Abstract
Muscle cells express a distinct splice variant of acetylcholinesterase (AChET), but the specific mechanisms governing this restricted expression remain unclear. In these cells, a fraction of AChE subunits is associated with a triple helical collagen, ColQ, each strand of which can recruit a tetramer of AChET. In the present study, we examined the expression of the various splice variants of AChE by transfection in the mouse C2C12 myogenic cells in vitro, as well as in vivo by injecting plasmid DNA directly into tibialis anterior muscles of mice and rats. Surprisingly, we found that transfection with an ACHEH cDNA, generating a glycophosphatidylinositol-anchored enzyme species, produced much more activity than transfection with AChET cDNA in both C2C12 cells and in vivo. This indicates that the exclusive expression of AChET in mature muscle is governed by specific splicing. Interaction of AChET subunits with the complete collagen tail ColQ increased enzyme activity in cultured cells, as well as in muscle fibers in vivo. Truncated ColQ subunits, presenting more or less extensive C-terminal deletions, also increased AChE activity and secretion in C2C12 cells, although the triple helix could not form in the case of the larger deletion. This suggests that heteromeric associations are stabilized compared with isolated AChET subunits. Coinjections of AChETand ColQ resulted in the production and secretion of asymmetric forms, indicating that assembly, processing, and externalization of these molecules can occur outside the junctional region of muscle fibers and hence does not require the specialized junctional Golgi apparatus.
Keywords: acetylcholinesterase, collagen tail, C2C12 muscle cells, skeletal muscle, alternative splicing, synaptic proteins, neuromuscular junctions
Acetylcholinesterase (AchE) (EC3.1.1.7.) is concentrated at neuromuscular junctions. Although both muscle fibers and motoneurons synthesize this enzyme in vivo (Brimijoin, 1979; Couraud and Di Giamberardino, 1980;Anglister, 1991), there is currently more information available on the pattern of AChE expression in muscle cells.
In mammals, alternative splicing of the AChE gene produces several types of subunits possessing identical catalytic activity (Massoulié et al., 1998). The AChE catalytic domain, which is common to all subunit types, is encoded by exons 2, 3, and 4, whereas alternative C-terminal peptides are generated by splicing of exons 5 [hydrophobic (H)] and 6 [tailed (T)] to yield the AChEH and AChET catalytic subunits, respectively. A third class of mRNA in which the intron after the invariant exon 4 is retained has been identified inTorpedo, mouse and rat, but not in man (Sikorav et al., 1988; Legay et al., 1993; Li et al., 1993). This latter mRNA species has been designated as the Readthrough (R) transcript (Taylor and Radic, 1994).
AChE exists as a family of molecular forms that may be classified as amphiphilic and nonamphiphilic according to their hydrophobic interactions and as homomeric and heteromeric according to their quaternary structure. The homomeric forms include amphiphilic and nonamphiphilic monomers (G1a, G1na), dimers (G2a, G2na), and tetramers (G4a, G4na). Heteromeric membrane-bound and collagen-tailed molecules result from the association of tetramers of AChET subunits with an hydrophobic anchoring subunit (P) or with a triple helical collagen tail (ColQ) (Massoulié et al., 1998). In collagen-tailed or asymmetric forms (A4, A8, A12), the catalytic subunits are attached to the N terminus of each collagen strand through a proline-rich attachment domain (PRAD) (Bon et al., 1997). In the adult, AChEH subunits are exclusively expressed in tissues of hematopoietic origin in which they anchored at the cell surface by a glycophosphatidylinositol (GPI). The AChET subunit is expressed in both muscle and neuronal cells.
The muscle-specific expression of AChET catalytic subunits may result from alternative splicing, mRNA stabilization, and differential post-translational processing. For example, the commitment of myogenic cells to the T splicing pattern occurs early during muscle differentiation (Legay et al., 1995). After fusion of these mononucleated cells into myotubes, T and R mRNAs coexist, but in vivo only T transcripts remain expressed after innervation of the muscle. Expression of AChET subunits is enhanced during myogenic differentiation in mammals as a result of mRNA stabilization rather than an increase in transcription (Fuentes and Taylor, 1993). Other regulatory events have been implicated because transfection of cDNAs, under the control of nonspecific viral promoters, led to a tissue-specific expression of different types of AChE subunits (Seidman et al., 1995). Post-translational mechanisms may therefore contribute to the preferential expression of AChET subunits in muscle cells. In the present study, we examined how several AChE mRNA constructs are expressed after transfection in C2C12 cells in culture.
MATERIALS AND METHODS
Cultures. C2C12 cells were seeded on Matrigel-coated (Collaborative Research, Bedford, MA) 35 mm culture plates and maintained at 37°C in a water-saturated atmosphere containing 5% CO2. Myoblasts were grown in a proliferation medium consisting of DMEM supplemented with 20% horse serum, 10% fetal calf serum, 292 ng/ml l-glutamine, and 100 U/ml of penicillin–streptomycin. Once the cells reached ∼90% confluence, the amount of serum in the media was reduced (5% horse serum) to stimulate differentiation into myotubes.
cDNA constructs. cDNA clones encoding the AChE catalytic subunits were from rat (Legay et al., 1993). They contained no 3′ untranslated region and only 12 bp from the mouse 5′ untranslated sequence (GTCCTGGCAGTC) to facilitate translation initiation. The RHT minigene contained the three common exons, followed by the 3′ alternative splicing domain (Fig.1A). The RH construct also contained the three common exons, followed by intron R and the coding region of exon H. Both RH and RHT sequences were obtained by reverse transcription-PCR using total RNA isolated from rat spleen. The cDNA clone encoding the collagen tail (tQ1) was fromTorpedo (Krejci et al., 1991). Mutagenesis of tQ1 was performed by PCR, using a forward common oligonucleotide and various mutagenized 3′ oligonucleotides. The structure of the constructs is shown in Figure 5A. All constructs were inserted at theXbaI site of the pEF-BOS vector, under the control of the promoter of elongation factor EF-1α (Mizushima and Nagata, 1990). Plasmid DNA was prepared using the Qiagen (Chatsworth, CA) mega-prep procedure.
