Abstract
Eosinophils are circulating granulocytes that have pleiotropic effects in response to inflammatory signals in the body. In response to allergens or pathogens, exposure eosinophils are recruited in various organs that execute pathological immune responses. IL-5 plays a key role in the differentiation, development, and survival of eosinophils. Eosinophils are involved in a variety of allergic diseases including asthma, dermatitis and various gastrointestinal disorders (EGID). IL-5 signal transduction involves JAK-STAT-p38MAPK-NFkB activation and executes extracellular matrix remodeling, EMT transition and immune responses in allergic diseases. IL-18 is a classical cytokine also involved in immune responses and has a critical role in inflammasome pathway. We recently identified the IL-18 role in the generation, transformation, and maturation of (CD101+CD274+) pathogenic eosinophils. In, addition, several other cytokines like IL-2, IL-4, IL-13, IL-21, and IL-33 also contribute in advancing eosinophils associated immune responses in innate and adaptive immunity. This review discusses with a major focus (1) Eosinophils and its constituents, (2) Role of IL5 and IL18 in eosinophils development, transformation, maturation, signal transduction of IL5 and IL18, (3) the role of eosinophils in allergic disorders and (4) the role of several other associated cytokines in promoting eosinophils mediated allergic diseases.
Keywords: Allergy, Eosinophils, IL-5, IL-18, Immune responses, Th2 responses
Graphical Abstract
Fig. Synergy of Interleukin (IL)-5 and IL-18 in eosinophil mediated pathogenesis of allergic diseases
1. Introduction
Eosinophils are likely first observed in 1846 by Wharton Jones and named by Paul Ehrlich in 1879, with time they have traditionally considered effector cells in the innate immune system. Eosinophils are granulocytic leukocytes that develop in the bone marrow from pluripotent progenitors and share many features with neutrophils, including growth and development in the bone marrow. In contrast with neutrophils, eosinophils exit the bone marrow and reside in the bloodstream with a half-life of ~18 hrs and migrate to the thymus, gastrointestinal tract under homeostatic conditions. Eosinophils arise from hematopoietic CD34+ progenitor cells in the bone marrow and are of 5 % of total white blood cells in healthy subjects. Eosinophils are distinguished phenotypically by their bilobed nuclei and cytoplasmic granules filled with cytotoxic and immunoregulatory proteins [1]. Mature eosinophils in the blood are less than 400 per mm3. Eosinophils respond to signals from cytokines, chemokines, and other proinflammatory mediators via cell-specific receptors most notably by cytokine interleukin-5 (IL-5) and the eotaxin chemokines. During eosinophilopoiesis, eosinophils acquire IL-5Rα and IL-18Rα on the cell surface and differentiate under the influence of both IL-5 and IL-18 [2]. Transcription factors play a crucial role in eosinophilic lineage commitment in the bone marrow to maturation and development of eosinophils. Eosinophilic progenitors express C/EBPα, C/EBPε, PU.1, and GATA-1 and GATA-2 [3]. The increased production of eosinophils is observed in various allergic conditions, skin diseases, parasitic infections, and reactions to drugs. Peripheral blood and tissue eosinophilia are hallmarks of parasitic helminth infection chronic asthma and allergic diseases and play a role in host defense [4]. Eosinophils migrate from bone marrow to the specific tissues on exposure with allergens and execute immune responses [5]. Eosinophils display pleiotropic effects such as lymphocyte homeostasis, PMN activation, basophil degranulation, mastocytosis, macrophage phagocytosis, Th2/DC’s stabilization, epithelial cell differentiation, neurite outgrowth, fat tissue homeostasis, glucose metabolism, tissue repair and fibrosis, cardiovascular morbidity, bronchospasm, pathogen defense, antigen presentation, extracellular pathogen trap, parasite antibody-dependent cell mediated cytotoxicity [6].
Eosinophils are key innate regulator cells. Under homeostasis, eosinophils are distributed in the blood, lung, thymus, uterus, adipose tissues, mammary gland, spleen and the lamina propria of the gastrointestinal (GI) tract [7]. However, under the allergic condition, the mature eosinophils under the influence of IL-5 and eotaxin are released from bone marrow to bloodstream and recruit target tissue and modulate immune reactions. Eosinophils also regulate adaptive immune reactions and modulate lymphocyte recruitment and homeostasis. Th2 cells produce the cytokine IL-5, which promotes eosinophil production, priming, and survival, and IL-13, which induces local cells to produce eotaxins, which attract circulating eosinophils into their niche eosinophils and are critical for T-cell homing in the lung. Eosinophil-deficient ΔdblGATA-1 mice reduced Peyer’s patch development, and T helper cell cytokine production was observed, highlighting the promoting role of eosinophils on lymphocyte homeostasis [8-10]. Bone marrow eosinophils co-localize with plasma cells during their maturation, secrete the cytokines APRIL and IL-6 and contribute to the survival of bone marrow plasma cells. B cell process of IgA class switching was recently found to be positively regulated by GI eosinophils in the intestinal tissue[8, 10]. Eosinophils promote B cell proliferation upon eosinophil activation in mice [11].
Eosinophils act as antigen-presenting cells. Eosinophils can present antigen to T cells. GM-CSF–treated eosinophils induce antigen-specific T-cell clone proliferation [12]. Upon allergen exposure, eosinophils present antigens by costimulation of MHC class II [13, 14]. Eosinophils in the airway lumen migrate to the draining lymph node and promote antigen-specific T-cell proliferation ex vivo. Eosinophils, alone or via dendritic cells, drive Th2 polarization. Upon allergen stimulation, eosinophils produce Th2 cytokines (IL-4, IL-5, and IL-13). Eosinophils regulate dendritic cells and Th2 pulmonary immune responses [15]. Eosinophils suppress Th17 and Th1 responses via DC regulation. Eosinophil products can serve as Th2 adjuvants via DC regulation.
Eosinophils also regulate T-cell development in the thymus. Eosinophils recognize PAMPs and defense against viral, parasitic and bacterial infection. Eosinophils and mast cells mutually potentiate in asthma and eosinophilic esophagitis. Mast cells support the survival and activation of eosinophils by secreting IL-5. Eosinophil MBP activates mast cells and basophils, and release cytokines, histamine, and TNF-α [6]. Eosinophils play a key role in tissue destruction and repair. Epithelial cells of various tissues stimulate eosinophil secretion of TGF-β and fibroblast growth factors and play a role in tissue repair and also promote wound healing [16-19].
Adipose tissue residential eosinophils regulate local cytokine milieu and glucose homeostasis. Eosinophils play a role in adipose tissue macrophage polarization and glucose metabolism critical for maintaining adipose tissue M2 macrophages [20]. Human eosinophils promoted dorsal root ganglia neuron branching. Peripheral dorsal root ganglia and airway neurons produce eotaxins to chemoattract eosinophils [21, 22]. Eosinophils producing nerve growth factor and neurotrophins regulate neuronal activity [23]. Eosinophils in the GI tract play a role in homeostasis and disease contexts, such as in EGID promoting IgA class-switching, enhancing intestinal mucus secretions, determining intestinal microbiota and inducing the development of Peyer’s patches [8, 10].
The current review summarizes the most recent advances in our understanding on the contributions of IL-5 and IL-18 to the growth, transformation, and maturation of eosinophils to the maintenance of innate and adaptive immune responses in various allergic diseases. The review also briefs the current pharmacotherapy available to treat various eosinophil-mediated allergic diseases.
2. Eosinophilic constituents
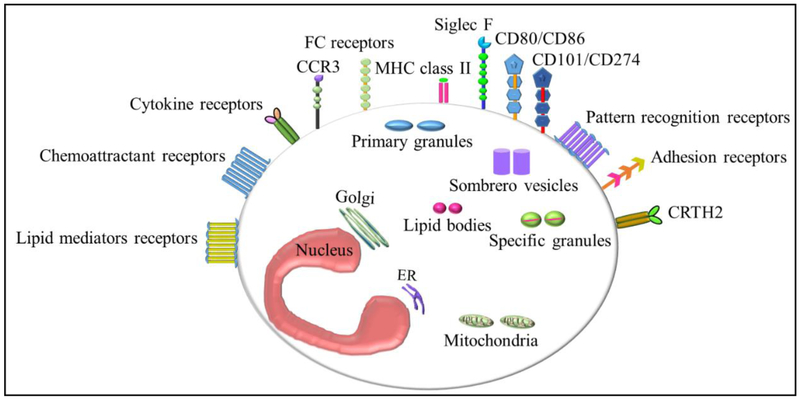
Eosinophil constitutes a wide variety of molecules such as adhesion molecules, adhesion receptors, apoptosis and signaling factors, chemoattractant receptors, chemokines, cytokine and receptors, Fc receptors, growth factors and its receptors, lipid body contents, lipid mediators and its receptors, pattern recognition receptors, granule-derived receptors, eosinophil-derived granule proteins, enzymes and reactive oxygen intermediates. Eosinophils morphology, cell-surface receptors, and intracellular contents differ between species. Particularly interleukin-5 receptor subunit-α (IL-5Rα) and CC-chemokine receptor 3 (CCR3), as well as sialic acid binding immunoglobulin-like lectin 8 (SIGLEC-8) in humans, and SIGLEC-F (also known as SIGLEC-5) in mice. In addition, eosinophil also has CRTH2 receptor that helps in the recruitment of eosinophils in allergic condition via bindings its ligands [24]. Eosinophilic granular proteins comprises, major basic protein-1 and −2 (MBP-1, MBP-2), eosinophil peroxidase (EPX), eosinophil cationic protein (ECP), eosinophil-derived neurotoxin (EDN), Charcot-Leyden crystal protein/Galectin-10 (CLC/Gal-10). These are routinely studied as markers of eosinophil activation, and we recently reported that IL-18 influence in the increase production of these toxic proteins during allergic condition [2].
2.1. Major basic protein
MBP comprised more than 50 % of the granule protein and localized in the core of the eosinophil secondary granules. MBP-1 damages cells by disrupting the lipid bilayer membrane or altering the activity of enzymes within tissues [25]. MBP-1 stimulates mediator (histamine) release from basophils and mast cells, activates neutrophils and platelets, and augments superoxide generation by alveolar macrophages. MBP-2 is expressed only in eosinophils and exhibits similar functions in vitro like MBP-1 [26-28].
2.2. Eosinophil peroxidase (EPX/EPO)
EPO is a heme peroxidase found in eosinophils, and its gene located on chromosome 17. Its activities include the oxidation of halide ions to bactericidal ROS, the cationic disruption of bacterial cell walls, and the post-translational modification of protein amino acid residues [29-31]. EPO also mediates protein nitration in allergic airway inflammation in mice. 3-nitrotyrosine (3NT) formation after allergen challenge is dependent on EPO activity, particularly in eosinophils [32].
2.3. Eosinophil cationic protein (ECP)
ECP is a basic cationic protein located in the eosinophil primary matrix and is released during degranulation of eosinophils. ECP possess helminth-toxic and ribonucleolytic activities. ECP is assayed as one of the predictive markers of eosinophil activation in biological fluids in diseases like asthma, bullous pemphigoid severity and outcome [32, 33]. ECP exerts cytotoxicity to parasites, bacteria, and virus and make pores on the cell membrane and kill the cell by osmotic lysis by the passage of water and other small molecules [34].
2.4. Eosinophil-derived neurotoxin (EDN)
EDN is released from eosinophil granules by cytokines and other proinflammatory mediators [35]. EDN is an active ribonuclease and capable of generating soluble ribonucleotides from insoluble tRNA [36]. Eosinophils upon activation with bacteria release EDN. Eosinophils in response to Staphylococcus aureus, release EDN [37]. EDN induces production of MMP-9 from the nasal epithelium and involves in the pathogenesis of chronic eosinophilic rhinosinusitis [38]. Eosinophil-derived neurotoxin correlated with the disease severity of AD and considered as a serum biomarker for disease severity in atopic dermatitis [39].
2.5. The Charcot-Leyden crystal protein/Galectin-10 (CLC/Gal-10)
CLC expression was increased in asthma, allergic reactions, and fungal and helminthic infections and related to the histological grade and with the number of eosinophils in the lesion of celiac disease patients and also higher in eosinophilic cystitis [40, 41]. CLCs upon their phagocytosis by primary human macrophages induce the release of the IL-1β and activate the NLRP3 Inflammasome. CLC functions as a T cell-suppressive molecule in eosinophils [42]. Neutralization of galectin-10 partially abrogated the suppressive function of the eosinophils, and recombinant galectin-10 was able to suppress T cell proliferation [43]. The components of eosinophils and their functions are described in Fig. 1 and Table 1.
Fig.1.
Eosinophil constituents.
Table.1.
Eosinophil constituents and function.
| Eosinophil constituents |
Molecules | Function | Reference |
|---|---|---|---|
| Adhesion molecules | Integrins, β1, β2, β7, selectins | eosinophil recruitment, T cell proliferation | [4, 44-46] |
| Adhesion receptors | LFA1, CR3, CR4, VLA4, CD44, CD62L, PSGL1, CD34 | ||
| Apoptosis and signaling factors | CD30, CD45, CD52, CD69, CD95 | Eosinophil survival and apoptotic cell death | [47, 48] |
| Chemoattractant receptors | CCR1, CCR3, CCR4, CCR5, CCR6, CCR8, CCR9, CXCR2, CXCR3, CXCR4, C5aR, C3aR, FPR1 | Adhesion of eosinophils to the endothelial cells, Chemoattractant to immune cells and the site of allergic inflammation. | [49, 50] |
| Chemokines | CCL3, CCL5, CCL7, CCL8, CCL11, CCL13, CXCL1, CXCL10, CXCL11, CXCL12 | ||
| Cytokines and receptors | IL1α, IL1β, IL2, IL-3, IL-4, IL-5, IL-6, Il-10, IL11, IL-12, IL-13, IL16, IL17, IL25, IFN-γ, GM-CSF, TNF, IL-2R, IL-3R, IL-4R, IL-5R, IL-9R, IL-10R, IL-13R, IL-17R, IL-23R, IL-27R, IL-31R, IL-33R, IFNγR, GM-CSFR, | Activation, proliferation, and maturation of eosinophils. Induction of inflammatory response, induce overexpression of adhesion molecules and promote airway remodeling. | [51] |
| Fc receptors | FcαR, FcγRII, FcεRI, FcεRII, | eosinophil survival, degranulation, and generation of leukotrienes | [46, 52] |
| Growth factors and receptors | NGF, PDGF, SCF, VEGF, EGF, TGFα, TGFβ, APRIL, TSLPR, KIT, TGFβR | Play role in pulmonary hypertension, allergic asthma, allergic rhinitis, and allergic urticarial-angioedema, allergic inflammation Stimulates T cells, B cell proliferation and differentiation, and eosinophil differentiation from peripheral progenitors, growth and differentiation of mast cell progenitors residing in tissues, wound-healing and tissue remodeling, fibrosis, and tissue repair, extracellular matrix protein deposition, stromal fibrosis and basement membrane thickening. | [53-62] |
| Lipid body contents | Leukotriene C4, leukotriene D4, leukotriene E4, Thromboxane B2, Prostaglandin E1, Prostaglandin E2, 15-hydroxyeicosatetraenoic acid, Platelet-activating factor | Activation of platelets, neutrophils and leukocyte recruitment and, increase airway reactivity, vascular permeability and induction of bronchoconstriction and mediates asthma and allergic rhinitis. | [49, 63] |
| Lipid mediators | Leukotriene C4/D4/E4, Eotoxin C4/D4/E4, Prostaglandin E1, E2, F1α, 5-HETE, 5,15-and 8,15-diHETE, platelet activating factor, Thromboxane B2 | ||
| Lipid mediators receptors | DP2 prostaglandin receptor (CRTH2), DP1 prostaglandin receptor, EP2 prostaglandin receptor, Leukotriene B4 receptor, Platelet-activating factor receptor | ||
| Pattern recognition receptors | Dectin-1, TLR1, TLR2, TLR3, TLR4, TLR5, TLR6, TLR7, TLR9, TLR 10, NOD1, NOD2, RIG-1, RAGE | Recognition of self, bacterial, viral and fungal signals. Activation of immune responses and release of pro-inflammatory chemokines, cytokines, granule proteins, leukotrienes, and reactive oxygen species. Activation of the adhesion system and cellular trafficking. Play a role in airway inflammation and hyper-reactivity. | [64] |
| Granule-derived proteins | Major basic protein (MBP)-1, MBP-2, Eosinophil peroxidase (EPO), Eosinophil-derived neurotoxin (EDN or RNase2), Eosinophil cationic protein (ECP or RNase3) | Local tissue damage, mast cell and basophil degranulation, airway mucus production and elevation of reactive oxygen species | [26, 51] |
| Enzymes | β-glucuronidase, matrix metalloproteinase 9, α-mannosidase, Phospholipase A2, 5-lipoxygenase, 15-lipoxygenase, leukotriene C4 synthase, lysozyme, NADPH oxidase, Acid phosphatase, collagenase, arylsulphatase B, histaminase, phospholipase D, catalase, nonspecific esterase, | Aid in the migration of eosinophils through basement membranes, tissue remodeling, degrade phospholipids of Gram-negative bacteria and to cause airway inflammation and smooth muscle contraction, defense of microorganisms | [48, 65] |
| Reactive oxygen intermediates | O2, H2O2, hydroxyl radicles, singlet oxygen | Induce inflammatory response and T cell proliferation | [66] |
3. IL5 mediated signal transduction and eosinophils development and survival
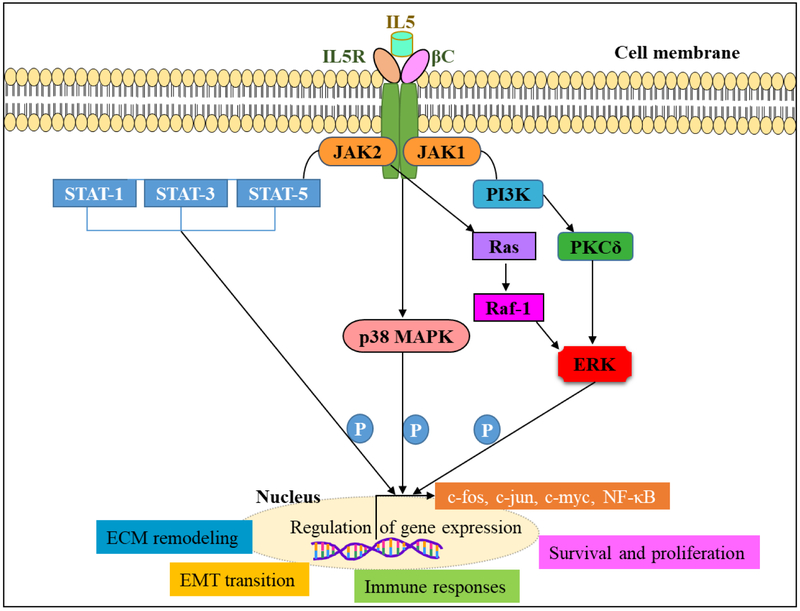
Interleukin 5 (IL-5) plays a vital role in the activation, terminal differentiation, growth and survival of eosinophils at the early stage and the sites of /inflammation. Eosinophils highly express the interleukin-5Rα on their surface and also release significant amounts of IL-5 [67]. IL-5 signals via the IL-5 receptor (R) α chain and β chain (βc) complex [68]. Upon activation, IL-5 signaling is initiated by the α-chain that binds IL-5 with low affinity, and dimerization with the βc-subunit forms high-affinity receptor [69]. IL-5 activation induces phosphorylation of a series of cellular proteins such as βc, HS1, Shc, Btk, JAK1, JAK2, STAT1, STAT3, STAT5, PI3K, MAPKs. Phosphorylation of these proteins activates down-stream molecules such as Pim-1, c-fos, c-jun, NFκb, and thus induce cell survival and proliferation, immune responses and eosinophil number [70-76]. JAK2 is associated with hIL-5Rα, whereas JAK1 is associated with hIL-5Rα and forms. JAK1 and JAK2 are activated upon IL-5 stimulation. JAK2 and STAT5 activation are essential for IL-5-dependent signal transduction in eosinophils and B cells [77, 78]. IL-5 also activates, SHP2 and Raf-1 and Raf-1 regulate degranulation. Ras GTPase-ERK activation is vital for IL-5 mediated cell survival, and proliferation [79]. The inhibitors for kinases demonstrated the role of kinases in eosinophils activation [80]. In eosinophils p38 MAP kinases are activated, whereas its activation is inhibited in dying eosinophils [81]. The inhibitor for p38 MAP kinase SB239063 abolish lung eosinophilia in mice, another inhibitor SB203580 enhances apoptosis of eosinophils [82, 83]. However, there is a concern that these inhibitors might have side effects as they target signaling mechanisms in various cell types. In addition to eosinophil survival, IL-5 also inhibits eosinophil apoptosis, via NF-kB mediated induction of BCL-XL and play a role in the survival of eosinophils [84, 85] (Fig. 2.).
