Abstract
Atherosclerosis is a chronic inflammation of the arterial vessel wall that arises from an imbalanced lipid metabolism. A growing body of literature describes leukocyte recruitment as a critical step in the initiation and progression of lesion development. By contrast, the role of leukocytes during plaque regression has been described in less detail. Leukocyte egress might be an important step to resolving chronic inflammation and therefore it may be a promising target for limiting advanced lesion development. This review aims to summarize our current knowledge of leukocyte recruitment to the arterial vessel wall. We will discuss mechanisms of leukocyte egress from the lesion site, as well as potential therapeutic strategies to promote atherosclerotic regression.
Keywords: Atherosclerosis, Chemokines, Inflammation, Monocytes
Introduction
The immune system consists of different specialized leukocytes to protect the organism against pathogens. Beside pathogens, also host-associated danger molecules are able to activate the immune system and cause inflammation. An imbalanced immune response can result in chronic inflammatory diseases such as atherosclerosis. Atherosclerosis is an inflammatory disorder of the arterial vessel wall characterized by failure to resolve inflammation. Clinical consequences of atherosclerosis, e.g., myocardial infarction or stroke, are major contributors to morbidity and mortality in Western societies [1].
Atherosclerosis develops over decades and is initiated by endothelial dysfunction at arterial branch points followed by extravasation and intimal retention of low-density lipoprotein (LDL) [2]. Monocytes bind to adhesion molecules on the activated endothelium, thus paving the way for monocyte transmigration into the intima. Lesional macrophages, derived from circulating transmigrated monocytes, phagocyte cell debris or oxLDL under hypercholesterolemic conditions and finally differentiate into foam cells [3]. Those macrophages release proinflammatory cytokines, thereby enhancing the activation of endothelial cells and leading to upregulation of endothelial adhesion molecules [4]. As a consequence, leukocytes adhere frequently [5]. Among all leukocyte subsets, macrophages are the most abundant cells within atherosclerotic lesions [6]. Activated lesional macrophages release cytokines, chemokines, and matrix metalloproteinases, with the latter digesting extracellular matrix components and thus leading to plaque destabilization. Finally, macrophages die as a consequence of intracellular lipid accumulation and hence contribute to necrotic core formation in advanced plaques [3, 7]. The persistent inflammation is a key characteristic of atherosclerosis and an important factor of leukocyte recruitment for the initiation and progression of lesion development. Atherosclerosis can be limited by prevention of continuous leukocyte recruitment and premature macrophage apoptosis or by promotion of leukocyte egress [8, 9, 10, 11].
This review summarizes the state of the art of leukocyte adhesion and recruitment to atherosclerotic lesions, as well as their egress and possible therapeutic strategies. However, most of the studies reviewed here are find ings in animal models, making a translation into human pathogenesis sometimes far fetched. Thus, proof of those assumptions is needed to show that these mechanisms occur in humans during atherosclerosis.
Mechanisms of Arterial Leukocyte Entry
Leukocyte recruitment is characterized by an interplay of different cell subsets and promigratory factors (cell adhesion molecules, selectins, and chemokines), a process overall known as the leukocyte recruitment cascade. This leukocyte recruitment cascade describes different steps including leukocyte rolling, activation, adhesion, and finally transmigration [12].
As a consequence of the disturbed blood flow, activated and dysfunctional endothelial cells upregulate the adhesion molecules E-selectin, P-selectin, intercellular cell adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1) on their cell surface and reallocate junctional adhesion molecule A to facilitate leukocyte entry at sites of atherosclerosis [5, 13]. In addition, endothelial cells covering atherosclerotic lesions have a higher propensity for plasma leakage, thus permitting exudation and retention of lipoproteins [14]. Endothelial cells, neutrophils, or tissue-resident macrophages oxidatively modify retained LDL [15]. The resulting oxLDL trigger further adhesion molecule upregulation on endothelial cells and chemokine release [16]. Circulating myeloid cells express very late antigen-4 (VLA-4) and P-selectin glycoprotein ligand 1 (PSGL1), which recognizes VCAM and selectins on activated endothelial cells, respectively, and induce leukocyte rolling and integrin activation [17, 18]. Furthermore, under high shear stress, neutrophils stabilize the rolling by forming slings that express a high density of PSGL-1 [19]. Leukocyte recruitment is controlled by chemokines immobilized on endothelial cells that promote cell arrest by integrin activation and adhesion strengthening. These chemokines, such as CCL5, CXCL1, and CCL2, can be produced either by endothelial cells or by subendothelial macrophages, neutrophils, and monocytes [20, 21]. In addition, activated leukocytes release granular proteins, such as CRAMP, MPO, and cathepsin G, which can bind to the endothelium and attract leukocytes [22, 23].
