Abstract
Aerobic energy metabolism uses glucose and oxygen to produce all the energy needs of the brain. Several studies published over the last 13 years challenged the assumption that the activated brain increases its oxidative glucose metabolism to meet the increased energy demands. Neuronal function in rat hippocampal slices supplied with 4 mm glucose could tolerate a 15 min activation by a 5 mm concentration of the excitatory neurotransmitter glutamate (Glu), whereas slices supplied with 10 mm glucose could tolerate a 15 min activation by 20 mm Glu. However, in slices in which neuronal lactate use was inhibited by the lactate transporter inhibitor a-cyano-4-hydroxycinnamate (4-CIN), activation by Glu elicited a permanent loss of neuronal function, with a twofold to threefold increase in tissue lactate content. Inhibition of glycolysis with the glucose analog 2-deoxy-d-glucose (2DG) during the period of exposure to Glu diminished normal neuronal function in the majority of slices and significantly reduced the number of slices that exhibited neuronal function after activation. However, when lactate was added with 2DG, the majority of the slices were neuronally functional after activation by Glu. NMDA, a nontransportable Glu analog by the glial glutamate transporter, could not induce a significant increase in slice lactate level when administered in the presence of 4-CIN. It is suggested that the heightened energy demands of activated neurons are met through increased glial glycolytic flux. The lactate thus formed is a crucial aerobic energy substrate that enables neurons to endure activation.
Keywords: hippocampal slices, energy metabolism, glutamate excitation, lactate transport, glial glycolytic flux, neuronal function
Normally, the substrates of cerebral energy metabolism are glucose and oxygen, and its products are ATP, carbon dioxide, and water (Clarke and Sokoloff, 1994). Moreover, it has been a long-held opinion that glucose is an obligatory energy substrate in the brain. However, the assumption that the heightened energy needs of activated brain tissue are answered via oxidative metabolism of glucose was challenged more than a decade ago (Fox and Raichle, 1986;Fox et al., 1988), initiating an ongoing controversy (Hyder et al., 1996; Malonek and Grinvald, 1996). Although many studies, using imaging techniques such as functional magnetic resonance imaging (fMRI) and blood oxygenation level-dependent fMRI, appear to support a transient uncoupling between cerebral blood flow (CBF) and oxidative metabolism after brain activation (Ogawa et al., 1992; Kim et al., 1994; Ramsey et al., 1996; Kim and Ugurbil, 1997), other studies, using the very same techniques, claim to observe a tight coupling between CBF and oxidative metabolism (Buxton and Frank, 1997; Malonek et al., 1997).
For years, elevated tissue lactate levels have been considered to signal the existence of hypoxia and anaerobic energy metabolism (Friedmann and Barborka, 1941; Haljamae, 1987). Although substantial evidence has been accumulated to indicate that large amounts of lactate can be produced in many tissues under fully aerobic conditions (Haljamae, 1987; Brooks, 1987), brain tissue has been presumed an exception. Thus, lactate production has been promoted as a major detrimental factor in ischemic brain damage (Siesjö, 1981).
Many studies now suggest that the brain is not necessarily different from other tissues, because it does produce lactate aerobically when stimulated in both humans (Fox and Raichle, 1986; Fox et al., 1988;Prichard et al., 1991; Raichle, 1991; Sappey-Marinier et al., 1992) and animals (Fellows et al., 1993; Fray et al., 1996; Hyder et al., 1996; Hu and Wilson, 1997). These results indicate that phasic changes in neural activity are supported by glycolysis. Nevertheless, other studies claim to demonstrate that oxidative metabolism of glucose meets all the energy needs of the activated brain (Hyder et al., 1996;Malonek and Grinvald, 1996).
In vitro research has shown that brain tissue produces lactate under aerobic conditions and has demonstrated the ability of this tissue to respire when lactate is the energy source (McIlwain, 1953a,b, 1956). Our studies and those of others indicated that lactate alone can support synaptic function in rat hippocampal slices (Schurr et al., 1988; Stittsworth and Lanthorn, 1993; Izumi et al., 1994). Lactate is a preferred substrate over glucose in sympathetic ganglia (Larrabee, 1995, 1996). Cerebellar slices prepared from adult rats had an increased ability to oxidize lactate to CO2 over slices prepared from neonates (Bueno et al., 1994). Studies with primary cultures of astrocytes (Pellerin and Magistretti, 1994; Magistretti et al., 1995) demonstrated the ability of glutamate (Glu) to stimulate glycolysis. These authors hypothesized that synaptically released Glu, taken up by astrocytes, is the signal that couples neuronal activity to glucose use. According to this hypothesis, astrocytic Glu uptake occurs via Na+ cotransport. The ensuing outward Na+ pumping activity is fueled by glycolysis. The lactate thus released from astrocytes is taken up by neurons as an aerobic energy substrate. This mechanism may explain the observed increase in glucose use and lactate production, as short-lived as they may be, without a concomitant increase in oxygen consumption after brain stimulation. Obviously, oxygen consumption should quickly resume for the oxidation of lactate by neurons. Unlike Glu, its analog NMDA is not taken up by glia because it is not recognized by the Glu transporter (Brew and Attwell, 1987; Rosenberg and Aizenmann, 1989;Rosenberg et al., 1992; Irwin et al., 1994).
