Abstract
In the last few decades, hyaluronic acid (HA) has become increasingly employed as a biomaterial in both clinical and research applications. The abundance of HA in many tissues, together with its amenability to chemical modification, has made HA an attractive material platform for a wide range of applications including regenerative medicine, drug delivery, and scaffolds for cell culture. HA has traditionally been appreciated to modulate tissue mechanics and remodeling through its distinctive biophysical properties and ability to organize other matrix proteins. However, HA can influence cell behavior in much more direct and specific ways by engaging cellular HA receptors, which can trigger signals that influence cell survival, proliferation, adhesion, and migration. In turn, cells modify HA by regulating synthesis and degradation through a dedicated arsenal of enzymes. Optimal design of HA-based biomaterials demands full consideration of these diverse modes of regulation. This review summarizes how HA-based signaling regulates cell behavior and discusses how these signals can be leveraged to create cell-instructive biomaterials.
Keywords: extracellular matrix, mechanobiology, motility, CD44, RHAMM, hyaluronidase
Graphical Abstract
Introduction:
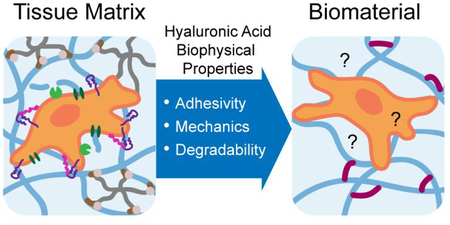
Hyaluronic acid (HA, also called hyaluronan) is a linear polysaccharide expressed in almost all bodily tissues and fluids at a concentration and molecular weight (MW) that varies by tissue type.1 The nearly ubiquitous expression of HA is suggestive of both its biological importance as well as its potential for clinical application. HA is amenable to a variety of chemical modifications through three orthogonal functional moieties (hydroxyl, carboxyl, and amide), facilitating its use for numerous applications requiring conjugation or crosslinking.2,3 While often incorrectly portrayed as an inert or non-adhesive scaffold, HA actually provides a rich abundance of mechanical and biological signals to surrounding cells and tissues.4,5 Cell surface receptors specific for HA enable cells to respond to the biophysical properties of HA, which can be modulated in vivo by controlling HA abundance, MW, and other factors.6,7 Cues from HA within the extracellular matrix (ECM) influence cell adhesion, migration, and downstream cell signaling (Fig. 1). In turn, cells modify and regulate the HA in the ECM through synthesis, degradation, and organization.8,9 HA-based signaling is especially important in development, wound healing, and metastatic disease.10–13 Resultant biological signals are critically dependent on the biophysical properties of HA, and thus require consideration in biomaterial design.
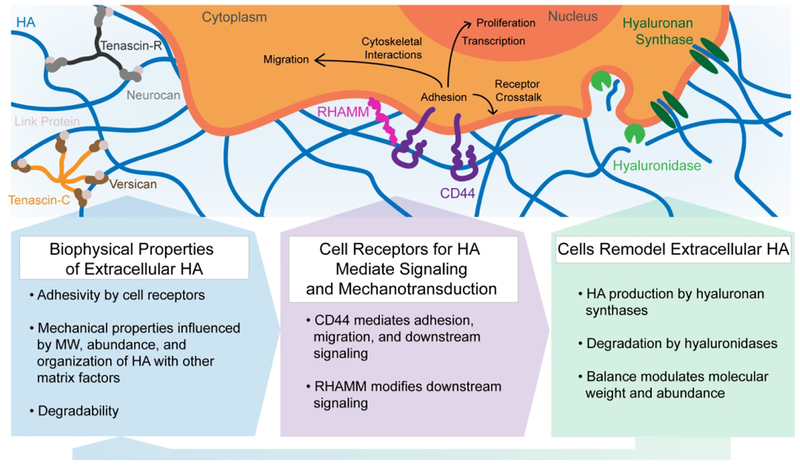
Figure 1.
Cells sense biophysical properties of extracellular HA (adhesivity, mechanical properties, and degradability) through surface receptors such as CD44 and RHAMM. These biophysical properties influence cell adhesion, migration, and proliferation through cytoskeletal interactions, transcription, and receptor crosstalk. In turn, cells remodel extracellular HA through synthesis by hyaluronan synthases and degradation by hyaluronidases.
Cells bind to HA directly through membrane receptors resulting in transduction of biochemical signals and reinforcement of mechanical linkages that directly mediate adhesion and motility.6,14 The most studied of these HA cell receptors are CD44 and the Receptor for HA-Mediated Motility (RHAMM) (Fig. 1). CD44 is a transmembrane receptor that binds to extracellular HA through a single binding domain and links indirectly to the actin cytoskeleton by way of ezrin, moesin, or radixin (ERM) family proteins or to the spectrin cytoskeleton through ankyrin proteins.14 RHAMM contains two HA-binding domains in which HA is bound less tightly than in the HA-binding domain of CD44.15,16 RHAMM is not a transmembrane receptor, and can exist intracellularly or on the extracellular cell surface in complex with other receptors such as CD44.16 The reported relationship between RHAMM and CD44 in mediating cell adhesion to HA has been somewhat contradictory and may be context dependent. For example, Lokeshwar and colleagues found RHAMM to be the main mediator of HA binding in primary human endothelial cells.17 In contrast, Savani and colleagues found that anti-CD44 but not anti-RHAMM antibodies inhibited adhesion of endothelial cells to HA.18 Similarly conflicting observations have been reported in glioblastomas (GBMs).19,20 These findings may potentially be reconciled by the fact that RHAMM modifies signaling through CD44, with the degree of modification depending strongly on context. For example, studies of invasive breast cancer cells demonstrate that CD44 and RHAMM coordinate to regulate ERK1/2 signaling and cell motility.21 Overall, the role of RHAMM and CD44 interactions in cell motility and dependence on the microenvironment remains an open question.
Independent of its relationship with RHAMM, CD44 plays a critical role in cell motility.14 For example, CD44 protein expression is increased in highly invasive and/or metastatic cells.22,23 In GBMs, high CD44 protein levels correlate with the most rapidly invading cell populations,24 and neutralization or knockdown of CD44 significantly impairs GBM invasion in animal models.25 CD44 can directly support adhesion and migration, likely through its intracellular cytoskeletal linkages. For example, human prostate cancer cells expressing CD44 mutants lacking the ankyrin binding domain do not adhere to HA.26 However, the relative contributions of ERM and ankyrin binding to CD44-dependent signaling remains poorly understood and are likely to be context-dependent. CD44 can also complement and potentiate signaling from other surface receptors; for example, Chopra and colleagues found that HA-CD44 binding can increase integrin signaling resulting in cell spreading.27
Growing evidence demonstrates that CD44, like integrins, is involved in sensing mechanical signals from the HA matrix. Our laboratory demonstrated that CD44-mediated adhesion and migration depend on the storage modulus of crosslinked HA hydrogels.20 One possible mechanism governing CD44-mediated mechanosensitivity is that CD44 can undergo force-dependent switching between low affinity and high affinity HA-binding states. The crystal structure of the CD44-HA complex supports this idea, revealing that there are at least two binding conformations.28 Similarly, molecular dynamics simulations suggest that HA can bind to CD44 in three different conformations, two of which are metastable states that enable low affinity binding.29 DeGrendele et al. demonstrated that leukocytes adopt a high-affinity state for HA binding during rolling, when adhesive tethers are stressed.30 Suzuki et al. showed that force experienced by leukocytes during rolling could convert HA-CD44 binding from a low affinity to high affinity state.31 While these studies differ on the number of proposed binding states, they together strongly suggest that CD44 exhibits force-dependent changes in HA-binding affinity and therefore mechanosensitivity. Shedding of CD44 is also important for CD44-mediated functions, but the role in mechanosensitivity is poorly understood.32 While there are still numerous open questions regarding CD44-mediated mechanosensitivity and motility, these findings underscore the biological importance of HA mechanics within the ECM.
Several other cell receptors have been reported to bind to HA, although the relative affinities for HA, mechanical roles, and resulting downstream signaling are incompletely understood. Lymphatic Vessel Endothelial Hyaluronan Receptor 1 (LYVE-1) is a lymphatic-specific HA receptor that may play an important immunological function.33,34 Layilin is a transmembrane protein reported to bind to HA extracellularly and to radixin and merlin proteins intracellularly, but the function is poorly understood.35,36 HA signaling can also be mediated by Toll like receptors 2 and 4 (TLR2/4),37–39 but more recent evidence suggests that the signaling effects may not act through a direct ligand-receptor interaction.40 Finally, tumor necrosis factor-stimulated gene 6 (TSG-6), is a signaling factor that can bind with HA and may enhance CD44-based signaling.41,42 Elevated levels of TSG-6 have been observed in the central nervous system following injury.43
The biophysical properties of HA can greatly impact the nature of HA-induced cell signaling such that optimized biomaterial design is necessary for appropriate downstream effects. In this review, we begin by discussing key biophysical properties of HA most pertinent to biomaterial design, broadly defined as mechanics, adhesivity, and degradability. We focus on how HA mechanics vary by tissue type and state, and how adhesivity and degradability relate to mechanics. These properties can profoundly influence cell and tissue homeostasis and disease, and we present selected examples from development, wound healing, and tumor progression. Within a biomaterial, the biophysical properties of HA are critically dependent on fabrication methods. Thus, in the second part we discuss how these biophysical properties can be incorporated in biomaterial design, along with the benefits and limitations of various strategies for doing so. As a whole, this review should provide guidance in selecting and achieving optimal biophysical design criteria for a given application.
PART I: HA Biophysical Regulation of Cell Behavior within Tissue
HA is a critical driver of a variety of normal and disease processes, including development, wound healing, tissue maintenance, inflammation, and metastasis.4,9–13,44 HA properties, particularly MW and abundance, undergo characteristic changes that support and drive tissue remodeling and homeostasis.1,8,45 For example, HA levels in tissue tend to be higher during development and play a particularly prominent role in the hematopoietic stem cell niche and central nervous system.11,46,47 HA is dynamically activated in the early stages of wound healing during which it may promote matrix organization, fibroblast migration, or tissue hydration.12,44 HA and associated regulatory enzymes are abnormally overexpressed in a variety of tumor types.10,13 This section will cover the biophysical properties of HA pertaining to adhesivity, organization, and mechanics with a discussion of their interdependency and select examples of biological impact.
HA Adhesivity and Organization Influences Mechanics
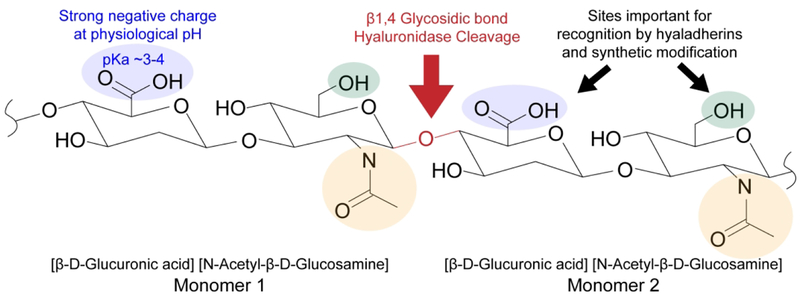
HA is a linear and negatively charged polysaccharide composed of disaccharide repeats of D-glucuronic acid and N-acetyl-D-glucosamine (Fig. 2).48 It is unique among glycosaminoglycans in that it is not a proteoglycan, is synthesized at the plasma membrane instead of the Golgi apparatus, and remains unsulfated and as an unbranched structure within the ECM.49 Each monomer contains one carboxylic acid, one primary alcohol, and one amide moiety, which are important for biological function and available for chemical modification. The carboxylic acid of the glucuronic acid subunit is effectively deprotonated at physiological pH, giving rise to a polyanionic character.50
Figure 2.
