Abstract
The experience of motherhood is most often emotionally positive and rewarding, but for many new mothers suffering from postpartum depression (PPD) this is not the case. Preclinical and clinical research has sought to uncover brain changes underlying PPD in order to gain a better understanding of how this disorder develops. This review focuses on the mesolimbic dopamine system, particularly the ventral tegmental area-nucleus accumbens pathway which has been implicated in the regulation of critical functions disrupted in PPD including mood, motivation and mothering. Specifically, we discuss normative changes in the mesolimbic system during motherhood in both rodents and humans and how these are impacted in PPD. We also consider modulation of mesolimbic dopamine by the hypothalamic neuropeptide oxytocin and how oxytocin-dopamine interactions regulate mood and mothering during the postpartum period. In addition to providing an overview of reward mechanisms in PPD, our goal is to highlight open questions which warrant further research.
Keywords: depression, dopamine, maternal, mesolimbic, nucleus accumbens, oxytocin, pregnancy, postpartum, striatum
Introduction
Becoming a mother is usually regarded as one of life’s most emotionally positive and rewarding experiences. However, for a significant number of women, the postpartum period can instead be a difficult time accompanied by mental illness. Indeed, recent analyses indicate that at least 15% of new mothers worldwide each year are affected by postpartum depression (PPD) making it the most common complication of childbirth. (Wisner et al. 2013). PPD is detrimental to maternal well-being and is one of the leading causes of maternal mortality resulting from suicide (Lindahl 2005; Osborne and Monk 2013). Further, PPD can compromise mother-infant interactions and as a result, negatively impact the development of the offspring (Grace et al. 2003; Letourneau et al. 2013; Verbeek et al. 2012; Hoffman et al. 2017) which carries a significant economic and social long-term cost to society (Baurer et al. 2015).
Although PPD has been deemed a major public health concern due to its prevalence and the risks it poses to mothers and their children (Wisner et al. 2006; Chesney et al. 2014; Meaney 2018), our current understanding of the underlying neurobiology of PPD remains limited. However, interest in PPD has been growing (Figure 1) with an increasing number of preclinical and clinical studies turning their attention to addressing this important issue. To date, findings from this emerging body of work have revealed that PPD is accompanied by dysregulation of mood-related neural circuits that have also been implicated in maternal caregiving (Pawluski et al. 2017). One such circuit is the mesolimbic reward system, which is the focus of this review (for other circuits, see reviews by Moses-Kolko et al. 2014; Duan et al. 2017; Pawluksi et al. 2017). Specifically, we discuss normative changes in the mesolimbic system during motherhood in both rodents and humans and how these are impacted in PPD. We also consider modulation of mesolimbic dopamine (DA) by the hypothalamic neuropeptide oxytocin (OT) and how OT-DA interactions regulate mood and mothering during the postpartum period. Aside from providing an overview of reward mechanisms in PPD, we highlight areas where further research is necessary.
Figure 1.
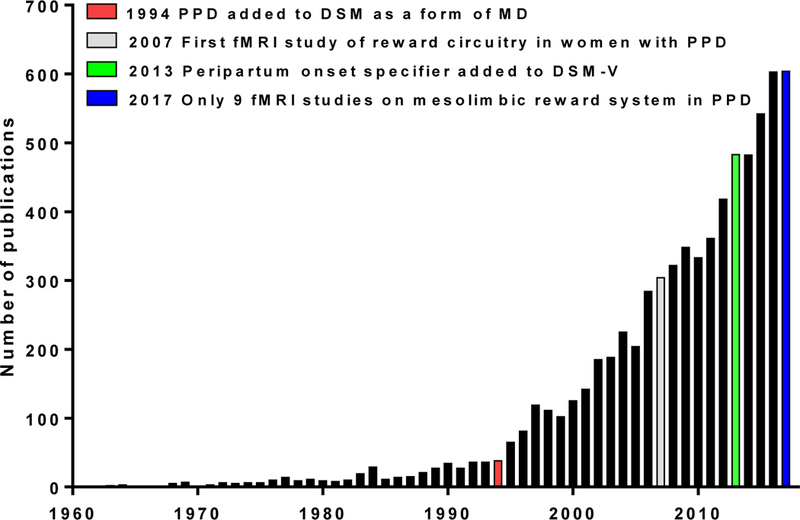
Representation of the number of studies published on postpartum depression (PPD) from 1960–2017. Numbers were generated using “postpartum depression” as a search term in PubMed. The red bar indicates when PPD was added to the DSM in 1994 as a form of major depression (MD). The gray bar represents when functional magnetic resonance imaging (fMRI) was first utilized to study the mesolimbic reward system in PPD (Silverman et al. 2007). In 2013 (green bar), a peripartum onset specifier was added to the DSM-V. As of 2017 (blue bar), there have only been approximately 20 fMRI studies completed on mothers suffering from PPD. Of these, nine showed reward system changes with PPD. Adapted from Li and Chou, 2016.
Defining PPD
After birth, mild and transient disruptions in mood are normative and characteristic of ‘postpartum blues’. PPD however is a clinical condition that is more severe and which, if left untreated, can be long-lasting persisting for many months or even longer as some women with PPD continue to experience elevated levels of depressive symptoms years after childbirth (Vliegen et al. 2014; Netsi et al. 2018). PPD was formally recognized in 1994 when the fourth edition of the Diagnostic and Statistical Manual of Mental Health Disorders (DSM-IV) classified PPD as major depression (MD) with postpartum onset, defined as within the four weeks after delivery. The diagnostic classification of PPD did not change until 2013 when the DSM-V introduced a peripartum onset specifier to account for antenatal onset of depression during pregnancy as well as after birth. Although generally regarded as an improvement, recent data suggests that PPD beginning during pregnancy maybe a distinguishable subtype from PPD that manifests postnatally as there are differences in their symptomology and severity (Altemus et al. 2012; Putnam et al. 2018). Further, many researchers and clinicians consider the four week postpartum onset specifier to be too conservative because depression that begins later than four weeks after delivery may still negatively impact mothers and their children (Murray et al 2011; Verbeek et al., 2012; Letourneau et al. 2013; Stein et al. 2014; Hoffman et al. 2017; Meaney, 2018). As such, in spite of current DSM-V guidelines, time frames that range up to one year postpartum are commonly used in research studies and clinical practice (Gaynes et al. 2005; Wisner et al. 2010; O’Hara and McCabe 2013).
PPD is characterized by low mood and sadness accompanied by anhedonia, impaired concentration, disrupted sleep and appetite, psychomotor disturbance, feelings of worthlessness or guilt, social withdrawal, and recurrent suicidal ideation (Meltzer-Brody et al. 2018). Because these symptoms mimic those of MD, whether PPD is a distinct disorder remains controversial (O’Hara and McCabe 2013; DiFloro and Metlzer-Brody 2015; Pawluski et al. 2017). Several features of PPD however do suggest a certain degree of distinctiveness. First, PPD occurs during a unique time physiologically when there are dramatic endocrine alterations involving steroid and peptide hormones (i.e. estrogens, progesterone, glucocorticoids, OT) as well as significant shifts in the immune profile (Robinson and Klein, 2012; Schiller et al. 2015; Brummelte and Galea 2016). Second, PPD presents with greater co-morbid anxiety with postpartum anxiety often preceding the onset of depression (Prenoveau et al. 2013; Wisner et al. 2013; Farr et al. 2014; Fox et al. 2018). Lastly, and perhaps most importantly, PPD strikes at a critical time when there is the added responsibility of caring for an infant. This can be challenging for depressed mothers who are more likely to partake in unhealthy feeding and sleep practices as compared to mothers without PPD (Field 2010). Along with such compromised caregiving activities, maternal depression can be damaging to mother-infant interactions. Thus, while non-depressed mothers exhibit positive, warm and sensitive caregiving, those with PPD interact with their infant in a way that is either withdrawn, passive, and under-stimulating or intrusive, controlling, and over-stimulating (Jones et al. 2001). Depressed mothers also tend to be more irritable and hostile, less affectionate and less sensitively attuned to their infants (Lovejoy et al. 2000). Maternal interactions in PPD are further characterized by reduced vocal and visual communication, less touch, and less smiling (Righetti-Veltema et al. 2003; Herrera et al. 2004; Granat et al. 2017) which likely contributes to difficulties bonding and disrupted synchrony (i.e. a mother’s capacity to coordinate her behavior with infant signals) (Feldman, 2007). Given that these disturbances occur when attachment processes and the mother-infant relationship shape the cognitive, emotional, and social development of the offspring, the children of depressed mothers are at high risk for experiencing negative outcomes in these domains and these can extend beyond infancy into childhood and late adolescence (Murray et al 2011; Verbeek et al., 2012; Letourneau et al. 2013; Stein et al. 2014; Hoffman et al. 2017; Meaney 2018). The detrimental effects of PPD on offspring have been well studied (Drury et al. 2016), but far less research has investigated the neurobiological sequelae of PPD in the mother.
Animal Models of PPD
Animal models represent a valuable translational tool that have been widely used to investigate the neurobiology of psychiatric disorders, although their use in studies of PPD is far less compared to other conditions such as MD (Perani and Slattery 2014; Li and Chou 2016). One approach to modeling mental illness in rodents is to incorporate known biological, psychosocial and/or other (i.e. environmental, genetic) risk factors. For PPD, endocrine events occurring during the perinatal period are considered to be among the biological factors that contribute to increased susceptibility in some women. These include alterations in the ovarian hormones, estrogen and progesterone, as well as dysregulation of the hypothalamic-pituitary-adrenal (HPA) axis (Glynn et al. 2013; Schiller et al. 2014). As such, there are models of PPD which employ peripartum-related hormonal manipulations involving either withdrawal from ovarian steroids (Galea et al. 2001; Green and Galea 2008; Suda et al. 2008; Navarre et al. 2010; Schiller et al. 2013; Baka et al. 2017) or administration of high dose corticosterone postpartum (Brummelte and Galea 2010) as well as other strategies to interfere with HPA axis function (Melón et al. 2018).
