Abstract
Chronic rhinosinusitis (CRS) is one of the most common types of respiratory disease and affects a large proportion of the population worldwide. The clinical differences between CRS with nasal polyps (CRSwNP) and CRS without nasal polyps (CRSsNP) facilitate studies on the development of polyps. In the present study, next-generation sequencing was performed to identify differentially expressed microRNAs (miRNAs/miRs) in CRSwNP vs. CRSsNP tissues and subsequently validated the expression of selected genes using reverse transcription-quantitative polymerase chain reaction analysis. In total, 6 miRNAs were identified to be downregulated in the CRSwNP samples compared with those in the CRSsNP samples. The predicted targets of these miRNAs were determined to be enriched in a number of signaling pathways, including the ErbB, Ras, cyclic adenosine monophosphate and Janus kinase (Jak)/signal transducer and activator of transcription (STAT) pathways. The expression of miR-4492 and that if its targets predicted by a bioinformatics analysis, tumor necrosis factor α (TNF-α) and interleukin (IL)-10, was validated in 96 clinical specimens. miR-4492 was downregulated and IL-10 was upregulated in CRSwNP vs. CRSsNP tissues, and an inverse correlation between miR-4492 and IL-10 was determined in CRS tissues; however no difference was identified in the expression of TNF-α between the different groups. The present results indicate that the miR-4492/IL-10 interaction in the Jak/STAT signaling pathway may be one of the key mechanisms in CRSwNP.
Keywords: chronic rhinosinusitis, nasal polyps, microRNA sequencing, microRNA-4492, interleukin 10
Introduction
Chronic rhinosinusitis (CRS) is a heterogenous disease of the sinuses and upper airways that affects a large proportion of the population worldwide (1,2). CRS is classified into CRS with nasal polyps (CRSwNP) and CRS without nasal polyps (CRSsNP), and is typically characterized by two or more of the following symptoms: Nasal obstruction, drainage, loss of sense of smell, headaches and facial pain or pressure (3,4). CRSsNP is associated with the symptoms of headache and facial pain or pressure, whereas CRSwNP is more closely associated with the symptoms of nasal obstruction and loss of sense of smell (5–7). In the clinic, CRSwNP is frequently resistant to medical therapy, and therefore, management of patients with CRSwNP is costly. Furthermore, a proportion of patients with CRSwNP do not benefit from topical corticosteroids, long-term systemic glucocorticosteroids and repeated sinus surgery (8–10). Thus, a better understanding of the reason why CRSwNP is not controlled by current approaches and the underlying molecular mechanisms is required.
MicroRNAs (miRNAs/miRs) are a class of small non-coding RNAs that are 21–24 nucleotides in length and have key roles in the pathogenesis of numerous diseases. miRNAs either suppress target transcripts or inhibit translation in order to post-transcriptionally regulate protein expression (11–13). Aberrant miRNA expression is a common feature of a diverse range of diseases (14,15). Previous studies indicate a distinct expression pattern of inflammatory and re-modeling mediators in CRSwNP (8). With the development of high-throughput gene detection techniques and bioinformatics, a new era of miRNA investigation has rapidly developed for numerous types of allergic airway disease, including allergic rhinitis (16) and asthma (17,18). However, the genome-scale miRNA expression pattern of CRSwNP remains to be fully elucidated.
In the present study, the different miRNA expression patterns of CRSwNP and CRSsNP were investigated using next-generation sequencing, and the expression of a candidate miRNA and its predicted targets was subsequently validated in CRSwNP using reverse transcription-quantitative polymerase chain reaction (RT-qPCR) analysis.
Materials and methods
Patients
A total of 100 patients requiring endoscopic sinus surgery were recruited at The First Affiliated Hospital of Xinjiang Medical University (Urumqi, China) between October 2016 and February 2017, including 61 patients with CRSwNP (38 male and 23 female patients; median age, 44 years old; age range, 23–63 years old) and 39 patients with CRSsNP (27 male and 12 female patients; median age, 41 years old; age range, 20–65 years old). All subjects met the criteria for CRS as defined by the European position paper on rhinosinusitis and NPs 2012 (10). An additional 35 patients (22 male and 13 female patients; median age, 39 years old; age range, 19–57 years old) that required septoplasty and did not have any inflammatory nasal disease were enrolled as control subjects. Patients that were pregnant or dependent on systemic steroid therapy were excluded. During surgery, ethmoidal mucosa or polyp tissues were collected from the patients with CRS and inferior turbinate tissues were obtained from the control patients. The specimens from 5 of the patients with CRSwNP, 4 of the patients with CRSsNP and 4 of the control patients were used for miRNA sequencing. Subsequently, the RNA of 26 of the patients was excluded due to low quality (Fig. S1). Finally, the RNA of 96 of the patients was used in the RT-qPCR validation assay.
Total RNA extraction and complementary (c)DNA synthesis
Total RNA was extracted from each specimen using TRIzol (Thermo Fisher Scientific, Inc.) according to the manufacturer's protocol. Small RNAs with 18–30 nt fragments were enriched using denaturing polyacrylamide gel electrophoresis (15% gel). According to the manufacturer's protocol, the fragments were ligated to 5 and 3 adaptors and reverse-transcribed into cDNA using a Mir-X™ miRNA First-Strand Synthesis kit (cat. no. 638313; Takara Bio Inc.) after purification. The quality of these RNA samples was assessed using an Agilent 2100 Bioanalyzer (Agilent Technologies, Inc.). For mRNA quantification, total RNA was subjected to reverse transcription using a PrimeScript™ 1st Strand cDNA Synthesis Kit (cat. no. 6110A; Takara Bio Inc.). Only samples meeting the criteria of optical density ratio at 260/280 nm ≥1.8 and RNA integrity number ≥8.0 were used for all of the analyses. The RNA integrity of each sample was also assessed using a denaturing agarose gel in order to determine the intensity ratio of the 28S and 18S ribosomal RNA bands.
miRNA sequencing (miRNA-seq)
Following RT of the RNA into cDNA, the cDNA was subjected to deep sequencing, which was performed by Genesky Biotechnologies. The small RNA libraries were evaluated using FastQC software (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/). After removing adaptor and low-quality sequences, the remaining high-quality sequences were analyzed with the miRBase (19) (http://www.mirbase.org/) and Rfam (20) (http://rfam.xfam.org/) databases.
