Abstract
Small-diameter sensory neurons that are primarily nociceptors can be divided neurochemically into two populations: isolectin B4 (IB4)-positive nonpeptidergic neurons, and IB4-negative peptidergic neurons. It has been shown that IB4-positive neurons depend on glial-derived neurotrophic factor (GDNF), whereas IB4-negative neurons depend on NGF for survival during postnatal development (Molliver et al., 1997). Furthermore, these two populations of nociceptors terminate in distinct regions of the superficial spinal cord. To date, however, no evidence exists that indicates whether these two groups of nociceptors have distinct functional roles in the process of nociception (Snider and McMahon, 1998). To search for functional differences, we performed whole-cell voltage and current-clamp recordings on acutely isolated adult mouse dorsal root ganglion neurons that were labeled with fluorescent IB4. We found that IB4-positive neurons have longer-duration action potentials, higher densities of TTX-resistant sodium currents, and smaller noxious heat-activated currents than IB4-negative neurons. Furthermore, we show that NGF, but not GDNF, directly increases the number of neurons that respond to noxious heat. The different electrophysiological properties expressed by IB4-positive and -negative small neurons, including their different heat sensitivities, indicates that they may relay distinct aspects of noxious stimuli both acutely and after injury in vivo.
Keywords: sensory neurons, isolectin B4, calcitonin gene-related peptide, substance P, patch clamp, pain, electrophysiology
Most small-diameter sensory neurons of the dorsal root ganglia (DRG) detect stimuli that may lead to pain. Nagy and Hunt first showed in the early 1980s that small-diameter sensory neurons could be divided into two major neurochemical subtypes. One group contains neuropeptides such as calcitonin-gene related neuropeptide (CGRP) and substance P, whereas the other group lacks peptides but contains fluoride-resistant acid phosphatase activity (FRAP) and binds the plant lectin isolectin B4(IB4) (Nagy and Hunt, 1982; Silverman and Kruger, 1990). These two populations also differ anatomically in that they each terminate in distinct but overlapping regions of the superficial dorsal horn of the spinal cord. Peptide-containing neurons project to lamina I and outer lamina II, whereas IB4- and FRAP-positive neurons terminate predominantly in inner lamina II (Coimbra et al., 1974;Silverman and Kruger, 1990). Recently, this neurochemical and neuroanatomical classification has gained more functional relevance because evidence demonstrates that these two populations are regulated by distinct neurotrophic factors during development. IB4-positive neurons express the glial-derived neurotrophic factor (GDNF) receptor complex, including c-ret, GFRα-1 and GFRα-2, and respond to GDNF in vitro and in vivo(Molliver et al., 1997; Bennett et al., 1998). Conversely, CGRP-containing neurons express the high-affinity NGF receptor trkA and respond to NGF (Verge et al., 1989; Averill et al., 1995; Michael et al., 1997). Further evidence for functional differences is the fact that the P2X3 receptor is predominantly localized to the IB4-positive population but not the IB4-negative/CGRP-containing population (Bradbury et al., 1998; Vulchanova et al., 1998). Moreover, CGRP-positive neurons sprout extensively in the dorsal horn in response to dorsal root rhizotomy, whereas IB4-positive neurons maintain their somatotopic distribution and do not sprout (Belyantseva and Lewin, 1999). Despite this range of data indicating that these two sets of nociceptive neurons may be distinct (Snider and McMahon, 1998), no evidence has yet been presented that these neurons are in any way functionally different.
In this study we have specifically searched for electrophysiological differences between these two types of neurons that may be particularly relevant to the detection and processing of nociceptive stimuli. We found that IB4-positive neurons had longer-duration action potentials (APs) and a corresponding higher density of voltage-gated TTX-resistant Na+ channels. In contrast, IB4-negative neurons expressed larger heat-evoked currents. Furthermore, we demonstrate for the first time that NGF can directly sensitize some neurons to noxious heat. Our results indicate that IB4-positive and -negative neurons possess distinct electrophysiological characteristics that are relevant to the detection and processing of nociceptive stimuli.
MATERIALS AND METHODS
Neuronal cultures. DRG from all spinal levels were removed from adult C57/Bl6 mice, and neurons were isolated and cultured as described previously (Lindsay, 1988). The DRGs were incubated with 1 mg/ml collagenase IV (Sigma, St. Louis, MO) and 0.05% trypsin (Sigma) for 30 min each at 37°C. The DRGs were suspended in DMEM/Hams-F12 medium (Life Technologies, Gaithersburg, MD) containing 10% heat-inactivated horse serum (Biochrom), 20 mm glutamine, 0.8% glucose, 100 U penicillin, and 100 μg/ml streptomycin (Life Technologies). DRGs were dissociated with fire-polished Pasteur pipettes, and cells were plated on poly-l-lysine (200 μg/ml)-coated coverslips (1000–2000 cells/coverslip) and maintained at 37°C, 5% CO2. In some cultures, NGF (mouse 7S, Boehringer Mannheim, Indianapolis, IN; 100 ng/ml), anti-NGF antibodies (anti-mouse, Boehringer Mannheim; 50 ng/ml) or GDNF (human, Pepro Tech Inc.; 50 ng/ml) were added. Long-term maintenance of neurons in culture (several days to weeks) has been shown to significantly alter the phenotype of neurons, particularly with respect to TTX and capsaicin sensitivity (Aguayo and White, 1992; Bevan and Winter, 1995). Therefore, we maintained the neurons in our study for the shortest period possible to keep the experiments as similar to in vivo as possible. Most recordings were made within 24 hr of isolation (mean time at recording 27 ± 0.5 hr; range 4–48 hr;n = 357). One mouse was used for each culture, and 86 neurons from 24 cultures were used for the action potential recordings, 37 neurons from 14 cultures were used for the Na+current recordings, and 235 neurons from 32 cultures were used for heat tests. On each experimental day, an approximately equal number of IB4-positive and -negative neurons were recorded. For experiments with added growth factors, a similar number of neurons were recorded from control cultures treated with no growth factor or with anti-NGF antibodies.
Electrophysiology. Whole-cell recordings were made using fire-polished glass electrodes (3–5 MΩ resistance) pulled from borosilicate glass (Hilgenberg, Malsfeld, Germany) on a laser micropipette puller (P-2000, Sutter Instruments, Novato, CA). The recording chamber (volume of 500 μl) was superfused continuously (2–3 ml/min) with extracellular solution containing (in mm): NaCl 154, KCl 5.6, CaCl2 2, MgCl2 1, HEPES 10, glucose 8, pH 7.4; osmolarity = 325 mOsm. Electrodes were filled with solution containing (in mm): KCl 122, Na+ 10, MgCl21, EGTA, 1, HEPES 10, pH 7.3; osmolarity = 290 mOsm. Neurons were visualized at 63× magnification with a Leica DMIRB inverted microscope. The diameter of each soma was calculated from the mean of the longest and shortest diameters. Neurons were treated with the plant lectin IB4, either immediately before or after recordings by incubation with 10 μg/ml IB4 conjugated directly to fluorescein isothiocyanate (IB4-FITC) for 10 min and then rinsed for 5 min in extracellular solution. IB4-FITC staining was visualized with standard FITC filters.