In addition to hormones, numerous psychosocial risk factors for PPD have been identified such as prior history of depression, marital problems, low socioeconomic status, lack of social support/social isolation, history of trauma, and other adverse life events (Robertson et al. 2004; Milgrom et al. 2008; Yim et al. 2015; Biaggi et al. 2016). Common to all of these is the psychological experience of stress and thus, stress-based PPD models have also been developed. These apply various types of stressors (i.e. restraint; restraint and overcrowding or bright light; chronic variable stress), typically for 7–14 days, during pregnancy (Smith et al. 2004; Champagne and Meaney 2006; O’Mahony et al. 2006; Hillerer et al. 2011; Haim et al. 2014; Leuner et al. 2014, 2016; Vanmierlo et al. 2018) or during the postpartum period in the form of repeated maternal separation (Boccia et al. 2007) or social stress (Nephew and Bridges 2011). In another stress-based PPD model, exposure to early life stressful experience is utilized (Nephew et al. 2017a). Recent attempts have also been made to develop models of maternal depression based on high-fat diet/obesity, another factor which increases risk for PPD (Perani et al. 2015; Bolton et al. 2017). Each of these models induces one or more critical aspects of postpartum depressive-like symptomology including behavioral despair, anhedonia, anxiety-like behavior, and/or impaired maternal care which like the human condition can negatively impact offspring neurodevelopment (Smith et al. 2004; Champagne and Meaney 2006; Brummelte et al. 2006; Babb et al. 2014). Some rodent models, like the Flinders sensitive line (FSL) of rats, don’t rely on risk factors but instead these animals are bred for depressive-like behavior which for postpartum females, is accompanied by deficits in maternal care and impaired maternal motivation (Lavi-Avnon et al. 2005, 2008). Lastly, different inbred mouse strains, which exhibit variations in their emotional and maternal phenotype, have been employed to investigate PPD (Avraham et al. 2017).
Like models for other complex psychiatric disorders, those for PPD are not necessarily intended to recapitulate all possible risk factors and the entire symptomology. Nonetheless, they can be used to study certain aspects of the disorder, particularly at cellular, neurochemical, and molecular levels of analyses that may not be as readily feasible in humans. Due to the conservation of major neural, neurotransmitter and neuromodulatory systems between rodents and humans, it is expected that novel mechanistic insights gained from animal models of PPD will be relevant to human mothers.
The Mesolimbic Reward System
The brain undergoes dramatic alterations during pregnancy and the postpartum period that are essential for optimizing emotional well-being and caregiving abilities (Barrett and Fleming 2011; Rilling 2013; Kim et al. 2016; Swain and Ho 2017). Many brain regions and systems exhibit modifications although there is considerable overlap in the neural circuits which regulate mood and various aspects of mothering (Pawluski et al. 2017). Thus, a possible way both depression and maternal disturbances might arise during the postpartum period would be if the normative, adaptive changes in these overlapping circuits failed to occur (Hillerer et al. 2012; Duan et al. 2017). Few studies have explicitly tested this hypothesis by performing direct comparisons of non-mothers to mothers with and without PPD. Even so, the available evidence points to key neural changes during motherhood and in PPD with both human and animal studies implicating the mesolimbic system (Pechtel et al. 2013; Moses-Kolko et al. 2014; Duan et al. 2017; Pawluski et al. 2017).
The mesolimbic system consists primarily of dopaminergic neurons in the ventral tegmental area (VTA) of the midbrain that project to the nucleus accumbens (NAc), part of the ventral striatum. The VTA-NAc circuit plays a well-established role in processing rewards and motivated behavior. DA neurons in the VTA also innervate cortical areas, the amygdala and hippocampus and in doing so link reward processes to cognitive and emotional function (Russo and Nestler 2013). The mesolimbic reward system has been a target in neurobiological investigations of PPD mechanisms based on known dopaminergic/reward changes which occur after parturition, the role of this system healthy caregiving (Stolzenberg and Numan 2011; Moses-Kolko et al. 2014; Swain and Ho 2017) and also because of convergent findings of reward system dysfunction and diminished DA in MD (Dunlop and Nemeroff 2007; Russo and Nestler 2013; Admon and Pizzagalli 2015).
The Reward System in PPD: Humans
The neural correlates of PPD have been investigated using functional magnetic resonance imaging (fMRI) approaches. The first fMRI study of PPD was done by Silverman et al. in 2007 and since that time approximately 20 others have been published (Fiorelli et al. 2015; Duan et al., 2017; Pawluski et al., 2017). Although not all of these were designed to assess the reward system, nine studies to date have identified mesolimbic dysfunction in depressed mothers (Table 1). Consistent with the anhedonia features of PPD, some of this work has provided evidence for a blunted striatal response to positive, rewarding stimuli. For example, positive words (Silverman et al. 2007) were shown to elicit less activation of the striatum in mothers with PPD relative to healthy mothers. Similarly, in response to positive faces, mothers with higher levels of depressive symptoms exhibit reduced striatal activity (Morgan et al., 2017). In other work using a monetary reward task, Moses-Kolko et al. (2011) found that although initial activation of the ventral striatum was similar in depressed and healthy mothers, depressed mothers’ responses rapidly attenuated to baseline while healthy mothers had a sustained response to reward. This rapid attenuation suggests blunted reward function which could contribute to decreased motivation in PPD. However, this may not be the case for all mothers with PPD as a more recent study found that decreased reward-related ventral striatal activity doesn’t generalize to young mothers with less severe depressive symptoms (Moses-Kolko et al. 2016).
Table 1.
Findings of functional magnetic resonance imaging (fMRI) and positron emission tomography (PET) studies on the mesolimbic reward system in PPD. Abbreviations: NAc, nucleus accumbens; MAO-A, monoamine oxidase-A.
| Year | Authors | Sample | Reward region Method Stimulus | Result in mothers with PPD (relative to healthy mothersunless otherwise noted) |
|---|---|---|---|---|
| 2007 | Silverman et al. | PPD women 6–8 weeks postpartum Healthy women 6–8 weeks postpartum |
Striatum fMRI Positive words |
↓ activation |
| 2011 | Moses-Kolko et al. | PPD women <10 weeks postpartum Healthy women <10 weeks postpartum |
Striatum fMRI Monetary reward task |
= Initial activation but ↑ attenuation to baseline |
| 2012 | Moses-Kolko et al. | PPD women early postpartum Healthy women early postpartum Non-postpartum depressed Non-postpartum healthy |
Striatum PET | ↓ D2/3 receptor binding with postpartum state but no differences between depressed and healthy postpartum women |
| 2012 | Laurent and Ablow | Depressed mothers 18 months postpartum Non-depressed mothers 18 months postpartum |
NAc, striatum, OFC fMRIOwn infant cry |
↓ Activation |
| 2013 | Laurent and Ablow | Depressed mothers 18 months postpartum Non-depressed 18 months postpartum |
Striatum, OFC fMRI Own infant joy faces |
↓ Activation |
| 2015 | Sacher et al. | PPD women <18 months postpartum Healthy postpartum women who cry due to sad mood <18 months postpartum Asymptomatic healthy postpartum women <18 months postpartum Healthy non-postpartum women |
Striatum PET | ↑ Increased MAO-A in PPD women and postpartum women who cry |
| 2016 | Moses-Kolko et al. | 17–20 year old mothers and non-mothers varying in levels of depressive symptoms 16 weeks postpartum | Striatum fMRI Monetary reward | ↑ Activity in non-mothers, but ≠ in mothers, with depressive symptoms |
| 2017 | Ho and Swain | Depressed mothers Non-depressed mothers | Functional connectivity fMRI Baby cry task | ↓ Functional connectivity between the NAc and the amygdala in depressed mothers, ↑ in healthy mothers |
| 2017 | Morgan et al. | 18–22 year old mothers varying in levels of depressive symptoms 17 weeks postpartum | Dorsal and ventral striatum fMRI Positive (i.e. happy) adult faces | ↓ Activation in mothers with higher depressive symptoms; for mothers with higher depressive symptoms, ↑ response associated with more positive caregiving, opposite pattern for mothers with lower symptoms |
Another strategy to evaluate the reward system in PPD has been to examine neural responses to positive infant cues – a more motivationally relevant stimulus for mothers. A number of postpartum neuroimaging studies have shown that healthy mothers display increased activation to the smiling face of their own infant as compared to an unknown infant in reward areas including the VTA, ventral striatum as well as the orbitofrontal cortex (OFC) (Bartels and Zeki 2004; Nitschke et al., 2004; Strathearn et al. 2008; Rilling 2013). In contrast, mothers meeting the diagnostic criteria for PPD with ongoing high depressive symptoms were shown to have reduced responses to their own infant’s joy faces in striatal areas and the OFC (Laurent and Ablow 2013). Depressed mothers have difficulty identifying happy affect in their infant’s facial expression which may be a contributing factor (Arteche et al. 2011). Accordingly, mothers struggling with PPD may be less able to respond to their infant’s joy because they experience it as less rewarding which could underlie diminished maternal responsiveness (Laurnet and Ablow 2013). Some evidence suggests that healthy and depressed mothers also process negative infant cues differently. For example, healthy mothers show activation of reward pathways in response to their own infant’s cry (Lorberbaum et al. 2002; Noriuchi et al. 2008), while mothers with PPD exhibit attenuated responses to their own infant cry in the NAc, striatum, and OFC (Laurent and Ablow 2012). As such, depressed mothers may also have a blunted motivational response to approach their crying infants which would further derail mothering.