Recently, we reported that glial lactate produced during hypoxia is an obligatory aerobic neuronal energy substrate after hypoxia (Schurr et al., 1997a,b,c).
Here, we tested the hypothesis that activation by Glu induces an increase in glial glycolytic flux to meet increased neuronal energy demands. We also explored the role of lactate, produced during such activation, in sustaining neuronal function after activation.
MATERIALS AND METHODS
Adult (200–350 gm) male Sprague Dawley rats were maintained and used according to the guidelines of the Institutional Animal Care and Use Committee. Hippocampal slices (400-μm-thick) were prepared using a McIlwain tissue chopper. Slices were placed in a dual incubation–recording chamber (12–15 slices per compartment) (Schurr et al., 1985) where the temperature was maintained at 34 ± 0.3°C, supplied with a humidified gas mixture (95% O2–5% CO2), and perfused with artificial CSF (aCSF) (60 ml/hr) of the following composition (in mm): NaCl 124, KCl 5, NaH2PO43, CaCl2 2.5, MgSO4 2, NaHCO3 23, and d-glucose 10 or 4, as indicated. The pH of the aCSF was 7.3–7.4. Where indicated, d-glucose was replaced with 10 mm 2-deoxy-d-glucose (2DG). Glu, NMDA, lactate (sodium salt), or α-cyano-4-hydroxycinnamate (4-CIN) was added via the aCSF. All chemicals were obtained from Sigma (St. Louis, MO).
Extracellular recordings of evoked population spikes (neuronal function) were made using borosilicate micropipettes (2–5 MΩ). Bipolar stimulating electrodes were placed in the Schaffer collaterals (orthodromic stimulation), and stimulus pulses of 0.1 msec in duration and amplitude of 8–10 V were applied once per minute. A waveform analysis program was used to determine the amplitude of the evoked response. Any slice that exhibited a population spike with an amplitude of ≥3 mV was considered to be neuronally functional (Schurr et al., 1997a,b,c).
Lactate and glucose were measured using the enzymatic kits of Sigma, as described elsewhere (Schurr et al., 1997a,b,c).
Each data point in the experiments described in this study was repeated at least three times unless indicated otherwise. Values are mean ± SD. Statistical analyses for significant differences were performed using the paired t test for biochemical data and the χ2 test for electrophysiological data.
RESULTS
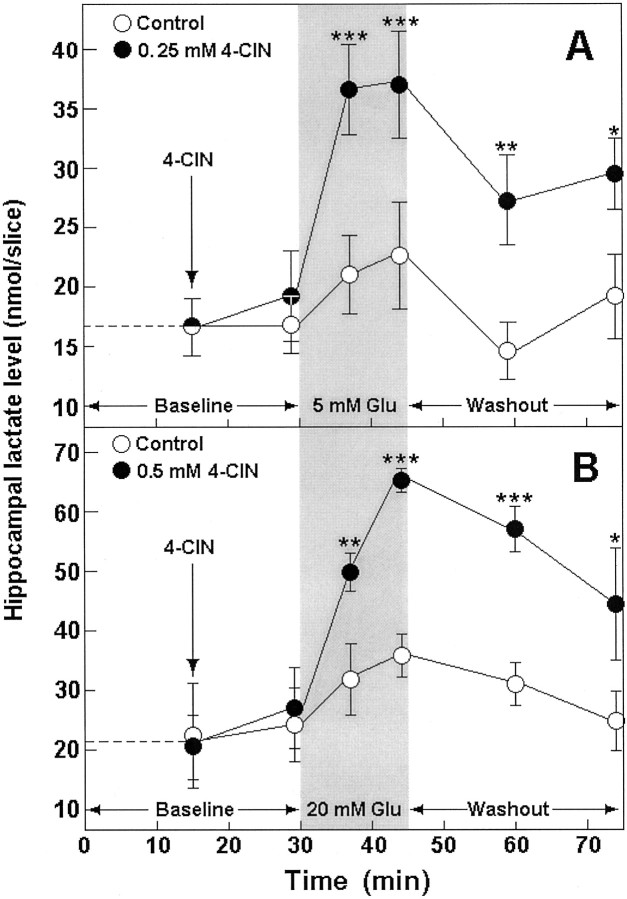
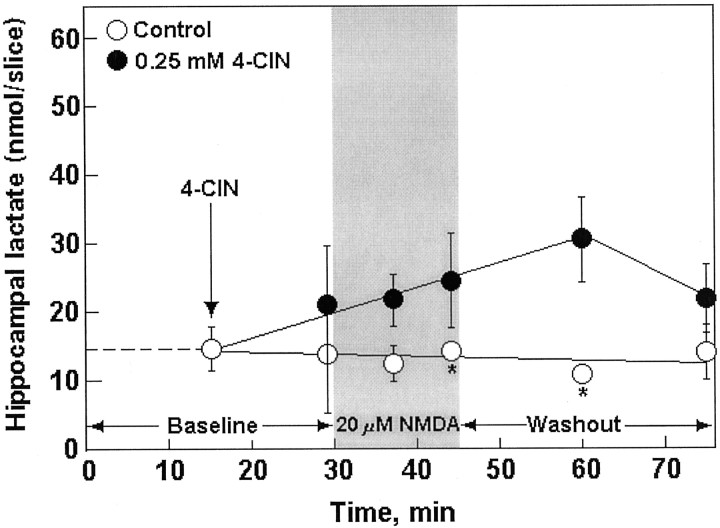
Because glucose is the main fuel of brain tissue during both rest and activation, we performed all the Glu experiments using two different glucose concentrations, 4 and 10 mm, in the aCSF that perfused the slices. This was done with the assumption that the higher the glucose concentration during activation, the higher the level of lactate produced by glia, and thus, the higher the concentration of Glu that slices would tolerate. Table1 (treatments 1, 2, 7, 8) shows the electrophysiological outcome, and Figure1 illustrates the biochemical outcome of exposing hippocampal slices to either 5 or 20 mm Glu in the absence or presence of the lactate transporter inhibitor 4-CIN (0.25 or 0.5 mm, respectively). This inhibitor was shown to inhibit neuronal lactate uptake in hippocampal slices (Schurr et al., 1997b) but not glial export of lactate (Volk et al., 1997). After 30 min of Glu washout, the majority of control slices