Abstract
These updated guidelines from the Infectious Diseases Community of Practice of the American Society of Transplantation review the epidemiology, diagnosis, prevention, and management of infection due to Arenaviruses and West Nile Virus (WNV) in the pre- and post-transplant period. Arenaviruses and WNV have been identified as causes of both donor-derived and post-transplant infection. Most data related to these infections have been published in case reports and case series. Transplant recipients may become infected with Arenaviruses if they, or their donors, are exposed to wild rodents or infected pet rodents. Lymphocytic choriomeningitis virus is the most commonly recognized Arenavirus among transplant recipients, and should be considered when transplant recipients present with fever, hepatitis, meningitis/encephalitis, and/or multisystem organ failure. WNV is a mosquito borne virus, and as such, its incidence varies yearly depending on environmental conditions. WNV in transplant recipients typically presents with fever, myalgias, and rash; approximately 1 in 40 develop neuroinvasive disease. Due to its morbidity, the Organ Procurement and Transplantation Network recently mandated that transplant centers screen living donors for WNV infection in endemic areas. Little is known about the optimal treatment of Arenaviruses or WNV; reduction in immunosuppression and supportive care are the mainstays of management at present.
Introduction
Arenaviruses and West Nile virus (WNV) have been identified as sources of both donor-derived and post-transplant infection. Most data related to these infections have been published in case reports and case series, and the majority of these reports focus on either lymphocytic choriomeningitis virus (LCMV) or WNV. Herein, we present discussions of Arenaviruses, with a particular focus on LCMV due to a lack of data on other Arenaviruses, and WNV infections in solid organ transplant (SOT) recipients. We describe their epidemiology, clinical manifestations, diagnosis, treatment, and prevention.
Arenaviruses
Description of Pathogens
Arenaviruses are single-stranded enveloped RNA viruses that are transmitted from infected rodents to humans. New Arenavirus viral particles (or virions) are formed by budding from the surface of the host’s cells; the viral particles are spherical, and their interiors contain variable numbers of dense granules that are host cell ribosomes. These structures, which resemble grains of sand, give this family its name (Latin arena, or “sand”).
The first Arenavirus to be identified was LCMV, which was reported in 1933 as a cause of aseptic meningitis. Since then, several additional Arenaviruses have been identified, including several that cause a hemorrhagic fever syndrome: Lassa virus, which has caused outbreaks of hemorrhagic fever in Africa; and several Arenaviruses causing hemorrhagic fever in South America. Arenaviruses are divided into two groups: the “Old World” or “LCMV/Lassa complex,” which includes LCMV and Lassa virus; and the “New World” or “Tacaribe complex,” which includes Junin, Machupo, Guanarito and Sabiá viruses, which are also referred to as the “South American hemorrhagic fever viruses.”
Rodents are the natural reservoir of Arenaviruses. The viruses exhibit high species specificity, with each virus having a single rodent species as the natural reservoir. The geographic distribution of the respective rodent species, in turn, determines the regional distribution of the disease. LCMV differs from other Arenaviruses in that common house mice (Mus domesticus and Mus musculus), rodents with global distribution as opposed to geographically restricted field mice, are its natural reservoir1. Other rodents, such as pet and laboratory rodents (including rats, mice, guinea pigs, and hamsters), are not natural reservoirs but can be infected by LCMV if they come in contact with infected house mice. The rodents have an asymptomatic chronic infection and shed the virus into excreta, especially urine. Transmission among rodents can occur horizontally or vertically during pregnancy.
Epidemiology and Risk Factors
Infection with LCMV occurs worldwide with occasional outbreaks reported. Seroprevalence studies show that up to 5% of adults in the US have evidence of prior infection with LCMV1,2. Infection with Lassa virus occurs in West Africa, particularly in Sierra Leone, Liberia, Guinea, and Nigeria. Studies in West African populations have shown a Lassa virus seroprevalence ranging from 10–58%3–5. Infections with the South American hemorrhagic fever viruses occur sporadically; the seroprevalence of Junin virus in rural populations in Argentina has been reported to be 12%6.
Human transmission of LCMV occurs through contact with feces or urine from infected rodents or by inhalation of dust soiled with rodent urine, saliva, or feces. Lower socioeconomic status, substandard housing, and agricultural activities have been associated with rodent infestation and a higher risk of infection7. Transplant recipients may become infected with Arenaviruses via (1) donor-derived infection or (2) exposure to wild rodents or infected pet rodents8–12. Isolated cases of LCMV infection have been reported in laboratory personnel after contact with infected hamsters or infected rodent cell lines13,14. Outbreaks of LCMV in employees of rodent breeding facilities have also been reported15. Person-to-person transmission of LCMV has only occurred through maternal-fetal transmission16 and donor-derived transmission in organ transplantation8–12. Person-to-person transmission of LCMV is generally associated with severe disease, with congenital infection resulting in birth defects, including hydrocephalus, chorioretinitis, optic atrophy, and microcephaly; and transmission through organ transplantation resulting in multisystem organ failure and death in the majority of cases.
Person-to-person transmission can occur with Lassa fever and some South American viral hemorrhagic fevers via (1) aerosol spread, (2) contact with infected fluid, (3) sexual contact, and (4) breastfeeding, even during recovery from acute illness. There are no published reports of infection with Lassa fever or the South American viral hemorrhagic fevers in transplant recipients.
Table 1 summarizes each of the Arenaviruses, including the disease caused by the virus, the year of discovery, the geographic distribution, and the incubation period.
Table 1:
Overview of Arenaviruses
| Arenavirus† | Disease | Year of discovery | Geographic distribution | Incubation period |
|---|---|---|---|---|
| LCMV | Lymphocytic choriomeningitis | 1933 | Worldwide | 1–3 weeks |
| Lassa virus | Lassa fever | 1969 | West Africa | 3–21 days |
| South American HF viruses | South America | 7–14 days | ||
| Junin | Argentine HF | 1958 | North-central Argentina | |
| Machupo | Bolivian HF | 1963 | Northeast Bolivia | |
| Guanarito | Venezuelan HF | 1989 | Central Venezuela | |
| Sabia | Brazilian HF | 1993 | Brazil | |
| Chapare | Chapare HF | 2004 | Cochabamba region of Bolivia | |
| Lujo | Lujo HF | 2008 | Southern Africa | 7–13 days |
Adapted from https://www.cdc.gov/vhf/virusfamilies/arenaviridae.html (accessed May 25, 2018)
Abbreviations: HF, hemorrhagic fever; LCMV, lymphocytic choriomeningitis virus
Clinical Manifestations
LCMV
LCMV infection is typically asymptomatic or only mildly symptomatic in immunocompetent individuals. When symptomatic, the illness is often subtle with self-limited symptoms of fever, malaise, headache, photophobia, listlessness, myalgia, confusion, memory deficits, and abdominal pain. In more severe cases, the infection may progress to meningitis, encephalitis, and/or other central nervous system manifestations, but overall case fatality rate is < 1%. The incidence of each of the different clinical syndromes caused by LCMV is unknown, since diagnostic testing is rarely requested.
Until recently, LCMV in SOT recipients had only been described in the setting of donor-derived infections (Table 2). Six clusters of transmission of LCMV and LCMV-like Arenavirus via organ transplantation have been reported with 21 affected organ recipients8–12. The majority of these organ recipients presented with symptoms in the first month post-transplant, and with severe illness, characterized by elevated transaminases, coagulopathy, and dysfunction of the transplanted organ. Additional signs and symptoms included fever, localized rash, abdominal pain, diarrhea, hyponatremia, thrombocytopenia, hypoxia, and acute kidney injury. Fifteen of the 21 (71%) organ recipients with donor-derived LCMV died from multisystem organ failure, with LCMV-associated hepatitis as a prominent feature. Delayed diagnosis, often only determined post-mortem, likely contributed to the high mortality rate.
Table 2:
Donor-derived cases of LCMV in solid organ transplant recipients
| Year, Location | Donor risk factors and testing | Cause of donor death | Organ donated | Onset of symptoms post-transplant | Symptoms | Recipient testing | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|
| 2003 Wisconsin9,94,95 | No risk factors identified.
