Abstract
Early life adversity (ELA) is a risk factor for major depressive disorder (MDD), however the underlying mechanisms are not well understood. Clinical studies suggest that negative affective biases (the process, whereby cognitive processes such as learning and memory and decision-making are modified by emotional state) represent a vulnerability factor for MDD. In this study we investigate the impact of ELA on affective biases and reward-associated behaviours in rats. Sprague Dawley rat pups underwent 14 days of postnatal maternal separation (180 min/day from postnatal day 1: MS180) whilst control pups remained unhandled. In adulthood, affective biases associated with reward learning and decision-making were assessed using the affective bias test (ABT), or judgement bias task (JBT) respectively. Changes in motivation and reward sensitivity were tested in a progressive ratio (PR) schedule of operant responding and the sucrose preference test (SPT) respectively. We observed that MS180 animals expressed enhanced negative biases in response to acute corticosterone treatment but without effects on antidepressant-induced positive biases. ELA animals were impaired in their ability to develop appropriate biases in response to changes in reward value in a modified ABT but in the absence of any changes in reward sensitivity or motivation. No effects on decision-making were observed in the JBT but MS180 animals failed to develop the same more optimistic behavioural profile as controls in response to an increase in reward value. These findings suggest that ELA in rats increases vulnerability to negative affective biases and impairs animals’ ability to appropriately learn reward value, independent of a reward sensitivity or changes in motivation. These data provide important evidence linking ELA with relevant neuropsychological impairments that may explain increased risk of developing MDD.
Subject terms: Depression, Emotion
Introduction
Major depressive disorder (MDD) is one of the most prevalent mental health conditions affecting modern society and it is projected to become the leading cause of global disability adjusted life years by 2030 [1]. Our understanding of the aetiology of MDD is limited, and whilst epidemiological studies have implicated risk factors including early life adversity, chronic stress, and family history [2–4], how these relate to vulnerability are relatively unknown. In 1967, Beck first proposed that adverse experiences in early life contribute to the development of negative schemata that ultimately lead to negative biases in the processing of emotional information [5]. More recently, studies in clinical populations suggest a key role of negative affective biases in learning, memory and decision-making in the development, maintenance and treatment of MDD and have led to a renewed interest in a cognitive neuropsychological hypothesis of depression and antidepressant action [6–8]. Negative affective biases across a number of cognitive domains have been observed in depressed patients, including negative interpretation of ambiguous information and a reduced memory for positively valenced stimuli [9–11]. Not only do these biases tend to persist into clinical remission and have been linked to an increased risk of relapse, but they have also been observed in non-depressed individuals who are at high risk of developing MDD [12–15]. This evidence indicates that, rather than being a simple marker of low mood, negative affective biases may represent a vulnerability factor for MDD.
The use of human neuropsychological tests to assay objective measures of affective biases in the clinic has provided us with the opportunity to use ‘reverse translation’ to evaluate similar neuropsychological processes in laboratory animals (for review see Hales et al. [16]). In particular we have developed the Affective Bias Test (ABT) to study biases in reward-learning and memory [17, 18]. This task uses associative learning between specific cues (digging substrates) and reward (food pellet) to test the influence of affective state at the time of learning on the subsequent relative valuation of that reward-association. Validation experiments have shown that acute changes in affective state at the time of learning biases subsequent choice behaviour in this assay [17]. We have recently used a modified version of this task where animals are trained to associated one digging substrate with a higher value reward which, during the preference test, induces a bias towards that substrate, referred to as a “reward-induced positive bias”. We have shown that animals in putative depression-like states fail to develop this bias independent of effects on simple hedonic responses [19]. The judgement bias task (JBT) is used to assay affective biases linked to decision-making behaviour, and test animal’s interpretation of ambiguous information within the context of positive versus negative/less positive associations [20, 21]. Together with other research groups, we have shown that animals in putative negative affective states make more pessimistic choices in response to ambiguous cues [20–23].
On the basis of these initial findings we designed a series of studies to investigate the hypothesis that early life adversity [24, 25], is associated with negative affective biases in these tasks and this underlies vulnerability to MDD. Repeated maternal separation in rats is commonly used as a model of early life adversity, with animals demonstrating exaggerated responses to stress and depression-like behaviours in adulthood [26, 27]. Our initial studies were designed to assess the impact of early life adversity on the development of stress- and reward-induced biases in the ABT, as well as interpretation biases in the JBT. We also assay consummatory anhedonia in the sucrose preference test (SPT), and reward motivation in an operant progressive ratio (PR) task, to compare the effects of early life adversity on distinct measures of reward processing.
Methods and Materials
Maternal separation
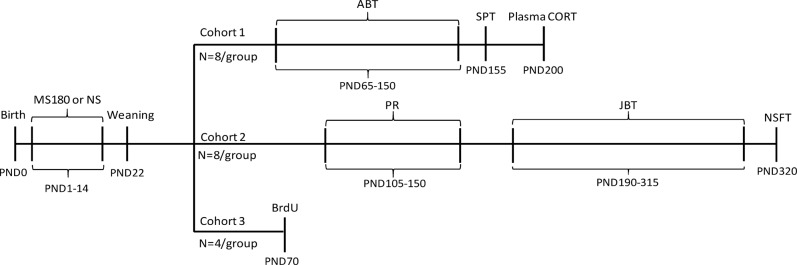
The maternal separation procedure was adapted from Mirescu et al. [28]. Litters were bred in-house from 7 female and 2 male Sprague Dawley rats, and standardised to 10 pups (6–7 males, 3–4 females) on postnatal day (PND) 1. Litters were assigned to one of two rearing conditions from PND 1–14: handled with 180 mins maternal separation per day (MS180) or no maternal separation or handling (NS) (full details provided in Supplementary Material). On PND22, all rats were weaned and housed as same-sex and same-litter pairs. All experiments were carried out from PND 65 in male rats only. Three cohorts of adult male Sprague Dawley rats were used in these studies (outlined in Fig. 1) with representation from different litters distributed across the cohorts. We also ran additional statistical analysis to check that there was no significant difference between litters within each rearing group (2-way ANOVA with REARING and LITTER as factors). Although the number of litters and therefore parental backgrounds were relatively small and are a limitation, our data suggest that the results were not influenced by litter effects. An example of the individual data for animals from the different litters is shown in the supplementary material (Supplementary Fig. S2). Validation of the MS180 model was achieved by measuring stress responsiveness (plasma corticosterone), neurogenesis (BrdU immunohistochemistry), novelty suppressed feeding and consummatory anhedonia (sucrose preference test).
