Abstract
Background:
This research describes the incidence and factors associated with opportunistic infections in rheumatoid arthritis (RA) patients treated with biological disease-modifying antirheumatic drugs (bDMARDs).
Methods:
A retrospective longitudinal study was carried out from 2007 to 2018. We included RA patients treated with a tumor necrosis factor (TNF)-targeted bDMARD or non-TNF-targeted bDMARD from the start of bDMARDs. An independent variable was the development of an indicator of opportunistic infection after biological (IOIb) treatment. Secondary variables included sociodemographic, clinical, and treatments. We used survival techniques to estimate the incidence of IOIb, per 1000 patient-years (95% CI). We performed a Cox multivariate regression analysis model to compare the risk of IOIb. Results were expressed as a hazard ratio (HR).
Results:
A total of 441 RA patients were included, that started 761 different courses of bDMARDs. A total of 81% were women with a mean age at first bDMARD of 57.3 ± 14 years. A total of 71.3% of the courses were TNF-targeted bDMARDs and 28.7% were non-TNF-targeted bDMARDs. There were 37 IOIb (25 viral, 6 fungal, 5 bacterial, 1 parasitic). Nine of these required hospitalization and one died. The global incidence of IOIb was 23.2 (16.8–32). TNF-targeted bDMARDs had 25 IOIb, incidence 20.5 (13.9–30.4), and non-TNF-targeted bDMARDs had 12 IOIb, incidence 31.7 (18–55.9). In the multivariate analysis, glucocorticosteroids (HR 2.17, p = 0.004) and lower lymphocyte count increased the risk for IOIb (HR 0.99, p = 0.005).
Conclusions:
The incidence of IOIb due to bDMARDs was 23 cases per 1000 patient-years. Close monitoring should be taken in the RA patients treated with bDMARDs and glucocorticosteroids, mainly in elderly patients and those with a low total lymphocyte count at the beginning of bDMARD treatment.
Keywords: biological therapy, opportunistic infections, rheumatoid arthritis
Introduction
Treatment of rheumatoid arthritis (RA) with biological disease-modifying antirheumatic drugs (bDMARDs) has brought a substantial improvement in disease activity and thus in the natural progression of the disease. Despite the advantages of the introduction of these drugs, there is a concern that they could generate immunological alterations in the context of immunosuppressive therapy.1,2 In fact, one of the notable problems with biological therapy is the risk of infection.3 Recently, the concept of indicator opportunistic infection after biological treatment (IOIb) has been raised according to consensus recommendations of the presence, or specific presentation, of a pathogen that suggests a greater probability of an alteration in the immunity in a host under treatment with bDMARDs.4
A recent meta-analysis of 70 clinical trials, identified an overall increased risk of IOIbs at 1.7 excess infections per 1000 patients treated with bDMARDs.5 Most information about safety on bDMARDs is extracted from clinical trials, with the limitations of the extrapolation to real-world patients. Some observational studies from clinical practice have also been published,6 but information about IOIbs over the long-term is scarce. Moreover, most of the available data on infection risk of targeted therapies concern inhibitors of tumor necrosis factor alpha (TNF-α), which have been in clinical use the longest, while information on the newer biologicals is much more limited. In addition, considering that more than half of IOIbs occurred among individuals who had been previously on a biological agent for over 1 year,7 these cases might not have been detected in many clinical trials that have shorter follow-up periods.
Thus, our aim was to describe the incidence of IOIb in RA patients taking bDMARDs and compare the risk of development between TNF-targeted and non-TNF-targeted biologicals in the long-term real-world setting.
Patients and methods
Study design, patient sample, and data collection
This study was carried out in one of the tertiary public health Hospitals of the Community of Madrid (Hospital Clínico San Carlos), an area of approximately 400,000 people. An observational retrospective longitudinal study was performed. All patients attending the rheumatology outpatient clinic of our center, with medical diagnosis (according to ICD-10) of RA, aged ⩾18 years who started treatment with bDMARDs between 1 January 2007 and 1 February 2017, were recruited. Patients were included from the start of treatment with bDMARDs between February 2007 and December 2017, and the maximum follow-up was in February 2018 (end of the study).
The patient data in this project were obtained during routine clinical practice by the rheumatologists for 10 years with the informed consent of patients to be treated in a service that has clinical assistance and research work. The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practices and was approved by the Hospital Clínico San Carlos institutional ethics committee (approval number 12/235-E).