Chemical structure of HA. The carboxylic acid (blue) and primary alcohol (green) are important for both recognition by hyaladherins and for chemical modification. The amide (yellow) also supports adhesion but is less commonly modified.
The mass of an average human adult consists of ~15 g of HA throughout the body, with ~30% of this total turned over daily.8,49 While some HA is found in virtually all tissue ECMs in the body, the abundance, organization, and MW of HA are strongly tissue-dependent.1 Solid tissues in rabbit have been reported to have a range from 1 – 500 μg HA /g of wet tissue, while human cartilage contains as much as 2500 μg HA /g of wet tissue.51,52 While the concentration of HA in most fluids is in the ng/mL to low μg/mL range, the concentration of the vitreous humor is as much as 200 μg/mL53 and that of the synovial joint is as much as 2–3 mg/mL.54 HA is traditionally regarded as an extracellular polymer; very little is understood about the intracellular role of HA.9,55 We focus exclusively on extracellular HA in this review based on its relevance for biomaterial design.
In fluids, HA does not exhibit a well-defined network structure but instead forms entangled networks that contribute to fluid viscosity particularly at high molecular weight (HMW) or with light crosslinking.56,57 The persistence length of HMW hyaluronan has been estimated to be ~10 nm, approximately 10 monomers, which is around the same length of HA that can bind to a single HA-binding domain.58 Proteins with HA-binding domains (hyaladherins) contribute to non-covalent assembly of HA in vivo, and aspects of this assembly can be mimicked in vitro. In the presence of aggrecan, HA forms more ordered structures in solution with higher packing densities. leading to an increase in viscosity.59 In synovial joint fluid, assembly of these dense, viscous complexes are widely regarded as important for maintaining shear flow while resisting osmotic compression and absorbing compressive force.59–61
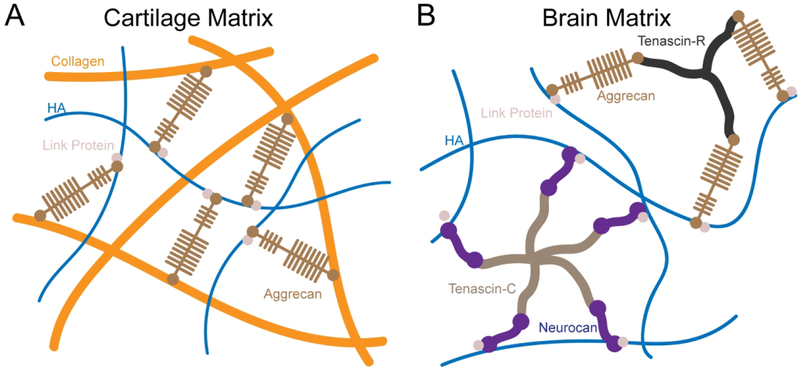
In solid tissues, HA is non-covalently assembled into a network by a subset of proteoglycans with HA binding domains.13,49,62 The organization varies by tissue type as well as the local cellular microenvironment (Fig. 3). In the brain, tenascins organize with link proteins and chondroitin sulfate (CS) proteoglycans such as versican, neurocan, and aggrecan to stabilize entangled networks of HA.62,63 These networks can form perineuronal nets that surround the cell membrane.64 HA-matrix organization dominates the intraparenchymal space of brain ECM, which is particularly high in HA content and low in fibrous proteins such as collagen I.65 In cartilage, HA is also bound and organized by proteoglycans but assembles into an interpenetrating network with collagen fibrils.66,67 HA-CS binding is mechanically reinforced by complexation with link proteins, which contain binding domains for both HA and CS.68 The organization and mechanical reinforcement of HA with other proteins is thus important for the mechanical properties of the overall matrix.
Figure 3.
Matrix organization of HA varies by tissue type and cell microenvironment. A) HA organizes as an interpenetrating network that interacts with mechanically-reinforcing collagen fibers in cartilage tissue. B) In contrast, intraparenchymal regions of brain tissue are generally devoid of collagen fibers and HA organizes primarily with chondroitin sulfates.
Hyaladherin-HA binding is generally based on a conserved mechanism involving electrostatic interactions. HA-binding domains, both in matrix proteins and cell receptors, contain positively-charged lysine and arginine residues, which coordinate with the negatively-charged HA backbone and bind 3–6 monomers depending on the hyaladherin type.69,70 Bano and colleagues investigated hyaladherin-HA affinity by measuring the rupture forces of HA and various hyaladherin binding domains using atomic force microscopy.71 The rupture force roughly correlated with the number of HA monomers bound by the hyaladherin and ranged from 24–52 pN. Remarkably, reinforcing aggrecan-HA binding by complexing with cartilage link protein effectively increased the binding force above that measured for streptavidin-biotin bonds. This result further supports the idea that HA-binding affinity depends on the length of the HA segment bound as well as underscores the role of HA in supporting ECM mechanical integrity.
Remodeling of HA Alters Mechanics
The MW of HA in the human body varies widely, from tetramers of around 1 kDa to HMW species of around 2 MDa.45 Changes in MW distribution affect both the physical properties of HA within the ECM as well as cellular biochemical signaling. The effects of MW on the physical properties of ECM stem largely from its contributions to mechanics. Within solutions, increasing MW greatly increases viscosity of HA, reflective of greater entanglement.72 The mechanical properties of HMW HA are key to the proper function of synovial joint fluid by resisting compressive forces while allowing shear thinning.73,74 Increases in low molecular weight (LMW) HA are associated with pathological conditions; for example, osteoarthritis patients exhibit a higher ratio of LMW to HMW HA in synovial fluid compared to healthy patients.75 Similarly, LMW HA is not commonly found in solid tissue unless the tissue is undergoing either a physiological or pathological remodeling process.1,8,45 Elevations and other alterations in LMW HA species have been observed in cartilage during aging,76 as well as in a variety of tumors.77–79 Broadly, these studies suggest that a shift from HMW to LMW species is associated with plasticity in ECM mechanics and potentially loss of structural integrity.
HA MW can also influence biological processes through biochemical signaling. LMW HA generally stimulates an inflammatory response while HMW HA induces an anti-inflammatory response.45 In macrophages, LMW HA fragments upregulate inflammatory gene expression contributing to polarization toward a tissue-destructive state.80 Later work showed that while LMW and HMW HA both activate macrophages, LMW HA induces a pro-inflammatory gene expression profile whereas HMW induces a pro-healing gene expression profile.81 The mechanisms by which cells sense and respond to MW of HA remain unclear, but experimental studies support several possibilities. It is possible that HA MW may affect cell signaling indirectly through changes in matrix mechanical properties such as increased viscous behavior resulting from increased chain entanglement, but the relative importance of this effect has not been directly demonstrated in vivo. More directly, HMW HA can induce multivalent binding and receptor clustering. Yang et al. showed that HMW HA induces CD44 clustering while HA oligomers of 3 – 10 monosaccharides inhibit clustering, with each reagent exerting differential effects on downstream ERK signaling.82 From a physical perspective, higher MWs stabilize binding to CD44 such that LMW HA binding is reversible while HMW binding is essentially irreversible.83 The cumulative effects of binding time and stability could have a range of effects on cell motility and downstream signaling. MW may also affect cellular uptake and downstream intracellular signaling, but this process and mechanistic effects are not well understood.84
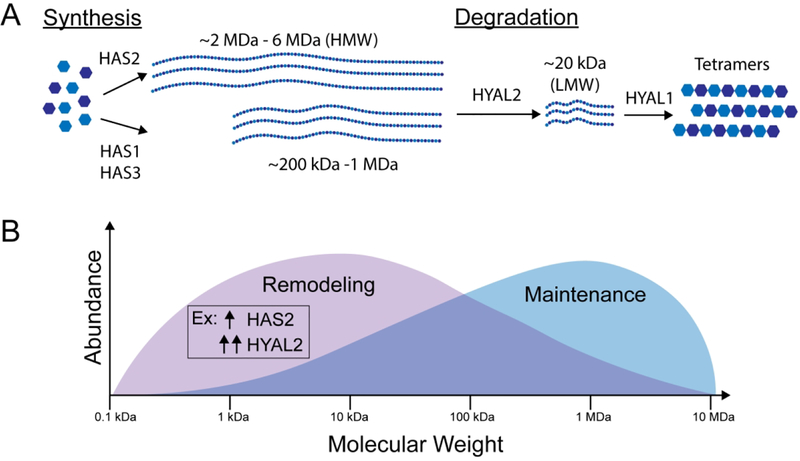
The MW and abundance of HA are mediated by the activity of hyaluronan synthases and hyaluronidases (Fig. 4A). There are three hyaluronan synthases (HAS1, HAS2, HAS3), all of which are multifold transmembrane receptors that vary in expression, rate, and MW of the HA produced. HAS1 has a slower rate of synthesis than HAS2 and HAS3. HAS1 and HAS3 produce lower MW species, while HAS2 can produce very HMW species.85,86 HAS2 seems to play a particularly significant role in cell invasion and cancer progression. Its expression is elevated in diffusely infiltrating astrocytomas and serves as a prognostic factor.87 Elevated HAS2 correlates with lower survival in breast cancer78 and primary brain cancers.87
Figure 4.
