Abstract
Background
Retrospective studies have reported an association between cancer and arterial thromboembolic event (ATE) risk.
Objectives
We sought to confirm this in a prospective cohort with adjudicated outcomes.
Methods
We evaluated participants enrolled in the REGARDS (REasons for Geographic and Racial Differences in Stroke) study with Medicare coverage for 365 days before their baseline visit (2003‐2007). Medicare claims were used to identify new cancer diagnoses during follow‐up. Using incidence‐density sampling, participants who developed cancer were matched by age, sex, race, and education 1:4 to control participants who had not developed cancer. Participants were prospectively followed through 2015 for an expert‐adjudicated ATE, defined as acute myocardial infarction or ischemic stroke. Cox regression was performed to evaluate the association between incident cancer and subsequent ATE.
Results
In this analysis, 836 REGARDS participants with incident cancer were matched to 3339 control participants without cancer. In the 30 days after cancer diagnosis, 0.60% (n = 5) of the participants had an ATE; most of these events occurred near the time of cancer diagnosis. After adjustment for demographics, geographic region, and cardiovascular risk factors, compared to the noncancer controls, participants with incident cancer had an increased risk of ATE in the first 30 days after diagnosis (hazard ratio, 5.8; 95% confidence interval, 2.1‐15.9). There was no association between cancer diagnosis and ATE beyond 30 days. Cancers with known metastases and types considered high risk for venous thromboembolism had the strongest associations with ATE.
Conclusions
Incident cancer is associated with an increased short‐term risk of ATE independent of vascular risk factors.
Keywords: cancer, myocardial infarction, neoplasms, stroke, thromboembolism
Essentials.
In retrospective studies, arterial thromboembolism risk appears to increase with incident cancer.
We aimed to confirm this finding in a prospective cohort (REGARDS) with adjudicated outcomes.
Subjects with incident cancer had an increased short‐term risk of arterial thromboembolic events.
The increased risk was highest with metastases and cancers associated with venous thrombosis.
1. INTRODUCTION
It is estimated that 14.1 million people worldwide receive a new cancer diagnosis each year, and this is projected to increase 68% by 2030.1 Besides reduced survival, patients with cancer face an increased risk of disability through secondary complications. This includes venous thromboembolism, which has a well‐known relationship with cancer, and is increased approximately 7‐fold in newly diagnosed cases.2, 3 Consequently, newly diagnosed cancer patients often have risk stratification and close monitoring for venous thromboembolism, and some high‐risk ambulatory patients may even be treated with prophylactic anticoagulation.4, 5
The association between cancer and arterial thromboembolism is less established. Recently, several cohort studies reported that incident cancer is associated with a roughly 2 times higher risk of arterial thromboembolism in the 6 months after diagnosis.6, 7, 8 However, these studies relied on claims data for identifying outcome events. Therefore, the reported associations could have been biased by misclassification, especially because metastases can mimic arterial thromboembolic events, and many outcomes in these studies occurred in patients with known metastases. Furthermore, these prior claims‐based studies were unable to control for lifestyle factors, including smoking and alcohol abuse, which increase the risks of both cancer and arterial thromboembolism and therefore could confound the association between diseases.9, 10, 11
To address the limitations of prior analyses, we recently published a study using prospectively collected data, including adjudicated stroke diagnoses, from the population‐based REGARDS (REasons for Geographic and Racial Differences in Stroke) cohort to confirm that incident cancer, particularly when metastatic, is associated with a substantially increased short‐term risk of ischemic or hemorrhagic cerebrovascular events independent of vascular risk factors.12 In this follow‐up analysis, we examined whether incident cancer is also an independent risk factor for arterial thromboembolic events (ATEs) more broadly, which we defined as fatal or nonfatal acute myocardial infarction or ischemic stroke.
2. METHODS
2.1. Design
This was a matched cohort study that used linked data from the REGARDS study and Medicare claims. REGARDS is an ongoing, nationwide, population‐based, prospective cohort study with adjudicated ascertainment of cerebrovascular and coronary heart disease events.13 Between 2003 and 2007, REGARDS study investigators enrolled 30 239 participants aged ≥45 years. Blacks and adults living in the Stroke Belt, a region in the southeastern United States with increased stroke mortality, were oversampled.14 Medicare is the federal health insurance program for US adults aged ≥65 years and younger adults with select comorbidities or disabilities. Participating institutions approved the REGARDS study, and all participants provided written informed consent, including for data linkage with Medicare claims.
2.2. Population
This analysis included participants who had continuous fee‐for‐service Medicare coverage (Parts A and B but not C) for at least 1 year before their REGARDS baseline in‐home study visit. Most REGARDS participants (67%) had some linked Medicare data. Baseline characteristics were similar between participants linked and not linked to Medicare, except those not linked were less often women and white.15 Upon enrollment into the REGARDS study, participants completed a phone interview to provide information on demographics, socioeconomic status, cardiovascular risk factors, and medical history. This was followed by an in‐home study visit, which included vital sign measurements, electrocardiography, and urine/blood sample collection.
To focus on first‐time events, participants with prevalent coronary heart disease, cerebrovascular disease, or cancer at baseline were excluded from the current analysis. History of coronary heart disease was determined through the baseline study interview (self‐report of a prior diagnosis of coronary heart disease ever) or the presence of any inpatient or outpatient Medicare International Classification of Diseases, 9th Revision, Clinical Modification (ICD‐9‐CM) diagnosis code for coronary heart disease (410.xx‐414.xx) in the year before baseline study visit. History of cerebrovascular disease was determined through the baseline study interview (self‐report of a prior diagnosis of stroke or transient ischemic attack ever) or the presence of any inpatient or outpatient Medicare ICD‐9‐CM diagnosis code for cerebrovascular disease (430.xx‐438.xx) in the year before baseline study visit. History of cancer was determined through the baseline study interview (self‐report of a prior diagnosis of cancer ever) or through the following Medicare ICD‐9‐CM code algorithm for cancer in the year before the baseline study visit: any inpatient or outpatient emergency department claim with an ICD‐9‐CM code diagnosis of 140.xx‐172.xx, 174.xx‐208.xx, or 209.0‐209.3 in any diagnosis position; any inpatient or outpatient claim with ICD‐9‐CM, Healthcare Common Procedure Coding System (HCPCS), or Current Procedural Terminology (CPT) codes for chemotherapy, radiation, or hormone therapy; ≥2 outpatient claims with an ICD‐9‐CM code diagnosis of 140.xx‐172.xx, 174.xx‐208.xx, or 209.0‐209.3 in any diagnosis position associated with physician evaluation and management (E&M) codes 30‐365 days apart; or any inpatient, outpatient emergency department, or outpatient claim associated with a physician E&M code for the ICD‐9‐CM diagnosis of V10.xx (history of malignant neoplasm).