exhibited normal neuronal function (population spike amplitude, ≥3 mV). Only 3 of 77 slices that were exposed to Glu in the presence of 4-CIN were neuronally functional after Glu washout (Table 1, treatments 2, 8). Biochemically, Glu was found to induce the accumulation of lactate in 4-CIN-treated hippocampal slices. In contrast, such accumulation was not observed in control slices (Fig. 1A,B). In both sets of slices, tissue glucose levels had not changed significantly during the exposure to Glu (results not shown). NMDA (20 μm) had a similar effect to that of Glu on neuronal function of 4 mm glucose-perfused hippocampal slices, i.e., an observable decline in population spike amplitude during 15 min of NMDA perfusion, with the majority of slices exhibiting preservation of neuronal function 30 min after NMDA washout (Table 1, treatment 5). Higher concentrations of NMDA (50–100 μm) diminished neuronal function in all slices, even after its washout, whereas a lower NMDA concentration (10 μm) was innocuous (results not shown). None of the slices that were exposed to 20 μmNMDA with 0.25 mm 4-CIN exhibited neuronal function 30 min after NMDA washout (Table 1, treatment 6). However, in contrast to the effect of Glu, when slices were perfused with NMDA (20 μm) in the presence of 4-CIN (0.25 mm), slice lactate level did not increase significantly (Fig.2).
Table 1.
The ratios of rat hippocampal slices incubated in either 4 or 10 mm glucose–aCSF that were neuronally functional (showing an orthodromically evoked CA1 population spike of an amplitude ≥3 mV) after 15 min perfusion with Glu (5 or 20 mm) or NMDA (20 μm) and 30 min of its washout
| Treatment | Number of neuronally functional slices/total | % of functional slices ± SD | p |
|---|---|---|---|
| 4 mm Glucose–aCSF | |||
| 1. Control Glu (5 mm)1-a | 37 /38 | 97.4 ± 4.4 | — |
| 2. Glu (5 mm) + 4-CIN (0.25 mm)1-b | 0 /38 | 0.0 | <0.0001* |
| 3. Glu (5 mm) + 2DG (10 mm)1-c (no glucose) | 16 /39 | 41.0 ± 11.8 | — |
| 4. Glu (5 mm) + 2DG (10 mm) + lactate (8 mm)1-d (no glucose) | 35 /37 | 94.7 ± 4.6 | <0.0001** |
| 5. NMDA (20 μm) | 31 /35 | 88.9 ± 19.2 | — |
| 6. NMDA (20 μm) + 4-CIN (0.25 mm) | 0 /35 | 0.0 | <0.0001*** |
| 10 mm Glucose–aCSF | |||
| 7. Control Glu (20 mm)1-a | 36 /39 | 91.9 ± 8.3 | — |
| 8. Glu (20 mm) + 4-CIN (0.5 mm)1-b | 3 /39 | 7.9 ± 8.4 | <0.0001**** |
| 9. Glu (20 mm) + 2DG (10 mm)1-c (no glucose) | 0 /38 | 0.0 | — |
| 10. Glu (20 mm) + 2DG (10 mm)1-d + lactate (20 mm) (no glucose) | 20 /38 | 52.8 ± 6.2 | <0.0001***** |
Slices were perfused with either 4 or 10 mm glucose–aCSF for 30 min before perfusion for 15 min with the same aCSF containing either 5 or 20 mm glutamate, respectively, followed by 30 min of washout with the same respective aCSF without glutamate. At the end of the washout period, slices were tested for the presence of neuronal function.
Similar to treatments 1 or 7, respectively, except that 4-CIN was added to the aCSF beginning 15 min before and during glutamate perfusion and during the glutamate washout period.
Similar to treatments 1 or 7, respectively, except that glucose was replaced by 2DG in the aCSF for the duration of glutamate perfusion (15 min).
Similar to treatments 3 or 9, respectively, except that lactate was added with 2DG for the duration of glutamate perfusion.
