Abstract
Phototransduction in Drosophila is mediated by a G-protein-coupled phospholipase C transduction cascade in which each absorbed photon generates a discrete electrical event, the quantum bump. In whole-cell voltage-clamp recordings, cAMP, as well as its nonhydrolyzable and membrane-permeant analogs 8-bromo-cAMP (8-Br-cAMP) and dibutyryl-cAMP, slowed down the macroscopic light response by increasing quantum bump latency, without changes in bump amplitude or duration. In contrast, cGMP or 8-Br-cGMP had no effect on light response amplitude or kinetics. None of the cyclic nucleotides activated any channels in the plasma membrane. The effects of cAMP were mimicked by application of the non-specific phosphodiesterase inhibitor IBMX and the adenylyl cyclase activator forskolin; zaprinast, a specific cGMP-phosphodiesterase inhibitor, was ineffective. Bump latency was also increased by targeted expression of either an activated Gs α subunit, which increased endogenous adenylyl cyclase activity, or an activated catalytic protein kinase A (PKA) subunit. The action of IBMX was blocked by pretreatment with the PKA inhibitor H-89. The effects of cAMP were abolished in mutants of the ninaC gene, suggesting this nonconventional myosin as a possible target for PKA-mediated phosphorylation. Dopamine (10 μm) and octopamine (100 μm) mimicked the effects of cAMP. These results indicate the existence of a G-protein-coupled adenylyl cyclase pathway in Drosophilaphotoreceptors, which modulates the phospholipase C-based phototransduction cascade.
Keywords: phototransduction, neuromodulation, TRP, photoreceptor, dopamine, calcium, cross-talk, adenylyl cyclase, phospholipase C
Phototransduction in vertebrate and invertebrate photoreceptors is believed to be mediated by a dedicated G-protein-coupled signaling pathway. For example, inDrosophila, which is a widely studied model for invertebrate phototransduction (for review, see Hardie and Minke, 1995; Zuker, 1996;Scott and Zuker, 1998b), a Gq-protein-coupled phospholipase C (PLC) is obligatory for excitation (Bloomquist et al., 1988; Minke and Selinger, 1992). Similarly, in vertebrates, excitation appears to be quantitatively accounted for by activation of transducin and phosphodiesterase (PDE) (Lamb and Pugh, 1992; Lamb, 1996). Nevertheless, reflecting the situation in the majority of cell types, both vertebrate and invertebrate photoreceptors contain biochemical machinery associated with other signal transduction pathways. For example, rod outer segments contain a light-activated phosphoinositide-specific PLC (Ferreira and Pak, 1994; Jiang et al., 1996) and a soluble guanylyl cyclase (Koch et al., 1994). However, the roles of these signaling pathways in photoreceptor function and their potential for cross-talk with the phototransduction cascade remain primarily obscure.
The best documented example of cross-talk within photoreceptors is in the Limulus lateral eye in which sensitivity to light is under control of a circadian rhythm. Octopamine, released by CNS efferents (Battelle et al., 1982), leads to structural changes in the retina and to biochemical changes, resulting in increased gain, lower noise, and more rapid dark adaptation (for review, see Barlow, 1987;Battelle, 1991). Most probably, the effects are mediated by a G-protein-coupled adenylyl cyclase pathway (Kaupp et al., 1982), and recently, an unconventional myosin III showing homology to theDrosophila NINAC protein was implicated as a phosphoprotein in this pathway (Battelle et al., 1998).
To our knowledge, in neither Drosophila nor other insects have there been reports of cAMP involvement in photoreceptor function outside of development (Strutt et al., 1995); however, a number of reports have suggested that cGMP may play a role in transduction. Thus, a cGMP-gated channel is expressed in Drosophila eyes (Baumann et al., 1994), along with a soluble guanylate cyclase (Yoshikawa et al., 1993; disputed by Liu et al., 1995), and cGMP has been reported to activate the migration of pigment granules in housefly photoreceptors (Hanyu and Franceschini, 1993). Nitric oxide, possibly released from second-order interneurons, has been reported to raise photoreceptor cGMP levels in locusts (Bicker and Schmachtenberg, 1997). Although, in Drosophila, such an action appears to be restricted to pupal stages and has been implicated in axonal pathfinding (Gibbs and Truman, 1998), Bacigalupo et al. (1995) reported that a membrane-permeant cGMP analog activated an inward current inDrosophila photoreceptors, suggesting a role in excitation or modulation. In the present study, we have investigated the possible involvement of cyclic nucleotides in Drosophilaphotoreceptors, using a variety of pharmacological and genetic approaches. We found that cGMP neither activated any channels nor had any effect on light responses; however, cAMP significantly slowed the kinetics of phototransduction. This effect could be mimicked pharmacologically or genetically via stimulation of Gs, adenylyl cyclase, and protein kinase A (PKA). Finally, the cAMP-induced effects were blocked in ninaCmutants, suggesting that the NINAC protein may play a key role in mediating modulation of the PLC-based phototransduction cascade.
MATERIALS AND METHODS
Flies were reared at 25°C in the dark. All experiments were performed on newly eclosed (<2 hr) adults of Drosophila melanogaster. Wild-type (WT) flies were Oregon R (OR) on a white (w) eye background. As described previously, a construct encoding an activated Gs α subunit (Gsα*) was made by substitution of leucine 215 with glutamine 215 in the DrosophilaGs sequence. With this substitution, the α subunit binds GTP but is unable to hydrolyze it, resulting in maintained maximal activity (Quan et al., 1991; Wolfgang et al., 1996). This construct was targeted to the photoreceptors using the Rh1 opsin promoter. The full genotype of these flies was as follows: cn; P[ Rh1-Gsα*], ry506 (hereafter referred to as Gsα*). The progenitor stock,cn;ry506,es, was used for controls.
To target expression of activated PKA catalytic subunits (PKAact) to photoreceptors, we used the UAS-Gal4 system (Brand and Perrimon, 1993). Flies expressing Gal4 under control of the opsin (Rh1) promoter (provided by C. Desplan, Rockefeller University, New York, NY) were crossed to two independent UAS-PKAact lines (PKAact1 and PKAact2 obtained from D. Kalderon, Columbia University, New York, NY). Other strains used included the following: trpCM (transient receptor potential), a functionally null allele of the gene encoding the light-sensitive TRP channel (Cosens and Manning, 1969; Reuss et al., 1997); trpl302(trp-like), a null allele of the second class of light-sensitive channel (Niemeyer et al., 1996);ninaCP235 (no inactivation no after potential), a null allele of the non-conventional myosin kinase (Matsumoto et al., 1987; Montell and Rubin, 1988; Porter and Montell, 1993); P[ninaCΔ132] and P[ninacΔ174], which are transformant flies expressing ninaC constructs lacking either the cytosolic (132 kDa) or rhabdomeric (174 kDa)ninaC transcripts on the nullninaCp235 background (provided by C. Montell, Johns Hopkins University, Baltimore, MD) (Porter et al., 1992). For Ca2+ measurements, we used a null rhodopsin mutant, ninaEI117. Finally, we also recorded from flies expressing a truncated rhodopsin lacking C-terminal phosphorylation sites,P[ninaEΔ356], on the same null rhodopsin background (provided by C. Zuker, University of California San Diego, La Jolla, CA) (Vinos et al., 1997).
Electrophysiological recordings and stimulation.Dissociated ommatidia were prepared as described previously (Hardie, 1991). In brief, whole retinas were dissected out in Ca2+ free Ringer’s solution under red light, triturated in fetal calf serum supplemented medium, and ommatidia then transferred to the bottom of a recording chamber on an inverted Nikon (Tokyo, Japan) Diaphot microscope. Whole-cell voltage-clamp recordings were made using electrodes of resistance of 5–10 MΩ. Cells were held at −70 mV unless otherwise stated. Series resistance values were generally below 25 MΩ and were routinely compensated by 80%. Data were collected and analyzed using an Axopatch 1-D amplifier and pCLAMP 6 software (Axon Instruments, Foster City CA).
Responses to brief 10 msec light flashes were collected between 2 and 4 min after establishing whole-cell configuration. This interval allows intracellular contents to equilibrate with pipette solutions and at the same time avoids changes in response kinetics occurring after prolonged whole-cell recordings. To avoid series resistance errors, light intensity was adjusted to elicit relatively small responses with peak amplitudes in the 200–400 pA range. To elicit single bumps, shorter (1 msec) flashes were delivered with intensity adjusted to elicit bumps with ∼70% success rate. Calcium measurements using INDO-1 (100 μm in the patch pipette; Molecular Probes, Eugene, OR) were performed as described previously (Hardie, 1996).