Differentially expressed gene (DEG) screening
The R package edgeR (http://www.bioconductor.org/packages/2.4/bioc/html/edgeR.html) was used to identify DEGs, with the cut-off values of |log2 fold change (FC)|≥1 and adjusted P-value of <0.05.
2-Dimensional (2D) hierarchical clustering analysis
A gene expression matrix of the DEGs was prepared and analyzed with the pheatmap package (http://CRAN.R-project.org/package=pheatmap) in order to perform unsupervised clustering in R after normalization.
Target prediction and functional enrichment
The miRWalk database version 2.0 (mirwalk.uni-hd.de/) was used for predicting miRNA-target interactions (21). Subsequently, all of the predicted targets were subjected to gene ontology (GO) (22) and Kyoto Encyclopedia of Genes and Genomes (KEGG) (23) pathway enrichment analysis in WebGestalt (http://www.webgestalt.org/), with a criterion of P<0.01.
qPCR
In order to investigate the expression of miR-4492, interleukin (IL)-10 and tumor necrosis factor α (TNF-α) in the specimens, qPCR was performed using SYBR Advantage qPCR Premix (cat. no. 638322; Takara Bio Inc.) on an ABI 7300 Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific, Inc.). GAPDH served as a housekeeping gene for standardization of protein-coding gene expression, and U6 served as the reference for standardization of miRNA expression. All assays were performed at least in triplicate and gene expression was quantified using the 2−∆∆Cq method (24). The sequences of the primer pairs were as follows: miR-4492 forward, 5′-CTGGGCGCGCGCCAAA-3′; IL-10 forward, 5′-TCAAGGCGCATGTGAACTCC-3′ and reverse, 5′-CCACGGCCTTGCTCTTGTTT-3′; TNF-α forward, 5′-TTCTGCCTGCTGCACTTTGG-3′ and reverse, 5′-TGTCACTCGGGGTTCGAGAAG-3′. The universal miRNA reverse primer was in the Mir-X miRNA First-Strand Synthesis kit.
Statistical analysis
Statistical analysis was performed with SPSS software version 16.0 (SPSS Inc.). All of the assays were performed at least in triplicate and values are expressed in bar charts as the mean ± standard error of the mean. Significant differences between groups were analyzed using Student's t-test or one-way analysis of variance followed by Duncan's multiple-comparisons test. P<0.05 was considered to indicate a statistically significant difference. Pearson correlation analysis was performed in order to assess the co-expression of miR-4492 and IL-10 in the 96 specimens.
Results
Identification of differentially expressed miRNAs in CRSwNP
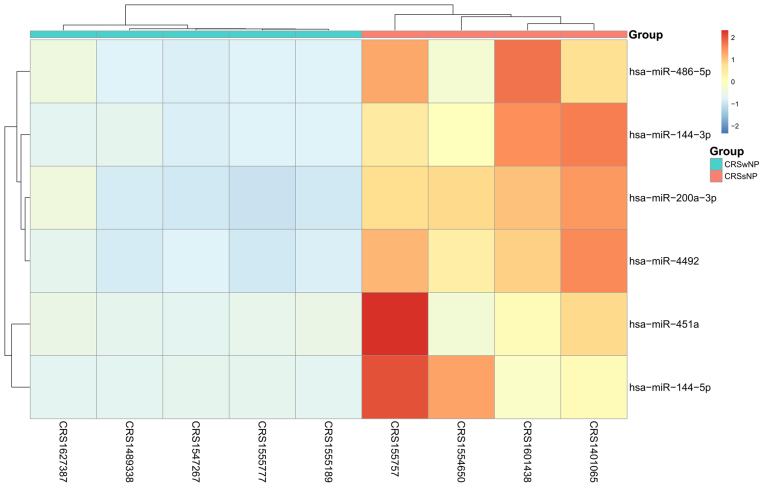
A total of 6 DEGs (6 downregulated miRNAs) were identified between CRSwNP and CRSsNP. One DEG (1 downregulated miRNA) was identified between CRSwNP and the control, and no DEGs were identified between CRSsNP and the control. The details of the DEGs are presented in Table I. Subsequently, the expression data of six DEGs were subjected to 2D hierarchical clustering analysis. The distinctive clusters for CRSwNP and CRSsNP were segregated for all of the CRS specimens (Fig. 1), revealing differences in the miRNA expression profiles of the two subgroups of CRS.
Table I.
Details of aberrantly expressed miRNAs.
| CRSwNP vs. CRSsNP | CRSwNP vs. NC | CRSsNP vs. NC | ||||
|---|---|---|---|---|---|---|
| Gene names | logFC | FDR | logFC | FDR | logFC | FDR |
| hsa-miR-144-3p | −3.065465067 | 0.002555901 | −1.820765547 | 0.821307633 | 0.857082137 | 0.996058565 |
| hsa-miR-144-5p | −3.160003078 | 0.003750538 | −1.835835772 | 0.821307633 | 0.856915634 | 0.996058565 |
| hsa-miR-486-5p | −2.135055827 | 0.013129379 | −1.258258512 | 0.939213466 | 0.713031482 | 0.996058565 |
| hsa-miR-200a-3p | −2.187602496 | 0.013129379 | −0.284138738 | 0.998572989 | 1.406274811 | 0.996058565 |
| hsa-miR-451a | −2.09844785 | 0.015772606 | −1.231243617 | 0.998572989 | 0.720894 | 0.996058565 |
| hsa-miR-4492 | −1.745117094 | 0.019422342 | −1.513875624 | 0.012444032 | 0.284184 | 0.996058565 |
miR, microRNA; hsa, Homo sapiens; CRSwNP, chronic rhinosinusitis with nasal polyps; CRSsNP, chronic rhinosinusitis without nasal polyps; FDR, false discovery rate; FC, fold change.
