Abstract
Background
Red blood cell exchange (RCE) transfusions are a mainstay in the treatment of sickle cell anemia (SCA), and allow a temporary restoration of physiological parameters with respect to erythrocyte oxygen carrying capacity and systems metabolism. Recently, we noted that (i) RCE significantly impact recipients’ metabolism in SCA; (ii) fresh and end of storage red blood cell (RBC) units differently impact systems of metabolism in healthy autologous recipients; and (iii) phosphate/inosine/pyruvate/adenine (PIPA) solution reverses the metabolic age of stored RBCs. Therefore, we hypothesized that RCE with PIPA treated RBC units could further increase the metabolic benefits of RCE in SCA patients.
Study design and Methods
Circulating plasma and erythrocytes were collected from patients with SCA before and after RCE, with either conventional or PIPA-treated RBC units, prior to metabolomics analyses.
Results
Consistent with prior work, RCE significantly decreased circulating levels of markers of systemic hypoxemia (lactate, succinate) and decreased plasma levels of acyl-carnitines and amino acids. However, PIPA-treated exchanges were superior to untreated RCEs, with a higher energy state and an increased capacity to activate the pentose phosphate pathway and glutamine metabolism. In addition, RBCs and plasma from recipients of PIPA-treated RBC units resulted in significantly decreased levels of post-transfusion plasticizers, though at the expenses of higher circulating levels of oxidized purines (hypoxanthine, xanthine and the antioxidant urate).
Conclusion
Transfusion of PIPA-treated RBCs further increases the metabolic benefits of RCE to patients with SCA, significantly reducing the levels of post-transfusion plasticizers.
Keywords: Mass spectrometry, metabolomics, blood storage, transfusion medicine
Introduction
Red blood cell (RBC) storage in the blood bank is characterized by the progressive accumulation of a series of biochemical and morphological alterations, collectively referred to as the storage lesion1. During storage in the blood bank, RBCs experience stressors beyond normal physiological conditions, such as refrigerated storage temperatures2, exposure to storage additives3,4 characterized by supraphysiological levels of substrates, and exposure to non-biological compounds such as phthalate plasticizers5. Collectively, these factors contribute to a progressive deregulation of energy6,7 and redox balance8,9 and, ultimately compromise the morphology and stiffness of the stored erythrocyte10, all factors increasing its propensity to hemolyze in vitro and, upon transfusion, in vivo11.
Over the last decade, omics tools have been extensively used to investigate the severity and progression of such lesions12. Early storage promotes the activation of the pentose phosphate pathway (PPP), a critical antioxidant pathway that generates the reducing cofactor NADPH that is critical for the reduction of oxidized glutathione and other NADPH-dependent antioxidant enzymes.13 Storage-induced activation of the PPP is triggered by the reversible oxidation of Cys152 in the active site of glyceraldehyde 3-phosphate dehydrogenase, a critical enzyme in glycolysis14. However, despite this mechanism, late storage RBCs progressively lose the capacity to activate the PPP15, resulting in a compromised antioxidant capacity. When these protection mechanisms fail16 , alternative pathways activate to promote repair17 or degradation18 of oxidatively damaged components. Altogether, combined with recent evidence from randomized clinical trials (reviewed here19), these studies have suggested that the chronological age of the unit (i.e., days elapsed since donation) may be irrelevant, while the metabolic age of the unit may be a more poignant parameter to monitor as a predictor of transfusion efficacy20.
In addition to providing significant advancements in our understanding of RBC storage biology as a function of storage additives3, omics tools are increasingly contributing to our understanding of the role of donor and recipient biology on blood storability and transfusion outcomes. Recently, we reported the impact on the plasma metabolome of healthy volunteers receiving autologous transfusions of fresh and end of storage blood21. Results indicated the transfusion of old blood significantly increased the circulating levels of oxidized purines, including hypoxanthine and oxidized lipids - metabolic marker of the storage lesion and post-transfusion recovery22. Similarly, transfusion of end of storage units significantly depleted the levels of arginine, suggestive of potential alterations of the transfused RBC capacity to transport and/or scavenge the vasodilatory mediator nitric oxide. Moreover, post-transfusion plasma was significantly contaminated with plasticizers – even if a single split (half of normal volume) unit was transfused21.
The impact of transfusion on the metabolic phenotypes of plasma and RBCs in the recipient is even more accentuated during red blood cell exchange (RCE) transfusion23, a therapeutic intervention based on the administration of >10 units of packed RBCs (with an approximate total volume of >4.5L) to replace the recipient’s diseased hemoglobin S (HbS)-containing erythrocytes with transfused, hemoglobin A (HbA)-containing RBCs. This intervention is critical in patients with sickle cell anemia (SCA) who require chronic transfusions for secondary stroke prevention or other indications.24 In SCA patients, RBCs are characterized by a defective hemoglobin (HbS) with impaired capacity to transport and deliver oxygen and increased likelihood to polymerize, altering RBC morphology and triggering vaso-occlusive crises25. Removal of diseased RBCs containing HbS and transfusion of RBC units containing HbA can ameliorate negative clinical outcomes of SCA, but is associated with acute and chronic procedural complications, including iron overload, iron-mediated hypercoagulability or progressive development of alloimmunization – proportional to the percentage of transfused RBCs that lyses or is otherwise sequestered by the reticuloendothelial system for phagocytosis26,27. Such percentage is proportional to the severity of the storage lesion, which is – as summarized above – recapitulated by the metabolic age of the unit. As such, strategies have been designed to metabolically rejuvenate stored units, by incubating the erythrocytes at 37 °C with a solution containing the substrates phosphate, inosine, pyruvate, and adenine(“PIPA” solutions such as Rejuvesol® Red Blood Cell Processing Solution, Zimmer Biomet Warsaw, IN) to fuel antioxidant and energy metabolism28,29. After establishing the benefits of rejuvenation on stored RBC metabolism28,29, we aimed to understand whether such benefits had any measurable impact on transfusion efficacy with respect to recipients’ plasma and RBC metabolism. As such, in the present study we performed metabolomics analyses of plasma and RBCs from patients with SCA receiving RCE with either untreated or rejuvenated RBCs.
