Abstract
Background:
Estimates of autism prevalence have increased dramatically over the past two decades. Evidence suggests environmental factors may contribute to the etiology of the disorder.
Objectives:
This scoping review aimed to identify and categorize primary research and reviews on the association between prenatal and early postnatal exposure to environmental chemicals and the development of autism in epidemiological studies and rodent models of autism.
Methods:
PubMed was searched through 8 February 2018. Included studies assessed exposure to environmental chemicals prior to 2 months of age in humans or 14 d in rodents. Rodent studies were considered relevant if they included at least one measurement of reciprocal social communicative behavior or repetitive and stereotyped behavior. Study details are presented in interactive displays using Tableau Public.
Results:
The search returned 21,603 unique studies, of which 54 epidemiological studies, 46 experimental rodent studies, and 50 reviews were deemed relevant, covering 152 chemical exposures. The most frequently studied exposures in humans were particulate matter (), mercury (), nonspecific air pollution (), and lead (). In rodent studies, the most frequently studied exposures were chlorpyrifos (), mercury (), and lead ().
Discussion:
Although research is growing rapidly, wide variability exists in study design and conduct, exposures investigated, and outcomes assessed. Conclusions focus on recommendations to guide development of best practices in epidemiology and toxicology, including greater harmonization across these fields of research to more quickly and efficiently identify chemicals of concern. In particular, we recommend chlorpyrifos, lead, and polychlorinated biphenyls (PCBs) be systematically reviewed in order to assess their relationship with the development of autism. There is a pressing need to move forward quickly and efficiently to understand environmental influences on autism in order to answer current regulatory questions and inform treatment and prevention efforts. https://doi.org/10.1289/EHP4386
Introduction
Autism and autism spectrum disorder comprise a broad array of conditions that impact an individual's social communication and behavior. The Diagnostic and Statistical Manual of Mental Disorders (DSM; American Psychiatric Association 2013) has three main diagnostic criteria for autism: a) persistent deficits in social communication and social interaction across multiple contexts; b) restricted, repetitive patterns of behavior, interests, or activities; and c) the presence of symptoms in the early developmental period, although they may not become apparent until social demands exceed the individual’s capacities (American Psychiatric Association 2013). Individuals with autism may express or experience these to varying degrees, resulting in a wide range of abilities from extremely gifted to severely challenged (American Psychiatric Association 2013; Centers for Disease Control and Prevention 2017a). Autism can have profound impacts on families and individuals, and also has widespread social and economic effects. In the United States, costs associated with autism are estimated at annually due primarily to medical care, special education, and lost parental productivity (Buescher et al. 2014; Lavelle et al. 2014).
The prevalence of autism has increased from 1 in 150 in 2002 to 1 in 59 in 2014 (Autism and Developmental Disabilities Monitoring Network Surveillance Year 2002 Principal Investigators 2007; Baio et al. 2018; Centers for Disease Control and Prevention 2017b). Although genetics are an important risk factor, they would not account for the dramatic rise in prevalence during this span in time, nor do changes in diagnostic criteria (Hertz-Picciotto and Delwiche 2009; Hertz-Picciotto et al. 2018b; King and Bearman 2009). Growing evidence suggests environmental factors and gene–environment interactions contribute to the etiology of the disorder (Frazier et al. 2014; Hallmayer et al. 2011). Some autism-related genes may be targeted by environmental pollutants, including pesticides, heavy metals, bisphenol A (BPA), phthalates, and many other chemicals in food, cosmetics, or household products (Carter and Blizard 2016). Further, evidence suggests that autism diagnosis is associated with variants in genes involved in the elimination of toxic chemicals from the body, which potentially results in a higher body burden of toxic chemicals (Rossignol et al. 2014). Investigating the contribution of environmental chemicals to autism offers an opportunity for intervention by reducing such exposures.
The early developmental period, and specifically the prenatal period, is a sensitive time when the developing brain is particularly susceptible to disruptions from environmental chemicals. This is supported by recent evidence indicating that subtle signs of autism can be detected as early as 9 months (Christensen et al. 2016a). Furthermore, autism disproportionately impacts males relative to females with a rate of diagnosis 3 to 4.5 times higher in males (Christensen et al. 2016b; Zablotsky et al. 2017), suggesting the developing endocrine system may be etiologically important (Baron-Cohen 2002). This is supported by studies comparing the length of the index finger to the ring finger, which is an established marker of fetal testosterone concentrations. Such studies have found that a decreased second digit-to-fourth digit ratio, indicating increased fetal testosterone exposure, is associated with an autism spectrum disorder diagnosis (Teatero and Netley 2013). The role and potential mechanisms of environmental endocrine disruptors in the etiology of autism has been discussed in earlier reviews (Moosa et al. 2018; Schwartzer et al. 2013).
Animal models can support the epidemiological evidence reporting associations between environmental chemical exposures and autism. Animal models, for example, allow for the investigation of the complex neurobiological events that occur during early development that may be etiologically important for autism. In addition, due to animals’ shorter lifespans, studies conducted in animal models may allow for more rapid assessment of a wide variety of environmental chemicals. Ideally, animal models accurately mimic the observed clinical phenotype, can be induced by the same biological or genetic mechanisms known to contribute to the etiology of autism in children, and have a similar response to treatments that prevent or treat autism in children (Crawley 2012). To the first point, significant progress has been made to outline specific rodent behavioral tests that address the two core behavioral features of a DSM diagnosis in children: persistent deficits in reciprocal social communication and restricted, repetitive patterns of behavior, interests, or activities (American Psychiatric Association 2013; Bey and Jiang 2014; Chang et al. 2017; Crawley 2012). The mouse and rat have been the best described and most utilized animal species to model autism to date. Recognizing the important contribution that animal models have, this scoping review considers the evidence that environmental chemical exposures are associated with autism in rodents alongside that in humans, which, to our knowledge, has not been done in previous reviews on the topic (e.g., Ng et al. 2017; Rossignol et al. 2014).
As a scoping review, the goal was to identify and categorize the peer-reviewed literature on the association between prenatal and early postnatal exposure to environmental chemicals and the development of autism in epidemiological studies and rodent models of autism. It was not the goal to draw conclusions about specific chemicals and their hazards. Specifically, we aimed to identify environmental chemical exposures during early life stages that could be further explored via systematic review. We also made recommendations to address research gaps, guide best practices, and prioritize future research.
Methods
A protocol for conducting this scoping review was prepared a priori but was not made publicly available, as there was no known venue for publication of protocols for scoping reviews at the time. A literature search strategy was crafted for PubMed and executed on 2 November 2016. Search strings were developed to address relevant populations, comparisons, and outcomes [three of the four components of a PECO (populations, exposures, comparators, outcomes) statement] (Table 1). The complete search logic is available in Excel Table S1. A combination of medical subject headings and free text words were used for the following concepts: autism spectrum disorder, prenatal or developmental life stages, human or rodent. There were no restrictions on exposures, language, or publication date. A search update was performed in PubMed on 8 February 2018 using the same search logic. During data extraction, reference lists of included studies were also hand screened to identify any additional studies that were not retrieved by the literature search.
Table 1.
PECO statement.
| PECO element | Evidence |
|---|---|
| Population | Human populations or rodent models of autism spectrum disorder. |
| Exposure | Exposure occurring during prenatal or early life period. Exposure should begin prior to 2 months of age for humans and postnatal day 14 for rats and mice. Exposure can be either gestational (i.e., via maternal exposure) or directly to the offspring. Only environmental chemical exposures are included (e.g., air pollution, pesticides, flame retardants, heavy metals, etc.). |
| Comparators | Comparison group with lower exposure or no exposure. |
| Outcome | Autism spectrum disorder or indicators of autism spectrum disorder. Rodent studies need to report at least one reciprocal social communicative behavior or one repetitive and stereotyped behavior. |
Note: PECO, populations, exposures, comparators, outcome.
Results of the PubMed search were uploaded to Sciome Workbench for Interactive computer-Facilitated Text-mining (SWIFT, Beta Test version) Active Screener (Sciome), a text mining and machine learning program. In this program, users are first presented with random studies to screen, and the program adaptively learns from the choices made by the users. Thus, as screening progresses, the remaining unreviewed studies are automatically prioritized, and the most relevant studies are presented for screening first, with the purpose of allowing screening to be stopped at a predefined estimated recovery rate for relevant studies.
Title and abstract screening was performed in SWIFT Active Screener by two reviewers (K.E.P. and A.L.B) until an estimated recall of 90% was achieved (i.e., the text mining and machine learning algorithms of SWIFT Active Screener estimated that at least 90% of the relevant studies were identified). The decision to cease screening upon reaching an estimated recall of 90% was determined a priori based on methods recommended by the U.S. National Toxicology Program as appropriate for scoping reviews (A.A. Rooney, personal communication). Further, we hand searched reference lists to capture important studies that might have been missed in the screening process. Discrepancies between reviewers were tracked within SWIFT Active Screener and resolved through discussion.