Figure 1.
Heat map of the differentially expressed miRs in the different groups. The lateral axis represents the differentially expressed miRs, and the longitudinal axis represents the samples. Red indicates upregulation, blue indicates downregulation, pink indicates CRSwNP specimens and cerulean indicates CRSsNP specimens. miR, microRNA; hsa, Homo sapiens; CRSwNP, chronic rhinosinusitis with nasal polyps; CRSsNP, chronic rhinosinusitis without nasal polyps.
Gene enrichment and functional annotation of the predicted targets of the DEGs
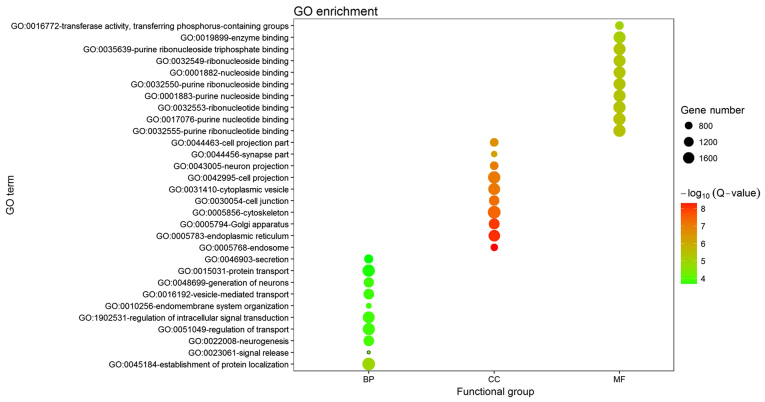
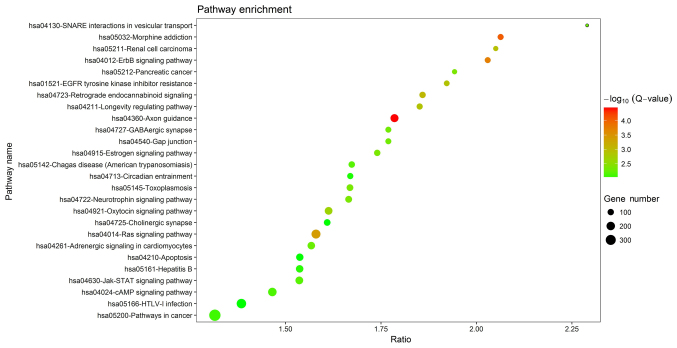
A total of 4,395 predicted target genes of the six DEGs were obtained from the miRWalk database. Among these genes, a diverse range of GO terms were significantly enriched in the following three categories: Biological process (BP), cellular component (CC) and molecular function (MF). The top 5 enriched terms in each category are provided in Fig. 2, of which establishment of protein localization, endosome and purine ribonucleotide binding were the most significant terms in each group, respectively. The KEGG pathway enrichment analysis identified a total of 26 pathways as being enriched by the DEGs (Fig. 3), several of which are associated with the ErbB, Ras, cyclic adenosine monophosphate and Janus kinase (Jak)/signal transducer and activator of transcription (STAT) signaling pathways. These pathways my therefore be involved in the development of CRSwNP.
Figure 2.
Visualization of GO enrichment for the predicted targets of the DEGs in CRSwNP. The detailed GO enrichment information is presented in a bubble diagram. The Y-axis represents the GO terms and the X-axis represents the categories BP, CC and MF. The number of genes enriched in a GO term is represented by the size of the node. The significance of the GO terms is represented by the colour, with red indicating the highest significance. GO, gene ontology; BP, biological process; CC, cellular component; MF, molecular function.
Figure 3.
Visualization of KEGG enrichment for the predicted targets of the differentially expressed miRs in chronic rhinosinusitis with nasal polyps vs. chronic rhinosinusitis with nasal polyps. The detailed KEGG enrichment information is presented as a bubble diagram. The Y-axis represents the KEGG pathways and the X-axis represents the enrichment factor (gene ratio). The number of genes classified into the pathway is represented by the size of the node. The significance of the pathway is represented by the colour, with red indicating the highest significance. hsa, Homo sapiens; KEGG, Kyoto Encyclopedia of Genes and Genomes; Jak, Janus kinase; STAT, signal transducer and activator of transcription; cAMP, cyclic adenosine monophosphate; hTLV, human T-lymphocyte virus; EGFR, epidermal growth factor receptor.
Validation of the miR-4492 expression profile in CRS
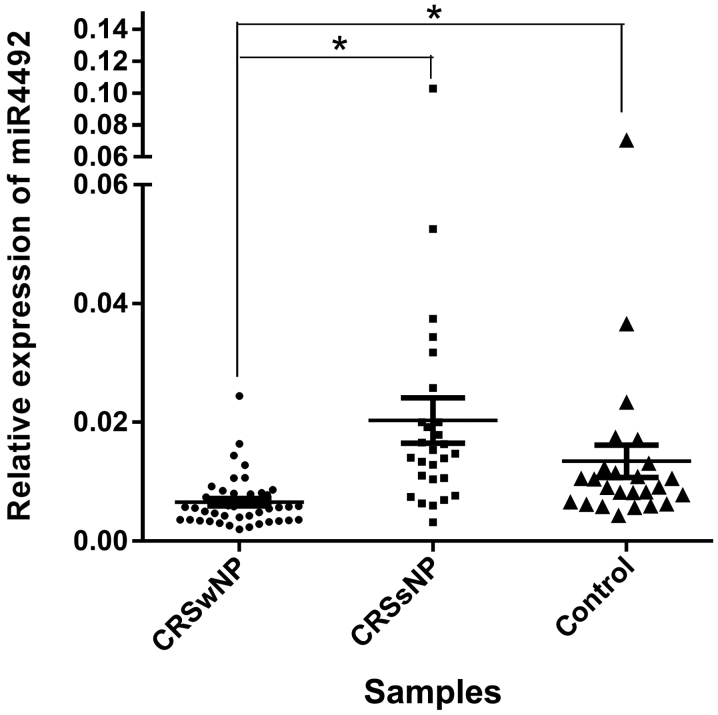
RNA samples from 122 patients were prepared for RT-qPCR. A total of 26 samples were removed due to a lack of RNA integrity (Fig. S1). The remaining 96 RNA samples all passed the quality control checks for RNA integrity and purity. As presented in Fig. 4, the expression levels of miR-4492 were significantly reduced in the CRSwNP samples compared with those in the CRSsNP samples (P=3.17×10−5). Similarly, miR-4492 was also markedly downregulated in the CRSwNP group compared with the control group (P=2.86×10−3). The difference between the miR-4492 expression levels of the CRSsNP group and the control group was not significant.