*Significantly different from treatment 1; **significantly different from treatment 3; ***significantly different from treatment 5; ****significantly different from treatment 7; *****significantly different from treatment 9.
Fig. 1.
Tissue content of lactate before (Baseline) and during (shaded area) exposure to Glu and during Glu washout (Washout) in control (open symbols) and 4-CIN-treated (filled symbols) rat hippocampal slices perfused with either 4 (A) or 10 mm(B) glucose–aCSF. *p < 0.03; **p = 0.01; ***p < 0.004, significantly different from control.
Fig. 2.
Tissue content of lactate before (Baseline) and during (shaded area) exposure to NMDA and during NMDA washout (Washout) in control (open symbols) and 4-CIN-treated (filled symbols) rat hippocampal slices perfused with 4 mm glucose–aCSF. Asterisks indicate that assay was done in duplicates only.
When 4 mm glucose was replaced with its nonmetabolizable analog 2DG during the period of 5 mm Glu perfusion (Table1, treatment 3), <50% of the slices exhibited neuronal function after 30 min of washout. When the same experiment was done with slices perfused with 10 mm glucose and 20 mm Glu (Table 1, treatment 9), none of the slices were neuronally functional after 30 min of Glu washout. However, when lactate was supplemented with 2DG (Table 1, treatments 4, 10), the majority of the slices were neuronally functional at the end of the Glu washout period.
DISCUSSION
We suggest that the higher level of lactate found in slices perfused with 10 mm glucose compared with those perfused with 4 mm glucose (Fig. 1, baseline) is responsible for the ability of the former to tolerate a higher concentration of Glu. This conclusion stems from the fact that 20 mm Glu, although having no effect on slices maintained on 10 mm glucose, completely diminished the neuronal function of slices maintained on 4 mm glucose (results not shown). Although the concentrations of Glu used in this study (5 and 20 mm) could be considered excitotoxic rather than stimulating, slices incubated with high enough glucose levels (4 and 10 mm, respectively) showed no ill effects after Glu washout. Moreover, excitotoxicity could very well be considered a case of overactivation. After all, any interference with normal Glu uptake by glia through a disturbance in energy metabolism (hypoxia, inhibition of glycolysis, etc.) enhances the excitotoxic effects of Glu (Novelli et al., 1988; Henneberry, 1989; Schurr et al., 1989). We interpreted the results shown in Figure 1 to indicate that under control conditions any lactate formed during exposure to Glu was immediately consumed by neurons as an aerobic energy substrate. The majority of lactate formation is most probably extraneuronal, i.e., glial. Two separate results support this conclusion. First, lactate transport into neurons is inhibited by 4-CIN (Schurr et al., 1997b), an inhibitor that was found to be ineffective in inhibiting glial export of lactate (Volk et al., 1997). If the majority of the lactate produced during exposure to Glu was neuronal in origin, 4-CIN would be without an effect. By inhibiting lactate transport into neurons, 4-CIN prevented its use and, eventually, the preservation of neuronal function after Glu washout. This is despite the ample supplies of glucose throughout the duration of the experiment. A 60 min perfusion of slices with 4-CIN (0.25 or 0.5 mm) without Glu did not change the levels of either glucose or lactate compared with untreated slices and had no effect on neuronal function, indicating that any increase in lactate level as shown in Figure 1 was induced by Glu rather than by the lactate transporter inhibitor. Nevertheless, Izumi and colleagues (1997) demonstrated that when glucose supplies are very limited, synaptic function is sustained by monocarboxylates, because inhibition of monocarboxylate transport with 4-CIN depressed neuronal EPSP of hippocampal slices and produced significant neuronal damage.
The second result in support of the postulate that glia are the main source of glycolytic lactate is the outcome of the experiments with NMDA (Fig. 2; Table 1, treatments 5, 6). NMDA, a Glu analog that is not recognized by the glial Glu transporter (Brew and Attwell, 1987;Rosenberg and Aizenmann, 1989; Rosenberg et al., 1992; Irwin et al., 1994), could not induce a significant increase in slice lactate level in the presence of 4-CIN. The fact that slices treated with 20 μm NMDA in the presence of 0.25 mm 4-CIN could not preserve neuronal function after washout of the excitotoxin highlights the importance of the basal level of lactate (15–20 nmol/slice) in protecting neurons from the damaging effects of activation. When the neuronal uptake of this basal lactate was blocked with 4-CIN, neuronal function was not protected from the toxic effect of NMDA. Moreover, this basal level of lactate is apparently insufficient for protection of neuronal function against the toxic effect of 50 or 100 μm NMDA. The fact that NMDA did not induce a significant increase in lactate production also argues against the possibility that this excitotoxin evokes the release of Glu, because such release would result in lactate production.
If glycolytic activity is of paramount importance during activation of brain tissue by Glu, one could speculate that blockade of glycolysis would weaken the ability of hippocampal slices to maintain neuronal function during 15 min exposure to Glu. Moreover, one could expect a diminishment in neuronal function after blockade of glycolysis in the presence of Glu, even after washout of the excitotoxin. Precisely this occurred when glucose was replaced with 2DG, a nonmetabolizable glucose analog and thus a glycolytic inhibitor, during the period of activation by Glu (Table 1, treatments 3, 9). Lactate supplementation with 2DG (Table 1, treatments 4, 10) preserved neuronal function in the majority of the slices at the end of the Glu washout period. This outcome clearly indicates that lactate plays a crucial role in assuring the preservation of neuronal viability during periods of brain tissue activation.
