Abstract
The neural mechanisms and durability of Δ9-tetrahydrocannabinol (THC) impact on threat processing in humans are not fully understood. Herein, we used functional MRI and psychophysiological tools to examine the influence of THC on the mechanisms of conditioned threat extinction learning, and the effects of THC on extinction memory retention when assessed 1 day and 1 week from learning. Healthy participants underwent threat conditioning on day 1. On day 2, participants were randomized to take one pill of THC or placebo (PBO) 2-h before threat extinction learning. Extinction memory retention was assessed 1 day and 1 week after extinction learning. We found that THC administration increased amygdala and ventromedial prefrontal cortex (vmPFC) activation during early extinction learning with no significant impact on skin conductance responses (SCR). When extinction memory retention was tested 24 h after learning, the THC group exhibited lower SCRs to the extinguished cue with no significant extinction-induced activations within the extinction network. When extinction memory retention was tested 1 week after learning, the THC group exhibited significantly decreased responses to the extinguished cues within the vmPFC and amygdala, but significantly increased functional coupling between the vmPFC, hippocampus, and dorsal anterior cingulate cortex during this extinction retention test. Our results are the first to report a long-term impact of one dose of THC on the functional activation of the threat extinction network and unveil a significant change in functional connectivity emerging after a week from engagement. We highlight the need for further investigating the long-term impact of THC on threat and anxiety circuitry.
Subject terms: Cognitive neuroscience, Emotion
Introduction
The recreational use of cannabis within the United states is becoming more mainstream, especially with the recent legalization of its use across many States. Its use worldwide has also been on the rise. In addition, cannabis use for clinical purposes is becoming more accepted. In turn, research efforts have been on the rise to try to understand and characterize the impact of Δ9-tetrahydrocannabinol (THC) use on the brain and behaviors, especially those associated with the impact of THC on emotion regulation [1]. There is an accumulating mass of rodent and human literature on the mechanisms by which THC could modulate neural correlates of emotion regulation. One key aspect that remains to be explored is the long-term impact of THC use on the functional activation of brain regions involved in affect regulation [1].
A plethora of studies now show that the amygdala, hippocampus, ventromedial prefrontal cortex (vmPFC), and dorsal anterior cingulate cortex (dACC) are part of a network critical for detecting and responding to threatening encounters, encoding contextual information about threat and safety, and retaining and expressing extinction memories [2]. We refer to these brain regions henceforth as the threat network. For example, preclinical studies have implicated the endocannabinoid system as a modulator of the threat extinction network. Many of these studies show that enhancing the cannabinoid system engages the threat extinction network [3–9]. The expression of cannabinoid type-1 (CB1) receptors is highly concentrated in the amygdala-hippocampus-corticostriatal circuit [10]. THC, which is the major psychoactive constituent in cannabis, is a non-specific CB1/CB2 ligand that exerts its central effects mainly though agonism of the CB1 receptor [11]. Animal studies indicate that manipulation of the CB1 receptor could modulate memory consolidation associated with threat learning and inhibition. For example, blocking CB1 receptors in mice resulted in failure to extinguish conditioned threat [12], whereas administering CB1 receptor agonists prior to extinction enhanced extinction learning and long-term retention of extinction memory in a drug-free state [13]. Aside from influencing extinction memory consolidation, CB1 receptor agonists appear to block reconsolidation of aversive 1-day and 7-day old memories when given directly after retrieval [14].
Translational work is starting to uncover a similar role for the endocannabinoid system in emotion regulation in the human brain. In healthy humans, THC administration influences the activation of the threat network, generally enhancing threat memory extinction. In prior studies, we found that THC attenuates amygdala activation in response to fearful stimuli [15], increases functional coupling between amygdala and medial prefrontal cortex [16], and reduces subgenual ACC reactivity to negative cues [17]. At the psychophysiological level, THC reduced conditioned skin conductance responses (SCRs) during extinction learning, but did not affect fear-potentiated startle [11]. We have previously examined the effects of THC on threat extinction learning and memory in healthy humans using psychophysiological indices for conditioned threat, and found that a low-dose of THC administered to healthy adults prior to extinction learning enhanced the threat extinction memory compared to placebo [18]. However, no effect of THC was observed during the within-session extinction learning phase as indexed by SCR [18]. In a follow-up study with the same design, we showed that THC administration attenuated amygdala reactivity to the conditioned stimulus during early extinction, and increased activation in the vmPFC and hippocampus during extinction memory testing [19]. The parallel between the rodent and human data suggests a role for THC in threat processes and extinction retention, though the long-term mechanisms of the modulation and durability of THC’s effects on threat extinction retention and retrieval are still unclear.
The longevity of the effects of THC on the neural correlates of memory of extinction learning and its psychophysiological indices has not been examined. In the present study, our first objective was to attempt to replicate the findings from the Rabinak et al. [19] study using a different paradigm that adds contextual manipulation into threat conditioning, extinction learning and extinction memory retention. The second objective was to test the neural mechanisms and interactions of the threat extinction network when extinction memory retention is retrieved one week after learning. This latter test is to evaluate the influence of THC on long-term extinction memory retention. To achieve our objectives, we randomized (double-blind) a sample of healthy humans into two groups: THC and Placebo. Participants ingested either an oral dose of synthetic THC or a placebo pill prior to extinction learning in a 4-day Pavlovian threat extinction paradigm. Outcome measures were skin conductance level and blood oxygenation level dependent (BOLD) response. We anticipated that THC would enhance extinction memory retention when tested after a short and long delay and we expected a significant activation of the threat-extinction network during the long-term extinction retention test. We submit that a test conducted one week after learning is still relatively short and does not capture the impact of THC use months and years after its use on emotion regulation. Also, the experimental design used in the present study examines the effects of a single dose of THC, which is unlikely to be the same effect as that used after multiple or chronic use. Nonetheless, the data to be obtained would be the first to show any potential impact of a single dose beyond the customary within-session or 24-h test points.