The title and abstract screening of potentially relevant primary studies and reviews performed in SWIFT Active Screener was very broad. For the initial screening, reviewers asked, “Does this reference discuss prenatal or very early life exposures and autism spectrum disorder in humans or rodent models?” There were no limitations on the types of exposure in this level. When it was unclear if a study met the inclusion criteria based on review of the title and abstract, it was moved forward to the next screening stage and later confirmed during full-text review.
Studies included after title and abstract screening in SWIFT Active Screener were then uploaded to DistillerSR (Evidence Partners) for initial tagging by publication type (primary research or review), evidence stream (epidemiological study or experimental rodent study), and type of exposure (air pollution, alcohol, assisted reproduction, drugs of abuse, endogenous hormones, environmental chemicals, folic acid, heavy metals, infant feeding, medication/pharmaceutical, minerals/trace elements, research compounds, smoking, vaccines/immunoglobulins, valproic acid, vitamin D, other/not sure).
Studies tagged as air pollution, environmental chemicals, metals, and those where it was unclear what the exposure was from the title and abstract screening (tagged as “other/not sure”) were reviewed at the full-text level. Full-text review of studies was carried out in DistillerSR. Studies had to be available in English language to be included at the stage of full-text review. For both epidemiological and experimental rodent studies to be included, there had to be exposure to environmental chemicals at an early developmental age. As this is a scoping review intended to cast a wide net, exposures that could possibly be proxy measurements of environmental chemical exposures were also included. Given that significant neurodevelopment occurs postnatally in rodents, exposure had to occur on or before postnatal day (PND) 14 to be included in this scoping review. This is a time approximately equivalent to 2 months of age in humans based on various neurodevelopmental events (Clancy et al. 2007; Workman et al. 2013). Thus, epidemiological studies had to assess environmental exposures at or before 2 months of age to be included in this scoping review. All epidemiological studies that reported an outcome of autism diagnosis, regardless of how a diagnosis was defined, were included. Rodent studies had to report at least one reciprocal social communicative behavior or one repetitive and stereotyped behavior to be included. Table 2 provides specific examples of rodent outcomes and how they were categorized for analysis. The outcomes and their classification as reciprocal social communicative behaviors or repetitive and stereotyped behaviors are based on those recommended in Bey and Jiang (2014); Chang et al. (2017); Crawley (2012), and are meant to reflect the diagnostic requirements in humans as outlined in the DSM-5 (American Psychiatric Association 2013). Reviews were included if they addressed developmental exposure to environmental chemicals and autism in humans, or autism-related behaviors in rodents.
Table 2.
Experimental rodent outcomes.
| Animal outcome categories | Specific examples of rodent outcomes |
|---|---|
| Reciprocal social communicative behaviorsa | Three-chambered test, open field test with social component, play behaviors measurements, ultrasonic vocalizations, nest seeking response, social choice, social discrimination, other measurements of social behaviors other than aggression |
| Repetitive and stereotyped behaviorsa | Classic stereotyped behaviors such as gnawing, circling, or rearing; repetitive behaviors measured by maze apparatus such as T, Y, or Morris water mazes; other stereotypical behavioral measurements |
| Comorbidities | Anxiety measurements such as elevated plus maze, light dark box, prepulse inhibition, elevated food test, or zero maze; other comorbid behaviors |
| Mechanistic outcomes | Neuropathology, neuropsychological functioning, neurochemical alterations |
Inclusion criteria.
Data extraction from full-text documents was carried out in DistillerSR. Bibliographic citation information was recorded for all included studies. The following information was recorded for epidemiological studies: study type, name of study if provided, geographic location, overall sample size, sex of included participants, how autism was diagnosed, the age range of diagnosis, the year of birth, what the exposure was, if it was an occupational or a general population exposure, how the exposure was measured, and age when exposure was assessed. Initially, the specific exposures that were assessed in each study were extracted and listed. Upon the completion of data extraction, it became necessary to broadly classify the long list of exposures that had been captured. It should be noted that some chemicals may be classified in more than one broad category. For example, all studies on mercury, regardless of its source, are found in the broad category “metals & semi-metals,” but only those studies where it was investigated as an air pollutant are also found in the broad category “air pollutants.” Likewise, it became necessary to broadly categorize how autism was diagnosed. After consultation with experts in the field, we broadly categorized studies as: using specific diagnostic tools, using specific screening tools, and/or stating that children met either the DSM or International Statistical Classification of Diseases and Related Health Problems (ICD) criteria. It should be noted that diagnostic tools and screening tools are used by practitioners to reach a DSM or ICD diagnosis, but stating that a DSM or ICD diagnosis was given does not indicate which specific diagnostic or screening tools were used to reach that conclusion. Further, not all diagnostic and screening tools may be comparable (Randall et al. 2018).
The following information was recorded for rodent studies: the strain and species, the environmental exposure, age of exposure (categorized as gestational, postnatal, or both, which is referred to as “developmental”), route of exposure, whether or not the outcome occurred spontaneously or was induced (for example, by apomorphine or amphetamine), the timing of outcome assessment [categorized as neonatal (0–14 d), juvenile (15–40 d), adult ()], and the outcome. Comorbidities and mechanistic outcomes were also recorded if noted within the included studies (Table 2). During full-text review, the included reviews were characterized as narrative, systematic, scoping, or meta-analytic reviews, as per how the authors identified the publications. Data on the evidence stream (epidemiological or experimental rodent) and exposures studied were also extracted. All extracted information was exported from DistillerSR to Microsoft Excel and was subsequently visualized using Tableau Desktop Professional Edition (version 2018.3.1; Tableau, https://public.tableau.com/profile/the.endocrine.disruption.exchange#!/).
Results
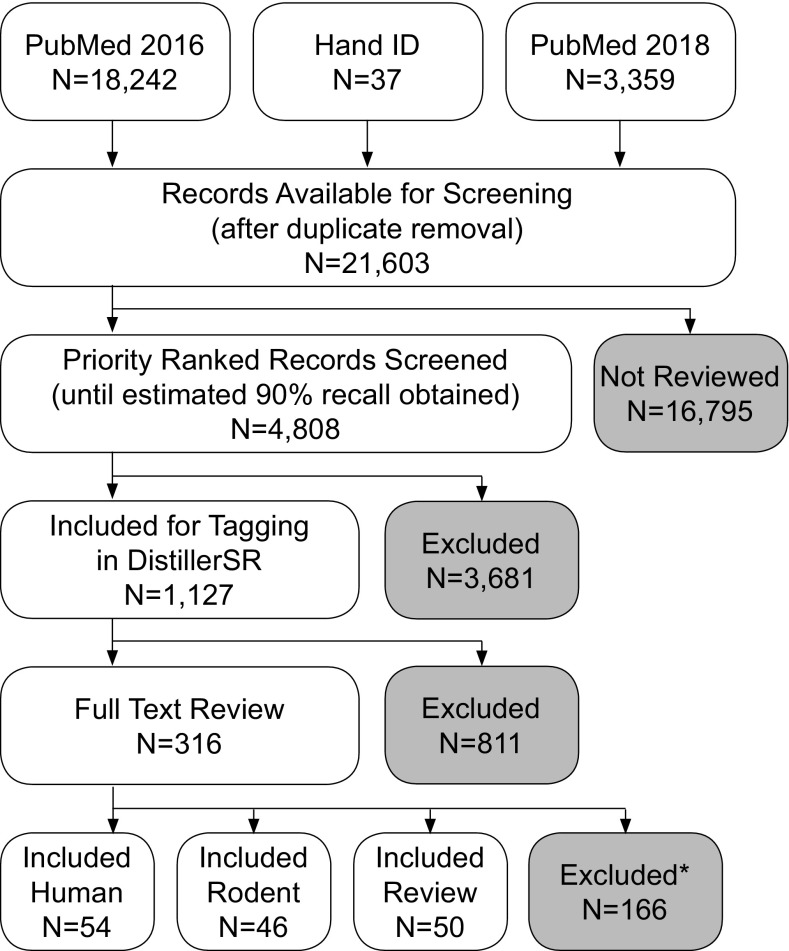
The PubMed search retrieved 18,242 studies (Figure 1). After duplicate removal, 18,218 studies were uploaded to SWIFT Active Screener for title and abstract screening. The literature update retrieved an additional 3,359 studies, 11 of which were duplicates from the original search. The studies retrieved from the update were processed identically to the initial search. An estimated recall of 90% was achieved when 4,000 studies had been screened in SWIFT Active Screener. Likewise, estimated recall of 90% was achieved when 808 studies of the search update had been screened. Hand searching the bibliographies of included references resulted in an additional 37 studies to screen. In total, 1,127 studies were considered relevant after title and abstract screening and moved to DistillerSR for further categorization. Full text was reviewed for the 316 studies that were tagged as including an exposure to an environmental chemical, air pollutants, or metals, and that appeared relevant based on the title and abstract. Reasons for exclusion during full-text review are provided in Excel Table S2.
Figure 1.
