Abstract
A circadian clock modulates the functional organization of the Japanese quail retina. Under conditions of constant darkness, rods dominate electroretinogram (ERG) b-wave responses at night, and cones dominate them during the day, yielding a circadian rhythm in retinal sensitivity and rod–cone dominance. The activity of tyrosine hydroxylase, the rate-limiting enzyme in dopamine synthesis, also exhibits a circadian rhythm in the retina with approximately threefold higher levels during the day than at night. The rhythm of tyrosine hydroxylase activity is opposite in phase to the circadian activity of tryptophan hydroxylase, the first enzyme in the melatonin biosynthetic pathway. We tested whether dopamine may be related to the physiological rhythms of the retina by examining the actions of pharmacological agents that effect dopamine receptors. We found that blocking dopamine D2 receptors in the retina during the day mimics the nighttime state by increasing the amplitude of the b-wave and shifting the retina to rod dominance. Conversely, activating D2 receptors at night mimics the daytime state by decreasing the amplitude of the b-wave and shifting the retina to cone dominance. A selective antagonist for D1 dopamine receptors has no effect on retinal sensitivity or rod–cone dominance. Reducing retinal dopamine partially abolishes rhythms in sensitivity and yields a rod-dominated retina regardless of the time of day. These results suggest that dopamine, under the control of a circadian oscillator, has a key role in modulating sensitivity and rod–cone dominance in the Japanese quail retina.
Keywords: dopamine, circadian rhythm, retina, ERG, quail, rod–cone dominance
Animals adapt their visual sensitivity to function optimally under a variety of lighting conditions. Adaptation in all animals begins at the earliest stages of light detection in the retina, using mechanisms that respond to changes in ambient light intensity (Dowling, 1987). Many animals have evolved additional mechanisms to modulate retinal sensitivity in the absence of changes in ambient lighting. For example, some have coupled retinal processes with circadian oscillators to adapt visual sensitivity in anticipation of changes in ambient intensity (Barlow et al., 1989;Remé et al., 1991). Others have developed centrifugal pathways that innervate the retina and modulate its sensitivity via efferent signals transmitted from the brain (Uchiyama, 1989).
The Japanese quail is an excellent model for studying both circadian and efferent modulation of retinal sensitivity. As in other avian visual systems, the quail retina receives a centrifugal input from the isthmo-optic nucleus of the brain. The efferent optic nerve activity triggered by this nucleus transiently enhances retinal ganglion cell responses to dynamic visual stimuli (Uchiyama and Barlow, 1994). The topographic organization of the centrifugal pathway and its relatively brief modulation of retinal sensitivity (∼300 msec) may serve to focus the attention of the quail to relevant visual targets.
An endogenous circadian oscillator also modulates the sensitivity of the quail retina. Under conditions of constant darkness, the sensitivity of the retina anticipates the day–night changes in ambient light intensity. At approximately the time of sundown, retinal sensitivity increases, stays high throughout the night, decreases near dawn, and remains low during the day (Manglapus et al., 1998a). These changes in the functional organization of the retina may be mediated by a circadian clock located with the eye (Underwood et al., 1988, 1990). Specifically, the ocular clock shifts rod–cone dominance so that rod signals dominate at night and cone signals dominate during the day (Manglapus et al., 1998a).
Here we present evidence that dopamine is the putative transmitter of these circadian rhythms in the quail retina. First, we show that synthesis in the retina of the precursor to dopamine, 3,4-dihydroxyphenylalanine (DOPA), exhibits a circadian rhythm. Second, we found that application of dopamine agonists at night shifts the retina from rod to cone dominance and that blockade of dopamine receptors during the day has the reverse effect. Finally, we show that depletion of dopaminergic cells in the retina abolishes circadian rhythms detected by the b-wave.
The action of dopamine on retinal function is not unique to the Japanese quail. The catecholamine seems to be a ubiquitous modulator of retinal processing (Dowling, 1991; Besharse and Iuvone, 1992; Witkovsky and Dearry, 1992). For example, it uncouples retinal horizontal cells in the fish retina (Lasater and Dowling, 1985) and mediates rod–cone coupling in Xenopus (Witkovsky et al., 1996). In goldfish, dopamine and a circadian oscillator modulate rod–cone inputs to cone horizontal cells (Wang and Mangel, 1996). Dopamine also influences brightness perception in goldfish (Lin and Yazulla, 1994). This suggests that dopamine may have a modulatory role in an avian retina.
MATERIALS AND METHODS
Animals. Sexually mature Japanese quail (Coturnix coturnix japonica) were purchased from Bruckner poultry laboratory (Cornell University, Ithaca, NY) and maintained on a 12:12 hr light/dark cycle for at least 1 week before experimentation. Methods for preparing animals for electrophysiological recordings have been described (Manglapus et al., 1998a). Briefly, quail are anesthetized [rompun (2 mg/kg); ketamine (5 mg/kg); urethane (10%; 1.0 ml/100 gm); and curare (0.1%; 0.3–0.4 ml/100 gm)], immobilized, and maintained on a heating pad (38–40°) in a light-tight shielded cage with constant moisturized air flowing through their lungs (95% O2/5% CO2; 140 ml/min).
Recording technique. We used the amplitude of the b-wave of the electroretinogram (ERG) as a convenient measure of retinal sensitivity (Dowling, 1960, 1987). To record the ERG, we sutured open the experimental eye and inserted a teflon-coated silver chloride electrode (0.005 inch bare; A-M Systems) through a small hole in the sclera into the vitreous. We then covered the eye with clear silicone to prevent drying (Dow Corning) and inserted a second electrode into the other eye to serve as a reference. Stimuli were generated with both xenon (Oriel Corporation) and tungsten light sources and were delivered to the eye with a light pipe (4 mm in diameter). At the surface of the cornea, the xenon light source gave an unattenuated output (indicated in figures as Log I = 0.0) via the light pipe of 1014 photons sec−1cm−2 between 470 and 610 nm (model S370; Graseby Optronics). The tungsten source, used only for tracking retinal responses at 610 nm, yielded an unattenuated output (Log I = 0.0) of 3.7 × 1015 photons sec−1 cm−2.
Pharmacological agents. The following dopaminergic ligands were purchased from Research Biochemicals (Natick, MA): haloperidol, a general blocker of dopamine receptors; SCH 23390, an antagonist of the D1 dopamine receptor; SKF 38393, an agonist of the D1 receptor; eticlopride, an antagonist of the D2 receptor; and quinpirole, an agonist of the D2 receptor. The antagonists were injected into the vitreous during an animal’s subjective day, and the agonists were injected during its subjective night. Haloperidol was dissolved in either 0.4% lactic acid or 0.2% tartaric acid. All other chemicals were dissolved in distilled water.
All pharmacological agents (10 μl final volume) were injected through the sclera into the vitreous of the experimental eye under dim red light with a Hamilton microliter syringe fitted with a 30 gauge needle. We estimate that 10 μl is ∼10% of the vitreal volume. Thus, pharmacological agents are diluted 10:1 in the vitreous, but it is not yet possible to estimate with precision the final concentrations at specific receptor sites. The effect of eticlopride was robust at a concentration of 5 mm but not at 50 μm. Similarly, the effect of quinpirole was clear at a concentration of 50 μm but not at 5 μm. An intensity–response function of the ERG b-wave was measured before an injection and at least 30 min after the injection. On occasion, a second injection of the same pharmacological agent was repeated within 2 hr of the first. Control studies showed that intravitreal injection of the vehicle (10 μl of distilled water) alone did not effect the waveform of the ERG, retinal sensitivity, or rod–cone dominance.
We selectively lesioned dopaminergic cells in the retina with injections of 6-hydroxydopamine (6-OHDA). In a given experiment, we injected 6-OHDA (25 μg/ml; 10 μl; 0.9% NaCl) into the vitreous on day 1 and repeated the injection a week later. Three days later (day 10), we assessed the sensitivity and rod–cone dominance of the retina by recording the ERG b-wave.
Measurement of in vivo tyrosine hydroxylase and tryptophan hydroxylase activity. The activities of tyrosine hydroxylase (TH) and tryptophan hydroxylase (TPH) can be estimatedin vivo by measuring the accumulation of their respective reaction products, DOPA and 5-hydroxytryptophan (5-HTP), after inhibition of aromatic l-amino acid decarboxylase with m-hydroxybenzylhydrazine (Carlsson et al., 1972). TH and TPH are key regulatory enzymes in the synthesis of dopamine and melatonin, respectively (e.g., Iuvone et al. 1978;Cahill and Besharse, 1990; Thomas and Iuvone, 1991), and accumulations of DOPA and 5-HTP are useful indices of dopamine and melatonin biosynthesis in the avian retina (Kazula et al., 1993; Thomas et al., 1993). DOPA and 5-HTP levels were measured at defined intervals during a 24 hr period of diurnal lighting and for 3 d in constant darkness. Animals were injected withm-hydroxybenzylhydrazine (150 mg/kg of body weight, i.p.). Thirty minutes later, the animals were killed by decapitation, and retinas were removed and frozen on solid CO2. Each retina was homogenized in cold 0.1 m HClO4 [20 vol (w/v)] containing 10 μm ascorbic acid, 0.1 mm EDTA, and 20 ng/ml 3,4-dihydroxybenzylamine as an internal standard. After centrifugation, DOPA and 5-HTP concentrations of the supernatant fractions were measured by HPLC with amperometric detection as described by Thomas and Iuvone (1991), except that the mobile phase contained 0.45 mm sodium octylsulfate.