Materials and methods
Participants
Fifty-two healthy participants were recruited from the community. Individuals reporting use of marijuana more than 10 times in life, use within the past 30 months, adverse reaction to cannabinoid substances, use of psychoactive or psychotropic medications or medication that would interact with dronabinol, medical illness, DSM-IV lifetime Axis I disorder or MRI exclusion criteria were not eligible for participation.
After excluding individuals that did not complete all experimental days or showed excessive head motion during scanning, 40 participants remained in the final sample. Participants were randomized to receive THC (n = 20) or placebo (n = 20). Description of all demographics can be found in Supplementary Table 1. Self-reported drug effects questionnaires and the results obtained from them, can be found in Supplemental Methods and Results sections. To reduce any between-group effects of sex hormones on our experimental outcomes, all women (22) participants underwent the experiment within approximately 1 week prior to menses onset. Of the 22 women, only 5 were on oral contraceptives (3 in THC group and 2 in PBO group). The University of Illinois-Chicago Institutional Review Board approved study procedures.
Threat conditioning and extinction procedure
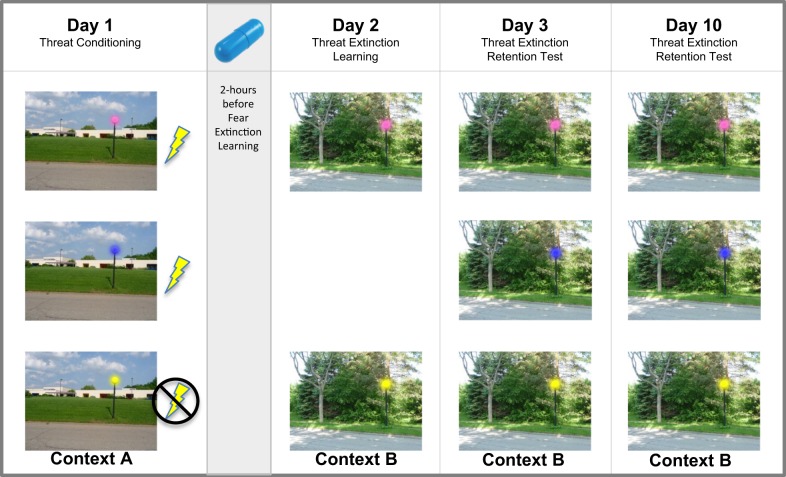
The experimental protocol was similar to that used in our prior studies [19, 20], took place over 10 days and included 4 visits (Fig. 1). On Day-1, participants completed threat conditioning training in context A, in the absence of any drug. Participants were presented with three cues, two of which were terminated with an unconditioned stimulus (termed CS+ vs. cue not followed by shock will be termed CS−). The unconditioned stimulus was an aversive mild electric shock to the left ankle that lasted 0.5 s and with maximum intensity 4.4 mA. We used a partial reinforcement rate of 60%. All participants were asked to select a shock level that was “highly annoying, but not painful”. There were 40 CS+ trials and 20 CS− trials. On Day 2, participants ingested an opaque gelatin capsule (size 00) with dextrose filler that contained either synthetic THC (generic dronabinol 7.5 mg; PAR Pharmaceutical) or dextrose alone (PBO). This dose has been shown to affect threat extinction processes in humans [19] and it is the lowest effective dose found to produce behavioral and subjective effects [15, 17]. Approximately 2-h after ingesting the capsule, participants completed threat extinction learning in context B. Timing of the session was designed to match the expected window of peak subjective effects and plasma levels of THC and matches our prior pharmacological-fMRI design [19]. During extinction learning, participants viewed 20 trials each of two cues: one of the CS+ cues, now labeled CS+ E and the CS−.
Fig. 1.
Threat conditioning and extinction paradigm. Threat conditioning occurred on Day 1 in context A by pairing a mild shock (unconditioned stimulus) to the lamp light colors pink and blue (termed conditioned stimuli or CS+), but not yellow (termed non-conditioned stimulus or CS-). THC (dronabinol 7.5 mg) or placebo was administered 2-h before threat extinction learning on Day-2, where one CS+ (pink light, now termed extinguished CS+ or CSE) and the CS− are shown in context B. Extinction memory retention was tested one day (Day 3) and one week (Day 10) after extinction learning, where both CS+s (pink light, CSE and the blue light, now termed unextinguished CS+ or CSU) and the CS− are shown
24-hours after extinction learning (Day 3) and 1-week after extinction learning (Day 10), memory of extinction learning was tested in context B. In these two sessions, participants viewed all three cues: the CS+E, CS−, and the other CS+, now CS+U that was not extinguished on Day 2. Participants were given one unsignaled shock prior to the onset of extinction and of the test phases (in the absence of a CS) and were told that they may or may not receive shocks during the task.
For each of the phases the visual context (A or B) was presented for 3–8 s, followed by presentation of the CS (within the visual context) for 4 s. All trials were interleaved with a fixation cross for 4–9 s and the task was presented over the course of two runs. Total task time for Conditioning, Day 3 and Day 10 was 15-min; Extinction was 10-min.
Skin conductance response (SCR) analysis
For details regarding the SCR data acquisition, see Supplemental Methods. For the conditioning phase, SCRs for all the CS+trials not followed by the US and all CS-s were analyzed to test for differential threat conditioning within and between groups using two-way ANOVA [21, 22]. For extinction learning, given that extinction occurs very quickly and skin responses decrease rapidly in human experiments [23], we focused on analyses of extinction learning on the first 10 trials (first 5 trials, block1, and trials 6–10, block 2). We conducted a group (THC vs. PBO) × trial × CS type ANOVA examining the first 5 trials which correspond to the same period interrogated by our fMRI analysis. During Days 3 and Day 10 tests, we compared SCRs during the last 4 trials from extinction learning and first 4 trials from recall (for CS+E) [24]. For statistical analysis of this measure, we used one-tailed Student t-test, given our a priori hypothesis that THC would reduce the response to threat during the extinction memory retention test, based on two already published studies from our group [18, 19].
