Abstract
Background:
A causal link between outdoor air pollution and childhood leukemia has been proposed, but some older studies suffer from methodological drawbacks. To the best of our knowledge, no systematic reviews have summarized the most recently published evidence and no analyses have examined the dose–response relation.
Objective:
We investigated the extent to which outdoor air pollution, especially as resulting from traffic-related contaminants, affects the risk of childhood leukemia.
Methods:
We searched all case–control and cohort studies that have investigated the risk of childhood leukemia in relation to exposure either to motorized traffic and related contaminants, based on various traffic-related metrics (number of vehicles in the closest roads, road density, and distance from major roads), or to measured or modeled levels of air contaminants such as benzene, nitrogen dioxide, 1,3-butadiene, and particulate matter. We carried out a meta-analysis of all eligible studies, including nine studies published since the last systematic review and, when possible, we fit a dose–response curve using a restricted cubic spline regression model.
Results:
We found 29 studies eligible to be included in our review. In the dose–response analysis, we found little association between disease risk and traffic indicators near the child’s residence for most of the exposure range, with an indication of a possible excess risk only at the highest levels. In contrast, benzene exposure was positively and approximately linearly associated with risk of childhood leukemia, particularly for acute myeloid leukemia, among children under 6 y of age, and when exposure assessment at the time of diagnosis was used. Exposure to nitrogen dioxide showed little association with leukemia risk except at the highest levels.
Discussion:
Overall, the epidemiologic literature appears to support an association between benzene and childhood leukemia risk, with no indication of any threshold effect. A role for other measured and unmeasured pollutants from motorized traffic is also possible. https://doi.org/10.1289/EHP4381
Introduction
Acute leukemia is the most frequent childhood cancer (Kehm et al. 2018a; Siegel et al. 2016). Its age-standardized incidence has been increasing by 0.6% per year from 1975 through most recent years (Isaevska et al. 2017; Noone et al. 2018), up to about five cases per 100,000 population in the United States and other high-income countries (Barrington-Trimis et al. 2017; Isaevska et al. 2017; Noone et al. 2018). The etiology of childhood leukemia is largely unknown, although there is increasing evidence that environmental determinants may play a major role (Metayer et al. 2016a). In addition to established and putative environmental risk factors such as ionizing and nonionizing radiation (Amoon et al. 2018; Bartley et al. 2010), infections (Kreis et al. 2019; Marcotte et al. 2014), pesticides (Malagoli et al. 2016), parental smoking (Heck et al. 2016), diet (Henshaw and Suk 2015), and occupation (Spycher et al. 2017), there is concern about outdoor air pollution and, in particular, exposure to contaminants released by motorized traffic, a nearly ubiquitous exposure (De Donno et al. 2018; Landrigan et al. 2018; Montero-Montoya et al. 2018; Suk et al. 2016). In recent years, five systematic reviews have evaluated the association between traffic-related air pollution and acute childhood leukemia (Boothe et al. 2014; Carlos-Wallace et al. 2016; Filippini et al. 2015; IARC 2016; Sun et al. 2014) and one has evaluated the association between benzene exposure and disease (IARC 2018). All but one (Sun et al. 2014) indicated some association. For instance, the 2016 IARC-WHO review of air pollution noted that weak associations with childhood leukemia (especially acute lymphoblastic leukemia) could not be ruled out based on epidemiological studies. Such associations were defined as “suggestive” but “inconsistent” (IARC 2016). However, most of these reviews relied on simple metrics of traffic density, and none addressed the dose–response relation between pollutants and leukemia. Furthermore, several recently published studies were not included in those reviews. In the present review, we include nine additional studies (Houot et al. 2015; Janitz et al. 2016, 2017; Lavigne et al. 2017; Magnani et al. 2016; Raaschou-Nielsen et al. 2018; Spycher et al. 2015; Symanski et al. 2016; Tamayo-Uria et al. 2018). In addition to analyses comparing highest versus lowest categories of exposure, we conducted a dose–response meta-analysis. The dose–response analysis incorporated several proxies to assess exposure to air pollutants besides modeling levels of air contaminants.
Methods
Search Strategy
We performed a systematic PubMed, Web of Science, and Embase literature database search formatting the research question according to the PECOS statement (Population, Exposure, Comparator(s), Outcomes, and Study design) (Morgan et al. 2018). Full details of the definition of the research question and related database search strings are reported in Table S1. In our latest database search, we looked at all papers up to 20 March 2019, without limiting the literature search to specific languages. We also used extensive search techniques to identify additional references of potential interest, including “snowballing” methods, that is, screening the reference lists of eligible or key articles (backward citations), checking which other articles have cited the eligible or key articles (forward citations), and using the “similar articles” function of online databases, which identifies articles similar to a selected paper [Booth 2008; European network for Health Technology Assessment (EUnetHTA) 2017; Vinceti et al. 2017].
Study Selection and Review
All titles and abstracts were screened by two authors (TF and MV). When there was disagreement or when the abstract was missing, both authors reviewed the full texts to determine eligibility for inclusion. Full-text evaluation of all potentially eligible studies was performed in duplicate, with controversies discussed by the two authors (TF and MV). Inclusion criteria were a) epidemiologic case–control or cohort studies, b) childhood population, c) any type of assessment of exposure to traffic from motorized vehicles, d) reporting of risk estimates or ability to compute them from the reported data. Exclusion criteria included a) ecologic study design, b) studies on only adult populations, c) exposure assessment limited to only occupational activities, and d) a lack of reporting of risk estimates for childhood leukemia.
We then examined all retrieved studies assessing outdoor air pollutant exposure through traffic density, air monitoring data, and dispersion air pollutant models based on motorized traffic. Traffic density included metrics such as traffic count (the estimated number of vehicles per day in the roads within a defined distance from the residence), road density (the sum of the length of roads within a defined area around the residence), and residential distance (the distance between the residence and a major road). We evaluated both maternal exposure during pregnancy and child’s exposure at birth, time of diagnosis, and average exposure during the child’s lifetime, based on residential addresses during pregnancy, at birth, or at the time of diagnosis. The effect of these different exposure windows was compared in analyses stratified into peri- and postnatal periods. We (TF and AC) also extracted other details of interest such as study size and characteristics, age at diagnosis, and leukemia subtype, that is, acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML). We (TF and MV) assessed the characteristics and quality of included studies through the Newcastle-Ottawa Scale (NOS). Briefly, the NOS scale is based on a “star” system in which a study is assessed on three broad perspectives: the “selection” of the study groups, the “comparability” of the groups, and the ascertainment of either the “exposure” or “outcome” of interest for case–control or cohort studies, respectively (Wells et al. 2019). High-quality answers to each NOS scale question are identified with an asterisk/star. In Table S2, we reported details used for the evaluation. Total score is the sum of the score for each answer to the NOS scale. A high score indicates that the study is of high quality.
Statistical Analysis
In our meta-analysis, the ranges of exposure levels for most studies were roughly comparable, although cut points for exposure category identification differed across studies to some extent. We extracted from all studies the risk ratio (RR) in each exposure category, by abstracting the odds ratios in case–control studies and hazard ratios or rate ratios in cohort studies, as well as the number of cases or events and controls or person-years in each exposure category. We used a random-effects model to estimate a summary RR for childhood leukemia, comparing the highest and lowest exposure categories for each metric of interest.
We also performed a dose–response meta-analysis to understand the shape of the curve relating air pollution and disease risk. To do that, we used methodology developed by Greenland and Longnecker (1992) and Orsini et al. (2012) that has been applied in other contexts (Crippa et al. 2018b; Vinceti et al. 2016) to estimate the trend from the RRs across categories of pollutant exposure levels and their approximate pointwise 95% confidence intervals (CIs) based on asymptotic normality. For each of the exposure strata, we abstracted the mean or, if means were unavailable, the median. If neither mean nor median were stated, we used the midpoint. When the highest and lowest exposure categories were “open,” that is, mean or median or extreme values were not presented, we entered a value that was 20% higher or lower than the closest cut point. We based this decision on the studies reporting both cut point values and median/mean values for the extreme categories and which found a difference ranging between 15% and 20%. We excluded from this dose–response meta-analysis the studies not reporting any exposure category cut points for the investigated pollutant and the studies providing only RR estimates based on 1-unit increment in exposure based on a linear model because these studies could not contribute to the assessment of departure from linearity. However, all those studies were included in the analysis of overall studies comparing highest versus lowest exposure. We then investigated the shape of the relation between traffic or pollutant exposures and childhood leukemia risk with either one-stage and two-stage dose–response meta-analysis, using restricted cubic splines with 3 knots at fixed percentiles (10, 50, and 90%) of the exposure distribution (Crippa et al. 2018a; Orsini et al. 2012). The restricted cubic spline model was fit with a generalized least-squares regression taking into account the correlation within each set of published RRs, and combining the study-specific estimates using the restricted maximum likelihood method in a multivariable random-effects meta-analysis (Greenland and Longnecker 1992; Jackson et al. 2010; Orsini et al. 2006, 2012). This dose–response meta-analysis was applied to three traffic-related metrics (traffic count, road density, and residential distance from a major road) and to the only two pollutants for which enough data for measured or monitored air levels were available [benzene and nitrogen dioxide ()]. For one study (Heck et al. 2014), some data needed for the meta-analysis were not reported in the publication but were provided by two coauthors (JEH and ASP). In these analyses, estimates for benzene were adjusted for 1,3-butadiene (and vice versa).
Heterogeneity was taken into consideration in the estimation of the random-effects model in the highest versus lowest analysis. We reported the and measures for each analysis. In order to explore the source of heterogeneity, we conducted stratified analyses, by leukemia subtype (ALL and AML), age at diagnosis ( and ), exposure time window (residence during pregnancy, at birth, and/or diagnosis), and continent. Because the fixed effect (average regression coefficients across studies) and variance/covariance structure of the random effects in spline models are not easily interpreted, we provided a graphical overlay of the marginal and conditional (best linear unbiased prediction of study-specific) dose–response trends we computed for each study (Crippa et al. 2018a). We also re-ran the analyses repeatedly, each time without one of the studies, to assess the missing study’s influence and to characterize the source and magnitude of any heterogeneity of results.
Finally, we checked for the possible presence of publication bias using funnel plots for studies reporting highest versus lowest exposure. We used Stata software (release 15.1; Stata Corp.) for all data analyses and specifically the “metan,” “metaninf,” “metafunnel,” “mkspline,” and “drmeta” routines.
Results
Description of Included Studies
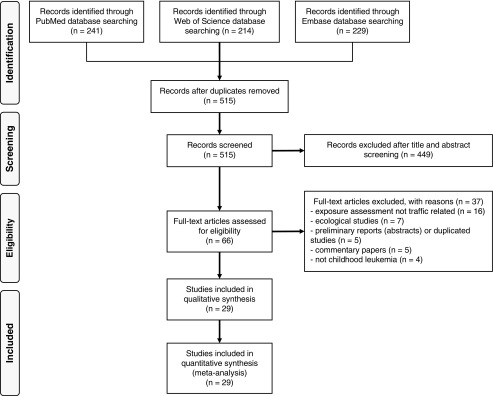
Figure 1 reports the flowchart of the literature search. We retrieved 29 papers eligible for this review, including 26 case–control and three cohort studies. Detailed characteristics of included studies are summarized in Table 1. Overall, they included over 13,000 cases and (for the case–control studies) 145,000 controls worldwide. The year of diagnosis of the cases ranged from 1960 to 2012. Seven studies limited their study population to children y of age (Ghosh et al. 2013; Heck et al. 2013, 2014; Lavigne et al. 2017; Reynolds et al. 2001, 2004; Symanski et al. 2016), and seven presented age-stratified analyses (Badaloni et al. 2013; Houot et al. 2015; Janitz et al. 2016, 2017; Savitz and Feingold 1989; Spycher et al. 2015; Vinceti et al. 2012).
Figure 1.
Flowchart of literature search and identification through 20 March 2019.
Table 1.