Methods
After receiving written, informed consent in conformity with the Declarations of Helsinki under protocol approved by the Duke University Medical Center Institutional Review Board, blood samples were collected from established SCA patients maintained free from prior vaso-occlusive crisis on a monthly RCE program24 using the automated Optia system (Terumo BCT, Lakewood, CO). The 4 patients were clinically stable, African American males with HbSS disease, ages 19–32 years old. Rejuvenated or conventional exchanges were planned in an alternating sequence. . For rejuvenated exchanges, only the final four RBC units to be exchanged were rejuvenated. Blood was drawn from each patient at baseline and post-exchange, prior to or 5h after the start of RCE (Figure 1.A) with either conventional (storage day 16.0 ± 5.3) or rejuvenated RBCs (storage day 22.0 ± 13.5) with collection in EDTA tubes and gentle centrifugation to sort plasma and RBCs (1500–2000 g × 10 min at 4°C). Separated RBC and plasma samples were stored at −80°C prior to shipping to Denver on dry ice.
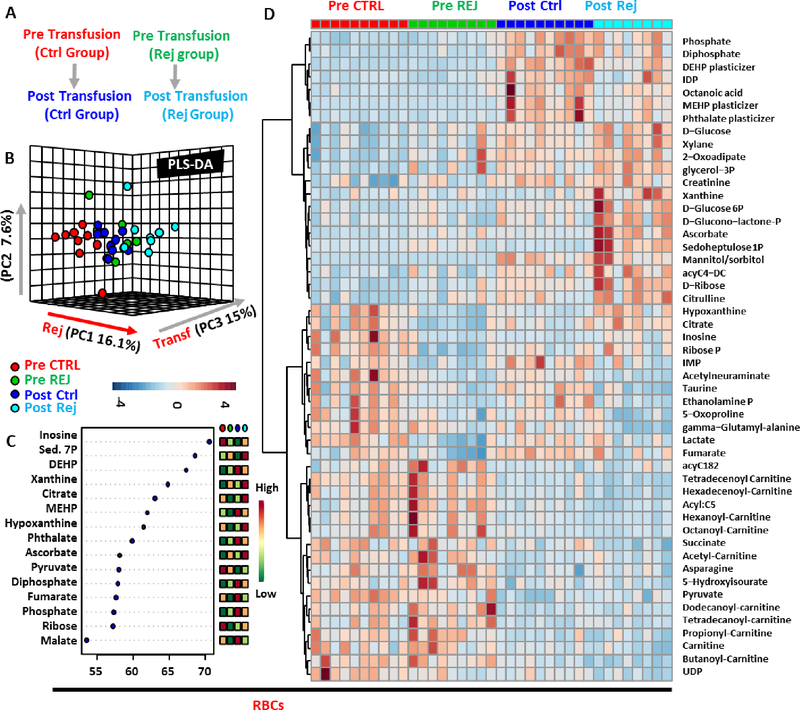
Figure 1– Metabolic changes in RBCs prior to and after RCE with conventional or rejuvenated red blood cells.
An overview of the experimental design (A) and partial least square-discriminant analysis (PLS-DA) of red blood cell (RBC – B) metabolic phenotypes in sickle cell anemia (SCA) patients prior to or 5h after RCE with standard or rejuvenated RBCs. In C, top 15 metabolites from the Variabile Importance in Projection plot. In D, top 50 metabolic changes by ANOVA.
Commercial reagents were purchased from Sigma-Aldrich (Saint Louis, MO) unless otherwise noted. Plasma and RBCs were separated by centrifugation at 2500 × g for 10 min at 4°C. Rejuvenation protocols (Zimmer Biomet, Braintree, MA, USA) were performed in the blood bank, as extensively described in prior work28,29, through approved FDA protocols. For logistical reasons, only the last 4 RBC units were rejuvenated, although the final units would be expected to comprise the majority of circulating, transfused RBCs remaining after an automated RCE.
Sample processing and metabolite extraction
RBC and plasma samples were extracted at 1:10 dilution (10 uL in 90 uL) and 1:25 dilution (20 uL and 480 uL), respectively, in ice-cold lysis/extraction buffer (methanol:acetonitrile:water 5:3:2). Samples were agitated (30 min, 4°C) followed by centrifugation (18,213 g, 10 min, 4 °C). Protein pellets were discarded, and supernatant extracts were stored at −80°C prior to metabolomic analyses.
UHPLC-MS metabolomics
Metabolomics analyses were performed as previously reported,55 with minor modifications. Extensive analytical protocols are provided in our recent method papers.30,31 Briefly, plasma and RBC extracts were injected (20 μL and 10 uL, respectively) into a Thermo Vanquish UHPLC system (San Jose, CA, USA) coupled to a Thermo Q Exactive mass spectrometer with electrospray ionization (Thermo Fisher, Bremen, Germany). Metabolites were separated on a Kinetex C18 column (150 × 2.1 mm, 1.7 μm – Phenomenex, Torrance, CA, USA ) at 45°C using a five minute gradient at 450 μl/min from 5% to 95% organic solvent B (mobile phases: A = water, 0.1% formic acid; B = acetonitrile, 0.1% formic acid in positive ion mode or mobile phases: A = 18 mΩ H2O, 1 mM ammonium acetate; B = acetonitrile, 1 mM ammonium acetate for negative ion mode). Technical mixes were generated by pooling aliquots of extracts, and were run every 12 analytical runs, to control for technical variability, as judged by coefficients of variation (CV). CV were determined by calculating the ratios of standard deviations divided by mean measurements for compounds of interest across all technical mix runs. MS analysis and data elaboration was performed as described.32 Metabolite assignments were performed using MAVEN (Princeton, NJ, USA), as described.33 Graphs and statistical analyses (either t-test) were prepared with GraphPad Prism 5.0 (GraphPad Software, Inc, La Jolla, CA) and Metaboanalyst 3.0, as extensively described34.
Results
Vital signs were stable throughout all RCEs, no transfusion reactions or de novo alloimmunization events were seen, and all participants remained crisis free during the study period.