Functional MRI analysis
For details regarding functional MRI data acquisition and scanner details, see supplemental methods. For each person, we created individual contrast maps for the following: CS+ vs. CS− during early (first 5 trials) and late (last 5 trials) extinction learning, early CS+E vs. CS+U during Day 3 and Day 10. The rationale for selecting these specific trials for each phase is based on prior fMRI studies showing distinct neural activations for these specific trials within each of the learning phases being examined [for review see ref. [25]. We had strong a priori hypotheses that extinction learning would engage the amygdala, anterior and posterior hippocampus, dACC and vmPFC, and that THC would modulate the activations of these regions. Therefore, we searched for significant activations that fell within our regions of interest (ROIs) defined as 5 mm radius spheres around peak activations found in our previous published study [19]. In addition, and since we are conducting de novo connectivity analysis on the effect of THC on the threat network, we used ROIs defined a priori using anatomical masks (MARINA: http://www.bion.de/Marina.htm) defined by atlas-based boundaries [26]. Within our ROIs (across Extinction and Day 3 and Day 10), activations surviving small volume correction (SVC) at p < 0.05 family-wise error (FWE) were considered significant.
Psychophysiological interaction analysis
Given that group differences, at the neural level during the threat extinction memory test, were found only in Day 10, we performed psychophysiological interaction analysis [gPPI; http://brainmap.wisc.edu/PPI; [27]] during this phase only. The seed of interest (SOI) in our analysis was a 5 mm radius sphere around the peak vmPFC activation during Day 10. The de-convolved time series from the SOI was extracted for each subject to create the physiological variable. The stimulus onset times for CS+E and CS+U were separately convolved with the canonical hemodynamic response function, creating the psychological regressors. The physiological variable was multiplied by the time series from the psychological regressors (CSs) to create the interaction terms (PPIs). Activity within the SOI was then regressed on a voxel-wise basis against the interaction. The physiological and psychological variables, as well as motion parameters, were included as regressors of no interest. We entered individual contrast images for CS+E vs. CS+U during the early part of that phase, into a 2nd-level between-group (PBO vs. THC) test to determine if there was any effect of THC on functional connectivity. We set the significance at p < 0.005 (uncorrected) with a cluster extent threshold greater than 10 contiguous voxels to balance between Type I and Type II errors [28].
Results
Threat extinction learning: SCR and BOLD
The focus of our study was to examine the impact of single dose of THC on extinction learning and its memory retention. However, it is important to show that all subjects showed comparable threat conditioning. To accomplish this, we analyzed the SCR during the threat conditioning phase of the study. We found that threat conditioning was successful in all participants as indicated by a significant difference between CS+ and CS− (main effect of CS: F(1,44) = 24.212, p < 0.0001). Importantly, the to-be PBO and to-be THC groups did not differ in conditioning as evidenced by the absence of main effect of group (F(1,44) = 1.036, p = 0.314) and of CS by group interaction (F(1,44) = 1.952, p = 0.17). Thus, these data indicate clear differential threat conditioning that did not differ between groups. Supplemental Fig. 1A depicts these results.
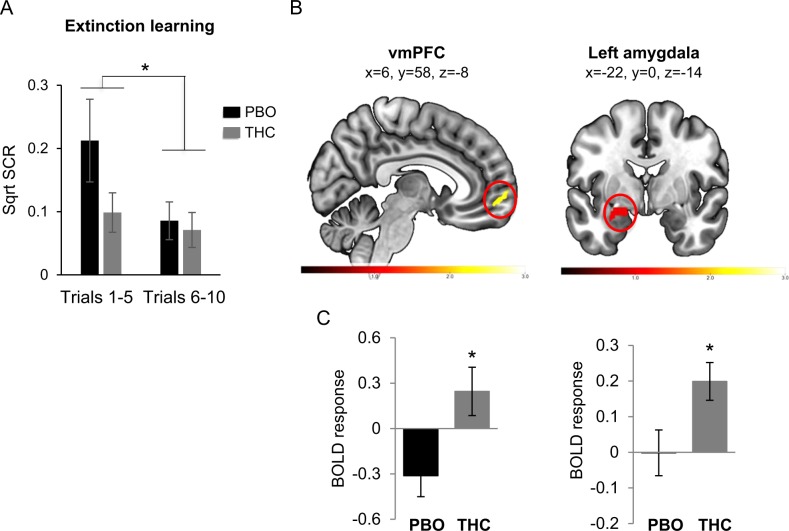
Regarding extinction learning, we first examined if extinction learning took place using SCR data. We focused on the first and second blocks of CS+ extinction for each group (Fig. 2a). This analysis showed a main effect of block (F(1,44) = 9.29, p = 0.004), no main effect of group (F(1,44) = 1.39, p = 0.24) and a significant block × group interaction (F(1,44) = 3.84, p = 0.05) indicating that extinction learning took place from block 1 to block 2 and hinting to potential impact of THC on extinction learning: either facilitating extinction learning or blunting SCR expression during early extinction learning. In an attempt to clarify this point, we conducted a trial-by-trial analysis for the first 5 extinction trials for the CS+. We found a main effect of trial (F(4,176) = 7.65, p < 0.001) and marginal effect of group (F(1,44) = 3.42, p = 0.071) (see Supplemental Results and Supplemental Fig. 1B), suggesting a potential marginal effect of THC on reducing the expression of SCR during early extinction learning. To test if THC had a non-specific effect on expression of SCR, we examined the expression of SCR during the very first 2 extinction trials (CS+ vs. CS−). We found that the two groups did not differ in the SCR expression at the beginning of extinction learning (see Supplemental Results and Supplemental Fig. 1C), arguing against a general impact of THC on SCR expression.
