Abstract
Neuronal α1E subunits are thought to form R-type Ca channels. When expressed in human embryonic kidney cells with M2 muscarinic acetylcholine receptors, Ca channels encoded by rabbit α1E exhibit striking biphasic modulation. Receptor activation first produces rapid inhibition of current amplitude and activation rate. However, in the continued presence of agonist, α1E currents subsequently increase. Kinetic slowing persists during this secondary stimulation phase. After receptor deactivation, kinetic slowing is quickly relieved, and current amplitude over-recovers before returning toward control levels. These features indicate that inhibition and stimulation of α1E are separate processes, with stimulation superimposed on inhibition. Pertussis toxin eliminates inhibition without affecting stimulation, demonstrating that inhibition and stimulation involve distinct signaling pathways. Neither inhibition nor stimulation is altered by coexpression of Ca channel β2a or β3 subunits. Stimulation is abolished by staurosporine and reduced by intracellular 5′-adenylylimidodiphosphate, suggesting that phosphorylation is required. However, stimulation does not seem to involve cAMP-dependent protein kinase, protein kinase C, cGMP-dependent protein kinase, tyrosine kinases, or phosphoinositide 3-kinases. Stimulation does not require a Ca signal, because it is not specifically altered by varying intracellular Ca buffering or by substituting Ba as the charge carrier. In contrast to those formed by α1E, Ca channels formed by α1A or α1B display only inhibition and no stimulation during prolonged activation of M2 receptors. The dual modulation of α1E may confer unique physiological properties on native R-type Ca channels. As one possibility, R-type channels may continue to mediate Ca influx during steady inhibition of N-type and P/Q-type channels by muscarinic or other receptors.
Keywords: α1A, α1B, R-type Ca channel, G-protein, ion channel modulation, neurosecretion, presynaptic inhibition, neuronal integration, HEK293 cells, electrophysiology, patch-clamp recording, phosphorylation, protein kinases
Ca influx through voltage-gated Ca channels triggers neurosecretion and influences neuronal membrane excitability, gene expression, and developmental events. Neurons express several different kinds of voltage-gated Ca channels, which are classified according to the primary structure of their pore-forming (α1) subunits. Class E (α1E) subunits are widely expressed in brain (Niidome et al., 1992; Soong et al., 1993; Wakamori et al., 1994;Yokoyama et al., 1995) and appear to localize primarily to neuronal soma and dendrites (Yokoyama et al., 1995; Westenbroek et al., 1998). Recent experiments indicate that α1E forms native “R-type” Ca channels in rat cerebellar granule neurons (Piedras-Rentería and Tsien, 1998). R-type channels are so named because they are resistant to known, selective Ca channel blockers (Randall and Tsien, 1995). Although the physiological functions of R-type Ca channels are mostly unknown, available evidence indicates that they contribute to neurotransmitter secretion at some central synapses (Turner et al., 1995; Wu et al., 1998) and to hormone secretion by certain types of neuroendocrine cells (Wang et al., 1998).
The functional activity of neuronal, high-voltage–activated Ca channels is controlled by biochemical cascades involving heterotrimeric G-proteins (Hille, 1994). Typically, activation of G-protein–coupled receptors produces inhibition of neuronal Ca channels. Inhibition can occur via membrane-delimited and/or cytoplasmic signaling pathways, and inhibition may be voltage dependent (Bean, 1989) or voltage independent (Luebke and Dunlap, 1994). Membrane-delimited, voltage-dependent inhibition of mammalian neuronal Ca channels seems to be mediated by G-protein βγ subunits [Herlitze et al. (1996); Ikeda (1996); but see Diversé-Pierluissi et al. (1997) regarding avian channels]. In the currently accepted hypothesis, certain Gβγ subunits interact directly with certain Ca channel α1 subunits. Recent studies have sought to identify structural regions of α1 involved in binding Gβγ; the results indicate that Gβγ can bind the intracellular I–II loop (De Waard et al., 1997; Zamponi et al., 1997; García et al., 1998b), the C terminal (Qin et al., 1997), and the N terminal (Page et al., 1998) of α1A, α1B, and α1E. On the single-channel level, Gβγ binding increases the first latency of channel opening, at least for N-type Ca channels (Carabelli et al., 1996; Patil et al., 1996).
Although cloned α1E and native R-type Ca channels are significantly modulated via G-protein–dependent pathways (Yassin et al., 1996; Jeong and Wurster, 1997; Qin et al., 1997; Meza and Adams, 1998; Page et al., 1998), the details of their modulation are unclear in comparison with the more extensively studied N-type and P/Q-type Ca channels (Jones and Elmslie, 1997). In this paper we report intriguing new findings concerning the receptor-mediated modulation of cloned α1E Ca channels. We show that sustained activation of M2 muscarinic receptors produces first inhibition and then stimulation of Ca channels encoded by α1E. Inhibition and stimulation are separate events, with stimulation superimposed on inhibition. Our results demonstrate that inhibition and stimulation result from distinct signaling pathways that couple to M2 receptors. The dual modulation of α1E may have important implications for the biological functions of native R-type Ca channels.
MATERIALS AND METHODS
Cell culture and transfection. Human embryonic kidney (HEK293) cells were maintained at 37°C in a humidified atmosphere of 95% O2 and 5% CO2. The culture medium contained 90% DMEM, 10% fetal bovine serum, and 50 μg/ml gentamycin. Every 2–3 d, the cells were briefly trypsinized and replated onto 12 mm round glass coverslips in 35 mm plastic culture dishes. Approximately 15 hr later, these replated cells were transfected via CaPO4 precipitation with expression plasmids encoding α1E (rabbit brain; accession numberX67856) (Niidome et al., 1992), α1A (rabbit brain; accession numberX57477) (Mori et al., 1991), or α1B (rabbit brain; accession numberD14157) (Fujita et al., 1993) Ca channel subunits at 1 μg per 35 mm culture dish. Unless noted, the transfection mixture also contained plasmids encoding Ca channel α2 (rat brain; accession number M86621) (Kim et al., 1992) and β3 (rabbit brain; accession number X64300) (Witcher et al., 1993) subunits at 1 μg per dish. In selected experiments the β subunit was omitted, or β2a (rat brain; accession number M80545) (Perez-Reyes et al., 1992) was used instead of β3. Transfection mixtures also included plasmids encoding the M2 muscarinic acetylcholine receptor (human; accession number X15264) (Peralta et al., 1987) at 0.05 μg of cDNA per dish (unless noted otherwise) and enhanced green fluorescent protein (jellyfish; accession number U55607; Clontech, Cambridge, UK) at 0.1 μg per dish. Successfully transfected cells were visually identified by their green fluorescence under ultraviolet illumination. Only green cells were used for electrophysiological experiments.
Expression plasmids. α1E was in the expression vector pcDNA3.1+ neo (Invitrogen, San Diego, CA). α1A and α1B were in pKCRH2 (Mishina et al., 1984); α2 was in pMT2 (Genetics Institute, Cambridge, MA); β3 was in pcDNA3 (Invitrogen); β2a was in p91023(b); the M2 receptor was in pRK5; and enhanced green fluorescent protein (EGFP) was in pEGFP-C3 (Clontech).
Voltage-clamp recordings. Large-bore patch pipettes were pulled from 100 μl borosilicate glass micropipettes (VWR Scientific) and filled with an intracellular solution containing (in mm): 155 CsCl, 10 Cs2EGTA, 4 MgATP, 0.32 TrisGTP, and 10 HEPES, pH 7.4 with CsOH. In selected experiments, Cs2EGTA was reduced to 0.1 mm or was replaced by either 0.1 or 20 mmCs4BAPTA. In some experiments, the pipette solution contained 20 mm BAPTA plus 10 mmCaCl2. Aliquots of pipette solutions were stored at −80°C, kept on ice after thawing, and filtered at 0.22 μm immediately before use. Pipette tips were dipped in molten paraffin to reduce capacitance and then fire-polished. Filled pipettes had DC resistances of 1.0–1.5 MΩ. The bath solution contained (in mm): 145 NaCl, 40 CaCl2, 2 KCl, and 10 HEPES, pH 7.4 with NaOH. After a gigaohm seal was formed, the residual pipette capacitance was compensated in the cell-attached configuration using the negative capacitance circuit of the patch-clamp amplifier. Ca currents were recorded using the whole-cell technique (Hamill et al., 1981). The steady holding potential was −90 mV. Test depolarizations were delivered every 1–15 sec; the stimulation rate was adjusted for individual cells to maximize sampling rate while minimizing cumulative inactivation. To minimize inactivation further, brief (5–10 msec for α1E currents) test depolarizations were used. Currents were filtered at 2–10 kHz using the built-in Bessel filter (four-pole low-pass) of an Axopatch 200A or 200B amplifier and sampled at 10–50 kHz using a Digidata 1200 analog-to-digital board installed in a Gateway 486 or Pentium I computer. The pCLAMP software programs Clampex and Clampfit (version 6.0.3) were used for data acquisition and analysis, respectively. Figures, linear regressions, and statistical comparisons were done using the software program Microcal Origin (version 5.0). Linear cell capacitance (C) was determined by integrating the area under the whole-cell capacity transient, evoked by clamping from −90 to −80 mV with the whole-cell capacitance compensation circuit of the amplifier turned off. The average value of C was 22 ± 1 pF (mean ± SEM;n = 318 cells). Series resistance (RS) was calculated as τ* (1/C), where τ is the time constant for decay of the whole-cell capacity transient.τ and RS were minimized using the series resistance compensation circuit of the amplifier. The average values of compensated τ andRS were 60 ± 3 μsec and 2.8 ± 0.1 MΩ, respectively (n = 318). Ca currents were evoked by step depolarizations to +30 mV, which is near the peak of the current–voltage (I–V) relationship under these ionic conditions (Meza and Adams, 1998). No corrections were made for liquid junction potentials. Maximal currents, measured at the time of peak inward current, were 1800 ± 100 pA (n = 318). The average maximal voltage error was 4.4 ± 0.2 mV (n = 318). The DC resistance of the whole-cell configuration was typically >500 MΩ. All currents were corrected for linear capacitance and leakage currents using −P/6 or −P/4 subtraction. To quantify macroscopic activation rates, we fit single-exponential functions to the activating segments of individual test currents. A single-exponential function provided a good-to-excellent fit, yielding a single time constant for activation. Carbachol (CCh) was dissolved directly in the bath solution; application of CCh was by bath exchange or local superfusion through a macropipette positioned close to the cell. Temperature (20–22°C) was continuously monitored using a miniature thermocouple placed in the recording chamber. Statistical comparisons were made using an unpairedt test or one-way ANOVA, as appropriate, withp < 0.05 considered significant.