Given the findings above, it seems plausible that PPD would be accompanied by dopaminergic dysregulation. Using positron emission tomography (PET), Moses-Kolko et al. (2012) investigated striatal DA functioning in PPD reporting lower D2/3 receptor binding with postpartum state but no differences between depressed and healthy postpartum women. The striatum was however identified in another PPD PET study as among the sites of increased monoamine oxidase A (MAO-A) levels which, given the role of this enzyme in monoamine catabolism, could lead to a deficiency in DA (Sacher et al. 2015). Genetic studies of PPD have further implicated MAO-A, as well as cathechol-O-methyltransferase (COMT), an enzyme that like MAO-A inactivates DA (Doornbos et al. 2009; Comasco et al., 2011; Alvim-Soares et al. 2013). More work is needed to further explore whether other aspects of DA signaling are impacted in PPD, perhaps in ways that differ from MD given the hormonal transition and behavioral adaptations unique to the postpartum period (Zsido et al. 2017).
Other studies have sought to establish functional significance of striatal/DA responses to infant stimuli by linking them with observations of mother-infant interactions. Studying non-depressed mothers, Atzil et al. (2011) found that synchronous mothers who coordinate their behavior with infant signals showed greater NAc activation when viewing positive mother-infant interaction clips. A similar pattern was not seen in intrusive mothers. In a subsequent study combining fMRI and PET, high synchronous mothers were shown to have a stronger DA response to their own infant in the NAc while low synchronous mothers did not (Atzil et al. 2017). These data may suggest that synchronous mothers experience the mother-infant interaction as more rewarding than intrusive/low synchronous mothers. Corroborating this work are findings from mothers with attachment disturbances who showed reduced striatal responses to infant stimuli (Strathearn et al., 2009). Recent evidence further indicates that mothers with greater reward circuitry function are those who are able to establish and maintain warm and nurturing relationships with their infant despite psychiatric symptoms (Morgan et al. 2017). Taken together, these findings underscore the importance of dopaminergic reward regions in positive maternal caregiving and maternal attachment while also providing support for the possibility that impaired mothering in PPD results from lower striatal activity/DA function.
One of the main limitations to neuroimaging research has been the focus on seed regions although network based approaches are becoming more prevalent. Functional connectivity analyses have been used to show that maternal bonding behavior relies on the synchronous firing of nucleus accumbens, amygdala and prefrontal cortex as a network (Atzil et al. 2011). Whether disruptions in this network contribute to bonding difficulties in PPD wasn’t examined. However, other work using motivationally salient baby cry stimuli found diminished functional connectivity between the NAc and the extended amygdala in depressed mothers while healthy mothers showed results in the opposite direction (Ho and Swain 2017). Given the roles of extended amygdala for threat processing and the NAc for reward processing, this could represent a biological mechanism underlying the difficulties of depressed mothers to integrate baby-cry distress signal processing with the reward processing needed for sensitive parenting behavior. Healthy mothers, on the other hand, may be better able to activate their NAc during baby-cry distress signaling to motivate caring behaviors for their baby. More studies are needed to better understand the connectivity of the reward network with other networks (Moses-Kolko et al. 2014; Duan et al. 2017).
Human neuroimaging studies of PPD underscore the intricate interplay among maternal mental health, the mother-infant relationship, and the reward system. It should be noted that depressive symptoms in MD are also related to blunted mesolimbic reward function in response to rewarding stimuli and low motivation and pleasure for positive events and interactions (Surguladze et al. 2005; Epsetin et al. 2006; Admon and Pizzagalli, 2015). On the surface this may suggest that at least within the reward system, the neurobiology of MD and PPD are similar. However, more subtle but meaningful, differences may exist. For example, currently available brain imaging methods do not have the resolution to differentiate cell types within reward regions showing activational changes and cannot distinguish between alterations in excitation or inhibition. Thus, the possibility remains that a differential mechanism might underlie reward system function in PPD.
The Reward System in PPD: Animal Models
Few studies using animal models of PPD have focused on the reward system. In some of the only work to date, Haim et al. (2014) found that depressive-like behavior in mothers exposed to chronic gestational stress was associated with compromised neuroplasticity in the NAc including reduced dendritic length, branching, and spine density. These results point to a potential mechanism underlying attenuated neuronal activation and changes in functional connectivity reported in the striatum of mothers with PPD discussed above. Importantly, the neuroplastic changes seen in gestationally stressed mothers differ from what has been reported in the NAc of males (more dendritic spines) and females (no postsynaptic effects) after stress and thus may be a unique feature associated with depressive behavior during the postpartum period (Christoffel et al. 2011; Bessa et al. 2013; Brancato et al. 2017). It was also shown that antidepressant treatment reversed the stress-induced behavioral and morphological alterations (Haim et al. 2014) suggesting that structural modifications in the NAc following gestational stress may contribute to the pathophysiology of PPD as well as its pharmacologically induced recovery.
Models of PPD can also be used to investigate the extent to which reward system dysfunction may contribute to maternal care deficits in PPD (Nephew et al. 2015). A large rodent literature indicates that maternal care is a highly rewarding, motivated behavior. Mother rats will bar press and develop a place preference for pups as they would other rewarding stimuli (Lee al. 2000; Mattson et al. 2001). For mothers, pups are so reinforcing that they are preferred over addictive drugs such as cocaine which have high reward value (Mattson et al. 2001; Ferris et al. 2005). Not surprisingly, reward circuit alterations enable maternal females to respond to offspring as rewarding (Lonstein and Morrell 2007; Pereira and Morrell 2011; Stolzenberg and Numan 2011). Indeed, numerous studies have shown that the mesolimbic system is activated in postpartum rats during maternal interactions (Fleming and Walsh 1994; Hernandez-Gonzalez et al. 2005; Febo 2011; Fang et al., 2018) and within the NAc, altered expression of various reward-related genes occurs postpartum (Zhao et al. 2014). Furthermore, in response to pup stimuli or the expression of maternal behavior, DA levels in the postpartum NAc increase and are correlated with the quality of maternal care displayed (Hansen et al. 1993; Champagne et al. 2004; Afonso et al. 2013; Shnitko et al. 2017). Conversely, maternal care is impaired following NAc or VTA lesions and after VTA inactivation (Hansen et al. 1991a, 1991b; Seip and Morrell 2009; Numan et al. 2009). Additional evidence that mesolimbic DA is essential for maternal responsiveness comes from work demonstrating that DA antagonists administered into the NAc disrupt maternal behavior (Numan et al. 2005). Since place preference for pups is also disrupted by blocking DA systemically or following VTA inactivation (Fleming et al. 1994; Seip and Morrell 2009), a major way DA within the reward system is thought to facilitate maternal behavior by enhancing the incentive value of pups. Together, these results suggest that disrupted maternal care seen in various PPD models may be due to mesolimbic dysregulation and a deficient pup-reward mechanism. Consistent with this are data from FSL mothers which show a lower DA response in the NAc while interacting with pups along with a failure to express a place preference for pups (Lavi-Avnon et al. 2008). Decreased striatal DA has also been observed in BALB/c mice, “poor mothers” that develop depressive-like behavior following pregnancy and delivery (Avraham et al. 2017). Other work using the gestational stress (Leuner et al. 2016) and high-fat diet (Bolton et al. 2017) models of PPD further point to altered DA signaling in the NAc and other VTA targets. DA dysfunction would also be predicted in endocrine models of PPD since sex steroid hormones and stress hormones modulate the reward system and influence reward behavior (Brummlete and Galea 2010; Montoya et al. 2014; Macoveanu et al. 2016), but this hasn’t been examined.
Like human research, most of the animal studies using PPD models focus on discrete brain regions. However, functional connectivity imaging methods have been recently applied to maternal rodents displaying caregiving deficits as a result of early life stress to investigate brain circuits in PPD. These results show reward-related connectivity changes in maternal females as a result of early life stress (Nephew et al. 2018). Early life stress produced different functional connectivity changes in virgin females providing additional support for the possibility of distinct neurobiological features in PPD (Nephew et al. 2017b).
Overall, findings from animal models complement the human literature and suggest that the reward circuit is impacted in PPD. Animal models of PPD further implicate changes in neuronal connectivity, altered neuroplasticity and dopaminergic dysfunction, and thus are beginning to shed light on more specific mechanisms that may be involved, some of which appear to be unique to postpartum females.
Oxytocin-Dopamine Interactions in PPD
The activity of the reward circuitry is regulated by a number of modulators including the neuropeptide OT which has several crucial functions during the peripartum period (Rilling and Young 2014). OT is synthesized in the hypothalamus and released from the pituitary into the periphery where it acts as a hormone at the end of pregnancy to stimulate parturition as well as during the postpartum period to promote lactation. Besides peripheral actions, OT is released intracerebrally (Leng et al. 2008) and via interactions with mesolimbic DA system regulates maternal care by making offspring rewarding (Stolzenberg and Numan 2011; Love 2014; Olazábal 2018). This is accomplished via projections from hypothalamic OT neurons to the VTA which expresses OT receptors (OTR) and where OT enhances the activity of DA neurons to stimulate DA release (Shahrokh et al. 2010; Beier et al. 2015; Song et al. 2016; Hung et al. 2017; Peris et al. 2017). Behavioral studies have provided a functional link among OT, DA, and maternal care − mother rats displaying greater levels of maternal care have more OT projections to the VTA whereas blockade of OTR in the VTA disrupts maternal behavior (Pedersen et al. 1994; Shahrokh et al. 2010).
Parallel investigations in humans have associated higher peripheral OT levels with better mothering (Levine et al. 2007; Galbally et al. 2011; Feldman and Bakermans-Kranenburg 2017; Kohlhoff et al. 2017) and genetic studies have linked variations in the OTR gene to maternal caregiving (Bakermans-Kranenburg et al. 2008; Feldmam et al 2012; Mileva-Seitz et al. 2013; Tombeau Cost et al. 2017). Several neuroimaging studies also suggest interactions between OT and the DA reward system in the regulation of mothering. For example, mothers that deliver vaginally, which involves a substantial OT surge and enhanced bonding, display increased neural activation in the striatum compared to mothers who deliver by Cesarean-section (Swain et al. 2008). Breastfeeding also increases OT levels and elevates striatal activity when mothers hear the cry of their own infant (Kim et al. 2011). Moreover, in synchronous mothers and those with greater maternal attachment, there is a positive correlation between levels of circulating OT and NAc activation when viewing infant stimuli (Strathearn et al. 2009; Atzil et al. 2011). OT administration has also been shown to increase activation of the VTA in response to infant stimuli (Gregory et al. 2015). Collectively, the human data align with the rodent findings by suggesting that OT via actions in the reward system supports maternal caregiving.