Fig. 2.
Skin conductance response and BOLD response during early extinction learning. a Skin conductance response during extinction learning to the conditioned stimulus (CS+) over trial blocks (5 trials per block) in both the PBO and THC groups. *p < 0.05. b BOLD signal for the early CS+ vs. early CS− (first 5 trials) during extinction learning. Results show a significant group difference in the following regions: vmPFC (left) and left amygdala (right). Color bars represent t-maps. c Extracted average beta-weights (and standard errors) from the significant cluster for the for each group (THC and PBO) with the vmPFC (left) and amygdala (right). Only regions with significant group differences that survive small volume correction are displayed. n(PBO) = 20 and n(THC) = 20. (vmPFC = ventromedial prefrontal cortex). *p < 0.05
Regarding the BOLD responses during extinction learning, we found that the group of individuals who received THC had significantly increased activation of the vmPFC (t = 2.60, k = 37, p = 0.03, Fig. 2b) and the left amygdala (t = 2.81, k = 96, p = 0.02, Fig. 2b). Extracted beta weights from these loci show that THC caused an increased relative activation in both the vmPFC and amygdala (Fig. 2c). As for late extinction learning, no significant between group differences were observed at either the SCR or BOLD responses (data not shown).
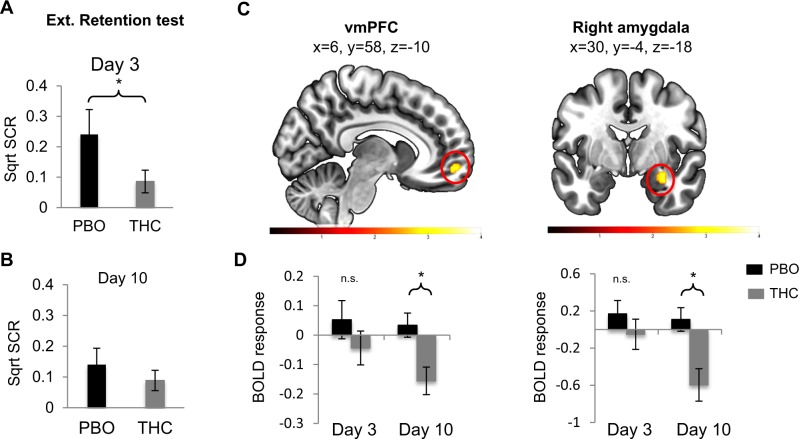
Extinction memory retention test on Day 3 and on Day 10
We first focused on analyzing the SCR data during extinction memory retention tests (Day 3 and Day 10). We subtracted SCRs to the last four trials of CS+E during extinction learning from the first four trials of CS+E in extinction memory test on Day 3 to specifically test the rebound of threat conditioned responses to the extinguished cue (CS+E). We observed a significant between-group difference (t(37) = 2.06, p = 0.04, Fig. 3a) showing that the THC group expressed significantly less SCR responses during this test point. For Day 10, no significant between-group differences were observed (t(37) = 1.28, p = 0.21, Fig. 3b). As for the BOLD responses during Day 3, we did not observe any significant between-group differences within our a priori ROIs. During Day 10, however, there was significantly higher activation by the PBO group within the vmPFC (t = 3.36, k = 14, p = 0.013) and right amygdala (t = 3.23, k = 54, p = 0.026, Fig. 3c). Extracted BOLD responses from these brain regions for both time points (Day 3 and Day 10) are shown in Fig. 3d. As can be noted from the figure, the THC group showed a significant reduction in activation within both the amygdala and vmPFC during the Day-10 test.
Fig. 3.
Skin conductance response and BOLD response during the first and second extinction memory tests (Day 3 and Day 10). a, b The retention test is calculated by subtracting the average SCR to the first 4 trials in the extinction memory tests from the average SCR to the last 4 trials of extinction. a Retention index shows significantly blunted return of fear on Day 3 in the THC group compared to the PBO group. *p < 0.05. b No significant group difference in retention index on Day 10. Bar graph showing mean+/− standard error. c BOLD signal for the early (first 5 trials) CS+E vs. early CS+U during extinction memory tests on Day 3 and Day 10. Results show significant group difference only on Day 10 in the following regions: vmPFC (left) and right amygdala (right). Color bars represent t-maps. d Extracted average beta-weights (and standard errors) from the significant cluster for the early CS+E vs. early CS+U contrast. Only regions with significant group differences that survive small volume correction are displayed. n(PBO) = 20 and n(THC) = 20 on Day 3, and 17 Day 10. (Ext., extinction; vmPFC, ventromedial prefrontal cortex). *p < 0.05
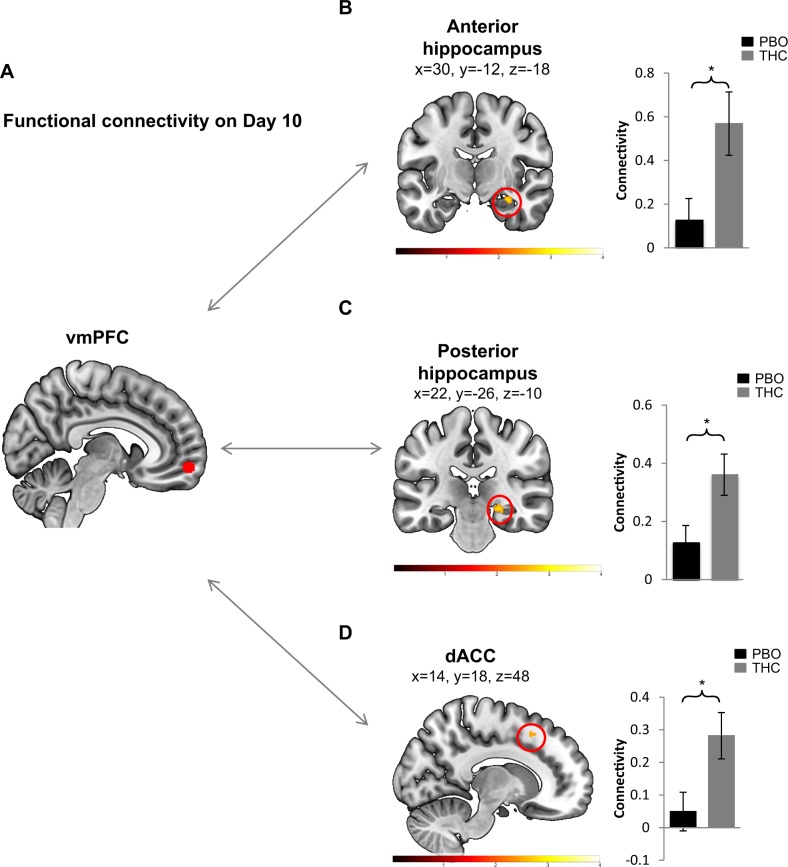
Long-term effects of THC on functional coupling
While there was no significant effect of THC use on the psychophysiological expression of extinction memory 10 days from THC administration, the BOLD data show a marked impact on the functional activation of two key nodes of the threat extinction network during this time point. To further examine the impact of THC on the threat network, we conducted a psychophysiological interaction analysis, taking vmPFC as the seed (Fig. 4a). Given that the effects of THC on vmPFC activation were on noted in Day 10 we focused the gPPI analysis during Day 10 only. This analysis revealed that the THC group had significantly greater connectivity between the vmPFC and the right anterior and posterior hippocampus (t = 2.99, k = 37, p = 0.02; t = 2.83, k = 26, p = 0.02, respectively Fig. 4b, c) and between the vmPFC and the right and left dACC (t = 2.77, k = 23, p = 0.025; t = 4.13, k = 271, p = 0.001 respectively, Fig. 4d-only right shown). These results suggest a long-term impact of one dose of THC on the functional coupling of the threat-extinction network.
Fig. 4.
