Abstract
Chronic adolescent stress alters behavior in a sex-specific manner at the end of adolescence and in adulthood. Although prolonged behavioral repercussions of chronic adolescent stress have been documented, the potential underlying mechanisms are incompletely understood. In this study we demonstrate that a history of chronic adolescent stress modified the adult stress response, as measured by corticosterone concentration, such that a history of chronic adolescent stress resulted in a blunted response to a novel acute stressor. In order to begin to address potential mechanistic underpinnings, we assessed the extent to which chronic adolescent stress impacted global DNA methylation. Reduced global hippocampal methylation was evident in females with a history of chronic adolescent stress; thus, it was possible that chronic adolescent stress altered global transcription in the whole hippocampi of adult male and female rats. In addition, because acute stress can stimulate a genomic response, we assessed the transcriptome following exposure to an acute novel stressor to determine the extent to which a history of chronic adolescent stress modifies the adult transcriptional response to an acute stressor in males and females. In addition to the reduction in global methylation, chronic adolescent stress resulted in distinct patterns of gene expression in the adult hippocampus that differentiated by sex. Furthermore, both sex and a history of chronic adolescent stress influenced the transcriptional response to an acute novel stressor in adulthood, suggesting both latent and functional effects of chronic adolescent stress at the level of gene transcription. Pathway analysis indicated that ESR1 and IFN-α may be particularly influential transcription factors mediating these transcriptional differences and suggest candidate mechanisms for future studies. Collectively, these studies demonstrate sex-specific and enduring effects of adolescent stress exposure that are more pronounced in females than in males.
Subject terms: Stress and resilience, Gene expression
Introduction
Women are more likely than men to develop stress-related disorders such as depression [1]. These sex differences in incidence of stress-related disorders begin during adolescence [2]. However, incomplete knowledge is currently available regarding the mechanisms which mediate the long-term impact of chronic adolescent stress on neural endpoints and the extent to which adolescent stress may differentially impact males and females. Extensive neuronal [3, 4] and neuroendocrine maturation occurs during adolescence, resulting in a vulnerable developmental period that may be prone to disruption by chronic stress exposure [5]. Evidence suggests that stress exposure during adolescence may be more behaviorally disruptive than exposure during adulthood [6, 7]. A model of chronic adolescent stress (CAS) exposure in rats has previously been shown to alter the adolescent stress response [8, 9] and increase anxiety-like and depressive-like behavior in adolescent and adult females, but not males [10]. These findings point to both short- and long-term consequences of CAS.
The hippocampus is involved in regulating feedback of the hypothalamic pituitary adrenal (HPA) axis [11, 12], is stress sensitive [13], and expresses receptors for stress and sex hormones [12, 14–16]. Previously, we have demonstrated that female rats that exhibit alterations in stress-sensitive gene expression in the hippocampus following CAS have a prolonged corticosterone response to a novel acute stress challenge at the end of adolescence [9]. However, it is not known if these effects are transient or sustained into adulthood at either the systemic level of corticosterone release or the level of altered gene expression. The CA3 region of the hippocampus has been implicated as a site of sex-specific alterations to the transcriptome following acute stressor exposure in adulthood [13]; however, the extent to which sex differences persist following CAS exposure, and the ability of adolescent stress to modify the adult transcriptional response to an acute stressor, have not yet been established. Though previous studies have focused on the effects of CAS on specific gene targets [9, 17], the impact of CAS is likely widespread, impacting numerous transcriptional networks.
Because of the previously observed effects of CAS on selected gene targets [9, 17], and because any changes observed in adult gene expression following CAS exposure have likely persisted since adolescent exposure to stress, we assessed DNA methylation to determine if a history of adolescent stress was sufficient to cause an enduring and sex-specific change in a global marker of epigenetic modulation. We measured global methylation and gene expression of DNA methyltransferases (DNMTs; enzymes that transfer a methyl group to DNA) in adult male and female rats with a history of CAS exposure. As this CAS paradigm has previously been documented to cause long-lasting changes in behavior [8], neuroinflammatory response to challenge in adulthood [17], and HPA axis function at the end of adolescence [9], it was hypothesized that CAS could also alter DNA methylation in adulthood.
In addition, due to the potential for reduced global methylation to impact gene transcription, we used the discovery-based method RNA sequencing to assess the global impact of CAS on the adult hippocampal transcriptome in male and female rats. RNA sequencing allows assessment of global transcriptional patterns and the opportunity to map altered genes to biological pathways to identify networks that are most profoundly impacted by CAS exposure in the whole hippocampus. In addition, previous studies have demonstrated that some effects of prior chronic stress exposure are latent and require an acute stressor challenge to be unmasked [9]. Therefore, to determine the extent to which prior exposure to CAS impacts adult stress responsivity, we probed hippocampal reactivity with an acute novel swim stressor in addition to assessing baseline global transcription. Collectively, the data presented here demonstrate that a history of chronic adolescent stress causes pervasive alterations in the genomics of the hippocampus, and that these effects interact with biological sex, as CAS effects are more pronounced in females than males.
Materials and methods
Animal husbandry
Male and female Wistar rats from Charles Rivers (Durham, NC) were bred to generate litters. Rats were housed on a 14:10 reverse light/dark cycle with standard rat chow and water available ad libitum. Litters were culled to eight pups (4 male, 4 female) on postnatal day (PND) 3, and rats were weaned on PND 21. Forty-eight male and female Wistar rats were used for the corticosterone assessment and RNA-sequencing studies at Emory University (n = 6/group). Additional rats were used in targeted real-time quantitative reverse transcription-PCR experiments (total non-stress (NS)-male: n = 9; NS-female: n = 10; CAS-male: n = 10; CAS-female: n = 10) at Emory University. A separate group of rats raised from timed-pregnant dams (Charles River, Durham, NC) at Virginia Commonwealth University was used for DNA methylation assessment and baseline corticosterone concentrations. Throughout all experiments, rats were housed in AALAC (American Association for Laboratory Animal Care)-approved facilities, and all studies were approved by the Institutional Animal Care and Use Committees at the respective universities.
Mixed-modality chronic adolescent stress paradigm
On PND 35, Wistar rats assigned to the CAS groups were individually housed. NS controls continued to be paired with same-sex littermates. From PND 38–49, the CAS groups were exposed to twelve alternating days of social defeat (6 days) and restraint (6 days) exposures as previously described (Fig. 1a) [8, 9]. For the restraint paradigm, each Wistar rat was placed in a clear plastic restraint tube (Braintree Scientific, Braintree, MA) for 1 h. For the social defeat paradigm, the adolescent Wistar rat was placed in the home cage of an adult Long Evans same-sex rat for a 2 min habituation period separated by a barrier that allows visual and olfactory cues, followed by 5 min of physical interaction or three pins, whichever comes first, and then an additional 25 min separated by the barrier. Male Long Evans rats were housed with ovariectomized female Long Evans rats as previously established [8, 9].