In addition to maternal behavior, OT has also been implicated in mood regulation (Neumann and Landgraf 2012). In rodents, OT has antidepressant and anxiolytic properties although the relationship between OT and emotion in humans remains unclear as discrepant findings have been reported (Slattery and Neumann 2010; McQuaid et al. 2014; Massey et al. 2016a). For PPD, some studies have shown an inverse relationship between peripheral OT levels and maternal depression such that mothers with higher OT levels during pregnancy or postpartum present less depressive symptoms (Skrundz et al. 2011; Apter-Levy et al. 2013; Stuebe et al. 2013, Eapen et al. 2014; Jobst et al. 2016). Depressed mothers often have difficulties nursing and are more likely to stop nursing earlier, which may indicate a common pathogenesis involving diminished OT (Steube et al. 2013). In other research, however, no association was found between OT and maternal depressive symptoms although higher OT was associated with lower depressive symptomology exclusively in mothers with greater psychosocial stress, suggesting that OT may protect women in stressful situations from developing depression (Garfield et al. 2015; Zelkowitz et al. 2014). Adding to the studies measuring peripheral OT levels, complementary studies have associated PPD with genetic and epigenetic variations in the genes for OT as well as the OTR (Apter-Levy et al. 2013; Jonas et al. 2013; Mileva-Setiz et al. 2013; Bell et al. 2015; Kimmel et al. 2016; King et al. 2017).
The limitation of all human OT studies is that analyses are done on peripheral samples which may not accurately reflect changes that occur in the brain. Non-invasive measurement of central OT isn’t feasible in humans and despite some recent advances, OTR currently cannot be quantified in the living human brain (Smith et al. 2016). With animal models however such analyses are possible and, as previously discussed, have provided much insight into the role of the central OT system in maternal care. Animal models are also beginning to shed light on OT mechanisms in PPD (Figure 2). In both gestational stress and chronic social stress rodent models of PPD, OT gene expression is reduced in the hypothalamus (Hillerer et al. 2011; Murgatroyd and Nephew 2013; Wang et al. 2018) suggestive of less OT availability in areas receiving OT input including the reward system. Fewer OT fibers and lower OTR expression has also been found in the VTA of gestationally stressed mothers (Leuner et al. 2016), another indicator of diminished OT signaling. Further research is necessary to more fully characterize how the OT system is affected in PPD (Moura et al. 2016) and how this in turn impacts reward system functioning to ultimately influence depressive and maternal behavior.
Figure 2.
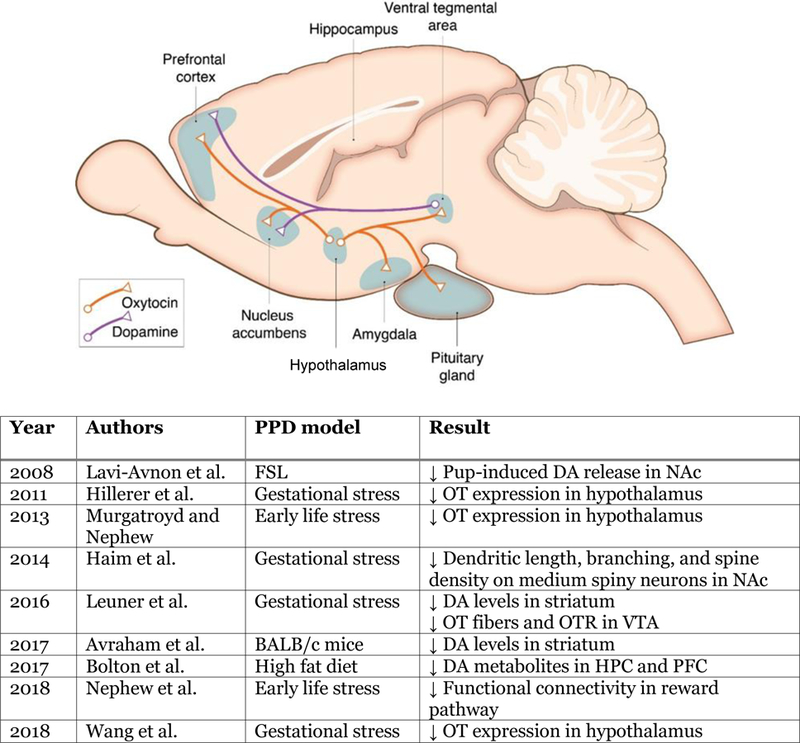
Simplified schematic diagram of dopamine (DA) and oxytocin (OT) neurocircuitry and findings from animal models of PPD investigating this circuit. Abbreviations: HPC, hippocampus; NAc, nucleus accumbens; PFC, Prefrontal cortex; VTA, ventral tegmental area.
Conclusions
Converging evidence from both clinical and preclinical studies implicate the mesolimbic dopaminergic system as a critical node of dysfunction in PPD. Consequently, treatments augmenting the reward system may be effective in improving mood and maternal functioning in mothers suffering with PPD and in doing so prevent the detrimental effects on the offspring. In this regard, OT has potential but its role as a therapeutic tool for the treatment of PPD is still unclear and requires further study (Kim et al. 2014; Mah 2016; Moura et al. 2016; Wang et al. 2018). Another potential strategy involves reward-based psychotherapy as behavioral therapy explicitly encouraging patients to engage in rewarding activities during treatment has been found to be effective in MD, potentially by affecting striatal response to reward (Dichter et al. 2009). Whether this treatment, like others (Swain et al., 2017), would be beneficial to women suffering from PPD hasn’t been examined but warrants investigation. It is also expected that as our understanding of the neurobiological underpinnings of PPD continues to grow, novel targets for intervention within the reward system will likely be uncovered.
Acknowledgments
Funding
This work was funded by The Eunice Kennedy Shriver National Institute of Child Health and Human Development (R21HD083791).
Footnotes
Conflict of Interest: The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
References
- Admon R, Pizzagalli DA (2015) Dysfunctional Reward Processing in Depression. Curr Opin Psychol 4:114–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Afonso VM, Shams WM, Jin D, Fleming AS (2013) Distal pup cues evoke dopamine responses in hormonally primed rats in the absence of pup experience or ongoing maternal behavior. J Neurosci 33(6):2305–2312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altemus M, Neeb CC, Davis A, Occhiogrosso M, Nguyen T, Bleiberg KL (2012) Phenotypic differences between pregnancy-onset and postpartum-onset major depressive disorder. J Clin Psychiatry 73(12):e1485–1491. [DOI] [PubMed] [Google Scholar]
- Alvim-Soares A, Miranda D, Campos SB, Figueira P, Romano-Silva MA, Correa H (2013) Postpartum depression symptoms associated with Val158Met COMT polymorphism. Arch Womens Ment Health 16(4):339–340. [DOI] [PubMed] [Google Scholar]
- Apter-Levy Y, Feldman M, Vakart A, Ebstein RP, Feldman R (2013) Impact of maternal depression across the first 6 Years of life on the child’s mental health, social engagement, and empathy: the moderating role of oxytocin. A J Psychiatry 170(10):1161–1168. [DOI] [PubMed] [Google Scholar]
- Arteche A, Joormann J, Harvey A, Craske M, Gotlib IH, Lehtonen A, Counsell N, Stein A (2011) The effects of postnatal maternal depression and anxiety on the processing of infant faces. J Affect Disord 133(1–2):197–203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atzil S, Hendler T, Feldman R (2011) Specifying the neurobiological basis of human attachment: brain, hormones, and behavior in synchronous and intrusive mothers. Neuropsychopharmacology 36(13):2603–2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atzil S, Touroutoglou A, Rudy T, Salcedo S, Feldman R, Hooker JM, Dickerson BC, Catana C, Barrett LF (2017) Dopamine in the medial amygdala network mediates human bonding. Proc Natl Acad Sci USA 114(9):2361–2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avraham Y, Hants Y, Vorobeiv L, Staum M, Abu Ahmad W, Mankuta D, Galun E, Arbel-Alon S (2017) Brain neurotransmitters in an animal model with postpartum depressive-like behavior. Behav Brain Res 326:307–321. [DOI] [PubMed] [Google Scholar]
- Babb JA, Carini LM, Spears SL, Nephew BC (2014) Transgenerational effects of social stress on social behavior, corticosterone, oxytocin, and prolactin in rats. Horm Behav 65(4):386–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baka J, Csakvari E, Huzian O, Dobos N, Siklos L, Leranth C, MacLusky NJ, Duman RS, Hajszan T (2017) Stress induces equivalent remodeling of hippocampal spine synapses in a simulated postpartum environment and in a female rat model of major depression. Neuroscience 343:384–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakermans-Kranenburg MJ, van Ijzendoorn MH (2008) Oxytocin receptor (OXTR) and serotonin transporter (5-HTT) genes associated with observed parenting. Soc Cogn Affect Neurosci 3(2):128–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett J, Fleming AS (2011) Annual Research Review: All mothers are not created equal: neural and psychobiological perspectives on mothering and the importance of individual differences. J Child Psychol Psychiatry 52(4):368–397. [DOI] [PubMed] [Google Scholar]
- Bartels A, Zeki S (2004) The neural correlates of maternal and romantic love. Neuroimage 21(3):1155–1166. [DOI] [PubMed] [Google Scholar]
- Baurer A, Knapp M, Parsonage M (2015) Lifetime costs of perintal anxiety and depression. J Affect Disord 192:83–90. [DOI] [PubMed] [Google Scholar]