Besides endothelial and myeloid cells, platelets contribute to leukocyte recruitment by delivering CCL5 and CXCL4 to the endothelium of atherosclerotic lesions [24]. Finally, adherent myeloid cells crawl along the endothelium in an integrin-dependent manner, looking for exit sites to transmigrate into the intima [25]. Howev er, most studies describing leukocyte recruitment have drawn conclusions from microcirculation, and more investigations are needed to confirm those findings in the macrocirculation. Nevertheless, the patrolling monocyte velocity is higher in the macrocirculation compared to the microcirculation, and it has been shown that monocyte adhesion to dysfunctional endothelium in large arteries is dependent on integrin-interactions [26]. The most abundant cells in atherosclerotic lesions are monocytes, macrophages, and smooth muscle cells (SMC). SMC apoptosis and SMC-derived macrophage-like cells promote the ongoing inflammation. SMC can phenotypically shift towards macrophage-like cells in a Klf4-dependent manner, and hence their contribution to lesion formation, composition, and stability may be underestimated [27]. Of note, SMC decrease the expression of their differentiation markers and increase the expression of the macrophage marker CD68 under atherosclerotic conditions.
However, monocytes are divided into 2 subsets called classical (inflammatory) and nonclassical (patrolling) monocytes. Interestingly, classical monocytes are more frequently recruited to atherosclerotic lesions compared to their nonclassical counterparts [28, 29]. In agreement herewith, a previous study showed that apolipoprotein E-deficient mice (Apoe–/–) depleted of classical monocytes exhibited less lesion development, while the absence of nonclassical monocytes in that model had no effect [30]. Furthermore, distinct chemokine receptors on monocyte subsets regulate lesion entry. CCR1/2/5 and CX3CR1 promote classical monocyte recruitment, whereas nonclassical monocyte recruitment depends on CCR5 [28, 30].
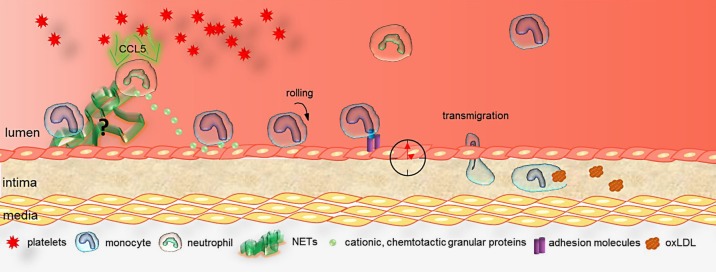
In chronic inflammation, platelets and neutrophils become activated to release proinflammatory factors. Simultaneous activation allows platelets and neutrophils to cooperate in monocyte recruitment. HNP-1 derived from activated neutrophils and platelet-derived CCL5 form heteromers, bind to the endothelium, and support firm monocyte adhesion [31]. In agreement with this, also other studies have highlighted the importance of the platelet-neutrophil interaction in monocyte recruitment during the initial phase of atherosclerosis. Besides heteromer formation of platelet-derived CCL5 with neutrophil HNP-1, CCL5 also activates neutrophils to release cathepsin G, which binds to endothelium and fosters monocyte adhesion [23]. Hypercholesterolemia-induced neutrophilia is positively correlated with the extent of early lesion development, while neutropenic mice have fewer lesional inflammatory monocytes and macrophages [20]. On the one hand, CCR1/5-dependent neutrophil infiltration promotes the release of inflammatory granule proteins to trigger further intimal monocyte recruitment (Fig. 1) [32]. On the other hand, neutrophils are able to form neutrophil extracellular traps (NET). Upon neutrophil activation by platelet-derived CXCL4/CCL5 heteromers, neutrophils release NET consisting of chromatin, histones, and cationic granule proteins [33]. Exposure of NET to endothelium causes endothelial activation and dysfunction [34, 35]. Notably, in vivo data represented less lesion formation in apolipoprotein E-deficient mice (Apoe–/–) that were pharmacologically treated with a NET formation blocker. Furthermore, NET are decorated with neutrophil granular proteins, which have been shown to trigger monocyte attraction [32].
Fig. 1.
Myeloid cell recruitment to atherosclerotic lesions. Imbalanced myeloid cell recruitment during lesion progression triggers chronic inflammation. Under inflammatory conditions, platelets secrete chemokine ligand 5 (CCL5), thus activating neutrophils to release cationic granular proteins or inducing neutrophil extracellular trap formation. Chemotactic, neutrophil-derived granule proteins (e.g., cathelicidin and cathepsin G) bind to endothelial cells and mediate monocyte adhesion. In addition, monocytes start to roll along adhesion molecules, which are presented on the endothelium and finally adhere via E-selectin-, P-selectin-, intercellular cell adhesion molecule-1-, or vascular cell adhesion molecule-1-mediated cell arrest. The endothelial cell adhesion molecule and chemokine expression as well as myeloid cell recruitment are regulated by circadian rhythmicity. Adherent monocytes try to find their way to transmigration through the endothelium into the intima, where monocyte-derived macrophages phagocyte oxLDL. NET, neutrophil extracellular traps.