RESULTS
Retinal levels of DOPA exhibit a circadian rhythm
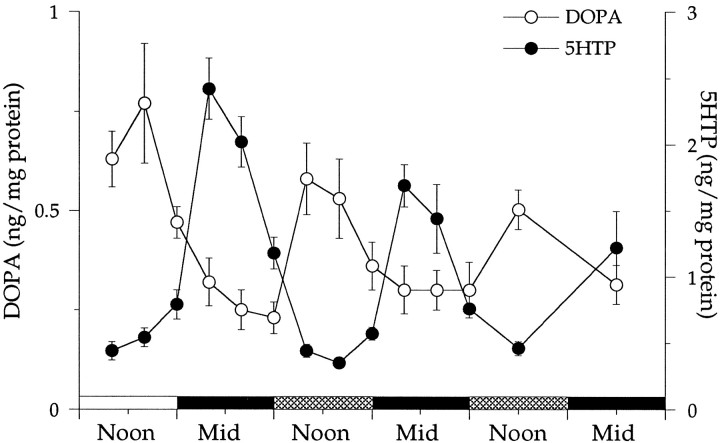
The activity of TH and, by inference, the rate of dopamine biosynthesis fluctuate in the retinas of animals exposed to cyclic lighting or maintained in constant darkness. Figure1 shows that under both conditions, DOPA accumulation is high during the daytime and low at night (open circles). The amplitude of the rhythm decreased ∼25% during the first 24 hr in constant darkness and continued to dampen on subsequent days. The changes in DOPA accumulation under constant darkness indicate that the synthesis of dopamine is controlled by an endogenous circadian pacemaker.
Fig. 1.
Plotted are the concentrations for DOPA (left y-axis) and 5-HTP (right y-axis) as functions of the time of subjective day and night. Noon (1200) is the middle of the subjective day, and midnight (Mid; 2400) is the middle of the subjective night. Concentrations were measured every 4 hr beginning at 0400 hr of the last day (open horizontal bar) that the animals were maintained in cyclic lighting. Measurements were continued for the next 2.5 d while the animals were maintained in darkness. Cross-hatched horizontal bars represent subjective day, and filled horizontal bars represent subjective night. Each point is the average of at least five measurements. Error bars indicate SEM, with some lying within the points.
Retinal 5-HTP accumulation also exhibits rhythmic changes when the animal is exposed to cyclic lighting or kept in constant darkness (Fig.1, filled circles). The rhythmic changes are opposite in phase to those detected in DOPA levels, that is, they are low during the day and high at night. As was the case with DOPA levels, the amplitude of the rhythm decreased during the first day in darkness and continued to dampen over the course of the measurements. The changes in 5-HTP levels shown in Figure 1 confirm the previous observation that a circadian clock modulates the synthesis of melatonin in the quail retina (Underwood et al., 1988, 1990).
What is the consequence of the circadian rhythms in retinal dopamine and melatonin? Are they related to the known circadian rhythms in retinal sensitivity and rod–cone dominance (Manglapus et al., 1998a)? We investigated the possible role of dopamine with pharmacological agents that eliminate dopaminergic cells and modulate D1 or D2 dopamine receptors.
Blocking dopamine receptors during the day shifts the retina to rod dominance
D2 receptors
Figure 2A plots the amplitude of the ERG b-wave response to 470 nm light flashes presented over a period of ∼20 hr in constant darkness. The amplitude is low (∼6 μV) during the late afternoon (1600–1800 hr), which is expected based on our previous studies of circadian rhythms in this retina (Manglapus et al., 1998a). The amplitude increases at approximately the time of dusk, reaches a peak of 15 μV near midnight, and declines in the early morning hours, returning to the low daytime level of ∼6 μV by 0800 hr. At 0935 hr, we injected eticlopride (5 mm; 10 μl), a D2-selective antagonist, into the vitreous. The b-wave amplitude increased rapidly after injection, nearly tripling in 1 hr to 15 μV, the same amplitude recorded the previous night. These data show that blocking D2 receptors during the daytime state of the retina increases the b-wave amplitude to nighttime levels. In other experiments (data not shown), eticlopride injection at night had no effect.
Fig. 2.
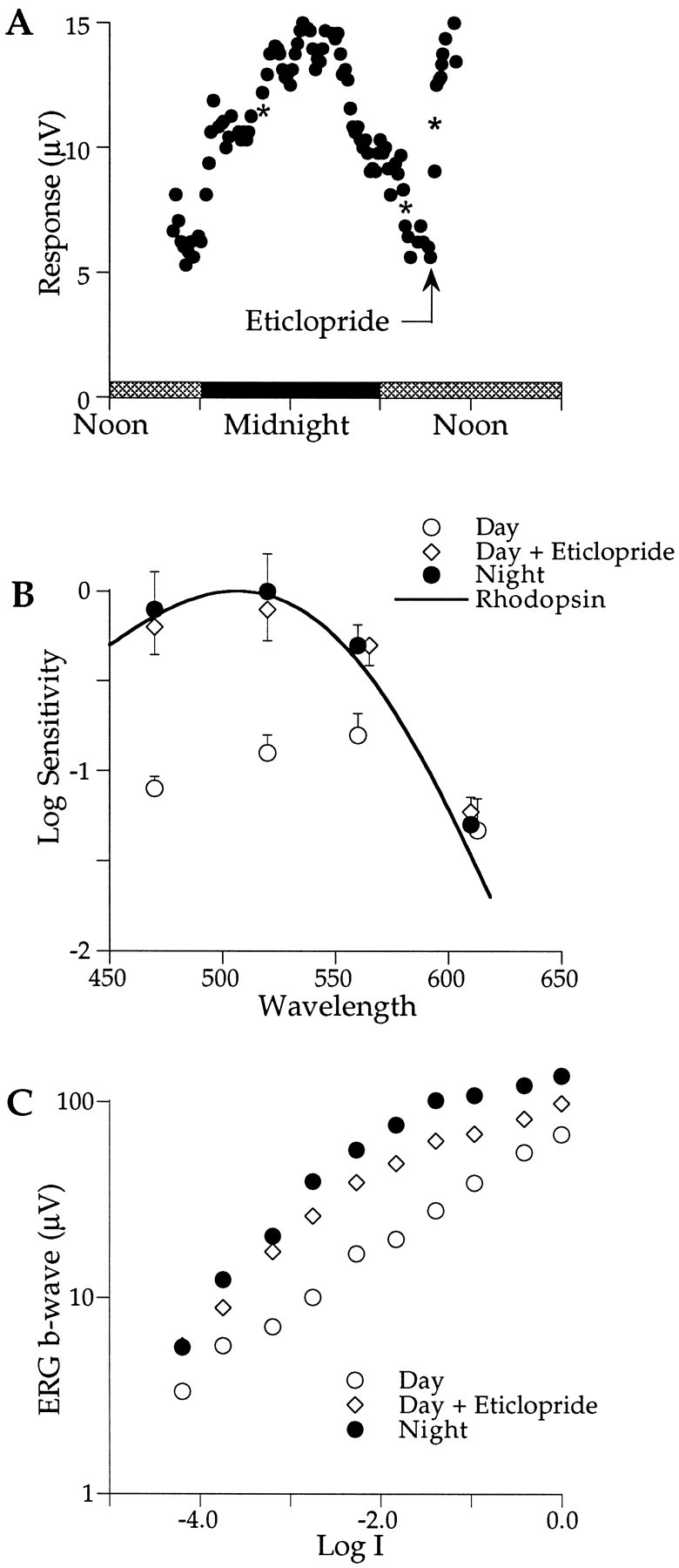
A, The amplitude of the ERG b-wave in response to 470 nm flashes is plotted as a function of time of day as described in Figure 1. The amplitude is low (∼5 μV) during the end of the first subjective day (cross-hatched horizontal bars), increases during the subjective night (filled horizontal bars), and decreases near the subjective dawn, reaching a low value (∼5 μV) by 0800 hr. Eticlopride, injected at 0935 hr (arrow), rapidly increased the b-wave amplitude to nighttime levels.Asterisks denote the time of spectral sensitivity measurements. B, Eticlopride, injected during the day, shifts the retina from cone dominance (open circles; λmax = ∼560 nm) to rod dominance (open diamonds; λmax = ∼500 nm) that matches the spectral sensitivity of the nighttime data (filled circles; λmax = ∼500 nm). Sensitivity does not change at the isosbestic point of 610 nm. The solid curve plots the rod nomogram for λmax = 506 nm. The criterion response was 10 μV (n = 3). Error bars indicate SEM. C, Intensity–response functions of the b-wave measured in constant darkness in response to 470 nm flashes are shown. The intensity–response function grows monotonically with increasing stimulus intensity at night (filled circles) but exhibits a characteristic plateau region at intermediate stim ulus intensities during the day (open circles). Eticlopride injection during the day abolishes the plateau, yielding a function that approximates that measured at night (open diamonds). At Log I = 0.0, each 50 msec flash delivered 5 × 1012 photons cm−2 at the cornea.