Solutions. Standard extracellular solution was composed of (in mm): 120 NaCl, 5 KCl, 10 TES, 4 MgCl2, 1.5 CaCl2, 25 proline, and 5 alanine. Intracellular solution was composed of (in mm): 140 K gluconate, 10 TES, 4 Mg ATP, 2 MgCl2, 1 NAD, and 0.4 Na GTP. All solutions were buffered to a pH of 7.15. Solutions containing cyclic nucleotides and their analogs were made from frozen 100 mm aqueous stocks and added to the pipette or bath solution as indicated. Phosphodiesterase inhibitors (IBMX and zaprinast), the adenylyl cyclase activator forskolin, the protein kinase A inhibitor H-89 (Biomol, Exeter, UK), as well as extracellular ligands dopamine, octopamine, tyramine, histamine, and serotonin, were added to the bath. In some experiments, test compounds were applied from a wide-bored (∼5 μm) puffer pipette positioned close to the cells. Unless otherwise stated, chemicals were obtained from Sigma (Poole, UK).
Immunocytochemistry. Frozen sections of fly heads were immunostained for Gsα as described previously (Wolfgang et al., 1990) using an affinity-purified antibody raised against a synthetic oligopeptide (residues 376–385 ofDrosophila Gs).
Adenylyl cyclase assay. GTP-dependent cyclase activity was assayed using dissected eye homogenates as described previously (Cassel and Selinger, 1977). Background activity was measured in the absence of any GTP; GTP-dependent activity was measured in the presence of 50 μm GTP. Maximal G-protein activation was achieved by addition of 10 μmGTPγS or 1 mm AlF4. The reaction was run for 15 min at 30° C.
RESULTS
cAMP modulates macroscopic response kinetics
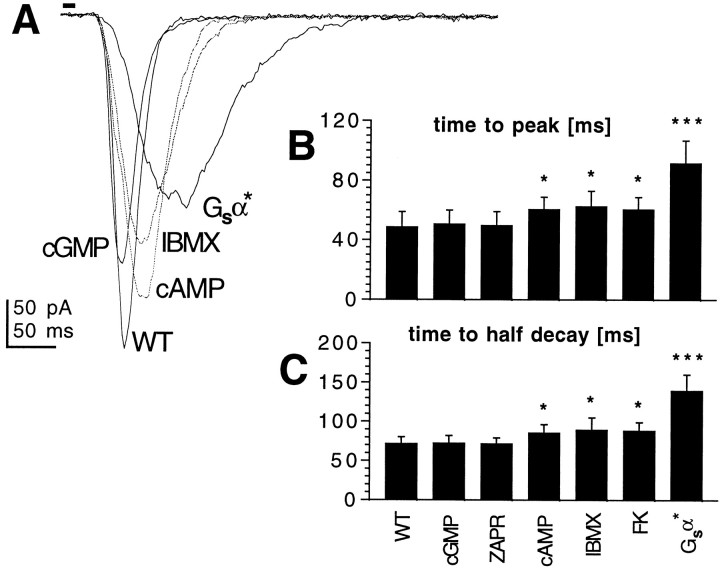
Experiments were performed using whole-cell recordings of dark-adapted photoreceptors from dissociated ommatidia voltage-clamped at resting potential (−70 mV). Under control conditions, photoreceptors respond to brief (10 msec) flashes of light with a stereotyped response with a time to peak of ∼50 msec and a time-to-half-decay (with respect to stimulus) of ∼70 msec. For responses up to at least 1 nA in amplitude, such macroscopic “flash responses” scale linearly with intensity, and their kinetics are invariant with intensity (Hardie, 1991). We tested the effects of cyclic nucleotides in this preparation by exposing the photoreceptors to cGMP, cAMP, or their nonhydrolyzable and membrane-permeant 8-bromo or dibutyryl analogs (8-Br-cGMP and 8-Br-AMP, db-cGMP and db-AMP). The cyclic nucleotides were applied by internal perfusion via the patch pipette or (for the case of membrane-permeant analogs) via bath application at concentrations up to 10 mm. When using cGMP or its analogs, in no case (n > 20) was any conductance activated nor were any effects detected on the sensitivity or kinetics of the flash response. In marked contrast, when cAMP was included in the patch electrode or when the analogs 8-Br-cAMP and db-cAMP were applied to the bath, the kinetics of the flash responses in WT flies were significantly slowed (Fig.1), both the time-to-peak of the flash response and the termination of the light-induced current being delayed by ∼10 msec compared with controls. When applied externally, the effect of db-cAMP (5 mm) developed within ∼30 sec of continuous puffer application (Fig.2) and was partially reversible (n = 4).
Fig. 1.
cAMP, but not cGMP, slows down macroscopic light response in wild-type photoreceptors. A, Responses to 10 msec flashes recorded in WT photoreceptors in controls and in the presence of 8-Br-cGMP (5 mm), 8-Br-cAMP (5 mm), IBMX (100 μm), and photoreceptors from flies expressing activated Gsα (Gsα*). Cyclic nucleotides were applied via the recording pipette, and all other drugs were added to the bath. B, C, Time-to-peak (B) and time-to-half-decay (C) of macroscopic light responses in control WT photoreceptors and in the presence of a cGMP analog (8-Br-cGMP; 5 mm), the specific cGMP-PDE inhibitor zaprinast (100 μm), 8-Br-cAMP (5 mm), IBMX (100 μm), forskolin (FK; 10 μm), and photoreceptors from Gsα* flies. Data are mean ± SD from between 5 and 20 cells. Statistically significant treatments are marked with asterisks (*p < 0.05; ***p < 0.001).
Fig. 2.
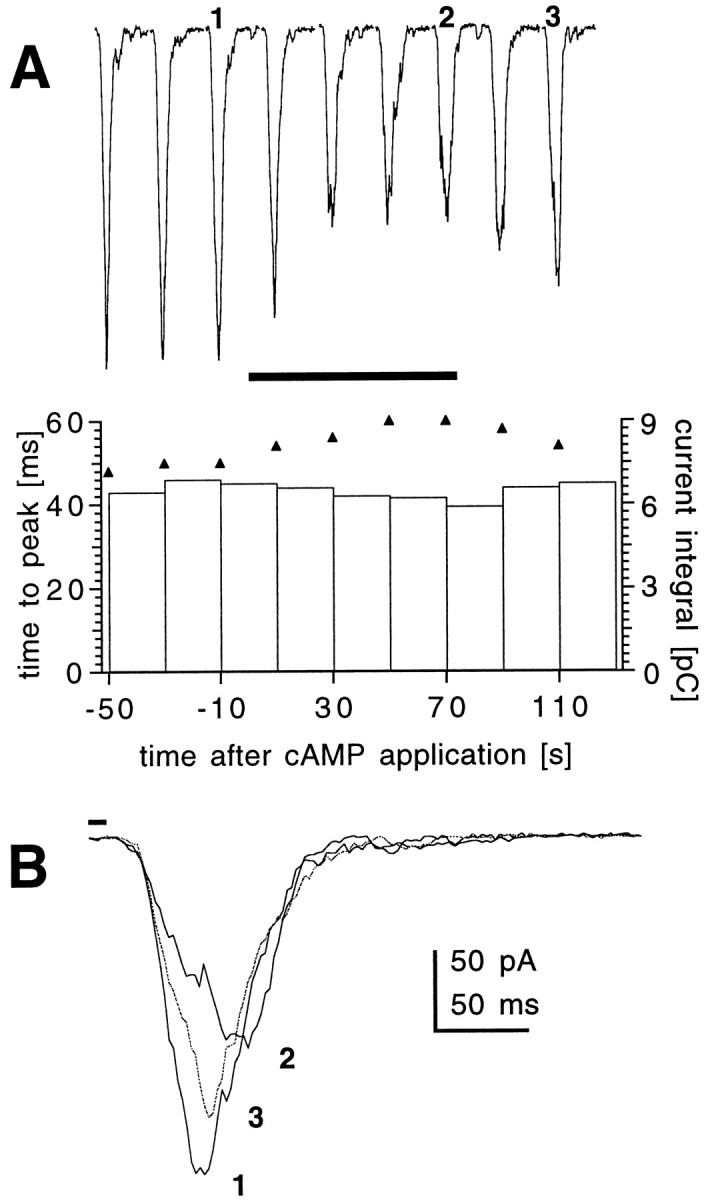
Time course of action of 8-Br-cAMP on response kinetics. A, Responses to 10 msec flashes recorded from a wild-type photoreceptor before, during (horizontal bar), and after application of 8-Br-cAMP (5 mm) from a puffer pipette placed near the recorded cell. Flashes were repeated, and responses were recorded at 20 sec intervals starting ∼2 min after establishing whole-cell configuration. Similar results were obtained on three other cells. Bottom, Changes in the values of time-to-peak (triangles) and current integrals (bars) of the macroscopic responses shown above. A gradual increase in the time-to-peak value was observed within 40 sec of 8-Br-cAMP application, with a partial recovery after washout. Although peak amplitudes decreased during application of cAMP, the current integral showed virtually no change. B, Individual macroscopic responses before (1), during (2), and after (3) 8-Br-cAMP application.
