Abstract
The proliferation and ectopic migration of neural precursor cells (NPCs) in response to ischemic brain injury was first reported two decades ago. Since then, studies of brain injury-induced subventricular zone cytogenesis, primarily in rodent models, have provided insight into the cellular and molecular determinants of this phenomenon and its modulation by various factors. However, despite considerable correlational evidence—and some direct evidence—to support contributions of NPCs to behavioral recovery after stroke, the causal mechanisms have not been identified. Here we discuss the subventricular zone cytogenic response and its possible roles in brain injury and disease, focusing on rodent models of stroke. Emerging evidence suggests that NPCs can modulate harmful responses and enhance reparative responses to neurologic diseases. We speculatively identify four broad functions of NPCs in the context of stroke: cell replacement, cytoprotection, remodeling of residual tissue, and immunomodulation. Thus, NPCs may have pleiotropic functions in supporting behavioral recovery after stroke.
Keywords: Cytogenesis, neural precursor cell, neurogenesis, recovery, stem cell, stroke
1. Introduction
The discovery of cell genesis in the adult mammalian brain displaced the belief that lost brain cells—due to stroke, for example—were irreplaceable. Indeed, stroke and other brain injuries instigate a cytogenic response characterized by increased cell proliferation and altered migration of new cells. While it is now appreciated that the subventricular zone (SVZ) cytogenic response to stroke is massively insufficient for complete repair, that new cells are formed and travel long distances to remodeling brain regions after injury is consistent with SVZ cytogenesis being an important repair process. Here we discuss this process, including whether newborn cells might meaningfully contribute to behavioral recovery after stroke and other brain injuries, and, if so, how. We consider evidence primarily from rodent stroke models examining the SVZ but also include some discussion on studies of other nervous system injury models, neural precursor cell transplantation, and injury-induced cytogenesis in the subgranular zone. In addition, we discuss several factors that modify the SVZ cytogenic response to stroke. While there has been a strong research focus on post-stroke neurogenesis, we suggest that neurogenesis is only one aspect of a diverse SVZ cytogenic response. Based on available evidence, we propose that multiple cell types arising from the SVZ contribute to stroke recovery through several mechanisms.
2. Subventricular zone cytogenesis in the adult brain
The formation of new brain cells in adulthood is a process conserved among mammals [1-5]. Neuro- and gliogenesis in the adult brain are largely restricted to the subventricular zone (SVZ) lining the lateral ventricles and the subgranular zone (SGZ) within the hippocampal dentate gyrus. There is also evidence of comparably sparse cell genesis in other regions of the intact and injured central nervous system [6-8]. We focus on the SVZ niche and refer to a broad population of cells, neural precursor cells (NPCs), which encompasses cellular subpopulations along a spectrum of differentiation – from stem cells to lineage-defined progenitors. Because dividing NPCs give rise to neurons and glia, we use the term “cytogenesis” (rather than “neurogenesis”) to refer to the formation of new cells from NPCs in the adult brain. Figure 1 illustrates SVZ cytogenesis in the adult brain and the cellular subpopulations involved.
Figure 1.
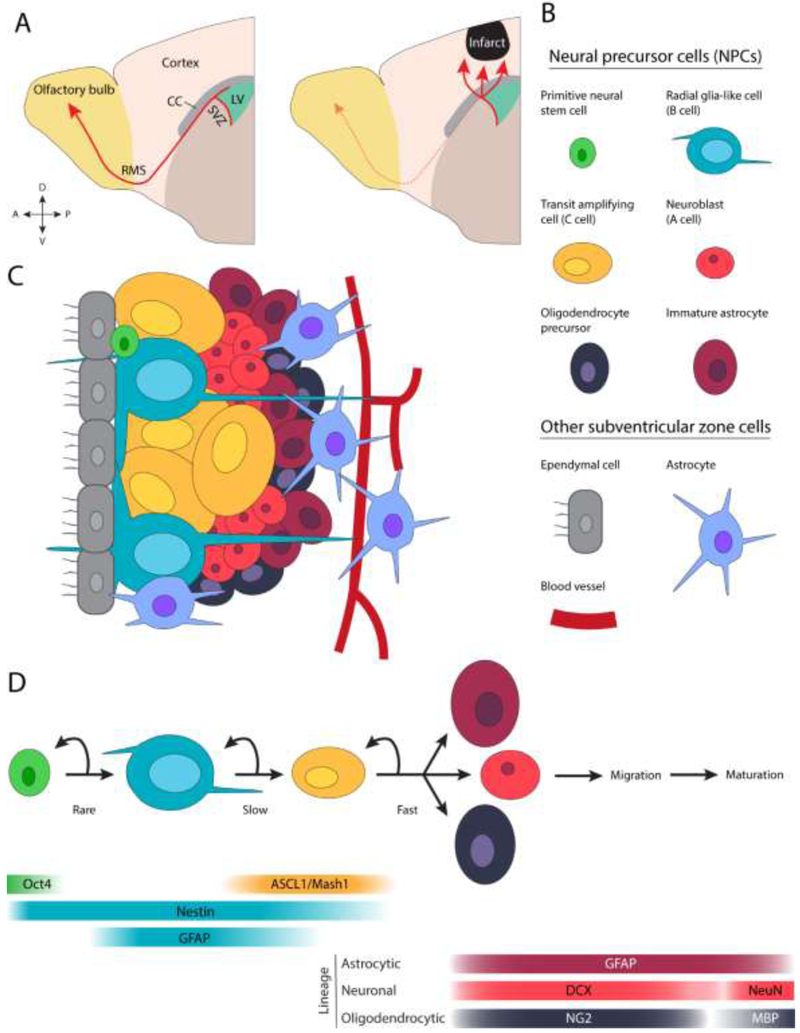
Cytogenesis in the adult subventricular zone. A) Depiction of NPC migration from the SVZ in the intact and injured brain. B and C) Selected cellular subpopulations of the SVZ. D) A lineage model of SVZ cytogenesis and molecular markers associated with each cellular subpopulation [12,19,30]. Note that other lineage models have been proposed [19,136]. ASCL1: Achaete-scute homolog 1, CC: corpus callosum, DCX: doublecortin, GFAP: glial fibrillary acidic protein, LV: lateral ventricle, MBP: myelin basic protein, NG2: neural/glial antigen 2, Oct4: octamer-binding transcription factor 4, RMS: rostral migratory stream.
Neural stem cells (type B cells in the SVZ) are radial glia-like, GFAP+ (glial fibrillary acidic protein), slowly dividing, multipotent cells [9,10]. They can divide symmetrically, forming two identical daughter cells, or asymmetrically, forming one stem cell and one differentiating cell. Symmetric division, the likely dominant type [11], includes self-renewal (two stem cell daughters) and differentiative (two non-stem cell daughters) division. Transit amplifying cells (type C cells) are rapidly dividing intermediates that give rise to immature glia and neurons (type A cells). In addition to these three subpopulations, recent evidence suggests that a scarce subpopulation of octamer-binding transcription factor 4+ (Oct4+) cells, termed primitive neural stem cells, exists in the adult SVZ [12,13]. The function of these cells in the intact brain is unclear, but they appear to serve as a reserve pool for replacing or expanding the GFAP+ type B cell population upon its depletion or after injury. Finally, while some have reported that SVZ ependymal cells may be a source of new cells after injury [14], there are reports to the contrary [15-17].
NPCs have a unique relationship with another cell population: blood vessels. In contrast to most of the brain, cytogenic niches have conspicuously bare vessels that are permissive to passage of small molecules [18]. Close association between NPCs and particularly permeable vessels positions nascent cells to integrate blood borne signals that regulate their behavior [19], and may allow NPCs to quickly identify and respond to distant or systemic stimuli, including noxious stimuli such as injury [20]. Close apposition to vasculature also gives NPCs privileged access to endothelial cell-derived soluble factors [21]. Outside of cytogenic regions, neuroblasts rely on blood vessels as a scaffold during migration [22]. As we discuss later, the intimate relationship between NPCs and vessels endures and transforms in the injured brain.
3. The NPC cytogenic response to stroke
In the intact brain, newborn cells migrate from the SVZ to the olfactory bulb via the rostral migratory stream. Injury and disease modify this process by increasing NPC proliferation and inducing ectopic migration (Figure 1A and 2). In this section we discuss the NPC response to stroke and its behavioral significance in the context of rodent models of ischemic stroke.
Figure 2.
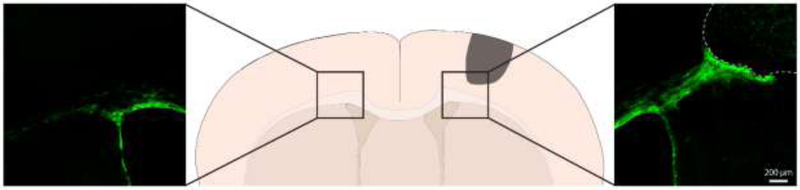
Migration of SVZ NPCs towards a unilateral cortical ischemic lesion. Expression of membrane-targeted GFP was induced in NPCs by administration of tamoxifen to an adult Nestin-CreERT2∷ Ai35D (cre-dependent archaerhodopsin/GFP fusion protein) mouse. Expression of this membrane-targeted fusion protein allows visualization of the full morphology of labeled NPCs. A photothrombotic infarct was induced in motor cortex two weeks later. The mouse was euthanized 8 weeks post-infarct. Note the significantly fewer NPCs in white matter and absence of NPCs in cortical grey matter in the contralesional hemisphere.
3.1. Stroke-induced SVZ NPC proliferation and migration
Stroke induces robust gene expression changes across cell types in surrounding regions beginning within hours [23,24]. Gene expression changes within SVZ cells recapitulate developmental programs that drive proliferation of neural stem cells and transit amplifying cells and migration of progenitors [25]. Within days after injury, cell proliferation is significantly increased within the SVZ across stroke models [26-32]. Stroke-induced NPC proliferation and migration has been observed in non-human primates [33] and humans [34-36] (cf. [37]). In rodent models, SVZ proliferation tends to peak within the first 2 weeks post-infarct [27]. Stroke-induced NPC proliferation is facilitated, at least in part, by enhanced vascular permeability within the SVZ (beyond the already unusually high permeability of SVZ vessels [18]), which allows endothelial cells and NPCs to respond to systemic signalling altered by stroke [20]. Indeed, stroke induces SVZ vessels to increase proliferation of closely associated stem cells via notch signalling [20]. The stroke-increased notch ligand expression in SVZ vessels is transient and coincides with the peak in NPC proliferation.
GFAP+ neural stem cells are the primary upstream source of new neurons in the SVZ of the intact and injured brain [9,38]. The stroke-initiated SVZ cytogenic response includes multiple lineages. After stroke, SVZ NPCs produce immature neurons (DCX+) and, predominantly, glia (GFAP+, NG2+, or OLIG2+) [30,39] (Figure 1). Multipotent NPCs also migrate to peri-infarct areas where they can proliferate and give rise to new astrocyte-lineage cells [40].
SVZ-derived NPCs migrate long distances rather quickly. By 7 days after distant cortical stroke in mice, thousands of neuroblasts are seen in peri-infarct cortex 2-4 mm from the SVZ [38]. We [41] and others [38] have observed NPCs reaching peri-infarct cortex within 3 days post-infarct in mice. The process of NPC migration is coupled to remodeling vasculature. Blood vessels act as a scaffold to facilitate NPC migration towards the lesion [42]. Within the peri-infarct region, remodeling vasculature expresses molecular cues (e.g., SFD-1, Ang1, EPO) necessary for directed NPC migration [38,43-45]. Once they reach peri-infarct vessels, newborn cells closely associate with them in a manner reminiscent of the SVZ vascular niche where vessels are uniquely permissive to passage of small molecules [18]. Close association with vasculature positions NPCs to respond to both circulating and local signals in the ectopic peri-infarct niche. Interestingly, migration of new cells towards cortical infarcts continues for at least many months, suggesting that injuries persistently alter the migratory goal of NPCs [27,46].