Neg IHC staining (heart, stomach, tongue, thyroid, kidney, prostate, cerebral cortex, midbrain, pons, medulla, cerebellum, spinal cord); Neg IgM and IgG (serum); Neg viral culture (serum, bone marrow and blood vessel) |
Head trauma/subdural hematoma | Kidney | Day 5 | Abdominal pain, fever, watery diarrhea, seizures, polymyoclonus | Pos IHC staining; neg IgM and IgG; pos viral culture | IS stopped, IVIG | Death on day 53 post-transplant |
| Kidney | Day 22 | Fever, leukopenia, peri-incisional erythema and tenderness, AMS, and seizures | Pos IHC staining; pos IgM; neg IgG; pos virus isolation | IS stopped, cidofovir, IVIG | Death on day 76 post-transplant | |||
| Liver | Early post-operatively | Fever, lethargy, hypotension, marked elevation of liver enzymes, respiratory failure, peri-incisional rash | Pos IHC staining. Serologies and virus isolation not performed | None | Death on day 17 post-transplant | |||
| Lung | Day 4 | Hypotension, bilateral pulmonary infiltrates, leukocytosis, fever, hypoxia | Pos IHC staining. Serologies and virus isolation not performed | None | Death on day 9 post-transplant | |||
| 2005 Rhode Island and Massachusetts9 |
Infected pet hamster. Neg IHC staining (CNS, heart, spleen, liver, pancreas, uterus, thyroid, gastrointestinal tract, muscle, skin, kidney); neg RT-PCR; neg IgM and IgG (blood and serum); neg viral culture | Right MCA stroke/IC hemorrhage | Kidney | Day 17 | Allograft tenderness, nausea, anorexia, diarrhea, fever, chills, erythema over allograft | Pos IHC stain (colon, kidney); pos RT-PCR; pos IgM; neg IgG; pos viral culture | IS stopped, IV ribavirin started day 26, followed by PO ribavirin for a total of 37 days | Survived |
| Kidney | Day 17 | Fever, allograft tenderness, lethargy, erythema over allograft, hepatitis | Pos IHC staining; pos RT-PCR; pos IgM; neg IgG; pos viral culture | None | Death on day 23 post-transplant | |||
| Liver | Early post-operatively | Headache, fever, abdominal and right shoulder pain, leukopenia, thrombocytopenia, elevation of liver enzymes, seizure | Pos IHC staining; pos RT-PCR; neg IgM; neg IgG; pos viral culture | High dose methylprednisolone and antithymocyte globulin due to concern for rejection | Death on day 26 post-transplant | |||
| Lung | Day 3 | Delirium, leukocytosis, thrombocytopenia, fever, pulmonary infiltrates, abdominal pain, respiratory distress | Pos IHC staining; pos RT-PCR; neg IgM and IgG; pos viral culture | High dose methylprenisolone for concern for rejection | Death on day 23 post-transplant | |||
| 2007 Australia11 |
None identified. Neg RT-PCR (serum, spleen, pancreas); pos IgM and IgG (serum) | IC hemorrhage | Kidney | Not described | Fever, sepsis, encephalopathy, acute tubular necrosis, graft rejection, chest infiltrates | Pos RT-PCR (plasma, CSF, urine, multiple tissues); neg IgM and IgG | None | Death on day 36 post-transplant |
| Liver | Not described | Fever, confusion, encephalopathy, myoclonus, chest infiltrates | Pos RT-PCR (plasma, serum, bronchoalveolar lavage), neg RT-PCR (CSF); pos IgM and IgG | None | Death on day 30 post-transplant | |||
| Kidney | Not described | Fever, graft rejection, encephalopathy | Pos RT-PCR (serum); neg IgM and IgG | None | Death on day 29 post-transplant | |||
| 2008 Massachusetts8 |
No risk factors identified. Pos IgM and IgG (serum) | Seizure, fever | Kidney | 3 weeks | Lethargy, anorexia, fever, shock, hepatic insufficiency, multisystem organ failure | Pos IHC staining; pos PCR; pos virus isolation | Not described | Death on day 28 post-transplant |
| Kidney | 2 weeks | Fever, severe hepatitis, multisystem organ failure | Pos IHC staining; pos PCR (whole blood); pos IgM (serum); virus isolation | IS stopped, IVIG, ribavirin 6 weeks after transplant | Death at 10 weeks post-transplant | |||
| 2011 Arkansas10 |
Possible rodent exposure. Pos RT-PCR (lymph node), neg IgM and IgG (serum) | Diabetic ketoacidosis, possible meningitis | Kidney | 1 week | Fever, nausea, vomiting, diarrhea, severe headache, fever, respiratory failure 3 weeks post-transplant | Pos RT-PCR (BAL); neg IgM and IgG | None | Death on day 30 post-transplant |
| Kidney | Day 2 | Fever, myalgia, severe headache, nausea, vomiting | Pos RT-PCR (CSF); neg IgM and IgG (CSF) | Reduction of IS, IV acyclovir for aseptic meningitis, concern for herpes simplex | Survived | |||
| Liver | Day 20 | Atrial fibrillation, AMS, elevated liver enzymes | Pos IHC staining (liver); pos RT-PCR (liver, serum); pos IgM and neg IgG (serum); virus isolation (serum) | Reduction of IS | Survived | |||
| Lung | Day 10 | Chills, dyspnea, fatigue, abdominal pain, nausea, vomiting, hepatitis | Pos IHC staining; pos RT-PCR (lung, liver) | None | Death on day 20 post-transplant | |||
| 2013 Iowa12 |
No risk factors identified. Pos RT-PCR (aortic endothelial cells) | IC hemorrhage | Cornea | N/A | Asymptomatic | Neg RT-PCR, neg IgM and IgG | None | Survived |
| Liver | Day 18–22 | Fever, abdominal pain, diarrhea, AMS, respiratory compromise | Pos RT-PCR; pos IgM | Reduction of IS, IV ribavirin on day 39, IVIG | Death on day 47 post-transplant | |||
| Kidney | Day 18–22 | Fever, abdominal pain, diarrhea, AMS | Pos RT-PCR; pos IgM | Reduction of IS, oral ribavin on day 38 | Survived, mild memory deficits | |||
| Kidney | Day 18–22 | Fever, abdominal pain, diarrhea, AMS, respiratory compromise | Pos RT-PCR; pos IgM | Reduction of IS, IV ribavin on day 39, IVIG | Survived, memory deficits, allograft failure |
Abbreviations: AMS, altered mental status; BAL, bronchoalveolar lavage; CNS, central nervous system; CSF, cerebrospinal fluid; IC, intracranial; IHC, immunohistochemistry; IS, immunosuppression; LCMV, lymphocytic choriomeningitis virus; MCA, middle cerebral artery; neg, negative; PCR, polymerase chain reaction; pos, positive; RT, reverse-transcriptase
A common donor, who transmitted the infection to multiple recipients, was recognized in each cluster. In the 2005 cluster, the donor had contact with a pet hamster infected with an LCMV strain identical to that detected in the organ recipients9. In other clusters, however, the implicated donor did not have any exposure to rodents identified. Several of the implicated donors died with intracranial hemorrhage and without any symptoms of infection.
The first reported case of LCMV infection in a transplant recipient that was not donor-derived was recently published: a kidney transplant recipient developed LCMV meningoencephalitis complicated by hydrocephalus after exposure to mice excreta17. The patient required ventriculoperitoneal shunt placement but survived to discharge.
LCMV is often under-recognized and under-diagnosed because the clinical characteristics of LCMV meningitis are non-specific. In addition, there is a lack of awareness of the virus among physicians, and the diagnostic assays are not commercially available, making it a difficult diagnosis to establish.
Lassa fever and South American viral hemorrhagic fevers
Lassa fever is mild or asymptomatic in most infected individuals. The initial symptoms of Lassa fever are non-specific and may include fever, headache, malaise, anorexia, and myalgias. Severe illness develops in approximately 20% of cases18,19 and is associated with abnormal bleeding, respiratory distress, hypotension, transaminitis, and encephalopathy, which may progress to multisystem organ failure with shock, coma, and/or death. The overall fatality rate of Lassa fever is 1%, but can be as high as 15–20% among patients who are hospitalized with severe illness18,19. The presentation of South American viral hemorrhagic fevers is similar, but with more frequent hemorrhagic and neurological complications. Approximately 30% of South American viral hemorrhagic fever infections become severe and, among those, the fatality rate is approximately 30%20. There have been no reports of infection with Lassa fever or South American hemorrhagic fever in SOT recipients to date, but cases are likely missed or not reported.
Diagnostic Strategies
The diagnosis of LCMV should be considered in SOT recipients presenting with fever, hepatitis, and/or multisystem organ failure. This clinical presentation in the first four weeks after organ transplantation should raise concern for donor-derived infection, particularly if similar signs and symptoms develop in multiple recipients with a common donor. LCMV should also be considered in the differential diagnosis of aseptic meningitis and encephalitis, whether or not there is a history of exposure to mice or pet rodents.
The laboratory diagnosis of LCMV can be made by the detection of LCMV-specific immunoglobulin (Ig) M and IgG antibodies in cerebrospinal fluid (CSF) and serum samples using immunofluorescence assay (IFA) or ELISA. Convalescent serologies can assist in confirming the diagnosis when LCMV remains a diagnostic consideration and an increase in titers is detected. LCMV can be detected by reverse transcription polymerase chain reaction (RT-PCR) or virus isolation in CSF, serum, and tissue specimens. Immunohistochemical staining of viral antigens in tissue specimens can be helpful in cases of negative serological assays. Serologic assays are available in few commercial laboratories. Serologies and other tests can be performed at state and public health reference laboratories, such as the US Centers for Disease Control and Prevention (CDC). Suspected cases of LCMV should be discussed with local health departments, who can provide guidance on where to submit clinical specimens. The sensitivity and specificity of serologies, PCR, and virus isolation are not known, and the sensitivity of serologies may be reduced in transplant recipients. To improve diagnostic yield, a combination of (1) serologic testing in serum and CSF; (2) PCR of serum, blood, and CSF; and (3) immunohistochemical staining of tissues should be obtained.
More recently, metagenomic deep sequencing has emerged as a potential diagnostic option for determining the etiology of viral encephalitis when initial testing is unrevealing. With this technology, RNA is extracted from CSF, reverse transcribed to single-stranded complementary DNA, and then the sequences are analyzed to identify potential pathogens based on the National Center for Biotechnology Information (NCBI) nucleotide reference database. This approach has been used retrospectively for the diagnosis of donor-derived LCMV11. Key limitations to the metagenomic deep sequencing approach include the need to sequence the human host background, the inadvertent detection of microbial contaminants, and the detection of clinically insignificant microbes (e.g. detection of low level herpesviruses that are not the culprit infection)21. The CSF metagenomics assay is offered for clinical testing at one Clinical Laboratory Improvement Amendments (CLIA)-certified laboratory in the US (at the University of California San Francisco) but has not been approved by the Federal Drug Administration (FDA)22.
Evaluating organ donors for LCMV presents several challenges. There are currently no FDA-approved screening tests for LCMV in organ donors. Further, deceased donors may be asymptomatic at the time of death, and prior reports have documented transmission from donors who had no evidence of infection by PCR or serological assays. With potential organ donors who died with aseptic meningitis or encephalitis of unknown etiology, the risks to transplant recipients should be carefully considered. There is no evidence at present to suggest that screening potential organ donors for LCMV should be undertaken, due to the low incidence of infection and lack of available rapid diagnostic testing with sufficient accuracy. In the clusters of donor-derived LCMV infection described in the literature, donor testing was performed retrospectively, after confirmation of recipient LCMV infection, and this testing was not universally positive, underscoring the limitations of current diagnostics.