RESULTS
α1E Ca channels exhibit biphasic, opposing modulation
Activation of M2 muscarinic acetylcholine receptors evoked a striking biphasic response of α1E Ca channels expressed in HEK293 cells. An experiment illustrating this phenomenon is depicted in Figure1A. Application of the acetylcholine receptor agonist CCh (50 μm) initially caused a rapid decrease in the macroscopic current amplitude and a slowing of activation kinetics (“inhibition”). Surprisingly, if the CCh application was maintained, α1E current amplitudes subsequently increased (“stimulation”). Kinetic slowing persisted during this secondary stimulation phase, as revealed by the time constants for activation (τact), which were 1.1 ± 0.04 msec (n = 42) before exposure to CCh (Fig.1A, point a), 1.7 ± 0.06 msec (n = 42) at the time of maximal inhibition (point b), and 1.6 ± 0.06 msec (n = 42) at the time of maximal stimulation (point c). After CCh washout, current amplitudes over-recovered beyond the initial control level (point d), and normal activation kinetics were restored (τact = 1.1 ± 0.04 msec;n = 38). The over-recovery after CCh washout was nearly as large (86 ± 4%; n = 38) as the initial inhibition measured in the same cell. After the over-recovery, current amplitudes gradually decreased toward the control level, with an approximately exponential time course.
Fig. 1.
Biphasic, opposing modulation of α1E Ca channels. A, The amplitudes of α1E Ca currents (mediated by α1E/α2/β3 channels) are shown plotted as a function of time during a representative experiment. The application of CCh (50 μm) is indicated by a horizontal black bar. Ca currents were evoked every 3 sec, using the voltage protocol shown (top right). Ca current waveforms, recorded at the indicated times (points a–d), are shown to the right of the time plot. Linear cell capacitance (C) was 23 pF; series resistance (RS) was 2.0 MΩ. File 98610007. B, Rapid application of CCh reveals distinct time courses for inhibition and stimulation. Ca currents were evoked at 1 Hz by short (10 msec) depolarizations to +30 mV from a holding potential of −90 mV. CCh (50 μm) was applied through a macropipette positioned within 2–3 mm of the cell; the perfusion apparatus allowed the medium surrounding the cell to be exchanged completely within 1–2 sec (Melliti et al., 1999).C = 16 pF; RS= 2.7 MΩ. File 99414033. C, α1A Ca currents do not exhibit stimulation. Currents mediated by α1A/α2/β3 were evoked every 2 sec using the voltage protocol diagrammed in A. C = 21 pF; RS = 4.2 MΩ. File 98904009. D, α1B Ca currents do not exhibit stimulation either. Currents mediated by α1B/α2/β3 were evoked every 5 sec using the voltage protocol diagrammed in A. C = 14 pF; RS = 2.5 MΩ. File 99205010. E, Average (± SEM) inhibition and stimulation of α1A, α1B, and α1E currents are shown. Data are from currents evoked by depolarizations to +30 mV. Percent inhibition (by 50 μm CCh) was calculated as the difference between the peak amplitude of the control current (recorded directly before CCh application) and the peak amplitude of the current during maximal inhibition divided by the control current amplitude. Percent stimulation was calculated as the difference between the peak current amplitude during maximal inhibition and the peak current amplitude at the height of stimulation divided by the control current amplitude. For the indicated vertical bars, atropine (+Atr; 50 μm) was present during the application of CCh. The number of cells in each group is given in parentheses in this and subsequent figures.
Two observations demonstrate that the secondary increase in α1E current amplitude (stimulation) does not reflect the relief of G-protein–mediated inhibition. First, kinetic slowing persisted during the stimulation phase. Kinetic slowing is a hallmark of membrane-delimited, voltage-dependent inhibition (Luebke and Dunlap, 1994), and its presence indicates that membrane-delimited inhibition was maintained during stimulation. Second, the over-recovery of current amplitude after CCh washout was opposite in direction, and similar in magnitude, to that of the initial inhibition. Thus, inhibition was present, mostly undiminished, throughout the application of CCh and was not relieved until washout. These observations suggest that inhibition and stimulation of α1E channels are separate events and that stimulation is superimposed on inhibition. Consistent with this interpretation, the time courses of inhibition and stimulation were distinctly different (Fig. 1B). When CCh was applied using a fast-perfusion apparatus and Ca currents were sampled at 1 Hz, inhibition could be seen to reach a stable plateau lasting several seconds before stimulation became apparent (Fig. 1B). When we measured from the last uninhibited current before CCh application (Fig. 1B, arrow), inhibition attained its maximal level in 4 ± 1 sec (n = 10), whereas stimulation required 47 ± 4 sec (n = 10) to reach its peak. In addition to their different time courses, there was no correlation between the magnitudes of inhibition and stimulation measured in the same cell (linear regression correlation coefficientr = 0.16; n = 42; p = 0.31). Together these observations suggest that inhibition and stimulation result from separate processes.
For comparative purposes, identical experiments were performed using α1A and α1B Ca channels, which encode P/Q-type and N-type Ca channels, respectively. As illustrated in Figure 1, C andD, currents mediated by α1A and α1B were steadily inhibited in the presence of CCh, and current amplitudes did not over-recover after CCh washout. No appreciable stimulation was observed in 11 experiments with α1A and 11 experiments with α1B. The steady inhibition of α1A and α1B channels is important because it demonstrates that M2 receptors did not desensitize within the time frame of our experiments.
Figure 1E compares the average modulation of α1A, α1B, and α1E Ca channels. The percentage of current inhibited by 50 μm CCh was greatest for α1B (77 ± 3%;n = 11), followed by α1A (53 ± 3%;n = 11) and α1E (40 ± 1%; n = 42). Only α1E displayed significant stimulation. Both inhibition and stimulation of α1E were blocked by the muscarinic antagonist atropine (50 μm), confirming that both phases of modulation were triggered via muscarinic acetylcholine receptors. CCh had no effects in cells that had not been transfected with M2 receptors (n = 8; data not shown), consistent with previous measurements of very low numbers (<200/cell) of endogenous muscarinic receptors in HEK293 cells (Peralta et al., 1987).
Neither inhibition nor stimulation of α1E was significantly altered after >5 min of intracellular dialysis in the whole-cell configuration (p = 0.51 and 0.23, respectively;n = 17). Furthermore, the magnitudes of inhibition and stimulation were uncorrelated with the initial α1E Ca current density (r = 0.09; n = 42; p = 0.58 for stimulation; r = 0.16; n = 42;p = 0.31 for inhibition). Inhibition of α1A and α1B were similarly independent of initial Ca current density (r = 0.18; n = 11; p = 0.59 for α1A; r = 0.33; n = 11;p = 0.38 for α1B). Thus, differences among cells in Ca channel expression level were not a significant variable in our experiments.
Pertussis toxin eliminates inhibition of α1E without affecting stimulation
We used pertussis toxin (PTX) to investigate which G-proteins underlie the inhibition and stimulation of α1E. As shown in Figure2, pretreatment with PTX (200 ng/ml for 24 hr) nearly abolished the M2 receptor–mediated inhibition of α1E currents, demonstrating that this inhibition is primarily mediated by Gαi/o proteins (West et al., 1985; Avigan et al., 1992). In contrast, PTX had no effect on the magnitude of stimulation (Fig. 2C). The τact of Ca currents in PTX-treated cells was 1.1 ± 0.1 msec before and 1.2 ± 0.1 msec during exposure to CCh (n = 7). Thus, Ca currents recorded from PTX-treated cells did not exhibit kinetic slowing (Fig. 2B), consistent with the absence of membrane-delimited, voltage-dependent inhibition after PTX treatment. Interestingly, in some cells (e.g., Fig. 2A) stimulation of α1E reached a peak and then spontaneously decreased even though CCh was still present. This behavior was observed, to varying degrees, in both PTX-treated and control cells (see and compare Figs.3D,E, 6B). Although we do not currently understand the mechanism, the spontaneous decrease in stimulation is unlikely to reflect receptor desensitization, because inhibition of α1A and α1B was well maintained during CCh applications of similar duration (Fig.1C,D). The over-recovery of α1E current amplitude after CCh washout (Fig. 1A) also argues against receptor desensitization. In summary, inhibition of α1E involves a PTX-sensitive G-protein, but stimulation does not. The biphasic, opposing modulation of α1E therefore requires at least two distinct signaling pathways that couple to M2 receptors.