- Beier KT, Steinberg EE, DeLoach KE, Xie S, Miyamichi K, Schwarz L, Gao XJ, Kremer EJ, Malenka RC, Luo L (2015) Circuit architecture of VTA dopamine neurons revealed by systematic input-output mapping. Cell 162(3):622–634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell AF, Carter CS, Steer CD, Golding J, Davis JM, Steffen AD, Rubin LH, Lillard TS, Gregory SP, Harris JC, Connelly JJ (2015) Interaction between oxytocin receptor DNA methylation and genotype is associated with risk of postpartum depression in women without depression in pregnancy. Front Genet 6:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biaggi A, Conroy S, Pawlby S, Pariante CM (2016) Identifying the women at risk of antenatal anxiety and depression: A systematic review. J Affect Disord 191:62–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bessa JM, Morais M, Marques F, Pinto L, Palha JA, Almeida OF, Sousa N (2013) Stress-induced anhedonia is associated with hypertrophy of medium spiny neurons of the nucleus accumbens. Transl Psychiatry 3:e266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boccia ML, Razzoli M, Vadlamudi SP, Trumbull W, Caleffie C, Pedersen CA (2007) Repeated long separations from pups produce depression-like behavior in rat mothers. Psychoneuroendocrinology 32(1):65–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolton JL, Wiley MG, Ryan B, Truong S, Strait M, Baker DC, Yang NY, Ilkayeva O, O’Connell TM, Wroth SW, Sánchez CL, Swamy G, Newgard C, Kuhn C, Bilbo SD, Simmons LA (2017) Perinatal western-type diet and associated gestational weight gain alter postpartum maternal mood. Brain Behav 7(10):e00828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brancato A, Bregman D, Ahn HF, Pfau ML, Menard C, Cannizzaro C, Russo SJ, Hodes GE (2017) Sub-chronic variable stress induces sex-specific effects on glutamatergic synapses in the nucleus accumbens. Neuroscience 350:180–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummelte S, Galea LA (2010) Chronic corticosterone during pregnancy and postpartum affects maternal care, cell proliferation and depressive-like behavior in the dam. Horm Behav 58(5):769–779. [DOI] [PubMed] [Google Scholar]
- Brummelte S, Galea LA (2016) Postpartum depression: etiology, treatment and consequences for maternal care. Horm Behav 77:153–166. [DOI] [PubMed] [Google Scholar]
- Champagne FA, Chretien P, Stevenson CW, Zhang TY, Gratton A, Meaney MJ (2004) Variations in nucleus accumbens dopamine associated with individual differences in maternal behavior in the rat. J Neurosci 24(17):4113–4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champagne FA, Meaney MJ (2006) Stress during gestation alters postpartum maternal care and the development of the offspring in a rodent model. Biol Psychiatry 59(12):1227–1235. [DOI] [PubMed] [Google Scholar]
- Chesney E, Goodwin GM, Fazel S (2014) Risks of all-cause and suicide mortality in mental disorders: a meta-review. World Psychiatry 13(2):153–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoffel DJ, Golden SA, Dumitriu D, Robison AJ, Janssen WG, Ahn HF, Krishnan V, Reyes CM, Han MH, Ables JL, Eisch AJ, Dietz DM, Ferguson D, Neve RL, Greengard P, Kim Y, Morrison JH, Russo SJ (2011) IκB kinase regulates social defeat stress-induced synaptic and behavioral plasticity. J Neurosci 31(1):314–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comasco E, Sylvén SM, Papadopoulos FC, Sundström-Poromaa I, Oreland L, Skalkidou A (2011) Postpartum depression symptoms: a case-control study on monoaminergic functional polymorphisms and environmental stressors. Psychiatr Genet 21(1):19–28. [DOI] [PubMed] [Google Scholar]
- Dichter GS, Felder JN, Petty C, Bizzell J, Ernst M, Smoski MJ (2009) The effects of psychotherapy on neural responses to rewards in major depression. Biol Psychiatry 66(9):886–897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Florio A, Meltzer-Brody S (2015) Is postpartum depression a distinct disorder? Curr Psychiatry Rep 17(10):76. [DOI] [PubMed] [Google Scholar]
- Doornbos B, Dijck-Brouwer DA, Kema IP, Tanke MA, van Goor SA, Muskiet FA, Korf J (2009) The development of peripartum depressive symptoms is associated with gene polymorphisms of MAOA, 5-HTT and COMT. Prog Neuropsychopharmacol Biol Psychiatry 33(7):1250–1254. [DOI] [PubMed] [Google Scholar]
- Drury SS, Scaramella L, Zeanah CH (2016) The Neurobiological Impact of Postpartum Maternal Depression: Prevention and Intervention Approaches. Child Adolesc Psychiatr Clin N Am 25(2):179–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duan C, Cosgrove J, Deligiannidis KM (2017) Understanding peripartum depression through neuroimaging: a review of structural and functional connectivity and molecular imaging research. Curr Psychiatry Rep 19(10):70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunlop BW, Nemeroff CB (2007) The role of dopamine in the pathophysiology of depression. Arch Gen Psychiatry 64(3):327–337. [DOI] [PubMed] [Google Scholar]
- Eapen V, Dadds M, Barnett B, Kohlhoff J, Khan F, Radom N, Silove DM (2014) Separation Anxiety, Attachment and Inter-Personal Representations: Disentangling the Role of Oxytocin in the Perinatal Period. PLoS One:9(9), e107745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein J, Pan H, Kocsis JH, Yang Y, Butler T, Chusid J, Hochberg H, Murrough J, Strohmayer E, Stern E, Silbersweig DA (2006) Lack of ventral striatal response to positive stimuli in depressed versus normal subjects. Am J Psychiatry 163(10):1784–1790. [DOI] [PubMed] [Google Scholar]
- Fang YY, Yamaguchi T, Song SC, Tritsch NX, Lin D (2018) A Hypothalamic Midbrain Pathway Essential for Driving Maternal Behaviors. Neuron 98(1):192–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farr SL, Dietz PM, O’Hara MW, Burley K, Ko JY (2014) Postpartum anxiety and comorbid depression in a population-based sample of women. J Womens Health 23(2):120–128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Febo M (2011) A bold view of the lactating brain: functional magnetic resonance imaging studies of suckling in awake dams. J Neuroendocrinol 23(11):1009–1119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedlman R (2007) Parent-infant synchrony and the construction of shared timing; physiological precursors, developmental outcomes, and risk conditions. J Child Psychol Psychiatry 48(3–4):329–54. [DOI] [PubMed] [Google Scholar]
- Feldman R, Zagoory-Sharon O, Weisman O, Schneiderman I, Gordon I, Maoz R, Shalev I, Ebstein RP (2012) Sensitive parenting is associated with plasma oxytocin and polymorphisms in the OXTR and CD38 genes. Biol Psychiatry 72(3):175–81. [DOI] [PubMed] [Google Scholar]
- Feldman R, Bakermans-Kranenburg MJ (2017) Oxytocin: a parenting hormone. Curr Opin Psychol 15:13–18. [DOI] [PubMed] [Google Scholar]
- Ferris CF, Kulkarni P, Sullivan JM Jr, Harder JA, Messenger TL, Febo M (2005) Pup suckling is more rewarding than cocaine: evidence from functional magnetic resonance imaging and three-dimensional computational analysis. J Neurosci 25(1):149–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field T (2010) Postpartum depression effects on early interactions, parenting, and safety practices: a review. Infant Behav Dev 33(1):1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorelli M, Aceti F, Marini I, Giacchetti N, Macci E, Tinelli E, Calistri V, Meuti V, Caramia F, Biondi M (2015) Magnetic Resonance Imaging Studies of Postpartum Depression: An Overview. Behav Neurol 2015:913843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming AS, Walsh C (1994) Neuropsychology of maternal behavior in the rat: c-fos expression during mother-litter interactions. Psychoneuroendocrinology 19(5 7):429–443. [DOI] [PubMed] [Google Scholar]
- Fleming AS, Korsmit M, Deller M (1994) Rat pups are potent reinforcers to the maternal animal: effects of experience, parity, hormones, and dopamine function. Psychobiol 22:44–53. [Google Scholar]
- Fox M, Sandman CA, Davis EP, Glynn LM (2018) A longitudinal study of women’s depression symptom profiles during and after the postpartum phase. Depress Anxiety 35(4):292–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea LA, Wide JK, Barr AM (2001) Estradiol alleviates depressive-like symptoms in a novel animal model of post-partum depression. Behav Brain Res 122(1):1–9. [DOI] [PubMed] [Google Scholar]
- Galbally M, Lewis AJ, van Ijzendoorn M, Permezel M (2011) The role of oxytocin in mother-infant relations: a systematic review of human studies. Harv Rev Psychiatry 19(1):1–14. [DOI] [PubMed] [Google Scholar]
- Garfield L, Giurgescu C, Carter CS, Holditch-Davis D, McFarlin BL, Schwertz D, Seng JS, White-Traut R (2015) Depressive symptoms in the second trimester relate to low oxytocin levels in African American women: a pilot. Arch Womens Ment Health 18(1): 123–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaynes BN, Gavin N, Meltzer-Brody S, Lohr KN, Swinson T, Gartlehner G, Brody S, Miller WC (2005) Perinatal depression: prevalence, screening accuracy, and screening outcomes. Evid Rep Technol Assess (Summ) 119:1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glynn LM, Davis EP, Sandman CA (2013) New insights into the role of perinatal HPA-axis dysregulation in postpartum depression. Neuropeptides 47(6):363–370. [DOI] [PubMed] [Google Scholar]
