Abstract
Although multiple determinants for establishing polarity in membranes of epithelial cells have been identified, the mechanism for maintaining apicobasal polarity is not fully understood. Here, we show that the conserved Hippo kinase pathway plays a role in the maintenance of apicobasal polarity in the developing intestine of Caenorhabditis elegans. We screened suppressors of the mutation in wts-1—the gene that encodes the LATS kinase homolog, deficiency of which leads to disturbance of the apicobasal polarity of the intestinal cells and to eventual death of the organism. We identified several alleles of yap-1 and egl-44 that suppress the effects of this mutation. yap-1 encodes a homolog of YAP/Yki, and egl-44 encodes a homolog of TEAD/Sd. WTS-1 bound directly to YAP-1 and inhibited its nuclear accumulation in intestinal cells. We also found that NFM-1, which is a homolog of NF2/Merlin, functioned in the same genetic pathway as WTS-1 to regulate YAP-1 to maintain cellular polarity. Transcriptome analysis identified several target candidates of the YAP-1-EGL-44 complex including TAT-2, which encodes a putative P-type ATPase. In summary, we have delineated the conserved Hippo pathway in C. elegans consisting of NFM-1-WTS-1-YAP-1-EGL-44 and proved that the proper regulation of YAP-1 by upstream NFM-1 and WTS-1 is essential for maintenance of apicobasal membrane identities of the growing intestine.
Keywords: Hippo kinase pathway, apicobasal polarity maintenance, membrane integrity, expanding membrane, C. elegans intestine
THE polarized plasma membrane of epithelial cells is subdivided into apical and basolateral membrane domains, which are separated from each other by cell–cell junctions. These domains are structurally and functionally distinct, and the establishment and maintenance of this apicobasal cell polarity during development is essential for organismal survival. Genetic screening has identified evolutionarily conserved polarity determinants including PAR/aPKC, Scribble/Disc Large and Crumbs complexes whose deficiencies perturb apicobasal cell polarity (Tepass et al. 1990; Izumi et al. 1998; Kuchinke et al. 1998; Tabuse et al. 1998; Hung and Kemphues 1999; Wodarz et al. 1999; Bilder and Perrimon 2000; Lin et al. 2000; Suzuki et al. 2001). These membrane-associated proteins define the identity of the membrane domains. Polarized sorting of vesicles that contain membrane-associated proteins into these distinct membrane domains is important for maintenance of apicobasal polarity in expanding membranes (Mellman and Nelson 2008; St Johnston and Ahringer 2010). Many polarity cues have been revealed, but how these cues are integrated during complex processes of tissue development is not well understood.
Several in vivo studies on the nematode Caenorhabditis elegans have shown that glycosphinglipids (GSLs) synthesis, clathrin and its AP-1 adaptor are essential for maintenance of the apicobasal polarity of the growing intestine, by allowing polarized targeting of multiple apical proteins (Seamen et al. 2009; Zhang et al. 2011; Shafaq-Zadah et al. 2012). In addition, in vitro studies on lumenogenesis using mammalian cell lines, Madin-Darby canine kidney, have found that vesicular trafficking could contribute to defining membrane domains by targeting polarity determinants including PARs and that the asymmetric distribution of lipids in the plasma membrane determines apical membrane identity (Bryant et al. 2010; Lu and Wilson 2016; Román-Fernández et al. 2018). These findings support the hypothesis that vesicles are not simply directed to deliver their cargo to the membrane domain, but can act upstream of asymmetric distribution of polarity determinants, and that the lipid composition of the membrane contributes to specification of apicobasal membrane.
The Hippo kinase pathway is a highly conserved signaling pathway that is present in a range of animals, from flies to mammals; it participates in regulation of tissue-size homeostasis (Harvey and Tapon 2007; Oh and Irvine 2010; Zhao et al. 2010a). During development, when cell number reaches a certain threshold, three membrane-associated proteins (Kibra, Merlin, and Expanded) activate the Hippo pathway, possibly by recruiting the pathway components at the subapical membrane and facilitating phosphorylation-mediated activation of the pathway (Boggiano and Fehon 2012; Enderle and McNeill 2013). As a result, activated LATS/Warts kinase phosphorylates the transcriptional coactivator YAP/Yki and inhibits its nuclear localization. In the nucleus, YAP/Yki interacts with the transcriptional factor TEAD/Sd to regulate the expression of several target genes that are involved mainly in cell proliferation and apoptosis.
The Hippo pathway has also been implicated in cell polarization. In fission yeast, a NDR/LATS kinase homolog, orb6, acts to maintain polarized cell growth during cell cycle by controlling localization of the small GTPase Cdc42, and also by positively regulating exocytosis (Verde et al. 1998; Das et al. 2009; Tay et al. 2019). In budding yeast, NDR/LATS kinase homolog Cbk1 functions in the polarized cell morphogenesis under the regulation by Kic1, the MST/Hpo kinase homolog (Bidlingmaier et al. 2001; Nelson et al. 2003; Brace et al. 2011). In Drosophila melanogaster, Hippo signaling has been shown to regulate apical domain size of wing imaginal disc epithelium; loss of Hippo signaling, or overexpression of Yki, leads to the expansion of apical domain, but does not affect the basolateral domain. It is also noteworthy that the apical polarity determinant Crumbs is required for this apical hypertrophy, which results from the loss of the Hippo pathway, but does not affect overgrowth phenotype, suggesting that the Hippo pathway regulates apical domain size in a manner that is independent of its functions in controlling organ size (Genevet et al. 2009; Hamaratoglu et al. 2009). Although how the Hippo pathway regulates apical domain size is still unclear, it was proposed that the pathway regulates membrane-protein turnover and endocytosis, and thereby affects the amount of apical protein transport. Furthermore, the fact that well-known polarity determinants including Crumbs, Lgl, and aPKC, have roles in activating the Hippo pathway also implies the crucial function of the Hippo pathway downstream, as well as upstream, of cell polarization (Chen et al. 2010; Grzeschik et al. 2010; Ling et al. 2010; Robinson et al. 2010).
Several components of the Hippo pathway and the genetic interactions among them are conserved in C. elegans (Cai et al. 2009; Kang et al. 2009; Iwasa et al. 2013); wts-1, yap-1, and egl-44 are some examples of this (Iwasa et al. 2013). A study showed that the subcellular localization of YAP-1, the worm homolog of YAP/Yki, is regulated by WTS-1, the worm homolog of LATS kinase, in the hypodermis. It also showed that YAP-1 binds to EGL-44, the worm homolog of TEAD/Sd (Iwasa et al. 2013). An interesting feature of the conservation of the Hippo pathway in worms is that it serves different purposes from its homologs reported in mammals and flies. The Hippo pathway in the hypodermis of C. elegans is involved in heat stress responses and in extending the health span (Iwasa et al. 2013). Another study showed that loss of WTS-1 activity causes defects in the maintenance of apicobasal polarity in the intestine, but this defective polarity is not a result of abnormalities in cell proliferation or apoptosis (Kang et al. 2009). It is worthwhile to note that the wts-1 mutant worms initially establish normal apicobasal polarity, but later begin to display a gradual misrouting of newly synthesized apical proteins to the basolateral region in a manner that is dependent on exocytosis, and, ultimately, die. This implies that the Hippo pathway is involved in maintaining, rather than establishing, the identities of growing membranes in C. elegans.
Here, to elucidate the molecular mechanism of WTS-1-involved maintenance of apicobasal polarity, we investigated the genes that act downstream of wts-1 by using forward genetic screening. We identified that mutations in yap-1 and egl-44 suppressed the defects in cellular polarity maintenance and the larval lethality of wts-1 mutants. We also found that NFM-1, the C. elegans homolog of NF2/Merlin, is also essential to maintain apicobasal polarity of intestinal cells and acts upstream of WTS-1 to regulate YAP-1. NF2/Merlin is the most well identified upstream factor of the Hippo pathway, so our genetic results showed that the conserved Hippo pathway is functional in C. elegans. We also tried to find transcriptional target genes of YAP-1 and EGL-44 that are responsible for membrane disintegrity. Microarray analysis isolated several candidate targets including TAT-2, which encodes P-type ATPase involved in regulatory roles in growing membrane. We also discuss the physiological and evolutionary significance of the maintenance of membrane integrity by the Hippo pathway.
Materials and Methods
Worm maintenance and strains
Worms were maintained at 20° and handled as previously described (Brenner 1974), unless noted otherwise. The Bristol strain N2 was used as the wild type. The following mutant strains were used: VC590 wts-1(ok753)/hT2[qIs48], wts-1(ok753); Ex[wts-1pro(2.7 kb)::wts-1::gfp, sur-5pro::sur-5::gfp], VC590; Ex[act-5pro::gfp::act-5] (Kang et al. 2009), wts-1(of1), yap-1(tm1416), yap-1(ys32), yap-1(ys33), yap-1(ys34), yap-1(ys37), yap-1(ys38), egl-44(n1080), egl-44(ys39), egl-44(ys41), tat-2(tm2332), VC555 nfm-1(ok754)/hT2[qIs48], and HT1593 unc-119(ed3). hT2[qIs48] has a pharyngeal GFP signal, and was used as a chromosome I and III balancer. The GFP signal was used as a marker to identify control heterozygous animals.
Molecular biology
For intestinal expression of YAP-1, the entire yap-1 was cloned into the pPD114.108 under the pept-1 promoter. For nfm-1, the full length of nfm-1a genomic DNA under its own 3-kb promoter was cloned into the GFP containing pPD95.75 vector. For WTS-1 expression, wts-1pro (2.7 kb)::wts-1::gfp was used (Kang et al. 2009).
Transgenic lines
For intestinal YAP-1 expression (Figure 3A), we introduced pept-1pro::yap-1::gfp constructs with UNC-119 wild-type copies into a unc-119(ed3) background using bombardment. We then isolated worms in which the uncoordinated phenotype was suppressed. The penetrance of transgenes was not 100%, so they may have been transferred as extrachromosomal arrays. For coexpression of intestinal YAP-1 and somatic nuclei marker SUR-5, we co-injected pept-1pro::yap-1::gfp constructs with sur-5pro::sur-5::mcherry at 50 ng/μl each with 100 ng/μl of pRF4, the injection marker. All other plasmids were injected into N2, VC590, and VC555 at 100 ng/μl with 100 ng/μl of pRF4 unless otherwise noted. By mating, transgenes were transferred to other mutant backgrounds.
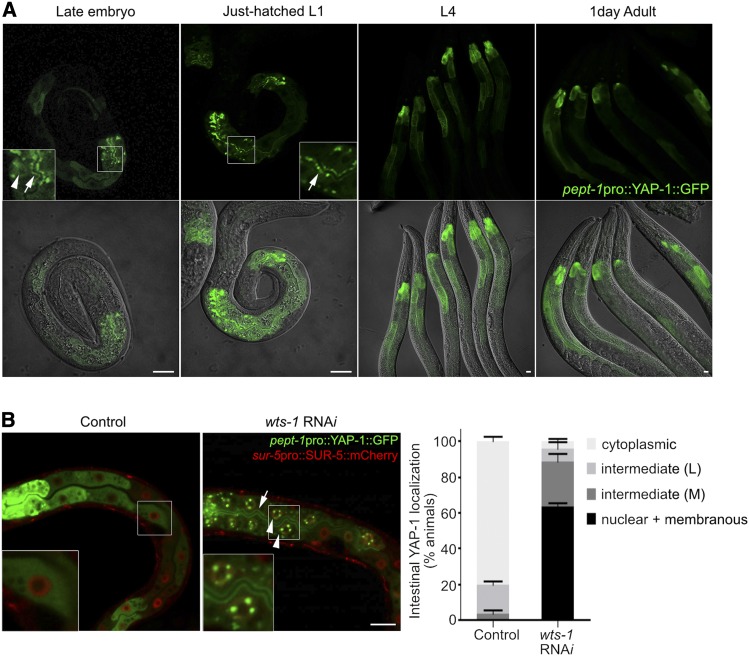
Figure 3.