2.3. Exposure
The exposure of interest was any new diagnosis of cancer during follow‐up except squamous or basal cell skin carcinomas. As in previous studies, new diagnoses of cancer were defined by ≥1 of the following Medicare claims algorithms: any inpatient or outpatient emergency department claim with ICD‐9‐CM diagnoses of 140.xx‐172.xx, 174.xx‐208.xx, or 209.0‐209.3 in any diagnosis position; any inpatient or outpatient claim with ICD‐9‐CM, HCPCS, or CPT codes for chemotherapy, radiation, or hormone therapy; or ≥2 outpatient claims with an ICD‐9‐CM diagnosis of 140.xx‐172.xx, 174.xx‐208.xx, or 209.0‐209.3 in any diagnosis position associated with physician E&M codes 30 to 365 days apart.12 In the algorithm requiring ≥2 outpatient claims, the cancer diagnosis was assigned the date of the second cancer claim. Prior investigations have reported that Medicare claims data can identify incident cancer with ≥98% specificity.16 A physician investigator (BBN) with expertise in claims‐based and cancer research determined specific cancer types after reviewing all inpatient and outpatient Medicare ICD‐9‐CM claims for cancer. Participants diagnosed with multiple cancer types during follow‐up were assigned the cancer type diagnosed first.
2.4. Outcomes
After their baseline visit, participants were contacted by telephone every 6 months to identify possible cardiovascular events. These telephone interviews included questioning about interim hospitalizations and general health status as well as the validated questionnaire for verifying stroke‐free status.17 When participants reported cardiovascular symptoms/diagnoses or the interviewer suspected a cardiovascular event, medical records were retrieved and reviewed by an expert panel for central adjudication. Outcome adjudicators were not blinded to the presence or absence of cancer.
The primary outcome was a REGARDS‐adjudicated ATE, defined as a composite of fatal or nonfatal acute myocardial infarction or ischemic stroke. The secondary outcomes were acute myocardial infarction alone and acute ischemic stroke alone. Myocardial infarction was defined according to published guidelines and required symptoms/signs of myocardial ischemia, a rising and/or falling pattern in cardiac troponin or creatinine kinase‐MB with a peak value at least twice the upper limit of normal over a period of ≥6 hours, and electrocardiogram changes consistent with myocardial ischemia.18 Ischemic stroke was defined per the World Health Organization (WHO) definition but also included cases with neurologic symptoms lasting <24 hours with brain imaging demonstrating acute ischemia and cases where the expert adjudicators believed the event was a likely ischemic stroke but clinical information was insufficient for the WHO or imaging‐based definitions.14, 19 Details of the REGARDS myocardial infarction and stroke definitions and adjudication processes have been described previously.14, 20
2.5. Analysis
We assembled a matched cohort design to evaluate ATE risk in participants with cancer. In this design, participants who developed cancer during follow‐up were matched 1:4 to control participants without cancer by age tertile (≤65 years, 66‐75 years, or >75 years), sex, race, and highest education achieved (less than college or some college or higher). These baseline factors were matched because a prior analysis demonstrated differences in these covariates between participants who did and did not develop cancer during follow‐up.12 To serve as a matched control, participants had to be cancer free at the time when their matched case was diagnosed with cancer. For 2 cancer cases, 4 control participants without cancer could not be identified; instead, 1 of these cases was matched to 2 controls and the other was matched to 1 control. Cancer cases and their matched controls without cancer both entered the study on the date of the case's cancer diagnosis. The sample size was based on available data from the REGARDS study. We used the Kaplan‐Meier method to calculate the cumulative incidence of ATEs and the log‐rank test to compare outcome rates between participants with and without cancer. Follow‐up was censored when participants experienced the primary outcome, developed cancer if serving as a cancer‐free control, withdrew from REGARDS, lost Medicare fee‐for‐service coverage, or on September 30, 2015.
To account for possible confounding by vascular risk factors, we also performed multivariable Cox proportional hazards analyses adjusting for age, region of residence, systolic and diastolic blood pressure, diabetes mellitus, atrial fibrillation, total and high‐density lipoprotein cholesterol, body mass index, self‐reported smoking history (current smoking [dichotomous variable] and smoking pack‐years [continuous variable]), socioeconomic status, antihypertensive medication use, left ventricular hypertrophy, estimated glomerular filtration rate, albuminuria, physical activity level, C‐reactive protein, and alcohol use. These covariates were selected based on the consensus opinion of the investigators prior to initiation of the analyses. Log‐log plots and visual inspection of cumulative incidence curves demonstrated that the proportional hazard assumption was violated. Therefore, hazard ratios were calculated during discrete time periods during which the assumption was met. These follow‐up time periods were 0 to 30 days, 31 to 90 days, and >90 days.
Using the final multivariable model, we performed secondary analyses restricting the study outcome to (1) acute fatal or nonfatal myocardial infarction and (2) acute fatal or nonfatal ischemic stroke. In addition, we performed several subgroup analyses evaluating ATE risk in select cancer populations. First, we restricted the cancer exposure to participants with solid tumor cancers. Second, we restricted the cancer exposure to new diagnoses of lung, colorectal, gastric, or pancreatic cancers because previous work has suggested that these cancer types confer the highest risks of arterial thromboembolism.6 Third, we restricted the cancer exposure to participants with claims for metastatic cancer (ICD‐9‐CM diagnosis codes 196.xx‐198.xx, 209.7x). Fourth, we restricted the cancer exposure to types considered high risk for venous thromboembolism (eg, pancreas, stomach, lung, gynecologic, bladder, or testicular cancers or lymphoma).4
We performed several sensitivity analyses. First, among participants diagnosed with cancer through outpatient diagnosis codes, we performed an analysis in which the date of the first outpatient claim for cancer was used as the date of cancer diagnosis. Second, instead of a matched cohort design, we modeled new diagnoses of cancer as a time‐dependent exposure, whereby participants who developed cancer during follow‐up contributed follow‐up time to both the cancer and noncancer groups. Similar to the primary analysis, multivariable Cox regression analyses adjusting for demographics, region of residence, and vascular risk factors were used to evaluate ATE risk among participants with incident cancer. Third, we used competing risk survival statistics accounting for the competing risk of death to evaluate the adjusted hazard for ATEs among participants with cancer. Fourth, we calculated propensity scores based on baseline demographic and clinical characteristics, matched participants with cancer to controls without cancer 1:4 based on the deciles of the score, and then compared the cumulative incidence of ATEs between groups. Analyses were performed using SAS version 9.4 (SAS Institute Inc., Cary, NC) and R version 3.5.0.
3. RESULTS
3.1. Characteristics
This matched cohort analysis comprised 4175 REGARDS study participants with linked Medicare claims data, including 836 participants with new diagnoses of cancer and 3339 control participants without cancer (Figure 1). At the time of cancer diagnosis, mean participant age was 75.5 years, 48% were women, and 69% were white. There was no evidence for differences between participants diagnosed with cancer and those not diagnosed with cancer, except that lower total cholesterol, current smoking, and higher smoking pack‐years were more common among the participants who subsequently developed cancer (Table 1). Diagnosed cancers included 640 solid‐tumor cancers, 71 hematologic cancers, 13 primary brain cancers, and 112 cancers of unknown primary type. The most frequent primary cancer types were prostate, breast, lung, colorectal, and bladder cancers, which combined, accounted for 59% of all diagnosed cancers (Table 2).
Figure 1.