Main characteristics of case–control and cohort studies on air pollution from motorized traffic and risk of childhood leukemia.
| Reference | Region | Study period | Type of cancer | Cases/controls | Age (y) | Exposure assessment | Methods | Type of residence | Case selection | Control selection | Matching variables | Adjustment factors |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Abdul Rahman et al. 2008 | Malaysia | 2001–2007 | All leukemias | 128/128 | 0–14 | Traffic density | Traffic density using distance from main road , ) | Residence at diagnosis | Histological confirmed diagnosis of acute leukemia in two Malaysian hospitals | Hospital selection from children received treatment in one of the two hospitals | None | Family income, paternal social contact, number of elder siblings, maternal age, paternal age, family history of cancer, paternal smoking, distance to main road, and distance to power line |
| Amigou et al. 2011 | France | 2003–2004 | All leukemias () | 763 | 0–14 | Traffic density estimated using three models and a composite of them, including estimated levels | Traffic density through proximity to main roads within area of using Navteq software function to characterize road classes on the basis of their importance and through density of heavy traffic roads within from the residence. Traffic-related levels | Residence at diagnosis | French National Cancer Registry of Childhood Hematopoietic Malignancies | Community recruitment with telephone interviews. Response rates for case/controls of 91% and 71% | Sex and age | Sex, age, and socioeconomic status |
| Badaloni et al. 2013 | Italy | 1998–2001 | All leukemias | 747/1,509 | 0–10 (0–4) | Traffic density using two models and pollutants exposure (, , , and ) | Traffic density using distance from main roads (, 50–150, ) and main roads’ length within area of . Pollutant exposures estimated using LUR model for , , , and divided into quartiles | Residence at birth | Italian Association of Pediatric Hematology and Oncology Registry | Community selection with face-to-face not-blinded interview. Response rates for case/controls of 91.4% and 69.2% | Sex and age | Sex, age, region of residence and parental education level |
| Crosignani et al. 2004 | Italy | 1978–1997 | All leukemias and ALL | 120/480 | 0–14 | Traffic density using distance from major roads and benzene exposure | Distance from major roads (, 20–150, ). Benzene estimated exposure in three categories (, 0.1–10, ) | Residence at diagnosis | Lombardy Cancer Registry | Community selection by Health Services archives of Varese | Sex and birth year | Sex, age, and socioeconomic status |
| Feychting et al. 1998 | Sweden | 1960–1985 | All leukemias | 39/151 | 0–15 | exposure using continuous values and in two categorical models | estimated levels through continuous scale () and three categories, using 50th (, 40–49, ) and 75th percentiles (, 50–79, ) as referent respectively | Residence for at least 1 y within from power lines | Swedish Cancer Registry linking medical records to verify diagnoses | Community selection from same area | Age, municipality and residence near the same high voltage power lines | Magnetic fields and socioeconomic status |
| Ghosh et al. 2013 | California | 1988–2008 | 0–5 | NO, , and exposure | Pollutant exposures estimated using LUR, unseasonalized, and seasonalized models | Residence at birth | California Cancer Registry | Community selection from birth certificates from mothers living in Los Angeles County | Sex and birth year | Sex, birth year, race/ethnicity, maternal education level, parity, prenatal care insurance type, and neighborhood socioeconomic index | ||
| Harrison et al. 1999 | United Kingdom | 1990–1994 | All leukemias | 130/251 | 0–15 | Traffic density as measured by weighted distance from major roads | Addresses within of major roads, using Geographic Information System | Residence at diagnosis | West Midland Cancer Intelligence Units, using ICD-9 | Solid tumor cases from the same register as the cases | None | None |
| Heck et al. 2013 | California | 1998–2007 | 0–5 | Traffic density, CO, and exposure estimated levels | Traffic density in vehicles per day within of the residence. Pollutant exposures estimated using CALINE dispersion model for CO; using measures from community air monitors | Residence at birth | California Cancer Registry | Community selection from birth certificates | Birth year | Birth year, maternal race/ethnicity, mother’s birth place, and neighborhood socioeconomic index | ||
| Heck et al. 2014 | California | 1990–2007 | 0–6 | Air toxic exposure | Pollutant exposures (total PAHs, benzene, ethyl-benzene, 1,3-butadiene) taken from air monitors within 2 or of residence | Residence at birth | California Cancer Registry | Community selection from birth certificates | Birth year | Birth year, maternal race/ethnicity, mother’s birth place, and neighborhood socioeconomic index | ||
| Houot et al. 2015 | France | 2002–2007 | All leukemias () | 2,760 , overall 517 , Île-de-France Region | 0–14 (0–4, 5–9, 10–14) | Traffic density estimated using two models, and benzene estimated levels | Traffic density through proximity to major roads within area of of residence and length of major roads within of residence using Navteq software function to characterize road classes on the basis of their importance. Traffic-related and benzene levels | Residence at diagnosis | French National Cancer Registry of Childhood Hematopoietic Malignancies | Community selection from National Institute for Statistics and Economic Studies | None | Age (and deprivation index only in road length analysis) |
| Janitz et al. 2016 | Oklahoma | 1997–2012 | All leukemias () | 307 | 0–19 (0–4, 5–9, 10–14, 15–19) | Traffic density using two models and exposure | Traffic density through proximity to major roads within area of 750 or of residence. Traffic-related levels using a satellite-based LUR model | Residence at birth | Oklahoma Central Cancer Registry | Community selection from birth certificates | Birth week | Age, maternal education (and urbanization) |
| Janitz et al. 2017, 2018 | Oklahoma | 1997–2012 | All leukemias () | 307 | 0–19 (0–4, 5–9, 10–14, 15–19) | Benzene exposure | Benzene exposure levels based on National-Scale Air to Toxics Assessment (NATA) 2005 models | Residence at birth | Oklahoma Central Cancer Registry | Community selection from birth certificates | Birth week | Age, maternal education and urbanization |
| Langholz et al. 2002 | California | 1978–1984 | All leukemias | 212/202 | 0–10 | Traffic density as vehicles per day estimated in two models | Traffic density at the longest-lived residence for all streets within 1,500-ft radius, divided into three categories (, 500–9,999 ) or in quintiles as cut points (, , , 13,264–28,497, ) | Residence of longest duration | Los Angeles Country Cancer Registry | Community selection by not-blinded telephone interviews with random digit dialing method | Sex and age | Wire-code |
| Lavigne et al. 2017 | Ontario, Canada | 1988–2012 | All leukemias ( | 941 ( (total cohort) | 0–5 | and exposure | Land-use regression model estimates of and temporally varying satellite-derived estimates of | Residence at birth | Pediatric Oncology Group of Ontario Networked Information System | Mother–baby Linked database of hospital admission records of deliveries across Ontario | Not applicable | Age at delivery, infant sex, parity, year of birth, maternal cigarette smoking during pregnancy, census tract median family income, census tract proportion of population who are visibly minority, and census tract proportion of the adult female population 25–64 y of age who completed postsecondary education |
| Magnani et al. 2016 | Italy | 1998–2001 | All leukemias () | 648 ( | 0–10 | Traffic density using main roads’ length | Traffic density using main roads’ length within area of in four categories as cut point (0, 10th, 50th, 90th percentiles) to four different types of residence | Residence at birth Residence of longest duration Residence for at least 50% of life Residence considered the highest exposed | Italian Association of Pediatric Hematology and Oncology Registry | Community selection with face-to-face not-blinded interview. Response rates for case/controls of 91.7% and 70.8% | Sex, age, and region | Sex, age, region, parental education, and parental smoking |
| Pearson et al. 2000 | Colorado | 1978–1983 | All leukemias | 97/259 | 0–14 | Traffic density in vehicles per day estimated in highest vs. lowest category: two different models | Traffic density within 750-ft distance-weighted traffic density using six categories: , 500–4,999, 5,000–9,999 10,000–14,999, 15,000–19,999, | Residence at diagnosis | Colorado Central Cancer Registry | Community selection by not-blinded telephone interviews with random digit dialing method | Sex, age, and telephone exchange area | None |
| Raaschou-Nielsen et al. 2001 | Denmark | 1968–1991 | All leukemias | 986/5,506 | 0–14 | Traffic density as vehicles per day, and pollutant (benzene and ) exposure during childhood and pregnancy | Traffic density estimated in vehicles/day using four categories: , , , in both childhood and pregnancy. Pollutant exposure using a modified version of the Operational Street Pollution Model and 50th, 90th, and 99th percentiles as cutoff points (data in ) resulted in four categories for benzene (, , , during childhood, , , , during pregnancy) and for (, , , during childhood, , , , during pregnancy) | Residence at diagnosis | Danish Cancer Registry | Danish Central Population Registry | Sex, age, and calendar time | Urban development, geographic region, type of residence, electromagnetic fields, mother’s age, and birth order |
| Raaschou-Nielsen et al. 2018 | Denmark | 1968–1991 | 0–14 | Benzene exposure during childhood and pregnancy | Pollution Model and 50th and 90th percentiles as cutoff points resulted in three categories for benzene during childhood (, , or , , ) and pregnancy (, , or , , ) | Residence at diagnosis | Danish Cancer Registry | Danish Central Population Registry | Sex, age, and calendar time | Degree of urbanization, geographic region, type of residence, electromagnetic fields, mother’s age, and birth order | ||
| Reynolds et al. 2001 | California | 1988–1994 | All leukemias | 90/349 | 0–5 | Traffic density using average number of cars per day (ADT) | ADT of road segments within a 550-ft radius from home address and divided into three categories: no ADT (reference), and percentiles | Residence at birth | California Cancer Registry | Community selection from birth certificates | Sex and birth year | Sex, birth year, ethnicity, and median child family income |
| Reynolds et al. 2004 | California | 1988–1997 | All leukemias (and ALL) | 1,728 (1,407)/3,456 | 0–4 | Traffic density and road density | Traffic density using combination of road length and vehicle traffic counts. Road density: summary of total road length (in miles per square mile) within 500-ft radius. For both, 25th, 50th, 75th, and 90th percentiles were used as cut points | Residence at birth | California Cancer Registry | Community selection from birth certificates | Sex and birth year | Sex, birth year, and ethnicity |
| Savitz and Feingold 1989 | Colorado | 1976–1983 | All leukemias (and ALL) | 98 (79)/262 | 0–14 (0–4, 5–14) | Traffic density in vehicles per day estimated in four different models | Traffic density at the address of residence using street maps of Denver Standard Metropolitan Statistical Area: vehicles/day divided into two or three categories: vs. or , 500–4,999, , or , 500–9,999, or vs. | Residence at diagnosis | Colorado Central Cancer Registry | Community recruitment by random digit dial, data collection via telephone interview. Overall response rate of 75% | Sex, age, and telephone exchange area | Sex, age, year of diagnosis, type of residence, location at birth, mother’s age, father’s education, and per capita income and wire configuration code at diagnosis |
| Spycher et al. 2015 | Switzerland | 1990–2008 | All leukemias (and ALL) | 532 (416)/2,096,402 (total cohort) | 0–15 (0–4) | Distance from heavy traffic roads | Distance to the nearest highway (, , , ) | Residence at diagnosis | Swiss Childhood Cancer Registry | Swiss resident population at the national censuses of 1990 and 2000 | Not applicable | Sex, birth year, urbanization, socioeconomic position, educational level of household head, number of persons per room, nationality, background ionizing radiation (terrestrial gamma and cosmic rays), distance to the nearest power line, and strength of electromagnetic fields from broadcast transmitters |
| Steffen et al. 2004 | France | 1995–1999 | All leukemias () | 280 | 0–14 | Traffic density using main roads’ density | Traffic density using presence of heavy traffic roads within from residence (all roads types, secondary road or main street, primary road and motorway or similar road) | Residence at diagnosis | Hospitals of Nancy, Lille, Lyon, and Paris | Hospital recruitment with face-to-face interviews to assess exposure | Sex, age, ethnic origin, and hospital | Sex, age, ethnic origin, and hospital |
| Symanski et al. 2016 | Texas | 1995–2011 | ALL | 1,248/12,172 | 0–4 | Benzene, 1,3-butadiene and POM exposure | Air pollutants (benzene, 1,3-butadiene, and POM) exposure levels based on 1996, 1999, 2002, and 2005 NATA models | Residence at birth | Texas Cancer Registry | Community controls from Texas Department of State Health Services vital statistics birth records | Birth year and month | Birth year and month, air pollutants (benzene, 1,3-butadiene, POM), census tract, maternal age, infant birth weight, sex, and maternal race/ethnicity |
| Tamayo-Uria et al. 2018 | Spain | 1996–2011 | All leukemias () | 1,061 | 0–14 | Traffic density using annual average daily traffic and distance to busy roads | Traffic density using Navteq software function to characterize road classes, their proximity and traffic density within multiple buffers (50, 100, 200, and ) from the residence | Residence at diagnosis for cases, at birth for controls | Spanish Registry of Childhood Tumours (RETI-SEHOP) | Community controls from Birth Registry of the Spanish Statistical Office | Sex, birth year, and, autonomous region of residence | Sex, birth year, autonomous region of residence, socioeconomic status, industrial pollution, and crop exposure |
| Vinceti et al. 2012 | Italy | 1998–2009 | All leukemias () | 83 | 0–14 (0–5, 5–14) | Benzene and exposure | Average and maximum hourly levels of benzene and estimated through the CALINE dispersion model and divided into quartiles: cut points for average benzene (, , , ) | Residence at diagnosis | Italian Association of Pediatric Hematology and Oncology Registry | Community selection from Local Health Units of Modena and Reggio Emilia | Sex, birth year, and province of residence | Benzene and average and maximum hourly levels |
| Visser et al. 2004 | Netherlands | 1989–1997 | ALL | 5/total population | 0–14 | Traffic density as residence along busiest main roads | Traffic density measured as residence along main roads with traffic identity score . Traffic density score was calculated using daily traffic intensity, counting cars for 1 and truck for 10 | Residence at 1998 or date of death | Amsterdam Cancer Registry | Amsterdam resident population | Not applicable | 5-y-of-age group and sex-specific standardized incidence rate |
| Von Behren et al. 2008 | California | 1995–2002 | ALL | 310/396 | 0–15 | Traffic density in total vehicle miles traveled per square mile | Traffic density in total vehicle miles traveled per square mile within 500-ft radius using 0th, 50th, and 75th percentiles as cut points (no roads, 1–38,499, 38,500–91,461, ) | Residence at birth, at diagnosis and the average lifetime | Hospitals participating in Northern California Childhood Leukemia Study | Community selection from birth certificates. Participation rates for cases/controls of 86% and 84%. | Sex, age, Hispanic ethnicity, and mother’s race | Sex, age, Hispanic ethnicity, mother’s race, and household income category |
| Weng et al. 2008 | Taiwan | 1995–2005 | All leukemias | 308/308 | 0–14 | levels from 66 air monitoring stations | levels divided into three categories: , 20.99–25.34, | Place of usual residence indicated in death certificate | Death certificates using ICD codes | Death certificates | Sex, birth year, and death year | Socioeconomic status |
Note: Metric conversion factors: 1 foot = 0.3048 meter; 1 mile = 1,609.34 meters. ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; ANLL, acute non-lymphoblastic leukemia; CALINE, California line source dispersion model; CO, carbon monoxide; LUR, land-use regression; NATA, National-Scale Air to Toxics Assessment; NO, nitric oxide; , nitrogen dioxide, , nitrogen oxides; , ozone; PAH, polycyclic aromatic hydrocarbons; PM, particulate matter; POM, polycyclic organic matter.