For heat tests, the temperature in the recording bath was monitored using a miniature thermocouple (time constant = 5 msec) (Physitemp, Clifton, NJ) that was placed within 1 mm of the recorded neuron. Heat ramp stimuli (24–49°C in 10 ± 1 sec) were applied by heating the extracellular solution immediately before it entered the bath. Bath temperature was otherwise maintained at 22–24°C.
To selectively record Na+ currents and minimize the contribution from Ca2+ and K+currents, the extracellular solution contained (in mm): NaCl 134, KCl 3, MgCl2 1, CaCl2 1, CdCl2 0.1, HEPES 10 mm, tetraethylammonium 20, and 4-aminopyridine 1), pH 7.4, osmolarity = 310 mOsm. To block tetrodotoxin-sensitive Na+ currents, 1 μm TTX was added to the extracellular solution. Electrodes were filled with solution containing (in mm): CsCl2 124, MgCl2 2, HEPES 10, EGTA 3, and tetraethylammonium 20, pH 7.2, osmolarity = 290 mOsm.
Data recording and analysis. Membrane voltage or current was clamped using an EPC-9 amplifier run by Tida 4.1 software for Windows 95 (HEKA Electronic, Lambrecht, Germany). Data were filtered with a four-pole Bessel filter (5.0 kHz), sampled at 20 kHz, and stored for off-line analysis. Whole-cell configuration was maintained at −60 mV. Seals ranged from 1.5 to 6.0 GΩ. Neurons were discarded if they had resting membrane potentials more positive than −40 mV, did not exhibit an action potential overshoot, or did not exhibit whole-cell currents after a heat test. For generating whole-cell voltage currents, neurons were prepulsed to −120 mV for 150 msec and depolarized from −50 to +50 mV in increments of 5 mV (40 msec test pulse duration). Voltage errors were minimized by using 70% series resistance compensation. Pipette and cell capacitance artifacts were estimated and corrected according to the procedures described by Sigworth (1995). Short trains of square-wave voltage pulses were applied, and the resulting capacitance transients were averaged, leak-subtracted, and then used to calculate the required corrections to the components of the compensation network. Whole-cell current–voltage (I–V) curves for individual neurons were generated by calculating the mean peak inward current at each test potential and correcting for cell capacitance (see above). TTX-sensitive currents were calculated by subtracting the TTX-resistant Na+currents from the total Na+ currents. APs were generated by injecting current from 0.02 to 1.2 nA for 40 msec. AP threshold was defined as the lowest current injected that evoked an AP with an overshoot. The duration of the AP was measured at 50 and 75% of the peak amplitude from resting potential because the 50% amplitude was close to the base of the AP, whereas 75% was near the inflection on the falling phase of the AP. Neurons were considered to be heat sensitive if heat elicited an inward current of ≥100 pA, and the threshold for a heat response was determined at the onset of the inward current. For statistical measures, groups were compared using Student’s t test or χ2 test. Unless stated otherwise, two-tailed comparisons were used. All error bars indicate SEM.
IB4 staining of fixed neurons. Cultures of DRG neurons from four adult mice were prepared separately and fixed 24 hr after isolation with 4% paraformaldehyde for 20 min. Cells were incubated with 10 μg/ml FITC-labeled IB4 in 0.1m PBS containing 0.1 mmCaCl2, 0.1 mm MgCl2, and 0.1 mm MnCl2 for 1 hr, rinsed, and inverted on a slide over a drop of Mowiol. Images of fields of cells (six fields per culture) were randomly collected at a magnification of 20× using Openlab software (Improvision). The perimeter of each neuron was traced, and the cross-sectional area and mean soma diameter were calculated. Neurons were also analyzed for mean brightness intensity of IB4 staining. For each culture, nonspecific staining was determined by sampling the brightness intensities of six large-diameter neurons that were clearly negative for IB4. Neurons with staining intensities 40% or more above this value were considered IB4-positive.
RESULTS
IB4-positive neurons have longer action potentials and larger TTX-resistant currents
We used IB4-FITC, which binds to living neurons, to distinguish small-diameter sensory neurons (soma diameter ≤26 μm) capable of binding this lectin from other small neurons. Figure1A shows fluorescent and phase-contrast images of the same field of DRG neurons stained with IB4-FITC as well as the distribution of IB4-positive neurons among neurons of all sizes. Approximately half (57.5%) of the smaller neurons are labeled with IB4 (range 53–63% over four cultures). During electrophysiological experiments, IB4-FITC was added to the neurons either immediately before or after the measurements were made. To verify that IB4-FITC added before recordings did not interfere with the electrophysiological properties of the neurons, we performed necessary control experiments. First, we compared the AP evoked in the cell soma by current injection before and after (up to 20 min) addition of IB4-FITC to neurons. Figure1B shows that no consistent changes occurred in the shape, duration, or amplitude of the AP evoked in the presence of IB4-FITC compared with those evoked before addition of IB4. In six IB4-positive neurons, the mean duration of the AP at 75% of the peak amplitude was 3.64 ± 0.37 msec before addition of IB4-FITC and 3.86 ± 0.36 msec 15 min after IB4-FITC. The mean amplitude of the AP in these neurons was 113.7 ± 4.8 mV before and 113.9 ± 4.6 mV 15 min after IB4-FITC. Second, the AP duration at 75% of the peak amplitude was measured in 29 neurons in the absence of IB4-FITC (4.38 ± 0.58 msec). These values were not different from those obtained from 66 neurons measured after addition of IB4-FITC (4.90 ± 0.37 msec). These results demonstrated that IB4-FITC did not alter the electrophysiological parameters and could be used to distinguish these two populations either before or after recordings were performed.
Fig. 1.
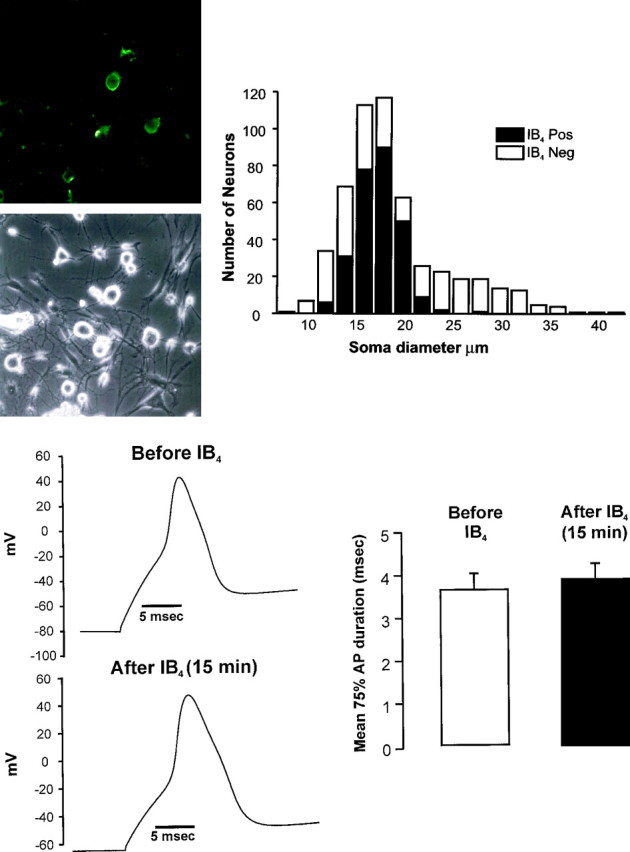
A, Left, Fluorescent and phase-contrast images of field of sensory neurons fixed after labeling with IB4 -FITC. Right, Distribution of IB4-positive and -negative sensory neurons by soma diameter. Dorsal root ganglion neurons from all spinal levels were isolated, placed in culture, and then fixed and stained 24 hr later (n = 4 mice, 530 neurons). B,Left, Profile of a somatic action potential (AP) before (top) and after (bottom) addition of IB4-FITC. This neuron was IB4 positive. Note that binding of IB4 did not alter the shape or duration of the AP. Right, In six IB4-positive neurons, IB4 binding did not alter the duration of the somatic AP.