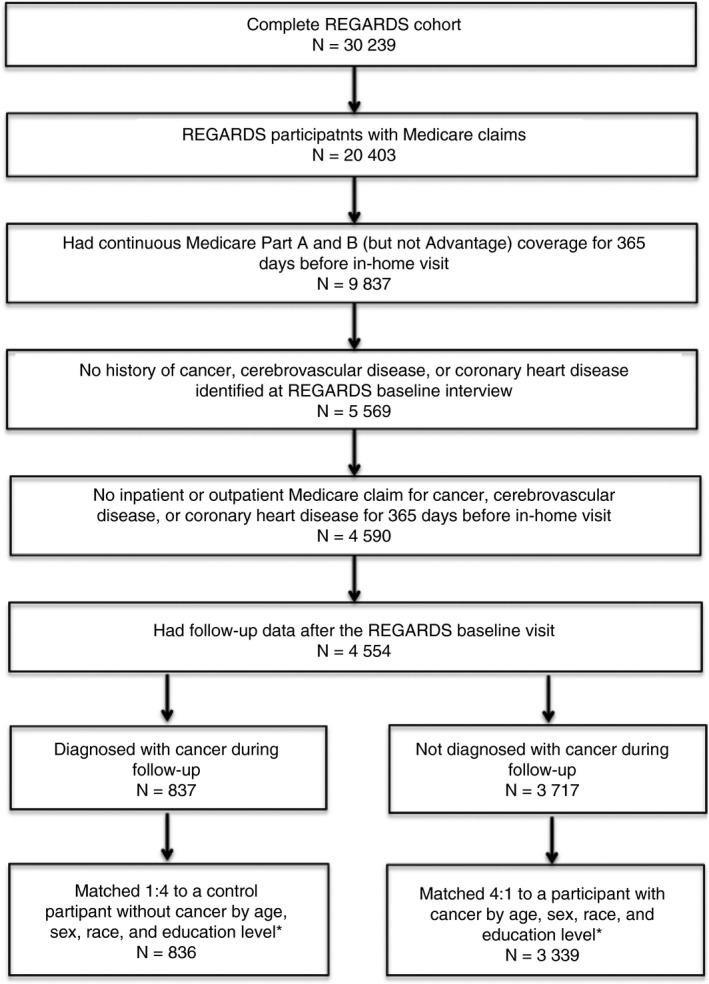
Study eligibility flow diagram. Flow diagram detailing REGARDS participants’ eligibility for this study. *For 2 cancer cases, 4 control participants without cancer could not be identified; instead, 1 of these cases was matched to 2 controls and the other was matched to 1 control
Table 1.
Participant characteristics at baseline REGARDS visit, stratified by the diagnosis of cancer during follow‐upa , b
| Characteristic | Cancer (N = 836) | No cancer (N = 3339) | P value |
|---|---|---|---|
| Age, mean (SD), y | 71.7 (6.6) | 71.6 (6.6) | 0.67 |
| Female | 402 (48) | 1606 (48) | 0.99 |
| Race | |||
| White | 578 (69) | 2309 (69) | 0.99 |
| Black | 258 (31) | 1030 (31) | |
| Annual income | |||
| <$20 000 | 125 (15) | 590 (18) | 0.24 |
| $20 000‐$34 999 | 239 (29) | 947 (28) | |
| $35 000‐$75 000 | 289 (35) | 1058 (32) | |
| >$75 000 | 79 (9) | 350 (11) | |
| Unknown | 104 (12) | 394 (12) | |
| Highest education level | |||
| Less than high school | 81 (10) | 335 (10) | 0.85 |
| High school | 212 (25) | 834 (25) | |
| Some college | 221 (26) | 926 (28) | |
| Higher than college | 322 (39) | 1244 (37) | |
| Urban/rural residencec | |||
| Urban | 561 (75) | 2303 (76) | 0.48 |
| Rural | 87 (12) | 344 (11) | |
| Mixed | 104 (14) | 369 (12) | |
| Region of residenced | |||
| Stroke Belt | 293 (35) | 1220 (37) | 0.46 |
| Stroke Buckle | 223 (27) | 823 (25) | |
| Stroke Nonbelt | 320 (38) | 1296 (39) | |
| Vascular risk factors | |||
| Systolic BP, mean (SD), mm Hg | 129 (16) | 129 (16) | 0.58 |
| Diastolic BP, mean (SD), mm Hg | 76 (9) | 76 (9) | 0.60 |
| Antihypertensive medication use ever | 407 (50) | 1620 (50) | 0.88 |
| Left ventricular hypertrophy | 76 (9) | 273 (8) | 0.37 |
| Diabetes mellitus | 132 (17) | 590 (18) | 0.25 |
| Atrial fibrillation | 48 (6) | 212 (6) | 0.55 |
| Total cholesterol, mean (SD), mg/dL | 189 (39) | 193 (38) | 0.01 |
| High‐density cholesterol, mean (SD), mg/dL | 53 (17) | 53 (17) | 0.83 |
| eGFR < 60, mL/min/1.73 m2 | 109 (14) | 375 (12) | 0.12 |
| Urinary albumin/creatinine ratio >30 mg/g | 121 (15) | 401 (13) | 0.04 |
| Physical activity | 538 (66) | 2267 (69) | 0.08 |
| Body mass index ≥30 kg/m2 | 244 (29) | 1008 (30) | 0.82 |
| Alcoholic drinks, wk (≥14 for M, ≥7 for F) | 171 (21) | 738 (23) | 0.34 |
| Current smoking | 95 (12) | 241 (7) | <0.01 |
| Smoking pack‐years, mean (SD), ye | 16 (24) | 13 (23) | <0.01 |
| Antithrombotic medication usef | |||
| Antiplatelet use | 373 (45) | 1,612 (48) | 0.06 |
| Anticoagulant use | 26 (3) | 110 (3) | 0.79 |
BP, blood pressure; eGFR, estimated glomerular filtration rate; F, female; M, male; SD, standard deviation.
All data are presented as n (%) unless otherwise specified.
Percentages may not add up to 100 because of rounding.
Size of census tract where the participant lives: rural = <25% urban, mixed = 25%‐75% urban, urban = >75% urban.
The Stroke Buckle includes coastal areas of North Carolina, South Carolina, and Georgia, while the Stroke Belt includes the rest of these states and Alabama, Mississippi, Louisiana, and Arkansas.
Among participants with any smoking history.
Patients were considered to use antiplatelets if they used any dose of aspirin or clopidogrel at least once in the 2 weeks before the baseline study visit, and anticoagulants if they used any dose of warfarin, enoxaparin, or tinzaparin at least once in the 2 weeks before the baseline study visit.
Table 2.
