Abstract
Vacuoles, cellular membrane-bound organelles, are the largest compartments of cells, occupying up to 90% of the volume of plant cells. Vacuoles are formed by the biosynthetic and endocytotic pathways. In plants, the vacuole is crucial for growth and development and has a variety of functions, including storage and transport, intracellular environmental stability, and response to injury. Depending on the cell type and growth conditions, the size of vacuoles is highly dynamic. Different types of cell vacuoles store different substances, such as alkaloids, protein enzymes, inorganic salts, sugars, etc., and play important roles in multiple signaling pathways. Here, we summarize vacuole formation, types, vacuole-located proteins, and functions.
Keywords: plant vacuole, lytic vacuole, protein storage vacuole, vacuole iron transporter
1. Discovery History of the Vacuoles
The term “vacuole” was first proposed by the famous French biologist Félix Dujardin and was used to represent the blank space of protozoan contractile vesicles [1]. A similar blank space was also observed in the leaves and roots of plants. Thus, the term was also adopted by plant biologists. In the early stages of vacuole research, methods of microscopic observation and neutral-red staining indicated that the vacuole was an acidic environment surrounded by membranes. At the end of the 19th century, de Vries believed that vacuoles were formed by special plastid-like precursors, called tonoplasts [2]. The vacuole is an important part of many cells. Mature cells of all terrestrial plants, most fungi, and algae (except prokaryotic cells) have vacuoles. However, there are no vacuoles in animal cells and in the immature plant cells, as well as some highly mature plant cells (such as stone cells). In mature plant cells, vacuoles can account for 90% of the cell’s volume. For a long time, the biochemical analysis of this organelle was hindered by a lack of technology, and the majority of knowledge came from the study of yeast bubbles. Some biochemical characterization of vacuole components and amino acid transport experiments were performed in yeast. In contrast, the study of plant vacuoles was limited to the localization of some vacuole components. In the early 1980s, the application of the method of separating vacuolar and purified vacuolar vesicles made biochemical and electrophysiological studies of plant vacuolar transporters feasible [3].
2. Formation Processes of Vacuoles
The early stages of vacuolar research were limited to examining vacuolar morphology, as described above. In recent years, however, improvements in technology have brought the research of vacuoles to the molecular level, including metabolomics, proteomics, T-DNA insertion mutants, and heterologous complementation.
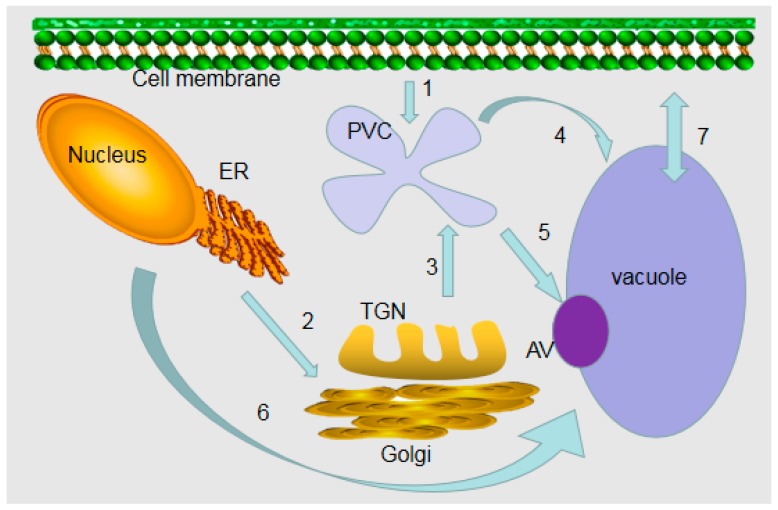
Experimental evidence indicates that the substances of the plant vacuole system come from the intracellular biosynthesis pathway and the endocytosis pathway. Biosynthesis pathways leading to vacuole formation include: (1) Early secretory pathway(from ER to late Golgi compartments): some proteins located in the vacuole are separated from the proteins transported to the cell surface; (2) Endocytosis of substances from the plasma membrane; (3) Autophagy; (4) Direct transmission of cytoplasm to vacuoles. Ultimately, specific proteins enter the vacuole and perform their functions through sorting and targeting mechanisms [4]. As in animal and yeast cells, the vacuolar transport pathway in plants begins with the endoplasmic reticulum (ER) (Figure 1). A conventional route of vacuole transport consists of a vesicle transport pathway including endoplasmic reticulum output mediated by vesicle and Golgi and post-Golgi transport [5,6]. For example, the tonoplast potassium channel AtTPK1 in Arabidopsis forms dimers, is controlled by ER quality control, and then reaches the vacuole through the Golgi apparatus [7]. An unconventional pathway of vacuolar transport is the direct transport from the ER to the vacuole without involving Golgi and Golgi-posterior vesicles [8,9]. Studies have shown that vacuole biogenesis and transport of plastid proteins and lipids can occur directly from the ER without Golgi involvement [10]. It is clear that these pathways are highly conserved in eukaryotes [11]. Recent studies have reviewed the commonalities and differences between different eukaryotic transport systems from the perspective of plants and have discussed plant vacuole substitution and trafficking pathways [9,12]. Proteins with N-terminal leading sequences or transmembrane domains are secreted by conventional protein secretion pathways. In contrast, proteins lacking signaling peptides are secreted by unconventional protein secretion pathways [8,13]. In addition, some studies have introduced two new glycosylated vacuolar GFP (green fluorescent protein) markers [14].
Figure 1.
Model for the formation of plant vacuoles. (1) Endocytosis from the cell surface to a prevacuolar compartment (PVC). (2) Early secretory pathway from the endoplasmic reticulum (ER) to the late Golgi compartment. (3) Proteins are sorted into the PVC by an early biosynthetic vacuolar pathway. The Golgi apparatus/trans-Golgi network (TGN) system is important for biosynthetic traffic. (4) PVC is transferred to vacuoles via the late biosynthetic vacuole pathway. (5) PVC enters vacuoles through autophagic vacuoles (AV) by degradation or biosynthetic pathways. (6) Direct transport from ER to vacuole. (7) Transport of ions and solutes on vacuole membrane.