To test whether photoreceptors contained endogenous enzymatic pathways for cAMP metabolism, we applied the nonspecific phosphodiesterase inhibitor IBMX (100 μm) and the adenylyl cyclase activator forskolin (10–100 μm). Both of these agents slowed down the kinetics of phototransduction in a manner indistinguishable from the effects of cAMP or its analogs. In contrast, a specific cGMP phosphodiesterase inhibitor, zaprinast (100 μm), was ineffective (Fig. 1).
Activated Gsα mimics effects of cAMP
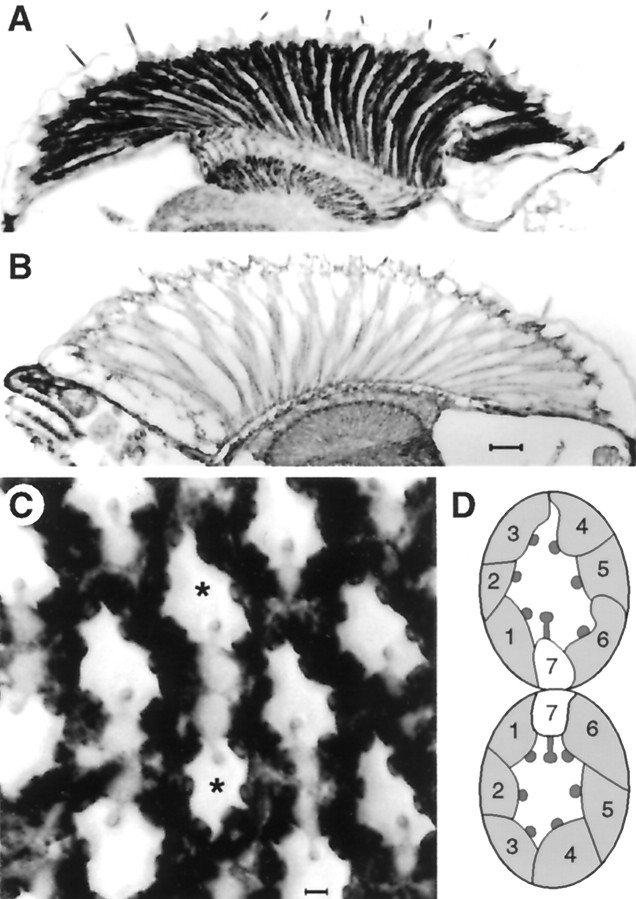
These results demonstrate that cAMP, as well as IBMX and forskolin, which are expected to increase endogenous cAMP levels, modulates the kinetics of the response to light. To complement these pharmacological data, we manipulated adenylyl cyclase activity genetically by overexpressing a DrosophilaGs α subunit, rendered constitutively active by substitution of leucine 215 by glutamine 215 (see Materials and Methods), targeted to the photoreceptors using the Rh1 opsin promoter. Successful and specific targeting of the construct to the photoreceptors was confirmed by immunocytochemistry, which revealed dense staining of the major R1–R6 class of photoreceptor, whereas R7, which expresses a different rhodopsin, was at most only weakly stained (Fig. 3).
Fig. 3.
Frozen sections of fly heads stained with antibodies for Drosophila Gsα.A, Transverse section through Gsα* flies showing high levels of Gs in the photoreceptor cell bodies and axon terminals in the lamina compared with control (B) untransformed flies. Scale bar, 20 μm.C, Tangential section through a Gsα* eye at the equator showing high levels of immunoreactivity in cell bodies of photoreceptors R1–R6 compared with the central cell R7. Scale bar, 2 μm. D, A schematic diagram of the twoasterisked ommatidia indicating photoreceptors 1–7 (8 is below the plane of section) within each unit. Note that the two ommatidia straddle the equator of the eye and so are mirror images of each other.
To determine whether the activated G-protein was indeed capable of activating an endogenous adenylyl cyclase, cyclase activity was assayed in an eye homogenate. GTP-dependent adenylyl cyclase activity was increased ∼10-fold in these flies, reaching levels close to those induced by maximal G-protein stimulation by AlF4or GTPγS (Table 1). Because the activated subunit was expressed only in photoreceptors, these data represent strong evidence for the presence of a functional G-protein-regulated adenylyl cyclase within the photoreceptors.
Table 1.
GTP-dependent adenylyl cyclase activity in control (cn, ry) flies and flies expressing the activated Gs α subunit
| Gsα* pmol/mg | Control prot/min | |
|---|---|---|
| Background | 90 ± 4 | 100 ± 9 |
| GTP | 720 ± 38** | 88 ± 8 |
| AlF4 | 1530 ± 67 | 1600 ± 72 |
| GTP-γS | 1030 ± 43 | 1250 ± 52 |
Cyclase activity was assayed in the absence (background) or presence of 50 μm GTP, 1mm AlF4, or 10 μm GTP-γS. The data are the mean ± SD of three determinations. The data are from a typical experiment that was repeated three times with essentially the same results. Note the greatly increased GTP-dependent activity in Gsα* flies (**p <0.001). Background activity and maximal activity (in the presence of AlF4 or GTP-γS) were unaffected.
When investigated in whole-cell patch clamp, photoreceptors from Gsα* flies appeared to mimic the pharmacological effects of cAMP, IBMX, and forskolin but in a more pronounced manner, with the macroscopic light response being greatly slowed during both activation and termination phases (Fig. 1). IBMX had no statistically significant effect on macroscopic light responses in these flies, suggesting that levels of cAMP and/or protein phosphorylation by cAMP-dependent kinase (see below) may be saturated (n = 7 cells) (data not shown).
Quantum bumps
At the intensities used in these experiments, the macroscopic flash response is the simple linear summation of responses to single photons of light, namely quantum bumps, which are generated with a finite but variable latency (Wong, 1982) (for review, see Hardie and Minke, 1995). The flash response waveform is thus completely described by the convolution of the bump waveform with the bump latency distribution (R. C. Hardie and S. R. Henderson, unpublished results). Determining which is responsible for the change in macroscopic response kinetics should provide insight into the underlying molecular mechanism(s), because bump latency inDrosophila is believed to be determined by early steps of the cascade (up to and including PLC), whereas the amplification responsible for the bump waveform is generated downstream of PLC (Pak et al., 1976; Scott and Zuker, 1998a) (for review, see Hardie and Minke, 1995).
To test whether bump latency, waveform, or both were affected by cAMP, we recorded individual quantum bumps in response to dim light under control and modulated conditions and generated average quantum bump waveforms by aligning bumps on their rising phases. Neither bump amplitude nor waveform recorded in the presence of IBMX or from flies expressing activated Gsα were significantly different from controls (n = 4–5 cells each) (Fig.4A), indicating that bump latency is the parameter that is modulated. The effect of cAMP-dependent modulation on quantum bump latency was illustrated more directly by the analysis of bumps elicited in responses to short (1 msec) flashes of light containing on average less than a single effective photon (Fig. 4B). In the presence of IBMX, mean bump latency was increased by ∼20 msec with respect to controls and >50 msec in the Gsα* transformants. The earliest bump latencies (∼20 msec) remained unaffected so that the effect on macroscopic kinetics was reflected in a shift in time-to-peak rather than in the timing of first detectable deviation from baseline.
Fig. 4.