Figure 4.
Differential expression of miR-4492 in patients with CRS. Reverse transcription polymerase chain reaction analysis of miR-4492 was performed and the expression levels were quantified relative to those of U6. miR-4492 expression in the nasal polyps was reduced to 32.3% of that in the CRSsNP group. *P<0.05. miR, microRNA; CRSwNP, chronic rhinosinusitis with nasal polyps; CRSsNP, chronic rhinosinusitis without nasal polyps.
IL-10 and TNF-α expression in CRS
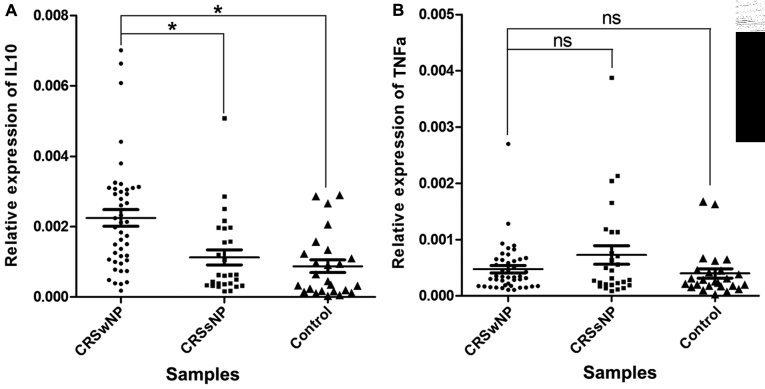
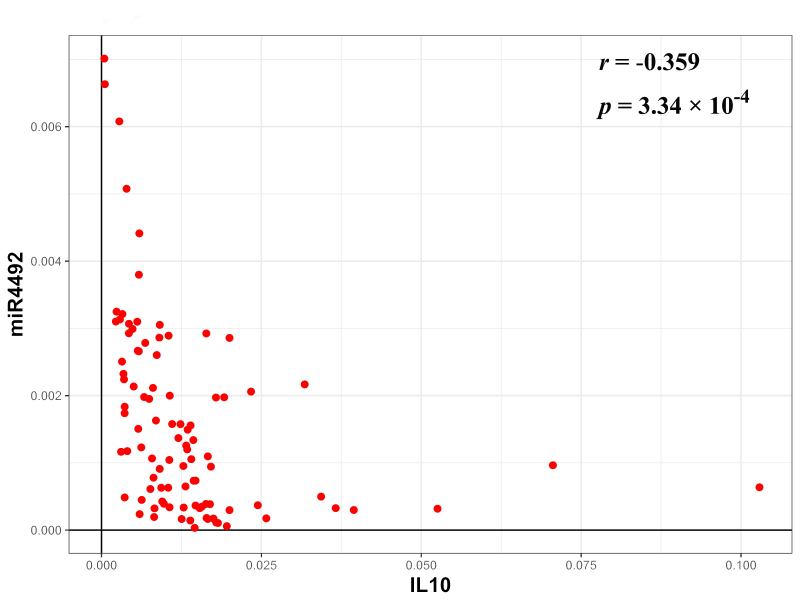
In order to investigate the targets of miR-4492, the protein-coding target genes were predicted using a Bioinformatics analysis. From the list of predicted targets of miR-4492, IL-10 and TNF-α were selected and their expression in the 96 samples was detected (Fig. 5). While the difference between the CRSsNP samples and the control samples was not significant, the expression levels of IL-10 were elevated in the CRSwNP samples compared with those in the CRSsNP samples (P=8.76×10−4) and the control samples (P=1.74×10−4). In addition, a negative correlation between the expression of miR-4492 and IL-10 expression was determined among the 96 specimens (Fig. 6). However, no significant correlation of miR-4492 with TNF-α expression was observed.
Figure 5.
Differential expression of IL10 in patients with CRS. Reverse transcription- polymerase chain reaction of IL10 and TNFa was performed and the expression levels of IL10 and TNFa were quantified relative to those of GAPDH. (A) IL10 in the nasal polyps was upregulated 2.0-fold of that in the CRSsNP group. (B) There was no significant difference in the expression levels of TNFa between the different groups. *P<0.05. ns, no significance; CRSwNP, chronic rhinosinusitis with nasal polyps; CRSsNP, chronic rhinosinusitis without nasal polyps; TNFa, tumor necrosis factor; IL, interleukin.
Figure 6.
Correlation of microRNA-4492 and interleukin-10 expression in the 96 samples. Pearson correlation analysis was performed to assess the correlation between miRNA4492 and IL10 expression. r, Pearson correlation coefficient; P, P-value. P<0.05 served as statistically significant.