Fig.2.
IL5 mediated cell signaling in eosinophils.
4. IL18 mediated signal transduction in inflammation
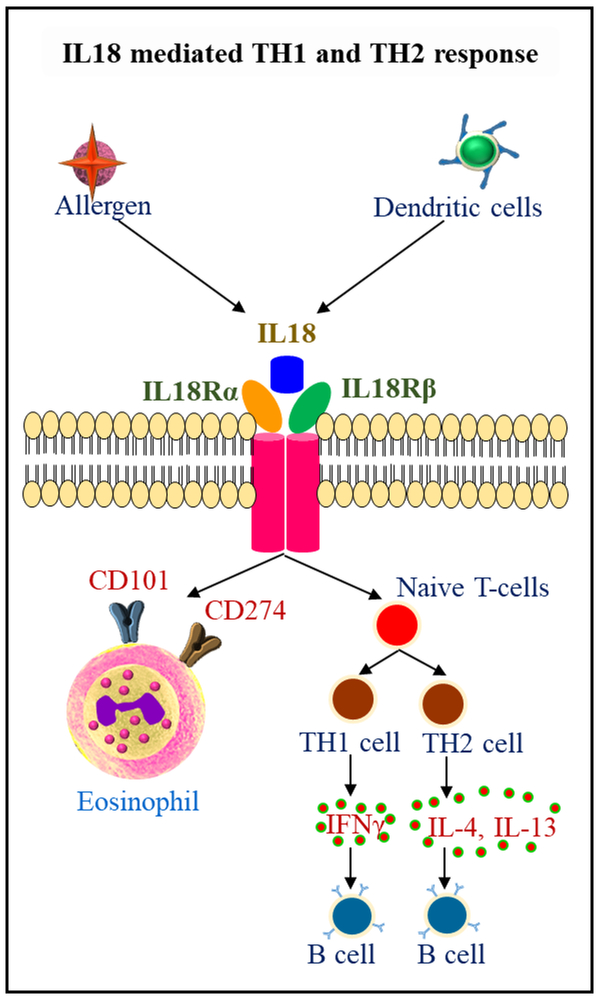
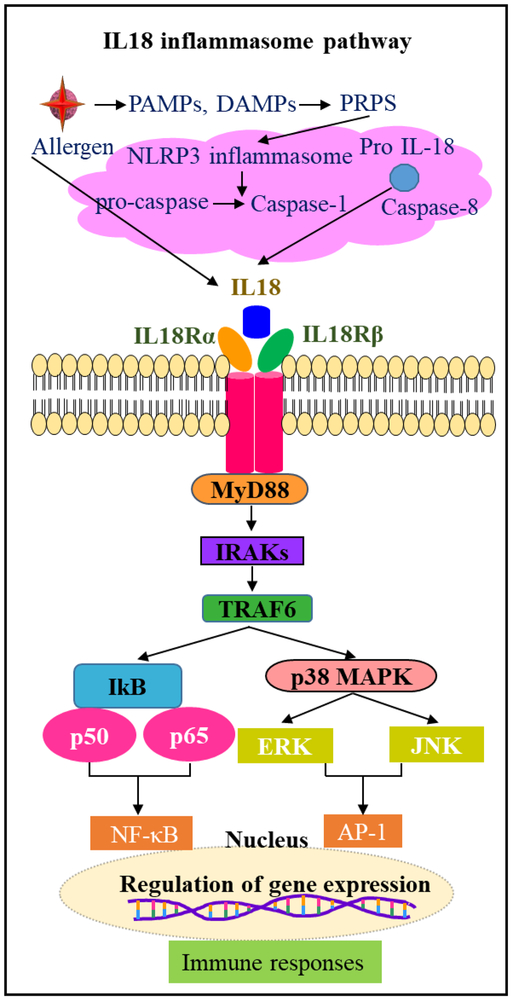
Interleukin-18 (IL-18) is an 18 kDa pro-inflammatory cytokine has been identified as an IFN-γ-inducing factor, and is a member of the IL-1 family of cytokines and display pleiotropic effects in inflammation and allergic diseases [86]. The pro-IL-18 expression observed in immune cells like monocytes, macrophages, dendritic cells, Kupffer cells and also in other cell types like keratinocytes, articular chondrocytes, synovial fibroblasts and osteoblasts [87]. Human eosinophils isolated from BALF and blood express IL-18 mRNA and protein [88]. Pro-IL-18 is cleaved by caspase1 and form IL-18. IL-18 recognizes heterodimeric IL18 receptors IL-18Rα and IL-18 Rβ, and signals via MyD88, IRAK, TRAF6 and degradation of IkB kinase and phosphorylates p50 and p65 complexes and induces NFkB signal transduction. On the other hand, IL18 mediated TRAF6 also phosphorylates MAPK, ERK, and JNK and induce AP-1 activation. These responses play a role in IL-18 mediated immune activation of T cells, B cells, NK cells, macrophages, mast cells and dendritic cells (Fig 4). IL-18 mediated Th1/Th2 response leads to B-cell activation via the release of cytokines IFNγ and IL-4 [89, 90]. Infection with pathogens like bacteria, virus, and fungi, and irritants exposure like silica, asbestos, and bleomycin, challenge activates NLRP3 inflammasome via PAMPS and DAMPs in immune cells and increase IL-1β secretion [91-94]. NLRP3 activation involves activation of adaptor protein ASC, and its sequestration in the nucleus to cytosol that interact with NLRP3 through its PYD and with caspase-1 through its CARD domains (9, 57). Activated caspase-1 cleaves IL-1β and IL-18, in the cytoplasm and these cytokines are released to the extracellular milieu and activate NLRP3-TLR-MYD88-NFkB signal transduction [95] (Fig 5). NLRP3 inflammasome responses drive steroid-resistant asthma [39]. NLRP3 inflammasome formation is observed in the development of COPD and many other inflammatory diseases [96].
Fig.4.
Role of IL18 in inflammation and eosinophil mediated allergic diseases
Fig.5.
Role of IL18 and inflammasome mediated NFκB activation and immune responses in inflammation
5. Role of IL18 in eosinophils development and maturity
Previously, Ougura et al. [97] described that IL18 induces neutrophils, eosinophils, and lymphocytes with an elevation in IL-5 and GM-CSF levels. Most recently, we first time implicated the role of IL-18 in eosinophils differentiation and transformation of IL-5 differentiated naïve eosinophils into the CD101+CD274+ pathogenic eosinophils [98]. We showed that eosinophil subsets are not present in naïve IL-18 gene-deficient mice and an increase in the human blood and tissues in diseases state. Importantly, Il-18 is induced in most allergic disease and all eosinophils accumulated in the tissues of allergic patients are expressing CD101+CD274 [98].
In addition, Kumano et al. [99] earlier showed that Interleukin-18 enhances eosinophil deposition in the airways of mice immunized and sensitized with oval albumin. Further reports showed the role of IL-18 in eosinophil-mediated tumoricidal activity against a Colo-205 carcinoma cell line by upregulating LFA-1 and ICAM-1, however neutralization of IL-18 reduced eosinophil-mediated Colo-205 apoptosis and inhibited cell-cell adhesion [100]. IL-18 is critical cytokine involved in activation of iNKT cells and ICAM in promoting human EoE, additionally IL-18 in vitro also stimulates human iNKT cells and endothelial cells and induce eosinophil-active cytokines IL-5 and IL-13 [101]. We previously reported that IL-18 induce iNKT cell activation to release the eosinophil activating cytokine IL-5, as IL-5-deficient mice and iNKT cell-deficient (CD1d null) mice do not induce EoE in response to intranasal IL-18 challenge. Thus allergen-induced IL-18 has a significant role in promoting IL-5 and iNKT dependent EoE pathogenesis in mice [102]. IL-18 overexpression induces Th1, Th2 cytokine-mediated airway inflammation in mice with an increase in IFN-γ, IL-13, eotaxin levels and CD4+ T cells in ova sensitized mice [103].
6. Importance of IL5 and IL18 in eosinophils biology
IL-5 is a well established eosinophils differentiation, growth, and survival factor for eosinophils. Previous studies demonstrated that IL-5 levels were increased in asthma, allergy, and inflammation [104-106]. Thus IL-5 selectively affects eosinophil biology and is involved in a wide variety of allergic/inflammatory diseases mediated by eosinophils and is a therapeutic target for eosinophil-associated diseases.
Role of IL-18 in asthma development was studied in a model of airway hyperresponsiveness, and peribronchial eosinophilic inflammation of mice. Administration of mice with anti-human IL-18 antibodies protected against eosinophil-mediated airway inflammation and demonstrated the role of IL-18 in the development of asthmatic inflammation [107]. The current review signifies the critical synergetic role of IL-5 and IL-18 in eosinophils transformation from naïve eosinophils to pathogenic eosinophils. These understandings will provide an important input in eosinphils biology and future therapeutic interventions for eosinophils associated allergic diseases.
7. Eosinophils mediate immune responses
In general eosinophils defense helminth parasites by the release of cytotoxic granular proteins via degranulation mechanism [108]. Neutrophils infiltrate to the site of allergen exposure or inflammation. Eosinophil major basic protein stimulates PI3K and PKCζ and releases superoxide via activation of NADPH oxidase [109]. MBP also enhance neutrophil adherence receptor CR3, thus enhance the inflammatory role of neutrophils in allergic diseases [110]. The transmembrane migration of eosinophils was observed upon coincubation of eosinophils with neutrophils and stimulation with IL-8, and this provides evidence that neutrophils migrate in response to IL-8 and leads to eosinophils accumulation in the airways in asthma [111]. Classical M1 type macrophages are proinflammatory and are activated by IFN-γ and LPS and release IL-12, IL-6, TNF-α, and CXCL10, CCL3. Alternatively, M2 macrophages exhibit anti-inflammatory activity and are activated by Th2 cytokines IL-4 and IL-13 via induction of STAT6 activation and release cytokines in asthma and allergic diseases [112, 113]. Depletion of alveolar macrophages increased eosinophils in bronchoalveolar lavage fluid of ovalbumin (OVA)-sensitized mice model of asthma, and transfer of unsensitized alveolar macrophages abrogated these changes [114]. IL-33 differentiates eosinophils from CD117+ progenitors in IL-5 dependent manner and enhances airway inflammation with an increase in eosinophils and macrophages [115].
Mast cells interact with eosinophils on IgE activation or dexamethasone challenge, and this interaction helps in eosinophils survival and communication and mediated by CD48, and 2B4 mediate mast cell–eosinophil interactions and increase eosinophil survival [116]. In another study of allergen-induced EOE model, Niranjan et al., [117] identified an increase in esophageal mast cell numbers in parallel with eosinophils and promoted muscle cell hyperplasia and hypertrophy. Mastocytosis and mast cell degranulation observed in the esophagi of patients with esophageal esophagitis [118]. Mast cell-mediated chemokine release leads to the recruitment of eosinophils and natural killer (NK) cells, through CCL11 and CXCL8 [119]. Mast cells alone are also able to mediate Th17 mediated allergic reactions independent of eosinophils via IL33 response in eosinophil-deficient ΔdblGATA mice model following OVA inhalation [120].
Basophils also play a key role in acute and chronic allergic reactions. Basophils secrete IL-4, and IL-13 and promote Th2 dependent immunity [121, 122]. Nakashima et al. [123] reported that basophils induce eosinophils upon interaction with mesenchymal fibroblasts in murine irritant contact dermatitis model via reactive oxygen species production. Upon allergen exposure, dendritic cells acquire the allergen and present to the draining lymph node, and the cells mature and induce T cell and B cell responses. Dendritic cell number increase with allergen exposure. Activation of TLR4 leads to DC migration and recruitment and induce Th2 responses [124]. On the other hand, eosinophil recruitment regulated by classical dendritic cells subsets. During memory stage of chronic asthma with allergen exposure in mouse cDC1s secrete CCL17 and CCL22 and recruit eosinophils and promote early eosinophil infiltration, as a part of type 2 immune response by lung CD24−CD11b+ DC2s by producing NO on day 1.5, and further this process is inhibited by releasing TGF-β1 on day 2.5 by CD24+ cDC2s [125].
Eosinophils also induce diverse immune responses upon allergen exposure and mediate cellular, humoral immune response and help defense the host immune system. Eosinophils induce Th2 immune response that involves the presentation of antigen to CD4+ T cells and Th2 differentiation, IL-4, IL-13 cytokine release within lymph nodes and tissues and secretion of EDN and recruitment of Th2 to cells under inflammation. Previous studies also demonstrated the central role of Th2 cells and eosinophils in allergic responses, and mice lacking IL-13, a Th2 cytokine and pre eosinophilic cytokine IL-5 neutralization displayed Aspergillus fumigatus clearance [126]. Eosinophils induce B cell proliferation, survival, antibody secretion and plasma cell maintenance, proliferation, and survival (Fig.3.). IL-5 Tg hypereosinophilic mice with profound B cell expansion showed a decrease in B cell lymphocytosis upon genetic deletion of eosinophils. Also, patients with idiopathic hypereosinophilic syndrome (HES) revealed a correlation between eosinophils levels and circulating B cell numbers [11, 127-129]
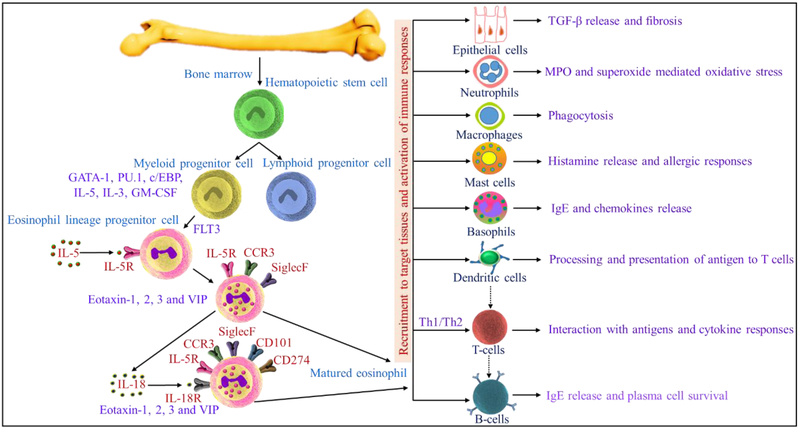
Fig.3.
Production, migration, and recruitment of eosinophils from bone marrow to targeted tissues in eosinophil mediated allergic diseases and activation of innate and adaptive immune responses.
8. Other cytokines associated involved in IL-5 and IL-18 mediated eosinophilic allergic diseases
Several cytokines play a critical role in allergic reactions, and contribute to disease pathology through the recruitment and activation of pro-inflammatory molecules and also mediated pro-fibrotic/remodeling events. T helper (Th) cells develop from naïve CD4+-T cells under lineage-specific culture conditions and release cytokines that play pleiotropic effects in allergic diseases. Th2 cells produce IL-4, IL-5, IL-9, and IL-13 and play a role in the protection against helminths. Th17 cells produce IL-17A, IL-17F, IL-22 and show activity on neutrophils, macrophages, B-cells and protect from bacteria, fungi and play a role in chronic inflammatory and autoimmune disorders [130, 131]. Th22 cells production of IL-22 plays a role in adaptive immune response [132]. IL-18 and IL-33 are involved in the activation of both Th1 and Th2 responses and have an important role in promoting adaptive immunity [133].
8.1. IL-4
IL-4 is produced by Th2 cells, basophils, mast cells, and eosinophils and regulates allergic conditions and the protective immune response against helminths and other extracellular parasites. IL-4+ TFH cell counts were increased in eosinophilic polyp tissues. IL-4 and IL-21 were involved in polyp TFH cell–induced IgE production from naive B cells, and nasal IL-4+ TFH cell counts correlated highly with local IgE levels in vivo, nasal IL-4+CXCR5+CD4+ T follicular helper cell counts correlate with local IgE production in eosinophilic nasal polyps [134]. ILC2s promote allergic inflammation, IL-4 production by IL-33-stimulated ILC2s blocks the generation of allergen-specific Treg cells and favors food allergy [135]. Thymic stromal lymphopoietin-elicited basophils promote epicutaneous sensitization to food antigens and subsequent IgE-mediated food allergy through Th2 polarization and IL-4 production [136]. Eosinophils were the major IL-4 expressing cell type in the heart during experimental autoimmune myocarditis. Eosinophils drive the progression of myocarditis to inflammatory dilated cardiomyopathy through IL-4 [137].