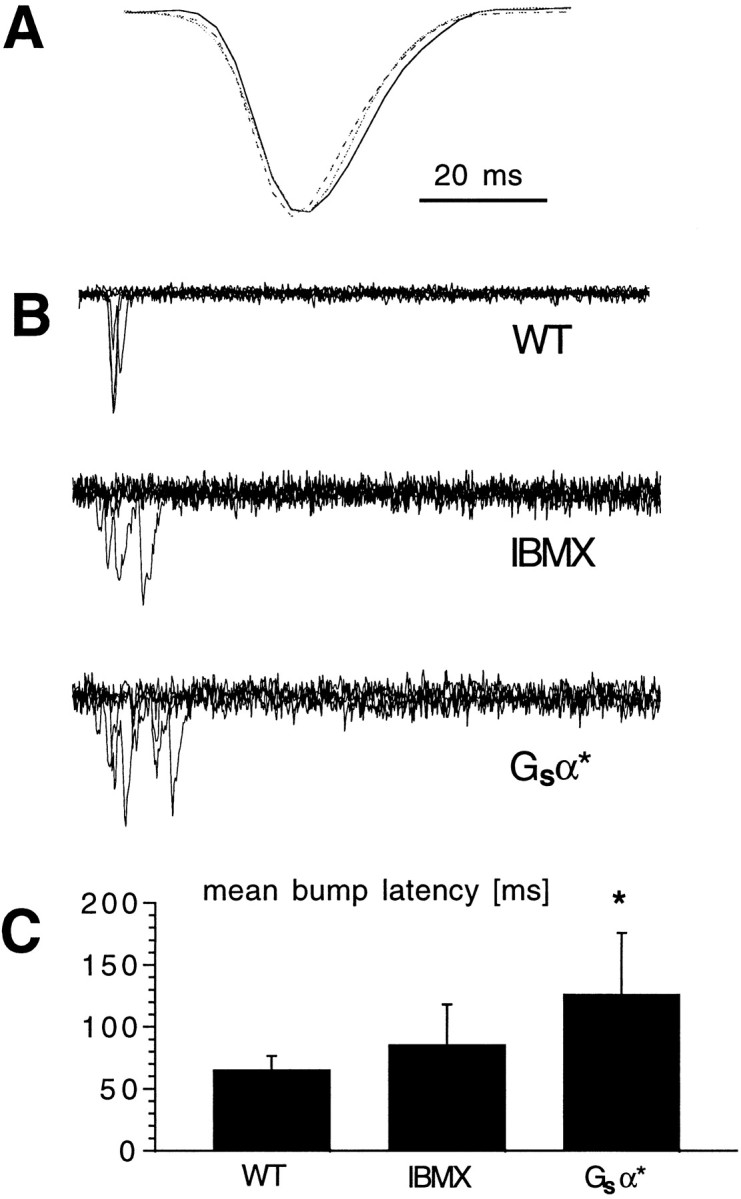
Quantum bump latency but not bump shape or amplitude change with cAMP modulation. A, Representative quantum bumps recorded in wild-type photoreceptors in the absence (WT; solid line) and presence (dotted line) of 100 μm IBMX and in Gsα* photoreceptors (broken line). Eachtrace is the average of at least 30 quantum bumps aligned by their rising phases and normalized, showing that their time courses are indistinguishable. Actual amplitudes were in the range of 10–12 pA and not statistically distinguishable (n= 4–5 cells for each condition). B, Quantum bumps elicited by brief (1 msec) flashes of light of intensity sufficient to evoke a single photon response ∼70% of time delivered at the start of each trace (superimposed). Records in WT photoreceptors in the absence and presence of IBMX (100 μm) and in photoreceptors from Gsα* flies. Note the longer latencies in the presence of IBMX and particularly in Gsα* flies. C, Mean quantum bump latencies recorded in WT photoreceptors in the absence and presence of IBMX and in photoreceptors from transgenic Gsα* flies. Mean ± SD from three to four cells in each case (latency from ∼10 bumps for each cell). *p < 0.05.
cAMP effects are mediated by protein kinase A
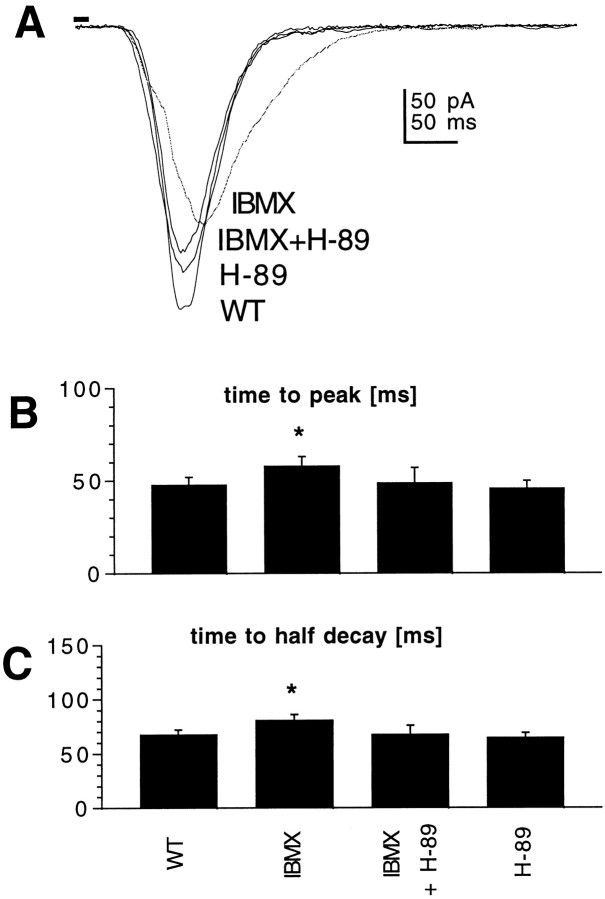
Most cellular effects of cAMP are mediated via PKA. To test whether this is also the case in Drosophila photoreceptors, we used two approaches. First, we attempted to pharmacologically inhibit PKA activation and therefore prevent cAMP modulatory effects. By itself, the specific protein kinase A inhibitor H-89 did not have any effect on macroscopic light response or quantum bump waveform. However, pretreatment (>5 min) with H-89 (1 μm) prevented the effect of IBMX on macroscopic light response (Fig. 5). H-89 did not have any effect on the macroscopic light response in Gsα* flies (data not shown), suggesting that endogenous phosphatase activity was not sufficient to reverse the effects of this transgene on the time scale (∼30–45 min) of the experiment.
Fig. 5.
cAMP modulation can be blocked by the protein kinase A inhibitor H-89. A, Responses to 10 msec flashes recorded in photoreceptors from WT photoreceptors in the absence and presence of phosphodiesterase inhibitor IBMX (100 μm), IBMX and protein kinase A inhibitor H-89 (1 μm), and H-89 alone (1 μm). B, C, Time-to-peak (B) and time-to-half-decay (C) of macroscopic light responses in control WT photoreceptors and in the presence of IBMX, IBMX and H-89, and H-89 alone. Although H-89 PKA inhibitor had no effect on its own, it eliminated the effect of IBMX on the response kinetics. Mean ± SD; n = 5–7 cells. *p < 0.05.
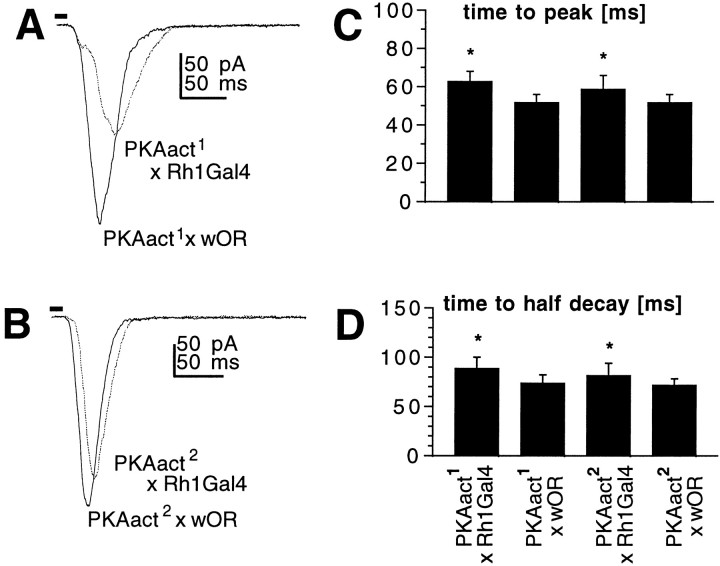
Second, we expressed a constitutively active catalytic subunit ofDrosophila PKA, in the expectation that this might mimic the effect of cAMP. To target the PKA construct to the photoreceptors, we used the UAS-Gal4 strategy (Brand and Perrimon, 1993), crossing flies transformed with UAS-PKAact constructs to lines expressing Gal4 under the control of the opsin promoter (Rh1Gal4). Two independent UAS-PKAact lines were tested in this way; both were found to have slower macroscopic kinetics, although bump waveforms were unaltered (data not shown), similar to the effects of cAMP and IBMX (Fig.6). The effect was significantly greater in one line (PKAact1) than the other (PKAact2); this is the same order of efficacy observed in an independent study in which the same UAS-PKA constructs were found to reduce the quantal amplitude of miniature excitatory junctional potentials (Davis et al., 1998). As controls for these flies we measured light responses in UAS-PKAact and Rh1-Gal4 flies crossed to WT; both had normal response kinetics (Fig. 6).
Fig. 6.
Flies expressing constitutively active protein kinase A in their photoreceptors exhibit slower light responses.A, B, Responses to 10 msec flashes recorded in photoreceptors from transgenic flies expressing constitutively active protein kinase A, PKAact1(A), and PKAact2(B). UAS-PKAact constructs were targeted to photoreceptors by crossing to flies expressing Gal4 under the control of the Rh1 opsin promoter (Rh1-Gal4). The control responses (PKAact × wOR) are from the progeny of the same UAS-PKAact lines crossed to WT (w Oregon R) flies. C,D, Time-to-peak (C) and time-to-half-decay (D) of macroscopic light responses in photoreceptors from PKAact1(A; n = 7 cells) and PKAact2 (B; n = 5) compared with responses from photoreceptors in the control flies (solid traces; n = 4). *p < 0.05.