RCE to SCA patients significantly impacts RBC and plasma metabolism
Metabolomics analyses were performed on RBCs and plasma from SCA patients receiving RCE with conventional RBCs (average storage age 16.0 ± 5.3) or rejuvenated RBCs (rejuvenation performed on storage day 22.0 ± 13.5). Results are extensively presented in Supplementary Table 1. Partial least square-discriminant analysis (PLS-DA) highlighted a significant impact of transfusion (explaining 15% of total variance on Principal Component 3 – PC3) and rejuvenation (16.1% on PC1) on the metabolic phenotype of RBCs (Figure 1.B). The top 15 metabolites from the Variable Importance in Projection (VIP) analysis are presented in Figure 1.C and include metabolites from the pentose phosphate pathway (PPP) such as sedoheptulose phosphate, purines (inosine, hypoxanthine), carboxylates (citrate, fumarate, malate) and phthalate plasticizers. An expanded view of the top 50 significant metabolic changes by ANOVA is provided in the heat map in Figure 1.D.
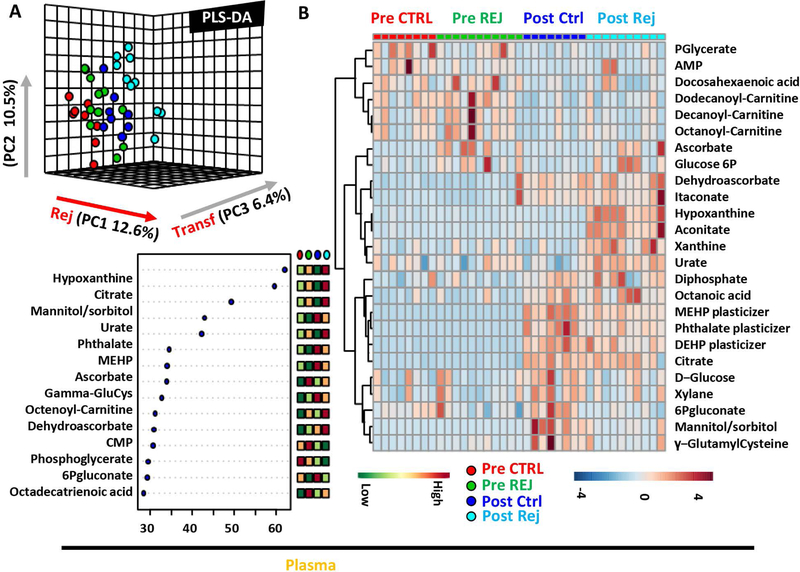
Similarly, RCE significantly impacted plasma metabolism in the recipient, as gleaned by PLS-DA (Figure 2.A), VIP scores (Figure 2.B) and ANOVA (heat map with the top 25 significant changes in Figure 2.C).
Figure 2– Metabolic changes in plasma prior to and after RCE with conventional or rejuvenated red blood cells.
Partial least square-discriminant analysis (PLS-DA) of red blood cell (RBC – A) metabolic phenotypes in sickle cell anemia (SCA) patients prior to or 5h after RCE with standard or rejuvenated RBCs. In B, top 15 metabolites from the Variabile Importance in Projection plot. In C, top 25 metabolic changes by ANOVA.
Upon transfusion, rejuvenated RBCs are characterized by higher antioxidant capacity than conventional stored RBCs
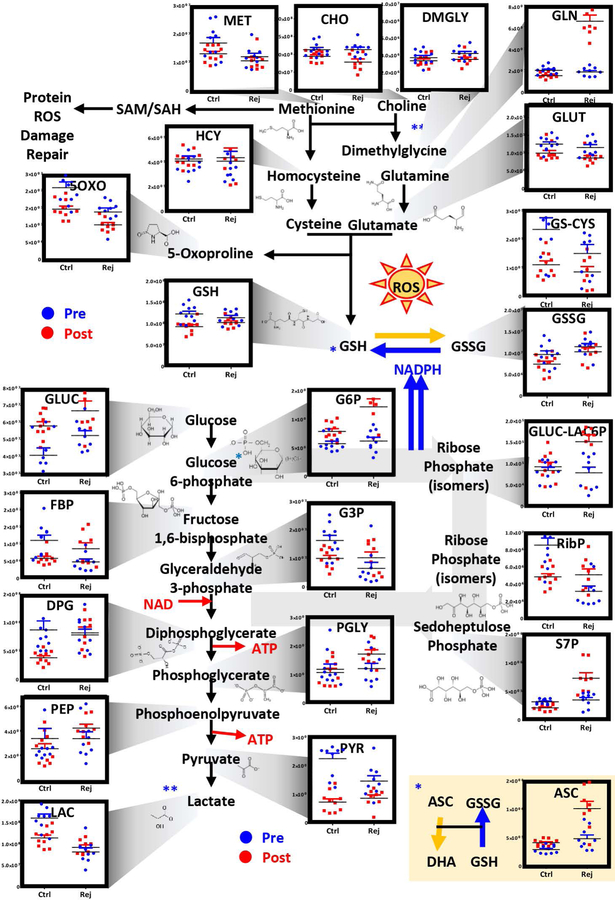
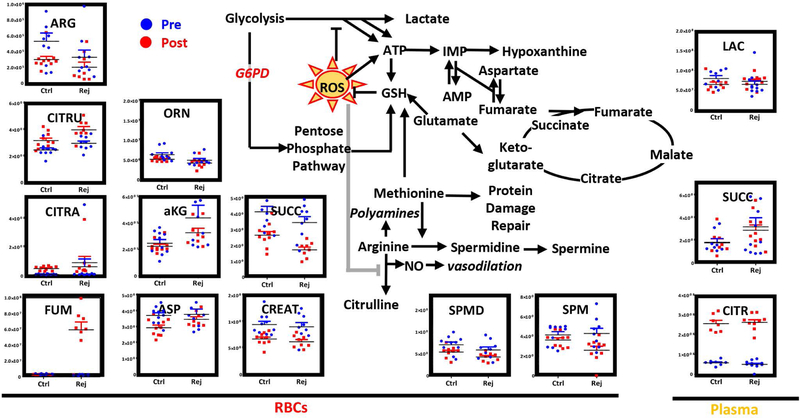
Based on the unsupervised metabolomics data, we focused on the top pathways impacted by transfusion and rejuvenation. Post-transfusion circulating RBCs – independently from rejuvenation – differed from the patients’ own RBCs prior to transfusion with respect to oxidant stress markers (5-oxoproline, glutathionyl-cysteine) and glycolytic byproducts (pyruvate and lactate – Figure 3). Transfusion of either conventional or rejuvenated RBCs normalized glycolysis, by decreasing the RBC levels of pyruvate and lactate. However, rejuvenated RBCs showed higher levels of DPG than conventional RBCs post-transfusion, suggestive of a superior oxygen off-loading capacity35. Of note, RCE with rejuvenated RBCs showed significantly higher levels of glucose 6-phosphate (hexose phosphate isobars) than pre-transfusion RBCs or post-transfusion conventional RBCs (Figure 3). Consistently, the PPP intermediates/byproducts gluconolactone 6-phosphate and sedoheptulose phosphate were significantly higher in the rejuvenated RBC group (Figure 3). Consistently, higher levels of glutamine and ascorbate in rejuvenated RBCs are suggestive of improved glutathione metabolism (Figure 3). Higher levels of reduced glutathione post-transfusion were noted in rejuvenated RBCs (levels that were comparable to those measured in the patients’ own RBCs prior to transfusion) in comparison to conventional RCEs (Figure 3).