Lassa fever and South American hemorrhagic fevers should be considered in travelers to endemic areas with a compatible clinical presentation and potential exposure to rodents or a person with a viral hemorrhagic fever. At present, there are no published reports documenting Lassa fever or South American hemorrhagic fever infections among transplant recipients, including no reported cases of donor-derived transmission of these infections. Any suspected cases of Lassa fever or other viral hemorrhagic fevers should be immediately reported to the local health department. In the US, the health department and the CDC Viral Special Pathogens Branch will decide if testing for Lassa fever or other viral hemorrhagic fevers should be performed. The diagnosis of Lassa fever can be made by the detection of viral antigen and Lassa virus-specific IgM and IgG. Detection of Lassa virus by RT-PCR has become the clinical diagnostic standard; however, false-negative results may occur due to the high degree of genetic diversity of the virus. Viral isolation in cell culture remains the “gold standard” for the diagnosis of Lassa fever but requires biosafety level 4 precautions and results are not available in a clinically useful time frame19.
Diagnosis of LCMV should be considered in transplant recipients presenting with fever, hepatitis, meningoencephalitis, and/or multisystem organ failure. The presence of these signs and symptoms in the first four weeks post-transplant should raise concern for donor-derived infection (strong, low).
If donor-derived infection with LCMV is suspected, clinicians should communicate with the local organ procurement organization (OPO) to (1) facilitate evaluation of the other recipients and (2) help to confirm the diagnosis (strong, low).
Diagnosis of LCMV requires a combination of testing modalities, including detection of LCMV-specific IgM and IgG in CSF and serum; detection of LCMV by RT-PCR or virus isolation in CSF, serum, and tissue; and immunohistochemical staining for viral antigens in tissue (strong, low). To improve diagnostic yield, testing of serum and CSF by serology and PCR should be performed (strong, low).
Screening of potential organ donors for LCMV is not recommended due to the low incidence of infection and limitations of current diagnostics (strong, low).
Treatment
Supportive care with meticulous fluid balance and electrolyte management remains the mainstay of therapy in Arenavirus infection. An effective antiviral agent for Arenaviruses has not been developed.
LCMV
The optimal management of LCMV infection in organ transplant recipients has not been established. Ribavirin possesses in vitro activity against LCMV, but its clinical efficacy remains unclear. In the reported clusters of donor-derived LCMV infections, strategies included reduction of immunosuppression, oral and intravenous ribavirin, and administration of intravenous immunoglobulin (IVIG). The survival rate among patients who were treated with ribavirin was 60% as compared to 19% among patients who did not receive ribavirin. This may have been confounded by the fact that ribavirin was employed more frequently in more recent years, when there was also an increased awareness of LCMV infection and other treatments, such as reduction of immunosuppression and IVIG, were utilized. Thus, although ribavirin is often used for SOT recipients with LCMV infection, it is difficult to know based on current data whether it truly affects mortality. The intravenous formulation of ribavirin is not FDA-approved, but can be obtained through an Emergency Investigational New Drug (EIND) application. Of note, ribavirin can cause significant hemolytic anemia, particularly when used intravenously.
As with other viral infections, reduction in immunosuppression may positively affect outcomes, particularly if done early in the course of illness. There are no available data on the anti-LCMV antibody concentrations in IVIG preparations so its benefit remains unclear.
Favipiravir, a new antiviral that is currently approved to treat influenza in Japan, has been shown to be broadly active against a wide range of RNA viruses including Arenaviruses. A study in mice with acute disseminated LCMV infection showed that treatment with favipiravir resulted in complete protection against mortality and reduction in viral loads23. There are no reports in humans as of yet determining its clinical efficacy.
Lassa fever
Intravenous ribavirin has been shown to reduce mortality from Lassa fever if administered within the first six days of illness24. Intravenous ribavirin is preferred to oral formulations in order to achieve higher serum concentrations. Oral ribavirin may be given if the intravenous formulation is not available, but optimal dosing is not known. Favipiravir successfully treated Lassa virus infection in macaques25, and has been used in combination with ribavirin in the treatment of two patients with Lassa fever26.
In the treatment of Arenavirus infections, immunosuppression should be reduced as much as possible (weak, low).
Intravenous ribavirin is the drug of choice for Lassa fever (strong, low).
The use of ribavirin, orally or intravenously, may be considered in LCMV and other South American hemorrhagic fevers, but its efficacy remains unclear (weak, low).
Prevention
Persons can minimize the risk of LCMV infection from pet rodents by being attentive to adequate hand hygiene and environmental cleaning27. Transplant recipients should avoid exposure to pet rodents. If that is not possible, they should defer cleaning of cages and care of pet rodents to another family member or friend, should observe proper hand hygiene after handling pet rodents, and should maintain adequate environmental cleaning. Human-to-human transmission of Lassa fever can occur via direct contact with blood, tissue, or other body fluids of an infected individual. Patients with Lassa fever, and potentially those with South American hemorrhagic fever, should be placed in airborne and contact isolation. Household members should avoid close physical contact with infected persons and their body fluids. Oral ribavirin may be considered for post-exposure prophylaxis of Lassa fever in healthcare workers and close contacts that were exposed to contaminated blood or body fluids. Currently, there are no vaccines for Lassa virus available. A live attenuated vaccine against Junin virus was found to be effective against Junin and Machupo viruses28,29. It is not available in the US. Since it is a live-attenuated vaccine, it is not recommended post-transplant and its efficacy in transplant recipients, if given pre-transplant, is not known.
Transplant recipients should avoid contact with house mice and wild or pet rodents (e.g., mice, hamsters, or guinea pigs). They should defer cleaning of cages and care of the pet to another family member or friend, should observe proper hand hygiene after handling pet rodents, and should maintain adequate environmental cleaning (strong, low).
Donors with suspected or proven Arenavirus infection should not be used for organ transplantation (strong, low).
Research and Future Areas of Investigation
Future research should focus on improved and commercially available diagnostics for Arenavirus infections, including diagnostic tests for the retrospective evaluation of organ donors when concern for donor-transmitted infection exists, development of effective and safe medications, and development of vaccines.
West Nile Virus
Description of Pathogen
WNV is a mosquito borne single-stranded RNA virus that belongs to the Flaviviridae family, which also includes St. Louis Encephalitis (SLEV), Japanese B encephalitis, Dengue, Zika, Yellow Fever, and Murray Valley encephalitis viruses. WNV strains show significant genetic diversity but form two main lineages (Lineage 1 and 2)30. Infected mosquitoes, most commonly of the Culex genera, acquire WNV through feeding on infected birds who serve as the primary amplifying hosts of WNV31. As the summer season progresses, a bird-mosquito enzootic cycle develops with increasing viral amplification and infectivity of “bridge vector” mosquitoes32. The net result is the successful transmission of WNV to incidental hosts, including humans. The incidence and geographic location of WNV varies yearly depending on environmental conditions such as the presence of Culex spp mosquitoes and their ability to grow in number and access bird vectors33.
Epidemiology and Risk Factors
In 1937, the first human case of WNV was reported in Uganda34. Since then, WNV outbreaks have occurred in Africa, Asia, Europe, and the Middle East where the virus is endemic. In 1999, the first outbreak of WNV in the Western hemisphere occurred in New York City35. Since then, WNV has spread westward over the continental US, northward to Canada, and southward to the Caribbean islands and Latin America31,36,37. WNV and SLEV are the only mosquito-borne flaviviridae endemic in the US. WNV has been reported in 48 states and the District of Columbia36,37. The majority of cases occur between July and October in the US, though earlier and later peaks have been noted in warmer states37.
In 2002 and 2003, WNV epidemics in the US and Canada identified non-mosquito borne transmission of WNV through SOT, blood transfusion, percutaneous injury in the laboratory, breast milk, and placental transmission during pregnancy38–44. Between 2002 and 2018, at least 20 cases of donor-derived transmission of WNV were identified (Table 3). In these cases, all of the donors were adults. Most of the implicated donors lived in areas of increased WNV activity and most likely acquired their infection from a mosquito bite. There were two cases in which the donor was likely exposed via blood transfusion44,45. Testing for WNV was performed pre-donation in only one of nine donors45. While donor-derived transmission of WNV has been of major concern, the majority of reports of WNV infection in transplant recipients are related to infected mosquito bites46.
