Abstract
Prolonged skeletal muscle inactivity (e.g. limb immobilization, bed rest, mechanical ventilation, spinal cord injury, etc.) results in muscle atrophy that manifests into a decreased quality of life and in select patient populations, a higher risk of morbidity and mortality. Thus, understanding the processes that contribute to muscle atrophy during prolonged periods of muscle disuse is an important area of research. In this regard, mitochondrial dysfunction has been directly linked to the muscle wasting that occurs during extended periods of skeletal muscle inactivity. While the concept that mitochondrial dysfunction contributes to disuse muscle atrophy has been contemplated for nearly 50 years, the mechanisms connecting mitochondrial signaling events to skeletal muscle atrophy remained largely unexplained until recently. Indeed, emerging evidence reveals that mitochondrial dysfunction and the associated mitochondrial signaling events are a requirement for several forms of inactivity-induced skeletal muscle atrophy. Specifically, inactivity-induced alterations in skeletal muscle mitochondria phenotype and increased ROS emission, impaired Ca2+ handling, and release of mitochondria-specific proteolytic activators are established occurrences that promote fiber atrophy during prolonged periods of muscle inactivity. This review highlights the evidence that directly connects mitochondrial dysfunction and aberrant mitochondrial signaling with skeletal muscle atrophy and discusses the mechanisms linking these interconnected phenomena.
Keywords: mitochondrial dysfunction, disuse muscle atrophy, reactive oxygen species, cell signaling, proteolysis, muscle wasting
1. Introduction
Maintaining healthy skeletal muscle mass is an essential component for sustaining quality of life through the performance of activities of daily living. Notably, maintaining a healthy skeletal muscle mass is associated with lower risk of all-cause mortality [1, 2]. Unfortunately, several health-related conditions (e.g., bone fractures, coma, etc.) can result in circumstances that induce prolonged inactivity of skeletal muscle resulting in muscle atrophy (e.g. limb immobilization, mechanical ventilation, etc). While it is well established that muscle atrophy occurs during inactivity due to alterations in the rates of both protein synthesis and protein degradation, the precise mechanisms that drive these alterations remain under investigation. In this regard, mounting evidence has revealed that mitochondria become damaged and dysfunctional during prolonged muscle inactivity, and that this mitochondrial dysfunction is a causal event in the initiation of muscle-inactivity induced atrophy [3, 4]. However, the specific mechanisms employed by dysfunctional mitochondria that enact signaling of inactivity-induced muscle atrophy have yet to be fully elucidated.
This review will discuss the key role that aberrant mitochondrial signaling plays in the development of inactivity-induced skeletal muscle atrophy. We begin with an overview of the events leading to inactivity-induced muscle atrophy. Then, we will briefly describe the processes required to maintain homeostatic mitochondrial function, followed by a discussion of the potential mechanisms that contribute to inactivity-induced mitochondrial dysfunction in muscle fibers. Finally, we will summarize the evidence linking mitochondrial dysfunction as a central player in the promotion of muscle atrophy during inactivity.
2. Inactivity-induced muscle atrophy: an introduction
2.1. Models of Inactivity
Unfortunately, there are numerous circumstances that result in prolonged inactivity of skeletal muscle, and, consequentially, muscle atrophy. For example, a broken bone resulting in limb immobilization (i.e., casting), prolonged bed rest, and the use of mechanical ventilation resulting in inactivity of inspiratory muscles are all events that reduce skeletal muscle activity and induce muscle atrophy. To investigate the events of inactivity-induced muscle atrophy, several experimental models have been employed to mimic the muscle atrophy conditions observed in clinical scenarios in order to elucidate relevant cellular and physiological processes. For instance, hindlimb unloading in rodents via tail suspension is often employed to unweight ambulatory muscles and mimic the inactivity-induced muscle atrophy that is observed during bed rest or space flight. Additionally, rodent models of limb immobilization can be used to study the inactivity-induced atrophy that occurs when a limb is casted for prolonged periods of time. Spinal cord isolation and muscle denervation are also experimentally induced in order to disable motor neuron activation of muscles in order to study the muscle atrophy that occurs as a result of inactivity due to spinal cord injury. Furthermore, a pre-clinical model of mechanical ventilation that is used to artificially support pulmonary gas exchange also induces muscle atrophy by reducing the activity of inspiratory muscles (e.g. diaphragm). Indeed, mechanical ventilation is often a life-saving intervention used to support respiration in critically ill patients or those undergoing surgery. However, patients who undergo prolonged mechanical ventilation often encounter difficulties in weaning due to the atrophy that occurs in respiratory muscles. Therefore, pre-clinical models of ventilator-induced respiratory muscle wasting are also extremely useful for investigating this unique type of muscle wasting. Collectively, these experimental models are important tools of research utilized to simulate the respective conditions that occur in humans.
In regard to the experimental selection of an inactivity-induced atrophy model, it is important to appreciate that the respective time point and model chosen in these experiments can impact the observation of the dependent variables measured. For instance, the muscle atrophy that occurs in diaphragm after 12 hours of mechanical ventilation requires 3 days of immobilization of the hindlimb in order to see similar atrophy in the gastrocnemius muscle [5-7]. Furthermore, the effects of disuse models can be compounding, as a combination of mechanical ventilation and denervation results in an amplified atrophic following 12 hours of mechanical ventilation [8]. Despite these differences, it is established that the muscle atrophy that occurs in these conditions is due to an imbalance in protein synthesis and protein degradation and a brief discussion of these processes follows.
2.2. Muscle atrophy occurs due to an imbalance between protein synthesis and protein degradation
Muscle protein balance is determined by the rates of protein synthesis and protein degradation. Specifically, muscle atrophy can occur due to decreases in protein synthesis, increases in protein degradation, or a combination of both. Indeed, studies reveal that during periods of prolonged inactivity, muscle atrophy occurs due to both a decrease in protein synthesis and accelerated proteolysis [9]. In regard to decreased protein synthesis during muscle inactivity, protein synthesis rates decrease due to a combination of decreased transcriptional activity, increased degradation of transcripts (e.g. mRNA stability), and decreased assembly of translational machinery (i.e., translation). In this regard, the PI3K/Akt/mTOR pathway is a key regulatory pathway involved in mediating the processes involved in protein synthesis in skeletal muscle and this pathway is down-regulated in muscle fibers during prolonged periods of inactivity [10-12]. Additionally, rates of protein synthesis can also be negatively impacted by a decreased abundance of myonuclei during prolonged muscle inactivity; this serves to decrease the transcriptional capacity of muscle [13]. As mentioned earlier, prolonged muscle inactivity is associated with increased proteolysis and muscle atrophy only occurs when rates of protein synthesis are below rates of protein degradation.
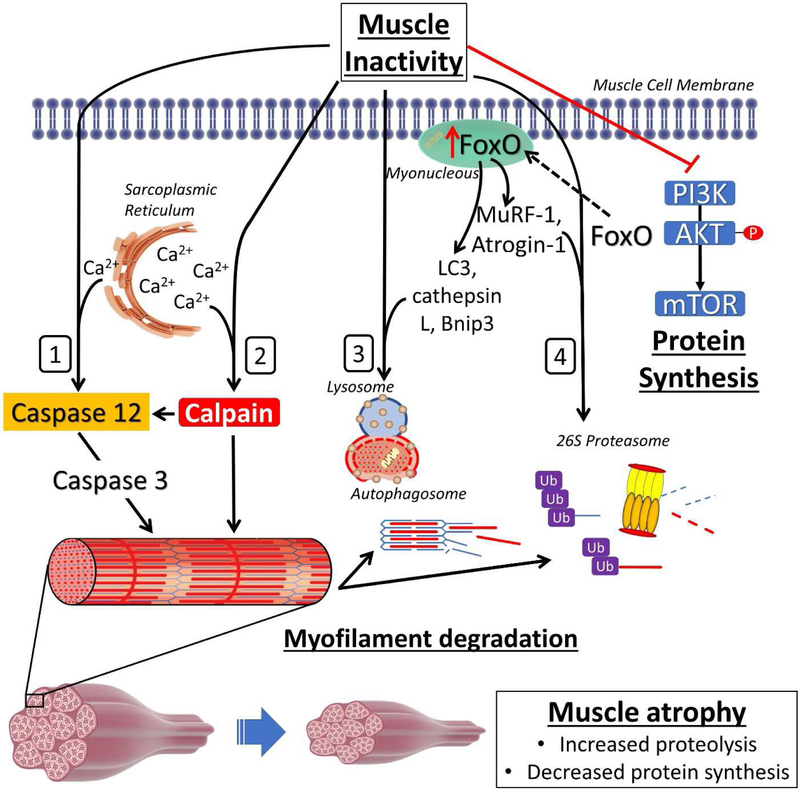
Protein degradation is a constitutively active process in muscle fibers that serves to recycle and remove damaged proteins. Four main proteolytic systems contribute to the degradation of proteins: 1) ubiquitin proteasome system (UPS); 2) calpain; 3) caspase-3; and 4) autophagy (Figure 1). Collectively, these proteolytic systems interact to maintain proteostasis in the fiber and prevent the aggregation of damaged protein structures. However, prolonged muscle inactivity results in signaling events that further activates these systems and, if left unchecked, can result in accelerated protein degradation of undamaged proteins resulting in a net loss of muscle protein. Importantly, autophagy, UPS, calpain, and the caspase-3 proteolytic systems have all been demonstrated to contribute to protein degradation during disuse muscle atrophy [14-16]. More details of these proteolytic systems follows.
Figure 1. Prolonged muscle inactivity activates proteolytic pathways and decreases protein synthesis pathways.
Muscle inactivity activates four proteolytic systems that mediate protein degradation: 1) caspase-3; 2) calpain; 3) Autophagy; 4) and the ubiquitin proteasome system (UBS). Additionally, prolonged muscle inactivity results in the inhibition of the PI3K/Akt/mTOR pathway which mediates protein synthesis. See text for more details. FoxO, forkhead box O; MuRF-1, muscle ring finger protein-1; LC3, microtubule-associated proteins 1A/1B light chain 3b; Bnip3, BCL2 interacting protein 3; PI3K, phosphatidylinositol 3-kinase; Akt, protein kinase B; mTOR, mammalian/mechanistic target of rapamycin.
The UPS functions to degrade damaged myofibrillar proteins within the muscle. The UPS is composed of numerous ubiquitin ligase enzymes and a large proteolytic complex termed the proteasome. Importantly, the UPS plays a large role in the protein degradation that occurs in skeletal muscle atrophy [17]. In this regard, ubiquitin ligase enzymes tag proteins that are damaged or deemed unnecessary with the protein ubiquitin; tagging of proteins with ubiquitin marks them for degradation by the proteasome. Specifically, two muscle specific E3 ligases, atrogin-1/MAFbx and muscle ring finger protein-1 (MuRF-1), are recognized in playing a significant role in UPS mediated protein degradation in skeletal muscle wasting [18]. Specifically, atrogin-1 is capable of ubiquitinating sarcomeric proteins (e.g. myosin light isoforms) as well as proteins involved in protein synthesis pathways [19]. Conversely, MuRF-1 is purported to play a larger role in the ubiquitination of thick filament components such as myosin heavy chain isoforms [20].
Importantly, the structural proteins that tether contractile elements (e.g. α-II-spectrin, titin, etc.) in skeletal muscle are cleaved by calpain/caspase-3-dependent proteolysis in order to free the thick and thin myofilaments [21]. However, the calpain and caspase-3 system may go beyond initial cleaving of large protein structures and serve as signaling effectors for protein degradation as well. Indeed, both the calpain and caspase-3 systems are implicated as critical proteolytic enzymes required for inactivity-induced atrophy to occur [22]. Both calpain and caspase-3 systems are allosterically regulated by Ca2+. Specifically, proteolysis in the caspase-3 system is mediated by activated caspase-3. In this regard, Ca2+-induced activation of caspase-12 or calpain results in activation of caspase-3 which can then cleave its target proteins. Moreover, caspase-3 activation may occur due to mitochondrial signaling pathways involving the release of cytochrome C (CytC) from the mitochondria [23].
The autophagy system has also been demonstrated to be a critical proteolytic system involved in inactivity-induced muscle atrophy [24]. Autophagy acts to degrade proteins and, in some cases, entire organelles. Autophagy occurs through the formation of an autophagosome which acts to engulf protein structures destined for degradation, and this autophagasomal degradation is facilitated through fusion of the autophagosome with lysosomes.