Fig. 1.
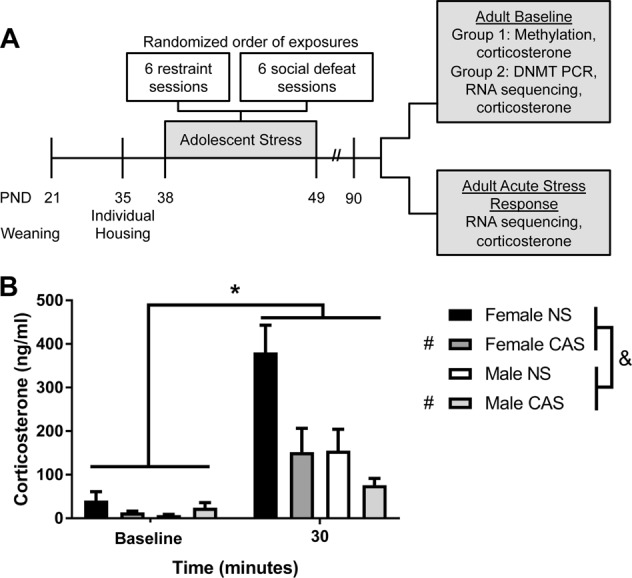
a Timeline of experiments. Rats were exposed to chronic adolescent stress (CAS) from postnatal day (PND) 38–49 and assessed during adulthood. Separate groups of rats were used for DNA methylation experiments and RNA sequencing, DNMT PCR, and corticosterone experiments. b Plasma corticosterone was assessed at baseline and following acute stressor exposure (30 min following acute swim stress). A three-way analysis of variance (ANOVA) (sex × adolescent stress × acute stress) revealed a significant main effect of acute stress (*), adolescent stress (#), and sex (&). Furthermore, there was a significant sex by acute stress interaction and a significant adolescent by acute stress interaction (p < 0.05). Data are represented as mean + SEM
Tissue collection, estrous cycle assessment, and corticosterone assessment
In adulthood (PND 90–120; balanced among sexes and groups), tissue and trunk blood were collected via rapid decapitation at baseline (no acute stressor exposure) or 30 min following the start of exposure to a novel 5 min swim stressor (acute stress challenge) [9]. Brains used in transcriptomic analyses were extracted, frozen on dry ice, and stored at −80 °C. Brains for methylation were fresh dissected. Uterine weights were acquired at collection and assessed for group differences indicative of variability in estrous cycle stage. Trunk blood was stored in microcentrifuge tubes at −80 °C until assayed for circulating plasma corticosterone concentrations (enzyme-linked immunosorbent assay (ELISA); Enzo Life Sciences, Farmingdale, NY, USA, cat. no. ADI−901-907) conducted per manufacturer’s protocol (sensitivity = 0.027 ng/µl; see Supplemental Methods).
DNA methylation
Genomic DNA was isolated from the left hemisphere of a freshly dissected adult rat hippocampus (NS-female: n = 5; NS-male: n = 6; CAS-female: n = 5; CAS-male: n = 6) using the DNEasy Blood & Tissue kit in combination with RNAse A (Qiagen Inc., Germantown, MD). DNA quality and concentration were assayed by Nanodrop One (Thermo Fisher Scientific, Waltham, MA). MethylFlash Methylated DNA 5-mC Quantification Kit (Colorimetric) (EpiGentek, Farmingdale, NY) was performed according to the manufacturer’s instructions (additional details in Supplementary Methods).
RNA isolation and sequencing
The left hemisphere of the whole hippocampus was dissected free-hand and used for RNA extraction. RNA-sequencing analysis was conducted at the Yerkes NHP Genomics Core Laboratory. Total RNA was prepared using QIAGEN RNeasy kits (Germantown, MD). The quality of total RNA was assessed on the Agilent bioanalyzer instrument (Santa Clara, CA). Available RIN values were >8. Polyadenylated transcripts were purified on oligo-dT magnetic beads, fragmented, reverse transcribed using random hexamers, and incorporated into barcoded complementary DNA libraries based on the Illumina TruSeq platform. Libraries were validated by microelectrophoresis, quantified, pooled, and clustered on Illumina TruSeq v3 flowcells (San Diego, CA). Clustered flowcells were sequenced to achieve target read depth of 15 million reads per sample on an Illumina HiSeq 1000 in 100-base paired end-read reactions.
RNA-sequencing analysis
Sequenced reads were aligned to the rat RefSeq rn5 genomic reference using the STAR aligner (v2.4.0g1) [18]. Counts of reads that uniquely map to genes in the rn5 reference annotation were accumulated using “htseq-count” (HTSeq 0.6.1p1) [19]. EdgeR [20, 21] was used to assess differential gene expression. In order to determine the long-term impact of exposure to chronic stress during adolescence on gene expression in the adult hippocampus, one set of comparisons contrasted gene expression of CAS vs. NS groups in adulthood at baseline with males and females compared separately. In order to determine the extent to which a history of chronic adolescent stress changed the genes expressed following an acute stressor in adulthood, CAS vs. NS groups were contrasted following an acute stressor in adulthood with males and females compared separately (30 min following forced swim). Groups compared for these differential expression analyses are detailed in Table 1 and Fig. 3. To determine the magnitude of the transcriptional response within NS and CAS groups in response to an acute stressor, baseline and 30 min post-acute stress gene expression were compared separately in rats without a history of chronic adolescent stress (NS) and in those with a history of CAS. Adult males and females were again compared separately—detailed in Table 1 and Fig. 4. To evaluate global shifts and pathway analyses following CAS, we used a significance criteria for differentially expressed genes (DEGs) of fold change (FC) of 1.3 (or 1/1.3) and uncorrected p value of 0.05 to assess global patterns in gene expression changes as previously performed [22, 23] (Figs. 3 and 4). FCs were expressed as log2(FC) for analyses and figures. Heatmap.2 in R was used to generate heatmaps of differential gene expression. GeneOverlap function in R [24] was used to perform Fisher’s exact test of significance in R with the total number of 17,089 background genes. Qiagen Ingenuity Pathway Analysis (IPA) was used for Upstream analyses. The significance cutoff for p values of overlap from IPA Upstream analysis is p < 0.01 according to Qiagen instructions. We confirmed our gene expression patterns by performing an additional analysis using false discovery rate (FDR) > 0.05 significance cutoff (Supplemental Figure 1).
Table 1.