8.2. IL-9
IL-9 is a Th2-derived cytokine and an important mediator of allergic inflammation. IL-9 overexpressing mice show increased mast cell numbers in the intestine with inflammation, paneth cell hyperplasia and up-regulation of IL-13-dependent innate immunity mediators in the intestinal mucosa [138]. IL-9 governs allergen-induced mast cell activation, and anti-IL-9 treatment protects from airway remodeling in asthma in mice [139]. Intestinal expression of IL-9 and mast cells are higher in patients with food allergy. In response to IL-33; IL-9-producing mucosal mast cells secrete increased amounts of IL-9 and IL-13 and play a key role in susceptibility to IgE-mediated food allergy [140].
8.3. IL-13
Overexpression of IL-13 in mice promotes asthma and EoE by an IL-5, eotaxin-1, and STAT6-dependent mechanism and increase eosinophils and epithelial cells hyperplasia [141]. However, it is also reorted that Il-5 has a critical role in promoting IL-13 induced disease pathogenesis [117], because IL-13 gene-deficient mice demonstrated development of allergen-induced EoE. Similarly, a report also indicates that esophageal eosinophilia is observedeven in IL-4/IL-13 double gene-deficient and STAT6 gene-deficient mice. Indicating, that anti-IL-4Rα therapy may not protect allergen or cytokine induced gastrointestinal disorders.
8.4. IL-17
IL-17A and IL-17F are Th17 cytokines produced by CD4+ T cells and implicated in the pathogenesis of various allergic and chronic inflammatory diseases. IL-17+ cell population was found exclusively in CD3+CD4+ T cells, and the percentage of Th17 cells increased in peripheral blood of AD patients [142]. IL-17 levels elevated in mouse upon allergen exposure, and IL-17 knockout mice attenuated inflammation with a significant decrease in eosinophils [143]. IL-17 levels elevated in mice infected with the respiratory syncytial virus during OVA-induced allergic airway inflammation [144].
8.5. IL-21
IL-21 is a member of the type I cytokine family and is produced by CD4+ T cells, natural killer T cells, and Th17 cells and plays a major role in the control of innate and adaptive immune reactions [145]. An increase in serum IL-21 levels in adult atopic dermatitis patients with acute skin lesions was observed in patients [146]. rIL-21 nasal administration ameliorated allergic rhinitis by prevention of IgE production by B cells, eotaxin production by fibroblasts and attenuation of eosinophil infiltration into nasal mucosa [147]. Indicating anti-IL-21 may be effective in eosinophilic allergic patients.
8.6. IL-22
IL-22 play a role in inflammations, allergic diseases, and autoimmunity. IL-22 is mainly produced by innate lymphoid cells, not by Th cells in the inflamed lungs [148]. IL-22 in lesioned skin of chronic AD is higher than in psoriatic lesions [149], IL-22 elevated in asthma patients and the mouse model of asthma [150, 151]. Allergen-sensitized IL-22-deficient mice suffer from significantly higher airway hyperreactivity upon airway challenge with an increase in eosinophil infiltration, lymphocyte invasion and production of CCL17 (TARC), IL-5 and IL-13 in the lung. IL-22 play a role in pathogen elimination and innate lung defense against A. fumigatus mediated by Dectin-1-dependent IL-22 production [152].
8.7. IL-25
IL-25-high subset people had high Th2 cytokine levels serum IgE, with airway and blood eosinophils, airway hyperresponsiveness, and subepithelial thickening [153]. IL-25 neutralization in allergen-exposed mice exhibited a reduction in pulmonary eosinophilia and levels of Th2 associated cytokines, IL-5 and IL-13 and decreased fibrosis, airway smooth muscle hyperplasia and airway hyperreactivity [154].
8.8. IL-33
IL-33 is a nuclear cytokine that belongs to the IL-1 family and plays a crucial role in innate immunity. IL-33 expressed in epithelial cells, lymphoid organs and its levels increased in allergic inflammation and bronchial asthma [155]. Upon allergen challenge IL-33, levels were increased with IL-5 and IL-13 production and airway eosinophilia without T or B cells via CD25+CD44hi lymphoid cells, and this innate type-2 response abolished in IL-33 gene-deficient mice [156].
9. Il-5 generated and Il-18 transformed pathogenic eosinophil mediated allergic diseases
Allergy is a condition that the body responds abnormally to foreign substances. This is due to the hypersensitivity of a person’s immune system to a particular or a variety of allergens in the environment. The common allergens include pollen, bee stings, certain foods, dust, mites, molds, drugs, latex, dander, exposure to animals, and seasonal climate change. The common symptoms associated with exposure to allergens include rashes on skins, eczema, hives, edema, inflammation, rhinitis, sneezing, itching, runny/stuffy nose, cough, chest tightness, wheezing or shortness of breath, watery, red or swollen eyes (conjunctivitis) and mucus formation.
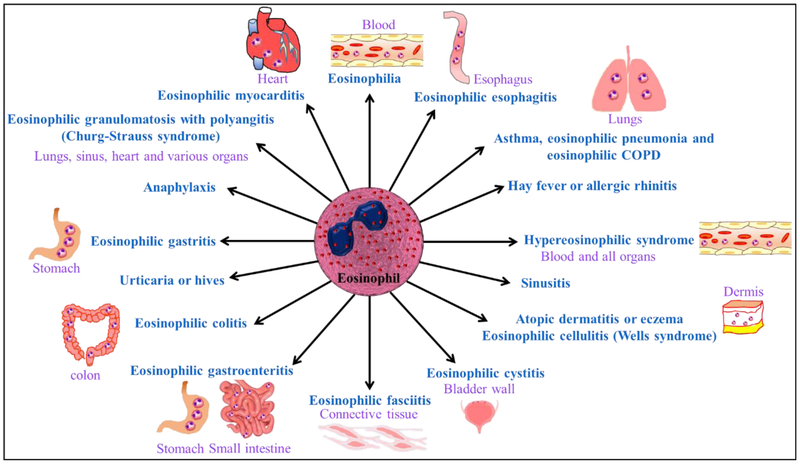
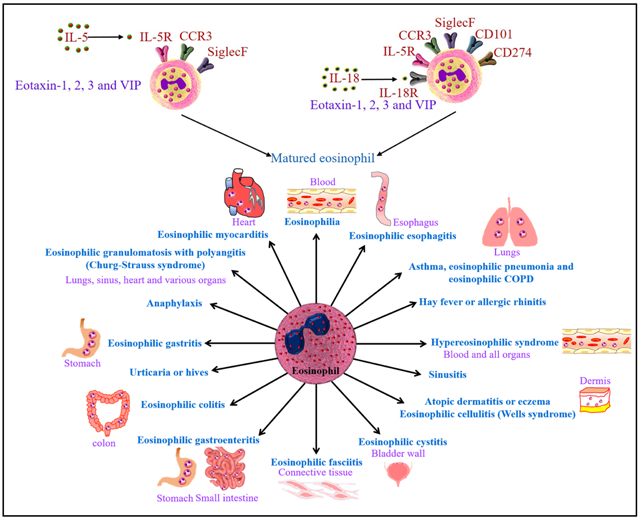
Allergic diseases affect approximately 50 million Americans and several people worldwide. The common allergic diseases include asthma, atopic dermatitis or eczema, anaphylaxis, hay fever, sinusitis, urticaria or hives, and eosinophilic esophagitis. Eosinophil mediated allergic diseases listed in Figure.6.
Fig.6.
Eosinophils associated allergic diseases
9.1. Asthma
Asthma is narrowing of airways due to inflammation and an excessive mucous generation that results in coughing, wheezing, chest tightness and shortness of breath. Eosinophilic function in asthma is not clear, and all the asthmatic patients are not eosinophilic. Preclinical models of lung allergy delineated that mice lacking eosinophils still develop airway hyperreactivity [157-159]. Several animal and human studies are correlated with eosinophils mediated asthma development with an increase in IL-5 and IL-18 levels. Eosinophil mediated asthma in humans account 20 % with refractory asthma [160]. Increase in IL-5 levels observed in asthma patients with acute respiratory, viral, mixed viral/bacterial, infections, pneumonia, bronchitis [161]. Increased IL-18 levels were observed in asthma patients and correlated with asthma exacerbations [162]. We most recently showed that CD101+CD274+ pathogenic eosinophils accumulate in the nasal lavages of asthma and esophageal biopsies of eosinophilic esophagitis patients, respectively. Additionally, these IL-18 responsive eosinophil subsets are also increased in the blood of asthma and eosinophilic esophagitis patients [2]. A similar observations are also reported in the mouse models of asthma and eosinophilic esophagitis.
9.2. Atopic dermatitis (AD) or eczema
Atopic dermatitis or eczema is one of the most prevalent skin disorder and often occurs in children and also may appear in adulthood. AD is genetical or occurs on exposure with allergens and or environmental factors that involve immune dysregulation and impaired skin barrier function and results in red itchy and dryness of the skin [163]. The allergens that enter the skin might be picked via IgE receptor by IgE specific antigen bound to Langerhans cells, and these cells migrate to lymph node and stimulate Th1 cells to differentiate to Th2 cells and promote differentiation of B cells to plasma cells and also induce chemokines, cytokines IL4, IL5 and IL-13 and IL-18 release and aid in eosinophil development and release [164]. Pathogenic eosinophilia has been shown to be present in patients with AD [165]. Activated eosinophils in turn also secrete cytokines and chemokines, such as IL-16, IL-12, TGF-β1, and IL-13 and induce immunological reactions. Eosinophil granular proteins deposition and its degranulation observed in AD patients [166]. Topical calcineurin inhibitors like tacrolimus, pimecrolimus, immunomodulatory agents prednisolone, systemic steroid-sparing agents azathioprine, methotrexate, cyclosporine, mycophenolate are available treatments for AD [167, 168].
9.3. Anaphylaxis
Anaphylaxis is a life-threatening hypersensitive reaction. The most common allergic reactions of anaphylaxis are exposure to wasp and bee venom, legumes, animal proteins, insect stings, and analgesic medications. The persons that come across anaphylaxis require immediate administration of epinephrine and medical attention. Mast cell signaling via FcεRI and IgE binds to FcεRI, and IgE and antigen-induced signaling events induce immunological reactions and mediate eosinophil-mediated allergic reactions [169]. Glucocorticosteroids, antihistamines, methylxanthines, venom immunotherapy considered for the management of anaphylaxis [170].
9.4. Hay fever or allergic rhinitis
Allergic rhinitis affects 400 million people worldwide [171]. On exposure with allergens such as pollens, grass, and dust mites result in a runny nose, and watery eyes with inflammation of the nose and mucous membranes, fever and the condition referred allergic rhinitis (AR). AR is part of a systemic inflammatory process and is associated with other inflammatory disorders of mucous membranes including asthma, rhinosinusitis, and allergic conjunctivitis. High prevalence of asthma is recorded in people with persistent and severe rhinitis [172]. Upon sensitization, to allergens, dendritic cells present allergens to T lymphocytes and promote Th2 responses and activate CD4+ T cells and releases IL-4, IL-5, IL-10, and IL-13 cytokines. IL-4 induces IgE class switch in B lymphocyte and release IgE in the bloodstream that binds with mast cells and basophils. Mast cells release histamine and mediate nasal response. Histamine and TNF-α, leukotriene C4 and prostaglandin D2 contribute to the influx of eosinophils, CD4+ T lymphocytes, and basophils by stimulation of expression of adhesion molecules on endothelial cells and cause severe allergic responses and nasal obstruction. CD4+ lymphocytes represent IL-5 that also play a role in allergic rhinitis by activation of eosinopoiesis, influx, and survival of eosinophils in nasal tissues [173-176].
Corticosteroids like fluticasone, mometasone, ciclesonide, triamcinolone, flunisolide, beclomethasone, betamethasone, antihistamines like azelastine, olopatadine, chromones like sodium cromoglicate, nedocromil sodium, anticholinergics like ipratropium bromide, decongestants like ephedrine, pseudoephedrine, xylometazoline; antihistamines like levocetirizine, cetirizine, desloratadine and loratadine, fexofenadine, acrivastine, rupatadine, carebastine and ebastine; corticosteroids like hydrocortisone, prednisolone; antileukotrienes like montelukast and zafirlukast and leukotriene synthesis inhibitors like zileuton; decongestants like pseudoephedrine are advised as a pharmacotherapy for allergic rhinitis. Apart from the medications immunotherapy is also a cure for people with allergic rhinitis [177].
9.5. Chronic hyperplastic eosinophilic sinusitis
Sinusitis is an inflammation of the sinuses that results in mucous formation. Acute sinusitis may be a result of allergens exposure and also results in fever, sore throat, headache and swelling of the face. Chronic sinuses are severe disease process associated with nasal polyposis and develop upon exposure with antigens, allergens, fungal, bacterial agents. Eosinophilic chronic rhinosinusitis is intractable and persistent disease and characterized by an inflammation of the nose and paranasal sinuses with nasal congestion and excessive mucus production and eosinophilic infiltration of nasal polyp tissue. IL-4, IL-5, or IL-1β may be critical for PDGFRα and play a pivotal role in the pathophysiology of eosinophilic and non-eosinophilic chronic rhinosinusitis with nasal polyps [178]. IL-16 may stimulate the migration and persistence of activated eosinophilic granulocytes in eosinophilic chronic rhinosinusitis [179]. Th2 mediated IL-25, IL-33, and IL-31, TGFα is up-regulated in eosinophilic chronic rhinosinusitis [180]. Higher leukotriene C4 levels can be an indicator of the risk of recurrence of nasal polyps in patients with recurrent sinonasal polyps after surgery. Leukotriene receptor antagonists like montelukast and zafirlukast and the leukotriene synthesis inhibitor zileuton are recommended therapeutic measures [180, 181]. Intranasal corticosteroids are also therapeutic strategies to modulate nasal mucosa, epithelial repair and regulating tissue remodeling markers, and to reduce tissue eosinophil infiltration [182].
9.6. Urticaria or hives
Body’s reactions to allergens cause rashes on the skin that turn to swollen pale red bumps that are itchy and sometimes causes burning, and the condition also referred to as hives. Food allergens, medications, exposure to cold or hot environmental conditions and insect stings are triggers for hives. Eosinophils are recruited upon allergen exposure in urticaria and contribute to the pathogenesis by the release of proinflammatory cytokines, tissue-damaging protein, and immunoregulatory molecules. Eosinophilic granular proteins MBP and ECP observed in urticaria with IL-3, IL-4, IL-5, IL-2, IL-10, IL-12, and GM-CSF, IFN-γ, eosinophilic infiltration with deposits of cationic proteins MBP and EDN and high levels of leukotrienes C4 are observed in the lesioned skin [183]. Medications for urticaria include epinephrine auto-injector, antihistamines, ultraviolet therapy, application of hydrophobic barrier creams, anti-IgE antibody Omalizumab administration [184-188].
9.7. Eosinophilia
The condition where eosinophils count increases <450 cells per μl of blood is eosinophilia [189]. Eosinophilia observed in a variety of allergic diseases that encountered as a result of increased eosinophil levels or cytokines such as IL-4, IL-5, and IL-13 that may also induce eosinophilia. Transgenic mice expressing IL-5 show eosinophilia [190]. The diagnosis of eosinophilia in patients characterized by an allergic condition like wheezing, rhinitis, or eczema [191]. The common factors that contribute to eosinophilia include exposure to helminth like schistosomiasis; the presence of a pet dog infected with Toxocara canis, and hypersensitivity to drugs. Eosinophilia observed in a variety of cancers [192, 193]. Parasitic eradication is the main cure for eosinophilia and albendazole is popularly used [194]. Therapeutics for eosinophilia with organ involvement include corticosteroids and IFN-α for the steroid-resistant disease. Other agents for the steroid-resistant disease, which are usually given as long-term maintenance regimens to control organ involvement, include the hydroxyurea, Chlorambucil, Vincristine, Cytarabine, 2-Chlorodeoxyadenosine (2-CdA) Etoposide, Cyclosporine [195].
9.8. Eosinophilic esophagitis (EoE)
EOE is one of the major cause of upper gastrointestinal morbidity with an estimated prevalence of 56.7/100,000 persons. Food sensitization is one of the main reasons for EOE and characterized by esophageal dysfunction with the formation of eosinophilic infiltrate in the esophageal mucosa [196]. Several eosinophils active Th2 cytokines, IL-4, IL-5, IL-13, and IL-18 shown to increase in EoE patients, and the overexpression of these mice promotes disease pathogenesis similar to the human. Our earlier study showed CD274 expressing and non-expressing eosinophils in pediatric and adult EoE patients. CD274 expression on blood eosinophils and blood mRNA expression levels were higher EoE patients and decreased following treatment [197]. Therapeutic approaches for EoE include chronic dietary elimination of allergic food, topical corticosteroid application, and esophageal dilation (Liacouras et al., 2011). Anti-IL-5, anti-IL-13 and anti-IL4 Ra clinical trials are in progress in most of the allergic diseases including EoE. Currently, the elimination diet and corticosteroids therapy are the best approaches to improve the EoE patients quality of life [198].
9.9. Eosinophilic cystitis (EC)
Eosinophilic cystitis characterized by infiltration of eosinophils and inflammation across the bladder wall. EC is characterized by injury of eosinophils to the bladder tissues resulting in gross hematuria, marked irritation of the lower urinary tract causing dysuria and frequency and fibrosis of the mucosa and muscularis necrosis of bladder wall [199]. Dysregulation of clonal eosinophilic proliferation, resulting in overproduction eosinophils in bladder mucosa with an elevation of IL-4, IL-5, IL-13, IFNγ, and TNFα observed in EC. Cyclophilin inhibitors and, cyclosporine identified as a promising strategy to treat EC [200].
9.10. Eosinophilic fasciitis (EF)
EF characterized by edema and induration of the arms and legs and infiltration of eosinophils. EF diagnosed as inflammation and thickening of collagen bundles in the superficial muscle fascia with infiltration of immune cells. Laboratory diagnosis EF includes eosinophils accumulation in blood, hypergammaglobulinemia, and an increase in erythrocyte sedimentation rate [201]. An increase in T cell population and IL-5 levels observed in EF patients, and the increase in IL-5 levels correlated with enhanced production of eosinophils, their maturation, and recruitment to the inflamed regions [202-205]. Therapy for EF includes administration of corticosteroids and sulfasalazine, cyclosporine, methotrexate, hydroxychloroquine [206].