Figure 3– Metabolic changes in RBC glycolysis, PPP, glutathione and methionine homeostasis.
prior to (blue) and after RCE (red) with conventional (left) or rejuvenated (right) red blood cell units.
Purine metabolism in RBCs and plasma upon transfusion with conventional and rejuvenated RBCs
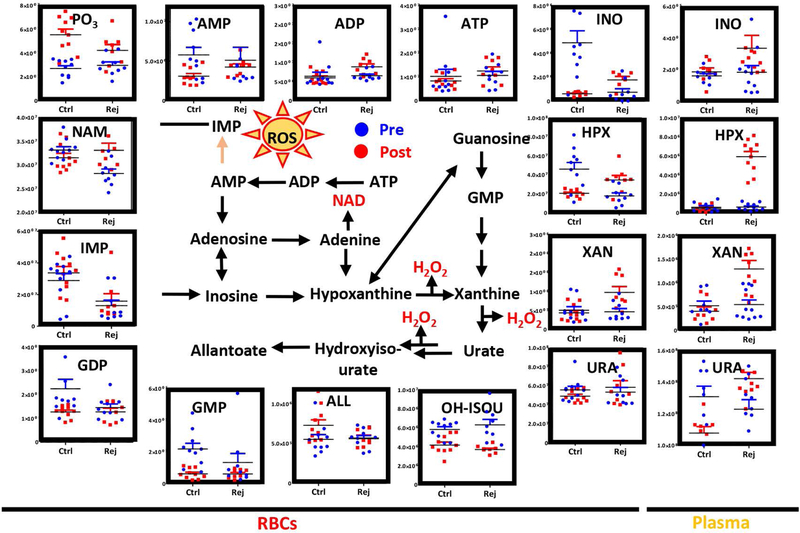
Consistent with a more active glycolysis, post-transfusion circulating RBCs from RCEs with rejuvenated units demonstrated lower levels of phosphate and higher levels of AMP, ADP and ATP than conventional RBCs upon transfusion – though no significant increases were noted in the levels of these metabolites in comparison to pre-transfusion values (Figure 4). On the other hand, rejuvenated RBCs showed higher levels of hypoxanthine and xanthine, but lower levels of hydroxyisourate and IMP, than conventional RBCs post-transfusion (Figure 4). However, plasma levels of these oxidized purines were significantly higher than pre-transfusion levels or post-transfusion levels in the conventional RBC arm of the study (Figure 4) – consistent with the presence of high-levels of inosine in the rejuvenation solution.
Figure 4– Metabolic changes in RBC and plasma purine metabolism.
prior to (blue) and after RCE (red) with conventional (left) or rejuvenated (right) red blood cell units.
Consistent with increased activation of purine oxidation and salvage reactions22, significant depletion of aspartate and accumulation of fumarate was noted in the conventional and rejuvenated RBC arm, respectively upon transfusion (Figure 5). Of note, another carboxylic acid - succinate (a marker of hypoxia) was significantly decreased in RBCs upon transfusion. On the other hand, plasma citrate increased in plasma in both groups upon transfusion, likely as a result of citrate-loaded RBC units (Figure 5).
Figure 5– Metabolic changes in RBC and plasma arginine and carboxylic acid metabolism.
prior to (blue) and after RCE (red) with standard (left) or rejuvenated (right) red blood cell units.
Arginine metabolism was significantly impacted by transfusion, with arginine consumed and citrulline generated in RBCs upon transfusion (Figure 5). Decreased post-transfusion levels of creatine and spermine suggest that arginine consumption was not attributable to polyamine synthesis or amino acid catabolism (Figure 5). On the other hand, given the absence of changes in ornithine levels, these changes in arginine levels suggest alterations in nitric oxide synthase rather than arginase activity21.
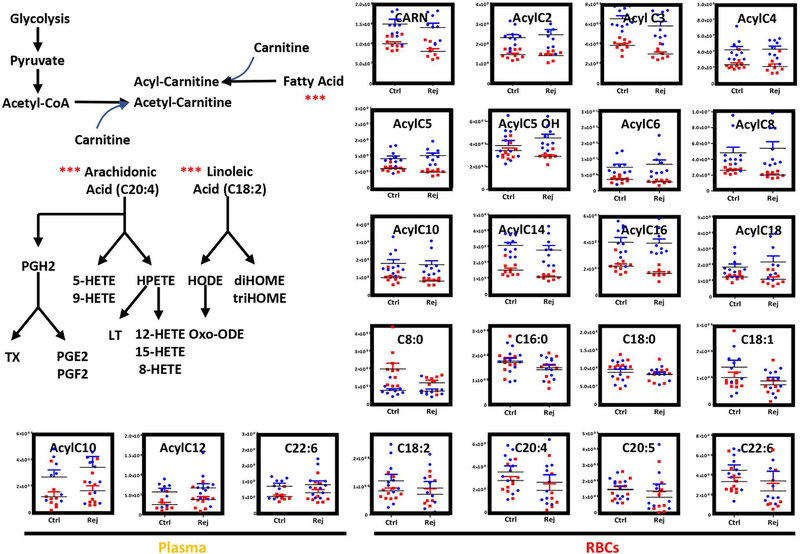
Transfusion – independent of rejuvenation – results in decreased RBC and plasma levels of acyl-carnitines
Consistent with our prior work23, transfusion to patients with SCA resulted in significant decreases in all acyl-carnitines in plasma and RBCs in comparison to pre-transfusion values (Figure 6). Rejuvenation did not significantly impact these parameters.
