Abstract
Purpose:
Children with epilepsy in low-income countries often go undiagnosed and untreated. We examine a portable, low-cost smartphone-based EEG technology in a heterogeneous pediatric epilepsy cohort in the West African Republic of Guinea.
Methods:
Children with epilepsy were recruited at the Ignace Deen Hospital in Conakry, 2017. Participants underwent sequential EEG recordings with an app-based EEG, the Smartphone Brain Scanner-2 (SBS2) and a standard Xltek EEG. Raw EEG data were transmitted via Bluetooth™ connection to an Android™ tablet and uploaded for remote EEG specialist review and reporting via a new, secure web-based reading platform, crowdEEG. The results were compared to same-visit Xltek 10–20 EEG recordings for identification of epileptiform and non-epileptiform abnormalities.
Results:
97 children meeting the International League Against Epilepsy’s definition of epilepsy (49 male; mean age 10.3 years, 29 untreated with an antiepileptic drug; 0 with a prior EEG) were enrolled. Epileptiform discharges were detected on 21 (25.3%) SBS2 and 31 (37.3%) standard EEG recordings. The SBS2 had a sensitivity of 51.6% (95%CI 32.4%, 70.8%) and a specificity of 90.4% (95%CI 81.4%, 94.4%) for all types of epileptiform discharges, with positive and negative predictive values of 76.2% and 75.8% respectively. For generalized discharges, the SBS2 had a sensitivity of 43.5% with a specificity of 96.2%.
Conclusions:
The SBS2 has a moderate sensitivity and high specificity for the detection of epileptiform abnormalities in children with epilepsy in this low-income setting. Use of the SBS2+crowdEEG platform permits specialist input for patients with previously poor access to clinical neurophysiology expertise.
Keywords: pediatric epilepsy, EEG, mHealth, Africa
Introduction
There are >40 million people with epilepsy (PWE) living in low-and middle-income countries (LMICs).[1] The incidence of active epilepsy in sub-Saharan Africa is particularly high, especially in children.[2,3] EEG can help classify an epilepsy syndrome and guide medication choice and is one of the basic care metrics set out in the American Academy of Neurology (AAN) epilepsy guidelines.[4] Large disparities exist in the availability of EEG for PWE between high-income countries compared to LMICs.[5–8] Many PWE are unable to access appropriate epilepsy care.[9] Thus, there is an urgent need for reliable, easily accessible, and cost-efficient EEG technologies in LMIC settings.
Digitization of EEG, coupled with technological advances in internet-based health platforms has allowed for digital transfer of neurophysiological signals in a similar fashion to radiological studies, facilitating remote interpretation.[10,11] Prior work by our group has found that the use of this technology is moderately sensitive (39.2%) and highly specific (94.8%) for the presence of epileptiform abnormalities in a heterogeneous group of PWE in Central Asia.[12] In this study, we validate the use of the same smartphone EEG technology in a new population, and focus on children with poorly controlled epilepsy in Sub-Saharan Africa. Utilizing free smartphone technology with compatible pre-placed electrode caps, we test whether this is a practical and reliable way to deliver EEG to a cohort of patients for whom EEG has not been previously available.
Methods
Ethical Approval
Study approval was granted by the ethical review boards of Ignace Deen Hospital (IDH) in the Republic of Guinea and Partners Healthcare in the USA. Approval was granted for the use of the crowdEEG platform from the Partners’ Research & Information Security Office. Parents or next of kin of each pediatric participant provided informed consent.
Participant Recruitment and Data Collection
Recruitment took place in November 2017 at IDH with patients from both rural and urban areas. Up to 100 participants who met the International League Against Epilepsy definition [13,14] of epilepsy were targeted for enrollment. Each participant was reimbursed the equivalent of 12 USD. At enrollment demographic and clinical data were collected via structured questionnaire. EEG data were collected at the same visit.