Possible mechanisms for cAMP-dependent modulation in photoreceptors
The finding that cAMP specifically affects quantum bump latency suggests that it modulates the early stages of signal transduction. Possible target sites might therefore include rhodopsin, Gq-protein, and PLC. Alternatively, its actions might be mediated indirectly, e.g., via regulation of Ca2+ levels, because reducing intracellular Ca2+ levels has been shown to increase bump latency, presumably by modulating one or more of these targets (Henderson and Hardie, unpublished results).
Rhodopsin
ninaEΔ356 is a truncated rhodopsin (Rh1) construct lacking the last 18 amino acid residues of its C terminus, including all potential serine and threonine phosphorylation sites (Vinos et al., 1997). The molecular identity of rhodopsin kinase (RK) has not been established inDrosophila, and although it would seem unlikely to be PKA, RK itself might also be subject to modulation, as is the case in vertebrate rods. Surprisingly, unlike the situation in vertebrates rods, phosphorylation of rhodopsin appears not to be required for response termination, because flies expressingninaEΔ356 instead of wild-type rhodopsin have been reported to show normal light responses (Vinos et al., 1997). To test whether rhodopsin phosphorylation might be responsible for the modulatory effects of cAMP, we applied IBMX to flies expressing ninaEΔ356. However, we found that macroscopic responses were still slowed in a manner indistinguishable from the effect of IBMX in WT flies, suggesting that rhodopsin is not a direct or indirect target for PKA modulation (n = 13 cells) (data not shown).
Calcium
Cytosolic Ca2+ levels were monitored using the ratiometric Ca2+ indicator INDO-1. Ca2+ levels were measured continuously, using null ninaE mutants lacking rhodopsin to eliminate responses to the measuring light (Hardie, 1996). Application of 5 mm 8-Br-cAMP (n= 2 cells) or 100 μm IBMX (n = 4 cells) by puffer pipette was not found to induce any significant change in baseline Ca2+ (data not shown). We cannot, however, exclude an effect on Ca2+ buffering capacity or localized changes that went undetected by our global Ca2+ measurements.
Light-sensitive ion channels TRP and TRPL
Although the light-sensitive channels would appear unlikely candidates for mediating an effect on bump latency, PKA consensus sequences are found in the amino acid sequences of the two light-sensitive channels, TRP and TRPL, and in vitroPKA-dependent phosphorylation of TRPL has been reported to modulate its ability to bind calmodulin (CaM) (Warr and Kelly, 1996). However, we found IBMX to be effective in slowing down light response kinetics in both trp and trpl mutants (n = 8 cells each) (data not shown), each lacking one or other of the two light-sensitive channels, indicating that neither channel alone was specifically responsible for mediating the effect.
ninaC is a putative target of cAMP modulation
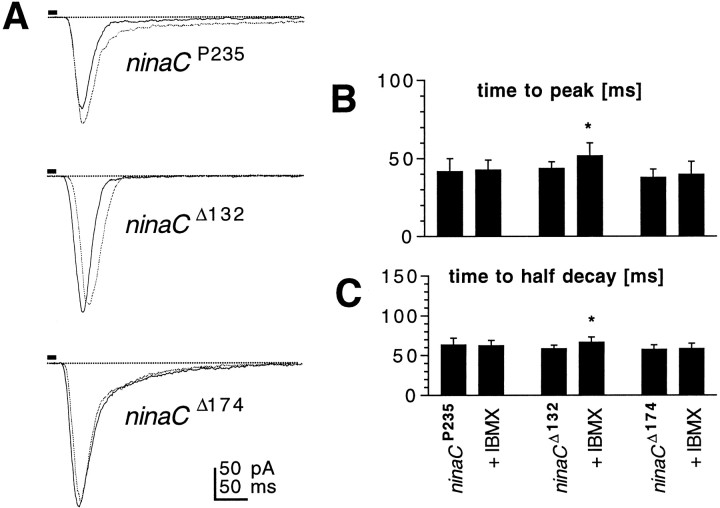
ninaC is a photoreceptor-specific gene encoding an unconventional myosin with a CaM binding domain, a protein kinase domain, and a myosin heavy chain head (Montell and Rubin, 1988). Alternative splicing of the gene generates two spatially segregated proteins: NINAC (132 kDa) found in photoreceptor cell bodies, and NINAC (174 kDa) found only in rhabdomeres. Null mutants lacking both proteins and mutants lacking the 174 kDa microvillar protein have specific defects in response deactivation and greatly reduced CaM levels in the microvilli (Porter et al., 1993, 1995; Hofstee et al., 1996). We hypothesized that the NINAC protein might be involved in the cAMP-dependent modulation, because, in Limulus lateral eye photoreceptors, which are also subject to modulation via a cAMP pathway, a homolog of NINAC has been shown to be phosphorylated by PKA (Battelle et al., 1998). We therefore tested the effect of both 8-Br-cAMP (5 mm) and IBMX (100 μm) on the kinetics of the macroscopic light response in various alleles of the ninaC gene. In P[ninaCΔ132], which lacks only the cytosolic 132 kDa protein, macroscopic response kinetics were slowed as in control WT flies without changes to bump waveform. However, neither 8Br-cAMP nor IBMX had any significant effect on the response kinetics in the null mutant (ninaCP235) or P[ninaCΔ174], lacking the microvillar 174 kDa protein (Fig. 7). These results indicate that the 174 kDa protein is required for the effects of cAMP to be manifested.
Fig. 7.
Drosophila NINAC is a putative target of cAMP modulation. A, Responses to 10 msec flashes recorded in photoreceptors from Drosophila mutantsninaCP235, ninaCΔ132, andninaCΔ174 in the absence (solid traces) and presence (dotted traces) of IBMX (100 μm, bath). Similar data were obtained with 8-Br-cAMP. B, C, Time-to-peak (B) and time-to-half-decay (C) of macroscopic light responses in photoreceptors from ninaC P235,ninaC Δ132, andninaCΔ174 in the absence and presence of 100 μm IBMX. Mean ± SD;n = 4–8 cells. *p < 0.05.
Biogenic amines mimic effects of cAMP
To investigate the possible identity of the putative in situ agonist that might be responsible for stimulating the cAMP pathway in Drosophila photoreceptors, we tested a number of potential neuromodulators. Histamine, the neurotransmitter released by the photoreceptors themselves (Hardie, 1987), had no effect at concentrations of 1 mm. Serotonin, which has been shown previously to modulate potassium currents inDrosophila photoreceptors (Hevers and Hardie, 1995), appeared to have a weak effect at concentrations of 1 mm, but this was not statistically significant. Another potential candidate would be octopamine, which is a widespread insect neurohormone and has been shown to modulate light responses inLimulus when released in a circadian manner from efferent nerve fibers (for review, see Barlow, 1987). Octopamine closely mimicked the effect of cAMP, reversibly slowing down the light-induced current without changing bump shape (Fig.8), but not at concentrations below 100 μm. Tyramine, an octopamine precursor and octopamine receptor agonist, also modulated the macroscopic light response in a similar manner, but its effects were only apparent at concentrations of 1 mm. The most potent agent tested was dopamine, which mimicked the effect of cAMP at concentrations down to 10 μm. Dopamine (1 μm), which would be expected to saturate known dopamine receptors in Drosophila (Sugamori et al., 1995; Feng et al., 1996) was ineffective, however, and the effect of dopamine was not blocked by butaclamol (10 μm), which has been reported to be an effective antagonist of a clonedDrosophila D1-like dopamine receptor (Sugamori et al., 1995) at submicromolar concentrations.
Fig. 8.