Studies on the survival of new neurons after experimental stroke suggest a bleak fate for newborn cells. Based on the number of immature neurons produced soon after stroke and thymidine analogue co-labeling at later survival times, it is estimated that <20% of newborn neurons survive to become mature neurons [28,29,38]. If the survival and integration of new neurons in peri-infarct regions supports post-stroke recovery, the failure for significant numbers of new neurons to survive could limit recovery of function in stroke survivors (for review, see [47]). Importantly, to our knowledge, no studies have assessed survival of new SVZ-derived glia in peri-infarct regions. Future studies of SVZ-derived cell survival could make use of indelible, inherited labeling systems [30] to track and manipulate both glial and neuronal lineages in peri-infarct tissue.
3.2. Behavioral significance of the SVZ cytogenic response to stroke
There is abundant correlational evidence to support a beneficial role for NPCs in stroke recovery. Generally, treatments or manipulations that enhance NPC proliferation or survival are associated with improved behavioral outcome, while manipulations with the opposite effects are typically associated with worsened outcome, as previously reviewed [48]. Here we focus on studies that have directly evaluated the contributions of NPCs to behavioral recovery in rodent models of cerebral ischemia (summarized in Table 1).
Table 1.
Chronological summary of studies that assessed behavioral outcome after NPC-specific manipulations in rodent models of stroke.
| Model | Species | Method | Region | Tests and outcome |
Ref. |
|---|---|---|---|---|---|
| bCCAo | Gerbil | Irradiation | SGZ | ↓MWM | [54] |
| pMCAo | Mouse | DCX-TK | SGZ, SVZ | ↓Rotarod ↓Limb placing ↓Elevated swing ↓Lesion size |
[51] |
| dMCAo, pMCAo | Mouse | DCX-TK | SGZ, SVZ | ↓Beam walking ↓ Corner turn ↓Rotarod ↓Limb placing ↓Elevated swing ↓Lesion size |
[50] |
| dMCAo | Mouse | DCX-TK | SGZ, SVZ | ↓Beam walking ~Corner turn ~Limb placing ↓Elevated swing ↓Lesion size |
[49] |
| dMCAo | Mouse | Nestin-TK | SGZ, SVZ | ~Ladder ~Catwalk ↓Barnes ~Lesion size |
[26] |
| pMCAo | Mouse | Nestin- CreERT2∷ NSE-DTA |
SGZ | ↑MWM ↑Context memory |
[58] |
| Cortical PT | Mouse | AAV-hSyn- TeNT |
SVZ | ↓Grid walking ↓Pasta handling |
[53] |
↑ = improved, ↓ = worsened, ~ = no change; abbreviations: AAV-hSyn-TeNT: adeno-associated virus – human synapsin – tetanus toxin, bCCAo: bilateral common carotid artery occlusion, dMCAo: distal middle cerebral artery occlusion, MWM: Morris water maze, NSE-DTA: neuron specific enolase – diphtheria toxin A, pMCAo: permanent middle cerebral artery occlusion, PT photothrombosis, tMCAo: transient middle cerebral artery occlusion
Several studies have directly examined contributions of SVZ NPCs to motor recovery in mouse models. In a series of studies, Jin and colleagues [49-51] found that ablation of neuroblasts in DCX-TK (doublecortin-thymidine kinase) mice prior to permanent or distal middle cerebral artery occlusion (MCAo) resulted in significantly larger lesion size and worse motor outcome on several tasks. However, Sun and colleagues [26] found no aggravation of lesion size or worsening of locomotor function in mice lacking NPCs (ablated with the Nestin-TK system) after distal MCAo. No studies have examined behavioral consequences of NPC ablation in focal stroke models that are most commonly used to model chronic post-stroke motor impairments, such as endothelin-1 or photothrombosis [52]. However, a recent study found that tetanus expression in new SVZ-derived neurons impaired motor recovery on the grid walking and pasta handling tasks following motor cortical photothrombotic lesions in mice [53]. Overall, stronger direct evidence that SVZ NPCs support motor behavioral recovery in stroke models is needed.
Together, these studies suggest that SVZ NPCs might support favorable outcomes from stroke; however, they have often used relatively insensitive tasks for probing motor function and have often not been able to discriminate between neuroprotective and other functions. In Section 5, we discuss possible causal mechanisms of SVZ NPC-mediated improvement of outcome after stroke.
3.3. Functional contributions of the SGZ cytogenic response to stroke
In addition to the SVZ, stroke potently stimulates neurogenesis within the SGZ. Studies on the functional impact of stroke-augmented SGZ cytogenesis have shown conflicting results. Two studies have assessed roles of SGZ NPCs in ischemia-induced cognitive impairment with NPC ablation methods. Raber and colleagues [54] subjected gerbils to x-ray irradiation to diminish SGZ proliferation two weeks prior to global ischemia induced by transient occlusion of both carotid arteries. This ischemia model most significantly damages vulnerable cell populations—including hippocampal CA1 pyramidal neurons—and produces memory deficits. Irradiation treatment exacerbated ischemia-induced spatial memory deficits as assayed with the Morris water maze [54]. Sun and colleagues [26] used Nestin-TK mice to ablate NPCs prior to distal MCAo. Mice lacking NPCs had impaired spatial learning and memory when tested on the Barnes maze beginning 8 weeks post-stroke [26]. Lastly, voluntary running was found to specifically increase SGZ neurogenesis and ameliorate cognitive deficits after transient MCAo in mice [55]. The number of new surviving dentate cells correlated with performance on the Morris water maze. These studies suggest that SGZ cytogenesis supports some aspects of cognitive recovery in models of stroke.
In contrast, some studies have reported morphological [56-58] and electrophysiological [59] abnormalities in new SGZ-derived neurons after stroke. These studies may suggest that stroke adversely impacts the integration of new neurons, at least within the hippocampus. Cuartero and colleagues [58] recently showed that post-MCAo increases in SGZ neurogenesis correlated with impairments in context and spatial memory. Further augmenting post-stroke SGZ neurogenesis with memantine or running exacerbated these deficits, whereas pharmacological or genetic inhibition of neurogenesis ameliorated them [58].These studies provide a cautionary note for neuron replacement strategies: integration of new neurons into existing circuits can go awry.
In summary, the NPC cytogenic response is often—but not exclusively—associated with behavioral benefits. In the next section, we describe several modifiers of stroke-induced cytogenesis.
4. Modifiers of stroke-induced SVZ cytogenesis
The unique microenvironment of the SVZ and the stroke-created niche in peri-infarct regions allows NPCs access to an assortment of regulatory signals that can modulate their behavior. In this section we discuss several factors that modify the SVZ cytogenic response to injury and the implications for stroke recovery and treatment. We direct readers to other works for reviews of molecular regulation of NPC fate and proliferation [19,21,60].
4.1. Age
Cytogenesis declines with age. Histological analysis of the mouse SVZ throughout aging reveals a progressive thinning of the structure, reduction in proliferation, and accumulation of astrocytes [61]. While the number of neural stem cells is stable with age (cf. [62]), beginning in middle age [63], neural stem cells adopt a quiescent state associated with changes in gene expression [64] and morphology [63], and a smaller progenitor pool. NPCs integrate many signals that control their behavior, including systemic, local, and intrinsic signals [65]. Remarkably, transplanting “old” neural stem cells into a young niche, or into an environment resembling a young niche, restores proliferation kinetics comparable to young stem cells in a young milieu [66,67]. Conversely, placing young stem cells in an “old” environment precipitates quiescence [66]. The role of systemic factors in regulating NPC behavior with aging is also elegantly demonstrated in the heterochronic parabiont model system in which the vascular systems of young and old mice are joined, thereby causing the exchange of circulating factors. Age-related diminishment of NPCs in the SVZ [68] and SGZ [69] is rescued in the old parabiont. Similarly, age-related vascular rarefication can be reversed [68]. These effects can be mimicked by injection of young plasma [69,70], or even a single “youthful” factor, GFD11 [68]. Correspondingly, exposure to an “aging” factor, CCL11, reduces neurogenesis in young mice [69]. These data implicate circulating and environmental factors, rather than cell-intrinsic signals, as the dominant signal encouraging age-related neural stem cell quiescence.
Cytogenesis is influenced by the interaction of age and injury. Although cell proliferation is reduced in the aged brain, its enhancement by ischemia is maintained [71]. Adamczak and colleagues [72] used longitudinal in vivo bioluminescence imaging to track neurogenesis in DCX-luciferase mice. They observed a ~50% decline in SVZ neurogenesis from 2 to 12 months of age. Following transient MCAo, neurogenesis increased ipsilaterally to lesions regardless of age, but the magnitude of the neurogenic response in 12 month-old mice was lower than in 3 month-old mice. Age-related blunting of the cytogenic response to ischemia has also been reported in the rat SVZ [71] and SGZ [73]. The Wnt signalling pathway is one regulator of cytogenesis that varies with age. Stroke increases local Wnt signalling that is paralleled by SVZ stem cell activation [66]. However, in the aged SVZ, Wnt signalling is reduced and not amplified by stroke, consistent with the relatively diminished cytogenic response. Therefore, both local and systemic signals likely play a role in age- and injury-dependent regulation of cytogenesis.
Aging has a marked dampening effect on cytogenesis. Beyond reducing the absolute number of NPCs, does aging affect the ability of NPCs to beneficially support function in the context of disease and injury? For example, might NPCs in the old brain secrete a different amount or assortment of intercellular signaling factors [74]? Given that stroke disproportionally affects older adults, understanding effects of aging on NPCs may have major clinical relevance.
4.2. Experience
The enhancement of SGZ neurogenesis by environmental enrichment and physical activity is well described [75,76]. In contrast, experience effects on SVZ cytogenesis in the intact and stroke brain are less clear. In one study, enriched environment housing of mice subjected to transient MCAo attenuated stroke-induced proliferation in the SVZ, as measured by BrdU (bromodeoxyurdine, administered daily during the first week post-infarct) and DCX immunolabeling at 4 weeks [77]. Moreover, there were half as many immature neurons and glia migrating towards the infarct in enriched mice [77]. In contrast, two studies showed that rats in enriched housing after cortical infarcts had increased proliferation in the SVZ 1 and 5 weeks post-stroke, as measured by DCX, BrdU (administered daily during the first week post-infarct), and Ki67 immunolabeling [78,79]. This increased proliferation was paralleled by improved motor function. These studies indicate that post-stroke environmental enrichment can have varying effects on SVZ cytogenesis. Several differences between studies could explain their conflicting results. The study finding reduced SVZ proliferation subjected mice to rather short (3 hours per day) bouts of enriched housing with 3-4 mice per cage in a stroke model that caused cortical and striatal damage [77]. The studies finding increased SVZ proliferation continuously housed 9-10 rats in enriched housing after infarcts restricted to cortex [78,79]. Therefore, the parameters of enrichment, species, and location of damage may influence the effects of environmental enrichment on post-stroke SVZ cytogenesis.
