuzaki D, Nishida N, Maeda S, Naito A, et al. Cell cycle-dependent Rho GTPase activity dynamically regulates cancer cell motility and invasion in vivo. PLoS One. 2013;8:e83629. doi: 10.1371/journal.pone.0083629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sveen A, Agesen TH, Nesbakken A, Rognum TO, Lothe RA, Skotheim RI. Transcriptome instability in colorectal cancer identified by exon microarray analyses: Associations with splicing factor expression levels and patient survival. Genome Med. 2011;3:32. doi: 10.1186/gm248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Agesen TH, Sveen A, Merok MA, Lind GE, Nesbakken A, Skotheim RI, Lothe RA. ColoGuideEx: A robust gene classifier specific for stage II colorectal cancer prognosis. Gut. 2012;61:1560–1567. doi: 10.1136/gutjnl-2011-301179. [DOI] [PubMed] [Google Scholar]
- 10.Liu YR, Hu Y, Zeng Y, Li ZX, Zhang HB, Deng JL, Wang G. Neurexophilin and PC-esterase domain family member 4 (NXPE4) and prostate androgen-regulated mucin-like protein 1 (PARM1) as prognostic biomarkers for colorectal cancer. J Cell Biochem. 2019;120:18041–18052. doi: 10.1002/jcb.29107. [DOI] [PubMed] [Google Scholar]
- 11.Pan F, Chen T, Sun X, Li K, Jiang X, Försti A, Zhu Y, Lai M. Prognosis prediction of colorectal cancer using gene expression profiles. Front Oncol. 2019;9:252. doi: 10.3389/fonc.2019.00252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song X, Tang T, Li C, Liu X, Zhou L. CBX8 and CD96 are important prognostic biomarkers of colorectal cancer. Med Sci Monit. 2018;24:7820–7827. doi: 10.12659/MSM.908656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yong L, YuFeng Z, Guang B. Association between PPP2CA expression and colorectal cancer prognosis tumor marker prognostic study. Int J Surg. 2018;59:80–89. doi: 10.1016/j.ijsu.2018.09.020. [DOI] [PubMed] [Google Scholar]
- 14.Li Y, Jiang Q, Ding Z, Liu G, Yu P, Jiang G, Yu Z, Yang C, Qian J, Jiang H, Zou Y. Identification of a common different gene expression signature in ischemic cardiomyopathy. Genes (Basel) 2018;9(pii):E56. doi: 10.3390/genes9010056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Smyth GK. Linear models and empirical bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004;3:Article3. doi: 10.2202/1544-6115.1027. [DOI] [PubMed] [Google Scholar]
- 16.Oliveros JC. VENNY. An interactive tool for comparing lists with Venn's diagrams. http://bioinfogp.cnb.csic.es/tools/venny/index.html 2007-2015
- 17.Ashburner M, Ball CA, Blake JA, Botstein D, Butler H, Cherry JM, Davis AP, Dolinski K, Dwight SS, Eppig JT, et al. Gene ontology: Tool for the unification of biology. The gene ontology consortium. Nat Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.The Gene Ontology Consortium, corp-author. The gene ontology resource: 20 years and still GOing strong. Nucleic Acids Res. 2019;47(D1):D330–D338. doi: 10.1093/nar/gky1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogata H, Goto S, Sato K, Fujibuchi W, Bono H, Kanehisa M. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 1999;27:29–34. doi: 10.1093/nar/27.1.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 21.Huang da W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Warde-Farley D, Donaldson SL, Comes O, Zuberi K, Badrawi R, Chao P, Franz M, Grouios C, Kazi F, Lopes CT, et al. The GeneMANIA prediction server: Biological network integration for gene prioritization and predicting gene function. Nucleic Acids Res. 2010;38((Web Server Issue)):W214–W220. doi: 10.1093/nar/gkq537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tang Z, Li C, Kang B, Gao G, Li C, Zhang Z. GEPIA: A web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017;45((W1)):W98–W102. doi: 10.1093/nar/gkx247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Subramanian A, Tamayo P, Mootha VK, Mukherjee S, Ebert BL, Gillette MA, Paulovich A, Pomeroy SL, Golub TR, Lander ES, Mesirov JP. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc Natl Acad Sci USA. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liberzon A, Subramanian A, Pinchback R, Thorvaldsdottir H, Tamayo P, Mesirov JP. Molecular signatures database (MSigDB) 3.0. Bioinformatics. 2011;27:1739–1740. doi: 10.1093/bioinformatics/btr260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shannon P, Markiel A, Ozier O, Baliga NS, Wang JT, Ramage D, Amin N, Schwikowski B, Ideker T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003;13:2498–2504. doi: 10.1101/gr.1239303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koehler JA, Kain T, Drucker DJ. Glucagon-like peptide-1 receptor activation inhibits growth and augments apoptosis in murine CT26 colon cancer cells. Endocrinology. 2011;152:3362–3372. doi: 10.1210/en.2011-1201. [DOI] [PubMed] [Google Scholar]
- 28.Schölzel S, Zimmermann W, Schwarzkopf G, Grunert F, Rogaczewski B, Thompson J. Carcinoembryonic antigen family members CEACAM6 and CEACAM7 are differentially expressed in normal tissues and oppositely deregulated in hyperplastic colorectal polyps and early adenomas. Am J Pathol. 2000;156:595–605. doi: 10.1016/S0002-9440(10)64764-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boguski MS. The turning point in genome research. Trends Biochem Sci. 1995;20:295–296. doi: 10.1016/S0968-0004(00)89051-9. [DOI] [PubMed] [Google Scholar]
- 30.Thompson J, Zimmermann W, Nollau P, Neumaier M, Weber-Arden J, Schrewe H, Craig I, Willcocks T. CGM2, a member of the carcinoembryonic antigen gene family is down-regulated in colorectal carcinomas. J Biol Chem. 1994;269:32924–32931. [PubMed] [Google Scholar]
- 31.Messick CA, Sanchez J, Dejulius KL, Hammel J, Ishwaran H, Kalady MF. CEACAM-7: A predictive marker for rectal cancer recurrence. Surgery. 2010;147:713–719. doi: 10.1016/j.surg.2009.10.056. [DOI] [PubMed] [Google Scholar]
- 32.Afratis N, Gialeli C, Nikitovic D, Tsegenidis T, Karousou E, Theocharis AD, Pavão MS, Tzanakakis GN, Karamanos NK. Glycosaminoglycans: Key players in cancer cell biology and treatment. FEBS J. 2012;279:1177–1197. doi: 10.1111/j.1742-4658.2012.08529.x. [DOI] [PubMed] [Google Scholar]
- 33.Mariman EC, Wang P. Adipocyte extracellular matrix composition, dynamics and role in obesity. Cell Mol Life Sci. 2010;67:1277–1292. doi: 10.1007/s00018-010-0263-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Järveläinen H, Sainio A, Koulu M, Wight TN, Penttinen R. Extracellular matrix molecules: Potential targets in pharmacotherapy. Pharmacol Rev. 2009;61:198–223. doi: 10.1124/pr.109.001289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Llacua LA, Faas MM, de Vos P. Extracellular matrix molecules and their potential contribution to the function of transplanted pancreatic islets. Diabetologia. 2018;61:1261–1272. doi: 10.1007/s00125-017-4524-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karamanos NK, Tzanakakis GN. Glycosaminoglycans: from ‘cellular glue’ to novel therapeutical agents. Curr Opin Pharmacol. 2012;12:220–222. doi: 10.1016/j.coph.2011.12.003. [DOI] [PubMed] [Google Scholar]
- 37.Theocharis AD, Tsolakis I, Tzanakakis GN, Karamanos NK. Chondroitin sulfate as a key molecule in the development of atherosclerosis and cancer progression. Adv Pharmacol. 2006;53:281–295. doi: 10.1016/S1054-3589(05)53013-8. [DOI] [PubMed] [Google Scholar]