EEG Data Acquisition and Technology
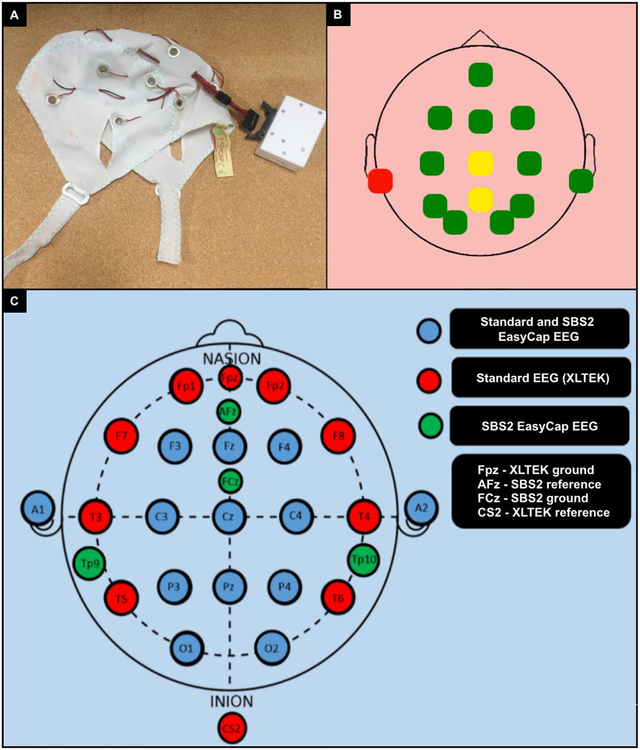
Participants had two sequentially-performed EEGs in no particular order: (1) a standard 21 lead (10–20 system) EEG utilizing Xltek technology performed by a U.S. board-certified EEG technologist, and (2) a Smartphone Brain Scanner-2 (SBS-2) EEG [15] using a 14-lead cap (Figure 1A) manufactured by EasyCap (https://www.easycap.de/wordpress/products/, Germany) and portable 14-lead capacity amplifier administered by African medical students and neurology residents after <1 hour of training. A blunt tip syringe was used to inject gel into the center of the electrode rings. The SBS-2 is an open-source software application for EEG that operates on mobile devices allowing data collection via a wireless connection to a tablet, smartphone, or laptop computer.[15] The EasyCap is a flexible multiple use and multi-channel EEG headset available in a range of head circumference sizes (36–56cm used here), containing ring electrodes aligned to the International 10–20 system. Head circumference of participants was matched to +/−2cm of the nearest available EasyCap size. The EasyCap ring electrodes were F3, C3, P3, O1, F4, C4, P4, O2, Fz, Pz, Fpz, Tp9 and Tp10 (Figure 1B). FCz and Afz served as reference and ground electrodes respectively.
Figure 1.
A. An EasyCap headset. B. Screenshot of SBS2 impedance check demonstrating good (green), moderate (yellow) and poor (red) impedance, respectively. C. Color-coded electrode map of the SBS2 and Xltek EEG.
For the SBS2 and standard Xltek EEG, raw EEG data were obtained at a sampling rate of 128 Hz. The software facilitates several data processing tasks including data acquisition, filtering, artifact removal and recording. It is available under Massachusetts Institute of Technology License and can be run in Windows, OSX, Linux and Android environments. The application is available under CERN Open Hardware License (https://github.com/SmartphoneBrainScanner). Nexus™ 7 (2013) Android tablets were used for data collection. Impedances prior to both recordings were 5 Ohms. For the SBS2-EEGs, an impedance electrode display was available on the Android tablet (Figure 1C). Standard EEG data was analyzed offline utilizing Natus Neuroworks software (Natus Medical Incorporated, Pleasanton, USA).
Web-based Reading Platform: crowdEEG
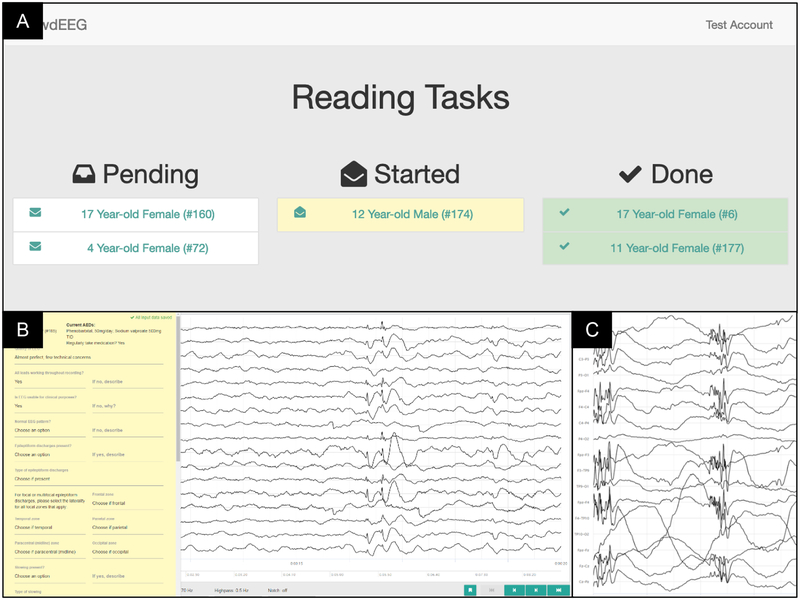
Data obtained through the SBS-2 EEG application were converted to European Data Format (EDF) then sent to the secure web-based platform, specifically designed for EEG reading (http://crowdeeg.ca). The platform allows for the presentation, annotation, and reporting of EEG data by remote international neurophysiologists via a central standardized portal. Each reader received a secure user account for EEG reading assignments. Upon log-in, readers can see their EEG assignments which can be divided into the categories of pending, in-progress, or completed reads. (Figure 2A).