Variables
The main outcome was the development of an opportunistic infection according to microbiological criteria: An IOIb according to consensus recommendations of the presence or specific presentation, of a pathogen that suggests a greater probability of an alteration in the immunity in a host under treatment with bDMARDs (I-V and NC: not clear).4
The independent variable was the use of TNF-targeted and non-TNF-targeted biologicals. They included the following anti-TNF biologicals etanercept (Etn), golimumab (Goli), certolizumab (Ctz), infliximab (Ifx), adalimumab (Ada) and other biologicals rituximab (Rtx), abatacept (Aba) and tocilizumab (Tzl).
The following predictive and confounding factors were considered. (1) Sociodemographic baseline variables including sex, age, marital status (married versus not married), an education level (any study degree versus no studies), job status (assessed as active, retired, housewife, student, unemployed). (2) Disease-related variables, including the date of RA onset and diagnosis, disease duration, erythrocyte sedimentation rate (ESR) (defined as mean value during the first year before first bDMARDs therapy), positive rheumatoid factor (RF), positive antibodies (anti-CCPs), comorbid baseline medical conditions, DAS28 and HAQ (both defined as mean value during the first year before first bDMARDs therapy), hemoglobin level, total lymphocyte count at the start of each bDMARD (at the time of starting ±1 month). (3) Baseline comorbid conditions (hypertension, hypercholesterolemia, cardiovascular disease, diabetes mellitus, depression, renal failure, osteoporosis, chronic obstructive pulmonary disease, and tobacco). (4) Other pharmacological variables including concomitant glucocorticosteroids [defined as a continuous-discrete quantitative variable (none, 0.1–7.49 mg, ⩾7.5 mg) with the mean during the first 3 months from the start of the bDMARD] and previous DMARDs and concomitant DMARDs (during the whole follow-up of the study). (5) Calendar time, dividing the start time of each bDMARD into 5 year intervals (from 1 January 2007 to 31 December 2012, from 1 January 2013 to 1 February 2017, etc., until end of follow-up).
Data sources
We retrospectively reviewed all the medical records (MRs) to obtain the variables, in a departmental electronic health record (MEDI <log>) used in our outpatient clinic.8 Data regarding IOIbs were reviewed in collaboration with two microbiologists, taking into account the causative pathogens with a positive culture, serology or both, their clinical manifestations, the different specimens cultured, imaging and histological data, main organs affected, and the treatment response.
Statistical analysis
A description of the sociodemographic and clinical characteristics of patients included was explored with frequency distribution and the mean and standard deviation or median and percentiles.
Incidence rates (IR) of IOIb were estimated using survival techniques, and results were expressed per 1000 patient-years with their respective confidence interval (95% CI). To explore IOIb, we included all the patients with RA and the time of exposure comprised the period from the baseline visit until the occurrence of any of the following cut-off points: loss of follow up, the main outcome, or the end of the study (February 2018). Exposure to biologicals was used in a time-dependent manner. Patients were included in different groups (TNF-targeted and non-TNF-targeted biological treatment) and contributed with patient-years at risk to both those exposed and those not exposed to these therapies.
Bivariate and Cox multivariate regression analyses were used to compare the different variables in the development of IOIb. In multivariate regression analysis, we included age, sex, calendar time, and all variables with a p < 0.2 in the bivariate analysis, to adjust for confounders. Results were expressed by hazard ratio (HR) and 95% CI. Proportional hazard assumption was tested using Schoenfeld residuals and the scaled Schoenfeld residuals. All analyses were performed in Stata v.13 statistical software (Stata Corp., College Station, TX, USA). A two-tailed p value under 0.05 was considered to indicate statistical significance.
Results
Patient’s baseline characteristics
A total of 441 patients were included in the study, who began 761 different courses of bDMARD treatment, with a total follow-up of 1592 patient-years. Table 1 includes a wide cohort description. Most of the patients were women with a mean age at diagnosis of 52.3 (±14.6) years, and the mean time to the first bDMARDs of 3.1 years. Most of the patients had at least moderate disease activity, with a slight level of disability. A total of 81.3% of the patients had at least one basal comorbid condition hypertension, hypercholesterolemia, and depression being the most prevalent ones. A total of 68% of the patients had positive RF and from those who had anti-CCP antibodies determination (n = 325), 62% were positive. Almost all patients were taking DMARDs at the start of the study (96%), and 90% of the patients were taking glucocorticosteroids, with a median dose at the beginning of the study of 7.5 (p25–75: 7.5–10) mg. The most frequently biological used was Ada, followed by Etn, Ifx, and Rtx. Interestingly, 92% of the treatment courses were in combined therapy with a synthetic classic DMARD.
Table 1.