Taken together, all four proteolytic systems have been demonstrated to be critically involved in skeletal muscle atrophy [14-16]. Thus, it is important to note that activation of the UPS, autophagy, calpain, or caspase-3 systems often coincides with activation of another: highlighting the extensive crosstalk that occurs between these systems. This observation is exemplified by the actions of a key transcriptional regulator of these systems: Forkhead box O (FoxO). FoxO has been recognized as an important transcriptional regulator during muscle inactivity-induced muscle wasting [25, 26]. In this regard, FoxO activation induces the transcription of UPS related genes (e.g. Atrogin-1 and MuRF-1) as well as autophagy related genes (e.g. microtubule-associated proteins 1A/1B light chain 3b (LC3), BCL2 interacting protein 3 (Bnip3), and cathepsin L) [27]. Moreover, the activation of the FoxO pathway during muscle inactivity also down-regulates PI3K/Akt/mTOR mediated protein synthesis [26]. Collectively, it is apparent that FoxO regulates the activation of multiple proteolytic systems and negatively impacts the systems involved in protein synthesis. The interconnected relationship between these systems also represents the possibility for a single governing regulator to exist that is capable of mediating atrophy programs during inactivity (e.g. mitochondrial dysfunction). Nonetheless, our understanding of the proteolytic systems involved in muscle atrophy have grown extensively. However, a large portion of the aforementioned knowledge of proteolysis in skeletal muscles has been derived from rodent experimental models and debate exists about the involvement of these systems in humans.
2.3. Controversy exists regarding the role that accelerated proteolysis plays in inactivity-induced atrophy in human skeletal muscle
Humans studies describing the rate of inactivity-induced muscle atrophy reveal that ≤14 days of bed rest or immobilization results in muscle loss at a rate ranging between 0.2%-2.3% of fiber cross sectional area per day [28]. Although it is clear that disuse muscle atrophy occurs due to both a decrease in protein synthesis and an increase in protein degradation in rodent experimental models [6, 29-32], debate exists on the role that proteolysis plays in mediating inactivity-induced muscle atrophy in humans. Indeed, it has been argued that in humans the key determinant of muscle atrophy during muscle inactivity is entirely due to decreased muscle protein synthesis [9]. However, the argument that a decrease in protein synthesis is the only factor driving muscle atrophy during inactivity may be flawed due to an inability to accurately measure rates of proteolysis in humans and therefore, this issue remains in debate [33]. While discrepancies exist on the relative contribution of protein synthesis and protein degradation to atrophy in humans, it is important to note that mitochondrial dysfunction is a hallmark trait that occurs during prolonged muscle inactivity in both animals and humans [34-37]. In the next section, we discuss the events involved in regulation of homeostatic mitochondrial function and how these are altered during muscle inactivity to induce mitochondrial dysfunction.
3. Mitochondrial morphology, proteins, protein import, and composition are disrupted during muscle inactivity
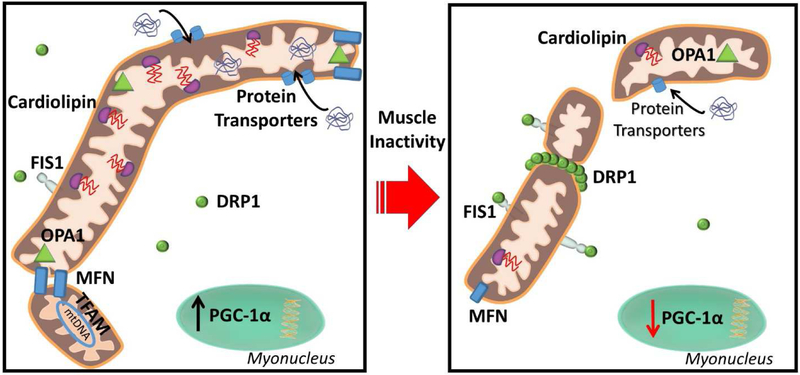
Mitochondrial dysfunction is a critical regulatory event for the activation of atrophic programs during inactivity-induced muscle atrophy [3, 4] and this key topic will be addressed in detail in a later section. Studies highlighting the mitochondrial dysfunction that occurs in skeletal muscles during periods of prolonged inactivity commonly measure mitochondrial respiratory function, reactive oxygen species (ROS) production, and morphology. In this regard, during prolonged muscle inactivity, mitochondria undergo rapid decrements of respiratory capacity and coupling, decreased mitochondrial volume, increased mitochondrial ROS emission, and morphological changes [34-39]. However, the factors that promote this mitochondrial dysfunction during prolonged muscle inactivity have not been fully elucidated. Nonetheless, in the next segments we will provide an overview of the established mechanisms that maintain mitochondrial function and how these processes are disrupted during inactivity (Figure 2).
Figure 2. Prolonged muscle inactivity induces mitochondrial dysfunction.
Mitochondria become dysfunctional during prolonged inactivity and demonstrate increased mitochondrial fission, decreased mitochondrial proteins, decreased mitochondrial protein import, and decreased cardiolipin. See text for more details. FIS1, mitochondrial fission 1; OPA1, optic atrophy 1; MFN, mitofusins; DRP1, dynamin related protein 1; PGC-1α, peroxisome proliferator-activated receptor gamma coactivator 1-alpha; TFAM, mitochondrial transcription factor A.
3.1. Mitochondrial morphology favors fission during muscle inactivity
The first report of on the existence of mitochondria was published in 1898, yet our understanding of the morphology/function of these organelles continues to expand [40]. Indeed, the discovery that mitochondria existed as a reticulum in skeletal muscle occurred eight decades later [41]. Importantly, the recent utilization of high-powered imaging techniques has provided an in-depth view on the extent of the mitochondrial reticulum [42]. These new findings provide the conceptual framework of mitochondria functioning as an interconnected “power grid”. For rapid ATP formation to occur in skeletal muscle during prolonged contractile activity, a high proton force must be maintained by the mitochondria to allow the transfer of electrons through the electron transport chain. When mitochondria exist in an elongated reticulum, sites near ATP consuming areas are aided by proton pumps distal from energy-requiring sites (e.g. Myosin ATPase, SERCA, etc.) acting to maintain a high proton gradient [42]. The regulation of this mitochondrial reticulum is an active process that involves frequent restructuring of mitochondrial organization in the forms of fusion (i.e. joining of adjacent mitochondria) and fission (i.e. separation of mitochondria). In this regard, fusion allows mitochondria to employ a larger breadth of machinery and increase ATP production capacity [42, 43]. Fission, however, serves as a process of quality control by separating damaged mitochondria from the existing reticulum, and can conversely act to create new mitochondrial networks [44]. In regard to muscle inactivity, mitochondria undergo a state of fission which may serve as a signaling effector for muscle atrophy due to a disruption in the ability to produce energy [39, 45, 46]. Thus, further understanding of the control mechanisms of fusion and fission is warranted.
The process of mitochondrial fusion and fission require elaborate fine-tuning due to the mitochondrial lipid bilayer membrane, thus necessitating coordination at both the inner and outer membrane. During fusion, the proteins mitofusin 1 and 2 (MFN1 and MFN2) mediate fusion by tethering the outer mitochondrial membrane to adjacent mitochondria, while the fusion of the inner membrane is mediated by optic atrophy 1 (OPA1) [47]. MFN 1 and 2 are also involved in mediating junctions between the sarcoplasmic reticulum creating membrane associated-sarcoplasmic reticulum membranes (MAM) which serve important roles in mitochondrial Ca2+ handling [48, 49]. A key regulatory element to regulation of fusion via MFN proteins is the E3 ligase Parkin, which ubiquitinates MFN1 and MFN2 leading to its extraction from the outer membrane of the mitochondria and subsequent degradation, thus serving to prevent mitochondrial fusion [50, 51]. Additionally, the mitochondrial proteases presenilins-associated rhomboid-like protein (PARL 1), mitochondrial ATPases associated with diverse cellular activities (m –AAA), and high temperature requiredA2 (HtrA2) can cleave OPA1 resulting in functionally different OPA1 isoforms that vary in their action (e.g. mediating fusion or cristae remodeling) [52].
Conversely, the process of fission is largely coordinated by dynamin related protein 1 (DRP1). DRP1 resides in the cytosol until signaling events induce its translocation toward the outer membrane of the mitochondria [53]. Currently, the best described regulatory actions of DRP1 translocation is phosphorylation at specific-serine sites. Phosphorylation at Ser616 is believed to activate DRP1, whereas phosphorylation at Ser637 and Ser693 appear to inactivate DRP1 and prevention of mitochondrial fission [54-56]. In addition, DRP1 activity can also be modified by sumoylation and S-nitrosylation [53]. Upon DRP1 recruitment to the mitochondria, DRP1 interacts with mitochondrial fission 1 (FIS1) on the outer mitochondrial membrane serving to anchor DRP1 to the mitochondria [57]. DRP1 oligomerizes with the mitochondria and multiple DRP1 dimers form a filament around the mitochondria leading to scission and fission of the mitochondria [58, 59]. Importantly, muscle inactivity results in alterations of the proteins involved in mediating both fusion and fission [39, 60].
During inactivity-induced muscle atrophy, total as well as phosphorylated (i.e. pDRP1-S616, active) DRP1 is increased in response to inactivity implicating DRP1 as an important mediator of the mitochondrial fragmentation that occurs with inactivity [39, 61]. Furthermore, levels of MFN1, MFN2, and OPA1 protein content are decreased, which reduces the capacity for mitochondrial fusion to occur [60]. Taken together, a concomitant decrease in fusion proteins along with an increased signaling for mitochondrial fission via DRP1 likely underlie mitochondrial fragmentation during muscle inactivity.
3.2. Key mitochondrial proteins are rapidly down-regulated during muscle inactivity
Intriguingly, decreases in the abundance of mitochondrial fusion proteins may be mediated, in part, by a decreased synthesis of mitochondrial proteins. Indeed, transcriptomic and proteomic studies reveal that pathways involved in mitochondrial biogenesis are downregulated during skeletal muscle inactivity [62, 63] and, in particular, these signaling pathways are the most negatively impacted transcriptional sub-group following only 48 hours of immobilization [38]. Regulation of mitochondrial proteostasis is unique due to the multi-faceted coordination of the nuclear and mitochondrial genome that operate synchronously to maintain functioning mitochondrial proteins. In this regard, the mitochondrial genome is responsible for encoding 37 proteins that are involved in mitochondrial maintenance and function, whereas over 1100 nuclear encoded proteins have currently been identified in the formation of human mitochondria [64].
Importantly, a key regulator of mitochondrial biogenesis, peroxisome proliferator-activated receptor gamma coactivator 1-alpha (PGC-1α), is significantly decreased during prolonged muscle inactivity [65] (Figure 2). PGC-1α is an important transcriptional coactivator that aides in the regulation of genes involved in mitochondrial biogenesis, fatty acid oxidation, and glycolysis. It follows that decreases in PGC-1α abundance results in impaired mitochondrial biogenesis resulting in a negative impact on mitochondrial structure and function [66]. Conversely, studies reveal that overexpression of PGC-1α preserves mitochondria and attenuates inactivity-induced muscle atrophy during limb immobilization, hindlimb suspension, and denervation [67-69]. Additionally, overexpression of PGC-1α ameliorates the decreases of MFN1, MFN2 and OPA1 that occur with muscle unloading [60], further highlighting the importance of maintaining upstream transcriptional activators. However, interpretation of the protective effects of PGC-1α in relation to mitochondrial dysfunction and the direct impact on muscle inactivity-induced atrophy is difficult. Indeed, PGC-1α commands a broad array of signaling and transcriptional events not all directly related to mitochondria [70]. Nonetheless, these PGC-1α overexpression studies add to the culminating evidence that protection of mitochondrial function is critical to attenuating inactivity induced muscle wasting.