Figure 2.
A. The CrowdEEG platform interface for readers with clear categories as to the stage of each study on their worklist. B. Portion of the standardized report template with drop down menus for readers as well as space for free text on the left-hand side of the screen, with a sample of 1–2Hz generalized slow spike and wave as seen on the recording. C. Screenshot of the EEG recording in a Bipolar Montage with a sample of generalized spike and polyspike and wave discharges.
The reading platform provided standardized montage settings (Common Average Reference, AP Bipolar, Transverse Bipolar, Ear Reference, Pz Reference) and filter options (low pass [70 Hz, 30 Hz, 15 Hz, off], high pass [10 Hz, 3 Hz, 1 Hz, 0.5 Hz, off], notch [60 Hz, 50 Hz, off]). Readers could adjust the gain/sensitivity of individual leads or all leads for the active montage through keyboard hotkeys.
The platform has the ability to facilitate the integration of the specific study report template into the reading platform (Figure 2B), enabling readers to enter observations while reading, and for all changes to be saved automatically to prevent any data loss. Readers could optionally localize features (i.e. interictal epileptiform discharges and seizures) by highlighting areas in the EEG signals.
EEG Interpretation
EEGs were assigned to U.S. and Canadian board-certified neurologists and clinical neurophysiologists for interpretation with the participant’s age and medications. Readers were blinded to other clinical information. Standard EEGs were read by a single EEG reader (JW), the SBS-2 EEGs were randomly assigned via the online reading platform to multiple readers (TF, DBH, ADL, AL, EL, TM, VK, TF, MV). All readers were asked to grade the quality of EEG recordings on a basic quality scale of 0 (worst) to 10 (best) recording (Supplementary Material Table 1). Studies were classified as either normal or abnormal, and when abnormal, the abnormalities were classified as either epileptiform or non-epileptiform. Further descriptions of the abnormalities (i.e. focal versus generalized) were also recorded. Both sets of readers were blinded to the interpretation of the other EEG study and the results from other readers.
Statistical Analysis
Descriptive statistics were calculated for participant age, EEG quality score, and length of recording time. The Xltek EEG was used as a comparative standard to evaluate the performance of the SBS-2 EEGs. The sensitivity, specificity and positive and negative predictive values were calculated for the SBS-2 EEG in relation to the Xltek EEG for all abnormalities: epileptiform and non-epileptiform. 95% confidence intervals were calculated using the normal approximation with continuity correction. Statistical analyses were carried out using Python 2.7.14.
Data availability
Data from this study will be made available to qualified investigators upon request.
Results
Participant demographic characteristics
97 participants were enrolled (49 male) with a mean age of 10.3 years (range 1.5–18 years). Mean age at seizure onset was 4.1 years (unavailable in n=3). Twenty-eight participants did not know the age at which they were diagnosed with epilepsy and, of the remaining 69, the average age at diagnosis was 6.3 years. Of the 82 participants who knew at what age they had first trialed an AED, the mean age of first AED intake was 6.1 years. At the time of enrollment, 29 (29.9%) participants were not on an AED, 64 (66.0%) were on monotherapy, and 4 (4.1%) were on dual therapy. Prescribed drugs were Phenobarbital (37, 38.1%), Valproate (20, 20.6%), Carbamazepine (13, 13.4%), and Clobazam (1, 1%). Nineteen (19.5%) had undergone neuroimaging with a CT scan. One had undergone a brain MRI. None had ever had an EEG. Data on self-reported epilepsy risk factors, seizure-related injuries and AED adherence are given in Supplementary Table 2.
