Abstract
Discovering that the anesthetic drug ketamine has rapidly acting antidepressant effects in many individuals with major depression is one of the most important findings in clinical psychopharmacology in recent decades. The initial report of these effects in human subjects was based on a foundation of rodent preclinical studies carried out in the 1990s, and subsequent investigation has included both further studies in individuals with depression, as well as reverse translational experiments in animal models, especially rodents. While there is general agreement in the rodent literature that ketamine has rapidly-acting, and generally sustained, antidepressant-like properties, there are also points of contention across studies, including the precise mechanism of action of this drug. In this review, we briefly summarize prominent yet variable findings regarding the mechanism of action. We also discuss a combination of similarities and variances in the rodent literature in the antidepressant-like effects of ketamine as a function of dose, species and strain, test, stressor, and presumably sex of the experimenter. We then present previously unpublished mouse strain comparison data suggesting that subanesthetic ketamine does not have robust antidepressant-like properties in unstressed animals, and may actually promote depression-like behavior, in contrast to widely reported findings. We conclude that the data best support the notion of ketamine action principally via NMDA receptor antagonism, transiently boosting glutamatergic (and possibly other) signaling in diverse brain circuits. We also suggest that future studies should address in greater detail the extent to which antidepressant-like properties of this drug are stress-sensitive, in an effort to better model major depression present in humans.
INTRODUCTION
Ketamine is currently gaining in clinical use for major depression. Interestingly, many features of ketamine action shown in human patients are also seen in rodents, and many neuroscientists are using rodent models of these effects to study the neurobiological mechanisms of ketamine action on the brain – to better understand both ketamine and depression-related biology itself. In this review we will focus on the rodent literature, with an initial brief background in human clinical findings. Of note, this review is composed of two parts, each relating to major points of variance in the realm of rodent ketamine research. The first part deals with variable results regarding the mechanism of action of ketamine in its antidepressant-like action – this point is of import for developing similar compounds in the future as therapeutics. The second part of this review is a meta-analysis of the consistency or variability among antidepressive-like behavioral outcomes published about rodents. This is done with a particular eye toward the role of psychosocial stress, given the importance of that variable in allowing translation. In covering these two major topics we attempt to discuss two major axes of difficulty in the field now. Throughout the rest of the text we will refer to “ketamine” as its typically administered racemic mixture, (R,S)-ketamine.
HISTORY, CLINICAL FINDINGS AND LINKAGES
Prior to delving into either major realm of rodent research described above, we will first give some background. Major depressive disorder (depression) is a neuropsychiatric disease often linked to psychological stress and for which pharmacological treatment options are limited (Bonde et al., 2016; Hosang, Shiles, Tansey, McGuffin, & Uher, 2014; Ruhe, Huyser, Swinkels, & Schene, 2006). Commonly used monoaminergic antidepressants, such as the SSRIs, SNRIs, and tricyclics, are not effective in all patients and their timecourse of therapeutic onset is usually slow, often requiring a week or longer for initial effects to become evident (Derivan, 1995; Henkel et al., 2009; Kudlow, Cha, & Mcintyre, 2012). Largely for that reason, there is growing interest in the use of rapidly-acting pharmacological agents with alternate underlying mechanisms to counteract depression, to both help patients who do not respond to other drugs and because these agents work faster. The most prominent of these treatments is the anesthetic (and drug of abuse), (R,S)-ketamine (Ebada, 2017; Rosenblat et al., 2019). This drug is already being used clinically worldwide to treat major depression, often being administered intravenously in hospitals and clinics. The recent FDA approval of a nasal spray formulation of its enantiomer (S)-ketamine (“esketamine”) has added to the feasibility of its widespread use (Targum, Daly, Fedgchin, Cooper, & Singh, 2019). The rapid onset of (R,S)-ketamine’s antidepressant effects, which occur within 1-4 hours of initial administration, as well as its ability to sustain these favorable effects for days or even weeks in some individuals, has captured the interest of clinicians and translational researchers alike (Berman et al., 2000; Chan et al., 2018; Zarate et al., 2006). Importantly, this rapid temporal profile of treatment effects implies that (R,S)-ketamine has a unique mechanism of action compared with conventional monoaminergic antidepressants. This then implies that understanding the effects of (R,S)-ketamine may inform us as to new elements of mood-related neurobiology – again provoking great scientific interest. In addition, in a subset of individuals with major depression, (R,S)-ketamine may have specific anti-suicidal properties (Machado-Vieira, Salvadore, DiazGranados, & Zarate, 2009).
It should also be noted that (R,S)-ketamine remains a popular drug of abuse worldwide, which poses a significant barrier to its use in the clinical treatment of major depression (Liao, Tang, & Hao, 2017). Because of its abuse potential and psychotomimetic properties, there remain some concerns by some in using it as a pharmacological agent for mainstream treatment of depression (Andreae et al., 2016). It indeed must be prescribed with caution to individuals with a history of substance abuse. Also, since it is administered in clinics in the United States, often intravenously over several hours, it is not as convenient to take as most oral antidepressants. For these reasons, there is great interest in understanding its mechanism of action in order to facilitate development of (R,S)-ketamine-like antidepressants that lack this drug’s dissociative properties and abuse potential (Duman, 2018).
Berman et al. (2000) provided the initial demonstration of the therapeutic efficacy of (R,S)-ketamine in human subjects with major depression (Berman et al., 2000). In that publication, the authors note that a number of rodent depression-related preclinical studies carried out in the 1990s, which used a wide range of NMDA receptor (NMDAR) antagonist drugs, motivated them to investigate whether this drug is an effective treatment of human depression. Zarate et al. (2006) performed another prominent, double-blind placebo-controlled study of (in this case, treatment-resistant) major depression, which generated further interest in (R,S)-ketamine as a rapidly acting antidepressant with sustained effects (Zarate et al., 2006). Since then, both preclinical and clinical researchers have focused on (R,S)-ketamine, more than other NMDAR antagonists, as a novel treatment for depression, perhaps partly because (R,S)-ketamine was and is already being used clinically as a surgical anesthetic, making its repurposing at a lower dose for neuropsychiatric disorders immediately feasible, at least in an off-label manner (Ebada, 2017).
In the rest of this article, we briefly review preclinical studies of (R,S)-ketamine as a rapidly acting antidepressant, with a focus on studies in mice and rats. This growing literature is helping us gain a greater understanding of the precise molecular- and circuit-based mechanisms of action of this drug, which aids in using it to more effectively counteract depression, while also driving further innovation in the discovery of the next generation of rapidly acting antidepressants (Gerhard & Duman, 2018). The precise mechanism of action of (R,S)-ketamine remains quite controversial, as there is indeed no consensus that it is acting exclusively or even principally as an NMDAR antagonist (Zanos & Gould, 2018; Zanos et al., 2016). There is, in contrast, a large number of rodent studies reporting that (R,S)-ketamine has antidepressant-like effects across many dimensions including: dose, specific rodent species and strain, type of depression-related test used, presence of and type of stressor, and presumably sex of the experimenter. The remainder of this review focuses on understanding this variability of both results and approaches in the literature.
MOLECULAR AND RECEPTOR-BASED MECHANISMS OF KETAMINE ACTION – AN AREA OF ACTIVE RESEARCH
While it has long been presumed that the well-documented NMDAR-antagonism is the primary mechanism of action of antidepressant dose (R,S)-ketamine given this drug’s receptor binding profile, that assumption is now coming into some question. Initial work presented strong evidence that (R,S)-ketamine is an antidepressant due to blockade of the NMDAR, ultimately leading to increased brain derived neurotrophic factor (BDNF) production in circuits such as the hippocampus (Autry et al., 2011). Surprisingly, subsequent work suggested that (R,S)-ketamine, which is typically administered as a balanced racemic mixture of its (R)- and (S)-ketamine enantiomers, actually achieves its antidepressant-like effects independently of NMDAR blockade through two of its pharmacologically active metabolites: (2S,6S)-hydroxynorketamine and (.2R,6R)-hydroxynorketamine (HNK). The latter of these has been suggested to therapeutically potentiate signaling at glutamatergic AMPA receptors (Zanos et al., 2016). On the other hand, later experiments then demonstrated that (2R,6R)-HNK, which had been described as the more behaviorally effective HNK metabolite, also blocks the NMDAR, and may thus produce any antidepressant-like effects through the same NMDAR-dependent pathway as (R,S)-ketamine (Suzuki, Nosyreva, Hunt, Kavalali, & Monteggia, 2017). The (2R,6R)-HNK hypothesis was further questioned based on the finding that (S)-ketamine, which is not metabolized into (2R,6R)-HNK in vivo, nonetheless has robust antidepressant effects in humans (Collingridge, Lee, Bortolotto, Kang, & Lodge, 2017; Singh et al., 2016). Additionally, numerous preclinical studies carried out (including in the 1990s) in rodents used a wide range of NMDAR antagonist compounds, which are thought to not be metabolized into (2R,6R)-HNK (or (2S,6S)-HNK), and nonetheless have robust antidepressant-like properties (Collingridge et al., 2017). Later published discussions (Zanos et al., 2017) countered these assertions by pointing out that the reported superiority of (R)-ketamine versus (S)-ketamine (C. Yang et al., 2015; J. C. Zhang, Li, & Hashimoto, 2014) is consistent with the (2R,6R)-HNK hypothesis, while also pointing out that the various preclinical non-ketamine NMDAR antagonist compounds noted above have only shown inconsistent antidepressant effects, including in humans (Kishimoto et al., 2016; Sanacora et al., 2014; Zarate et al, 2006). This debate over the mechanism of action of (R,S)-ketamine continues in more recent publications (Kavalali & Monteggia, 2018; Lumsden et al., 2019; Zanos, Highland, Liu, et al., 2019; Zanos, Highland, Stewart, et al., 2019), including the possibility that (2R,6R)-HNK is an open channel blocker of the NMDAR (Thu Ha Pham et al., 2018), or that (R,S)-ketamine acts through a non-NMDAR mechanism that is instead dependent on intracellular cAMP signaling (Wray, Schappi, Singh, Senese, & Rasenick, 2018).
Other studies have also weighed in on the (2R,6R)-HNK versus NMDAR antagonism question in various rodent publications. It has recently been suggested, for example, that (2R,6R)-HNK boosts downstream BDNF signaling, which is then necessary for the antidepressant-like effects of (R,S)-ketamine (Fukumoto et al., 2019). Furthermore, a single systemic administration of (2R,6R)-HNK itself can rapidly rescue chronic stress-induced depression-like behavior and persist for up to 21 days (Chou et al., 2018). In contrast, recent mouse (Yamaguchi et al., 2018) and rat (Shirayama & Hashimoto, 2018) studies by another group have suggested that whereas (R)-ketamine exhibits rapid antidepressant-like properties, its metabolite (2R,6R)-HNK does not. These researchers have additionally found that intracerebroventricular infusion of (2R,6R)-HNK lacks antidepressant-like effects in a chronic social defeat stress model (CSDS) (Zhang, Fujita, & Hashimoto, 2018). This group has also recently found that (S)-norketamine, a principal metabolite of (S)-ketamine that can be converted to (2S,6S)-HNK, has more favorable effects on prefrontal and hippocampal synaptic plasticity than (R)-norketamine, and AMPA receptor antagonists do not block its antidepressant-like effects (Yang et al., 2018). This group has also pointed out that in spite of the recent introduction of the esketamine nasal spray formulation to the market, preclinical data suggest that compared to (S)-ketamine, (R-ketamine has greater potency and longer duration antidepressant-like effects. In addition, its side effects are more mild than both S)-ketamine and (R,S)-ketamine (Hashimoto, 2019). That publication also provides a review on the topic of (R,S)-ketamine and its enantiomers as antidepressants.
Aside from the above experiments, glutamatergic signaling has long been suggested to play a role in (R,S)-ketamine’s mechanism of action in depression. A recent review on this topic compared evidence for inhibition of glutamatergic signaling versus its activation, and proposed a synaptic connectivity model of chronic stress pathology and suggested that “transient (prefrontal) glutamate postsynaptic activation” is a primary mechanism of (R,S)-ketamine (Abdallah, Sanacora, Duman, & Krystal, 2018). Subanesthetic doses of systemically administered (R,S)-ketamine are indeed known to increase medial prefrontal cortex (mPFC) glutamate release, whereas anesthetic doses do the opposite (Moghaddam, Adams, Verma, & Daly, 1997). A human magnetic resonance spectroscopy study found evidence for increased prefrontal glutamatergic signaling after (R,S)-ketamine intravenous infusion, which positively correlated with perceptual dissociation (Abdallah, De Feyter, et al., 2018). Regarding the case for glutamatergic inhibition by (R,S)-ketamine: it has been shown in rodents that inhibiting NMDARs or glutamate release can block chronic stress-induced dendritic atrophy (McEwen, 1999). It has also been demonstrated that transient exposure to (R,S)-ketamine upregulates GluR1 and GluR2 AMPA receptor subunits on human dopamine neurons, which may be involved in a downstream mechanism of action of this drug (Collo, Cavalleri, Chiamulera, & Pich, 2019).
Even if the principal, initial mechanism of action of (R,S)-ketamine is glutamatergic, a wide range of neurotransmitter systems and molecular pathways may be modulated by (R,S)-ketamine (Zanos & Gould, 2018; Zanos et al., 2018). We will briefly touch upon some of these, beginning with the brain’s principal inhibitory neurotransmitter, GABA. Dysfunction in GABAergic interneurons has been implicated in the pathophysiological effects of chronic stress and major depression itself (Fee, Banasr, & Sibille, 2017; Fogaça & Duman, 2019). Some studies have reported decreased brain GABA levels in depression (Hasler et al., 2007; Luscher & Fuchs, 2015), as well as reduced molecular markers of GABA in the default mode network during this disease (Gabbay et al., 2012). These studies and others, coupled with findings of enhanced glutamatergic transmission noted above, have implicated a high excitatory:inhibitory (E:I) ratio in many cases of human major depression (Fee et al., 2017), which (R,S)-ketamine may normalize in prefrontal circuits (Fogaça & Duman, 2019). Acute administration of (R,S)-ketamine to humans or rodents has been widely reported to enhance the power of electroencephalography (EEG) or local field potential (LFP) gamma oscillations in a number of circuits (Fitzgerald & Watson, 2019; Hakami et al., 2009; Hunt, Garcia, Large, & Kasicki, 2008; Lee, Hudson, O’Brien, Nithianantharajah, & Jones, 2017), and this property may underlie its antidepressant action (Amat-Foraster et al., 2018). There appears to be a relationship between the LFP (including gamma) and the E:I ratio (Watson, Ding, & Buzsaki, 2018), further suggesting a role for (R,S)-ketamine in modulating this ratio in depression (Fitzgerald & Watson, 2018).