Groups assessed in RNA-sequencing experiments
| Group paired comparisons | Included in Figure | Figure notation |
|---|---|---|
| Female-NS-baseline vs female-CAS-baseline | Fig. 3 | Female baseline |
| Female-NS-acute vs. female-CAS-acute | Fig. 3 | Female acute |
| Male-NS-baseline vs male-CAS-baseline | Fig. 3 | Male baseline |
| Male-NS-acute vs. male-CAS-acute | Fig. 3 | Male acute |
| Female-NS-baseline vs. female-NS-acute | Fig. 4 | Female NS |
| Female-CAS-baseline vs. female-CAS-acute | Fig. 4 | Female CAS |
| Male-NS-baseline vs. male-NS-acute | Fig. 4 | Male NS |
| Male-CAS-baseline vs. male-CAS-acute | Fig. 4 | Male CAS |
Male and female rats were exposed to CAS or NS control conditions during adolescence. In adulthood, hippocampal tissue was collected at baseline or 30 min following exposure to a novel acute stressor challenge
CAS chronic adolescent stress, NS non-stress
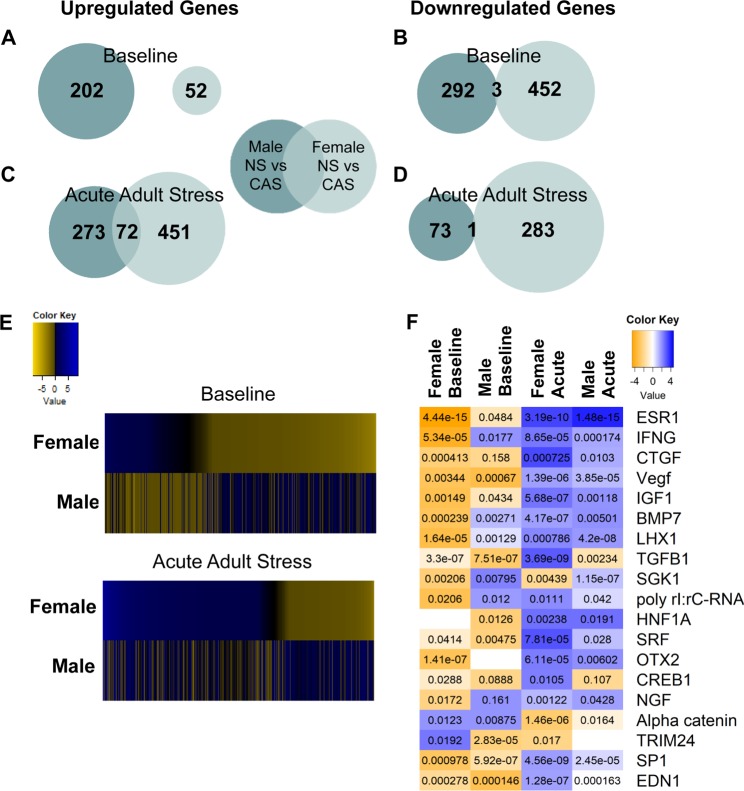
Fig. 3.
Paired comparison of gene expression data between rats exposed to chronic adolescent stress (CAS) and non-stressed (NS) controls within each sex and acute stress time point was assessed with uncorrected p value of 0.05 and fold change (FC) = 1.3. Differentially expressed genes (DEGs) were divided into groups that were up- or down-regulated in the CAS vs. NS condition. The number of upregulated and downregulated DEGs in males and females at baseline (a, b) or 30 min (acute) following exposure to a novel acute stressor (c, d) are shown in Venn diagrams. e DEGs in either paired comparison are represented in a heatmap as log2(FC). Fisher’s exact test of overlap was used to determine that up- and down-regulated DEGs in females and males do not significantly overlap (p > 0.05). In groups exposed to an acute adult stressor, upregulated genes significantly overlap (p = 1.3E−39) between males and females, but downregulated DEGs do not (p > 0.05). f Qiagen Ingenuity Pathway Analysis (IPA) was used to predict upstream regulators of transcriptional changes. The heatmap color represents uncorrected activation z-score, the predicted activity of transcription factors for each of the four paired comparisons (CAS vs. NS). The p value of significance of overlap of DEGs with lists of genes known to be altered by each upstream regulator are notated within each cell. IPA sets a significance cutoff of α = 0.01 for significance of upstream regulators. The heatmap is sorted top to bottom by z-score
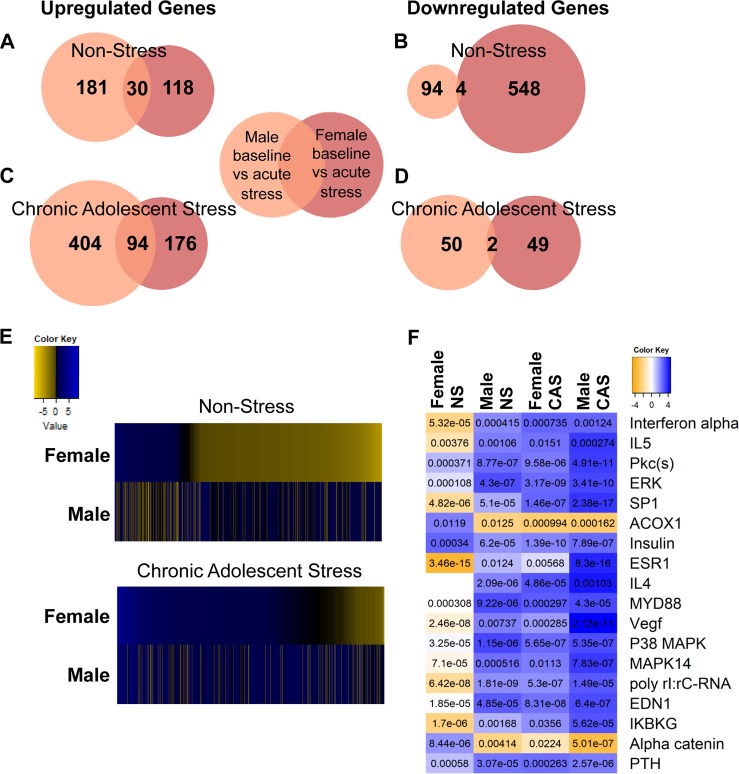
Fig. 4.