EEG Results
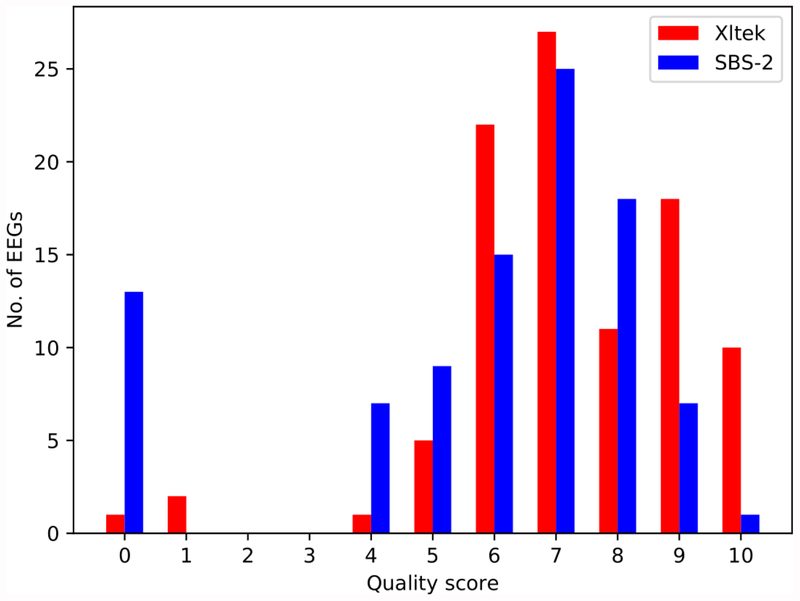
Mean recording time for standard and SBS-2 EEGs was 22.9 and 27.9 minutes respectively (Table 1). The mean and median quality score for the Xltek were 7.2 [standard deviation (SD)=1.9] and 7.0, respectively, versus 5.8 (SD=2.7) and 7.0 for the SBS-2 EEGs (Figure 3). Three Xltek EEGs (3.1%) and 11 (11.3%) SBS-2 EEGs were uninterpretable due to lead, myogenic, and/or sweat artifacts. Two patients had an Xltek EEG but not an SBS-2 EEG thus yielding 83 pairs of studies available for further analysis.
Table 1.
Characteristics of EEG Recordings
| Standard EEG (n=97) | SBS-2 EEG (n=95) | |
|---|---|---|
| Usable for clinical interpretation, n (%) | 96 (98.9) | 83 (87.4) |
| Mean Quality Score, points (Standard Deviation) | 7.2 (1.9) | 5.8 (2.7) |
| Median Quality Score (Interquartile Range) | 7.0 (3.0) | 7.0 (3.0) |
| Mean Recording Time, min (Standard Deviation) | 22.9 (3.8) | 27.9 (7.2) |
| Median Recording Time, min (Interquartile Range) | 22.0 (1.63) | 29.8 (6.0) |
| Number Abnormal (%) | 56 (67.4) | 31 (37.3) |
| Number with epileptiform discharges (%) | 31 (37.3) | 21 (25.3) |
| Number with non-epileptiform abnormalities (%) | 25 (30.1) | 10 (12.0) |
Figure 3.
Histogram of assigned EEG quality scores.
Fifty-six (67.4%) Xltek EEGs were abnormal compared to 31 (37.3%) SBS-2 EEGs (Table 2). The SBS-2 EEG had a sensitivity and specificity for any abnormality of 51.8% (95%CI 37.8%,65.8%) and 92.6% (95% CI, 80.1%, 100%). Epileptiform discharges were detected on 21 (25.3%) SBS-2 and 31 (37.3%) standard EEGs. The SBS-2 had a sensitivity of 51.6% (95% CI, 95% 32.4%, 70.8%) and a specificity of 90.4% (95% CI, 81.4%, 94.4%) for epileptiform discharges with positive and negative predictive values of 76.2% and 75.8% respectively. The Cohen’s kappa (κ) for agreement between the final interpretation of the SBS2 EEG and the standard EEG for abnormal versus normal was κ= 0.358 (95% CI, 0.199, 0.517) and for epileptiform abnormalities was κ=0.449 (95% CI, 0.25%,0.7%) (Table 3).
Table 2.
Interpretations of SBS2 and Standard (Xltek) EEGs.
| SBS2EEG | Cohen’s Kappa | ||||
|---|---|---|---|---|---|
| Abnormal | Normal | Cohen’s Kappa | 95% CI | ||
| Standard EEG | Abnormal | 29 | 27 | 0.358 | (0.199, 0.517) |
| Normal | 2 | 25 | |||
| No epileptiform | Epileptiform | ||||
| No epileptiform | 47 | 5 | 0.449 | (0.251, 0.647) | |
| Epileptiform | 15 | 16 | |||
| No non-epileptiform abnormalities | Non-epileptiform abnormalities | ||||
| No non-epileptiform abnormalities | 54 | 4 | 0.206 | (−0.004, 0.416) | |
| Non-epileptiform abnormalities | 19 | 6 | |||
Table 3.