- Grace SL, Evindar A, Steward DE (2003) The effect of postpartum depression on child cognitive development and behavior: a review and critical analysis of the literature. Arch Womens Ment Health 6(4):263–274. [DOI] [PubMed] [Google Scholar]
- Granat A, Gadassi R, Gilboa-Schechtman E, Feldman R (2017) Maternal depression and anxiety, social synchrony, and infant regulation of negative and positive emotions. Emotion 17(1):11–27. [DOI] [PubMed] [Google Scholar]
- Green AD, Galea LA (2008) Adult hippocampal cell proliferation is suppressed with estrogen withdrawal after a hormone-simulated pregnancy. Horm Behav 54:203–211. [DOI] [PubMed] [Google Scholar]
- Gregory R, Cheng H, Rupp HA, Sengelaub DR, Heiman JR (2015) Oxytocin increases VTA activation to infant and sexual stimuli in nulliparous and postpartum women. Horm Behav 69:82–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haim A, Sherer M, Leuner B (2014) Gestational stress induces persistent depressive-like behavior and structural modifications within the postpartum nucleus accumbens. Eur J Neurosci 40(12):3766–3773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen S, Harthon C, Wallin E, Löfberg L, Svensson K (1991a) The effects of 6-OHDA-induced dopamine depletions in the ventral or dorsal striatum on maternal and sexual behavior in the female rat. Pharmacol Biochem Behav 39(1):71–77. [DOI] [PubMed] [Google Scholar]
- Hansen S, Harthon C, Wallin E, Löfberg L, Svensson K (1991b) Mesotelencephalic dopamine system and reproductive behavior in the female rat: effects of ventral tegmental 6-hydroxydopamine lesions on maternal and sexual responsiveness. Behav Neurosci 105(4):588–598. [DOI] [PubMed] [Google Scholar]
- Hansen S, Bergvall AH, Nyiredi S (1993) Interaction with pups enhances dopamine release in the ventral striatum of maternal rats: a microdialysis study. Pharmacol Biochem Behav 45(3):673–676. [DOI] [PubMed] [Google Scholar]
- Hernández-González M, Navarro-Meza M, Prieto-Beracoechea CA, Guevara MA (2005) Electrical activity of prefrontal cortex and ventral tegmental area during rat maternal behavior. Behav Processes 70(2):132–143. [DOI] [PubMed] [Google Scholar]
- Herrera E, Reissland N, Shepherd J (2004) Maternal touch and maternal child-directed speech: effects of depressed mood in the postnatal period. J Affect Disord 81(1):29–39. [DOI] [PubMed] [Google Scholar]
- Hillerer KM, Reber SO, Neumann ID, Slattery DA (2011) Exposure to chronic pregnancy stress reverses peripartum-associated adaptations: implications for postpartum anxiety and mood disorders. Endocrinology 152(10):3930–3940. [DOI] [PubMed] [Google Scholar]
- Hillerer KM, Neumann ID, Slattery DA (2012) From stress to postpartum mood and anxiety disorders: how chronic peripartum stress can impair maternal adaptations. Neuroendocrinology 95(1):22–38. [DOI] [PubMed] [Google Scholar]
- Ho SS, Swain JE (2017) Depression alters maternal extended amygdala response and functional connectivity during distress signals in attachment relationship. Behav Brain Res 325(Pt B):290–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman C, Dunn DM, Njoroge WFM (2017) Impact of postpartum mental illness upon infant development. Curr Psychiatry Rep 19(12):100. [DOI] [PubMed] [Google Scholar]
- Hung LW, Neuner S, Polepalli JS, Beier KT, Wright M, Walsh JJ, Lewis EM, Luo L, Deisseroth K, Dölen G, Malenka RC (2017) Gating of social reward by oxytocin in the ventral tegmental area. Science 357(6358):1406–1411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jobst A, Krause D, Maiwald C, Härtl K, Myint AM, Kästner R, Obermeier M, Padberg F, Brücklmeier B, Weidinger E, Kieper S, Schwarz M, Zill P, Müller N (2016) Oxytocin course over pregnancy and postpartum period and the association with postpartum depressive symptoms. Arch Womens Ment Health 19(4):571–579. [DOI] [PubMed] [Google Scholar]
- Jonas W, Mileva-Seitz V, Girard AW, Bisceglia R, Kennedy JL, Sokolowski M, Meaney MJ, Fleming AS, Steiner M; MAVAN Research Team (2013) Genetic variation in oxytocin rs2740210 and early adversity associated with postpartum depression and breastfeeding duration. Genes Brain Behav 12(7):681–694. [DOI] [PubMed] [Google Scholar]
- Kim P, Feldman R, Mayes LC, Eicher V, Thompson N, Leckman JF, Swain JE (2011) Breastfeeding, brain activation to own infant cry, and maternal sensitivity. J Child Psychol Psychiatry 52(8):907–915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Soeken TA, Cromer SJ, Martinez SR, Hardy LR, Strathearn L (2014) Oxytocin and postpartum depression: delivering on what’s known and what’s not. Brain Res 1580:219–232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim P, Strathearn L, Swain JE (2016) The maternal brain and its plasticity in humans. Horm Behav 77:113–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimmel M, Clive M, Gispen F, Guintivano J, Brown T, Cox O, Beckmann MW, Kornhuber J, Fasching PA, Osborne LM, Binder E, Payne JL, Kaminsky Z (2016) Oxytocin receptor DNA methylation in postpartum depression. Psychoneuroendocrinology 69:150–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King L, Robins S, Chen G, Yerko V, Zhou Y, Nagy C, Feeley N, Gold I, Hayton B, Turecki G, Zelkowitz P (2017) Perinatal depression and DNA methylation of oxytocin-related genes: a study of mothers and their children. Horm Behav 96:84–94. [DOI] [PubMed] [Google Scholar]
- Kohlhoff J, Eapen V, Dadds M, Khan F, Silove D, Barnett B (2017) Oxytocin in the postnatal period: Associations with attachment and maternal caregiving. Compr Psychiatry 76:56–68. [DOI] [PubMed] [Google Scholar]
- Laurent HK, Ablow JC (2012) A cry in the dark: depressed mothers show reduced neural activation to their own infant’s cry. Soc Cogn Affect Neurosci 7(2):125–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurent HK, Ablow JC (2013) A face a mother could love: depression-related maternal neural responses to infant emotion faces. Soc Neurosci 8(3):228–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavi-Avnon Y, Shayit M, Yadid G, Overstreet HD, Weller A (2005) Immobility in the swim test and observations of maternal behavior in lactating Flinders sensitive line rats. Behav Brain Res 161(1):155–163. [DOI] [PubMed] [Google Scholar]
- Lavi-Avnon Y, Weller A, Finberg JP, Gispan-Herman I, Kinor N, Stern Y, Schroeder M, Gelber V, Bergman SY, Overstreet DH, Yadid G (2008) The reward system and maternal behavior in an animal model of depression: a microdialysis study. Psychopharmacology 196(2):281–291. [DOI] [PubMed] [Google Scholar]
- Lee A, Clancy S, Fleming AS (2000) Mother rats bar-press for pups: effects of lesions of the MPOA and limbic sites on maternal behavior and operant responding for pup-reinforcement. Behav Brain Res 100(1–2):15–31. [DOI] [PubMed] [Google Scholar]
- Leng G, Meddle SL, Douglas AJ (2008) Oxytocin and the maternal brain. Curr Opin Pharmacol 8(6):731–734. [DOI] [PubMed] [Google Scholar]
- Letourneau NL, Tramonte L, Willms JD (2013) Maternal depression, family functioning and children’s longitudinal development. J Pediatr Nurs 28(3): 223–234. [DOI] [PubMed] [Google Scholar]
- Leuner B, Fredericks PJ, Nealer C, Albin-Brooks C (2014) Chronic gestational stress leads to depressive-like behavior and compromises medial prefrontal cortex structure and function during the postpartum period. PLoS One 9(3):e89912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leuner B, Haim A, Albin-Brooks C, Julian D, Springer B, Brothers H (2016) Gestational stress effects on dopamine and oxytocin within the postpartum reward circuitry: implications for mood and mothering. Program No. 338.06, 2016 Neuroscience Meeting Planner San Diego, CA: Society for Neuroscience, Online. [Google Scholar]
- Levine A, Zagoory-Sharon O, Feldman R, Weller A (2007) Oxytocin during pregnancy and early postpartum: individual patterns and maternal–fetal attachment. Peptides 28(6):1162–1169. [DOI] [PubMed] [Google Scholar]
- Li M, Chou SY (2016) Modeling postpartum depression in rats: theoretic and methodological issues. Dongwuxue Yanjiu 37(4):229–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl V, Pearson JL, Colpe L (2005) Prevalence of suicidality during pregnancy and the postpartum. Arch Womens Ment Health 8(2):77–87. [DOI] [PubMed] [Google Scholar]
- Lonstein JS, Morrell JI (2007) Neuroendocrinology and Neurochemistry of Maternal Motivation and Behavior. Handbook of Neurochemistry and Molecular Neurobiology, pp.197–229.