Cohort description.
| Number of patients (n) | 441 |
| Treatment courses | 761 |
| Female (%) | 81.8 |
| Primary studies (%) | 51.4 |
| Active worker (%) | 52.99 |
| Age at diagnosis (years), mean ± SD | 52.3 ± 14.8 |
| Lag time to the first bDMARDS (years) median
(p25–p75) Age at bDMARD start (years), mean ± SD |
3.1 (0.5–4.6) 57.3 ± 14.9 |
| Positive RF (%) | 68.7 |
| Positive anti-CCP antibodies (%) | 62.4 |
| DAS28, median (p25–p75) | 4.3 (3.6–5.3) |
| HAQ, mean ± SD | 1 ± 1.1 |
| Baseline comorbid conditions,% | |
| Hypertension | 45.5 |
| Hypercholesterolemia | 49.8 |
| Cardiovascular disease | 9.18 |
| Diabetes Mellitus | 13.1 |
| Depression | 22.8 |
| Cancer history | 6.2 |
| bDMARDs used,% | |
| Adalimumab | 28.4 |
| Etanercept | 23.5 |
| Infliximab | 7 |
| Rituximab | 17.3 |
| Abatacept | 6.2 |
| Certolizumab | 10.6 |
| Tocilizumab | 5.3 |
| Golimumab | 1.7 |
bDMARD, biological disease-modifying antirheumatic drugs; HAQ, health assessment questionnaire; RF, rheumatoid factor.
We found a total of 37 IOIb were registered during the follow-up, 72.9% of them were in women, with a mean age at bDMARD start of 59.6 (±14) years, and mean lag time taking bDMARDs to IOIb of 3.5 (±2.8) years. At the time of infection, the mean ESR was 36 (±24), and the median total lymphocyte count was 1700 (p25 1400–p75 2300). Other characteristics are shown in Table 2. The IOIbs were classified as 26 viral infections, 6 fungal infections, 5 bacterial infections, and 1 parasitic infection (Table 3) Microbiologists included a level of evidence for each infection, according to the degree of association with the drug and in two cases (5%) the degree of association with the drug was not clear.4
Table 2.
IOIb cases description.
| IOIb (n) | 37 |
| Female (%) | 72.9 |
| Age at bDMARD start (years), mean ± SD | 59.6 ± 14 |
| Lag time taking bDMARD to IOIb (years), mean ± SD | 3.5 ± 2.8 |
| ESR, mean ± SD | 36.6 ± 24.1 |
| HAQ, mean ± SD | 0.5 ± 0.9 |
| Total lymphocyte count at IOIb occurrence (median, p25–p75) | 1700 (1400–2300) |
| Hospital admissions, n (%) | 9 (24) |
| Deaths, n (%) | 1 (2.6) |
bDMARD, biological disease-modifying antirheumatic drug; ESR, erythrocyte sedimentation rate; HAQ, health assessment questionnaire; IOIb, indicator of opportunistic infection after biological.
Table 3.
Incidence rate analysis.
| IOIb | n | Patients*year | Incidence rate | CI 95% |
|---|---|---|---|---|
| Total | 37 | 1592 | 23.22 | 16.8–32 |
| Severe IOIb | 9 | 1583 | 5.68 | 2.95–10.92 |
| Women | 27 | 1327 | 20.3 | 13.9–29.6 |
| Men | 10 | 265 | 37.7 | 20.2–70 |
| Age ⩽69 | 27 | 1253 | 21.5 | 14.7–31.4 |
| Age ⩾70 | 10 | 339 | 29.4 | 15.8–54.7 |
| Five-year period | ||||
| 2007–2012 | 30 | 1118 | 26.8 | 18.7–38.3 |
| 2013–2017 | 7 | 474 | 14.7 | 7.03–30.9 |
| bDMARD | ||||
| Etanercept | 9 | 434 | 20.7 | 10.7–39.8 |
| Infliximab | 3 | 113.9 | 26.3 | 8.4–81.6 |
| Adalimumab | 11 | 552.8 | 19.8 | 11–35.9 |
| Rituximab | 9 | 223.2 | 40.3 | 20.9–77.4 |
| Tocilizumab | 1 | 65.9 | 15.1 | 2.1–107.6 |
| Abatacept | 2 | 88.8 | 22.5 | 5.6–90 |
| Certolizumab | 2 | 86.1 | 23.2 | 5.8–92.8 |
| Golimumab | 0 | 27.9 | 0 | _ |
n: events; CI 95%: confidence interval.
Incidence rate is calculated as the number of new events calculated per 1000 patients per year.
bDMARD, biological disease-modifying antirheumatic drugs; CI, confidence interval; IOIb, incidence of opportunistic infection after biological.
Of the total IOIb, nine were considered serious and required hospital admission (23.3%) including four cases of invasive-oropharyngeal candidiasis, one legionellosis, one salmonellosis, one tuberculosis, one VHB reactivation, and one herpes zoster (HZ). A patient died directly attributed to an IOIb. It was disseminated candidiasis in a patient taking Ada (Table 2 and supplementary material).