Several studies have assessed the effects of use-dependent behavioral manipulations on cytogenesis. Four studies found that constraint-induced forced use of the stroke-impaired limb ameliorated functional deficits and increased SVZ neurogenesis in various rat models [80-83]. In two studies, this was coupled with increased angiogenesis [81,83]. Recently, Liang and colleagues [53] showed that overuse of the impaired forelimb induced by Botox injections in the less-impaired limb after photothrombosis in the forelimb area of motor cortex increased the number and survival of SVZ-derived cells in peri-infarct cortex, promoted their neuronal differentiation, and augmented synaptogenesis. Importantly, chemogenetically increasing activity in peri-infarct forelimb motor cortex (mimicking forelimb overuse) also increased the density of SVZ-derived cells, whereas chemogenetic inhibition of the same region had an opposite effect [53]. Interestingly, Liang and colleagues [53] also found that this activity-dependent modulation of post-stroke cytogenesis was region-specific: while impaired forelimb overuse increased the density of DCX+ cells in peri-infarct cortex, hindlimb overuse decreased peri-infarct DCX+ cell density. These studies demonstrate the sensitivity of post-stroke SVZ cytogenesis to activity-dependent modulation.
Voluntary wheel running induces changes in protein expression in peri-infarct cortex, including upregulation of proteins associated with cytogenesis [84]. Surprisingly, Komitova and colleagues [79] found that voluntary running in the first week post-infarct attenuated injury-induced SVZ stem cell and neuroblast proliferation in a rat cortical infarct model. Behavioral outcome was not affected. Interestingly, there was no effect of running on the total number of proliferating cells (Ki67+ or BrdU+) within the SVZ, perhaps indicating a shift towards gliogenesis. Another study found that treadmill running beginning two days after MCAo in rats increased the number of BrdU+ cells in peri-infarct regions relative to non-runners [85]. Together, these studies show that stroke and behavioral experience interact to modify SVZ cytogenesis.
4.3. Inflammation
Stroke triggers a significant inflammatory response involving innate and adaptive immunity [86,87]. Activated microglia expressing pro-cytogenic factors (e.g., IGF-1) accumulate in the SVZ after stroke, implying that they facilitate the NPC response [88]. Notably, microglia in the SVZ are morphologically and molecularly distinct from those in peri-infarct striatum [88]. This observation might reflect functionally separate subpopulations of microglia after stroke whereby a predominantly pro-cytogenic subpopulation is spatially and temporally restricted in the SVZ. To examine the role of inflammation in the cytogenic response, Chapman and colleagues [89] showed that striatal LPS injection induced an inflammatory response comparable to MCAo, but without overt cell death. The authors identified a distinct transcriptional program shared by microglia activated by LPS and ischemia. Critically, intrastriatal LPS caused a neurogenic response resembling that following focal ischemia, despite inflammation being the only noxious cue. These findings position acute inflammation as a contributing stimulus for the cytogenic response to stroke.
The poor survival of newborn cells after stroke has often been attributed to the harsh inflammatory environment within peri-infarct regions [28,43]. The inflammatory response to stroke is critical to limiting the spread of cytotoxic debris within the infarct [90]. However, properly timed anti-inflammatory treatment could be an effective strategy for limiting death of new cells. In support of this idea, post-infarct anti-inflammatory treatments promote the survival of new neurons in peri-infarct cortex [91] and the SGZ [92], and post-stroke NPC proliferation and survival are enhanced in immunodeficient mice [93].
Inflammation can positively and negatively regulate cytogenesis. A more refined understanding of the role of the inflammatory response in directing NPC behavior might uncover novel treatment approaches. We will discuss in the next section that the relationship between inflammation and SVZ cytogenesis is likely bidirectional; NPCs may have immunomodulatory functions.
The factors we have described, as well as others, that modify SVZ cytogenesis most certainly interact. For example, aging is associated with chronic, mild inflammation and a concomitant blunting of the acute inflammatory response [94]. Such interactions must be considered in studies examining modifiers of SVZ cytogenesis and during development of new therapies involving cytogenesis. Relative contributions of various cytogenesis-altering factors will be difficult to disentangle. Moreover, most studies focus on modification of neurogenesis, and some factors might differentially modify gliogenesis or bias cell fate decisions. Studies on modifiers of SVZ cytogenesis could benefit from a view that is less neuron-centric.
5. Possible functional contributions of neural precursor cells after stroke
A mechanistic understanding of the roles of NPCs in neurologic injury and disease is crucial to the development of novel treatment strategies aimed at exploiting the endogenous cytogenic response. In this section we discuss evidence from several models and manipulations, including NPC ablation and transplantation, which provide insight into potential functions of NPCs after stroke. While the pathobiology of stroke shares many commonalities with other forms of injury and disease, there are unique aspects for each condition that should be considered. Furthermore, because NPCs are modified by their environment and other factors, there are likely differences in the functions of transplanted versus endogenous NPCs. Nonetheless, NPCs appear to possess pleiotropic functions that render them beneficial across diverse disease states (Figure 3).
Figure 3.
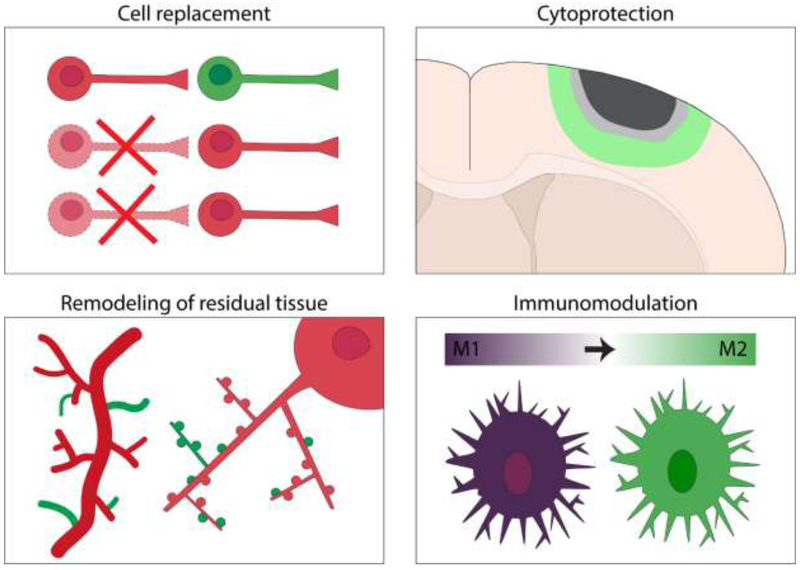
Summary of hypothesized beneficial functions of NPCs after stroke. NPCs might replace some cells lost to stroke (cell replacement), protect cells in peri-infarct regions from cell death (cytoprotection), support remodeling of residual tissue (e.g. by enhancing neuroplasticity of remaining neural circuits and angiogenesis of blood vessels), and modulate the immune response to stroke (immunomodulation).
5.1. Cell replacement
Perhaps the most obvious potential role of NPCs after stroke is cell replacement. As we discussed, the number of new cells produced in response to experimental stroke is considerably lower than the number killed, and relatively few new neurons appear to survive long-term [28,29]. However, a relatively small population of new cells could still have powerful effects. Although stroke kills neurons and glia, and both populations are produced from the SVZ, most studies of endogenous cell replacement after experimental ischemic injury have focused on new neurons. Young hippocampal neurons are unique in that they are highly excitable and plastic [95]. New neurons in peri-infarct cortex of mice are similarly excitable [39]. Of particular interest in the abnormal context of peri-infarct regions, where excessive GABA signalling is rampant [96], is that young neurons (in both the SVZ and SGZ) are initially excited by GABA, and GABA-induced depolarization promotes differentiation and survival of newborn neurons in the dentate gyrus and olfactory bulb [19,95]. Whether GABA similarly affects differentiation and survival of new neurons in peri-infarct regions is unclear, but new neurons in peri-infarct cortex receive largely GABAergic input 4 weeks after cortical infarcts in mice [39]. Intriguingly, there is some evidence that new hippocampal neurons are preferentially recruited into behaviorally-relevant circuits relative to older neurons [19,97]. After sensorimotor cortical infarcts in mice, conferring similar properties (preferential recruitment into circuits) to a small pool of residual neurons in peri-infarct cortex by virus-mediated CREB overexpression improves functional remapping and behavioral recovery [98]. Therefore, newborn neurons could disproportionately influence plasticity of the residual neural circuits that contribute to behavioral recovery [99]. However, the extent to which new peri-infarct neurons share the unique properties of new neurons in the intact brain is uncertain.
Some neuroblasts become mature neurons in the vicinity of the infarct [28,29,31,38] and can display ultrastructural features of synapses [100]. One study found that SVZ-derived neurons in peri-infarct striatum exhibited post-synaptic currents and action potentials 8 weeks after MCAo [101]. Similarly, Hou and colleagues [102] showed that SVZ-derived neuroblasts in peri-infarct regions developed increasingly complex morphology over time after MCAo. At 8 weeks post-infarct, new neurons in peri-infarct striatum were largely cholinergic or GABAergic, fired action potentials, and received mixed synaptic inputs. These studies indicate that new neurons can functionally integrate into peri-infarct circuits. A recent study by Kannangara and colleagues [39] found that new neurons in peri-infarct cortex 4 weeks after photothombotic cortical infarcts were predominantly GABAergic and fired action potentials, but were quite sparsely innervated relative to typical cortical GABAergic neurons. This finding suggests somewhat poor integration of new neurons into residual circuits, at least at the 4-week time point. Furthermore, this study raises the question of whether improving integration of new neurons would enhance behavioral recovery. A recent study by Liang and colleagues [53] showed that new SVZ-derived neurons express synaptic markers in peri-infarct cortex after photothrombosis. In addition, they found that overuse of the impaired forelimb greatly increased synaptogenesis and connectivity in new neurons measured two months post-stroke [53]. Importantly, blocking neurotransmitter release specifically from new neurons with tetanus toxin impaired long-term motor function [53].
Time is an important factor to consider in the potential contributions of cell replacement to behavioral recovery. Neuroblasts migrate from the SVZ towards the infarct, which can take over one week (and likely longer in humans) [38]. Then, they develop progressively more mature morphology and form connections over many weeks [102]. In rodent models, significant recovery of behavioral function typically occurs during the first month after injury [103], seemingly before significant functional integration of young neurons takes place, as measured by the low frequency of spontaneous post synaptic currents in new neurons 4 weeks post-infarct [39]. However, in the dentate gyrus and olfactory bulb, new neurons begin to form synapses by 2 weeks of age and can become well integrated by 4 weeks [104]. Interestingly, the electrophysiological maturation of new dentate granule cells is accelerated—and these new cells become hyperexcitable—after MCAo that does not directly damage the hippocampus [59]. The maturation and integration new neurons are seemingly altered depending on local circuit function and the presence of, and proximity to, injury. Together, these studies show that functional integration of new neurons can occur after stroke, but they do not address the utility of this process. To our knowledge, no study has directly tested behavioral contributions of the activity of newly integrated neurons after experimental stroke. Such experiments could involve chemo- or optogenetic manipulation of SVZ-derived neurons in behaving animals.
Although new glia may also be functionally significant, their contributions have received much less attention. A prominent reaction to stroke is reactive astrogliosis, whereby astrocytes alter gene expression and morphology and take part in scar formation [105]. Two studies indicate that SVZ-derived astrocytes have a role in the astrogliotic response to stroke. First, Benner and colleagues [106] observed thrombospondin-4 expressing reactive astrocytes arising from the SVZ in a postnatal ischemia model. Selective killing of these cells aggravated microvascular hemorrhage. Second, Faiz and colleagues [40] found that neural stem cells that left the SVZ after focal ischemia produced reactive astrocytes in peri-infarct cortex. Astrogliosis is a complex process that has functions both beneficial and detrimental to recovery [105]. More work is needed to clarify the role of SVZ-derived reactive astrocytes after stroke.
