Abstract
The studies presented here explore intracellular signals resulting from the action of repellents on growth cones. Growth cone challenge with thrombin or thrombin receptor-activating peptide (TRAP) triggers collapse via a receptor-mediated process. The results indicate that this involves activation of cytosolic phospholipase A2(PLA2) and eicosanoid synthesis. The collapse response to repellents targets at least two functional units of the growth cone, the actin cytoskeleton and substratum adhesion sites. We show in a cell-free assay that thrombin and TRAP cause the detachment of isolated growth cones from laminin. Biochemical analyses of isolated growth cones reveal that thrombin and TRAP stimulate cytosolic PLA2 but not phospholipase C. In addition, thrombin stimulates synthesis of 12- and 15-hydroxyeicosatetraenoic acid (HETE) from the released arachidonic acid via a lipoxygenase (LO) pathway. A selective LO inhibitor blocks 12/15-HETE synthesis in growth cones and inhibits thrombin-induced growth cone collapse. Exogenously applied 12(S)-HETE mimics the thrombin effect and induces growth cone collapse in culture. These observations indicate that thrombin-induced growth cone collapse occurs by a mechanism that involves the activation of cytosolic PLA2 and the generation of 12/15-HETE.
Keywords: growth cone, collapse, thrombin, signaling, phospholipase A2, lipoxygenase
Chemorepellents provide important guidance cues for growth cones during nervous system development (Tessier-Lavigne and Goodman, 1996). Thrombin, a serine protease, was the first neurite repellent identified (Monard et al., 1983; Gurwitz and Cunningham, 1988; Hawkins and Seeds, 1986, 1989). However, it initially was thought to affect growth cones strictly by digestion of extracellular ligand or matrix molecules via its protease activity (Monard, 1988). Recent observations (Jalink and Moolenaar, 1992; Suidan et al., 1992) establish that the effects of thrombin on neurites are receptor-mediated. Thrombin activates its receptors by proteolytically unmasking the “tethered ligand,” a peptide sequence near the N terminus of the receptor (Vu et al., 1991; Grand et al., 1996). Nonproteolytic thrombin receptor-activating peptides (TRAPs) representing the tethered ligand also can activate the receptor (Feng et al., 1995). At least two distinct thrombin receptors have been identified (Ishihara et al., 1997). That thrombin must play an important role in brain development and function is indicated by the finding that the genes encoding prothrombin, a thrombin receptor, and a thrombin antagonist (glial-derived nexin) are expressed in various brain regions, especially during development and in those regions known to exhibit plasticity (Mansuy et al., 1993; Niclou et al., 1994;Weinstein et al., 1995).
Maximum repellent stimulation causes growth cone “collapse,” the withdrawal of lamellipodia and filopodia to form a club-shaped terminal structure (Kapfhammer and Raper, 1987). How repellent receptor activation causes this morphological change is unclear. Heterotrimeric G-proteins have been implicated in repellent signaling (Schwab, 1990;Igarashi et al., 1993; Grand et al., 1996; Moolenaar et al., 1997;Schindelholz and Reber, 1997), and so have receptor and other tyrosine kinases (Schindelholz and Reber, 1997; Smalheiser and Ali, 1994;Drescher et al., 1995; Brennan et al., 1997). Small Rho-family G-proteins are critical for reshaping the actin cytoskeleton in various cells and growth cones (Jalink et al., 1994; Gebbink et al., 1997; Jin and Strittmatter, 1997; Lamoureux et al., 1997; Tapon and Hall, 1997;Zipkin et al., 1997; Gallo and Letourneau, 1998; Katoh et al., 1998). Yet, we do not know whether growth cone collapse is caused primarily by cytoskeletal retraction (Stossel, 1993) followed by passive filopodial detachment, or whether dissociation of adhesion sites is a separate process triggered by the repellents. Because any form of amoeboid locomotion necessitates the coordinated attachment and detachment of pseudopods (Stossel, 1993; Huttenlocher et al., 1995), the involvement of an active detachment mechanism must be considered.
Nerve growth cones are enriched in high molecular weight, cytosolic phospholipase A2 (cPLA2;Nègre-Aminou et al., 1996), which may be involved in signaling (Dennis, 1994). Interestingly, PLA2 and pseudopod activity may be correlated (Bar-Sagi and Feramisco, 1986; Bar-Sagi, 1989; Teslenko et al., 1997). In platelets, thrombin activates cPLA2 and causes spreading (Grand et al., 1996). Does thrombin also stimulate growth cone cPLA2, and is the cPLA2 product arachidonic acid (AA) or one of its eicosanoid derivatives involved in altering growth cone configuration?
Eicosanoids are synthesized through three different pathways involving cyclooxygenases (generating prostaglandins), cytochrome P-450 monooxygenases, or lipoxygenases [LOs; synthesizing hydroperoxyeicosatetraenoic acids (HPETEs) and leukotrienes; Smith, 1989; Capdevila et al., 1992; Yamamoto et al., 1997]. HPETEs are reduced to hydroxyeicosatetraenoic acids (HETEs). 12(S)-HETE is of particular interest, because several reports link its function to pseudopod activity or cell motility (Goetzl et al., 1977; Yoshino et al., 1993; Ferrante et al., 1994). In endothelial and certain cancer cells 12(S)-HETE induces disruption of adhesion sites and process retraction (Tang et al., 1993; de la Houssaye et al., 1997).
The present studies test the hypotheses that (1) cPLA2 activation and 12-HETE generation are necessary for thrombin-induced growth cone collapse; (2) 12-HETE mimics the thrombin response; and (3) a signaling pathway involving cPLA2 and 12/15-LO regulates growth cone detachment (de la Houssaye et al., 1997; Mikule et al., 1997).
MATERIALS AND METHODS
Materials. Free AA was purchased from Sigma (St. Louis, MO), andl-stearoyl-2-arachidonyl-sn-glycerol (DG) was from Avanti Polar Lipids (Alabaster, AL).l-α-Stearoyl-2-[14C]arachidonyl-phosphatidylinositol ([14C]AA-PI; 20–40 mCi/mmol), -phosphatidylethanolamine ([14C]AA-PE; 40–60 mCi/mmol), and -phosphatidylcholine ([14C]AA-PC; 40–60 mCi/mmol) were obtained from DuPont New England Nuclear (Boston, MA) as was [3H]AA. Reagents for chemiluminescence were from the same vendor. Analytical grade solvents for routine thin-layer-chromatography (TLC) analysis and tissue culture dishes were purchased from Fisher Scientific (Pittsburgh, PA). 2,3-Butanedione monoxime (BDM),N-Tris-[hydroxymethyl]-methyl-2-aminoethanesulfonic acid (TES), thrombin, and other chemicals were from Sigma. Aprotinin was from Calbiochem (San Diego, CA). Polyacrylamide, prestained molecular weight standards, and other reagents for SDS-PAGE were from Life Technologies (Grand Island, NY). Tissue culture media and laminin were also from Life Technologies. 12(S)- and 15(S)-HETE, nordihydroguaiaretic acid (NDGA), cinnamyl-3,4-dihydroxy-α-cyanocinnamate (CDC), and indomethacin were purchased from Biomol (Plymouth Meeting, PA). TRAP came from Peninsula Laboratories (San Carlos, CA). TLC plates were obtained from EM Science (Gibbstown, NJ) and Whatman (Clifton, NJ). Sprague Dawley rats were from Harlan (Indianapolis, IN). Solid phase extraction columns were purchased from J. T. Baker (Phillipsburg, NJ). Rabbit antibodies to platelet 12-LO and to leukocyte 12/15-LO were from Oxford Biomedical Research (Oxford, MI) and Cayman Chemical (Ann Arbor, MI), respectively. HRP-conjugated goat anti-rabbit antibody was purchased from Vector Laboratories (Burlingame, CA). Texas Red-conjugated phalloidin and Slow-Fade Light were products of Molecular Probes (Eugene, OR). Insulin and insulin-like growth factor 1 (IGF-1) were from Collaborative Biomedical Products (Bedford, MA), and brain-derived neurotrophic factor (BDNF) was from Amgen (Thousand Oaks, CA).
Neuron culture. Cerebral cortices were dissected from embryonic day 18 Sprague Dawley rats, cut into explants of <1mm3, and cultured on either poly-d-lysine-coated coverglass (Assistent) or poly-d-lysine-coated tissue culture plastic. After 24 hr in B27 neurobasal medium (NB) supplemented with 10% fetal bovine serum, cultures were switched to serum-free B27 NB. The cultures were incubated at 37°C in 5% CO2 in air. Sprouting neurons were observed readily on day 1. On day 2 or 3, long neurites were present, and the cultures were used for collapse experiments.
Actin staining. After experimentation, cultures were fixed by slow infusion of fixative (4% paraformaldehyde in 0.1 mphosphate buffer, pH 7.4) into the culture dishes over 10 min (Pfenninger and Maylié-Pfenninger, 1981). Thereafter, the fixative was removed by rinsing three times with PBS containing 1 mm glycine. Cultures were then blocked with PBS and 1% bovine serum albumin (BSA) and permeabilized by incubation in 0.02% Triton X-100 for 5 min. After three washes in PBS and BSA, cultures were incubated with Texas Red-conjugated phalloidin for 30 min. Unbound phalloidin was washed away by two PBS rinses, after which the coverglasses were mounted in Slow-Fade Light on microscope slides. Images were recorded on 35 mm film using a Zeiss (Thornwood, NY) Axiophot fluorescence microscope equipped with epiillumination.
Growth cone collapse assay. Before repellent factor treatment, some of the day 2–3 explant cultures were preincubated (at 37°C) with inhibitor or vehicle control (dimethylsulfoxide or ethanol) for 30–60 min. Cultures were then challenged with a repellent factor or vehicle control for 1–30 min. In some experiments, live cultures were photographed with a Zeiss IM 35 inverted photo microscope equipped with a stage heater, whereas in others cultures were fixed (as above) after 7 min of repellent factor treatment. Growth cone collapse was assessed quantitatively in aldehyde-fixed cultures by grading the degree of collapse in a large number of randomly selected growth cones. Because growth cones of control neurons exhibited a heterogenous phenotype and some of the collapse responses (at 7 min) were graded, binary assignments (collapsed or intact) were inadequate for these analyses. Therefore, we classified growth cones into one of four categories: fully spread, “veiled” growth cones (1, “spread and veiled”), spread-out growth cones with prominent filopodia but reduced veiling (2, “reduced veiling”), growth cones without lamellipodia and possessing only few filopodia (3, “partially collapsed”), and fully collapsed, club-shaped neurite termini (4, “club-shaped”). The numbers assigned to the classes were used to determine a “collapse index” for each experimental condition by calculating a weighted average for each growth cone population analyzed. The resulting collapse indices were averaged for the same condition, and SEMs were determined. Student's t test was used to assess statistical significance of values for different conditions.