What influence does eticlopride have on rod–cone dominance? Figure2B plots the spectral sensitivity of the ERG b-wave before and after the injection of eticlopride during the day. Before injection, spectral sensitivity peaks near 560 nm (open circles), characteristic of cone dominance. After injection, spectral sensitivity shifts to ∼500 nm (open diamonds), characteristic of rod dominance. The spectral shift is associated with approximately a sixfold (or ∼0.8 log unit) increase in sensitivity that is also indicative of a rod-dominated retina. The spectral sensitivity measured during an the animal’s subjective night (filled circles) virtually overlays that after eticlopride injection during the day. Both of these spectral sensitivities are well fit by a rhodopsin nomogram (λmax = 506 nm; solid curve), thus providing further evidence that the retina is rod dominated at night and that injecting a D2 receptor blocker during the day can convert the retina from cone to rod dominance.
Does eticlopride influence the intensity coding characteristics of the b-wave response? Previous research revealed a circadian rhythm in the intensity–response function of the b-wave that was especially prominent for short wavelength stimuli (Manglapus et al., 1998a). The amplitude of the b-wave grows monotonically with increasing light intensity at night (Fig. 2C, filled circles) but not during the day (open circles). Increasing the intensity of 470 nm flashes during the day under dark-adapted conditions yields a characteristic plateau region for b-wave responses in the range of 6–8 μV. The plateau represents a shift from rod-dominated responses at low intensities to cone-dominated ones at higher intensities. This Purkinje-like shift occurs just above the estimated cone threshold (Manglapus et al., 1998a). The injection of eticlopride (5 mm; 10 μl) abolished the plateau region, yielding a monotonically increasing function (open diamonds) that matches the nighttime function. Blocking D2 receptors during the day transforms the intensity–response properties of the b-wave to the nighttime state, providing further evidence of a role of dopamine in the circadian rhythms of the quail retina.
Haloperidol (30 mm; 10 μl), a general D1 and D2 receptor blocker, mimics the action of eticlopride (data not shown). Injected during the day, haloperidol increases the ERG b-wave amplitude to nighttime levels and shifts the retina from cone to rod dominance.
D1 receptors
To examine the underlying receptor mechanisms further, we assessed the effect of SCH 23390, a selective antagonist of dopamine D1 receptors. Figure 3A shows the typical circadian changes in b-wave responses to 470 nm flashes for an animal maintained in constant darkness. The time course of amplitude changes follows closely that shown in Figure 2A. After the b-wave amplitude had declined to low daytime levels (∼1000 hr), SCH 23390 (10 mm; 10 μl) was injected intravitreally. No significant change in b-wave amplitude was detectable over the 4 hr after injection. At approximately the time of sundown, the b-wave increased slightly and then gradually decayed during the second night in darkness as is commonly observed (Manglapus et al., 1998a). The decay appears to reflect a decline in the health of the preparation. The lack of an effect of SCH 23390 in this and other experiments strongly suggests that dopamine D1 receptors are not involved in the circadian rhythms of the quail retina.
Fig. 3.
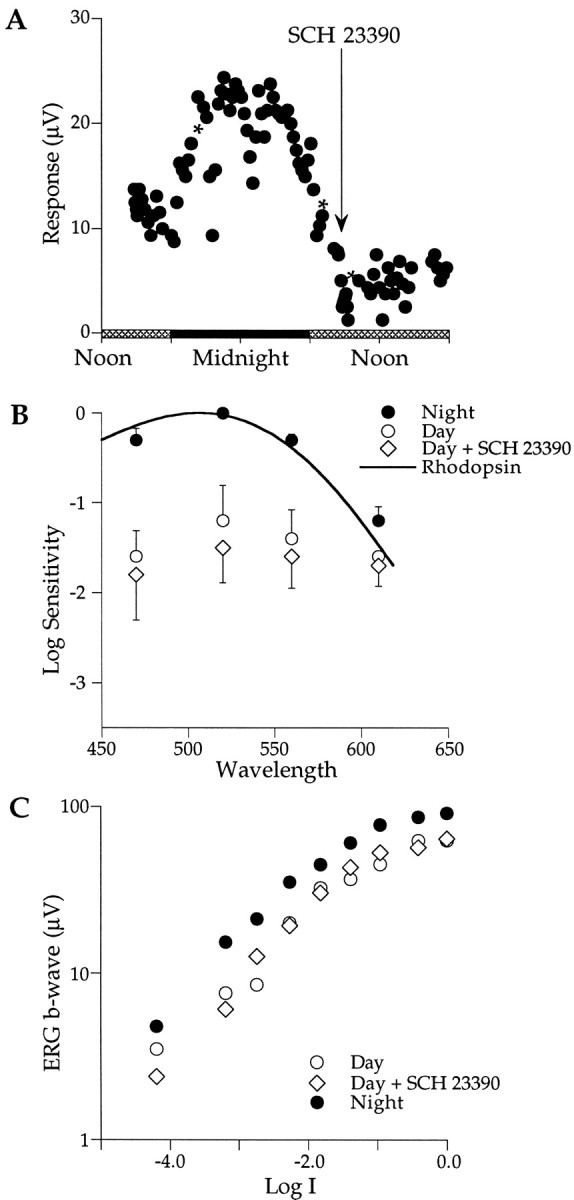
A, Injection of SCH 23390, a D1 receptor blocker, during the second subjective day (arrow; 0850 hr) does not affect the amplitude of the b-wave. B, SCH 23390 does not affect the spectral sensitivity of the retina (open diamonds) that remains in the cone-dominated daytime state. The criterion response was 10 μV (n = 3). C, SCH 23390, injected during the day, does not affect the intensity–response function.Symbols, asterisks, error bars, and the light cycle are described in Figure 2.
The spectral sensitivity of the retina is also unaffected by the injection of SCH 23390. Figure 3B summarizes the results of three experiments. As is commonly found, retinal sensitivity is rod dominated at night (filled circles) and fits well the rhodopsin nomogram (λ = 506 nm; solid curve). During the subjective day (open circles), the average retinal sensitivity decreased by ∼1.2 log units and shifted to cone dominance (λmax, ∼520–560 nm). Injecting SCH 23390 (10 mm; 10 μl) into the vitreous during the day had no effect (open diamonds); retinal sensitivity remained low and cone dominated (λmax, ∼520–560 nm), suggesting that D1 dopamine receptors are not involved in mediating the circadian changes in rod–cone dominance.
The b-wave intensity–response function was not significantly affected by injecting SCH 23390. Figure 3C shows that at night, b-wave responses grow monotonically with increasing intensity of 470 nm light flashes (filled circles). During the subjective day, the characteristic plateau region is detected in the range of 6–8 μV (open circles). The intensity–response function recorded after SCH 23390 injection (10 mm; 10 μl) nearly overlays the daytime data (open diamonds), providing further evidence that D1 receptors are not involved in the endogenous rhythms in the quail retina.
Comparing D1 and D2 effects
We explored further the effects of D1- and D2-selective antagonists on ERG b-wave amplitude by plotting the results from several experiments (Fig. 4). The b-wave responses more than double after injection of eticlopride, reaching nearly the same amplitude recorded the previous night within 30 min (Fig. 4, top). However, after injection of SCH 23390, the b-wave amplitude remains relatively unchanged or decreases (Fig. 4,bottom). The b-wave amplitude persists at low, daytime-like levels for up to 4 hr after injection (Fig. 4, bottom). These results support the idea that dopamine modulates circadian rhythms via a D2-like mechanism.
Fig. 4.