Discussion
CRS is an inflammatory upper airway disease with a high prevalence worldwide (1). Although CRSwNP is not the most common form of CRS, there is a higher proportion of patients with CRSwNP in tertiary care populations that have failed under medical management (25). A considerable number of studies have confirmed that miRNAs are involved in a diverse range of biological functions, including cell proliferation, differentiation, apoptosis, stress responses and signal transduction, and abnormal miRNA expression is considered to be a common feature of various diseases (26,27). The key role of miRNAs in inflammation is demonstrated by studies on the association of miRNA dysregulation with excessive inflammation and aberrant immune responses, including pulmonary fibrosis and asthma (17,18). More recently, several studies have identified that marked changes in miRNA expression, which is tightly regulated, serve essential roles in the sustained and exaggerated inflammation of CRS (26–30). In the present study, next-generation sequencing was performed in order to detect key miRNAs in CRSwNP. Compared to the CRSsNP group, six differentially expressed miRNAs, namely Homo sapiens (hsa)-miR-200a-3p, hsa-miR-144-5p, hsa-miR-144-3p, hsa-miR-486-5p, hsa-miR-4492 and hsa-miR-451a, were identified in the CRSwNP samples. Downregulation of miR-200 family members as part of the epithelial to mesenchymal transition has been previously implicated in cancer development and progression (31). Furthermore, previous studies have confirmed the involvement of miR-200 family members in inflammatory and immune responses (32). Downregulation of exosome miR-200 has been detected in the bronchoalveolar lavage fluid of patients with asthma (33). A recent study indicated that miR-144 is an important regulator of innate immune responses, and its major and minor product are involved in colorectal polyposis, which involves inflammatory polyps at a different anatomic site (34). Panganiban et al (35) detected the expression of 420 plasma miRNAs in healthy individuals and patients with allergic rhinitis (AR) and asthma using RT-qPCR. They revealed that miR-144 was commonly dysregulated in patients with AR and asthma, suggesting that miR-144 is a promising plasma biomarker in these diseases. Furthermore, a previous study suggested that miR-541a, another miRNA that was identified as aberrant in the present study, is also an asthma-associated miRNA (36). It is widely accepted that CRS and asthma are closely associated in numerous aspects and they have similar pathogenic mechanisms and therapeutic principles. Based on the above, it may be possible to speculate on a concordant function of miR-200a, miR-144 and miR-451a in these two diseases. miR-485 has also been demonstrated to be reduced in CRSwNP, as detected using a microarray-based approach (29). The differential expression of miR-4492 has been identified in a number of conditions, including responses to viral infection (37,38), necrosis (39), Hirschsprung's disease (40) and breast cancer (41). However, the exact functions and mechanisms require further investigation.
The mechanisms underlying the inflammation are different between the two types of CRS. CRSsNP involves more T helper type 1 cell (Th1) polarization and collagen deposition, whereas CRSwNP is characterized by more Th2 polarization and tissue eosinophilia (42,43). It has been reported that miRNA is involved in the regulation of innate immune cells that are recruited to infiltrate the inflamed tissue, as well as the polarization of Th2 cells (44). Although relatively few studies have assessed the miRNAs involved in CRS, a recent study indicated that miRNAs are associated with the inflammatory pattern of this disease (45). Previous studies have revealed that miR150-5p, miR-125b and miR-124 promote the development of CRS via regulating the immune response to inflammation (27,30,46). Xia et al (26) demonstrated that 7 miRNAs were differentially expressed in CRSwNP by using RT-qPCR, and the expression was correlated with the occurrence of NPs. Although these miRNAs were not among the DEGs identified in the present study, the disparity may be attributed to a number of reasons. First, different detecting platforms may partly account for the difference, as the RNA-seq data do not have complete genome coverage. In addition, in the present study, a relatively small number of samples were used for RNA-seq. Furthermore, the distinctive genetic backgrounds of the individuals and the tissue heterogeneity characteristic of this disease may have also affected the results. Therefore, a validation analysis of these DEGs was necessary in the present study. Of note, miR-4492, was the only differentially expressed miRNA identified between the CRSwNP samples and the control samples, and was downregulated in the polyps. Hence, the expression of miR-4492 was validated in the 96 specimens with acceptable RNA quality using RT-qPCR. The RT-qPCR analysis confirmed the downregulation of this miR in NP. Therefore, despite the lack of previous studies that support the involvement of this miRNA in NPs, the present results suggest that miR-4492 may have important roles in NPs according to the results of the present study.
Deregulation of a number of cytokines, including IL-1, IL-2, IL-10 and TNF-α, has been detected in a large number of diseases, including cancer, autoimmune diseases and inflammatory diseases. Increasing evidence suggests that cytokines are key mediators of the inflammation and immune response associated with NP, among which TNF-α and IL-10 are two predicted targets of miR-4492. Therefore, these two molecules were selected as candidates for validation in the present study. TNF-α is one of the most important pro-inflammatory cytokines produced by macrophages and T lymphocytes. Previous studies of Parkinson's disease demonstrate the importance of upregulated TNF-α in immune-mediated initiation of inflammation (47,48). Although differential expression of TNF-α has been reported in CRS with and without NP in previous studies, differences in the expression of TNF-α were not detected in the present study using RT-qPCR. A possible explanation for this discrepancy may be the heterogeneity of individuals from different regions. IL-10 is a pleiotropic cytokine with important immunoregulatory functions (49). Dysregulation of IL-10 is involved in the immunopathologic response to infection and the development of numerous autoimmune diseases (50). In the present study, elevated levels of IL-10 were detected in the CRSwNP samples, and IL-10 expression was negatively correlated with the expression of miR-4492 in CRS. Consistent with this, a previous study using clinical specimens demonstrated that increased expression levels of IL-10 have a pivotal role in the pathogenesis of CRSwNP (51). In a previous study by our group, dysregulation of miR-4492, miR-200-3p, TNF-α and IL-10 was observed in paraffin-embedded CRSwNP samples using RNA-seq and RT-qPCR (52), whereas differences in the expression of TNF-α were not detected in the present study. Differences in sample type and sample size may at least partly account for this discrepancy. In addition, Luo et al (53) indicated that miR-19a has a crucial role in nasal polyposis through the suppression of IL-10 in peripheral dendritic cells. Furthermore, in KEGG pathway analysis, IL-10 was enriched in the Jak/STAT signaling pathway. Combined with the results of the present study, it is indicated that hsa-miR-4492 may serve roles in nasal polyposis via the hsa-miR-4492/IL-10 interaction in the Jak/STAT signaling pathway (52). However, the present study has a number of limitations, including the relatively small number of samples for miRNA sequencing and lack of a functional validation assay. Therefore, further studies are required in order to elucidate the interactional mechanism of this pathway in CRSwNP.