The regulation and role of HA MW in biophysical signaling. A) MW is dependent on expression and activity of hyaluronan synthases and hyaluronidases. HMW HA is synthesized at lengths dependent on the hyaluronan synthase. HMW HA is degraded by HYAL2 to form ~20 kDa fragments which are then further degraded by other hyaluronidases, primarily HYAL1, into tetramer units. B) Human HA is present in a distribution of MWs varying from about 0.1 kDa to 2 MDa. LMW HA elicits a tissue remodeling response, while HMW promotes tissue maintenance. A shift from HMW species to LMW species can be induced by increased synthesis (HAS2) followed by greatly increased degradation (HYAL2) leading to the accumulation of HA fragments.78,79
Five hyaluronidases are encoded in the human genome (HYAL1, HYAL2, HYAL3, HYAL4, PH-20/SPAM1), and their expression and function differ by tissue type.88 Notably, PH-20/SPAM1 is expressed only in testes, while the rest of the hyaluronidases are expressed more broadly. Structures of human HYAL1 show that hyaluronidases bind tetrasaccharides, and the enrichment of arginine residues in the binding cleft suggest the importance of the carboxylic acid on HA for proper recognition.89 The hyaluronidases differ in the MW of HA they recognize as well as the MW of their cleavage products. Notably, HYAL2 cleaves HMW HA to ~20 kDa fragments, while other hyaluronidases cleave ~20 kDa fragments to tetrasaccharide products.88
Differential expression of enzymes with varying substrates, rates, and products provides a means by which cells can regulate the MW of HA within their environment and resulting shift between inflammatory/prometastatic and anti-inflammatory/anti-metastatic signals (Fig. 4B). While this balance remains poorly understood, recent studies are revealing the biological function of this balance. As previously described, tumors are often HA-rich. In vitro models suggest that glioblastoma cells upregulate HA synthesis if HA is lacking in the surrounding matrix,90 and that incorporation of HA into gelatin matrices alters inhibitor sensitivity and upregulates malignancy.91,92 Interestingly, both HYAL2 and HAS2 gene expression are increased in mesenchymal subtype tumors, and inhibition of HAS2 gene expression results in more dependence on focal adhesion-mediated invasion.93 A similar pattern of expression is observed in highly invasive breast cancers, which express abnormally high amounts of both HYAL2 and HAS2.94 This somewhat paradoxical increase in expression of both synthases and hyaluronidases enriches the microenvironment in short, loosely bound, HA fragments.79 Consistent with this finding, Wu and colleagues observed that LMW HA, but not total HA, correlated with lymph node metastasis and cell invasiveness, and that both hyaluronidases and hyaluronan synthases were overexpressed.78 Similarly, accumulation of LMW HA and the shift toward a metastatic phenotype results from upregulation of HA synthesis and degradation in prostate cancer.95,96
A notable recent study by Tian and colleagues demonstrated the relationship between very HMW HA (10 MDa) and cancer incidence in the naked mole rat, a species in which cancer is rarely observed.97 By perturbing the abundance of the HMW HA either through HAS2 knockdown or HYAL2 overexpression, naked mole rat cells became highly susceptible to malignant transformation. These results clearly demonstrate the important biological role of HA MW regulation and its therapeutic potential.
PART II: Incorporation of HA Biophysical Properties into Biomaterial Design
While HA provides a rich set of biological cues in vivo, the biophysical signals arising from HA in biomaterials may dramatically differ depending on the fabrication method. We consider these biophysical properties categorically as relating to either mechanics, adhesivity, or degradability (Table 1). We then discuss strategies to achieve these properties in various biomaterial applications with the potential advantages or disadvantages of each strategy.
Table 1:
Potential advantages and disadvantages of strategies used to incorporate key biophysical properties of HA into biomaterial design.
| Biophysical Property of HA in ECM | Strategy for Incorporating Property into HA Biomaterials | Potential Advantages and Disadvantages of Strategy |
|---|---|---|
| Mechanics | ||
| Change HA density | ||
| Change molecular weight | ||
| HA backbone modification and crosslinking | ||
| Incorporate interpenetrating / semi-interpenetrating networks | ||
| Adhesivity | ||
| Of HA backbone | HA backbone modification | |
| Of other ECM components | Peptide Conjugation |
|
| Form Interpenetrating network with other ECM components | ||
| Degradability | ||
| Of HA backbone | Backbone modification | |
| Crosslinking | ||
| Of CrossIinkers | MMP-cleavable crosslinks |
|
| Crosslinks degrade by hydrolysis (i.e. esters) | ||
Applications of HA-based biomaterials
One of the earliest clinical applications of HA was to restore lubrication and enhance stress dissipation (viscosupplementation) in joints as a therapeutic treatment for osteoarthritis.98 Not long after, HA became more widely used for viscosupplementation in ophthalmology, and eventually otology.98,99 Early work in these applications revealed that a main limitation of viscosupplementation was the rapid degradation (<1 day) of the injected HA, thereby reducing the therapeutic benefit.100 Chemical modification and crosslinking of HA was explored as a means to reduce degradation rates and extend treatment.101 As methods to chemically modify HA developed, the use of HA has expanded to dermal fillers, tissue regeneration, and drug delivery.102–106 In many of these applications, the anti-inflammatory, anti-tumorigenic properties of HMW HA have proven attractive. As a drug delivery vehicle, HA can be used to protect peptide or nucleotide therapeutics from rapid degradation or to target cells or tissues with high HA uptake.107 A number of excellent reviews have been written about the clinical applications of HA matrices.108–110
A developing application of HA is for tissue engineering.111 HA-mediated signaling, particularly that arising from HMW HA, supports survival, proliferation, and stemness. Thus, HA-based biomaterials show promise for encapsulating stem cells and supporting their directed differentiation. As an example, Gerecht and colleagues demonstrated that HA-based hydrogels can maintain stemness of human embryonic stem cells, but that the addition of soluble factors could still induce differentiation in a controllable manner.112 We have demonstrated that HA-based scaffolds support viability of implanted human pluripotent stem cell-derived dopaminergic neurons and neural progenitor cells for treatment of Parkinson’s disease.113,114 HA-based hydrogels have also been explored as scaffolds for adipose tissue115,116, cartilage117, and bone engineering.118 Because HA is a major component of endogenous ECM and the mechanics of HA can be tuned through a variety of parameters, HA-based biomaterials with controllable mechanics are also used as a research platform in mechanobiology.119–122
Central to the development of HA-based biomaterials is the presence of three functional moieties (primary alcohol, carboxylic acid, and amide) that can chemically and orthogonally modified, facilitating control of biophysical properties for the desired application.2,3,123–125 Most modifications are made to the carboxylic acid and the primary alcohol, but modifications can also be made to the amide.2 These modifications support a large backbone diversity, which can then be crosslinked to form a gel or conjugated with peptides, growth factors, or other matrix proteins.3 HA can also be crosslinked with other polymer backbones to form semi-interpenetrating networks.126–128 Several excellent reviews have detailed the various chemistries and methodologies used for HA modification.2,3,123
Incorporating HA Mechanics into Biomaterial Design
To control mechanical properties of HA-abundant fluids for applications such as viscosupplementation, the concentration and MW of HA are the most important parameters.56,57 Thus, the viscosity of soluble HA may be easily modulated simply by choosing an appropriate MW range and concentration. For applications requiring solid rather than fluid biomaterials, gelation must be induced through some form of crosslinking. In this case, the backbone MW, the degree of crosslinking, the chemistry of the modification and crosslinker, and the matrix density can all contribute to the bulk matrix properties. Bulk matrix properties can be engineered by tuning any of the aforementioned parameters, but some strategies may reduce cell viability or motility. Several studies have noted that high density HA matrices restrict cell migration and diffusion of biomacromolecules.129,130
One commonly used mechanical parameter of HA and other biological materials is the bulk storage modulus, which is widely understood to be an important effector of cell spreading and motility.131–133 The storage modulus varies widely by tissue type, from a few hundred Pa in soft tissues such as fat, marrow, and brain to tens of MPa in bone.134 HA materials are most easily fabricated with elastic moduli in the hundreds of Pa to tens of kPa, a range which encompasses most soft tissues.135 HA hydrogels are often limited for applications in regenerating hard tissues such as cartilage or bone regeneration due to the comparatively low elastic modulus of these materials. One strategy for augmenting the elasticity of HA matrices is to assemble composite polymer networks with stiffer materials. For example, Tavsanli and colleagues used an HA and poly(N,N-dimethylacrylamide) (PDMA) to create hydrogels with high strength and high compressive modulus (in the MPa range) necessary for load-bearing tissues.136 As described below, a number of investigators have also exploited mixed stiff HA/collagen and HA/gelatin scaffolds for cell culture applications.
While mechanical characterization of solid biomaterials often tends to focus on bulk storage modulus, tissues are typically viscoelastic rather than purely elastic, and this mixed character can greatly influence cell morphology and signaling. Dense HA networks crosslinked with covalent bonds typically exhibit high elasticity with very little viscosity. However, incorporating crosslinks that can dynamically switch between bound and unbound states over experimental time scales results in an increased viscous component. For example, HA viscoelastic properties may be controlled by conjugating cyclodextrins to the HA backbone, which enables supramolecular assembly into structures capable of both storing and dissipating mechanical stresses.137 Variation of viscoelastic properties in this way influences mesenchymal stem cell (MSC) viability.121 More recently, Lou et al. employed a dynamic hydrazone bond to crosslink HA polymers within an interpenetrating network of HA and collagen I in order to confer stress relaxation to the hydrogel. Varying the crosslinker affinity, MW of HA, and concentration of HA allowed for tuning of the relaxation time, with faster relaxation times promoting MSC spreading and focal adhesion formation.138
Tissue ECM is not spatially homogeneous but rather exhibits temporal and spatial variation in mechanics and composition. Efforts to recapitulate these variations for tissue engineering or mechanobiology research have focused on biomaterial patterning. Because HA modification and crosslinking chemistries are compatible with photoactivation, recent work has focused on developing HA biomaterials with photoresponsive patterned properties. Marklein and Burdick used photoactivated crosslinking to pattern the bulk modulus of a gel from 3 kPa to 100 kPa, a range over which human MSC spreading and proliferation was found to vary.139 Our own laboratory used orthogonal photoresponsive chemistries to pattern perpendicular gradients of adhesive peptide and increasing modulus into a single gel for a high-resolution investigation of cell response to microenvironment variation.124 Rosales and colleagues incorporated a photoswitchable azobenzene moiety that was capable of forming a complex with cyclodextrin in the trans conformation and not in the cis conformation, allowing for photo-reversible control over the viscoelastic properties of HA.140 Ongoing work involves investigating the role of dynamic mechanics on cell morphology.
Thin film HA hydrogels (<100 μm) offer the opportunity to apply these materials as interfacial coatings, which may be necessary when a different material is needed to provide basal structural or mechanical properties (e.g. orthopedic implants). For example, HA conjugated with immobilized arginine-glycine-aspartic acid-containing peptides can be coated onto titanium in a polyelectrolyte film with chitosan to improve osteoblast adhesion and reduce bacterial fouling.141 A number of groups have generated thin films through layer-by-layer deposition with HA and cationic polyelectrolytes such as chitosan and polylysine.142,143 The storage modulus of the films can be controlled over several orders of magnitude by secondary crosslinking in order to probe cell adhesion and mechanotransduction.144 For example, Richert et al. showed that the storage modulus of a film could increase from 20 kPa before additional crosslinking to 800 kPa after additional chemical crosslinking.145 Schneider et al. reported a similar magnitude of change in HA-chitosan films from an initial modulus of 15 kPa to 150 kPa after additional crosslinking, subsequently leading to more fibroblast spreading and adhesion.146
As previously mentioned, HA scaffolds within tissue are typically composed of very long HA chains, and use of HMW HA in biomaterials applications strongly influences HA-dependent adhesive signaling and can induce anti-inflammatory effects. However, the high viscosity of HMW HA solutions can make handling and mixing such solutions challenging, particularly in fabrication processes such as micromolding and 3D printing. To this end, supramolecular assembly of HA-based hydrogels has been exploited to enhance shear-thinning.137,147 Ouyang and colleagues utilized the orthogonal modification of the HA backbone to synthesize a gel that would undergo shear thinning to facilitate 3D printing but could subsequently be stabilized by covalent fixation.147 With this technology, higher MWs of HA can be incorporated into 3D printed scaffolds as well as other applications requiring rapid mixing or manipulation. Continued consideration of MW should enhance efforts to model tissue using HA-based biomaterials. At least one recent study has successfully incorporated HMW (500 – 750 kDa) HA into culture scaffolds to emulate the MW present in brain matix.148
Incorporating Adhesivity and Biodegradability into Biomaterial Design
As previously described, cells express HA-specific receptors that can bind directly to the HA backbone. Given that most solid HA-based biomaterials require modification of the HA backbone, an important question is how chemical modification alters adhesion and adhesion-dependent signaling. The adhesivity of the HA may be dependent on the type and degree of modification and seems to differentially affect specific receptors. For example, since receptor binding pockets typically accommodate around 4–6 HA monomers, it is likely that modifications on a low percentage of monomers (<15%) would only minimally affect HA adhesivity. As an example, modest aldehyde (10% of monomers) or thiol (25% of monomers) backbone modifications do not appear to significantly affect either aggrecan binding to the HA backbone or cell spreading and adhesion.149 However, increasing thiol functionalization of the carboxylic acid (from 20% to 40% of monomers) has been reported to reduce biodegradability and neurite extension of encapsulated cortical neurons.150 Bencherif and colleagues found that degree of methacrylation correlated inversely with cell adhesion and degradation.151 The sulfonation of hydroxyl groups on the HA backbone also leads to a decrease of platelet adhesion, suggesting the importance of the hydroxyl moiety for some functions.152 Thus, the changes in HA adhesivity due to backbone modification are nuanced and depend on the degree, type, and site of modification.