Paired comparison of gene expression between acute stress and baseline time points was assessed within sex and adolescent stress history group (non-stress (NS) and chronic adolescent stress (CAS)). Differentially expressed genes (DEGs) were split into up- and downregulated genes in animals without a history of CAS exposure (a, b) and those with prior CAS exposure (c, d) and shown in Venn diagrams. e Heatmaps show direction of gene expression change using log2(fold change (FC)) following acute stressor exposure in male and female rats with a history of CAS or NS control conditions. Genes that were significantly up- or downregulated in either males or females were included in heatmaps. Fisher’s exact test of overlap shows that upregulated genes in NS males and females significantly overlap (p = 4E−28) but downregulated genes do not (p > 0.05). In animals with a history of CAS, both upregulated (p = 2.3e-76) and downregulated (p = 0.011) genes significantly overlap between males and females. f Qiagen Ingenuity Pathway Analysis (IPA) upstream analysis was used to assess upstream regulators of transcriptional changes. The heatmap represents the activation z-score in each cell. The p value of overlap between significant DEGs in each paired comparison (acute stress vs baseline) and genes known to be regulated by each upstream regulator are notated within each cell; α = 0.01 for IPA Upstream Analyses
Statistical analysis
All statistics and graphing were performed using RStudio Version 0.99.456 and GraphPad Prism Version 7.02. Analysis of RNA-Seq results are discussed above. Parametric analysis used α = 0.05. Corticosterone concentrations were analyzed with a three-way analysis of variance (ANOVA) with the factors of sex, adolescent stress, and acute stress. Methylation data were analyzed with two-way ANOVA with factors of sex and adolescent stress. Power analyses were conducted for the methylation data to determine the likelihood of an underpowered examination falsely generating the observed sex difference. An a priori power analysis was completed using G*Power 3.1 [25] to determine the sample size necessary to detect statistical significance between the mean methylation values of non-stress control and chronic adolescent stress males in a two-sided t-test (α = 0.05). The potential for a correlation between methylation and basal corticosterone concentrations was assessed with Pearson's correlation. Uterine weights were normalized to terminal body mass and compared using a two-way ANOVA with the factors of adolescent and acute stress. Planned comparisons with Bonferroni corrections were used for posthoc assessment to identify individual group differences when a main effect or interaction was identified.
Results
A history of CAS blunts the corticosterone response to a novel stressor in adulthood
As expected, plasma corticosterone increased following acute stressor exposure (F1, 31 = 40.30, p = 4.57E−7). Sex dictated corticosterone response such that females exhibited higher plasma corticosterone following the acute stressor than males (F1, 31 = 9.00, p = 0.0053). In addition, rats with a history of CAS had a lower corticosterone response than rats that did not have a history of adolescent stress (F1, 31 = 9.92, p = 0.0036). Furthermore, sex and acute stress interacted to alter corticosterone (F1, 31 = 6.23, p = 0.018), and plasma corticosterone was also impacted by a significant adolescent stress by acute stress interaction (F1, 31 = 7.97, p = 0.0082) (Fig. 1b). Planned comparisons with Bonferroni correction (α = 0.0125) indicate that these interactions were driven by the effect of acute stress on plasma corticosterone in NS females (t8 = 5.198, p = 0.0008). Although the other group differences were directionally similar, statistical significance of the differences do not survive correction (α = 0.0125; male NS baseline vs. male NS acute: t7 = 2.671, p = 0.0319; female CAS baseline vs. female CAS acute: t10 = 2.528, p = 0.0300; male CAS baseline vs. male CAS acute: t6 = 2.638, p = 0.0386). These data suggest that a history of adolescent stress blunts the corticosterone response to a novel acute stressor in adult females.
A history of CAS reduced global methylation in the adult hippocampus
Due to the enduring consequences of CAS observed in previous studies and the alterations in the acute stress response in adulthood following a history of adolescent stress, we assessed DNA methylation in the left hemisphere of the whole hippocampus in order to understand the extent to which CAS alters a broad epigenetic marker in the hippocampus in a sex-specific and long-lasting manner. Prior exposure to CAS reduced global DNA methylation (Fig. 2a; F1, 18 = 5.404, p = 0.032). This effect was driven by a reduction in methylation in females with a history of CAS compared to NS females (t8 = 2.789, p = 0.0236); methylation in male rats did not differ based on whether or not they had a history of CAS (p > 0.05). Achieved power was computed via a post hoc power analysis between two independent means using G*Power 3.1 for each sex [25]. Results show a modest effect size within the males (d = 0.497, Ncontrol = 6, NCAS = 6, δ = 0.861, df = 10, critical t = 2.228, power = 0.112, α = 0.05). Achieved power within the females was calculated in a similar fashion, showing a stronger effect size and subsequently stronger power (d = 1.764, Ncontrol = 5, NCAS = 5, δ = 2.790, df = 8, critical t = 2.306, power = 0.687, α = 0.05). In order to obtain the recommend statistical power of 0.8 [26], a sample size of approximately 42 males would be needed in each of the groups (output parameters δ = 3.666, df = 82, critical t = 1.989).
Fig. 2.
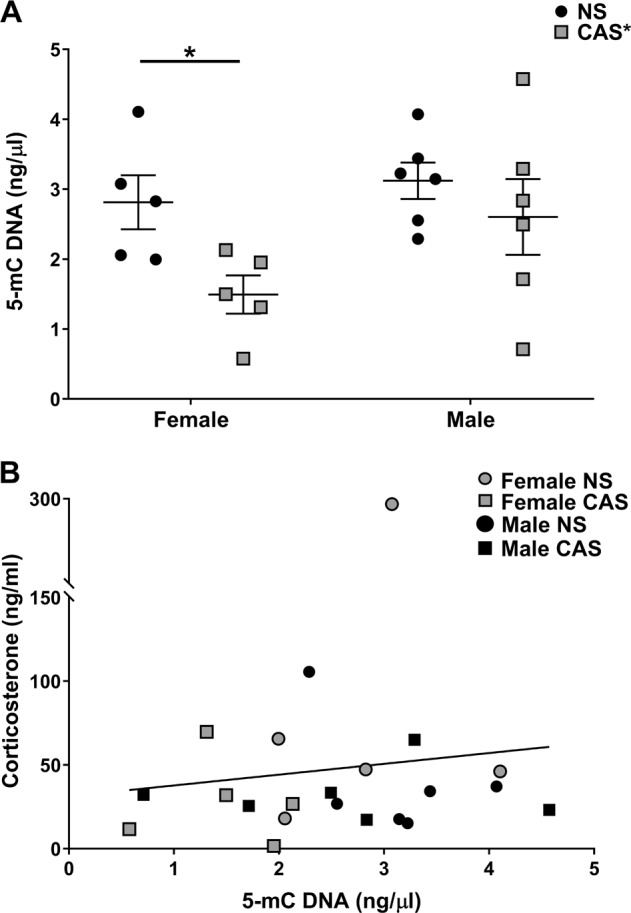
a Chronic adolescent stress (CAS) exposure reduced DNA methylation in adulthood, an effect that is more pronounced in females. Male and female adolescent rats were exposed to a mixed-modality CAS paradigm or non-stress (NS) control conditions. In adulthood at baseline, tissue was collected, and a MethylFlash Methylated DNA 5-mC Quantification Kit (Colorimetric) was used to assess DNA methylation. A two-way analysis of variance (ANOVA) revealed a significant main effect of stress (p < 0.05) and posthoc analysis attributed this effect to a difference between NS and CAS females (*p < 0.05). b Basal corticosterone concentration was not predictive of observed 5-mC DNA levels (p = 0.6193; R2 = 0.01257). Data are represented as mean ± SEM
Given the variability in methylation, we examined basal corticosterone to determine if it was predictive of methylation. Consistent with data presented in Fig. 1b, there were no group differences in basal corticosterone concentrations among rats used in the methylation endpoint (p > 0.05). In addition, basal corticosterone concentration was not predictive of observed 5-mC DNA levels (Fig. 2b; p = 0.6193; R2 = 0.01257). Furthermore, targeted PCR revealed that sex or a history of CAS did not alter gene expression of Dnmt1, Dnmt3a, Dnmt3b, or Mecp2 in the adult hippocampus (Supplemental Table 1).