Figure 6– Metabolic changes in RBC and plasma acyl-carnitines and free fatty acids.
prior to (blue) and after RCE (red) with conventional (left) or rejuvenated (right) red blood cell units.
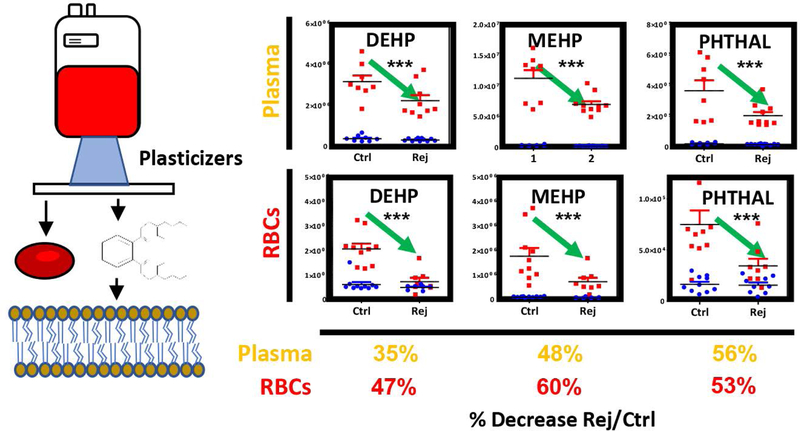
Transfusion of rejuvenated RBCs mitigates the transfusion-related increase in circulating levels of plasticizers
Transfusion of RBCs resulted in significant increases in the levels of phthalate plasticizers, including diethyhexyl-phthalate (DEHP), monoethyhexylphthalate (MEHP) and phthalate (Figure 7). However, both plasma and RBC levels of plasticizers were ~50% lower in patients receiving RCE with rejuvenated RBCs when compared to conventional products, despite the average storage duration of rejuvenated RBCs being one week longer than their conventional counterparts in this study and only the last four units being rejuvenated.
Figure 7– Metabolic changes in RBC and plasma phthalate plasticizers.
prior to (blue) and after RCE (red) with conventional (left) or rejuvenated (right) red blood cell units. Transfusion of rejuvenated RBCs significantly decreased the RBC and circulating levels of plasticizers in transfused patients.
Discussion
RBC storage is a logistic necessity to make >100 million units available for transfusion every year worldwide. Despite a century of advancements36,37, current storage strategies result in progressive biochemical and morphological changes that ultimately impact the quality of stored blood1. To address this issue, over the past decade, systematic efforts from our group and others focused on the characterization of RBC metabolism as a function of blood storage in all currently marketed storage additives, including MAP, SAGM, AS-1, AS-3, AS-5, PAGGSM3,4,38–42. Attention has recently shifted to other factors contributing to the metabolic heterogeneity of stored blood units43, such as the impact of processing strategies. Indeed, processing strategies can be leveraged to mitigate the severity of the storage lesion, or even reverse it to a certain extent. For example, rejuvenation of stored RBCs is a viable strategy to reverse at least in part the metabolic age of the erythrocyte – especially the storage-dependent consumption of 2,3-DPG - and, as a result, ameliorate the RBC capacity to transport and deliver oxygen upon transfusion28,29,35,44,45.
In an attempt to expand our understanding of transfusion biology, after extensive work on the molecular characterization of the blood product per se (i.e. using omics technologies to understand “what’s in the unit”46), we started a systematic effort to understand the impact of blood transfusion on systems metabolism in the recipient. First, we analyzed the impact of fresh versus old blood on autologous healthy volunteers21. This study confirmed that most of the storage-dependent metabolic changes to RBCs and supernatants are transferred from the unit to the bloodstream of the recipient. Such changes included post-transfusion increases in circulating levels of oxidized purines and lipids, decreases in vasodilatory nitric oxide-generating reactions, as well as significant increases in circulating levels of potentially harmful47–49 phthalate plasticizers. While studies of autologous transfusions in healthy volunteers may facilitate the interpretation of the impact of blood transfusion within the context of a controlled setting, understanding the impact of blood transfusion on the metabolism of a sick patient requiring transfusion is clearly more clinically relevant. As such, we recently investigated the impact of RCE on patients with SCA23, a category of recipients requiring lifelong chronic transfusion. Results suggested that RBCs stored for ~2 weeks (average storage duration for these exchanges at Duke University) were still capable of regenerating high energy phosphate compounds ATP and DPG within 5h from transfusion to levels above those measured in patients’ own sickle RBCs before transfusion23. This is relevant owing to the role of these metabolites in stabilizing the tense deoxygenated state of hemoglobin and promoting oxygen off-loading50, suggesting that RCE was effective in restoring systemic tissue oxygenation in patients with SCA. Despite these benefits, transfused stored RBCs appeared to have lower antioxidant capacity (e.g. lower levels of glutathione and pentose phosphate pathway) in comparison to pre-transfusion SCA, suggestive of (i) compensatory mechanisms in the sickle RBC to mitigate oxidative injury and/or (ii) a long lasting impact of storage-induced decreased in the total glutathione pool38,51,52 that persists within 5h from transfusion. In order to test the latter hypothesis, in the present study we leveraged metabolic rejuvenation of stored RBCs as a strategy to boost RBC energy and redox pathways28 by providing inosine, pyruvate and phosphate to fuel glycolysis and PPP prior to transfusion. As a result, transfusion of rejuvenated RBCs proved to be superior to transfusion of conventionally stored RBCs, in terms of post-transfusion RBCs with improved energy metabolism (glycolysis) and antioxidant metabolism (PPP at the oxidative and non-oxidative phase, glutathione, ascorbate and glutamine/transamination metabolism). Decreased oxidant stress after transfusion of rejuvenated RBCs appeared to also benefit the synthesis of the vasodilating agent nitric oxide through metabolism of arginine to citrulline by RBC nitric oxide synthase21, which is otherwise inhibited by oxidant stress. While therapies impacting nitric oxide synthesis in vivo can interfere with the generation of volatile organic compounds, detection of those compounds is not compatible with the workflows and instrumentation used in this study. However, transfusion of rejuvenated RBCs was associated with increased plasma levels of oxidized purines (hypoxanthine, xanthine, urate). While plasma urate may represent a potent antioxidant53,54, the very generation of this metabolism by catabolism of xanthine also results in the production of hydrogen peroxide.