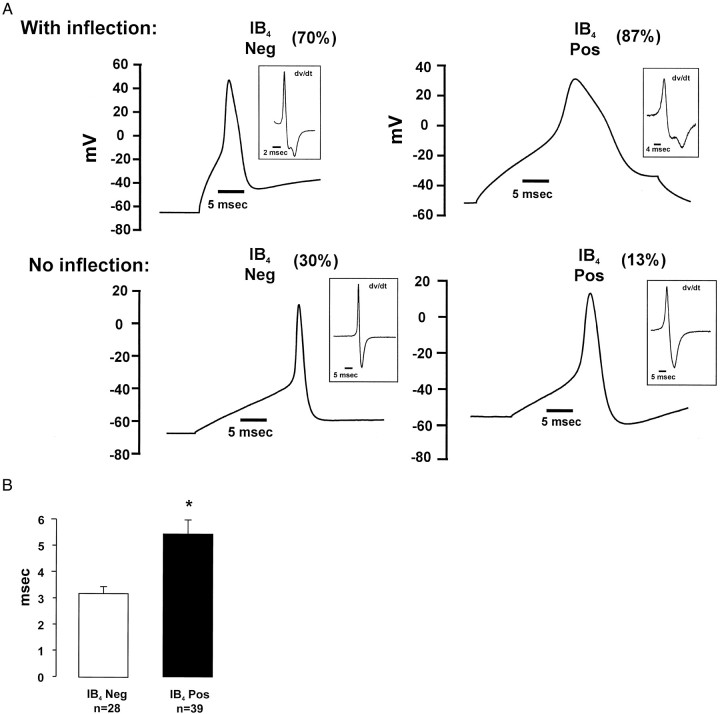
Sensory neurons with a small-diameter cell soma (≤26 μm) that have an inflection on the falling phase of the AP have a high probability of being nociceptors (Koerber et al., 1988; Traub and Mendell, 1988; Gold et al., 1996). Both IB4-positive and -negative neurons had APs with or without an inflection (Fig.2A); however, more IB4-positive neurons (87%) had APs with inflections than did IB4-negative neurons (70%; p < 0.05; one-tailed χ2). Among small neurons that had an inflection on the falling phase of the AP, the first difference was that IB4-positive neurons had APs that were on average twofold longer in duration than IB4-negative neurons. (Fig.2B, Table 1). In addition, the mean threshold for generation of an AP in IB4-positive neurons was significantly higher than for IB4-negative neurons (Table 1) (p < 0.05; one-tailed t test). Small neurons that had no inflection on the falling phase had shorter AP durations overall, and there was no difference in the mean duration between IB4-positive and -negative neurons (Table 1). The range of AP durations reported here is consistent with values previously reported for isolated rodent sensory neurons (Cardenas et al., 1995). APs recorded in isolated neurons tend to have longer durations and lack the pronounced afterhyperpolarization compared with APs recorded with sharp electrodes in vivo or in situ (Traub and Mendell, 1988; McCarthy and Lawson, 1997). Our data indicate that IB4-positive nociceptors have longer-duration APs than IB4-negative, peptidergic nociceptors.
Fig. 2.
A, Examples of APs in IB4-positive and -negative neurons with (top) and without (bottom) inflections on the falling phase. Insets show the first derivative of each spike and illustrate that the APs in the top have an inflection, whereas those in bottom do not. Note that IB4-positive neurons have longer duration APs than IB4-negative neurons. B, Mean duration of AP measured at 75% of spike amplitude. The asteriskindicates that the duration of APs in IB4-positive neurons is significantly longer than in IB4-negative neurons (p < 0.05; t test).
Table 1.
Action potential characteristics of small-diameter (≤26 μm) IB4-positive and -negative dorsal root ganglion neurons
| Soma diameter (μm) | Cell capacitance (pF) | Input resistance (mOhm) | Membrane potential (mV) | AP threshold (pA) | AP height (mV) | AP 50% width (msec) | AP 75% width (msec) | n | |
|---|---|---|---|---|---|---|---|---|---|
| IB4negative, with inflection | 21.8 ± 0.6 | 16.5 ± 1.1 | 437.3 ± 52.8 | −52.1 ± 2.4 | 209.3 ± 32.9* | 97.9 ± 4.7 | 6.11 ± 0.5** | 3.18 ± 0.3** | 29 |
| IB4 positive, with inflection | 22.2 ± 0.4 | 18.4 ± 0.8 | 344.6 ± 49.9 | −51.7 ± 1.4 | 299.5 ± 36.7 | 102.67 ± 2.93 | 9.56 ± 0.94 | 5.45 ± 0.53 | 39 |
| IB4 negative, no inflection | 23.6 ± 1.2 | 21.6 ± 3.0 | 333.5 ± 67.3 | −51.4 ± 2.1 | 306.7 ± 56.3 | 88.40 ± 5.04 | 5.63 ± 0.87 | 2.80 ± 0.51 | 12 |
| IB4 positive, no inflection | 22.8 ± 0.5 | 19.4 ± 3.1 | 340.8 ± 113.2 | −51.7 ± 3.7 | 176.7 ± 49.1 | 99.4 ± 11.1 | 8.82 ± 1.76 | 3.82 ± 0.46 | 6 |
Asterisks indicate IB4-negative group with action potential inflection is significantly different from IB4-positive group with inflection (**p < 0.05, two-tailed t test; *p < 0.05, one-tailed t test). The mean diameter of neurons analyzed electrophysiologically was somewhat larger than the mean diameter of neurons (≤26 μm) analyzed in the fixed cultures. This difference is likely attributable to systematic differences in the methods for analyzing the cell diameters on two different microscopes (see Materials and Methods) and to shrinkage of neurons that can occur with fixation.
We postulated that the differences in AP duration might reflect differential expression of voltage-gated Na+channels. Sensory neurons express both fast TTX-sensitive Na+ channels and slow TTX-resistant Na+ channels (Kostyuk et al., 1981). We recorded whole-cell currents under conditions that select for Na+ currents (see Materials and Methods). There was no significant difference in the peak amplitude of the total Na+ current in IB4-positive and -negative neurons. At −10 mV test potential, the mean current density was −82.0 ± 9.8 pA/pF (n = 22) for IB4-negative neurons and −97.7 ± 16.8 pA/pF (n = 15) for IB4-positive neurons (p > 0.1; t test). We then determined the portion of the Na+ current that was TTX sensitive and TTX resistant in these two populations of neurons. Figure 3A shows examples of Na+ currents in an IB4-negative and an IB4-positive neuron. Na+ currents were recorded in the absence of TTX (Total) and in the presence of 1 μm TTX (TTX-Resistant). The TTX-resistant currents were digitally subtracted from the total currents to reveal the TTX-sensitive component. All IB4-negative and -positive neurons expressed some TTX-sensitive Na+ current, and the I–V relationships for the TTX-sensitive current were identical in IB4-positive and -negative neurons (Fig.3B) (p > 0.5; t test). With respect to TTX-resistant currents, all IB4-positive neurons (n = 15) had some TTX-resistant component, whereas only 78% of small IB4-negative neurons (n = 17) had some TTX-resistant current (p < 0.05; χ2 test). When we compared the TTX-resistant I–V relationship for all neurons that had some TTX-resistant current, we found that the TTX-resistant component was markedly enhanced in IB4-positive neurons (Fig. 3C). The average TTX-resistant current density at −10 mV test potential in IB4-positive neurons was 2.1-fold larger than that in IB4-negative neurons (p < 0.05;t test). We propose that the larger slow TTX-resistant Na+ current in IB4-positive neurons contributes to the longer-duration APs that we observed in these neurons under current-clamp conditions.