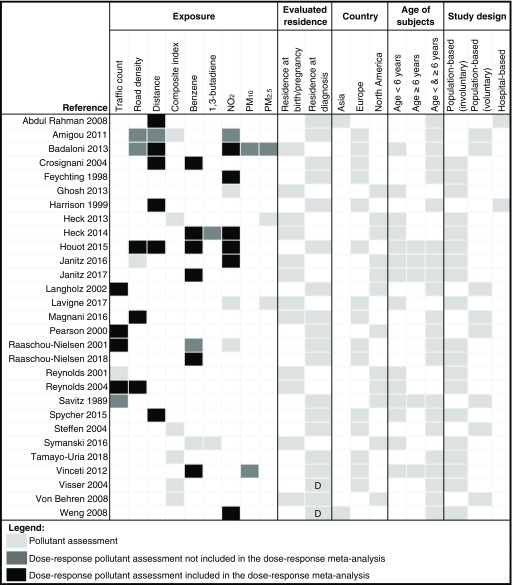
The various methods used in these studies for the assessment of exposure to traffic exhaust contaminants are summarized in Figure 2. Traffic density was assessed in 20 studies: as traffic count (), road density (), residential distance from a major road (), or a combination of them (). Another 16 studies utilized measured or modeled levels of traffic-related air contaminants, mainly benzene (), 1,3-butadiene (), (), and particulate matter (PM) (). Other pollutants considered in these studies were ozone, other nitrogen oxides, carbon monoxide, and polycyclic organic matter. These pollutants were generally included in a multivariate model with other pollutants. Sixteen studies utilized only one method of exposure assessment, whereas the remaining 13 studies used two or more methods. NOS scale scores of included studies are reported in Table S3. The median value of the total score was 9 for both case–control and cohort studies, the highest level of this scale, indicating a generally very good quality and a substantially low risk of bias. Most studies had a population-based design, with 2 being hospital-based. Among the 27 population-based studies overall, 19 (including all those assessing benzene exposure) did not depend on voluntary participation of children and their families. These studies performed an exposure assessment, without contacting the participants, based on modeling applied to location information for the residence of study participants.
Figure 2.
Characteristics of included studies according to traffic pollution assessment, and other characteristics. Composite index, combination of various measures of traffic density; D, residential address retrieved from death certificate; distance, distance between the residence and a major road; , nitrogen dioxide; PM, particulate matter; road density, sum of the length of roads within a defined area around the residence; traffic count, estimated number of vehicles per day in the roads within a defined distance from the residence. For population-based studies, we indicated whether exposure assessment depended upon acceptance to participate in the study (voluntary) or it was based solely on residential address, without contacting the participants (involuntary).
To assess exposure, most studies used either the residential address at the time of diagnosis or the address retrieved from the death certificate. Some studies used residential address during pregnancy or at birth; only two studies evaluated exposures both at and after birth (Figure 2).
Table 1 provides details of characteristics of each eligible study, including confounding factors they considered. All but two studies accounted for sex and age, generally as matching variables, and all but four included a measure of socioeconomic status in the regression model. The databases with extracted data used for both highest versus lowest (see Excel Table S1) and dose–response meta-analysis (see Excel Table S2) are included and described in the Supplemental Excel File.
Highest versus Lowest Meta-Analysis
Tables 2 and 3 present the summary RR and 95% CIs comparing the highest with lowest exposure categories for each exposure assessment method and the corresponding forest plots are shown in Figures S1–S15. As shown in Table 2, the summary RR comparing the highest to lowest exposure categories from studies using traffic density to assess air pollution exposure and leukemia risk was slightly elevated, being 1.09 (95% CI: 1.00, 1.20). Of the eight studies that investigated benzene exposure, two involved the same population. For the remaining seven, the summary 1.27 (95% CI: 1.03, 1.56). Of the eligible studies assessing exposure, two were updated versions of previous reports. The eight independent studies of exposure yielded a summary 1.04 (95% CI: 0.90, 1.19). We observed a moderate association for and childhood leukemia [two studies, summary 1.20 (95% CI: 0.70, 2.04)], but the association was much weaker for [three studies, summary 1.05 (95% CI: 0.94, 1.16)]. 1,3-butadiene was associated with increased disease risk, based on two studies [summary 1.45 (95% CI: 1.08, 1.95)] (Table 3).
Table 2.
Summary risk ratios (RRs) of childhood leukemia in the highest exposure category versus the lowest one for traffic density, benzene, and nitrogen dioxide () exposure, for all studies and stratified by age at diagnosis, leukemia subtype, exposure timing, and region.
| All children | Preschool children () | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Indicator | RR | 95% CI | (%) | RR | 95% CI | (%) | RR | 95% CI | (%) | ||||||
| Traffic density | |||||||||||||||
| All leukemia | 16 | 1.09 | 1.00, 1.20 | 56.2 | 0.012 | 7 | 1.00 | 0.93, 1.09 | 67.0 | 0.006 | 3 | 1.05 | 0.96, 1.15 | 10.0 | 0.002 |
| Subtype | |||||||||||||||
| ALL | 9 | 1.05 | 0.96, 1.16 | 34.7 | 0.005 | 3 | 1.02 | 0.99, 1.05 | 0.0 | 0.000 | 1 | 1.00 | 0.92, 1.09 | — | 0.000 |
| AML | 5 | 1.09 | 0.86, 1.38 | 70.0 | 0.034 | 2 | 1.03 | 0.77, 1.38 | 87.7 | 0.039 | 1 | 1.25 | 1.02, 1.53 | — | 0.000 |
| Exposure timing | |||||||||||||||
| At birth | 5 | 0.98 | 0.90, 1.06 | 30.9 | 0.003 | 4 | 0.95 | 0.85, 1.05 | 52.4 | 0.006 | 1 | 1.15 | 0.78, 1.70 | — | 0.000 |
| At diagnosis | 14 | 1.32 | 1.12, 1.55 | 50.7 | 0.033 | 3 | 1.27 | 0.95, 1.71 | 81.0 | 0.049 | 2 | 1.05 | 0.94, 1.17 | 24.6 | 0.004 |
| Region | |||||||||||||||
| Asia | 1 | 1.27 | 0.51, 3.17 | — | 0.000 | — | — | ||||||||
| Europe | 9 | 1.25 | 1.05, 1.49 | 50.4 | 0.026 | 3 | 1.05 | 0.87, 1.25 | 63.8 | 0.017 | 1 | 1.05 | 0.95, 1.17 | — | 0.000 |
| North America | 6 | 1.02 | 0.89, 1.16 | 65.4 | 0.014 | 4 | 0.98 | 0.84, 1.15 | 74.9 | 0.017 | 2 | 1.09 | 0.72, 1.64 | 17.4 | 0.032 |
| Benzene | |||||||||||||||
| All leukemia | 7 | 1.27 | 1.03, 1.56 | 52.4 | 0.043 | 4 | 1.39 | 1.03, 1.87 | 27.9 | 0.035 | 2 | 1.08 | 0.64, 1.82 | 0.0 | 0.000 |
| Subtype | |||||||||||||||
| ALL | 7 | 1.09 | 0.88, 1.36 | 51.8 | 0.034 | 3 | 1.19 | 1.00, 1.40 | 0.0 | 0.000 | 1 | 0.69 | 0.27, 1.78 | — | 0.000 |
| AML | 5 | 1.84 | 1.31, 2.59 | 0.0 | 0.000 | 2 | 3.21 | 1.39, 7.42 | 0.0 | 0.000 | 1 | 0.43 | 0.04, 4.79 | — | 0.000 |
| Exposure timing | |||||||||||||||
| At birth | 3 | 1.21 | 1.04, 1.41 | 0.0 | 0.000 | 3 | 1.22 | 1.03, 1.43 | 0.0 | 0.000 | 1 | 1.14 | 0.63, 2.08 | — | 0.000 |
| At diagnosis | 4 | 1.36 | 0.92, 2.00 | 65.2 | 0.125 | 1 | 3.30 | 1.03, 10.59 | — | 0.000 | 1 | 0.90 | 0.31, 2.60 | — | 0.000 |
| Region | |||||||||||||||
| Asia | — | — | — | ||||||||||||
| Europe | 4 | 1.36 | 0.92, 2.00 | 65.2 | 0.125 | 1 | 3.30 | 1.03, 10.59 | — | 0.000 | 1 | 0.90 | 0.31, 2.60 | — | 0.000 |
| North America | 3 | 1.21 | 1.04, 1.41 | 0.0 | 0.000 | 3 | 1.22 | 1.03, 1.43 | 0.0 | 0.000 | 1 | 1.14 | 0.63, 2.08 | — | 0.000 |
| All leukemia | 8 | 1.04 | 0.90, 1.19 | 55.5 | 0.023 | 4 | 1.03 | 0.90, 1.18 | 14.9 | 0.004 | 1 | 0.89 | 0.42, 1.89 | — | 0.000 |
| Subtype | |||||||||||||||
| ALL | 4 | 1.02 | 0.89, 1.18 | 55.6 | 0.011 | 2 | 1.10 | 0.92, 1.32 | 46.2 | 0.008 | — | ||||
| AML | 4 | 0.97 | 0.79, 1.19 | 0.0 | 0.000 | 2 | 0.86 | 0.60, 1.23 | 0.0 | 0.000 | — | ||||
| Exposure timing | |||||||||||||||
| At birth | 4 | 1.07 | 0.96, 1.19 | 0.0 | 0.000 | 4 | 1.03 | 0.90, 1.18 | 14.9 | 0.004 | 1 | 0.89 | 0.42, 1.89 | — | 0.000 |
| At diagnosis | 4 | 1.17 | 0.82, 1.67 | 74.9 | 0.093 | — | — | ||||||||
| Region | |||||||||||||||
| Asia | 1 | 2.29 | 1.44, 3.64 | — | 0.000 | — | — | ||||||||
| Europe | 4 | 0.91 | 0.82, 1.00 | 0.0 | 0.000 | 1 | 0.79 | 0.52, 1.20 | — | 0.000 | — | ||||
| North America | 3 | 1.06 | 0.95, 1.18 | 0.0 | 0.000 | 3 | 1.06 | 0.94, 1.19 | 1.7 | 0.000 | 1 | 0.89 | 0.42, 1.89 | — | 0.000 |
Note: —, data not available; ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; CI, confidence interval; , I-squared statistic; RR, risk ratio; , tau-squared statistic.