Regulation of the subcellular localization of YAP-1 in intestine. (A) Subcellular localization of intestinal YAP-1 in developing worms. Nuclear (arrowhead) and membranous (arrows) localization of YAP-1 was restricted to late embryos and early L1-stage worms. From L1 stage, intestinal YAP-1 was consistently localized in the cytoplasm. (B) WTS-1-mediated regulation of subcellular localization of YAP-1 in intestine. Nucleus was labeled with SUR-5::mCherry. Knockdown of WTS-1 by RNAi caused nuclear (arrowheads) and membranous (arrow) localization of intestinal YAP-1 in larva. Right graph: quantified results. “cytosplasmic”: worms that show exclusive expression of YAP-1::GFP in cytoplasm; “intermediate (L, low)”: worms that show nuclear and membranous YAP-1::GFP only in anterior intestine; “intermediate (M, medium)”: worms that have nuclear and membranous YAP-1::GFP in anterior intestine and some other intestinal cells; “nuclear + membranous”: worms that show nuclear and membranous YAP-1::GFP throughout the entire intestine. Data: average percentages of worms SD Bar, 10 μm.
Ethyl methane sulfonate mutagenesis and mapping of responsible genes
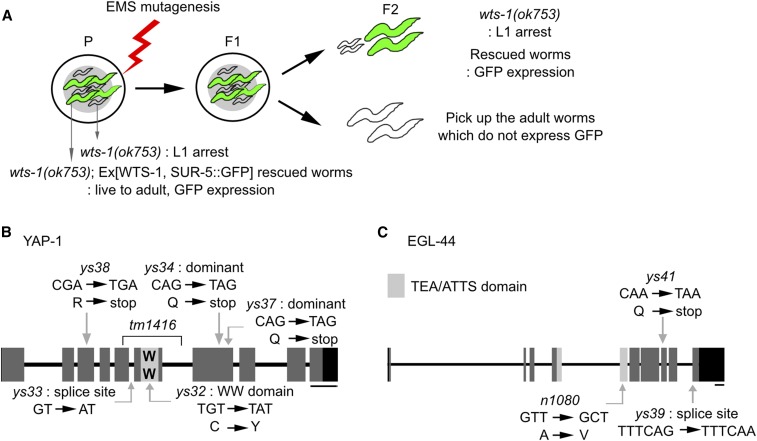
To isolate suppressors of wts-1(ok753) lethality, wts-1(ok753); Ex[wts-1pro::wts-1::gfp, sur-5pro::sur-5::gfp] animals were treated with 50 mM ethyl methane sulfonate (EMS), then ∼10,000 worms of the F1 generation were plated. Suppressor mutations were identified by the absence of the SUR-5::GFP. Fourteen independent suppressor lines were obtained and backcrossed to the parent line more than four times. Single-nucleotide polymorphism (SNP) mapping and manual sequencing of the YAP-1 and EGL-44 coding regions identified that five of these lines had mutations in the YAP-1, and two of them had mutations in the EGL-44 gene. To determine whether the suppressor mutation is dominant or recessive, we introduced one copy of each suppressor allele into wts-1(ok753) homozygote by mating, and observed that it is sufficient to rescue larval lethal phenotype of wts-1(ok753) without wild-type copy of WTS-1. Only wts-1(ok753); yap-1(ys34)/+, wts-1(ok753); yap-1(ys37)/+ survived. For the complementation test, yap-1(tm1416) or egl-44(ys39) were used to determine whether the mutations in the remaining suppressor lines are located in the yap-1 or in the egl-44 gene.
Fluorescence microscopy
To observe GFP::ACT-5 distribution and YAP-1::GFP subcellular localization, fluorescence microscopy (Axioplan2; Carl Zeiss) was used. All fluorescence images were acquired using the confocal microscope (ZEISS LSM700; Carl Zeiss) and ZEN software (Carl Zeiss).
Analysis of L1 viability
To measure L1 viability of worms, 20 day 1 gravid adult worms were transferred to a Nematode Growth Medium (NGM) agar plate and removed after 2–3 hr, leaving the laid eggs. We counted the number of embryos and, after 1 day, counted the number of unhatched embryos; 2 days later, we checked the number of adults. L1 viability was calculated as the ratio of the number of adults to the total number of hatched L1 individuals (total number of embryos – the number of unhatched embryos). We used same methods for phenotype analysis (Figure 6E). Experiments were repeated at least three times.
Figure 6.
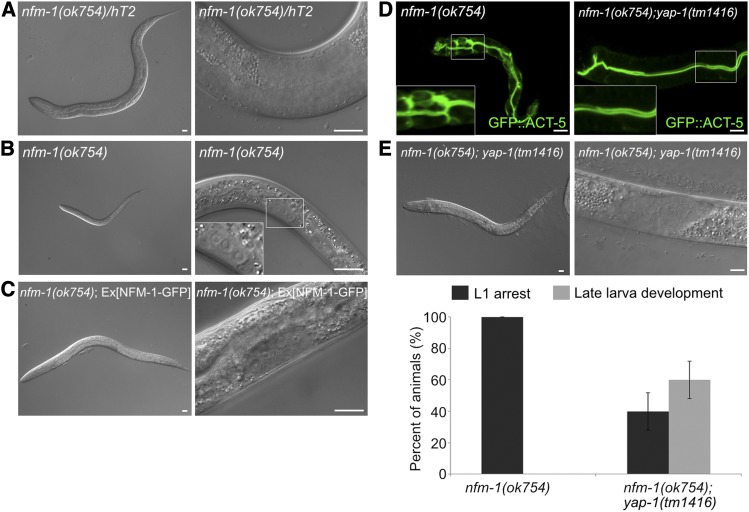
yap-1 suppresses polarity defects and larval lethality of the nfm-1 lacking mutant (A and B) Representative images of 3-day-old larvae of (A) nfm-1 heterozygous mutants and (B) nfm-1 homozygous mutants. nfm-1 homozygous mutants were arrested in the L1 stage, whereas heterozygous mutants developed to the L4 stage. Boxed region: magnification of somatic gonad precursor cells. (C) Rescue with wild-type copies of NFM-1 allowed only L3 development, possibly due to the germline defect in nfm-1(ok754). (D) Suppression of polarity defects in nfm-1(ok754) by introduction of yap-1 mutation. (E) Suppression of early larval arrest phenotype of nfm-1(ok754) by yap-1. yap-1 mutation also allowed only L3 development of nfm-1(ok754). Lower graph: average percentage of worms ± SD. Bar, 10 μm.
Analysis of cellular polarity
We monitored the sorting of apical proteins by observing the GFP::ACT-5 distribution. For each genotype, 20 day 1 adults were transferred to NGM plates and removed after 12 hr; 1–2 days later, the distribution of GFP::ACT-5 was observed. VC590 wts-1(ok753)/hT2[qIs48] or VC555 nfm-1(ok754)/hT2[qIs48] was maintained as heterozygotes, so, for cell polarity analysis of wts-1 and nfm-1, we inspected the newly hatched L1 and isolated control heterozygotes that expressed pharyngeal GFP, and mutant homozygotes that did not express pharyngeal GFP, then transferred them to new NGM plates separately. On the day that worms hatched, and 1–2 days later, L1-arrested wts-1 or nfm-1 homozygous mutants were used for analysis of the cellular polarity. Worms were classified into three groups; “apical,” which show apical localization of ACT-5; “+ basolateral,” which show apical and basolateral localization of ACT-5; and “+ bubble-like,” which have ACT-5 in bubble-like structures, in addition to the apical and basolateral sides. Experiments were repeated at least three times.
YAP-1 subcellular localization analysis
For wts-1 RNAi, four to six L4 worms were transferred to wts-1 RNAi plates and control plates, phenotype was scored in their F1 progeny at the L1 stage since wts-1 knockdown induces L1 arrest phenotype. To quantify subcellular localization of YAP-1::GFP, worms were classified into four groups; “cytoplasmic,” which shows exclusive localization of YAP-1::GFP in the cytoplasm; “intermediate (L, low),” which has nuclear and membranous YAP-1::GFP only in anterior intestine cells; “intermediate (M, medium),” which displays YAP-1::GFP in nucleus and membrane of several intestinal cells, in addition to the anterior intestine; and “nuclear + membranous,” which shows nuclear and membranous YAP-1::GFP expression throughout the entire intestine. Experiments were repeated three times.
RNAi feeding experiments
For wts-1 RNAi, wts-1 cDNA(bases 350–1550) was cloned into the L4440 vector (Kang et al. 2009). For nfm-1 RNAi, we used the construct from the C. elegans ORFome library. For mob-1, cst-1, cst-2 RNAi, entire cDNA region of each gene was cloned into the L4440 vector. For other genes RNAi, we used constructs from the J. Ahringer library. RNAi feeding experiments were performed using standard methods.
Yeast two-hybrid screen
The wts-1 bait (full length, 2727 nt) was cloned into BamHI/ PstI sites of the pGBKL vector. The construct was transformed into yeast strain PBN204; the resultant strain was tested for expression of wts-1 and absence of autoactivation. This strain was transformed using a C. elegans cDNA AD library in pPC86 vector (PanBioNet, Korea).
GST pulldown assay
yap-1 full-length cDNA was cloned into pGEX-4T1 vector, then introduced into BL21. GST-YAP-1 was incubated with glutathione sepharose beads (Amersham) in sonication buffer [20 mM HEPES-KOH/pH 7.6, 300 mM potassium acetate, 20% glycerol, 2 mM EDTA, 10 mM MgCl2, 1 mM DTT, 1 mM PMSF, protease inhibitor (Calbiochem)] + 1.5% Triton X-100 at 4°. Beads were washed three times with sonication buffer. wts-1 full-length, N-terminus, and C-terminus forms of cDNA were cloned into the pCDNA3.1 vector. To obtain mutant wts-1 construct on PPxY motif (Y-to-A mutation), a site-directed mutagenesis kit (Stratagene) was used. To obtain in vitro-translated WTS-1 proteins, these constructs were used as template for coupled in vitro transcription/translation (TNT; Promega), in the presence of 2 μCi [35S] methionine. The 1 μl of radiolabeled WTS-1 proteins were incubated with the GST-beads or GST-YAP-1-beads in GST pulldown buffer (20 mM Tris acetate/pH 7.9, 100 mM potassium acetate, 10% glycerol, 0.1 mM EDTA, 0.2% NP-40, 1 mM DTT, 1 mM PMSF, protease inhibitor) + 1% BSA at 4° overnight, washed three times with GST pulldown buffer, then analyzed using SDS-PAGE and autoradiography with a Fuji phosphorimager.
Gene expression comparison by microarray and data analysis
To collect embryos of N2 and yap-1(ys38), gravid adults from 10 plates of 10-cm NGM were bleached. Total RNA was extracted from three biological replicates using the freeze-thaw method. The isolated RNA was qualified and used as templates for cDNA synthesis in the Genome Research Facility of the Seoul National University, using an Affymetrix chip (Seolin bio, Seoul, Korea) containing 22,625 gene probes that target 22,150 genes from C. elegans. The chip data were analyzed using the Microarray Suite version 5.0 (MAS 5.0) with global scaling performed as the normalization method using Genplex ver.3.0 (istech). The results of one sample of N2 were significantly different from those of the other two replicates, and were therefore excluded from the analysis. Significant genes were obtained by calculating fold-change and Welch’s T-test (P-value ≤0.05) using a Volcano Plot and filtering absent (A) by detection call.