The functional contribution of cell replacement by SVZ NPCs remains unclear. Kolb and colleagues [107] massively improved the endogenous tissue regenerative response to pial devascularisation-induced cortical lesions in rats by sequential treatment with epidermal growth factor and erythropoietin to stimulate proliferation and neuronal differentiation of NPCs, respectively. This treatment prompted significant regeneration of cortical tissue (although regenerated tissue was non-laminar), which was accompanied by enhanced recovery of motor function. Removal of the regenerated cortex reinstated motor deficits, but, crucially, the behavioral effect was delayed: motor function was unaffected one week after removal of new tissue, but at three weeks post-removal there was a significant decline in function. This finding suggests that the activity of new cells was not directly responsible for functional improvements. Notably, many precursor cell transplantation studies report improved functional outcome despite poor differentiation or survival of transplanted cells [108-110]. In addition, administration of neural stem cell-derived extracellular vesicles—nanometer scale microvesicles containing proteins, RNAs, and lipids—improves outcome in several stroke models, despite the absence of cells [111-113]. These data support alternative mechanisms of NPCs that are not dependent on cell replacement per se.
5.2. Cytoprotection
Stroke creates a region of irreversible cell death—the ischemic core—surrounded by a region of ischemic tissue at risk, the penumbra. Significant effort has been directed at reducing the extent of the ischemic core and salvaging the penumbra. Considerable evidence that NPCs have cytoprotective functions comes from NPC transplantation studies. For example, Bacigallupi and colleagues [109] administered neural stem cells (from cultures of isolated adult mouse SVZ NPCs) intravenously 72 hours after transient MCAo in young adult mice. This treatment improved behavioral function, decreased peri-infarct apoptosis, and modestly reduced lesion size measured one month later. At the transcriptome level, stem cell transplantation regulated gene expression in a manner indicative of reduced inflammation and astrogliosis. These transcriptomic changes were corroborated by reduced numbers of activated microglia and infiltrated leukocytes in peri-infarct striatum. Given the changes in the brain microenvironment with age that we discussed earlier, a clinically important question is whether such a treatment would translate to the aged brain. Another study examined effects of intraparenchymal neural stem cell (from cultures of neural stem cells isolated from the telencephalon of embryonic mice) administration in young and aged rats after transient MCAo [114]. The results were auspicious: while there was an age-related aggravation of injury, stem cell transplantation was cytoprotective regardless of age. These studies suggest that, at least in some cases, transplanted NPCs exert protective effects; however, exogenously administered cells have experienced a vastly different environment than NPCs in vivo and therefore likely behave quite differently. The functions of transplanted NPCs may differ from the endogenous NPC response.
Evidence supporting cytoprotection by endogenous NPCs comes from NPC ablation experiments. In a series of studies, Jin and colleagues [49-51] used DCX-TK mice and large vessel occlusion stroke models to study contributions of neuroblasts to stroke outcome. They consistently found that neuroblast ablation prior to ischemia exacerbated infarct severity. However, it should be noted that no cytoprotective effect was found in a study that ablated NPCs in Nestin-TK mice (which ablates considerably more NPCs than the DCX-TK system [26,49,51,115]) prior to distal MCAo [26]. The basis for these inconsistent findings is unclear. The studies by Jin and colleagues [49-51] identify protective effects of neuroblasts, but they do not identify mechanisms underlying cytoprotection. A study by Butti and colleagues [116] showed that excitotoxic damage from 4-aminopyridine-induced seizures or transient MCAo was worsened in animals lacking NPCs (ablated by ganciclovir administration to nestin-TK mice or with the antimitotic agent ara-C). Importantly, the authors found that NPCs protect nearby striatal neurons through secretion of the endocannabinoid arachidonoyl ethanolamide, which regulates glutamatergic tone: production of arachidonoyl ethanolamide was increased in response to 4-aminopyridine, but this effect was abolished in mice lacking NPCs [116]. Pharmacologically restoring cannabinoid levels reversed the exacerbation of excitotoxic damage in mice lacking NPCs [116]. It is unclear whether this protective mechanism is generalized to more distant insults (e.g. cortical infarcts). Nonetheless, factors secreted by NPCs may have potent protective effects. As noted earlier, administration of NPC-derived extracellular vesicles reduces infarct size in murine and porcine stroke models [111,112]. Identification and administration of the specific factors mediating these effects is a promising treatment avenue.
5.3. Repair and remodeling of residual tissue
Functional improvements in animal stroke models have been linked with remodeling of peri-infarct tissue [117]. The remodeling process involves coordinated reorganization among many cell types, including neurons, blood vessels, and astrocytes [99,103]. NPCs migrate to areas of active remodeling after stroke, which positions them well to influence remodeling processes. NPCs could modify repair processes through cell-cell contact or through release of soluble or vesicle-contained factors (e.g. growth factors, miRNAs, cytokines) [43,118-120]. Hypoxia induces changes in the extracellular vesicle content of cultured neural stem cells [121], which suggests that the NPC secretome is adaptively varied. Characterization of factors transmitted by NPCs and how they vary in the context of neurologic disease and injury would provide substantial insight into NPC functions.
NPCs secrete neurotrophic factors that could facilitate remodelling of peri-infarct tissue [122,123]. However, the types and amounts of factors produced by NPCs varies greatly with the source and experiences of cells (e.g. immortalized cell lines vs. isolated NPCs vs. NPCs in vivo [124,125]). Nonetheless, neural stem cell transplantation studies support significant roles of transplanted cell-derived factors such as vascular endothelial growth factor (VEGF) and brain-derived neurotrophic factor (BDNF) in ameliorating injury and disease [110,126]. Andres and colleagues [110] transplanted human neural stem cells into peri-infarct cortex one week after distal MCAo in rats. NPC transplantation improved behavioral outcome without affecting lesion size, suggesting that behavioral benefits were not attributable to cytoprotection. Indeed, NPC transplantation bilaterally increased dendritic complexity and promoted axonal sprouting throughout the motor system. In addition, infarct-caused deficits in axonal transport were rescued by transplanted NPCs. In vitro experiments revealed several factors implicated in these effects on neural remodeling, including VEGF and thrombospondins. The majority of transplanted NPCs remained undifferentiated. This study provides strong support for the idea that NPC-secreted factors can facilitate reparative processes after stroke. Additionally, neural stem cell transplantation has been associated with increased angiogenesis in peri-infarct cortex, along with augmented expression of VEGF in resident astrocytes and vasculature [114]. Cell transplantation in models of Alzheimer’s [127] and Huntington’s [128] diseases have shown a functional role for NPC-derived BDNF. Whether the endogenous cytogenic response has comparable capabilities remains to be determined. Notably, in the intact SGZ, but not SVZ, NPCs secrete large amounts of VEGF [74].
These studies provide evidence that plastic responses to disease and injury might be beneficially supported by NPCs and identify mechanisms through which NPC might contribute to remodeling processes after stroke. However, to our knowledge, the contributions of endogenous cytogenesis to remodeling processes after stroke have not yet been addressed experimentally.
5.4. Immunomodulation
Inflammation after stroke is both beneficial and detrimental. As described earlier, inflammation regulates cytogenesis. However, the relationship is bidirectional [129]. In the SGZ, NPCs secrete several factors that regulate microglial behavior, including their proliferation, proclivity for phagocytosis, and secretion of cytokines and chemokines [130]. Substantial evidence, primarily from NPC transplantation studies (reviewed by [131,132]), supports immunomodulatory roles of NPCs in the context of acute and/or chronic inflammation. For example, intrastriatal transplantation of human NPCs reduced peri-infarct microglial accumulation at 6 and 14 weeks post transient MCAo in rats [133]. Bacigaluppi and colleagues [109] showed that intravenous delivery of adult mouse SVZ-derived NPCs three days after transient MCAo in mice reduced numbers of microglia and infiltrated leukocytes in peri-infarct regions. In a mouse model of spinal cord injury, Cusimano and colleagues [119] showed that NPC transplantation improved locomotor recovery and decreased reactive astrogliosis and the number of microglia at the site of injury. Importantly, the treatment markedly affected expression of genes implicated in inflammation and reduced the proportion of M1-like (generally pro-inflammatory) macrophages. Transplanted cells accumulated at the lesion border, largely undifferentiated, in close association with resident and infiltrated immune cells, commonly forming intercellular junctions. The latter observation might suggest direct cell-cell modulation. Another study by the same group revealed similar immunomodulatory mechanisms by endogenous spinal cord NPCs [134]. Mice with NPCs ablated prior to spinal cord injury had more severe lesions, worse locomotor recovery, decreased expression of trophic factors, and increased M1-like myeloid cells. The difference in lesion severity could be a contributing factor to the latter observations. Nonetheless, these studies suggest potent immunomodulatory effects of NPCs in acute and chronic inflammatory settings.
In summary, transplanted NPCs have an immunomodulatory role in stroke and nervous system injury models—for example, by supressing T-cell infiltration and promoting M2-like (generally protective) microglia/macrophage phenotypes over M1-like phenotypes [132]. These effects limit damage and may support reparative processes. Notably, secreted factors likely mediate these immunomodulatory effects because neural stem cell extracellular vesicles alone have a modulating effect on inflammation in a rodent stroke model [111]. At least in the spinal cord, endogenous NPCs regulate the inflammatory response to injury, but there is no direct evidence of the same effect of SVZ NPCs after stroke. Future work should directly examine whether endogenous NPCs have immunomodulatory functions in stroke models.
An important caveat of the studies we have discussed is that much of the data supporting various functions of NPCs arises from NPC transplantation studies. This gain-of-function strategy for probing NPC roles is imperfect because transplanted cells come from many sources, each a drastically different environment from the normal SVZ niche. Given, among other factors, the predilection of NPCs to adjust their behavior based on environmental cues, transplanted cells likely behave quite differently from endogenous NPCs. Additionally, these studies encompass diverse contexts (disease models), many of which are significantly different from human stroke. However, these studies provide an indispensable perspective on the flexibility of NPC functions. Moreover, data on endogenous NPC functions, although relatively scarce, seem to corroborate the pleiotropic effects of NPCs revealed in transplant studies.
6. Concluding remarks
Stroke prompts profound changes in the behavior of SVZ NPCs. The proliferation and migration of NPCs towards the insult makes it tempting to view NPC behavior as regenerative. However, only a meagre proportion of new cells appear to survive in the inhospitable milieu surrounding ischemic lesions. While the process of SVZ cytogenesis is demonstrably amenable to modification—for example by age, experience, and inflammation—functions of new cells have remained largely a mystery. In this review we have summarized data supporting several potential roles of NPCs.
We speculatively identified four functions of NPCs after stroke and possibly in other disease contexts: cell replacement, cytoprotection, remodeling of residual tissue, and immunomodulation (Figure 3). Although there is evidence that endogenous NPCs replace some neurons, the functional significance of this replacement may be minor because 1) few new neurons survive, 2) new neurons seemingly mature and integrate in peri-infarct regions over a time course delayed relative to behavioral recovery, and 3) some evidence suggests that integration of new neurons is poor [39]. However, the integration process could meaningfully influence plasticity of surviving neurons. Replacement of glia has been less studied. There is direct evidence that NPCs are cytoprotective in the context of excitotoxicity [116], including after infarcts encroaching upon the SVZ [49-51], but whether this protective mechanism functions over long distances (i.e. in a human brain) is unclear. Stem and progenitor cell transplantation studies support roles of NPCs in remodeling the injured brain and modulating the immune response, but evidence for these same functions in SVZ NPCs is needed.