Additionally, decreases in the mitochondrial transcription factor A (TFAM) that occur in response to prolonged muscle inactivity may also serve as an event dictating mitochondrial dysfunction [37, 65, 71]. A downstream target of PGC-1α, TFAM is an important regulator of transcription of mitochondrial DNA (mtDNA) encoded genes and acts to stabilize and protect mtDNA from damage and degradation [72]. Indeed, ablation of TFAM results in embryonic lethality [73]. During muscle inactivity, TFAM promoter activity is decreased by 30% a mere 8 hours following denervation and further decreased by 65% following 3 days [74]. Furthermore, denervation results in a significant decrease of TFAM localization within mitochondria and a corresponding decrease in mitochondrial copy number [74]. Thus, decreases in TFAM during disuse muscle atrophy may expose mtDNA to a higher risk of damage: resulting in a cascading effect of damage leading to mitochondrial signaling events that activate atrophic pathways [72]. Indeed, this is significant as mtDNA number decreases during prolonged muscle disuse [5, 37] which is likely a response to accumulation of damaged mtDNA and consequential removal. Nonetheless, direct interventions of modulating TFAM independently have not been performed in inactivity-induced atrophy and may warrant future research. Moreover, the reductions in the synthesis of mitochondrial proteins are further exacerbated by a decreased capacity for mitochondrial protein import.
3.3. Mitochondrial protein import and import machinery are disrupted during prolonged muscle inactivity
Reductions in the abundance of mitochondrial proteins can be further exacerbated by a decreased capacity for mitochondrial protein import. Mitochondria utilize specialized transporters to incorporate nuclear encoded proteins by shuttling them across the mitochondrial inner and outer membranes. Specifically, protein import requires a multitude of various membrane transporters, as well as processing enzymes to cleave the mitochondrial protein precursors responsible for guiding nuclear encoded proteins destined for mitochondrial import [75]. During prolonged muscle inactivity however, the capacity for mitochondrial protein import is decreased along with decreases in the associated proteins for mitochondrial import [76]. Indeed, the mitochondrial import translocase of the outer membrane (Tom20) and translocase of the inner membrane (Tim 23) are significantly reduced following muscle denervation in rodents [76]. These findings are likely important, as the decrease in the capacity for mitochondrial import following denervation was highly correlated with the extent of mitochondrial dysfunction. Furthermore, this disruption in mitochondrial protein import capacity is also likely translatable to humans as transcripts encoding for mitochondrial import machinery are also downregulated in human skeletal muscles during prolonged limb immobilization [38].
3.4. Prolonged skeletal muscle inactivity decreases mitochondrial cardiolipin
Cardiolipin is a critical component in the composition of the mitochondrial membrane. Specifically, the phospholipid cardiolipin is largely exclusive to the mitochondrial inner membrane. Cardiolipin functions to spatially orient mitochondrial cristae, trap protons in the intermembrane space to maintain a proton gradient, and provide a scaffold for the electron transfer complexes in the respiratory chain [77]. In order for mitochondria to incorporate cardiolipin, the molecule is synthesized by cardiolipin synthase (CLS) and then further converted into a mature form via remodeling of its four fatty acid chains by the enzymes tafazzin (Taz) and Acyl-CoA:lyscocardiolipin acyltransferase-1 (ALCAT1) [78].
During prolonged muscle inactivity, cardiolipin abundance decreases along with a congruent decrease in the relative composition of cardiolipin with linoleic acid (18:2n-6) fatty acid chains [79]. While the findings that cardiolipin abundance is altered during inactivity is still novel, this may be an important occurrence during muscle inactivity as alterations of cardiolipin abundance as well as the composition of its fatty acid chains can have direct effects on mitochondrial function [80]. In skeletal muscle, cardiolipin fatty acid chains are primarily composed of linoleic acids (18:2n-6) [81]. Of note, decreases in the relative composition of cardiolipin with linoleic acids is associated with decreased cytochrome c oxidase activity [82], thus may serve as one mechanism for mitochondrial dysfunction. Intriguingly however, a compensatory mechanism appears to take place for the loss of cardiolipin during muscle inactivity, as Taz protein expression and transcription of ALCAT1 and CLS are increased during muscle inactivity [78, 79]. While future research is required to fully understand the processes and extent of cardiolipin remodeling during disuse, this may play an important role in mitochondrial dysfunction as cardiolipin modeling impacts another key trait of mitochondrial dysfunction: increased ROS emission [14, 83]. Importantly, increased ROS emissions are a hallmark of mitochondrial dysfunction during prolonged muscle disuse and will be discussed in the subsequent section.
In summary, the mitochondrial dysfunction that occurs in skeletal muscle fibers during prolonged periods of inactivity likely occurs due to a combination of alterations in mitochondrial morphology, transcription and translation of mitochondrial proteins, protein import capacity, and changes in the membrane lipid profile. Indeed, mitochondria become fragmented during prolonged muscle inactivity by increases in activation of mitochondrial fission machinery along with decreases in fission proteins. Furthermore, decreases in the transcriptional and translational events for mitochondrial proteins reduce the ability for mitochondrial biogenesis and preservation of mtDNA. Additionally, a decreased capacity to import and incorporate these proteins into the mitochondria yields a lower ability to recycle damaged mitochondrial proteins and sustain adequate mitochondrial function. Alterations in the composition and content of cardiolipin in mitochondria may also serve to decrease the ability to trap protons in the intermitochondrial membrane space resulting in increased ROS emissions and reducing ATP production. These alterations that occur in skeletal muscle mitochondria during prolonged periods of inactivity highlight the extensive interruptions that occur in mitochondrial function and illustrate a path to dysfunction. The next section discusses the evidence that dysfunctional mitochondria directly contribute to activation of the signaling pathways that promote skeletal muscle atrophy.
4. Mechanisms of mitochondrial dysfunction-induced skeletal muscle atrophy
The final sections of this review will discuss the mechanisms involved in mediating mitochondrial dysfunction induced muscle atrophy during inactivity. We begin with an overview of the first evidence that stimulated interest in the role that mitochondria play in promoting skeletal muscle atrophy followed by direct evidence linking mitochondrial dysfunction with muscle fiber atrophy. The concept that mitochondrial dysfunction contributes to muscle atrophy during inactivity first appeared in the literature half a century ago in the 1960’s [84, 85]. However, direct evidence adjudging dysfunctional mitochondria as the nexus between inactivity and muscle atrophy was lacking until recently. Indeed, technological advancements in the tools capable of specifically targeting mitochondria have only recently become available, and even so the methodology employed must be selective in order to demonstrate direct causal evidence for this phenomena. For instance, the findings that overexpression of PGC-1α protect against mitochondrial dysfunction and muscle atrophy are useful [67], however PGC-1α affects a broad array of metabolic and anabolic pathways thus complicating the interpretation of these findings in relation to a direct role for mitochondria. In this regard, the first evidence suggesting that mitochondrial dysfunction mediates muscle atrophy was published by Romanello et al. [45], demonstrating that by knocking down proteins involved in mitochondrial fragmentation and degradation (FIS1 and Bnip3) skeletal muscle was partially protected against muscle atrophy due to denervation (~35% reductions in cross-sectional area vs ~50% in control animals). While these findings were critically important in providing evidence that mitochondrial dysfunction promotes inactivity-induced muscle atrophy, this methodology approached the problem from a perspective of protecting mitochondria against fragmentation and did not demonstrate full protection against muscle atrophy. In order for a causal link to be determined between mitochondrial dysfunction and inactivity-induced muscle atrophy, it was necessary to demonstrate that the pathways that dictate muscle atrophy are not activated in the absence of mitochondrial dysfunction.
In this regard, two key studies emerged demonstrating that dysfunctional mitochondria are a causal event in mediating the processes of muscle atrophy due to inactivity [3, 4]. In both of these studies, utilization of a mitochondrial targeted peptide, SS-31, protected against mitochondrial dysfunction and completely prevented muscle-inactivity induced atrophy [3, 4]. Administration of SS-31 protected mitochondrial function as demonstrated by preventing inactivity-induced decrements in mitochondrial coupling and elevations in mitochondrial ROS emissions [3, 4]. Consequently, protecting against mitochondrial dysfunction abolishes increased proteolysis in muscle fibers by prevention of inactivity-induced activation of proteolytic enzymes (i.e. calpain-1 and caspase-3), upregulation of atrogenes (i.e. Atrogin-1 and MuRF-1), and activation of the autophagy system (i.e. ratio of LC3 II/I and cathepsin mRNA) [86]. Furthermore, the anabolic signaling pathways promoting protein synthesis (i.e. p-mTOR/mTOR) are also protected against inactivity-induced decrements when mitochondria are protected [86].
The mechanisms of protection of skeletal muscle mitochondria against inactivity-induced dysfunction remain an active area of research. In this regard, SS-31 exhibits a high specificity for binding to cardiolipin within the mitochondria and does not exhibit off-target effects outside of the mitochondria [77]. While the exact mechanisms of SS-31 functions are not completely understood, SS-31 demonstrates high specificity for co-localization within mitochondria by binding and stabilizing cardiolipin, but the molecule also demonstrates antioxidant properties [77]. Indeed, the interactions of SS-31 with cardiolipin may have important implications on why elevated ROS emissions were prevented in inactive muscle mitochondria and this point will be discussed later. Nonetheless, the aforementioned studies demonstrate that protection of mitochondrial function ameliorates muscle atrophy during inactivity by preventing mitochondrial dysfunction-induced activation of proteolytic pathways and suppression of protein synthesis pathways [3, 4, 86].
A key question remains; how do dysfunctional mitochondria activate the signaling pathways that trigger accelerated proteolysis in skeletal muscle fibers? In the subsequent sections, we highlight the available knowledge on the signaling events involved in promoting muscle atrophy due to inactivity-induced mitochondrial dysfunction. In this regard, one of the most prominent components of mitochondrial dysfunction-induced muscle atrophy is the role of mitochondrial ROS emissions during inactivity [87].
4.1. Mitochondrial ROS trigger atrophy signaling pathways during prolonged muscle inactivity
Aberrant mitochondrial ROS emissions serve as important signaling effectors for muscle atrophy during inactivity (Figure 3). Although mitochondrial ROS emissions serve as signaling molecules in homeostatic regulation of muscle fibers, high levels of ROS production can negatively impact the pathways involved in maintaining muscle mass [87, 88]. In brief, unabated ROS production promotes expression of proteins involved in the UPS, the autophagy system, and activation of the calpain and caspase systems. Furthermore, ROS-induced oxidation of myofibrillar proteins increases their susceptibility to degradation by proteolytic systems. Protein synthesis pathways can also be negatively impacted by levels of ROS through inhibition of the initiation of mRNA translation [89]. Additionally, it has also been demonstrated that exposure of myocytes to high ROS levels promotes mitochondrial fission [90], which also has implications on inducing muscle atrophy [45] and the relationship between mitochondrial fission and muscle atrophy will be discussed in a later section.
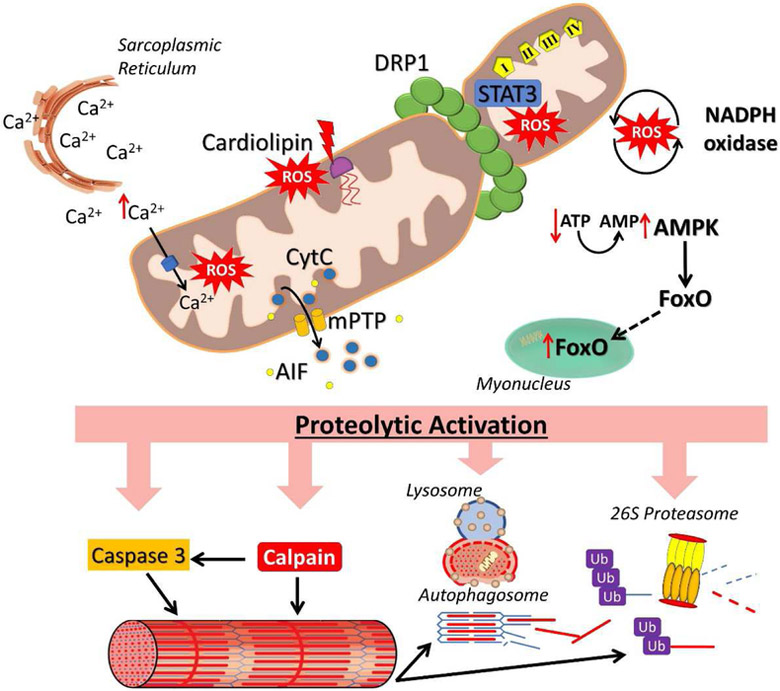
Figure 3. Dysfunctional mitochondria signal for proteolytic activation.