In addition, circadian rhythmicity represents another important aspect for control of leukocyte recruitment. The circadian clock has been described to influence leukocyte recruitment under physiological as well as inflammatory conditions. The expression of promigratory factors such as ICAM-1, VCAM-1, selectins, and CCL2 exhibited oscillations within 24 h, thereby regulating leukocyte trafficking [36, 37]. However, knowledge about the influence of circadian rhythmicity on leukocyte recruitment to atherosclerotic lesions is still unclear. Nevertheless, circadian rhythmicity should be taken into consideration for treatment strategies to prevent lesion development, due to the higher efficiency and fewer side effects by chronopharmacology.
To conclude, leukocyte recruitment plays an important role in atherogenesis. Different cell types but also their interactions with each other have been identified in the past to be involved in cell recruitment to atherosclerotic lesions and therefore foster lesion development. Finding specific markers that are mainly involved in lesion development is of particular clinical relevance in order to further improve treatment strategies without affecting cell recruitment in other organs.
Leukocyte Egress from Established Atherosclerotic Plaques
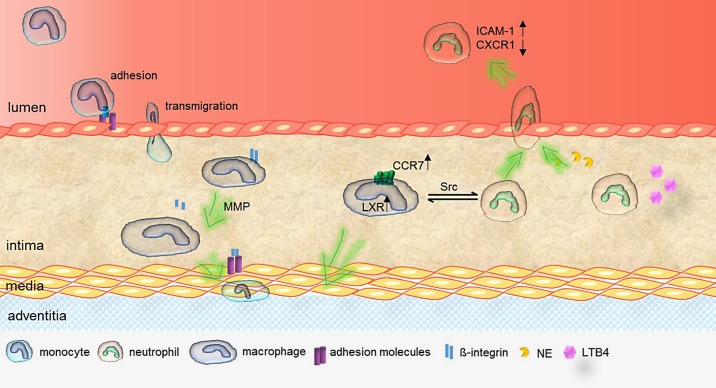
Acute inflammation is well controlled by phagocytic clearance of the initially recruited leukocytes, leading to a shift from a proinflammatory to a proresolving phenotype. In contrast, chronic inflammation appears for a long period of time to be regulated by the persistence of proinflammatory factors and failure to resolve inflammation. The immune system is not able to clear the instigating stimulus, e.g., in the event of hypercholesteremia. Thus, continued leukocyte infiltration causes progression of atherosclerosis. However, lesional leukocytes were long believed to migrate one-directionally, but there is now evidence that they are able to egress back into the circulation or to the lymphatic vessels. Leukocytes migrate via lymphatic vessels to lymph nodes to present antigens and therefore modulate the adaptive immune response. Macrophage egress has been described to be dependent on matrix metalloproteinase-mediated integrin-β2 cleavage [38]. Soluble integrin-β2 binds ICAM-1 and blocks the adhesion capacity of adhesion molecules, enabling macrophages to leave the site of inflammation and limiting further leukocyte infiltration. Therefore, the reduced leukocyte influx and the enhanced egress initiate the resolution of inflammation [38]. Besides the proteinase-dependent cleavage of integrin, it has been shown that liver X receptor (LXR) in interplay with CCR7 expression on monocyte-derived cells is a potent egress mediator during atherosclerosis regression in hypercholestolemic mice [39]. In addition, Potteaux et al. [8] showed a CCR7-independent reduction of lesional macrophages. In their study, macrophage apoptosis and limited monocyte recruitment were decisive mechanisms for the reduction of macrophage numbers in regressing lesions [8].
Apart from macrophage efflux to the lymphatic vessels, also reverse transendothelial migration of neutrophils can limit proinflammatory cell counts at the lesion site. Neutrophil reverse transendothelial migration and reentering into the circulation is encouraged by macrophage interaction via redox-regulated Src family kinase signaling in response to reactive oxygen species [40]. Moreover, macrophage-depletion shows an increased number of neutrophils under inflammatory conditions, pointing to uncontrolled neutrophil recruitment. However, the macrophage-neutrophil interplay was not necessary for neutrophil efflux in that study, demonstrating that other factors and mechanisms are involved in this process [40, 41]. Neutrophils are able to reenter the blood stream by passing endothelial cells. Therefore, they discharge neutrophil elastase in response to the lipid chemoattractant LTB4, which leads to proteolytic cleavage of junctional adhesion molecule-C (JAM-C), thus promoting neutrophil egress (Fig. 2) [42]. Tissue-experienced leukocytes can be identified by a high expression of ICAM-1 and a low expression of CXCR1 on their cell surface. Neutrophils expressing this profile have been detected in patients with chronic inflammatory diseases, e.g., atherosclerosis. Once they exit the site of inflammation, they migrate to the liver, spleen, or bone marrow, where they are cleared from the circulation [41, 43]. As mentioned above, reverse transmigrated leukocytes express an increased level of the integrin-β2 high-affinity ligand ICAM-1. Integrin-β2 is expressed on residential macrophages, and the elevated ICAM-1 expression on tissue-experienced cells could support the efficient removal of tissue-experienced neutrophils to resolve inflammation [43].