Flowchart of studies through the review process. This describes the number of studies evaluated at each step of the review process. “Hand ID” are the studies identified by scanning reference lists of included studies. Priority-ranked studies were screened at the title and abstract level in Sciome Workbench for Interactive computer-Facilitated Text-mining (SWIFT) Active Screener. Studies were excluded if they did not pertain to prenatal or very early life exposures and autism in humans or rodent models. At the full-text level, studies were excluded if there was not exposure to environmental chemicals prior to 2 months of age in humans or 14 d in rodents. *Note: Reasons for exclusion at the full-text level can be found in Excel Table S2.
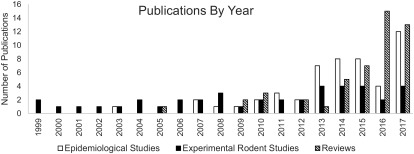
After full-text review, 150 studies were included: 54 epidemiological studies, 46 rodent studies, and 50 reviews (Figure 1). It should be noted that the direction of association (positive, negative, or not associated) of any particular exposure with autism was not captured in this scoping review. Overall, the rate of publication has been steadily increasing in the last 10 y, with a dramatic increase in epidemiological studies after 2012 (Figure 2). The number of reviews in 2016 () equaled the number from the prior 5 y combined.
Figure 2.
Number of studies published by year and type (1999–2017). The number of studies in each evidence stream are shown by year of publication. Note: Five experimental rodent studies published before 1999 are not shown in this figure. One paper was published in each of the following years: 1974, 1979, 1984, 1985, and 1995.
Extracted data from epidemiological and rodent studies is available in an interactive format in Figures S1 and S2 (see Supplemental Materials for more information on accessing and navigating the supplemental figures). In these interactive figures, the data can be filtered by environmental exposure and/or outcome. The epidemiological data can be additionally filtered by study type, and the rodent data can be additionally filtered by the timing of exposure or outcome assessment. Extracted data can also be viewed in Excel Table S3 (epidemiological data), Excel Table S4 (experimental rodent data), and Excel Table S5 (reviews). Results for epidemiological studies, experimental rodent studies, and reviews are presented and discussed below.
Epidemiological Studies
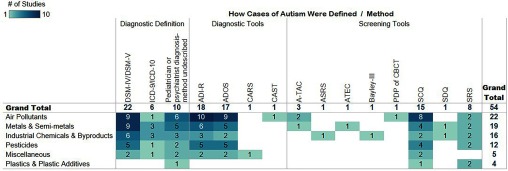
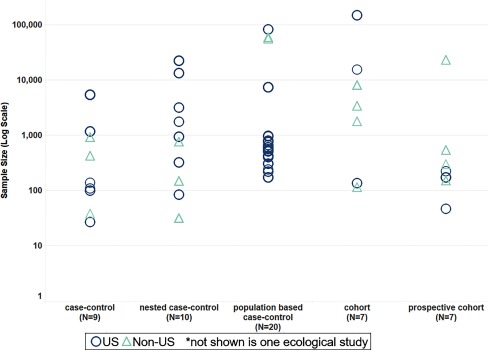
Fifty-four epidemiological studies were identified (Figure 3, Excel Table S3). Fifty studies investigated general population exposures, and five investigated occupational exposures. Most studies used a case–control study design (), including 20 population-based studies and 10 nested studies. Cohort (), prospective cohort (), and ecological () study designs were also used (Figure 4). Eleven of the epidemiological studies were from the Childhood Autism Risks from Genetics and Environment (CHARGE) project, a large population-based case–control study in California (Excel Table S3). The majority of the studies from CHARGE investigated the role of air pollutants (), but pesticides (), metals (), and occupational exposures () have also been studied in this project.
Figure 3.
Heat map of included epidemiological studies by exposure (in rows) and how autism was determined within the study (in columns). Numbers within cells indicate the number of studies for a given exposure and method of autism determination. Empty cells indicate a lack of studies. An interactive version of this figure is available at https://public.tableau.com/profile/the.endocrine.disruption.exchange#!/vizhome/Fig3_Enviornmentalchemicalsandautism-epidemiologicaldata/Interactive. For clarity, the different methods of autism determination were collapsed into the following broad categories: used specific diagnostic tools, used specific screening tools, and/or stated that children met either the DSM or ICD criteria. It should be noted that diagnostic tools and screening tools are used by practitioners to reach a DSM or ICD diagnosis, but stating that a DSM or ICD diagnosis was given does not indicate which specific tools were used to reach that conclusion. The 142 specific exposures assessed in the included studies were also collapsed into six broad categories for improved clarity. The categories for types of exposures can be fully expanded in the interactive version of the figure. Additional study information can be found in the interactive Figure S1 and in Excel Table S3. Note: A-TAC: autism tics, ADHD, and other comorbidities inventory; ADIR-R, Autism Diagnostic Interview–Revised; ADOS, Autism Diagnostic Observation Schedules; ASRS, Autism Spectrum Rating Scales; ATEC, Autism Treatment Evaluation Checklist; CARS, Childhood Autism Rating Scale; CAST, Childhood Autism Spectrum Test; DSM, Diagnostic and Statistical Manual of Mental Disorders; ICD, International Statistical Classification of Diseases and Related Health Problems; PDP of CBCT, Pervasive Developmental Problems subscale of the Child Behavior Checklist for Toddlers; SCQ, Social Communication Questionnaire; SDQ, Strengths and Difficulties Questionnaire; SRS, Social Responsiveness Scale.
Figure 4.
Sample size by epidemiological study type. Epidemiological studies are displayed in the scatterplot based on their study design on the x-axis (case–control, nested case–control, population-based case–control, cohort, or prospective cohort) and on the number of study participants on the y-axis (log scale). Studies conducted in the United States are shown as blue circles. Studies conducted outside of the United States are shown as light-teal triangles.
The included studies represent children born from 1967 to 2010. Studies were conducted in 15 countries, but most were located within the United States (Figure 4), with 23 studies (43% of all epidemiological studies) conducted in California (Excel Table S3). Findings from these studies may or may not be generalizable to the general population, given the variability in exposure to environmental chemicals across different geographical locations and during different time periods.
One hundred forty-two chemical exposures were catalogued and organized into six broader exposure categories (Figure 3, Figure S1, Excel Table S3). In some studies, exposures were described only in general terms (e.g., metals, pesticides, air pollutants). In others, specific chemicals or chemical groups were investigated [e.g., BPA, chlorpyrifos, polychlorinated biphenyls (PCBs)]. In some studies, both the general exposures and the individual chemicals were analyzed. On average, each paper explored six exposures ().
The broad category of air pollutants has been the most studied to date ( studies; Figure S1). Air pollutant studies ranged from general “air pollution” to one or more individual chemicals, spanning 56 chemicals across all air pollutant studies. Of these, 11 were metals or semimetals studied as air pollutants. The next-largest broad category of exposure studied was metals and semimetals with studies spanning 18 specific exposures. Industrial chemicals and by-products followed with studies spanning 20 specific exposures, and pesticides with studies spanning 40 specific exposures. The 40 specific pesticide exposures included 27 individual pesticides and 12 pesticide classes (e.g., described by study authors as “pyrethroids”), as well as the general term “pesticides.”
Specific exposures can be viewed by expanding the exposures display in the interactive Figure S1, and from there, the specific studies associated with each exposure can also be easily obtained. Note that as described in the “Methods” section, some specific exposures may appear in more than one broad category. For example, studies that evaluated lead or mercury are always displayed in the metals and semimetals broad category, and additionally appear in the air pollutants broad category when they were considered in the context of air pollution.
Under the broad category of air pollutants (), several specific air pollutants have been evaluated. The most studied were particulate matter (), nonspecific “air pollution” (mostly as near-roadway or traffic-related air pollution; ), nitrogen dioxide (), ozone (), lead (), nitrogen oxides (), manganese (), and cadmium (). PCBs are the most studied industrial chemicals and by-products (), though per- and polyfluoroalkyl substances and polybrominated diphenyl ethers (PBDEs) have been evaluated in studies each. Mercury (), lead (), manganese (), chromium (), cadmium (), nickel (), nonspecific “metals” (), and arsenic () have been the most studied metals and semimetals to date. As noted, some of these metals have been studied in the context of air pollution as well as from other sources (for example, mercury from dental amalgams). With the exception of organophosphates (), dichlorodiphenyldichloroethylene (), pyrethroids (), chlorpyrifos (), and trans-nonachlor (), most pesticides have only been evaluated in one or two papers each. Of the plastics and plastic additives, BPA has been studied the most (), with the phthalates and phthalate metabolites each being studied only once or twice each. Of all the 142 specific exposures catalogued, only 22% () have been investigated in at least one prospective study.
Environmental exposures were determined by a variety of measurements. Details on how and when exposure was assessed can be found in Excel Table S3 and by hovering over the study details in Figure S1. In some cases, there was direct measurement in a biological matrix (e.g., blood, breast milk, and urine); in other cases, the exposure was determined based on modeling data, taking into consideration maternal residence and factors such as local or regional air quality monitoring data, or known dates of pesticide application. The latter measurements are less sensitive and specific and rely upon several assumptions that could introduce artifacts capable of impacting the outcome of the study. There is also the possibility for lack of specificity in terms of exposure timing. Some of the exposure measurements may be considered proxies for prenatal exposure (e.g., hair from first baby haircut, baby teeth, and some of the modeling data), and it is not clear if these truly represent early developmental vs. infant or early childhood exposures.