Quantitative real-time PCR
Embryos of N2, yap-1(tm1416), egl-44(ys39), and egl-44(ys41) were obtained by the standard bleaching method. To collect wts-1(ok753) heterozygous and homozygous mutants, VC590 wts-1(ok753)/hT2[qIs48] gravid adults were bleached, then, 1 day later, hatched L1 worms were sorted based on pharyngeal GFP, which marked heterozygous mutants, using a Biosorter instrument (UNION BIOMETRICA). Total RNA of the obtained embryos or L1 larvae were extracted using TRI reagent (Molecular Research Center) by the freeze-thaw method. cDNA was synthesized with TOPscript reverse transcriptase (Enzynomics) using oligo(dT) primer. Quantitative real-time PCR was performed using Bio-Rad iQ SYBR Green supermix in Bio-Rad CFX connect as described in manufacturer’s manual. Primers used for the validation of the 20 top-ranked candidate genes are provided in Table 1. For all cases, the expression levels of act-1/3 genes were used as control. For act-1/3, we used forward: 5′-ACGCCAACACTGTTCTTTCC-3′, reverse: 5′-GATGATCTTGATCTTCATGGTTGA-3′ primers. At least three biological replicates were used for analysis.
Table 1. Primers used for validation of the candidate genes.
| ORF name | Gene name | Primers |
|---|---|---|
| F32A7.6 | aex-5 | F: 5′-GTTTGAGGCTCAGTGGAAGC-3′ |
| R: 5′-GCTTCCCGGATGTAAAGTGA-3′ | ||
| F15E11.13 | pud-1.1 | F: 5′-ATGGGATGCGGATAAGTCTG–3′ |
| R: 5′-CCGATTTGGTGACCTTTGTT-3′ | ||
| F37A4.3 | F: 5′-CAATTCTCCGGGATTTTCAC-3′ | |
| R: 5′-TTTCCGGCAAATACCTAACG-3′ | ||
| H22K11.4 | sgca-1 | F: 5′-TCGAACGTCGAAATCATTCA-3′ |
| R: 5′-TGTGACCGGTGTGCCTATTA-3′ | ||
| Y4C6B.3 | F:5′-GTGAGCGGAGTGATCTGTGA-3′ | |
| R: 5′-TTTCCAAGCTCTTTGGCACT-3′ | ||
| F28H6.1 | akt-2 | F: 5′-CTTTTGCGGAACACCAGAAT-3′ |
| R: 5′-TTCGAAGAGTTTGCCGTTCT-3′ | ||
| F58G11.1 | letm-1 | F: 5′-CTAGATGGTGCATTCGCTGA-3′ |
| R: 5′-CAAATCAACGCTGTTCTCCA-3′ | ||
| F54F2.7 | F: 5′-GGATTTTGGAACGACGAAGA-3′ | |
| R: 5′-GAACACCTGCTCTCACGACA-3′ | ||
| C16A11.9 | fbxc-43 | F: 5′-ATCGGATAACCCCCTAATCG-3′ |
| R: 5′-TATTCCCGGTCTTGTTGGAG-3′ | ||
| C52E12.4 | ist-6 | F: 5′-AAGGTGGAGGACACAATTCG-3′ |
| R: 5′-GTTGTGCCGAGTAACGGAAT-3′ | ||
| F11C1.1 | F: 5′-ACACCAACCCGAATGATGTT-3′ | |
| R: 5′-CACTCCATAAACGCCATCAA-3′ | ||
| H13N06.2 | F: 5′-TGGGAAATCGAAGAAACCTG-3′ | |
| R: 5′-TTTTGCCATTTGGTTCTTCC-3′ | ||
| K10B2.2 | ctsa-1 | F: 5′-AGCGACCAAGTTGGAAAGAA-3′ |
| R: 5′-GCCTCGTTCACTTTCTCCTG-3 | ||
| Y39B6A.33 | F: 5′-CCGAAATACGGAAAAGACGA-3′ | |
| R: 5′-ATGTGAATCCTGCTCCTTGG-3′ | ||
| H06H21.10 | tat-2 | F: 5′-CTGTTTTGGCAATGGGATCT-3′ |
| R: 5′-TGCGTTGTAAAATGCACCAT-3′ | ||
| ZC434.3 | F:5′-ATGTTGGAACCGACCCATTA-3′ | |
| R: 5′-AATCAAATCTCCAGCCATGC-3′ | ||
| T19D12.7 | oig-8 | F: 5′-TCCGCTCGTTTCAGAAAGTT-3′ |
| R: 5′-TCCAGGCATTTCATCAACAA-3′ | ||
| F56C9.7 | F: 5′-TTGGCAATTGGAACGTGTAA-3′ | |
| R: 5′-TTGAACGAATTGCTGACGAG-3′ | ||
| R193.2 | F: 5′-AGTCGGTCCATCTTCCACAC-3′ | |
| R: 5′-TGCCAAATCTGCGTCAGTAG-3′ | ||
| D1025.4 | nspc-20 | F: 5′-GTTTTGTGCAGGTGTCAACG-3′ |
| R: 5′-AGGTAACGACGATTGCATCC-3′ |
Data availability
Strains and plasmids used in this study are available upon request. Supplemental files are available at FigShare. Supplemental Material, Table S1 contains results of yeast two hybrid screening using WTS-1 as a bait. Table S2 is the list of genes whose expression was affected in the absence of YAP-1. Figure S1 shows that WTS-1 and YAP-1 bind to each other. Figure S2 shows subcellular localization of intestinal YAP-1 in worms in which Hpo pathway components were knocked down. Figure S3 shows larva arrest phenotype of wts-1(of1) which has a point mutation in the 863rd threonine residue of WTS-1. Figure S4 shows the validation of candidates for target genes of YAP-1 and EGL-44 complex. Figure S5 shows tat-2 as a candidate of yap-1 and egl-44 target. Figure S6 shows sphingolipid deficiency-mediated phenotype analysis of yap-1(tm1416). The raw data of the microarray experiment were deposited in the Gene Expression Omnibus (GEO) under the Subseries GSE133667. Supplemental material available at FigShare: https://doi.org/10.25386/genetics.8993831.
Results
Identification of yap-1 and egl-44 as genetic suppressors of the wts-1 lethality phenotype
Worms that lack WTS-1 display lethality in early larval stage because of defects in apicobasal polarity in their intestinal membranes (Kang et al. 2009) (Figure 2, A and B). They establish normal apicobasal polarity at the beginning, but they fail to maintain it and gradually begin to show basolateral and bubble-like distribution of apical proteins, forming multiple ectopic lumen-like structures. To elucidate how WTS-1 participates in maintenance of cellular polarity in the expanding membrane, we performed a forward genetic screening to identify factors downstream of WTS-1. wts-1 mutants of which lethality was rescued by expressing wild-type copies of WTS-1 fused GFP with SUR-5::GFP as a transgene marker were treated with EMS to create mutants. In the F2 generation, we isolated worms that survived without transgene expression (Figure 1A); 10,000 F1 screenings yielded 14 independent suppressor lines, which were then maintained. SNP mapping and manual sequencing demonstrated that five of them had mutations in yap-1, whereas two of them had mutations in egl-44 (Figure 1, B and C). yap-1 and egl-44 have been suggested to be C. elegans homologs of YAP/Yki and TEAD/Sd, respectively, which are highly conserved downstream factors of LATS/Warts kinase (Iwasa et al. 2013). Of the five alleles of the mutant yap-1, yap-1(ys34), and yap-1(ys37), which produced truncated YAP-1 proteins containing a WW domain, were dominant whereas other alleles were recessive (Figure 1B). By a complementation test, we determined that four of the remaining seven suppressor lines had mutations in yap-1, and three had mutations in egl-44, not in yap-1. The fact that yap-1 and egl-44 are the only two genes whose mutations were recovered from the genetic screen strongly suggests that they are the major, if not the only, downstream effectors of wts-1 in these phenomena.
Figure 2.
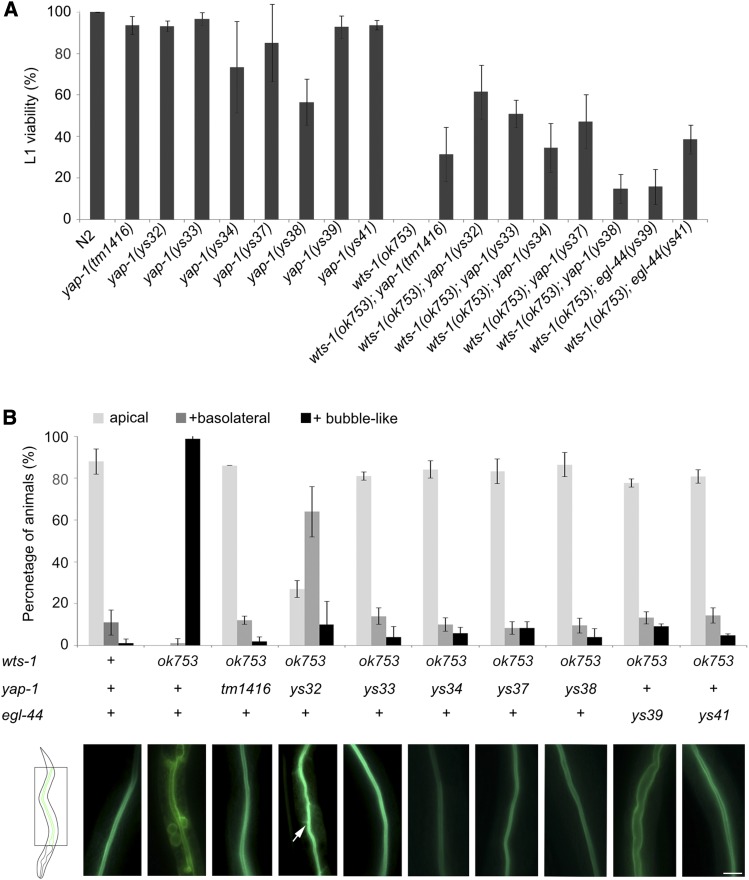
Mutations in yap-1 or egl-44 suppress wts-1 mutant phenotypes (A) Results of phenotypic analyses of wts-1, yap-1, egl-44, wts-1; yap-1, wts-1; egl-44 mutant worms. L1 viability is calculated as the ratio of the number of adult worms to total number of hatched L1 worms. (B) GFP::ACT-5 localization in the wild type N2, wts-1 mutant, and double mutant with wts-1 and various alleles of yap-1 or egl-44. Upper panel: “+ basolateral” = worms that showed apical and basolateral localization of the protein; “+ bubble-like” = worms that had proteins localized in bubble-like structures, in addition to the apical and basolateral sides. Two days after hatching, ACT-5 localization was measured. At the time of observation, wts-1 mutants were arrested at the L1 stage. The wild type, wts-1; yap-1, and wts-1; egl-44 worms were at the L4 stage. Lower panel: representative images of GFP::ACT-5 distribution in each genotype of the worms. Arrow: laterally localized GFP::ACT-5. Data: average percentages of worms SD Bar, 10 μm.
Figure 1.