Sensitivity, specificity, and positive and negative predictive value analyses for SBS-2 EEG and Standard (Xltek) EEG.
| Normal EEG | 95% CI | All abnormalities (Epileptiform or non-epileptiform) | 95% CI | Epileptiform discharges | 95% CI | Non-epileptiform abnormalities | 95% CI | |
|---|---|---|---|---|---|---|---|---|
| Sensitivity | 0.926 | (0.809, 1.0) | 0.518 | (0.378, 0.658) | 0.516 | (0.324, 0.708) | 0.240 | (0.053, 0.427) |
| Specificity | 0.518 | (0.378, 0.658) | 0.926 | (0.809, 1.0) | 0.904 | (0.814, 0.994) | 0.931 | (0.857, 1.0) |
| Positive predictive value | 0.481 | (0.335, 0.626) | 0.935 | (0.833, 1.0) | 0.762 | (0.556, 0.968) | 0.600 | (0.246, 0.954) |
| Negative predictive value | 0.935 | (0.833, 1.0) | 0.481 | (0.335, 0.626) | 0.758 | (0.643, 0.873) | 0.740 | (0.632, 0.847) |
| Positive percent agreement | 92.593 | (80.862, 100.0) | 51.786 | (37.805, 65.766) | 51.613 | (32.408, 70.818) | 24.00 | (5.258, 42.742) |
| Negative percent agreement | 51.786 | (37.805, 65.766) | 92.593 | (80.862, 100.0) | 90.385 | (81.41, 99.359) | 93.103 | (85.72, 100.0) |
Further evaluation of the 31 standard EEGs and 21 SBS2 EEGs with epileptiform abnormalities was performed. Among standard EEGs, 26 were focal and 5 generalized epileptiform abnormalities occurred compared to 12 focal and 9 generalized for SBS2 EEGs. For focal abnormalities the SBS-2 EEG had a sensitivity of 23.1%, a specificity of 100%, a positive predictive value of 100%, and negative predictive value of 71.4%. For generalized discharges, the SBS-2 had a sensitivity of 43.5% with a specificity of 96.2%. The positive predictive value was 90.9% and negative predictive value was 65.8%. The positive percentage agreement (ppa) for focal discharges was 23.1% and negative percentage agreement (npa) was 100%. For generalized discharges the ppa was 43.5% and npa was 96.2%. Of the 21 epileptiform abnormalities identified by the SBS-2 EEG 19 were on anti-epileptic medication. In the two patients who were not on an anti-epileptic medication, the availability of the SBS-2 EEG could have changed management._A further 7 patients who_were not taking any anti-epileptic medication had epileptiform discharges identified on standard EEG recordings that were not present on the SBS-2 EEG.
Of the 26 focal epileptiform abnormalities identified on the standard EEG, 14 were not identified by the SBS-2 EEG. These 14 were located in the temporal (7), frontotemporal (2), frontal (2), parietal (2), and parieto-temporal (1) regions. Thus 11 out of these 14 were in the frontal, temporal, or frontotemporal regions.
Discussion
We demonstrate the use of portable EEG technology in conjunction with a secure data exchange platform allowing for remote specialist interpretation of a previously unavailable neurodiagnostic test. Using a pragmatic study design, we test whether a low-cost, mobile EEG by minimally trained health care workers can meet the parameters of a standard EEG and certified EEG technician when performed on the same participants at the same low-income sub-Saharan African hospital site.[10] Our findings also have relevance to certain high income countries where recruitment of clinical neurophysiologists in some locations is inadequate.[11]
Remote interpretation of EEG is not a new concept. “Over the last decade there has been an increase in the number of commercially available mobile EEG systems, for example OpenBCI (https://shop.openbci.com/collections) and Emotiv produced mobile EEG technology (https://www.emotiv.com/emotivpro/) to name a small sample of the growing number of mobile EEG systems available for purchase at varying price points. Importantly, research is lacking on how mobile EEG technology could be applied in LMICs to improve access to neuro-diagnostics for PWE, particularly children. There are few comparative real world clinical studies examining their quality and effectiveness when compared to standard Xltek.[15] Our study helps address this important gap in the literature by demonstrating the SBS-2 EEG device is poorly sensitive yet highly specific for the detection of EEG and epileptiform abnormalities in a heterogeneous pediatric epilepsy population who previously had no access to EEG. Notably quality scores for the two types of recordings were comparable; however, more SBS-2 EEGs were unusable. This work confirms our prior work in the Kingdom of Bhutan[12] that found a 39% sensitivity and 95% specificity of epileptiform discharges for SBS2 compared to Xltek in PWE or suspected epilepsy of any age. In the present cohort, these same parameters were 51% sand 90%; however, more EEGs were overall unusable in Guinean children, potentially due to the overall younger age group; higher prevalence of developmental delay and poorly controlled seizures; and the very hot, humid, and crowded environment in Guinea. Artifacts due to motion and sweat were notable on both technologies.