A recent study found that the opioid receptor antagonist drug, naltrexone, blocked the therapeutic effect of (R,S)-ketamine in treatment-resistant depression (Williams et al., 2018). Also, a mu opioid receptor agonist, tramadol, has been found to facilitate the antidepressant-like effect of a sub-effective dose of (R,S)-ketamine in the mouse FST (Ostadhadi S et al., 2017). On the other hand, another study reports that blockade, partial agonism and low-affinity agonism of the opioid receptor long-term does not affect the efficacy of (R,S)-ketamine for depressive symptoms in human subjects (Marton, Barnes, Wallace, & Woolley, 2019). Moreover, a pilot study in humans with major depression and concurrent alcohol use disorder found that pretreatment with naltrexone did not interfere with the antidepressant effects of (R,S)-ketamine (Yoon, Petrakis, & Krystal, 2019). Recent rodent data also found that naltrexone did not block the antidepressant-like effect of (R,S)-ketamine in CSDS or lipopolysaccharide models (K. Zhang & Hashimoto, 2019). One reason for differences in results in opioid system-related studies could be that short-term alteration can interfere with the effects of ketamine whereas chronic alteration does not.
Serotonergic signaling may also play a role in (R,S)-ketamine action. Such modulation has been demonstrated in rodent models for 5-HT1A, 5-HT3, and 5-HT6 receptors, for example (Fukumoto, Iijima, Funakoshi, & Chaki, 2018; Kordjazy et al., 2016; Suárez-Santiago, Briones-Aranda, Espinosa-Raya, & Picazo, 2017). Also, serotonin depletion with the drug para-chlorophenylalanine (PCPA) can block the antidepressant-like effect of (R,S)-ketamine (du Jardin et al., 2016). A recent mouse study, however, found that serotonin depletion with PCPA did not block the antidepressant-like effect of (R)-ketamine in a CSDS model (K. Zhang, Dong, Fujita, Fujita, & Hashimoto, 2018).
Noradrenergic signaling is another candidate contributor to the mechanism of action of (R,S)-ketamine. In two microdialysis studies in freely moving rats, it was shown that mPFC release of norepinephrine is dose-dependently enhanced by systemic (R,S)-ketamine, with an anesthetic dose (100 mg/kg) producing greater synaptic levels than subanesthetic doses (Kubota, Anzawa, et al., 1999; Kubota, Hirota, et al., 1999). It was also demonstrated that this enhanced mPFC level of norepinephrine was counteracted by the drug clonidine, an inhibitor of presynaptic norepinephrine release. This pair of studies may suggest a previously undescribed role for norepinephrine, at high concentrations, in promoting a state of anesthesia rather than alertness or arousal (Fitzgerald & Watson, 2019). A human brain imaging study has demonstrated a decrease in resting state functional connectivity from the noradrenergic locus coeruleus to the thalamus following (R,S)-ketamine administration (Liebe et al., 2018). An additional point that may implicate norepinephrine in (R,S)-ketamine’s mechanism of action: like (R,S)-ketamine itself, norepinephrine boosting antidepressants such as reboxetine acutely enhance the power of gamma oscillations (Fitzgerald & Watson, 2018; Hajós, Hoffmann, Robinson, Yu, & Va Hajó S-Korcsok, 2003).
In addition to its effects on neurotransmitter systems, administration of (R,S)-ketamine, not surprisingly, modulates a number of downstream intracellular molecular pathways (Duman, Li, Liu, Duric, & Aghajanian, 2012; Zanos & Gould, 2018). For example, systemic (R,S)-ketamine has been shown to activate mechanistic target of rapamycin (mTOR) signaling, and prefrontal or intracerebroventricular infusion of rapamycin blocks the antidepressant-like effects of (R,S)-ketamine (Abelaira et al., 2017; Li et al., 2011; Thelen et al., 2019). Another group, however, has found that rapamycin blocks the antidepressant-like effect of (S)-ketamine but not (R)-ketamine in a mouse CSDS model, suggesting mTOR signaling is not necessary for the therapeutic effects of (R)-ketamine (Yang, Ren, et al., 2018). Recent data from human subjects suggest that rapamycin actually prolongs, rather than blocks, the antidepressant effect of (R,S)-ketamine (Abdallah, Averill, et al., 2018).
As mentioned above, the Monteggia group has implicated BDNF signaling in the therapeutic effects of (R,S)-ketamine (Autry et al., 2011), and other groups have also suggested a similar role for this molecule, through both hippocampal and mPFC circuits (Garcia et al., 2008; Lepack, Fuchikami, Dwyer, Banasr, & Duman, 2015). These molecular pathways and others, such as Ras/MAPK, may play a critical role in the downstream synaptic plasticity mechanisms thought to underlie the antidepressant-like effects of (R,S)-ketamine (Duman et al., 2012; W. Liu etal., 2017).
Thus clearly a variety of studies by a range of groups have found a remarkable range of correlates with ketamine action. The factors contributing to the variance in these data may range from experimental details to differential outcome measures to use of multiple species to a drug that engages much of the brain. Is a broad brain engagement key to a mechanism of action or a signature of efficacy? As a community we will need to determine which of these effects may be causal – and consistently causal - in the action of ketamine.
BRAIN REGION-BASED MECHANISMS OF ACTION
Regardless of molecular mechanism, changes in neural activity in specific brain regions may be a final common pathway for the behavioral effects of (R,S)-ketamine. Yet multiple regions have been implicated in (R,S)-ketamine action, including the mPFC, ventral hippocampus and habenula. How these regions cooperate is not clear and each seems to demonstrate at least sufficiency in replicating behavioral depression-related effects in rodents.
The mPFC is a region frequently implicated in mood and emotional regulation, where microinfusion of (R,S)-ketamine or optogenetic stimulation of the infralimbic subdivision of mPFC produces antidepressant-like effects similar to systemic administration of (R,S)-ketamine in rats (Fuchikami et al., 2015). In rats exposed to chronic unpredictable stress there are decreases in the expression of synaptic proteins and spine numbers in mPFC layer 5 pyramidal cells, and these deficits are rapidly reversed by (R,S)-ketamine (Li et al., 2011). A recent study in mice found that chronic exposure to the stress molecule, corticosterone, in the drinking water promotes stress-like behavioral changes and elimination of dendritic spines on mPFC projection neurons, and (R,S)-ketamine reverses these synaptic and behavioral effects (Moda-Sava et al., 2019).
The ventral CA3 region of the hippocampus has also been implicated in the therapeutic effects of (R,S)-ketamine, particularly its prophylactic effects against social defeat stress in mice, partly mediated by expression of the protein ΔFosB (Mastrodonato et al., 2018). A series of pharmacological, optogenetic, and chemogenetic experiments in rats demonstrated that a ventral hippocampus-mPFC circuit is both necessary and sufficient for the antidepressant-like behavioral effects of (R,S)-ketamine (Carreno et al., 2016). Bilateral infusion of the enantiomer (R-ketamine into infralimbic (but not prelimbic) cortex, CA3, or dentate gyrus of the hippocampus each produced antidepressant-like effects in a rat learned helplessness model (Shirayama & Hashimoto, 2017).
Other work has suggested that neuronal bursting activity in the lateral habenula (which has been termed the “anti-reward center”) promotes depression-like behavior (Cui et al., 2018; Y. Yang et al., 2018). These papers suggest that (R,S)-ketamine blocks this bursting and may disinhibit downstream monoaminergic reward centers, where bursting is bidirectionally modulated by the astroglial potassium channel Kir4.1 (Cui et al., 2018; Y. Yang et al., 2018). Yang et al. (2018) also found that systemic administration of the T-type calcium channel blocker ethosuximide has (R,S)-ketamine-like rapid antidepressant-like effects, but this was not replicated in a recent study using the chronic social defeat stress (CSDS) behavioral model of stress (Tian, Dong, Zhang, Chang, & Hashimoto, 2018). Another study by the latter group also found that Kir4.1 channel inhibitors did not produce rapid and sustained antidepressant-like effects in a CSDS model (Xiong et al., 2019).
Perhaps relatedly, (R,S)-ketamine and its metabolite (2R,6R)-HNK have been shown in mice to impair long-term potentiation in the nucleus accumbens. (R,S)-ketamine inhibits phosphorylation of the GluA1 AMPA receptor subunit in this circuit, where these synaptic effects may modulate reward-related behaviors (Yao, Skiteva, Zhang, Svenningsson, & Chergui, 2018).
Moving forward to fully characterize the mechanism of action of ketamine may require a focus on the circuit level effects of (R,S)-ketamine – given the clear involvement of many brain regions. One region may drive others, or all may be driven by (R,S)-ketamine. Furthermore, their interaction may or may not be crucial, independent of the individual effects in single regions. Targeted multisite recording and stimulation methods may be crucial here to clarify these multiple findings – and again may both help us understand (R,S)-ketamine actions and new neurobiology.
In summary, we need still to determine whether (R,S)-ketamine has multiple effects simultaneously and at what levels those effects are brought about – action at multiple receptors, multiple downstream intracellular effectors or final common networks. The complexities raised by these pharmacologic studies demonstrate the need for a great deal of further research. In addition, it should be remembered that large-scale effects from any one system can induce changes in other neurotransmitters or other signaling systems. Thus, an important future focus may be on specifically assessing causal relationships in both directions between treatment and effect, as well as at smaller scales – for example examining whether changes in one system are actually induced by (R,S)-ketamine versus induction by downstream effects of this drug.
HYPOTHESES ON MECHANISM OF ACTION
While there is much variability in the data as summarized above, which requires further experimentation, other ideas may have sufficiently emerged at this point to allow us to integrate and speculate in ways that may guide future hypothesis testing. A broad range of brain regions, neurotransmitter systems (not limited to glutamatergic signaling) and downstream molecular pathways are recruited and altered by a single, systemic administration of (R,S)-ketamine. Regarding the NMDAR antagonism versus AMPA potentiation by (2R,6R)-HNK question: we suggest here that NMDAR blockade is more likely to be the primary initial mechanism of action, if only due to the large number of studies showing this mechanism in ketamine itself and with a variety of drugs that are thought to be NMDAR antagonists (Layer, Popik, Olds, & Skolnick, 1995; Meloni et al., 1993; Moryl, Danysz, & Quack, 1993; Papp & Moryl, 1994, 1996; Przegaliński, Tatarczyńska, Dereń-Wesołek, & Chojnacka-Wójcik, 1997; Trullas & Skolnick, 1990). These drugs are unlikely to all have (2R,6R)- or (2S,6R)-HNK as metabolites, suggesting the parsimonious hypothesis that (R,S)-ketamine and all of these other drugs are acting at least mainly through NMDAR antagonism. If (2R,6R-HNK is potentiating AMPAR signaling (i.e., a facilitation of glutamatergic transmission), then this effect could still add to the “glutamatergic burst” that (R,S)-ketamine may principally evoke through NMDAR antagonism, where this burst may involve inhibition of GABAergic interneurons bearing NMDA receptors, leading to disinhibition of glutamatergic neurons and increased release of synaptic glutamate (Duman et al., 2012). Additionally, both AMPAR- and NMDAR-based mechanisms will necessarily involve each other due to positive feedback in recurrent mostly-glutamatergic neural networks such as those in the cortex and the hippocampal CA3 region. Further, as noted above, even if the principal, initial mechanism of action of (R,S)-ketamine is NMDAR antagonism, a wide range of neurotransmitter systems, molecular pathways, and brain regions may be modulated by (R,S)-ketamine due to downstream crosstalk.
We suggest here that the “glutamatergic burst” which follows acute administration of (R,S)-ketamine is accompanied by elevated synaptic norepinephrine (Kubota, Anzawa, et al., 1999; Kubota, Hirota, et al., 1999), where the initial release of these two neurotransmitters is interrelated and can be accompanied by the dissociative effects of this drug. In the minutes to hours that follow, both in rodents and humans, compensatory mechanisms are induced to suppress this excessive neural activity (including suppression of noradrenergic and glutamatergic signaling), which decreases the E:I ratio in a number of mood-related circuits and thereby has an antidepressant-like effect that is maintained through many of the molecular and synaptic mechanisms noted above. We draw an analogy here between subanesthetic (R,S)-ketamine and electroconvulsive therapy (ECT): both may have an initially “excitatory” mechanism that is followed by suppression of neural activity in diverse circuits (Kheirabadi, Vafaie, Kheirabadi, Mirlouhi, & Hajiannasab, 2019; Kohtala et al., 2018; Mickey et al., 2018; Tadler & Mickey, 2018). Finally, if glutamatergic and noradrenergic signaling are indeed intertwined in the therapeutic effects of (R,S)-ketamine, then norepinephrine-lowering drugs may also have rapidly acting antidepressant properties, which has already been demonstrated for the drug guanfacine in rodents (Mineur et al., 2015, 2018).
It is difficult to speculate how the multiple brain regions thus far implicated in the mechanism of action of (R,S)-ketamine interact, but mPFC, habenula and hippocampus all have been shown to be of import using causal experimental methodologies. Given that multi-region interactions are frequently observed in many brain phenomena using fMRI and other whole-brain imaging techniques, it is likely that these and possibly other regions interact to enable the effects of (R,S)-ketamine. It may actually specifically be the re-balancing of the relative activities of these regions that is the key to (R,S)-ketamine function. That said, the sufficiency of manipulation of each region to control ketamine effects suggests that these individual nodes of this potential network are each able to induce relatively full responses across the network. This is a case where in vivo circuit activity measurement - and not just causal experimentation - may shed the most light.