Fig. 3.
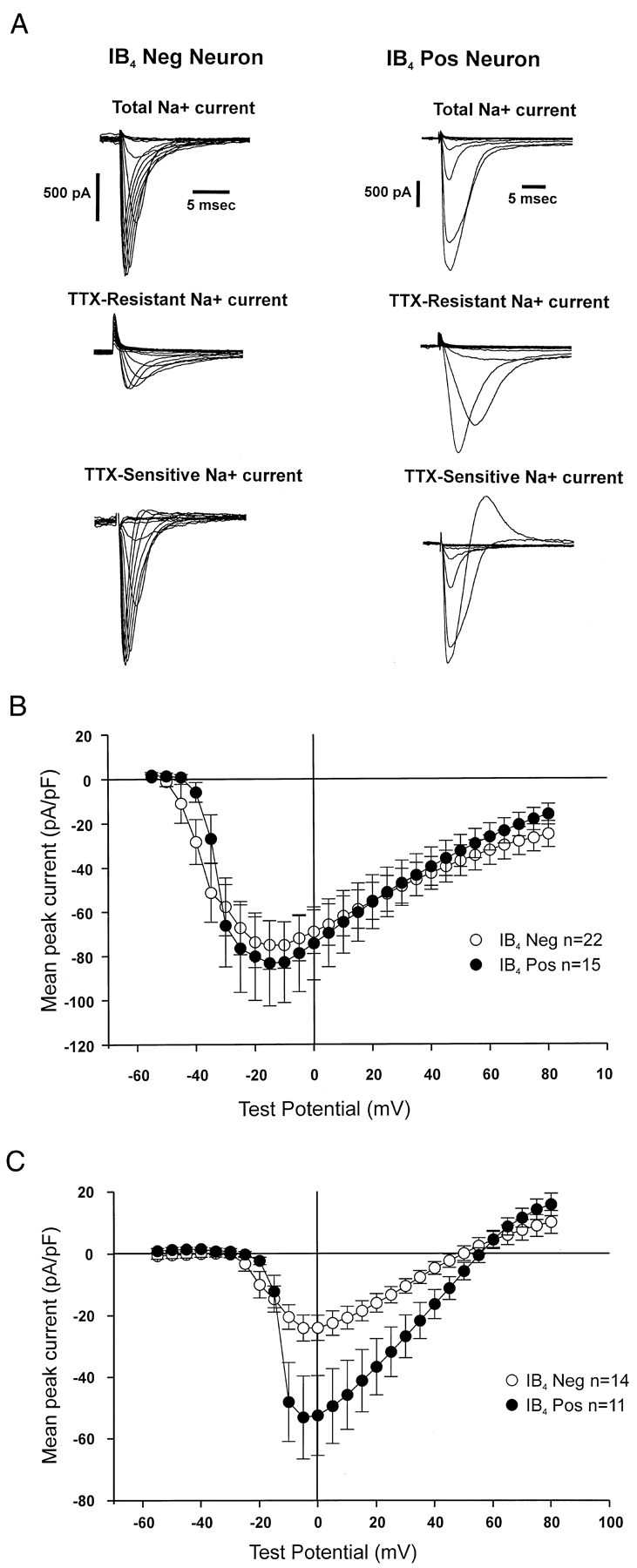
A, Examples of whole-cell voltage-clamp recordings under conditions selective for Na+ currents in an IB4-negative and an IB4-positive neuron. TTX-resistant Na+current was recorded in the presence of 1 μm TTX, and TTX-sensitive current was acquired by electronically subtracting the TTX-resistant current from the Total Na+current. The overshoot at one test-pulse in the calculated TTX-sensitive component of the IB4-positive neuron was caused by variability in the voltage clamp and electronically subtracting the TTX-resistant current from the total current.B, Mean TTX-sensitive Na+ current density for all neurons at different test potentials. Peak TTX-sensitive currents were divided by total cell capacitance to give the current density. C, Mean TTX-resistant Na+ current density for all neurons. TTX-resistant currents were recorded in the presence of 1 μm TTX, and peak currents were corrected for cell capacitance.
IB4-negative neurons have larger heat currents
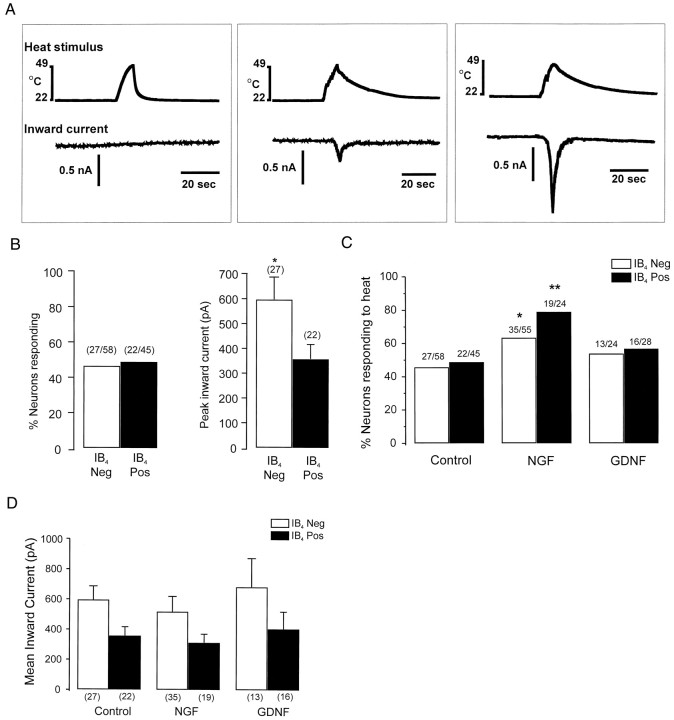
Noxious heat applied directly to the membrane of isolated small-diameter primary afferent neurons activates a cation current (Cesare and McNaughton, 1996). This conductance likely underlies the sensitivity of many polymodal nociceptors to noxious heat. Because 40–50% of C-fiber nociceptors that innervate skin in rodents respond to noxious heat (Koltzenburg et al., 1997) and because IB4labels half of the small-diameter sensory neurons, it was logical to investigate whether heat sensitivity correlates with IB4binding. Figure 4Ashows voltage-clamp recordings from three different small-diameter neurons and their response to a noxious heat ramp (25–49°C in 9–11 sec). Some small neurons did not respond to heat, whereas others responded with inward currents of magnitudes from 110 to 1920 pA (n = 49). The characteristics of these currents in isolated mouse sensory neurons are comparable to those described in isolated rat sensory neurons (Cesare and McNaughton, 1996). We found that IB4 binding is not correlated with heat sensitivity. Approximately 45% of IB4-positive and -negative neurons responded with an inward current to the noxious heat stimulus (Fig.4B). This percentage of heat-responsive neurons in isolated neurons is consistent with the percentage of identified cutaneous C-fiber nociceptors in mice that respond to noxious heat (Koltzenburg et al., 1997). However, the amplitude of the heat-evoked response in IB4-positive and -negative neurons was different. IB4-negative neurons exhibited inward currents that were on average 70% larger than those in IB4-positive neurons (Fig. 4B). There was no difference in the mean soma diameter of IB4-positive and -negative neurons tested for heat responses (IB4 positive: 22.6 ± 0.4 μm; IB4 negative: 23.6 ± 0.7 μm;p > 0.1; Student’s t test). There was also no difference in the diameter of IB4-positive and -negative neurons that responded to heat (IB4 positive: 22.8 ± 0.5 μm; IB4 negative: 24.6 ± 0.8 μm;p > 0.05; Student’s t test). These data show that it is unlikely that differences in cell size account for the difference in the amplitude of the heat-evoked currents in the two groups. Likewise, there were no significant differences between the two groups in whole-cell capacitance, input resistance, or resting membrane potential among either neurons tested for heat or neurons that responded to heat (data not shown). The heat threshold was also not different between the groups (IB4 positive: 39.0 ± 1.6°C; IB4 negative: 41.6 ± 1.2°C).