Frequency of specific cancer types stratified by the subsequent development of an arterial thromboembolic eventa
| Cancer type | Total (n = 836) | Arterial thromboembolic event (n = 63)b | No arterial thromboembolic event (n = 773) |
|---|---|---|---|
| Prostate | 175 (21%) | 17 (27%) | 158 (20%) |
| Breast | 124 (15%) | 10 (16%) | 114 (15%) |
| Unknown primary | 112 (13%) | 10 (16%) | 102 (13%) |
| Lung | 89 (11%) | 8 (13%) | 81 (10%) |
| Colorectal | 64 (8%) | 5 (8%) | 59 (8%) |
| Bladder | 41 (5%) | 2 (3%) | 39 (5%) |
| Leukemia | 29 (3%) | 2 (3%) | 27 (3%) |
| Non‐Hodgkin lymphoma | 26 (3%) | 0 (0%) | 26 (3%) |
| Melanoma | 22 (3%) | 0 (0%) | 22 (3%) |
| Kidney | 20 (2%) | 2 (3%) | 18 (2%) |
| Head and neck | 18 (2%) | 2 (3%) | 16 (2%) |
| Ovarian | 16 (2%) | 1 (2%) | 15 (2%) |
| Primary brain | 13 (2%) | 0 (0%) | 13 (2%) |
| Pancreas | 12 (1%) | 0 (0%) | 12 (2%) |
| Multiple myeloma | 12 (1%) | 1 (2%) | 11 (1%) |
| Uterine | 11 (1%) | 1 (2%) | 10 (1%) |
| Gastric | 10 (1%) | 1 (2%) | 9 (1%) |
| Esophageal | 7 (1%) | 0 (0%) | 7 (1%) |
| Liver | 6 (1%) | 0 (0%) | 6 (1%) |
| Thyroid | 6 (1%) | 0 (0%) | 6 (1%) |
| Gallbladder/biliary tract | 4 (0%) | 0 (0%) | 4 (1%) |
| Hodgkin lymphoma | 4 (0%) | 0 (0%) | 4 (1%) |
| Bone | 3 (0%) | 0 (0%) | 3 (0%) |
| Cervical | 2 (0%) | 0 (0%) | 2 (0%) |
| Penile | 2 (0%) | 1 (2%) | 1 (0%) |
| Sarcoma | 2 (0%) | 0 (0%) | 2 (0%) |
| Small bowel | 2 (0%) | 0 (0%) | 2 (0%) |
| Adrenal | 1 (0%) | 0 (0%) | 1 (0%) |
| Primary peritoneal | 1 (0%) | 0 (0%) | 1 (0%) |
| Pleural | 1 (0%) | 0 (0%) | 1 (0%) |
| Vaginal | 1 (0%) | 0 (0%) | 1 (0%) |
Arterial thromboembolic events were a composite of fatal/nonfatal myocardial infarction or ischemic stroke.
Due to rounding, percentages do not add up to 100.
3.2. Primary and secondary analyses
Among participants diagnosed with cancer, median follow‐up from time of cancer diagnosis to ATE or end of follow‐up was 2.9 years (interquartile range [IQR], 1.0‐6.3), and during this period, 63 participants (7.5%) had an acute myocardial infarction (fatal, n = 17; nonfatal, n = 19) or ischemic stroke (fatal, n = 2; nonfatal, n = 25). Most ATEs occurred among participants with solid‐tumor cancers, particularly prostate (n = 17), breast (n = 10), or lung (n = 8) cancers, although 3 participants with hematologic cancers (leukemia, n = 2; multiple myeloma, n = 1) also had events. There were no ATEs diagnosed in participants with primary brain cancers. There were 691 participants with cancer (83% of cohort) who had at least 1 surgical procedure during follow‐up (median number of procedures, 6; IQR, 3‐12), and 29 (4.2%) participants had an ATE in the 30 days after a procedure. Overall, 46% of cancer participants with an ATE (n = 63) had an event within 30 days after a surgical procedure. Meanwhile, among matched control participants not diagnosed with cancer, median follow‐up time was 3.6 years (IQR, 1.7‐6.5), and 216 (6.5%) were diagnosed with an acute myocardial infarction (fatal n = 52, nonfatal n = 76) or ischemic stroke (fatal n = 8, nonfatal n = 80).
The estimated cumulative incidence of ATEs was numerically higher in participants with cancer vs. those without (P = 0.06 for log‐rank test; Figure 2). The annual incidence rate of ATEs was 2.01 (95% confidence interval [CI], 1.57‐2.58) per 100 person‐years among participants with cancer and 1.53 (95% CI, 1.34‐1.75) per 100 person‐years among participants without cancer. In the first 30 days after cancer diagnosis, participants with cancer had an increased hazard for ATEs as compared to matched controls without cancer (hazard ratio [HR], 5.2; 95% CI, 2.1‐12.7) (Table 3). This association persisted after multivariable adjustment for demographics, region of residence, and vascular risk factors (HR, 5.8; 95% CI, 2.1‐12.9). Furthermore, the association was enhanced when the outcome was restricted to fatal/nonfatal acute ischemic stroke (adjusted HR, 9.0; 95% CI, 2.7‐29.4), while it was attenuated and not significant when restricted to fatal/nonfatal acute myocardial infarction (adjusted HR, 2.7; 95% CI, 0.4‐19.9). Beyond 30 days, cancer was not associated with an increased hazard for ATEs; however, there was a nonsignificant trend in the 31‐ to 90‐day period after cancer diagnosis (adjusted HR, 1.5; 95% CI, 0.4‐6.1). Data on censoring and the timing of ATEs are presented in Table 4.
Figure 2.
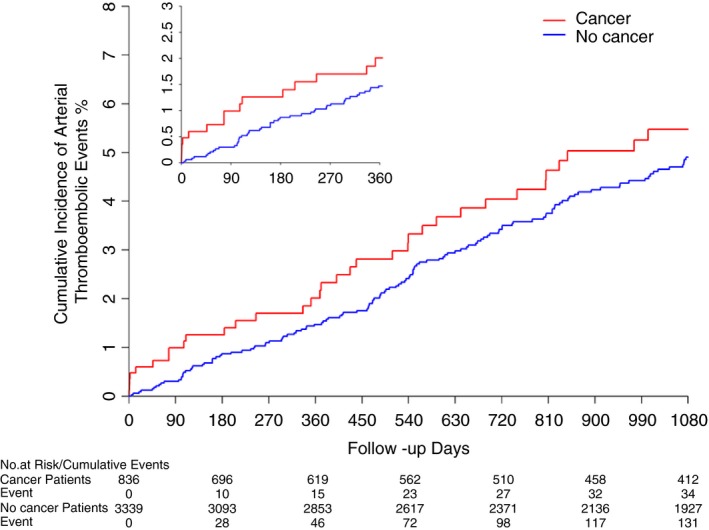
Cumulative incidence of arterial thromboembolic events among participants with and without a new diagnosis of cancer. Participants who developed cancer during follow‐up were matched 1:4 (except 2 cases) to control participants without cancer by age tertile, sex, race, and education level. Cancer cases and their matched controls without cancer both entered the study on the date of the cancer case's cancer diagnosis. Kaplan‐Meier statistics were used to calculate the cumulative incidence of arterial thromboembolic events (composite of myocardial infarction or ischemic stroke). Follow‐up was censored when participants experienced an outcome, developed cancer (if a cancer‐free control), withdrew from REGARDS, lost Medicare fee‐for‐service coverage, or on September 30, 2015. The inset shows a magnified view of the first 360 d of follow‐up.