Many questions remain surrounding the cytogenic response to injury. The rerouting of NPCs towards a lesion is long-lasting [27,46]. However, due to the time-variant nature of reparative and deleterious processes after stroke [103], is there a critical window during which NPCs are beneficial? Does this time window depend on factors intrinsic (e.g. location, severity) or extrinsic (e.g. age) to the cytogenic stimulus? Other uncertainties concern the types of cells involved in the cytogenic response. The NPC population that responds to stroke is heterogeneous. While most studies focus on the neurogenic response to stroke, there is a significantly larger glial component [30,39]. Furthermore, undifferentiated cells migrate into peri-infarct tissue [40]. Do the functions of these nascent cells vary depending on their phenotype? If so, could we regulate NPC fate to promote phenotype-specific functions that may be differentially favorable depending on the disease context? Ultimately, many of the beneficial functions of NPCs likely depend on their ability to interact with other cell types, including through secreted factors [108,111-113,122,131]. Therefore, characterizing the secretome of NPCs could yield significant insights into their functions. In particular, we need to understand how the NPC secretome varies with injury and disease, and whether it can be manipulated as a therapeutic avenue. The answers to these questions could have significant clinical implications. The emergence of tools that, for example, allow in vivo cell-type specific, temporally controllable manipulation and labelling (e.g. inducible cre/loxP [135]) of NPCs and large scale “omic” profiling will allow the behavioral contributions and underlying molecular mechanisms of NPCs in neurologic disease to be elucidated.
A variety of neurologic diseases and injuries induce NPC proliferation and migration, and this response is typically associated with repair and recovery. The cytogenic response is discernibly modified by many factors—including age, experience, and inflammation—that interact complexly with one another as well as with disease states. Here, we described four potential mechanisms by which NPCs may contribute to repair and recovery from stroke and other diseases: cell replacement, cytoprotection, remodeling of residual tissue, and immunomodulation. Significant experimental effort will be needed to clarify the functions of the cytogenic response, and to therapeutically leverage it, but we believe that the clinical impact will justify these efforts.
Acknowledgements
This work was supported by Canadian Institutes of Health Research Doctoral Award DFS-157838 to MRW, National Institutes of Health R37 NS056839, R21 NS101564 to TAJ, and R01 MH102595, R01 MH117426 to MRD. We thank two anonymous reviewers for their constructive feedback.
Abbreviations:
- ara-C
cytosine arabinoside
- BrdU
bromodeoxyuridine
- DCX
doublecortin
- GABA
gamma-aminobutyric acid
- GFAP
glial fibrillary acidic protein
- LPS
lipopolysaccharide
- MCAo
middle cerebral artery occlusion
- NPCs
neural precursor cells
- Oct4
octamer-binding transcription factor 4
- SGZ
subgranular zone
- SVZ
subventricular zone
- TK
thymidine kinase
- VEGF
vascular endothelial growth factor
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Cameron HA, Woolley CS, McEwen BS, Gould E, Differentiation of newly born neurons and glia in the dentate gyms of the adult rat, Neuroscience. 56 (1993) 337–344. doi: 10.1016/0306-4522(93)90335-D. [DOI] [PubMed] [Google Scholar]
- [2].Gould E, Reeves AJ, Fallah M, Tanapat P, Gross CG, Fuchs E, Hippocampal neurogenesis in adult Old World primates., Proc. Natl. Acad. Sci. U. S. A. 96 (1999) 5263–7. doi: 10.1073/pnas.96.9.5263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Kornack DR, Rakic P, Continuation of neurogenesis in the hippocampus of the adult macaque monkey, Proc. Natl. Acad. Sci 96 (1999) 5768–5773. doi: 10.1073/pnas.96.10.5768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Boldrini M, Fulmore CA, Tartt AN, Simeon LR, Pavlolva F, Poposka V, Rosoklija GB, Stankov A, Arango V, Dwork AJ, Hen R, Mann JJ, Human Hippocampal Neurogenesis Persists throughout Aging, Cell Stem Cell. 22 (2018) 589–599. doi: 10.1016/j.stem.2018.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Eriksson PS, Perfilieva E, Bjork-Eriksson T, Albom A-M, Nordborg C, Peterson DA, Gage FH, Neurogenesis in the adult human hippocampus, Nat. Med 4 (1998) 1313–1317. doi: 10.1038/3305. [DOI] [PubMed] [Google Scholar]
- [6].Gould E, How widespread is adult neurogenesis in mammals?, Nat. Neurosci 8 (2007) 481–488. [DOI] [PubMed] [Google Scholar]
- [7].Martino G, Pluchino S, Bonfanti L, Schwartz M, Brain Regeneration in Physiology and Pathology: The Immune Signature Driving Therapeutic Plasicity of Neural Stem Cells, Physiol Rev. 91 (2011) 1281–1304. doi: 10.1152/physrev.00032.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Nemirovich-Danchenko NM, Khodanovich MY, New Neurons in the Post-ischemic and Injured Brain: Migrating or Resident?, Front. Neurosci 13 (2019) 588. doi: 10.3389/fnins.2019.00588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Garcia ADR, Doan NB, Imura T, Bush TG, V Sofroniew M, GFAP-expressing progenitors are the principal source of constitutive neurogenesis in adult mouse forebrain, Nat. Neurosci 7 (2004) 1233–1241. doi: 10.1038/nn1340. [DOI] [PubMed] [Google Scholar]
- [10].Doetsch F, Caille I, Lim DA, Garcı JM, Alvarez-buylla A, Subventricular Zone Astrocytes Are Neural Stem Cells in the Adult Mammalian Brain, Cell. 97 (1999) 703–716. [DOI] [PubMed] [Google Scholar]
- [11].Obemier K, Cebrian-Silla A, Thomson M, Parraguez JI, Anderson R, Guinto C, Rodas Rodriguez J, Garcia-Verdugo JM, Alvarez-Buylla A, Adult Neurogenesis Is Sustained by Symmetric Self-Renewal and Differentiation, Cell Stem Cell. (2018) 221–234.e8. doi: 10.1016/j.stem.2018.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Reeve RL, Yammine SZ, Morshead CM, van der Kooy D, Quiescent Oct4+ Neural Stem Cells (NSCs) Repopulate Ablated Glial Fibrillary Acidic Protein+ NSCs in the Adult Mouse Brain, Stem Cells. 35 (2017) 2071–2082. [DOI] [PubMed] [Google Scholar]
- [13].Sachewsky N, Leeder R, Xu W, Rose KL, Yu F, van der Kooy D, Morshead CM, Primitive Neural Stem Cells in the Adult Mammalian Brain Give Rise to GFAP-Expressing Neural Stem Cells, Stem Cell Reports. 2 (2014) 810–824. doi: 10.1016/j.stemcr.2014.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Carlén M, Meletis K, Göritz C, Darsalia V, Evergren E, Tanigaki K, Amendola M, Bamabé-Heider F, Yeung MSY, Naldini L, Honjo T, Kokaia Z, Shupliakov O, Cassidy RM, Lindvall O, Frisén J, Forebrain ependymal cells are Notch-dependent and generate neuroblasts and astrocytes after stroke, Nat. Neurosci 12 (2009) 259–267. doi: 10.1038/nn.2268. [DOI] [PubMed] [Google Scholar]
- [15].Muthusamy N, Brumm A, Zhang X, Carmichael ST, Ghashghaei HT, Foxj1 expressing ependymal cells do not contribute new cells to sites of injury or stroke in the mouse forebrain, Sci. Rep 8 (2018) 1–9. doi: 10.1038/s41598-018-19913-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Ren Y, Ao Y, O’Shea TM, Burda JE, Bernstein AM, Brumm AJ, Muthusamy N, Ghashghaei HT, Carmichael ST, Cheng L, Sofroniew MV, Ependymal cell contribution to scar formation after spinal cord injury is minimal, local and dependent on direct ependymal injury, Sci. Rep 7 (2017) 1–16. doi: 10.1038/srep41122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Shah PT, Stratton JA, Stykel MG, Abbasi S, Sharma S, Mayr KA, Koblinger K, Whelan PJ, Biernaskie J, Single-Cell Transcriptomics and Fate Mapping of Ependymal Cells Reveals an Absence of Neural Stem Cell Function Matters Arising Single-Cell Transcriptomics and Fate Mapping of Ependymal Cells Reveals an Absence of Neural Stem Cell Function, Cell. 173 (2018) 1045–1052.e9. doi: 10.1016/j.cell.2018.03.063. [DOI] [PubMed] [Google Scholar]
- [18].Tavazoie M, Van Der Veken L, Silva-vargas V, Louissaint M, Colonna L, Zaidi B, Garcia-verdugo JM, Doetsch F, A Specialized Vascular Niche for Adult Neural Stem Cells, Cell Stem Cell. 3 (2008) 279–288. doi: 10.1016/j.stem.2008.07.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Ming G, Song H, Adult Neurogenesis in the Mammalian Brain: Significant Answers and Significant Questions, Neuron. 70 (2011) 687–702. doi: 10.1016/j.neuron.2011.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lin R, Cai J, Kenyon L, Iozzo R, Rosenwasser R, Iacovitti L, Systemic factors trigger vasculature cells to drive Notch signaling and neurogenesis in neural stem cells in the adult brain, Stem Cells. 37 (2019) 395–406. doi: 10.1002/stem.2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Aimone JB, Li Y, Lee SW, Clemenson GD, Deng W, Gage FH, Regulation and Function of Adult Neurogenesis: From Genes to Cognition, Physiol Rev. 94 (2018) 991–1026. doi: 10.1152/physrev.00004.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bovetti S, Hsieh Y, Bovolin P, Perroteau I, Kazunori T, Puche AC, Blood Vessels Form a Scaffold for Neuroblast Migration in the Adult Olfactory Bulb, J. Neurosci 27 (2007) 5976–5980. doi: 10.1523/JNEUROSCI.0678-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Carmichael ST, Archibeque I, Luke L, Nolan T, Momiy J, Li S, Growth-associated gene expression after stroke : Evidence for a growth-promoting region in peri-infarct cortex, Exp. Neurol 193 (2005) 291–311. doi: 10.1016/j.expneurol.2005.01.004. [DOI] [PubMed] [Google Scholar]
- [24].Carmichael ST, Gene expression changes after focal stroke, traumatic brain and spinal cord injuries, Curr. Opin. Neurol 16 (2003) 699–704. doi: 10.1097/01.wco.0000102621.38669.77. [DOI] [PubMed] [Google Scholar]
- [25].Liu XS, Zhang ZG, Zhang RL, Gregg S, Morris DC, Wang Y, Chopp M, Stroke induces gene profile changes associated with neurogenesis and angiogenesis in adult subventricular zone progenitor cells, J. Cereb. Blood Flow Metab 27 (2007) 564–574. doi: 10.1038/sj.jcbfm.9600371. [DOI] [PubMed] [Google Scholar]