Fig. 2.
Myeloid cell egress. Macrophages, derived from transmigrated monocytes, release matrix metalloproteinases (MMP) which degrade extracellular matrix components and integrins on macrophages. Soluble integrins-β2 block adhesion molecules, which enable macrophages to leave atherosclerotic plaques through the media to adventitial lymphatic vessels. Macrophage liver X receptor (LXR) activation increases chemokine receptor 7 (CCR7) expression and fosters the reverse transmigration of macrophages. Neutrophil reentry into the circulation is caused by macrophage interaction via redox-regulated Src family kinase signaling. To reverse transmigration through endothelial cells, neutrophils release neutrophil elastase (NE) in response to leukotriene B4 (LTB4). NE cleaves endothelial cell junction adhesion molecule-C (JAM-C). Altered JAM-C distribution enables neutrophils to leave the lesion site and reenter into circulation. Tissue-experienced neutrophils express high levels of intercellular adhesion molecule-1 (ICAM-1) and low levels of chemokine receptor CXCR1 on their cell surface.
Nevertheless, the egress of leukocytes is controversially discussed in the literature. While some studies describe leukocyte egress as a beneficial effect for solving atherosclerosis [39, 44, 45], others point out that reversed migrated neutrophils promote systemic inflammation [42, 43, 46]. Apoptosis of reversed transmigrated neutrophils is delayed, meaning that those cells can circulate for a prolonged time. Interestingly, these neutrophils are characterized by a high ability to release reactive oxygen species, causing tissue damage [42]. Furthermore, increased levels of soluble JAM-C can be detected in trauma patients and have been correlated with multiorgan failure [46]. However, not only the effect of leukocyte egress but also whether leukocyte egress affects atherosclerosis regression is controversially discussed in the literature [8, 39, 46, 47, 48]. The elimination of macrophages from lesions is a logical approach to limiting atherosclerosis, due to their abundance in atherosclerotic lesions. Depletion of lesional macrophages can be driven by egress, apoptosis, and limited cell recruitment. Evidence of the relative importance of each of these mechanisms is currently not available and may very much depend on the mouse model used.
In summary, leukocyte egress has been shown to be important for atherosclerotic regression. The interaction of different cell types, to foster reverse transmigration, is of high interest for the treatment of atherosclerosis. Besides studying the mechanism of leukocyte egress, it will also be important to figure out how tissue-experienced leukocytes affect the whole body and if they might cause systemic inflammation.
Therapeutic Implications
Atherosclerosis is diagnosed at a clinical stage, and for this reason its prevention is an important research field with growing interest. Resolving inflammatory pathways during advanced atherosclerosis can be driven by stimulating cholesterol efflux, neutralizing cytokines, inhibiting leukocyte recruitment, or promoting leukocyte egress [49, 50, 51, 52, 53]. The current therapeutic strategies target the well-known atherosclerotic risk factors, e.g., hypercholesterolemia and hypertension. However, treatment with statins or high-blood-pressure-regulating drugs (β- blockers) is not aimed at decreasing chronic inflammation. Atherosclerosis is nowadays well described as a chronic inflammatory disease, and targeting the imbalanced inflammatory response might be an improvement of atherosclerotic treatment in combination with the gold standard of cardiovascular disease therapy. Studies focusing on anti-inflammatory approaches have shown a beneficial immune modulating effect of cytokine-inhibitors [54]. A reduction of cardiovascular events was achieved by specific neutralization of the proinflammatory cytokine IL-1β [55]. Besides cytokine-inhibitory strategies, also the disruption of leukocyte recruitment into lesions is believed to be a potent therapeutic target [53, 56]. Thus, a study focusing on blocking CCL5 signaling as a treatment showed a reduced atherosclerotic progression in mice [56], resulting in a reduced macrophage plaque content and a smaller lesion size. In agreement with that previous study, Ravindran et al. [51] showed similar results by treating mice with the chemokine-binding protein M3. M3 is able to bind CCL2, CCL5, and CX3CL1, thus reducing leukocyte recruitment and improving plaque vulnerability by increasing the SMC content and limiting leukocyte adhesion. Leukocyte adhesion and transmigration is one of the major contributors to atherosclerosis progression. Therefore, the genetic deletion of CX3CR1 results in a 50% decrease in monocyte and macrophage content in the lesion. Moreover, simultaneous inhibition of the chemokines CCL2 and CCR5 and the chemokine receptor CX3CR1 in hypercholesterolemic apoe-deficient mice results in a 90% reduction of atherosclerotic lesions [9, 53]. Similar antiatherogenic effects were observed by therapeutically blocking the receptor of ligand CX3CL1, i.e., CX3CR1 [57]. Nevertheless, neutralization of chemokines impairs the overall leukocyte recruitment, leading to a weakened immune defense, similar to what was observed in the CANTOS study, where a specific neutralization of the proinflammatory cytokine IL-1β led to a higher incidence of infection and sepsis [55].