Fig. 1.

A is a schematic representation of the various cDNAs encoding AChE splice variants. See Materials and Methods for details. B shows AChE activity associated with the myotubes and in the media after transfection of the different constructs. For each construct, a minimum of six distinct cultures (in triplicate) were analyzed. AChE activity is expressed in relation to a cotransfected LacZ construct used to control for transfection efficiency. The values are given as mean ± SEM.
Fig. 5.
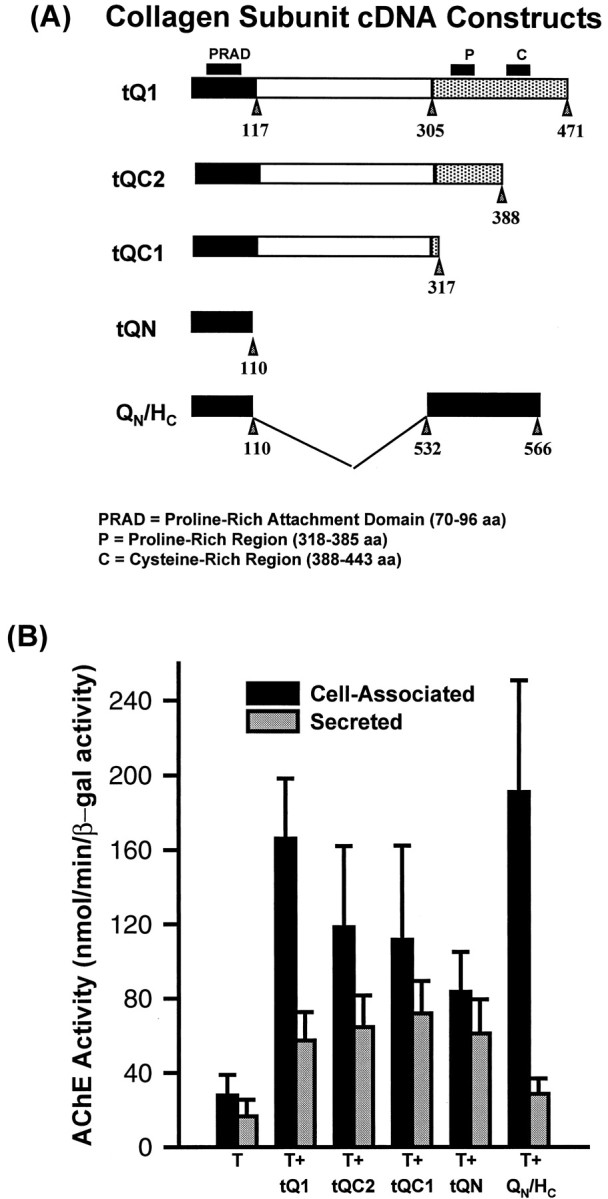
A is a schematic representation of the various cDNA constructs encoding the collagenic subunit, as well as the chimeric QN/HC cDNA.B shows AChE activity associated with the myotubes and in the media after transfection and cotransfection of the different constructs. Note that cotransfection with any of the structural subunits resulted in significant increases in AChE enzyme activity (p < 0.05). For each construct, a minimum of six distinct cultures (in triplicate) were analyzed. AChE activity is expressed in relation to a cotransfected LacZ construct used to control for transfection efficiency.
Transfection of cultured cells. At ∼50% confluence, C2C12 myoblasts were transfected using the mammalian transfection system–calcium phosphate kit (Promega, Madison, WI) as described previously (Gramolini et al., 1998). Briefly, cells were incubated for 6 hr with 5 μg of DNA encoding AChE and 5 μg of DNA encoding the various collagen subunits, together with 3 μg of a cytomegalovirus-LacZ plasmid (Clontech, Palo Alto, CA), which was included to quantify transfection efficiency. They were then shocked with DMEM containing 15% glycerol for 2 min. After transfection, myoblasts were returned to the proliferation medium (see above) until they reached ∼90% confluence. The medium was then replaced by the differentiation medium (see above), and the cells were allowed to differentiate into myotubes for 3 d before analysis.
In vivo gene transfer. These experiments were performed using the tibialis anterior muscles of mouse, as described previously (Chan et al., 1999). Briefly, 25 μl of a DNA solution containing 2.5 μg/ml of the appropriate plasmids were injected directly into TA muscles of 4-week-old rats or mice. Seven to 14 d later, injected muscles were excised and rapidly frozen in isopentane precooled with liquid nitrogen. Cryostat tissue sections (10 μm) were processed histochemically for the simultaneous detection of β-galactosidase and AChE activity (Gramolini et al., 1998).
AChE extraction and biochemical analysis. Three-day-old myotubes were washed twice in PBS and scraped into 400 μl (per 35 mm plate) of a high-salt detergent buffer containing 10 mm Tris-HCl, pH 7.0, 10 mmEDTA, 1 m NaCl, 1% Triton X-100 or Brij-96, and 1.0 mg/ml of bacitracin and 0.25 mg/ml of aprotinin, as protease inhibitors. Cells were homogenized in a Polytron set at maximum speed, twice for 15 sec. After low-speed centrifugation, supernatants were collected, transferred to fresh tubes, and stored at −80°C for further analysis. For some experiments, cells were first harvested in PBS and immediately frozen at −80°C.
Total AChE activity was determined using the spectrophotometric method of Ellman et al. (1961) as described elsewhere (Jasmin and Gisiger, 1990; Duval et al., 1992). The various AChE molecular forms were separated by velocity sedimentation in 5–20% sucrose gradients in the presence of 1% Brij-96 or Triton X-100. Amphiphilic forms were characterized by the fact that their sedimentation coefficient was lower in the presence of Brij-96 than in the presence of Triton X-100, whereas nonamphiphilic forms were not affected by the detergents (Bon et al., 1991). Treatment of the extract with phosphatidylinositol-phospholipase C (PI-PLC) was performed as described by Legay et al. (1993). The amount of protein present in each sample was determined using the bicinchoninic acid protein assay reagent (Pierce, Rockford, Il). β-Galactosidase activity was assessed using the β-Galactosidase enzyme assay system (Promega).