Fig. 1.
Outline of the study protocol. Three cohorts of rats acquired from the maternal separation protocol were used for the behavioural and immunohistological studies. The above schematic outlines the time points of each experimental procedure. PND: postnatal day, MS180: Maternal separation 180 min/day procedure, NS non-separated control animals, ABT affective bias test, SPT sucrose preference test, CORT corticosterone, JBT judgement bias task, PR progressive ratio, NFST novelty suppressed feeding test
Rats in cohort 1 weighed ~320–350 g at the start of behavioural training for the ABT (PND65) and rats in cohort 2 weighed ~410–470 g at the start of PR training (PND105). Rats in cohort 3 weighed ~330–350 g at the time of BrdU injections (PND70). There was no significant difference in body weight at any of the time points post-weaning (Table S3). During behavioural testing rats were maintained at approximately 90% of their free-feeding weight by restricting access to laboratory chow (Purina, UK) to ~18 g per rat per day. Water was provided ad libitum. All rats were maintained in temperature-controlled conditions on a 12:12 h light–dark cycle (lights off at 07:00 h), and behavioural testing was carried out between 09:00–17:00 during the animals’ active phase. All procedures received ethical approval by the UK Home Office and were conducted in adherence to the regulations of the 1986 Animals (Scientific Procedures) Act, EU Directive 2010/63/EU and ARRIVE guidelines [29].
Validation of the ELA model
Plasma corticosterone
Blood samples were taken from the lateral tail vein immediately following removal of the animal from the home cage (baseline stress level), or on a separate occasion following 20 min in a restraint tube. Plasma corticosterone was determined by radioimmunoassay. Full methods are described in the Supplementary Material.
Novelty suppressed feeding test (NSFT)
Rats were food deprived for 24 h before being placed at the edge of a circular, opaque test arena (diameter: 70 cm, height: 50 cm) lined with sawdust and containing a ceramic bowl (diameter: 10 cm) at the centre filled with standard laboratory chow. Latency to approach the food and latency to feed were recorded and the animal was returned to the home cage either once feeding commenced, or after a maximum of 15 min.
BrdU immunohistochemistry and quantification
Cell proliferation was assessed by staining for the exogenous thymidine analogue 5-bromodeoxyuridine (BrdU). Animals received 4 injections of BrdU (50 mg/kg i.p.) at 2-hour intervals and were sacrificed 24 h after the last injection. BrdU-positive cells in the hippocampus were visualised using a DAB-staining protocol (Supplementary Material). BrdU-positive labelled cells were counted in the subgranular zone of the hippocampus from 6 bregma levels at 40-times magnification (−2.76, −3.24, −3.76, −5.40, −5.64, −6.00). Results were analysed as the total number of counted cells.
Sucrose preference test (SPT)
Animals were acclimated to drinking a 1% sucrose solution from two drip-resistant water bottles for 48 h (Ancare, USA). On the test day, animals were water restricted for 4 h and moved into individual clean cages for 30 min before testing. The rats were then given a 1 h two-bottle choice sucrose consumption test (one bottle of water and one bottle containing a 1% sucrose solution).
Affective bias test (ABT)
General Protocol
A detailed description of the training and testing procedure is provided in the Supplementary Material and in Stuart et al. [17]. Animals were first trained to dig in bowl containing sawdust to retrieve a food reward (45 mg rodent tablet, TestDiet, Sandown Scientific UK). Training was complete once each rat was able to find the pellets on 12 consecutive trials within 20 sec for each trial. Animals then underwent a discrimination session consisting of discrete trials where the animal was placed into the test arena and allowed to approach and explore two bowls: one ‘rewarded’ substrate (CS+), and one ‘blank’ unrewarded substrate. (CS-). Once the animal started digging in one bowl, the other was removed by the experimenter. Animals were required to achieve 6 consecutive correct trials from a maximum of 20 trials to progress to testing.
Drug-induced bias studies
These studies followed a standard protocol of four pairing sessions followed by a preference test session on the fifth day. Each of the pairing sessions followed the same protocol as the discrimination session described above. A within-subject design was used wherein each animal learned to associate two different digging substrates (CS+A or CS+B) with a food pellet reward during pairing sessions. Independent pairing sessions (CS+A vs CS− or CS+B vs CS−) were carried out on days 1–4 (Supplementary Fig. S1A) and, on the fifth day, the rats were presented with both previously reinforced substrates together for the first time (CS + A vs CS+B) and their choices over 30 trials recorded. Drug-induced affective bias is established by pairing one session with pre-treatment of the test drug (either corticosterone or venlafaxine), and the other session with pre-treatment of vehicle. Treatment groups are outlined in Tables S1 and S2. We tested both corticosterone and venlafaxine to determine whether animals showed an exaggerated response to negative biases specifically or whether ELA caused a more general increase in affective biases irrespective of the valence. Choice bias was calculated as the proportion of choices made for the drug-paired substrate vs. the total number of trials (drug-paired substrate + vehicle-paired substrate). A value of 50 was then subtracted from the choice bias score to give a % choice bias where a bias towards the drug-paired substrate gave a positive value and a bias towards the vehicle-paired substrate gave a negative value.