9.11. Eosinophilic granulomatosis with polyangiitis (EGPA), aka Churg-Strauss syndrome
EGPA is a systemic small- vessel eosinophilic vasculitis observed in association with asthma and eosinophilia. It is characterized by the presence of anti-myeloperoxidase (MPO), anti-neutrophil antibodies in the cytoplasm of 30–38% of patients and most frequently involves peripheral nerves and skin [207]. Apart from genetic background associated with HLA-DRB4 alleles the disease can be triggered by exposure to allergens, infections, vaccinations or drugs such as macrolide antibiotics, leukotriene receptor antagonists and exposure to particulate silica. EGPA is a Th2 mediated disease and the T cells produced Th2 cytokines such as IL-4, IL-13, IL-5, and CD294+ T cells observed with an increase in CCL17 and IL17A and a decrease in TREG cells. Clinical manifestation and laboratory findings demonstrate asthma and rhino- sinusitis, peripheral eosinophilia lung infiltrates due to bronchial plugging by mucus formation is also observed in patients [208]. Cyclophosphamide, Methotrexate, Rituximab, Mepolizumab suggested treatment strategy for EGPA [209, 210].
9.12. Eosinophilic pneumonia (EP)
Eosinophilic pneumonia is a pulmonary disease characterized by diffuse pulmonary infiltrates and an increase in eosinophils in the lungs and bronchoalveolar lavage fluid. EP’s are due to lung infections, or drug-induced and off acute and chronic types. Several studies reported that cigarette smoking linked with the development of acute eosinophilic pneumonia. Chronic eosinophilic pneumonia characterized by radiographic evidence of bilateral peripherally located opacities with shortness of breath for several months. Peripheral blood eosinophilia with blood eosinophil count >1000/mL and increase in serum IgE level, erythrocyte sedimentation rate, C-reactive protein, and platelet counts [211, 212]. Corticosteroid treatment with intravenous methylprednisolone or oral prednisone and omalizumab were considered an effective strategy for EP [213, 214].
9.13. Hypereosinophilic syndrome (HES)
HES is characterized by a marked increase in eosinophils (>1500 eosinophils/mm3) and its associated pathology with eosinophil-mediated end-organ damage. HES is also associated with cardiovascular complications with endomyocardial fibrosis and thrombosis [215]. HES patients showed clonal T-cell receptor rearrangements and IL-5 producing T cells characterized by CD3− CD4+ T cells [216]. HES also may be due to interstitial chromosomal deletion on 4q12 leading to the creation of the FIP1L1-PDGFRA fusion gene (F/P+variant) [217]. Corticosteroids, hydroxyl urea, vincristine, prednisone, hydroxycarbamide, and interferon-α, mepolizumab (anti–IL-5 mAb), imatinib mesylate, cyclophosphamide, 6-thioguanine, methotrexate, cytarabine, 2-CDA, alemtuzumab, cyclosporine, intravenous immunoglobulin are suggested therapy for HES [218, 219],
9.14. Eosinophilic COPD
Chronic obstructive pulmonary disease is a lethal disease and is the 4th leading cause of deaths worldwide (WHO 2015). IL-18 overexpression is linked with emphysematous lesions and mediates Th1, Th2, and Th17 cytokine responses [220], Increased expression of IL18 was observed in COPD patients and correlated with disease severity [221-223]. Due to the pathogenic role of eosinophils in COPD clinical results demonstrated the benefits of monoclonal antibodies targeting IL-5 in severe eosinophilic COPD. Mepolizumab, a humanized monoclonal antibody, reduces eosinophil counts in blood and tissues by blocking IL-5, through binding to eosinophil surface receptors [67, 224]. Mepolizumab at a dose of 100 mg was associated with a lower annual rate of moderate or severe exacerbations than placebo among patients with COPD and an eosinophilic phenotype and suggested that eosinophilic airway inflammation contributes to COPD exacerbations[225].
9.15. Eosinophilic myocarditis (EM)
EM is an acute life-threatening cardiac inflammatory disease that is characterized by eosinophilic infiltration and has a poor prognosis and requires endomyocardial biopsy for definitive diagnosis. EM often accompanied by eosinophilia and is associated with immune dysfunctions. The clinical observation of patients with EM is with abrupt impairment of left ventricular ejection fraction and high risk of malignant arrhythmias. Thus, corticosteroid therapy, some form of inotropes and mechanical circulatory support for recovery in patients with EM was supportive and beneficial. Elevated IL-5 levels were observed in patients with EM and anti-IL5 therapy by administration of Mepolizumab improved cardiac function [226]. Despite the progress in understanding the EM further clinical trials and awareness of disease progress and treatment are required for treating patients with EM [227, 228]. Induction of EM in hypereosinophilic IL-5Tg mice resulted in eosinophilic myocarditis with ventricular and atrial inflammation and progress to dilated cardiomyopathy [137].
9.16. Eosinophilic cellulitis (ECE)/ Wells syndrome
ECE also was known as Wells syndrome is a hypersensitivity of the dermis against a variety of exogenous and endogenous antigenic stimuli and is usually seen in adults and rarely in children. ECE represents acute pruritic dermatosis, with scarring and flame figures of the skin with an accumulation of inflammatory cells and eosinophilic debris. IL-5 overproduction might contribute to eosinophil accumulation [229]. CD4+CD7−T cells may be implicated in IL-5 production and recruitment of dysregulated eosinophils in ECE [230]. IL-4 and IL-13 mediated activation of CD163+ M2 macrophages might also contribute to the recruitment of eosinophils in ECE [231]. IL-2 mediated platelet activation stimulated activation CD25 expressing eosinophils degranulation and release of eosinophilic cationic protein and tissue damage documented in ECE [232]. Treatment measures of ECE include corticosteroids, dapsone, antihistamines, colchicine, interferon- α, antimalarial drugs, and immunosuppressive agents like cyclosporine and azathioprine, and tacrolimus [233-236].
9.17. Pulmonary eosinophilia (PE)
Following allergen exposure in pulmonary eosinophilia characterized by eosinophilia, goblet cell metaplasia, and increased Th1, Th2, Th17 mediated cytokine production. Particularly, Th17 mediated IL-17 play a key role in immune reactions in pulmonary eosinophilia following Aspergillus fumigatus exposure in mice. Inflammation attenuated in IL-17 knockout mice challenged with A. fumigatus with a significant decrease in eosinophils, conidial clearance, and the early transient peak of CD4+ CD25+ FoxP3+ cells was blunted explaining the role for IL-17 in driving Th2-type inflammation to repeated inhalation of fungal conidia [143]. Therapy for PE includes prednisolone, methylprednisolone, and oral glucocorticoids, inhaled glucocorticoids [237]. The JAK-3 inhibitor CP-690550 acts as an anti-inflammatory agent for pulmonary eosinophilia in a mouse model [238].
9.18. Eosinophilic gastrointestinal disorders (EGID)
EGID’s selectively affect the gastrointestinal tract with inflammation and accumulation of eosinophils in the absence of known causes for eosinophilia like drug reactions, parasitic infections, and malignancy. The common factors for EGID’s are genetic, food sensitization and persons with EGID often have an atopic disease. EGID symptoms include difficulty in swallowing, vomiting, reflux, abdominal and chest pain. IL-5, IL-13, and IL-18 implicated in the EGID pathogenesis. The diagnosis for EGID is through biopsies from endoscopy and/or colonoscopy [239, 240]. The common EGID’s described below.
9.18.1. Eosinophilic colitis (ECO)
It’s a rare form of the eosinophilic gastrointestinal disease and observed in neonates and also in young adults. The pathophysiology associated with ECO is altered hypersensitivity and associated with food allergy in infants, and in adults, it is T lymphocyte-associated. Common symptoms include abdominal pain, weight loss, and diarrhea, mucosal injury and malabsorption [241]. ECO characterized by eosinophilic infiltration in the colon, which is also associated with high serum IgE, and IL-5 levels. Treatment for ECO includes administration with glucocorticoids, antihistamines, leukotriene receptors antagonists and drugs that target IL-5 and IgE levels [242].
9.18.2. Eosinophilic gastritis (EG)
EG is commonly confined to stomach eosinophilic inflammation; however it also represents coexisting eosinophilic accumulation in the esophagus. Blood eosinophil counts, and accumulation of MIB-1+, CD117+ mast cells, and FOXP3+ T-reg cells, activated T cells, are increased in patients with EG. Cytokine-like IL-4, IL-5, IL-13, IL-17, IL-18 and CCL26 was also significantly increased in EG patients that may correlate with elevated eosinophil levels or vice versa [243]. Therapies for EG include a dietary restriction in patients allergic to milk, egg, soy, wheat, nuts, seafood, red meats and other foods [244].
9.18.3. Eosinophilic gastroenteritis
EGE is commonly associated with food allergy and characterized by abdominal pain, dysphagia, nausea, vomiting, and diarrhea. EGE characterized by the eosinophilic accumulation in the gastrointestinal tract, serum IgE levels are also observed in EGE patients [245]. IL-3, IL-5 and GM-CSF accumulation observed along with eosinophil levels in EGE. Eotoxin associated eosinophil accumulation seen in lamina propria of stomach and intestine [246]. Treatment for EGE includes administration of corticosteroids like fluticasone, prednisolone and budesonide and mast cell stabilizer cromolyn, leukotriene receptor antagonists like montelukast [247].
10. Conclusion and future perspectives
Eosinophils are normal constituents of the GI tract, mammary glands, and bone marrow, and play roles in development and homeostasis of these organs. In the present review, we discussed the evolution of the eosinophil as a multi-factorial leukocyte with pleiotropic functions, and the significance of IL-5, IL-18 and other cytokines in eosinophil associated allergic diseases. Although historically eosinophils are considered as cells that exhibit defense against parasites, the vast majority of data supports that eosinophils are pleiotropic multifunctional leukocytes that promote allergy and allergic diseases and elicit innate and adaptive immune responses upon hyperactivity to allergens. In Th2 type inflammation/allergy IL-4, IL-5, IL-9, IL-13, IL-18, IL-25, and IL-31 cytokines play a critical role. Several studies carried out in humans, and animal models of eosinophil allergic diseases revealed that among these cytokines IL-5 and IL-18 play a major role in eosinophil differentiation, maturation, development, transformation, migration, accumulation and elicit immune reactions. Small molecule antagonists of CCR3, CRTH2 or eosinophil inhibitory receptors might also be an effective strategy to treat eosinophils in allergic diseases. Therapies with molecules that block IL-5 or and IL-18 signaling might be promising to treat eosinophil-mediated allergic diseases. Although anti-IL-5 antibody therapy for allergic diseases remains elusive, a potential of humanized anti-human-IL-5Rα mAb treatment or anti-hIL-18Rα mAb treatment or in combination for asthmatic and allergic disease patients may be beneficial as a therapeutic strategy in the treatment to eliminate and control eosinophils and subsets of eosinophilic phenotypes that mediate allergic diseases. Blockade of eosinophils production in bone marrow, transformation into pathogenic eosinophils, inhibition of eosinophil activation and factors that contribute to eosinophil apoptosis is also interesting strategies for eosinophil depletion in allergic diseases. In summary, we propose the initiation of the anti-IL-18 clinical trial to block the transformation of naïve eosinophils into the pathogenic eosinophils. Anti-IL-18 therapy may maintain the eosinophils associated innate immunity and restrict disease pathogenesis by restricting the maturation of pathogenic eosinophils. Thus, we need additional studies using genetically engineered IL-5 and IL-18 double gene-deficient mice for in vivo, characterization of the immunobiology eosinophils associated disease pathogenesis.
Highlights.
Eosinophils and its constituents
Role of IL5 and IL18 in eosinophils development, transformation, maturation
Signal transduction of IL5 and IL18
The role of eosinophils in allergic disorders
The role of several other associated cytokines in promoting eosinophils mediated allergic diseases
Acknowledgments
Dr. Mishra is the Endowed Schlieder Chair; therefore, authors thank Edward G. Schlieder Educational Foundation for the support. The work is partially supported, by NIH R01 AI080581 grant funding (AM).
Abbreviations:
- 2-CDA
2-Chlorodeoxyadenosine
- AD
Atopic dermatitis
- AP-1
Activator protein 1
- AR
allergic rhinitis
- APRIL
A proliferation-inducing ligand
- ASC
adaptor protein
- BCL-XL
B-cell lymphoma-extra large
- Btk
Bruton's tyrosine kinase
- C/EBP
CCAAT/enhancer binding protein
- CARD
C-terminal caspase-recruitment domain
- CCL
Chemokine (C-C motif) ligand
- CCR3
CC-chemokine receptor 3
- CD
Cluster of differentiation
- c-fos
FBJ Murine Osteosarcoma Viral Oncogene Homolog
- c-jun
proto-oncogene
- CLC
Charcot-Leyden crystal protein
- COPD
Chronic obstructive pulmonary disease
- CXCL
chemokine (C-X-C motif) ligand
- CXCR
CXC chemokine receptor
- DAMPs
Damage-associated molecular patterns
- DC
Dendritic cell
- EC
Eosinophilic cystitis
- ECE
Eosinophilic cellulitis
- ECO
Eosinophilic colitis
- ECP
eosinophil cationic protein
- EDN
eosinophil-derived neurotoxin
- EF
Eosinophilic fasciitis
- EG
Eosinophilic gastritis
- EGE
Eosinophilic gastroenteritis
- EGF
Epidermal growth factor
- EGID
Eosinophilic gastrointestinal disorders
- EGPA
Eosinophilic granulomatosis with polyangiitis
- EM
Eosinophilic myocarditis
- EOE
Eosinophilic esophagitis
- EP
Eosinophilic pneumonia
- EPX
eosinophil peroxidase
- ERK
Extracellular signal-regulated kinase
- FcεRI
Fc Fragment of IgE receptor I
- FGF
Fibroblast growth factor
- FIP1L1-PDGFRA
Factor interacting with PAPOLA and CPSF1-like-1–platelet-derived growth factor receptor
- FOXP3
Forkhead box P3
- Gal-10
Galectin-10
- GATA
Globin transcription factor
- GI
Gastrointestinal
- GM-CSF
Granulocyte macrophage-colony stimulating factor
- GTPase
Guanine binding protein Rac1
- HES
Hypereosinophilic syndrome
- hIL-5Rα
Human interleukin-5 receptor alpha
- HLA- DRB4
HLA Class II histocompatibility antigen-DR Beta 4 Chain
- HS1
Hematopoietic lineage cell-specific protein
- ICAM-1
Intercellular adhesion molecule 1
- IFN-γ
Interferon gamma
- IgE
Immunoglobulin E
- IkB kinase
Inhibitor of nuclear factor kappa-B kinase
- IL
Interleukin
- ILC2s
Type 2 innate lymphoid cells
- iNKT
Invariant natural killer T cells
- IRAK
Interleukin-1 receptor-associated kinase 1
- JAK
Janus kinase
- JNK
c-Jun N-terminal protein kinase
- LFA-1
Lymphocyte function-associated antigen 1
- MAPK
Mitogen-activated protein kinases
- MBP
major basic protein
- MPO
myeloperoxidase
- MyD88
Myeloid Differentiation Primary Response 88
- NADPH
Nicotinamide adenine dinucleotide phosphate
- NFκb
Nuclear factor kappa B subunit
- NK
natural killer
- NLRP3
NLR family pyrin domain containing 3
- OVA
ovalbumin
- p50
Nuclear factor kappa-B P50 Subunit
- p65
Transcription factor p65
- PAMPs
Pathogen-associated molecular patterns
- PDGFRα
Platelet-derived growth factor receptor alpha
- PE
Pulmonary eosinophilia
- PI3K
Phosphoinositide-3-Kinase
- Pim-1
Proto-oncogene serine/threonine-protein kinase-1 Pim-1
- PKCζ
Protein kinase C zeta
- PMN
Polymorphonuclear neutrophils
- PU.1
Transcription factor PU.1
- PYD
Pyrin domain
- Raf-1
Raf-1 proto-oncogene serine/threonine kinase
- Ras
Retrovirus-associated DNA sequences
- rIL-15
Recombinant interleukin-15
- rMIL-21
Recombinant mouse interleukin-15
- Shc
Src homology 2 domain containing transforming protein 1
- SHP2
protein-tyrosine phosphatase 2C (PTP-2C)
- SIGLEC
Sialic acid binding immunoglobulin-like lectin
- SIGLEC-F
Sialic acid binding Ig-like lectin
- STAT
Signal transducer and activator of transcription
- TARC
Thymus and activation-regulated chemokine
- TFH cell
Follicular helper T cells
- TGF-β1
Transforming growth factor beta 1
- Th2
T helper cell type 2
- TLR
Toll-like receptor
- TNF-α
Tumor necrosis factor alpha
- TRAF6
TNF receptor associated factor 6
- TREG cells
Regulatory T cells
- VEGF
Vascular endothelial growth factor
Biography

Dr. Hemanth Kumar Kandikattu, PhD is Postdoctoral Research Fellow in the Section of Pulmonary Diseases at Tulane School of Medicine, New Orleans, LA. Dr. Hemath K Kandikattu is working on role of eosinophils in allergic diseases, and on pancreatitis and its progression to pancreatic cancer. Dr. Hemanth had an exceptional background, foundation with his renowned education at Sri Venkateswara University, Tirupati, India where he graduated with Bachelor of Science in Biotechnology followed by Master of Science in Biochemistry. Having excelled in science education, he then undertook PhD training at Defence Food Research Laboratory and University of Mysore, Mysore, India. Dr. Hemanth completed his Post-Doctoral training in Cardiovascular Medicine from University of Missouri, Columbia, Missouri, USA. He has published over 35 articles, book chapters, and reviews. He is a member of American Academy of Allergy, Asthma & Immunology (AAAAI), Milwaukee, WI, USA, European Academy of Allergy and Clinical Immunology, Zurich, Switzerland, American Gastroenterological Association, Bethesda, MD, USA, National Environmental Science Academy (NESA), Delhi, India.