To assess secreted AChE activity produced by nontransfected and transfected myotubes, the differentiation media were prepared with horse serum that was pretreated with diisopropyl fluorophosphate (Sigma, St. Louis, MO) (Boudreau-Larivière and Jasmin, 1999).
AChE histochemical staining. Myotubes and cryostat muscle sections were fixed briefly with 4% paraformaldehyde and processed for AChE histochemistry, using the procedure of Karnovsky and Roots (1964).
Immunofluorescence. Myotubes were rinsed in PBS, fixed with 4% paraformaldehyde in PBS for 15 min at room temperature, and washed thoroughly with buffer A (0.5% glycine in PBS) with or without 0.1% Triton X-100. Blockade of nonspecific binding was achieved by incubating the cells in buffer A that contained 5% normal goat serum for 15 min. Cells were then incubated at room temperature with a rabbit anti-rat AChE antibody (A63, diluted 1/400) (Marsh et al., 1984) diluted in buffer A. One hour later, the myotubes were washed with buffer A and incubated for 1 hr at room temperature with Cy3- or fluorescein isothiocyanate-conjugated donkey anti-rabbit IgG antibodies (BioCan, Mississauga, Ontario, Canada). After extensive washing with PBS, the myotubes were mounted in Citifluor (Canterbury, UK) and observed with a Zeiss (Oberkochen, Germany) Axiophot photomicroscope equipped with epifluorescence.
RESULTS
Expression of AChE splice variants in C2C12 myotubes
C2C12 myoblasts were transiently transfected with various constructs encoding the distinct splice variants of AChE catalytic subunits (Fig. 1A). Total AChE activity was assayed in 3-d-old myotubes, and the pattern of AChE molecular forms was analyzed by velocity sedimentation in sucrose gradients. Nontransfected cells contained an extremely low level of AChE activity (Figs.1B, 2). This activity could not be detected histochemically under the conditions used for visualization of AChE in transfected cells (Fig.3). In agreement with recent findings (Luo et al., 1998; Boudreau-Larivière and Jasmin, 1999), no asymmetric forms of AChE could be detected in these myotubes. In contrast, total AChE activity produced in transfected cells was significantly higher (p < 0.05; Student’st test) and varied according to the particular construct used for transfection. For example, total enzyme activity per dish (cell-associated plus secreted) in cells transfected with constructs containing the H and T cDNAs was more than 30-fold and sixfold higher, respectively, than in nontransfected cells (Figs. 1, 3). In our experiments, transfected C2C12 cells showed no visible morphological difference from controls for any of the constructs that we used (Fig.3).
Fig. 2.

Representative examples of AChE molecular form profiles seen in myotubes, as well as in the media, after transfection with the different AChE cDNA constructs. For some experiments, the cell extracts were treated with PI-PLC before velocity sedimentation to determine whether the molecular forms contained a GPI-anchor. The data are expressed in arbitrary units.
Fig. 3.
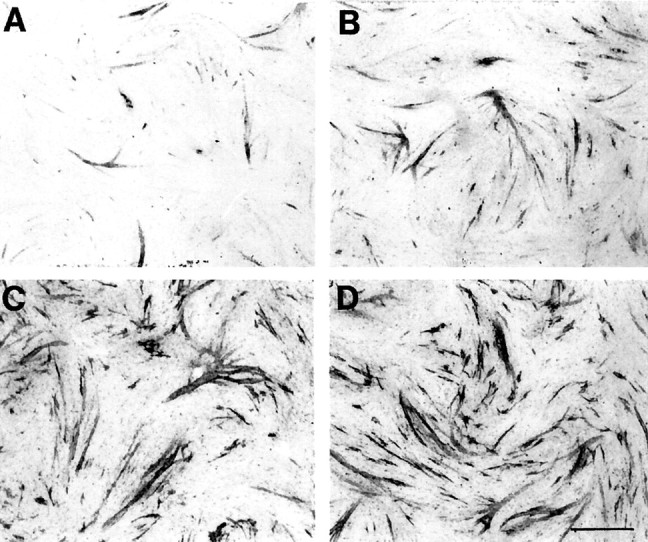
Histochemical analysis of AChE expression in transfected myotubes. A shows that transfection of the AChET cDNA resulted in modest levels of enzyme activity in a few myotubes, as revealed by histochemical staining using the method of Karnovsky and Roots (1964) (see Materials and Methods).B demonstrates that cotransfection of the AChET construct with ColQ increased enzyme activity. Consistent with our biochemical analysis, transfection of AChEH cDNAs (C) and cotransfection of AChET with QN/HC(D) resulted in the most dramatic increase in staining intensity. In parallel experiments, we observed no detectable staining of untransfected cells (data not shown). Note that the morphology of the myotubes is not affected by the expression of these various constructs. Scale bar, 600 μm.
After 3 d of differentiation, C2C12 cells transfected with AChET cDNA contained mostly monomers (G1a), together with low levels of dimers (G2a) and tetramers (G4na), respectively, appearing as a shoulder and a minor peak in the sedimentation profile (Fig. 2). The corresponding media contained G1a and G4na in similar amounts, along with a lower proportion of G2a, as revealed by comparison of the molecular form profiles obtained in Triton X-100 versus Brij-96-containing sucrose gradients (data not shown). The minor 13 S component, which was occasionally observed in AChET-transfected COS cells (Bon and Massoulié, 1997), was never detected in transfected C2C12 cells. Production of this component seems to be correlated with high levels of AChET expression (Simon et al., 1998).
Analysis of the pattern of AChE molecular forms revealed also that C2C12 cells transfected with the H construct produced cell-associated G2a and secreted G2na (Fig. 2). As expected, the sedimentation of G2a was shifted after PI-PLC treatment, thereby demonstrating that these cell-associated dimers were GPI-anchored. In addition, these amphiphilic dimers were targeted to membrane of the muscle cells, as revealed by immunofluorescence staining of nonpermeabilized H-transfected C2C12 cells (Fig. 4A). Similarly, histochemical experiments, performed on cryostat sections, showed that the H construct could also be highly expressed along the sarcolemma of muscle fibers after in vivo gene transfer (Fig. 4B,C).