The global incidence of IOIb was 23.22 per 1000 patient-years (95% CI: 16.8–32). TNF-targeted bDMARDs had an incidence rate of 20.5 (13.9–30.4), and non-TNF-targeted bDMARDs had and incidence rate of 31.7 (18–55.9) (Figure 1). The incidence rate for severe IOIb was 5.6 (2.9–10.9). In Table 3, we also show the crude incidence rate by a different patient characteristic such as age or sex. It seemed to be higher in men and in older ages. In the first 5-year period (2007–2012) the incidence was about double that in the last 5-year period (27.2 versus 13.9). We also show the incidence by separate drugs and most notable was that Rtx seemed to have higher crude IR than others.
Figure 1.
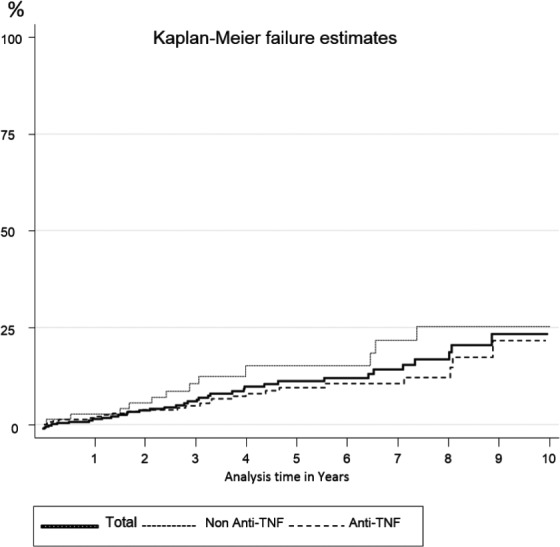
IOIb Cumulative incidence over time, for the total of the biological drugs and TNF-targeted treatments.
The bivariate analysis show that older ages [HR: 1.02 (1.01–1.05), p = 0.04] had increased risk to present IOIbs. Regarding concomitant therapy, glucocorticosteroids increased the risk [HR: 2.26 (1.34–2.82), p = 0.002]. Other factors such as lymphocyte count [HR: 0.99 (0.99–0.99), p = 0.002] achieved statistical significance (supplementary material). For non-TNF-targeted bDMARD compared with TNF-targeted bDMARD, the hazard ratio was not significant [HR: 1.78 (0.83–3.81), p = 0.13].
In the multivariate analysis (Table 4), we did not find statistical difference between type of targeted bDMARDs [HR 1.11 (0.46–2.69), p = 0.8], whereas glucocorticosteroids use [HR 2.17 (1.28–3.67), p = 0.004] and lower lymphocyte count [HR 0.99 (0.99–0.99), p = 0.005] increased the risk for IOIb with statistical significance. Older age [HR 1.02 (0.99–1.05), p = 0.07] showed a tendency to increase the risk of IOIb (Table 4). Proportionality of these the regression models was tested with a p > = 0.45.
Table 4.
Multivariate regression analysis.
| HR | CI 95% | p | |
|---|---|---|---|
| Male gender | 1.74 | 0.80–3.80 | 0.15 |
| Age at diagnosis | 1.02 | 0.99–1.05 | 0.07 |
| Type of bDMARD* | 1.11 | 0.46–2.69 | 0.80 |
| First versus subsequent bDMARD | 0.79 | 0.37–1.69 | 0.55 |
| Five-year period# | 0.28 | 0.08–0.95 | 0.04 |
| Glucocorticoids | 2.17 | 1.28–3.67 | 0.004 |
| Lymphocyte count | 0.99 | 0.99–0.99 | 0.005 |
Analysis adjusted by age, sex, educational level, tobacco, RF, ESR, Hemoglobin, calendar time, duration of RA, other DMARDs.
no Anti-TNF versus Anti-TNF; #2013-2017 versus 2007-2012.
bDMARD, biological disease-modifying rheumatic drugs; HR, hazard ratio.
CI, confidence interval; HR, Hazard ratio.
Discussion
This study showed that, in a real-world setting, there is a low incidence of IOIb infections in patients with RA treated concurrently with a bDMARD. The results are consistent with those from previous retrospective cohort studies assessing infection rates in patients with RA treated with bDMARDs.3,9–11 We show an incidence of IOIb in approximately 23 cases per 1000 patient-years. Crude incidence was higher for non-TNF-targeted bDMARD compared with TNF-targeted bDMARD. Nevertheless, this difference was not maintained in the multivariate model, reflecting that many of the variables in the patient’s risk of IOIbs developing were determined by other factors than the biological target exposure.