3. Different Types of the Vacuoles
Vacuoles account for most of the volume of mature cells in plants. Two types of vacuole, lytic vacuoles (LV) and protein storage vacuoles (PSV), appear sequentially during embryogenesis [15,16]. LVs contain hydrolases to degrade unwanted cellular substances, while PSVs accumulate large amounts of defense and storage proteins. Because of the degradation and storage functions exhibited by LVs and PSVs, respectively, some labeled proteins are used to distinguish them [15,17]. Recent studies have indicated that these two types of vacuoles share a transport protein [18,19]. They also noticed that in maturing seeds, the LVs were turned into PSVs, which was reversed during germination [20]. Most vacuolar soluble proteins are synthesized into larger precursors in the ER and then transported into vacuoles, in which become mature following the intervention of vacuolar processing enzymes (VPE) [21]. Most stored proteins are synthesized as precursors on ribosomes, processed by ER and Golgi apparatus, and transported to specialized vacuoles to perform their functions. This process usually requires proteolysis to promote stable storage [22,23]. PSVs have been shown to arise de novo in the cotyledons of the developing pea (Pisum sativum), and a similar mechanism may operate in Medicago truncatula embryos [24]. The results in Arabidopsis indicate that PSV arise by the remodeling of preexisting vacuoles rather than by de novo biogenesis of PSV [25]. In addition, the LVs play an important role in ion storage and homeostasis, cell degradation, stress buffering, and defense against pathogens. The rapid absorption or release of ions and water in the vacuole cavity enables plants to respond quickly and effectively to various environmental challenges [26].
4. Vacuole-Localized Proteins
Plant or animal cells contain up to 10,000 different types of proteins, while yeast cells contain about 5000. Each protein must be located in a precise intracellular compartment, cell membrane, or organelle to function properly [27]. Vacuolar-located proteins make up a very low amounts (1%) of total proteins [28]. Some transmembrane proteins and peripheral proteins are involved in vacuole activities, such as proton pumps, channel proteins, transport proteins, and solution carriers. Compared with some other organelles (such as mitochondria and chloroplasts), plant vacuoles are very fragile and difficult to isolate using traditional methods [29]. Large-scale isolation and identification of organelles, as well as qualitative and quantitative analysis of subcellular pathways, will contribute to a better understanding of the plant system.
Vacuoles were first isolated from protoplasts of young tomato root tip tissue by osmotic shock [30]. At present, there are two main strategies for the separation and enrichment of vacuolar proteins. One consists in preparing protoplasts, separating complete vacuoles from protoplasts, and then extracting vacuolar protein samples [31]. This technique is a key step in characterizing vacuolar transporters. It allows to study vacuoles from different plants and analyze their vacuole contents. Second, density gradients combined with isotope tagging can be used to distinguish membrane proteins from different organelles [32]. Besides, the patch clamp technology is commonly used to study the electrophysiological properties of vacuolar channels and transporters [33]. The first vacuole channel identified was the slow vacuole channel, which was later renamed TPC on the basis of its animal homolog. This approach showed that H+-ATPase and H+-PPase are localized on the same vacuole, and both pumps can acidify the vacuole cavity [34].
As an important model plant, Arabidopsis has been used for the study of biological functions. At present, there are reports on the isolation of vacuoles by osmotic shock of Arabidopsis leaves and cultured cell protoplasts [35,36]. In addition to Arabidopsis, other plant vacuoles have also been isolated, which will contribute to a better understanding of their functions (Table 1). In recent years, some intramembrane proteins have been characterized in the seed vacuoles [37]. Intracellular markers, called tonoplast intrinsic proteins (TIPs), have been used for vacuole biogenesis and identification [38].
Table 1.
Partially localized proteins on vacuoles.
| Classification | Name | Functions | References |
|---|---|---|---|
| Proton Pumps | Vacuolar-type H+-pumping ATP hydrolase (H+-ATPase, VHA) | For the acidification of the vacuole. | [39,40] |
| H+-pumping pyrophosphatase (H+-PPase, AVP1) | For the acidification of vacuoles and the control of auxin transport. | [39,40,41] | |
| Proton antiporters | Cation (Na+/K+) proton antiporters (NHXs) | To change the color of flowers. | [42] |
| Na+/H+ antiporter (AtNHX1) | To mediate Na+ isolation in vacuoles and improve plant salt tolerance. | [43] | |
| Ca2+/H+ antiporters | To regulate plant processes, including ionic homeostasis and development. | [44] | |
| The characterization of the copper transporter COPT5 | To export copper in vacuoles. | [45] | |
| Vacuolar anion exchanger AtCLCa, AtALMT9 | Stomatal regulation and vacuole delivery of their anions. | [46,47] | |
| ATP-binding cassette (ABC) transporters | MRPs, AtTAP2 | For transporting glutathione conjugates and glucosidic acid conjugates. | [35,48] |
| Multidrug and toxic compound extrusion (MATE) transporters | SbMATE2 | To transport secondary compounds such as alkaloids, cyano glucoside, and some flavonoids. | [49,50,51] |
| Heavy Metal Transporters | Vacuole iron transporter (VIT) | To regulate the synthesis of anthocyanins; resistance to heavy metal ions; to regulate cytosolic iron homeostasis. | [52,53] |
| BnMEB2 | Resistance to heavy metal ions | [54] | |
| Mn2+ transporters | Resistance to heavy metal ions | [55,56] | |
| Vacuolar Sugar Transporters | AtSuc4 | Resistance to heavy metal ions | [57] |
5. Multifaceted Roles of Plant Vacuoles
Plant vacuoles are multifunctional organelles with differences not only in some basic properties but also in morphology and dynamics in different cell types and conditions [58]. Similar to the lysosomes of animal cells, they contain digestive hydrolases functioning in the degradation of extracellular and intracellular components [59,60]. In addition, vacuoles also transport a variety of secondary metabolites such as organic acids, glycosides and glutathione conjugates, alkaloids, and anthocyanin [58].
5.1. Vacuoles Can Be Used as Professional Repositories
Vacuoles are reservoirs of many metabolites, such as inorganic substances, organic acids, amino acids, and sugar in seeds and nutrient tissues [61,62]. Special cells in these organs accumulate proteins primarily as amino acid stores. The most common storage proteins are globulin, found in embryos, and glutenin, specific to cereal endosperm [63,64]. The results show that prolamins accumulation in the ER is an important step for the subsequent accumulation in storage vacuoles [63].