Fig. 4.
A, Examples of whole-cell voltage-clamp recordings from three different small-diameter neurons and their responses to a ramp of heated buffer applied to cell membrane (24–49°C in 10 ± 1 sec). Neuron that did not respond to heat (left) was IB4 negative, neuron with small inward current (middle) was IB4 positive, and neuron with large inward current (right) was IB4 negative. B, Left, Percentage of IB4-positive and -negative neurons that responded to a noxious heat stimulus. Right, Magnitude of heat-evoked currents. The asterisk indicates that currents in IB4-negative neurons were significantly larger than those in IB4-positive neurons (p < 0.05; χ2 test).C, Cultures of neurons were treated with no growth factor (Control), NGF (100 ng/ml), or GDNF (50 ng/ml) for 24 hr and then tested for responses to noxious heat. Values in parenthesesindicate the number of responding cells per number of cells tested for each group. Single asterisk indicates that percentage of responding cells in NGF-treated IB4-negative neurons is significantly different from that of IB4-negative control neurons. Double asterisk indicates that percentage of responding cells in NGF-treated IB4-positive neurons is significantly different from that of IB4-positive control neurons. D, Mean inward currents evoked in neurons treated with no growth factor, NGF, or GDNF for 24 hr. Values inparentheses indicate the number of responding cells in each group.
NGF increases number of heat-sensitive nociceptors
Because NGF potently induces heat hyperalgesia in rats (Lewin et al., 1993), we asked whether stimulation of isolated sensory neuronsin vitro with NGF is capable of sensitizing the heat-activated current. Control cultures were treated in parallel with anti-NGF antibodies to ensure that endogenous NGF was removed from the system. There was no difference in the percentage of neurons that responded to heat or the amplitude of the heat-evoked currents in the anti-NGF-treated cultures compared with the untreated cultures; therefore, all control values were combined in Figure 4C. Treatment with NGF (100 ng/ml) for 24 hr increased the percentage of IB4-negative neurons that responded to heat from 46 to 64% (p < 0.05; one-tailed χ2). NGF also increased the percentage of IB4-positive neurons that responded to heat from 46 to 79% (p < 0.01; χ2; 15 NGF-treated cultures) (Fig. 4C). A difference in soma size could not account for the NGF-induced increase in responsiveness because there was no difference in the size of NGF-treated neurons tested compared with control neurons (NGF-treated IB4 negative, 24.3 ± 0.6 μm vs control 23.6 ± 0.7 μm; NGF-treated IB4 positive, 23.4 ± 0.6 μm vs control 22.6 ± 0.4 μm). Despite the NGF-induced increase in the number of responsive neurons, NGF had no effect on the mean amplitude of heat-activated currents in either IB4-positive or -negative neurons (Fig. 4D). Thus, there was a dissociation between the amplitude of the heat-induced current and the fraction of neurons that responded to heat. There was no change in the mean threshold for a response to heat (IB4 negative: untreated = 41.6 ± 1.2°C; NGF-treated = 41.0 ± 1.0°C; IB4 positive: untreated = 39.0 ± 1.6°C; NGF-treated = 40.0 ± 1.3°C).
To determine whether the effect of NGF on the heat responsiveness was specific, we tested another neurotrophin GDNF because IB4-positive neurons express receptors for GDNF (Molliver et al., 1997; Bennett et al., 1998). Incubation with GDNF (50 ng/ml) for 24 hr had no effect on the percentage of neurons that responded to heat or the amplitude of the heat-activated current in either IB4-positive or -negative neurons (Figs. 4C,D) (12 GDNF-treated cultures). GDNF also had no effect on the threshold for a response (IB4 negative: GDNF-treated = 43.3 ± 2.1; IB4 positive: GDNF-treated = 40.2 ± 2.1).
DISCUSSION
This study provides the first evidence that IB4-positive and -negative small-diameter sensory neurons have distinct electrophysiological properties. The first difference was that APs in IB4-positive neurons were longer in duration than those in IB4-negative neurons. A long-duration AP with an inflection on the falling phase is a characteristic feature of nociceptive sensory neurons in vivo (Koerber et al., 1988;Traub and Mendell, 1988; Djouhri et al., 1998). In contrast, low-threshold mechanoreceptive neurons with small cell bodies, for example D-hair afferent neurons, always display a very narrow spike with no inflection on the falling phase of the AP (Koerber et al., 1988; Djouhri et al., 1998). It is unlikely that the difference we found in the duration of the AP between IB4-positive and -negative small neurons is attributable to the inclusion of non-nociceptive D-hair neurons because we excluded cells lacking an inflection on the AP. In a previous study of C-fiber nociceptorsin vivo, no correlation between the duration of the somatic AP and response modality or target innervation was found (Traub and Mendell, 1988). However, our data indicate that a relationship does exist between AP duration and neurochemical phenotype in that IB4-positive nonpeptidergic neurons have APs with longer duration than do IB4-negative peptidergic neurons. Similarly, in a recent report in vivo, some nonpeptidergic C-fibers were shown that had APs with longer duration than any peptidergic C-fibers (McCarthy and Lawson, 1997).