Although the IOIb seems to be low, patients with RA and taking bDMARDs will carry a higher risk for opportunistic infection compared with those without bDMARDs and RA.3 Studies and screening guidelines are crucial to prevent or minimize the development of these. In this sense, many studies have been published in the last decade, mainly related to TNF-targeted agents.
There has been a clear increase of tuberculosis (TB) in patients treated with TNF-targeted bDMARDs, and the later strategies have been developed to treat latent TB infections.12–14 We only included the TB patients with extra-pulmonary presentations, confirmed with a positive culture.
The incidence of IOIb in the first 5 years of the study was about twice that of the last 5 years. This falling incidence observed in our cohort in the calendar time analysis is likely to reflect improved screening and treatment of latent TB following the introduction of official recommendations on latent tuberculosis infections, which has led to a significant reduction in the incidence of TB among patients receiving biological therapy. In addition, because of such proactive patient monitoring, infections occurring in this patient population may have been less likely to require hospital admission, therefore, lowering the number of serious infections observed.
Maybe this closer control has been possible by the implementation of our more advanced departmental electronic health record with a software system ‘Reporting and Analysis for Incident Learning and Adverse Events’. This is a web platform, integrated and connected to our departmental electronic health record that works as an alert system, warning about probable risk factors for the development of IOIb.15
TNF-targeted treatment plays an important role in the host antiviral response, so TNF-targeted treatments may probably increase the reactivation risk of chronic viral infections. Hepatitis B and hepatitis C reactivation under biological therapy have been described and several retrospective studies reported reactivations in a series of patients treated with biological therapies.16–18 Smitten and colleagues19 reported an increased risk for the development of HZ skin infections in patients receiving TNF-targeted treatment, suggesting that vaccination before starting treatment with these agents would be necessary.19,20 HZ was the most common IOIb seen in our study, with 18 cases recorded. There was no difference in the rate of serious HZ by drug type in all different bDMARD, compared with the study by Yun and colleagues9 who revealed that the incidence of HZ was similar among the patient groups treated with the different biologic agents, which included the five TNF-targeted bDMARDs, and Aba, Rtx, and Tzl. Furthermore, it is uncertain what impact sequential biological exposure has on infection risk.
In this sense it is clear that RA patients with varying degrees of immunosuppression and glucocorticoid use may have different risk profiles for HZ.9,21 This risk may be particularly associated with older patients, and the mean age at bDMARDs start of our cohort was 57.3 years, considered another important factor that contributes to the HZ risk rates.22–24 We are aware that patients with small molecule Janus-associated kinase inhibitors (JAKi) were not included in this study, where specifically the reactivation of varicella zoster virus leading to HZ is the most characteristic infectious complication,25,26 even though how JAKi increases the risk of HZ reactivation is unclear.27,28
Nevertheless, studies related to bacterial opportunistic infections are scarce. Legionellosis might be increased among patients treated with TNF-targeted bDMARDs as described in the study of Tubach and colleagues.29 A number of case reports indicate that treatment with TNF-targeted bDMARDS may lead to an increased susceptibility for infection with different salmonella species.30–33 In the case of parasitic infections, it seems to be unusual, but in our study, we found one Leishmania, which is probably related because the Mediterranean area is endemic for Leishmania.
Although there is little literature on the IOIb possible associated factors, some studies have found results similar to ours, and thus, in the RATIO study, glucocosticosteroids were independent predictors of the risk of opportunistic infections.34 In addition, glucocorticosteroids use and increasing age were independent predictors of HZ reactivation in some previous studies.21,35
A low lymphocyte count at the beginning of the biological therapy was found as a predictor of the development of IOIb, so rheumatologists should be alert to patients with low lymphocyte counts, according to lymphopenia moderate defined by OMERACT criteria (⩽1500 cells/mm3).36
Access to detailed clinical information is a strength of our study, which provides a unique evaluation of a cohort of patients with RA in a real-world clinical practice setting. In our study, the patient’s full MRs were used to gather details surrounding potential events of interest. Further, patients taking a bDMARDs were routinely asked about recent infections, hospitalizations, and use of antibiotics at each clinic visit, which maximized the total number of events identified. In addition, having two microbiologists confirming the outcomes resulting in a consensus definition allowed for consistency and reproducibility of outcomes. Moreover, one of the greatest strengths of this study is the long-term follow-up of non-selected patients that reflects clinical practice, adjusted for confounders.
The results of our study should be interpreted in light of particular limitations. First, we have to take into account the retrospective nature of the study and that our patients were treated at a single center. This, associated with the fact that data was recorded during routine consultations, in an environment with a heavy workload, makes it easier to collect incomplete and nonrecoverable information. Consequently, to minimize missing values, we had to perform an averaged measure of these variables for the first year at different cut-off points of follow-up. In addition, we could not evaluate the relative risk of hospitalized infection in the newly approved treatments (JAKi).