Exposure to CAS sex-specifically altered baseline gene expression in the adult hippocampus as compared to stress-naive controls
Given the enduring impact of CAS on DNA methylation and its potential to impact gene expression, we used RNA sequencing to assess the whole hippocampal transcriptome to identify candidate pathways and gene targets that were impacted by exposure to CAS. In order to assess global effects of CAS on adult hippocampal transcription, we used significance criteria of FC = 1.3 or (1/1.3) and uncorrected p = 0.05 as previously performed [22, 23] (Figs. 3 and 4). These cutoffs allowed assessment of global shifts in gene expression in adulthood due to prior exposure to CAS and were appropriate for IPA Upstream Analysis.
We performed a direct comparison of the CAS to NS groups at baseline and within sex in order to assess the impact of CAS exposure on the adult hippocampal transcriptome (Fig. 3). These comparisons are detailed in Table 1 (female-NS-baseline vs. female-CAS-baseline; male-NS-baseline vs. male-CAS-baseline). Genes were divided into up- and downregulated DEGs in order to show the directional impact of stress exposure on gene expression (Fig. 3; genes identified in Supplemental Table 2). Few DEGs driven by a history of CAS compared to NS controls were overlapped between males and females at baseline. In fact, zero overlap was evident in upregulated genes (Fig. 3a, e; p > 0.05, Fisher’s exact test of overlap) and only 3 of the same genes were downregulated in both males and females (Fig. 3b, e; p > 0.05, Fisher’s exact test of overlap). Specific genes are identified in Supplemental Table 3.
Upstream Analysis in IPA was used to predict upstream regulators that may mediate the measured transcriptional patterns (α = 0.01; Fig. 3f, Supplemental Table 4). Upstream Analysis revealed significant overlap with estrogen receptor 1 (ESR1)-regulated genes in females at baseline (bias corrected z = −2.750; z = −4.588, p = 4.44E−15) and predicted reduced ESR1 activity in females with a history of CAS exposure as compared to NS females at baseline. This overlap with ESR1 genes was specific to females as males did not exhibit significant overlap with genes regulated by ESR1 (α = 0.01; p = 0.048) or significant predicted altered activity.
The adult transcription following a novel acute stressor is altered by a history of CAS and differentiates by sex
In the comparisons following acute stressor exposure (female-NS-acute vs. female-CAS-acute; male-NS-acute vs. male-CAS-acute), only genes that were upregulated in rats with a history of CAS significantly overlapped between males and females (Fig. 3c, e; p = 1.3E−39; genes identified in Supplemental Table 4) with 72 genes upregulated by CAS following acute adult stress in both sexes. This was specific to upregulated genes as genes that had reduced expression in CAS rats did not significantly overlap between males and females after acute stressor exposure (Fig. 3d, e; p > 0.05), and only 1 gene was downregulated in both sexes. Following acute stress, CAS-altered genes (compared to same-sex NS group after the acute stress) significantly overlapped with ESR1-regulated genes in females (Fig. 3f; p = 3.19E−10) and males (Fig. 3f; p = 1.48E−15) suggesting increased predicted activity of ESR1 for both sexes in acute stress is experienced following CAS exposure.
The transcriptional response to an acute stressor in adulthood diverges between males and females when there is no history of CAS
In order to assess DEGs that were altered following the novel stressor exposure in adulthood in NS males and females, gene expression after acute stress was directly contrasted to baseline gene expression (female-NS-baseline vs. female-NS-acute; male-NS-baseline vs. male-NS-acute; Fig. 4; genes altered provided in Supplemental Table 5). Although the majority of DEGs did not overlap between males and females, genes that were upregulated following acute stressor exposure overlapped between NS males and NS females to a greater degree (30 genes; 9% overlap, Fisher’s exact p = 4E−28) than downregulated genes (4 genes; 0.6%, Fisher’s exact p > 0.05) (Fig. 4a, b; specific genes provided in Supplemental Table 5). Furthermore, NS females exhibited enhanced repression of genes following acute stressor exposure (548 genes) compared to males (94 genes). IPA Upstream Analysis revealed overlap with genes regulated by multiple transcription factors including interferon-α (IFN-α), interleukin 5, Pkc(s), extracellular signal-regulated kinase, and SP1 (Fig. 4f; Supplemental Table 6). Upstream Analysis revealed significant overlap with IFN-α-regulated genes in NS females (bias corrected z = −0.715; z = −2.391, p = 5.32E−5) and predicted reduced activity in NS females following acute stress exposure. There was an effect of acute adult stress on IFN-α-regulated genes in males (with or without a history of CAS) and females with a history of CAS, but in these cases, exposure to acute stress in adulthood resulted in an upregulation of IFN-α-regulated genes.
CAS reduces sex differences in the transcriptional response to a novel acute stressor
Rats with a history of CAS (female-CAS-baseline vs. female-CAS-acute; male-CAS-baseline vs. male-CAS-acute) exhibited more upregulated genes in the acute stress response (404 genes in males, 176 genes in females, and 94 overlapping genes; Fig. 4c) and reduced repression of gene expression (50 genes in males, 49 in females, and 2 overlapping; Fig. 4d) relative to NS controls (contrasting Fig. 4a, b to Fig. 4c, d). The proportion of genes that were upregulated was higher in males and females exposed to CAS than in same-sex NS controls. Furthermore, male and female rats with a history of CAS exhibited a reduction in downregulation of genes following acute stressor exposure in contrast to NS controls with the most pronounced effect in females (548 unique genes downregulated by acute stress in NS females; 49 genes downregulated by acute stress in CAS females). Interestingly, males and females with a history of CAS exhibited similar acute transcriptional responses to stressor exposure indicated by significant overlap between genes with increased (Fisher’s exact p = 2.3E−76) and decreased expression (Fisher’s exact p = 0.011) illustrated in Fig. 4a, b.
FDR significance threshold further confirms profound sex differences in hippocampal transcriptome
We further confirmed that the impact of CAS and acute stressor exposure on global gene expression by using an FDR significance threshold of 0.05 (Supplemental Figure 1, Supplemental Table 7). CAS (Supplemental Figure 1A) altered gene expression differently in males and females such that females did not have genes that passed correction for multiple comparisons at baseline but males did have genes that passed correction for multiple comparisons at baseline. The transcriptional response to acute stressor exposure differed between males and females with a history of CAS (Supplemental Figure 1B, Supplemental Table 7), but results of multiple comparison correction were in line with the less stringent criteria used to identify global patterns of gene expression changes.