YAP-1 and EGL-44 are downstream targets of WTS-1 (A) Schematic diagram of the EMS mutagenesis screening to obtain wts-1 suppressors in the wts-1(ok753); Ex[wts-1pro::wts-1, sur-5pro::sur-5::gfp] background. In the parents, extrachromosomal WTS-1 rescues wts-1(ok753) mutant from larval lethality. From the F2 generation, we isolated adult worms that did not express SUR-5::GFP; this result indicates that the mutation of the suppressor gene overcame the mutant wts-1 larval arrest phenotype. (B) Structure of yap-1. Dark gray boxes: exons; connecting lines: introns; black boxes: untranslated regions. ys32 is a missense mutation in the WW domain, caused by a C to Y change. ys33 disrupts the splice acceptor site of the fifth intron. ys34 and ys37 are dominant mutations that have premature stop codons after the WW domain. ys38 changes R to a stop codon at the N-terminus of YAP-1. tm1416 is almost completely deleted from the fifth to the sixth exon including the WW domain. (C) The egl-44 structure. ys39 and ys41 abrogate the C terminal region of EGL-44. ys39 ruins the 3′ splicing site ahead of the 10th exon. ys41 creates a premature stop codon in the eighth exon. n1080 is a missense mutation in the TEA/ATTS domain. Bar, 200 bp
WTS-1 interacts directly with WW domain of YAP-1 through PPxY motifs
To determine whether WTS-1 interacts with YAP-1 directly, we performed a GST pull-down assay. WTS-1 bound GST fused YAP-1, but not GST alone; this result indicates that WTS-1 interacts directly with YAP-1(Figure S1C). The N-terminus region of WTS-1, including two putative PPxY motifs and a N-terminal regulatory domain (NTR), bound to YAP-1, but the C-terminus region did not; this region includes the kinase domain and following hydrophobic motif (HM; Figure S1, A and C). This result is consistent with the notion that the PPxY motif in LATS kinase is essential to bind YAP. To test whether putative PPxY motifs in the N-terminal region of WTS-1 are necessary for binding with YAP-1, we abrogated the motifs by mutating Y residues to A. The Y398A mutation in WTS-1 did not affect the interaction. In contrast, Y221A mutation reduced, but did not completely abolish, the interaction; the double mutation at Y221A and Y398A abolished the interaction between WTS-1 and YAP-1 almost completely. These results indicate that both of these Y residues are involved in the binding between WTS-1 and YAP-1, with Y221 as a major site (Figure S1C).
We identified 10 putative binding partners (Table S1), including YAP-1, by performing unbiased yeast two-hybrid screens using full-length WTS-1 as a bait (Table S1). Consistent with the previous report that WTS-1 interacts with WW domain of YAP-1 (Iwasa et al. 2013), eight different preys contained parts of the YAP-1 protein with the WW domain (Figure S1B). These data indicate that WTS-1 binds directly to the WW domain of YAP-1 through its PPxY motif.
Mutations of yap-1 and egl-44 suppress polarity defects of wts-1 mutant animals
yap-1 and egl-44 mutations can suppress the early larval arrest phenotype of wts-1 mutants. In addition to the five alleles that were obtained from our screen, a putative null mutation of yap-1(tm1416) also allowed a wts-1 mutant to develop to a postlarval stage (Figure 2A). For egl-44, the conventional mutant egl-44(n1080) failed to suppress early larval lethality in wts-1(ok753) (data not shown), and we excluded this allele from our analysis (see Discussion). yap-1 and egl-44 mutations that suppress the lethality also suppressed the polarity defects of wts-1 mutants. Double mutants with wts-1 and either a yap-1 or an egl-44 mutant allele displayed normal distribution of apical protein ACT-5 on the luminal membrane of the intestine (Figure 2B). Although wts-1; yap-1(ys32) mutants showed abnormal distribution of ACT-5 in the basolateral region, they retained more intact membrane than did wts-1 single mutants, and were able to overcome lethality (Figure 2, A and B). These data suggest that induction of polarity defects and eventual death in wts-1 mutants requires both YAP-1 and EGL-44. Thus, appropriate regulation of YAP-1 and EGL-44 by WTS-1 during development is essential for the polarized sorting of newly synthesized proteins and for maintaining apicobasal polarity in expanding membranes.
WTS-1 regulates subcellular localization of YAP-1 in the intestine
During development, subcellular localization of YAP is tightly regulated by the upstream components of the Hippo pathway, whereas TEAD is constitutively localized in the nucleus (Vassilev et al. 2001). Activated LATS kinase phosphorylates YAP and inhibits its function as a transcriptional coactivator in the nucleus. Phosphorylated YAP is sequestered in the cytoplasm by interaction with the 14-3-3 complex (Vassilev et al. 2001; Zhao et al. 2007) or undergoes phosphodegron-mediated degradation (Zhao et al. 2010b). Loss of LATS kinase activity induces nuclear accumulation of YAP and abrogates the transcriptional regulation of target genes (Hao et al. 2008). In C. elegans, YAP-1 is expressed in various epithelial tissues including the hypodermis and the intestine. In the hypodermis, yap-1 is localized in the nuclei at the beginning of ventral enclosure. Afterward, YAP-1 is sequestered in the hypodermal cytoplasm throughout the larval stages in a manner that is dependent on WTS-1 (Iwasa et al. 2013). EGL-44 expressed more widely than YAP-1 and is observed in the nuclei in various sites including intestine, hypodermis, and neuronal cells throughout development (Wu et al. 2001). These distributions are consistent with the regulatory modalities of YAP/Yki and TEAD/Sd in other organisms.
To maintain intestinal apicobasal polarity, WTS-1 acts cell-autonomously. Intestinal expression of WTS-1 is sufficient to prevent larval lethality of wts-1 mutants (Kang et al. 2009). To investigate the subcellular localization of YAP-1 and its regulatory mechanisms in the intestine, we expressed GFP-fused YAP-1 in intestinal cells by using the intestine-specific promoter pept-1. From the L1 stage onward, intestinal YAP-1 was sequestered in the cytoplasm (Figure 3A). Only in late embryos and just-hatched L1 worms, YAP-1 was detected in the nucleus and luminal membrane of the intestine. Although the localization pattern of intestinal YAP-1 seemed to be highly mosaic in individual animals, anterior cells including int1 largely showed nuclear and membranous localization of YAP-1. This localization was not observed during other developmental stages (Figure 3A). Knockdown of WTS-1 activity by RNAi induced L1 larval arrest phenotype as previously reported (Kang et al. 2009) and led to the accumulation of YAP-1 in the nuclei and luminal membranes of intestinal cells in the arrested larva (Figure 3B). These data suggest that WTS-1 is involved in the regulation of the subcellular localization of YAP-1 in the postembryonic intestine, too.
NFM-1, the NF2/Merlin homolog, is also involved in the polarity maintenance
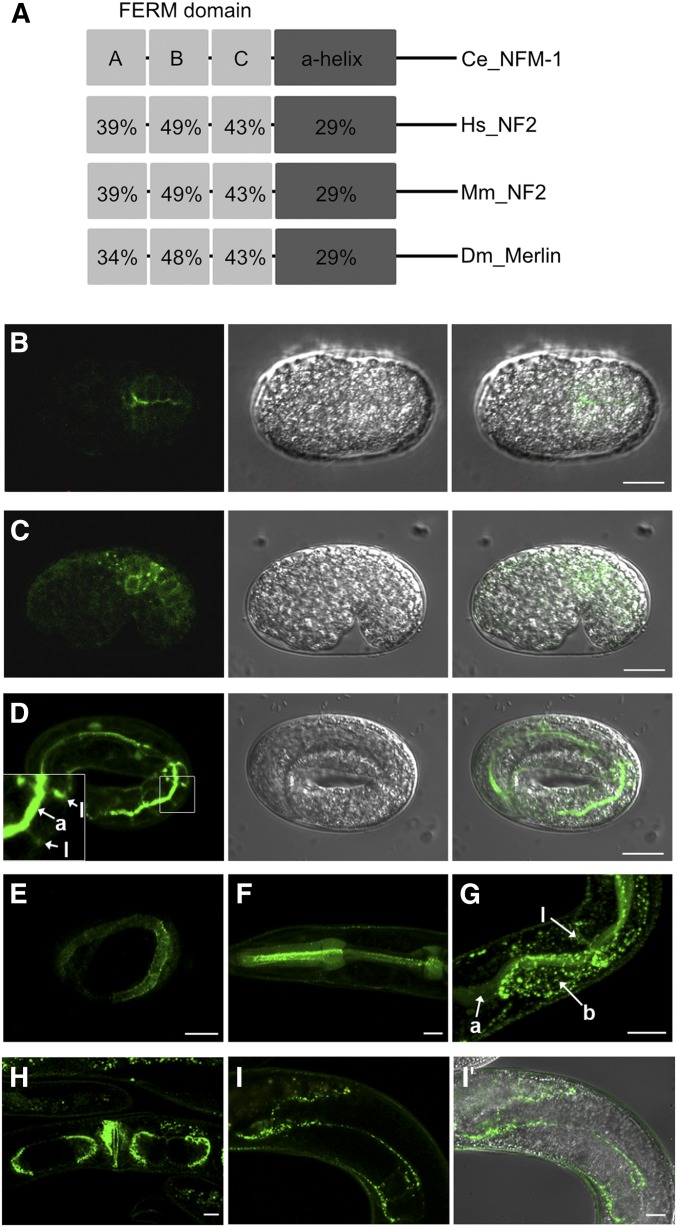
Merlin/NF2 has been suggested to act upstream of the core kinase components of the Hippo pathway in a cooperative manner with Expanded and Kibra, but the molecular mechanism of how these upstream components activate downstream kinase cascades was not clear (Hamaratoglu et al. 2006; Baumgartner et al. 2010; Genevet et al. 2010; Yu et al. 2010; Zhang et al. 2010). A recent study shows that Merlin and Kibra colocalize at the apical membrane of epithelial cells and recruit Hpo kinase to the apical membrane for activation in parallel to Extended (Su et al. 2017). C. elegans has one homolog of NF2/Merlin, NFM-1 (Göbel et al. 2004; Josephson et al. 2017). NFM-1 shares some characteristics of the Merlin/NF2, including the FERM domain at the N-terminus following the α helix domain (Figure 4A). Previous studies showed that NFM-1 is expressed in several tissues including intestine, body wall muscle, and hypodermis, and has a cell nonautonomous function in migration of Q neuroblasts (Josephson et al. 2017). Within cells, NFM-1 is localized in the basolateral domain of epithelial tissues, but its precise function in the epithelial cell has not yet been determined (Göbel et al. 2004). We observed that NFM-1 translational reporter showed expression in various epithelial tissues, including intestine and hypodermis, as well as in gonad sheath cells (Figure 4, B–I). It was also localized subcellularly in both apical and basolateral membranes of the intestine (Figure 4, D and G).
Figure 4.
NFM-1, the NF2/Merlin homolog, is expressed in various tissues including intestine (A) The structure comparison of NF2/Merlin and NFM-1 genes. Numbers: amino acid identity with NFM-1. (B–I, I′) Expression pattern of translational reporter of NFM-1. (B–D) NFM-1::GFP expression in intestine of embryos. NFM-1 localized at apical (a) and lateral (l) membrane. (E) NFM-1::GFP expression in the hypodermal membrane of embryos. (F–I) NFM-1::GFP was expressed in the (F) pharynx, (G) intestine, (H) vulva and uterus, and (I, I′) gonad sheath cell at postembryonic stage. “a,” “b,” and “l” mean apical, basal, and lateral membrane in the intestine, respectively, in (G). Bar, 10 µm.