In conclusion, many of the variability in patient’s risk of IOIbs development was explained by factors other than biological agent exposure. In response to our finding on the importance of age and total lymphocyte count, it would, therefore, be desirable to estimate the pretreatment risk in individual patients and to classify patients according to risk profile, in order to make decisions about (comparative) risk with biological therapy. Close monitoring should be taken in those RA patients treated with bDMARDs and glucocorticosteroids, mainly in elderly patients and those with a low total lymphocyte count at the start of these treatments.
Supplemental Material
Supplemental material, Supplementary_table1_rev for Indicator opportunistic infections after biological treatment in rheumatoid arthritis, 10 years follow-up in a real-world setting by Leticia Leon, Marina Peñuelas, Francisco Javier Candel, Dalifer Freites, Luis Rodriguez-Rodriguez, Benjamin Fernandez-Gutierrez, Juan Angel Jover and Lydia Abasolo in Therapeutic Advances in Musculoskeletal Disease
Supplemental Material
Supplemental material, Supplementary_table2_rev for Indicator opportunistic infections after biological treatment in rheumatoid arthritis, 10 years follow-up in a real-world setting by Leticia Leon, Marina Peñuelas, Francisco Javier Candel, Dalifer Freites, Luis Rodriguez-Rodriguez, Benjamin Fernandez-Gutierrez, Juan Angel Jover and Lydia Abasolo in Therapeutic Advances in Musculoskeletal Disease
Footnotes
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Instituto de Salud Carlos III (ISCIII), Ministry of Health, Spain (Miguel Servet research contract: CP11/00189 to Lydia Abasolo; Fondo de Investigaciones Sanitarias: PI18/01188; and Red de Investigación en Inflamación y Enfermedades Reumáticas: RD16/0012/0014).
Conflict of interest statement: The authors declare that there is no conflict of interest.
ORCID iDs: Leticia Leon  https://orcid.org/0000-0001-7142-0545
https://orcid.org/0000-0001-7142-0545
Benjamin Fernandez-Gutierrez  https://orcid.org/0000-0002-6126-8786
https://orcid.org/0000-0002-6126-8786
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Leticia Leon, Instituto de Investigación Sanitaria del Hospital Clínico San Carlos (IDISSC), Hospital Clínico San Carlos, Calle Martín Lagos, s/n, Madrid, 28040, Spain; Universidad Camilo Jose Cela, Madrid, Spain.
Marina Peñuelas, Clinical Microbiology and Infectious Diseases Department and IDISSC, Hospital Clínico San Carlos, Madrid, Spain.
Francisco Javier Candel, Clinical Microbiology and Infectious Diseases Department and IDISSC, Hospital Clínico San Carlos, Madrid, Spain; Transplant Coordination Unit, Hospital Clínico San Carlos, Madrid, Spain.
Dalifer Freites, Rheumatology Department and IDISSC, Hospital Clínico San Carlos, Madrid, Spain.
Luis Rodriguez-Rodriguez, Rheumatology Department and IDISSC, Hospital Clínico San Carlos, Madrid, Spain.
Benjamin Fernandez-Gutierrez, Rheumatology Department and IDISSC, Hospital Clínico San Carlos, Madrid, Spain.
Juan Angel Jover, Rheumatology Department and IDISSC, Hospital Clínico San Carlos, Madrid, Spain; Medicine Department, Universidad Complutense, Madrid, Spain.
Lydia Abasolo, Rheumatology Department and IDISSC, Hospital Clínico San Carlos, Madrid, Spain.