Second, IB4-positive neurons expressed 2.1-fold larger voltage-gated TTX-resistant Na+ currents than IB4-negative neurons. Large-diameter sensory neurons that are probably low-threshold mechanoreceptors have only fast TTX-sensitive Na+ channels; however, small-diameter neurons, which are predominantly nociceptors, express both fast TTX-sensitive channels and slow TTX-resistant Na+channels (Kostyuk et al., 1981; Roy and Narahashi, 1992). The higher density of TTX-resistant Na+ currents in IB4-positive neurons may determine the longer-duration APs in these neurons. A higher portion of TTX-resistant Na+ channels has been demonstrated to be responsible for longer-duration APs (Matsuda et al., 1978; Fukuda and Kameyama, 1980). Until recently, it was thought that the TTX-resistant Na+ currents in primary sensory neurons are carried only through the SNS/PN3 channel. This channel activates at more positive membrane potentials and has a slower rate of activation and inactivation than TTX-sensitive Na+ channels (Akopian et al., 1996; Sangameswaran et al., 1996). However, a novel TTX-resistant Na+ channel (NaN or SNS2) that has only 50% homology to SNS/PN3 has recently been cloned (Dib-Hajj et al., 1998; Tate et al., 1998). The threshold for activation and the kinetics of inactivation for SNS2 are very similar to those of TTX-sensitive Na+ channels. The threshold for activation and the voltage-dependent amplitude of the TTX-resistant Na+ currents recorded in our study are similar to the current properties reported for the SNS/PN3 channel (Akopian et al., 1996; Sangameswaran et al., 1996; Tate et al., 1998). Because inactivation of the SNS/PN3 channel is slower than any of the other Na+ channels, enhanced function of this channel would prolong the AP. Therefore, the longer AP observed in IB4-positive neurons could result from enhanced function or expression of the SNS/PN3 channel.
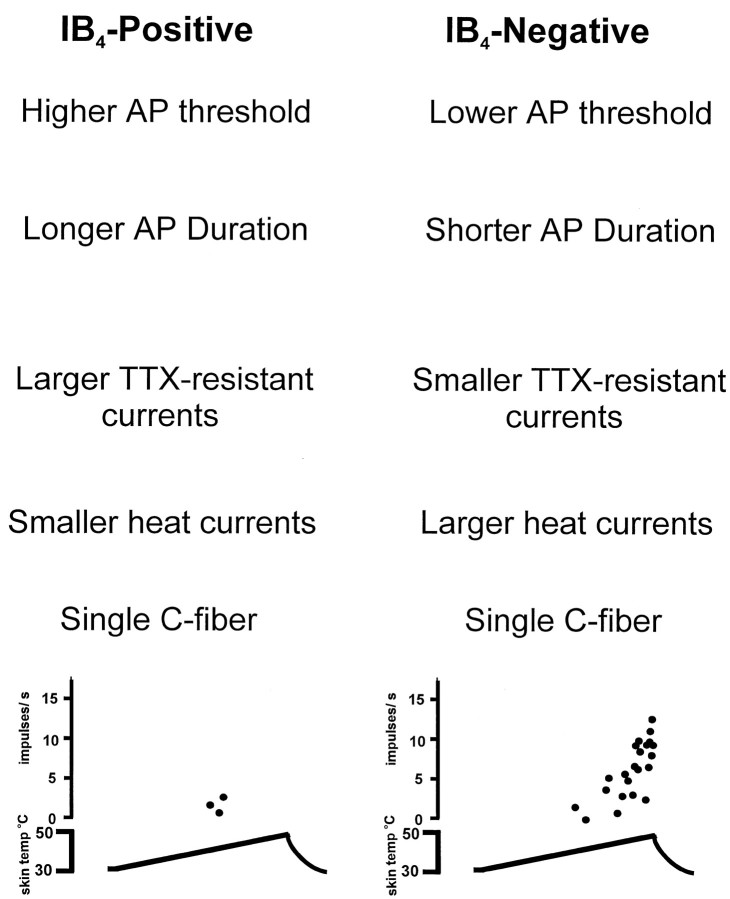
To determine whether certain sensory modalities are conveyed differently by IB4-positive and -negative neurons, we examined the heat-activated inward current in these two groups. Because 40–50% of cutaneous C-fibers in mice respond to noxious heat (Koltzenburg et al., 1997), we investigated whether the heat-responsive population corresponds to either the IB4-positive or -negative population. Our data clearly show that there is a correlation between heat sensitivity and neurochemical phenotype in terms of the amplitude of the heat-induced inward current but not in the percentage of responding neurons. IB4-negative neurons consistently displayed larger heat-activated currents than did IB4-positive neurons. Such heterogeneity in the magnitude of heat responses has also been found in nociceptors recorded extracellularly in vivo and in situ. Some C-fibers respond to a noxious heat ramp with only two to three APs, whereas other nociceptors respond with 20–30 APs (Lynn and Carpenter, 1982). We propose that IB4-positive neurons that have small heat-induced currents correspond with C-fiber nociceptors that respond to heat with only a few APs, and IB4-negative neurons that have large heat-induced currents correlate with C-fibers that respond with a large burst of APs (Fig. 5). The smaller heat-activated inward currents displayed by IB4-positive neurons together with their tendency to require injection of more current for initiation of an action potential suggest that IB4-positive neurons display poor heat sensitivity in vivo. These results indicate that IB4-negative neurons play a greater role in acute responses to noxious heat than do IB4-positive neurons.
Fig. 5.
Putative roles of IB4-positive and -negative neurons in nociceptive function.
It has been postulated that the recently cloned capsaicin receptor vanilloid receptor-1 (VR-1) may be the native heat sensor in C-fiber nociceptors because VR-1 induces novel responses to noxious heat in human embryonic kidney-derived cells (Caterina et al., 1997). Anatomical studies indicate that up to 80% of IB4-positive and -negative sensory neurons express the VR-1 receptor (Tominaga et al., 1998). Our finding that only 45% of IB4-positive and -negative neurons responded to heat suggests that levels of VR-1 higher than those detected immunocytochemically are required for a functional heat response. Alternatively, VR-1 could constitute only part of the endogenous heat receptor, and other proteins may be required to interact with or modulate VR-1 to make a fully functional receptor. Given the larger heat-evoked currents in IB4-negative neurons, it would be interesting to determine whether levels of VR-1 expression per neuron are higher in IB4-negative neurons than in IB4-positive neurons.
A single, systemic injection of NGF in adult rodents produces within minutes a long-lasting hyperalgesia to heat in vivo (Lewin et al., 1993). This NGF-induced hyperalgesia is physiologically meaningful because increased NGF production in the periphery after inflammatory injury is necessary for the hyperalgesia that follows (Lewin et al., 1994; Woolf et al., 1994; Snider and McMahon, 1998). NGF can sensitize nociceptors to heat when it is directly applied to their receptive fields in situ (Rueff and Mendell, 1996). However, the acute sensitizing effects of NGF (minutes to hours) have been shown to be caused entirely by the degranulation of mast cells and subsequent effects of released inflammatory chemicals on sensory neurons (Lewin et al., 1994; Rueff and Mendell, 1996). Our data show for the first time that NGF can directly sensitize primary afferent neurons to heat in the absence of mast cells. This direct sensitization could contribute to the long-lasting late phase of NGF-induced hyperalgesia that has been shown to be completely independent of mast cells (Lewin et al., 1994). Surprisingly, a significant number of IB4-positive neurons were sensitized to heat. In fact, studies from different groups have shown that up to 25% of IB4-positive neurons express trkA receptors (Averill et al., 1995; Molliver et al., 1995; Michael et al., 1997) and these cells may be particularly susceptible to sensitization by NGF. If NGF acts to unmask heat sensitivity in IB4-positive/Trk A-positive sensory neurons, this would represent a new mechanism whereby NGF exerts its heat hyperalgesic effects. Enhancement of heat-responsiveness was specific for NGF because GDNF did not sensitize the heat-activated current even in IB4-positive neurons, which express receptors for GDNF (Molliver et al., 1997). This result is consistent with the finding that GDNF does not appear to induce behavioral hyperalgesia to acute noxious thermal stimuli (Bennett et al., 1998).