CONSISTENCY OF REPORTED ANTIDEPRESSANT-LIKE BEHAVIORAL EFFECTS
The second half of this paper addresses another intriguing aspect of the (R,S)-ketamine literature: the consistent positive reporting of antidepressant-like responses to (R,S)-ketamine across dose, mouse or rat species and strain, type of test used, presence or type of stressor, and even the sex of the experimenter. While the similarity of reporting is striking, we specifically analyze the publication record and reporting patterns and ask whether stress does or does not play into various reports and whether it should or should not be considered a major axis of study in the rodent field.
The relatively remarkable reporting of similar antidepressant-like effects across very different tests (tail suspension, sucrose preference, as well as FST) and a wide range of mouse and rat strains (Autry et al., 2011; Cunha et al., 2015; Mishra, Kumar, Behar, & Patel, 2018; Neis et al., 2016) suggests the utility of rodent research for understanding this drug that also appears to help many patients with a variety of clinical depression subtypes. But on the other hand, this finding on animal strain is somewhat surprising, given that the behavior of different strains of mice and rats varies greatly upon exposure to monoaminergic antidepressants (Dulawa, Holick, Gundersen, & Hen, 2004) and in fear-related behavior (Fitzgerald et al., 2014; Hefner et al., 2008), as well as in other tests commonly used in behavioral neuroscience (Fitzpatrick et al., 2013; Gileta et al., 2018; Mozhui et al., 2010). Additionally, while in human populations, (R,S)-ketamine is given to individuals with depression, many rodent studies report that (R,S)-ketamine has antidepressant-like effects in unstressed animals.
To further investigate this topic, on January 16, 2019 we systematically searched PubMed with the following terms: ketamine AND ("forced swim" OR "forced swimming" OR antidepressant-like OR antidepressant-related OR depression-like OR depression-related). This search of 328 papers yielded 52 that met our criteria to be included in Table 1. This table summarizes all studies from this search that used a single intraperitoneal injection of racemic (R,S)-ketamine and measured immobility in the FST 24 hours or more later. Importantly, we chose not to focus on the acute (i.e., minutes to several hours) effects of (R,S)-ketamine in this test, which we suggest may be associated with psychotomimetic or dissociative effects of this drug rather than its sustained antidepressant-like qualities that emerge later. Inspection of Table 1 suggests that across a wide range of variables—species and strain, sex, dose, time delay between injection and start of FST, type (or absence) of stressor, and possibly even the sex of the experimenter—(R,S)-ketamine displays similar effects in the FST, namely an antidepressant-like decrease in immobility. There are some exceptions to this such as our own recent study (Fitzgerald, Yen, & Watson, 2019), which indicates that stress strongly modulates the effect of (R,S)-ketamine.
Table 1.
Summary of literature on sustained effects of (R,S)-ketamine in the rodent forced swim test (FST). Criteria for studies to be included in this table. 1) wild type mouse or rat, 2) single intraperitoneal injection of 1-30 mg/kg (R,S)-ketamine, 3) FST carried out 24 hours or more post-injection. “Sex” refers to sex of the animals used. “Change in Immobility” column indicates whether (R,S)-ketamine decreased or increased this measure relative to vehicle-injected animals subjected to the same stress condition (i.e., stressed or unstressed); “Stat Sig” column indicates whether the difference between (R,S)-ketamine and vehicle immobility was statistically significant, for p < 0.05. Other abbreviations are defined within the table when they first appear. All experiments that used chronic stress are marked in red. Experiments that showed a statistically significant decrease in immobility are marked in dark green, whereas those with a decrease that was not significant are marked in light green. Experiments that exhibited a statistically significant increase in immobility are colored dark blue, whereas those with an increase (or “no change”) that was not significant are light blue.
| Publication | Species | Strain | Sex | Dose(s) (mg/kg) |
Time Delay |
Stressor/Unstressed | Δ Immobility | Stat Sig |
|---|---|---|---|---|---|---|---|---|
| (Autry et al., 2011) | Mouse | C57BL/6 | M | 3 | 24 hr | Unstressed | Decrease | Yes |
| C57BL/6 | M | 3 | 7 days | Unstressed | Decrease | Yes | ||
| (Liu et al., 2012) | Mouse | C57BL/6 | M | 10 | 24 hr | Unstressed | Decrease | Yes |
| (Ma et al., 2013) | Mouse | C57BL/6J | M | 10 | 48 hr | Chronic Mild Stress (CMS) | Decrease | Yes |
| C57BL/6J | M | 10 | 48 hr | Unstressed (control) | Decrease | No | ||
| (Pozzi et al., 2014) | Mouse | C57BL/6N | M | 3 | 24 hr | Unstressed | Decrease | Yes |
| C57BL/6N | M | 3 | 7 days | Unstressed | Decrease | No | ||
| (Gideons et al., 2014) | Mouse | C57BL/6 | M | 3 | 24 hr | Unstressed | Decrease | Yes |
| (Nosyreva et al., 2014) | Mouse | C57BL/6 | M | 3 | 24 hr | Unstressed | Decrease | Yes |
| (Franceschelli et al., 2015) | Mouse | C57BL/6J | M | 3 | 24 hr | Unstressed | Decrease | No |
| C57BL/6J | M | 5 | 24 hr | Unstressed | Decrease | No | ||
| C57BL/6J | M | 10 | 24 hr | Unstressed | Decrease | Yes | ||
| C57BL/6J | F | 3 | 24 hr | Unstressed | Decrease | No | ||
| C57BL/6J | F | 5 | 24 hr | Unstressed | Decrease | Yes | ||
| C57BL/6J | F | 10 | 24 hr | Unstressed | Decrease | Yes | ||
| C57BL/6J | M | 10 | 24 hr | CMS | Decrease | Yes | ||
| C57BL/6J | M | 10 | 7 days | CMS | Decrease | Yes | ||
| C57BL/6J | F | 10 | 24 hr | CMS | Decrease | Yes | ||
| C57BL/6J | F | 10 | 7 days | CMS | Decrease | No | ||
| C57BL/6J | F | 10 | 5 days | CMS | Decrease | Yes | ||
| (Dong et al., 2017) | Mouse | C57BL/6 | M | 10 | 48 hr | Social Defeat Stress | Decrease | Yes |
| (Zhang et al., 2018) | Mouse | C57BL/6 | M | 10 | 24 hr | Chronic Unpredictable Stress (CUS) | Decrease | Yes |
| C57BL/6 | M | 10 | 3 days | CUS | Decrease | Yes | ||
| C57BL/6 | M | 10 | 5 days | CUS | Decrease | Yes | ||
| C57BL/6 | M | 10 | 7 days | CUS | Decrease | Yes | ||
| (Fukumoto et al., 2018) | Mouse | C57BL/6J | M | 30 | 24 hr | Unstressed | Decrease | Yes |
| (Wang et al., 2018a) | Mouse | C57BL/6 | M | 30 | 28 days | Unstressed | Decrease | Yes |
| C57BL/6 | M | 30 | 28 days | Unpredictable Chronic Mild Stress (UCMS) | Decrease | No | ||
| (Shen et al., 2018) | Mouse | C57BL/6J | M | 10 | 5 days | Chronic Social Defeat Stress (CSDS) | Decrease | Yes |
| (Huang et al., 2019) | Mouse | C57BL/6 | M | 10 | 25 hr | Lipopolysaccharide (Induced Inflammation) | Decrease | Yes |
| (Hare et al., 2019) | Mouse | C57BL/6J | M & F | 10 | 24 hr | Unstressed | Decrease | No |
| (Fitzgerald et al., 2019) | Mouse | C57BL/6J | M | 10 | 24 hr | Unstressed | Increase | Yes |
| C57BL/6J | M | 30 | 24 hr | Unstressed | Increase | Yes | ||
| C57BL/6J | M | 10 | 7 days | Unstressed | Increase | No | ||
| C57BL/6J | M | 30 | 7 days | Unstressed | Decrease | No | ||
| C57BL/6J | M | 10 | 24 hr | UCMS | Increase | No | ||
| C57BL/6J | M | 30 | 24 hr | UCMS | Decrease | No | ||
| C57BL/6J | M | 10 | 7 days | UCMS | Increase | No | ||
| C57BL/6J | M | 30 | 7 days | UCMS | Decrease | No | ||
| (Wang et al., 2018b) | Mouse | ICR | M | 10 | 24 hr | Unstressed | Decrease | Yes |
| (Botanas et al., 2017) | Mouse | ICR | M | 5 | 24 hr | Unstressed | Decrease | Yes |
| ICR | 10 | 24 hr | Unstressed | Decrease | Yes | |||
| (Lin et al., 2016) | Mouse | ICR | M | 3 | 7 days | Unstressed | Decrease | No |
| ICR | M | 10 | 7 days | Unstressed | Decrease | Yes | ||
| ICR | M | 15 | 7 days | Unstressed | Decrease | Yes | ||
| (Pham et al., 2017) | Mouse | BALB/cJ | M | 10 | 24 hr | Unstressed | Decrease | Yes |
| (Wu et al., 2017) | Mouse | BALB/cJ | M and F | 30 | 24 hr | Offspring of Female Mice with Post Partum Depression (PPD) | Decrease | Yes |
| (Pham et al., 2018) | Mouse | BALB/cJ | M | 10 | 24 hr | Unstressed | Decrease | Yes |
| (Xia et al., 2016) | Mouse | BALB/cJ | F | 30 | 24 hr | Prepregnancy Stress and Parturition (SP) | Decrease | Yes |
| (Tao et al., 2014) | Mouse | Kunming | M | 30 | 24 hr | Unstressed | Decrease | Yes |
| (Tang et al., 2015) | Mouse | Kunming | M | 30 | 24 hr | CMS | Decrease | Yes |
| (Popik et al., 2017) | Mouse | CD-1 | M | 5 | 24 hr | Unstressed | Increase | No |
| CD-1 | M | 10 | 24 hr | Unstressed | Increase | No | ||
| CD-1 | M | 15 | 24 hr | Unstressed | Decrease | No | ||
| CD-1 | M | 25 | 24 hr | Unstressed | Increase | No | ||
| (Clarke et al., 2017) | Mouse | CD-1 | M | 10 | 2 days | Unstressed | Decrease | No |
| CD-1 | M | 10 | 5 days | Unstressed | Decrease | No | ||
| CD-1 | M | 10 | 8 days | Unstressed | Decrease | No | ||
| (Bechtholt-Gompf et al., 2011) | Mouse | CD-1 | M | 2.5 | 7 days | Unstressed | Decrease | No |
| CD-1 | M | 12.5 | 7 days | Unstressed | Decrease | No | ||
| (Zanos et al., 2015) | Mouse | CD-1 | M | 10 | 24 hr | Unstressed | Decrease | Yes |
| (Zanos et al., 2016) | Mouse | CD-1 | M | 1 | 24 hr | Unstressed | Decrease | No |
| CD-1 | M | 3 | 24 hr | Unstressed | Decrease | No | ||
| CD-1 | M | 10 | 24 hr | Unstressed | Decrease | Yes | ||
| CD-1 | M | 30 | 24 hr | Unstressed | Decrease | No | ||
| CD-1 | F | 1 | 24 hr | Unstressed | Decrease | No | ||
| CD-1 | F | 3 | 24 hr | Unstressed | Decrease | Yes | ||
| CD-1 | F | 10 | 24 hr | Unstressed | Decrease | Yes | ||
| (Yuen et al., 2017) | Mouse | NIH- Swiss | M | 17 | 24 hr | Unstressed | Increase | No |
| (Popik et al., 2008) | Mouse | Albino Swiss | M | 1.25 | 14 days | Unstressed | Decrease | No |
| Albino Swiss | M | 2.5 | 14 days | Unstressed | Decrease | No | ||
| Albino Swiss | M | 5 | 14 days | Unstressed | Decrease | No | ||
| Albino Swiss | M | 10 | 14 days | Unstressed | Decrease | No | ||
| (Maeng et al., 2008) | Mouse | Not Specified | M | 2.5 | 14 days | Unstressed | Decrease | Yes |
| (Wang et al., 2011) | Rat | Sprague-Dawley | M | 10 | 24 hr | Spared Nerve Injury (SNI) | Decrease | Yes |
| (Gigliucci et al., 2013) | Rat | Sprague-Dawley | M | 25 | 24 hr | Unstressed | Decrease | Yes |
| Sprague-Dawley | M | 25 | 24 hr | Restraint Stress | Increase | No | ||
| (Koike & Chaki, 2014) | Rat | Sprague-Dawley | M | 10 | 24 hr | Unstressed | Decrease | Yes |
| (Lepack et al., 2015) | Rat | Sprague-Dawley | M | 10 | 24 hr | Unstressed | Decrease | Yes |
| (Dwyer et al., 2015) | Rat | Sprague-Dawley | M | 10 | 24 hr | Unstressed | Decrease | Yes |
| (Jett et al., 2015) | Rat | Sprague-Dawley | M | 10 | 7 days | Unstressed | Decrease | Yes |
| (Sun et al., 2016) | Rat | Sprague-Dawley | M | 10 | 72 hr | UCMS | Decrease | Yes |
| (Sarkar & Kabbaj, 2016) | Rat | Sprague-Dawley | M | 2.5 | 3 days | Isolation Stress (IS) | Decrease | No |
| Sprague-Dawley | M | 5 | 3 days | IS | Decrease | Yes | ||
| Sprague-Dawley | M | 2.5 | 3 days | Unstressed (Pair Housed) | Decrease | No | ||
| Sprague-Dawley | M | 5 | 3 days | Unstressed | Decrease | Yes | ||
| Sprague-Dawley | F | 2.5 | 3 days | IS | Decrease | No | ||
| Sprague-Dawley | F | 5 | 3 days | IS | Decrease | Yes | ||
| Sprague-Dawley | F | 2.5 | 3 days | Unstressed | Decrease | Yes | ||
| Sprague-Dawley | F | 5 | 3 days | Unstressed | Decrease | Yes | ||
| (Chowdhury et al., 2017) | Rat | Sprague-Dawley | M | 3 | 24 hr | Unstressed | Decrease | No |
| Sprague-Dawley | M | 30 | 24 hr | Unstressed | Decrease | Yes | ||
| (Podkowa et al., 2016) | Rat | Sprague-Dawley | M | 3 | 24 hr | Unstressed | Decrease | No |
| Sprague-Dawley | M | 10 | 24 hr | Unstressed | Decrease | Yes | ||
| Sprague-Dawley | M | 30 | 24 hr | Unstressed | Decrease | No | ||
| (Zhang et al., 2016) | Rat | Sprague-Dawley | M | 20 | 24 hr | CFA | Decrease | Yes |
| (Donegan & Lodge, 2017) | Rat | Sprague Dawley | M | 10 | 7 days | Unstressed | Decrease | Yes |
| (Xie et al., 2017) | Rat | Sprague-Dawley | M | 20 | 48 hr | Unstressed | No change | No |
| Sprague-Dawley | M | 20 | 48 hr | SNI | Decrease | Yes | ||
| (Jiang et al., 2017) | Rat | Sprague-Dawley | M | 10 | 24 hr | CUS | Decrease | No |
| Sprague-Dawley | M | 10 | 7 days | CUS | Decrease | Yes | ||
| (Hou et al., 2018) | Rat | Sprague-Dawley | M | 5 | 23.5 hr | SPS&S (Single Prolonged Shock and Electric Foot Shock) | Decrease | No |
| Sprague-Dawley | M | 10 | 23.5 hr | SPS&S | Decrease | Yes | ||
| Sprague-Dawley | M | 15 | 23.5 hr | SPS&S | Decrease | Yes | ||
| Sprague-Dawley | M | 20 | 23.5 hr | SPS&S | Decrease | No | ||
| (Shepard et al., 2018) | Rat | Sprague-Dawley | M | 20 | 24 hr | Unstressed (Postnatal (P)42-P50) | Decrease | No |
| Sprague-Dawley | M | 20 | 24 hr | Maternal Deprivation (MD) | Decrease | Yes | ||
| Sprague-Dawley | M | 20 | 24 hr | Unstressed (P21-P28) | Increase | No | ||
| Sprague-Dawley | M | 20 | 24 hr | MD | Increase | Yes | ||
| (Pałucha-Poniewiera et al., 2019) | Rat | Sprague-Dawley | M | 3 | 24 hr | Unstressed | Decrease | No |
| (Maciel et al., 2018) | Rat | Wistar | M | 15 | 8 days | CMS | Decrease | Yes |
| Wistar | M | 15 | 8 days | Maternal Deprivation | Decrease | Yes | ||
| (Réus et al., 2014) | Rat | Wistar | M | 15 | 23.5 hr | Unstressed | Decrease | Yes |
| (Chindo et al., 2012) | Rat | Wistar | M | 30 | 24 hr | Unstressed | No change | No |
| (Liu et al., 2013) | Rat | Not Specified | M | 10 | 24 hr | Unstressed | Decrease | Yes |
| Not Specified | M | 10 | 7 days | Unstressed | Decrease | Yes | ||
| Not Specified | M | 10 | 14 days | Unstressed | Increase | No | ||
| Not Specified | M | 1 | 24 hr | Unstressed | Increase | No |
While the data in Table 1 follow the general trend of demonstrating (R,S)-ketamine induced reduction of FST immobility, closer inspection of the table may reveal some more subtle differences with respect to these variables. For example, C57BL/6 and BALB/cJ mice tend to show stronger antidepressant-like responses to (R,S)-ketamine, whereas CD-1 mice show more variable responses. Within several studies, the unstressed groups of rodents did not respond significantly to a certain dose, whereas the stressed animals responded to that same dose, suggesting some degree of stress-sensitivity (Ma et al., 2013; Shepard et al., 2018; Xie et al., 2017). However, a fairly similar proportion of studies that used stress (19/22 = 0.86) versus no stress (28/40 = 0.70) reported a significant decrease in immobility, suggesting a lack of prominent stress sensitivity to (R,S)-ketamine in the FST. Mice and rats showed a significant decrease in immobility in a similar proportion of studies (mice: 26/36 = 0.72, rats: 22/30 = 0.73), even though brain-body scaling factors (i.e., larger animals require lower mg/kg ratio dose to receive same effect) would suggest that rats should receive a lower mg/kg dose than mice. For comparison, a dose of 0.5 mg/kg (i.v.) is often used as an efficacious antidepressant dose in humans. The mice and rat studies summarized in Table 1 suggest (R,S)-ketamine has antidepressant-like effects in rodents over a wide range of doses (1-30 mg/kg), and the field has apparently settled on 10 mg/kg as the most frequently used dose.