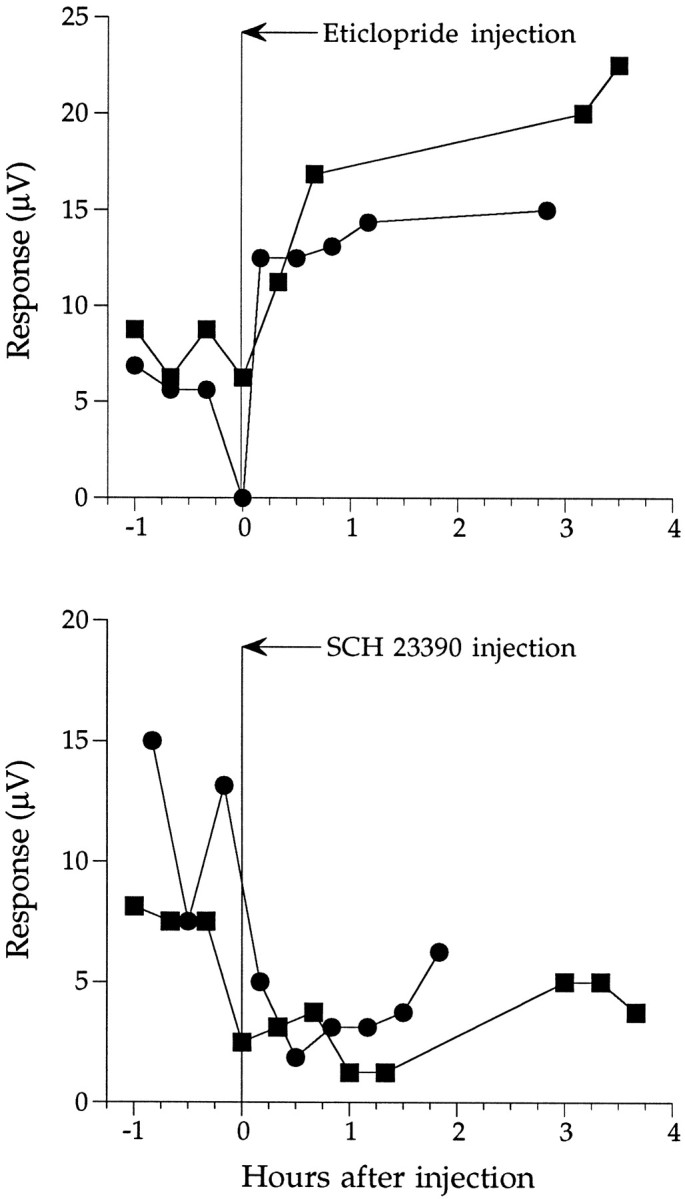
Top, Injection of eticlopride during the day increases the b-wave amplitude. The figure plots the results of two experiments. After injection of eticlopride, the b-wave responses more than double within 30 min, reaching nearly the same amplitude recorded the previous night. Bottom, The b-wave amplitude remains relatively unchanged or decreases after injection of SCH 23390. The results of two experiments are plotted. The b-wave amplitude persists at low, daytime-like levels for up to 4 hr after injection.
Activating dopamine receptors at night shifts the retina to cone dominance: D2 receptors
Figure 5A plots b-wave amplitude in response to 470 nm flashes for an animal maintained in constant darkness. For the experiments shown in Figures2A and 3A, the b-wave amplitude possesses a circadian rhythm. At the time of dusk, the b-wave amplitude began increasing from a low daytime value of ∼10 μV and leveled off at ∼30 μV at 2100 hr (Fig. 5A). Quinpirole (10 μl; 50 μm), a dopamine D2 receptor agonist, was injected into the vitreous at 2130 hr. Almost immediately the amplitude began to decrease. Within 1 hr (2230 hr), it reached ∼10 μV that is equal to that measured ∼7 hr earlier during the day. By midnight, the quinpirole effect was no longer detectable because the b-wave amplitude increased, reaching high nighttime levels at ∼0100 hr. Shortly after dawn (0600 hr), the b-wave amplitude began to decrease to low daytime levels as expected. These data show that at night a D2 receptor agonist can reduce retinal responses to daytime levels. In other experiments (data not shown), quinpirole injection during the day had no effect.
Fig. 5.
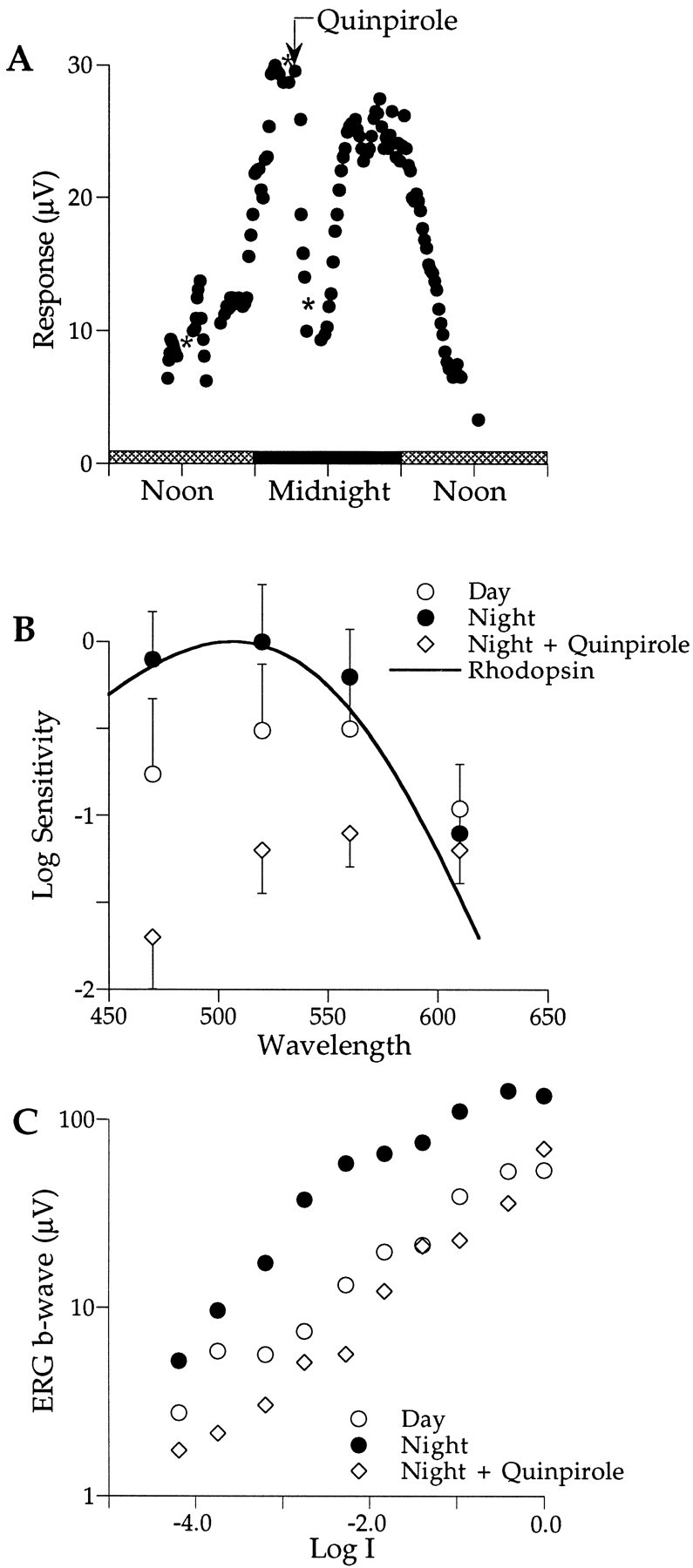
A, Quinpirole, injected at night (arrow; 2150 hr), reduces the b-wave amplitude to low daytime levels within ∼1 hr (2230 hr). After midnight, the quinpirole effect reverses, and the b-wave amplitude returns to its high nighttime state. Shortly after dawn, the b-wave decreases to the low daytime levels. B, Quinpirole, injected at night, reduces sensitivity and shifts the retina from rod (filled circles) to cone (open diamonds) dominance. Sensitivities fell well below those measured during the preceding day (open circles). The criterion responses were 10 μV (Day, n = 4; Night + Quinpirole, n = 7). C, Quinpirole, injected at night, reduces the b-wave response to all test intensities (open diamonds). Some are smaller than those measured during the preceding day (open circles).Symbols, asterisks, error bars, and the light cycle are described in Figure 2.
Figure 5B shows that quinpirole can also shift the spectral sensitivity of the retina. Nighttime spectral sensitivity (filled circles) peaks at ∼500 nm and is well fit by a rhodopsin nomogram (λmax = 506 nm; solid curve), indicating rod dominance. After activation of dopamine D2 receptors at night with quinpirole, retinal sensitivity decreases ∼30-fold, and peak spectral sensitivity shifts to ∼550 nm, signifying cone dominance (open diamonds). The spectral shift induced by quinpirole at night resembles that recorded during the subjective day (open circles). Light adaptation at night can also shift the retina from rod to cone dominance (Manglapus et al., 1998a). These data suggest that activation of dopamine D2 receptors at night can mimic the effects of light by shifting the retina to the daytime, cone-dominated state.
Figure 5C shows that quinpirole can also influence the intensity–response properties of the b-wave. The characteristic plateau region is apparent during the day (open circles) but not at night (filled circles; λ = 470 nm). Injection of quinpirole (50 μm; 10 μl) at night (open diamonds) reduced the amplitude of the b-wave at all test intensities to below those measured during the day. The shape of the intensity–response function changed significantly with just a suggestion of a plateau in the range of Log I = −3.0 to −2.0. These data are consistent with those in Figure 5, A and B, and provide further evidence that activating D2 dopamine receptors at night affects both retinal sensitivity and rod–cone dominance by shifting the retina to the daytime state.