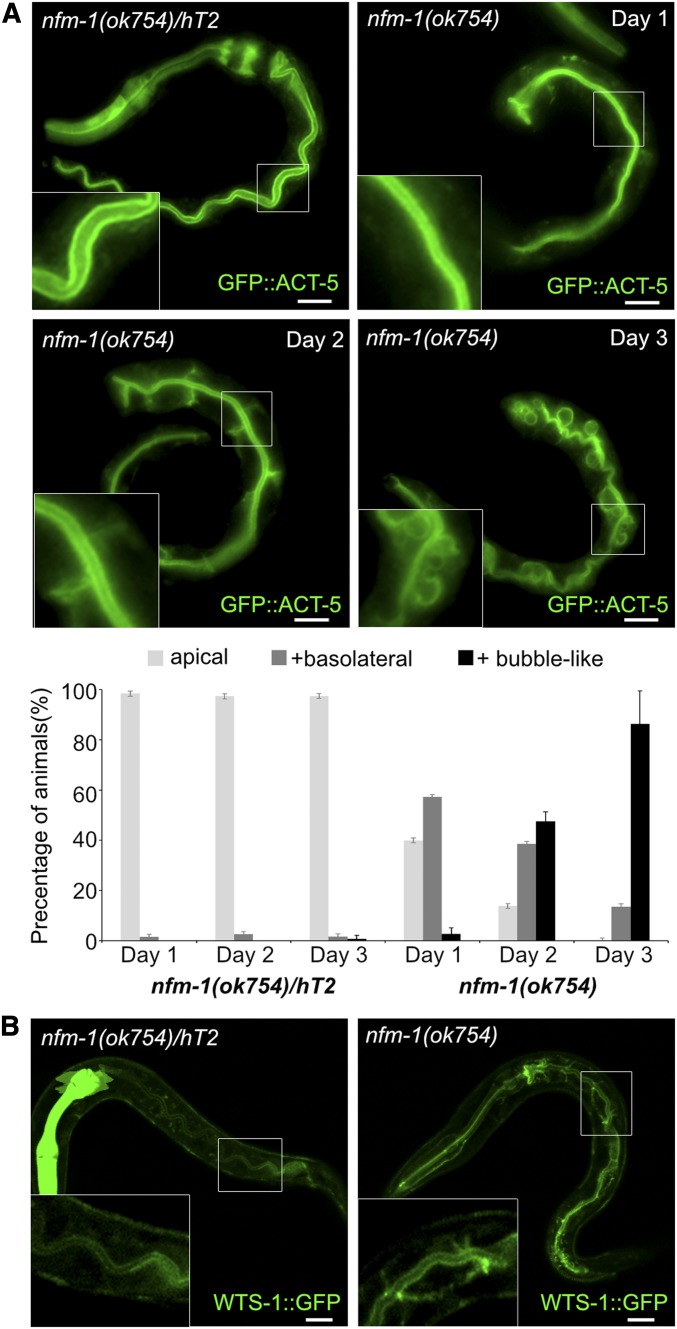
Remarkably, nfm-1(ok754), similar to wts-1(ok753), displayed a gradual conversion of apicobasal polarity and became lethal during the first larval stage (Figure 5A and Figure 6, A and B). In nfm-1 homozygote worms, intestinal cells initially showed the normal distribution of ACT-5 at the apical membrane, but gradually displayed basolateral and bubble-like distribution. This indicates that, similar to wts-1, nfm-1 activity is required to maintain intestinal polarity.
Figure 5.
NFM-1, which may be the upstream regulator of WTS-1, is involved in the maintenance of intestinal membrane polarity. (A) Defective GFP::ACT-5 localization in nfm-1 mutant. nfm-1 mutant initially established normal apical polarity, but gradually lost the polarized distribution of GFP::ACT-5 at the apical domain. Lower graph: quantified results. Worms were categorically scored and quantified as done in Figure 2B. (B) WTS-1 subcellular localization in nfm-1(ok754)/+ and nfm-1(ok754) intestines. (A and B) hT2[qIS48], which has a pharyngeal GFP signal, was used as a dominant balancer for nfm-1. The GFP signal was used as a marker to identify control heterozygotes. Bar, 10 µm.
To determine whether the polarity defects and larval lethality of nfm-1 are also consequences of misregulated YAP-1, we introduced a yap-1 mutation into nfm-1(ok754), and found that loss of yap-1 led to post-L1 development of nfm-1 mutants (Figure 6E). About 60% of nfm-1(ok754); yap-1(tm1416) double mutants developed until the late L3 stage, in which intestinal polarity defects were successfully rescued (n = 48, apical: 95.8%, apical + basolateral: 4.2%; Figure 6D), but these mutants failed to develop to the adult stage possibly because of defects in germline development (Figure 6E). Consistent with these findings, extrachromosomal expression of NFM-1 in nfm-1 mutants allowed only post-L1 development. Rescued worms developed until the late L3 stages and then died, similar to the nfm-1; yap-1 double mutants (Figure 6C). nfm-1 may have additional and essential roles for the post L3 development in the germline, and the failure to rescue might have come from transgene silencing in the germline of worms (Kelly et al. 1997). In addition, the functions of nfm-1 in the germline may be independent of YAP-1 activity. Nevertheless, our genetic data indicate that NFM-1 acts upstream of YAP-1 to maintain intestinal polarity.
To determine how NFM-1 regulates YAP-1, we observed WTS-1 localization in nfm-1(ok754). Normally, WTS-1 is present only at the subapical region of intestinal cells (Kang et al. 2009) (n = 122, apical = 100%; Figure 5B). However, in nfm-1(ok754), we found that WTS-1 was also localized in the basolateral domain of intestine (n = 75, apical = 8%, apical and basolateral = 92%; Figure 5B). This result suggests that NFM-1 acts upstream of WTS-1 to regulate YAP-1, and that the conserved NFM-1-WTS-1-YAP-1-EGL-44 pathway functions in maintaining apicobasal polarity in C. elegans intestine.
Hpo kinase-dependent phosphorylation of WTS-1 may be conserved in worms
The highly conserved MST/Hippo(Hpo) kinase functions as one of the core Hippo pathway components along with LATS/Warts and YAP/Yki. Whether the entire Hippo pathway is conserved in C. elegans remains controversial (Yang and Hata 2013). In the hypodermis, WTS-1 was the only component of the Hippo pathway that controlled YAP-1; therefore, LATS kinase, YAP, and TEAD, but no other components, have been identified as possible conserved signaling components in C. elegans (Iwasa et al. 2013; Yang and Hata 2013). RNAi against each worm homolog of the Hippo pathway components except WTS-1 failed to induce nuclear accumulation of YAP-1 in the intestine (Figure 3B and Figure S2); this observation is consistent with the results in the hypodermis. Knockdown of CST-1 and CST-2—the worm homologs of MST/Hpo kinase—only partially triggered the nuclear localization of YAP-1 in the intestine. Furthermore, a recent study shows that simultaneous loss of both CST-1 and CST-2 does not result in organismal death (Feng et al. 2017). We assumed that the loss of the worm homolog of Hippo kinase, the upstream regulator of WTS-1, should lead to polarity defects and larval lethality, if conserved. Thus, CST-1 and CST-2 do not appear to be parts of the Hippo pathway in C. elegans. To identify the C. elegans Hpo kinase that acts in the developing intestine, we screened all putative Ste20-like kinases of worms that are expressed in epithelial tissues, including the intestine, that are essential for viability whose deficiency triggers larval lethality. The genes that meet these criteria are gck-3, gck-4, and kin-18. The loss of yap-1 activity could not suppress the lethal phenotype of any of the mutants. However, independent genetic screening for lethal mutations detected a single point mutation (T863I) in the hydrophobic motif of WTS-1, caused early larval lethality (Figure S3, A and B). T863, along with S700, is one of two evolutionarily conserved phosphorylation residues of LATS/Warts; these two sites are essential for phosphorylation-mediated activation by MST/Hpo kinase (Chan et al. 2005; Kang et al. 2009). Although we have not identified the Hpo ortholog in C. elegans, the genetic results suggest that the phosphorylation-mediated regulatory mechanism of WTS-1 by a Hpo kinase may be conserved in the nematode.
Identification of YAP-1 and EGL-44 target genes
Our genetic data suggest that the constitutive localization of YAP-1 in the intestinal nuclei of wts-1 mutants leads to apicobasal polarity defects and organismal lethality in a manner that is dependent on EGL-44. This inference implies that the misregulated target genes of YAP-1 and EGL-44 are responsible for the polarity defects and the eventual lethality of wts-1 mutants. In other organisms, YAP/Yki and TEAD/Sd complexes in the nucleus regulate the expression of several genes like cyclin E, diap, and bantam, which promote organ growth (Harvey et al. 2003; Hu et al. 2004; Wei et al. 2007).
To identify target genes of the Hippo pathway for cellular polarity maintenance, we compared the expression levels of all the genes of yap-1(ys38) mutants to those of wild-type worms by using a microarray experiment. Nuclear localization of endogenous YAP-1 is limited to embryos and very early L1 stages (Figure 3A) (Iwasa et al. 2013), so we collected the embryos of each genotype for mRNA extraction. We found that 29 genes were significantly downregulated, and 51 were upregulated in the absence of YAP-1 activity (Table S2). TEAD/Sd is usually involved in YAP/Yki-mediated gene inductions (Lin et al. 2000), so we focused on the genes that were downregulated in yap-1(ys38). To select candidates for the Hippo pathway target genes, we measured the expression levels of these genes in the different mutant alleles of yap-1(tm1416), egl-44(ys39), and egl-44(ys41). Eight genes of the top-ranked 20 genes were consistently downregulated in all yap-1 and egl-44 mutants (Figures S4 and S5, A and B). Among the eight genes, TAT-2 was the only candidate for which the intestinal function is known and mutants were available. In addition, TAT-2 expression level was increased in larva that lacked wts-1 (Figure S5C).
The tat-2 mutant was previously isolated by genetic screening for suppressors of sphingolipid deficiency-mediated larval death (Seamen et al. 2009). sptl-1 encodes a putative serine palmitoyltransferase, and its activity is required for sphingolipid synthesis (Zhang et al. 2011). Knockdown of sptl-1 by RNAi caused defects in polarized protein sorting in the growing intestine, which eventually led to early larval death (Seamen et al. 2009; Zhang et al. 2011). Introduction of a tat-2 mutation into this sphingolipid-deficient worm caused it to bypass organismal lethality without restoration of lipid synthesis (Seamen et al. 2009). Interestingly, we found that the loss of YAP-1 also partially suppressed lethality of sphingolipid deficiency (Figure S6). These results indicate that TAT-2 is one of the possible target genes of YAP-1 and EGL-44 responsible for defective cell polarity.
We speculated that ectopically increased TAT-2 was responsible for the disruption of the apicobasal membrane polarity, and consequent early larval lethality of wts-1 mutants. To test this hypothesis, we introduced a tat-2 mutation into wts-1 mutants. However, the loss of tat-2 alone failed to suppress the larval lethality of wts-1 mutant; this result indicates that tat-2 is not the only target gene. It suggests that defective polarity of the intestinal membrane requires upregulation of other target genes than tat-2 by improperly activated YAP-1.
Discussion
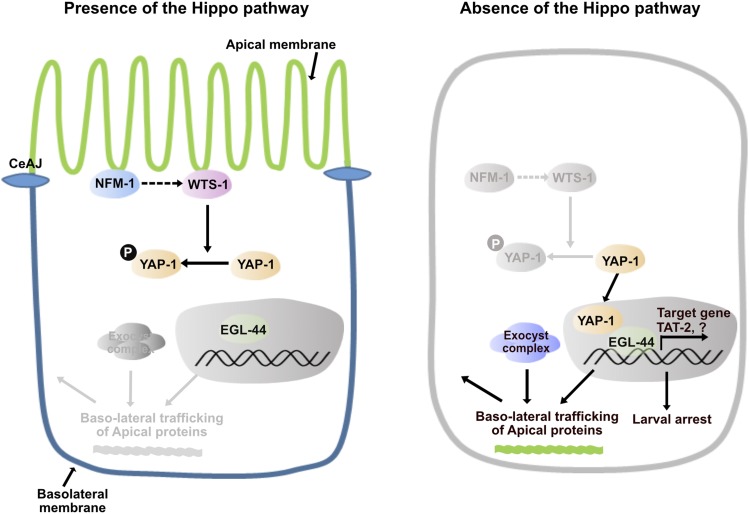
In expanding epithelial membranes, the polarized sorting of newly synthesized proteins to distinct membrane domains is essential for the maintenance of structural integrity of the membranes and for the survival of the organism. In the developing intestine of C. elegans, WTS-1, the worm homolog of LATS kinase, is necessary for sorting of newly produced apical proteins to the apical membrane domain (Kang et al. 2009). Loss of WTS-1 activity causes basolateral sorting of several apical proteins, resulting in membrane disintegration and organismal death. This abrogated protein sorting occurs in an exocytosis-dependent manner since knockdown of the exocyst complex genes suppresses protein mislocalization of wts-1 mutant (Kang et al. 2009). In this study, we have shown that the conserved Hippo pathway, consisting of NFM-1, WTS-1, YAP-1, and EGL-44, is functional in worms and that the spatio-temporal regulation of YAP-1, by upstream signaling through NFM-1 and WTS-1, is essential to maintain apicobasal membrane polarity in the developing intestine. In the absence of the Hippo pathway, disinhibited YAP-1 enters the nucleus and causes malfunctions in the EGL-44-mediated transcriptional regulation. As a result, dysregulated targets including TAT-2 may lead to basolateral trafficking of newly synthesized apical proteins possibly by affecting membrane identities of growing intestine and result in consequent failure of lumenogenesis and death of the organism (Figure 7).