Many reports indicate that toxic pollutants in the environment have a serious negative impact on a variety of organisms [65]. Metals and metalloid pollutants come from natural or anthropogenic factors [66]. One study focused on the tolerance and accumulation mechanism of the heavy metals cadmium (Cd) and arsenic (As) and discussed how to use the knowledge collected on this subject to develop pollution-free crops and utilize phytoremediation [67]. Iron (Fe) is an essential micronutrient for both plant growth and human health. It can be used as a necessary cofactor for the electron transport chain of cell redox reactions involved in DNA biosynthesis, respiration, photosynthesis, and other reactions [68,69]. Iron deficiency leads to yellowing and growth retardation of young leaves, which leads to a decrease in photosynthetic efficiency and crop productivity [70]. Micronutrient malnutrition undermines the health and well-being of women and pre-school children in particular [71]. Conversely, excessive iron can cause severe dysfunction and cell damage, which is harmful to cells and organisms [72]. Metal contamination and toxicity in soil limits food production. Scientific research shows that biofortification is a way to solve hidden hunger by increasing the dietary iron content in staple food crops [73].
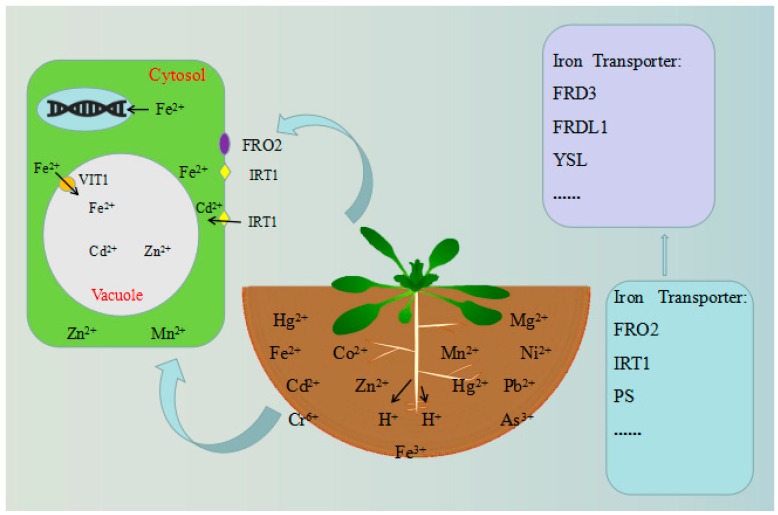
The storage of iron in seeds is a good example of plant vacuole storage of different heavy metals. At least 95% of the iron in Arabidopsis seeds is stored in vacuoles. However, in other seeds (such as P. sativum), the iron content of the vacuole is very low [74,75,76]. Iron is a rich element in most soils, but its solubility is low in aerobic environments, especially in alkaline calcareous soils [77]. Plants use two different strategies to absorb iron. In strategy I, plants, including dicots (such as cassava and Arabidopsis) and non-grass monocots, rely on the following processes: (1) plants secrete protons into the rhizosphere to reduce the pH of the soil, thereby increasing the solubility of ferric iron complexes (Fe3+); (2) the root protein ferric chelate reductase (FRO2) reduces Fe3+ into the more soluble Fe2+ (ferrous ion) on the root surface [78]; (3) the iron-regulated transporter 1 (IRT1)-type ferric transporter, of the zinc-iron transporter (ZIP) family, moves Fe2+ onto the cortical membrane of the root epidermal plasma membrane; (4) flavins are secreted to further facilitate the solubilization of ferric iron [79]. Under the condition of iron deficiency, the four mechanisms are upregulated in the root system. Incredibly, graminaceous plants have another unique strategy for absorbing iron, i.e. strategy II. This strategy has been described as a “chelation” strategy, similar to that used by many bacteria and fungi [80], and may result from adaptation to alkaline soils [81]. The plant secretes phytosiderophore (PS) into the rhizosphere to form the chelating complex Fe3+–PS, which is then absorbed into root cells by the yellow stripe 1 (YS1) transporter [82,83]. Iron is then transported from the roots through the xylem to the shoots, such as branches, leaves, and seeds, for use [69]. To reach its final destination, iron must also be transported to the appropriate cell compartments for use (Figure 2).
Figure 2.
Tolerance mechanisms of model plants. Under the condition of iron deficiency, the four depicted mechanisms are upregulated in the root system. After that, iron is transported from the roots, through the xylem, to the shoots. Additionally, iron must also be transported to the cell compartments for utilization.
5.2. The Roles of Plant Vacuoles in Protein Degradation
Autophagy is the primary mechanism to control the degradation and the recycling of cellular components. As a degradation pathway, autophagy plays an important role in protein and organelle turnover [84]. It is involved in vacuolation and cell differentiation, which are essential for survival under stress conditions like nutritional deficiencies. On the other hand, autophagy is involved in some trafficking events [85]. Autophagy is also used to transport other types of cargo and vesicles produced by the ER, such as rubber and anthocyanins [86,87].
Studies have shown that the trans-Golgi network (TGN), provacuoles, and autophagosomes exhibit an acidic environment and contain lysosomal acid hydrolases [4]. Vacuoles usually contain high concentrations of phosphate, malate, citrate, aspartic acid, and glutamic acid [88]. The cytoplasm in autophagosomes degrades after being completely blocked. It is speculated that with the destruction of the inner boundary membrane, digestive enzymes are released. Digestive activity is restricted to the vacuole, which forms a tonoplast. After autophagy digestion is completed, autophagic vacuoles are incorporated into the central vacuole. However, when autophagic digestion is inhibited, autophagic vacuoles remain in the cytoplasm [4,89,90]. Studies have shown that the induction of autophagy is affected by the mitochondria and respiratory substrate supply, rather than by reducing the concentrations of sucrose and hexose phosphate [88]. The formation of autophagy vesicles is related to an increase in the intracellular proteolysis rate and decomposition of membrane polar lipids [91,92].