Drugs
Corticosterone was purchased from Sigma Aldrich, UK, dissolved in a 5% DMS0, 95% sesame oil vehicle, and administered at a dose range of 0–30 mg/kg via the subcutaneous (s.c.) route. Vehicle-treated animals received an s.c. injection of the DMSO/sesame oil vehicle. Venlafaxine was purchased from Hello Bio, UK, dissolved in 0.9% saline, and administered at a dose range of 0–30 mg/kg via the intraperitoneal (i.p.) route using a modified handling technique to minimise stress (Stuart and Robinson, 2015). Vehicle-treated animals in this study received 0.9% saline i.p. All doses were administered at a volume of 1 ml/kg, 30 mins before ABT sessions. The dose ranges were selected based on previously published data using the ABT [17, 18].
Reward-induced positive bias
Each animal underwent pairing sessions as previously described, however one substrate (CS+A or CS+B) was paired with a single food pellet reward, and the other paired with 2 food pellets (Supplementary Fig. S1B) in the absence of any drug treatment. Preference test trials were run with a single pellet using the random reinforcement protocol.
Judgement Bias Task (JBT)
General protocol
Rats were trained in an operant version of the JBT where they learned to make an active response (lever press) to an auditory tone predicting a positive ‘P’ outcome (receiving food reward) and to make an active avoidance response (mount platform) to a light cue predicting a combined positive and negative ‘P/N’ outcome (receiving food reward and avoiding footshock). Previous studies have used cues which predict either obtaining reward or punishment avoidance which are thought to results in animals developing a positive valence and negative valence respectively [21, 30, 31]. The avoidance response is time consuming to train and requires relatively high shock intensities risking development of learned helplessness, therefore, in this study we used a reward plus punishment avoidance association for the lower value association. Full details of the training and testing procedure are provided in the Supplementary Material. Briefly, training and testing sessions were carried out in six computer-controlled standard operant boxes (MedAssociates, Sandown Scientific, UK) fitted with a lever and custom-built insulated platform. K-Limbic software (Conclusive Solutions Ltd., UK) was used to program behavioural protocols and for data acquisition. Animals underwent a progressive training procedure involving initial training to the positive’P’ cue (2 kHz, 75 dB) where a lever press response resulted in delivery of a food reward (45 mg rodent tablet, TestDiet, Sandown Scientific UK). Once animals reached 70% accuracy they progressed to positive and negative ‘P/N’-cue training. For the P/N cue (yellow house light), an active response involved mounting the platform which avoided footshock and delivered a food pellet reward. Footshock was delivered at 0.1 mA and increased by 0.01 mA each day until animals achieved a criterion of 70% active avoidance. The final footshock amplitude was 0.2 mA, and the maximum duration was 30 s of 13 pulses [0.2 s shock ON, 1.8 s shock OFF] if the animals did not subsequently avoid the shock by mounting the platform. A summary of the stages used for training, number of sessions and trials at each stage are given in Table 1. There were no differences in training between the groups (RMANOVA with session and group as factors). The final stage of training involved a discrimination stage where rats were trained to discriminate between the P and P/N cues. Animals were considered trained once they achieved > 70% accuracy over three consecutive days.
Table 1.
Summary of JBT training procedure
| Stage | Details | Number of sessions to criteria | Number of trials per session | Group difference |
|---|---|---|---|---|
| 1 | Reward training | 16 | 100 trials | none |
| 2 | Punishment training stage 1 | 5 | 50 trials | none |
| 3 | Punishment training stage 2 | 6 | 30 trials | none |
| 4 | ‘P’ vs ‘P’ discrimination, Lever - 1 pellet vs platform - 1 pellet | 8 | 100 trials | none |
| 5 | ‘P’ vs ‘P/N’ discrimination, Lever - 1 pellet vs platform - 1 pellet and footshock avoidance | 12 | 100 trials | none |
| 6 | ‘P’ vs ‘P/N’ discrimination, Lever - 2 pellets vs platform - 1 pellet and footshock avoidance | 3 | 100 trials | none |
Ambiguous (compound) cue testing
Each testing session consisted of 40 trials each of the P and P/N cues, and 20 ambiguous (compound) cue trials. Each cue (20 s presentation) was presented in randomised order and separated by a 10 s ITI. A lever or platform response during compound cue trials resulted in random P or P/N outcomes. A lack of response during the 20 s cue presentation was marked as an omission. Cognitive bias index (CBI) was calculated as the proportion of P/N responses to the ambiguous cue subtracted from the proportion of P responses, creating values in the range of −1 and 1. Negative values indicated a negative judgement bias and positive values indicated a positive judgement bias.
Animals were initially tested under the same contingencies as training however the results showed very negative biases in each group suggesting the relative saliency of the two outcomes were not appropriately balanced, resulting in a potential floor effect. To address this, animals were re-trained to new contingencies with the ‘P’ response paired with 2 food pellets and ‘N’ response paired with 1 food pellet and the avoidance of footshock. Animals were then re-tested using an ambiguous probe session.
Progressive ratio operant responding
General protocol
Operant training was carried out in 8 identical computer-controlled operant chambers (dimensions 30.5 × 24.1 × 21.0 cm, MedAssociates, Sandown Scientific, UK) inside light- and sound-attenuating boxes. Only one lever (left or right, counter-balanced across all animals) was active during this experiment. The reinforcer was a single reward pellet (45 mg rodent tablet, TestDiet, Sandown Scientific UK). Rats were initially trained on a fixed ratio (FR) 1 schedule then progressed to a gradual PR series: 1, 1, 2, 2, 3, 3, 4, 4, etc. The breakpoint for each animal was defined as the final completed ratio before responding ceased for a period of 5 min or more.