Table 1 includes only a limited number of studies in female animals, so studying sex differences (of the animals used) is an important future direction. These data may nonetheless suggest that female rodents are somewhat more sensitive to (R,S)-ketamine than males, considering that all five studies that used groups that were purely female found a significant antidepressant-like effect. These studies include one with both stressed and unstressed experiments whose authors suggest this may be due to hormonal, serotonergic, or glutamatergic sex differences in circuits such as prefrontal cortex and hippocampus (Franceschelli, Sens, Herchick, Thelen, & Pitychoutis, 2015). These authors also suggest that female rodents are more sensitive to earlier (up to 24 hours) effects of this drug, but then these effects wear off sooner than in males (Franceschelli et al., 2015). Although human females are more frequently afflicted with major depression than males, it has been suggested that (R,S)-ketamine is similarly effective in both sexes (Williams & Trainor, 2018). Table 1 also illustrates the sustained nature of (R,S)-ketamine’s antidepressant-like effects: many studies report a sustained and significant decrease in depression-like behavior after 7 days and 14 days, for example. One study even shows a significant decrease in immobility after 28 days (Y. Wang et al., 2018).
KETAMINE ACTION IN UNSTRESSED RODENTS
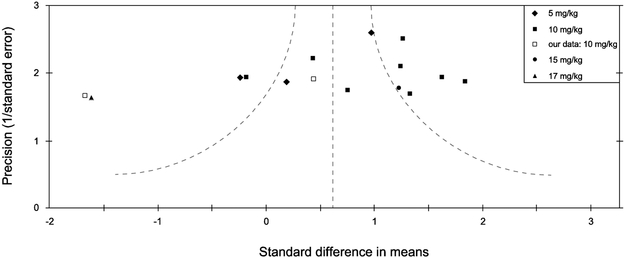
Figure 1 represents an effort to “meta-analyze” the data from the field gathered in the literature search described above. It shows a funnel plot, summarizing effect size distribution in a subset of the studies listed in Table 1 - specifically the 13 studies for which we were able to obtain immobility data in unstressed mice (various strains) at the 24 hour post-injection timepoint, in a moderate subanesthetic dose range between 5-20 mg/kg (for which the highest dose that met our criteria was 17 mg/kg). We chose to plot only unstressed mice in Figure 1 for several reasons: 1) “stressed” animals in the various studies from Table 1 were given a very wide range of stress types across studies, so combining those data in one plot may be confusing; 2) unstressed animals are widely reported to exhibit antidepressant-like responses to (R,S)-ketamine in these studies, which may seem surprising since these animals were presumably not in a depression-like state and this should be investigated further; 3) we sought to compare these various unstressed studies with our own unstressed mouse strain comparison data (Figure 2).
Figure 1.
Funnel plot “meta analysis” of a subset of 13 unstressed forced swim studies from Table 1. The abscissa is effect size which quantifies the difference in immobility between the (R,S)-ketamine and vehicle groups in a given study, whereas the ordinate is the “precision” also known as the inverse standard error, a measure of sample size. The curved lines represent the 95% confidence interval. Cohen’s d was used to determine effect sizes. Positive effect sizes represent decreases in immobility induced by (R,S)-ketamine, whereas negative values indicate increases in immobility on this drug. As noted in the legend, our own data (Fitzgerald et al. 2019) are depicted as open squares in this graph. This figure only includes studies of mice (various strains) at the 24 hour post-injection timepoint, in a moderate subanesthetic dose range between 5-20 mg/kg (see legend).
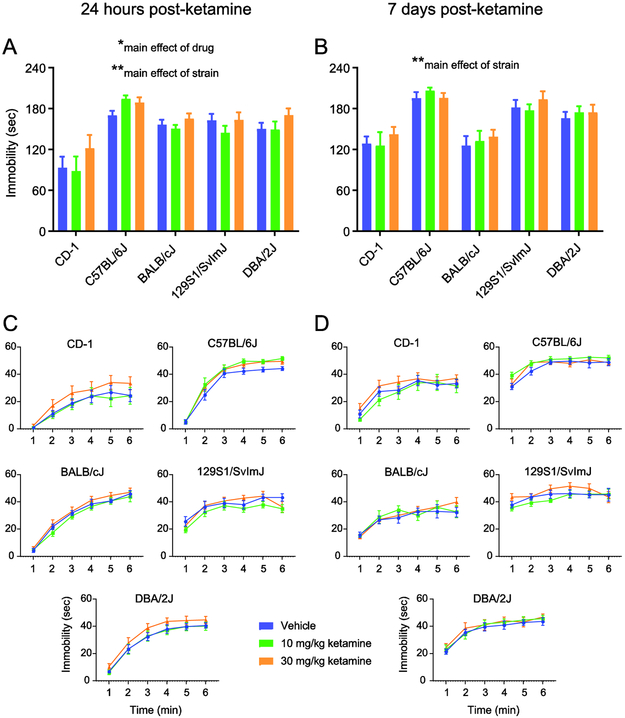
Figure 2.
Strain comparison data reveal increased forced swim immobility after (R,S)-ketamine in unstressed mice. Immobility in this test: A, 24 hours after a single injection of (R,S)-ketamine, averaged across the last four minutes of the six minute test; B, the same animals were tested again seven days after this injection, also averaged across the last four minutes; C, 24 hour post-injection data binned in one minute intervals; D, 7 day post-injection data binned in one minute intervals. Strain-by-drug two-way ANOVAs reveal a main effect of strain at both timepoints in A and B, and a main effect of drug only at the 24 hour point. Vehicle solution is 0.9% saline. Bars and points in A-D depict mean ± standard error of mean (SEM). Significance indicators for the two-way ANOVAs (centered over each graph) are marked by *p < 0.05, **p < 0.01. See Fitzgerald et al. (2019) for detailed methodology.
In Figure 1 we also included data from two of our own cohorts that used 10 mg/kg (R,S)-ketamine in male C57BL/6J mice (see Legend), obtained from our previous publication (Fitzgerald, Yen, & Watson, 2019). The “meta analysis” data shown in Figure 1 have more points on the right side of the graph, which means that more published studies show a decrease in immobility in the FST under these conditions. One of our two cohorts falls closer to the center of the distribution, with the other farther to the left, where far leftward points represent a paradoxical increase in immobility. Quantitatively, the distribution of the standard difference in means has a skewness (measured by the adjusted Fisher-Pearson standardized moment coefficient) of −0.9085, which indicates that it is moderately negatively skewed and that the majority of points fall to the right of the expected value. This may be due to the presence of an underlying non-gaussian, and possibly multi-modal, distribution or a variety of other factors. Finding more points to the right in Figure 1 is consistent with findings from the larger number of studies we summarized in Table 1, which also reported a high proportion of decreases in immobility in the FST after a single injection of (R,S)-ketamine. It remains unclear where the spread of data comes from and whether the increased numbers of points toward the right of the graph compared to the left is worth specific consideration. One possibility is that “unstressed” conditions vary in different labs’ experimental procedures or housing (lighting, staff handling, enrichment, etc.), and that such differences could drive variability with respect to the mean observed in Figure 1. Also, opposing effects of (R,S)-ketamine based on the stress state of mice would be represented as both leftward and rightward points in the funnel plot. This may be expected given that a recent study in human subjects found opposing effects of (R,S)-ketamine based on whether the individuals had major depression or not, since depression can be induced by psychological stress (Nugent et al., 2018). If there is an under-representation of negative (i.e., no change in immobility) or contrary (i.e., paradoxical increase in immobility) results in the published literature, it may be because it is more difficult to publish these types of results at this stage since the decrease in immobility result is well-established.
To further investigate how (R,S)-ketamine and stress effects may vary across individuals, we compared FST effects in different mouse strains. Figure 2 shows strain comparison data from our lab, previously unpublished except for the C57BL/6J data that were contained in Fitzgerald et al. (2019) and for that mouse strain represent the first of our two experiments described in the previous paragraph (Figure 1, our leftmost point), with the addition of C57BL/6J mice that received a 30 mg/kg dose (Fitzgerald et al., 2019). The data in Figure 2 depict FST immobility in five strains of unstressed mice, averaged over the last four minutes of the six minute test (Fig. 2A,B), and also binned by minute (Fig. 2C,D) for these two post-injection tests (see Table S1 for a statistical summary). Since different strains of mice are known to exhibit widely differing amounts of immobility in the FST, it is not surprising that at both the 24 hour post-injection timepoint (Fig. 2A) and when the same animals were tested again 7 days post-injection (Fig. 2B), there is a main effect of strain in the two-way (strain x drug) ANOVA (24 hours: F(4,105) = 21.06, p < 0.01; 7 days: F(4,105) = 21.9, p < 0.01) (see Table S1 for a summary of which strains differed significantly from one another). However, at the 24 hour timepoint there is also a main effect of drug (F(2,105) = 3.3, p < 0.05), with 30 mg/kg (R,S)-ketamine on average showing a trend toward an increase in immobility relative to vehicle (Tukey’s multiple comparisons test; p = 0.089) and the 10 mg/kg (p = 0.059) groups, where the latter two groups tend to be similar to one another across strains. When we parsed the data into one minute bins for the 24 hour post-ketamine test (Fig. 2C) and 7 days post-ketamine test (Fig. 2D), repeated measures ANOVA did not reveal a significant effect of drug or a drug-by-time interaction (each p > 0.05) for any mouse strain, but there was a significant effect of time for all strains (each p < 0.0001). The results in Figure 2 are perhaps paradoxical and suggest (R,S)-ketamine is not antidepressant-like in the FST across a range of mouse strains that have not been subjected to marked stress, extending findings that we previously reported for C57BL/6J mice (Fitzgerald et al., 2019). It is also interesting to note that while there was no strain-by-drug interaction in the two-way ANOVA, the C57BL/6J strain seems to differ from the other strains in that both the 10 and 30 mg/kg doses increase immobility 24 hours post-injection ( Fitzgerald et al., 2019).