Studies differed in how autism cases were diagnosed and/or defined (Figure 3; Figure S1). The DSM and the ICD criteria are the two most widely used manuals for defining autism. The DSM was updated from DSM-IV(American Psychiatric Association 1994) to DSM-V (American Psychiatric Association 2013) in 2013, and the ICD was updated from ICD-9 (Centers for Disease Control and Prevention 2013) to ICD-10 (WHO 2016) in 2015, and both versions were used by the studies included in this review. Twenty-two studies reportedly used the DSM criteria, and six used the ICD criteria. The specific evaluative measures administered by trained professionals for reaching a diagnosis based on the DSM or ICD manuals are referred to as diagnostic tools. The specific diagnostic tools used were reported in 23 studies (Figure S1; Excel Table S3). In comparison, screening tools are quick methods to identify someone who may have autism and who should be referred for more in-depth diagnostic testing (Children's Hospital of Philadelphia 2016). Screening tools are also used in the research setting to confirm controls are properly classified. Screening tools were used in 26 studies (Figure S1, Excel Table S3), indicating that many studies either do not thoroughly screen controls to ensure they are classified appropriately, or do not report it.
Of the 39 case–control studies, nearly all stated that cases received a DSM-IV or DSM-V diagnosis (), an ICD-9 or ICD-10 diagnosis (), or a diagnosis from a psychiatrist or physician (with no criteria provided) (), but most did not specify which specific screening or diagnostic tools were used to reach the diagnosis. Further, many of the case–control studies relied on previous diagnosis of autism as obtained from medical/administrative records, as opposed to performing diagnosis on the individual children enrolled in the studies. The exception is that the 11 reports from the population based case–control CHARGE study reported only the specific screening (Social Communication Questionnaire) and diagnostic tests used [Autism Diagnostic Interview-Revised (ADI-R) and Autism Diagnostic Observation Schedule (ADOS)], without specifying that case children met the DSM or ICD criteria, though that is implied. In contrast, the cohort studies relied mainly on screening tools, with the Social Responsiveness Scale (SRS) used most frequently (). The three largest cohort studies, however, did not use a screening tool (Gong et al. 2017; Kalkbrenner et al. 2014; von Ehrenstein et al. 2014). Instead, DSM-IV cases were identified by examining records from records-based surveillance programs. Whether it is appropriate to include and/or combine data from studies using different diagnostic and screening tools for autism should be addressed in future reviews.
Half of the studies did not report the age of autism diagnosis. Of those that did (), the range of age at diagnosis across the studies was from 0.3 to 12 y of age. Studies in which children are diagnosed at younger ages may miss cases that, for various reasons, are not diagnosed until the children are older. On the other hand, studies that include children diagnosed at later, preteen ages may be susceptible to misdiagnosis with other phenotypically similar disorders such as attention deficit hyperactivity disorder (ADHD) (Bal et al. 2019; Grzadzinski et al. 2016; Hommer and Swedo 2015). In either case, the field would benefit from better reporting of the age of diagnosis.
Experimental Rodent Studies
Forty-six studies met the inclusion criteria for rodent studies [i.e., the study had to report assessment of at least one reciprocal social communicative behavior or repetitive and stereotyped behavior (Table 2), and exposure had to occur prior to PND 14]. It should be noted that it was not required that studies deem the behaviors to be altered in a way that suggests an autism-like phenotype; rather, we only required the behavior to have been studied. Exposures occurred either gestationally (), postnatally (), or developmentally (exposure occurring during both pregnancy and lactation; ). In Figure S2, these different exposure periods are represented by different colors and can also be found by hovering over the study details. Two studies evaluated the impact of different exposure timing scenarios on the behavioral outcomes in order to more clearly define the critical period of exposure (Venerosi et al. 2006; Zaidi et al. 1985). Animals were exposed to the chemicals via inhalation, subcutaneously (by injection or implantation of osmotic minipump or silastic capsule), by intraperitoneal injection, or orally (via feed or diet, drinking water or gavage).
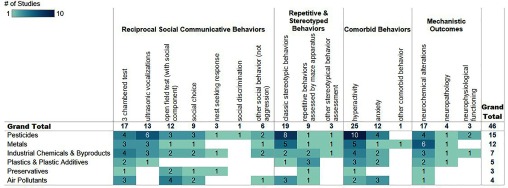
There were 25 unique exposures captured (Figure 5, Figure S2, Excel Table S4). Pesticides and metals were the most explored with chlorpyrifos (), mercury (), and lead () being the specific chemicals most studied. PCBs and particulate matter were each investigated in three studies. The remaining exposures were studied in one () or two () studies each.
Figure 5.
Heat map of included rodent studies by exposure (in rows) and types of rodent outcomes (in columns). Numbers within cells indicate the number of studies for a given exposure and outcome. Empty cells indicate a lack of studies. An interactive version of this figure is available at https://public.tableau.com/profile/the.endocrine.disruption.exchange#!/vizhome/SupplementalFigure2_Environmentalchemicalsandautism-rodentdata/Interactive. For clarity, the different outcomes have been collapsed to the four types of outcomes that were captured in this report. Additional study information can be found in the interactive Figure S2 and in Excel Table S4.
In humans, an autism diagnosis is based on the presence of persistent deficits in social communication and social interaction and restricted, repetitive patterns of behavior, interests, or activities (American Psychiatric Association 2013). Thirty-one rodent studies reported reciprocal social communicative behaviors, and 28 studies reported repetitive and stereotyped behaviors (Figure S2). A challenge in using animals to study autism is assessing the model’s face validity, or in other words, determining if the behavioral features are at least conceptually analogous to core diagnostic symptoms in humans. To this end, 13 studies reported on both types of behaviors in the same study and therefore may have stronger face validity. Arsenic, butyl paraben, cadmium, chlorpyrifos, di(2-ethylhexyl) phthalate, lead, manganese, mercury, ozone, particulate matter, PBDEs, PCBs, and trihalomethanes and perchloroethylene mixture were the only exposures in which reciprocal social communicative behaviors and repetitive and stereotyped behaviors were reported in the same study. Of these, only chlorpyrifos and particulate matter were reported in more than one study with both types of behaviors. Assessing both types of behaviors within a study would better reflect human diagnostic criteria.
Tests for reciprocal social communicative behavior assess how a rodent interacts with and/or communicates with another individual in a social setting, and may represent the persistent deficits in social communication and social interaction diagnostic of autism in humans (Roullet and Crawley 2011). The most commonly reported tests for reciprocal social communicative behavior were the three-chambered test (), ultrasonic vocalizations (), and the open field with social component test () (Figure S2, Excel Table S4). The three-chambered test and open field test with a social component specifically assess aspects of sociability (Bey and Jiang 2014). Assessment of ultrasonic vocalizations specifically assesses communication behavior. Ultrasonic vocalizations are often evaluated by removing a neonatal pup from the dam and littermates, but intentional communication may be evaluated in juvenile or adult animals (Bey and Jiang 2014; Roullet and Crawley 2011). Assessment of repetitive and stereotyped behaviors in rodents represents the restricted, repetitive patterns of behavior, interests, or activities that are diagnostic of autism in humans (Crawley 2012). Assessment of classic stereotyped and repetitive behaviors (e.g., gnawing, circling, rearing) was reported in 19 studies and was the most common assessment of repetitive and stereotyped behaviors. The dopamine agonist apomorphine or the stimulant amphetamine was used to induce repetitive behaviors in rodents in nine of the 19 stereotypy studies. Data suggests induced and spontaneous stereotypies may be mediated by different mechanisms, although there is, as of yet, no clear guidance as to which assessment is more directly applicable in rodent models of autism (Lewis et al. 2007; Presti et al. 2002). It should be noted that the animal evidence may be underestimated in this scoping review because we did not include search terms for each specific behavioral test.
In Figure S2, the different outcome assessment periods are represented by different shapes and can also be found by hovering over the study details. The timing of the outcome assessment was largely dependent on the outcome being measured. Some outcomes, such as ultrasonic vocalizations, can be evaluated for both pups and adults, although they need to be interpreted differently (Roullet and Crawley 2011). Eighteen studies evaluated end points in neonates (14 d old or younger) (Figure S2). End points measured at this time were primarily ultrasonic vocalizations and nest-seeking response. Twenty-one studies evaluated end points in juveniles (15–40 d old), and 36 studies evaluated end points in adults (). Combinations of exposure and outcome timing can be explored in Figure S2.
Anxiety and hyperactivity are associated features supporting diagnosis of autism (American Psychiatric Association 2013; Bey and Jiang 2014). Thirty-one studies reported at least one comorbid behavior, with most () reporting a measurement of neonatal, juvenile, or adult hyperactivity, and some () reporting a measurement of juvenile or adult anxiety. Reporting of comorbid behaviors can further support the face validity of rodent models when the phenotype observed is analogous to human clinical presentation.