Fig. 2.
PTX abolishes M2 receptor–mediated inhibition of α1E without reducing stimulation. A, Maximal Ca currents recorded from a PTX-pretreated cell plotted as a function of time. The horizontal bar indicates application of CCh (50 μm). Currents were evoked every 5 sec, using the voltage protocol illustrated in B. B, Ca current waveforms recorded at the times(points a–c) indicated in A.C = 22 pF; RS = 1.5 MΩ. File 98O02022. C, Average (± SEM) inhibition and stimulation in control cells (n = 42) and PTX-pretreated cells (n = 7; p= 0.27 for stimulation).
Fig. 3.
Effects of coexpressing Ca channel β subunits.A, Average current densities (left) and extent of inactivation (right) in cells transfected with α1E and α2 alone (−β) or with α1E and α2 plus β2a or β3 subunits are shown. Data are from currents evoked by depolarizations from −90 to +30 mV. Inactivation was quantified as the percentage of peak current remaining at the end of a 500 msec test pulse.B, Coexpression of β2a or β3 does not significantly alter the voltage dependence of α1E currents. EachI–V plot represents normalized, averaged data from three to five cells in each group. C, Coexpression of the rat brain β2a subunit slows macroscopic inactivation. Current amplitudes have been scaled to facilitate comparison. −β cell: C = 21 pF;RS = 2.1 MΩ. File 99129008. β2a cell:C = 49 pF; RS = 1.8 MΩ. File 99128006. β3 cell: C = 27 pF;RS = 2.6 MΩ. File 99127009.D, Exogenous Ca channel β subunits are not required for stimulation of α1E. The horizontal bar indicates CCh application. Currents in a cell transfected with α1E and α2 alone were evoked by depolarizations to +30 mV every 2 sec.C = 48 pF; RS= 4.5 MΩ. File 98701008. E, Coexpression of the rat brain β2a subunit does not prevent inhibition or stimulation of the rabbit α1E Ca channel. Currents in a cell transfected with α1E, α2, and rat brain β2a were evoked every 2 sec.C = 39 pF; RS= 3.1 MΩ. File 98619010. F, The average magnitudes of inhibition and stimulation of α1E are unaffected by coexpression of rat brain β2a or rabbit brain β3 subunits.
Fig. 6.
Stimulation of α1E does not involve tyrosine kinases or phosphoinositide 3-kinases. A, Genistein (hatched horizontal bar; 100 μm) inhibits α1E current but does not prevent stimulation. The solid horizontal bar indicates CCh application. C= 52 pF; RS = 2.5 MΩ. File 98814002.B, Daidzein (100 μm) produces less inhibition than does genistein. C = 27 pF;RS = 2.1 MΩ. File 98911030.C, Summary of results, including data from cells exposed to 200 nm wortmannin for at least 2 hr before and during experiments.
Although native neurotransmitter receptors have been shown to couple to both PTX-sensitive and PTX-insensitive pathways in neurons (Hay and Kunze, 1994; Choi and Lovinger, 1996; Kammermeier and Ikeda, 1999), it is conceivable that M2 receptors couple to the PTX-insensitive pathway only when they are expressed at higher concentrations than are endogenous Gαi/o proteins. To investigate this possibility, we decreased the level of receptor expression by including reduced amounts of M2 receptor plasmid in our transfection mixture. Neither inhibition (35.0 ± 2.4%; n = 10) nor stimulation (27.4 ± 5.7%; n = 10) of α1E was significantly reduced in cells transfected with 1/2 the normal amount of receptor plasmid, suggesting that M2 receptors were in fact expressed at saturating levels in our experiments. However, in cells transfected with 1/10 the normal amount of receptor plasmid, inhibition was reduced to 16.5 ± 5.7% (n = 10). This ∼60% reduction in inhibition presumably means that the cells’ capacity to supply endogenous Gαi/o proteins had not been exceeded. Importantly, stimulation was reduced to a comparable degree in these cells, to 12.9 ± 3.7% (n = 10). These results indicate that M2 receptors couple to the PTX-insensitive pathway even when these receptors are expressed at nonsaturating levels.
We next used cholera toxin (CTX) to explore whether Gαs participates in the stimulation of α1E. CTX produces tonic activation of Gαs that is followed by downregulation of the Gαs protein within 8 hr (Gill and Meren, 1978; Chang and Bourne, 1989). Stimulation of α1E currents was reduced to 10 ± 2% (n = 10) in cells exposed to CTX (500 ng/ml for 10–18 hr), compared with 25 ± 2% (n = 42) stimulation in untreated control cells (p = 0.001). However, CTX also reduced the inhibition of α1E to 24 ± 2% (n = 10), compared with 40 ± 1% (n = 42) inhibition in control cells. Because inhibition of α1E is mediated primarily by Gαi/o (Fig. 2), these results suggest that CTX has additional effects. As one possibility, CTX may decrease the number of functionally expressed M2 receptors (MacKenzie and Milligan, 1991). We conclude that Gαs is not a key component of the pathway producing stimulation of α1E Ca channels.
Modulation of α1E is unaltered by coexpression of Ca channel β subunits
Previous studies have found that G-protein–dependent inhibition of native neuronal Ca channels is enhanced after depletion of Ca channel β subunits (Campbell et al., 1995). Furthermore, G-protein–mediated inhibition of cloned rat α1A Ca channels expressed in Xenopus oocytes is decreased by coexpression of exogenous Ca channel β subunits (Bourinet et al., 1996). Recently, it was reported that coexpression of rat brain β2a subunits occludes (Qin et al., 1997) or significantly reduces (Qin et al., 1998) the M2 receptor–mediated inhibition of human α1E Ca channels expressed in Xenopus oocytes. These and other findings have suggested that G-proteins and Ca channel β subunits compete for similar binding sites on Ca channel α1 subunits (De Waard et al., 1997; Zamponi et al., 1997).
To determine whether stimulation of α1E could be antagonized by coexpression of Ca channel β subunits, we compared the modulation of currents produced by coexpression of α1E and α2 alone with currents recorded from cells expressing α1E, α2, and either β3 (from rabbit brain) or β2a (from rat brain). As shown in Figure3A, the average current density was significantly higher (87 ± 10 pA/pF;n = 42) in cells cotransfected with rabbit β3 than in cells cotransfected with rat β2a (21 ± 3 pA/pF;n = 13). In contrast with this result, Jones et al. (1998) found that rat β2a and rat β3 were equally effective in enhancing the current density of the rat α1E. However, in their experiments the α2 subunit was omitted, and their α1E and β subunits were from rat rather than from rabbit. In our experiments, cells not transfected with an exogenous β subunit had an average α1E current density of 19 ± 2 pA/pF (n = 17); this is an overestimation, however, because these 17 cells were selected for experiments on the basis of their relatively large Ca currents. We found that cells transfected with α1E and α2 alone tended to express either very small or reasonably large Ca current densities, raising the possibility that some (but not all) HEK293 cells express endogenous β subunits. We found that the current–voltage (I–V) relationship was unaffected by coexpression of either β2a or β3 (Fig. 3B). In contrast, several other previous studies have found that β subunit coexpression produces a negative shift in the voltage dependence of activation of α1E (Wakamori et al., 1994; Williams et al., 1994; Parent et al., 1997; Stephens et al., 1997; Jones et al., 1998).
Coexpression of rat brain β2a significantly slowed macroscopic inactivation (Fig. 3C). Inactivation was quantified by measuring the percentage of current remaining at the end of a 500 msec depolarization to +30 mV (Fig. 3A). This percentage was 28.2 ± 5.1% for β2a cells (n = 7), 6.0 ± 1.1% for β3 cells (n = 7), and 11.4 ± 3.7% for cells not transfected with a β subunit (n = 7). These changes in current density and inactivation rate demonstrate that both β2a and β3 were functionally expressed.
Biphasic modulation of α1E did not require coexpression of an exogenous β subunit (Fig. 3D). Furthermore, coexpression of rat brain β2a neither occluded nor reduced inhibition of α1E (Fig. 3E). In fact, the average magnitudes of inhibition and stimulation were not significantly altered by coexpression of either β2a or β3 (Fig. 3F). These findings differ markedly from those obtained by Qin et al. (1997) for the human α1E Ca channel. However, they agree with recent results obtained by Page et al. (1998) for rat α1E. Together with the latter study, our observations seem inconsistent with the hypothesis that Ca channel β subunits compete with Gβγ subunits for binding sites on neuronal Ca channels.
Stimulation of α1E involves phosphorylation
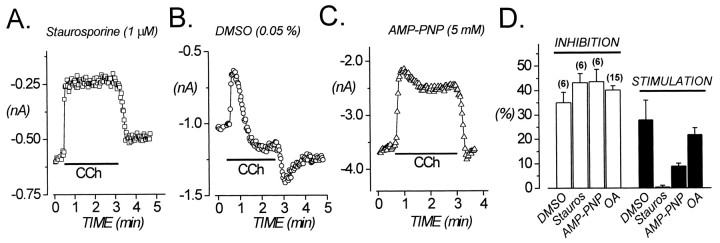
The relatively slow time course of stimulation (Fig.1B) suggested the involvement of a cytosolic signaling pathway. The rabbit α1E subunit contains several phosphorylation consensus sites (Niidome et al., 1992), and α1E has been demonstrated to be a substrate in vitro for phosphorylation by cAMP-dependent protein kinase (PKA), protein kinase C (PKC), cGMP-dependent protein kinase (PKG), and CaMKII (Yokoyama et al., 1995). To explore whether stimulation of α1E requires phosphorylation, we exposed cells to staurosporine, a broad-spectrum kinase inhibitor. As shown in Figure4, stimulation was completely blocked by 1 μm staurosporine, whereas inhibition was unaffected. Stimulation was also substantially reduced (to 6.1 ± 1.6%; n = 4) by a much lower concentration of staurosporine (50 nm), consistent with a specific action of this compound on protein kinases. Application of the vehicle (DMSO) alone had no effect (Fig. 4B). To determine further the involvement of phosphorylation in stimulation of α1E, we dialyzed cells with the nonhydrolyzable ATP analog 5′-adenylylimidodiphosphate (AMP-PNP; 5 mm) for >5 min before applying CCh. As shown in Figure 4C, stimulation was significantly reduced (p = 0.004) in cells dialyzed with AMP-PNP, whereas inhibition was unaffected (Fig. 4D). Together these results suggest that stimulation of α1E involves phosphorylation by a protein kinase.