Operant testing under a progressive ratio schedule
Once animals reached a stable breakpoint (<10% change across 3 consecutive sessions), the motivational load of the task was increased for the testing sessions using the exponential PR schedule: 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, …. This is derived from the formula [(5 × e0.2n)−5], rounded to the nearest integer, where n is the position in the sequence of ratios [32, 33]. Animals were tested across 3 consecutive sessions under food restriction (high motivational state), and then given food ad libitum for 2 days before being tested in another 3 sessions under free feeding conditions (low motivational state).
Statistical analysis
All studies were performed with the experimenter blind to rearing group and drug treatment until the end of the study. All doses and other experimental factors were fully counter-balanced to avoid bias. A mixed-model ANOVA was used to analyse % choice bias data from dose-response experiments in the ABT (between-subject factor: GROUP, within-subject factor: DRUG) as well as % positive responses, response latency and % omissions in the JBT (between-subject factor: GROUP, within-subject factor: CUE). The % choice bias data from the reward-induced positive bias study, as well as CBI, sucrose preference, NSFT and plasma corticosterone data were analysed with an unpaired t-test comparing MS180 vs. NS group. Post hoc analysis for each treatment used a one-sample t-test against a theoretical mean of 0% choice bias where 0% is equivalent to 15 choices for the treatment- (drug or high reward) paired substrate and 15 choices for the control- (vehicle or low reward) paired substrate [17, 19]. Post hoc analysis was made using pairwise comparisons between MS180 and NS groups if a significant main effect or interaction (p < 0.05) was observed. Bonferroni correction was applied for multiple pairwise comparisons, Huynh-Feldt correction was used to adjust for violations of the sphericity assumption, and Levene’s test was used to correct for inequality of variances. Statistical analyses were performed using SPSS Statistics 23 and graphs created using GraphPad Prism v7.
Results
Validation of the ELA model
Plasma corticosterone
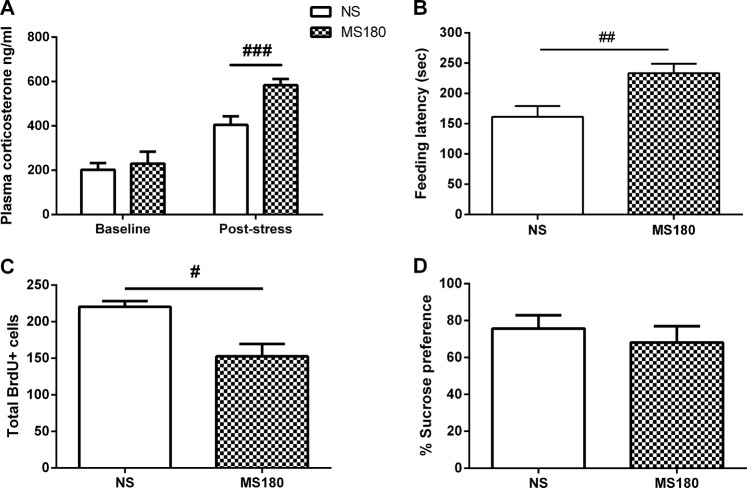
There was no difference in plasma corticosterone levels at baseline. In the restraint stress experiment the MS180 had significantly higher corticosterone levels (unpaired t test: t9 = 6.16, p = 0.0002, n = 4–6/group; three blood samples from the NS group and two from the MS180 group could not be obtained, and one data point from the NS group was an outlier [2 SD from the mean] and so excluded; Fig. 2a).
Fig. 2.
Validation of the ELA model. Maternal separation (MS180) increases the corticosterone response to acute restraint stress (a), increases the feeding latency in a novel environment (b), and reduces the total number of BrdU-positive cells in the hippocampus (c) compared to non-separated (NS) animals. MS180 animals do not show reduced sucrose preference in the SPT (d). Data represents the mean ± SEM, #p < 0.05, ###p < 0.001; n = 4–6/group (CORT response), n = 8/group (NSFT), n = 4/group (BrdU), n = 8/group (SPT)
NSFT
MS180 animals had a longer latency to feed compared to NS animals in the NFST (unpaired t-test: t12 = 3.06, p = 0.01, n = 8/group; Fig. 2b). There was no difference in approach latency between groups (Supplementary Fig. S3).
Hippocampal BrdU
MS180 animals showed a reduction in the number of total BrdU + cells in the hippocampus compared to NS animals (unpaired t-test, t5 = 3.17, p = 0.025, n = 4/group; Fig. 2c).
SPT
The MS180 and NS animals had a preference for a 1% sucrose solution over water, but there was no difference between groups (Fig. 2d). There was no group difference in total fluid consumption (Supplementary Fig. S4).