Reduction of retinal dopamine abolishes circadian rhythms
Rod–cone shift requires dopamine
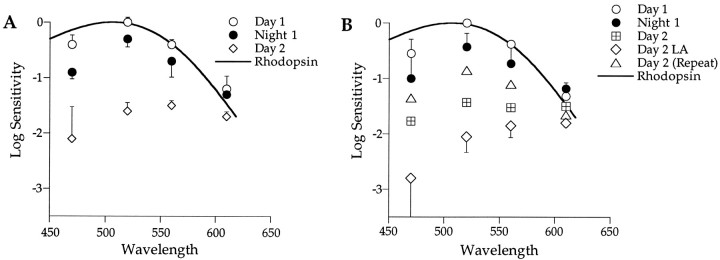
Intravitreal injection of 6-OHDA ablates dopaminergic cells in the vertebrate retina (Ehinger and Nordenfelt, 1977). Spectral sensitivity recorded from Japanese quail subjected to 6-OHDA reveals a nighttime-like, rod-dominated retina both day and night (Fig.6A). During the first day in darkness, retinal sensitivity is high and peaks in the range of 500–520 nm (open circles). As the animal enters subjective night, sensitivity remained high with no significant shift in spectral sensitivity; the retina remains rod dominated (λmax, ∼506 nm; filled circles). A rod nomogram fits well the spectral sensitivities measured during the first day and night (solid curve). During the second day in darkness, retinal sensitivity decreases, and its maximum spectral sensitivity shifts to longer wavelengths (λmax, ∼560–600 nm;open diamonds), indicative of cone contributions. This is a consistent finding in 6-OHDA–treated animals. Why their retinas partially shift toward cone dominance after 2 d in darkness is not known. This is not the case for untreated animals that exhibit clear circadian rhythms in rod–cone dominance throughout a 2 d period in darkness (Manglapus et al., 1998a). To explore these apparent rod–cone shifts in 6-OHDA–treated animals further, we measured their spectral sensitivities under conditions of both light and dark adaption after 2 d in darkness.
Fig. 6.
6-OHDA partially blocks circadian rhythms of rod–cone dominance. A, Spectral sensitivity of 6-OHDA–treated animals (n = 3) does not exhibit a rod–cone shift the first day in constant darkness. During Day 1, spectral sensitivity is high, rod dominated (open circles; λmax, ∼520 nm), and fit well by a rod nomogram (λmax = 506 nm). During Night 1, retinal sensitivity remains high and rod dominated (filled circles). Spectral sensitivity decreases and shifts to cone dominance (λmax, ∼560–600 nm) during Day 2 in constant darkness (open diamonds). Error bars indicate SEM. B, Dopamine is not required for light to shift the retina to cone dominance. As described in Figure 6A, spectral sensitivity does not change significantly from Day 1 (open circles) to Night 1 (filled circles) in 6-OHDA–treated animals. By Day 2 in constant darkness (crossed squares), retinal sensitivity decreases and shifts to longer wavelengths (λmax, ∼520–610 nm). Light adaptation (LA) during day 2 (Day 2 LA; open diamonds) further shifts the retina toward cone dominance (λmax, ∼560–610 nm). Dark adaptation during day 2 [Day 2 (Repeat); open triangles] increases retinal sensitivity and shifts it back to rod dominance (λmax, ∼520 nm). Under these conditions, the spectral sensitivity resembles that measured the first 24 hr and fits a rhodopsin nomogram reasonably well (solid curve; λmax = 506 nm). Error bars indicate SD (n = 2).
Light adaptation can override the clock
Figure 6B plots spectral sensitivities of the b-wave responses recorded during the first day in darkness from animals treated with 6-OHDA (open circles). Their retinas are rod dominated and remain so the following night (filled circles). A rhodopsin nomogram fits both sets of data reasonably well (λmax = 506 nm; solid curve). However, during the second day in constant darkness (crossed squares), retinal sensitivity decreases by ∼1 log unit, and its spectral sensitivity broadens and shifts toward longer wavelengths (λmax = 520–610 nm). As noted above, this long wavelength shift suggests a cone contribution to the generation of the b-wave in 6-OHDA–treated retinas after 24 hr in darkness. Light adaptation on day 2 (open diamonds) further reduces their sensitivity (0.4 log units) and with a clear shift toward cone dominance (λmax, ∼560–610 nm). A previous study showed that light adaptation of an untreated retina at night can override the clock’s influence and shift its sensitivity to cone dominance (Manglapus et al., 1998a) as we observe here for 6-OHDA–treated retinas. Dark adaptation of these retinas during day 2 (open triangles) increases their sensitivity by ∼1 log unit and shifts them back toward rod dominance. Under these conditions, the spectral sensitivity of the retina fits a rhodopsin nomogram reasonably well and is similar to that recorded during the first 24 hr in darkness. These experiments suggest that reduction of retinal dopamine partially blocks circadian rhythms in retinal sensitivity and rod–cone dominance. The neuromodulator however does not seem to be required for a light-induced shift to cone dominance.
DISCUSSION
Circadian rhythms characterize a wide range of visual systems
Circadian rhythms have been reported in the visual systems of a range of vertebrate retinas including goldfish, Xenopus,Anolis, rabbit, quail, rats, and humans (Katz et al., 1975;Brandenburg et al., 1983; Fowlkes et al., 1984; Pierce and Besharse, 1985; Terman and Terman, 1985; Bassi and Powers, 1986, 1987; Wang and Mangel, 1996; Barlow et al., 1997; Manglapus et al., 1998a). Effects range from circadian changes in intraocular pressure and mechanical movements of photoreceptors to changes in rod–cone dominance, dopamine synthesis, and visual sensitivity. Circadian rhythms also characterize many invertebrates and are generally associated with day–night changes in retinal sensitivity (Page, 1981;Barlow et al., 1989).
In most cases, the action of circadian clocks on retinal sensitivity is to increase it at night. Our results indicate that the clock in the Japanese quail retina acts to decrease its sensitivity during the day. A similar result was recently reported for zebrafish. Using a behavioral measure of avoidance to visual stimuli, Li and Dowling (1998) found evidence that a circadian oscillator decreases the visual sensitivity of zebrafish during the day rather than increasing it at night.
We have found that circadian changes in the sensitivity of the Japanese quail retina are associated with changes in rod–cone dominance such that rods dominate the b-wave responses at night and cones dominate them during the day (Manglapus et al., 1998a). In this study we examine the clock mechanism(s) that mediates these circadian changes in the retina.
Circadian rhythm of dopamine synthesis
The activity of TH, the rate-limiting enzyme of dopamine synthesis, exhibits a robust rhythm in the Japanese quail retina (Fig.1). Activity is high during the day and low at night. This rhythm persists in constant darkness, suggesting that a circadian oscillator may play an important role in controlling dopamine synthesis. Dopamine synthesis and release exhibit a circadian rhythm in the retinas of these vertebrates [rat (Wirz-Justice et al., 1984); fish (McCormack and Burnside, 1993); and pigeon (Adachi et al., 1998)]. In all cases dopamine synthesis is high during the day and low at night. Illumination of the retina at night, however, can override the clock and increase dopamine synthesis (Iuvone et al., 1978). We found that illumination of the Japanese quail retina at night can also override the circadian modulation of rod–cone dominance and shift the retina to daytime cone dominance (Manglapus et al., 1998a). Does dopamine under control of a circadian clock regulate the functional organization of the quail retina?
Dopamine modulates retinal sensitivity
The rhythm in the synthesis of a dopamine precursor is correlated with circadian changes in retinal sensitivity and rod–cone dominance (Figs. 2, 5), suggesting that dopamine is the circadian modulator. At night, dopamine levels are low, and the retina is rod dominated. During the day, dopamine levels are high, and the retina is cone dominated. Blocking dopamine receptors during the day shifts the retina to the rod-dominated nighttime state, and activating dopamine receptors at night shifts the retina to the cone-dominated daytime state. It appears that the circadian clock uses dopamine to change the functional organization of the retina. The release of dopamine during the day appears to block rod signals at the outer retina, allowing only cone signals to be transmitted to the inner retina. What receptor mechanisms mediate these dopamine effects?
Dopamine modulates circadian rhythms via a D2-like mechanism
Quinpirole activates dopamine D2-like receptors, and eticlopride blocks them. Injected at night, quinpirole decreases the sensitivity of the retina and shifts it to a cone-dominated daytime state (λmax = 550–560 nm; Fig. 5). Injected during the day, eticlopride has the opposite effect; it increases retinal sensitivity and shifts it to the nighttime state (Fig. 2). Modulation of these receptors alters the sensitivity and rod–cone dominance of the retina (Figs. 2, 5). Haloperidol, a general blocker of both D1 and D2 receptors, mimics the effects of eticlopride by shifting the retina to the rod-dominated nighttime state (data not shown). A selective antagonist of dopamine D1 receptors, SCH 23390, injected during the day does not influence either retinal sensitivity or rod–cone dominance (Fig. 3A,B). In sum, dopamine acts via D2-like receptors in the Japanese quail retina to change its sensitivity and rod–cone dominance.