Figure 7.
The working model of the Hippo pathway in the cellular polarity maintenance based on our genetic studies After the C. elegans adherens junction (CeAJ) forms, WTS-1 is localized to the subapical membrane, possibly in a manner that depends on NFM-1, and may be activated. Activated WTS-1 phosphorylates YAP-1 and inhibits its nuclear localization. In the nucleus, EGL-44 alone cannot induce expression of its target genes. In the absence of the Hippo pathway (e.g., in nfm-1, wts-1 mutants), YAP-1 entry to the nucleus, and interaction of YAP-1 with EGL-44, are not restricted. YAP-1-EGL-44 ectopically induces target gene expression, including TAT-2. Misregulated target genes may influence the identities of the plasma membrane domain; consequently, polarized sorting of newly produced proteins to the distinct domain is abrogated. Exocytosis-mediated abnormal sorting of apical proteins such as ACT-5 to the basolateral region leads to disintegration of intestinal membranes and organismal death.
Our genetic screens repeatedly identified mutations in yap-1 and egl-44 as downstream suppressors of the wts-1 mutation. Although one cannot rule out the possibility that unrevealed WTS-1 downstream factors other than yap-1 and egl-44 exist because larval viability was not fully restored in the suppressor lines, it is obvious that YAP-1 and EGL-44 are the major, if not sole, effectors of WTS-1 in the maintenance of apicobasal polarity. YAP-1 and EGL-44 are worm homologs of YAP/Yki and TEAD/Sd, which are known downstream factors of LATS/Warts kinase. A deletion mutation in yap-1, tm1416, also suppresses the phenotype of wts-1 mutants. However, egl-44(n1080), in which EGL-44-mediated neurogenesis is defective (Vassilev et al. 2001), failed to suppress the wts-1 mutant phenotype. The C-terminal region of TEAD/Sd is responsible for binding to YAP/Yki (Hao et al. 2008). Therefore, an EGL-44 protein with an abrogated C terminal in egl-44(ys39) and egl-44(ys41) might be more defective in binding with YAP-1 than is an EGL-44 that has a missense mutation in its fifth exon in egl-44(n1080).
The WW domain of YAP is a well-known protein motif that binds the PPxY motif found in several components of the Hippo pathway, including LATS kinase. These proteins bind and inactivate YAP in manners that are both phosphorylation-dependent (Huang et al. 2005) and phosphorylation-independent (Oh et al. 2009). In our suppressor lines ys34 and ys37, which lack WTS-1, the imperfect YAP-1 that contains the WW domain might compete with the intact YAP-1 for binding with the PPxY motif of other unknown binding factors and display dominant effects. Consistently, cell-line experiments showed that C terminal YAP that contains the WW domain has dominant negative effects (Yagi et al. 1999). All of these data suggest the presence of unknown binding partners containing PPxY motifs that positively regulate YAP-1. While several inhibitory upstream regulators of YAP/Yki have been identified, including components of the Hippo pathway, positive regulators of YAP/Yki are relatively unknown. Angiomotin (Amot), a member of motin family, interacts directly with the WW domain of YAP through the PPxY motif, and positively regulates YAP by inhibiting the interaction with LATS1 (Yi et al. 2013). Since Amot has also been suggested to function upstream of the Hippo pathway as a negative regulator of YAP (Zhao et al. 2011), the proposed function of Amot as a cofactor of YAP-TEAD complex seems controversial. The mode of regulation of YAP by Amot may depend on the context. Overall, our results suggest that C. elegans has unknown factors that act as positive regulators by direct interaction with YAP-1.
Activated LATS kinase is known to phosphorylate and inhibit YAP by sequestering it in the cytoplasm (Zhao et al. 2007). In organisms studied so far, nuclear localization of YAP is restricted to early developmental stages. In C. elegans, intestinal YAP-1 was transiently observed in the nucleus only in late embryos and early larvae, then sequestered in the cytoplasm during the larval stages. We did not observe any significant phenotypic defects in yap-1 or egl-44 single mutants, possibly due to genetic redundancy.
Loss of WTS-1 activity led to abnormal accumulation of YAP-1 in the intestinal nuclei and the luminal membranes. An interesting feature of nuclear YAP-1 is that it was highly observed in clusters. Although the physiological meaning of its nuclear clustering is not clear, a recent study using super-resolution microscopy showed that, in several mammalian cells, nuclear YAP is distributed mainly in the clusters, and upstream signal weaken clustering and transcriptional activity of YAP (Gao et al. 2017). This suggests subnuclear distribution of YAP as a new layer of the regulatory mechanism of YAP, and our observation implies that this mechanism might exist in worms.
Polarity defects and lethality in wts-1 mutants require the activity of EGL-44, the C. elegans homolog of TEAD/Sd, so dysregulation of YAP-1 and EGL-44 target genes in the nucleus is responsible for these defects. A comparative gene expression study identified 29 genes that showed decreased expression in the absence of YAP-1. Among these genes, tat-2 was proposed to function in the regulation of postembryonic growth (Seamen et al. 2009), thus it was suspected to be a target of YAP-1 and EGL-44. Consistently, expression of tat-2 was decreased in yap-1 and egl-44 mutants, and increased in the absence of WTS-1. In addition, similarly to tat-2(tm2332), yap-1(tm1416) suppressed larval lethality mediated by sphingolipid deficiency, suggesting that the effect of yap-1 loss is similar to that of tat-2 loss. Although our genetic data showed that loss of tat-2 alone was not enough to suppress larval lethality of wts-1, tat-2 is still one of target genes of YAP-1 and EGL-44. Tight regulation of TAT-2 expression by WTS-1 may allow animals to maintain apicobasal polarity of the intestinal membrane; this polarity is essential for the survival of the organism.
Subcellular localization of YAP-1 implies that YAP-1 has functions in the plasma membrane as well as in the nucleus. In embryos, YAP-1 is localized in the membrane and in the nucleus, and loss of WTS-1 triggered localization of YAP-1 in both membranes and nuclei. Alternatively, membrane localization of YAP-1 could be a result of a WTS-1-independent inhibitory mechanism. Amot has been known to inhibit YAP in two different ways; by phosphorylation and sequential activation of the Hippo pathway, or by direct binding to YAP and its sequestration of it in the membrane (Zhao et al. 2011). Studies to identify upstream factors or unknown binding partners of YAP-1 that may interact with its WW domain will help to clarify the functions of YAP-1 in the membrane.
Studies of the Hippo pathway in C. elegans have found that YAP-1 and EGL-44 act downstream of WTS-1 in C. elegans hypodermis, and that YAP-1 is involved in heat-shock response and health-span regulation (Iwasa et al. 2013). However, the conservation of the other components of the Hippo pathway was unclear (Iwasa et al. 2013; Yang and Hata 2013). We observed that NFM-1 is also involved in the maintenance of cellular polarity along with WTS-1 to regulate YAP-1; this relationship suggests extended evolutionary conservation of the Hippo pathway. Loss of NFM-1 caused defective apicobasal polarity and eventual larval death similar to the phenotypes of wts-1 mutants, in a manner that is dependent on YAP-1. Neither introduction of a yap-1 mutation nor introduction of wild-type copy of NFM-1 fully restored postlarval growth; this observation implies that NFM-1 has functions in germline development in a manner that is independent of the Hippo pathway. A recent report showed NFM-1, possibly in muscles, genetically interacts with SLT-1/Slit to guide Q neuroblast migration. Intriguingly, a fosmid-derived wild-type copy of NFM-1 could fully restore viability of the nfm-1 mutant (Josephson et al. 2017). This inconsistency may occur because fosmid-derived constructs tend to be more resistant to germline suppression than a transgene array (Sarov et al. 2012)
The core kinase of the Hippo pathway is the MST/Hpo kinase, which receives signals from upstream and phosphorylates and activates LATS/warts kinase. However, loss of Hpo kinase homologs, CST-1 and CST-2, only partially induced the nuclear localization of YAP-1 and did not lead to larval death. We screened several Ste20-like kinases for which activity deficit results in larval death; the loss of yap-1 could not suppress the lethality of any mutants. Despite many attempts, we were unable to identify the worm homolog of the Hpo kinase that acts in maintenance of membrane polarity. However, the evolutionary conservation of the phosphorylation residue in WTS-1 implies that an unknown Hpo kinase homolog functions in activation of WTS-1 by phosphorylation. Alternatively, NFM-1 may regulate WTS-1 directly. We showed that loss of NFM-1 causes abnormal distributions of WTS-1 in the plasma membrane. Our observation is consistent with the previous observation in mammals and Drosophila, in which NF2/Merlin directly binds to and recruits LATS/Warts kinase to the membrane, leading to the phosphorylation and activation of LATS/Warts (Yin et al. 2013). It is also possible that mislocalization of WTS-1 in nfm-1 mutant is a indirect consequence resulted from defective membrane polarity rather than a direct outcome of the loss of nfm-1. Therefore, further studies should be conducted to identify the factors that phosphorylate and activate WTS-1 in C. elegans. Nevertheless, evidence from our genetic studies demonstrates the developmental function of the conserved Hippo pathway in maintaining the cellular polarity of expanding membranes. Our comparative gene expression analysis also suggests a molecular mechanism for the maintenance of membrane-domain identities.
The function of the Hippo pathway in the apicobasal polarity regulation suggests several clinical implications in polarity-associated diseases. First, worms that are deficient in the Hippo pathway have a multiple lumen-like structure phenotype, which is similar to those in patients of microvilli inclusion disease (MVID). Two disease responsible genes, myosin VB (MYO5B) and syntaxin 3 (STX3), are required for apical exocytosis of several apical proteins. More importantly, Myo5bKD human colon cells leads to nuclear retention of YAP and to crypt-like phenotype (Wiegerinck et al. 2014; Kravtsov et al. 2016; Vogel et al. 2017). These observations imply the involvement of the Hippo pathway in MVID. Second, the Hippo pathway is required for proper development of kidneys, and its deficiency is relevant to various kidney diseases including polycystic kidney disease (PKD), in which epithelial cysts replace normal renal tubes (Happé et al. 2011; Reginensi et al. 2013; Seo et al. 2016; Jiang et al. 2017; Kunnen et al. 2018; Xu et al. 2018). Patients with autosomal dominant (AD) PKD show defective membrane polarity, mislocalization of apical proteins to the basolateral domain, and mislocalization of normally basolateral proteins to the apical, luminal membrane (Wilson et al. 1991; Du and Wilson 1995; Lebeau et al. 2002). Two genes, PKD1 and PKD2 are responsible for ADPKD, and a recent study identified the RhoA-YAP1-c-Myc axis as a direct effector of PKD1 deficiency in ADPKD (Cai et al. 2018). We have observed that the conserved Hippo pathway acts in the maintenance of apicobasal polarity of epithelial tissues by regulating several putative target genes; this information will help to develop understanding of the mechanism of ADPKD pathogenesis, and may guide identification of therapeutic targets.