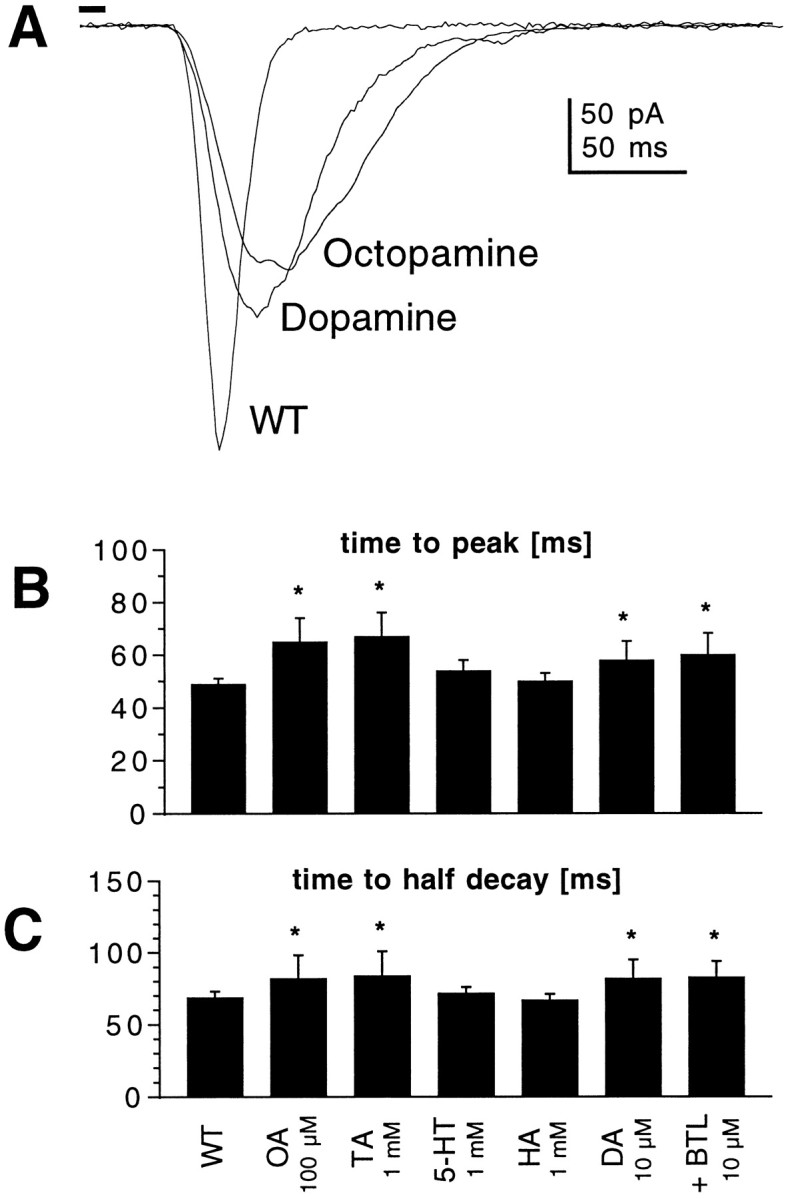
Biogenic amines mimic the effects of cAMP.A, Responses to 10 msec flashes recorded in photoreceptors from WT flies in the absence and presence of dopamine (DA; 10 μm) and octopamine (OA; 100 μm). B,C, Time-to-peak (B) and time-to-half-decay (C) of macroscopic light responses in WT photoreceptors under control conditions and in the presence of octopamine (OA; 100 μm;n = 16), tyramine (TA; 1 mm; n = 8), serotonin (5-HT; 1 mm; n = 9), histamine (HA; 1 mm; n = 5), dopamine (DA; n = 13), and dopamine plus butaclamol (BTL; 10 μm plus 10 μm dopamine; n = 4). *p < 0.05.
DISCUSSION
This study has shown that cAMP can modulate the light response and provided evidence for the existence of many components of a functioning G-protein-coupled adenylyl cyclase signaling cascade inDrosophila photoreceptors. In contrast, we found no evidence for any role of cGMP in either activating light sensitive channels or modulating the response to light. On the one hand, the negative results obtained using cGMP or its analogs serve as a control for the specificity of the actions of cAMP. On the other hand, the reproducible effects of cAMP and its analogs serve as a positive control highlighting the inability of cGMP to activate channels or influence the kinetics of phototransduction. These results contradict an earlier study by Bacigalupo et al. (1995), who reported that cGMP activated an inward current and enhanced responses to light. We cannot unequivocally resolve this discrepancy, but note that inspection of Bacigalupo et al.’s published traces indicates that many recordings were made from cells exhibiting spontaneous activity of the light-sensitive channels typical of a rundown state (cf. Hardie and Minke, 1994).
Although cAMP has been implicated in developmental processes inDrosophila retina (Strutt et al., 1995), to our knowledge, there has been no other evidence for involvement of cAMP metabolism in adult Drosophila photoreceptor function, althoughSchraermeyer et al. (1995) presented histochemical evidence for the existence of adenylyl cyclase in microvillar membrane of the blowflyCalliphora. In the present study, strong evidence for the presence of a functional G-protein-dependent adenylyl cyclase inDrosophila photoreceptors has been provided by the finding of increased cyclase activity in eye homogenates from flies in which an activated Gsα subunit was targeted specifically to the photoreceptors. Gsα* flies have a characteristic phenotype of increased quantum bump latency, which was also mimicked by application of cAMP or forskolin to WT flies. These data implicate this putative adenylyl cyclase in cross-talk with the PLC-based phototransduction cascade. A cAMP-dependent PDE is implicated by the action of IBMX, which is a nonspecific PDE inhibitor, and the lack of effect of a specific cGMP PDE inhibitor, zaprinast, at concentrations 10–100 times its reported IC50(Burns et al., 1992). Two independent lines of evidence support the involvement of PKA in mediating the response: namely, the ability of the specific PKA inhibitor H-89 to block the effect of IBMX, and the ability of a transgenically expressed constitutively active PKA subunit to mimic the prolongation of the light response. Finally, we have found that external application of some biogenic amines, including dopamine and octopamine, also mimic the effects of cAMP. Because of the relatively high concentrations required to elicit modulation (10–100 μm) and the lack of effect of a dopamine receptor antagonist, there remains doubt as to the identity of the naturally occurring agonist and receptors; however, the efficacy of dopamine and octopamine suggests the existence of membrane receptors which couple to an endogenous Gs-protein. Overall, our results present a consistent body of evidence supporting the existence of a receptor and G-protein-coupled adenylyl cyclase and PKA capable of modulating the PLC-based phototransduction cascade.
Potential PKA targets
The finding that the modulation could be accounted for by a specific lengthening of quantum bump latency suggests action, either directly or via an intermediate, at an early stage in the cascade (up to and including PLC). This is because hypomorphic mutations in Gq (Scott et al., 1995) or PLC (Pak et al, 1976;Scott and Zuker, 1998a) have been reported to result in increases in bump latency without affecting bump shape or amplitude, implying that these early stages determine latency, whereas events downstream of PLC are responsible for amplification and bump waveform. Phosphorylation of rhodopsin, either directly or via modulation of RK, seems an unlikely mechanism because the modulatory effect of cAMP remained intact in flies expressing a truncated rhodopsin lacking the C-terminal phosphorylation sites. In principle, either Gq or PLC might be targets; however, because neither sequence contains consensus PKA phosphorylation sites one, would need to hypothesize that the proteins might be modulated by an intermediate. A precedent is suggested in vertebrate photoreceptors in which the activity of G-protein (transducin) can be regulated by PKA-dependent phosphorylation of phosducin, which binds to and inactivates the βγ subunits of transducin only in the unphosphorylated state (Willardson et al., 1996).
Another possible intermediate target was suggested by the recent finding that a ninaC homolog in Limulus lateral eye can be phosphorylated by PKA, leading to the suggestion that this might mediate some of the effects of the adenylyl cyclase believed to underlie the circadian changes initiated by octopamine (Battelle et al., 1998). The Drosophila NINAC protein (Montell and Rubin, 1988) is a multifunctional protein that is required for normal response termination, but its mechanism of action remains obscure. The two NINAC splice variants are multifunctional chimeric proteins that contain a myosin heavy chain head domain believed to interact with the central actin filament in each microvillus, a kinase domain (the substrate for which is unknown), and a CaM binding domain that is required for targeting CaM to the rhabdomeres (Porter et al., 1993). Many of the electrophysiological consequences of deletion of the rhabdomeric NINAC isoform (174 kDa) appear to be explained by the reduced CaM levels, because they can be mimicked by transgenic flies expressing ninaC constructs lacking only the CaM binding domain (Porter et al., 1995). Interestingly, we found that deletion of the 174 kDa microvillar ninaC splice variant (but not the 132 kDa cytosolic form) abolished the ability of both cAMP and IBMX to slow the response to light. This suggests that the microvillar NINAC protein might be a PKA target responsible for mediating this effect. This possibility is supported by the presence of two putative PKA phosphorylation sites in the NINAC174 sequence, but which are missing in NINAC132. One can only speculate as to how phosphorylation of NINAC might result in the specific change in bump latency. Possibilities include modulation of its own kinase activity, which might then result in a change in phosphorylation state of a further target such as Gq or PLC, or changes in its affinity for CaM, which might result in regulation of further Ca–CaM-dependent processes within the microvilli.
Functional considerations and comparison with other species
Bump latency is presumed to decrease during light adaptation so that the increase in bump latency seen in response to cAMP can be interpreted as enhanced dark adaptation. Although the modulated responses did not appear to be associated with any increase in sensitivity per se (as bump amplitude remains unaffected), theoretical considerations suggest that information capacity of photoreceptors will be enhanced at low-light levels if signal power is concentrated at low temporal frequencies (van Hateren, 1992). It therefore seems reasonable to suggest that dopamine (or some other neuromodulator) might be released, in either a circadian manner, as in Limulus, or in direct response to ambient illumination, to predispose the eye for vision in dim light.