Table 3.
| Model and time period following cancer diagnosisc | Hazard ratio (95% CI) |
|---|---|
| All cancers (n = 836) | |
| Unadjusted | |
| 0‐30 d | 5.2 (2.1‐12.7) |
| 31‐90 d | 1.6 (0.5‐4.9) |
| >90 d | 1.2 (0.9‐1.6) |
| Adjustment for demographics, region of residence, and vascular risk factorsd | |
| 0‐30 d | 5.8 (2.1‐15.9) |
| 31‐90 d | 1.5 (0.4‐6.0) |
| >90 d | 1.1 (0.8‐1.6) |
| Cancers considered high risk for venous thromboembolism (n = 210)e | |
| Unadjusted | |
| 0‐30 d | 17.8 (6.2‐50.7) |
| 31‐90 d | –f |
| >90 d | 1.2 (0.6‐2.3) |
| Adjustment for demographics, region of residence, and vascular risk factorsd | |
| 0‐30 d | 18.5 (5.1‐66.9) |
| 31‐90 d | –f |
| >90 d | 1.3 (0.6‐3.0) |
| Cancers with known metastases (n = 230) | |
| Unadjusted | |
| 0‐30 d | 14.1 (4.3‐46.6) |
| 31‐90 d | –f |
| >90 d | 1.2 (0.6‐2.4) |
| Adjustment for demographics, region of residence, and vascular risk factorsd | |
| 0‐30 d | 14.4 (4.0‐52.2) |
| 31‐90 d | –f |
| >90 d | 1.0 (0.4‐2.4) |
CI, confidence interval.
Cancer cases were matched 1:4 (except 2 cases) to control participants without cancer by age tertile, sex, race, and education level. To be a control, participants had to be cancer free at the time when their matched case developed cancer.
Arterial thromboembolic events comprised fatal/nonfatal myocardial infarction or ischemic stroke.
Hazard ratios were calculated at discrete time periods to fulfill the proportional hazard assumption. The reference group is participants without a diagnosis of cancer.
Vascular risk factors included systolic and diastolic blood pressure, diabetes mellitus, atrial fibrillation, total and high‐density cholesterol, coronary heart disease, smoking and alcohol history, annual income, highest education level, antihypertensive medication use, left ventricular hypertrophy, estimated glomerular filtration rate, urine albumin‐creatinine ratio, physical activity, and obesity.
Cancers considered high risk for venous thromboembolism were pancreas, gastric, lung, gynecologic, bladder, or testicular cancers or lymphoma.
Too few data points to calculate a hazard ratio.
Table 4.
Crude number and percentage of arterial thromboembolic events stratified by diagnosis of cancer and time perioda , b , c
| Time period | Cancer diagnosis | No cancer diagnosis |
|---|---|---|
| Days 0‐30 | ||
| Total at riskc | 836 | 3339 |
| ATE | 5 (0.60%) | 4 (0.12%) |
| No ATE | 831 (99.40%) | 3335 (99.88%) |
| Days 31‐90 | ||
| Total at risk | 792 | 3299 |
| ATE | 3 (0.38%) | 6 (0.18%) |
| No ATE | 789 (99.62%) | 3293 (99.82%) |
| Days 91‐end of follow‐up | ||
| Total at risk | 745 | 3218 |
| ATE | 55 (7.38%) | 206 (6.40%) |
| No ATE | 690 (92.62%) | 3012 (93.60%) |
ATE, arterial thromboembolic event.
Cancer cases and their matched controls without cancer both entered the study on the date of the cancer case's cancer diagnosis. Kaplan‐Meier statistics were used to calculate the cumulative incidence of arterial thromboembolic events, defined as a composite of fatal or nonfatal acute myocardial infarction or ischemic stroke. Follow‐up was censored when participants experienced an arterial thromboembolic event, developed cancer if serving as a cancer‐free control, withdrew from the REGARDS study, lost Medicare fee‐for‐service coverage, or on September 30, 2015.
Median follow‐up time was 2.9 years (interquartile range, 1.0‐6.3) in the cancer group and 3.6 years (interquartile range, 1.7‐6.5) in the noncancer group.
Refers to the number of participants at risk for arterial thromboembolic events at the beginning of each time period. The numbers at risk do not include participants who had an arterial thromboembolic event or were censored for death, loss of Medicare coverage, or end of study in previous time periods.
3.3. Subgroup analyses
Among participants with any solid‐tumor cancer (n = 640), the adjusted HR for ATEs in the first 30 days after cancer diagnosis was 7.6 (95% CI, 2.8‐21.1). When restricted to participants with lung, colorectal, gastric, or pancreatic cancers (n = 175), the adjusted HR for ATEs in the first 30 days after cancer diagnosis was 15.6 (95% CI, 3.3‐73.3). Among participants with known metastatic cancer (n = 230), the adjusted HR for ATEs in the first 30 days after cancer diagnosis was 14.4 (95% CI, 4.0‐52.2). Among participants with cancer types considered high risk for venous thromboembolism (n = 210), the adjusted HR for ATEs in the first 30 days after cancer diagnosis was 18.5 (95% CI, 5.1‐66.9). Beyond 30 days from cancer diagnosis, these cancer subgroups were not associated with an increased hazard for ATEs.
3.4. Sensitivity analyses
The study's main results were materially unchanged when the date of the first outpatient cancer claim was used as the date of cancer diagnosis (adjusted HR, 5.8; 95% CI, 2.1‐15.8; Table 5). When incident cancer was modeled as a time‐dependent exposure, cancer remained associated with a short‐term increased risk for ATEs (Table 6). When accounting for the competing risk of death, incident cancer was associated with subsequent ATEs (adjusted HR during first 30 days, 5.9; 95% CI, 2.1‐16.2). In an analysis matching on propensity scores, new diagnoses of cancer were associated with an increased risk for ATEs in the first 30 days after diagnosis (adjusted HR, 6.2; 95% CI, 2.3‐16.7). Incident cancer was not associated with a statistically significant increased risk for ATEs beyond 30 days in any of the aforementioned sensitivity analyses.
Table 5.
Cox models evaluating the association between a new cancer diagnosis and arterial thromboembolic events: sensitivity analysis using a different outpatient coding schema to identify new cancer diagnosesa
| Model and time period following cancer diagnosisb | Hazard ratio (95% CI) |
|---|---|
| Unadjusted | |
| 0‐30 d | 5.2 (2.1‐12.7) |
| 31‐90 d | 1.6 (0.5‐4.9) |
| >90 d | 1.2 (0.9‐1.6) |
| Adjustment for demographics, region of residence, and vascular risk factorsc | |
| 0‐30 d | 5.8 (2.1‐15.8) |
| 31‐90 d | 1.5 (0.4‐6.0) |
| >90 d | 1.1 (0.8‐1.6) |
CI, confidence interval.
For the primary analysis, new diagnoses of cancer were defined by at least 1 of the following Medicare claims algorithms: any inpatient or outpatient emergency department claim with International Classification of Diseases, 9th Revision, Clinical Modification (ICD‐9‐CM) diagnoses of 140.xx‐172.xx, 174.xx‐208.xx, or 209.0‐209.3 in any diagnosis position; any inpatient or outpatient claim with ICD‐9‐CM, Healthcare Common Procedure Coding System (HCPCS), or Current Procedural Terminology (CPT) codes for chemotherapy, radiation, or hormone therapy; or ≥2 outpatient claims with an ICD‐9‐CM diagnosis of 140.xx‐172.xx, 174.xx‐208.xx, or 209.0‐209.3 in any diagnosis position associated with physician evaluation and management codes 30‐365 d apart. In this sensitivity analysis, for those diagnosed through outpatient codes, the date of the first outpatient claim was taken to be the date of cancer diagnosis.