Dr. Sathisha Upparahalli Venkateshaiah, PhD is Postdoctoral Research Fellow in the Section of Pulmonary Diseases at Tulane School of Medicine, New Orleans, LA. Dr. Sathisha UV had an exceptional background, foundation with his renowned education at University of Mysore, Mysore, India where he graduated with Bachelor of Science. Having excelled in science education, he then undertook PhD training at University of Mysore, Mysore, India. Dr. Sathisha UV skilled in Cancer Biology and Molecular Biology training from an esteemed Central food Technological Research Institute (CSIR), Mysore, India. Dr. Sathisha UV completed his postdoctoral training at Myeloma Institute for Research and Therapy, Winthrop P. Rockefeller Cancer Institute, UAMS, Little Rock, AR, USA, Tulane School of Medicine, New Orleans, LA. Dr. Sathisha UV most important contribution was role of bone marrow microenvironment in Myeloma tumorigenesis. Dr. Sathisha UV’s most important contribution was IL-15′s protective role in the pathogenesis of asthma, Journal of Allergy and Clinical Immunology (PMID: 28606589); Critical role for IL-18 in transformation and maturation of naive eosinophils to pathogenic eosinophils, J Allergy Clin Immunology (PMID: 29499224).

Dr. Anil Mishra, PhD is Edward G. Schlieder Educational Foundation Endowed Chair and Professor of Medicine. He is also the Director of Tulane Eosinophilic Disorder Center in the Section of Pulmonary Diseases at Tulane University School of Medicine. Dr. Mishra's research established that eosinophils are the resident cells of the gastrointestinal tract that home prenatally (Mishra et al., J. Clin. Invest. 1999). He showed that eosinophil active chemokine eotaxin-1 constitutively expressed and has significant role for eosinophils homing into the gastrointestinal tract. He developed the first murine model of eosinophilic esophagitis (EoE) (Mishra et al., J. Clin. Invest. 2001). His findings implicated aeroallergen in the etiology of EoE and suggested that esophageal eosinophilic inflammation is mechanistically regulated by IL-5, IL-13 and eotaxin (J. Immunology 2002, and Gastroenterology, 2003). Furthermore, we also indicated that T cell subset iNKT cells are critical and the source of eosinophil active cytokines in human EoE, and targeting the cell surface receptors of iNKT cells improve EoE in experimental model of EoE (Clinical and Translational Immunology, 2014). Most recently, the laboratory of Dr. Mishra has reported that rIL-15 is a therapeutic molecule for the allergen-induced airway hyperactivity and fibrosis for chronic asthma and other pulmonary functional impairment (J. Allergy Clin. Immunol. 2017). Dr. Mishra is an elected fellow of the American Academy of Allergy Asthma Immunology (FAAAAI) and the American Gastrointestinal Association (FAGA). He has published over 85 articles, book chapters and reviews on molecular mechanisms of pulmonary and gastrointestinal, allergic responses in high impact factor journals. Dr. Mishra’ research is supported by TheNational Institutes of Health via NIDDK and NIAID institutes Dr Mishra is also a member of several NIH study sections and serving Editor and Editorial Board member in a number of international journals.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest
No author has any conflict of interest.
References
- [1].Fulkerson PC, Rothenberg ME, Eosinophil Development, Disease Involvement, and Therapeutic Suppression, Adv Immunol 138 (2018) 1–34. [DOI] [PubMed] [Google Scholar]
- [2].Venkateshaiah SU, Mishra A, Manohar M, Verma AK, Rajavelu P, Niranjan R, Wild LG, Parada NA, Blecker U, Lasky JA, Mishra A, A critical role for IL-18 in transformation and maturation of naive eosinophils to pathogenic eosinophils, J Allergy Clin Immunol 142(1) (2018) 301–305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Fulkerson PC, Transcription Factors in Eosinophil Development and As Therapeutic Targets, Front Med (Lausanne) 4 (2017) 115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Rosenberg HF, Phipps S, Foster PS, Eosinophil trafficking in allergy and asthma, J Allergy Clin Immunol 119(6) (2007) 1303–10; quiz 1311–2. [DOI] [PubMed] [Google Scholar]
- [5].Uhm TG, Kim BS, Chung IY, Eosinophil development, regulation of eosinophil-specific genes, and role of eosinophils in the pathogenesis of asthma, Allergy Asthma Immunol Res 4(2) (2012) 68–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Wen T, Rothenberg ME, The Regulatory Function of Eosinophils, Microbiol Spectr 4(5) (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Rothenberg ME, Hogan SP, The eosinophil, Annu Rev Immunol 24 (2006) 147–74. [DOI] [PubMed] [Google Scholar]
- [8].Chu VT, Beller A, Rausch S, Strandmark J, Zanker M, Arbach O, Kruglov A, Berek C, Eosinophils promote generation and maintenance of immunoglobulin-A-expressing plasma cells and contribute to gut immune homeostasis, Immunity 40(4) (2014) 582–93. [DOI] [PubMed] [Google Scholar]
- [9].Jacobsen EA, Ochkur SI, Pero RS, Taranova AG, Protheroe CA, Colbert DC, Lee NA, Lee JJ, Allergic pulmonary inflammation in mice is dependent on eosinophil-induced recruitment of effector T cells, J Exp Med 205(3) (2008) 699–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Jung Y, Wen T, Mingler MK, Caldwell JM, Wang YH, Chaplin DD, Lee EH, Jang MH, Woo SY, Seoh JY, Miyasaka M, Rothenberg ME, IL-1beta in eosinophil-mediated small intestinal homeostasis and IgA production, Mucosal Immunol 8(4) (2015) 930–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wong TW, Doyle AD, Lee JJ, Jelinek DF, Eosinophils regulate peripheral B cell numbers in both mice and humans, J Immunol 192(8) (2014) 3548–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Del Pozo V, De Andres B, Martin E, Cardaba B, Fernandez JC, Gallardo S, Tramon P, Leyva-Cobian F, Palomino P, Lahoz C, Eosinophil as antigen-presenting cell: activation of T cell clones and T cell hybridoma by eosinophils after antigen processing, Eur J Immunol 22(7) (1992) 1919–25. [DOI] [PubMed] [Google Scholar]
- [13].Akuthota P, Melo RC, Spencer LA, Weller PF, MHC Class II and CD9 in human eosinophils localize to detergent-resistant membrane microdomains, Am J Respir Cell Mol Biol 46(2) (2012) 188–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Shi HZ, Humbles A, Gerard C, Jin Z, Weller PF, Lymph node trafficking and antigen presentation by endobronchial eosinophils, J Clin Invest 105(7) (2000) 945–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Jacobsen EA, Zellner KR, Colbert D, Lee NA, Lee JJ, Eosinophils regulate dendritic cells and Th2 pulmonary immune responses following allergen provocation, J Immunol 187(11) (2011) 6059–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Horiuchi T, Weller PF, Expression of vascular endothelial growth factor by human eosinophils: upregulation by granulocyte macrophage colony-stimulating factor and interleukin-5, Am J Respir Cell Mol Biol 17(1) (1997) 70–7. [DOI] [PubMed] [Google Scholar]
- [17].Ohno I, Nitta Y, Yamauchi K, Hoshi H, Honma M, Woolley K, O'Byrne P, Dolovich J, Jordana M, Tamura G, et al. , Eosinophils as a potential source of platelet-derived growth factor B-chain (PDGF-B) in nasal polyposis and bronchial asthma, Am J Respir Cell Mol Biol 13(6) (1995) 639–47. [DOI] [PubMed] [Google Scholar]
- [18].Stenfeldt AL, Wenneras C, Danger signals derived from stressed and necrotic epithelial cells activate human eosinophils, Immunology 112(4) (2004) 605–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Todd R, Donoff BR, Chiang T, Chou MY, Elovic A, Gallagher GT, Wong DT, The eosinophil as a cellular source of transforming growth factor alpha in healing cutaneous wounds, Am J Pathol 138(6) (1991) 1307–13. [PMC free article] [PubMed] [Google Scholar]
- [20].Wu D, Molofsky AB, Liang HE, Ricardo-Gonzalez RR, Jouihan HA, Bando JK, Chawla A, Locksley RM, Eosinophils sustain adipose alternatively activated macrophages associated with glucose homeostasis, Science 332(6026) (2011) 243–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Foster EL, Simpson EL, Fredrikson LJ, Lee JJ, Lee NA, Fryer AD, Jacoby DB, Eosinophils increase neuron branching in human and murine skin and in vitro, PLoS One 6(7) (2011) e22029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Fryer AD, Stein LH, Nie Z, Curtis DE, Evans CM, Hodgson ST, Jose PJ, Belmonte KE, Fitch E, Jacoby DB, Neuronal eotaxin and the effects of CCR3 antagonist on airway hyperreactivity and M2 receptor dysfunction, J Clin Invest 116(1) (2006) 228–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Kobayashi H, Gleich GJ, Butterfield JH, Kita H, Human eosinophils produce neurotrophins and secrete nerve growth factor on immunologic stimuli, Blood 99(6) (2002) 2214–20. [DOI] [PubMed] [Google Scholar]
- [24].Verma AK, Manohar M, Venkateshaiah SU, Blecker U, Collins MH, Mishra A, Role of Vasoactive Intestinal Peptide in Promoting the Pathogenesis of Eosinophilic Esophagitis (EoE), Cell Mol Gastroenterol Hepatol 5(1) (2018) 99–100 e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Gleich GJ, Adolphson CR, Leiferman KM, The biology of the eosinophilic leukocyte, Annu Rev Med 44 (1993) 85–101. [DOI] [PubMed] [Google Scholar]
- [26].Acharya KR, Ackerman SJ, Eosinophil granule proteins: form and function, J Biol Chem 289(25) (2014) 17406–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Puxeddu I, Berkman N, Nissim Ben Efraim AH, Davies DE, Ribatti D, Gleich GJ, Levi-Schaffer F, The role of eosinophil major basic protein in angiogenesis, Allergy 64(3) (2009) 368–74. [DOI] [PubMed] [Google Scholar]
- [28].Xue A, Wang J, Sieck GC, Wylam ME, Distribution of major basic protein on human airway following in vitro eosinophil incubation, Mediators Inflamm 2010 (2010) 824362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Kandikattu HK, Mishra A, Immunomodulatory effects of tacrolimus (FK506) for the treatment of allergic diseases, Int. J Cell Biol. Physiol 1 (2018) 5–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].O'Brien PJ, Peroxidases, Chem Biol Interact 129(1–2) (2000) 113–39. [DOI] [PubMed] [Google Scholar]
- [31].Wang J, Slungaard A, Role of eosinophil peroxidase in host defense and disease pathology, Arch Biochem Biophys 445(2) (2006) 256–60. [DOI] [PubMed] [Google Scholar]
- [32].Duguet A, Iijima H, Eum SY, Hamid Q, Eidelman DH, Eosinophil peroxidase mediates protein nitration in allergic airway inflammation in mice, Am J Respir Crit Care Med 164(7) (2001) 1119–26. [DOI] [PubMed] [Google Scholar]
- [33].Koh GC, Shek LP, Goh DY, Van Bever H, Koh DS, Eosinophil cationic protein: is it useful in asthma? A systematic review, Respir Med 101(4) (2007) 696–705. [DOI] [PubMed] [Google Scholar]
- [34].Venge P, Bystrom J, Carlson M, Hakansson L, Karawacjzyk M, Peterson C, Seveus L, Trulson A, Eosinophil cationic protein (ECP): molecular and biological properties and the use of ECP as a marker of eosinophil activation in disease, Clin Exp Allergy 29(9) (1999) 1172–86. [DOI] [PubMed] [Google Scholar]
- [35].Rosenberg HF, Dyer KD, Foster PS, Eosinophils: changing perspectives in health and disease, Nat Rev Immunol 13(1) (2013) 9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Slifman NR, Loegering DA, Mckean DJ, Gleich GJ, Ribonuclease-Activity Associated with Human Eosinophil-Derived Neurotoxin and Eosinophil Cationic Protein, Journal of Immunology 137(9) (1986) 2913–2917. [PubMed] [Google Scholar]
- [37].Hosoki K, Nakamura A, Kainuma K, Sugimoto M, Nagao M, Hiraguchi Y, Tanida H, Tokuda R, Wada H, Nobori T, Suga S, Fujisawa T, Differential activation of eosinophils by bacteria associated with asthma, Int Arch Allergy Immunol 161 Suppl 2 (2013) 16–22. [DOI] [PubMed] [Google Scholar]
- [38].Tsuda T, Maeda Y, Nishide M, Koyama S, Hayama Y, Nojima S, Takamatsu H, Okuzaki D, Kinehara Y, Kato Y, Nakatani T, Obata S, Akazawa H, Shikina T, Takeda K, Hayama M, Inohara H, Kumanogoh A, Eosinophil-derived neurotoxin enhances airway remodeling in eosinophilic chronic rhinosinusitis and correlates with disease severity, Int Immunol (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kim HS, Kim JH, Seo YM, Chun YH, Yoon JS, Kim HH, Lee JS, Kim JT, Eosinophil-derived neurotoxin as a biomarker for disease severity and relapse in recalcitrant atopic dermatitis, Ann Allergy Asthma Immunol 119(5) (2017) 441–445. [DOI] [PubMed] [Google Scholar]
- [40].Staribratova D, Belovejdov V, Staikov D, Dikov D, Demonstration of Charcot-Leyden crystals in eosinophilic cystitis, Arch Pathol Lab Med 134(10) (2010) 1420. [DOI] [PubMed] [Google Scholar]
- [41].De Re V, Simula MP, Cannizzaro R, Pavan A, De Zorzi MA, Toffoli G, Canzonieri V, Galectin-10, eosinophils, and celiac disease, Ann N Y Acad Sci 1173 (2009) 357–64. [DOI] [PubMed] [Google Scholar]
- [42].Rodriguez-Alcazar JF, Ataide MA, Engels G, Schmitt-Mabmunyo C, Garbi N, Kastenmuller W, Latz E, Franklin BS, Charcot-Leyden Crystals Activate the NLRP3 Inflammasome and Cause IL-1beta Inflammation in Human Macrophages, J Immunol 202(2) (2019) 550–558. [DOI] [PubMed] [Google Scholar]
- [43].Lingblom C, Andersson J, Andersson K, Wenneras C, Regulatory Eosinophils Suppress T Cells Partly through Galectin-10, J Immunol 198(12) (2017) 4672–4681. [DOI] [PubMed] [Google Scholar]
- [44].Wang H, Rudd CE, SKAP-55, SKAP-55-related and ADAP adaptors modulate integrin-mediated immune-cell adhesion, Trends Cell Biol 18(10) (2008) 486–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Mishra A, Hogan SP, Brandt EB, Wagner N, Crossman MW, Foster PS, Rothenberg ME, Enterocyte expression of the eotaxin and interleukin-5 transgenes induces compartmentalized dysregulation of eosinophil trafficking, J Biol Chem 277(6) (2002) 4406–12. [DOI] [PubMed] [Google Scholar]
- [46].Liao W, Long H, Chang CC, Lu Q, The Eosinophil in Health and Disease: from Bench to Bedside and Back, Clin Rev Allergy Immunol 50(2) (2016) 125–39. [DOI] [PubMed] [Google Scholar]
- [47].Shen ZJ, Malter JS, Determinants of eosinophil survival and apoptotic cell death, Apoptosis 20(2) (2015) 224–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Goldsmith KC, Gross M, Peirce S, Luyindula D, Liu X, Vu A, Sliozberg M, Guo R, Zhao H, Reynolds CP, Hogarty MD, Mitochondrial Bcl-2 family dynamics define therapy response and resistance in neuroblastoma, Cancer Res 72(10) (2012) 2565–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Leiferman KM, Beck LA Gleich GJ, Regulation of the production and activation of eosinophils, Rev Immunol 24 (2006) 147–74. [Google Scholar]
- [50].Lee SC, Brummet ME, Shahabuddin S, Woodworth TG, Georas SN, Leiferman KM, Gilman SC, Stellato C, Gladue RP, Schleimer RP, Beck LA, Cutaneous injection of human subjects with macrophage inflammatory protein-1 alpha induces significant recruitment of neutrophils and monocytes, J Immunol 164(6) (2000) 3392–401. [DOI] [PubMed] [Google Scholar]
- [51].Michael BD, Griffiths MJ, Granerod J, Brown D, Davies NW, Borrow R, Solomon T, Characteristic Cytokine and Chemokine Profiles in Encephalitis of Infectious, Immune-Mediated, and Unknown Aetiology, PLoS One 11(1) (2016) e0146288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Giembycz MA, Lindsay MA, Pharmacology of the eosinophil, Pharmacol Rev 51(2) (1999) 213–340. [PubMed] [Google Scholar]
- [53].Bonini S, Lambiase A, Bonini S, Angelucci F, Magrini L, Manni L, Aloe L, Circulating nerve growth factor levels are increased in humans with allergic diseases and asthma, Proc Natl Acad Sci U S A 93(20) (1996) 10955–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Broudy VC, Stem cell factor and hematopoiesis, Blood 90(4) (1997) 1345–64. [PubMed] [Google Scholar]
- [55].Davoine F, Lacy P, Eosinophil cytokines, chemokines, and growth factors: emerging roles in immunity, Front Immunol 5 (2014) 570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [56].Hartman M, Piliponsky AM, Temkin V, Levi-Schaffer F, Human peripheral blood eosinophils express stem cell factor, Blood 97(4) (2001) 1086–91. [DOI] [PubMed] [Google Scholar]