Estrous cycle stage was not primary driver of observed differences in females
Uterine weight was used as a proxy for estrous cycle staging. Uterine weight did not differ among groups at time congruent with transcriptome analysis, suggesting that estrous cycle-dependent differences in ovarian hormones did not drive changes in predicted estrogen receptor activity (p > 0.05) (Supplemental Table 8) [27].
Discussion
Collectively, these data provide new evidence that chronic stress exposure during adolescence is sufficient to confer long-lasting, sex-specific, epigenetic, and transcriptional changes in the hippocampus that persist into adulthood. These studies establish that perturbations during adolescence are sufficient to substantially change global transcriptional regulation into adulthood and that these effects interact with adult exposures. Rats with a history of chronic adolescent stress have a blunted corticosterone response to a novel stressor in adulthood and reduced DNA methylation, effects that are more pronounced in females than males. In addition, although both males and females demonstrate enduring genomic effects of CAS, genomic effects of an acute adult stress, and genomic implications of the combination of these stressor exposures, these responses are sex dependent and generally more pronounced in females. Finally, we identified ESR1 as a potential driver of the enduring baseline sex-specific transcriptional changes of CAS and IFN-α as a candidate for sex differences in the adult acute stress response. These sex-specific, stress-dependent, modifications in gene expression may contribute to the divergence of stress-related disorders and their symptoms in males and females.
To better understand how CAS impacted epigenetic regulation through the lifespan, we assessed the extent to which CAS altered global DNA methylation and expression of DNA methyltransferases. Prior exposure to CAS reduced global DNA methylation; however, this effect was limited to female groups upon posthoc analysis and male groups would need to consist of 42 rats/group in order to provide statistical power to detect a difference. Although we cannot completely rule out an effect in males, this sex-specific lack of power suggests that either the effect is more pronounced in females or that an additional variable is modulating the effect in males. Therefore, we conclude that adult female rats exposed to CAS had reduced global methylation, suggesting that regulation of DNA methylation may be impacted by CAS in a sex-specific manner with potential to underlie the observed changes in hippocampal gene transcription. Despite previous reports that found a role of DNMT3a in susceptibility to stress exposure and nucleus accumbens transcription [23], we found no effect of either sex or CAS history on expression of hippocampal DNA methyltransferases, including Dnmt3a, suggesting a disconnect between adult gene expression of DNMTs and adult global methylation. Reduced global methylation could contribute to the enhanced upregulation of gene expression observed in CAS but not NS females following acute stressor exposure if the methylation state primes the system to promote enhanced transcription following acute stress. However, future work is necessary to determine the localization of altered methylation to establish the functional implications as reduced methylation at specific genes has been found to increase psychiatric disease risk [28]. Furthermore, the location of DNA methylation within a gene impacts whether DNA methylation increases or decreases gene expression [29, 30]. While our results suggest that the reduced DNA methylation we observed is in line with the overall upregulation of gene expression in animals with a history of CAS exposure following acute stressor exposure, we did not specifically address the localization of the alterations in DNA methylation and are unable to draw these specific conclusions.
In order to determine if the enduring effects of CAS on DNA methylation impacted genomics, we used RNA sequencing to assess the extent to which global hippocampal transcription was impacted in a sex-specific manner and to identify networks of genes that were impacted by prior exposure to CAS. Previous work has assessed the effect of adolescent stress exposure on expression of specific targeted genes and found a gene-specific impact of adolescent stress in adolescence [9, 31] and adulthood [17, 31, 32] at baseline, following acute stress, and after lipopolysaccharide challenge. We extend the appreciation of the lasting consequences of CAS exposure on gene transcription to the global scale and show that CAS-induced alterations to gene transcription are widespread and sex specific. In this dataset, we observed global shifts in gene expression such that CAS altered different patterns of genes in males and females at baseline and following exposure to a subsequent novel acute stressor in adulthood. Previous work has shown sex differences in the nucleus accumbens transcriptome following subchronic variable stress exposure [23] and in the ventromedial prefrontal cortex and nucleus accumbens following chronic variable stress [33] in mice. However, these sequencing studies occurred in adults shortly after stressor exposure in contrast to the lasting effect we observe in the current data. Therefore, we extend the previous findings to show that exposure to stressors during adolescence alters the transcriptome in a lasting manner, months removed from stressor exposure.
Remarkably, a history of CAS modifies sex differences in the adult genomic response to acute stress. We assessed the extent to which CAS impacted the transcriptional response to an acute stressor by directly comparing global gene expression following exposure to a novel acute stressor against baseline gene expression. Males and females without CAS exposure exhibited little overlap in the transcriptional response to an acute stressor, indicating widespread sex differences in the transcriptional response to acute stressor exposure. Sex differences in the global transcriptional response following an acute stressor exposure have been previously reported in adult mice in the CA3 region of the hippocampus in which acute stress alters a greater number of genes in females than males [13]. Males and females with a history of CAS exposure upregulated a greater number of genes following acute stressor exposure than same-sex controls and downregulated fewer genes following acute stressor exposure than same-sex controls. Interestingly, the sex difference in the genomic response to an acute adult stressor was reduced if the rats had a history of adolescent stress exposure. Males and females with a history of CAS exhibited more similar transcriptional profiles to one another as compared to the substantial sex difference between NS controls following the acute stress in adulthood (Fig. 4). Specifically, NS females exhibited reduced expression of 548 unique genes after acute stressor exposure, as compared to 94 downregulated genes in males, whereas CAS females had 49 downregulated genes and CAS males had 50 downregulated genes. Consistent with these findings, developmental stress has previously been reported to induce masculinizing effects on behavior and on the endocrine system, and in altering sex hormone receptors in the hippocampus in female guinea pigs [34]. Furthermore, as demonstrated in the corticosterone data presented here, the adult acute stress response in females with a history of chronic adolescent stress is more similar to the male response to an acute stressor than to that of females without a history of chronic stress.
To extend the global transcriptional findings to biologically relevant networks of genes, IPA was used to assess upstream regulators that are impacted by CAS exposure (Fig. 3f). IPA Upstream Analysis identified genes regulated by estrogen receptor alpha (ESR1) to be significantly altered by CAS exposure (compared to NS controls). Adult females, but not males, with a history of CAS exposure exhibited reduced predicted ESR1 activity at baseline. Conversely, both males and females exposed to CAS exhibited enhanced predicted ESR1 activity following acute stressor exposure compared to NS controls. The protective effects of estrogen on mood disorders have been extensively studied [35–37], and sex differences have been reported in the specific role of each estrogen receptor in the hippocampus [38]. More specifically, a deficiency in estrogen signaling was implicated in sex differences in affective behavior following chronic stress exposure in mice [39]. Furthermore, following chronic social defeat stress, susceptible mice exhibited reduced predicted upstream activity of ESR1 in the nucleus accumbens compared to resilient mice [40]. We show here that CAS exposure alters ESR1 in the hippocampus with a pattern consistent with the pro-susceptible activity pattern observed previously. Uterine weight at collection was used as a proxy for estrous cycle staging [27]. There was no significant effect of CAS or acute stress on uterine weight, suggesting that there were not group-specific differences in estrous cycle stage. The absence of a group effect on uterine weight indicates that the observed effect of CAS on predicted ESR1 activity is not a function of different estrous cycle stages among groups when tissue was collected.