Fig. 4.
Stimulation of α1E involves phosphorylation. Ca currents were evoked every 2–10 sec by step depolarizations to +30 mV.A, Staurosporine (1 μm) blocks stimulation of α1E without affecting inhibition. The staurosporine solution contained 0.05% DMSO. The horizontal bar indicates CCh application. C = 11 pF;RS = 4.5 MΩ. File 98625008.B, DMSO alone (0.05%) has no effect.C = 48 pF; RS= 3.6 MΩ. File 98624003. C, Stimulation is reduced by intracellular dialysis with 5 mm AMP-PNP.C = 36 pF; RS= 2.6 MΩ. File 98903001. D, Summary of results. Control data were from cells exposed to 0.05% DMSO. OA, Okadaic acid (100 nm); Stauros, staurosporine.
If stimulation involves phosphorylation, then recovery from stimulation should involve dephosphorylation. To explore this issue, we exposed cells to 100 nm okadaic acid for at least 2 hr before and throughout the experiments. As summarized in Figure4D, okadaic acid did not change the magnitude of stimulation. Okadaic acid did not prevent recovery from stimulation (data not shown), suggesting that phosphatases 1 and 2A are not required for this event. Unfortunately, we were unable to determine whether okadaic acid altered the time course or extent of recovery.
Stimulation of α1E does not involve PKA, PKC, or PKG
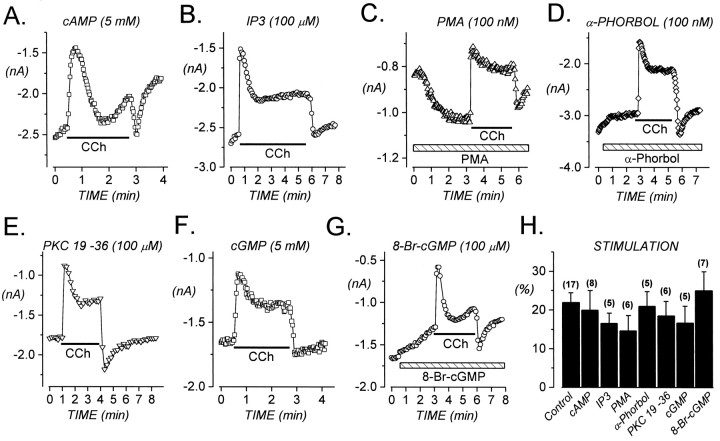
M2 muscarinic acetylcholine receptors couple efficiently to Gαi, and activation of these receptors can inhibit adenylyl cyclase and thereby reduce the intracellular concentration of cAMP (Ashkenazi et al., 1987; Peralta et al., 1988). To investigate the possibility that stimulation of α1E results, either directly or indirectly, from a decline in intracellular cAMP, we dialyzed cells with a pipette solution containing 5 mm cAMP for at least 5 min before applying CCh. As shown in Figure5A, this treatment had no effect on the magnitude of stimulation. We also found that stimulation was unaffected by dialyzing cells with 100 μmprotein kinase inhibitor (6-22) amide, a specific peptide inhibitor of PKA (n = 3; data not shown). These results suggest that changes in intracellular cAMP or phosphorylation by PKA do not account for stimulation of α1E.
Fig. 5.
Stimulation of α1E does not involve PKA, IP3, PKC, or PKG. Ca currents were evoked every 2–10 sec by depolarizations to +30 mV. Cells were dialyzed with cAMP, IP3, PKC 19–36, or cGMP for 5–10 min before applying CCh (indicated by solid horizontal bar).A, Intracellular cAMP (5 mm) does not reduce stimulation. C = 17 pF;RS = 2.8 MΩ. File 98731007.B, Intracellular IP3 does not reduce stimulation. C = 19 pF;RS = 3.0 MΩ. File 99121011.C, The phorbol ester PMA (100 nm) does not reduce stimulation. C = 18 pF;RS = 2.6 MΩ. File 98820015.D, Effects of the inactive α-phorbol (100 nm) are shown. C = 15 pF;RS = 4.2 MΩ. File 98908001.E, Intracellular application of PKC 19–36 (100 μm), a pseudosubstrate inhibitor of PKC, does not reduce stimulation. C = 11 pF;RS = 3.1 MΩ. File 98626003.F, Intracellular cGMP (5 mm) does not reduce stimulation. C = 37 pF;RS = 4.6 MΩ. File 98730022.G, Bath application of membrane-permeant 8-Br-cGMP (indicated by hatched horizontal bar; 100 μm) does not reduce stimulation.C = 19 pF; RS= 2.0 MΩ. File 98925012. H, Summary of results (p = 0.62, ANOVA).
M2 receptors weakly stimulate the production of phosphoinositides (Ashkenazi et al., 1987), suggesting that these receptors may also couple to Gαq proteins, although with lower efficiency than they couple to Gαi/o (Peralta et al., 1988). In agreement with this possibility, Berstein et al. (1992) observed that M2 receptors stimulate GTP-γ-S binding by Gαq reconstituted into lipid vesicles, although M2 receptors are only 10% as effective as M1 receptors in this regard. Classically, Gαq subunits stimulate phospholipase Cβ1, resulting in the production of inositol trisphosphate and diacylglycerol (DAG). Inclusion of d-myo-inositol 1,4,5-trisphosphate (IP3; 100 μm) in the standard 10 mm EGTA–containing pipette solution had no effect (Fig. 5B), suggesting that signaling by IP3 is not involved in producing stimulation of α1E.
Some forms of PKC are activated by DAG. To investigate the potential involvement of PKC in producing stimulation of α1E, we exposed cells to 100 nm phorbol 12-myristate 13-acetate (PMA) before and during CCh application. As illustrated in Figure 5C, Ca current amplitudes slowly increased during PMA exposure, presumably reflecting the PKC-dependent phosphorylation of α1E channels or associated proteins. In contrast, the inactive 4α-phorbol produced a slight decrease in Ca current amplitudes (Fig. 5D). These results confirm previously demonstrated effects of PMA on α1E Ca channels (Stea et al., 1995). Despite its ability to increase baseline α1E currents, PMA did not occlude stimulation of α1E by CCh (Fig.5H). Interestingly, PMA did reduce the magnitude of α1E inhibition to 28.5 ± 2.7% (n = 6), compared with 40.0 ± 1.4% inhibition in control cells (n = 42). In additional experiments, we dialyzed cells with PKC 19–36, a pseudosubstrate peptide inhibitor of PKC, for >5 min before applying CCh. As shown in Figure 5E, the PKC 19–36 peptide had no effect on stimulation. Inhibition in cells dialyzed with PKC 19–36 was also identical (40.2 ± 6.0%;n = 6) to that in control cells. Thus, PKC-dependent phosphorylation does not seem to be involved in the M2 receptor–mediated stimulation of α1E.
We next examined whether stimulation of α1E involves PKG. Intracellular dialysis with cGMP (5 mm) did not reduce the magnitude of stimulation (Fig. 5F). Application of 8-bromo-cGMP (8-Br-cGMP; 100 μm), a membrane-permeant, hydrolysis-resistant analog of cGMP, consistently produced a slow decrease in the amplitude of α1E currents (Fig.5G). However, 8-Br-cGMP failed to alter the magnitude of CCh-induced stimulation (Fig. 5H). In summary, these experiments with activators and inhibitors of various protein kinases suggest that PKA, PKC, and PKG are not responsible for the M2 receptor–mediated stimulation of α1E.
Stimulation of α1E does not involve tyrosine kinases or phosphoinositide 3-kinases
Previous studies have shown that G-protein–coupled receptors can modulate ion channels via activation of tyrosine kinases (Huang et al., 1993; Diversé-Pierluissi et al., 1997; Felsch et al., 1998). We used genistein, a broad-spectrum tyrosine kinase inhibitor, to examine whether tyrosine kinase–dependent phosphorylation underlies stimulation of α1E. As shown in Figure6A, genistein caused a substantial inhibition of α1E currents under control conditions. This effect of genistein may result from inhibition of basally active tyrosine kinases or direct block of α1E channels. In support of the latter possibility, genistein can directly block voltage-gated Na channels (Paillart et al., 1997), GABAA channels (Huang et al., 1999), and T-type Ca channels (U. Meza and B. Adams, unpublished observations). However, the fact that daidzein (a weakly active genistein analog) produced a much smaller decrease in α1E currents than did genistein (Fig. 6B) is consistent with inhibition of basally active tyrosine kinases. As summarized in Figure 6C, neither genistein nor daidzein significantly altered the CCh-induced modulation of α1E currents. Intracellular application of genistein (100 μm) through the patch pipette, either alone or in combination with external genistein application, also failed to alter the inhibition or stimulation of α1E (data not shown). Although these experiments are not exhaustive, they indicate that genistein-sensitive tyrosine kinases are not responsible for M2 receptor–mediated stimulation of α1E.