5.3. The Role of Vacuoles in Plant Metabolism
The vacuole is often enriched in plant specialized metabolites (PSMs), which contain more than 200,000 compounds [93]. Secondary metabolites were the first compounds found in vacuoles. Although most of the secondary compounds produced to prevent harmful environmental conditions, such as fighting pathogens and herbivores, are stable, they are converted into toxic compounds when cells are destroyed. For example, cyanoglucosinolates or glucosinolates are hydrolyzed to protect plants from their toxicity [50,94]. To some extent, these alterations are dependent on vacuolar transporters. In recent years, a detailed review of the vacuolar transport mechanisms of metabolites [93,95] and of the transport of flavonoids and alkaloids [95,96] has been carried out. Despite extensive research on glucosinolates, no vacuolar proteins have been found to transport these secondary compounds, and little is known about secondary compounds exported from vacuoles.
6. Our Prospect of Vacuole Research
Plant vacuoles are high dynamic and polymorphic, and their size depends on the cell type and growth conditions. Vacuolar fusion leads to increased longevity, and V-ATPase is an important regulator of pH homeostasis, multiple metabolic pathways, and longevity [97]. Many vacuolar transporters are transported from the ER through the Golgi apparatus [4]. This membrane transport can be accelerated during the closing of the stomata. When the stomata are opened, the number of vacuoles decreases, while their size increases. From a single confocal image slice, it was found that small bubbles fuse to form larger vacuoles when the stomata are opened [98]. Homogeneous membrane fusion steps are essential in vacuolar biosynthesis. Under some circumstances, two different types of vacuoles, protein storage vacuoles and lytic vacuoles, can also fuse to produce large central vacuoles [99]. Vacuoles distinguish different cellular components, such as proteins, sugars, tannins, organic acids, ions, and other secondary metabolites, and play a key role in plant response to different biological/abiotic signal transduction pathways. At present, many different intracellular transport pathways have been mapped and provide a structural framework for the existing concepts in vacuole physiology. Recent articles have also summarized two different approaches for vacuole defense against pathogens and discussed how plants use vacuole cell death to attack invading pathogens [100,101]. Recently, researchers have increased the research on the transport mechanisms of vacuolar transporters. It is also clear that plant cells have multiple mechanisms and transport pathways to sort vacuolar proteins. Nevertheless, there are still a lot of unresolved issues [102]. At present, our laboratory is studying the function of vacuole iron transporter in Brassica napus L. In addition, it is still difficult to detect the activity of vacuolar membranes because a certain degree of their enrichment in the corresponding cell fraction is required. Therefore, more work needs to be done to molecularly characterize vacuoles more accurately.
Author Contributions
X.T. (Xiaona Tan) and J.C. designed and supervised the review, X.T. (Xiaoli Tan), K.L., Z.W., K.Z., and J.C. performed the review and wrote the manuscript. All authors read and approved the manuscript.
Funding
This project is supported by grants from the National Key Research and Development Program of China (2016YFD0101904 and 2016YFD0100305) and the National Science Foundation of China (No. 31871655).
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Dujardin F. Histoire Naturelle des Zoophytes: Infusoires. Librairie Encyclopédique de Roret; Paris, France: 1841. [Google Scholar]
- 2.DeVries H. Plasmolytische studien über die wand der vakuolen. Jahrb Bot. 1885;16:465–598. [Google Scholar]
- 3.Blumwald E., Poole R.J. Na+/H+ Antiport in Isolated Tonoplast Vesicles from Storage Tissue of Beta vulgaris. Plant Physiol. 1985;78:163–167. doi: 10.1104/pp.78.1.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marty F. Plant vacuoles. Plant Cell. 1999;11:587–600. doi: 10.1105/tpc.11.4.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xiang L., Etxeberria E., Van den Ende W. Vacuolar protein sorting mechanisms in plants. FEBS J. 2013;280:979–993. doi: 10.1111/febs.12092. [DOI] [PubMed] [Google Scholar]
- 6.Viotti C. ER to Golgi-Dependent Protein Secretion: The Conventional Pathway. Methods Mol. Biol. 2016;1459:3–29. doi: 10.1007/978-1-4939-3804-9_1. [DOI] [PubMed] [Google Scholar]
- 7.Maîtrejean M., Wudick M.M., Voelker C., Prinsi B., Mueller-Roeber B., Czempinski K., Pedrazzini E., Vitale A. Assembly and sorting of the tonoplast potassium channel AtTPK1 and its turnover by internalization into the vacuole. Plant Physiol. 2011;156:1783–1796. doi: 10.1104/pp.111.177816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bellucci M., Marchis F.D., Pompa A. The endoplasmic reticulum is a hub to sort proteins toward unconventional traffic pathways and endosymbiotic organelles. J. Exp. Bot. 2017;69:7–20. doi: 10.1093/jxb/erx286. [DOI] [PubMed] [Google Scholar]
- 9.Sansebastiano G.P.D., Barozzi F., Piro G., Denecke J., Lousa C.D.M. Trafficking routes to the plant vacuole: Connecting alternative and classical pathways. J. Exp. Bot. 2017;69:79–90. doi: 10.1093/jxb/erx376. [DOI] [PubMed] [Google Scholar]