Affective Bias Test
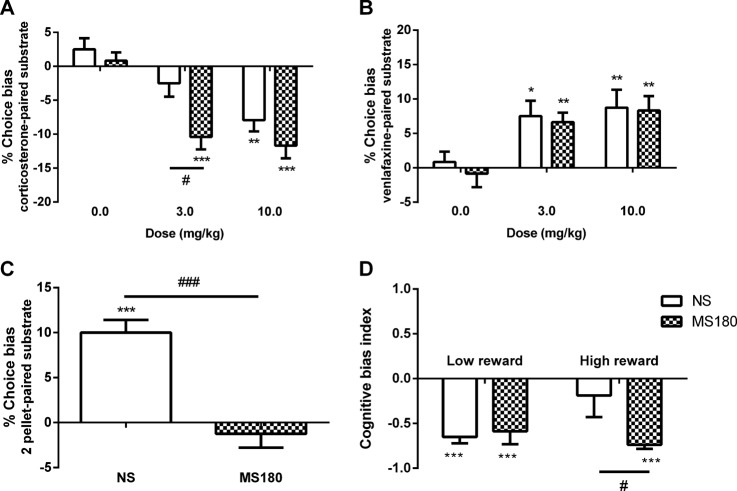
Corticosterone induced a dose-dependent negative affective bias in both groups (RM ANOVA, DRUG: F2,28 = 22.8, p < 0.001; GROUP: F1,14 = 10.7, p = 0.006; DRUGxGROUP: F2,28 = 1.66, p = 0.208, n = 8/group; Fig. 3a). However, at the lower dose of 10 mg/kg, MS180 rats showed a greater negative bias than the NS group (unpaired t test: t14 = 2.95, p = 0.01). Venlafaxine induced a dose-dependent positive affective bias with no group difference (DRUG: F2,28 = 16.6, p < 0.0001, GROUP: F1,14 = 0.21, p = 0.66, DRUGxGROUP: F2,28 = 0.08, p = 0.923, n = 8/group; Fig. 3b). In the modified version of the ABT, NS rats developed a reward-induced positive bias for the higher value substrate, an effect that was not observed in the MS180 group (unpaired t test: t14 = 5.4, p < 0.0001, n = 8/group; Fig. 3c).
Fig. 3.
ELA increases corticosterone-induced negative affective bias and impairs reward-induced positive bias in the ABT and animals show impaired responses to an increase in reward in the JBT. Acute treatment with corticosterone induces a negative affective bias in rats in the ABT, with maternal separation (MS180) animals showing a greater bias at a lower dose compared to non-separated (NS) animals (a), however there was no effect of ELA on positive bias induced by the antidepressant, venlafaxine (b). MS180 animals show a significant deficit in reward-associated positive bias (c). Both MS180 and NS groups show a negative cognitive bias index (CBI) when the positive (P) cue predicts a low value of reward in the JBT (d). When the reward value is increased, NS but not MS180 animals shift towards a less negative CBI, indicating a less pessimistic judgement bias. Data represent the mean ± SEM, *p < 0.05, **p < 0.01, ***p < 0.001 vs 0% choice bias, #p < 0.05, ###p < 0.001 vs NS; n = 16/group (ABT), n = 7 per group (low reward JBT), n = 8 per group (high reward JBT)
Judgement bias task (JBT)
There was no difference in CBI between the MS180 and NS animals in the initial JBT with both groups exhibiting a negative CBI (one sample t-test vs. CBI = 0.0: [NS: t7 = 9.19, p < 0.0001], [MS180: t6 = 7.07, p = 0.004], n = 8/group; Fig. 3d). There was a main effect of CUE for the percentage of positive lever presses (RM ANOVA, F2,26 = 629, p < 0.0001), showing the animals were able to discriminate between previously learned cues, however there was no effect of GROUP or GROUP × CUE interaction (Supplementary Fig. S5). There were no main effects of ELA on response latency or omissions (Supplementary Fig. S6). After the relative value of the outcomes was altered, the MS animals failed to shift their bias in the same, more positive direction as the controls suggesting a failure to integrate the new reward information resulting in a relatively higher level of anticipation of negative events compared to controls. A significant effect on CBI was observed, with the NS animals showing a less negative score compared to the MS180 group (unpaired t-test, t14 = 2.23, p = 0.043; Fig. 3d). There was a main effect of GROUP (RM ANOVA, F1,14 = 5.95, p = 0.029) and GROUP × CUE interaction (RM ANOVA, F2,28 = 3.46, p = 0.046; Supplementary Fig. S7) on the percentage of positive lever presses. Post-hoc analysis revealed that NS animals made a greater percentage of responses on the positive lever in response to the ambiguous, compound cue compared to MS180 animals (post hoc pairwise comparisons, p = 0.043; Supplementary Fig. S7). The NS group also showed a trend towards greater responding on the positive lever in response to the N cue (post hoc pairwise comparison, p = 0.066; Supplementary Fig. S6). There was no effect of group on response latencies or omissions (Supplementary Fig. S8).
Progressive ratio operant responding
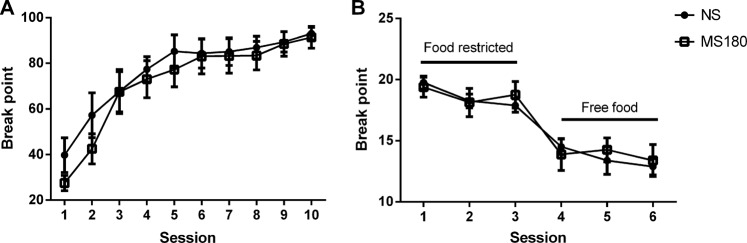
There was no effect of ELA on the break point of responding across the 10 training sessions analysed (Fig. 4a). There was also no observed difference between MS180 and NS animals under conditions of food restriction or free food (Fig. 4b).
Fig. 4.
ELA has no effect on motivation to obtain reward in an operant progressive ratio task. Maternal separation (MS180) animals show no difference from non-separated (NS) controls in performance during training on a progressive ratio schedule (gradual PR series: 1, 1, 2, 2, 3, 3, 4, 4, etc) of reward (left panel). Using the more challenging test schedule (1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50 etc), no effect was observed during either a state of high motivation (food restriction), or low motivation (free food) (right panel). Data shown as mean ± SEM, n = 8/group
Discussion
In the present study, we show that repeated maternal separation of rat pups induces behavioural and physiological changes in adulthood that are characteristic of an animal model of early life adversity. MS180 animals demonstrate an exaggerated corticosterone response to acute restraint stress, as well as increased hyponeophagia compared to control animals. The number of litters we have sampled from may present a limitation to the behavioural data, however, these effects have been well described in the literature and have also been seen in other animal models of depression, including chronic mild stress [27, 34, 35]. We also show that the model causes a decrease in hippocampal neurogenesis consistent with previous studies in rats [28]. These findings suggest that our MS180 method induced the expected phenotype and confirmed the presence of a deficit in adult animals.