- Lorberbaum JP, Newman JD, Horwitz AR, Dubno JR, Lydiard RB, Hamner MB, Bohning DE, George MS (2002) A potential role for thalamocingulate circuitry in human maternal behavior. Biol Psychiatry 51: 431–445. [DOI] [PubMed] [Google Scholar]
- Love TM (2014) Oxytocin, motivation and the role of dopamine. Pharmacol Biochem Behav 119:49–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovejoy MC, Graczyk PA, O’Hare E, Neuman G (2000) Maternal depression and parenting behavior: a meta-analytic review. Clin Psychol Rev 20(5):561–592. [DOI] [PubMed] [Google Scholar]
- Macoveanu J, Henningsson S, Pinborg A, Jensen P, Knudsen GM, Frokjaer VG, Siebner HR (2016) Sex-steroid hormone manipulation reduces brain response to reward. Neuropsychopharmacology 41, 1047–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mah BL (2016) Oxytocin, postnatal pepression, and parenting: a systematic review. Harv Rev Psychiatry 24(1):1–13. [DOI] [PubMed] [Google Scholar]
- Massey SH, Backes KA, Schuette SA (2016a) Plasma oxytocin concentration and depressive symptoms: a review of current evidence and directions for future research. Depress Anxiety 33(4):316–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massey SH, Schuette SA, Pournajafi-Nazarloo H, Wisner KL, Carter CS (2016b) Interaction of oxytocin level and past depression may predict postpartum depressive symptom severity. Arch Womens Ment Health 19(5):799–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson BJ, Williams S, Rosenblatt JS, Morrell JI (2001) Comparison of two positive reinforcing stimuli: pups and cocaine throughout the postpartum period. Behav Neurosci 115(3):683–694. [DOI] [PubMed] [Google Scholar]
- McQuaid RJ, McInnis OA, Abizaid A, Anisman H (2014) Making room for oxytocin in understanding depression. Neurosci Biobehavl Rev 45: 305–322. [DOI] [PubMed] [Google Scholar]
- Meaney MJ (2018) Perinatal maternal depressive symptoms as an issue for population health. Am J Psychiatry, in press. [DOI] [PubMed]
- Melón LC, Hooper A, Yang X, Moss SJ, Maguire J (2018) Inability to suppress the stress-induced activation of the HPA axis during the peripartum period engenders deficits in postpartum behaviors in mice. Psychoneuroendocrinology 90:182–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer-Brody S, Howard LM, Bergink V, Vigod S, Jones I, Munk-Olsen T, Honikman S, Milgrom J (2018) Postpartum psychiatric disorders. Nat Rev Dis Primers 4:18022. [DOI] [PubMed] [Google Scholar]
- Mileva-Seitz V, Steiner M, Atkinson L, Meaney MJ, Levitan R, Kennedy JL, Sokolowski MB, Fleming AS (2013) Interaction between oxytocin genotypes and early experience predicts quality of mothering and postpartum mood. PLoS One 8(4):e61443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milgrom J, Gemmill AW, Bilszta JL, Hayes B, Barnett B, Brooks J, Ericksen J, Ellwood D, Buist A (2008) Antenatal risk factors for postnatal depression: a large prospective study. J Affect Disord 108(1–2):147–157. [DOI] [PubMed] [Google Scholar]
- Montoya ER, Bos PA, Terburg D, Rosenberger LA, van Honk J (2014) Cortisol administration induces global down-regulation of the brain’s reward circuitry. Psychoneuroendocrinology 47:31–42. [DOI] [PubMed] [Google Scholar]
- Morgan JK, Guo C, Moses-Kolko EL, Phillips ML, Stepp SD, Hipwell AE (2017) Postpartum depressive symptoms moderate the link between mothers’ neural response to positive faces in reward and social regions and observed caregiving. Soc Cogn Affect Neurosci 12(10):1605–1613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses-Kolko EL, Fraser D, Wisner KL, James JA, Saul AT, Fiez JA, Phillips ML (2011) Rapid habituation of ventral striatal response to reward receipt in postpartum depression. Biol Psychiatry 70(4):395–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses-Kolko EL, Price JC, Wisner KL, Hanusa BH, Meltzer CC, Berga SL, Grace AA, di Scalea TL, Kaye WH, Becker C, Drevets WC (2012) Postpartum and depression status are associated with lower [[¹¹C]raclopride BP(ND) in reproductive-age women. Neuropsychopharmacology 37(6):1422–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses-Kolko EL, Horner MS, Phillips ML, Hipwell AE, Swain JE (2014) In search of neural endophenotypes of postpartum psychopathology and disrupted maternal caregiving. J Neuroendocrinol 26(10):665–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moses-Kolko EL, Forbes EE, Stepp S, Fraser D, Keenan KE, Guyer AE, Chase HW, Phillips ML, Zevallos CR, Guo C, Hipwell AE (2016) The influence of motherhood on neural systems for reward processing in low income, minority, young women. Psychoneuroendocrinology 66:130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moura D, Canavarro MC, Figueiredo-Braga M (2016) Oxytocin and depression in the perinatal period-a systematic review. Arch Womens Ment Health 19(4):561–70. [DOI] [PubMed] [Google Scholar]
- Murgatroyd CA, Nephew BC (2013) Effects of early life social stress on maternal behavior and neuroendocrinology. Psychoneuroendocrinology 38(2):219–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray L, Arteche A, Fearon P, Halligan S, Goodyer I, Cooper P (2011) Maternal postnatal depression and the development of depression in offspring up to 16 years of age. J Am Acad Child Adolesc Psychiatry 50(5):460–467 [DOI] [PubMed] [Google Scholar]
- Navarre BM, Laggart JD, Craft RM (2010) Anhedonia in postpartum rats. Physiol Behav 99(1):59–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nephew BC, Bridges RS (2011) Effects of chronic social stress during lactation on maternal behavior and growth in rats. Stress 14(6):677–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nephew BC, Murgatroyd C, Pittet F, Febo M (2015) Brain reward pathway dysfunction in maternal depression and addiction: a present and future transgenerational risk. J Reward Defic Syndr 1(3):105–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nephew BC, Carini LM, Sallah S, Cotino C, Alyamani RAS, Pittet F, Bradburn S, Murgatroyd C (2017a) Intergenerational accumulation of impairments in maternal behavior following postnatal social stress. Psychoneuroendocrinology 82:98–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nephew BC, Huang W, Poirier GL, Payne L, King JA (2017b) Altered neural connectivity in adult female rats exposed to early life social stress. Behav Brain Res 316:225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nephew BC, Febo M, Huang W, Colon-Perez LM, Payne L, Poirier GL, Greene O, King JA (2018) Early life social stress and resting state functional connectivity in postpartum rat anterior cingulate circuits. J Affect Disord 229:213–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netsi E, Pearson RM, Murray L, Cooper P, Craske MG, Stein A (2018) Association of persistent and severe postnatal depression with child outcomes. JAMA Psychiatry 75(3):247–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann ID, Landgraf R (2012) Balance of brain oxytocin and vasopressin: implications for anxiety, depression, and social behaviors. Trends Neurosci 35(11):649–59. [DOI] [PubMed] [Google Scholar]
- Nitschke JB1, Nelson EE, Rusch BD, Fox AS, Oakes TR, Davidson RJ (2004) Orbitofrontal cortex tracks positive mood in mothers viewing pictures of their newborn infants. Neuroimage 21(2):583–592. [DOI] [PubMed] [Google Scholar]
- Numan M, Numan MJ, Pliakou N, Stolzenberg DS, Mullins OJ, Murphy JM, Smith CD (2005) The effects of D1 or D2 dopamine receptor antagonism in the medial preoptic area, ventral pallidum, or nucleus accumbens on the maternal retrieval response and other aspects of maternal behavior in rats. Behav Neurosci 119(6):1588–1604. [DOI] [PubMed] [Google Scholar]
- Numan M, Stolzenberg DS, Dellevigne AA, Correnti CM, Numan MJ (2009) Temporary inactivation of ventral tegmental area neurons with either muscimol or baclofen reversibly disrupts maternal behavior in rats through different underlying mechanisms. Behav Neurosci 123(4):740–751. [DOI] [PubMed] [Google Scholar]
- Olazábal DE (2018) Role of oxytocin in parental behaviour. J Neuroendocrinol 30:e12594. [DOI] [PubMed] [Google Scholar]
- O’Hara MW, McCabe JE (2013) Postpartum depression: current status and future directions. Annu Rev Clin Psychol 9:379–407. [DOI] [PubMed] [Google Scholar]
- O’Mahony SM, Myint AM, van de Hove D, Desbonnet L, Steinbusch H, Leonard BE (2006) Gestational stress leads to depressive-like behavioural immunological changes in the rat. Neuroimmunomodulation 13(2):82–88. [DOI] [PubMed] [Google Scholar]
- Osborne LM, Monk C (2013) Perinatal depression–the fourth inflammatory morbidity of pregnancy? : Theory and literature review. Psychoneuroendocrinology 38:1929–1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawluski JL, Lonstein JS, Fleming AS (2017) The neurobiology of postpartum anxiety and depression. Trends Neurosci 40(2):106–120. [DOI] [PubMed] [Google Scholar]
- Pechtel P, Murray LMM, Brumariu LE, Lyons-Ruth K (2013) Reactivity, regulation, and reward responses to infant cues among mothers with and without psychopathology: an fMRI review. Transl Dev Psychiatry 1:19673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen CA, Caldwell JD, Walker C, Ayers G, Mason GA (1994) Oxytocin activates the postpartum onset of rat maternal behavior in the ventral tegmental and medial preoptic areas. Behav Neurosci 108(6):1163–1171. [DOI] [PubMed] [Google Scholar]
- Perani CV, Slattery DA (2014) Using animal models to study postpartum psychiatric disorders. Br J Pharmacol 171(20):4539–4555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perani CV, Neumann ID, Reber SO, Slattery DA (2015) High-fat diet prevents adaptive peripartum-associated adrenal gland plasticity and anxiolysis. Sci Rep 5:14821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira M, Morrell JI (2011) Functional mapping of the neural circuitry of rat maternal motivation: effects of site-specific transient neural inactivation. J Neuroendocrinol 23(11):1020–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peris J, MacFadyen K, Smith JA, de Kloet AD, Wang L, Krause EG (2017) Oxytocin receptors are expressed on dopamine and glutamate neurons in the mouse ventral tegmental area that project to nucleus accumbens and other mesolimbic targets. J Comp Neurol 525(5):1094–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prenoveau J, Craske M, Counsell N, West V, Davies B, Cooper P, Rapa E, Stein A (2013) Postpartum GAD is a risk factor for postpartum MDD: the course and longitudinal relationships of postpartum GAD and MDD. Depress Anxiety ;30(6):506–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putnam KT, Wilcox M, Robertson-Blackmore E, Sharkey K, Bergink V, Munk-Olsen T, Deligiannidis KM, Payne J, Altemus M, Newport J, Apter G, Devouche E, Viktorin A, Magnusson P, Penninx B, Buist A, Bilszta J, O’Hara M, Stuart S, Brock R, Roza S, Tiemeier H, Guille C, Epperson CN, Kim D, Schmidt P, Martinez P, Di Florio A, Wisner KL, Stowe Z, Jones I, Sullivan PF, Rubinow D, Wildenhaus K, Meltzer-Brody S; Postpartum Depression: Action Towards Causes and Treatment (PACT) Consortium (2017) Clinical phenotypes of perinatal depression and time of symptom onset: analysis of data from an international consortium. Lancet Psychiatry 4(6):477–485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Righetti-Veltema M, Bousquet A, Manzano J (2003) Impact of postpartum depressive symptoms on mother and her 18-month-old infant. Eur Child Adolesc Psychiatry 12(2):75–83. [DOI] [PubMed] [Google Scholar]