The chemistry of the modification may also have specific, context-dependent effects. Increasing divinyl sulfone crosslinking can induce a subcutaneous inflammatory response in vivo, apparently offsetting the anti-inflammatory properties of HMW HA.153 Both deacetylation of the amide moiety and sulfation of the alcohol moiety of HA can reduce CD44-mediated adhesion to HA, with dual modification further reducing adhesion.154 While the degree to which modification of the carboxylic acid moiety affects CD44 adhesion is not well known, crystallographic studies suggest that the negative charge and orientation of the carboxylic acid is important for binding to CD44.28 Modification would likely disrupt rather than enhance this binding. In a similar manner, Lord et al. found that serum proteins were more loosely bound on sulfated photoreactive HA versus non-sulfated HA, and that fibronectin orientation changed with sulfation to affect the degree of cell adhesion.155
While the HA backbone can intrinsically support cell adhesion, engagement of integrins is often an important biomaterial design goal, e.g. to promote cell spreading.156,157 To include these functionalities, peptides or recombinant proteins can be conjugated to the HA backbone which in turn affect cell morphology.158,159 However, protein conjugation generally requires backbone modification which reduces hyaladherin adhesivity based on the aforementioned studies. Alternatively, other matrix factors can be incorporated into HA-based materials as interpenetrating networks, particularly collagen, Matrigel, and gelatin.117,128,160,161 The inclusion of other matrix factors adds other types of adhesivity, but can lead to steric hindrance or matrix interactions that change other material properties of the hydrogel.128
A variety of studies suggest that while some degree of hyaluronidase recognition and degradation of HA is retained after backbone modification and gelation, these rates are reduced in a manner that depends on the modification site, the degree of modification, and the chemistry of the new functional moiety.162,163 Acrylation of the primary alcohol of HA has been reported to reduce hyaluronidase-mediated digestion of HA in solution by ~70%, implying that the modification interferes with enzyme binding or activity.159 While these studies clear indicate that hyaluronidase degradation of matrices is possible, the mechanism by which cells degrade HA-based biomaterials and the relationship with HA MW is poorly understood.
Because the carboxylic acid moiety on HA is important for hyaluronidase recognition, carboxylic acid modifications would be expected to inhibit HA degradation. To this end, complete esterification of the carboxylic group has been observed to prevent degradation by hyaluronidase, while partial esterification of the backbone reduced degradation rate.164 In one study, HA degradation rate was observed to depend critically on the degree of adipic dihydrazide modification of HA, with 65% modification reducing the rate nearly ten-fold.165 In another study, a high degree of biotinylation of HA and other chondroitin sulfates at the carboxylic acid disrupted degradation by hyaluronidases, but partial biotinylation enabled some hyaluronidase-based degradation.166 Furthermore, increasing crosslink hydrophobicity via the use of hydrazide chemistry reduces hyaluronidase degradation rate.167 These results together suggest that degradability by hyaluronidase is subject to the modification and crosslinking chemistry, and thus should be a key consideration when designing biomaterials for tissue regeneration or engineering as well as for research platforms in mechanistic studies.
While most modification strategies can be used to reduce HA-based biomaterial degradation, it may be more challenging to retain degradability in applications where both robust mechanics and degradability are desirable. Both properties are valuable in tissue engineering scaffolds and HA-based research platforms in which cells may need to be robustly organized but also be able to modify the microenvironment. The simplest strategy is to minimize the degree of modification to only modestly reduce HA bioactivity. To this end, the degree of modification is controllable to some degree by tailoring reaction conditions.168 Alternatively, the degradability can be incorporated in the crosslinks through some non-hyaluronidase based degradation mechanism. For example, Sahoo and colleagues used a crosslinking strategy to form an ester linkage with HA that could be rapidly hydrolyzed to yield the native HA backbone structure.169 Further work showed that the degradation rate could be extended by using a more hydrophobic polycaprolactone-based crosslinker.170 Several groups have also used matrix metalloproteases (MMP)-degradable peptide crosslinkers.157,171 MSCs cultured in HA with MMP-sensitive crosslinkers exhibit more rapid sprouting and matrix deposition.158
Another option is to not modify the HA backbone at all, but instead rely on non-covalent methods for gelation. For example, HA can be incorporated into an interpenetrating network with collagen in which electrostatic forces result in an HA coating over collagen fibrils.115 An alternative option that has yet to be explored in great depth is to use native CS or CS mimics to assemble HA matrices. As one example of this possibility, Bernhard and Panitch developed an aggrecan-mimetic peptide that increased the storage modulus of gels for cartilage engineering applications.172 While the high bond strength between HA and the CS-link protein complex suggests such binding is possible, it is unclear whether this means of crosslinking would be practical for any of the current applications.
Conclusions and Future Outlook:
Based on its bioactivity and versatility, HA is an attractive material platform for a variety of research and technological applications. By carefully considering how HA signaling influences cells and tissues, researchers and engineers can create HA formulations to meet a wide range of design requirements. Central to HA biophysical signaling is its mechanical properties, adhesivity, and degradability. In addition, HA MW has key implications for biophysical signaling, with HMW HA being associated with homeostasis and LMW HA being associated with tissue remodeling.
Various strategies exist for modifying the biophysical cues of HA, each with advantages and limitations that depend on the application. Modification of the HA backbone is a powerful and the most common way to control mechanics, conjugate adhesive ligands, or control degradability. However, backbone modification or crosslinking can reduce the adhesivity of the HA to HA-specific receptors such as CD44 or hamper degradation by hyaluronidases. The degree of modification is still difficult to precisely control using current synthetic methods. Even with these modifications, no strategies to date have captured the complexities of HA organization with other matrix factors and resulting mechanics observed in vivo. As a whole, HA is not well suited to applications requiring truly inert or non-degradable biomaterials due to its significant influence on cell signaling and matrix remodeling.
As the field’s understanding and appreciation of HA biology continues to expand, future work on HA-based biomaterials should focus on incorporating critical features of HA into biomaterial design and thorough characterization of the downstream effects. First, more attention to HA MW is warranted, given the importance of this parameter to both HA viscoelastic properties and biological effects. Second, the biological importance of HA organization within the ECM remains an open question in the field. As new studies seek to the address this question, chemistries and methodologies should expand to emulate key features of HA organization within biomaterial design. Third, the role of HA degradation in biomaterial performance remains understudied and needs to be addressed for both clinical and research applications. In each of these key areas, the biological effects of HA must be validated to ensure that HA is serving the expected or desired role within the context of the specific biomaterial formulation. With continued progress in all of these areas, the field will be poised to precisely tailor HA formulation for specific applications and better predict how these manipulations influence biological function.
Acknowledgements:
The authors gratefully acknowledge financial support from the following sources: National Science Foundation (Graduate Research Fellowship to K.W.); National Institutes of Health (Ruth L. Kirschstein Predoctoral Individual National Research Service Award No. F31CA228317 to K.W.; R21EB025017, R56DK118940, and R01CA227136 to S.K.).