Prolonged inactivity induces mitochondrial dysfunction that activates proteolytic signaling. Increased intramitochondrial Ca2+, disruptions in cardiolipin, STAT3 translocation, and NADPH oxidase crosstalk increase ROS emissions. Dysfunctional mitochondria also release CytC and AIF that activate proteolytic pathways. Mitochondrial fission also results in disrupted energy production and activates FoxO via AMPK. See text for more details. DRP1, dynamin related protein 1; STAT3, signal transducer and activator of transcription 3; AMPK, AMP-activated protein kinase; CytC, cytochrome C; AIF, apoptosis inducing factor; FoxO, forkhead box O; mPTP, mitochondrial permeable transition pore. I, II, III, IV, denote complex I, complex II, complex III, and complex IV of the electron transport chain.
The occurrence of oxidative stress (i.e. an imbalance between oxidants and antioxidants) occurs in muscle fibers during prolonged muscle inactivity and plays a significant role in mediating muscle atrophy [87]. In this regard, the delivery of exogenous antioxidant treatment to animals has been shown to attenuate proteolysis and muscle atrophy in preclinical models of immobilization and mechanical ventilation-induced muscle wasting [91-93]. While numerous sources of ROS production exist in skeletal muscle, mitochondria are a primary source of ROS production in skeletal muscle during prolonged periods of inactivity [87]. Indeed, overexpression of a mitochondrial specific antioxidant, peroxiredoxin, has been shown to provide partial protection against disuse atrophy in diaphragm muscle fibers following 6 hours of mechanical ventilation [37]. Moreover, mitochondrial dysfunction-induced muscle atrophy is further supported by the observation that the treatment of animals with the mitochondrial-targeted peptide SS-31 prevents the muscle inactivity-induced increases in mitochondrial ROS emission and protects against oxidative stress [3, 4]. However, the specific mechanisms that promote increased mitochondrial ROS emissions during prolonged muscle inactivity remain unclear.
One potential mechanism to explain increased mitochondrial ROS emissions during prolonged muscle inactivity is alterations of mitochondrial levels of cardiolipin. Given that cardiolipin abundance and composition are disrupted in skeletal muscle mitochondria during prolonged inactivity [79], the observation that the mitochondrial peptide SS-31 preserves mitochondrial function by binding to cardiolipin implicates the importance of conserving cardiolipin in skeletal muscle mitochondria during long periods of contractile inactivity. Indeed, the SS-31/cardiolipin complex has been shown to protect against peroxidation of cardiolipin by cytochrome c peroxidase, resulting in protection against alterations in mitochondrial cristae that occur during prolonged ischemia [94]. Further, cardiolipin plays an important role in scaffolding for electron transport chain enzymes and thus, changes in cardiolipin structure or abundance can be deleterious for mitochondrial function by negatively affecting the ability of cardiolipin to perform this role [77, 83]. It follows that protecting cardiolipin during inactivity may be a critical factor for protecting skeletal muscle mitochondria during prolonged periods of muscle inactivity. Clearly, future research is warranted to better understand the involvement of cardiolipin alterations in the development of mitochondrial dysfunction during prolonged periods of muscle inactivity. In this regard, cardiolipin’s role in mitochondrial ROS production is currently being evaluated in Barth’s syndrome which occurs due to a mutation in tafazzin, an important protein involved in cardiolipin metabolism; this research will likely lead to an improved understanding of the role that cardiolipin plays in mitochondrial function [83].
Alterations in mitochondrial-Ca2+ handling have also been posited to induce the aberrant mitochondrial ROS emissions observed during prolonged muscle inactivity. Importantly, mitochondria act as a Ca2+ sink and mitochondrial play an integrative role in cellular Ca2+ handling and signaling. Moreover, mitochondrial respiration is also regulated, in part, by mitochondrial Ca2+ levels [95, 96]. For instance, Ca2+ is an allosteric activator of citric acid cycle enzymes (e.g. pyruvate dehydrogenase, isocitrate dehydrogenase, etc.,) and electron transport chain components (e.g. ATP synthase) [97]. However, the dynamic relationship between mitochondria and Ca2+ signaling is altered during prolong muscle inactivity and this may play a role in precipitating the mitochondrial dysfunction that promotes disuse muscle atrophy. In this regard, muscle cytosolic Ca2+ levels increase during prolonged periods of muscle inactivity. Because mitochondria function as a Ca2+ sink, intramitochondrial Ca2+ levels rise in response to elevated cytosolic Ca2+ during muscle inactivity; this increase in mitochondrial Ca2+ level may promote an increased production of ROS. Indeed, Ca2+ activates mitochondrial ROS producing enzymes such as α-ketoglutarate dehydrogenases and glycerol phosphate dehydrogenase [98]. Furthermore, Ca2+ stimulation of citric acid cycle and electron transport enzymes increases the proton motive force within mitochondria. A high proton motive force in mitochondria results in greater ROS production [99]; this is problematic during prolonged inactivity of skeletal muscle where ATP turnover is decreased and a high proton motive force is sustained.
Of note, a previous study has demonstrated that the sarcoplasmic reticulum calcium handler, ryanodine receptor 1 (RyR1), is oxidized during muscle inactivity causing a Ca2+ leak from the sarcoplasmic reticulum (SR) due to disassociation of the protein calstabin1 [100]. Intriguingly, pharmacologic stabilization of the calstabin1-RyR1 complex reduced the Ca2+ leak from the RyR1 and results in protection against inactivity-induced muscle atrophy [100]. Thus, Ca2+ leak from the SR could trigger an increase in Ca2+-stimulated mitochondrial ROS production. Nonetheless, this study did not assess mitochondrial function and therefore, the impact of reducing the Ca2+ leak from the RyR1 in these experiments remains unknown. Given that mitochondrial ROS emissions could also play a role in the oxidation of the RyR1, it would be interesting to assess the relationship between these events and determine if prevention of the Ca2+ leak from the SR would also confer protection to the mitochondria.
The entry of cytosolic Ca2+ into the mitochondria is largely mediated by the mitochondrial Ca2+ uniporter (MCU). Intriguingly, overexpression of MCU has been shown to provide protection against disuse muscle atrophy in a denervation model of muscle wasting [101]. At first glance, the observation that overexpression of MCU protects against atrophy may seem paradoxical due to a greater capacity of Ca2+ entry into mitochondria, which could result in a higher Ca2+ abundance in mitochondria and promote an increase in mitochondrial ROS emission. However, a recent study may help explain this finding with evidence suggesting that a lack of a Ca2+ transient during inactivity may trigger the onset of mitochondrial dysfunction via a lack of mitochondrial Ca2+ uptake [102]. These experiments revealed that the increased mitochondrial ROS generation in denervated muscle was attenuated when the muscle was stimulated to contract via electrical stimulation. Yet, the protective effects of electrical stimulation against denervation-induced increases in mitochondrial ROS was lost when mitochondrial Ca2+ uptake was prevented by pharmacological blockade of the MCU (Ru360). Given the positive regulatory role Ca2+ plays in mitochondrial energetics [96], it is possible that prevention of mitochondrial Ca2+ uptake will disrupt the energy producing capacity and initiate mitochondrial dysfunction. Thus, a physiological Ca2+ transient between the cytosol and mitochondria is likely important for maintaining normal mitochondrial function by regulating mitochondrial ROS production. Future studies are required to provide the molecular basis responsible for the mitochondrial response to Ca2+ transients.
A third mechanism that may contribute to elevated mitochondrial ROS emission during prolonged muscle inactivity is the translocation of signal transducer and activator of transcription 3 (STAT3) into mitochondria. Activated STAT3 binds to the mitochondrial complex I subunit, GRIM-19, and results in increased mitochondrial ROS emissions [103]. During mechanical ventilation-induced diaphragm inactivity, STAT3 becomes phosphorylated and translocates into mitochondria. However, prevention of STAT3 activation with the usage of a Janus kinase (JAK) inhibitor prevented inactivity-induced increases in mitochondrial ROS emissions and diaphragm muscle atrophy [104]. While the beneficial effects of blocking STAT3 signaling has only been demonstrated in diaphragm muscle, the JAK/STAT pathway is also activated in limb muscle during inactivity and may also contribute to disuse muscle atrophy in limb muscle as well [105]. Currently, the mechanisms responsible for increased mitochondrial ROS emissions due to STAT3 translocation to mitochondria is not fully understood. In this regard, it has been shown that translocation of STAT3 to mitochondria can increase activity of the electron transport chain [103]. It is possible that STAT3 translocation may induce ROS formation by this increase in electron flux within the transport chain. This would be deleterious in inactive muscle where mitochondria are at a basal state of respiration (i.e. state 4) where ROS emissions are higher due to the reduced state of electron carriers in the electron transport chain [35]. Further elucidation of the role that STAT3 plays in mitochondrial dysfunction may lead to therapeutic targets for preventing inactivity-induced muscle atrophy.
Lastly, accumulating evidence also reveals that cross-talk exists between mitochondrial ROS production and activation of ROS generating enzymes such as NADPH oxidase [106]. This factor may be important during prolonged muscle inactivity whereby increases in mitochondrial ROS formation or activation of NADPH oxidase results in a viscous cycle of ROS formation between mitochondrial and NADPH oxidases. Indeed, mitochondria are prone to damage during oxidative stress which can result in further elevated production of ROS due to damaged mitochondrial machinery [107]. A viscous cycle of ROS production would then lead to uncompensated oxidative stress and activation of the proteolytic systems. In this regard, pharmacological inhibition of NADPH oxidase has been shown to attenuate muscle-inactivity induced atrophy [108].
In summary, mitochondrial ROS emissions can indirectly and directly activate proteolytic systems and promote muscle atrophy during prolonged periods of muscle inactivity. However, the mechanisms that induce elevated ROS emission during inactivity are not fully understood. Alterations in cardiolipin content and abundance, altered Ca2+ handling, STAT3 translocation, and NADPH oxidase cross-talk may all be involved in mediating aberrant mitochondrial ROS emission. Certainly mitochondrial ROS emissions play a major role in activation of proteolytic systems, however dysfunctional mitochondria may also promote muscle atrophy programs through other means separate from ROS activation and these programs will be discussed in the following segments.
4.2. Mitochondrial dysfunction results in release of mitochondrial-specific atrophic signaling effectors
In addition to increased ROS production, damaged and dysfunctional mitochondria can also release mitochondrial-specific signaling molecules that activate atrophy pathways in skeletal muscle. Specifically, the release of the mitochondrial proteins apoptosis inducing factor (AIF) and CytC into the cytosol results in the activation of caspase-3 which can promote actin/myosin breakdown and trigger myonuclear apoptosis which lowers the transcriptional capacity of myofibers [23]. The role of CytC and AIF in prolonged inactivity-induced muscle atrophy has been confirmed by a double knockout model of two proteins that permeabilize the mitochondrial membrane: Bax and Bak [61]. Knockout of Bax and Bak resulted in a blunted release of AIF and, consequentially, attenuated inactivity-induced muscle atrophy [61]. However, mitochondria were not protected against inactivity-induced dysfunction (as determined by dysfunctional mitochondrial respiration and increased ROS emission) and this likely explains why fiber atrophy was only partially attenuated in these experiments. Nonetheless, the release of AIF and CytC from mitochondria play an important role in signaling for muscle atrophy via activation of proteolytic systems and likely act in concert with other mitochondria signaling effectors to induce the full extent of muscle atrophy that occurs in response to prolonged periods of inactivity.
The release of AIF and CytC can occur via permeabilization of the mitochondrial membrane or opening of the mitochondrial permeable transition pore (mPTP) allowing for AIF and CytC to enter into the cytosol. The mPTP is a non-specific channel that, when opened, is permeable to molecules <1.5kDa. Intriguingly, the specific proteins that form the mPTP have not been concisely agreed upon. Currently, the mPTP is suggested to consist of the adenine nucleotide transporter (ANT), phosphate carrier (PiC), and FoF1 ATP synthase [109]. The opening of the mPTP is largely regulated by Ca2+ present within the mitochondrial matrix and opens in response to a Ca2+ overload. In this regard, mPTP sensitivity to Ca2+ induced opening is subject to modulation and can be increased by factors such as oxidative stress and phosphate [109]. During inactivity, the sensitivity of mPTP opening is increased in skeletal muscle and may serve an important role in the release of CytC and AIF from mitochondria [110]. Given that prolonged muscle disuse promotes an increase in cytosolic Ca2+ abundance, a heightened sensitivity to mPTP may serve as an important mechanism for the release of AIF and CytC and the subsequent activation of proteolytic pathways leading to muscle atrophy.