Likewise, mechanistic end points in rodent studies can support the validity of the model, for example, if molecular effects are the same as those observed in humans with autism spectrum disorders. A major advantage to using rodent models is that mechanistic end points, such as changes in neural cell populations, can more easily be studied than in humans. Nearly half of the rodent studies () included further investigation of at least one mechanistic end point, with the most common being neurochemical alterations () such as differences in dopamine or serotonin levels. Fewer studies () reported changes in neural structures or architecture, such as alterations in cell number or cell structure. Three studies reported alterations in neurophysiological functioning (acetylcholinesterase activity). De Felice et al. (2016) and Sadowski et al. (2014) published follow-up studies with mechanistic outcomes.
The experimental rodent studies were split fairly evenly between rat () and mouse () model systems. While there are many similarities, there are more options for exploring the role of genetics when using mice due to the availability of a wider variety of genetically modified mouse models than rat models. This is apparent by the lack of genetically modified strains of rat and the use of genetically modified strains of mice in the included studies. Ellenbroek and Youn (2016) discuss notable species differences as they pertain to models of neuropsychiatric disorders. These include functional differences in brain structure and differences in social structure that in turn affect social behavior (Ellenbroek and Youn 2016). They conclude that rats may be the preferred model for assessing social behaviors, as mice display less social interaction and receptiveness. It should also be noted that strain differences are likely to exist within each species as well (Spearow et al. 1999). While our search of animal models of autism was limited to rat and mouse models, other animal models exist and should be considered in future work on this topic. For example, primates and rodent models other than mice and rats, [e.g., Microtus (vole) and Peromyscus (deer mouse)] have been used to study environmental chemicals (Patisaul et al. 2018). Although they may not be as well characterized or utilized as rat and mouse models for studying autism, they have unique advantages (Watson and Platt 2012). For example, the vole is more social than either the rat or the mouse (Beery and Kaufer 2015), and the mechanism underlying their prosocial traits is well characterized, making them an excellent animal model for studying the effects of environmental chemicals on neurodevelopmental disorders like autism (Patisaul et al. 2018).
Reviews
Fifty reviews were identified, covering 57 exposures (Excel Table S5). As in the primary literature, air pollution, mercury, and pesticides were the most frequently discussed exposures in the reviews. Of the various review types, systematic reviews and meta-analyses are generally regarded as the best suited for establishing the strength of the evidence between environmental exposures and autism (Gopalakrishnan and Ganeshkumar 2013; Mandrioli and Silbergeld 2016; Woodruff and Sutton 2014). Nine systematic reviews (as identified by the review authors) and three meta-analyses have been conducted since 2014; they included only human evidence. Note that we did not evaluate the quality of the systematic reviews to ascertain whether they were indeed systematic according to current standards such as ROSES [RepOrting standards for Systematic Evidence Syntheses (Haddaway et al. 2017)]. Only mercury (from air pollution) (Yoshimasu et al. 2014), particulate matter (Flores-Pajot et al. 2016; Lam et al. 2016), nitrogen dioxide (Flores-Pajot et al. 2016), and ozone (Flores-Pajot et al. 2016) have been evaluated by meta-analysis. Systematic reviews of the epidemiological evidence have been conducted on particulate matter (Lam et al. 2016; Morales-Suárez-Varela et al. 2017), air pollutants (Flores-Pajot et al. 2016; Fordyce et al. 2018; Lam et al. 2016; Rossignol et al. 2014), chromium and nickel (McDermott et al. 2015), mercury (from air pollution) (Yoshimasu et al. 2014), neonicotinoid pesticides (Cimino et al. 2016), and phthalate esters (Jeddi et al. 2016). Though significant effects were noted for mercury, and there was limited evidence that developmental exposure to air pollutants was associated with autism, overall, the systematic reviews highlighted the need for more and better observational human studies. Authors of the systematic reviews largely declared that there was not enough evidence, or the evidence was too heterogeneous, to declare strong support for an association between the reviewed exposures and autism.
The remaining reviews were narrative and addressed human epidemiological studies () and rodent studies (). Some were more general (e.g., Fluegge 2016; Heyer and Meredith 2017), for example, discussing autism and other neurological diseases and disorders (e.g., ADHD), and others were more narrowly focused on autism (e.g., Lyall et al. 2017; Ye et al. 2017). Yet other reviews drew mechanistic links between environmental exposures and autism and used the primary literature to support their proposed hypotheses (e.g., Moosa et al. 2018). Most reviews discussed five or fewer environmental chemical exposures.
Discussion
The aims of this scoping review were to identify research gaps, make recommendations to help guide the field and prioritize future research, and propose specific topics that we deemed ready for systematic review. In this review, we identified 152 exposures across the epidemiological and experimental rodent studies. Importantly, this surveys only a small fraction of the chemicals manufactured or processed, which is estimated to be upwards of 85,000 in the United States (U.S. EPA 2016).
Epidemiological Studies
When assessing a body of evidence, particularly in a systematic review with meta-analysis, a major challenge is combining studies that used very different methods. In this review, the 54 epidemiological studies varied widely in terms of study design, specific exposures assessed, how and when exposures were evaluated, and how autism cases were defined. We recommend the following in order to reduce this heterogeneity and improve research methods in this field of study:
Autism diagnosis.
Based on our experience conducting this scoping review, the field would benefit from more thorough reporting in the primary research of how autism cases and controls are defined, specifically which diagnostic and/or screening tools are used to reach a DSM or ICD diagnosis. There are numerous tools available to screen and diagnose autism, some more specific and sensitive than others (Falkmer et al. 2013; Randall et al. 2018), and whenever possible, we recommend researchers use the most rigorous methods of diagnosing autism [e.g., ADOS and ADI-R (Randall et al. 2018)]. Further, in our opinion, attention should be paid to appropriately screening participants to minimize cases being inappropriately classified as controls. Additionally, the age of autism diagnosis should be better reported, as it is important in evaluating the likelihood of an accurate diagnosis. These issues are particularly problematic in studies that rely solely on record review for DSM or ICD diagnosis.
Exposure assessment.
Exposure assessment methods and timing varied widely between chemicals and even for the same chemical. The reliance in the past on less sensitive and precise measures of exposure levels and timing [e.g., those based on data modeled from historical Toxic Release Inventory data (Lam et al. 2016) or baby’s first haircut] allowed for the early exploration of associations with autism, but in our opinion, will be less useful moving forward. More sensitive and direct exposure assessment methods that determine individual levels of exposure are now available. For example, biological samples, personal exposure monitoring systems, and exposomic analyses may provide more accurate and comprehensive exposure information (Turner et al. 2018; Vineis et al. 2017). In addition, for chemicals for which exposure levels are known to fluctuate over time [e.g., pesticides (Arcury et al. 2009; Li et al. 2014)] or that are rapidly metabolized [e.g., BPA (Thayer et al. 2015; Ye et al. 2011)], studies should include exposure assessments at multiple time points.
Study location.
The geographical distribution of studies identified in this review was highly skewed toward the United States, and specifically California. It is known that environmental chemical exposures vary between time and place, due to different environmental chemical regulations (e.g., for pesticides), regional weather patterns (e.g., air pollution), differences in local industries (e.g., manufacturing), and other variables. Further, it is also known that autism diagnosis rates vary geographically, and this may be related to different environmental exposures across regions (Hoffman et al. 2017). It may be important to establish studies in more diverse locations to better understand the influence of environmental chemical exposures on autism.
Study design.
There were only seven prospective studies identified in this scoping review. Prospective studies that enroll families during early pregnancy or even preconception, and track exposures through early postnatal life, provide the most direct evidence linking environmental exposures to autism. It may be helpful to establish studies similar to the Health Outcomes and Measures of the Environment (HOME) study, a prospective cohort study set in Cincinnati, Ohio (Braun et al. 2017), in diverse geographic locations. On the other hand, additional population based case–control studies similar to the CHARGE study in California (Hertz-Picciotto et al. 2006) would more quickly grow the evidence base for chemicals that currently only have one or two studies.
Experimental Rodent Studies
Although animal models will never fully mimic real-world exposures and the complex set of demographic, environmental, and social factors that go into a condition like autism, results from animal model studies can contribute valuable information to the body of evidence linking environmental chemicals to autism. In part, this is due to the ability to control the chemicals, doses, and timing of exposure in animal studies. Animal studies can also shed light on biological mechanisms critical to ensuring proper development, such as neurotransmitter and hormone signaling. Understanding these processes as they are related to autism may help identify opportunities for prevention or treatment. Further, for chemicals deemed to be associated with autism, animal studies will likely be necessary to satisfy current regulatory risk assessment methods that set safe levels of exposure (U.S. EPA 1998). Based on the 46 experimental rodent studies identified in this scoping review, we make the following recommendations:
Harmonized efforts.
As the field moves forward, better harmonization between the chemicals that are studied in humans and those studied in animals is recommended. Many chemicals were only studied in one body of evidence or the other. It would be helpful for future animal studies to focus on addressing questions identified by epidemiological studies (e.g., regarding dose, timing, and mechanisms of action) to help identify causal relationships. For example, there are 22 epidemiological studies evaluating over 50 different components of air pollution, yet there are only four rodent studies. More research using rodent models could improve our understanding of causative chemicals in such complex mixtures. In addition, chemicals currently identified only in animal studies (e.g., butyl paraben, diethylhexyl phthalate, dichlorvos, fenvalerate, glufosinate ammonium, isobutyl paraben, propionic acid, and tungsten) should be evaluated for possible inclusion in epidemiological studies to better understand their relevance to humans.