In summary, this study provides the first electrophysiological evidence that IB4-positive and -negative nociceptors are functionally distinct. They display different densities of voltage-gated TTX-resistant Na+ channels, different somatic APs, and different responses to noxious heat. These properties have important consequences for the transduction of nociceptive information in vivo, for both the initiation of impulses at the peripheral receptive terminal as well as the transfer of information at the first central synapse. IB4-negative neurons would be more important in the transduction of information about tissue-damaging heat stimuli to the spinal cord. Alternatively, at the level of the spinal cord, the higher density of TTX-resistant Na+ channels and longer-duration APs of IB4-positive neurons could have important consequences for the transmission of information at the first central synapse. It is known that longer-duration APs in sensory neurons can lead to a more efficient influx of calcium into the presynaptic terminal, resulting in more reliable transmitter release from the terminal (Park and Dunlap, 1988). Thus, it is conceivable that IB4-positive neurons mediate a more reliable synaptic connection in inner lamina II compared with IB4-negative peptidergic nociceptors, which terminate in lamina I and outer lamina II. Because IB4-positive and -negative neurons terminate in distinct regions of the superficial dorsal horn, regions that have in turn been implicated in different aspects of nociceptive behavior (Malmberg et al., 1997; Mantyh et al., 1997), differences in the quality of the nociceptive information that they receive and transmit is undoubtedly functionally significant. Functional differences between IB4-positive and -negative neurons may become particularly important after chronic injury, where IB4-negative (peptidergic) neurons sprout more vigorously (Belyantseva and Lewin, 1999). Thus the nature of the persistent pain after injury may crucially depend on functional differences between these two sets of nociceptors.
Footnotes
This work was supported by Deutsche Forschungsgemeinschaft Grant SPP 322-1026. We thank Iska Liebner for technical assistance and Drs. Frank Pfrieger and Alex Verkhratsky for advice and critical comments on this manuscript.
Correspondence should be addressed to Dr. Gary R. Lewin, Growth Factors and Regeneration Group, Max-Delbrück Center for Molecular Medicine, Robert-Rössle-Strasse 10, Berlin-Buch D-13122, Germany.
Dr. Stucky’s current address: Department of Cell Biology, Neurobiology and Anatomy, Medical College of Wisconsin, 8701 Watertown Plank Road, Milwaukee, WI 53226.
REFERENCES
- 1.Aguayo LG, White G. Effects of nerve growth factor on TTX- and capsaicin-sensitivity in adult rat sensory neurons. Brain Res. 1992;570:61–67. doi: 10.1016/0006-8993(92)90564-p. [DOI] [PubMed] [Google Scholar]
- 2.Akopian AN, Sivilotti L, Wood JN. A tetrodotoxin-resistant voltage-gated sodium channel expressed by sensory neurons. Nature. 1996;379:257–262. doi: 10.1038/379257a0. [DOI] [PubMed] [Google Scholar]
- 3.Averill S, McMahon SB, Clary DO, Reichardt LF, Priestley JV. Immunocytochemical localization of trkA receptors in chemically identified subgroups of adult rat sensory neurons. Eur J Neurosci. 1995;7:1484–1494. doi: 10.1111/j.1460-9568.1995.tb01143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Belyantseva IA, Lewin GR. Stability and plasticity of primary afferent projections following nerve regeneration and central degeneration. Eur J Neurosci. 1999;11:457–469. doi: 10.1046/j.1460-9568.1999.00458.x. [DOI] [PubMed] [Google Scholar]
- 5.Bennett DLH, Michael GJ, Ramachandran N, Muson JB, Averill S, Yan Q, McMahon SB, Priestley JV. A distinct subgroup of small DRG cells express GDNF receptor components and GDNF is protective for these neurons after nerve injury. J Neurosci. 1998;18:3059–3072. doi: 10.1523/JNEUROSCI.18-08-03059.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bevan S, Winter J. Nerve growth factor (NGF) differentially regulates the chemosensitivity of adult rat cultured sensory neurons. J Neurosci. 1995;15:4918–4926. doi: 10.1523/JNEUROSCI.15-07-04918.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bradbury EJ, Burnstock G, McMahon SB. The expression of P2X3 purinoreceptors in sensory neurons: effects of axotomy and glial-derived neurotrophic factor. Mol Cell Neurosci. 1998;12:256–268. doi: 10.1006/mcne.1998.0719. [DOI] [PubMed] [Google Scholar]
- 8.Cardenas CG, Del Mar LP, Scroggs RS. Variation in serotonergic inhibition of calcium channel currents in four types of rat sensory neurons differentiated by membrane properties. J Neurophysiol. 1995;74:1870–1879. doi: 10.1152/jn.1995.74.5.1870. [DOI] [PubMed] [Google Scholar]
- 9.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. 1997;389:816–824. doi: 10.1038/39807. [DOI] [PubMed] [Google Scholar]
- 10.Cesare P, McNaughton P. A novel heat-activated current in nociceptive neurons and its sensitization by bradykinin. Proc Natl Acad Sci USA. 1996;93:15435–15439. doi: 10.1073/pnas.93.26.15435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Coimbra A, Sodré-Borges BP, Magalhães MM. The substantia gelatinosa Rolandi of the rat. Fine structure, cytochemistry (acid phosphatase) and changes after dorsal root section. J Neurocytol. 1974;3:199–217. doi: 10.1007/BF01098389. [DOI] [PubMed] [Google Scholar]
- 12.Dib-Hajj SD, Tyrrell L, Black JA, Waxman SG. NaN, a novel voltage-gated Na channel, is expressed preferentially in peripheral sensory neurons and down-regulated after axotomy. Proc Natl Acad Sci USA. 1998;95:8963–8968. doi: 10.1073/pnas.95.15.8963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Djouhri L, Bleazard L, Lawson SN. Association of somatic action potential shape with sensory receptive properties in guinea-pig dorsal root ganglion neurones. J Physiol (Lond) 1998;513:857–872. doi: 10.1111/j.1469-7793.1998.857ba.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fukuda J, Kameyama M. Tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels in tissue-cultured spinal ganglion neurons from adult mammals. Brain Res. 1980;182:191–197. doi: 10.1016/0006-8993(80)90844-6. [DOI] [PubMed] [Google Scholar]
- 15.Gold MS, Dastmalchi S, Levine JD. Co-expression of nociceptor properties in dorsal root ganglion neurons from the adult rat in vitro. Neuroscience. 1996;71:265–275. doi: 10.1016/0306-4522(95)00433-5. [DOI] [PubMed] [Google Scholar]
- 16.Koerber HR, Druzinsky RE, Mendell LM. Properties of somata of spinal dorsal root ganglion cells differ according to peripheral receptor innervated. J Neurophysiol. 1988;60:1584–1596. doi: 10.1152/jn.1988.60.5.1584. [DOI] [PubMed] [Google Scholar]