Growth cone particle isolation. Growth cone particles (GCPs) were prepared as described by Pfenninger et al. (1983)and modified by Lohse et al. (1996). Briefly, 18 day fetal brains were homogenized in ∼8 volumes of 0.32 m sucrose containing 1 mm MgCl2, 2 mm TES buffer, pH 7.3, and 3 μm aprotinin. A low-speed supernatant (1660 × g for 15 min) was loaded onto a discontinuous sucrose density gradient with steps of 0.83 and 2.66m sucrose containing MgCl2and TES. The gradients were spun to equilibrium at 242,000 ×g for 40 min in a vertical rotor (Vti50; Beckman Instruments, Palo Alto, CA). The GCP fraction at the 0.32–0.83m sucrose interface was collected and used for the experiments described below.
GCP release assay. Petri dishes (35 mm diameter) were coated with 10 μg of mouse laminin in PBS by incubation at room temperature for 60 min with shaking. Dishes were subsequently rinsed three times with PBS to remove unbound laminin and then blocked with 5% nonfat dry milk (Carnation) in PBS for 30 min. After three PBS rinses to remove the blocking agent, the dishes were ready for the addition of GCPs. Before plating, the GCP fraction was slowly added at 4°C to an equivalent volume of 2× concentrated, modified Krebs' buffer (22 mm sucrose, 50 mm NaCl, 5 mm KCl, 22 mm HEPES, pH 7.4, 10 mm glucose, 1.2 mm NaH2PO4, 1.2 mm MgCl2, and 2 mmCaCl2). After incubation for 5 min at 37°C, this buffered GCP preparation was added to the laminin-coated Petri dishes. Contact with the substratum was facilitated by centrifuging the dishes for 15 min at 5000 × g (Beckman JS5.2 rotor) at room temperature, followed by incubation at 37°C to allow adhesion site formation. After 10 min, unattached GCPs were removed by rinsing the dishes twice with 1× modified Krebs' buffer. Plated GCPs were then challenged with repellent factor (TRAP or thrombin) for 20 min at 37°C. The supernatant containing released GCPs was collected and centrifuged for protein determination of the pellet. GCPs that remained attached after repellent treatment were removed with 5% SDS for protein assay. Protein was measured according to the method of Lowry et al. (1951) as modified by Peterson (1983). Results of these experiments were expressed as the percentage of released versus total GCP protein initially attached minus release in untreated samples.
Gel electrophoresis and Western blotting. SDS-PAGE was performed according to the method of Laemmli (1970) using 5–15% acrylamide gradients. The GCP fraction was diluted threefold to fourfold with 0.32 m sucrose buffer and spun for 30 min at 39,800 × g. Protein amounts in the pellets were determined by dye-binding assay (Bradford, 1976). Polypeptides of pelleted GCPs (30 μg/lane) were resolved by SDS-PAGE. Prestained standards were used to determine apparent molecular mass. Resolved proteins were transferred to nitrocellulose essentially as described byTowbin et al. (1979), with a semidry blotting apparatus for 60 min at 300 mA for a 14 × 17 cm gel. Ponceau S staining served to monitor the efficiency of protein transfer. Blots were then rinsed in PBS and distilled water and dried. Before incubation with primary antibody, blots were blocked with 5% nonfat milk powder and 0.02% Tween 20 in PBS for 2 hr at room temperature. Blots were probed in the same blocking solution and conditions with either polyclonal rabbit anti-platelet or polyclonal rabbit anti-leukocyte 12-LO antibody at 1:2000 or 1:5000, respectively. Blots were washed five times in blocking solution, followed by incubation with secondary antibody (HRP-conjugated goat anti-rabbit antibody at 1:5000) for 1 hr in blocking solution. After extensive washing, bound antibody was detected by enhanced chemiluminescence according to the manufacturer's directions (New England Nuclear) by contact-exposing x-ray film (X-OMAT Blue XB-1; Eastman Kodak, Rochester, NY).
Phospholipase assays. For PLA2 assays, GCPs suspended in modified Ca2+-free Krebs' buffer (10–30 μg protein/assay) were first incubated for 10 min on ice in the presence or absence of effectors. Subsequently, phospholipid substrate (PC, PE, or PI) containing radiolabeled AA (7–14 μm; 50,000–100,000 cpm/assay) was added, and the reaction was performed at 37°C for 10 min. Typically, assay mixtures contained 10 μm CaCl2; specific modifications are described in the figure legends. For phospholipase C (PLC) assays, conditions were the same when run in parallel with PLA2 assays. When PLC assays were run independently, Ca2+ was increased to 1 mm. Reactions were terminated by the addition of 3.75 volumes of cold chloroform/methanol (1:2). PLA2assays also were performed on neurons dissociated from fetal rat cerebral cortex. The assay conditions were the same as those just described for GCPs.
Extraction of phospholipase products was performed according to the method of Bligh and Dyer (1959). Products recovered in chloroform were loaded onto Silica Gel 60 TLC plates and developed as described earlier (Nègre-Aminou and Pfenninger, 1993) in hexane/ether/acetic acid (40:60:1). AA and DG were identified on TLC by co-migration with authentic standards. The appropriate bands were excised into scintillation fluid and counted on a Beckman LS 1801 scintillation spectrometer. All assays were run in triplicate.
Eicosanoid assays. Eicosanoid production in GCPs was measured by metabolic labeling from 3H-AA or14C-AA-PC or by mass spectrometric detection. To determine HETE synthesis by radiolabeling, assays were run essentially as for PLA2 except that phospholipid substrate was increased to 100,000 cpm/assay, and assay time at 37°C was 15 min. To measure LO activity directly, without PLA2involvement, 3H-AA was the substrate of choice. Reactions were stopped by the addition of chloroform/methanol as for the phospholipase assays. Reaction products were extracted as described by Birkle et al. (1988) and spotted on preactivated Silica Gel 60 TLC plates, and the plates were developed in the upper phase of ethyl acetate/isooctane/acetic acid/water (100:60:20:100). AA and HETEs were identified by co-migration with standards. Appropriate bands were counted by liquid scintillation spectrometry.
For identification of HETEs we used a liquid chromatography–tandem mass spectrometry (LC–MS–MS) strategy. The reaction substrate consisted of 3.5 μm AA, with the addition of 3.5 μm deuterated AA (D8-AA) plus 1 μCi [3H]AA to track reaction products by mass and radioactivity, respectively. Reactions were stopped with ice-cold ethanol; the mixtures were kept overnight at −20°C under N2; and the proteins were spun out. The supernatants were diluted with H2O and loaded onto 1 ml C18 Sep-Pak columns (Varian, Palo Alto, CA). After washing with H2O, eicosanoid was eluted with 2 ml of methanol. These samples were resolved by reverse-phase HPLC, and the eluted fractions were analyzed on-line by a tandem quadrupole mass spectrometer (API-III+; Perkin-Elmer Sciex, Thornhill, Ontario, Canada; MacMillan and Murphy, 1995). Collision-induced decomposition of HETE regioisomers produced characteristic fragment ions that were identified at the second MS stage.
RESULTS
Growth cone collapse in culture
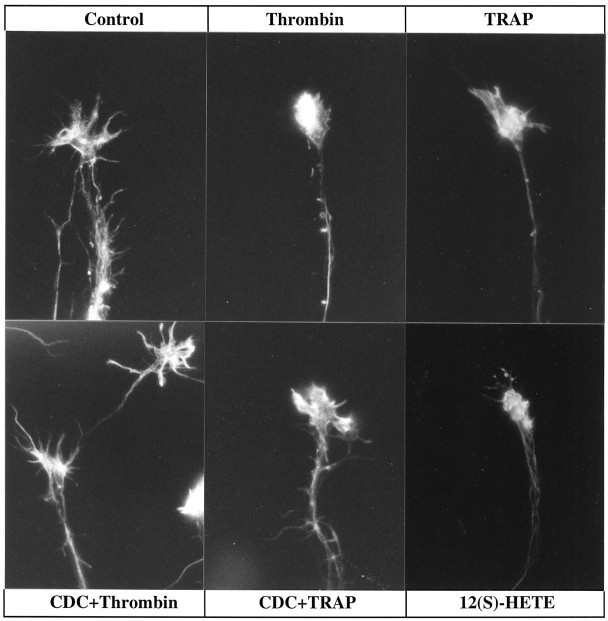
Experiments were performed on growth cones in cultures of rat cerebral cortex to assess qualitatively and quantitatively the collapsing effects of thrombin and the nonproteolytic TRAP, with or without inhibitors of AA metabolism. Figure1 shows the effect of thrombin treatment on growth cones at different times. Almost immediately thrombin causes disappearance of lamellipodia and, after several minutes, filopodia. After 5–10 min, most growth cones are fully collapsed, and neurite length is reduced. By 30 min neurite beading is observed (results not shown). TRAP was used at a concentration ∼1000 times higher than that of thrombin, consistent with data on receptor binding and TRAP activation in other systems (Feng et al., 1995; also seeNataranjan et al., 1995; Grand et al., 1996). TRAP has qualitatively the same effect as thrombin, but the change is not as pronounced, and neurite beading is not seen routinely. The collapse phenomenon is shown at higher magnification in Figure 2, after staining of filamentous actin with Texas Red-conjugated phalloidin. Control growth cones exhibit spread-out veils and/or filopodia with filamentous actin abundant in the periphery, whereas thrombin or TRAP treatment causes withdrawal of lamellipodia and filopodia and actin redistribution from the peripheral to the proximal growth cone.
Fig. 1.
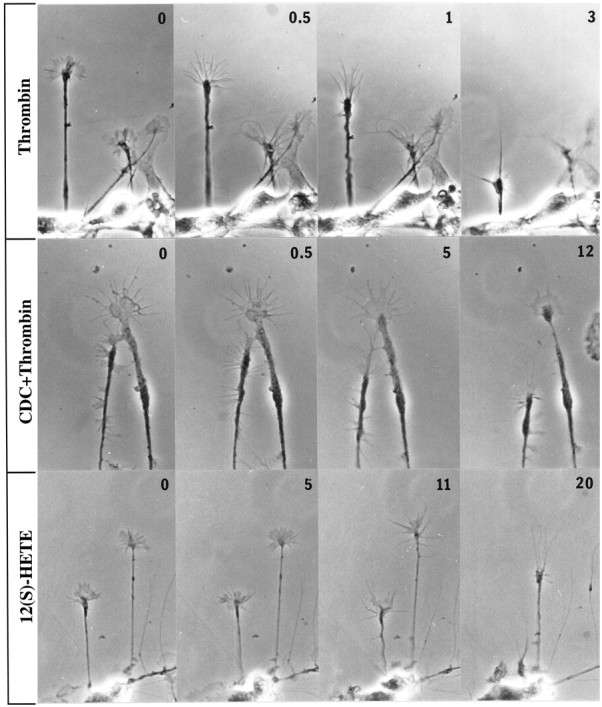
Effects of thrombin, thrombin plus LO inhibitor, and 12(S)-HETE on growth cone configuration. Phase-contrast micrographs are shown of cultures of embryonic day 18 rat cortical neurons that were either pretreated with vehicle alone or inhibitor for 45 min before challenge with thrombin or 12(S)-HETE. Numbers in thetop right corners indicate time in minutes of exposure to collapsing reagent. Top to bottom, 100 nm thrombin, 10 μm CDC pretreatment (30 min) followed by 100 nm thrombin, and 10−7m 12(S)-HETE. The large growth cone in CDC+Thrombin exhibits at 0 min (which is a CDC-alone control) and at 0.5 min vertically extended veils. By 5 min they have disappeared.