Recent experiments by Viard et al. (1999) have shown that vascular L-type Ca channels are stimulated via a pathway involving Gβγ-activated phosphoinositide 3-kinases (PI3-K). We used wortmannin, a cell-permeant, irreversible PI3-K inhibitor, to evaluate the potential involvement of these kinases in stimulation of α1E. Cells were exposed to 200 nm wortmannin for at least 2 hr before and throughout the experiments. Inhibition and stimulation in wortmannin-treated cells were 36.0 ± 2.3% (n = 10) and 21.5 ± 4.3% (n = 10), respectively, not significantly different from that of control cells (Fig.6C). Thus, wortmannin-sensitive PI3-kinases do not appear to be responsible for stimulation of α1E.
Stimulation of α1E does not require a Ca signal
In rat sympathetic ganglion neurons, activation of endogenous muscarinic receptors produces biphasic (i.e., fast and slow) inhibition of N-type Ca channels. The fast inhibition occurs via a membrane-delimited pathway, whereas the slow inhibition occurs via a cytosolic pathway that does not seem to involve PKA, PKC, or PKG (Bernheim et al., 1991). Inhibition of N-type channels via the slow pathway is blocked by high intracellular concentrations of BAPTA or EGTA (Beech et al., 1991), suggesting that this slow pathway involves a Ca signal. If stimulation of α1E also involves a Ca signal, then this signal might be increased, and stimulation consequently enhanced, by reducing intracellular Ca buffering. However, we found that both inhibition and stimulation were significantly reduced (to 27.1 ± 2.4 and 14.3 ± 2.7%, respectively; n = 10) when the pipette solution contained a reduced concentration (0.1 mm) of EGTA (Fig.7A). Similar results were obtained using a pipette solution containing 0.1 mm BAPTA (25.2 ± 2.3% inhibition and 12.8 ± 2.2% stimulation; n = 10). Thus, reducing intracellular Ca buffering clearly did not enhance stimulation. The decreased modulation of α1E channels with 0.1 mm intracellular EGTA or BAPTA is unexplained and is at odds with the finding of Beech et al. (1991) that modulation of native N-type channels is greatest in the presence of low intracellular concentrations of Ca buffers.
Fig. 7.
Stimulation does not require a Ca signal.A, Average magnitudes of inhibition and stimulation with different concentrations of Ca buffers in the pipette solution are shown. The pipette solutions contained (in mm): 10 EGTA, 0.1 EGTA, 0.1 BAPTA, 20 BAPTA, or 20 BAPTA plus 10 Ca.B, Stimulation of α1E is not prevented by 20 mm intracellular BAPTA. C = 14 pF;RS = 2.3 MΩ. File 99106001.C, Stimulation of α1E does not require Ca influx. For these experiments, the bath solution initially contained 40 mm Ca. Directly before CCh application (solid horizontal bar), the bath solution was switched to one containing 40 mm Ba (hatched horizontal bar). C = 30 pF;RS = 4.4 MΩ. File 98O09014. Cells were dialyzed for >5 min in the whole-cell configuration with BAPTA-containing pipette solutions before applying CCh. Currents were evoked every 5 sec (B) or 2 sec (C) by depolarizations to +30 mV.
Our standard pipette solution contained 10 mm EGTA, which is expected to produce effective steady-state buffering of intracellular Ca. However, EGTA has a relatively slow on-rate and hence may not prevent rapid Ca transients. To examine whether stimulation of α1E involves a rapid Ca signal, we used the fast Ca buffer BAPTA. As shown in Figure 7B, stimulation of α1E was not prevented by 20 mm intracellular BAPTA. As was found for 0.1 mm EGTA and BAPTA, both inhibition and stimulation were reduced to similar degrees by 20 mm BAPTA (Fig. 7A). To determine whether these effects of high BAPTA concentration stemmed from buffering intracellular Ca at extremely low levels (cf. Cruzblanca et al., 1998), we used a pipette solution containing 20 mm BAPTA plus 10 mm added Ca. This solution is predicted to have a free Ca concentration of ∼140 nm (Beech et al., 1991), very close to the resting cytoplasmic Ca concentration measured in HEK293 cells (Tong et al., 1999). As summarized in Figure 7A, inhibition and stimulation of α1E were similarly reduced by 20 mm BAPTA even in the presence of physiological free Ca. These results are consistent with the previously suggested (Beech et al., 1991) possibility that BAPTA has intracellular effects unrelated to its Ca-chelating properties.
To test whether influx of extracellular Ca is required for stimulation of α1E, we substituted equimolar Ba for Ca as the charge carrier. For these experiments, a nominally Ca-free pipette solution (20 mm BAPTA and no added Ca) was used. As shown in Figure7C, Ba currents through α1E channels exhibited biphasic, opposing modulation. The magnitude of stimulation for Ba currents was 14.3 ± 1.3% (n = 6), similar to stimulation of Ca currents (11.8 ± 1.9%; n = 15) recorded using the same pipette solution. In summary, these experiments with BAPTA, EGTA, and Ba suggest that stimulation of α1E does not require Ca influx, a transient rise in intracellular Ca concentration, or the participation of a Ca-activated signaling molecule (e.g., calmodulin, calcineurin, or CaM kinases).
DISCUSSION
We have demonstrated that α1E Ca channels are simultaneously inhibited and stimulated during activation of M2 muscarinic acetylcholine receptors. Inhibition has a relatively fast onset and is associated with kinetic slowing, suggesting that it occurs via a membrane-delimited pathway. In contrast, stimulation is considerably slower in onset and seems to require phosphorylation, suggesting that it occurs via a cytosolic, kinase-dependent pathway. Kinetic slowing is maintained during stimulation, and after CCh washout, both kinetic slowing and inhibition of current amplitude are rapidly relieved. The over-recovery of current amplitude after CCh washout is approximately equal to the magnitude of inhibition, indicating that inhibition is maintained at a relatively constant level during stimulation. These observations demonstrate that inhibition and stimulation of α1E are separate events, with stimulation superimposed on inhibition.
Inhibition and stimulation involve at least two distinct signaling pathways coupled to M2 receptors, because inhibition depends on PTX-sensitive G-proteins whereas stimulation does not. This dual coupling occurs even when M2 receptors are expressed at nonsaturating levels. The coupling of M2 receptors to more than one pathway is not likely to be an artifact of heterologous expression, because endogenous metabotropic glutamate receptors have also been shown to couple to both PTX-sensitive and PTX-insensitive pathways in neurons (Hay and Kunze, 1994; Choi and Lovinger, 1996; Kammermeier and Ikeda, 1999). Previous studies have found that HEK293 cells express Gαi(1–3), Gαo, Gαq, and Gαs proteins (Law et al., 1993; Kim et al., 1994; Offermanns et al., 1994; Yamauchi et al., 1999). When expressed in HEK293 cells, M2 receptors are thought to couple to Gαi/o proteins with high efficiency and possibly also to Gαq proteins with much lower efficiency (Ashkenazi et al., 1987; Peralta et al., 1988). Our experiments with PTX and CTX indicate that Gαi/o or Gαs are not responsible for stimulating α1E. Thus, by elimination Gαq seems the most likely candidate. However, Gαq is classically thought to activate phospholipases, resulting in the liberation of IP3 and DAG, which trigger intracellular Ca release and activation of PKC, respectively. Because our results indicate that stimulation of α1E does not involve signaling by IP3, PKC, or Ca, it is currently unclear how Gαq might produce stimulation. Potentially, βγ dimers released from Gαq could activate small GTPases (e.g., Ras) that in turn could activate MAP kinases (Crespo et al., 1994), and MAP kinase–dependent phosphorylation might directly or indirectly produce stimulation of α1E. However, MAP kinases are activated by phorbol esters (Dulin et al., 1999; Zhang et al., 1999), and we found that stimulation was unaltered by PMA (Fig. 5). These considerations suggest that MAP kinases are unlikely to be responsible for stimulating α1E. Our experiments additionally suggest that stimulation does not involve phosphorylation by tyrosine kinases or PI3-kinases (Fig. 6).
Membrane-delimited inhibition is hypothesized to result from direct binding of Gβγ subunits to neuronal α1A, α1B, and α1E subunits [De Waard et al. (1997); Zamponi et al. (1997); but seeDiversé-Pierluissi et al. (1997)]. If this hypothesis is correct, then our data suggest that Gβγ subunits remain bound to α1E during stimulation. However, because stimulation can occur in the absence of inhibition (Fig. 2), Gβγ binding to α1E may not be a prerequisite for stimulation. Conversely, if stimulation involves phosphorylation of α1E itself, then such phosphorylation must not reduce Gβγ binding or counteract its effects on channel gating. For α1A and α1B Ca channels, PKC-dependent phosphorylation of specific amino acids within the I–II loop reduces binding of Gβγ subunits and thereby antagonizes Gβγ-mediated channel inhibition (De Waard et al., 1997; Zamponi et al., 1997). Reduction of N-type Ca channel inhibition can also occur via PKC-dependent phosphorylation of neurotransmitter receptors (García et al., 1998a). In both cases, PKC-dependent phosphorylation actually decreases the Gβγ-mediated inhibition of α1A and α1B. These two types of “cross-talk” between kinase-dependent and G-protein–dependent pathways are distinct from the biphasic, opposing modulation of α1E described here, in which stimulation develops in the continued presence of inhibition and does not substantially reduce the extent of inhibition.