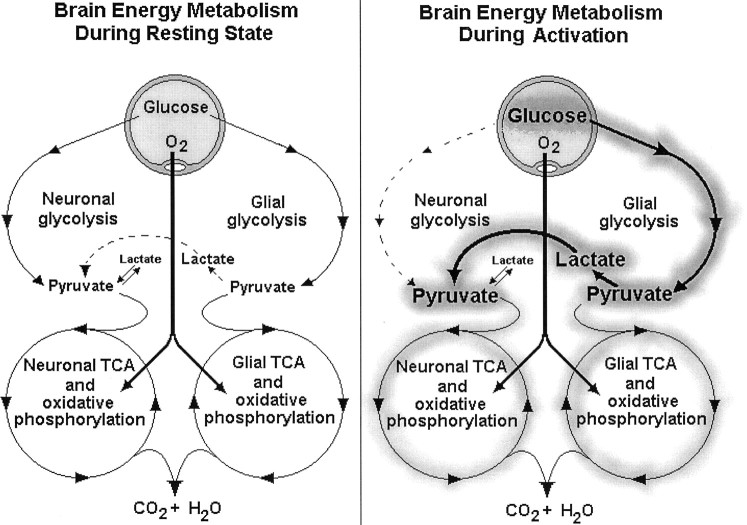
Based on the results of this investigation, we offer the following scenario (Fig. 3) for brain energy metabolism during a resting state and during activation (exposure to Glu) using the dual compartment model of Pellerin and Magistretti (1994). Under resting conditions, most of the glucose taken up by the brain from the blood supply is metabolized oxidatively in both the glial and neuronal compartments. Most of the basal lactate produced in glia is transported into neurons where it directly enters the neuronal TCA. After activation (exposure to Glu), an immediate glial Glu uptake is initiated, accompanied by Na+ transport (Pellerin and Magistretti, 1994, 1997; Magistretti et al., 1995). The need to pump out this extra Na+ brings about a dramatic increase in glial Na-K-ATPase activity and thus an equally dramatic increase in glucose consumption, most of which is glycolytic, as was evidenced by the large increase in lactate production in the presence of 4-CIN. A plausible explanation for the observed increase in this nonoxidative lactate production could be the existence of a separate glycolytic pathway, the sole purpose of which is to provide the glial Na-K-ATPase system with its own ATP supply (Lipton and Robacker, 1983). Because such a pathway does not require oxygen for its activity, no increase in oxygen consumption would be observed after its activation. The large amount of glial lactate produced under conditions of activation (Fig. 1) is transported out of glia by a specific glial lactate transporter (Bröer et al., 1997; Volk et al., 1997) and into neurons via a specific neuronal transporter. In neurons, lactate becomes the main aerobic energy substrate because of the concomitant increase in neuronal lactate/glucose ratio (Larrabee, 1995,1996). The increase in neuronal lactate use is accompanied by a decrease in neuronal glucose use or a reduced neuronal glycolytic flux (Larrabee, 1995, 1996). This scenario would explain the observation made by many investigators that the stimulation of brain tissue increases glucose uptake and consumption without a concomitant increase in O2 consumption (Fox and Raichle, 1986; Prichard et al., 1991; Raichle, 1991; Sappey-Marinier et al., 1992; Fellows et al., 1993; Magistretti et al., 1995), an increase that could be blunted by the decrease in neuronal glucose consumption as neurons shift into lactate use. Moreover, in vivo studies of energy metabolism of activated brain tissue (Fox and Raichle, 1986; Prichard et al., 1991; Raichle, 1991; Sappey-Marinier et al., 1992; Ogawa et al., 1992;Fellows et al., 1993; Kim et al., 1994; Magistretti et al., 1995; Hyder et al., 1996; Malonek and Grinvald, 1996; Ramsey et al., 1996; Kim and Ugurbil, 1997) cannot separate between the glial and the neuronal compartments and thus measure the sum of changes of both oxidative and nonoxidative glucose metabolism in both compartments. It is possible that a significant elevation in glial glucose consumption via increased glycolytic flux is masked by a significant decrease in neuronal oxidative use of glucose given that neuronal TCA flux during activation is supported almost entirely by consumption of glial lactate. After activation, this scenario thus envisions a total increase in glucose consumption, primarily glial and nonoxidative, and a decrease in neuronal oxidative glucose consumption, which is being replaced by an oxidative use of glial lactate. In other words, in each compartment, the normal resting stoichiometry of O2/glucose could change dramatically after activation. The glial compartment would exhibit a decrease in O2/glucose because of an increase in glycolytic flux, whereas the neuronal compartment would exhibit an increase in O2/glucose because of a reduction in glucose consumption and a significant increase in oxidative use of glial lactate. Consequently, the measured sum of the O2/glucose stoichiometry of both compartments, as is the case in vivo, could remain close to the normal value. Recently, Sibson et al. (1998) determined that the stoichiometry between oxidative glucose metabolism and glutamatergic neuronal activity in the cortex in vivo is close to 1:1, implying that the majority of the energy produced during activation supports glutamatergic neuronal function. Moreover, these authors envisioned that during neuronal Glu release and its glial uptake astrocytic glycolysis is the main glucose consumer, and neuronal lactate use is the main oxygen consumer.