Fig. 2.
Effects of thrombin, TRAP, thrombin or TRAP plus LO inhibitor, and 12(S)-HETE on filamentous actin in growth cones. Cultured El8 rat cortical neurons were fixed and stained with Texas Red-conjugated phalloidin after the following experimental treatments: control (vehicle alone), thrombin (100 nm) for 7 min, TRAP (100 μm) for 7 min, 45 min pretreatment with CDC (10 μm) followed by thrombin (100 nm) or TRAP (100 μm) for 7 min, and 12(S)-HETE (10−7m) for 12 min. Thrombin and TRAP cause collapse, but the TRAP effect, although clear-cut, is not as dramatic as that of thrombin; CDC inhibits or significantly attenuates collapse, and 12(S)-HETE mimics the effects of thrombin and TRAP.
To test for the putative role of PLA2 in thrombin and TRAP signaling, one would ideally inhibit or activate growth cone PLA2. However, the molecular identities of growth cone PLA2s have not been established, and selective inhibitors that block these enzymes are not known. Therefore, we proceeded with experiments involving inhibitors of the metabolism of the PLA2 product AA. Indomethacin is a specific blocker of cyclooxygenases, thereby inhibiting the synthesis of prostaglandins and related eicosanoids (Salari et al., 1984), and has no effect on control or thrombin treatment of growth cones (data not shown). CDC (Cho et al., 1991; Wen et al., 1996) is a selective inhibitor of LOs, including 12-LO, which catalyzes the synthesis of 12(S)- and some 15(S)-HETE. CDC alone does not alter growth cone structure qualitatively (Fig. 1,CDC+Thrombin, 0 min) or quantitatively (the collapse index for CDC alone, 30 min, is 1.42 for 100 growth cones vs 1.34 for controls; see Table 1). However, pretreatment with CDC inhibits the thrombin effect on growth cones (Figs. 1, 2). Although thrombin collapses growth cones even after incubation with indomethacin, CDC-pretreated growth cones retain their spread-out appearance. A more potent but less selective inhibitor of all LOs, NDGA (Salari et al., 1984), also protects growth cones from thrombin- or TRAP-induced collapse (data not shown).
Table 1.
Comparison of growth cone collapse indices
| Condition | Collapse index ± SEM1-a |
|---|---|
| Control | 1.34 ± 0.05 |
| Thrombin | 3.22 ± 0.06* |
| CDC/thrombin | 2.32 ± 0.16** |
| TRAP | 2.83 ± 0.12* |
| 12(S)-HETE | 2.92 ± 0.12* |
Data are calculated from those shown in Figure 4. Values close to 1.0 indicate intact growth cones; those close to 4.0 indicate completely collapsed growth cones.
*Values significantly different from control (p< 10−4 in Student's t test).
**Value significantly different from thrombin treatment alone (p < 0.0075 in Student's ttest).
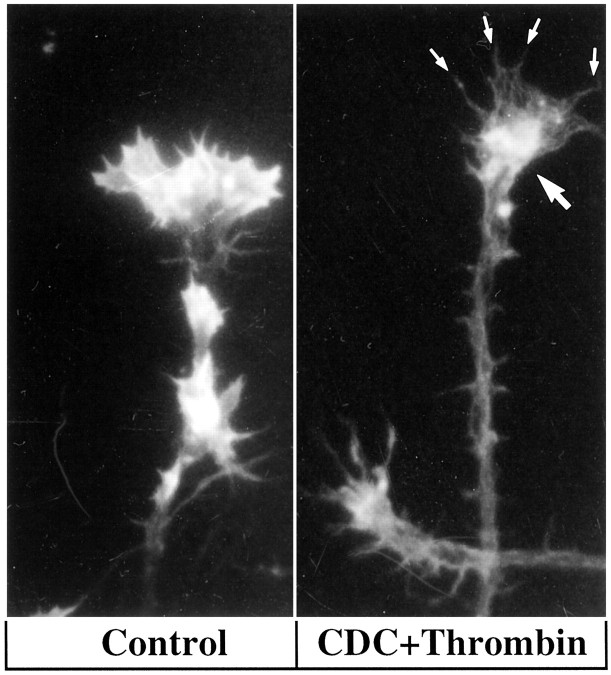
Figures 2 and 3 allow for comparison of phalloidin-stained growth cones in control cultures and in cultures challenged with thrombin or TRAP after CDC incubation. Again, growth cones treated with CDC alone (data not shown) are not distinguishable from controls. CDC-pretreated growth cones challenged with thrombin typically retain their spread-out shape with actin-rich, attached filopodia and lamellipodia. In some cases, however, we observed fully spread growth cones with filamentous actin largely depleted from lamellipodia and filopodia and clumped in the proximal body (Fig. 3). Some actin depolymerization may have taken place as well.
Fig. 3.
The LO inhibitor CDC inhibits thrombin-induced shape change of the growth cone but may allow redistribution of the actin cytoskeleton. A, Growth cones fixed and stained with Texas Red-conjugated phalloidin after control (vehicle alone) incubation. B, 30 min CDC (10 μm) pretreatment followed by thrombin challenge (100 nm) for 7 min. Small arrows indicate intact filopodia with diminished actin staining. The large arrow denotes “clumped” actin filaments in the proximal growth cone.
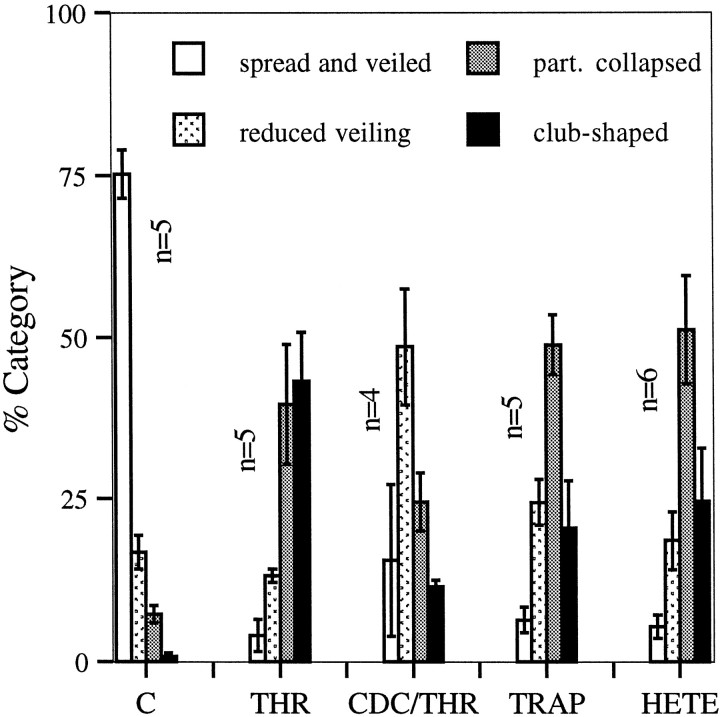
The results of these experiments have been analyzed quantitatively. At least 50 randomly selected growth cones in each of four to six independent experiments were classified into four different categories: spread and veiled, reduced veiling, partially collapsed, and club-shaped (see Materials and Methods). These categories are exemplified by the main growth cone in Figure 1 (thrombin treatment) at 0–3 min. Figure 4 shows a shift of growth cones from predominantly intact (controls) to predominantly collapsed (thrombin or TRAP) and the strong attenuation of this effect by CDC. Collapse indices for the different experimental conditions are shown in Table 1 and were used to determine that thrombin- and TRAP-induced collapse was highly significant, as was its attenuation by CDC.
Fig. 4.
Quantitative analysis of growth cone collapse induced by thrombin (with or without CDC pretreatment), TRAP, or 12(S)-HETE. Collapse status was assessed on fixed neural cultures having undergone the following treatments:C, control, vehicle alone; THR, thrombin (100 nm) for 7 min; CDC/THR, CDC (10 μm) pretreatment for 30 min, followed by thrombin (100 nm) for 7 min; TRAP (100 μm) for 7 min; and 12(S)-HETE (10−7m) for 10 min. For each condition at least 200 growth cones from at least four independent experiments were scored as described in Materials and Methods. Data are presented as percent of total growth cones observed. Error bars indicate SEM; n, number of independent experiments.
If 12-LO is necessary for at least a part of the collapsing action of thrombin and TRAP, as the experiments with CDC suggest, then the primary 12-LO product 12(S)-HETE should mimic the effect of thrombin and TRAP. This is shown qualitatively in Figures 1and 2 and quantitatively in Figure 4 and Table 1. In this system at 10−7m, 12(S)-HETE does indeed cause growth cone collapse that is morphologically very similar to the effect of thrombin and TRAP. 12(S)-HETE-induced collapse is highly significant but less dramatic than that of thrombin (see Fig. 4, Table1), and the neurite beading seen after 30 min of maximum thrombin exposure is observed only rarely. The collapse effect of 12(S)-HETE is not affected by growth cone pretreatment with the LO inhibitor CDC (data not shown).
Growth cone detachment
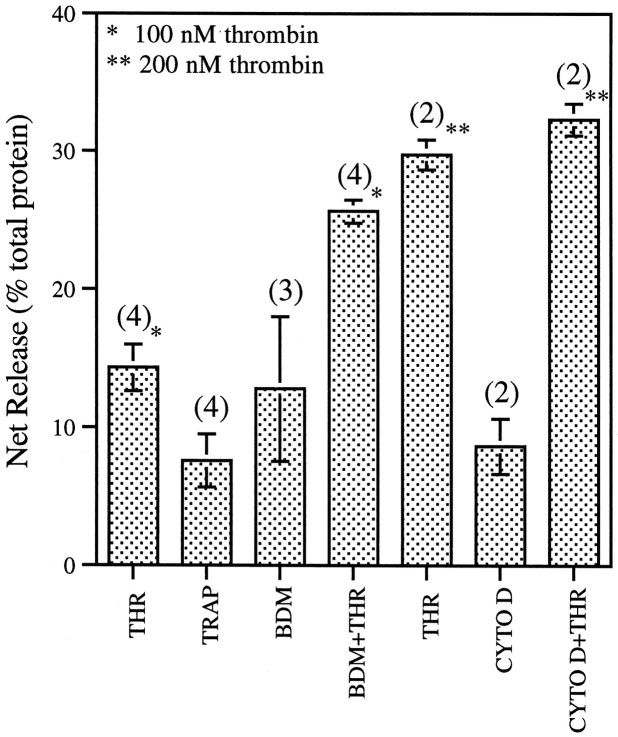
Collapsing factors must affect the cytoskeleton of the growth cone as well as filopodial and lamellipodial attachment to the substratum. To analyze this latter phenomenon, we designed a cell-free substrate detachment assay involving so-called GCPs isolated from fetal rat brain (Pfenninger et al., 1983). GCPs contain a full complement of growth cone organelles, are viable for at least 1 hr after isolation (Lockerbie et al., 1991), and have been shown to be derived primarily from axonal growth cones (Lohse et al., 1996). When GCPs attached to laminin were exposed to thrombin, many of them detached from the substratum and could be collected in the supernatant (Fig.5). Thrombin-induced detachment was detectable at ≥50 nm and reached a plateau between 200 and 300 nm (data not shown), indicating dose dependence. TRAP mimicked the thrombin effect, demonstrating that protease activity is not required for detachment.