Another poorly understood factor at this time may be how sex of the (human) experimenter may interact with sex of the animals being tested in the FST. This phenomenon has already been repeatedly demonstrated in psychological studies investigating behavior in human subjects (Chapman, Benedict, & Schiöth, 2018), a concept that may extend to rodent experiments (Georgiou et al., 2018). Since (R,S)-ketamine treatment has different effects on male versus female mice and rats (Carrier & Kabbaj, 2013; Franceschelli et al., 2015; Thelen, Sens, Mauch, Pandit, & Pitychoutis, 2016), perhaps experimenter sex could interact with these pharmacological effects. The potential effects of sex of the experimenter are, however, not well characterized at this time and future studies should address whether it has significant effects on rodent behavior.
Animal age is an additional factor to consider in rodent antidepressant studies, since these drugs can be less effective in elderly human populations (Patel, Abdool, Rajji, & Mulsant, 2017). Another variable, or set of variables, in rodent drug studies comprises the setting or context of the injection itself. For example, is the room in which the injection takes place the same as the testing room? After the injection and before the test, are the animals placed in a cage with other vehicle only animals, or instead in a cage with only animals that received drug? These factors could contribute to the variability that we report here in the ketamine literature.
In summary, there is some degree of consistency in the reported antidepressant-like effects of (R,S)-ketamine across a range of differing variables, including in the rodent FST (Table 1) but we may need to pay specific attention to whether animals are stressed or unstressed. Our brief “meta analysis” of a subset of these data (Figure 1) suggests that, in animals not subjected to marked psychosocial stress, negative results or paradoxical increases in immobility have not been emphasized in the literature. Our own mouse strain comparison data (Figure 2) suggest that a number of unstressed strains show mild increases in immobility in the 24-hour post-injection FST, after a single injection of 30 mg/kg (R,S)-ketamine.
SUMMARY AND CONCLUSIONS
Here we have reviewed two intriguing topics in the (R,S)-ketamine literature: first, the putative antidepressant-like mechanism of action of this drug, and second, its widely reported similar therapeutic effects across a range of factors and testing parameters. We suggest that (R,S)-ketamine’s antidepressant effects (in humans and rodents) require an initial glutamatergic (and possibly noradrenergic) “excitatory burst”, whether mediated principally by NMDAR antagonism or partly by AMPAR potentiation. This burst is followed by compensatory suppression of neural activity that decreases the E:I ratio to achieve its antidepressant-related effects. This effect may be spread across multiple brain regions simultaneously and each may be crucial. In the second portion of the paper, we suggest that there has been an under-emphasis of the potential stress-sensitivity of (R,S)-ketamine in rodent depression-related tests, including the FST. Gaining a greater understanding of the experimental conditions under which this drug exhibits antidepressant-like properties (or depression-like ones) has translational relevance for modeling major depression in human subjects (Fitzgerald et al., 2019), where opposing effects of (R,S)-ketamine have recently been reported based on whether the individuals were suffering from major depression or not (Nugent et al., 2018). Many studies of this drug have been conducted only in unstressed animals, so including animals that have been exposed to chronic psychosocial stress, such as CSDS, to induce a depression-like state may be critical for understanding the therapeutic properties of (R,S)-ketamine (Bale et al., 2019; Hashimoto & Shirayama, 2018).
We suggest that researchers view the variability and current lack of clarity on many points in the field as potentially helpful in the long term. If we can understand the source of variance in these data we can better study the complex biology we are attempting to understand. The variance is there, most likely, due to a combination of “noise” but also possibly some systematic differences in factors such as animal housing, drug administration, animal handling, recording methodologies, analytical techniques, animal strain, sex, dosing and test timing, circadian differences and others. Human psychiatric conditions are also expressed in individuals within a context that includes huge variability in background conditions. If, as a field, we can understand how the variance in our laboratories influences the effects of (R,S)-ketamine and other drugs, we stand a better chance of understanding variability among individuals in patient populations. Considering variability as not a barrier but as a tool can lead to more effective and often personalized interventions on our way to better understanding neurobiology.
Supplementary Material
Acknowledgments
Funding sources: NIH K08 MH107662, Neuroscience Fellows at the University of Michigan, Frances and Kenneth Eisenberg Scholar Award, a NARSAD Young Investigator Award; all for B.W. The authors report no financial conflicts of interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abdallah CG, Averill LA, Gueorguieva R, Goktas S, Purohit P, Ranganathan M, … Krystal JH (2018). Rapamycin, an Immunosuppressant and mTORC1 Inhibitor, Triples the Antidepressant Response Rate of Ketamine at 2 Weeks Following Treatment: A double-blind, placebo-controlled, cross-over, randomized clinical trial. Biorxiv. 10.1101/500959 [DOI] [Google Scholar]
- Abdallah CG, De Feyter HM, Averill LA, Jiang L, Averill CL, Chowdhury GMI, … Mason GF (2018). The effects of ketamine on prefrontal glutamate neurotransmission in healthy and depressed subjects. Neuropsychopharmacology. 10.1038/s41386-018-0136-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abdallah CG, Sanacora G, Duman RS, & Krystal JH (2018). The neurobiology of depression, ketamine and rapid-acting antidepressants: Is it glutamate inhibition or activation? Pharmacology and Therapeutics. 10.1016/j.pharmthera.2018.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abelaira HM, Réus GZ, Ignácio ZM, dos Santos MAB, de Moura AB, Matos D, … Quevedo J (2017). Effects of ketamine administration on mTOR and reticulum stress signaling pathways in the brain after the infusion of rapamycin into prefrontal cortex. Journal of Psychiatric Research. 10.1016/j.jpsychires.2016.12.002 [DOI] [PubMed] [Google Scholar]
- Amat-Foraster M, Jensen AA, Plath N, Herrik KF, Celada P, & Artigas F (2018). Temporally dissociable effects of ketamine on neuronal discharge and gamma oscillations in rat thalamo-cortical networks. Neuropharmacology. 10.1016/j.neuropharm.2018.04.022 [DOI] [PubMed] [Google Scholar]
- Andreae MH, Rhodes E, Bourgoise T, Carter GM, White RS, Indyk D, … Rhodes R (2016). An Ethical Exploration of Barriers to Research on Controlled Drugs. American Journal of Bioethics, 16(4), 36–47. 10.1080/15265161.2016.1145282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autry AE, Adachi M, Nosyreva E, Na ES, Los MF, Cheng PF, … Monteggia LM (2011). NMD A receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature. 10.1038/nature10130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bale TL, Abel T, Akil H, Carlezon WA, Moghaddam B, Nestler EJ, … Thompson SM (2019). The critical importance of basic animal research for neuropsychiatric disorders. Neuropsychopharmacology, 44(8), 1349–1353. 10.1038/s41386-019-0405-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechtholt-Gompf AJ, Smith KL, John CS, Kang HH, Carlezon WA, Cohen BM, & Öngür D (2011). CD-1 and Balb/cJ mice do not show enduring antidepressant-like effects of ketamine in tests of acute antidepressant efficacy. Psychopharmacology. 10.1007/s00213-011-2169-8 [DOI] [PubMed] [Google Scholar]
- Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, & Krystal JH (2000). Antidepressant Effects of Ketamine in Depressed Patients. Biol Psychiatry, 47, 351–354. [DOI] [PubMed] [Google Scholar]
- Bonde JP, Utzon-Frank N, Bertelsen M, Borritz M, Eller NH, Nordentoft M, … Rugulies R (2016). Risk of depressive disorder following disasters and military deployment: Systematic review with meta-analysis. British Journal of Psychiatry. 10.1192/bjp.bp.114.157859 [DOI] [PubMed] [Google Scholar]
- Botanas CJ, Bryan de la Peña J, Custodio RJ, Joy dela Peña I, Kim M, Woo T, … Cheong JH (2017). Methoxetamine produces rapid and sustained antidepressant effects probably via glutamatergic and serotonergic mechanisms. Neuropharmacology, 126, 121–127. 10.1016/j.neuropharm.2017.08.038 [DOI] [PubMed] [Google Scholar]
- Carreno FR, Donegan JJ, Boley AM, Shah A, DeGuzman M, Frazer A, & Lodge DJ (2016). Activation of a ventral hippocampus-medial prefrontal cortex pathway is both necessary and sufficient for an antidepressant response to ketamine. Molecular Psychiatry, 21(9), 1298–1308. 10.1038/mp.2015.176 [DOI] [PubMed] [Google Scholar]
- Carrier N, & Kabbaj M (2013). Sex differences in the antidepressant-like effects of ketamine. Neuropharmacology. 10.1016/j.neuropharm.2012.12.009 [DOI] [PubMed] [Google Scholar]
- Chan LF, Eu CL, Soh SY, Maniam T, Kadir ZS, Chong BTW, … Kahn DA (2018). Is Ketamine the Future Clozapine for Depression? A Case Series and Literature Review on Maintenance Ketamine in Treatment-resistant Depression with Suicidal Behavior. Journal of Psychiatric Practice, 24(4), 279–291. 10.1097/PRA.0000000000000316 [DOI] [PubMed] [Google Scholar]
- Chapman CD, Benedict C, & Schiöth HB (2018). Experimenter gender and replicability in science. Science Advances. 10.1126/sciadv.1701427 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chindo BA, Adzu B, Yahaya TA, & Gamaniel KS (2012). Ketamine-enhanced immobility in forced swim test: A possible animal model for the negative symptoms of schizophrenia. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 38(2), 310–316. 10.1016/j.pnpbp.2012.04.018 [DOI] [PubMed] [Google Scholar]
- Chou D, Peng HY, Lin T. Bin, Lai CY, Hsieh MC, Wen YC, … Ho YC (2018). (2R,6R)-hydroxynorketamine rescues chronic stress-induced depression-like behavior through its actions in the midbrain periaqueductal gray. Neuropharmacology. 10.1016/j.neuropharm.2018.06.033 [DOI] [PubMed] [Google Scholar]
- Chowdhury GMI, Zhang J, Thomas M, Banasr M, Ma X, Pittman B, … Sanacora G (2017). Transiently increased glutamate cycling in rat PFC is associated with rapid onset of antidepressant-like effects. Molecular Psychiatry, 22(1), 120–126. 10.1038/mp.2016.34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke M, Razmjou S, Prowse N, Dwyer Z, Litteljohn D, Pentz R, … Hayley S (2017). Ketamine modulates hippocampal neurogenesis and pro-inflammatory cytokines but not stressor induced neurochemical changes. Neuropharmacology. 10.1016/j.neuropharm.2016.04.021 [DOI] [PubMed] [Google Scholar]
- Collingridge GL, Lee Y, Bortolotto ZA, Kang H, & Lodge D (2017, April 15). Antidepressant Actions of Ketamine Versus Hydroxynorketamine. Biological Psychiatry, Vol. 81, pp. e65–e67. 10.1016/j.biopsych.2016.06.029 [DOI] [PubMed] [Google Scholar]
- Collo G, Cavalleri L, Chiamulera C, & Pich EM (2019). Ketamine increases the expression of GluR1 and GluR2 α-amino-3-hydroxy-5-methy-4-isoxazole propionate receptor subunits in human dopaminergic neurons differentiated from induced pluripotent stem cells. NeuroReport, 30, 207–212. 10.1097/WNR.0000000000001185 [DOI] [PubMed] [Google Scholar]
- Cui Y, Yang Y, Ni Z, Dong Y, Cai G, Foncelle A, … Hu H (2018). Astroglial Kir4.1 in the lateral habenula drives neuronal bursts in depression. Nature, 554(7692), 323–327. 10.1038/nature25752 [DOI] [PubMed] [Google Scholar]
- Cunha MP, Pazini FL, Rosa JM, Ramos-Hryb AB, Oliveira Á, Raster MP, & Rodrigues ALS (2015). Creatine, similarly to ketamine, affords antidepressant-like effects in the tail suspension test via adenosine A1and A2Areceptor activation. Purinergic Signalling. 10.1007/s11302-015-9446-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derivan AT (1995). Antidepressants: can we determine how quickly they work? Issues from the literature. Psychopharmacol Bull, 31, 23–28. [PubMed] [Google Scholar]
- Donegan JJ, & Lodge DJ (2017). Hippocampal perineuronal nets are required for the sustained antidepressant effect of ketamine. International Journal of Neuropsychopharmacology, 20(4), 354–358. 10.1093/ijnp/pyw095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong C, Zhang JC, Yao W, Ren Q, Ma M, Yang C, … Hashimoto (2017). Rapid and Sustained Antidepressant Action of the mGlu2/3 Receptor Antagonist MGS0039 in the Social Defeat Stress Model: Comparison with Ketamine. International Journal of Neuropsychopharmacology. 10.1093/ijnp/pyw089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- du Jardin KG, Liebenberg N, Müller HK, Elfving B, Sanchez C, & Wegener G (2016). Differential interaction with the serotonin system by S-ketamine, vortioxetine, and fluoxetine in a genetic rat model of depression. Psychopharmacology. 10.1007/s00213-016-4327-5 [DOI] [PubMed] [Google Scholar]
- Dulawa SC, Holick KA, Gundersen B, & Hen R (2004). Effects of chronic fluoxetine in animal models of anxiety and depression. Neuropsychopharmacology 10.1038/sj.npp.1300433 [DOI] [PubMed] [Google Scholar]
