Paleolithic cave dwellers in Israel consumed “canned food” some 400,000 years ago, demonstrating advanced planning skills.
Abstract
Bone marrow and grease constitute an important source of nutrition and have attracted the attention of human groups since prehistoric times. Marrow consumption has been linked to immediate consumption following the procurement and removal of soft tissues. Here, we present the earliest evidence for storage and delayed consumption of bone marrow at Qesem Cave, Israel (~420 to 200 ka). By using experimental series controlling exposure time and environmental parameters, combined with chemical analyses, we evaluated bone marrow preservation. The combination of archaeological and experimental results allowed us to isolate specific marks linked to dry skin removal and determine a low rate of marrow fat degradation of up to 9 weeks of exposure. This is the earliest evidence of such previously unidentified behavior, and it offers insights into the socio-economy of the human groups who lived at Qesem and may mark a threshold to new modes of Palaeolithic human adaptation.
INTRODUCTION
Animal fat constitutes a significant source for human nutrition [e.g., (1, 2)]. Its calorific value is much higher than that of protein or carbohydrates; therefore, fat sources are of special significance to communities who are dependent almost exclusively on animal products with little or no source of carbohydrates (3, 4).
The significance of bone marrow and grease is further highlighted by the fact that bone fat contains a higher quality of fat [greater percentage of fatty acids (FAs)] than that found in the rest of an animal carcass (2). The mandible and most appendicular elements contain medullary cavities filled with marrow. This soft tissue can be removed by cracking the bone with heavy hammers and extracting it by hand, by using an implement, or by sucking. Fat can also be recovered from within spongy, cancellous bone, which makes up much of the axial skeleton and appendicular epiphyses. This is often referred to as bone grease. Unlike bone marrow, bone grease extraction requires major efforts. Ethnographic data indicate that the cancellous portion of the bone must be broken into small fragments, destroying the structure of the trabecular bone so the fragments can be boiled. Upon cooling, the grease hardens and can be removed mechanically (4, 5). Given the relatively low nutritional yield of bone grease in relation to its extraction costs, it has been argued that grease rendering represents a significant form of resource intensification [(6), but see also Baker (5), who argues that grease rendering is not always related to stress].
Many studies have focused on documenting the processing of bone grease and its detection in the fossil record [e.g., (7, 8)], but the possibility of its preservation in archaeological sites of early prehistoric periods remains practically unexplored. Perhaps the best ethnographic data on delayed consumption of bone grease is from historic time cultures of the Great Plains, actively involved in the production of pemmican, a substance composed of dried meat and fat (5). This product had a high nutritional value and could be stored for up to 3 years. Pemmican was often produced in concert with the large fall harvest and the processing of bison, and it was critical for survival during the winter months. Although ethnographic accounts refer to the production of pemmican using both bone marrow and grease, the data point to the fact that the production of bone grease was particularly valued because of its high quality in terms of essential FA content (5).
One thought-provoking noteworthy case relates to the Nunamiut Eskimo communities. Binford (1) reported that bones were often stored throughout the winter months to be processed in large batches for grease and marrow consumption. From a microbiological perspective, marrow could be relatively safe compared to meat because the bone casing offers protection from microbes, although bacteria injected into the circulatory system could, in theory, enter the bone through the nutrient artery (9). At this point, we wondered whether the storage of certain bones for delayed marrow consumption may leave sufficiently specific and recognizable taphonomic signatures in the archaeological record and whether the unique damage patterns on fallow deer bones that we observed at Qesem Cave, Israel [420 to 200 thousand years (ka)] were related to such an option. If the answer was positive, then the question would be for how long would such storage allow marrow preservation in good consumable condition in various environments. In this study, we try to answer these questions based on the fact that specific butchery techniques may provide archaeologically identifiable signatures of the exploitation of particular types of fat. Thus, our efforts here focus on exploring the role that specific nutrients—in this case, bone marrow—play in food preservation and storage during the Middle Pleistocene human occupation site of Qesem Cave. The results provide the first archaeological and experimental evidence supporting the significant role preservation and delayed consumption of food resources have had in Middle Pleistocene times. Our study has relevant implications for the economic, social, and cognitive transformations that occurred in the Middle Pleistocene Levant, which, in turn, set the stage for a new mode of human adaptation that followed during later stages of the Pleistocene.
RESULTS
Qesem faunal assemblages
A total of 81,898 faunal specimens [number of specimens (NSP)] were analyzed: 59,681 from Amudian, blade-dominated contexts and 22,217 from Yabrudian, scraper-dominated contexts. Among the total faunal fragments recovered, only 8.46% were taxonomically identifiable because of the high degree of fragmentation; most of the bones analyzed were less than 20 mm long, with percentages ranging from 65.6% from the sediments close to the wall of the cave to 92.9% in the south-western area. In addition, most of the shafts showed less than one-quarter of their original circumference, especially in the case of the Amudian contexts (NSP = 9447 of 10,875 long bone fragments more than 20 mm in length, 86.9%). The bone breakage analysis indicates that longitudinal fractures (n = 12,683 of 31,118 breakage planes analyzed; 41%), oblique angles (n = 12,417, 40%), and smooth edges (n = 25,245, 81%) are predominant across the sequence, coinciding with a green fracture of most long bones of more than 20 mm in length. In the case of deer metapodials, we also found a major presence of longitudinal planes (n = 1597 of 3516, 45%), oblique angles (n = 1403; 40%), and smooth edges (n = 2825, 80%), and 93.9% of shafts with less than two surfaces were represented.
The faunal assemblages consist of 14 taxa, including ungulates, birds, tortoises, and, very sporadically, carnivores (cf. Hyaenidae). Fallow deer (Dama cf. mesopotamica) is the main taxa in all layers, with [number of identified specimens (NISP)] percentages of representation between 75.8 and 79% (Table 1). The %MAU (minimum animal units) indicates a biased skeletal representation characterized by a predominance of mandibles, stylopodials, zeugopodials, and metapodials and a low representation of axial bones (vertebrae and ribs), pelvises, and phalanges. This fact is particularly conspicuous for size class 2 (small-sized animals such as Dama cf. mesopotamica) and size class 3 (medium-sized animals such as Cervus cf. elaphus). Size class 4 (large-sized ungulates such as Bos primigenius or Equus ferus) differ in the metapodial quantities, showing a considerably lower representation, or in some cases, a total absence (Fig. 1). Because of this significant bias in anatomical profiles, the assemblages were tested in a first stage for possible differential bone destruction. The correlation between %MAU and bone mineral density points to a weak linear correlation for size class 3 (rs = 0.487, P = 0.066) and no significant correlation for size classes 2 and 4 (rs = 0.170, P = 0.545 and rs = 0.063, P = 0.824, respectively), indicating a minimal impact of the destructive processes associated with mineral density but providing no major explanation for the anatomical profile recorded at the site. The %MAU was subsequently correlated with the utility index (UI) (10) and the unsaturated marrow index (UMI) (11), showing that ungulate body part representation at Qesem correlates positively with the UI bone marrow (large-sized, rs = 0.588, P = 0.271; medium-sized, rs = 0.788, P = 0.0008; and small-sized, rs = 0.748, P = 0.0021) (Table 2) and the UMI (large-sized, rs = 0.6695, P = 0.049; medium-sized, rs = 0.711, P = 0.032; and small-sized, rs = 0.798, P = 0.001).
Table 1. NSP, NISP, MNE, MNI, and bone damage from Amudian and Yabrudian archaeological contexts of Qesem Cave.