Fig. 5.
Thrombin- and TRAP-induced detachment of GCPs from a laminin substratum. GCPs plated on laminin were exposed for 20 min to thrombin (THR, 100 or 200 nm), to vehicle with or without cytochalasin D (CYTO D, 1 μm) or BDM (20 mm) pretreatment, or to TRAP (100 μm). Detachment was measured as pelletable protein collected in the supernatant. Data are net increases ± SEM above untreated controls, expressed as percent of total GCP protein bound initially. In control conditions detachment was 15.2 ± 6.9% (average ± SD) of total GCPs plated. Numbers inparentheses indicate numbers of independent experiments.
GCPs contain a substantial actin meshwork (Pfenninger et al., 1983). Despite their small size (∼0.3–0.5 μm diameter), contraction of the actin–myosin system could be responsible for at least some of the detachment effect observed in the assay. Therefore, laminin-attached GCPs were first treated for 20 min at 37°C with 1 μmcytochalasin D to depolymerize the actin cytoskeleton or with 20 mm BDM to inhibit selectively myosin ATPase (Cramer and Mitchison, 1995). Both reagents caused considerable release of GCPs by themselves (Fig. 5), consistent with known effects on cell spreading (Mooney et al., 1995; Sanders et al., 1999). However, thrombin challenge increased detachment further (p < 0.018 for BDM; p < 0.0047 for cytochalasin D). The release by the combined treatments equals the sum (for BDM) or is within the error range of the sum (for cytochalasin D) of the separate treatments.
Phospholipase activation
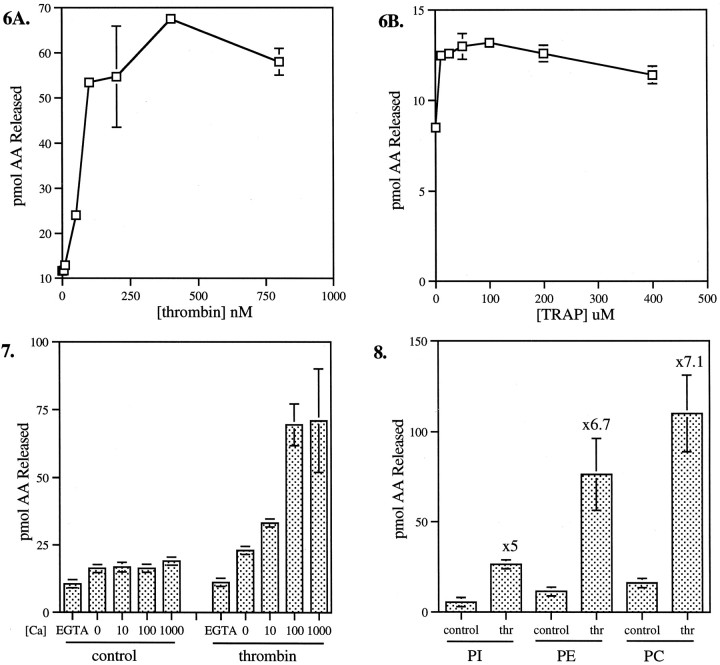
Our hypothesis predicts that one of the important steps in thrombin-induced growth cone collapse is the activation of cytosolic PLA2. Therefore, we studied PLA2 activation in primary neurons and in GCPs isolated from fetal rat brain. Because these assays have to be performed on cellular systems with endogenous phospholipid present, one cannot readily measure absolute enzyme activities. However, comparisons of the hydrolysis of exogenous, radiolabeled substrates enable one to determine relative PLA2 activities in comparable experiments. In PLA2 assays performed on primary neurons dissociated from cerebral cortex, thrombin greatly stimulated AA release from PE or PI, as shown in Table2. Because of the specific effect of thrombin on growth cones and because of the enrichment of PI-selective PLA2 in growth cones (Nègre-Aminou et al., 1996), we proceeded with the more detailed analysis of PLA2 activation in GCPs. Data are shown primarily for PI as a substrate, because PI is the phospholipid with the highest level of AA turnover in growth cones (Nègre-Aminou and Pfenninger, 1993). Table 2 shows that, under comparable experimental conditions, thrombin stimulates PLA2 in GCPs to much higher levels of free AA than in whole neurons. Figure6 shows the release of AA from PI as a function of increasing concentrations of thrombin or TRAP. We observed a high level of activation (from fivefold to sevenfold) saturating at ∼250 nm for thrombin. TRAP also clearly stimulated PLA2, but to a considerably lesser degree and at a ∼1000-fold higher concentration.
Table 2.
PLA2 activity in neurons and growth cones: whole neurons versus GCPs
| Condition | Concentration (nm) | Substrate | Net activity (pmol · min−1 · mg protein−1) | |
|---|---|---|---|---|
| Intact neurons | GCPs | |||
| Control | PI | 0.6 ± 0.45 | 37.9 ± 1.1 | |
| PE | 4.4 ± 0.99 | 25.7 ± 2.3 | ||
| Thrombin | 200 | PI | 42.5 ± 16 | 262 ± 47 |
| PE | 26.8 ± 1.9 | 210.2 ± 4.8 | ||
Data are from representative experiment done in triplicate (values are means ± SD). All measurements were obtained in the same experiment and share the control indicated.
Fig. 6.
Dose–response curves of PLA2activation by thrombin (A) and TRAP (B) in GCPs. GCPs were incubated for 10 min on ice with varying concentrations of thrombin (A) or TRAP (B) and then for 10 min at 37°C in the reaction mixture in the presence of 10 μmCaCl2. The substrate was [14C]AA-PI. The data presented are net values in triplicate. Error bars represent SD; where not present error bars were too small to be indicated.
We had determined previously that GCPs contain very little, if any, secreted PLA2 (Nègre-Aminou et al., 1996). To exclude the possibility that thrombin stimulated the release of a secreted PLA2 we performed PLA2 assays in the presence of reducing agent, which inactivates the catalytic domain of all secreted forms of the enzyme. As Table 3 shows, dithiothreitol (DTT) does not reduce the control or thrombin-stimulated levels of PLA2 activity. Data are shown for PI, but the same observations were made for PE and PC (data not shown). This confirms the role of high molecular weight PLA2 in the cytosol in a thrombin-activated pathway.
Table 3.
PLA2 activity in neurons and growth cones: effect of reduction on PLA2 activity in GCPs
| Condition | Concentration (nm) | Substrate | Net activity (pmol · min−1 · mg protein−1) | |
|---|---|---|---|---|
| No DTT | 50 nm DTT | |||
| Control | PI | 41.9 ± 1.1 | 66.0 ± 4.7 | |
| Thrombin | 100 | PI | 221 ± 39.9 | 223 ± 53.4 |
Data are from representative experiment done in triplicate (values are means ± SD). All measurements were obtained in the same experiment and share the control indicated.
The PLA2 response to thrombin is calcium-dependent in permeabilized GCPs, as shown in Figure7. In the presence of EGTA, there is no stimulation of enzyme activity, whereas the thrombin response reaches a maximum at ∼100 μm Ca2+, without much change in basal, nonstimulated conditions.
Fig. 7.
Effects of calcium on control and thrombin-stimulated levels of PLA2 in GCPs. GCPs were incubated alone (control) or in the presence of 100 nm thrombin for 10 min on ice and then combined with substrate ([14C]AA-PI) for 10 min at 37°C in the presence of EGTA or varying micromolar concentrations of CaCl2 (0 [Ca], no addition of Ca2+ or EGTA). AA release was measured as described in Materials and Methods. Data presented are in triplicate. Error bars indicate SD.
In a previous publication (Nègre-Aminou et al., 1996), we reported that GCPs contain at least two biochemically separable PLA2 activities selective for PI and PE, respectively. Under experimental conditions used at the time, PC hydrolysis in GCPs was at background level without stimulation. However, Figure 8 shows that thrombin stimulates AA release from all three phospholipid substrates but at different levels, ranging from approximately fivefold for PI to approximately sevenfold for PC. These values do not take into account, however, known differences in the abundance of endogenous phospholipid substrates and/or potential competition among the PLA2s for a given substrate.
Fig. 8.
Substrate selectivity of thrombin-stimulated growth cone PLA2. GCPs were assayed in the absence (control) or presence of 100 nmthrombin (thr) with 10 μmCaCl2 and equal concentrations of [14C]AA-PI, -PE, or -PC as substrate.Numbers above thrombin columns indicate increase in activity relative to control levels. Absolute values for PE, relative to those for PI, are higher in these experiments compared with those shown in Tables 2-4, because the PE substrate concentration was lower in the latter experiments. However, the degrees of thrombin-induced stimulation were very similar. Data are averages of three experiments, all done in triplicate, ± SEM.