Transfusion of rejuvenated RBCs normalized (i.e. decreased) the circulating levels of acyl-carnitines, as did conventional stored RBCs. Though potentially irrelevant in the sickle cell population, this observation may be relevant in bleeding patients, in the light of the anticoagulant effect of acyl-carnitines55,56. On the other hand, rejuvenated RBCs significantly decrease (on average, by cutting in half) the RBC and circulating levels of post-transfusion plasticizers, potentially harmful compounds that have been previously associated with prostate cancer and fertility issues in males57 and reproductive outcomes in female in laboratory studies49,58. Notably, plasticizers from the initial conventional units transfused to the rejuvenated group, likely remained in circulation despite subsequent erythrocytapheresis, reducing the effectiveness of the post-rejuvenation washing step in minimizing phthalate exposure. During storage, phthalate plasticizers leach progressively from the plastic bags into the supernatants and intercalate into RBC membranes, while reaching up to mM levels by the end of the shelf-life of the unit5. In this view, RCE with rejuvenated RBCs may limit the exposure to phthalate plasticizers, a potentially beneficial effect of particular relevance in chronically transfused patients subject to this therapy since childhood.
Conclusion
Improving RBC storage quality through data-driven approaches is an achievable goal with current omics tools and systems biology approaches59. However, such goal can be met only once three components are systemically characterized: (i) the blood product, involving the impact of current processing strategies and storage additives; (ii) the donor, involving the impact of donor biology – age, sex, ethnicity, donation frequency, diet, exercise or other environmental factors such as smoking; (iii) the recipient(s), involving the heterogeneity of biomedical conditions and metabolic demands of the recipients requiring transfusion and the best way to correct such demand. In this view, although a patient with SCA and another with active bleeding and coagulopathy experience hypoxia, they may have different metabolic needs – as data from independent studies would suggest60,61.
While the advent of high-throughput omics technologies62 will facilitate progress in the characterization of large donor and recipient populations, in the present study we provide the first preliminary clinical evidence that a processing strategy using metabolic rejuvenation of stored RBC units, can contribute to maximizing the metabolic benefits of RCE in patients with SCA. The benefits include boosting transfused RBC energy, redox and arginine/nitric oxide metabolism, normalization of circulating levels of acyl-carnitines and decreased exposure to phthalate plasticizers.
Supplementary Material
Acknowledgments
Research reported in this publication was supported in part by funds from the Boettcher Webb-Waring Biomedical Research Award – Early Career grant (ADA) and Zimmer Biomet (AD and IW), NIH R01HL121232–01 (IJW), NHLBI R01 HL146442 (AD) and RM1GM131968 (AD) from the National Institute of General Medical Sciences (NIGMS).
Disclosure of Conflict of interest ADA is a founder of Omix Technologies Inc. ADA is a consultant for Hemanext Inc. AG and ML are employees of Zimmer Biomet. All the other authors disclose no conflict of interest relevant to this study. IJW is funded by NIH R01HL121232–01. ADA is a recipient of the Boettcher Webb-Waring Early Career 2017 RM1GM131968 from the National Institute of General Medical Sciences (NIGMS). This study was supported in part by funds from Zimmer Biomet to AD and IW.
References
- 1.Yoshida T, Prudent M, D’alessandro A. Red blood cell storage lesion: causes and potential clinical consequences. Blood Transfus. Trasfus. Sangue 2019;17(1):27–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yurkovich JT, Zielinski DC, Yang L, et al. Quantitative time-course metabolomics in human red blood cells reveal the temperature dependence of human metabolic networks. J. Biol. Chem 2017;292(48):19556–19564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.D’Alessandro A, Reisz JA, Culp-Hill R, et al. Metabolic effect of alkaline additives and guanosine/gluconate in storage solutions for red blood cells. Transfusion . 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rolfsson Ó, Sigurjonsson ÓE, Magnusdottir M, et al. Metabolomics comparison of red cells stored in four additive solutions reveals differences in citrate anticoagulant permeability and metabolism. Vox Sang. 2017;112(4):326–335. [DOI] [PubMed] [Google Scholar]
- 5.D’Alessandro A, Nemkov T, Hansen KC. Rapid detection of DEHP in packed red blood cells stored under European and US standard conditions. Blood Transfus. 2016;14(2):140–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bordbar A, Johansson PI, Paglia G, et al. Identified metabolic signature for assessing red blood cell unit quality is associated with endothelial damage markers and clinical outcomes. Transfusion . 2016;56(4):852–862. [DOI] [PubMed] [Google Scholar]
- 7.D’Alessandro A, Nemkov T, Kelher M, et al. Routine storage of red blood cell (RBC) units in additive solution-3: a comprehensive investigation of the RBC metabolome. Transfusion . 2015;55(6):1155–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wither M, Dzieciatkowska M, Nemkov T, et al. Hemoglobin oxidation at functional amino acid residues during routine storage of red blood cells. Transfusion . 2016;56(2):421–426. [DOI] [PubMed] [Google Scholar]
- 9.Reisz JA, Wither MJ, Dzieciatkowska M, et al. Oxidative modifications of glyceraldehyde 3-phosphate dehydrogenase regulate metabolic reprogramming of stored red blood cells. Blood. 2016;blood-2016–05-714816. [DOI] [PubMed] [Google Scholar]