Besides chemokines, also selectins play a crucial role in atherosclerosis development. P-selectins are highly expressed on endothelial cells in advanced atherosclerosis and they are responsible for leukocyte recruitment into the vessel wall [5]. Neutralization of P-selectin shows reduced tissue damage after cardiovascular events [58]. Furthermore, the development of nanoparticle-based RNA interference downregulates P-selectins, E-selectins, ICAM-1, ICAM-2, and VCAM1, thereby reducing leukocyte recruitment to ischemic myocardium in a mouse model, and limits plaque destabilization [59]. However, it has also been shown that monocyte recruitment to the lesion is important in the context of atherosclerosis egress after lowering lipid levels. Ly6Chi monocytes are recruited into advanced lesions and their polarization towards M2 macrophages contributes to atherosclerosis regression [60]. The absence of M2 macrophages impairs plaque regression, highlighting similarities in atherosclerosis regression and wound healing [50]. Thus, such studies indicate that therapeutic manipulation of myeloid cell recruitment into atherosclerosis is complex and may require stage-dependent regimens.
While influencing leukocyte recruitment might be a promising treatment for delaying atherosclerosis, promoting leukocyte exit could be a potent treatment for its resolution as well. As discussed above, LXR is a potent egress mediator during lesion progression [39]. For that reason, its agonist R211945 induces plaque regression in rabbits [44]. More clues on cell egress can be derived from findings of the developing nervous system. The neuronal guiding molecule netrin-1 can serve, depending on its receptor expression, as a migrating or emigrating signal. Activated macrophages express netrin-1 as an arrest molecule. Thus, inhibition of netrin-1 leads to an enhanced macrophage egress independently of ongoing hypercholesterolemia and provides atherosclerosic regression [45].
Besides inducing macrophage egress, also the phenotype shift from proinflammatory (M1) to proresolving macrophages (M2) by resolving lipid mediators can limit atherosclerosis [61, 62]. Resolving lipid mediators, e.g., maresin 1 or resolvin D2, might be a useful tool to resolve atherosclerosis [61] by encouraging apoptosis or stimulating efferocytic cell clearance without affecting the normal immune response or taking the risk of causing systemic inflammation of atherosclerotic-experienced egressed cells.
Therapies that specifically reduce leukocyte recruitment or induce leukocyte egress from atherosclerotic plaques and resolve lipid mediators could be a useful synergy with currently used cholesterol-lowering therapies to improve the disease outcome in established atherosclerosis.
Conclusion
The initiation and progression of atherosclerosis are mediated by a continuous recruitment of leukocytes to lesions [30, 63]. They release proinflammatory factors, such as chemokines and granular proteins, promoting endothelial cell activation. Adhesion molecule expression is upregulated on activated endothelial cells, and thus leukocytes adhere and transmigrate through the endothelium. Therefore, infiltrated leukocytes release their cellular content and influence plaque stability by enzymatically degrading the extracellular matrix and promoting inflammation [17, 64]. Limiting leukocyte influx or enhancing their egress might be promising targets to stabilize or induce the regression of lesions even at a clinical stage of atherosclerosis [8, 39]. Therefore, the identification of molecular patterns specifically causing leukocyte recruitment to lesions has been very well documented during the last decades [12, 29, 65, 66]. Besides studying leukocyte adhesion and recruitment to lesions, leukocyte egress is also a promising research field for future investigation that can generate new antiatherogenetic therapeutic strategies. Nevertheless, leukocyte egress as a therapeutic approach is challenged by studies showing systemic inflammation as a result of leukocyte reverse transmigration.
Acknowledgement
O.S. is supported by the DFG (SFB914 TP B8, SFB1123 TP A6, B5, SO876/6-1, and SO876/11-1), the Vetenskapsrådet (2017-01762), and the European Union's Horizon 2020 Research and Innovation Programme under Marie Skłodowska-Curie grant agreement No. 675111.