- [26].Sun C, Sun H, Wu S, Lee CC, Akamatsu Y, Wang RK, Kemie SG, Liu J, Conditional Ablation of Neuroprogenitor Cells in Adult Mice Impedes Recovery of Poststroke Cognitive Function and Reduces Synaptic Connectivity in the Perforant Pathway, J. Neurosci 33 (2013) 17314–17325. doi: 10.1523/JNEUROSCI.2129-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Thored P, Arvidsson A, Cacci E, Ahlenius H, Kallur T, Darsalia V, Ekdahl CT, Kokaia Z, Lindvall O, Persistent Production of Neurons from Adult Brain Stem Cells During Recovery after Stroke, Stem Cells. 24 (2006) 739–747. doi: 10.1634/stemcells.2005-0281. [DOI] [PubMed] [Google Scholar]
- [28].Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O, Neuronal replacement from endogenous precursors in the adult brain after stroke, Nat. Med 8 (2002) 963–970. doi: 10.1038/nm. [DOI] [PubMed] [Google Scholar]
- [29].Parent JM, Vexler ZS, Gong C, Derugin N, Ferriero DM, Rat Forebrain Neurogenesis and Striatal Neuron Replacement after Focal Stroke, Ann. Neurol 52 (2002) 802–813. [DOI] [PubMed] [Google Scholar]
- [30].Li L, Harms KM, Ventura PB, Lagace DC, Eisch AJ, Cunningham LA, Ventura PB, Lagace DC, Eisch AJ, Cunningham LA, Ventura PB, Lagace DC, Eisch AJ, Cunningham LA, Focal Cerebral Ischemia Induces a Multilineage Cytogenic Response from Adult Subventricular Zone that is Predominantly Gliogenic, Glia. 58 (2010) 1610–1619. doi: 10.1002/glia.21033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Zhang RL, Zhang ZG, Zhang L, Chopp MY, Proliferation and Differentiation of Progenitor Cells in the Cortex and the Subventricular Zone in the Adult Rat after Focal Cerebral Ischemia, Neuroscience. 105 (2001) 33–41. [DOI] [PubMed] [Google Scholar]
- [32].Jin K, Minami M, Lan JQ, Mao XO, Batteur S, Simon RP, Greenberg DA, Neurogenesis in dentate subgranular zone and rostral subventricular zone after focal cerebral ischemia in the rat, Proc. Natl. Acad. Sci. U. S. A. 98 (2001) 4710–4715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Tonchev AB, Yamashima T, Zhao L, Okano HJ, Okano H, Proliferation of neural and neuronal progenitors after global brain ischemia in young adult macaque monkeys, Mol. Cell. Neurosci 23 (2003) 292–301. doi: 10.1016/S1044-7431(03)00058-7. [DOI] [PubMed] [Google Scholar]
- [34].Jin K, Wang X, Xie L, Mao XO, Zhu W, Wang Y, Shen J, Mao Y, Banwait S, D A. Greenberg, Evidence for stroke-induced neurogenesis in the human brain, Proc. Natl. Acad. Sci 103 (2006) 13198–13202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Marti-Fabregas J, Romaguera-Ros M, Pinedo-Gomez U, Martinez-Ramirez S, Jiminez-Xarrie E, Marin R, Marto-Vilalta J-L, Garcia-Verdugo J-M, Proliferation in the human ipsilateral subventricular zone after ischemic stroke, Neurology. 74 (2010) 357–65. [DOI] [PubMed] [Google Scholar]
- [36].Macas J, Nern C, Plate KH, Momma S, Increased Generation of Neuronal Progenitors after Ischemic Injury in the Aged Adult Human Forebrain, J. Neurosci 26 (2006) 13114–13119. doi: 10.1523/JNEUROSCI.4667-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Huttner HB, Bergmann O, Salehpour M, Rácz A, Tatarishvili J, Lindgren E, Csonka T, Csiba L, Hortobágyi T, Méhes G, Englund E, Solnestam BW, Zdunek S, Scharenberg C, Ström L, Ståhl P, Sigurgeirsson B, Dahl A, Schwab S, Possnert G, Bernard S, Kokaia Z, Lindvall O, Lundeberg J, Frisén J, The age and genomic integrity of neurons after cortical stroke in humans, Nat. Neurosci 17 (2014) 801–803. doi: 10.1038/nn.3706. [DOI] [PubMed] [Google Scholar]
- [38].Ohab JJ, Fleming S, Blesch A, Carmichael ST, A neurovascular niche for neurogenesis after stroke, J. Neurosci 26 (2006) 13007–13016. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kannangara TS, Carter A, Xue Y, Dhaliwal JS, Béïque J-C, Lagace DC, Excitable Adult-Generated GABAergic Neurons Acquire Functional Innervation in the Cortex after Stroke, Stem Cell Reports. 0(2018) 1–10. doi: 10.1016/j.stemcr.2018.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Faiz M, Sachewsky N, Gascon S, Bang KWA, Morshead CM, Nagy A, Adult Neural Stem Cells from the Subventricular Zone Give Rise to Reactive Astrocytes in the Cortex after Stroke, Cell Stem Cell. 17 (2015) 624–634. doi: 10.1016/j.stem.2015.08.002. [DOI] [PubMed] [Google Scholar]
- [41].Williamson MR, Dunn AK, Drew MR, Jones TA, Post-stroke Vascular Remodeling and the Role of Neural Precursor Cells, in: Int. Stroke Conf, 2019. doi: 10.1161/str.50.suppl_1.WP149. [DOI] [Google Scholar]
- [42].Kojima T, Hirota Y, Ema M, Takahashi S, Miyoshi I, Okano H, Sawamoto K, Subventricular Zone-Derived Neural Progenitor Cells Migrate Along a Blood Vessel Scaffold Toward the Post-stroke Striatum, Stem Cells. 28 (2010) 545–554. doi: 10.1002/stem.306. [DOI] [PubMed] [Google Scholar]
- [43].Ohab JJ, Carmichael ST, Poststroke Neurogenesis: Emerging Principles of Migration and Localization of Immature Neurons, Neurosci. 14 (2008) 369–380. doi: 10.1177/1073858407309545. [DOI] [PubMed] [Google Scholar]
- [44].Dibajnia P, Morshead CM, Role of neural precursor cells in promoting repair following stroke, Acta Pharmacol. Sin 34 (2012) 78–90. doi: 10.1038/aps.2012.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Tsai PT, Ohab JJ, Kertesz N, Groszer M, Matter C, Gao J, Liu X, Wu H, Carmichael ST, A Critical Role of Erythropoietin Receptor in Neurogenesis and Post-Stroke Recovery, J. Neurosci 26 (2006) 1269–1274. doi: 10.1523/JNEUROSCI.4480-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Osman AM, Porritt MJ, Nilsson M, Kuhn HG, Long-Term Stimulation of Neural Progenitor Cell Migration After Cortical Ischemia in Mice, Stroke. 42 (2011) 3559–3565. doi: 10.1161/STROKEAHA.111.627802. [DOI] [PubMed] [Google Scholar]
- [47].Turnley AM, Basrai HS, Christie KJ, Is integration and survival of newborn neurons the bottleneck for effective neural repair by endogenous neural precursor cells?, Front. Neurosci 8 (2014) 29. doi: 10.3389/fnins.2014.00029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Lagace DC, Does the endogenous neurogenic response alter behavioral recovery following stroke?, Behav. Brain Res 227 (2012) 426–432. doi: 10.1016/j.bbr.2011.08.045. [DOI] [PubMed] [Google Scholar]
- [49].Wang X, Mao X, Xie L, Sun F, Greenberg DA, Jin K, Conditional depletion of neurogenesis inhibits long-term recovery after experimental stroke in mice, PLoS One. 7 (2012) e38932. doi: 10.1371/journal.pone.0038932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Sun F, Wang X, Mao X, Xie L, Jin K, Ablation of Neurogenesis Attenuates Recovery of Motor Function after Focal Cerebral Ischemia in Middle-Aged Mice, PLoS One. 7 (2012) e46326. doi: 10.1371/journal.pone.0046326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Jin K, Wang X, Xie L, Mao XO, Greenberg DA, Transgenic ablation of doublecortin-expressing cells suppresses adult neurogenesis and worsens stroke outcome in mice., Proc. Natl. Acad. Sci. U. S. A. 107 (2010) 7993–8. doi: 10.1073/pnas.1000154107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Kleim JA, Boychuk JA, Adkins DL, Rat Models of Upper Extremity Impairment in Stroke, ILAR J. 48 (2007) 374–384. [DOI] [PubMed] [Google Scholar]
- [53].Liang H, Zhao H, Gleichman A, Machnicki M, Telang S, Tang S, Rshtouni M, Ruddell J, Carmichael ST, Region-specific and activity-dependent regulation of SVZ neurogenesis and recovery after stroke, PNAS. 116 (2019) 13621–13630. doi: 10.1073/pnas,1811825116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Raber J, Fan Y, Matsumori Y, Liu Z, Weinstein PR, Fike JR, Liu J, Irradiation Attenuates Neurogenesis and Exacerbates Ischemia-Induced Deficits, Ann. Neurol 55 (2004) 381–389. [DOI] [PubMed] [Google Scholar]
- [55].Luo CX, Jiang J, Zhou QG, Zhu XJ, Wang W, Zhang ZJ, Han X, Zhu DY, Voluntary Exercise-Induced Neurogenesis in the Postischemic Dentate Gyrus Is Associated With Spatial Memory Recovery From Stroke, J. Neurosci. Res 85 (2007) 1637–1646. doi: 10.1002/jnr.21317. [DOI] [PubMed] [Google Scholar]
- [56].Niv F, Keiner S, Krishna-K, Witte OW, Lie DC, Redecker C, Aberrant neurogenesis after stroke: A retroviral cell labeling study, Stroke. 43 (2012) 2468–2475. doi: 10.1161/STROKEAHA.112.660977. [DOI] [PubMed] [Google Scholar]
- [57].Woitke F, Ceanga M, Rudolph M, Niv F, Witte OW, Redecker C, Kunze A, Keiner S, Adult hippocampal neurogenesis poststroke : More new granule cells but aberrant morphology and impaired spatial memory, PLoS One. 12 (2017) e0183463. doi: 10.1371/journal.pone.0183463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Cuartero MI, Lizasoain I, Ángeles M, Cuartero MI, De Parra J, Pérez-ruiz A, Bravo-ferrer I, Durán-laforet V, Lizasoain I, Moro MÁ, Abolition of aberrant neurogenesis ameliorates cognitive impairment after stroke in mice Find the latest version : Abolition of aberrant neurogenesis ameliorates cognitive impairment after stroke in mice, 129 (2019) 1536–1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Ceanga M, Keiner S, Grünewald B, Haselmann H, Frahm C, Couillard-Despres S, Witte OW, Redecker C, Geis C, Kunze A, Stroke accelerates and uncouples intrinsic and synaptic excitability maturation of mouse hippocampal DCX + adult-born granule cells, J. Neurosci 39 (2019) 1755–1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Yao B, Christian KM, He C, Jin P, Ming GL, Song H, Epigenetic mechanisms in neurogenesis, Nat. Rev. Neurosci 17 (2016) 537–549. doi: 10.1038/nrn.2016.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Luo J, Daniels SB, Lennington JB, Noth RQ, Conover JC, The aging neurogenic subventricular zone, Aging Cell. (2006) 139–152. doi: 10.1111/j.l474-9726.2006.00197.x. [DOI] [PubMed] [Google Scholar]
- [62].Maslov AY, Barone TA, Plunkett RJ, Pruitt SC, Neural Stem Cell Detection , Characterization , and Age-Related Changes in the Subventricular Zone of Mice, J. Neurosci 24 (2004) 1726–1733. doi: 10.1523/JNEUROSCI.4608-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Bouab M, Paliouras GN, Aumont A, Berard-Forest K, Fernandes KJL, Aging of the Subventricular Zone Neural Stem Cell Niche: Evidence for Quiescence-Associated Changes Between Early and Mid-Adulthood, Neuroscience. 173 (2011) 135–149. doi: 10.1016/j.neuroscience.2010.11.032. [DOI] [PubMed] [Google Scholar]