- Duman RS (2018). Ketamine and rapid-acting antidepressants: a new era in the battle against depression and suicide. F1000Research, 7, 659 10.12688/f1000research.14344.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duman RS, Li N, Liu RJ, Duric V, & Aghajanian G (2012). Signaling pathways underlying the rapid antidepressant actions of ketamine. Neuropharmacology. 10.1016/j.neuropharm.2011.08.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer JM, Maldonado-Avilés JG, Lepack AE, DiLeone RJ, & Duman RS (2015). Ribosomal protein S6 kinase 1 signaling in prefrontal cortex controls depressive behavior. Proceedings of the National Academy of Sciences, 112(19), 6188–6193. 10.1073/pnas.1505289112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebada ME (2017, November 1). Drug repurposing may generate novel approaches to treating depression. Journal of Pharmacy and Pharmacology, Vol. 69, pp. 1428–1436. 10.1111/jphp.12815 [DOI] [PubMed] [Google Scholar]
- Fee C, Banasr M, & Sibille E (2017). Somatostatin-Positive Gamma-Aminobutyric Acid Interneuron Deficits in Depression: Cortical Microcircuit and Therapeutic Perspectives. Biological Psychiatry. 10.1016/j.biopsych.2017.05.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald PJ, & Watson BO (2018). Gamma oscillations as a biomarker for major depression: an emerging topic. Translational Psychiatry. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald Paul J., & Watson BO (2019). In vivo electrophysiological recordings of the effects of antidepressant drugs. Experimental Brain Research. 10.1007/s00221-019-05556-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald Paul J., Whittle N, Flynn SM, Graybeal C, Pinard CR, Gunduz-Cinar O, … Holmes A (2014). Prefrontal single-unit firing associated with deficient extinction in mice. Neurobiology of Learning and Memory. 10.1016/j.nlm.2013.11.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald Paul J., Yen JY, & Watson BO (2019). Stress-sensitive antidepressant-like effects of ketamine in the mouse forced swim test. PLoS ONE, 14(4). 10.1371/journal.pone.0215554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick CJ, Gopalakrishnan S, Cogan ES, Yager LM, Meyer PJ, Lovic V, … Morrow JD (2013). Variation in the Form of Pavlovian Conditioned Approach Behavior among Outbred Male Sprague-Dawley Rats from Different Vendors and Colonies: Sign-Tracking vs. Goal-Tracking. PLoS ONE. 10.1371/journal.pone.0075042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogaça MV, & Duman RS (2019). Cortical GABAergic Dysfunction in Stress and Depression: New Insights for Therapeutic Interventions. Frontiers in Cellular Neuroscience, 13 10.3389/fncel.2019.00087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franceschelli A, Sens J, Herchick S, Thelen C, & Pitychoutis PM (2015). Sex differences in the rapid and the sustained antidepressant-like effects of ketamine in stress-naive and “depressed” mice exposed to chronic mild stress. Neuroscience. 10.1016/j.neuroscience.2015.01.008 [DOI] [PubMed] [Google Scholar]
- Fuchikami M, Thomas A, Liu R, Wohleb ES, Land BB, DiLeone RJ, … Duman RS (2015). Optogenetic stimulation of infralimbic PFC reproduces ketamine’s rapid and sustained antidepressant actions. Proceedings of the National Academy of Sciences. 10.1073/pnas.1414728112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto K, Fogaç MV, Liu R-J, Duman C, Kato T, Li X-Y, & Duman RS (2019). Activity-dependent brain-derived neurotrophic factor signaling is required for the antidepressant actions of (2R,6R)-hydroxynorketamine. Proc Natl Acad Sci U S A. 116, 297–302. 10.1073/pnas.1814709116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukumoto K, Iijima M, Funakoshi T, & Chaki S (2018). Role of 5-HT1A Receptor Stimulation in the Medial Prefrontal Cortex in the Sustained Antidepressant Effects of Ketamine. International Journal of Neuropsychopharmacology. 10.1093/ijnp/pyx116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabbay V, Mao X, Klein RG, Ely BA, Babb JS, Panzer AM, … Shungu DC (2012). Anterior cingulate cortex γ-aminobutyric acid in depressed adolescents: Relationship to anhedonia. Archives of General Psychiatry. 10.1001/archgenpsychiatry.2011.131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia LS, Comim CM, Valvassori SS, Réus GZ, Andreazza AC, Stertz L, … Quevedo J (2008). Chronic administration of ketamine elicits antidepressant-like effects in rats without affecting hippocampal brain-derived neurotrophic factor protein levels. Basic and Clinical Pharmacology and Toxicology. 10.1111/j.1742-7843.2008.00210.x [DOI] [PubMed] [Google Scholar]
- Georgiou P, Zanos P, Jenne C, Highland J, Gerhard D, Duman R, & Gould T (2018). Human experimenter sex modulates mouse behavioral responses to stress and to the antidepressant ketamine. Biological Psychiatry, 83(9, supplement), S277 10.1016/j.biopsych.2018.02.715 [DOI] [Google Scholar]
- Gerhard DM, & Duman RS (2018). Rapid-Acting Antidepressants: Mechanistic Insights and Future Directions. Current Behavioral Neuroscience Reports, 5(1), 36–47. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/30034992 [PMC free article] [PubMed] [Google Scholar]
- Gideons ES, Kavalali ET, & Monteggia LM (2014). Mechanisms underlying differential effectiveness of memantine and ketamine in rapid antidepressant responses. Proceedings of the National Academy of Sciences, 111(23), 8649–8654. 10.1073/pnas.1323920111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigliucci V, O’Dowd G, Casey S, Egan D, Gibney S, & Harkin A (2013). Ketamine elicits sustained antidepressant-like activity via a serotonin-dependent mechanism. Psychopharmacology, 228(1), 157–166. 10.1007/s00213-013-3024-x [DOI] [PubMed] [Google Scholar]
- Gileta AF, Fitzpatrick CJ, Chitre AS, St Pierre CL, Joyce EV, Maguire RJ, … Palmer AA (2018). Genetic characterization of outbred Sprague Dawley rats and utility for 4 genome-wide association studies. 10.1101/412924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajós M, Hoffmann WE, Robinson DD, Yu JH, & Va Hajó S-Korcsok É (2003). Norepinephrine but not Serotonin Reuptake Inhibitors Enhance Theta and Gamma Activity of the Septo-Hippocampal System. Neuropsychopharmacology, 28(5), 857–864. 10.1038/sj.npp.1300116 [DOI] [PubMed] [Google Scholar]
- Hakami T, Jones NC, Tolmacheva EA, Gaudias J, Chaumont J, Salzberg M, … Pinault D (2009). NMDA receptor hypofunction leads to generalized and persistent aberrant γ oscillations independent of hyperlocomotion and the state of consciousness. PLoS ONE. 10.1371/journal.pone.0006755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare BD, Shinohara R, Liu RJ, Pothula S, DiLeone RJ, & Duman RS (2019). Optogenetic stimulation of medial prefrontal cortex Drd1 neurons produces rapid and long-lasting antidepressant effects. Nature Communications, 10(1). 10.1038/s41467-018-08168-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K (2019). Rapid-acting Antidepressant Ketamine, Its Metabolites and Other Candidates: A Historical Overview and Future Perspective. Psychiatry and Clinical Neurosciences. 10.1111/pcn.12902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto K, & Shirayama Y (2018). What Are the Causes for Discrepancies of Antidepressant Actions of (2R,6R)-Hydroxynorketamine? Biological Psychiatry. 10.1016/j.biopsych.2017.12.007 [DOI] [PubMed] [Google Scholar]
- Hasler G, Van Der Veen JW, Tumonis T, Meyers N, Shen J, & Drevets WC (2007). Reduced Prefrontal Glutamate/Glutamine and-Aminobutyric Acid Levels in Major Depression Determined Using Proton Magnetic Resonance Spectroscopy. In Arch Gen Psychiatry (Vol. 64). [DOI] [PubMed] [Google Scholar]
- Hefner K, Whittle N, Juhasz J, Norcross M, Karlsson R-M, Saksida LM, … Holmes A (2008). Impaired Fear Extinction Learning and Cortico-Amygdala Circuit Abnormalities in a Common Genetic Mouse Strain. Journal of Neuroscience. 10.1523/JNEUROSCI.4904-07.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkel V, Seemiüiller F, Obermeier M, Adli M, Bauer, Mundt C, … Riedel M (2009). Does early improvement triggered by antidepressants predict response/remission? - Analysis of data from a naturalistic study on a large sample of inpatients with major depression. Journal of Affective Disorders, 115(3), 439–449. 10.1016/j.jad.2008.10.011 [DOI] [PubMed] [Google Scholar]
- Hosang GM, Shiles C, Tansey KE, McGuffin P, & Uher R (2014). Interaction between stress and the BDNF Val66Met polymorphism in depression: A systematic review and meta-analysis. BMC Medicine. 10.1186/1741-7015-12-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou L, Qi Y, Sun H, Wang G, Li Q, Wang Y, … Sun L (2018). Applying ketamine to alleviate the PTSD-like effects by regulating the HCN1-related BDNF. Progress in Neuro- Psychopharmacology and Biological Psychiatry, 86, 313–321. 10.1016/j.pnpbp.2018.03.019 [DOI] [PubMed] [Google Scholar]
- Huang N, Hua D, Zhan G, Li S, Zhu B, Jiang R, … Yang C (2019). Role of Actinobacteria and Coriobacteriia in the antidepressant effects of ketamine in an inflammation model of depression. Pharmacology Biochemistry and Behavior, 176, 93–100. 10.1016/j.pbb.2018.12.001 [DOI] [PubMed] [Google Scholar]
- Hunt MJ, Garcia R, Large CH, & Kasicki S (2008). Modulation of high-frequency oscillations associated with NMDA receptor hypofunction in the rodent nucleus accumbens by lamotrigine. Progress in Neuro-Psychopharmacology and Biological Psychiatry. 10.1016/j.pnpbp.2008.04.009 [DOI] [PubMed] [Google Scholar]
- Jett JD, Boley AM, Girotti M, Shah A, Lodge DJ, & Morilak DA (2015). Antidepressant-like cognitive and behavioral effects of acute ketamine administration associated with plasticity in the ventral hippocampus to medial prefrontal cortex pathway. Psychopharmacology, 232(17), 3123–3133. 10.1007/s00213-015-3957-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang Y, Wang Y, Sun X, Lian B, Sun H, Wang G, … Sun L (2017). Short- and long-term antidepressant effects of ketamine in a rat chronic unpredictable stress model. Brain and Behavior. 10.1002/brb3.749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavalali E, & Monteggia L (2018). The Ketamine Metabolite 2R,6RHydroxynorketamine Blocks NMDA Receptors and Impacts Downstream Signaling Linked to Antidepressant Effects. Neuropsychopharmacology, 43(1), 221–222. 10.1038/npp.2017.192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kheirabadi G, Vafaie M, Kheirabadi D, Mirlouhi Z, & Hajiannasab R (2019). Comparative Effect of Intravenous Ketamine and Electroconvulsive Therapy in Major Depression: A Randomized Controlled Trial. Advanced Biomedical Research, 8(1), 25 10.4103/abr.abr_166_18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishimoto T, Chawla JM, Hagi K, Zarate CA, Kane JM, Bauer M, & Correll CU (2016). Single-dose infusion ketamine and non-ketamine N-methyl-d-aspartate receptor antagonists for unipolar and bipolar depression: A meta-analysis of efficacy, safety and time trajectories. Psychological Medicine, 46(7), 1459–1472. 10.1017/S0033291716000064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohtala S, Theilmann W, Rosenholm M, Penna L, Karabulut G, Uusitalo S, … Rantamaki T (2018, June 1). Cortical Excitability and Activation of TrkB Signaling During Rebound Slow Oscillations Are Critical for Rapid Antidepressant Responses. Molecular Neurobiology. 10.1007/s12035-018-1364-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koike H, & Chaki S (2014). Requirement of AMPA receptor stimulation for the sustained antidepressant activity of ketamine and LY341495 during the forced swim test in rats. Behavioural Brain Research, 271, 111–115. 10.1016/j.bbr.2014.05.065 [DOI] [PubMed] [Google Scholar]
- Kordjazy N, Haj-Mirzaian A, Amiri S, Ostadhadi S, Amini-Khoei H, & Dehpour AR (2016). Involvement of N-methyl-d-aspartate receptors in the antidepressant-like effect of 5-hydroxytryptamine 3 antagonists in mouse forced swimming test and tail suspension test. Pharmacology Biochemistry and Behavior. 10.1016/j.pbb.2015.11.009 [DOI] [PubMed] [Google Scholar]
- Kubota T, Anzawa N, Hirota K, Yoshida HM, Kushikata T, & Matsuki A (1999). Effects of ketamine and pentobarbital on noradrenaline release from the medial prefrontal cortex in rats. Canadian Journal of Anaesthesiology, 46(4), 388–392. [DOI] [PubMed] [Google Scholar]
- Kubota T, Hirota K, Anzawa N, Yoshida H, Kushikata T, & Matsuki A (1999). Physostigmine antagonizes ketamine-induced noradrenaline release from the medial prefrontal cortex in rats. Brain Research. 10.1016/S0006-8993(99)01793-X [DOI] [PubMed] [Google Scholar]
- Kudlow PA, Cha DS, & Mcintyre RS (2012). Predicting Treatment Response in Major Depressive Disorder: The Impact of Early Symptomatic Improvement. Can J Psychiatry, 57, 782–788. Retrieved from www.TheCJP.ca [DOI] [PubMed] [Google Scholar]
- Layer RT, Popik P, Olds T, & Skolnick P (1995). Antidepressant-like actions of the polyamine site NMDA antagonist, eliprodil (SL-82.0715). Pharmacology, Biochemistry and Behavior. 10.1016/0091-3057(95)00155-P [DOI] [PubMed] [Google Scholar]
- Lee J, Hudson MR, O’Brien TJ, Nithianantharajah J, & Jones NC (2017). Local NMDA receptor hypofunction evokes generalized effects on gamma and high-frequency oscillations and behavior. Neuroscience. 10.1016/j.neuroscience.2017.06.039 [DOI] [PubMed] [Google Scholar]