Fig. 3.
Schematic diagram of the two main pathways of energy metabolism, glycolysis, and oxidative phosphorylation in two brain tissue compartments, neuronal and glial, during resting state (left) and during a state of activation (right). For more details, see Results.
In summary, our results strongly support the hypothesis that the energy needs of activated brain tissue are met through glial nonoxidative glucose consumption, which significantly increases lactate production. This extra lactate becomes a major neuronal oxidative energy substrate that significantly reduces the neuronal glucose consumption.
These findings could have broad implications for the understanding of the coupling between brain stimulation and energy metabolism and for several major brain disorders.
Footnotes
We thank C. Maldonado and H. L. Neibergs for helpful discussions and P. Bensinger for excellent editorial expertise.
Correspondence should be addressed to Avital Schurr, Brain Attack Research Laboratory, Department of Anesthesiology, University of Louisville, School of Medicine, Louisville, KY 40292.
REFERENCES
- 1.Brew H, Attwell D. Electrogenic glutamate uptake is a major current carrier in the membrane of axolotl retinal glial cells. Nature. 1987;327:707–709. doi: 10.1038/327707a0. [DOI] [PubMed] [Google Scholar]
- 2.Bröer S, Rahman B, Pellegri G, Pellerin L, Martin J-L, Verleysdonk S, Hamprecht B, Magistretti PJ. Comparison of lactate transport in astroglial cells and monocarboxylate transporter 1 (MCT 1) expressing Xenopus laevis oocytes. J Biol Chem. 1997;272:30096–30102. doi: 10.1074/jbc.272.48.30096. [DOI] [PubMed] [Google Scholar]
- 3.Brooks GA. Lactate production under fully aerobic conditions: the lactate shuttle during rest and exercise. Fed Proc. 1987;45:2924–2929. [PubMed] [Google Scholar]
- 4.Bueno D, Azzolin IR, Perry MLS. Ontogenic study of glucose and lactate utilisation by rat cerebellum slices. Med Sci Res. 1994;22:631–632. [Google Scholar]
- 5.Buxton RB, Frank LR. A model for the coupling between cerebral blood flow and oxygen metabolism during neural stimulation. J Cereb Blood Flow Metab. 1997;17:64–72. doi: 10.1097/00004647-199701000-00009. [DOI] [PubMed] [Google Scholar]
- 6.Clarke DD, Sokoloff L. Circulation and energy metabolism of the brain. In: Siegle GJ, Agranoff BW, Albers RW, Molinoff PB, editors. Basic neurochemistry. Raven; New York: 1994. pp. 645–680. [Google Scholar]
- 7.Fellows LK, Boutelle MG, Fillenz M. Physiological stimulation increases nonoxidative glucose metabolism in the brain of the freely moving rat. J Neurochem. 1993;60:1258–1263. doi: 10.1111/j.1471-4159.1993.tb03285.x. [DOI] [PubMed] [Google Scholar]
- 8.Fox PT, Raichle ME. Focal physiological uncoupling of cerebral blood flow and oxidative metabolism during somatosensory stimulation in human subjects. Proc Natl Acad Sci USA. 1986;83:1140–1144. doi: 10.1073/pnas.83.4.1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fox PT, Raichle ME, Mintun MA, Dence C. Nonoxidative glucose consumption during focal physiologic neural activity. Science. 1988;241:462–464. doi: 10.1126/science.3260686. [DOI] [PubMed] [Google Scholar]
- 10.Fray AE, Forsyth RJ, Boutelle MG, Fillenz M. The mechanisms controlling physiologically stimulated changes in rat brain glucose and lactate: a microdialysis study. J Physiol (Lond) 1996;496:49–57. doi: 10.1113/jphysiol.1996.sp021664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Friedmann TE, Barborka C. The significance of the ratio of lactic acid to pyruvic acid in blood after exercise. J Biol Chem. 1941;141:993–994. [Google Scholar]
- 12.Haljamae H. Lactate metabolism. Intens Care World. 1987;4:118–121. [Google Scholar]
- 13.Henneberry RC. The role of neuronal energy in the neurotoxicity of excitatory amino acids. Neurobiol Aging. 1989;10:611–613. doi: 10.1016/0197-4580(89)90149-8. [DOI] [PubMed] [Google Scholar]
- 14.Hu Y, Wilson GS. A temporary lacal energy pool coupled to neuronal activity: fluctuations of extracellular lactate levels in rat brain monitored with rapid-response enzyme-based sensor. J Neurochem. 1997;69:1484–1490. doi: 10.1046/j.1471-4159.1997.69041484.x. [DOI] [PubMed] [Google Scholar]