References:
- (1).Cowman MK; Lee HG; Schwertfeger KL; McCarthy JB; Turley EA The Content and Size of Hyaluronan in Biological Fluids and Tissues. Front. Immunol 2015, 6, 261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Schanté CE; Zuber G; Herlin C; Vandamme TF Chemical Modifications of Hyaluronic Acid for the Synthesis of Derivatives for a Broad Range of Biomedical Applications. Carbohydr. Polym 2011, 85 (3), 469–489. [Google Scholar]
- (3).Highley CB; Prestwich GD; Burdick JA Recent Advances in Hyaluronic Acid Hydrogels for Biomedical Applications. Curr. Opin. Biotechnol 2016, 40, 35–40. [DOI] [PubMed] [Google Scholar]
- (4).Dicker KT; Gurski LA; Pradhan-Bhatt S; Witt RL; Farach-Carson MC; Jia X Hyaluronan: A Simple Polysaccharide with Diverse Biological Functions. Acta Biomater. 2014, 10 (4), 1558–1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Girish KS; Kemparaju K The Magic Glue Hyaluronan and Its Eraser Hyaluronidase: A Biological Overview. Life Sci. 2007, 80 (21), 1921–1943. [DOI] [PubMed] [Google Scholar]
- (6).Toole BP Hyaluronan and Its Binding Proteins, the Hyaladherins. Curr. Opin. Cell Biol 1990, 2 (5), 839–844. [DOI] [PubMed] [Google Scholar]
- (7).Day AJ; Prestwich GD Hyaluronan-Binding Proteins: Tying up the Giant. J. Biol. Chem 2002, 277 (7), 4585–4588. [DOI] [PubMed] [Google Scholar]
- (8).Stern R Hyaluronan Catabolism: A New Metabolic Pathway. Eur. J. Cell Biol 2004, 83 (7), 317–325. [DOI] [PubMed] [Google Scholar]
- (9).Lee JY; Spicer AP Hyaluronan: A Multifunctional, MegaDalton, Stealth Molecule. Curr. Opin. Cell Biol 2000, 12 (5), 581–586. [DOI] [PubMed] [Google Scholar]
- (10).Chanmee T; Ontong P; Itano N Mini-Review Hyaluronan: A Modulator of the Tumor Microenvironment. Cancer Lett. 2016, 375, 20–30. [DOI] [PubMed] [Google Scholar]
- (11).Khaing ZZ; Seidlits SK Hyaluronic Acid and Neural Stem Cells: Implications for Biomaterial Design. J. Mater. Chem. B 2015, 3 (40), 7850–7866. [DOI] [PubMed] [Google Scholar]
- (12).Manuskiatti W; Maibach HI Hyaluronic Acid and Skin: Wound Healing and Aging . Int. J. Dermatol 1996, 35 (8), 539–544. [DOI] [PubMed] [Google Scholar]
- (13).Toole BP Hyaluronan: From Extracellular Glue to Pericellular Cue. Nat. Rev. Cancer 2004, 4 (7), 528–539. [DOI] [PubMed] [Google Scholar]
- (14).Ponta H; Sherman L; Herrlich PA CD44: From Adhesion Molecules to Signalling Regulators. Nat. Rev. Mol. Cell Biol 2003, 4 (1), 33–45. [DOI] [PubMed] [Google Scholar]
- (15).Ziebell MR; Prestwich GD Interactions of Peptide Mimics of Hyaluronic Acid with the Receptor for Hyaluronan Mediated Motility (RHAMM). J. Comput. Aided. Mol. Des 2004, 18 (10), 597–614. [DOI] [PubMed] [Google Scholar]
- (16).Maxwell CA; McCarthy J; Turley E; Göttlicher M; Sleeman J; Plug R; Howells N; von Stein O; Ponta H; Herrlich P Cell-Surface and Mitotic-Spindle RHAMM: Moonlighting or Dual Oncogenic Functions? J. Cell Sci 2008, 121 (Pt 7), 925–932. [DOI] [PubMed] [Google Scholar]
- (17).Lokeshwar VB; Selzer MG Differences in Hyaluronic Acid-Mediated Functions and Signaling in Arterial, Microvessel and Vein-Derived Human Endothelial Cells. J. Biol. Chem 2000, 275 (36), 27641–27649. [DOI] [PubMed] [Google Scholar]
- (18).Savani RC; Cao G; Pooler PM; Zaman A; Zhou Z; DeLisser HM Differential Involvement of the Hyaluronan (HA) Receptors CD44 and Receptor for HA-Mediated Motility in Endothelial Cell Function and Angiogenesis. J. Biol. Chem 2001, 276 (39), 36770–36778. [DOI] [PubMed] [Google Scholar]
- (19).Akiyama Y; Jung S; Salhia B; Lee S; Hubbard S; Taylor M; Mainprize T; Akaishi K; van Furth W; Rutka JT Hyaluronate Receptors Mediating Glioma Cell Migration and Proliferation. J. Neurooncol 2001, 53 (2), 115–127. [DOI] [PubMed] [Google Scholar]
- (20).Kim Y; Kumar S CD44-Mediated Adhesion to Hyaluronic Acid Contributes to Mechanosensing and Invasive Motility. Mol. Cancer Res 2014, 12 (10), 1416–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Hamilton SR; Fard SF; Paiwand FF; Tolg C; Veiseh M; Wang C; Mccarthy JB; Bissell MJ; Koropatnick J; Turley EA The Hyaluronan Receptors CD44 and Rhamm (CD168) Form Complexes with ERK1,2 That Sustain High Basal Motility in Breast Cancer Cells. J. Biol. Chem 2007, 282 (22), 16667–16680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (22).Toole BP Hyaluronan-CD44 Interactions in Cancer: Paradoxes and Possibilities. Clin. Cancer Res 2009, 15 (24), 7462–7468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Zöller M CD44: Can a Cancer-Initiating Cell Profit from an Abundantly Expressed Molecule? Nat. Rev. Cancer 2011, 11 (4), 254–267. [DOI] [PubMed] [Google Scholar]
- (24).Yoshida T; Matsuda Y; Naito Z; Ishiwata T CD44 in Human Glioma Correlates with Histopathological Grade and Cell Migration. Pathol. Int 2012, 62 (7), 463–470. [DOI] [PubMed] [Google Scholar]
- (25).Breyer R; Hussein S; Radu DL; Pütz KM; Gunia S; Hecker H; Samii M; Walter GF; Stan AC Disruption of Intracerebral Progression of Rat C6 Glioblastoma by in Vivo Treatment with Anti-CD44 Monoclonal Antibody. J. Neurosurg 2009, 92 (1), 140–149. [DOI] [PubMed] [Google Scholar]
- (26).Zhu D; Bourguignon LY Interaction between CD44 and the Repeat Domain of Ankyrin Promotes Hyaluronic Acid-Mediated Ovarian Tumor Cell Migration. J. Cell. Physiol 2000, 183 (2), 182–195. [DOI] [PubMed] [Google Scholar]
- (27).Chopra A; Murray ME; Byfield FJ; Mendez MG; Halleluyan R; Restle DJ; Raz-Ben Aroush D; Galie PA; Pogoda K; Bucki R; Marcinkiewicz C; Prestwich GD; Zarembinski TI; Chen CS; Puré E; Kresh JY; Janmey PA Augmentation of Integrin-Mediated Mechanotransduction by Hyaluronic Acid. Biomaterials 2014, 35 (1), 71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Banerji S; Wright AJ; Noble M; Mahoney DJ; Campbell ID; Day AJ; Jackson DG Structures of the CD44–hyaluronan Complex Provide Insight into a Fundamental Carbohydrate-Protein Interaction. Nat. Struct. Mol. Biol 2007, 14 (3), 234–239. [DOI] [PubMed] [Google Scholar]
- (29).Vuorio J; Vattulainen I; Martinez-Seara H Atomistic Fingerprint of Hyaluronan–CD44 Binding. PLOS Comput. Biol 2017, 13 (7), e1005663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).DeGrendele HC; Estess P; Siegelman MH Requirement for CD44 in Activated T Cell Extravasation into an Inflammatory Site. Science. 1997, 278 (5338), 672–675. [DOI] [PubMed] [Google Scholar]
- (31).Suzuki T; Suzuki M; Ogino S; Umemoto R; Nishida N; Shimada I Mechanical Force Effect on the Two-State Equilibrium of the Hyaluronan-Binding Domain of CD44 in Cell Rolling. Proc. Natl. Acad. Sci 2015, 112 (22), 6991–6996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Stamenkovic I; Yu Q Shedding Light on Proteolytic Cleavage of CD44: The Responsible Sheddase and Functional Significance of Shedding. J. Invest. Dermatol 2009, 129 (6), 1321–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Banerji S; Ni J; Wang SX; Clasper S; Su J; Tammi R; Jones M; Jackson DG LYVE-1, a New Homologue of the CD44 Glycoprotein, Is a Lymph-Specific Receptor for Hyaluronan. J. Cell Biol 1999, 144 (4), 789–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Jackson DG Immunological Functions of Hyaluronan and Its Receptors in the Lymphatics. Immunol. Rev 2009, 230 (1), 216–231. [DOI] [PubMed] [Google Scholar]
- (35).Bono P; Rubin K; Higgins JMG; Hynes RO Layilin, a Novel Integral Membrane Protein, Is a Hyaluronan Receptor. Mol. Biol. Cell 2001, 12 (4), 891–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Bono P; Cordero E; Johnson K; Borowsky M; Ramesh V; Jacks T; Hynes RO Layilin, a Cell Surface Hyaluronan Receptor, Interacts with Merlin and Radixin. Exp. Cell Res 2005, 308 (1), 177–187. [DOI] [PubMed] [Google Scholar]
- (37).Scheibner KA; Lutz MA; Boodoo S; Fenton MJ; Powell JD; Horton MR Hyaluronan Fragments Act as an Endogenous Danger Signal by Engaging TLR2. J. Immunol 2006, 177 (2), 1272–1281. [DOI] [PubMed] [Google Scholar]
- (38).Termeer C; Benedix F; Sleeman J; Fieber C; Voith U; Ahrens T; Miyake K; Freudenberg M; Galanos C; Simon JC Oligosaccharides of Hyaluronan Activate Dendritic Cells via Toll-like Receptor 4. J. Exp. Med 2002, 195 (1), 99–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (39).Gariboldi S; Palazzo M; Zanobbio L; Selleri S; Sommariva M; Sfondrini L; Cavicchini S; Balsari A; Rumio C Low Molecular Weight Hyaluronic Acid Increases the Self-Defense of Skin Epithelium by Induction of Beta-Defensin 2 via TLR2 and TLR4. J. Immunol 2008, 181 (3), 2103–2110. [DOI] [PubMed] [Google Scholar]
- (40).Ebid R; Lichtnekert J; Anders H-J Hyaluronan Is Not a Ligand but a Regulator of Toll-like Receptor Signaling in Mesangial Cells: Role of Extracellular Matrix in Innate Immunity. ISRN Nephrol. 2014, 2014, 714081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Mahoney DJ; Blundell CD; Day AJ Mapping the Hyaluronan-Binding Site on the Link Module from Human Tumor Necrosis Factor-Stimulated Gene-6 by Site-Directed Mutagenesis. J. Biol. Chem 2001, 276 (25), 22764–22771. [DOI] [PubMed] [Google Scholar]
- (42).Parkar AA; Day AJ Overlapping Sites on the Link Module of Human TSG-6 Mediate Binding to Hyaluronan and Chondroitin-4-Sulphate. FEBS Lett. 1997, 410 (2–3), 413–417. [DOI] [PubMed] [Google Scholar]
- (43).Coulson-Thomas VJ; Lauer ME; Soleman S; Zhao C; Hascall VC; Day AJ; Fawcett JW Tumor Necrosis Factor-Stimulated Gene-6 (TSG-6) Is Constitutively Expressed in Adult Central Nervous System (CNS) and Associated with Astrocyte-Mediated Glial Scar Formation Following Spinal Cord Injury. J. Biol. Chem 2016, 291 (38), 19939–19952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Weigel PH; Frost SJ; McGary CT; LeBoeuf RD The Role of Hyaluronic Acid in Inflammation and Wound Healing. Int. J. Tissue React 1988, 10 (6), 355–365. [PubMed] [Google Scholar]