Fig. 4.

AChEH cDNA can be expressed in muscles cells. A, Transfected muscle cells in culture stained by immunofluorescence with the A63 antibody directed against AChE.B, C, Cryostat sections from in vivo injected whole muscle stained with the histochemical staining procedure of Karnovsky and Roots (1964). Note that, as expected, the labeling appears associated with the sarcolemma because the H construct contained a GPI addition signal. Scale bars:A, B, 50 μm; C, 100 μm.
We designed two constructs to examine whether alternative splicing is specifically directed toward the T exon in myogenic cells and whether the H exon may be used in the absence of the downstream region. The RH construct contained the common exons 2, 3, and 4 encoding the catalytic domain, followed by intron R and the coding sequence of exon 5; in addition, the RHT construct contained intron 5′ and exon 6, i.e., the entire alternative splicing domain (see Fig. 1A). As shown in Figure 1B, total AChE activity was significantly higher (p < 0.05; Student’st test) in cells transfected with the T cDNA compared with that seen in RH-transfected cells, although a larger proportion of AChE activity was secreted with the latter construct (∼47% in the case of RH-transfected cells vs ∼37% for T-transfected cells). Transfection of the RHT construct into C2C12 cells yielded ∼20% more activity than for AChET-transfected cells (Fig.1B).
C2C12 cells transfected with RH constructs produced and secreted G1na (4.8 S in Triton X-100) together with a lower amount of cell-associated GPI-linked G2a. In the presence of Brij-96, the intact G2aform sedimented at the same position as the nonamphiphilic G1na monomers (4.5 S), but PI-PLC treatment produced nonamphiphilic dimers (G2na) that sedimented at 6.5 S and therefore appeared as a distinct shoulder. Amphiphilic and nonamphiphilic molecules were also characterized by comparison of their sedimentation coefficients in the presence of Triton X-100 and Brij-96 (data not shown).
RHT-transfected cells produced a similar pattern of AChE forms as AChET-transfected cells but with a slightly higher proportion of the G1a form. In these cells, the sedimentation profiles were clearly not affected by incubating the cell extract with PI-PLC, indicating that the 4.5 S peak does not correspond to GPI-anchored dimers. The sedimentation profiles obtained for RHT-transfected cells did not reveal any detectable nonamphiphilic monomers.
Influence of ColQ subunit on AChE expression
We transfected C2C12 cells with cDNAs encoding AChET alone or together with tQ1 (Krejci et al., 1991). Total AChE activity (cell-associated and secreted) was significantly higher (p < 0.05; Student’st test) in cotransfected cells, indicating that a fraction of the enzyme was stabilized by its association with the collagen subunit (Fig. 5B). As expected, these cotransfections resulted in the production of asymmetric forms of AChE. The sedimentation profiles show a major peak at 16 S, corresponding to the A12 form, as well as minor peaks at 14 and ∼9–10 S, probably representing A8 and a mixture of A4 and G4 forms (Fig. 6). The G4 form may be generated by proteolysis of collagen-tailed molecules. Although the sedimentation profiles do not exclude the presence of globular 13 S molecules, their formation is extremely unlikely because they were not observed when AChET was expressed alone.
Fig. 6.

Representative examples of AChE molecular form profiles seen in myotubes, as well as in the media, after cotransfection with the different AChE and collagenic subunit cDNA constructs. The data are expressed in arbitrary units. A small peak, which was observed in the culture medium, sedimented slightly faster than the cellular A12 form, most likely because the differentiation media contained trace amounts of collagenase.
The N-terminal domain of ColQ (tQN) (Fig.5B) was sufficient to increase the total activity by threefold to fourfold. Cotransfection with the chimeric protein QN/HC in which the N-terminal of ColQ is linked to a GPI-addition signal peptide also increased by more than fivefold total AChE activity. Similar to what was observed in COS cells, these cotransfected subunits resulted in the production of GPI-anchored G4a together with more modest levels of G1a and G2a (Fig. 6).
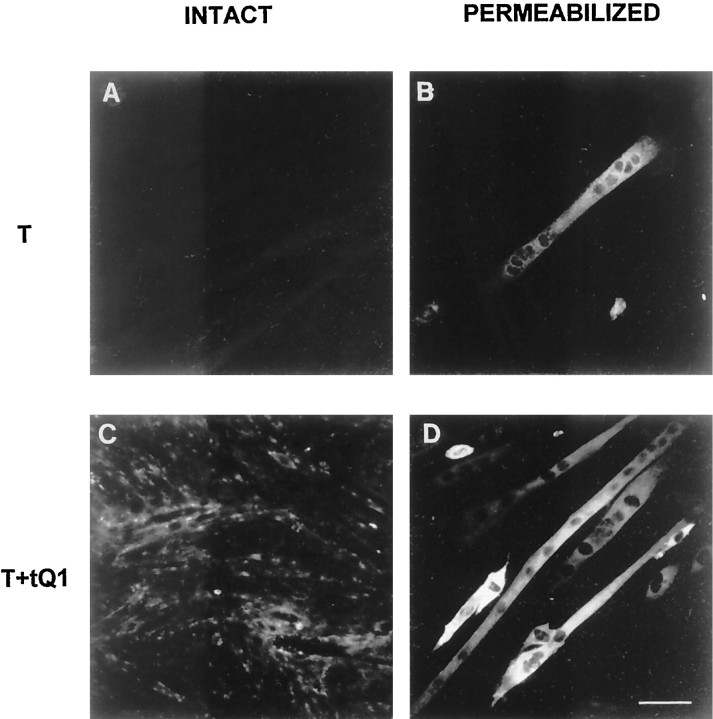
To examine whether coexpression with ColQ modified the subcellular distribution of AChE, as well as the total activity, we performed immunofluorescence experiments on cotransfected C2C12 cells using a rabbit polyclonal antibody raised against rat AChE (Marsh et al., 1984). As shown in Figure 7, a strong labeling was seen in permeabilized cells cotransfected with the AChET and tQ1 constructs but not in intact nonpermeabilized cells. As shown in Figures 6 and 7, the cells secreted a significant amount of asymmetric forms that did not attach to the cell surface. In agreement with these findings, treatment with collagenase did not release a significant amount of AChE activity from these cells (data not shown). In vivo, coinjections of AChET and ColQ cDNAs resulted in AChE staining around the cells in extrasynaptic regions (Fig.8).