In addition, IPA Upstream Analysis identified genes regulated by IFN-α to be significantly downregulated by adult acute stress in NS control females, whereas adult acute stress upregulated IFN-α-related genes in males after adult acute stress, regardless of their adolescent stress exposure, and in females with a history of CAS (Fig. 4f). This is consistent with the overall pattern observed in the data set which suggests that CAS exposure in females results in the hippocampal genomic response to be more similar to that of male rats than in control females. This is particularly evident in the predicted patterns of upstream regulators in non-stressed males and females compared to CAS males and females (Fig. 4f, comparing the first two heatmap columns to the second two heatmap columns). INF-α is of particular interest because it has been previously linked to manifestation of depression following usage in humans [41], and depressive-like behaviors have been observed following the CAS paradigm employed here [10]. In addition, IFN-α has been demonstrated to alter the electrophysiological properties of the hippocampus [42], the brain region of interest in this study. Interestingly, sex differences in the impact of interferons have been reported in the context of diabetes susceptibility [43]. Future work will be necessary to determine if the observed changes in IFN-α expression patterns in the hippocampus play a functional role in previously observed phenotypes following CAS exposure.
An important consideration in interpretation of the data presented here is the potential influence of temporal variability in the timing of puberty between male and female rats. Although our chronic stress paradigm spans multiple developmental days that fall within the peri-pubertal period of both males and females [7], the maturation of all processes in the body is not uniform between males and females. This is highlighted by work regarding the maturation of microglia [44] which illustrates differences in developmental timelines between the sexes. In addition, previous work in male rats has demonstrated the importance of timing during the adolescent period and the altered outcomes based on stressor timing [45, 46]. Given the length of the stressors employed in this study, it appears implausible that timing alone dictates the sex differences observed, but this possibility cannot be ruled out without future studies that systematically manipulate the developmental days included in the stress paradigm for all possible permutations.
Collectively, the data presented here demonstrate profound and prolonged effects of chronic adolescent stress on the HPA axis, methylation in the hippocampus, and the transcriptome of the hippocampus. Although these effects are most pronounced in females, remarkable alterations in the transcriptome of male rats are also demonstrated. Furthermore, a history of CAS exposure interacts with the acute stress response such that both the sex differences in the HPA axis response and the hippocampal transcriptome are reduced following CAS. Finally, ESR1 and IFN-α are identified as a potential candidates for the enduring and sex-specific effects of chronic adolescent stress exposure. These data highlight the importance of considering the impact of adolescent stress exposure for adult outcomes relative to the hippocampus and stress response in both males and females.
Funding and disclosure
This work was supported by the National Institutes of Health National Institute of Nursing Research (NR014886; to GNN). The Yerkes NHP Genomics Core is supported in part by ORIP/OD P51OD011132. SAR was supported by training grant T32-GM008602. GS was supported by training grant 2R25GM090084-09. MMH was supported by training grant K12GM093857. The authors declare no competing interests.
Supplementary information
Acknowledgements
RNA-sequencing data were included in abstracts presented as posters at the International Society of Psychoneuroendocrinology meeting in 2016 and the Society of Biological Psychiatry meeting in 2016. These data were included in a dissertation for Emory University.
Footnotes
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Supplementary Information accompanies this paper at (10.1038/s41386-019-0321-z).
References
- 1.Pratt LA, Brody DJ. Depression in the U.S. Household Population, 2009–2012. NCHS Data Brief, no 172. Hyattsville: National Center for Health Statistics; 2014. [PubMed] [Google Scholar]
- 2.Nolen-Hoeksema S, Girgus JS. The emergence of gender differences in depression during adolescence. Psychol Bull. 1994;115:424–43. doi: 10.1037/0033-2909.115.3.424. [DOI] [PubMed] [Google Scholar]
- 3.Gourley SL, et al. Arg kinase regulates prefrontal dendritic spine refinement and cocaine-induced plasticity. J Neurosci. 2012;32:2314–23. doi: 10.1523/JNEUROSCI.2730-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andersen SL, Teicher MH. Stress, sensitive periods and maturational events in adolescent depression. Trends Neurosci. 2008;31:183–91. doi: 10.1016/j.tins.2008.01.004. [DOI] [PubMed] [Google Scholar]
- 5.Fuhrmann D, Knoll LJ, Blakemore SJ. Adolescence as a sensitive period of brain development. Trends Cogn Sci. 2015;19:558–66. doi: 10.1016/j.tics.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 6.Stone EA, Quartermain D. Greater behavioral effects of stress in immature as compared to mature male mice. Physiol Behav. 1997;63:143–5. doi: 10.1016/S0031-9384(97)00366-1. [DOI] [PubMed] [Google Scholar]
- 7.Spear LP. The adolescent brain and age-related behavioral manifestations. Neurosci Biobehav Rev. 2000;24:417–63. doi: 10.1016/S0149-7634(00)00014-2. [DOI] [PubMed] [Google Scholar]
- 8.Bourke CH, Neigh GN. Behavioral effects of chronic adolescent stress are sustained and sexually dimorphic. Horm Behav. 2011;60:112–20. doi: 10.1016/j.yhbeh.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bourke CH, et al. Glucocorticoid sensitizers Bag1 and Ppid are regulated by adolescent stress in a sex-dependent manner. Psychoneuroendocrinology. 2013;38:84–93. doi: 10.1016/j.psyneuen.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bourke CH, Neigh GN. Behavioral effects of chronic adolescent stress are sustained and sexually dimorphic. Horm Behav. 2011;60:112–120. doi: 10.1016/j.yhbeh.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sapolsky RM, Krey LC, McEwen BS. Glucocorticoid-sensitive hippocampal neurons are involved in terminating the adrenocortical stress response. Proc Natl Acad Sci USA. 1984;81:6174–7. doi: 10.1073/pnas.81.19.6174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sapolsky RM, Meaney MJ, McEwen BS. The development of the glucocorticoid receptor system in the rat limbic brain. III. Negative-feedback regulation. Brain Res. 1985;350:169–73. doi: 10.1016/0165-3806(85)90261-5. [DOI] [PubMed] [Google Scholar]
- 13.Marrocco J, et al. A sexually dimorphic pre-stressed translational signature in CA3 pyramidal neurons of BDNF Val66Met mice. Nat Commun. 2017;8:808. doi: 10.1038/s41467-017-01014-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiland NG, et al. Distribution and hormone regulation of estrogen receptor immunoreactive cells in the hippocampus of male and female rats. J Comp Neurol. 1997;388:603–12. doi: 10.1002/(SICI)1096-9861(19971201)388:4<603::AID-CNE8>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 15.Guerra-Araiza C, et al. Changes in progesterone receptor isoforms content in the rat brain during the oestrous cycle and after oestradiol and progesterone treatments. J Neuroendocrinol. 2003;15:984–90. doi: 10.1046/j.1365-2826.2003.01088.x. [DOI] [PubMed] [Google Scholar]