In conclusion, the present study demonstrated that miR-4492 is downregulated and IL-10 is upregulated in NPs, and suggests that the involvement of the hsa-miR-4492/IL-10-axis in the Jak/STAT signaling pathway may be a key mechanism in CRSwNP.
Supplementary Material
Acknowledgements
Not applicable.
Funding
The present study was supported in part by the National Natural Science Foundation of China (grant no. 81460094) and the Natural Science Foundation of Xinjiang Province (grant no. 2015211C076).
Availability of data and materials
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
LL and JYo conceived and designed the study. LL, JF, DZ, YW, JYa and RH performed the data analysis. LL wrote the manuscript. LL and JYo edited the manuscript. All authors reviewed the manuscript and approved the final version.
Ethics approval and informed consent
The present study was approved by the Ethics Committee of the First Affiliated Hospital of Xinjiang Medical University (Urumqi, China; permit no. 20160114-05) and it was performed with written informed consent from the participants.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.DeConde AS, Soler ZM. Chronic rhinosinusitis: Epidemiology and burden of disease. Am J Rhinol Allergy. 2016;30:134–139. doi: 10.2500/ajra.2016.30.4297. [DOI] [PubMed] [Google Scholar]
- 2.Hoffmans R, Schermer T, van der Linde K, Bor H, van Boven K, van Weel C, Fokkens W. Rhinosinusitis in morbidity registrations in dutch general practice: A retro-spective case-control study. BMC Fam Pract. 2015;16:120. doi: 10.1186/s12875-015-0332-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meltzer EO, Hamilos DL, Hadley JA, Lanza DC, Marple BF, Nicklas RA, Bachert C, Baraniuk J, Baroody FM, Benninger MS, et al. Rhinosinusitis: Establishing definitions for clinical research and patient care. J Allergy Clin Immunol. 2004;114((6 Suppl)):S155–S212. doi: 10.1016/j.jaci.2004.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Benninger MS, Ferguson BJ, Hadley JA, Hamilos DL, Jacobs M, Kennedy DW, Lanza DC, Marple BF, Osguthorpe JD, Stankiewicz JA, et al. Adult chronic rhinosinusitis: Definitions, diagnosis, epidemiology, and pathophysiology. Otolaryngol Head Neck Surg. 2003;129((3 Suppl)):S1–S32. doi: 10.1053/hn.2003.v128.amhn0312811. [DOI] [PubMed] [Google Scholar]
- 5.Banerji A, Piccirillo JF, Thawley SE, Levitt RG, Schechtman KB, Kramper MA, Hamilos DL. Chronic rhinosinusitis patients with polyps or polypoid mucosa have a greater burden of illness. Am J Rhinol. 2007;21:19–26. doi: 10.2500/ajr.2007.21.2979. [DOI] [PubMed] [Google Scholar]
- 6.Koskinen A, Numminen J, Markkola A, Karjalainen J, Karstila T, Seppälä M, Julkunen A, Lemmetyinen R, Pekkanen J, Rautiainen M, et al. Diagnostic accuracy of symptoms, endoscopy, and imaging signs of chronic rhinosinusitis without nasal polyps compared to allergic rhinitis. Am J Rhinol Allergy. 2018;32:121–131. doi: 10.1177/1945892418762891. [DOI] [PubMed] [Google Scholar]
- 7.Dietz de Loos DA, Hopkins C, Fokkens WJ. Symptoms in chronic rhinosinusitis with and without nasal polyps. Laryngoscope. 2013;123:57–63. doi: 10.1002/lary.23671. [DOI] [PubMed] [Google Scholar]
- 8.Avdeeva K, Fokkens W. Precision medicine in chronic rhinosinusitis with nasal polyps. Curr Allergy Asthma Rep. 2018;18:25. doi: 10.1007/s11882-018-0776-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, Cohen N, Cervin A, Douglas R, Gevaert P, et al. European position paper on rhinosinusitis and nasal polyps 2012. Rhinol. 2012;(Suppl 23):1–298. doi: 10.4193/Rhino50E2. 3 p preceding table of contents. [DOI] [PubMed] [Google Scholar]
- 10.Fokkens WJ, Lund VJ, Mullol J, Bachert C, Alobid I, Baroody F, Cohen N, Cervin A, Douglas R, Gevaert P, et al. EPOS 2012: European position paper on rhinosinusitis and nasal polyps 2012. A summary for otorhinolaryngologists. Rhinology. 2012;50:1–12. doi: 10.4193/Rhino50E2. [DOI] [PubMed] [Google Scholar]
- 11.Vishnoi A, Rani S. MiRNA biogenesis and regulation of diseases: An overview. Methods Mol Biol. 2017;1509:1–10. doi: 10.1007/978-1-4939-6524-3_1. [DOI] [PubMed] [Google Scholar]
- 12.Ni WJ, Leng XM. miRNA-dependent activation of mRNA translation. Microrna. 2016;5:83–86. doi: 10.2174/2211536605666160825151201. [DOI] [PubMed] [Google Scholar]
- 13.Beilharz TH, Humphreys DT, Preiss T. miRNA effects on mRNA closed-loop formation during translation initiation. Prog Mol Subcell Biol. 2010;50:99–112. doi: 10.1007/978-3-642-03103-8_7. [DOI] [PubMed] [Google Scholar]
- 14.Fleshner M, Crane CR. Exosomes, DAMPs and miRNA: Features of stress physiology and immune homeostasis. Trends Immunol. 2017;38:768–776. doi: 10.1016/j.it.2017.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Foley NH, O'Neill LA. miR-107: A toll-like receptor-regulated miRNA dysregulated in obesity and type II diabetes. J Leukoc Biol. 2012;92:521–527. doi: 10.1189/jlb.0312160. [DOI] [PubMed] [Google Scholar]