Table 3.
Summary risk ratios (RRs) for association of childhood leukemia with particulate matter () and 1,3-butadiene comparing the highest versus the lowest exposure categories for all studies and stratified by age at diagnosis, leukemia subtype, exposure timing, and region.
| All children | Preschool children () | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Indicator | RR | 95% CI | (%) | RR | 95% CI | (%) | RR | 95% CI | (%) | ||||||
| All leukemia | 3 | 1.05 | 0.94, 1.16 | 0.0 | 0.000 | 3 | 1.04 | 0.94, 1.16 | 0.0 | 0.000 | — | ||||
| Subtype | |||||||||||||||
| ALL | 2 | 1.11 | 0.95, 1.31 | 0.0 | 0.000 | 2 | 1.11 | 0.95, 1.31 | 0.0 | 0.000 | — | ||||
| AML | 2 | 1.00 | 0.87, 1.13 | 0.0 | 0.000 | 2 | 1.00 | 0.87, 1.13 | 0.0 | 0.000 | — | ||||
| Exposure timing | |||||||||||||||
| At birth | 3 | 1.05 | 0.94, 1.16 | 0.0 | 0.000 | 3 | 1.04 | 0.94, 1.16 | 0.0 | 0.000 | — | ||||
| At diagnosis | — | — | — | ||||||||||||
| Region | |||||||||||||||
| Europe | 1 | 1.00 | 0.72, 1.39 | — | 0.000 | 1 | 0.94 | 0.62, 1.43 | — | 0.000 | — | ||||
| North America | 2 | 1.05 | 0.94, 1.17 | 0.0 | 0.000 | 2 | 1.05 | 0.94, 1.17 | 0.0 | 0.000 | — | ||||
| All leukemia | 2 | 1.20 | 0.70, 2.04 | 43.5 | 0.075 | 2 | 1.09 | 0.66, 1.80 | 12.3 | 0.028 | 1 | 1.50 | 0.48, 4.70 | — | 0.000 |
| Subtype | |||||||||||||||
| ALL | 1 | 1.45 | 0.73, 2.87 | — | 0.000 | 1 | 1.50 | 0.52, 4.33 | — | 0.000 | 1 | 1.39 | 0.54, 3.57 | — | 0.000 |
| AML | 1 | 1.30 | 0.41, 4.14 | — | 0.000 | 1 | 1.21 | 0.18, 8.16 | — | 0.000 | 1 | 1.18 | 0.25, 5.56 | — | 0.000 |
| Exposure timing | |||||||||||||||
| At birth | 1 | 1.00 | 0.70, 1.42 | — | 0.000 | 1 | 0.97 | 0.62, 1.51 | — | 0.000 | — | ||||
| At diagnosis | 1 | 1.80 | 0.82, 3.97 | — | 0.000 | 1 | 1.90 | 0.60, 6.01 | — | 0.000 | 1 | 1.50 | 0.48, 4.70 | — | 0.000 |
| Region | |||||||||||||||
| Europe | 2 | 1.20 | 0.70, 2.04 | 43.5 | 0.075 | 2 | 1.09 | 0.66, 1.80 | 12.3 | 0.028 | 1 | 1.50 | 0.48, 4.70 | — | 0.000 |
| North America | — | — | — | ||||||||||||
| 1,3—Butadiene | |||||||||||||||
| All leukemia | 2 | 1.45 | 1.08, 1.95 | 28.0 | 0.025 | 2 | 1.45 | 1.08, 1.95 | 28.0 | 0.025 | — | ||||
| Subtype | |||||||||||||||
| ALL | 2 | 1.31 | 1.11, 1.54 | 0.0 | 0.000 | 2 | 1.31 | 1.11, 1.54 | 0.0 | 0.000 | — | ||||
| AML | 1 | 2.35 | 1.02, 5.40 | — | 0.000 | 1 | 2.35 | 1.02, 5.40 | — | 0.000 | — | ||||
| Exposure timing | |||||||||||||||
| At birth | 2 | 1.45 | 1.08, 1.95 | 28.0 | 0.025 | 2 | 1.45 | 1.08, 1.95 | 28.0 | 0.025 | |||||
| At diagnosis | — | — | |||||||||||||
| Region | |||||||||||||||
| Europe | — | — | — | ||||||||||||
| North America | 2 | 1.45 | 1.08, 1.95 | 28.0 | 0.025 | 2 | 1.45 | 1.08, 1.95 | 28.0 | 0.025 | — | ||||
Note: —, data not available; ALL, acute lymphoblastic leukemia; AML, acute myeloid leukemia; CI, confidence interval; , I-squared statistic; RR, risk ratio; , tau-squared statistic.
In analyses stratified by disease subtype (Tables 2 and 3; see also Figures S1–S6), RR estimates were found to differ markedly only for benzene exposure, for which the summary RR became 1.09 (95% CI: 0.88, 1.36) for ALL, based on seven studies, and 1.84 (95% CI: 1.31, 2.59) for AML, based on five studies. No such change in RR according to leukemia subtype was found for traffic indicators, for or for PM. 1,3-Butadiene exposure was associated with a higher RR for AML compared with ALL, but the summary estimates were statistically unstable, being based on two studies only. For the aforementioned pollutants, RRs according to disease subtype were roughly the same after restricting the analysis to children y of age, with the exception of the summary RRs associated with benzene exposure, showing a larger difference between AML and ALL in this younger subgroup (Tables 2 and 3; see also Figures S7–S11). Data for older children, however, were based on one study only, and the RR was imprecise (Tables 2 and 3; see also Figures S12–S15). In general, for other air pollution exposure metrics, results were somewhat stronger among younger children, but there were few studies with data on children , and the latter results were statistically unstable.
In analyses stratified by study region, the North American studies showed a summary 1.02 (95% CI: 0.89, 1.16) and 1.21 (95% CI: 1.04, 1.41) for traffic density and benzene exposure, respectively (Tables 2 and 3; see also Figures S1–S6). For European studies, the corresponding estimates were higher, that is, 1.25 (95% CI: 1.05, 1.49) for traffic indicators and 1.36 (95% CI: 0.92, 2.00) for benzene exposure. When exposure timing in relation to leukemia occurrence was taken into account, studies assessing exposure on the basis of either child’s residence at diagnosis or the longest place of residence yielded a higher summary RR for traffic density of 1.32 (95% CI: 1.12, 1.55) and for benzene 1.36 (95% CI: 0.92, 2.00). When exposure assessment was based on maternal residence at birth or during pregnancy, the 0.98 (95% CI: 0.90, 1.06) for traffic indicators and 1.21 (95% CI: 1.04, 1.41) for benzene.