Ctm, cut marks; BBr, bone breakage (only diagnostic elements included); Burn, burnt bones; MNI, minimum number of individuals; MNE, minimum number of elements.
| Taxa/size body class | Amudian | Yabrudian | ||||||||||||
| n | n | |||||||||||||
| NSP | NISP | MNE | MNI | Ctm | BBr | Burn | NSP | NISP | MNE | MNI | Ctm | BBr | Burn | |
| Carnivora | 2 | 2 | 2 | 2 | 2 | 10 | 10 | 7 | 1 | 3 | 1 | 3 | ||
| Stephanorhinus hemitoechus | 20 | 20 | 8 | 6 | 19 | 19 | 4 | 3 | 1 | |||||
| Equus ferus | 125 | 125 | 30 | 11 | 1 | 26 | 19 | 19 | 9 | 5 | 4 | |||
| Equus hydruntinus | 18 | 18 | 10 | 3 | 1 | 4 | ||||||||
| Sus scrofa | 56 | 56 | 18 | 9 | 1 | 4 | 21 | 21 | 11 | 4 | 4 | |||
| Cervidae | 30 | 30 | 15 | 2 | 2 | 10 | ||||||||
| Dama cf. mesopotamica | 4033 | 4033 | 2018 | 76 | 458 | 186 | 1129 | 1387 | 1387 | 468 | 35 | 139 | 51 | 473 |
| Cervus cf. elaphus | 380 | 380 | 158 | 17 | 32 | 13 | 100 | 160 | 160 | 61 | 9 | 14 | 5 | 41 |
| Bos primigenius | 220 | 220 | 45 | 18 | 2 | 1 | 18 | 65 | 65 | 16 | 9 | 7 | ||
| Capra aegagrus | 9 | 9 | 8 | 4 | 1 | 13 | 13 | 9 | 3 | 2 | 3 | |||
| cf. Capreolus capreolus | 36 | 36 | 13 | 5 | 2 | 2 | 28 | 28 | 18 | 5 | 1 | 9 | ||
| Testudo sp. | 165 | 165 | 33 | 14 | 10 | 2 | 60 | 106 | 106 | 80 | 10 | 3 | 1 | 34 |
| Large bird | 2 | 2 | 2 | 1 | 1 | 1 | ||||||||
| Cygnus sp. | 1 | 1 | 1 | 1 | 1 | 1 | ||||||||
| Corvus ruficollis | 3 | 3 | 3 | 1 | 1 | |||||||||
| Columba sp. | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | |||||
| Aves, unident. | 2 | 2 | 2 | 2 | ||||||||||
| Very large size | 4 | 1 | 1 | 23 | 6 | 8 | ||||||||
| Large size | 3322 | 64 | 63 | 71 | 1014 | 1420 | 24 | 21 | 33 | 622 | ||||
| Medium size | 9295 | 122 | 133 | 220 | 3719 | 1940 | 42 | 49 | 65 | 797 | ||||
| Small size | 38985 | 439 | 379 | 472 | 12041 | 15577 | 194 | 139 | 112 | 5684 | ||||
| Unident. | 2972 | 2 | 19 | 816 | 1428 | 1 | 4 | 598 | ||||||
| Total | 59681 | 5103 | 2993 | 173 | 1090 | 985 | 18948 | 22217 | 1829 | 950 | 85 | 372 | 272 | 8288 |
Fig. 1. %MAU distribution by skeletal element and weight size categories split by archaeological contexts (Amudian and Yabrudian).

Size classes 5 [very large (<1000 kg)] and 1a [very small (<20 kg)] were excluded, as their low number of elements could lead to distorted outcomes.
Table 2. General utility rate grouped by body size classes for Qesem Cave faunal assemblages.
| Utility rate* | Amudian | Yabrudian | |||||
| Large size | Medium size | Small size | Large size | Medium size | Small size | ||
| General utility | rs | 0.31602 | 0.07481 | −0.03080 | 0.22898 | −0.07481 | −0.15198 |
| P value | 0.27100 | 0.79940 | 0.91670 | 0.43100 | 0.79940 | 0.60400 | |
| Food utility | rs | 0.19006 | −0.04180 | −0.09461 | 0.05642 | −0.20022 | −0.24559 |
| P value | 0.51520 | 0.88720 | 0.74770 | 0.84810 | 0.49250 | 0.39740 | |
| Bone fat | rs | 0.11934 | −0.12981 | −0.14301 | −0.06084 | −0.22662 | −0.23238 |
| P value | 0.68450 | 0.56830 | 0.62570 | 0.83630 | 0.43590 | 0.42400 | |
| Bone marrow | rs | 0.58758 | 0.78809 | 0.74835 | 0.62375 | 0.53201 | 0.69172 |
| P value | 0.02713 | 0.00081 | 0.00208 | 0.01714 | 0.05020 | 0.00613 | |
*Data taken from Emerson (10).
All the Qesem assemblages included damage caused during anthropogenic bone breakage [e.g., (12)]. Long bone shafts showed a higher proportion of alterations than metaphyses and/or flat bones (NSP = 739, 58.8%). Bone surface damage comprised percussion pits (n = 33; 2.5%), notches (n = 333, 25.2%), impact flakes (n = 888, 67.2%; cortical flakes and scars included), counterblows (n = 16, 1.2%), and peeling (n = 11, 0.8%). In the case of metapodials, 53 specimens showed intentional bone breakage (Amudian, n = 19; Yabrudian, n = 34), and notches were the dominant damage observed (n = 34, 64.1%). Metapodials exhibited blows with a preference to the lateral/medial sides of the shafts (only 11.7% showed impact points on the dorsal and palmar sides).
Regarding cut marks, most were documented on limb bones (n = 1273, 87.1%), with a slightly higher proportion on intermediate appendicular bones (tibia and radius) from Yabrudian layers (43.9%); 80% of the cut marks were on shafts, and only 19.9% were on portions near or on the epiphysis. These frequencies and their distributions on “hot zones” have been related to early access to the fleshed carcasses [e.g., (13)]. In the case of cervid cut-marked metapodials (n = 195, 12.4%), we found a double pattern with similar proportions between the marks that appeared on the metaphyses/proximal epiphyses and the diaphyses. Most of the metapodials registered cut marks on the diaphysis and on the proximal epiphysis (and metaphysis); however, the type of marks varied considerably depending on the anatomical portion and the side (Fig. 2). Proximal epiphyses and metaphyses showed slicing and sawing marks with straight delineation and transverse orientation (n = 73; fig. S1), while the diaphyses bore oblique slicing marks on their medial and lateral sides (n = 49, 37.9%). These, in turn, contrasted with the marks located on the anterior and posterior sides of the diaphyses, representing very different morphologies from the classic incisions, with shapes similar to cortical scars and chop marks (n = 15, 19.5% of cut-marked anterior/posterior shafts) sometimes combined with short, parallel incisions and sawing marks (n = 75, 58.1%) (Fig. 3). If we look at these “atypical” marks in detail, we can see that the direction of the cut or blow is usually oblique, with an inclination almost parallel to the bone. Following the same trend observed in the epiphyses and proximal metaphyses of the metapodials, 43.42% of carpals and tarsals also had transverse and oblique incisions on one or two lateral sides (fig. S1).
Fig. 2. Bar diagrams showing data on cut mark type, orientation and length in Qesem Cave, and experimental samples.
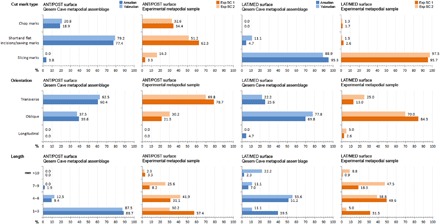
Note that only data from metapodial shafts are shown. Percentages were calculated relative to the total number of cut marks per bone surface [anterior (ANT)/posterior (POST) and lateral (LAT)/medial (MED)].
Fig. 3. Archaeological (Qesem Cave) and experimental [outdoor scenarios (SC 1 and SC 2)] damage on metapodials.