a Ventromedial prefrontal cortex (vmPFC) region (obtained from the between-group contrast shown in Fig. 3c) used as a seed for the psychophysiological interactions (PPI) during extinction memory test on Day 10. b–d Regions of connectivity, t-map color bar, along with bar graphs representing extracted average beta-weights (and standard error) that show the direction of connectivity. Only regions with significant differences between groups are shown. n(PBO) = 20 and n(THC) = 17. *p < 0.05
Discussion
In the current study, we administered THC to healthy participants prior to extinction learning and tested extinction memory retention after a 1-day and 1-week delay. Our functional imaging data during extinction learning revealed that the group receiving THC showed significant amygdala and vmPFC activation, consistent with the possibility of enhanced extinction learning and perhaps memory consolidation. During the short-term extinction memory test (1-day after extinction learning), we observed enhanced extinction retention in the THC group (indexed by SCR). One week later, we found no between-group differences at the SCR level. However, functional imaging data during this phase revealed the PBO group activated the vmPFC and amygdala more than the THC group, a surprising and apparently contradictory finding to our hypothesis. Another novel finding in our study is that a single dose of THC has caused a long-term effect on the functional coupling between the vmPFC, bilateral dACC, and the right anterior and posterior hippocampus, key nodes of the threat extinction network (results summarized in Table 1). The results reveal a distinct impact on neural circuits of threat-extinction and the expression of this extinction memory indicating an intricate effect of THC on threat extinction memory and its expression.
Table 1.
Summary table of the influence of THC on SCR and BOLD response and PPI during threat extinction learning, extinction retention tests Day 3 and Day 10

|
vmPFC ventromedial prefrontal cortex, dACC dorsal anterior cingulate cortex
During extinction learning, THC did not seem to impact the expression of SCR in the very first two extinction trials (see supplemental Fig. 1C) but marginally reduced SCR during the first 5 extinction trials. THC, however, had a more significant impact on brain activations. We found that the THC group displayed greater activations in the amygdala, and vmPFC in the early trials of extinction learning. Early trials of extinction typically involve the recall of the conditioned memory and therefore display high levels of threat detection [25]. In our study, THC appears to reduce the threat response while possibly at the same time accelerating extinction learning and consolidation. A potential mechanism for this effect is that THC induces increased amygdala activation during early extinction thus leading to accelerated mechanisms of extinction learning consolidation. Evidence from animal studies show that CB1 receptor activation in mice impairs the retrieval of aversive memories, and local infusion of a CB1 agonist within the amygdala interferes with reconsolidation of aversive memories [12, 29]. In addition to the amygdala, THC enhanced vmPFC activation compared to PBO. Translational work in threat extinction research suggests a role for the vmPFC in processing and the consolidation of new extinction memories during the late trials of extinction learning [20, 21]. Here, THC induced a significant increase in vmPFC activation during early extinction, suggesting that THC might accelerate the consolidation process of extinction.
The lower psychophysiological threat expression in THC group observed during the first extinction retention test (Day 3) is consistent with enhanced extinction memory by THC, further supporting and replicating prior reports [19]. The lack of between-group difference (at the psychophysiological level) during the long-term extinction memory retention test (Day 10) might be due to a practice effect; subjects were exposed to too many extinction trials leading to a floor effect. At the neurobiological level, however, we found that the PBO group activated the vmPFC and the amygdala more than the THC group to the CS+E vs. CS+U contrast, which is the opposite of our hypothesis. A plausible explanation for this apparent discrepancy is that during extinction learning, THC generated a generalized extinction memory to both CS+E and CS+U. The activation of the vmPFC and amygdala during extinction learning may have contributed to forming generalized representations of all events in a specific context. We speculate that the PBO group increased activation of both the vmPFC and the amygdala in order to learn that CS+U is safe and in order to consolidate that safety memory, considering that these processes during extinction learning were not as robust as they were in the THC group. In support of this idea, several animal studies have shown that manipulation of the vmPFC during extinction learning is critical for the consolidation and expression of the extinction memory after a delay [for review see ref. [25]].