- [57].Levi-Montalcini R, Skaper SD, Dal Toso R, Petrelli L, Leon A, Nerve growth factor: from neurotrophin to neurokine, Trends Neurosci 19(11) (1996) 514–20. [DOI] [PubMed] [Google Scholar]
- [58].Matsuda H, Coughlin MD, Bienenstock J, Denburg JA, Nerve growth factor promotes human hemopoietic colony growth and differentiation, Proc Natl Acad Sci U S A 85(17) (1988) 6508–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Minshall EM, Leung DY, Martin RJ, Song YL, Cameron L, Ernst P, Hamid Q, Eosinophil-associated TGF-beta1 mRNA expression and airways fibrosis in bronchial asthma, Am J Respir Cell Mol Biol 17(3) (1997) 326–33. [DOI] [PubMed] [Google Scholar]
- [60].Powell PP, Klagsbrun M, Abraham JA, Jones RC, Eosinophils expressing heparin-binding EGF-like growth factor mRNA localize around lung microvessels in pulmonary hypertension, Am J Pathol 143(3) (1993) 784–93. [PMC free article] [PubMed] [Google Scholar]
- [61].Solomon A, Aloe L, Pe'er J, Frucht-Pery J, Bonini S, Bonini S, Levi-Schaffer F, Nerve growth factor is preformed in and activates human peripheral blood eosinophils, Journal of Allergy and Clinical Immunology 102(3) (1998) 454–460. [DOI] [PubMed] [Google Scholar]
- [62].Wong DTW, Weller PF, Galli SJ, Elovic A, Rand TH, Gallagher GT, Chiang T, Chou MY, Matossian K, Mcbride J, Todd R, Human Eosinophils Express Transforming Growth Factor-Alpha, Journal of Experimental Medicine 172(3) (1990) 673–681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Feltenmark S, Gautam N, Brunnstrom A, Griffiths W, Backman L, Edenius C, Lindbom L, Bjorkholm M, Claesson HE, Eoxins are proinflammatory arachidonic acid metabolites produced via the 15-lipoxygenase-1 pathway in human eosinophils and mast cells, Proc Natl Acad Sci U S A 105(2) (2008) 680–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Kvarnhammar AM, Cardell LO, Pattern-recognition receptors in human eosinophils, Immunology 136(1) (2012) 11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Stahle-Backdahl M, Parks WC, 92-kd gelatinase is actively expressed by eosinophils and stored by neutrophils in squamous cell carcinoma, Am J Pathol 142(4) (1993) 995–1000. [PMC free article] [PubMed] [Google Scholar]
- [66].Yagisawa M, Yuo A, Yonemaru M, ImajohOhmi S, Kanegasaki S, Yazaki Y, Takaku F, Superoxide release and NADPH oxidase components in mature human phagocytes: Correlation between functional capacity and amount of functional proteins, Biochemical and Biophysical Research Communications 228(2) (1996) 510–516. [DOI] [PubMed] [Google Scholar]
- [67].Varricchi G, Bagnasco D, Borriello F, Heffler E, Canonica GW, Interleukin-5 pathway inhibition in the treatment of eosinophilic respiratory disorders: evidence and unmet needs, Curr Opin Allergy Clin Immunol 16(2) (2016) 186–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Adachi T, Alam R, The mechanism of IL-5 signal transduction, Am J Physiol 275(3 Pt 1) (1998) C623–33. [DOI] [PubMed] [Google Scholar]
- [69].Stein ML, Munitz A, Targeting interleukin (IL) 5 for asthma and hypereosinophilic diseases, Recent Pat Inflamm Allergy Drug Discov 4(3) (2010) 201–9. [DOI] [PubMed] [Google Scholar]
- [70].Kouro T, Kikuchi Y, Kanazawa H, Hirokawa K, Harada N, Shiiba M, Wakao H, Takaki S, Takatsu K, Critical proline residues of the cytoplasmic domain of the IL-5 receptor alpha chain and its function in IL-5-mediated activation of JAK kinase and STAT5, International Immunology 8(2) (1996) 237–245. [DOI] [PubMed] [Google Scholar]
- [71].Ogata N, Kouro T, Yamada A, Koike M, Hanai N, Ishikawa T, Takatsu K, JAK2 and JAK1 constitutively associate with an interleukin-5 (IL-5) receptor alpha and beta c subunit, respectively, and are activated upon IL-5 stimulation, Blood 91(7) (1998) 2264–2271. [PubMed] [Google Scholar]
- [72].Pazdrak K, Adachi T, Alam R, Src homology 2 protein tyrosine phosphatase (SHPTP2)/Src homology 2 phosphatase 2 (SHP2) tyrosine phosphatase is a positive regulator of the interleukin 5 receptor signal transduction pathways leading to the prolongation of eosinophil survival, J Exp Med 186(4) (1997) 561–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Pazdrak K, Olszewska-Pazdrak B, Stafford S, Garofalo RP, Alam R, Lyn, Jak2, and Raf-1 kinases are critical for the antiapoptotic effect of interleukin 5, whereas only Raf-1 kinase is essential for eosinophil activation and degranulation, Journal of Experimental Medicine 188(3) (1998) 421–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [74].Sato N, Sakamaki K, Terada N, Arai K, Miyajima A, Signal transduction by the high-affinity GM-CSF receptor: two distinct cytoplasmic regions of the common beta subunit responsible for different signaling, EMBO J 12(11) (1993) 4181–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Sato S, Katagiri T, Takaki S, Kikuchi Y, Hitoshi Y, Yonehara S, Tsukada S, Kitamura D, Watanabe T, Witte O, Takatsu K, Il-5 Receptor-Mediated Tyrosine Phosphorylation of Sh2/Shs-Containing Proteins and Activation of Bruton Tyrosine and Janus-2 Kinases, Journal of Experimental Medicine 180(6) (1994) 2101–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Takaki S, Kanazawa H, Shiiba M, Takatsu K, A Critical Cytoplasmic Domain of the Interleukin-5 (Il-5) Receptor-Alpha Chain and Its Function in Il-5-Mediated Growth Signal-Transduction, Molecular and Cellular Biology 14(11) (1994) 7404–7413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Horikawa K, Kaku H, Nakajima H, Davey HW, Hennighausen L, Iwamoto I, Yasue T, Kariyone A, Takatsu K, Essential role of Stat5 for IL-5-dependent IgH switch recombination in mouse B cells, J Immunol 167(9) (2001) 5018–26. [DOI] [PubMed] [Google Scholar]
- [78].Kagami S, Nakajima H, Suzuki K, Saito Y, Takatsu K, Leonard W, Iwamoto I, Both Stat5a and Stat5b are required for antigen-induced eosinophil and T cell recruitment into the tissue, Journal of Allergy and Clinical Immunology 105(1) (2000) S355–S355. [PubMed] [Google Scholar]
- [79].Hall DJ, Cui J, Bates ME, Stout BA, Koenderman L, Coffer PJ, Bertics PJ, Transduction of a dominant-negative H-Ras into human eosinophils attenuates extracellular signal-regulated kinase activation and interleukin-5-mediated cell viability, Blood 98(7) (2001) 2014–21. [DOI] [PubMed] [Google Scholar]
- [80].Chung KF, p38 mitogen-activated protein kinase pathways in asthma and COPD, Chest 139(6) (2011) 1470–1479. [DOI] [PubMed] [Google Scholar]
- [81].Park YM, Bochner BS, Eosinophil survival and apoptosis in health and disease, Allergy Asthma Immunol Res 2(2) (2010) 87–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Kankaanranta H, De Souza PM, Barnes PJ, Salmon M, Giembycz MA, Lindsay MA, SB 203580, an inhibitor of p38 mitogen-activated protein kinase, enhances constitutive apoptosis of cytokine-deprived human eosinophils, J Pharmacol Exp Ther 290(2) (1999) 621–8. [PubMed] [Google Scholar]
- [83].Underwood DC, Osborn RR, Kotzer CJ, Adams JL, Lee JC, Webb EF, Carpenter DC, Bochnowicz S, Thomas HC, Hay DW, Griswold DE, SB 239063, a potent p38 MAP kinase inhibitor, reduces inflammatory cytokine production, airways eosinophil infiltration, and persistence, J Pharmacol Exp Ther 293(1) (2000) 281–8. [PubMed] [Google Scholar]
- [84].Amruta N, Kandikattu HK, Apoptosis of inflammatory cells in Asthma, Int J Cell Biol Physiol 1 (2018) 1–6. [Google Scholar]
- [85].Schwartz C, Willebrand R, Huber S, Rupec RA, Wu D, Locksley R, Voehringer D, Eosinophil-specific deletion of IkappaBalpha in mice reveals a critical role of NF-kappaB-induced Bcl-xL for inhibition of apoptosis, Blood 125(25) (2015) 3896–904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Dinarello CA, Novick D, Kim S, Kaplanski G, Interleukin-18 and IL-18 binding protein, Front Immunol 4 (2013)289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [87].Dinarello CA, Interleukin-18, Methods 19(1) (1999) 121–32. [DOI] [PubMed] [Google Scholar]
- [88].Mathur SK, Sedgwick JB, Vrtis RF et al. ,, Human eosinophils express IL-18 mRNA and protein, J. Allergy Clin. Immunol 113 (2004) S166–7. [Google Scholar]
- [89].Nakanishi K, Unique Action of Interleukin-18 on T Cells and Other Immune Cells, Front Immunol 9 (2018) 763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Sandersa NL, Venkateshaiah SU, Manohar M, Verma AK, Kandikattu HK, Mishra A, Interleukin-18 has an Important Role in Differentiation and Maturation of Mucosal Mast Cells, J Mucosal Immunol Res 2(1) (2018). [PMC free article] [PubMed] [Google Scholar]
- [91].Dostert C, Petrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J, Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica, Science 320(5876) (2008) 674–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Gasse P, Mary C, Guenon I, Noulin N, Charron S, Schnyder-Candrian S, Schnyder B, Akira S, Quesniaux VF, Lagente V, Ryffel B, Couillin I, IL-1R1/MyD88 signaling and the inflammasome are essential in pulmonary inflammation and fibrosis in mice, J Clin Invest 117(12) (2007) 3786–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Hornung V, Bauernfeind F, Halle A, Samstad EO, Kono H, Rock KL, Fitzgerald KA, Latz E, Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization, Nat Immunol 9(8) (2008) 847–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Xu JF, Washko GR, Nakahira K, Hatabu H, Patel AS, Fernandez IE, Nishino M, Okajima Y, Yamashiro T, Ross JC, Estepar RS, Diaz AA, Li HP, Qu JM, Himes BE, Come CE, D'Aco K, Martinez FJ, Han MK, Lynch DA, Crapo JD, Morse D, Ryter SW, Silverman EK, Rosas IO, Choi AM, Hunninghake GM, C.O. Investigators, Statins and pulmonary fibrosis: the potential role of NLRP3 inflammasome activation, Am J Respir Crit Care Med 185(5) (2012) 547–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].dos Santos G, Kutuzov MA, Ridge KM, The inflammasome in lung diseases, Am J Physiol Lung Cell Mol Physiol 303(8) (2012) L627–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Yang W, Ni H, Wang H, Gu H, NLRP3 inflammasome is essential for the development of chronic obstructive pulmonary disease, Int J Clin Exp Pathol 8(10) (2015) 13209–16. [PMC free article] [PubMed] [Google Scholar]
- [97].Ogura T, Ueda H, Hosohara K, Tsuji R, Nagata Y, Kashiwamura S, Okamura H, Interleukin-18 stimulates hematopoietic cytokine and growth factor formation and augments circulating granulocytes in mice, Blood 98(7) (2001) 2101–7. [DOI] [PubMed] [Google Scholar]
- [98].Mesnil C, Raulier S, Paulissen G, Xiao X, Birrell MA, Pirottin D, Janss T, Starkl P, Ramery E, Henket M, Schleich FN, Radermecker M, Thielemans K, Gillet L, Thiry M, Belvisi MG, Louis R, Desmet C, Marichal T, Bureau F, Lung-resident eosinophils represent a distinct regulatory eosinophil subset, J Clin Invest 126(9) (2016) 3279–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Kumano K, Nakao A, Nakajima H, Hayashi F, Kurimoto M, Okamura H, Saito Y, Iwamoto I, Interleukin-18 enhances antigen-induced eosinophil recruitment into the mouse airways, Am J Respir Crit Care Med 160(3) (1999) 873–8. [DOI] [PubMed] [Google Scholar]
- [100].Gatault S, Delbeke M, Driss V, Sarazin A, Dendooven A, Kahn JE, Lefevre G, Capron M, IL-18 Is Involved in Eosinophil-Mediated Tumoricidal Activity against a Colon Carcinoma Cell Line by Upregulating LFA-1 and ICAM-1, J Immunol 195(5) (2015) 2483–92. [DOI] [PubMed] [Google Scholar]
- [101].Niranjan R, Rajavelu P, Ventateshaiah SU, Shukla JS, Zaidi A, Mariswamy SJ, Mattner J, Fortgang I, Kowalczyk M, Balart L, Shukla A, Mishra A, Involvement of interleukin-18 in the pathogenesis of human eosinophilic esophagitis, Clin Immunol 157(2) (2015) 103–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Dutt P, Shukla JS, Ventateshaiah SU, Mariswamy SJ, Mattner J, Shukla A, Mishra A, Allergen-induced interleukin-18 promotes experimental eosinophilic oesophagitis in mice, Immunol Cell Biol 93(10) (2015) 849–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Sawada M, Kawayama T, Imaoka H, Sakazaki Y, Oda H, Takenaka S, Kaku Y, Azuma K, Tajiri M, Edakuni N, Okamoto M, Kato S, Hoshino T, IL-18 induces airway hyperresponsiveness and pulmonary inflammation via CD4+ T cell and IL-13, PLoS One 8(1) (2013) e54623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Brusselle GG, Maes T, Bracke KR, Eosinophils in the spotlight: Eosinophilic airway inflammation in nonallergic asthma, Nat Med 19(8) (2013) 977–9. [DOI] [PubMed] [Google Scholar]
- [105].Tanaka H, Komai M, Nagao K, Ishizaki M, Kajiwara D, Takatsu K, Delespesse G, Nagai H, Role of interleukin-5 and eosinophils in allergen-induced airway remodeling in mice, Am J Respir Cell Mol Biol 31(1) (2004) 62–8. [DOI] [PubMed] [Google Scholar]
- [106].Turkeli A, Yilmaz O, Taneli F, Horasan GD, Kanik ET, Kizilkaya M, Gozukara C, Yuksel H, IL-5, IL-8 and MMP −9 levels in exhaled breath condensate of atopic and nonatopic asthmatic children, Respir Med 109(6) (2015) 680–8. [DOI] [PubMed] [Google Scholar]
- [107].Kuroda-Morimoto M, Tanaka H, Hayashi N, Nakahira M, Imai Y, Imamura M, Yasuda K, Yumikura-Futatsugi S, Matsui K, Nakashima T, Sugimura K, Tsutsui H, Sano H, Nakanishi K, Contribution of IL-18 to eosinophilic airway inflammation induced by immunization and challenge with Staphylococcus aureus proteins, Int Immunol 22(7) (2010) 561–70. [DOI] [PubMed] [Google Scholar]
- [108].Shamri R, Xenakis JJ, Spencer LA, Eosinophils in innate immunity: an evolving story, Cell and Tissue Research 343(1) (2011) 57–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Shenoy NG, Gleich GJ, Thomas LL, Eosinophil major basic protein stimulates neutrophil superoxide production by a class IA phosphoinositide 3-kinase and protein kinase C-zeta-dependent pathway, J Immunol 171(7) (2003) 3734–41. [DOI] [PubMed] [Google Scholar]
- [110].Moy JN, Thomas LL, Whisler LC, Eosinophil major basic protein enhances the expression of neutrophil CR3 and p150,95, J Allergy Clin Immunol 92(4) (1993) 598–606. [DOI] [PubMed] [Google Scholar]
- [111].Kikuchi I, Kikuchi S, Kobayashi T, Hagiwara K, Sakamoto Y, Kanazawa M, Nagata M, Eosinophil trans-basement membrane migration induced by interleukin-8 and neutrophils, Am J Respir Cell Mol Biol 34(6) (2006) 760–5. [DOI] [PubMed] [Google Scholar]
- [112].Moreira AP, Hogaboam CM, Macrophages in allergic asthma: fine-tuning their pro- and anti-inflammatory actions for disease resolution, J Interferon Cytokine Res 31(6) (2011) 485–91. [DOI] [PubMed] [Google Scholar]
- [113].Murray PJ, Wynn TA, Protective and pathogenic functions of macrophage subsets, Nat Rev Immunol 11(11) (2011) 723–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Bang BR, Chun E, Shim EJ, Lee HS, Lee SY, Cho SH, Min KU, Kim YY, Park HW, Alveolar macrophages modulate allergic inflammation in a murine model of asthma, Exp Mol Med 43(5) (2011) 275–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Stolarski B, Kurowska-Stolarska M, Kewin P, Xu D, Liew FY, IL-33 exacerbates eosinophil-mediated airway inflammation, J Immunol 185(6) (2010) 3472–80. [DOI] [PubMed] [Google Scholar]
- [116].Elishmereni M, Alenius HT, Bradding P, Mizrahi S, Shikotra A, Minai-Fleminger Y, Mankuta D, Eliashar R, Zabucchi G, Levi-Schaffer F, Physical interactions between mast cells and eosinophils: a novel mechanism enhancing eosinophil survival in vitro, Allergy 66(3) (2011) 376–85. [DOI] [PubMed] [Google Scholar]
- [117].Niranjan R, Mavi P, Rayapudi M, Dynda S, Mishra A, Pathogenic role of mast cells in experimental eosinophilic esophagitis, Am J Physiol Gastrointest Liver Physiol 304(12) (2013) G1087–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Abonia JP, Blanchard C, Butz BB, Rainey HF, Collins MH, Stringer K, Putnam PE, Rothenberg ME, Involvement of mast cells in eosinophilic esophagitis, J Allergy Clin Immunol 126(1) (2010) 140–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Burke SM, Issekutz TB, Mohan K, Lee PW, Shmulevitz M, Marshall JS, Human mast cell activation with virus-associated stimuli leads to the selective chemotaxis of natural killer cells by a CXCL8-dependent mechanism, Blood 111(12) (2008) 5467–76. [DOI] [PubMed] [Google Scholar]