- Lepack AE, Fuchikami M, Dwyer JM, Banasr M, & Duman RS (2015). BDNF release is required for the behavioral actions of ketamine. International Journal of Neuropsychopharmacology. 10.1093/ijnp/pyu033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, … Duman RS (2011). Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biological Psychiatry. 10.1016/j.biopsych.2010.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liao Y, Tang Y.lang, & Hao W (2017, September 3). Ketamine and international regulations. American Journal of Drug and Alcohol Abuse, Vol. 43, pp. 495–504. 10.1080/00952990.2016.1278449 [DOI] [PubMed] [Google Scholar]
- Liebe T, Li M, Colic L, Munk MHJ, Sweeney-Reed CM, Woelfer M, … Walter M (2018). Ketamine influences the locus coeruleus norepinephrine network, with a dependency on norepinephrine transporter genotype – a placebo controlled fMRI study. Neuroimage: Clinical, 20, 715–723. 10.1016/j.nicl.2018.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin JC, Lee MY, Chan MH, Chen YC, & Chen HH (2016). Betaine enhances antidepressant-like, but blocks psychotomimetic effects of ketamine in mice. Psychopharmacology, 233(17), 3223–3235. 10.1007/s00213-016-4359-x [DOI] [PubMed] [Google Scholar]
- Liu RJ, Fuchikami M, Dwyer JM, Lepack AE, Duman RS, & Aghajanian GK (2013). GSK-3 inhibition potentiates the synaptogenic and antidepressant-like effects of subthreshold doses of ketamine. Neuropsychopharmacology, 38(11), 2268–2277. 10.1038/npp.2013.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RJ, Lee FS, Li XY, Bambico F, Duman RS, & Aghajanian GK (2012). Brain-derived neurotrophic factor Val66Met allele impairs basal and ketamine-stimulated synaptogenesis in prefrontal cortex. Biological Psychiatry, 71(11), 996–1005. https://doi.Org/10.1016/j.biopsych.2011.09.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Ge T, Leng Y, Pan Z, Fan J, Yang W, & Cui R (2017). The Role of Neural Plasticity in Depression: From Hippocampus to Prefrontal Cortex. Neural Plasticity, 2017, 1–11. 10.1155/2017/6871089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumsden EW, Troppoli TA, Myers SJ, Zanos P, Aracava Y, Kehr J, … Gould TD (2019). Antidepressant-relevant concentrations of the ketamine metabolite (2 R ,6 R )-hydroxynorketamine do not block NMDA receptor function. Proceedings of the National Academy of Sciences, 116(11), 5160–5169. 10.1073/pnas.1816071116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luscher B, & Fuchs T (2015). GABAergic control of depression-related brain states. Advances in Pharmacology. 10.1016/bs.apha.2014.11.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma XC, Dang YH, Jia M, Ma R, Wang F, Wu J, … Hashimoto K (2013). Long-Lasting Antidepressant Action of Ketamine, but Not Glycogen Synthase Kinase-3 Inhibitor SB216763, in the Chronic Mild Stress Model of Mice. PLoS ONE. 10.1371/journal.pone.0056053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado-Vieira R, Salvadore G, DiazGranados N, & Zarate CA (2009, August). Ketamine and the next generation of antidepressants with a rapid onset of action. Pharmacology and Therapeutics, Vol. 123, pp. 143–150. 10.1016/j.pharmthera.2009.02.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maciel AL, Abelaira HM, de Moura AB, de Souza TG, Rosa T, Matos D, … Réus GZ (2018). Acute treatment with ketamine and chronic treatment with minocycline exert antidepressant-like effects and antioxidant properties in rats subjected different stressful events. Brain Research Bulletin. 10.1016/j.brainresbull.2017.12.005 [DOI] [PubMed] [Google Scholar]
- Maeng S, Zarate CA, Du J, Schloesser RJ, McCammon J, Chen G, & Manji HK (2008). Cellular Mechanisms Underlying the Antidepressant Effects of Ketamine: Role of a-Amino-3-Hydroxy-5-Methylisoxazole-4-Propionic Acid Receptors. Biological Psychiatry, 63(4), 349–352. 10.1016/j.biopsych.2007.05.028 [DOI] [PubMed] [Google Scholar]
- Marton T, Barnes DE, Wallace A, & Woolley JD (2019). Concurrent Use of Buprenorphine, Methadone, or Naltrexone Does Not Inhibit Ketamine’s Antidepressant Activity. Biological Psychiatry. 10.1016/j.biopsych.2019.02.008 [DOI] [PubMed] [Google Scholar]
- Mastrodonato A, Martinez R, Pavlova IP, LaGamma CT, Brachman RA, Robison AJ, & Denny CA (2018). Ventral CA3 Activation Mediates Prophylactic Ketamine Efficacy Against Stress-Induced Depressive-like Behavior. Biological Psychiatry, 84(11), 846–856. 10.1016/j.biopsych.2018.02.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS (1999). STRESS AND HIPPOCAMPAL PLASTICITY. Annual Review of Neuroscience, 22(1), 105–122. 10.1146/annurev.neuro.22.1.105 [DOI] [PubMed] [Google Scholar]
- Meloni D, Carla Gambarana I, Graziella Montis MDE, Dal Pra P, Taddei I, Tagliamonte A, … Tagliamonte A (1993). Dizocilpine Antagonizes the Effect of Chronic Imipramine on Learned Helplessness in Rats. In Pharmacology Biochemistry and Behavior (Vol. 46). [DOI] [PubMed] [Google Scholar]
- Mickey BJ, White AT, Arp AM, Leonardi K, Torres MM, Larson AL, … Tadler SC (2018). Propofol for treatment-resistant depression: A pilot study. International Journal of Neuropsychopharmacology, 21(12), 1079–1089. 10.1093/ijnp/pyy085 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineur YS, Bentham MP, Zhou WL, Plantenga ME, McKee SA, & Picciotto MR (2015). Antidepressant-like effects of guanfacine and sex-specific differences in effects on c-fos immunoreactivity and paired-pulse ratio in male and female mice. Psychopharmacology. 10.1007/s00213-015-4001-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mineur YS, Cahuzac EL, Mose TN, Bentham MP, Plantenga ME, Thompson DC, & Picciotto MR (2018). Interaction between noradrenergic and cholinergic signaling in amygdala regulates anxiety- and depression-related behaviors in mice. Neuropsychopharmacology. 10.1038/s41386-018-0024-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra PK, Kumar A, Behar KL, & Patel AB (2018). Subanesthetic ketamine reverses neuronal and astroglial metabolic activity deficits in a social defeat model of depression. Journal of Neurochemistry. 10.1111/jnc.14544 [DOI] [PubMed] [Google Scholar]
- Moda-Sava RN, Murdock MH, Parekh PK, Fetcho RN, Huang BS, Huynh TN, … Liston C (2019). Sustained rescue of prefrontal circuit dysfunction by antidepressant-induced spine formation. Science (New York, N.Y.), 364(6436), eaat8078 10.1126/science.aat8078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moghaddam B, Adams B, Verma A, & Daly D (1997). Activation of Glutamatergic Neurotransmission by Ketamine: A Novel Step in the Pathway from NMDA Receptor Blockade to Dopaminergic and Cognitive Disruptions Associated with the Prefrontal Cortex. Journal of Neuroscience, 17(8), 2921–2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moryl E, Danysz W, & Quack G (1993). Potential Antidepressive Properties of Amantadine, Memantine and Bifemelane. Pharmacology & Toxicology. 10.1111/j.1600-0773.1993.tb01351.x [DOI] [PubMed] [Google Scholar]
- Mozhui K, Karlsson RM, Kash TL, Ihne J, Norcross M, Patel S, … Holmes A (2010). Strain Differences in Stress Responsivity Are Associated with Divergent Amygdala Gene Expression and Glutamate-Mediated Neuronal Excitability. Journal of Neuroscience. 10.1523/JNEUROSCI.5017-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neis VB, Bettio LEB, Moretti M, Rosa PB, Ribeiro CM, Freitas AE, … Rodrigues (2016). Acute agmatine administration, similar to ketamine, reverses depressive-like behavior induced by chronic unpredictable stress in mice. Pharmacology Biochemistry and Behavior. 10.1016/j.pbb.2016.10.004 [DOI] [PubMed] [Google Scholar]
- Nosyreva E, Autry AE, Kavalali ET, & Monteggia LM (2014). Age dependence of the rapid antidepressant and synaptic effects of acute NMDA receptor blockade. Frontiers in Molecular Neuroscience, 7 10.3389/fnmol.2014.00094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nugent AC, Ballard ED, Gould TD, Park LT, Moaddel R, Brutsche NE, & Zarate CA Jr (2018). Ketamine has distinct electrophysiological and behavioral effects in depressed and healthy subjects. Molecular Psychiatry. 10.1038/s41380-018-0028-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostadhadi S, Norouzi-Javidan A, Chamanara M, Akbarian R, Imran-Khan M, Ghasemi M, & Dehpour AR. (2017). Involvement of NMDA receptors in the antidepressant-like effect of tramadolin the mouse forced swimming test. Brain Res Bulletin, 134, 136–141. [DOI] [PubMed] [Google Scholar]
- Pałucha-Poniewiera A, Podkowa K, & Pile A (2019). Role of AMPA receptor stimulation and TrkB signaling in the antidepressant-like effect of ketamine co-administered with a group II mGlu receptor antagonist, LY341495, in the forced swim test in rats. Behavioural Pharmacology, 1. 10.1097/fbp.0000000000000471 [DOI] [PubMed] [Google Scholar]
- Papp M, & Moryl E (1994). Antidepressant activity of non-competitive and competitive NMDA receptor antagonists in a chronic mild stress model of depression. European Journal of Pharmacology. 10.1016/0014-2999(94)90516-9 [DOI] [PubMed] [Google Scholar]
- Papp M, & Moryl E (1996). Antidepressant-like effects of 1-aminocyclopropanecarboxylic acid and D-cycloserine in an animal model of depression. European Journal of Pharmacology. 10.1016/S0014-2999(96)00675-9 [DOI] [PubMed] [Google Scholar]
- Patel K, Abdool PS, Rajji TK, & Mulsant BH (2017, April 13). Pharmacotherapy of major depression in late life: what is the role of new agents? Expert Opinion on Pharmacotherapy, Vol. 18, pp. 599–609. 10.1080/14656566.2017.1308484 [DOI] [PubMed] [Google Scholar]
- Pham TH, Mendez-David I, Defaix C, Guiard BP, Tritschler L, David DJ, & Gardier AM (2017). Ketamine treatment involves medial prefrontal cortex serotonin to induce a rapid antidepressant-like activity in BALB/cJ mice. Neuropharmacology, 112, 198–209. 10.1016/j.neuropharm.2016.05.010 [DOI] [PubMed] [Google Scholar]
- Pham Thu Ha, Defaix C, Xu X, Deng SX, Fabresse N, Alvarez JC, … Gardier AM (2018, July 1). Common Neurotransmission Recruited in (R,S)-Ketamine and (2R,6R)-Hydroxynorketamine-Induced Sustained Antidepressant-like Effects. Biological Psychiatry, Vol. 84, pp. e3–e6. 10.1016/j.biopsych.2017.10.020 [DOI] [PubMed] [Google Scholar]
- Podkowa K, Pochwat B, Brański P, Pile A, & Pałucha-Poniewiera A (2016). Group II mGlu receptor antagonist LY341495 enhances the antidepressant-like effects of ketamine in the forced swim test in rats. Psychopharmacology, 233(15–16), 2901–2914. 10.1007/s00213-016-4325-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popik P, Hołuj M, Kos T, Nowak G, Librowski T, & Sałat K (2017). Comparison of the Psychopharmacological Effects of Tiletamine and Ketamine in Rodents. Neurotoxicity Research, 32(4), 544–554. 10.1007/s12640-017-9759-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popik P, Kos T, Sowa-Kucma M, & Nowak G (2008). Lack of persistent effects of ketamine in rodent models of depression. Psychopharmacology. 10.1007/s00213-008-1158-z [DOI] [PubMed] [Google Scholar]
- Pozzi L, Dorocic IP, Wang X, Carlén M, & Meletis K (2014). Mice lacking NMDA receptors in parvalbumin neurons display normal depression-related behavior and response to antidepressant action of NMDAR antagonists. PLoS ONE. 10.1371/journal.pone.0083879 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Przegaliński E, Tatarczyńska E, Dereń-Wesołek A, & Chojnacka-Wójcik E (1997). Antidepressant-like effects of a partial agonist at strychnine-insensitive glycine receptors and a competitive NMDA receptor antagonist in the forced swimming test in rats. Neuropharmacology, 36, 31–37. [DOI] [PubMed] [Google Scholar]
- Réus GZ, Vieira FG, Abelaira HM, Michels M, Tomaz DB, dos Santos MAB, … Quevedo J (2014). MAPK signaling correlates with the antidepressant effects of ketamine. Journal of Psychiatric Research, 55(1), 15–21. 10.1016/j.jpsychires.2014.04.010 [DOI] [PubMed] [Google Scholar]
- Rosenblat JD, Carvalho AF, Li M, Lee Y, Subramanieapillai M, & McIntyre RS (2019). Oral Ketamine for Depression. The Journal of Clinical Psychiatry, 80(3). 10.4088/JCP.18r12475 [DOI] [PubMed] [Google Scholar]
- Ruhe HG, Huyser J, Swinkels JA, & Schene AH (2006). Switching antidepressants after a first selective serotonin reuptake inhibitor in major depressive disorder: a systematic review. J Clin Psychiatry, 67, 1836–1855. [DOI] [PubMed] [Google Scholar]
- Sanacora G, Smith MA, Pathak S, Su HL, Boeijinga PH, McCarthy DJ, & Quirk MC (2014). Lanicemine: A low-trapping NMDA channel blocker produces sustained antidepressant efficacy with minimal psychotomimetic adverse effects. Molecular Psychiatry, 19(9), 978–985. 10.1038/mp.2013.130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarkar A, & Kabbaj M (2016). Sex Differences in Effects of Ketamine on Behavior, Spine Density, and Synaptic Proteins in Socially Isolated Rats. Biological Psychiatry, 80(6), 448–456. 10.1016/j.biopsych.2015.12.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen M, Lv D, Liu X, Li S, Chen Y, Zhang Y, … Wang C (2018). Essential roles of neuropeptide VGF regulated TrkB/mTOR/BICC1 signaling and phosphorylation of AMPA receptor subunit GluAl in the rapid antidepressant-like actions of ketamine in mice. Brain Research Bulletin, 143, 58–65. 10.1016/j.brainresbull.2018.10.004 [DOI] [PubMed] [Google Scholar]