Fig. 7.
Immunofluorescence experiments using the A63 antibody were performed to localize exogenous expression of AChE in transfected myotubes. AChE expression after transfection with AChET alone was restricted to the intracellular compartment of selected myotubes (A, B). Cotransfection of AChET with tQ1 (C,D) resulted in a much higher expression of AChE activity. Scale bar, 200 μm.
Fig. 8.
In vivo expression of AChET constructs coinjected directly into skeletal muscle, along with tQ1 and LacZ. Serial cryostat sections were histochemically processed for β-galactosidase (A) and AChE enzyme activity (B). Note the coexpression of the two enzymes in some fibers, indicating successful transfection. These pictures were taken in extrajunctional regions of skeletal muscle fibers. No labeling was observed when only AChET cDNAs were injected. Also, note that not all LacZ-positive fibers expressed AChE activity, because it is expected that fibers do not necessarily uptake all injected constructs (Jones et al., 1997; Decrouy et al., 1998). Scale bar, 100 μm.
The complete ColQ subunits are thus able to recruit the catalytic AChET subunits and target them to the cell surface. We tried to define the respective roles of C-terminal peptide motifs in the trimerization and secretion of ColQ. We thus generated three mutants of tQ1, termed tQC2, tQC1, and tQN, as shown in Figure5A, by deletions of the C terminus. In cotransfection experiments, the highest level of total AChE activity (cell-associated and secreted) was observed when the AChET cDNA was transfected together with the wild-type collagen subunit, whereas the lowest amount of activity was detected with cotransfection of the tQN mutant that contains the PRAD domain (Fig. 5B).
The tQC2 mutant, which lacks the cysteine-rich domain, nonetheless allowed the formation of the asymmetric forms A12, A8, and A4 when cotransfected with the AChE construct (Fig. 6). The AChE sedimentation profiles for both cell-associated and secreted enzymes were identical in tQC2- and tQ1-transfected cells (Fig. 6). With these two constructs, C2C12 cells secreted only asymmetric forms of AChE. In the tQC1 construct, the C-terminal region was nearly completely deleted, except for two cysteines that follow the collagen domain and can form disulfide bonds between the strands in the triple helix. In the tQN construct, the collagen and C-terminal regions were deleted, leaving only the N-terminal domain, including the PRAD. These two constructs did not produce A forms (Fig. 6). Only G4 was produced and secreted by these cells along with low amounts of G1 and G2, indicating therefore that the truncated tQC1 and tQN subunits recruited most of the G1subunits into tetramers.
DISCUSSION
We have examined some of the events that govern expression of AChET catalytic subunits in muscle by expressing several AChE cDNA constructs corresponding to various domains of the alternatively spliced sequence in transfected C2C12 cells and in musclein vivo. Transfection of the AChETconstruct (T) into C2C12 cells resulted in the production of molecular forms that were basically identical to those seen previously in COS cells (Bon and Massoulié, 1997), although they were present in slightly different proportions. A small component of G4a was occasionally observed in these cell extracts that could represent the association of catalytically active tetramers with endogenous hydrophobic proteins, such as the 20 kDa subunit, in agreement with the existence of membrane-bound tetramers (G4a) in muscle (Fernandez et al., 1996). Although the H transcript is only weakly expressed in C2C12 myoblasts and is absent from mature myotubes (Legay et al., 1995), transfected H transcripts were translated and processed efficiently in muscle cells, and the resulting GPI-linked dimers became anchored to the sarcolemma, both in culture and in vivo. Therefore, these results indicate that specific expression of AChET subunits in muscle originates from the regulation of the splice choice from pre-mRNAs and not from a post-transcriptional elimination of H mRNAs or AChEH subunits. In the present study, we observed that myogenic C2C12 cells express approximately fivefold higher levels of active AChEH subunits compared with AChET subunits when transfected with the corresponding H and T cDNAs. In contrast, COS cells produced twice as much activity with AChET as with AChEH when transfected with the same constructs (Bon et al., 1997). This indicates that the processing and stabilization of the two types of subunits are cell-specific processes.
The restricted secretion of unassembled subunits and the preferential externalization of cysteine-bound dimers from overexpressing human embryonic kidney cells have led to the proposal that the T peptide acts as a retention signal (Velan et al., 1991; Kerem et al., 1993). It is not clear whether the free cysteine of the T peptide plays a crucial role in the ER retention. In this respect, it is noteworthy that, in the present study, we observed an active secretion of AChET monomers by transfected C2C12 cells, as reported previously in the case of COS cells (Bon and Massoulié, 1997). Thus, oligomerization is not an absolute prerequisite for externalization of AChE. In C2C12 cells, coexpression of the AChET subunit along with various constructs containing the PRAD increased the level of AChE activity, suggesting that the formation of PRAD-linked tetramers stabilized catalytic subunits that would otherwise be degraded. The association of the PRAD with tetramers could therefore mask a degradation signal.
We also examined the splicing choice made by C2C12 cells when transfected with either RH or RHT constructs. When the noncoding region after exon H was absent from the construct (RH), C2C12 cells produced mostly G1na, corresponding to the translation product of R transcripts, and a small but significant percentage of GPI-linked dimers that represented splicing toward the H exon. When the 3′ region downstream of exon H was present (RHT), C2C12 cells produced mostly AChETsubunits. Based on the present results, it appears therefore that the H acceptor splice site is weaker than the T site in myogenic cells. The absence of the T splicing site and of the upstream intronic sequence did not force the splicing choice toward exon H. Using a human construct similar to our rat RH clone, Seidman et al. (1995) failed to detect the expression of the R and H subunits in transgenicXenopus muscle. This apparent discrepancy with our results may originate from the species specificity of splicing factors and from intrinsic species-specific nucleotide sequences. For example, transfected mouse blood cells produced R transcripts with the mouse AChE gene but not with the human gene (Li et al., 1993). It is noteworthy that the noncoding region located between the H and T coding sequences contains a 100 nucleotide pyrimidine stretch between the putative branch points and the 3′ splice junction, similar in length to those found upstream of exons that are specifically spliced in muscle, such as in the β-tropomyosin gene (Goux-Pelletan et al., 1990). Therefore, the structure of the sequence upstream of the T exon may reinforce the preferential splicing choice in C2C12 cells toward this exon.