Improved model characterization.
Currently, resources are available that describe how different rodent behavioral assays contribute to the face validity of the model (Roullet and Crawley 2011). However, there has not been, as of yet, clear guidance on which specific behavioral tests or combinations of tests provide the most valid and reliable model of autism. Moving forward, we recommend that studies specifically aiming to model autism in animals should measure both reciprocal social communicative and stereotyped and repetitive behaviors. Measurements of comorbid behaviors and mechanistic outcomes should also be included in future studies in order to more fully characterize the phenotype. Furthermore, the utility of other animal models, including nonhuman primates and voles, should continue to be explored, as they may offer unique similarities to the human condition.
Reviews
We identified 50 reviews, including nine systematic reviews and three meta-analyses. The reviews varied greatly in their depth. None of the systematic reviews or meta-analyses in this field have incorporated evidence from animal models. One of the benefits of systematic review methods designed for environmental health research (Rooney et al. 2014; Woodruff and Sutton 2014) is that they provide specific guidance on how to integrate evidence across human and animal studies, as well as how to incorporate mechanistic evidence. As such, these frameworks allow stronger conclusions to be drawn about hazards posed by environmental chemicals. Other strengths of these systematic review frameworks are that they feature risk of bias evaluations, inclusion of positive and negative findings, and transparent decision-making in order to arrive at the most robust conclusions. Based on our experience conducting this scoping review, we recommend the following:
Future systematic reviews.
There is no clear guidance on what makes a topic ready for systematic review and meta-analysis. Although, technically, a systematic review can be conducted on a minimum of two studies, typically they include more, as they are designed to tease apart more complex research questions in environmental health. In conducting scoping reviews, we base our recommendations for systematic review on the number of studies we identify in the epidemiological and animal literature, the presence or absence of other literature reviews, insight gained during the scoping process, and other contextual factors. As a result of this scoping review of autism, we recommend the following chemicals for systematic review:
Chlorpyrifos: We identified three epidemiological studies and nine experimental rodent studies that investigated the relationship between the pesticide chlorpyrifos and autism. The epidemiological studies (but not the rodent studies) were previously reviewed by Lam et al. (2016), indicating a trend towards a positive association. Notably, chlorpyrifos was one of the few chemicals we reviewed that included rodent studies with behaviors addressing both diagnostic criteria for autism. Chlorpyrifos’s association with autism has not yet been evaluated by meta-analysis. Given current efforts to determine whether U.S. Environmental Protection Agency should regulate chlorpyrifos due to its effects on neurodevelopment (Hertz-Picciotto et al. 2018a; U.S. EPA 2017), we believe a systematic review considering both the epidemiological and experimental rodent evidence is warranted and timely.
Lead: We identified ten epidemiological studies on lead, six of which were related to lead as an air pollutant (reviewed by Lam et al. with mixed results) (Lam et al. 2016). It may be fruitful to review lead again, adding the three epidemiological studies where lead was investigated as a biomarker of exposure (in baby teeth) and the four experimental rodent studies. Although efforts are underway to bring lead exposure to “near zero” levels (Bellinger et al. 2017), understanding specifically whether it contributes to the incidence of autism is an important question to address, as it may have relevance for treatment and prevention efforts.
PCBs: Another potential candidate for systematic review is the group of industrial pollutants, PCBs. We identified eight epidemiological studies and three experimental rodent studies on PCBs. Although they have been largely regulated as hazardous chemicals, whether PCBs contribute to the etiology of autism in humans remains to be determined, as they have not yet been evaluated by systematic review or meta-analysis.
Search terms.
It should be noted that the animal evidence in this scoping review may be underestimated because we did not include search terms for specific behavioral tests such as ultrasonic vocalizations or the three-chamber test. It is likely that these specific tests have been utilized in developmentally exposed animals outside of the context of autism, for example, in studies exploring effects of developmental chemical exposures on social behavior [e.g., Wolstenholme et al. (2011)] or in studies modeling other neurobehavioral disorders. Future reviews would benefit from the refinement of relevant literature search terms for specific animal behavioral tests. To some extent, this may occur as journals begin publishing protocols developed a priori for scoping and systematic reviews, which allows for input on the search strategy during peer review and also provides an opportunity for public review. Toward this end, all of the data evaluated in this scoping review are publicly available in easy-to-use, interactive figures that allow further exploration and analysis of the research that has been conducted to date.
Supplementary Material
Acknowledgments
We gratefully acknowledge H. Patisaul, S. Degroote, A. Halladay, L. Wiederlight, S. Bernard, J. Rochester, and K. Schultz for helpful conversations, K. Rose from Tableau Service Corps for help creating the interactive web browser, and C. Ribbens for help in literature procurement and cataloguing. Funding was provided by the Arkansas Community Foundation, Winslow Foundation, and Tides Foundation.
References
- American Psychiatric Association. 1994. Diagnostic and Statistical Manual of Mental Disorders. 4th ed Arlington, VA:American Psychiatric Publishing, Inc. [Google Scholar]
- American Psychiatric Association. 2013. Diagnostic and Statistical Manual of Mental Disorders. 5th ed Washington, DC:American Psychiatric Association. [Google Scholar]
- Arcury TA, Grzywacz JG, Isom S, Whalley LE, Vallejos QM, Chen H, et al. 2009. Seasonal variation in the measurement of urinary pesticide metabolites among Latino farmworkers in eastern North Carolina. Int J Occup Environ Health 15(4):339–350, PMID: 19886344, 10.1179/oeh.2009.15.4.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Autism and Developmental Disabilities Monitoring Network Surveillance. 2007. Prevalence of autism spectrum disorders–autism and developmental disabilities monitoring network, 14 sites, United States, 2002. MMWR Surveill Summ 56(1):12–28, PMID: 17287715 https://www.cdc.gov/mmwr/preview/mmwrhtml/ss5601a2.htm. [PubMed] [Google Scholar]
- Baio J, Wiggins L, Christensen DL, Maenner MJ, Daniels J, Warren Z, et al. 2018. Prevalence of autism spectrum disorder among children aged 8 years - autism and developmental disabilities monitoring network, 11 sites, United States, 2014. MMWR Surveill Summ 67(6):1–23, PMID: 29701730, 10.15585/mmwr.ss6706a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bal VH, Kim SH, Fok M, Lord C. 2019. Autism spectrum disorder symptoms from ages 2 to 19 years: implications for diagnosing adolescents and young adults. Autism Res 12(1):89–99, PMID: 30101492, 10.1002/aur.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron-Cohen S. 2002. The extreme male brain theory of autism. Trends Cogn Sci 6(6):248–254, PMID: 12039606, 10.1016/S1364-6613(02)01904-6. [DOI] [PubMed] [Google Scholar]
- Beery AK, Kaufer D. 2015. Stress, social behavior, and resilience: insights from rodents. Neurobiol Stress 1:116–127, PMID: 25562050, 10.1016/j.ynstr.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellinger DC, Chen A, Lanphear BP. 2017. Establishing and achieving national goals for preventing lead toxicity and exposure in children. JAMA Pediatr 171(7):616–618, PMID: 28505218, 10.1001/jamapediatrics.2017.0775. [DOI] [PubMed] [Google Scholar]
- Bey AL, Jiang YH. 2014. Overview of mouse models of autism spectrum disorders. Curr Protoc Pharmacol 66(1):5.66.1–5.66.26, PMID: 25181011, 10.1002/0471141755.ph0566s66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun JM, Kalloo G, Chen A, Dietrich KN, Liddy-Hicks S, Morgan S, et al. 2017. Cohort profile: the Health Outcomes and Measures of the Environment (HOME) study. Int J Epidemiol 46(1):24, PMID: 27006352, 10.1093/ije/dyw006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buescher AV, Cidav Z, Knapp M, Mandell DS. 2014. Costs of autism spectrum disorders in the United Kingdom and the United States. JAMA Pediatr 168(8):721–728, PMID: 24911948, 10.1001/jamapediatrics.2014.210. [DOI] [PubMed] [Google Scholar]
- Carter CJ, Blizard RA. 2016. Autism genes are selectively targeted by environmental pollutants including pesticides, heavy metals, bisphenol A, phthalates and many others in food, cosmetics or household products. Neurochem Int 101:83–109, PMID: 27984170, 10.1016/j.neuint.2016.10.011. [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention. 2013. International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM). http://www.cdc.gov/nchs/icd/icd9cm.htm [accessed 6 February 2019].
- Centers for Disease Control and Prevention. 2017a. Facts About Autism Spectrum Disorder (ASD). https://www.cdc.gov/ncbddd/autism/facts.html [accessed 23 August 2018].
- Centers for Disease Control and Prevention. 2017b. Data & Statistics on Autism Spectrum Disorder. https://www.cdc.gov/ncbddd/autism/data.html [accessed 20 December 2017].