4.3. Mitochondrial fission disrupts energy homeostasis and activates atrophy during inactivity
The mitochondrial fission that occurs during prolonged muscle inactivity may also play a role in signaling to promote muscle atrophy [39, 45, 46]. While fission itself can have implications on multiple aspects of mitochondrial function such as ROS formation, fission can result in decreases in ATP production and therefore, impact muscle atrophy. Indeed, another mechanism that may contribute to inactivity-induced muscle atrophy due to mitochondrial dysfunction is lowered ATP output resulting in increases in the abundance of AMP. A rise in cellular AMP levels results in activation of AMP-activated protein kinase (AMPK) which can trigger atrophic signaling by activation of the transcriptional factor, FoxO3 [45, 111]. Indeed, activation of AMPK in muscle fibers during prolonged inactivity is closely associated with the activation of FoxO3 [45, 60, 68]. Activation of FoxO3 promotes muscle wasting by promoting an increased expression of atrogenes involved in both the ubiquitin proteasome system and autophagy [112, 113]. In regard to AMPK activation of FoxO3, AMPK can phosphorylate FoxO3 on its Ser588 site, resulting in the increased transcriptional activity of the proteolytic target genes regulated by FoxO3 [114].
Nonetheless, it is noteworthy that conflicting evidence exists on the role that AMPK activation plays in inactivity-induced muscle atrophy [39, 45, 60, 115]. Indeed, AMPK activation may occur divergently depending on the experimental model used to study to inactivity-induced muscle atrophy. For example, it has been predicted that a hypermetabolic state occurs during muscle inactivity and that the AMPK pathway is not activated in diaphragm muscle during prolonged mechanical ventilation [37]. In this regard, it is possible that AMPK activation in inactive skeletal muscle muscles may require several days of disuse for sufficient levels of AMP to accumulate resulting in AMPK activation. It follows that AMPK activation may not occur in diaphragm muscle during the 12-24 hours of study that is commonly employed in mechanical ventilation studies. In contrast to this mechanical ventilation study, a recent study in limb muscle suggests that AMPK signaling plays a key role in inactivity-induced atrophy. Specifically, a study of transgenic mice expressing a dominant negative mutant of AMPK reveals that disuse-induced muscle atrophy was reduced by ~50% in the soleus muscle, whereas muscle atrophy was unaffected in both the gastrocnemius and plantaris muscles; these results suggest that the role of AMPK-mediated atrophy may differ between muscle fiber types [115]. Thus, it is plausible that mitochondrial fission-induced AMPK activation during muscle inactivity may be one mechanism mitochondrial dysfunction employs to promote muscle atrophy.
In regard to mitochondrial fission and muscle atrophy, overexpression of a mutant FIS1 (FIS1k148R), the anchoring protein for DRP1, results in mitochondrial fission but does not promote muscle atrophy or demonstrate increased transcription of FoxO3 target genes [45]. This observation suggests that fission itself is not responsible for the activation of muscle atrophy. Intriguingly, blocking fission through the use of a dominant negative mutant DRP1 (DRP1K38A, inhibits DRP1 ability to bind GTP and causes loss of DRP1 function) attenuates skeletal muscle atrophy induced by transfection of a constitutively active FoxO3 (c.a.FoxO3) gene [45]. This finding is consistent with the notion that fission mediated by DRP1 serves an important signaling event in promoting disuse muscle atrophy. In this regard, the expression of DRP1K38A gene also prevents c.a.FoxO3 induced activation of a MuRF-1 reporter gene, demonstrating that crosstalk exists between the mitochondrial network and nuclear transcription (i.e. mitochondrial fission mediated by DRP1 is required for FoxO3 upregulation of nuclear target genes). Hence, it is feasible that mitochondrial fission promotes muscle atrophy through crosstalk between the mitochondrial network and nuclear transcription by means outside of energy disruption and AMPK-FoxO3 mediating signaling. Clearly, additional research is required to fully unravel the mechanisms responsible for fission-mediated muscle atrophy.
5. Conclusions
Direct evidence confirms that prolonged periods of skeletal muscle inactivity results in alterations in mitochondrial morphology, protein synthesis, protein import, and biochemical composition that result in mitochondrial dysfunction. Importantly, this inactivity-induced dysfunction in skeletal muscle mitochondria plays a causal role in disuse muscle atrophy likely through elevations in mitochondrial ROS emission, release of proteolytic-activating mitochondrial proteins, and cell signaling events caused by mitochondrial fragmentation. However, several questions remain unanswered regarding the specific biochemical links between prolonged muscle inactivity and mitochondrial dysfunction. For example, what are the biochemical forces behind the reductions in key mitochondrial proteins? Which factors are responsible for the inactivity-induced decrease in mitochondrial cardiolipin content? How do changes in mitochondria Ca2+ signaling contribute to muscle inactivity-induced mitochondrial dysfunction? These unanswered questions and others remain as an important area for research in an effort to understand the details of the relationship between mitochondrial dysfunction and inactivity-induced muscle atrophy. Importantly, it is predicted that future research elucidating the specific mechanisms responsible for inactivity-induced mitochondrial dysfunction will inevitably lead to the development of novel therapeutic strategies to protect against inactivity-induced muscle wasting. Indeed, it is feasible that pharmaceutical approaches toward protecting mitochondria against damage during prolonged periods of muscle inactivity will become an important therapeutic strategy for prevention of disuse muscle atrophy.
Acknowledgements
This work was supported by a grant from the National Institutes of Health (NIH R01 AR064189 awarded to SKP).
Abbreviations:
- PI3K
phosphatidylinositol 3-kinase
- Akt
protein kinase B
- mTOR
mammalian/mechanistic target of rapamycin
- UPS
ubiquitin proteasome system
- FoxO
forkhead box O
- MuRF-1
muscle ring finger protein-1
- LC3
microtubule-associated proteins 1A/1B light chain 3b
- Bnip3
BCL2 interacting protein 3
- DRP1
dynamin related protein 1
- STAT3
signal transducer and activator of transcription 3
- AMPK
AMP-activated protein kinase
- CytC
cytochrome C
- AIF
apoptosis inducing factor
- FoxO
forkhead box O
- mPTP
mitochondrial permeable transition pore
- PGC-1α
peroxisome proliferator-activated receptor gamma coactivator 1-alpha
- TFAM
mitochondrial transcription factor A
- mtDNA
mitochondrial DNA
- SR
sarcoplasmic reticulum
- RyR1
ryanodine receptor 1
- MCU
mitochondrial calcium uniporter
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Newman AB, Kupelian V, Visser M, Simonsick EM, Goodpaster BH, Kritchevsky SB, et al. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci 2006;61(1):72–7. Epub 2006/02/04. PubMed PMID: 16456196. [DOI] [PubMed] [Google Scholar]
- 2.Srikanthan P, Karlamangla AS. Muscle mass index as a predictor of longevity in older adults. Am J Med 2014;127(6):547–53. Epub 2014/02/25. doi: 10.1016/j.amjmed.2014.02.007. PubMed PMID: 24561114; PubMed Central PMCID: PMCPMC4035379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Powers SK, Hudson MB, Nelson WB, Talbert EE, Min K, Szeto HH, et al. Mitochondria-targeted antioxidants protect against mechanical ventilation-induced diaphragm weakness. Crit Care Med 2011;39(7):1749–59. Epub 2011/04/05. doi: 10.1097/CCM.0b013e3182190b62. PubMed PMID: 21460706; PubMed Central PMCID: PMCPMC4995067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Min K, Smuder AJ, Kwon OS, Kavazis AN, Szeto HH, Powers SK. Mitochondrial-targeted antioxidants protect skeletal muscle against immobilization-induced muscle atrophy. J Appl Physiol (1985). 2011;111(5):1459–66. Epub 2011/08/06. doi: 10.1152/japplphysiol.00591.2011. PubMed PMID: 21817113; PubMed Central PMCID: PMCPMC3220313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chacon-Cabrera A, Lund-Palau H, Gea J, Barreiro E. Time-Course of Muscle Mass Loss, Damage, and Proteolysis in Gastrocnemius following Unloading and Reloading: Implications in Chronic Diseases. PLoS One. 2016;11(10):e0164951. Epub 2016/10/30. doi: 10.1371/journal.pone.0164951. PubMed PMID: 27792730; PubMed Central PMCID: PMCPMC5085049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thomason DB, Biggs RB, Booth FW. Protein metabolism and beta-myosin heavy-chain mRNA in unweighted soleus muscle. Am J Physiol 1989;257(2 Pt 2):R300–5. Epub 1989/08/01. doi: 10.1152/ajpregu.1989.257.2.R300. PubMed PMID: 2764153. [DOI] [PubMed] [Google Scholar]
- 7.McClung JM, Kavazis AN, Whidden MA, DeRuisseau KC, Falk DJ, Criswell DS, et al. Antioxidant administration attenuates mechanical ventilation-induced rat diaphragm muscle atrophy independent of protein kinase B (PKB Akt) signalling. J Physiol 2007;585(Pt 1):203–15. Epub 2007/10/06. doi: 10.1113/jphysiol.2007.141119. PubMed PMID: 17916612; PubMed Central PMCID: PMCPMC2375462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smuder AJ, Gonzalez-Rothi EJ, Kwon OS, Morton AB, Sollanek KJ, Powers SK, et al. Cervical spinal cord injury exacerbates ventilator-induced diaphragm dysfunction. J Appl Physiol (1985). 2016;120(2):166–77. Epub 2015/10/17. doi: 10.1152/japplphysiol.00488.2015. PubMed PMID: 26472866; PubMed Central PMCID: PMCPMC4719055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rennie MJ, Selby A, Atherton P, Smith K, Kumar V, Glover EL, et al. Facts, noise and wishful thinking: muscle protein turnover in aging and human disuse atrophy. Scandinavian journal of medicine & science in sports. 2010;20(1):5–9. Epub 2009/06/30. doi: 10.1111/j.1600-0838.2009.00967.x. PubMed PMID: 19558380. [DOI] [PubMed] [Google Scholar]
- 10.Haddad F, Adams GR, Bodell PW, Baldwin KM. Isometric resistance exercise fails to counteract skeletal muscle atrophy processes during the initial stages of unloading. J Appl Physiol (1985). 2006;100(2):433–41. Epub 2005/10/22. doi: 10.1152/japplphysiol.01203.2005. PubMed PMID: 16239603. [DOI] [PubMed] [Google Scholar]