- 10.D’Alessandro A, D’Amici GM, Vaglio S, Zolla L. Time-course investigation of SAGM-stored leukocyte-filtered red bood cell concentrates: from metabolism to proteomics. Haematologica. 2012;97(1):107–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rapido F, Brittenham GM, Bandyopadhyay S, et al. Prolonged red cell storage before transfusion increases extravascular hemolysis. J. Clin. Invest 2017;127(1):375–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.D’Alessandro A, Kriebardis AG, Rinalducci S, et al. An update on red blood cell storage lesions, as gleaned through biochemistry and omics technologies. Transfusion . 2015;55(1):205–219. [DOI] [PubMed] [Google Scholar]
- 13.Karafin MS, Fu X, D’Alessandro A, et al. The clinical impact of glucose-6-phosphate dehydrogenase deficiency in patients with sickle cell disease. Curr. Opin. Hematol 2018;25(6):494–499. [DOI] [PubMed] [Google Scholar]
- 14.Reisz JA, Wither MJ, Dzieciatkowska M, et al. Oxidative modifications of glyceraldehyde 3-phosphate dehydrogenase regulate metabolic reprogramming of stored red blood cells. Blood. 2016;128(12):e32–42. [DOI] [PubMed] [Google Scholar]
- 15.Tzounakas VL, Kriebardis AG, Georgatzakou HT, et al. Glucose 6-phosphate dehydrogenase deficient subjects may be better “storers” than donors of red blood cells. Free Radic. Biol. Med 2016;96:152–165. [DOI] [PubMed] [Google Scholar]
- 16.D’Alessandro A, Hansen KC, Eisenmesser EZ, Zimring JC. Protect, repair, destroy or sacrifice: a role of oxidative stress biology in inter-donor variability of blood storage? Blood Transfus. Trasfus. Sangue 2019;1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reisz JA, Nemkov T, Dzieciatkowska M, et al. Methylation of protein aspartates and deamidated asparagines as a function of blood bank storage and oxidative stress in human red blood cells. Transfusion . 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.D’Alessandro A, Dzieciatkowska M, Nemkov T, Hansen KC. Red blood cell proteomics update: is there more to discover? Blood Transfus. 2017;15(2):182–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Belpulsi D, Spitalnik SL, Hod EA. The controversy over the age of blood: what do the clinical trials really teach us? Blood Transfus. 2017;15(2):112–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.D’Alessandro A, Zimring JC, Busch M. Chronological storage age and metabolic age of stored red blood cells: are they the same? Transfusion . 2019; [DOI] [PubMed] [Google Scholar]
- 21.D’Alessandro A, Reisz JA, Zhang Y, et al. Effects of aged stored autologous red blood cells on human plasma metabolome. Blood Adv. 2019;3(6):884–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nemkov T, Sun K, Reisz JA, et al. Hypoxia modulates the purine salvage pathway and decreases red blood cell and supernatant levels of hypoxanthine during refrigerated storage. Haematologica. 2018;103(2):361–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Culp-Hill R, Srinivasan J, Gehrke S, et al. Effects of red blood cell (RBC) transfusion on sickle cell disease recipient plasma and RBC metabolism. Transfusion . 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yawn BP, Buchanan GR, Afenyi-Annan AN, et al. Management of sickle cell disease: summary of the 2014 evidence-based report by expert panel members. JAMA. 2014;312(10):1033–1048. [DOI] [PubMed] [Google Scholar]
- 25.Goodman SR, Pace BS, Hansen KC, et al. Minireview: Multiomic candidate biomarkers for clinical manifestations of sickle cell severity: Early steps to precision medicine. Exp. Biol. Med. Maywood NJ 2016;241(7):772–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wun T, Hassell K. Best practices for transfusion for patients with sickle cell disease. Hematol. Rev 2010;1(2):. [Google Scholar]
- 27.Shah N, Welsby IJ, Fielder MA, Jacobsen WK, Nielsen VG. Sickle cell disease is associated with iron mediated hypercoagulability. J. Thromb. Thrombolysis 2015;40(2):182–185. [DOI] [PubMed] [Google Scholar]
- 28.D’Alessandro A, Gray AD, Szczepiorkowski ZM, et al. Red blood cell metabolic responses to refrigerated storage, rejuvenation, and frozen storage. Transfusion . 2017;57(4):1019–1030. [DOI] [PubMed] [Google Scholar]
- 29.Gehrke S, Srinivasan AJ, Culp-Hill R, et al. Metabolomics evaluation of early-storage red blood cell rejuvenation at 4°C and 37°C. Transfusion . 2018;58(8):1980–1991. [DOI] [PubMed] [Google Scholar]
- 30.Reisz JA, Zheng C, D’Alessandro A, Nemkov T. Untargeted and Semi-targeted Lipid Analysis of Biological Samples Using Mass Spectrometry-Based Metabolomics. Methods Mol. Biol. Clifton NJ 2019;1978:121–135. [DOI] [PubMed] [Google Scholar]
- 31.Nemkov T, Reisz JA, Gehrke S, Hansen KC, D’Alessandro A. High-Throughput Metabolomics: Isocratic and Gradient Mass Spectrometry-Based Methods. Methods Mol. Biol. Clifton NJ 2019;1978:13–26. [DOI] [PubMed] [Google Scholar]
- 32.D’Alessandro A, Nemkov T, Yoshida T, et al. Citrate metabolism in red blood cells stored in additive solution-3. Transfusion . 2017;57(2):325–336. [DOI] [PubMed] [Google Scholar]
- 33.Nemkov T, Hansen KC, D’Alessandro A. A three-minute method for high-throughput quantitative metabolomics and quantitative tracing experiments of central carbon and nitrogen pathways. Rapid Commun. Mass Spectrom. RCM 2017;31(8):663–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xia J, Sinelnikov IV, Han B, Wishart DS. MetaboAnalyst 3.0—making metabolomics more meaningful. Nucleic Acids Res. 2015;43(Web Server issue):W251–W257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Inglut C, Kausch K, Gray A, Landrigan M. Rejuvenation of Stored Red Blood Cells Increases Oxygen Release Capacity. Blood. 2016;128(22):4808–4808. [Google Scholar]
- 36.Hess JR. An update on solutions for red cell storage. Vox Sang. 2006;91(1):13–19. [DOI] [PubMed] [Google Scholar]
- 37.Greenwalt TJ A short history of transfusion medicine. Transfusion . 2003;37(5):550–563. [DOI] [PubMed] [Google Scholar]