- 10.Viotti C., Krüger F., Krebs M., Neubert C., Fink F., Lupanga U., Scheuring D., Boutté Y., Frescatada-Rosa M., Wolfenstetter S., et al. The endoplasmic reticulum is the main membrane source for biogenesis of the lytic vacuole in Arabidopsis. Plant cell. 2013;25:3434–3449. doi: 10.1105/tpc.113.114827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sanderfoot A.A., Raikhel N.V. The specificity of vesicle trafficking: Coat proteins and SNAREs. Plant Cell. 1999;11:629–642. doi: 10.1105/tpc.11.4.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goring D.R., Sansebastiano G.P.D. Protein and membrane trafficking routes in plants: Conventional or unconventional? J. Exp. Bot. 2018;69:1–5. doi: 10.1093/jxb/erx435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang X., Chung K.P., Lin W., Jiang L. Protein secretion in plants: Conventional and unconventional pathways and new techniques. J. Exp. Bot. 2017;69:21–37. doi: 10.1093/jxb/erx262. [DOI] [PubMed] [Google Scholar]
- 14.Stigliano E., Faraco M., Neuhaus J.M., Montefusco A., Dalessandro G., Piro G., Di Sansebastiano G.P. Two glycosylated vacuolar GFPs are new markers for ER-to-vacuole sorting. Plant Physiol. Biochem. 2013;73:337–343. doi: 10.1016/j.plaphy.2013.10.010. [DOI] [PubMed] [Google Scholar]
- 15.Jauh G., Phillips T., Rogers J. Tonoplast intrinsic protein isoforms as markers for vacuolar functions. Plant Cell. 1999;11:1867–1882. doi: 10.1105/tpc.11.10.1867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zouhar J., Rojo E. Plant vacuoles: Where did they come from and where are they heading? Curr. Opin. Plant Biol. 2009;12:677–684. doi: 10.1016/j.pbi.2009.08.004. [DOI] [PubMed] [Google Scholar]
- 17.Paris N., Stanley C.M., Jones R.L., Rogers J.C. Plant cells contain two functionally distinct vacuolar compartments. Cell. 1996;85:563–572. doi: 10.1016/S0092-8674(00)81256-8. [DOI] [PubMed] [Google Scholar]
- 18.Shimada T., Koumoto Y., Li L., Yamazaki M., Kondo M., Nishimura M., Hara-Nishimura I. AtVPS29, a putative component of a retromer complex, is required for the efficient sorting of seed storage proteins. Plant Cell Physiol. 2006;47:1187–1194. doi: 10.1093/pcp/pcj103. [DOI] [PubMed] [Google Scholar]
- 19.Yamazaki M., Shimada T., Takahashi H., Tamura K., Kondo M., Nishimura M., Hara-Nishimura I. Arabidopsis VPS35, a retromer component, is required for vacuolar protein sorting and involved in plant growth and leaf senescence. Plant Cell Physiol. 2008;49:142–156. doi: 10.1093/pcp/pcn006. [DOI] [PubMed] [Google Scholar]
- 20.Kruger F., Schumacher K. Pumping up the volume-vacuole biogenesis in Arabidopsis thaliana. Semin. Cell Dev. Biol. 2018;80:106–112. doi: 10.1016/j.semcdb.2017.07.008. [DOI] [PubMed] [Google Scholar]
- 21.Hara-Nishimura I., Inoue K., Nishimura M. A unique vacuolar processing enzyme responsible for conversion of several proprotein precursors into the mature forms. FEBS Lett. 1991;294:89–93. doi: 10.1016/0014-5793(91)81349-D. [DOI] [PubMed] [Google Scholar]
- 22.Chrispeels M.J., Schaewen A.V. Sorting of proteins in the secretory system of plant cells. Antonie Van Leeuwenhoek. 1992;61:161–165. doi: 10.1007/BF00580624. [DOI] [PubMed] [Google Scholar]
- 23.Herman E.M., Larkins B.A. Protein storage bodies and vacuoles. Plant Cell. 1999;11:601–614. doi: 10.1105/tpc.11.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frigerio L., Hinz G., Robinson D.G. Multiple vacuoles in plant cells: Rule or exception? Traffic. 2008;9:1564–1570. doi: 10.1111/j.1600-0854.2008.00776.x. [DOI] [PubMed] [Google Scholar]
- 25.Feeney M., Kittelmann M., Menassa R., Hawes C., Frigerio L. Protein Storage Vacuoles Originate from Remodeled Preexisting Vacuoles in Arabidopsis thaliana. Plant Physiol. 2018;177:241–254. doi: 10.1104/pp.18.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Viotti C. ER and vacuoles: Never been closer. Front Plant Sci. 2014;5:20. doi: 10.3389/fpls.2014.00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vitale A., Galili G. The endomembrane system and the problem of protein sorting. Plant Physiol. 2001;125:115–118. doi: 10.1104/pp.125.1.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martinoia E. Vacuolar Transporters-Companions on a Longtime Journey. Plant Physiol. 2018;176:1384–1407. doi: 10.1104/pp.17.01481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Robert S., Zouhar J., Carter C., Raikhel N. Isolation of intact vacuoles from Arabidopsis rosette leaf-derived protoplasts. Nat. Protoc. 2007;2:259–262. doi: 10.1038/nprot.2007.26. [DOI] [PubMed] [Google Scholar]
- 30.Cocking E.C. A Method for the Isolation of Plant Protoplasts and Vacuoles. Nature. 1960;187:962–963. doi: 10.1038/187962a0. [DOI] [Google Scholar]
- 31.Buser-Suter C., Wiemken A., Matile P. A malic Acid permease in isolated vacuoles of a crassulacean Acid metabolism plant. Plant Physiol. 1982;69:456–459. doi: 10.1104/pp.69.2.456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schmidt U.G., Endler A., Schelbert S., Brunner A., Schnell M., Neuhaus H.E., Marty-Mazars D., Marty F., Baginsky S., Martinoia E. Novel tonoplast transporters identified using a proteomic approach with vacuoles isolated from cauliflower buds. Plant Physiol. 2007;145:216–229. doi: 10.1104/pp.107.096917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schroeder J.I., Hedrich R., Fernandez J.M. Potassium-selective single channels in guard cell protoplasts of Vicia faba. Nature. 1984;312:361–362. doi: 10.1038/312361a0. [DOI] [Google Scholar]