Acknowledgments
We thank A. Fire for the worm expressing vectors, and J. Ahringer and M. Vidal for RNAi plasmids. We also thank the Caenorhabditis Genetics Center and the National BioResource Project for providing strains. This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) (NRF-2014R1A4A1005259) and by the Samsung Science and Technology Foundation under Project Number SSTF-BA1501-04. H.L. was supported by a scholarship for basic research, Seoul National University, Seoul, Korea.
Footnotes
Supplemental material available at FigShare: https://doi.org/10.25386/genetics.8993831.
Communicating editor: B. Goldstein
Literature Cited
- Baumgartner R., Poernbacher I., Buser N., Hafen E., and Stocker H., 2010. The WW domain protein Kibra acts upstream of Hippo in Drosophila. Dev. Cell 18: 309–316. 10.1016/j.devcel.2009.12.013 [DOI] [PubMed] [Google Scholar]
- Bidlingmaier S., Weiss E. L., Seidel C., Drubin D. G., and Snyder M., 2001. The Cbk1p pathway is important for polarized cell growth and cell separation in Saccharomyces cerevisiae. Mol. Cell. Biol. 21: 2449–2462. 10.1128/MCB.21.7.2449-2462.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilder D., and Perrimon N., 2000. Localization of apical epithelial determinants by the basolateral PDZ protein Scribble. Nature 403: 676–680. 10.1038/35001108 [DOI] [PubMed] [Google Scholar]
- Boggiano J. C., and Fehon R. G., 2012. Growth control by committee: intercellular junctions, cell polarity, and the cytoskeleton regulate Hippo signaling. Dev. Cell 22: 695–702. 10.1016/j.devcel.2012.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brace J., Hsu J., and Weiss E. L., 2011. Mitotic exit control of the Saccharomyces cerevisiae Ndr/LATS kinase Cbk1 regulates daughter cell separation after cytokinesis. Mol. Cell. Biol. 31: 721–735. 10.1128/MCB.00403-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brenner S., 1974. The genetics of Caenorhabditis elegans. Genetics 77: 71–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant D. M., Datta A., Rodriguez-Fraticelli A. E., Peranen J., Martin-Belmonte F. et al. , 2010. A molecular network for de novo generation of the apical surface and lumen. Nat. Cell Biol. 12: 1035–1045. 10.1038/ncb2106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J., Song X., Wang W., Watnick T., Pei Y. et al. , 2018. A RhoA-YAP-c-Myc signaling axis promotes the development of polycystic kidney disease. Genes Dev. 32: 781–793. 10.1101/gad.315127.118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai Q., Wang W., Gao Y., Yang Y., Zhu Z. et al. , 2009. Ce-wts-1 plays important roles in Caenorhabditis elegans development. FEBS Lett. 583: 3158–3164. 10.1016/j.febslet.2009.09.002 [DOI] [PubMed] [Google Scholar]
- Chan E. H., Nousiainen M., Chalamalasetty R. B., Schafer A., Nigg E. A. et al. , 2005. The Ste20-like kinase Mst2 activates the human large tumor suppressor kinase Lats1. Oncogene 24: 2076–2086. 10.1038/sj.onc.1208445 [DOI] [PubMed] [Google Scholar]
- Chen C. L., Gajewski K. M., Hamaratoglu F., Bossuyt W., Sansores-Garcia L. et al. , 2010. The apical-basal cell polarity determinant Crumbs regulates Hippo signaling in Drosophila. Proc. Natl. Acad. Sci. USA 107: 15810–15815. 10.1073/pnas.1004060107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das M., Wiley D. J., Chen X., Shah K., and Verde F., 2009. The conserved NDR kinase Orb6 controls polarized cell growth by spatial regulation of the small GTPase Cdc42. Curr. Biol. 19: 1314–1319. 10.1016/j.cub.2009.06.057 [DOI] [PubMed] [Google Scholar]
- Du J., and Wilson P. D., 1995. Abnormal polarization of EGF receptors and autocrine stimulation of cyst epithelial growth in human ADPKD. Am. J. Physiol. 269: C487–C495. 10.1152/ajpcell.1995.269.2.C487 [DOI] [PubMed] [Google Scholar]
- Enderle L., and McNeill H., 2013. Hippo gains weight: added insights and complexity to pathway control. Sci. Signal. 6: re7 10.1126/scisignal.2004208 [DOI] [PubMed] [Google Scholar]
- Feng G., Zhu Z., Li W. J., Lin Q., Chai Y. et al. , 2017. Hippo kinases maintain polarity during directional cell migration in Caenorhabditis elegans. EMBO J. 36: 334–345. 10.15252/embj.201695734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao J., He L., Shi Y., Cai M., Xu H. et al. , 2017. Cell contact and pressure control of YAP localization and clustering revealed by super-resolution imaging. Nanoscale 9: 16993–17003. 10.1039/C7NR05818G [DOI] [PubMed] [Google Scholar]
- Genevet A., Polesello C., Blight K., Robertson F., Collinson L. M. et al. , 2009. The Hippo pathway regulates apical-domain size independently of its growth-control function. J. Cell Sci. 122: 2360–2370. 10.1242/jcs.041806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genevet A., Wehr M. C., Brain R., Thompson B. J., and Tapon N., 2010. Kibra is a regulator of the Salvador/Warts/Hippo signaling network. Dev. Cell 18: 300–308. 10.1016/j.devcel.2009.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Göbel V., Barrett P. L., Hall D. H., and Fleming J. T., 2004. Lumen morphogenesis in C. elegans requires the membrane-cytoskeleton linker erm-1. Dev. Cell 6: 865–873. 10.1016/j.devcel.2004.05.018 [DOI] [PubMed] [Google Scholar]
- Grzeschik N. A., Parsons L. M., Allott M. L., Harvey K. F., and Richardson H. E., 2010. Lgl, aPKC, and crumbs regulate the salvador/Warts/Hippo pathway through two distinct mechanisms. Curr. Biol. 20: 573–581. 10.1016/j.cub.2010.01.055 [DOI] [PubMed] [Google Scholar]
- Hamaratoglu F., Willecke M., Kango-Singh M., Nolo R., Hyun E. et al. , 2006. The tumour-suppressor genes NF2/Merlin and Expanded act through Hippo signalling to regulate cell proliferation and apoptosis. Nat. Cell Biol. 8: 27–36. 10.1038/ncb1339 [DOI] [PubMed] [Google Scholar]
- Hamaratoglu F., Gajewski K., Sansores-Garcia L., Morrison C., Tao C. et al. , 2009. The Hippo tumor-suppressor pathway regulates apical-domain size in parallel to tissue growth. J. Cell Sci. 122: 2351–2359. 10.1242/jcs.046482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao Y., Chun A., Cheung K., Rashidi B., and Yang X., 2008. Tumor suppressor LATS1 is a negative regulator of oncogene YAP. J. Biol. Chem. 283: 5496–5509. 10.1074/jbc.M709037200 [DOI] [PubMed] [Google Scholar]
- Happé H., van der Wal A. M., Leonhard W. N., Kunnen S. J., Breuning M. H. et al. , 2011. Altered Hippo signalling in polycystic kidney disease. J. Pathol. 224: 133–142. 10.1002/path.2856 [DOI] [PubMed] [Google Scholar]
- Harvey K., and Tapon N., 2007. The Salvador-Warts-Hippo pathway - an emerging tumour-suppressor network. Nat. Rev. Cancer 7: 182–191. 10.1038/nrc2070 [DOI] [PubMed] [Google Scholar]
- Harvey K. F., Pfleger C. M., and Hariharan I. K., 2003. The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell 114: 457–467. 10.1016/S0092-8674(03)00557-9 [DOI] [PubMed] [Google Scholar]
- Hu H., Columbus J., Zhang Y., Wu D., Lian L. et al. , 2004. A map of WW domain family interactions. Proteomics 4: 643–655. 10.1002/pmic.200300632 [DOI] [PubMed] [Google Scholar]
- Huang J. B., Wu S., Barrera J., Matthews K., and Pan D. J., 2005. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila homolog of YAP. Cell 122: 421–434. 10.1016/j.cell.2005.06.007 [DOI] [PubMed] [Google Scholar]
- Hung T. J., and Kemphues K. J., 1999. PAR-6 is a conserved PDZ domain-containing protein that colocalizes with PAR-3 in Caenorhabditis elegans embryos. Development 126: 127–135. [DOI] [PubMed] [Google Scholar]
- Iwasa H., Maimaiti S., Kuroyanagi H., Kawano S., Inami K. et al. , 2013. Yes-associated protein homolog, YAP-1, is involved in the thermotolerance and aging in the nematode Caenorhabditis elegans. Exp. Cell Res. 319: 931–945. 10.1016/j.yexcr.2013.01.020 [DOI] [PubMed] [Google Scholar]
- Izumi Y., Hirose T., Tamai Y., Hirai S., Nagashima Y. et al. , 1998. An atypical PKC directly associates and colocalizes at the epithelial tight junction with ASIP, a mammalian homologue of Caenorhabditis elegans polarity protein PAR-3. J. Cell Biol. 143: 95–106. 10.1083/jcb.143.1.95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L., Sun L., Edwards G., Manley M. Jr., Wallace D. P. et al. , 2017. Increased YAP activation is associated with hepatic cyst epithelial cell proliferation in ARPKD/CHF. Gene Expr. 17: 313–326. 10.3727/105221617X15034976037343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Josephson M. P., Aliani R., Norris M. L., Ochs M. E., Gujar M. et al. , 2017. The Caenorhabditis elegans NF2/merlin molecule NFM-1 nonautonomously regulates neuroblast migration and interacts genetically with the guidance cue SLT-1/slit. Genetics 205: 737–748. 10.1534/genetics.116.191957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J., Shin D., Yu J. R., and Lee J., 2009. Lats kinase is involved in the intestinal apical membrane integrity in the nematode Caenorhabditis elegans. Development 136: 2705–2715. 10.1242/dev.035485 [DOI] [PubMed] [Google Scholar]
- Kelly W. G., Xu S., Montgomery M. K., and Fire A., 1997. Distinct requirements for somatic and germline expression of a generally expressed Caernorhabditis elegans gene. Genetics 146: 227–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kravtsov D. V., Ahsan M. K., Kumari V., van Ijzendoorn S. C., Reyes-Mugica M. et al. , 2016. Identification of intestinal ion transport defects in microvillus inclusion disease. Am. J. Physiol. Gastrointest. Liver Physiol. 311: G142–G155. 10.1152/ajpgi.00041.2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuchinke U., Grawe F., and Knust E., 1998. Control of spindle orientation in Drosophila by the Par-3-related PDZ-domain protein Bazooka. Curr. Biol. 8: 1357–1365. 10.1016/S0960-9822(98)00016-5 [DOI] [PubMed] [Google Scholar]
- Kunnen S. J., Malas T. B., Formica C., Leonhard W. N., ’t Hoen P. A. C. et al. , 2018. Comparative transcriptomics of shear stress treated Pkd1−/− cells and pre-cystic kidneys reveals pathways involved in early polycystic kidney disease. Biomed. Pharmacother. 108: 1123–1134. 10.1016/j.biopha.2018.07.178 [DOI] [PubMed] [Google Scholar]
- Lebeau C., Hanaoka K., Moore-Hoon M. L., Guggino W. B., Beauwens R. et al. , 2002. Basolateral chloride transporters in autosomal dominant polycystic kidney disease. Pflugers Arch. 444: 722–731. 10.1007/s00424-002-0880-3 [DOI] [PubMed] [Google Scholar]
- Lin D., Edwards A. S., Fawcett J. P., Mbamalu G., Scott J. D. et al. , 2000. A mammalian PAR-3-PAR-6 complex implicated in Cdc42/Rac1 and aPKC signalling and cell polarity. Nat. Cell Biol. 2: 540–547. 10.1038/35019582 [DOI] [PubMed] [Google Scholar]