- Rilling JK (2013) The neural and hormonal bases of human parental care. Neuropsychologia 51(4):731–747. [DOI] [PubMed] [Google Scholar]
- Rilling JK, Young LJ (2014) The biology of mammalian parenting and its effect on offspring social development. Science 345(6198):771–776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson E, Grace S, Wallington T, Stewart DE (2004) Antenatal risk factors for postpartum depression: a synthesis of recent literature. Gen Hosp Psychiatry 26(4):289–295. [DOI] [PubMed] [Google Scholar]
- Robinson DP, Klein SL (2012) Pregnancy and pregnancy-associated hormones alter immune responses and disease pathogenesis. Horm Behav 62(3):263–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Nestler EJ (2013) The brain reward circuitry in mood disorders. Nat Rev Neurosci 14(9):609–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sacher J, Rekkas PV, Wilson AA, Houle S, Romano L, Hamidi J, Rusjan P, Fan I, Stewart DE, Meyer JH (2015) Relationship of monoamine oxidase-A distribution volume to postpartum depression and postpartum crying. Neuropsychopharmacology 40(2):429–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller CE, O’Hara MW, Rubinow DR, Johnson AK (2013) Estradiol modulates anhedonia and behavioral despair in rats and negative affect in a subgroup of women at high risk for postpartum depression. Physiol Behav 119:137–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller CE, Schmidt PJ, Rubinow DR (2014) Allopregnanolone as a mediator of affective switching in reproductive mood disorders. Psychopharmacology 231(17):3557–3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller CE, Meltzer-Brody S, Rubinow DR (2015) The role of reproductive hormones in postpartum depression. CNS Spectr 20(1):48–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seip KM, Morrell JI (2009) Transient inactivation of the ventral tegmental area selectively disrupts the expression of conditioned place preference for pup- but not cocaine-paired contexts. Behav Neurosci 123(6):1325–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahrokh DK, Zhang TY, Diorio J, Gratton A, Meaney MJ (2010) Oxytocin-dopamine interactions mediate variations in maternal behavior in the rat. Endocrinology 151(5):2276–2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shnitko TA1, Mace KD, Sullivan KM, Martin WK, Andersen EH, Williams Avram SK, Johns JM, Robinson DL (2017) Use of fast-scan cyclic voltammetry to assess phasic dopamine release in rat models of early postpartum maternal behavior and neglect. Behav Pharmacol 28(8):648–660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skrundz M, Bolten M, Nast I, Hellhammer DH, Meinlschmidt G (2011) Plasma oxytocin concentration during pregnancy is associated with development of postpartum depression. Neuropsychopharmacology 36(9):1886–1893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman ME, Loudon H, Safier M, Protopopescu X, Leiter G, Liu X, Goldstein M (2007) Neural dysfunction in postpartum depression: an fMRI pilot study. CNS Spectr 12(11):853–862. [DOI] [PubMed] [Google Scholar]
- Slattery DA, Neumann ID (2010) Oxytocin and major depressive disorder: experimental and clinical evidence for links to aetiology and possible treatment. Pharmaceuticals 3:702–724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JW, Seckl JR, Evans AT, Costall B, Smythe JW (2004) Gestational stress induces post-partum depression-like behavior and alters maternal care in rats. Psychoneuroendocrinology 29(2):227–244. [DOI] [PubMed] [Google Scholar]
- Smith AL, Freeman SM, Barnhart TE, Abbott DH, Ahlers EO, Kukis DL, Bales KL, Goodman MM, Young LJ (2016) Initial investigation of three selective and potent small molecule oxytocin receptor PET ligands in New World monkeys. Bioorg Med Chem Lett 26(14):3370–3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song Z, Borland JM, Larkin TE, O’Malley M, Albers HE (2016) Activation of oxytocin receptors, but not arginine-vasopressin V1a receptors, in the ventral tegmental area of male Syrian hamsters is essential for the reward-like properties of social interactions Psychoneuroendocrinology 74:164–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein A, Pearson RM, Goodman SH, Rapa E, Rahman A, McCallum M, Howard LM, Pariante CM (2014) Effects of perinatal mental disorders on the fetus and child. Lancet 384(9956):1800–1819. [DOI] [PubMed] [Google Scholar]
- Stolzenberg DS, Numan M (2011) Hypothalamic interaction with the mesolimbic DA system in the control of the maternal and sexual behaviors in rats. Neurosci Biobehav Rev 35(3):826–847. [DOI] [PubMed] [Google Scholar]
- Strathearn L, Li J, Fonagy P, Montague PR (2008) What’s in a smile? Maternal brain responses to infant facial cues. Pediatrics 122(1):40–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strathearn L, Fonagy P, Amico J, Montague PR (2009) Adult attachment predicts maternal brain and oxytocin response to infant cues. Neuropsychopharmacology 34(13):2655–2666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuebe AM, Grewen K, Meltzer-Brody S (2013) Association between maternal mood and oxytocin response to breastfeeding. Journal of Women’s Health 22(4):352–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suda S, Segi-Nishida E, Newton SS, Duman RS (2008) A postpartum model in rat: behavioral and gene expression changes induced by ovarian steroid deprivation. Biol Psychiatry 64(4):311–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surguladze S, Brammer MJ, Keedwell P, Giampietro V, Young AW, Travis MJ, Williams SC, Phillips ML (2005) A differential pattern of neural response toward sad versus happy facial expressions in major depressive disorder. Biol Psychiatry 57(3):201–209. [DOI] [PubMed] [Google Scholar]
- Swain JE, Tasgin E, Mayes LC, Feldman R, Constable RT, Leckman JF (2008) Maternal brain response to own baby-cry is affected by cesarean section delivery. J Child Psychol Psychiatry 49(10):1042–1052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE, Ho SS (2017) Neuroendocrine mechanisms for parental sensitivity: overview, recent advances and future directions. Curr Opin Psychol 15:105–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain JE, Ho SS, Rosenblum KL, Morelen D, Dayton CJ, Muzik M (2017) Parent-child intervention decreases stress and increases maternal brain activity and connectivity during own baby-cry: An exploratory study. Dev Psychopathol 29(2):535–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tombeau Cost K, Unternaehrer E, Plamondon A, Steiner M, Meaney M, Atkinson L, Kennedy JL, Fleming AS; MAVAN Research Team (2017) Thinking and doing: the effects of dopamine and oxytocin genes and executive function on mothering behaviours. Genes Brain Behav 16(2):285–295. [DOI] [PubMed] [Google Scholar]
- Vanmierlo T, De Vry J, Nelissen E, Sierksma A, Roumans N, Steinbusch HWM, Wennogle LP, van den Hove D, Prickaerts J (2018) Gestational stress in mouse dams negatively affects gestation and postpartum hippocampal BDNF and P11 protein levels. Mol Cell Neurosci 88:292–299. [DOI] [PubMed] [Google Scholar]
- Verbeek T, Bockting CL, van Pampus MG, Ormel J, Meijer JL, Hartman CA, Burger H (2012) Postpartum depression predicts offspring mental health problems in adolescence independently of parental lifetime psychopathology. J Affect Disord 136(3):948–954. [DOI] [PubMed] [Google Scholar]
- Vliegen N, Casalin S, Luyten P (2014) The course of postpartum depression: a review of longitudinal studies. Harv Rev Psychiatry 22(1):1–22. [DOI] [PubMed] [Google Scholar]
- Wang T, Shi C, Li X, Zhang P, Liu B, Wang H, Wang Y, Yang Y, Wu Y, Li H, Xu ZD (2018) Injection of oxytocin into paraventricular nucleus reverses depressive-like behaviors in the postpartum depression rat model. Behav Brain Res 336:236–243. [DOI] [PubMed] [Google Scholar]
- Wisner KL, Chambers C, Sit DK (2006) Postpartum depression: a major public health problem. JAMA 296(21):2616–2618. [DOI] [PubMed] [Google Scholar]
- Wisner KL, Moses-Kolko E, Sit D (2010) Postpartum depression: a disorder in search of a definition. Arch Womens Ment Health 13:37–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisner KL, Sit DK, McShea MC, Rizzo DM, Zoretich RA, Hughes CL, Eng HF, Luther JF, Wisniewski SR, Costantino ML, Confer AL, Moses-Kolko EL, Famy CS, Hanusa BH (2013) Onset timing, thoughts of self-harm, and diagnoses in postpartum women with screen-positive depression findings. JAMA Psychiatry 70(5):490–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim IS, Tanner Stapleton LR, Guardino CM, Hahn-Holbrook J, Dunkel Schetter C (2015) Biological and psychosocial predictors of postpartum depression: systematic review and call for integration. Annu Rev Clin Psychol 11:99–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelkowitz P, Gold I, Feeley N, Hayton B, Carter CS, Tulandi T, Abenhaim HA, Levin P (2014) Psychosocial stress moderates the relationships between oxytocin, perinatal depression, and maternal behavior. Horm Behav 66(2):351–360. [DOI] [PubMed] [Google Scholar]
- Zhao C, Eisinger BE, Driessen TM, Gammie SC (2014) Addiction and reward-related genes show altered expression in the postpartum nucleus accumbens. Front Behav Neurosci 8:388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zsido RG, Villringer A, Sacher J (2017) Using positron emission tomography to investigate hormone-mediated neurochemical changes across the female lifespan: implications for depression. Int Rev Psychiatry 29(6):580–596. [DOI] [PubMed] [Google Scholar]