Chop marks, cortical scars, and chipped marks on the anterior (C and G) and posterior (A, B, D, E, and F) surface of metapodial shafts. Note the short and slight chop marks combined with flat incisions/sawing marks in (F) and the inclination angle in the mark section almost parallel to the bone on posterior surfaces of metapodials in (A), (F), and (G). Experimental bones in the image are labeled as “EXP” followed by the abbreviation of scenario (SC 1 or SC 2) and exposure week. The 3D images and details were generated by a KH-8700 3D digital microscope. Photo credits: R. Blasco.
Experimental series
In the experimental series, we controlled both bone exposure time and environmental parameters using three different scenarios [two outdoors (scenarios 1 and 2) and one indoor (scenario 3)] applied to red deer (C. elaphus) metapodial bones. The objectives were to evaluate the preservation of bone marrow encapsulated in the metapodials after a period (up to 9 weeks) of subaerial exposure, determine by chemical analysis from which point in time its value would cease to be nutritionally attractive, and, lastly, detect the taphonomic signature of the secondary (post-storage) processing of the bones for marrow extraction (see the “Experimental approach” section in Materials and Methods for details).
A total of 273 fragments corresponding to 37 metapodials of the outdoor experimental series (scenarios 1 and 2) were analyzed. Before the start of the experiment, we recorded the cut marks inflicted by rangers using modern steel knives when separating the metapodials from the rest of the carcass. These marks were observed on the basipodials (in the metapodials that conserved them, e.g., second week of scenario 1) and/or on the proximal epiphyses/metaphyses. In total, 18 metapodials showed disarticulation marks with straight delineation and transverse orientation. In 44.4% of the cases, this damage covered more than one side of the bone.
Skinning metapodials was carried out following each week of exposure and resulted in different types of marks. Short incisions, both shallow and deep incisions (n = 197, 65.9%), and short sawing marks (n = 64, 21.4%) were identified. Chops and chipped marks were detected sporadically from the second week of exposure and systematically from the seventh week in scenario 1. These marks were not abundant (n = 38, 12.7%), although they were recorded on both the anterior and posterior sides in 92.1% of cases. This type of damage differs from that documented in other experimental works in which the extraction of skin and tendons was performed in fresh state, producing short, transverse, and deep cut marks, as well as long longitudinal marks on the grooves of metapodials [e.g., (14)]. It is worth noting that from the fourth week, the number of cut marks (incisions and sawing) increased considerably, and inclinations in the sections of the marks started to appear, representing transversal use of the tool with an inclination almost parallel to the bone (n = 44 bone fragments showed cut marks representing parallel or almost parallel inclinations, 68.7%) (Fig. 3 and figs. S2 and S3). These occurred when the experimenter vertically or horizontally placed the metapodial to make it easier to remove the skin and tendon.
The tendons and skin were removed together on all occasions, especially after the third week when the skin was dry and began to bind more strongly to the rest of the tissues. On these occasions, cuts were made on one end of the tendon, and once the skin and tendon were slightly separated from the bone, both tissues were pulled strongly by hand in the opposite direction, combining this action with cuts to help detach the skin. The result was an increase in marks with parallel inclination. This differs from the removal of the tendon during the first week, performed with one cut in the proximal portion and another in the distal portion, which helped to completely detach it from the bone in the two outdoor series (fig. S3).
Only two fragments with scraping marks were recorded in the fifth week of scenario 1, and these were linked to specific movements of the butcher to accelerate the skinning process. Oblique slicing marks on the medial and lateral sides of the diaphyses were only registered in the first week.
In scenario 3 (indoor), no processing of the bones was performed, since this series only aimed to analyze the sequence of marrow degradation in a similar environment to that of Israel. It is important to note that the skinless metapodials had marrow that was more gelatinous, harder, and pinker than those exposed with skin, which had a more liquid yellowish marrow.
After the skinning in scenarios 1 and 2, the metapodials were broken to extract the marrow by hammerstone percussion (fig. S3). This generated percussion notches (n = 15, 5.5%) and impact flakes (n = 19, 6.9%) that were more evident in the first 2 weeks. From the third week, the notches were not so well defined, but the impact zone now showed percussion pits associated with cortical flaking and longitudinal or slightly curved fractures. Percussion damage usually occurred between the proximal metaphysis and diaphysis, with no preference to either side.
In the outdoor experiments, the number of fragments after percussion impacts to access the marrow tended to increase in line with the exposure time (R2 = 0.762, P = 0.0013). The greatest increase was observed from the seventh week in scenario 1 and progressively in scenario 2.
The bone breakage analysis of metapodials indicates similar proportions for both outdoor scenarios (1 and 2), with a predominance of longitudinal and curved/V-shaped fractures (SC 1, n = 739 of 919 breakage planes analyzed, 80.4%; SC 2, n = 347 of 444 breakage planes analyzed, 78.1%), oblique angles (SC 1, n = 511, 55.6%; SC 2, n = 247, 55.6%), and smooth edges (SC 1, n = 791, 86%; SC 2, n = 396, 89.1%) (fig. S4).
Marrow chemical analyses
Dry matter (DM) content of marrow was very high (96.5 ± 3.19%), and its main component is fat (96.3 ± 3.2%). Only one sample had less than 90% of DM, and it could already be classified as very liquid. It emitted bad odor at the extraction. Excluding this sample, there was a linear relationship between the week of conservation and DM content (+1.4% DM/week; P < 0.05). The marrow’s weight and energetic value were analyzed to obtain the nutrient value of the bones. According to these values, the marrow mean energetic content was 8.7 kcal/g. Quadratic coefficients of the regression of marrow by week of conservation were not statistically different from zero, and no differences between intercepts were detected according to the scenario of conservation (P = 0.868). The marrow percentage from fresh bones was estimated at 8.1 ± 0.75%, and indoor and outdoor (spring) scenarios had a significant decrease in marrow percentage per week (−1.0 ± 0.4% and − 1.4 ± 0.3% per week, respectively). The outdoor (autumn) scenario showed no decrease from 0 to 9 weeks of conservation (slope not significantly different from 0; −0.2 ± 0.3) (Fig. 4).
Fig. 4. Temporal evolution of marrow percentage in metapodials according to week and scenario.
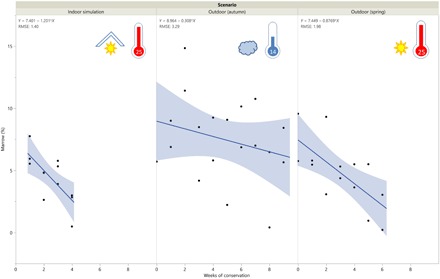
RMSE, root mean square error.
Marrow composition was mostly unsaturated FA (78%), especially monounsaturated (74%), and only 22% comprised saturated fats (table S1). Oleic (C18:1n-9) was the most abundant FA in marrow (36% in week 0), with a significant decrease per week (−0.7 ± 0.14%; P < 0.001). Other FA, like palmitoleic (C16:1n-7), palmitic (C16:0), and vaccenic (C18:1), had lower percentages (10 to 16%) and remained constant over time.
The energy value of marrow obtained from metapodial bones ranged from 123 kcal (bone from week 2 in the outdoor autumn scenario) to 2.7 kcal (bone from week 6 in the outdoor spring scenario). The energy contained in one bone in good conservation conditions (i.e., up to 9 weeks in the outdoor autumn scenario or the first few weeks in the outdoor spring scenario) could be comparable to the crude energy content of 25 g of fresh meat.
The comparison of the preservation of the marrow between exposed metapodials with skin and those exposed after they had been skinned showed a larger decrease in marrow percentage over time, i.e., per week of conservation (−1.07 ± 0.4%/week and − 1.45 ± 0.6%/week for nonskinned and skinned bones, respectively). Nevertheless, this difference was not statistically significant (P = 0.63; table S2).