- [120].Cho KA, Suh JW, Sohn JH, Park JW, Lee H, Kang JL, Woo SY, Cho YJ, IL-33 induces Th17-mediated airway inflammation via mast cells in ovalbumin-challenged mice, Am J Physiol Lung Cell Mol Physiol 302(4) (2012) L429–40. [DOI] [PubMed] [Google Scholar]
- [121].Otsuka A, Nakajima S, Kubo M, Egawa G, Honda T, Kitoh A, Nomura T, Hanakawa S, Sagita Moniaga C, Kim B, Matsuoka S, Watanabe T, Miyachi Y, Kabashima K, Basophils are required for the induction of Th2 immunity to haptens and peptide antigens, Nat Commun 4 (2013) 1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Perrigoue JG, Saenz SA, Siracusa MC, Allenspach EJ, Taylor BC, Giacomin PR, Nair MG, Du Y, Zaph C, van Rooijen N, Comeau MR, Pearce EJ, Laufer TM, Artis D, MHC class II-dependent basophil-CD4+ T cell interactions promote T(H)2 cytokine-dependent immunity, Nat Immunol 10(7) (2009) 697–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Nakashima C, Otsuka A, Kitoh A, Honda T, Egawa G, Nakajima S, Nakamizo S, Arita M, Kubo M, Miyachi Y, Kabashima K, Basophils regulate the recruitment of eosinophils in a murine model of irritant contact dermatitis, J Allergy Clin Immunol 134(1) (2014) 100–7. [DOI] [PubMed] [Google Scholar]
- [124].Gill MA, The role of dendritic cells in asthma, J Allergy Clin Immunol 129(4) (2012) 889–901. [DOI] [PubMed] [Google Scholar]
- [125].Yi S, Zhai J, Niu R, Zhu G, Wang M, Liu J, Huang H, Wang Y, Jing X, Kang L, Song W, Shi Y, Tang H, Eosinophil recruitment is dynamically regulated by interplay among lung dendritic cell subsets after allergen challenge, Nat Commun 9(1) (2018) 3879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Porter P, Susarla SC, Polikepahad S, Qian Y, Hampton J, Kiss A, Vaidya S, Sur S, Ongeri V, Yang T, Delclos GL, Abramson S, Kheradmand F, Corry DB, Link between allergic asthma and airway mucosal infection suggested by proteinase-secreting household fungi, Mucosal Immunol 2(6) (2009) 504–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Chu VT, Berek C, Immunization induces activation of bone marrow eosinophils required for plasma cell survival, Eur J Immunol 42(1) (2012) 130–7. [DOI] [PubMed] [Google Scholar]
- [128].Chu VT, Frohlich A, Steinhauser G, Scheel T, Roch T, Fillatreau S, Lee JJ, Lohning M, Berek C, Eosinophils are required for the maintenance of plasma cells in the bone marrow, Nat Immunol 12(2) (2011) 151–9. [DOI] [PubMed] [Google Scholar]
- [129].Wong TW, Kita H, Hanson CA, Walters DK, Arendt BK, Jelinek DF, Induction of malignant plasma cell proliferation by eosinophils, PLoS One 8(7) (2013) e70554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Cosmi L, Liotta F, Maggi E, Romagnani S, Annunziato F, Th17 cells: new players in asthma pathogenesis, Allergy 66(8) (2011) 989–98. [DOI] [PubMed] [Google Scholar]
- [131].Kandikattu HK, Rachitha P, Jayashree GV, Krupashree K, Sukhith M, Majid A, Amruta N, Khanum F, Anti-inflammatory and anti-oxidant effects of Cardamom (Elettaria repens (Sonn.) Baill) and its phytochemical analysis by 4D GCXGC TOF-MS, Biomed Pharmacother 91 (2017) 191–201. [DOI] [PubMed] [Google Scholar]
- [132].Jia L, Wu C, The biology and functions of Th22 cells, Adv Exp Med Biol 841 (2014) 209–30. [DOI] [PubMed] [Google Scholar]
- [133].Arend WP, Palmer G, Gabay C, IL-1, IL-18, and IL-33 families of cytokines, Immunol Rev 223 (2008) 20–38. [DOI] [PubMed] [Google Scholar]
- [134].Zhang YN, Song J, Wang H, Wang H, Zeng M, Zhai GT, Ma J, Li ZY, Liao B, Wang BF, Zhen Z, Wang N, Cao PP, Lin P, Ning Q, Liu Z, Nasal IL-4(+)CXCR5(+)CD4(+) T follicular helper cell counts correlate with local IgE production in eosinophilic nasal polyps, J Allergy Clin Immunol 137(2) (2016) 462–73. [DOI] [PubMed] [Google Scholar]
- [135].Noval Rivas M, Burton OT, Oettgen HC, Chatila T, IL-4 production by group 2 innate lymphoid cells promotes food allergy by blocking regulatory T-cell function, J Allergy Clin Immunol 138(3) (2016) 801–811 e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Hussain M, Borcard L, Walsh KP, Pena Rodriguez M, Mueller C, Kim BS, Kubo M, Artis D, Noti M, Basophil-derived IL-4 promotes epicutaneous antigen sensitization concomitant with the development of food allergy, J Allergy Clin Immunol 141(1) (2018) 223–234 e5. [DOI] [PubMed] [Google Scholar]
- [137].Diny NL, Rose NR, Cihakova D, Eosinophils in Autoimmune Diseases, Frontiers in Immunology 8 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Steenwinckel V, Louahed J, Lemaire MM, Sommereyns C, Warnier G, McKenzie A, Brombacher F, Van Snick J, Renauld JC, IL-9 promotes IL-13-dependent paneth cell hyperplasia and up-regulation of innate immunity mediators in intestinal mucosa, J Immunol 182(8) (2009) 4737–43. [DOI] [PubMed] [Google Scholar]
- [139].Kearley J, Erjefalt JS, Andersson C, Benjamin E, Jones CP, Robichaud A, Pegorier S, Brewah Y, Burwell TJ, Bjermer L, Kiener PA, Kolbeck R, Lloyd CM, Coyle AJ, Humbles AA, IL-9 governs allergen-induced mast cell numbers in the lung and chronic remodeling of the airways, Am J Respir Crit Care Med 183(7) (2011) 865–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Chen CY, Lee JB, Liu B, Ohta S, Wang PY, Kartashov AV, Mugge L, Abonia JP, Barski A, Izuhara K, Rothenberg ME, Finkelman FD, Hogan SP, Wang YH, Induction of Interleukin-9-Producing Mucosal Mast Cells Promotes Susceptibility to IgE-Mediated Experimental Food Allergy, Immunity 43(4) (2015) 788–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Liu J, Harberts E, Tammaro A, Girardi N, Filler RB, Fishelevich R, Temann A, Licona-Limon P, Girardi M, Flavell RA, Gaspari AA, IL-9 regulates allergen-specific Th1 responses in allergic contact dermatitis, J Invest Dermatol 134(7) (2014) 1903–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Koga C, Kabashima K, Shiraishi N, Kobayashi M, Tokura Y, Possible pathogenic role of Th17 cells for atopic dermatitis, J Invest Dermatol 128(11) (2008) 2625–30. [DOI] [PubMed] [Google Scholar]
- [143].Murdock BJ, Falkowski NR, Shreiner AB, Sadighi Akha AA, McDonald RA, White ES, Toews GB, Huffnagle GB, Interleukin-17 drives pulmonary eosinophilia following repeated exposure to Aspergillus fumigatus conidia, Infect Immun 80(4) (2012) 1424–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [144].Newcomb DC, Boswell MG, Reiss S, Zhou W, Goleniewska K, Toki S, Harintho MT, Lukacs NW, Kolls JK, Peebles RS Jr., IL-17A inhibits airway reactivity induced by respiratory syncytial virus infection during allergic airway inflammation, Thorax 68(8) (2013) 717–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Akdis M, Burgler S, Crameri R, Eiwegger T, Fujita H, Gomez E, Klunker S, Meyer N, O'Mahony L, Palomares O, Rhyner C, Ouaked N, Schaffartzik A, Van De Veen W, Zeller S, Zimmermann M, Akdis CA, Interleukins, from 1 to 37, and interferon-gamma: receptors, functions, and roles in diseases, J Allergy Clin Immunol 127(3) (2011) 701–21 e1–70. [DOI] [PubMed] [Google Scholar]
- [146].Mizutani H, Tamagawa-Mineoka R, Nakamura N, Masuda K, Katoh N, Serum IL-21 levels are elevated in atopic dermatitis patients with acute skin lesions, Allergol Int 66(3) (2017) 440–444. [DOI] [PubMed] [Google Scholar]
- [147].Hiromura Y, Kishida T, Nakano H, Hama T, Imanishi J, Hisa Y, Mazda O, IL-21 administration into the nostril alleviates murine allergic rhinitis, J Immunol 179(10) (2007) 7157–65. [DOI] [PubMed] [Google Scholar]
- [148].Taube C, Tertilt C, Gyulveszi G, Dehzad N, Kreymborg K, Schneeweiss K, Michel E, Reuter S, Renauld JC, Arnold-Schild D, Schild H, Buhl R, Becher B, IL-22 is produced by innate lymphoid cells and limits inflammation in allergic airway disease, PLoS One 6(7) (2011) e21799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Nograles KE, Zaba LC, Shemer A, Fuentes-Duculan J, Cardinale I, Kikuchi T, Ramon M, Bergman R, Krueger JG, Guttman-Yassky E, IL-22-producing "T22" T cells account for upregulated IL-22 in atopic dermatitis despite reduced IL-17-producing TH17 T cells, J Allergy Clin Immunol 123(6) (2009) 1244–52 e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Besnard AG, Sabat R, Dumoutier L, Renauld JC, Willart M, Lambrecht B, Teixeira MM, Charron S, Fick L, Erard F, Warszawska K, Wolk K, Quesniaux V, Ryffel B, Togbe D, Dual Role of IL-22 in allergic airway inflammation and its cross-talk with IL-17A, Am J Respir Crit Care Med 183(9) (2011) 1153–63. [DOI] [PubMed] [Google Scholar]
- [151].Farfariello V, Amantini C, Nabissi M, Morelli MB, Aperio C, Caprodossi S, Carlucci A, Bianchi AM, Santoni G, IL-22 mRNA in peripheral blood mononuclear cells from allergic rhinitic and asthmatic pediatric patients, Pediatr Allergy Immunol 22(4) (2011) 419–23. [DOI] [PubMed] [Google Scholar]
- [152].Gessner MA, Werner JL, Lilly LM, Nelson MP, Metz AE, Dunaway CW, Chan YR, Ouyang W, Brown GD, Weaver CT, Steele C, Dectin-1-dependent interleukin-22 contributes to early innate lung defense against Aspergillus fumigatus, Infect Immun 80(1) (2012) 410–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [153].Cheng D, Xue Z, Yi L, Shi H, Zhang K, Huo X, Bonser LR, Zhao J, Xu Y, Erle DJ, Zhen G, Epithelial interleukin-25 is a key mediator in Th2-high, corticosteroid-responsive asthma, Am J Respir Crit Care Med 190(6) (2014) 639–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [154].Gregory LG, Jones CP, Walker SA, Sawant D, Gowers KH, Campbell GA, McKenzie AN, Lloyd CM, IL-25 drives remodelling in allergic airways disease induced by house dust mite, Thorax 68(1) (2013) 82–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [155].Cayrol C, Girard JP, IL-33: an alarmin cytokine with crucial roles in innate immunity, inflammation and allergy, Curr Opin Immunol 31 (2014) 31–7. [DOI] [PubMed] [Google Scholar]
- [156].Bartemes KR, Iijima K, Kobayashi T, Kephart GM, McKenzie AN, Kita H, IL-33-responsive lineage- CD25+ CD44(hi) lymphoid cells mediate innate type 2 immunity and allergic inflammation in the lungs, J Immunol 188(3) (2012) 1503–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [157].Fattouh R, Al-Garawi A, Fattouh M, Arias K, Walker TD, Goncharova S, Coyle AJ, Humbles AA, Jordana M, Eosinophils are dispensable for allergic remodeling and immunity in a model of house dust mite-induced airway disease, Am J Respir Crit Care Med 183(2) (2011) 179–88. [DOI] [PubMed] [Google Scholar]
- [158].Humbles AA, Lloyd CM, McMillan SJ, Friend DS, Xanthou G, McKenna EE, Ghiran S, Gerard NP, Yu C, Orkin SH, Gerard C, A critical role for eosinophils in allergic airways remodeling, Science 305(5691) (2004) 1776–9. [DOI] [PubMed] [Google Scholar]
- [159].Lloyd CM, Saglani S, Eosinophils in the spotlight: Finding the link between obesity and asthma, Nat Med 19(8) (2013) 976–7. [DOI] [PubMed] [Google Scholar]
- [160].Haldar P, Pavord ID, Shaw DE, Berry MA, Thomas M, Brightling CE, Wardlaw AJ, Green RH, Cluster analysis and clinical asthma phenotypes, Am J Respir Crit Care Med 178(3) (2008) 218–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [161].Giuffrida MJ, Valero N, Mosquera J, Duran A, Arocha F, Chacin B, Espina LM, Gotera J, Bermudez J, Mavarez A, Alvarez-Mon M, Increased Systemic Cytokine/Chemokine Expression in Asthmatic and Non-asthmatic Patients with Bacterial, Viral or Mixed Lung Infection, Scand J Immunol 85(4) (2017) 280–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [162].Tanaka H, Miyazaki N, Oashi K, Teramoto S, Shiratori M, Hashimoto M, Ohmichi M, Abe S, IL-18 might reflect disease activity in mild and moderate asthma exacerbation, J Allergy Clin Immunol 107(2) (2001) 331–6. [DOI] [PubMed] [Google Scholar]
- [163].Wallach D, Taieb A, Atopic dermatitis/atopic eczema, Chem Immunol Allergy 100 (2014) 81–96. [DOI] [PubMed] [Google Scholar]
- [164].Werfel T, Allam JP, Biedermann T, Eyerich K, Gilles S, Guttman-Yassky E, Hoetzenecker W, Knol E, Simon HU, Wollenberg A, Bieber T, Lauener R, Schmid-Grendelmeier P, Traidl-Hoffmann C, Akdis CA, Cellular and molecular immunologic mechanisms in patients with atopic dermatitis, J Allergy Clin Immunol 138(2) (2016) 336–49. [DOI] [PubMed] [Google Scholar]
- [165].Celakovska J, Bukac J, Ettler K, Vaneckova J, Krcmova I, Ettlerova K, Krejsek J, Evaluation of Peripheral Blood Eosinophilia in Adolescent and Adult Patients Suffering from Atopic Dermatitis and the Relation to the Occurrence of Allergy to Aeroallergens, Indian Journal of Dermatology 64(1) (2019) 34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [166].Rasheed Z, Zedan K, Saif GB, Salama RH, Salem T, Ahmed AA, El-Moniem AA, Elkholy M, Al Robaee AA, Alzolibani AA, Markers of atopic dermatitis, allergic rhinitis and bronchial asthma in pediatric patients: correlation with filaggrin, eosinophil major basic protein and immunoglobulin E, Clin Mol Allergy 16 (2018)23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [167].Hall A, Atopic Dermatitis (Atopic Eczema), Atlas of Male Genital Dermatology (2019) 41–43. [Google Scholar]
- [168].Kandikattu HKM, A., Oxido-nitrosative stress and antioxidants in asthma, Int J Basic Clin Immonol 1(9–12) (2018). [Google Scholar]
- [169].Kalesnikoff J, Galli SJ, Anaphylaxis: mechanisms of mast cell activation, Chem Immunol Allergy 95 (2010)45–66. [DOI] [PubMed] [Google Scholar]
- [170].Dhami S, Panesar SS, Roberts G, Muraro A, Worm M, Bilo MB, Cardona V, Dubois AE, DunnGalvin A, Eigenmann P, Fernandez-Rivas M, Halken S, Lack G, Niggemann B, Rueff F, Santos AF, Vlieg-Boerstra B, Zolkipli ZQ, Sheikh A, Allergy EF, Anaphylaxis Guidelines G, Management of anaphylaxis: a systematic review, Allergy 69(2) (2014) 168–75. [DOI] [PubMed] [Google Scholar]
- [171].Bousquet J, Khaltaev N, Cruz AA, Denburg J, Fokkens WJ, Togias A, Zuberbier T, Baena-Cagnani CE, Canonica GW, van Weel C, Agache I, Ait-Khaled N, Bachert C, Blaiss MS, Bonini S, Boulet LP, Bousquet PJ, Camargos P, Carlsen KH, Chen Y, Custovic A, Dahl R, Demoly P, Douagui H, Durham SR, van Wijk RG, Kalayci O, Kaliner MA, Kim YY, Kowalski ML, Kuna P, Le LT, Lemiere C, Li J, Lockey RF, Mavale-Manuel S, Meltzer EO, Mohammad Y, Mullol J, Naclerio R, O'Hehir RE, Ohta K, Ouedraogo S, Palkonen S, Papadopoulos N, Passalacqua G, Pawankar R, Popov TA, Rabe KF, Rosado-Pinto J, Scadding GK, Simons FE, Toskala E, Valovirta E, van Cauwenberge P, Wang DY, Wickman M, Yawn BP, Yorgancioglu A, Yusuf OM, Zar H, Annesi-Maesano I, Bateman ED, Ben Kheder A, Boakye DA, Bouchard J, Burney P, Busse WW, Chan-Yeung M, Chavannes NH, Chuchalin A, Dolen WK, Emuzyte R, Grouse L, Humbert M, Jackson C, Johnston SL, Keith PK, Kemp JP, Klossek JM, Larenas-Linnemann D, Lipworth B, Malo JL, Marshall GD, Naspitz C, Nekam K, Niggemann B, Nizankowska-Mogilnicka E, Okamoto Y, Orru MP, Potter P, Price D, Stoloff SW, Vandenplas O, Viegi G, Williams D, O. World Health, Galen, AllerGen, Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen), Allergy 63 Suppl 86 (2008) 8–160. [DOI] [PubMed] [Google Scholar]
- [172].Bousquet J, Van Cauwenberge P, Khaltaev N, Aria Workshop G, O. World Health, Allergic rhinitis and its impact on asthma, J Allergy Clin Immunol 108(5 Suppl) (2001) S147–334. [DOI] [PubMed] [Google Scholar]
- [173].Kato A, Schleimer RP, Beyond inflammation: airway epithelial cells are at the interface of innate and adaptive immunity, Curr Opin Immunol 19(6) (2007) 711–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [174].Miyata M, Hatsushika K, Ando T, Shimokawa N, Ohnuma Y, Katoh R, Suto H, Ogawa H, Masuyama K, Nakao A, Mast cell regulation of epithelial TSLP expression plays an important role in the development of allergic rhinitis, Eur J Immunol 38(6) (2008) 1487–92. [DOI] [PubMed] [Google Scholar]