References
- 1.Swirski FK, Nahrendorf M. Leukocyte behavior in atherosclerosis, myocardial infarction, and heart failure. Science. 2013;339:161–166. doi: 10.1126/science.1230719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473:317–325. doi: 10.1038/nature10146. [DOI] [PubMed] [Google Scholar]
- 3.Moore KJ, Tabas I. Macrophages in the pathogenesis of atherosclerosis. Cell. 2011;145:341–355. doi: 10.1016/j.cell.2011.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Zhang J, et al. Regulation of endothelial cell adhesion molecule expression by mast cells, macrophages, and neutrophils. PLoS One. 2011;6:e14525. doi: 10.1371/journal.pone.0014525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huo Y, Ley K. Adhesion molecules and atherogenesis. Acta Physiol Scand. 2001;173:35–43. doi: 10.1046/j.1365-201X.2001.00882.x. [DOI] [PubMed] [Google Scholar]
- 6.Ley K, Miller YI, Hedrick CC. Monocyte and macrophage dynamics during atherogene sis. Arterioscler Thromb Vasc Biol. 2011;31:1506–1516. doi: 10.1161/ATVBAHA.110.221127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Silvestre-Roig C, et al. Atherosclerotic plaque destabilization: mechanisms, models, and therapeutic strategies. Circ Res. 2014;114:214–226. doi: 10.1161/CIRCRESAHA.114.302355. [DOI] [PubMed] [Google Scholar]
- 8.Potteaux S, et al. Suppressed monocyte recruitment drives macrophage removal from atherosclerotic plaques of Apoe–/– mice during disease regression. J Clin Invest. 2011;121:2025–2036. doi: 10.1172/JCI43802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Combadiere C, et al. Combined inhibition of CCL2, CX3CR1, and CCR5 abrogates Ly6C(hi) and Ly6C(lo) monocytosis and almost abolishes atherosclerosis in hypercholesterolemic mice. Circulation. 2008;117:1649–1657. doi: 10.1161/CIRCULATIONAHA.107.745091. [DOI] [PubMed] [Google Scholar]
- 10.Arai S, et al. A role for the apoptosis inhibitory factor AIM/Spalpha/Api6 in atherosclerosis development. Cell Metab. 2005;1:201–213. doi: 10.1016/j.cmet.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 11.Viola J, Soehnlein O. Atherosclerosis: a matter of unresolved inflammation. Semin Immunol. 2015;27:184–193. doi: 10.1016/j.smim.2015.03.013. [DOI] [PubMed] [Google Scholar]
- 12.Nahrendorf M, Swirski FK. Immunology: neutrophil-macrophage communication in inflammation and atherosclerosis. Science. 2015;349:237–238. doi: 10.1126/science.aac7801. [DOI] [PubMed] [Google Scholar]
- 13.Schmitt MM, et al. Endothelial junctional adhesion molecule-a guides monocytes into flow-dependent predilection sites of atherosclerosis. Circulation. 2014;129:66–76. doi: 10.1161/CIRCULATIONAHA.113.004149. [DOI] [PubMed] [Google Scholar]
- 14.Lundeberg E, et al. Assessing large-vessel endothelial permeability using near-infrared fluorescence imaging - brief report. Arterioscler Thromb Vasc Biol. 2015;35:783–786. doi: 10.1161/ATVBAHA.114.305131. [DOI] [PubMed] [Google Scholar]
- 15.Yoshida H, Kisugi R. Mechanisms of LDL oxidation. Clin Chim Acta. 2010;411:1875–1882. doi: 10.1016/j.cca.2010.08.038. [DOI] [PubMed] [Google Scholar]
- 16.Tabas I, Williams KJ, Boren J. Subendothelial lipoprotein retention as the initiating process in atherosclerosis: update and therapeutic implications. Circulation. 2007;116:1832–1844. doi: 10.1161/CIRCULATIONAHA.106.676890. [DOI] [PubMed] [Google Scholar]
- 17.Ley K, et al. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat Rev Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 18.Stadtmann A, et al. The PSGL-1-L-selectin signaling complex regulates neutrophil adhesion under flow. J Exp Med. 2013;210:2171–2180. doi: 10.1084/jem.20130664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sundd P, et al. “Slings” enable neutrophil rolling at high shear. Nature. 2012;488:399–403. doi: 10.1038/nature11248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Drechsler M, et al. Hyperlipidemia-triggered neutrophilia promotes early atherosclerosis. Circulation. 2010;122:1837–1845. doi: 10.1161/CIRCULATIONAHA.110.961714. [DOI] [PubMed] [Google Scholar]
- 21.Zhou Z, et al. Lipoprotein-derived lysophosphatidic acid promotes atherosclerosis by releasing CXCL1 from the endothelium. Cell Metab. 2011;13:592–600. doi: 10.1016/j.cmet.2011.02.016. [DOI] [PubMed] [Google Scholar]
- 22.Wantha S, et al. Neutrophil-derived cathelicidin promotes adhesion of classical monocytes. Circ Res. 2013;112:792–801. doi: 10.1161/CIRCRESAHA.112.300666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ortega-Gomez A, et al. Cathepsin G controls arterial but not venular myeloid cell recruitment. Circulation. 2016;134:1176–1188. doi: 10.1161/CIRCULATIONAHA.116.024790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.von Hundelshausen P, et al. RANTES deposition by platelets triggers monocyte arrest on inflamed and atherosclerotic endothelium. Circulation. 2001;103:1772–1777. doi: 10.1161/01.cir.103.13.1772. [DOI] [PubMed] [Google Scholar]