DISCUSSION
Taking into account the scarcity of postdepositional taphonomic damage and the low influence of mineral density-mediated attrition processes at Qesem, the hominid transport decisions and the ravaging by carnivores were considered as candidates in the search for the main factors to explain the bias of the anatomical profile [e.g., (15, 16)]. Destruction and subsequent ravaging are closely linked to the mineral density of the bones and their portions in the case of carnivores [e.g., (17, 18)]. For example, Madrigal and Holt (19) argued that if the limb bones are processed, the isolated shafts tend to survive carnivore ravaging, while cancellous bone portions will be removed by ravaging carnivores. The scarcity of the epiphyses of long bones, especially the least dense epiphyseal portions, such as the proximal humerus, distal femur, and proximal tibia at Qesem, could raise the possibility of carnivore attrition. However, an underrepresentation of spongy bone is not necessarily only due to carnivore attrition but may be also the result of other causes, including anthropogenic processing, such as bone grease production, or the use of bone as fuel (20). As argued in several previous works, the impact of carnivores on the faunal assemblages at Qesem is minimal [e.g., (15, 16)]; thus, the inspection of the relationship between the anatomical profile and the economic utility of elements in this case becomes relevant to the assessment of economic transport strategies.
The skeletal representation at Qesem is biased toward the high utility elements, with a predominance of limbs and mandibles compared to skulls and axial bones. The ungulate body part profile correlates positively with the UI bone marrow and UMI, pointing to the importance of marrow in hominin transport decisions. However, some specific differences between weight sizes are worth highlighting, since they precisely relate to the representativeness of the metapodials. The %MAU shows very low proportions for the metapodials of large-sized ungulates (e.g., aurochs and horse) with values between 0 and 9.7%. The trend changes completely in the case of small- and medium-sized species (e.g., fallow deer and red deer) with percentages between 65.4 and 84.6. This composition was already detected in the faunal assemblage of the central hearth area and interpreted on the basis of ethnographic parallels once postdepositional processes and carnivore ravaging were ruled out (16). According to some modern ethnographic descriptions, the pattern of disarticulation is highly variable among different hunter-gatherer groups and species. Domínguez-Rodrigo (21) documented an example of variation in the pattern of dismembering in the case of the Maasai people, which differs from the one observed by Gifford-Gonzalez (22). The ethnic group from Peninj (Tanzania) usually severs metapodials from the limbs after the first step of skinning; however, the Massai from the south-east of Kenya remove complete limbs first (without disarticulating them) after evisceration. Among the Hadza or the San, it is repeatedly observed that the preparation of carcasses for transport may involve the consumption of some viscera and marrow from long bones, especially in large ungulates (21, 23). These episodes would lead to the breaking of some marrow-rich bones, such as the metapodials, at the kill site or hunting stations for marrow extracting and immediate consumption. This internal resource would provide an extra nutritional supplement for hunters while they process the carcass and prepare it for transport (7, 24). Marrow extraction is a low-cost activity relative to fat removal in that it only requires a few minutes to completely process a bone, particularly if the bone is not covered by flesh, as is the case of metapodials (17). This phenomenon could explain why the metapodials of large-sized ungulates at Qesem were scarce compared to the quantity of the rest of the limb bones. That is, the initial consumption has been able to condition the variety of bones that were transported to the base camp. A carcass can be conceptualized as a patch of skeletal elements, each with a pursuit and handling cost (25). Nevertheless, we must take into account that other variables could also affect transport decisions and generate different body part profiles, e.g., the distance from the hunting area to the home base, the number of animals harvested simultaneously, the number of participants in the hunting party, the location and time of day when the animals are acquired (e.g., 26, 27), the technological state of development (28), the condition of the animals (7), and the risk of predation by other carnivores (29). The dynamics of carcass transport are complex, and although the degree of difficulty is evident and can vary with each carcass or situational event, major trends can emerge.
O’Connell et al. (30) documented that the abandonment or processing of some limb bones at kill sites is often contingent on prey size. The metapodials of small- and medium-sized ungulates are well represented in both the Amudian and Yabrudian of Qesem Cave contexts, and they correlate with the other limb bones, showing relatively similar quantities. Thus, there seems to be a differential treatment according to weight size as a general trend in Qesem, where small- and medium-sized animals are mainly transported as field-butchered units to base camp. The presence of transverse cut marks on the basipodia and proximal epiphyses/metaphyses of the metapodials suggests that they were almost systematically separated from the intermediate appendicular bones (radius-ulna and tibia). This butchery pattern seems similar to that performed with the metapodials of large-sized ungulates at the kill sites, but now it was performed at the cave after the limb bones were transported whole. However, how can we know if skinning and bone breakage (and the subsequent marrow consumption) were immediate or delayed?
The metapodials of medium- and small-sized animals show the typical signs of intentional percussion to access the marrow, and therefore, a priori, we could consider immediate consumption of the marrow as a snack or additional nutrient during processing or as one of the final stages of the sequence after the extraction of the animal’s external resources. However, our experimental series do not show any differences in the morphology or location of the notches during the first 2 weeks of exposure that would enable us to identify whether the consumption was immediate or slightly delayed. The notable difference takes place from the third week onward, when the notches are less well defined and are replaced by percussion pits associated with cortical flaking and longitudinal or slightly curved fractures. Given the high level of bone fragmentation in the Qesem assemblages, and because of anthropogenic and postdepositional processes (different types of pressure loading, such as trampling and/or soil compaction), metapodial fragments do not always register the impact points (notches or pits), and therefore, our attention must turn back to the fracture planes looking for clues to the condition of the bones at the time they were broken for marrow extraction.
By applying the criteria of Villa and Mahieu (31), the metapodials in Qesem appear to mainly register characteristics of a fresh fracture, with a preference to oblique angles, longitudinal delineations, and smooth surfaces. However, these bones can remain fresh over time, as they maintain not only their collagen in high proportions but also their nutritional values, such as fat and protein (32). In relation to this, the analysis of the fractures in the experimental series revealed that the angles, outlines, and surfaces were similar to those generated by fresh breakage even in weeks 6 to 9 in natural outdoor conditions (scenarios 1 and 2). At this point, we needed to explore more variables.
Obviously, before the metapodials were fractured, they had to be skinned. The cut marks could provide us with data on the state of the skin when it was removed, since the effort to remove this tissue varies depending on whether it is fresh or dry, a circumstance that would also result in a different taphonomic signature. The same situation can be observed when the dried flesh is removed from the bone because the cut marks’ frequency and morphology can vary depending on factors such as the state and weight of attached flesh at the time butchery is undertaken [e.g., (33)]. Dry flesh is more attached to the bone, which is why more effort is required to remove it than when it is fresh, as is the case when the tool reaches the muscular insertions or tendons firmly attached to the bone. This leads not only to a greater number of marks but also to a different pattern with different morphologies and orientations from those observed in the defleshing of large, fresh muscle bundles or when the butchery is performed with a specific purpose, such as extracting long cuts or slices of flesh of roughly standardized shape (i.e., fillets) for drying [e.g., (34)].