Recently, Taylor and colleagues compared the splice choices operated by C2C12 cells that were transfected with a mouse genomic AChE construct or with a construct in which the constitutive introns were deleted (Luo et al., 1998). The latter construct, termed Δi2–3,3–4, is in fact similar to our RHT construct except that it contains the endogenous AChE promoter and a 5′ untranslated region. When transfected into C2C12 cells, this construct produced R, T, and H transcripts in decreasing order, whereas the genomic construct only produced the T transcript. In our experiments, we could not detect any H or R subunits in RHT-transfected cells. In their work, Luo et al. (1998) suggested that the third constitutive intron (intron 3′) containscis-elements that influence the downstream splicing. The difference between our results and those of Luo et al. may be related to the fact that they used the mouse gene with its own promoter, whereas we used the rat gene with the EF-1α promoter or that their transfections were performed 1 d after induction of differentiation, whereas we performed transfections on myoblasts and induced differentiation the next day.
Cysteine- and proline-rich subdomains, which are conserved among vertebrates, have been characterized in the C-terminal sequence of the collagen subunit (Krejci et al., 1991, 1997). Deletion of the more distal cysteine-rich subdomain did not prevent the production of asymmetric forms of AChE, indicating that this region is not essential for the formation of the triple helix. On the other hand, deletions of both cysteine- and proline-rich subdomains did not allow association of the three strands of collagen. Recently, in a genetic analysis of patients presenting a disabling congenital myasthenic syndrome, Ohno and collaborators identified a mutation at a position equivalent to nucleotide 1133 in the Torpedo collagen subunit that resulted in the absence of asymmetric forms (Ohno et al., 1998). Altogether, these data indicate that integrity of the entire proline-rich domain is essential for initiating the triple helix assembly, which is known to proceed from the C to the N termini (Prockop and Kivirikko, 1995).
When the AChET construct was cotransfected with tQC1, only tetramers were secreted in the medium, suggesting that these tetramers are assembled by a single strand of truncated collagen. It has been shown that single strands of collagen are normally degraded within the cell (Nakai et al., 1992). The PRAD domain must be present in the secreted tetramers (Bon et al., 1997), but we do not know whether the entire collagen domain is conserved in these forms. One hypothesis may be that the association of AChETsubunit with the N-terminal region of the collagen subunit prevents the intracellular degradation of at least the N-terminal domain of ColQ, thereby allowing secretion of hetero-oligomers. However, the absence of the C-terminal domains in tQC2, tQC1, and tQN mutants decreased the total AChE activity in cotransfected cells compared with wild-type tQ1, suggesting that the truncated molecules are less stable but more efficiently secreted. Some natural variants lacking part of the ColQ C terminus have been found in rat (Krejci et al., 1997). Our results imply that these ColQ variants would still associate with tetramers and would be secreted at the neuromuscular junction in vivo. However, they would not become anchored in the basal lamina because the C-terminal region of ColQ appears necessary for a stable interaction with the extracellular matrix (Donger et al., 1998).
Coinjection experiments in tibialis anterior muscles showed that the presence of ColQ in muscle fibers may direct the secretion of AChET subunits, even in extrajunctional domains of the fibers. This suggests that the endplate versus nonendplate specialization of the Golgi apparatus (Jasmin et al., 1989,1995) and other elements of the secretory pathway (Jasmin et al., 1990) observed previously in skeletal muscle fibers do not affect the capacity of these cells to secrete hetero-oligomers along their entire length. Accordingly, this indicates that the presence of hetero-oligomers in muscle fibers relies exclusively on the expression of AChET subunits (Michel et al., 1994;Chan et al., 1999) and ColQ subunits (E. Krejci, C. Legay, S. Thomine, J. Sketelj, and J. Massoulié, unpublished observations).
Finally, it should be noted that only asymmetric but not globular forms of AChE were secreted from C2C12 cells cotransfected with AChET and tQ1 or tQC2 constructs. The latter result is, in fact, in agreement with recent findings showing that no AChE activity is detected in the synaptic cleft of ColQ-deficient mice, suggesting that the secreted AChE essentially corresponds to asymmetric forms in vivo (Feng et al., 1999).
Footnotes
This work was supported by the Medical Research Council of Canada (MRC), the Centre National de la Recherche Scientifique, the Direction des Forces et de la Prospective, the European Community, and the Association Française contre les Myopathies (AFM). F.A.M. was supported by a Ministry of Ontario Graduate Studentship. B.J.J. is an MRC Scientist. We thank Dr. Roxanne Chan for fruitful discussions and critical reading of this manuscript. Also, we thank Monique Lambergeon and John Lunde for expert technical assistance.
Correspondence should be addressed to Dr. Claire Legay, Laboratoire de Neurobiologie Moléculaire et Cellulaire, Centre National de la Recherche Scientifique, Unité Mixte de Recherche 8544, Ecole Normale Supérieure, 46 rue d’Ulm, 75005 Paris, France.