- 17.Koltzenburg M, Stucky CL, Lewin GR. Receptive properties of mouse sensory neurons innervating hairy skin. J Neurophysiol. 1997;78:1841–1850. doi: 10.1152/jn.1997.78.4.1841. [DOI] [PubMed] [Google Scholar]
- 18.Kostyuk PG, Veselovsky NS, Tsydrenko AY. Ionic currents in somatic membrane of rat dorsal root ganglion neurons. I: sodium currents. Neuroscience. 1981;6:2423–2430. doi: 10.1016/0306-4522(81)90088-9. [DOI] [PubMed] [Google Scholar]
- 19.Lewin GR, Ritter AM, Mendell LM. Nerve growth factor-induced hyperalgesia in the neonatal and adult rat. J Neurosci. 1993;13:2136–2148. doi: 10.1523/JNEUROSCI.13-05-02136.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lewin GR, Rueff A, Mendell LM. Peripheral and central mechanisms of NGF-induced hyperalgesia. Eur J Neurosci. 1994;6:1903–1912. doi: 10.1111/j.1460-9568.1994.tb00581.x. [DOI] [PubMed] [Google Scholar]
- 21.Lindsay RM. Nerve growth factors (NGF, BDNF) enhance axonal regeneration but are not required for survival of adult sensory neurons. J Neurosci. 1988;8:2394–2405. doi: 10.1523/JNEUROSCI.08-07-02394.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lynn B, Carpenter SE. Primary afferent units from the hairy skin of the rat hind limb. Brain Res. 1982;238:29–43. doi: 10.1016/0006-8993(82)90768-5. [DOI] [PubMed] [Google Scholar]
- 23.Malmberg AB, Chen C, Tonegawa S, Basbaum AI. Preserved acute pain and reduced neuropathic pain in mice lacking PKCγ. Science. 1997;278:279–283. doi: 10.1126/science.278.5336.279. [DOI] [PubMed] [Google Scholar]
- 24.Mantyh PW, Rogers SD, Honore P, Allen BJ, Ghilardi JR, Li J, Daughters RS, Lappi DA, Wiley RG, Simone DA. Inhibition of hyperalgesia by ablation of lamina I spinal neurons expressing the substance P receptor. Science. 1997;278:275–279. doi: 10.1126/science.278.5336.275. [DOI] [PubMed] [Google Scholar]
- 25.Matsuda Y, Yoshida S, Yonezawa T. Tetrodotoxin sensitivity and Ca component of action potentials of mouse dorsal root ganglion cells cultured in vitro. Brain Res. 1978;154:69–82. doi: 10.1016/0006-8993(78)91052-1. [DOI] [PubMed] [Google Scholar]
- 26.McCarthy PW, Lawson SN. Differing action potential shapes in rat dorsal root ganglion neurones related to their substance P and calcitonin gene-related peptide immunoreactivity. J Comp Neurol. 1997;388:541–549. [PubMed] [Google Scholar]
- 27.Michael GJ, Averill S, Nitkunan A, Rattray M, Bennett DLH, Yan Q, Priestley JV. Nerve growth factor treatment increases brain-derived neurotrophic factor selectively in trkA-expressing dorsal root ganglion cells and in their central terminations within the spinal cord. J Neurosci. 1997;17:8476–8490. doi: 10.1523/JNEUROSCI.17-21-08476.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Molliver DC, Radeke MJ, Feinstein SC, Snider WD. Presence or absence of trkA protein distinguishes subsets of small sensory neurons with unique cytochemical characteristics and dorsal horn projections. J Comp Neurol. 1995;361:404–416. doi: 10.1002/cne.903610305. [DOI] [PubMed] [Google Scholar]
- 29.Molliver DC, Wright DE, Leitner ML, Parsadanian AS, Doster K, Wen D, Yan Q, Snider WD. IB4-binding DRG neurons switch from NGF to GDNF dependence in early postnatal life. Neuron. 1997;19:849–861. doi: 10.1016/s0896-6273(00)80966-6. [DOI] [PubMed] [Google Scholar]
- 30.Nagy JI, Hunt SP. Fluoride-resistant acid phosphatase-containing neurones in dorsal root ganglia are separate from those containing substance P or somatostatin. Neuroscience. 1982;7:89–97. doi: 10.1016/0306-4522(82)90155-5. [DOI] [PubMed] [Google Scholar]
- 31.Park D, Dunlap K. Dynamic regulation of calcium influx by G-proteins, action potential waveform, and neuronal firing frequency. J Neurosci. 1988;18:6757–6766. doi: 10.1523/JNEUROSCI.18-17-06757.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Roy ML, Narahashi T. Differential properties of tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels in rat dorsal root ganglion neurons. J Neurosci. 1992;12:2104–2111. doi: 10.1523/JNEUROSCI.12-06-02104.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rueff A, Mendell LM. Nerve growth factor and NT-5 induce increased thermal sensitivity of cutaneous nociceptors in vitro. J Neurophysiol. 1996;76:3593–3596. doi: 10.1152/jn.1996.76.5.3593. [DOI] [PubMed] [Google Scholar]
- 34.Sangameswaran L, Delgado SG, Fish LM, Koch BD, Jakeman LB, Stewart GR, Sze P, Hunter JC, Eglen RM, Herman RC. Structure and function of a novel voltage-gated, tetrodotoxin-resistant sodium channel specific to sensory neurons. J Biol Chem. 1996;271:5953–5956. doi: 10.1074/jbc.271.11.5953. [DOI] [PubMed] [Google Scholar]
- 35.Sigworth FJ. Electronic design of the patch clamp. In: Sakmann B, Neher E, editors. Single channel recording. Plenum Press; New York: 1995. pp. 3–35. [Google Scholar]
- 36.Silverman JD, Kruger L. Selective neuronal glycoconjugate expression in sensory and autonomic ganglia: relation of lectin reactivity to peptide and enzyme markers. J Neurocytol. 1990;19:789–801. doi: 10.1007/BF01188046. [DOI] [PubMed] [Google Scholar]
- 37.Snider WD, McMahon SB. Tackling pain at the source: new ideas about nociceptors. Neuron. 1998;20:629–632. doi: 10.1016/s0896-6273(00)81003-x. [DOI] [PubMed] [Google Scholar]
- 38.Tate S, Benn S, Hick C, Trezise D, John V, Mannion RJ, Costigan M, Plumpton C, Grose D, Gladwell Z, Kendall G, Dale K, Bountra C, Woolf CJ. Two sodium channels contribute to the TTX-R sodium current in primary sensory neurons. Nature Neurosci. 1998;1:653–655. doi: 10.1038/3652. [DOI] [PubMed] [Google Scholar]
- 39.Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21:531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 40.Traub RJ, Mendell LM. The spinal projection of individual identified A-delta- and C-fibers. J Neurophysiol. 1988;59:41–55. doi: 10.1152/jn.1988.59.1.41. [DOI] [PubMed] [Google Scholar]
- 41.Verge VMK, Richardson PM, Benoit R, Riopelle RJ. ) Histochemical characterization of sensory neurons with high-affinity receptors for nerve growth factor. J Neurocytol. 1989;18:583–591. doi: 10.1007/BF01187079. [DOI] [PubMed] [Google Scholar]
- 42.Vulchanova L, Riedl MS, Shuster SJ, Stone LS, Hargreaves KM, Buell G, Surprenant A, North RA, Elde R. P2X3 is expressed by DRG neurons that terminate in inner lamina II. Eur J Neurosci. 1998;10:3470–3478. doi: 10.1046/j.1460-9568.1998.00355.x. [DOI] [PubMed] [Google Scholar]
- 43.Woolf CJ, Safieh-Garabedian B, Ma QP, Crilly P, Winter J. Nerve growth factor contributes to the generation of inflammatory sensory hypersensitivity. Neuroscience. 1994;62:327–331. doi: 10.1016/0306-4522(94)90366-2. [DOI] [PubMed] [Google Scholar]