The modulation reported here in Drosophila shows intriguing similarities with that reported in the Limulus lateral eye, in particular the involvement of biogenic amines, adenylyl cyclase, PKA, and an unconventional myosin (for review, see Barlow, 1987;Battelle, 1991); however, there are also significant differences. InLimulus, the overall gain of phototransduction is increased and spontaneous noise caused by thermal isomerizations of rhodopsin is reduced (Barlow et al., 1987), neither of which were observed in Drosophila. Also, although the kinetics of the light response are also slowed down in Limulus, this appears to be primarily by an increase in bump duration rather than bump latency (Kaplan and Barlow, 1990). Finally, it is interesting to note that dopamine has also been reported to modulate responses of vertebrate photoreceptors, although in this case indirectly by modulation of voltage-sensitive ion channels (Akopian and Witkovsky, 1996), Na-K ATPase pumps (Shulman and Fox, 1996), or rod–cone coupling (Krizaj et al., 1998).
Footnotes
This work was supported by grants from the Biotechnology and Biological Sciences Research Council (R.C.H., S.C., W.H.), the United States–Israel Science Foundation and the Minerva Center for Visual Transduction (Z.S.), and the National Institutes of Health (M.F., W.J.W.).
Correspondence should be addressed to Roger C. Hardie, Cambridge University, Department of Anatomy, Downing Street, Cambridge CB2 3DY, United Kingdom.
Dr. Hevers’s present address: University of Mainz, Clinical Research Group, Department of Psychiatry, Untere Zahlbacher Strasse 8 D-55131 Mainz, Germany
REFERENCES
- 1.Akopian A, Witkovsky P. D2 dopamine receptor-mediated inhibition of a hyperpolarization- activated current in rod photoreceptors. J Neurophysiol. 1996;76:1828–1835. doi: 10.1152/jn.1996.76.3.1828. [DOI] [PubMed] [Google Scholar]
- 2.Bacigalupo J, Bautista DM, Brink DL, Hetzer JF, Oday PM. Cyclic GMP enhances light-induced excitation and induces membrane currents in Drosophila retinal photoreceptors. J Neurosci. 1995;15:7196–7200. doi: 10.1523/JNEUROSCI.15-11-07196.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barlow RB. Circadian rhythms in the invertebrate retina. In: Stavenga DG, Hardie RC, editors. Facets of vision. Springer; New York: 1987. pp. 257–280. [Google Scholar]
- 4.Barlow RBJ, Kaplan E, Renninger GH, Saito T. Circadian rhythms in Limulus photoreceptors. I. Intracellular studies. J Gen Physiol. 1987;96:353–378. doi: 10.1085/jgp.89.3.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Battelle BA. Regulation of retinal functions by octopaminergic efferent neurons in Limulus. Prog Retinal Res. 1991;10:333–355. [Google Scholar]
- 6.Battelle BA, Evans JA, Chamberlain SC. Efferent fibers to Limulus eyes synthesize and release octopamine. Science. 1982;216:1250–1252. doi: 10.1126/science.6123151. [DOI] [PubMed] [Google Scholar]
- 7.Battelle BA, Andrews AW, Calman BG, Sellers JR, Greenberg RM, Smith WC. A myosin III from Limulus eyes is a clock-regulated phosphoprotein. J Neurosci. 1998;18:4548–4559. doi: 10.1523/JNEUROSCI.18-12-04548.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Baumann A, Frings S, Godde M, Seifert R, Kaupp UB. Primary structure and functional expression of a Drosophila cyclic nucleotide-gated channel present in eyes and antennae. EMBO J. 1994;13:5040–5050. doi: 10.1002/j.1460-2075.1994.tb06833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bicker G, Schmachtenberg O. Cytochemical evidence for nitric oxide/cyclic GMP signal transmission in the visual system of the locust. Eur J Neurosci. 1997;9:189–193. doi: 10.1111/j.1460-9568.1997.tb01366.x. [DOI] [PubMed] [Google Scholar]
- 10.Bloomquist BT, Shortridge RD, Schneuwly S, Pedrew M, Montell C, Steller H, Rubin G, Pak WL. Isolation of putative phospholipase C gene of Drosophila, norpA, and its role in phototransduction. Cell. 1988;54:723–733. doi: 10.1016/s0092-8674(88)80017-5. [DOI] [PubMed] [Google Scholar]
- 11.Brand AH, Perrimon N. Targeted gene expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- 12.Burns F, Rodger IW, Pyne NJ. The catalytic subunit of protein kinase A triggers activation of the type V cyclic GMP-specific phosphodiesterase from guinea-pig lung. Biochem J. 1992;283:487–491. doi: 10.1042/bj2830487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cassel D, Selinger Z. Activation of Turkey erythrocyte adenylate cyclase and blocking of the catecholamine-stimulated GTPase by guanosine 5-(γ-thio) triphosphate. Biochem Biophys Res Commun. 1977;77:868–873. doi: 10.1016/s0006-291x(77)80058-2. [DOI] [PubMed] [Google Scholar]
- 14.Cosens DJ, Manning A. Abnormal electroretinogram from a Drosophila mutant. Nature. 1969;224:285–287. doi: 10.1038/224285a0. [DOI] [PubMed] [Google Scholar]
- 15.Davis GW, DiAntonio A, Petersen SA, Goodman CS. Postsynaptic PKA controls quantal size and reveals a retrograde signal that regulates presynaptic transmitter release in Drosophila. Neuron. 1998;20:305–315. doi: 10.1016/s0896-6273(00)80458-4. [DOI] [PubMed] [Google Scholar]
- 16.Feng GP, Hannan F, Reale V, Hon YY, Kousky CT, Evans PD, Hall LM. Cloning and functional characterization of a novel dopamine receptor from Drosophila melanogaster. J Neurosci. 1996;16:3925–3933. doi: 10.1523/JNEUROSCI.16-12-03925.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferreira PA, Pak WL. Bovine phospholipase C highly homologous to the norpA protein of Drosophila is expressed specifically in cones. J Biol Chem. 1994;269:3129–3131. [PubMed] [Google Scholar]
- 18.Gibbs SM, Truman JW. Nitric oxide and cyclic GMP regulate retinal patterning in the optic lobe of Drosophila. Neuron. 1998;20:83–93. doi: 10.1016/s0896-6273(00)80436-5. [DOI] [PubMed] [Google Scholar]
- 19.Hanyu Y, Franceschini N. Pigment granule migration and phototransduction are triggered by separate pathways in fly photoreceptor cells. NeuroReport. 1993;4:215–218. doi: 10.1097/00001756-199302000-00026. [DOI] [PubMed] [Google Scholar]
- 20.Hardie RC. Is histamine a neurotransmitter in insect photoreceptors? J Comp Physiol [A] 1987;161:201–213. doi: 10.1007/BF00615241. [DOI] [PubMed] [Google Scholar]
- 21.Hardie RC. Whole-cell recordings of the light-induced current in Drosophila photoreceptors: evidence for feedback by calcium permeating the light sensitive channels. Proc R Soc Lond B Biol Sci. 1991;245:203–210. [Google Scholar]
- 22.Hardie RC. INDO-1 measurements of absolute resting and light-induced Ca2+ concentration in Drosophila photoreceptors. J Neurosci. 1996;16:2924–2933. doi: 10.1523/JNEUROSCI.16-09-02924.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hardie RC, Minke B. Spontaneous activation of light-sensitive channels in Drosophila photoreceptors. J Gen Physiol. 1994;103:389–407. doi: 10.1085/jgp.103.3.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hardie RC, Minke B. Phosphoinositide-mediated phototransduction in Drosophila photoreceptors: the role of Ca2+ and trp. Cell Calcium. 1995;18:256–274. doi: 10.1016/0143-4160(95)90023-3. [DOI] [PubMed] [Google Scholar]
- 25.Hevers W, Hardie RC. Serotonin modulates the voltage dependence of delayed rectifier and Shaker potassium channels in Drosophila photoreceptors. Neuron. 1995;14:845–856. doi: 10.1016/0896-6273(95)90228-7. [DOI] [PubMed] [Google Scholar]
- 26.Hofstee CA, Henderson S, Hardie RC, Stavenga DG. Differential effects of ninaC proteins (p132 and p174) on light- activated currents and pupil mechanism in Drosophila photoreceptors. Vis Neurosci. 1996;13:897–906. doi: 10.1017/s0952523800009147. [DOI] [PubMed] [Google Scholar]