Table 3:
Donor-derived cases of WNV in solid organ transplant recipients
| Year, Location |
Donor
risk factors and testing |
Organ donated |
Onset of
symptoms post-transplant |
Recipient serum testing |
Recipient CSF testing | Treatment | Outcome |
|---|---|---|---|---|---|---|---|
| 2011 US45,96 |
Increased WNV activity in donor region Serum IgM and IgG pos, serum NAT neg, LN PCR pos, spleen PCR pos |
Kidney | Day 10 | NAT pos |
NAT pos, IgM neg | IVIG, IFN α2b | Remained in coma, developed status epilepticus, died on day 23 |
| Kidney | Day 17 | NAT pos, IgM pos | NAT pos, IgM neg | IVIG, IFN α2b, FFP containing WNV IgG |
Resolved, no neurologic deficits | ||
| Bilateral lungs | Day 20 | NAT pos | NAT pos, IgM pos | IVIG, IFN α2b | Complete flaccid paralysis, multiple seizures, died on day 38 | ||
| Liver | Day 18 | NAT neg, IgM neg, IgG pos | NAT pos, IgM neg | Oral ribavirin, IVIG | Resolved, asymptomatic | ||
| 2011 Italy97,98 |
Donor mosquito
bite NAT neg, IgM pos, IgG pos |
Kidney | Day 10 | NAT pos, IgM pos, IgG pos | NAT pos, IgM pos, IgG pos |
WNV IgG-pos FFP | Neuroinvasive disease, remained in coma |
| Kidney | Day 10 | NAT pos, IgM pos, IgG pos | NAT pos, IgM pos, IgG pos |
None | Neuroinvasive disease | ||
| Liver | Asymptomatic | NAT neg, IgM pos, IgG pos | None | None | Asymptomatic infection | ||
| Single Lung | Asymptomatic | NAT pos, IgM pos, IgG pos | None | None | Asymptomatic infection | ||
| Heart | Asymptomatic | NAT neg, IgM neg, IgG neg | None | None | Not infected | ||
| 2010 California89 |
Donor mosquito
bite Serum NAT pos, IgM neg, IgG pos |
Kidney | Day 8 | IgM pos, IgG pos | NAT neg, IgM pos | Supportive care | Neuroinvasive disease, died |
| Kidney | Asymptomatic | NAT pos, IgM pos, IgG pos | Not obtained | None | Asymptomatic | ||
| Liver | Asymptomatic | NAT neg, IgM neg, IgG pos | Not obtained | None | Asymptomatic | ||
| 2009 Italy45,57 |
Donor mosquito bite Serum NAT pos |
Liver | Asymptomatic | NAT pos, IgM pos | Not obtained |
Prophylaxis with WNV IgG+ FFP, Omr-IgG-am | Asymptomatic |
| 2009 (Unpublished CDC data)89 |
Donor mosquito
bite NAT pos, IgM pos, IgG equivocal |
Kidney | Not described | Not described | Not described | Not described | Neuroinvasive disease, resolved |
| Kidney | Asymptomatic | Not described | Not described | Not described | Asymptomatic | ||
| Liver | Asymptomatic | Not described | Not described | Not described | Asymptomatic | ||
| 2009 California48 |
Possible donor
mosquito bite Serum NAT pos, IgM neg |
Liver | Day 15 | NAT neg, IgM pos, IgG neg 3 weeks later: IgG pos |
IgM pos | IVIG | Neuroinvasive disease, resolved |
| 2008 Louisiana44,89 |
Donor blood transfusion Serum NAT neg, IgM neg, IgG neg; one blood donor for organ donor IgM pos |
Heart | Day 8 | IgM pos | IgM pos | Supportive care |
Neuroinvasive disease, survived |
| 2005 New York, Pennsylvania92 |
Probable donor mosquito
bite Serum NAT neg, IgM pos, IgG pos |
Liver | Day 13 | IgM pos | NAT pos, IgM pos |
Omr-IgG-am |
Neuroinvasive disease, coma |
| Lung | Day 17 | Day 19: IgM neg Day 23: IgM pos, IgG pos |
Day 24: NAT neg, IgM neg Day 27: IgM pos, IgG pos |
Omr-IgG-am | Neuroinvasive disease, coma | ||
| Kidney | Asymptomatic | NAT pos, IgM neg, IgG pos | Not obtained |
Prophylactic Omr-IgG-am |
Asymptomatic | ||
| Kidney | Asymptomatic | IgM neg, IgG neg, NAT neg |
Not obtained |
Prophylactic Omr-IgG-am |
Not infected | ||
| 2002 Georgia, Florida33,99,100 |
Donor blood
transfusion Serum NAT pos |
Kidney | Day 13 | Day 22: IgM equivocal,IgG equivocal Day 42: IgM pos |
IgM pos | None | Neuroinvasive disease, survived |
| Kidney | Day 17 | IgM neg, IgG neg | IgM neg, IgG neg | None | Neuroinvasive disease, died | ||
| Heart | Day 8 | NAT pos, IgM pos | IgM pos | None | Neuroinvasive disease, improved | ||
| Liver | Day 7 | IgM pos | Not obtained | None | Resolved |
Abbreviations: d, days; FFP, fresh frozen plasma; IFN, interferon; IVIG, Intravenous immunoglobulin; LN, lymph node; mpNAT, minipool nucleic acid-amplification test; Neg, negative; PCR, polymerase chain reaction; Pos, positive; QOD, every other day; US, United States
Clinical Manifestations
The incubation period for WNV is between 3 and 14 days (mean of 6 days)32. Approximately 80% of immunocompetent individuals remain asymptomatic with WNV infection32. Of those who develop symptoms, the majority develop an acute systemic febrile illness (West Nile fever) that includes fever, myalgias, malaise, nausea, vomiting, diarrhea, and transient rash31,47. Less than 1% of infected immunocompetent individuals develop neuroinvasive disease, which can include meningitis, encephalitis, meningoencephalitis, or a poliomyelitis-like flaccid paralysis31,32. Studies have reported that up to 50% of patients with neuroinvasive disease have residual symptoms at one-year post-infection, including movement disorders, headaches, fatigue, and cognitive complaints32,47.
Groups at higher risk for the development of neuroinvasive disease include older individuals and those that are immunosuppressed, such as SOT recipients32,46,48 and recipients of chemotherapy including rituximab and B-cell depleting agents49,50. Neuroinvasive disease is estimated to occur in approximately 1 in 40 SOT recipients infected with WNV via mosquito bite, though some series have reported rates of neuroinvasive disease as high as 40%46,51. When transmitted via blood or deceased organ donation, the incidence of neuroinvasive disease is significantly higher, ranging between 50 and 75%38,43.
To date, there are 23 reported transplant recipients who have received organs from donors with WNV infection. Twenty of these (87%) became infected with WNV45. The mean incubation period was 13 days (range 7–17 days)45,52. Fourteen of the 20 (70%) recipients developed encephalitis45. In a study of kidney recipients with donor-derived WNV, the 1-year survival rate was 69%; the primary causes of death were encephalitis and meningitis53. Of note, there are no reported cases of WNV transmission via living donor transplantation at present54.
Diagnostic Strategies
The diagnosis of WNV depends on a high index of suspicion and laboratory testing31. The clinician should consider WNV in the differential diagnosis of a patient presenting with fevers, altered mental status, lower extremity paralysis, Parkinsonian cogwheel rigidity, or other neurologic symptoms during the “typical WNV season”, defined as May 1 to November 30 in the US37,55. To assist the clinician in determining WNV activity, local and state health departments and the CDC report cases of WNV infections in mosquitos, birds, and/or humans in specific locations (see Arbonet www.cdc.gov/ncidod/dvbid/westnile/index.htm).
Laboratory studies that can be used for diagnosis include serum and CSF WNV IgM and IgG antibodies and viral nucleic acid testing (NAT). Interpretation of the results is facilitated by review of the timeline of WNV infection (Figure 1)56. In most cases, WNV-infected mosquito bites are followed by an average incubation period of 6 days. After the incubation period, asymptomatic viremia lasting 5–14 days can be identified by serum and CSF WNV NAT testing. Longer periods of viremia may occur, especially in immunocompromised patients57,58. Patients with defective humoral immunity, including transplant recipients or those treated with rituximab, may be unable to produce WNV IgM or IgG antibodies and may have a persistent WNV viremia49,50. Therefore, serum and CSF NAT testing may be the primary means of diagnosing WNV infection in the transplant population53,54.
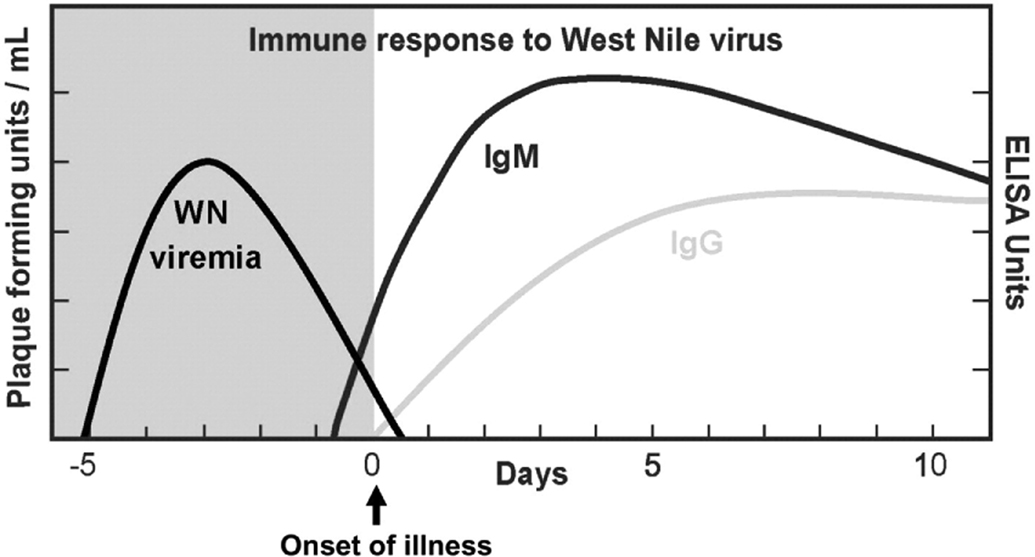
Figure 1. Immune response to WNV.
The phases of WNV viremia, the onset of illness, and the immune response to a WNV infection (Zhang et al.56, copied with permission, © 2009 Elsevier Inc.). There may be variability in the timeline in transplant recipients.
Commonly, decline in WNV viremia is followed by the production of IgM antibodies. IgM is typically produced within 8 days after the initial WNV exposure and an average of 3.9 days after the onset of viremia51,59. Serum WNV IgG is then produced within the following 3.4 days, or 7.7 days from the onset of viremia59,60. Serum IgM may persist for several months or years and thus may not be indicative of acute infection61. If a patient presents to care more than a week into his/her illness, both IgM and IgG antibodies may be present. If a patient presents within one week of symptom onset, the absence of virus-specific IgM does not rule out the diagnosis of WNV infection. Repeating the IgM and IgG serologies (or obtaining acute and convalescent serologies) may therefore be helpful in interpreting the results of the initial testing.