We then carried out a series of studies that have revealed some important characteristics of the model in relation to affective biases. We have shown that animals subjected to ELA appear to have an increased sensitivity to corticosterone-induced negative bias in adulthood. The dose-response curve is shifted such that these animals show a negative affective bias at a dose of the drug which does not induce a negative bias in control animals. This effect may relate to the exaggerated responses to stressors characteristic of ELA models and links this vulnerability to an increased propensity to negative affective biases. Using the modified version of the ABT, we are able to investigate the effects of ELA on reward-related learning and memory and subsequent anticipation of reward. In this assay the animals must make a decision about which substrate-reward cue to select based on their prior experience of the associated reward value. We show that normal rats develop a positive bias towards the substrate that has previously been paired with a higher value of reward. This is consistent with studies showing that rodents will learn to associate a cue with a higher value reward and subsequently demonstrate a preference for that cue over one that predicts a lower value reward [36–38]. This reward-induced positive bias is lost in the ELA model which we suggest reflects a failure to appropriately anticipate the greater value of the substrate paired with the higher value reward. We have previously shown that animals treated chronically with ‘pro-depressant’ drugs, ie. drugs that are known to increase the risk of negative affective states such as MDD in humans, results in a similar deficit in reward-induced positive bias [19]. Interestingly our data show that this effect occurs in the absence of a reduced preference for a 1% sucrose solution vs. water, replicating findings from other groups that fail to show an effect of ELA in the SPT [26, 39]. Together with our current data, these findings would suggest that the reward deficits observed in the ABT are independent of the hedonic response as measured by the SPT. While tasks like the SPT are able to quantify consummatory aspects of reward, and how animals experience pleasure at the time of consumption, the deficits in human depression are more complex [40, 41]. Depression is more commonly associated with impairments in anticipation of reward which we propose represent an interaction between cues which predict reward, activation of memory processes, and subsequent recall of expected reward value, which then drive goal-directed behaviour. Whilst various methods involving chronic stress have been shown to decrease sucrose preference in rodents [42], several researchers have been unable to replicate these findings [43–45]. These results from human and animal studies suggest that perhaps the SPT is less suitable as an assessment of anhedonia relevant to depressive disorders, and we propose that our current work adds to previous observations that reward deficits measured using the modified ABT may be more relevant to the reward deficits seen in MDD.
Previous studies using the JBT have shown that chronic stressors induce enduring negative biases in decision-making [22, 23]. In the first study, no differences were observed between groups however, the groups both showed very negative CBIs which may have resulted in a floor effect. As this was a new version of the task involving reward plus punishment avoidance, we cannot be sure whether the relative valence and value was learnt in line with our predictions. After the relative value of the outcomes was altered, the MS animals failed to shift their bias in the same, more positive direction as the controls suggesting a failure to integrate the new reward information resulting in a relatively higher level of anticipation of negative events compared to controls. As the animals were re-trained to new contingencies, the effect may have been related to a failure of the MS animals to update cue-reward associations in order to drive appropriate decision-making behaviour. When the value of the cue predicting a positive outcome is increased, normal animals modify their behaviour and show a less pessimistic interpretation of an ambiguous cue. The failure of animals that have undergone ELA to similarly shift their responses in this task could be due to a failure to integrate new information about the value of a reward-associated cue into their decisions.
It is important to note that the pessimistic judgement biases exhibited by both treatment groups is characteristic of reward vs. punishment versions of the JBT [20]. In a more recent version of the task where the two learned cue-outcome associations are linked to different values of reward (high reward vs low reward), rats instead show a positive judgement bias that becomes more negative after chronic restraint stress [21]. This version may therefore be useful in further investigating the effects of ELA on judgment biases in rats.
Clinical Implications
Together our findings support the evidence from clinical studies that stress in early life leads to lasting changes in neuropsychological processes relevant to MDD. ELA represents a strong vulnerability factor for MDD and our work suggests that it causes increased sensitivity to acute stress-induced negative affective biases in learning, memory and pessimistic decision-making. These may play a key role in the development of MDD in adulthood. Indeed prospective studies in patients have shown that negative processing biases, for example when interpreting ambiguous stimuli, predict negative affect in response to stress [46].
Our work has also revealed key differences between reward-related learning and memory deficits in the ABT and behaviours measured using the SPT and PR tasks, suggesting a distinction between hedonic and motivational deficits compared with cognitive aspects of reward processing. The deficits observed in the ABT and the ELA model compare favourably with findings in MDD patients who demonstrate a deficit in goal-directed behaviours linked to the anticipation of reward in the absence of consummatory anhedonia [40, 41]. Further studies to test whether antidepressant treatment is able to remediate the deficits in reward processing present in ELA animals is warranted to determine a causal relationship of negative affective biases in MDD. Overall our findings support a neuropsychological hypothesis in the vulnerability to MDD and why at-risk individuals who have previously experienced ELA may be more likely to exhibit changes in reward processing and develop negative biases during periods of stress.
Supplementary information
Funding and Disclosure
This research was funded by the Medical Research Council (MR/L011212/1). JKH is funded by a University of Bristol PhD Studentship. ESJR has recieved academic research funding from Boehringer Ingelheim, Eli Lilly, Pfizer, MSD and undertaken contract research for SmallPharma. None of these companies have been directly involved in the research published in this article. The authors declare no competing interests.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41386-019-0388-6).