REFERENCES
- 1.Anglister L. Acetylcholinesterase from the motor nerve terminal accumulates on the synaptic basal lamina of the myofiber. J Cell Biol. 1991;115:755–764. doi: 10.1083/jcb.115.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bon S, Massoulié J. Quaternary associations of acetylcholinesterase. I. Oligomeric associations of T subunits with and without the amino-terminal domain of the collagen tail. J Biol Chem. 1997;272:3007–3015. doi: 10.1074/jbc.272.5.3007. [DOI] [PubMed] [Google Scholar]
- 3.Bon S, Rosenberry TL, Massoulié J. Amphiphilic, glycophosphatidylinositol-specific phospholipase C (PI-PLC)-insensitive monomers and dimers of acetylcholinesterase. Cell Mol Neurobiol. 1991;11:157–172. doi: 10.1007/BF00712807. [DOI] [PubMed] [Google Scholar]
- 4.Bon S, Coussen F, Massoulié J. Quaternary associations of acetylcholinesterase. II. the polyproline attachment domain of the collagen tail. J Biol Chem. 1997;272:3016–3021. doi: 10.1074/jbc.272.5.3016. [DOI] [PubMed] [Google Scholar]
- 5.Boudreau-Larivière C, Jasmin BJ. Calcitonin gene-related peptide decreases expression of acetylcholinesterase in mammalian myotubes. FEBS Lett. 1999;444:22–26. doi: 10.1016/s0014-5793(99)00015-0. [DOI] [PubMed] [Google Scholar]
- 6.Brimijoin S. Axonal transport and subcellular distribution of molecular forms of acetylcholinesterase in rabbit sciatic nerve. Mol Pharmacol. 1979;15:641–648. [PubMed] [Google Scholar]
- 7.Chan RY, Boudreau-Larivière C, Angus LM, Mankal FA, Jasmin BJ. An intronic enhancer containing an N-box motif is required for synapse- and tissue-specific expression of the acetylcholinesterase gene in skeletal muscle fibers. Proc Natl Acad Sci USA. 1999;96:4627–4632. doi: 10.1073/pnas.96.8.4627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Couraud JY, Di Giamberardino L. Axonal transport of the molecular forms of acetylcholinesterase in chick sciatic nerve. J Neurochem. 1980;35:1053–1066. doi: 10.1111/j.1471-4159.1980.tb07859.x. [DOI] [PubMed] [Google Scholar]
- 9.Decrouy A, Renauds J-M, Lunde JA, Dickson G, Jasmin BJ. Mini- and full-length dystrophin induces the recovery of nitric oxide synthase at the sarcolemma of mdx4cv skeletal fibers. Gene Ther. 1998;5:59–64. doi: 10.1038/sj.gt.3300553. [DOI] [PubMed] [Google Scholar]
- 10.Donger C, Krejci E, Pou Serradell A, Eymard B, Bon S, Nicole S, Chateau D, Gary F, Fardeau M, Massoulié J, Guicheney P. Mutation in the human acetylcholinesterase-associated collagen gene, ColQ, is responsible for congenital myasthenic syndrome with end-plate acetylcholinesterase deficiency (type Ic). Am J Hum Genet. 1998;63:967–975. doi: 10.1086/302059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duval N, Massoulié J, Bon S. H and T subunits of acetylcholinesterase from Torpedo, expressed in COS cells, generate all types of globular forms. J Cell Biol. 1992;118:641–653. doi: 10.1083/jcb.118.3.641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ellman GL, Courtney KD, Andres V, Featherstone RM. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 13.Feng G, Krejci E, Molgo J, Cunningham JM, Massoulié J, Sanes JR. Genetic analysis of collagen Q: role in acetylcholinesterase and butyrylcholinesterase assembly and in synaptic structure and function. J Cell Biol. 1999;144:1349–1360. doi: 10.1083/jcb.144.6.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernandez HL, Moreno RD, Inestrosa NC. Tetrameric (G4) acetylcholinesterase: structure, localization, and physiological regulation. J Neurochem. 1996;66:1335–1346. doi: 10.1046/j.1471-4159.1996.66041335.x. [DOI] [PubMed] [Google Scholar]
- 15.Fuentes ME, Taylor P. Control of acetylcholinesterase gene expression during myogenesis. Neuron. 1993;10:679–687. doi: 10.1016/0896-6273(93)90169-r. [DOI] [PubMed] [Google Scholar]
- 16.Goux-Pelletan M, Libri D, d’Aubenton-Carafa Y, Fiszman M, Brody E, Marie J. In vitro splicing of mutually exclusive exons from the chicken β-tropomyosin gene: role of the branch point location and very long pyrimidine stretch. EMBO J. 1990;9:241–249. doi: 10.1002/j.1460-2075.1990.tb08101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gramolini AO, Burton EA, Tinsley JM, Ferns MJ, Cartaud A, Cartaud J, Davies KE, Lunde JA, Jasmin BJ. Muscle and neural isoforms of agrin increase utrophin expression in cultured myotubes via a transcriptional regulatory mechanism. J Biol Chem. 1998;273:736–743. doi: 10.1074/jbc.273.2.736. [DOI] [PubMed] [Google Scholar]
- 18.Jasmin BJ, Gisiger V. Regulation by exercise of the pool of G4 acetylcholinesterase characterizing fast muscles: opposite effects of running training in antagonist muscles. J Neurosci. 1990;10:1444–1454. doi: 10.1523/JNEUROSCI.10-05-01444.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jasmin BJ, Cartaud J, Bornens M, Changeux JP. Golgi apparatus in chick skeletal muscle: changes in its distribution during end plate development and after denervation. Proc Natl Acad Sci USA. 1989;86:7216–7222. doi: 10.1073/pnas.86.18.7218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jasmin BJ, Changeux JP, Cartaud J. Compartimentalization of cold-stable and acetylated microtubules in the subsynaptic domain of chick skeletal muscle fibre. Nature. 1990;344:673–675. doi: 10.1038/344673a0. [DOI] [PubMed] [Google Scholar]
- 21.Jasmin BJ, Antony C, Changeux JP, Cartaud J. Nerve-dependent plasticity of the Golgi complex in skeletal muscle fibres: compartimentalization within the subneural sarcoplasm. Eur J Neurosci. 1995;7:470–479. doi: 10.1111/j.1460-9568.1995.tb00343.x. [DOI] [PubMed] [Google Scholar]