Acute WNV infection is likely present when: (1) there is a positive serum NAT test, regardless of serum IgM and IgG results; or (2) there is a positive serum IgM, regardless of serum NAT or IgG testing. In these cases, the results can be confirmed by neutralizing antibody testing of acute- and convalescent-phase serum specimens at a state public health laboratory or the CDC. Acute WNV infection is unlikely when: (1) Serum NAT, IgM, and IgG are all negative and the patient is more than one week into his/her illness; or (2) only the serum IgG is positive (as this likely reflects past infection).
For the diagnosis of WNV neuroinvasive disease, CSF should be obtained for cell counts with differential, protein, glucose, WNV IgM/IgG, and WNV NAT. Studies of SOT recipients with naturally occurring WNV disease have reported CSF pleocytosis ranging from 5–540 white blood cells with half of cases showing a lymphocytic predominance and the other half demonstrating a neutrophilic predominance. CSF protein levels ranged between 41–142 and the majority of patients had normal CSF glucose levels46,48. Similar CSF profiles have been described in cases of donor-derived WNV neuroinvasive disease45. Neuroinvasive WNV infection is confirmed when: (1) CSF NAT testing is positive (regardless of CSF IgM and IgG results); or (2) CSF WNV IgM is positive, since the IgM antibody does not cross the blood brain barrier (regardless of CSF WNV IgG or NAT results). Presence of only WNV IgG in the CSF suggests either prior disease or may be a false-positive due to cross-reactivity from a distinct etiology.
A major limitation in the interpretation of WNV serologies is the cross-reactivity with other flaviviridae, including SLEV, Japanese Encephalitis, Zika, and Dengue viruses62–64. Further, the Yellow Fever vaccine may result in false-positive serologies for WNV65. To assist in differentiation, the CDC utilizes IgM-ELISA microsphere assays that are specific to the different flaviviridae. For specific confirmation, plaque reduction neutralization testing (PRNT) may be obtained through the CDC66, although results are not likely to be available prior to organ recovery.
More recently, metagenomic deep sequencing has emerged as a potential diagnostic option. This approach has been shown in case reports to be capable of identifying cases of WNV encephalitis among transplant recipients when standard serology and NAT testing were negative22. As described in the Arenaviruses section, this technology is not yet FDA-approved and still has several limitations, including the possibility of inadvertent detection of contaminants.
CT imaging has been reportedly normal with WNV meningoencephalitis67,68. In contrast, diffusion and T2-weighted MRI imaging is often helpful by showing enhancement that is similar to other forms of acute or chronic demyelinating processes. Both symmetric and asymmetric enhancement have been reported in the leptomeninges, brainstem, basal ganglia, thalami, pons, and parietal and frontal lobes68. T2-weighted enhancement of the spinal cord has been reported with acute flaccid paralysis68. These radiographic findings have been observed in over 70% of transplant recipients as compared to only a third of immunocompetent patients52,69. If the initial MRI of the brain is unremarkable but the index of suspicion for WNV is high, a repeat MRI of the brain may be considered after 24–48 hours to evaluate for progression of disease. Electromyelographic studies may show findings of anterior horn cell disease48,68.
The laboratory diagnosis of WNV is made using serum and CSF WNV IgM, IgG, and NAT (strong, high). The diagnosis of acute WNV infection is likely when: (1) serum or CSF WNV NAT is positive (regardless of serology results); or (2) serum or CSF WNV IgM is positive (regardless of NAT or IgG results). Confirmation can be obtained through PRNT offered by the CDC.
Treatment
The primary treatment of WNV is supportive care, including ventilatory support as needed. Temporary reduction in immunosuppression should be considered in order to allow for restoration of natural immunity to WNV. There are no clinical trials to support specific antiviral agents for treatment, though several management strategies have been published in case reports and case series70.
Intravenous immunoglobulin (IVIG)
IVIG containing WNV-specific antibodies is the most commonly employed therapeutic agent45,48,57,58,71. WNV appears to have greater susceptibility to humoral, rather than cell-mediated, immunity49. In a mouse model, WNV infection was lessened or completely aborted in a dose-dependent manner with transfer of passive antibodies72. In case reports and case series in humans, passive transfer of monoclonal or polyclonal virus-specific antibodies have had variable outcomes, ranging from complete recovery to progressive disease and death45,48,57,58,71.
The presence of adequate WNV antibodies in the IVIG product initially required use of high titer WNV-specific immunoglobulin (Omr-IgG-am, OMRIX Biopharmaceuticals, Israel) from the Middle East, where there are areas of high endemicity for WNV59,72, and was granted orphan drug status by the FDA in 200773. However, the seroprevalence of WNV in the US has increased, resulting in the presence of high titer WNV antibodies in US plasma-derived products. though the concentrations may vary by region depending on WNV endemicity71. A small randomized controlled trial of Omr-IgG-am versus standard IVIG failed to show a clinical benefit in adults with symptomatic disease74. Successful use of US-derived IVIG for the treatment of acute WNV infection has been reported in both immunocompetent and immunosuppressed individuals45,48. Doses have varied: 0.4–0.5 g/kg administered every 1–4 days for variable durations43,46,75; 1000 mg/kg/day for 2 days76; 1000 mg/kg followed by 500 mg/kg on two subsequent days72. IVIG has been used alone or in combination with fresh frozen plasma, interferon, or ribavirin in case reports45. Mouse models have suggested that early administration of IVIG, ideally during the time of viremia, may improve the outcome of WNV infection70,77. Further studies are needed to determine the efficacy of IVIG and optimal dosing strategies.
Interferon α−2b
Interferons restrict viral replication by activation of cytotoxic T-cell responses and may restrict WNV neuroinvasion by tightening of the blood-brain barrier78. Animal studies have suggested improved survival with WNV infection when interferon is employed79. In an unblinded clinical trial of 23 patients with WNV neuroinvasive disease, 15 of whom were given interferon α−2b, there was significantly greater improvement in neurological status in those that received the treatment, but these data remain unpublished80. Reports of successful treatment with interferon α−2b at a dose of 3 million units daily for 14 days have been published with full, or nearly full, neurologic recovery among the patients81–84. However, there are other case reports and case series that document no improvement after treatment with interferon α−2b and ultimately death for the described patients due to their WNV infection58,85. In a case series where three SOT recipients with WNV infection were treated with IFN α, one recovered, but two had progressive disease and died45. There is also significant concern that interferon administration to SOT recipients may precipitate organ rejection86. Thus, because of the insufficient evidence of efficacy and the risk for rejection in SOT, its use in transplant recipients has not been formally studied and is not currently recommended.
Ribavirin
Ribavirin has demonstrated in vitro activity against WNV infection87. There is limited clinical experience however. In a case series of SOT recipients with WNV, of which two were treated with ribavirin, the authors reported that one had progressive disease and died, while the other survived with partial neurologic recovery46. In another case series, there was a single transplant recipient with WNV infection treated with ribavirin, who recovered from the disease45. Ribavirin was also administered to 37 patients during a WNV outbreak in Israel in 2000, of which an unspecified number were SOT recipients67. In this report, ribavirin use was associated with an increased risk of death on bivariable analysis, though this may have been due to confounding by indication and was not significant on multivariable analysis67. Taken together, there is not sufficient clinical evidence to suggest efficacy, and ribavirin is not currently recommended for the treatment of WNV infection among SOT recipients.
Treatment of WNV infection includes supportive care, reduction in immunosuppression, and the consideration of IVIG (weak, moderate).
Prevention
Prevention of donor-derived WNV infection
Screening for WNV infection among organ donors has not been extensively studied, and most practices are extrapolated from blood bank screening policies88,89. Blood banks in the US screen year-round for WNV using NAT testing for WNV RNA. Blood donors are tested in minipools. If one or more minipool is positive by NAT, the blood bank begins screening individual samples90,91. In February 2013, the Organ Procurement and Transplantation Network (OPTN) mandated that transplant centers start screening living donors for WNV infection in endemic areas54. Year-round screening has not been adopted due to the seasonality of WNV infections. Rather, it has been recommended that screening of living donors occur (1) when local blood banks switch to individual sample screening, or (2) when bird or mosquito pools are positive as reported by local health departments54. For most US regions, the period between May 1 and November 30 would encompass the time of highest WNV activity each year and would represent a reasonable screening period.
For laboratory screening, living donors should be screened with WNV NAT within 7–14 days of donation since actively viremic individuals (i.e. serum NAT-positive) are most likely to transmit WNV by blood and organ donation. There are currently two FDA-approved donor screening NAT assays utilized by screening laboratories: (1) Procleix transcription mediated amplification (TMA) WNV assay (Gen-Probe, Inc; San Diego, CA, USA); (2) Cobas TaqScreen WNV Test utilizing RT-PCR (Roche Molecular Systems, Inc; Pleasanton, CA, USA).
Though donor screening for WNV is recommended by OPTN for all living donors in endemic areas, there are no formal recommendations for deceased donors and practices vary by OPO. In areas of high endemicity, it is reasonable to consider screening for deceased donors during times of increased WNV activity, though there are several practical limitations. More specifically: (1) Testing requires specialized laboratory facilities that are not logistically available for all OPOs so that NAT results may not be available prior to transplant. (2) WNV NAT testing is performed on large platforms and is not conducive to single donor sampling. Efforts are being made to provide smaller donor sampling testing capabilities by billing only for portions used (RTI Biologics Inc). (3) NAT-negative donors may transmit WNV. Though uncommon, WNV infection was unexpectedly transmitted in 2005 to three of four SOT recipients from a donor who was seropositive for WNV IgM and IgG but had a negative WNV NAT92. In 2008, a donor who was WNV IgM, IgG, and NAT negative had received a WNV IgM positive/NAT negative blood donation and transmitted WNV to the heart transplant recipient42,89 (Table 3). These two episodes suggest that the virus may remain in tissue and red blood cell compartments after the viremia clears93 or that the RNA copy number may have been below the level of detection of the NAT assay. (4) The WNV NAT has a high false-positive rate. In a study of blood donor screening, 47% of the initial positive WNV NAT tests were found to be false-positives (based on nonreactive TMA and PCR results in all additional testing, and negative WNV serology testing on the donation sample and/or follow up samples)90. With a high false-positive rate, routine WNV screening could result in unnecessary organ loss. FDA-approved confirmatory testing is available for blood donor screening for the Procleix TIGRIS platform but is not available for organ donation.