Dose–Response Meta-Analysis
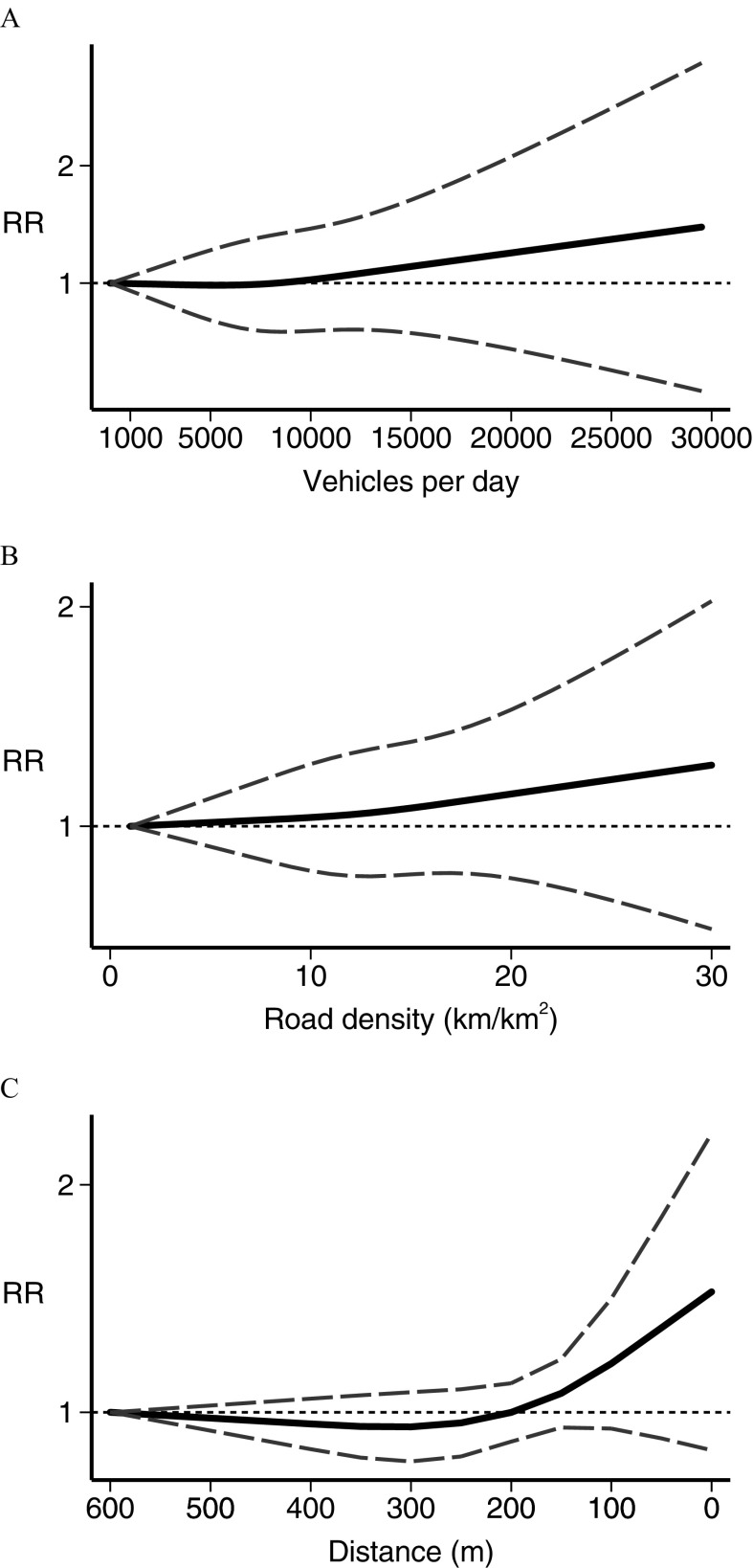
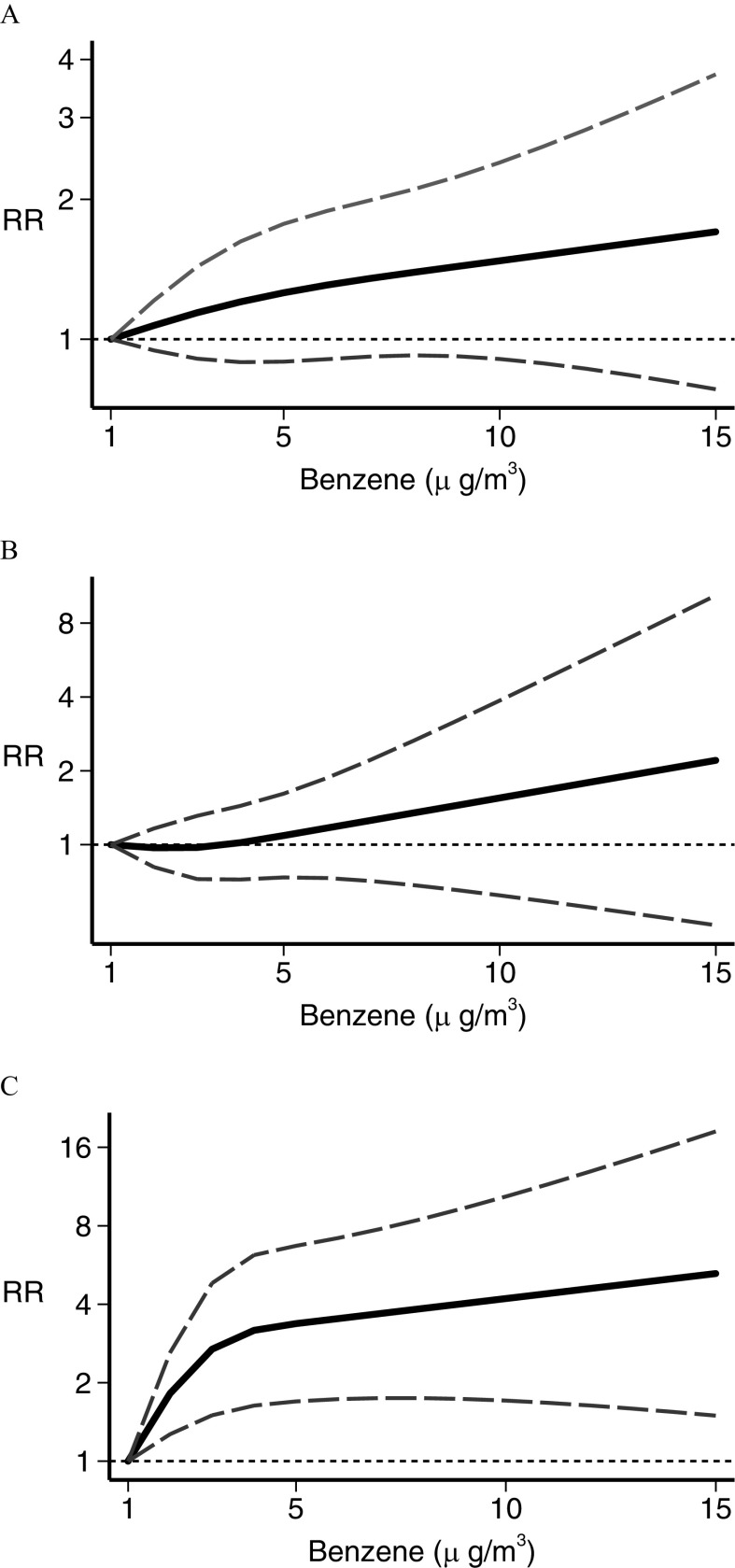
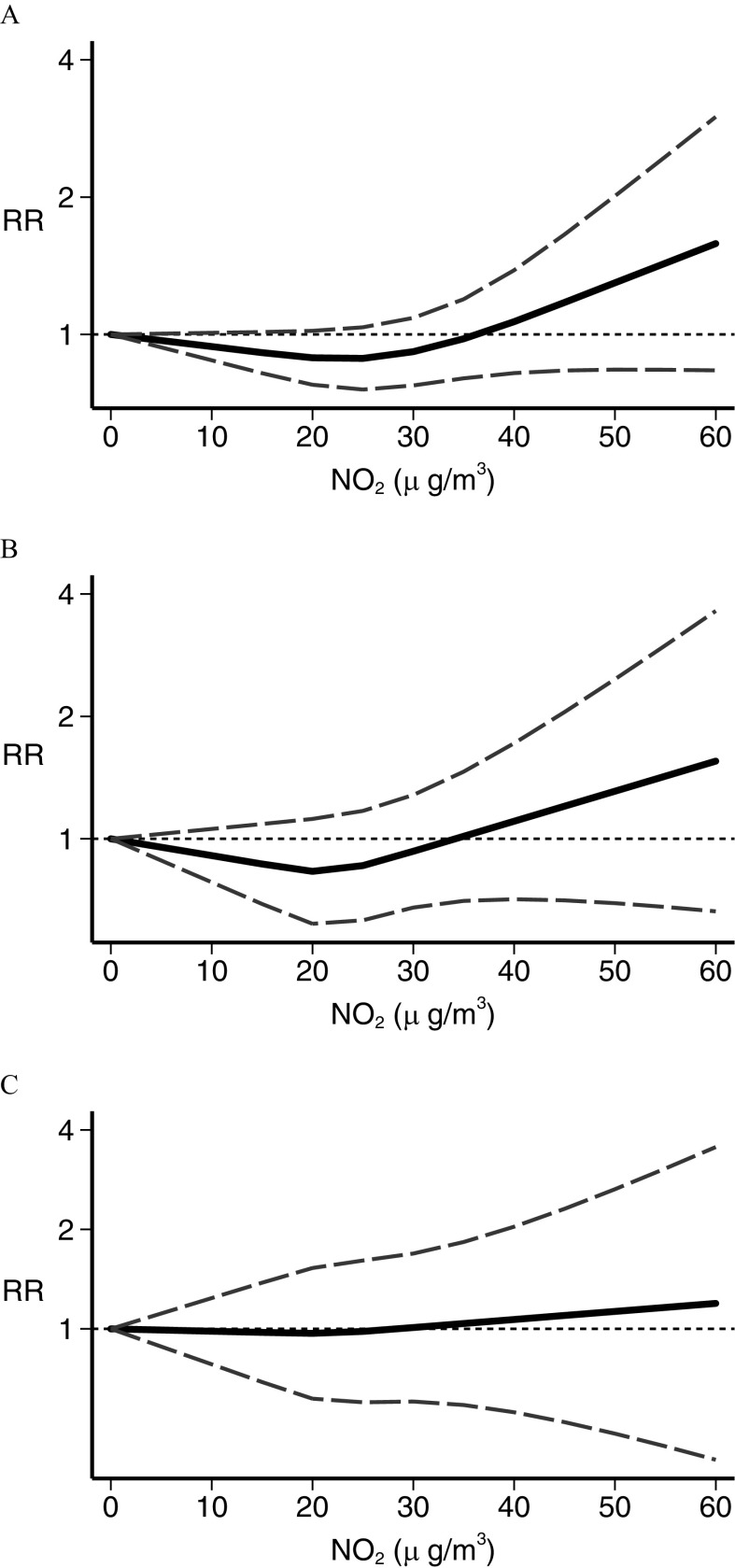
In the dose–response meta-analysis, five studies (Abdul Rahman et al. 2008; Harrison et al. 1999; Houot et al. 2015; Janitz et al. 2016; Langholz et al. 2002) had no value assigned to open-ended categories at the end of the exposure scale. For these we entered a value 20% higher or lower than the closest cut points. In the analysis of traffic density indicators, the restricted cubic spline analysis (Figure 3) showed little association between leukemia RR and the number of vehicles per day (Langholz et al. 2002; Pearson et al. 2000; Raaschou-Nielsen et al. 2001; Reynolds et al. 2004) in the street closest to the child’s residence, except at the highest exposure levels where a small and statistically imprecise excess risk emerged (Figure 3A). Three studies used a similar measure of road density around the child’s residence (Houot et al. 2015; Magnani et al. 2016; Reynolds et al. 2004), yielding results comparable with those obtained for the vehicles per day metric (Figure 3B). Distance from a major nearby road (Abdul Rahman et al. 2008; Badaloni et al. 2013; Crosignani et al. 2004; Harrison et al. 1999; Houot et al. 2015; Spycher et al. 2015) showed little association with RR until from the road’s edge, below which an indication of a higher RR emerged and increased steeply with decreasing distance (Figure 3C). For benzene (Crosignani et al. 2004; Heck et al. 2014; Houot et al. 2015; Janitz et al. 2017; Raaschou-Nielsen et al. 2018; Vinceti et al. 2012), there was an approximately linear increase in estimated risk starting from the lowest levels of benzene exposure (), as shown in Figure 4. After stratifying by leukemia subtype, we found a considerably stronger association between benzene exposure and risk of AML (Heck et al. 2014; Houot et al. 2015; Janitz et al. 2017; Raaschou-Nielsen et al. 2018; Vinceti et al. 2012) than we found for ALL (Crosignani et al. 2004; Heck et al. 2014; Houot et al. 2015; Janitz et al. 2017; Raaschou-Nielsen et al. 2018; Vinceti et al. 2012). Concerning (Badaloni et al. 2013; Feychting et al. 1998; Heck et al. 2014; Houot et al. 2015; Janitz et al. 2016; Weng et al. 2008), the dose–response meta-analysis showed some evidence of an excess risk starting from to the maximum amount investigated, , although the increase was statistically unstable (Figure 5). Subgroup analysis according to disease type showed that this excess risk at the higher exposure levels was limited to ALL (Figure 5). The dose–response analysis could not be carried out for 1,3-butadiene or for PM, due to the limited number of studies reporting the information needed for such an analysis.
Figure 3.
Dose–response meta-analysis of childhood leukemia risk from traffic indicators using (A) vehicles per day count (Langholz et al. 2002; Pearson et al. 2000; Raaschou-Nielsen et al. 2001, Reynolds et al. 2004), (B) road density in (Houot et al. 2015; Magnani et al. 2016; Reynolds et al. 2004), and (C) residential distance from a major road in meters (Abdul Rahman et al. 2008; Badaloni et al. 2013; Crosignani et al. 2004; Harrison et al. 1999; Houot et al. 2015; Spycher et al. 2015). Spline curve (black solid line) with 95% confidence limits (gray dashed lines). RR, risk ratio.
Figure 4.
Dose–response meta-analysis of childhood leukemia risk from benzene exposure for (A) all leukemias (Crosignani et al. 2004; Heck et al. 2014; Houot et al. 2015; Janitz et al. 2017; Raaschou-Nielsen et al. 2018; Vinceti et al. 2012), (B) acute lymphoblastic leukemia only (Crosignani et al. 2004; Heck et al. 2014; Houot et al. 2015; Janitz et al. 2017; Raaschou-Nielsen et al. 2018; Vinceti et al. 2012), and (C) acute myeloid leukemia only (Heck et al. 2014; Houot et al. 2015; Janitz et al. 2017; Raaschou-Nielsen et al. 2018; Vinceti et al. 2012). Spline curve (black solid line) with 95% confidence limits (gray dashed lines). RR, risk ratio.
Figure 5.
Dose–response meta-analysis of childhood leukemia risk from exposure for (A) all leukemias (Badaloni et al. 2013; Feychting et al. 1998; Heck et al. 2014; Houot et al. 2015; Janitz et al. 2016; Weng et al. 2008), (B) acute lymphoblastic leukemia only (Heck et al. 2014; Houot et al. 2015; Janitz et al. 2016), and (C) acute myeloid leukemia only (Heck et al. 2014; Houot et al. 2015; Janitz et al. 2016). Spline curve (black solid line) with 95% confidence limits (gray dashed lines). RR, risk ratio.