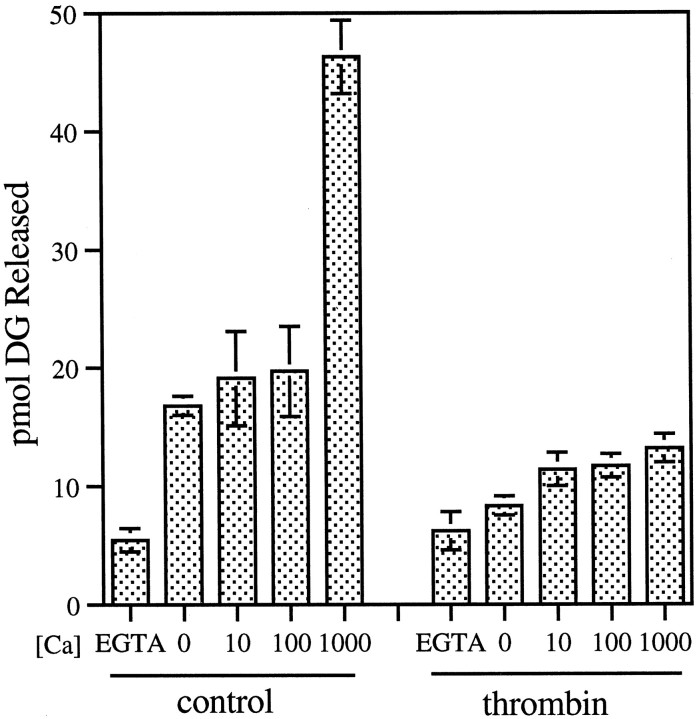
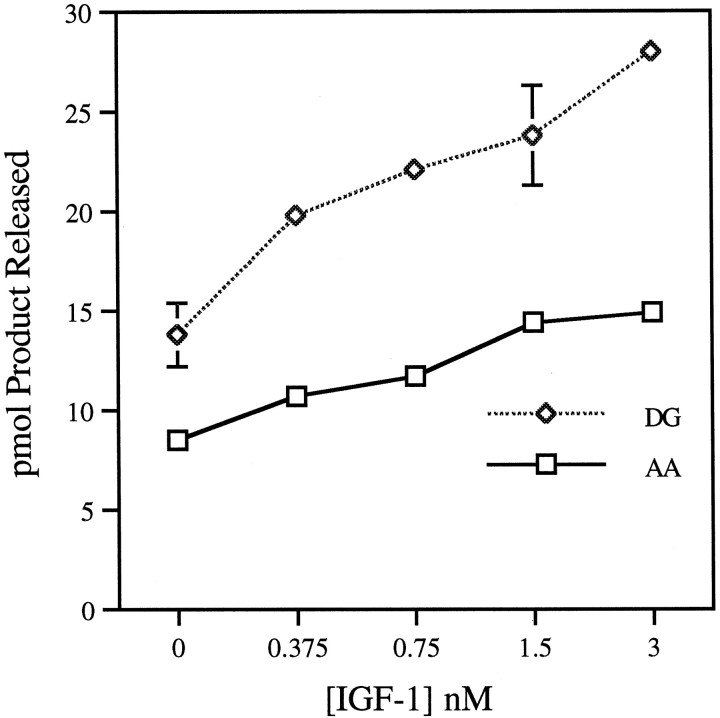
In other systems, such as platelets, thrombin has been reported to activate PLC (Crouch and Lapetina, 1988; Huang et al., 1991). Therefore, we measured DG release from PI in parallel to AA release. Figure 9 shows that thrombin inhibits DG release, rather than stimulating it, and that the effect is calcium-dependent in a manner similar to the stimulation of PLA2. To ascertain the authenticity of thrombin-induced PLC inhibition, we compared the effects of thrombin and IGF-1 on PLA2 and PLC in GCPs. GCPs are known to be rich in IGF-1 receptors (Quiroga et al., 1995), and IGF-1 is known to activate PLC in many systems (Poiraudeau et al., 1997). Figure10 shows AA and DG release from PI (at 10 μm free Ca2+) in response to different concentrations of IGF-1. In the low nanomolar range, IGF-1 does indeed stimulate DG release in GCPs approximately twofold (Fig.10). IGF-1 also stimulates AA release from PI, but only ∼1.7-fold. This activation of PLA2 is a much weaker response than that observed with thrombin (see Figs. 6, 8).
Fig. 9.
Thrombin inhibits growth cone PLC in a calcium-dependent manner. GCPs were incubated alone (control) or in the presence of 100 nm thrombin for 10 min on ice and then combined with substrate ([14C]AA-PI) for 10 min at 37°C in the presence of EGTA or varying micromolar concentrations of CaCl2 (0 [Ca], no addition of Ca2+ or EGTA). DG release was measured as described in Materials and Methods. Data presented are triplicates ± SD.
Fig. 10.
IGF-1 stimulation of PLC and PLA2 in growth cones. GCPs were assayed for both PLA2 and PLC activity as described, in the presence of 10 μmCaCl2 and varying concentrations of IGF-1, using [14C]AA-PI as substrate and recovering [14C]AA and [14C]AA-DG as products. Data are from a representative experiment done in triplicate. Error bars indicate SD; where no error bars appear they were too small to register.
Because of the mild stimulation of PLA2 by IGF-1, we assayed for PLA2 activation by other trophic factors whose receptors are known to be present on growth cones or to be linked to PLA2 stimulation in other cellular systems. (In these experiments, shown in Tables 2-4, net control levels of PLA2 activity in GCPs range from 38 to 107 pmol AA released · min−1 · mg of protein−1, probably because of slight variations in the GCP fraction. However, data sets are shown always with controls measured in the same experimental series.) TrkB, the receptor for BDNF, is readily detectable by immunoblot and enriched in GCPs isolated from whole fetal brain (K. H. Pfenninger and S. Ross, unpublished results). However, BDNF did not significantly stimulate PLA2 activity in GCPs (Table4). BDNF combined with IGF-1 or insulin alone also failed to stimulate PLA2 significantly in our assays (Table 4). When these experiments were performed with PE or PC as substrates, AA release again was only marginally or not at all increased (data not shown).
Table 4.
PLA2 activity in neurons and growth cones: response of GCPs to different factors
| Condition | Concentration (nm) | Substrate | Net activity (pmol · min−1 · mg protein−1) |
|---|---|---|---|
| Control | PI | 107 ± 4 | |
| BDNF | 100 | PI | 112 ± 7 |
| IGF-1 | 0.75 | PI | 125 ± 10 |
| BDNF + IGF-1 | 100/0.75 | PI | 102 ± 8 |
| Insulin | 1 | PI | 106 ± 6 |
Data are from representative experiment done in triplicate (values are means ± SD). All measurements were obtained in the same experiment and share the control indicated.
Generation of eicosanoids
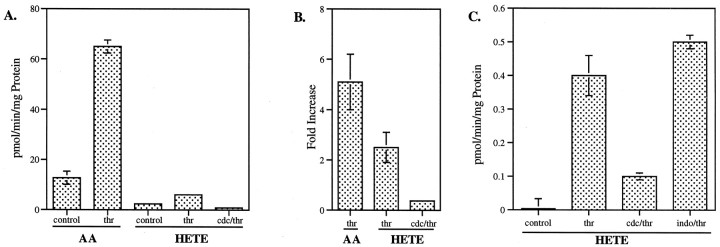
The effect of CDC in collapse and growth cone detachment assays suggests that eicosanoid synthesis from AA is an important step in thrombin and TRAP signaling. Lipid extracts prepared in acid conditions can be resolved by TLC to separate 12/15-HETE (which co-migrate) from the other compounds, including AA, DG, and 5-HETE (Birkle et al., 1988). With this approach we studied the generation of HETE in GCPs, without or with thrombin stimulation. Figure11A shows, in assays involving [14C]AA-PC as a substrate, the generation of AA and of a compound co-migrating in TLC with 12- or 15-HETE. As seen already, there is substantial stimulation of AA release by thrombin. Radioactivity co-migrating with 12-HETE also is increased, ∼2.5-fold above control (Fig. 11B). The selective 12-LO inhibitor CDC reduces thrombin-stimulated HETE levels to below control, suggesting that this compound is indeed 12- and/or 15-HETE. This experiment indicates that thrombin stimulates HETE synthesis in GCPs, but it does not discriminate between the activation of PLA2 (shown by increased AA release) and the possible stimulation of LO activity.
Fig. 11.
Thrombin-stimulated release of AA and HETE. GCPs were assayed for both PLA2 and LO activity as described. Inhibitor (0.156 μm CDC or 0.2 μmindomethacin) was introduced to some GCP samples for 15 min on ice before the addition of 100 nm thrombin. After an additional 10 min incubation on ice, the GCP mixture was then incubated with either [14C]AA-PC (A, B) or [3H]AA (C) in the presence of 10 μm CaCl2 for 15 min at 37°C.A, B, Thrombin-stimulated release of AA and HETE in assays using the PC substrate, shown as actual amounts (A) and fold increase (B).C, Thrombin stimulation of HETE directly from the AA substrate. In A and B, values for control and thrombin-stimulated conditions were averaged from six experiments, all done in triplicate, whereas the values for CDC and indomethacin-treated GCPs were averaged from two experiments, done in triplicate. Data in C are from one representative experiment, done in triplicate. Error bars indicate SD; where not seen, they were too small to be shown.
To determine whether thrombin regulates LO activity, analogous assays were performed with [3H]AA as the substrate (total AA concentration, ∼8 μm). As shown in Figure 11C, thrombin does indeed stimulate 12/15-HETE production from AA, and the effect is strongly inhibited by CDC but not by the cyclooxygenase blocker indomethacin. This suggests thrombin activation of 12-LO, in addition to PLA2. However, the amount of 12/15-HETE generated with thrombin stimulation is only a small fraction of the amount of AA released (Fig.11A).
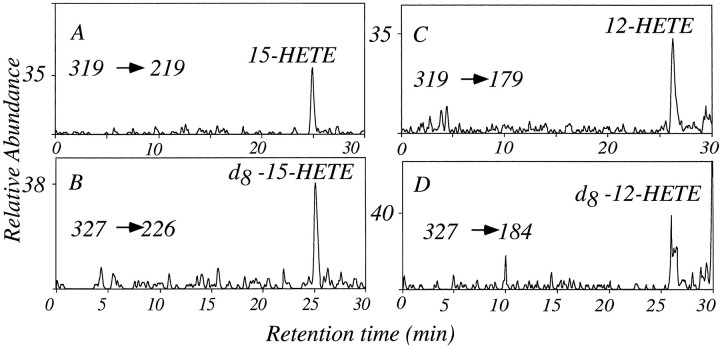
Although TLC is suitable for isolating eicosanoids, identification of the compounds is tentative only because it is based on co-migration with standards. Therefore, we performed LC–MS–MS to detect specifically the generation of HETE isomers present in GCP extracts after incubation with equal molar quantities of unlabeled and D8-AA. The collisional activation of the common carboxylate anion for the unlabeled isomers (m/z319) and labeled isomers (m/z 327) of HETE was performed, and specific ion transitions were monitored to indicate the elution of each HETE species not only by the ion transition but also by the characteristic HPLC retention time. For example, the carboxylate anion from unlabeled 12-HETE was collisionally activated to yield abundant ion at m/z 179, whereas the carboxylate anion from labeled 12-HETE was collisionally activated to yield abundant fragmention at m/z 184. The retention times of the elution of these two components (26.1 min) were virtually identical. Using this multiple reaction monitoring technique, 12- and 15-HETE were both found to elute at the appropriate retention times along with the corresponding deuterated species (Fig.12). The relative quantities of deuterium-labeled species were similar to those of the labeled HETE regioisomers. This supports the conclusion that the origin of recovered HETEs was the exogenous AA added to the GCPs. In separate analyses, known quantities of synthetic 12- and 15-HETE were added to sample aliquots before LC–MS–MS analysis. The corresponding transitions at the appropriate retention time increased for each of the expected HETE regioisomers (data not shown). These techniques revealed the generation in GCPs of both 12- and 15-HETE but could not assign the chirality of each regioisomer.
Fig. 12.
Growth cones synthesize 12- and 15-HETE, as demonstrated by LC–MS–MS. Lipid extracts from growth cones incubated with equal molar quantities of unlabeled and deuterium-labeled (d8) arachidonic acid were resolved by reverse-phase HPLC, and the eluted fractions were analyzed by tandem MS. A, LC–MS–MS corresponding to the collisional activation of m/z 319, yielding the ion at m/z 219, which corresponds to 15-HETE.B, Collisional activation ofm/z 327, yielding product ions atm/z 226, which correspond to D8-15-HETE. C, Collisional activation ofm/z 319, yielding product ions atm/z 179, which correspond to 12-HETE.D, Collisional activation ofm/z 327, yielding product ions atm/z 184, which correspond to D8-12-HETE.