- 15.Hyder F, Chase JR, Behar KL, Mason GF, Siddeek M, Rothman DL, Shulman RG. Increased tricarboxylic acid cycle flux in rat brain during forepaw stimulation detected with 1H[13C]NMR. Proc Natl Acad Sci USA. 1996;93:7612–7617. doi: 10.1073/pnas.93.15.7612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Irwin RP, Lin S-Z, Paul SM. N-methyl-d-aspartate induces a rapid, reversible, and calcium-dependent intracellular acidosis in cultured fetal rat hippocampal neurons. J Neurosci. 1994;14:1352–1357. doi: 10.1523/JNEUROSCI.14-03-01352.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Izumi Y, Benz AM, Zorumski CF, Olney JW. Effects of lactate and pyruvate on glucose deprivation in rat hippocampal slices. NeuroReport. 1994;5:617–620. doi: 10.1097/00001756-199401000-00021. [DOI] [PubMed] [Google Scholar]
- 18.Izumi Y, Benz AM, Katsuki H, Zorumski CF. Endogenous monocarboxylates sustain hippocampal synaptic function and morphological integrity during energy deprivation. J Neurosci. 1997;17:9448–9457. doi: 10.1523/JNEUROSCI.17-24-09448.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim S-G, Ugurbil K. Comparison of blood oxygenation and cerebral blood flow effects in fMRI: estimation of relative oxygen consumption change. Magn Reson Med. 1997;38:59–65. doi: 10.1002/mrm.1910380110. [DOI] [PubMed] [Google Scholar]
- 20.Kim S-G, Hendrich K, Hu X, Merkle H, Ugurbil K. Potential pitfalls of functional MRI using conventional gradient-recalled echo techniques. NMR Biomed. 1994;7:69–74. doi: 10.1002/nbm.1940070111. [DOI] [PubMed] [Google Scholar]
- 21.Larrabee MG. Lactate metabolism and its effects on glucose metabolism in an excised neural tissue. J Neurochem. 1995;64:1734–1741. doi: 10.1046/j.1471-4159.1995.64041734.x. [DOI] [PubMed] [Google Scholar]
- 22.Larrabee MG. Partitioning of CO2 production between glucose and lactate in excised sympathetic ganglia, with implications for brain. J Neurochem. 1996;67:1726–1734. doi: 10.1046/j.1471-4159.1996.67041726.x. [DOI] [PubMed] [Google Scholar]
- 23.Lipton P, Robacker K. Glycolysis and brain function: [K+]o stimulation of protein synthesis and K+ uptake require glycolysis. Fed Proc. 1983;12:2875–2880. [PubMed] [Google Scholar]
- 24.Magistretti PJ, Pellerin L, Martin JL. Brain energy metabolism: an integrated cellular perspective. In: Bloom FE, Kupfer DJ, editors. Psychopharmacology: the Fourth Generation of Progress. Raven; New York: 1995. pp. 657–670. [Google Scholar]
- 25.Malonek D, Grinvald A. Interactions between electrical activity and cortical microcirculation revealed by imaging spectroscopy: implications for functional brain mapping. Science. 1996;272:551–554. doi: 10.1126/science.272.5261.551. [DOI] [PubMed] [Google Scholar]
- 26.Malonek D, Dirnagl U, Lindauer U, Yamada K, Kanno I, Grinvald A. Vascular imprints of neuronal activity: relationships between the dynamics of cortical blood flow, oxygenation, and volume changes following sensory stimulation. Proc Natl Acad Sci USA. 1997;94:14826–14831. doi: 10.1073/pnas.94.26.14826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McIlwain H. Glucose level, metabolism, and response to electrical impulses in cerebral tissues from man and laboratory animals. Biochem J. 1953a;55:618–624. doi: 10.1042/bj0550618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.McIlwain H. Substances which support respiration and metabolic response to electrical impulses in human cerebral tissues. J Neurol Neurosurg Psychiatry. 1953b;16:257–266. doi: 10.1136/jnnp.16.4.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McIlwain H. Electrical influences and speed of chemical change in the brain. Physiol Rev. 1956;36:355–375. doi: 10.1152/physrev.1956.36.3.355. [DOI] [PubMed] [Google Scholar]
- 30.Novelli A, Reilly JA, Lysko PG, Henneberry RC. Glutamate becomes neurotoxic via the N-methyl-d-aspartate receptor when intracellular energy levels are reduced. Brain Res. 1988;451:205–212. doi: 10.1016/0006-8993(88)90765-2. [DOI] [PubMed] [Google Scholar]
- 31.Ogawa S, Tank DW, Menon RS, Ellermann JM, Kim S-G, Merkle H, Ugurbil K. Intrinsic signal changes accompanying sensory stimulation: functional brain mapping using MRI. Proc Natl Acad Sci USA. 1992;89:5951–5955. doi: 10.1073/pnas.89.13.5951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pellerin L, Magistretti PJ. Glutamate uptake into astrocytes stimulates aerobic glycolysis: a mechanism coupling neuronal activity to glucose utilization. Proc Natl Acad Sci USA. 1994;91:10625–10629. doi: 10.1073/pnas.91.22.10625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pellerin L, Magistretti PJ. Glutamate uptake stimulates Na+,K+-ATPase activity in astrocytes via activation of a distinct subunit highly sensitive to ouabain. J Neurochem. 1997;69:2132–2137. doi: 10.1046/j.1471-4159.1997.69052132.x. [DOI] [PubMed] [Google Scholar]