- (45).Stern R; Asari AA; Sugahara KN Hyaluronan Fragments: An Information-Rich System. Eur. J. Cell Biol 2006, 85 (8), 699–715. [DOI] [PubMed] [Google Scholar]
- (46).Marei WF; Ghafari F; Fouladi-Nashta AA Role of Hyaluronic Acid in Maturation and Further Early Embryo Development of Bovine Oocytes. Theriogenology 2012, 78 (3), 670–677. [DOI] [PubMed] [Google Scholar]
- (47).Haylock DN; Nilsson SK The Role of Hyaluronic Acid in Hemopoietic Stem Cell Biology. Regen. Med 2006, 1 (4), 437–445. [DOI] [PubMed] [Google Scholar]
- (48).Meyer K Chemical Structure of Hyaluronic Acid. Fed. Proc 1958, 17 (4), 1075–1077. [PubMed] [Google Scholar]
- (49).Fraser JRE; Laurent TC; Laurent UBG Hyaluronan: Its Nature, Distribution, Functions and Turnover. J. Intern. Med 1997, 242 (1), 27–33. [DOI] [PubMed] [Google Scholar]
- (50).Park JW; Chakrabarti B Optical Characteristics of Carboxyl Group in Relation to the Circular Dichroic Properties and Dissociation Constants of Glycosaminoglycans. Biochim. Biophys. Acta 1978, 544 (3), 667–675. [DOI] [PubMed] [Google Scholar]
- (51).Laurent UBG; Tengblad A Determination of Hyaluronate in Biological Samples by a Specific Radioassay Technique. Anal. Biochem 1980, 109 (2), 386–394. [DOI] [PubMed] [Google Scholar]
- (52).Armstrong SE; Bell DR Measurement of High-Molecular-Weight Hyaluronan in Solid Tissue Using Agarose Gel Electrophoresis. Anal. Biochem 2002, 308 (2), 255–264. [DOI] [PubMed] [Google Scholar]
- (53).Osterlin S On the Molecular Biology of the Vitreous in the Aphakic Eye. Acta Ophthalmol. 1977, 55 (3), 353–361. [DOI] [PubMed] [Google Scholar]
- (54).Dahl LB; Dahl IM; Engström-Laurent A; Granath K Concentration and Molecular Weight of Sodium Hyaluronate in Synovial Fluid from Patients with Rheumatoid Arthritis and Other Arthropathies. Ann. Rheum. Dis 1985, 44 (12), 817–822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).Hascall VC; Majors AK; de la Motte CA; Evanko SP; Wang A; Drazba JA; Strong SA; Wight TN Intracellular Hyaluronan: A New Frontier for Inflammation? Biochim. Biophys. Acta - Gen. Subj 2004, 1673 (1–2), 3–12. [DOI] [PubMed] [Google Scholar]
- (56).Krause WE; Bellomo EG; Colby RH Rheology of Sodium Hyaluronate under Physiological Conditions. Biomacromolecules 2001, 2 (1), 65–69. [DOI] [PubMed] [Google Scholar]
- (57).Milas M; Rinaudo M; Roure I; Al-Assaf S; Phillips GO; Williams PA Comparative Rheological Behavior of Hyaluronan from Bacterial and Animal Sources with Cross-Linked Hyaluronan (Hylan) in Aqueous Solution. Biopolymers 2001, 59 (4), 191–204. [DOI] [PubMed] [Google Scholar]
- (58).Oelschlaeger C; Cota Pinto Coelho M; Willenbacher N Chain Flexibility and Dynamics of Polysaccharide Hyaluronan in Entangled Solutions: A High Frequency Rheology and Diffusing Wave Spectroscopy Study. Biomacromolecules 2013, 14 (10), 3689–3696. [DOI] [PubMed] [Google Scholar]
- (59).Horkay F; Basser PJ; Hecht A-M; Geissler E Gel-like Behavior in Aggrecan Assemblies. J. Chem. Phys 2008, 128 (13), 135103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Zhu W; Mow VC; Rosenberg LC; Tang L-H Determination of Kinetic Changes of Aggrecan-Hyaluronan Interactions in Solution from Its Rheological Properties. J. Biomech 1994, 27 (5), 571–579. [DOI] [PubMed] [Google Scholar]
- (61).Nishimura M; Yan W; Mukudai Y; Nakamura S; Nakamasu K; Kawata M; Kawamoto T; Noshiro M; Hamada T; Kato Y Role of Chondroitin Sulfate–Hyaluronan Interactions in the Viscoelastic Properties of Extracellular Matrices and Fluids. Biochim. Biophys. Acta - Gen. Subj 1998, 1380 (1), 1–9. [DOI] [PubMed] [Google Scholar]
- (62).Rauch U Brain Matrix: Structure, Turnover and Necessity. Biochem. Soc. Trans 2007, 35 (Pt 4), 656–660. [DOI] [PubMed] [Google Scholar]
- (63).Lundell A; Olin AI; Mörgelin M; al-Karadaghi S; Aspberg A; Logan DT Structural Basis for Interactions between Tenascins and Lectican C-Type Lectin Domains: Evidence for a Crosslinking Role for Tenascins. Structure 2004, 12 (8), 1495–1506. [DOI] [PubMed] [Google Scholar]
- (64).Galtrey CM; Fawcett JW The Role of Chondroitin Sulfate Proteoglycans in Regeneration and Plasticity in the Central Nervous System. Brain Res. Rev 2007, 54 (1), 1–18. [DOI] [PubMed] [Google Scholar]
- (65).Zimmermann DR; Dours-Zimmermann MT Extracellular Matrix of the Central Nervous System: From Neglect to Challenge. Histochem Cell Biol 2008, 130, 635–653. [DOI] [PubMed] [Google Scholar]
- (66).Dijkgraaf LC; de Bont LG; Boering G; Liem RS Normal Cartilage Structure, Biochemistry, and Metabolism: A Review of the Literature. J. Oral Maxillofac. Surg 1995, 53 (8), 924–929. [DOI] [PubMed] [Google Scholar]
- (67).Roughley PJ; Lee ER Cartilage Proteoglycans: Structure and Potential Functions. Microsc. Res. Tech 1994, 28 (5), 385–397. [DOI] [PubMed] [Google Scholar]
- (68).Tang L-H; Buckwalter JA; Rosenberg LC Effect of Link Protein Concentration on Articular Cartilage Proteoglycan Aggregation. J. Orthop. Res 1996, 14 (2), 334–339. [DOI] [PubMed] [Google Scholar]
- (69).Bajorath J; Greenfield B; Munro SB; Day AJ; Aruffo A Identification of CD44 Residues Important for Hyaluronan Binding and Delineation of the Binding Site. J. Biol. Chem 1998, 273 (1), 338–343. [DOI] [PubMed] [Google Scholar]
- (70).Kahmann JD; O ‘brien R; Werner JM; Heinegård D; Ladbury JE; Campbell ID; Day AJ Localization and Characterization of the Hyaluronan-Binding Site on the Link Module from Human TSG-6. Structure 2000, 8 (7), 763–774 [DOI] [PubMed] [Google Scholar]
- (71).Bano F; Tammi MI; Kang DW; Harris EN; Richter RP Single-Molecule Unbinding Forces between the Polysaccharide Hyaluronan and Its Binding Proteins. Biophys. J 2018, 114 (12), 2910–2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (72).Kobayashi Y; Okamoto A; Nishinari K Viscoelasticity of Hyaluronic Acid with Different Molecular Weights. Biorheology 1994, 31 (3), 235–244. [DOI] [PubMed] [Google Scholar]
- (73).Schurz J; Ribitsch V Rheology of Synovial Fluid. Biorheology 1987, 24 (4), 385–399. [DOI] [PubMed] [Google Scholar]
- (74).Zhang Z; Christopher GF The Nonlinear Viscoelasticity of Hyaluronic Acid and Its Role in Joint Lubrication. Soft Matter 2015, 11 (13), 2596–2603. [DOI] [PubMed] [Google Scholar]
- (75).Bjelle A; Andersson T; Granath K Molecular Weight Distribution of Hyaluronic Acid of Human Synovial Fluid in Rheumatic Diseases. Scand. J. Rheumatol 1983, 12 (2), 133–138. [DOI] [PubMed] [Google Scholar]
- (76).Holmes MW; Bayliss MT; Muir H Hyaluronic Acid in Human Articular Cartilage. Age-Related Changes in Content and Size. Biochem. J 1988, 250 (2), 435–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (77).Lokeshwar VB; Rubinowicz D; Schroeder GL; Forgacs E; Minna JD; Block NL; Nadji M; Lokeshwar BL Stromal and Epithelial Expression of Tumor Markers Hyaluronic Acid and HYAL1 Hyaluronidase in Prostate Cancer. J. Biol. Chem 2001, 276 (15), 11922–11932. [DOI] [PubMed] [Google Scholar]
- (78).Wu M; Cao M; He Y; Liu Y; Yang C; Du Y; Wang W; Gao F A Novel Role of Low Molecular Weight Hyaluronan in Breast Cancer Metastasis. FASEB J. 2015, 29 (4), 1290–1298. [DOI] [PubMed] [Google Scholar]
- (79).McAtee CO; Barycki JJ; Simpson MA Emerging Roles for Hyaluronidase in Cancer Metastasis and Therapy. Adv. Cancer Res 2014, 123, 1–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (80).McKee CM; Penno MB; Cowman M; Burdick MD; Strieter RM; Bao C; Noble PW Hyaluronan (HA) Fragments Induce Chemokine Gene Expression in Alveolar Macrophages. The Role of HA Size and CD44. J. Clin. Invest 1996, 98 (10), 2403–2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (81).Rayahin JE; Buhrman JS; Zhang Y; Koh TJ; Gemeinhart RA High and Low Molecular Weight Hyaluronic Acid Differentially Influence Macrophage Activation. ACS Biomater. Sci. Eng 2015, 1 (7), 481–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (82).Yang C; Cao M; Liu H; He Y; Xu J; Du Y; Liu Y; Wang W; Cui L; Hu J; Gao F The High and Low Molecular Weight Forms of Hyaluronan Have Distinct Effects on CD44 Clustering. J. Biol. Chem 2012, 287 (51), 43094–43107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (83).Wolny PM; Banerji S; Gounou C; Brisson AR; Day AJ; Jackson DG; Richter RP Analysis of CD44-Hyaluronan Interactions in an Artificial Membrane System: Insights into the Distinct Binding Properties of High and Low Molecular Weight Hyaluronan. J. Biol. Chem 2010, 285 (39), 30170–30180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (84).Cyphert JM; Trempus CS; Garantziotis S Size Matters: Molecular Weight Specificity of Hyaluronan Effects in Cell Biology. Int. J. Cell Biol 2015, 2015, 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (85).Itano N; Kimata K Mammalian Hyaluronan Synthases. IUBMB Life (International Union Biochem. Mol. Biol. Life) 2002, 54 (4), 195–199. [DOI] [PubMed] [Google Scholar]
- (86).Itano N; Sawai T; Yoshida M; Lenas P; Yamada Y; Imagawa M; Shinomura T; Hamaguchi M; Yoshida Y; Ohnuki Y; Miyauchi S; Spicer AP; McDonald JA; Kimata K Three Isoforms of Mammalian Hyaluronan Synthases Have Distinct Enzymatic Properties. J. Biol. Chem 1999, 274 (35), 25085–25092. [DOI] [PubMed] [Google Scholar]
- (87).Valkonen M; Haapasalo H; Rilla K; Tyynelä-Korhonen K; Soini Y; Pasonen-Seppänen S Elevated Expression of Hyaluronan Synthase 2 Associates with Decreased Survival in Diffusely Infiltrating Astrocytomas. BMC Cancer 2018, 18 (1), 664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (88).Stern R; Jedrzejas MJ Hyaluronidases: Their Genomics, Structures, and Mechanisms of Action. Chem. Rev 2006, 106 (3), 818–819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (89).Chao KL; Muthukumar L; Herzberg O Structure of Human Hyaluronidase-1, a Hyaluronan Hydrolyzing Enzyme Involved in Tumor Growth and Angiogenesis. Biochemistry 2007, 24 (23), 6911–6920. [DOI] [PubMed] [Google Scholar]