The generation of 12- and 15-HETE by growth cones, the inhibition of this synthesis by CDC (at 0.156 μm), and the inhibition of the thrombin effect on growth cone morphology by CDC suggest that growth cones contain one or several forms of 12-LO. To test for this possibility, we analyzed GCPs by Western blot with the two 12-LO antibodies currently available, specific for platelet 12-LO and leukocyte 12/15-LO, respectively. Gels contained equal amounts of protein from fetal-brain low-speed supernatant (LSS, the crude parent fraction of GCPs) and GCPs. The blots in Figure13 indicate that GCPs are significantly enriched, relative to LSS, in a protein of ∼75 kDa and reactive with the antibody to leukocyte 12/15-LO. No immunoreactivity was detected with anti-platelet 12-LO antibody (data not shown).
Fig. 13.
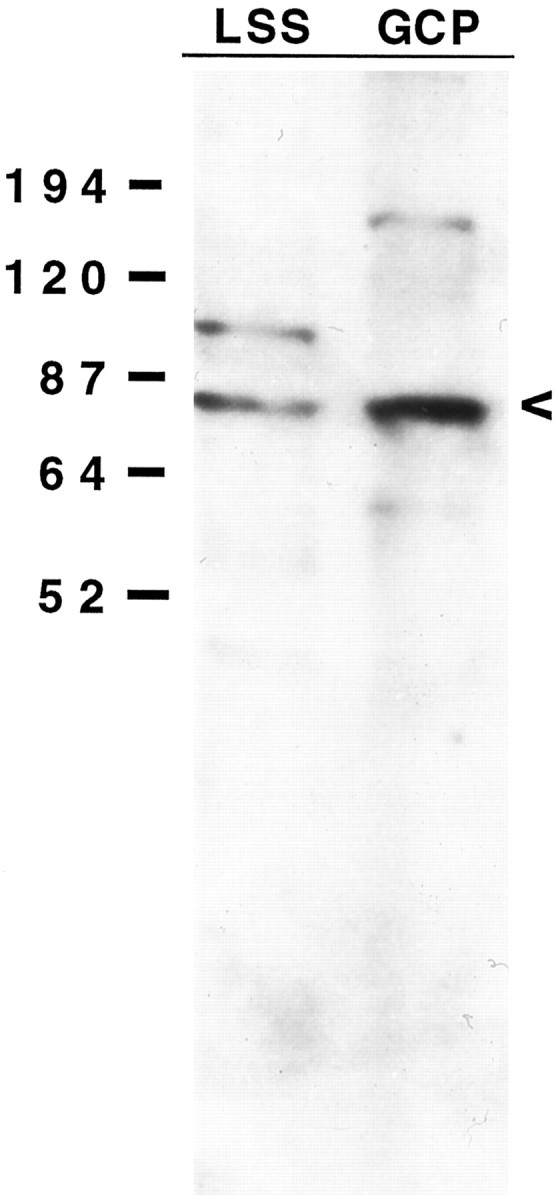
Western blot of GCP polypeptides probed with an antibody to leukocyte 12/15-LO. Equal amounts of protein (30 μg/lane) of LSS and pelleted GCPs were analyzed. The arrowheadindicates a 75 kDa band corresponding to the molecular weight of 12/15-LO. Note that the 75 kDa band is enriched in the GCP lane.
DISCUSSION
Thrombin as a growth cone repellent
Thrombin receptor activation leads to diverse cellular responses, such as secretion and pseudopod spreading in platelets and locomotion and mitogenesis in fibroblasts. However, it causes cell rounding in endothelial and neural cells and pseudopod withdrawal in certain cancer cells (Jalink and Moolenaar, 1992; Grand et al., 1996; Vouret-Craviari et al., 1998) (S. Ross, B. Essary, B. A. de la Houssaye, X. Pan, K. Mikule, O. Mubarak, and K. H. Pfenninger, unpublished results). In neurons, thrombin induces growth cone collapse comprising redistribution and partial depolymerization of the actin skeleton as well as detachment of filopodia and lamellipodia. This is very similar to collapse induced by other factors (Kapfhammer and Raper, 1987; Fan et al., 1993). TRAP mimics the effect of thrombin, indicating that this is a receptor-mediated event, not dependent on proteolysis of adhesion molecules or their extracellular ligands (Jalink and Moolenaar, 1992; Suidan et al., 1992). Thus, thrombin qualifies as a bona-fide collapsing factor and repellent.
Activation of phospholipase and lipoxygenase
In platelets, thrombin activates both cPLA2and PLC (for review, see Grand et al., 1996). It seemed logical, therefore, to assay for these enzymes in cultures of thrombin-responsive primary neurons. Isolated growth cones were of particular interest in view of the enrichment of at least two different cPLA2s in them (Nègre-Aminou et al., 1996; whether the two enzymes occur separately in distinct growth cone populations or together in all GCPs is unknown).
Thrombin and, to a lesser extent, TRAP stimulate PLA2 activity in primary neurons and GCPs, with the highest levels of free AA achieved in GCPs. Growth factors whose receptors are present on GCPs, such as BDNF, NGF, insulin, and IGF-1, stimulate growth cone cPLA2 only weakly or not at all, indicating that thrombin activation is selective. Thrombin increases AA release from phospholipid from sevenfold to fivefold, with the apparent substrate preference PC > PE > PI. Again, this result does not take into account differences in endogenous substrate concentration and/or possible competition among the PLA2s for the substrate. Resting as well as stimulated levels of PLA2 activity are resistant to reducing agents, excluding a contribution of secreted PLA2. PC hydrolysis had not previously been detected in unstimulated GCPs (Nègre-Aminou et al., 1996). Thus, present results suggest that a third, PC-selective cPLA2 may possibly exist in GCPs. We show that thrombin activation of cPLA2 requires Ca2+. However, Nègre-Aminou et al. (1996) reported PE- and PI-selective cPLA2s to be Ca2+-independent. Therefore, Ca2+ is likely to be necessary for a step upstream of cPLA2.
Although growth cones and platelets share receptor-mediated thrombin stimulation of cPLA2, they are different with regard to PLC regulation. Thrombin stimulates platelet PLC, causing the release of inositol trisphosphate and subsequent efflux of Ca2+ from intracellular stores. In GCPs, however, thrombin inhibits this phosphoinositidase, suggesting that intracellular release of Ca2+ is not important for the collapse response. Thrombin-induced Ca2+ transients have been reported for platelets (Grand et al., 1996) and neuroblastoma cells (Jalink and Moolenaar, 1992), but they were shown not to be necessary for thrombin-induced rounding of neuroblastoma and endothelial cells (Jalink and Moolenaar, 1992; Vouret-Craviari et al., 1998). Therefore, our findings are consistent with these observations as well as the fact that (at least so far) Ca2+ transients have not been detectable in nerve growth cones treated with repellents (Ivins et al., 1991; Ming et al., 1997). The second consequence of PLC stimulation, the release of DG, activates PKC in platelets. Lack of PLC stimulation in thrombin-treated GCPs, however, does not rule out a role for PKC in repellent action.
cPLA2s cleave phospholipids into an unsaturated fatty acid, in brain typically AA, and a lysophospholipid. Whether lyso-PI, -PE, and -PC generated in growth cones have functional role(s) is not known. Most released AA is rapidly reincorporated into phospholipid (Nègre-Aminou and Pfenninger, 1993), but AA may directly influence growth cone functions and/or may be converted into one or more eicosanoids (Smith, 1989; Shimizu and Wolfe, 1990). Our biochemical studies show that thrombin stimulates in growth cones not only the release of AA but also the synthesis of an AA-derived compound that co-extracts and co-migrates in TLC with 12- or 15-HETE. The generation of this product is reduced by the selective 12-LO inhibitor CDC but not by the cyclooxygenase inhibitor indomethacin. Finally, LC–MS–MS analysis of GCP extracts definitively demonstrates GCP synthesis of primarily 12- and also 15-HETE.
In sum, our results demonstrate strong and selective thrombin stimulation of one or multiple cPLA2s in growth cones, followed by conversion of some of the released AA into 12/15-HETE. We estimate the conversion to amount to ∼1 pmol, or 5%, of ∼20 pmol of AA released in the same assay (assuming equal recoveries). Although we cannot exclude a role of 15-LO in GCPs, HETEs are likely to be synthesized by 12-LO because of the selective inhibitory effect of CDC at 0.156 μm (Cho et al., 1991) and a report that leukocyte 12/15-LO also generates 15-HETE (Yamamoto et al., 1997). This view is consistent with the observation that growth cones are enriched in a polypeptide that co-migrates in SDS-PAGE with 12-LO (at 75 kDa) and immuno-cross-reacts with an antibody to leukocyte-type 12/15-LO (but not platelet 12-LO).
It is generally assumed that eicosanoid synthesis, including that of 12-HETE, is regulated by the supply of AA, and that 12-LO is constitutively active (Smith, 1989; Shimizu and Wolfe, 1990; Yamamoto et al., 1997). However, we observed that thrombin stimulates not only cPLA2 but also the 12-LO in GCPs.
Functional role of lipid messengers in growth cone collapse
The data discussed so far correlate the collapsing effect of thrombin with cPLA2 activation and eicosanoid synthesis. To determine whether a causal relationship exists, inhibitor experiments were performed. Selective inhibitors of most or all growth cone cPLA2 activity are not known, but specific eicosanoid synthesis inhibitors exist. The cyclooxygenase inhibitor indomethacin does not interfere with thrombin-induced growth cone collapse. However, the general LO inhibitor NDGA and the specific 12-LO inhibitor CDC block thrombin- and TRAP-induced collapse, as well as LO activity in biochemical assays. Conversely, exogenous 12(S)-HETE added to cultures mimics the effects of thrombin or TRAP. These results are consistent with the observed thrombin stimulation of 12/15-HETE synthesis and link cPLA2 activation and 12/15-HETE synthesis functionally to the collapse mechanism.
Growth cone collapse involves reorganization of the actin cytoskeleton as well as filopodial and lamellipodial detachment. Our detachment assay demonstrates that thrombin and TRAP trigger growth cone dissociation from the laminin substratum, even in the absence of a functional actin cytoskeleton. Disassembly of the actin cytoskeleton with cytochalasin D or inhibition of myosin ATPase with BDM interferes to some degree with GCP adhesion. However, release induced by thrombin plus blocker of the actin cytoskeleton approximates the sum of thrombin alone plus the actin function blocker alone. Thus, BDM and cytochalasin D do not block thrombin-induced detachment. This observation is complemented by the finding that growth cones pretreated with CDC and then challenged with thrombin sometimes contain peripherally depleted and proximally condensed F-actin, even though they retain their spread-out, attached filopodia and overall shape. Together these findings may suggest that cPLA2 and leukocyte 12/15-LO are involved primarily in triggering detachment rather than cytoskeletal redistribution.