Longitudinal and oblique incisions on the lateral sides of the metapodials similar to those that would occur when the skin is in a fresh state have been identified in Qesem. These marks were also occasionally observed in the experimental series, although they were only recorded in the first week of exposure. From the second week, the short (shallow and deep) incisions and sawing marks were predominant, with special relevance on the anterior and posterior surfaces (where the tendons are found), and it is from the fourth week onward that the number of these marks increased along with a variation in the inclination of the sections toward an almost flat oblique position. These types of marks are precisely the ones that predominate in Qesem (77.9% of the anterior/posterior surfaces of metapodial shafts showing cut marks), which would lead us to consider a possible delayed secondary skinning (by at least 2 weeks according to our experiments). Nevertheless, despite the similarity to the experimental marks, we cannot rule out equifinality, i.e., other processes could produce similar cut marks. For instance, we cannot rule out the existence of cultural patterns in processing techniques that give rise to marks with these characteristics. These specific “ways of doing” could be perpetuated over time and materialize in the archaeological record in patterns or in what Yellen (26) called “style” in the butchery among the!Kung Bushmen. However, other types of marks that could be diagnosed with possible secondary processing exist. These are the cortical scars associated with chop marks (or chipped marks), which are sometimes combined with prominent incisions and sawing marks on the anterior/posterior side, showing the same orientation and inclination almost parallel to the bone. These marks were also sporadically generated at the experimental level from the second week and systematically from the seventh week in scenario 1. This atypical damage was caused by the difficulty of removing the dry skin and tendons that remained strongly attached to the bone after outdoor exposure. The presence of these alterations does seem to suggest that some Qesem metapodials could have been processed subsequently (after 2 to 7 weeks), and it also makes the previous type of marks more relevant for this interpretation.
According to the nutritional analyses of the experimental sample, the marrow of the metapodials was conserved in good condition in the outdoor autumn scenario (scenario 1), preserving useful nutrients until the ninth week; however, in the indoor and outdoor spring series (scenarios 2 and 3), the marrow showed a significant decrease week by week, which was particularly noticeable from the third week. Thus, seasonality seems to be an important variable when assessing marrow degradation. This fact is interesting because in Qesem Cave, seasonal hunting peaks have been detected that specifically include late summer through autumn, during and/or after the rutting time (16, 35).
From a microbiological perspective, the delayed consumption of marrow also seems to be relatively safer than consuming dry meat, since the marrow remains encapsulated by the bone, offering protection against microbes, even when the bacteria have been injected into the circulatory system and have reached the marrow via the nutrient artery (9). The study by Smith et al. (9) showed that, in raw meat, all bacterial populations grew rapidly within 24 hours; in contrast, the number of colony-forming units in samples taken from marrow inside the bone was consistently low.
Apart from bone coverage, the skin could also provide insulation or have a protective effect against insects and/or bacteria. Insects play an important role in carcass decomposition processes. By transporting microbes and producing young that tunnel and aerate the tissues of the carcasses, insects alter the microbial and physical nature of the carrion in such a way that they promote bacterial growth (36). In the case of the metapodials, the skin and tendons are in direct contact with the bone, and in the absence of soft tissues (such as flesh) susceptible to being rapidly colonized by bacteria, they could offer preservation advantages in the case of outdoor exposure. Although this hypothesis seems logical, the truth is that in the experimental level, the metapodials exposed without skin in scenario 3 did not show statistically significant differences in nutritional degradation compared to those exposed with skin. Despite this, during the preparation of samples for chemical analysis, a different aspect was detected in the marrow that came from the skinless metapodials, which had a more gelatinous, harder, and pinker appearance. In any case, Qesem’s metapodials register marks that indicate that they were accumulated with skin to be processed secondarily and later in time in an attempt to preserve the bone marrow.
Accumulating bones for delayed consumption of grease and marrow has been documented ethnographically among Nunamiut Eskimo communities, where the bones are stored during the winter months to be processed in large batches (1). The Loucheux people also process the bones secondarily and with a slight delay, although normally they do not exceed 3 days of outdoor exposure; once the grease/fat is extracted, these groups store it inside the stomach of caribou (converted into bags), where they claim that it stays in good condition for 2 or 3 years (24). Another example of the use of ungulate organs to store bone grease after rendering comes from the Comanche and Blackfoot people, who store dried meat mixed with bone grease and marrow in stomachs, intestines, and rawhide bags sealed airtight with tallow [e.g., (37)].
Ethnographic studies have shown that a significant number of nonagrarian peoples engage in some sort of delayed consumption [e.g., (38)]. This practice often requires the development of preservation techniques (mainly in the case of meat), which can vary depending on factors such as geographical area, environmental conditions, seasonality, and/or technological capabilities [e.g., (39–41)]. Drying meat under natural temperatures, humidity, and air circulation, including direct sunlight, is perhaps one of the simplest methods. This presumably applies to smoking too, as it also involves the removal of moisture from the meat (40). Smoking meat has an added preservative effect, apart from surface drying, in that the smoke from the sawdust contains bactericidal agents, such as formaldehyde, and also inhibits fat oxidation (41). During colder seasons in northern environments, freezing is another method that would allow preservation of internal and external resources (i.e., meat and fat/grease) without much effort, permitting entire articulated carcasses (or with minimal field butchery) to be cached after skinning and gutting (39).
Hunter-gatherer food storage is considered a “risk-reducing mechanism” designed to offset seasonal downturns in resource availability and is typically seen as evidence of intensified subsistence activities [e.g., (42)]. Recently, Speth (43) argued the potential use of fermented and deliberately rotted meat and fish in forager diets throughout the arctic and subarctic, concluding that putrefied food was widely used as a desirable and nutritionally important component of human diets (and not solely as starvation food). Fermentation is a widespread technique used for food preparation and preservation. These types of “processed” foods can also have dietary benefits and are even considered delicious (instead of unpleasant) by people who grow up eating them (44). Speth (43) extended this approach to the Eurasian Middle Palaeolithic hominids who inhabited analogous environments, suggesting the possibility of delayed consumption among the Middle and early Late Pleistocene populations. At this point, it can be assumed that bone marrow could also have been part of this pack of resources susceptible to being processed secondarily over time. Marrow FA composition evolves with time of conservation, showing a decrease of monounsaturated FAs presumably due to its oxidation into shorter chain products, including dicarboxylic acids and short-chain FAs. These products could make fats taste and smell rancid. It is difficult to know if this rancidity could have impaired the consumption of aged marrow, but, as in the case of dry meat, we could assume that the preference for this type of aging depends on the consumer and/or group traditions (44, 45).
It is also worth mentioning that besides its dietary importance, marrow also has many other artisanal uses. For instance, the Nunamiut use the marrow of ungulates’ distal members to waterproof skins and treat bowstrings (1). It can also be used as fuel for lighting (46) and can even be used in the tanning process, as reported by the traditional peoples of Siberia (47). Whether it was consumed or used for other purposes, the important point here is the capacity to plan and forecast that arises from this fact. The deliberate accumulation of metapodials implies an anticipated concern for future needs and a capacity for “temporal displacement” that surpasses the “here and now” as a means of subsistence (34). Therefore, the study of the preservation or delayed consumption of resources, as well as possible storage systems, has great potential for detecting not only economical but also social and cognitive changes among Middle Pleistocene populations.
MATERIALS AND METHODS
Geological, chronological, and archaeological setting: Qesem Cave, Israel
Qesem Cave is located on the western slopes of the Samaria Hills, about 12 km east of Tel Aviv, Israel, and 90 meters above sea level (m asl). Its stratigraphic sequence (still incomplete, as bedrock has not yet been reached) is divided into two main parts: the lower (ca. 6.5 m thick) consisting of sediments with clastic content, gravel, and clays and the upper (ca. 4.5 m thick) composed of cemented sediment with a large ash component. The lower part was deposited in a closed karstic chamber, while the presence of calcified rootlets in the upper part points toward a more open environment (48). The stratigraphic profile has been dated by several methods [uranium-thorium (U/Th), thermoluminescence (TL), electron spin resonance (ESR), and ESR/U series] to 420 to 200 ka.
The entire stratigraphic sequence was assigned to the late Lower Palaeolithic Acheulo-Yabrudian Cultural Complex (AYCC), which is a local cultural entity differing from the preceding Acheulean and the following Mousterian. Qesem contains two of the three AYCC industries: the blade-dominated Amudian and the scraper-dominated Yabrudian. Biface production continued in the AYCC, but bifaces are extremely rare at the site. Recycling flint is a clear component of the assemblages throughout the cave’s sequence and indicates well-established technological trajectories for the production of designated types of specific sharp flakes and blades for targeted purposes [e.g., (49)].