References
- 1. Crum NF, Lederman ER, Wallace MR. Infections associated with tumor necrosis factor-alpha antagonists. Medicine (Baltimore) 2005; 84: 291–302. [DOI] [PubMed] [Google Scholar]
- 2. Cunnane G, Doran M, Bresnihan B. Infections and biological therapy in rheumatoid arthritis. Best Pract Res Clin Rheumatol 2005; 17: 345–363. [DOI] [PubMed] [Google Scholar]
- 3. Lahiri M, Dixon WG. Risk of infection with biologic antirheumatic therapies in patients with rheumatoid arthritis. Best Pract Res Clin Rheumatol 2015; 29: 290–305. [DOI] [PubMed] [Google Scholar]
- 4. Winthrop KL, Novosad SA, Baddley JW, et al. Opportunistic infections and biologic therapies in immune-mediated inflammatory diseases: consensus recommendations for infection reporting during clinical trials and postmarketing surveillance. Ann Rheum Dis 2015; 74: 2107–2116. [DOI] [PubMed] [Google Scholar]
- 5. Kourbeti IS, Ziakas PD, Mylonakis E. Biologic therapies in rheumatoid arthritis and the risk of opportunistic infections: a meta-analysis. Clin Infect Dis 2014; 58: 1649–1657. [DOI] [PubMed] [Google Scholar]
- 6. Ichinose K, Shimizu T, Umeda M, et al. Frequency of hospitalized infections is reduced in rheumatoid arthritis patients who received biological and targeted synthetic disease-modifying antirheumatic drugs after 2010. J Immunol Res 2018; 14: 6259010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rutherford AI, Patarata E, Subesinghe S, et al. Opportunistic infections in rheumatoid arthritis patients exposed to biologic therapy: results from the British Society for Rheumatology Biologics Register for Rheumatoid Arthritis. Rheumatology (Oxford) 2018; 57: 997–1001. [DOI] [PubMed] [Google Scholar]
- 8. Leon L, Jover JA, Loza E, et al. Health-related quality of life as a main determinant of access to rheumatologic care. Rheumatol Int 2013; 33: 1797–1804. [DOI] [PubMed] [Google Scholar]
- 9. Yun H, Xie F, Delzell E, et al. Risks of herpes zoster in patients with rheumatoid arthritis according to biologic disease-modifying therapy. Arthritis Care Res (Hoboken) 2015; 67: 731–736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Carmona L, Gonzalez-Alvaro I, Balsa A, et al. Rheumatoid arthritis in Spain: occurrence of extra-articular manifestations and estimates of disease severity. Ann Rheum Dis 2003; 62: 897–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lampropoulos C, Orfanos P, Bournia V, et al. Adverse events and infections in patients with rheumatoid arthritis treated with conventional drugs or biologic agents: a real world study. Clin Exp Rheumatol 2015; 33: 216–224. [PubMed] [Google Scholar]
- 12. Dixon WG, Hyrich KL, Watson KD, et al. Drug-specific risk of tuberculosis in patients with rheumatoid arthritis treated with anti-TNF therapy: results from the British Society for Rheumatology Biologics Register (BSRBR). Ann Rheum Dis 2010; 69: 522–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Keane J, Gershon S, Wise RP, et al. Tuberculosis associated with infliximab, a tumor necrosis factor alpha neutralizing agent. N Engl J Med 2001; 345: 1098–1104. [DOI] [PubMed] [Google Scholar]
- 14. Carmona L, Gómez-Reino JJ, Rodriguez-Valverde V, et al. Effectiveness of recommendations to prevent reactivation of latent tuberculosis infection in patients treated with tumor necrosis factor antagonists. Arthritis Rheum 2005; 52: 1766–1772. [DOI] [PubMed] [Google Scholar]
- 15. Abasolo L, Leon L, Rodriguez-Rodriguez L, et al. Safety of disease-modifying antirheumatic drugs and biologic agents for rheumatoid arthritis patients in real-life conditions. Semin Arthritis Rheum 2015; 44: 506–513. [DOI] [PubMed] [Google Scholar]
- 16. Carroll MB, Bond MI. Use of tumor necrosis factor-alpha inhibitors in patients with chronic hepatitis B infection. Semin Arthritis Rheum 2008; 38: 208–217. [DOI] [PubMed] [Google Scholar]
- 17. Peterson JR, Hsu FC, Simkin PA, et al. Effect of tumour necrosis factor alpha antagonists on serum transaminases and viraemia in patients with rheumatoid arthritis and chronic hepatitis C infection. Ann Rheum Dis 2003; 62: 1078–1082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ferri C, Ferraccioli G, Ferrari D, et al. Safety of anti-tumor necrosis factor-alpha therapy in patients with rheumatoid arthritis and chronic hepatitis C virus infection. J Rheumatol 2008; 35: 1944–1949. [PubMed] [Google Scholar]
- 19. Smitten AL, Choi HK, Hochberg MC, et al. The risk of herpes zoster in patients with rheumatoid arthritis in the United States and the United Kingdom. Arthritis Rheum 2007; 57: 1431–1438. [DOI] [PubMed] [Google Scholar]