The proportional hazard assumption was violated for the entirety of patient follow‐up. Therefore, hazard ratios were calculated at discrete time periods when the assumption was met.
Vascular risk factors included systolic blood pressure, diastolic blood pressure, diabetes mellitus, atrial fibrillation, total and high‐density cholesterol, coronary heart disease, smoking history, annual income, highest education level, antihypertensive medication use, left ventricular hypertrophy, estimated glomerular filtration rate, urine albumin‐creatinine ratio, physical activity, body mass index, and alcoholic drink consumption.
Table 6.
Sensitivity analysis for which new cancer diagnoses were modeled as a time‐dependent exposure to evaluate their association with arterial thromboembolic eventsa , b
| Model and time period following cancer diagnosisc | Hazard ratio (95% CI) |
|---|---|
| Unadjusted besides matching factors | |
| 0‐30 d | 5.3 (2.2‐12.9) |
| 31‐90 d | 1.8 (0.6‐5.4) |
| >90 d | 1.2 (0.9‐1.6) |
| Additionally adjusted for region of residence and vascular risk factorsd | |
| 0‐30 d | 5.0 (1.9‐13.5) |
| 31‐90 d | 1.4 (0.4‐5.7) |
| >90 d | 1.0 (0.7‐1.4) |
CI, confidence interval.
Time of study entry for all participants was the date of the baseline, in‐home, REGARDS study visit, which occurred between 2003 and 2007, when the REGARDS cohort was enrolled. New diagnoses of cancer were modeled as a time‐dependent exposure. Therefore, participants who developed cancer during study follow‐up contributed follow‐up time to both the cancer and noncancer groups. Specifically, they contributed follow‐up time to the noncancer group before their cancer diagnosis and to the cancer group after their cancer diagnosis. Follow‐up was censored when participants had an arterial thromboembolic event, withdrew from the REGARDS study, lost Medicare fee‐for‐service insurance coverage, or on September 30, 2015.
Arterial thromboembolic events were defined as a composite of fatal or nonfatal myocardial infarction or ischemic stroke.
The proportional hazard assumption was violated for the entirety of patient follow‐up. Therefore, hazard ratios were calculated at discrete time periods when the assumption was met. The reference group is participants without a diagnosis of cancer.
Vascular risk factors included systolic blood pressure, diastolic blood pressure, diabetes mellitus, atrial fibrillation, total and high‐density cholesterol, coronary heart disease, smoking history, annual income, antihypertensive medication use, left ventricular hypertrophy, estimated glomerular filtration rate, urine albumin‐creatinine ratio, physical activity, body mass index, and alcoholic drink consumption.
4. DISCUSSION
In a prospective cohort study with adjudicated cardiovascular diagnoses and multivariable adjustment for vascular risk factors, participants’ risk of an ATE in the first 30 days after cancer diagnosis was increased 5‐fold as compared to matched controls without cancer. This increased short‐term risk was highest in participants whose cancers were considered high risk for venous thromboembolism or who had known metastases. Furthermore, incident cancer was more strongly associated with ischemic stroke than myocardial infarction, although there were more myocardial infarctions than ischemic strokes among the cancer group. Beyond 30 days after cancer diagnosis, the risk of an ATE was no longer statistically significantly increased for cancer participants. However, participants with cancer had numerically higher rates of ATEs during all studied time periods and at the end of follow‐up than matched controls without cancer. Therefore, we cannot rule out a long‐term increased risk.
These findings substantiate retrospective studies that reported an association between cancer and short‐term arterial thromboembolism risk.6, 7, 8, 21 In a recent analysis of 279 719 US patients with incident cancer identified through registry data, the risk of ATEs was increased 5‐fold in the first 30 days after cancer diagnosis.6 Beyond 30 days, excess risk precipitously declined, although it remained elevated for about 9 months. Furthermore, at 6 months after cancer diagnosis, patients’ absolute risk of ATEs was more than double that of cancer‐free controls. Similarly, analyses of over 820 000 Swedish patients with cancer demonstrated comparable findings, with most cancers conferring an increased short‐term risk of acute coronary disease and ischemic stroke events that dissipated or resolved after 6 months.7, 8 The current study builds on these retrospective analyses by comprehensively adjusting for vascular risk factors, including lifestyle factors such as alcohol and smoking use, which are not reliably captured in claims data and are common in persons with cancer. Also, by using prospectively adjudicated outcomes to identify ATEs, the current analysis is less prone to misclassification error than claims‐based diagnoses.11 With this more rigorous design, this study confirms that a new diagnosis of cancer is associated with a substantially increased short‐term risk of ATEs. However, this study also had fewer participants than in the aforementioned claims‐based studies, resulting in less statistical power, and therefore it was unable to reliably determine the duration of excess risk associated with cancer, which may persist for longer than 1 month from diagnosis.
We previously reported that among participants enrolled in the prospective REGARDS cohort study, a diagnosis of cancer was associated with an increased short‐term risk of cerebrovascular events.12 In this follow‐up study, we found that incident cancer was also an independent short‐term risk factor for ATEs more broadly, defined as fatal/nonfatal myocardial infarction or ischemic stroke. In addition to this broader outcome, the novel aspects of the current study include a different eligibility criterion (ie, patients with prevalent coronary disease were also excluded), a longer time period and duration of follow‐up, and a more robust primary analytical technique: a matched cohort design with incidence density sampling instead of Cox hazards regression with cancer evaluated as a time‐dependent covariate. The current study also corroborates the findings of a recent prospective analysis of 1880 patients with cancer, among whom vascular risk factors (eg, age, hypertension, and smoking) and cancers considered high risk for venous thromboembolism (eg, lung and kidney cancers) were associated with a higher arterial thromboembolism risk.22
There are multiple possible explanations for the increased risk of ATEs with cancer. Cancer can produce a hypercoagulable state through its effects on the coagulation cascade, platelet function, and vessel wall endothelial integrity.23, 24, 25 This includes the release of tumor microparticles that circulate in the bloodstream and promote thrombosis through tissue factor and non–tissue factor pathways.26 Additionally, cancer can activate the innate immune system through neutrophil extracellular trap formation, which can trigger thrombosis.27 These cancer‐mediated hypercoagulable phenomena could lead to ATEs through in situ thromboses within the cardiac and cerebral vasculature or by embolization from thromboses on cardiac valves (ie, marantic endocarditis) or deep veins (ie, paradoxical embolization). Cancer treatments may also contribute to the increased risk of ATEs. Chemotherapy, particularly platinum‐based and antiangiogenesis treatments, have been linked to ATEs.28, 29 In addition, radiotherapy can cause vasculopathy and accelerated atherosclerosis, although this typically occurs years after treatment, and therefore it is unlikely to explain the increased short‐term risk seen in this study.23 Surgery, which is common after cancer diagnosis, could cause ATEs through direct injury of blood vessels, heightened inflammation, and disruption of thrombosis and hemostatic pathways. Furthermore, cancer treatments, particularly surgery and chemotherapy, sometimes require temporary interruption of antithrombotic and statin medicines, which could precipitate events.23 Stress, which is common with cancer, is associated with an increased risk of ATEs.30 Another possible explanation for the increased short‐term risk of ATEs in persons with newly diagnosed cancer is residual confounding from shared risk factors such as smoking and atrial fibrillation, although these factors were adjusted for in our multivariable analyses. Additionally, detection bias is possible, as many of the early ATEs in the cancer group were diagnosed at or near the time of cancer diagnosis.23 It is possible that in some cancer patients, an ATE was diagnosed because of increased surveillance for cancer staging, or conversely, an ATE led to a concomitant cancer diagnosis because of findings on diagnostic evaluation.