- 34.Hedrich R., Kurkdjian A., Guern J., Flugge U.I. Comparative studies on the electrical properties of the H+ translocating ATPase and pyrophosphatase of the vacuolar-lysosomal compartment. EMBO J. 1989;8:2835–2841. doi: 10.1002/j.1460-2075.1989.tb08430.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shimaoka T., Ohnishi M., Sazuka T., Mitsuhashi N., Hara-Nishimura I., Shimazaki K., Maeshima M., Yokota A., Tomizawa K., Mimura T. Isolation of intact vacuoles and proteomic analysis of tonoplast from suspension-cultured cells of Arabidopsis thaliana. Plant Cell Physiol. 2004;45:672–683. doi: 10.1093/pcp/pch099. [DOI] [PubMed] [Google Scholar]
- 36.Jaquinod M., Villiers F., Kieffer-Jaquinod S., Hugouvieux V., Bruley C., Garin J., Bourguignon J. A proteomics dissection of Arabidopsis thaliana vacuoles isolated from cell culture. Mol. Cell. Proteom. 2007;6:394–412. doi: 10.1074/mcp.M600250-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Melroy D.L., Herman E.M. TIP, an integral membrane protein of the protein-storage vacuoles of the soybean cotyledon undergoes developmentally regulated membrane accumulation and removal. Planta. 1991;184:113–122. doi: 10.1007/BF00208244. [DOI] [PubMed] [Google Scholar]
- 38.Maeshima M. Characterization of the major integral protein of vacuolar membrane. Plant Physiol. 1992;98:1248–1254. doi: 10.1104/pp.98.4.1248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hedrich R., Schroeder J.I. The Physiology of ION Channels and Electrogenic Pumps in Higher Plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1989;40:539–569. doi: 10.1146/annurev.pp.40.060189.002543. [DOI] [Google Scholar]
- 40.Sze H., Li X., Palmgren M.G. Energization of plant cell membranes by H+-pumping ATPases. Regulation and biosynthesis. Plant Cell. 1999;11:677–690. doi: 10.1105/tpc.11.4.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li J., Yang H., Peer W.A., Richter G., Blakeslee J., Bandyopadhyay A., Titapiwantakun B., Undurraga S., Khodakovskaya M., Richards E.L., et al. Arabidopsis H+-PPase AVP1 regulates auxin-mediated organ development. Science. 2005;310:121–125. doi: 10.1126/science.1115711. [DOI] [PubMed] [Google Scholar]
- 42.Yoshida K., Kawachi M., Mori M., Maeshima M., Kondo M., Nishimura M., Kondo T. The involvement of tonoplast proton pumps and Na+(K+)/H+ exchangers in the change of petal color during flower opening of Morning Glory, Ipomoea tricolor cv. Heavenly Blue. Plant Cell Physiol. 2005;46:407–415. doi: 10.1093/pcp/pci057. [DOI] [PubMed] [Google Scholar]
- 43.Blumwald E. Sodium transport and salt tolerance in plants. Curr. Opin. Cell Biol. 2000;12:431–434. doi: 10.1016/S0955-0674(00)00112-5. [DOI] [PubMed] [Google Scholar]
- 44.Cheng N.H., Pittman J.K., Barkla B.J., Shigaki T., Hirschi K.D. The Arabidopsis cax1 mutant exhibits impaired ion homeostasis, development, and hormonal responses and reveals interplay among vacuolar transporters. Plant Cell. 2003;15:347–364. doi: 10.1105/tpc.007385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klaumann S., Nickolaus S.D., Furst S.H., Starck S., Schneider S., Ekkehard Neuhaus H., Trentmann O. The tonoplast copper transporter COPT5 acts as an exporter and is required for interorgan allocation of copper in Arabidopsis thaliana. New Phytol. 2011;192:393–404. doi: 10.1111/j.1469-8137.2011.03798.x. [DOI] [PubMed] [Google Scholar]
- 46.Wege S., De Angeli A., Droillard M.J., Kroniewicz L., Merlot S., Cornu D., Gambale F., Martinoia E., Barbier-Brygoo H., Thomine S., et al. Phosphorylation of the vacuolar anion exchanger AtCLCa is required for the stomatal response to abscisic acid. Sci. Signal. 2014;7:ra65. doi: 10.1126/scisignal.2005140. [DOI] [PubMed] [Google Scholar]
- 47.De Angeli A., Zhang J.B., Meyer S., Martinoia E. AtALMT9 is a malate-activated vacuolar chloride channel required for stomatal opening in Arabidopsis. Nat. Commun. 2013;4:1804. doi: 10.1038/ncomms2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tommasini R., Vogt E., Fromenteau M., Hortensteiner S., Matile P., Amrhein N., Martinoia E. An ABC-transporter of Arabidopsis thaliana has both glutathione-conjugate and chlorophyll catabolite transport activity. Plant J. 1998;13:773–780. doi: 10.1046/j.1365-313X.1998.00076.x. [DOI] [PubMed] [Google Scholar]
- 49.Takanashi K., Yamada Y., Sasaki T., Yamamoto Y., Sato F., Yazaki K. A multidrug and toxic compound extrusion transporter mediates berberine accumulation into vacuoles in, coptis japonica. Phytochemistry. 2017;138:76–82. doi: 10.1016/j.phytochem.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 50.Shitan N., Yazaki K. New insights into the transport mechanisms in plant vacuoles. Int. Rev. Cell Mol. Biol. 2013;305:383–433. doi: 10.1016/B978-0-12-407695-2.00009-3. [DOI] [PubMed] [Google Scholar]
- 51.Darbani B., Motawia M.S., Olsen C.E., Nour-Eldin H.H., Moller B.L., Rook F. The biosynthetic gene cluster for the cyanogenic glucoside dhurrin in Sorghum bicolor contains its co-expressed vacuolar MATE transporter. Sci. Rep. 2016;6:37079. doi: 10.1038/srep37079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Momonoi K., Tsuji T., Kazuma K., Yoshida K. Specific expression of the vacuolar iron transporter, TgVit, causes iron accumulation in blue-colored inner bottom segments of various tulip petals. Biosci. Biotechnol. Biochem. 2012;76:319–325. doi: 10.1271/bbb.110708. [DOI] [PubMed] [Google Scholar]
- 53.Zhang Y., Xu Y., Yi H., Gong J. Vacuolar membrane transporters OsVIT1 and OsVIT2 modulate iron translocation between flag leaves and seeds in rice. Plant J. 2012;72:400–410. doi: 10.1111/j.1365-313X.2012.05088.x. [DOI] [PubMed] [Google Scholar]