- 20. Galloway JB, Mercer LK, Moseley A, et al. Risk of skin and soft tissue infections (including shingles) in patients exposed to anti-tumour necrosis factor therapy: results from the British Society for Rheumatology Biologics Register. Ann Rheum Dis 2013; 72: 229–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. McDonald JR, Zeringue AL, Caplan L, et al. Herpes zoster risk factors in a national cohort of veterans with rheumatoid arthritis. Clin Infect Dis 2009; 48: 1364–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hope-Simpson RE. The nature of herpes zoster: a long-term study and a new hypothesis. Proc R Soc Med 1965; 58: 9–20. [PMC free article] [PubMed] [Google Scholar]
- 23. Weller TH. Varicella and herpes zoster: changing concepts of the natural history, control, and importance of a not-so-benign virus. N Engl J Med 1983; 309: 1434–1440. [DOI] [PubMed] [Google Scholar]
- 24. Oxman MN, Levin MJ, Johnson GR, et al. A vaccine to prevent herpes zoster and postherpetic neuralgia in older adults. N Engl J Med 2005; 352: 2271–2284. [DOI] [PubMed] [Google Scholar]
- 25. Winthrop KL, Yamanaka H, Valdez H, et al. Herpes zoster and tofacitinib therapy in patients with rheumatoid arthritis. Arthritis Rheumatol 2014; 66: 2675–2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Bechman K, Subesinghe S, Norton S, et al. A systematic review and meta-analysis of infection risk with small molecule JAK inhibitors in rheumatoid arthritis. Rheumatology. Epub ahead of print 14 April 2019. DOI: 10.1093/rheumatology/kez087/5456863 [DOI] [PubMed] [Google Scholar]
- 27. Ghoreschi K, Laurence A, O’Shea JJ. Janus kinases in immune cell signalling. Immunol Rev 2009; 228: 273–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Abendroth A, Arvin AM. Immune evasion as a pathogenic mechanism of varicella zoster virus. Semin Immunol 2001; 13: 27–39. [DOI] [PubMed] [Google Scholar]
- 29. Tubach F, Ravaud P, Salmon-Céron D, et al. Emergence of Legionella pneumophila pneumonia in patients receiving tumor necrosis factor-antagonists. Clin Infect Dis 2006; 43: 95–100. [DOI] [PubMed] [Google Scholar]
- 30. Netea MG, Radstake T, Joosten LA, et al. Salmonella septicemia in rheumatoid arthritis patients receiving anti-tumor necrosis factor therapy: association with decreased interferon-gamma production and Toll-like receptor 4 expression. Arthritis Rheum 2003; 48: 1853–1857. [DOI] [PubMed] [Google Scholar]
- 31. Rijkeboer A, Voskuyl A, Van AM. Fatal Salmonella enteritidis septicaemia in a rheumatoid arthritis patient treated with a TNF-alpha antagonist. Scand J Infect Dis 2007; 39: 80–83. [DOI] [PubMed] [Google Scholar]
- 32. Bassetti M, Nicco E, Delfino E, et al. Disseminated Salmonella paratyphi infection in a rheumatoid arthritis patient treated with infliximab. Clin Microbiol Infect 2010; 16: 84–85. [DOI] [PubMed] [Google Scholar]
- 33. Pena-Sagredo JL, Farinas MC, Perez-Zafrilla B, et al. Non-typhi Salmonella infection in patients with rheumatic diseases on TNF-alpha antagonist therapy. Clin Exp Rheumatol 2009; 27: 920–925. [PubMed] [Google Scholar]
- 34. Salmon-Ceron D, Tubach F, Lortholary O, et al. Drug-specific risk of non-tuberculosis opportunistic infections in patients receiving anti-TNF therapy reported to the 3-year prospective French RATIO registry. Ann Rheum Dis 2011; 70: 616–623. [DOI] [PubMed] [Google Scholar]
- 35. Strangfeld A, Listing J, Herzer P, et al. Risk of herpes zoster in patients with rheumatoid arthritis treated with anti-TNF alpha agents. JAMA 2009; 301: 737–744. [DOI] [PubMed] [Google Scholar]
- 36. Woodworth TG, Furst DE, Strand V, et al. Standardizing assessment of adverse effects in rheumatology clinical trials. Status of OMERACT Toxicity Working Group March 2000: towards a common understanding of comparative toxicity/safety profiles for antirheumatic therapies. J Rheumatol 2001; 28: 1163–1169. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Supplementary_table1_rev for Indicator opportunistic infections after biological treatment in rheumatoid arthritis, 10 years follow-up in a real-world setting by Leticia Leon, Marina Peñuelas, Francisco Javier Candel, Dalifer Freites, Luis Rodriguez-Rodriguez, Benjamin Fernandez-Gutierrez, Juan Angel Jover and Lydia Abasolo in Therapeutic Advances in Musculoskeletal Disease
Supplemental material, Supplementary_table2_rev for Indicator opportunistic infections after biological treatment in rheumatoid arthritis, 10 years follow-up in a real-world setting by Leticia Leon, Marina Peñuelas, Francisco Javier Candel, Dalifer Freites, Luis Rodriguez-Rodriguez, Benjamin Fernandez-Gutierrez, Juan Angel Jover and Lydia Abasolo in Therapeutic Advances in Musculoskeletal Disease