Another novel finding we report in the present study, and that supports the long-term effects of THC on the threat network, pertains to long-term changes in functional coupling induced by a single dose of THC. We found that during the 1-week test of extinction memory, THC induced an increase in functional connectivity between the vmPFC and anterior and posterior hippocampus. Published data support a bidirectional flow of information between the vmPFC and different parts of the hippocampus that serves in the formation and retrieval of memories [reviewed in the ref. [30]]. Specifically, the anterior hippocampus is responsible for initiating prefrontal control over memories [30]. Whereas the posterior hippocampus is more involved in retaining and recalling fine details of events [31, 32]. In addition, the functional coupling between the vmPFC and hippocampus is believed to be important in first, resolving conflict around existing memories and new events as they are processed, second, consolidation of new memories and third, in the expression of these memories [30]. Following this narrative and building on our findings, THC might be enhancing the functional connectivity between these key regions, thus promoting a more efficient extinction memory that requires less engagement of the threat extinction network. This is supported by our data showing less activation of the vmPFC and amygdala observed during the long-term extinction memory test in a drug-free state. In addition to increasing connectivity between the vmPFC and the hippocampus, THC also increased functional coupling between the vmPFC and the dACC. This is a novel and exciting finding given that the dACC is a brain region known to be modulator of expression of threat detection [33–36]. By increasing functional coupling with the vmPFC, THC could be enhancing prefrontal control over the expression of threat conditioning while also engaging the hippocampus to promote the extinction memory and its contextualization as previously suggested [37].
There may be apparent inconsistencies between our current findings and those reported by our group previously [19] that we attribute to subtle but significant paradigm differences. In the present study, THC induced a quick effect on extinction learning that appears to have caused a marked improvement on short-term extinction retention, without the need to activate vmPFC during the extinction memory retention test itself. In contrast, Rabinak et al. [19] reported no THC effect on neural correlates of extinction learning but enhanced vmPFC activation during the extinction memory retention, along with reduced SCR. These differences may be due to contextual manipulation differences between the studies. Specifically, the current paradigm uses contextual manipulations, whereas the prior study used simple colored squares as the cues with no context shifts. Perhaps the use of more complex cues during the experimental manipulation leads to more rapid engagement of the extinction network thus leading to accelerated impact of THC on emotion regulation. Testing the impact and interactions of THC administration on extinction learning and contextual manipulations could be tested in prospective preclinical studies. One important aspect to note is that the overall effect of THC on threat extinction is consistent across the two studies; timing of the effect is the key variant. Thus, combined from the two studies, we propose that THC has a significant impact on extinction memory retention and its expression; its engagement of the vmPFC could be either during extinction learning or during extinction memory retention.
Here we note some limitations in this study. First, the THC group is more likely to know that they had the active pill and not placebo. This aspect of the study is difficult, if not impossible, to mitigate (we used a low dose of THC). It is important to note, however, that our findings were independent of any subjective reports but the subjective feelings and their impact on our findings cannot be fully ruled out. A second limitation in the study is that we did not take into consideration sex differences or the impact of hormonal variance and its potential interactions with THC use. Future studies should control for the effect of sex hormones, as studies have shown that estradiol particularly influences threat extinction learning and its memory retention [38, 39]. The interpretation of the PPI results should be considered with caution. Here, we infer that the changes in connectivity between the brain regions interrogated reflect a long-term impact of THC on the connectivity of the brain nodes examined. However, PPI analyses use activation data induced by the task per se and thus do not necessarily measure task-independent connectivity. Moreover, while our Day 10 test reflect a long-time window between THC use and the test, it is a not real long-term measure as in months or years of THC use, nor does it reflect the multiple doses or chronic THC use. Future studies should take into consideration all of these critical aspects of THC use on neural circuits of emotion regulation.
In sum, our results are the first to examine the long-term impact of a single dose administration of THC on the functional activation of the threat extinction network. Our findings show a significant effect on the functional connectivity of threat-detection network that emerged after a week from engagement. These data highlight the need to further investigate the long-term influence of THC on threat and anxiety circuitry. Specifically, THC, or compounds with comparable impact on CB1 receptors (e.g., cannabidiol) could be used as adjuncts to extinction-based therapies for PTSD and anxiety disorders. This is especially relevant to PTSD treatment given that threat extinction learning and extinction memory retention has been shown to be deficient in PTSD patients. Moreover, the neural correlates related to PTSD psychopathology are comparable to those engaged by THC in the present study.
Funding and disclosure
Research reported in this presentation was supported by the National Institute of Mental Health of the National Institutes of Health under Award Number R21MH093917 (to KLP). Other support for this work was by the University of Illinois at Chicago (UIC) Center for Clinical and Translational Science (CCTS) award number UL1RR029879 from the National Center for Research Resources. The authors declare no competing interests.
Supplementary information
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41386-019-0416-6).