- (90).Chen JWE; Pedron S; Harley BAC The Combined Influence of Hydrogel Stiffness and Matrix-Bound Hyaluronic Acid Content on Glioblastoma Invasion. Macromol. Biosci 2017, 17 (8), 1700018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (91).Pedron S; Hanselman JS; Schroeder MA; Sarkaria JN; Harley BAC Extracellular Hyaluronic Acid Influences the Efficacy of EGFR Tyrosine Kinase Inhibitors in a Biomaterial Model of Glioblastoma. Adv. Healthc. Mater 2017, 6 (21), 1700529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (92).Pedron S; Becka E; Harley BAC Regulation of Glioma Cell Phenotype in 3D Matrices by Hyaluronic Acid. Biomaterials 2013, 34 (30), 7408–7417. [DOI] [PubMed] [Google Scholar]
- (93).Cha J; Kang SG; Kim P Strategies of Mesenchymal Invasion of Patient-Derived Brain Tumors: Microenvironmental Adaptation. Sci. Rep 2016, 6 (1), 24912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (94).Heldin P; Basu K; Olofsson B; Porsch H; Kozlova I; Kahata K Deregulation of Hyaluronan Synthesis, Degradation and Binding Promotes Breast Cancer. J. Biochem 2013, 154 (5), 395–408. [DOI] [PubMed] [Google Scholar]
- (95).Bharadwaj AG; Kovar JL; Loughman E; Elowsky C; Oakley GG; Simpson MA Spontaneous Metastasis of Prostate Cancer Is Promoted by Excess Hyaluronan Synthesis and Processing. Am. J. Pathol 2009, 174 (3), 1027–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (96).Kovar JL; Johnson MA; Volcheck WM; Chen J; Simpson MA Hyaluronidase Expression Induces Prostate Tumor Metastasis in an Orthotopic Mouse Model. Am. J. Pathol 2006, 169 (4), 1415–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (97).Tian X; Azpurua J; Hine C; Vaidya A; Myakishev-Rempel M; Ablaeva J; Mao Z; Nevo E; Gorbunova V; Seluanov A High-Molecular-Mass Hyaluronan Mediates the Cancer Resistance of the Naked Mole Rat. Nature 2013, 499 (7458), 346–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (98).Balazs EA Therapeutic Use of Hyaluronan. Struct. Chem 2009, 20 (2), 341–349. [Google Scholar]
- (99).Balazs EA; Denlinger JL Clinical Uses of Hyaluronan. Ciba Found. Symp 1989, 143, 265–75; [DOI] [PubMed] [Google Scholar]
- (100).Brown T; Laurent U; Fraser. Turnover of Hyaluronan in Synovial Joints: Elimination of Labelled Hyaluronan from the Knee Joint of the Rabbit. Exp. Physiol 1991, 76 (1), 125–134. [DOI] [PubMed] [Google Scholar]
- (101).Adams ME An Analysis of Clinical Studies of the Use of Crosslinked Hyaluronan, Hylan, in the Treatment of Osteoarthritis. J. Rheumatol. Suppl 1993, 39, 16–18. [PubMed] [Google Scholar]
- (102).Collins MN; Birkinshaw C Hyaluronic Acid Based Scaffolds for Tissue Engineering-A Review. Carbohydr. Polym 2013, 92, 1262–1279. [DOI] [PubMed] [Google Scholar]
- (103).Prestwich GD Hyaluronic Acid-Based Clinical Biomaterials Derived for Cell and Molecule Delivery in Regenerative Medicine. J. Control. Release 2011, 155 (2), 193–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (104).Arsiwala SZ Current Trends in Facial Rejuvenation with Fillers. J. Cutan. Aesthet. Surg 2015, 8 (3), 125–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (105).Tezel A; Fredrickson GH The Science of Hyaluronic Acid Dermal Fillers. J. Cosmet. Laser Ther 2008, 10 (1), 35–42. [DOI] [PubMed] [Google Scholar]
- (106).Price RD; Berry MG; Navsaria HA Hyaluronic Acid: The Scientific and Clinical Evidence. J. Plast. Reconstr. Aesthetic Surg 2007, 60 (10), 1110–1119. [DOI] [PubMed] [Google Scholar]
- (107).Oh EJ; Park K; Kim KS; Kim J; Yang J-A; Kong J-H; Lee MY; Hoffman AS; Hahn SK Target Specific and Long-Acting Delivery of Protein, Peptide, and Nucleotide Therapeutics Using Hyaluronic Acid Derivatives. J. Control. Release 2010, 141 (1), 2–12. [DOI] [PubMed] [Google Scholar]
- (108).Köwitsch A; Zhou G; Groth T Medical Application of Glycosaminoglycans: A Review. J. Tissue Eng. Regen. Med 2018, 12 (1), e23–e41. [DOI] [PubMed] [Google Scholar]
- (109).Selyanin MA; Boykov PY; Khabarov VN Medical Applications of Hyaluronan In Hyaluronic Acid; Production, Properties, Application in Biology and Medicine; Polyak F, Eds; John Wiley & Sons, Ltd: Chichester, UK, 2015; 143–192. [Google Scholar]
- (110).Kogan G; Šoltés L; Stern R; Gemeiner P Hyaluronic Acid: A Natural Biopolymer with a Broad Range of Biomedical and Industrial Applications. Biotechnol. Lett 2006, 29 (1), 17–25. [DOI] [PubMed] [Google Scholar]
- (111).Burdick JA; Prestwich GD Hyaluronic Acid Hydrogels for Biomedical Applications. Adv. Mater 2011, 23 (12), H41–H56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (112).Gerecht S; Burdick JA; Ferreira LS; Townsend SA; Langer R; Vunjak-Novakovic G Hyaluronic Acid Hydrogel for Controlled Self-Renewal and Differentiation of Human Embryonic Stem Cells. Proc. Natl. Acad. Sci. U. S. A 2007, 104 (27), 11298–11303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (113).Adil MM; Vazin T; Ananthanarayanan B; Rodrigues GMC; Rao AT; Kulkarni RU; Miller EW; Kumar S; Schaffer DV Engineered Hydrogels Increase the Post-Transplantation Survival of Encapsulated HESC-Derived Midbrain Dopaminergic Neurons. Biomaterials 2017, 136, 1–11. [DOI] [PubMed] [Google Scholar]
- (114).Adil MM; Rao AT; Ramadoss GN; Chernavsky NE; Kulkarni RU; Miller EW; Kumar S; Schaffer DV Dopaminergic Neurons Transplanted Using Cell-Instructive Biomaterials Alleviate Parkinsonism in Rodents. Adv. Funct. Mater 2018, 1804144. [Google Scholar]
- (115).Xin X; Borzacchiello A; Netti PA; Ambrosio L; Nicolais L Hyaluronic-Acid-Based Semi-Interpenetrating Materials. J. Biomater. Sci. Polym. Ed 2004, 15 (9), 1223–1236. [DOI] [PubMed] [Google Scholar]
- (116).Tan H; Rubin JP; Marra KG Injectable in Situ Forming Biodegradable Chitosan-Hyaluronic Acid Based Hydrogels for Adipose Tissue Regeneration. Organogenesis 6 (3), 173–180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (117).Tan H; Wu J; Lao L; Gao C Gelatin/Chitosan/Hyaluronan Scaffold Integrated with PLGA Microspheres for Cartilage Tissue Engineering. Acta Biomater. 2009, 5 (1), 328–337. [DOI] [PubMed] [Google Scholar]
- (118).Kim J; Kim IS; Cho TH; Lee KB; Hwang SJ; Tae G; Noh I; Lee SH; Park Y; Sun K Bone Regeneration Using Hyaluronic Acid-Based Hydrogel with Bone Morphogenic Protein-2 and Human Mesenchymal Stem Cells. Biomaterials 2007, 28 (10), 1830–1837. [DOI] [PubMed] [Google Scholar]
- (119).Rape A; Ananthanarayanan B; Kumar S Engineering Strategies to Mimic the Glioblastoma Microenvironment. Adv. Drug Deliv. Rev 2014, 79–80, 172–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (120).Boudou T; Crouzier T; Nicolas C; Ren K; Picart C Polyelectrolyte Multilayer Nanofilms Used as Thin Materials for Cell Mechano-Sensitivity Studies. Macromol. Biosci 2011, 11 (1), 77–89. [DOI] [PubMed] [Google Scholar]
- (121).Eddhahak A; Zidi M Influence of Viscoelastic Properties of an Hyaluronic Acid-Based Hydrogel on Viability of Mesenchymal Stem Cells. Biomed. Mater. Eng 2015, 26 (3–4), 103–114. [DOI] [PubMed] [Google Scholar]
- (122).Cha J; Kim P Biomimetic Strategies for the Glioblastoma Microenvironment. Front. Mater 2017, 4, 45. [Google Scholar]
- (123).Volpi N; Schiller J; Stern R; Soltes L Role, Metabolism, Chemical Modifications and Applications of Hyaluronan. Curr. Med. Chem 2009, 16 (14), 1718–1745. [DOI] [PubMed] [Google Scholar]
- (124).Rape AD; Zibinsky M; Murthy N; Kumar S A Synthetic Hydrogel for the High-Throughput Study of Cell–ECM Interactions. Nat. Commun 2015, 6, 8129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (125).Lim HJ; Perera TH; Wilems TS; Ghosh S; Zheng YY; Azhdarinia A; Cao Q; Callahan LAS Response to Di-Functionalized Hyaluronic Acid with Orthogonal Chemistry Grafting at Independent Modification Sites in Rodent Models of Neural Differentiation and Spinal Cord Injury. J. Mater. Chem. B 2016, 4, 6865. [DOI] [PubMed] [Google Scholar]
- (126).Nistor MT; Chiriac AP; Nita LE; Vasile C; Bercea M Semi-Interpenetrated Polymer Networks of Hyaluronic Acid Modified with Poly(Aspartic Acid). J. Polym. Res 2013, 20 (2), 86. [Google Scholar]
- (127).Chen Q; Passos A; Balabani S; Chivu A; Zhao S; Azevedo HS; Butler P; Song W Semi-Interpenetrating Network Hyaluronic Acid Microgel Delivery Systems in Micro-Flow. J. Colloid Interface Sci 2018, 519, 174–185. [DOI] [PubMed] [Google Scholar]
- (128).Brigham MD; Bick A; Lo E; Bendali A; Burdick JA; Khademhosseini A Mechanically Robust and Bioadhesive Collagen and Photocrosslinkable Hyaluronic Acid Semi-Interpenetrating Networks. Tissue Eng. Part A 2009, 15 (7), 1645–1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (129).Erickson IE; Huang AH; Sengupta S; Kestle S; Burdick JA; Mauck RL Macromer Density Influences Mesenchymal Stem Cell Chondrogenesis and Maturation in Photocrosslinked Hyaluronic Acid Hydrogels. Osteoarthr. Cartil 2009, 17 (12), 1639–1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (130).Ananthanarayanan B; Kim Y; Kumar S Elucidating the Mechanobiology of Malignant Brain Tumors Using a Brain Matrix-Mimetic Hyaluronic Acid Hydrogel Platform. Biomaterials 2011, 32 (31), 7913–7923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (131).Ulrich TA; Pardo EMDJ; Kumar S The Mechanical Rigidity of the Extracellular Matrix Regulates the Structure, Motility, and Proliferation of Glioma Cells. Cancer Res 2009, No. 10, 4167–4175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (132).Keung AJ; de Juan-Pardo EM; Schaffer DV; Kumar S Rho GTPases Mediate the Mechanosensitive Lineage Commitment of Neural Stem Cells. Stem Cells 2011, 29 (11), 1886–1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (133).Pathak A; Kumar S Independent Regulation of Tumor Cell Migration by Matrix Stiffness and Confinement. Proc. Natl. Acad. Sci. U. S. A 2012, 109 (26), 10334–10339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (134).Handorf AM; Zhou Y; Halanski MA; Li W-J Tissue Stiffness Dictates Development, Homeostasis, and Disease Progression. Organogenesis 2015, 11 (1), 1–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (135).Discher DE; Janmey P; Wang Y-L Tissue Cells Feel and Respond to the Stiffness of Their Substrate. Science 2005, 310 (5751), 1139–1143. [DOI] [PubMed] [Google Scholar]