- 16.Hou M, Li W, Xie Z, Ai J, Sun B, Tan G. Effects of anticholinergic agent on miRNA profiles and transcriptomes in a murine model of allergic rhinitis. Mol Med Rep. 2017;16:6558–6569. doi: 10.3892/mmr.2017.7411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Solberg OD, Ostrin EJ, Love MI, Peng JC, Bhakta NR, Hou L, Nguyen C, Solon M, Nguyen C, Barczak AJ, et al. Airway epithelial miRNA expression is altered in asthma. Am J Respir Crit Care Med. 2012;186:965–974. doi: 10.1164/rccm.201201-0027OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu F, Qin HB, Xu B, Zhou H, Zhao DY. Profiling of miRNAs in pediatric asthma: Upregulation of miRNA-221 and miRNA-485-3p. Mol Med Rep. 2012;6:1178–1182. doi: 10.3892/mmr.2012.1030. [DOI] [PubMed] [Google Scholar]
- 19.Kozomara A, Griffiths-Jones S. miRBase: Annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 2014;42:D68–D73. doi: 10.1093/nar/gkt1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kalvari I, Argasinska J, Quinones-Olvera N, Nawrocki EP, Rivas E, Eddy SR, Bateman A, Finn RD, Petrov AI. Rfam 13.0: Shifting to a genome-centric resource for non-coding RNA families. Nucleic Acids Res. 2018;46:D335–D342. doi: 10.1093/nar/gkx1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dweep H, Gretz N, Sticht C. miRWalk database for miRNA-target interactions. Methods Mol Biol. 2014;1182:289–305. doi: 10.1007/978-1-4939-1062-5_25. [DOI] [PubMed] [Google Scholar]
- 22.The Gene Ontology Consortium, corp-author. Expansion of the gene ontology knowledgebase and resources. Nucleic Acids Res. 2017;45:D331–D338. doi: 10.1093/nar/gkw1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kanehisa M, Goto S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 25.Lal D, Scianna JM, Stankiewicz JA. Efficacy of targeted medical therapy in chronic rhinosinusitis, and predictors of failure. Am J Rhinol Allergy. 2009;23:396–400. doi: 10.2500/ajra.2009.23.3334. [DOI] [PubMed] [Google Scholar]
- 26.Xia G, Bao L, Gao W, Liu S, Ji K, Li J. Differentially expressed miRNA in inflammatory mucosa of chronic rhinosinusitis. J Nanosci Nanotechnol. 2015;15:2132–2139. doi: 10.1166/jnn.2015.9161. [DOI] [PubMed] [Google Scholar]
- 27.Ma Z, Shen Y, Zeng Q, Liu J, Yang L, Fu R, Hu G. MiR-150-5p regulates EGR2 to promote the development of chronic rhinosinusitis via the DC-Th axis. Int Immunopharmacol. 2018;54:188–197. doi: 10.1016/j.intimp.2017.11.011. [DOI] [PubMed] [Google Scholar]
- 28.Li FM, Zheng JF. Application and progress of miRNA in chronic rhinosinusitis. Lin Chuang Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2016;30:1656–1658. doi: 10.13201/j.issn.1001-1781.2016.20.019. (In Chinese) [DOI] [PubMed] [Google Scholar]
- 29.Ma ZX, Tan X, Shen Y, Ke X, Yang YC, He XB, Wang ZH, Dai YB, Hong SL, Hu GH. MicroRNA expression profile of mature dendritic cell in chronic rhinosinusitis. Inflamm Res. 2015;64:885–893. doi: 10.1007/s00011-015-0870-5. [DOI] [PubMed] [Google Scholar]
- 30.Zhang XH, Zhang YN, Li HB, Hu CY, Wang N, Cao PP, Liao B, Lu X, Cui YH, Liu Z. Overexpression of miR-125b, a novel regulator of innate immunity, in eosinophilic chronic rhinosinusitis with nasal polyps. Am J Respir Crit Care Med. 2012;185:140–151. doi: 10.1164/rccm.201103-0456OC. [DOI] [PubMed] [Google Scholar]
- 31.Izumchenko E, Chang X, Michailidi C, Kagohara L, Ravi R, Paz K, Brait M, Hoque MO, Ling S, Bedi A, Sidransky D. The TGFβ-miR200-MIG6 pathway orchestrates the EMT-associated kinase switch that induces resistance to EGFR inhibitors. Cancer Res. 2014;74:3995–4005. doi: 10.1158/0008-5472.CAN-14-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zidar N, Boštjančič E, Jerala M, Kojc N, Drobne D, Štabuc B, Glavač D. Down-regulation of microRNAs of the miR-200 family and up-regulation of Snail and Slug in inflammatory bowel diseases-hallmark of epithelial-mesenchymal transition. J Cell Mol Med. 2016;20:1813–1820. doi: 10.1111/jcmm.12869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Levänen B, Bhakta NR, Torregrosa Paredes P, Barbeau R, Hiltbrunner S, Pollack JL, Sköld CM, Svartengren M, Grunewald J, Gabrielsson S, et al. Altered microRNA profiles in bronchoalveolar lavage fluid exosomes in asthmatic patients. J Allergy Clin Immunol. 2013;131:894–903. doi: 10.1016/j.jaci.2012.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wu CW, Cao X, Berger CK, Foote PH, Mahoney DW, Simonson JA, Anderson BW, Yab TC, Taylor WR, Boardman LA, et al. Novel approach to fecal occult blood testing by assay of erythrocyte-specific microRNA markers. Dig Dis Sci. 2017;62:1985–1994. doi: 10.1007/s10620-017-4627-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Panganiban RP, Wang Y, Howrylak J, Chinchilli VM, Craig TJ, August A, Ishmael FT. Circulating microRNAs as biomarkers in patients with allergic rhinitis and asthma. J Allergy Clin Immunol. 2016;137:1423–1432. doi: 10.1016/j.jaci.2016.01.029. [DOI] [PubMed] [Google Scholar]