Two general classes of dopamine receptors have been localized in the vertebrate retina (Kebabian and Calne, 1979). A D1-like receptor has been found on horizontal cells, and a D2-like receptor has been found on both photoreceptors and amacrine cells (Besharse and Witkovsky, 1992; Behrens and Wagner, 1995; Rohrer and Stell, 1995; for review, see Djamgoz and Wagner, 1992; Witkovsky and Dearry, 1992). The existence of dopamine receptors in the outer retina is consistent with our finding that dopamine acts at this level to modulate the transmission of rod signals to the inner retina.
What is the source of retinal dopamine?
Thus far amacrine cells are the only dopaminergic cell type identified in the avian retina [chick (Djamgoz and Wagner, 1992) and quail (M. K. Manglapus, unpublished observation)]. If they are the source of the circadian changes in retinal dopamine, then the dopamine they release must diffuse to the outer retina where it can modulate the transmission of photoreceptor signals to the inner retina (Witkovsky et al., 1993).
Dopamine effects in other retinas
Dopamine has been shown to exert both morphological and physiological effects in a range of vertebrate retinas. It can induce light-adaptive cone contraction via a D2-like receptor mechanism inXenopus (Pierce and Besharse, 1985) and fish (Dearry and Burnside, 1986; McCormack and Burnside, 1993); however, its removal does not inhibit circadian retinomotor movements in fish (Douglas et al., 1992). Dopamine, possibly acting at D1-like receptors, mediates a circadian rhythm in spinule formation in fish horizontal cells (Wagner et al., 1992). Also acting via D1 receptors, it decreases gap junctional coupling between horizontal cells in the fish retina (Lasater and Dowling, 1985). Mangel and Wang (1996) reported that light-evoked responses of cone horizontal cells in the fish retina exhibit a circadian rhythm; cone responses predominate during the day, and rod responses predominate at night. Presumably dopamine acts via D2 and D4 receptors on inner segments of photoreceptor cells in the fish retina, increasing cone input to horizontal cells (Mangel and Wang, 1996). Reduction of retinal dopamine or blockade of its receptors during the day increases rod input to the cone horizontal cells (Mangel and Wang, 1996). Similarly, both light and dopamine modify photoreceptor input to horizontal cells in the Xenopusretina (Krizaj and Witkovsky, 1993). Dopamine acting via D2 receptors regulates gap junctions between rods and cones and thereby influences rod–cone coupling (Witkovsky et al., 1988, 1996; Krizaj and Witkovsky, 1993).
We have no direct evidence that the mechanisms described above function in the quail retina, but it is not unreasonable to suggest that dopamine modulates photoreceptor signals at the outer plexiform layer of the quail retina by affecting the coupling of rods and cones as well as their input to horizontal cells. Whatever the mechanism(s) underlying the endogenous rod–cone shift is, it must be (1) located in the outer plexiform layer because a rod–cone shift is detected in the responses of ON bipolar cells (b-waves) and not in those of photoreceptors [Manglapus et al. (1998a), their Fig. 5, PIII]; (2) triggered by cone responses because rod signals are not blocked below the predicted cone threshold during the day [Manglapus et al. (1998a), their Fig. 8]; and (3) mediated by dopamine via D2 receptors (Figs. 2, 5).
Our working hypothesis is that melatonin, under control of a circadian clock, reduces dopamine levels and shifts the retina to rod dominance and increases its sensitivity at night. With this scheme, dopamine reduces the sensitivity of the retina and prepares the retina for visual processing during the day.
What cells mediate these modulatory mechanisms? One possible way for cones to influence rod signals is by direct rod–cone coupling. Functional coupling of rods and cones has been detected in many vertebrate retinas [salamander (Yang and Wu, 1989); rabbit (DeVries and Baylor, 1995); cat (Nelson, 1977); and primate (Schneeweis and Schnapf, 1995)]. Indeed, Witkovsky et al. (1988, 1996) showed that dopamine can modify rod–cone coupling such that cone signals are enhanced and rod signals are suppressed. Similarly, Yang and Wu (1989)reported that light mimics the effects of dopamine in the salamander retina. Because D2 receptors have been detected on photoreceptor inner segments in chick, the same may be true for quail so that they can detect dopamine released by amacrine cells during the day. Activation of D2 receptors may then increase rod–cone coupling and enable cones to shunt rod signals, effectively blocking their transmission to the inner retina. This would continue to be the case when dopamine levels are high during the day and reverse at night. The expected changes in spectral sensitivity are exactly what we observe (Manglapus et al., 1998a) (Figs. 2, 3, 5, open circles).
Feedback from horizontal cells that receive input from both rods and cones may provide an alternative mechanism for cones to block rod signals. Mariani (1987) has reported such a horizontal cell in the avian retina but also reports three other types of horizontal cell that do not receive mixed inputs. It is therefore difficult to conceive of a mechanism involving horizontal cells that would allow cones to block completely transmission of rod signals to the inner retina during the day.
What retinal cells contain the clock(s)?
Many, perhaps all, vertebrate eyes contain endogenous circadian oscillators [Xenopus (Besharse and Iuvone, 1983); rat (Remé et al., 1991); quail (Underwood et al., 1988,1990); and hamster (Tosini and Menaker, 1996)], but what retinal cells contain the oscillator(s)? A reduced Xenopusretina, one devoid of most all postphotoreceptor cells, generates a circadian rhythm in melatonin synthesis, strongly indicating that the clock may reside in photoreceptor cells (Cahill and Besharse, 1993). A similarly reduced chick retina also maintains rhythmic melatonin synthesis, again suggesting that the photoreceptor cells may be the site of circadian oscillators (Thomas et al., 1993). Vertebrate photoreceptors can also mediate circadian regulation of gene expression. Specifically, mRNAs for chicken serotoninN-acetyltransferase and Xenopus TPH that express a circadian rhythm are localized to photoreceptor cells (Green et al., 1995a,b; Bernard et al., 1997). Additionally, iodopsin, the red cone visual pigment, exhibits a circadian rhythm in quail and chick retinal cultures (Pierce et al., 1993). Finally, a circadian rhythm of photoreceptor responses (PIII) may reflect the action of circadian oscillators within the photoreceptors themselves (Manglapus et al., 1998a). Taken together, these data point to photoreceptors as the loci of circadian oscillators.
What is the role of melatonin?
Although dopamine is the focus of this paper, it is interesting that the circadian rhythm of its precursor DOPA is reciprocally related to that of the precursor for melatonin (Fig. 1). It is also interesting that two studies have associated melatonin with retinal function in darkness. In one, Pierce and Besharse (1985) found that melatonin mimics the effect of darkness on cone elongation. In the other, Mangel and Wang (1996) found that melatonin applied to the goldfish retina in vitro shifts the responses of cone horizontal cells to rod dominance. Inspired by their results, we injected melatonin into the vitreous of the quail retina following the procedures outlined above. Although these experiments are preliminary, we have yet to detect an effect of melatonin on either retinal sensitivity or rod–cone dominance (Manglapus et al., 1998b). This may not be a valid test for melatonin because we do not know whether our method delivers melatonin to appropriate sites of action in the retina. This leaves open the question of whether melatonin has a direct role in the circadian rhythms of the quail retina.
Do dopamine and melatonin interact?
Perhaps melatonin has an indirect role in the generation of retinal circadian rhythms in the quail. Indeed, rhythms in melatonin synthesis have been detected in a number of retinas (Hamm and Menaker, 1980; Underwood et al., 1988; Adachi et al., 1995; Tosini and Menaker, 1996) and are directly regulated by circadian oscillators in bothXenopus and chicken retinas (Green et al., 1995b; Bernard et al., 1997; Chong et al., 1999). Melatonin inhibits dopamine release in rabbit, Xenopus, and chick retinas (Dubocovich, 1983, 1988;Boatright et al., 1994), and conversely, dopamine inhibits melatonin release in Xenopus and chicken retinas (Iuvone, 1986;Zawilska and Iuvone, 1989; Cahill and Besharse, 1991; Zawilska et al., 1994). Thus, these two neuromodulators form a mutual inhibitory or “push–pull” biosynthetic mechanism in the chick andXenopus. If this were the case in quail retina, then the interacting neuromodulators would enhance the functional reorganization of the retina at dawn and dusk. Because dopamine and melatonin have been detected in the retinas of many species, the circadian mechanisms we report here for the quail retina may be widespread in the animal kingdom.
Footnotes
This research was supported in part by Research to Prevent Blindness, National Institutes of Health (NIH) Grant EY 00667 and National Science Foundation Grant IBN 9696208 to R.B.B., NIH Grant EY 04864 to P.M.I., NIH Grant EY 10672 to M.E.P., and NIH Grant NS 20961 to H.U. We thank Neal Buelow for technical assistance.
Correspondence should be addressed to Dr. Mary K. Manglapus, Center for Vision Research, State University of New York Health Science Center, 750 East Adams Street, Syracuse, NY 13210.