Potential Clinical Utility
In 2009, it was reported that there were 267 neurologists in all of sub-Saharan Africa, with no available figures for neurophysiologists or EEG technologists. In 2016, several sub-Saharan African countries still lacked structured neurological training programs and any advanced training in EEG for neurologists-in-training.[7,16] The World Health Organization recommends the implementation of collaborative programs between specialist and non-specialist health care providers for certain conditions, including epilepsy.[17] Administration remains problematic in several LMICs locations globally.[18] Increased use of smartphone technology has expanded and changed traditional health care delivery, with growing acceptance of the digitization of medicine.[19] The SBS-2 EEG holds promise as portable, easy to administer, battery-operated, reusable, and low cost (~350 USD per unit) compared to standard EEG technology.
While the identification of focal epileptiform abnormalities by SBS2 was poor, with a sensitivity of 23%, there was an extremely high specificity of 100% with a positive predictive value also of 100%. Even with the low sensitivity, the high specificity means that any abnormalities found via the SBS-2 EEG were confirmed by the traditional Xltek EEG. In practical terms, this could aid clinicians to both diagnose and tailor antiepileptic treatment and prompt further diagnostic investigations.
In cases of primary generalized epilepsies, carbamazepine (the third most commonly prescribed medication in our cohort) can worsen seizures, as can phenytoin and phenobarbital, particularly in children with absence epilepsy.[20–23] The SBS-2 reliably identified 9 participants with generalized epileptiform discharges. Six were taking phenobarbital, 1 valproate, 1 carbamazepine, and 1 was not taking any medication. In these patients, the availability of EEG via the SBS-2 would have allowed the clinicians more diagnostically useful information, which could have altered treatment choice and, in the case of the patient not taking any antiepileptic medication, commencement of therapy. In cases of first seizure, it is well established that there is a higher rate of seizure recurrence if the initial EEG is abnormal, information which could be utilized to give patients and their families more clarity and to help stratify risk of recurrence in the real world.[24]. “While 5 of these generalized abnormalities were also confirmed on the standard Xltek EEG recording, 4 were not. There are several possible explanations for this: firstly, the unpredictable nature of inter-ictal epileptiform abnormalities, coupled with the fact that EEGs were performed sequentially and not simultaneously, may account for some of this difference. Secondly, the mean recording for the SBS-2 EEG was on average 5 minutes longer than the standard EEG (27.9 mins versus 22.9 mins). Finally, the SBS2 detections may be false positives.”
Given that the sensitivity for focal abnormalities is 23.1% on SBS-2, this would not be robust enough for a screening tool in this population; however, when present, the specificity and positive predictive value are 100% which is convincing. Thus where a focal abnormality is identified, it is a real finding and could help persuade clinicians to pursue neuroimaging to look for structural pathology. This may be particularly useful in many LMICs where head imaging for PWE is unusual unless recommended strongly by a physician.
Study Strengths and Limitations
There is a paucity of literature from African or other LMIC settings on children with epilepsy using mobile EEG technology. We compare SBS-2 to the current standard 10–20 Xltek EEG and demonstrate that portable EEG technology can be administered easily and reliably after brief training. We extend this data collection study to include data transmitted via an Android™ tablet and interpreted by remote specialists via a secure novel online platform, crowdEEG. As data are transmitted via a wireless network, EEG diagnostics became available for participants without need for access to proprietary reading software on site. The crowdEEG platform presents one central, standardized point of entry for all readers, enabling researchers to manage a pool of EEG readers effectively and monitor the project’s progress without the need for distribution of individual expensive commercial software. The platform also facilitated the integration of a single reading template for the input of clinically relevant observations during an EEG interpretation, as well as the standardization of montage and filter options for all readers in a centralized manner.