For N-type Ca channels, the first latency of single-channel opening is increased by G-protein–dependent, membrane-delimited inhibition (Carabelli et al., 1996; Patil et al., 1996). If the same molecular mechanism applies to G-protein–inhibited α1E Ca channels, then the persistence of kinetic slowing during the secondary stimulation phase predicts that first latencies remain long during stimulation. Our results therefore suggest that stimulation of α1E reflects decreased single-channel closed time, increased channel open time, or an increase in the number of functional channels (Yang and Tsien, 1993). Further experiments using single-channel recordings will be necessary to discriminate among these possibilities.
Stimulation of α1E does not require coexpression of an exogenous Ca channel β subunit (Fig. 3). Furthermore, inhibition of α1E was not reduced by coexpression of β2a or β3. A similar observation was made previously by Page et al. (1998), who found that rat brain β2a subunits did not antagonize the receptor-mediated inhibition of rat α1E expressed in Xenopus oocytes or COS-7 cells. In contrast, Qin et al. (1997, 1998) found that coexpression of rat brain β2a occludes, and coexpression of β1b or β3 reduces, inhibition of human α1E expressed in Xenopus oocytes. The rat brain β2a subunit possesses two N-terminal cysteine residues that can be palmitoylated in a dynamic manner (Chien et al., 1996, 1998), and it has been proposed (Qin et al., 1998) that cysteines 3 and 4 within rat brain β2a must be palmitoylated for β2a to antagonize the G-protein–mediated inhibition of α1E. Although we did not confirm palmitoylation of rat brain β2a expressed in our HEK293 cells, such palmitoylation seems likely because Chien et al. (1996) demonstrated palmitoylation of this same β2a subunit expressed in tsA201 cells (a clone of HEK293 cells stably expressing SV40 large T antigen). Thus, our results and those of Page et al. (1998) seem inconsistent with the hypothesis that Ca channel β subunits compete with G-proteins for binding to Ca channel α1 subunits (Campbell et al., 1995: Bourinet et al., 1996).
Previous studies have reported complex Ca channel modulation that is similar to the biphasic, opposing modulation of α1E described here. Thus, in neuroblastoma cells recovering from PTX treatment, Friederich et al. (1993) observed Ca channel stimulation during activation of receptors that otherwise cause inhibition. Zong and Lux (1994) found that intracellular dialysis of chick dorsal root ganglion neurons with GTP-γ-S produced first inhibition and then stimulation of mixed whole-cell Ba currents. They also observed kinetic slowing during the secondary stimulation phase, indicating that stimulation was superimposed on inhibition. However, because more than one Ca channel type contributed to the whole-cell currents recorded in their experiments, it is unknown whether a single Ca channel type was both inhibited and stimulated. Wang and Lipsius (1998) also observed biphasic, opposing effects of genistein on native L-type Ca channels in feline atrial myocytes. They concluded that genistein affected two different kinds of tyrosine kinases to produce inhibition and stimulation.
Our present results demonstrate that cloned α1E channels can be simultaneously inhibited and stimulated during activation of a single type of neurotransmitter receptor. Our data further suggest that biphasic, opposing modulation is unique to α1E, because α1A and α1B showed only inhibition during sustained activation of M2 receptors. However, our results do not exclude the possibility that α1A and α1B exhibit more complex modulation under different circumstances. Our experiments were conducted in HEK293 cells expressing cloned M2 receptors and Ca channels, and in many respects this is an artificial system. However, M2 receptors are widely expressed throughout the brain (Peralta et al., 1987; Buckley et al., 1988) where they are essential for numerous neurological functions (Gomeza et al., 1999). Additionally, M2 receptors and α1E channels may be colocalized, because both are expressed on neuronal cell bodies and dendrites (Hersch et al., 1994; Yokoyama et al., 1995; Westenbroek et al., 1998). It therefore seems reasonable to predict that biphasic, opposing modulation of α1E Ca channels occurs in neurons.
To understand the physiological significance of our observations, it will be necessary to study native α1E Ca channels in cells in which their functional roles are known. However, we speculate that dual modulation of α1E confers unique functional properties on native R-type Ca channels. As one possibility, the secondary stimulation of α1E may enable R-type channels to mediate Ca influx during tonic or repetitive activation of muscarinic (or other) receptors. Because N-type and P/Q-type channels would tend to be inhibited under these conditions, maintained Ca influx through R-type channels might generate significant spatial and/or temporal differences in Ca signals within a single neuron. Such Ca signals could be important in producing subcellular differences in gene expression, membrane excitability, or secretion.
Footnotes
This work was supported by National Institutes of Health Grant NS34423 and a grant-in-aid from the American Heart Association to B.A. U.M. was the recipient of a Consejo Nacional de Ciencia y Tecnologia fellowship.
Correspondence should be addressed to Dr. Brett Adams, Department of Biology, 5305 Old Main Hill, Utah State University, Logan, UT 84322-5305. E-mail: brett@biology.usu.edu.
REFERENCES
- 1.Ashkenazi A, Winslow JW, Peralta EG, Peterson GL, Schimerlik MI, Capon DJ, Ramachandran J. An M2 muscarinic receptor subtype coupled to both adenylyl cyclase and phosphoinositide turnover. Science. 1987;238:672–675. doi: 10.1126/science.2823384. [DOI] [PubMed] [Google Scholar]
- 2.Avigan J, Murtagh JJ, Stevens LA, Angus CW, Moss J, Vaughan M. Pertussis toxin-catalyzed ADP-ribosylation of Goα with mutations at the carboxyl terminus. Biochemistry. 1992;31:7736–7740. doi: 10.1021/bi00148a039. [DOI] [PubMed] [Google Scholar]
- 3.Bean BP. Neurotransmitter inhibition of neuronal calcium currents by changes in channel voltage dependence. Nature. 1989;340:153–156. doi: 10.1038/340153a0. [DOI] [PubMed] [Google Scholar]
- 4.Beech DJ, Bernheim L, Mathie A, Hille B. Intracellular Ca buffers disrupt muscarinic suppression of Ca current and M current in rat sympathetic neurons. Proc Natl Acad Sci USA. 1991;88:652–656. doi: 10.1073/pnas.88.2.652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernheim L, Beech DJ, Hille B. A diffusible second messenger mediates one of the pathways coupling receptors to calcium channels in rat sympathetic neurons. Neuron. 1991;6:859–867. doi: 10.1016/0896-6273(91)90226-p. [DOI] [PubMed] [Google Scholar]
- 6.Berstein G, Blank JL, Smrcka AV, Higashijima T, Sternweis PC, Exton JH, Ross EM. Reconstitution of agonist-stimulated phosphatidylinositol 4,5-bisphosphate hydrolysis using purified m1 muscarinic receptor, Gq/11, and phospholipase C-β1. J Biol Chem. 1992;267:8081–8088. [PubMed] [Google Scholar]
- 7.Bourinet E, Soong TW, Stea A, Snutch TP. Determinants of the G protein-dependent opioid modulation of neuronal calcium channels. Proc Natl Acad Sci USA. 1996;93:1486–1491. doi: 10.1073/pnas.93.4.1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buckley NJ, Bonner TI, Brann MR. Localization of a family of muscarinic receptor mRNAs in rat brain. J Neurosci. 1988;8:4646–4652. doi: 10.1523/JNEUROSCI.08-12-04646.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell V, Berrow NS, Fitzgerald EM, Brickley K, Dolphin AC. Inhibition of the interaction of G protein Go with calcium channels by the calcium channel β-subunit in rat neurones. J Physiol (Lond) 1995;485:365–372. doi: 10.1113/jphysiol.1995.sp020735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carabelli V, Lovallo M, Magnelli V, Zucker H, Carbone E. Voltage-dependent modulation of single N-Type Ca channel kinetics by receptor agonists in IMR32 cells. Biophys J. 1996;70:2144–2154. doi: 10.1016/S0006-3495(96)79780-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chang FH, Bourne HR. Cholera toxin induces cAMP-independent regulation of Gs. J Biol Chem. 1989;264:5352–5357. [PubMed] [Google Scholar]
- 12.Chien AJ, Carr KM, Shirokov RE, Rios E, Hosey MM. Identification of palmitoylation sites within the L-type calcium channel β2a subunit and effects on channel function. J Biol Chem. 1996;271:26465–26468. doi: 10.1074/jbc.271.43.26465. [DOI] [PubMed] [Google Scholar]