- 27.Jiang HP, Lyubarsky A, Dodd R, Vardi N, Pugh E, Baylor D, Simon MI, Wu DQ. Phospholipase-C β-4 is involved in modulating the visual response in mice. Proc Natl Acad Sci USA. 1996;93:14598–14601. doi: 10.1073/pnas.93.25.14598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kaplan E, Barlow RB., Jr Circadian rhythm in Limulus photoreceptors. II. Quantum Bumps. J Gen Physiol. 1990;96:665–685. doi: 10.1085/jgp.96.3.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kaupp UB, Malbon CC, Battelle BA, Brown JE. Octopamine stimulated rise of cAMP in Limulus ventral photoreceptors. Vision Res. 1982;22:1503–1506. doi: 10.1016/0042-6989(82)90216-4. [DOI] [PubMed] [Google Scholar]
- 30.Koch KW, Lambrecht HG, Haberecht M, Redburn D, Schmidt H. Functional coupling of a Ca2+/calmodulin-dependent nitric oxide synthase and a soluble guanylyl cyclase in vertebrate photoreceptor cells. EMBO J. 1994;13:3312–3320. doi: 10.1002/j.1460-2075.1994.tb06633.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Krizaj D, Gabriel R, Owen WG, Witkovsky P. Dopamine D2 receptor-mediated modulation of rod-cone coupling in the Xenopus retina. J Comp Neurol. 1998;398:529–538. [PMC free article] [PubMed] [Google Scholar]
- 32.Lamb TD. Gain and kinetics of activation in the G-protein cascade of phototransduction. Proc Natl Acad Sci USA. 1996;93:566–570. doi: 10.1073/pnas.93.2.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lamb TD, Pugh E., Jr A quantitative account of the activation steps involved in phototransduction in amphibian photoreceptors. J Physiol (Lond) 1992;449:719–758. doi: 10.1113/jphysiol.1992.sp019111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Liu WC, Yoon J, Burg M, Chen L, Pak WL. Molecular characterization of 2 Drosophila guanylate cyclases expressed in the nervous system. J Biol Chem. 1995;270:12418–12427. doi: 10.1074/jbc.270.21.12418. [DOI] [PubMed] [Google Scholar]
- 35.Matsumoto H, Isono K, Pye Q, Pak WL. Gene encoding cytoskeletal proteins in Drosophila rhabdomeres. Proc Natl Acad Sci USA. 1987;84:985–989. doi: 10.1073/pnas.84.4.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Minke B, Selinger Z. The inositol–lipid pathway is necessary for light excitation in fly photoreceptors. In: Corey D, Roper SD, editors. Sensory transduction. Rockefeller UP; New York: 1992. pp. 201–217. [PubMed] [Google Scholar]
- 37.Montell C, Rubin GM. The Drosophila ninaC locus encodes two photoreceptor cell specific proteins with domains homologous to protein kinases and the myosin heavy chain head. Cell. 1988;52:757–772. doi: 10.1016/0092-8674(88)90413-8. [DOI] [PubMed] [Google Scholar]
- 38.Niemeyer BA, Suzuki E, Scott K, Jalink K, Zuker CS. The Drosophila light-activated conductance is composed of the two channels TRP and TRPL. Cell. 1996;85:651–659. doi: 10.1016/s0092-8674(00)81232-5. [DOI] [PubMed] [Google Scholar]
- 39.Pak WL, Ostroy SE, Deland MC, Wu CF. Photoreceptor mutant of Drosophila: is protein involved in intermediate steps of phototransduction? Science. 1976;194:956–959. doi: 10.1126/science.824732. [DOI] [PubMed] [Google Scholar]
- 40.Porter JA, Montell C. Distinct roles of the Drosophila ninaC kinase and myosin domains revealed by systematic mutagenesis. J Cell Biol. 1993;122:601–612. doi: 10.1083/jcb.122.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Porter JA, Hicks JL, Williams DS, Montell C. Differential localizations of and requirements for the two Drosophila ninaC kinase/myosins in photoreceptor cells. J Cell Biol. 1992;116:683–693. doi: 10.1083/jcb.116.3.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Porter JA, Yu M, Doberstein SK, Pollard TD, Montell C. Dependence of calmodulin localization in the retina on the NINAC unconventional myosin. Science. 1993;262:1038–1042. doi: 10.1126/science.8235618. [DOI] [PubMed] [Google Scholar]
- 43.Porter JA, Minke B, Montell C. Calmodulin binding to Drosophila NinaC required for termination of phototransduction. EMBO J. 1995;14:4450–4459. doi: 10.1002/j.1460-2075.1995.tb00124.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Quan F, Thomas L, Forte M. Drosophila stimulatory G-protein α-subunit activates mammalian adenylyl cyclase but interacts poorly with mammalian receptors—implications for receptor–G-protein interaction. Proc Natl Acad Sci USA. 1991;88:1898–1902. doi: 10.1073/pnas.88.5.1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Reuss H, Mojet MH, Chyb S, Hardie RC. In vivo analysis of the Drosophila light-sensitive channels, TRP and TRPL. Neuron. 1997;19:1249–1259. doi: 10.1016/s0896-6273(00)80416-x. [DOI] [PubMed] [Google Scholar]
- 46.Schraermeyer U, Stieve H, Rack M. Cytochemical localization of guanylate and adenylate cyclase in photoreceptor cells of the fly. Z Naturforsch [C] 1995;50:695–698. [Google Scholar]
- 47.Scott K, Zuker CS. Assembly of the Drosophila phototransduction cascade into a signalling complex shapes elementary responses. Nature. 1998a;395:805–808. doi: 10.1038/27448. [DOI] [PubMed] [Google Scholar]
- 48.Scott K, Zuker CS. TRP, TRPL and trouble in photoreceptor cells. Curr Opin Neurobiol. 1998b;8:383–388. doi: 10.1016/s0959-4388(98)80065-2. [DOI] [PubMed] [Google Scholar]
- 49.Scott K, Becker A, Sun Y, Hardy R, Zuker C. G(α) protein function in vivo: genetic dissection of its role in photoreceptor cell physiology. Neuron. 1995;15:919–927. doi: 10.1016/0896-6273(95)90182-5. [DOI] [PubMed] [Google Scholar]
- 50.Shulman LM, Fox DA. Dopamine inhibits mammalian photoreceptor Na+,K+-ATPase activity via a selective effect on the α3 isozyme. Proc Natl Acad Sci USA. 1996;93:8034–8039. doi: 10.1073/pnas.93.15.8034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Strutt DI, Wiersdorff V, Mlodzik M. Regulation of furrow progression in the Drosophila eye by cAMP-dependent protein kinase-A. Nature. 1995;373:705–709. doi: 10.1038/373705a0. [DOI] [PubMed] [Google Scholar]
- 52.Sugamori KS, Demchyshyn LL, McConkey F, Forte MA, Niznik HB. A primordial dopamine D1-like adenylyl cyclase-linked receptor from Drosophila melanogaster displaying poor affinity for benzazepines. FEBS Lett. 1995;362:131–138. doi: 10.1016/0014-5793(95)00224-w. [DOI] [PubMed] [Google Scholar]
- 53.van Hateren JH. Real and optimal neural images in early vision. Nature. 1992;360:68–70. doi: 10.1038/360068a0. [DOI] [PubMed] [Google Scholar]
- 54.Vinos J, Jalink K, Hardy RW, Britt SG, Zuker CS. A G protein-coupled receptor phosphatase required for rhodopsin function. Science. 1997;277:687–690. doi: 10.1126/science.277.5326.687. [DOI] [PubMed] [Google Scholar]
- 55.Warr CG, Kelly LE. Identification and characterization of two distinct calmodulin-binding sites in the Trpl ion-channel protein of Drosophila melanogaster. Biochem J. 1996;314:497–503. doi: 10.1042/bj3140497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Willardson BM, Wilkins JF, Yoshida T, Bitensky MW. Regulation of phosducin phosphorylation in retinal rods by Ca2+/calmodulin-dependent adenylyl cyclase. Proc Natl Acad Sci USA. 1996;93:1475–1479. doi: 10.1073/pnas.93.4.1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wolfgang WJ, Quan F, Goldsmith P, Unson C, Spiegel A, Forte M. Immunolocalization of G-protein α-subunits in the Drosophila CNS. J Neurosci. 1990;10:1014–1024. doi: 10.1523/JNEUROSCI.10-03-01014.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wolfgang WJ, Roberts IJH, Quan F, O’Kane C, Forte M. Activation of protein kinase A-dependent pathways by Gs-α in Drosophila. Proc Natl Acad Sci USA. 1996;93:14542–14547. doi: 10.1073/pnas.93.25.14542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wong F. Adapting bump model for ventral photoreceptors of Limulus. J Gen Physiol. 1982;79:1089–1114. doi: 10.1085/jgp.79.6.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yoshikawa S, Miyamoto I, Aruga J, Furuichi T, Okano H, Mikoshiba K. Isolation of a Drosophila gene encoding a head-specific guanylyl cyclase. J Neurochem. 1993;60:1570–1573. doi: 10.1111/j.1471-4159.1993.tb03324.x. [DOI] [PubMed] [Google Scholar]
- 61.Zuker CS. The biology of vision in Drosophila. Proc Natl Acad Sci USA. 1996;93:571–576. doi: 10.1073/pnas.93.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]