Our study captured a “real word” scenario and participants were selected from a clinic of heterogenous active epilepsy patients. This allowed enrolment of participants with both focal and generalized epilepsy patterns due to a variety of etiologies. We have demonstrated that delivering diagnostic technology in such a way to this population is feasible.
There were also multiple notable limitations to this study: recordings took place sequentially so direct comparison between simultaneous raw EEG data was not possible. We assumed there was a random nature of epileptiform discharges, such that there was equal likelihood that a random epileptiform discharge would appear on either technology. We also acknowledge that even amongst experienced neurologists there can be high inter-rater variability with interpretations of EEG data.[25] Our measurement of agreement was relatively low (<0.5) which is not atypical for EEG readings between expert raters. The next phase of this project aims to evaluate automatic interpretations of EEGs as well as determine the value of a multiple longer SBS-2 EEGs in a single participant over time.
The most notable weakness was that 14 focal abnormalities were not captured by the SBS-2 when compared to Xltek. Eleven of these were in the frontotemporal or temporal chain. While the SBS-2 has some temporal coverage via two sub-temporal electrodes at Tp9 and Tp10, there is not full frontotemporal coverage like there is on a standard 10–20 EEG. We were limited to 14 electrodes as this was the capacity of the portable EEG amplifier. Future work will concentrate on the expansion of the capacity of the digital amplifier. In this study, the technology was limited to the capacity of 14 electrodes. This was likely a major contributor to the reduced sensitivity of the SBS-2 EEG for focal epileptiform abnormalities compared to the standard 21-electrode EEG.
It is conceivable that the SBS-2 EEG could be integrated into the local care model for children with epilepsy, including in field settings with minimally trained health care workers with adequate training.[26] The low cost of an SBS-2 and its ease of application make it a useful device to assist the diagnosis of epilepsy, direct appropriate patients to neuroimaging, and at times initiate or select a different antiepileptic medication. A major consideration in LMICs is whether SBS-2 and EEG itself can actually assist the diagnosis and clinical acumen of frontline healthcare workers. Presently, the use of EEG is absent. Whereas in some cases, this is entirely appropriate and does not change management in LMICs, it is also possible that some children with epilepsy would benefit. This would require thorough educational initiatives on the interpretation of a normal EEG as consistent with the diagnosis of epilepsy; the neuroanatomical basis of focal discharges and how it may prompt imaging in poor patients; the treatment approaches to generalized epilepsies; and particularly in locations that have neurocysticercosis, the treatment and prevention of underlying etiologies.
With continued modifications, the SBS-2 app could be easily translated into additional languages for new locations, facilitating its use among frontline healthcare workers. Future clinical uses could include inpatient settings, neonatal intensive care units, and at home and facility-based sleep and overnight recordings.
Highlights:
There are limited data on portable EEG technologies in low-income populations.
We test a smartphone-based EEG in children with epilepsy compared to standard EEG.
97 children with epilepsy were enrolled in the West African Republic of Guinea.
Epileptiform discharges were detected on 25% of smartphone and 37% of standard EEGs.
Smartphone EEG had a sensitivity of 52% and specificity of 90% for epileptiform discharges compared to standard EEG.
Acknowledgements
We would like to thank patients, their parents and volunteers for their time and participation in this work. We would also like to thank Mr. Per Baekgaard, Dr. Andreas Trier Poulsen, and Professor Lars Kai Hansen of the Danish Technical University in Copenhagen for their technical assistance with data processing.
Funding
This work was supported by National Institutes of Health (NINDS and Fogarty International Center grant number R21NS098886), the Charles Hood Foundation, NSERC CHRP (grant number CHRP 478468–15), and CIHR CHRP (grant number CPG-140200). The design, conduct, and reporting were entirely the responsibility of the authors, independent from all funders.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests: All authors declare no conflicts of interest in relation to this work.
References Cited:
- [1].No authors listed. World Health Organization. Atlas: epilepsy care in the world. 2005.