References
- 1.Volkow Nora D., Swanson James M., Evins A. Eden, DeLisi Lynn E., Meier Madeline H., Gonzalez Raul, Bloomfield Michael A. P., Curran H. Valerie, Baler Ruben. Effects of Cannabis Use on Human Behavior, Including Cognition, Motivation, and Psychosis: A Review. JAMA Psychiatry. 2016;73(3):292. doi: 10.1001/jamapsychiatry.2015.3278. [DOI] [PubMed] [Google Scholar]
- 2.Pitman Roger K., Rasmusson Ann M., Koenen Karestan C., Shin Lisa M., Orr Scott P., Gilbertson Mark W., Milad Mohammed R., Liberzon Israel. Biological studies of post-traumatic stress disorder. Nature Reviews Neuroscience. 2012;13(11):769–787. doi: 10.1038/nrn3339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chhatwal Jasmeer P, Davis Michael, Maguschak Kimberly A, Ressler Kerry J. Enhancing Cannabinoid Neurotransmission Augments the Extinction of Conditioned Fear. Neuropsychopharmacology. 2004;30(3):516–524. doi: 10.1038/sj.npp.1300655. [DOI] [PubMed] [Google Scholar]
- 4.Chhatwal Jasmeer P, Gutman Alisa R, Maguschak Kimberly A, Bowser Michael E, Yang Yong, Davis Michael, Ressler Kerry J. Functional Interactions between Endocannabinoid and CCK Neurotransmitter Systems May Be Critical for Extinction Learning. Neuropsychopharmacology. 2008;34(2):509–521. doi: 10.1038/npp.2008.97. [DOI] [PubMed] [Google Scholar]
- 5.Reich CG, Mohammadi MH, Alger BE. Endocannabinoid modulation of fear responses: learning and state-dependent performance effects. J Psychopharmacol. 2008;22:769–77. doi: 10.1177/0269881107083999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gunduz-Cinar O, MacPherson K P, Cinar R, Gamble-George J, Sugden K, Williams B, Godlewski G, Ramikie T S, Gorka A X, Alapafuja S O, Nikas S P, Makriyannis A, Poulton R, Patel S, Hariri A R, Caspi A, Moffitt T E, Kunos G, Holmes A. Convergent translational evidence of a role for anandamide in amygdala-mediated fear extinction, threat processing and stress-reactivity. Molecular Psychiatry. 2012;18(7):813–823. doi: 10.1038/mp.2012.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Micale Vincenzo, Stepan Jens, Jurik Angela, Pamplona Fabricio A., Marsch Rudolph, Drago Filippo, Eder Matthias, Wotjak Carsten T. Extinction of avoidance behavior by safety learning depends on endocannabinoid signaling in the hippocampus. Journal of Psychiatric Research. 2017;90:46–59. doi: 10.1016/j.jpsychires.2017.02.002. [DOI] [PubMed] [Google Scholar]
- 8.Cavener VS, Gaulden A, Pennipede D, Jagasia P, Uddin J, Marnett LJ, et al. Inhibition of diacylglycerol lipase impairs fear extinction in mice. Front Neurosci. 2018;12:479. [DOI] [PMC free article] [PubMed]
- 9.Morena Maria, Berardi Andrea, Colucci Paola, Palmery Maura, Trezza Viviana, Hill Matthew N, Campolongo Patrizia. Enhancing Endocannabinoid Neurotransmission Augments The Efficacy of Extinction Training and Ameliorates Traumatic Stress-Induced Behavioral Alterations in Rats. Neuropsychopharmacology. 2017;43(6):1284–1296. doi: 10.1038/npp.2017.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kelmendi Benjamin, Adams Thomas G., Yarnell Stephanie, Southwick Steven, Abdallah Chadi G., Krystal John H. PTSD: from neurobiology to pharmacological treatments. European Journal of Psychotraumatology. 2016;7(1):31858. doi: 10.3402/ejpt.v7.31858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klumpers Floris, Denys Damiaan, Kenemans J Leon, Grillon Christian, van der Aart Jasper, Baas Johanna MP. Testing the effects of Δ9-THC and D-cycloserine on extinction of conditioned fear in humans. Journal of Psychopharmacology. 2012;26(4):471–478. doi: 10.1177/0269881111431624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marsicano Giovanni, Wotjak Carsten T., Azad Shahnaz C., Bisogno Tiziana, Rammes Gerhard, Cascio Maria Grazia, Hermann Heike, Tang Jianrong, Hofmann Clementine, Zieglgänsberger Walter, Di Marzo Vincenzo, Lutz Beat. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418(6897):530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- 13.Pamplona FA, Bitencourt RM, Takahashi RN. Short- and long-term effects of cannabinoids on the extinction of contextual fear memory in rats. Neurobiol Learn Mem. 2008;90:290–3. doi: 10.1016/j.nlm.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 14.Stern Cristina A J, Gazarini Lucas, Takahashi Reinaldo N, Guimarães Francisco S, Bertoglio Leandro J. On Disruption of Fear Memory by Reconsolidation Blockade: Evidence from Cannabidiol Treatment. Neuropsychopharmacology. 2012;37(9):2132–2142. doi: 10.1038/npp.2012.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Phan K. L., Angstadt M., Golden J., Onyewuenyi I., Popovska A., de Wit H. Cannabinoid Modulation of Amygdala Reactivity to Social Signals of Threat in Humans. Journal of Neuroscience. 2008;28(10):2313–2319. doi: 10.1523/JNEUROSCI.5603-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gorka Stephanie M, Phan K Luan, Lyons Maryssa, Mori Shoko, Angstadt Mike, Rabinak Christine A. Cannabinoid Modulation of Frontolimbic Activation and Connectivity During Volitional Regulation of Negative Affect. Neuropsychopharmacology. 2015;41(7):1888–1896. doi: 10.1038/npp.2015.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rabinak Christine A., Sripada Chandra Sekhar, Angstadt Mike, de Wit Harriet, Phan K. Luan. Cannabinoid modulation of subgenual anterior cingulate cortex activation during experience of negative affect. Journal of Neural Transmission. 2011;119(6):701–707. doi: 10.1007/s00702-011-0747-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rabinak Christine A., Angstadt Mike, Sripada Chandra S., Abelson James L., Liberzon Israel, Milad Mohammed R., Phan K. Luan. Cannabinoid facilitation of fear extinction memory recall in humans. Neuropharmacology. 2013;64:396–402. doi: 10.1016/j.neuropharm.2012.06.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rabinak Christine A., Angstadt Mike, Lyons Maryssa, Mori Shoko, Milad Mohammed R., Liberzon Israel, Luan Phan K. Cannabinoid modulation of prefrontal–limbic activation during fear extinction learning and recall in humans. Neurobiology of Learning and Memory. 2014;113:125–134. doi: 10.1016/j.nlm.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Milad Mohammed R., Wright Christopher I., Orr Scott P., Pitman Roger K., Quirk Gregory J., Rauch Scott L. Recall of Fear Extinction in Humans Activates the Ventromedial Prefrontal Cortex and Hippocampus in Concert. Biological Psychiatry. 2007;62(5):446–454. doi: 10.1016/j.biopsych.2006.10.011. [DOI] [PubMed] [Google Scholar]