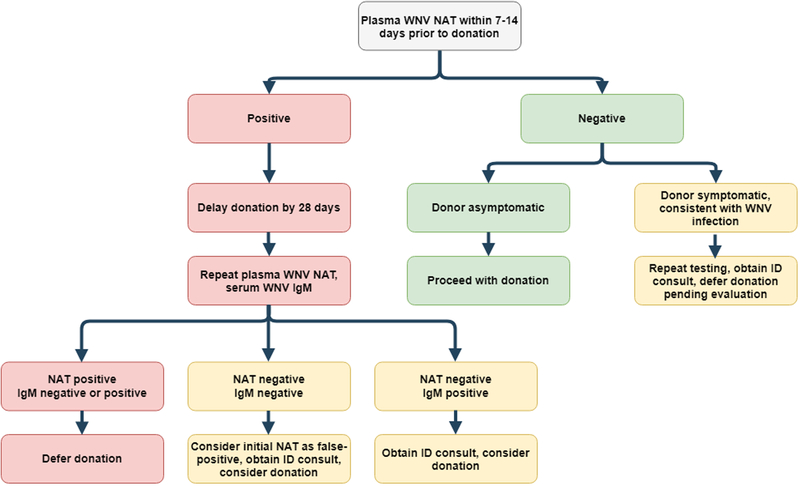
Patients with positive WNV testing are asked to defer blood product donations for 120 days. Though there are no studies evaluating organ donation after WNV, we recommend deferring donation for at least 28 days with live donors, at which point repeat NAT testing as well as IgM testing should be obtained54. If the NAT and IgM are negative, this is consistent with a false-positive NAT test and donation can be considered. If the NAT is negative and IgM is positive, this likely reflects viral clearance and organ donation can be considered. If the NAT remains positive, organ donation should be deferred once again54. (See Figure 2 for a living donor screening algorithm, adapted from Levi et al54). For deceased donors, on whom testing may or may not be performed or available within the needed timeframe, we would recommend: deferring donors with (1) known WNV infection, (2) positive WNV NAT testing, or (3) clinical meningitis, encephalitis, or flaccid paralysis of unknown etiology if from a region with reported WNV activity.
Figure 2. Living donor screening recommendations for WNV.
Adapted from Levi et al54 with permission (© Copyright 2014 The American Society of Transplantation and the American Society of Transplant Surgeons, Wiley Periodicals Inc.). Abbreviations: ID, Infectious Diseases; NAT, Nucleic acid test; WNV, West Nile Virus.
Prevention of WNV infection in the post-transplant population
In the post-transplant period, WNV infection may be prevented by avoiding mosquito bites. In particular, patients should be counseled to use long sleeves and long pants and apply topical insecticides on exposed skin, such as DEET, picardin, oil of lemon eucalyptus, or IR3535 in concentrations between 10–50%. As Culex spp mosquitoes are most active in the evenings, patients should avoid outdoor activities from dusk to dawn if possible.
Serum WNV NAT screening is recommended for living donors in endemic areas during times of increased WNV activity (see Figure 2 for details) (weak, moderate).
Living donors with positive WNV NAT testing should defer donation for at least 28 days, at which point repeat NAT testing as well as IgM testing should be obtained. If the NAT and IgM are negative, this is consistent with a false-positive NAT test and donation can be considered (see Figure 2) (weak, moderate).
There are no established recommendations for screening deceased donors, and practices vary by OPO.
Deceased donors should be deferred if they have known WNV infection; a positive WNV NAT; or clinical meningitis, encephalitis, or flaccid paralysis of unknown etiology (strong, high).
Research and Future Areas of Investigation
Future research is needed in many areas related to WNV infection among SOT recipients. Major issues that remain unresolved include: (1) determinants of neuroinvasive disease among SOT recipients; (2) optimal screening practices for deceased donors in areas that are endemic for WNV; (3) timing of donation with a living donor who has had WNV infection; (4) improving diagnostics to reduce turnaround time and cross-reactivity; and (5) expanding potential therapeutic options.
Acknowledgements
This manuscript was modified from the Guideline included in the 3rd Edition of the AST Infectious Diseases Community of Practice Guidelines written by Neeraj Singh and Marilyn E. Levi published in the American Journal of Transplantation 2013;13 (Suppl 4): 361–371, and endorsed by the American Society of Transplantation.
Conflict of interest:
Dr. Judith Anesi receives grant funding from the National Institutes of Health (grant number K01-AI137317 to J.A.A.) and the Transplant Foundation’s Innovative Research Grant Program, an affiliate of the Gift of Life Donor Program (Donation and Transplantation Grant to J.A.A.). Dr. Fernanda Silveira receives research support from Shire, Whiscon, and Qiagen. None of these conflicts are relevant to this manuscript.
References
- 1.Childs JE, Glass GE, Ksiazek TG, Rossi CA, Oro JG, Leduc JW. Human-rodent contact and infection with lymphocytic choriomeningitis and Seoul viruses in an inner-city population. Am J Trop Med Hyg. 1991;44(2):117–121. [DOI] [PubMed] [Google Scholar]
- 2.Park JY, Peters CJ, Rollin PE, et al. Age distribution of lymphocytic choriomeningitis virus serum antibody in Birmingham, Alabama: evidence of a decreased risk of infection. Am J Trop Med Hyg. 1997;57(1):37–41. [DOI] [PubMed] [Google Scholar]
- 3.Kerneis S, Koivogui L, Magassouba N, et al. Prevalence and risk factors of Lassa seropositivity in inhabitants of the forest region of Guinea: a cross-sectional study. PLoS Negl Trop Dis. 2009;3(11):e548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sogoba N, Rosenke K, Adjemian J, et al. Lassa Virus Seroprevalence in Sibirilia Commune, Bougouni District, Southern Mali. Emerg Infect Dis. 2016;22(4):657–663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tobin E, Asogun D, Akpede N, Adomeh D, Odia I, Gunther S. Lassa fever in Nigeria: Insights into seroprevalence and risk factors in rural Edo State: A pilot study. Journal of Medicine in the Tropics. 2015;17(2):51–55. [Google Scholar]
- 6.Weissenbacher MC, Sabattini MS, Avila MM, et al. Junin virus activity in two rural populations of the Argentine hemorrhagic fever (AHF) endemic area. J Med Virol. 1983;12(4):273–280. [DOI] [PubMed] [Google Scholar]
- 7.Ter Meulen J, Lukashevich I, Sidibe K, et al. Hunting of peridomestic rodents and consumption of their meat as possible risk factors for rodent-to-human transmission of Lassa virus in the Republic of Guinea. Am J Trop Med Hyg. 1996;55(6):661–666. [DOI] [PubMed] [Google Scholar]
- 8.Brief report: Lymphocytic choriomeningitis virus transmitted through solid organ transplantation--Massachusetts, 2008. MMWR Morb Mortal Wkly Rep. 2008;57(29):799–801. [PubMed] [Google Scholar]
- 9.Fischer SA, Graham MB, Kuehnert MJ, et al. Transmission of lymphocytic choriomeningitis virus by organ transplantation. N Engl J Med. 2006;354(21):2235–2249. [DOI] [PubMed] [Google Scholar]
- 10.Macneil A, Stroher U, Farnon E, et al. Solid organ transplant-associated lymphocytic choriomeningitis, United States, 2011. Emerg Infect Dis. 2012;18(8):1256–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Palacios G, Druce J, Du L, et al. A new arenavirus in a cluster of fatal transplant-associated diseases. N Engl J Med. 2008;358(10):991–998. [DOI] [PubMed] [Google Scholar]
- 12.Schafer IJ, Miller R, Stroher U, Knust B, Nichol ST, Rollin PE. Notes from the field: a cluster of lymphocytic choriomeningitis virus infections transmitted through organ transplantation - Iowa, 2013. MMWR Morb Mortal Wkly Rep. 2014;63(11):249. [PMC free article] [PubMed] [Google Scholar]
- 13.Baum SG, Lewis AM Jr., Rowe WP, Huebner RJ. Epidemic nonmeningitic lymphocytic-choriomeningitis-virus infection. An outbreak in a population of laboratory personnel. N Engl J Med. 1966;274(17):934–936. [DOI] [PubMed] [Google Scholar]
- 14.Deibel R, Woodall JP, Decher WJ, Schryver GD. Lymphocytic choriomeningitis virus in man. Serologic evidence of association with pet hamsters. JAMA. 1975;232(5):501–504. [PubMed] [Google Scholar]
- 15.Knust B, Stroher U, Edison L, et al. Lymphocytic choriomeningitis virus in employees and mice at multipremises feeder-rodent operation, United States, 2012. Emerg Infect Dis. 2014;20(2):240–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bonthius DJ. Lymphocytic choriomeningitis virus: an underrecognized cause of neurologic disease in the fetus, child, and adult. Semin Pediatr Neurol. 2012;19(3):89–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanveer F, Younas M, Fishbain J. Lymphocytic choriomeningitis virus meningoencephalitis in a renal transplant recipient following exposure to mice. Transpl Infect Dis. 2018;20(6):e13013. [DOI] [PubMed] [Google Scholar]
- 18.Houlihan C, Behrens R. Lassa fever. BMJ. 2017;358:j2986. [DOI] [PubMed] [Google Scholar]
- 19.Raabe V, Koehler J. Laboratory Diagnosis of Lassa Fever. J Clin Microbiol. 2017;55(6):1629–1637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Enria DA, Pinheiro F. Rodent-borne emerging viral zoonosis. Hemorrhagic fevers and hantavirus infections in South America. Infect Dis Clin North Am. 2000;14(1):167–184, x. [DOI] [PubMed] [Google Scholar]