- 38.Burnham CE, Amlal H, Wang Z, Shull GE, Soleimani M. Cloning and functional expression of a human kidney Na+:HCO3- cotransporter. J Biol Chem. 1997;272:19111–19114. doi: 10.1074/jbc.272.31.19111. [DOI] [PubMed] [Google Scholar]
- 39.Abuladze N, Lee I, Newman D, Hwang J, Boorer K, Pushkin A, Kurtz I. Molecular cloning, chromosomal localization, tissue distribution, and functional expression of the human pancreatic sodium bicarbonate cotransporter. J Biol Chem. 1998;273:17689–17695. doi: 10.1074/jbc.273.28.17689. [DOI] [PubMed] [Google Scholar]
- 40.Choi I, Romero MF, Khandoudi N, Bril A, Boron WF. Cloning and characterization of a human electrogenic Na+-HCO-3 cotransporter isoform (hhNBC) Am J Physiol. 1999;276:C576–C584. doi: 10.1152/ajpcell.1999.276.3.C576. [DOI] [PubMed] [Google Scholar]
- 41.Sun XC, Bonanno JA. Identification and cloning of the Na/HCO(3-) cotransporter (NBC) in human corneal endothelium. Exp Eye Res. 2003;77:287–295. doi: 10.1016/S0014-4835(03)00150-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Demirci FY, Chang MH, Mah TS, Romero MF, Gorin MB. Proximal renal tubular acidosis and ocular pathology: A novel missense mutation in the gene (SLC4A4) for sodium bicarbonate cotransporter protein (NBCe1) Mol Vis. 2006;12:324–330. [PubMed] [Google Scholar]
- 43.Hong Y, Downey T, Eu KW, Koh PK, Cheah PY. A ‘metastasis-prone’ signature for early-stage mismatch-repair proficient sporadic colorectal cancer patients and its implications for possible therapeutics. Clin Exp Metastasis. 2010;27:83–90. doi: 10.1007/s10585-010-9305-4. [DOI] [PubMed] [Google Scholar]
- 44.Skrzypczak M, Goryca K, Rubel T, Paziewska A, Mikula M, Jarosz D, Pachlewski J, Oledzki J, Ostrowski J. Modeling oncogenic signaling in colon tumors by multidirectional analyses of microarray data directed for maximization of analytical reliability. PLoS One. 2010;5(pii):e13091. doi: 10.1371/journal.pone.0013091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gaedcke J, Grade M, Jung K, Camps J, Jo P, Emons G, Gehoff A, Sax U, Schirmer M, Becker H, et al. Mutated KRAS results in overexpression of DUSP4, a MAP-kinase phosphatase, and SMYD3, a histone methyltransferase, in rectal carcinomas. Genes Chromosomes Cancer. 2010;49:1024–1034. doi: 10.1002/gcc.20811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Notterman DA, Alon U, Sierk AJ, Levine AJ. Transcriptional gene expression profiles of colorectal adenoma, adenocarcinoma, and normal tissue examined by oligonucleotide arrays. Cancer Res. 2001;61:3124–3130. [PubMed] [Google Scholar]
- 47.Kaiser S, Park YK, Franklin JL, Halberg RB, Yu M, Jessen WJ, Freudenberg J, Chen X, Haigis K, Jegga AG, et al. Transcriptional recapitulation and subversion of embryonic colon development by mouse colon tumor models and human colon cancer. Genome Biol. 2007;8:R131. doi: 10.1186/gb-2007-8-7-r131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sabates-Bellver J, Van der Flier LG, de Palo M, Cattaneo E, Maake C, Rehrauer H, Laczko E, Kurowski MA, Bujnicki JM, Menigatti M, et al. Transcriptome profile of human colorectal adenomas. Mol Cancer Res. 2007;5:1263–1275. doi: 10.1158/1541-7786.MCR-07-0267. [DOI] [PubMed] [Google Scholar]
- 49.Janssen P, Rotondo A, Mulé F, Tack J. Review article: A comparison of glucagon-like peptides 1 and 2. Aliment Pharmacol Ther. 2013;37:18–36. doi: 10.1111/apt.12092. [DOI] [PubMed] [Google Scholar]
- 50.Cho YM, Fujita Y, Kieffer TJ. Glucagon-like peptide-1: Glucose homeostasis and beyond. Annu Rev Physiol. 2014;76:535–559. doi: 10.1146/annurev-physiol-021113-170315. [DOI] [PubMed] [Google Scholar]