Sensitivity Analyses and Publication Bias
We repeated all analyses after systematically excluding each study in turn from the meta-analysis (see Table S4–S5 and Figures S16–S18). None of these analyses appreciably changed the various summary estimates. Use of alternative estimates for the highest and lowest exposure categories with unknown mean/median values, that is, entering a value instead of with relation to the closest (lower and upper) available boundary, also had little effect on the results (see Figures S19–S21). Funnel plots based on the different exposure assessment methods showed a slightly asymmetric distribution, especially for traffic and benzene, and therefore we could not entirely rule out the occurrence of publication bias (Figure S22). Finally, we report study-specific dose–response trends in addition to the overall dose–response meta-analyses in Figures S23–S25.
Discussion
The reviewed literature of epidemiologic studies and the combined dose–response meta-analysis appear to support a relation of air pollution, and particularly benzene emissions from motorized traffic, with the risk of childhood leukemia (Table 2 and Figure 4); the strongest associations were found with AML and there was little evidence of any threshold of exposure. For exposure to , little evidence for an association was found except at the highest levels of exposure (Table 3 and Figure 5). Based on a limited number of studies, there was some association with 1,3-butadiene (Table 3) and a weak association with (Table 3), although the dose–response curve could not be assessed for these contaminants.
Air pollution has not been unanimously considered to be associated with childhood leukemia (Sun et al. 2014) due to some inconsistencies across epidemiologic studies (Schüz and Erdmann 2016), although most of the recent reviews tend to support such a relation (Boothe et al. 2014; Carlos-Wallace et al. 2016; Filippini et al. 2015; IARC 2016, 2018; Spycher et al. 2017; Steinmaus and Smith 2017). In some instances, the reevaluation of previous databases by conducting stratified analyses for leukemia subtypes has identified associations between benzene exposure from motorized traffic and disease risk, particularly AML, that had previously gone undetected (Raaschou-Nielsen et al. 2001, 2018). In the present review, we took advantage of the availability of nine recently published studies, a reanalysis of a former study (Heck et al. 2014), and a new method for dose–response spline regression analysis (Crippa et al. 2018a) to assess for the first time, to the best of our knowledge, the shape of the relation of exposure to benzene, , and traffic density with childhood leukemia risk. We also attempted to identify thresholds of exposure, timing of exposure, and the roles of some potential effect modifiers.
A role of outdoor air pollution in childhood leukemia etiology is supported by several laboratory studies, which have provided biological plausibility for this association (Andreoli et al. 2012; Arayasiri et al. 2010; Bollati et al. 2007; Buthbumrung et al. 2008; Carlos-Wallace et al. 2016; D’Andrea and Reddy 2018; Elliott et al. 2017; Jiang et al. 2016; Loomis et al. 2013; McKenzie et al. 2017; Suk et al. 2016; Vattanasit et al. 2014). A review published by the IARC in 2016 defined air pollution as carcinogenic due to its ability to induce lung cancer in humans. This review reported evidence from animal and mechanistic studies supporting the carcinogenicity of air pollution for lung cancer and identified the several inorganic and organic components of air pollution classified as established, probable, or possible carcinogens (IARC 2016). However, with reference to the above evidence, it should be noted that many of the laboratory studies were carried out using mixtures, precluding identification of the specific pollutants responsible for the increased risk. Evidence from animal carcinogenicity data and mechanistic data has also recently been made available, specifically for benzene (IARC 2018), showing the capacity of this compound to generate reactive electrophilic metabolites, induce oxidative stress, and DNA damage as well as immunotoxic and hematotoxic effects.
Although exposure to benzene, an established leukemogen in adults for the acute myeloid form (IARC 2018), was associated with disease risk in our analysis, exposure assessed through traffic indicators such as road density or distance showed limited association with childhood leukemia (Figure 3). This discrepancy may indicate that traffic-related metrics are less sensitive as a measure of outdoor air pollutants than modeled levels of carcinogenic pollutants such as benzene and 1,3-butadiene, as has been reported elsewhere (Wu et al. 2011). This would explain the smaller estimates of effect seen when using surrogate measures of exposure (Table 2). The higher risk observed for families living within of a major road is of considerable interest, however, because it suggests a distance potentially usable by policymakers considering placement of schools or other facilities for children. This corridor is characterized by high levels of carcinogenic air pollutants, including heavy metals and benzene, with a sudden drop in their concentration outside that range (Karner et al. 2010).
A few of the studies we reviewed have suggested that other air pollutants, such as 1,3-butadiene and selenium (Heck et al. 2014; Knox 2006; Symanski et al. 2016; Vinceti et al. 2012), may be associated with childhood leukemia risk. However, few studies have controlled for other pollutants simultaneously (Heck et al. 2014; Symanski et al. 2016). Therefore, we acknowledge that an etiologic relation between air pollution and childhood leukemia risk, which is supported by our findings and appears to be mainly attributable to benzene, might be at least partly due to other pollutants in outdoor air that covary with benzene emissions (Ghosh et al. 2012; Heck et al. 2014; Vinceti et al. 2012; Wilhelm et al. 2011), acting either alone or as mixtures. In the two published studies on 1,3-butadiene, correlations with benzene were high (Heck et al. 2014; Symanski et al. 2016). Exposure assessment methods based on denser air pollution monitoring networks or more sophisticated air pollution models may be better able to differentiate between the effects of benzene and 1,3-butadiene and, more generally, to tease apart the influence of each toxic agent.
Our meta-analysis suggested a differential effect of traffic-related benzene exposure according to the clinical subtype of leukemia, that is, a much higher risk associated with AML compared with ALL (Table 2 and Figure 3). Conversely, little evidence of such a differential relation with disease type emerged when assessing exposure through traffic indicators and PM levels. For , a pollutant so far not recognized as a carcinogen, the association appeared to be limited to ALL and to the highest exposure levels only (Table 3 and Figure 5).
We observed higher RRs associated with benzene exposure in the postnatal period compared with the perinatal window of exposure (Table 2). We found higher estimates for benzene exposure in younger () compared with older children, although for the latter, little data were available. There was also an indication of an interaction between exposure window and age given that the excess disease risk associated with benzene exposure at diagnosis compared with exposure at birth was limited to the youngest children, although based on only one study. A previous meta-analysis investigating the association between traffic exposure and childhood leukemia risk found similar results, that is, a higher summary RR for exposure at diagnosis than for exposure at birth or gestation; effect–measure modification by age was not investigated in that study (Carlos-Wallace et al. 2016).
We also detected slightly stronger RRs for benzene exposure in Europe compared with North America (Table 2). This difference might result from misclassification of exposure stemming from greater residential mobility in North America (Lupo et al. 2010; Tee Lewis et al. 2019; Urayama et al. 2009) or by the larger influence of postnatal exposure assessment in European studies. We also note that there is a much larger percentage of diesel cars in Europe than in North America (Neumaier 2014), possibly reflecting differences in pollutant exposure characterizing the two regions. Another potential source of discrepancy might be the use in some of the U.S. studies of methods based on a “nearest-neighbor” exposure assessment that has been carried out at the census tracts level or using a limited number of air monitors (Heck et al. 2014; Symanski et al. 2016). These methods likely yield a lower spatial resolution compared with person-level exposure data based on air pollution dispersion modeling at the residential address, thus adding to exposure misclassification and likely diluting the RRs.
Some degree of unmeasured confounding may have occurred in the studies, due to sources of outdoor air pollution such as oil and gas development (Elliott et al. 2017; McKenzie et al. 2017) and industrial sources (García-Pérez et al. 2015; Park et al. 2017), indoor air pollution from heating sources and dust (Whitehead et al. 2011, 2015), passive smoking (Metayer et al. 2016b; Pyatt and Hays 2010), magnetic field exposure (Amoon et al. 2018), and socioeconomic factors (Kehm et al. 2018b). However, most of these factors are unlikely to play a major role in disease etiology, and some studies we included in the meta-analysis, particularly the most recent ones, took into account several potential confounding factors, yielding results in line with the overall meta-analysis (Table 1 shows the confounding factors considered in each study). In addition, we systematically used the most adjusted RRs to carry out our meta-analysis. The occurrence of selection bias could also be ruled out particularly for the most recent studies, including all those assessing benzene exposure in relation to disease risk given that they were population-based and not dependent on voluntary participation.
In conclusion, in this systematic review and dose–response meta-analysis, we found that traffic-related air pollution, particularly exposure to benzene, was associated with excess risk of childhood leukemia. No apparent minimal threshold of exposure emerged for benzene, whereas analyses for traffic density and gave evidence of such a threshold. Disease subtype, windows of exposure and child’s age appeared to modify these associations.
Supplementary Material
Acknowledgments
This Associazione Sostegno Ematologia Oncologia Pediatrica (ASEOP) of Modena, Italy (grants RIMB 2/2017 and RIMB 3/2018 to M.V.) and by the National Institutes of Health (grant R21ES018960 to J.E.H.).