- 21.Gu W, Miller S, Chiu CY. Clinical Metagenomic Next-Generation Sequencing for Pathogen Detection. Annu Rev Pathol. 2019;14:319–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson MR, Zimmermann LL, Crawford ED, et al. Acute West Nile Virus Meningoencephalitis Diagnosed Via Metagenomic Deep Sequencing of Cerebrospinal Fluid in a Renal Transplant Patient. Am J Transplant. 2017;17(3):803–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hickerson BT, Westover JB, Jung KH, Komeno T, Furuta Y, Gowen BB. Effective Treatment of Experimental Lymphocytic Choriomeningitis Virus Infection: Consideration of Favipiravir for use with Infected Organ Transplant Recipients. J Infect Dis. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bausch DG, Rollin PE, Demby AH, et al. Diagnosis and clinical virology of Lassa fever as evaluated by enzyme-linked immunosorbent assay, indirect fluorescent-antibody test, and virus isolation. J Clin Microbiol. 2000;38(7):2670–2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenke K, Feldmann H, Westover JB, et al. Use of Favipiravir to Treat Lassa Virus Infection in Macaques. Emerg Infect Dis. 2018;24(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raabe VN, Kann G, Ribner BS, et al. Favipiravir and Ribavirin Treatment of Epidemiologically Linked Cases of Lassa Fever. Clin Infect Dis. 2017;65(5):855–859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Martins AF, Kuchenbecker RS, Pilger KO, Pagano M, Barth AL, Force C-PST High endemic levels of multidrug-resistant Acinetobacter baumannii among hospitals in southern Brazil. Am J Infect Control. 2012;40(2):108–112. [DOI] [PubMed] [Google Scholar]
- 28.Maiztegui JI, McKee KT Jr., Barrera Oro JG, et al. Protective efficacy of a live attenuated vaccine against Argentine hemorrhagic fever. AHF Study Group. J Infect Dis. 1998;177(2):277–283. [DOI] [PubMed] [Google Scholar]
- 29.Peters CJ, Jahrling PB, Liu CT, Kenyon RH, McKee KT Jr., Barrera Oro JG. Experimental studies of arenaviral hemorrhagic fevers. Curr Top Microbiol Immunol. 1987;134:5–68. [DOI] [PubMed] [Google Scholar]
- 30.Bakonyi T, Ivanics E, Erdelyi K, et al. Lineage 1 and 2 strains of encephalitic West Nile virus, central Europe. Emerg Infect Dis. 2006;12(4):618–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dauphin G, Zientara S, Zeller H, Murgue B. West Nile: worldwide current situation in animals and humans. Comp Immunol Microbiol Infect Dis. 2004;27(5):343–355. [DOI] [PubMed] [Google Scholar]
- 32.Petersen LR, Marfin AA. West Nile virus: a primer for the clinician. Ann Intern Med. 2002;137(3):173–179. [DOI] [PubMed] [Google Scholar]
- 33.Brubaker SA, Robert Rigney P. West Nile Virus workshop: scientific considerations for tissue donors. Cell Tissue Bank. 2012;13(3):499–511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smithburn KC, Hughes TP, Burke AW, Paul JH. A neurotropic virus isolated from the blood of a native of Uganda. American Journal of Tropical Medicine. 1940;20:471–492. [Google Scholar]
- 35.Nash D, Mostashari F, Fine A, et al. The outbreak of West Nile virus infection in the New York City area in 1999. N Engl J Med. 2001;344(24):1807–1814. [DOI] [PubMed] [Google Scholar]
- 36.Goetz AM, Goldrick BA. West Nile virus: a primer for infection control professionals. Am J Infect Control. 2004;32(2):101–105. [DOI] [PubMed] [Google Scholar]
- 37.Centers for Disease Control and Prevention CDC. West Nile virus disease cases reported to CDC by state of residence, 1999–2017. https://www.cdc.gov/westnile/statsmaps/cumMapsData.html.
- 38.Pealer LN, Marfin AA, Petersen LR, et al. Transmission of West Nile virus through blood transfusion in the United States in 2002. N Engl J Med. 2003;349(13):1236–1245. [DOI] [PubMed] [Google Scholar]
- 39.Centers for Disease C, Prevention. Possible West Nile virus transmission to an infant through breast-feeding--Michigan, 2002. MMWR Morb Mortal Wkly Rep. 2002;51(39):877–878. [PubMed] [Google Scholar]
- 40.Centers for Disease C, Prevention. Interim guidelines for the evaluation of infants born to mothers infected with West Nile virus during pregnancy. MMWR Morb Mortal Wkly Rep. 2004;53(7):154–157. [PubMed] [Google Scholar]
- 41.From the Centers for Disease Control and Prevention. Laboratory-acquired West Nile virus infections--United States, 2002. JAMA. 2003;289(4):414–415. [PubMed] [Google Scholar]
- 42.Fonseca K, Prince GD, Bratvold J, et al. West Nile virus infection and conjunctival exposure. Emerg Infect Dis. 2005;11(10):1648–1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Iwamoto M, Jernigan DB, Guasch A, et al. Transmission of West Nile virus from an organ donor to four transplant recipients. N Engl J Med. 2003;348(22):2196–2203. [DOI] [PubMed] [Google Scholar]
- 44.Centers for Disease C, Prevention. West Nile virus transmission via organ transplantation and blood transfusion - Louisiana, 2008. MMWR Morb Mortal Wkly Rep. 2009;58(45):1263–1267. [PubMed] [Google Scholar]
- 45.Winston DJ, Vikram HR, Rabe IB, et al. Donor-derived West Nile virus infection in solid organ transplant recipients: report of four additional cases and review of clinical, diagnostic, and therapeutic features. Transplantation. 2014;97(9):881–889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kumar D, Prasad GV, Zaltzman J, Levy GA, Humar A. Community-acquired West Nile virus infection in solid-organ transplant recipients. Transplantation. 2004;77(3):399–402. [DOI] [PubMed] [Google Scholar]
- 47.Sejvar JJ. Clinical manifestations and outcomes of West Nile virus infection. Viruses. 2014;6(2):606–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rhee C, Eaton EF, Concepcion W, Blackburn BG. West Nile virus encephalitis acquired via liver transplantation and clinical response to intravenous immunoglobulin: case report and review of the literature. Transpl Infect Dis. 2011;13(3):312–317. [DOI] [PubMed] [Google Scholar]
- 49.Levi ME, Quan D, Ho JT, Kleinschmidt-Demasters BK, Tyler KL, Grazia TJ. Impact of rituximab-associated B-cell defects on West Nile virus meningoencephalitis in solid organ transplant recipients. Clin Transplant. 2010;24(2):223–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mawhorter SD, Sierk A, Staugaitis SM, et al. Fatal West Nile Virus infection after rituximab/fludarabine--induced remission for non-Hodgkin’s lymphoma. Clin Lymphoma Myeloma. 2005;6(3):248–250. [DOI] [PubMed] [Google Scholar]
- 51.Kumar D, Drebot MA, Wong SJ, et al. A seroprevalence study of west nile virus infection in solid organ transplant recipients. Am J Transplant. 2004;4(11):1883–1888. [DOI] [PubMed] [Google Scholar]
- 52.Centers for Disease C, Prevention. Update: Investigations of West Nile virus infections in recipients of organ transplantation and blood transfusion. MMWR Morb Mortal Wkly Rep. 2002;51(37):833–836. [PubMed] [Google Scholar]
- 53.Shingde R, Habachou LI, Calisa V, et al. Unexpected donor-derived infectious transmissions by kidney transplantation: A systematic review. Transpl Infect Dis. 2018;20(2):e12851. [DOI] [PubMed] [Google Scholar]
- 54.Levi ME, Kumar D, Green M, et al. Considerations for screening live kidney donors for endemic infections: a viewpoint on the UNOS policy. Am J Transplant. 2014;14(5):1003–1011. [DOI] [PubMed] [Google Scholar]
- 55.Freifeld AG, Meza J, Schweitzer B, Shafer L, Kalil AC, Sambol AR. Seroprevalence of West Nile virus infection in solid organ transplant recipients. Transpl Infect Dis. 2010;12(2):120–126. [DOI] [PubMed] [Google Scholar]
- 56.Zhang W, Wu J, Li Y, Li F, Njoo H. Rapid and accurate in vitro assays for detection of West Nile virus in blood and tissues. Transfus Med Rev. 2009;23(2):146–154. [DOI] [PubMed] [Google Scholar]
- 57.Morelli MC, Sambri V, Grazi GL, et al. Absence of neuroinvasive disease in a liver transplant recipient who acquired West Nile virus (WNV) infection from the organ donor and who received WNV antibodies prophylactically. Clin Infect Dis. 2010;51(4):e34–37. [DOI] [PubMed] [Google Scholar]
- 58.Penn RG, Guarner J, Sejvar JJ, et al. Persistent neuroinvasive West Nile virus infection in an immunocompromised patient. Clin Infect Dis. 2006;42(5):680–683. [DOI] [PubMed] [Google Scholar]
- 59.Busch MP, Kleinman SH, Tobler LH, et al. Virus and antibody dynamics in acute west nile virus infection. J Infect Dis. 2008;198(7):984–993. [DOI] [PubMed] [Google Scholar]
- 60.Hazell SL. Serological diagnosis of West Nile virus. MLO Med Lab Obs. 2004;36(6):10–12, 16; quiz 18–19. [PubMed] [Google Scholar]
- 61.Papa A, Anastasiadou A, Delianidou M. West Nile virus IgM and IgG antibodies three years post- infection. Hippokratia. 2015;19(1):34–36. [PMC free article] [PubMed] [Google Scholar]
- 62.Papa A, Karabaxoglou D, Kansouzidou A. Acute West Nile virus neuroinvasive infections: cross-reactivity with dengue virus and tick-borne encephalitis virus. J Med Virol. 2011;83(10):1861–1865. [DOI] [PubMed] [Google Scholar]
- 63.Hartmann CA, Vikram HR, Seville MT, et al. Neuroinvasive St. Louis Encephalitis Virus Infection in Solid Organ Transplant Recipients. Am J Transplant. 2017;17(8):2200–2206. [DOI] [PubMed] [Google Scholar]
- 64.Rabe IB, Staples JE, Villanueva J, et al. Interim Guidance for Interpretation of Zika Virus Antibody Test Results. MMWR Morb Mortal Wkly Rep. 2016;65(21):543–546. [DOI] [PubMed] [Google Scholar]
- 65.Kayser M, Klein H, Paasch I, Pilaski J, Blenk H, Heeg K. Human antibody response to immunization with 17D yellow fever and inactivated TBE vaccine. J Med Virol. 1985;17(1):35–45. [DOI] [PubMed] [Google Scholar]
- 66.Centers for Disease Control and Prevention CDC. WNV Antibody Testing. https://www.cdc.gov/westnile/healthcareproviders/healthCareProviders-Diagnostic.html.