- 11.Kelleher AR, Kimball SR, Dennis MD, Schilder RJ, Jefferson LS. The mTORC1 signaling repressors REDD1/2 are rapidly induced and activation of p70S6K1 by leucine is defective in skeletal muscle of an immobilized rat hindlimb. Am J Physiol Endocrinol Metab 2013;304(2):E229–36. Epub 2012/11/30. doi: 10.1152/ajpendo.00409.2012. PubMed PMID: 23193052; PubMed Central PMCID: PMCPMC3543567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelleher AR, Pereira SL, Jefferson LS, Kimball SR. REDD2 expression in rat skeletal muscle correlates with nutrient-induced activation of mTORC1: responses to aging, immobilization, and remobilization. Am J Physiol Endocrinol Metab 2015;308(2):E122–9. Epub 2014/11/20. doi: 10.1152/ajpendo.00341.2014. PubMed PMID: 25406262; PubMed Central PMCID: PMCPMC4297780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allen DL, Linderman JK, Roy RR, Bigbee AJ, Grindeland RE, Mukku V, et al. Apoptosis: a mechanism contributing to remodeling of skeletal muscle in response to hindlimb unweighting. Am J Physiol 1997;273(2 Pt 1):C579–87. Epub 1997/08/01. doi: 10.1152/ajpcell.1997.273.2.C579. PubMed PMID: 9277355. [DOI] [PubMed] [Google Scholar]
- 14.Powers SK, Wiggs MP, Duarte JA, Zergeroglu AM, Demirel HA. Mitochondrial signaling contributes to disuse muscle atrophy. Am J Physiol Endocrinol Metab 2012;303(1):E31–9. Epub 2012/03/08. doi: 10.1152/ajpendo.00609.2011. PubMed PMID: 22395111; PubMed Central PMCID: PMCPMC3404565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jackman RW, Kandarian SC. The molecular basis of skeletal muscle atrophy. Am J Physiol Cell Physiol 2004;287(4):C834–43. Epub 2004/09/10. doi: 10.1152/ajpcell.00579.2003. PubMed PMID: 15355854. [DOI] [PubMed] [Google Scholar]
- 16.Romanello V, Sandri M. Mitochondrial Quality Control and Muscle Mass Maintenance. Front Physiol 2015;6:422. Epub 2016/01/23. doi: 10.3389/fphys.2015.00422. PubMed PMID: 26793123; PubMed Central PMCID: PMCPMC4709858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bilodeau PA, Coyne ES, Wing SS. The ubiquitin proteasome system in atrophying skeletal muscle: roles and regulation. Am J Physiol Cell Physiol 2016;311(3):C392–403. Epub 2016/08/12. doi: 10.1152/ajpcell.00125.2016. PubMed PMID: 27510905. [DOI] [PubMed] [Google Scholar]
- 18.Rom O, Reznick AZ. The role of E3 ubiquitin-ligases MuRF-1 and MAFbx in loss of skeletal muscle mass. Free Radic Biol Med 2016;98:218–30. Epub 2016/01/08. doi: 10.1016/j.freeradbiomed.2015.12.031. PubMed PMID: 26738803. [DOI] [PubMed] [Google Scholar]
- 19.Lokireddy S, Wijesoma IW, Sze SK, McFarlane C, Kambadur R, Sharma M. Identification of atrogin-1-targeted proteins during the myostatin-induced skeletal muscle wasting. Am J Physiol Cell Physiol 2012;303(5):C512–29. Epub 2012/06/08. doi: 10.1152/ajpcell.00402.2011. PubMed PMID: 22673621. [DOI] [PubMed] [Google Scholar]
- 20.Cohen S, Brault JJ, Gygi SP, Glass DJ, Valenzuela DM, Gartner C, et al. During muscle atrophy, thick, but not thin, filament components are degraded by MuRF1-dependent ubiquitylation. J Cell Biol 2009;185(6):1083–95. Epub 2009/06/10. doi: 10.1083/jcb.200901052. PubMed PMID: 19506036; PubMed Central PMCID: PMCPMC2711608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tidball JG, Spencer MJ. Expression of a calpastatin transgene slows muscle wasting and obviates changes in myosin isoform expression during murine muscle disuse. J Physiol 2002;545(Pt 3):819–28. Epub 2002/12/17. PubMed PMID: 12482888; PubMed Central PMCID: PMCPMC2290726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Talbert EE, Smuder AJ, Min K, Kwon OS, Powers SK. Calpain and caspase-3 play required roles in immobilization-induced limb muscle atrophy. J Appl Physiol (1985). 2013;114(10):1482–9. Epub 2013/03/09. doi: 10.1152/japplphysiol.00925.2012. PubMed PMID: 23471945. [DOI] [PubMed] [Google Scholar]
- 23.Adhihetty PJ, Ljubicic V, Menzies KJ, Hood DA. Differential susceptibility of subsarcolemmal and intermyofibrillar mitochondria to apoptotic stimuli. Am J Physiol Cell Physiol 2005;289(4):C994–C1001. Epub 2005/05/20. doi: 10.1152/ajpcell.00031.2005. PubMed PMID: 15901602. [DOI] [PubMed] [Google Scholar]
- 24.Smuder AJ, Sollanek KJ, Nelson WB, Min K, Talbert EE, Kavazis AN, et al. Crosstalk between autophagy and oxidative stress regulates proteolysis in the diaphragm during mechanical ventilation. Free Radic Biol Med 2018;115:179–90. Epub 2017/12/05. doi: 10.1016/j.freeradbiomed.2017.11.025. PubMed PMID: 29197632; PubMed Central PMCID: PMCPMC5767544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gomes MD, Lecker SH, Jagoe RT, Navon A, Goldberg AL. Atrogin-1, a muscle-specific F-box protein highly expressed during muscle atrophy. Proc Natl Acad Sci U S A. 2001;98(25):14440–5. Epub 2001/11/22. doi: 10.1073/pnas.251541198. PubMed PMID: 11717410; PubMed Central PMCID: PMCPMC64700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, et al. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117(3):399–412. Epub 2004/04/28. PubMed PMID: 15109499; PubMed Central PMCID: PMCPMC3619734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mammucari C, Milan G, Romanello V, Masiero E, Rudolf R, Del Piccolo P, et al. FoxO3 controls autophagy in skeletal muscle in vivo. Cell metabolism. 2007;6(6):458–71. Epub 2007/12/07. doi: 10.1016/j.cmet.2007.11.001. PubMed PMID: 18054315. [DOI] [PubMed] [Google Scholar]
- 28.Wall BT, Dirks ML, van Loon LJ. Skeletal muscle atrophy during short-term disuse: implications for age-related sarcopenia. Ageing research reviews. 2013;12(4):898–906. Epub 2013/08/21. doi: 10.1016/j.arr.2013.07.003. PubMed PMID: 23948422. [DOI] [PubMed] [Google Scholar]
- 29.Shanely RA, Van Gammeren D, Deruisseau KC, Zergeroglu AM, McKenzie MJ, Yarasheski KE, et al. Mechanical ventilation depresses protein synthesis in the rat diaphragm. Am J Respir Crit Care Med 2004;170(9):994–9. Epub 2004/08/07. doi: 10.1164/rccm.200304-575OC. PubMed PMID: 15297271. [DOI] [PubMed] [Google Scholar]
- 30.Loughna PT, Goldspink DF, Goldspink G. Effects of hypokinesia and hypodynamia upon protein turnover in hindlimb muscles of the rat. Aviat Space Environ Med 1987;58(9 Pt 2):A133–8. Epub 1987/09/01. PubMed PMID: 3675479. [PubMed] [Google Scholar]
- 31.Sandri M. Protein breakdown in muscle wasting: role of autophagy-lysosome and ubiquitin-proteasome. Int J Biochem Cell Biol 2013;45(10):2121–9. Epub 2013/05/15. doi: 10.1016/j.biocel.2013.04.023. PubMed PMID: 23665154; PubMed Central PMCID: PMCPMC3775123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bodine SC, Latres E, Baumhueter S, Lai VK, Nunez L, Clarke BA, et al. Identification of ubiquitin ligases required for skeletal muscle atrophy. Science. 2001;294(5547):1704–8. Epub 2001/10/27. doi: 10.1126/science.1065874. PubMed PMID: 11679633. [DOI] [PubMed] [Google Scholar]
- 33.Reid MB, Judge AR, Bodine SC. CrossTalk opposing view: The dominant mechanism causing disuse muscle atrophy is proteolysis. J Physiol 2014;592(24):5345–7. Epub 2014/12/17. doi: 10.1113/jphysiol.2014.279406. PubMed PMID: 25512436; PubMed Central PMCID: PMCPMC4270496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yajid F, Mercier JG, Mercier BM, Dubouchaud H, Prefaut C. Effects of 4 wk of hindlimb suspension on skeletal muscle mitochondrial respiration in rats. J Appl Physiol (1985). 1998;84(2):479–85. Epub 1998/02/26. doi: 10.1152/jappl.1998.84.2.479. PubMed PMID: 9475856. [DOI] [PubMed] [Google Scholar]
- 35.Kavazis AN, Talbert EE, Smuder AJ, Hudson MB, Nelson WB, Powers SK. Mechanical ventilation induces diaphragmatic mitochondrial dysfunction and increased oxidant production. Free Radic Biol Med 2009;46(6):842–50. Epub 2009/02/03. doi: 10.1016/j.freeradbiomed.2009.01.002. PubMed PMID: 19185055; PubMed Central PMCID: PMCPMC2906125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Muller FL, Song W, Jang YC, Liu Y, Sabia M, Richardson A, et al. Denervation-induced skeletal muscle atrophy is associated with increased mitochondrial ROS production. Am J Physiol Regul Integr Comp Physiol 2007;293(3):R1159–68. Epub 2007/06/23. doi: 10.1152/ajpregu.00767.2006. PubMed PMID: 17584954. [DOI] [PubMed] [Google Scholar]
- 37.Picard M, Jung B, Liang F, Azuelos I, Hussain S, Goldberg P, et al. Mitochondrial dysfunction and lipid accumulation in the human diaphragm during mechanical ventilation. Am J Respir Crit Care Med 2012;186(11):1140–9. Epub 2012/10/02. doi: 10.1164/rccm.201206-0982OC. PubMed PMID: 23024021. [DOI] [PubMed] [Google Scholar]
- 38.Abadi A, Glover EI, Isfort RJ, Raha S, Safdar A, Yasuda N, et al. Limb immobilization induces a coordinate down-regulation of mitochondrial and other metabolic pathways in men and women. PLoS One. 2009;4(8):e6518. Epub 2009/08/06. doi: 10.1371/journal.pone.0006518. PubMed PMID: 19654872; PubMed Central PMCID: PMCPMC2716517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Picard M, Azuelos I, Jung B, Giordano C, Matecki S, Hussain S, et al. Mechanical ventilation triggers abnormal mitochondrial dynamics and morphology in the diaphragm. J Appl Physiol (1985). 2015;118(9):1161–71. Epub 2015/03/15. doi: 10.1152/japplphysiol.00873.2014. PubMed PMID: 25767033. [DOI] [PubMed] [Google Scholar]
- 40.Benda C. Ueber die spermatogenese der vertebraten und hoherer evertebraten, II. Theil: Die histiogenese der spermien. Arch Anat Physiol 1898;73:393–8. [Google Scholar]
- 41.Bakeeva LE, Chentsov Yu S, Skulachev VP. Mitochondrial framework (reticulum mitochondriale) in rat diaphragm muscle. Biochim Biophys Acta. 1978;501(3):349–69. Epub 1978/03/13. PubMed PMID: 629958. [DOI] [PubMed] [Google Scholar]
- 42.Glancy B, Hartnell LM, Malide D, Yu ZX, Combs CA, Connelly PS, et al. Mitochondrial reticulum for cellular energy distribution in muscle. Nature. 2015;523(7562):617–20. doi: 10.1038/nature14614. PubMed PMID: 26223627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rambold AS, Kostelecky B, Elia N, Lippincott-Schwartz J. Tubular network formation protects mitochondria from autophagosomal degradation during nutrient starvation. Proc Natl Acad Sci U S A. 2011;108(25):10190–5. Epub 2011/06/08. doi: 10.1073/pnas.1107402108. PubMed PMID: 21646527; PubMed Central PMCID: PMCPMC3121813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Youle RJ, van der Bliek AM. Mitochondrial fission, fusion, and stress. Science. 2012;337(6098):1062–5. Epub 2012/09/01. doi: 10.1126/science.1219855. PubMed PMID: 22936770; PubMed Central PMCID: PMCPMC4762028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Romanello V, Guadagnin E, Gomes L, Roder I, Sandri C, Petersen Y, et al. Mitochondrial fission and remodelling contributes to muscle atrophy. EMBO J. 2010;29(10):1774–85. doi: 10.1038/emboj.2010.60. PubMed PMID: 20400940; PubMed Central PMCID: PMCPMC2876965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kang C, Yeo D, Ji LL. Muscle immobilization activates mitophagy and disrupts mitochondrial dynamics in mice. Acta physiologica (Oxford, England). 2016;218(3):188–97. Epub 2016/04/17. doi: 10.1111/apha.12690. PubMed PMID: 27083499. [DOI] [PubMed] [Google Scholar]