- 54.Zhu W., Zuo R., Zhou R., Huang J., Tang M., Cheng X., Liu Y., Tong C., Xiang Y., Dong C., et al. Vacuolar Iron Transporter BnMEB2 Is Involved in Enhancing Iron Tolerance of Brassica napus. Front. Plant Sci. 2016;7:1353. doi: 10.3389/fpls.2016.01353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sinclair S.A., Kramer U. The zinc homeostasis network of land plants. Biochim. Biophys. Acta. 2012;1823:1553–1567. doi: 10.1016/j.bbamcr.2012.05.016. [DOI] [PubMed] [Google Scholar]
- 56.Delhaize E., Kataoka T., Hebb D.M., White R.G., Ryan P.R. Genes encoding proteins of the cation diffusion facilitator family that confer manganese tolerance. Plant Cell. 2003;15:1131–1142. doi: 10.1105/tpc.009134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Endler A., Meyer S., Schelbert S., Schneider T., Weschke W., Peters S.W., Keller F., Baginsky S., Martinoia E., Schmidt U.G. Identification of a vacuolar sucrose transporter in barley and Arabidopsis mesophyll cells by a tonoplast proteomic approach. Plant Physiol. 2006;141:196–207. doi: 10.1104/pp.106.079533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wink M. The Plant Vacuole: A Multifunctional Compartment. J. Exp. Bot. 1993;44:231–246. [Google Scholar]
- 59.Boller T., Kende H. Hydrolytic enzymes in the central vacuole of plant cells. Plant Physiol. 1979;63:1123–1132. doi: 10.1104/pp.63.6.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pedrazzini E., Caprera A., Fojadelli I., Stella A., Rocchetti A., Bassin B., Martinoia E., Vitale A. The Arabidopsis tonoplast is almost devoid of glycoproteins with complex N-glycans, unlike the rat lysosomal membrane. J. Exp. Bot. 2016;67:1769–1781. doi: 10.1093/jxb/erv567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Müntz K. Deposition of storage proteins. Plant Mol. Biol. 1998;38:77–99. doi: 10.1023/A:1006020208380. [DOI] [PubMed] [Google Scholar]
- 62.Staswick P.E. Storage Proteins of Vegetative Plant Tissues. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1994;45:303–322. doi: 10.1146/annurev.pp.45.060194.001511. [DOI] [Google Scholar]
- 63.Mainieri D., Morandini F., Maîtrejean M., Saccani A., Pedrazzini E., Alessandro V. Protein body formation in the endoplasmic reticulum as an evolution of storage protein sorting to vacuoles: Insights from maize γ-zein. Front Plant Sci. 2014;5:331. doi: 10.3389/fpls.2014.00331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vitale A., Hinz G. Sorting of proteins to storage vacuoles: how many mechanisms? Trends Plant Sci. 2015;10:316–323. doi: 10.1016/j.tplants.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 65.Bartrons M., Catalan J., Penuelas J. Spatial and Temporal Trends of Organic Pollutants In Vegetation From Remote And Rural Areas. Sci. Rep. 2016;6:25446. doi: 10.1038/srep25446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fasani E., Manara A., Martini F., Furini A., DalCorso G. The potential of genetic engineering of plants for the remediation of soils contaminated with heavy metals. Plant Cell Environ. 2018;41:1201–1232. doi: 10.1111/pce.12963. [DOI] [PubMed] [Google Scholar]
- 67.Zhang J., Martinoia E., Lee Y. Vacuolar transporters for cadmium and arsenic in plants and their applications in phytoremediation and crop development. Plant Cell Physiol. 2018;59:1317–1325. doi: 10.1093/pcp/pcy006. [DOI] [PubMed] [Google Scholar]
- 68.Curie C., Briat J. Iron transport and signaling in plants. Ann. Rev. Plant Biol. 2003;54:183–206. doi: 10.1146/annurev.arplant.54.031902.135018. [DOI] [PubMed] [Google Scholar]
- 69.Conte S., Walker E. Transporters contributing to iron trafficking in plants. Mol. Plant. 2011;4:464–476. doi: 10.1093/mp/ssr015. [DOI] [PubMed] [Google Scholar]
- 70.Wu H., Ji Y., Du J., Kong D., Liang H., Ling H. ClpC1, an ATP-dependent Clp protease in plastids, is involved in iron homeostasis in Arabidopsis leaves. Ann. Bot. 2010;105:823–833. doi: 10.1093/aob/mcq051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Haddad L., Ross J., Oshaug A., Torheim L.E., Cogill B. 5th Report on the World Nutrition Situation: Nutrition for Improved Development Outcomes. Volume 39. SCN; Geneva, Switzerland: 2004. pp. 257–258. [Google Scholar]
- 72.Briat J., Ravet K., Arnaud N., Duc C., Boucherez J., Touraine B., Cellier F., Gaymard F. New insights into ferritin synthesis and function highlight a link between iron homeostasis and oxidative stress in plants. Ann. Bot. 2010;105:811–822. doi: 10.1093/aob/mcp128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Nestel P., Bouis H., Meenakshi J., Pfeiffer W. Biofortification of staple food crops. J. Nutr. 2006;136:1064–1067. doi: 10.1093/jn/136.4.1064. [DOI] [PubMed] [Google Scholar]
- 74.Kim S., Punshon T., Lanzirotti A., Li L., Alonso J., Ecker J.R., Kaplan J., Guerinot M.L. Localization of iron in Arabidopsis seed requires the vacuolar membrane transporter VIT1. Science. 2006;314:1295–1298. doi: 10.1126/science.1132563. [DOI] [PubMed] [Google Scholar]
- 75.Briat J., Curie C., Gaymard F. Iron utilization and metabolism in plants. Curr. Opin. Plant Biol. 2007;10:276–282. doi: 10.1016/j.pbi.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 76.Curie C., Mari S. New routes for plant iron mining. New Phytol. 2017;214:521–525. doi: 10.1111/nph.14364. [DOI] [PubMed] [Google Scholar]
- 77.Guerinot M., Yi Y. Iron: Nutritious, Noxious, and Not Readily Available. Plant Physiol. 1994;104:815–820. doi: 10.1104/pp.104.3.815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Robinson N., Procter C., Connolly E., Guerinot M. A ferric-chelate reductase for iron uptake from soils. Nature. 1999;397:694–697. doi: 10.1038/17800. [DOI] [PubMed] [Google Scholar]