REFERENCES
- 1.Adachi A, Hasegawa M, Ebihara S. Measurement of circadian rhythms of ocular melatonin in the pigeon by in vivo microdialysis. NeuroReport. 1995;7:286–288. [PubMed] [Google Scholar]
- 2.Adachi A, Nogi T, Ebihara S. Phase-relationship and mutual effects between circadian rhythms of ocular melatonin and dopamine in the pigeon. Brain Res. 1998;792:361–369. doi: 10.1016/s0006-8993(98)00206-6. [DOI] [PubMed] [Google Scholar]
- 3.Barlow RB, Chamberlain SC, Lehman HK. Circadian rhythms in the invertebrate retina. In: Stavenga D, Hardie R, editors. Facets of vision. Springer; Berlin: 1989. pp. 257–280. [Google Scholar]
- 4.Barlow RB, Boudreau EA, Moore DC, Huckins SC, Lindstrom AM. Glucose and time of day modulate human contrast sensitivity and fMRI signals from visual cortex. Invest Ophthalmol. 1997;38:S735. [Google Scholar]
- 5.Bassi CJ, Powers MK. Daily fluctuations in the detectability of dim lights by humans. Physiol Behav. 1986;38:871–877. doi: 10.1016/0031-9384(86)90056-9. [DOI] [PubMed] [Google Scholar]
- 6.Bassi CJ, Powers MK. Circadian rhythm in goldfish visual sensitivity. Invest Ophthalmol. 1987;28:1811–1815. [PubMed] [Google Scholar]
- 7.Behrens UD, Wagner H-J. Localization of dopamine D1-receptors in vertebrate retinae. Neurochem Int. 1995;27:497–507. doi: 10.1016/0197-0186(95)00044-9. [DOI] [PubMed] [Google Scholar]
- 8.Bernard M, Klein DC, Zatz M. Chick pineal clock regulates serotonin N-acetyltransferase mRNA rhythm in culture. Proc Natl Acad Sci USA. 1997;94:304–309. doi: 10.1073/pnas.94.1.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Besharse J, Iuvone PM. Circadian clock in Xenopus eye controlling retinal serotonin N-acetyltransferase. Nature. 1983;305:133–135. doi: 10.1038/305133a0. [DOI] [PubMed] [Google Scholar]
- 10.Besharse J, Iuvone PM. Is dopamine a light-adaptive or a dark-adaptive modulator in retina? Neurochem Int. 1992;20:193–199. doi: 10.1016/0197-0186(92)90167-p. [DOI] [PubMed] [Google Scholar]
- 11.Besharse JC, Witkovsky P. Light-evoked contraction of red absorbing cones in the Xenopus retina is maximally sensitive to green light. Vis Neurosci. 1992;8:243–249. doi: 10.1017/s0952523800002893. [DOI] [PubMed] [Google Scholar]
- 12.Boatright J, Rubim NM, Iuvone PM. Regulation of endogenous dopamine release in amphibian retina by melatonin: the role of GABA. Vis Neurosci. 1994;11:1013–1018. doi: 10.1017/s0952523800003941. [DOI] [PubMed] [Google Scholar]
- 13.Brandenburg J, Bobbert AC, Eggelmeyer F. Circadian changes in the response of the rabbit’s retina to flashes. Behav Brain Res. 1983;7:113–123. doi: 10.1016/0166-4328(83)90008-6. [DOI] [PubMed] [Google Scholar]
- 14.Cahill G, Besharse JC. Circadian regulation of melatonin in the retina of Xenopus laevis: limitation by serotonin availability. J Neurochem. 1990;54:716–719. doi: 10.1111/j.1471-4159.1990.tb01932.x. [DOI] [PubMed] [Google Scholar]
- 15.Cahill G, Besharse JC. Circadian clock functions localized in Xenopus retinal photoreceptors. Neuron. 1993;10:573–577. doi: 10.1016/0896-6273(93)90160-s. [DOI] [PubMed] [Google Scholar]
- 16.Cahill GM, Besharse JC. Resetting the circadian clock in cultured Xenopus eyecups: regulation of retinal melatonin rhythms by light and D2 dopamine receptors. J Neurosci. 1991;11:2959–2971. doi: 10.1523/JNEUROSCI.11-10-02959.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carlsson A, Davis JN, Kehr W, Lundqvist M, Atack CV. Simultaneous measurement of tyrosine and tryptophan hydroxylase activities in brain in vivo using an inhibitor of the aromatic amino acid decarboxylase. Naunyn Schmiedebergs Arch Pharmacol. 1972;275:153–168. doi: 10.1007/BF00508904. [DOI] [PubMed] [Google Scholar]
- 18.Chong NW, Cassone VM, Bernard M, Klein DC, Iuvone PM (1999) Circadian expression of tryptophan hydroxylase mRNA in chicken retina. Mol Brain Res, in press. [DOI] [PubMed]
- 19.Dearry A, Burnside B. Dopaminergic regulation of cone retinomotor movement in isolated teleost retinas. I. Induction of cone contraction is mediated by D2 receptors. J Neurochem. 1986;46:1006–1021. doi: 10.1111/j.1471-4159.1986.tb00612.x. [DOI] [PubMed] [Google Scholar]
- 20.DeVries SH, Baylor DA. An alternative pathway for signal flow from rod photoreceptors to ganglion cells in mammalian retina. Proc Natl Acad Sci USA. 1995;92:10658–10662. doi: 10.1073/pnas.92.23.10658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Djamgoz M, Wagner H-J. Localization and function of dopamine in the adult vertebrate retina. Neurochem Int. 1992;20:139–191. doi: 10.1016/0197-0186(92)90166-o. [DOI] [PubMed] [Google Scholar]
- 22.Douglas RH, Wagner H-J, Zaunreiter M, Behrens UD, Djamgoz MBA. The effect of dopamine depletion of light-evoked and circadian retinomotor movements in the teleost retina. Vis Neurosci. 1992;9:335–343. doi: 10.1017/s0952523800010749. [DOI] [PubMed] [Google Scholar]
- 23.Dowling J. The chemistry of visual adaptation in the rat. Nature. 1960;188:114–118. doi: 10.1038/188114a0. [DOI] [PubMed] [Google Scholar]
- 24.Dowling J. The retina: an approachable part of the brain. Harvard UP; Cambridge, MA: 1987. [Google Scholar]
- 25.Dowling J. Retinal neuromodulation: the role of dopamine. Vis Neurosci. 1991;7:87–97. doi: 10.1017/s0952523800010968. [DOI] [PubMed] [Google Scholar]
- 26.Dubocovich M. Melatonin is a potent modulator of dopamine release in the retina. Nature. 1983;306:782–784. doi: 10.1038/306782a0. [DOI] [PubMed] [Google Scholar]
- 27.Dubocovich M. Role of melatonin in retina. In: Osborne NN, Chader GJ, editors. Progress in retinal research. Pergamon; Oxford: 1988. pp. 129–151. [Google Scholar]
- 28.Ehinger B, Nordenfelt L. Destruction of retinal dopamine-containing neurons in rabbit and goldfish. Exp Eye Res. 1977;24:170–187. doi: 10.1016/0014-4835(77)90258-5. [DOI] [PubMed] [Google Scholar]
- 29.Fowlkes D, Karwoski CJ, Proenza LM. Endogenous circadian rhythm in electroretinogram of free-moving lizards. Invest Ophthalmol. 1984;25:121–124. [PubMed] [Google Scholar]
- 30.Green C, Cahill G, Besharse JC. Regulation of tryptophan hydroxylase expression by a retinal circadian oscillator in vitro. Brain Res. 1995a;667:283–290. doi: 10.1016/0006-8993(95)00166-n. [DOI] [PubMed] [Google Scholar]
- 31.Green C, Cahill G, Besharse JC. Tryptophan hydroxylase is expressed by photoreceptors in Xenopus laevis retina. Vis Neurosci. 1995b;12:663–670. doi: 10.1017/s0952523800008956. [DOI] [PubMed] [Google Scholar]
- 32.Hamm H, Menaker M. Retinal rhythms in chicks: circadian variation in melatonin and serotonin N-acetyltransferase activity. Proc Natl Acad Sci USA. 1980;77:4998–5002. doi: 10.1073/pnas.77.8.4998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iuvone PM. Evidence for a D2-dopamine receptor in frog retina that decreases cyclic AMP accumulation and serotonin N-acetyltransferase activity. Life Sci. 1986;38:331–342. doi: 10.1016/0024-3205(86)90080-9. [DOI] [PubMed] [Google Scholar]
- 34.Iuvone PM, Galli CL, Garisson-Gund CK, Neff NH. Light stimulates tyrosine hydroxylase activity and dopamine synthesis in retinal amacrine neurons. Science. 1978;202:901–902. doi: 10.1126/science.30997. [DOI] [PubMed] [Google Scholar]
- 35.Katz RS, Heinkind P, Weitzman ED. The circadian rhythm of the intraocular pressure in the New Zealand white rabbit. Invest Ophthalmol. 1975;14:775–780. [PubMed] [Google Scholar]
- 36.Kazula A, Nowak JZ, Iuvone PM. Regulation of melatonin and dopamine biosynthesis in chick retina: the role of GABA. Vis Neurosci. 1993;10:621–629. doi: 10.1017/s0952523800005320. [DOI] [PubMed] [Google Scholar]