- [64].Codega P, Silva-vargas V, Paul A, Maldonado-Soto AR, Deleo AM, Pastrana E, Doetsch F, Prospective Identification and Purification of Quiescent Adult Neural Stem Cells from Their In Vivo Niche, Neuron. 82 (2014) 545–559. doi: 10.1016/j.neuron.2014.02.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Decarolis NA, Kirby ED, Wyss-Coray T, Palmer TD, The role of the microenvironmental niche in declining stem-cell functions associated with biological aging, Cold Spring Harb. Perspect. Med 5 (2015). doi: 10.1101/cshperspect.a025874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Piccin D, Tufford A, Morshead CM, Neural stem and progenitor cells in the aged subependyma are activated by the young niche, Neurobiol. Aging. 35 (2014) 1669–1679. doi: 10.1016/j.neurobiolaging.2014.01.026. [DOI] [PubMed] [Google Scholar]
- [67].Ruddy RM, Morshead CM, Home sweet home: the neural stem cell niche throughout development and after injury, Cell Tissue Res. (2017). doi: 10.1007/s00441-017-2658-0. [DOI] [PubMed] [Google Scholar]
- [68].Katsimpardi L, Litterman NK, Schein PA, Miller CM, Loffredo FS, Wojtkiewicz GR, Chen JW, Lee RT, Wagers AJ, Rubin LL, Vascular and Neurogenic Rejuvenation of the Aging Mouse Brain by Young Systemic Factors, Science (80-,). 344 (2014) 630–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Villeda SA, Luo J, Mosher KI, Zou B, Britschgi M, Bieri G, Stan TM, Fainberg N, Ding Z, Eggel A, Lucin KM, Czirr E, Park J-S, Couillard-Despres S, Li G, Peskind ER, Kaye JA, Quinn JF, Galasko DR, Xie XS, Rando TA, Wyss-Coray T, The ageing systemic milieu negatively regulates neurogenesis and cognitive function, Nature. 477 (2011) 90–94. doi: 10.1038/nature10357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Villeda SA, Plambeck KE, Middeldorp J, Castellano JM, Mosher KI, Luo J, Smith LK, Bieri G, Lin K, Berdnik D, Wabl R, Udeochu J, Wheatley EG, Zou B, Simmons DA, Xie XS, Longo FM, Wyss-Coray T, Young blood reverses age-related impairments in cognitive function and synaptic plasticity in mice, Nat. Med 20 (2014) 659–663. doi: 10.1038/nm.3569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Jin K, Minami M, Xie L, Sun Y, Mao XO, Wang Y, Simon RP, Greenberg DA, Ischemia-induced neurogenesis is preserved but reduced in the aged rodent brain, Aging Cell. (2004) 373–377. doi: 10.1111/j.1474-9728.2004.00131.x. [DOI] [PubMed] [Google Scholar]
- [72].Adamczak J, Aswendt M, Kreutzer C, Rotheneichner P, Riou A, Selt M, Beyrau A, Uhlenkiiken U, Diedenhofen M, Nelles M, Aigner L, Couillard-Despres S, Hoehn M, Neurogenesis upregulation on the healthy hemisphere after stroke enhances compensation for age-dependent decrease of basal neurogenesis, Neurobiol. Dis 99 (2017) 47–57. doi: 10.1016/j.nbd.2016.12.015. [DOI] [PubMed] [Google Scholar]
- [73].Darsalia V, Heldmann U, Lindvall O, Kokaia Z, Stroke-Induced Neurogenesis in Aged Brain, Stroke. (2005) 1790–1795. doi: 10.1161/01.STR.0000173151.36031.be. [DOI] [PubMed] [Google Scholar]
- [74].Kirby ED, Kuwahara AA, Messer RL, Wyss-Coray T, Adult hippocampal neural stem and progenitor cells regulate the neurogenic niche by secreting VEGF, Proc. Natl. Acad. Sci 112 (2015) 4128–4133. doi: 10.1073/pnas,1422448112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Van Praag H, Kempermann G, Gage FH, Running increases cell proliferation and neurogenesis in the adult mouse dentate gyrus, Nat. Neurosci 2 (1999) 266–270. [DOI] [PubMed] [Google Scholar]
- [76].Kempermann G, Kuhn HG, Gage FH, More hippocampal neurons in adults mice living in an enriched environment, Nature. 386 (1997) 493–495. [DOI] [PubMed] [Google Scholar]
- [77].Nygren J, Wieloch T, Pesic J, Brundin P, Deierborg T, Enriched Environment Attenuates Cell Genesis in Subventricular Zone After Focal Ischemia in Mice and Decreases Migration of Newborn Cells to the Striatum, Stroke. 37 (2006) 2824–2829. [DOI] [PubMed] [Google Scholar]
- [78].Komitova M, Mattsson Β, Johansson ΒΒ, Eriksson PS, Enriched Environment Increases Neural Stem/Progenitor Cell Proliferation and Neurogenesis in the Subventricular Zone of Stroke-Lesioned Adult Rats, Stroke. 36 (2005) 1278–1282. doi: 10.1161/01.STR.0000166197.94147.59. [DOI] [PubMed] [Google Scholar]
- [79].Komitova M, Zhao LR, Gido G, Johansson BB, Eriksson P, Postischemic exercise attenuates whereas enriched environment has certain enhancing effects on lesion- induced subventricular zone activation in the adult rat, Eur. J. Neurosci 21 (2005) 2397–2405. doi: 10.1111/j.1460-9568.2005.04072.x. [DOI] [PubMed] [Google Scholar]
- [80].Qu HL, Zhao M, Zhao SS, Xiao T, Song CG, Cao YP, Jolkkonen J, Zhao CS, Forced Limb-use enhanced neurogenesis and behavioral recovery after stroke in the aged rats, Neuroscience. 286 (2015) 316–324. doi: 10.1016/j.neuroscience.2014.11.040. [DOI] [PubMed] [Google Scholar]
- [81].Li C, Zhang B, Zhu Y, Li Y, Liu P, Gao B, Tian S, Du L, Bai Y, Post-stroke Constraint-induced Movement Therapy Increases Functional Recovery, Angiogenesis, and Neurogenesis with Enhanced Expression of HIF-1α and VEGF, Curr. Neurovasc. Res 14 (2017) 368–377. [DOI] [PubMed] [Google Scholar]
- [82].Zhao S, Zhao Y, Xiao T, Zhao M, Jolkkonen J, Zhao C, Increased Neurogenesis Contributes to the Promoted Behavioral Recovery by Constraint-Induced Movement Therapy after Stroke in Adult Rats, CNS Neurosci. Ther 19 (2013) 194–196. doi: 10.1111/cns.12058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Zhai Z-Y, Feng J, Constraint-induced movement therapy enhances angiogenesis and neurogenesis after cerebral ischemia/reperfusion, Neural Regen. Res 14 (2015) 1743–1754. doi: 10.4103/1673-5374.257528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Mizutani K, Sonoda S, Yamada K, Beppu H, Shimpo K, Alteration of protein expression profile following voluntary exercise in the perilesional cortex of rats with focal cerebral infarction, Brain Res. 1416 (2011) 61–68. doi: 10.1016/j.brainres.2011.08.012. [DOI] [PubMed] [Google Scholar]
- [85].Pang Q, Zhang H, Chen Z, Wu Y, Bai M, Liu Y, Zhao Y, Tu F, Liu C, Chen X, Role of caveolin-1 / vascular endothelial growth factor pathway in basic fibroblast growth factor-induced angiogenesis and neurogenesis after treadmill training following focal cerebral ischemia in rats, Brain Res. 1663 (2017) 9–19. doi: 10.1016/j.brainres.2017.03.012. [DOI] [PubMed] [Google Scholar]
- [86].Covacu R, Brundin L, Effects of Neuroinflammation on Neural Stem Cells, Neurosci. 23 (2017) 27–39. doi: 10.1177/1073858415616559. [DOI] [PubMed] [Google Scholar]
- [87].Qin C, Zhou L-Q, Ma X-T, Hu Z-W, Yang S, Chen M, Bosco DB, Wu L-J, Tian D-S, Dual Functions of Microglia in Ischemic Stroke, Neurosci. Bull, epub (2019). doi: 10.1007/s12264-019-00388-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Thored P, Heldmann U, Gomes-Leal W, Gisler R, Darsalia V, Taneera J, Nygren JM, Jacobsen S-EW, Ekdahl CT, Kokaia Z, Lindvall O, Long-Term Accumulation of Microglia with Proneurogenic Phenotype Concomitant with Persistent Neurogenesis in Adult Subventricular Zone After Stroke, Glia. 849 (2009) 835–849. doi: 10.1002/glia.20810. [DOI] [PubMed] [Google Scholar]
- [89].Chapman KZ, Ge R, Monni E, Tatarishvili J, Ahlenius H, Arvidsson A, Ekdahl CT, Lindvall O, Kokaia Z, Inflammation without neuronal death triggers striatal neurogenesis comparable to stroke, Neurobiol. Dis 83 (2015) 1–15. doi: 10.1016/j.nbd.2015.08.013. [DOI] [PubMed] [Google Scholar]
- [90].Tobin MK, Bonds JA, Minshall RD, Pelligrino DA, Testai FD, Lazarov O, Neurogenesis and inflammation after ischemic stroke: what is known and where we go from here, J. Cereb. Blood Flow Metab 06 (2014) 1573–1584. doi: 10.1038/jcbfm.2014.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Hoehn BD, Palmer TD, Steinberg GK, Neurogenesis in Rats After Focal Cerebral Ischemia is Enhanced by Indomethacin, Stroke. 36 (2005) 2718–2724. doi: 10.1161/01.STR.0000190020.30282.cc. [DOI] [PubMed] [Google Scholar]
- [92].Liu Z, Fan Y, Won SJ, Neumann M, Hu D, Zhou L, Weinstein PR, Liu J, Chronic Treatment With Minocycline Preserves Adult New Neurons and Reduces Functional Impairment After Focal Cerebral Ischemia, Stroke. (2006) 146–152. doi: 10.1161/01.STR.0000251791.64910.cd. [DOI] [PubMed] [Google Scholar]
- [93].Saino O, Taguchi A, Nakagomi T, Nakano-Doi A, Kashiwamura SI, Doe N, Nakagomi N, Soma T, Yoshikawa H, Stem DM, Okamura H, Matsuyama T, Immunodeficiency reduces neural stem/progenitor cell apoptosis and enhances neurogenesis in the cerebral cortex after stroke, J. Neurosci. Res 88 (2010) 2385–2397. doi: 10.1002/jnr.22410. [DOI] [PubMed] [Google Scholar]
- [94].Franceschi C, Campisi J, Chronic inflammation (Inflammaging) and its potential contribution to age-associated diseases, Journals Gerontol. - Ser. A Biol. Sci. Med. Sci 69 (2014) S4–S9. doi: 10.1093/gerona/glu057. [DOI] [PubMed] [Google Scholar]
- [95].Doetsch F, Hen R, Young and excitable: the function of new neurons in the adult mammalian brain, Curr. Opin. Neurobiol 15 (2005) 121–128. [DOI] [PubMed] [Google Scholar]
- [96].Clarkson AN, Huang BS, Macisaac SE, Mody I, Carmichael ST, Reducing excessive GABA-mediated tonic inhibition promotes functional recovery after stroke., Nature. 468 (2010) 305–309. doi: 10.1038/nature09511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [97].Ramirez-Amaya V, Marrone DF, Gage FH, Worley PF, Barnes CA, Integration of New Neurons into Functional Neural Networks, J. Neurosci 26 (2006) 12237–12241. doi: 10.1523/JNEUROSCI.2195-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [98].Caracciolo L, Marosi M, Mazzitelli J, Latifi S, Sano Y, Galvan L, Kawaguchi R, Holley S, Levine MS, Coppola G, Portera-Cailliau C, Silva AJ, Carmichael ST, CREB controls cortical circuit plasticity and functional recovery after stroke, Nat. Commun 9 (2018). doi: 10.1038/s41467-018-04445-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Kim SY, Hsu JE, Husbands LC, Kleim JA, Jones TA, Coordinated Plasticity of Synapses and Astrocytes Underlies Practice-Driven Functional Vicariation in Peri-Infarct Motor Cortex, J. Neurosci 38 (2018) 93–107. doi: 10.1523/JNEUROSCI.1295-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [100].Yamashita T, Ninomiya M, Hernandez Acosta P, Garcia-Verdugo JM, Sunabori T, Sakaguchi M, Adachi K, Kojima T, Hirota Y, Kawase T, Araki N, Abe K, Okano H, Sawamoto K, Subventricular Zone-Derived Neuroblasts Migrate and Differentiate into Mature Neurons in the Post-Stroke Adult Striatum, J. Neurosci 26 (2006) 6627–6636. doi: 10.1523/JNEUROSCI.0149-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Lai B, Ou X, Xie L, Jin K, Greenberg DA, Electrophysiological neurodifferentiation of subventricular zone-derived precursor cells following stroke, Neurosci. Lett 442 (2008) 305–308. doi: 10.1016/j.neulet.2008.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [102].Hou S-W, Wang Y-Q, Xu M, Shen D-H, Wang J-J, Huang F, Yu Z, Sun F-Y, Functional Integration of Newly Generated Neurons Into Striatum After Cerebral Ischemia in the Adult Rat Brain, Stroke. 39 (2008) 2837–2844. doi: 10.1161/STROKEAHA.107.510982. [DOI] [PubMed] [Google Scholar]