- 13.Chien AJ, Gao T, Perez-Reyes E, Hosey MM. Membrane targeting of L-type calcium channels. J Biol Chem. 1998;273:23590–23597. doi: 10.1074/jbc.273.36.23590. [DOI] [PubMed] [Google Scholar]
- 14.Choi S, Lovinger DM. Metabotropic glutamate receptor modulation of voltage-gated Ca2+ channels involves multiple receptor subtypes in cortical neurons. J Neurosci. 1996;16:36–45. doi: 10.1523/JNEUROSCI.16-01-00036.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Crespo P, Ningzhi X, Simonds WF, Gutkind JS. Ras-dependent activation of MAP kinase pathway mediated by G-protein βγ subunits. Nature. 1994;369:418–420. doi: 10.1038/369418a0. [DOI] [PubMed] [Google Scholar]
- 16.Cruzblanca H, Koh D-S, Hille B. Bradykinin inhibits M current via phospholipase C and Ca release from IP3-sensitive stores in rat sympathetic neurons. Proc Natl Acad Sci USA. 1998;95:7151–7156. doi: 10.1073/pnas.95.12.7151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.De Waard M, Liu H, Walker D, Scott VE, Gurnett CA, Campbell KP. Direct binding of G-protein betagamma complex to voltage-dependent calcium channels. Nature. 1997;385:446–450. doi: 10.1038/385446a0. [DOI] [PubMed] [Google Scholar]
- 18.Diversé-Pierluissi M, Remmers AE, Neubig RR, Dunlap K. Novel form of crosstalk between G protein and tyrosine kinase pathways. Proc Natl Acad Sci USA. 1997;94:5417–5421. doi: 10.1073/pnas.94.10.5417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dulin NO, Sorokin A, Reed E, Elliott S, Kehrl JH, Dunn MJ. RGS3 inhibits G protein-mediated signaling via translocation to the membrane and binding to Gα11. Mol Cell Biol. 1999;19:714–723. doi: 10.1128/mcb.19.1.714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Felsch JS, Cachero TG, Peralta EG. Activation of protein tyrosine kinase PYK2 by the m1 muscarinic acetylcholine receptor. Proc Natl Acad Sci USA. 1998;95:5051–5056. doi: 10.1073/pnas.95.9.5051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Friederich P, Nurnberg B, Schultz G, Hescheler J. Inversion of Ca current modulation during recovery of neuroblastoma cells from pertussis toxin pretreatment. FEBS Lett. 1993;334:322–326. doi: 10.1016/0014-5793(93)80703-w. [DOI] [PubMed] [Google Scholar]
- 22.Fujita Y, Mynlieff M, Dirksen RT, Kim MS, Niidome T, Nakai J, Friedrich T, Iwabe N, Miyata T, Furuichi T, Furutama D, Mikoshiba K, Mori Y, Beam KG. Primary structure and functional expression of the ω-conotoxin-sensitive N-type calcium channel from rabbit brain. Neuron. 1993;10:585–598. doi: 10.1016/0896-6273(93)90162-k. [DOI] [PubMed] [Google Scholar]
- 23.García DE, Brown S, Hille B, Mackie K. Protein kinase C disrupts cannabinoid actions by phosphorylation of the CB1 cannabinoid receptor. J Neurosci. 1998a;18:2834–2841. doi: 10.1523/JNEUROSCI.18-08-02834.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.García DE, Li B, García-Ferreiro RE, Hernández-Ochoa EO, Yang K, Gautam N, Catterall WA, Mackie K, Hille B. G-protein β-subunit specificity in the fast membrane-delimited inhibition of Ca channels. J Neurosci. 1998b;18:9163–9170. doi: 10.1523/JNEUROSCI.18-22-09163.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gill DM, Meren R. ADP-ribosylation of membrane proteins catalyzed by cholera toxin: basis of the activation of adenylate cyclase. Proc Natl Acad Sci USA. 1978;75:3050–3054. doi: 10.1073/pnas.75.7.3050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gomeza J, Shannon H, Kostenis E, Felder C, Zhang L, Brodkin J, Grinberg A, Sheng H, Wess J. Pronounced pharmacological deficits in M2 muscarinic acetylcholine receptor knockout mice. Proc Natl Acad Sci USA. 1999;96:1692–1697. doi: 10.1073/pnas.96.4.1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 28.Hay M, Kunze DL. Glutamate metabotropic receptor inhibition of voltage-gated calcium currents in visceral sensory neurons. J Neurophysiol. 1994;72:421–430. doi: 10.1152/jn.1994.72.1.421. [DOI] [PubMed] [Google Scholar]
- 29.Herlitze S, Garcia DE, Mackie K, Hille B, Scheuer T, Catterall WA. Modulation of Ca channels by G-protein βγ subunits. Nature. 1996;380:258–262. doi: 10.1038/380258a0. [DOI] [PubMed] [Google Scholar]
- 30.Hersch SM, Gutekunst CA, Rees HD, Heilman CJ, Levey AI. Distribution of m1–m4 muscarinic receptor proteins in the rat striatum: light and electron microscopic immunocytochemistry using subtype-specific antibodies. J Neurosci. 1994;14:3351–3363. doi: 10.1523/JNEUROSCI.14-05-03351.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hille B. Modulation of ion-channel function by G-protein-coupled receptors. Trends Neurosci. 1994;17:531–536. doi: 10.1016/0166-2236(94)90157-0. [DOI] [PubMed] [Google Scholar]
- 32.Huang X-Y, Morielli AD, Peralta EG. Tyrosine kinase-dependent suppression of a potassium channel by the G protein-coupled m1 muscarinic acetylcholine receptor. Cell. 1993;75:1145–1156. doi: 10.1016/0092-8674(93)90324-j. [DOI] [PubMed] [Google Scholar]
- 33.Huang RQ, Fang MJ, Dillon GH. The tyrosine kinase inhibitor genistein directly inhibits GABAA receptors. Brain Res Mol Brain Res. 1999;67:177–183. doi: 10.1016/s0169-328x(99)00061-3. [DOI] [PubMed] [Google Scholar]
- 34.Ikeda SR. Voltage-dependent modulation of N-type calcium channels by G-protein βγ subunits. Nature. 1996;380:255–258. doi: 10.1038/380255a0. [DOI] [PubMed] [Google Scholar]
- 35.Jeong S-W, Wurster RD. Muscarinic receptor activation modulates Ca channels in rat intracardiac neurons via a PTX- and voltage-sensitive pathway. J Neurophysiol. 1997;78:1476–1490. doi: 10.1152/jn.1997.78.3.1476. [DOI] [PubMed] [Google Scholar]
- 36.Jones LP, Wei S-K, Yue DT. Mechanism of auxiliary subunit modulation of neuronal α1E calcium channels. J Gen Physiol. 1998;112:125–143. doi: 10.1085/jgp.112.2.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jones SW, Elmslie KS. Transmitter modulation of neuronal calcium channels. J Membr Biol. 1997;155:1–10. doi: 10.1007/s002329900153. [DOI] [PubMed] [Google Scholar]
- 38.Kammermeier PJ, Ikeda SR. Expression of RGS2 alters the coupling of metabotropic glutamate receptor 1a to M-type K+ and N-type Ca2+ channels. Neuron. 1999;22:819–829. doi: 10.1016/s0896-6273(00)80740-0. [DOI] [PubMed] [Google Scholar]
- 39.Kim G-D, Carr IC, Anderson LA, Zabavnik J, Eidne KA, Milligan G. The long isoform of the rat thyrotropin-releasing hormone receptor down-regulates Gq proteins. J Biol Chem. 1994;269:19933–19940. [PubMed] [Google Scholar]
- 40.Kim HL, Kim H, Lee P, King RG, Chin H. Rat brain expresses an alternatively spliced form of the dihydropyridine-sensitive L-type calcium channel α2 subunit. Proc Natl Acad Sci USA. 1992;89:3251–3255. doi: 10.1073/pnas.89.8.3251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Law SF, Yasuda K, Bell GI, Reisine T. Giα3 and Goα selectively associate with the cloned somatostatin receptor subtype SSTR2. J Biol Chem. 1993;268:10721–10727. [PubMed] [Google Scholar]
- 42.Luebke JI, Dunlap K. Sensory neuron N-type calcium currents are inhibited by both voltage-dependent and -independent mechanisms. Pflügers Arch. 1994;428:499–507. doi: 10.1007/BF00374571. [DOI] [PubMed] [Google Scholar]
- 43.MacKenzie FR, Milligan G. Cholera toxin impairment of opioid-mediated inhibition of adenylate cyclase in neuroblastoma x glioma cells is due to a toxin-induced decrease in opioid receptor levels. Biochem J. 1991;275:175–181. doi: 10.1042/bj2750175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Melliti K, Meza U, Fisher R, Adams B. Regulators of G protein signaling attenuate the G protein-mediated inhibition of N-type Ca channels. J Gen Physiol. 1999;113:97–109. doi: 10.1085/jgp.113.1.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Meza U, Adams B. G-protein-dependent facilitation of neuronal α1A, α1B, and α1E Ca channels. J Neurosci. 1998;18:5240–5252. doi: 10.1523/JNEUROSCI.18-14-05240.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mishina M, Kurosaki T, Tobimatsu T, Morimoto Y, Noda M, Yamamoto T, Terao M, Lindstrom J, Takahashi T, Kuno M, Numa S. Expression of functional acetylcholine receptor from cloned cDNAs. Nature. 1984;307:604–608. doi: 10.1038/307604a0. [DOI] [PubMed] [Google Scholar]
- 47.Mori Y, Friedrich T, Kim MS, Mikami A, Nakai J, Ruth P, Bosse E, Hofmann F, Flockerzi V, Furuichi T, Mikoshiba K, Imoto K, Tanabe T, Numa S. Primary structure and functional expression from complementary DNA of a brain calcium channel. Nature. 1991;350:398–402. doi: 10.1038/350398a0. [DOI] [PubMed] [Google Scholar]