- 21.Phelps Elizabeth A., Delgado Mauricio R., Nearing Katherine I., LeDoux Joseph E. Extinction Learning in Humans. Neuron. 2004;43(6):897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 22.Harnett Nathaniel G., Shumen Joshua R., Wagle Pooja A., Wood Kimberly H., Wheelock Muriah D., Baños James H., Knight David C. Neural mechanisms of human temporal fear conditioning. Neurobiology of Learning and Memory. 2016;136:97–104. doi: 10.1016/j.nlm.2016.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Milad Mohammed R., Orr Scott P., Pitman Roger K., Rauch Scott L. Context modulation of memory for fear extinction in humans. Psychophysiology. 2005;42(4):456–464. doi: 10.1111/j.1469-8986.2005.00302.x. [DOI] [PubMed] [Google Scholar]
- 24.Coelho CA, Dunsmoor JE, Phelps EA. Compound stimulus extinction reduces spontaneous recovery in humans. Learn Mem. 2015;22:589–93. doi: 10.1101/lm.039479.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Milad MR, Quirk GJ. Fear extinction as a model for translational neuroscience: ten years of progress. Annu Rev Psychol. 2012;63:129–151. doi: 10.1146/annurev.psych.121208.131631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tzourio-Mazoyer N., Landeau B., Papathanassiou D., Crivello F., Etard O., Delcroix N., Mazoyer B., Joliot M. Automated Anatomical Labeling of Activations in SPM Using a Macroscopic Anatomical Parcellation of the MNI MRI Single-Subject Brain. NeuroImage. 2002;15(1):273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- 27.McLaren Donald G., Ries Michele L., Xu Guofan, Johnson Sterling C. A generalized form of context-dependent psychophysiological interactions (gPPI): A comparison to standard approaches. NeuroImage. 2012;61(4):1277–1286. doi: 10.1016/j.neuroimage.2012.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lieberman MD, Cunningham WA. Type I and Type II error concerns in fMRI research: re-balancing the scale. Soc Cogn Affect Neurosci. 2009;4:423–8. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lin HC, Mao SC, Gean PW. Effects of intra-amygdala infusion of CB1 receptor agonists on the reconsolidation of fear-potentiated startle. Learn Mem. 2006;13:316–21. doi: 10.1101/lm.217006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Preston AR, Eichenbaum H. Interplay of hippocampus and prefrontal cortex in memory. Curr Biol. 2013;23:R764–73. doi: 10.1016/j.cub.2013.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liang JC, Wagner AD, Preston AR. Content representation in the human medial temporal lobe. Cereb Cortex. 2013;23:80–96. doi: 10.1093/cercor/bhr379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Evensmoen Hallvard Røe, Ladstein Jarle, Hansen Tor Ivar, Møller Jarle Alexander, Witter Menno P., Nadel Lynn, Håberg Asta K. From details to large scale: The representation of environmental positions follows a granularity gradient along the human hippocampal and entorhinal anterior-posterior axis. Hippocampus. 2014;25(1):119–135. doi: 10.1002/hipo.22357. [DOI] [PubMed] [Google Scholar]
- 33.Marin Marie-France, Song Huijin, VanElzakker Michael B., Staples-Bradley Lindsay K., Linnman Clas, Pace-Schott Edward F., Lasko Natasha B., Shin Lisa M., Milad Mohammed R. Association of Resting Metabolism in the Fear Neural Network With Extinction Recall Activations and Clinical Measures in Trauma-Exposed Individuals. American Journal of Psychiatry. 2016;173(9):930–938. doi: 10.1176/appi.ajp.2015.14111460. [DOI] [PubMed] [Google Scholar]
- 34.Milad Mohammed R., Quirk Gregory J., Pitman Roger K., Orr Scott P., Fischl Bruce, Rauch Scott L. A Role for the Human Dorsal Anterior Cingulate Cortex in Fear Expression. Biological Psychiatry. 2007;62(10):1191–1194. doi: 10.1016/j.biopsych.2007.04.032. [DOI] [PubMed] [Google Scholar]
- 35.Linnman Clas, Zeidan Mohamed A., Pitman Roger K., Milad Mohammed R. Resting cerebral metabolism correlates with skin conductance and functional brain activation during fear conditioning. Biological Psychology. 2012;89(2):450–459. doi: 10.1016/j.biopsycho.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Marin Marie-France, Zsido Rachel G., Song Huijin, Lasko Natasha B., Killgore William D. S., Rauch Scott L., Simon Naomi M., Milad Mohammed R. Skin Conductance Responses and Neural Activations During Fear Conditioning and Extinction Recall Across Anxiety Disorders. JAMA Psychiatry. 2017;74(6):622. doi: 10.1001/jamapsychiatry.2017.0329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Liberzon I, Abelson JL. Context processing and the neurobiology of post-traumatic stress disorder. Neuron. 2016;92:14–30. doi: 10.1016/j.neuron.2016.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Milad M.R., Igoe S.A., Lebron-Milad K., Novales J.E. Estrous cycle phase and gonadal hormones influence conditioned fear extinction. Neuroscience. 2009;164(3):887–895. doi: 10.1016/j.neuroscience.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Graham BM, Milad MR. Blockade of estrogen by hormonal contraceptives impairs fear extinction in female rats and women. Biol Psychiatry. 2013;73:371–8. doi: 10.1016/j.biopsych.2012.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.