References
- 1.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heim C, Nemeroff CB. The role of childhood trauma in the neurobiology of mood and anxiety disorders: preclinical and clinical studies. Biol Psychiatry. 2001;49:1023–39. doi: 10.1016/S0006-3223(01)01157-X. [DOI] [PubMed] [Google Scholar]
- 3.Hammen C. Stress and depression. Annu Rev Clin Psychol. 2005;1:293–319. doi: 10.1146/annurev.clinpsy.1.102803.143938. [DOI] [PubMed] [Google Scholar]
- 4.Sullivan PF, Neale MC, Kendler KS. Genetic epidemiology of major depression: review and meta-analysis. Am J Psychiatry. 2000;157:1552–62. doi: 10.1176/appi.ajp.157.10.1552. [DOI] [PubMed] [Google Scholar]
- 5.Beck AT. Depression: clinical, experimental, and theoretical aspects. Hoeber Medical Division; New York, 1967.
- 6.Elliott R, Zahn R, Deakin JF, Anderson IM. Affective cognition and its disruption in mood disorders. Neuropsychopharmacology. 2011;36:153–82. doi: 10.1038/npp.2010.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Roiser JP, Elliott R, Sahakian BJ. Cognitive mechanisms of treatment in depression. Neuropsychopharmacology. 2012;37:117–36. doi: 10.1038/npp.2011.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harmer CJ, Goodwin GM, Cowen PJ. Why do antidepressants take so long to work? A cognitive neuropsychological model of antidepressant drug action. Br J Psychiatry. 2009;195:102–8. doi: 10.1192/bjp.bp.108.051193. [DOI] [PubMed] [Google Scholar]
- 9.Bouhuys AL, Geerts E, Gordijn MC. Depressed patients’ perceptions of facial emotions in depressed and remitted states are associated with relapse: a longitudinal study. J Nerv Ment Dis. 1999;187:595–602. doi: 10.1097/00005053-199910000-00002. [DOI] [PubMed] [Google Scholar]
- 10.Gur RC, Erwin RJ, Gur RE, Zwil AS, Heimberg C, Kraemer HC. Facial emotion discrimination: II. Behavioral findings in depression. Psychiatry Res. 1992;42:241–51. doi: 10.1016/0165-1781(92)90116-K. [DOI] [PubMed] [Google Scholar]
- 11.Surguladze SA, Young AW, Senior C, Brébion G, Travis MJ, Phillips ML. Recognition accuracy and response bias to happy and sad facial expressions in patients with major depression. Neuropsychology. 2004;18:212–8. doi: 10.1037/0894-4105.18.2.212. [DOI] [PubMed] [Google Scholar]
- 12.Hayward G, Goodwin GM, Cowen PJ, Harmer CJ. Low-dose tryptophan depletion in recovered depressed patients induces changes in cognitive processing without depressive symptoms. Biol Psychiatry. 2005;57:517–24. doi: 10.1016/j.biopsych.2004.11.016. [DOI] [PubMed] [Google Scholar]
- 13.Chan SW, Goodwin GM, Harmer CJ. Highly neurotic never-depressed students have negative biases in information processing. Psychol Med. 2007;37:1281–91. doi: 10.1017/S0033291707000669. [DOI] [PubMed] [Google Scholar]
- 14.Joormann J, Talbot L, Gotlib IH. Biased processing of emotional information in girls at risk for depression. J Abnorm Psychol. 2007;116:135–43. doi: 10.1037/0021-843X.116.1.135. [DOI] [PubMed] [Google Scholar]
- 15.Dearing KF, Gotlib IH. Interpretation of ambiguous information in girls at risk for depression. J Abnorm Child Psychol. 2009;37:79–91. doi: 10.1007/s10802-008-9259-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hales CA, Stuart SA, Anderson MH, Robinson ES. Modelling cognitive affective biases in major depressive disorder using rodents. Br J Pharmacol. 2014;171:4524–38. doi: 10.1111/bph.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stuart SA, Butler P, Munafò MR, Nutt DJ, Robinson ES. A translational rodent assay of affective biases in depression and antidepressant therapy. Neuropsychopharmacology. 2013;38:1625–35. doi: 10.1038/npp.2013.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stuart SA, Robinson ES. Reducing the stress of drug administration: implications for the 3Rs. Sci Rep. 2015;5:14288. doi: 10.1038/srep14288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stuart SA, Wood CM, Robinson ESJ. Using the affective bias test to predict drug-induced negative affect: implications for drug safety. Br J Pharmacol. 2017;174:3200–10. doi: 10.1111/bph.13972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anderson MH, Munafò MR, Robinson ES. Investigating the psychopharmacology of cognitive affective bias in rats using an affective tone discrimination task. Psychopharmacology. 2013;226:601–13. doi: 10.1007/s00213-012-2932-5. [DOI] [PubMed] [Google Scholar]
- 21.Hales CA, Robinson ES, Houghton CJ. Diffusion modelling reveals the decision making processes underlying negative judgement bias in rats. PLoS ONE. 2016;11:e0152592. doi: 10.1371/journal.pone.0152592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Papciak J, Popik P, Fuchs E, Rygula R. Chronic psychosocial stress makes rats more ‘pessimistic’ in the ambiguous-cue interpretation paradigm. Behav Brain Res. 2013;256:305–10. doi: 10.1016/j.bbr.2013.08.036. [DOI] [PubMed] [Google Scholar]
- 23.Rygula R, Papciak J, Popik P. Trait pessimism predicts vulnerability to stress-induced anhedonia in rats. Neuropsychopharmacology. 2013;38:2188–96. doi: 10.1038/npp.2013.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heim C, Nemeroff CB. Neurobiology of early life stress: clinical studies. Semin Clin Neuropsychiatry. 2002;7:147–59. doi: 10.1053/scnp.2002.33127. [DOI] [PubMed] [Google Scholar]