- 36.Liu Z, Zhang XH, Callejas-Diaz B, Mullol J. MicroRNA in united airway diseases. Int J Mol Sci. 2016;17:E716. doi: 10.3390/ijms17050716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xun M, Ma CF, Du QL, Ji YH, Xu JR. Differential expression of miRNAs in enterovirus 71-infected cells. Virol J. 2015;12:56. doi: 10.1186/s12985-015-0288-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Egaña-Gorroño L, Guardo AC, Bargalló ME, Planet E, Vilaplana E, Escribà T, Pérez I, Gatell JM, García F, Arnedo M, et al. MicroRNA profile in CD8+ T-Lymphocytes from HIV-infected individuals: Relationship with antiviral immune response and disease progression. PLoS One. 2016;11:e0155245. doi: 10.1371/journal.pone.0155245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ahn SH, Ahn JH, Ryu DR, Lee J, Cho MS, Choi YH. Effect of Necrosis on the miRNA-mRNA regulatory network in CRT-MG human astroglioma cells. Cancer Res Treat. 2018;50:382–397. doi: 10.4143/crt.2016.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gao ZG, Chen QJ, Shao M, Qian YZ, Zhang LF, Zhang YB, Xiong QX. Preliminary identification of key miRNAs, signaling pathways, and genes associated with hirschsprung's disease by analysis of tissue microRNA expression profiles. World J Pediatr. 2017;13:489–495. doi: 10.1007/s12519-017-0064-z. [DOI] [PubMed] [Google Scholar]
- 41.Boo L, Ho WY, Ali NM, Yeap SK, Ky H, Chan KG, Yin WF, Satharasinghe DA, Liew WC, Tan SW, et al. MiRNA transcriptome profiling of spheroid-enriched cells with cancer stem cell properties in human breast MCF-7 cell line. Int J Biol Sci. 2016;12:427–445. doi: 10.7150/ijbs.12777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wei CH, Phan L, Feltz J, Maiti R, Hefferon T, Lu Z. tmVar 2.0: Integrating genomic variant information from literature with dbSNP and clinvar for precision medicine. Bioinformatics. 2018;34:80–87. doi: 10.1093/bioinformatics/btx541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Cheng KJ, Xu YY, Zhou ML, Zhou SH, Wang SQ. Role of local allergic inflammation and staphylococcus aureus enterotoxins in Chinese patients with chronic rhinosinusitis with nasal polyps. J Laryngol otol. 2017;131:707–713. doi: 10.1017/S0022215117001335. [DOI] [PubMed] [Google Scholar]
- 44.Mattes J, Collison A, Plank M, Phipps S, Foster PS. Antagonism of microRNA-126 suppresses the effector function of TH2 cells and the development of allergic airways disease. Proc Natl Acad Sci USA. 2009;106:18704–18709. doi: 10.1073/pnas.0905063106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang XH, Zhang YN, Liu Z. MicroRNA in chronic rhinosinusitis and allergic rhinitis. Curr Allergy Asthma Rep. 2014;14:415. doi: 10.1007/s11882-013-0415-3. [DOI] [PubMed] [Google Scholar]
- 46.Liu CC, Xia M, Zhang YJ, Jin P, Zhao L, Zhang J, Li T, Zhou XM, Tu YY, Kong F, et al. Micro124-mediated AHR expression regulates the inflammatory response of chronic rhinosinusitis (CRS) with nasal polyps. Biochem Biophys Res Commun. 2018;500:141–151. doi: 10.1016/j.bbrc.2018.03.204. [DOI] [PubMed] [Google Scholar]
- 47.Nishimura M, Mizuta I, Mizuta E, Yamasaki S, Ohta M, Kaji R, Kuno S. Tumor necrosis factor gene polymorphisms in patients with sporadic Parkinson's disease. Neurosci Lett. 2001;311:1–4. doi: 10.1016/S0304-3940(01)02111-5. [DOI] [PubMed] [Google Scholar]
- 48.Lindenau JD, Altmann V, Schumacher-Schuh AF, Rieder CR, Hutz MH. Tumor necrosis factor alpha polymorphisms are associated with Parkinson's disease age at onset. Neurosci Lett. 2017;658:133–136. doi: 10.1016/j.neulet.2017.08.049. [DOI] [PubMed] [Google Scholar]
- 49.Mocellin S, Marincola F, Rossi CR, Nitti D, Lise M. The multifaceted relationship between IL-10 and adaptive immunity: Putting together the pieces of a puzzle. Cytokine Growth Factor Rev. 2004;15:61–76. doi: 10.1016/j.cytogfr.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 50.Iyer SS, Cheng G. Role of interleukin 10 transcriptional regulation in inflammation and autoimmune disease. Crit Rev Immunol. 2012;32:23–63. doi: 10.1615/CritRevImmunol.v32.i1.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Xu J, Han R, Kim DW, Mo JH, Jin Y, Rha KS, Kim YM. Role of interleukin-10 on nasal polypogenesis in patients with chronic rhinosinusitis with nasal polyps. PLoS One. 2016;11:e0161013. doi: 10.1371/journal.pone.0161013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li LG, Ma T, YR C. MiR-200A-3P and miR4492 involved in regulation of chronic rhinosinusitis with nasal polyps. Int J Genet. 2018;41:108–114. [Google Scholar]
- 53.Luo XQ, Shao JB, Xie RD, Zeng L, Li XX, Qiu SQ, Geng XR, Yang LT, Li LJ, Liu DB, et al. Micro RNA-19a interferes with IL-10 expression in peripheral dendritic cells of patients with nasal polyposis. Oncotarget. 2017;8:48915–48921. doi: 10.18632/oncotarget.16555. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.