- Chang YC, Cole TB, Costa LG. 2017. Behavioral phenotyping for autism spectrum disorders in mice. Curr Protoc Toxicol 72(1):11.22.1–11.22.21, PMID: 28463420, 10.1002/cptx.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Children's Hospital of Philadelphia. 2016. Diagnosis. https://www.carautismroadmap.org/category/diagnosis/ [accessed 24 May 2018].
- Christensen DL, Bilder DA, Zahorodny W, Pettygrove S, Durkin MS, Fitzgerald RT, et al. 2016a. Prevalence and characteristics of autism spectrum disorder among 4-year-old children in the autism and developmental disabilities monitoring network. J Dev Behav Pediatr 37(1):1–8, 10.1097/DBP.0000000000000235. [DOI] [PubMed] [Google Scholar]
- Christensen DL, Braun KVN, Baio J, Bilder D, Charles J, Constantino JN, et al. 2016b. Prevalence and characteristics of autism spectrum disorder among children aged 8 years–autism and developmental disabilities monitoring network, 11 sites, United States, 2012. MMWR Surveill Summ 65(13):1–23, PMID: 30439868, 10.15585/mmwr.ss6513a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimino AM, Boyles AL, Thayer KA, Perry MJ. 2016. Effects of neonicotinoid pesticide exposure on human health: a systematic review. Environ Health Perspect 125(2):155–162, PMID: 27385285, 10.1289/EHP515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clancy B, Finlay BL, Darlington RB, Anand KJ. 2007. Extrapolating brain development from experimental species to humans. Neurotoxicology 28(5):931–937, PMID: 17368774, 10.1016/j.neuro.2007.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN. 2012. Translational animal models of autism and neurodevelopmental disorders. Dialogues Clin Neurosci 14(3):293–305, PMID: 23226954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Felice A, Greco A, Calamandrei G, Minghetti L. 2016. Prenatal exposure to the organophosphate insecticide chlorpyrifos enhances brain oxidative stress and prostaglandin E2 synthesis in a mouse model of idiopathic autism. J Neuroinflammation 13(1):149, PMID: 27301868, 10.1186/s12974-016-0617-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenbroek B, Youn J. 2016. Rodent models in neuroscience research: is it a rat race? Dis Model Mech 9(10):1079–1087, PMID: 27736744, 10.1242/dmm.026120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkmer T, Anderson K, Falkmer M, Horlin C. 2013. Diagnostic procedures in autism spectrum disorders: a systematic literature review. Eur Child Adolesc Psychiatry 22(6):329–340, PMID: 23322184, 10.1007/s00787-013-0375-0. [DOI] [PubMed] [Google Scholar]
- Flores-Pajot MC, Ofner M, Do MT, Lavigne E, Villeneuve PJ. 2016. Childhood autism spectrum disorders and exposure to nitrogen dioxide, and particulate matter air pollution: a review and meta-analysis. Environ Res 151:763–776, PMID: 27609410, 10.1016/j.envres.2016.07.030. [DOI] [PubMed] [Google Scholar]
- Fluegge K. 2016. Does environmental exposure to the greenhouse gas, N2O, contribute to etiological factors in neurodevelopmental disorders? A mini-review of the evidence. Environ Toxicol Pharmacol 47:6–18, PMID: 27566494, 10.1016/j.etap.2016.08.013. [DOI] [PubMed] [Google Scholar]
- Fordyce TA, Leonhard MJ, Chang ET. 2018. A critical review of developmental exposure to particulate matter, autism spectrum disorder, and attention deficit hyperactivity disorder. J Environ Sci Health A Tox Hazard Subst Environ Eng 53(2):174–204, PMID: 29157090, 10.1080/10934529.2017.1383121. [DOI] [PubMed] [Google Scholar]
- Frazier TW, Thompson L, Youngstrom EA, Law P, Hardan AY, Eng C, et al. 2014. A twin study of heritable and shared environmental contributions to autism. J Autism Dev Disord 44(8):2013–2025, PMID: 24604525, 10.1007/s10803-014-2081-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong T, Dalman C, Wicks S, Dal H, Magnusson C, Lundholm C, et al. 2017. Perinatal exposure to traffic-related air pollution and autism spectrum disorders. Environ Health Perspect 125(1):119–126, PMID: 27494442, 10.1289/EHP118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gopalakrishnan S, Ganeshkumar P. 2013. Systematic reviews and meta-analysis: understanding the best evidence in primary healthcare. J Family Med Prim Care 2(1):9–14, PMID: 24479036, 10.4103/2249-4863.109934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grzadzinski R, Dick C, Lord C, Bishop S. 2016. Parent-reported and clinician-observed autism spectrum disorder (ASD) symptoms in children with attention deficit/hyperactivity disorder (ADHD): implications for practice under DSM-5. Mol Autism 7:7, PMID: 26788284, 10.1186/s13229-016-0072-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haddaway NR, Macura B, Whaley P, Pullin AS. 2017. ROSES: Reporting standards for systematic evidence syntheses. https://www.roses-reporting.com/ [accessed 11 February 2019].
- Hallmayer J, Cleveland S, Torres A, Phillips J, Cohen B, Torigoe T, et al. 2011. Genetic heritability and shared environmental factors among twin pairs with autism. Arch Gen Psychiatry 68(11):1095–1102, PMID: 21727249, 10.1001/archgenpsychiatry.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Croen LA, Hansen R, Jones CR, van de Water J, Pessah IN. 2006. The CHARGE study: an epidemiologic investigation of genetic and environmental factors contributing to autism. Environ Health Perspect 114(7):1119–1125, PMID: 16835068, 10.1289/ehp.8483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Delwiche L. 2009. The rise in autism and the role of age at diagnosis. Epidemiology 20(1):84–90, PMID: 19234401, 10.1097/EDE.0b013e3181902d15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Sass JB, Engel S, Bennett DH, Bradman A, Eskenazi B, et al. 2018a. Organophosphate exposures during pregnancy and child neurodevelopment: recommendations for essential policy reforms. PLoS Med 15(10):e1002671, PMID: 30356230, 10.1371/journal.pmed.1002671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertz-Picciotto I, Schmidt RJ, Krakowiak P. 2018b. Understanding environmental contributions to autism: causal concepts and the state of science. Autism Res 11(4):554–586, PMID: 29573218, 10.1002/aur.1938. [DOI] [PubMed] [Google Scholar]
- Heyer DB, Meredith RM. 2017. Environmental toxicology: sensitive periods of development and neurodevelopmental disorders. Neurotoxicology 58:23–41, PMID: 27825840, 10.1016/j.neuro.2016.10.017. [DOI] [PubMed] [Google Scholar]
- Hoffman K, Weisskopf MG, Roberts AL, Raz R, Hart JE, Lyall K, et al. 2017. Geographic patterns of autism spectrum disorder among children of participants in Nurses' Health Study II. Am J Epidemiol 186(7):834–842, PMID: 28525627, 10.1093/aje/kwx158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hommer RE, Swedo SE. 2015. Schizophrenia and autism-related disorders. Schizophr Bull 41(2):313–314, PMID: 25634913, 10.1093/schbul/sbu188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeddi MZ, Janani L, Memari AH, Akhondzadeh S, Yunesian M. 2016. The role of phthalate esters in autism development: a systematic review. Environ Res 151:493–504, PMID: 27567353, 10.1016/j.envres.2016.08.021. [DOI] [PubMed] [Google Scholar]
- Kalkbrenner AE, Windham GC, Serre ML, Akita Y, Wang X, Hoffman K, et al. 2014. Particulate matter exposure, prenatal and postnatal windows of susceptibility, and autism spectrum disorders. Epidemiology 26(1):30–42, PMID: 25286049, 10.1097/EDE.0000000000000173. [DOI] [PubMed] [Google Scholar]
- King M, Bearman P. 2009. Diagnostic change and the increased prevalence of autism. Int J Epidemiol 38(5):1224–1234, PMID: 19737791, 10.1093/ije/dyp261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lam J, Sutton P, Kalkbrenner A, Windham G, Halladay A, Koustas E, et al. 2016. A systematic review and meta-analysis of multiple airborne pollutants and autism spectrum disorder. PloS One 11(9):e0161851, PMID: 27653281, 10.1371/journal.pone.0161851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lavelle TA, Weinstein MC, Newhouse JP, Munir K, Kuhlthau KA, Prosser LA. 2014. Economic burden of childhood autism spectrum disorders. Pediatrics 133(3):e520–e529, PMID: 24515505, 10.1542/peds.2013-0763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis MH, Tanimura Y, Lee LW, Bodfish JW. 2007. Animal models of restricted repetitive behavior in autism. Behav Brain Res 176(1):66–74, PMID: 16997392, 10.1016/j.bbr.2006.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Ma H, Lydy MJ, You J. 2014. Occurrence, seasonal variation and inhalation exposure of atmospheric organophosphate and pyrethroid pesticides in an urban community in South China. Chemosphere 95:363–369, PMID: 24125706, 10.1016/j.chemosphere.2013.09.046. [DOI] [PubMed] [Google Scholar]