- Shepard RD, Langlois LD, Browne CA, Berenji A, Lucki I, & Nugent FS (2018). Ketamine Reverses Lateral Habenula Neuronal Dysfunction and Behavioral Immobility in the Forced Swim Test Following Maternal Deprivation in Late Adolescent Rats. Frontiers in Synaptic Neuroscience. 10.3389/finsyn.2018.00039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirayama Y, & Hashimoto K (2017). Effects of a single bilateral infusion of R-ketamine in the rat brain regions of a learned helplessness model of depression. European Archives of Psychiatry and Clinical Neuroscience, 267(2), 177–182. 10.1007/s00406-016-0718-1 [DOI] [PubMed] [Google Scholar]
- Shirayama Y, & Hashimoto K (2018). Lack of Antidepressant Effects of (2R,6R)-Hydroxynorketamine in a Rat Learned Helplessness Model: Comparison with (R)-Ketamine. International Journal of Neuropsychopharmacology. 10.1093/ijnp/pyx108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh JB, Fedgchin M, Daly E, Xi L, Melman C, De Bruecker G, … Van Nueten L (2016). Intravenous Esketamine in Adult Treatment-Resistant Depression: A Double-Blind, Double-Randomization, Placebo-Controlled Study. . Biological Psychiatry, 80(6), 424–431. 10.1016/j.biopsych.2015.10.018 [DOI] [PubMed] [Google Scholar]
- Suárez-Santiago JE, Briones-Aranda A, Espinosa-Raya J, & Picazo O (2017). Agonist E-6837 and antagonist SB-271046 of 5-HT 6 receptors both reverse the depressive-like effect induced in mice by subchronic ketamine administration. Behavioural Pharmacology. 10.1097/FBP.0000000000000327 [DOI] [PubMed] [Google Scholar]
- Sun HL, Zhou ZQ, Zhang GF, Yang C, Wang XM, Shen JC, … Yang JJ (2016). Role of hippocampal p11 in the sustained antidepressant effect of ketamine in the chronic unpredictable mild stress model. Translational Psychiatry, 6, e741 10.1038/tp.2016.21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki K, Nosyreva E, Hunt K, Kavalali E, & Monteggia L (2017). Effects of a ketamine metabolite on synaptic NMDAR function. Nature, 546, E1–3. 10.1038/nature22084 [DOI] [PubMed] [Google Scholar]
- Tadler SC, & Mickey BJ (2018, August 1). Emerging evidence for antidepressant actions of anesthetic agents. Current Opinion in Anaesthesiology, Vol. 31, pp. 439–445. 10.1097/ACO.0000000000000617 [DOI] [PubMed] [Google Scholar]
- Tang J, Xue W, Xia B, Ren L, Tao W, Chen C, … Chen G (2015). Involvement of normalized NMDA receptor and mTOR-related signaling in rapid antidepressant effects of Yueju and ketamine on chronically stressed mice. Scientific Reports. 10.1038/srep13573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao W, Zhang H, Xue W, Ren L, Xia B, Zhou X, … Chen G (2014). Optimization of supercritical fluid extraction of oil from the fruit of Gardenia jasminoides and its antidepressant activity. Molecules, 19(12), 19350–19360. 10.3390/molecules191219350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Targum SD, Daly E, Fedgchin M, Cooper K, & Singh JB (2019). Comparability of blinded remote and site-based assessments of response to adjunctive esketamine or placebo nasal spray in patients with treatment resistant depression. Journal of Psychiatric Research, 111, 68–73. 10.1016/j.jpsychires.2019.01.017 [DOI] [PubMed] [Google Scholar]
- Thelen C, Flaherty E, Saurine J, Sens J, Mohamed S, & Pitychoutis PM (2019). Sex Differences in the Temporal Neuromolecular and Synaptogenic Effects of the Rapid-acting Antidepressant Drug Ketamine in the Mouse Brain. Neuroscience, 398, 182–192. 10.1016/j.neuroscience.2018.11.053 [DOI] [PubMed] [Google Scholar]
- Thelen C, Sens J, Mauch J, Pandit R, & Pitychoutis PM (2016). Repeated ketamine treatment induces sex-specific behavioral and neurochemical effects in mice. Behavioural Brain Research. 10.1016/j.bbr.2016.06.041 [DOI] [PubMed] [Google Scholar]
- Tian Z, Dong C, Zhang K, Chang L, & Hashimoto K (2018). Lack of Antidepressant Effects of Low-Voltage-Sensitive T-Type Calcium Channel Blocker Ethosuximide in a Chronic Social Defeat Stress Model: Comparison with (R)-Ketamine. International Journal of Neuropsychopharmacology, 21(11), 1031–1036. 10.1093/ijnp/pyy072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trullas R, & Skolnick P (1990). Functional antagonists at the NMDA receptor complex exhibit antidepressant actions. European Journal of Pharmacology. 10.1016/0014-2999(90)90204-J [DOI] [PubMed] [Google Scholar]
- Wang J, Goffer Y, Xu D, Tukey DS, Shamir DB, Eberle SE, … Ziff EB (2011). A Single Subanesthetic Dose of Ketamine Relieves Depression-like Behaviors Induced by Neuropathic Pain in Rats. Anesthesiology, 115, 812–821. Retrieved from www.FAER.org. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XD, Yang G, Bai Y, Feng YP, & Li H (2018). The behavioral study on the interactive aggravation between pruritus and depression. Brain and Behavior, 8(6). 10.1002/brb3.964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Xie L, Gao C, Zhai L, Zhang N, & Guo L (2018). Astrocytes activation contributes to the antidepressant-like effect of ketamine but not scopolamine. Pharmacology Biochemistry and Behavior. 10.1016/j.pbb.2018.05.001 [DOI] [PubMed] [Google Scholar]
- Watson BO, Ding M, & Buzsaki G (2018). Temporal coupling of field potentials and action potentials in the neocortex. Eur J Neuroscience. 10.1111/ejn.13807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams NR, Heifets BD, Blasey C, Sudheimer K, Pannu J, Pankow H, … Schatzberg AF (2018). Attenuation of Antidepressant Effects of Ketamine by Opioid Receptor Antagonism. American Journal of Psychiatry. 10.1176/appi.ajp.2018.18020138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams AV, & Trainor BC (2018, July 1). The impact of sex as a biological variable in the search for novel antidepressants. Frontiers in Neuroendocrinology, Vol. 50, pp. 107–117. 10.1016/j.yfrne.2018.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wray NH, Schappi JM, Singh H, Senese NB, & Rasenick MM (2018, June 12). NMDAR-independent, cAMP-dependent antidepressant actions of ketamine. Molecular Psychiatry, pp. 1–11. 10.1038/s41380-018-0083-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu R, Zhang H, Xue W, Zou Z, Lu C, Xia B, … Chen G (2017). Transgenerational impairment of hippocampal Akt-mTOR signaling and behavioral deficits in the offspring of mice that experience postpartum depression-like illness. Progress in Neuro-Psychopharmacology and Biological Psychiatry, 73, 11–18. 10.1016/j.pnpbp.2016.09.008 [DOI] [PubMed] [Google Scholar]
- Xia B, Chen C, Zhang H, Xue W, Tang J, Tao W, … Chen G (2016). Chronic stress prior to pregnancy potentiated long-lasting postpartum depressive-like behavior, regulated by Akt-mTOR signaling in the hippocampus. Scientific Reports, 6 10.1038/srep35042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie ZM, Wang XM, Xu N, Wang J, Pan W, Tang XH, … Yang JJ (2017). Alterations in the inflammatory cytokines and brain-derived neurotrophic factor contribute to depression-like phenotype after spared nerve injury: Improvement by ketamine. Scientific Reports, 7(1). 10.1038/s41598-017-03590-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Z, Zhang K, Ishima T, Ren Q, Ma M, Pu Y, … Hashimoto K (2019). Lack of rapid antidepressant effects of Kir4.1 channel inhibitors in a chronic social defeat stress model: Comparison with (R)-ketamine. Pharmacology Biochemistry and Behavior, 176, 57–62. 10.1016/j.pbb.2018.11.010 [DOI] [PubMed] [Google Scholar]
- Yamaguchi JI, Toki H, Qu Y, Yang C, Koike H, Hashimoto K, … Chaki S (2018). (2R,6R)-Hydroxynorketamine is not essential for the antidepressant actions of (R)-ketamine in mice. Neuropsychopharmacology. 10.1038/s41386-018-0084-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Shirayama Y, Zhang JC, Ren Q, Yao W, Ma M, … Hashimoto K (2015). R-ketamine: A rapid-onset and sustained antidepressant without psychotomimetic side effects. Translational Psychiatry. 10.1038/tp.2015.136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Chun, Kobayashi S, Nakao K, Dong C, Han M, Qu Y, … Hashimoto K (2018). AMPA Receptor Activation–Independent Antidepressant Actions of Ketamine Metabolite (S)-Norketamine. Biological Psychiatry. 10.1016/j.biopsych.2018.05.007 [DOI] [PubMed] [Google Scholar]
- Yang Chun, Ren Q, Qu Y, Zhang JC, Ma M, Dong, & Hashimoto K (2018). Mechanistic Target of Rapamycin–Independent Antidepressant Effects of (R)-Ketamine in a Social Defeat Stress Model. Biological Psychiatry, 83(1), 18–28. 10.1016/j.biopsych.2017.05.016 [DOI] [PubMed] [Google Scholar]
- Yang Y, Cui Y, Sang K, Dong Y, Ni Z, Ma S, & Hu H (2018). Ketamine blocks bursting in the lateral habenula to rapidly relieve depression. Nature, 554(7692), 317–322. 10.1038/nature25509 [DOI] [PubMed] [Google Scholar]
- Yao N, Skiteva O, Zhang X, Svenningsson P, & Chergui K (2018). Ketamine and its metabolite (2R,6R)-hydroxynorketamine induce lasting alterations in glutamatergic synaptic plasticity in the mesolimbic circuit. Molecular Psychiatry, 23(10), 2066–2077. 10.1038/mp.2017.239 [DOI] [PubMed] [Google Scholar]
- Yoon G, Petrakis IL, & Krystal JH (2019, March 1). Association of Combined Naltrexone and Ketamine with Depressive Symptoms in a Case series of Patients with Depression and Alcohol Use Disorder. JAMA Psychiatry, Vol. 76, pp. 337–338. 10.1001/jamapsychiatry.2018.3990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen E, Swanson S, & Witkin JM (2017). Prediction of human efficacious antidepressant doses using the mouse forced swim test. Pharmacology Biochemistry and Behavior, 161, 22–29. 10.1016/j.pbb.2017.09.002 [DOI] [PubMed] [Google Scholar]
- Zanos P, & Gould TD (2018). Mechanisms of ketamine action as an antidepressant. Molecular Psychiatry, 23(4), 801–811. 10.1038/mp.2017.255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos P, Piantadosi SC, Wu H-Q, Pribut HJ, Dell MJ, Can A, … Gould TD (2015). The Prodrug 4-Chlorokynurenine Causes Ketamine-Like Antidepressant Effects, but Not Side Effects, by NMDA/GlycineB-Site Inhibition. Journal of Pharmacology and Experimental Therapeutics, 355(1), 76–85. 10.1124/jpet.115.225664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos Panos, Highland JN, Liu X, Troppoli TA, Georgiou P, Lovett J, … Gould TD (2019). ( R )- ketamine exerts antidepressant actions partly via conversion to ( 2R,6R )-hydroxynorketamine, while causing adverse effects at sub- anesthetic doses. British Journal of Pharmacology. 10.1111/bph.14683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos Panos, Highland JN, Stewart BW, Georgiou P, Jenne CE, Lovett J, … Gould TD (2019). ( 2R,6R )-hydroxynorketamine exerts mGlu 2 receptor-dependent antidepressant actions. Proceedings of the National Academy of Sciences, 116(13), 6441–6450. 10.1073/pnas.1819540116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos Panos, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, … Gould TD (2016). NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature. 10.1038/nature17998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos Panos, Moaddel R, Morris PJ, Riggs LM, Highland JN, Georgiou P, … Gould TD (2018). Ketamine and Ketamine Metabolite Pharmacology: Insights into Therapeutic Mechanisms. Pharmacological Reviews. 10.1124/pr.117.015198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanos Panos, Moaddel R, Morris P, Wainer I, Albuquerque E, Thompson S, … Gould T (2017). Reply to: Antidepressant Actions of Ketamine Versus Hydroxynorketamine. Biological Psychiatry, 81(8), e69–e71. 10.1016/j.biopsych.2016.06.029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zarate CA, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, … Manji HK (2006). A Randomized Trial of an N-methyl-D-aspartate Antagonist in Treatment-Resistant Major Depression. Arch Gen Psychiatry, 856–864. [DOI] [PubMed] [Google Scholar]
- Zarate et al. (2006). A Double-Blind, Placebo-Controlled Study of Memantine in the Treatment of Major Depression. Am J Psychiatry, 153–155. [DOI] [PubMed] [Google Scholar]
- Zhang GF, Wang J, Han JF, Guo J, Xie ZM, Pan W, … Sun KJ (2016). Acute single dose of ketamine relieves mechanical allodynia and consequent depression-like behaviors in a rat model. Neuroscience Letters, 631, 7–12. 10.1016/j.neulet.2016.08.006 [DOI] [PubMed] [Google Scholar]
- Zhang JC, Li SX, & Hashimoto K (2014). R (−)-ketamine shows greater potency and longer lasting antidepressant effects than S (+)-ketamine. Pharmacology Biochemistry and Behavior. 10.1016/j.pbb.2013.11.033 [DOI] [PubMed] [Google Scholar]
- Zhang K, Dong C, Fujita Y, Fujita A, & Hashimoto K (2018). 5-Hydroxytryptamine-Independent antidepressant actions of (R)-Ketamine in a chronic social defeat stress model. International Journal of Neuropsychopharmacology, 21(2), 157–163. 10.1093/ijnp/pyx100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, Fujita Y, & Hashimoto K (2018). Lack of metabolism in (R)-ketamine’s antidepressant actions in a chronic social defeat stress model. Scientific Reports, 8(1). 10.1038/s41598-018-22449-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, & Hashimoto K (2019, March 15). Lack of Opioid System in the Antidepressant Actions of Ketamine. Biological Psychiatry, Vol. 85, pp. e25–e27. 10.1016/j.biopsych.2018.11.006 [DOI] [PubMed] [Google Scholar]
- Zhang W. jie, Wang H. hua, Lv Y. dong, Liu C. cai, Sun W. yao, & Tian L. jun. (2018). Downregulation of Egr-1 Expression Level via GluN2B Underlies the Antidepressant Effects of Ketamine in a Chronic Unpredictable Stress Animal Model of Depression. Neuroscience. 10.1016/j.neuroscience.2017.12.045 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.