- 37.Kebabian JW, Calne DB. Multiple receptors for dopamine. Nature. 1979;277:493–498. doi: 10.1038/277093a0. [DOI] [PubMed] [Google Scholar]
- 38.Krizaj D, Witkovsky P. Effects of submicromolar concentration of dopamine on photoreceptor to horizontal cell communication. Brain Res. 1993;627:122–128. doi: 10.1016/0006-8993(93)90755-c. [DOI] [PubMed] [Google Scholar]
- 39.Lasater E, Dowling JE. Dopamine decreases conductance of the electrical junctions between cultured retinal horizontal cells. Proc Natl Acad Sci USA. 1985;82:3025–3029. doi: 10.1073/pnas.82.9.3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li L, Dowling JE. Zebrafish visual sensitivity is regulated by a circadian clock. Vis Neurosci. 1998;15:851–857. doi: 10.1017/s0952523898155050. [DOI] [PubMed] [Google Scholar]
- 41.Lin ZS, Yazulla S. Depletion of retinal dopamine increases brightness perception in goldfish. Vis Neurosci. 1994;11:683–693. doi: 10.1017/s0952523800002996. [DOI] [PubMed] [Google Scholar]
- 42.Mangel SC, Wang Y. Melatonin acts as a circadian clock regulator of rod and cone pathways in fish retina. Soc Neurosci Abstr. 1996;22:2017. [Google Scholar]
- 43.Manglapus M, Uchiyama H, Buelow N, Barlow R. Circadian rhythms of rod–cone dominance in the Japanese quail retina. J Neurosci. 1998a;18:4775–4784. doi: 10.1523/JNEUROSCI.18-12-04775.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Manglapus M, Pierce M, Barlow R. Rhythmic expression of melatonin does not influence rod-cone dominance in the quail retina. Soc Neurosci Abstr. 1998b;24:1872. [Google Scholar]
- 45.Mariani AP. Neuronal and synaptic organization of the outer plexiform layer of the pigeon retina. Am J Anat. 1987;179:25–39. doi: 10.1002/aja.1001790105. [DOI] [PubMed] [Google Scholar]
- 46.McCormack CA, Burnside B. Light and circadian modulation of teleost retinal tyrosine hydroxylase activity. Invest Ophthalmol. 1993;34:1853–1860. [PubMed] [Google Scholar]
- 47.Nelson R. Cat cones have rod input: a comparison of response properties of cones and horizontal cell bodies in the retina of the cat. J Comp Neurol. 1977;172:109–136. doi: 10.1002/cne.901720106. [DOI] [PubMed] [Google Scholar]
- 48.Page TL. Neural and endocrine control of circadian rhythmicity in invertebrates. In: Aschoff J, editor. Handbook of behavioral neurobiology, Vol 4, Biological rhythms. Plenum; New York: 1981. pp. 145–172. [Google Scholar]
- 49.Pierce M, Besharse JC. Circadian regulation of retinomotor movements I: interaction of melatonin and dopamine in the control of cone length. J Gen Physiol. 1985;86:671–689. doi: 10.1085/jgp.86.5.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pierce ME, Sheshberadaran H, Zhang Z, Fox LE, Applebury ML, Takahashi JS. Circadian regulation of iodopsin gene expression in embryonic photoreceptors in retinal cell culture. Neuron. 1993;10:579–584. doi: 10.1016/0896-6273(93)90161-j. [DOI] [PubMed] [Google Scholar]
- 51.Remé C, Wirz-Justice A, Terman M. The visual input stage of the mammalian circadian pacemaking system. I. Is there a clock in the mammalian eye? J Biol Rhythms. 1991;6:5–29. doi: 10.1177/074873049100600104. [DOI] [PubMed] [Google Scholar]
- 52.Rohrer B, Stell WK. Localization of putative dopamine D2-like receptors in the chick retina, using in situ hybridization and immunocytochemistry. Brain Res. 1995;695:110–116. doi: 10.1016/0006-8993(95)00700-z. [DOI] [PubMed] [Google Scholar]
- 53.Schneeweis DM, Schnapf JL. Photovoltage of rods and cones in the macaque retina. Science. 1995;268:1053–1056. doi: 10.1126/science.7754386. [DOI] [PubMed] [Google Scholar]
- 54.Terman M, Terman J. A circadian pacemaker for visual sensitivity? Ann NY Acad Sci. 1985;453:147–161. doi: 10.1111/j.1749-6632.1985.tb11807.x. [DOI] [PubMed] [Google Scholar]
- 55.Thomas KB, Iuvone PM. Circadian rhythm of tryptophan hydroxylase activity in chicken retina. Cell Mol Neurobiol. 1991;11:511–527. doi: 10.1007/BF00734813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Thomas KB, Tigges M, Iuvone PM. Melatonin synthesis and circadian tryptophan hydroxylase activity in chicken retina following destruction of serotonin immunoreactive amacrine and bipolar cells by kainic acid. Brain Res. 1993;601:303–307. doi: 10.1016/0006-8993(93)91725-8. [DOI] [PubMed] [Google Scholar]
- 57.Tosini G, Menaker M. Circadian rhythms in cultured mammalian retina. Science. 1996;272:419–421. doi: 10.1126/science.272.5260.419. [DOI] [PubMed] [Google Scholar]
- 58.Uchiyama H. Centrifugal pathways to the retina: influence of the optic tectum. Vis Neurosci. 1989;3:183–206. doi: 10.1017/s0952523800009950. [DOI] [PubMed] [Google Scholar]
- 59.Uchiyama H, Barlow RB. Centrifugal inputs enhance responses of retinal ganglion cells in the Japanese quail without changing their spatial coding properties. Vision Res. 1994;34:2189–2194. doi: 10.1016/0042-6989(94)90101-5. [DOI] [PubMed] [Google Scholar]
- 60.Underwood H, Siopes T, Barrett RK. Does a biological clock reside in the eye of quail? J Biol Rhythms. 1988;3:323–331. doi: 10.1177/074873048800300402. [DOI] [PubMed] [Google Scholar]
- 61.Underwood H, Barrett RK, Siopes T. The quail’s eye: a biological clock. J Biol Rhythms. 1990;5:257–265. doi: 10.1177/074873049000500307. [DOI] [PubMed] [Google Scholar]
- 62.Wagner HJ, Behrens UD, Zaunreiter M, Douglas RH. The circadian component of spinule dynamics in teleost retinal horizontal cells is dependent on the dopaminergic system. Vis Neurosci. 1992;9:345–351. doi: 10.1017/s0952523800010750. [DOI] [PubMed] [Google Scholar]
- 63.Wang Y, Mangel S. A circadian clock regulates rod and cone input to fish retinal cone horizontal cells. Proc Natl Acad Sci USA. 1996;93:4655–4660. doi: 10.1073/pnas.93.10.4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wirz-Justice A, Da Prada M, Reme C. Circadian rhythm in rat retinal dopamine. Neurosci Lett. 1984;45:21–25. doi: 10.1016/0304-3940(84)90323-9. [DOI] [PubMed] [Google Scholar]
- 65.Witkovsky P, Dearry A. Functional roles of dopamine in the vertebrate retina. In: Osborne NN, Chader GJ, editors. Progress in retinal research. Pergamon; Oxford: 1992. pp. 247–292. [Google Scholar]
- 66.Witkovsky P, Stone S, Besharse JC. Dopamine modifies the balance of rod and cone inputs to horizontal cells of the Xenopus retina. Brain Res. 1988;449:332–336. doi: 10.1016/0006-8993(88)91048-7. [DOI] [PubMed] [Google Scholar]
- 67.Witkovsky P, Nicholson C, Rice M, Bohmmaker K, Meller E. Extracellular dopamine concentration in the retina of the clawed frog, Xenopus laevis. Proc Natl Acad Sci USA. 1993;90:5667–5671. doi: 10.1073/pnas.90.12.5667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Witkovsky P, Gabriel R, Krizaj D. Modulation of rod-cone coupling in the Xenopus retina. Invest Ophthalmol. 1996;37:S675. [PMC free article] [PubMed] [Google Scholar]
- 69.Yang X-L, Wu S. Modulation of rod-cone coupling by light. Science. 1989;224:352–354. doi: 10.1126/science.2711185. [DOI] [PubMed] [Google Scholar]
- 70.Zawilska JB, Iuvone PM. Catecholamine receptors regulating serotonin N-acetyltransferase activity and melatonin content of chicken retina and pineal gland: D2-dopamine receptors in retina and alpha-2 adrenergic receptors in pineal gland. J Pharmacol Exp Ther. 1989;250:86–92. [PubMed] [Google Scholar]
- 71.Zawilska JB, Derbiszewska T, Nowak JZ. Clozapine and other neuroleptic drugs antagonize the light-evoked suppression of melatonin biosynthesis in chick retina: involvement of the D4-like dopamine receptor. J Neural Transm. 1994;97:107–117. doi: 10.1007/BF01277947. [DOI] [PubMed] [Google Scholar]