- 25.Vestweber D. How leukocytes cross the vascular endothelium. Nat Rev Immunol. 2015;15:692–704. doi: 10.1038/nri3908. [DOI] [PubMed] [Google Scholar]
- 26.Quintar A, et al. Endothelial protective monocyte patrolling in large arteries intensified by Western diet and atherosclerosis. Circ Res. 2017;120:1789–1799. doi: 10.1161/CIRCRESAHA.117.310739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shankman LS, et al. KLF4-dependent phenotypic modulation of smooth muscle cells has a key role in atherosclerotic plaque pathogenesis. Nat Med. 2015;21:628–637. doi: 10.1038/nm.3866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tacke F, et al. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swirski FK, et al. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soehnlein O, et al. Distinct functions of chemokine receptor axes in the atherogenic mobilization and recruitment of classical monocytes. EMBO Mol Med. 2013;5:471–481. doi: 10.1002/emmm.201201717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alard JE, et al. Recruitment of classical monocytes can be inhibited by disturbing heteromers of neutrophil HNP1 and platelet CCL5. Sci Transl Med. 2015;7 doi: 10.1126/scitranslmed.aad5330. 317ra196. [DOI] [PubMed] [Google Scholar]
- 32.Soehnlein O, et al. Neutrophil secretion products pave the way for inflammatory monocytes. Blood. 2008;112:1461–1471. doi: 10.1182/blood-2008-02-139634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rossaint J, et al. Synchronized integrin engagement and chemokine activation is crucial in neutrophil extracellular trap-mediated sterile inflammation. Blood. 2014;123:2573–2584. doi: 10.1182/blood-2013-07-516484. [DOI] [PubMed] [Google Scholar]
- 34.Knight JS, et al. Peptidylarginine deiminase inhibition reduces vascular damage and modulates innate immune responses in murine models of atherosclerosis. Circ Res. 2014;114:947–956. doi: 10.1161/CIRCRESAHA.114.303312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Quillard T, et al. TLR2 and neutrophils potentiate endothelial stress, apoptosis and detachment: implications for superficial erosion. Eur Heart J. 2015;36:1394–1404. doi: 10.1093/eurheartj/ehv044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nguyen KD, et al. Circadian gene Bmal1 regulates diurnal oscillations of Ly6C(hi) inflammatory monocytes. Science. 2013;341:1483–1488. doi: 10.1126/science.1240636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Scheiermann C, et al. Adrenergic nerves govern circadian leukocyte recruitment to tissues. Immunity. 2012;37:290–301. doi: 10.1016/j.immuni.2012.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gomez IG, et al. Metalloproteinase-mediated shedding of integrin beta2 promotes macrophage efflux from inflammatory sites. J Biol Chem. 2012;287:4581–4589. doi: 10.1074/jbc.M111.321182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feig JE, et al. LXR promotes the maximal egress of monocyte-derived cells from mouse aortic plaques during atherosclerosis regression. J Clin Invest. 2010;120:4415–4424. doi: 10.1172/JCI38911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tauzin S, et al. Redox and Src family kinase signaling control leukocyte wound attraction and neutrophil reverse migration. J Cell Biol. 2014;207:589–598. doi: 10.1083/jcb.201408090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang J, et al. Visualizing the function and fate of neutrophils in sterile injury and repair. Science. 2017;358:111–116. doi: 10.1126/science.aam9690. [DOI] [PubMed] [Google Scholar]
- 42.Colom B, et al. Leukotriene B4-neutrophil elastase axis drives neutrophil reverse transendothelial cell migration in vivo. Immunity. 2015;42:1075–1086. doi: 10.1016/j.immuni.2015.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Buckley CD, et al. Identification of a phenotypically and functionally distinct population of long-lived neutrophils in a model of reverse endothelial migration. J Leukoc Biol. 2006;79:303–311. doi: 10.1189/jlb.0905496. [DOI] [PubMed] [Google Scholar]