References
- Abdul Rahman HI, Shah SA, Alias H, Ibrahim HM. 2008. A case-control study on the association between environmental factors and the occurrence of acute leukemia among children in Klang Valley, Malaysia. Asian Pac J Cancer Prev 9(4):649–652, PMID: 19256754. [PubMed] [Google Scholar]
- Amigou A, Sermage-Faure C, Orsi L, Leverger G, Baruchel A, Bertrand Y, et al. 2011. Road traffic and childhood leukemia: the ESCALE study (SFCE). Environ Health Perspect 119(4):566–572, PMID: 21147599, 10.1289/ehp.1002429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amoon AT, Crespi CM, Ahlbom A, Bhatnagar M, Bray I, Bunch KJ, et al. 2018. Proximity to overhead power lines and childhood leukaemia: an international pooled analysis. Br J Cancer 119(3):364–373, PMID: 29808013, 10.1038/s41416-018-0097-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreoli R, Protano C, Manini P, De Palma G, Goldoni M, Petyx M, et al. 2012. Association between environmental exposure to benzene and oxidative damage to nucleic acids in children. Med Lav 103(5):324–337, PMID: 23077793. [PubMed] [Google Scholar]
- Arayasiri M, Mahidol C, Navasumrit P, Autrup H, Ruchirawat M. 2010. Biomonitoring of benzene and 1,3-butadiene exposure and early biological effects in traffic policemen. Sci Total Environ 408(20):4855–4862, PMID: 20627202, 10.1016/j.scitotenv.2010.06.033. [DOI] [PubMed] [Google Scholar]
- Badaloni C, Ranucci A, Cesaroni G, Zanini G, Vienneau D, Al-Aidrous F, et al. 2013. Air pollution and childhood leukaemia: a nationwide case-control study in Italy. Occup Environ Med 70(12):876–883, PMID: 24142970, 10.1136/oemed-2013-101604. [DOI] [PubMed] [Google Scholar]
- Barrington-Trimis JL, Cockburn M, Metayer C, Gauderman WJ, Wiemels J, McKean-Cowdin R. 2017. Trends in childhood leukemia incidence over two decades from 1992 to 2013. Int J Cancer 140(5):1000–1008, PMID: 27778348, 10.1002/ijc.30487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartley K, Metayer C, Selvin S, Ducore J, Buffler P. 2010. Diagnostic X-rays and risk of childhood leukaemia. Int J Epidemiol 39(6):1628–1637, PMID: 20889538, 10.1093/ije/dyq162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollati V, Baccarelli A, Hou L, Bonzini M, Fustinoni S, Cavallo D, et al. 2007. Changes in DNA methylation patterns in subjects exposed to low-dose benzene. Cancer Res 67(3):876–880, PMID: 17283117, 10.1158/0008-5472.CAN-06-2995. [DOI] [PubMed] [Google Scholar]
- Booth A. 2008. Unpacking your literature search toolbox: on search styles and tactics. Health Info Libr J 25(4):313–317, PMID: 19076679, 10.1111/j.1471-1842.2008.00825.x. [DOI] [PubMed] [Google Scholar]
- Boothe VL, Boehmer TK, Wendel AM, Yip FY. 2014. Residential traffic exposure and childhood leukemia: a systematic review and meta-analysis. Am J Prev Med 46(4):413–422, PMID: 24650845, 10.1016/j.amepre.2013.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buthbumrung N, Mahidol C, Navasumrit P, Promvijit J, Hunsonti P, Autrup H, et al. 2008. Oxidative DNA damage and influence of genetic polymorphisms among urban and rural schoolchildren exposed to benzene. Chem Biol Interact 172(3):185–194, PMID: 18282563, 10.1016/j.cbi.2008.01.005. [DOI] [PubMed] [Google Scholar]
- Carlos-Wallace FM, Zhang L, Smith MT, Rader G, Steinmaus C. 2016. Parental, in utero, and early-life exposure to benzene and the risk of childhood leukemia: a meta-analysis. Am J Epidemiol 183(1):1–14, PMID: 26589707, 10.1093/aje/kwv120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crippa A, Discacciati A, Bottai M, Spiegelman D, Orsini N. 2018a. One-stage dose-response meta-analysis for aggregated data. Stat Methods Med Res, PMID: 29742975, 10.1177/0962280218773122. [DOI] [PubMed] [Google Scholar]
- Crippa A, Larsson SC, Discacciati A, Wolk A, Orsini N. 2018b. Red and processed meat consumption and risk of bladder cancer: a dose-response meta-analysis of epidemiological studies. Eur J Nutr 57(2):689–701, PMID: 28070638, 10.1007/s00394-016-1356-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crosignani P, Tittarelli A, Borgini A, Codazzi T, Rovelli A, Porro E, et al. 2004. Childhood leukemia and road traffic: a population-based case-control study. Int J Cancer 108(4):596–599, PMID: 14696126, 10.1002/ijc.11597. [DOI] [PubMed] [Google Scholar]
- D’Andrea MA, Reddy GK. 2018. Health risks associated with benzene exposure in children: a systematic review. Glob Pediatr Health 5:2333794X18789275, PMID: 30148190, 10.1177/2333794X18789275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Donno A, De Giorgi M, Bagordo F, Grassi T, Idolo A, Serio F, et al. 2018. Health risk associated with exposure to PM10 and benzene in three Italian towns. Int J Environ Res Public Health 15(8):E1672, PMID: 30082675, 10.3390/ijerph15081672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elliott EG, Trinh P, Ma X, Leaderer BP, Ward MH, Deziel NC. 2017. Unconventional oil and gas development and risk of childhood leukemia: assessing the evidence. Sci Total Environ 576:138–147, PMID: 27783932, 10.1016/j.scitotenv.2016.10.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EUnetHTA (European network for Health Technology Assessment). 2017. Process of Information Retrieval for Systematic Reviews and Health Technology Assessments on Clinical Effectiveness. Version 1.2, December 2017. Diemen, Netherlands:EUnetHTA. [Google Scholar]
- Feychting M, Svensson D, Ahlbom A. 1998. Exposure to motor vehicle exhaust and childhood cancer. Scand J Work Environ Health 24(1):8–11, PMID: 9562395, 10.5271/sjweh.272. [DOI] [PubMed] [Google Scholar]
- Filippini T, Heck JE, Malagoli C, Del Giovane C, Vinceti M. 2015. A review and meta-analysis of outdoor air pollution and risk of childhood leukemia. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev 33(1):36–66, PMID: 25803195, 10.1080/10590501.2015.1002999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Pérez J, López-Abente G, Gómez-Barroso D, Morales-Piga A, Romaguera EP, Tamayo I, et al. 2015. Childhood leukemia and residential proximity to industrial and urban sites. Environ Res 140:542–553, PMID: 26025512, 10.1016/j.envres.2015.05.014. [DOI] [PubMed] [Google Scholar]
- Ghosh JK, Heck JE, Cockburn M, Su J, Jerrett M, Ritz B. 2013. Prenatal exposure to traffic-related air pollution and risk of early childhood cancers. Am J Epidemiol 178(8):1233–1239, PMID: 23989198, 10.1093/aje/kwt129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh JK, Wilhelm M, Su J, Goldberg D, Cockburn M, Jerrett M, et al. 2012. Assessing the influence of traffic-related air pollution on risk of term low birth weight on the basis of land-use-based regression models and measures of air toxics. Am J Epidemiol 175(12):1262–1274, PMID: 22586068, 10.1093/aje/kwr469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenland S, Longnecker MP. 1992. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol 135(11):1301–1309, PMID: 1626547, 10.1093/oxfordjournals.aje.a116237. [DOI] [PubMed] [Google Scholar]
- Harrison RM, Leung PL, Somervaille L, Smith R, Gilman E. 1999. Analysis of incidence of childhood cancer in the West Midlands of the United Kingdom in relation to proximity to main roads and petrol stations. Occup Environ Med 56(11):774–780, PMID: 10658564, 10.1136/oem.56.11.774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck JE, Contreras ZA, Park AS, Davidson TB, Cockburn M, Ritz B. 2016. Smoking in pregnancy and risk of cancer among young children: a population-based study. Int J Cancer 139(3):613–616, PMID: 27016137, 10.1002/ijc.30111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck JE, Park AS, Qiu J, Cockburn M, Ritz B. 2014. Risk of leukemia in relation to exposure to ambient air toxics in pregnancy and early childhood. Int J Hyg Environ Health 217(6):662–668, PMID: 24472648, 10.1016/j.ijheh.2013.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heck JE, Wu J, Lombardi C, Qiu J, Meyers TJ, Wilhelm M, et al. 2013. Childhood cancer and traffic-related air pollution exposure in pregnancy and early life. Environ Health Perspect 121(11–12):1385–1391, PMID: 24021746, 10.1289/ehp.1306761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henshaw DL, Suk WA. 2015. Diet, transplacental carcinogenesis, and risk to children. BMJ 351:h4636, PMID: 26318965, 10.1136/bmj.h4636. [DOI] [PubMed] [Google Scholar]
- Houot J, Marquant F, Goujon S, Faure L, Honoré C, Roth MH, et al. 2015. Residential proximity to heavy-traffic roads, benzene exposure, and childhood leukemia—the GEOCAP Study, 2002–2007. Am J Epidemiol 182(8):685–693, PMID: 26377958, 10.1093/aje/kwv111. [DOI] [PubMed] [Google Scholar]
- IARC (International Agency for Research on Cancer). 2016. Outdoor air pollution. Vol 109, IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Lyon, France:IARC; https://monographs.iarc.fr/wp-content/uploads/2018/06/mono109.pdf [accessed 2 April 2019]. [Google Scholar]
- IARC. 2018. Benzene. Vol 120, IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. Lyon, France:IARC; http://publications.iarc.fr/_publications/media/download/5074/69ba844af3f3018bc06e63dee8a52c2e4494a771.pdf [accessed 2 April 2019]. [Google Scholar]
- Isaevska E, Manasievska M, Alessi D, Mosso ML, Magnani C, Sacerdote C, et al. 2017. Cancer incidence rates and trends among children and adolescents in Piedmont, 1967–2011. PLoS One 12(7):e0181805, PMID: 28742150, 10.1371/journal.pone.0181805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson D, White IR, Thompson SG. 2010. Extending DerSimonian and Laird’s methodology to perform multivariate random effects meta-analyses. Stat Med 29(12):1282–1297, PMID: 19408255, 10.1002/sim.3602. [DOI] [PubMed] [Google Scholar]
- Janitz AE, Campbell JE, Magzamen S, Pate A, Stoner JA, Peck JD. 2016. Traffic-related air pollution and childhood acute leukemia in Oklahoma. Environ Res 148:102–111, PMID: 27038831, 10.1016/j.envres.2016.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janitz AE, Campbell JE, Magzamen S, Pate A, Stoner JA, Peck JD. 2017. Benzene and childhood acute leukemia in Oklahoma. Environ Res 158:167–173, PMID: 28645022, 10.1016/j.envres.2017.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janitz AE, Campbell JE, Magzamen S, Pate A, Stoner JA, Peck JD. 2018. Corrigendum to ‘Benzene and childhood acute leukemia in Oklahoma.’ Environ Res 165:505–506, PMID: 29525036, 10.1016/j.envres.2018.02.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang WC, Wu SY, Ke YB. 2016. Association of exposure to environmental chemicals with risk of childhood acute lymphocytic leukemia [in Chinese]. Zhonghua Yu Fang Yi Xue Za Zhi 50(10):893–899, PMID: 27686768, 10.3760/cma.j.issn.0253-9624.2016.10.011. [DOI] [PubMed] [Google Scholar]
- Karner AA, Eisinger DS, Niemeier DA. 2010. Near-roadway air quality: synthesizing the findings from real-world data. Environ Sci Technol 44(14):5334–5344, PMID: 20560612, 10.1021/es100008x. [DOI] [PubMed] [Google Scholar]
- Kehm RD, Osypuk TL, Poynter JN, Vock DM, Spector LG. 2018a. Do pregnancy characteristics contribute to rising childhood cancer incidence rates in the United States? Pediatr Blood Cancer 65(3):e26888, PMID: 29160610, 10.1002/pbc.26888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehm RD, Spector LG, Poynter JN, Vock DM, Osypuk TL. 2018b. Socioeconomic status and childhood cancer incidence: a population-based multilevel analysis. Am J Epidemiol 187(5):982–991, PMID: 29036606, 10.1093/aje/kwx322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knox EG. 2006. Roads, railways, and childhood cancers. J Epidemiol Community Health 60(2):136–141, PMID: 16415262, 10.1136/jech.2005.042036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreis C, Doessegger E, Lupatsch JE, Spycher BD. 2019. Space–time clustering of childhood cancers: a systematic review and pooled analysis. Eur J Epidemiol 34(1):9–21, PMID: 30446850, 10.1007/s10654-018-0456-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landrigan PJ, Fuller R, Hu H, Caravanos J, Cropper ML, Hanrahan D, et al. 2018. Pollution and global health—an agenda for prevention. Environ Health Perspect 126(8):084501, PMID: 30118434, 10.1289/EHP3141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langholz B, Ebi KL, Thomas DC, Peters JM, London SJ. 2002. Traffic density and the risk of childhood leukemia in a Los Angeles case-control study. Ann Epidemiol 12(7):482–487, PMID: 12377426, 10.1016/S1047-2797(01)00317-9. [DOI] [PubMed] [Google Scholar]
- Lavigne É, Bélair MA, Do MT, Stieb DM, Hystad P, van Donkelaar A, et al. 2017. Maternal exposure to ambient air pollution and risk of early childhood cancers: a population-based study in Ontario, Canada. Environ Int 100:139–147, PMID: 28108116, 10.1016/j.envint.2017.01.004. [DOI] [PubMed] [Google Scholar]
- Loomis D, Grosse Y, Lauby-Secretan B, El Ghissassi F, Bouvard V, Benbrahim-Tallaa L, et al. 2013. The carcinogenicity of outdoor air pollution. Lancet Oncol 14(13):1262–1263, PMID: 25035875, 10.1016/S1470-2045(13)70487-X. [DOI] [PubMed] [Google Scholar]
- Lupo PJ, Symanski E, Chan W, Mitchell LE, Waller DK, Canfield MA, et al. 2010. Differences in exposure assignment between conception and delivery: the impact of maternal mobility. Paediatr Perinat Epidemiol 24(2):200–208, PMID: 20415777, 10.1111/j.1365-3016.2010.01096.x. [DOI] [PubMed] [Google Scholar]
- Magnani C, Ranucci A, Badaloni C, Cesaroni G, Ferrante D, Miligi L, et al. 2016. Road traffic pollution and childhood leukemia: a nationwide case-control study in Italy. Arch Med Res 47(8):694–705, PMID: 28476197, 10.1016/j.arcmed.2017.02.001. [DOI] [PubMed] [Google Scholar]
- Malagoli C, Costanzini S, Heck JE, Malavolti M, De Girolamo G, Oleari P, et al. 2016. Passive exposure to agricultural pesticides and risk of childhood leukemia in an Italian community. Int J Hyg Environ Health 219(8):742–748, PMID: 27693118, 10.1016/j.ijheh.2016.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcotte EL, Ritz B, Cockburn M, Yu F, Heck JE. 2014. Exposure to infections and risk of leukemia in young children. Cancer Epidemiol Biomarkers Prev 23(7):1195–1203, PMID: 24793957, 10.1158/1055-9965.EPI-13-1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenzie LM, Allshouse WB, Byers TE, Bedrick EJ, Serdar B, Adgate JL. 2017. Childhood hematologic cancer and residential proximity to oil and gas development. PLoS One 12(2):e0170423, PMID: 28199334, 10.1371/journal.pone.0170423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metayer C, Dahl G, Wiemels J, Miller M. 2016a. Childhood leukemia: a preventable disease. Pediatrics 138(Suppl 1):S45–S55, PMID: 27940977, 10.1542/peds.2015-4268H. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metayer C, Petridou E, Aranguré JM, Roman E, Schüz J, Magnani C, et al. 2016b. Parental tobacco smoking and acute myeloid leukemia: the Childhood Leukemia International Consortium. Am J Epidemiol 184(4):261–273, PMID: 27492895, 10.1093/aje/kww018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montero-Montoya R, López-Vargas R, Arellano-Aguilar O. 2018. Volatile organic compounds in air: sources, distribution, exposure and associated illnesses in children. Ann Glob Health 84(2):225–238, PMID: 30873816, 10.29024/aogh.910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan RL, Whaley P, Thayer KA, Schünemann HJ. 2018. Identifying the PECO: a framework for formulating good questions to explore the association of environmental and other exposures with health outcomes. Environ Int 121(Pt 1):1027–1031, PMID: 30166065, 10.1016/j.envint.2018.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumaier C. 2014. Eco-friendly versus cancer causing: perceptions of diesel cars in West Germany and the United States 1970–1990. Technol Cult 55(2):429–460, PMID: 25265652, 10.1353/tech.2014.0043. [DOI] [PubMed] [Google Scholar]
- Noone AM, Howlader N, Krapcho M, Miller D, Brest A, Yu M, et al. 2018. SEER Cancer Statistics Review (CSR) 1975–2015. https://seer.cancer.gov/csr/1975_2015/ [accessed 2 April 2019].