- [175].Nakamura Y, Miyata M, Ohba T, Ando T, Hatsushika K, Suenaga F, Shimokawa N, Ohnuma Y, Katoh R, Ogawa H, Nakao A, Cigarette smoke extract induces thymic stromal lymphopoietin expression, leading to T(H)2-type immune responses and airway inflammation, J Allergy Clin Immunol 122(6) (2008) 1208–14. [DOI] [PubMed] [Google Scholar]
- [176].van den Oord RA, Sheikh A, Filaggrin gene defects and risk of developing allergic sensitisation and allergic disorders: systematic review and meta-analysis, BMJ 339 (2009) b2433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [177].Greiner AN, Hellings PW, Rotiroti G, Scadding GK, Allergic rhinitis, Lancet 378(9809) (2011) 2112–22. [DOI] [PubMed] [Google Scholar]
- [178].Lin H, Lin D, Xiong XS, Dai XX, Lin T, Role of platelet-derived growth factor-alpha in eosinophilic and non-eosinophilic chronic rhinosinusitis with nasal polyps, Int Forum Allergy Rhinol 4(11) (2014) 909–14. [DOI] [PubMed] [Google Scholar]
- [179].Lackner A, Raggam RB, Stammberger H, Beham A, Braun H, Kleinhappl B, Buzina W, Kittinger C, Reinisch S, Berghold A, Freudenschuss K, Barth S, Marth E, The role of interleukin-16 in eosinophilic chronic rhinosinusitis, Eur Arch Otorhinolaryngol 264(8) (2007) 887–93. [DOI] [PubMed] [Google Scholar]
- [180].Shah SA, Ishinaga H, Takeuchi K, Pathogenesis of eosinophilic chronic rhinosinusitis, J Inflamm (Lond) 13 (2016) 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [181].Steinke JW, Kennedy JL, Leukotriene Inhibitors in Sinusitis, Curr Infect Dis Rep (2012). [DOI] [PubMed] [Google Scholar]
- [182].de Borja Callejas F, Martinez-Anton A, Picado C, Alobid I, Pujols L, Valero A, Roca-Ferrer J, Mullol J, Corticosteroid treatment regulates mucosal remodeling in chronic rhinosinusitis with nasal polyps, Laryngoscope 125(5) (2015) E158–67. [DOI] [PubMed] [Google Scholar]
- [183].Antia C, Baquerizo K, Korman A, Bernstein JA, Alikhan A, Urticaria: A comprehensive review: Epidemiology, diagnosis, and work-up, J Am Acad Dermatol 79(4) (2018) 599–614. [DOI] [PubMed] [Google Scholar]
- [184].Kreft B, Wohlrab J, Marsch WC, [Aquagenic urticaria. A case report], Hautarzt 65(2) (2014) 130–2. [DOI] [PubMed] [Google Scholar]
- [185].Kursbaum A, Spalter L, Ben-Assuly S, [A dramatic presentation of primary idiopathic cold urticaria], Harefuah 104(9) (1983) 393–4. [PubMed] [Google Scholar]
- [186].Mazarakis A, Bardousis K, Almpanis G, Mazaraki I, Markou S, Kounis NG, Kounis syndrome following cold urticaria: the swimmer's death, Int J Cardiol 176(2) (2014) e52–3. [DOI] [PubMed] [Google Scholar]
- [187].Trevisonno J, Balram B, Netchiporouk E, Ben-Shoshan M, Physical urticaria: Review on classification, triggers and management with special focus on prevalence including a meta-analysis, Postgrad Med 127(6) (2015) 565–70. [DOI] [PubMed] [Google Scholar]
- [188].Zuberbier T, Bindslev-Jensen C, Canonica W, Grattan CE, Greaves MW, Henz BM, Kapp A, Kozel MM, Maurer M, Merk HF, Schafer T, Simon D, Vena GA, Wedi B, Eaaci/Ga2Len/Edf, EAACI/GA2LEN/EDF guideline: definition, classification and diagnosis of urticaria, Allergy 61(3) (2006) 316–20. [DOI] [PubMed] [Google Scholar]
- [189].Fulkerson PC, Rothenberg ME, Targeting eosinophils in allergy, inflammation and beyond, Nat Rev Drug Discov 12(2) (2013) 117–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [190].Dent LA, Strath M, Mellor AL, Sanderson CJ, Eosinophilia in transgenic mice expressing interleukin 5, J Exp Med 172(5) (1990) 1425–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [191].Rothenberg ME, Eosinophilia N Engl J Med 338(22) (1998) 1592–600. [DOI] [PubMed] [Google Scholar]
- [192].Davis BP, Rothenberg ME, Eosinophils and cancer, Cancer Immunol Res 2(1) (2014) 1–8. [DOI] [PubMed] [Google Scholar]
- [193].Manohar M, Verma AK, Venkateshaiah SU, Mishra A, Significance of Eosinophils in Promoting Pancreatic malignancy, J Gastroenterol Pancreatol Liver Disord 5(1) (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [194].Insiripong S, Siriyakorn N, Treatment of eosinophilia with albendazole, Southeast Asian J Trop Med Public Health 39(3) (2008) 517–20. [PubMed] [Google Scholar]
- [195].Michaelann L, Erik Zeger L, Palaniandy K, Eosinophilia Treatment & Management, Hematology (2018). [Google Scholar]
- [196].Dellon ES, Jensen ET, Martin CF, Shaheen NJ, Kappelman MD, Prevalence of eosinophilic esophagitis in the United States, Clin Gastroenterol Hepatol 12(4) (2014) 589–96 e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [197].Venkateshaiah SU, Manohar M, Verma AK, Blecker U, Mishra A, Possible Noninvasive Biomarker of Eosinophilic Esophagitis: Clinical and Experimental Evidence, Case Rep Gastroenterol 10(3) (2016) 685–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [198].Sgouros SN, Bergele C, Mantides A, Eosinophilic esophagitis in adults: a systematic review, Eur J Gastroenterol Hepatol 18(2) (2006) 211–7. [DOI] [PubMed] [Google Scholar]
- [199].Li G, Cai B, Song H, Yang Z, Clinical and radiological character of eosinophilic cystitis, Int J Clin Exp Med 8(1) (2015) 533–9. [PMC free article] [PubMed] [Google Scholar]
- [200].Aleem S, Kumar B, Fasano MB, Takacs E, Azar AE, Successful use of cyclosporine as treatment for eosinophilic cystitis: a case report, World Allergy Organ J 9 (2016) 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [201].Pinal-Fernandez I, Selva-O' Callaghan A, Grau JM, Diagnosis and classification of eosinophilic fasciitis, Autoimmun Rev 13(4–5) (2014) 379–82. [DOI] [PubMed] [Google Scholar]
- [202].Antic M, Lautenschlager S, Itin PH, Eosinophilic fasciitis 30 years after - what do we really know? Report of 11 patients and review of the literature, Dermatology 213(2) (2006) 93–101. [DOI] [PubMed] [Google Scholar]
- [203].Dziadzio L, Kelly EA, Panzer SE, Jarjour N, Huttenlocher A, Cytokine abnormalities in a patient with eosinophilic fasciitis, Ann Allergy Asthma Immunol 90(4) (2003) 452–5. [DOI] [PubMed] [Google Scholar]
- [204].Enokihara H, Furusawa S, Nakakubo H, Kajitani H, Nagashima S, Saito K, Shishido H, Hitoshi Y, Takatsu K, Noma T, et al. , T cells from eosinophilic patients produce interleukin-5 with interleukin-2 stimulation, Blood 73(7) (1989) 1809–13. [PubMed] [Google Scholar]
- [205].French LE, Shapiro M, Junkins-Hopkins JM, Wolfe JT, Rook AH, Eosinophilic fasciitis and eosinophilic cellulitis in a patient with abnormal circulating clonal T cells: increased production of interleukin 5 and inhibition by interferon alfa, J Am Acad Dermatol 49(6) (2003) 1170–4. [DOI] [PubMed] [Google Scholar]
- [206].Bischoff L, Derk CT, Eosinophilic fasciitis: demographics, disease pattern and response to treatment: report of 12 cases and review of the literature, Int J Dermatol 47(1) (2008) 29–35. [DOI] [PubMed] [Google Scholar]
- [207].Mouthon L, Dunogue B, Guillevin L, Diagnosis and classification of eosinophilic granulomatosis with polyangiitis (formerly named Churg-Strauss syndrome), J Autoimmun 48–49 (2014) 99–103. [DOI] [PubMed] [Google Scholar]
- [208].Vaglio A, Buzio C, Zwerina J, Eosinophilic granulomatosis with polyangiitis (Churg-Strauss): state of the art, Allergy 68(3) (2013) 261–73. [DOI] [PubMed] [Google Scholar]
- [209].Mohammad AJ, Hot A, Arndt F, Moosig F, Guerry MJ, Amudala N, Smith R, Sivasothy P, Guillevin L, Merkel PA, Jayne DR, Rituximab for the treatment of eosinophilic granulomatosis with polyangiitis (Churg-Strauss), Ann Rheum Dis 75(2) (2016) 396–401. [DOI] [PubMed] [Google Scholar]
- [210].Wechsler ME, Akuthota P, Jayne D, Khoury P, Klion A, Langford CA, Merkel PA, Moosig F, Specks U, Cid MC, Luqmani R, Brown J, Mallett S, Philipson R, Yancey SW, Steinfeld J, Weller PF, Gleich GJ, Team EMS, Mepolizumab or Placebo for Eosinophilic Granulomatosis with Polyangiitis, N Engl J Med 376(20) (2017) 1921–1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [211].Allen J, Wert M, Eosinophilic Pneumonias J Allergy Clin Immunol Pract 6(5) (2018) 1455–1461. [DOI] [PubMed] [Google Scholar]
- [212].Marchand E, Cordier JF, Idiopathic chronic eosinophilic pneumonia, Orphanet J Rare Dis 1 (2006) 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [213].Kaya H, Gumus S, Ucar E, Aydogan M, Musabak U, Tozkoparan E, Bilgic H, Omalizumab as a steroid-sparing agent in chronic eosinophilic pneumonia, Chest 142(2) (2012) 513–516. [DOI] [PubMed] [Google Scholar]
- [214].Rhee CK, Min KH, Yim NY, Lee JE, Lee NR, Chung MP, Jeon K, Clinical characteristics and corticosteroid treatment of acute eosinophilic pneumonia, Eur Respir J 41(2) (2013) 402–9. [DOI] [PubMed] [Google Scholar]
- [215].Ogbogu PU, Rosing DR, Horne MK 3rd, Cardiovascular manifestations of hypereosinophilic syndromes, Immunol Allergy Clin North Am 27(3) (2007) 457–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [216].Helbig G, Wieczorkiewicz A, Dziaczkowska-Suszek J, Majewski M, Kyrcz-Krzemien S, T-cell abnormalities are present at high frequencies in patients with hypereosinophilic syndrome, Haematologica 94(9) (2009) 1236–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [217].Roufosse FE, Goldman M, Cogan E, Hypereosinophilic syndromes, Orphanet J Rare Dis 2 (2007) 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [218].Gleich GJ, Leiferman KM, Pardanani A, Tefferi A, Butterfield JH, Treatment of hypereosinophilic syndrome with imatinib mesilate, Lancet 359(9317) (2002) 1577–8. [DOI] [PubMed] [Google Scholar]
- [219].Klion AD, Bochner BS, Gleich GJ, Nutman TB, Rothenberg ME, Simon HU, Wechsler ME, Weller PF, G. The Hypereosinophilic Syndromes Working, Approaches to the treatment of hypereosinophilic syndromes: a workshop summary report, J Allergy Clin Immunol 117(6) (2006) 1292–302. [DOI] [PubMed] [Google Scholar]
- [220].Dima E, Koltsida O, Katsaounou P, Vakali S, Koutsoukou A, Koulouris NG, Rovina N, Implication of Interleukin (IL)-18 in the pathogenesis of chronic obstructive pulmonary disease (COPD), Cytokine 74(2) (2015)313–7. [DOI] [PubMed] [Google Scholar]
- [221].Imaoka H, Hoshino T, Takei S, Kinoshita T, Okamoto M, Kawayama T, Kato S, Iwasaki H, Watanabe K, Aizawa H, Interleukin-18 production and pulmonary function in COPD, Eur Respir J 31(2) (2008) 287–97. [DOI] [PubMed] [Google Scholar]
- [222].Nakajima T, Owen CA, Interleukin-18: the master regulator driving destructive and remodeling processes in the lungs of patients with chronic obstructive pulmonary disease?, Am J Respir Crit Care Med 185(11) (2012) 1137–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [223].Wang J, Liu X, Xie M, Xie J, Xiong W, Xu Y, Increased expression of interleukin-18 and its receptor in peripheral blood of patients with chronic obstructive pulmonary disease, COPD 9(4) (2012) 375–81. [DOI] [PubMed] [Google Scholar]
- [224].Hart TK, Cook RM, Zia-Amirhosseini P, Minthorn E, Sellers TS, Maleeff BE, Eustis S, Schwartz LW, Tsui P, Appelbaum ER, Martin EC, Bugelski PJ, Herzyk DJ, Preclinical efficacy and safety of mepolizumab (SB-240563), a humanized monoclonal antibody to IL-5, in cynomolgus monkeys, J Allergy Clin Immunol 108(2) (2001) 250–7. [DOI] [PubMed] [Google Scholar]
- [225].Pavord ID, Chanez P, Criner GJ, Kerstjens HAM, Korn S, Lugogo N, Martinot JB, Sagara H, Albers FC, Bradford ES, Harris SS, Mayer B, Rubin DB, Yancey SW, Sciurba FC, Mepolizumab for Eosinophilic Chronic Obstructive Pulmonary Disease, N Engl J Med 377(17) (2017) 1613–1629. [DOI] [PubMed] [Google Scholar]
- [226].Song T, Jones DM, Homsi Y, Therapeutic effect of anti-IL-5 on eosinophilic myocarditis with large pericardial effusion, BMJ Case Rep 2017 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- [227].Brambatti M, Matassini MV, Adler ED, Klingel K, Camici PG, Ammirati E, Eosinophilic Myocarditis: Characteristics, Treatment, and Outcomes, J Am Coll Cardiol 70(19) (2017) 2363–2375. [DOI] [PubMed] [Google Scholar]
- [228].Cooper LT Jr., Eosinophilic Myocarditis as a Cause of Acute Cardiac Syndromes: The Importance of Awareness, J Am Coll Cardiol 70(19) (2017) 2376–2377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [229].Espana A, Sanz ML, Sola J, Gil P, Wells' syndrome (eosinophilic cellulitis): correlation between clinical activity, eosinophil levels, eosinophil cation protein and interleukin-5, Br J Dermatol 140(1) (1999) 127–30. [DOI] [PubMed] [Google Scholar]
- [230].Yagi H, Tokura Y, Matsushita K, Hanaoka K, Furukawa F, Takigawa M, Wells' syndrome: a pathogenic role for circulating CD4+CD7− T cells expressing interleukin-5 mRNA, Br J Dermatol 136(6) (1997) 918–23. [PubMed] [Google Scholar]
- [231].Fujimura T, Kambayashi Y, Furudate S, Kakizaki A, Aiba S, A possible mechanism in the recruitment of eosinophils and Th2 cells through CD163(+) M2 macrophages in the lesional skin of eosinophilic cellulitis, Eur J Dermatol 24(2) (2014) 180–5. [DOI] [PubMed] [Google Scholar]
- [232].Simon HU, Plotz S, Simon D, Seitzer U, Braathen LR, Menz G, Straumann A, Dummer R, Levi-Schaffer F, Interleukin-2 primes eosinophil degranulation in hypereosinophilia and Wells' syndrome, Eur J Immunol 33(4) (2003) 834–9. [DOI] [PubMed] [Google Scholar]
- [233].Caputo R, Marzano AV, Vezzoli P, Lunardon L, Wells syndrome in adults and children: a report of 19 cases, Arch Dermatol 142(9) (2006) 1157–61. [DOI] [PubMed] [Google Scholar]
- [234].Cherng E, McClung AA, Rosenthal HM, Hicks J, Levy ML, Wells' syndrome associated with parvovirus in a 5-year old boy, Pediatr Dermatol 29(6) (2012) 762–4. [DOI] [PubMed] [Google Scholar]
- [235].Gilliam AE, Bruckner AL, Howard RM, Lee BP, Wu S, Frieden IJ, Bullous "cellulitis" with eosinophilia: case report and review of Wells' syndrome in childhood, Pediatrics 116(1) (2005) e149–55. [DOI] [PubMed] [Google Scholar]
- [236].Ohtsuka T, Oral tacrolimus treatment for refractory eosinophilic cellulitis, Clin Exp Dermatol 34(8) (2009)e597–8. [DOI] [PubMed] [Google Scholar]
- [237].Katz U, Shoenfeld Y, Pulmonary eosinophilia, Clin Rev Allergy Immunol 34(3) (2008) 367–71. [DOI] [PubMed] [Google Scholar]
- [238].Kudlacz E, Conklyn M, Andresen C, Whitney-Pickett C, Changelian P, The JAK-3 inhibitor CP-690550 is a potent anti-inflammatory agent in a murine model of pulmonary eosinophilia, Eur J Pharmacol 582(1–3) (2008) 154–61. [DOI] [PubMed] [Google Scholar]
- [239].Rothenberg ME, Eosinophilic gastrointestinal disorders (EGID), J Allergy Clin Immunol 113(1) (2004) 11–28; quiz 29. [DOI] [PubMed] [Google Scholar]
- [240].Straumann A, Eosinophil-Associated Gastrointestinal Disorders, In Clinical Immunology (2019) 633–640. [Google Scholar]
- [241].Alfadda AA, Storr MA, Shaffer EA, Eosinophilic colitis: epidemiology, clinical features, and current management, Therap Adv Gastroenterol 4(5) (2011) 301–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [242].Uppal V, Kreiger P, Kutsch E, Eosinophilic Gastroenteritis and Colitis: a Comprehensive Review, Clin Rev Allergy Immunol 50(2) (2016) 175–88. [DOI] [PubMed] [Google Scholar]
- [243].Caldwell JM, Collins MH, Stucke EM, Putnam PE, Franciosi JP, Kushner JP, Abonia JP, Rothenberg ME, Histologic eosinophilic gastritis is a systemic disorder associated with blood and extragastric eosinophilia, TH2 immunity, and a unique gastric transcriptome, J Allergy Clin Immunol 134(5) (2014) 1114–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [244].Ko HM, Morotti RA, Yershov O, Chehade M, Eosinophilic gastritis in children: clinicopathological correlation, disease course, and response to therapy, Am J Gastroenterol 109(8) (2014) 1277–85. [DOI] [PubMed] [Google Scholar]
- [245].Baig MA, Qadir A, Rasheed J, A review of eosinophilic gastroenteritis, J Natl Med Assoc 98(10) (2006) 1616–9. [PMC free article] [PubMed] [Google Scholar]
- [246].Desreumaux P, Bloget F, Seguy D, Capron M, Cortot A, Colombel JF, Janin A, Interleukin 3, granulocyte-macrophage colony-stimulating factor, and interleukin 5 in eosinophilic gastroenteritis, Gastroenterology 110(3) (1996) 768–74. [DOI] [PubMed] [Google Scholar]
- [247].Ingle SB, Hinge Ingle CR, Eosinophilic gastroenteritis: an unusual type of gastroenteritis, World J Gastroenterol 19(31) (2013) 5061–6. [DOI] [PMC free article] [PubMed] [Google Scholar]