- 44.Vucic E, et al. Regression of inflammation in atherosclerosis by the LXR agonist R211945: a noninvasive assessment and comparison with atorvastatin. JACC Cardiovasc Imaging. 2012;5:819–828. doi: 10.1016/j.jcmg.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.van Gils JM, et al. The neuroimmune guidance cue netrin-1 promotes atherosclerosis by inhibiting the emigration of macrophages from plaques. Nat Immunol. 2012;13:136–143. doi: 10.1038/ni.2205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Woodfin A, et al. The junctional adhesion molecule JAM-C regulates polarized transendothelial migration of neutrophils in vivo. Nat Immunol. 2011;12:761–769. doi: 10.1038/ni.2062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Randolph GJ. Mechanisms that regulate macrophage burden in atherosclerosis. Circ Res. 2014;114:1757–1771. doi: 10.1161/CIRCRESAHA.114.301174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gautier EL, et al. Local apoptosis mediates clearance of macrophages from resolving inflammation in mice. Blood. 2013;122:2714–2722. doi: 10.1182/blood-2013-01-478206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chistiakov DA, et al. The phenomenon of atherosclerosis reversal and regression: lessons from animal models. Exp Mol Pathol. 2017;102:138–145. doi: 10.1016/j.yexmp.2017.01.013. [DOI] [PubMed] [Google Scholar]
- 50.Fisher EA. Regression of atherosclerosis: the journey from the liver to the plaque and back. Arterioscler Thromb Vasc Biol. 2016;36:226–235. doi: 10.1161/ATVBAHA.115.301926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ravindran D, et al. Chemokine binding protein “M3” limits atherosclerosis in apo lipoprotein E–/– mice. PLoS One. 2017;12:e0173224. doi: 10.1371/journal.pone.0173224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Favari E, et al. Cholesterol efflux and reverse cholesterol transport. Handb Exp Pharmacol. 2015;224:181–206. doi: 10.1007/978-3-319-09665-0_4. [DOI] [PubMed] [Google Scholar]
- 53.Combadiere C, et al. Decreased atherosclerotic lesion formation in CX3CR1/apolipoprotein E double knockout mice. Circulation. 2003;107:1009–1016. doi: 10.1161/01.cir.0000057548.68243.42. [DOI] [PubMed] [Google Scholar]
- 54.Ridker PM. From C-reactive protein to interleukin-6 to interleukin-1: moving upstream to identify novel targets for atheroprotection. Circ Res. 2016;118:145–156. doi: 10.1161/CIRCRESAHA.115.306656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ridker PM, et al. Antiinflammatory therapy with canakinumab for atherosclerotic disease. N Engl J Med. 2017;377:1119–1131. doi: 10.1056/NEJMoa1707914. [DOI] [PubMed] [Google Scholar]
- 56.Braunersreuther V, et al. A novel RANTES antagonist prevents progression of established atherosclerotic lesions in mice. Arterioscler Thromb Vasc Biol. 2008;28:1090–1096. doi: 10.1161/ATVBAHA.108.165423. [DOI] [PubMed] [Google Scholar]
- 57.Poupel L, et al. Pharmacological inhibition of the chemokine receptor, CX3CR1, reduces atherosclerosis in mice. Arterioscler Thromb Vasc Biol. 2013;33:2297–2305. doi: 10.1161/ATVBAHA.112.300930. [DOI] [PubMed] [Google Scholar]
- 58.Tardif JC, et al. Effects of the P-selectin antagonist inclacumab on myocardial damage after percutaneous coronary intervention for non-ST-segment elevation myocardial infarction: results of the SELECT-ACS trial. J Am Coll Cardiol. 2013;61:2048–2055. doi: 10.1016/j.jacc.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 59.Sager HB, et al. RNAi targeting multiple cell adhesion molecules reduces immune cell recruitment and vascular inflammation after myocardial infarction. Sci Transl Med. 2016;8 doi: 10.1126/scitranslmed.aaf1435. 342ra80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Rahman K, et al. Inflammatory Ly6Chi monocytes and their conversion to M2 macrophages drive atherosclerosis regression. J Clin Invest. 2017;127:2904–2915. doi: 10.1172/JCI75005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Viola JR, et al. Resolving lipid mediators maresin 1 and resolvin D2 prevent atheroprogression in mice. Circ Res. 2016;119:1030–1038. doi: 10.1161/CIRCRESAHA.116.309492. [DOI] [PubMed] [Google Scholar]
- 62.Fredman G, et al. An imbalance between specialized pro-resolving lipid mediators and pro-inflammatory leukotrienes promotes instability of atherosclerotic plaques. Nat Commun. 2016;7:12859. doi: 10.1038/ncomms12859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wezel A, et al. Mast cells mediate neutrophil recruitment during atherosclerotic plaque progression. Atherosclerosis. 2015;241:289–296. doi: 10.1016/j.atherosclerosis.2015.05.028. [DOI] [PubMed] [Google Scholar]
- 64.Newby AC. Metalloproteinases promote plaque rupture and myocardial infarction: a persuasive concept waiting for clinical translation. Matrix Biol. 2015:44–46. doi: 10.1016/j.matbio.2015.01.015. 157–166. [DOI] [PubMed] [Google Scholar]
- 65.Landsman L, et al. CX3CR1 is required for monocyte homeostasis and atherogenesis by promoting cell survival. Blood. 2009;113:963–972. doi: 10.1182/blood-2008-07-170787. [DOI] [PubMed] [Google Scholar]
- 66.Woollard KJ, Geissmann F. Monocytes in atherosclerosis: subsets and functions. Nat Rev Cardiol. 2010;7:77–86. doi: 10.1038/nrcardio.2009.228. [DOI] [PMC free article] [PubMed] [Google Scholar]