The faunal assemblage is dominated by fallow deer and supplemented by red deer. Horse, aurochs, wild pig, and wild ass are also present, as well as other small ungulates, such as goat and roe deer. In contrast, carnivores are extremely rare in the entire sequence. Zooarchaeological analyses suggest that cooperative hunting strategies focused mainly on fallow deer and the transport of selected ungulate body parts to the cave, where hominins carried out food-processing activities and the last phases of carcass processing (15, 16, 35). Twenty-four bone fragments from the Amudian contexts and 16 from the Yabrudian contexts show percussion marks related to their use as bone retouchers for shaping stone tools.
The use of fire is present in the earliest levels of the cave and is evident throughout the sequence, both directly by the presence of a central hearth and large amounts of wood ash and indirectly by the high quantity of burnt flint and bones (48, 49).
Qesem has also yielded 13 human teeth from different parts of the stratigraphic profile. Data provided by morphometrical analysis and three-dimensional (3D) scanning point to the fact that the teeth from Qesem are not of Homo erectus (sensu lato) but bear similarities with the Late Pleistocene local populations of Skhul and Qafzeh, as well as some Neanderthal affinities (50). Therefore, the human fossils may belong to a yet unknown local hominin lineage of the Levant.
Skeletal and taphonomic analyses
Beyond the general subdivision of the sedimentary column of Qesem Cave (upper and lower sequences) by Karkanas et al. (48) and the subdivision according to elevations (units I and II for the upper part and units III to V for the lower part) by Stiner et al. (15), here, we present faunal data from specific archaeological contexts; they are named by acronyms mainly after their sedimentary characteristics and grouped into AYCC units (Amudian and Yabrudian).
The data analyzed for each faunal specimen were skeletal element, taxon/body size class, portion, surface, and age at death. We established NSP [including anatomic and taxonomically identifiable bone fragments, as well as fragments not attributed to a body size class; see (16) for body size classes], NISP, MNE, MNI, and %MAU. Several researchers have shown that the interpretation of skeletal part frequencies in relation to economic utility was severely compromised by density-mediated destruction of bone [e.g., (51)]. Non-nutritive processes of bone destruction include those processes that are not the result of animals or humans attempting to derive nutrition, e.g., chemical leaching, sediment compaction, trampling, burning, and any other mechanical or chemical process that destroys bone [p. 34 of (17)]. It is widely assumed that these phenomena are density mediated, meaning that the degree of damage is negatively related to the skeletal mineral density [e.g., (17, 51)]. The data of (51) and (52) were used to calculate the relationship between %MAU and the mineral density of portion-specific values of bones (Spearman’s rank). To explore hypotheses related to hominin decisions about marrow procurement, the %MAU was subsequently correlated with the UI of (10) and the UMI of (11).
The methods of analysis were based on published standards for taphonomy, with a special focus on anthropogenic damage. Bone surfaces were macro- and microscopically examined under a stereo light microscope (with a magnification of up to 120), and some selected specimens were also investigated using a KH-8700 3D digital microscope. Cut marks were identified on the basis of the criteria of several authors [e.g., (51)]. Type, morphology, number of striations, location, and orientation regarding the longitudinal axis of the bone were noted. As for orientation, we used the ranges proposed by Soulier and Morin (34): longitudinal (0° to 15° and 165° to 180°), oblique (15° to 75° and 105° to 165°), and transverse (75° to 105°). We also searched for surface damage caused during bone breakage, such as percussion pits, notches, impact flakes, counterblows, and peeling [e.g., (12)]. The location and distribution of percussion modifications were noted in terms of anatomical area, portion, and surface. Bone fragments longer than 20 mm were also analyzed in terms of breakage (outline, fracture angle, and edge) according to the criteria developed by Villa and Mahieu (31).
Experimental approach
The aim of the experiment was to test whether bone marrow could be preserved without preparation (simply encapsulated in the bone) for a prolonged period of time. This required subsequent secondary processing (skinning and bone breakage) to finally achieve a delayed consumption of the marrow. This study aimed to observe the marrow degradation process, determine from which point its consumption would cease to be nutritionally attractive (profitable), and detect the taphonomic signature of its secondary processing according to exposure time.
We used adult or prime-adult red deer (C. elaphus) metapodials from the Boumourt National Game Reserve (Pallars Jussà, Lleida, Spain), which were systematically separated from the forelimbs and hindlimbs at the carpals and tarsals. This procedure is common among the reserve’s rangers when carrying out spring and winter population checks to prepare the carcasses for meat consumption; the metapodials are systematically rejected, since they contain no meat. In total, 79 metapodials (38 metacarpals and 41 metatarsals) were used, divided into three experimental series corresponding to three different environmental scenarios. The first two were performed in natural outdoor conditions in autumn [mean temperature from 21 September to 23 November: 13.3°C; relative humidity (RH): 64%] and spring (mean temperature from 27 April to 8 June: 18.25°C; RH: 64%; data from the Catalonia Meteorological Service) in a Mediterranean Pyrenean location (42.41°N, 0.74°E, 857 m asl). In the first two series (scenarios 1 and 2), the metapodials were exposed for a minimum period of 1 week and a maximum period of 9 weeks. Therefore, the experiment’s main variables were exposure time and environmental conditions (seasonality).
The third scenario was aimed at reproducing Israel’s Mediterranean environmental conditions and was conducted in an indoor simulation of climate conditions (accelerated weathering chamber) at the Museo Nacional de Ciencias Naturales (MNCN) in Madrid, Spain, for a minimum period of 1 week and a maximum period of 4 weeks [mean temperature, 20.2°C; RH, 67%; data from the Israel Meteorological Service (Average climatic parameters for Tel Aviv 1916–2007)]. In this last scenario, the aim was to only analyze the sequence of marrow degradation in a similar environment to that of Israel. Apart from the use of environmental simulation equipment, the main difference from the previous series was the introduction of the “skinless” variant. This new variable was included with the aim of chemically comparing any differences in the nutritional preservation of the marrow between the exposed metapodials with skin and those exposed after they had been skinned.
To correlate the marrow degradation with the marks derived from the secondary processing of the metapodials, each week, up to five metapodials were removed from the subaerial exposure: two to perform chemical analyses on the nutritional values of the marrow (see proceedings below) and two/three for processing (skinning and breaking the bone open for the marrow). This was performed systematically in the first two series in outdoor conditions. Skin/hide extraction was performed with flint flakes, and the marrow was accessed using hammerstone percussion with quartzite percussion tools. The secondary processing of the metapodials was always performed by the same individual, with no guidelines on how to extract the marrow.
Biochemical analyses
The nutrient value of bones was obtained by analyzing the marrow’s weight and energy value. Temporal variation of nutrient value was assessed using the red deer (C. elaphus) metapodial bones conserved during different time periods (exposure time), from 0 (fresh) to 9 weeks. Three conservation condition scenarios were evaluated. Marrow content was extracted from two to three bones for each scenario each week. The bones were cut, discarding the epiphyses, and the diaphyses were flayed. The diaphyses were weighed without tendons and mechanically broken to extract all marrow content. Marrow composition was obtained using AOAC (Association of Official Analytical Chemists) method 920.39, and its energy value was calculated assuming a value of 9.4 kcal/g of fat. FA analysis of marrow was analyzed in duplicate in samples obtained from scenario 1 at 0, 2, 4, 6, and 8 weeks of conservation. Marrow FA composition was determined by capillary gas chromatography of the fatty acid methyl esters [FAMEs; (53)].
Temporal changes of marrow percentages (marrow/diaphysis weight) were analyzed using regression, where scenario affects intercept (% at week 0) and linear and quadratic coefficients. Regression was implemented using GLM (General Linear Model) procedures of SAS (Cary, NC).