- 47.Cipolat S, Martins de Brito O, Dal Zilio B, Scorrano L. OPA1 requires mitofusin 1 to promote mitochondrial fusion. Proc Natl Acad Sci U S A. 2004;101(45):15927–32. Epub 2004/10/29. doi: 10.1073/pnas.0407043101. PubMed PMID: 15509649; PubMed Central PMCID: PMCPMC528769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.de Brito OM, Scorrano L. Mitofusin-2 regulates mitochondrial and endoplasmic reticulum morphology and tethering: the role of Ras. Mitochondrion. 2009;9(3):222–6. Epub 2009/03/10. doi: 10.1016/j.mito.2009.02.005. PubMed PMID: 19269351. [DOI] [PubMed] [Google Scholar]
- 49.Eisner V, Csordas G, Hajnoczky G. Interactions between sarco-endoplasmic reticulum and mitochondria in cardiac and skeletal muscle - pivotal roles in Ca(2)(+) and reactive oxygen species signaling. J Cell Sci 2013;126(Pt 14):2965–78. Epub 2013/07/12. doi: 10.1242/jcs.093609. PubMed PMID: 23843617; PubMed Central PMCID: PMCPMC3711195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Glauser L, Sonnay S, Stafa K, Moore DJ. Parkin promotes the ubiquitination and degradation of the mitochondrial fusion factor mitofusin 1. J Neurochem 2011;118(4):636–45. Epub 2011/05/28. doi: 10.1111/j.1471-4159.2011.07318.x. PubMed PMID: 21615408. [DOI] [PubMed] [Google Scholar]
- 51.Gegg ME, Cooper JM, Chau KY, Rojo M, Schapira AH, Taanman JW. Mitofusin 1 and mitofusin 2 are ubiquitinated in a PINK1/parkin-dependent manner upon induction of mitophagy. Human molecular genetics. 2010;19(24):4861–70. Epub 2010/09/28. doi: 10.1093/hmg/ddq419. PubMed PMID: 20871098; PubMed Central PMCID: PMCPMC3583518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Alavi MV, Fuhrmann N. Dominant optic atrophy, OPA1, and mitochondrial quality control: understanding mitochondrial network dynamics. Mol Neurodegener. 2013;8:32. Epub 2013/09/27. doi: 10.1186/1750-1326-8-32. PubMed PMID: 24067127; PubMed Central PMCID: PMCPMC3856479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chang CR, Blackstone C. Dynamic regulation of mitochondrial fission through modification of the dynamin-related protein Drp1. Ann N Y Acad Sci 2010;1201:34–9. Epub 2010/07/24. doi: 10.1111/j.1749-6632.2010.05629.x. PubMed PMID: 20649536; PubMed Central PMCID: PMCPMC5585781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chou CH, Lin CC, Yang MC, Wei CC, Liao HD, Lin RC, et al. GSK3beta-mediated Drp1 phosphorylation induced elongated mitochondrial morphology against oxidative stress. PLoS One. 2012;7(11):e49112. Epub 2012/11/28. doi: 10.1371/journal.pone.0049112. PubMed PMID: 23185298; PubMed Central PMCID: PMCPMC3502545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chang CR, Blackstone C. Cyclic AMP-dependent protein kinase phosphorylation of Drp1 regulates its GTPase activity and mitochondrial morphology. J Biol Chem 2007;282(30):21583–7. Epub 2007/06/08. doi: 10.1074/jbc.C700083200. PubMed PMID: 17553808. [DOI] [PubMed] [Google Scholar]
- 56.Taguchi N, Ishihara N, Jofuku A, Oka T, Mihara K. Mitotic phosphorylation of dynamin-related GTPase Drp1 participates in mitochondrial fission. J Biol Chem 2007;282(15):11521–9. Epub 2007/02/16. doi: 10.1074/jbc.M607279200. PubMed PMID: 17301055. [DOI] [PubMed] [Google Scholar]
- 57.Mozdy AD, McCaffery JM, Shaw JM. Dnm1p GTPase-mediated mitochondrial fission is a multistep process requiring the novel integral membrane component Fis1p. J Cell Biol 2000;151(2):367–80. Epub 2000/10/19. PubMed PMID: 11038183; PubMed Central PMCID: PMCPMC2192649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Frohlich C, Grabiger S, Schwefel D, Faelber K, Rosenbaum E, Mears J, et al. Structural insights into oligomerization and mitochondrial remodelling of dynamin 1-like protein. EMBO J. 2013;32(9):1280–92. Epub 2013/04/16. doi: 10.1038/emboj.2013.74. PubMed PMID: 23584531; PubMed Central PMCID: PMCPMC3642683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tilokani L, Nagashima S, Paupe V, Prudent J. Mitochondrial dynamics: overview of molecular mechanisms. Essays in biochemistry. 2018;62(3):341–60. Epub 2018/07/22. doi: 10.1042/EBC20170104. PubMed PMID: 30030364; PubMed Central PMCID: PMCPMC6056715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cannavino J, Brocca L, Sandri M, Grassi B, Bottinelli R, Pellegrino MA. The role of alterations in mitochondrial dynamics and PGC-1alpha over-expression in fast muscle atrophy following hindlimb unloading. J Physiol 2015;593(8):1981–95. Epub 2015/01/08. doi: 10.1113/jphysiol.2014.286740. PubMed PMID: 25565653; PubMed Central PMCID: PMCPMC4405755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.O'Leary MF, Vainshtein A, Carter HN, Zhang Y, Hood DA. Denervation-induced mitochondrial dysfunction and autophagy in skeletal muscle of apoptosis-deficient animals. Am J Physiol Cell Physiol 2012;303(4):C447–54. Epub 2012/06/08. doi: 10.1152/ajpcell.00451.2011. PubMed PMID: 22673615. [DOI] [PubMed] [Google Scholar]
- 62.Chen YW, Gregory CM, Scarborough MT, Shi R, Walter GA, Vandenborne K. Transcriptional pathways associated with skeletal muscle disuse atrophy in humans. Physiological genomics. 2007;31(3):510–20. Epub 2007/09/07. doi: 10.1152/physiolgenomics.00115.2006. PubMed PMID: 17804603. [DOI] [PubMed] [Google Scholar]
- 63.Brocca L, Pellegrino MA, Desaphy JF, Pierno S, Camerino DC, Bottinelli R. Is oxidative stress a cause or consequence of disuse muscle atrophy in mice? A proteomic approach in hindlimb-unloaded mice. Experimental physiology. 2010;95(2):331–50. Epub 2009/10/13. doi: 10.1113/expphysiol.2009.050245. PubMed PMID: 19819934. [DOI] [PubMed] [Google Scholar]
- 64.Calvo SE, Clauser KR, Mootha VK. MitoCarta2.0: an updated inventory of mammalian mitochondrial proteins. Nucleic Acids Res 2016;44(D1):D1251–7. Epub 2015/10/10. doi: 10.1093/nar/gkv1003. PubMed PMID: 26450961; PubMed Central PMCID: PMCPMC4702768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kang C, Ji LL. Muscle immobilization and remobilization downregulates PGC-1alpha signaling and the mitochondrial biogenesis pathway. J Appl Physiol (1985). 2013;115(11):1618–25. Epub 2013/08/24. doi: 10.1152/japplphysiol.01354.2012. PubMed PMID: 23970536. [DOI] [PubMed] [Google Scholar]
- 66.Zechner C, Lai L, Zechner JF, Geng T, Yan Z, Rumsey JW, et al. Total skeletal muscle PGC-1 deficiency uncouples mitochondrial derangements from fiber type determination and insulin sensitivity. Cell metabolism. 2010;12(6):633–42. Epub 2010/11/27. doi: 10.1016/j.cmet.2010.11.008. PubMed PMID: 21109195; PubMed Central PMCID: PMCPMC2999961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kang C, Goodman CA, Hornberger TA, Ji LL. PGC-1alpha overexpression by in vivo transfection attenuates mitochondrial deterioration of skeletal muscle caused by immobilization. FASEB J. 2015;29(10):4092–106. Epub 2015/07/17. doi: 10.1096/fj.14-266619. PubMed PMID: 26178167; PubMed Central PMCID: PMCPMC4566942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cannavino J, Brocca L, Sandri M, Bottinelli R, Pellegrino MA. PGC1-alpha over-expression prevents metabolic alterations and soleus muscle atrophy in hindlimb unloaded mice. J Physiol 2014;592(20):4575–89. Epub 2014/08/17. doi: 10.1113/jphysiol.2014.275545. PubMed PMID: 25128574; PubMed Central PMCID: PMCPMC4287741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Vainshtein A, Desjardins EM, Armani A, Sandri M, Hood DA. PGC-1alpha modulates denervation-induced mitophagy in skeletal muscle. Skelet Muscle. 2015;5:9. Epub 2015/04/04. doi: 10.1186/s13395-015-0033-y. PubMed PMID: 25834726; PubMed Central PMCID: PMCPMC4381453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chan MC, Arany Z. The many roles of PGC-1alpha in muscle--recent developments. Metabolism. 2014;63(4):441–51. Epub 2014/02/25. doi: 10.1016/j.metabol.2014.01.006. PubMed PMID: 24559845; PubMed Central PMCID: PMCPMC4040247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Adhihetty PJ, O'Leary MF, Chabi B, Wicks KL, Hood DA. Effect of denervation on mitochondrially mediated apoptosis in skeletal muscle. J Appl Physiol (1985). 2007;102(3):1143–51. Epub 2006/11/24. doi: 10.1152/japplphysiol.00768.2006. PubMed PMID: 17122379. [DOI] [PubMed] [Google Scholar]
- 72.Theilen NT, Kunkel GH, Tyagi SC. The Role of Exercise and TFAM in Preventing Skeletal Muscle Atrophy. J Cell Physiol 2017;232(9):2348–58. Epub 2016/12/15. doi: 10.1002/jcp.25737. PubMed PMID: 27966783; PubMed Central PMCID: PMCPMC5444986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Larsson NG, Wang J, Wilhelmsson H, Oldfors A, Rustin P, Lewandoski M, et al. Mitochondrial transcription factor A is necessary for mtDNA maintenance and embryogenesis in mice. Nature genetics. 1998;18(3):231–6. Epub 1998/03/21. doi: 10.1038/ng0398-231. PubMed PMID: 9500544. [DOI] [PubMed] [Google Scholar]
- 74.Tryon LD, Crilly MJ, Hood DA. Effect of denervation on the regulation of mitochondrial transcription factor A expression in skeletal muscle. Am J Physiol Cell Physiol 2015;309(4):C228–38. Epub 2015/06/13. doi: 10.1152/ajpcell.00266.2014. PubMed PMID: 26063705; PubMed Central PMCID: PMCPMC4537931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Schmidt O, Pfanner N, Meisinger C. Mitochondrial protein import: from proteomics to functional mechanisms. Nature reviews. 2010;11(9):655–67. Epub 2010/08/24. doi: 10.1038/nrm2959. PubMed PMID: 20729931. [DOI] [PubMed] [Google Scholar]
- 76.Singh K, Hood DA. Effect of denervation-induced muscle disuse on mitochondrial protein import. Am J Physiol Cell Physiol 2011;300(1):C138–45. Epub 2010/10/15. doi: 10.1152/ajpcell.00181.2010. PubMed PMID: 20943961. [DOI] [PubMed] [Google Scholar]
- 77.Birk AV, Chao WM, Bracken C, Warren JD, Szeto HH. Targeting mitochondrial cardiolipin and the cytochrome c/cardiolipin complex to promote electron transport and optimize mitochondrial ATP synthesis. Br J Pharmacol 2014;171(8):2017–28. Epub 2013/10/19. doi: 10.1111/bph.12468. PubMed PMID: 24134698; PubMed Central PMCID: PMCPMC3976619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ostojic O, O'Leary MF, Singh K, Menzies KJ, Vainshtein A, Hood DA. The effects of chronic muscle use and disuse on cardiolipin metabolism. J Appl Physiol (1985). 2013;114(4):444–52. Epub 2012/12/12. doi: 10.1152/japplphysiol.01312.2012. PubMed PMID: 23221956. [DOI] [PubMed] [Google Scholar]
- 79.Fajardo VA, Mikhaeil JS, Leveille CF, Saint C, LeBlanc PJ. Cardiolipin content, linoleic acid composition, and tafazzin expression in response to skeletal muscle overload and unload stimuli. Sci Rep 2017;7(1):2060. Epub 2017/05/19. doi: 10.1038/s41598-017-02089-1. PubMed PMID: 28515468; PubMed Central PMCID: PMCPMC5435726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Dudek J. Role of Cardiolipin in Mitochondrial Signaling Pathways. Front Cell Dev Biol 2017;5:90. Epub 2017/10/17. doi: 10.3389/fcell.2017.00090. PubMed PMID: 29034233; PubMed Central PMCID: PMCPMC5626828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Acehan D, Vaz F, Houtkooper RH, James J, Moore V, Tokunaga C, et al. Cardiac and skeletal muscle defects in a mouse model of human Barth syndrome. J Biol Chem 2011;286(2):899–908. Epub 2010/11/12. doi: 10.1074/jbc.M110.171439. PubMed PMID: 21068380; PubMed Central PMCID: PMCPMC3020775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Yamaoka S, Urade R, Kito M. Mitochondrial function in rats is affected by modification of membrane phospholipids with dietary sardine oil. J Nutr 1988;118(3):290–6. Epub 1988/03/01. doi: 10.1093/jn/118.3.290. PubMed PMID: 3351630. [DOI] [PubMed] [Google Scholar]