- 25.Danese A, Moffitt TE, Harrington H, Milne BJ, Polanczyk G, Pariante CM, et al. Adverse childhood experiences and adult risk factors for age-related disease: depression, inflammation, and clustering of metabolic risk markers. Arch Pedia Adolesc Med. 2009;163:1135–43. doi: 10.1001/archpediatrics.2009.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Matthews K, Robbins TW. Early experience as a determinant of adult behavioural responses to reward: the effects of repeated maternal separation in the rat. Neurosci Biobehav Rev. 2003;27:45–55. doi: 10.1016/S0149-7634(03)00008-3. [DOI] [PubMed] [Google Scholar]
- 27.Aisa B, Tordera R, Lasheras B, Del Río J, Ramírez MJ. Cognitive impairment associated to HPA axis hyperactivity after maternal separation in rats. Psychoneuroendocrinology. 2007;32:256–66. doi: 10.1016/j.psyneuen.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 28.Mirescu C, Peters JD, Gould E. Early life experience alters response of adult neurogenesis to stress. Nat Neurosci. 2004;7:841–6. doi: 10.1038/nn1290. [DOI] [PubMed] [Google Scholar]
- 29.McGrath JC, Lilley E. Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol. 2015;172:3189–93. doi: 10.1111/bph.12955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Enkel T, Gholizadeh D, von Bohlen Und Halbach O, Sanchis-Segura C, Hurlemann R, Spanagel R, et al. Ambiguous-cue interpretation is biased under stress- and depression-like states in rats. Neuropsychopharmacology. 2010;35:1008–15. doi: 10.1038/npp.2009.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harding EJ, Paul ES, Mendl M. Animal behaviour: cognitive bias and affective state. Nature. 2004;427:312. doi: 10.1038/427312a. [DOI] [PubMed] [Google Scholar]
- 32.Roberts D, Richardson N. Self-administration of psychomotor stimulants using progressive ratio schedules of reinforcement. Totowa: Humana; 1992.
- 33.Rickard JF, Body S, Zhang Z, Bradshaw CM, Szabadi E. Effect of reinforcer magnitude on performance maintained by progressive-ratio schedules. J Exp Anal Behav. 2009;91:75–87. doi: 10.1901/jeab.2009.91-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, et al. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry. 2011;69:754–61. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McCormick CM, Smith C, Mathews IZ. Effects of chronic social stress in adolescence on anxiety and neuroendocrine response to mild stress in male and female rats. Behav Brain Res. 2008;187:228–38. doi: 10.1016/j.bbr.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 36.Vogel JR, Mikulka PJ, Spear NE. Effects of shifts in sucrose and saccharine concentrations on licking behavior in the rat. J Comp Physiol Psychol. 1968;66:661–6. doi: 10.1037/h0026556. [DOI] [PubMed] [Google Scholar]
- 37.Lattal KA, Gleeson S. Response acquisition with delayed reinforcement. J Exp Psychol Anim Behav Process. 1990;16:27–39. doi: 10.1037/0097-7403.16.1.27. [DOI] [PubMed] [Google Scholar]
- 38.Giertler C, Bohn I, Hauber W. The rat nucleus accumbens is involved in guiding of instrumental responses by stimuli predicting reward magnitude. Eur J Neurosci. 2003;18:1993–6. doi: 10.1046/j.1460-9568.2003.02904.x. [DOI] [PubMed] [Google Scholar]
- 39.Shalev U, Kafkafi N. Repeated maternal separation does not alter sucrose-reinforced and open-field behaviors. Pharm Biochem Behav. 2002;73:115–22. doi: 10.1016/S0091-3057(02)00756-6. [DOI] [PubMed] [Google Scholar]
- 40.Berlin I, Givry-Steiner L, Lecrubier Y, Puech AJ. Measures of anhedonia and hedonic responses to sucrose in depressive and schizophrenic patients in comparison with healthy subjects. Eur Psychiatry. 1998;13:303–9. doi: 10.1016/S0924-9338(98)80048-5. [DOI] [PubMed] [Google Scholar]
- 41.Dichter GS, Smoski MJ, Kampov-Polevoy AB, Gallop R, Garbutt JC. Unipolar depression does not moderate responses to the Sweet Taste Test. Depress Anxiety. 2010;27:859–63. doi: 10.1002/da.20690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Willner P, Muscat R, Papp M. Chronic mild stress-induced anhedonia: a realistic animal model of depression. Neurosci Biobehav Rev. 1992;16:525–34. doi: 10.1016/S0149-7634(05)80194-0. [DOI] [PubMed] [Google Scholar]
- 43.Forbes NF, Stewart CA, Matthews K, Reid IC. Chronic mild stress and sucrose consumption: validity as a model of depression. Physiol Behav. 1996;60:1481–4. doi: 10.1016/S0031-9384(96)00305-8. [DOI] [PubMed] [Google Scholar]
- 44.Harris RB, Zhou J, Youngblood BD, Smagin GN, Ryan DH. Failure to change exploration or saccharin preference in rats exposed to chronic mild stress. Physiol Behav. 1997;63:91–100. doi: 10.1016/S0031-9384(97)00425-3. [DOI] [PubMed] [Google Scholar]
- 45.Reid I, Forbes N, Stewart C, Matthews K. Chronic mild stress and depressive disorder: a useful new model? Psychopharmacol (Berl) 1997;134:365–7. doi: 10.1007/s002130050471. [DOI] [PubMed] [Google Scholar]
- 46.Pury Cynthia L.S. Information-processing predictors of emotional response to stress. Cognition & Emotion. 2002;16(5):667–683. doi: 10.1080/02699930143000400. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.