- 79.Rodríguez-Celma J., Schmidt W. Reduction-based iron uptake revisited: On the role of secreted iron-binding compounds. Plant Signal. Behav. 2013;8:1473–1485. doi: 10.4161/psb.26116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Miethke M., Marahiel M. Siderophore-based iron acquisition and pathogen control. Microbiol. Mol. Biol. Rev. 2007;71:413–451. doi: 10.1128/MMBR.00012-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ma J.F., Nomoto K. Effective regulation of iron acquisition in graminaceous plants. The role of mugineic acids as phytosiderophores. Physiol. Plant. 2010;97:609–617. doi: 10.1111/j.1399-3054.1996.tb00522.x. [DOI] [Google Scholar]
- 82.Takagi S., Nomoto K., Takemoto T. Physiological aspect of mugineic acid, a possible phytosiderophore of graminaceous plants. J. Plant Nutr. 2008;7:469–477. doi: 10.1080/01904168409363213. [DOI] [Google Scholar]
- 83.Curie C., Panaviene Z., Loulergue C., Dellaporta S., Briat J., Walker E. Maize yellow stripe1 encodes a membrane protein directly involved in Fe(III) uptake. Nature. 2001;409:346–349. doi: 10.1038/35053080. [DOI] [PubMed] [Google Scholar]
- 84.Liu Y., Bassham D.C. Autophagy: Pathways for self-eating in plant cells. Annu. Rev. Plant Biol. 2012;63:215–237. doi: 10.1146/annurev-arplant-042811-105441. [DOI] [PubMed] [Google Scholar]
- 85.Michaeli S., Avin-Wittenberg T., Galili G. Involvement of autophagy in the direct ER to vacuole protein trafficking route in plants. Front Plant Sci. 2014;5:134. doi: 10.3389/fpls.2014.00134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chanoca A., Kovinich N., Burkel B., Stecha S., Bohorquez-Restrepo A., Ueda T., Eliceiri K.W., Grotewold E., Otegui M.S. Anthocyanin Vacuolar Inclusions Form by a Microautophagy Mechanism. Plant Cell. 2015;27:2545–2559. doi: 10.1105/tpc.15.00589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Herman E., Schmidt M. Endoplasmic reticulum to vacuole trafficking of endoplasmic reticulum bodies provides an alternate pathway for protein transfer to the vacuole. Plant Physiol. 2004;136:3440–3446. doi: 10.1104/pp.104.051722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Martinoia E., Meyer S., De Angeli A., Nagy R. Vacuolar Transporters in Their Physiological Context. Ann. Rev. Plant Biol. 2012;63:183–213. doi: 10.1146/annurev-arplant-042811-105608. [DOI] [PubMed] [Google Scholar]
- 89.Ludevid D., Hofte H., Himelblau E., Chrispeels M.J. The Expression Pattern of the Tonoplast Intrinsic Protein gamma-TIP in Arabidopsis thaliana Is Correlated with Cell Enlargement. Plant Physiol. 1992;100:1633–1639. doi: 10.1104/pp.100.4.1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Maurel C. Aquaporins and Water Permeability of Plant Membranes. Ann. Rev. Plant Physiol. Plant Mol. Biol. 1997;48:399–429. doi: 10.1146/annurev.arplant.48.1.399. [DOI] [PubMed] [Google Scholar]
- 91.Aubert S., Gout E., Bligny R., Marty-Mazars D., Barrieu F., Alabouvette J., Marty F., Douce R. Ultrastructural and biochemical characterization of autophagy in higher plant cells subjected to carbon deprivation: Control by the supply of mitochondria with respiratory substrates. J. Cell Biol. 1996;133:1251–1263. doi: 10.1083/jcb.133.6.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Moriyasu Y., Ohsumi Y. Autophagy in Tobacco Suspension-Cultured Cells in Response to Sucrose Starvation. Plant Physiol. 1996;111:1233–1241. doi: 10.1104/pp.111.4.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Francisco R.D.B., Martinoia E. The Vacuolar Transportome of Plant Specialized Metabolites. Plant Cell Physiol. 2018;59:1326–1336. doi: 10.1093/pcp/pcy039. [DOI] [PubMed] [Google Scholar]
- 94.Martinoia E., Maeshima M., Neuhaus H.E. Vacuolar transporters and their essential role in plant metabolism. J. Exp. Bot. 2007;58:83–102. doi: 10.1093/jxb/erl183. [DOI] [PubMed] [Google Scholar]
- 95.Shitan N. Secondary metabolites in plants: Transport and self-tolerance mechanisms. Biosci. Biotechnol. Biochem. 2016;80:1283–1293. doi: 10.1080/09168451.2016.1151344. [DOI] [PubMed] [Google Scholar]
- 96.Zhao J. Flavonoid transport mechanisms: How to go, and with whom. Trends Plant Sci. 2015;20:576–585. doi: 10.1016/j.tplants.2015.06.007. [DOI] [PubMed] [Google Scholar]
- 97.Aufschnaiter A., Büttner S. The vacuolar shapes of ageing: From function to morphology. Biochim. Biophys. Acta Mol. Cell Res. 2019;1866:957–970. doi: 10.1016/j.bbamcr.2019.02.011. [DOI] [PubMed] [Google Scholar]
- 98.Tanaka Y., Kutsuna N., Kanazawa Y., Kondo N., Hasezawa S., Sano T. Intra-vacuolar reserves of membranes during stomatal closure: The possible role of guard cell vacuoles estimated by 3-D reconstruction. Plant Cell Physiol. 2007;48:1159–1169. doi: 10.1093/pcp/pcm085. [DOI] [PubMed] [Google Scholar]
- 99.Zheng H., Staehelin L. Protein storage vacuoles are transformed into lytic vacuoles in root meristematic cells of germinating seedlings by multiple, cell type-specific mechanisms. Plant Physiol. 2011;155:2023–2035. doi: 10.1104/pp.110.170159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Shimada T., Takagi J., Ichino T., Shirakawa M., Hara-Nishimura I. Plant Vacuoles. Annu Rev. Plant Biol. 2018;69:123–145. doi: 10.1146/annurev-arplant-042817-040508. [DOI] [PubMed] [Google Scholar]
- 101.Martinoia E., Mimura T., Hara-Nishimura I., Shiratake K. The Multifaceted Roles of Plant Vacuoles. Plant Cell Physiol. 2018;59:1285–1287. doi: 10.1093/pcp/pcy113. [DOI] [PubMed] [Google Scholar]
- 102.Pedrazzini E., Komarova N.Y., Rentsch D., Vitale A. Traffic routes and signals for the tonoplast. Traffic. 2013;14:622–628. doi: 10.1111/tra.12051. [DOI] [PubMed] [Google Scholar]