- 48.Niidome T, Kim M-S, Friedrich T, Mori Y. Molecular cloning and characterization of a novel calcium channel from rabbit brain. FEBS Lett. 1992;308:7–13. doi: 10.1016/0014-5793(92)81038-n. [DOI] [PubMed] [Google Scholar]
- 49.Offermanns ST, Wieland T, Homann D, Sandmann J, Bombien E, Spicher K, Schultz G, Jakobs KH. Transfected muscarinic acetylcholine receptors selectively couple to Gi-type G proteins and Gq/11. Mol Pharmacol. 1994;45:890–898. [PubMed] [Google Scholar]
- 50.Page KM, Cantí C, Stephens GJ, Berrow NS, Dolphin AC. Identification of the amino terminus of neuronal Ca channel α1 subunits α1B and α1E as an essential determinant of G-protein modulation. J Neurosci. 1998;18:4815–4824. doi: 10.1523/JNEUROSCI.18-13-04815.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Paillart C, Carlier E, Guedin D, Dargent B, Couraud F. Direct block of voltage-sensitive sodium channels by genistein, a tyrosine kinase inhibitor. J Pharmacol Exp Ther. 1997;280:521–526. [PubMed] [Google Scholar]
- 52.Parent L, Schneider T, Moore CP, Talwar D. Subunit regulation of the human brain α1E calcium channel. J Membr Biol. 1997;160:127–140. doi: 10.1007/s002329900302. [DOI] [PubMed] [Google Scholar]
- 53.Patil PG, de Leon M, Reed RR, Dubel S, Snutch TP, Yue DT. Elementary events underlying voltage-dependent G-protein inhibition of N-type calcium channels. Biophys J. 1996;71:2509–2521. doi: 10.1016/S0006-3495(96)79444-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Peralta EG, Ashkenazi A, Winslow JW, Smith DH, Ramachandran J, Capon DJ. Distinct primary structures, ligand-binding properties and tissue-specific expression of four human muscarinic acetylcholine receptors. EMBO J. 1987;6:3923–3929. doi: 10.1002/j.1460-2075.1987.tb02733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Peralta EG, Ashkenazi A, Winslow JW, Ramachandran J, Capon DJ. Differential regulation of PI hydrolysis and adenylyl cyclase by muscarinic receptor subtypes. Nature. 1988;334:434–437. doi: 10.1038/334434a0. [DOI] [PubMed] [Google Scholar]
- 56.Perez-Reyes E, Castellano A, Kim HS, Bertrand P, Baggstrom E, Lacerda AE, Wei XY, Birnbaumer L. Cloning and expression of a cardiac/brain beta subunit of the L-type calcium channel. J Biol Chem. 1992;267:1792–1797. [PubMed] [Google Scholar]
- 57.Piedras-Rentería ES, Tsien RW. Antisense oligonucleotides against α1E reduce R-type calcium currents in cerebellar granule cells. Proc Natl Acad Sci USA. 1998;95:7760–7765. doi: 10.1073/pnas.95.13.7760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Qin N, Platano D, Olcese R, Stefani E, Birnbaumer L. Direct interaction of Gβγ with a C-terminal Gβγ-binding domain of the Ca channel α1 subunit is responsible for channel inhibition by G protein-coupled receptors. Proc Natl Acad Sci USA. 1997;94:8866–8871. doi: 10.1073/pnas.94.16.8866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Qin N, Platano D, Olcese R, Costantin JL, Stefani E, Birnbaumer L. Unique regulatory properties of the type 2a Ca channel β subunit caused by palmitoylation. Proc Natl Acad Sci USA. 1998;95:4690–4695. doi: 10.1073/pnas.95.8.4690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Randall A, Tsien RW. Pharmacological dissection of multiple types of Ca channel currents in rat cerebellar granule neurons. J Neurosci. 1995;15:2995–3012. doi: 10.1523/JNEUROSCI.15-04-02995.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Soong TW, Stea A, Hodson CD, Dubel SJ, Vincent SR, Snutch TP. Structure and functional expression of a member of the low voltage-activated calcium channel family. Science. 1993;260:1133–1136. doi: 10.1126/science.8388125. [DOI] [PubMed] [Google Scholar]
- 62.Stea A, Soong TW, Snutch TP. Determinants of PKC-dependent modulation of a family of neuronal calcium channels. Neuron. 1995;15:929–940. doi: 10.1016/0896-6273(95)90183-3. [DOI] [PubMed] [Google Scholar]
- 63.Stephens GJ, Page KM, Burley JR, Berrow NS, Dolphin AC. Functional expression of rat brain cloned α1E calcium channels in COS-7 cells. Pflügers Arch. 1997;433:523–532. doi: 10.1007/s004240050308. [DOI] [PubMed] [Google Scholar]
- 64.Tong J, McCarthy TV, MacLennan DH. Measurement of resting cytosolic Ca concentrations and Ca store size in HEK-293 cells transfected with malignant hyperthermia or central core disease mutant Ca release channels. J Biol Chem. 1999;274:693–702. doi: 10.1074/jbc.274.2.693. [DOI] [PubMed] [Google Scholar]
- 65.Turner TJ, Lampe RA, Dunlap K. Characterization of presynaptic calcium channels with ω-conotoxin MVIIC and ω-grammotoxin SIA: role for a resistant calcium channel type in neurosecretion. Mol Pharmacol. 1995;47:348–353. [PubMed] [Google Scholar]
- 66.Viard P, Exner T, Maier U, Mironneau J, Nurnberg B, Nacrez N. Gβγ dimers stimulate vascular L-type Ca2+ channels via phosphoinositide 3-kinase. FASEB J. 1999;13:685–694. doi: 10.1096/fasebj.13.6.685. [DOI] [PubMed] [Google Scholar]
- 67.Wakamori M, Niidome T, Furutama D, Furuichi T, Mikoshiba K, Fujita Y, Tanaka I, Katayama K, Yatani A, Schwartz A, Mori Y. Distinctive functional properties of the neuronal BII (class E) calcium channel. Receptors Channels. 1994;2:303–314. [PubMed] [Google Scholar]
- 68.Wang G, Dayanithi G, Urge L, Newcomb R, Lemos JR. R-type Ca currents specifically regulate oxytocin release from neurohypophysial terminals. Soc Neurosci Abstr. 1998;24:A1023. [Google Scholar]
- 69.Wang YG, Lipsius SL. Genistein elicits biphasic effects on L-type Ca current in feline atrial myocytes. Am J Physiol. 1998;275:H204–H212. doi: 10.1152/ajpheart.1998.275.1.H204. [DOI] [PubMed] [Google Scholar]
- 70.West RE, Moss J, Vaughan M, Liu T, Liu T-Y. Pertussis toxin-catalyzed ADP-ribosylation of transducin: cysteine 347 is the ADP-ribose acceptor site. J Biol Chem. 1985;260:14428–14430. [PubMed] [Google Scholar]
- 71.Westenbroek RE, Hoskins L, Catterall WA. Localization of Ca channel subtypes on rat spinal motor neurons, interneurons, and nerve terminals. J Neurosci. 1998;18:6319–6330. doi: 10.1523/JNEUROSCI.18-16-06319.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Williams ME, Marubio LM, Deal CR, Hans M, Brust PF, Philipson LH, Miller RJ, Johnson EC, Harpold MM, Ellis SB. Structure and functional characterization of neuronal α1E calcium channel subtypes. J Biol Chem. 1994;269:22347–22357. [PubMed] [Google Scholar]
- 73.Witcher DR, De Waard M, Sakamoto J, Franzini-Armstrong C, Pragnell M, Kahl SD, Campbell KP. Subunit identification and reconstitution of the N-type Ca channel complex purified from brain. Science. 1993;261:486–489. doi: 10.1126/science.8392754. [DOI] [PubMed] [Google Scholar]
- 74.Wu LG, Borst JGG, Sakmann B. R-type Ca currents evoke transmitter release at a rat central synapse. Proc Natl Acad Sci USA. 1998;95:4720–4725. doi: 10.1073/pnas.95.8.4720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yamauchi J, Kaziro Y, Itoh H. Differential regulation of mitogen-activated protein kinase kinase 4 (MKK4) and 7 (MKK7) by signaling from G protein βγ subunit in human embryonal kidney 293 cells. J Biol Chem. 1999;274:1957–1965. doi: 10.1074/jbc.274.4.1957. [DOI] [PubMed] [Google Scholar]
- 76.Yang J, Tsien RW. Enhancement of N- and L-type calcium channel currents by protein kinase C in frog sympathetic neurons. Neuron. 1993;10:127–136. doi: 10.1016/0896-6273(93)90305-b. [DOI] [PubMed] [Google Scholar]
- 77.Yassin M, Zong S, Tanabe T. G-protein modulation of neuronal class E (α1E) calcium channel expressed in GH3 cells. Biochem Biophys Res Commun. 1996;220:453–458. doi: 10.1006/bbrc.1996.0426. [DOI] [PubMed] [Google Scholar]
- 78.Yokoyama CT, Westenbroek RE, Hell JW, Soong TW, Snutch TP, Catterall WA. Biochemical properties and subcellular distribution of the neuronal class E calcium channel α1 subunit. J Neurosci. 1995;15:6419–6432. doi: 10.1523/JNEUROSCI.15-10-06419.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zamponi GW, Bourinet E, Nelson D, Nargeot J, Snutch TP. Crosstalk between G proteins and protein kinase C mediated by the calcium channel α1 subunit. Nature. 1997;385:442–446. doi: 10.1038/385442a0. [DOI] [PubMed] [Google Scholar]
- 80.Zhang Y, Neo SY, Han J, Yaw LP, Lin S-C. RGS16 attenuates Gαq-dependent p38 mitogen-activated protein kinase activation by platelet-activating factor. J Biol Chem. 1999;274:2851–2857. doi: 10.1074/jbc.274.5.2851. [DOI] [PubMed] [Google Scholar]
- 81.Zong X, Lux HD. Augmentation of calcium channel currents in response to G-protein activation by GTPγS in chick sensory neurons. J Neurosci. 1994;14:4847–4853. doi: 10.1523/JNEUROSCI.14-08-04847.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]