- 38.D’Alessandro A, Nemkov T, Kelher M, et al. Routine Storage of Red Blood Cell Units in Additive Solution-3: a comprehensive investigation of the RBC metabolome. Transfusion . 2015;55(6):1155–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yachie-Kinoshita A, Nishino T, Shimo H, Suematsu M, Tomita M. A Metabolic Model of Human Erythrocytes: Practical Application of the E-Cell Simulation Environment. J. Biomed. Biotechnol 2010;2010:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gevi F, D’Alessandro A, Rinalducci S, Zolla L. Alterations of red blood cell metabolome during cold liquid storage of erythrocyte concentrates in CPD-SAGM. J. Proteomics 2012;76 Spec No.:168–180. [DOI] [PubMed] [Google Scholar]
- 41.D’Alessandro A, Hansen KC, Silliman CC, et al. Metabolomics of AS-5 RBC supernatants following routine storage. Vox Sang. 2014;108(2):131–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bordbar A, Johansson PI, Paglia G, et al. Identified metabolic signature for assessing red blood cell unit quality is associated with endothelial damage markers and clinical outcomes. Transfusion . 2016;56(4):852–862. [DOI] [PubMed] [Google Scholar]
- 43.D’Alessandro A, Culp-Hill R, Reisz JA, et al. Heterogeneity of blood processing and storage additives in different centers impacts stored Red Blood Cell metabolism as much as storage time: lessons from REDS III – Omics. Transfusion . 2018; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meyer EK, Dumont DF, Baker S, Dumont LJ. Rejuvenation capacity of red blood cells in additive solutions over long-term storage. Transfusion . 2011;51(7):1574–1579. [DOI] [PubMed] [Google Scholar]
- 45.Valeri CR, Zaroulis CG, Vecchione JJ, et al. Therapeutic effectiveness and safety of outdated human red blood cells rejuvenated to improve oxygen transport function, frozen for about 1.5 years at 80 C, washed, and stored at 4 C for 24 hours prior to rapid infusion. Transfusion . 1980;20(3):263–276. [DOI] [PubMed] [Google Scholar]
- 46.Spitalnik SL, Triulzi D, Devine DV, et al. 2015 proceedings of the National Heart, Lung, and Blood Institute’s State of the Science in Transfusion Medicine symposium. Transfusion . 2015;55(9):2282–2290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hannon PR, Brannick KE, Wang W, Gupta RK, Flaws JA. Di(2-ethylhexyl) phthalate inhibits antral follicle growth, induces atresia, and inhibits steroid hormone production in cultured mouse antral follicles. Toxicol. Appl. Pharmacol 2015;284(1):42–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.D’alessandro A, Nemkov T, Hansen KC. Rapid detection of DEHP in packed red blood cells stored under European and US standard conditions. Blood Transfus. Trasfus. Sangue 2016;14(2):140–144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Niermann S, Rattan S, Brehm E, Flaws JA. Prenatal exposure to di-(2-ethylhexyl) phthalate (DEHP) affects reproductive outcomes in female mice. Reprod. Toxicol 2015;53:23–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sun K, Zhang Y, D’Alessandro A, et al. Sphingosine-1-phosphate promotes erythrocyte glycolysis and oxygen release for adaptation to high-altitude hypoxia. Nat. Commun 2016;7:12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.D’Alessandro A, Nemkov T, Yoshida T, et al. Citrate metabolism in red blood cells stored in additive solution-3. Transfusion . 2017;57(2):325–336. [DOI] [PubMed] [Google Scholar]
- 52.Whillier S, Raftos JE, Sparrow RL, Kuchel PW. The effects of long-term storage of human red blood cells on the glutathione synthesis rate and steady-state concentration. Transfusion . 2011;51(7):1450–1459. [DOI] [PubMed] [Google Scholar]
- 53.Tzounakas VL, Georgatzakou HT, Kriebardis AG, et al. Uric acid variation among regular blood donors is indicative of red blood cell susceptibility to storage lesion markers: A new hypothesis tested. Transfusion . 2015;55(11):2659–2671. [DOI] [PubMed] [Google Scholar]
- 54.Tzounakas VL, Karadimas DG, Anastasiadi AT, et al. Donor-specific individuality of red blood cell performance during storage is partly a function of serum uric acid levels. Transfusion . 2018;58(1):34–40. [DOI] [PubMed] [Google Scholar]
- 55.Deguchi H, Banerjee Y, Trauger S, et al. Acylcarnitines are anticoagulants that inhibit factor Xa and are reduced in venous thrombosis, based on metabolomics data. Blood. 2015;126(13):1595–1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zimring JC. Does AC stand for acylcarnitine, anticoagulant, or both? Blood. 2015;126(13):1524–1525. [DOI] [PubMed] [Google Scholar]
- 57.Kismali G, Yurdakok-Dikmen B, Kuzukiran O, Arslan P, Filazi A. Phthalate induced toxicity in prostate cancer cell lines and effects of alpha lipoic acid. Bratisl. Lek. Listy 2017;118(8):460–466. [DOI] [PubMed] [Google Scholar]
- 58.Kay VR, Chambers C, Foster WG. Reproductive and developmental effects of phthalate diesters in females. Crit. Rev. Toxicol 2013;43(3):200–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yurkovich JT, Bordbar A, Sigurjónsson ÓE, Palsson BO. Systems biology as an emerging paradigm in transfusion medicine. BMC Syst. Biol 2018;12:. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sun K, D’Alessandro A, Ahmed MH, et al. Structural and Functional Insight of Sphingosine 1-Phosphate-Mediated Pathogenic Metabolic Reprogramming in Sickle Cell Disease. Sci. Rep 2017;7(1):15281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Peltz ED, D’Alessandro A, Moore EE, et al. Pathologic metabolism: an exploratory study of the plasma metabolome of critical injury. J. Trauma Acute Care Surg. 2015;78(4):742–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nemkov T, Hansen KC, D’Alessandro A. A three-minute method for high-throughput quantitative metabolomics and quantitative tracing experiments of central carbon and nitrogen pathways. Rapid Commun. Mass Spectrom. RCM 2017;31(8):663–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.