- [2].Preux PM, Druet-Cabanac M. Epidemiology and aetiology of epilepsy in sub-Saharan Africa. The Lancet Neurology 2005;4:21–31. [DOI] [PubMed] [Google Scholar]
- [3].Mung’ala-Odera V, White S, Meehan R, et al. Prevalence, incidence and risk factors of epilepsy in older children in rural Kenya. Seizure : the journal of the British Epilepsy Association 2008;17:396–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Fountain NB, Van Ness PC, Swain-Eng R, et al. Quality improvement in neurology: AAN epilepsy quality measures: Report of the Quality Measurement and Reporting Subcommittee of the American Academy of Neurology. Neurology 2011;76:94–99. [DOI] [PubMed] [Google Scholar]
- [5].Idro R, Newton C, Kiguli S, Kakooza-Mwesige A. Child neurology practice and neurological disorders in East Africa. Journal of child neurology 2010;25:518–524. [DOI] [PubMed] [Google Scholar]
- [6].Bower JH, Zenebe G. Neurologic services in the nations of Africa. Neurology 2005;64:412–415. [DOI] [PubMed] [Google Scholar]
- [7].No authors listed. A new voice for global neurology? The Lancet Neurology 2010;9:1. [DOI] [PubMed] [Google Scholar]
- [8].McLane HC, Berkowitz AL, Patenaude BN, et al. Availability, accessibility, and affordability of neurodiagnostic tests in 37 countries. Neurology 2015;85:1614–1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Jang M, Sakadi F, Tassiou NR, et al. Impact of poorly controlled epilepsy in the Republic of Guinea. Seizure : the journal of the British Epilepsy Association 2018;61:71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lemesle M, Kubis N, Sauleau P, N’Guyen The Tich S, Touzery-de Villepin A. Tele-transmission of EEG recordings. Neurophysiologie clinique = Clinical neurophysiology 2015;45:121–130. [DOI] [PubMed] [Google Scholar]
- [11].Coates S, Clarke A, Davison G, Patterson V. Tele-EEG in the UK: a report of over 1,000 patients. J Telemed Telecare 2012;18:243–246. [DOI] [PubMed] [Google Scholar]
- [12].McKenzie ED, Lim AS, Leung EC, et al. Validation of a smartphone-based EEG among people with epilepsy: A prospective study. Scientific reports 2017;7:45567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Fisher RS, Acevedo C, Arzimanoglou A, et al. ILAE official report: a practical clinical definition of epilepsy. Epilepsia 2014;55:475–482. [DOI] [PubMed] [Google Scholar]
- [14].Falco-Walter JJ, Scheffer IE, Fisher RS. The new definition and classification of seizures and epilepsy. Epilepsy Res 2018;139:73–79. [DOI] [PubMed] [Google Scholar]
- [15].Stopczynski A, Stahlhut C, Larsen JE, Petersen MK, Hansen LK. The smartphone brain scanner: a portable real-time neuroimaging system. PloS one 2014;9:e86733–e86733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mateen FJ, Clark SJ, Borzello M, Kabore J, Seidi O. Neurology Training in sub-Saharan Africa: A survey of people in training from 19 countries. Annals of neurology 2016;79:871–881. [DOI] [PubMed] [Google Scholar]
- [17].No authors listed. Neurology in sub-Saharan Africa—WHO cares? The Lancet Neurology 2006;5:637. [DOI] [PubMed] [Google Scholar]
- [18].Mbuba CK, Ngugi AK, Newton CR, Carter JA. The epilepsy treatment gap in developing countries: a systematic review of the magnitude, causes, and intervention strategies. Epilepsia 2008;49:1491–1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Krishna S, Boren SA, Balas EA. Healthcare via cell phones: a systematic review. Telemedicine journal and e-health : the official journal of the American Telemedicine Association 2009;15:231–240. [DOI] [PubMed] [Google Scholar]
- [20].Perucca E, Gram L, Avanzini G, Dulac O. Antiepileptic drugs as a cause of worsening seizures. Epilepsia 1998;39:5–17. [DOI] [PubMed] [Google Scholar]
- [21].Chaves J, Sander JW. Seizure aggravation in idiopathic generalized epilepsies. Epilepsia 2005;46 Suppl 9:133–139. [DOI] [PubMed] [Google Scholar]
- [22].Berkovic SF. Aggravation of generalized epilepsies. Epilepsia 1998;39 Suppl 3:S11–14. [DOI] [PubMed] [Google Scholar]
- [23].French JA. Seizure exacerbation by antiepileptic drugs. Epilepsy currents 2005;5:192–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Berg AT, Shinnar S. The risk of seizure recurrence following a first unprovoked seizure: a quantitative review. Neurology 1991;41:965–972. [DOI] [PubMed] [Google Scholar]
- [25].Grant AC A-B S, Weedon J, et al. EEG interpretation reliability and interpreter confidence: a large single-center study. Epilepsy Behav 2014:102–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Labrique AB, Wadhwani C, Williams KA, et al. Best practices in scaling digital health in low and middle income countries. Globalization and Health 2018;14:103. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data from this study will be made available to qualified investigators upon request.