Supplementary Material
Acknowledgments
We thank Y. Fernández-Jalvo from the Museo Nacional de Ciencias Naturales (MNCN, Madrid, Spain) and J. Fàbregas for valuable help during the process. Metapodial samples were provided by the Cos d’Agents Rurals from the Departament d’Agricultura, Ramaderia, Pesca i Alimentació of the Generalitat de Catalunya. Funding: The Qesem Cave excavation project is supported by the Israel Science Foundation, the CARE Archaeological Foundation, the Leakey Foundation, the Wenner-Gren Foundation, the Dan David foundation, and the Thyssen Foundation and by a Deutsche Forschungsgemeinschaft (DFG) research grant (UT 41/4-1). J.R. and R.Bl. develop their work within the Spanish MINECO/FEDER (projects CGL2015-65387-C3-1-P, CGL2016-80000-P, and CGL2015-68604-P) and the Generalitat de Catalunya (project 2017 SGR 836 and CLT009/18/00055). M.A. is the beneficiary of a research fellowship (FI) from AGAUR (2017FI-B-00096), and A.M. was supported by a Ramón y Cajal research contract by the Ministry of Economy and Competitiveness (RYC-2012-11867). Author contributions: R.Bl., J.R., M.A., A.G., R.Ba., A.M., and D.V. conceived the project. R.Bl., J.R., and M.A. performed the experiments. A.M. and D.V. executed biochemical analyses. R.Bl., J.R., M.A., A.G., R.Ba., A.M., and D.V. interpreted the data. R.Bl. and J.R. wrote the manuscript with the support of A.M., D.V., M.A., A.G., and R.Ba. All authors contributed to the manuscript and approved the final version, and all authors qualifying for authorship are listed. Competing interests: All authors declare that they have no competing interests. Data and materials availability: All data needed to evaluate the conclusions in the paper are present in the paper and/or the Supplementary Materials. Additional data related to this paper may be requested from the authors.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/10/eaav9822/DC1
Fig. S1. Examples of cut marks associated to disarticulation and/or skinning from Amudian and Yabrudian levels of Qesem Cave.
Fig. S2. Test of normality and graphs showing the number of cut marks with inclination almost parallel to the bone and weeks of conservation by scenarios (SC 1 and SC 2).
Fig. S3. Examples of different actions (skinning, tendon removal, and bone breakage) during the development of the SC 1.
Fig. S4. Ternary plots showing analysis of bone break planes (outline, angle, and surface edge) of metapodials with more than 20 mm length from experimental series [outdoor (autumn and spring) scenarios] and Qesem Cave faunal assemblage following the criteria established by Villa and Mahieu (31).
Table S1. Variation on FAME (%) composition according to the week of conservation in the outdoor (autumn) scenario (SC 1).
Table S2. Weight and energy data (kcal) from the metapodial bones by experimental scenario and exposure time.
REFERENCES AND NOTES
- 1.L. R. Binford, Nunamiut Ethnoarchaeology (Academic Press, 1978). [Google Scholar]
- 2.Brink J. W., Fat content in leg bones of Bison bison, and applications to archaeology. J. Archaeol. Res. 24, 259–274 (1997). [Google Scholar]
- 3.J. F. Mead, R. B. Alfin-Slater, D. R. Howton, G. Popjak, Lipids: Chemistry, Biochemistry, and Nutrition (Plenum Press, 1986). [Google Scholar]
- 4.Outram A. K., A new approach to identifying bone marrow and grease exploitation: Why the “Indeterminate” fragments should not be ignored. J. Archaeol. Sci. 28, 401–410 (2001). [Google Scholar]
- 5.J. D. Baker, thesis, University of Tennessee (2009). [Google Scholar]
- 6.Church R. R., Lyman R. L., Small fragments make small differences in efficiency when rendering grease from fractured artiodactyl bones by boiling. J. Archaeol. Sci. 30, 1077–1084 (2003). [Google Scholar]
- 7.Speth J. D., Middle paleolithic subsistence in the Near East: Zooarchaeological perspectives-past, present, and future. Before Farming 2, 1–45 (2012). [Google Scholar]
- 8.Speth J. D., When did humans learn to boil? PaleoAnthropol. 2015, 54–67 (2015). [Google Scholar]
- 9.Smith A. R., Carmody R. N., Dutton R. J., Wrangham R. W., The significance of cooking for early hominin scavenging. J. Hum. Evol. 84, 62–70 (2015). [DOI] [PubMed] [Google Scholar]
- 10.A. M. Emerson, in From Bones to Behavior: Ethnoarchaeological and Experimental Contributions to the Interpretation of Faunal Remains, J. Hudson, Ed. (University at Carbondale, 1993), pp. 138–155. [Google Scholar]
- 11.Morin E., Fat composition and nunamiut decision-making: A new look at the marrow and bone grease indices. J. Archaeol. Sci. 34, 69–82 (2007). [Google Scholar]
- 12.Pickering T. R., Egeland C. P., Experimental patterns of hammerstone percussion damage on bones: Implications for inferences of carcass processing by humans. J. Archaeol. Sci. 33, 459–469 (2006). [Google Scholar]
- 13.Domínguez-Rodrigo M., Bunn H. T., Mabulla A. Z. P., Baquedano E., Uribelarrea D., Pérez-González A., Gidna A., Yravedra J., Diez-Martin F., Egeland C. P., Barba R., Arriaza M. C., Organista E., Ansón M., On meat eating and human evolution: A taphonomic analysis of BK4b (Upper Bed II, Olduvai Gorge, Tanzania), and its bearing on hominin megafaunal consumption. Quat. Int. 322-323, 129–152 (2014). [Google Scholar]
- 14.Soulier M.-C., Costamagno S., Let the cutmarks speak! Experimental butchery to reconstruct carcass processing. J. Archaeol. Sci. Rep. 11, 787–802 (2017). [Google Scholar]
- 15.Stiner M. C., Gopher A., Barkai R., Hearth-side socioeconomics, hunting and paleoecology during the late Lower Paleolithic at Qesem Cave, Israel. J. Hum. Evol. 60, 213–233 (2011). [DOI] [PubMed] [Google Scholar]
- 16.Blasco R., Rosell J., Gopher A., Barkai R., Subsistence economy and social life: A zooarchaeological view from the 300 kya central hearth at Qesem Cave, Israel. J. Anthropol. Archaeol. 35, 248–268 (2014). [Google Scholar]
- 17.Marean C. W., Cleghorn N., Large mammal skeletal element transport: Applying foraging theory in a complex taphonomic system. J. Taphonomy 1, 15–42 (2003). [Google Scholar]
- 18.Marean C. W., Spencer L. M., Blumenschine R. J., Capaldo S. D., Captive hyena bone choice and destruction, the schlepp effect, and Olduvai archaeofaunas. J. Archaeol. Sci. 19, 101–121 (1992). [Google Scholar]
- 19.Madrigal T. C., Holt J. Z., White-tailed deer meat and marrow return rates and their application to Eastern Woodlands archaeology. Am. Antiq. 67, 745–759 (2002). [Google Scholar]
- 20.E. Morin, in The taphonomy of Burned Organic Residues and Combustion Features in Archaeological Contexts, I. Théry-Parisot, L. Chabal, S. Costamagno, Eds. (CEPAM, P@lethnology, ed. 2, 2010), pp. 209–217. [Google Scholar]
- 21.Domínguez-Rodrigo M., The study of skeletal part profiles: an ambiguous taphonomic tool for Zooarchaeology. Complutum 19, 15–24 (1999). [Google Scholar]