- 67.Chowers MY, Lang R, Nassar F, et al. Clinical characteristics of the West Nile fever outbreak, Israel, 2000. Emerg Infect Dis. 2001;7(4):675–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Sejvar JJ, Haddad MB, Tierney BC, et al. Neurologic manifestations and outcome of West Nile virus infection. JAMA. 2003;290(4):511–515. [DOI] [PubMed] [Google Scholar]
- 69.Kleinschmidt-DeMasters BK, Marder BA, Levi ME, et al. Naturally acquired West Nile virus encephalomyelitis in transplant recipients: clinical, laboratory, diagnostic, and neuropathological features. Arch Neurol. 2004;61(8):1210–1220. [DOI] [PubMed] [Google Scholar]
- 70.Roehrig JT, Staudinger LA, Hunt AR, Mathews JH, Blair CD. Antibody prophylaxis and therapy for flavivirus encephalitis infections. Ann N Y Acad Sci. 2001;951:286–297. [DOI] [PubMed] [Google Scholar]
- 71.Planitzer CB, Modrof J, Kreil TR. West Nile virus neutralization by US plasma-derived immunoglobulin products. J Infect Dis. 2007;196(3):435–440. [DOI] [PubMed] [Google Scholar]
- 72.Saquib R, Randall H, Chandrakantan A, Spak CW, Barri YM. West Nile virus encephalitis in a renal transplant recipient: the role of intravenous immunoglobulin. Am J Kidney Dis. 2008;52(5):e19–21. [DOI] [PubMed] [Google Scholar]
- 73.Shimoni Z, Niven MJ, Pitlick S, Bulvik S. Treatment of West Nile virus encephalitis with intravenous immunoglobulin. Emerg Infect Dis. 2001;7(4):759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hart J Jr., Tillman G, Kraut MA, et al. West Nile virus neuroinvasive disease: neurological manifestations and prospective longitudinal outcomes. BMC Infect Dis. 2014;14:248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Yango AF, Fischbach BV, Levy M, et al. West Nile virus infection in kidney and pancreas transplant recipients in the Dallas-Fort Worth Metroplex during the 2012 Texas epidemic. Transplantation. 2014;97(9):953–957. [DOI] [PubMed] [Google Scholar]
- 76.Li J, Loeb JA, Shy ME, et al. Asymmetric flaccid paralysis: a neuromuscular presentation of West Nile virus infection. Ann Neurol. 2003;53(6):703–710. [DOI] [PubMed] [Google Scholar]
- 77.Ben-Nathan D, Lustig S, Tam G, Robinzon S, Segal S, Rager-Zisman B. Prophylactic and therapeutic efficacy of human intravenous immunoglobulin in treating West Nile virus infection in mice. J Infect Dis. 2003;188(1):5–12. [DOI] [PubMed] [Google Scholar]
- 78.Lazear HM, Daniels BP, Pinto AK, et al. Interferon-lambda restricts West Nile virus neuroinvasion by tightening the blood-brain barrier. Sci Transl Med. 2015;7(284):284ra259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Samuel MA, Diamond MS. Alpha/beta interferon protects against lethal West Nile virus infection by restricting cellular tropism and enhancing neuronal survival. J Virol. 2005;79(21):13350–13361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Wehbeh W Treatment of West Nile Virus Central Nervous System Infections with Interferon Alpha-2b. Paper presented at: 44th ICAAC meeting of the American Society for Microbiology2004; Washington, DC. [Google Scholar]
- 81.Hwang J, Ryu HS, Kim H, Lee SA. The first reported case of West Nile encephalitis in Korea. J Korean Med Sci. 2015;30(3):343–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Kalil AC, Devetten MP, Singh S, et al. Use of interferon-alpha in patients with West Nile encephalitis: report of 2 cases. Clin Infect Dis. 2005;40(5):764–766. [DOI] [PubMed] [Google Scholar]
- 83.Lewis M, Amsden JR. Successful treatment of West Nile virus infection after approximately 3 weeks into the disease course. Pharmacotherapy. 2007;27(3):455–458. [DOI] [PubMed] [Google Scholar]
- 84.Sayao AL, Suchowersky O, Al-Khathaami A, et al. Calgary experience with West Nile virus neurological syndrome during the late summer of 2003. Can J Neurol Sci. 2004;31(2):194–203. [DOI] [PubMed] [Google Scholar]
- 85.Chan-Tack KM, Forrest G. Failure of interferon alpha-2b in a patient with West Nile virus meningoencephalitis and acute flaccid paralysis. Scand J Infect Dis. 2005;37(11–12):944–946. [DOI] [PubMed] [Google Scholar]
- 86.Stravitz RT, Shiffman ML, Sanyal AJ, et al. Effects of interferon treatment on liver histology and allograft rejection in patients with recurrent hepatitis C following liver transplantation. Liver Transpl. 2004;10(7):850–858. [DOI] [PubMed] [Google Scholar]
- 87.Jordan I, Briese T, Fischer N, Lau JY, Lipkin WI. Ribavirin inhibits West Nile virus replication and cytopathic effect in neural cells. J Infect Dis. 2000;182(4):1214–1217. [DOI] [PubMed] [Google Scholar]
- 88.Rosen A, Ison MG. Screening of living organ donors for endemic infections: Understanding the challenges and benefits of enhanced screening. Transpl Infect Dis. 2016. [DOI] [PubMed] [Google Scholar]
- 89.Nett RJ, Kuehnert MJ, Ison MG, Orlowski JP, Fischer M, Staples JE. Current practices and evaluation of screening solid organ donors for West Nile virus. Transpl Infect Dis. 2012;14(3):268–277. [DOI] [PubMed] [Google Scholar]
- 90.Orton SL, Stramer SL, Dodd RY. Self-reported symptoms associated with West Nile virus infection in RNA-positive blood donors. Transfusion. 2006;46(2):272–277. [DOI] [PubMed] [Google Scholar]
- 91.Busch MP, Caglioti S, Robertson EF, et al. Screening the blood supply for West Nile virus RNA by nucleic acid amplification testing. N Engl J Med. 2005;353(5):460–467. [DOI] [PubMed] [Google Scholar]
- 92.Centers for Disease C, Prevention. West Nile virus infections in organ transplant recipients--New York and Pennsylvania, August-September, 2005. MMWR Morb Mortal Wkly Rep. 2005;54(40):1021–1023. [PubMed] [Google Scholar]
- 93.Lanteri MC, Lee TH, Wen L, et al. West Nile virus nucleic acid persistence in whole blood months after clearance in plasma: implication for transfusion and transplantation safety. Transfusion. 2014;54(12):3232–3241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Centers for Disease C, Prevention. Lymphocytic choriomeningitis virus infection in organ transplant recipients--Massachusetts, Rhode Island, 2005. MMWR Morb Mortal Wkly Rep. 2005;54(21):537–539. [PubMed] [Google Scholar]
- 95.Amman BR, Pavlin BI, Albarino CG, et al. Pet rodents and fatal lymphocytic choriomeningitis in transplant patients. Emerg Infect Dis. 2007;13(5):719–725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Blau DM, Rabe IB, Bhatnagar J, et al. West Nile virus RNA in tissues from donor associated with transmission to organ transplant recipients. Emerg Infect Dis. 2013;19(9):1518–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Inojosa WO, Scotton PG, Fuser R, et al. West Nile virus transmission through organ transplantation in north-eastern Italy: a case report and implications for pre-procurement screening. Infection. 2012;40(5):557–562. [DOI] [PubMed] [Google Scholar]
- 98.Costa AN, Capobianchi MR, Ippolito G, et al. West Nile virus: the Italian national transplant network reaction to an alert in the north-eastern region, Italy 2011. Euro Surveill. 2011;16(41). [PubMed] [Google Scholar]
- 99.Centers for Disease C, Prevention. West Nile virus infection in organ donor and transplant recipients--Georgia and Florida, 2002. MMWR Morb Mortal Wkly Rep. 2002;51(35):790. [PubMed] [Google Scholar]
- 100.From the Centers for Disease Control and Prevention. West Nile virus infection in organ donor and transplant recipients--Georgia and Florida, 2002. JAMA. 2002;288(12):1465–1466. [PubMed] [Google Scholar]