- 83.Saric A, Andreau K, Armand AS, Moller IM, Petit PX. Barth Syndrome: From Mitochondrial Dysfunctions Associated with Aberrant Production of Reactive Oxygen Species to Pluripotent Stem Cell Studies. Front Genet 2015;6:359. Epub 2016/02/03. doi: 10.3389/fgene.2015.00359. PubMed PMID: 26834781; PubMed Central PMCID: PMCPMC4719219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kohn RR. Denervation Muscle Atrophy: An Autolytic System in Vitro. Am J Pathol 1965;47:315–23. Epub 1965/08/01. PubMed PMID: 14333520; PubMed Central PMCID: PMCPMC1920431. [PMC free article] [PubMed] [Google Scholar]
- 85.Carafoli E, Margreth A, Buffa P. Early Biochemical Changes in Mitochondria from Denervated Muscle and Their Relation to the Onset of Atrophy. Exp Mol Pathol 1964;3:171–81. Epub 1964/04/01. PubMed PMID: 14165610. [DOI] [PubMed] [Google Scholar]
- 86.Talbert EE, Smuder AJ, Min K, Kwon OS, Szeto HH, Powers SK. Immobilization-induced activation of key proteolytic systems in skeletal muscles is prevented by a mitochondria-targeted antioxidant. J Appl Physiol (1985). 2013;115(4):529–38. Epub 2013/06/15. doi: 10.1152/japplphysiol.00471.2013. PubMed PMID: 23766499. [DOI] [PubMed] [Google Scholar]
- 87.Powers SK, Smuder AJ, Judge AR. Oxidative stress and disuse muscle atrophy: cause or consequence? Curr Opin Clin Nutr Metab Care. 2012;15(3):240–5. Epub 2012/04/03. doi: 10.1097/MCO.0b013e328352b4c2. PubMed PMID: 22466926; PubMed Central PMCID: PMCPMC3893113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Powers SK, Morton AB, Ahn B, Smuder AJ. Redox control of skeletal muscle atrophy. Free Radic Biol Med 2016;98:208–17. doi: 10.1016/j.freeradbiomed.2016.02.021. PubMed PMID: 26912035; PubMed Central PMCID: PMCPMC5006677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Shenton D, Smirnova JB, Selley JN, Carroll K, Hubbard SJ, Pavitt GD, et al. Global translational responses to oxidative stress impact upon multiple levels of protein synthesis. J Biol Chem 2006;281(39):29011–21. Epub 2006/07/20. doi: 10.1074/jbc.M601545200. PubMed PMID: 16849329. [DOI] [PubMed] [Google Scholar]
- 90.Fan X, Hussien R, Brooks GA. H2O2-induced mitochondrial fragmentation in C2C12 myocytes. Free Radic Biol Med 2010;49(11):1646–54. Epub 2010/08/31. doi: 10.1016/j.freeradbiomed.2010.08.024. PubMed PMID: 20801212; PubMed Central PMCID: PMCPMC2970628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Appell HJ, Duarte JA, Soares JM. Supplementation of vitamin E may attenuate skeletal muscle immobilization atrophy. Int J Sports Med 1997;18(3):157–60. Epub 1997/04/01. PubMed PMID: 9187967. [DOI] [PubMed] [Google Scholar]
- 92.McClung JM, Whidden MA, Kavazis AN, Falk DJ, Deruisseau KC, Powers SK. Redox regulation of diaphragm proteolysis during mechanical ventilation. Am J Physiol Regul Integr Comp Physiol 2008;294(5):R1608–17. Epub 2008/03/07. doi: 10.1152/ajpregu.00044.2008. PubMed PMID: 18321950. [DOI] [PubMed] [Google Scholar]
- 93.Shibaguchi T, Yamaguchi Y, Miyaji N, Yoshihara T, Naito H, Goto K, et al. Astaxanthin intake attenuates muscle atrophy caused by immobilization in rats. Physiol Rep 2016;4(15). Epub 2016/08/03. doi: 10.14814/phy2.12885. PubMed PMID: 27482075; PubMed Central PMCID: PMCPMC4985550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Birk AV, Liu S, Soong Y, Mills W, Singh P, Warren JD, et al. The mitochondrial-targeted compound SS-31 re-energizes ischemic mitochondria by interacting with cardiolipin. J Am Soc Nephrol 2013;24(8):1250–61. Epub 2013/07/03. doi: 10.1681/ASN.2012121216. PubMed PMID: 23813215; PubMed Central PMCID: PMCPMC3736700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.McCormack JG, Halestrap AP, Denton RM. Role of calcium ions in regulation of mammalian intramitochondrial metabolism. Physiol Rev 1990;70(2):391–425. Epub 1990/04/01. doi: 10.1152/physrev.1990.70.2.391. PubMed PMID: 2157230. [DOI] [PubMed] [Google Scholar]
- 96.Glancy B, Willis WT, Chess DJ, Balaban RS. Effect of calcium on the oxidative phosphorylation cascade in skeletal muscle mitochondria. Biochemistry. 2013;52(16):2793–809. Epub 2013/04/04. doi: 10.1021/bi3015983. PubMed PMID: 23547908; PubMed Central PMCID: PMCPMC4157357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Brookes PS, Yoon Y, Robotham JL, Anders MW, Sheu SS. Calcium, ATP, and ROS: a mitochondrial love-hate triangle. Am J Physiol Cell Physiol 2004;287(4):C817–33. Epub 2004/09/10. doi: 10.1152/ajpcell.00139.2004. PubMed PMID: 15355853. [DOI] [PubMed] [Google Scholar]
- 98.Kowaltowski AJ, de Souza-Pinto NC, Castilho RF, Vercesi AE. Mitochondria and reactive oxygen species. Free Radic Biol Med 2009;47(4):333–43. Epub 2009/05/12. doi: 10.1016/j.freeradbiomed.2009.05.004. PubMed PMID: 19427899. [DOI] [PubMed] [Google Scholar]
- 99.Brand MD, Affourtit C, Esteves TC, Green K, Lambert AJ, Miwa S, et al. Mitochondrial superoxide: production, biological effects, and activation of uncoupling proteins. Free Radic Biol Med 2004;37(6):755–67. Epub 2004/08/12. doi: 10.1016/j.freeradbiomed.2004.05.034. PubMed PMID: 15304252. [DOI] [PubMed] [Google Scholar]
- 100.Matecki S, Dridi H, Jung B, Saint N, Reiken SR, Scheuermann V, et al. Leaky ryanodine receptors contribute to diaphragmatic weakness during mechanical ventilation. Proc Natl Acad Sci U S A. 2016;113(32):9069–74. doi: 10.1073/pnas.1609707113. PubMed PMID: 27457930; PubMed Central PMCID: PMCPMC4987795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Mammucari C, Gherardi G, Zamparo I, Raffaello A, Boncompagni S, Chemello F, et al. The mitochondrial calcium uniporter controls skeletal muscle trophism in vivo. Cell Rep 2015;10(8):1269–79. Epub 2015/03/04. doi: 10.1016/j.celrep.2015.01.056. PubMed PMID: 25732818; PubMed Central PMCID: PMCPMC4351162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Karam C, Yi J, Xiao Y, Dhakal K, Zhang L, Li X, et al. Absence of physiological Ca(2+) transients is an initial trigger for mitochondrial dysfunction in skeletal muscle following denervation. Skelet Muscle. 2017;7(1):6. Epub 2017/04/12. doi: 10.1186/s13395-017-0123-0. PubMed PMID: 28395670; PubMed Central PMCID: PMCPMC5387329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Yang R, Rincon M. Mitochondrial Stat3, the Need for Design Thinking. Int J Biol Sci 2016;12(5):532–44. Epub 2016/03/29. doi: 10.7150/ijbs.15153. PubMed PMID: 27019635; PubMed Central PMCID: PMCPMC4807418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Smith IJ, Godinez GL, Singh BK, McCaughey KM, Alcantara RR, Gururaja T, et al. Inhibition of Janus kinase signaling during controlled mechanical ventilation prevents ventilation-induced diaphragm dysfunction. FASEB J. 2014;28(7):2790–803. doi: 10.1096/fj.13-244210. PubMed PMID: 24671708; PubMed Central PMCID: PMCPMC4062832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Salah H, Fury W, Gromada J, Bai Y, Tchkonia T, Kirkland JL, et al. Muscle-specific differences in expression and phosphorylation of the Janus kinase 2/Signal Transducer and Activator of Transcription 3 following long-term mechanical ventilation and immobilization in rats. Acta physiologica (Oxford, England). 2018;222(3). Epub 2017/10/17. doi: 10.1111/apha.12980. PubMed PMID: 29032602. [DOI] [PubMed] [Google Scholar]
- 106.Ferreira LF, Laitano O. Regulation of NADPH oxidases in skeletal muscle. Free Radic Biol Med 2016;98:18–28. Epub 2016/05/18. doi: 10.1016/j.freeradbiomed.2016.05.011. PubMed PMID: 27184955; PubMed Central PMCID: PMCPMC4975970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kowaltowski AJ, Vercesi AE. Mitochondrial damage induced by conditions of oxidative stress. Free Radic Biol Med 1999;26(3–4):463–71. Epub 1999/01/23. PubMed PMID: 9895239. [DOI] [PubMed] [Google Scholar]
- 108.McClung JM, Van Gammeren D, Whidden MA, Falk DJ, Kavazis AN, Hudson MB, et al. Apocynin attenuates diaphragm oxidative stress and protease activation during prolonged mechanical ventilation. Crit Care Med 2009;37(4):1373–9. Epub 2009/02/27. doi: 10.1097/CCM.0b013e31819cef63. PubMed PMID: 19242334; PubMed Central PMCID: PMCPMC2909189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Halestrap AP, Richardson AP. The mitochondrial permeability transition: a current perspective on its identity and role in ischaemia/reperfusion injury. J Mol Cell Cardiol 2015;78:129–41. Epub 2014/09/03. doi: 10.1016/j.yjmcc.2014.08.018. PubMed PMID: 25179911. [DOI] [PubMed] [Google Scholar]
- 110.Csukly K, Ascah A, Matas J, Gardiner PF, Fontaine E, Burelle Y. Muscle denervation promotes opening of the permeability transition pore and increases the expression of cyclophilin D. J Physiol 2006;574(Pt 1):319–27. Epub 2006/05/06. doi: 10.1113/jphysiol.2006.109702. PubMed PMID: 16675492; PubMed Central PMCID: PMCPMC1817793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Tong JF, Yan X, Zhu MJ, Du M. AMP-activated protein kinase enhances the expression of muscle-specific ubiquitin ligases despite its activation of IGF-1/Akt signaling in C2C12 myotubes. Journal of cellular biochemistry. 2009;108(2):458–68. Epub 2009/07/30. doi: 10.1002/jcb.22272. PubMed PMID: 19639604. [DOI] [PubMed] [Google Scholar]
- 112.Smuder AJ, Sollanek KJ, Min K, Nelson WB, Powers SK. Inhibition of forkhead boxO-specific transcription prevents mechanical ventilation-induced diaphragm dysfunction. Crit Care Med 2015;43(5):e133–42. doi: 10.1097/CCM.0000000000000928. PubMed PMID: 25746508; PubMed Central PMCID: PMCPMC4400222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Senf SM, Dodd SL, Judge AR. FOXO signaling is required for disuse muscle atrophy and is directly regulated by Hsp70. Am J Physiol Cell Physiol 2010;298(1):C38–45. Epub 2009/10/30. doi: 10.1152/ajpcell.00315.2009. PubMed PMID: 19864323; PubMed Central PMCID: PMCPMC2806148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Sanchez AM, Csibi A, Raibon A, Cornille K, Gay S, Bernardi H, et al. AMPK promotes skeletal muscle autophagy through activation of forkhead FoxO3a and interaction with Ulk1. Journal of cellular biochemistry. 2012;113(2):695–710. Epub 2011/10/19. doi: 10.1002/jcb.23399. PubMed PMID: 22006269. [DOI] [PubMed] [Google Scholar]
- 115.Egawa T, Goto A, Ohno Y, Yokoyama S, Ikuta A, Suzuki M, et al. Involvement of AMPK in regulating slow-twitch muscle atrophy during hindlimb unloading in mice. Am J Physiol Endocrinol Metab 2015;309(7):E651–62. Epub 2015/08/06. doi: 10.1152/ajpendo.00165.2015. PubMed PMID: 26244519. [DOI] [PubMed] [Google Scholar]