- [103].Murphy TH, Corbett D, Plasticity during stroke recovery: from synapse to behaviour., Nat. Rev. Neurosci 10 (2009) 861–872. doi: 10.1038/nrn2735. [DOI] [PubMed] [Google Scholar]
- [104].Toni N, Schinder AF, Maturation and Functional Integration of New Granule Cells into the Adult Hippocampus, Cold Spring Harb. Perspect. Biol 8 (2015) aO 18903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Sofroniew MV, Molecular dissection of reactive astrogliosis and glial scar formation, Trends Neurosci. (2009) 638–647. doi: 10.1016/j.tins.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Benner EJ, Luciano D, Jo R, Abdi K, Paez-Gonzalez P, Sheng H, Warner D, Liu C, Eroglu C, Kuo CT, Post-injury protective astrogenesis from SVZ niche is controlled by Notch modulator Thbs4, Nature. 497 (2013) 369–373. doi: 10.1038/nature12069.Post-injury. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Kolb B, Morshead C, Gonzalez C, Kim M, Gregg C, Shingo T, Weiss S, Growth factor-stimulated generation of new cortical tissue and functional recovery after stroke damage to the motor cortex of rats., J. Cereb. Blood Flow Metab 27 (2007) 983–997. doi: 10.1038/sj.jcbfm.9600402. [DOI] [PubMed] [Google Scholar]
- [108].Martino G, Pluchino S, The therapeutic potential of neural stem cells, Nat. Rev. Neurosci 7 (2006) 395–406. [DOI] [PubMed] [Google Scholar]
- [109].Bacigaluppi M, Pluchino S, Jametti LP, Kilic E, Salani G, Brambilla E, West MJ, Comi G, Delayed post-ischaemic neuroprotection following systemic neural stem cell transplantation involves multiple mechanisms, Brain. 132 (2009) 2239–2251. doi: 10.1093/brain/awpl74. [DOI] [PubMed] [Google Scholar]
- [110].Andres RH, Horie N, Slikker W, Keren-Gill H, Zhan K, Sun G, Manley NC, Pereira MP, Sheikh LA, Mcmillan EL, Schaar BT, Svendsen CN, Bliss TM, Steinberg GK, Human neural stem cells enhance structural plasticity and axonal transport in the ischaemic brain, Brain. 134 (2011) 1777–1789. doi: 10.1093/brain/awr094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [111].Webb RL, Kaiser EE, Scoville SL, Thompson TA, Fatima S, Pandya C, Sriram K, Swetenburg RL, Vaibhav K, Arbab AS, Baban B, Dhandapani KM, Hess DC, Hoda MN, Stice SL, Human Neural Stem Cell Extracellular Vesicles Improve Tissue and Functional Recovery in the Murine Thromboembolic Stroke Model, Transl. Stroke Res 9 (2018) 530–539. doi: 10.1007/s12975-017-0599-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [112].Webb RL, Kaiser EE, Jurgielewicz BJ, Spellicy S, Scoville SL, Thompson TA, Swetenburg RL, Hess DC, West FD, Stice SL, Human Neural Stem Cell Extracellular Vesicles Improve Recovery in a Porcine Model of Ischemic Stroke, Stroke. 49 (2018) 1248–1256. doi: 10.1161/STROKEAHA.117.020353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Otero-Ortega L, Laso-García F, Del Carmen Gómez-De Frutos M, Rodríguez-Frutos B, Pascual-Guerra J, Fuentes B, Díez-Tejedor E, Gutiérrez-Femández M, White matter repair after extracellular vesicles administration in an experimental animal model of subcortical stroke, Sci. Rep 7 (2017) 1–11. doi: 10.1038/srep44433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Tang Y, Wang J, Lin X, Wang L, Shao B, Jin K, Wang Y, Yang GY, Neural stem cell protects aged rat brain from ischemia-reperfusion injury through neurogenesis and angiogenesis, J. Cereb. Blood Flow Metab 34 (2014) 1138–1147. doi: 10.1038/jcbfm.2014.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Singer BH, Jutkiewicz EM, Fuller CL, Lichtenwalner RJ, Zhang H, Velander AJ, Li X, Gnegy ME, Burant CF, Parent JM, Conditional Ablation and Recovery of Forebrain Neurogenesis in the Mouse, J. Comp. Neurol 582 (2009) 567–582. doi: 10.1002/cne.22052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [116].Butti E, Bacigaluppi M, Rossi S, Cambiaghi M, Bari M, Silla AC, Brambilla E, Musella A, De Ceglia R, Teneud L, De Chiara V, Adamo PD, Garcia-verdugo JM, Comi G, Muzio L, Quattrini A, Leocani L, Maccarrone M, Centonze D, Martino G, Subventricular zone neural progenitors protect striatal neurons from glutamatergic excitotoxicity, Brain. (2012) 3320–3335. doi: 10.1093/brain/aws194. [DOI] [PubMed] [Google Scholar]
- [117].Jones TA, Adkins DL, Motor System Reorganization After Stroke: Stimulating and Training Toward Perfection, Physiology. 30 (2015) 358–370. doi: 10.1152/physiol.00014.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Butti E, Cusimano M, Bacigaluppi M, Martino G, Neurogenic and non-neurogenic functions of endogenous neural stem cells, Front. Neurosci 8 (2014) 92. doi: 10.3389/fnins.2014.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [119].Cusimano M, Biziato D, Brambilla E, Doneg M, Alfaro-Cervello C, Snider S, Salani G, Pucci F, Comi G, Garcia-Verdugo JM, De Palma M, Martino G, Pluchino S, Transplanted neural stem/precursor cells instruct phagocytes and reduce secondary tissue damage in the injured spinal cord, Brain. 135 (2012) 447–460. doi: 10.1093/brain/awr339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Zhang ZG, Chopp M, Promoting brain remodeling to aid in stroke recovery, Trends Mol. Med 21 (2015) 543–548. doi: 10.1016/j.molmed.2015.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Zhang G, Chen L, Guo X, Wang H, Chen W, Wu G, Gu B, Miao W, Kong J, Jin X, Yi G, You Y, Su X, Gu N, Comparative analysis of microRNA expression profiles of exosomes derived from normal and hypoxic preconditioning human neural stem cells by next generation sequencing, J. Biomed. Nanotechnol 14 (2018) 1075–1089. doi: 10.1166/jbn.2018.2567. [DOI] [PubMed] [Google Scholar]
- [122].Drago D, Cossetti C, Iraci N, Gaude E, Musco G, Bachi A, Pluchino S, The stem cell secretome and its role in brain repair, Biochimie. 95 (2013) 2271–2285. doi: 10.1016/j.biochi.2013.06.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Ottoboni L, Merlini A, Martino G, Neural Stem Cell Plasticity: Advantages in Therapy for the Injured Central Nervous System, Front. Cell Dev. Biol 5 (2017) 52. doi: 10.3389/fcell.2017.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Lu P, Jones LL, Snyder EY, Tuszynski MH, Neural stem cells constitutively secrete neurotrophic factors and promote extensive host axonal growth after spinal cord injury, Exp. Neurol 181 (2003) 115–129. doi: 10.1016/S0014-4886(03)00037-2. [DOI] [PubMed] [Google Scholar]
- [125].Mi R, Luo Y, Cai J, Limke TL, Rao MS, Hoke A, Immortalized neural stem cells differ from nonimmortalized cortical neurospheres and cerebellar granule cell progenitors, Exp. Neurol 194 (2005) 301–319. doi: 10.1016/j.expneurol.2004.07.011. [DOI] [PubMed] [Google Scholar]
- [126].Ploughman M, Windle V, MacLellan CL, White N, Doré JJ, Corbett D, Brain-derived neurotrophic factor contributes to recovery of skilled reaching after focal ischemia in rats, Stroke. 40 (2009) 1490–1495. doi: 10.1161/STROKEAHA.108.531806. [DOI] [PubMed] [Google Scholar]
- [127].Blurton-Jones M, Kitazawa M, Martinez-coria H, Castello NA, Mu F-J, Loring JF, Yamasaki TR, Poon WW, Green KN, LaFerla FM, Neural stem cells improve cognition via BDNF in a transgenic model of Alzheimer disease, 106 (2009) 13594–13599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [128].Ryu JK, Kim J, Cho SJ, Hatori K, Nagai A, Choi HB, Lee MC, McLamon JG, Kim SU, Proactive transplantation of human neural stem cells prevents degeneration of striatal neurons in a rat model of Huntington disease, Neurobiol. Dis 16 (2004) 68–77. doi: 10.1016/j.nbd.2004.01.016. [DOI] [PubMed] [Google Scholar]
- [129].Ziv Y, Avidan H, Pluchino S, Martino G, Schwartz M, Synergy between immune cells and adult neural stem/progenitor cells promotes functional recovery from spinal cord injury, Proc. Natl. Acad. Sci. U. S. A. 103 (2006) 13174–13179. doi: 10.1073/pnas.0603747103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Mosher KI, Andres RH, Fukuhara T, Bieri G, Hasegawa-Moriyama M, He Y, Guzman R, Wyss-Coray T, Neural progenitor cells regulate microglia functions and activity, Nat. Neurosci 15 (2012) 1485–1487. doi: 10.1038/nn.3233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Kokaia Z, Martino G, Schwartz M, Lindvall O, Cross-talk between neural stem cells and immune cells: the key to better brain repair?, Nat. Neurosci 15 (2012) 1078–1087. doi: 10.1038/nn.3163. [DOI] [PubMed] [Google Scholar]
- [132].Boshuizen MCS, Steinberg GK, Stem Cell-Based Immunomodulation After Stroke, Stroke. 49 (2018) 1563–1570. doi: 10.1161/STROKEAHA.117.020465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [133].Mine Y, Tatarishvili J, Oki K, Monni E, Kokaia Z, Lindvall O, Neurobiology of Disease Grafted human neural stem cells enhance several steps of endogenous neurogenesis and improve behavioral recovery after middle cerebral artery occlusion in rats, Neurobiol. Dis 52 (2013) 191–203. doi: 10.1016/j.nbd.2012.12.006. [DOI] [PubMed] [Google Scholar]
- [134].Cusimano M, Brambilla E, Capotondo A, De Feo D, Tomasso A, Comi G, Adamo PD, Muzio L, Martino G, Selective killing of spinal cord neural stem cells impairs locomotor recovery in a mouse model of spinal cord injury, J. Neuroinflammation. 15 (2018) 58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Huckleberry KA, Shue F, Copeland T, Chitwood RA, Yin W, Drew MR, Dorsal and ventral hippocampal adult-born neurons contribute to context fear memory, Neuropsychopharmacology. 43 (2018) 2487–2496. doi: 10.1038/s41386-018-0109-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Nishiyama A, Komitova M, Suzuki R, Zhu X, Polydendrocytes (NG2 cells): multifunctional cells with lineage plasticity, Nat. Rev. Neurosci 10 (2009) 9–22. doi: 10.1038/nrn2495. [DOI] [PubMed] [Google Scholar]