This study has several limitations. First, Medicare claims data were used to identify new cancer diagnoses. This could have led to incorrect or missed cancer diagnoses, although Medicare claims algorithms have been shown to identify incident cancer with sensitivity and specificity of greater than 90%.16 Second, by relying on claims data, cancer diagnosis dates could have been incorrect, which could have affected the temporal associations between cancer and ATEs. Furthermore, the exact timing of when cancers became biologically active is unknown, and it is possible that some cancers affected arterial thromboembolism risk before diagnosis, which could have biased the study toward the null. Third, we did not have detailed data on cancer stage, histology, or treatments. Also, we lacked data on antithrombotic drug use during study follow‐up and differences in medication use could have affected the study's estimated hazard for ATEs after cancer diagnosis. Fourth, this study included white and black Americans with Medicare health insurance enrolled in the REGARDS study, and therefore its results may not generalize to other populations. Fifth, in this analysis, we aimed to prospectively validate prior retrospective studies that reported an association between cancer and arterial thromboembolism risk; therefore, we investigated the REGARDS cohort because it is a large prospective cohort study systematically assessing for incident stroke and myocardial infarction. However, despite using data from the REGARDS cohort, which included 836 participants with a cancer exposure, the multivariable analyses may have been underpowered, as indicated by the wide confidence intervals, thereby limiting our ability to detect potential associations with ATE risk beyond 30 days from cancer diagnosis when effect sizes were smaller. This was particularly evident in the analysis of secondary outcomes. Furthermore, because of the small number of outcome events, especially during the first 30 days of follow‐up, replication of the current results is recommended before definite conclusions are made.
5. CONCLUSION
A new diagnosis of cancer, particularly when metastatic or a type considered high risk for venous thromboembolism, is associated with an increased short‐term risk of ATEs independent of vascular risk factors. An association beyond 30 days, as seen in larger retrospective studies, was not confirmed in this prospective study. Future investigations are needed to delineate the underlying mechanisms responsible for the increased risk of ATEs in persons with cancer, to identify biomarkers that can discriminate especially high‐risk persons, and to determine the optimal treatment strategies to prevent myocardial infarction and stroke in the cancer population.
RELATIONSHIP DISCLOSURE
GH serves on an executive committee for Bayer HealthCare and previously served as a member of a data and safety monitoring board for a randomized trial sponsored by Alexion. MSVE received compensation for providing consultative services for Abbott, Biotelemetry/Cardionet, BMS‐Pfizer Alliance for Eliquis, Boehringer‐Ingelheim, Sanofi‐Regeneron Partnership, and Vascular Dynamics; received compensation for serving as an expert witness in litigation for BMS‐Sanofi, Merck/Organon, Hi‐Tech Pharmaceuticals, Auxilium, and Sorin; received research support from the BMS‐Pfizer Alliance for Eliquis and Roche; received royalties from UpToDate for chapters related to stroke; and serves on the National, Founders Affiliate, and New York City chapter boards of the American Heart Association/American Stroke Association. CI served on the scientific advisory board for Broadview Ventures. LMD served on scientific advisory boards for Juno Therapeutics, Sapience, Roche, Tocagen, and BTG International. HK received compensation for serving on the advisory board for Roivant Sciences, the steering committee for Medtronic, the deputy editor for JAMA Neurology, and research support from BMS and Roche. MC provided consultative services for Merck and received honorarium for speaking from Diazyme and Diadexus. MS received research funding from Amgen and Diadexus. PM received research funding from Amgen Inc. The other authors report nothing to disclose.
AUTHOR CONTRIBUTIONS
BBN conceived and designed the research, analyzed and interpreted the data, handled funding and supervision, drafted the manuscript, and made critical revision of the manuscript for important intellectual content. GH conceived and designed the research, acquired the data, analyzed and interpreted the data, and made critical revision of the manuscript for important intellectual content. VJH acquired the data, handled funding and supervision, and made critical revision of the manuscript for important intellectual content. HZ analyzed and interpreted the data, performed statistical analysis, and made critical revision of the manuscript for important intellectual content. SEJ acquired the data and made critical revision of the manuscript for important intellectual content. MSVE analyzed and interpreted the data, handled funding and supervision, and made critical revision of the manuscript for important intellectual content. CI handled funding and supervision and made critical revision of the manuscript for important intellectual content. LMD conceived and designed the research, handled funding and supervision, and made critical revision of the manuscript for important intellectual content. HK conceived and designed the research, analyzed and interpreted the data, and made critical revision of the manuscript for important intellectual content. PMO made critical revision of the manuscript for important intellectual content. SG analyzed and interpreted the data, and made critical revision of the manuscript for important intellectual content. EZS acquired the data and made critical revision of the manuscript for important intellectual content. MC acquired the data, handled funding and supervision, and made critical revision of the manuscript for important intellectual content. MS analyzed and interpreted the data, and made critical revision of the manuscript for important intellectual content. PM analyzed and interpreted the data, handled funding and supervision, and made critical revision of the manuscript for important intellectual content.
Supporting information
ACKNOWLEDGMENTS
This research project is supported by cooperative agreement U01NS041588 cofunded by the National Institute of Neurological Disorders and Stroke (NINDS) and the National Institute on Aging (NIA), National Institutes of Health, Department of Health and Human Service. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NINDS or the NIA. Representatives of the NINDS were involved in the review of this manuscript but were not directly involved in the collection, management, analysis, or interpretation of the data. Additional funding was from National Institutes of Health grants K23NS091395 (Navi) and R01HL080477 (Safford, Muntner), and the Florence Gould Endowment for Discovery in Stroke (Navi). The authors thank the other investigators, the staff, and the participants of the REGARDS study for their valuable contributions. A full list of participating REGARDS investigators and institutions can be found at http://www.regardsstudy.org.
Navi BB, Howard G, Howard VJ, et al. The risk of arterial thromboembolic events after cancer diagnosis. Res Pract Thromb Haemost. 2019;3:639–651. 10.1002/rth2.12223
Contributor Information
Babak B. Navi, Email: ban9003@med.cornell.edu.
Suzanne E. Judd, @suzjudd.
Susan Gilchrist, @fit_cardio_onc.
Mary Cushman, @MaryCushmanMD.
Paul Muntner, @muntnerpaul.
REFERENCES
- 1. Cancer Research UK . Worldwide Cancer Statistics. Available from https://www.cancerresearchuk.org/health-professional/cancer-statistics/worldwide-cancer#heading-zero [Accessed 2018 September 5].