In conclusion, our data indicate that thrombin is a growth cone repellent that triggers not only redistribution of the actin cytoskeleton but also detachment of filopodia and lamellipodia. Our results indicate further that thrombin-induced growth cone collapse occurs by a mechanism that involves cPLA2 and 12/15-LO. The mode of action of 12/15-HETE is not known, but our preliminary data suggest the involvement of PKC activation and phosphorylation of the adhesion site-associated protein, MARCKS (myristoylated alanine-rich C-kinase substrate), which may trigger disassembly of attachment sites (de la Houssaye et al., 1997; Mikule et al., 1997).
Footnotes
This work was supported by National Institutes of Health Grant R01 NS24672 to K.H.P. We thank Dr. Colin Funk (University of Pennsylvania) for lipoxygenase antibodies and for helpful discussions regarding eicosanoid synthesis. We are particularly indebted to Dr. Robert Murphy, Charis Johnson, and Wesley Martin (National Jewish Center and the University of Colorado) and to Drs. Brad Bendiak and Tammy Fang (University of Colorado) for their assistance with the eicosanoid analysis by mass spectroscopy. Brain-derived neurotrophic factor was the generous gift of Amgen (Thousand Oaks, CA). We also thank David Oquist and Dot Dill for expert assistance with the preparation of this manuscript.
B.A.d.l.H. and K.M. contributed equally to this work.
Correspondence should be addressed to Dr. Karl H. Pfenninger, University of Colorado Health Science Center, Campus Box B-111, Cellular and Structural Biology, 4200 East Ninth Avenue, Denver, CO 80262. E-mail: Karl.pfenninger@UCHSC.edu.
Dr. Nikolic's present address: University of Belgrade School of Medicine, 11000 Belgrade, Yugoslavia.
REFERENCES
- 1.Bar-Sagi D. Phospholipase A2 in ras-transformed cells. In: Dennis EA, Hunter T, Berridge M, editors. Cell activation and signal initiation: receptor and phospholipase control of inositol phosphate, PAF, and eicosanoid production. Liss; New York: 1989. pp. 331–335. [Google Scholar]
- 2.Bar-Sagi D, Feramisco JR. Induction of membrane ruffling and fluid-phase pinocytosis in quiescent fibroblasts by ras proteins. Science. 1986;233:1061–1068. doi: 10.1126/science.3090687. [DOI] [PubMed] [Google Scholar]
- 3.Birkle DL, Bazan EP, Bazan NG. Analysis of prostaglandins, leukotrienes, and related compounds in retina and brain. In: Boulton AA, Baker GB, Horrocks LA, editors. Neuromethods, Vol 7. Humana; Totowa: NJ: 1988. pp. 227–244. [Google Scholar]
- 4.Bligh EG, Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 5.Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 6.Brennan C, Monschau B, Lindberg R, Guthrie B, Drescher U, Bonhoeffer, Holder N. Two Eph receptor tyrosine kinase ligands control axon growth and may be involved in the creation of the retinotectal map in the zebrafish. Development. 1997;124:655–664. doi: 10.1242/dev.124.3.655. [DOI] [PubMed] [Google Scholar]
- 7.Capdevila JH, Falck JR, Estabrook RW. Cytochrome P450 and the arachidonate cascade. FASEB J. 1992;6:731–736. doi: 10.1096/fasebj.6.2.1537463. [DOI] [PubMed] [Google Scholar]
- 8.Cho H, Ueda M, Tamaoka M, Hamaguchi M, Aisaka K, Kiso Y, Inoue T, Ogino R, Tatsuoka T, Ishihara T, Noguchi T, Morita I, Murota S. Novel caffeic acid derivatives: extremely potent inhibitors of 12-lipoxygenase. J Med Chem. 1991;34:1503–1505. doi: 10.1021/jm00108a039. [DOI] [PubMed] [Google Scholar]
- 9.Cramer LP, Mitchison TJ. Myosin is involved in postmitotic cell spreading. J Cell Biol. 1995;131:179–189. doi: 10.1083/jcb.131.1.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Crouch MF, Lapetina EG. A role for Gi in control of thrombin receptor-phospholipase C coupling in human platelets. J Biol Chem. 1988;263:3363–3371. [PubMed] [Google Scholar]
- 11.de la Houssaye BA, Mikule K, Ross S, Pfenninger KH. Thrombin-triggered pseudopod detachment in nerve growth cones and motile cancer cells. Mol Biol Cell. 1997;8:265a. [PubMed] [Google Scholar]
- 12.Dennis EA. Diversity of group types, regulation, and function of phospholipase A2. J Biol Chem. 1994;269:13057–13060. [PubMed] [Google Scholar]
- 13.Drescher U, Kremoser C, Handwerker C, Loschinger J, Noda M, Bonhoeffer F. In vitro guidance of retinal ganglion cell axons by RAGS, a 25 kDa tectal protein related to ligands for Eph receptor tyrosine kinases. Cell. 1995;82:359–370. doi: 10.1016/0092-8674(95)90425-5. [DOI] [PubMed] [Google Scholar]
- 14.Fan J, Mansfield G, Redmond T, Gordon-Weeks PR, Raper JA. The organization of F-actin and microtubules in growth cones exposed to a brain-derived collapsing factor. J Cell Biol. 1993;121:867–878. doi: 10.1083/jcb.121.4.867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Feng D-M, Veber DF, Connolly TM, Condra C, Tang M-J, Nutt RF. Development of a potent thrombin receptor ligand. J Med Chem. 1995;38:4125–4130. doi: 10.1021/jm00020a029. [DOI] [PubMed] [Google Scholar]
- 16.Ferrante A, Goh D, Harvey D, Robinson B, Hii CS, Bates E, Hardy SJ, Johnson DW, Poulos A. Neurtophil migration inhibitory properties of polyunsaturated fatty acids. J Clin Invest. 1994;93:1063–1070. doi: 10.1172/JCI117056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gallo G, Letourneau PC. Axon guidance: GTPases help axons reach their targets. Curr Biol. 1998;8:R80–R82. doi: 10.1016/s0960-9822(98)70051-x. [DOI] [PubMed] [Google Scholar]
- 18.Gebbink M, Kranenburg O, Poland M, van Horck FPG, Houssa B, Moolenaar WH. Identification of a novel, putative Rho-specific GDP/GTP exchange factor and a RhoA-binding protein: control of neuronal morphology. J Cell Biol. 1997;137:1603–1613. doi: 10.1083/jcb.137.7.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goetzl EJ, Woods JM, Gorman RR. Stimulation of human eosinophil and neutrophil polymorphonuclear leukocyte chemotaxis and random migration by 12-l-hydroxy-5,8,10,14-eicosatetraenoic acid. J Clin Invest. 1977;59:179–183. doi: 10.1172/JCI108617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Grand RJA, Turnell AS, Grabham PW. Cellular consequences of thrombin-receptor activation. Biochem J. 1996;313:353–368. doi: 10.1042/bj3130353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gurwitz D, Cunningham DD. Thrombin modulates and reverses neuroblastoma neurite outgrowth. Proc Natl Acad Sci USA. 1988;85:3440–3444. doi: 10.1073/pnas.85.10.3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hawkins RL, Seeds NW. Effect of proteases and their inhibitors on neurite outgrowth from neonatal mouse sensory ganglia in culture. Brain Res. 1986;398:63–70. doi: 10.1016/0006-8993(86)91250-3. [DOI] [PubMed] [Google Scholar]
- 23.Hawkins RL, Seeds NW. Protease inhibitors influence the direction of neurite outgrowth. Dev Brain Res. 1989;45:203–209. doi: 10.1016/0165-3806(89)90039-4. [DOI] [PubMed] [Google Scholar]
- 24.Huang R, Sorisky A, Church WR, Simons ER, Rittenhouse SE. “Thrombin” receptor-directed ligand accounts for activation by thrombin of platelet phospholipase C and accumulation of 3-phosphorylated phosphoinositides. J Biol Chem. 1991;266:18435–18438. [PubMed] [Google Scholar]
- 25.Huttenlocher A, Sandborg R, Horwitz A. Adhesion in cell migration. Curr Opin Cell Biol. 1995;7:697–706. doi: 10.1016/0955-0674(95)80112-x. [DOI] [PubMed] [Google Scholar]
- 26.Igarashi M, Strittmatter SM, Vartanian T, Fishman MC. Mediation by G proteins of signals that cause collapse of growth cones. Science. 1993;259:77–79. doi: 10.1126/science.8418498. [DOI] [PubMed] [Google Scholar]
- 27.Ishihara H, Connolly AJ, Zeng D, Kahn ML, Zheng YW, Timmons C, Tram T, Coughlin SR. Protease-activated receptor 3 is a second thrombin receptor in humans. Nature. 1997;386:502–506. doi: 10.1038/386502a0. [DOI] [PubMed] [Google Scholar]
- 28.Ivins JK, Raper JA, Pittman RN. Intracellular calcium levels do not change during contact-mediated collapse of chick DRG growth cone structure. J Neurosci. 1991;11:1597–1608. doi: 10.1523/JNEUROSCI.11-06-01597.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jalink K, Moolenaar WH. Thrombin receptor activation causes rapid neural cell rounding and neurite retraction independent of classic second messengers. J Cell Biol. 1992;118:411–419. doi: 10.1083/jcb.118.2.411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Jalink K, van Corven EJ, Hengeveld T, Morii N, Narumiya S, Moolenaar WH. Inhibition of lysophosphatidate- and thrombin-induced neurite retraction and neuronal cell rounding by ADP ribosylation of the small GTP-binding protein Rho. J Cell Biol. 1994;126:801–810. doi: 10.1083/jcb.126.3.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jin Z, Strittmatter SM. Rac1 mediates collapsin-1-induced growth cone collapses. J Neurosci. 1997;17:6256–6263. doi: 10.1523/JNEUROSCI.17-16-06256.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kapfhammer JP, Raper JA. Interactions between growth cones and neurites growing from different neural tissues in culture. J Neurosci. 1987;7:1595–1600. doi: 10.1523/JNEUROSCI.07-05-01595.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Katoh H, Aoki J, Ichikawa A, Negishi M. p160 RhoA-binding kinase ROKa Induces Neurite Retraction. J Biol Chem. 1998;273:2489–2492. doi: 10.1074/jbc.273.5.2489. [DOI] [PubMed] [Google Scholar]
- 34.Laemmli UK. Cleavage of structural proteins during assembly of the head of the bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 35.Lamoureux P, Altun-Gultekin ZF, Lin C, Wagner JA, Heidemann SR. Rac is required for growth cone function but not neurite assembly. J Cell Sci. 1997;110:635–641. doi: 10.1242/jcs.110.5.635. [DOI] [PubMed] [Google Scholar]