- 16.Moghadami S, et al. Gonadectomy reduces the density of androgen receptor-immunoreactive neurons in male rat’s hippocampus: testosterone replacement compensates it. Behav Brain Funct. 2016;12:5. doi: 10.1186/s12993-016-0089-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pyter LM, et al. Sex differences in the effects of adolescent stress on adult brain inflammatory markers in rats. Brain Behav Immun. 2013;30:88–94. doi: 10.1016/j.bbi.2013.01.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dobin A, et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anders S, Pyl PT, Huber W. HTSeq--a Python framework to work with high-throughput sequencing data. Bioinformatics. 2015;31:166–9. doi: 10.1093/bioinformatics/btu638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Robinson MD, McCarthy DJ, Smyth GK. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics. 2010;26:139–40. doi: 10.1093/bioinformatics/btp616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McCarthy DJ, Chen Y, Smyth GK. Differential expression analysis of multifactor RNA-Seq experiments with respect to biological variation. Nucleic Acids Res. 2012;40:4288–97. doi: 10.1093/nar/gks042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bagot RC, et al. Ketamine and imipramine reverse transcriptional signatures of susceptibility and induce resilience-specific gene expression profiles. Biol Psychiatry. 2017;81:285–295. doi: 10.1016/j.biopsych.2016.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hodes GE, et al. Sex differences in nucleus accumbens transcriptome profiles associated with susceptibility versus resilience to subchronic variable stress. J Neurosci. 2015;35:16362–76. doi: 10.1523/JNEUROSCI.1392-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shen L, Sinai M (2013). GeneOverlap: test and visualize gene overlaps. http://shenlab-sinai.github.io/shenlab-sinai/.
- 25.Faul F, et al. G*Power 3: a flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–91. doi: 10.3758/BF03193146. [DOI] [PubMed] [Google Scholar]
- 26.Lakens D. Calculating and reporting effect sizes to facilitate cumulative science: a practical primer for t-tests and ANOVAs. Front Psychol. 2013;4:863. doi: 10.3389/fpsyg.2013.00863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harrell CS, et al. Ovarian steroids influence cerebral glucose transporter expression in a region- and isoform-specific pattern. J Neuroendocrinol. 2014;26:217–25. doi: 10.1111/jne.12139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klengel T, et al. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nat Neurosci. 2013;16:33–41. doi: 10.1038/nn.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yang X, et al. Gene body methylation can alter gene expression and is a therapeutic target in cancer. Cancer Cell. 2014;26:577–90. doi: 10.1016/j.ccr.2014.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baylin SB, Jones PA. A decade of exploring the cancer epigenome - biological and translational implications. Nat Rev Cancer. 2011;11:726–34. doi: 10.1038/nrc3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wulsin AC, et al. Adolescent chronic stress causes hypothalamo-pituitary-adrenocortical hypo-responsiveness and depression-like behavior in adult female rats. Psychoneuroendocrinology. 2016;65:109–17. doi: 10.1016/j.psyneuen.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelly SD, Harrell CS, Neigh GN. Chronic stress modulates regional cerebral glucose transporter expression in an age-specific and sexually-dimorphic manner. Physiol Behav. 2014;126:39–49. doi: 10.1016/j.physbeh.2013.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Labonte B, et al. Sex-specific transcriptional signatures in human depression. Nat Med. 2017;23:1102–1111. doi: 10.1038/nm.4386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kaiser S, et al. Early social stress in female guinea pigs induces a masculinization of adult behavior and corresponding changes in brain and neuroendocrine function. Behav Brain Res. 2003;144:199–210. doi: 10.1016/S0166-4328(03)00077-9. [DOI] [PubMed] [Google Scholar]
- 35.Walf AA, Paris JJ, Frye CA. Chronic estradiol replacement to aged female rats reduces anxiety-like and depression-like behavior and enhances cognitive performance. Psychoneuroendocrinology. 2009;34:909–16. doi: 10.1016/j.psyneuen.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Walf AA, Frye CA. Estradiol reduces anxiety- and depression-like behavior of aged female mice. Physiol Behav. 2010;99:169–74. doi: 10.1016/j.physbeh.2009.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schmidt PJ, et al. Estrogen replacement in perimenopause-related depression: a preliminary report. Am J Obstet Gynecol. 2000;183:414–20. doi: 10.1067/mob.2000.106004. [DOI] [PubMed] [Google Scholar]
- 38.Oberlander JG, Woolley CS. 17beta-Estradiol acutely potentiates glutamatergic synaptic transmission in the hippocampus through distinct mechanisms in males and females. J Neurosci. 2016;36:2677–90. doi: 10.1523/JNEUROSCI.4437-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hu Y, et al. Hippocampal nitric oxide contributes to sex difference in affective behaviors. Proc Natl Acad Sci USA. 2012;109:14224–9. doi: 10.1073/pnas.1207461109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lorsch ZS, et al. Estrogen receptor alpha drives pro-resilient transcription in mouse models of depression. Nat Commun. 2018;9:1116. doi: 10.1038/s41467-018-03567-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Musselman DL, et al. Paroxetine for the prevention of depression induced by high-dose interferon alfa. N Engl J Med. 2001;344:961–6. doi: 10.1056/NEJM200103293441303. [DOI] [PubMed] [Google Scholar]
- 42.Mendoza-Fernandez V, Andrew RD, Barajas-Lopez C. Interferon-alpha inhibits long-term potentiation and unmasks a long-term depression in the rat hippocampus. Brain Res. 2000;885:14–24. doi: 10.1016/S0006-8993(00)02877-8. [DOI] [PubMed] [Google Scholar]
- 43.Carrero JA, et al. Type I and II interferon receptors differentially regulate type 1 diabetes susceptibility in male versus female NOD mice. Diabetes. 2018;67:1830–1835. doi: 10.2337/db18-0331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kopec AM, et al. Microglial dopamine receptor elimination defines sex-specific nucleus accumbens development and social behavior in adolescent rats. Nat Commun. 2018;9:3769. doi: 10.1038/s41467-018-06118-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lui P, et al. Divergent stress-induced neuroendocrine and behavioral responses prior to puberty. Physiol Behav. 2012;107:104–11. doi: 10.1016/j.physbeh.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Romeo RD, et al. Pubertal shifts in adrenal responsiveness to stress and adrenocorticotropic hormone in male rats. Psychoneuroendocrinology. 2014;42:146–52. doi: 10.1016/j.psyneuen.2014.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.