- Orsini N, Bellocco R, Greenland S. 2006. Generalized least squares for trend estimation of summarized dose–response data. Stata J 6(1):40–57, 10.1177/1536867X0600600103. [DOI] [Google Scholar]
- Orsini N, Li R, Wolk A, Khudyakov P, Spiegelman D. 2012. Meta-analysis for linear and nonlinear dose-response relations: examples, an evaluation of approximations, and software. Am J Epidemiol 175(1):66–73, PMID: 22135359, 10.1093/aje/kwr265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park AS, Ritz B, Ling C, Cockburn M, Heck JE. 2017. Exposure to ambient dichloromethane in pregnancy and infancy from industrial sources and childhood cancers in California. Int J Hyg Environ Health 220(7):1133–1140, PMID: 28720343, 10.1016/j.ijheh.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson RL, Wachtel H, Ebi KL. 2000. Distance-weighted traffic density in proximity to a home is a risk factor for leukemia and other childhood cancers. J Air Waste Manag Assoc 50(2):175–180, PMID: 10680346, 10.1080/10473289.2000.10463998. [DOI] [PubMed] [Google Scholar]
- Pyatt D, Hays S. 2010. A review of the potential association between childhood leukemia and benzene. Chem Biol Interact 184(1–2):151–164, PMID: 20067778, 10.1016/j.cbi.2010.01.002. [DOI] [PubMed] [Google Scholar]
- Raaschou-Nielsen O, Hertel O, Thomsen BL, Olsen JH. 2001. Air pollution from traffic at the residence of children with cancer. Am J Epidemiol 153(5):433–443, PMID: 11226975, 10.1093/aje/153.5.433. [DOI] [PubMed] [Google Scholar]
- Raaschou-Nielsen O, Hvidtfeldt UA, Roswall N, Hertel O, Poulsen AH, Sørensen M. 2018. Ambient benzene at the residence and risk for subtypes of childhood leukemia, lymphoma and CNS tumor. Int J Cancer 143(6):1367–1373, PMID: 29633247, 10.1002/ijc.31421. [DOI] [PubMed] [Google Scholar]
- Reynolds P, Elkin E, Scalf R, Von Behren J, Neutra RR. 2001. A case-control pilot study of traffic exposures and early childhood leukemia using a geographic information system. Bioelectromagnetics 22(Suppl 5):S58–S68, PMID: 11170118, . [DOI] [PubMed] [Google Scholar]
- Reynolds P, Von Behren J, Gunier RB, Goldberg DE, Hertz A. 2004. Residential exposure to traffic in California and childhood cancer. Epidemiology 15(1):6–12, PMID: 14712141, 10.1097/01.ede.0000101749.28283.de. [DOI] [PubMed] [Google Scholar]
- Savitz DA, Feingold L. 1989. Association of childhood cancer with residential traffic density. Scand J Work Environ Health 15(5):360–363, PMID: 2477895, 10.5271/sjweh.1848. [DOI] [PubMed] [Google Scholar]
- Schüz J, Erdmann F. 2016. Environmental exposure and risk of childhood leukemia: an overview. Arch Med Res 47(8):607–614, PMID: 28476188, 10.1016/j.arcmed.2016.11.017. [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A. 2016. Cancer statistics, 2016. CA Cancer J Clin 66(1):7–30, PMID: 26742998, 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- Spycher BD, Feller M, Röösli M, Ammann RA, Diezi M, Egger M, et al. 2015. Childhood cancer and residential exposure to highways: a nationwide cohort study. Eur J Epidemiol 30(12):1263–1275, PMID: 26520639, 10.1007/s10654-015-0091-9. [DOI] [PubMed] [Google Scholar]
- Spycher BD, Lupatsch JE, Huss A, Rischewski J, Schindera C, Spoerri A, et al. 2017. Parental occupational exposure to benzene and the risk of childhood cancer: a census-based cohort study. Environ Int 108:84–91, PMID: 28802171, 10.1016/j.envint.2017.07.022. [DOI] [PubMed] [Google Scholar]
- Steffen C, Auclerc MF, Auvrignon A, Baruchel A, Kebaili K, Lambilliotte A, et al. 2004. Acute childhood leukaemia and environmental exposure to potential sources of benzene and other hydrocarbons; a case-control study. Occup Environ Med 61(9):773–778, PMID: 15317919, 10.1136/oem.2003.010868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinmaus C, Smith MT. 2017. Steinmaus and Smith respond to “Proximity to gasoline stations and childhood leukemia.” Am J Epidemiol 185(1):5–7, PMID: 27923799, 10.1093/aje/kww133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suk WA, Ahanchian H, Asante KA, Carpenter DO, Diaz-Barriga F, Ha EH, et al. 2016. Environmental pollution: an under-recognized threat to children’s health, especially in low- and middle-income countries. Environ Health Perspect 124(3):A41–A45, PMID: 26930243, 10.1289/ehp.1510517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun XX, Zhang SS, Ma XL. 2014. No association between traffic density and risk of childhood leukemia: a meta-analysis. Asian Pac J Cancer Prev 15(13):5229–5232, PMID: 25040979, 10.7314/APJCP.2014.15.13.5229. [DOI] [PubMed] [Google Scholar]
- Symanski E, Tee Lewis PG, Chen TY, Chan W, Lai D, Ma X. 2016. Air toxics and early childhood acute lymphocytic leukemia in Texas, a population based case control study. Environ Health 15(1):70, PMID: 27301866, 10.1186/s12940-016-0154-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamayo-Uria I, Boldo E, García-Pérez J, Gómez-Barroso D, Romaguera EP, Cirach M, et al. 2018. Childhood leukaemia risk and residential proximity to busy roads. Environ Int 121(Pt 1):332–339, PMID: 30241021, 10.1016/j.envint.2018.08.056. [DOI] [PubMed] [Google Scholar]
- Tee Lewis PG, Chen TY, Chan W, Symanski E. 2019. Predictors of residential mobility and its impact on air pollution exposure among children diagnosed with early childhood leukemia. J Expo Sci Environ Epidemiol, PMID: 30770842, 10.1038/s41370-019-0126-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urayama KY, Von Behren J, Reynolds P, Hertz A, Does M, Buffler PA. 2009. Factors associated with residential mobility in children with leukemia: implications for assigning exposures. Ann Epidemiol 19(11):834–840, PMID: 19364662, 10.1016/j.annepidem.2009.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vattanasit U, Navasumrit P, Khadka MB, Kanitwithayanun J, Promvijit J, Autrup H, et al. 2014. Oxidative DNA damage and inflammatory responses in cultured human cells and in humans exposed to traffic-related particles. Int J Hyg Environ Health 217(1):23–33, PMID: 23567252, 10.1016/j.ijheh.2013.03.002. [DOI] [PubMed] [Google Scholar]
- Vinceti M, Filippini T, Cilloni S, Platt J, Jess C. 2017. Implications of different methods for literature searching and assessment in systematic reviews and meta-analyses: a case study [Abstract]. Poster 2085. Abstracts of the Global Evidence Summit, Cape Town, South Africa Cochrane Database Syst Rev 9(Suppl 1). 10.1002/14651858.CD201702 [accessed 2 April 2019]. [DOI] [Google Scholar]
- Vinceti M, Filippini T, Crippa A, de Sesmaisons A, Wise LA, Orsini N. 2016. Meta-analysis of potassium intake and the risk of stroke. J Am Heart Assoc 5(10):e004210, PMID: 27792643, 10.1161/JAHA.116.004210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinceti M, Rothman KJ, Crespi CM, Sterni A, Cherubini A, Guerra L, et al. 2012. Leukemia risk in children exposed to benzene and PM10 from vehicular traffic: a case–control study in an Italian population. Eur J Epidemiol 27(10):781–790, PMID: 22892901, 10.1007/s10654-012-9727-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visser O, van Wijnen JH, van Leeuwen FE. 2004. Residential traffic density and cancer incidence in Amsterdam, 1989–1997. Cancer Causes Control 15(4):331–339, PMID: 15141134, 10.1023/B:CACO.0000027480.32494.a3. [DOI] [PubMed] [Google Scholar]
- Von Behren J, Reynolds P, Gunier RB, Rull RP, Hertz A, Urayama KY, et al. 2008. Residential traffic density and childhood leukemia risk. Cancer Epidemiol Biomarkers Prev 17(9):2298–2301, PMID: 18768496, 10.1158/1055-9965.EPI-08-0338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells GA, Shea B, O’Connell D, Peterson J, Welch V, Losos M, et al. 2019. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp [accessed 4 March 2019].
- Weng HH, Tsai SS, Chen CC, Chiu HF, Wu TN, Yang CY. 2008. Childhood leukemia development and correlation with traffic air pollution in Taiwan using nitrogen dioxide as an air pollutant marker. J Toxicol Environ Health Part A 71(7):434–438, PMID: 18306090, 10.1080/15287390701839042. [DOI] [PubMed] [Google Scholar]
- Whitehead TP, Crispo Smith S, Park JS, Petreas MX, Rappaport SM, Metayer C. 2015. Concentrations of persistent organic pollutants in California women’s serum and residential dust. Environ Res 136:57–66, PMID: 25460621, 10.1016/j.envres.2014.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitehead T, Metayer C, Gunier RB, Ward MH, Nishioka MG, Buffler P, et al. 2011. Determinants of polycyclic aromatic hydrocarbon levels in house dust. J Expo Sci Environ Epidemiol 21(2):123–132, PMID: 20040932, 10.1038/jes.2009.68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilhelm M, Ghosh JK, Su J, Cockburn M, Jerrett M, Ritz B. 2011. Traffic-related air toxics and preterm birth: a population-based case-control study in Los Angeles County, California. Environ Health 10:89, PMID: 21981989, 10.1186/1476-069X-10-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Wilhelm M, Chung J, Ritz B. 2011. Comparing exposure assessment methods for traffic-related air pollution in an adverse pregnancy outcome study. Environ Res 111(5):685–692, PMID: 21453913, 10.1016/j.envres.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.