- 22.Jones G, Meier T, Lichtsteiner M, Witzemann W, Sakmann B, Brenner HR. Induction by agrin of ectopic and functional postsynaptic-like membrane in innervated muscle. Proc Natl Acad Sci USA. 1997;94:2654–2659. doi: 10.1073/pnas.94.6.2654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karnovsky MJ, Roots L. A direct-coloring thiocholine method for cholinesterases. J Histochem Cytochem. 1964;12:219–221. doi: 10.1177/12.3.219. [DOI] [PubMed] [Google Scholar]
- 24.Kerem A, Kronman C, Bar-Nun S, Shafferman A, Velan B. Interrelations between assembly and secretion of recombinant human acetylcholinesterase. J Biol Chem. 1993;268:180–184. [PubMed] [Google Scholar]
- 25.Krejci E, Coussen F, Duval N, Chatel JM, Legay C, Puype M, Vandekerckhove J, Cartaud J, Bon S, Massoulié J. Primary structure of a collagenic tail peptide of Torpedo acetylcholinesterase: co-expression with catalytic subunit induces the production of collagen-tailed forms in transfected cells. EMBO J. 1991;10:1285–1293. doi: 10.1002/j.1460-2075.1991.tb08070.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krejci E, Thomine S, Boschetti N, Legay C, Sketelj J, Massoulié J. The mammalian gene of acetylcholinesterase-associated collagen. J Biol Chem. 1997;272:22840–22847. doi: 10.1074/jbc.272.36.22840. [DOI] [PubMed] [Google Scholar]
- 27.Legay C, Bon S, Massoulié J. Expression of a cDNA encoding the glycolipid-anchored form of rat acetylcholinesterase. FEBS Lett. 1993;315:163–166. doi: 10.1016/0014-5793(93)81155-s. [DOI] [PubMed] [Google Scholar]
- 28.Legay C, Huchet M, Massoulié J, Changeux JP. Developmental regulation of acetylcholinesterase transcripts in the mouse diaphragm: alternative splicing and focalization. Eur J Neurosci. 1995;7:1803–1809. doi: 10.1111/j.1460-9568.1995.tb00699.x. [DOI] [PubMed] [Google Scholar]
- 29.Li Y, Camp S, Taylor P. Tissue-specific expression and alternative mRNA processing of the mammalian acetylcholinesterase gene. J Biol Chem. 1993;268:5790–5797. [PubMed] [Google Scholar]
- 30.Luo ZD, Camp S, Mutero A, Taylor P. Splicing of 5′ introns dictates alternative splice selection of acetylcholinesterase pre-mRNA and specific expression during myogenesis. J Biol Chem. 1998;273:28486–28495. doi: 10.1074/jbc.273.43.28486. [DOI] [PubMed] [Google Scholar]
- 31.Marsh D, Grassi J, Vigny M, Massoulié J. An immunological study of rat acetylcholinesterase: comparison with acetylcholinesterases from other vertebrates. J Neurochem. 1984;43:204–213. doi: 10.1111/j.1471-4159.1984.tb06698.x. [DOI] [PubMed] [Google Scholar]
- 32.Massoulié J, Anselmet A, Bon S, Krejci E, Legay C, Morel N, Simon S. Acetylcholinesterase: C-terminal domains, molecular forms and functional localization. J Physiol (Paris) 1998;92:183–190. doi: 10.1016/s0928-4257(98)80007-7. [DOI] [PubMed] [Google Scholar]
- 33.Michel RN, Vu CQ, Tetzlaff W, Jasmin BJ. Neural regulation of acetylcholinesterase mRNAs at mammalian neuromuscular synapses. J Cell Biol. 1994;127:1061–1069. doi: 10.1083/jcb.127.4.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mizushima S, Nagata S. pEF-BOS, a powerful mammalian expression vector. Nucleic Acids Res. 1990;18:5322. doi: 10.1093/nar/18.17.5322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakai A, Satoh M, Hirayoshi K, Nagata K. Involvement of the stress protein HSP47 in procollagen processing in the endoplasmic reticulum. J Cell Biol. 1992;117:903–914. doi: 10.1083/jcb.117.4.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ohno K, Brengman J, Tsujino A, Engel AG. Human endplate acetylcholinesterase deficiency caused by mutations in the collagen-like (ColQ) of the asymmetric enzyme. Proc Natl Acad Sci USA. 1998;95:9654–9659. doi: 10.1073/pnas.95.16.9654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prockop DJ, Kivirikko KI. Collagens: molecular biology, diseases, and potentials for therapy. Annu Rev Biochem. 1995;64:403–434. doi: 10.1146/annurev.bi.64.070195.002155. [DOI] [PubMed] [Google Scholar]
- 38.Seidman S, Sternfeld M, Ben Aziz-Aloya R, Timberg R, Kaufer-Nachum D, Soreq H. Synaptic and epidermal accumulations of human acetylcholinesterase are encoded by alternative 3′-terminal exons. Mol Cell Biol. 1995;1555:2993–3002. doi: 10.1128/mcb.15.6.2993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sikorav JL, Duval N, Anselmet A, Bon S, Krejci E, Legay C, Osterlund M, Reimund B, Massoulié J. Complex alternative splicing of acetylcholinesterase transcripts in Torpedo electric organ; primary structure of the precursor of the glycolipid-anchored dimeric form. EMBO J. 1988;7:2983–2993. doi: 10.1002/j.1460-2075.1988.tb03161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Simon S, Krejci E, Massoulié J. A four-to-one association between peptide motifs: four C-terminal domains from cholinesterase assemble with one proline-rich attachment domain (PRAD) in the secretory pathway. EMBO J. 1998;17:6178–6187. doi: 10.1093/emboj/17.21.6178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Taylor P, Radic Z. The cholinesterases: from genes to proteins. Annu Rev Pharmacol Toxicol. 1994;34:281–320. doi: 10.1146/annurev.pa.34.040194.001433. [DOI] [PubMed] [Google Scholar]
- 42.Velan B, Grosfeld H, Kronman C, Leitner M, Gozes Y, Lazar A, Flashner Y, Marcus D, Cohen S, Shafferman A. The effect of elimination of intersubunit disulfide bonds on the activity, assembly, and secretion of recombinant human acetylcholinesterase. Expression of acetylcholinesterase Cys-580→Ala mutant. J Biol Chem. 1991;266:23977–23984. [PubMed] [Google Scholar]