- 2. Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA. 2005;293:715–22. [DOI] [PubMed] [Google Scholar]
- 3. Gomes M, Khorana AA. Risk assessment for thrombosis in cancer. Semin Thromb Hemost. 2014;40:319–24. [DOI] [PubMed] [Google Scholar]
- 4. Khorana AA, Kuderer NM, Culakova E, Lyman GH, Francis CW. Development and validation of a predictive model for chemotherapy‐associated thrombosis. Blood. 2008;111:4902–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kimpton M, Wells PS, Carrier M. Apixaban for the prevention of venous thromboembolism in high‐risk ambulatory cancer patients receiving chemotherapy: rational and design of the AVERT trial. Thromb Res. 2018;164(suppl 1):S124–9. [DOI] [PubMed] [Google Scholar]
- 6. Navi BB, Reiner AS, Kamel H, Iadecola C, Okin PM, Elkind MSV, et al. Risk of arterial thromboembolism in patients with cancer. J Am Coll Cardiol. 2017;70:926–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zoller B, Ji J, Sundquist J, Sundquist K. Risk of haemorrhagic and ischaemic stroke in patients with cancer: a nationwide follow‐up study from Sweden. Eur J Cancer. 2012;48:1875–83. [DOI] [PubMed] [Google Scholar]
- 8. Zoller B, Ji J, Sundquist J, Sundquist K. Risk of coronary heart disease in patients with cancer: a nationwide follow‐up study from Sweden. Eur J Cancer. 2012;48:121–8. [DOI] [PubMed] [Google Scholar]
- 9. Goff DC Jr, Lloyd‐Jones DM, Bennett G, Coady S, D'Agostino RB Sr, Gibbons R, et al.; American College of Cardiology/American Heart Association Task Force on Practice Guidelines . 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2935–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Parkin DM, Boyd L, Walker LC. 16. The fraction of cancer attributable to lifestyle and environmental factors in the UK in 2010. Br J Cancer. 2011;105(suppl 2):S77–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Desai RJ, Solomon DH, Shadick N, Iannaccone C, Kim SC. Identification of smoking using Medicare data – a validation study of claims‐based algorithms. Pharmacoepidemiol Drug Saf. 2016;25:472–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Navi BB, Howard G, Howard VJ, Zhao H, Judd SE, Elkind MSV, et al. New diagnosis of cancer and the risk of subsequent cerebrovascular events. Neurology. 2018;90:e2025–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Howard VJ, Cushman M, Pulley L, Gomez CR, Go RC, Prineas RJ, et al. The reasons for geographic and racial differences in stroke study: objectives and design. Neuroepidemiology. 2005;25:135–43. [DOI] [PubMed] [Google Scholar]
- 14. Howard VJ, Kleindorfer DO, Judd SE, McClure LA, Safford MM, Rhodes JD, et al. Disparities in stroke incidence contributing to disparities in stroke mortality. Ann Neurol. 2011;69:619–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xie F, Colantonio LD, Curtis JR, Safford MM, Levitan EB, Howard G, et al. Linkage of a population‐based cohort with primary data collection to Medicare claims: the reasons for geographic and racial differences in stroke study. Am J Epidemiol. 2016;184:532–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Setoguchi S, Solomon DH, Glynn RJ, Cook EF, Levin R, Schneeweiss S. Agreement of diagnosis and its date for hematologic malignancies and solid tumors between Medicare claims and cancer registry data. Cancer Causes Control. 2007;18:561–9. [DOI] [PubMed] [Google Scholar]
- 17. Jones WJ, Williams LS, Meschia JF. Validating the Questionnaire for Verifying Stroke‐Free Status (QVSFS) by neurological history and examination. Stroke. 2001;32:2232–6. [DOI] [PubMed] [Google Scholar]
- 18. Luepker RV, Apple FS, Christenson RH, Crow RS, Fortmann SP, Goff D, et al.; AHA Council on Epidemiology and Prevention, AHA Statistics Committee, World Heart Federation Council on Epidemiology and Prevention, European Society of Cardiology Working Group on Epidemiology and Prevention, Centers for Disease Control and Prevention, National Heart, Lung, and Blood Institute . Case definitions for acute coronary heart disease in epidemiology and clinical research studies: a statement from the AHA Council on Epidemiology and Prevention; AHA Statistics Committee; World Heart Federation Council on Epidemiology and Prevention; the European Society of Cardiology Working Group on Epidemiology and Prevention; Centers for Disease Control and Prevention; and the National Heart, Lung, and Blood Institute. Circulation. 2003;108:2543–9. [DOI] [PubMed] [Google Scholar]
- 19. Stroke–1989. Recommendations on stroke prevention, diagnosis, and therapy. Report of the WHO Task Force on Stroke and other Cerebrovascular Disorders. Stroke. 1989;20:1407–31. [DOI] [PubMed] [Google Scholar]
- 20. Levitan EB, Olubowale OT, Gamboa CM, Rhodes JD, Brown TM, Muntner P, et al. Characteristics and prognosis of acute myocardial infarction by discharge diagnosis: the Reasons for Geographic and Racial Differences in Stroke study. Ann Epidemiol. 2015;25:499–504.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Navi BB, Reiner AS, Kamel H, Iadecola C, Elkind MS, Panageas KS, et al. Association between incident cancer and subsequent stroke. Ann Neurol. 2015;77:291–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Grilz E, Konigsbrugge O, Posch F, Schmidinger M, Pirker R, Lang IM, et al. Frequency, risk factors, and impact on mortality of arterial thromboembolism in patients with cancer. Haematologica. 2018;103:1549–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Navi BB, Iadecola C. Ischemic stroke in cancer patients: a review of an underappreciated pathology. Ann Neurol. 2018;83:873–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Caine GJ, Stonelake PS, Lip GY, Kehoe ST. The hypercoagulable state of malignancy: pathogenesis and current debate. Neoplasia. 2002;4:465–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Eichinger S. Cancer associated thrombosis: risk factors and outcomes. Thromb Res. 2016;140(suppl 1):S12–7. [DOI] [PubMed] [Google Scholar]
- 26. Bang OY, Chung JW, Lee MJ, Kim SJ, Cho YH, Kim GM, et al. Cancer cell‐derived extracellular vesicles are associated with coagulopathy causing ischemic stroke via tissue factor‐independent way: the OASIS‐CANCER study. PLoS ONE. 2016;11:e0159170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Demers M, Wagner DD. NETosis: a new factor in tumor progression and cancer‐associated thrombosis. Semin Thromb Hemost. 2014;40:277–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Li SH, Chen WH, Tang Y, Rau KM, Chen YY, Huang TL, et al. Incidence of ischemic stroke post‐chemotherapy: a retrospective review of 10,963 patients. Clin Neurol Neurosurg. 2006;108:150–6. [DOI] [PubMed] [Google Scholar]
- 29. Ranpura V, Hapani S, Chuang J, Wu S. Risk of cardiac ischemia and arterial thromboembolic events with the angiogenesis inhibitor bevacizumab in cancer patients: a meta‐analysis of randomized controlled trials. Acta Oncol. 2010;49:287–97. [DOI] [PubMed] [Google Scholar]
- 30. Dimsdale JE. Psychological stress and cardiovascular disease. J Am Coll Cardiol. 2008;51:1237–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