- 36.Lockerbie RO, Miller VE, Pfenninger Regulated plasmalemmal expansion in nerve growth cones. J Cell Biol. 1991;112:1215–1227. doi: 10.1083/jcb.112.6.1215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lohse K, Helmke SM, Wood MR, Quiroga S, de la Houssaye B, Miller VE, Nègre-Aminou P, Pfenninger KH. Axonal origin and purity of growth cones isolated from fetal rat brain. Dev Brain Res. 1996;96:83–96. doi: 10.1016/0165-3806(96)00076-4. [DOI] [PubMed] [Google Scholar]
- 38.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 39.MacMillan DK, Murphy RC. Analysis of lipid hydroperoxides and long-chain conjugated keto acids by negative ion electrospray mass spectrometry. J Am Soc Mass Spectrom. 1995;6:1190–1201. doi: 10.1016/1044-0305(95)00505-6. [DOI] [PubMed] [Google Scholar]
- 40.Mansuy IM, van der Putten H, Schmid P, Meins M, Botteri FM, Monard D. Variable and multiple expression of protease nexin-1 during mouse organogenesis and nervous system development. Development. 1993;119:1119–1134. doi: 10.1242/dev.119.4.1119. [DOI] [PubMed] [Google Scholar]
- 41.Mikule K, de la Houssaye BA, Pfenninger KH. Collapsin signalling involves phospholipase A2 activation. Soc Neurosci Abstr. 1997;23:600. [Google Scholar]
- 42.Ming GL, Song HJ, Berninger B, Holt C, Tessier-Lavigne M, Poo M-m. cAMP-dependent growth cone guidance by netrin-1. Neuron. 1997;19:1225–1235. doi: 10.1016/s0896-6273(00)80414-6. [DOI] [PubMed] [Google Scholar]
- 43.Monard D. Cell-derived proteases and protease inhibitors as regulators of neurite outgrowth. Trends Neurosci. 1988;11:541–544. doi: 10.1016/0166-2236(88)90182-8. [DOI] [PubMed] [Google Scholar]
- 44.Monard D, Niday E, Limat A, Solomon F. Inhibition of protease activity can lead to neurite extension in neuroblastoma cells. Prog Brain Res. 1983;58:359–364. doi: 10.1016/S0079-6123(08)60037-0. [DOI] [PubMed] [Google Scholar]
- 45.Moolenaar WH, Kranenburg O, Postma FR, Zondag GCM. Lysophosphatidic acid: G-protein signalling and cellular responses. Curr Opin Cell Biol. 1997;9:168–173. doi: 10.1016/s0955-0674(97)80059-2. [DOI] [PubMed] [Google Scholar]
- 46.Mooney DJ, Langer R, Ingber DE. Cytoskeletal filament assembly and the control of cell spreading and function by extracellular matrix. J Cell Sci. 1995;108:2311–2320. doi: 10.1242/jcs.108.6.2311. [DOI] [PubMed] [Google Scholar]
- 47.Nataranjan S, Riexinger D, Peluso M, Sieler SM. Tethered ligand derived pentapeptide agonists of thrombin receptor: a study of side chain requirements for human platelet activation and GTPase stimulation. Int J Peptide Res. 1995;45:145–151. doi: 10.1111/j.1399-3011.1995.tb01033.x. [DOI] [PubMed] [Google Scholar]
- 48.Nègre-Aminou P, Pfenninger KH. Arachidonic acid turnover and phospholipase A2 activity in neuronal growth cones. J Neurochem. 1993;60:1126–1136. doi: 10.1111/j.1471-4159.1993.tb03263.x. [DOI] [PubMed] [Google Scholar]
- 49.Nègre-Aminou P, Nemenoff RA, Wood MR, de la Houssaye BA, Pfenninger KH. Characterization of phospholipase A2 activity enriched in the nerve growth cone. J Neurochem. 1996;67:2599–2608. doi: 10.1046/j.1471-4159.1996.67062599.x. [DOI] [PubMed] [Google Scholar]
- 50.Niclou S, Suidan HS, Brown-Luedi M, Monard D. Expression of the thrombin receptor mRNA in rat brain. Cell Mol Biol. 1994;40:421–428. [PubMed] [Google Scholar]
- 51.Peterson GL. Determination of total protein. Methods Enzymol. 1983;91:95–119. doi: 10.1016/s0076-6879(83)91014-5. [DOI] [PubMed] [Google Scholar]
- 52.Pfenninger KH, Maylié-Pfenninger M-F. Lectin labelling of sprouting neurons. I. Regional distribution of surface glycoconjugates. J Cell Biol. 1981;89:536–546. doi: 10.1083/jcb.89.3.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Pfenninger KH, Ellis L, Johnson MP, Friedman LB, Somlo S. Nerve growth cones isolated from fetal rat brain: subcellular fractionation and characterization. Cell. 1983;35:573–584. doi: 10.1016/0092-8674(83)90191-5. [DOI] [PubMed] [Google Scholar]
- 54.Poiraudeau S, Lieberherr M, Kergosie N, Corvol MT. Different mechanisms are involved in intracellular calcium increase by insulin-like growth factors 1 and 2 in articular chondrocytes: voltage-gated calcium channels, and/or phospholipase C coupled to a pertussis-sensitive G-protein. J Cell Biochem. 1997;64:414–422. [PubMed] [Google Scholar]
- 55.Quiroga S, Garofalo R, Pfenninger KH. Insulin-like growth factor I receptors of fetal brain are eniched in nerve growth cones and contain a β-subunit variant. Proc Natl Acad Sci USA. 1995;92:4309–4312. doi: 10.1073/pnas.92.10.4309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Salari H, Braquet P, Borgeat P. Comparative effects of indomethacin, acetylenic acids, 15-HETE, nordihydroguaiaretic acid and BW755C on the metabolism of arachidonic acid in human leukocytes and platelets. Prostaglandins Leukot Med. 1984;13:53–60. doi: 10.1016/0262-1746(84)90102-1. [DOI] [PubMed] [Google Scholar]
- 57.Sanders LC, Matsumura F, Bokoch GM, de Lanerolle P. Inhibition of myosin light chain kinase by p21-activated kinase. Science. 1999;283:2083–2085. doi: 10.1126/science.283.5410.2083. [DOI] [PubMed] [Google Scholar]
- 58.Schindelholz B, Reber BFX. Bradykinin-induced collapse of rat pheochromocytoma (PC12) cell growth cones: a role for tyrosine kinase activity. J Neurosci. 1997;17:8391–8401. doi: 10.1523/JNEUROSCI.17-21-08391.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Schwab ME. Myelin-associated inhibitors of neurite growth and regeneration in the CNS. Trends Neurosci. 1990;13:452–456. doi: 10.1016/0166-2236(90)90098-u. [DOI] [PubMed] [Google Scholar]
- 60.Shimizu T, Wolfe LS. Arachidonic acid cascade and signal transduction. J Neurochem. 1990;55:1–15. doi: 10.1111/j.1471-4159.1990.tb08813.x. [DOI] [PubMed] [Google Scholar]
- 61.Smalheiser NR, Ali JY. Acute neurite retraction triggered by lysophosphatidic acid: timing of the inhibitory effects of genistein. Brain Res. 1994;600:309–318. doi: 10.1016/0006-8993(94)91304-8. [DOI] [PubMed] [Google Scholar]
- 62.Smith W. Eicosanoids and their biochemical mechanisms of action. Biochem J. 1989;259:315–324. doi: 10.1042/bj2590315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Stossel TP. On the crawling of animal cells. Science. 1993;260:1086–1094. doi: 10.1126/science.8493552. [DOI] [PubMed] [Google Scholar]
- 64.Suidan HS, Stone SR, Hemmings BA, Monard D. Thrombin causes neurite retraction in neuronal cells through activation of cell surface receptors. Neuron. 1992;8:363–375. doi: 10.1016/0896-6273(92)90302-t. [DOI] [PubMed] [Google Scholar]
- 65.Tang DG, Timar J, Grossi IM, Renaud C, Kimler VA, Diglio CA, Taylor JD, Honn KV. The lipoxygenase metabolite, 12(S)-HETE, induces a protein kinase C-dependent cytoskeletal rearrangement and retraction of microvascular endothelial cells. Exp Cell Res. 1993;207:361–375. doi: 10.1006/excr.1993.1203. [DOI] [PubMed] [Google Scholar]
- 66.Tapon N, Hall A. Rho, Rac and Cdc42 GTPases regulate the organization of the actin cytoskeleton. Curr Opin Cell Biol. 1997;9:86–92. doi: 10.1016/s0955-0674(97)80156-1. [DOI] [PubMed] [Google Scholar]
- 67.Teslenko V, Rogers M, Lefkowith JB. Macrophage arachidonate release via both the cytosolic Ca2+-dependent and -independent phospholipases is necessary for cell spreading. Biochim Biophys Acta. 1997;1344:189–199. doi: 10.1016/s0005-2760(96)00137-3. [DOI] [PubMed] [Google Scholar]
- 68.Tessier-Lavigne M, Goodman CS. The molecular biology of axon guidance. Science. 1996;274:1123–1133. doi: 10.1126/science.274.5290.1123. [DOI] [PubMed] [Google Scholar]
- 69.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Vouret-Craviari V, Boquet P, Pouysségur J, Van Obberghen-Schilling E. Regulation of the actin cytoskeleton by thrombin in human endothelial cells: role of Rho proteins in endothelial barrier function. Mol Biol Cell. 1998;9:2639–2653. doi: 10.1091/mbc.9.9.2639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Vu TK, Hung DT, Wheaton VI, Coughlin SR. Molecular cloning of a functional thrombin receptor reveals a novel proteolytic mechanism of receptor activation. Cell. 1991;64:1057–1068. doi: 10.1016/0092-8674(91)90261-v. [DOI] [PubMed] [Google Scholar]
- 72.Weinstein JR, Gold SJ, Cunningham DD, Gall CM. Cellular localization of thrombin receptor mRNA in rat brain: expression by mesencephalic dopaminergic neurons and codistribution with prothrombin mRNA. J Neurosci. 1995;15:2906–2919. doi: 10.1523/JNEUROSCI.15-04-02906.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wen Y, Nadler JL, Gonzales N, Scott S, Clauser E, Natarangan R. Mechanisms of ANG II-induced mitogenic responses: role of 12-lipoxygenase and biphasic MAP kinase. Am J Physiol. 1996;271:C1212–C1220. doi: 10.1152/ajpcell.1996.271.4.C1212. [DOI] [PubMed] [Google Scholar]
- 74.Yamamoto S, Suzuki H, Ueda N. Arachidonate 12-lipoxygenases. Prog Lipid Res. 1997;36:23–41. doi: 10.1016/s0163-7827(97)00002-7. [DOI] [PubMed] [Google Scholar]
- 75.Yoshino H, Kitayama S, Morita K, Okamoto H, Tsujimoto A, Dohi T. 12-Lipoxygenase product as an inhibitor of the action of chemoattractant peptide fMet-Leu-Phe in rat neutrophils. Gen Pharmacol. 1993;24:1249–1251. doi: 10.1016/0306-3623(93)90376-9. [DOI] [PubMed] [Google Scholar]
- 76.Zipkin LD, Kindt RM, Kenyon CJ. Role of a new Rho family member in cell migration an axon guidance in C. elegans. Cell. 1997;90:883–894. doi: 10.1016/s0092-8674(00)80353-0. [DOI] [PubMed] [Google Scholar]