- 51.Larsson SC, Orsini N, Wolk A. Diabetes mellitus and risk of colorectal cancer: A meta-analysis. J Natl Cancer Inst. 2005;97:1679–1687. doi: 10.1093/jnci/dji375. [DOI] [PubMed] [Google Scholar]
- 52.Jiang Y, Ben Q, Shen H, Lu W, Zhang Y, Zhu J. Diabetes mellitus and incidence and mortality of colorectal cancer: A systematic review and meta-analysis of cohort studies. Eur J Epidemiol. 2011;26:863–876. doi: 10.1007/s10654-011-9617-y. [DOI] [PubMed] [Google Scholar]
- 53.Deng L, Gui Z, Zhao L, Wang J, Shen L. Diabetes mellitus and the incidence of colorectal cancer: An updated systematic review and meta-analysis. Dig Dis Sci. 2012;57:1576–1585. doi: 10.1007/s10620-012-2055-1. [DOI] [PubMed] [Google Scholar]
- 54.Krämer HU, Schöttker B, Raum E, Brenner H. Type 2 diabetes mellitus and colorectal cancer: Meta-analysis on sex-specific differences. Eur J Cancer. 2012;48:1269–1282. doi: 10.1016/j.ejca.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 55.Zanders MM, Vissers PA, Haak HR, van de Poll-Franse LV. Colorectal cancer, diabetes and survival: Epidemiological insights. Diabetes Metab. 2014;40:120–127. doi: 10.1016/j.diabet.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 56.Rowland KJ, Trivedi S, Lee D, Wan K, Kulkarni RN, Holzenberger M, Brubaker PL. Loss of glucagon-like peptide-2-induced proliferation following intestinal epithelial insulin-like growth factor-1-receptor deletion. Gastroenterology. 2011;141:2166–2175.e7. doi: 10.1053/j.gastro.2011.09.014. [DOI] [PubMed] [Google Scholar]
- 57.Shi X, Li X, Wang Y, Zhang K, Zhou F, Chan L, Li D, Guan X. Glucagon-like peptide-2-stimulated protein synthesis through the PI 3-kinase-dependent Akt-mTOR signaling pathway. Am J Physiol Endocrinol Metab. 2011;300:E554–E563. doi: 10.1152/ajpendo.00620.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.L'Heureux MC, Brubaker PL. Glucagon-like peptide-2 and common therapeutics in a murine model of ulcerative colitis. J Pharmacol Exp Ther. 2003;306:347–354. doi: 10.1124/jpet.103.051771. [DOI] [PubMed] [Google Scholar]
- 59.Rowland KJ, Brubaker PL. Life in the crypt: A role for glucagon-like peptide-2? Mol Cell Endocrinol. 2008;288:63–70. doi: 10.1016/j.mce.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 60.Thulesen J, Hartmann B, Hare KJ, Kissow H, Ørskov C, Holst JJ, Poulsen SS. Glucagon-like peptide 2 (GLP-2) accelerates the growth of colonic neoplasms in mice. Gut. 2004;53:1145–1150. doi: 10.1136/gut.2003.035212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gruber AD, Elble RC, Ji HL, Schreur KD, Fuller CM, Pauli BU. Genomic cloning, molecular characterization, and functional analysis of human CLCA1, the first human member of the family of Ca2+-activated Cl- channel proteins. Genomics. 1998;54:200–214. doi: 10.1006/geno.1998.5562. [DOI] [PubMed] [Google Scholar]
- 62.Yang B, Cao L, Liu B, McCaig CD, Pu J. The transition from proliferation to differentiation in colorectal cancer is regulated by the calcium activated chloride channel A1. PLoS One. 2013;8:e60861. doi: 10.1371/journal.pone.0060861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yang B, Cao L, Liu J, Xu Y, Milne G, Chan W, Heys SD, McCaig CD, Pu J. Low expression of chloride channel accessory 1 predicts a poor prognosis in colorectal cancer. Cancer. 2015;121:1570–1580. doi: 10.1002/cncr.29235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li X, Hu W, Zhou J, Huang Y, Peng J, Yuan Y, Yu J, Zheng S. CLCA1 suppresses colorectal cancer aggressiveness via inhibition of the Wnt/beta-catenin signaling pathway. Cell Commun Signal. 2017;15:38. doi: 10.1186/s12964-017-0192-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.