- Ling C., Zheng Y. G., Yin F., Yu J. Z., Huang J. A. et al. , 2010. The apical transmembrane protein Crumbs functions as a tumor suppressor that regulates Hippo signaling by binding to Expanded. Proc. Natl. Acad. Sci. USA 107: 10532–10537. 10.1073/pnas.1004279107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu R., and Wilson J. M., 2016. Rab14 specifies the apical membrane through Arf6-mediated regulation of lipid domains and Cdc42. Sci. Rep. 6: 38249 10.1038/srep38249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellman I., and Nelson W. J., 2008. Coordinated protein sorting, targeting and distribution in polarized cells. Nat. Rev. Mol. Cell Biol. 9: 833–845. 10.1038/nrm2525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson B., Kurischko C., Horecka J., Mody M., Nair P. et al. , 2003. RAM: a conserved signaling network that regulates Ace2p transcriptional activity and polarized morphogenesis. Mol. Biol. Cell 14: 3782–3803. 10.1091/mbc.e03-01-0018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh H., and Irvine K. D., 2010. Yorkie: the final destination of Hippo signaling. Trends Cell Biol. 20: 410–417. 10.1016/j.tcb.2010.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh H., Reddy B. V., and Irvine K. D., 2009. Phosphorylation-independent repression of Yorkie in Fat-Hippo signaling. Dev. Biol. 335: 188–197. 10.1016/j.ydbio.2009.08.026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reginensi A., Scott R. P., Gregorieff A., Bagherie-Lachidan M., Chung C. et al. , 2013. Yap- and Cdc42-dependent nephrogenesis and morphogenesis during mouse kidney development. PLoS Genet. 9: e1003380 10.1371/journal.pgen.1003380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson B. S., Huang J., Hong Y., and Moberg K. H., 2010. Crumbs regulates Salvador/Warts/Hippo signaling in Drosophila via the FERM-domain protein Expanded. Curr. Biol. 20: 582–590. 10.1016/j.cub.2010.03.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Román-Fernández A., Roignot J., Sandilands E., Nacke M., Mansour M. A. et al. , 2018. The phospholipid PI(3,4)P2 is an apical identity determinant. Nat. Commun. 9: 5041 10.1038/s41467-018-07464-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarov M., Murray J. I., Schanze K., Pozniakovski A., Niu W. et al. , 2012. A genome-scale resource for in vivo tag-based protein function exploration in C. elegans. Cell 150: 855–866. 10.1016/j.cell.2012.08.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seamen E., Blanchette J. M., and Han M., 2009. P-type ATPase TAT-2 negatively regulates monomethyl branched-chain fatty acid mediated function in post-embryonic growth and development in C. elegans. PLoS Genet. 5: e1000589 10.1371/journal.pgen.1000589 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seo E., Kim W. Y., Hur J., Kim H., Nam S. A. et al. , 2016. The Hippo-Salvador signaling pathway regulates renal tubulointerstitial fibrosis. Sci. Rep. 6: 31931 10.1038/srep31931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafaq-Zadah M., Brocard L., Solari F., and Michaux G., 2012. AP-1 is required for the maintenance of apico-basal polarity in the C. elegans intestine. Development 139: 2061–2070. 10.1242/dev.076711 [DOI] [PubMed] [Google Scholar]
- St Johnston D., and Ahringer J., 2010. Cell polarity in eggs and epithelia: parallels and diversity. Cell 141: 757–774. 10.1016/j.cell.2010.05.011 [DOI] [PubMed] [Google Scholar]
- Su T., Ludwig M. Z., Xu J. J., and Fehon R. G., 2017. Kibra and Merlin activate the hippo pathway spatially distinct from and independent of expanded. Dev. Cell 40: 478–490.e3. 10.1016/j.devcel.2017.02.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki A., Yamanaka T., Hirose T., Manabe N., Mizuno K. et al. , 2001. Atypical protein kinase C is involved in the evolutionarily conserved PAR protein complex and plays a critical role in establishing epithelia-specific junctional structures. J. Cell Biol. 152: 1183–1196. 10.1083/jcb.152.6.1183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabuse Y., Izumi Y., Piano F., Kemphues K. J., Miwa J. et al. , 1998. Atypical protein kinase C cooperates with PAR-3 to establish embryonic polarity in Caenorhabditis elegans. Development 125: 3607–3614. [DOI] [PubMed] [Google Scholar]
- Tay Y. D., Leda M., Spanos C., Rappsilber J., Goryachev A. B. et al. , 2019. Fission yeast NDR/LATS kinase Orb6 regulates exocytosis via phosphorylation of the exocyst complex. Cell Rep. 26: 1654–1667.e7. 10.1016/j.celrep.2019.01.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tepass U., Theres C., and Knust E., 1990. Crumbs encodes an EGF-like protein expressed on apical membranes of Drosophila epithelial cells and required for organization of epithelia. Cell 61: 787–799. 10.1016/0092-8674(90)90189-L [DOI] [PubMed] [Google Scholar]
- Vassilev A., Kaneko K. J., Shu H., Zhao Y., and DePamphilis M. L., 2001. TEAD/TEF transcription factors utilize the activation domain of YAP65, a Src/Yes-associated protein localized in the cytoplasm. Genes Dev. 15: 1229–1241. 10.1101/gad.888601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verde F., Wiley D. J., and Nurse P., 1998. Fission yeast orb6, a ser/thr protein kinase related to mammalian rho kinase and myotonic dystrophy kinase, is required for maintenance of cell polarity and coordinates cell morphogenesis with the cell cycle. Proc. Natl. Acad. Sci. USA 95: 7526–7531. 10.1073/pnas.95.13.7526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel G. F., van Rijn J. M., Krainer I. M., Janecke A. R., Posovszky C. et al. , 2017. Disrupted apical exocytosis of cargo vesicles causes enteropathy in FHL5 patients with Munc18–2 mutations. JCI Insight 2: 94564 10.1172/jci.insight.94564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei X., Shimizu T., and Lai Z. C., 2007. Mob as tumor suppressor is activated by Hippo kinase for growth inhibition in Drosophila. EMBO J. 26: 1772–1781. 10.1038/sj.emboj.7601630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegerinck C. L., Janecke A. R., Schneeberger K., Vogel G. F., van Haaften-Visser D. Y. et al. , 2014. Loss of syntaxin 3 causes variant microvillus inclusion disease. Gastroenterology 147: 65–68.e10. 10.1053/j.gastro.2014.04.002 [DOI] [PubMed] [Google Scholar]
- Wilson P. D., Sherwood A. C., Palla K., Du J., Watson R. et al. , 1991. Reversed polarity of Na(+) -K(+) -ATPase: mislocation to apical plasma membranes in polycystic kidney disease epithelia. Am. J. Physiol. 260: F420–F430. [DOI] [PubMed] [Google Scholar]
- Wodarz A., Ramrath A., Kuchinke U., and Knust E., 1999. Bazooka provides an apical cue for Inscuteable localization in Drosophila neuroblasts. Nature 402: 544–547. 10.1038/990128 [DOI] [PubMed] [Google Scholar]
- Wu J., Duggan A., and Chalfie M., 2001. Inhibition of touch cell fate by egl-44 and egl-46 in C. elegans. Genes Dev. 15: 789–802. 10.1101/gad.857401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D., Lv J., He L., Fu L., Hu R. et al. , 2018. Scribble influences cyst formation in autosomal-dominant polycystic kidney disease by regulating Hippo signaling pathway. FASEB J. 32: 4394–4407. 10.1096/fj.201701376RR [DOI] [PubMed] [Google Scholar]
- Yagi R., Chen L. F., Shigesada K., Murakami Y., and Ito Y., 1999. A WW domain-containing yes-associated protein (YAP) is a novel transcriptional co-activator. EMBO J. 18: 2551–2562. 10.1093/emboj/18.9.2551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., and Hata Y., 2013. What is the Hippo pathway? Is the Hippo pathway conserved in Caenorhabditis elegans? J. Biochem. 154: 207–209. 10.1093/jb/mvt060 [DOI] [PubMed] [Google Scholar]
- Yi C., Shen Z., Stemmer-Rachamimov A., Dawany N., Troutman S. et al. , 2013. The p130 isoform of angiomotin is required for Yap-mediated hepatic epithelial cell proliferation and tumorigenesis. Sci. Signal. 6: ra77 10.1126/scisignal.2004060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin F., Yu J., Zheng Y., Chen Q., Zhang N. et al. , 2013. Spatial organization of Hippo signaling at the plasma membrane mediated by the tumor suppressor Merlin/NF2. Cell 154: 1342–1355. 10.1016/j.cell.2013.08.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Zheng Y., Dong J., Klusza S., Deng W. M. et al. , 2010. Kibra functions as a tumor suppressor protein that regulates Hippo signaling in conjunction with Merlin and Expanded. Dev. Cell 18: 288–299. 10.1016/j.devcel.2009.12.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H., Abraham N., Khan L. A., Hall D. H., Fleming J. T. et al. , 2011. Apicobasal domain identities of expanding tubular membranes depend on glycosphingolipid biosynthesis. Nat. Cell Biol. 13: 1189–1201. 10.1038/ncb2328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N., Bai H., David K. K., Dong J., Zheng Y. et al. , 2010. The Merlin/NF2 tumor suppressor functions through the YAP oncoprotein to regulate tissue homeostasis in mammals. Dev. Cell 19: 27–38. 10.1016/j.devcel.2010.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B., Wei X., Li W., Udan R. S., Yang Q. et al. , 2007. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes Dev. 21: 2747–2761. 10.1101/gad.1602907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B., Li L., Lei Q. Y., and Guan K. L., 2010a The Hippo-YAP pathway in organ size control and tumorigenesis: an updated version. Genes Dev. 24: 862–874. 10.1101/gad.1909210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B., Li L., Tumaneng K., Wang C. Y., and Guan K. L., 2010b A coordinated phosphorylation by Lats and CK1 regulates YAP stability through SCF beta-TRCP. Genes Dev. 24: 72–85. 10.1101/gad.1843810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B., Li L., Lu Q., Wang L. H., Liu C. Y. et al. , 2011. Angiomotin is a novel Hippo pathway component that inhibits YAP oncoprotein. Genes Dev. 25: 51–63. 10.1101/gad.2000111 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Strains and plasmids used in this study are available upon request. Supplemental files are available at FigShare. Supplemental Material, Table S1 contains results of yeast two hybrid screening using WTS-1 as a bait. Table S2 is the list of genes whose expression was affected in the absence of YAP-1. Figure S1 shows that WTS-1 and YAP-1 bind to each other. Figure S2 shows subcellular localization of intestinal YAP-1 in worms in which Hpo pathway components were knocked down. Figure S3 shows larva arrest phenotype of wts-1(of1) which has a point mutation in the 863rd threonine residue of WTS-1. Figure S4 shows the validation of candidates for target genes of YAP-1 and EGL-44 complex. Figure S5 shows tat-2 as a candidate of yap-1 and egl-44 target. Figure S6 shows sphingolipid deficiency-mediated phenotype analysis of yap-1(tm1416). The raw data of the microarray experiment were deposited in the Gene Expression Omnibus (GEO) under the Subseries GSE133667. Supplemental material available at FigShare: https://doi.org/10.25386/genetics.8993831.