- (136).Tavsanli B; Can V; Okay O Mechanically Strong Triple Network Hydrogels Based on Hyaluronan and Poly(N,N-Dimethylacrylamide). Soft Matter 2015, 11 (43), 8517–8524. [DOI] [PubMed] [Google Scholar]
- (137).Rodell CB; Kaminski AL; Burdick JA Rational Design of Network Properties in Guest–Host Assembled and Shear-Thinning Hyaluronic Acid Hydrogels. Biomacromolecules 2013, 14 (11), 4125–4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (138).Lou J; Stowers R; Nam S; Xia Y; Chaudhuri O Stress Relaxing Hyaluronic Acid-Collagen Hydrogels Promote Cell Spreading, Fiber Remodeling, and Focal Adhesion Formation in 3D Cell Culture. Biomaterials 2018, 154, 213–222. [DOI] [PubMed] [Google Scholar]
- (139).Marklein RA; Burdick JA Spatially Controlled Hydrogel Mechanics to Modulate Stem Cell Interactions. Soft Matter 2010, 6 (1), 136–143. [Google Scholar]
- (140).Rosales AM; Rodell CB; Chen M; Burdick JA; Anseth KS Supramolecular Hyaluronic Acid-Based Hydrogels with Dynamic Viscoelasticity. Front. Bioeng. Biotechnol. 10th World Biomaterials Congress, 2016, 4. [Google Scholar]
- (141).Chua PH; Neoh KG; Kang ET; Wang W Surface Functionalization of Titanium with Hyaluronic Acid/Chitosan Polyelectrolyte Multilayers and RGD for Promoting Osteoblast Functions and Inhibiting Bacterial Adhesion. Biomaterials 2008, 29 (10), 1412–1421. [DOI] [PubMed] [Google Scholar]
- (142).Picart C; Lavalle P; Hubert P; Cuisinier FJG; Decher G; Schaaf P; Voegel J-C Buildup Mechanism for Poly(l-lysine)/Hyaluronic Acid Films onto a Solid Surface. Langmuir 2001, 17 (23), 7414–7424. [Google Scholar]
- (143).Salomäki M; Kankare J Influence of Synthetic Polyelectrolytes on the Growth and Properties of Hyaluronan−Chitosan Multilayers. Biomacromolecules 2009, 10 (2), 294–301. [DOI] [PubMed] [Google Scholar]
- (144).Schneider A; Francius G; Obeid R; Schwinté P; Hemmerlé J; Frisch B; Schaaf P; Voegel JC; Senger B; Picart C Polyelectrolyte Multilayers with a Tunable Young’s Modulus: Influence of Film Stiffness on Cell Adhesion. Langmuir 2006, 22 (3), 1193–1200. [DOI] [PubMed] [Google Scholar]
- (145).Richert L; Engler AJ; Discher DE; Picart C Elasticity of Native and Cross-Linked Polyelectrolyte Multilayer Films. Biomacromolecules 2004, 5 (5), 1908–1916. [DOI] [PubMed] [Google Scholar]
- (146).Schneider A; Richert L; Francius G; Voegel J-C; Picart C Elasticity, Biodegradability and Cell Adhesive Properties of Chitosan/Hyaluronan Multilayer Films. Biomed. Mater 2007, 2 (1), S45–S51. [DOI] [PubMed] [Google Scholar]
- (147).Ouyang L; Highley CB; Rodell CB; Sun W; Burdick JA 3D Printing of Shear-Thinning Hyaluronic Acid Hydrogels with Secondary Cross-Linking. ACS Biomater. Sci. Eng 2016, 2 (10), 1743–1751. [DOI] [PubMed] [Google Scholar]
- (148).Xiao W; Zhang R; Sohrabi A; Ehsanipour A; Sun S; Liang J; Walthers CM; Ta L; Nathanson DA; Seidlits SK Brain-Mimetic 3D Culture Platforms Allow Investigation of Cooperative Effects of Extracellular Matrix Features on Therapeutic Resistance in Glioblastoma. Cancer Res. 2018, 78 (5), 1358–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (149).Köwitsch A; Yang Y; Ma N; Kuntsche J; Mäder K; Groth T Bioactivity of Immobilized Hyaluronic Acid Derivatives Regarding Protein Adsorption and Cell Adhesion. Biotechnol. Appl. Biochem 2011, 58 (5), 376–389. [DOI] [PubMed] [Google Scholar]
- (150).Eng D; Caplan M; Preul M; Panitch A Hyaluronan Scaffolds: A Balance between Backbone Functionalization and Bioactivity. Acta Biomater. 2010, 6 (7), 2407–2414. [DOI] [PubMed] [Google Scholar]
- (151).Bencherif SA; Srinivasan A; Horkay F; Hollinger JO; Matyjaszewski K; Washburn NR Influence of the Degree of Methacrylation on Hyaluronic Acid Hydrogels Properties. Biomaterials 2008, 29 (12), 1739–1749. [DOI] [PubMed] [Google Scholar]
- (152).Cen L; Neoh KG; Li Y; Kang ET Assessment of in Vitro Bioactivity of Hyaluronic Acid and Sulfated Hyaluronic Acid Functionalized Electroactive Polymer. Biomacromolecules 2004, 5 (6), 2238–2246. [DOI] [PubMed] [Google Scholar]
- (153).Ibrahim S; Kang QK; Ramamurthi A The Impact of Hyaluronic Acid Oligomer Content on Physical, Mechanical, and Biologic Properties of Divinyl Sulfone-Crosslinked Hyaluronic Acid Hydrogels. J. Biomed. Mater. Res. Part A 2010, 94 (2), 355–370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (154).Bhattacharya DS; Svechkarev D; Souchek JJ; Hill TK; Taylor MA; Natarajan A; Mohs AM Impact of Structurally Modifying Hyaluronic Acid on CD44 Interaction. J. Mater. Chem. B 2017, 5, 8183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (155).Lord MS; Pasqui D; Barbucci R; Milthorpe BK Protein Adsorption on Derivatives of Hyaluronic Acid and Subsequent Cellular Response. J. Biomed. Mater. Res. Part A 2009, 91A (3), 635–646. [DOI] [PubMed] [Google Scholar]
- (156).Zhu X; Gojgini S; Chen TH; Fei P; Dong S; Ho C-M; Segura T Directing Three-Dimensional Multicellular Morphogenesis by Self-Organization of Vascular Mesenchymal Cells in Hyaluronic Acid Hydrogels. J. Biol. Eng 2017, 11 (1), 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (157).Kim J; Park Y; Tae G; Lee KB; Hwang SJ; Kim IS; Noh I; Sun K Synthesis and Characterization of Matrix Metalloprotease Sensitive-Low Molecular Weight Hyaluronic Acid Based Hydrogels. J. Mater. Sci. Mater. Med 2008, 19 (11), 3311–3318. [DOI] [PubMed] [Google Scholar]
- (158).Lei Y; Gojgini S; Lam J; Segura T The Spreading, Migration and Proliferation of Mouse Mesenchymal Stem Cells Cultured inside Hyaluronic Acid Hydrogels. Biomaterials 2011, 32 (1), 39–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (159).Park YD; Tirelli N; Hubbell JA Photopolymerized Hyaluronic Acid-Based Hydrogels and Interpenetrating Networks. Biomaterials 2003, 24, 893–900. [DOI] [PubMed] [Google Scholar]
- (160).Kutty JK; Cho E; Soo Lee J; Vyavahare NR; Webb K The Effect of Hyaluronic Acid Incorporation on Fibroblast Spreading and Proliferation within PEG-Diacrylate Based Semi-Interpenetrating Networks. Biomaterials 2007, 28 (33), 4928–4938. [DOI] [PubMed] [Google Scholar]
- (161).Yang Y; Sun C; Wilhelm ME; Fox LJ; Zhu J; Kaufman LJ Influence of Chondroitin Sulfate and Hyaluronic Acid on Structure, Mechanical Properties, and Glioma Invasion of Collagen I Gels. Biomaterials 2011, 32 (31), 7932–7940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (162).Nimmo CM; Owen SC; Shoichet MS Diels-Alder Click Cross-Linked Hyaluronic Acid Hydrogels for Tissue Engineering. Biomacromolecules 2011, 12, 824–830. [DOI] [PubMed] [Google Scholar]
- (163).Oh EJ; Kang S; Kim B; Jiang G; Cho IH; Hahn SK Control of the Molecular Degradation of Hyaluronic Acid Hydrogels for Tissue Augmentation. J. Biomed. Mater. Res. Part A 2008, 86A (3), 685–693. [DOI] [PubMed] [Google Scholar]
- (164).Zhong SP; Campoccia D; Doherty PJ; Williams RL; Benedetti L; Williams DF Biodegradation of Hyaluronic Acid Derivatives by Hyaluronidase. Biomaterials 1994, 15 (5), 359–365. [DOI] [PubMed] [Google Scholar]
- (165).Bulpitt P; Aeschlimann D New Strategy for Chemical Modification of Hyaluronic Acid: Preparation of Functionalized Derivatives and Their Use in the Formation of Novel Biocompatible Hydrogels. J. Biomed. Mater. Res 1999, 47 (2), 152–169. [DOI] [PubMed] [Google Scholar]
- (166).Altgärde N; Nilebäck E; de Battice L; Pashkuleva I; Reis RL; Becher J; Möller S; Schnabelrauch M; Svedhem S Probing the Biofunctionality of Biotinylated Hyaluronan and Chondroitin Sulfate by Hyaluronidase Degradation and Aggrecan Interaction. Acta Biomater. 2013, 9 (9), 8158–8166. [DOI] [PubMed] [Google Scholar]
- (167).Prestwich GD; Marecak DM; Marecek JF; Vercruysse KP; Ziebell MR Controlled Chemical Modification of Hyaluronic Acid: Synthesis, Applications, and Biodegradation of Hydrazide Derivatives. J. Control. Release 1998, 53 (1–3), 93–103. [DOI] [PubMed] [Google Scholar]
- (168).Oudshoorn MHM; Rissmann R; Bouwstra JA; Hennink WE Synthesis of Methacrylated Hyaluronic Acid with Tailored Degree of Substitution. Polymer. 2007, 48 (7), 1915–1920. [Google Scholar]
- (169).Sahoo S; Chung C; Khetan S; Burdick JA Hydrolytically Degradable Hyaluronic Acid Hydrogels with Controlled Temporal Structures. Biomacromolecules 2008, 9 (4), 1088–1092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (170).Chung C; Beecham M; Mauck RL; Burdick JA The Influence of Degradation Characteristics of Hyaluronic Acid Hydrogels on in Vitro Neocartilage Formation by Mesenchymal Stem Cells. Biomaterials 2009, 30 (26), 4287–4296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (171).Wei Z; Lewis DM; Xu Y; Gerecht S Dual Cross-Linked Biofunctional and Self-Healing Networks to Generate User-Defined Modular Gradient Hydrogel Constructs. Adv. Healthc. Mater 2017, 6 (16), 1700523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (172).Bernhard JC; Panitch A Synthesis and Characterization of an Aggrecan Mimic. Acta Biomater. 2012, 8 (4), 1543–1550. [DOI] [PubMed] [Google Scholar]