- 22.D. Gifford-Gonzalez, “Observations of modern human settlements as an aid to archaeological interpretation,” thesis, University of California, Berkeley (1977). [Google Scholar]
- 23.O’Connell J. F., Hawkes K., Blurton Jones N. G., Patterns in the distribution, site structure and assemblage composition of Hadza kill-butchering sites. J. Archeol. Sci. 19, 319–345 (1992). [Google Scholar]
- 24.A. K. Outram, thesis, University of Durham, England (1998). [Google Scholar]
- 25.H.T. Bunn, in Breathing Life into Fossils: Taphonomic Studies in Honor of C.K. “Bob” Brain, T. Pickering, K. Schick, N. Toth, Eds. (Stone Age Institute Press, 2007), pp. 269–279. [Google Scholar]
- 26.J. E. Yellen, in Experimental Archaeology, D. Ingersoll, J. E. Yellen, W. MacDonald, Eds. (Columbia Univ. Press, 1977), pp. 271–331. [Google Scholar]
- 27.Faith J. T., Domínguez-Rodrigo M., Gordon A. D., Long-distance carcass transport at Olduvai Gorge? A quantitative examination of Bed I skeletal element abundances. J. Hum. Evol. 56, 247–256 (2009). [DOI] [PubMed] [Google Scholar]
- 28.J. S. Oliver, in From Bones to Behavior: Ethnoarchaeological and Experimental Contributions to the Interpretation of Faunal Remains, J. Hudson, Ed. (University at Carbondale, 1993), pp. 200–227. [Google Scholar]
- 29.Monahan C. M., The Hadza carcass transport debate revisited and its archaeological implications. J. Archaeol. Sci. 25, 405–424 (1998). [Google Scholar]
- 30.O’Connell J. F., Hawkes K., Blurton Jones N., Reanalysis of large mammal body transport among the Hadza. J. Anthropol. Res. 17, 301–316 (1990). [Google Scholar]
- 31.Villa P., Mahieu E., Breakage patterns of human long bones. J. Hum. Evol. 21, 27–48 (1991). [Google Scholar]
- 32.Margalida A., Villalba D., The importance of the nutritive value of old bones in the diet of Bearded vultures Gypaetus barbatus. Sci. Rep. 7, 8061 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Domínguez-Rodrigo M., On cut marks and statistical inferences: Methodological comments on lupo & O’Connell (2002). J. Archaeol. Sci. 30, 381–386 (2003). [Google Scholar]
- 34.Soulier M.-C., Morin E., Cutmark data and their implications for the planning depth of Late Pleistocene societies. J. Hum. Evol. 97, 37–57 (2016). [DOI] [PubMed] [Google Scholar]
- 35.Blasco R., Rosell J., Sañudo P., Gopher A., Barkai R., What happens around a fire: Faunal processing sequences and spatial distribution at Qesem Cave (300 ka), Israel, Israel. Quat. Int. 398, 190–209 (2016). [Google Scholar]
- 36.DeVault T. L., Rhodes O. E. Jr., Shivik J. A., Scavenging by vertebrates: Behavioral, ecological, and evolutionary perspectives on an important energy transfer pathway in terrestrial ecosystems. Oikos 102, 225–234 (2003). [Google Scholar]
- 37.S. Graff, E. Rodríguez-Alegría, The Menial Art of Cooking: Archaeological Studies of Cooking and Food Preparation (University Press of Colorado, 2012). [Google Scholar]
- 38.P. Rowley-Conwy, M. Zvelebil, in Bad Year Economics: Cultural Responses to Risk and Uncertainty, P. Halstead, J. M. O’Shea, Ed. (Cambridge Univ. Press, 1989), pp. 40–56.
- 39.Friesen T. M., Stewart A., To freeze or to dry: Seasonal variability in caribou processing and storage in the barrenlands of Northern Canada. Anthropozoologica 48, 89–109 (2013). [Google Scholar]
- 40.Henrikson L. S., Bison freezers and hunter-gatherer mobility: Archaeological analysis of cold lava tube caves on idaho’s snake river plain. Plains Anthropol. 48, 263–285 (2017). [Google Scholar]
- 41.Rixson D., Butchery evidence on animal bones. Circaea 6, 49–62 (1989). [Google Scholar]
- 42.Sanger M. C., Evidence for significant subterranean storage at two hunter-gatherer sites: The presence of a mast-based economy in the late archaic coastal american southeast. Am. Antiq. 82, 50–70 (2017). [Google Scholar]
- 43.Speth J. D., Putrid meat and fish in the eurasian middle and upper paleolithic: Are we missing a key part of neanderthal and modern human diet? PaleoAnthropology 5, 44–72 (2017). [Google Scholar]
- 44.J. Rottman, J. M. DeJesus, E. Gerdin, in The Moral Psychology of Disgust, N. Strohminger, V. Kumar, Eds. (Rowman & Littlefield International, 2018), pp. 27–52. [Google Scholar]
- 45.Sitz C. R., Calkins D. M., Feuz W. J., Umberger K. M., Eskridge, Consumer sensory acceptance and value of wet-aged and dry-aged beef steaks. J. Anim. Sci. 84, 1221–1226 (2006). [DOI] [PubMed] [Google Scholar]
- 46.Burch E. S., Jr., The caribou-wild reindeer as a human resource. Am. Antiq. 37, 339–368 (1972). [Google Scholar]
- 47.M. G. Levin, Potapov, L. P. The Peoples of Siberia (Chicago Univ. Press, 1964). [Google Scholar]
- 48.Karkanas P., Shahack-Gross R., Ayalon A., Bar-Matthews M., Barkai R., Frumkin A., Gopher A., Stiner M. C., Evidence for habitual use of fire at the end of the Lower Paleolithic: site-formation processes at Qesem Cave, Israel. J. Hum. Evol. 53, 197–212 (2007). [DOI] [PubMed] [Google Scholar]
- 49.Barkai R., Rosell J., Blasco R., Gopher A., Fire for a reason: Barbecue at Middle Pleistocene Qesem Cave, Israel. Curr. Anthropol. 58 ( suppl. 16), S314–S328 (2017). [Google Scholar]
- 50.Hershkovitz I., Weber G. W., Fornai C., Gopher A., Barkai R., Slon V., Quam R., Gabet Y., Sarig R., New Middle Pleistocene dental remains from Qesem Cave (Israel). Quat. Int. 398, 148–158 (2016). [Google Scholar]
- 51.R. L. Lyman, Vertebrate Taphonomy (Cambridge Univ. Press, 1994). [Google Scholar]
- 52.Lam Y. M., Chen X., Pearson O. M., Intertaxonomic variability in patterns of Bone density and the differential representation of bovid, cervid, and equid elements in the archaeological Record. Am. Antiq. 64, 343–362 (1999). [Google Scholar]
- 53.Álvarez-Rodríguez J., Villalba D., Cubiló D., Babot D., Tor M., Organic practices and gender are effective strategies to provide healthy pork loin. J. Integr. Agric. 15, 608–617 (2016). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/5/10/eaav9822/DC1
Fig. S1. Examples of cut marks associated to disarticulation and/or skinning from Amudian and Yabrudian levels of Qesem Cave.
Fig. S2. Test of normality and graphs showing the number of cut marks with inclination almost parallel to the bone and weeks of conservation by scenarios (SC 1 and SC 2).
Fig. S3. Examples of different actions (skinning, tendon removal, and bone breakage) during the development of the SC 1.
Fig. S4. Ternary plots showing analysis of bone break planes (outline, angle, and surface edge) of metapodials with more than 20 mm length from experimental series [outdoor (autumn and spring) scenarios] and Qesem Cave faunal assemblage following the criteria established by Villa and Mahieu (31).
Table S1. Variation on FAME (%) composition according to the week of conservation in the outdoor (autumn) scenario (SC 1).
Table S2. Weight and energy data (kcal) from the metapodial bones by experimental scenario and exposure time.