- 34.Prichard J, Rothman D, Novotny E, Petroff O, Kuwabara T, Avison M, Howseman A, Hanstock C, Shulman R. Lactate rise detected by 1H NMR in human visual cortex during physiologic stimulation. Proc Natl Acad Sci USA. 1991;88:5829–5831. doi: 10.1073/pnas.88.13.5829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Raichle ME. The metabolic requirements of functional activity in the human brain: a positron emission tomography study. Adv Exp Med Biol. 1991;291:1–4. doi: 10.1007/978-1-4684-5931-9_1. [DOI] [PubMed] [Google Scholar]
- 36.Ramsey NF, Kirby BS, Van Gelderen P, Berman KF, Duyn JH, Frank JA, Mattay VS, van Horn JD, Eposito G, Moonen CTW, Weinberger DR. Functional mapping of human sensorimotor cortex with 3D Bold fMRI correlates highly with H215O PET rCBF. J Cereb Blood Flow Metab. 1996;16:755–764. doi: 10.1097/00004647-199609000-00001. [DOI] [PubMed] [Google Scholar]
- 37.Rosenberg PA, Aizenmann E. Hundred-fold increase in neuronal vulnerability to glutamate toxicity in astrocytes-poor cultures of rat cerebral cortex. Neurosci Lett. 1989;103:162–168. doi: 10.1016/0304-3940(89)90569-7. [DOI] [PubMed] [Google Scholar]
- 38.Rosenberg PA, Amin S, Leitner M. Glutamate uptake disguises neurotoxic potency of glutamate agonists in cerebral cortex in dissociated cell culture. J Neurosci. 1992;12:56–61. doi: 10.1523/JNEUROSCI.12-01-00056.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sappey-Marinier D, Calabrese G, Fein G, Hugg JW, Biggins C, Weiner MW. Effect of photic stimulation on human visual cortex lactate and phosphates using 1H and 31P magnetic resonance spectroscopy. J Cereb Blood Flow Metab. 1992;12:584–592. doi: 10.1038/jcbfm.1992.82. [DOI] [PubMed] [Google Scholar]
- 40.Schurr A, Reid KH, Tseng MT, Edmonds HL, Jr, Rigor BM. A dual chamber for comparative studies using the brain slice preparation. Comp Biochem Physiol. 1985;82A:701–704. doi: 10.1016/0300-9629(85)90454-2. [DOI] [PubMed] [Google Scholar]
- 41.Schurr A, West CA, Rigor BM. Lactate-supported synaptic function in the rat hippocampal slice preparation. Science. 1988;240:1326–1328. doi: 10.1126/science.3375817. [DOI] [PubMed] [Google Scholar]
- 42.Schurr A, West CA, Rigor BM. Electrophysiology of energy metabolism and neuronal function in the hippocampal slice preparation. J Neurosci Methods. 1989;28:7–13. doi: 10.1016/0165-0270(89)90004-6. [DOI] [PubMed] [Google Scholar]
- 43.Schurr A, Payne RS, Miller JJ, Rigor BM. Brain lactate, not glucose, fuels the recovery of synaptic function from hypoxia upon reoxygenation: an in vitro study. Brain Res. 1997a;744:105–111. doi: 10.1016/s0006-8993(96)01106-7. [DOI] [PubMed] [Google Scholar]
- 44.Schurr A, Payne RS, Miller JJ, Rigor BM. Brain lactate is an obligatory aerobic energy substrate for functional recovery after hypoxia: further in vitro validation. J Neurochem. 1997b;69:423–426. doi: 10.1046/j.1471-4159.1997.69010423.x. [DOI] [PubMed] [Google Scholar]
- 45.Schurr A, Payne RS, Miller JJ, Rigor BM. Glia are the main source of lactate utilized by neurons for recovery of function posthypoxia. Brain Res. 1997c;774:221–224. doi: 10.1016/s0006-8993(97)81708-8. [DOI] [PubMed] [Google Scholar]
- 46.Sibson NR, Dhankhar A, Mason GF, Rothman DL, Behar KL, Shulman RG. Stoichiometric coupling of brain glucose metabolism and glutamatergic neuronal activity. Proc Natl Acad Sci USA. 1998;95:316–321. doi: 10.1073/pnas.95.1.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Siesjö BK. Cell damage in the brain: a speculative synthesis. J Cereb Blood Flow Metab. 1981;1:155–185. doi: 10.1038/jcbfm.1981.18. [DOI] [PubMed] [Google Scholar]
- 48.Stittsworth JD, Jr, Lanthorn TH. Lactate mimics only some effects of d-glucose on epileptic depolarization and long-term synaptic failure. Brain Res. 1993;630:21–27. doi: 10.1016/0006-8993(93)90637-3. [DOI] [PubMed] [Google Scholar]
- 49.Volk C, Kempski B, Kempski OS. Inhibition of lactate export by quercetin acidifies rat glial cells in vitro. Neurosci Lett. 1997;223:121–124. doi: 10.1016/s0304-3940(97)13420-6. [DOI] [PubMed] [Google Scholar]