- Lyall K, Croen L, Daniels J, Fallin MD, Ladd-Acosta C, Lee BK, et al. 2017. The changing epidemiology of autism spectrum disorders. Annu Rev Public Health 38:81–102, PMID: 28068486, 10.1146/annurev-publhealth-031816-044318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandrioli D, Silbergeld EK. 2016. Evidence from toxicology: the most essential science for prevention. Environ Health Perspect 124(1):6–11, PMID: 26091173, 10.1289/ehp.1509880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDermott S, Salzberg DC, Anderson AP, Shaw T, Lead J. 2015. Systematic review of chromium and nickel exposure during pregnancy and impact on child outcomes. J Toxicol Environ Health A 78(21–22):1348–1368, PMID: 26571332, 10.1080/15287394.2015.1090939. [DOI] [PubMed] [Google Scholar]
- Moosa A, Shu H, Sarachana T, Hu VW. 2018. Are endocrine disrupting compounds environmental risk factors for autism spectrum disorder? Horm Behav 101:13–21, PMID: 29042182, 10.1016/j.yhbeh.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales-Suárez-Varela M, Peraita-Costa I, Llopis-González A. 2017. Systematic review of the association between particulate matter exposure and autism spectrum disorders. Environ Res 153:150–160, PMID: 27984759, 10.1016/j.envres.2016.11.022. [DOI] [PubMed] [Google Scholar]
- Ng M, de Montigny JG, Ofner M, Do MT. 2017. Environmental factors associated with autism spectrum disorder: a scoping review for the years 2003–2013. Health Promot Chronic Dis Prev Can 37(1):1–23, PMID: 28102992, 10.24095/hpcdp.37.1.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patisaul HB, Fenton SE, Aylor D. 2018. Animal models of endocrine disruption. Best Practice & Best Pract Res Clin Endocrinol Metab 32(3):283–297, PMID: 29779582, 10.1016/j.beem.2018.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presti MF, Powell SB, Lewis MH. 2002. Dissociation between spontaneously emitted and apomorphine-induced stereotypy in Peromyscus maniculatus bairdii. Physiol Behav 75(3):347–353, PMID: 11897261, 10.1016/S0031-9384(02)00641-8. [DOI] [PubMed] [Google Scholar]
- Randall M, Egberts KJ, Samtani A, Scholten R, Hooft L, Livingstone N, et al. 2018. Diagnostic tests for autism spectrum disorder (ASD) in preschool children. Cochrane Database Syst Rev 7:CD009044, PMID: 30075057, 10.1002/14651858.CD009044.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rooney AA, Boyles AL, Wolfe MS, Bucher JR, Thayer KA. 2014. Systematic review and evidence integration for literature-based environmental health science assessments. Environ Health Perspect 122(7):711–718, PMID: 24755067, 10.1289/ehp.1307972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossignol DA, Genuis SJ, Frye RE. 2014. Environmental toxicants and autism spectrum disorders: a systematic review. Transl Psychiatry 4:e360, PMID: 24518398, 10.1038/tp.2014.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roullet FI, Crawley JN. 2011. Mouse models of autism: testing hypotheses about molecular mechanisms. Curr Top Behav Neurosci 7:187–212, PMID: 21225409, 10.1007/7854_2010_113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadowski RN, Wise LM, Park PY, Schantz SL, Juraska JM. 2014. Early exposure to bisphenol A alters neuron and glia number in the rat prefrontal cortex of adult males, but not females. Neuroscience 279:122–131, PMID: 25193849, 10.1016/j.neuroscience.2014.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartzer JJ, Koenig CM, Berman RF. 2013. Using mouse models of autism spectrum disorders to study the neurotoxicology of gene-environment interactions. Neurotoxicol Teratol 36:17–35, PMID: 23010509, 10.1016/j.ntt.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spearow JL, Doemeny P, Sera R, Leffler R, Barkley M. 1999. Genetic variation in susceptibility to endocrine disruption by estrogen in mice. Science 285(5431):1259–1261, PMID: 10455051, 10.1126/science.285.5431.1259. [DOI] [PubMed] [Google Scholar]
- Teatero ML, Netley C. 2013. A critical review of the research on the extreme male brain theory and digit ratio (2D:4D). J Autism Dev Disord 43(11):2664–2676, PMID: 23575643, 10.1007/s10803-013-1819-6. [DOI] [PubMed] [Google Scholar]
- Thayer KA, Doerge DR, Hunt D, Schurman SH, Twaddle NC, Churchwell MI, et al. 2015. Pharmacokinetics of bisphenol A in humans following a single oral administration. Environ Int 83:107–115, PMID: 26115537, 10.1016/j.envint.2015.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner MC, Vineis P, Seleiro E, Dijmarescu M, Balshaw D, Bertollini R, et al. 2018. EXPOsOMICS: final policy workshop and stakeholder consultation. BMC Public Health 18(1):260, PMID: 29448939, 10.1186/s12889-018-5160-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. EPA (Environmental Protection Agency). 1998. Guidelines for Neurotoxicity Risk Assessment. EPA/630/R-95/001F Washington, DC:United States Environmental Protection Agency. [Google Scholar]
- U.S. EPA. 2016. About the TSCA Chemical Substance Inventory. https://www.epa.gov/tsca-inventory/about-tsca-chemical-substance-inventory [accessed 11 February 2019].
- U.S. EPA. 2017. Revised Human Health Risk Assessment on Chlorpyrifos. https://www.epa.gov/ingredients-used-pesticide-products/revised-human-health-risk-assessment-chlorpyrifos [accessed 12 February 2019].
- Venerosi A, Calamandrei G, Ricceri L. 2006. A social recognition test for female mice reveals behavioral effects of developmental chlorpyrifos exposure. Neurotoxicol Teratol 28(4):466–471, PMID: 16814983, 10.1016/j.ntt.2006.05.003. [DOI] [PubMed] [Google Scholar]
- Vineis P, Chadeau-Hyam M, Gmuender H, Gulliver J, Herceg Z, Kleinjans J, et al. 2017. The exposome in practice: design of the EXPOsOMICS project. Int J Hyg Environ Health 220(2 Pt A):142–151, PMID: 27576363, 10.1016/j.ijheh.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Ehrenstein OS, Aralis H, Cockburn M, Ritz B. 2014. In utero exposure to toxic air pollutants and risk of childhood autism. Epidemiology 25(6):851–858, 10.1097/EDE.0000000000000150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson KK, Platt ML. 2012. Of mice and monkeys: using non-human primate models to bridge mouse- and human-based investigations of autism spectrum disorders. J Neurodev Disord 4(1):21, PMID: 22958282, 10.1186/1866-1955-4-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO (World Health Organization). 2016. International Statistical Classification of Diseases and Related Health Problems, 10th Revision. http://apps.who.int/classifications/icd10/browse/2016/en [accessed 6 February 2019].
- Wolstenholme JT, Taylor JA, Shetty SR, Edwards M, Connelly JJ, Rissman EF. 2011. Gestational exposure to low dose bisphenol A alters social behavior in juvenile mice. PLoS One 6(9):e25448, PMID: 21980460, 10.1371/journal.pone.0025448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff TJ, Sutton P. 2014. The Navigation Guide systematic review methodology: a rigorous and transparent method for translating environmental health science into better health outcomes. Environ Health Perspect 122(10):1007–1014, PMID: 24968373, 10.1289/ehp.1307175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman AD, Charvet CJ, Clancy B, Darlington RB, Finlay BL. 2013. Modeling transformations of neurodevelopmental sequences across mammalian species. J Neurosci 33(17):7368–7383, PMID: 23616543, 10.1523/JNEUROSCI.5746-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye BS, Leung AOW, Wong MH. 2017. The association of environmental toxicants and autism spectrum disorders in children. Environ Pollut 227:234–242, 10.1016/j.envpol.2017.04.039. [DOI] [PubMed] [Google Scholar]
- Ye X, Wong LY, Bishop AM, Calafat AM. 2011. Variability of urinary concentrations of bisphenol A in spot samples, first morning voids, and 24-hour collections. Environ Health Perspect 119(7):983–988, PMID: 21406337, 10.1289/ehp.1002701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshimasu K, Kiyohara C, Takemura S, Nakai K. 2014. A meta-analysis of the evidence on the impact of prenatal and early infancy exposures to mercury on autism and attention deficit/hyperactivity disorder in the childhood. Neurotoxicology 44:121–131, PMID: 24952233, 10.1016/j.neuro.2014.06.007. [DOI] [PubMed] [Google Scholar]
- Zablotsky B, BL I, Blumberg SJ. 2017. Estimated prevalence of children with diagnosed developmental disabilities in the United States, 2014–2016. NCHS Data Brief (291):1–8. https://www.cdc.gov/nchs/products/databriefs/db291.htm. [PubMed] [Google Scholar]
- Zaidi NF, Agrawal AK, Srivastava SP, Seth PK. 1985. Effect of gestational and neonatal styrene exposure on dopamine receptors. Neurobehav Toxicol Teratol 7(1):23–28, PMID: 4039799. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.